Patents
Literature
2911 results about "Implanted device" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
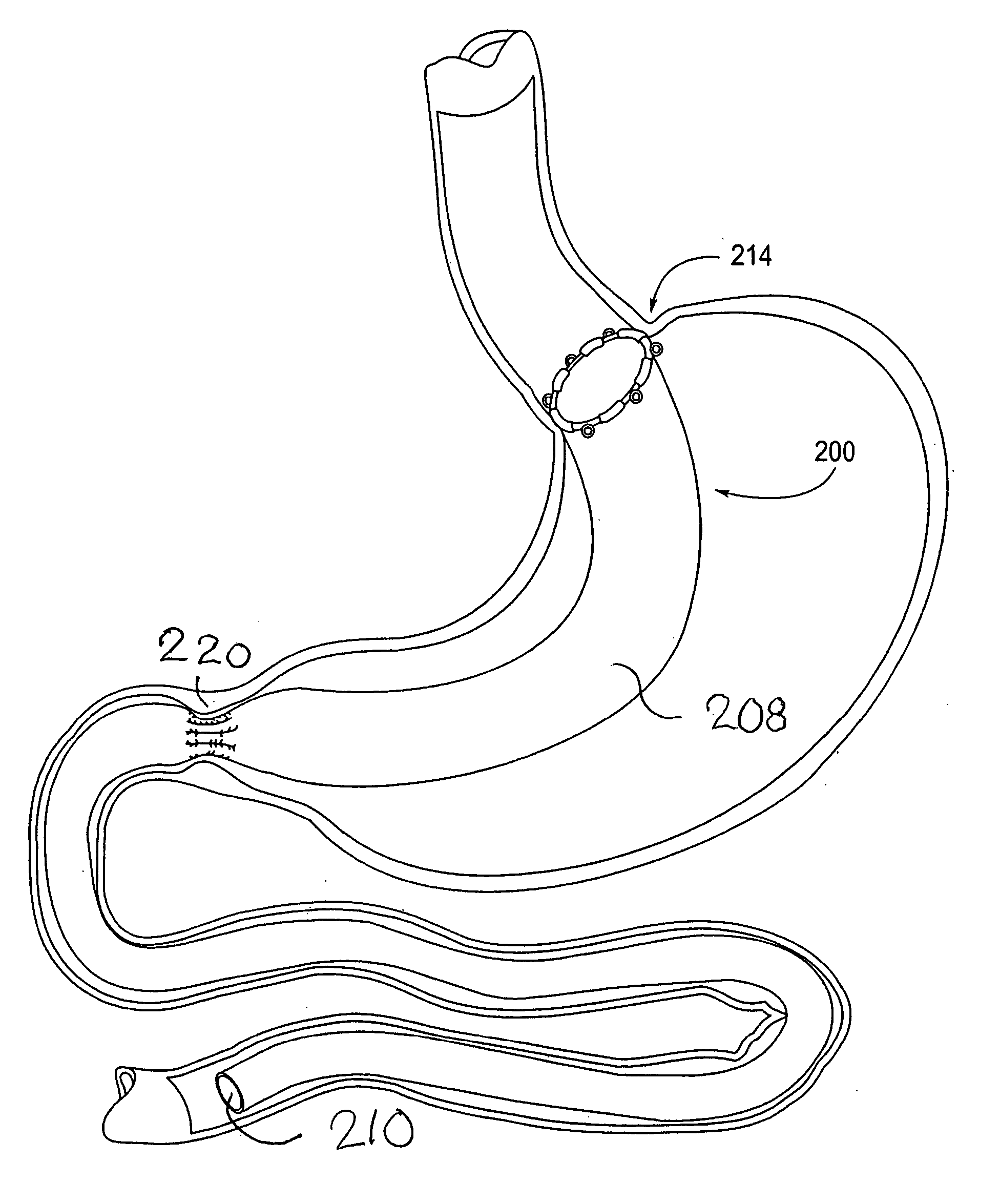
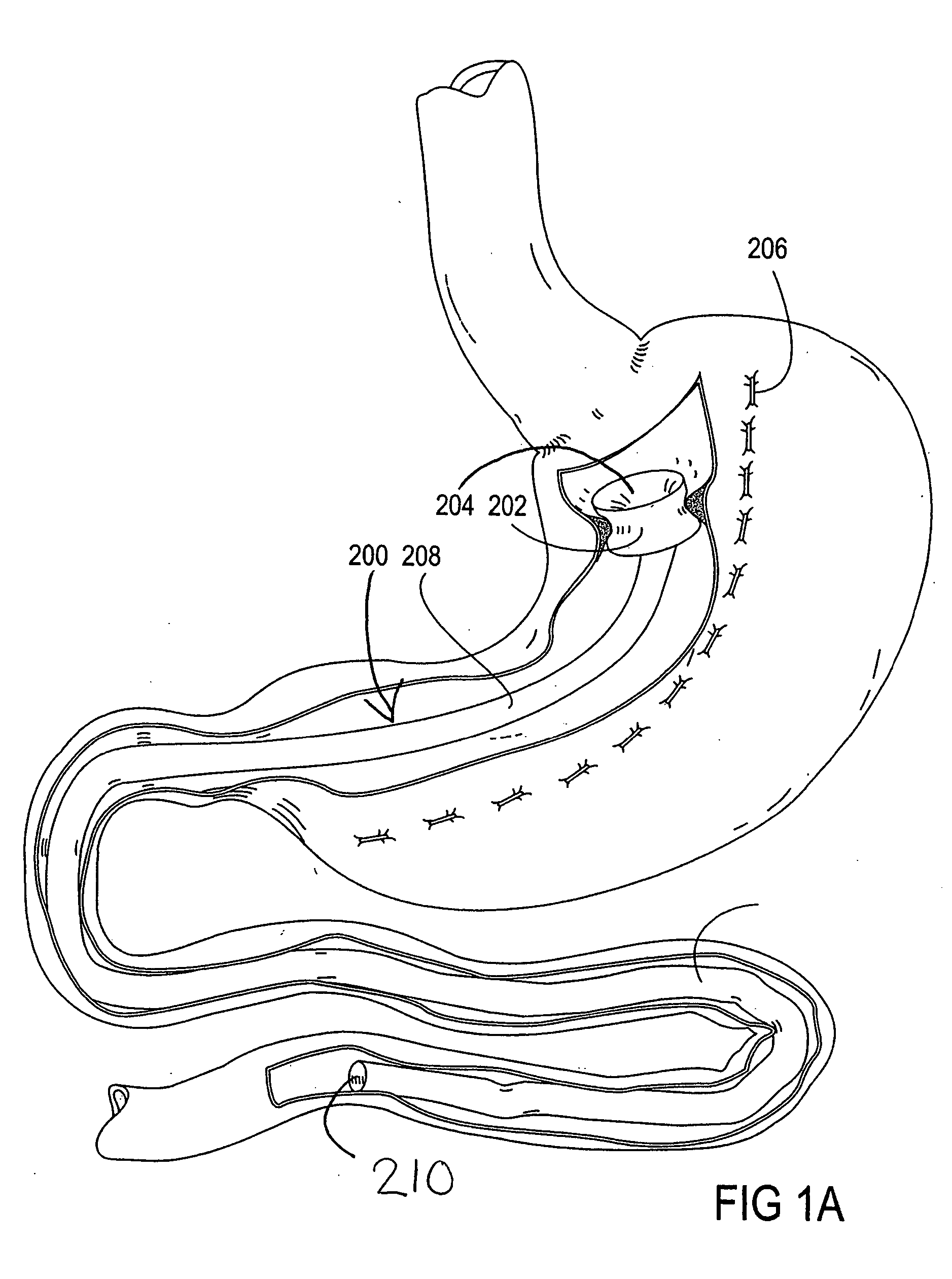
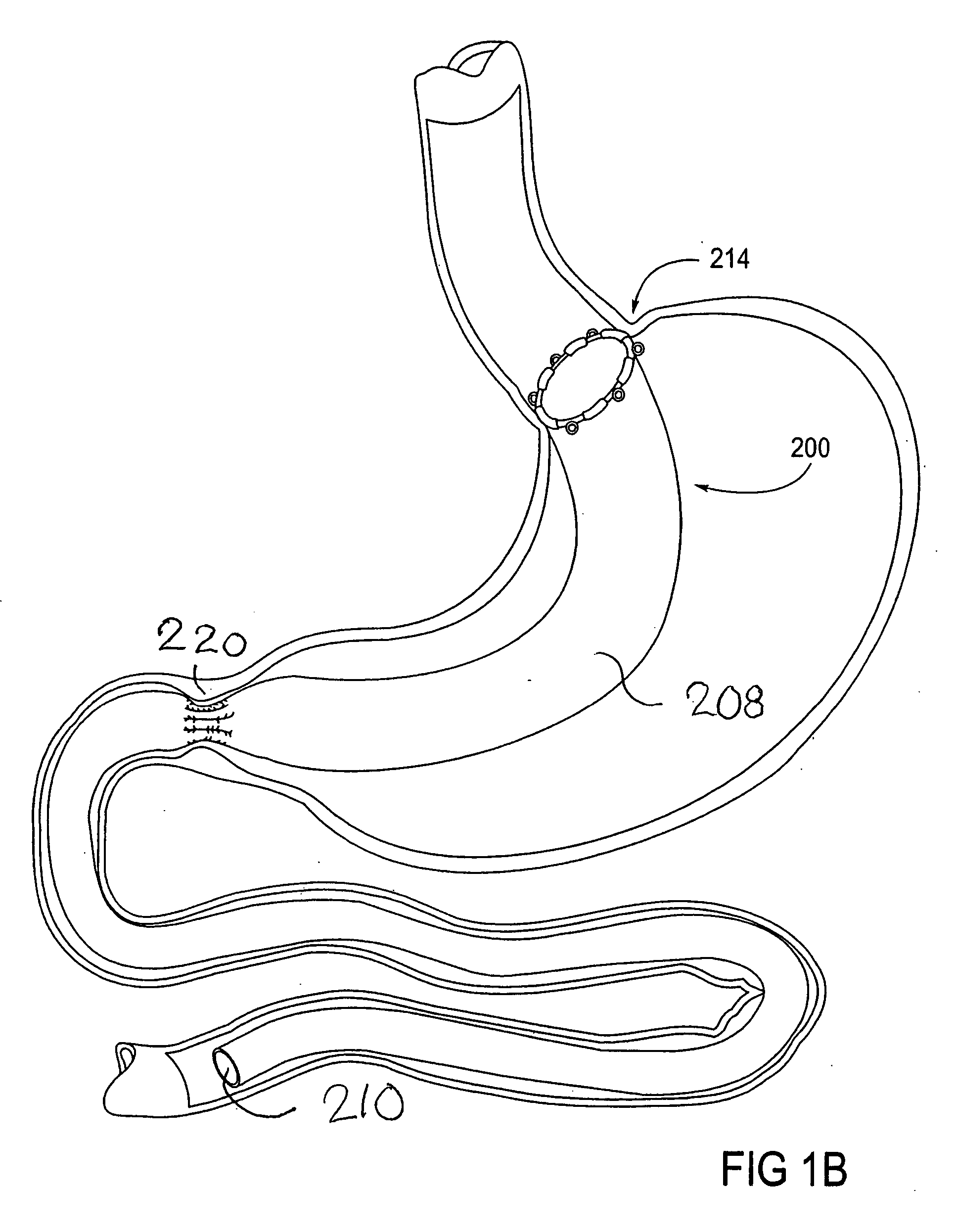
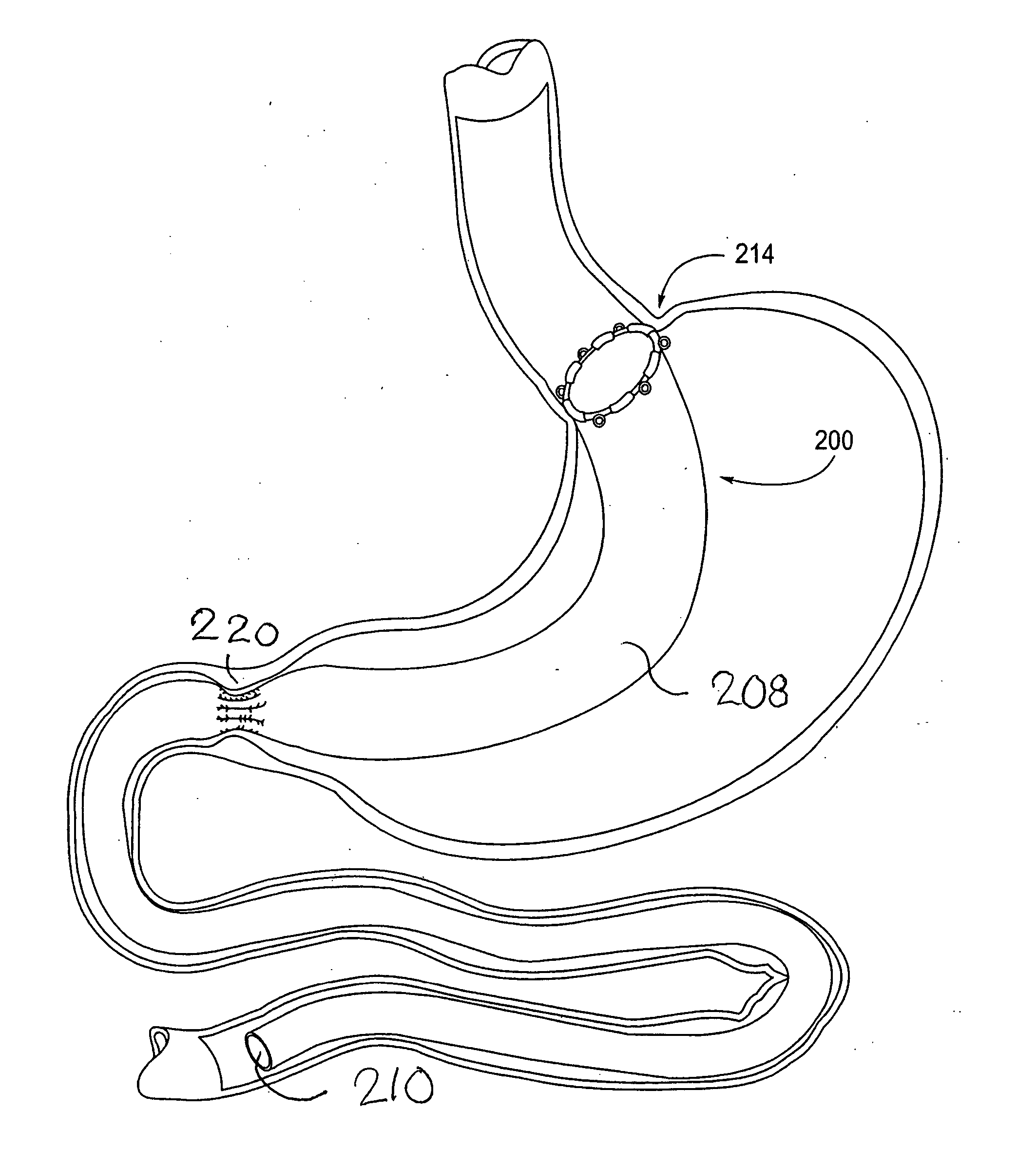
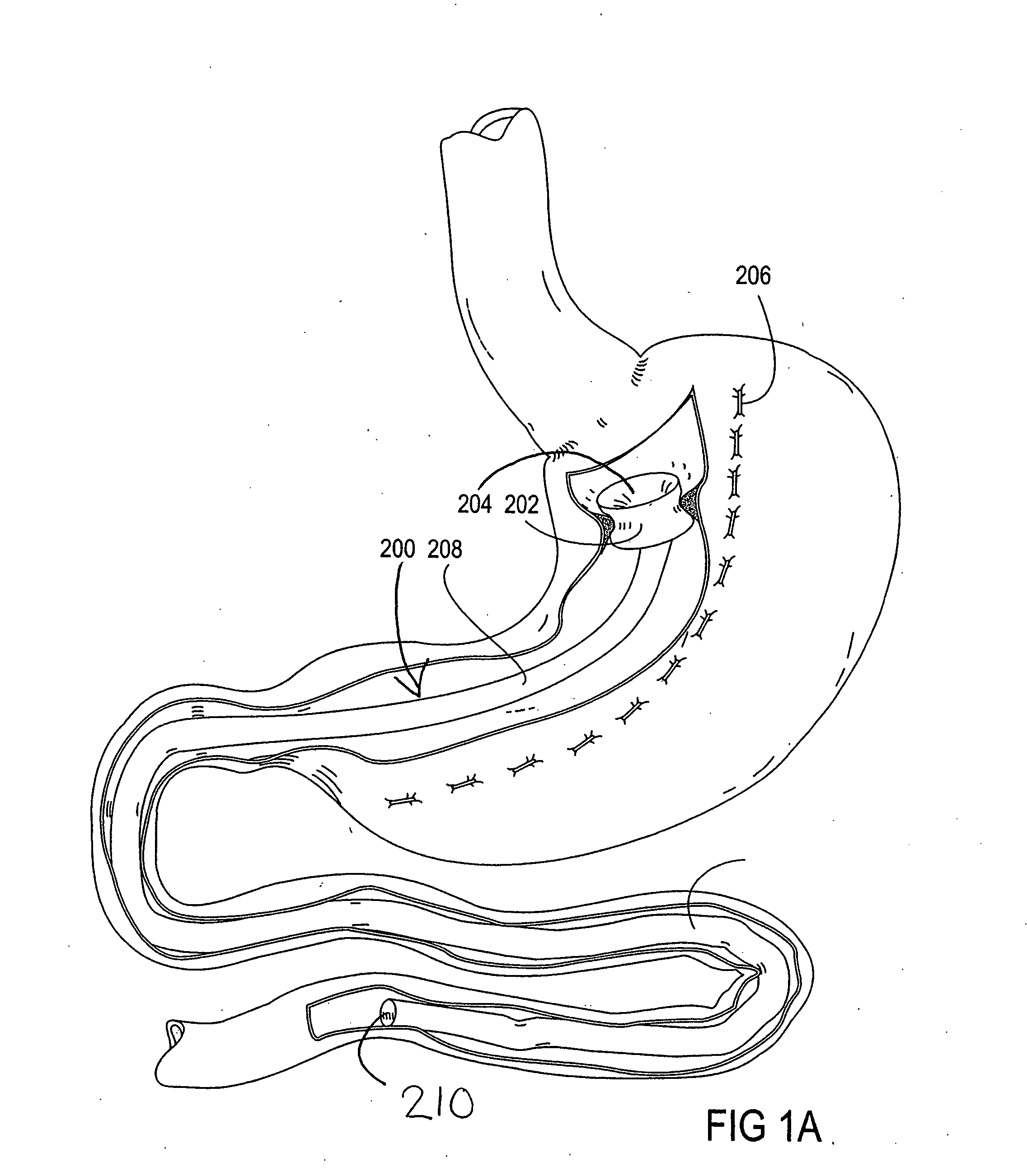
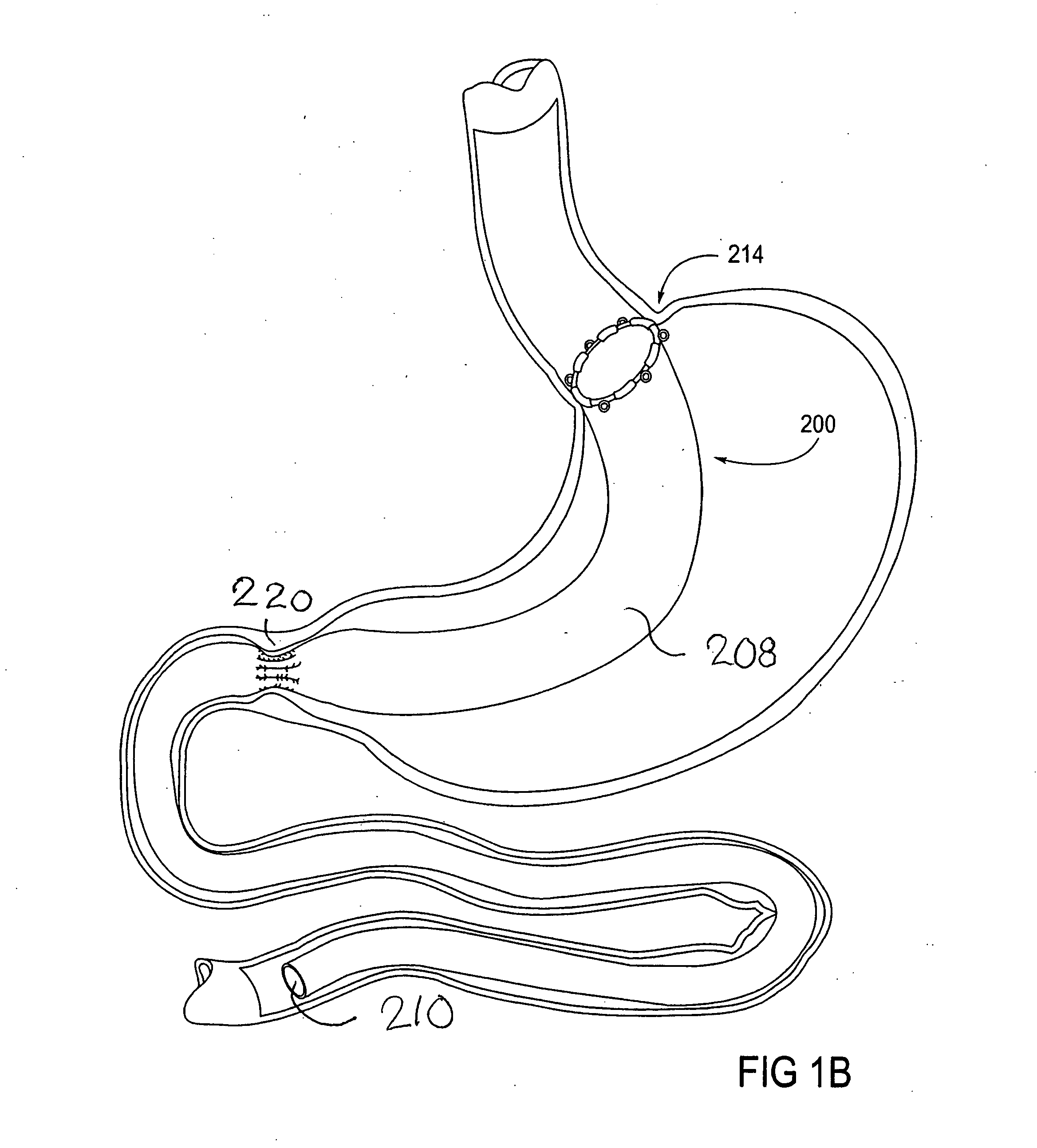
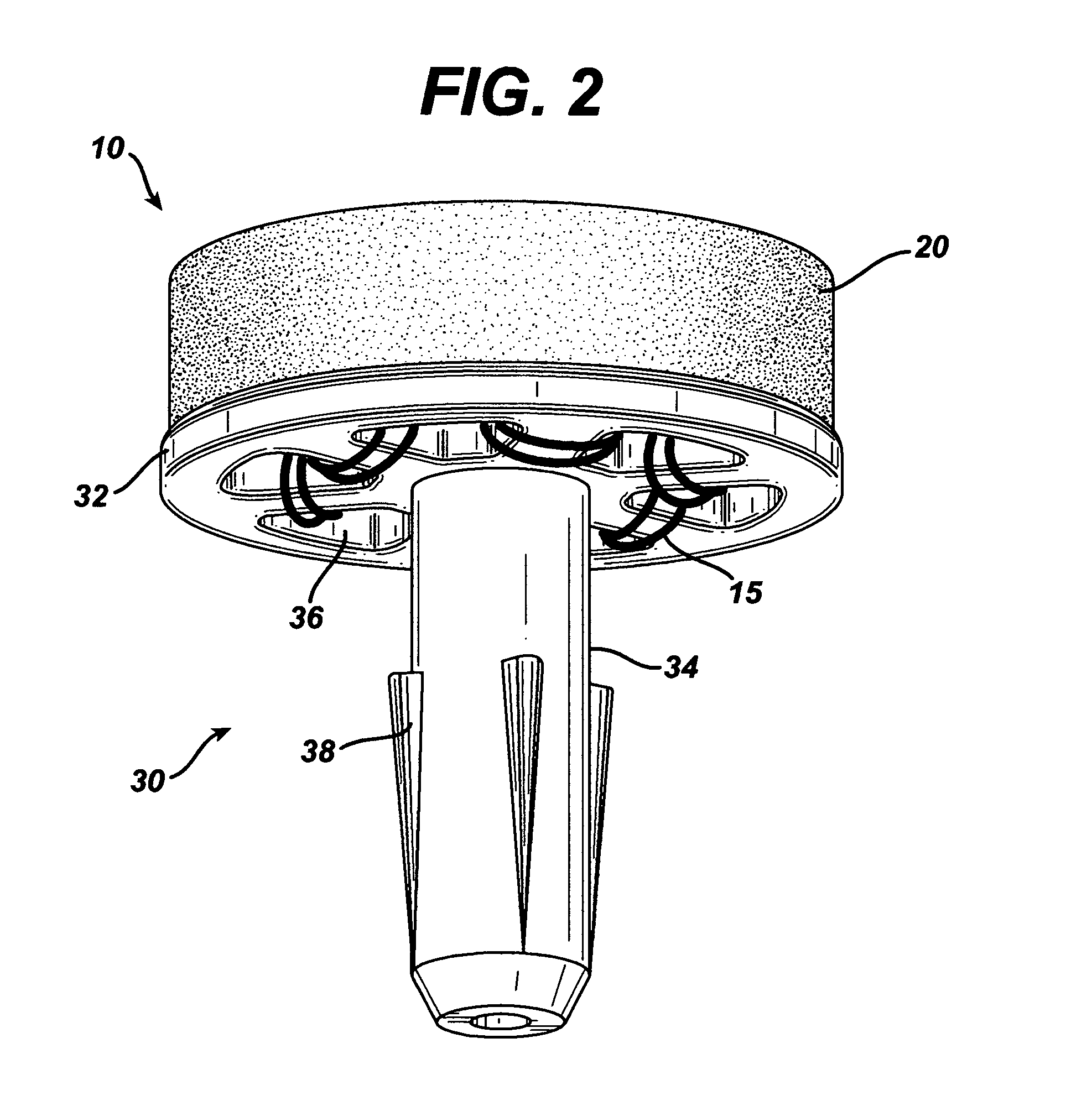
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
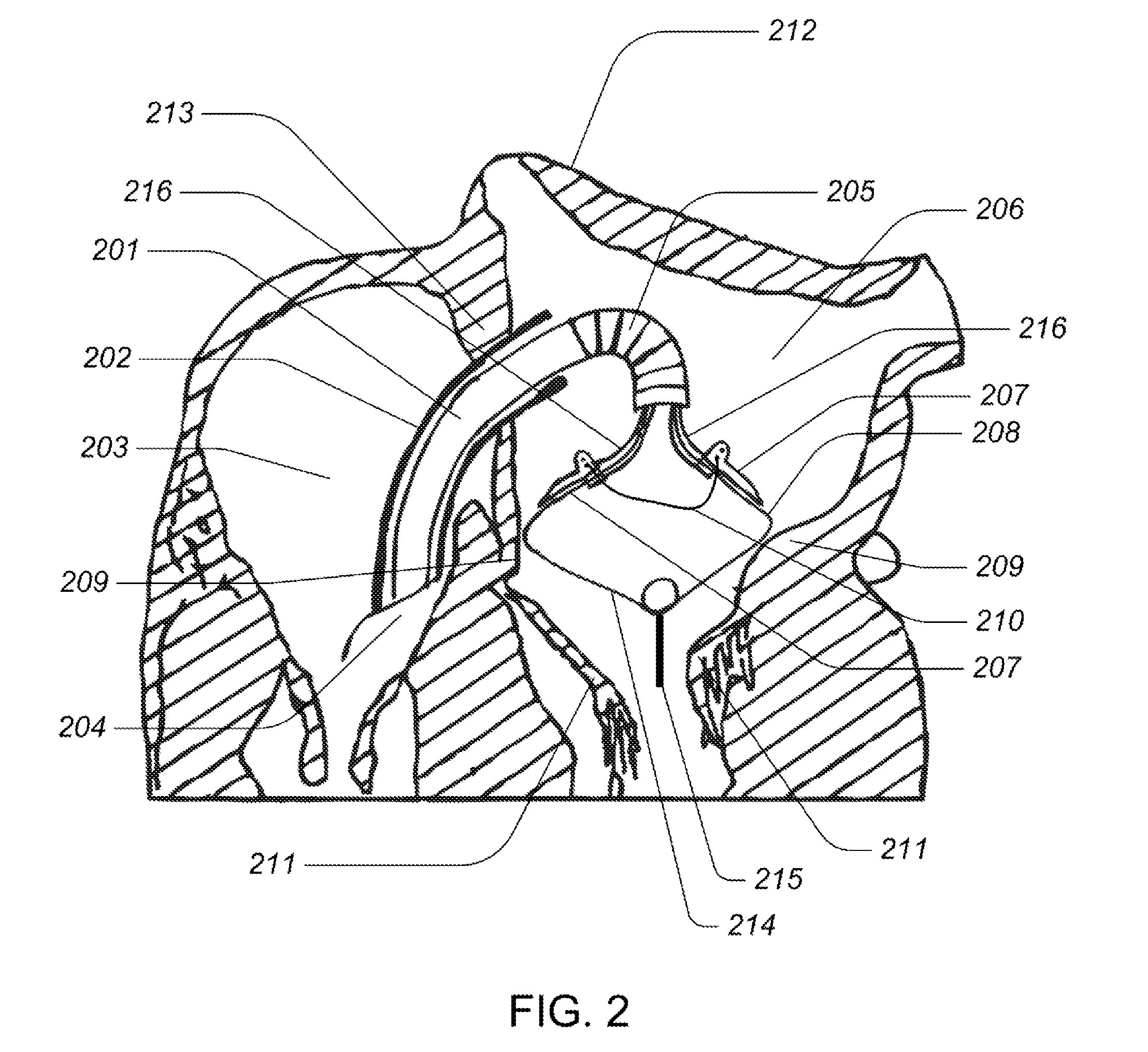
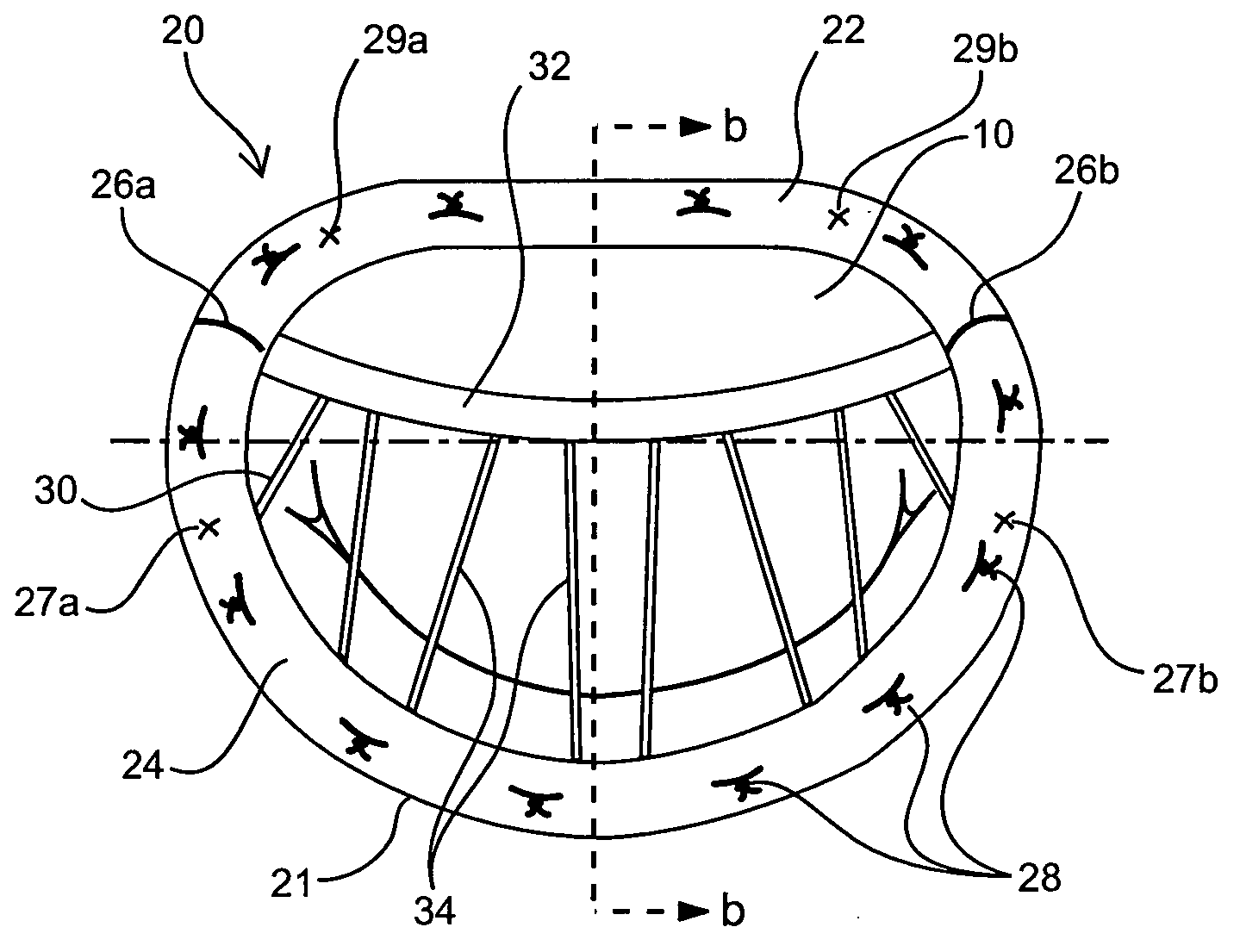
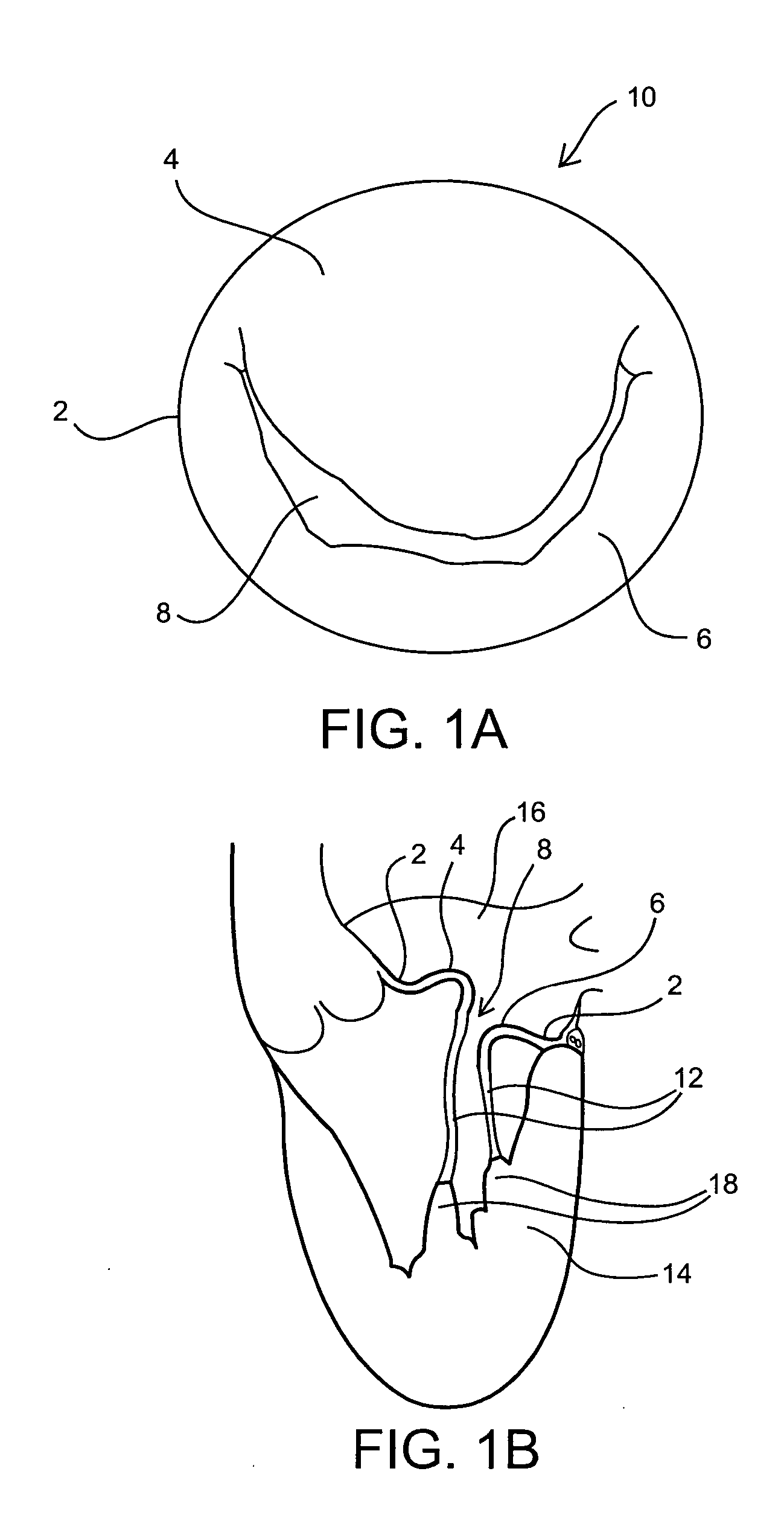
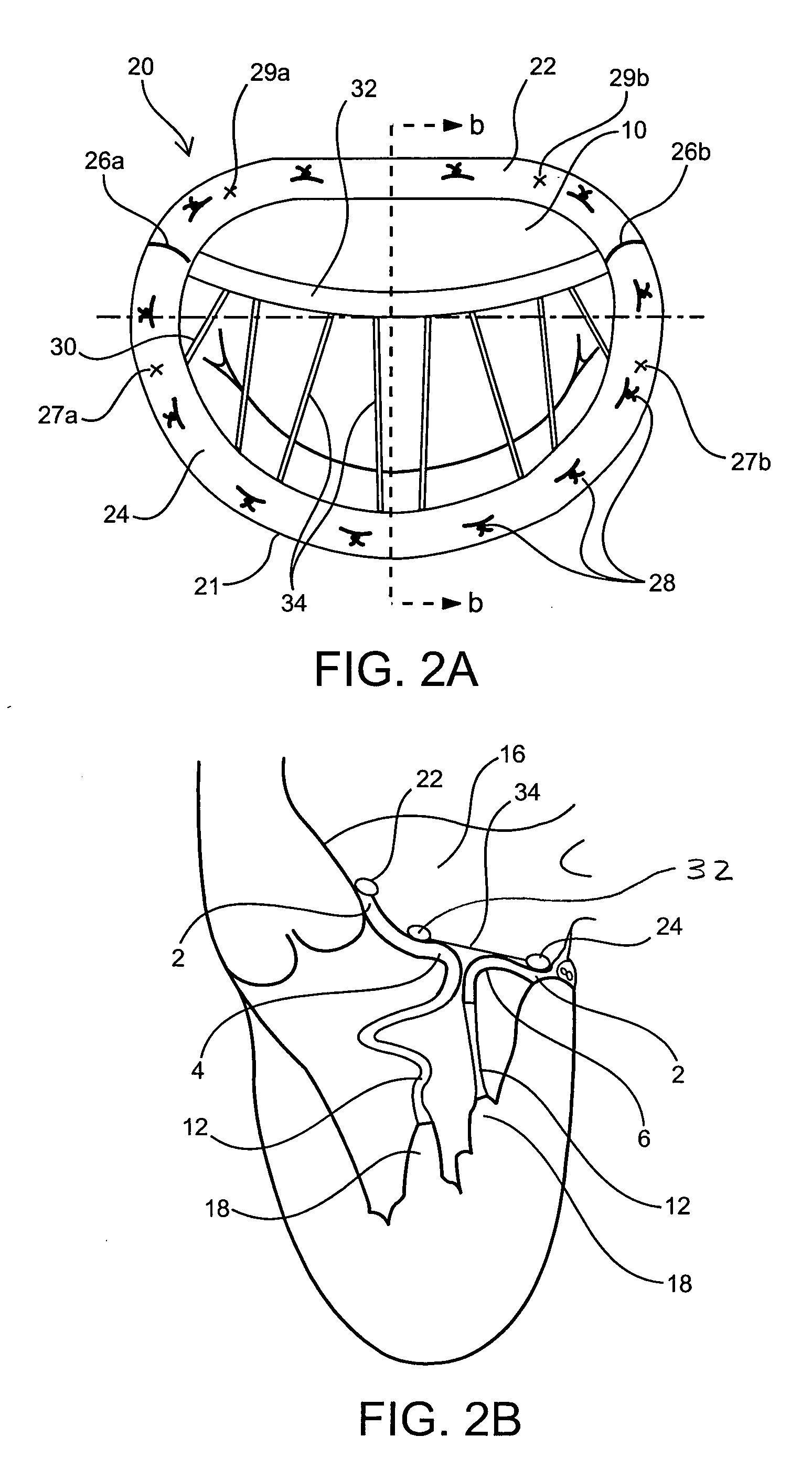
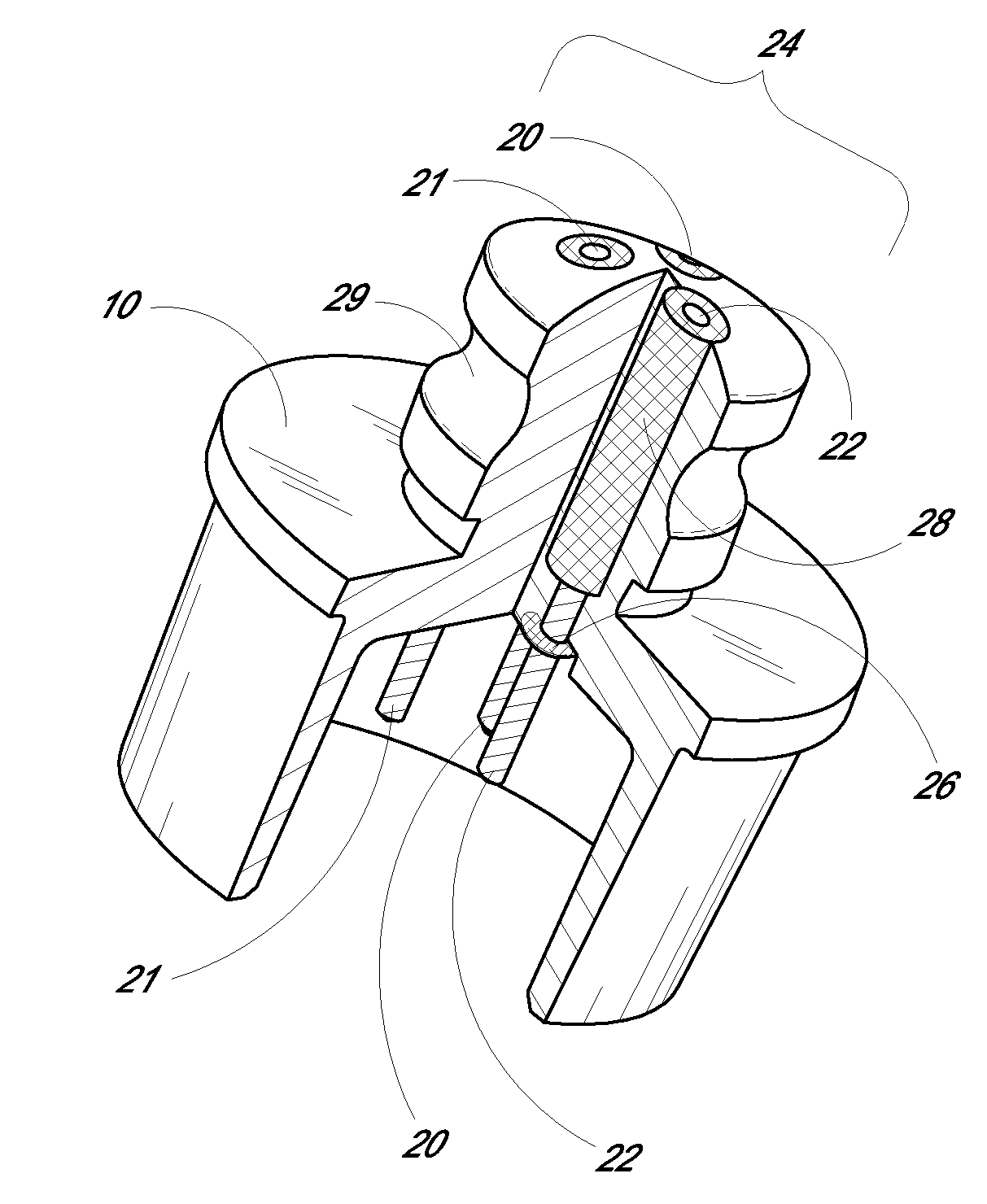
Medical device, kit and method for constricting tissue or a bodily orifice, for example, a mitral valve
InactiveUS20110082538A1Prevent retreatSuture equipmentsBone implantPosterior leafletAnnuloplasty rings
A device, kit and method may include or employ an implantable device (e.g., annuloplasty implant) and a tool operable to implant such. The implantable device is positionable in a cavity of a bodily organ (e.g., a heart) and operable to constrict a bodily orifice (e.g., a mitral valve). The tissue anchors may be guided into precise position by an intravascularly or percutaneously deployed anchor guide frame of the tool and embedded in an annulus of the orifice. Constriction of the orifice may be accomplished via a variety of structures, for example by cinching a flexible cable or via a anchored annuloplasty ring, the cable or ring attached to the tissue anchors. The annuloplasty ring may be delivered in an unanchored, generally elongated configuration, and implanted in an anchored generally arch, arcuate or annular configuration. Such may approximate the septal and lateral (clinically referred to as anterior and posterior) annulus of the mitral valve, to move the posterior leaflet anteriorly and the anterior leaflet posteriorly, thereby improving leaflet coaptation to eliminate mitral regurgitation.
Owner:KARDIUM
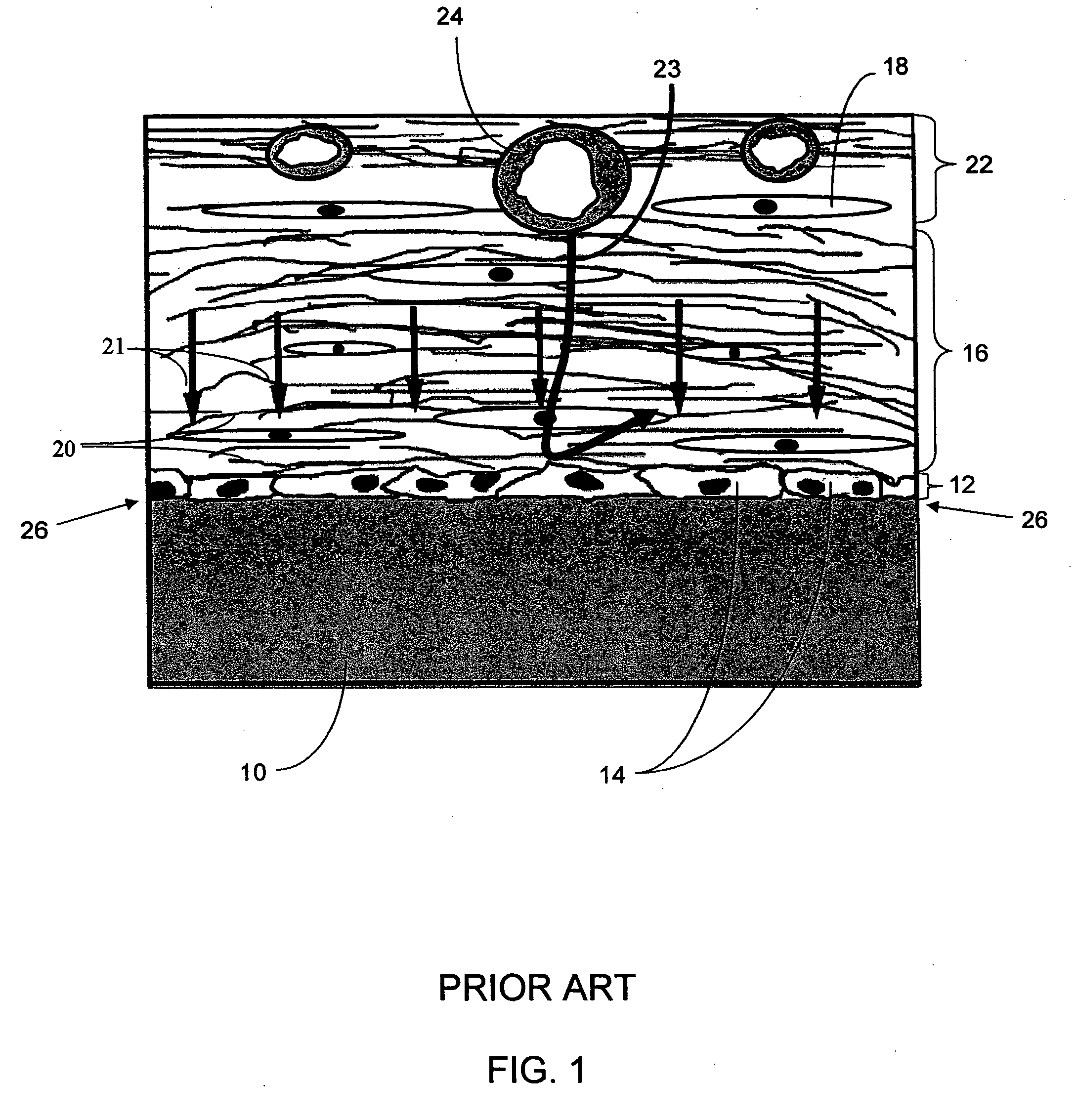
Biointerface membranes incorporating bioactive agents
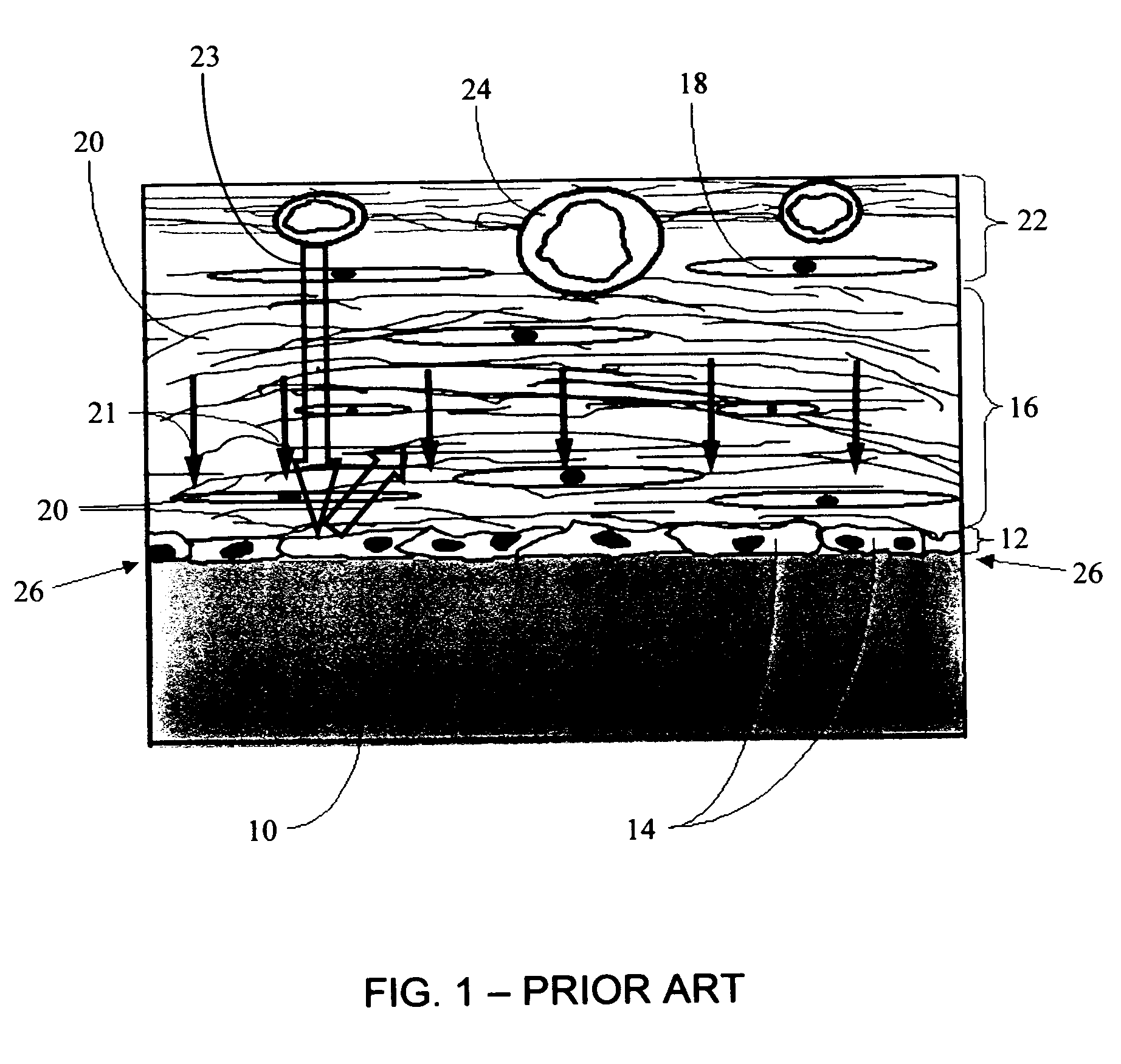
InactiveUS20050031689A1Improve performancePowder deliveryAdditive manufacturing apparatusBiointerfaceActive agent
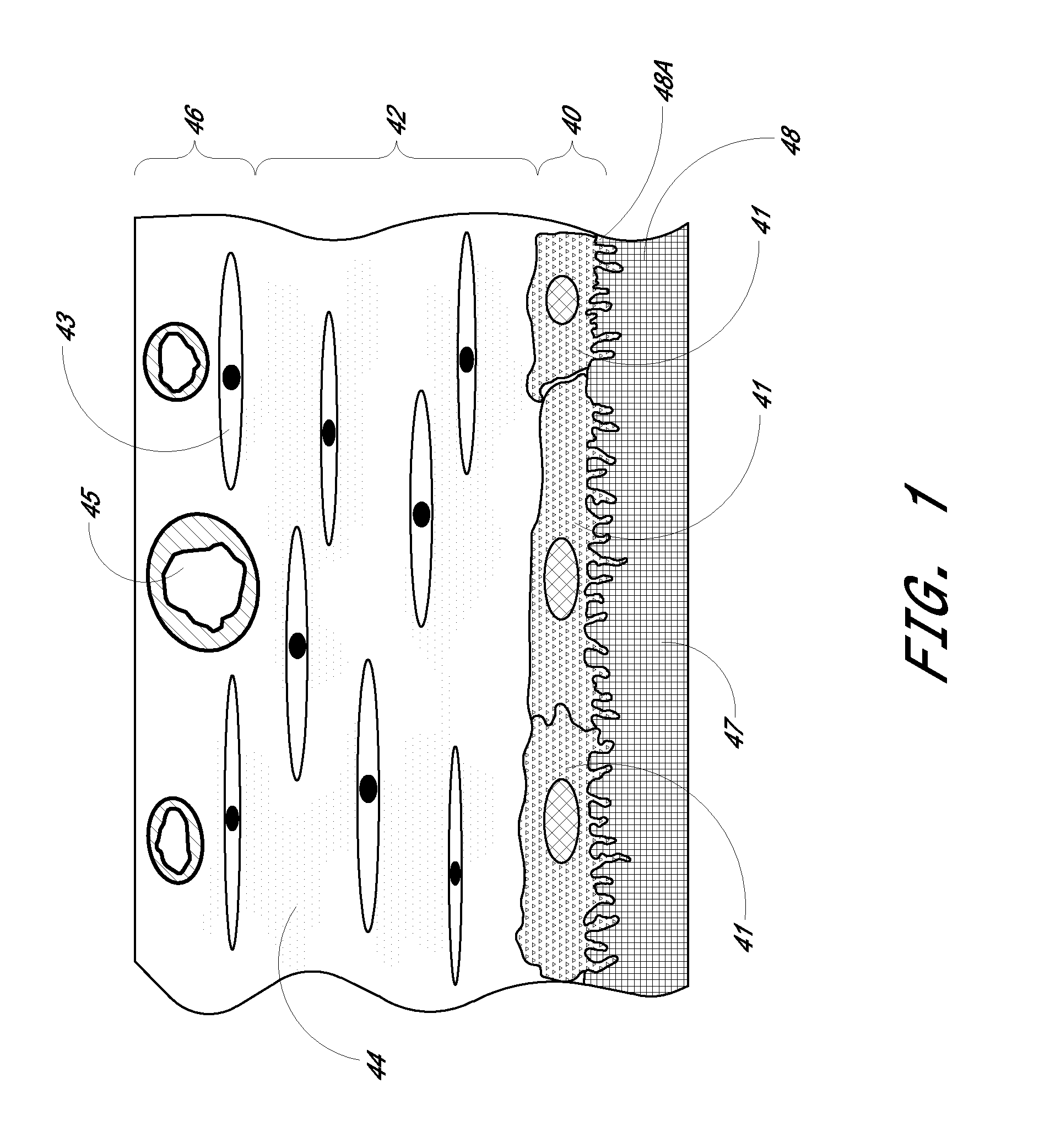
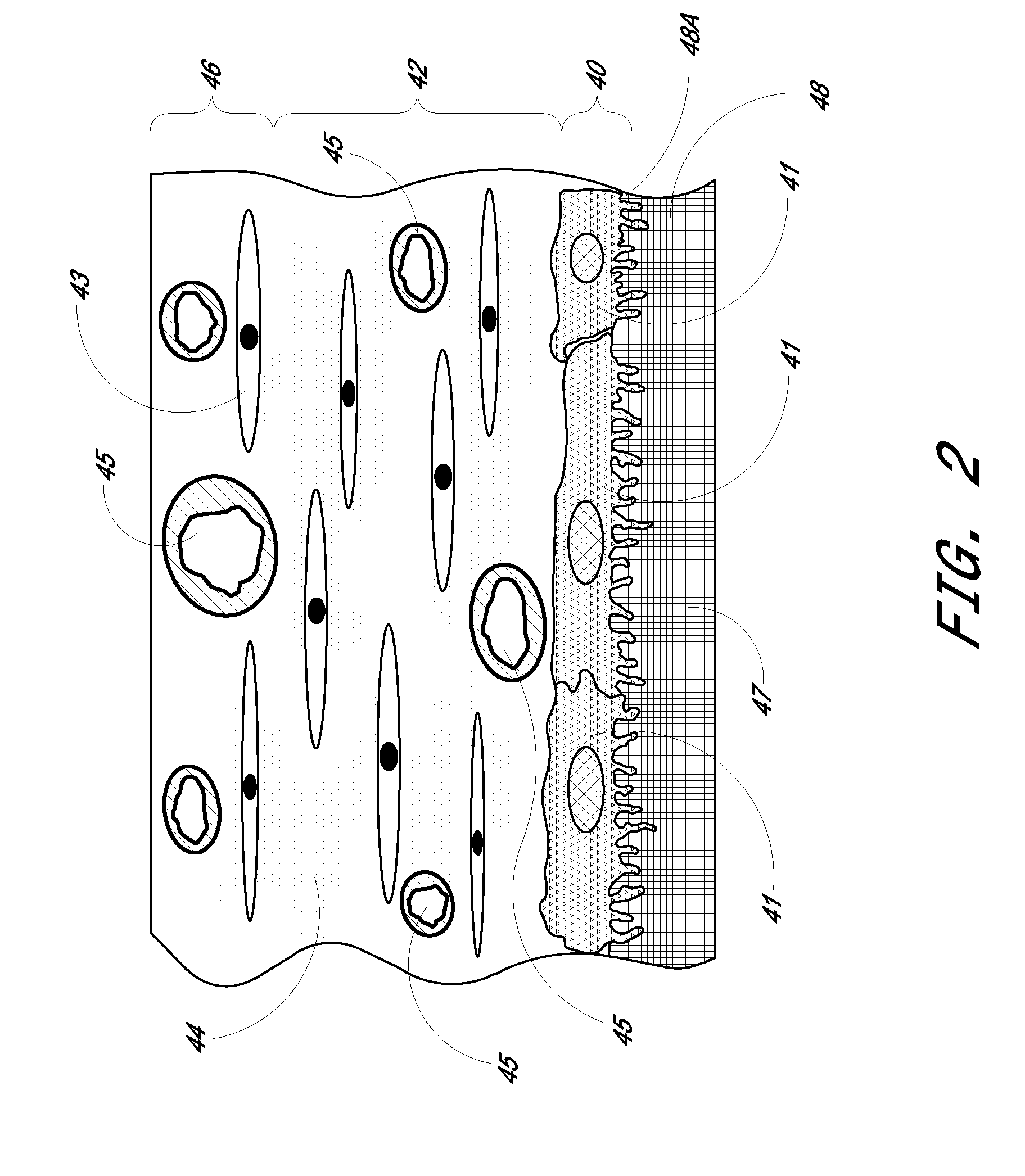
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM
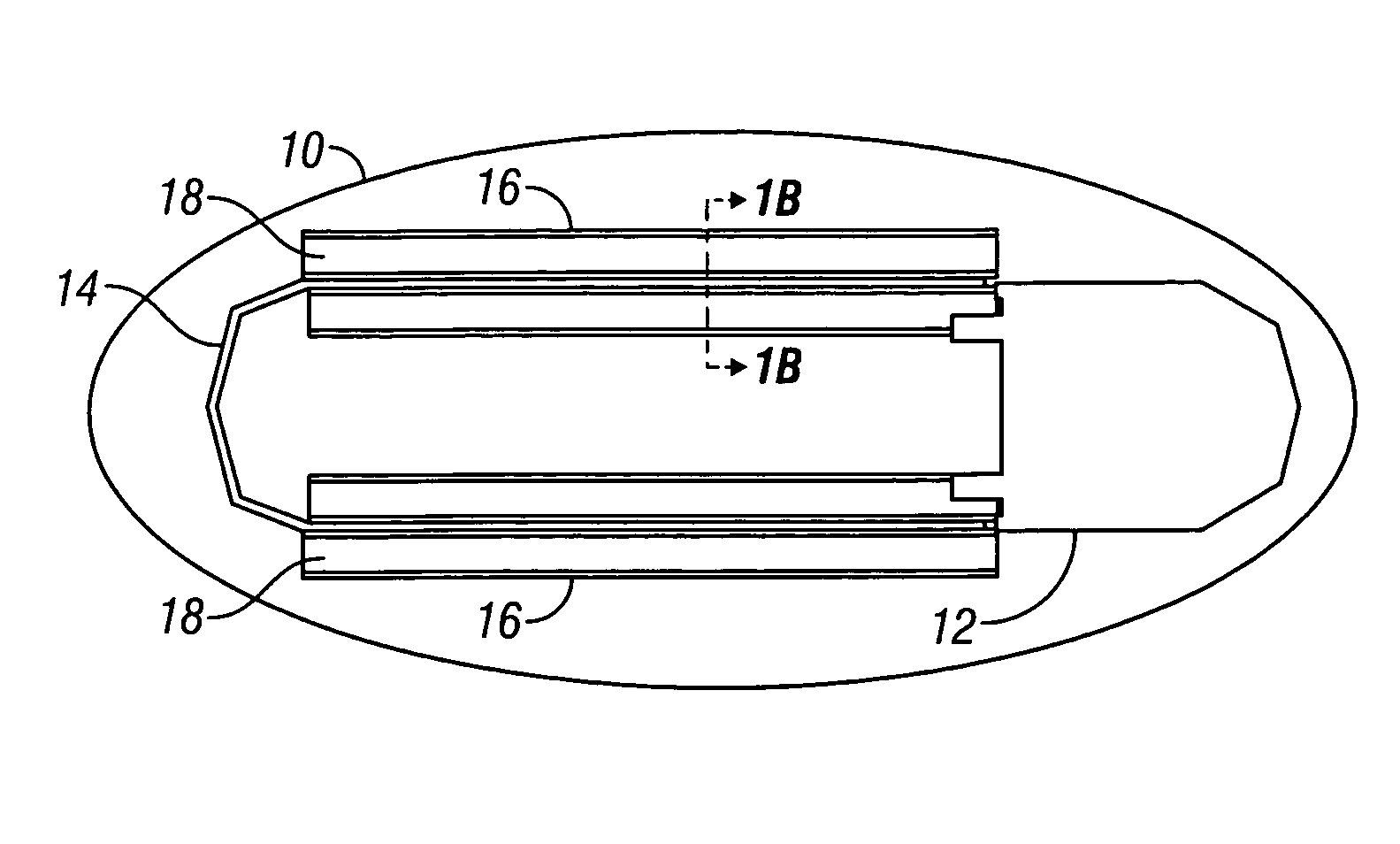
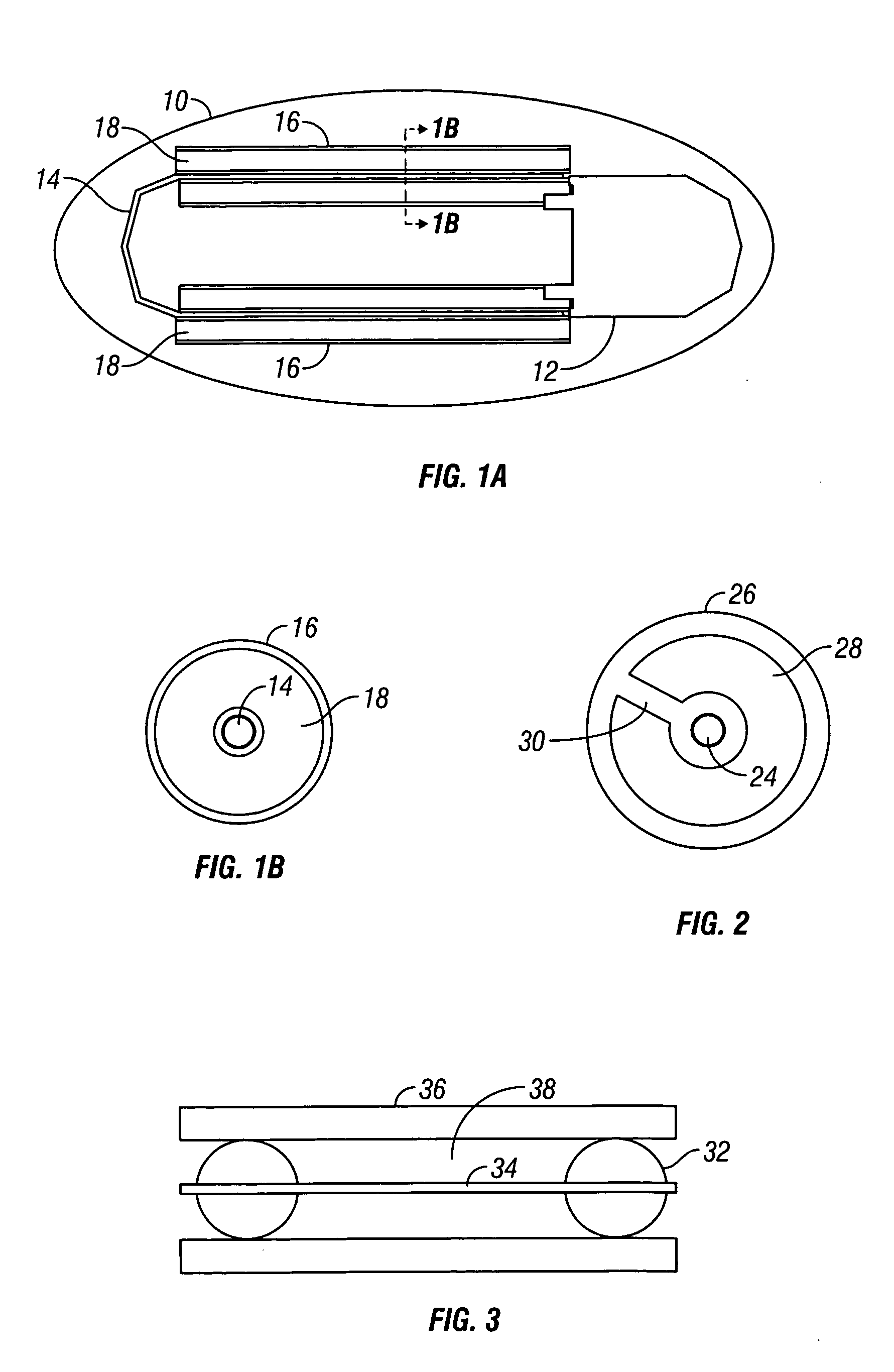
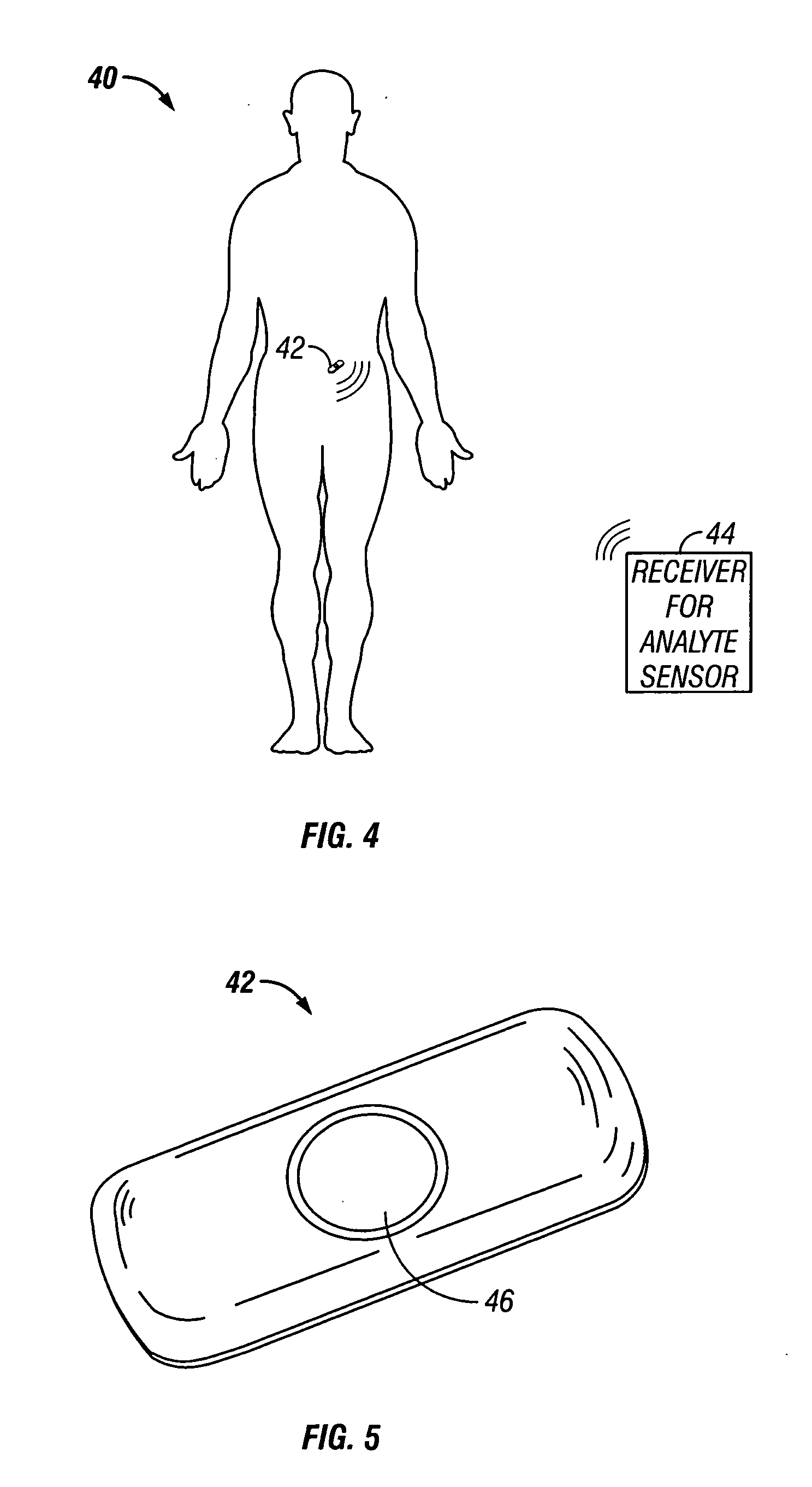
Device and method for determining analyte levels
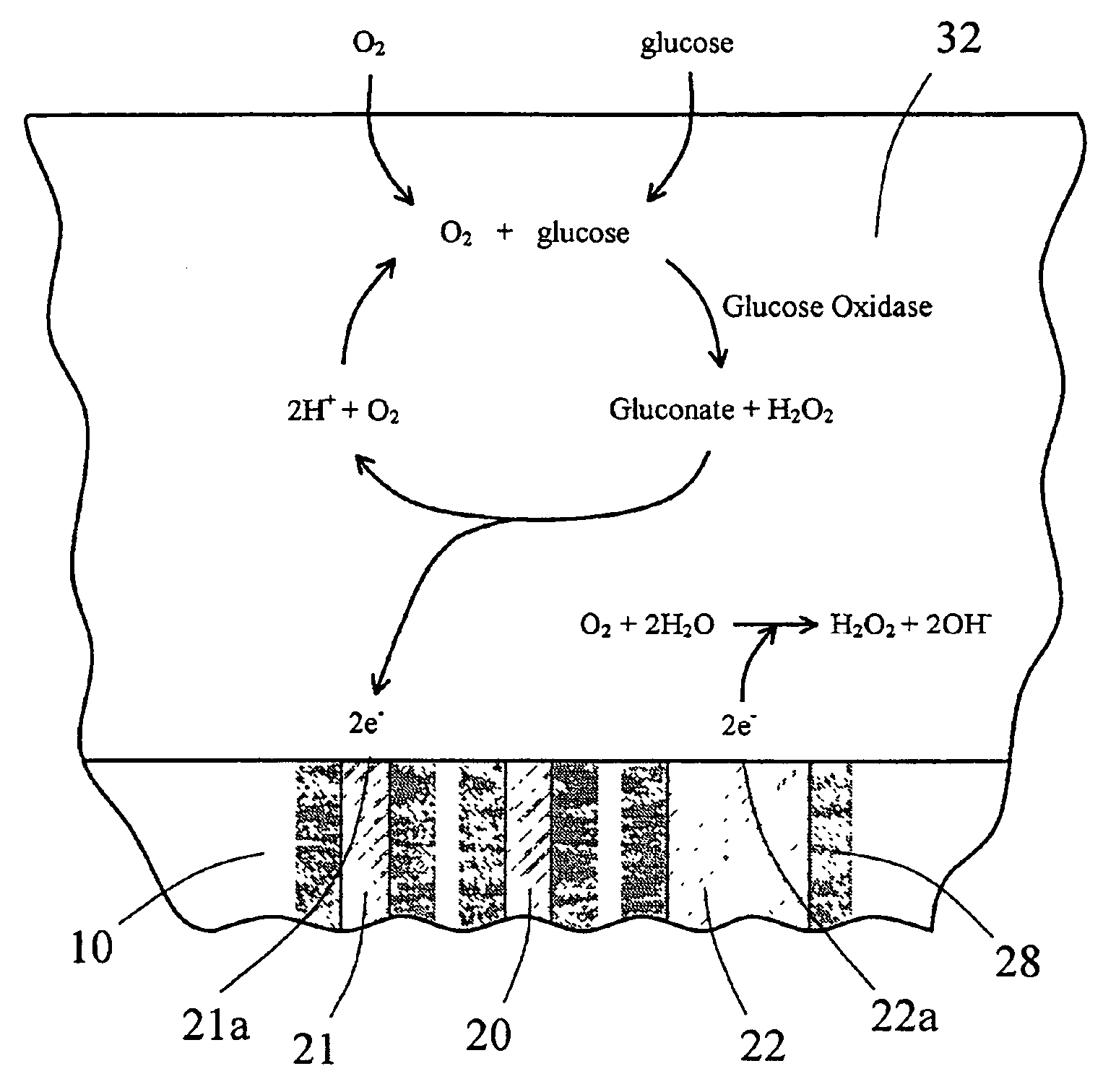
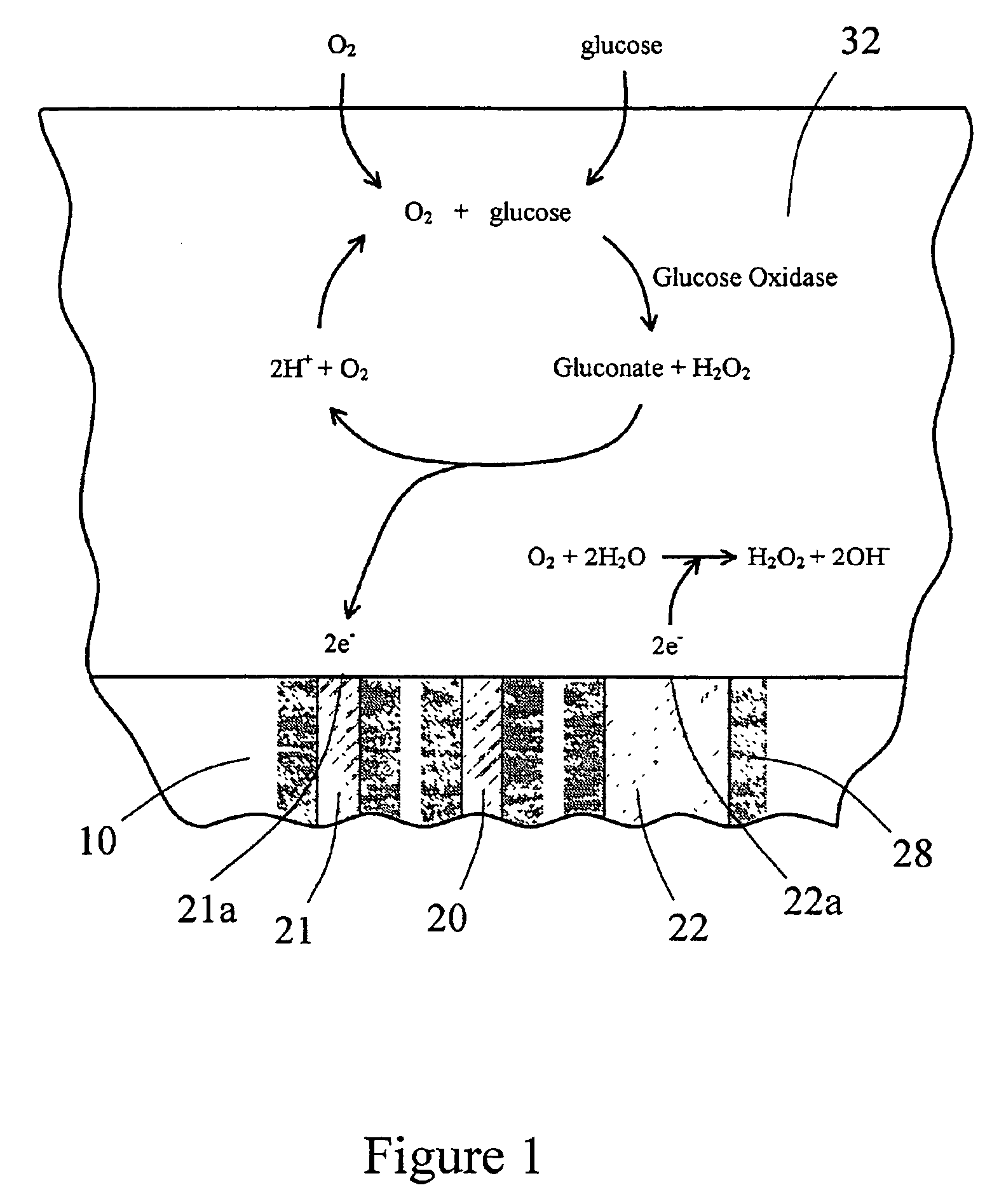
InactiveUS6862465B2Reduce in quantityControl volumeImmobilised enzymesBioreactor/fermenter combinationsAnalyteImplanted device
Devices and methods for determining analyte levels are described. The devices and methods allow for the implantation of analyte-monitoring devices, such as glucose monitoring devices that result in the delivery of a dependable flow of blood to deliver sample to the implanted device. The devices include unique architectural arrangement in the sensor region that allows accurate data to be obtained over long periods of time.
Owner:DEXCOM
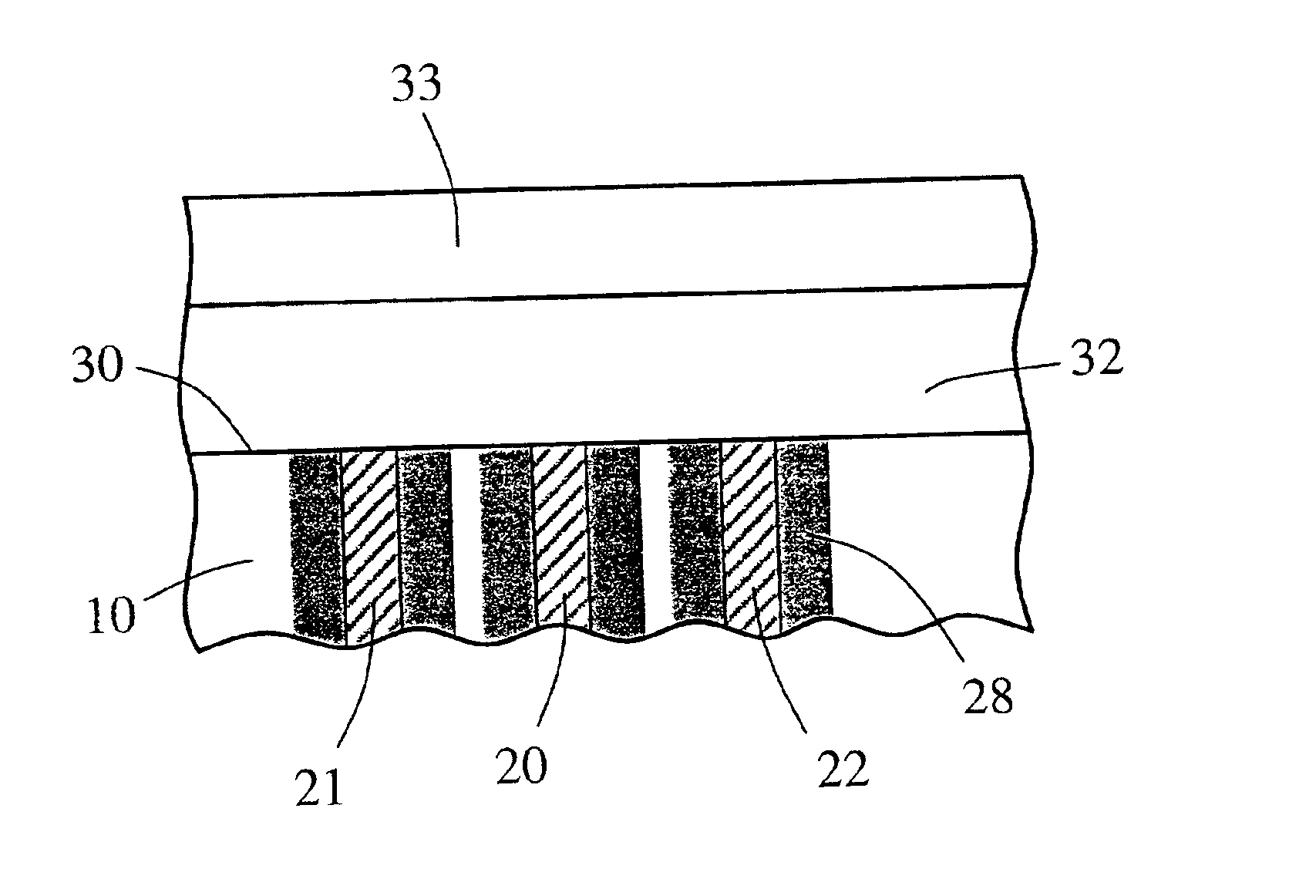
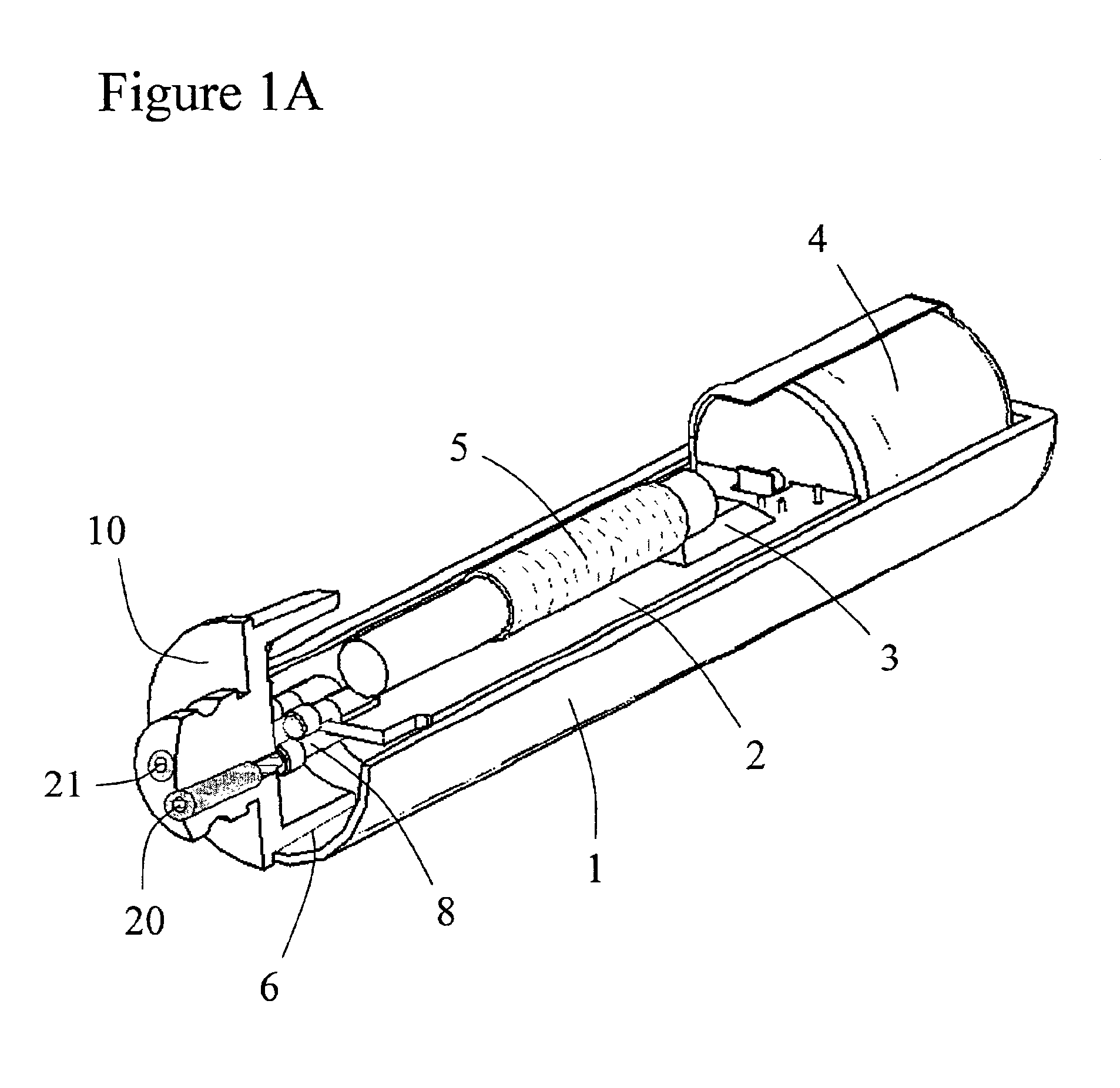
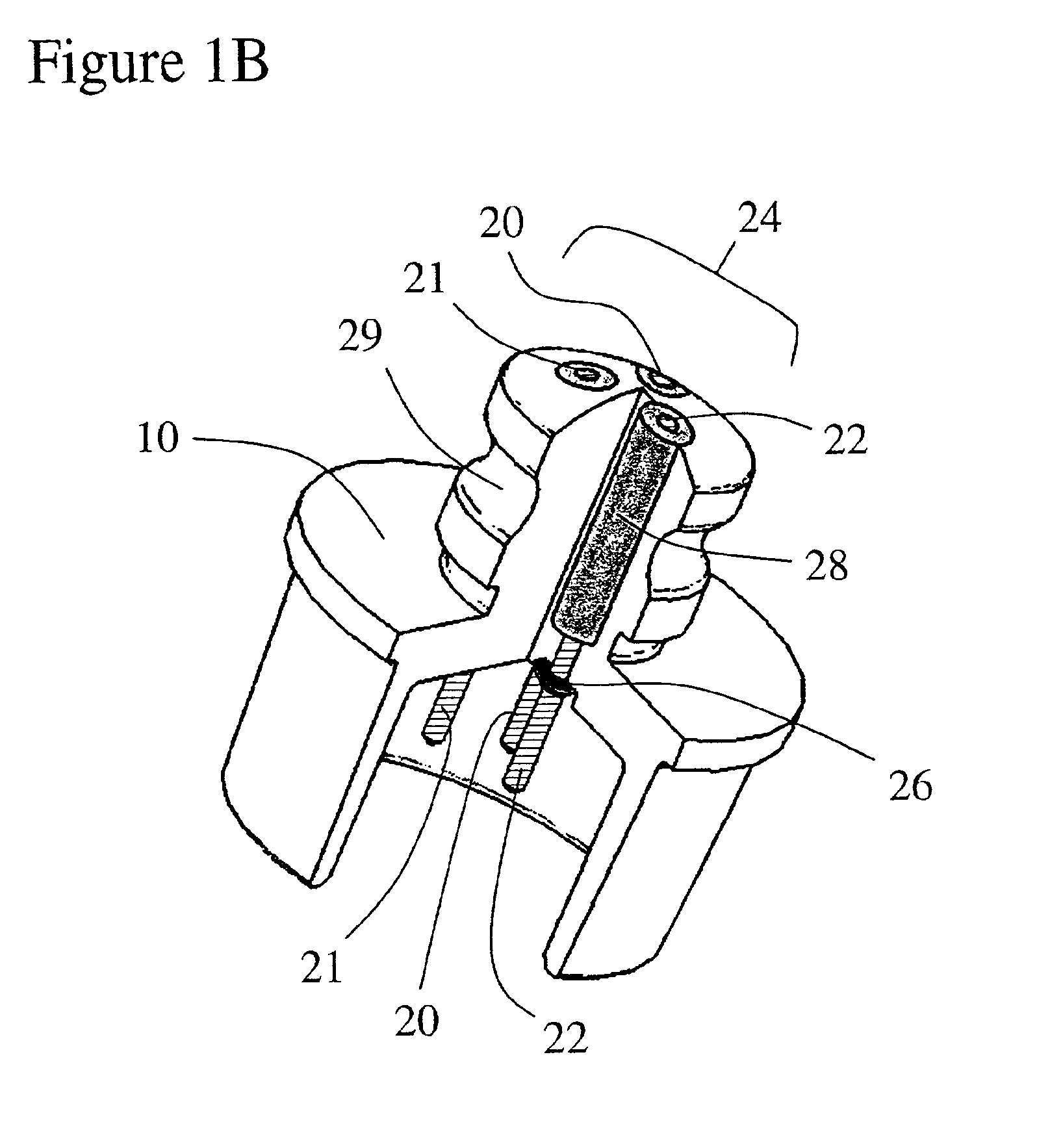
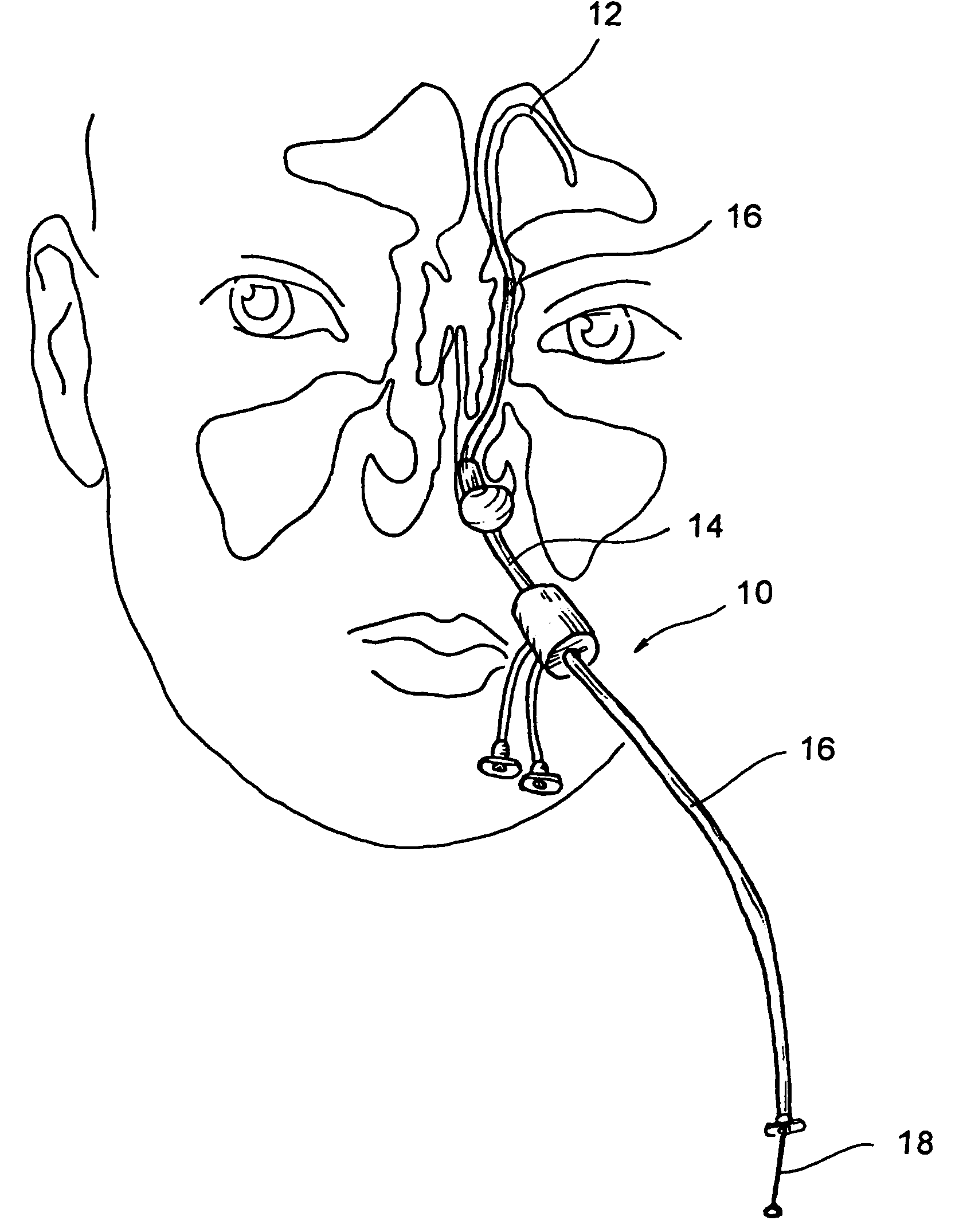
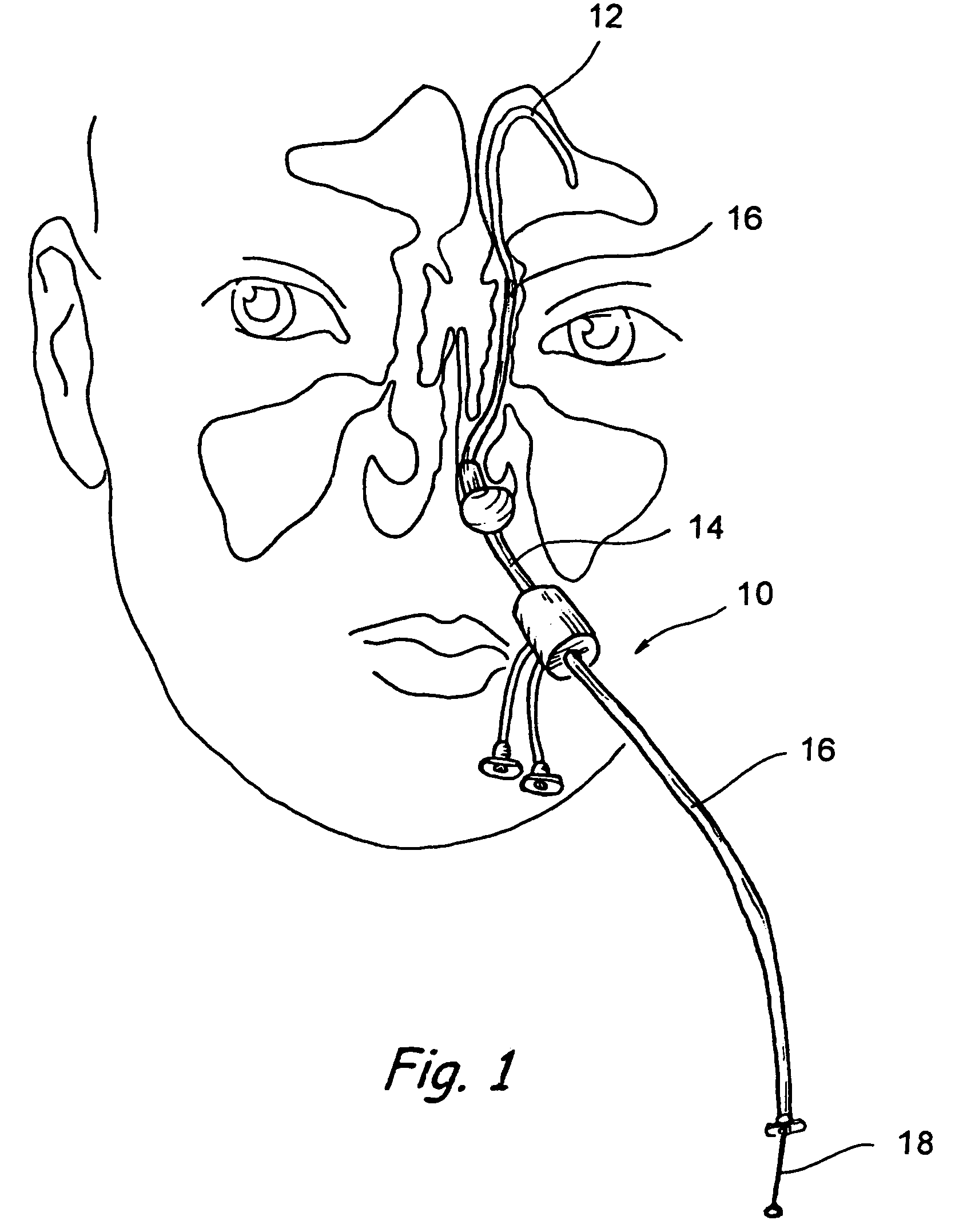
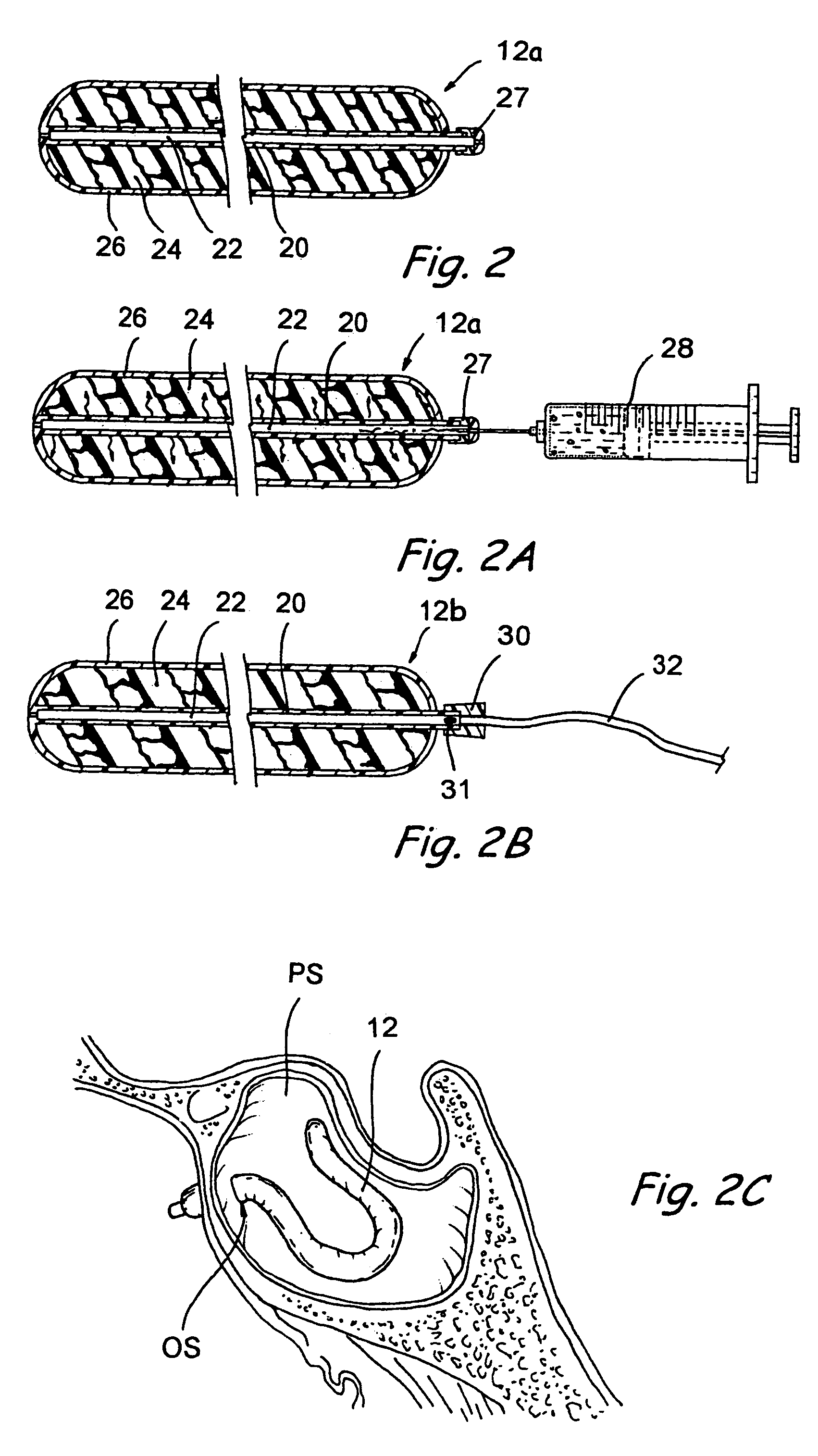
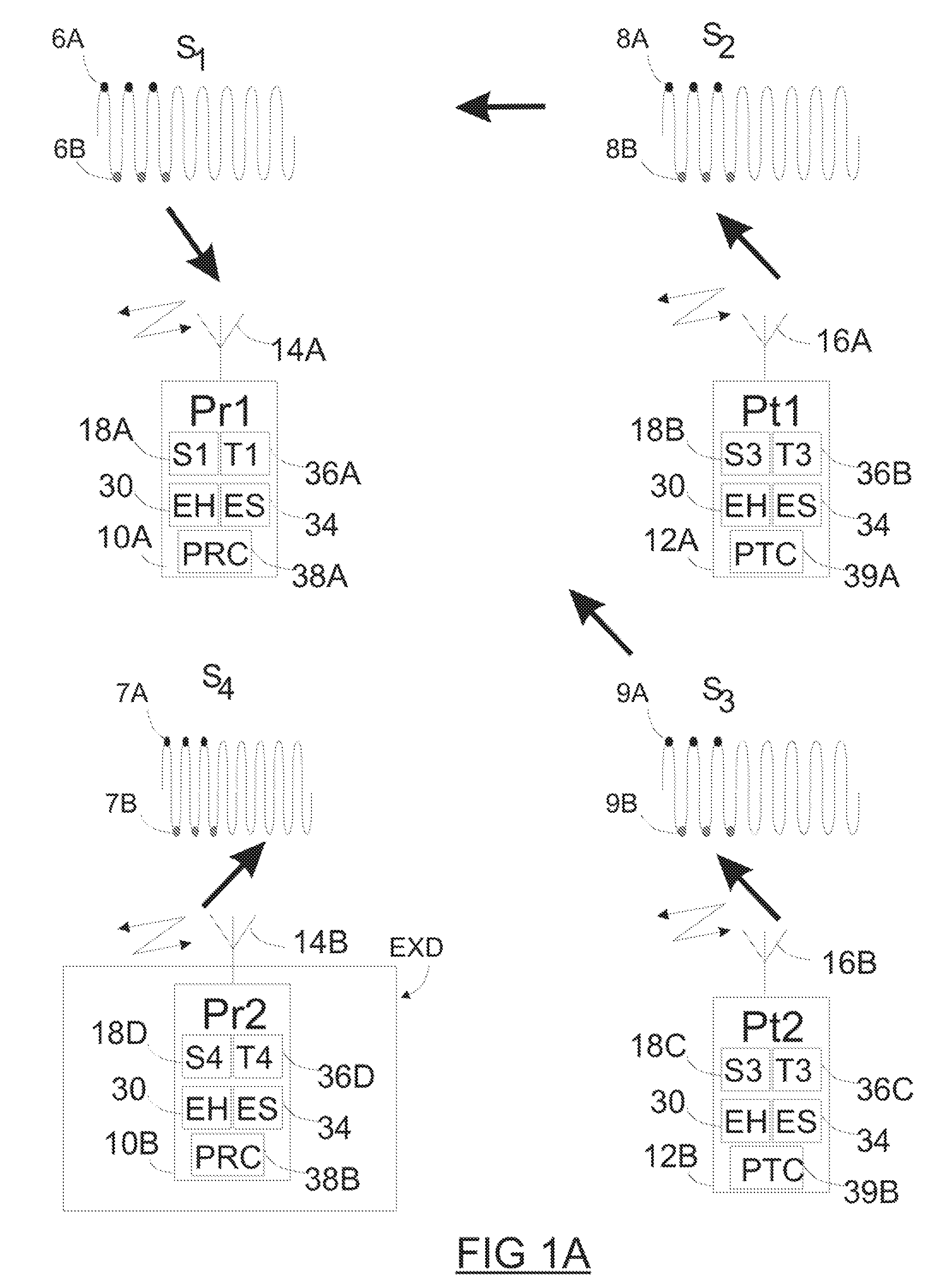
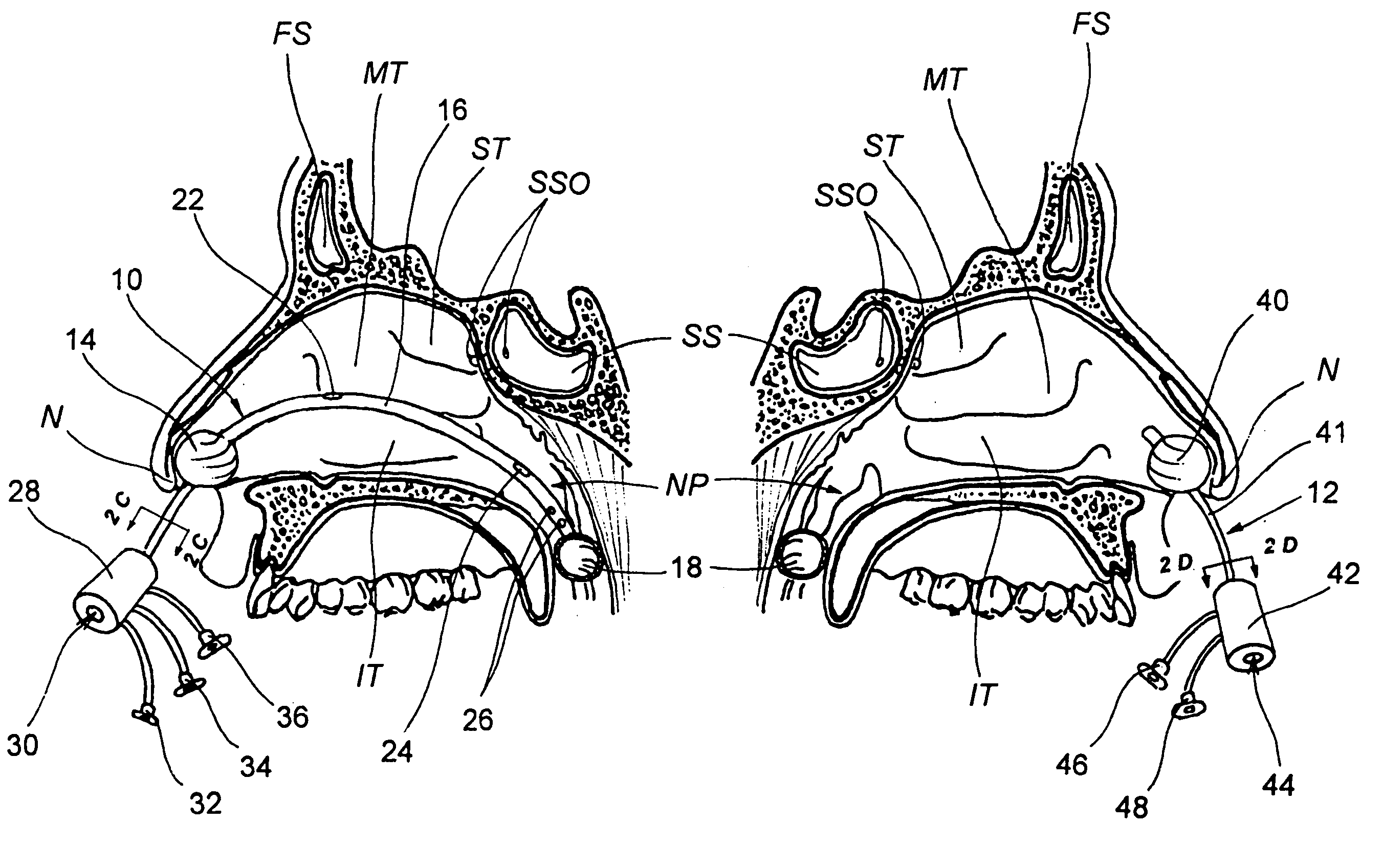
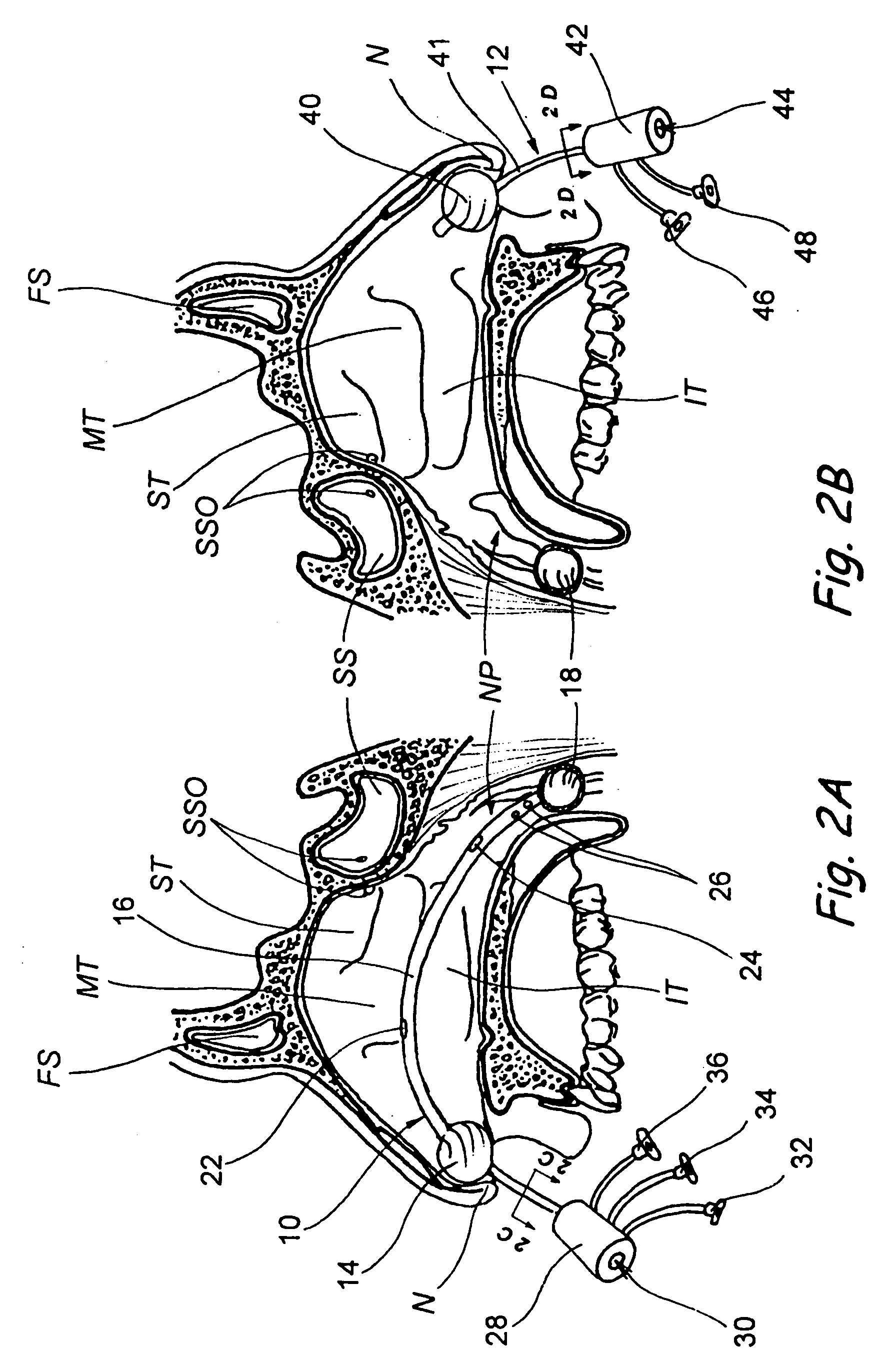
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
Attachment of absorbable tissue scaffolds to fixation devices
The present invention relates to tissue scaffold implant devices useful in the repair and / or regeneration of diseased and / or damaged musculoskeletal tissue and that include a tissue scaffold component fixedly attached to a scaffold fixation component via at least one of sutures, fabrics, fibers, threads, elastomeric bands, reinforcing elements and interlocking protrusions for engaging and maintaining the scaffold component fixedly attached to the fixation component.
Owner:ETHICON INC
Device and method for determining analyte levels
InactiveUS7110803B2Reducing and eliminating phenomenonEliminate and significantly delay environmental stress crackingMicrobiological testing/measurementWithdrawing sample devicesAnalyteImplanted device
Owner:DEXCOM
Sensor head for use with implantable devices
InactiveUS7471972B2Minimizing negative potential extremesImprove solubilityMicrobiological testing/measurementMaterial analysis by electric/magnetic meansElectrochemical responseAnalyte
The present invention provides a sensor head for use in an implantable device that measures the concentration of an analyte in a biological fluid which includes: a non-conductive body; a working electrode, a reference electrode and a counter electrode, wherein the electrodes pass through the non-conductive body forming an electrochemically reactive surface at one location on the body and forming an electronic connection at another location on the body, further wherein the electrochemically reactive surface of the counter electrode is greater than the surface area of the working electrode; and a multi-region membrane affixed to the nonconductive body and covering the working electrode, reference electrode and counter electrode. In addition, the present invention provides an implantable device including at least one of the sensor heads of the invention and methods of monitoring glucose levels in a host utilizing the implantable device of the invention.
Owner:DEXCOM INC
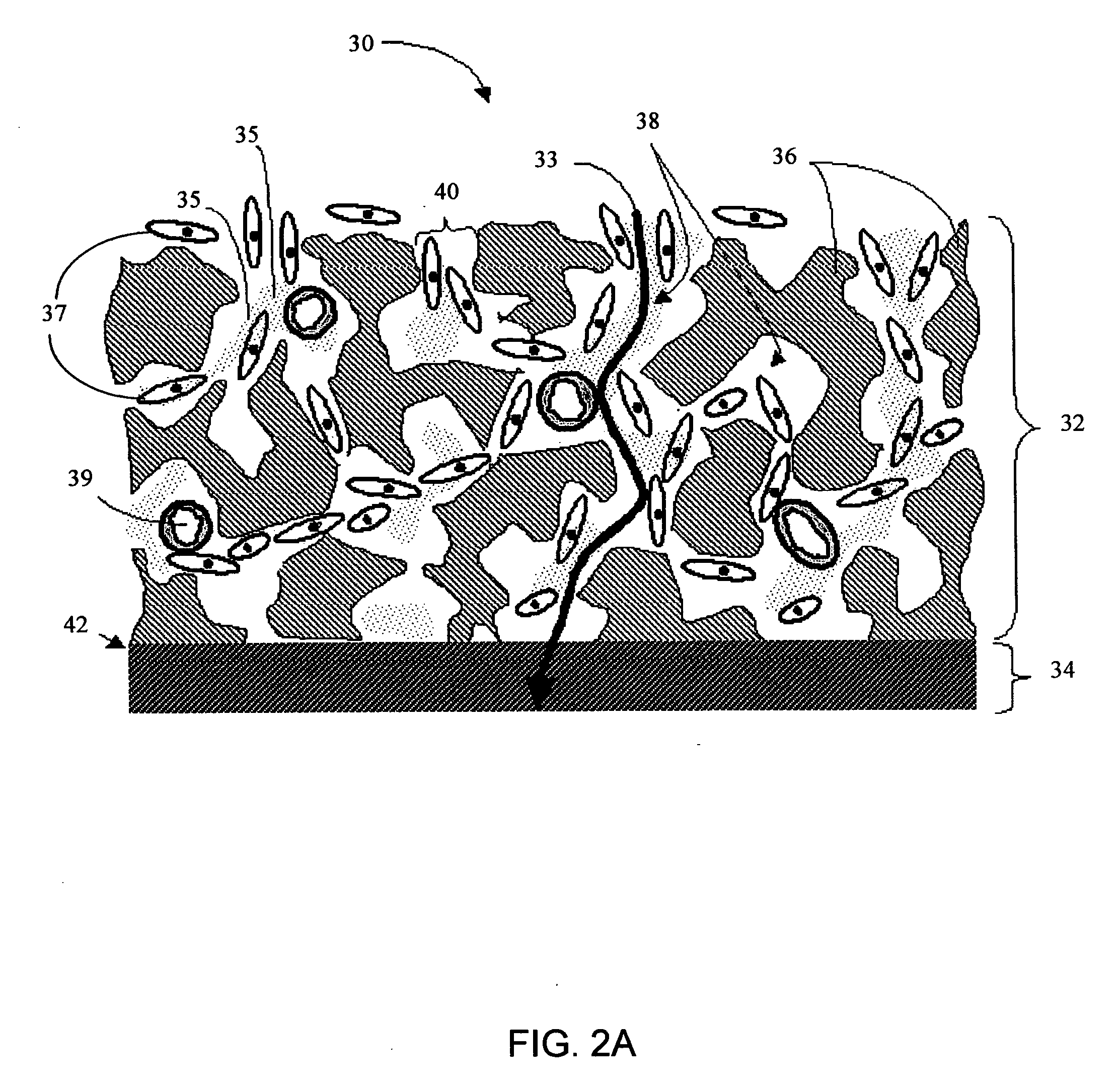
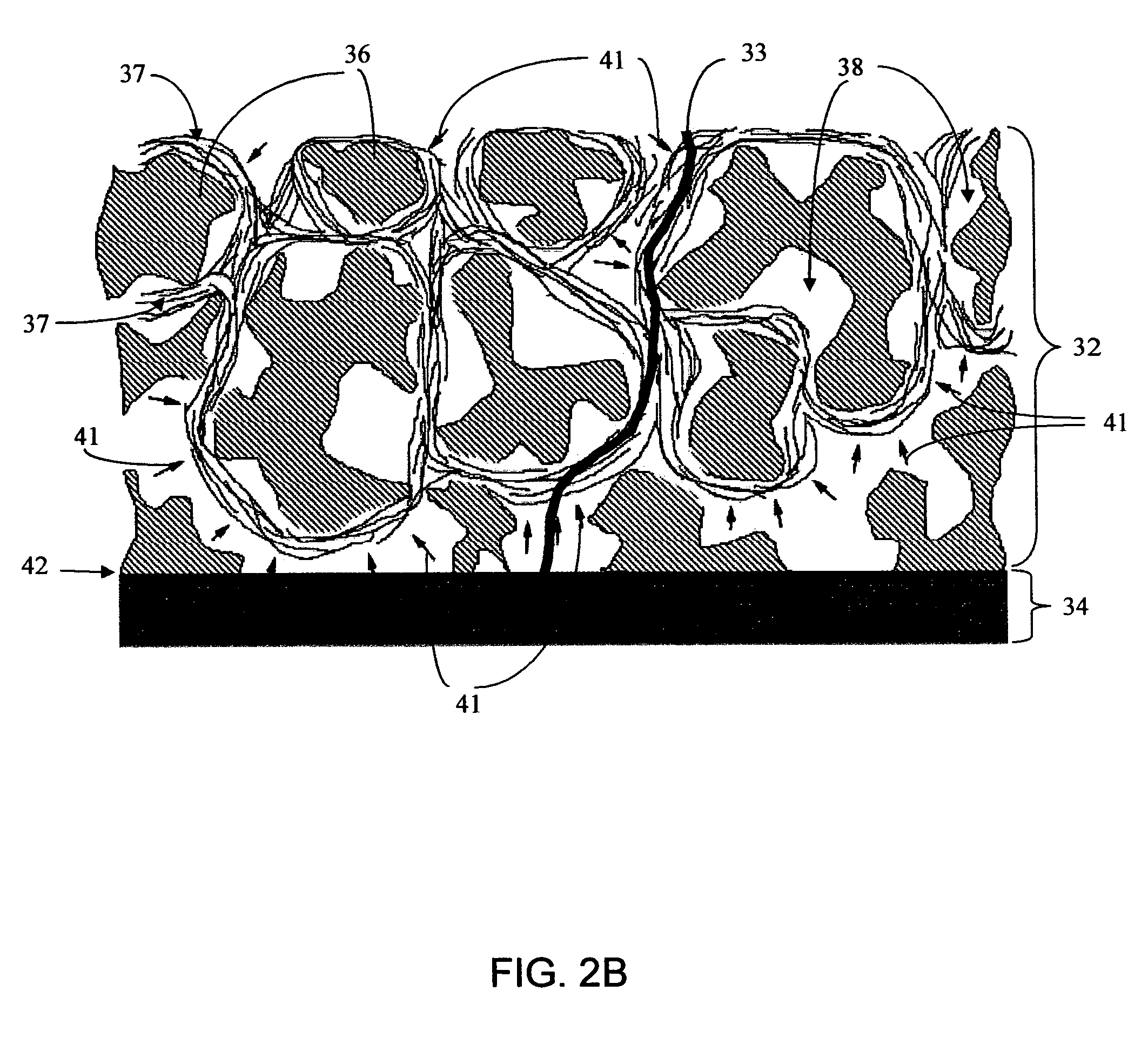
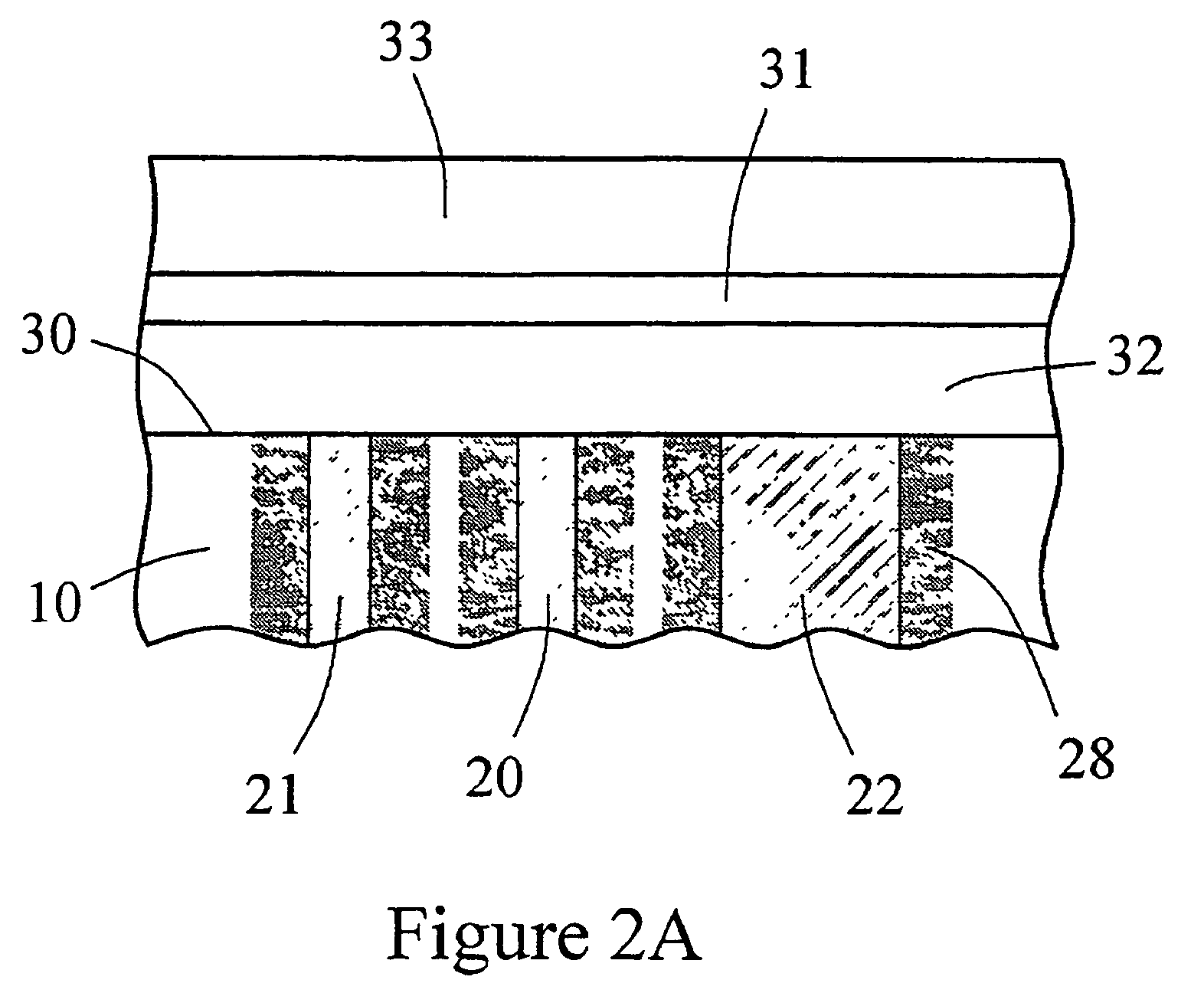
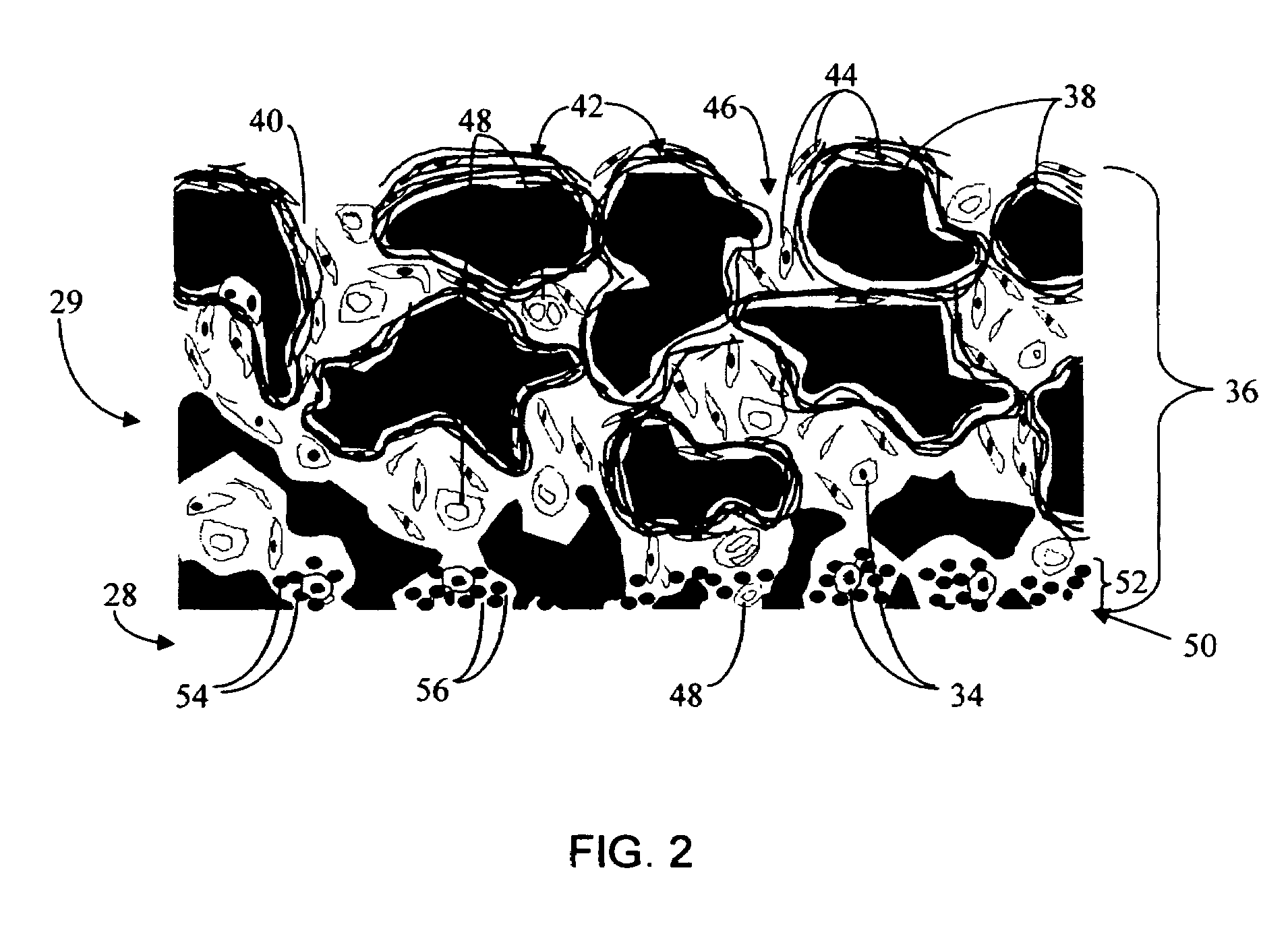
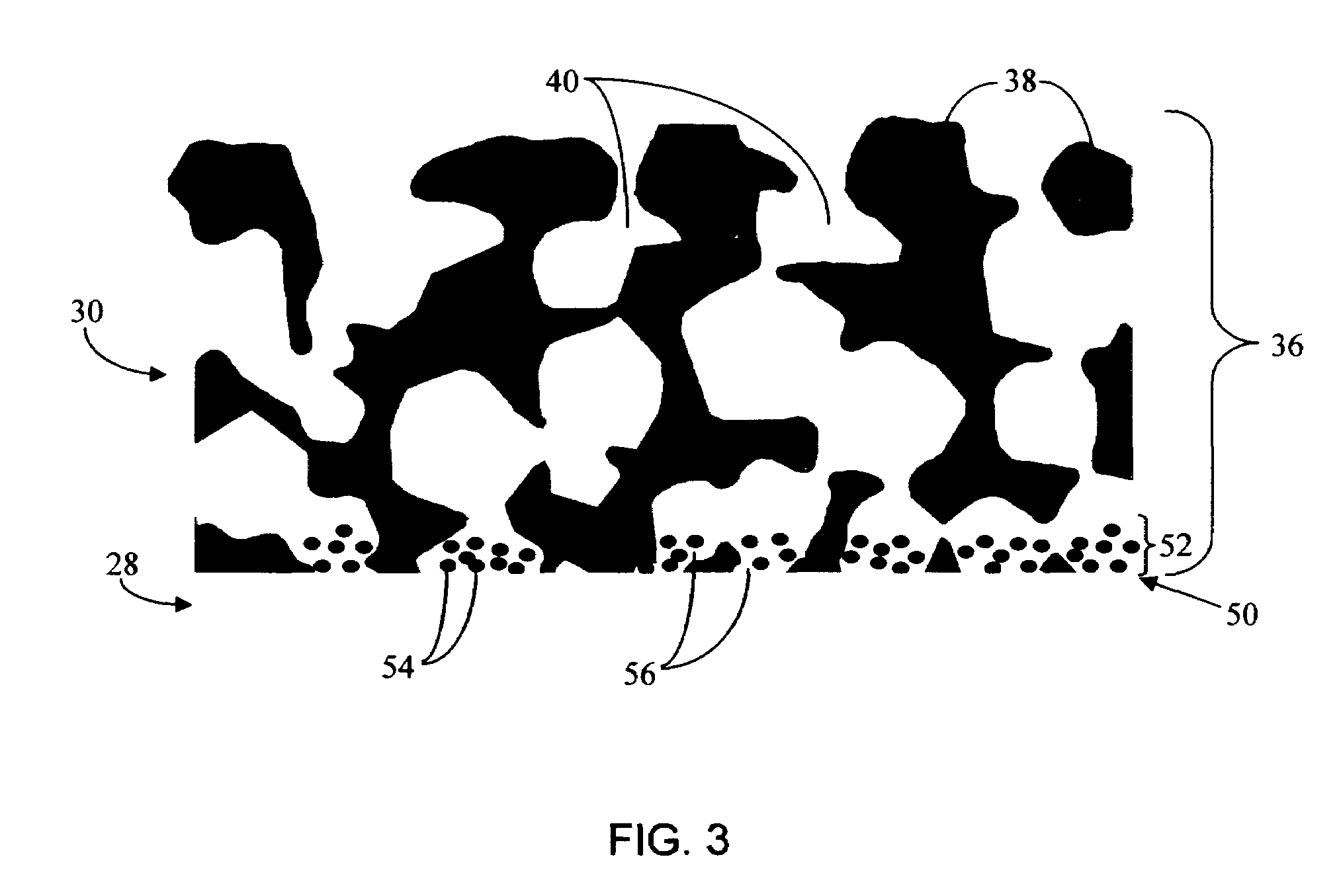
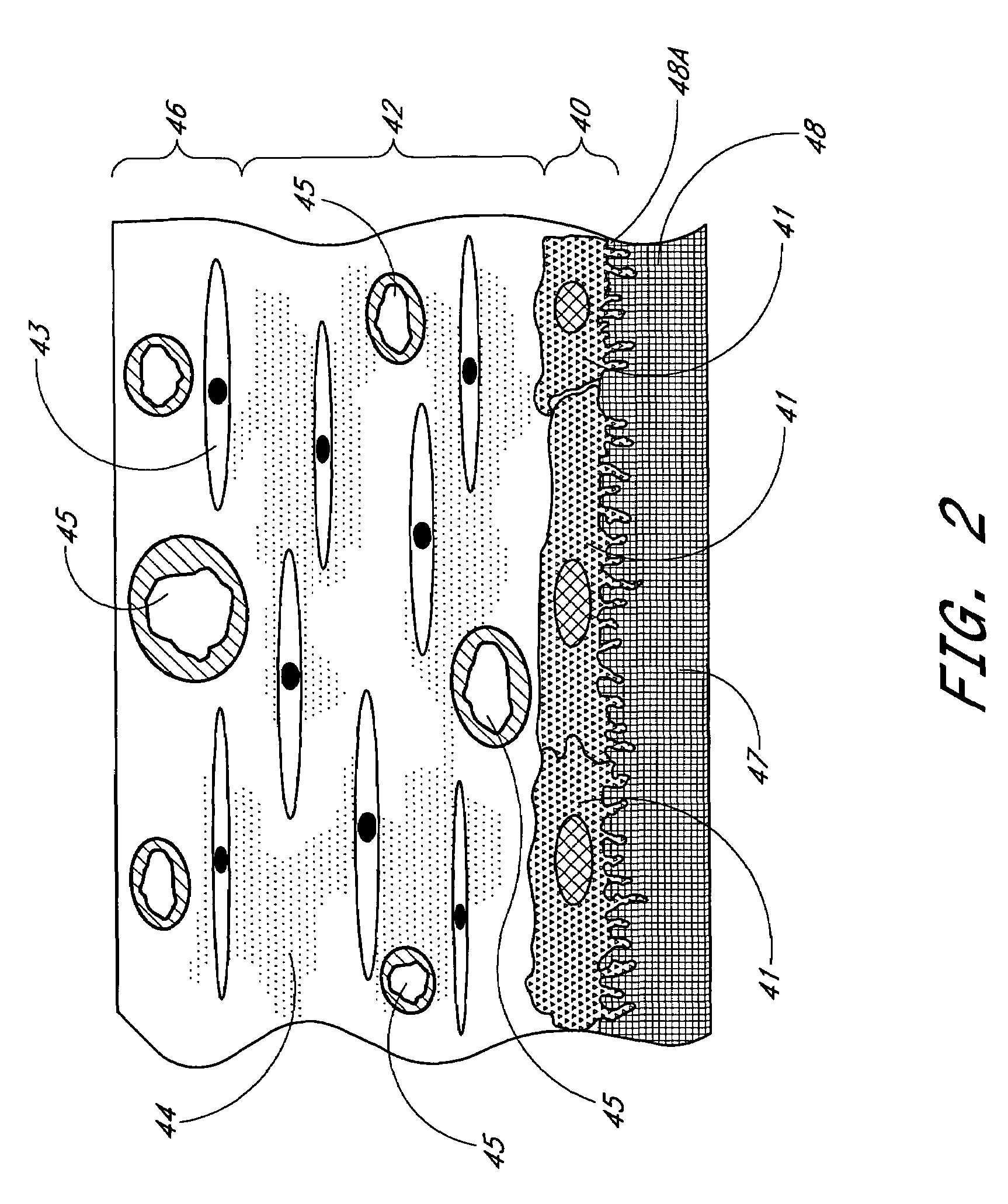
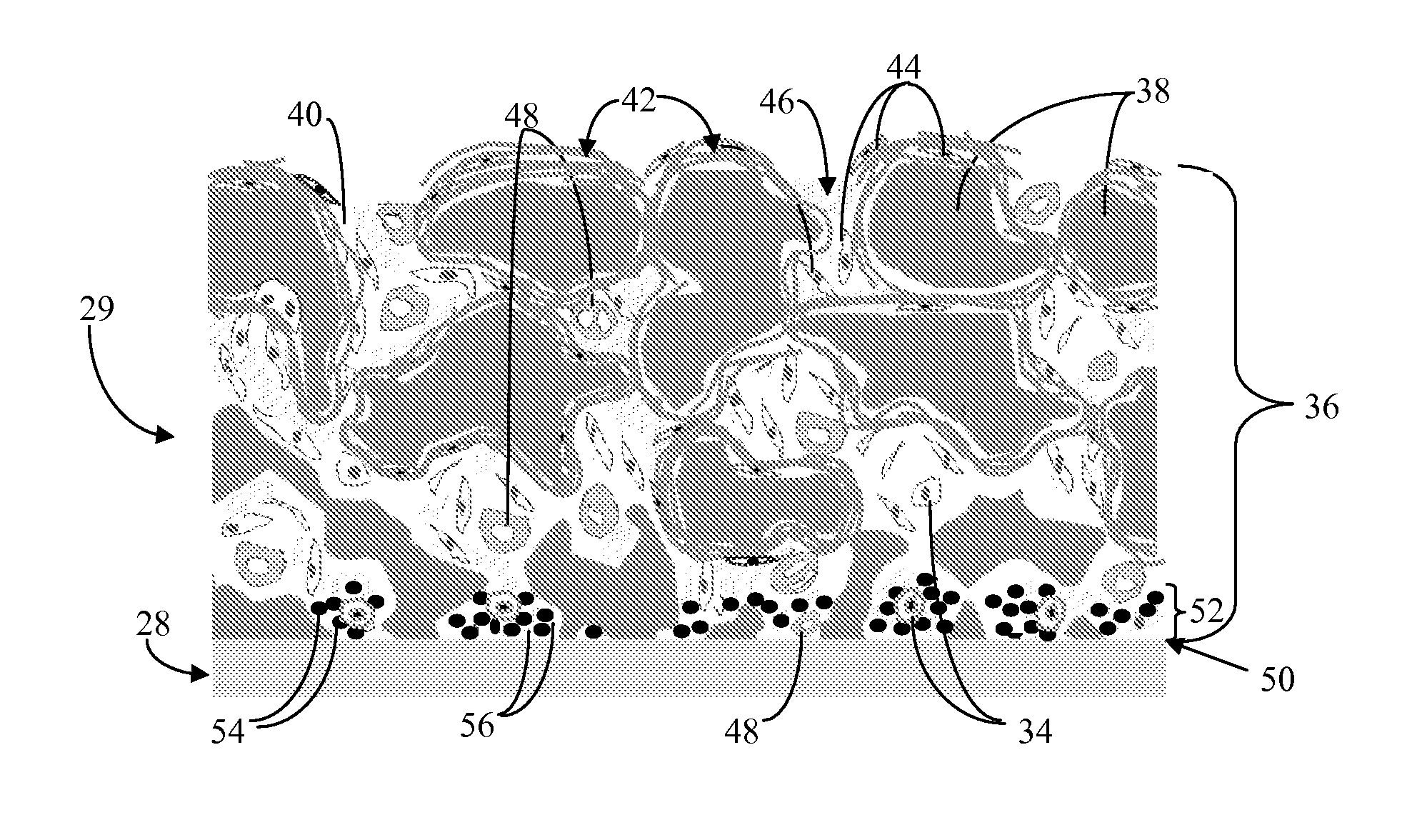
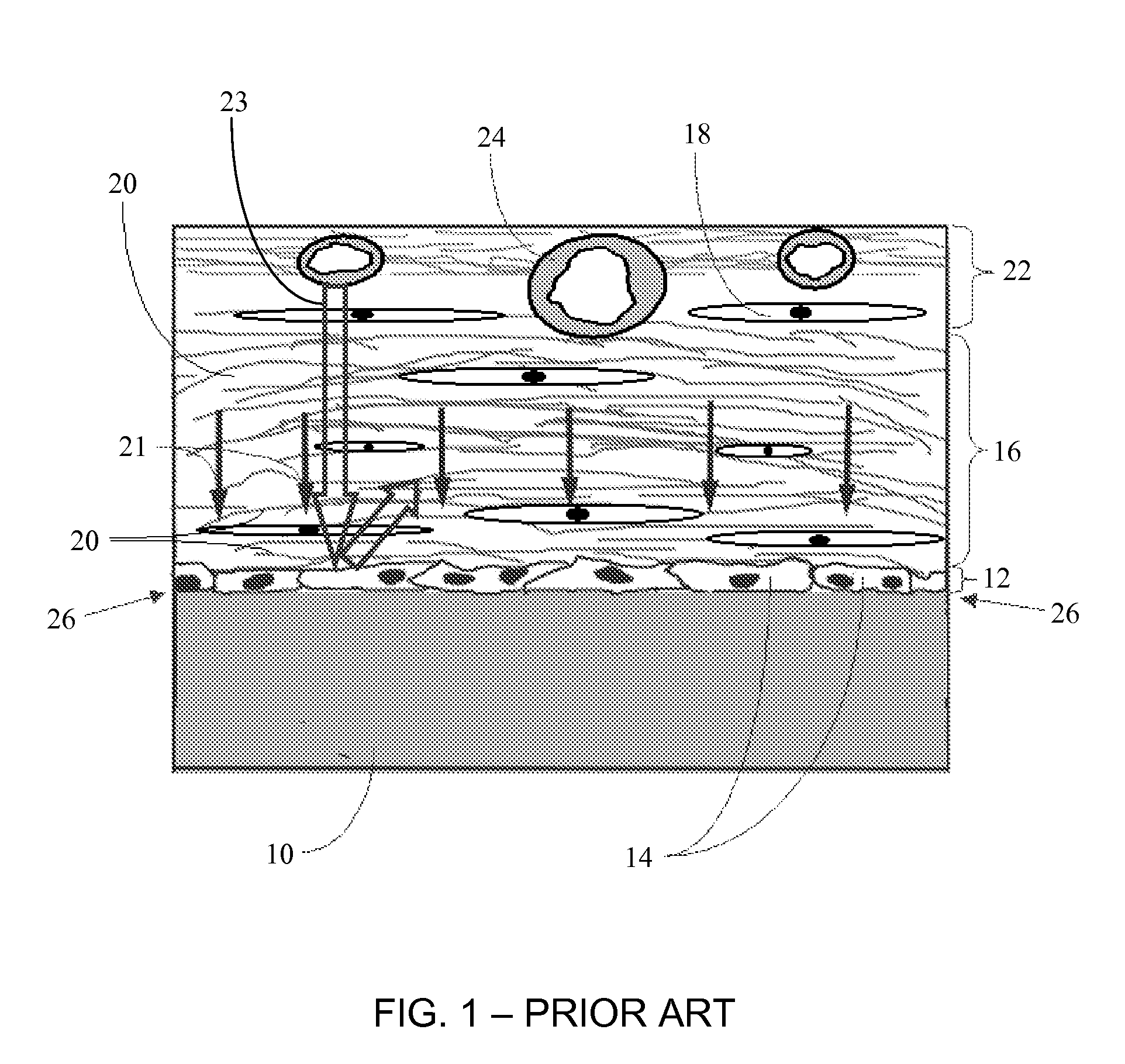
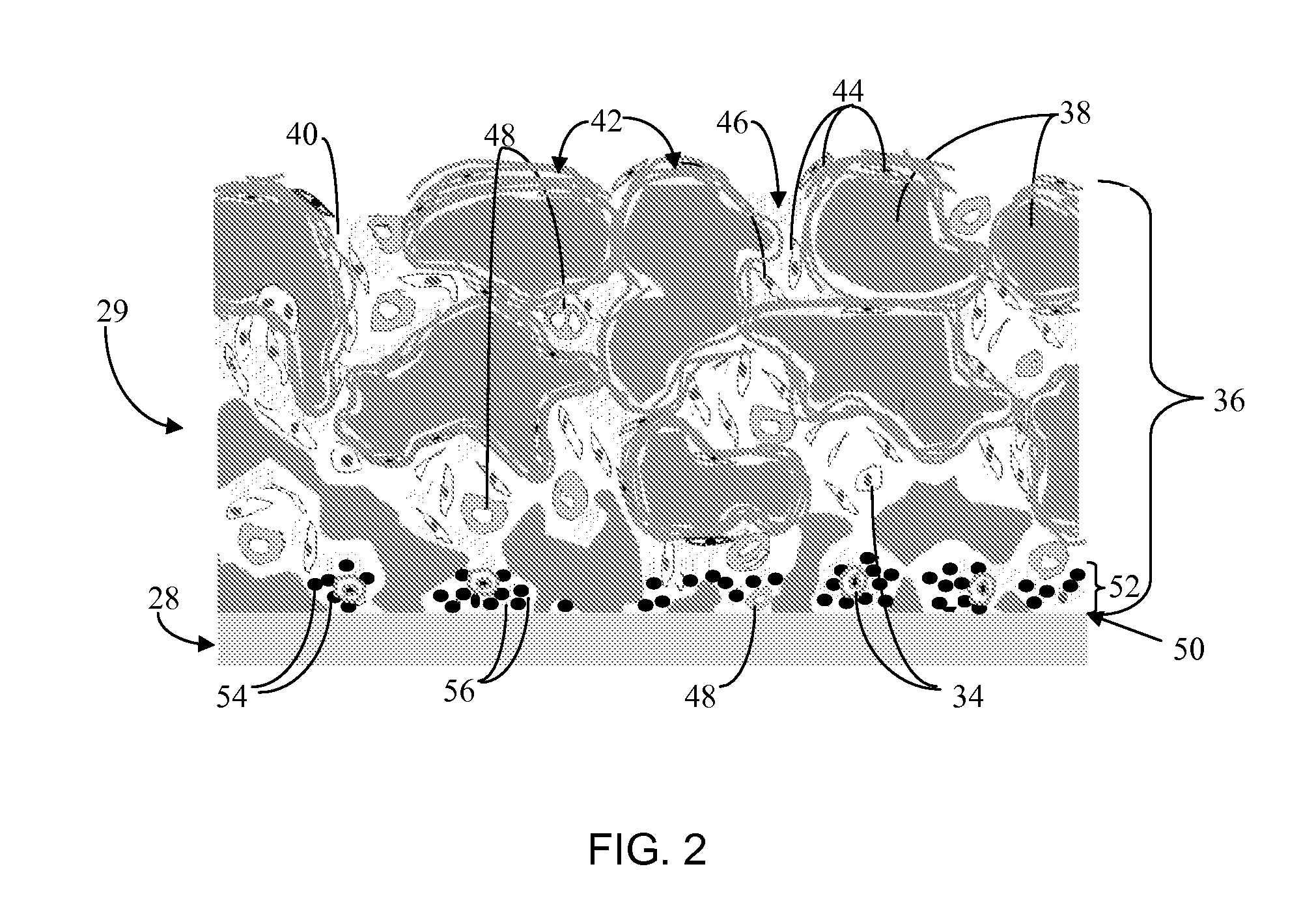
Biointerface membrane with macro-and micro-architecture
Disclosed herein are biointerface membranes including a macro-architecture and a micro-architecture co-continuous with and bonded to and / or located within at least a portion of the macro-architecture. The macro- and micro-architectures work together to manage and manipulate the high-level tissue organization and the low-level cellular organization of the foreign body response in vivo, thereby increasing neovascularization close to a device-tissue interface, interfering with barrier cell layer formation, and providing good tissue anchoring, while reducing the effects of motion artifact, and disrupting the organization and / or contracture of the FBC. The biointerface membranes of the preferred embodiments can be utilized with implantable devices such as devices for the detection of analyte concentrations in a biological sample (for example, from a body), cell transplantation devices, drug delivery devices, electrical signal delivering or measuring devices, and / or combinations thereof.
Owner:DEXCOM
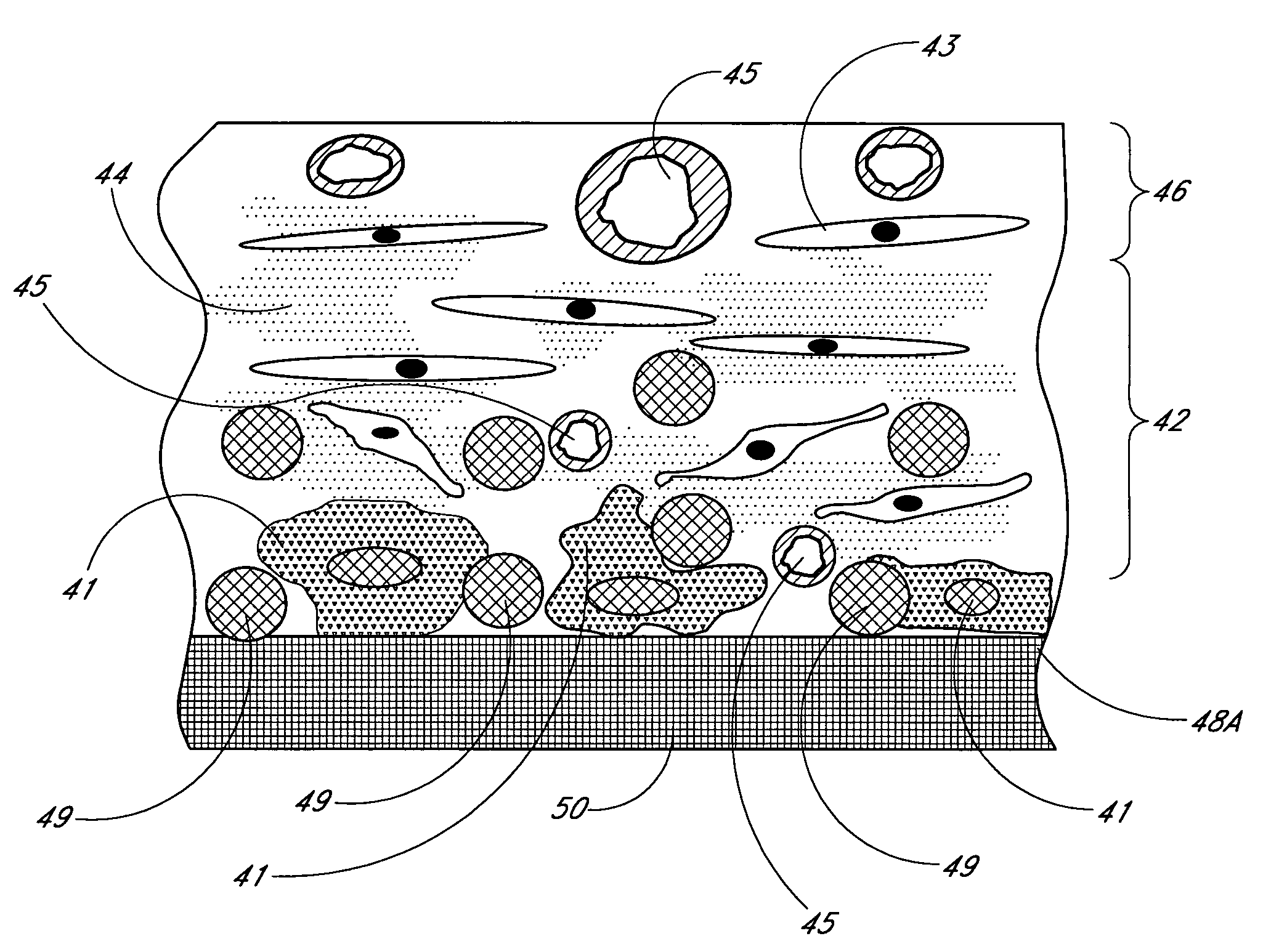
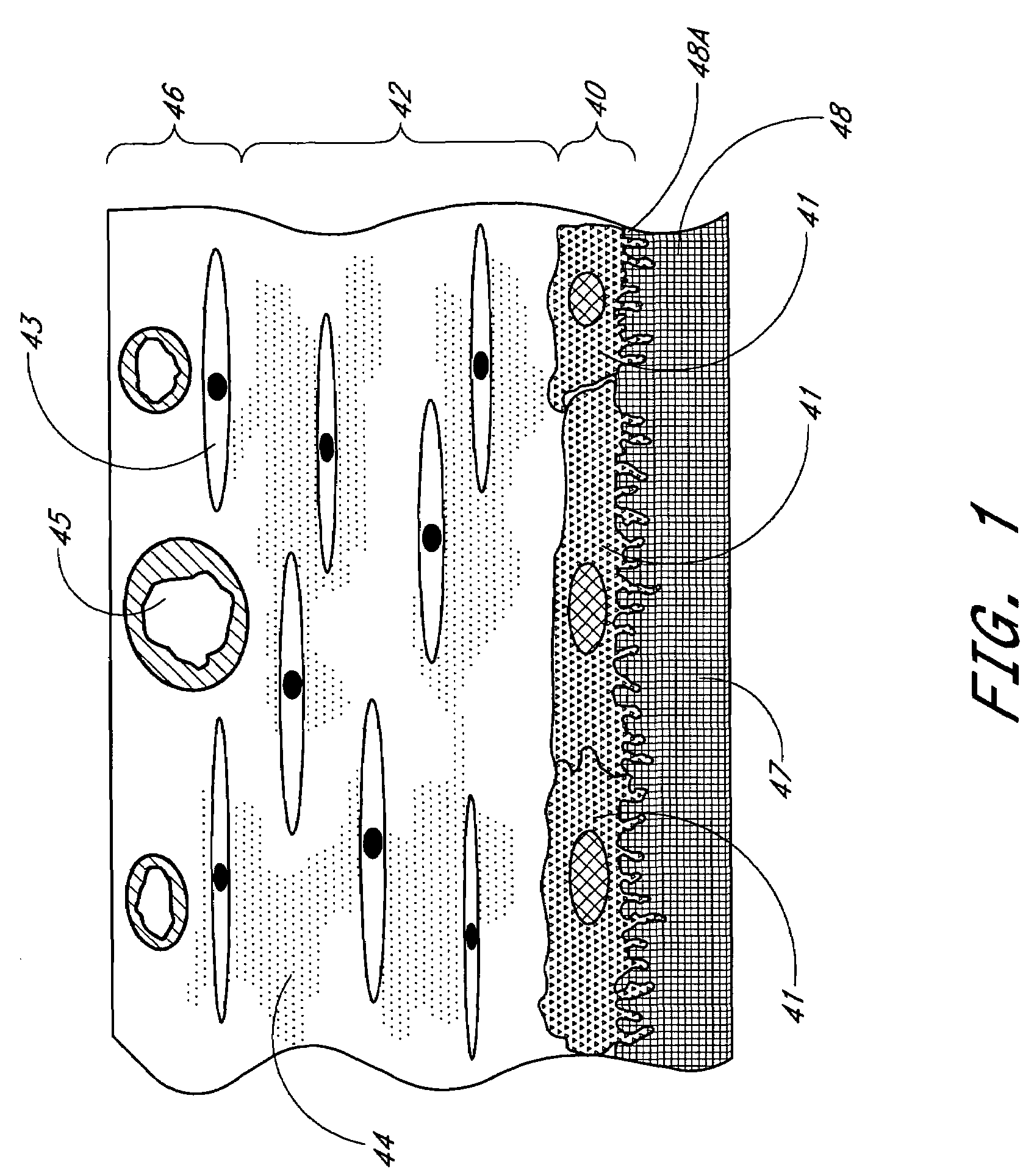
Membrane for use with implantable devices
The present invention provides a biointerface membrane for use with an implantable device that interferes with the formation of a barrier cell layer including; a first domain distal to the implantable device wherein the first domain supports tissue attachment and interferes with barrier cell layer formation and a second domain proximal to the implantable device wherein the second domain is resistant to cellular attachment and is impermeable to cells. In addition, the present invention provides sensors including the biointerface membrane, implantable devices including these sensors or biointerface membranes, and methods of monitoring glucose levels in a host utilizing the analyte detection implantable device of the invention. Other implantable devices which include the biointerface membrane of the present invention, such as devices for cell transplantation, drug delivery devices, and electrical signal delivery or measuring devices are also provided.
Owner:DEXCOM
Implantable devices having swellable grip members
The present disclosure relates to implantable medical devices including swellable tissue gripping elements and methods of forming such devices. The implantable medical device may comprise a biocompatible substrate having a surface comprising at least one swellable grip member. The implantable medical device may take on the form of a surgical mesh, patch, buttress, or pledget and the swellable member may comprise spikes and / or spiked naps. The implantable medical device may also contain a bioactive agent.
Owner:TYCO HEALTHCARE GRP LP
Composite material for implantable device
Devices suitable for implantation in a body of a host and systems and methods for their manufacture are provided. The implantable devices include a composite material formed at least from a matrix material and hollow gas-filled beads. In preferred embodiments, the composite material includes a polymeric matrix mixed with hollow air-filled glass beads, which are mixed and cured to form at least a portion of the body of the implantable device. Implantable devices including this composite material have decreased weight and / or overall density as compared to implantable devices without the beads incorporated therein, which is believed to improve the acceptance and function of the implantable device in vivo. Additionally, implantable devices concerned with transmitting and receiving via RF are believed to achieve improved RF performance due to a reduced dielectric constant provided by the incorporation of beads within the composite material.
Owner:DEXCOM INC
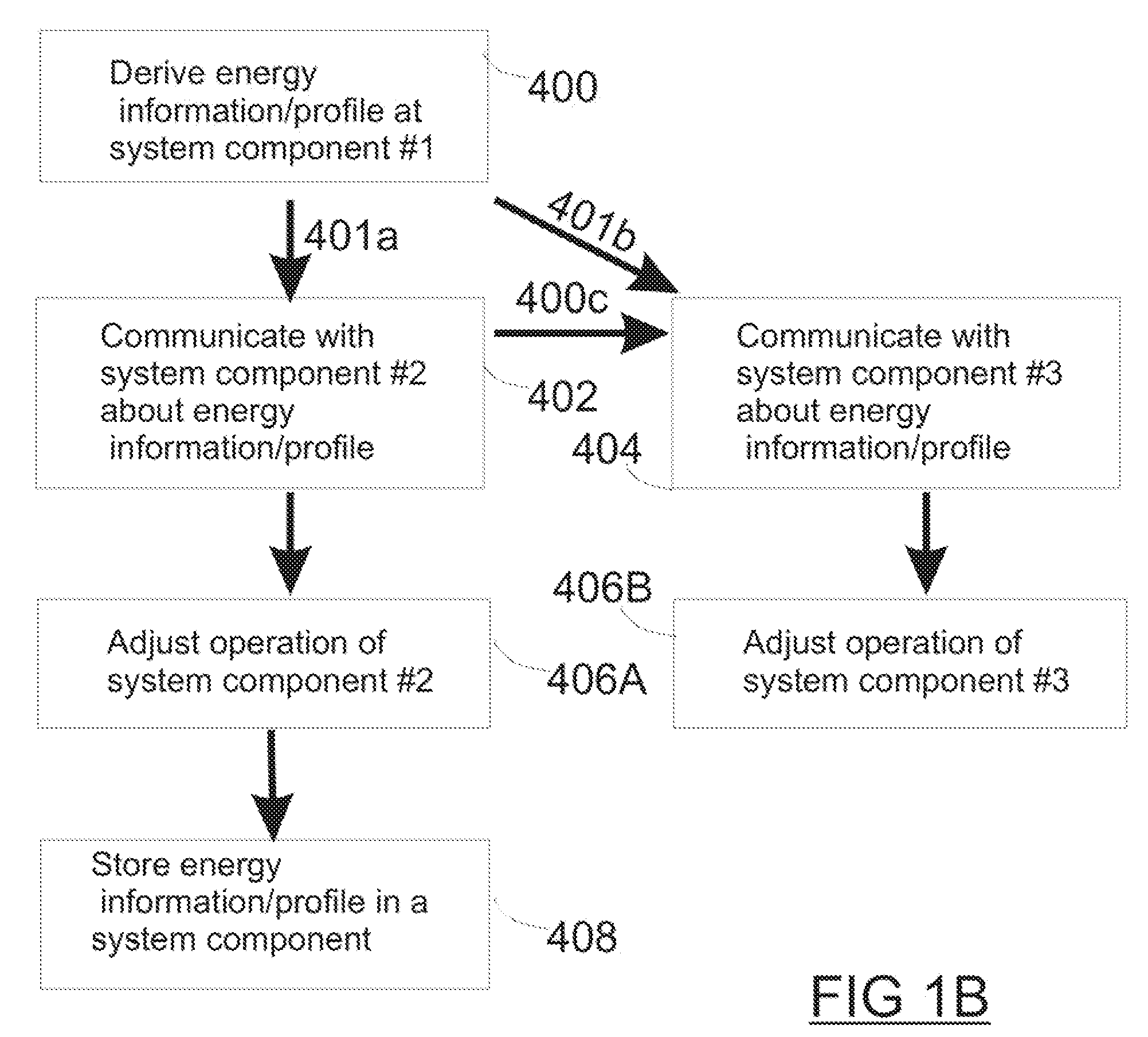
Systems and Methods for Wireless Power
ActiveUS20090058361A1Extend the lifespanImprove performanceElectrotherapyBatteries circuit arrangementsElectric power transmissionElectric power system
The present invention is a wireless power system which includes components which can be recharged by harvesting wireless power, wireless power transmitters for transmitting the power, and devices which are powered from the components. Features such as temperature monitoring, tiered network protocols including both data and power communication, and power management strategies related to both charging and non-charging operations, are used to improve performance of the wireless network. Rechargeable batteries which are configured to be recharged using wireless power have unique components specifically tailored for recharging operations rather than for providing power to a device. A wireless power supply for powering implanted devices benefits from an external patient controller which contains features for adjusting both power transmission and harvesting provided by other components of the wireless power network.
Owner:WITRICITY CORP
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Oxygen enhancing membrane systems for implantable devices
ActiveUS20050054909A1Immobilised enzymesBioreactor/fermenter combinationsImplanted deviceOxygen enhanced
The present invention relates generally to systems and methods for increasing oxygen availability to implantable devices. The preferred embodiments provide a membrane system configured to provide protection of the device from the biological environment and / or a catalyst for enabling an enzymatic reaction, wherein the membrane system includes a polymer formed from a high oxygen soluble material. The high oxygen soluble polymer material is disposed adjacent to an oxygen-utilizing source on the implantable device so as to dynamically retain high oxygen availability to the oxygen-utilizing source during oxygen deficits. Membrane systems of the preferred embodiments are useful for implantable devices with oxygen-utilizing sources and / or that function in low oxygen environments, such as enzyme-based electrochemical sensors and cell transplantation devices.
Owner:DEXCOM
Annuloplasty rings and methods for repairing cardiac valves
InactiveUS20050004668A1Facilitate customized remodelingAccurate shapeBone implantAnnuloplasty ringsStructure functionImplanted device
Owner:FLEXCOR
Membrane for use with implantable devices
ActiveUS20100087724A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteBiointerface
The present invention provides a biointerface membrane for use with an implantable device that interferes with the formation of a barrier cell layer including; a first domain distal to the implantable device wherein the first domain supports tissue attachment and interferes with barrier cell layer formation and a second domain proximal to the implantable device wherein the second domain is resistant to cellular attachment and is impermeable to cells. In addition, the present invention provides sensors including the biointerface membrane, implantable devices including these sensors or biointerface membranes, and methods of monitoring glucose levels in a host utilizing the analyte detection implantable device of the invention. Other implantable devices which include the biointerface membrane of the present invention, such as devices for cell transplantation, drug delivery devices, and electrical signal delivery or measuring devices are also provided.
Owner:DEXCOM INC
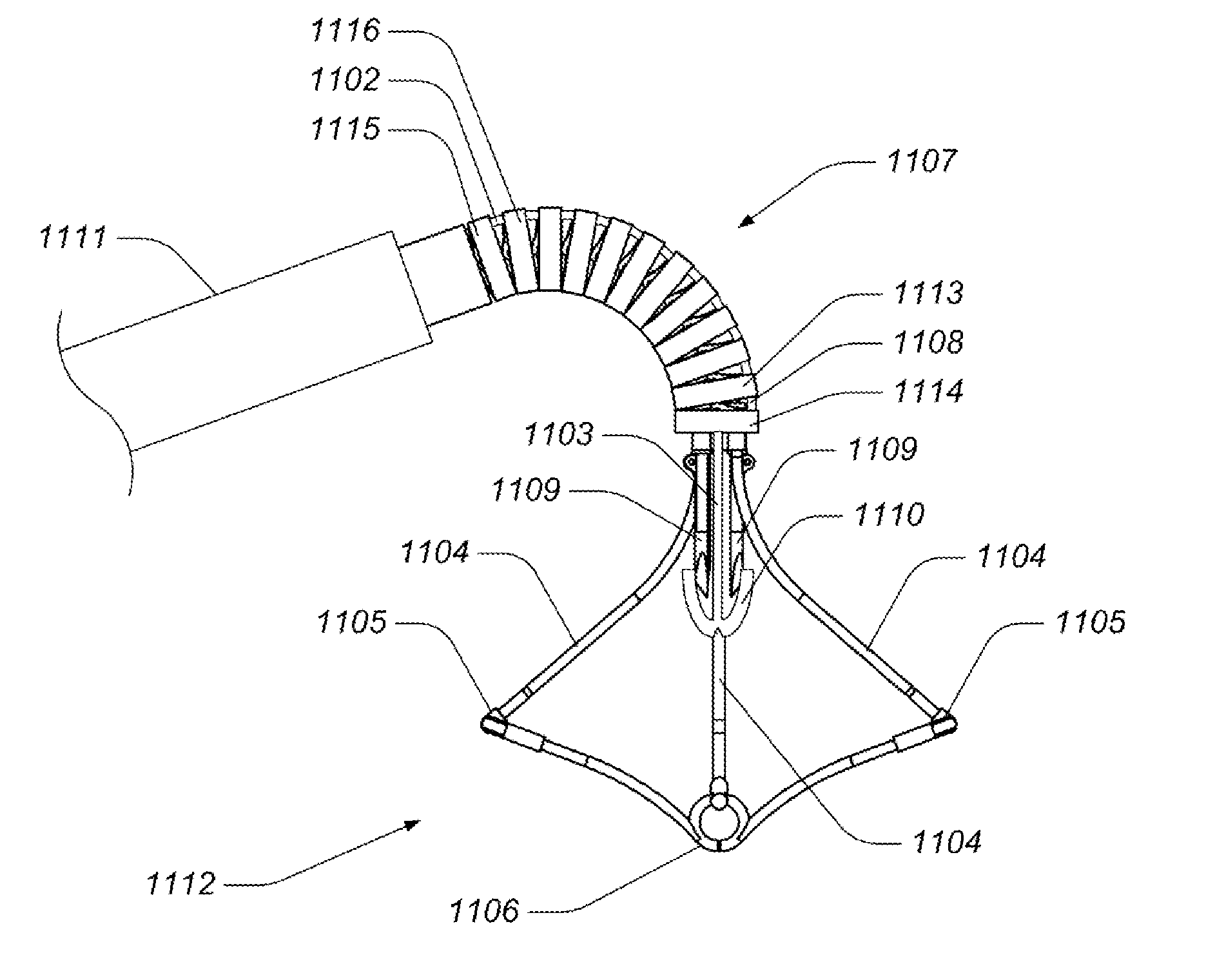
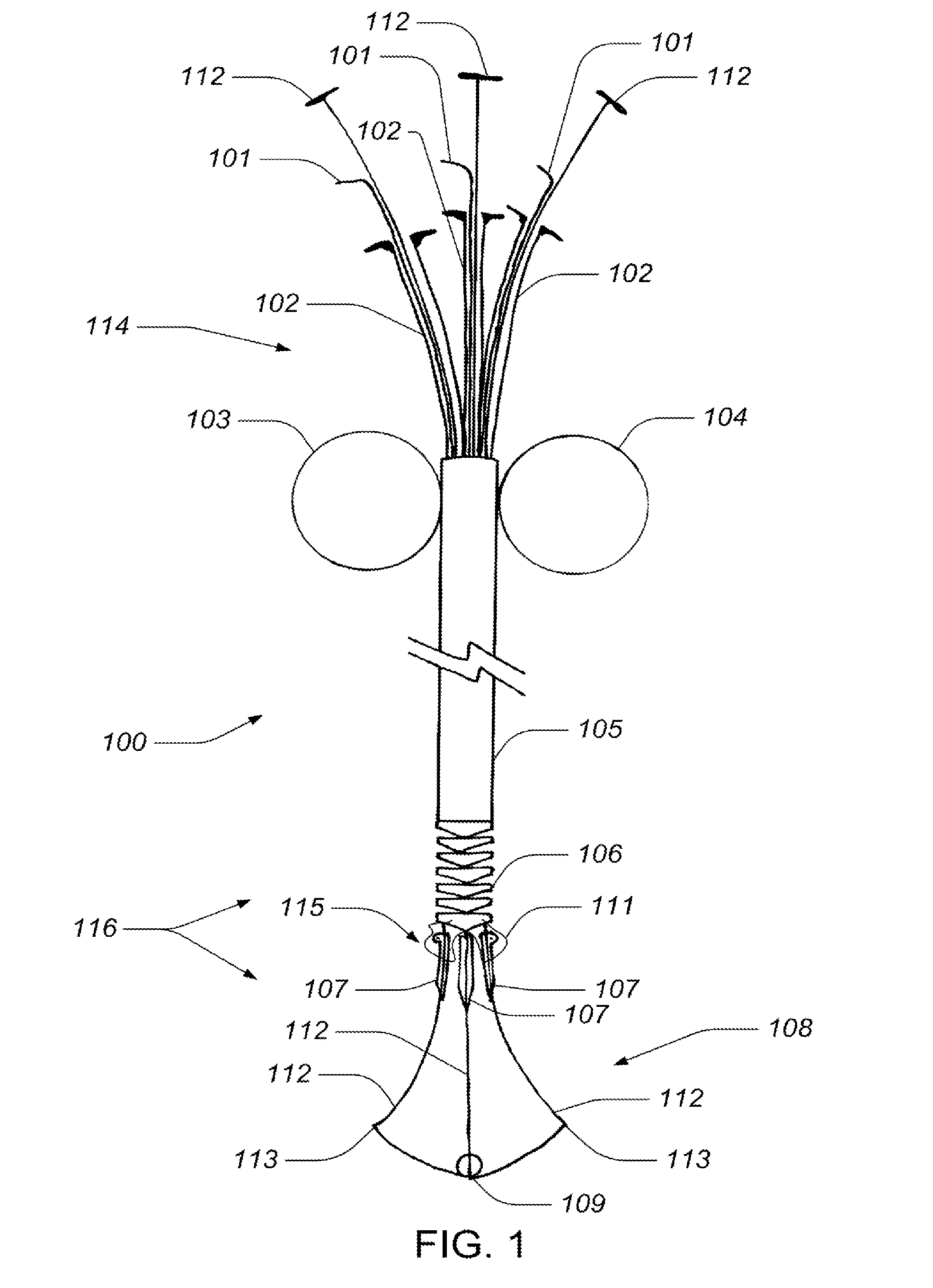
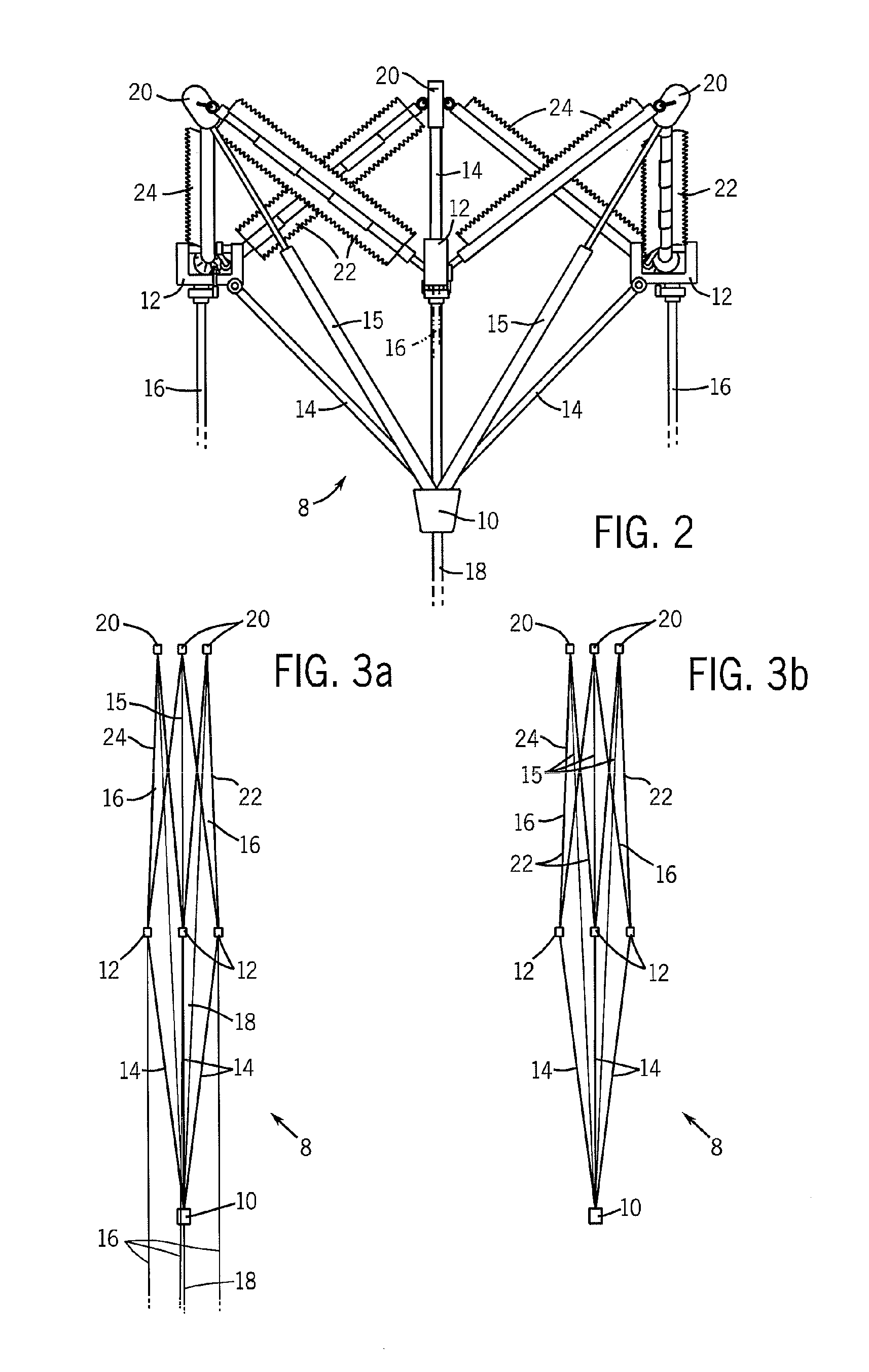
Valve implanting device
ActiveUS6951571B1Easy to replaceIncreased durabilityVenous valvesBlood vesselsImplanted deviceGuide wires
Disclosed is a valve implanting device comprising a collapsible frame, inner and outer guide wires removably connected to the collapsible frame, and a plurality of valve flaps attached to the collapsible frame. The collapsible frame is inserted into a patient's femoral vein or artery, guided to a deployment position using the guide wires, expanded using the guide wires, and stabilized using the guide wires to manipulate fixating hubs on the collapsible frame. The collapsible frame includes a central hub, a plurality of spokes, fixating hubs, gripping members, and a plurality of valve flaps.
Owner:SRIVASTAVA ROHIT
Biointerface with macro- and micro-architecture
Owner:DEXCOM INC
Systems and methods for wireless power
ActiveUS8115448B2Extend the lifespanImprove performanceNear-field transmissionBatteries circuit arrangementsElectric power transmissionElectric power system
The present invention is a wireless power system which includes components which can be recharged by harvesting wireless power, wireless power transmitters for transmitting the power, and devices which are powered from the components. Features such as temperature monitoring, tiered network protocols including both data and power communication, and power management strategies related to both charging and non-charging operations, are used to improve performance of the wireless network. Rechargeable batteries which are configured to be recharged using wireless power have unique components specifically tailored for recharging operations rather than for providing power to a device. A wireless power supply for powering implanted devices benefits from an external patient controller which contains features for adjusting both power transmission and harvesting provided by other components of the wireless power network.
Owner:WITRICITY CORP
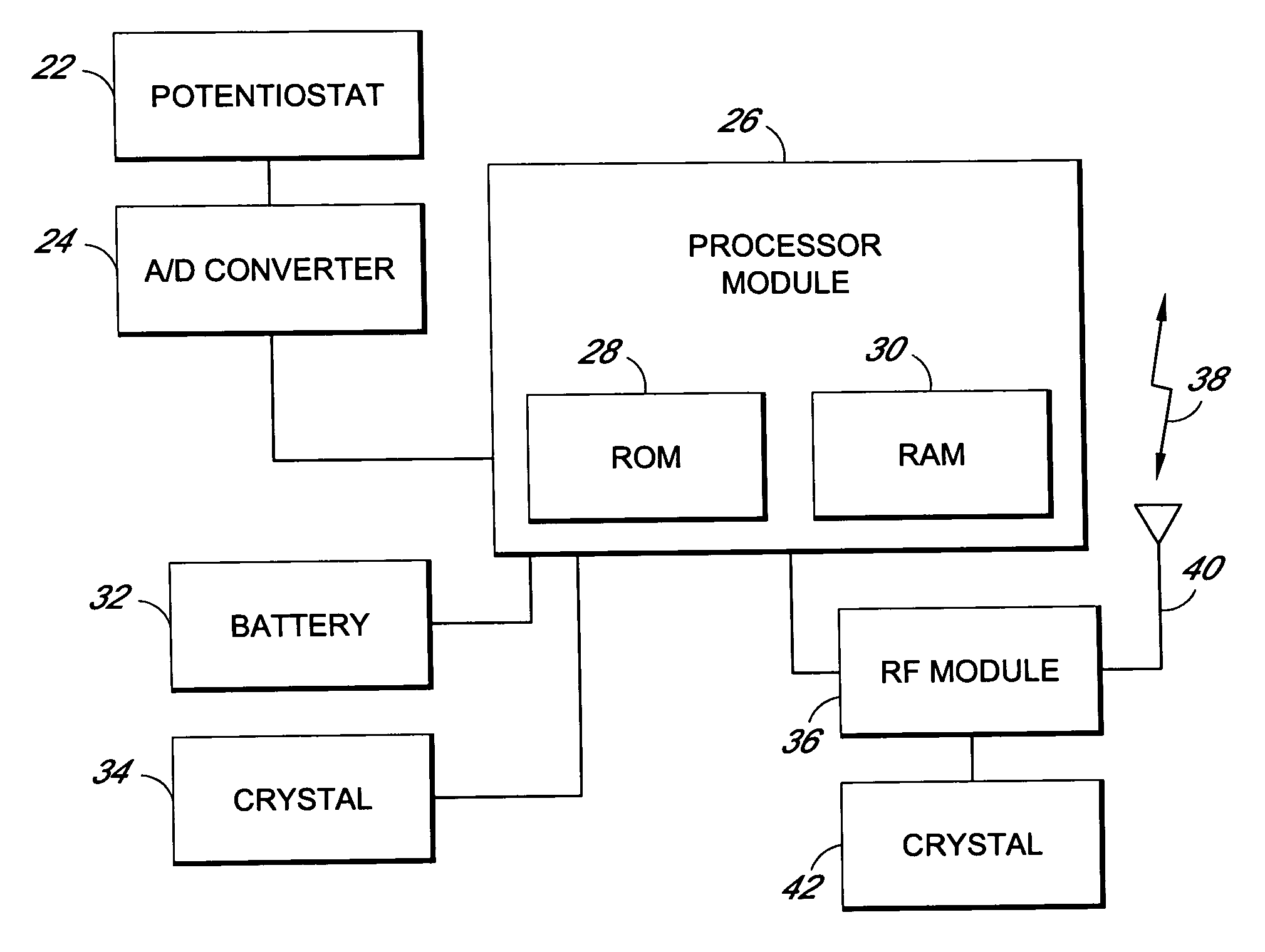
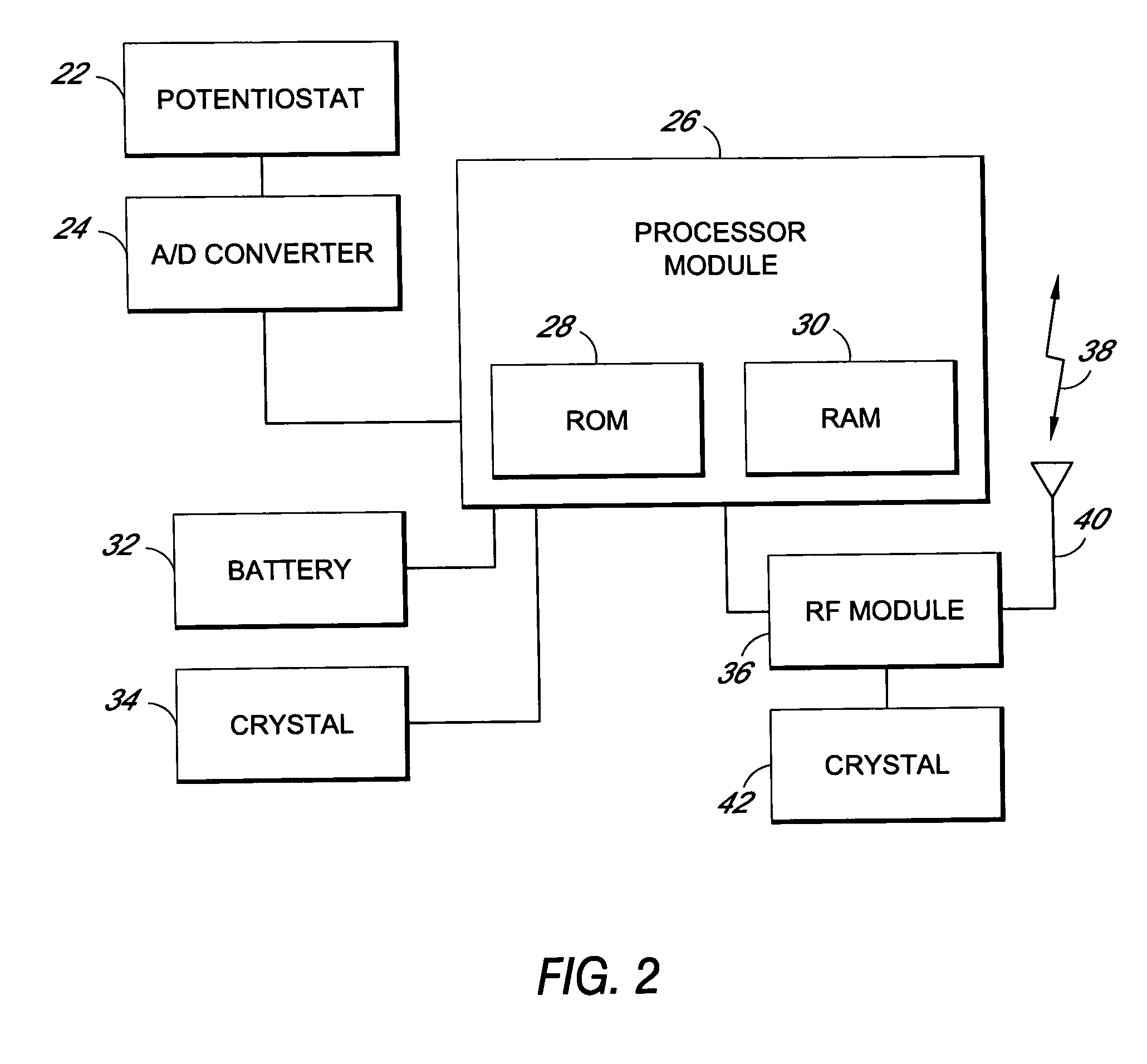
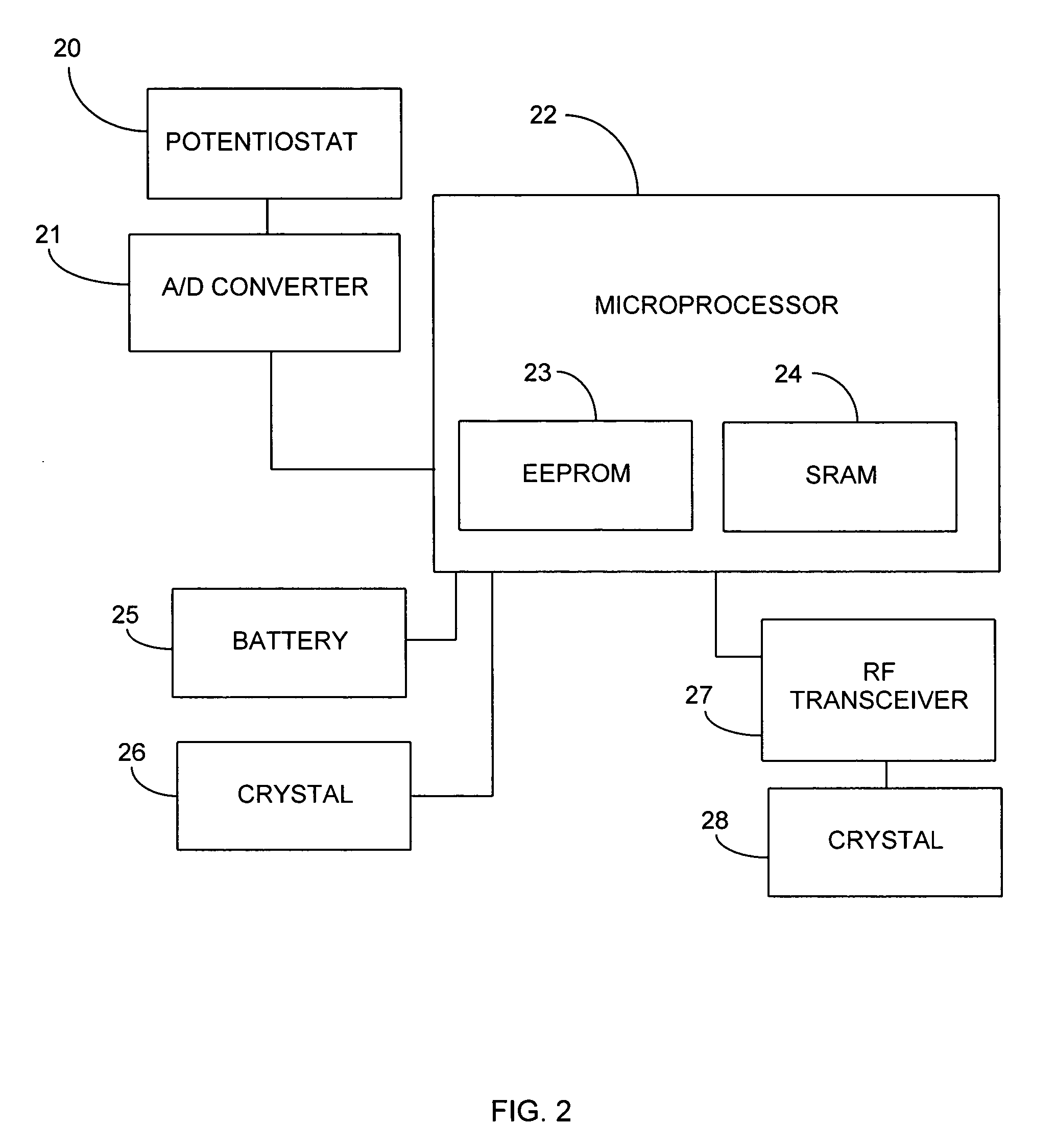
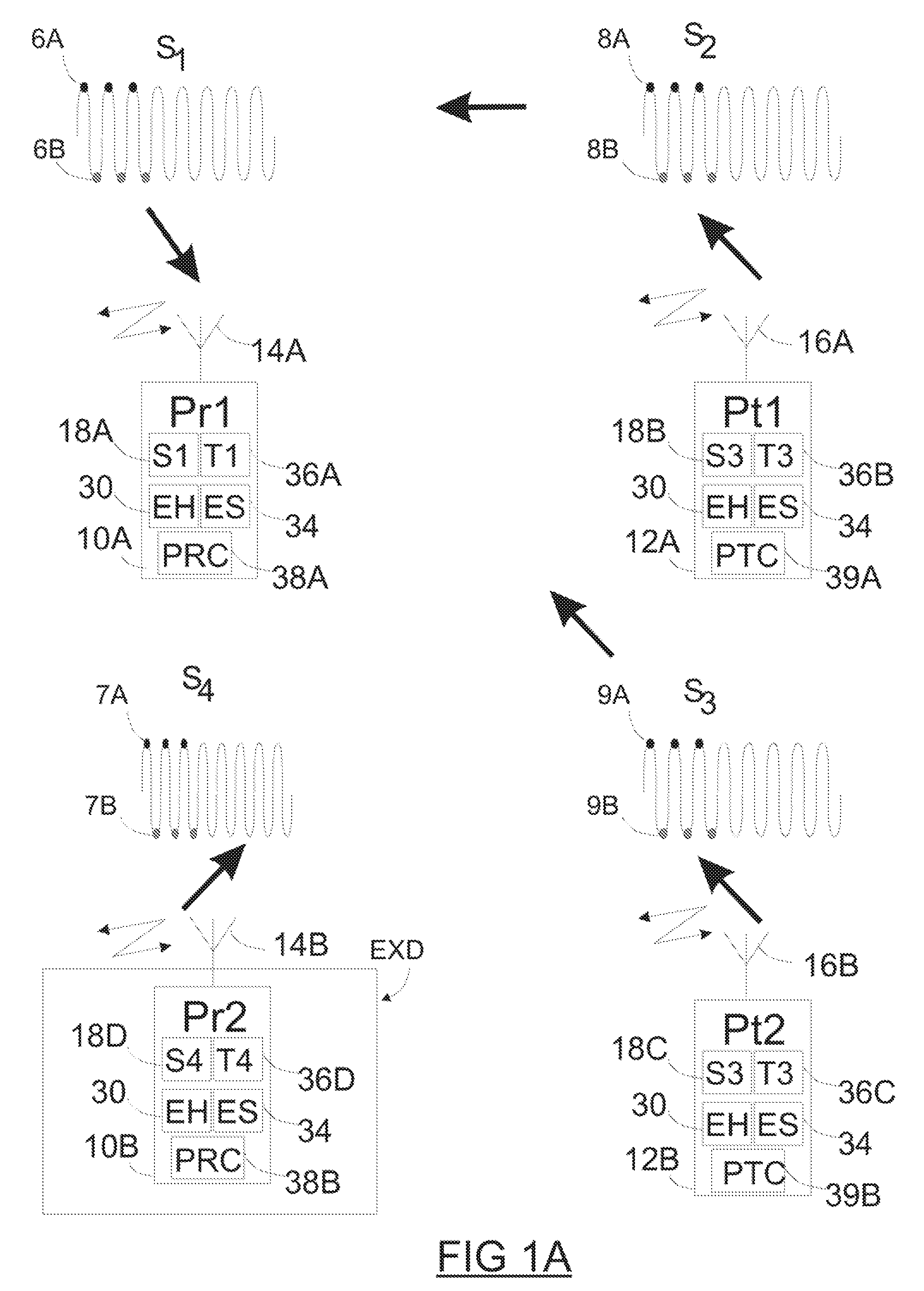
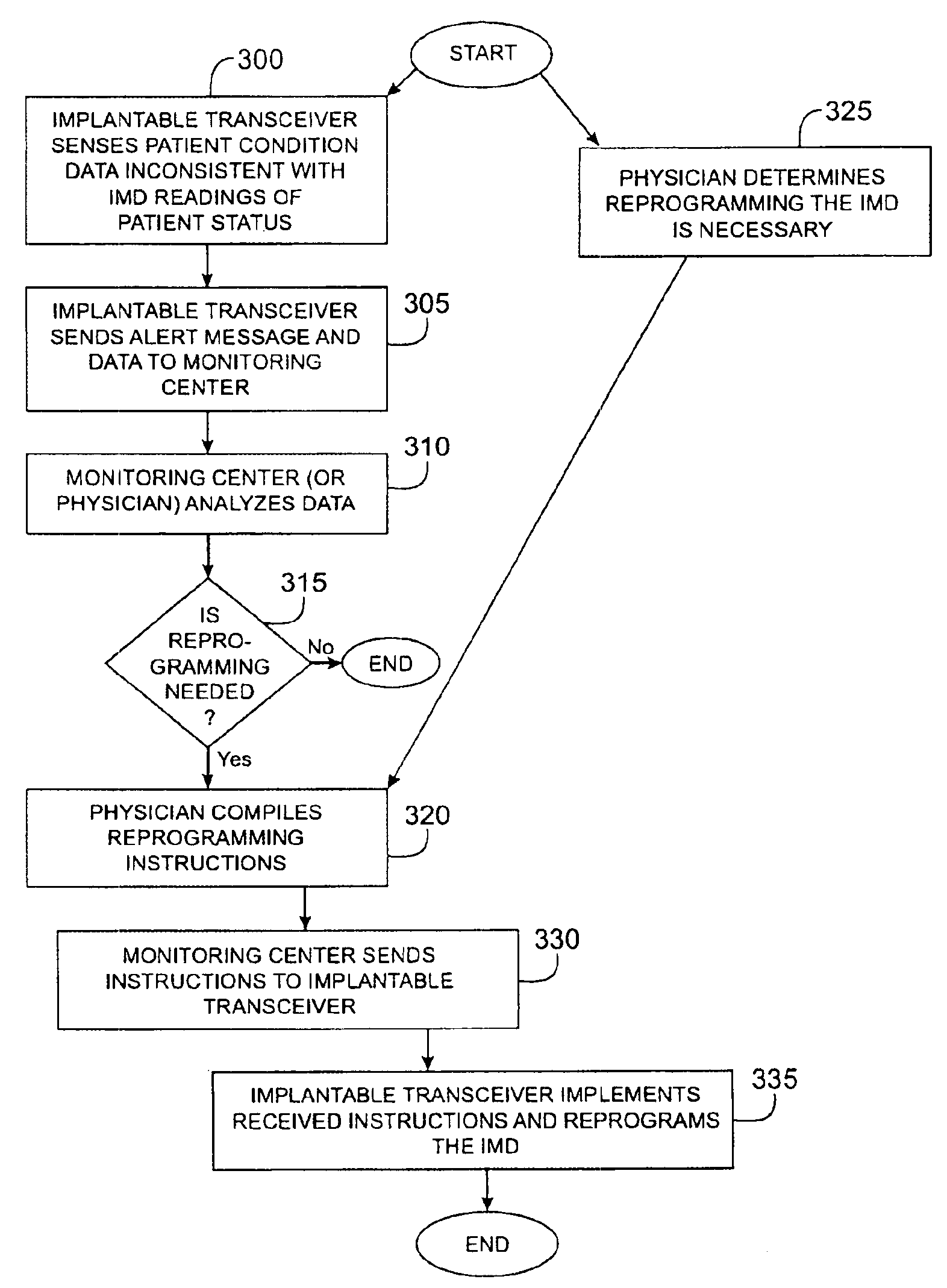
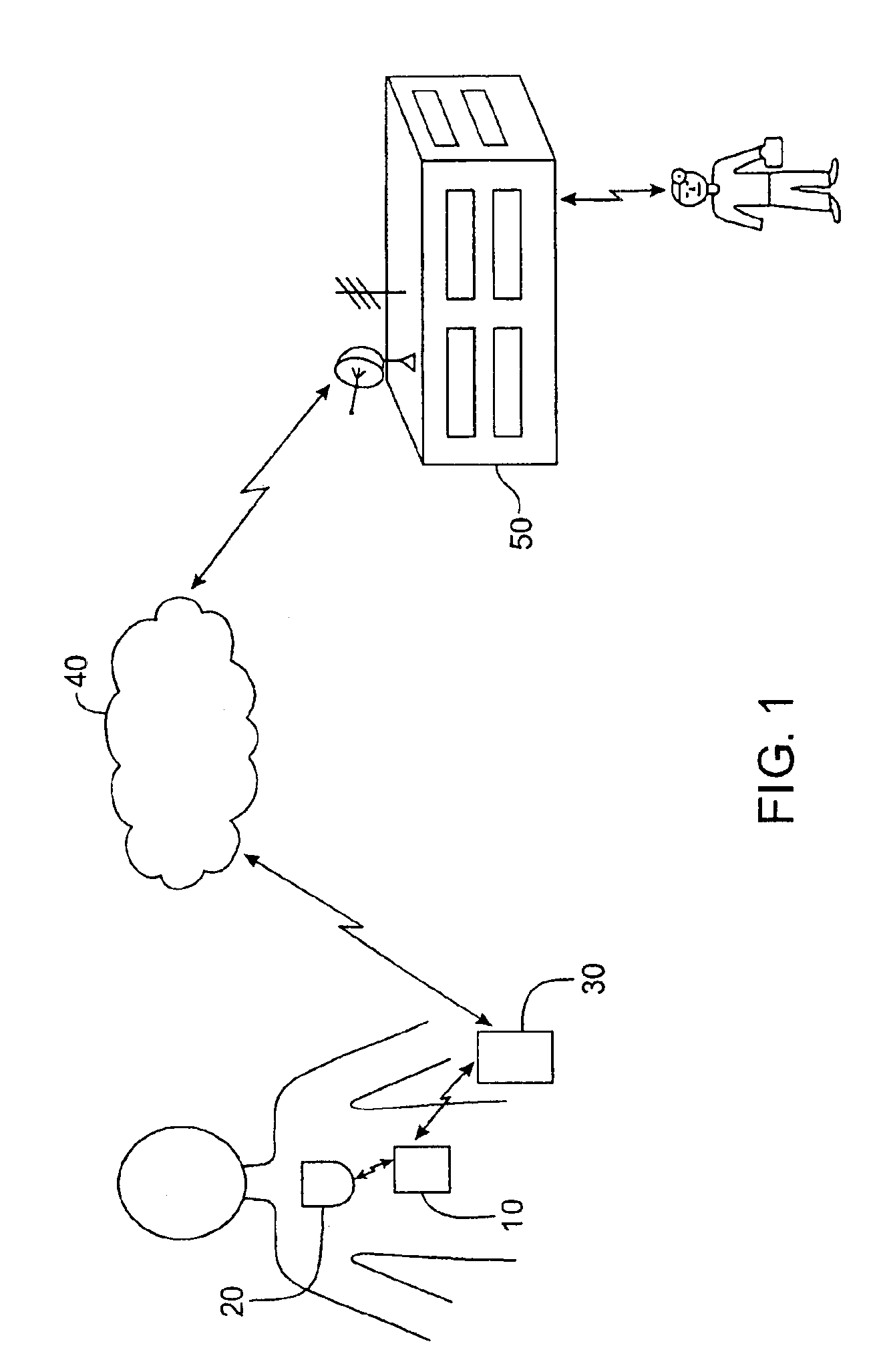
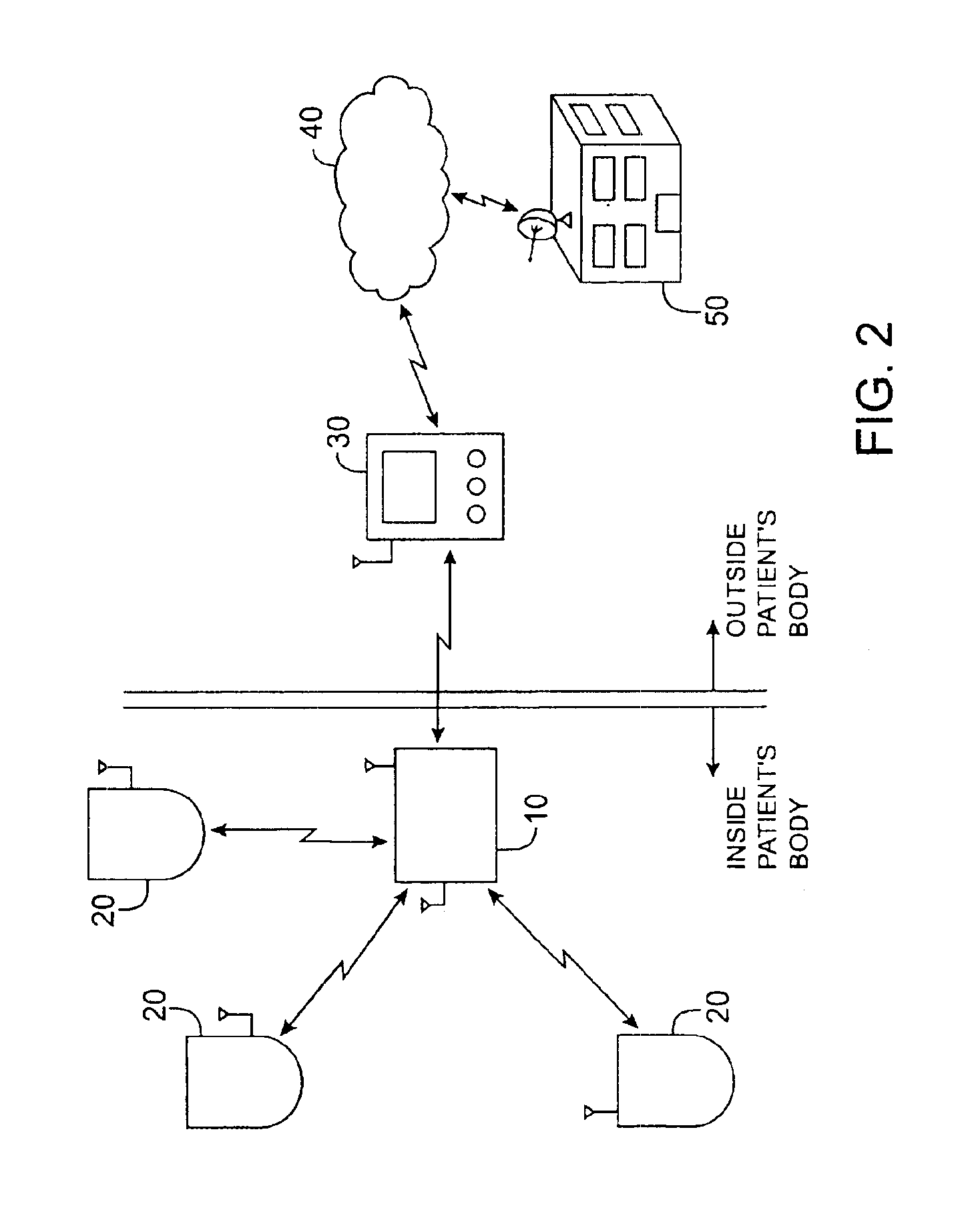
Method and apparatus for monitoring and communicating with an implanted medical device
InactiveUS6957107B2Simplify the surgical processMinimize impactElectrotherapySurgeryCommunication interfaceDevice implant
A method and apparatus for communicating with and monitoring the operation of a device implanted within a patient. A transceiver capable of being implanted within a patient provides a communication interface between an implanted medical device and a monitor external to the patient's body. The external monitor can communicate with a remote monitoring center over a communication network. The external monitor also provides control signals to the implanted device via the transceiver unit. The transceiver apparatus is capable of two-way communication between the implanted device and the external monitor. The transceiver apparatus is also capable of detecting actions performed by the implanted device and physiological signals directly from the patient's body. Thus, the transceiver apparatus provides circuitry for determining whether an implanted medical device is operating properly. The transceiver apparatus provides a way to remotely reprogram one or more implanted medical devices.
Owner:BRAEMAR MFG
Methods of forming a coating for a prosthesis
InactiveUS6503556B2Increase the amount addedIncrease the number ofRadiation applicationsGlovesProsthesisImplanted device
Methods of forming a coating onto an implantable device or endoluminal prosthesis, such as a stent, are provided. The coating may be used for the delivery of an active ingredient. The coating may have a selected pattern of interstices for allowing a fluid to seep through the coating in the direction of the pattern created.
Owner:ABBOTT CARDIOVASCULAR
Polyaxial pedicle screw having a threaded and tapered compression locking mechanism
InactiveUSRE37665E1Improve locking effectEasy to compressInternal osteosythesisJoint implantsCouplingLocking mechanism
A polyaxial orthopedic device for use with rod implant apparatus includes a screw having a curvate head, a two-piece interlocking coupling element which mounts about the curvate head, and a rod receiving cylindrical body member having a tapered socket into which both the screw and the interlocking coupling element are securely nested. The interlocking coupling element includes a socket portion which is slotted and tapered so that when it is radially compressed by being driven downwardly into the tapered socket in the cylindrical body it crush locks to the screw. The securing of the rod in the body member provides the necessary downward force onto the socket portion through a contact force on the top of the cap portion. Prior to the rod being inserted, therefore, the screw head remains polyaxially free with respect to the coupling element and the body. In a preferred embodiment, the cap portion and the socket portion are formed and coupled in such a way that when the cap portion is compressed toward the socket portion, there is an additional inward radial force applied by the cap portion to the socket portion, thereby enhancing the total locking force onto the head of the screw.
Owner:FASTENETIX L L C
Implantable device with improved radio frequency capabilities
Owner:GRIFFIN ADAM +2
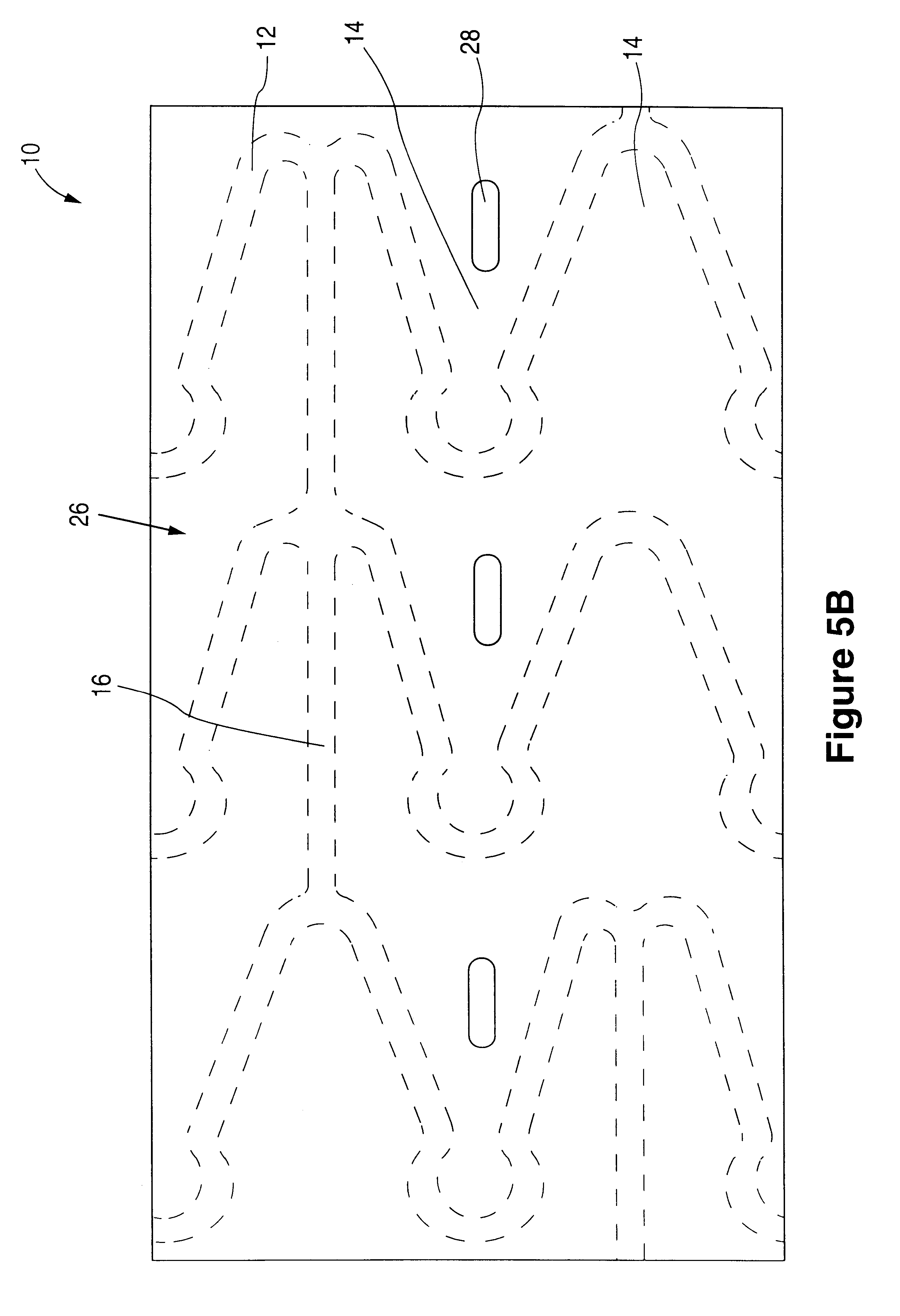
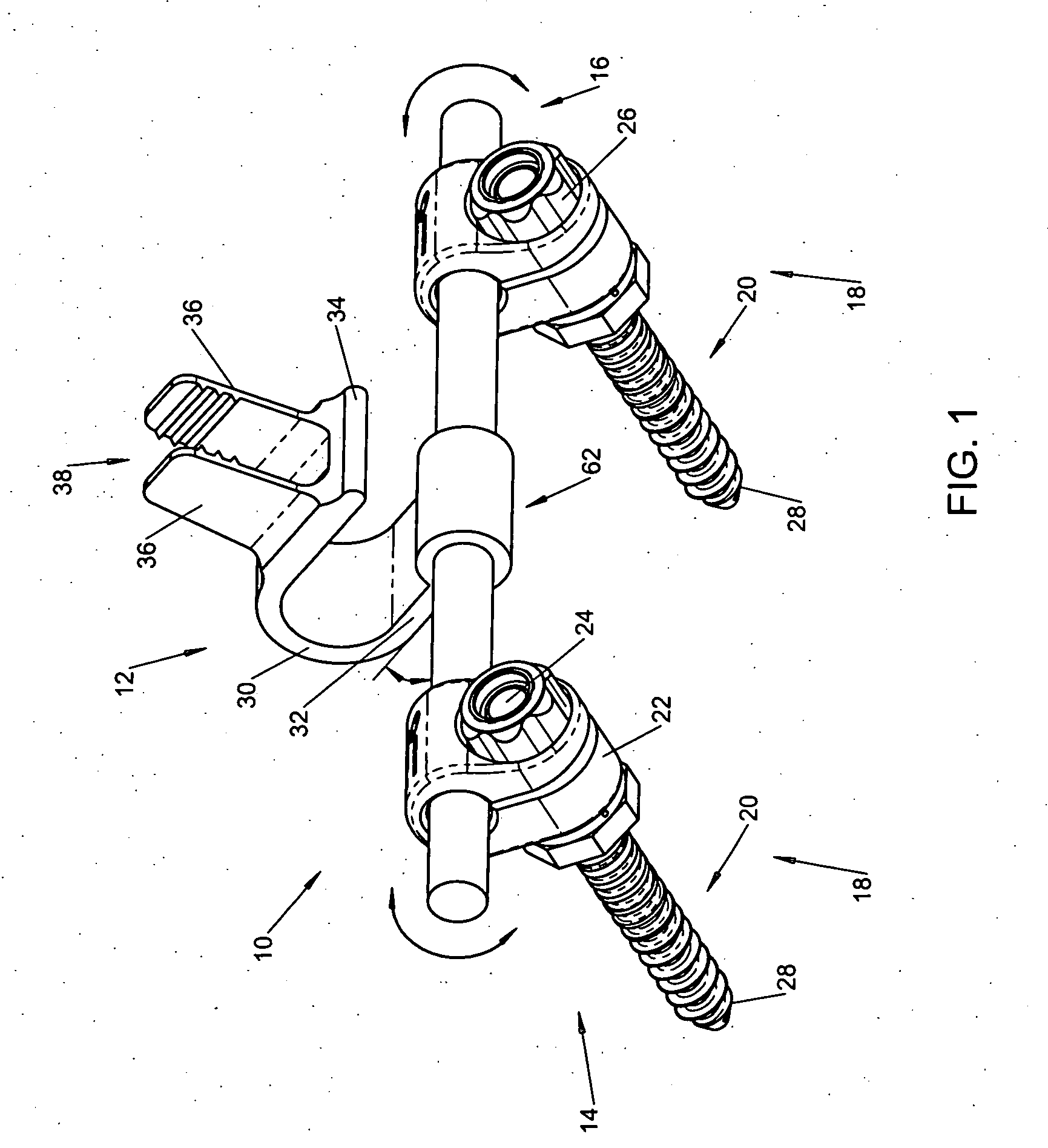
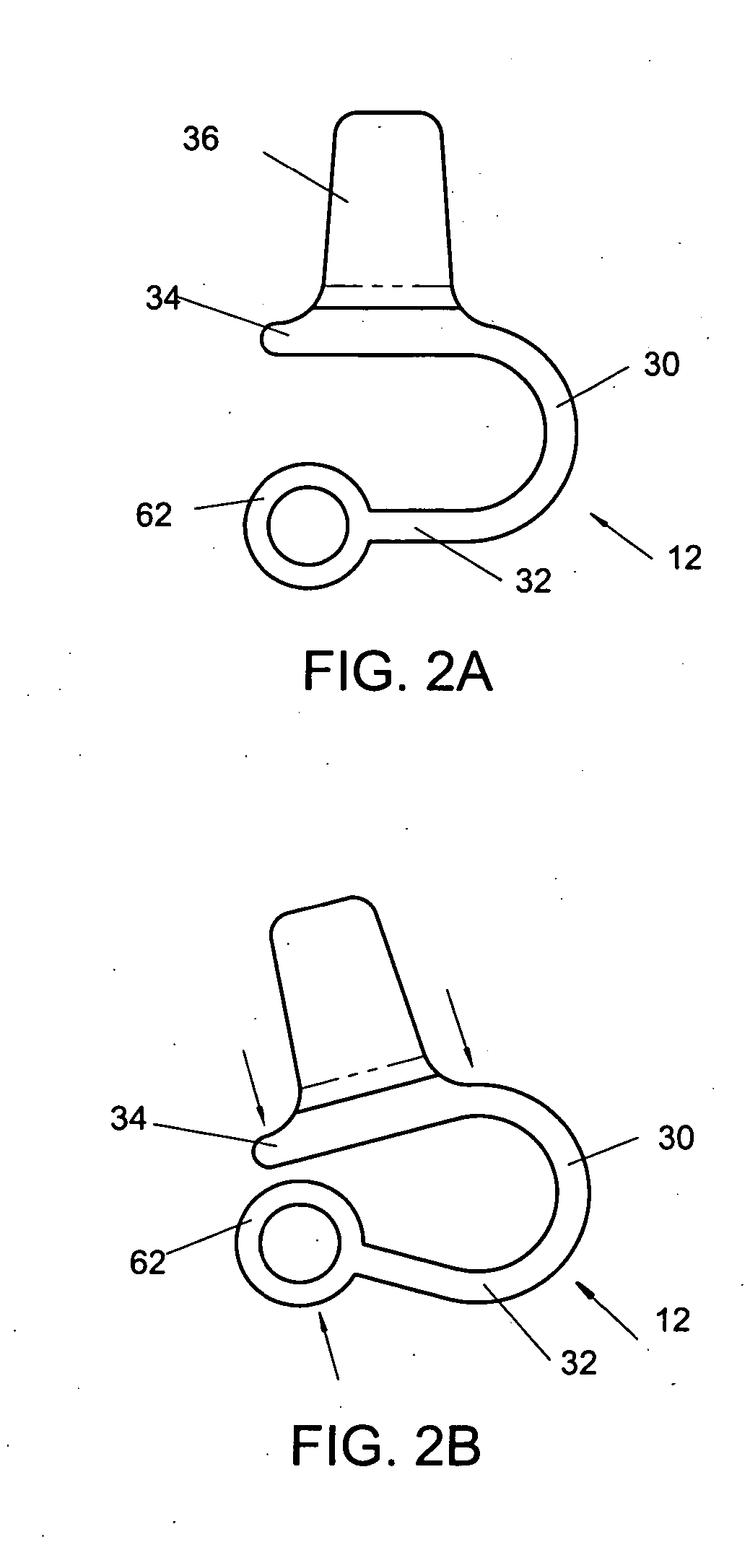
Interspinous vertebral and lumbosacral stabilization devices and methods of use
Implantable devices are provided for stabilizing adjacent vertebrae and the lumbosacral region of a patient. The devices can comprise an interspinous flexible spacer body having a substantially U-shape comprising a superior section, inferior section, and a midsection extending therebetween. The superior and / or inferior sections can include a pair of lateral walls configured to engage a spinous process of a vertebra. Fixation caps can be provided for securing a spinous process of a vertebra to the flexible spacer body. To secure the flexible spacer body between the lumbar vertebra and an adjacent vertebra, an anchor assembly is provided. Also provided are methods of using the implantable devices to stabilize a patient's spine.
Owner:PARADIGM SPINE LLC
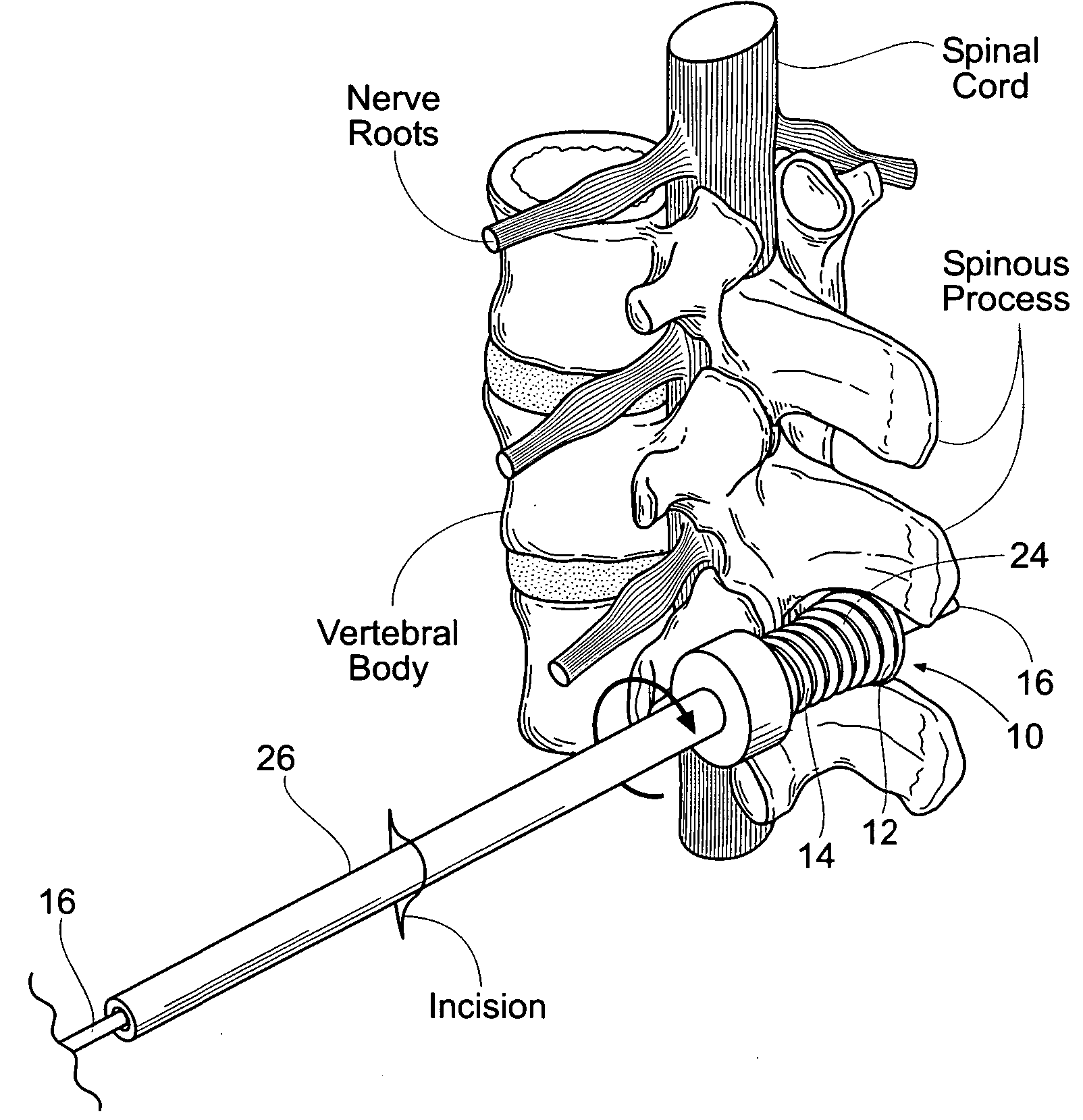
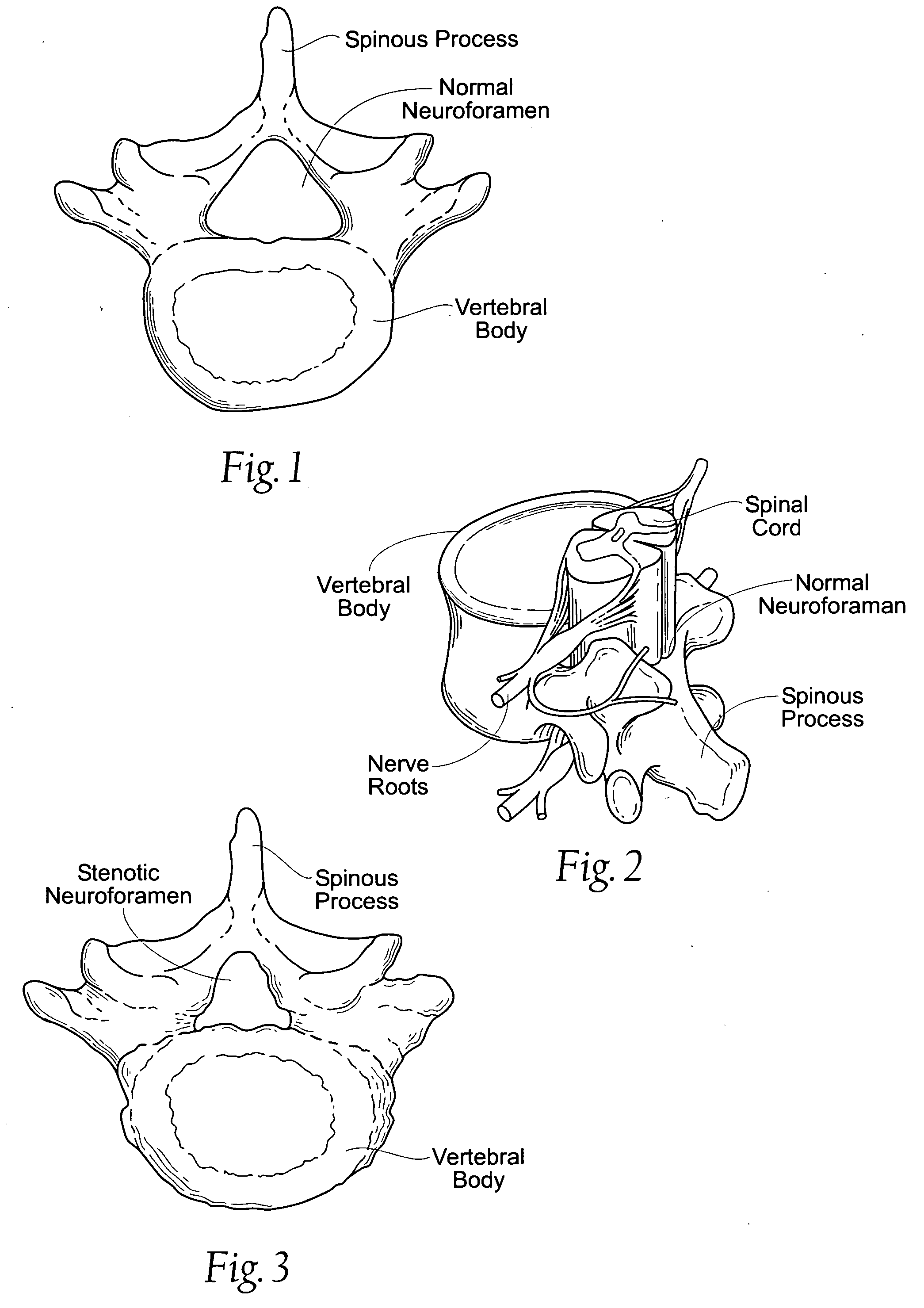
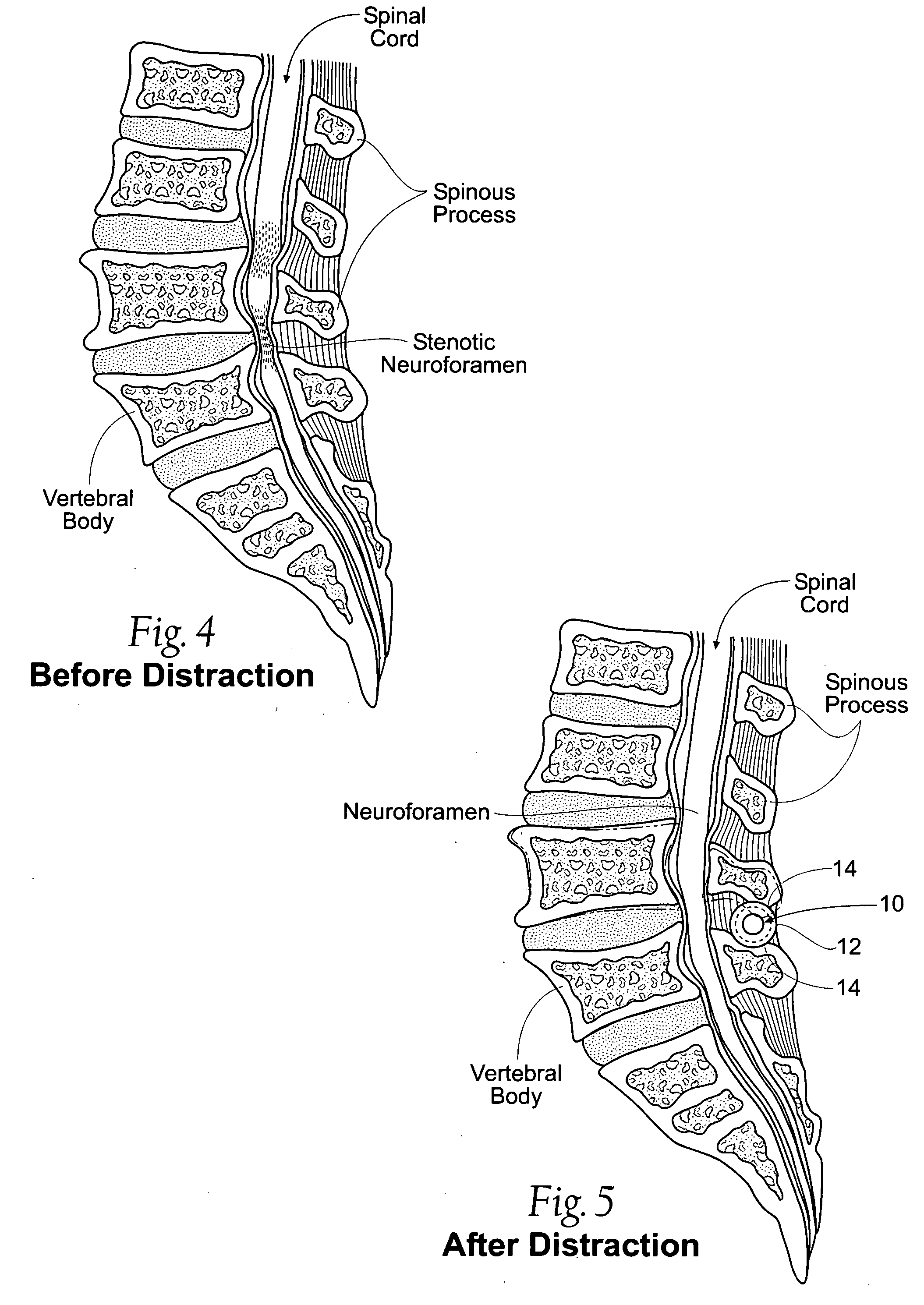
Percutaneous spine distraction implant systems and methods
Systems and methods for treating spinal stenosis insert a guide element percutaneously into proximity with the adjacent spinous processes. The systems and methods direct an implant device over the guide element to a position resting between the adjacent spinous processes. The device is sized and configured to distend the adjacent spinous processes. The implant device itself can be variously constructed. It can, e.g., possess threaded lands and / or a notched region in which a spinous process can rest. The implant device has a lumen to accommodate passage of the guide element, so that the device can be passed percutaneously over the guide element for implantation between adjacent spinous processes.
Owner:REILEY MARK A
Devices, systems and methods for treating disorders of the ear, nose and throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
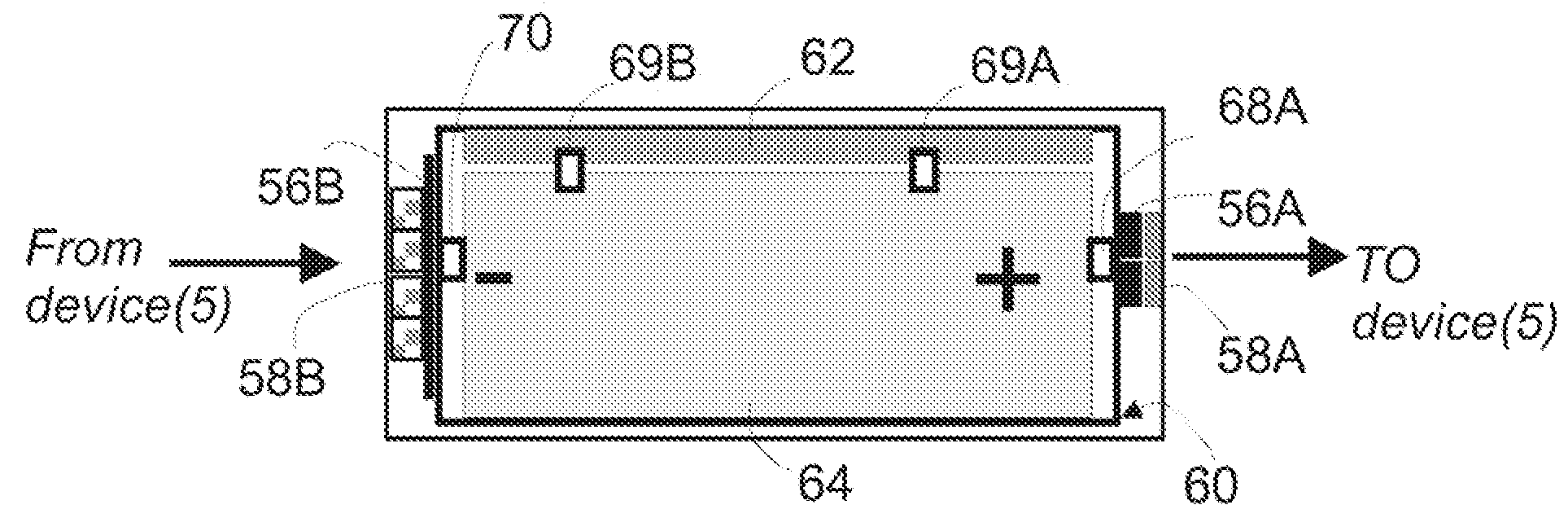
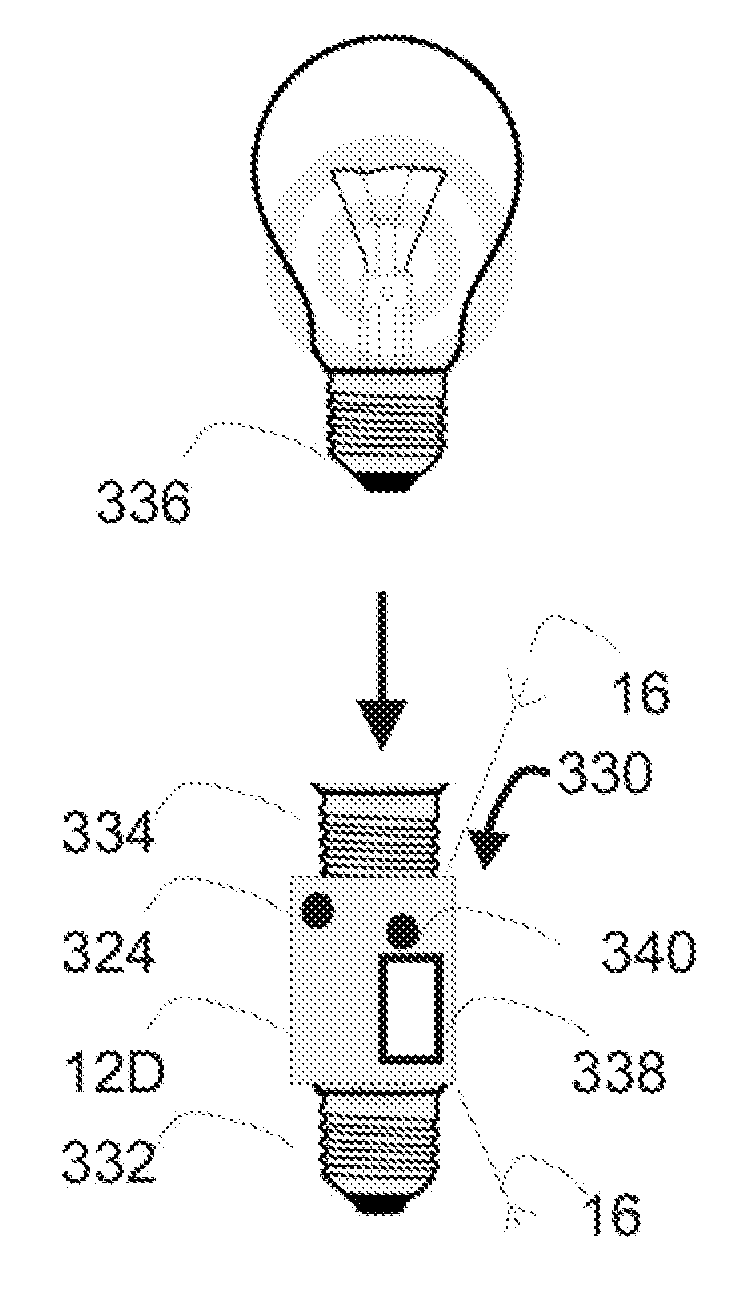
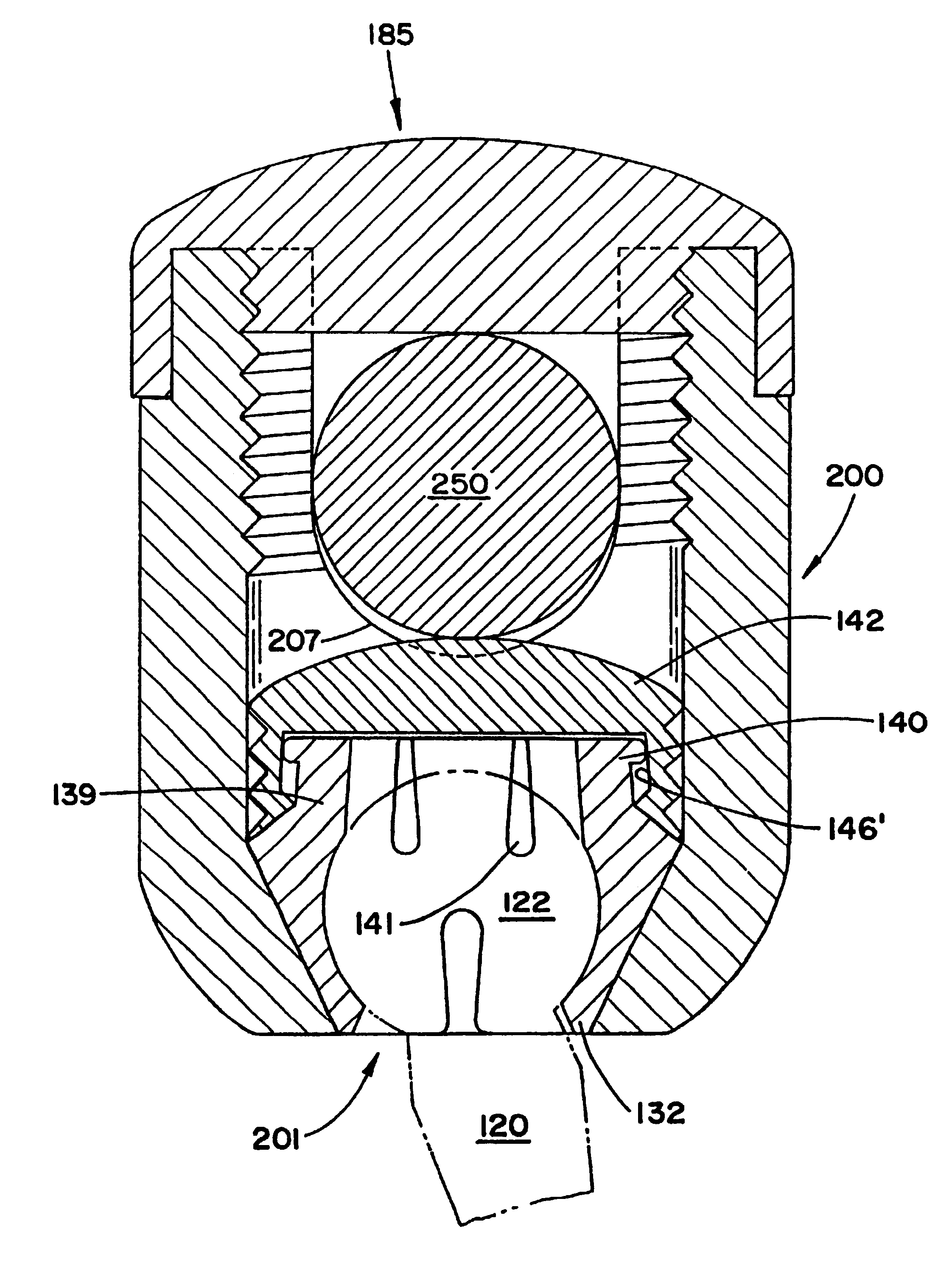
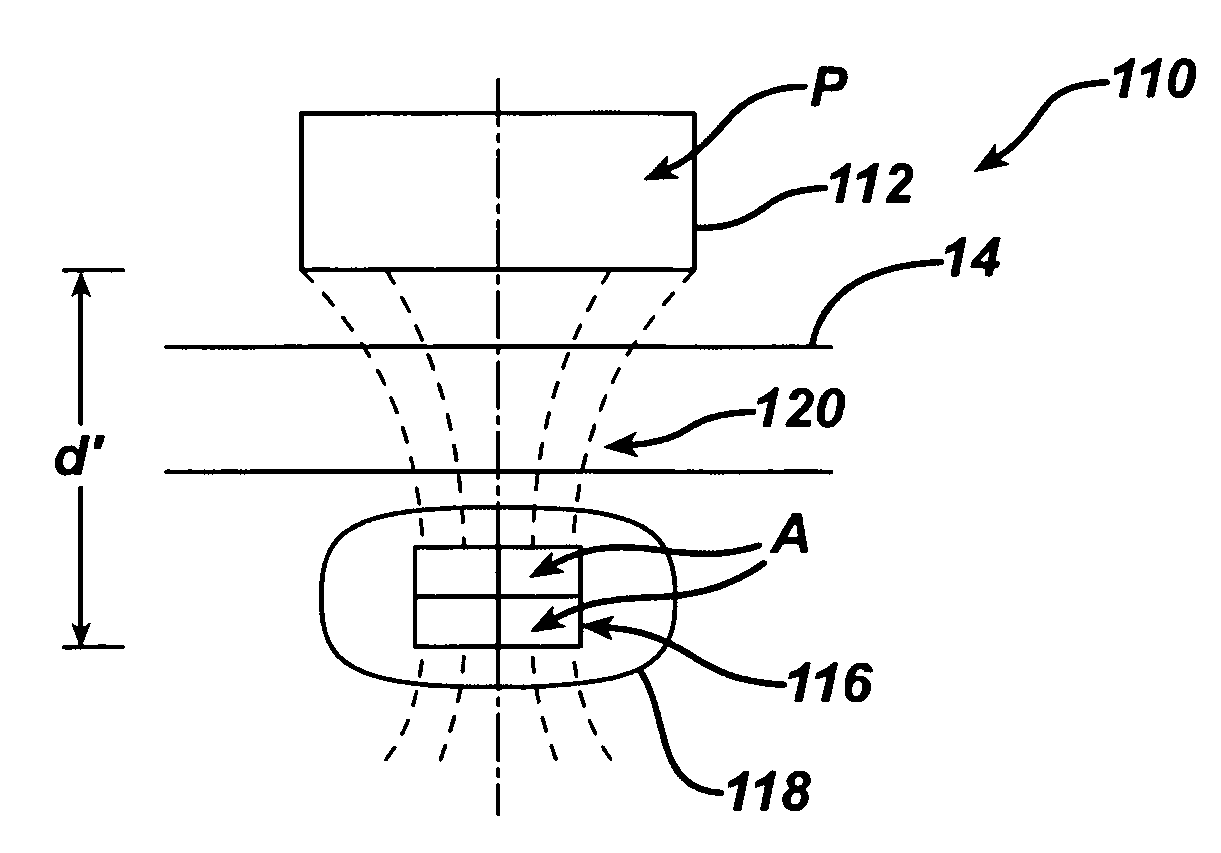
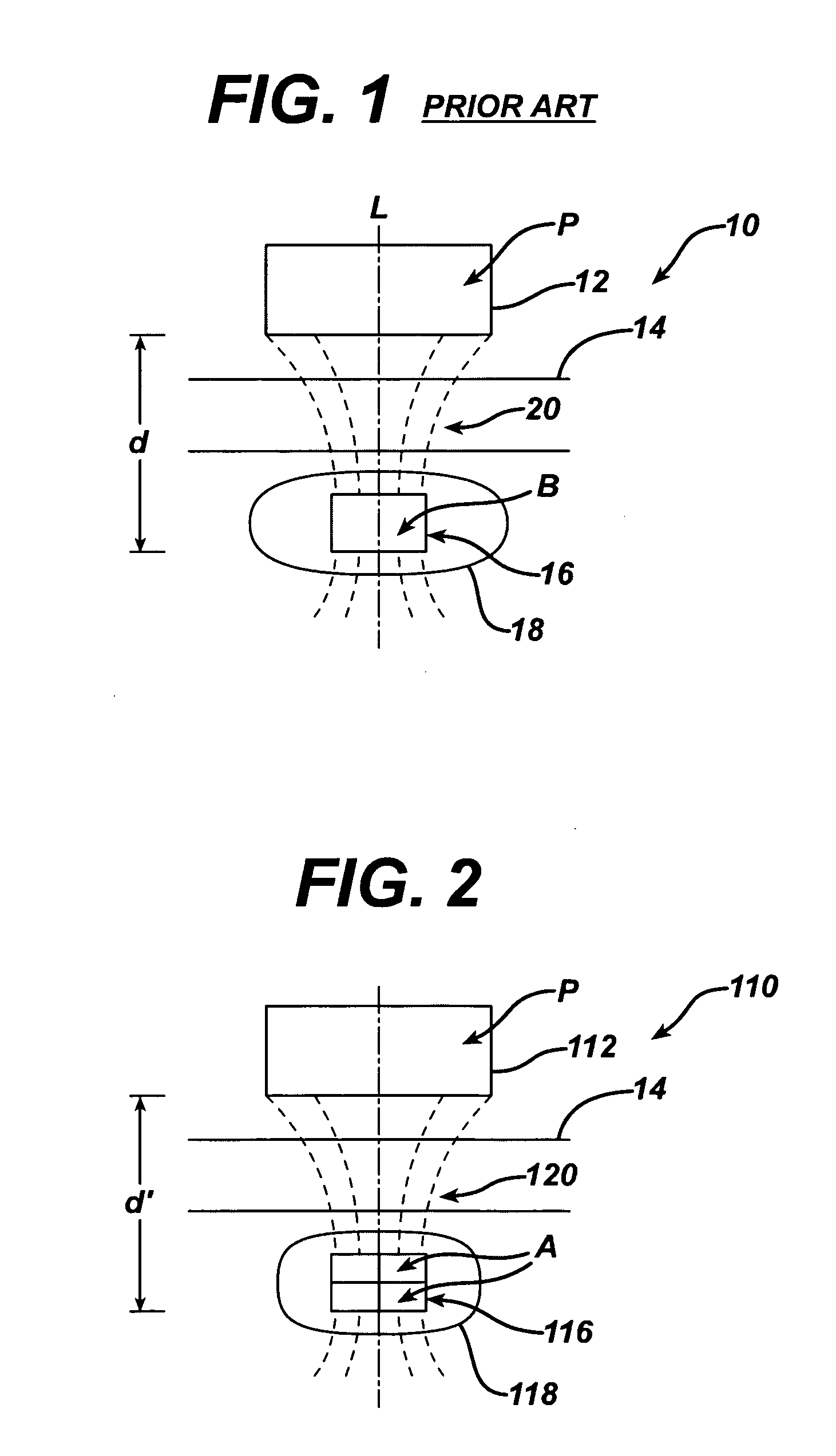
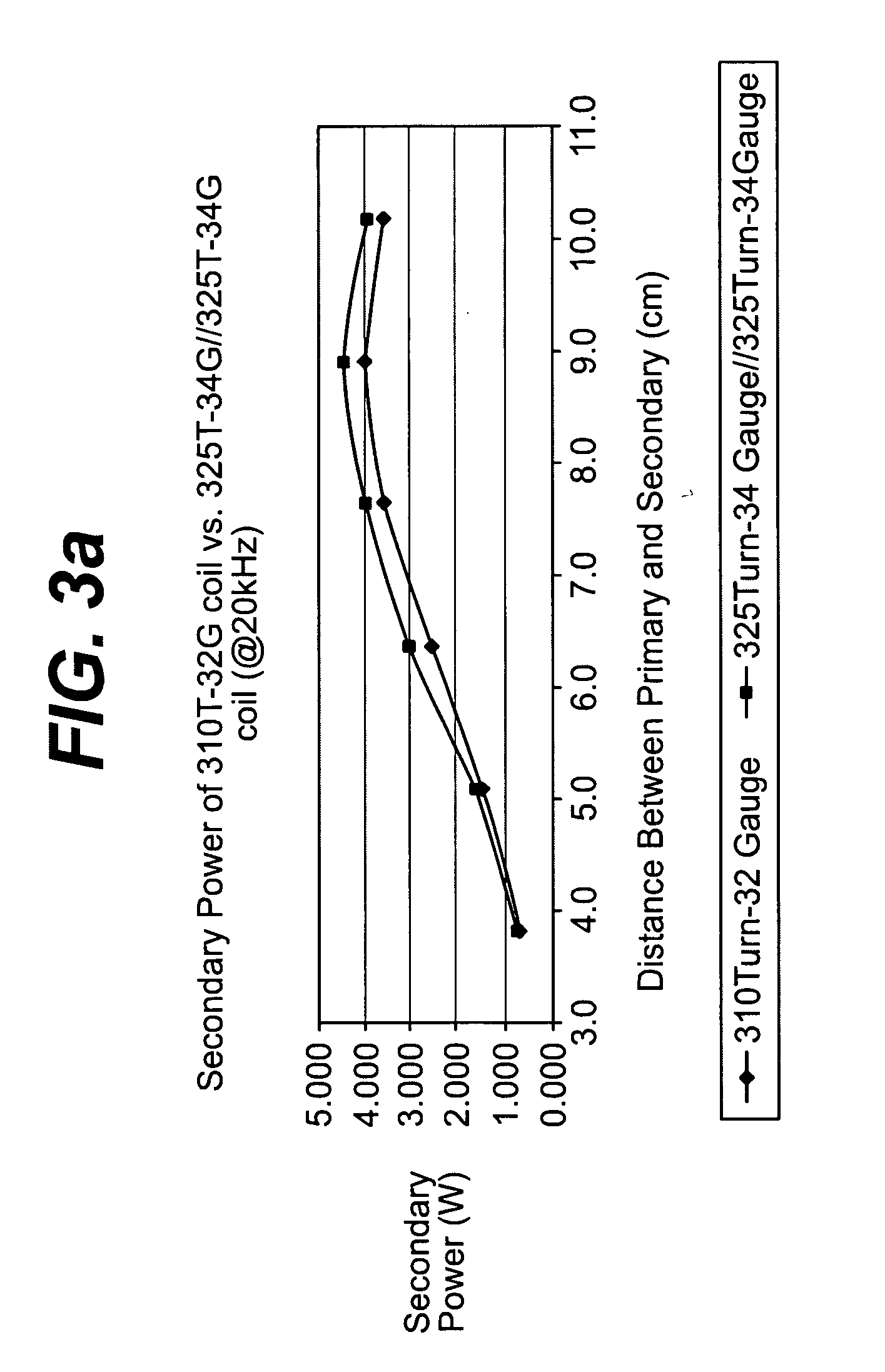
Spatially decoupled twin secondary coils for optimizing transcutaneous energy transfer (TET) power transfer characteristics
ActiveUS7191007B2Raise transfer toImplantation is simpleElectrotherapyConversion without intermediate conversion to dcEnergy transferImplanted device
An implantable device, such as an infuser device for bidirectional hydraulically controlling a medical artificial sphincter, enhances power transfer characteristics to a secondary coil thereby allowing implantation to greater physical depths and / or enclosing the secondary coil within a housing of the infuser device. The enhanced power transfer is achieved with multiple coils that are longitudinally aligned and physical and electrical parallel to form the secondary loop of a transcutaneous energy transfer system (TET) instead of a single coil. It better optimizes the power transfer from a parallel tuned tank circuit primary coil to an implanted secondary series tuned tank circuit coil.
Owner:ETHICON ENDO SURGERY INC
Total joint arthroplasty system
ActiveUS20090131941A1Precise alignmentImprove visualizationMedical simulationProgramme controlJoint arthroplastyTotal hip arthroplasty
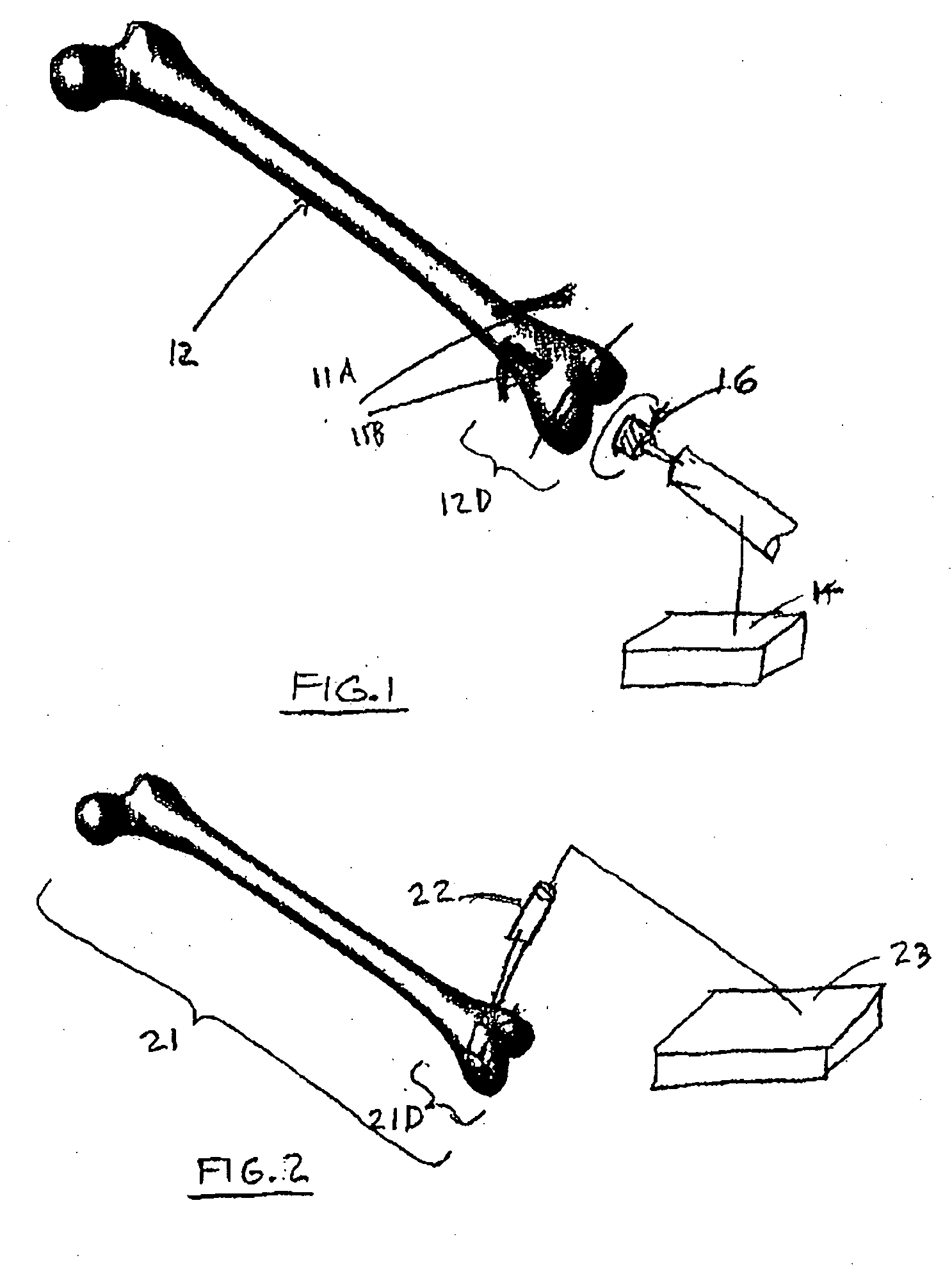
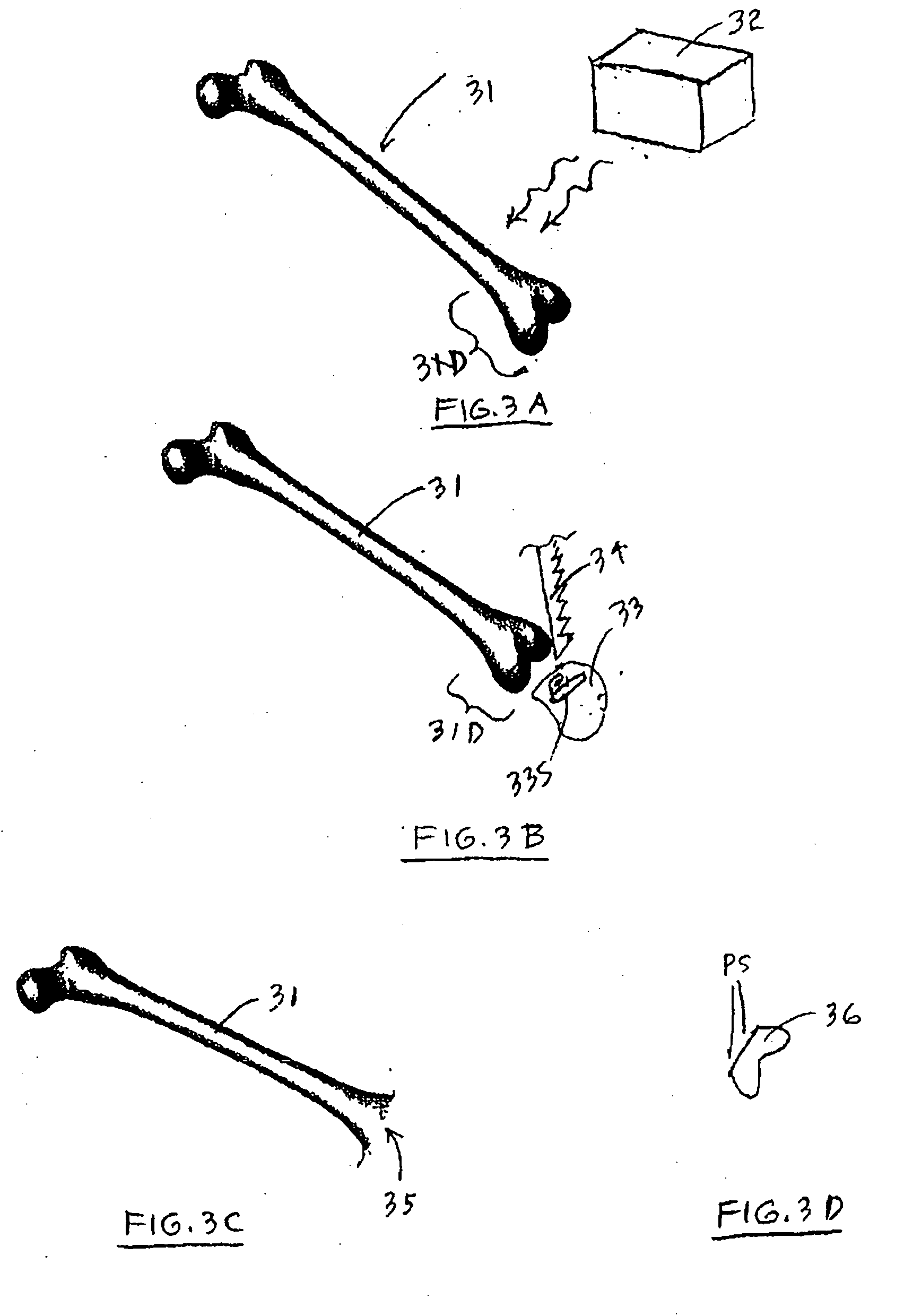
A method and system for performing a total joint arthroplasty procedure on a patient's damaged bone region. A CT image or other suitable image is formed of the damaged bone surfaces, and location coordinate values (xn,yn,zn) are determined for a selected sequence of bone surface locations using the CT image data. A mathematical model z=f(x,y) of a surface that accurately matches the bone surface coordinates at the selected bone spice locations, or matches surface normal vector components at selected bone surface locations, is determined. The model provides a production file from which a cutting jig and an implant device (optional), each patient-specific and having controllable alignment, are fabricated for the damaged bone by automated processing. At this point, the patient is cut open (once), the cutting jig and a cutting instrument are used to remove a selected portion of the bone and to provide an exposed planar surface, the implant device is optionally secured to and aligned with the remainder of the bone, and the patient's incision is promptly repaired.
Owner:HOWMEDICA OSTEONICS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com