Patents
Literature
1889 results about "Dextran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
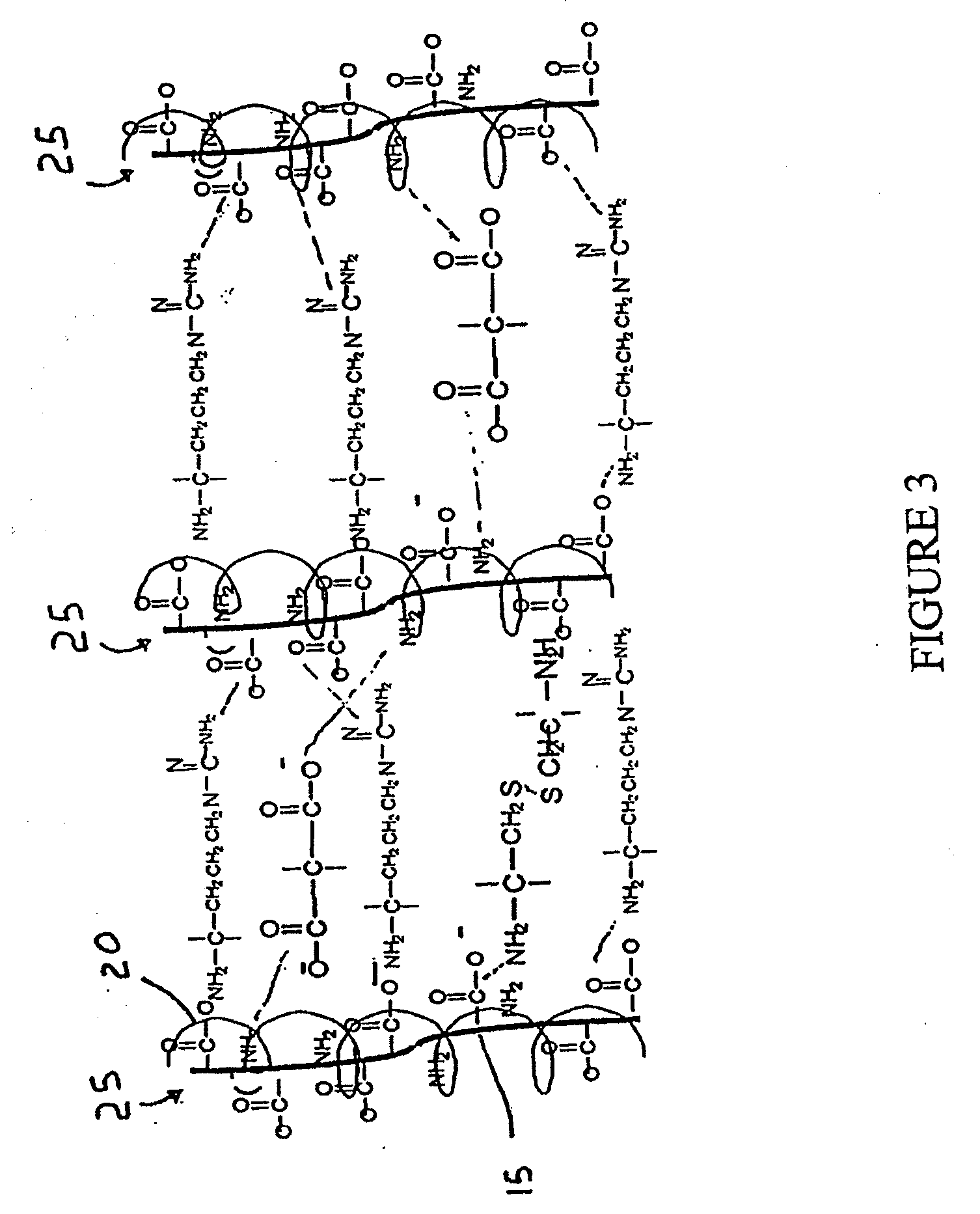
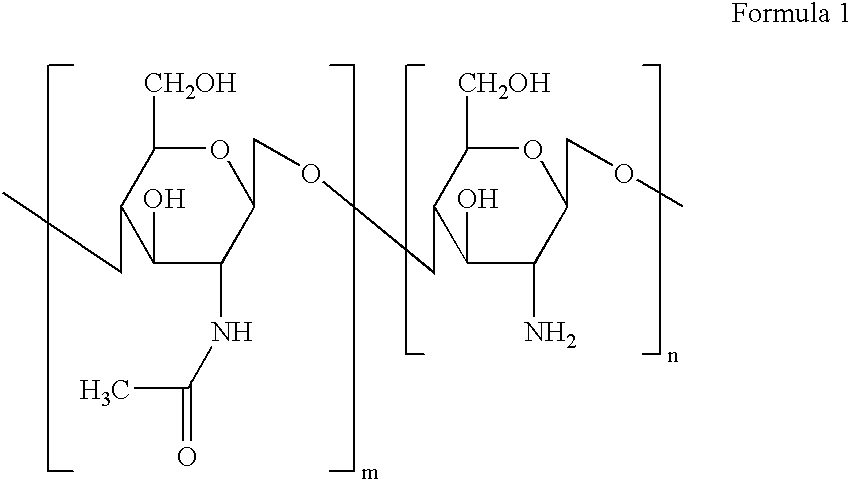
Dextran is a complex branched glucan (polysaccharide derived from the condensation of glucose). IUPAC defines dextrans as "Branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 → C-6". Dextran chains are of varying lengths (from 3 to 2000 kilodaltons).
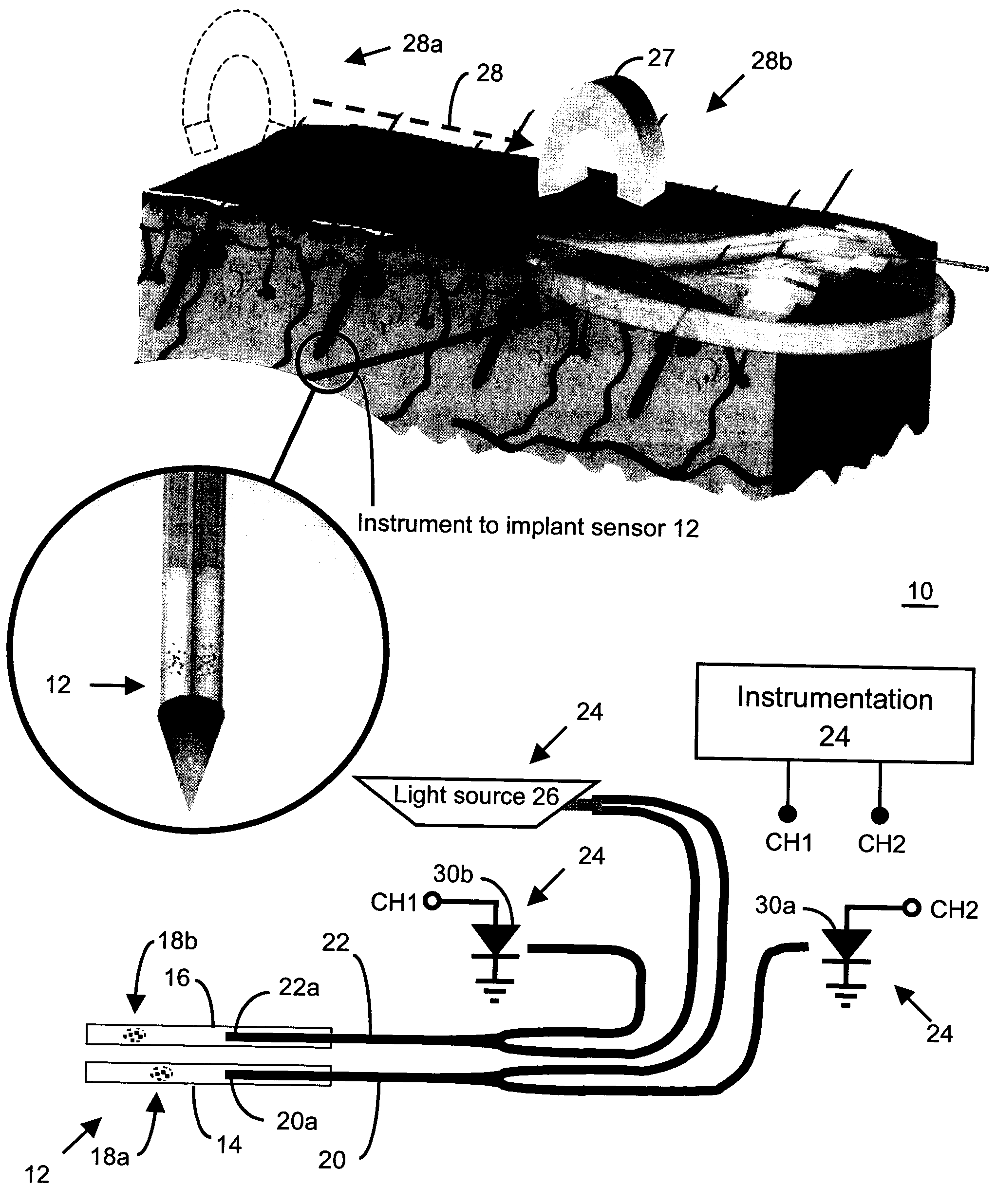
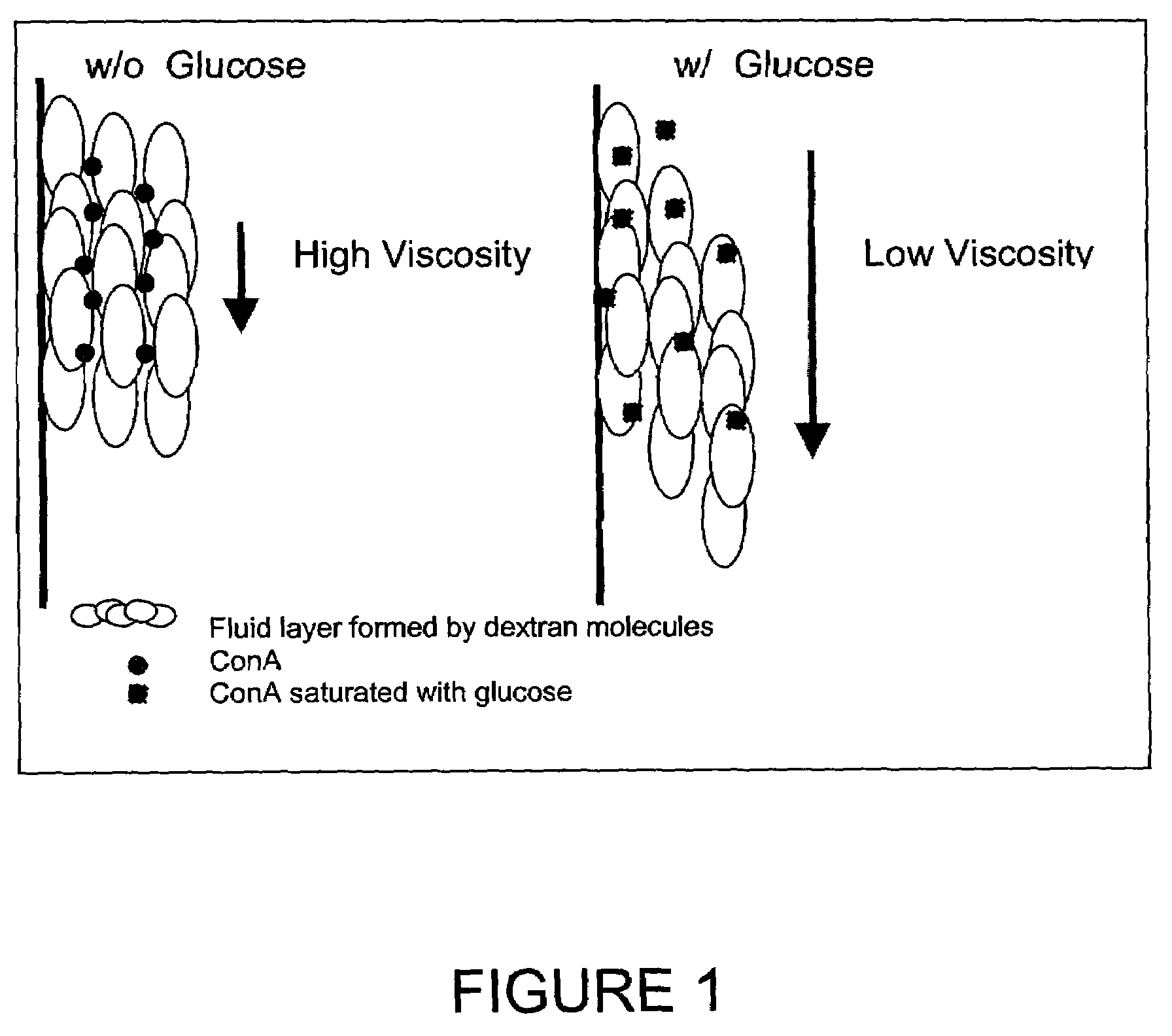
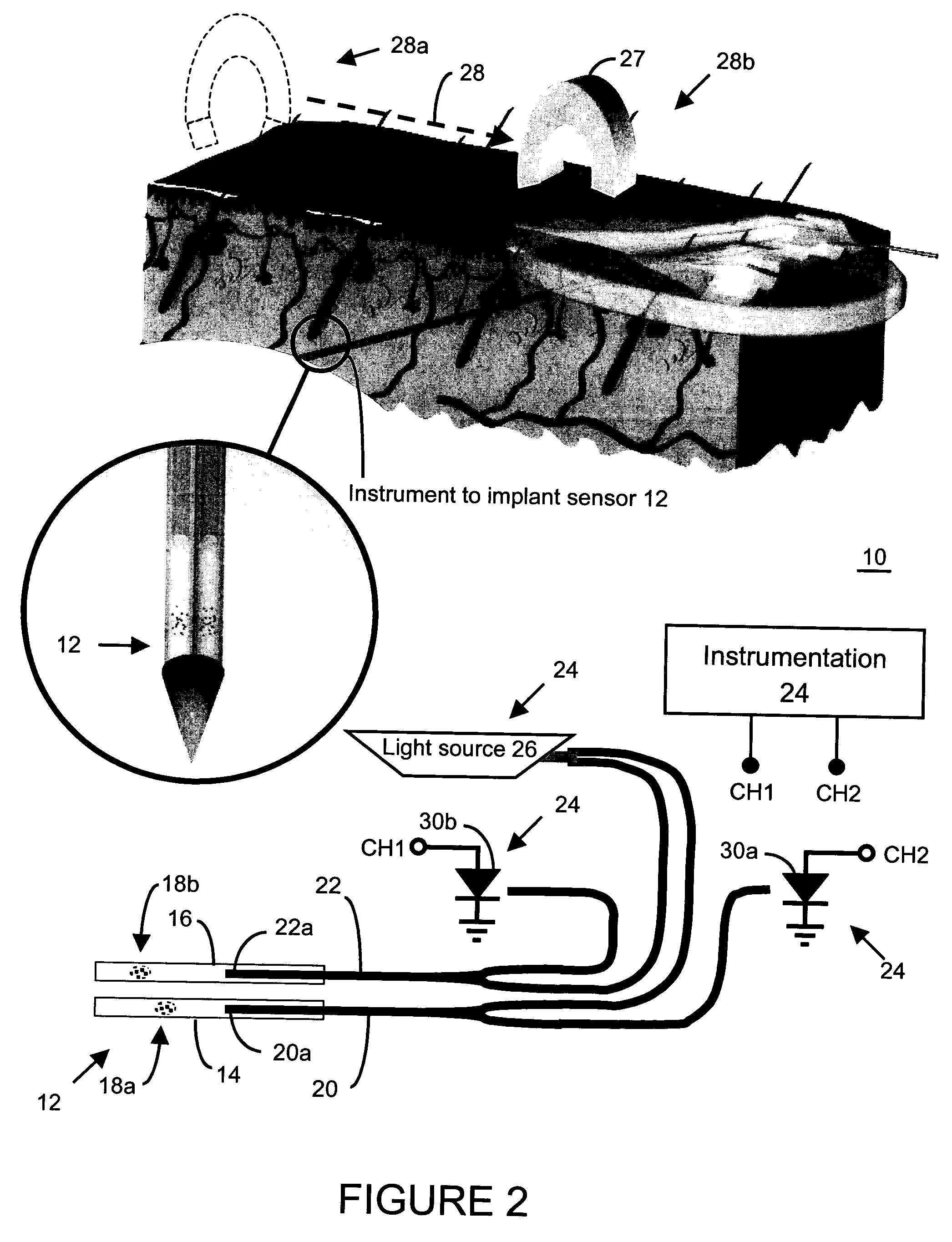
Method and apparatus for analyte sensing
InactiveUS7226414B2Reducing dispersion viscosityLow viscosityCatheterDiagnostic recording/measuringRare-earth elementEngineering
In one aspect, the present invention is directed to a glucose sensing device for implantation within subcutaneous tissue of an animal body. In one embodiment, the glucose sensing device includes a first chamber containing first magnetic particles and a hydrocolloid solution (for example, ConA-dextran hydrocolloid) wherein the first magnetic particles are dispersed in the hydrocolloid solution. In operation, glucose within the animal may enter and exit the first chamber and the hydrocolloid solution changes in response to the presence or concentration of glucose within the first chamber. The sensing device also includes a reference chamber containing second magnetic particles and a reference solution wherein the second magnetic particles are dispersed in the reference solution. The reference solution (for example, oil or alcohol compounds) includes a known or fixed viscosity. The reference solution may also be a hydrocolloid solution (for example, ConA-dextran hydrocolloid). The first and / or second magnetic particles may include amine-terminated particles, at least one rare earth element (for example, neodymium or samarium), and / or a ferromagnetic material.
Owner:BIOTEX
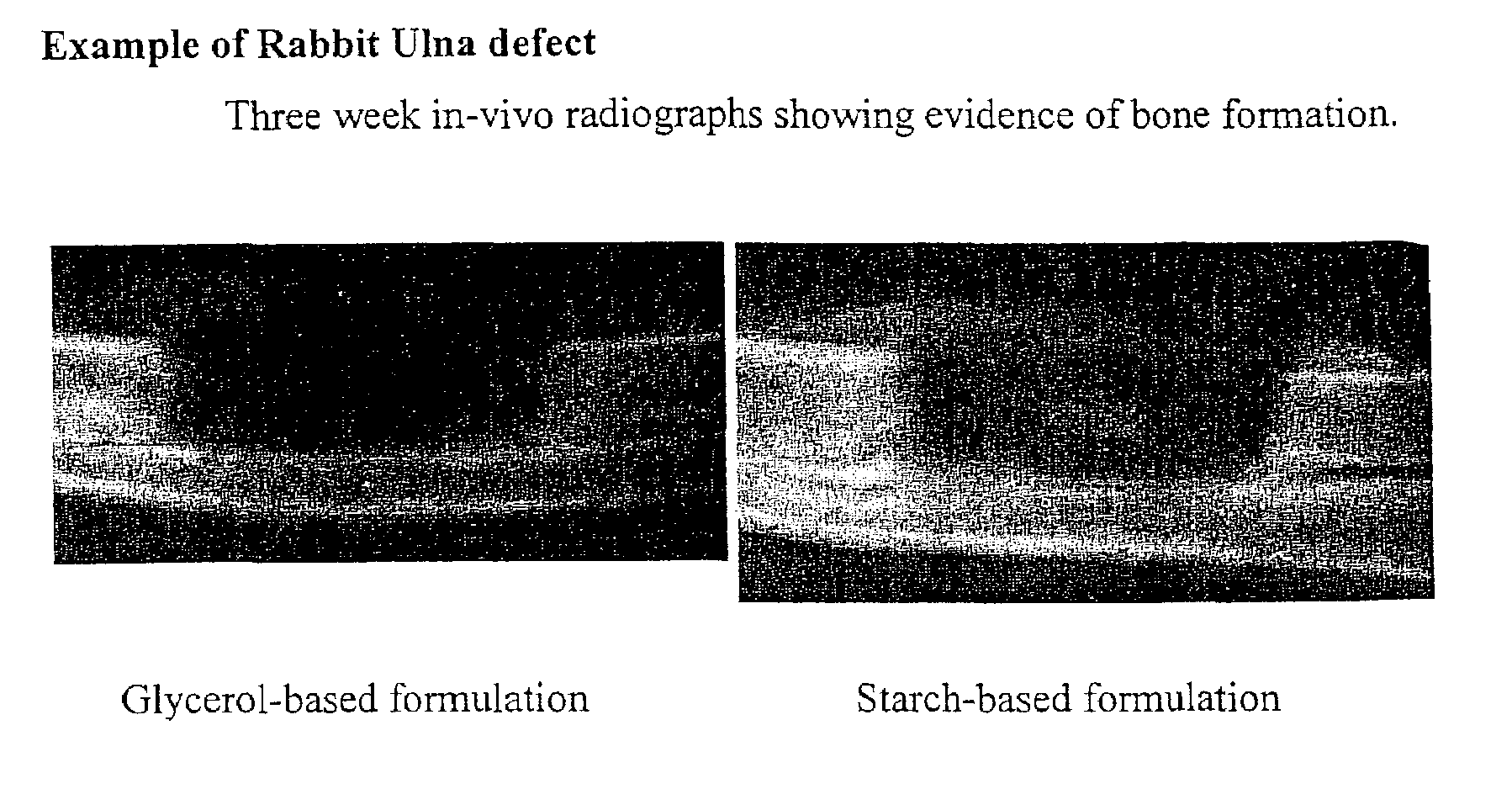
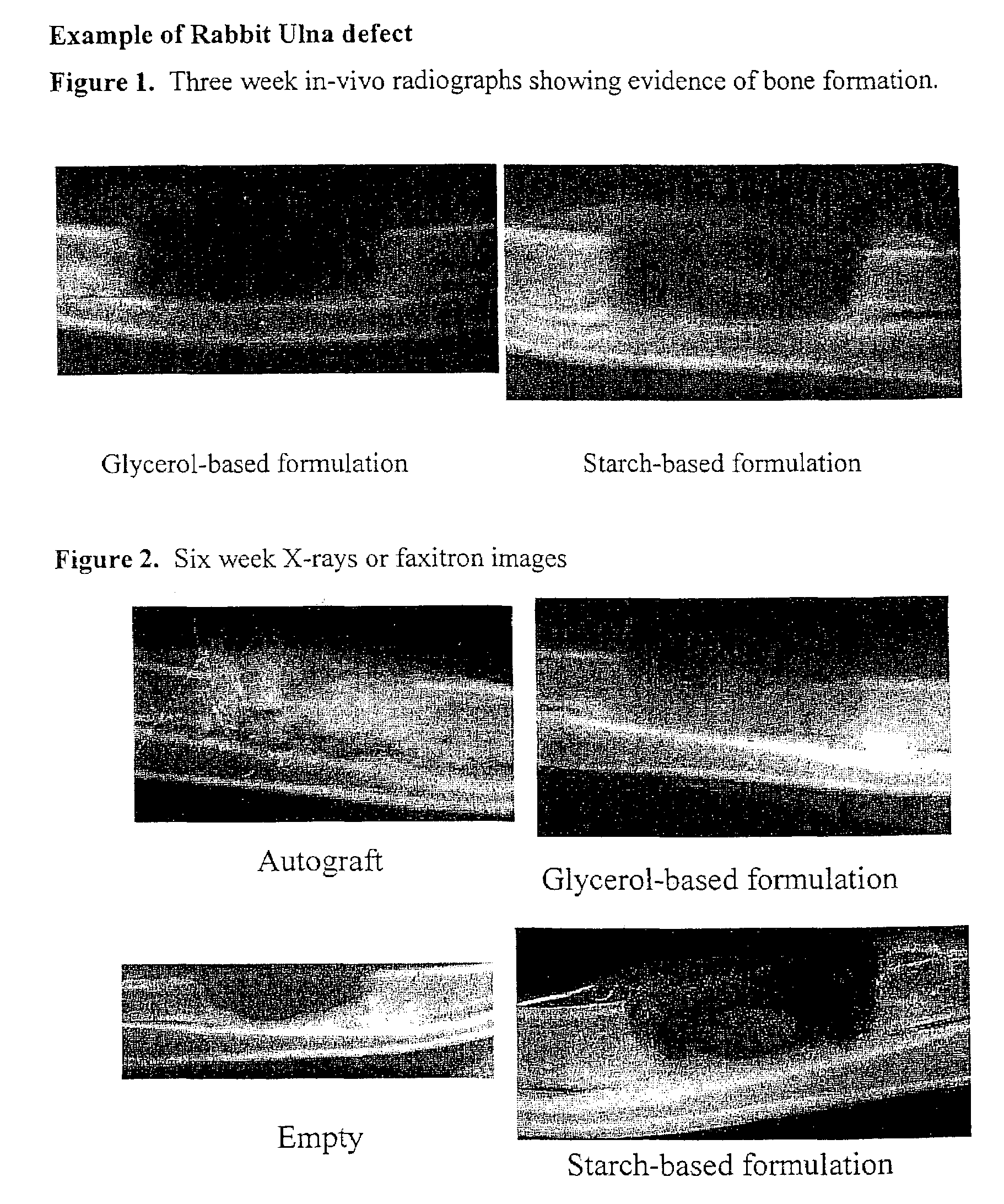
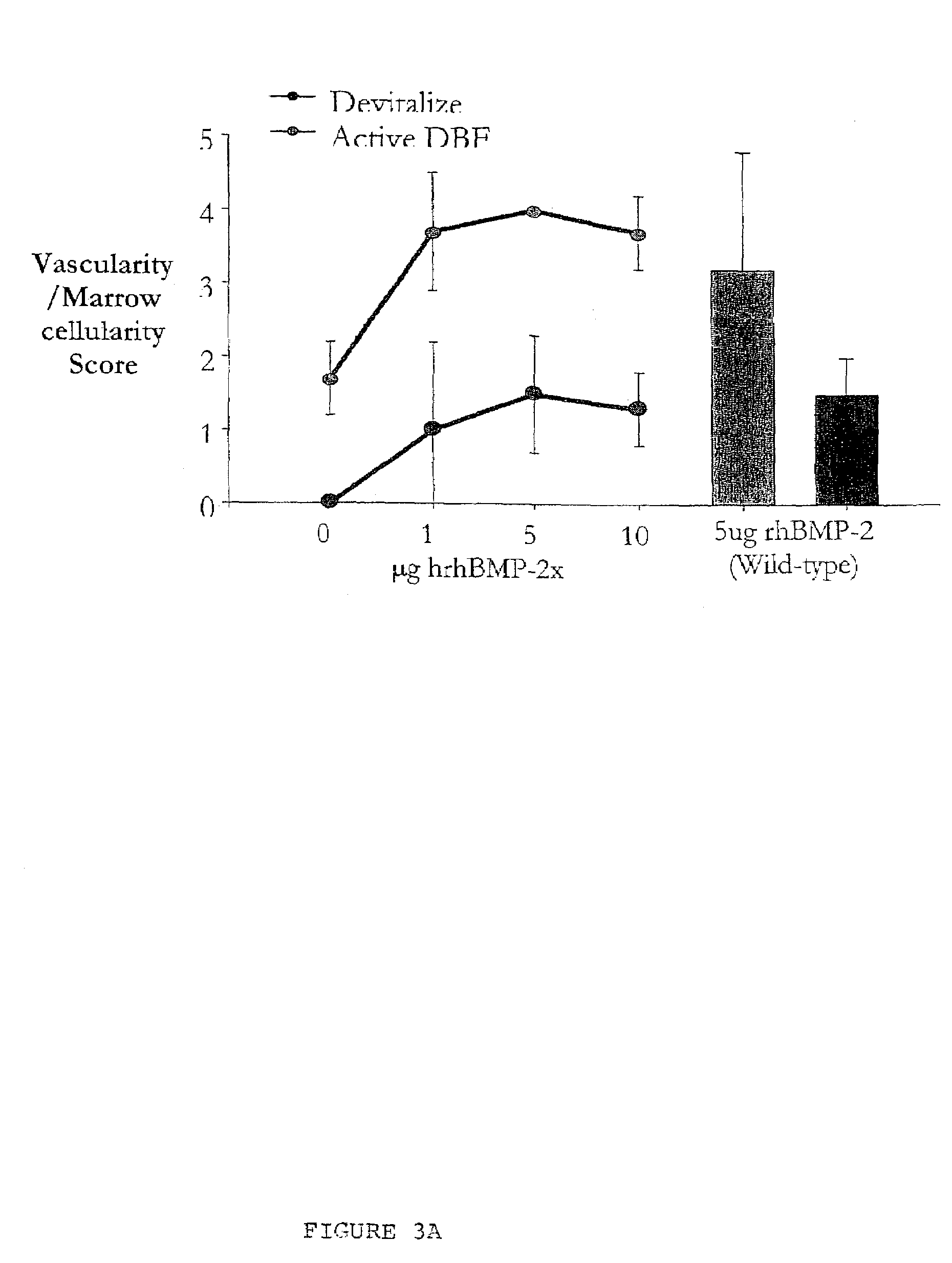
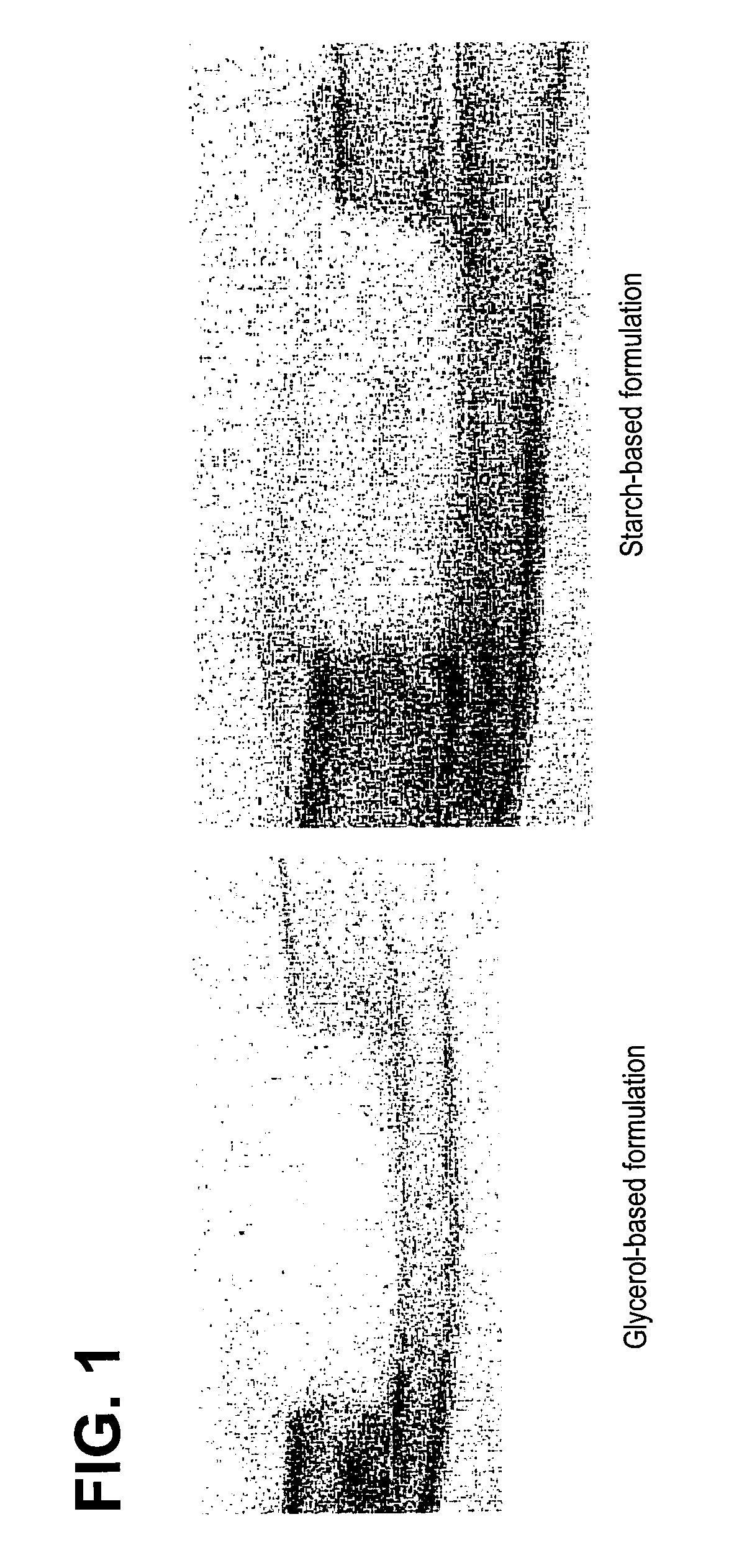
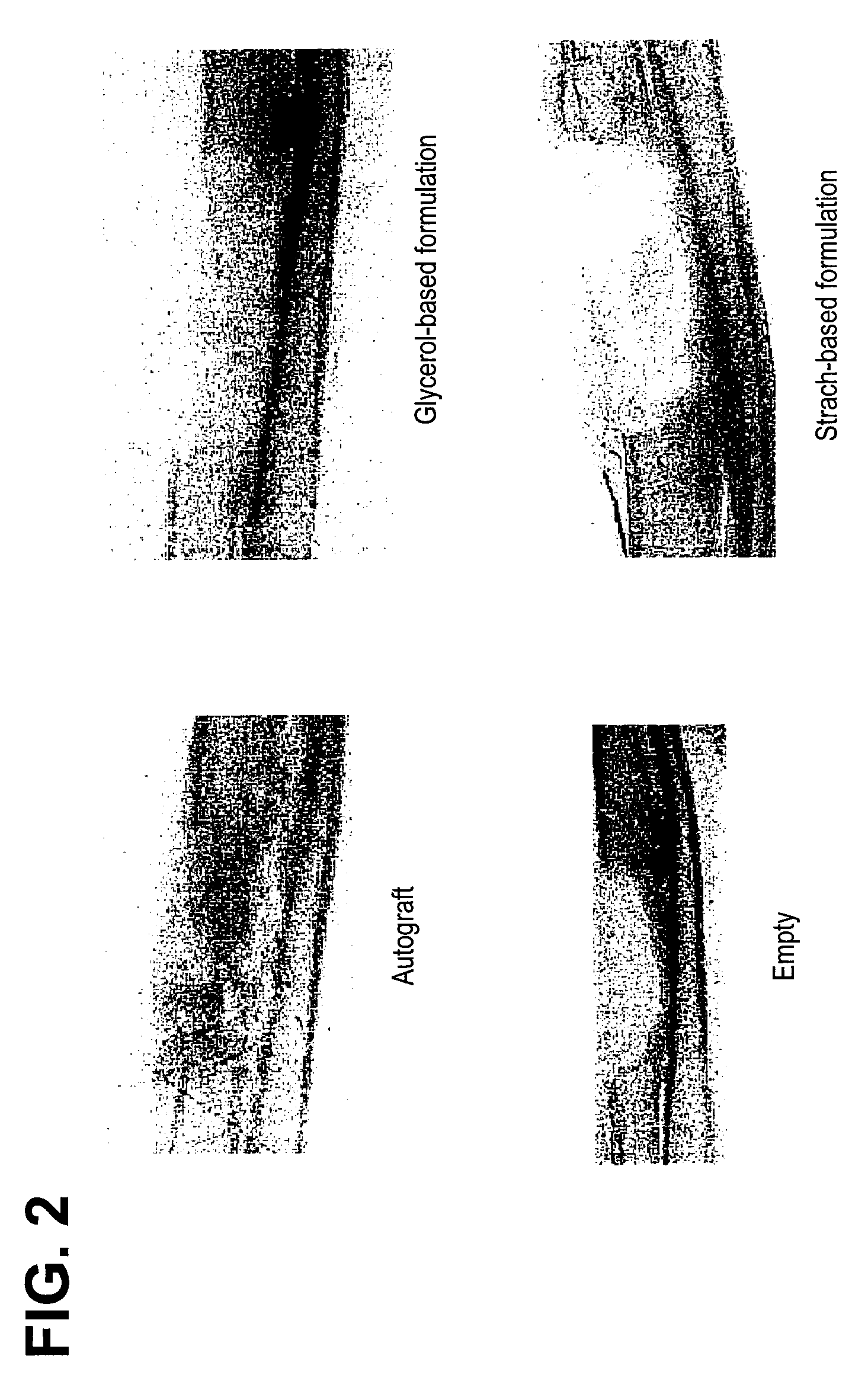
Bone graft
ActiveUS7163691B2Fast reduction in osteoinductiveGood curative effectOrganic active ingredientsImpression capsOSTEOINDUCTIVE FACTORIn vivo
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-β, BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
Compositions And Methods For Inhibiting Adhesions
InactiveUS20080069857A1Fast cross-linkingLimitation on parameterPowder deliveryOrganic active ingredientsActive agentMicroparticle
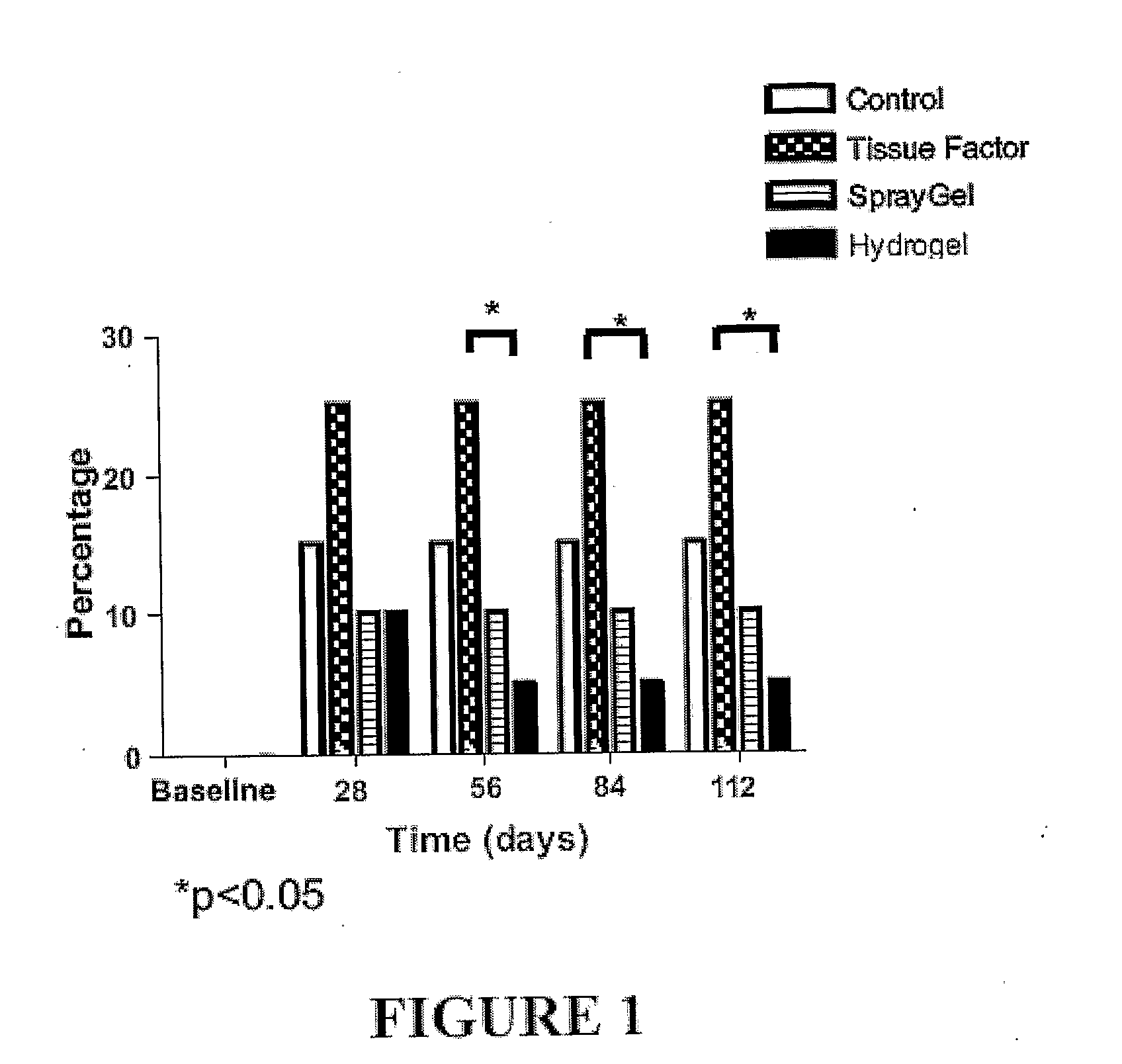
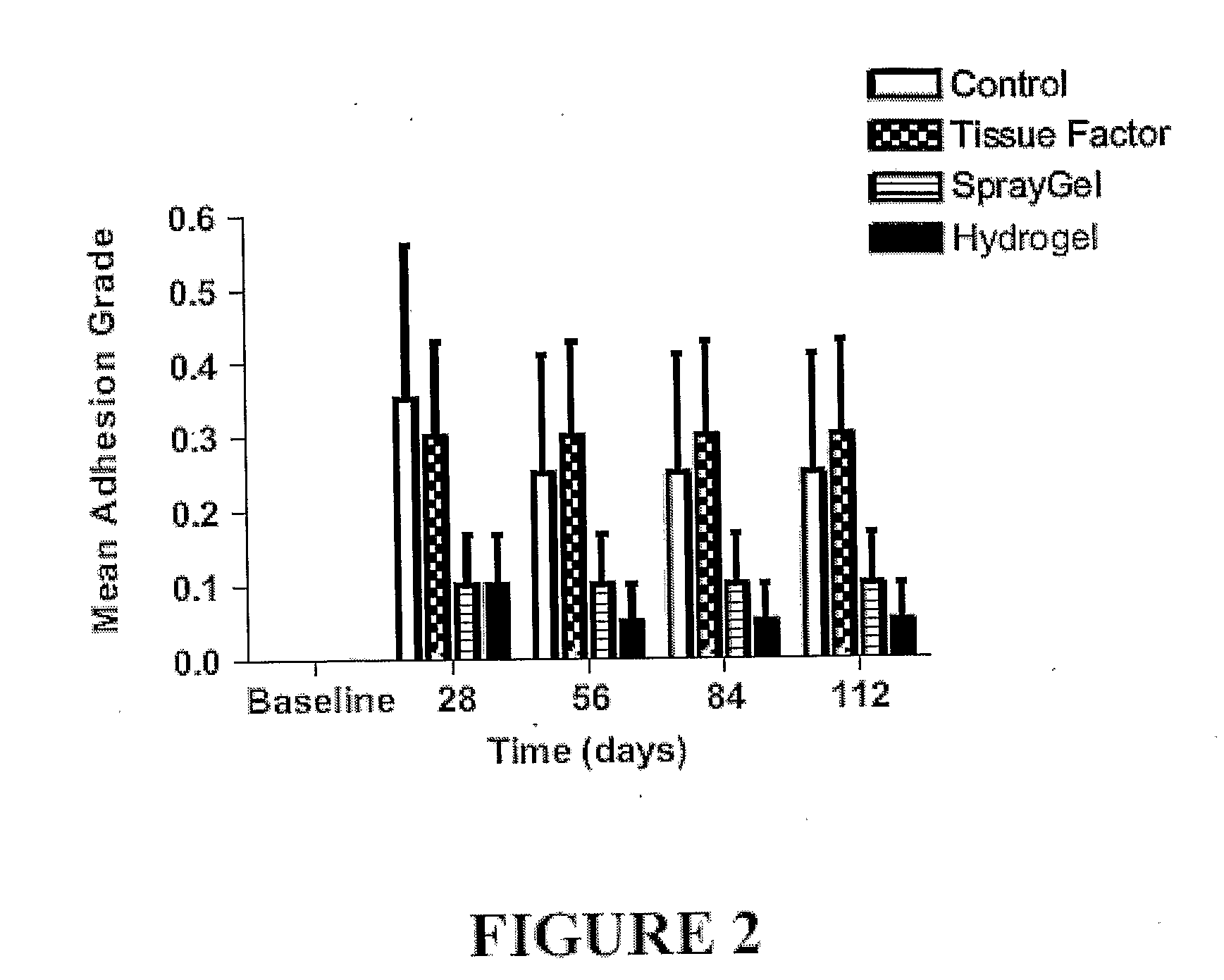
The present invention provides compositions and methods for inhibiting adhesions. The methods involve administering solutions containing hydrogel precursors such as polysaccharide derivatives, e.g., derivatives of hyaluronic acid, cellulose, or dextran, to a subject at a site where adhesions may form, e.g., as a consequence of surgery, injury, or infection. The hydrogel precursors, e.g., polysaccharide derivatives, become crosslinked following their administration to form a hydrogel that maintains tissue separation. In certain embodiments of the invention one or both solutions contains particles, e.g., polymeric nanoparticles or microparticles, so that a composite hydrogel containing the particles is formed. The solution(s), particle(s), or both, may contain a biologically active agent such as an agent that contributes to inhibiting adhesions. The biologically active agent may be covalently attached to a hydrogel precursor.
Owner:EI DU PONT DE NEMOURS & CO +2
Stabilized Glycosaminoglycan Preparations and Related Methods
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
Purification of immunoglobulins
ActiveUS20060134805A1Faster and economic purificationIncrease capacitySolid sorbent liquid separationImmunoassaysCross-linkChemical physics
The present invention relates to a separation matrix comprised of porous particles to which antibody-binding protein ligands have been immobilised, wherein the ligand density is in the range of 5.0-10 mg / ml; the gel phase distribution coefficient of the particles expressed as Kav for a dextran of size 110 kDa is above 0.65 and the median particle diameter is between 65-84 μm. The carbohydrate material is preferably highly cross-linked agarose.
Owner:CYTIVA BIOPROCESS R&D AB
Sealing material which swells when treated with water
InactiveUS6358580B1Safely preventingRapid and sizable and controlled swellingCosmetic preparationsOrganic detergent compounding agentsElastomerCallose
The invention relates to an optionally foamed sealing composition for preformed seals which can swell when treated with water. The invention also relates to a method for the production thereof out of natural rubber and / or elastomers with a matrix comprised of natural rubber / elastomer components and particle-shaped water absorbing material stored therein. The water absorbing material is a combination of (A) polysaccharide(s) selected from cellulose, starch, starch derivatives removed from grafted starch, amylose, amylopectin, dextran, pectin, inulin, chitin, xanthan, alginic acid, alginates, carrageenan, pustulan, callose, laminarin, guluronic acid, pullulan, lichenin or mixtures of the same with (B) a highly water absorbent synthetic polymer selected from polymers based on (meth)acrylate, poly(meth)acrylic acid and the salts thereof, polyacrylamide, polyalcohols or copolymers of said synthetic polymers. The invention also relates to additional cross-linking and processing auxiliary agents and to property improving agents. It is possible to securely seal superstructures, substructures, tunnels and canals with the assistance of the inventive sealing compositions.
Owner:DAETWYLER AG CH +1
Glue for cartilage repair
ActiveUS7067123B2Promote migrationIncreased proliferationBiocidePeptide/protein ingredientsMedicineBone marrow cell
The invention is directed toward a sterile cartilage defect implant material comprising milled lyophilized allograft cartilage pieces ranging from 0.01 mm to 1.0 mm in size in a bioabsorbable carrier taken from a group consisting of sodium hyaluronate, hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran or polymers with allogenic chondrocytes or bone marrow cells in an amount exceeding the natural occurrence of same in hyaline cartilage and adding a cell growth additive.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
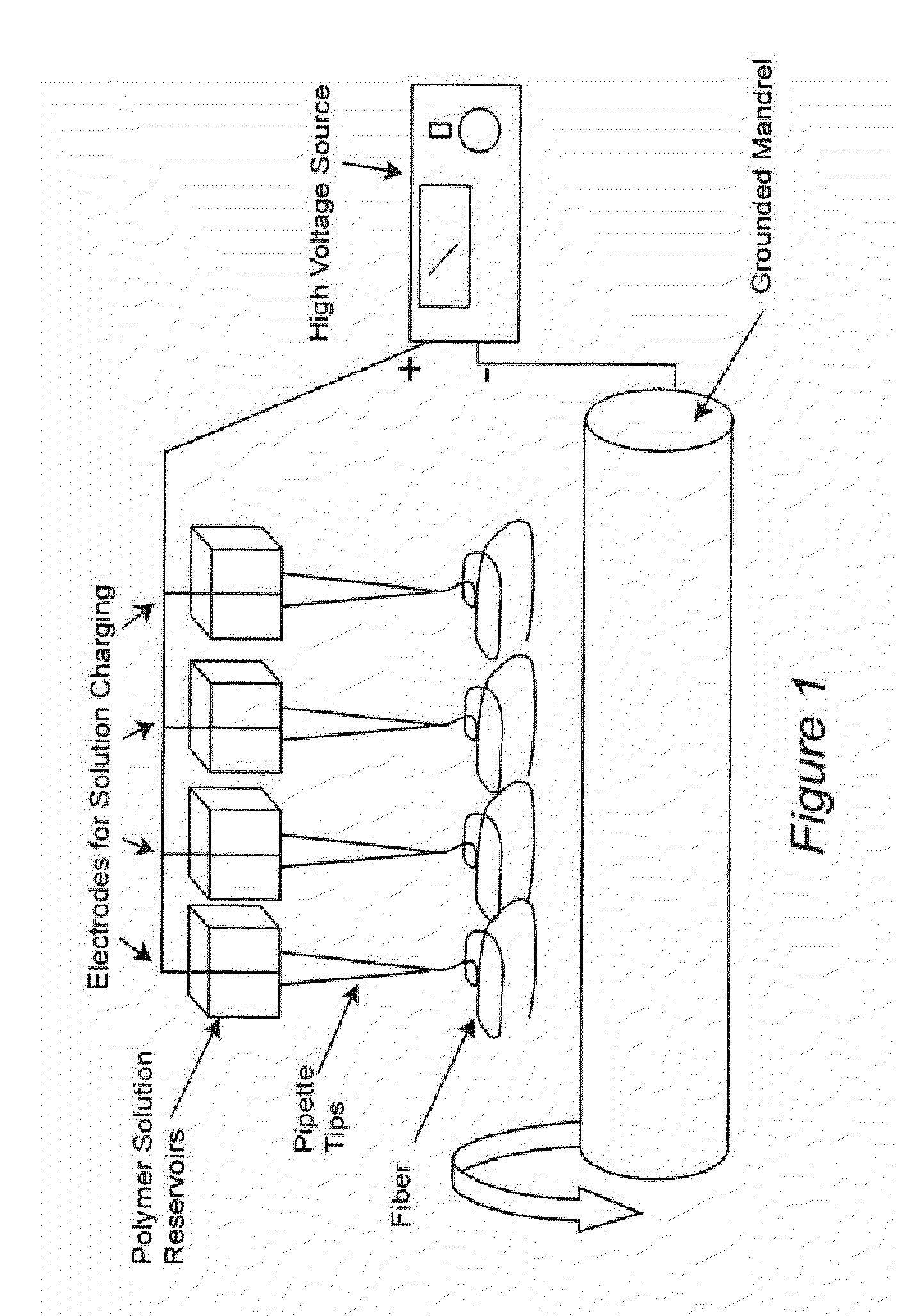
Electrospun dextran fibers and devices formed therefrom
The invention generally relates to dextran fibers which are preferably electrospun and devices formed from such fibers. In particular, such devices may include substances of interest (such as therapeutic substances) associated with the electrospun fibers. Upon exposure to a liquid the electrospun fibers dissolve immediately and the substances of interest are released into the liquid. Exemplary devices include bandages formed from electrospun dextran fibers and associated agents that promote hemostasis, such as thrombin and fibrinogen.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Methods and compositions based on inhibition of cell invasion and fibrosis by anionic polymers
Owner:TRIAD
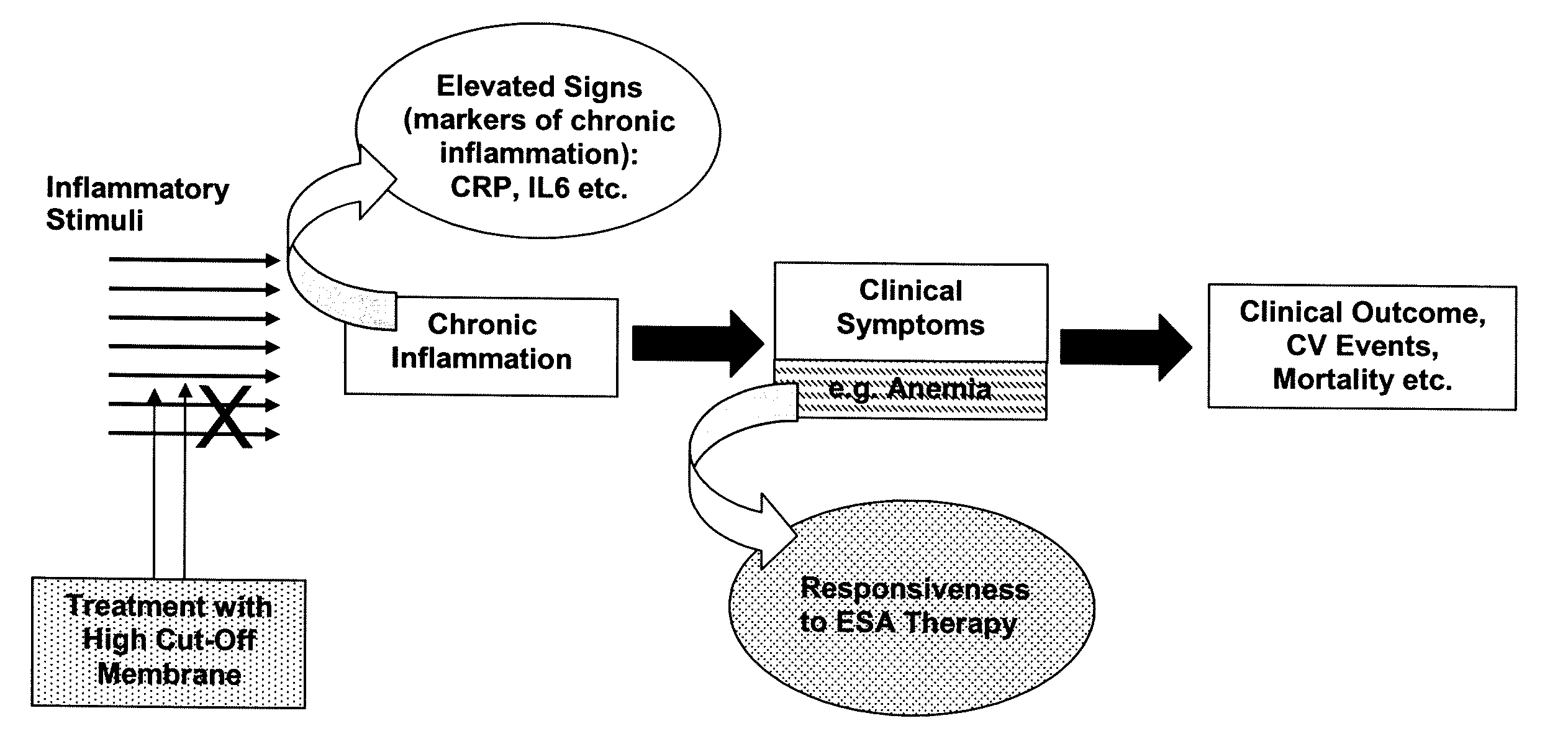
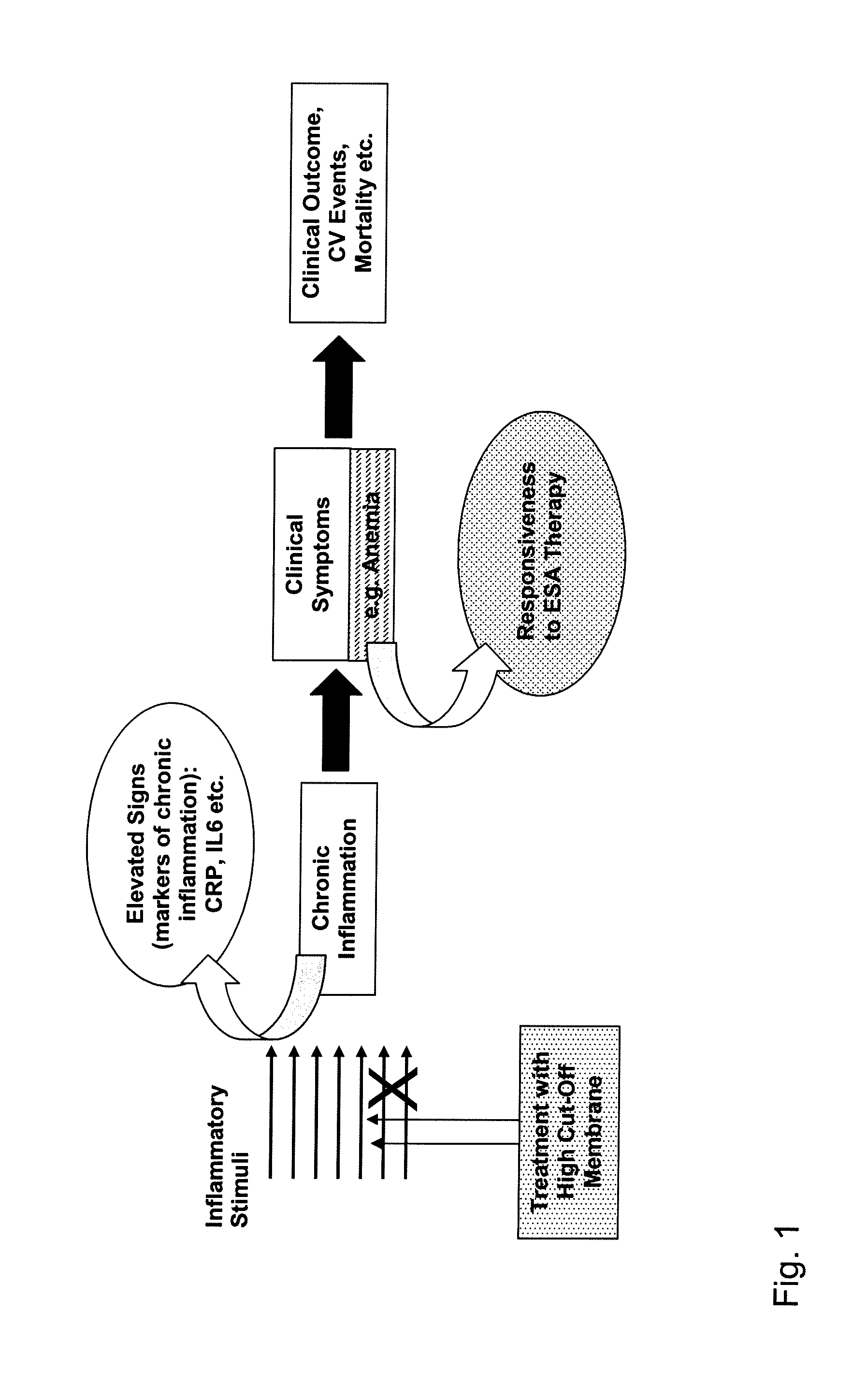
Method for Treating Anemia in Hemodialysis Patients
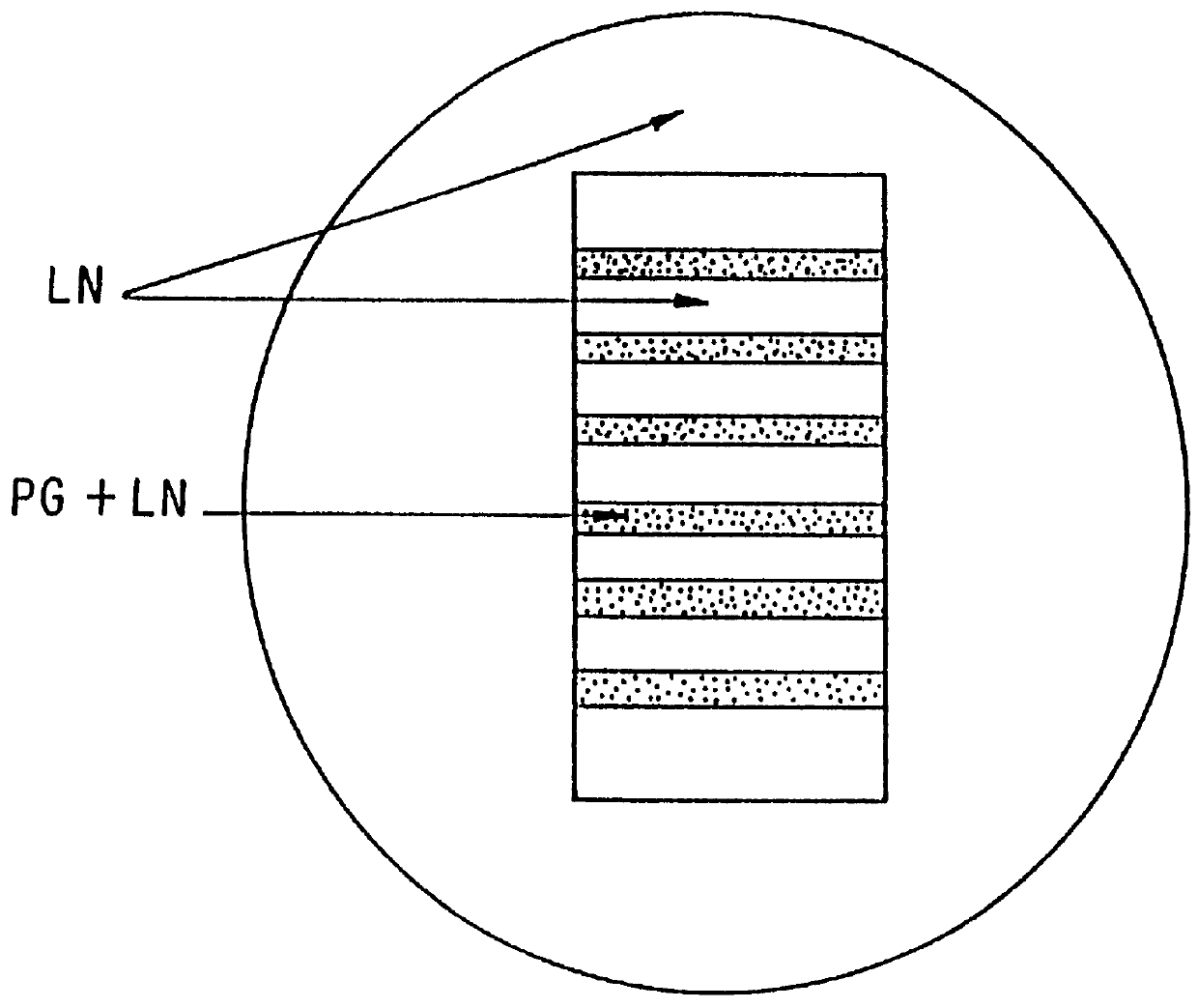
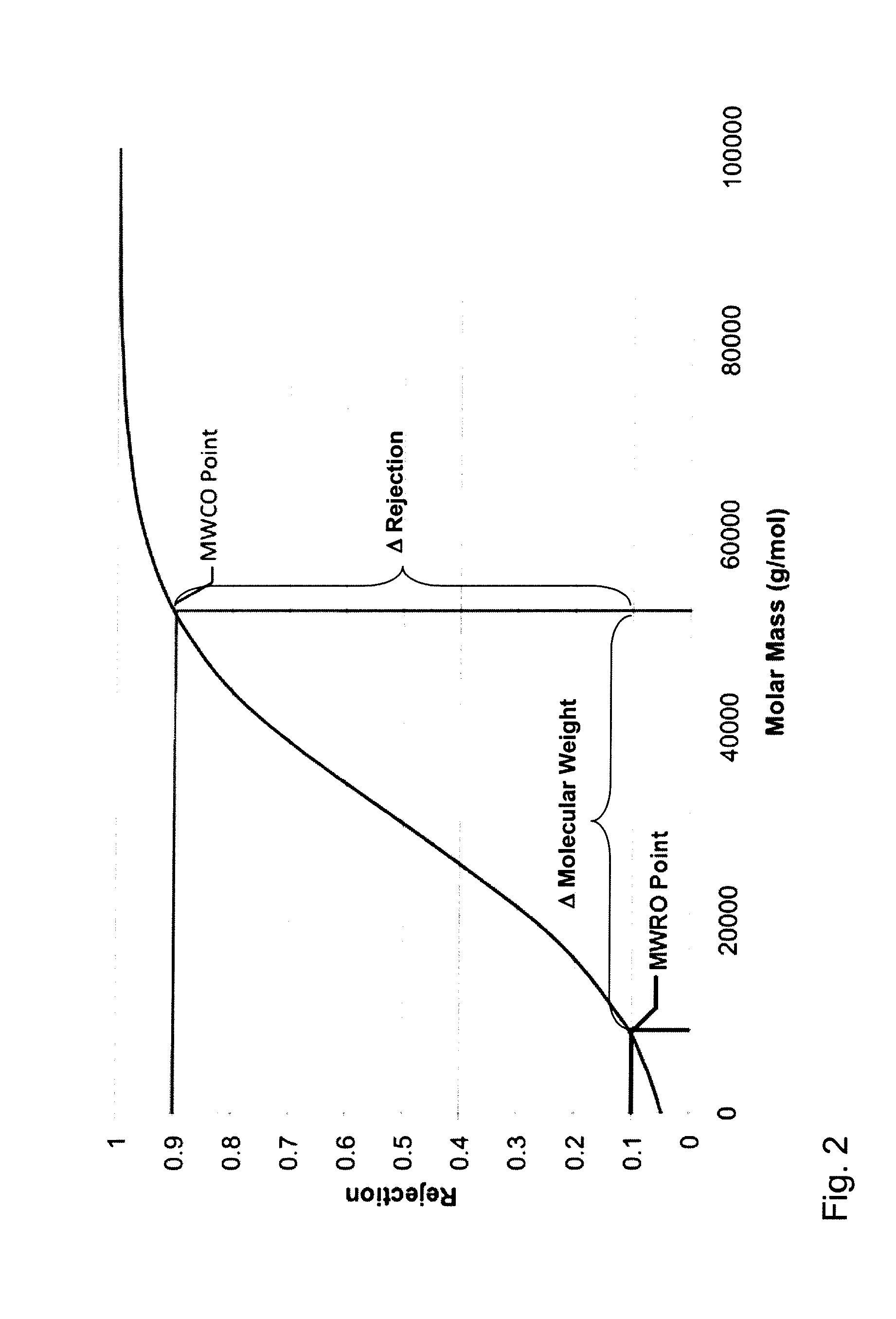
The present invention relates to a method of treating anemia especially in an EPO resistant hemodialysis patient, comprising hemodialysis with a high cut-off dialysis membrane, wherein the hemodialysis membrane is characterized in that it has a molecular weight cut-off in water, based on dextran sieving coefficients, of between 90 and 200 kD and a molecular weight retention onset in water, based on dextran sieving coefficients, of between 10 and 20 kD, and a ΔMW of between 90 and 170 kD. The invention further relates to a high cut-off hemodialysis membrane for the treatment of anemia in hemodialysis patients, especially EPO resistant hemodialysis patients.
Owner:GAMBRO LUNDIA AB
Methods and Compositions for Regenerating Connective Tissue
Connective tissue regenerative compositions and methods of repairing and regenerating connective tissue using such compositions are provided. The compositions generally comprise a bioactive hydrogel matrix comprising a polypeptide, such as gelatin, and a long chain carbohydrate, such as dextran. The hydrogel matrix may further include polar amino acids, as well as additional beneficial additives. Advantageously, the compositions include further components, such as osteoinductive or osteoconductive materials, medicaments, stem or progenitor cells, and three-dimensional structural frameworks. The compositions are useful for regenerating connective tissue, and can be administered to an area having injury to, or a loss of, connective tissue, such as bone, cartilage, tendon, and ligament.
Owner:PIONEER SURGICAL TECH INC
Dry hemostatic compositions and methods for their preparation
InactiveUS20080085316A1Simple compositionPowder deliverySurgical adhesivesCross-linkPolyethylene glycol
Dry cross-linked gelatin compositions are prepared that rapidly re-hydrate to produce gelatin hydrogels suitable as hemostatic sealants. Gelatin is cross-linked in the presence of certain re-hydration aids, such as polyethylene glycol, polyvinylprovidone, and dextran, in order to produce a dry cross-linked gelatin powder. The use of the re-hydration aids has been found to substantially increase the re-hydration rate in the presence of an aqueous re-hydration medium, typically thrombin-containing saline.
Owner:BAXTER INT INC +1
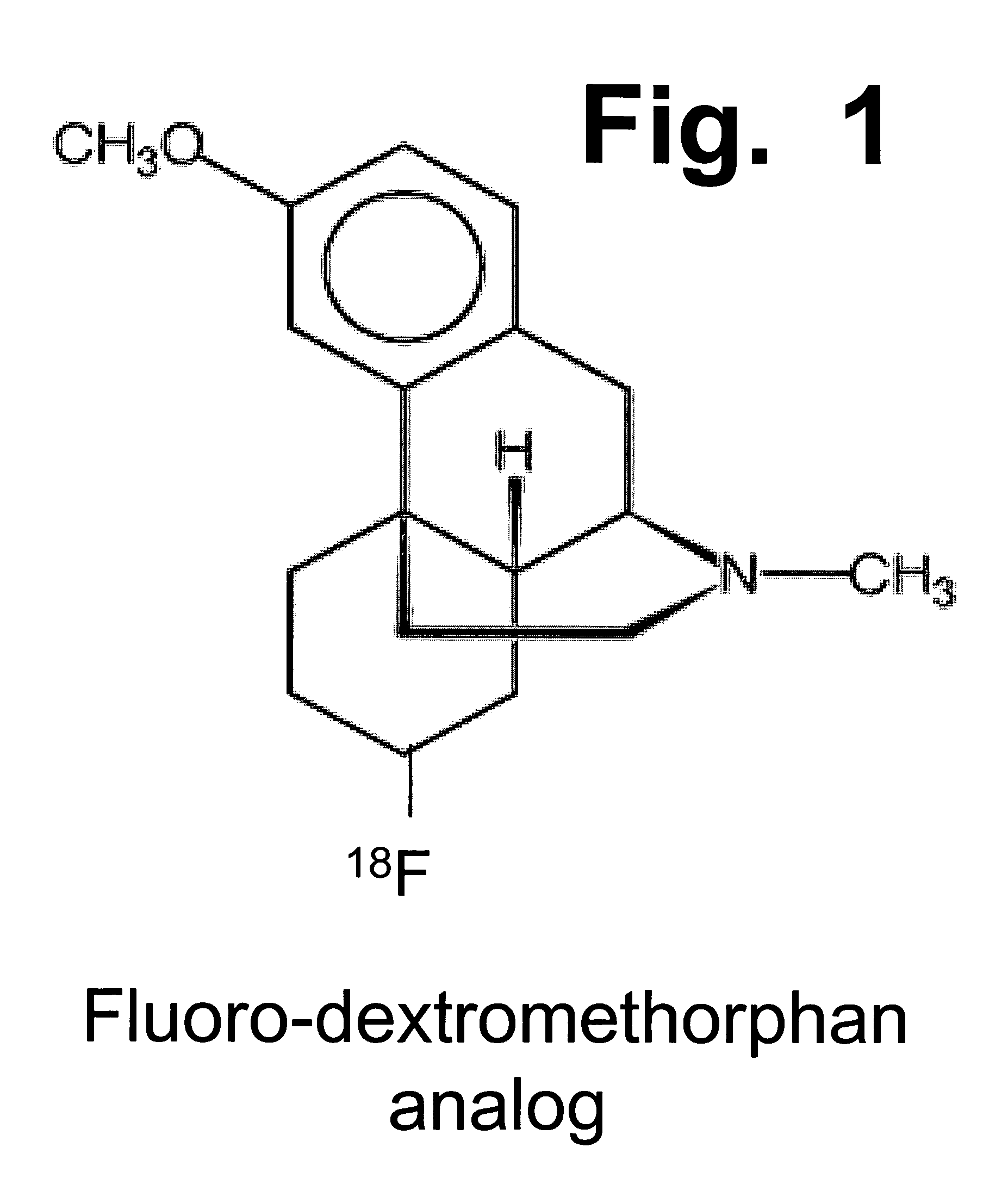
Enhancement of impaired motor and mental functions, using dextromethorphan and oxidase enzyme inhibitor
InactiveUS20070191411A1Improve abilitiesImprove motor controlBiocideNervous disorderDiseaseClinical trial
During clinical trials on patients suffering from neurological disorders, it has been observed that some patients obtain dramatic improvements in motor control and / or higher mental functioning, when they receive a combination of dextromethorphan and quinidine, at suitable dosages. Improved motor control has been exemplified to date by improved ability to swallow and / or speak, among victims of stroke, head injury, or ALS. Improved higher mental functioning has been exemplified better job performance, increased ability to analyze and solve problems, and increased ability to have successful and satisfying interactions with other people. These types of effects can be seen in a relatively brief time period, such as within several days to a week.
Owner:CENT FOR NEUROLOGIC STUDY
Composite biological adsorption and preparing method thereof
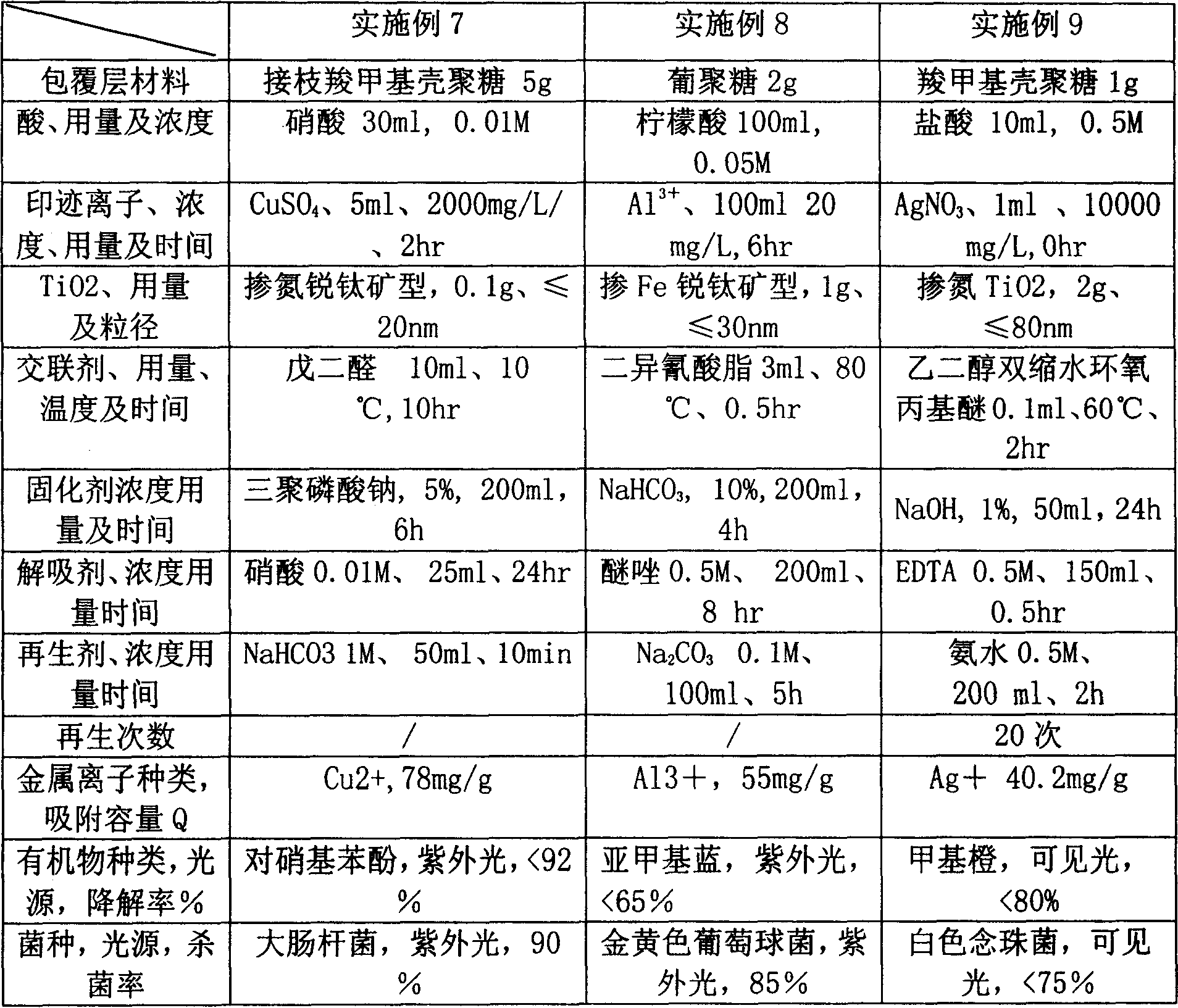
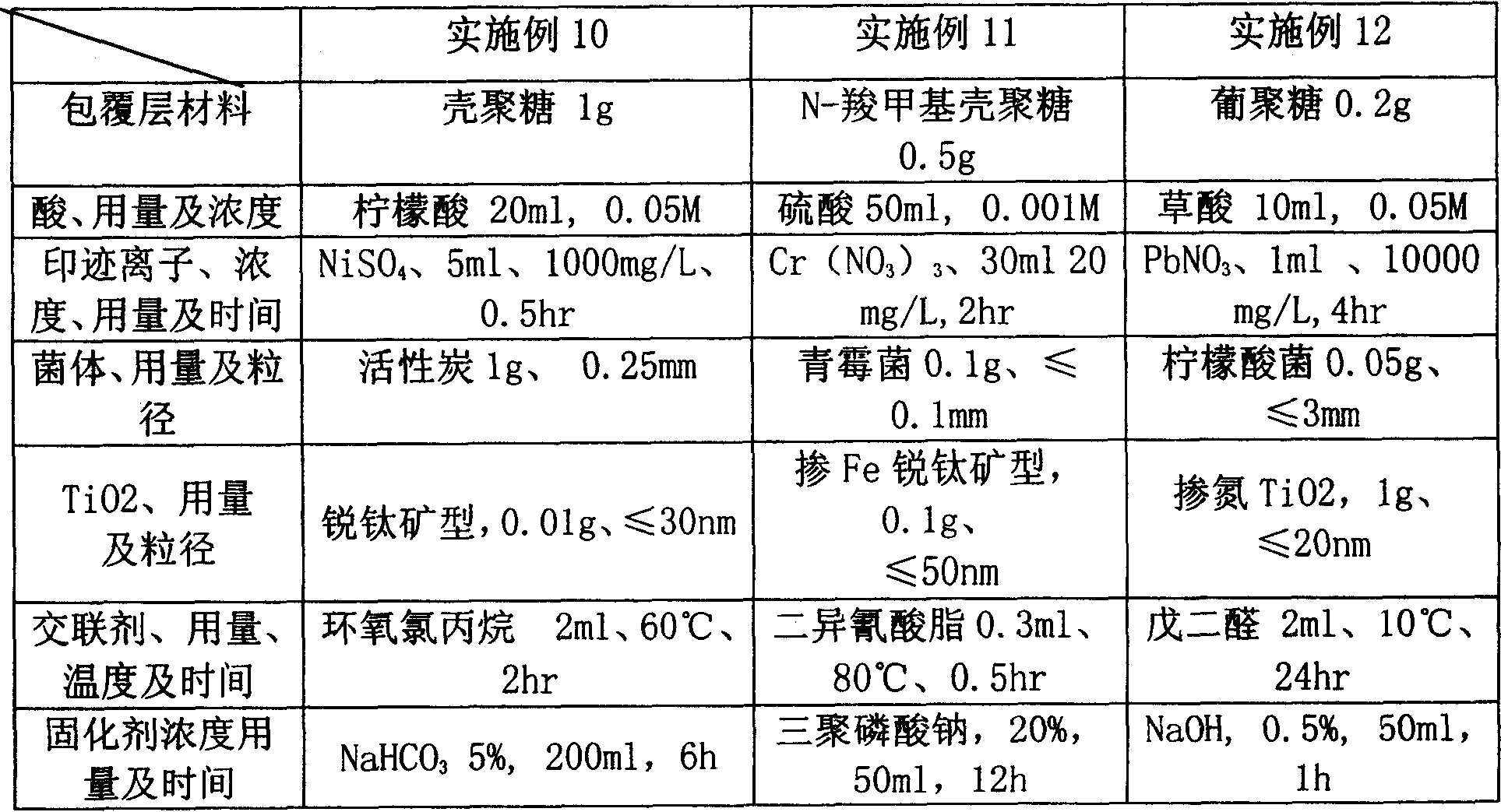
InactiveCN101077795ARealize multi-functional compositeImprove adsorption capacityWater/sewage treatment by sorptionDextranBiology
The present invention relates to one kind of composite biological adsorbent and its preparation process. The adsorbent is chitosan or dextran cross-linked resin with supported titania photocatalyst and surface heavy metal ion blotting, and has the functions of adsorbing heavy metal ion, and degrading and eliminating organic pollutant, pathogen and microbe from water solution. The adsorbent is prepared through cross-linking and supporting titania photocatalyst onto chitosan and other saccharide biomass, coupling molecular engram with nanometer titania photocatalyst to form surface heavy metal ion blotting in the surface. The adsorbent has high adsorption capacity, high heavy metal selectivity, and high capacity of degrading and eliminating organic pollutant, pathogen and microbe.
Owner:BEIJING UNIV OF CHEM TECH
Polymer blends that swell in an acidic environment and deswell in a basic environment
InactiveUS6537584B1Improve propertiesControlling the swelling and/or deswelling behaviorBiocidePowder deliveryChemical structurePolymer dissolution
A polymer blend is prepared by dissolving chitosan and a second polymer in an acidic aqueous solution to form an aqueous polymer blend, dehydrating said aqueous polymer blend, and recovering said polymer blend. The second polymer may be selected from the group consisting of polyether glycols including polyethylene glycols; cellulose esters including cellulose acetate; poloxamers; polysaccharides including dextran and guar; polyvinylpyrrolidones; polyvinyl alcohols; and mixtures or copolymers thereof. These polymer blends swell in an acidic environment and deswell in a more neutral or basic environment. This technology is valuable for the dispensing of biologically active material or drugs into a surrounding environment, especially the environment as is found in the gastrointestinal tract. Since the various polymer blends of the present invention are not covalently or ionically crosslinked, but are physically combined, each polymer in the physical blend maintains its original chemical structure, and therefore, is safe for oral administration.
Owner:BTG INT LTD
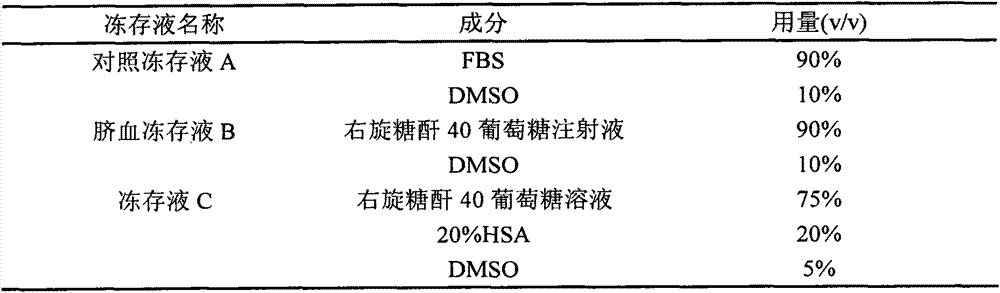
Cell freezing medium
The invention provides a cell freezing medium which can be clinically safely used and is used for freezing human cells. The cell freezing medium comprises: 2-8wt% of human albumin, 5-10 volume% of dimethyl sulfoxide, 3.5-5.1wt% of dextranum-40 and 2.75-4.25wt% of glucose.
Owner:BEIJING YONGTAI IMMUNITY APPL TECH
Cartilage repair mixture containing allograft chondrocytes
InactiveUS20060210643A1Promote migrationIncreased proliferationPeptide/protein ingredientsSurgeryCartilage repairDextran
The invention is directed toward a sterile cartilage defect implant material comprising milled lyophilized allograft cartilage pieces ranging from 0.01 mm to 1.0 mm in size in a bioabsorbable carrier taken from a group consisting of sodium hyaluronate, hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran or mixed polymers with allograft chondrocytes added in an amount ranging from 2.5×105 to 2.5×107.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
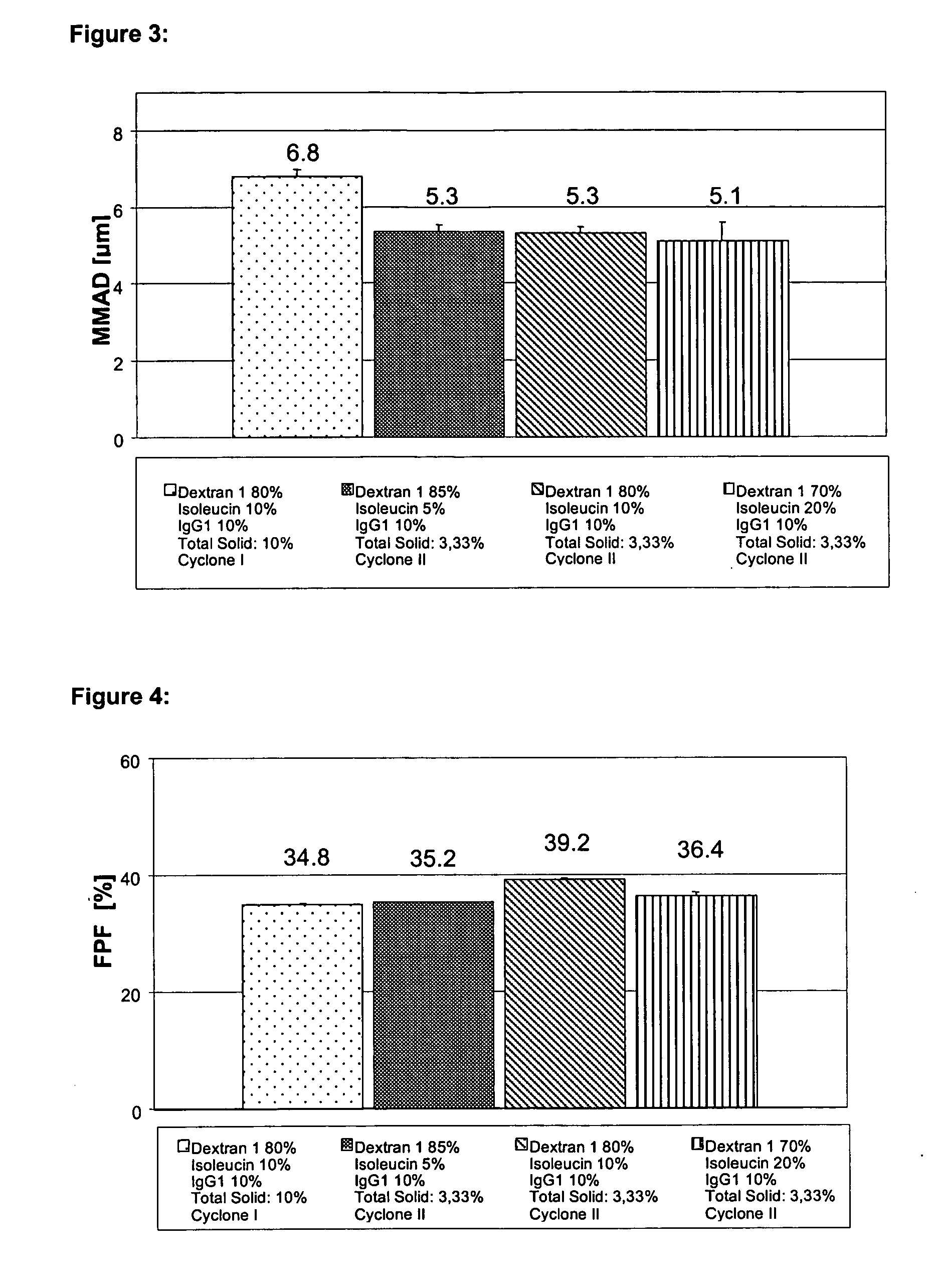
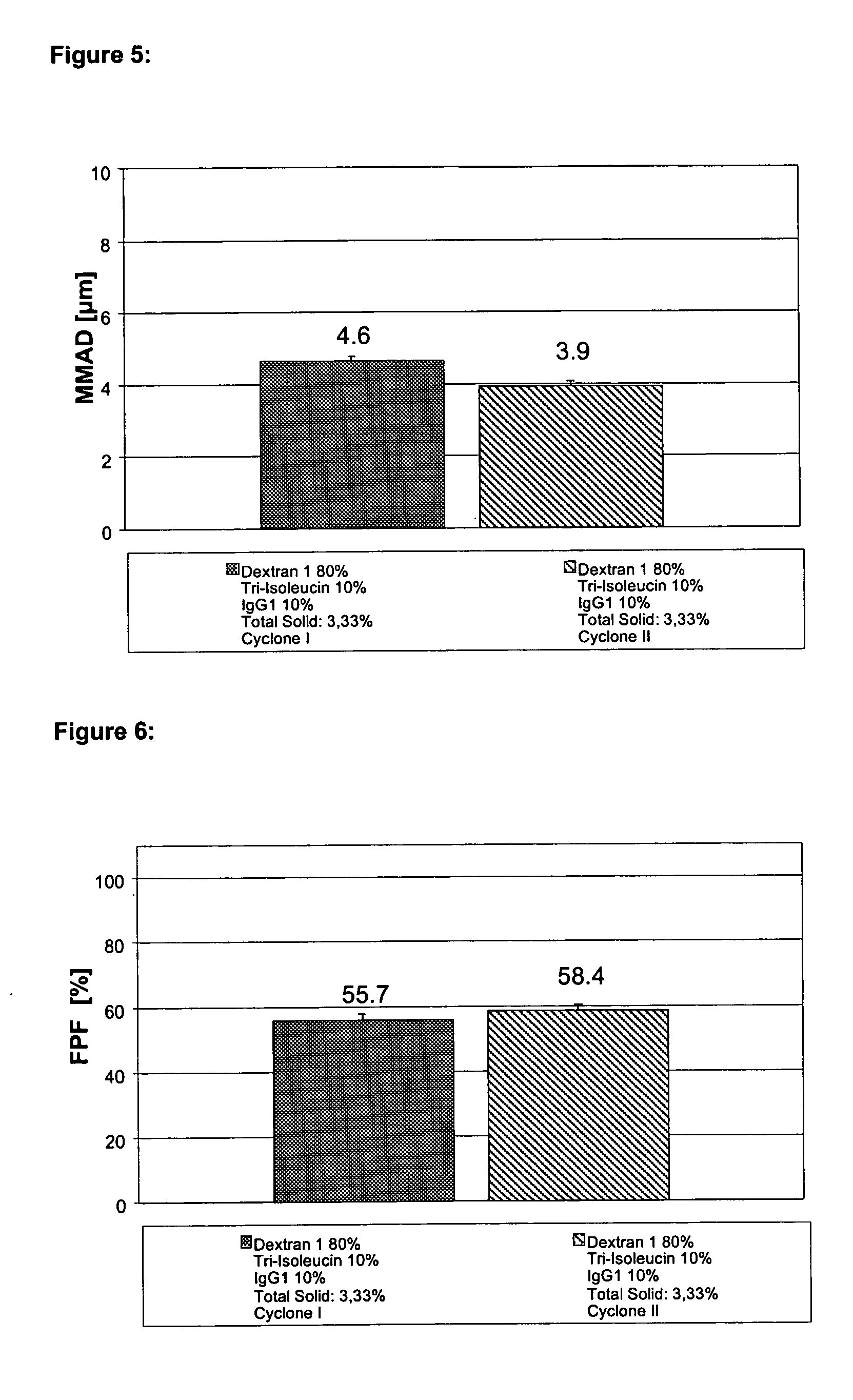
Powders comprising low molecular dextran and methods of producing those powders
Disclosed are powders, preferably spray-dried powders, which contain a pharmaceutical active substance and low-molecular dextran as excipient. Also disclosed are processes for preparing such powders and methods of administering them by inhalation.
Owner:BOEHRINGER INGELHEIM PHARM KG
Surgical hydrogel
InactiveUS20100291055A1Avoid stickingAffect haemostasisOrganic active ingredientsBiocideCross-linkWound healing
The invention provides a hydrogel suitable for use in wound healing, particularly for reducing post-surgical adhesions. The hydrogel comprises cross-linked derivatives of chitosan and dextran polymers. The hydrogel forms when solutions of the polymers are combined.
Owner:MEDTRONIC XOMED INC
Dextran starch and flocculant combination for improving red mud clarification
InactiveUS6726845B1Easy to separateGallium/indium/thallium compoundsCentrifugal force sediment separationFiltrationCentrifugation
The claimed invention is a method for separating Bayer process red mud from a Bayer process liquor which comprises adding to a Bayer process liquor containing red mud an effective amount of a water soluble synthetic flocculant, dextran and starch combination. The flocculant is added anywhere in the slurry containing the red mud suspended in Bayer process liquor, or in a liquor slurry containing bauxite prior to or during digestion. Once the flocculant combination is added, it is mixed sequentially with the Bayer process liquor and the red mud contained in the Bayer process liquor is removed by sedimentation, centrifugation or filtration.
Owner:ECOLAB USA INC
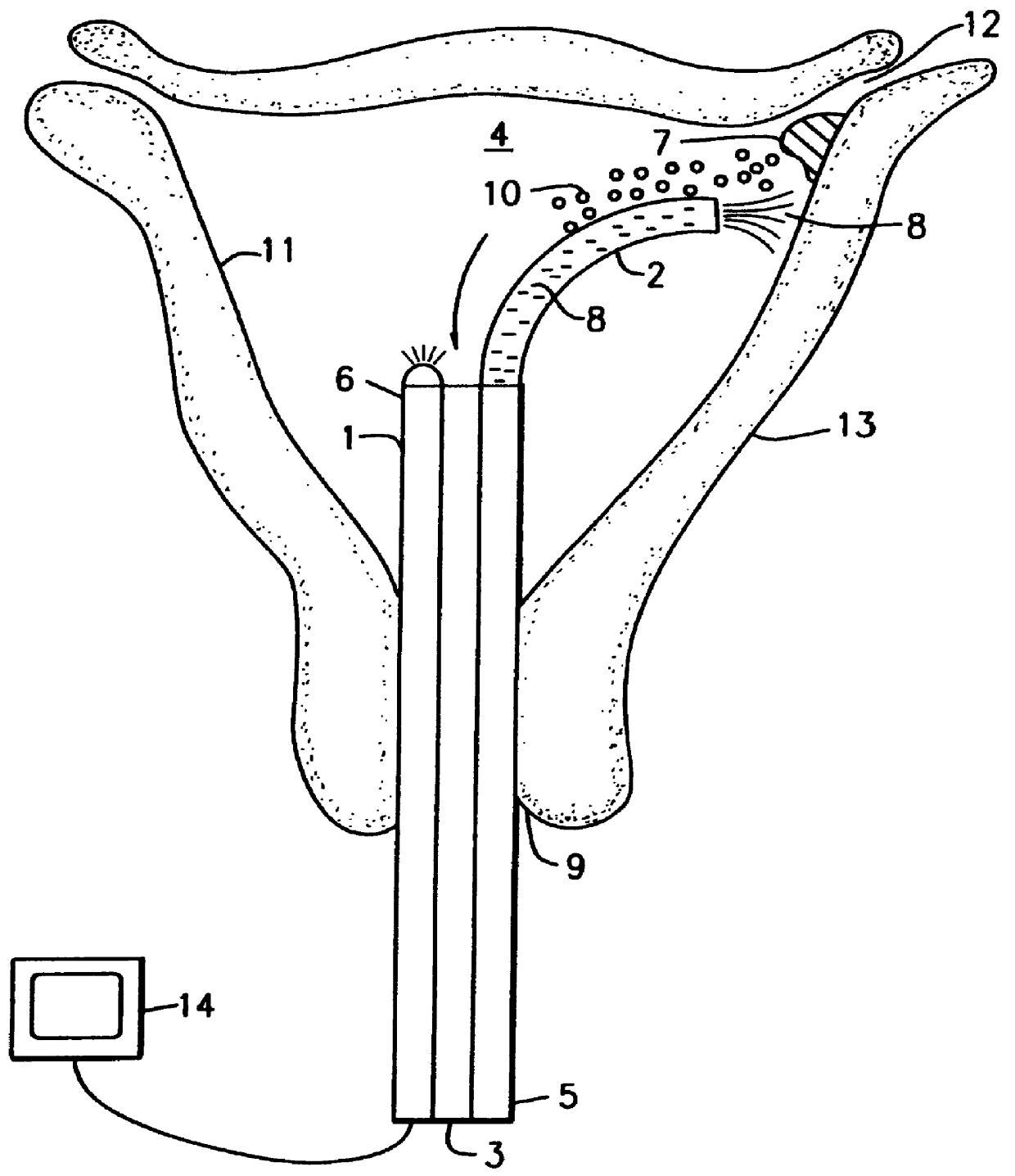
Intrauterine chemical necrosing method, composition, and apparatus
InactiveUS6165492ARapidly rendered non-causticNot to damageOrganic active ingredientsAerosol deliveryGynecologyAqueous solution
A method and composition for effecting chemical necrosis of a tissue lining of a mammalian body cavity, particularly a uterine endometrium, by delivering a caustic tissue necrosing composition, e.g., a silver nitrate and dextran paste, to the tissue to be necrosed and allowing the paste to remain in contact with the target tissue for a period of time sufficient to chemically necrose substantially the entirety of the tissue lining, and then contacting the caustic composition with a deactivating agent, e.g., an aqueous sodium chloride solution, thereby rendering the caustic composition non-caustic, and then rinsing the cavity. Compositions and methods for delivering medicaments are also disclosed.
Owner:NEUWIRTH ROBERT S
Methods and compositions for regenerating connective tissue
InactiveUS20080145404A1Halting progressionAntibacterial agentsPowder deliveryProgenitorLigament structure
Owner:PIONEER SURGICAL TECH INC
Amplification for solid phase immunoassay
ActiveUS20100081125A1High sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteDextran
The present invention is directed to immunoassays for detecting one or more target analytes in a fluid sample wherein the detection reaction occurs on a solid support and involves an amplification system. In particular, the invention is directed to making and using a test device having at least one site for detecting the presence of at least one target analyte, wherein a conjugate comprising dextran-polystreptavidin is immobilized at the test site(s) as a capture reagent for a complex containing the target analyze.
Owner:REMEL
Modified membrane fabric, preparation method and application thereof
ActiveCN103976886AStrong residentReduce evaporationCosmetic preparationsAntipyreticPullulanCarrageenan
The invention provides a modified membrane fabric and a preparation method and application of the modified membrane fabric. The surface of the modified membrane fabric is coated with a polymer or polymer dispersion, and the polymer in the polymer or polymer dispersion may be polysaccharide, polypeptide and protein, may be an artificial polymerized polymer or may be a modified natural high polymer material or a mixture thereof, including but not limited to one or more selected from the group consisting of gelatins (such as gelatin and gelatin hydrolysate), cellulose ethers (e.g., carboxymethyl cellulose and hydroxyethyl methyl cellulose), modified starches (e.g., pullulan and hydroxypropyl starch), PVP, PVA, hyaluronic acids, albumin, chitosan, dextran, Arabic gum, xanthan gum, carrageenan, pectin, konjac gum, agar, Carbomer, carrageenin, polyvinylpyrrolidone, polyacrylamide, polyacrylate and polyacrylic acid and its derivatives. When the modified membrane fabric comes across water in use, the polymer on the surface of the modified membrane fabric can bond with water molecules to form a water retention system which has better retention performance compared with pure water and enables an evaporation speed to be slower and retention performance on the skin to be better.
Owner:李和伟
Bone Graft
ActiveUS20080145392A1Good curative effectSimple compositionAdditive manufacturing apparatusBone implantOSTEOINDUCTIVE FACTORIn vivo
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-.beta., BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions
Owner:WARSAW ORTHOPEDIC INC
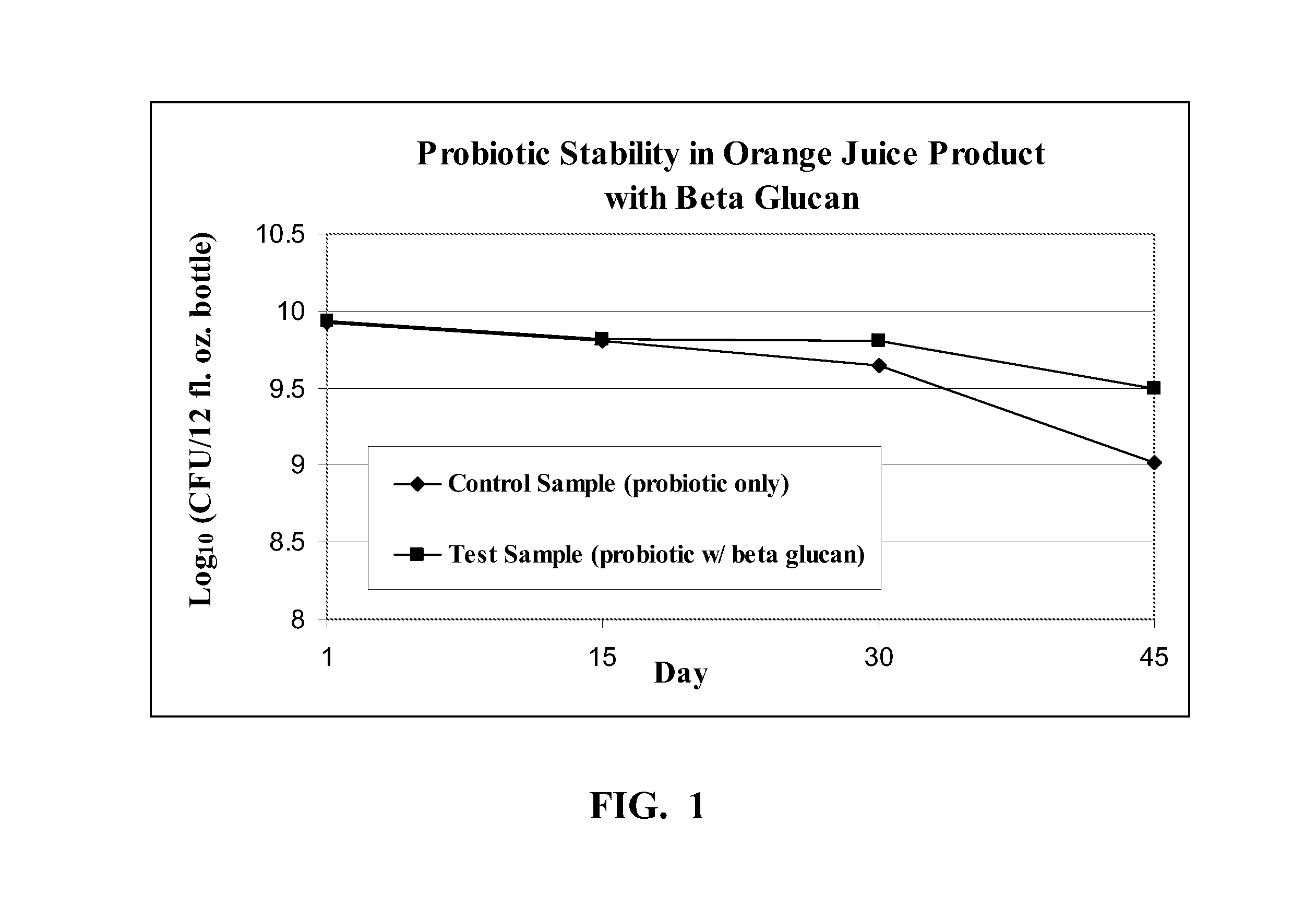
High acid beverage products and methods to extend probiotic stability
InactiveUS20110123677A1Improve survivabilityIncreased number and percentMilk preparationAcidic food ingredientsFruit juiceBeta-glucan
Beverage products are disclosed comprising at least one fruit juice, at least one sweetener, probiotic bacteria, and beta-glucan, where the beverage product has a pH of at most 4.5 and an acid level of 0.5%-1.0%. In certain exemplary and non-limiting embodiments, the beverage product has the characteristic that if tested after 45 days of storage in hermetically sealed individually sized 12 fl. oz. PET vessels stored in the dark or in otherwise UV shielded conditions at a refrigeration temperature of 35° F. the beverage product has an increased shelf life when compared to the same beverage product without beta glucan. Methods are provided for making such beverage products with extended probiotic stability.
Owner:TROPICANA PROD INC
Injectable hydrogel microspheres from aqueous two-phase system
InactiveUS7776240B2Big lossSustained releaseLiquid surface applicatorsPeptide/protein ingredientsSolubilityEmulsion
Injectable hydrogel microspheres are prepared by forming an emulsion where hydrogel precursors are in a disperse aqueous phase and polymerizing the hydrogel precursors. In a preferred case, the hydrogel precursors are poly(ethylene glycol) diacrylate and N-isopropylacrylamide and the continuous phase of the emulsion is an aqueous solution of dextran and a dextran solubility reducer. The microspheres will load protein, e.g., cytokines, from aqueous solution.
Owner:CORNELL RES FOUNDATION INC
Cell cryopreservation liquid, application thereof and cryopreservation method of megakaryocyte progenitor cells
ActiveCN105076116AVitality has little effectReduce harmDead animal preservationCryopreservationCellular viability
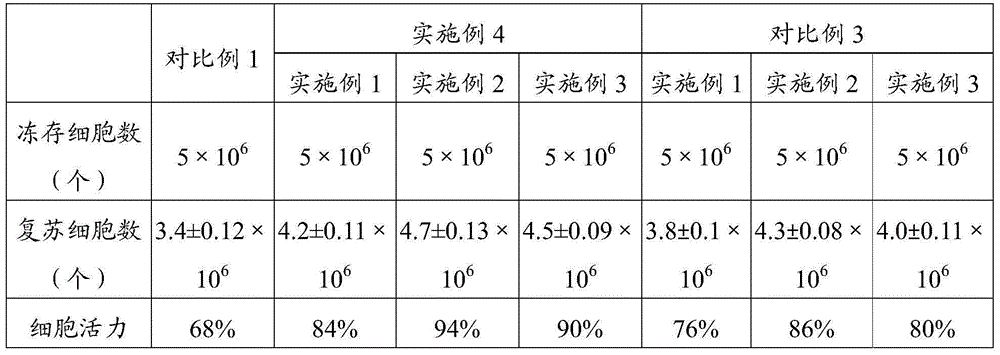
The invention relates to the field of cell culture, in particular to cell cryopreservation liquid, application thereof and a cryopreservation method of megakaryocyte progenitor cells. The cell cryopreservation liquid comprises DMSO, fetal calf serum, dextran, trehalose and albumin. By means of the cell cryopreservation liquid, the cytoactive of the megakaryocyte progenitor cells can be well maintained during the cryopreservation period, and damage to cells in the cryopreservation and resuscitation processes is lowered. According to the cell cryopreservation liquid, application thereof and the cryopreservation method of the megakaryocyte progenitor cells, the cryopreservation and temperature reduction processes are softer, the cells are stored in liquid nitrogen after the temperature reduction process is conducted, the influence on the cytoactive is small, and damage to the cells in the cryopreservation process is lowered. It is indicated by experiments that the vigour of the resuscitated cells can reach 94% one month after the megakaryocyte progenitor cells are cryopreservated in the cryopreservation liquid, and multiplication activity is good, and the cell cryopreservation liquid is significantly superior to the prior art.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Iron-dextran compound for use as a component in a therapeutical composition for prophylaxis or treatment of iron-deficiency
An iron-dextran compound for parenteral treatment of iron-deficiency anemia comprises hydrogenated dextran having a weight average molecular weight (Mw) between 700 and 1,400 Daltons, preferably approximately 1,000 Daltons, a number average molecular weight (Mn) of 400 to 1,400 Daltons and wherein 90% by weight of the dextran has molecular weights less than 2,700 Daltons and the Mw of the 10% by weight fraction of the dextran having the highest molecular weights is below 3,200 Daltons, said hydrogenated dextran having been subjected to purification by membrane processes having a cut-off value between 340 and 800 Daltons, in stable association with ferric oxyhydroxide. The compound is produced by using membrane processes to eliminate dextrans of higher molecular weights than approximately 2,700 Daltons and membrane processes to remove saccharides of molecular weights below approximately 340 Daltons from hydrogenated dextran before precipitating ferric hydroxide in the presence of said dextran followed by heat treatment and purification.
Owner:PHARMACOSMOS HLDG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com