Patents
Literature
10784results about "Skeletal/connective tissue cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasma protein matrices and methods for their preparation
InactiveUS7009039B2Rapid cell growthRapid vascularizationBiocidePeptide/protein ingredientsBiological propertyFreeze-drying
A freeze dried biocompatible matrix comprising plasma proteins, useful as implants for tissue engineering as well as in biotechnology, and methods of producing the matrix are provided. Mechanical and physical parameters can be controlled by use of auxiliary components or additives which may be removed after the matrix is formed in order to improve the biological properties of the matrix. The matrices according to the present invention may be used clinically per se, or as a cell-bearing implant.
Owner:PROCHON BIOTECH
Generation of adipose tissue and adipocytes
The invention provides novel methods by which adipose tissue, preadipocytes, and adipocytes can be generated for research purposes, and methods for identifying cell populations that can proliferate and differentiate into adipocytes in vivo. The invention further provides a means for the in vivo derivation of “designer” or “customized” adipose tissue, preadipocytes, and adipocytes. Also provided are methods for identifying agents that affect adipocytes and adipose tissue, as well as the agents themselves. In particular, the present invention allows for creation of tissues and cells that can be used to screen for agents useful for treating human disorders associated with adipose tissue, including obesity, metabolic syndrome, and diabetes.
Owner:LOREM VASCULAR PTE LTD
Methods and materials for the growth of primate-derived primordial stem cells in feeder-free culture
Methods and materials for culturing primate-derived primordial stem cells are described. In one embodiment, a cell culture medium for growing primate-derived primordial stem cells in a substantially undifferentiated state is provided which includes a low osmotic pressure, low endotoxin basic medium that is effective to support the growth of primate-derived primordial stem cells. The basic medium is combined with a nutrient serum effective to support the growth of primate-derived primordial stem cells and a substrate selected from the group consisting of feeder cells and an extracellular matrix component derived from feeder cells. The medium further includes non-essential amino acids, an anti-oxidant, and a first growth factor selected from the group consisting of nucleosides and a pyruvate salt.
Owner:ASTERIAS BIOTHERAPEUTICS INC
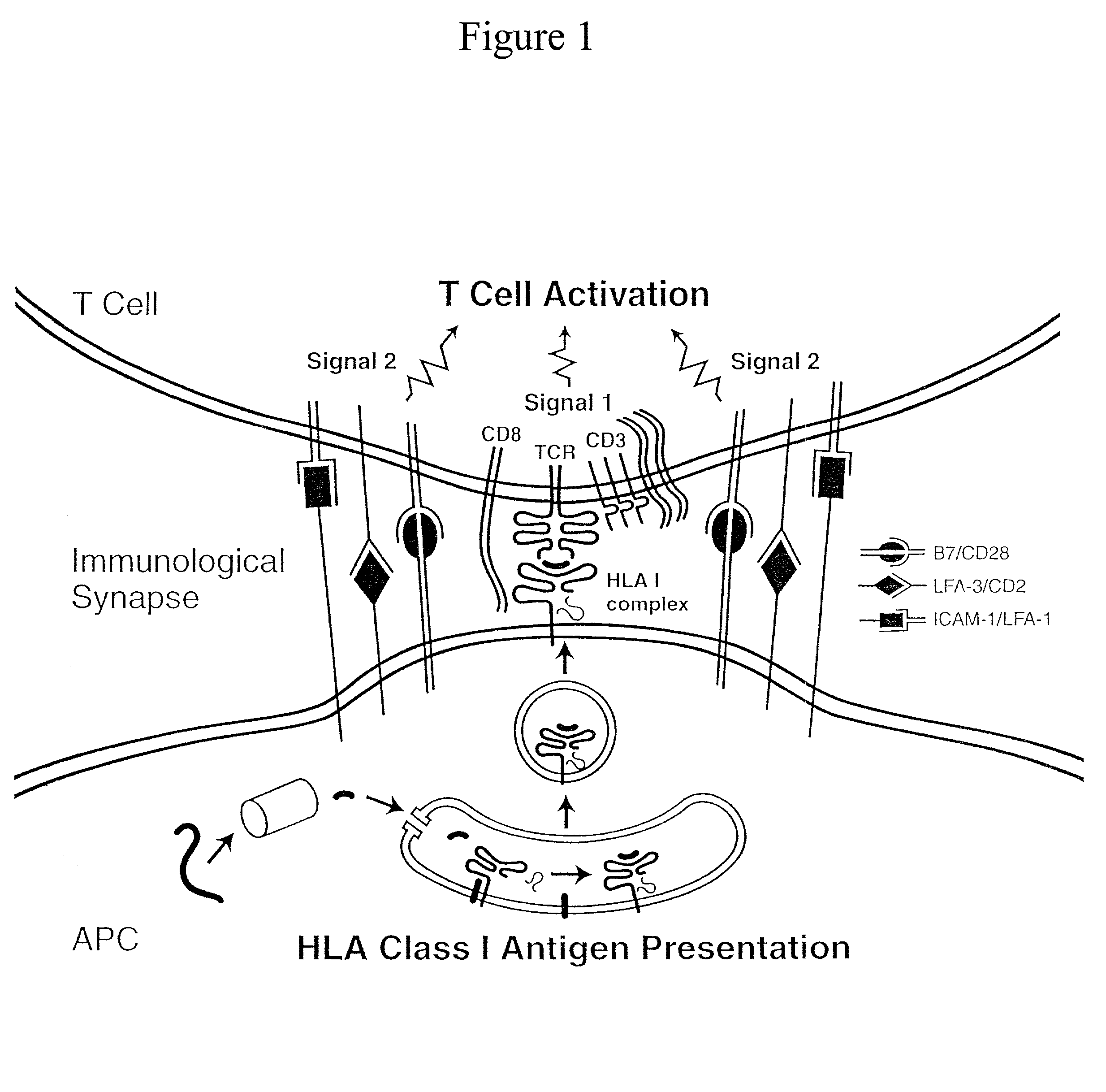
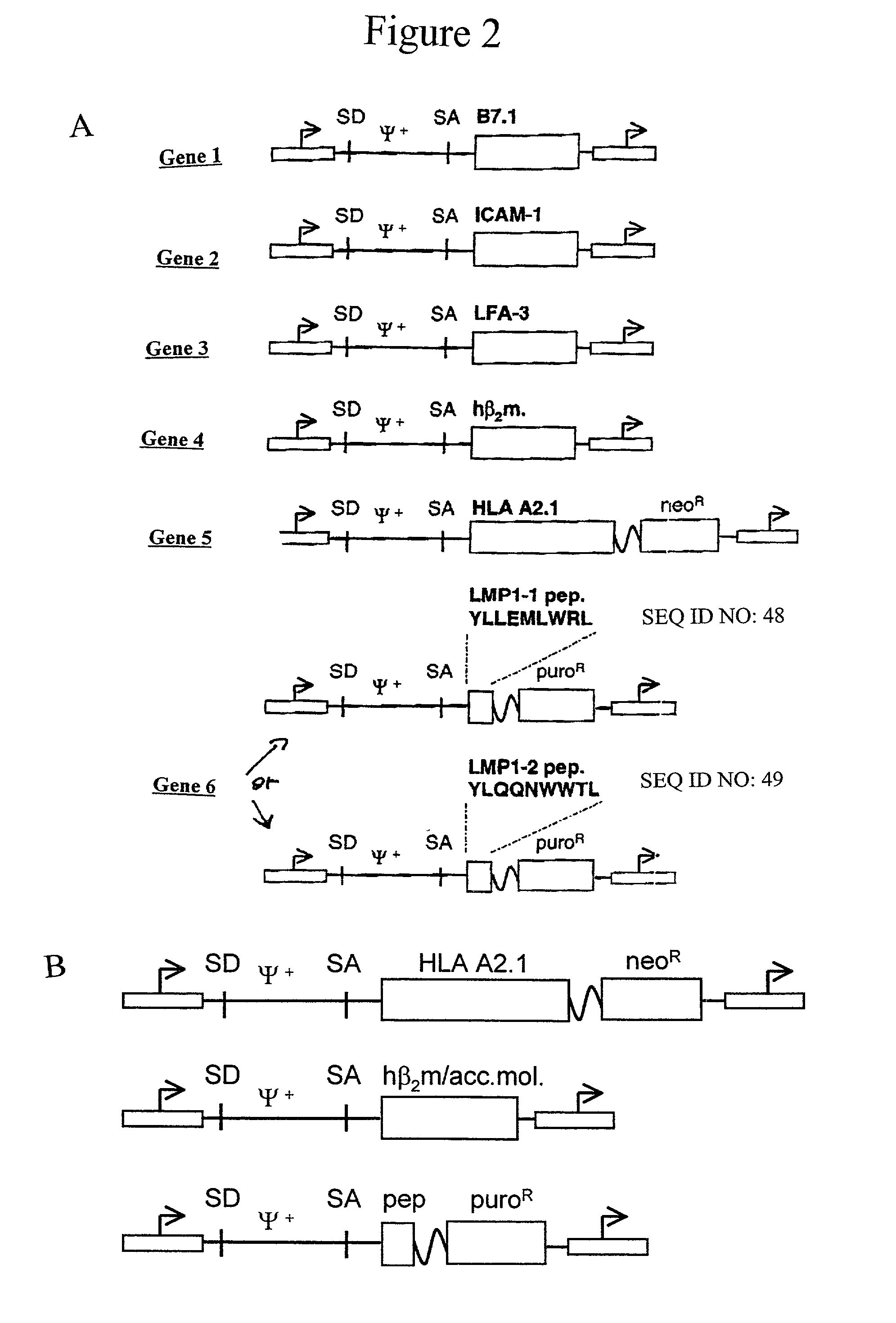
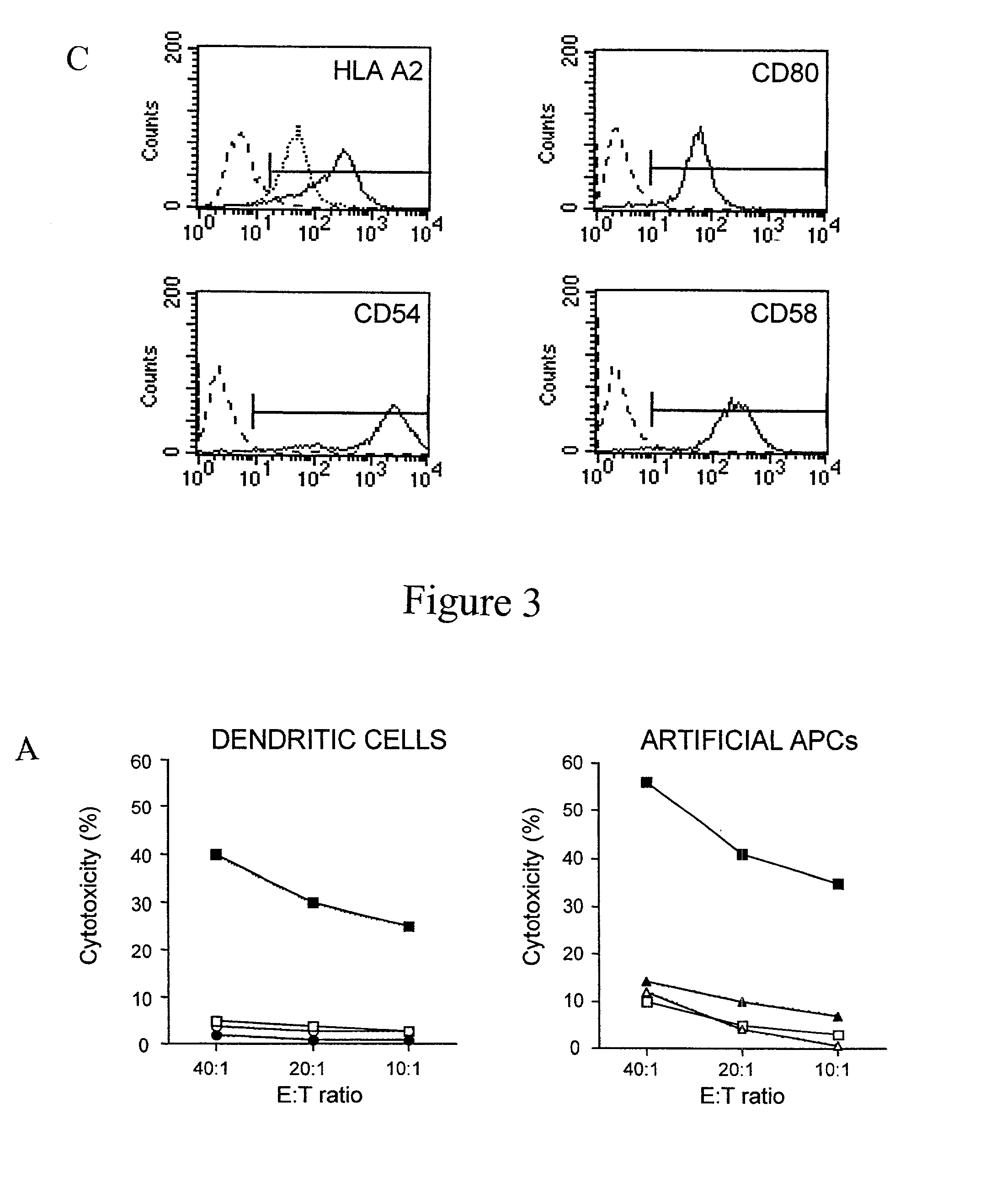
Artificial antigen presenting cells and methods of use thereof
InactiveUS20020131960A1Palliating their conditionReduce riskBiocideCompound screeningEpitopeAccessory molecule
The invention provides an artificial antigen presenting cell (AAPC) comprising a eukaryotic cell expressing an antigen presenting complex comprising a human leukocyte antigen (HLA) molecule of a single type, at least one exogenous accessory molecule and at least one exogenous T cell-specific epitope. Methods of use for activation of T lymphocytes are also provided.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
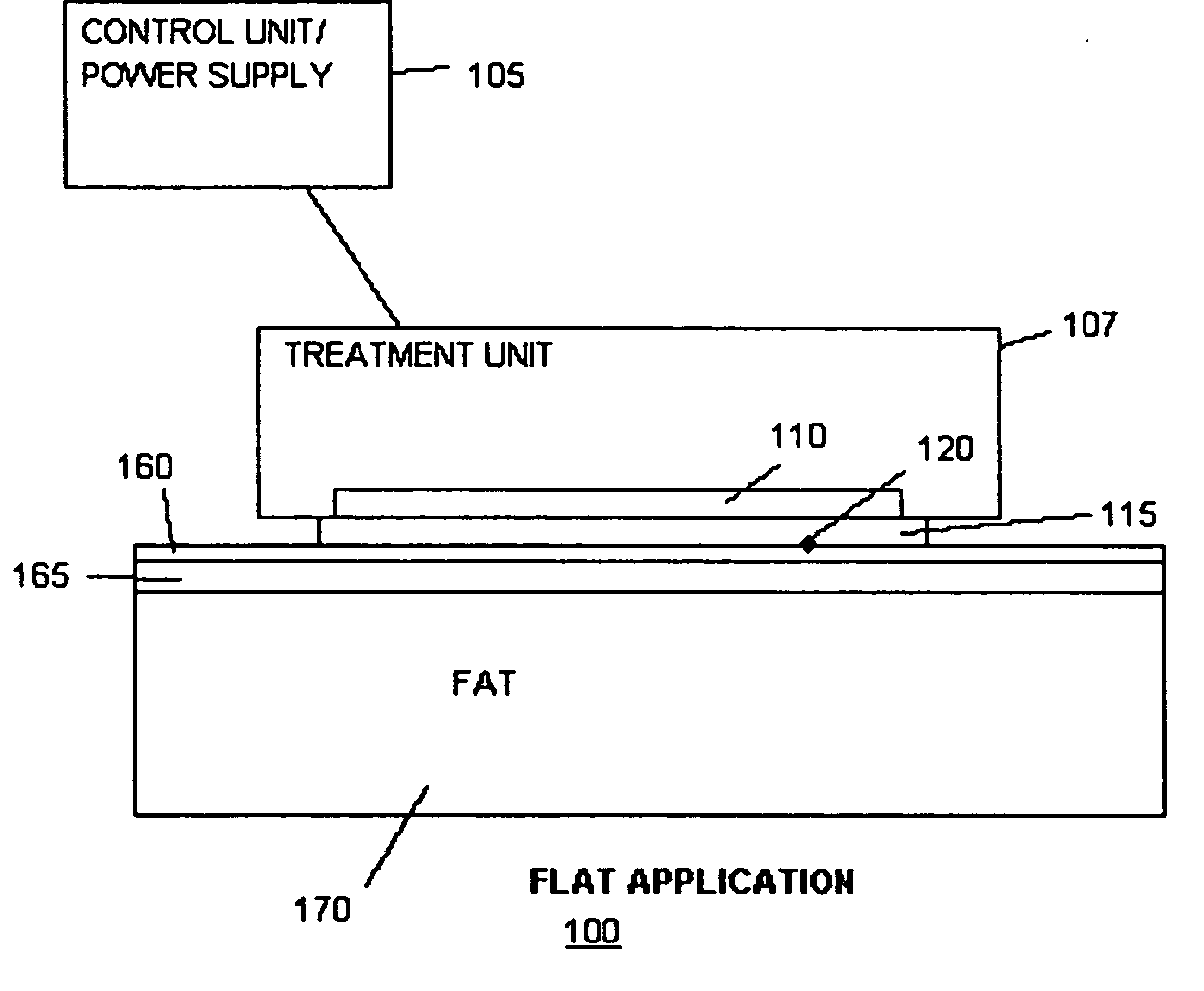
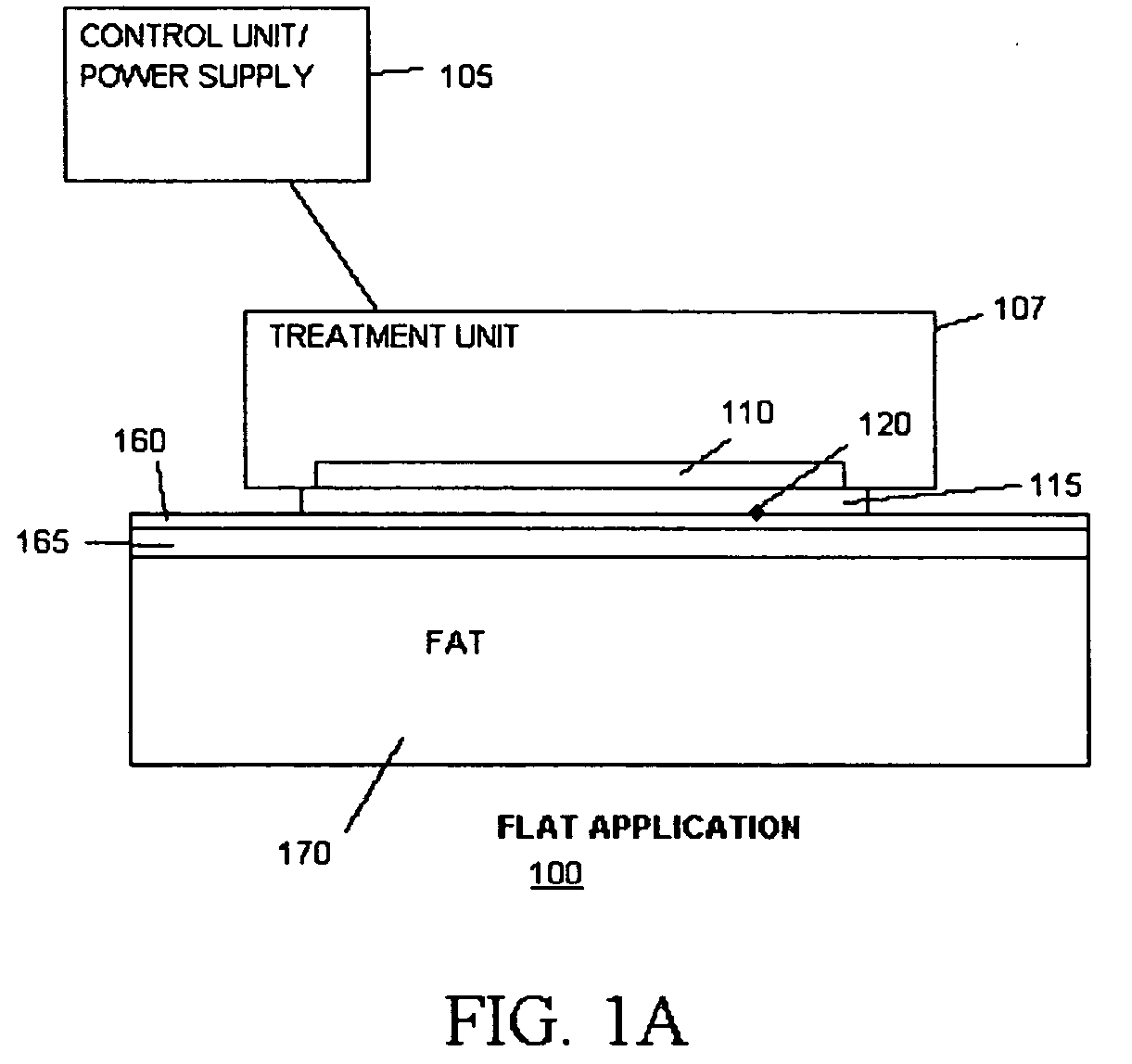
Methods and devices for detection and control of selective disruption of fatty tissue during controlled cooling
ActiveUS20050251120A1Ultrasonic/sonic/infrasonic diagnosticsSkeletal/connective tissue cellsMedicineAdipose tissue
Owner:THE GENERAL HOSPITAL CORP
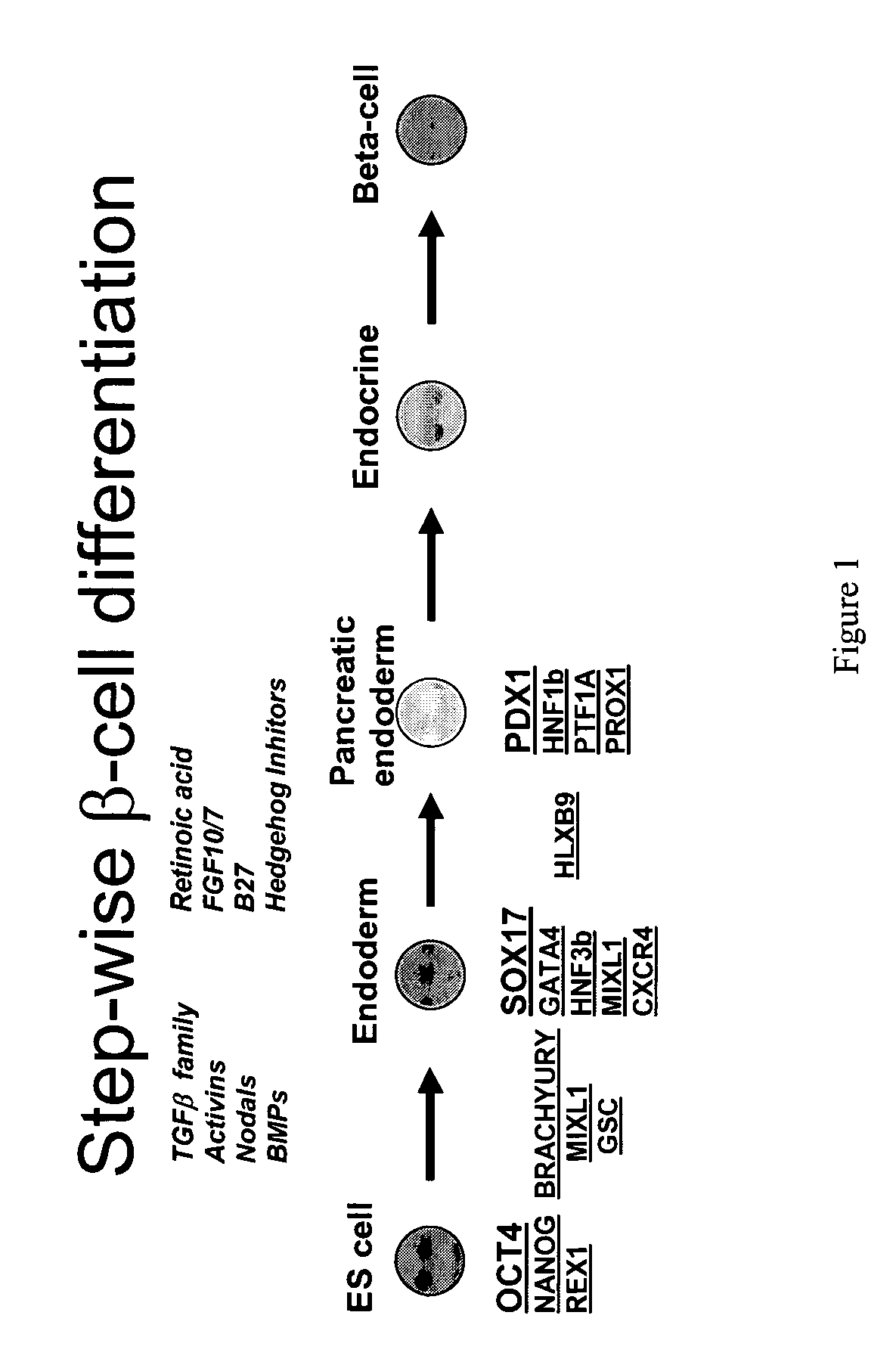
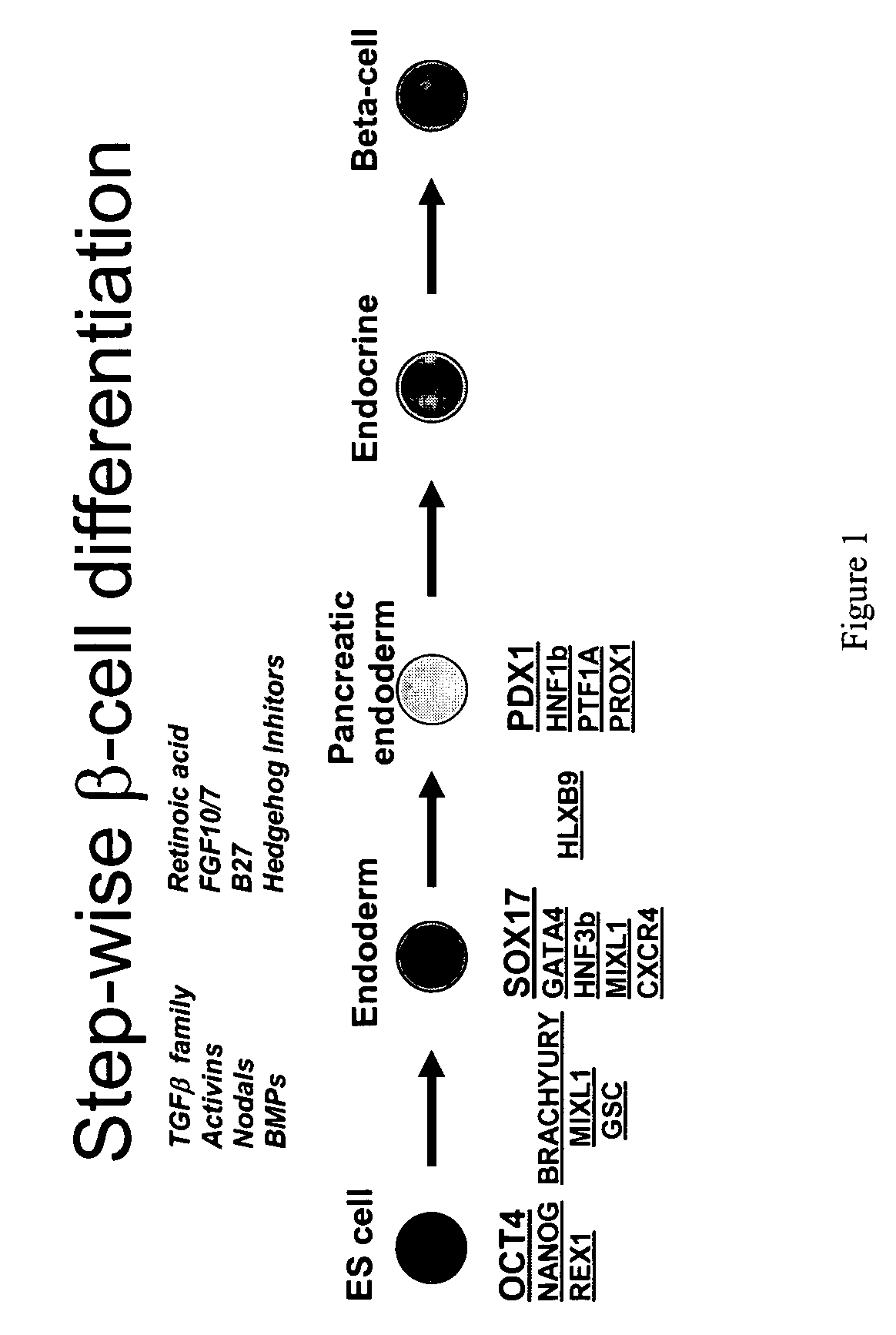
PDX1 expressing endoderm
InactiveUS20050266554A1Increase differentiationIncrease productionGastrointestinal cellsDiagnosticsGerm layerCell type
Disclosed herein are cell cultures comprising PDX1-positive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified PDX1-positive endoderm cells as well as methods for enriching, isolating and purifying PDX1-positive endoderm cells from other cell types. Methods of identifying differentiation factors capable of promoting the differentiation of endoderm cells, such as PDX1-positive foregut endoderm cells and PDX1-negative definitive endoderm cells, are also disclosed.
Owner:CYTHERA
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Defined media for stem cell culture
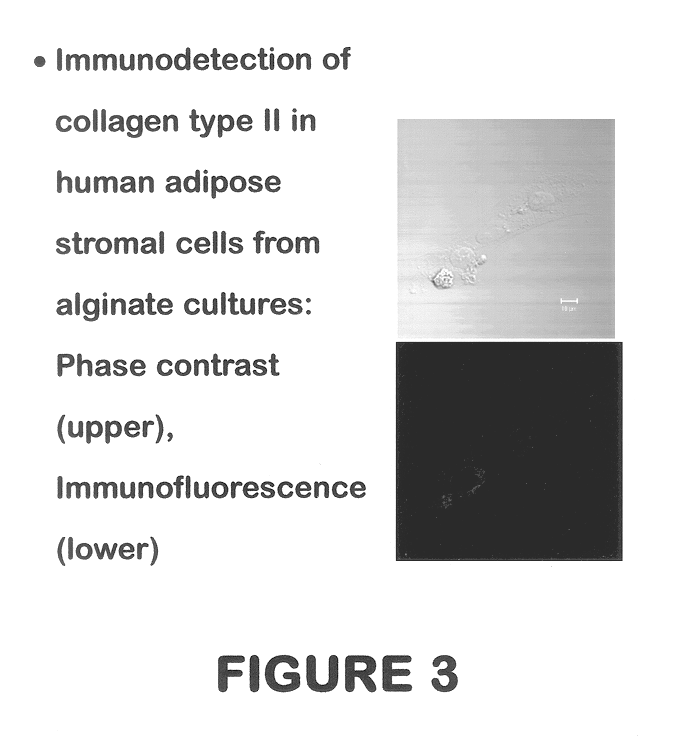
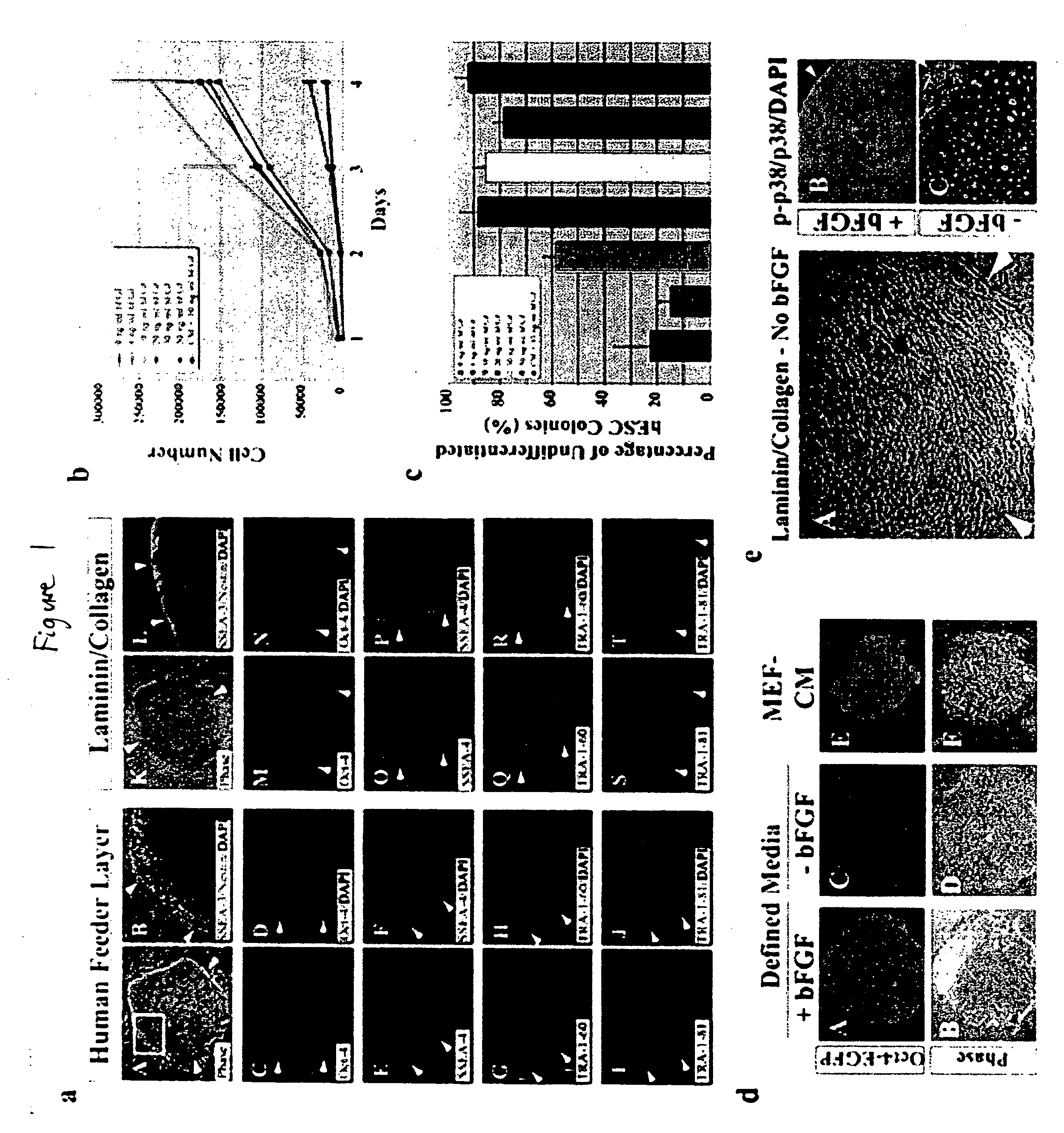
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Multiple mesodermal lineage differentiation potentials for adipose tissue-derived stromal cells and uses thereof
InactiveUS6555374B1Enhance and inhibit differentiationIncrease differentiationMicrobiological testing/measurementMammal material medical ingredientsGerm layerSmooth muscle
The invention relates to methods and compositions for the differentiation of stromal cells from adipose tissue into hematopoietic supporting stromal cells and myocytes of both the skeletal and smooth muscle type. The cells produced by the methods are useful in providing a source of fully differentiated and functional cells for research, transplantation and development of tissue engineering products for the treatment of human diseases and traumatic tissue injury repair.
Owner:COGNATE BIOSERVICES
Defined media for pluripotent stem cell culture
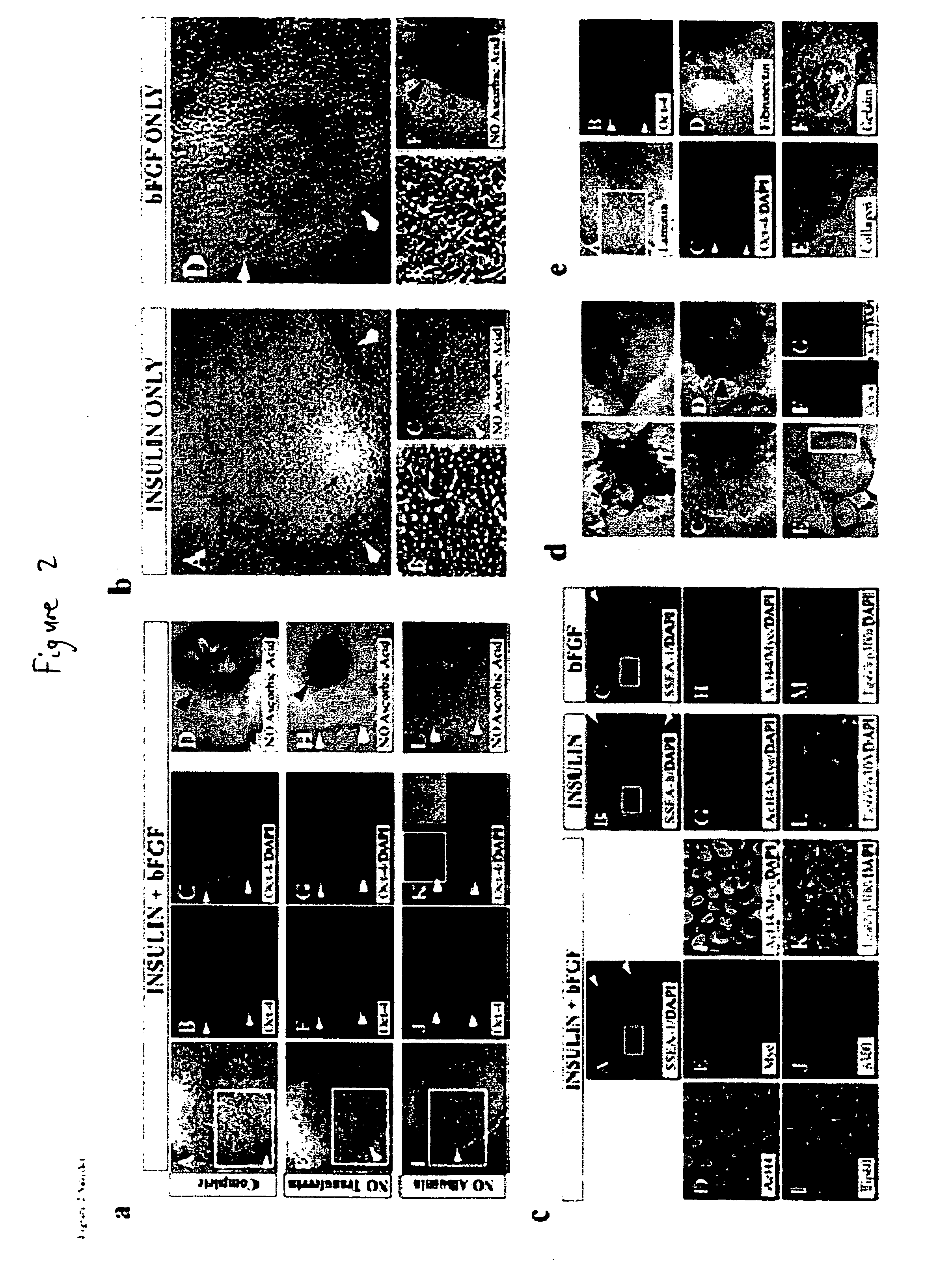
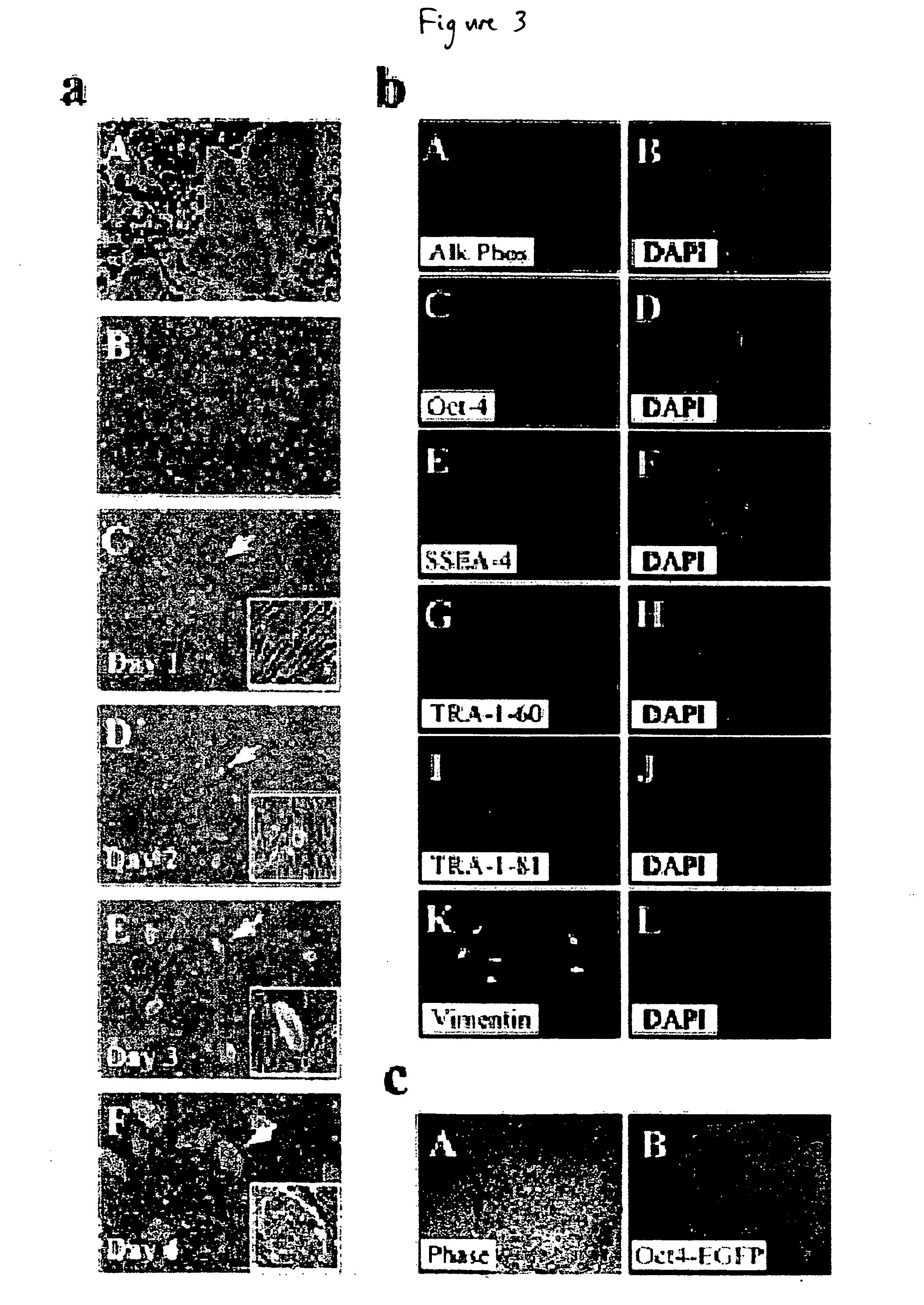
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Human cord blood as a source of neural tissue for repair of the brain and spinal cord
InactiveUS20020028510A1Easy to distinguishImprove neurological dysfunctionNervous disorderCell differentiationDiseaseCord blood stem cell
The present invention relates to the use of umbilical cord blood cells from a donor or patient to provide neural cells which may be used in transplantation. The isolated cells according to the present invention may be used to effect autologous and allogeneic transplantation and repair of neural tissue, in particular, tissue of the brain and spinal cord and to treat neurodegenerative diseases of the brain and spinal cord.
Owner:SANERON CCEL THERAPEUTICS +1
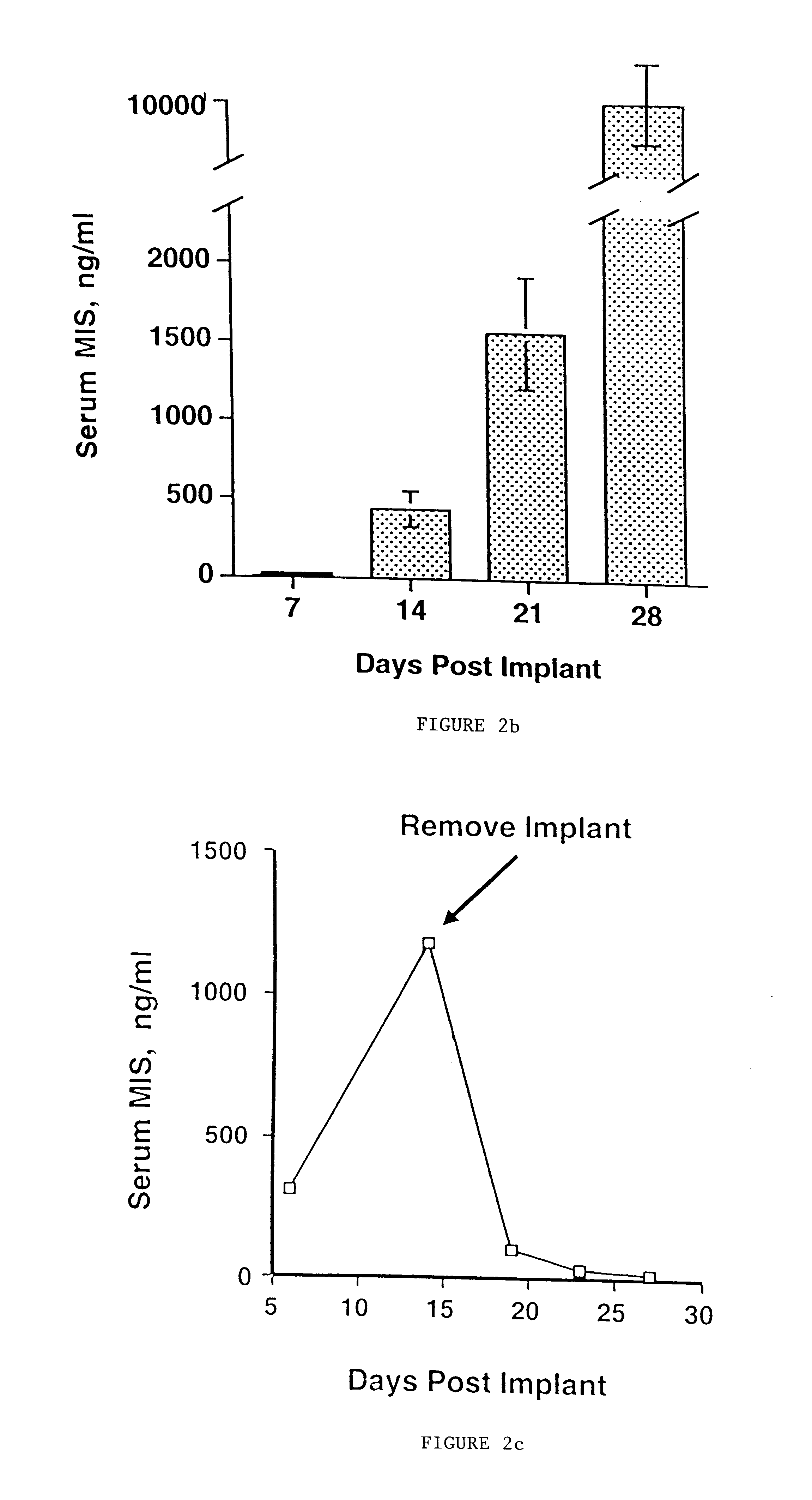
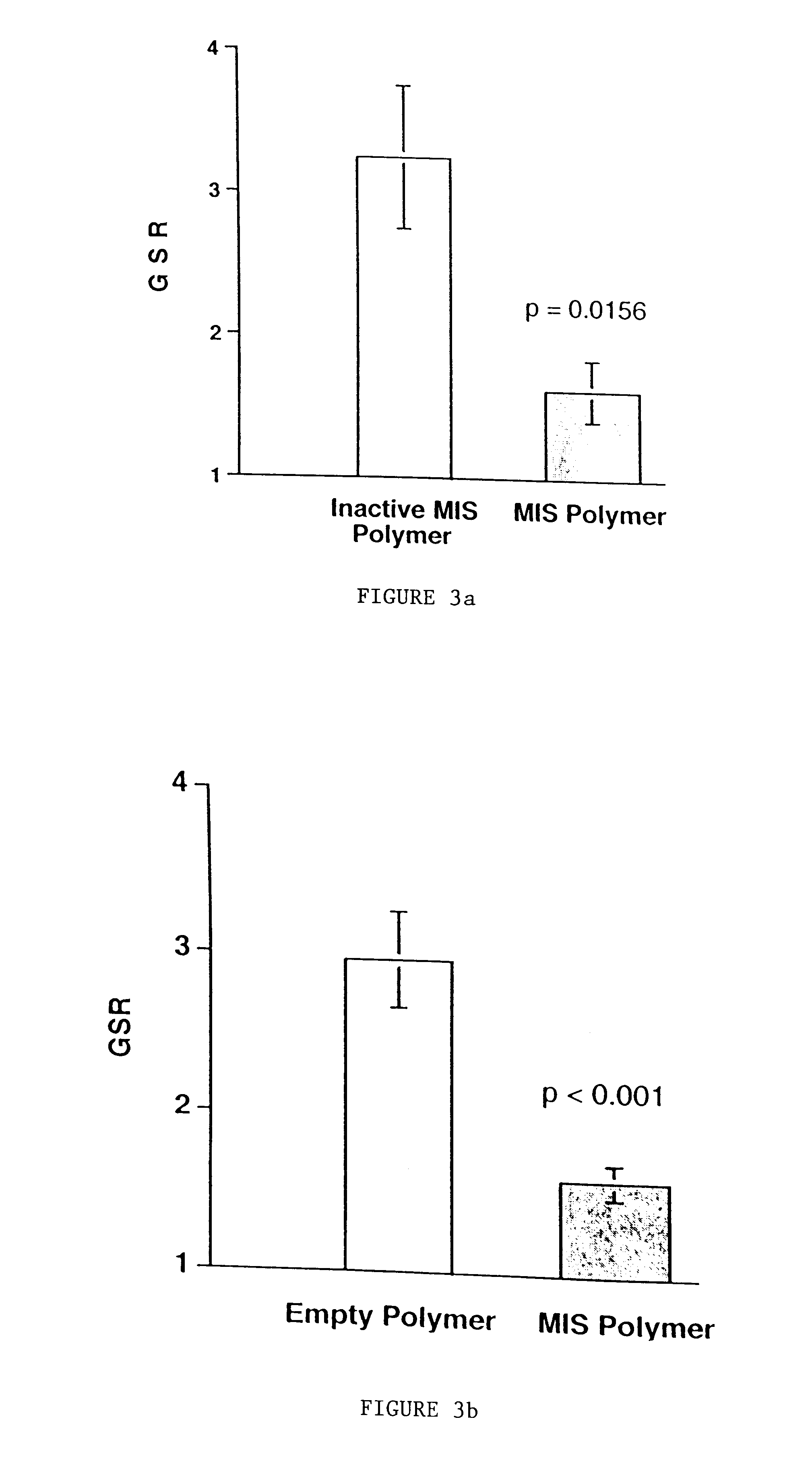
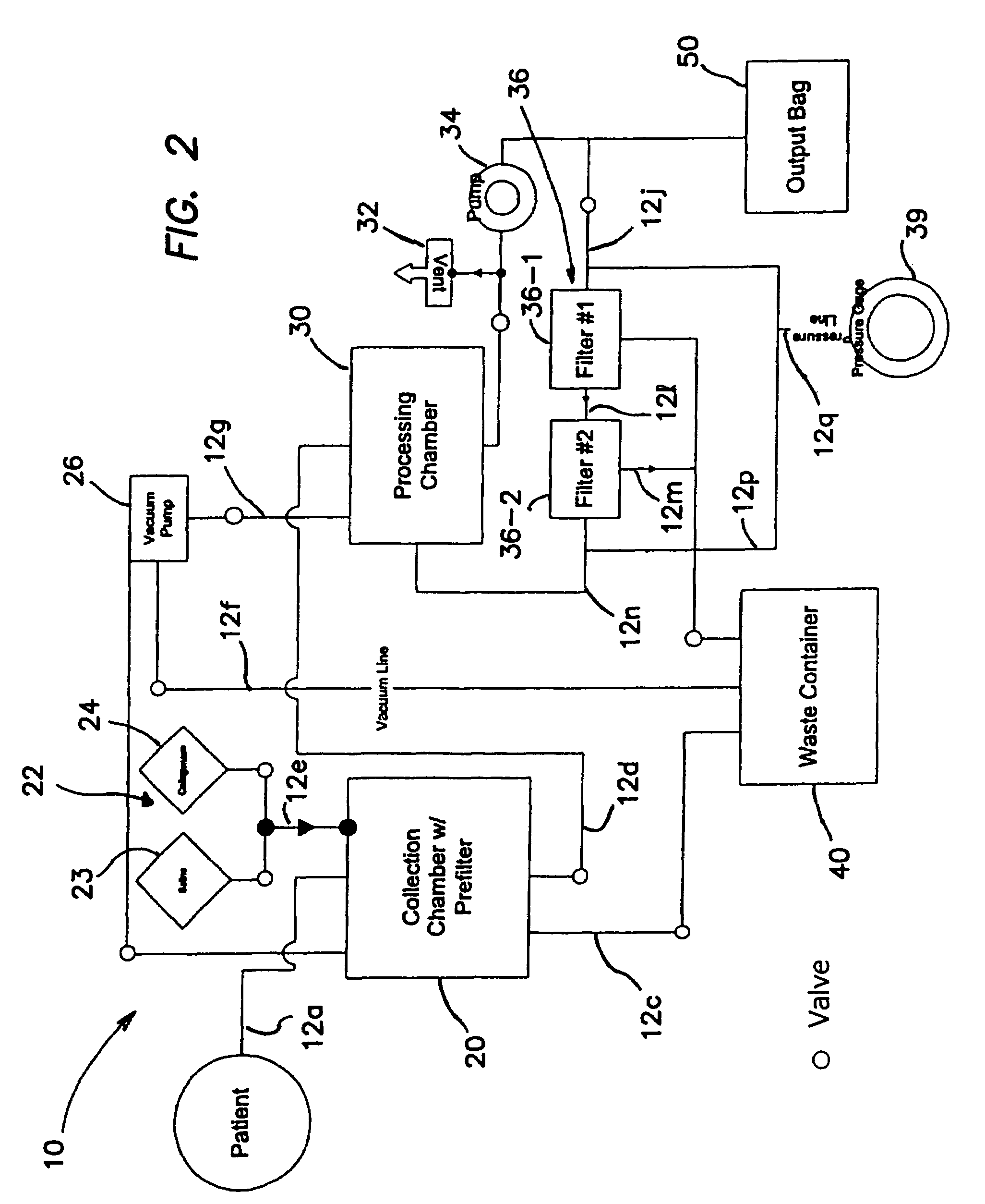
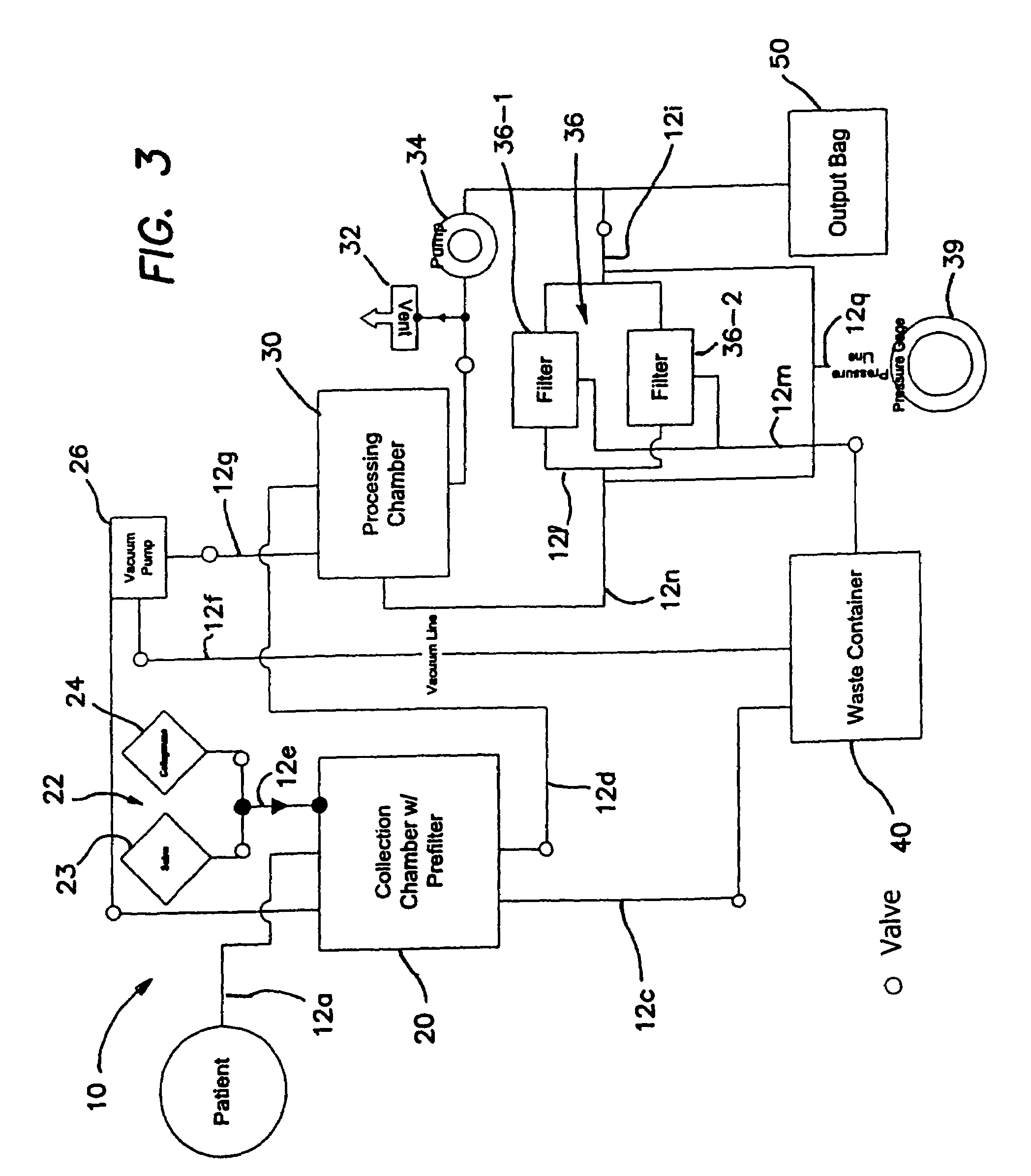
Delivery of therapeutic biologicals from implantable tissue matrices
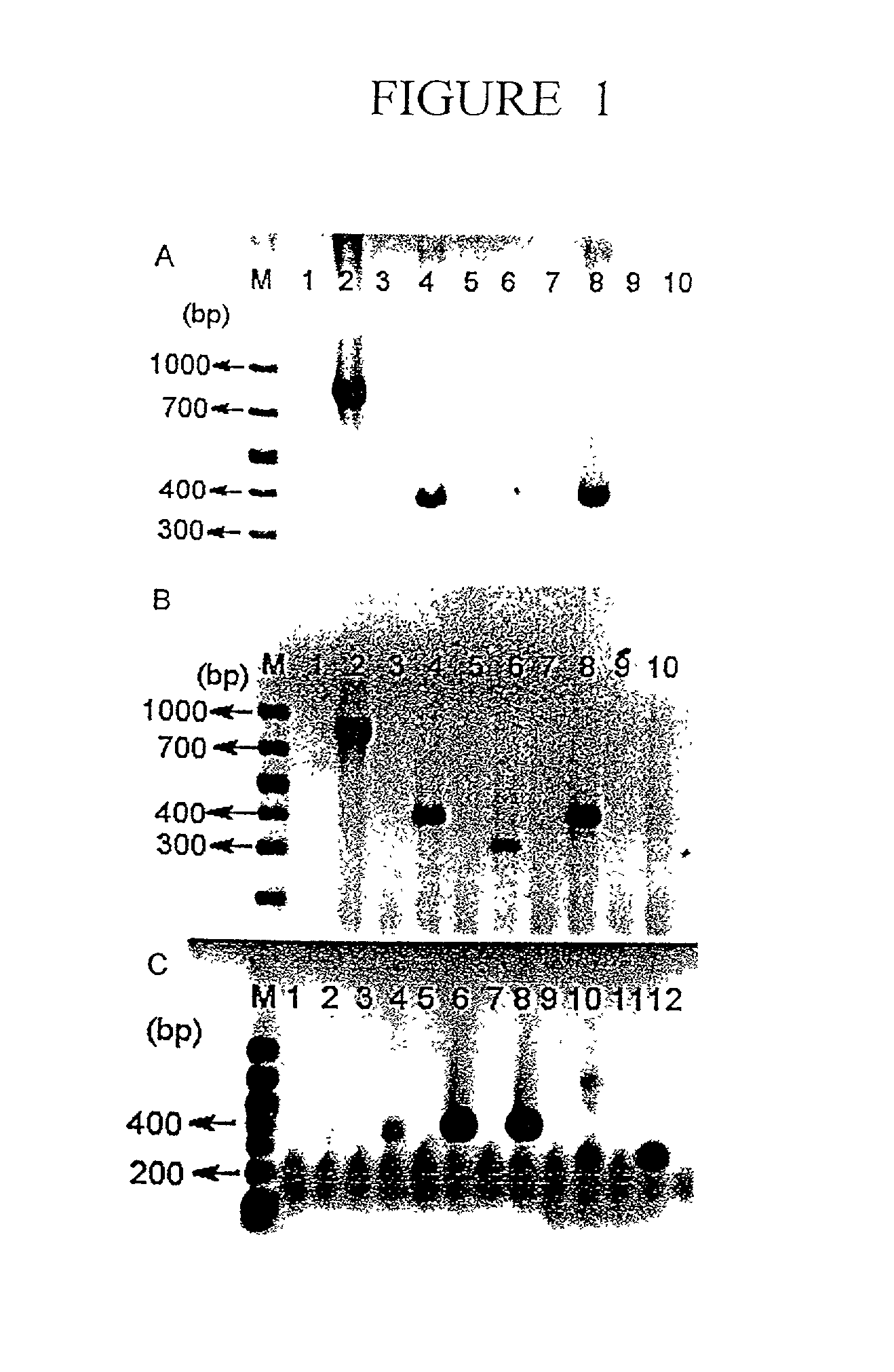
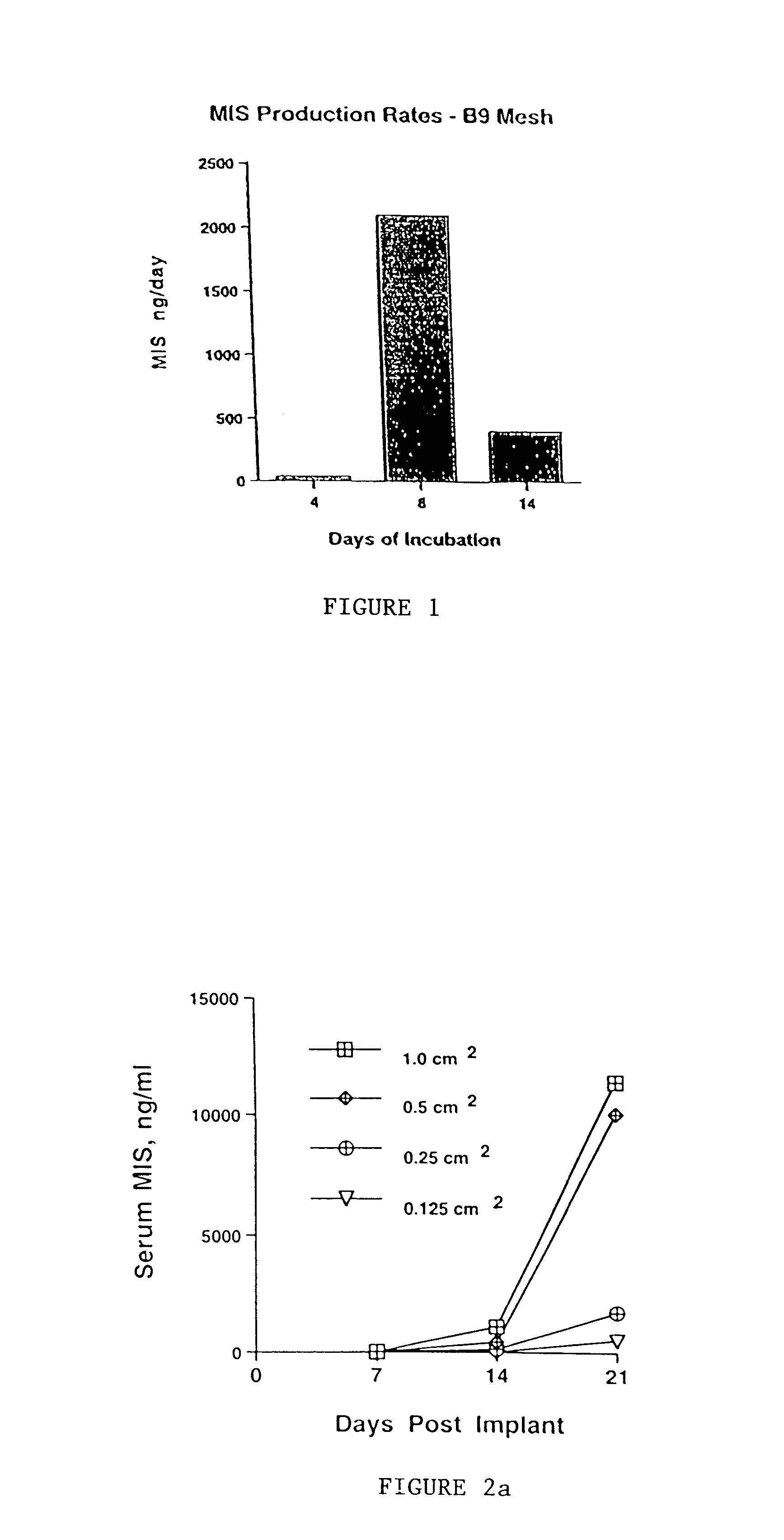
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Nanofibrillar structure and applications including cell and tissue culture
ActiveUS20050095695A1Reduce usageBioreactor/fermenter combinationsNanostructure manufactureLipid formationNanofiber
A nanofibrillar structure for cell culture and tissue engineering is disclosed. The nanofibrillar structure can be used in a variety of applications including methods for proliferating and / or differentiating cells and manufacturing a tissue. Also disclosed is an improved nanofiber comprising a lipid, lipophilic molecule, or chemically modified surface. The nanofibers can be used in a variety of applications including the formation of nanofibrillar structures for cell culture and tissue engineering.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Isolation, cultivation and uses of stem/progenitor cells
The present invention relates to a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The isolated stem cell cells can have embryonic stem cell-like properties and can be used for various therapeutic purposes. In one embodiment, the invention relates to the isolation and cultivation of stem cells such as epithelial and / or mesenchymal stem / progenitor cells under conditions allowing the cells to undergo mitotic expansion. Furthermore, the invention is directed to a method for the differentiation of the isolated stem / progenitor cells into epithelial and / or mesenchymal cells.
Owner:CELLRESEARCH CORP PTE LTD
Biodegradable polyurethanes and use thereof
InactiveUS20050013793A1Improve responseIncrease ratingsCell culture supports/coatingSkeletal/connective tissue cellsPolymer scienceDrug biological activity
A biodegradable and biocompatible polyurethane composition synthesized by reacting isocyanate groups of at least one multifunctional isocyanate compound with at least one bioactive agent having at least one reactive group —X which is a hydroxyl group (—OH) or an amine group (—NH2). The polyurethane composition is biodegradable within a living organism to biocompatible degradation products including the bioactive agent. Preferably, the released bioactive agent affects at least one of biological activity or chemical activity in the host organism. A biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is preferably derived from a diurea diol or a diester diol and is preferably biodegradable into biomolecule degradation products or into biomolecule degradation products and a biocompatible diol. Another biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is derived from a diurethane diol and is biodegradable into biomolecule degradation products.
Owner:CARNEGIE MELLON UNIV +1
Oligopeptide-free cell culture media
InactiveUS20070212770A1Efficient expression of recombinantEfficient productionFactor VIIBacteriaCulture cellCell culture media
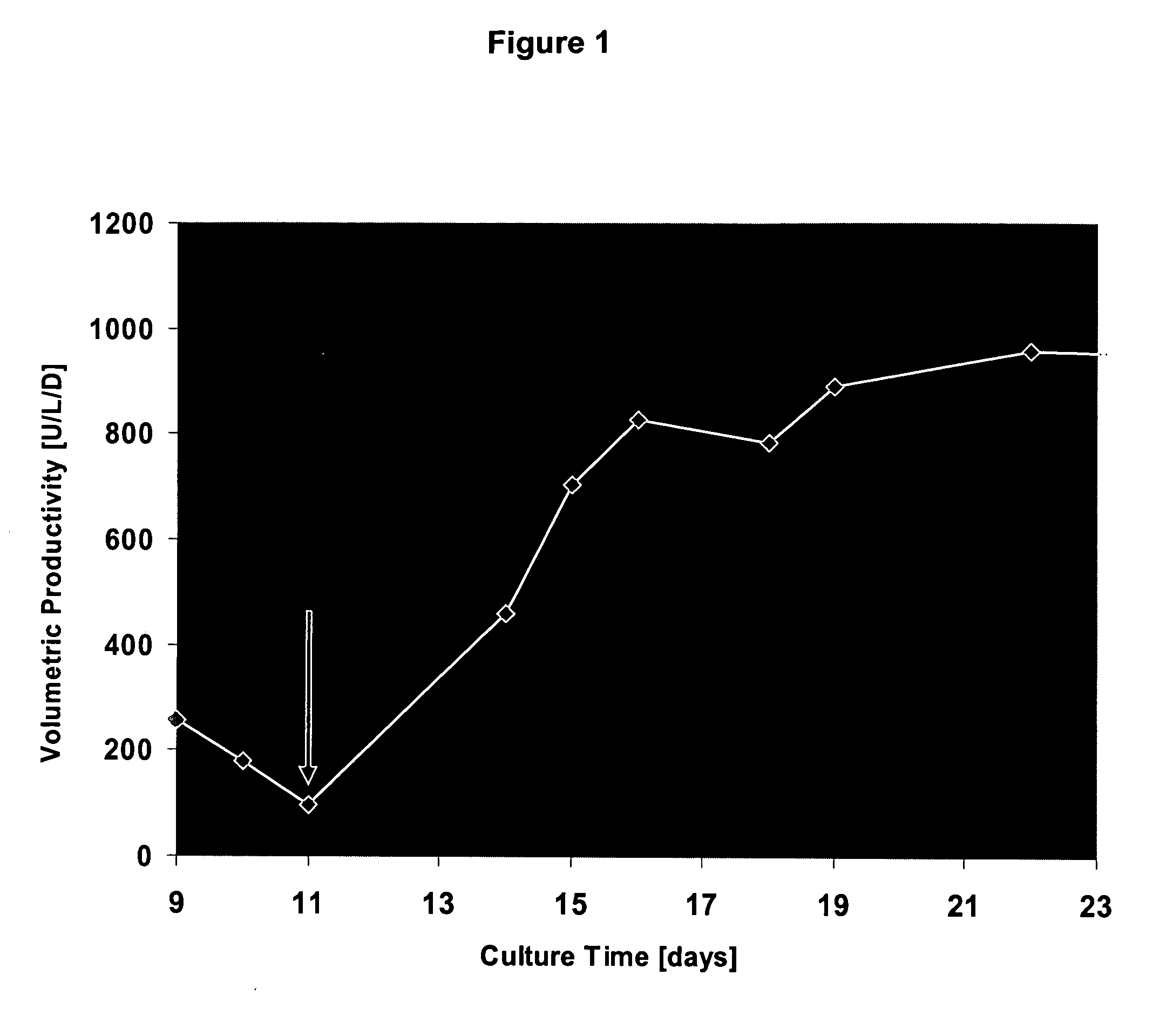
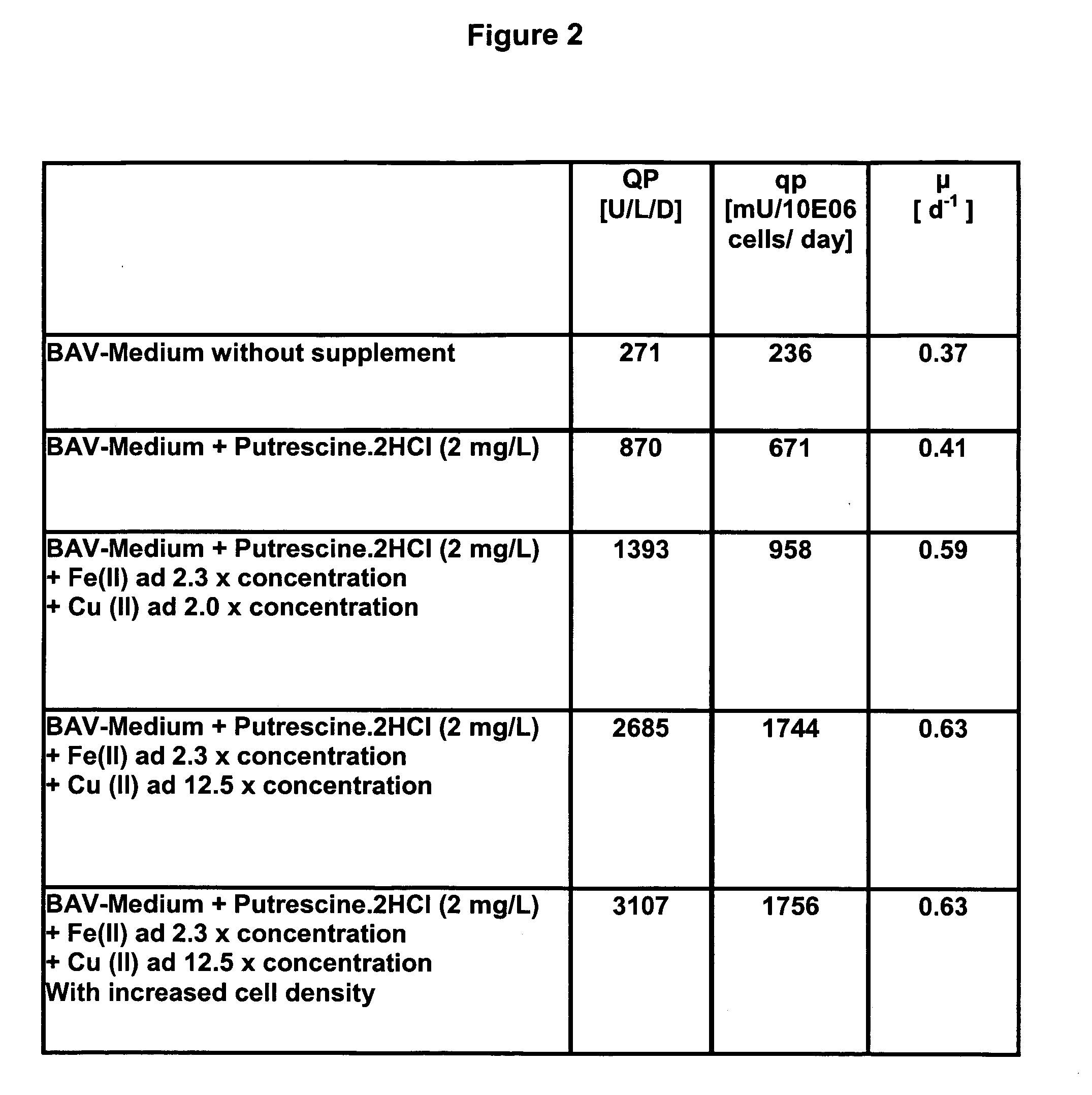
The present invention relates to oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine and to methods for cultivating cells in said oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine. The invention also relates to methods for expressing at least one protein in a medium comprising at least 0.5 mg / L of a polyamine and to methods for producing at least one virus in a medium comprising at least 0.5 mg / L of a polyamine.
Owner:BAXTER HEALTHCARE SA +1
Bone marrow cells as a source of neurons for brain and spinal cord repair
Owner:SOUTH FLORIDA UNIVESITY OF
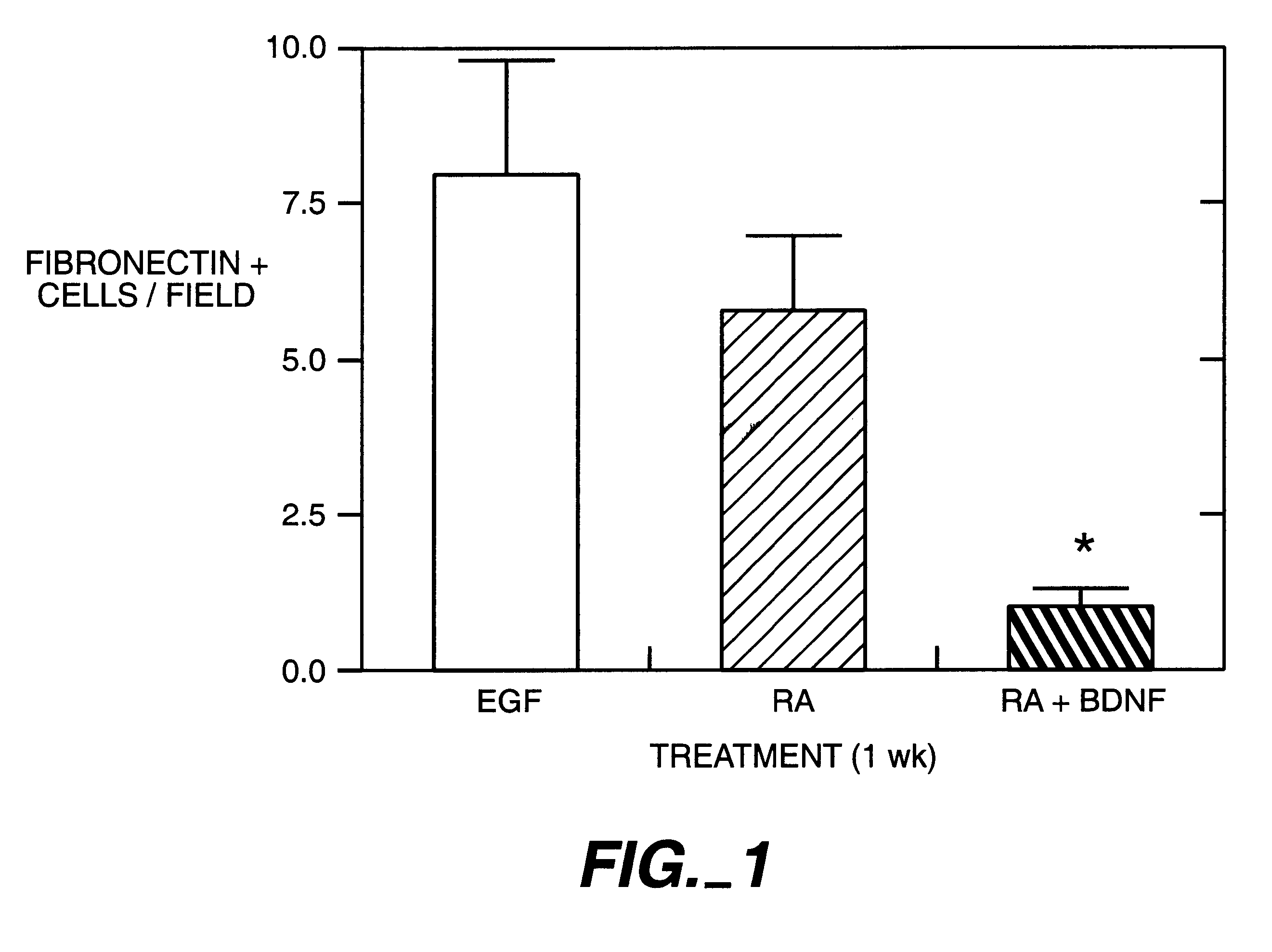
Methods and compositions for the repair and/or regeneration of damaged myocardium
InactiveUS20020061587A1Restoring functional integrityRestoring structuralBiocidePeptide/protein ingredientsCardiac muscleCytokine
Owner:NEW YORK MEDICAL COLLEGE
Formable and settable polymer bone composite and method of production thereof
ActiveUS20050008672A1Reduce molecular weightEasy to insertBiocideSurgical adhesivesBone implantEngineering
A composite osteoimplant. The osteoimplant includes a polymer and bone-derived particles. The composite is adapted and constructed to be formable during or immediately prior to implantation and to be set after final surgical placement.
Owner:WARSAW ORTHOPEDIC INC
Methods of using adipose tissue-derived cells in augmenting autologous fat transfer
ActiveUS20050025755A1Rapid and reliableHigh yieldBiocideElectrotherapyBreast augmentationAutologous Fat Transfer
Methods of treating patients for conditions such as breast augmentation, soft tissue defects, and urinary incontinence, are described. The methods include removing adipose tissue from a patient, processing a portion of the adipose tissue to obtain a substantially isolated population of regenerative cells, mixing the regenerative cells with another portion of adipose tissue to form a composition, and administering the composition to the patient from which the adipose tissue was removed.
Owner:LOREM VASCULAR PTE LTD
Cell-culture and polymer constructs
InactiveUS6378527B1Efficient disseminationPerfect cartilage repair surgeryDiagnosticsSurgeryImplanted deviceTissue replacement
Cells grown on a microcarrier are separated from the microcarrier by enzymatically digesting the microcarrier. More specifically, chondrocytes may be grown on dextran microcarrier beadlets and then the beadlets digested using dextranase to separate the chondrocytes from the carrier. Cells can also be grown on chitosan microcarriers to be used for implantation. In addition, cells can be grown on polysaccharide polymers to be used as implant devices. Various polymers serve as scaffolds for cells to be used for implantation. The polymers can be used for cell culture as well as for preparing scaffolds useful for tissue replacement such as cartilage tissue.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Autogenic living scaffolds and living tissue matrices: methods and uses thereof
ActiveUS20050226856A1Preventing host rejectionThicker and strongBiocideSkin implantsTransdifferentiationOrganism
A 3-dimensional structure comprising suitable cells (or entities) and the ECM (or matrix) that has been completely produced and arranged by these cells (or entities) that promotes the differentiation, dedifferentiation and / or transdifferentiation of cells and / or formation of tissue in vitro and in vivo, while at the same time promoting cell growth, proliferation, migration, acquisition of in vivo-like morphology, or combinations thereof, and that 1. provides structural and / or nutritional support to cells, tissue, organs, or combinations thereof, termed an “Autogenic Living Scaffold” (ALS); or 2. is capable of being transformed into a more complex tissue (or matrix) or a completely different type of tissue (or matrix), termed a “Living Tissue Matrix” (LTM). Autogenic means it is self-produced. The living cells that produce the LTM or ALS, or are added to Autogenic Living Scaffolds, may be genetically engineered or otherwise modified. The matrix component of the ALS or LTM provides a structural framework for cells that guide their direction of growth, enables them to be correctly spaced, prevents overcrowding, enables cells to communicate between each other, transmit subtle biological signals, receive signals from their environment, form bonds and contacts that are required for proper functioning of all cells within a unit such as a tissue, or combinations thereof. The ALS or LTM may thus provide proper or supporting mechanical and chemical environments, signals, or stimuli to other cells, to the cells that produce the ALS, to surrounding tissue at an implantation site, to a wound, for in vitro and ex vivo generation and regeneration of cells, tissue and organs, or combinations thereof. They may also provide other cells with nutrients, growth factors, and / or other necessary or useful components. They may also take in or serve as buffers for certain substances in the environment, and have also some potential at adapting to new environments.
Owner:GENESIS TECH LTD
Adipose tissue-derived stromal cell that expresses characteristics of a neuronal cell
The invention is in the area of pleuripotent stem cells generated from adipose tissue-derived stromal cells and uses thereof. In particular, the invention includes isolated adipose tissue derived stromal cells that have been induced to express at least one phenotypic characteristic of a neuronal, astroglial, hematopoietic progenitor, or hepatic cell. The invention also includes an isolated adipocyte tissue-derived stromal cell that has been dedifferentiated such that there is an absence of adipocyte phenotypic markers.
Owner:VETSTEM BIOPHARMA INC
Methods for identifying factors for differentiating definitive endoderm
Disclosed herein are methods of identifying one or more differentiation factors that are useful for differentiating cells in a cell population comprising definitive endoderm cells into cells which are capable of forming tissues and / or organs that are derived from the gut tube.
Owner:VIACYTE INC
Isolation of bone marrow fraction rich in connective tissue growth components and the use thereof to promote connective tissue formation
InactiveUS20050130301A1Peptide/protein ingredientsSkeletal disorderConnective tissue fiberTissue defect
A bone marrow isolate rich in one or more connective tissue growth components, methods of forming the isolate, and methods of promoting connective tissue growth using the isolate are described. A biological sample comprising bone marrow is centrifuged to separate the sample into fractions including a fraction rich in connective tissue growth components. The fraction rich in connective tissue growth components is then isolated from the separated sample. The isolate can be used directly or combined with a carrier and implanted into a patient at a tissue (e.g., bone) defect site. The biological sample can comprise bone marrow and whole blood. The isolate can be modified (e.g., by transfection with a nucleic acid encoding an osteoinductive polypeptide operably linked to a promoter) prior to application to the tissue defect site. The isolate can be made and applied to the tissue defect site in a single procedure (i.e., intraoperatively).
Owner:MCKAY WILLIAM F +1
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Method for processing and using adipose-derived stem cells
The present invention relates to a device comprising a cell carrier portion containing regenerative cells, e.g., stem and progenitor cells, and a cell carrier containment portion. The device is useful for the treatment of bone related disorders, including spinal fusion related disorders and long bone or flat bone related defects. The device may be used in conjunction with disclosed automated systems and methods for separating and concentrating regenerative cells.
Owner:LOREM VASCULAR PTE LTD
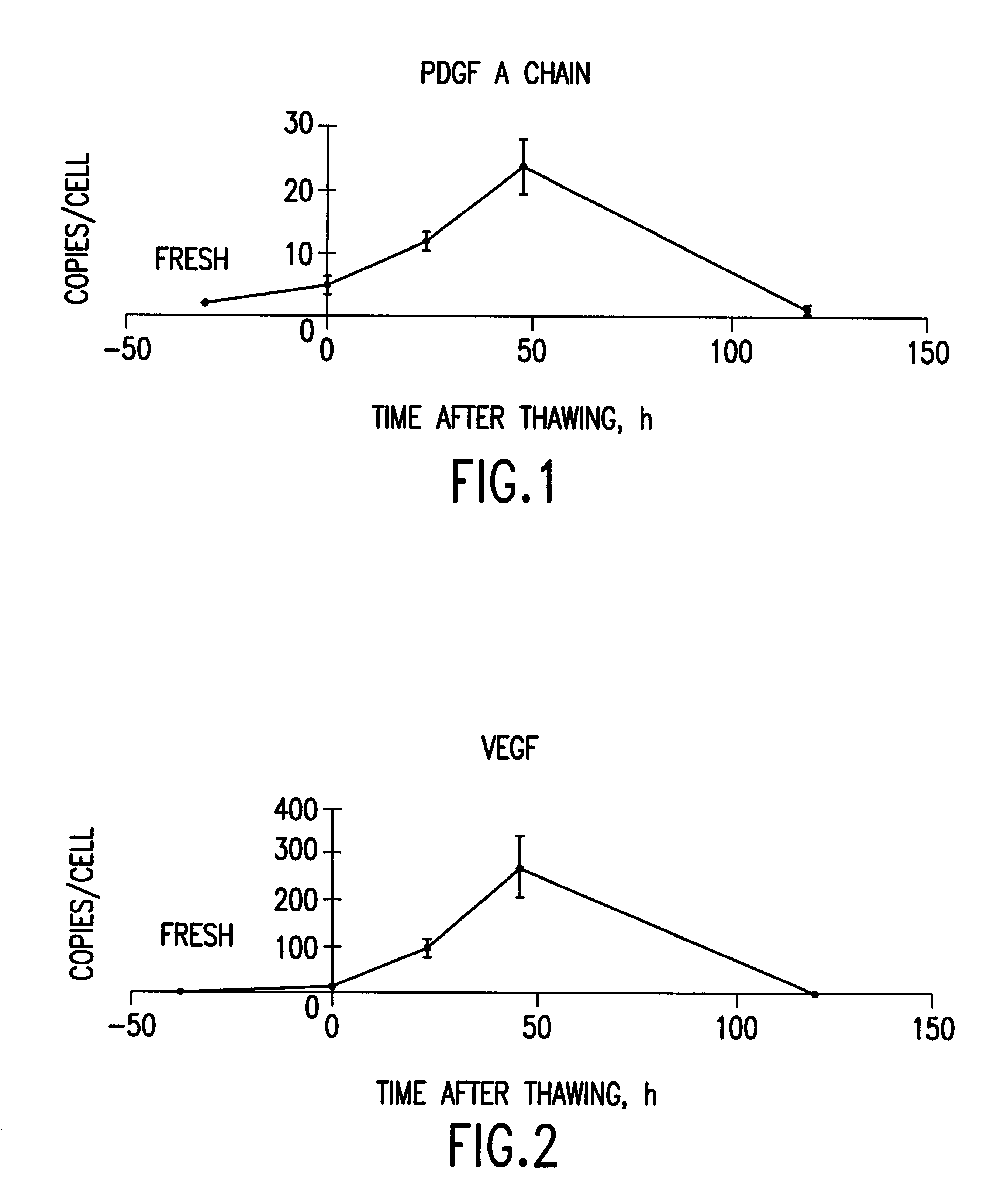
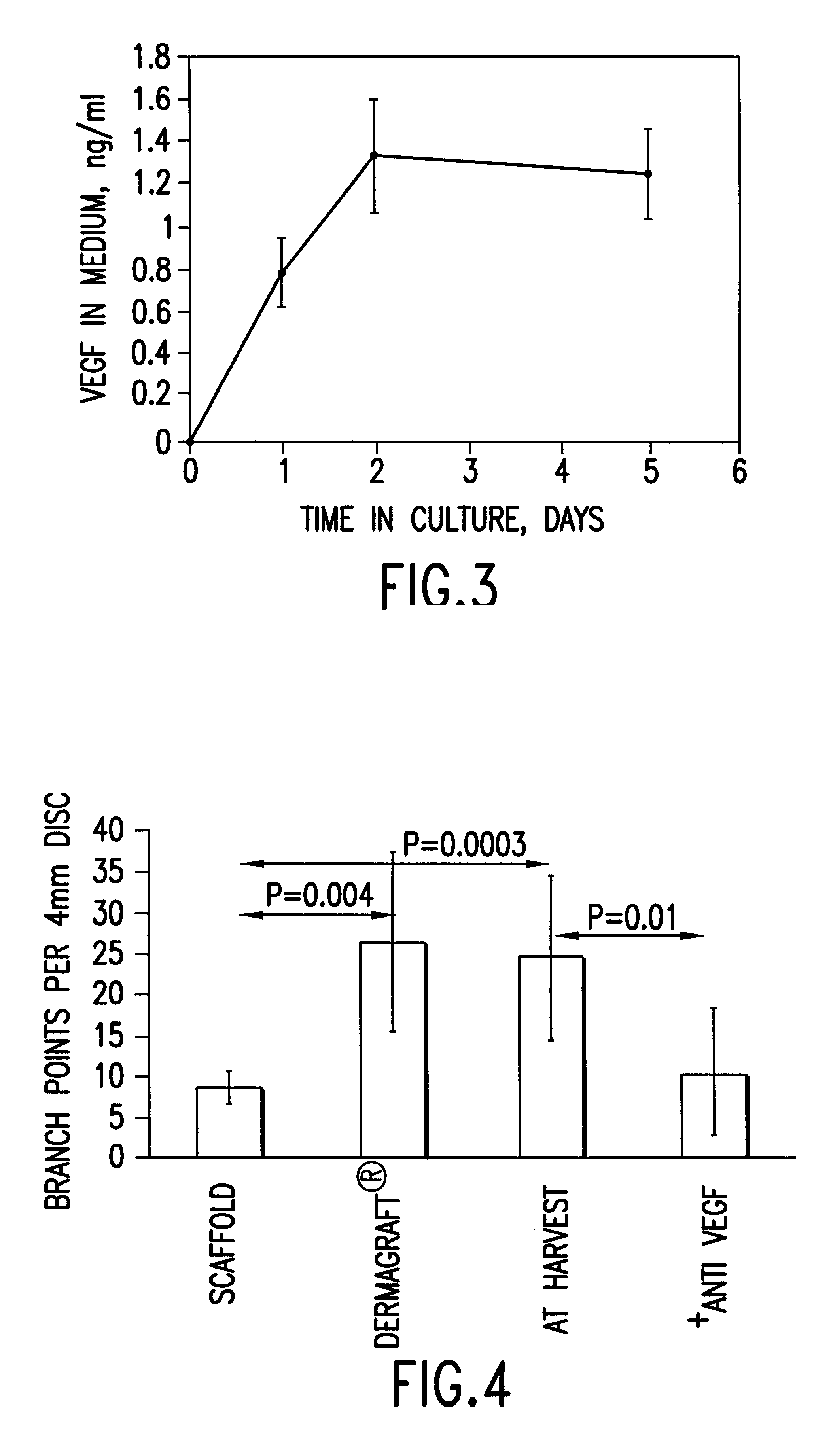
Cells or tissues with increased protein factors and methods of making and using same
InactiveUS6291240B1Induce productionAvoid damageGenetic material ingredientsMammal material medical ingredientsVascular tissueTissue defect
The invention relates to cells or tissues having an increased amount of regulatory proteins, including cytokines, growth factors, angiogenic factors and / or stress proteins, and methods of producing and using those cells or tissues. The invention is based on the discovery that the production of regulatory proteins is induced in cells or tissue constructs following cryopreservation and subsequent thawing of the cells or constructs. The compositions and methods of this invention are useful for the treatment of wound healing and the repair and / or regeneration of other tissue defects including those of skin, cartilage, bone, and vascular tissue as well as for enhancing the culture and / or differentiation of cells and tissues in vitro.
Owner:ORGANOGENESIS
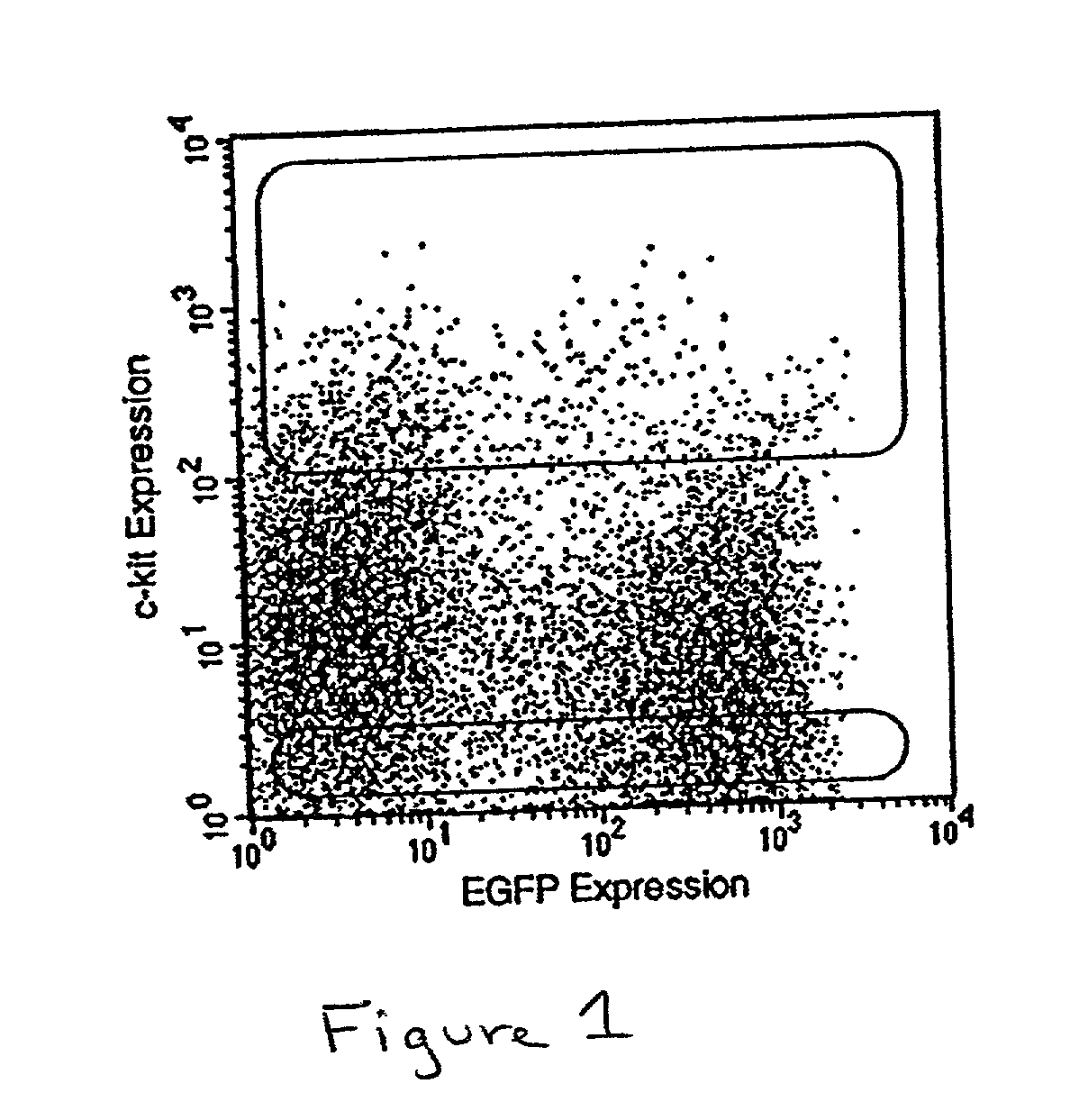
Method of producing undifferentiated hemopoietic stem cells using a stationary phase plug-flow bioreactor
A method of expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells by obtaining undifferentiated hemopoietic stem cells or progenitor cells; and either seeding the undifferentiated hemopoietic stem cells or progenitor cells into a stationary phase plug-flow bioreactor in which a three-dimensional stromal cell culture has been pre-established on a substrate in the form of a sheet, the substrate including a non-woven fibrous matrix forming a physiologically acceptable three-dimensional network of fibers, thereby expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells, or culturing the undifferentiated hemopoietic stem cells or progenitor cells in conditioned medium obtained from such a reactor.
Owner:PLURISTEAM LTD +1
Regeneration and augmentation of bone using mesenchymal stem cells
InactiveUS6863900B2Reduce functionReduce the numberBiocideBioreactor/fermenter combinationsMedicineBiopolymer
Disclosed are compositions and methods for augmenting bone formation by administering isolated human mesenchymal stem cells (hMSCs) with a ceramic material or matrix or by administering hMSCs; fresh, whole marrow; or combinations thereof in a resorbable biopolymer which supports their differentiation into the osteogenic lineage. Contemplated is the delivery of (i) isolated, culture-expanded, human mesenchymal stem cells; (ii) freshly aspirated bone marrow; or (iii) their combination in a carrier material or matrix.
Owner:MESOBLAST INT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com