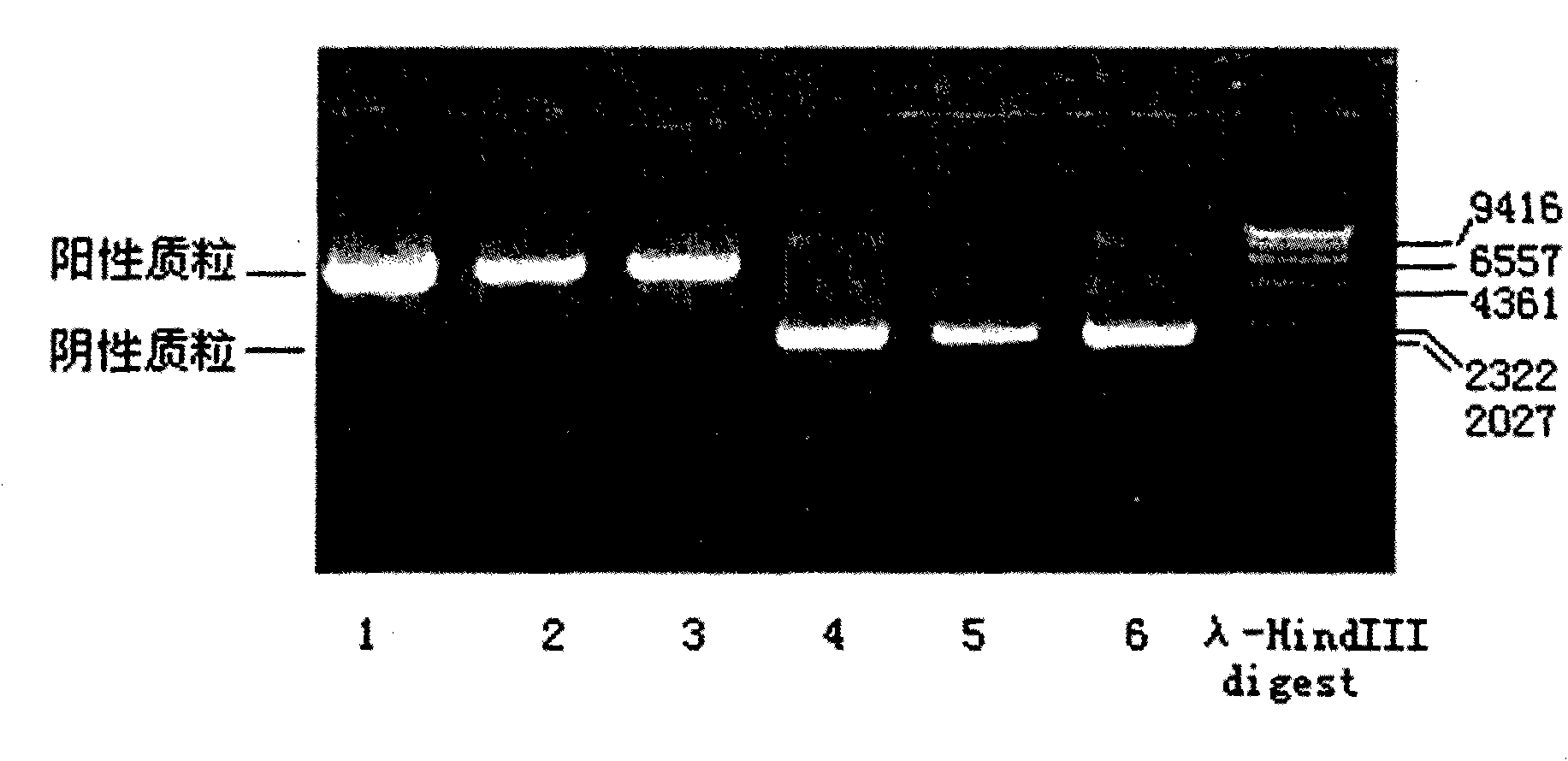
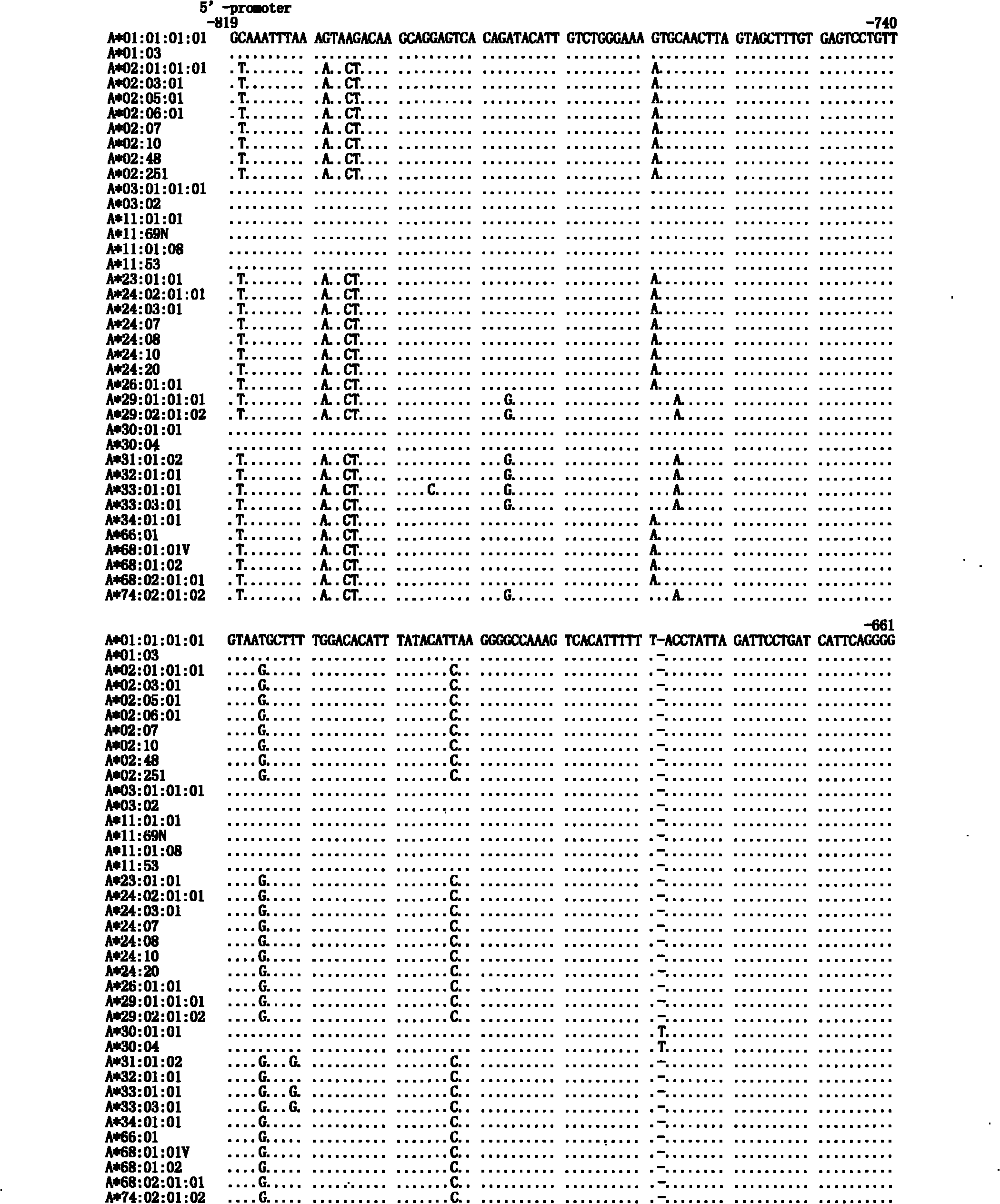
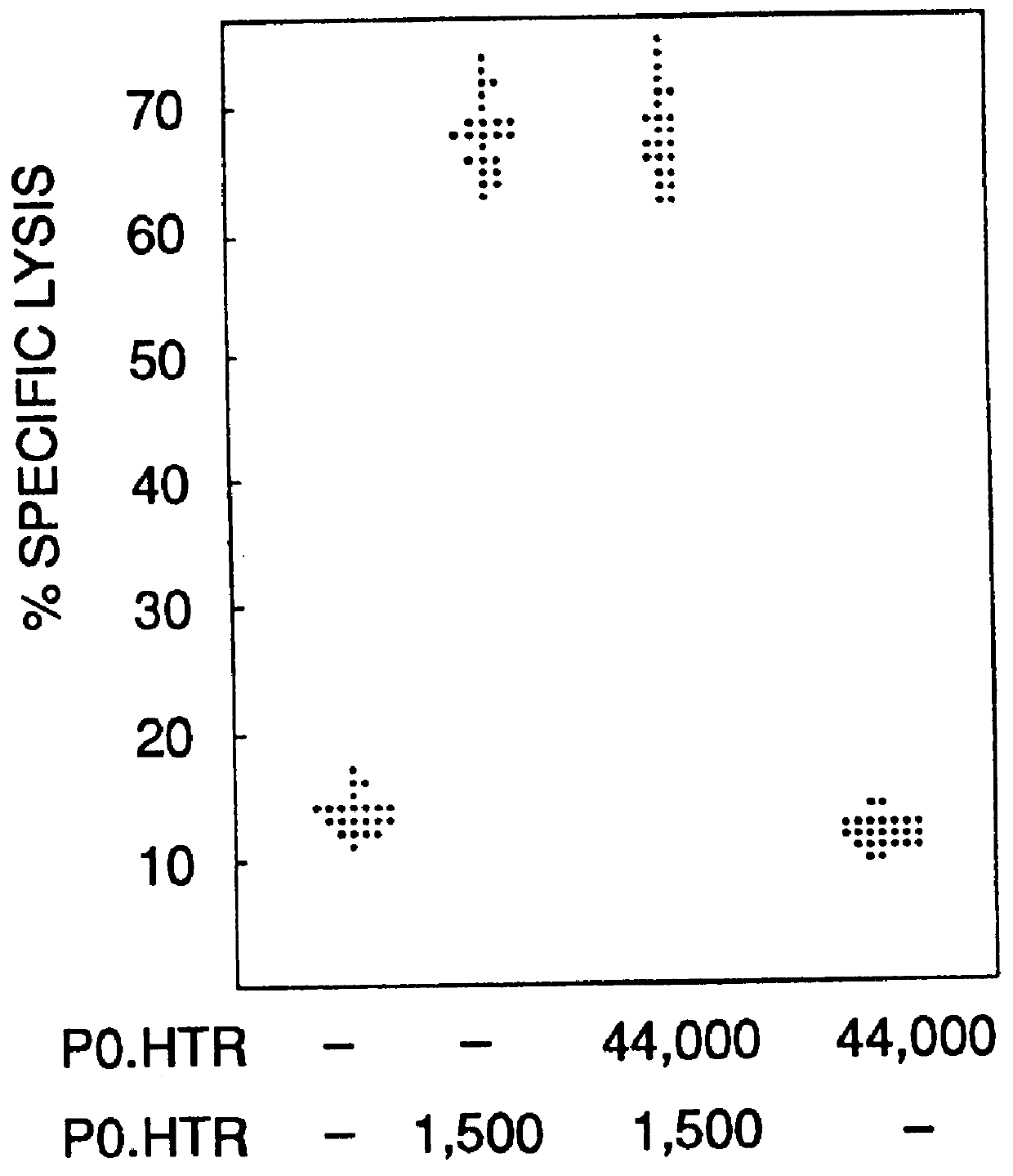
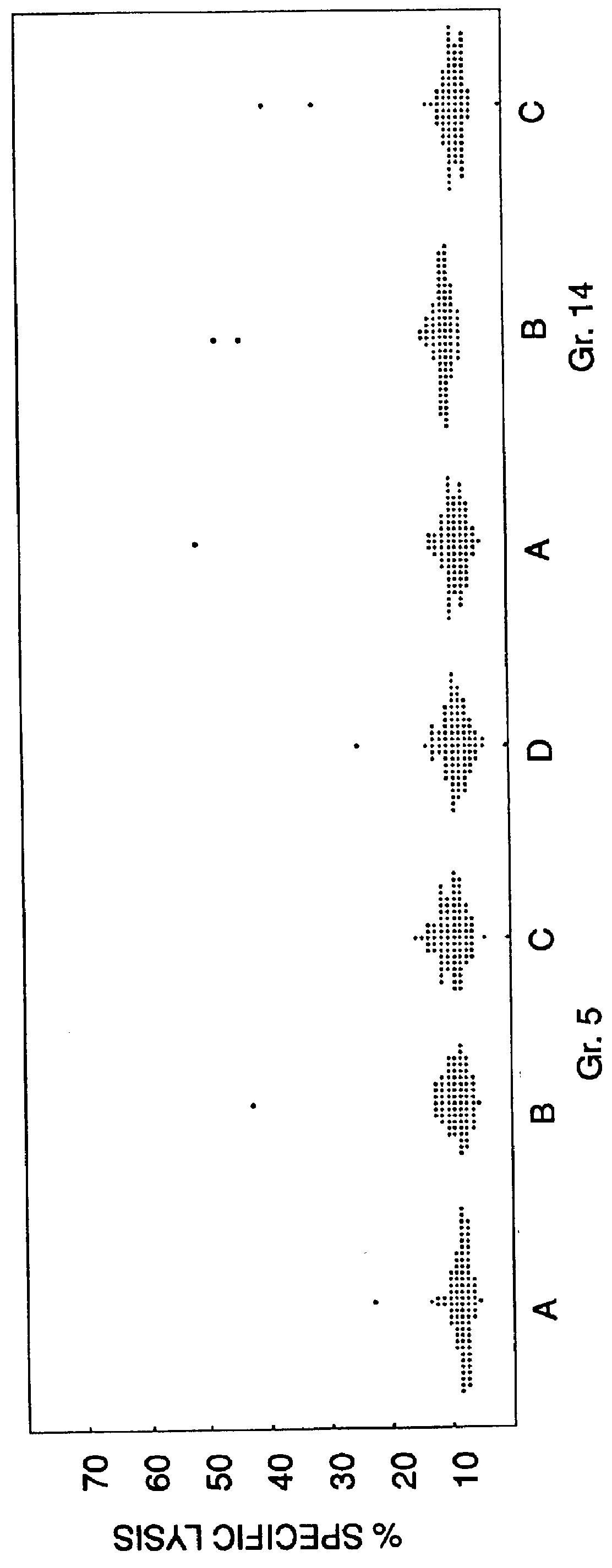
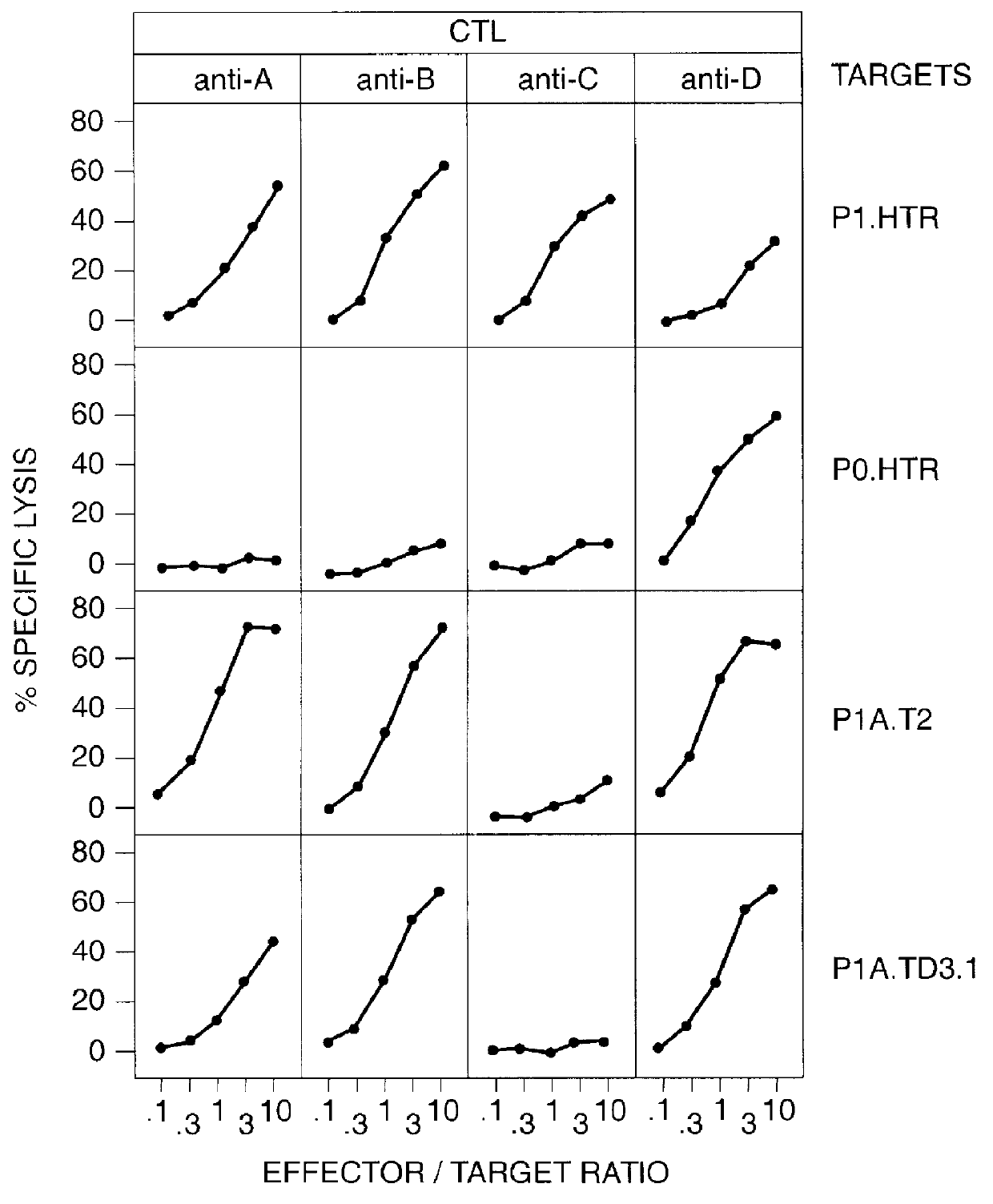
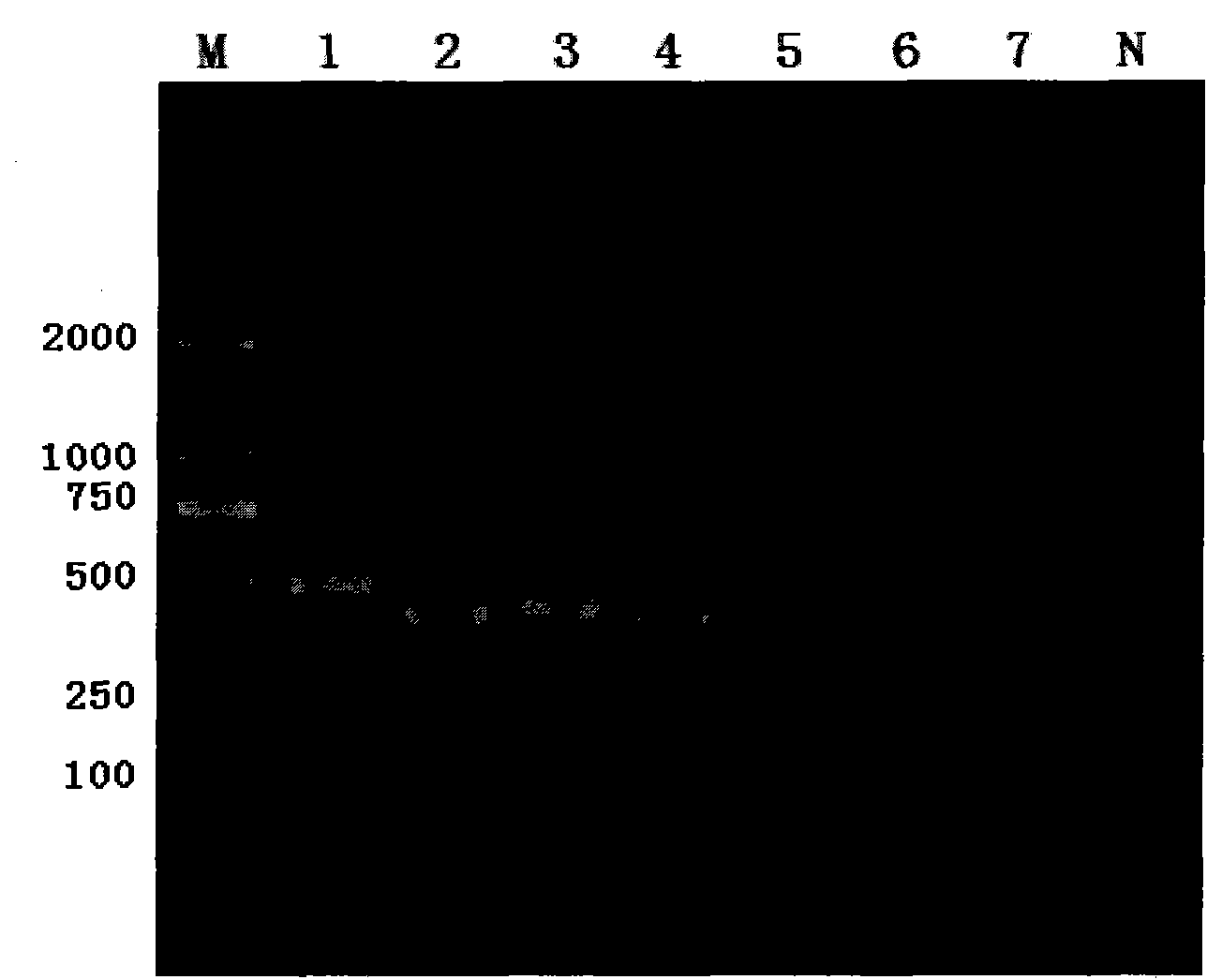
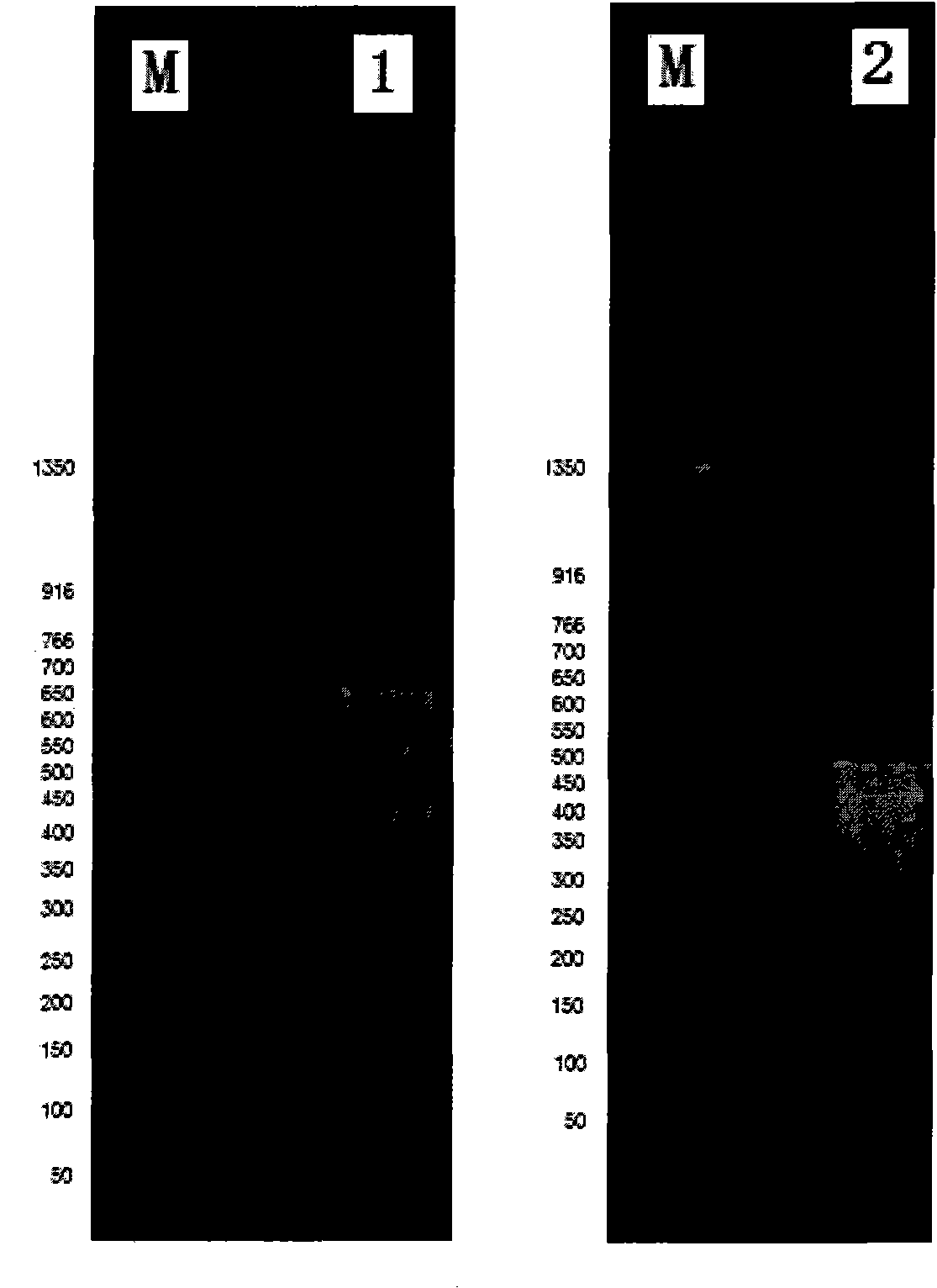
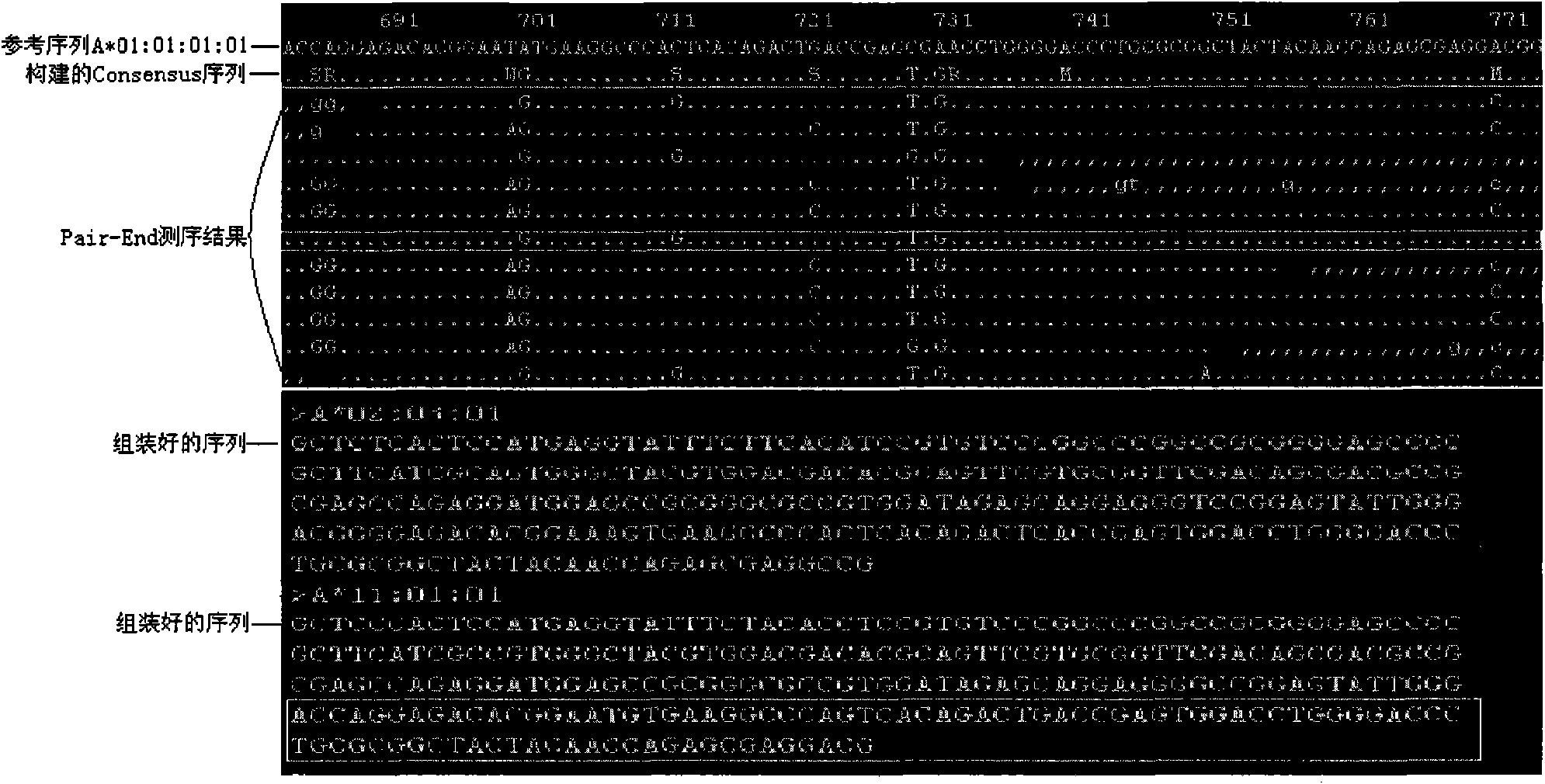
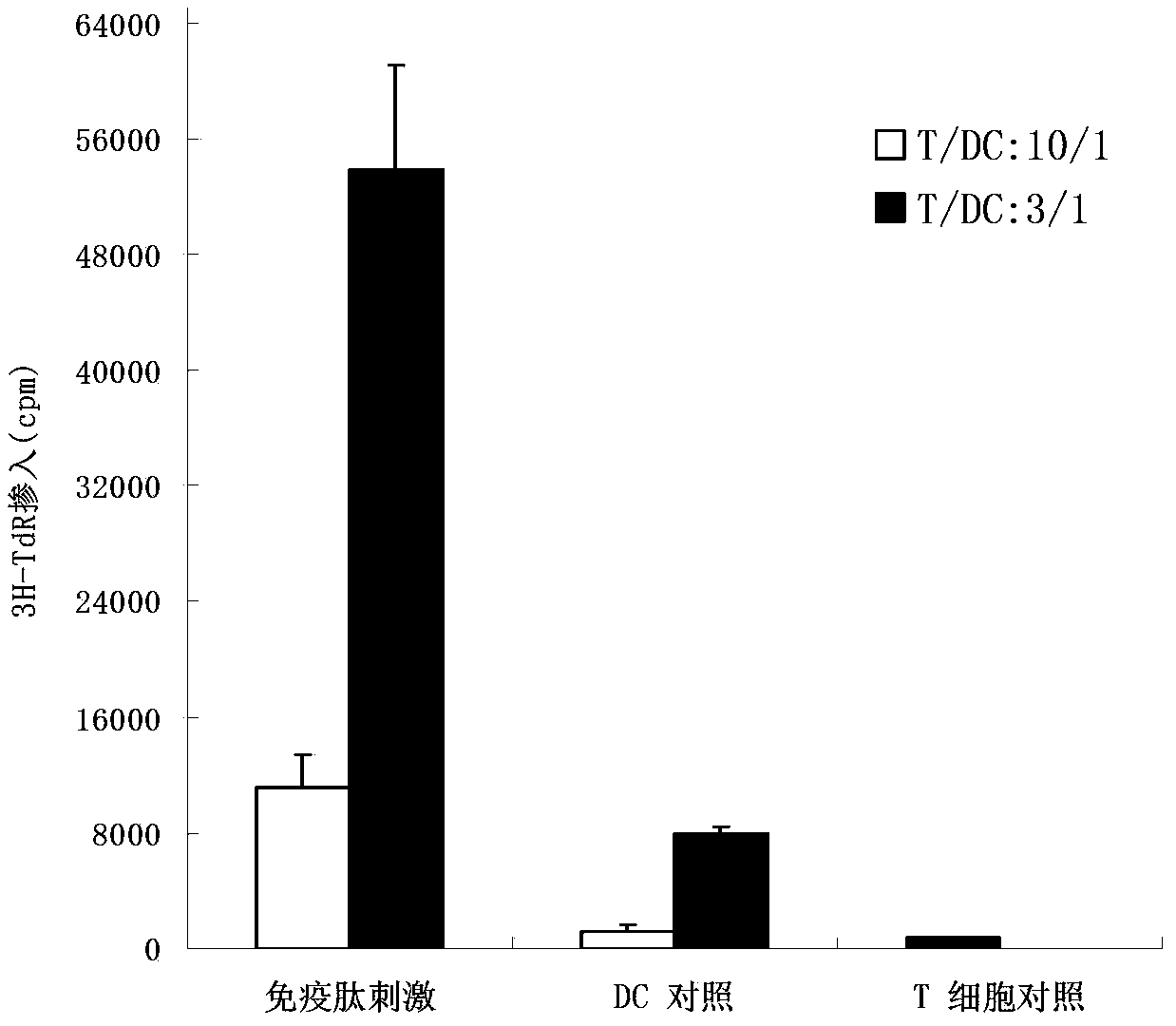
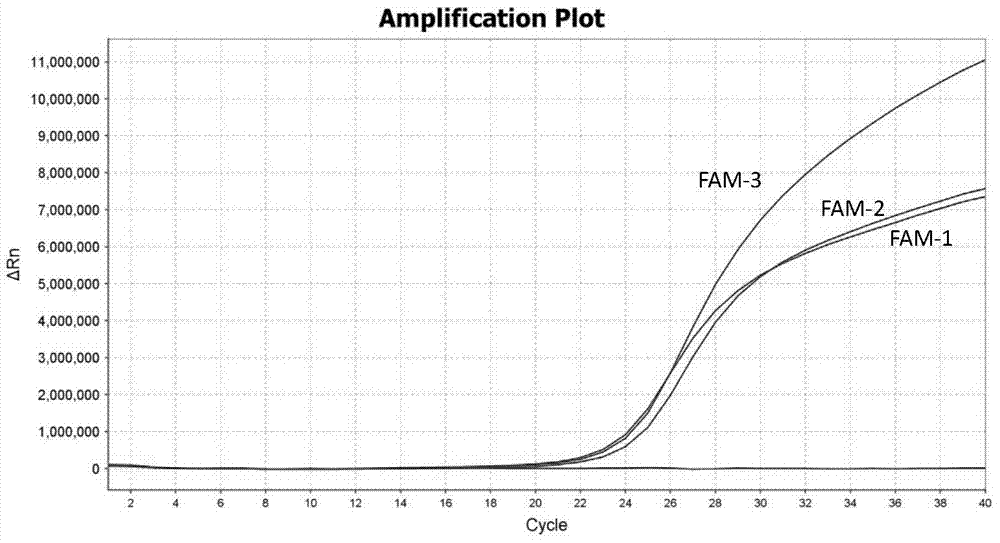
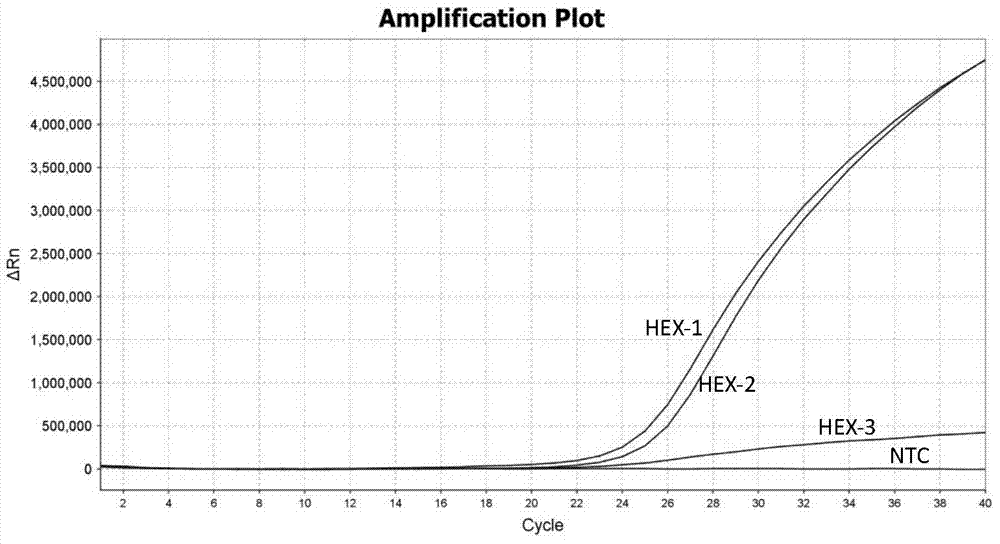
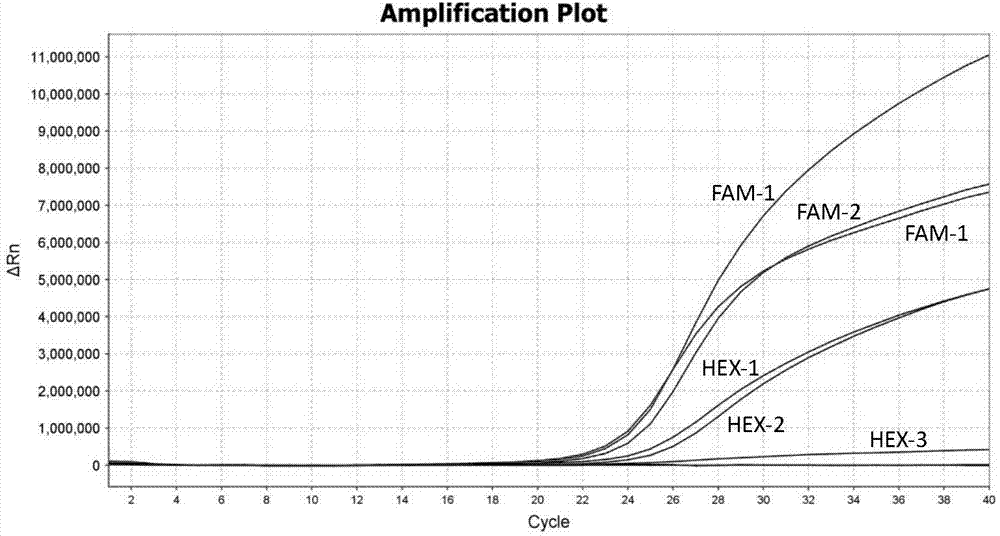
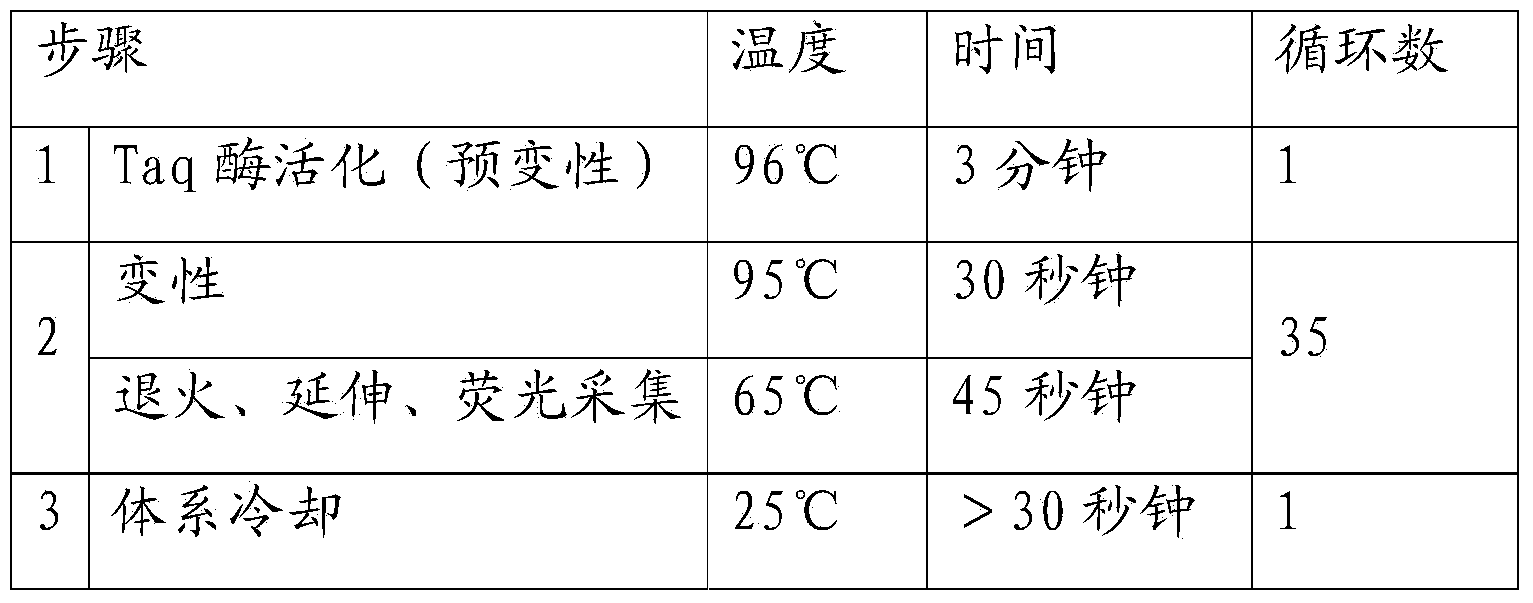
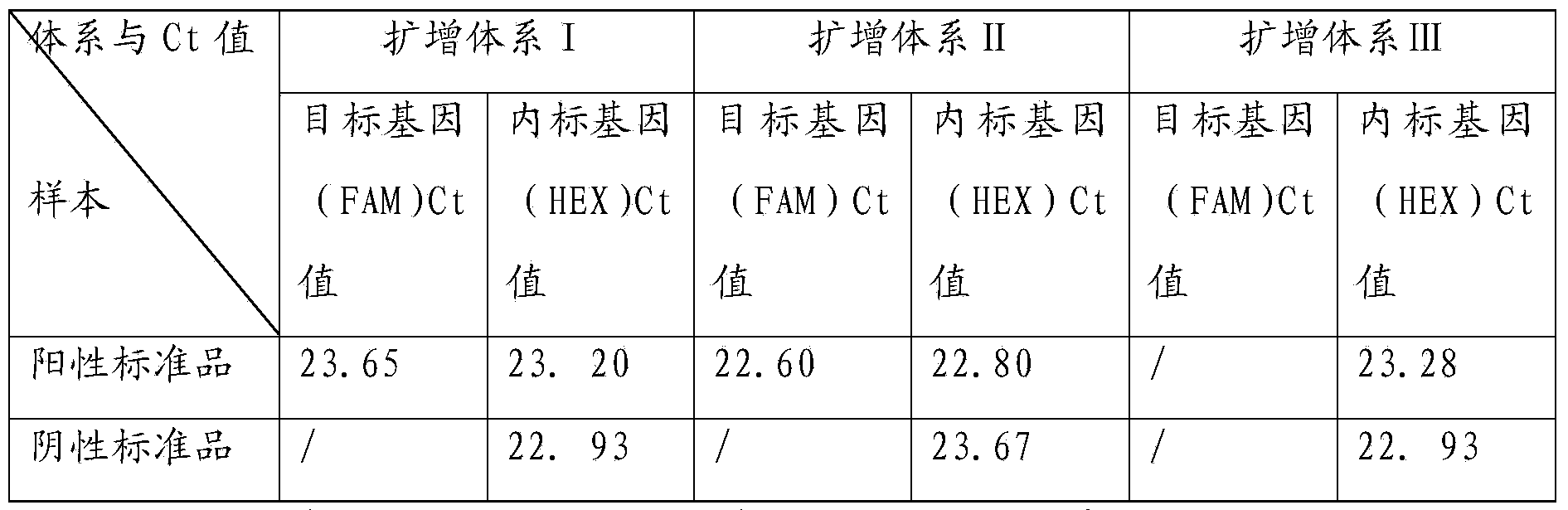
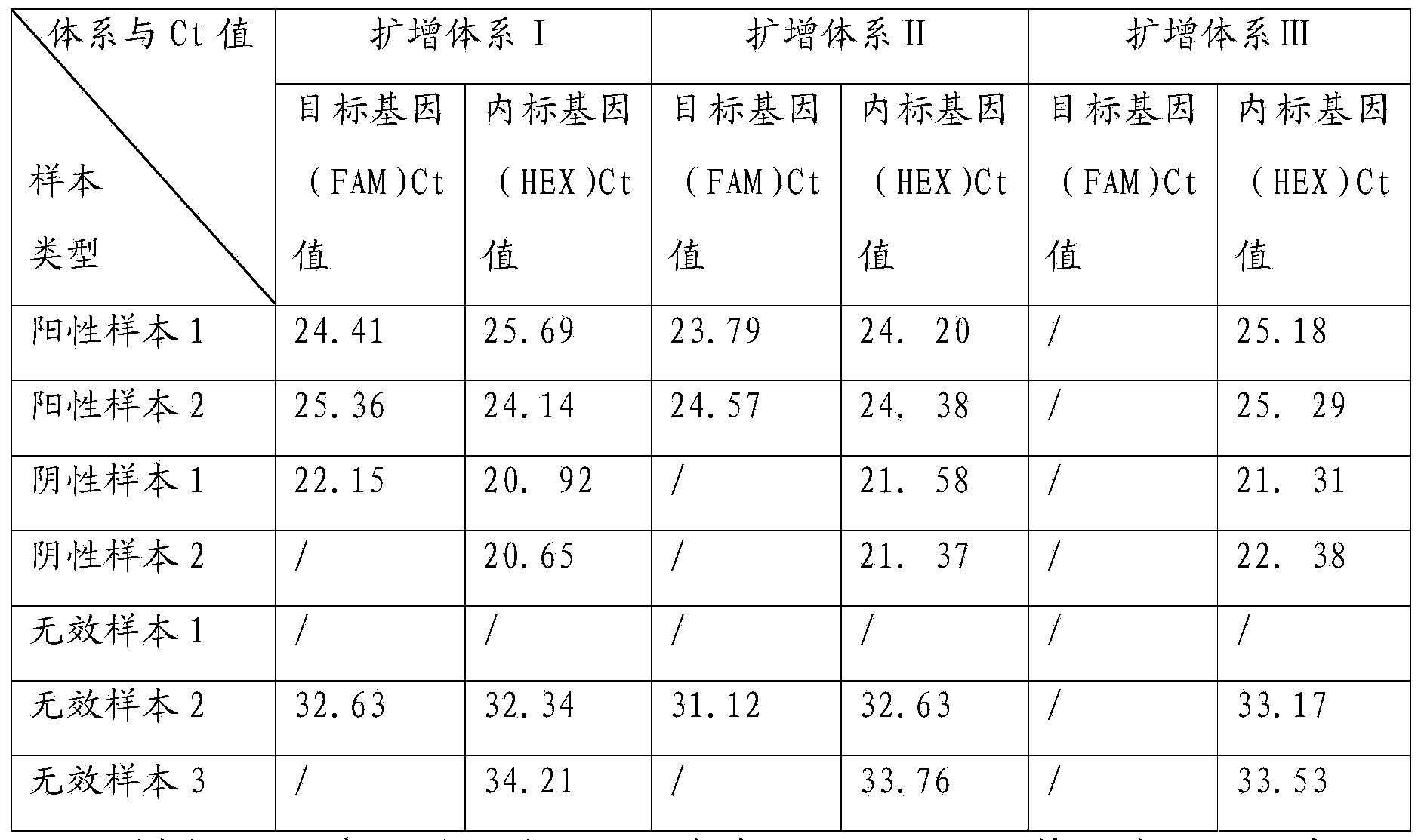
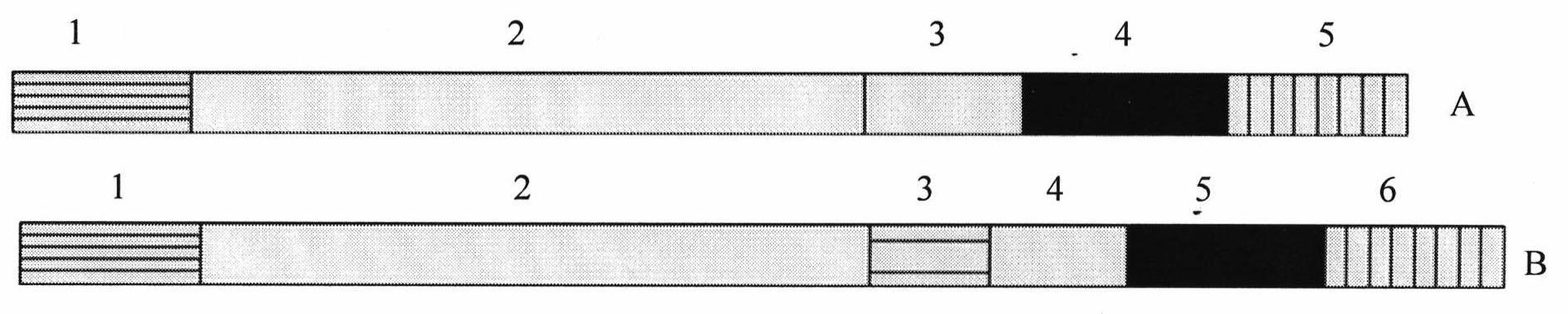
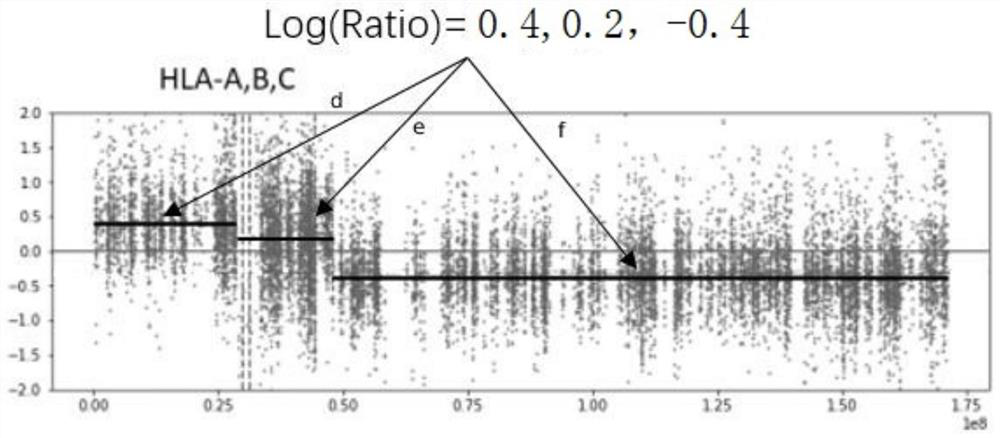
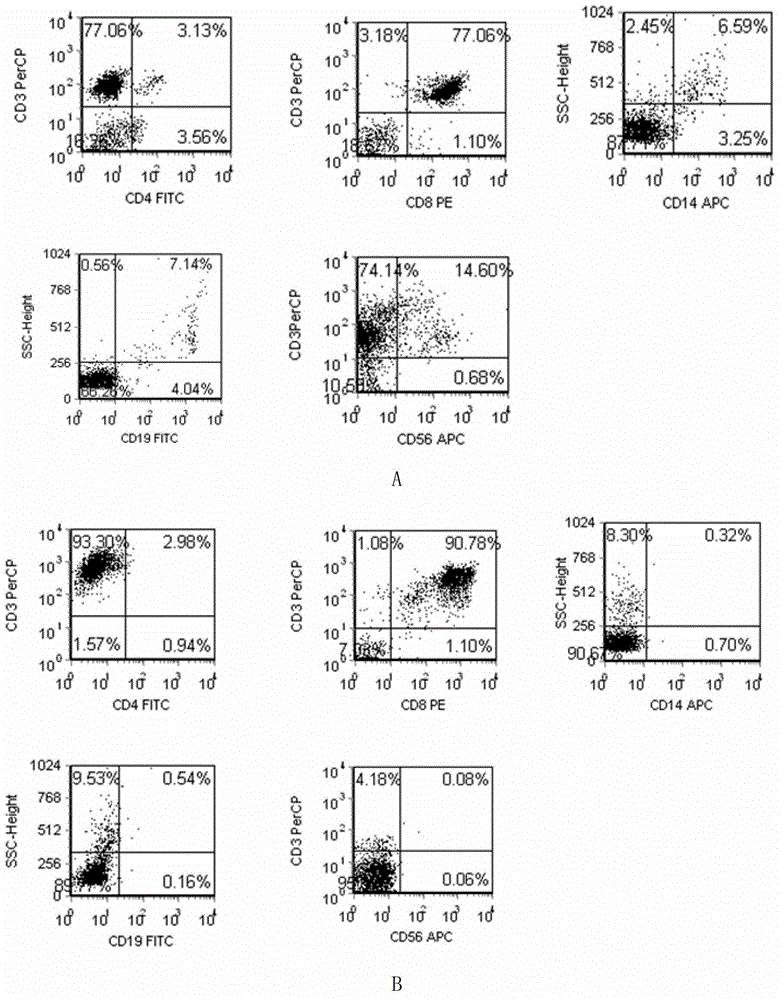
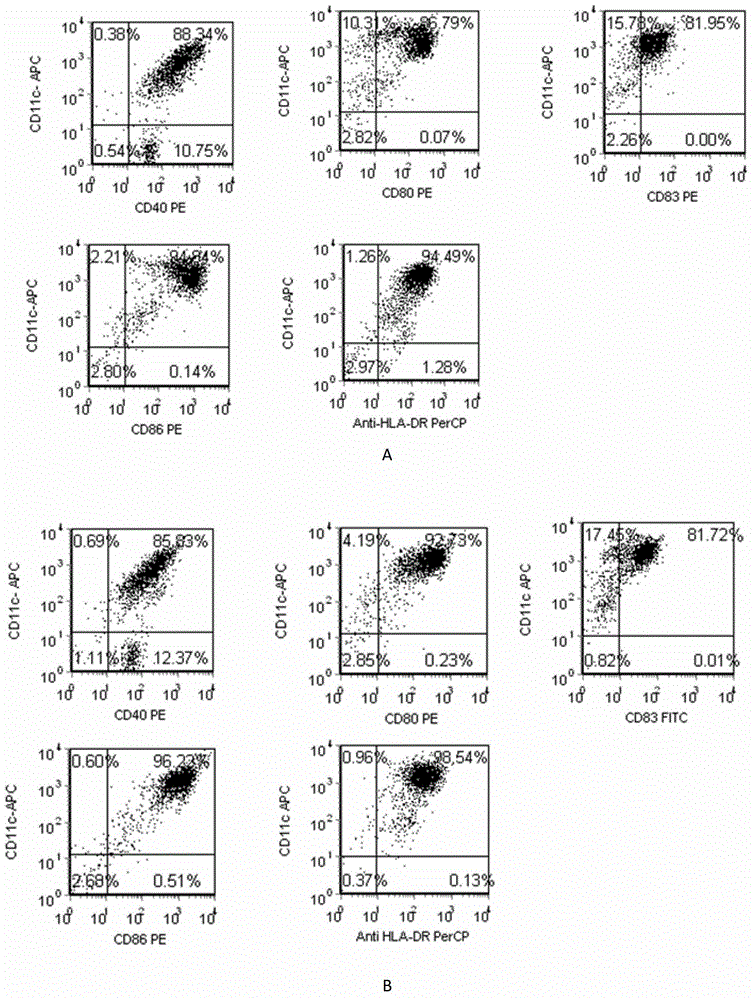
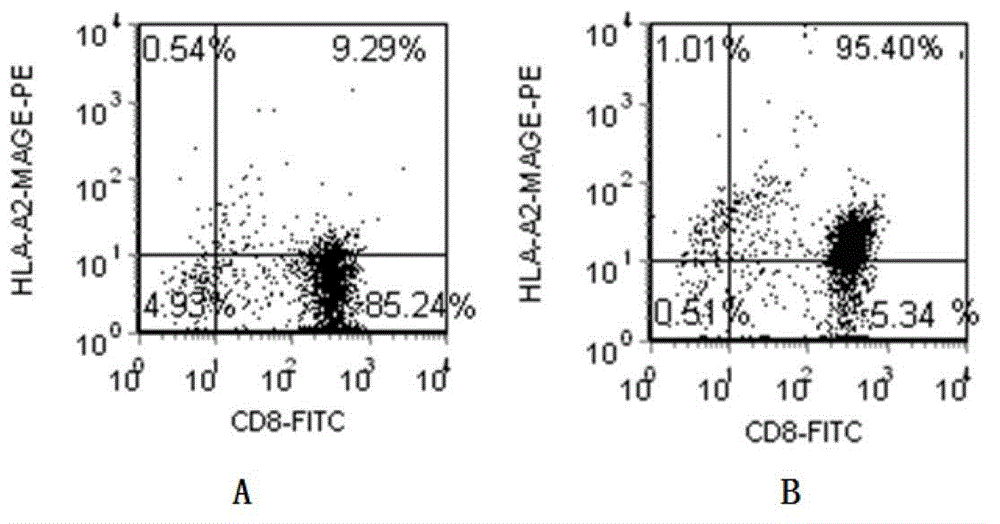
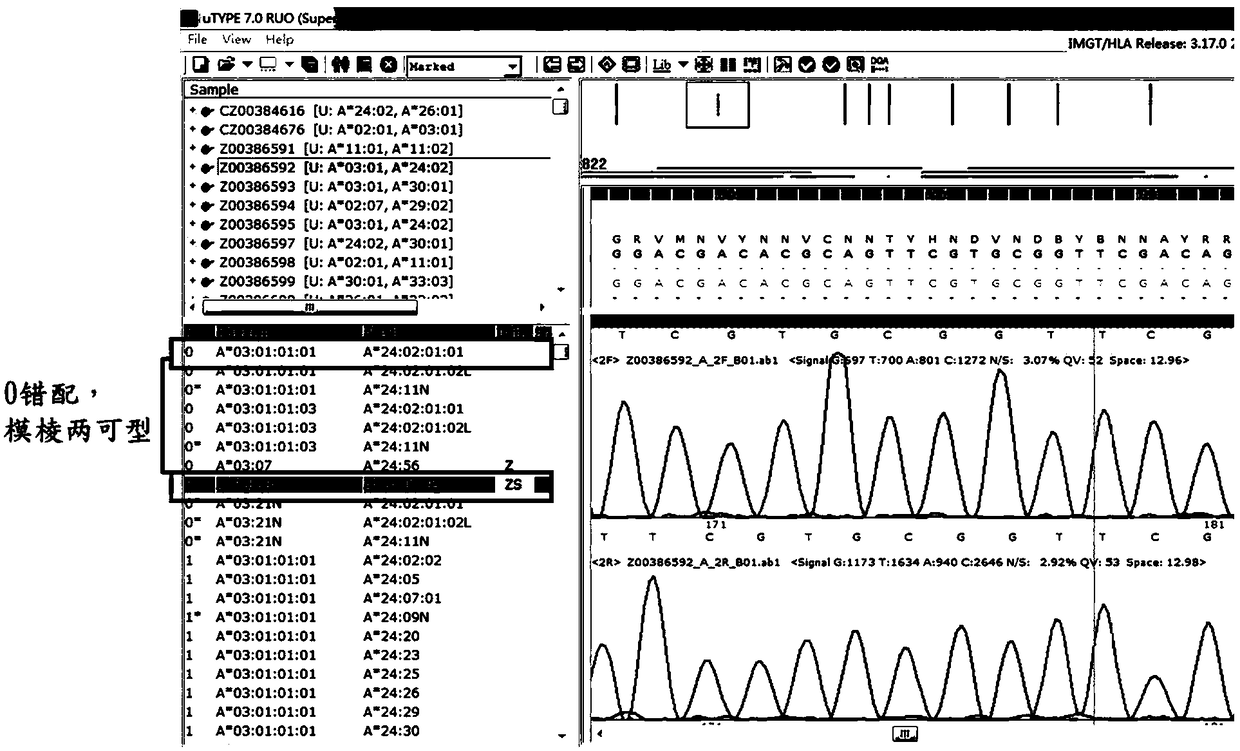
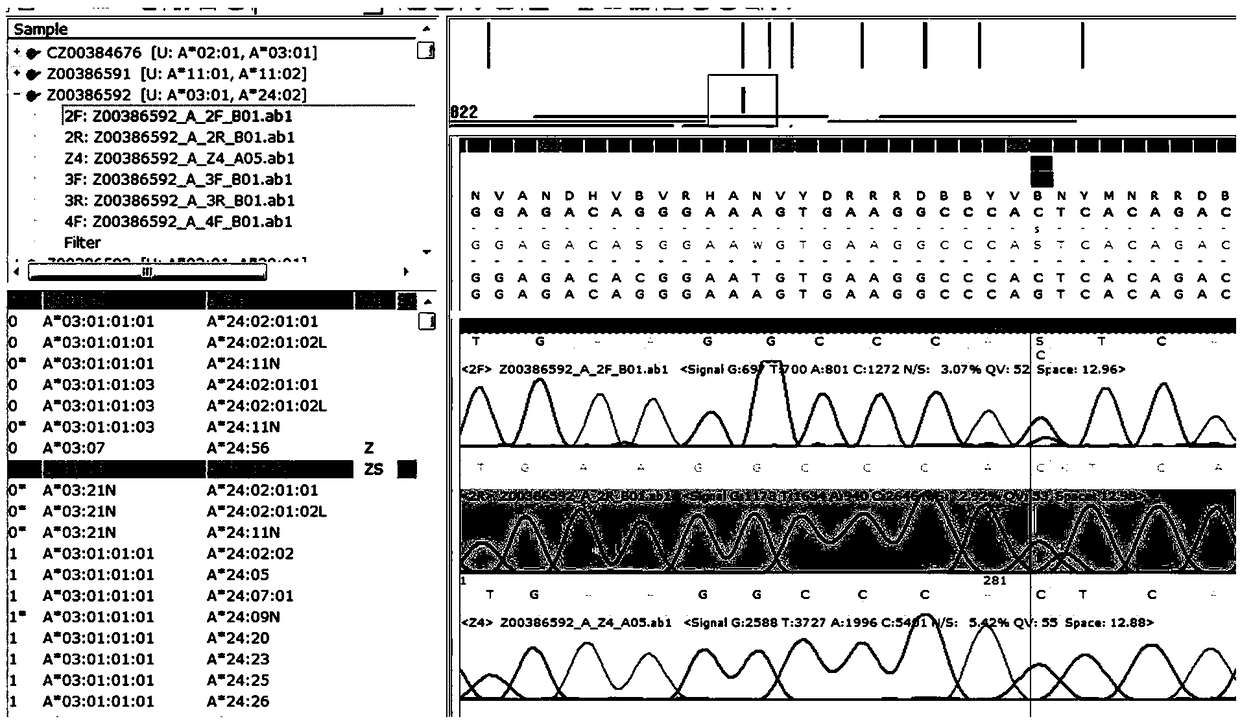
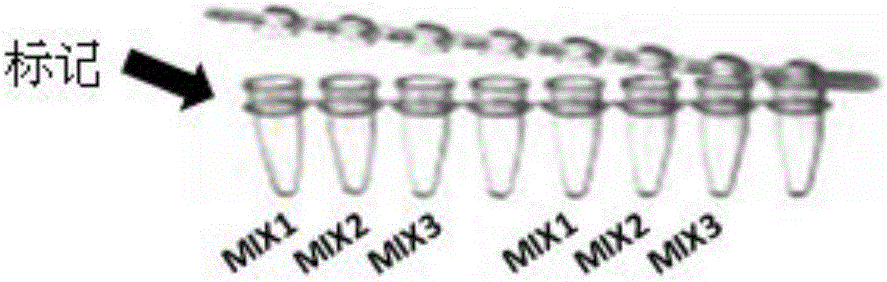
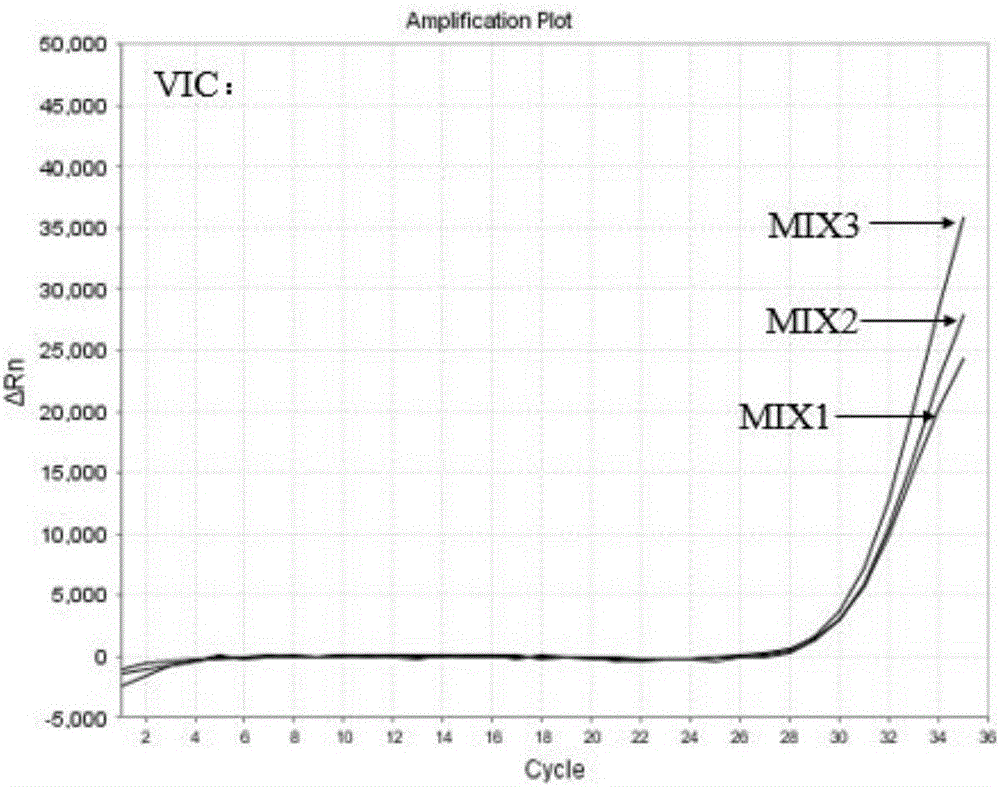
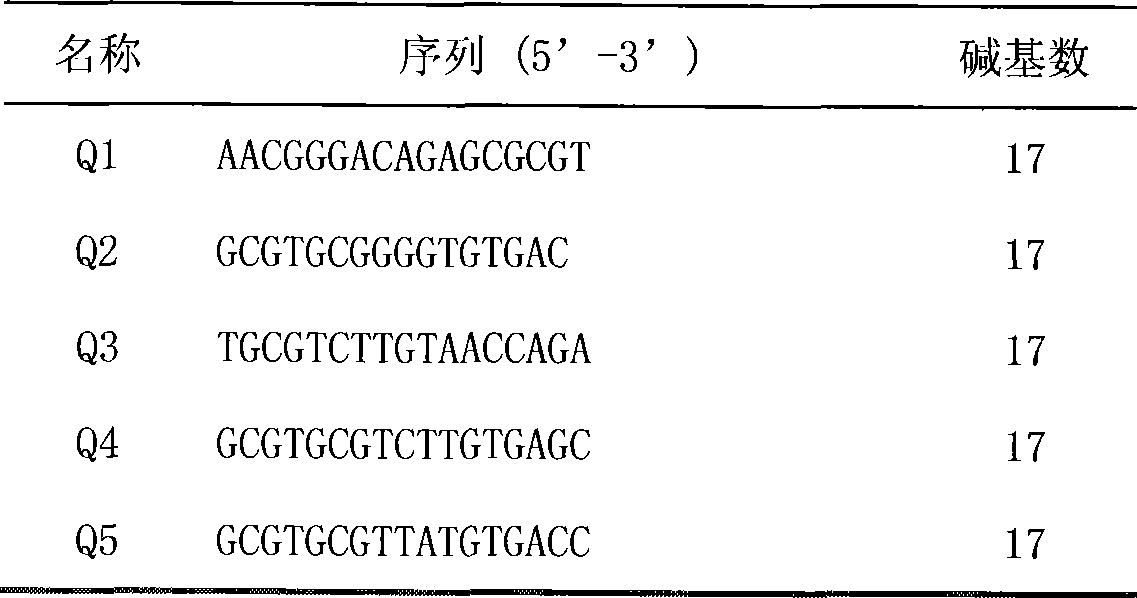
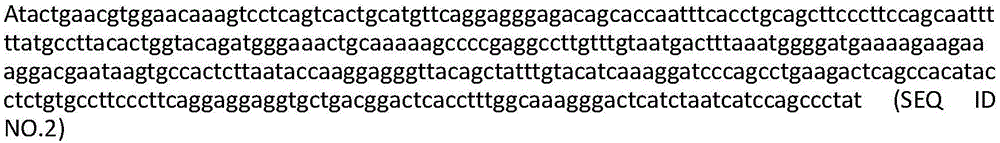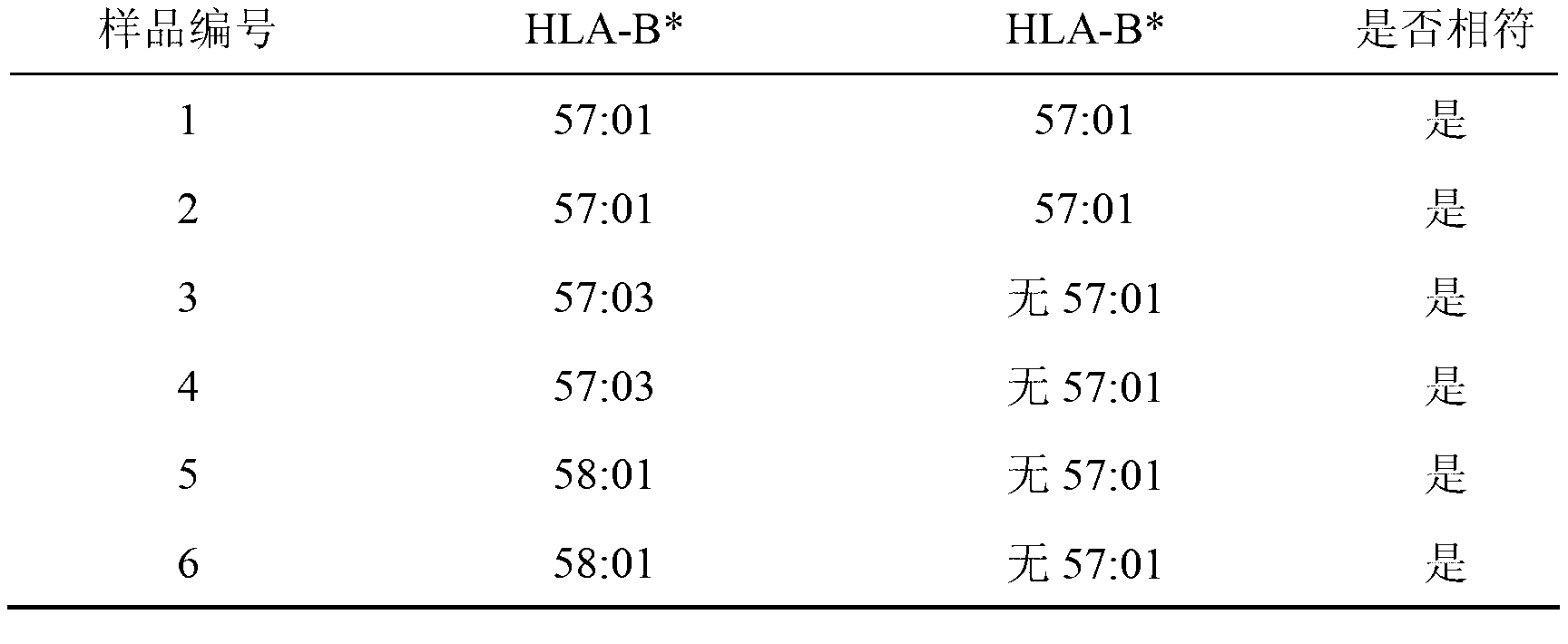Patents
Literature
101 results about "Human leukocyte antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The human leukocyte antigen (HLA) system or complex is a gene complex encoding the major histocompatibility complex (MHC) proteins in humans. These cell-surface proteins are responsible for the regulation of the immune system in humans. The HLA gene complex resides on a 3 Mbp stretch within chromosome 6p21. HLA genes are highly polymorphic, which means that they have many different alleles, allowing them to fine-tune the adaptive immune system. The proteins encoded by certain genes are also known as antigens, as a result of their historic discovery as factors in organ transplants. Different classes have different functions...
Isolated nucleic acid molecules coding for tumor rejection antigen precursor MAGE-3 and uses thereof
InactiveUS6235525B1BacteriaPeptide/protein ingredientsHuman leukocyte antigenTumor rejection antigen
Owner:LUDWIG INST FOR CANCER RES
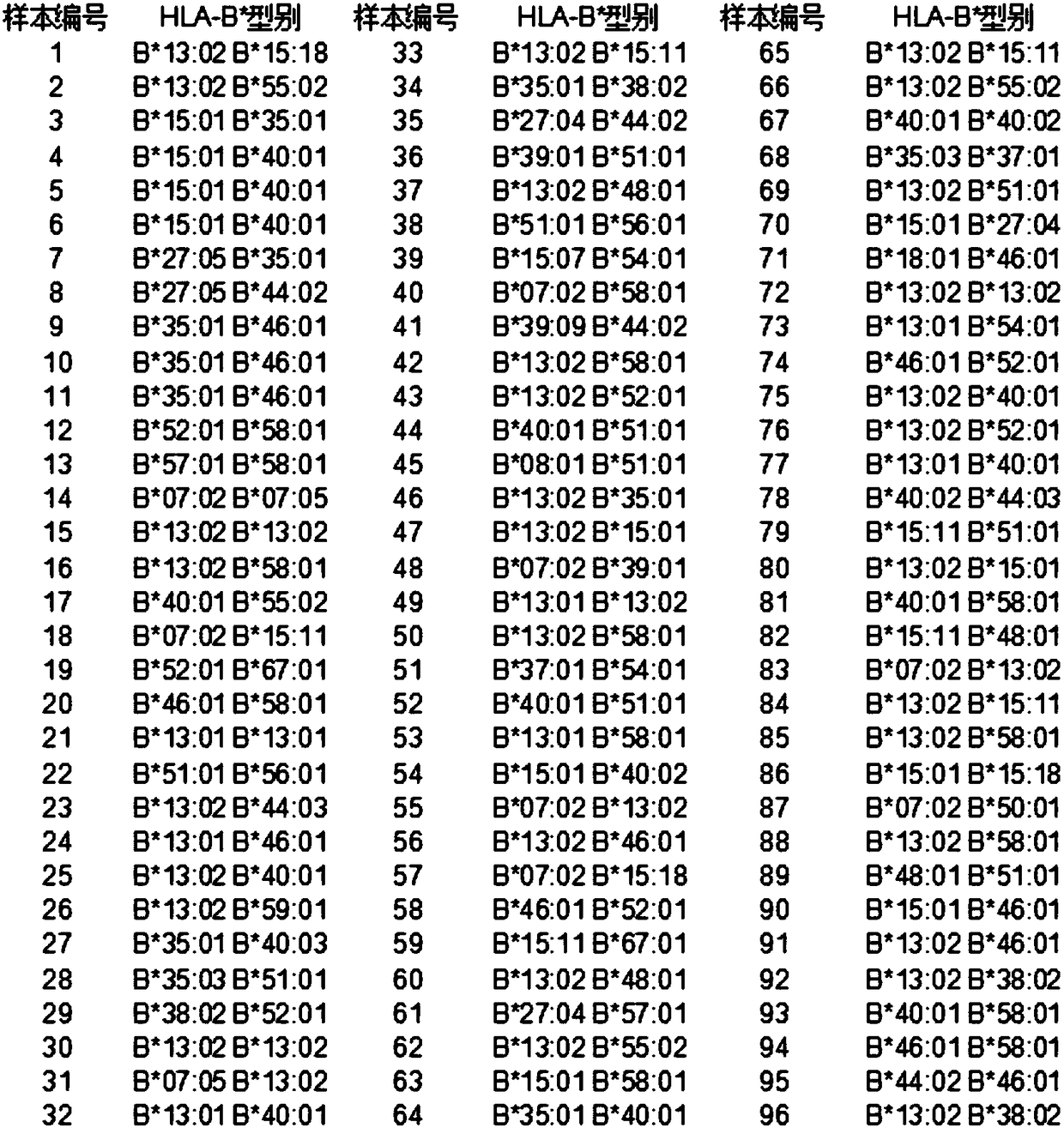
Human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and HLA gene sequencing and typing method
InactiveCN101962676AWide detection coverageResolving Ambiguous Results IssuesMicrobiological testing/measurementDNA/RNA fragmentationTyping methodsHLA-B gene
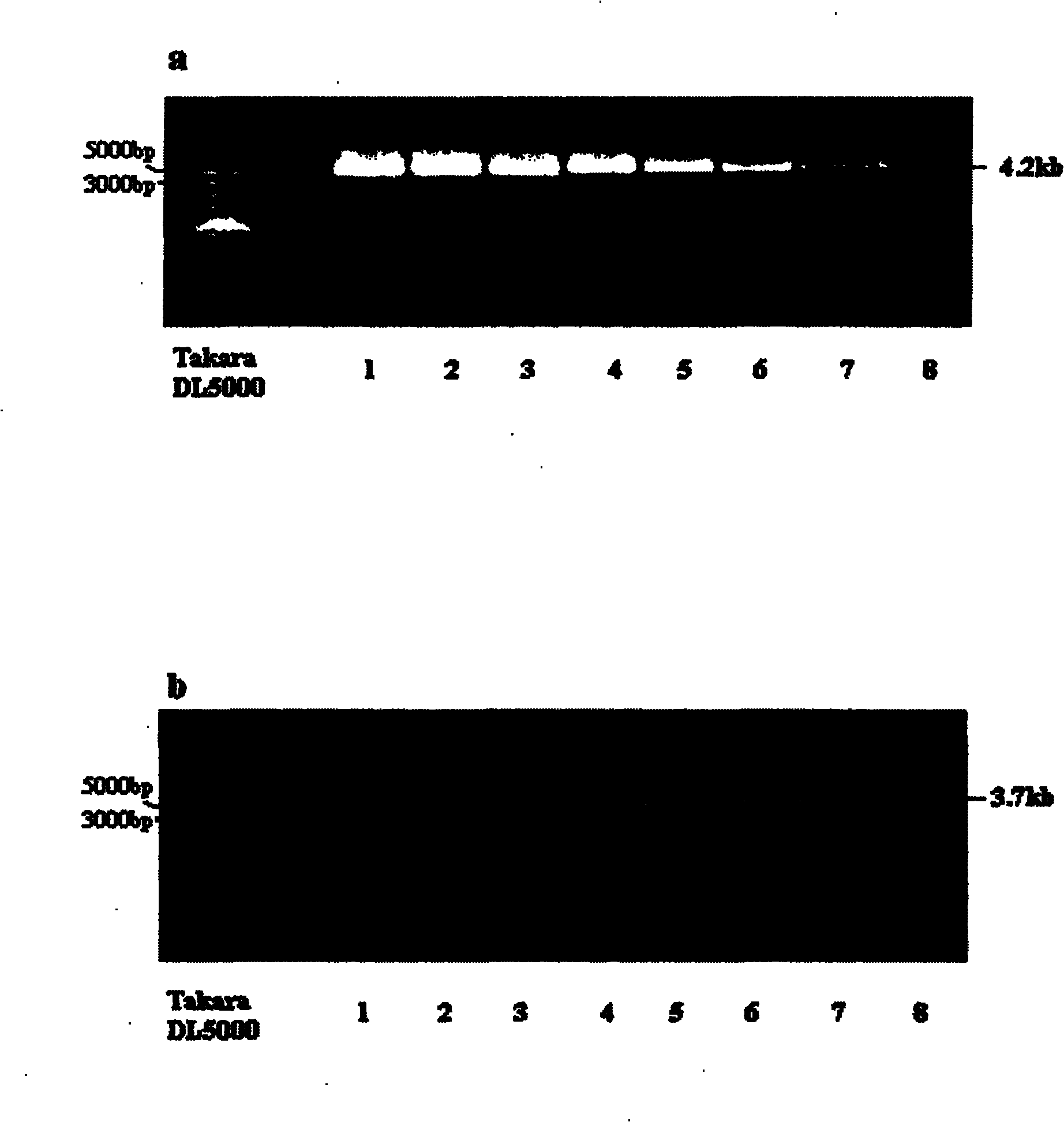
The invention discloses a human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and an HLA gene sequencing and typing method. The HLA-A and HLA-B gene full-length sequencing method comprises the following steps of: a, performing PCR amplification on about 4kb full-length sequences of HLA-A and HLA-B genes by using a pair of primers respectively; and b, cloning the amplification products to a pGEM-Tea sy vector, sequencing the full-length sequences by using ten walking sequencing primers in positive and negative directions respectively, and totally obtaining 38 allele 4.3kb full-length sequences of the HLA-A and 30 allele 3.7kb full-length sequences of the HLA-B. The HLA-A and HLA-B sequencing and typing method comprises the following steps of: performing PCR amplification on typing target areas of the HLA-A and HLA-B by using two pairs of primers respectively; and performing two directional sequencing on products by using fourteen sequencing primers respectively, wherein the HLA-DRB1 and HLA-DQB1 sequencing and typing method comprises the following steps of: amplifying sequences of second and third exons of DRB1 and DQB1 by adopting group specificity primers respectively; performing two directional sequencing on the second and third exons of the DRB1 by adopting eight group specificity primers and three sequencing primers; and performing two directional sequencing on the second and third exons of the DQB1 by adopting four sequencing primers respectively.
Owner:SHENZHEN BLOOD CENT
Isolated nucleic acid molecules coding for tumor rejection antigen precursor mage-3 and uses thereof
The invention relates to nucleic acid molecules which code for the tumor rejection antigen precursor MAGE-3. Also disclosed are vectors, cell lines, and so forth, which utilize the nucleic acid molecule, and optionally, molecules coding for human leukocyte antigen HLA-A1. Uses of these materials in therapeutic and diagnostic contexts are also a part of the invention.
Owner:LUDWIG INST FOR CANCER RES
Detection of surface-associated human leukocyte elastase
InactiveUS6858400B2Microbiological testing/measurementBiological material analysisPhagocyteBinding site
In order to accurately and reliably quantitate HLE on the plasma membranes of the lymphocytes and mononuclear phagocytes, a test sample containing the lymphocytes and mononuclear phagocytes is initially treated with a first antiserum specific for CD4 receptors on the plasma membrane or with a second antiserum specific for chemokine receptors on the plasma membrane. Once the CD4 or chemokine receptors have been rendered non-reactive (competitive) relative to the HLE receptors (also “binding sites”) on the plasma membrane, the test sample is contacted with an immunoreagent specific for interaction with one or more of the HLE receptors on the plasma membranes of the lymphocytes and mononuclear phagocytes. The immunoreagent forms a complex with the HLE binding sites and produces a characteristic physical change in the lymphocytes and mononuclear phagocytes that can be monitored by any one of a number of standard techniques, (e.g., confocal laser scanning microscopy and flow cytometry).
Owner:BRISTOW CYNTHIA L
HLA gene specific PCR amplification primer, HLA typing method and kit
InactiveCN103045591ANo StrapReasonable and accurateMicrobiological testing/measurementDNA/RNA fragmentationNucleotideTyping methods
The invention discloses an HLA (human leukocyte antigen) gene specific PCR (polymerase chain reaction) amplification primer, an HLA typing method and an HLA typing kit. A sequence of the PCR amplification primer comprises a primer sequences shown as SEQ ID No. (sequence identification number) 1-2, 3-4, 5-6, 7-8 and 9-10. The HLA gene typing method comprises the steps that the HLA gene specific PCR amplification primer is used for conducting PCR amplification and purification on sample DNA (deoxyribonucleic acid); a gene exon sequencing primer is used for conducting sequencing PCR amplification, purification and sequencing on an HLA gene amplification product, the sequence of the HLA gene amplification product is compared with a standard sequence; and the type of the HLA gene is determined. The HLA gene typing kit comprises the PCR amplification primer and the sequencing primer. A qualitative leap in aspects of detection flux, data quality, cost control and the like is achieved; data is more reliable and realer; and the problem that typing cannot be conducted when nucleotide of certain alleles is positioned outside an amplification area is solved.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
HLA (Human Leukocyte Antigen)-A,B genotyping PCR (Polymerase Chain Reaction) primer and application method thereof
ActiveCN101921842AImprove featuresImproved conservatismMicrobiological testing/measurementDNA/RNA fragmentationHla genesBiology
The invention provides an HLA (Human Leukocyte Antigen)-A,B genotyping PCR (Polymerase Chain Reaction) primer, and a method of applying the PCR primer to HLA gene analysis based on a sequencing method.
Owner:BGI GENOMICS CO LTD
Tumor antigenic polypeptide and application thereof as tumor vaccine
The invention provides a human mucin-1 tumor antigenic polypeptide with an amino acid sequence shown in SEQ ID NO: 1 or variants thereof, wherein the polypeptide can be combined with an HLA (Human Leukocyte Antigen) I and can be recognized by a cell CD8<+>T. The invention further provides nucleic acids coding the polypeptide. The invention further provides an antigen presenting cell capable of presenting the polypeptide on the surface of the cell and an immune effector cell capable of recognizing the polypeptide or antigen presenting cell. The invention further provides application of the polypeptide or variants thereof, the nucleic acids, the antigen presenting cell or the immune effector cell in the preparation of vaccines or pharmaceutical compositions for treating or preventing cancer. Tumor vaccines provided by the invention have a good treatment effect on relatively large crowds and are particularly applicable to the Asian, e.g. Chinese.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +1
TaqMan probe real-time fluorescence PCR (Polymerase Chain Reaction) method for detecting HLA (Human Leukocyte Antigen)-B*5801 alleles
ActiveCN104232781ASave consumablesShorten the timeMicrobiological testing/measurementDNA/RNA fragmentationReference genesFluorescence
The invention discloses a primer probe assembly for high-specificity amplification HLA (Human Leukocyte Antigen)-B*5801 alleles on the basis of a TaqMan probe detection method. The primer probe assembly comprises an upstream primer Fp: 5'-AGGGGCCGGAGTATTGGGATG-3', a downstream primer Rp: 5'-TTGGCCTCAACTGAAAATGAAAC-3' and a probe: 5'-HEX-TCAGGGAGGCGGATCTCGGAC-BHQ2-3'. Primers and probes of reference genes ACTB are used simultaneously, target genes and the reference genes are added into a tube for dual-channel fluorescent quantitative PCR reaction, and a result is analyzed through an amplification curve. The method has the characteristics of simplicity, convenience, flexibility, quickness, high specificity, high flux, zero pollution, high resolution and the like, and is applicable to detection of the HLA-B*5801 alleles of a whole-genome DNA (Deoxyribonucleic Acid) sample in the peripheral blood and the saliva of a human body.
Owner:SHANXI LIFEGEN
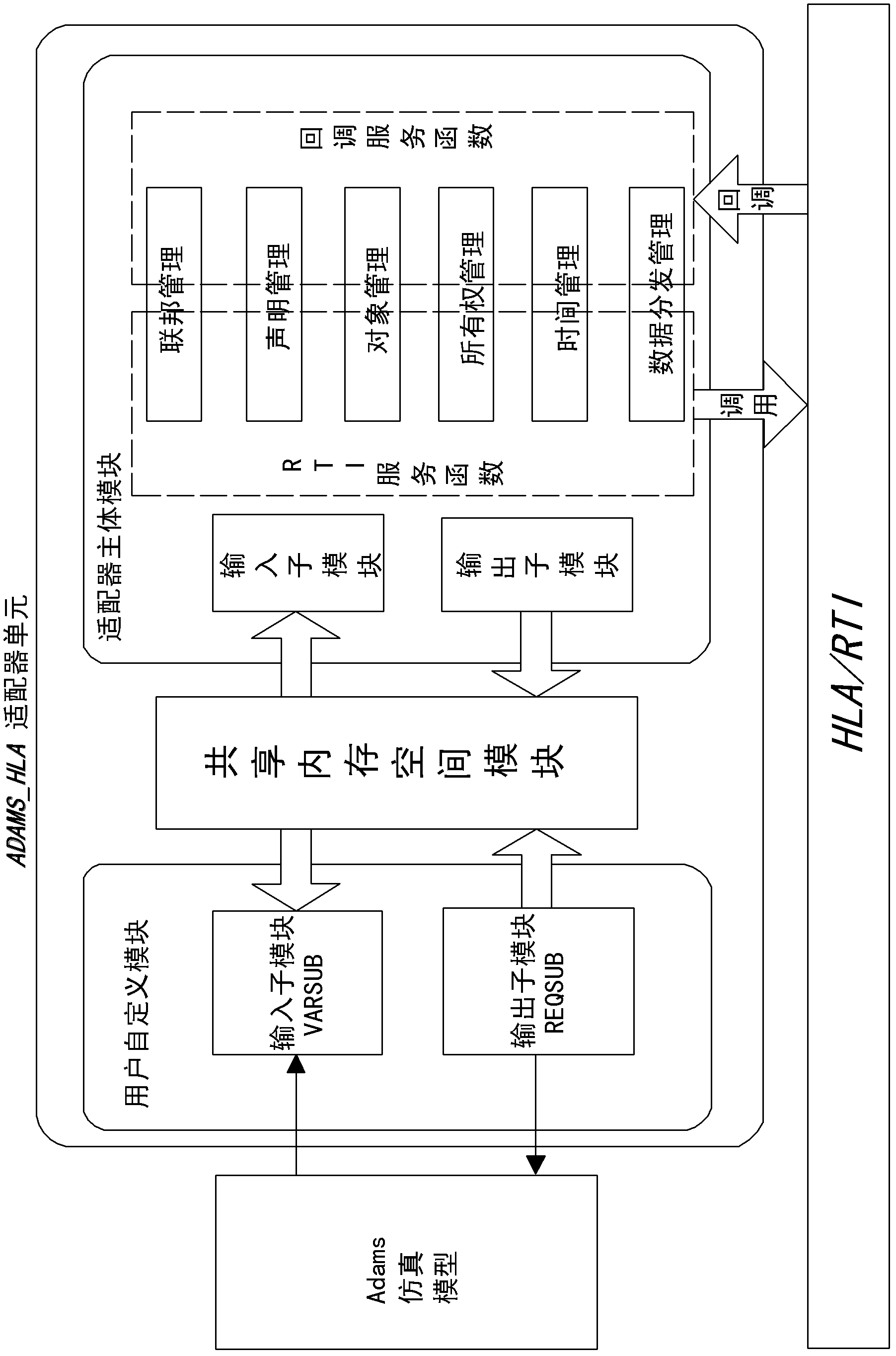
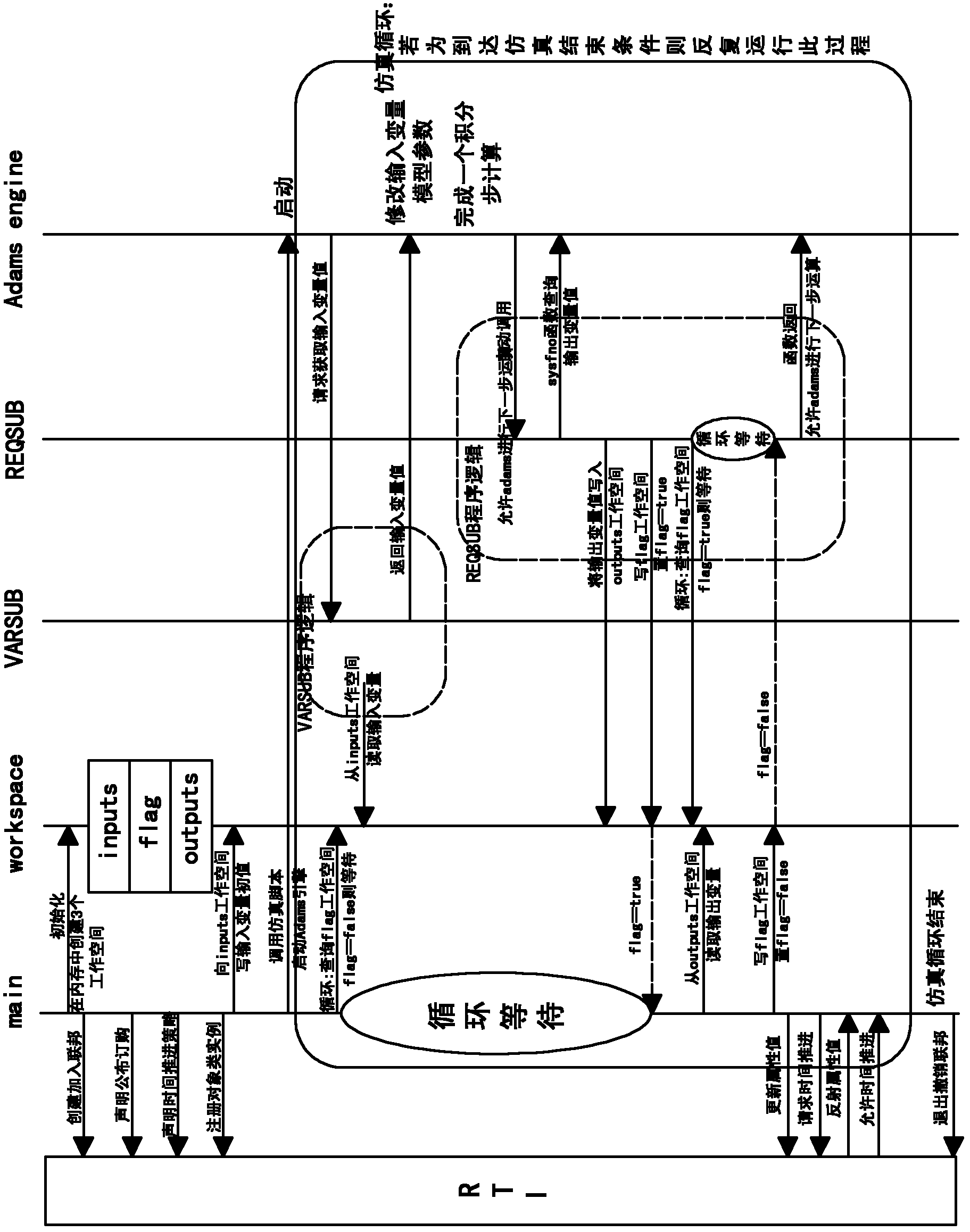
HLA (Human Leukocyte Antigen)-based Adams simulation model integrated platform and method
InactiveCN102915386AEasy to useGood effectSpecial data processing applicationsReusabilityHuman leukocyte antigen
The invention discloses an HLA (Human Leukocyte Antigen)-based Adams simulation model integrated platform, to solve the defect in the prior art that an Adams simulation model is hard to be subjected to HLA integration. The HLA-based Adams simulation model integrated platform comprises a simulation model unit, an adaptor unit and an HLA system, wherein the simulation model unit comprises at least one Adams simulation module; the adaptor unit comprises a user defined module, a shared memory space module and a main body module; and the user defined module comprises an input sub-module and an output sub-module. The invention discloses an HLA-based Adams simulation model integrated method. With the adoption of the HLA-based Adams simulation model integrated method, the advantages of a commercial simulation software solver are maintained, the packaging of an Adams model is realized under the condition that the simulation model needs not to be modified or is slightly configured, and the reusability of the Adams model is greatly improved.
Owner:TSINGHUA UNIV
TCR (T Cell Receptor) for identifying MAGE-A1 (Melanoma antigen A1) oligopeptides
ActiveCN106749620AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenOligopeptide
The invention provides a TCR (T Cell Receptor) which is capable of specifically binding oligopeptides KVLEYVIKV derived from an MAGE-A1 (Melanoma antigen A1). A compound can be formed by the oligopeptides KVLEYVIKV derived from the MAGE-A1 and an HLA (Human Leukocyte Antigen) A0201, and the oligopeptides KVLEYVIKV and the HLA A0201 can be transferred to cell surfaces together. The invention also provides a nucleic acid molecule for coding the TCR and a carrier comprising the nucleic acid molecule. In addition, the invention also provides a cell for transducing the TCR.
Owner:XLIFESC LTD
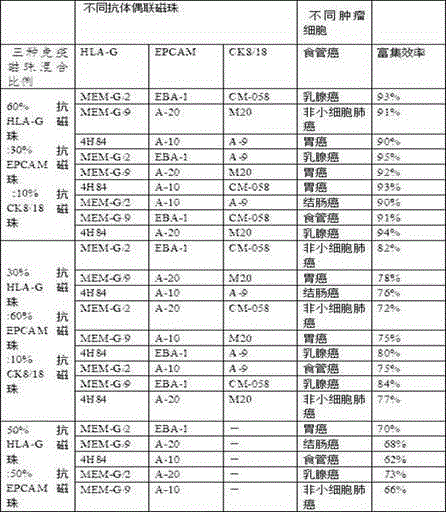
Joint application of three types of monoclonal antibody-coupled immunomagnetic beads to sorting tumor cells
ActiveCN105087493AIncrease coverageHigh efficiency in sorting tumorsTumor/cancer cellsAntiendomysial antibodiesOncology
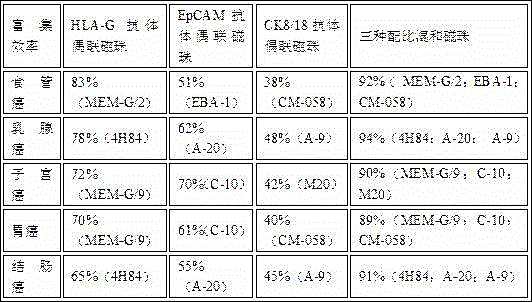
The invention discloses joint application of three types of monoclonal antibody-coupled immunomagnetic beads to sorting tumor cells and a method for sorting breast cancer tumor cells. The three types of monoclonal antibody-coupled immunomagnetic beads include anti-HLA-G (human leukocyte antigen-G) monoclonal antibody-coupled immunomagnetic beads, anti-EpCAM (epithelial cell adhesion molecule) monoclonal antibody-coupled immunomagnetic beads and anti-CK8 / 18 (casein kinase 8 / 18) monoclonal antibody-coupled immunomagnetic beads. The joint application and the method have the advantages that the three types of jointly applied antibody-coupled immunomagnetic beads are high in breast cancer tumor cell enrichment ratio and good in sensitivity and repeatability, and the relevant tumor cells can be detected; the three types of immunomagnetic beads (the anti-EpCAM immunomagnetic beads, the anti-HLA-G immunomagnetic beads and the anti-CK8 / 18 immunomagnetic beads) are jointly applied to enriching the tumor cells, so that the circulating tumor cell coverage rate can be increased to the greatest extent, the enrichment ratio can reach 80%, and a technology is international pioneer; the tumor cell enrichment specificity and sensitivity can be particularly guaranteed owing to usage of the anti-HLA-G monoclonal antibody-coupled immunomagnetic beads.
Owner:济南红枫叶生物科技有限公司
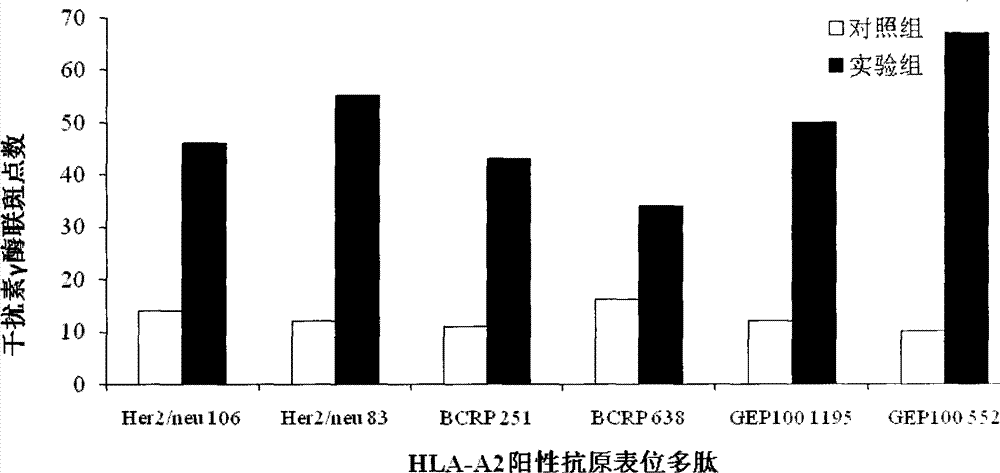
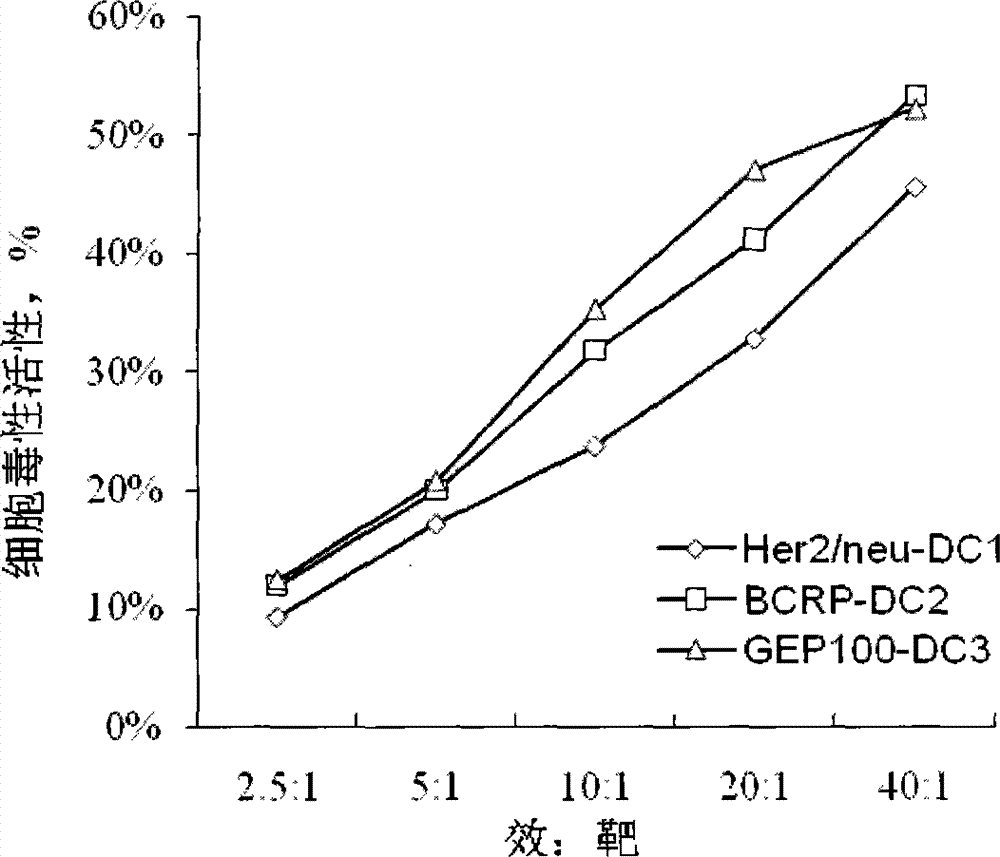
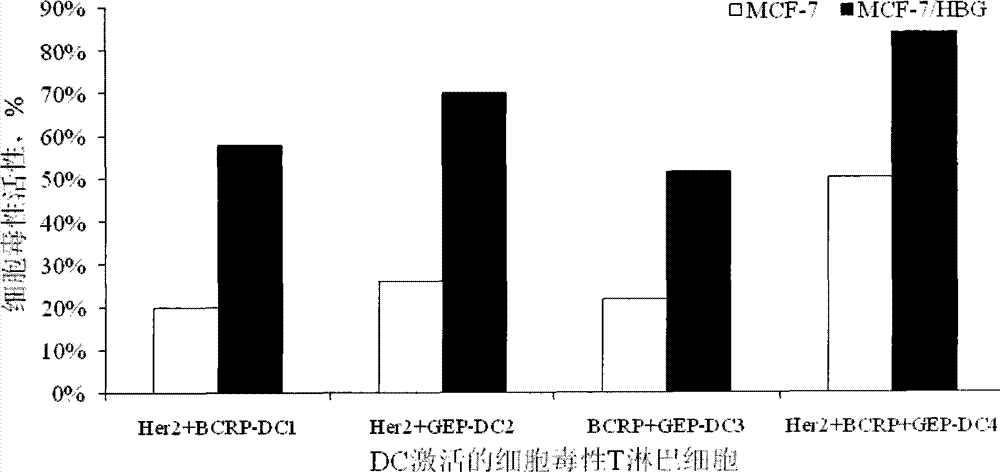
Preparation method of breast cancer-specific epitope polypeptide-loaded dendritic cell vaccine and kit thereof
InactiveCN103784950ACure recurrenceCure metastasesBlood/immune system cellsAntibody medical ingredientsWhite blood cellT lymphocyte
The invention provides a preparation method of a breast cancer-specific epitope polypeptide-loaded dendritic cell vaccine, and the method comprises the following steps: loading dendritic cells with identified human leukocyte antigen A201 positive epitope polypeptide of human epidermal growth factor receptor 2, breast cancer drug resistance protein and IQ motif and Sec7 structural domain 1 protein which specifically express in breast cancer (namely polypeptide comprising the amino acid sequence shown in any of SEQ ID No.1-6) to prepare into the dendritic cell vaccine which can induce strong breast cancer-targeting cytotoxic T lymphocyte response. The invention also provides a kit comprising the breast cancer-specific epitope polypeptide-loaded dendritic cell vaccine.
Owner:BEIJING HONGRUNYUAN BIOLOGICAL TECH
Detection method and kit of HLA (Human Leukocyte Antigen)-B*58:01 allele
The invention relates to a detection method of HLA (Human Leukocyte Antigen)-B*58:01 allele. The detection method comprises the following steps: providing a mixture of a sample to be tested, a nucleic acid amplification system and a fluorescence detection system; circularly amplifying target polynucleotide through an amplified reaction; indirectly combining a fluorescence generating group and an amplified target polynucleotide sequence; and detecting the fluorescent amount generated by the fluorescence generating group so as to determine existence of the target polynucleotide and the relative amount thereof. The invention further discloses a kit for detecting the allele. The kit comprises a plurality of sealed centrifuge tubes which are respectively filled with a reaction liquid 1, a reaction liquid 2, a reaction liquid 3, an EZ Taq enzyme mixed liquid, a standard liquid 1 and a standard liquid 2 as well as the kit which separates and packages the centrifuge tubes in a centralized manner. The kit and the detection method provided by the invention are simple and convenient to operate, short in time consumed, strong in specificity and high in sensitivity. The kit and the detection device can be widely applied to detecting the HLA-B*58:01 allele and clinically avoiding severe untoward effects of skins of the HLA-B*58:01 allele patients caused by using allopurinol.
Owner:GUANGDONG UNITY BIOTECH
Chimeric antibody and immunocyte
InactiveCN102120772ASpecific immune functionSpecific cleavageMicroorganismsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSpecific immunity
The invention discloses a chimeric antibody and an immunocyte. The chimeric antibody provided by the invention has an amino acid sequence containing a target antibody, a transmembrane domain segment of a HLA-A2 (Human Leukocyte Antigen-A2) molecule and an intracellular domain segment of a CD3 compound protein zeta chain in sequence from a terminal N to a terminal C. The chimeric antibody can be expressed on the surfaces of any cells, when the chimeric antibody is expressed on the surface of an immunocyte, the immunocyte is endowed with a specific immunity by virtue of the target antibody on the chimeric antibody, and thus, the chimeric antibody provided by the invention can be specifically combined with a plurality of antigens along with different target antibodies in the chimeric antibody. The chimeric antibody, a single-domain antibody and an immunocyte for expressing the chimeric antibody, provided by the invention, are about to have broad application prospects in the cellular immunotherapy.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
HLA (human leucocyte antigen) typing method based on PacBio RS II sequencing platform
The invention discloses an HLA (human leucocyte antigen) typing method based on a PacBio RS II sequencing platform. A collected sample is subjected to DNA extraction and then to PCR (polymerase chain reaction) amplification, PCR products are mixed to establish a 10k library, and PacBio RS II sequencing is performed; then, original data obtained by sequencing are corrected, and HLA typing is performed with software programs. Compared with existing HLA typing methods, the HLA typing method has super-high resolution and is of important value to applications such as clinical graft tissue matching, population genetics, anthropology and evolutiology, as well as basic research work.
Owner:BEIJING GRANDOMICS BIOTECH
NK (Natural Killer) cell new technology for treating tumor
InactiveCN103372029AEasy accessLarge sourceMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthLymphatic Spread
Hematopoietic stem cells of healthy volunteers are separated and purified in a 100 grade GMP (Good Manufacturing Practice) plant, and are induced to divide to NK cells under a special condition of culture. Qualified NK cells are put in storage or KIR-matched with HLA (Human Leukocyte Antigen) and KN cells of the patients. The qualified NK cells are intravenously infused to tumor patients to prevent recurrence and metastasis of cancer cells of the tumor patients. The hematopoietic stem cells used by the method have the advantages of abundant source, material object storage and the like, are free from toxic and side effects and adverse reaction for treating tumor, and remarkably improve the survival quality of the tumor patients.
Owner:孙勇
Genetic testing for risk of causing serious adverse reactions of skin of carbamazepine
InactiveCN102108382AMicrobiological testing/measurementFluorescence/phosphorescenceAdverse drug reactionAntiepileptic drug
The invention discloses a genetic testing method for risk of causing serious adverse reactions of carbamazepine. The genetic testing method comprises the following steps: collecting oral mucosa cells of a subject, extracting genome DNA (deoxyribonucleic acid) of the oral mucosa cells, testing HLA (human leukocyte antigen)-B*1502 allelotype of the genome DNA and evaluating the risk of causing the serious adverse reactions of the skin of an anti-epileptic medicament-carbamazepine, thereby providing reference basis for clinical individual medication.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Method for modulating HLA class ii tumor cell surface expression with a cytokine mixture
InactiveUS20070166279A1Peptide/protein ingredientsAntineoplastic agentsAbnormal tissue growthHla class ii
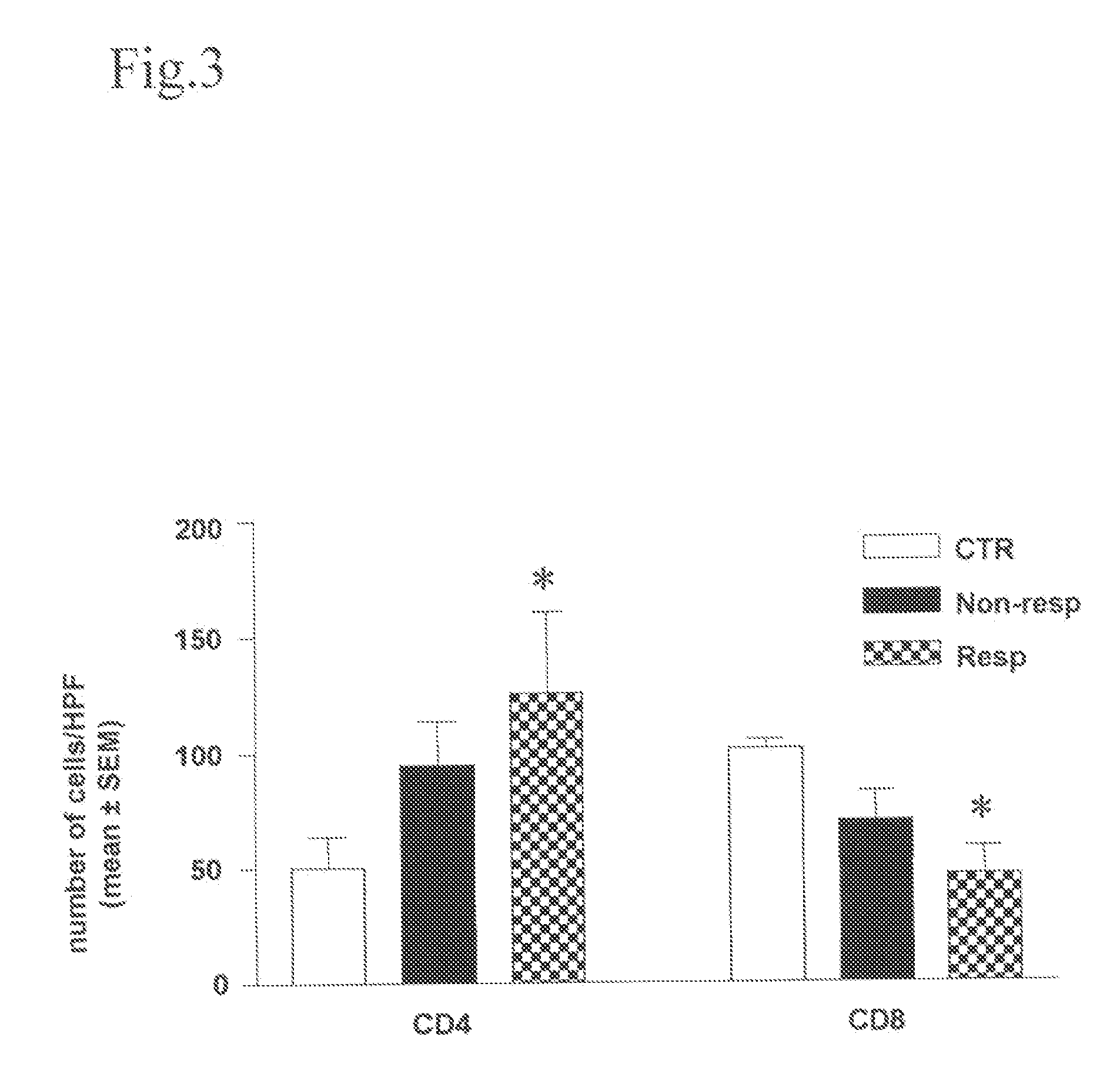
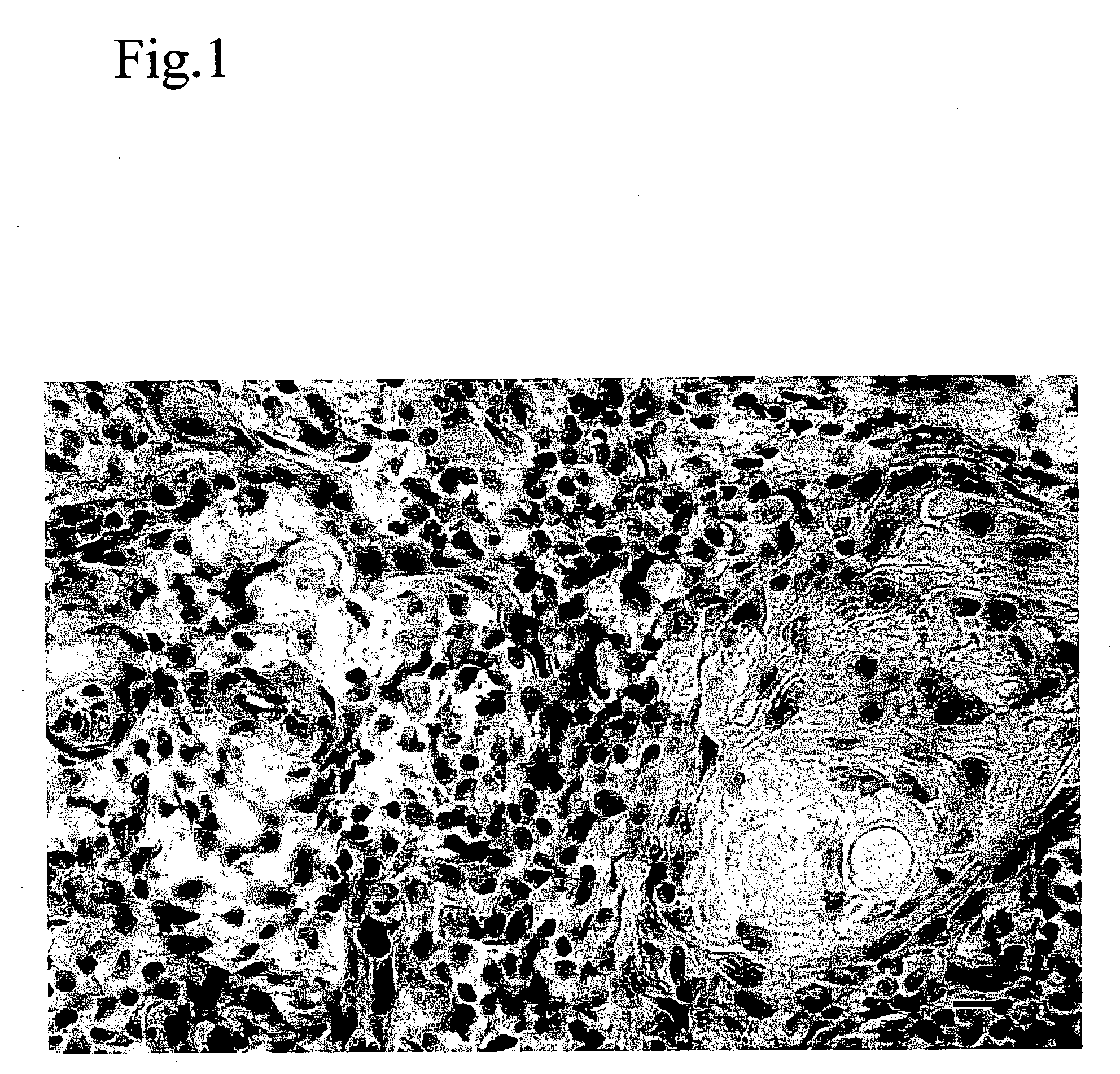
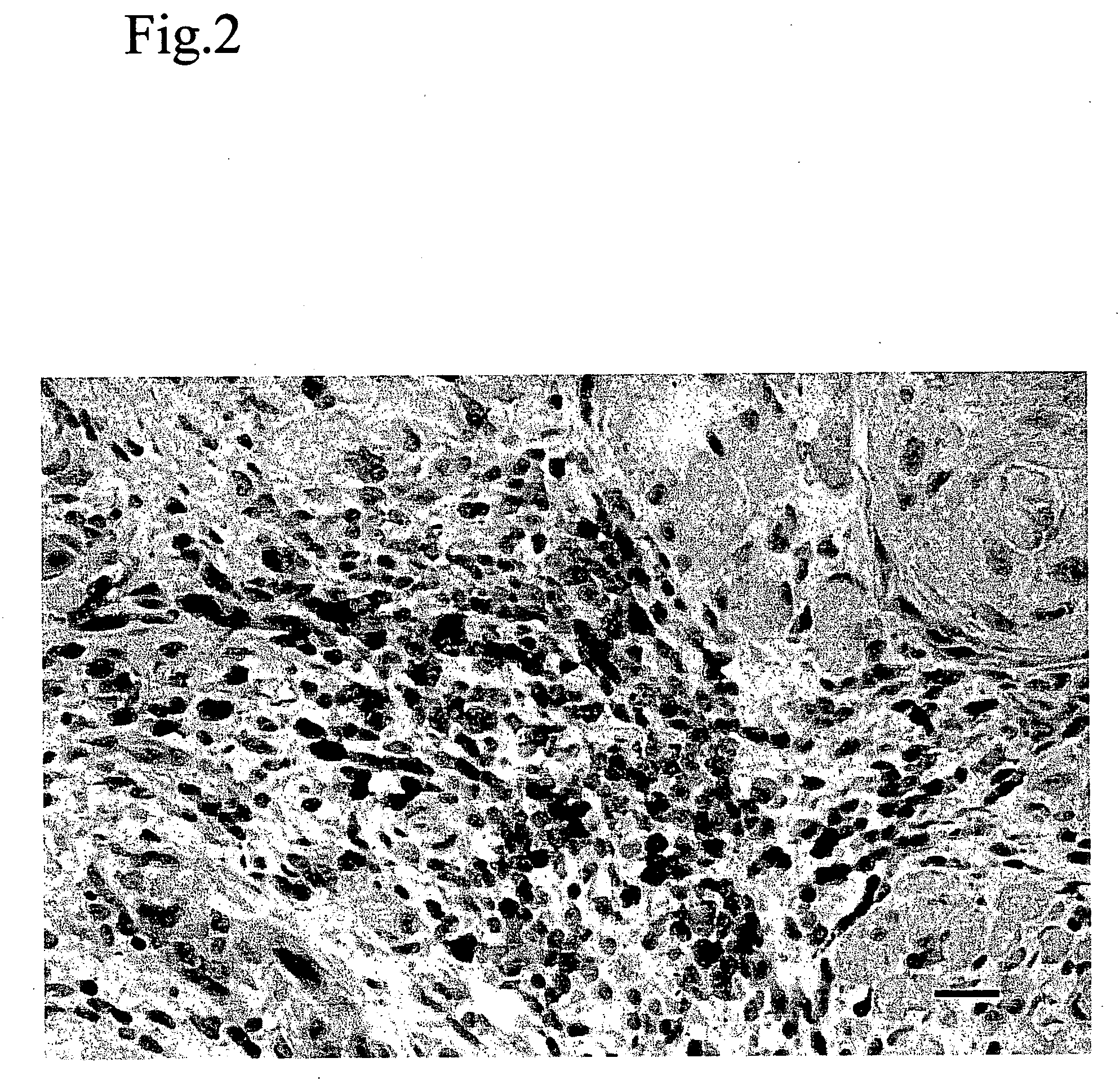
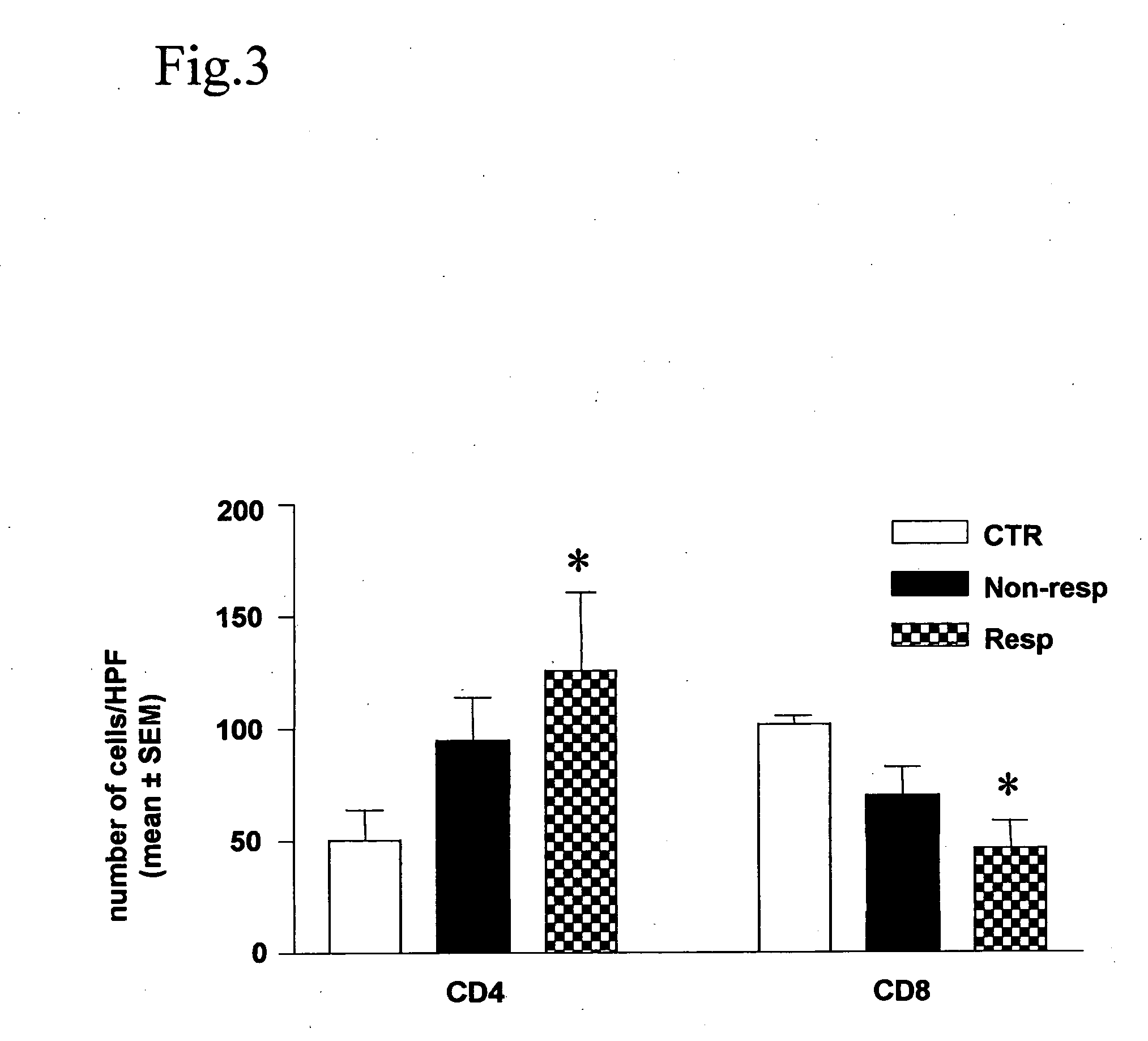
A method for altering the composition of tumor infiltrating mononuclear cells, increasing CD4+ / CD8+ ratio, increasing tumor stroma / epithelial ratio and modulating HLA (Human Leukocyte Antigen) class II expression on a tumor cell surface with a serum-free and mitogen-free mixture having specific cytokine ratios from the group of IL-1β, TNF-α, IFN-γ, GM-CSF, and Interleukin-2 (IL-2) with specific ratios of IL-1β, TNF-α, IFN-γ, GM-CSF to IL-2, respectively. The serum-free and mitogen-free mixtures comprised of cytokine ratios include Leukocyte Interleukin Injection (LI) or Multikine®, which can be further used alone or in combination with other drugs for the treatment of cancer thereby increasing the success of cancer treatment and the disease free survival of cancer patients.
Owner:TALOR EYAL
Method and system for detecting HLA heterozygosity deficiency
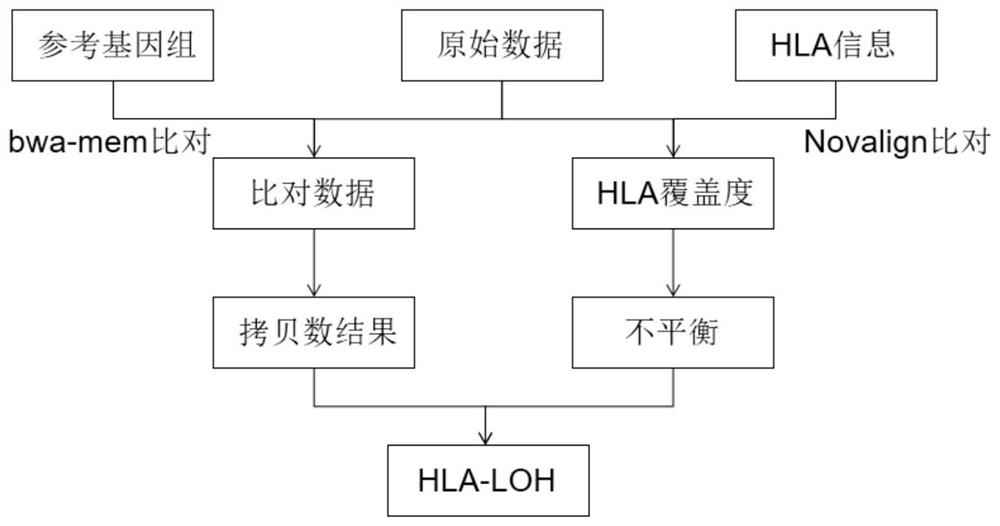
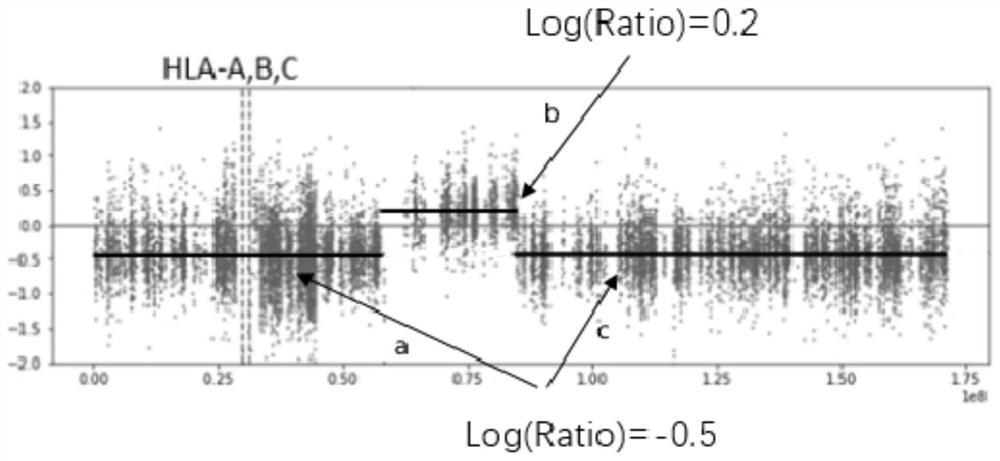
The invention provides a method and a system for detecting HLA (human leukocyte antigen) heterozygosity deficiency. The method comprises the following steps: data acquisition: acquiring sequencing data of a tumor sample and a control sample; hLA typing detection: detecting HLA molecular types of the tumor sample and the contrast sample; HLA allele imbalance detection: comparing the sequencing data with an HLA typing result to obtain an HLA allele imbalance detection result; copy number variation detection: performing copy number variation detection on all the target areas to obtain a copy number variation detection result of the HLA gene loci; and HLA heterozygosity deletion judgment: judging whether HLA heterozygosity deletion exists or not according to an HLA allele imbalance detection result and a copy number variation detection result. According to the invention, the sequence information on the HLA gene is used independently, and the sequence information near the HLA gene is also combined, so that an accurate HLA LOH result is obtained.
Owner:深圳裕策生物科技有限公司
Preparation method of HLA-A0201 (Human Leukocyte Antigen-A0201) restrictive anti-MAGE (MicroArray and Gene Expression) antigenic specificity CTL (Cytotoxic T Lymphocyte)
ActiveCN102719401AHigh purityImprove proliferative abilityBlood/immune system cellsDendritic cellPeripheral blood mononuclear cell
The invention discloses a preparation method of an HLA-A0201 (Human Leukocyte Antigen-A0201) restrictive anti-MAGE (Micro Array and Gene Expression) antigenic specificity CTL (Cytotoxic T Lymphocyte). The method comprises the following steps of: carrying out hemapheresis to collect peripheral blood mononuclear cells; enriching and purifying CD8+T lymphocytes; utilizing a mature dendritic cell loaded with an HLA-A0201 restrictive anti-MAGE antigen polypeptide to stimulate the CD8+T lymphocytes; utilizing rhIL-2 and rhIL-7 to accelerate the T cells to grow; utilizing a Tetramer marking method and a flow type cell separation technology to purify the target CTL; utilizing an anti-human CD3 monoclonal antibody covered by a solid phase and an IL-2 to stimulate the target CTL to grow; adding an autologous PBMC (Peripheral Blood Mononuclear Cell) radiated by gamma rays to enhance the activation of the target CTL; adding rhIL-15 to cultivate and reproduce; and collecting and identifying. The prepared target CTL has high purity, high reproduction capability, high killing activity and high ratio CTL-CM, and can be used for immune treatment of tumors and the like.
Owner:江苏得康生物科技有限公司
Method of detecting common ambiguous types of HLA (human leukocyte antigen) typing locus A
InactiveCN109355367AAccurate and preciseLow costMicrobiological testing/measurementDNA/RNA fragmentationTypingHuman leukocyte antigen
The invention discloses a method of detecting common ambiguous types of HLA (human leukocyte antigen) typing locus A. The method includes: (1) acquiring ambiguous types of locus A through HLA typing results, and selecting specific primers necessary to distinguish the ambiguous types, wherein the specific primers are shown as SEQ ID NO. 1 to 9; (2) using the selected specific primers to perform sequencing PCR (polymerase chain reaction) on the purified HLA-A gene amplification product; (3) purifying the post-sequencing product, acquiring a product sequence through a sequencer, comparing the product sequence with the ambiguous types so as to distinguish the ambiguous types, and finally determining HLA-A type information. Compared with traditional PCR-SSP (PCR-sequence specific primer) methods to determine gene ambiguous types, the method herein can accurately and precisely judge the types, has greatly reduced cost, is simple to perform, can easily provide high throughput, and is a time-saving economical option for large-scale donor HLA typing.
Owner:银丰基因科技有限公司 +1
Primer group and reagent box used for detecting HLA-B* (human leukocyte antigen)-1502 allelic genes
ActiveCN106119362AMicrobiological testing/measurementDNA/RNA fragmentationHLA-BHuman leukocyte antigen
The invention provides a PCR (polymerase chain reaction) amplification primer group and Taqm probes used for detecting HLA (human leukocyte antigen)-B*1502 allelic genes. The PCR amplification primer group and Taqm probes include two pairs of primers designed according to specific sequence of HLA-B*1502 and two corresponding probes, further include one pair of probes used for filtering out false positive results of the HLA-B*1502 and one corresponding probe, and further include one pair of internal-contract primers and one corresponding probe. The primer group has the technical advantages that the genes of the HLA-B*1502 are positive or not are determined through two reaction tubes, the false positive result of the HLA-B*1502 is eliminated through one reaction tube, and the false positive results of the genes of the sampled HLA-B*1502 are eliminated by adding the internal-control primers and the probes in reaction holes.
Owner:JIANGSU WEIHE BIOTECH
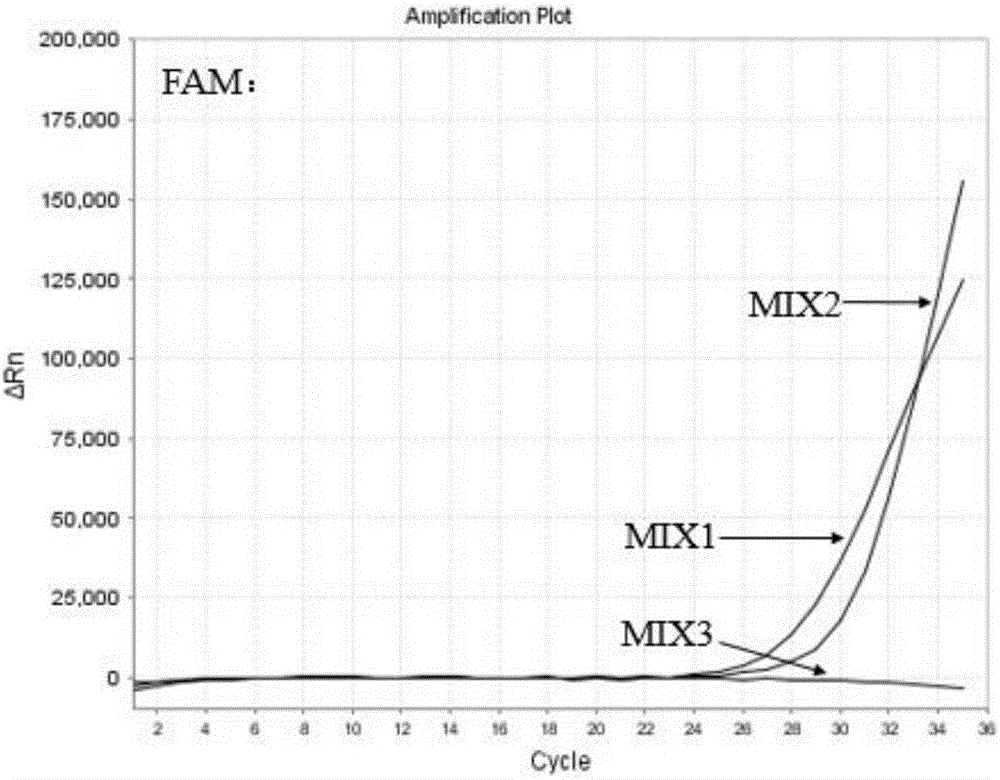
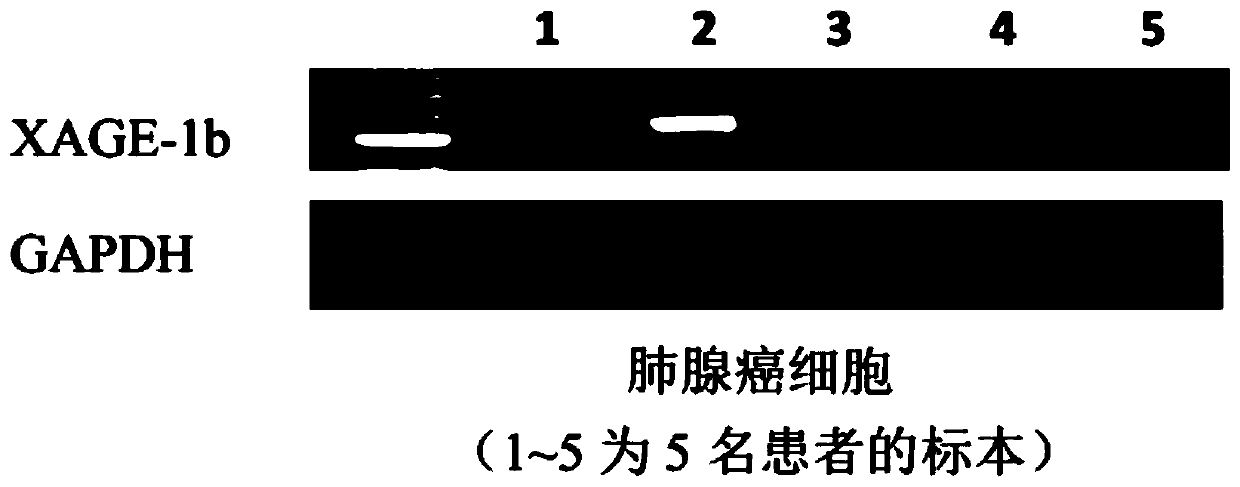
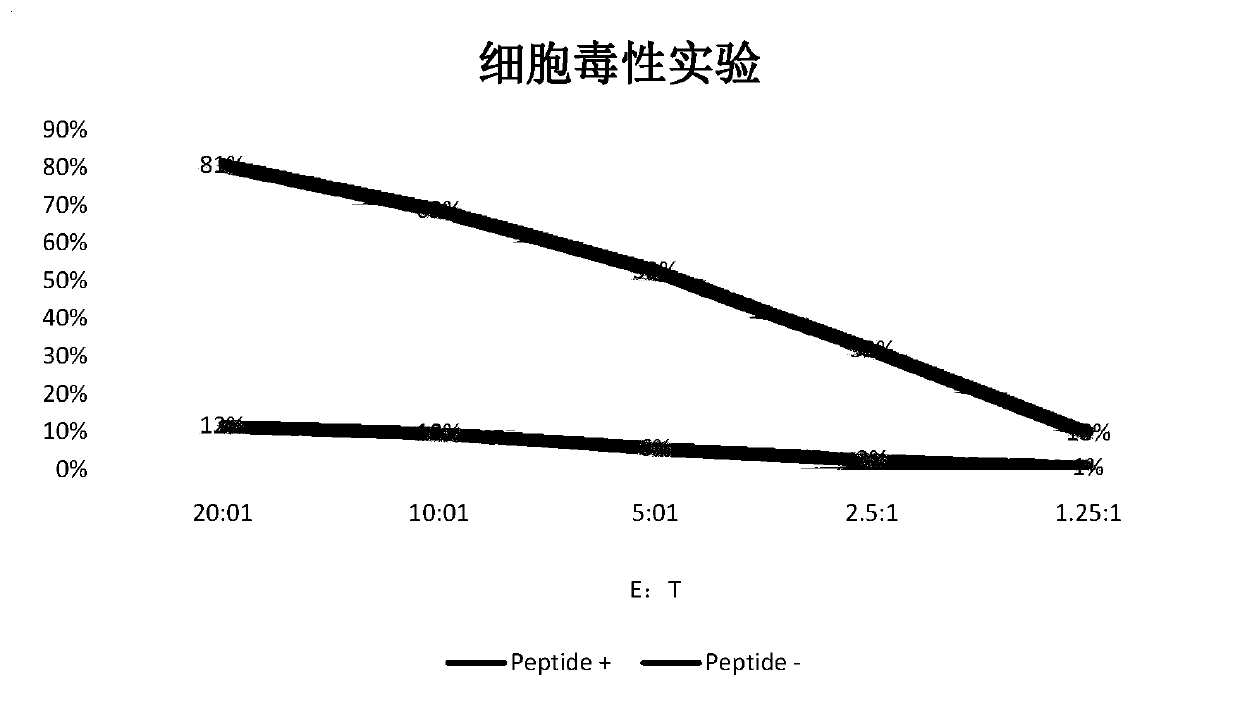
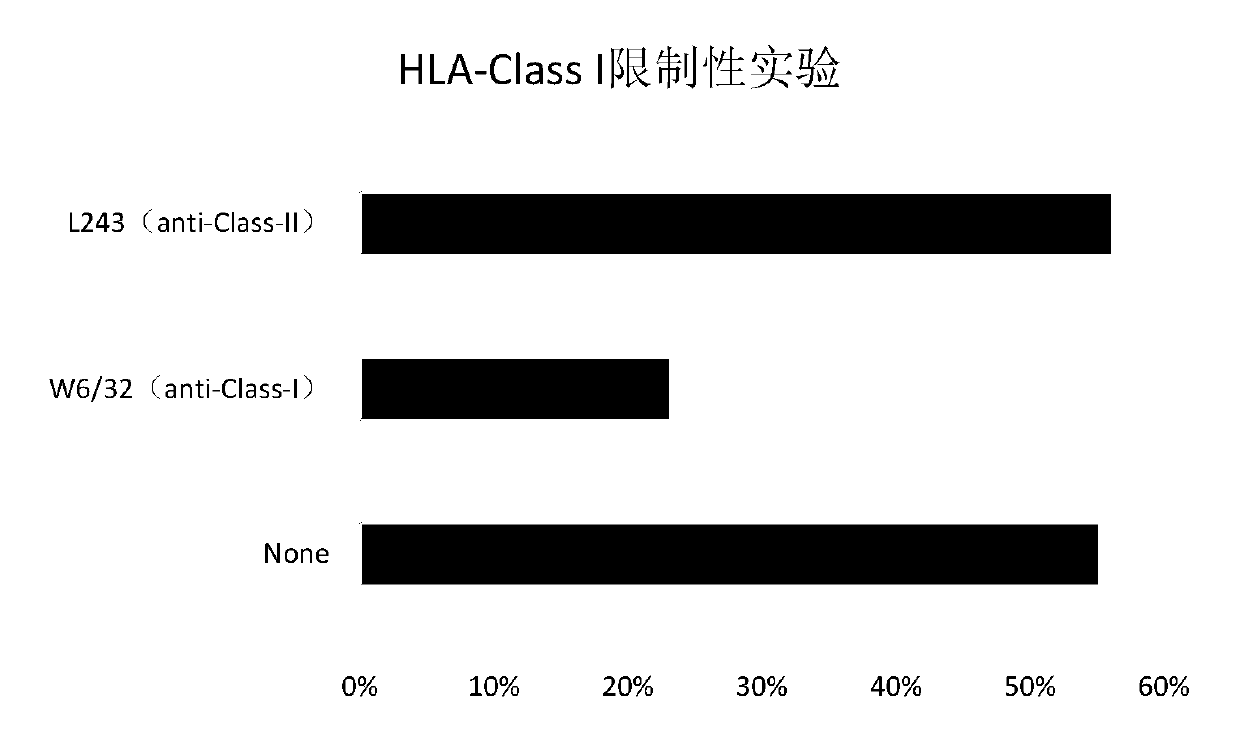
Tumor-associated antigen XAGE-1b nonapeptide and application thereof
InactiveCN109970846AGood specific killing effectPromote clinical translationTumor rejection antigen precursorsBiological material analysisT lymphocyteCD8
The invention discloses tumor-associated antigen XAGE-1b nonapeptide and application thereof. The sequence of XAGE-1b oligopeptide is RQKKIRIQL. CTLs (cytotoxic T lymphocytes) induced by the XAGE-1b antigen peptide do not produce immune responses to testicular cells, but only kill tumor cells. Induced CTL clones have a good tumor cell specificity lethal effect. In the process of establishing the CTLs, the XAGE-1b antigen peptide screened out by the inventor has proper affinity with HLAs (human leukocyte antigens) on DC cells and can effectively stimulate and induce the specific CTLs, thereby having a good potential of serving as a polypeptide vaccine and a DC vaccine. Virus carriers are created by cloning specific TCR (T cell receptor) genes of the XAGE-1b antigen peptide, and peripheral circulation CD8+T cells are transduced to obtain clones of TCR gene modified T cells (TCR-T); the clones also have the tumor cell specificity lethal effect same as the parental CTLs, thereby having a good prospect in clinical transformation and practical application.
Owner:广州美萨生物科技有限公司
HLA gene amplification primer, kit, sequencing library construction method and sequencing method
PendingCN113817725AConvenient experimentSimple processMicrobiological testing/measurementLibrary creationMedicineHuman leukocyte antigen
The invention discloses an HLA (human leukocyte antigen) gene amplification primer. The HLA gene amplification primer comprises a mixture consisting of any one or more of the following nine primer groups of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4; SEQ ID NO: 5 and SEQ ID NO: 6; SEQ ID NO: 7 and SEQ ID NO: 8; SEQ ID NO: 9 and SEQ ID NO: 10; SEQ ID NO: 11 and SEQ ID NO: 12; SEQ ID NO: 12 and SEQ ID NO: 13; SEQ ID NO: 14 and SEQ ID NO: 15; SEQ ID NO: 16, SEQ ID NO: 17 and SEQ ID NO: 18. The invention also discloses a kit for sequencing the HLA gene, and the kit comprises the HLA gene amplification primer. The invention also discloses a construction method of a gene sequencing library based on the HLAHLA amplification primer. The invention also discloses an HLA gene sequencing method based on the construction method of the HLA gene sequencing library.
Owner:XIAN HAORUI GENE TECH LTD
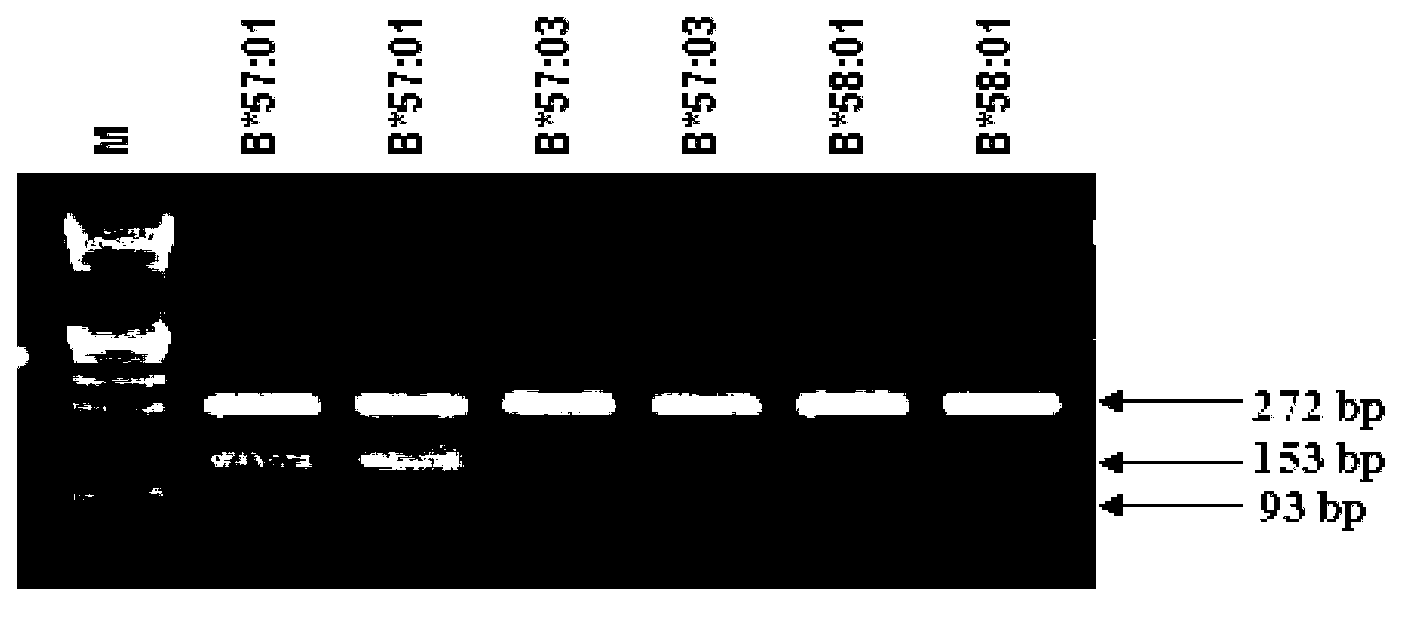
Assay kit for detecting human leukocyte antigen-B (HLA-B)*57:01 and HLA complex P5 (HCP5) alleles
InactiveCN103215356ASave operating timeSave operating workloadMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceGenotype
The invention discloses an assay kit for detecting human leukocyte antigen-B (HLA-B)*57:01 and HLA complex P5 (HCP5) alleles. The assay kit comprises a polymerase chain reaction (PCR) reaction liquid containing primers for detecting HLA-B*57:01 and HCP5, wherein the primers for detecting HLA-B*57:01 are primers B57F, B57R1 and B57R2; the primer B57F has a sequence shown in SEQIDNO:1, the primer B57R1 has a sequence shown in SEQIDNO:2, and the primer B57R2 has a sequence shown in SEQIDNO:3; and the primers for detecting the HCP5 are primers 631R and 568GF, wherein the primer 631R has a sequence shown in SEQIDNO:4, and the primer 568GF has a sequence shown in SEQIDNO:5. The assay kit has high detection sensitivity and good specificity, and can be used for accurately separating genotypes without sequencing.
Owner:上海血液生物医药有限责任公司
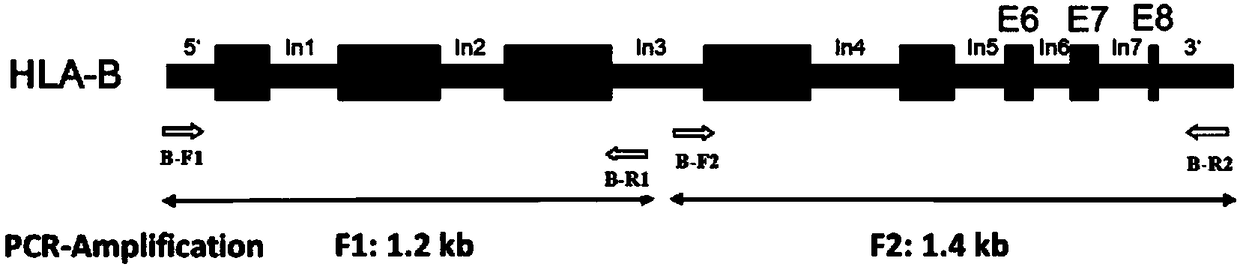
Primer group, kit and method for amplification of human leukocyte antigen-B (HLA)-B gene, and primer group, kit and method for genotyping of HLA-B gene
ActiveCN108531568AIncrease heightShorten the lengthMicrobiological testing/measurementDNA/RNA fragmentationPatient survivalOrgan transplantation
The invention discloses a primer group, a kit and a method for amplification of a human leukocyte antigen-B (HLA)-B gene, and the primer group, the kit and the method for genotyping of the HLA-B gene,belonging to the field of gene detection. According to the primer group, provided by the invention, for the amplification of the HLA-B gene, at least two sets of primers are designed according to theeight exon regions of the HLA-B gene; the primers are used for performing polymerase chain reaction (PCR) amplification on the HLA-B gene, so that a product having a gene fragment length of less than1.5 kb is obtained; after the HLA-B gene is subjected to the PCR amplification by the primers, the gene fragment length of the amplified product is less than 1.5 kb, so that the requirement for template integrity is reduced, the amplification efficiency is high, amplification reaction time is short, and the cost is low. The primer groups, the kits and the methods can meet the needs of amplification or genotyping of the HLA-B gene in mass samples, provide a more accurate basis for clinical HLA matching, provide suitable transplant donors for patients, reduce the rejection in a transplantationprocess and improve the success rate of organ transplantation and the patient survival rate.
Owner:BEIJING NUOSHI KANGYING MEDICAL TECH
Primers, kit and method for HLA (human leukocyte antigen) genotyping
InactiveCN105861673AEfficient specificityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationTypingGene engineering
The invention belongs to the technical field of gene engineering, and discloses a primer combination for HLA (human leukocyte antigen) genotyping, a kit containing the primer combination and a method for HLA genotyping. Thus, the sequence-specific HLA genotyping (GSA-SBT) technique is quick, simple, accurate and visual. The primer combination can be synchronously used for amplification and sequencing with the commercialized kit, thereby lowering the experimentation cost. Besides, the specific design of mismatched bases are introduced to the 3' terminal of the primer, thereby enhancing the specificity of the primer amplification and ensuring the accuracy of the typing result.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
HLA-DQB1 gene typing DNA micro-array chip reagent kit
The invention relates to a gene detecting kit that is used in clinical detection, in particular to a DNA microarray chip detecting kit, which can realize subtype detection of human leukocyte antigen DQB1(HLA-DQB1) gene in high flux, high efficiency and high specificity.
Owner:GUANGZHOU DARUI BIOTECH
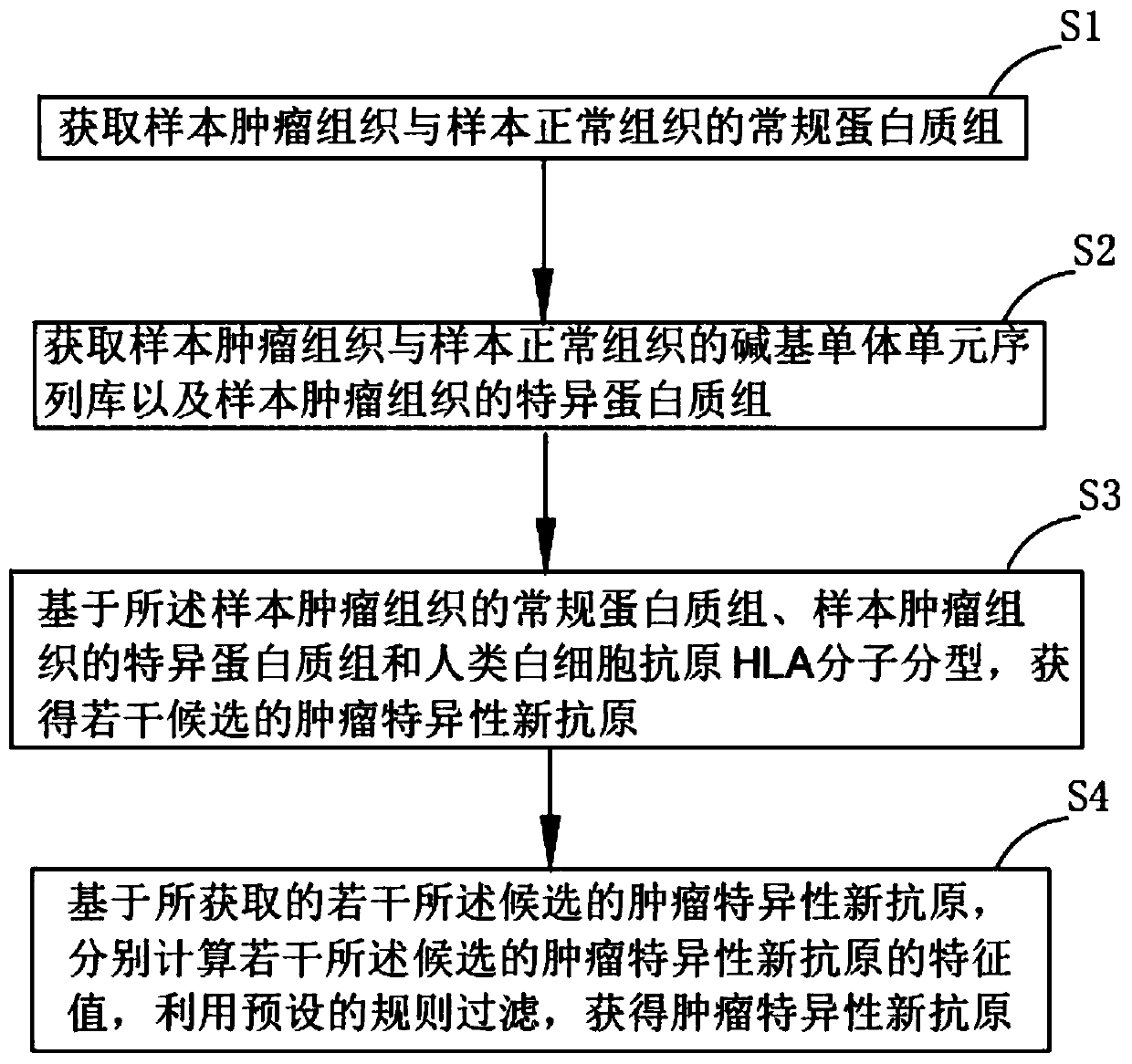
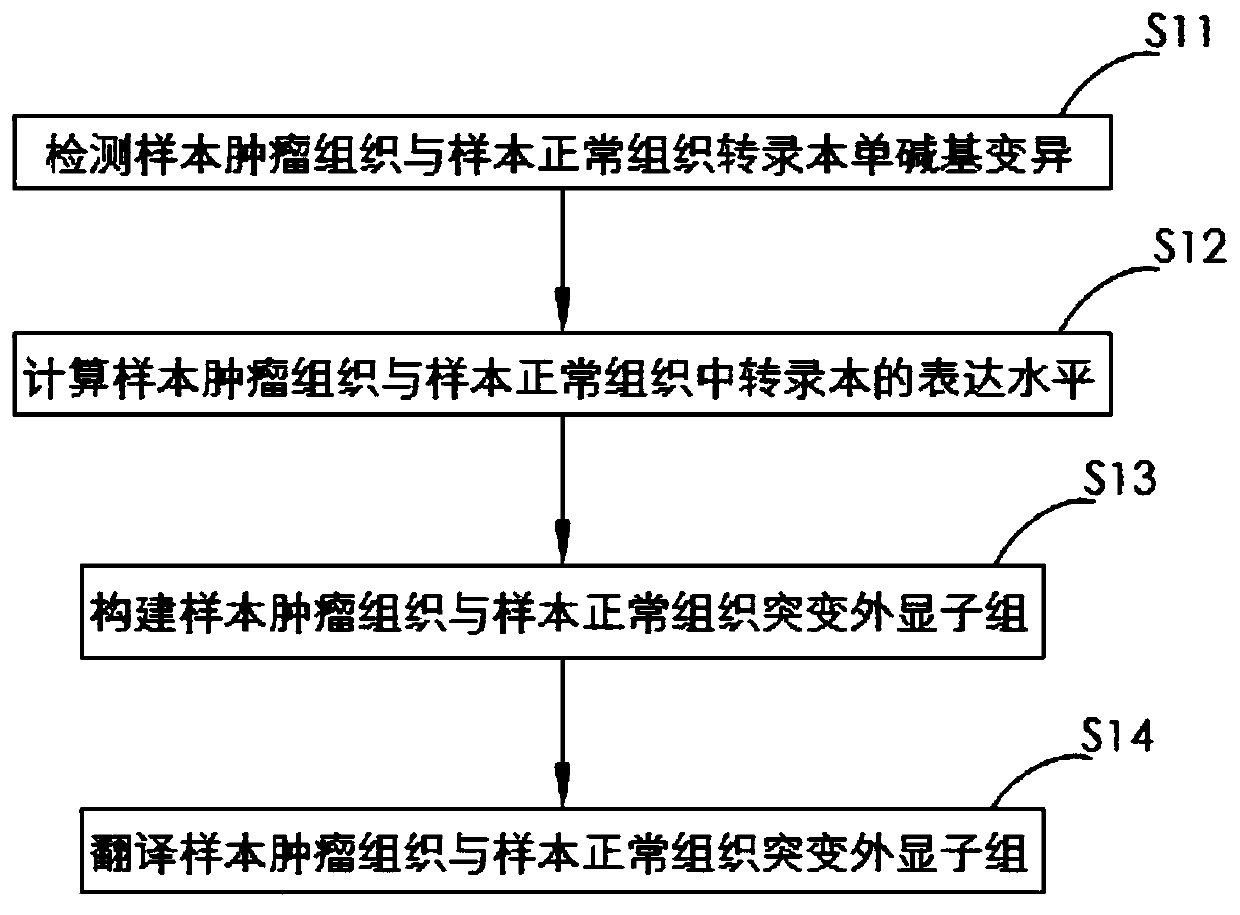
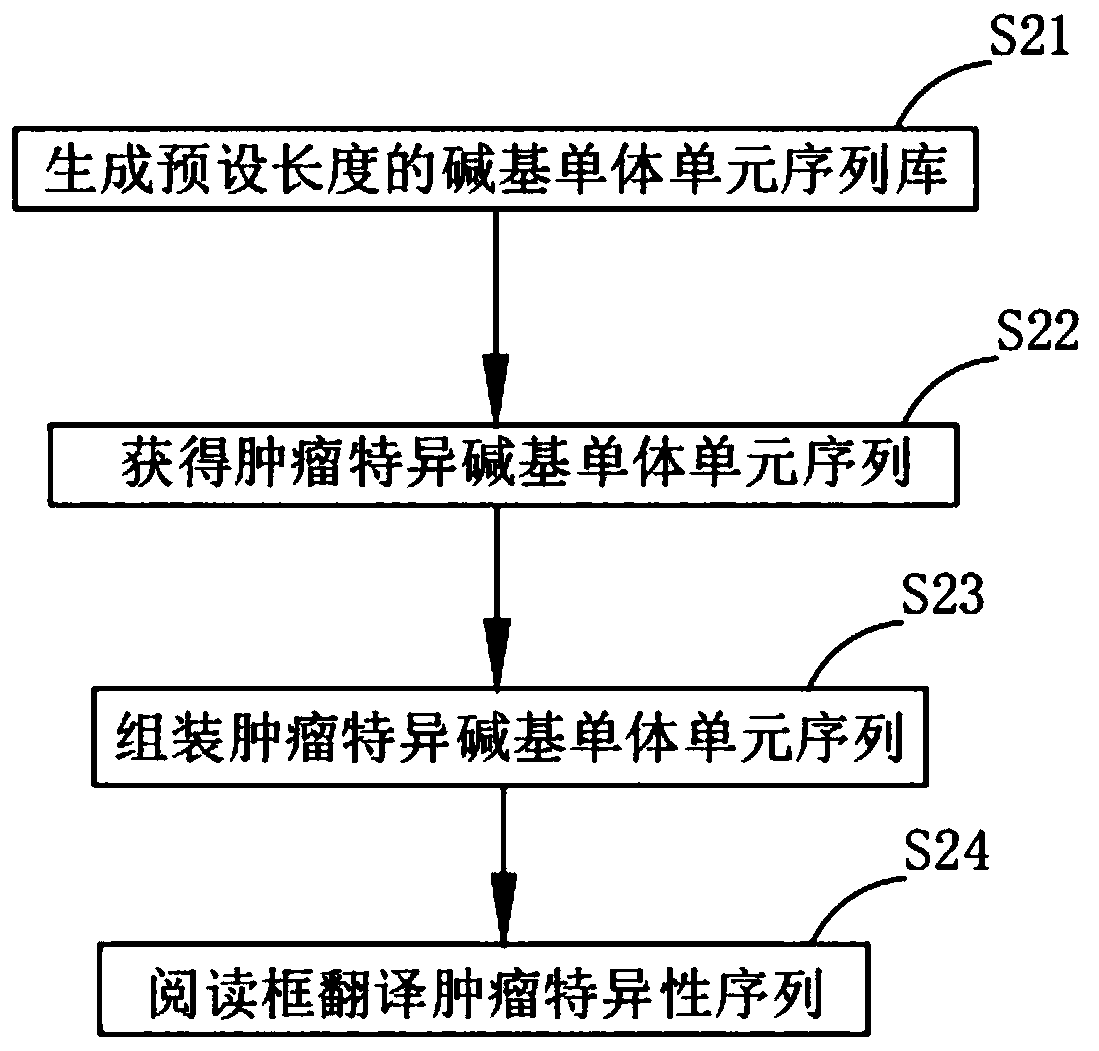
Method and system for extracting immunotherapy neoantigen
The invention discloses a method and a system for extracting an immunotherapy neoantigen. The method comprises the following steps: S1, acquiring conventional proteomes of a tumor tissue and a normaltissue of a sample; S2, obtaining a base monomer unit sequence library of a sample tumor tissue and a sample normal tissue and a specific proteome of the sample tumor tissue; S3, based on the conventional proteome of the sample tumor tissue, the specific proteome of the sample tumor tissue and human leukocyte antigen (HLA) molecular typing, obtaining a plurality of candidate tumor specific neoantigens; S4, based on the plurality of obtained candidate tumor specific neoantigens, respectively calculating the existence conditions in a sample tumor tissue conventional proteome, a sample normal tissue conventional proteome, a sample tumor tissue basic group monomer unit sequence library and a sample normal tissue basic group monomer unit sequence library, and taking the existence conditions andgene expression change multiples as filtering rules to obtain the tumor specific neoantigen. From the aspect of source, the tumor specific neoantigen part discovered by the scheme of the invention comes from a genome non-coding region and is not limited to a coding region, so that more neoantigens can be discovered.
Owner:NEOCURA BIO-MEDICAL TECH CO LTD
Method for modulating HLA class II tumor cell surface expression with a cytokine mixture
InactiveUS20060257357A1Peptide/protein ingredientsAntineoplastic agentsAbnormal tissue growthHla class ii
A method for altering the composition of tumor infiltrating mononuclear cells, increasing CD4+ / CD8+ ratio, increasing tumor stroma / epithelial ratio and modulating HLA (Human Leukocyte Antigen) class II expression on a tumor cell surface with a serum-free and mitogen-free mixture having specific cytokine ratios from the group of IL-1β, TNF-α, IFN-γ, GM-CSF, and Interleukin-2 (IL-2) with specific ratios of IL-1β, TNF-α, IFN-γ, GM-CSF to IL-2, respectively. The serum-free and mitogen-free mixtures comprised of cytokine ratios include Leukocyte Interleukin Injection (LI) or Multikine®, which can be further used alone or in combination with other drugs for the treatment of cancer thereby increasing the success of cancer treatment and the disease free survival of cancer patients.
Owner:CEL SCI CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com