Methods and Systems for Facilitating Clinical Trials
a clinical trial and clinical technology, applied in the field of methods and systems for facilitating clinical trials, can solve the problems of affecting the clinical effect of clinical trials,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
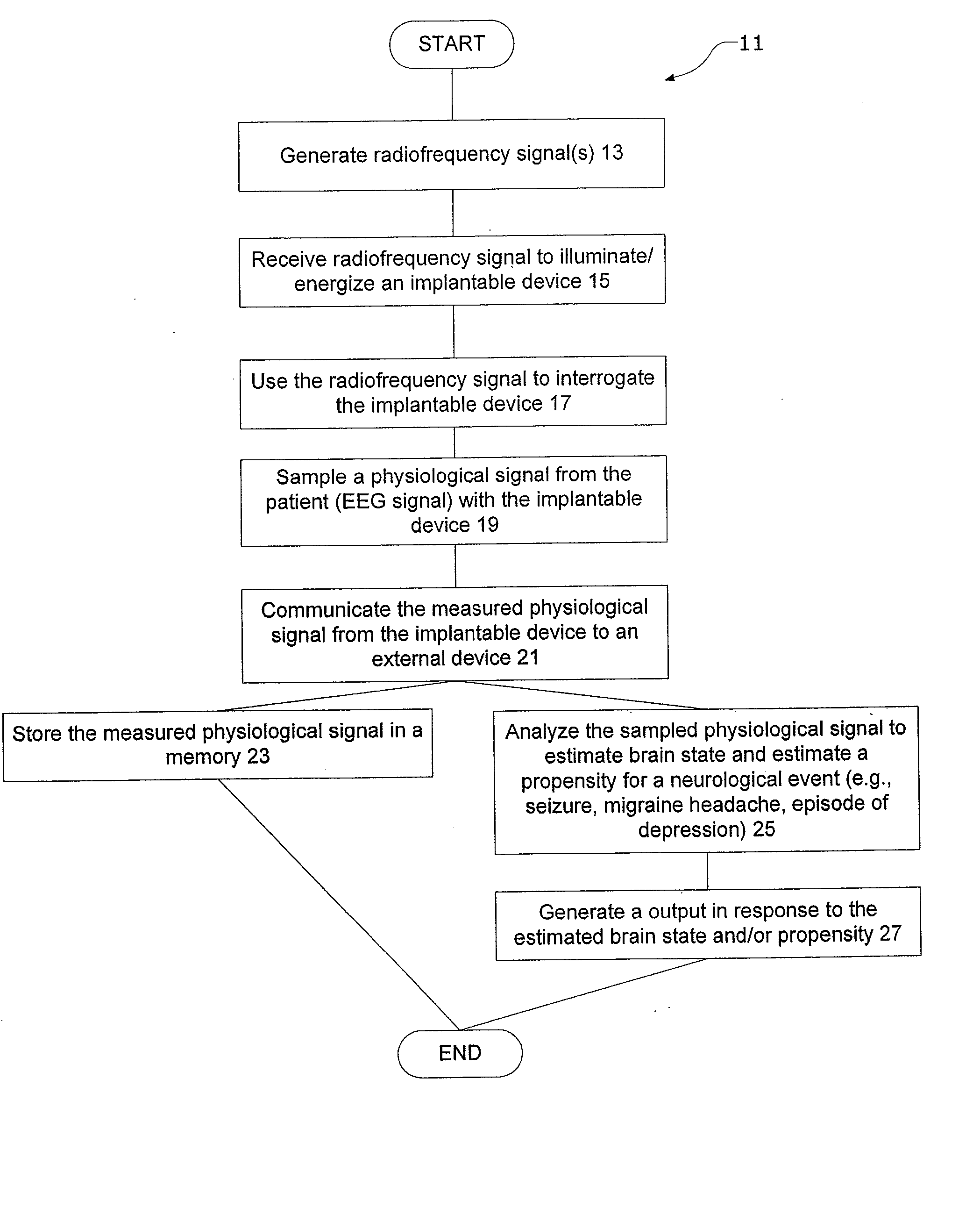
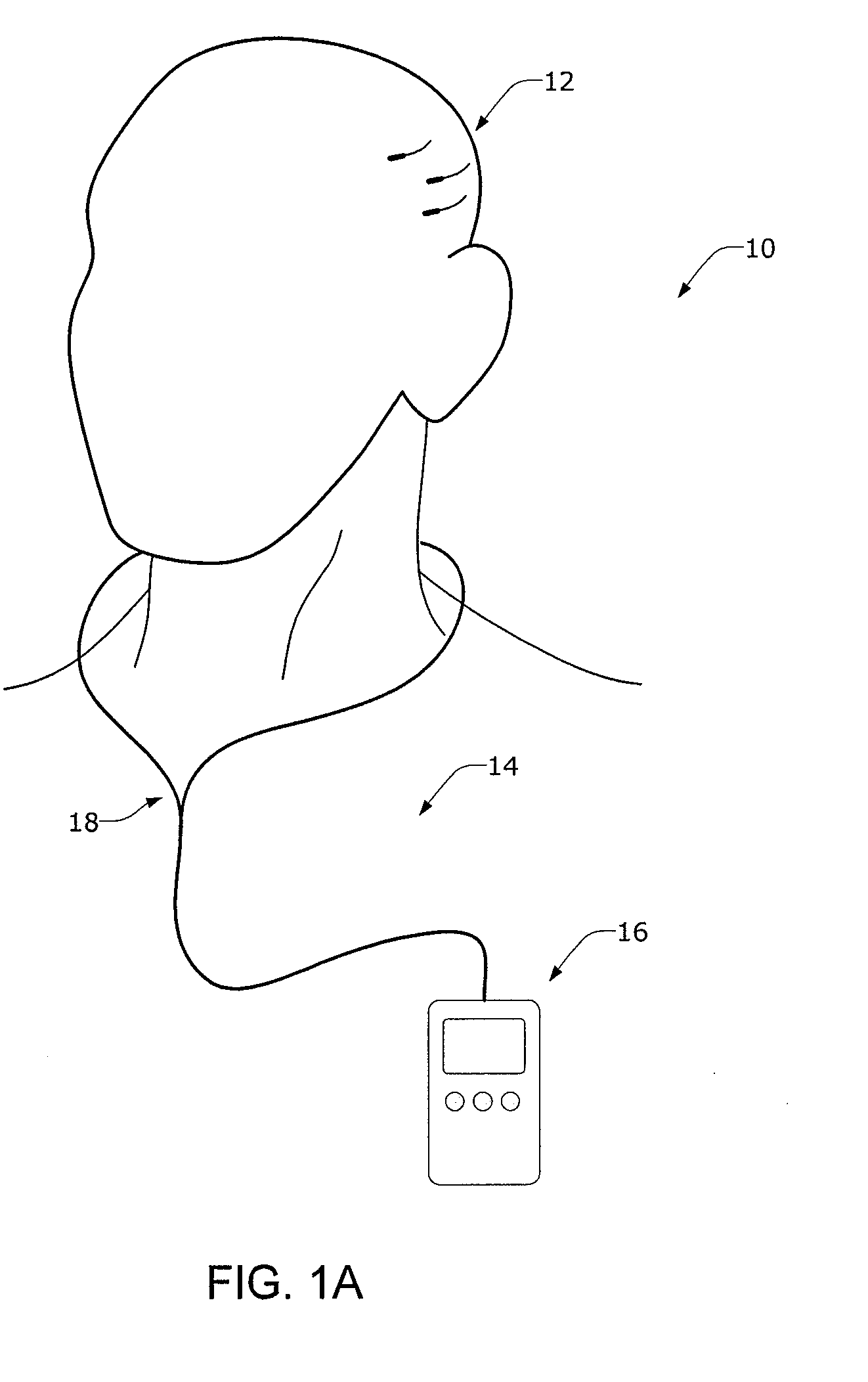
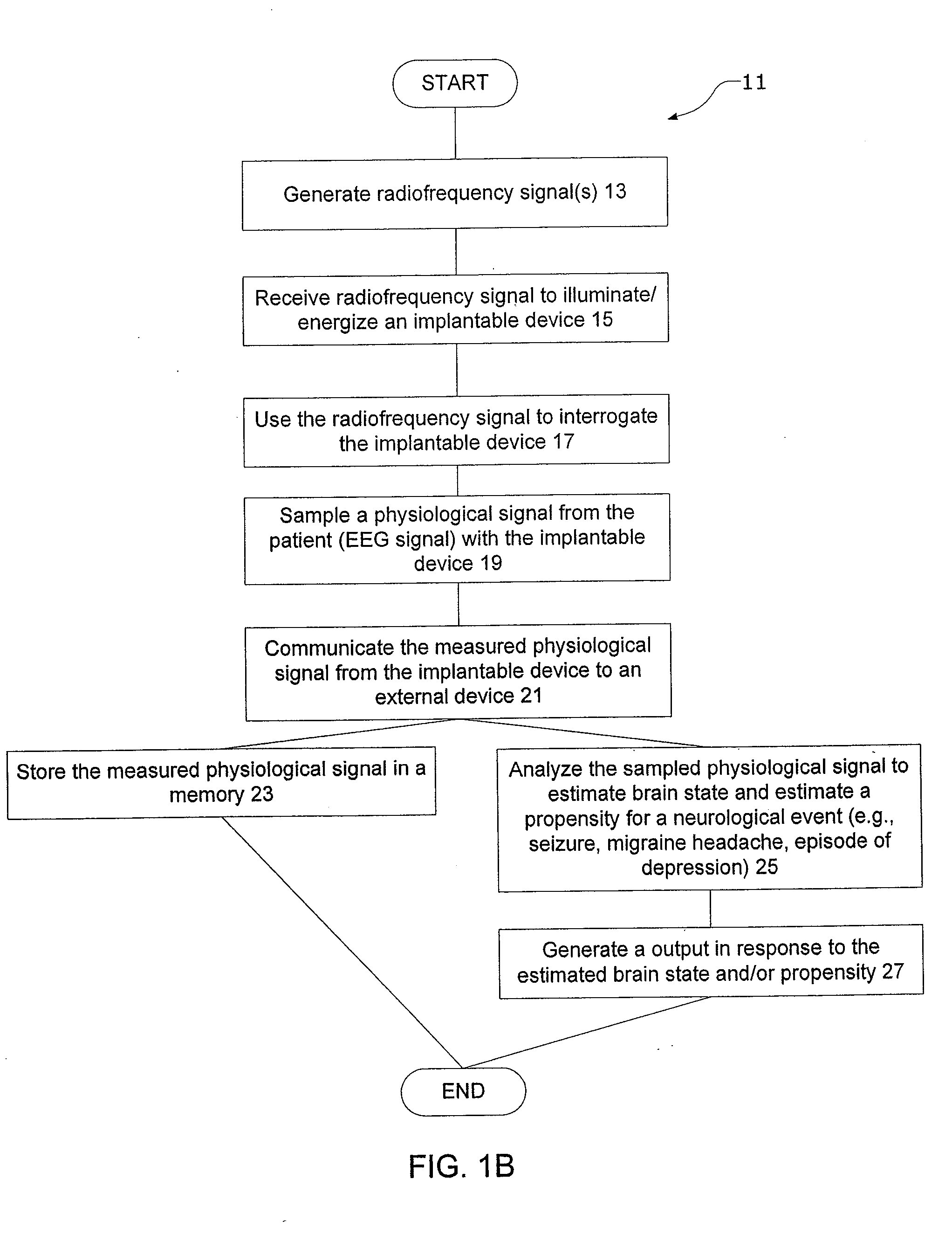
[0049] Certain specific details are set forth in the following description and figures to provide an understanding of various embodiments of the invention. Certain well-known details, associated electronics and devices are not set forth in the following disclosure to avoid unnecessarily obscuring the various embodiments of the invention. Further, those of ordinary skill in the relevant art will understand that they can practice other embodiments of the invention without one or more of the details described below. Finally, while various processes are described with reference to steps and sequences in the following disclosure, the description is for providing a clear implementation of particular embodiments of the invention, and the steps and sequences of steps should not be taken as required to practice this invention.
[0050] The term “condition” is used herein to generally refer to the patient's underlying disease or disorder—such as epilepsy, depression, Parkinson's disease, headac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com