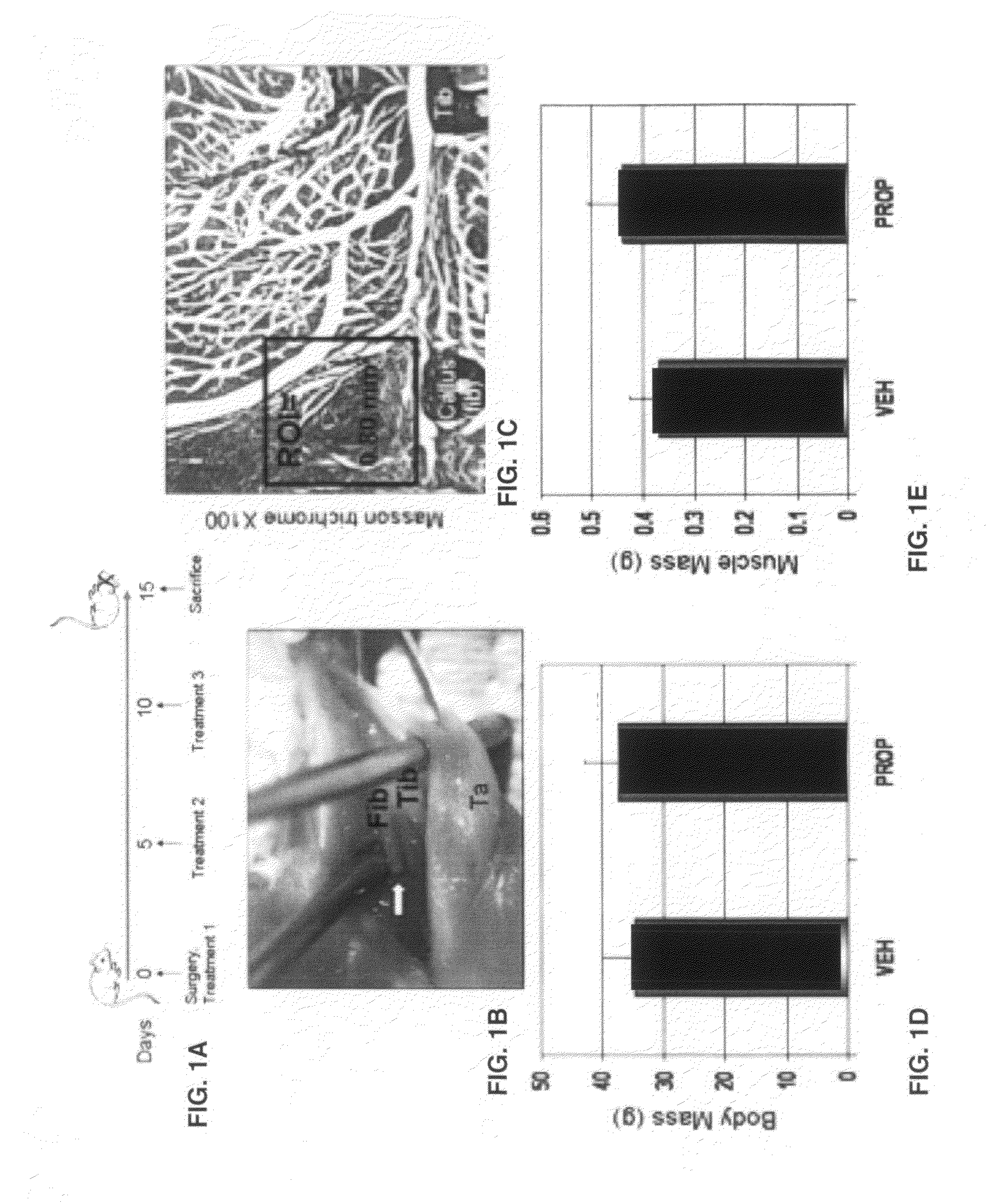
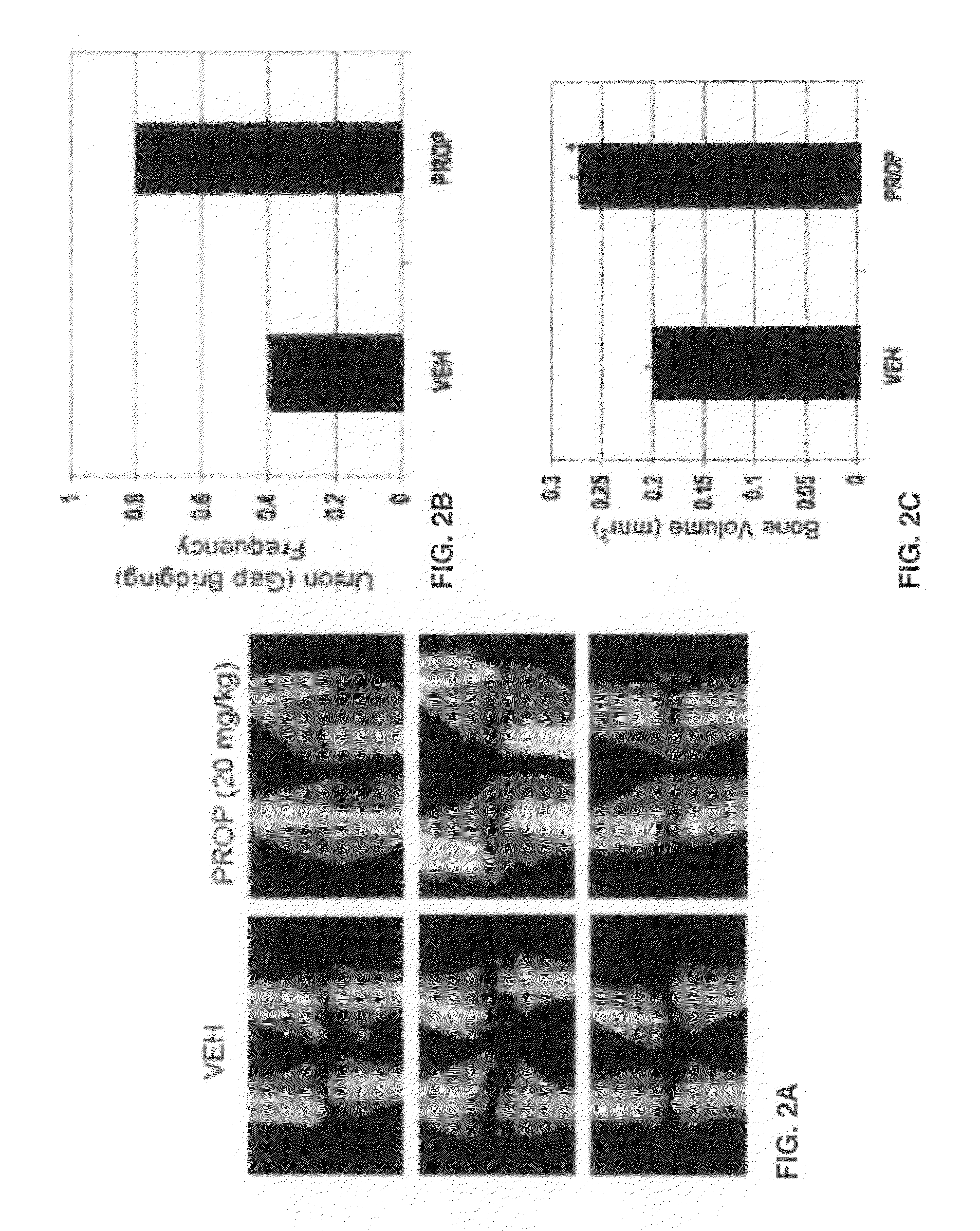
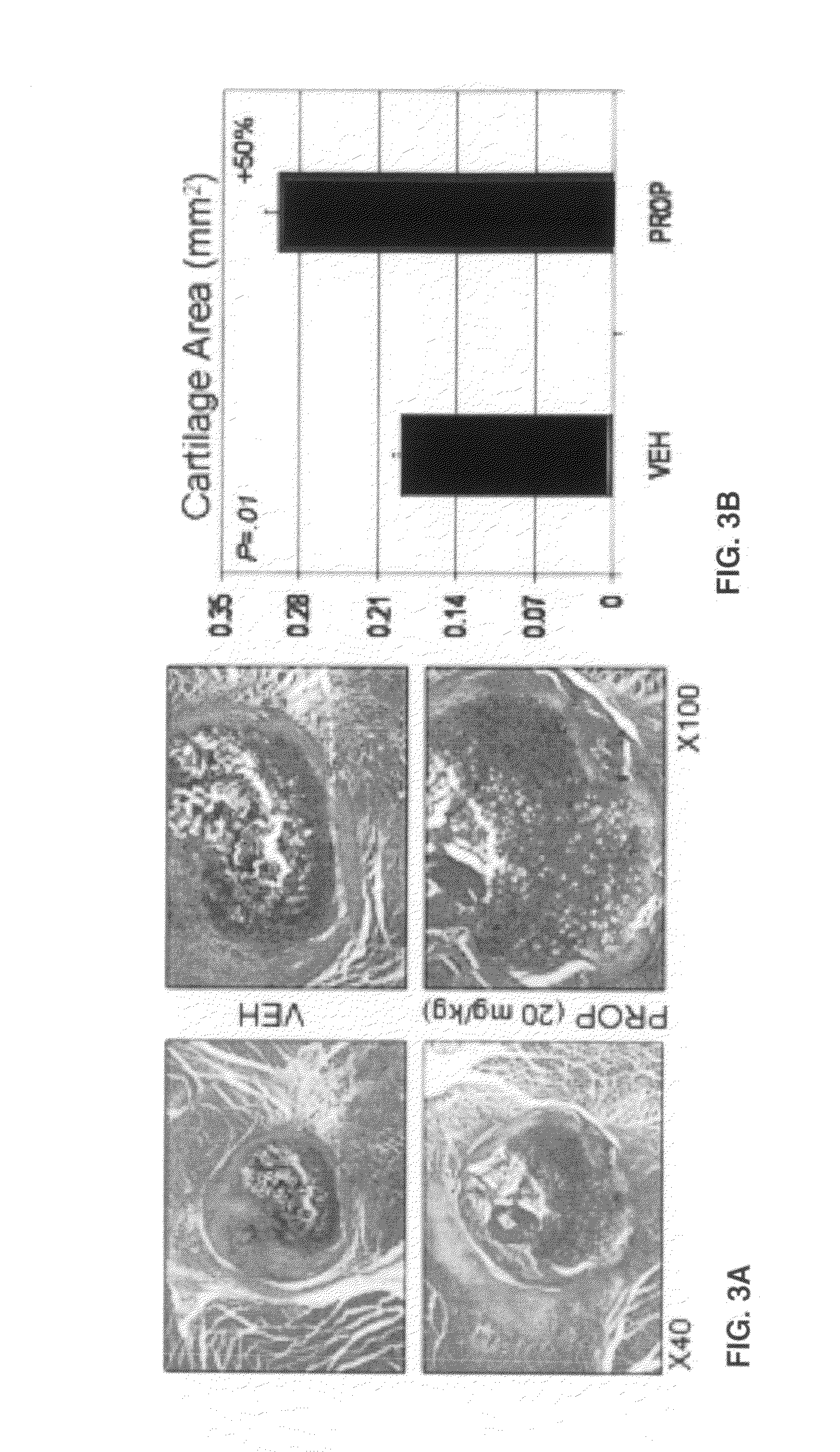
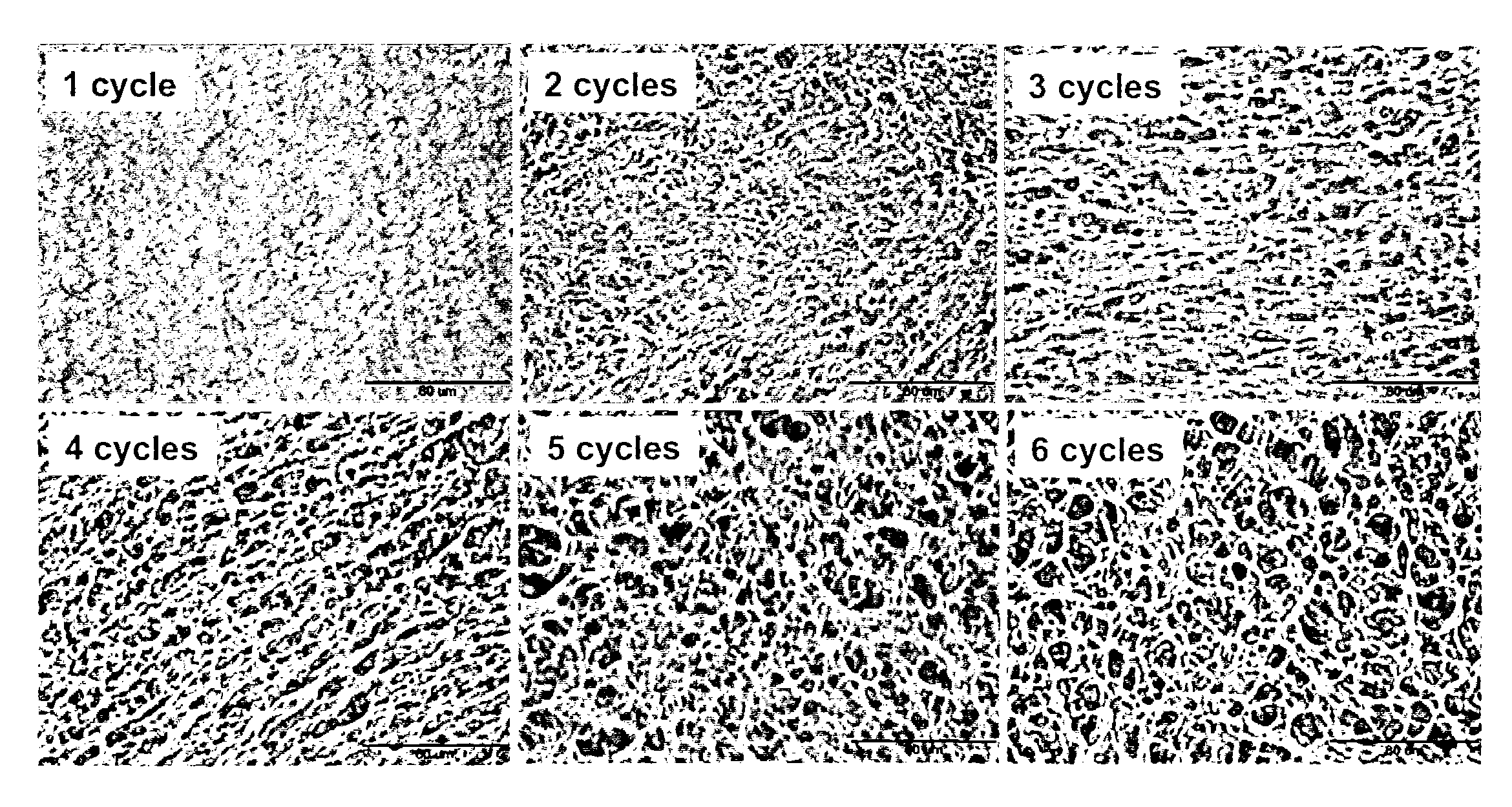
Patents
Literature
42 results about "Musculoskeletal tissue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
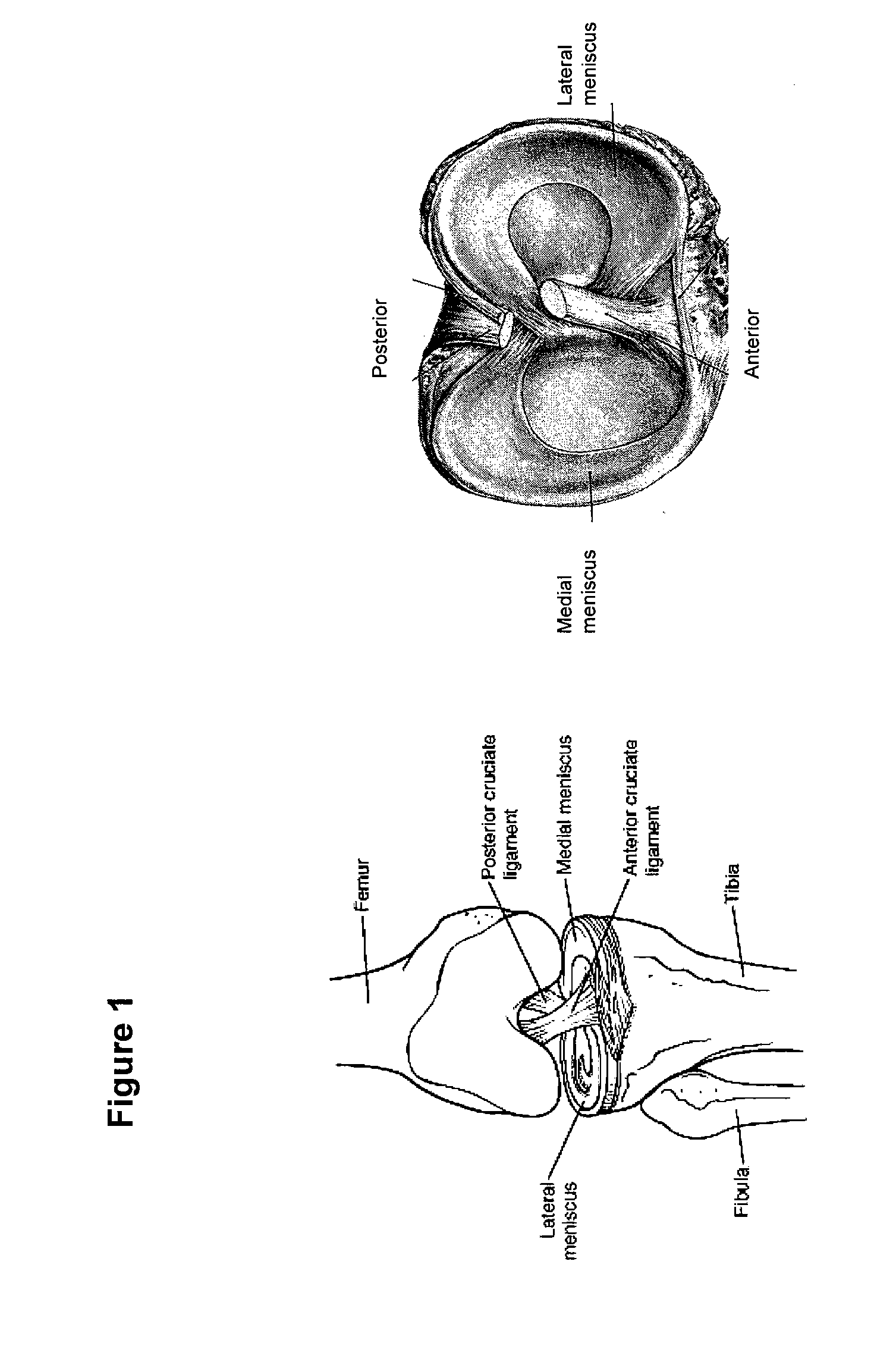
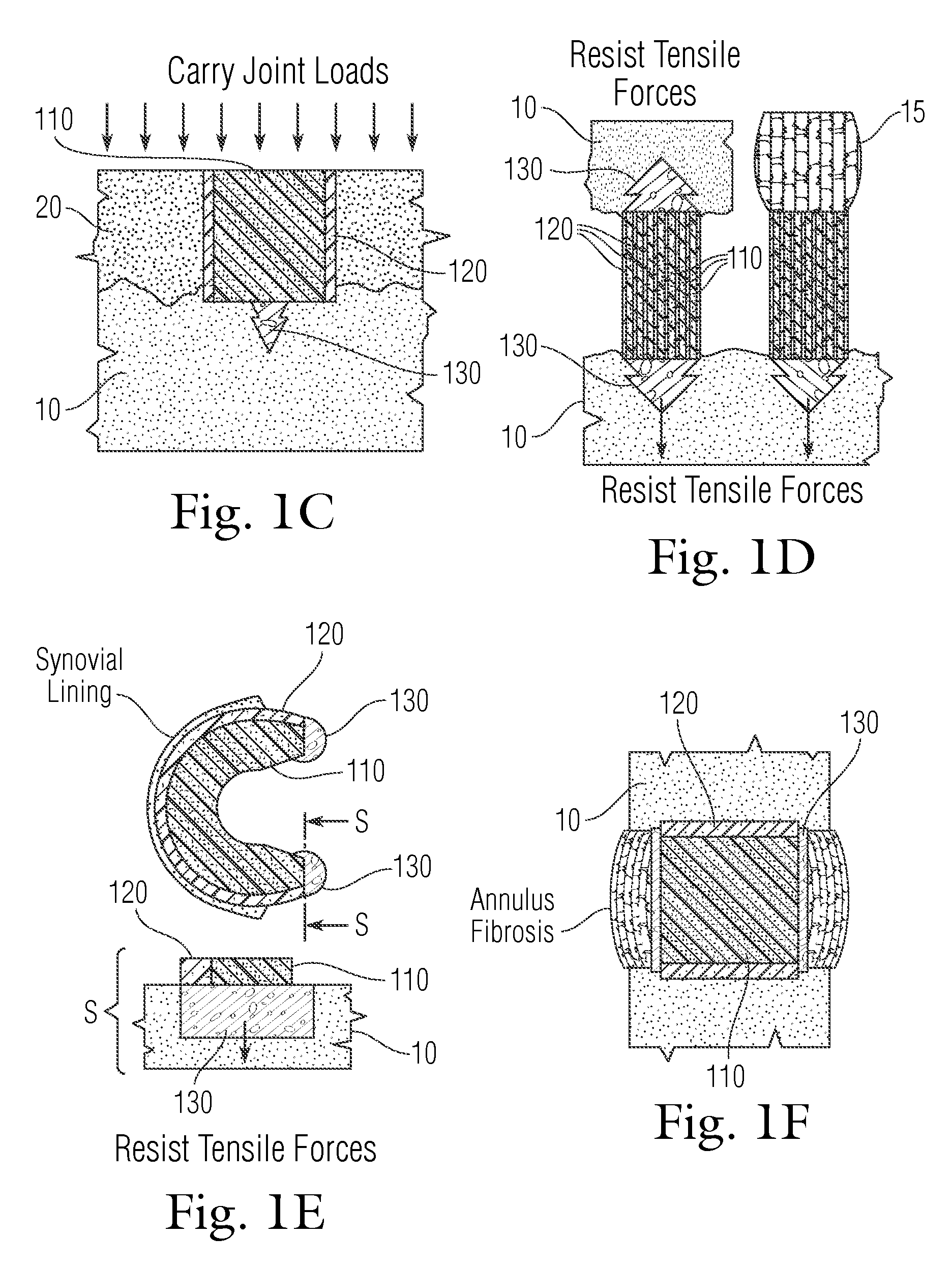
Musculoskeletal tissue includes all the tissue types in the human body that support the bone structure and form of the body directly, such as tendons, ligaments, and cartilage. They also include types of tissue that give shape to key features, such as collagen and surface muscle groups, and bone itself.
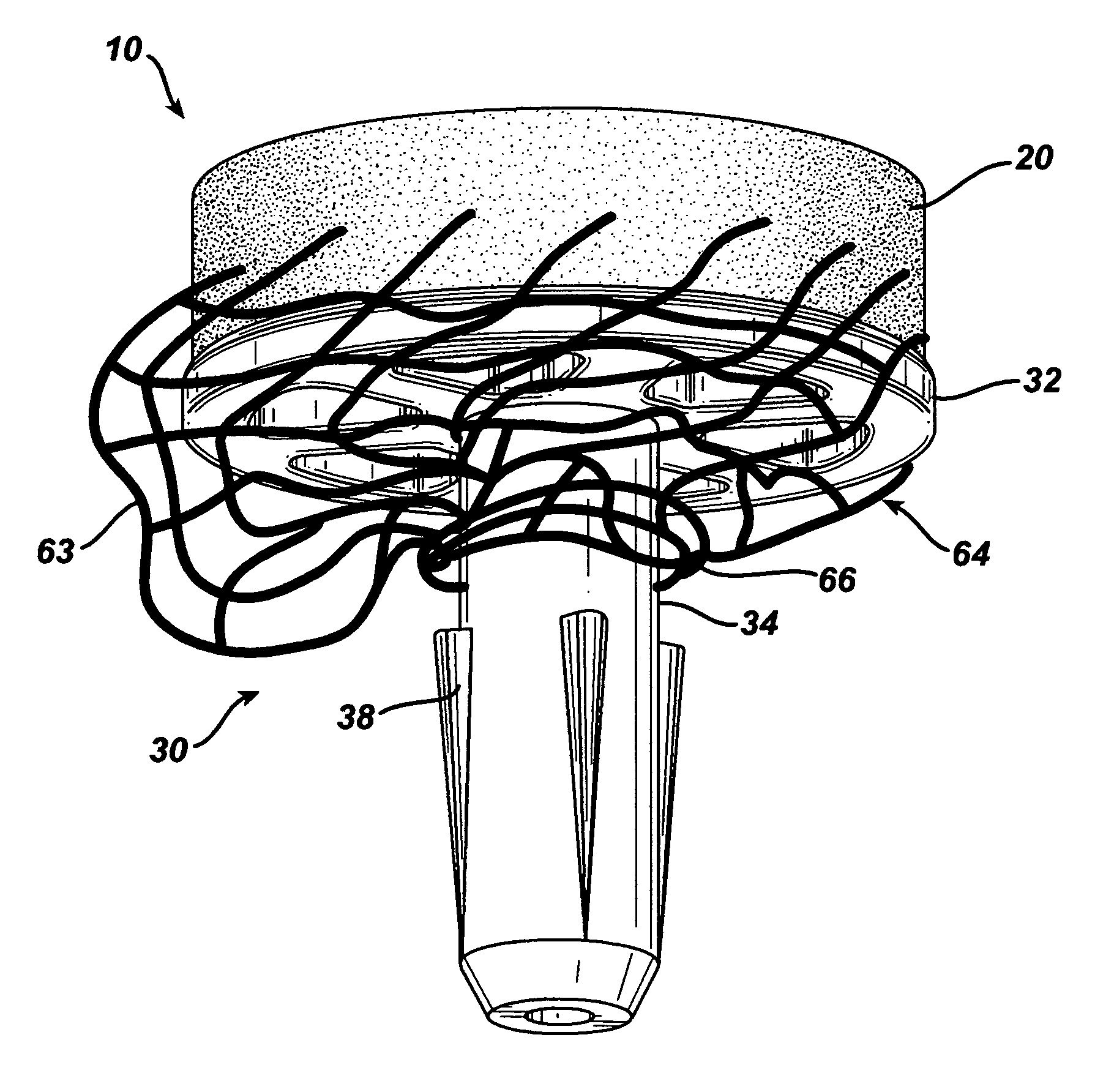
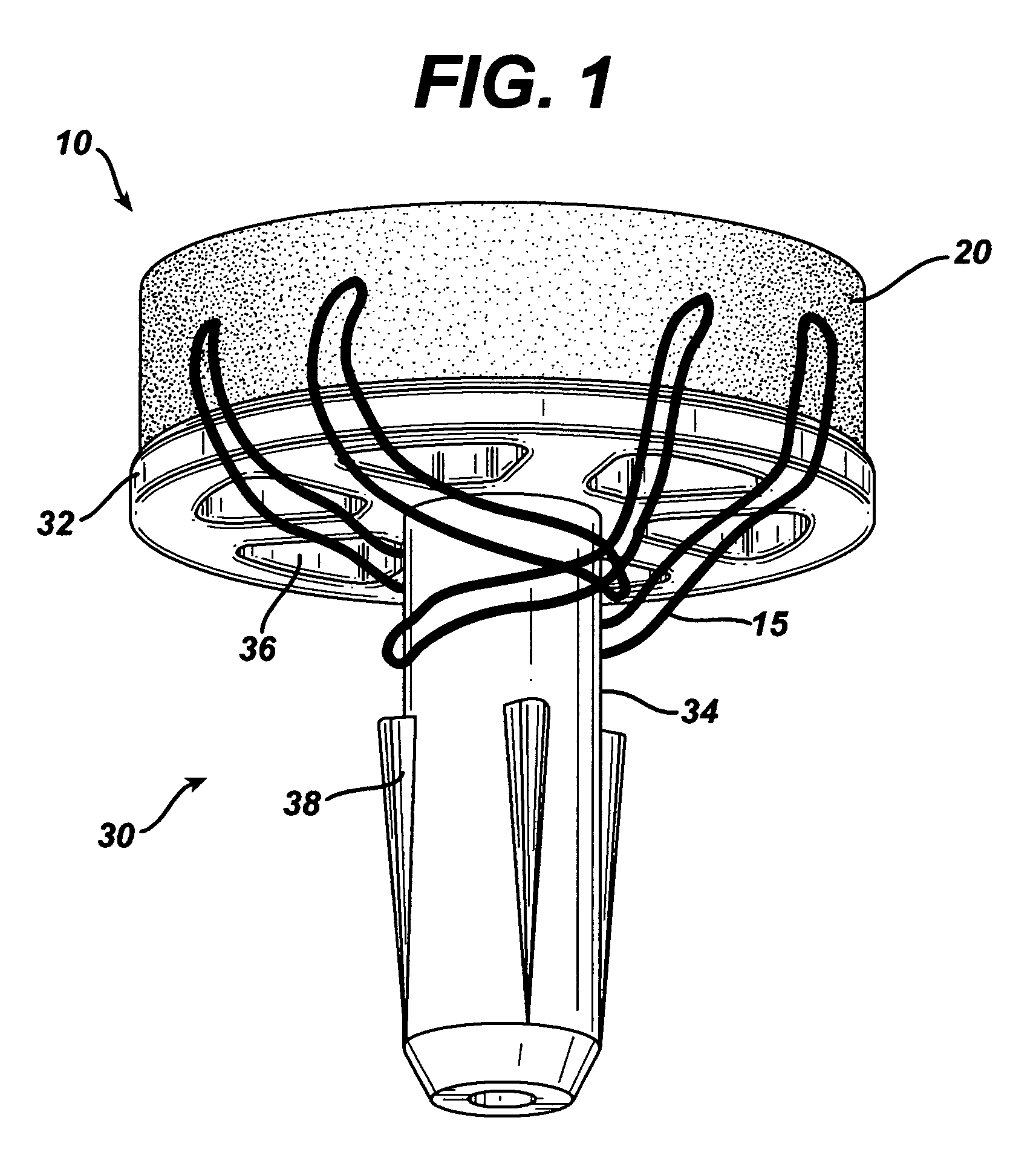
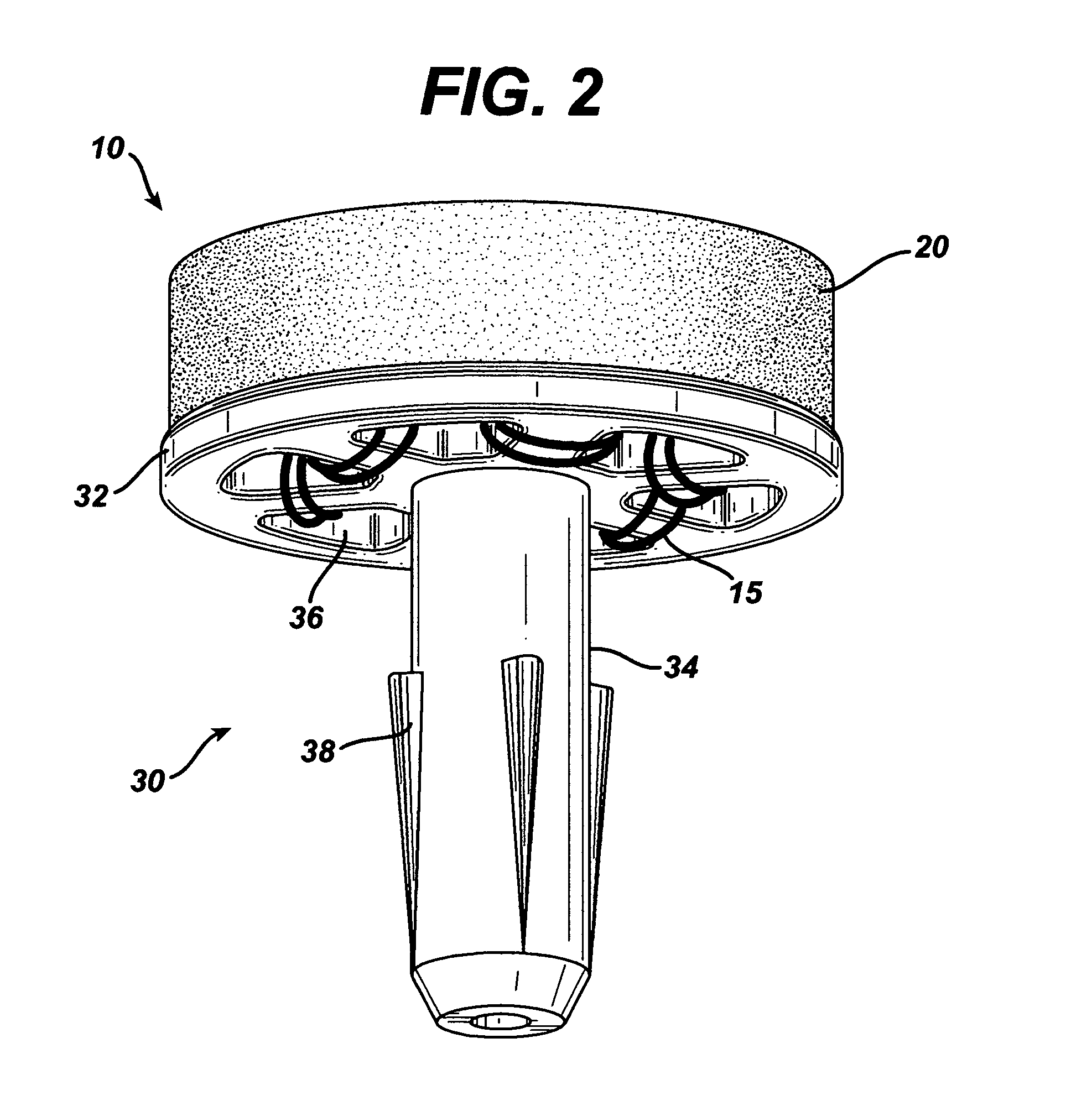
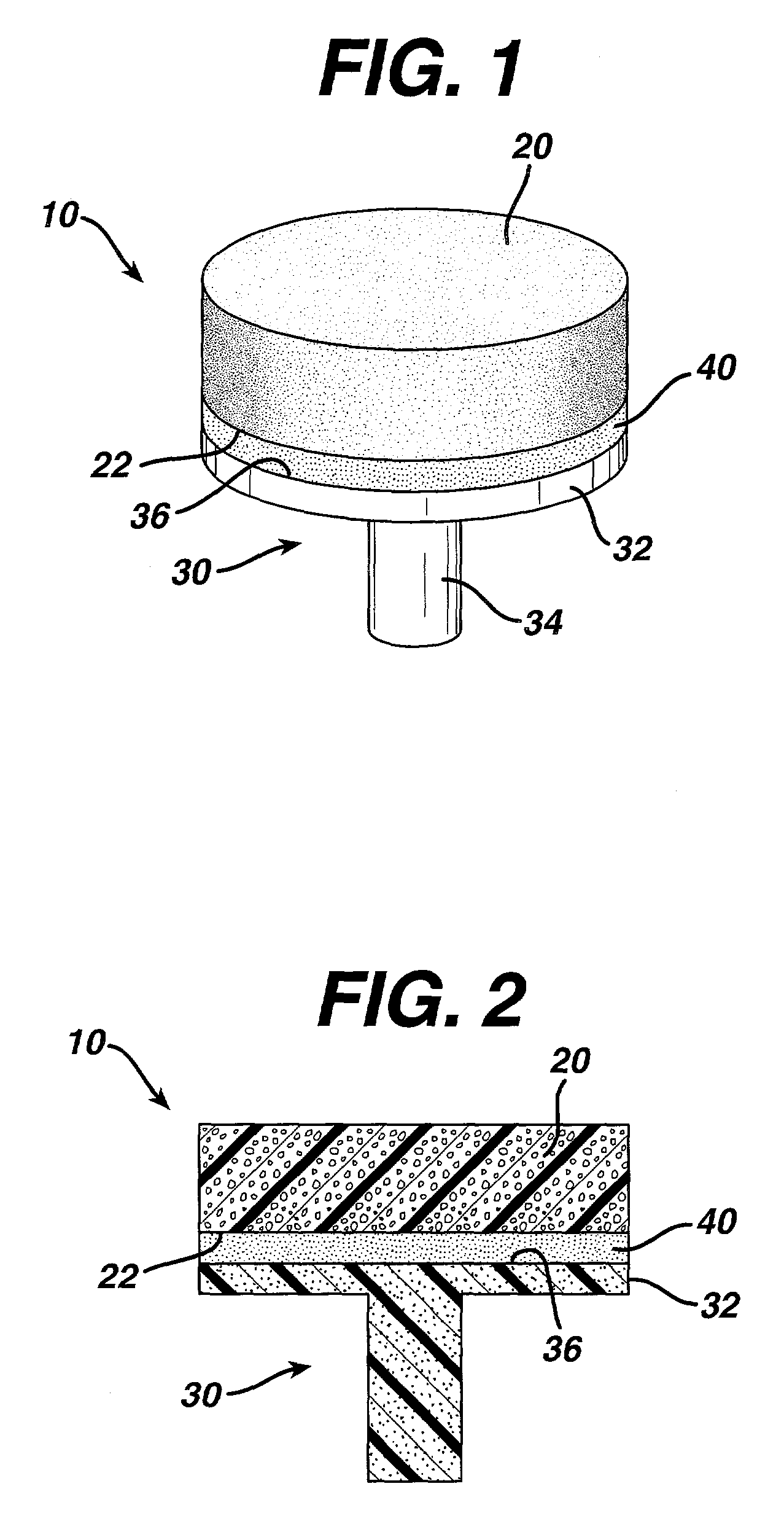
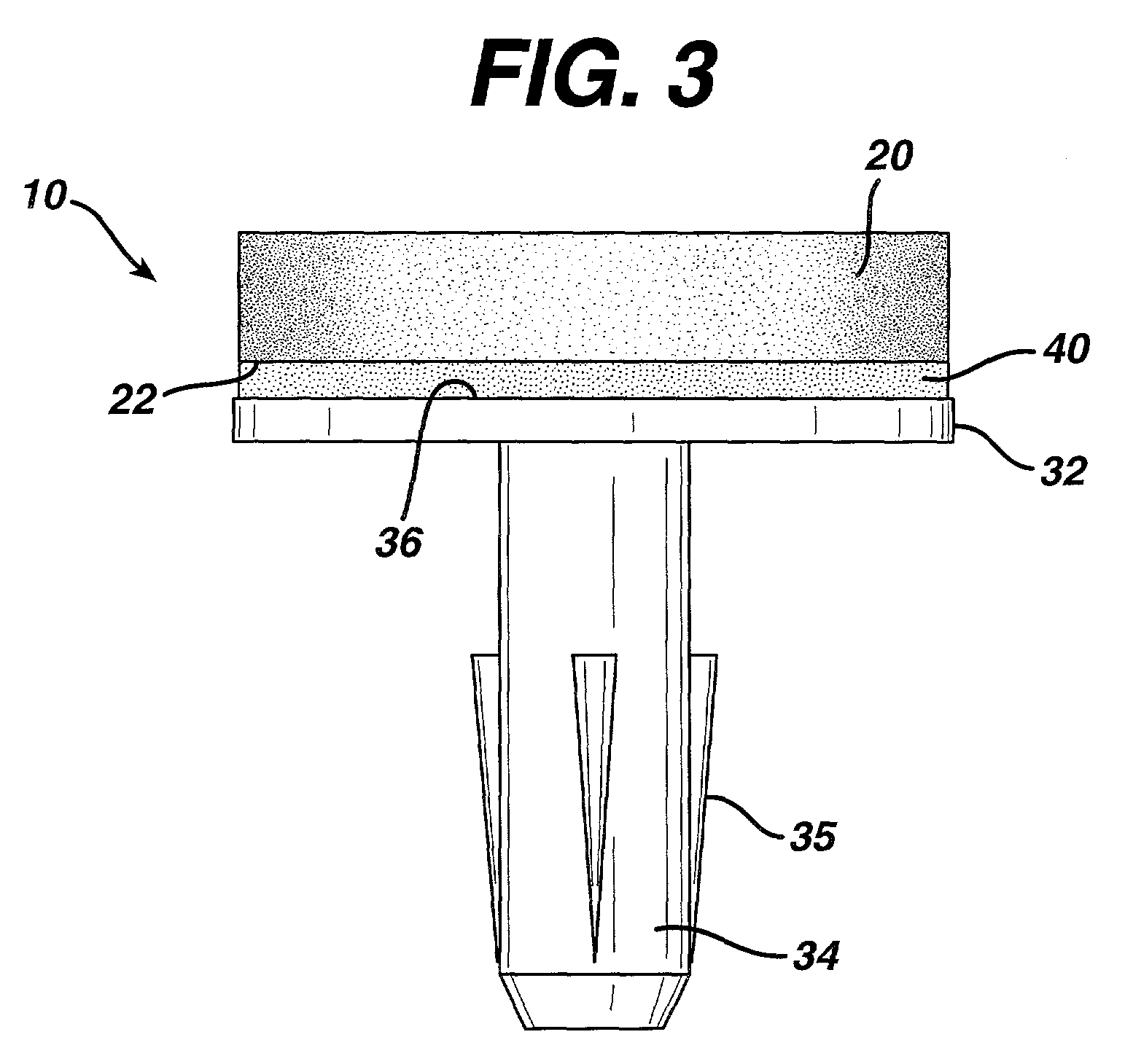
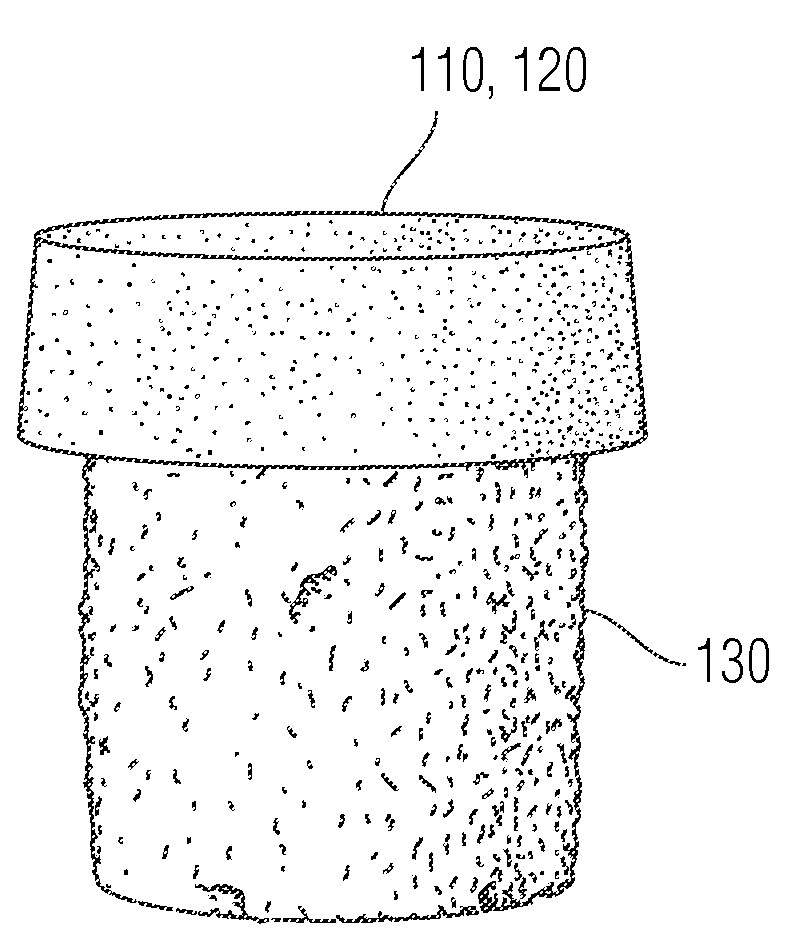
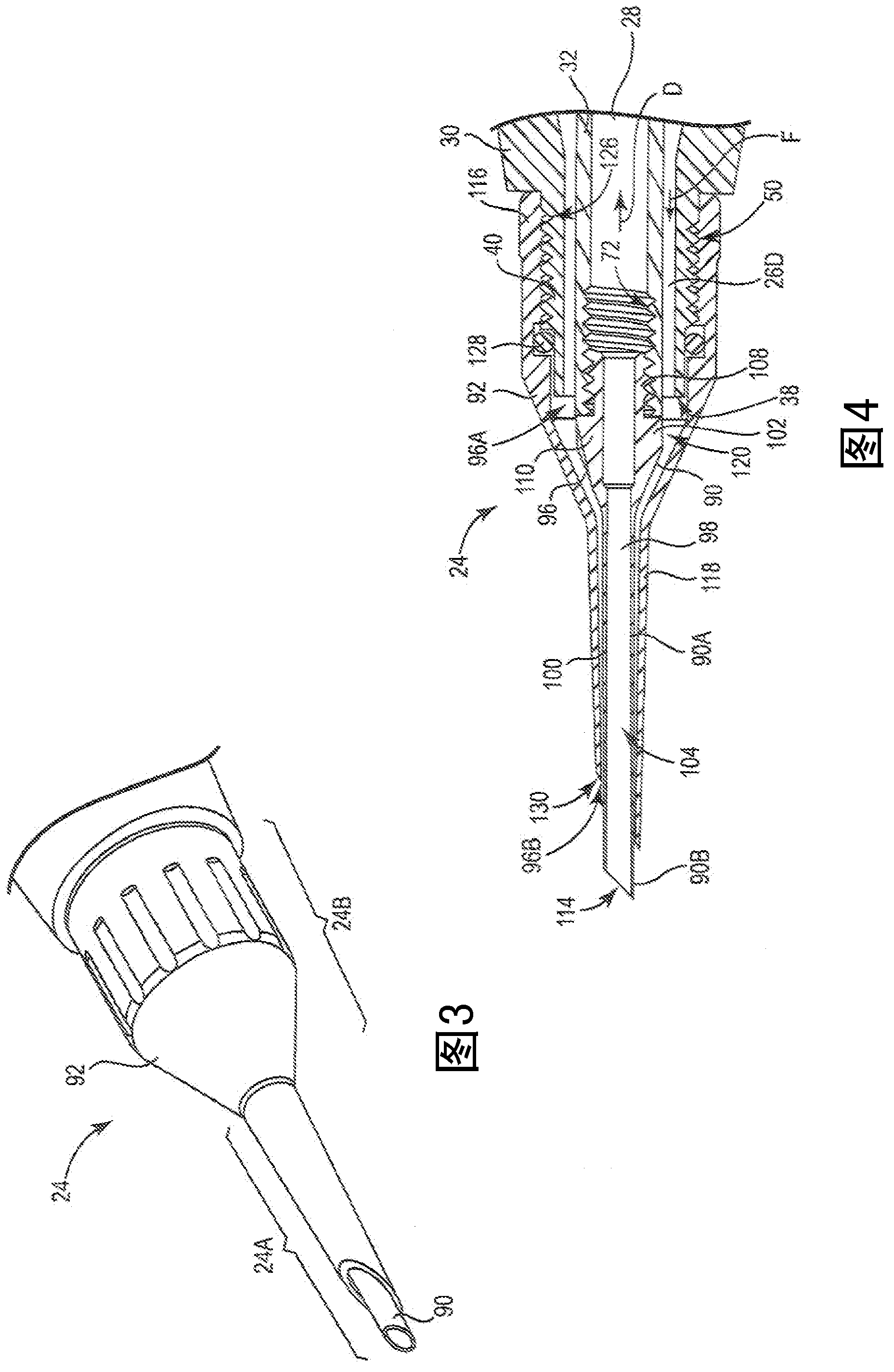
Attachment of absorbable tissue scaffolds to fixation devices
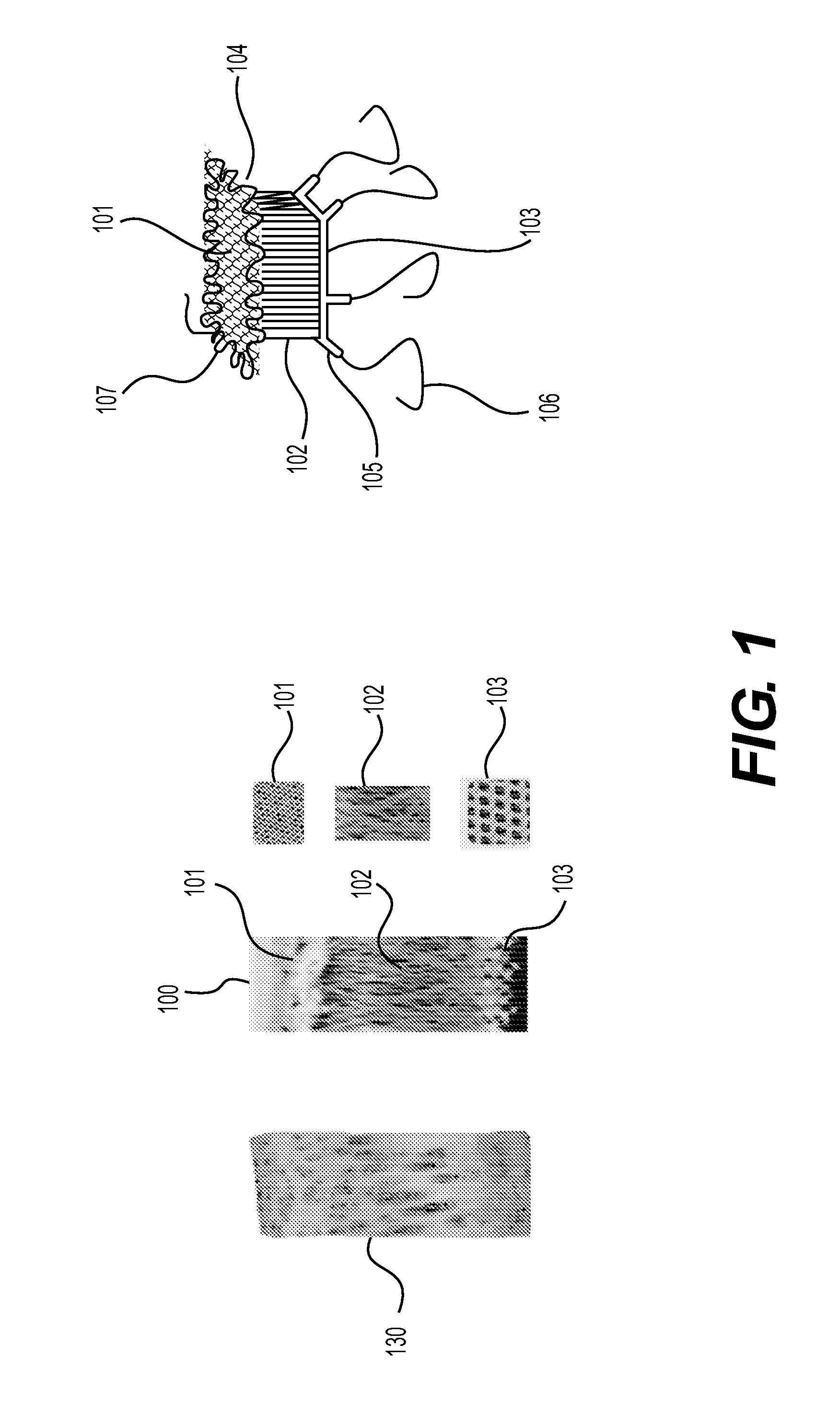
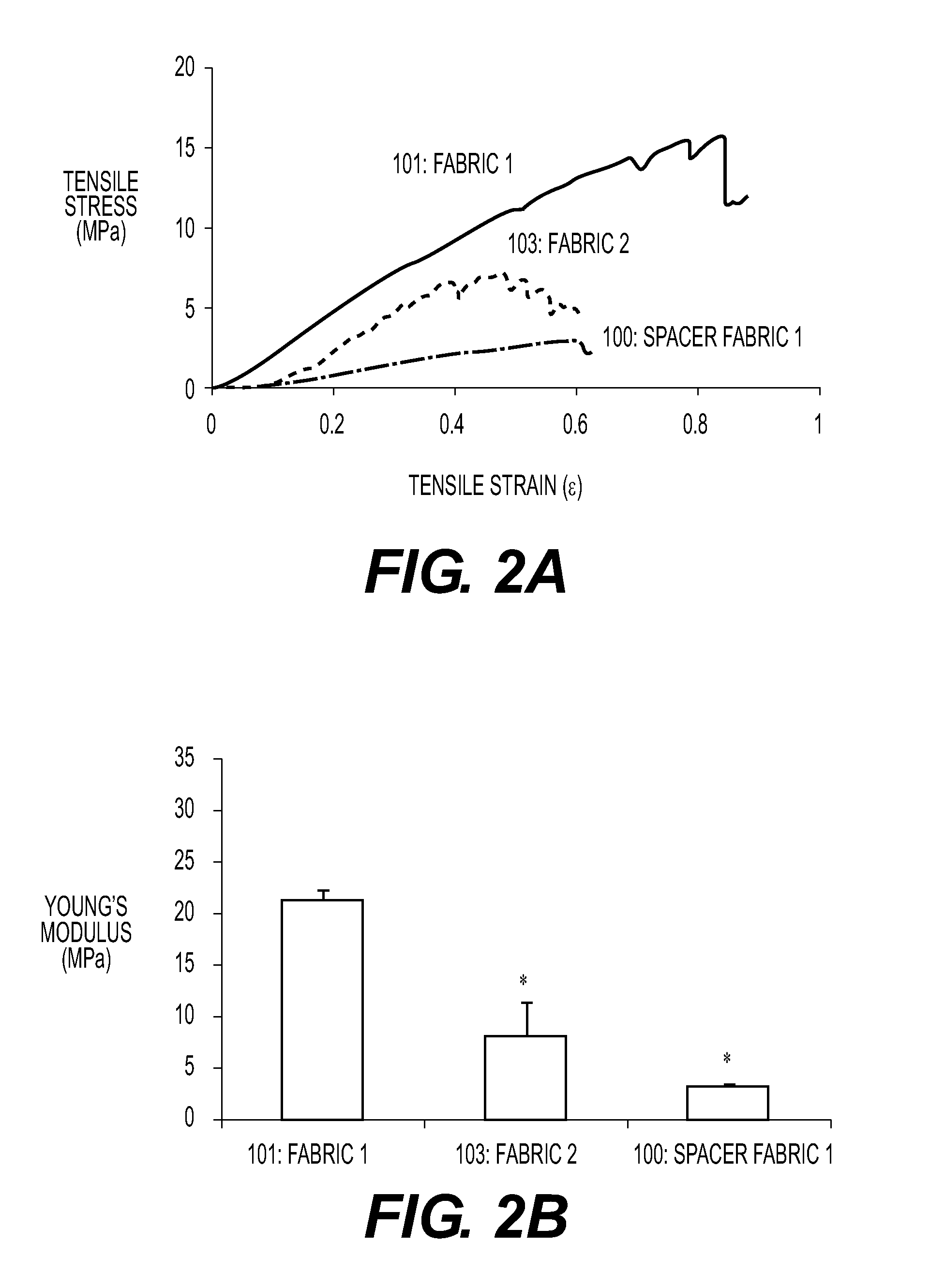
The present invention relates to tissue scaffold implant devices useful in the repair and / or regeneration of diseased and / or damaged musculoskeletal tissue and that include a tissue scaffold component fixedly attached to a scaffold fixation component via at least one of sutures, fabrics, fibers, threads, elastomeric bands, reinforcing elements and interlocking protrusions for engaging and maintaining the scaffold component fixedly attached to the fixation component.
Owner:ETHICON INC
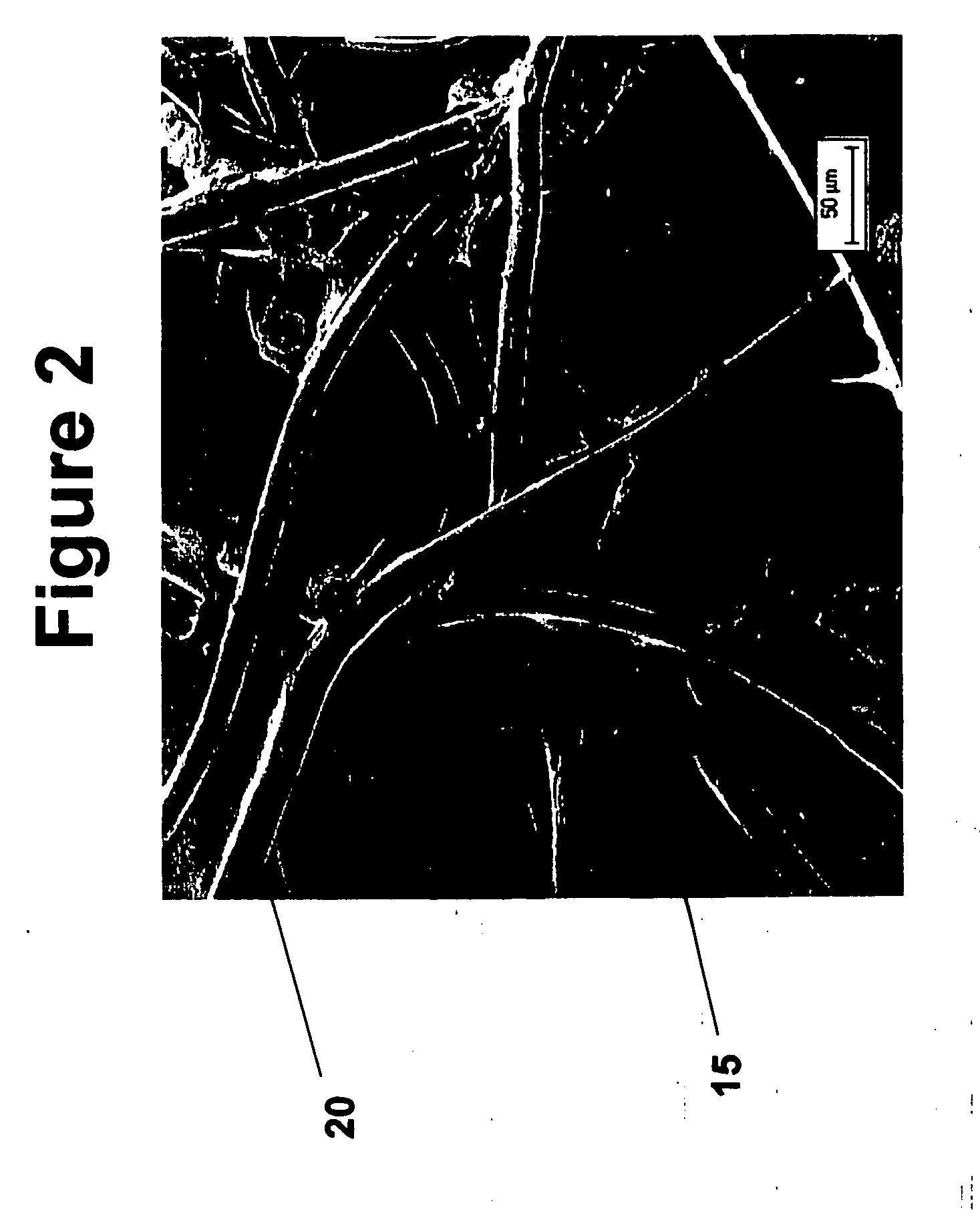
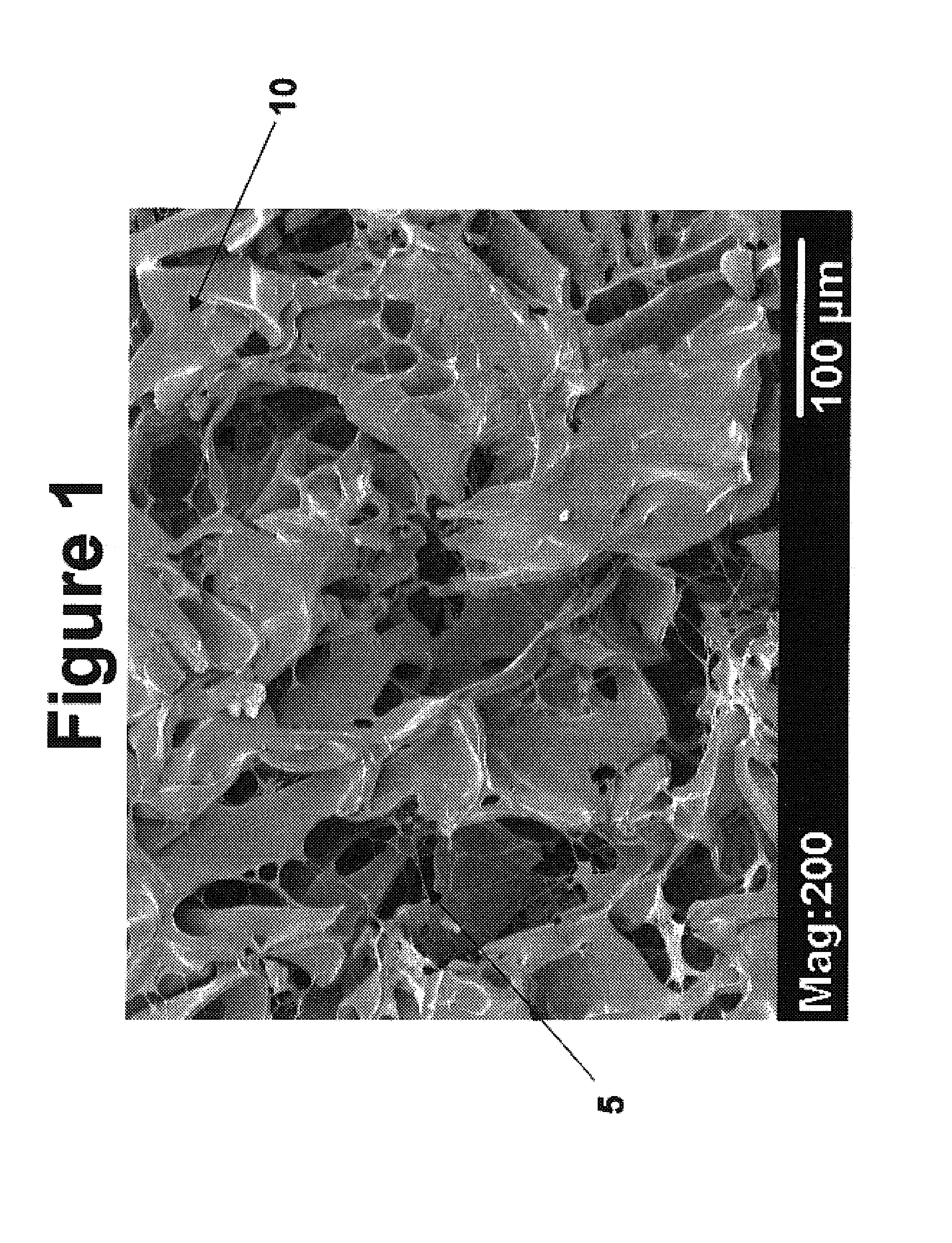
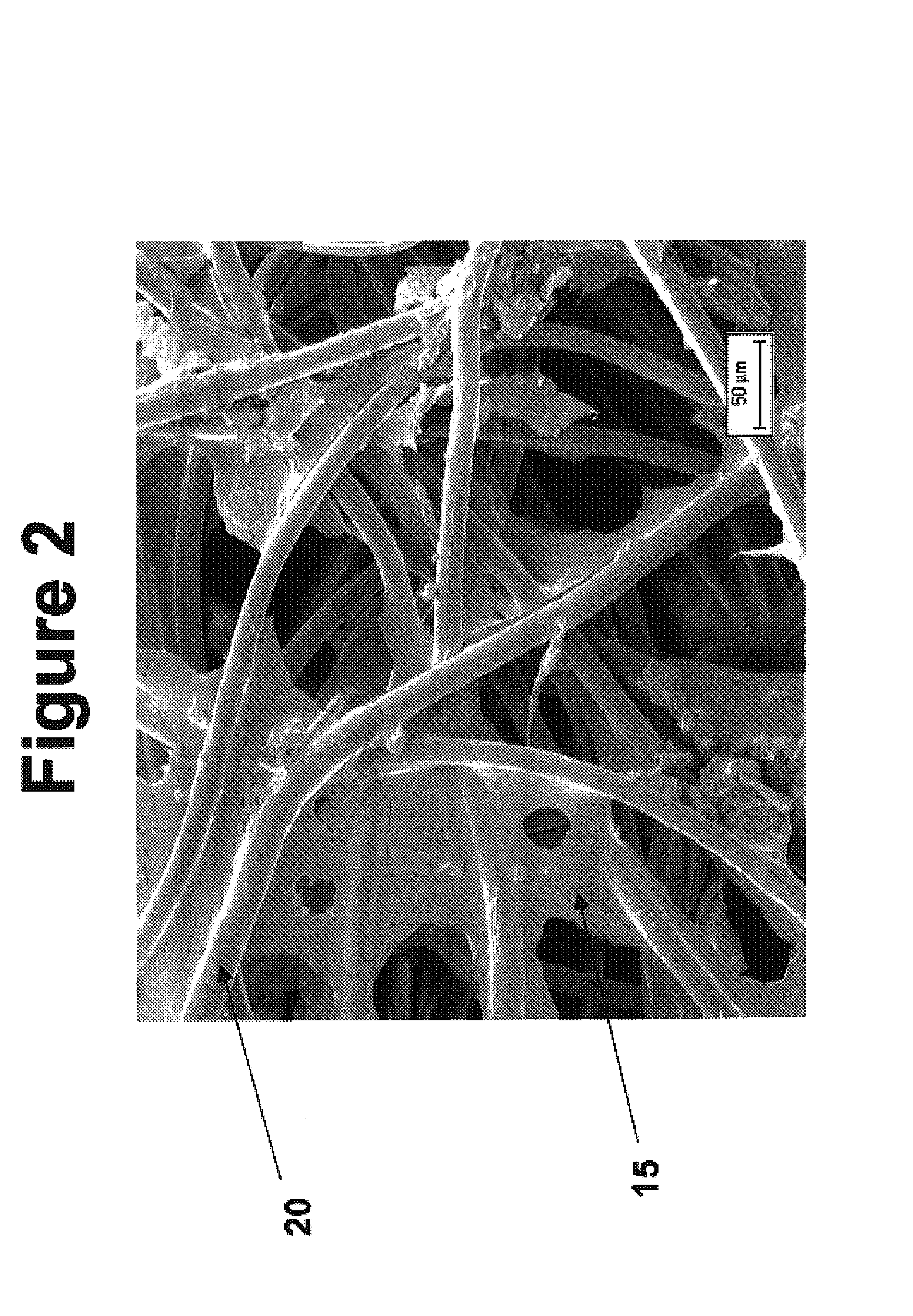
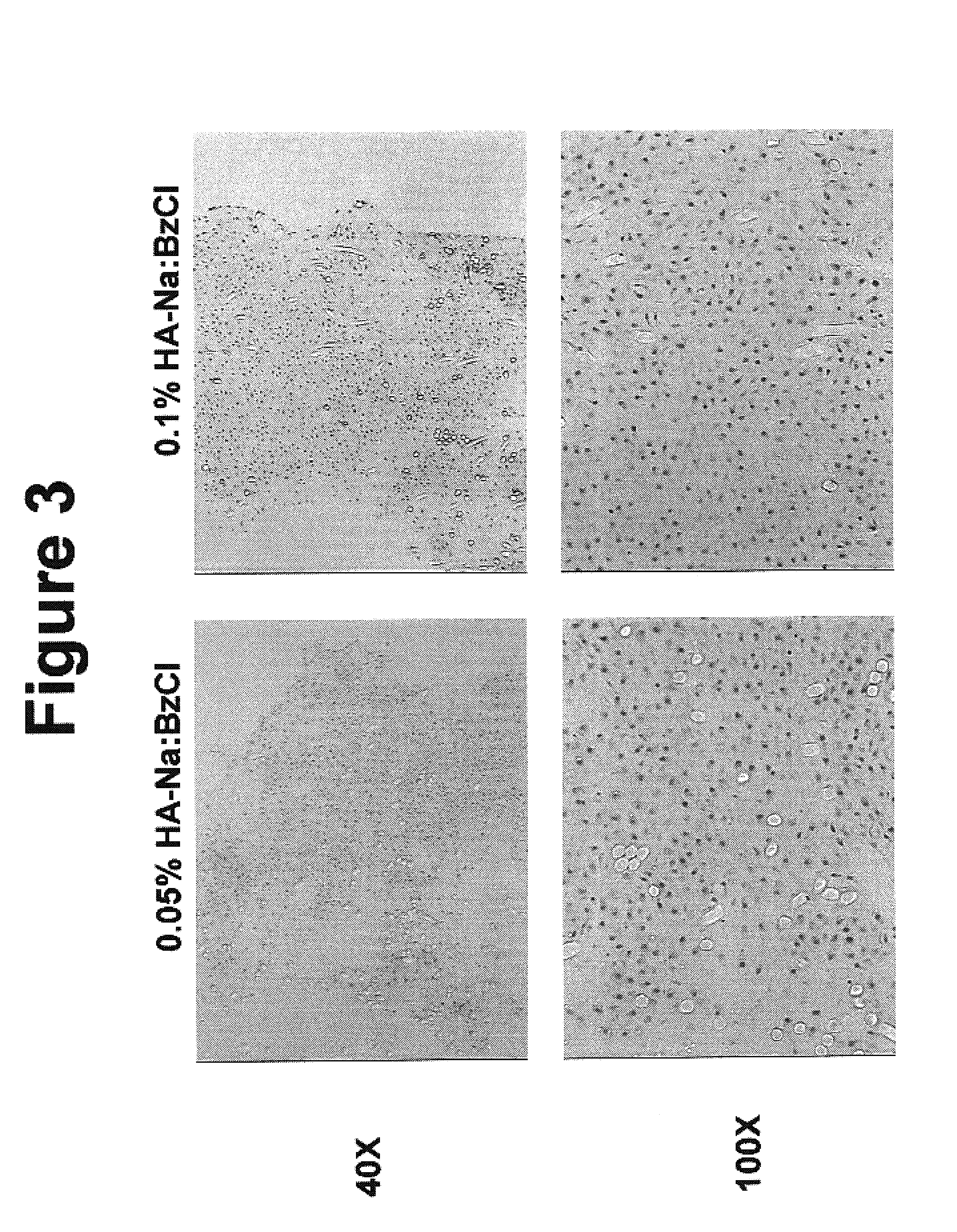
Modified hyaluronic acid for use in musculoskeletal tissue repair
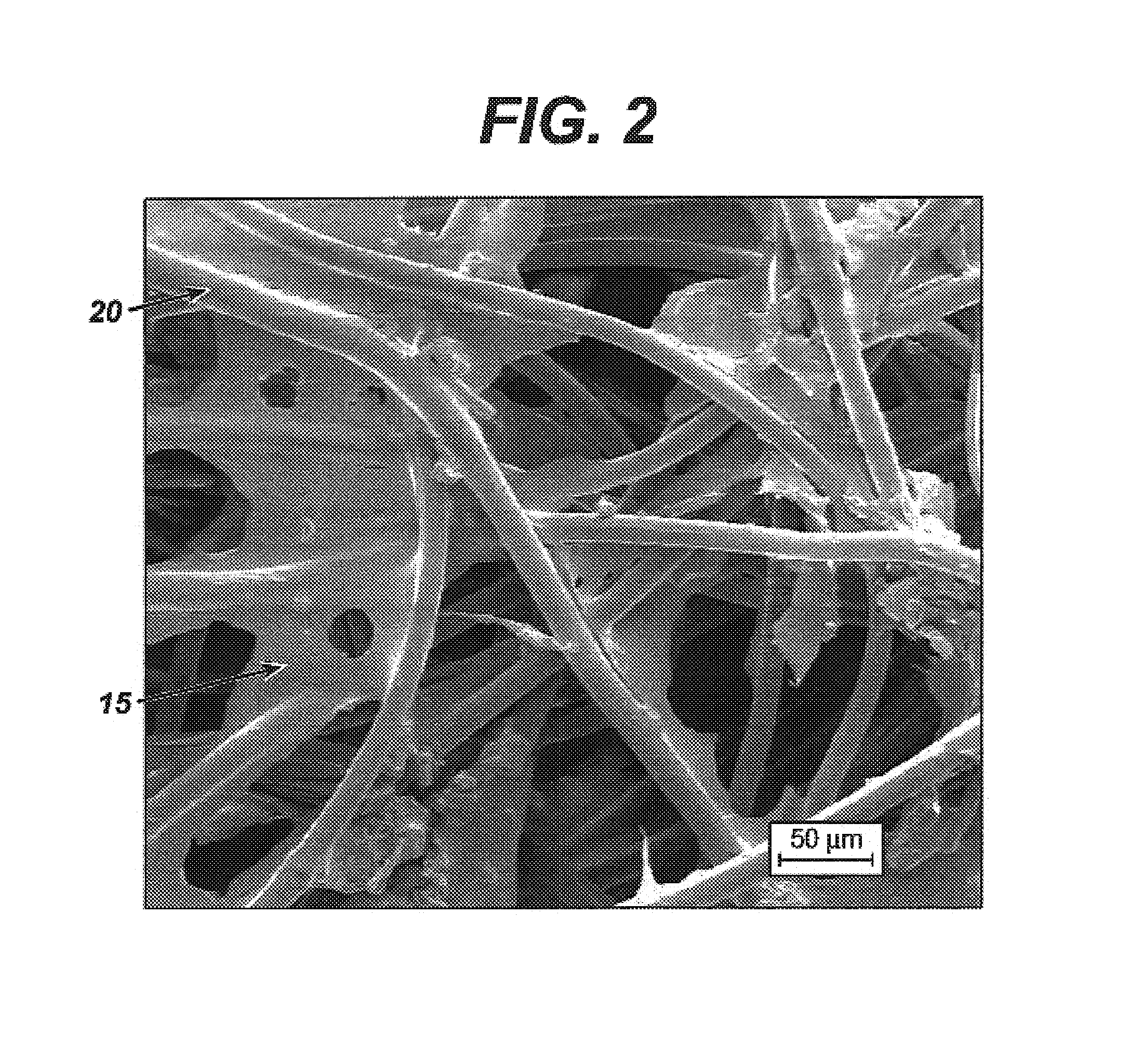
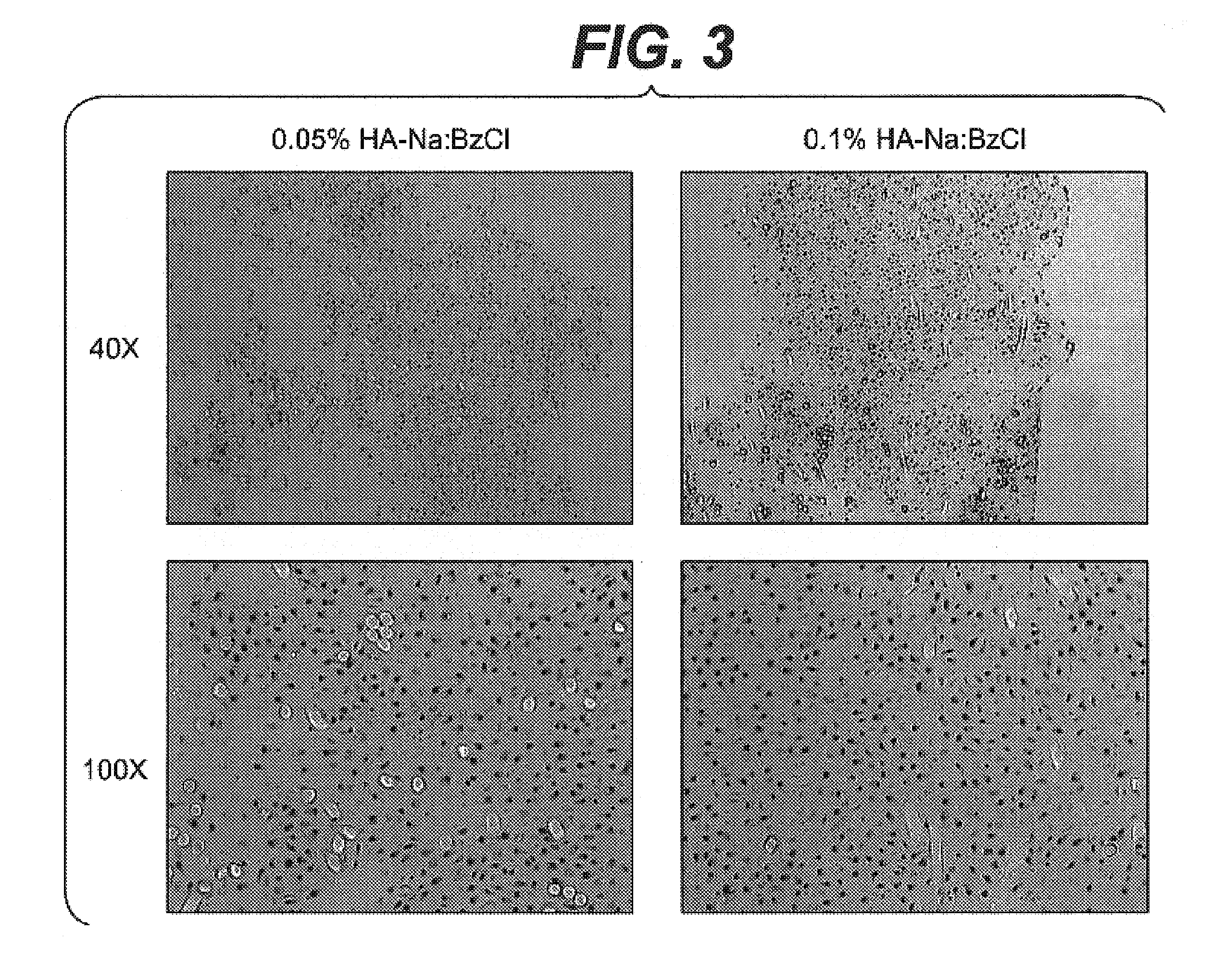
The present invention includes hyaluronic acid complexes of a monovalent alkali metal salt of hyaluronic acid and a tetra alkyl ammonium halide that are suitable for incorporation with tissue scaffolds that are suitable for use in repair and / or regeneration of muscoloskeletal tissue and that include a biodegradable, porous substrate made from a biodegradable, hydrophobic polymer, where the hyaluronic acid complex is substantially insoluble in water at room temperature, yet soluble in mixtures of organic and aqueous solvents in which the selected hydrophobic polymer is soluble.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Methods and devices to treat compressive neuropathy and other diseases
InactiveUS20100125266A1Relieve pressureInhibit swellingEndoscopesBlunt dissectorsDiseaseCTS - Carpal tunnel syndrome
Methods, systems and devices for treatment of musculoskeletal tissue may include one or more of stretching, scoring, cutting, and cryogenic cooling of the tissue. Exemplary usage includes the treatment of compressive neuropathy such as in carpal tunnel syndrome and plantar fasciitis. Other musculoskeletal tissues such as those in the hip, shoulder, and other regions of the body may also be treated.
Owner:THE FOUNDY LLC
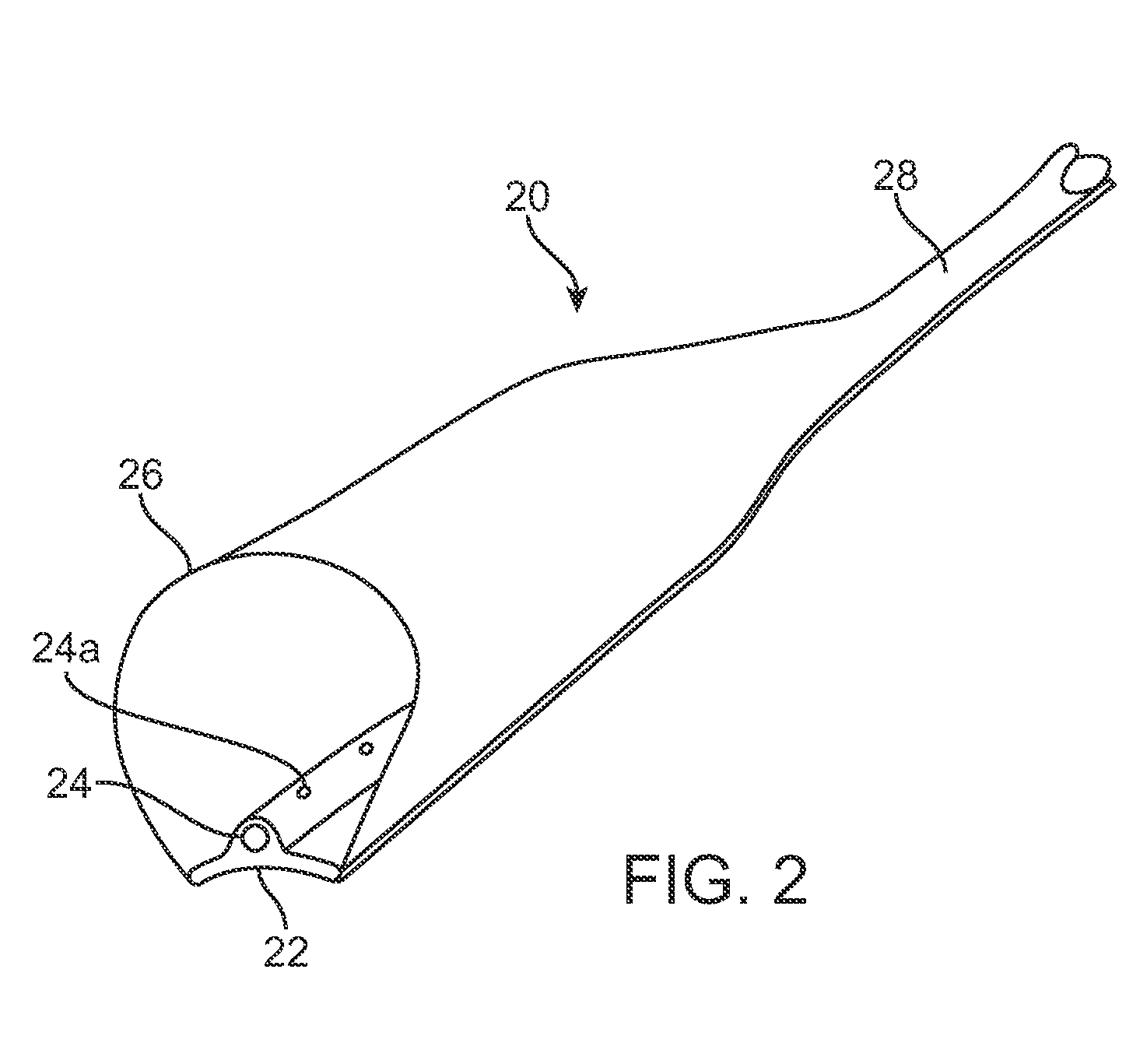
Graft collar and scaffold apparatuses for musculoskeletal tissue engineering and related methods
This application describes apparatuses and methods for musculoskeletal tissue engineering. Specifically, graft collar and scaffold apparatuses are provided for promoting fixation of musculoskeletal soft tissue to bone.This application provides for graft collars comprising biopolymer mesh and / or polymer-fiber mesh for fixing tendon to bone. In one aspect, the graft collar comprises more than one region, wherein the regions can comprise different materials configured to promote integration of and the regeneration of the interfacial region between tendon and bone.This application also provides for scaffold apparatuses and methods for fixing musculoskeletal soft tissue to bone. The scaffold apparatus is multiphasic, preferably triphasic, and each phase is configured promote growth and proliferation of a different cell and its associated tissue. In one aspect, the scaffold apparatus is triphasic, with phases comprising materials to promote growth and proliferation of fibroblasts, chondroblasts, and osteoblasts. In addition, an apparatus comprising two portions, each of said portion being the scaffold apparatus described above is provided, wherein each of said portion encases one end of a soft tissue graft. Further, a triphasic interference screw is provided.This application further provides apparatuses and methods for inducing formation of fibrocartilage comprising wrapping a graft collar with polymer-fiber mesh configured to apply compression to the graft collar. In another aspect, the polymer-fiber is applied directly to the graft to apply compression to the graft.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Implantable Devices for Musculoskeletal Repair and Regeneration
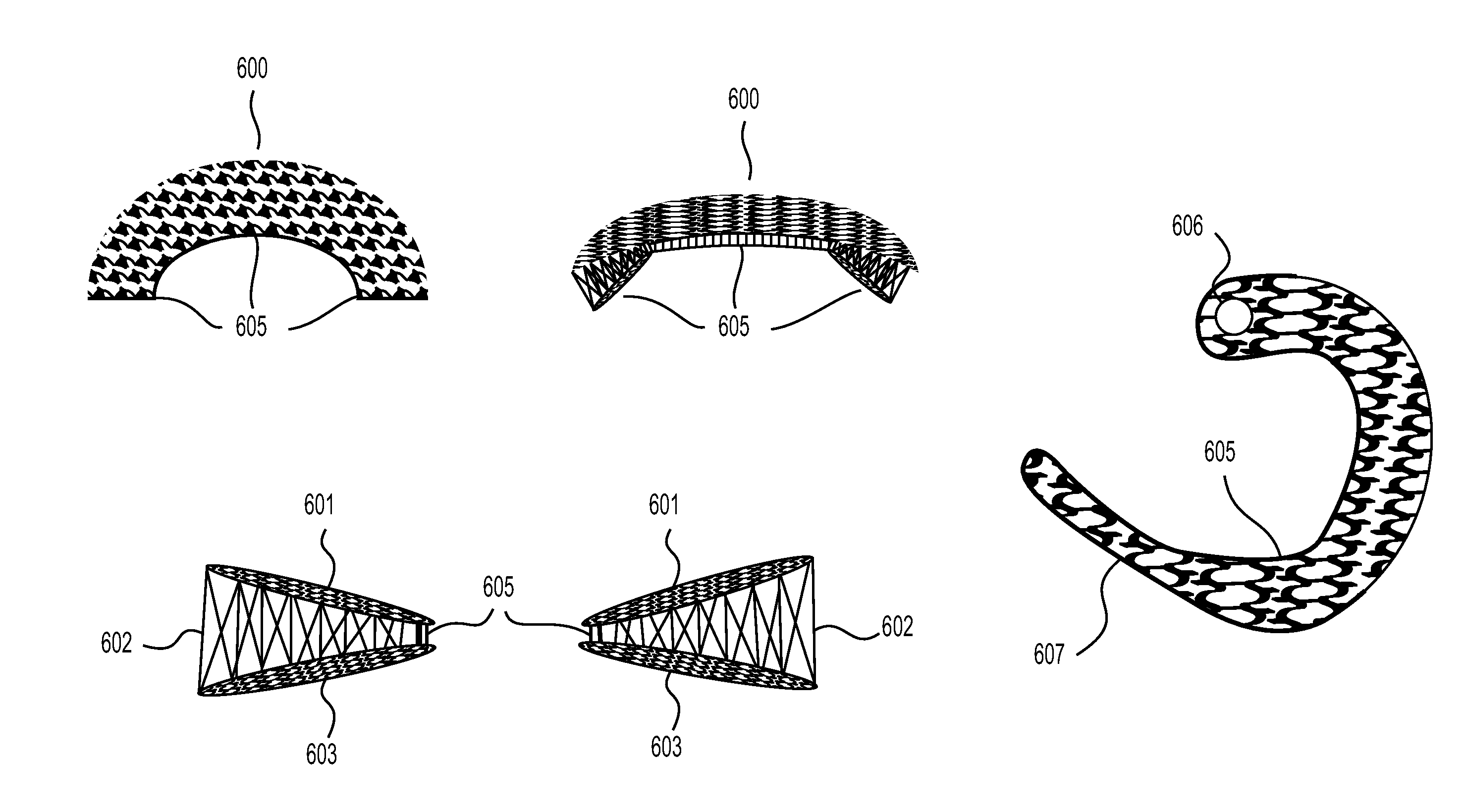
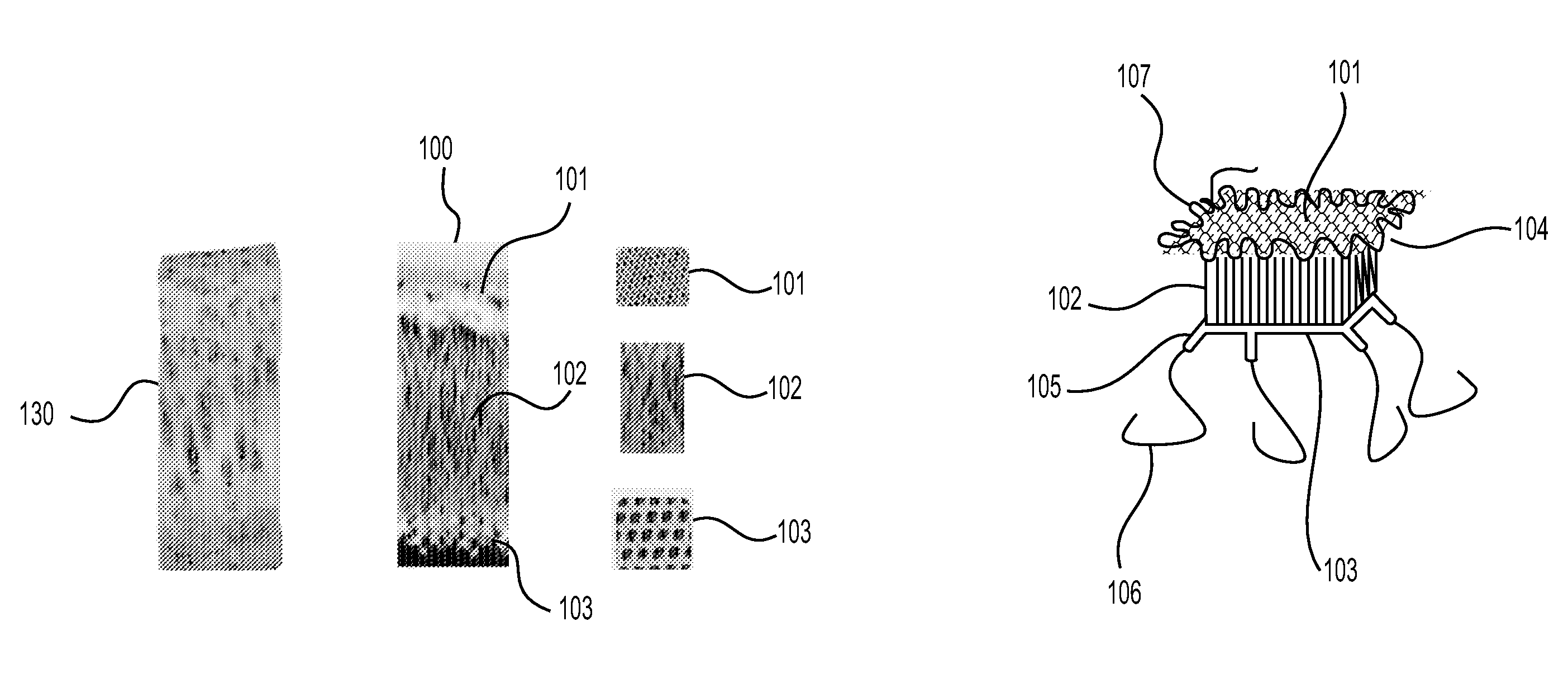
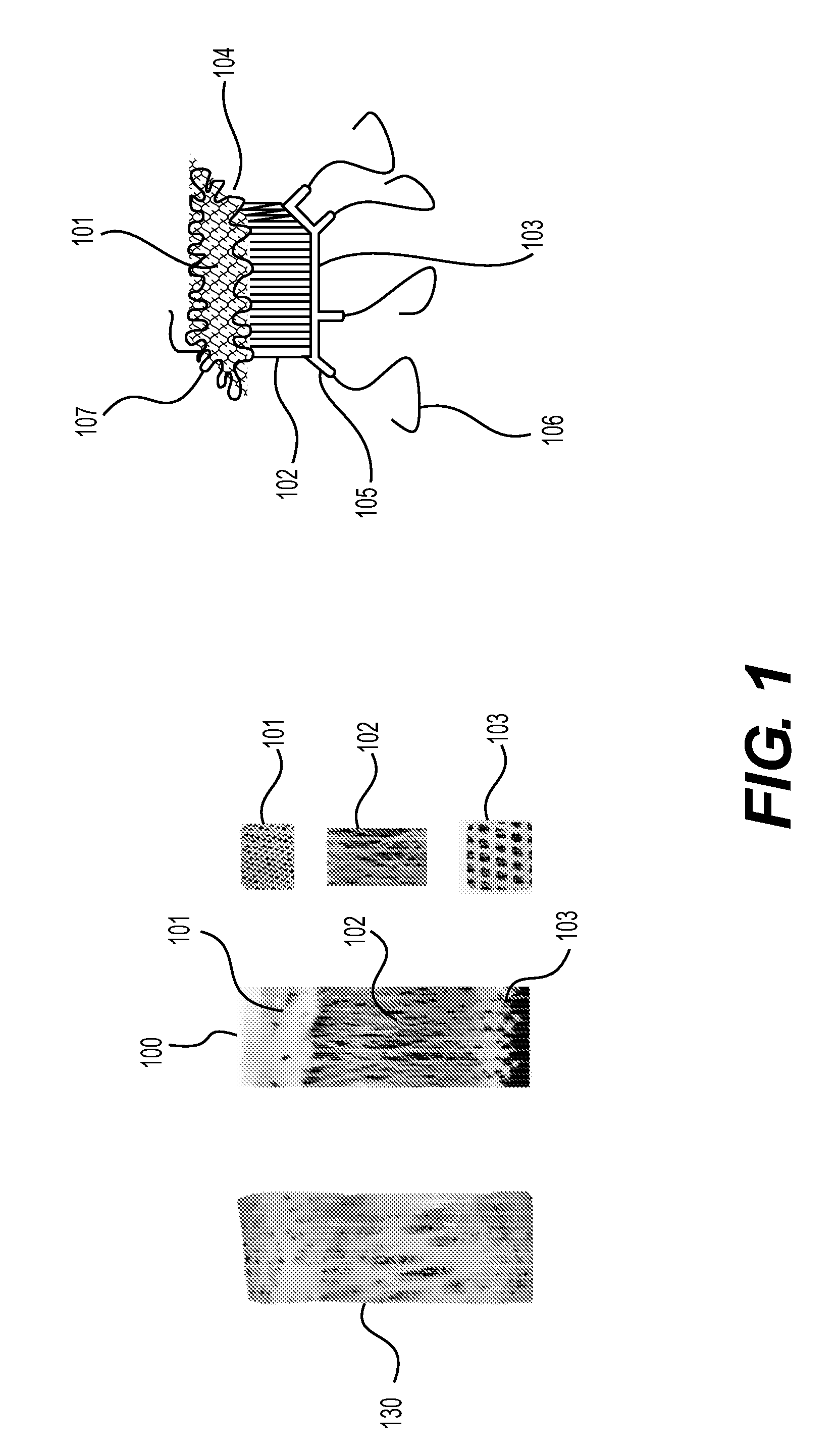
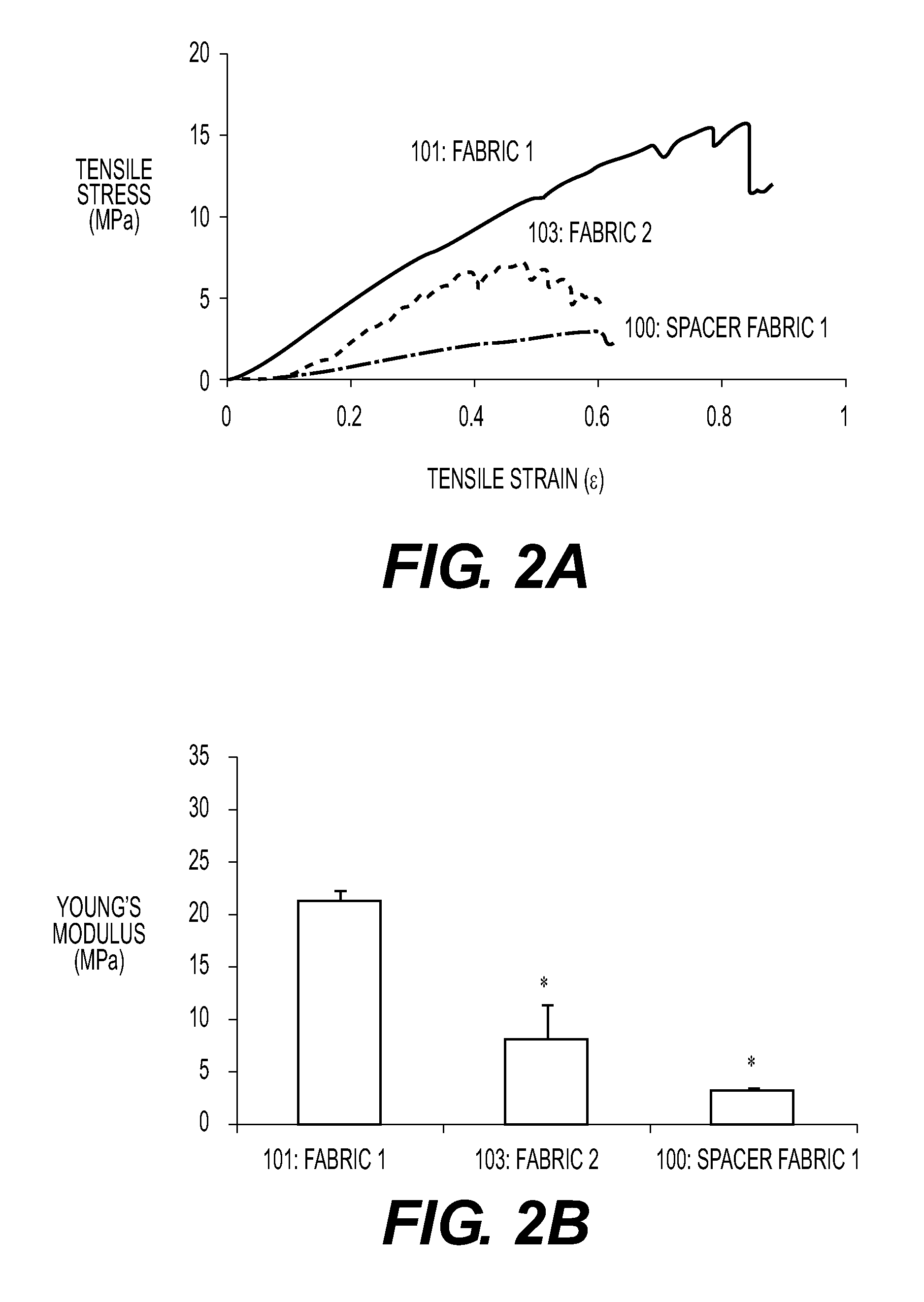
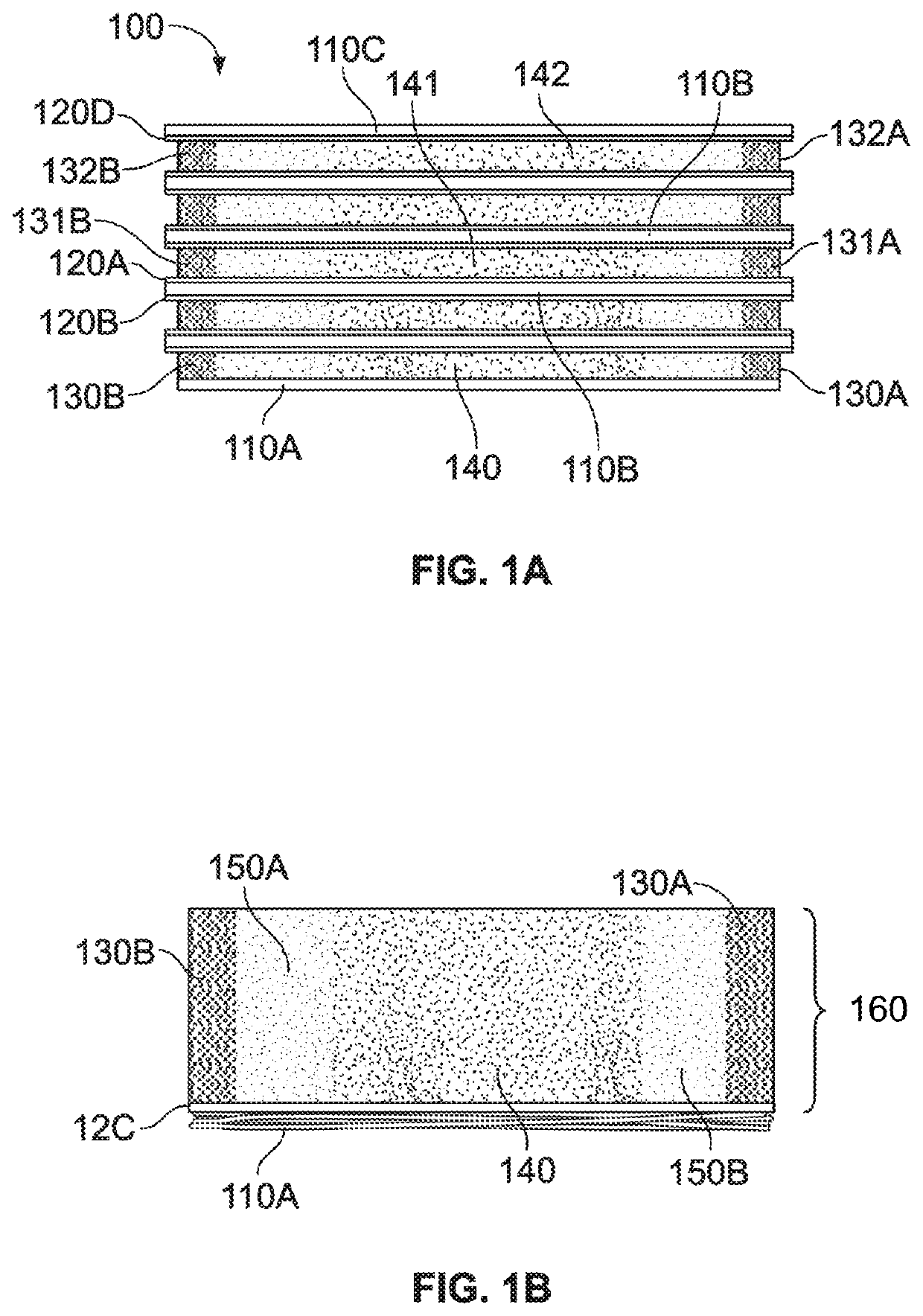
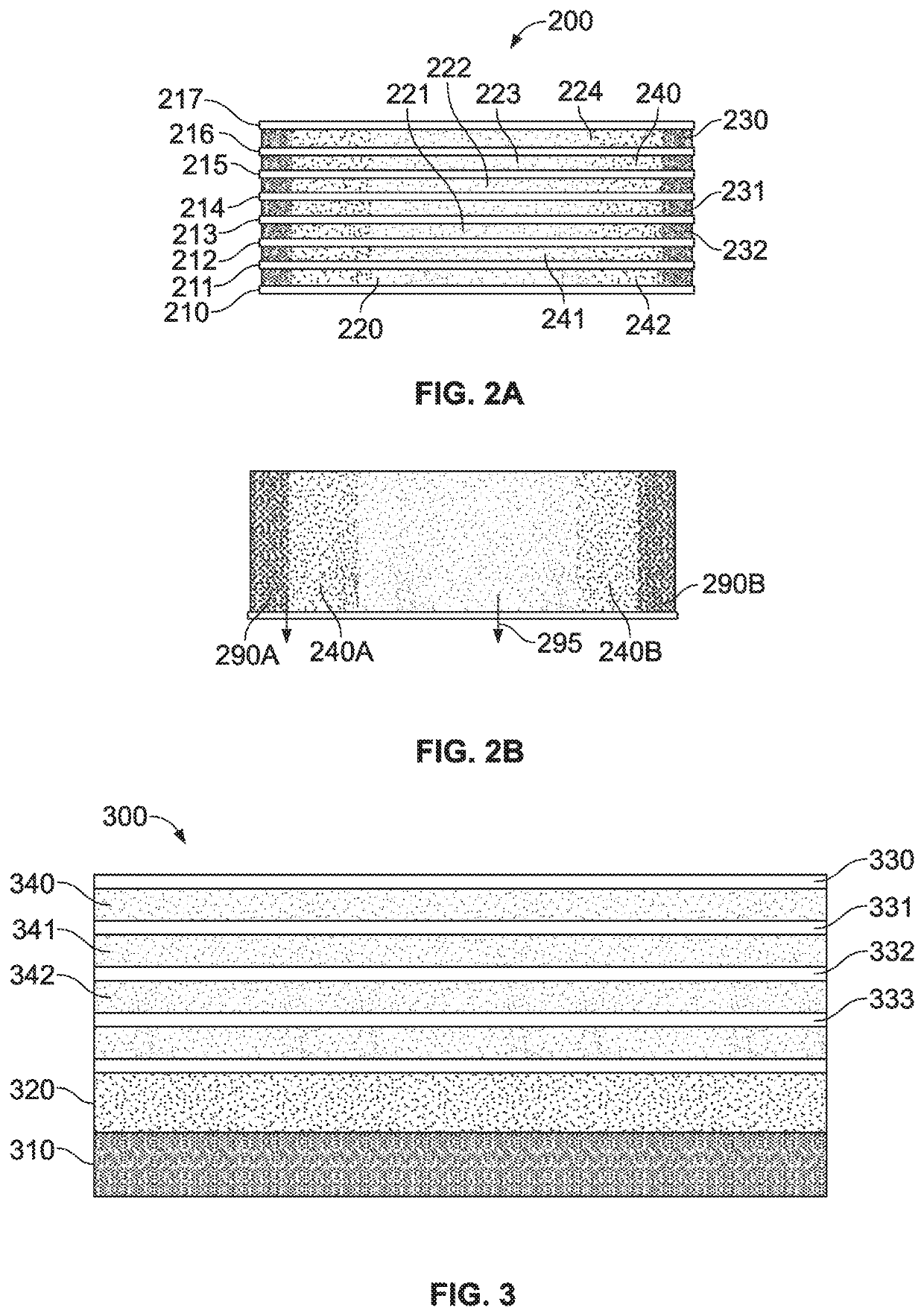
This application describes an implantable device for tissue repair comprising at least two fabrics with interconnecting spacer elements transversing, connecting, and separating the fabrics, forming the device. Some embodiments have fixation points which can be an extension of at least one of the fabrics. The implantable device allows modification of the two fabrics having varying constructions, chemistries, and physical properties. The spacer elements create a space between the two fabrics, which can be used for the loading of biological materials (peptides, proteins, cells, tissues), offer compression resistance (i.e. stiffness), and compression recovery (i.e., return to original dimensions) following deformation and removal of deforming load. The inclusive fixation points of the fabrics are designed to allow for fine adjustment of the sizing and tension of the device to promote integration with the surrounding tissues as well as maximize the compressive resistance. The fixation points can include either the first fabric, the second fabric, or the combination of both fabrics. This device is suitable for soft and hard tissue regeneration or replacement with a preference for musculoskeletal tissues including but not limited to cartilage (including hyaline (referred to as articular, e.g. cartilage on the ends of long bones), fibrous (e.g. meniscus or intervertebral discs), elastic (e.g. ear, epiglottis)), bone, muscle, tendon, ligament, and fat.
Owner:MCCULLEN SETH
Multi-component non-biodegradable implant, a method of making and a method of implantation
ActiveUS20140324169A1Maximizes integrationEasy to integrateBone implantSurgerySacroiliac jointMusculoskeletal tissue
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
Tissue scaffolds for use in muscoloskeletal repairs
The present invention includes a tissue scaffold suitable for use in repair and / or regeneration of musculoskeletal tissue when implanted in a body that includes a porous substrate made from a biodegradable, hydrophobic polymer having a modified hyaluronic acid complex dispersed on the surface of and / or, where the modified hyaluronic acid complex is insoluble in water, yet soluble in mixtures of organic and aqueous solvents in which the hydrophobic polymer is soluble.
Owner:ETHICON ENDO SURGERY INC
Tissue scaffolds for use in muscoloskeletal repairs
The present invention includes a tissue scaffold suitable for use in repair and / or regeneration of musculoskeletal tissue when implanted in a body that includes a porous substrate made from a biodegradable, hydrophobic polymer having a modified hyaluronic acid complex dispersed on the surface of and / or, where the modified hyaluronic acid complex is insoluble in water, yet soluble in mixtures of organic and aqueous solvents in which the hydrophobic polymer is soluble.
Owner:ETHICON ENDO SURGERY INC
Myostatin inhibitor enhancement of musculoskeletal repair
InactiveUS20100331252A1Promote repairPromote regenerationPowder deliveryPeptide/protein ingredientsMyostatinNon union
The methods and compositions of this invention provide a means of regenerating injured musculoskeletal tissue by inhibition of myostatin function. The invention provides methods of treating a nonunion fracture in an individual comprising delivering to the fracture via a delivery system comprising biodegradable hydrogel, a pharmacological amount of myostatin propeptide effective to inhibit myostatin function. The invention also teaches compositions useful for the treatment of non-union fracture in an individual, said compositions comprising a pharmacological amount of myostatin propeptide effective to inhibit myostatin function; and a delivery system for delivering said myostatin propeptide to said fracture, wherein the delivery system comprises a biodegradable hydrogel or biodegradable nanobeads.
Owner:GEORGIA HEALTH SCI UNIV RES INST
Medicated orthopedic support structures for treatment of damaged musculoskeletal tissue
A system and method for treating damaged musculoskeletal tissue includes an orthopedic support structure and a nitroglycerin-containing composition. The orthopedic support structure provides mechanical support to the damaged tissue and maintains the nitroglycerin-containing composition in contact with the skin of a subject wearing using the system. The nitroglycerin-containing composition can be integrated into the orthopedic support structure or the support structure can be configured to contain removable doses of the nitroglycerin-containing composition. The system and method provides relief of pain and improved function of the musculoskeletal tissue.
Owner:CURE THERAPEUTICS
Fiber-Hydrogel Composite for Tissue Replacement
InactiveUS20110288199A1Suitable for implantationSwelling of the composite is minimizedImpression capsSurgical adhesivesFibrocartilageTissues types
The present invention includes tailored fiber-reinforced hydrogel composites for implantation into a subject. The present invention also includes systems and methods for controlling the relative percent volume of the hydrogel and fibers, cross-linking, fiber orientation, weave and density, such that the material properties of the composite can be controlled and / or customized to match particular tissue types. The composites of the present invention are suitable for repairing or replacing musculoskeletal tissues and / or fibrocartilage, such as the meniscus, ligaments and tendons.
Owner:DREXEL UNIV +1
Compound betamethasone suspension injection
InactiveCN101167730A"block" does not appearGood injectabilityOrganic active ingredientsComponent separationSolubilityCollagen disease
The invention provides a compound betamethasone suspension injection, which is a compound preparation composed of low-solubility betamethasone dipropionate and high-solubility betamethasone sodium phosphate. Long-lasting, it can be used for the treatment of musculoskeletal and cartilage tissue diseases, allergic diseases, skin diseases, collagen diseases, tumors and other clinical diseases.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Implantable devices for musculoskeletal repair and regeneration
This application describes an implantable device for tissue repair comprising at least two fabrics with interconnecting spacer elements transversing, connecting, and separating the fabrics, forming the device. Some embodiments have fixation points which can be an extension of at least one of the fabrics. The implantable device allows modification of the two fabrics having varying constructions, chemistries, and physical properties. The spacer elements create a space between the two fabrics, which can be used for the loading of biological materials (peptides, proteins, cells, tissues), offer compression resistance (i.e. stiffness), and compression recovery (i.e., return to original dimensions) following deformation and removal of deforming load. The inclusive fixation points of the fabrics are designed to allow for fine adjustment of the sizing and tension of the device to promote integration with the surrounding tissues as well as maximize the compressive resistance. The fixation points can include either the first fabric, the second fabric, or the combination of both fabrics. This device is suitable for soft and hard tissue regeneration or replacement with a preference for musculoskeletal tissues including but not limited to cartilage (including hyaline (referred to as articular, e.g. cartilage on the ends of long bones), fibrous (e.g. meniscus or intervertebral discs), elastic (e.g. ear, epiglottis)), bone, muscle, tendon, ligament, and fat.
Owner:MCCULLEN SETH
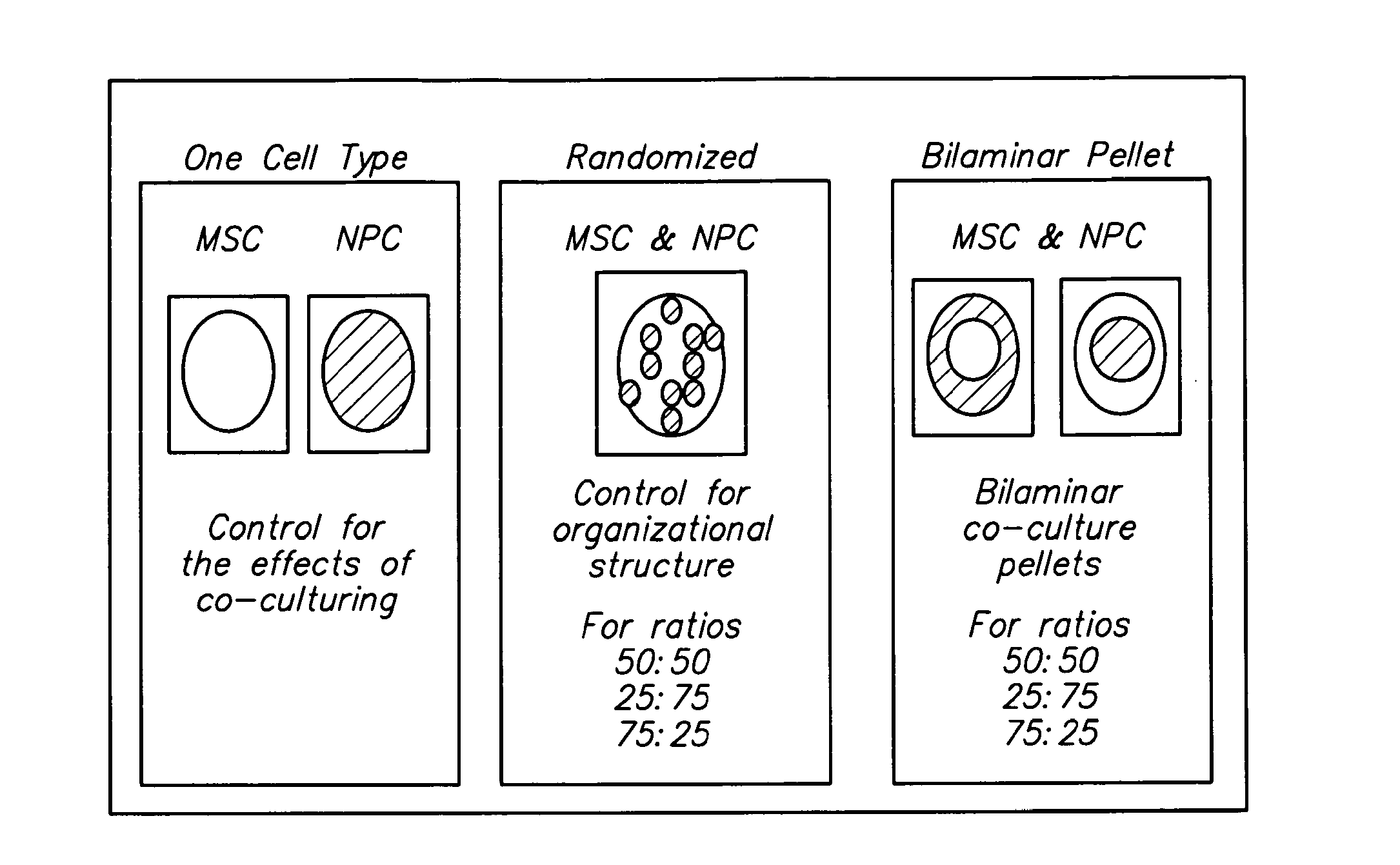
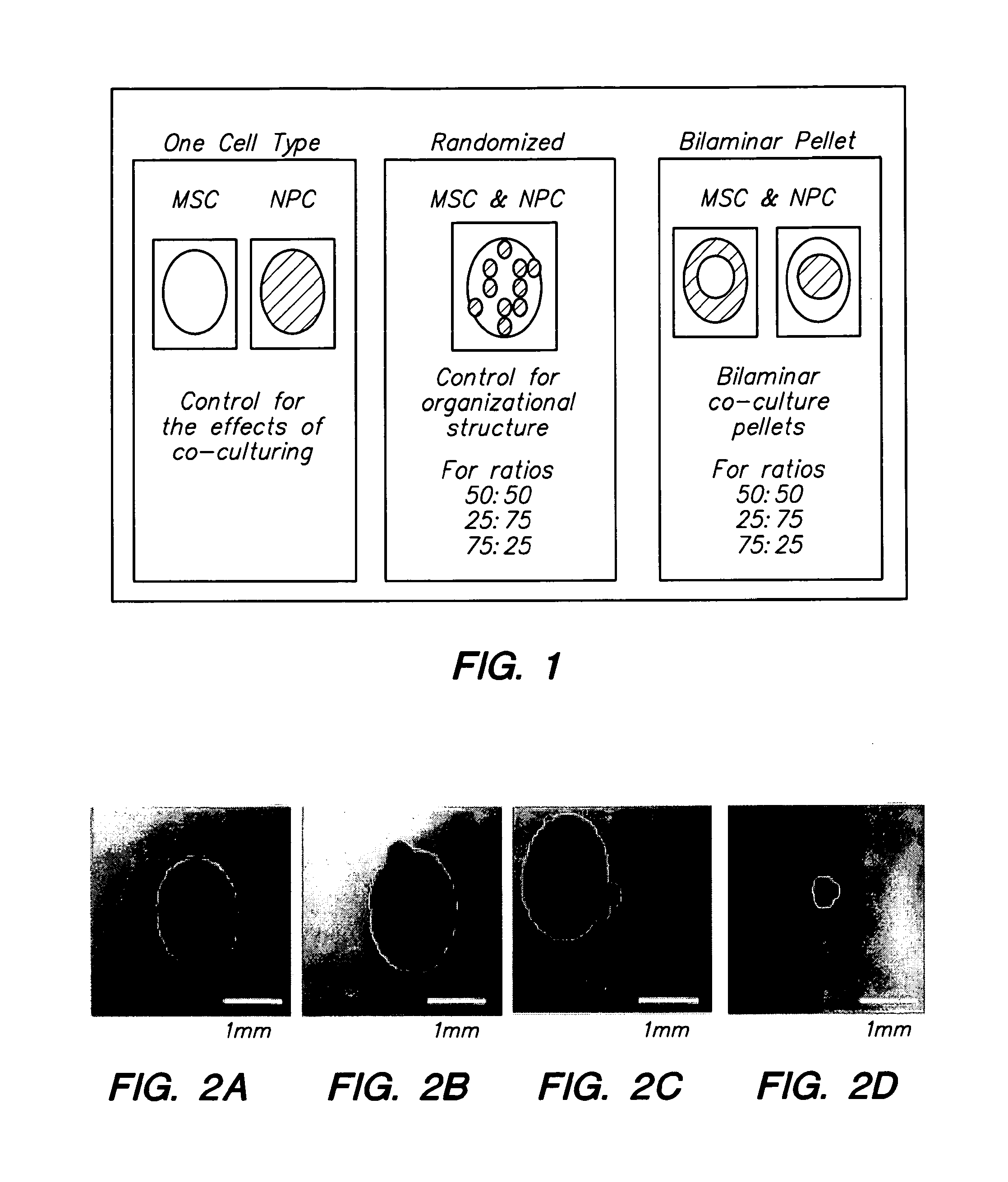
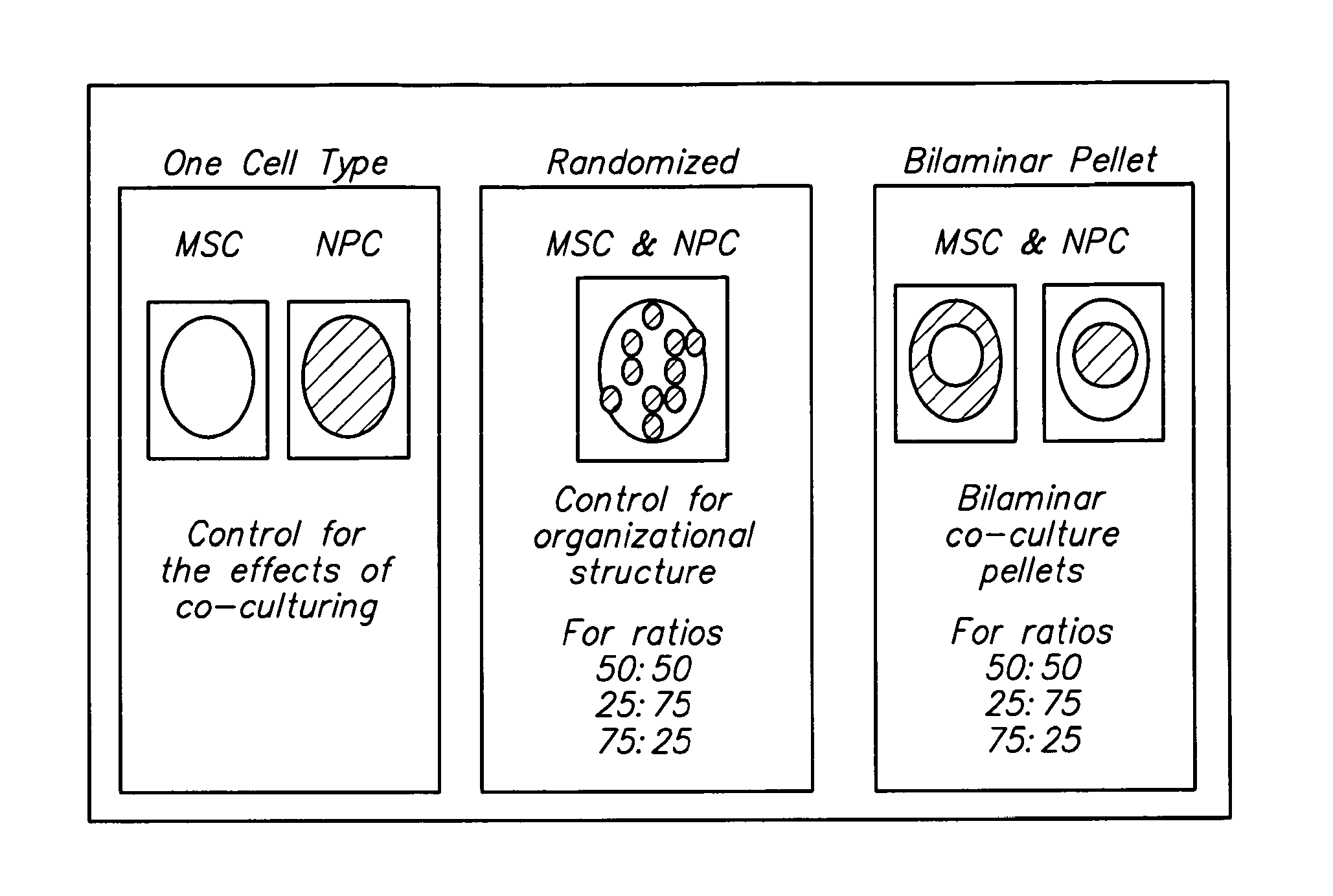
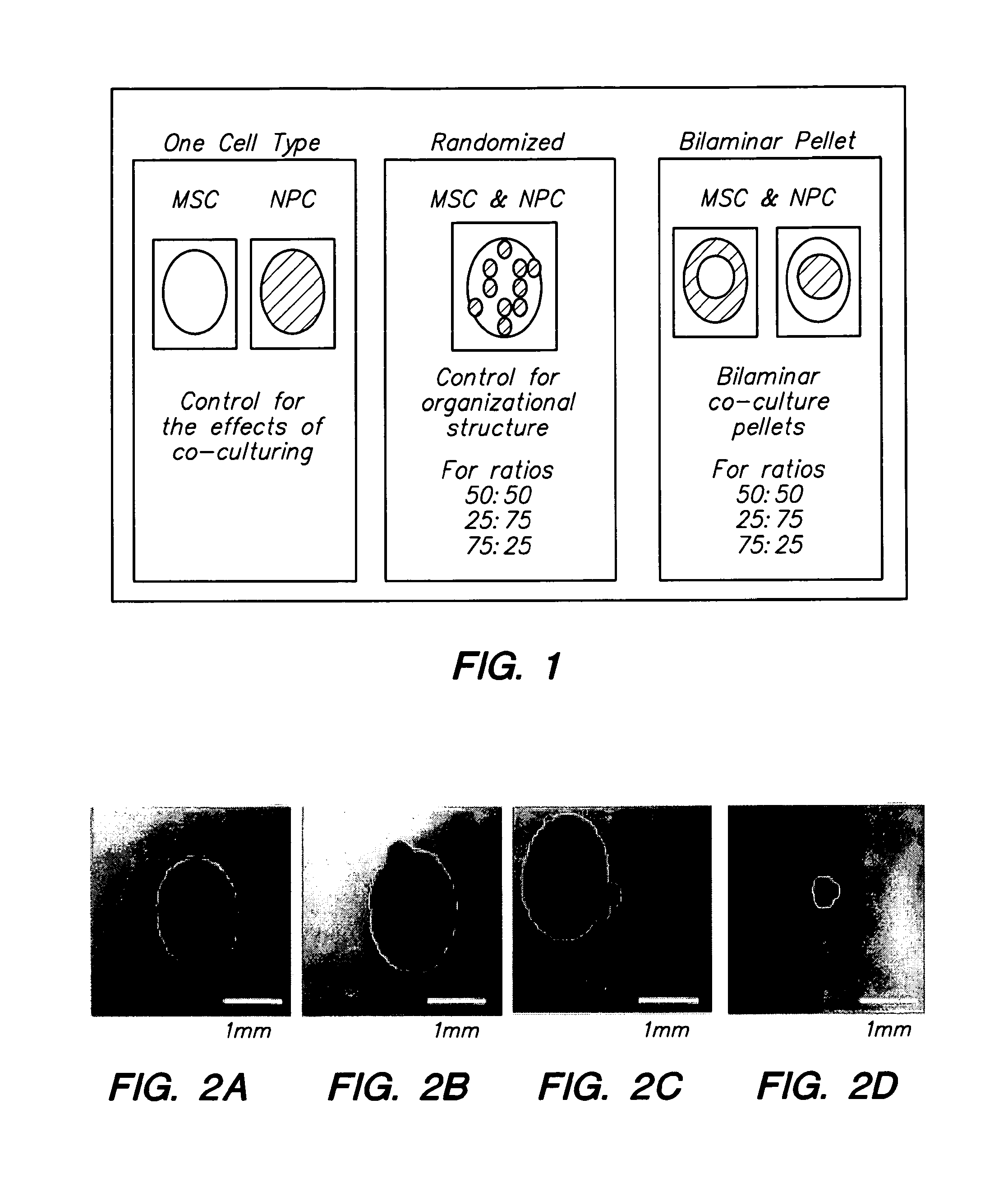
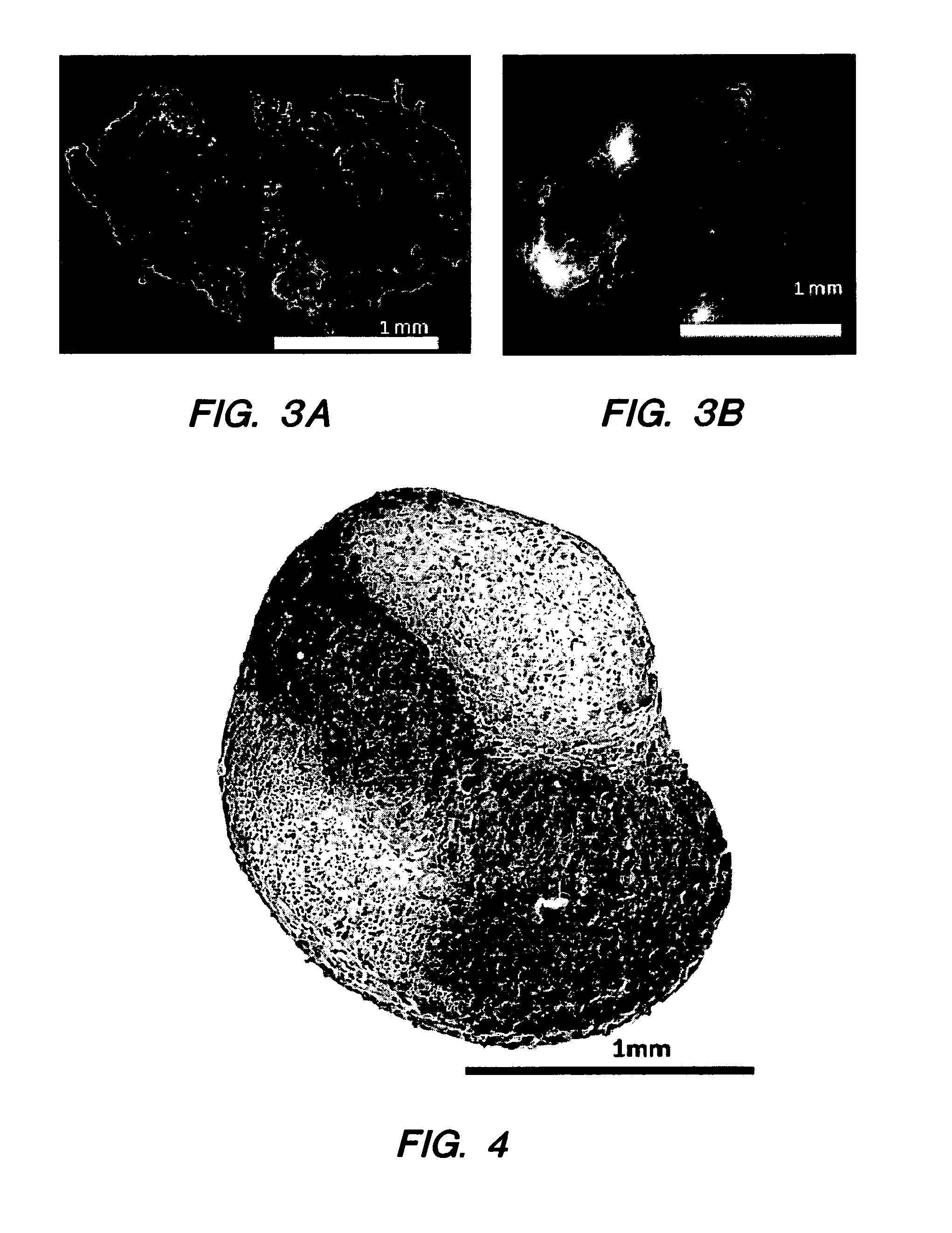
Compositions and Methods for Generating Musculoskeletal Tissue
ActiveUS20110177132A1BiocidePharmaceutical delivery mechanismIntervertebral discMesenchymal stem cell
The present disclosure provides compositions comprising musculoskeletal cells and mesenchymal stem cells in discrete regions. The present disclosure provides systems comprising a subject composition; and methods of using a subject composition to generate cartilage, bone, tendon, muscle, intervertebral disc, or other musculoskeletal tissues.
Owner:RGT UNIV OF CALIFORNIA
Penetrating antibiotic gel for soft tissue diseases
A penetrating antibiotic gel for treating pain, inflammation and other pathological conditions affecting musculoskeletal tissues and other soft tissues of the body. The composition includes an antibiotic compound and a mobilizing agent in an amount sufficient to enable the antimicrobial compound to penetrate into the sub-dermal soft tissues. The antimicrobial compound may be a macrolide antibiotic compound such as azithromycin, erythromycin or roxithromycin, for example, and the mobilizing agent may be an organogel compound, such as pluronic lecithin liposomal organogel (PLO). The composition may further include a penetration enhancing adjuvant, such as d-limonene, for example. The composition may be applied topically so as to penetrate into the sub-dermal soft tissues, or may injected so as to be absorbed into the soft tissues locally.
Owner:ALLEN DAVID M
Interconnected porous non-degradable poly(VINYL) alcohol implant and method of manufacture
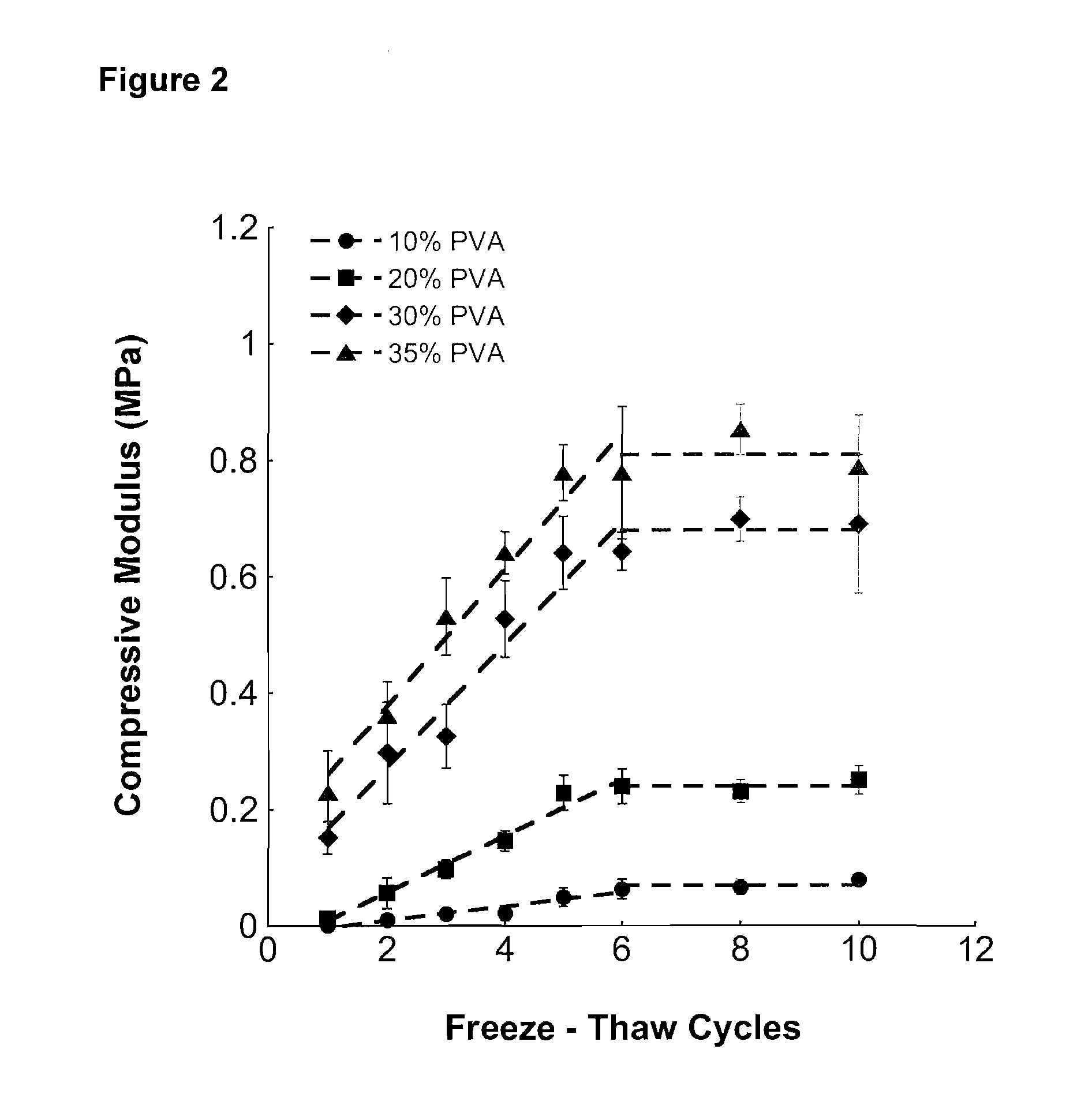
The present invention relates to a method of manufacture of an interconnected porous non-biodegradable polymer implant suitable for implantation into a mammal for the treatment, repair or replacement of defects or injury in musculoskeletal tissue, wherein the mechanical properties of the implant can be controlled by varying the concentration of the non-biodegradable polymer and / or varying the duration and number of freeze-thaw cycles and the interconnected porous non-biodegradable polymer implant has sufficient percent porosity and pore diameter to facilitate integration of cells and attachment within the mammal via ingrowth of surrounding tissue. The present invention also relates to an implant manufactured by the method.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
Attachment of absorbable tissue scaffolds to fixation devices
The present invention relates to tissue scaffold implant devices useful in the repair and / or regeneration of diseased and / or damaged musculoskeletal tissue and that include a tissue scaffold component fixedly attached to a scaffold fixation component via a polymeric adhesive layer, and to methods of making such tissue scaffold implant devices.
Owner:ETHICON ENDO SURGERY INC
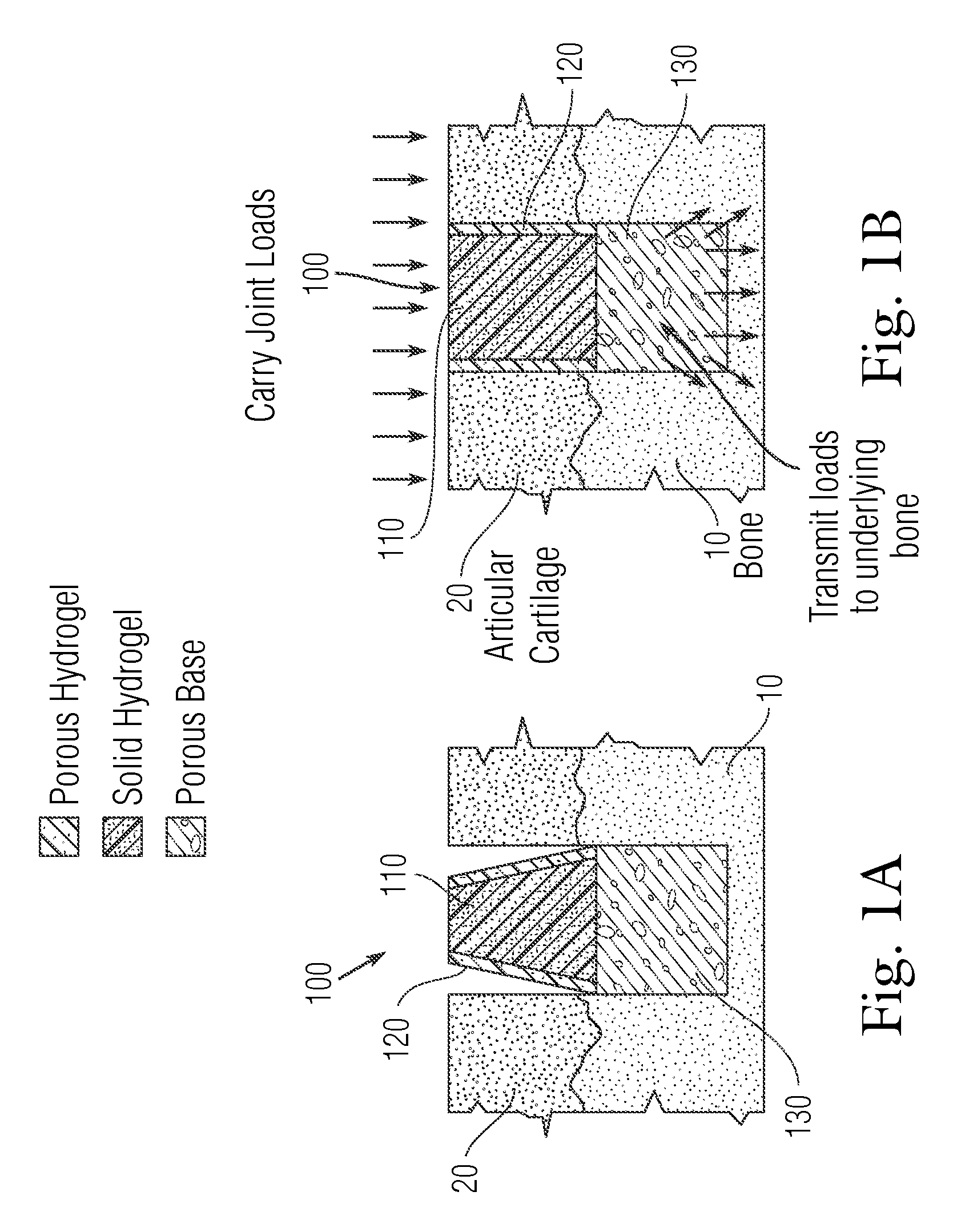
Multi-component non-biodegradable implant, a method of making and a method of implantation
ActiveUS9545310B2Maximizes integrationHigh and low viscosityBone implantSurgeryBiodegradable implantsBiomedical engineering
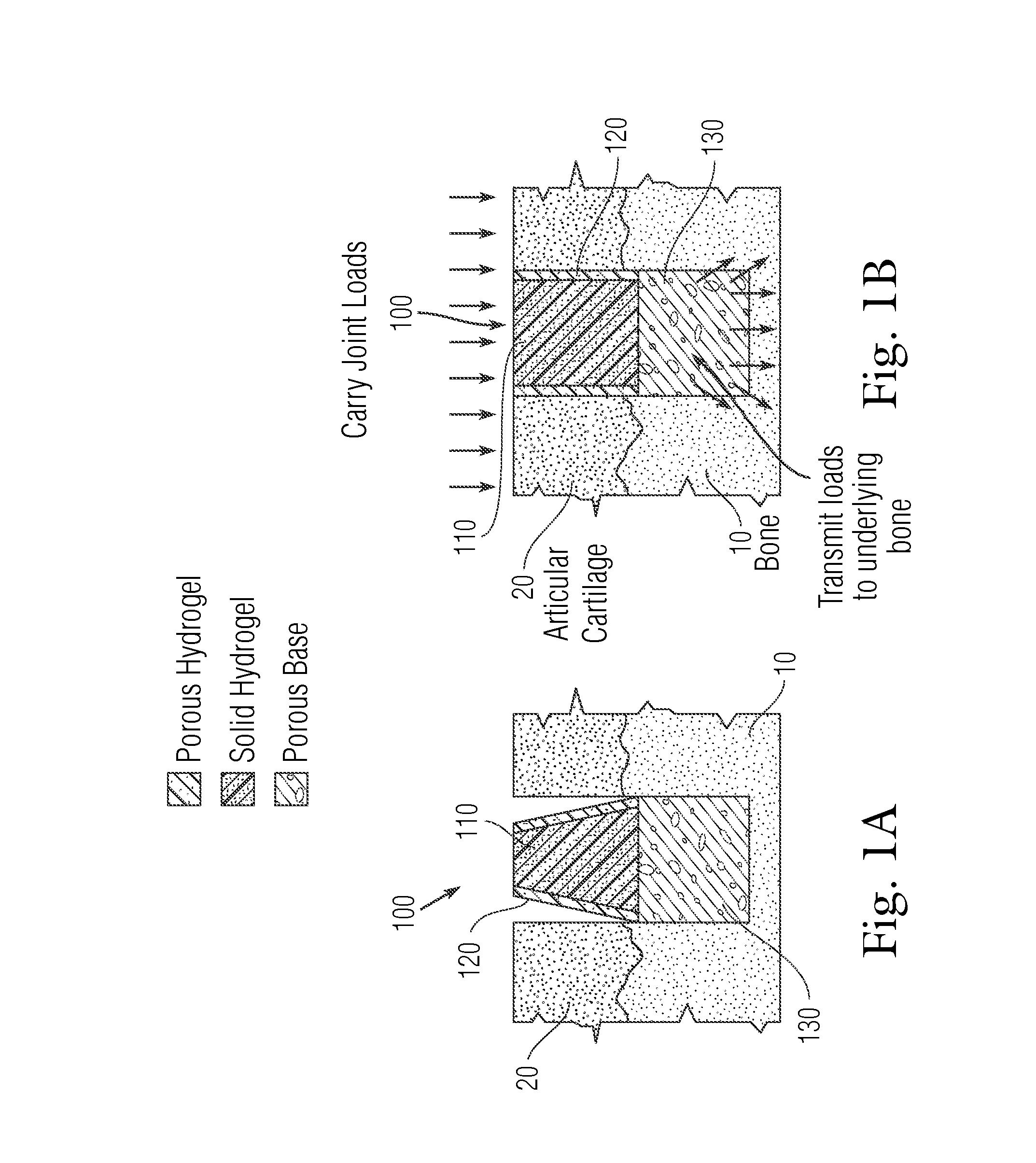
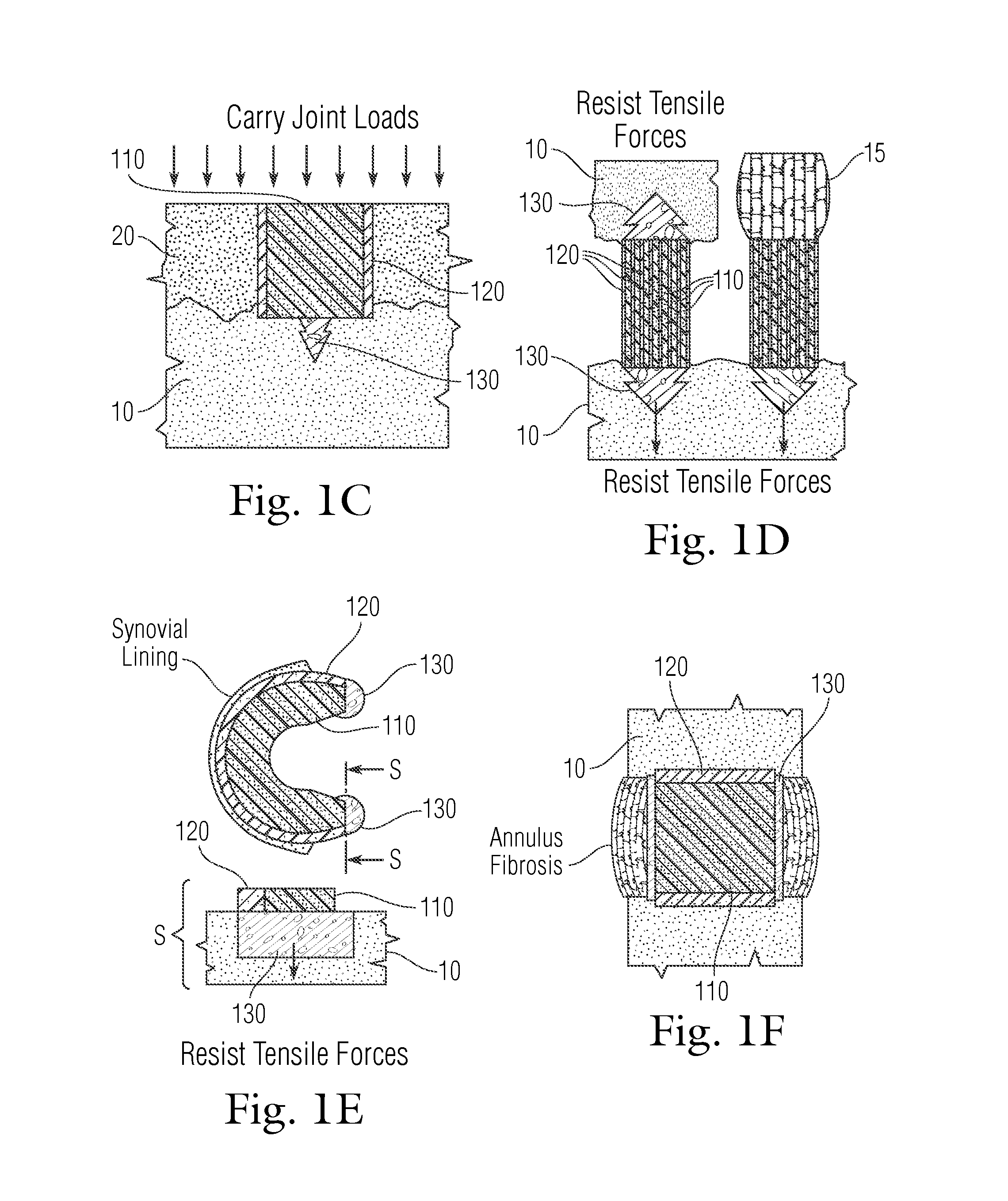
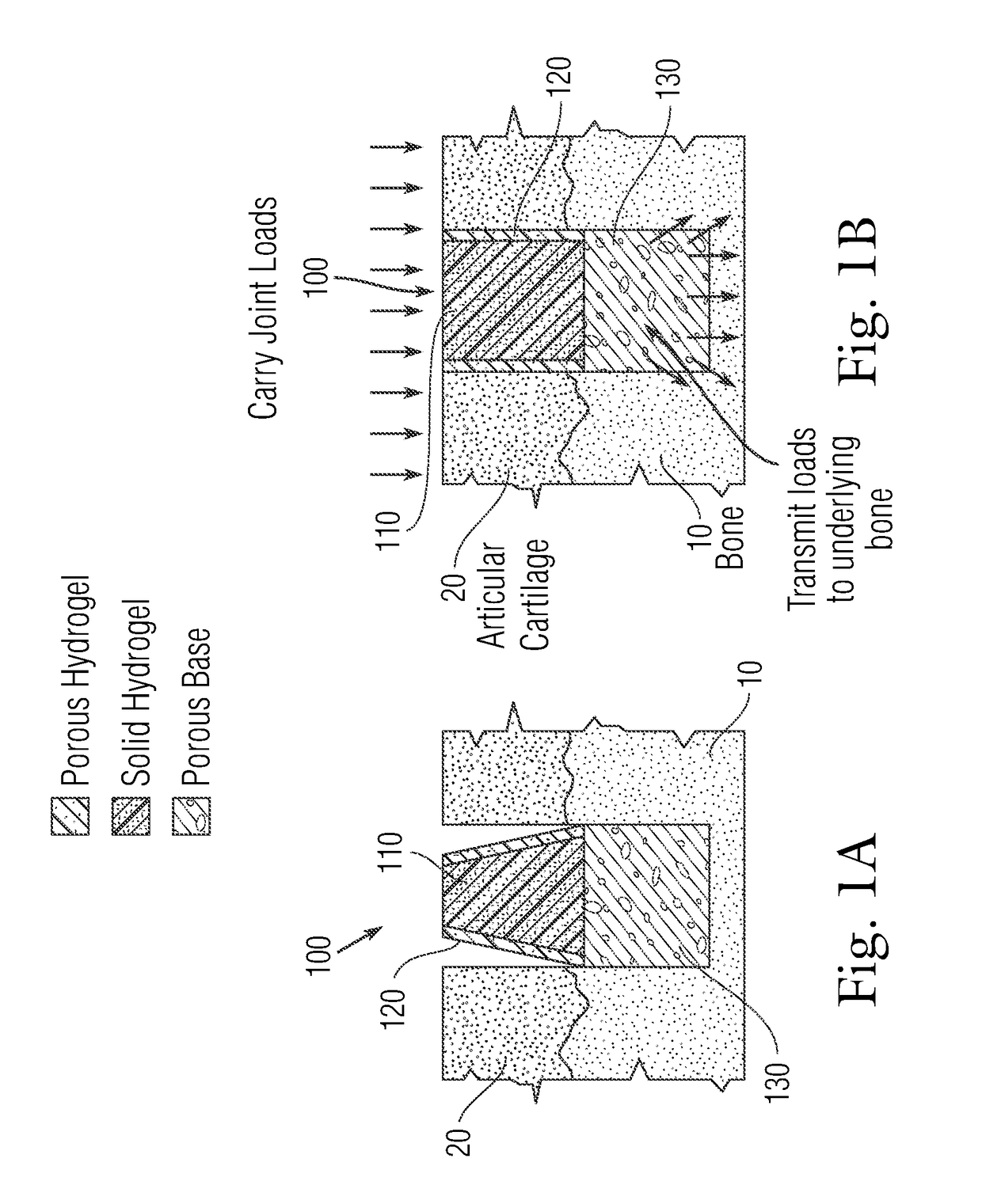
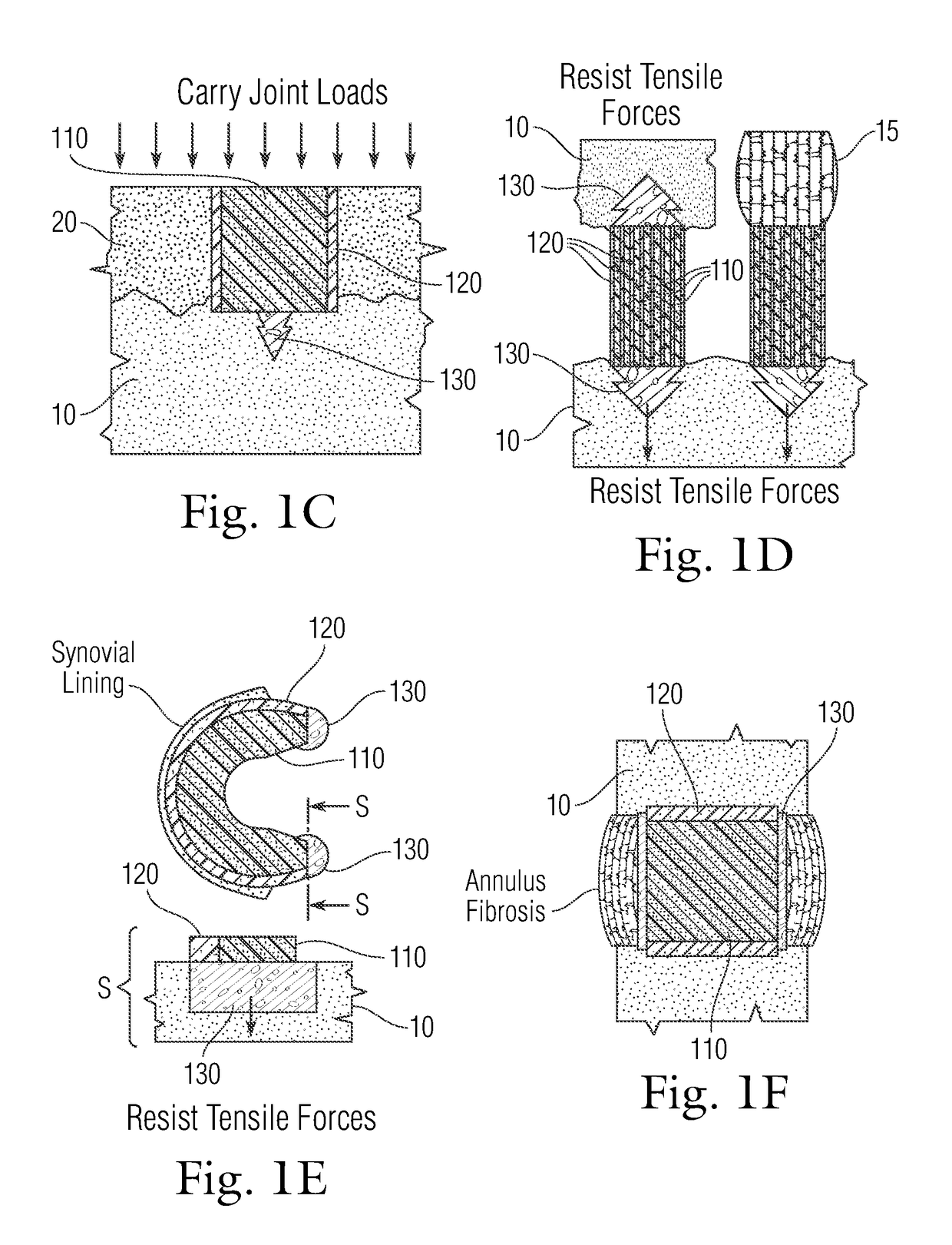
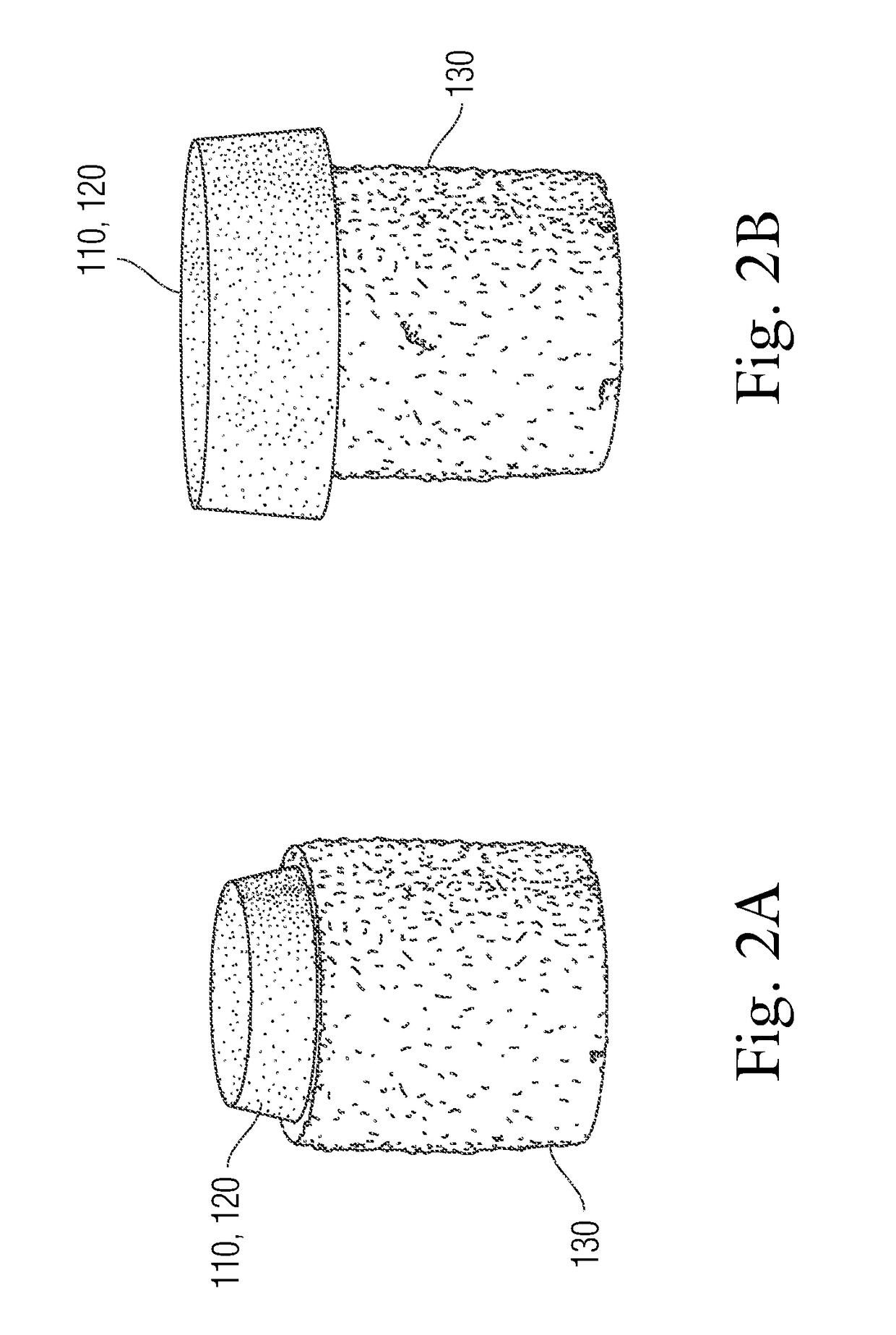
An implant having at least three components, namely, a solid hydrogel, a porous hydrogel adjacent to or surrounding the solid hydrogel (together considered “the hydrogel”), and a porous rigid base. The solid hydrogel and porous rigid base carry joint load, and the porous hydrogel layer and the porous rigid base allow for cellular migration into and around the implant. The invention is also a novel method of manufacturing the implant, a novel method of implanting the implant, and a method of treating, repairing or replacing biological tissue, more preferably musculoskeletal tissue, with the implant.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
In vitro mechanical loading of musculoskeletal tissues
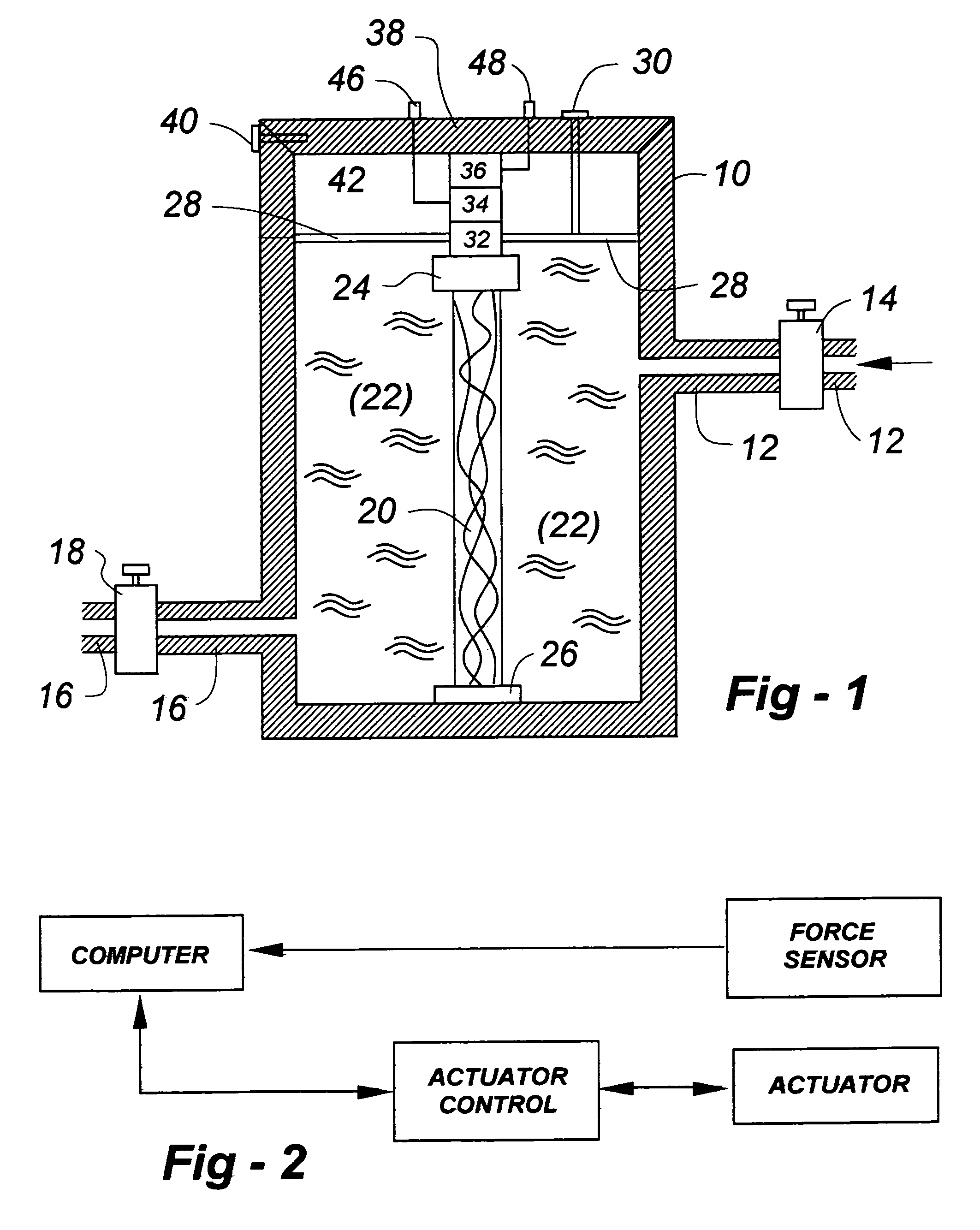
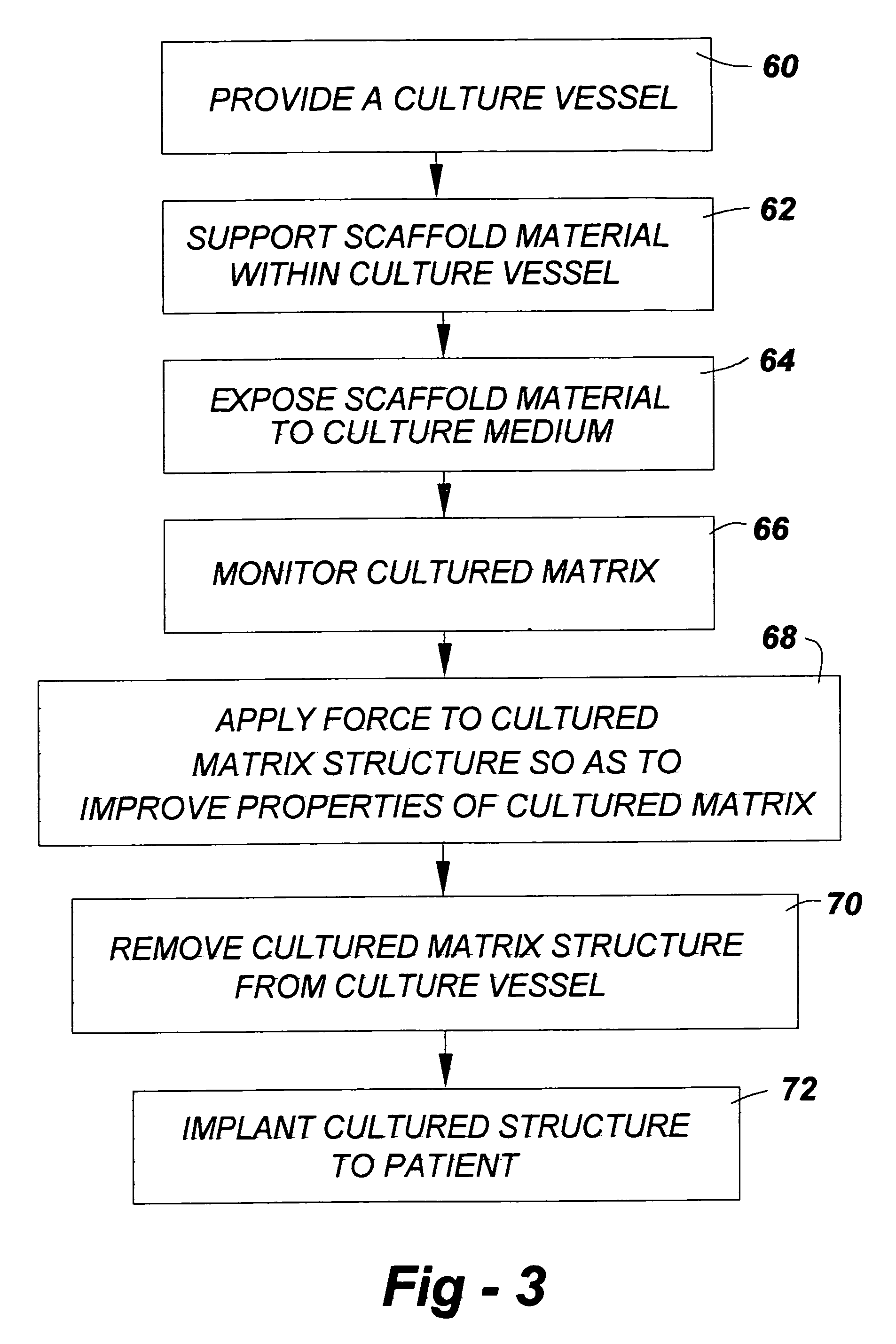
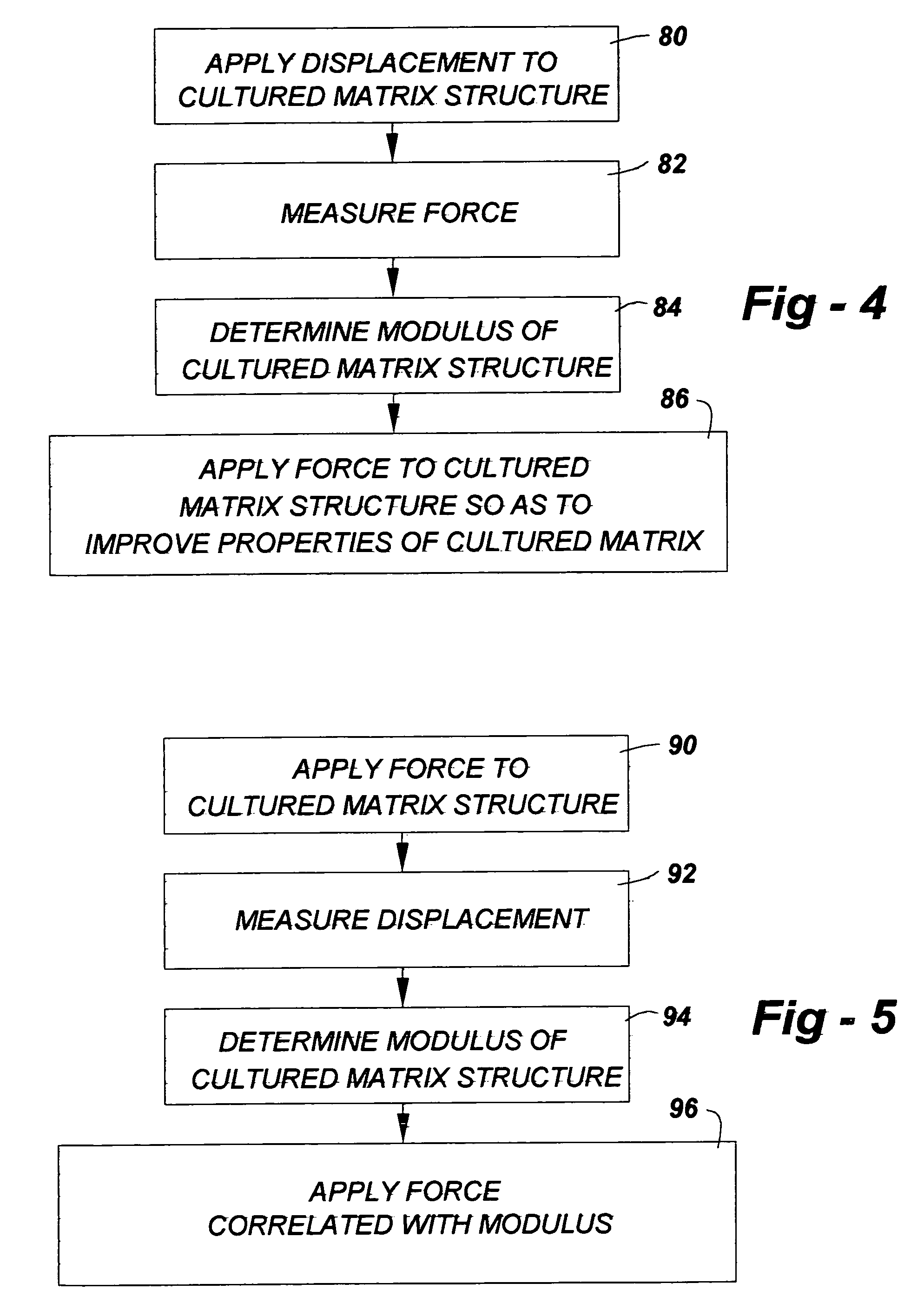
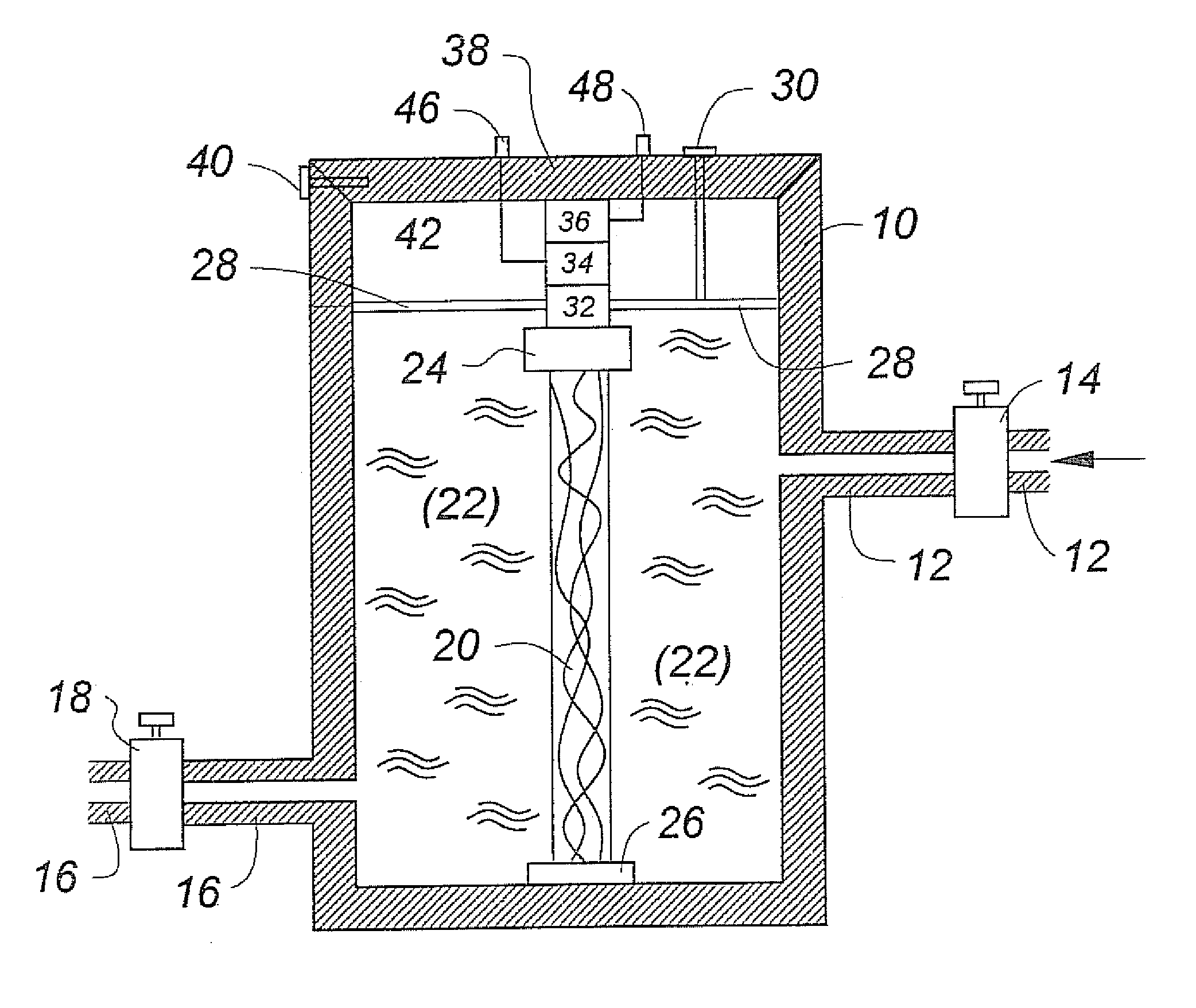
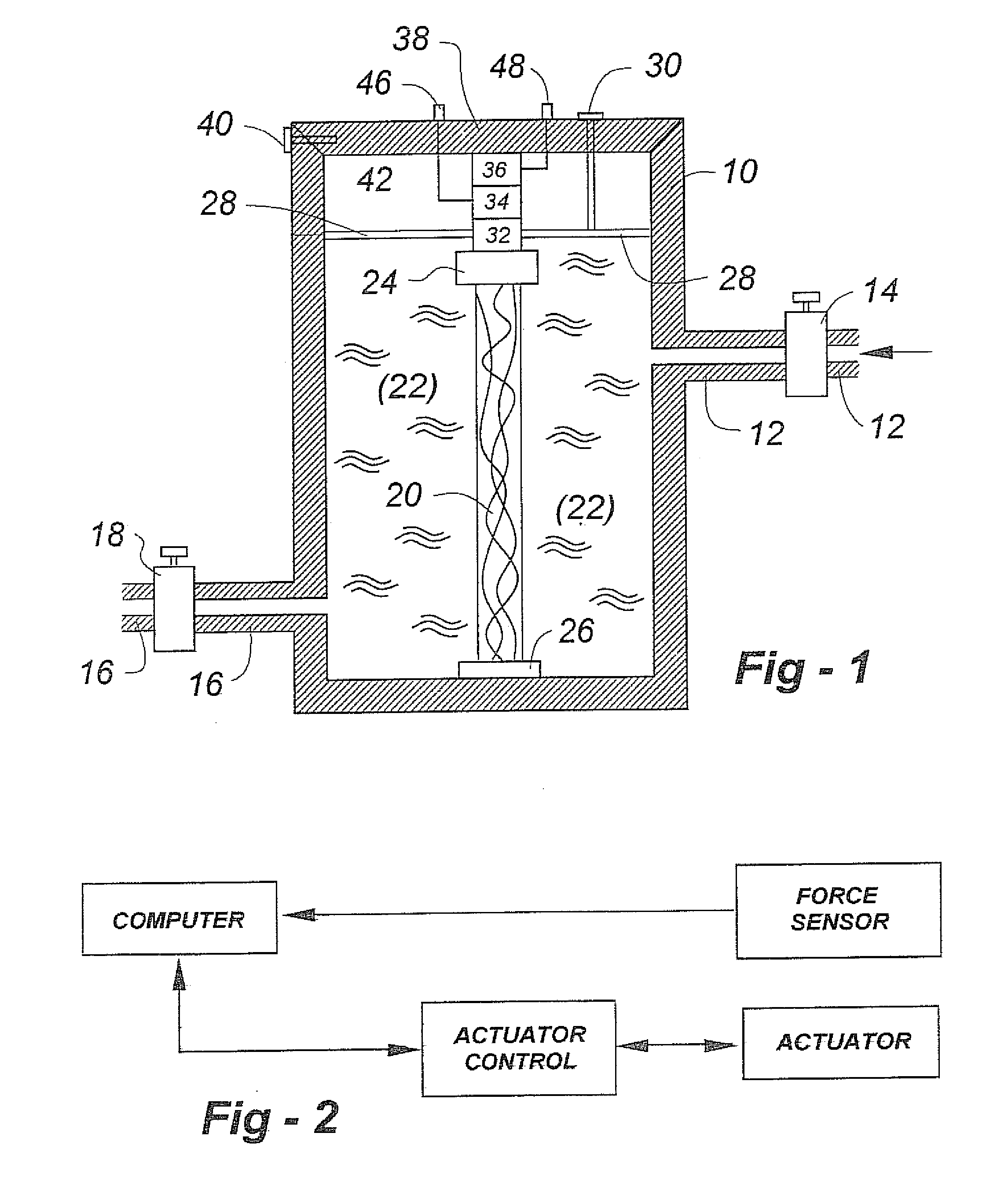
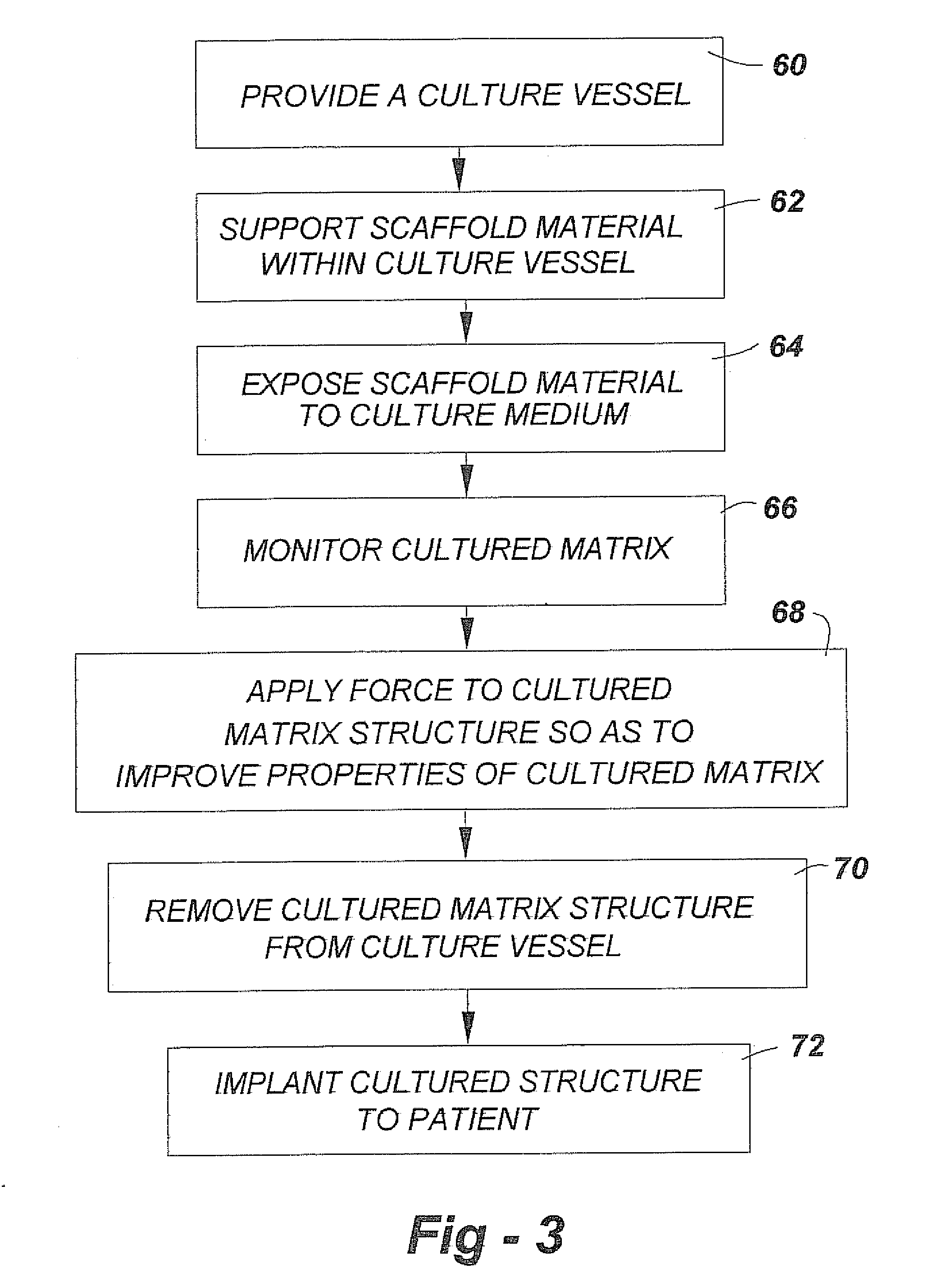
Musculoskeletal tissues produced in vitro are optimized in response to an externally applied mechanical load. The load applied may vary from tissue to tissue, depending upon the response desired, and may include intermittent axial, torsional, and bending loads to produce cortical structures. Compression alone is preferably applied to produce cancellous bone. A method according to the invention for culturing bone in vitro comprises: providing a culture vessel providing a scaffold material, supporting the scaffold material within the tissue culture vessel so as to be exposed to a tissue culture medium, and exerting a force on the scaffold material during growth of a bone construct (a cultured bone growth) around the scaffold material. Applicable apparatus preferably includes a culture vessel, holders for holding a scaffold within the tissue culture vessel, means for introducing a tissue culture medium to the tissue culture vessel, and an actuator adapted to apply a force to developing bone during the in vitro culture of the tissue, whether bone, cartilage, ligament, or composites thereof.
Owner:MEDIDEA
In-vitro mechanical loading of musculoskeletal tissues
InactiveUS20090325295A1Skeletal/connective tissue cellsTissue/virus culture apparatusLigament structureBone growth
Musculoskeletal tissues produced in vitro are optimized in response to an externally applied mechanical load. The load applied may vary from tissue to tissue, depending upon the response desired, and may include intermittent axial, torsional, and bending loads to produce cortical structures. Compression alone is preferably applied to produce cancellous bone. A method according to the invention for culturing bone in vitro comprises: providing a culture vessel providing a scaffold material, supporting the scaffold material within the tissue culture vessel so as to be exposed to a tissue culture medium, and exerting a force on the scaffold material during growth of a bone construct (a cultured bone growth) around the scaffold material. Applicable apparatus preferably includes a culture vessel, holders for holding a scaffold within the tissue culture vessel, means for introducing a tissue culture medium to the tissue culture vessel, and an actuator adapted to apply a force to developing bone during the in vitro culture of the tissue, whether bone, cartilage, ligament, or composites thereof.
Owner:MEDIDEA
Compositions and methods for generating musculoskeletal tissue
The present disclosure provides compositions comprising musculoskeletal cells and mesenchymal stem cells in discrete regions. The present disclosure provides systems comprising a subject composition; and methods of using a subject composition to generate cartilage, bone, tendon, muscle, intervertebral disc, or other musculoskeletal tissues.
Owner:RGT UNIV OF CALIFORNIA
System and method for minimally invasive tissue treatment
Delivering ultrasonic energy to a target musculoskeletal tissue site includes connecting a delivery device to a vacuum source, a fluid source, and a power signal source. The delivery device has a housing portion maintaining an ultrasound transducer and a tip portion having a sleeve and a cannula. The cannula is coupled to the ultrasound transducer and received in the sleeve to define a covered portion and an exposed portion. Ultrasonic energy is generated by sending a power signal from the power signal source to the ultrasound transducer. The ultrasonic energy is transmitted from the ultrasound transducer to the cannula, such that the exposed portion of the cannula delivers ultrasonic energy at a frequency that is pre-selected to debride musculoskeletal tissue upon percutaneous insertion of the tip portion.
Owner:TENEX HEALTH
Scaffolds for Bone-Soft Tissue Interface and Methods of Fabricating the Same
PendingUS20200163752A1Additive manufacturing apparatusBone implantMechanical integritySkeletal tissues
A device for regenerating musculoskeletal tissue having a scaffold comprised of fiber layers adapted to provide mechanical integrity to the scaffold in the form of increased tensile and compressive resistance and one or more other layers adapted to provide mechanical integrity and to provide a suitable biochemical environment.
Owner:STC UNM
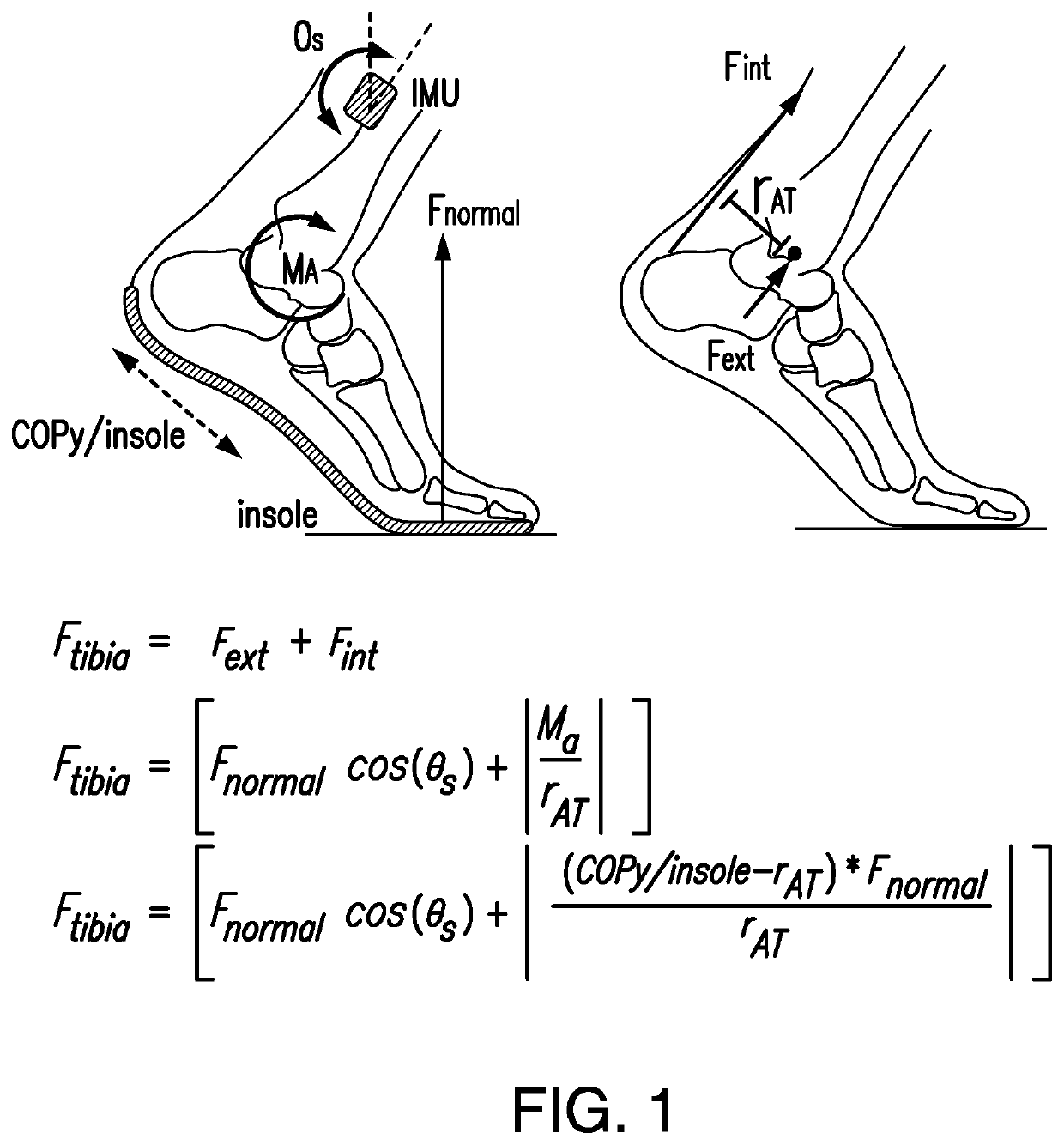
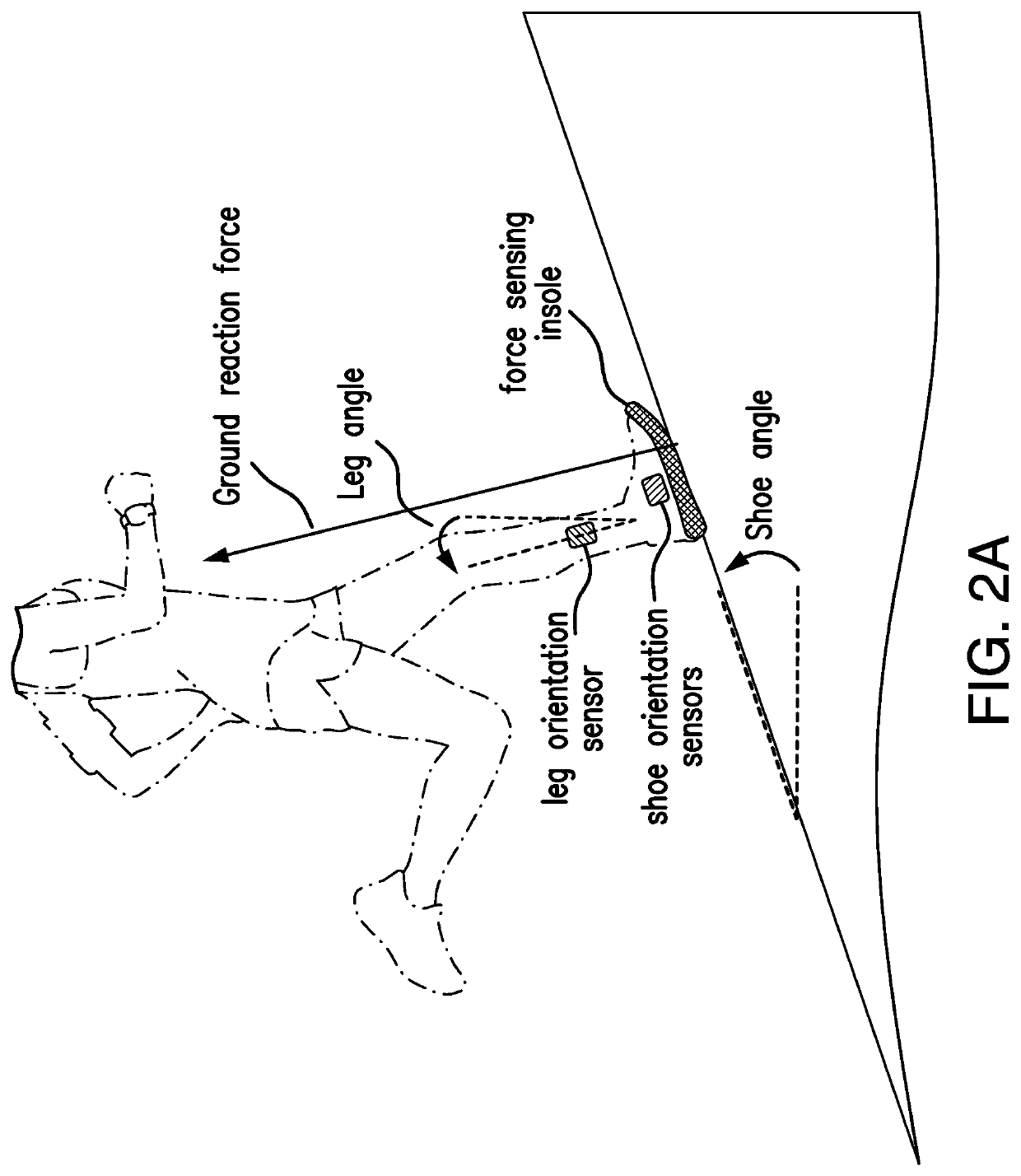
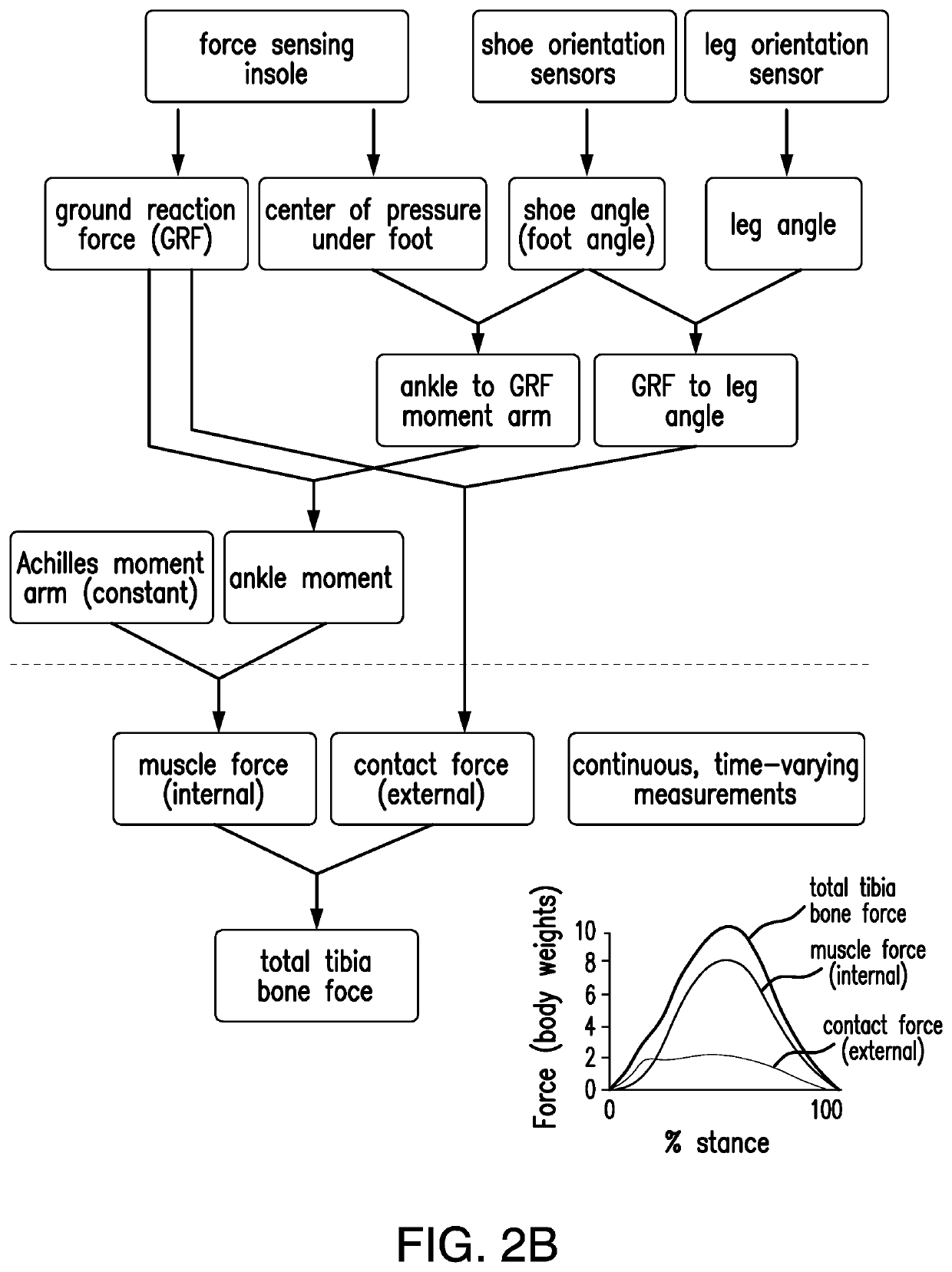
Wearable device to monitor musculoskeletal loading, estimate tissue microdamage and provide injury risk biofeedback
PendingUS20210236020A1Reduce the risk of injuryMusculoskeletal system evaluationInertial sensorsBiomechanicsEngineering
A wearable device operably worn by a user for monitoring musculoskeletal loading on structure inside the body of the user includes a plurality of sensors, each sensor operably worn by the user at a predetermined location and configured to detect information about a biomechanical activity of musculoskeletal tissues, a limb segment orientation, and / or a loading magnitude or location thereon; and a processing unit in communication with the plurality of sensors and configured to process the detected information by the plurality of sensors to estimate the musculoskeletal loading, and communicate the estimated musculoskeletal loading to the user and / or a party of interest.
Owner:VANDERBILT UNIV
Carbon nanotubes and graphene patches and implants for biological tissue
InactiveUS20160030640A1Prevent relapseEasy transferBiocideMaterial nanotechnologyCuticleCarbon nanotube
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
System and method for three dimensional printed implantation guides
InactiveUS20150351916A1Accurate placementSurgical needlesVaccination/ovulation diagnosticsDiagnostic Radiology ModalityCt arthrography
A process for fabricating size-specific, customized bio-printed musculoskeletal tissue using three dimensional data collected from radiologic imaging is provided. Also, provided is a guide that is created from radiological imaging that demarcates the area of surgical interest. The guide is 3D printed according to guide dimensions collected from radiological imaging, including, but not limited to, CT imaging scans, CT arthrography, ultrasound, MRI, MR arthrography, or any other imaging modality used to image the musculoskeletal system.
Owner:KOSAREK FR J +1
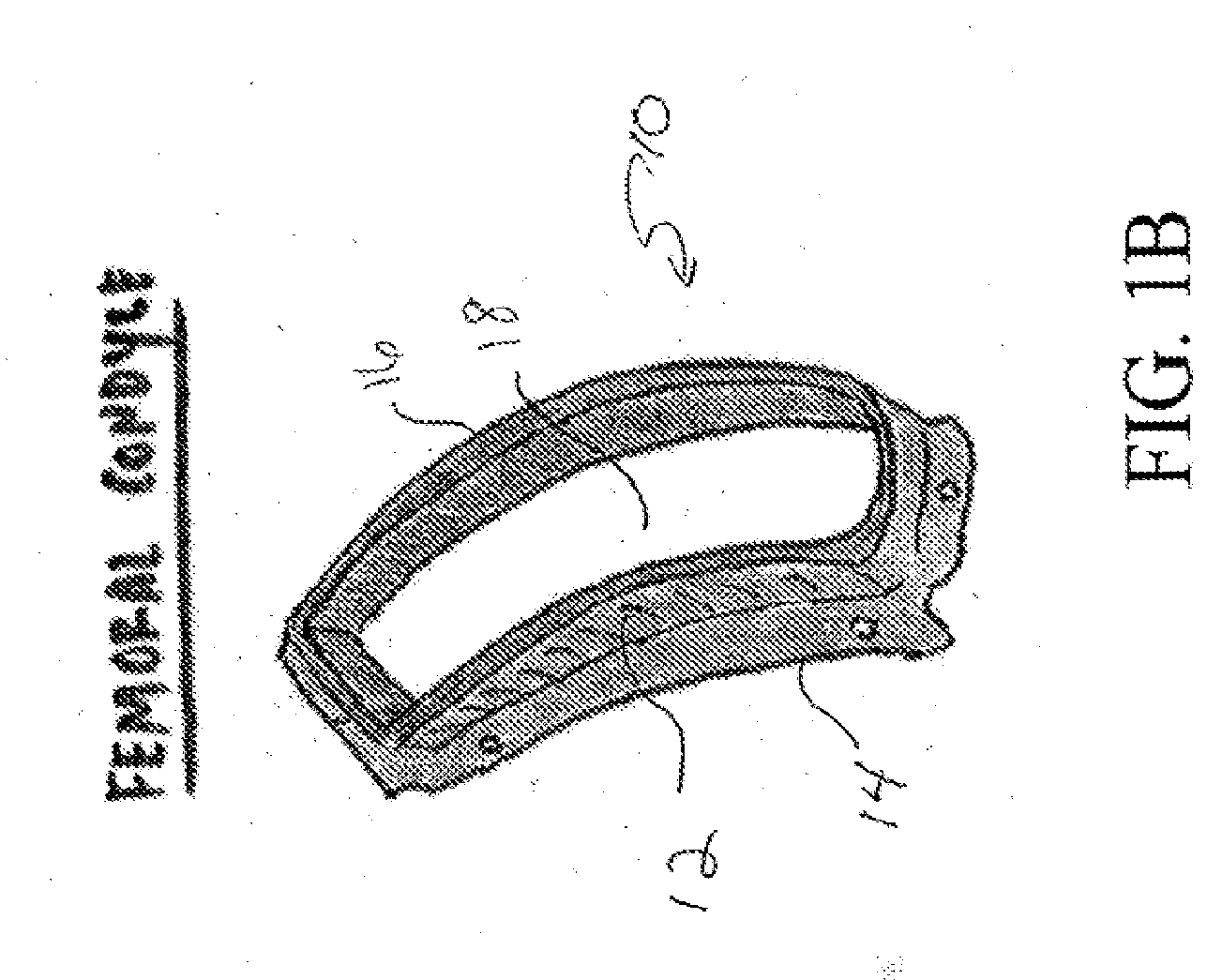
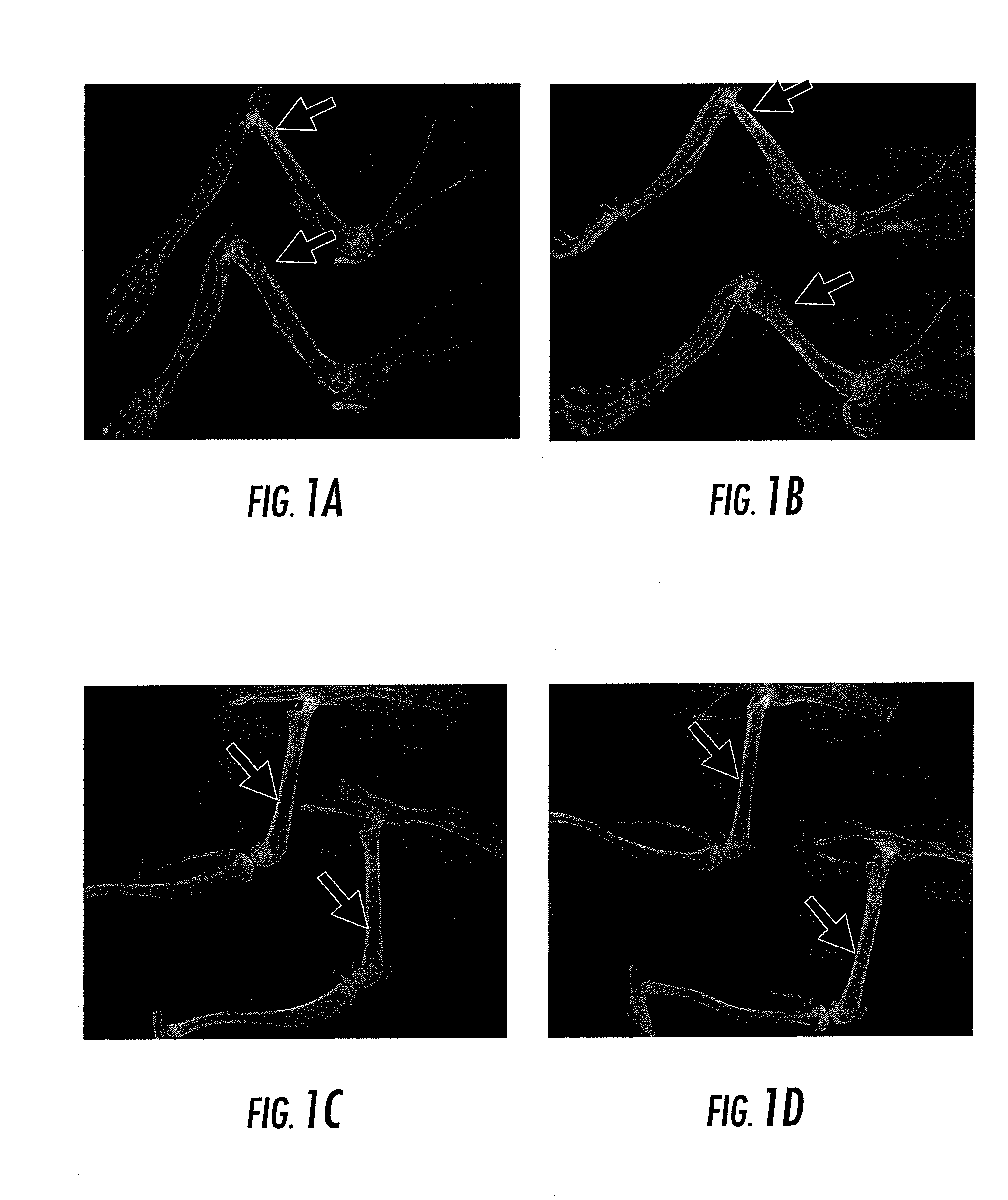
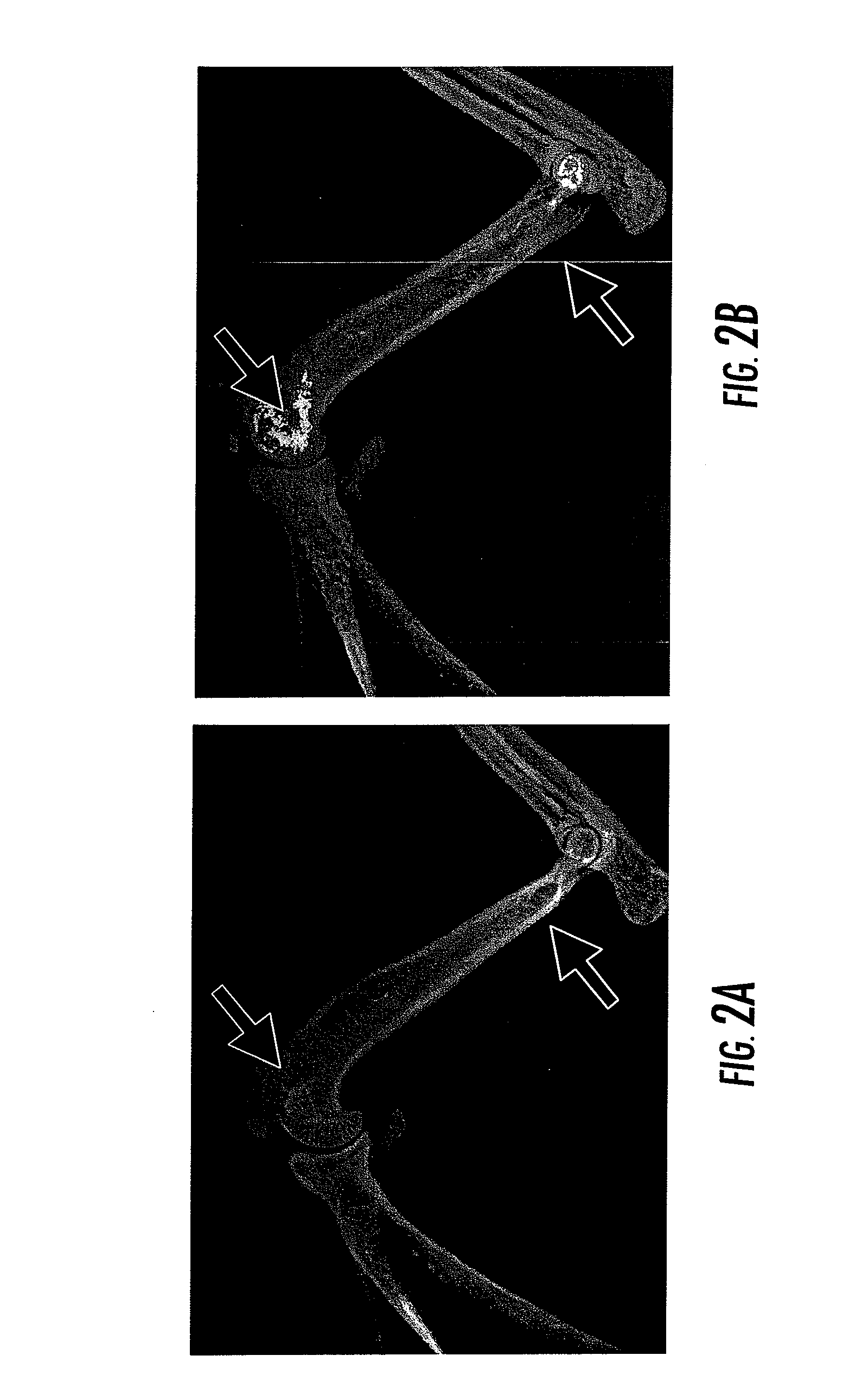
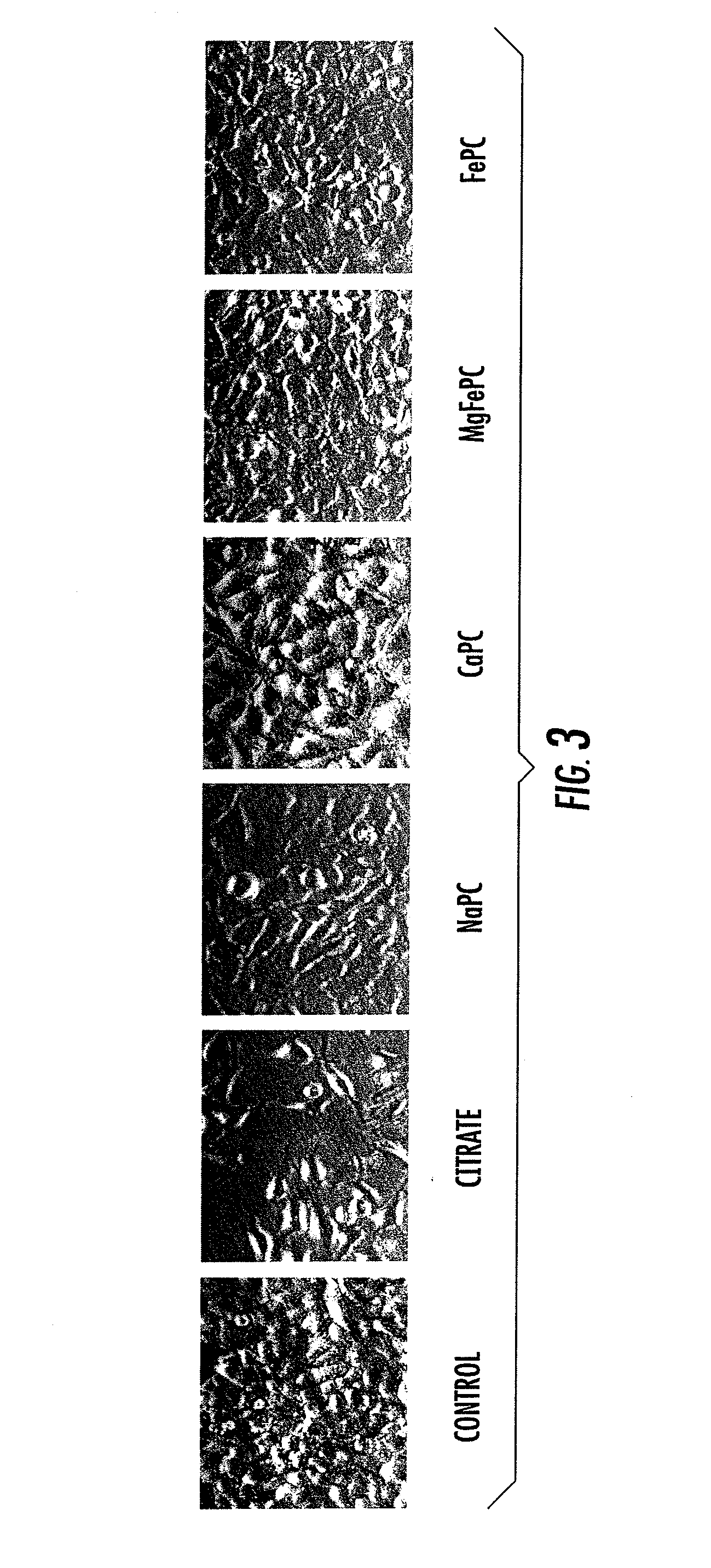
Compositions and Methods for the Treatment of Musculoskeletal Related Diseases and Disorders Using Metal Ion-Citrate Analog Complexes
InactiveUS20120238521A1Prevent degradationSuppression problemBiocideMuscular disorderDiseasePhenyl group
The invention is directed to a composition and associated method for the treatment of musculoskeletal related disorders and diseases. In particular, the present invention provides a class of citrate compounds that can be used to for the treatment of diseases / disorders characterized by the degeneration of musculoskeletal tissues including menisci, bone, articular cartilage, and soft tissues. Examples of suitable citrate and citrate analog compounds include citrates having the following base formula (I):where X may be one of the following:wherein Y, Y′, and Y″ are independently a citrate moiety, O, O—Y, OH, NH, NH—Y, R and R—Y, with R being an alkyl, alkyene, ester, aryl, or phenyl group having from 1 to 6 carbon atoms.
Owner:CHARLOTTE MECKLENBURG HOSPITAL AUTHORITY
Medium for handling and storing biological tissues of the musculoskeletal system outside an organism
InactiveUS20110070204A1Maintain their viabilityBiocideDead animal preservationLigament structureOrganism
The present disclosure relates to a medium for handling, including storing, cultivating, coating, impregnating and / or preserving musculoskeletal tissues such as bone, cartilage, tendon, muscle, nerve, ligament, blood vessel, skin, fascia, bursa and joint capsule tissue or cells, wherein the medium is an aqueous solution comprising hyaluronan and a first saccharide, polyol, or combination thereof. Further disclosed are methods of handling musculoskeletal tissue or cells or preserving the viability of these tissues or cells using this medium as well as the preserved tissues or cells.
Owner:ELIAS ILAN
Multi-component non-biodegradable implant, a method of making and a method of implantation
ActiveUS20170209624A1Maximizes integrationHigh and low viscosityBone implantSurgeryEngineeringBiodegradable implants
An implant comprising at least three components, namely, a solid hydrogel, a porous hydrogel adjacent to or surrounding the solid hydrogel (together considered “the hydrogel”), and a porous rigid base. The solid hydrogel and porous rigid base carry joint load, and the porous hydrogel layer and the porous rigid base allow for cellular migration into and around the implant. The invention is also a novel method of manufacturing the implant, a novel method of implanting the implant, and a method of treating, repairing or replacing biological tissue, more preferably musculoskeletal tissue, with the implant.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
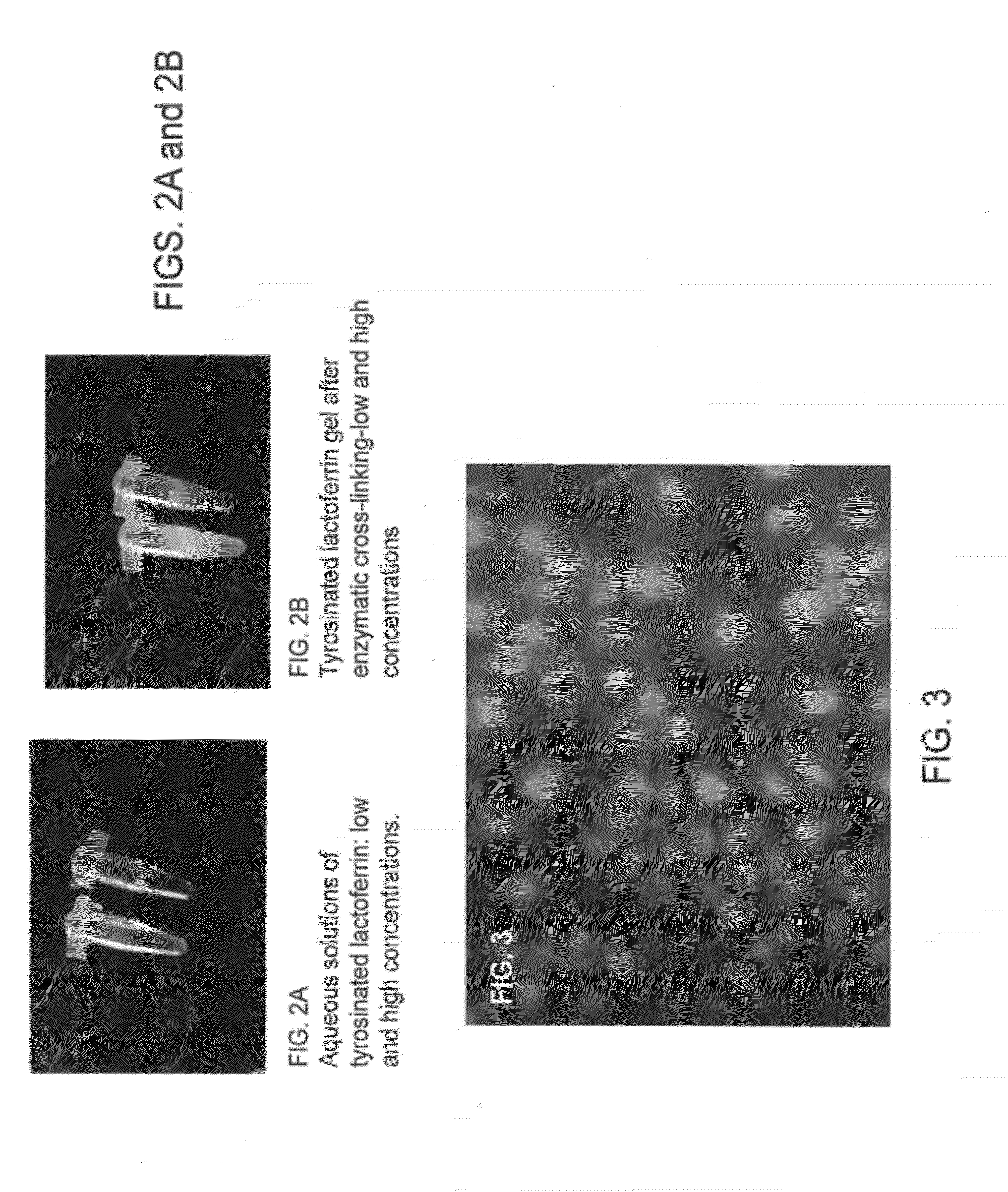
Lactoferrin-based biomaterials for tissue regeneration and drug delivery
The invention provides biomatrix compositions comprising cross-linked lactoferrin, either alone or in combination with other organic or inorganic components. Also provided are methods of making and using the biomatrix compositions. As described herein, cross-linked lactoferrin biomatrix retains the bioactivities of the lactoferrin molecule. The biomatrix composition can act as a matrix for cell adhesion and growth and is particularly useful in musculoskeletal tissue regeneration. The biomatrix compositions can be pre-formed or injectable and can act as a cell, drug or protein delivery vehicle.
Owner:UNIV OF CONNECTICUT
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com