Patents
Literature
1472 results about "Clinical trial" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial – their approval does not mean that the therapy is 'safe' or effective, only that the trial may be conducted.
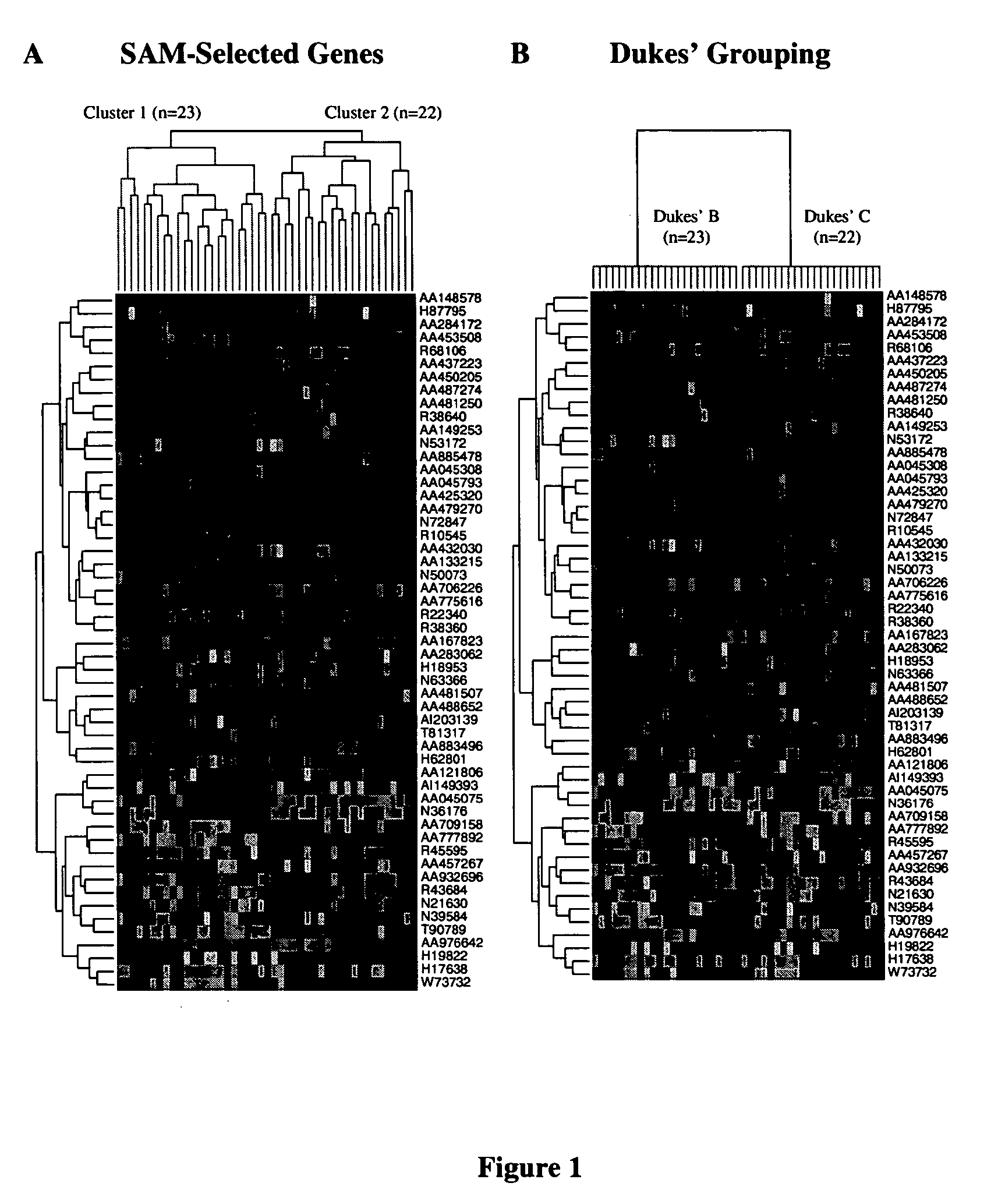
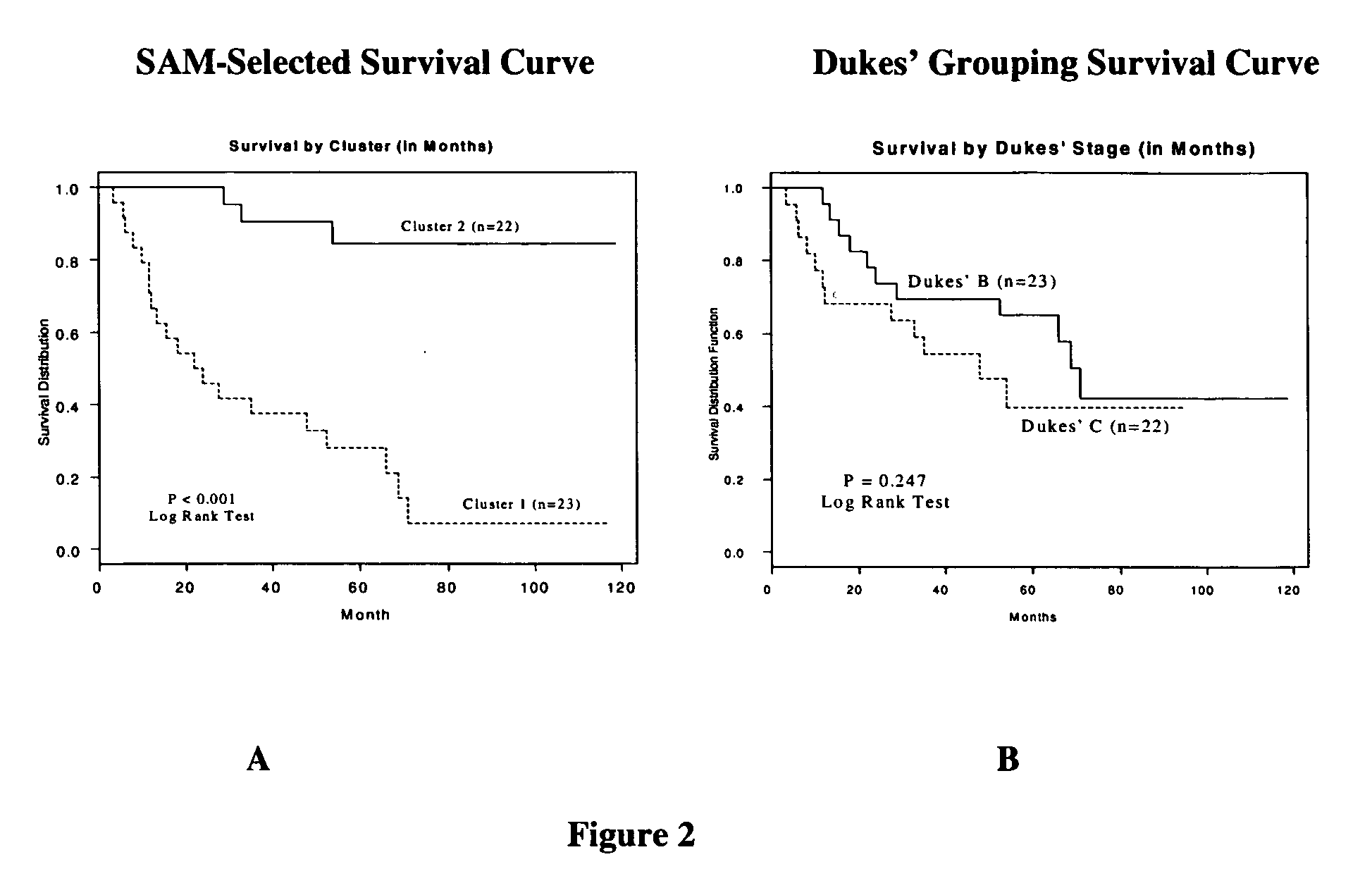
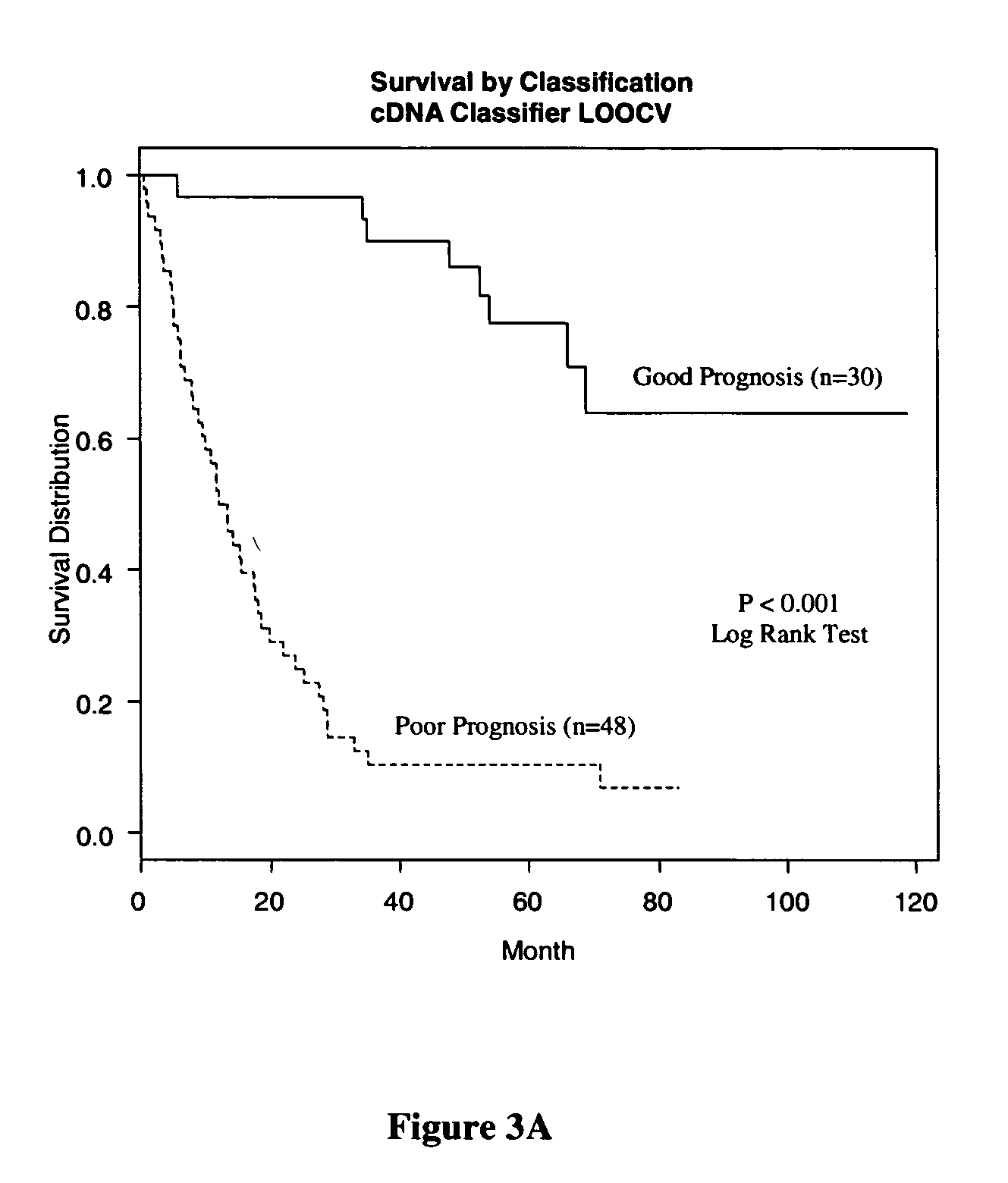
Methods and systems for predicting cancer outcome
The invention provides a molecular marker set that can be used for prognosis of colorectal cancer in a colorectal cancer patient. The invention also provides methods and computer systems for evaluating prognosis of colorectal cancer in a colorectal cancer patient based on the molecular marker set. The invention also provides methods and computer systems for determining chemotherapy for a colorectal cancer patient and for enrolling patients in clinical trials.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC +1
Methods for the treatment and diagnosis of cardiovascular disease
InactiveUS6156500AImprove throughputBacteriaPeptide/protein ingredientsClinical trialPercent Diameter Stenosis
The present invention relates to methods and compositions for the treatment and diagnosis of cardiovascular disease, including, but not limited to, atherosclerosis, ischemia / reperfusion, hypertension, restenosis, and arterial inflammation. Specifically, the present invention identifies and describes genes which are differentially expressed in cardiovascular disease states, relative to their expression in normal, or non-cardiovascular disease states, and / or in response to manipulations relevant to cardiovascular disease. Further, the present invention identifies and describes genes via the ability of their gene products to interact with gene products involved in cardiovascular disease. Still further, the present invention provides methods for the identification and therapeutic use of compounds as treatments of cardiovascular disease. Moreover, the present invention provides methods for the diagnostic monitoring of patients undergoing clinical evaluation for the treatment of cardiovascular disease, and for monitoring the efficacy of compounds in clinical trials. Additionally, the present invention describes methods for the diagnostic evaluation and prognosis of various cardiovascular diseases, and for the identification of subjects exhibiting a predisposition to such conditions.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Interactive psychophysiological profiler method and system
An efficient, objective, flexible and easily deployable system for conducting evaluations of mental and physiological state and recommending individualized treatment to improve said state is described. The method and system are based on commensurate measurement of mental functions, levels of stress and anxiety, and / or biologically active molecules such as neurotransmitters, immune markers including cytokines and hormones. The method and system are designed to assess an individual's cognitive function and the underlying physiology in order to delineate various disease processes, injuries, drug states, training stages, fatigue levels, stress levels, aging processes, predict susceptibility to stress and / or sleep deprivation, identify aptitude for training and / or characterize effects of any experimental conditions. The system and method may be used in recommending individualized treatment protocols, as well as to guide the treatment process by assessing the efficacy of such therapies in the clinical trials process.
Owner:ADVANCED BRAIN MONITORING
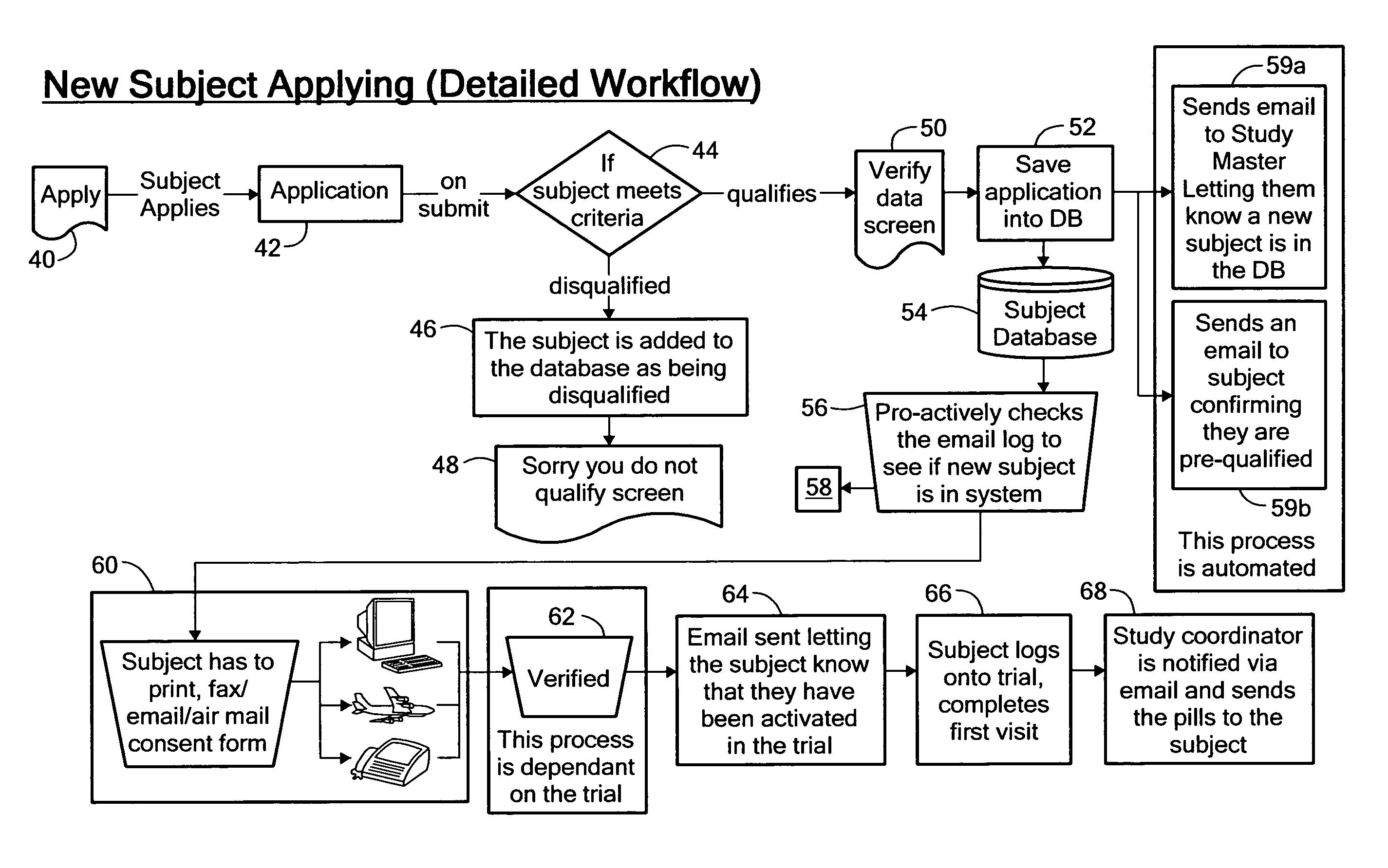
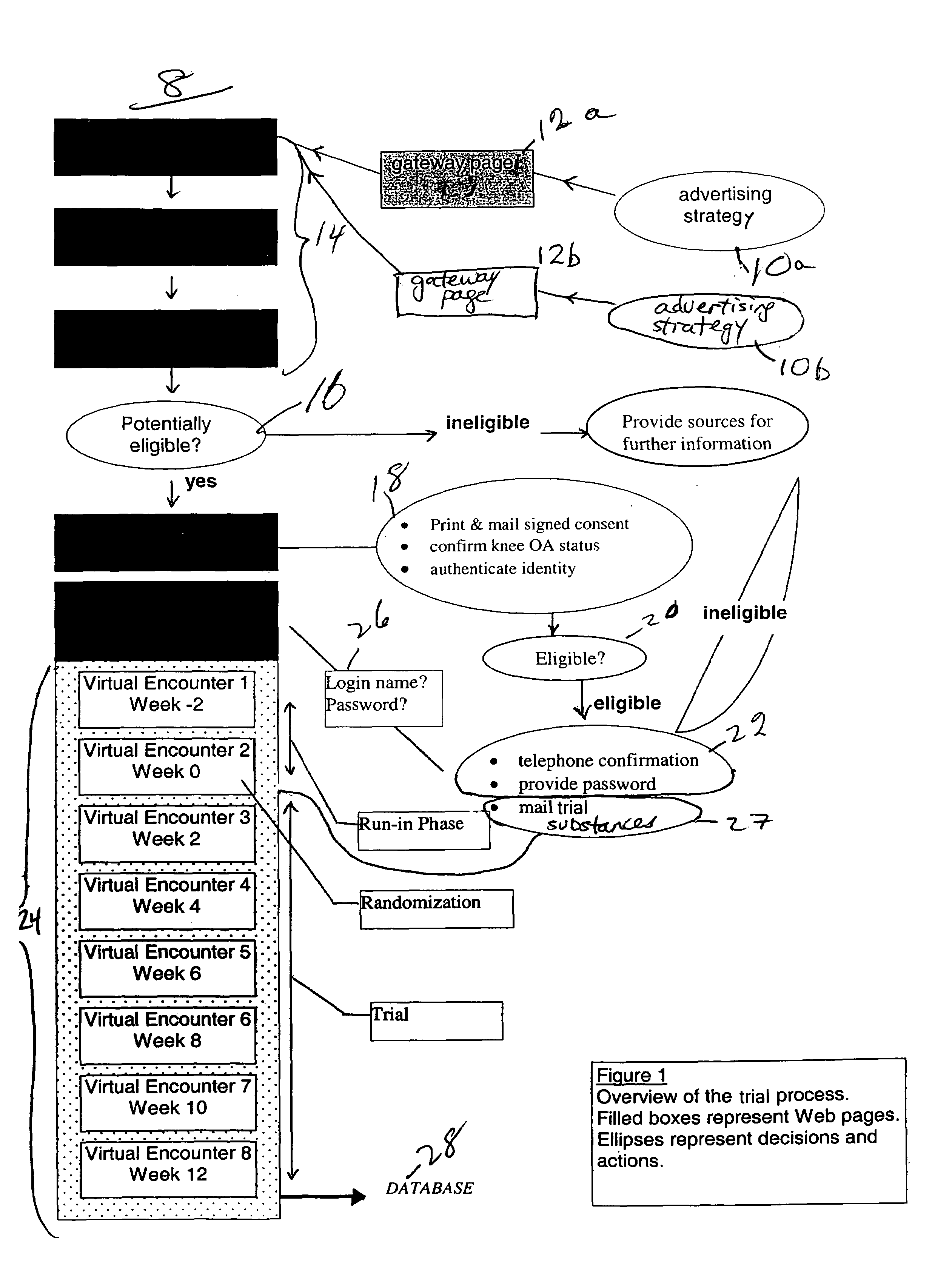
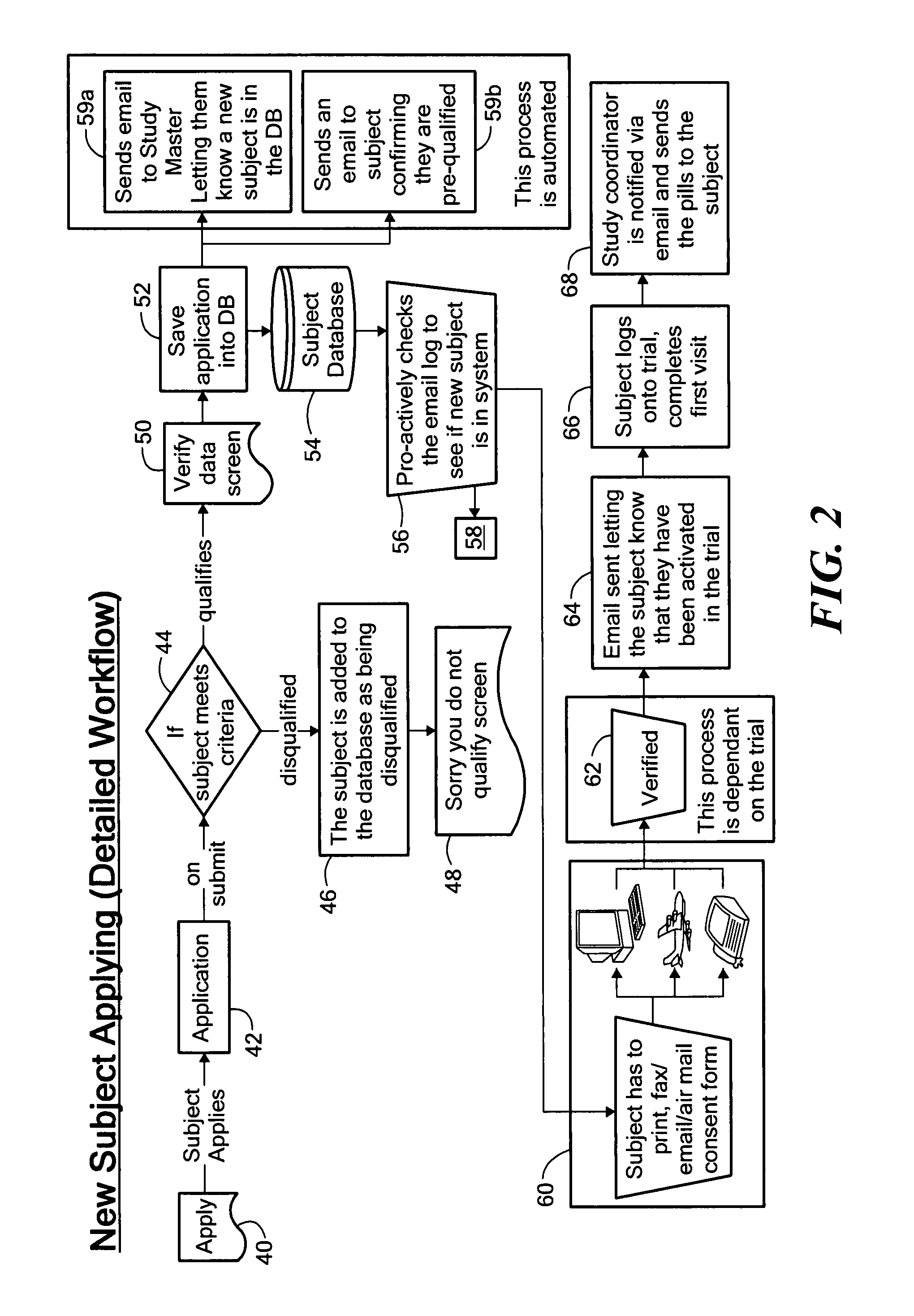
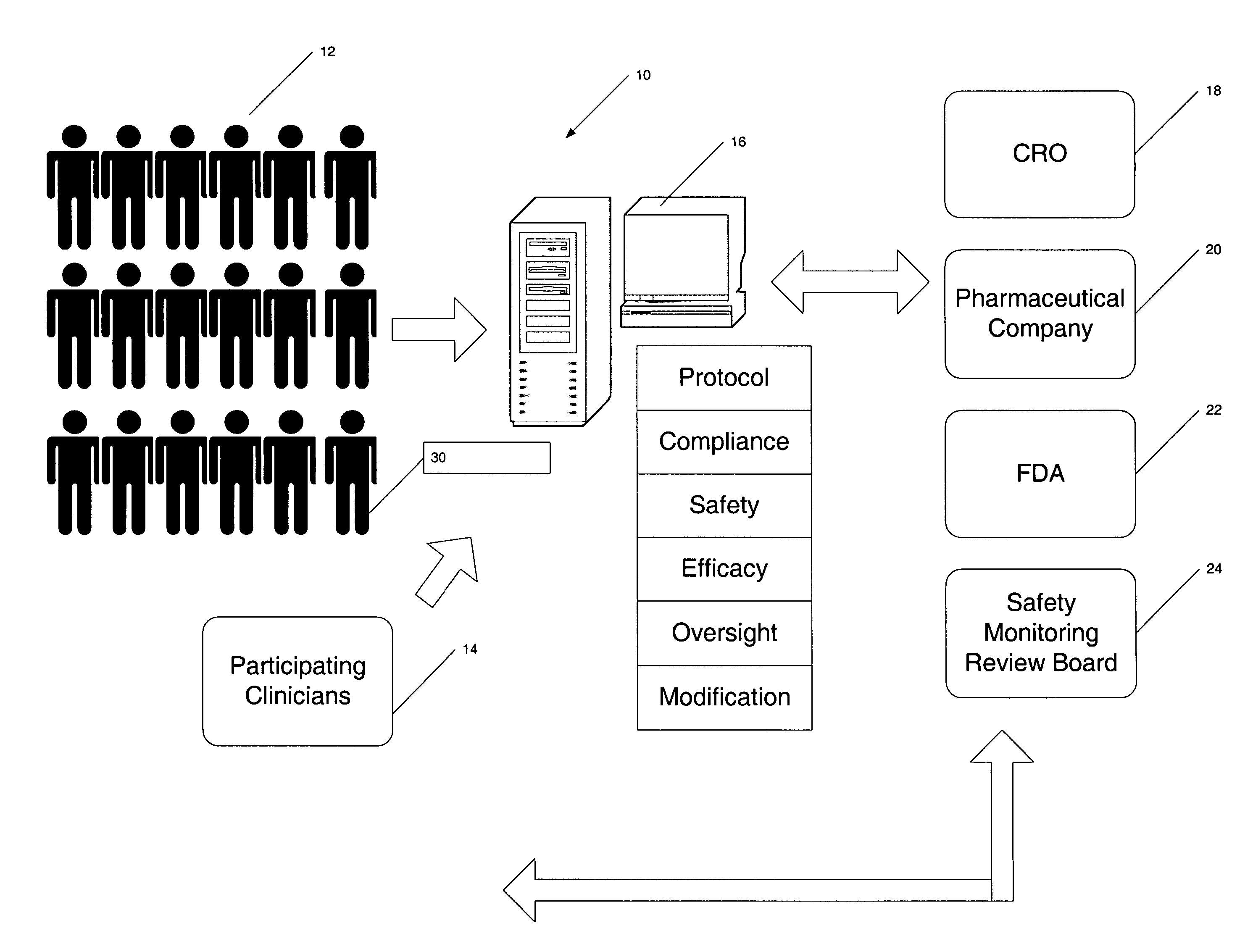
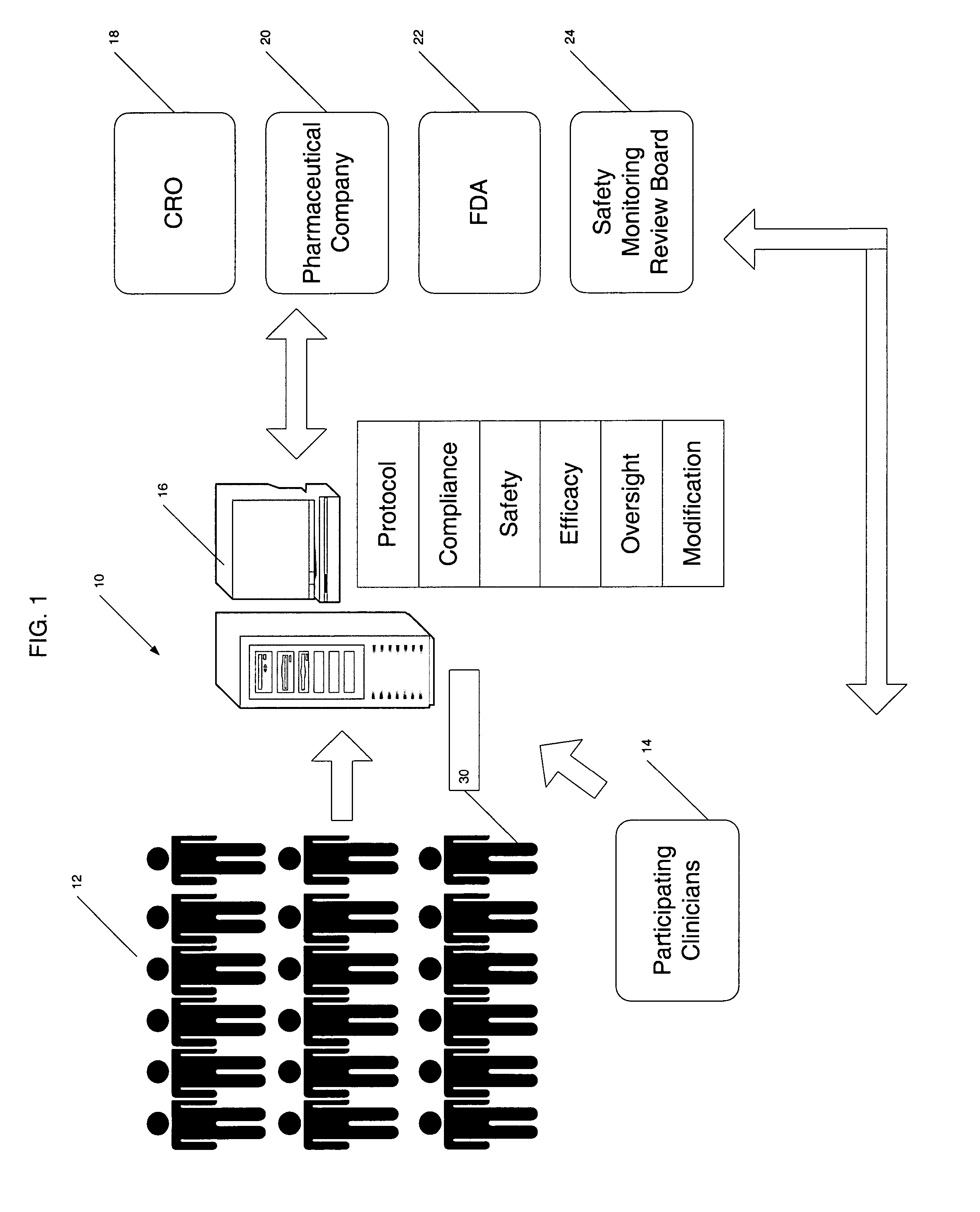
Method for conducting clinical trials over the internet
InactiveUS7251609B1Data processing applicationsComputer-assisted medical data acquisitionSubstance usePrimary sites
The invention encompasses a method of conducting a clinical trial of a test substance from a primary site, via the internet. The internet is used in various phases of a clinical trial, including: recruiting and screening for candidates who are eligible to participate in a clinical trial of a test substance using the internet; obtaining, directly from a participant at a remote site, personal information as well as information allowing a determination of any effect(s) of the test substance on the participant after use (e.g., by evaluation forms completed and transmitted over the internet); compiling data from multiple participants.
Owner:TRUSTEES OF BOSTON UNIV
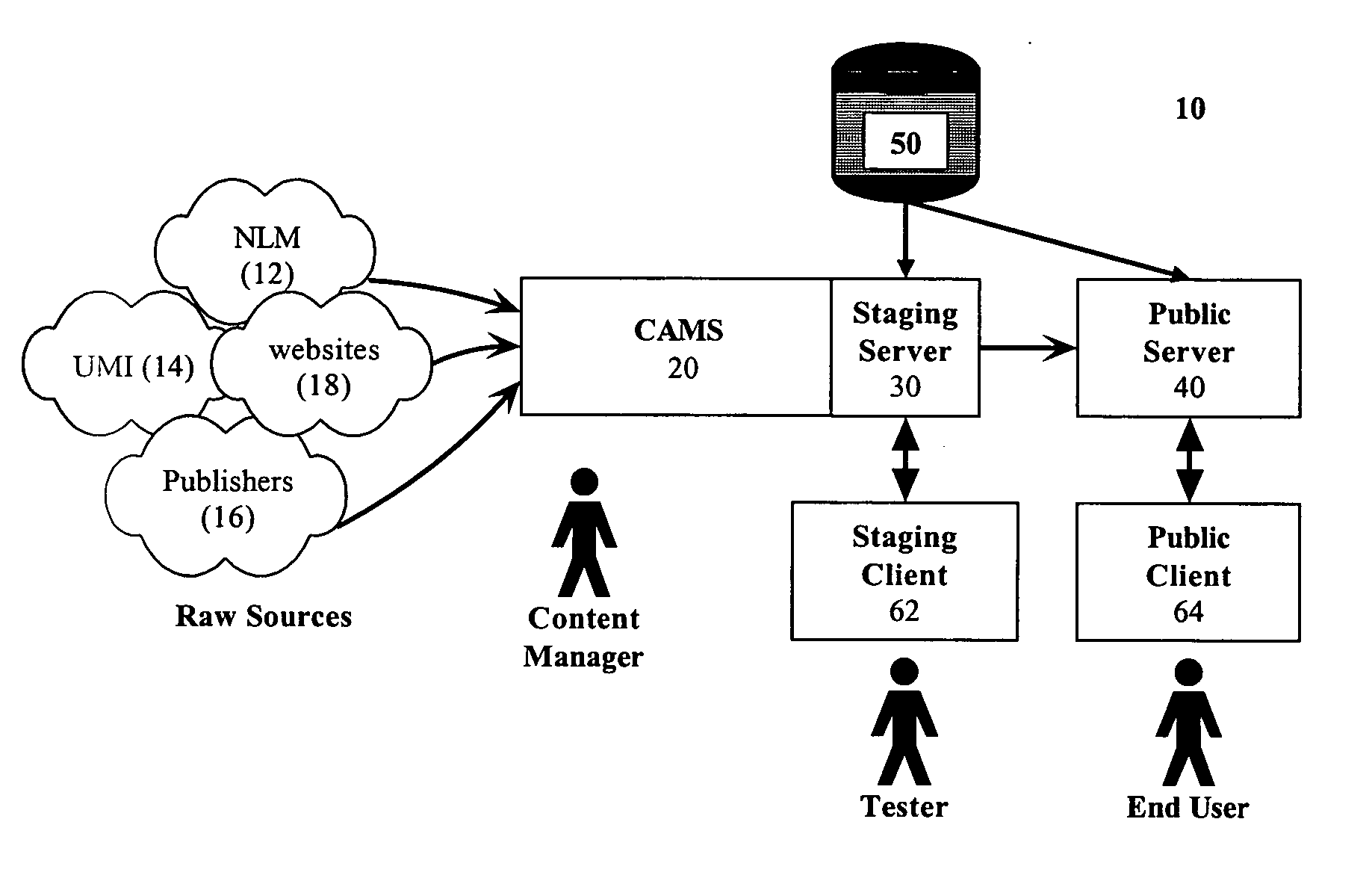
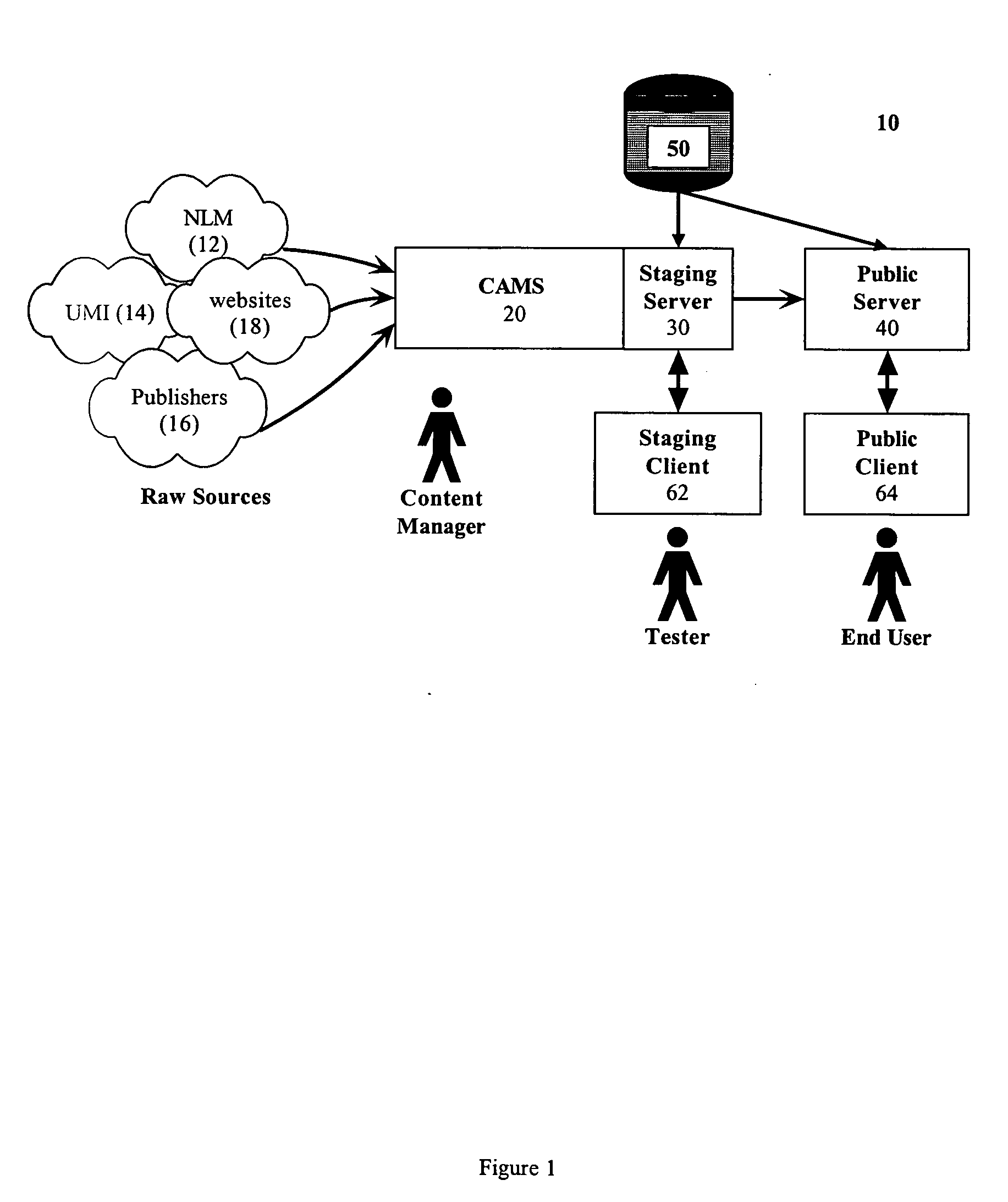
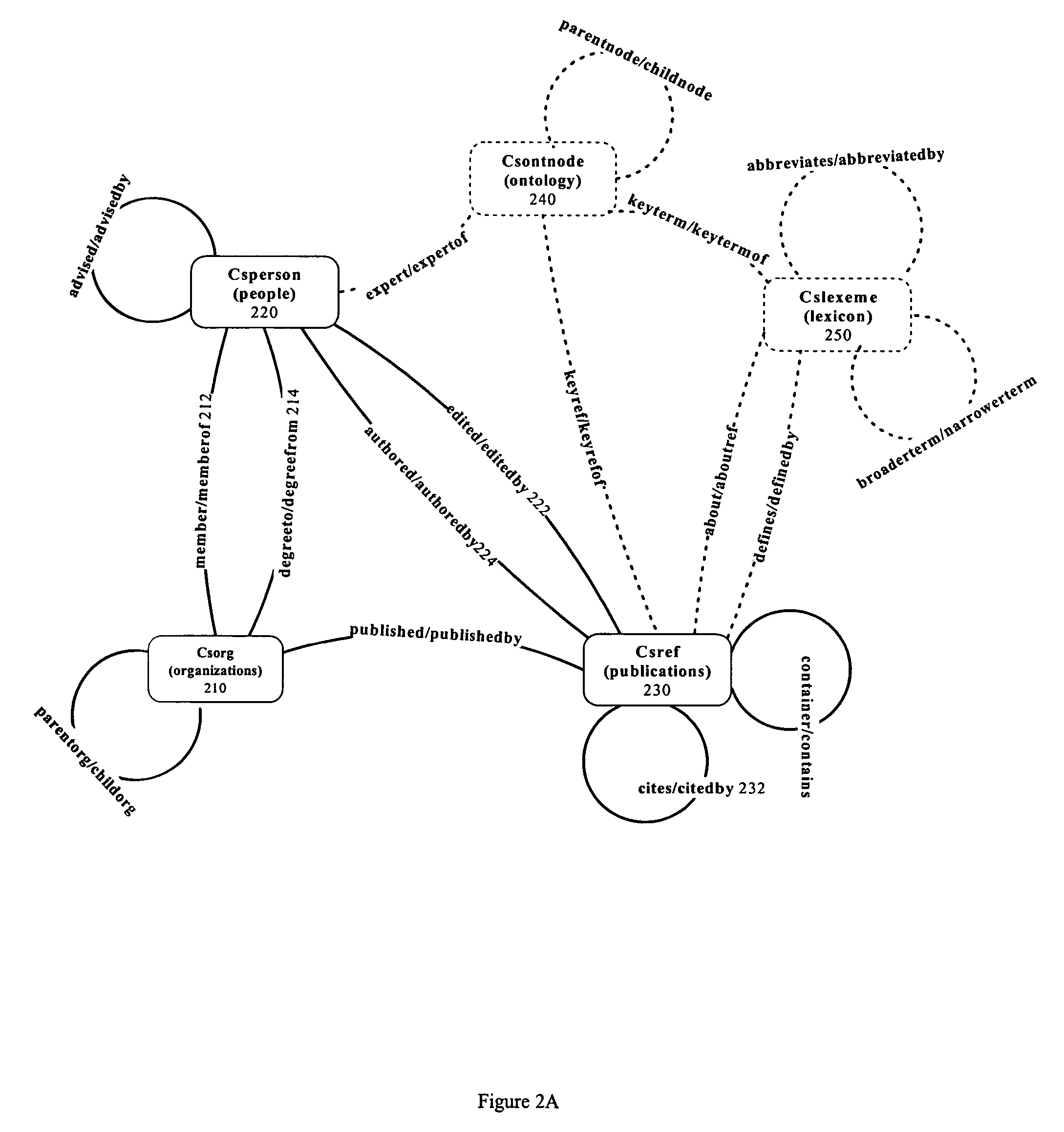
Systems and methods for creating and publishing relational data bases
InactiveUS20050149538A1Digital data processing detailsWebsite content managementEntity typeSource type
A searchable electronic database system that can return search results independent of reference source type. The electronic database system includes information that can be content or discipline specific. The database can be focused to allow research to be limited to the discipline specific universe of information. The database can include person, organization, publication, and other entity types. The publications can include journal articles, books, dissertations, grants, clinical trials, and web resources. The database can also include ontology and lexicon entities. The entities are interconnected through relationships. Searches performed on the database return results across all entity types. A single search can return results from each of the different publication types. Details of the results can be displayed. Dynamic links to one or more fields in a particular result detail can link to a result categorized according to the field.
Owner:SINGH SADANAND +5
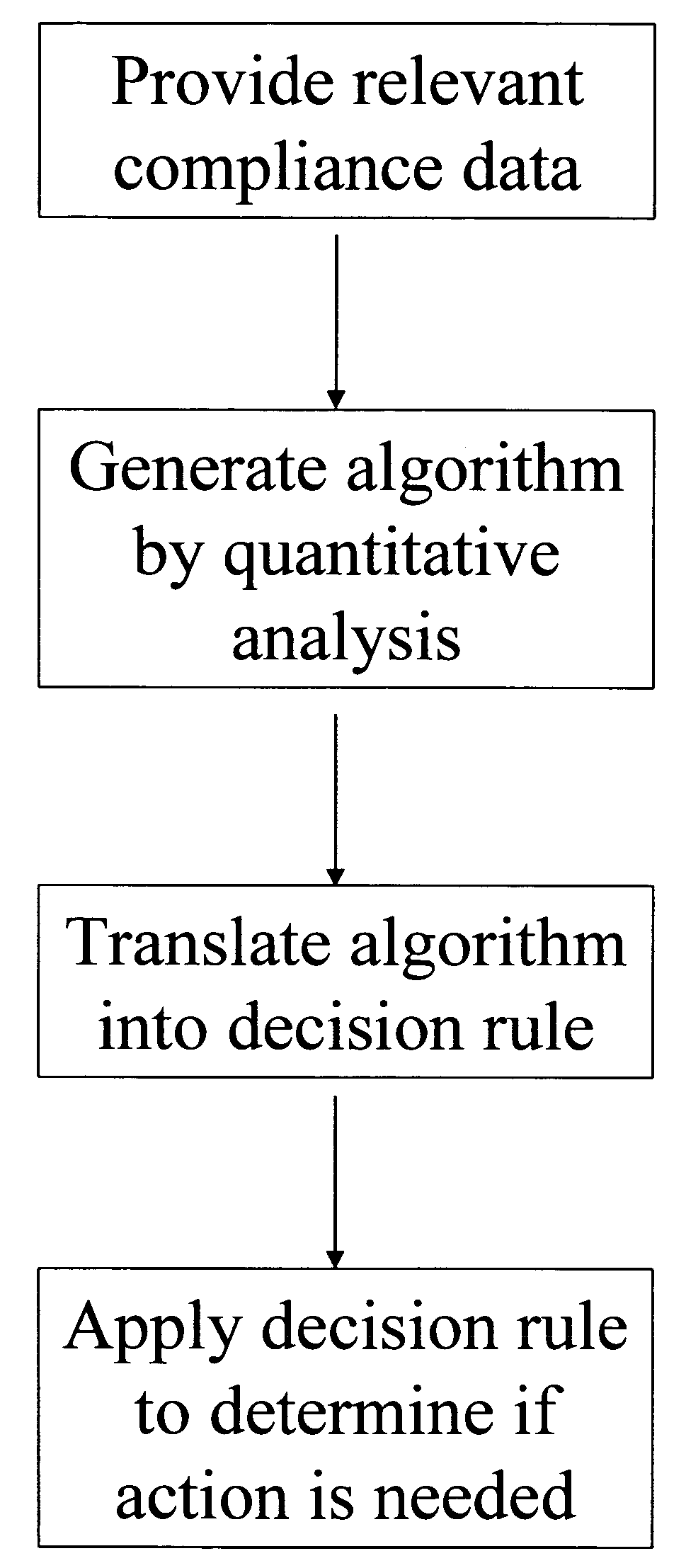
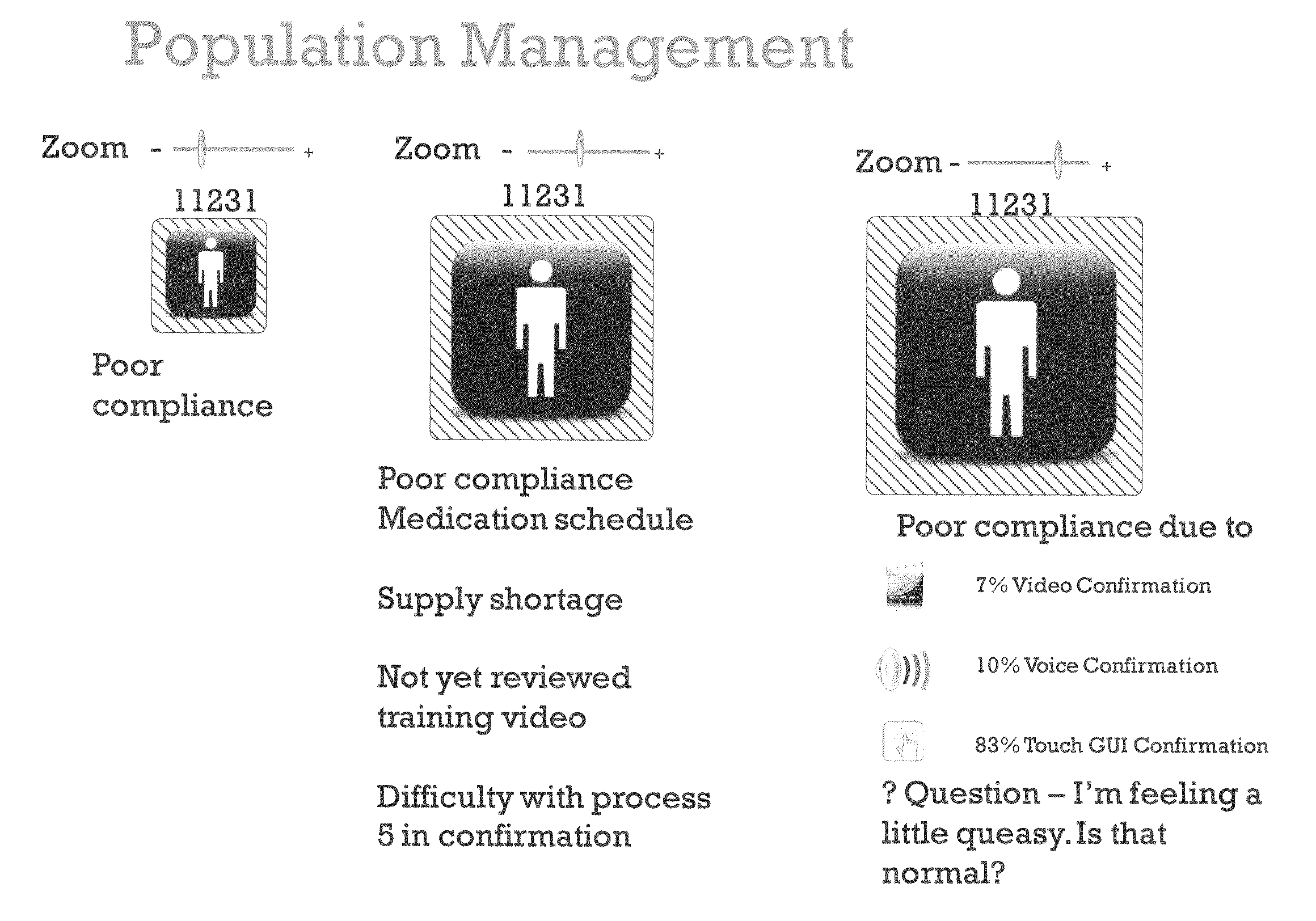
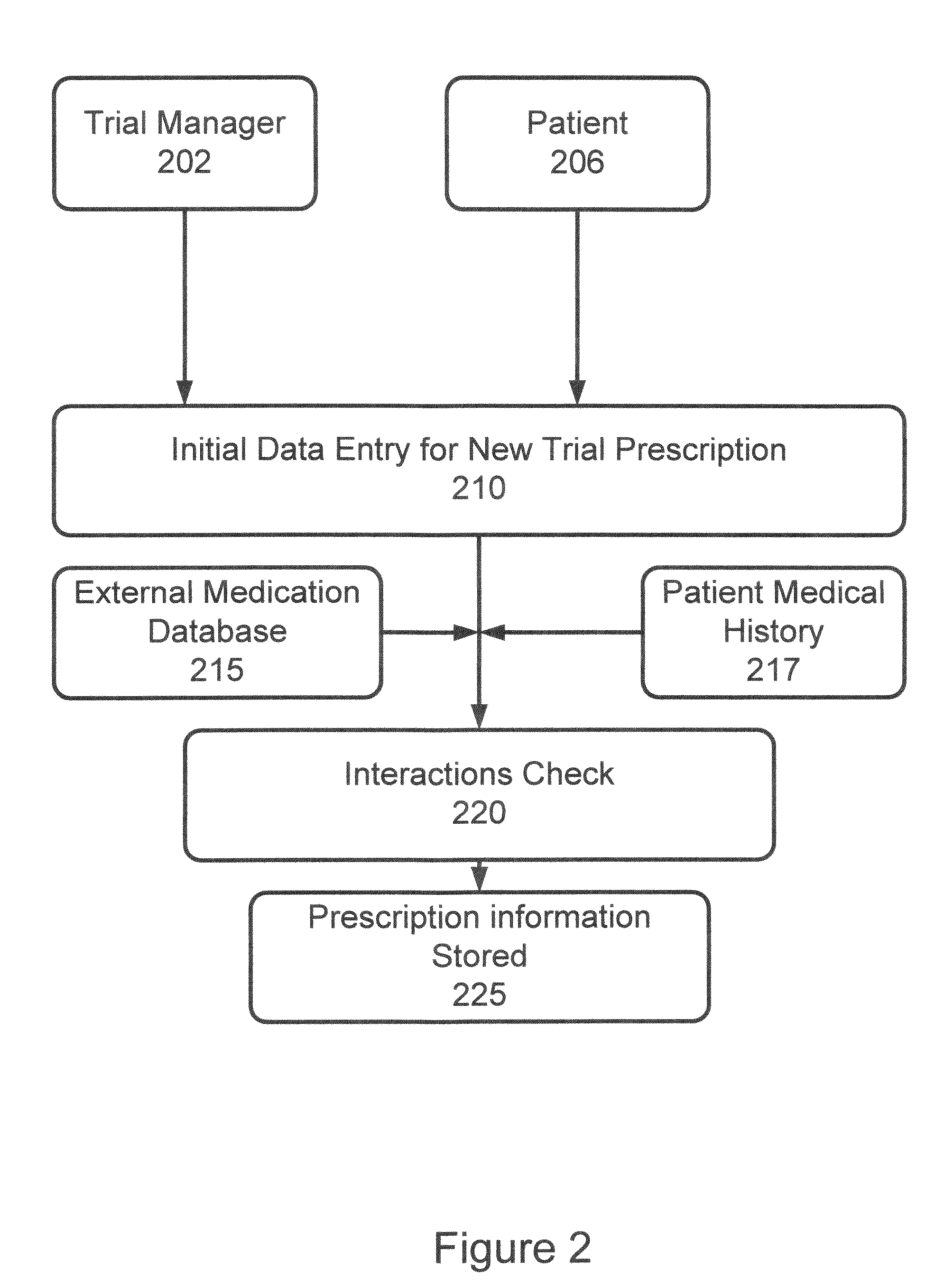
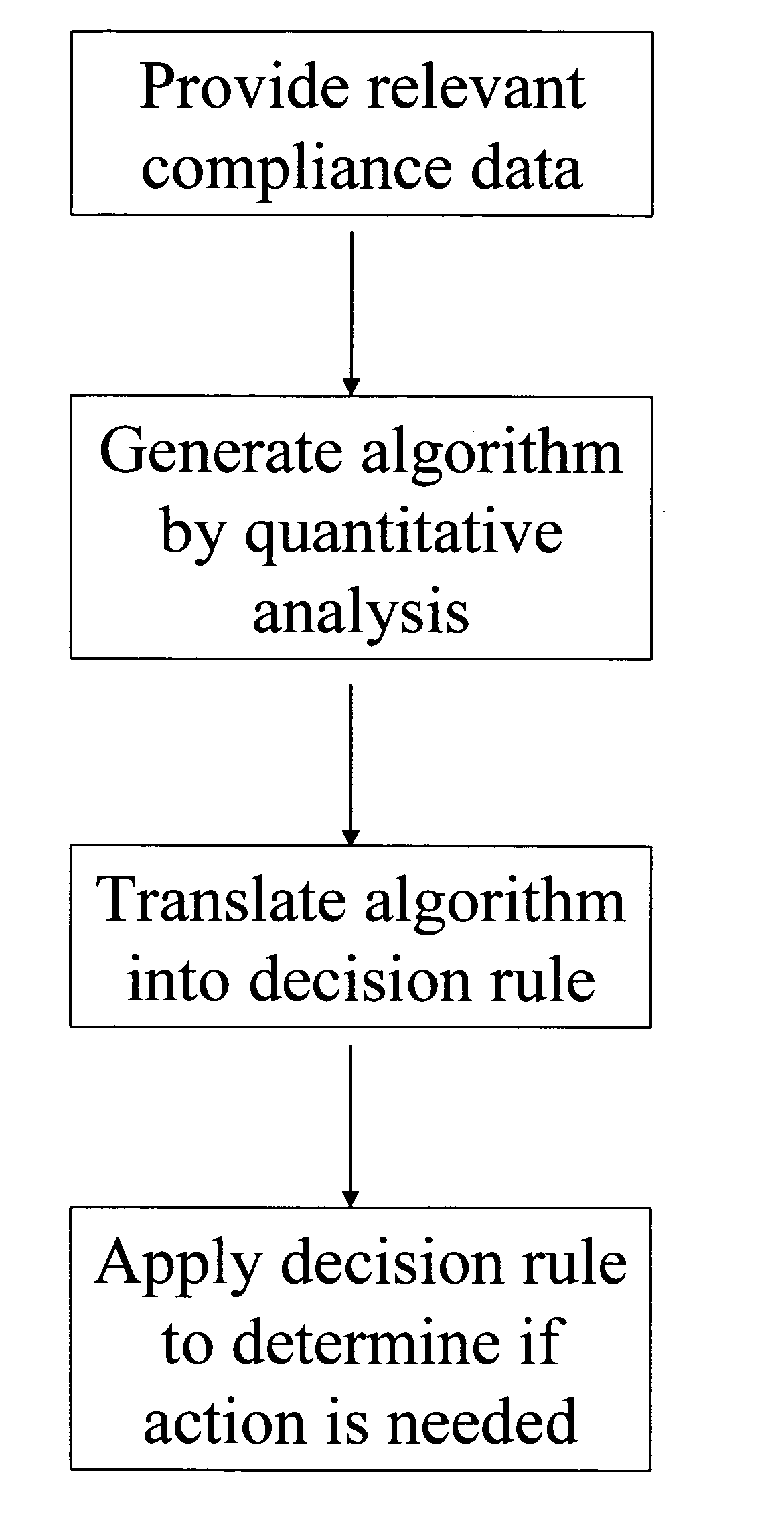
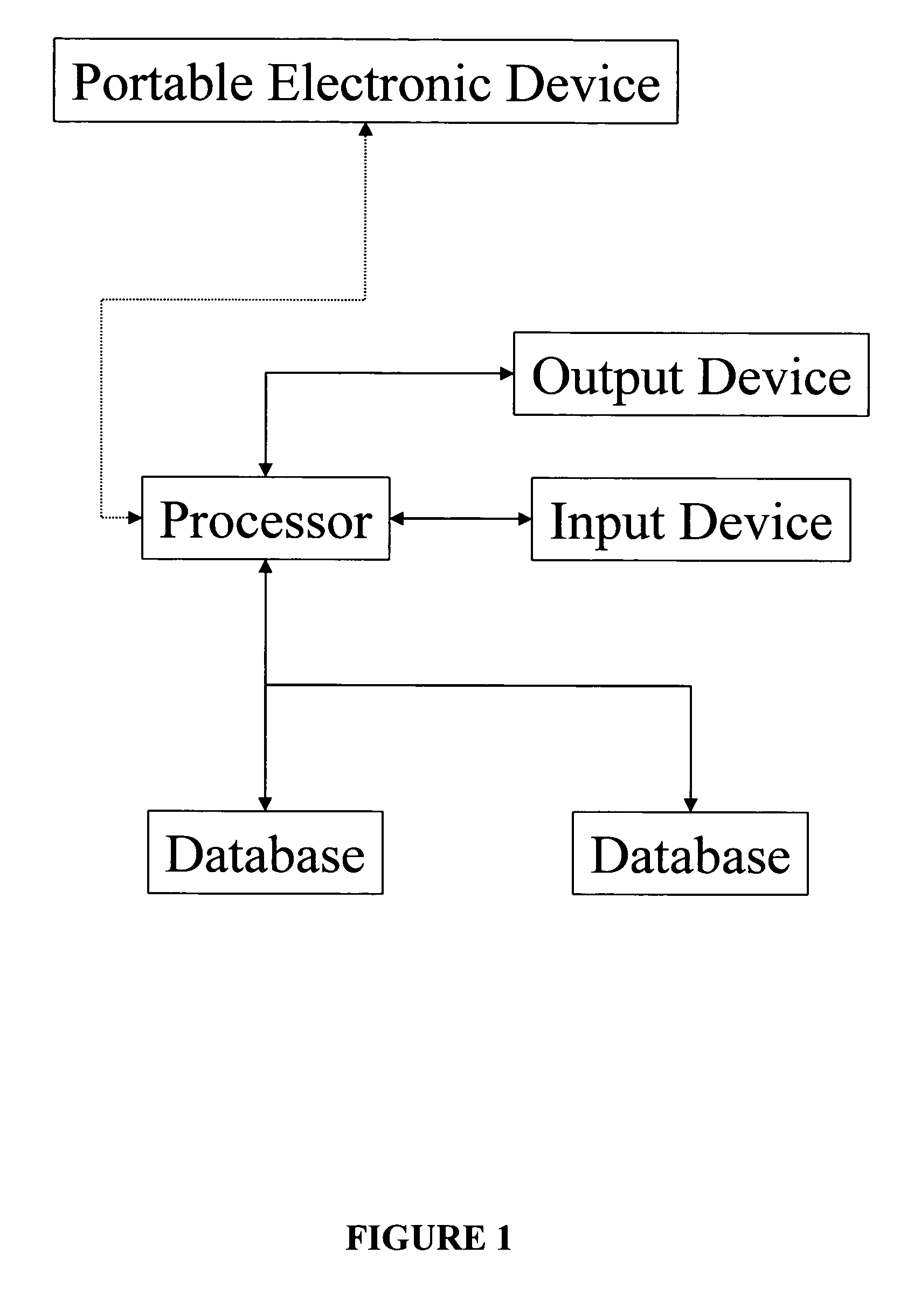
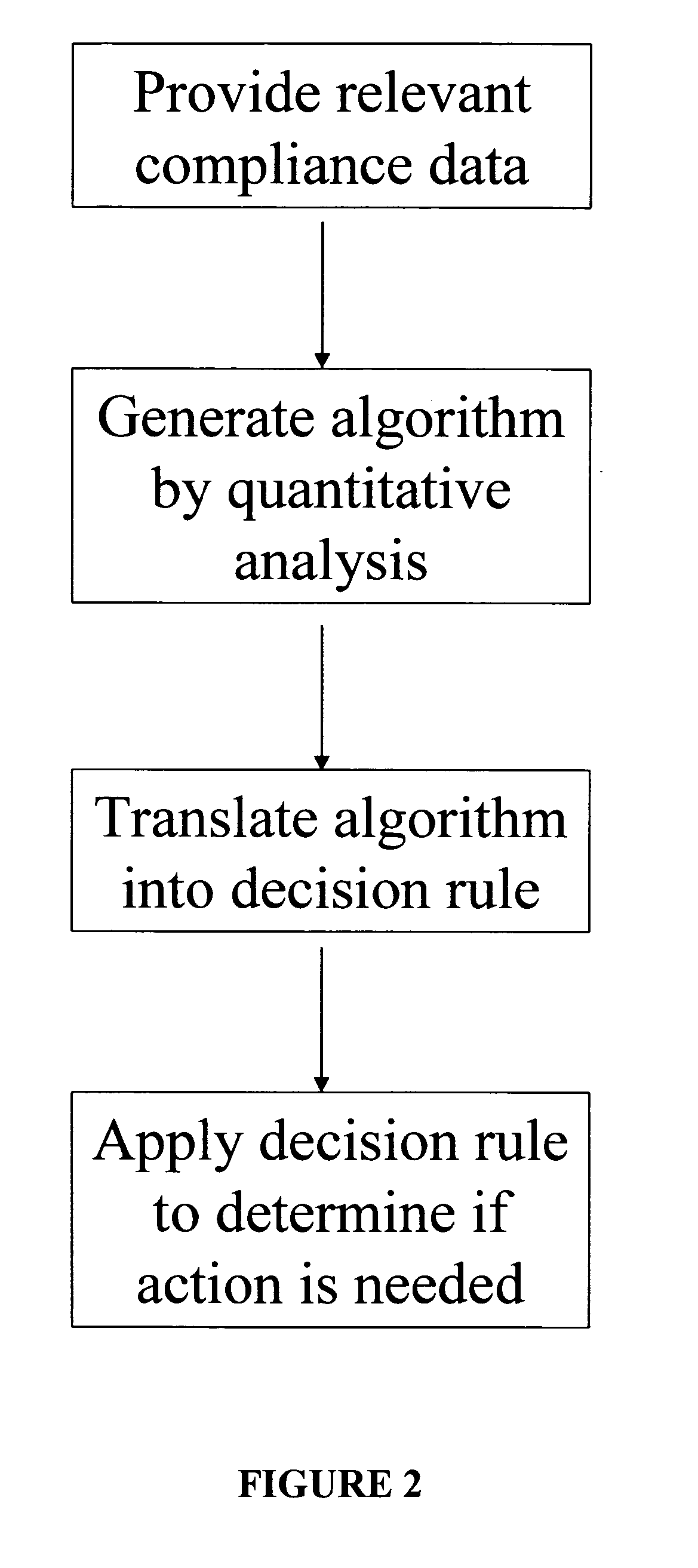
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS7415447B2Reduce testing costsImprove statistics performanceDigital computer detailsForecastingNon complianceClinical trial
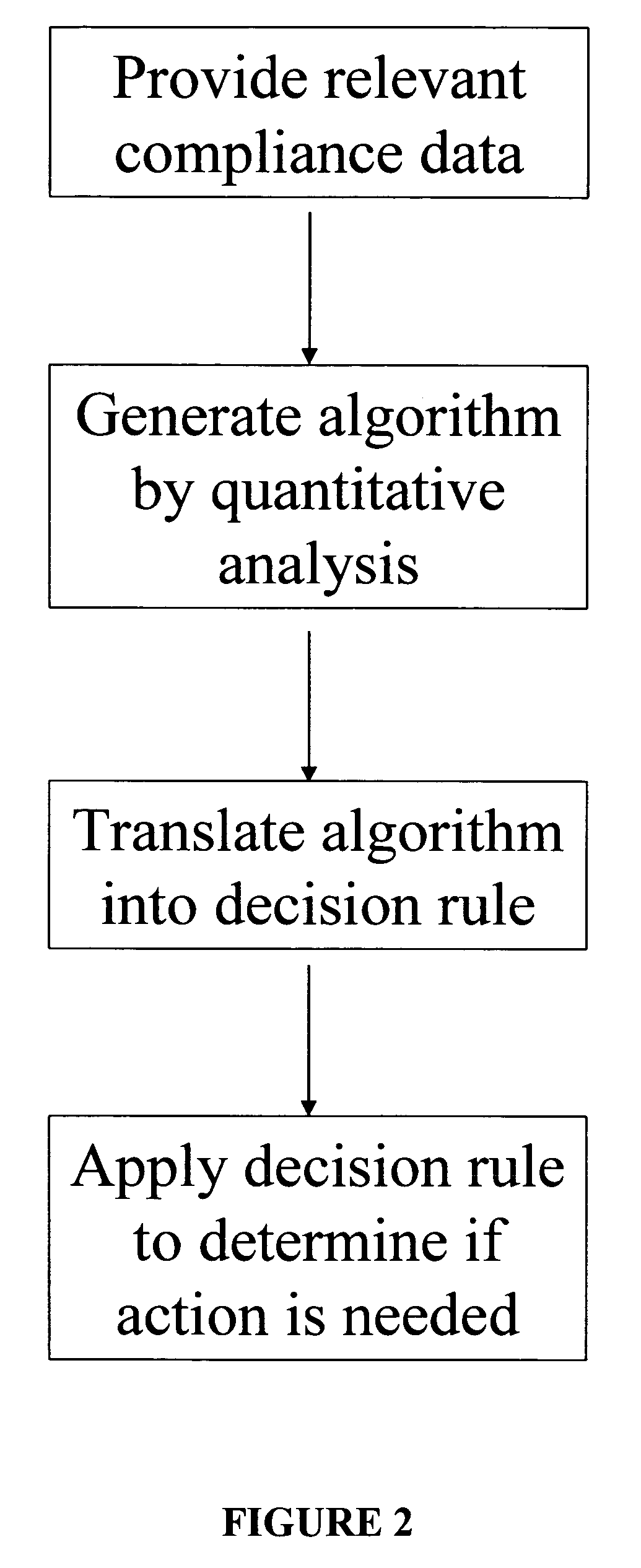
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
Personalized Monitoring and Healthcare Information Management Using Physiological Basis Functions
InactiveUS20110004110A1Accurate trackingAccurate classificationMedical data miningMedical automated diagnosisPersonalizationSide effect
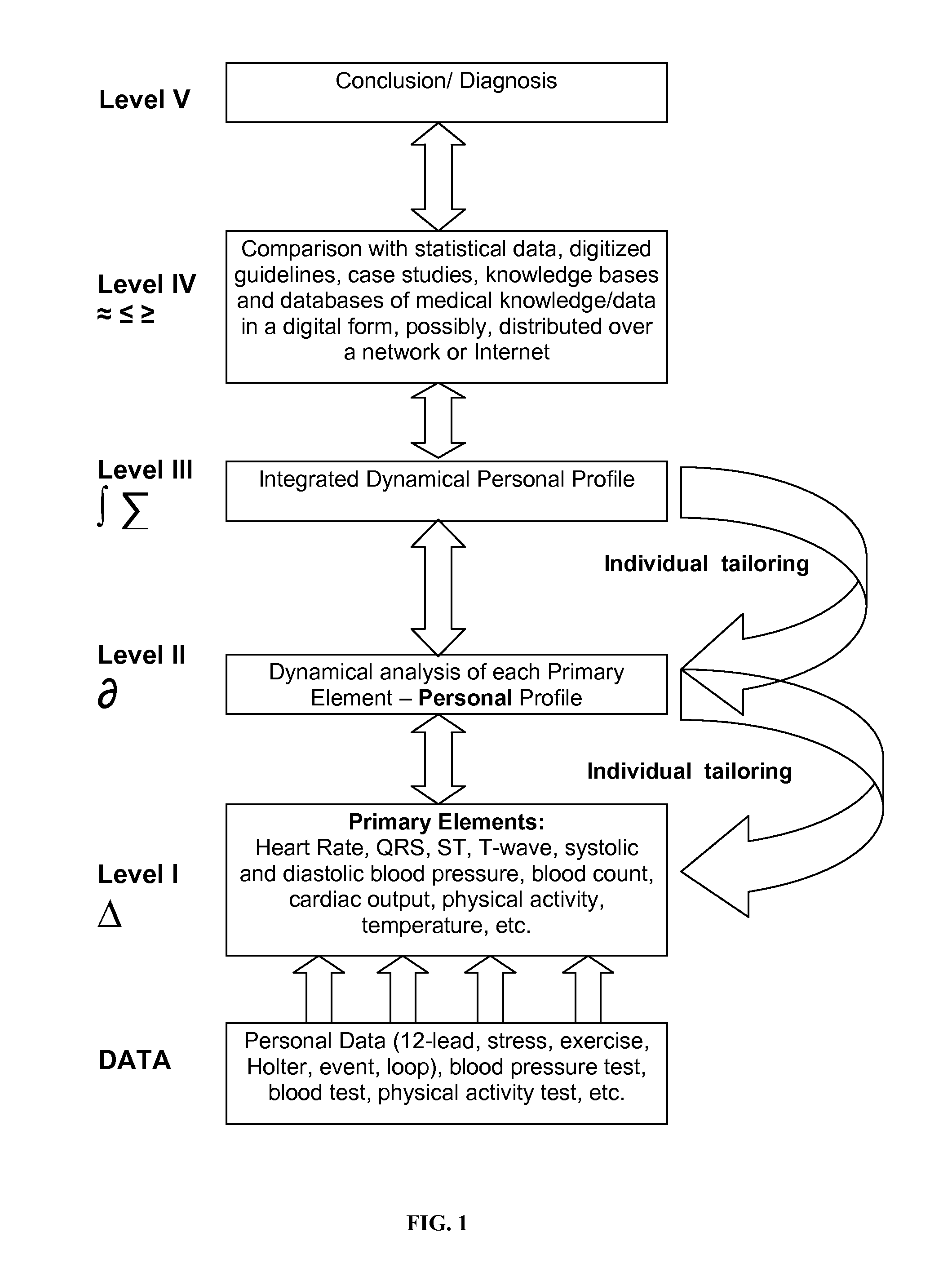
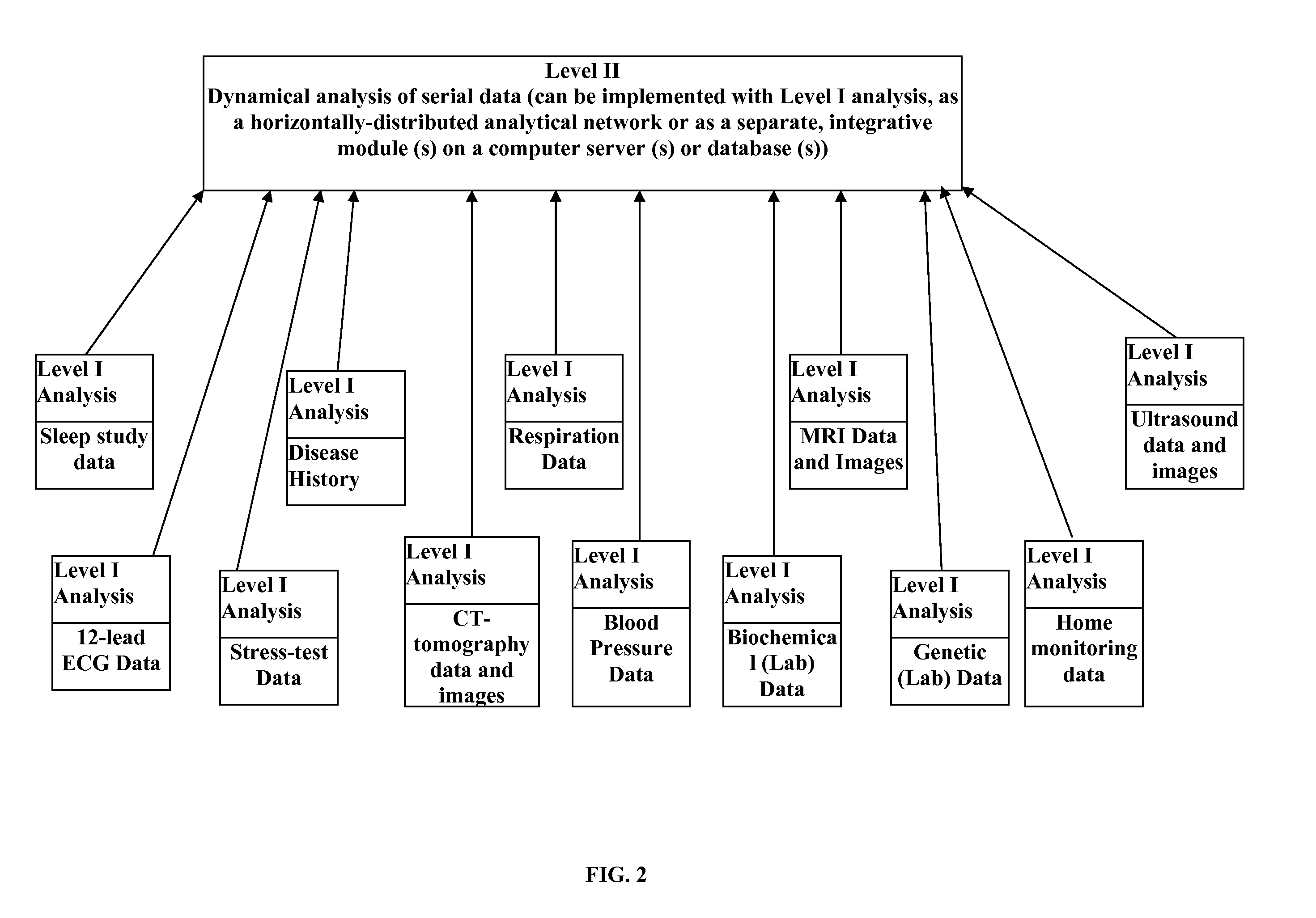
Analysis of individual's serial changes, also referred to as the physiological, pathophysiological, medical or health dynamics, is the backbone of medical diagnosis, monitoring and patient healthcare management. However, such an analysis is complicated by enormous intra-individual and inter-individual variability. To address this problem, a novel serial-analysis method and system based on the concept of personalized basis functions (PBFs) is disclosed. Due to more accurate reference information provided by the PBFs, individual's changes associated with specific physiological activity or a sequence, transition or combination of activities (for example, a transition from sleep to wakefulness and transition from rest to exercise) can be monitored more accurately. Hence, subtle but clinically important changes can be detected earlier than using other methods. A library of individual's PBFs and their transition probabilities (which can be described by Hidden Markov Models) can completely describe individual's physiological dynamics. The system can be adapted for healthcare information management, diagnosis, medical decision support, treatment and side-effect control. It can also be adapted for guiding health, fitness and wellness training, subject identification and more efficient management of clinical trials.
Owner:SHUSTERMAN VLADIMIR
Radiolabeling method using multivalent glycoligands as hepatic receptor imaging agent
InactiveUS20110097264A1Low toxicityImprove securityRadioactive preparation carriersSaccharide peptide ingredientsImaging agentClinical trial
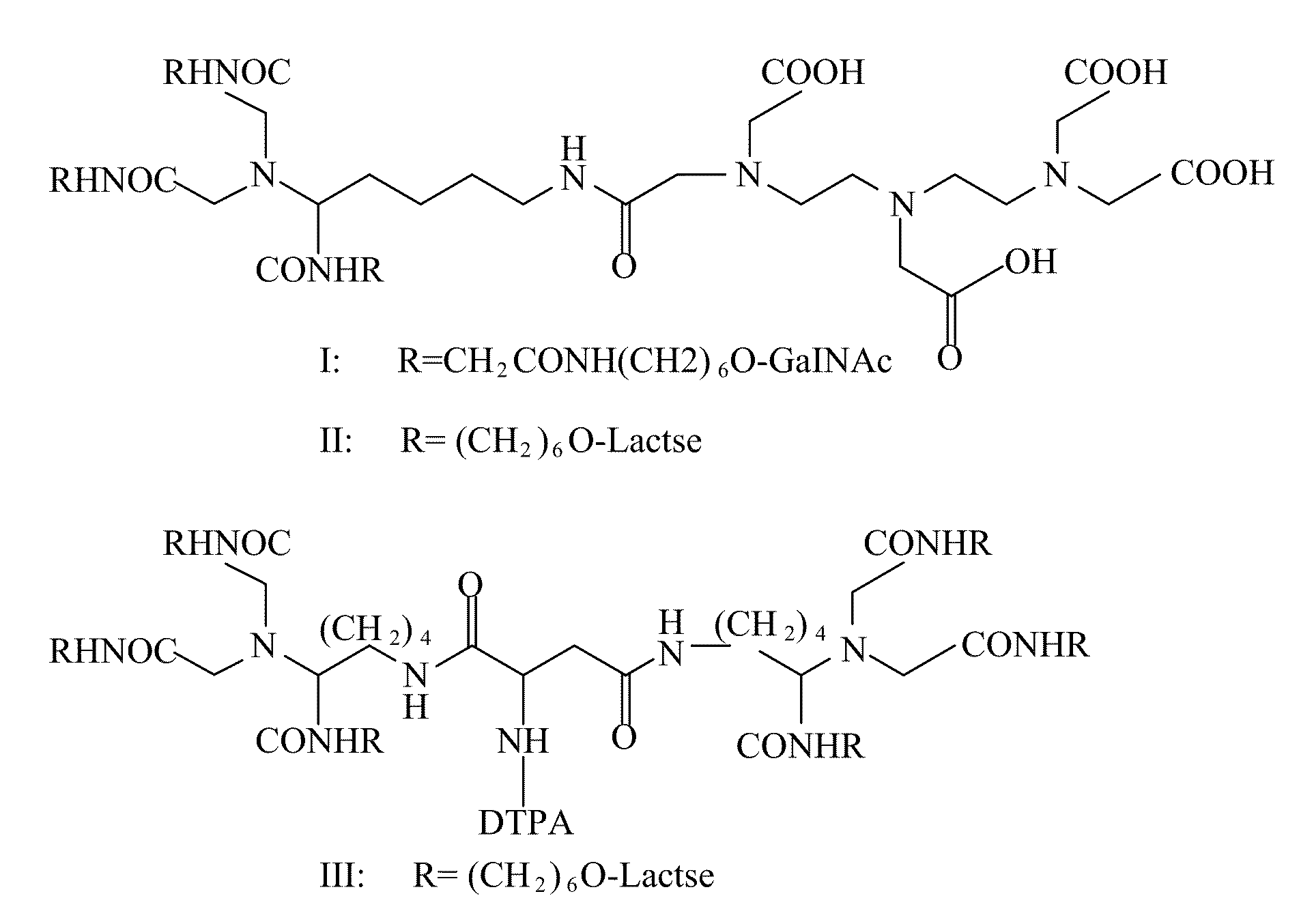
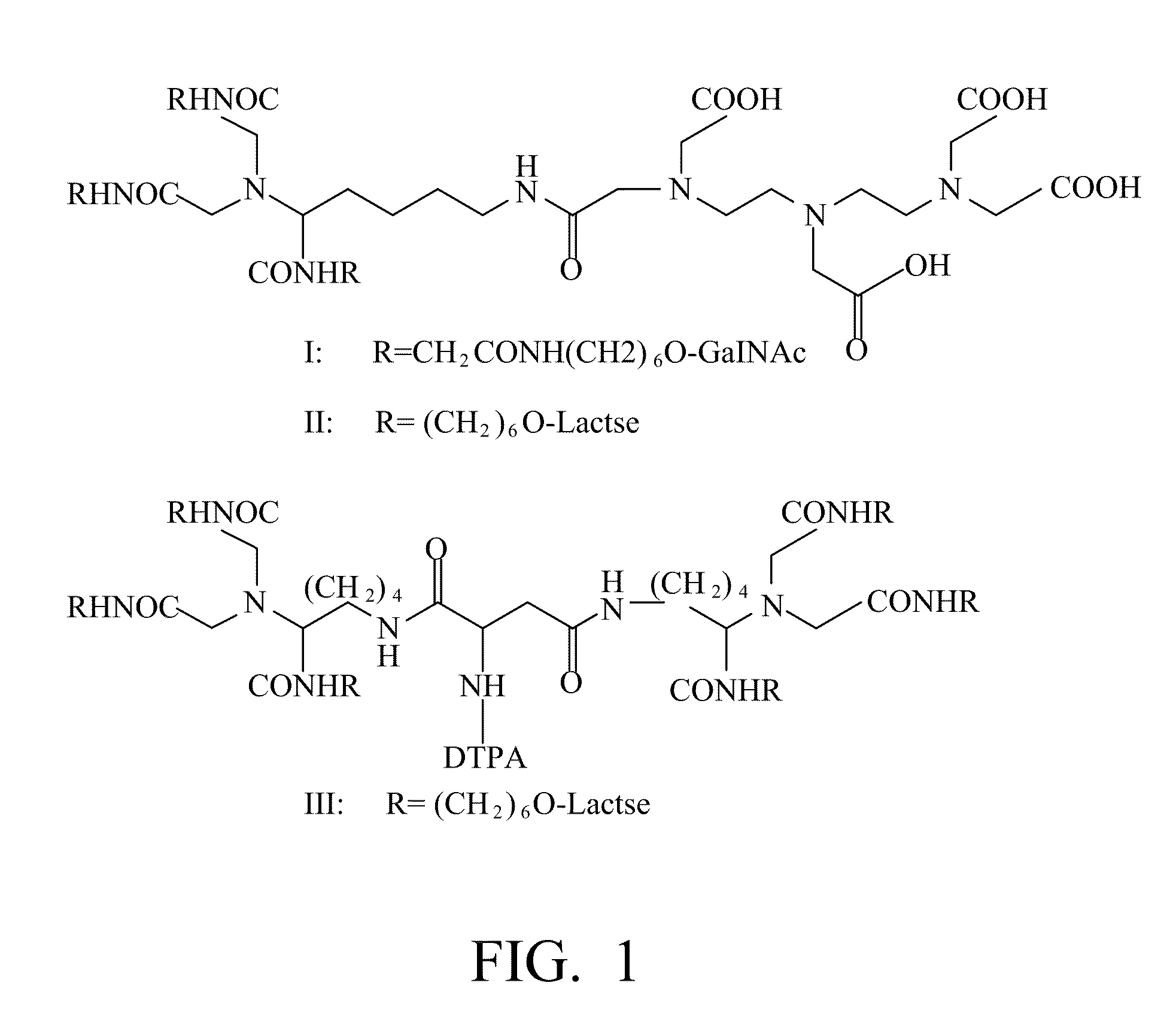
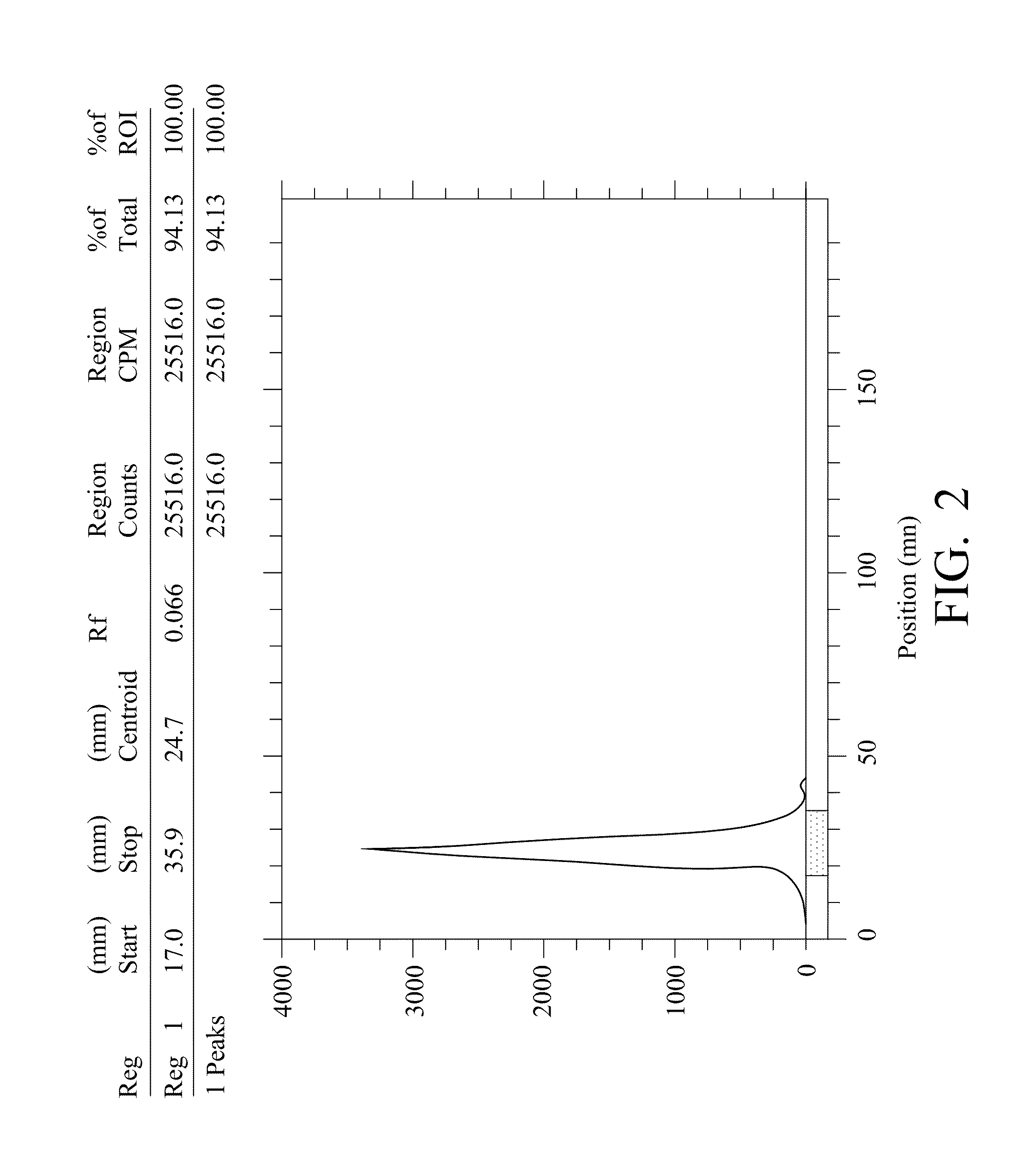
A radiolabeling method using a multivalent glycoligand as hepatic receptor imaging agent is provided. The multivalent glycoligand-DTPA derivatives (In-111-DTPA-hexa lactoside and In-111-DTPA-tri-galactosamine glycoside) labeled with In-111 are used as hepatic receptor imaging agent. The effects of imaging of a hepatic receptor in different species are evaluated, the lowest specific radioactivity values of hepatic receptor imaging required in different species are discovered. Since the specificity of the human ASGPR closely resembles that of the mouse. This kind of radiolabelling method, agent and related study about specific radioactivity could be used in clinical trial in the future.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Detection and treatment of autoimmune disorders
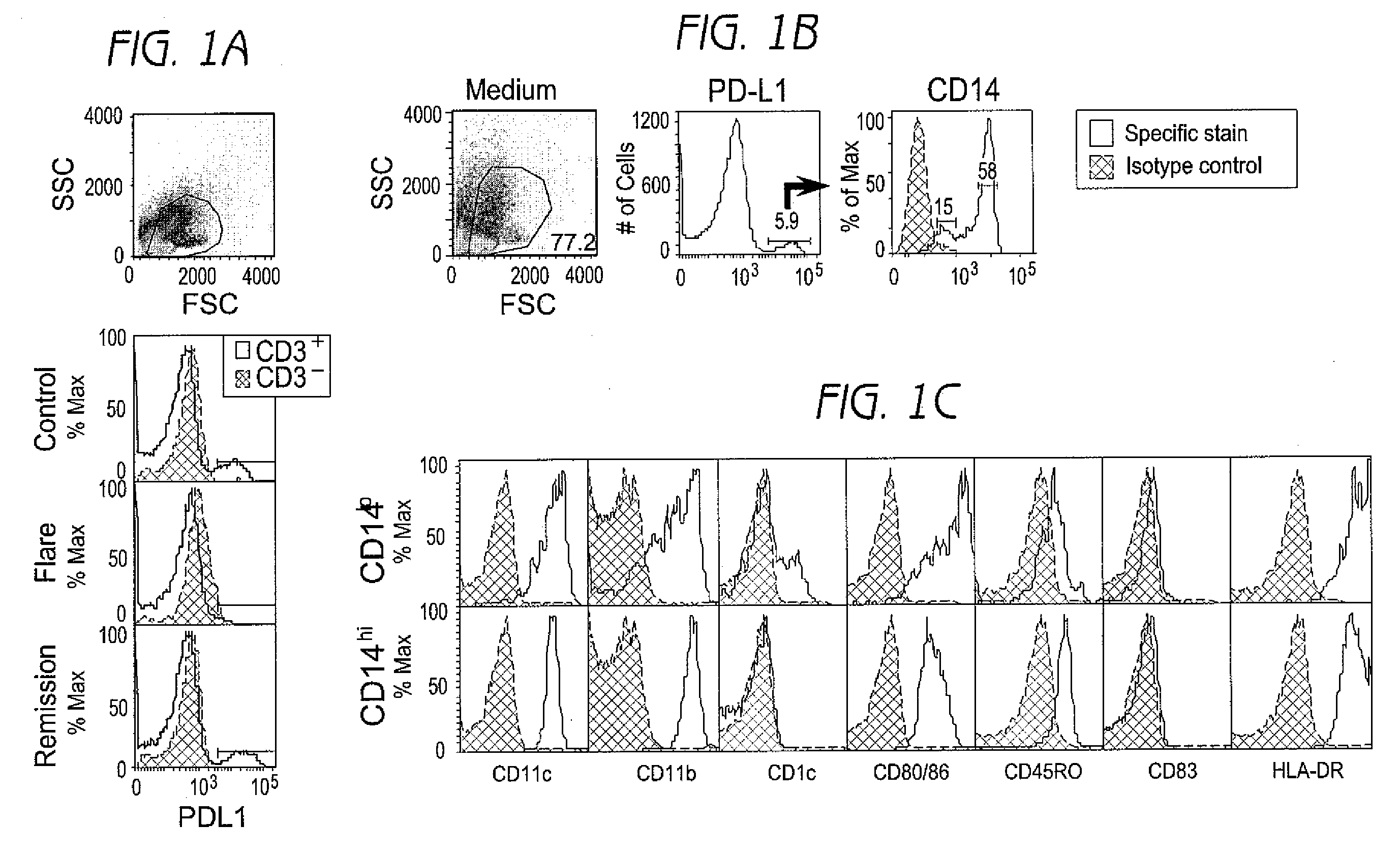
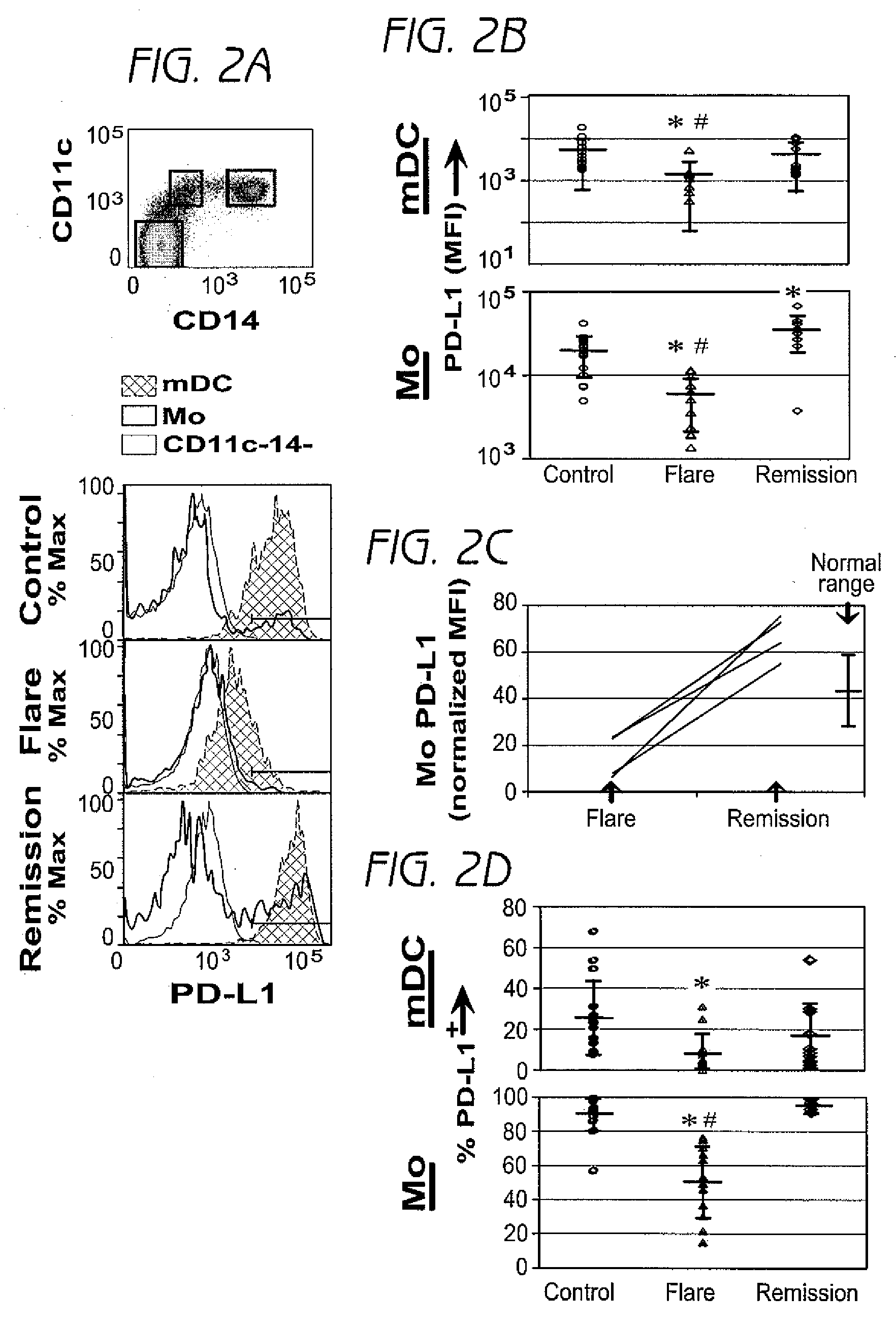
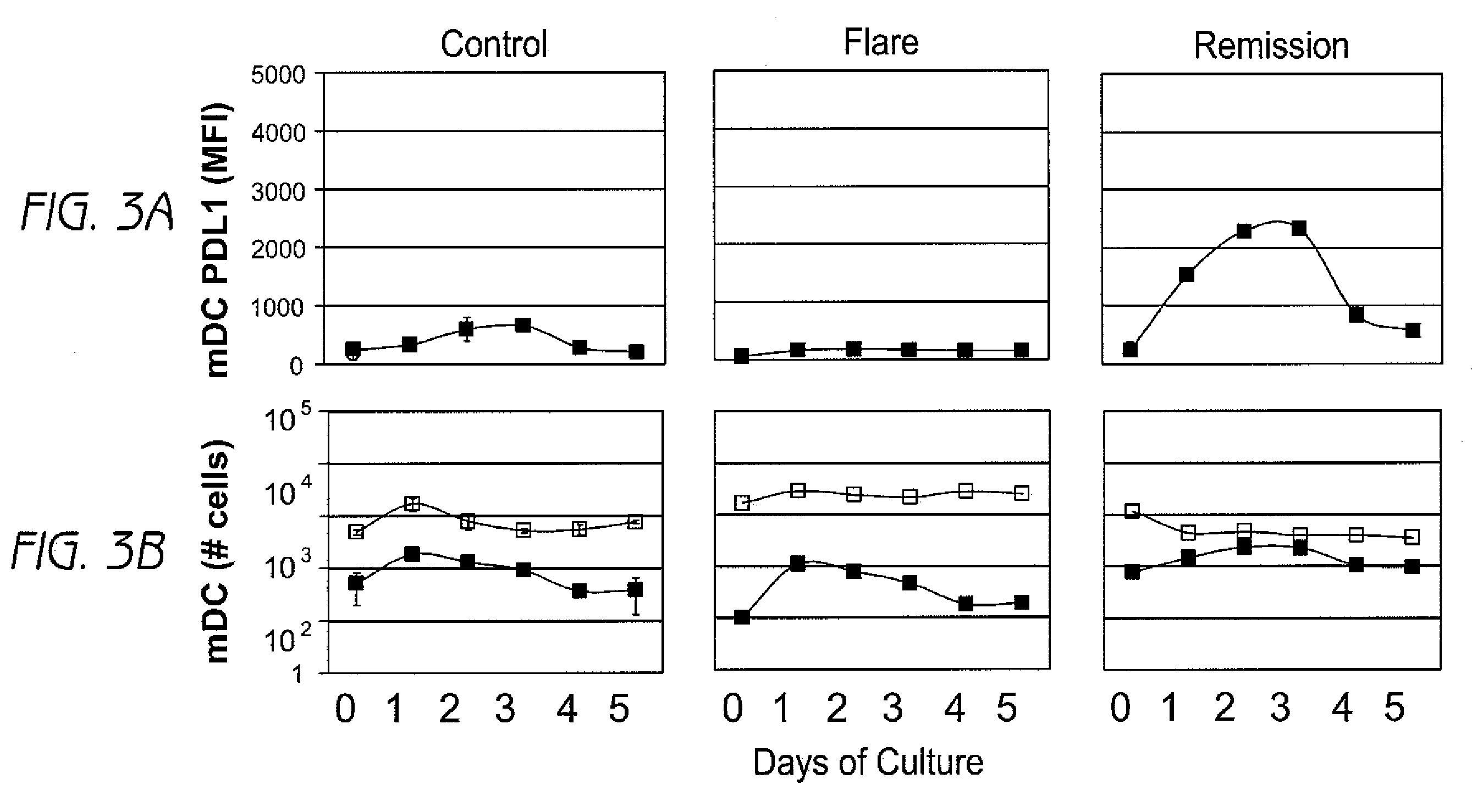
Disclosed herein are methods of treatment of autoimmune diseases such as systemic lupus erythematosus (SLE) as well as clinical assays for detection of autoimmune disease activity in patients utilizing the involving a PD1 ligand.
Owner:SEATTLE CHILDRENS HOSPITAL
Apparatus and methods for monitoring subjects
InactiveUS20060084848A1Improve battery lifeLow and controllable power consumptionPerson identificationInertial sensorsClinical trialComputer science
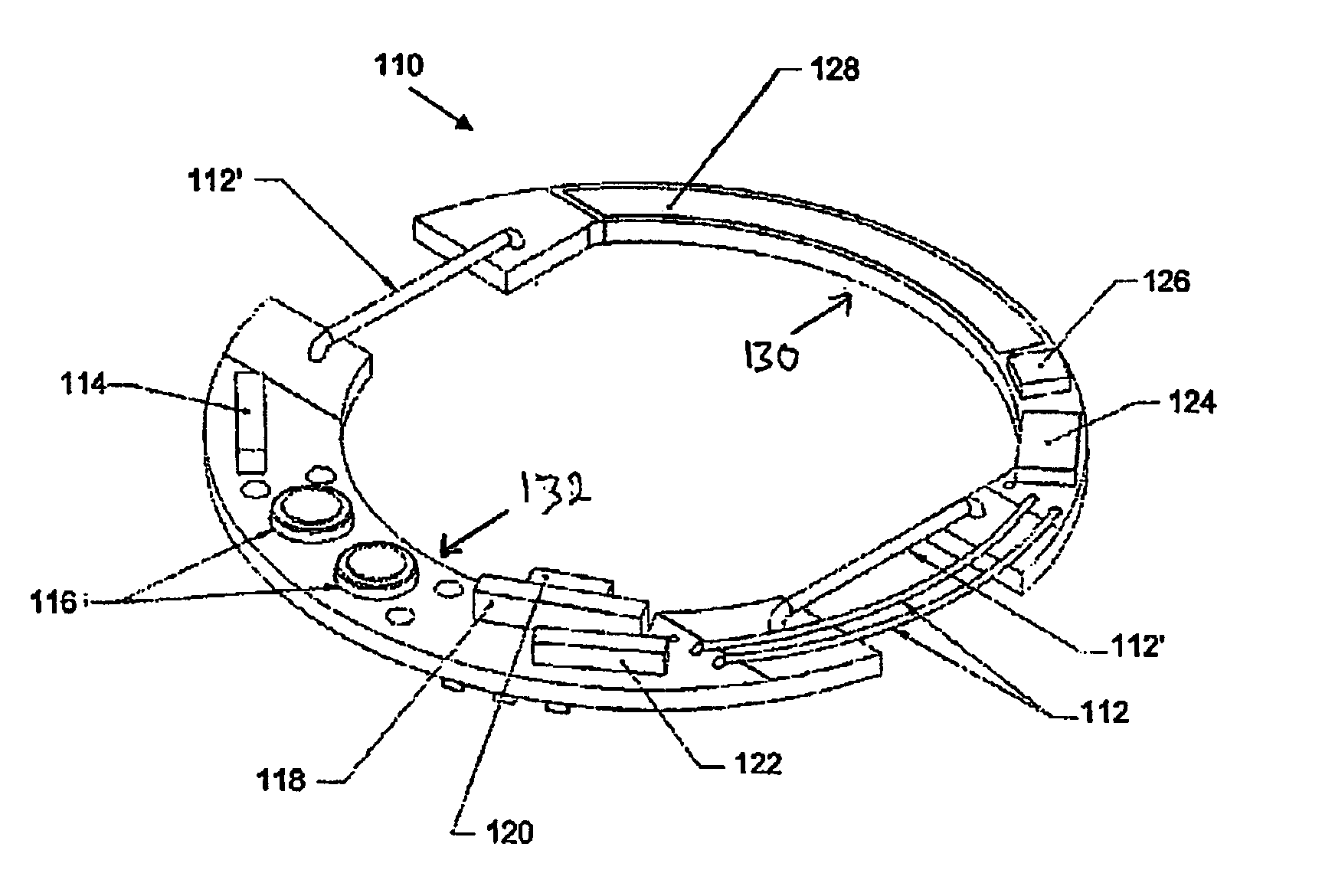
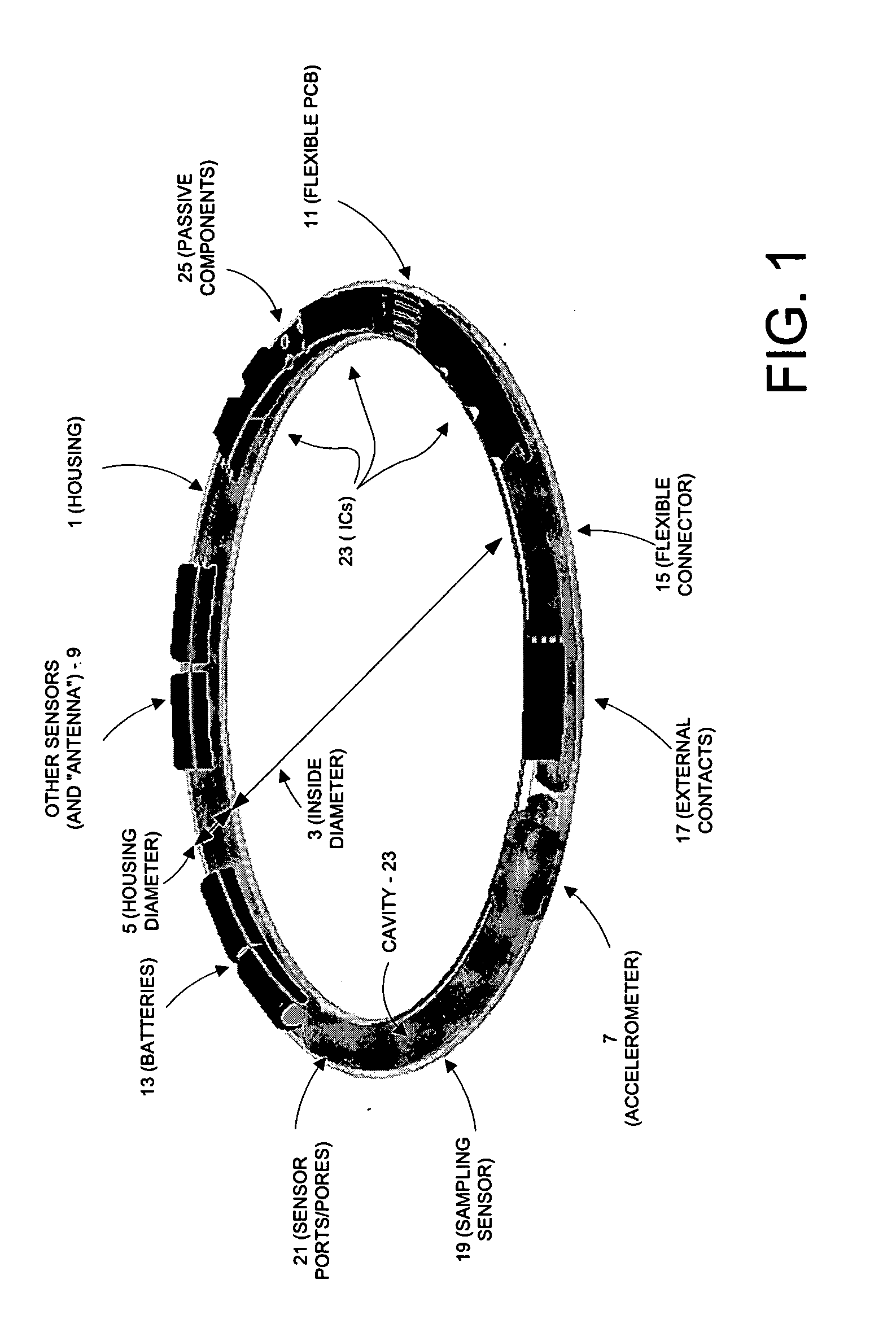
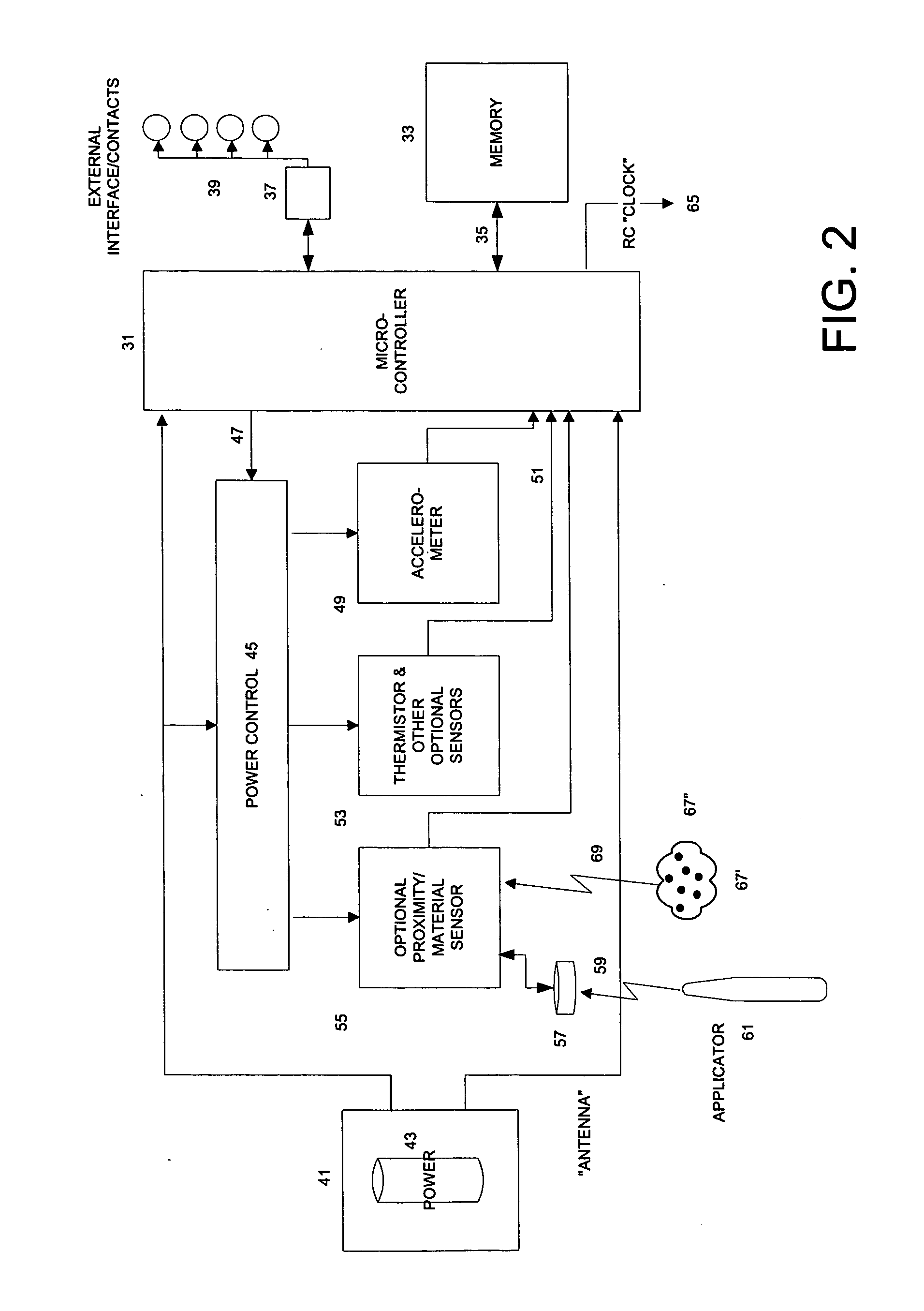
This invention provides a device, and method and system for its use, for monitoring participants in clinical trials so that participant self-reporting, which is known to be notoriously inaccurate, can be minimized or eliminated. In preferred embodiments, the device is self-contained and self-powered, resides on or in a body cavity of the participant, collects data monitoring medically relevant aspects of the participant's behavior and of the local device environment, and stores data in a memory on-board the device. An accompanying external station reads stored data and prepares it for use. Devices may include electrically-active sensors and non-electrical active sampling sensors. A preferred embodiment of the device is in clinical trials of microbicides inhibiting transmission of the HIV virus.
Owner:INT PARTNERSHIP FOR MICROBICIDES
Method of monitoring patient participation in a clinical study
InactiveUS20050182664A1Strengthen incentivesSpeed up the processComputer-assisted medical data acquisitionOffice automationClinical trialClinical study
A method is proposed for monitoring participation of a patient in at least one clinical trial. The method includes collecting data regarding at least one clinical trial for the patient. Using a computer device, the collected data is then compared to at least one threshold. Finally, the patient is rewarded upon the collected data at least meeting at least one threshold.
Owner:SIEMENS AG
Methods and Systems for Facilitating Clinical Trials
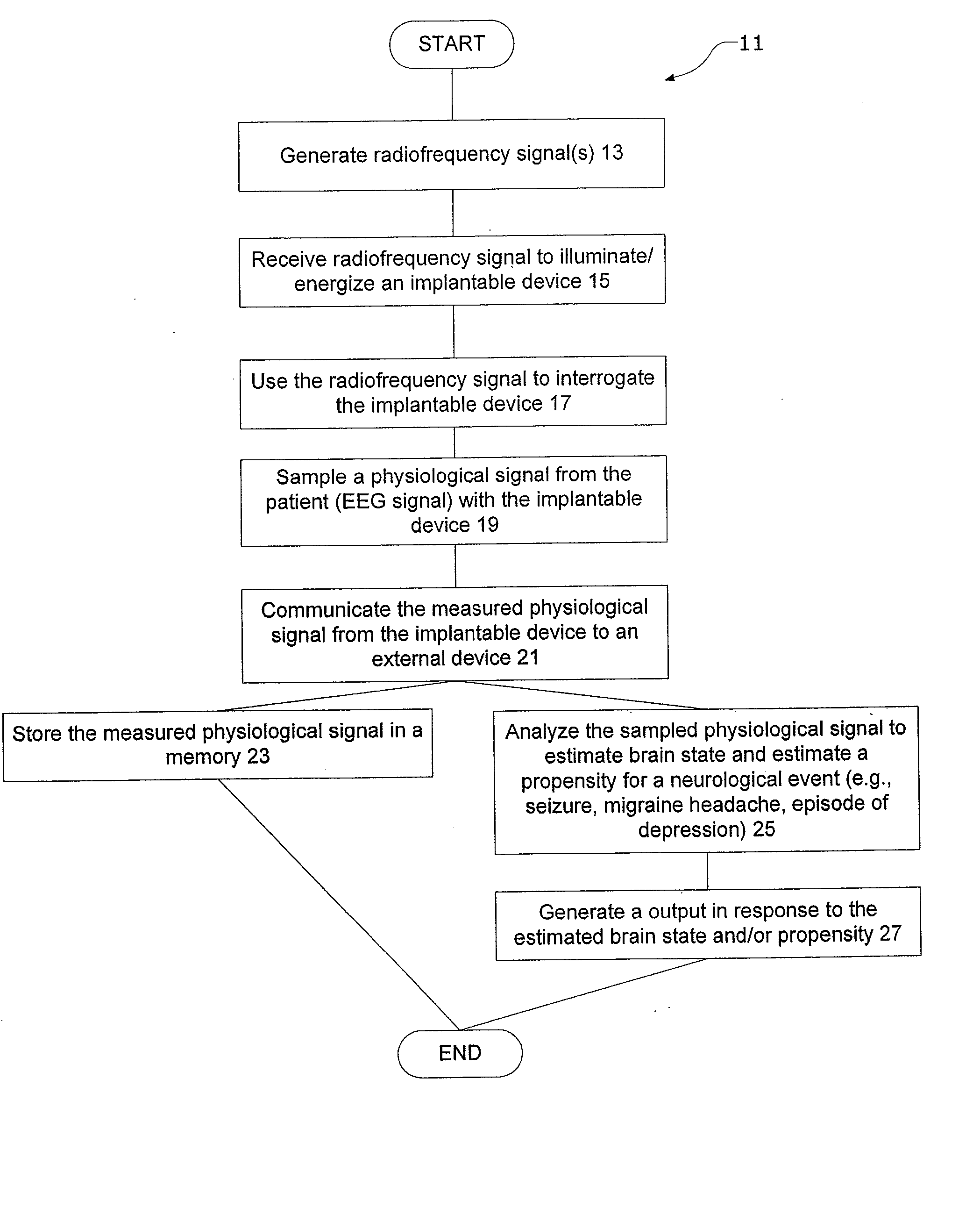
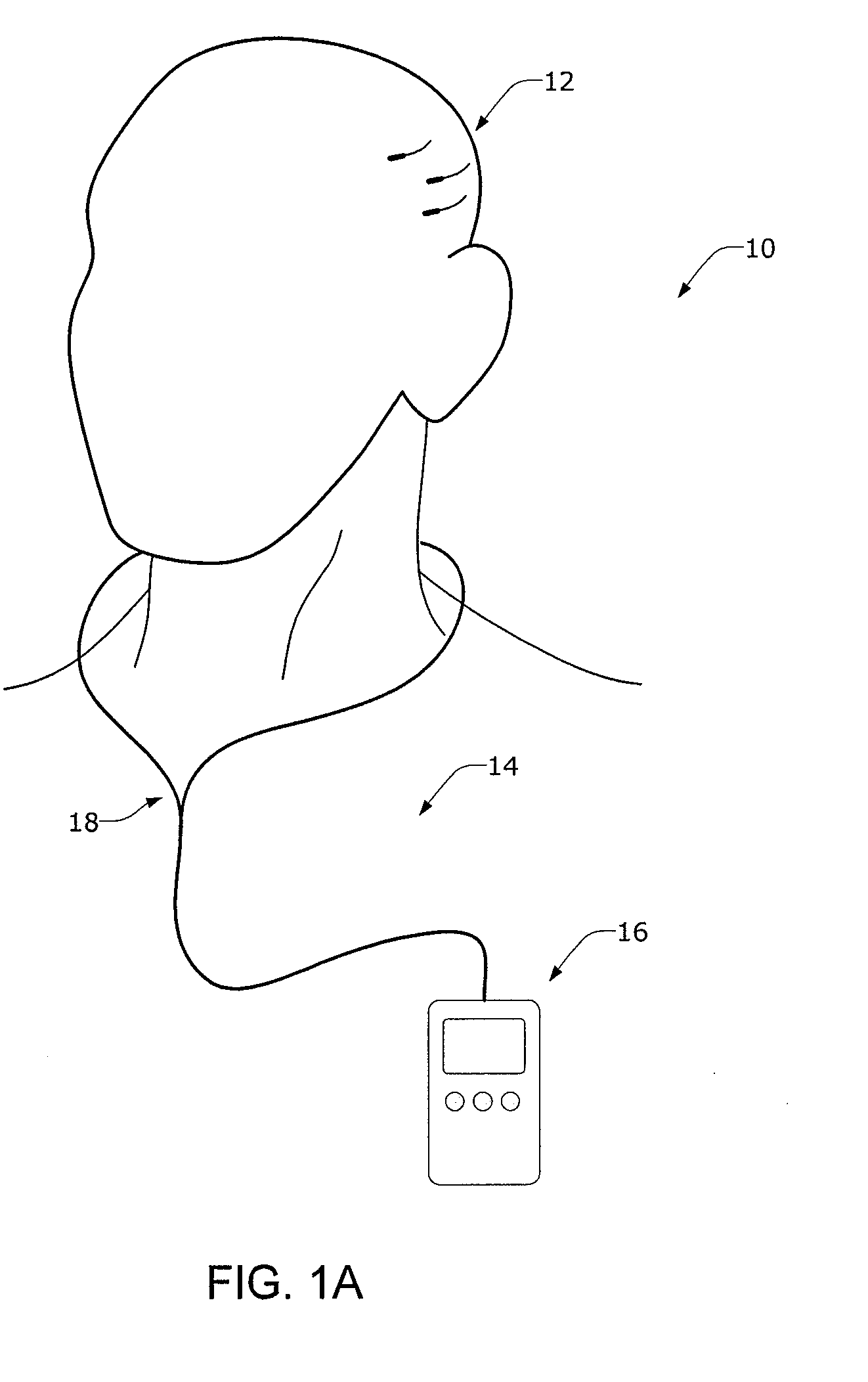
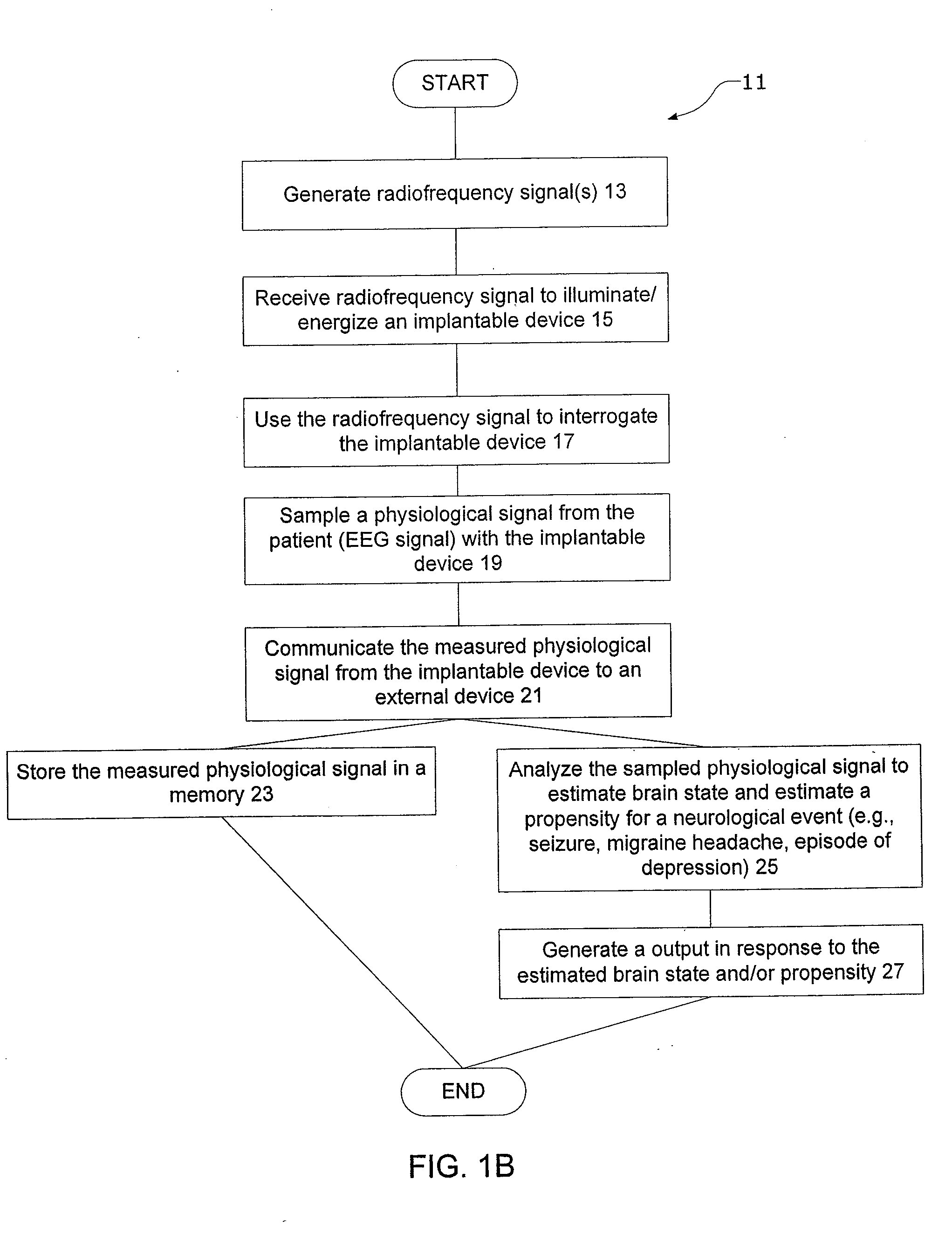
InactiveUS20080021341A1Easy data transferElectroencephalographyElectrotherapyClinical trialImplanted device
The present invention provides methods and systems for improving clinical trials of experimental therapies. The present invention uses minimally invasive, leadless implantable devices that facilitate long term monitoring of a physiological signal (e.g., neural signals) from a patient population which may provide an indication of the effect of the experimental therapy on the patient population. In preferred embodiments, neural signals are sampled from the patients in the patient population with an externally powered, leadless implanted device and are transmitted to an external device for further processing.
Owner:CYBERONICS INC
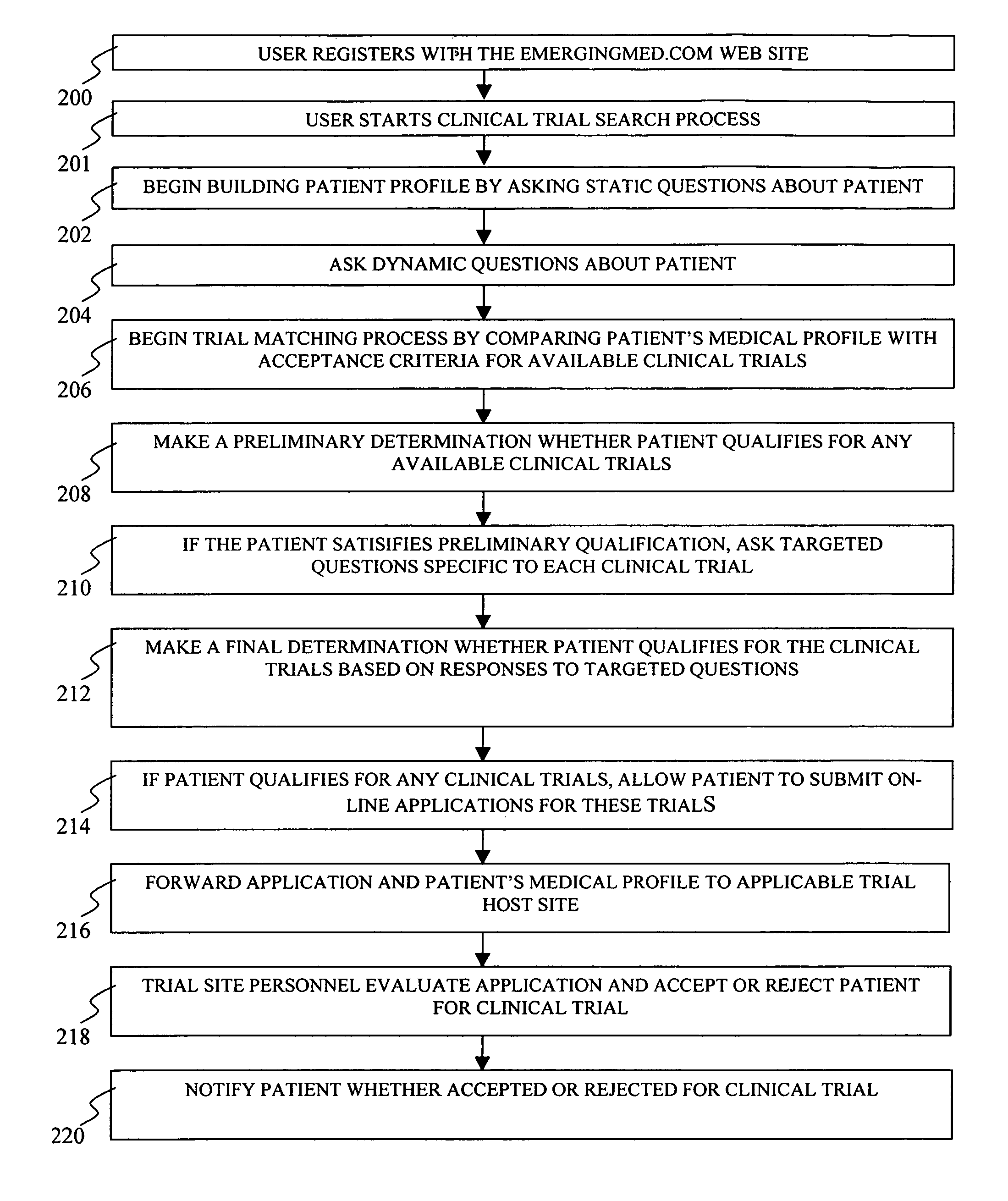
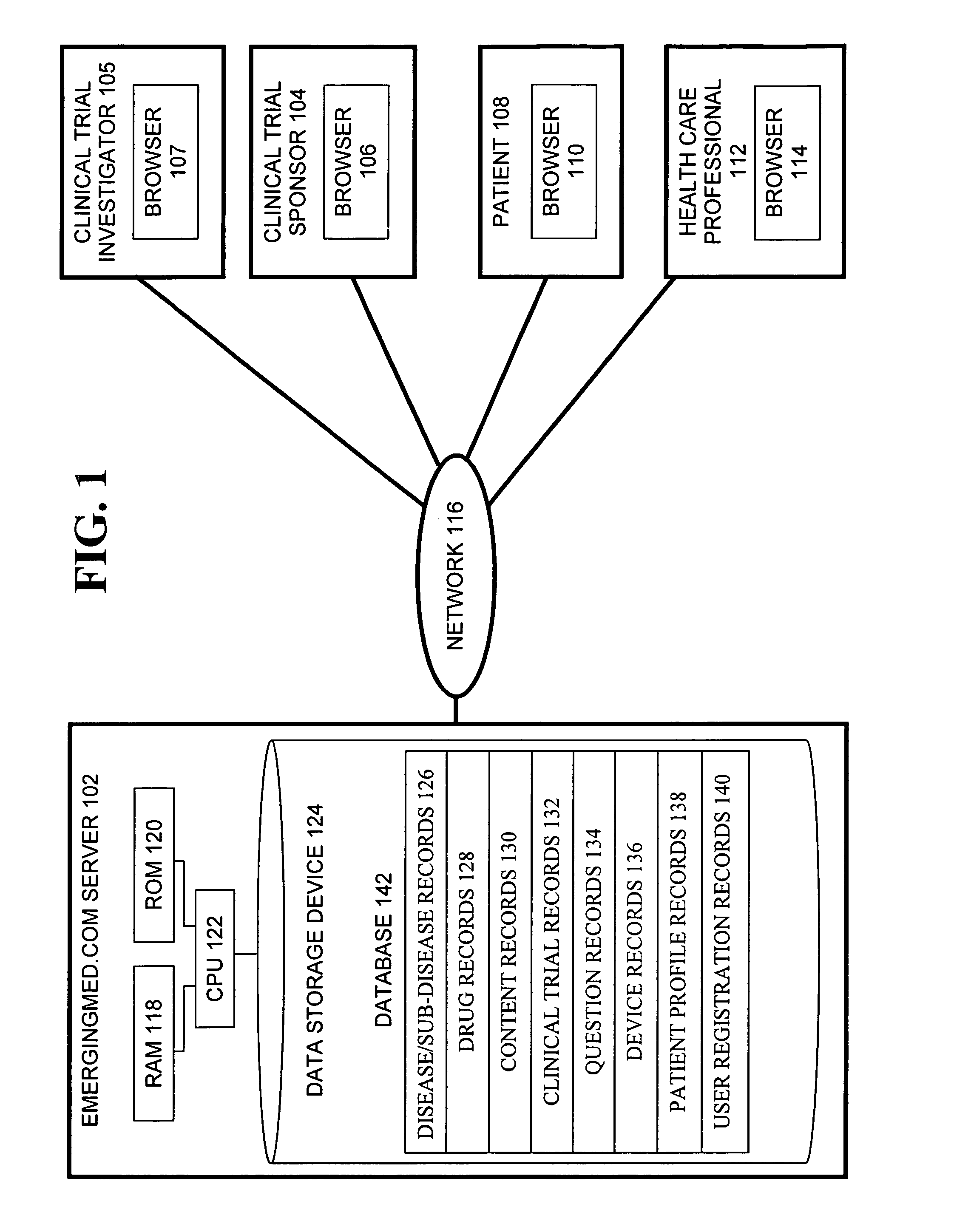
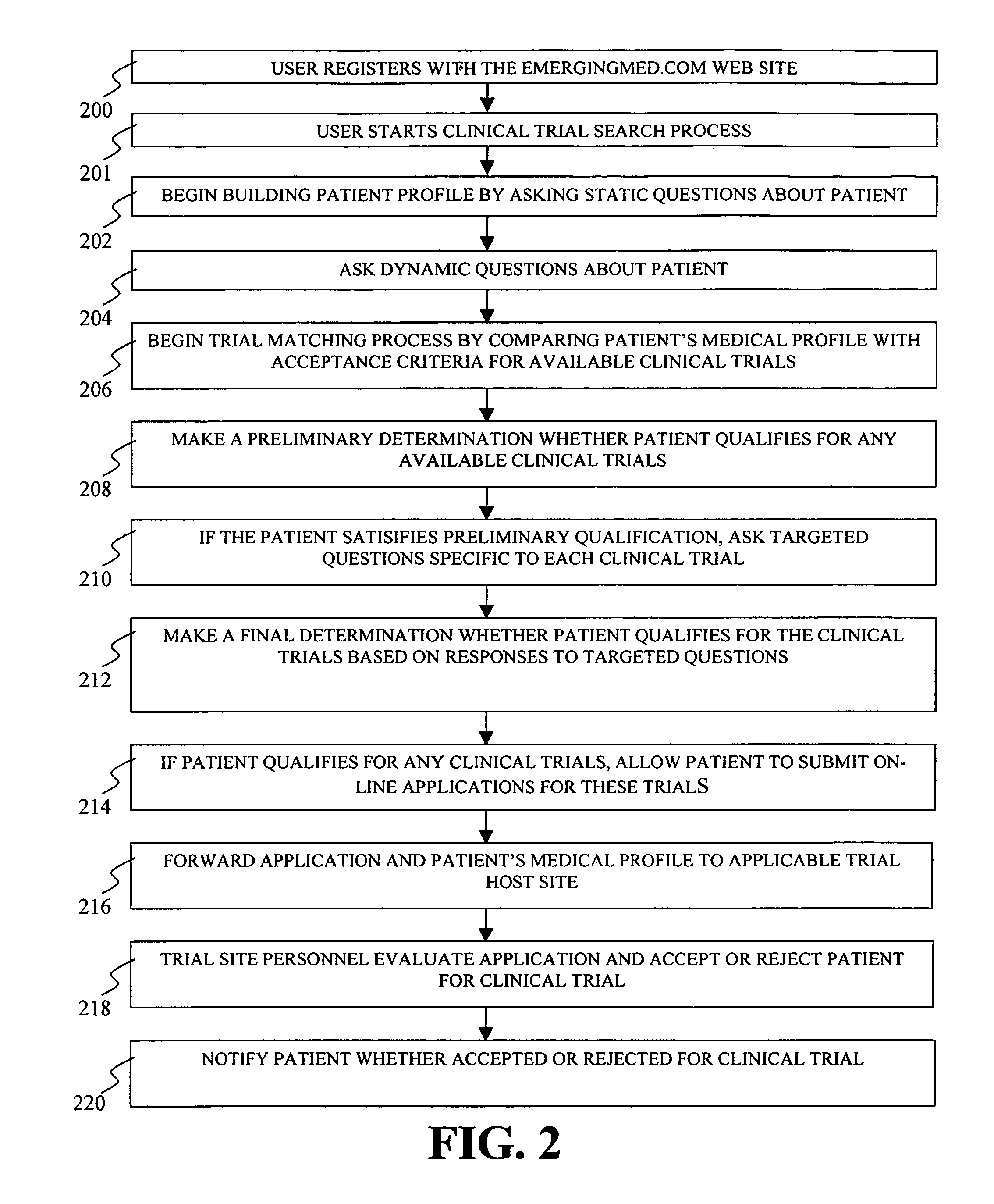
System and method for matching patients with clinical trials
InactiveUS7711580B1Privacy protectionComputer-assisted medical data acquisitionDiagnostic recording/measuringClinical trialTrial Site
A system and method for matching patients with clinical trials and particular trial sites, prequalifying patients for clinical trials and trial sites, and providing information to patients to allow them to inform themselves about available clinical trials and trial sites. The method comprises receiving patient profile information for a patient at a server connected to a computer network, the patient profile information submitted by a user at a terminal connected to the network, comparing the patient profile information with acceptance criteria for clinical trials stored in a database, the comparison performed by the server, determining whether the patient prequalifies for any of the clinical trials, and notifying the user and the trial site whether the patient has prequalified for any clinical trials.
Owner:EMERGINGMED COM
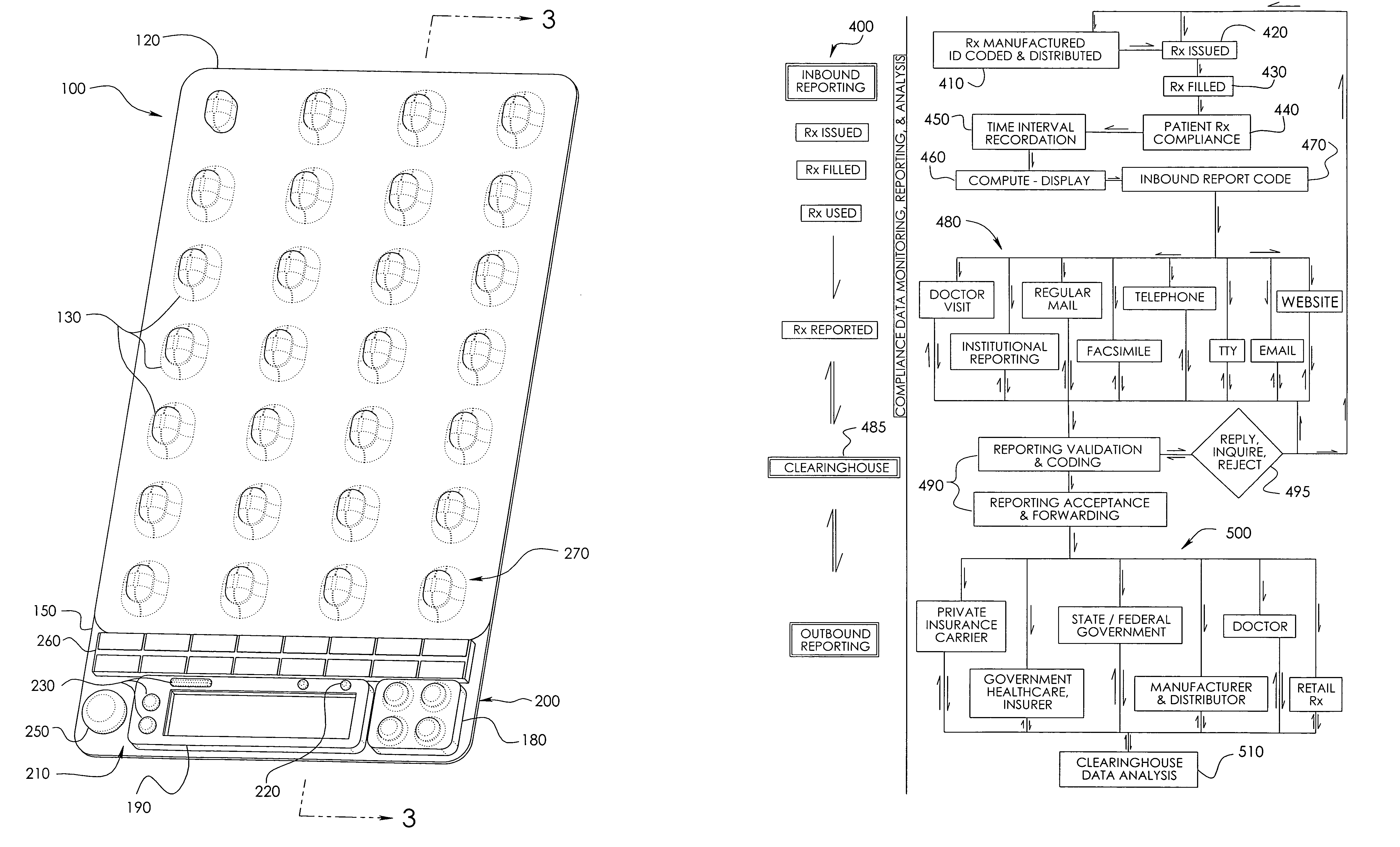
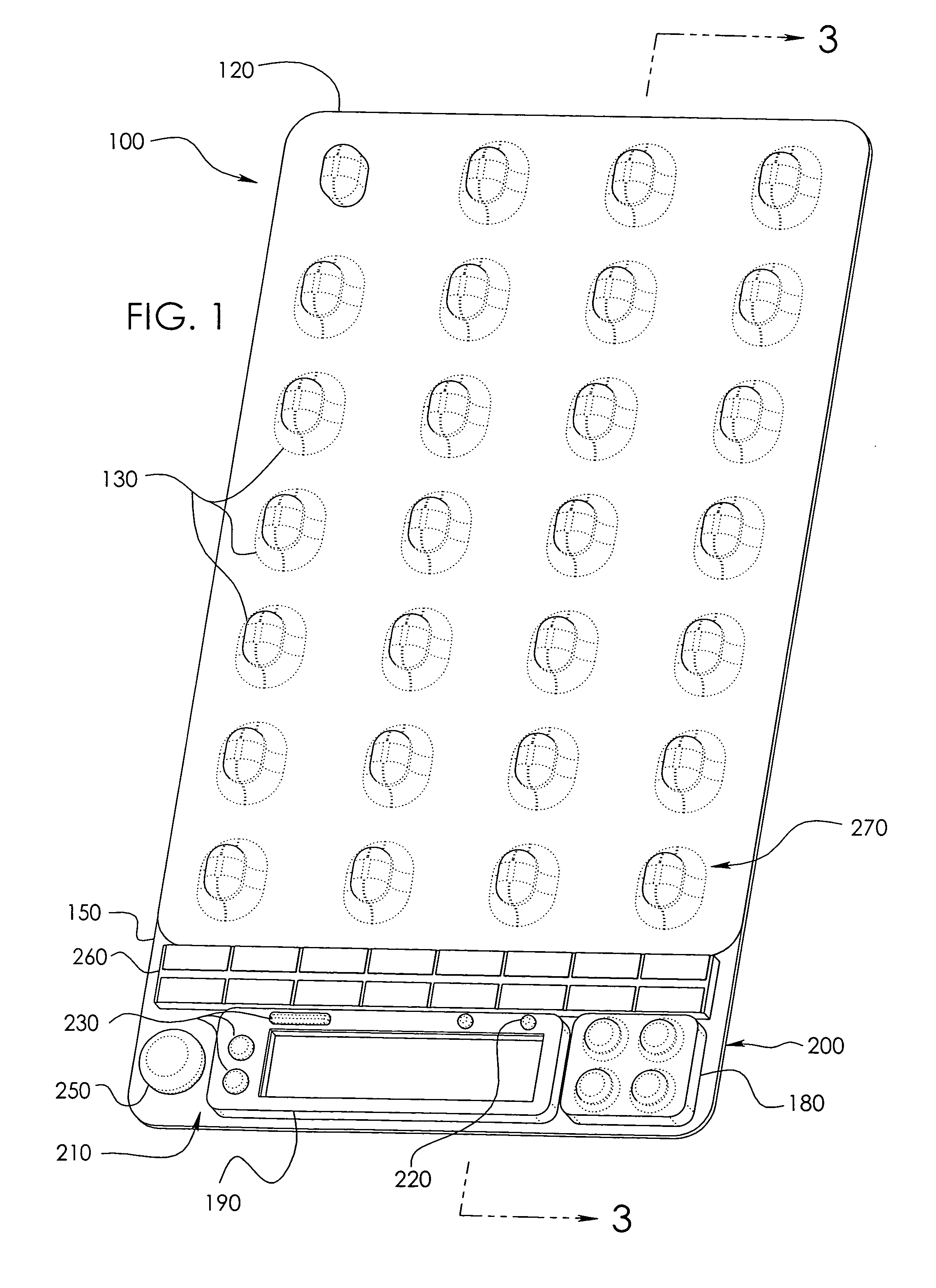
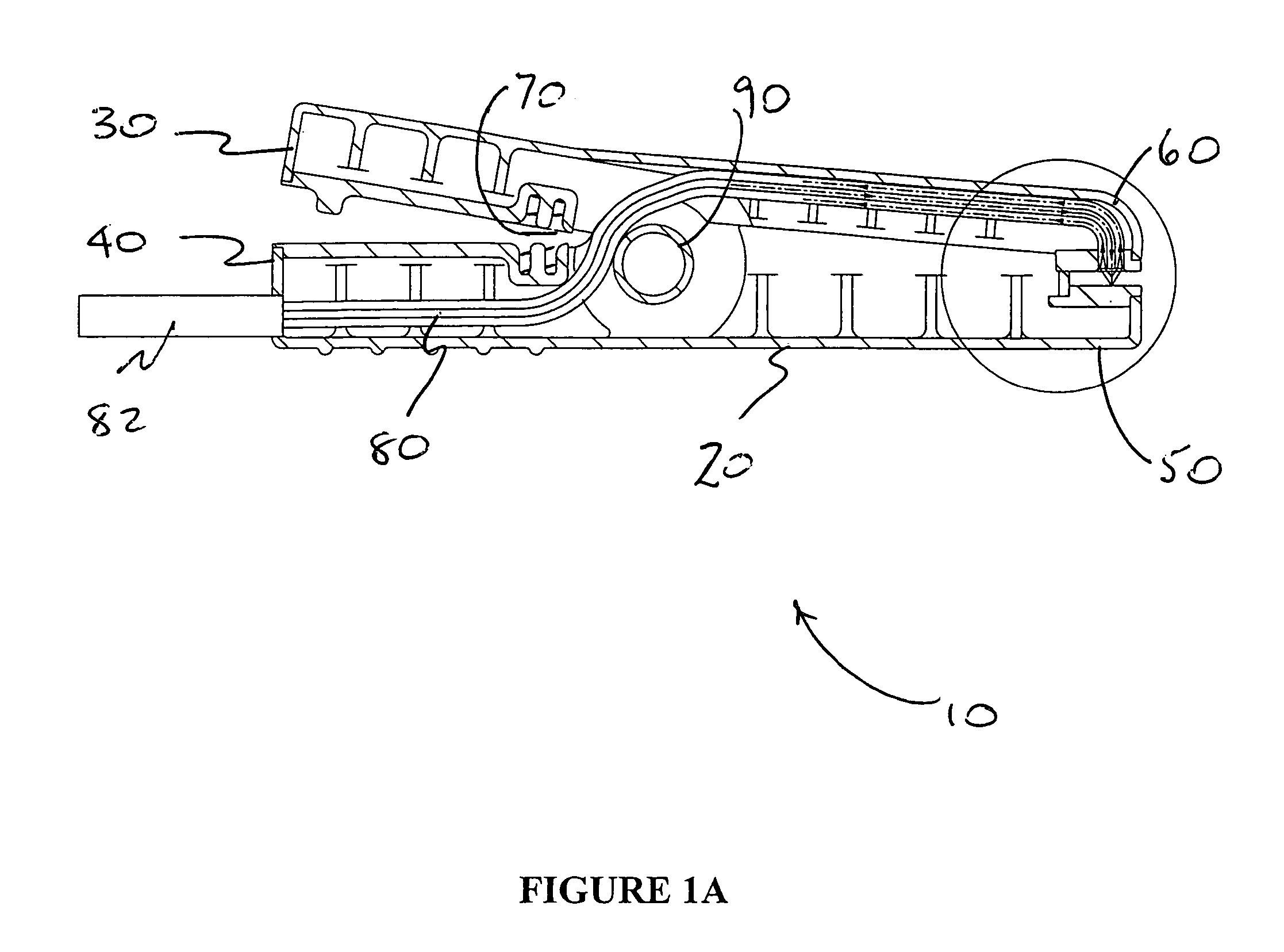
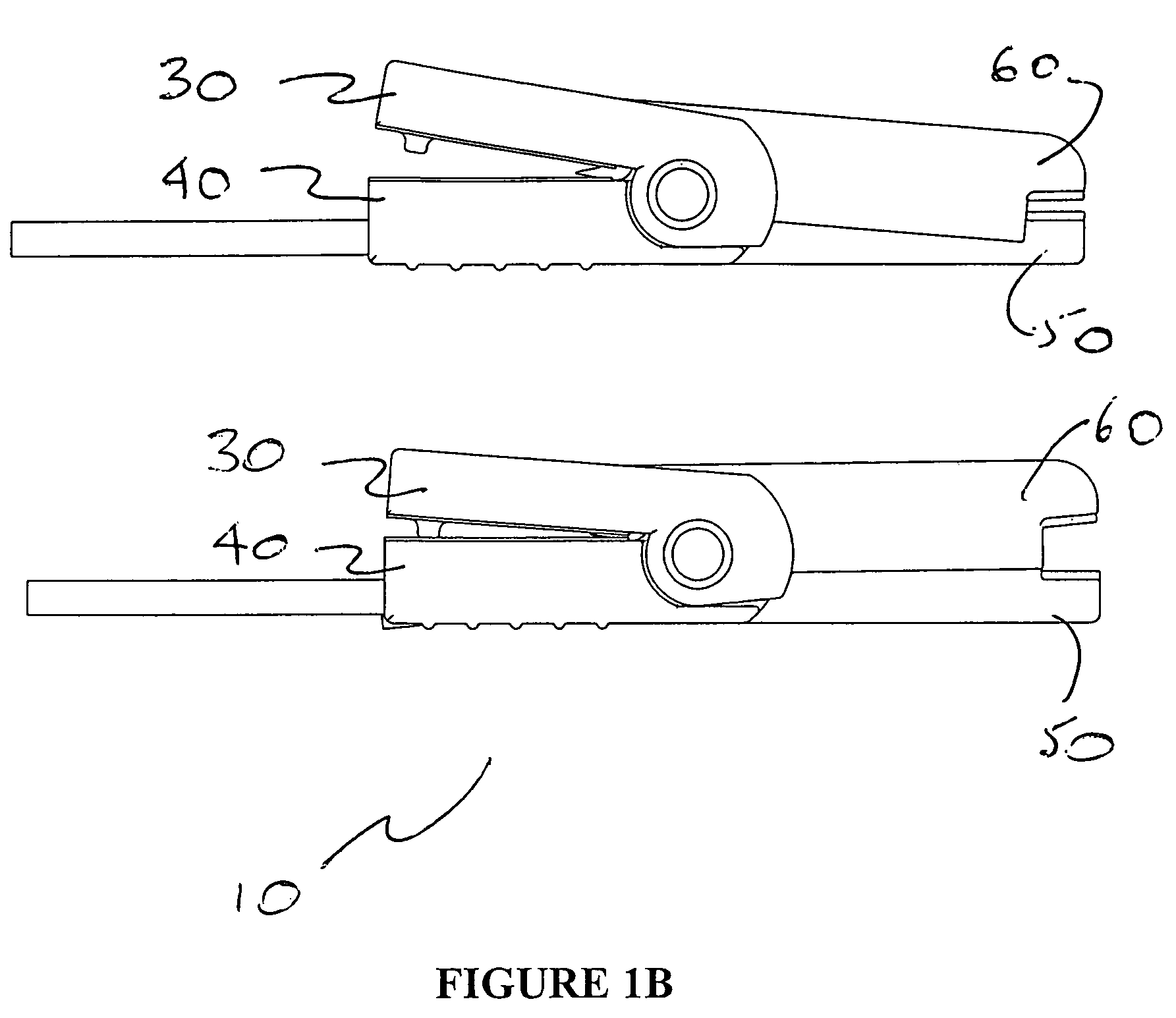
Unit dose compliance monitoring and reporting device and system
ActiveUS6973371B1Reduce pressureEffective mergerElectric signal transmission systemsMechanical clocksData displayCompliance Monitoring
A unit dose medication compliance monitoring and reporting apparatus and system that includes a dispenser shell formed with dose compartments. A retainer sheet affixed to the shell seals each compartment and partially bursts upon dispensing. A sensor network and monitoring and reporting circuitry records dispensing times and determines an average time interval, which can be reported with other data on an integral data display. The system can thereby monitor and report patient compliance with prescription regimens. Additional data can be recorded and displayed for augmented patient compliance assistance and analysis, which data can include customized informational messages, telephone and other patient support contact information, unit doses dispensed and remaining, reminder alarms, identification data, prescription regimens, among other data. In myriad variations and alternative preferred configurations, the devices and systems have demonstrated efficacy in minimalist to complex monitoring and reporting applications ranging from routine prescription monitoring to detailed clinical trial assessments.
Owner:BENOUALI NADIR
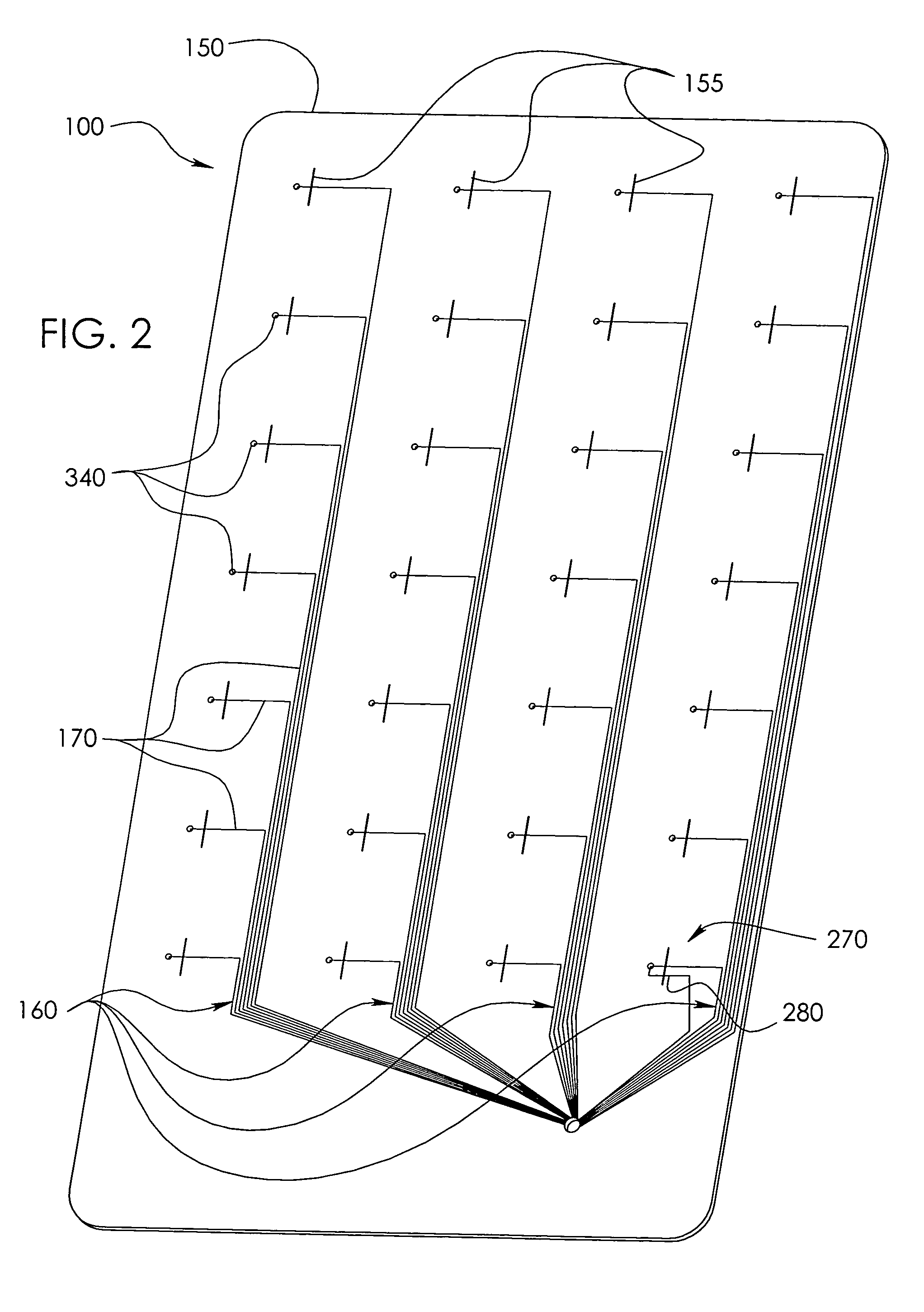
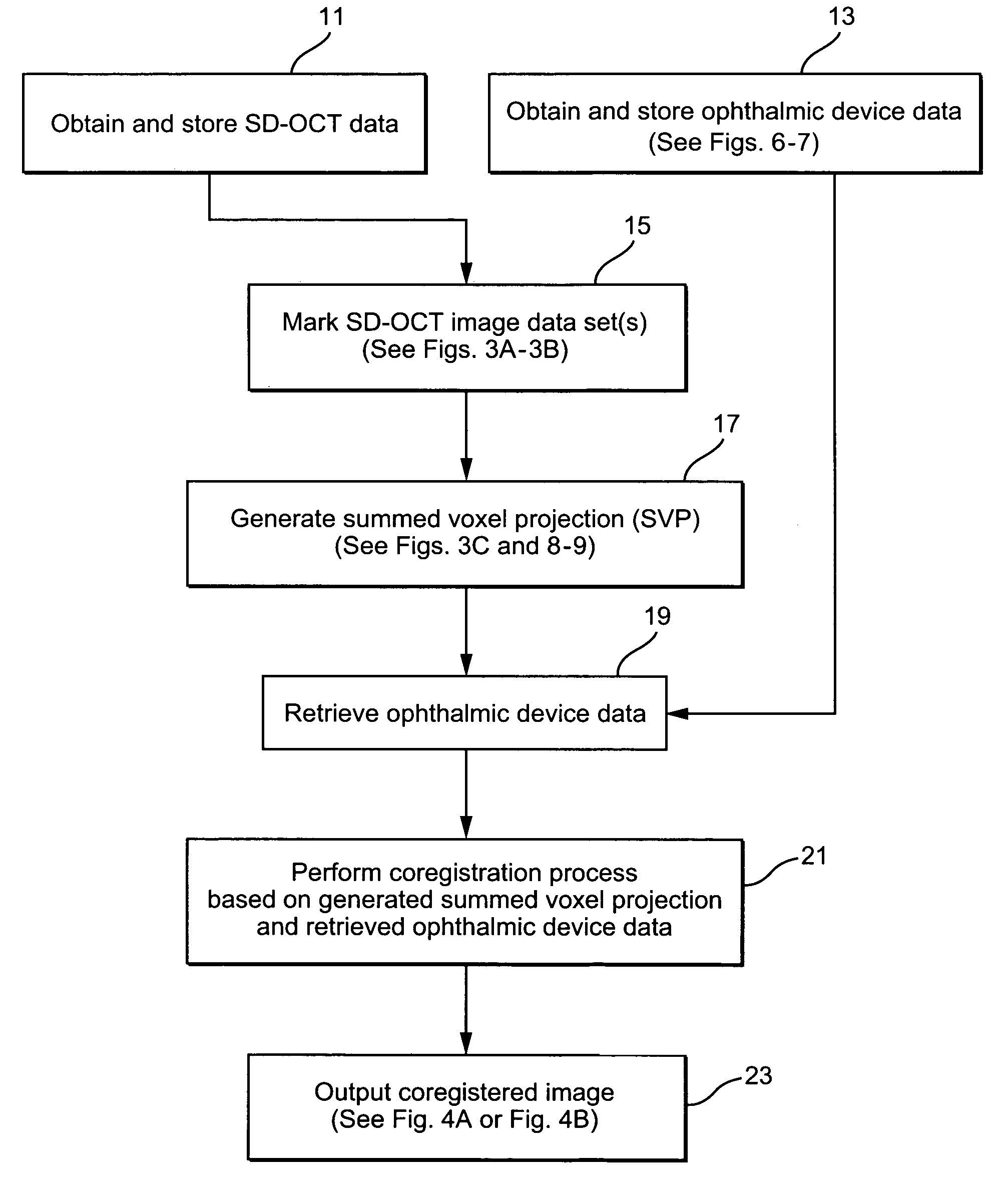
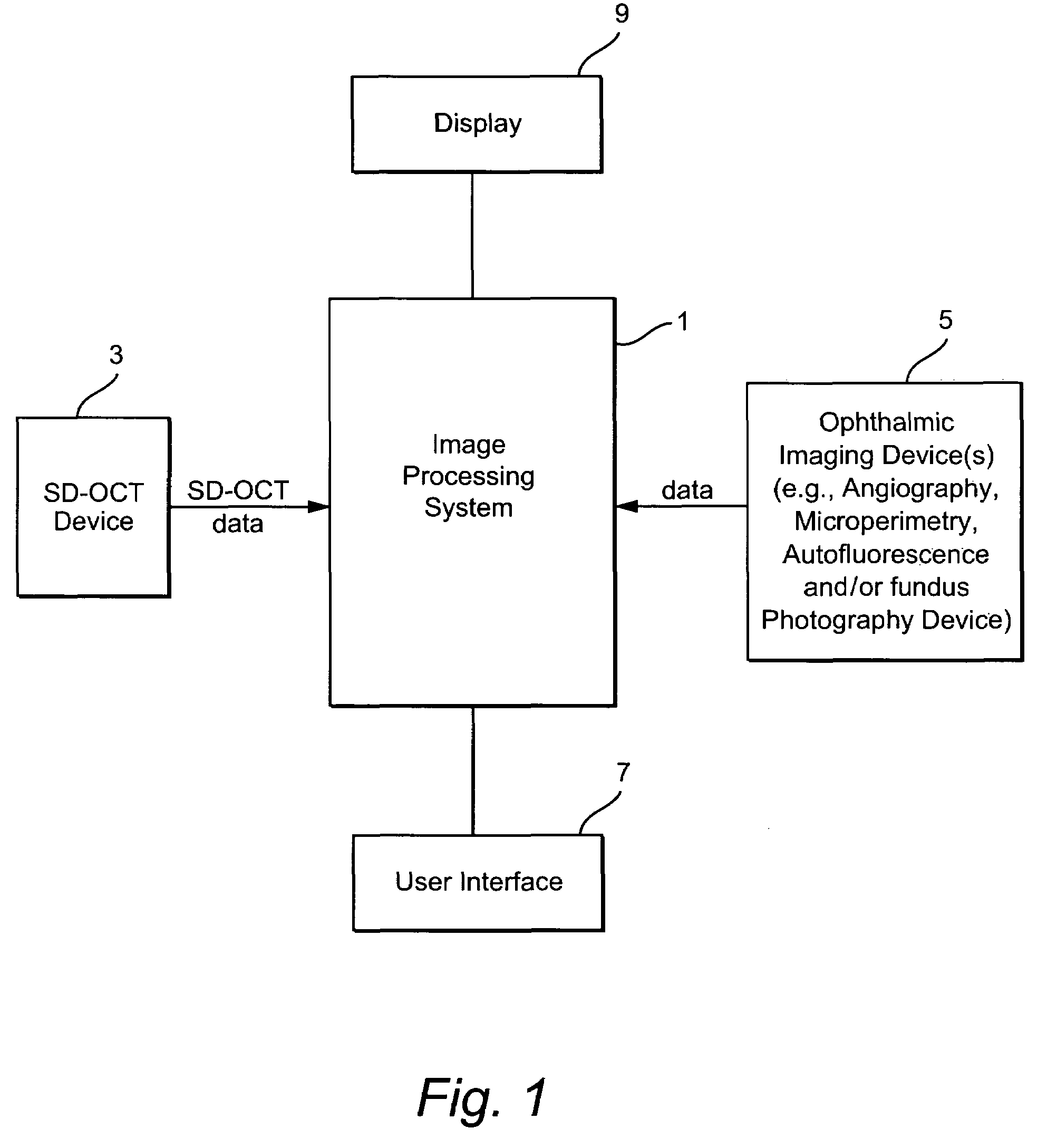
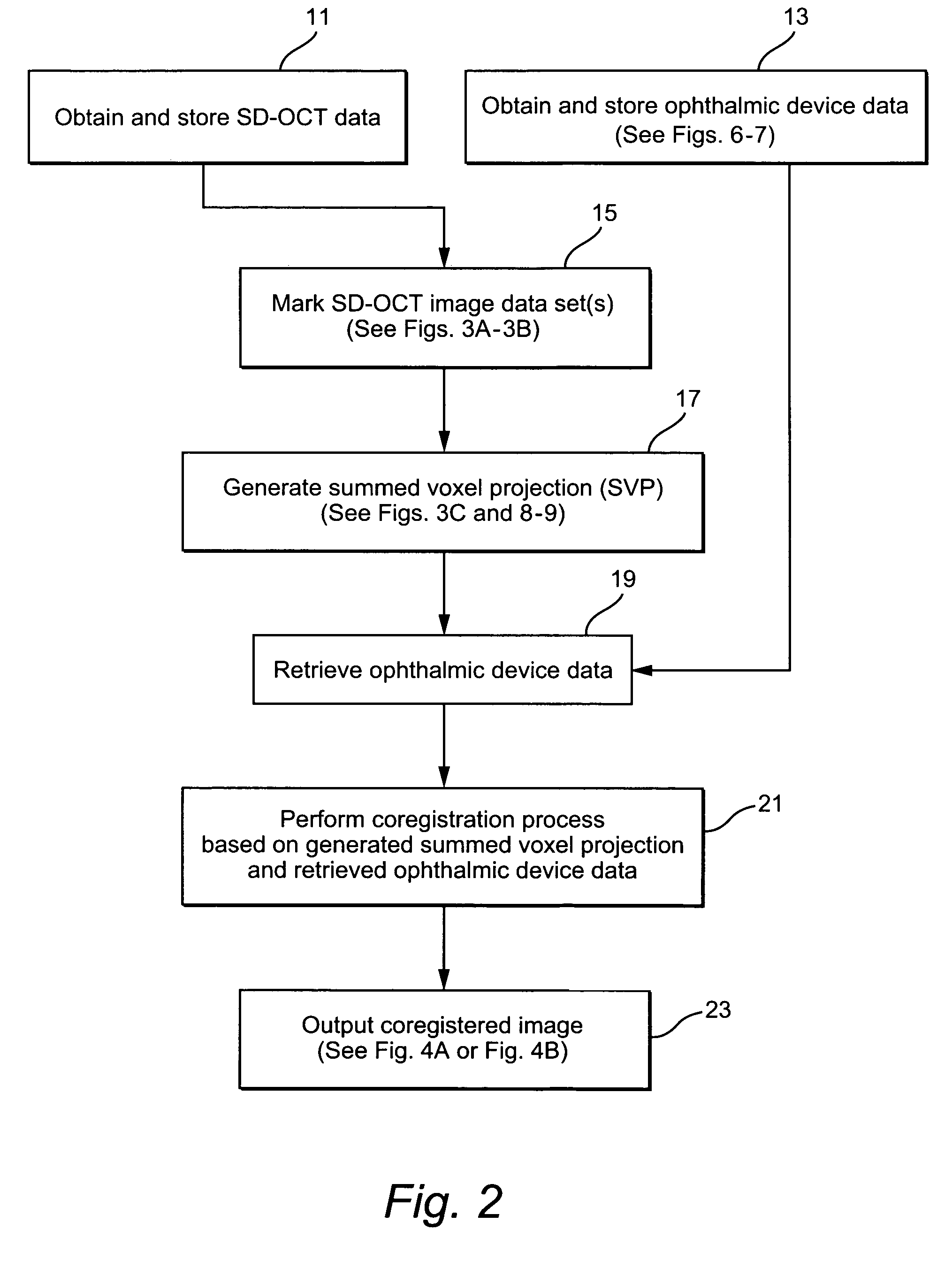
Method and system of coregistrating optical coherence tomography (OCT) with other clinical tests
ActiveUS7593559B2Improve signal-to-noise ratioReduce artifactsMaterial analysis using wave/particle radiationRadiation/particle handlingClinical testsClinical trial
Owner:DUKE UNIV
Near infrared risk assessment of diseases
The present invention provides an apparatus and a method for identifying the risk of a clinical condition in a human or animal by correlating Near Infrared (NIR) absorbance spectral data with one or several parameters including a concentration of one or more substances in the skin, a concentration of one or more substances in skin plus subdermal tissue, a score derived from one or more clinical tests like a stress test on a treadmill, coronary angiography, or intravascular coronary ultrasound. The method determines the concentration of a compound in the skin of a human or animal and comprises the steps of placing a part of the skin against a receptor, directing electromagnetic radiation (EMR) from the near-infrared spectrum onto the skin, measuring a quantity of EMR reflected by, or transmitted through, the skin with a detector; and performing a quantitative mathematical analysis of the quantity of EMR to determine the concentration of the compound, for example free and esterfied cholesterol. An example of a clinical condition is cardiovascular disease.
Owner:TYCO HEALTHCARE GRP LP
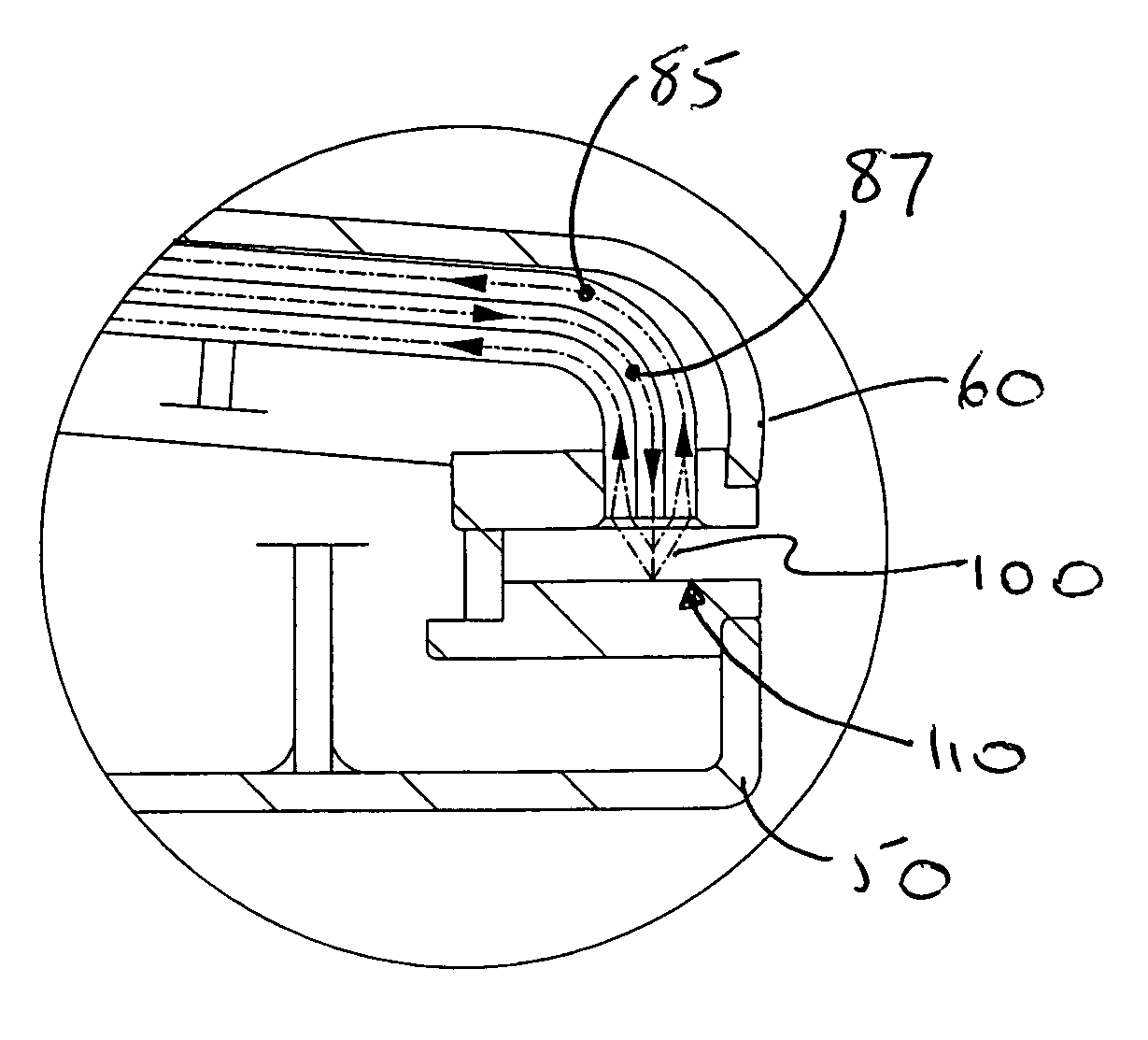
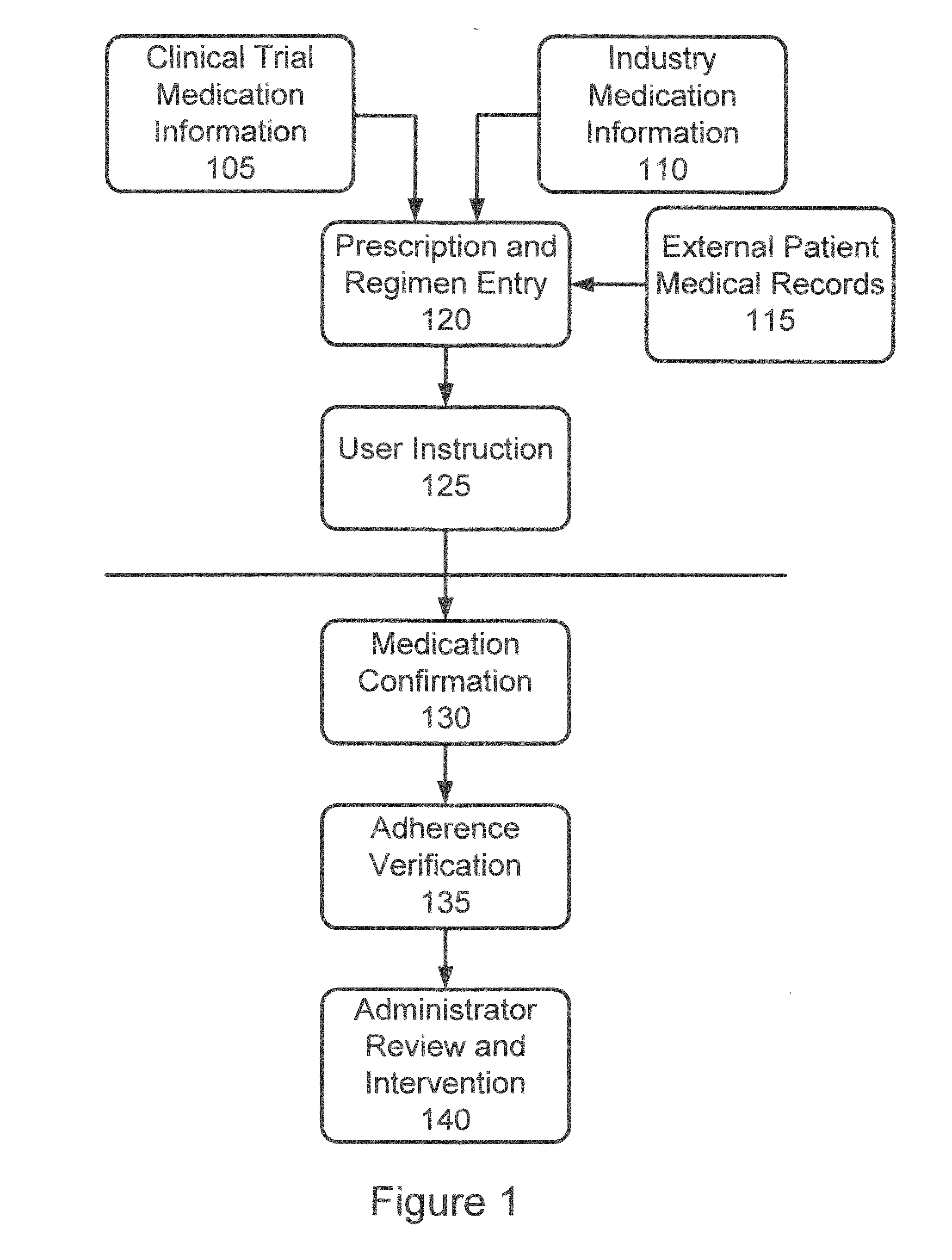
Method and Apparatus for Verification of Clinical Trial Adherence
InactiveUS20110153360A1Effective corporate compliance programMinimize forgetfulnessImage analysisDigital data processing detailsRegimenClinical trial
A system and method of confirming administration of medication in a clinical trial is provided. The method comprises the steps of receiving information identifying a particular medication prescription regimen in accordance with the clinical trial, determining one or more procedures for administering such prescription regimen and identifying one or more activity sequences associated with such procedures. Activity sequences of actual administration of such prescription regimen are captured and then compared to the identified activity sequences to determine differences therebetween. A notice is provided if differences are determined.
Owner:AI CURE TECH
Integrated data collection and analysis for clinical study
A data collection system includes remote, implantable sensors for monitoring one or more patient parameters, collecting and processing data from those sensors and utilizing that data in the performance of a clinical study of a drug or other pharmacological agent. The system assists with preparation of a protocol for a clinical trial; presentation of that protocol; assuring compliance with the protocol; and generating useful results from data collected via the system and externally for presentation to an approval forum.
Owner:MEDTRONIC INC
Compositions and methods for the treatment and diagnosis of immune disorders
InactiveUS6084083AReduce in quantityLower Level RequirementsAntibacterial agentsBacteriaClinical trialCell subpopulations
The present invention relates to methods and compositions for the treatment and diagnosis of immune disorders, especially T helper lymphocyte-related disorders. For example, genes which are differentially expressed within and among T helper (TH) cells and TH cell subpopulations, which include, but are not limited to TH0, TH1 and TH2 cell subpopulations are identified. Genes are also identified via the ability of their gene products to interact with gene products involved in the differentiation, maintenance and effector function of such TH cells and TH cell subpopulations. The genes identified can be used diagnostically or as targets for therapeutic intervention. In this regard, the present invention provides methods for the identification and therapeutic use of compounds as treatments of immune disorders, especially TH cell subpopulation-related disorders. Additionally, methods are provided for the diagnostic evaluation and prognosis of TH cell subpopulation-related disorders, for the identification of subjects exhibiting a predisposition to such conditions, for monitoring patients undergoing clinical evaluation for the treatment of such disorders, and for monitoring the efficacy of compounds used in clinical trials.
Owner:MILLENNIUM PHARMA INC
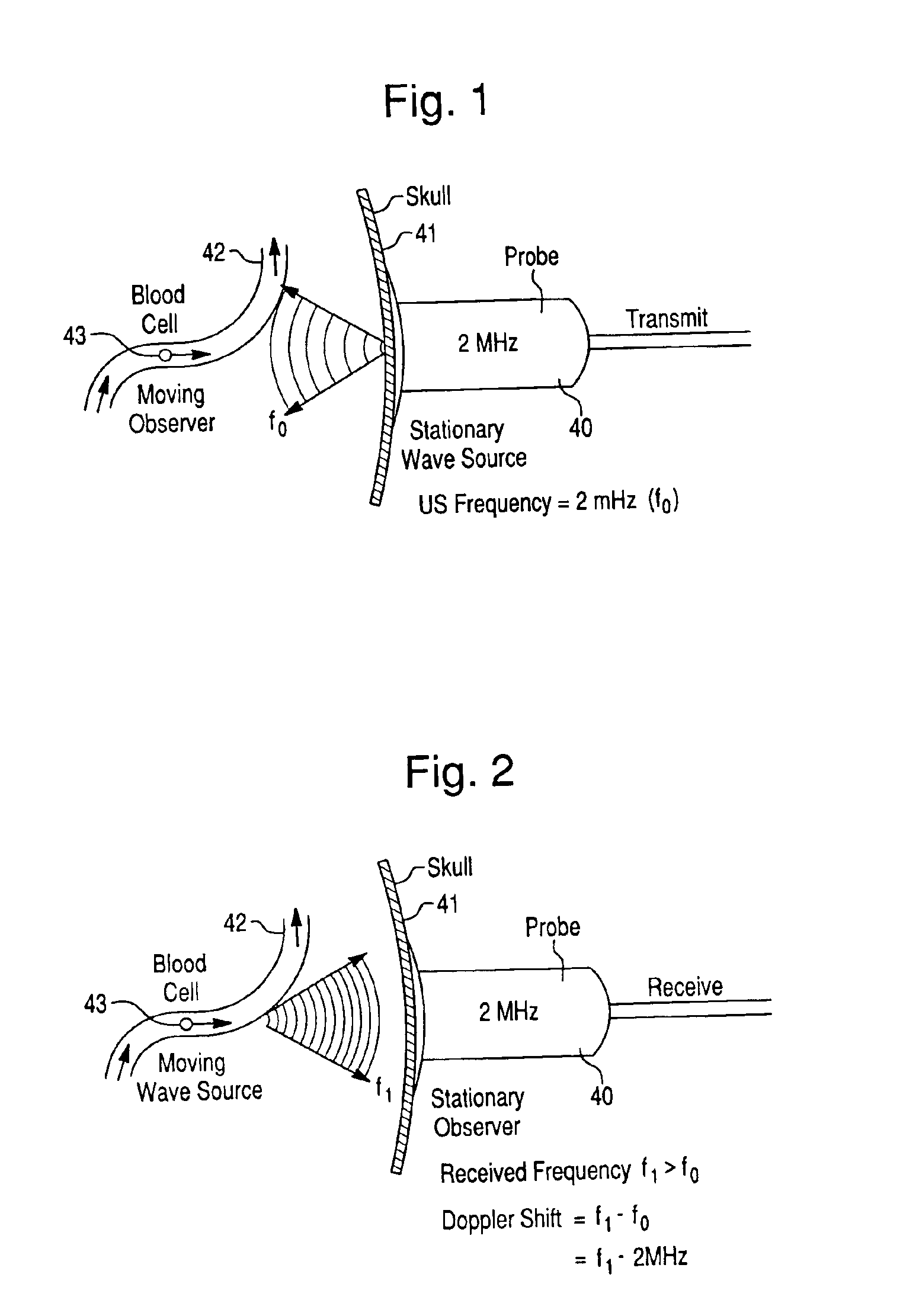
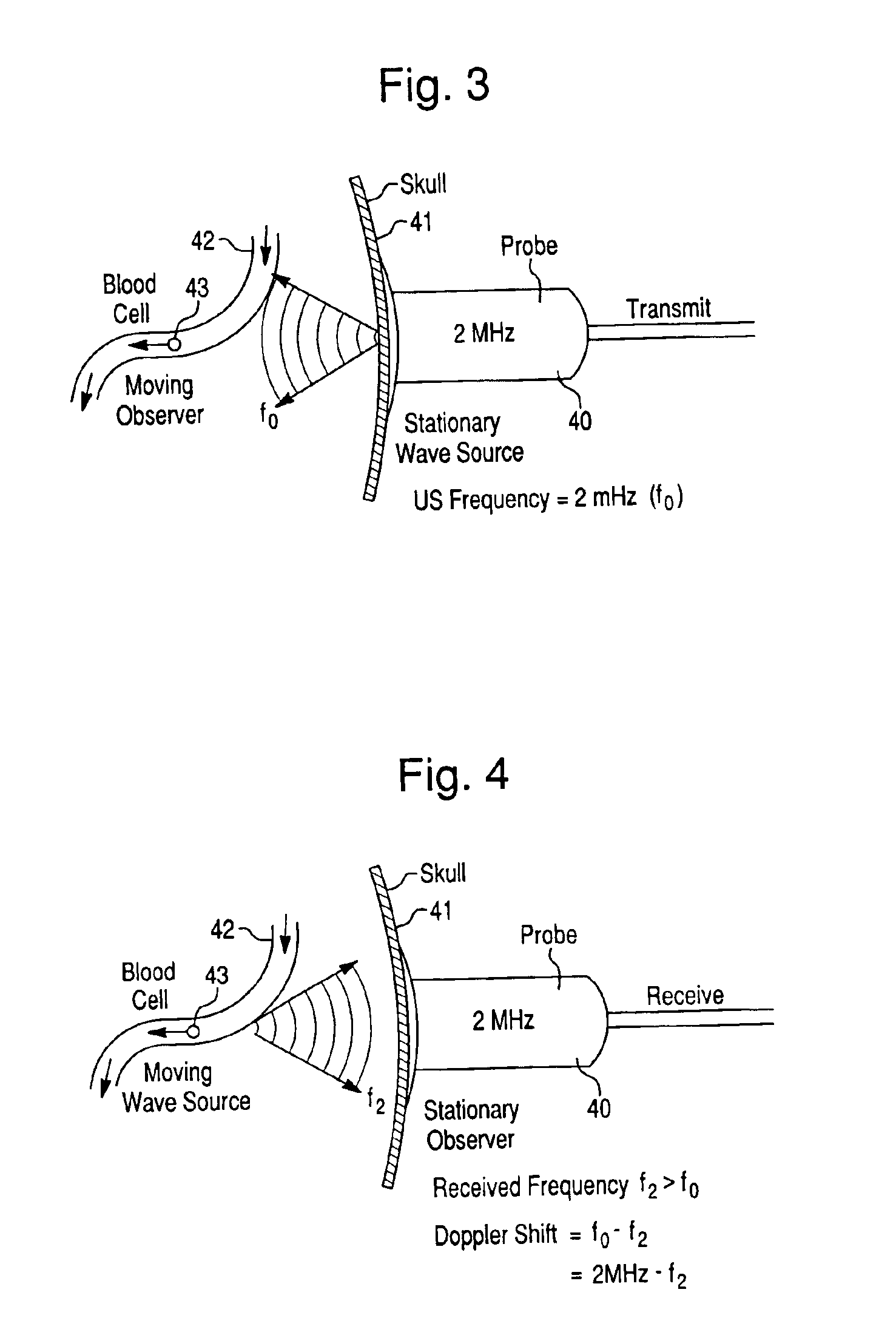
Precision brain blood flow assessment remotely in real time using nanotechnology ultrasound
InactiveUS6955648B2Rapid and inexpensive to performEfficient deliveryBlood flow measurement devicesMedical automated diagnosisClinical trialStreamflow
The present invention relates generally to systems and methods for assessing blood flow in blood vessels, for assessing vascular health, for conducting clinical trials, for screening therapeutic interventions for adverse effects, and for assessing the effects of risk factors, therapies and substances, including therapeutic substances, on blood vessels, especially cerebral blood vessels, all achieved by measuring various parameters of blood flow in one or more vessels and analyzing the results in a defined matter. The relevant parameters of blood flow include mean flow velocity, systolic acceleration, and pulsatility index. By measuring and analyzing these parameters, one can ascertain the vascular health of a particular vessel, multiple vessels and an individual. Such measurements can also determine whether a substance has an effect, either deleterious or advantageous, on vascular health. In one of many embodiments, the present invention further provides an expert system for achieving the above.
Owner:NEW HEALTH SCI
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS20060184493A1Improve statistics performanceReduce testing costsDigital computer detailsForecastingCardiovascular drugNon compliance
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
High throughput correlation of polymorphic forms with multiple phenotypes within clinical populations
A computer-assisted method of looking for pharmacologic targets, in which large numbers of persons are enrolled in drugh clinical trials, they are medically examined and documented, tissue samples are taken, the tissue samples are genotyped, and an examination is made of the genotypes to try to ascertain associations between the genotypes and the documented disease phenotypes of the patients.
Owner:SMITHKLINE BECKMAN CORP
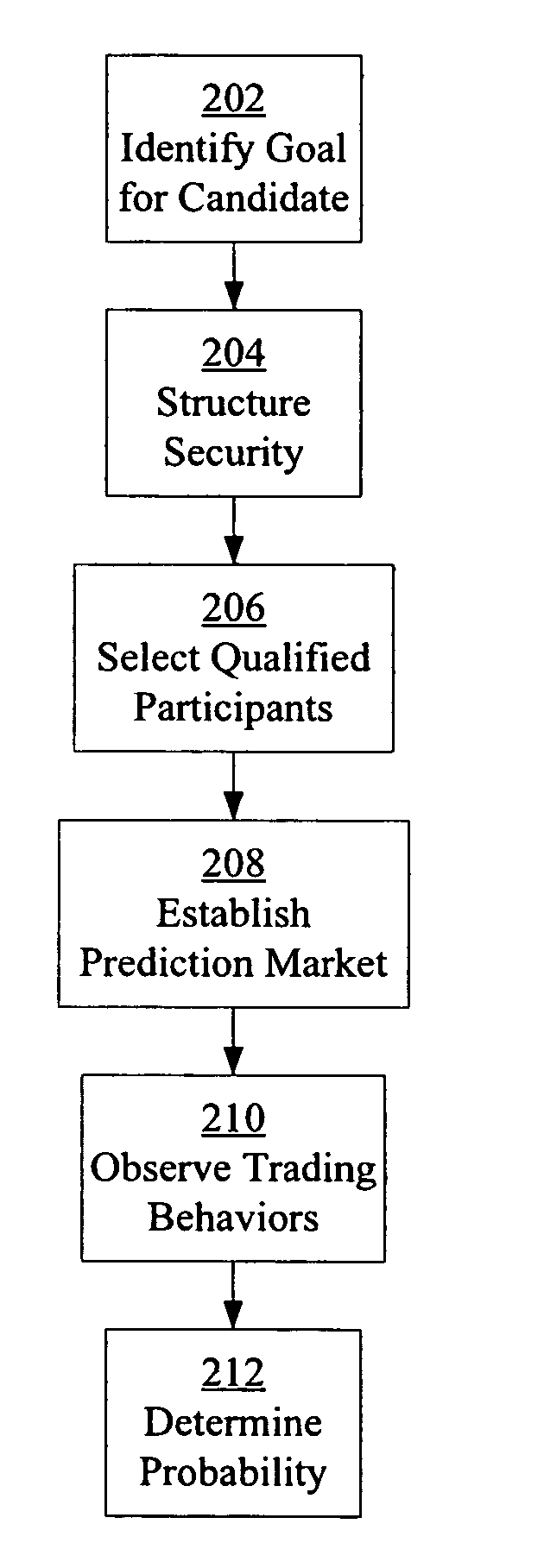
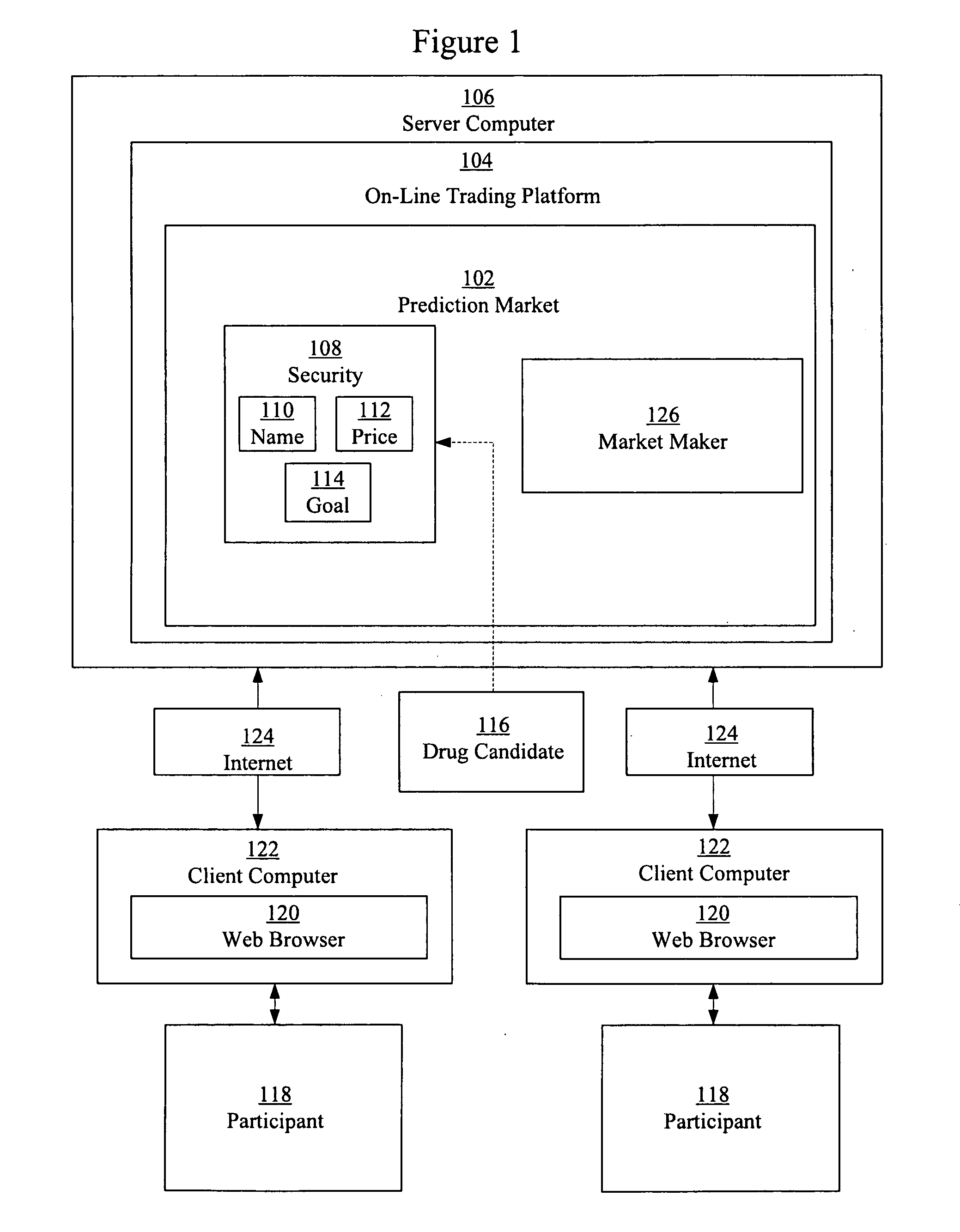
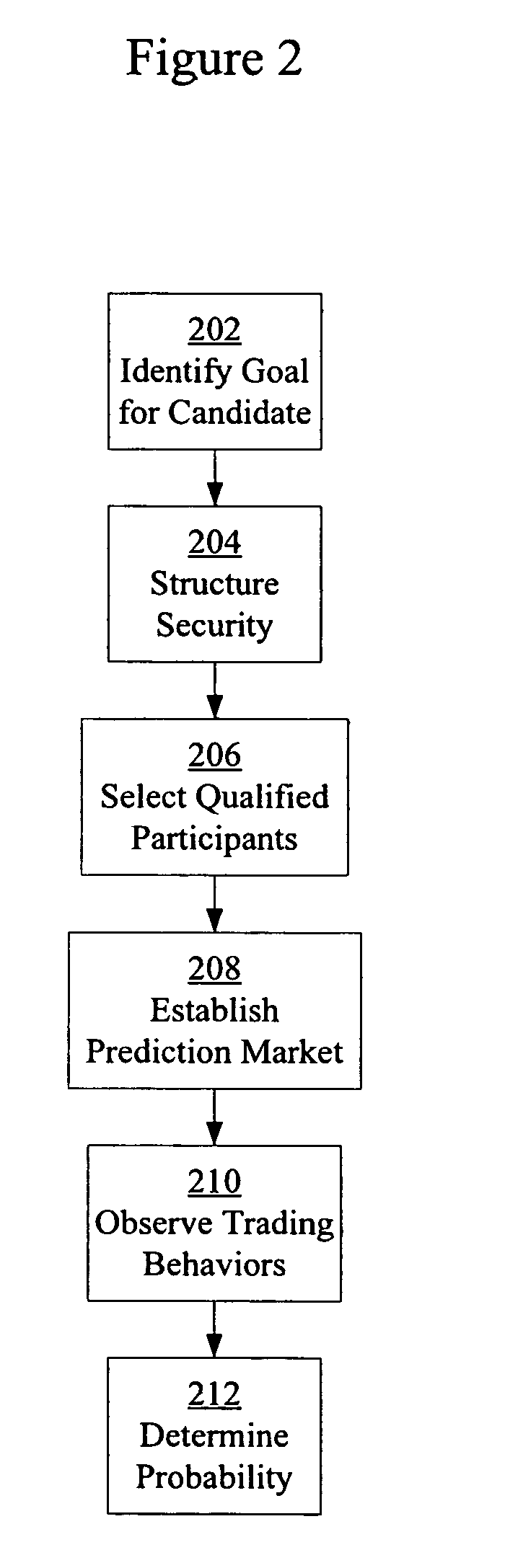
Prediction markets for assessing clinical probabilities of success
Prediction markets are used to determine the probability of an experimental therapeutic, diagnostic, or prophylactic candidate meeting clinical trial and post-trial goals, such as clinical trial endpoints and timelines. The prediction market processes buy and sell orders from market participants, while adjusting the prices of the securities according to the orders. The securities have specific meanings which correspond to goals in clinical trials or other outcomes in clinical candidate development. The price of a security determined by the market corresponds to the probability of the corresponding goal or outcome. The participants are selected for their expert knowledge of specific factors related to candidate development. Using appropriately selected securities and participants, the prediction market may be used to generate probabilities of success useful for long-range planning and valuation, determining production timelines and volumes, management of candidates in a development portfolio, and clinical management of patients by physicians.
Owner:CLINICAL FUTURES
Non-lethal conditioning methods for the treatment of acquired immunodeficiency syndrome
InactiveUS6039684AGood conditionPromote resultsPeptide/protein ingredientsEnergy modified materialsAcquired immunodeficiencyImmunodeficiency virus
The present invention relates to the use of a non-lethal preparative regimen for the treatment of a patient with human immunodeficiency virus (HIV) disease. In a clinical trial, an HIV-positive patient was conditioned by non-lethal doses of irradiation and a chemotherapeutic drug prior to receiving donor bone marrow cells from a baboon. While long-term engraftment of donor cells was not observed, the non-lethal preparative conditioning regimen was able to reduce the viral burden and improved the clinical outcome. Such method is useful for treatment of patients with advanced acquired immunodeficiency syndrome (AIDS).
Owner:ALLEGHENY UNIV OF THE HEALTH SCI +1
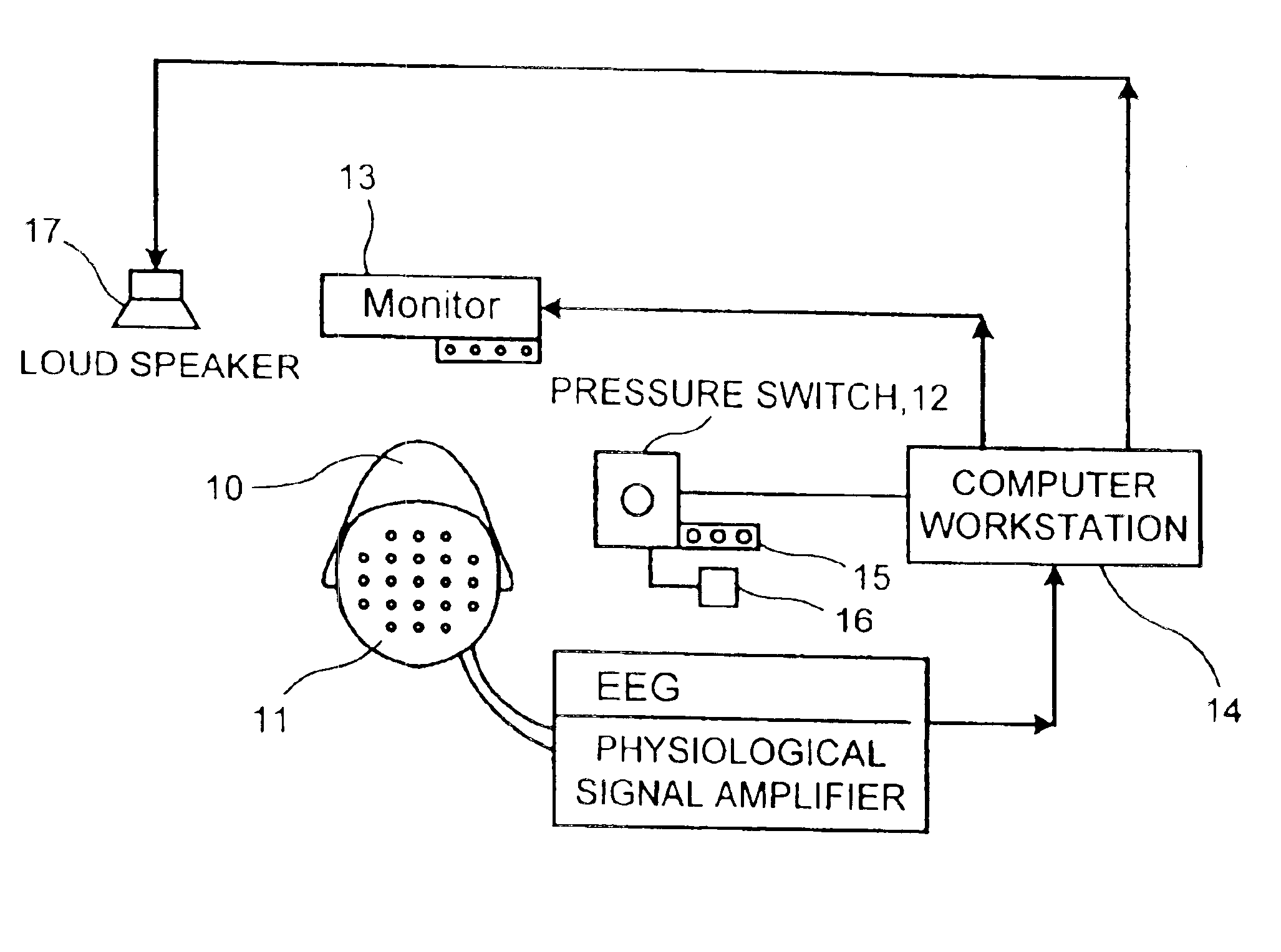
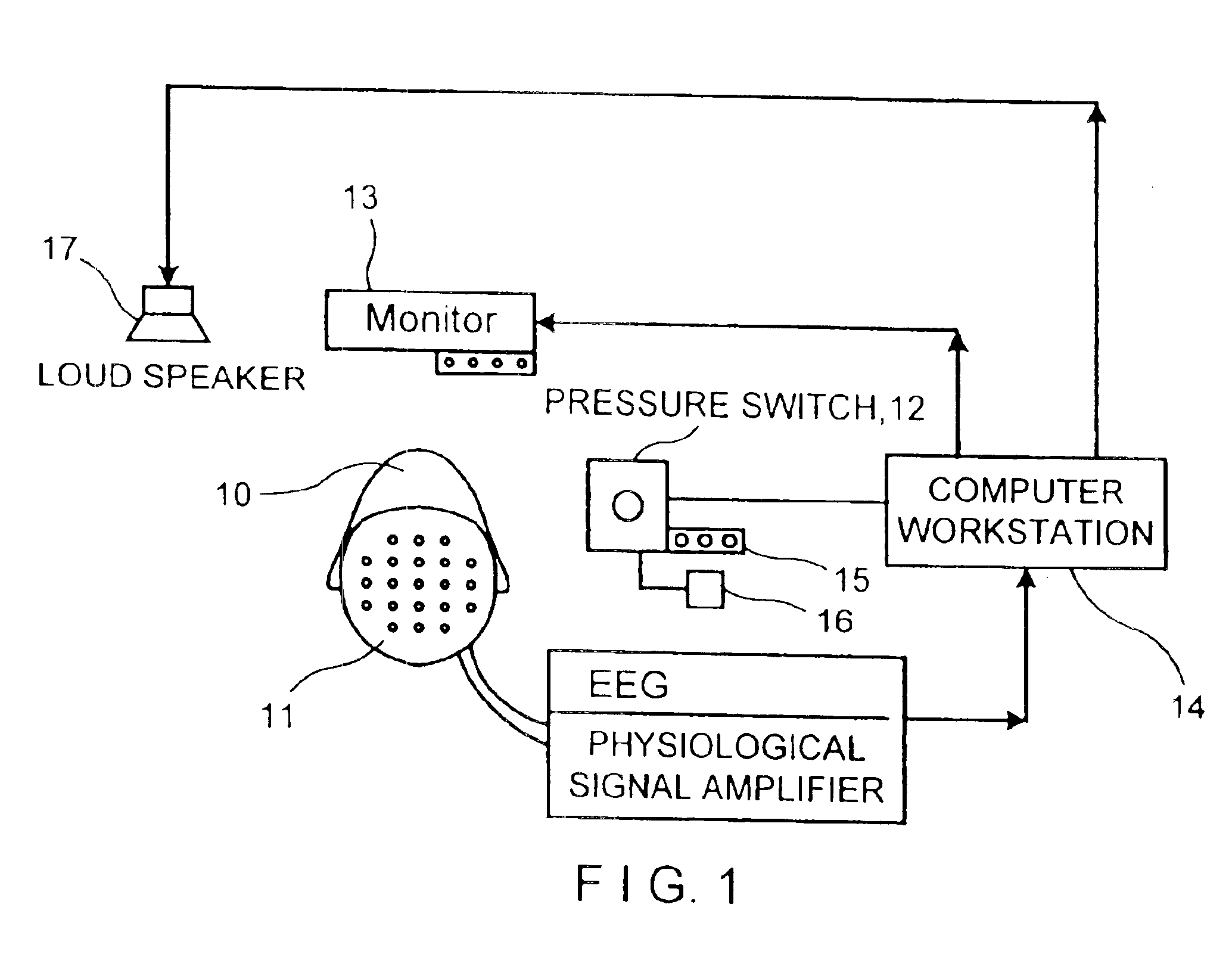
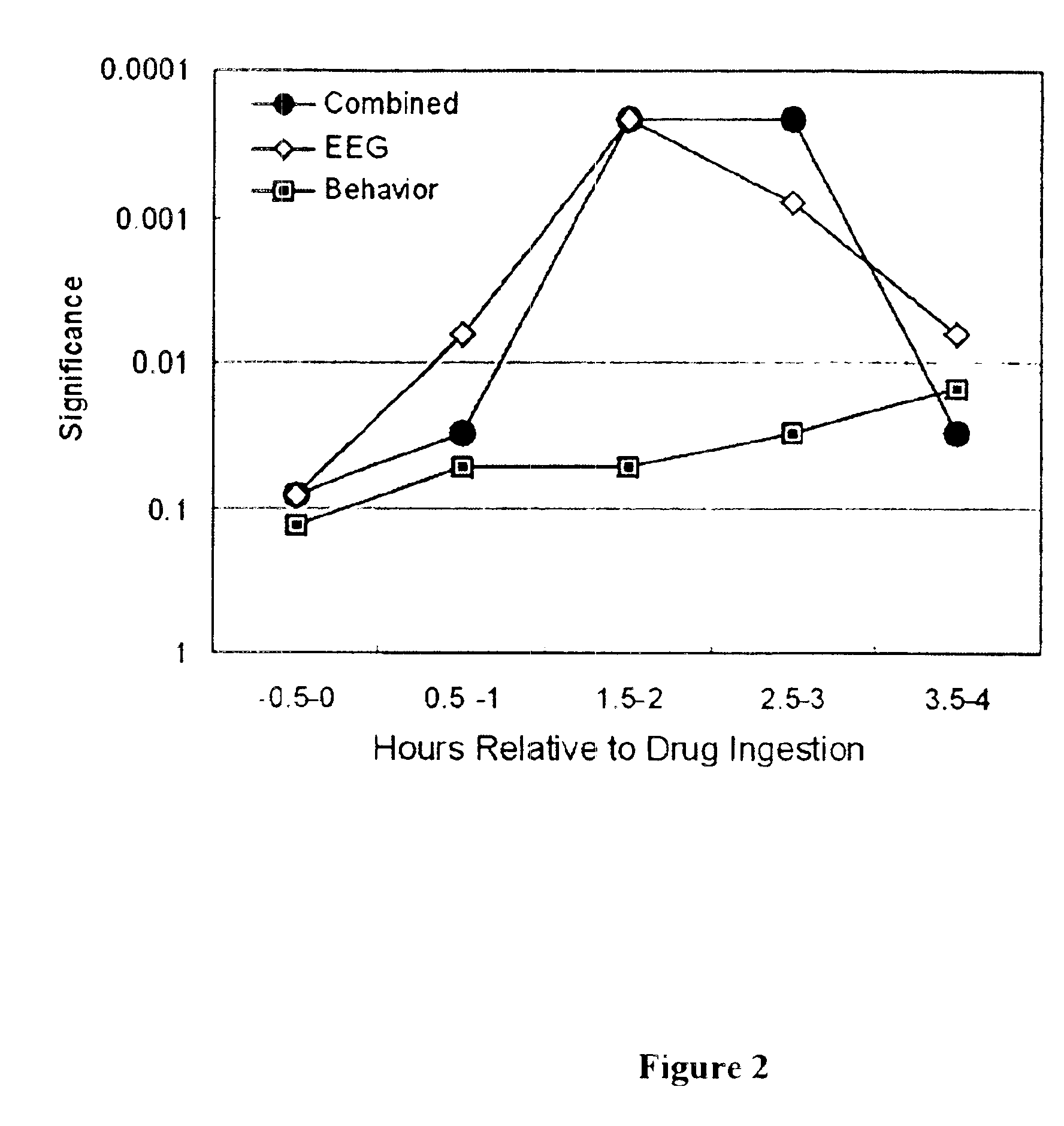
Neurocognitive function EEG measurement method and system
InactiveUS6947790B2Sufficient sensitivityHigh sensitivityElectroencephalographyHealth-index calculationDiseaseDrug approval
An efficient, objective testing method and system for evaluating changes in mental function is described. The method and system are based on measuring an individual's behavioral responses and brain function during a brief cognitive test battery and passive control conditions. The method and system is designed to assess an individual's fundamental cognitive functions, and whether those functions have been significantly affected by a variety of factors such as progressive disease processes, medication, stress, fatigue, training, or the passage of time. The method and system can be used to determine whether drugs being evaluated to treat diseases or conditions affecting cognitive brain function have a significant positive effect on delaying or improving the symptoms of such a disease or condition, especially during clinical trials for drug approval and subsequent marketing. The method and system may also be employed as part of the successful diagnosis or ongoing treatment of neurological diseases or conditions that directly or indirectly affect human neurocognitive performance. The method and system may also be used to determine transitory changes in overall cognitive function due to emotional stress or fatigue, and more long lasting changes in overall cognitive function following training and educational programs.
Owner:SAM TECHOLOGY
Interactive spoken dialogue interface for collection of structured data
InactiveUS20130339030A1Enhanced interactionMinimize effortTherapiesMedical automated diagnosisMedical recordSpoken language
A multimodal dialog interface for data capture at point of origin is disclosed. The interface is designed to allow loading of forms needed for task record keeping, and is therefore customizable to wide range of record keeping requirements such as medical record keeping or recording clinical trial. The interface has a passive mode that is able to capture data while the user is performing other tasks, and an interactive dialog mode that ensures completion of all required information.
Owner:NANT HLDG IP LLC
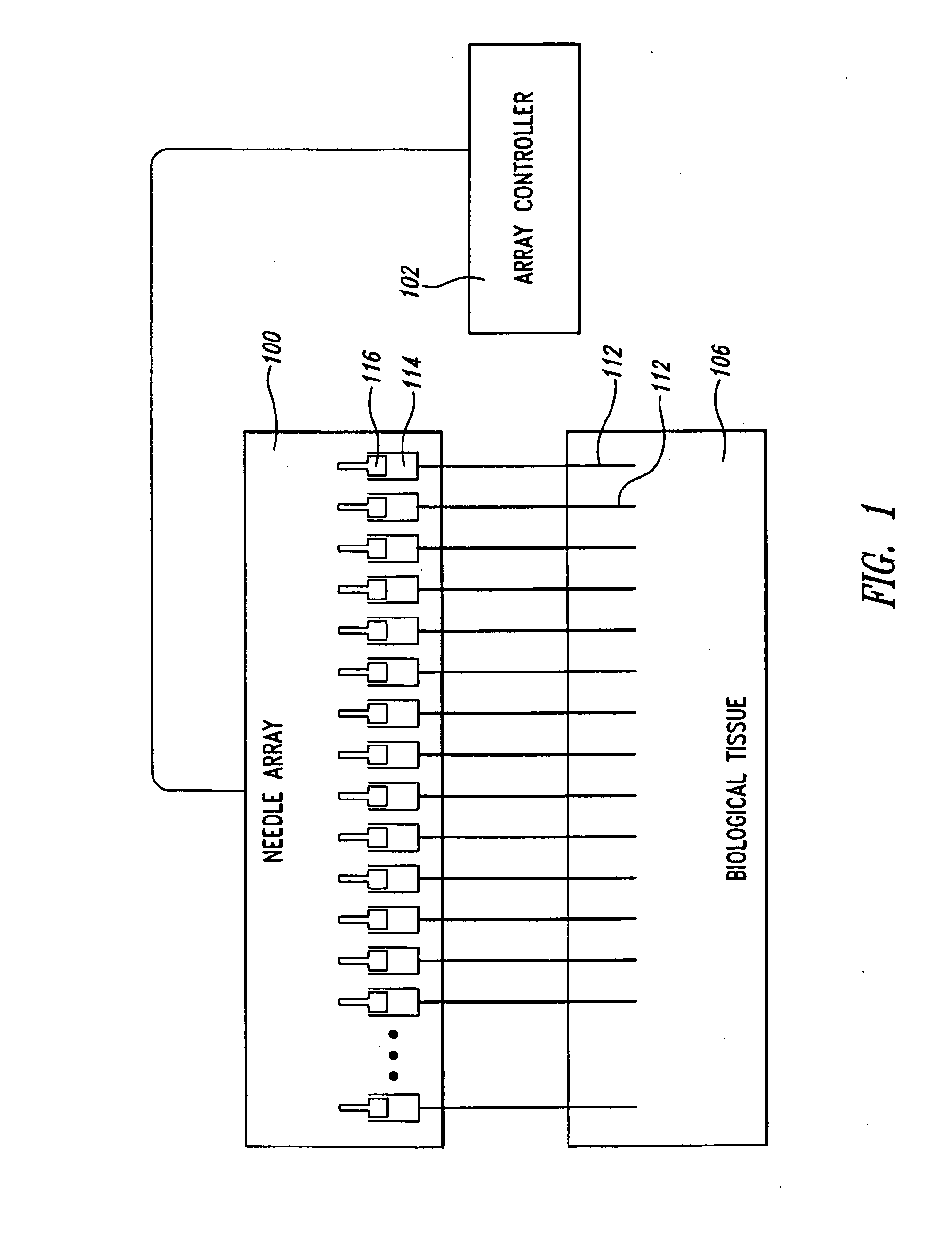
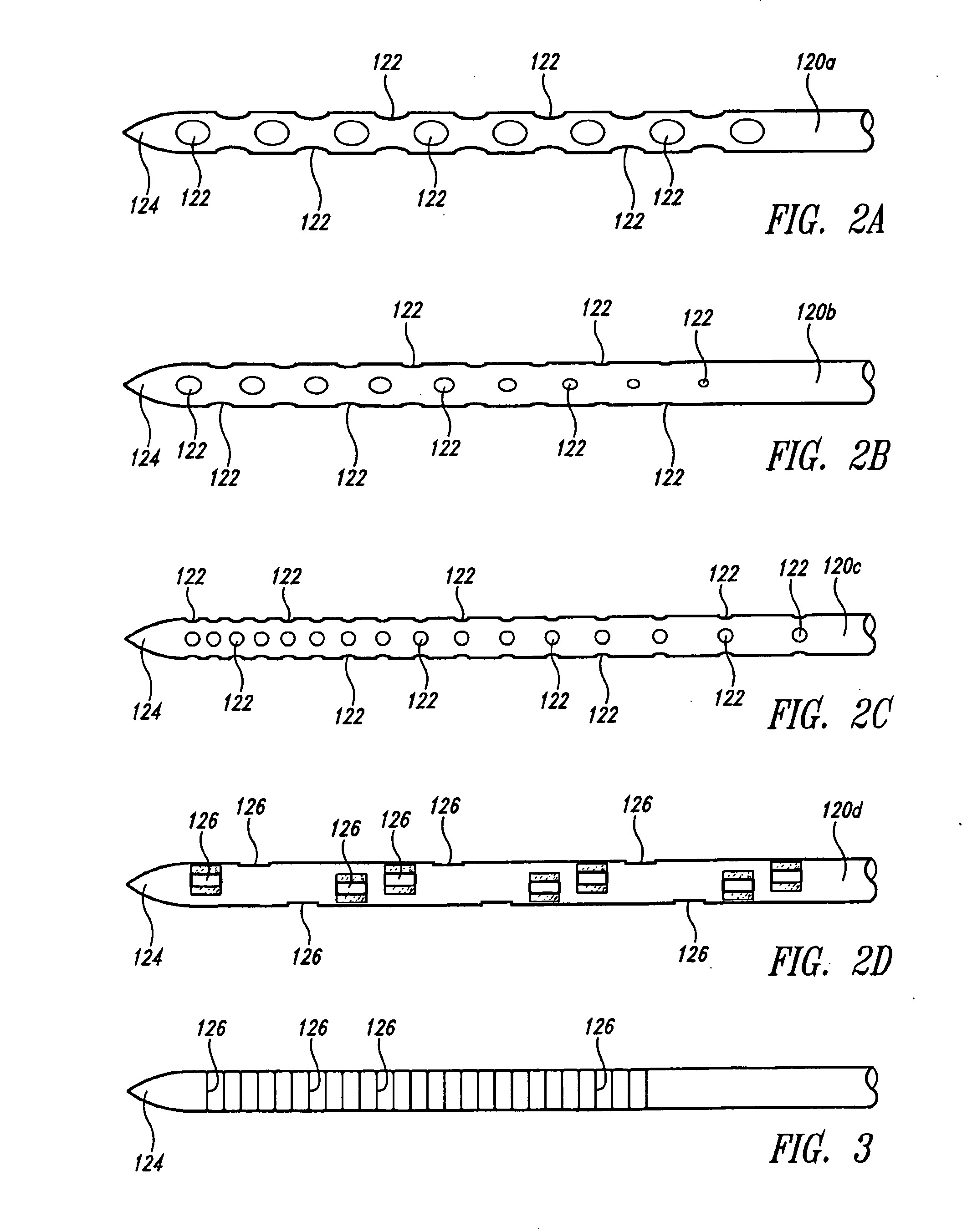
Needle array assembly and method for delivering therapeutic agents
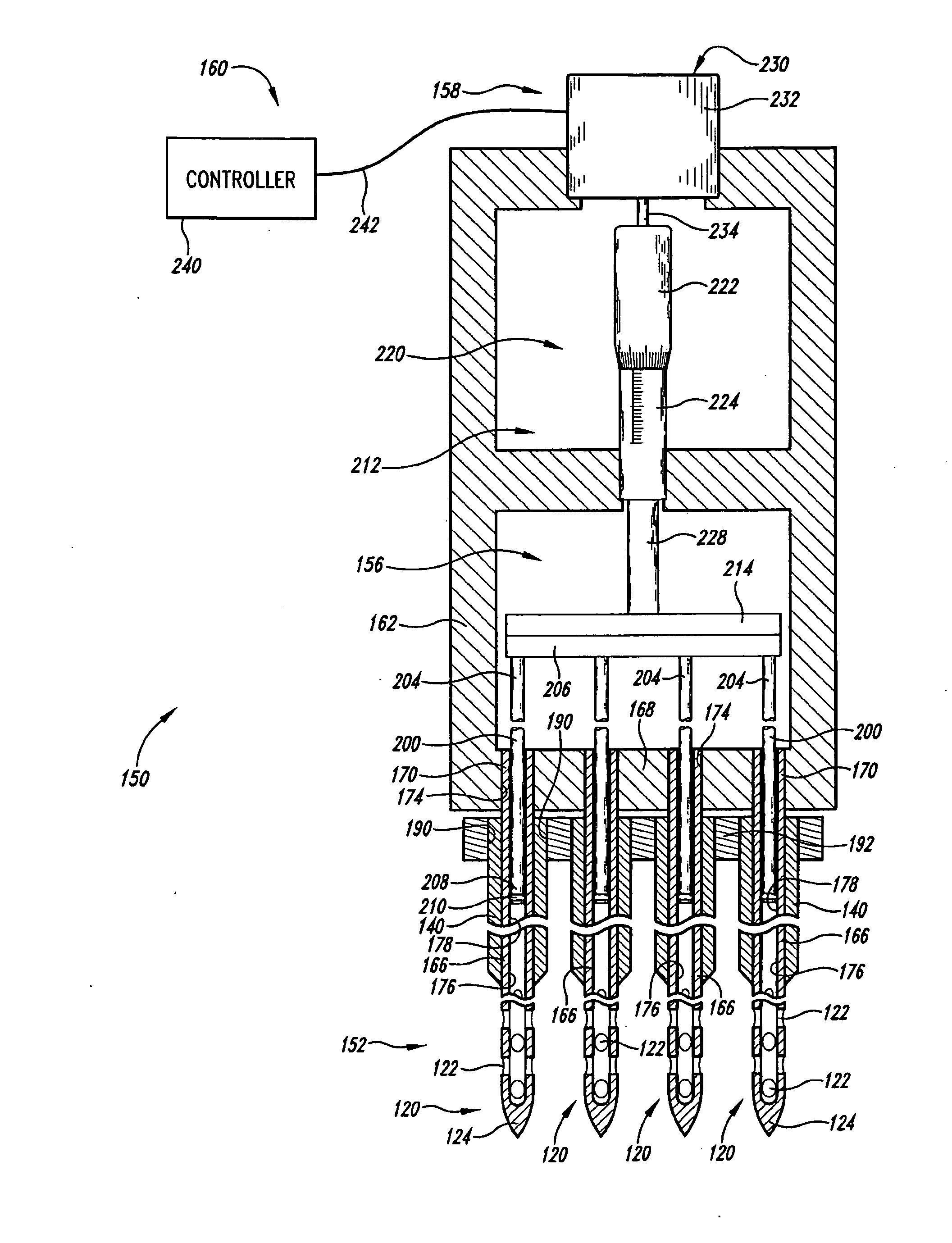
ActiveUS20100330589A1Automatic syringesMicrobiological testing/measurementSolid tissueClinical trial
A fluid delivery device includes an array of needles, each in fluid communication with a respective reservoir. Respective actuators are coupled so as to be operable to drive fluid from the reservoirs via needle ports. Each needle can have a plurality of ports, and the ports can be arranged to deliver a substantially equal amount of fluid at any given location along its length. A driver is coupled to the actuators to selectively control the rate, volume, and direction of flow of fluid through the needles. The device can simultaneously deliver a plurality of fluid agents along respective axes in solid tissue in vivo. If thereafter resected, the tissue can be sectioned for evaluation of an effect of each agent on the tissue, and based on the evaluation, candidate agents selected or deselected for clinical trials or therapy, and subjects selected or deselected for clinical trials or therapeutic treatment.
Owner:FRED HUTCHINSON CANCER RES CENT
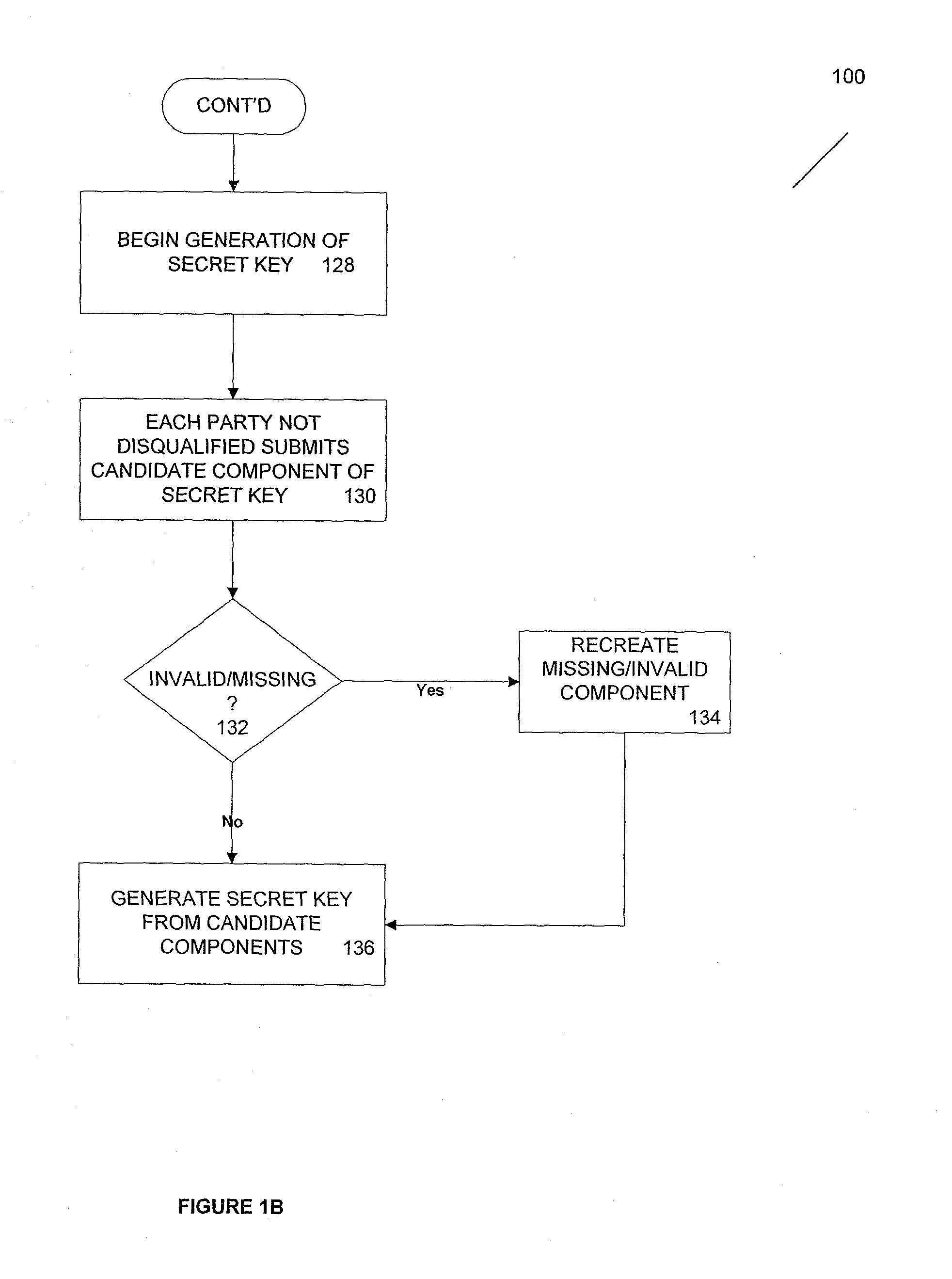
Method and apparatus for time-lapse cryptography
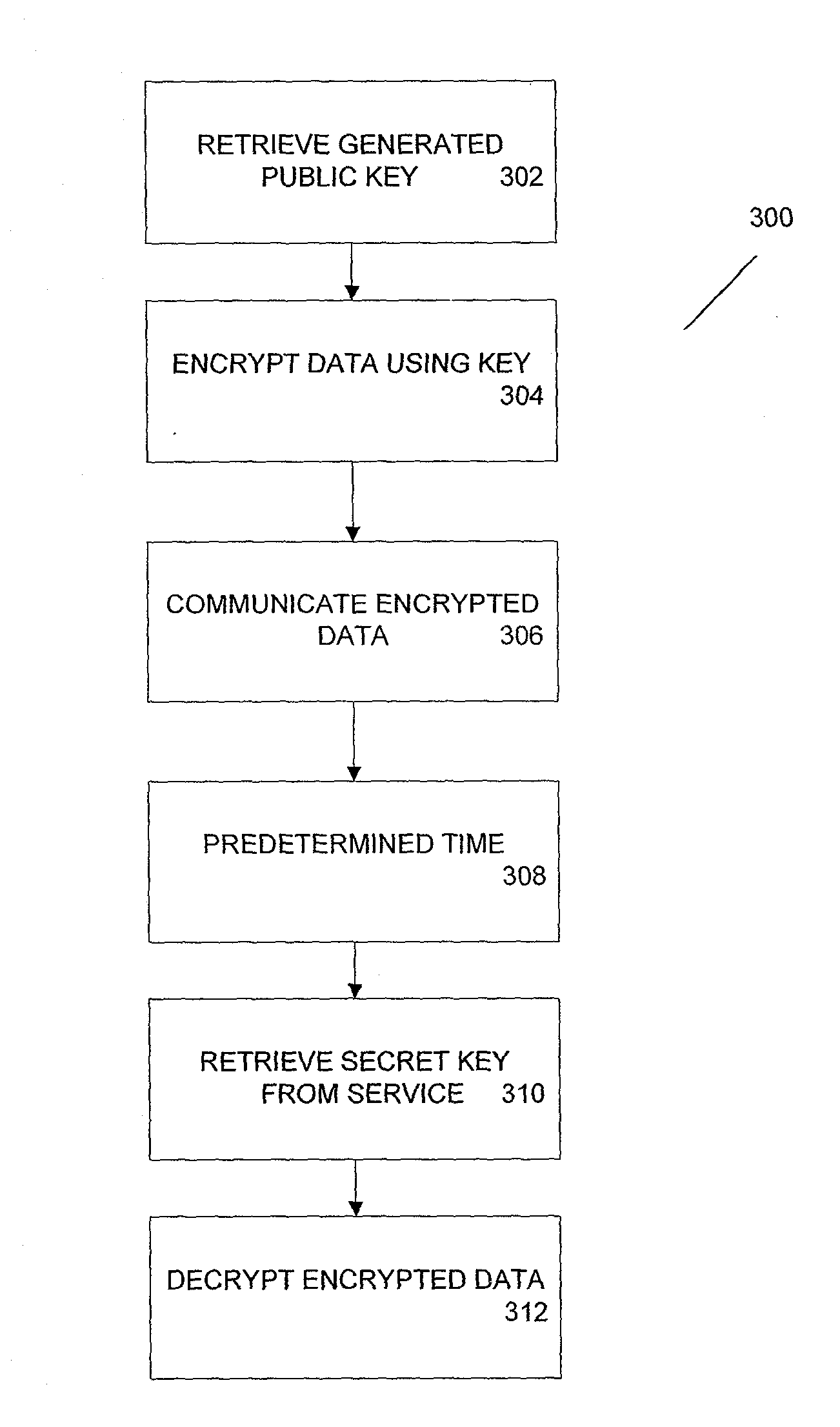
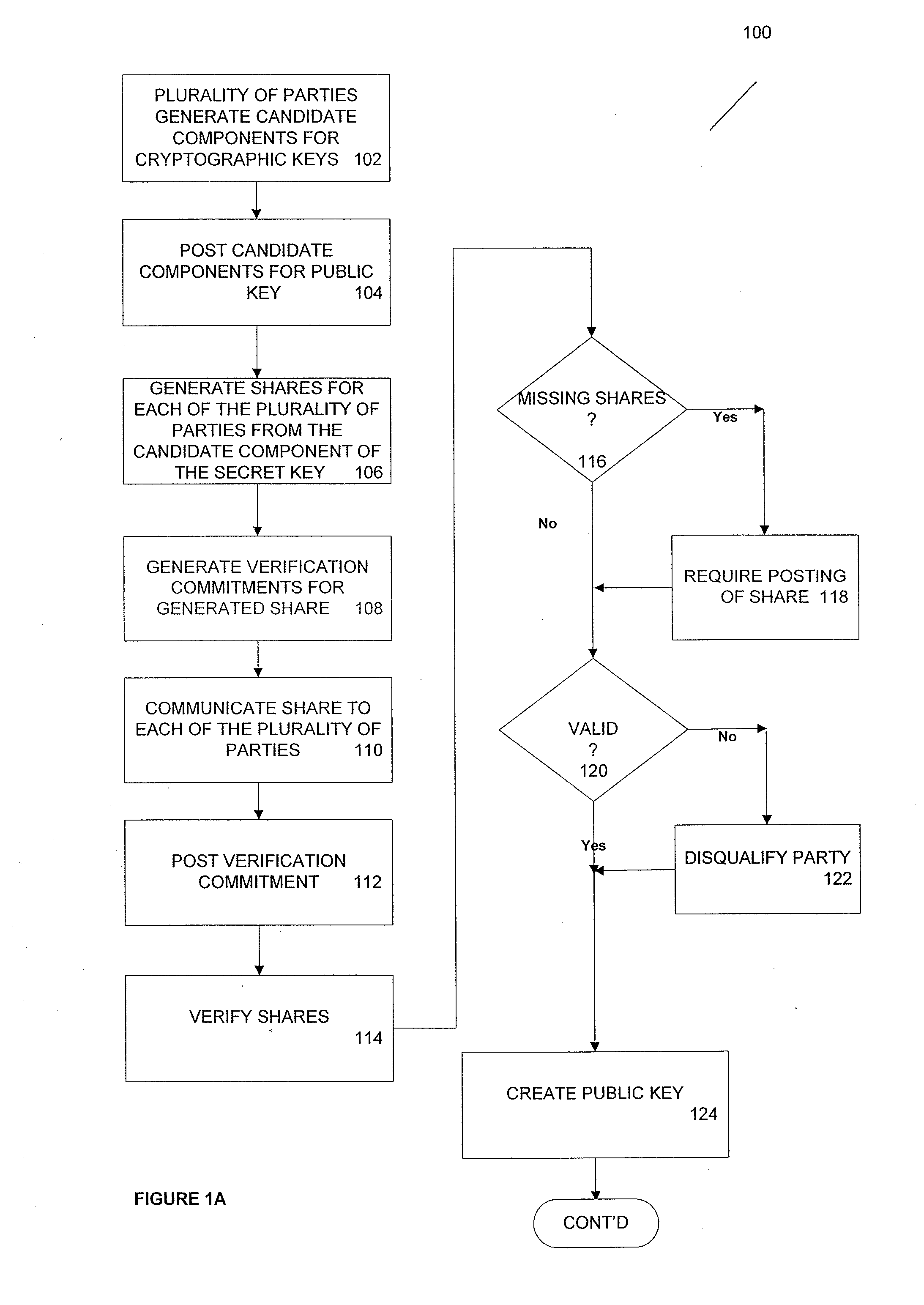
ActiveUS20100185863A1Key distribution for secure communicationMultiple keys/algorithms usageThird partyCiphertext
According to one aspect, provided is a construction and specification for an implementation of a new cryptographic primitive, “Time-Lapse Cryptography”, with which a sender can encrypt a message so that it is guaranteed to be revealed at an exact moment in the future, even if this revelation turns out to be undesirable to the sender. In one embodiment, a Time-Lapse Cryptography Service is provided (“the Service”) based on a network of parties. Senders encrypt their messages with this public key whose secret key is not known to anyone—not even a trusted third party—until a predefined and specific future time T+δ, at which point the secret key is constructed and published. In one example, the secret key can only be known after it is constructed. At or after that time, anyone can decrypt the cipher text using this secret key. Other embodiments describe other applications of such a service, for example, one embodiment is used in sealed bid auctions, others in insider stock sales, clinical trials, and electronic voting, among a variety of possible implementations. In one embodiment, a method for cryptographic encoding is provided, including generation of cryptographic key components by a plurality of parties, where participation of the parties is verified. A public key is constructed from a plurality of key components,
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
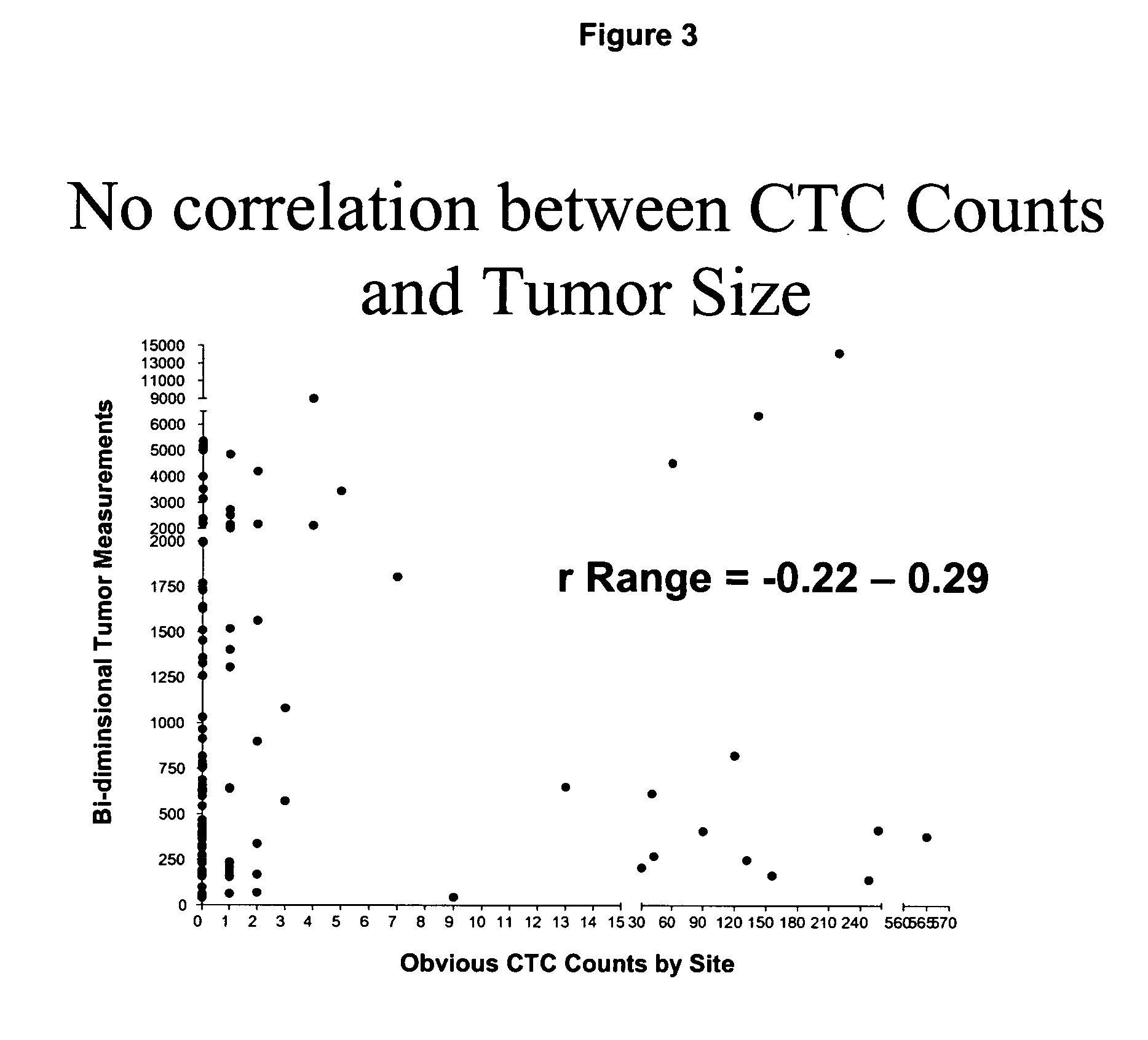
Circulating tumor cells (CTC's): early assessment of time to progression, survival and response to therapy in metastatic cancer patients
InactiveUS20070037173A1Less side effectsImprove the quality of lifeMicrobiological testing/measurementBiological testingClinical trialOncology
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:JANSSEN DIAGNOSTICS LLC
Methods and Systems for Clinical Trial Data Management
InactiveUS20060259783A1Unauthorized memory use protectionComputer-assisted medical data acquisitionGuidelineClinical trial
The invention provides systems and methods for creating certified copies of original information, including original hardcopy documents, in compliance with federal regulations and guidelines. The present invention also provides systems and methods of data management, and in particular, management of such certified copies. In some embodiments the invention relates to original clinical trial information such as source documents, and methods and systems for creating certified copies of such information to create an accessible central repository of such certified copies.
Owner:THERAPEIAS HEALTH MANAGEMENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com