Silk microspheres for encapsulation and controlled release
a microsphere and fibroblast technology, applied in the field of encapsulation and controlled release of microspheres, can solve the problems of reducing the usefulness of encapsulation vehicles for many of the smaller drug molecules, affecting the biological activity of incorporated protein drugs in the polymer matrix, and affecting the biological activity of incorporated protein drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
Materials
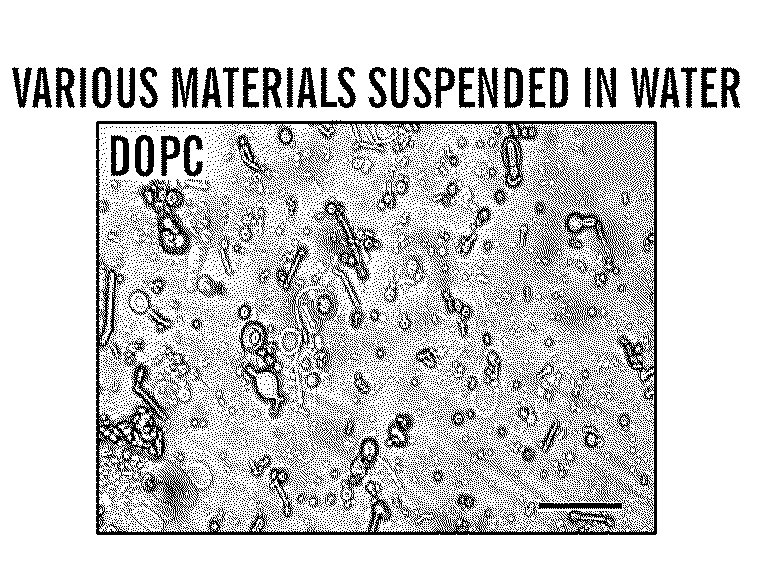
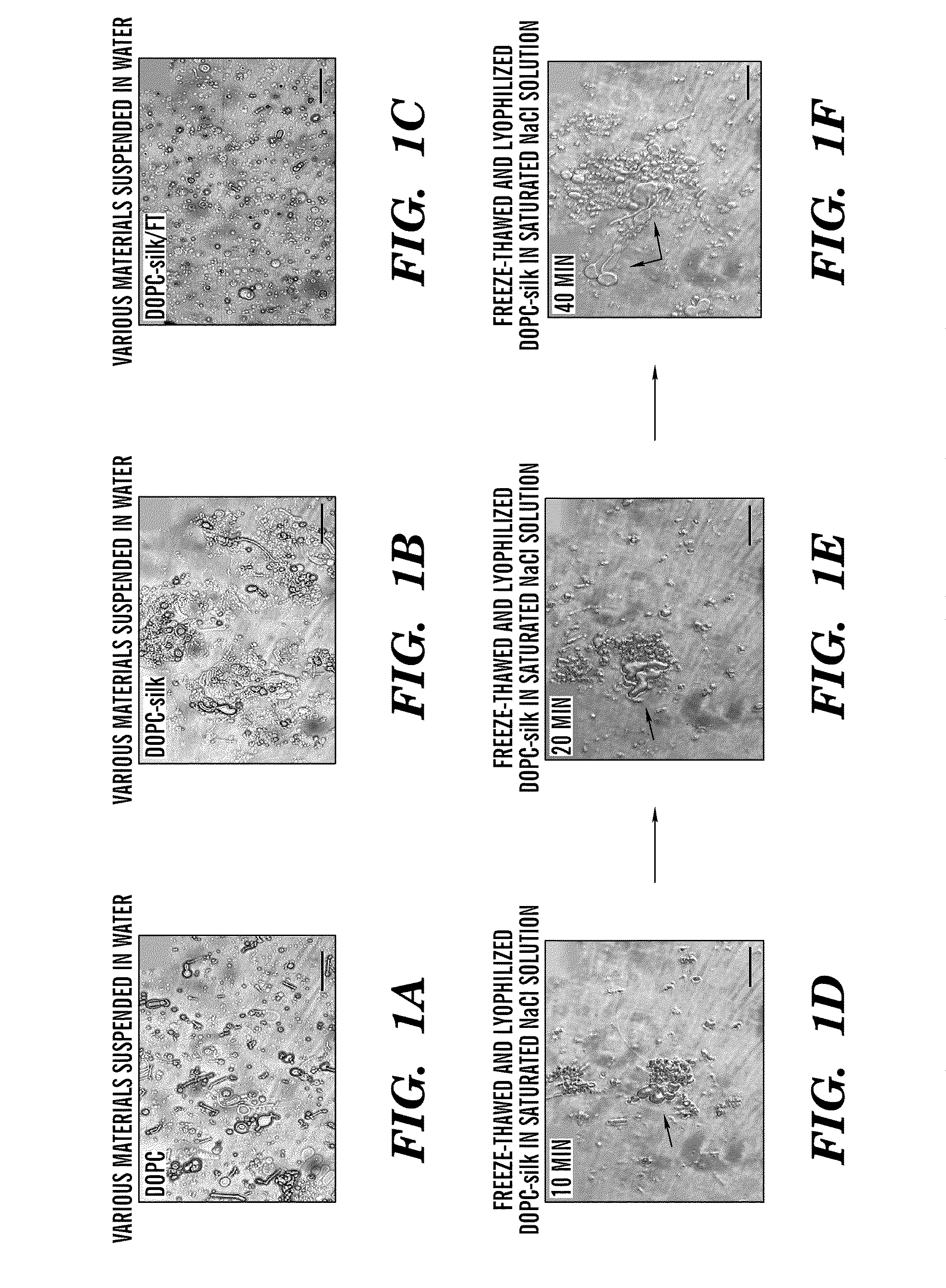
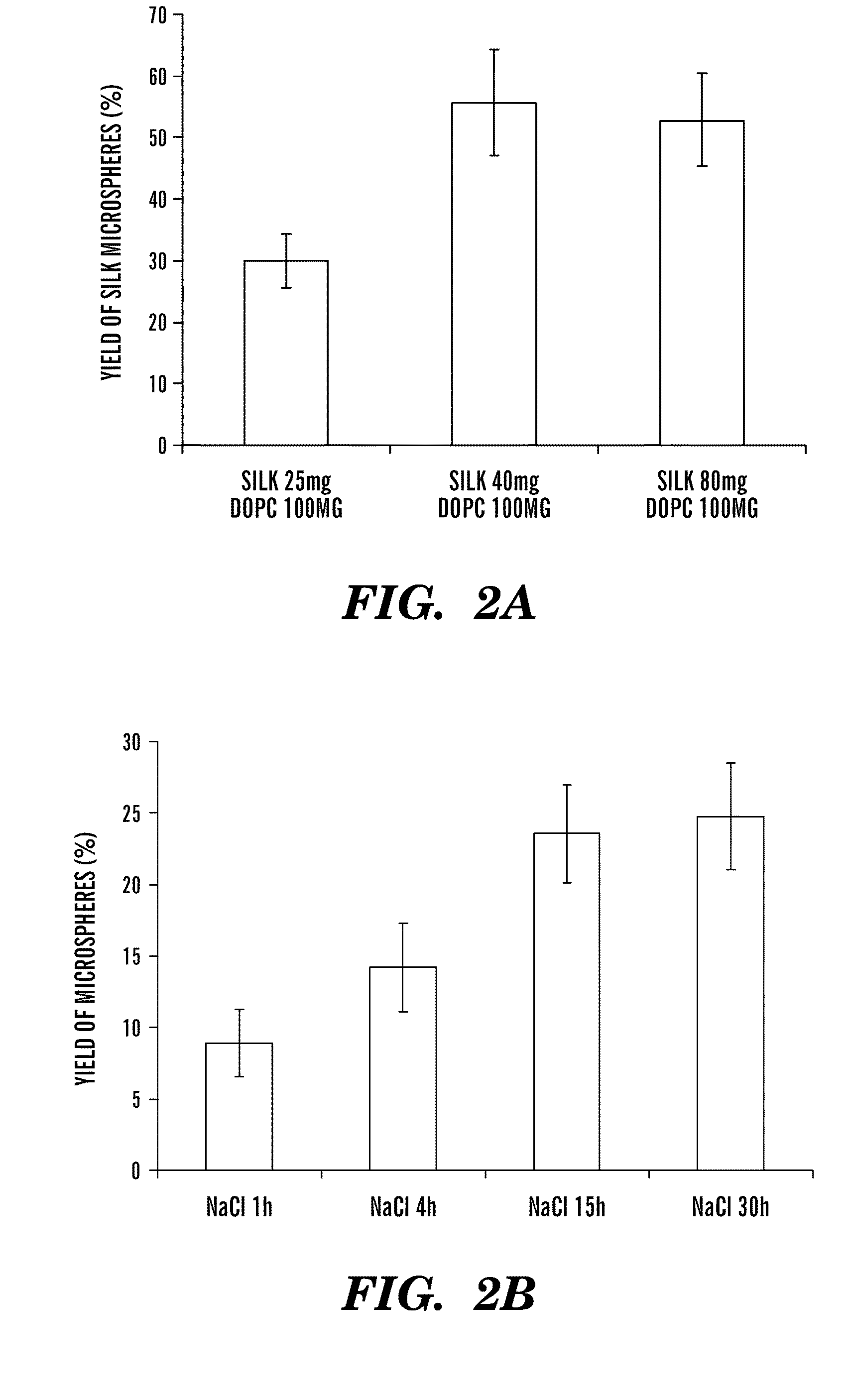
[0063]Cocoons of B. mori silkworm silk were supplied by M. Tsukada (Institute of Sericulture, Tsukuba, Japan). 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein) (fluorescein-DOPE) were purchased from Avanti Polar Lipids (Alabaster, Ala.). 5-(Aminoacetamido)fluorescein (fluoresceinyl glycine amide) was purchased from Molecular Probes (Carlsbad, Calif.). Rhodamine β isothiocyanate-Dextran (M.W. 40,000 Da), horseradish peroxidase (HRP), β-galactosidase, and other chemicals were obtained from Sigma Aldrich (St. Louis, Mo.). 3,3′5,5′ Tetramethylbenzidine (TMB) solution was purchased from BioFX laboratories (Owing Mills, Md.). 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and hydroxylamine hydrochloride were purchased from Pierce Biotechnology (Rockford, Ill.). All other chemicals were purchased from Sigma-Aldrich (St. Louis, Mo.).
Purification and Fluorescent La...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com