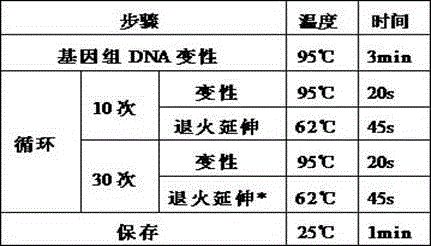
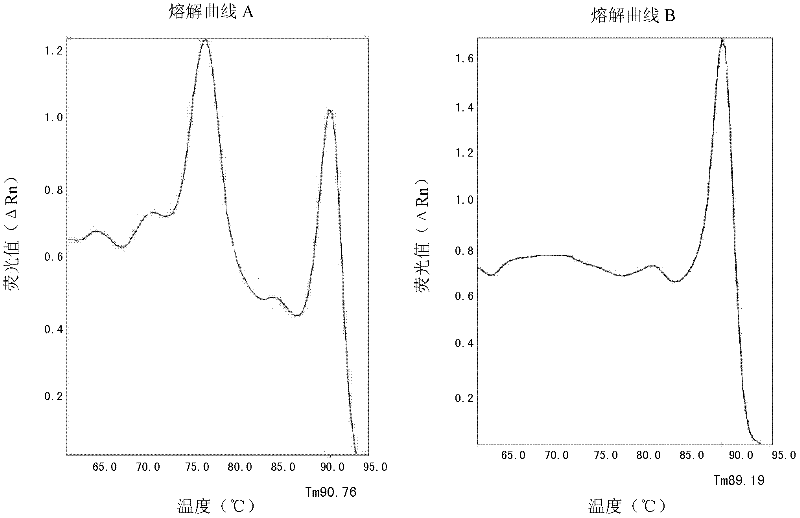
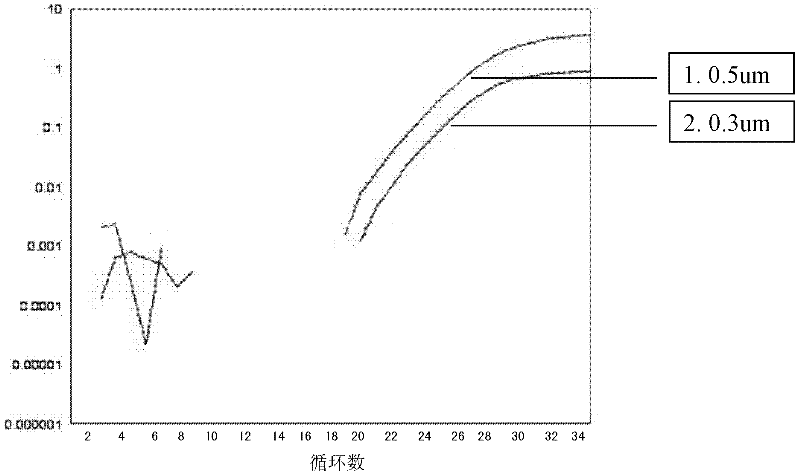
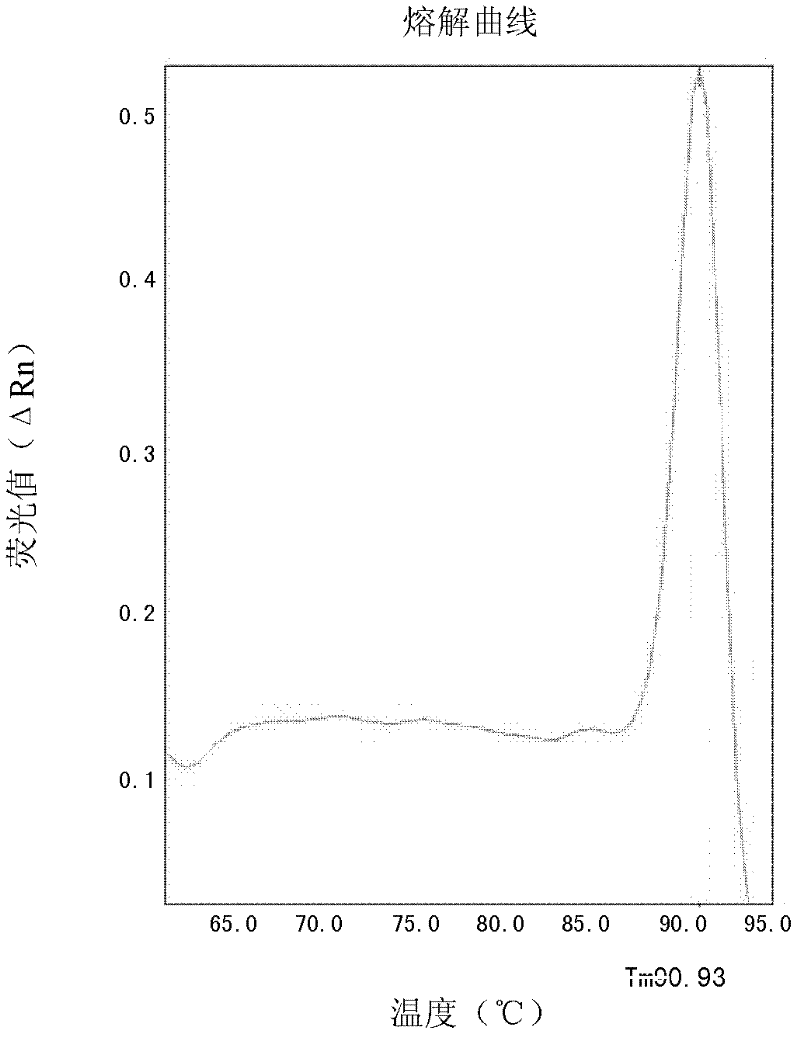
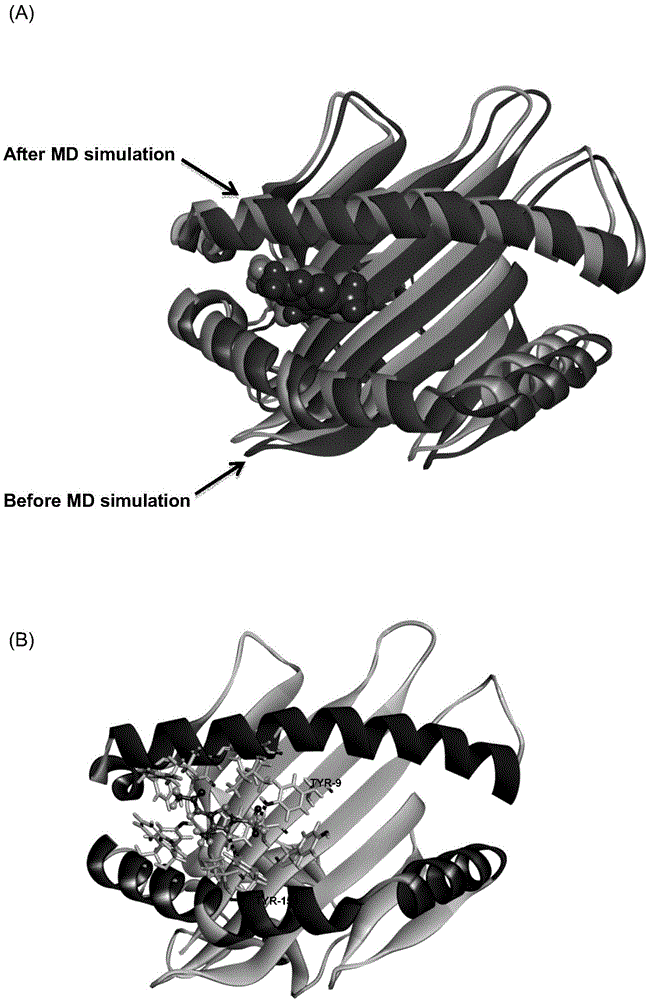
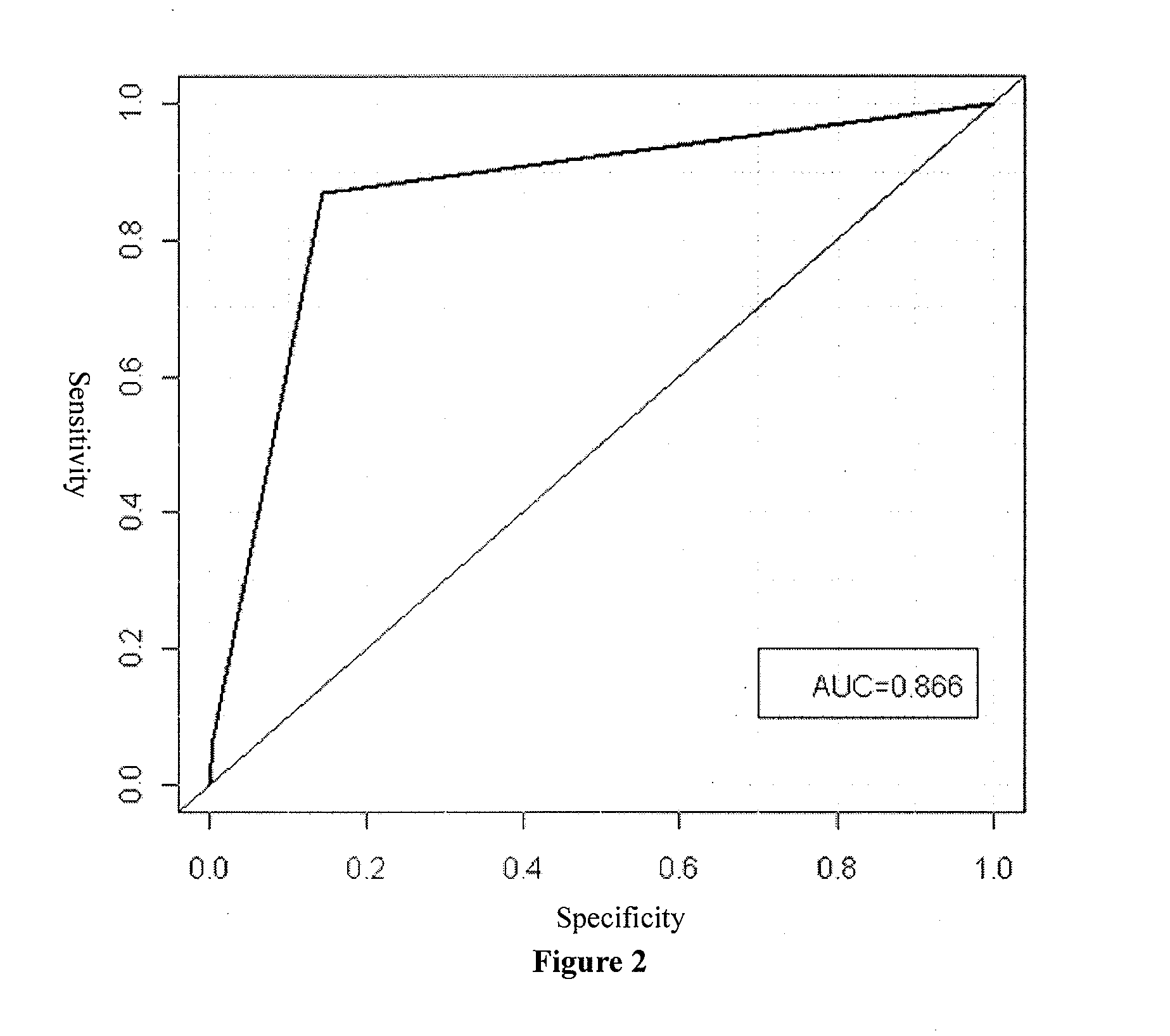
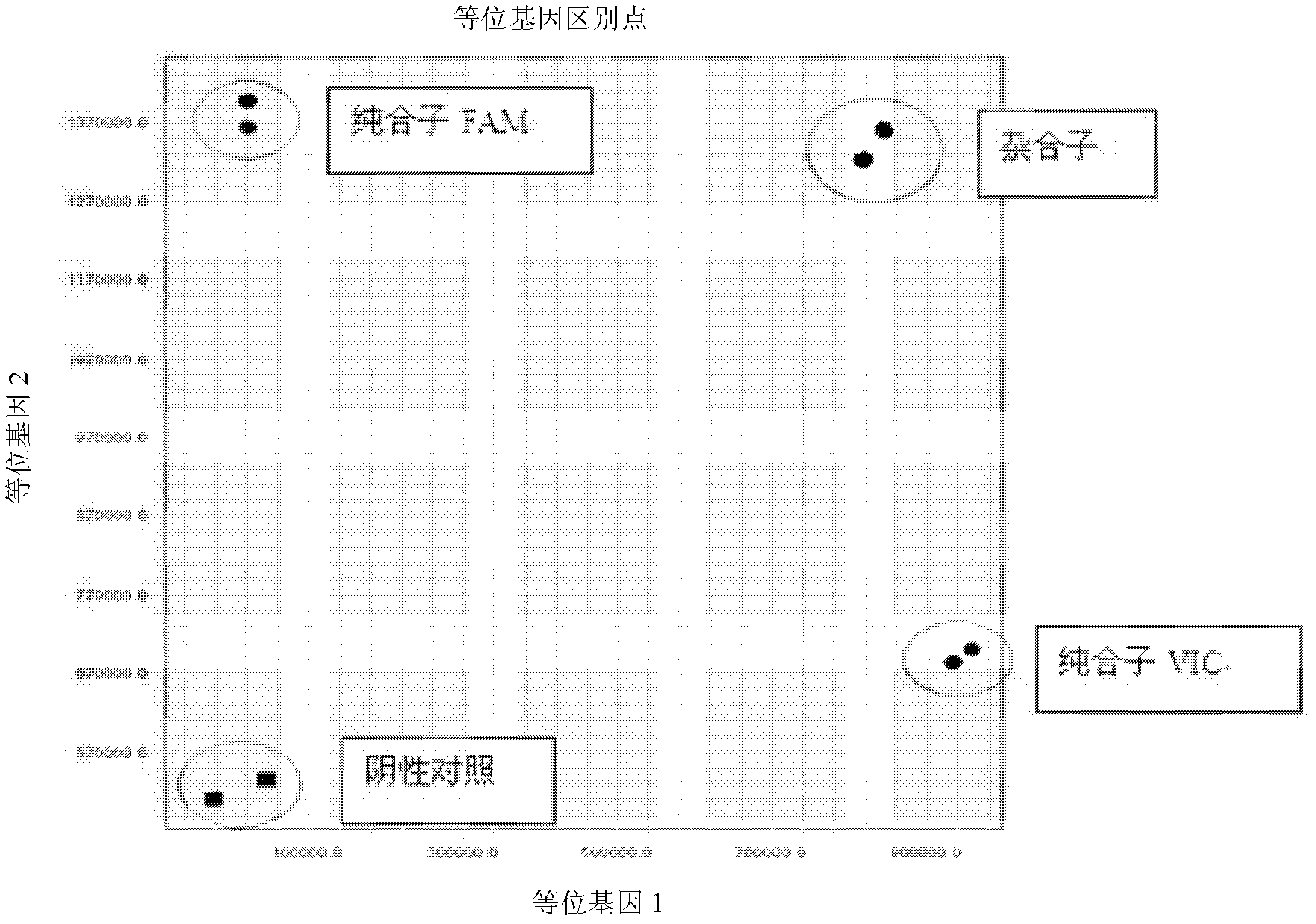
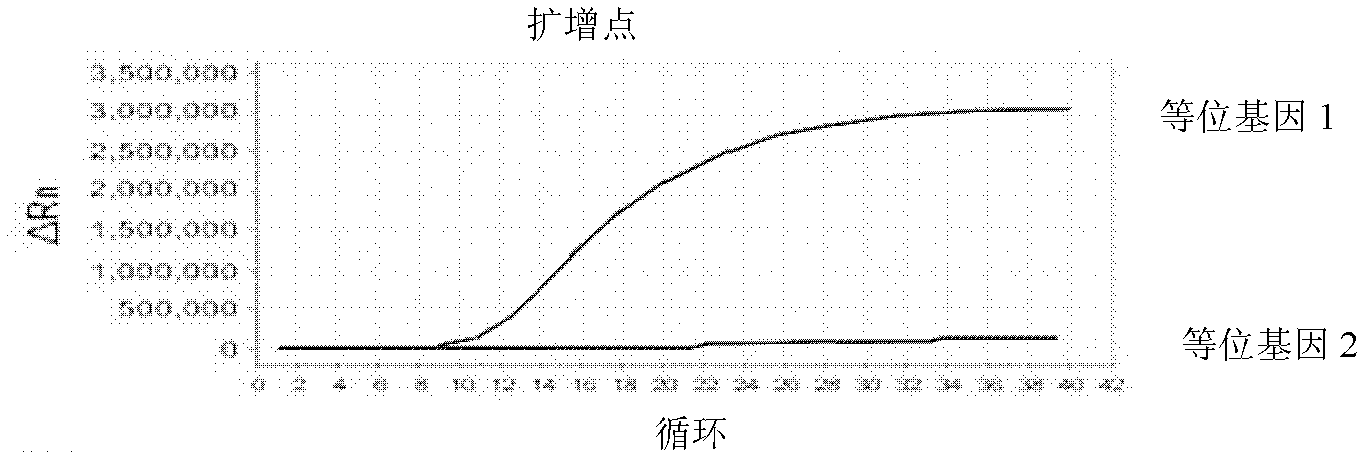
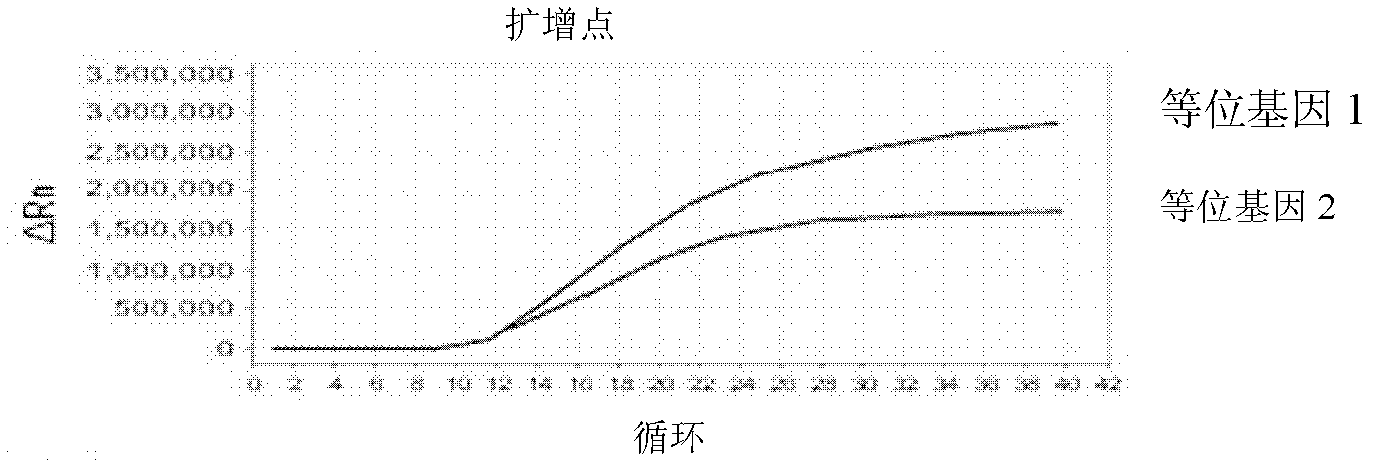
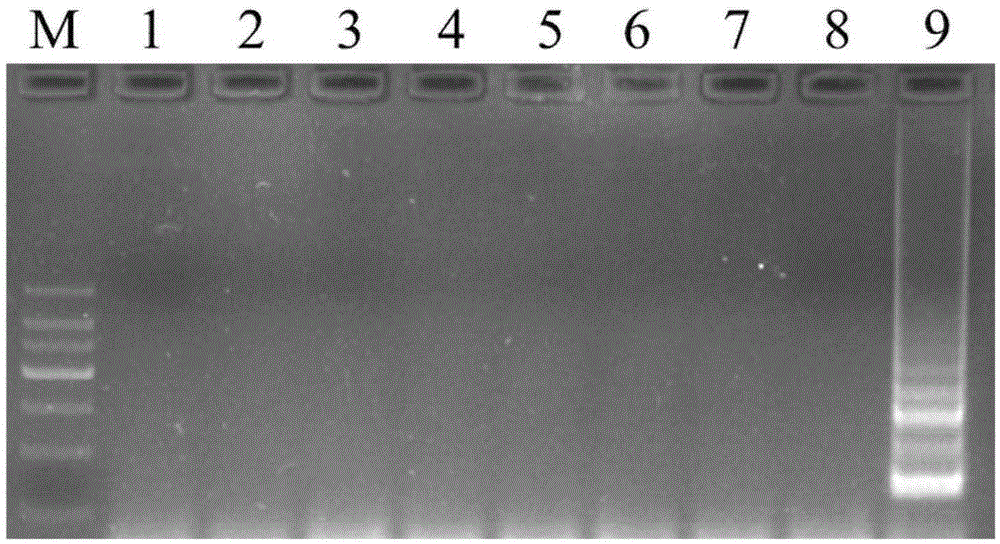
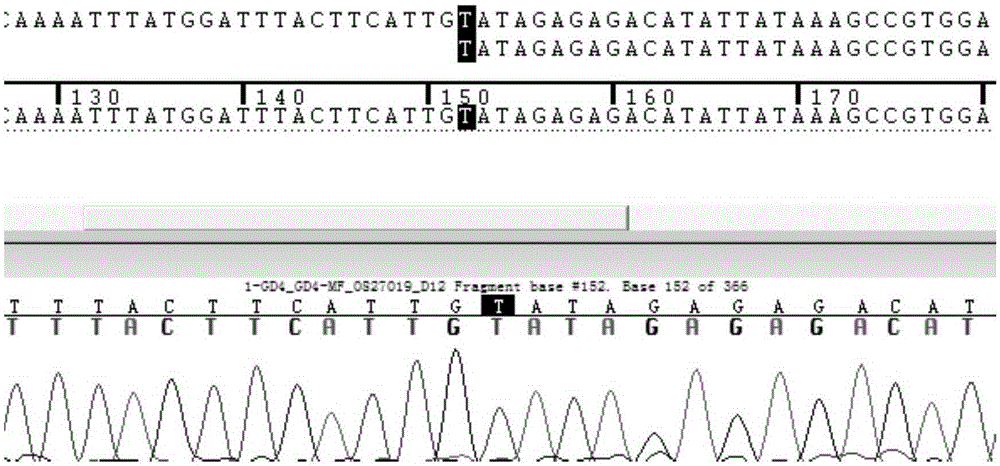
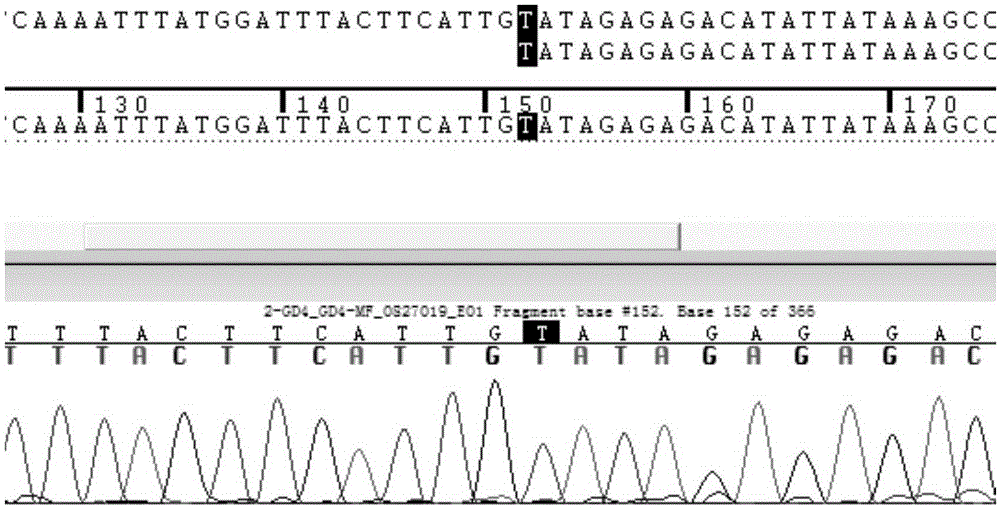
Patents
Literature
111 results about "HLA-B" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
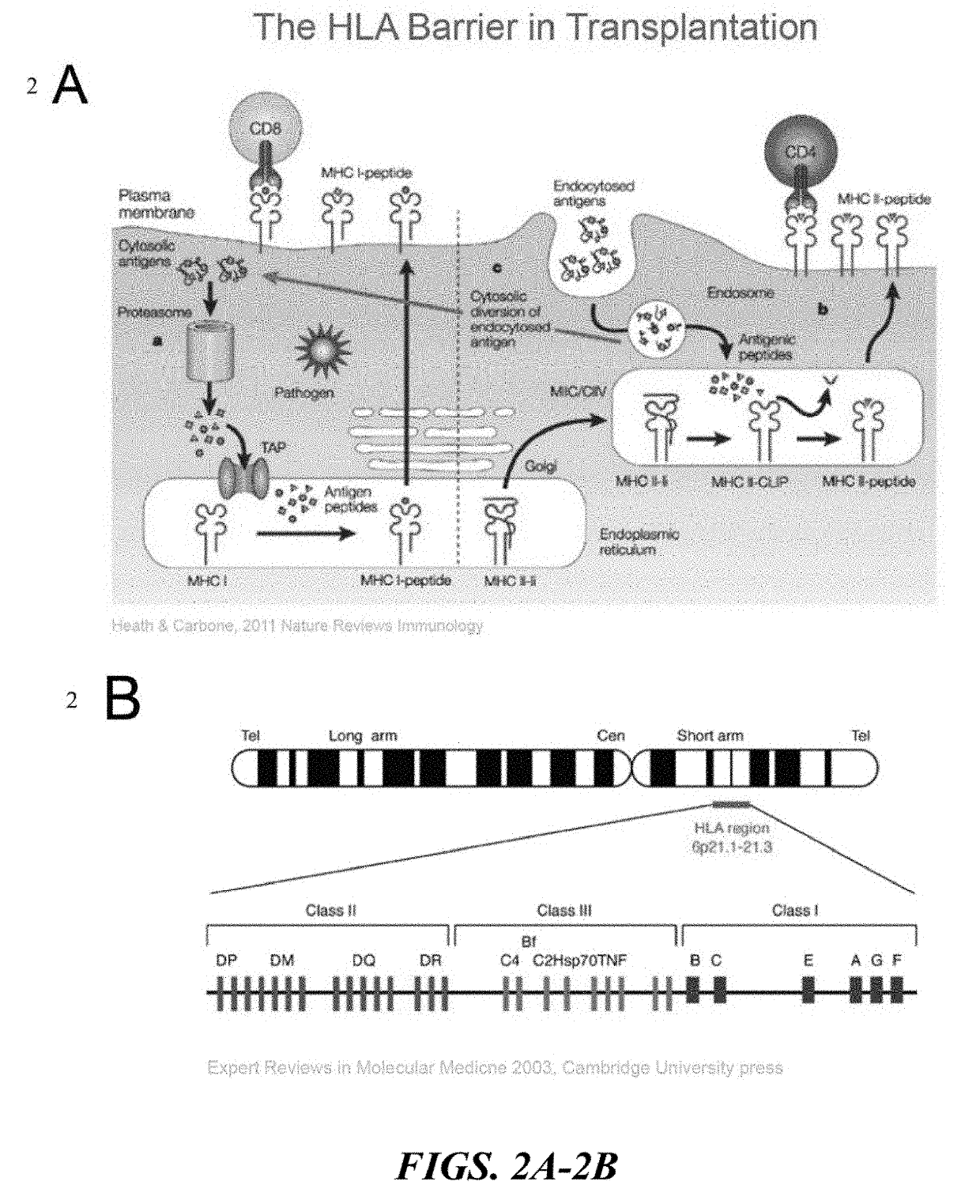
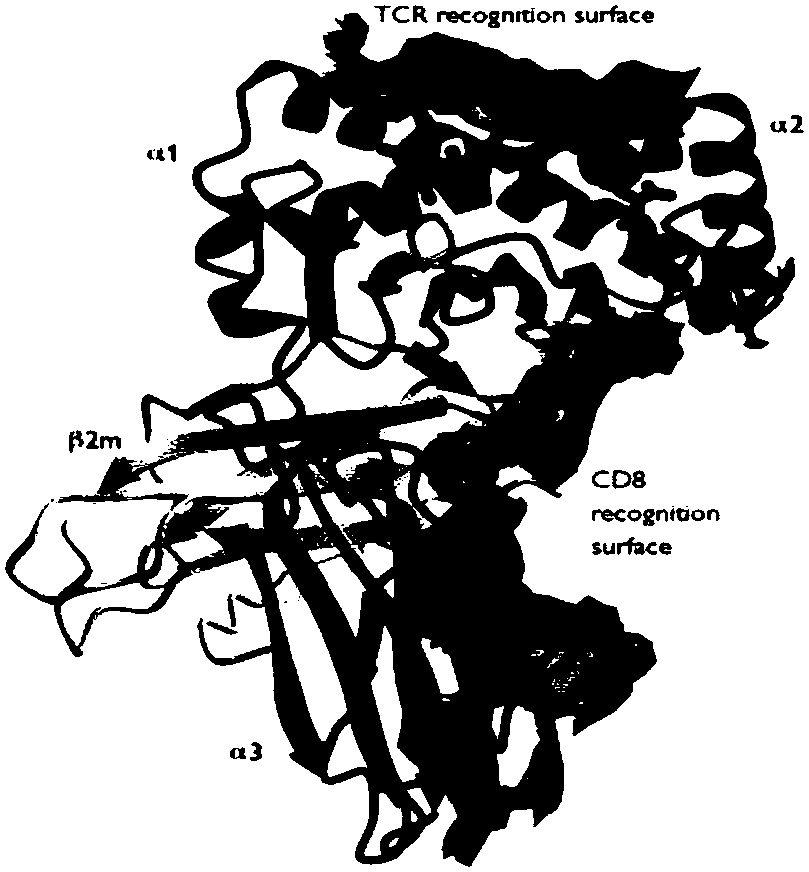
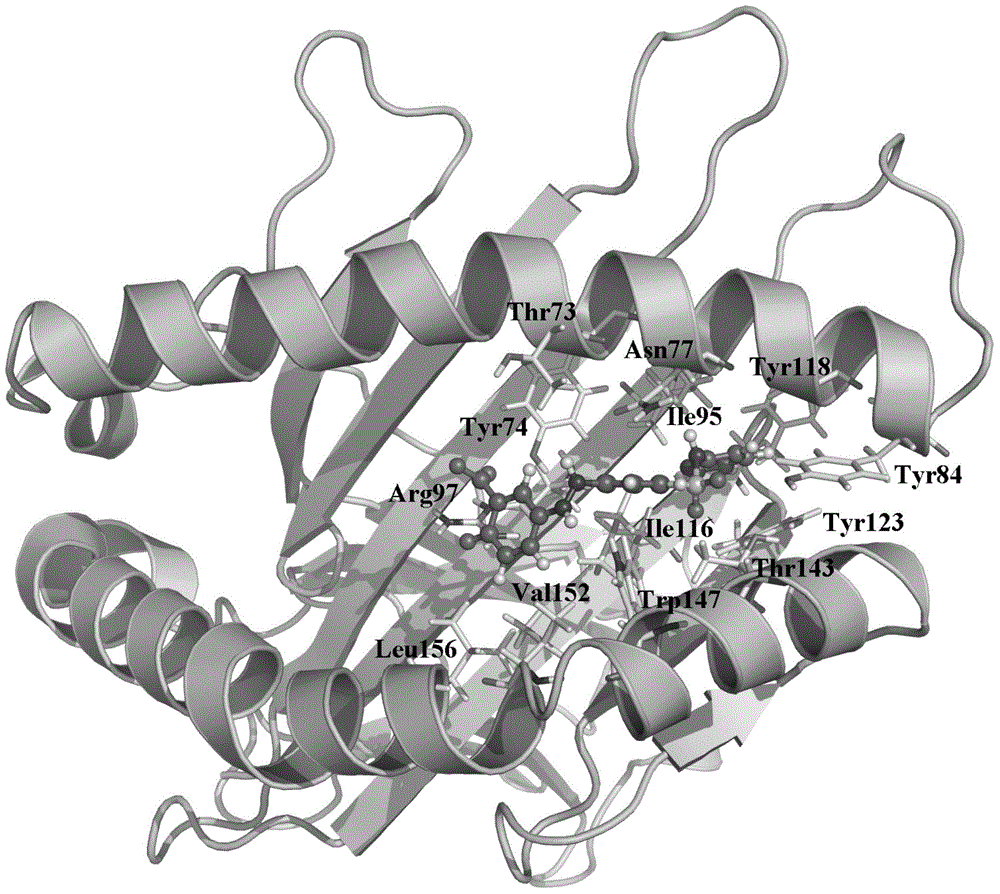
HLA-B (major histocompatibility complex, class I, B) is a human gene that provides instructions for making a protein that plays a critical role in the immune system. HLA-B is part of a family of genes called the human leukocyte antigen (HLA) complex. The HLA complex helps the immune system distinguish the body's own proteins from proteins made by foreign invaders such as viruses and bacteria.
Risk assessment for adverse drug reactions
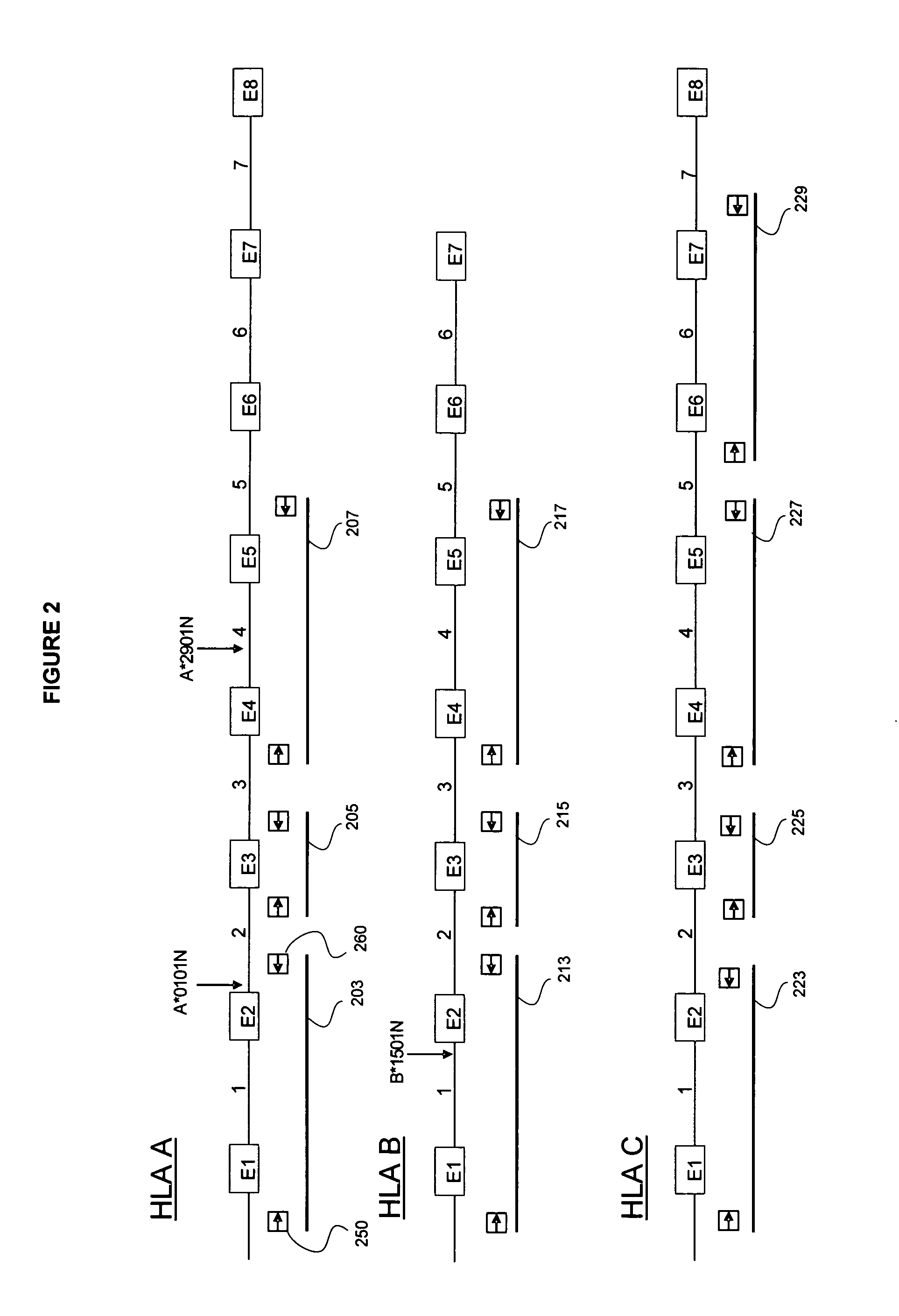
The present invention provides a method of predicting the risk of a patient for developing adverse drug reactions, particularly SJS or TEN. It was discovered that an HLA-B allele, HLA-B* 1502, is associated with SJS / TEN that is induced by a variety of drugs. The correlation with HLA-B* 1502 is most significant for carbamazepine-induced SJS / TEN, wherein all the patients tested have the HLA-B* 1502 allele. In addition, another HLA-B allele, HLA-B*5801, is particularly associated with SJS / TEN induced by allopurinol. Milder cutaneous reactions, such as maculopapular rash, erythema multiforme (EM), urticaria, and fixed drug eruption, are particularly associated with a third allele, HLA-B *4601. For any of the alleles, genetic markers (e.g., HLA markers, microsatellite, or single nucleotide polymorphism markers) located between DRB1 and HLA-A region of the specific HLA-B haplotype can also be used for the test.
Owner:ACAD SINIC
System and method for detection of HLA Variants
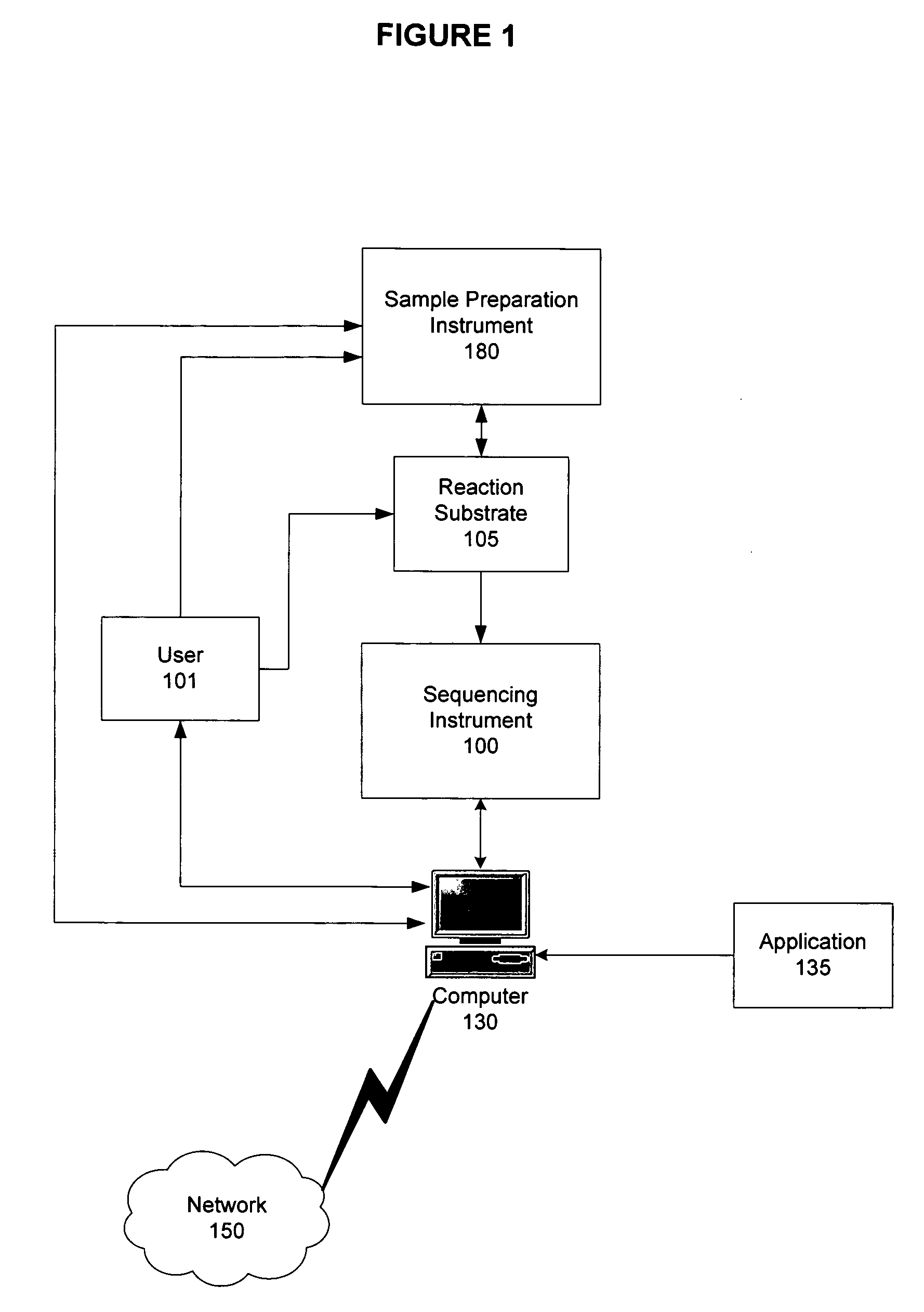
A method for detecting one or more HLA sequence types is described that comprises the steps of: amplifying a plurality of first amplicons from a double stranded nucleic acid sample, wherein the first amplicons are amplified with a plurality of pairs of nucleic acid primers that define exons 2 and 3 of both strands of HLA loci from the group consisting of HLA-A, HLA-B, and HLA-C; amplifying the first amplicons to produce a plurality of populations of second amplicons, wherein each population of second amplicons is clonally amplified from one of the first amplicons; sequencing the plurality of populations of second amplicons to generate a nucleic acid sequence composition for each of the plurality of second amplicons; and detecting variation in the sequence composition from one or more of the second amplicons for one or more of the HLA loci.
Owner:454 LIFE SCIENCES CORP
HPV epitopes targeted by T cells infiltrating cervical malignancies for use in vaccines
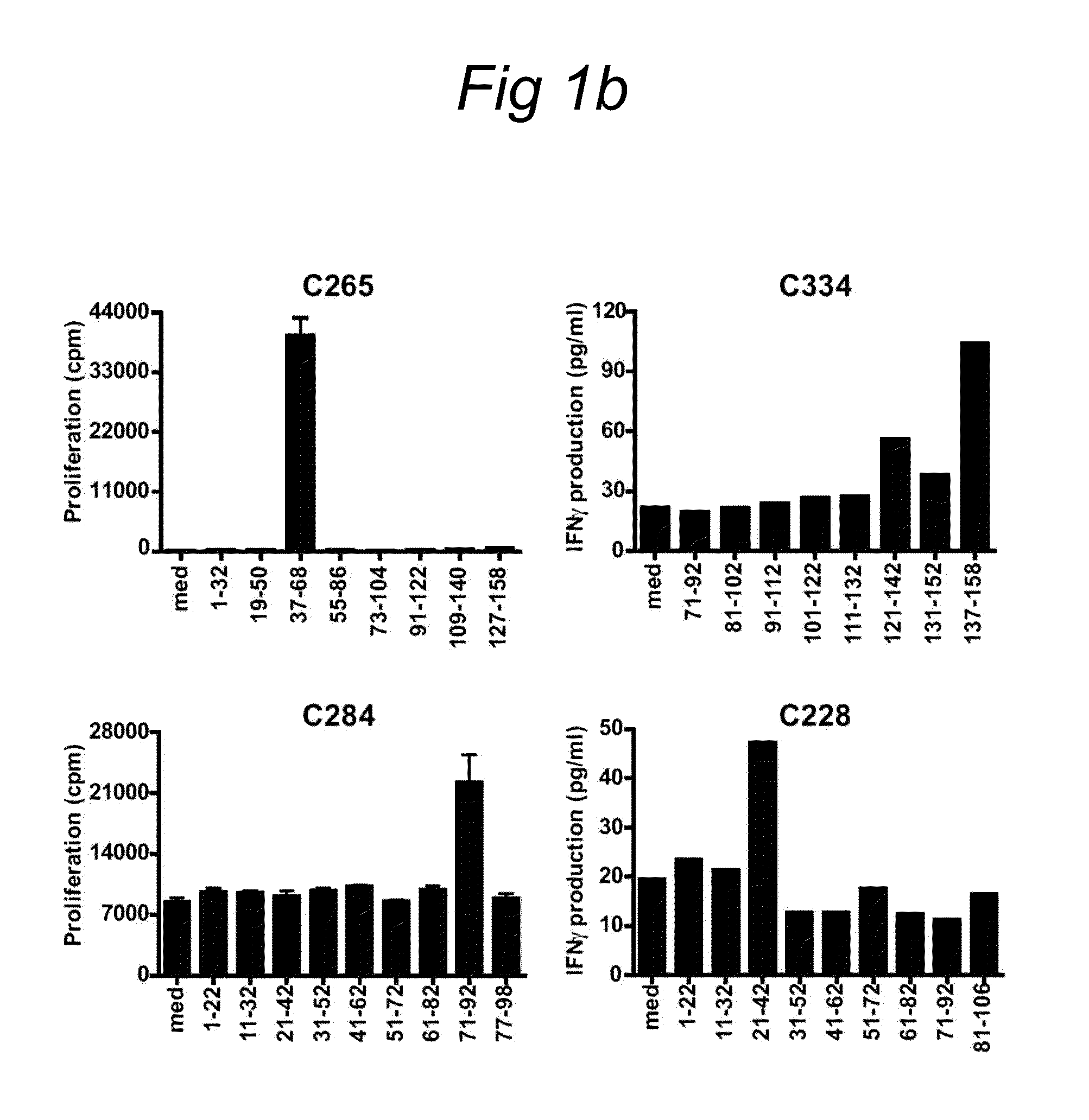
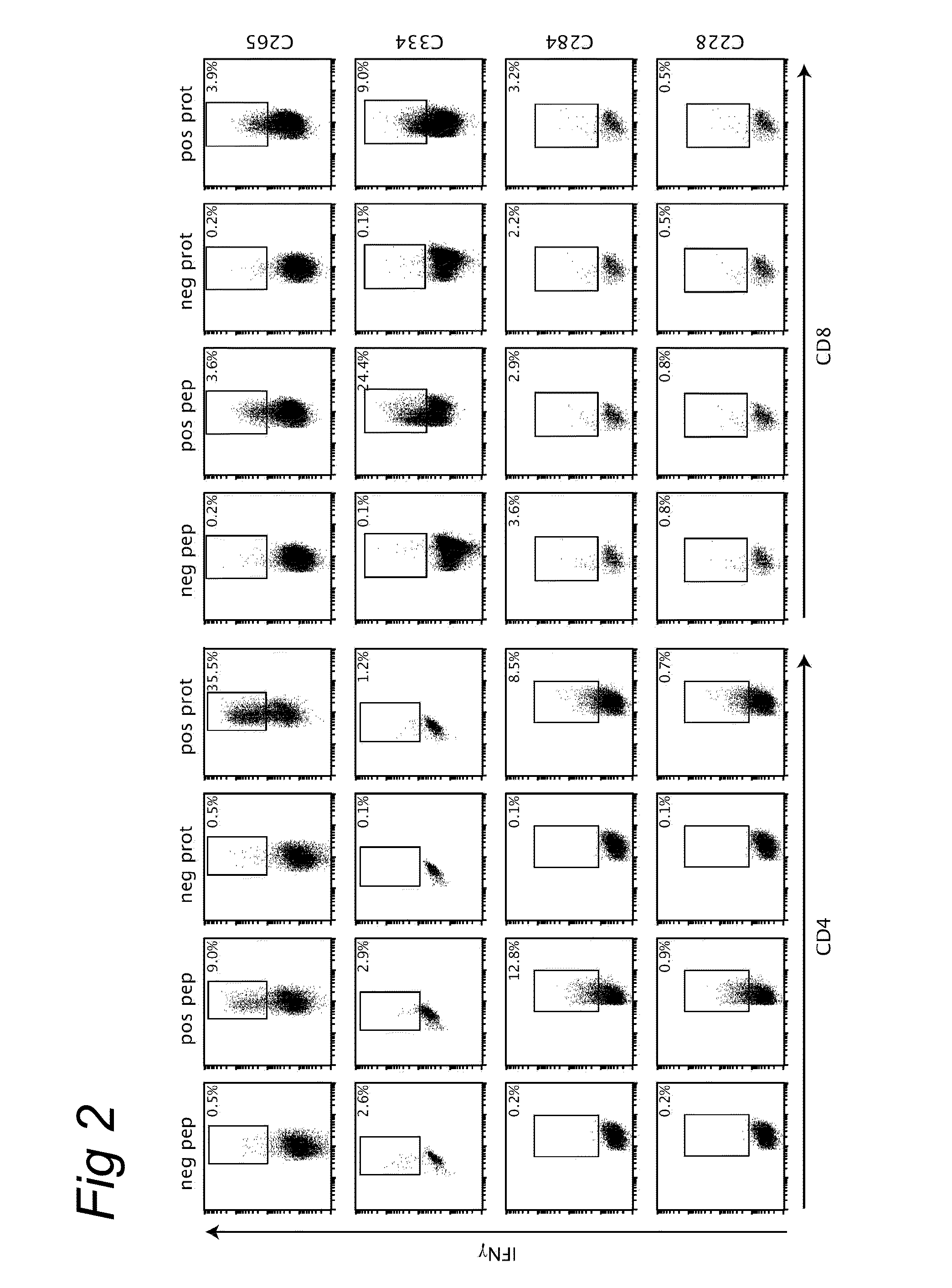
InactiveUS20100189742A1Improved and enhanced and prolonged CDConfirm the capability of peptidesPeptide/protein ingredientsVirus peptidesDiseaseHLA-B
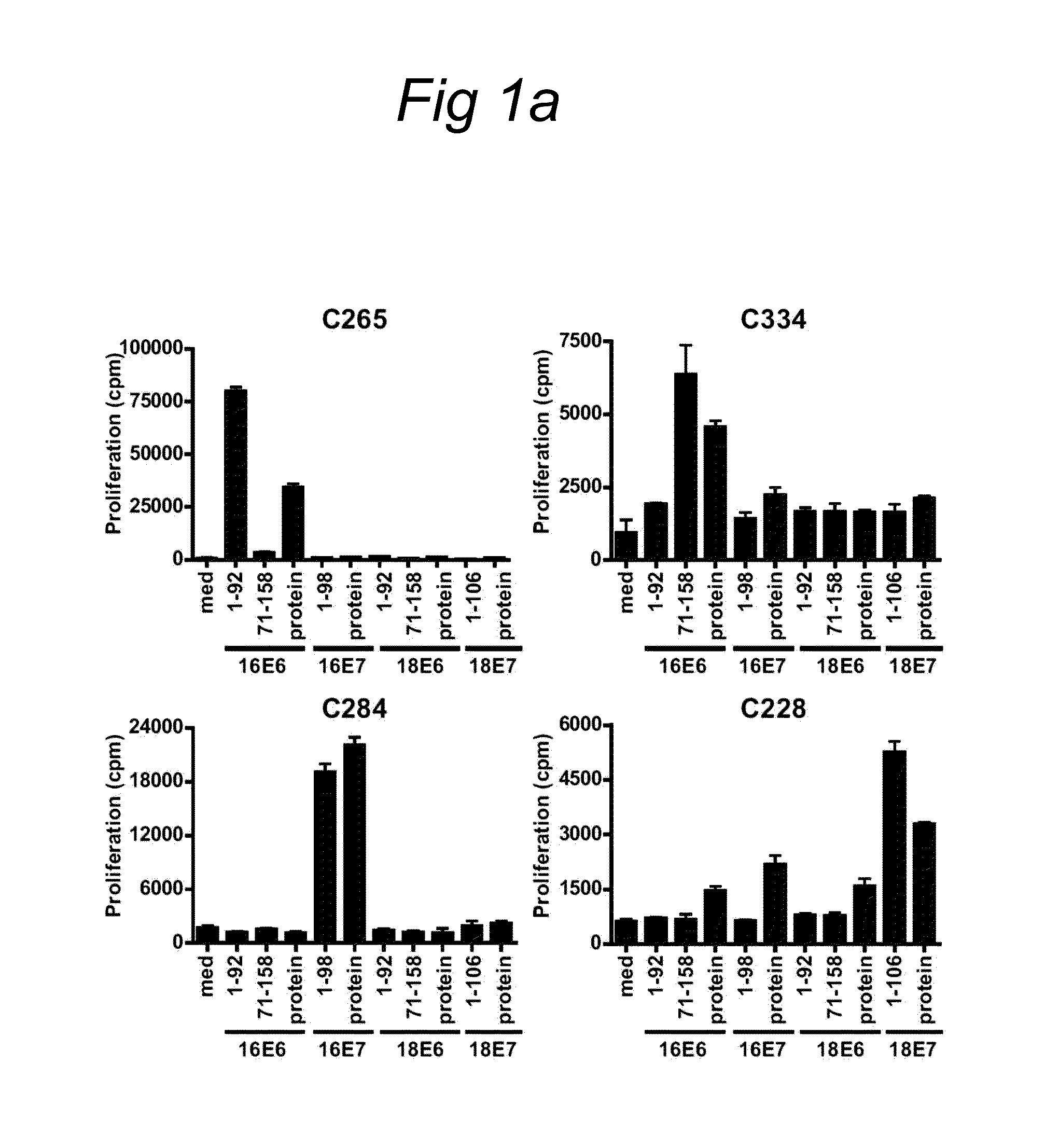
The present invention relates to novel CD4+ and CD8+ T cell epitopes that are specific for HPV-specific E6 and E7 oncoproteins, to peptides comprising these novel T cell epitopes, and to (vaccine) compositions comprising these peptides for use in methods for the prevention and / or treatment of HPV related diseases. Preferred epitopes are recognized by a T cell that infiltrates a cervical neoplastic lesion or by a T cell from a draining lymph node, and are presented by an HLA-DQ or HLA-DP molecule, or an HLA-B.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
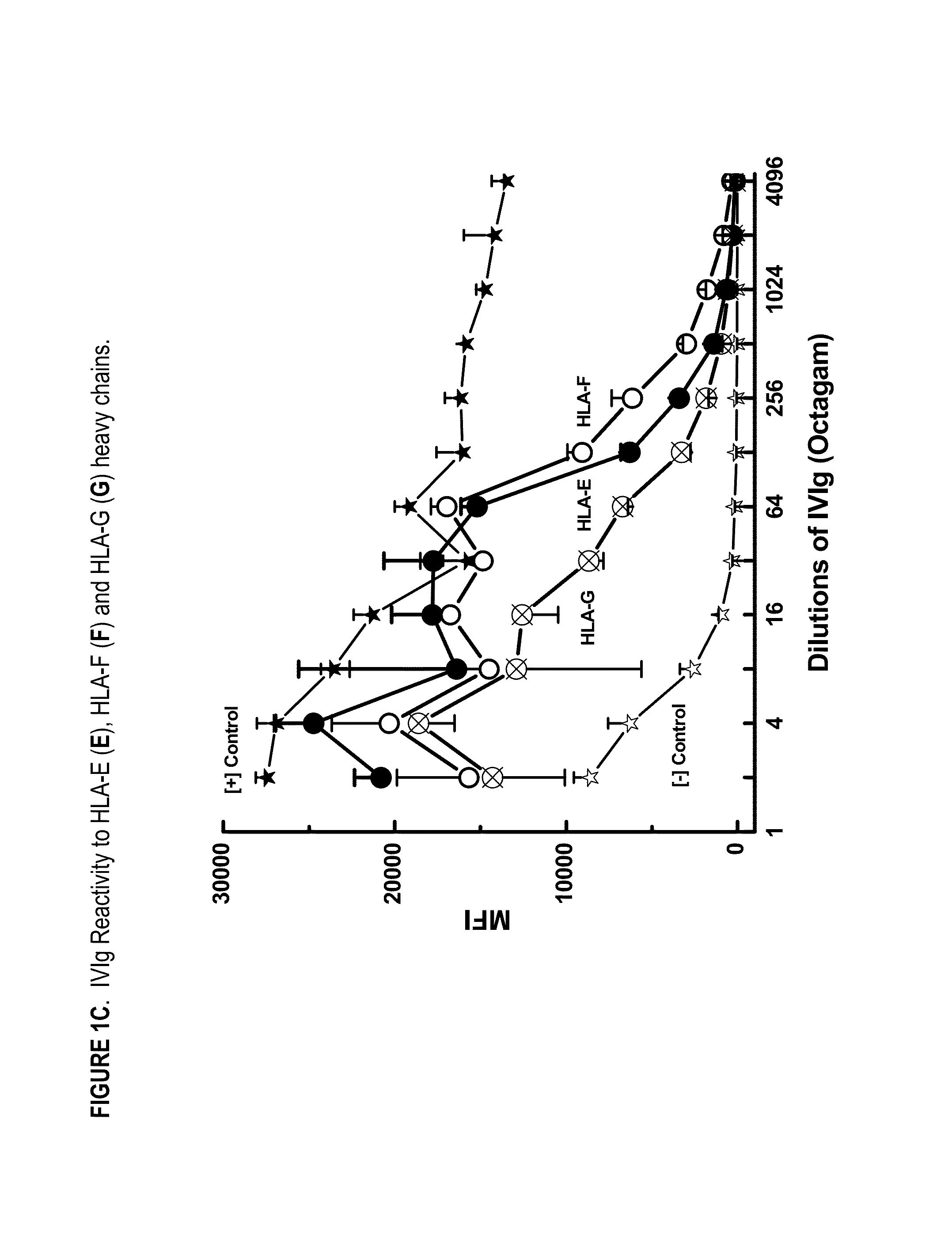
ANTI-HLA CLASS-Ib ANTIBODIES MIMIC IMMUNOREACTIVITY AND IMMUNOMODULATORY FUNCTIONS OF INTRAVENOUS IMMUNOGLOBULIN (IVIg) USEFUL AS THERAPEUTIC IVIg MIMETICS AND METHODS OF THEIR USE
InactiveUS20130177574A1Market price thereof has been risingMinimizing IVIg related side effectAntipyreticAnalgesicsDiseaseAntigen
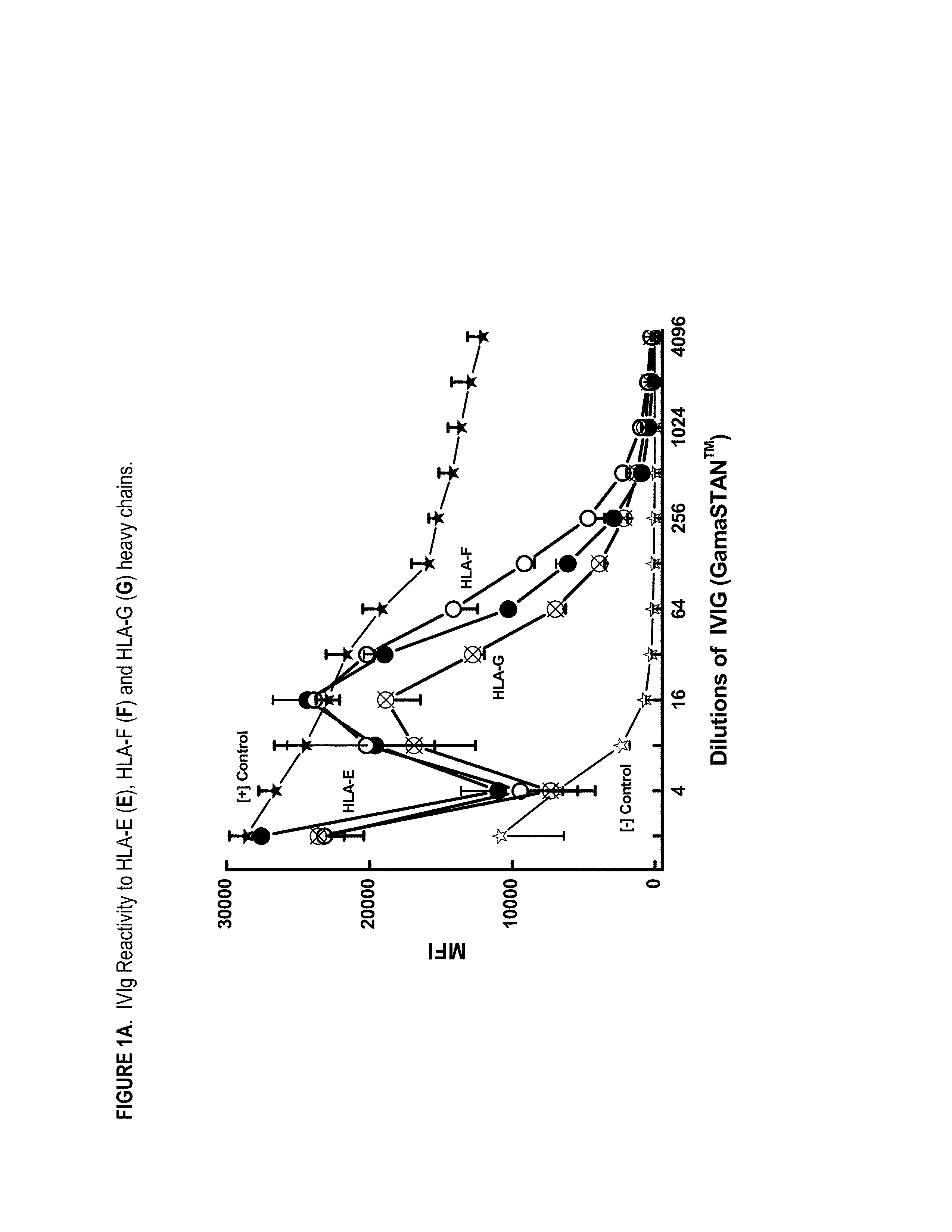
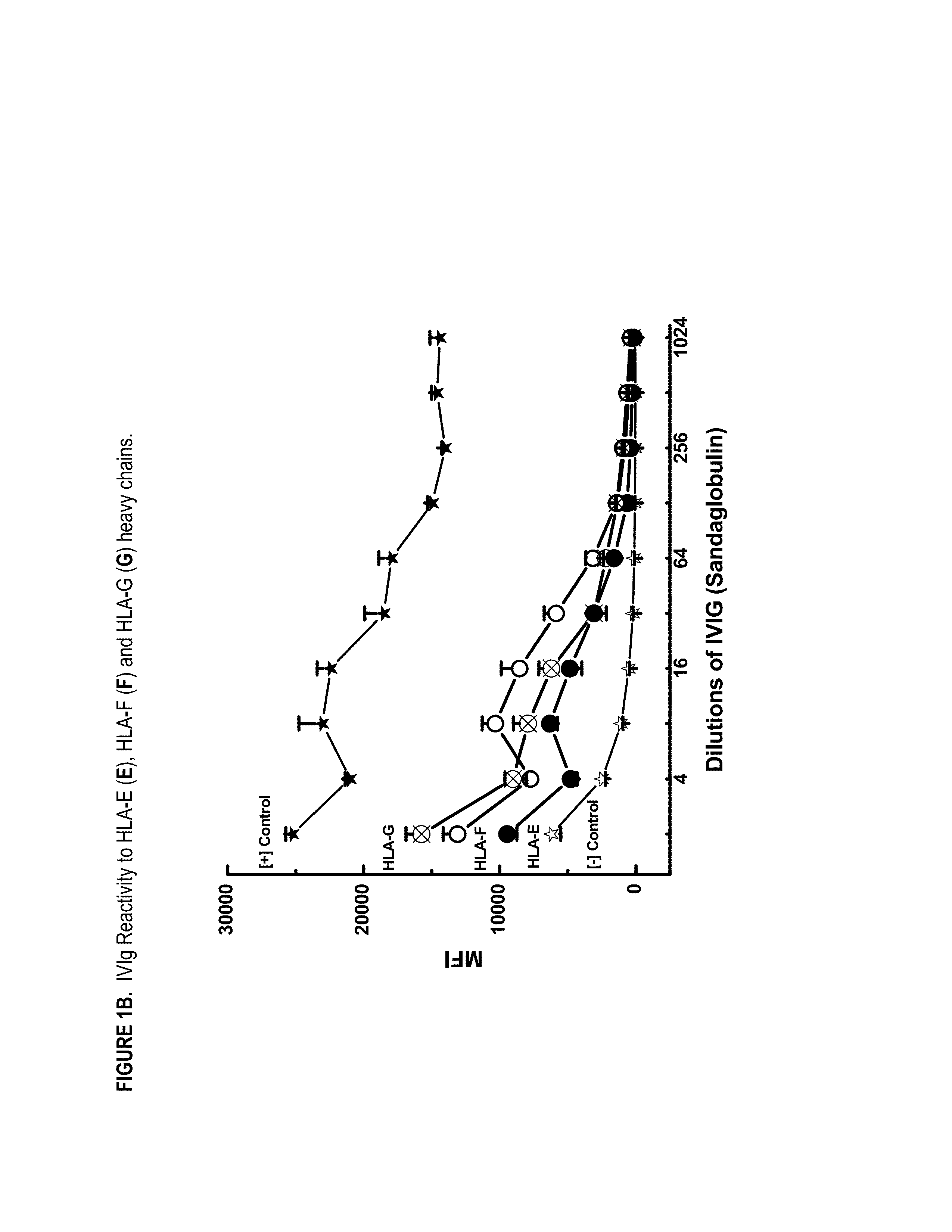
Provided herein are compositions comprising anti-HLA-Ib antibodies as IVIg mimetics and methods for using the same for the prevention, treatment, therapy and / or amelioration of inflammation induced diseases and allograft rejection. In certain embodiments, the anti-HLA-Ib antibodies (monoclonal antibodies or mixed monoclonal antibodies, recombinant or chimeric or humanized or human antibodies) strongly mimic IVIg in immunoreactivity to HLA class Ia (HLA-A, HLA-B and HLA-Cw) and Ib antigens (HLA-E, HLA-F and HLA-G). In certain embodiments, the anti-HLA-Ib antibodies (monoclonal or mixed monoclonal antibodies; recombinant, chimeric, humanized or human antibodies) strongly mimic IVIg in immunomodulatory or immunosuppressive activities. While anti-HLA-Ib mAbs can be used to restore anti-tumor activities of CD8+ T cells and Natural killer cells by passive therapy in cancer patients, methods are also provided herein to induce production of polyclonal anti-HLA-Ib antibodies in cancer patients for restoring anti-tumor activities of CD8+ T cells and NK cells, by active specific immunotherapy.
Owner:RAVINDRANATH MEPUR DR
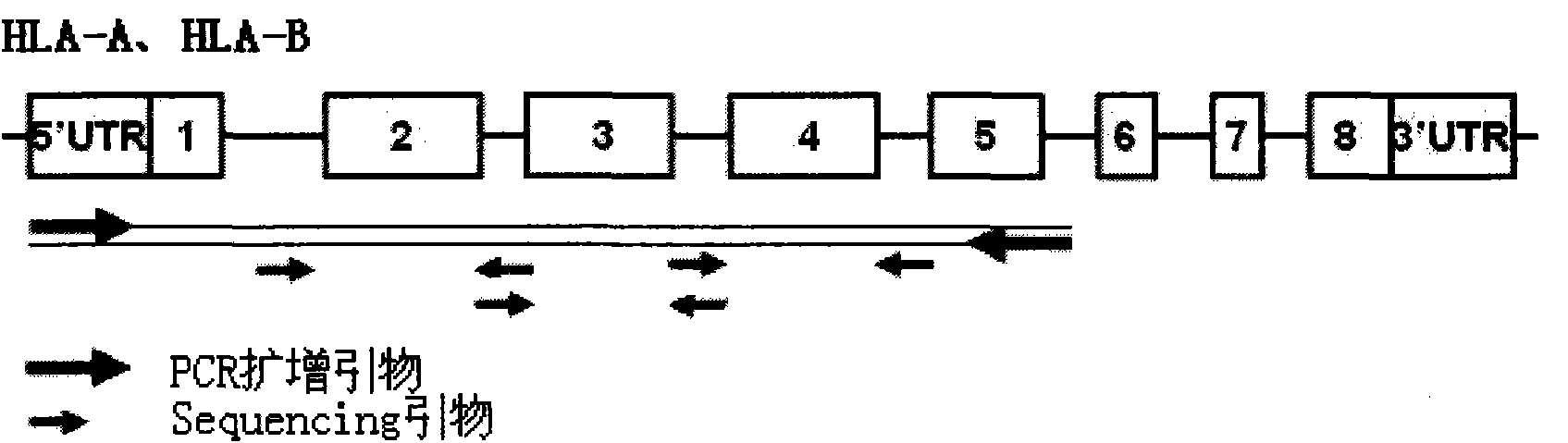
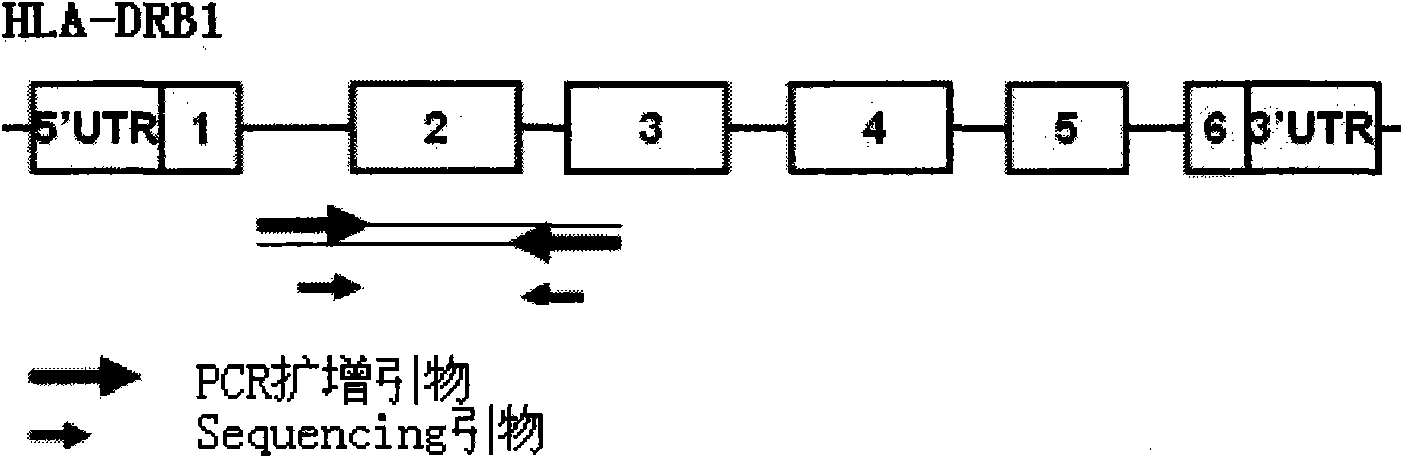
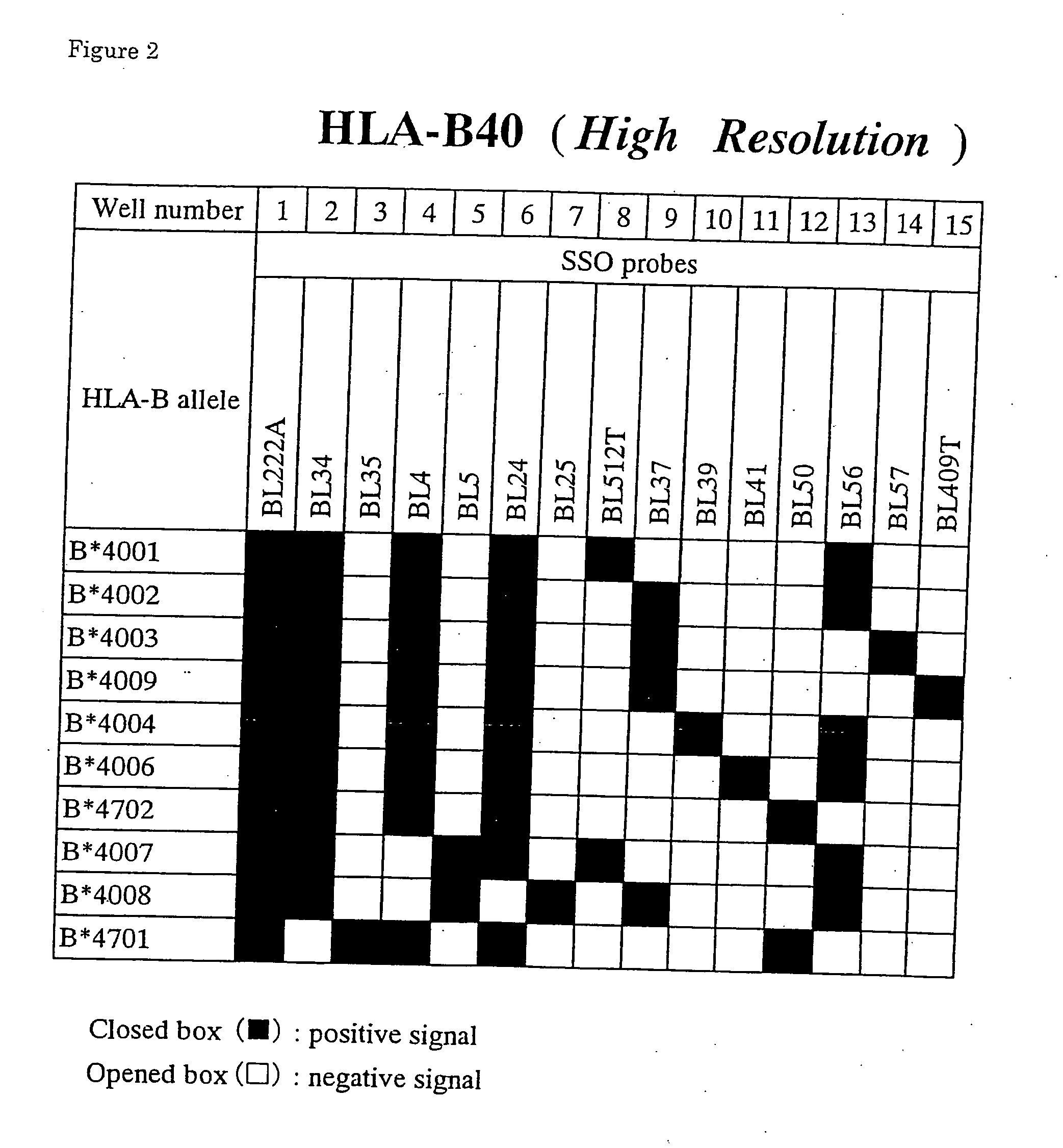
HLA high-resolution gene sequencing kit
InactiveCN101892317AAvoid problems that cannot be effectively typedHigh resolutionMicrobiological testing/measurementDNA/RNA fragmentationHLA-BExon
The invention discloses a parting method of leucocyte antigen gene of human being, comprising the following steps of: (1) extracting genome DNA to be tested by a regular technology, and amplifying a destination gene fragment to be analyzed by using PCR amplification primer: 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB; and (2) amplifying the PCR output obtained in the step (1) by using sequencing primer, amplifying the exon, sequencing the amplified exon and comparing the sequencing result with the standard sequence in a database to determine the gene parting result. As the 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB are effectively amplified as a result of optimized combination of the HLA gene sequencing kit and the test condition, and the corresponding exon is sequenced, the invention solves the problem that effective parting can not be performed when certain allelic gene nucleotide is located outside an amplification area during further parting, thereby improving the parting resolution and accuracy of the HLA gene.
Owner:SUZHOU UNIV +1
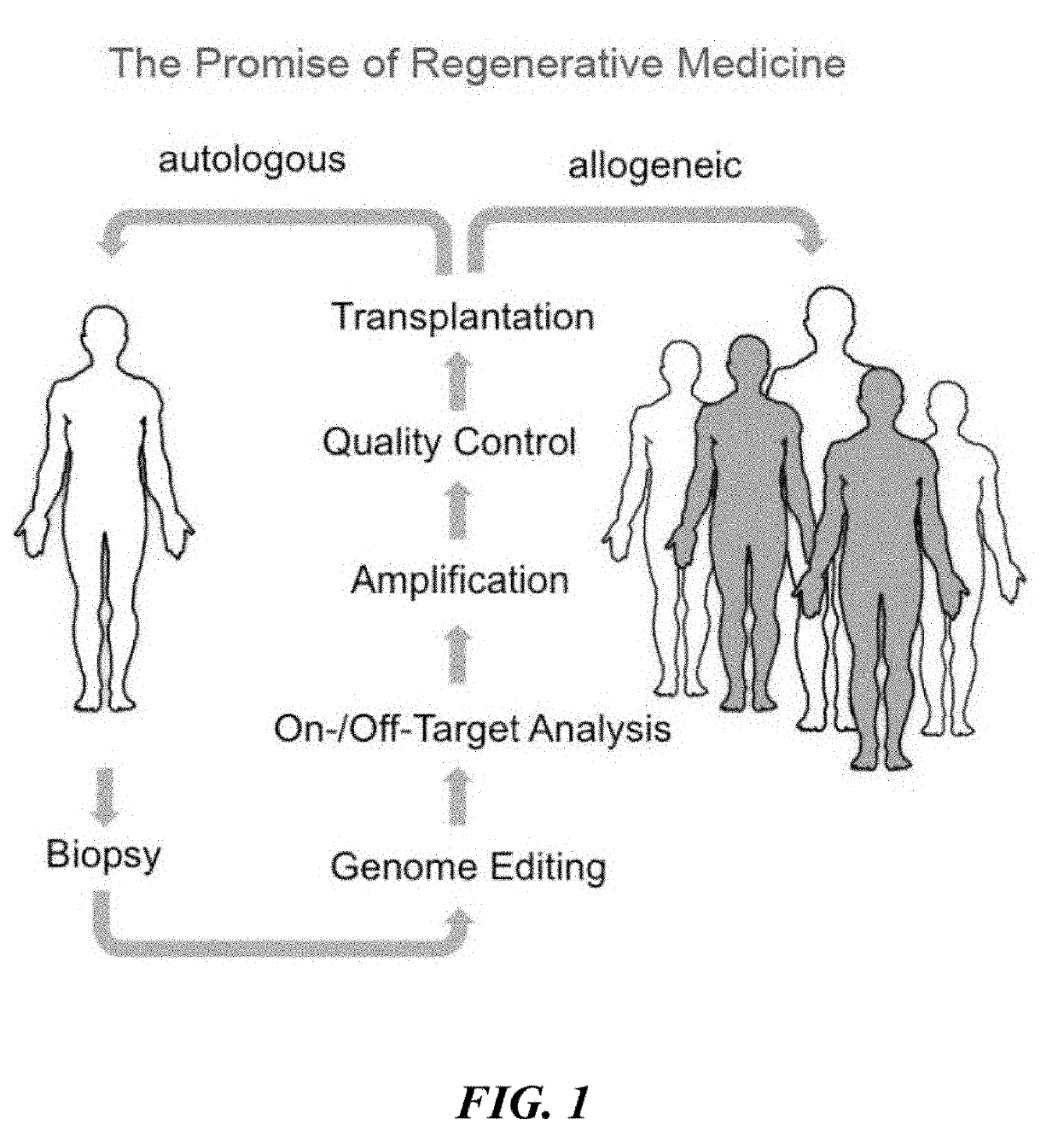
Universal donor stem cells and related methods
ActiveUS20190309259A1Reduce and eliminate and activityReduce and eliminate surface expressionGenetically modified cellsStable introduction of DNAHLA-BImmunogenicity
Disclosed herein are universal donor stem cells and related methods of their use and production. The universal donor stem cells disclosed herein are useful for overcoming the immune rejection in cell-based transplantation therapies. In certain embodiments, the universal donor stem cells disclosed herein do not express one or more MHC-I and MHC-II human leukocyte antigens. Similarly, in certain embodiments, the universal donor stem cells disclosed herein do not express one or more human leukocyte antigens (e.g., HLA-A, HLA-B and / or HLA-C) corresponding to MHC-I and MHC-II human leukocyte antigens, thereby rendering such cells hypoimmunogenic.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Methods of predicting responsiveness to interferon treatment and treating hepatitis c infection
InactiveUS20120009148A1Prediction of responsivenessPeptide/protein ingredientsMicrobiological testing/measurementInterferon therapyHLA-G
Provided are methods of predicting responsiveness of a subject to interferon treatment, comprising comparing a level of expression in a cell of the subject of at least one gene selected from the group consisting KIR3DL3, KIR3DL2, KIR3DL1, KIR2DL1, KIR2DL2, KIR2DL3, KLRG1, KIR3DS1, CD160, HLA-A, HLA-B, HLA-C, HLA-F, HLA-G and IFI27 to a reference expression data of the at least one gene obtained from at least one interferon responder subject and / or at least one interferon non-responder subject. Also provided are methods and pharmaceutical compositions for treating a subject in need of interferon treatment.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Human HLA-B*5801 gene polymorphism detection kit
InactiveCN106929591AAccurate detectionHigh sensitivityMicrobiological testing/measurementInternal standardHLA-B
The invention relates to a human HLA-B*5801 gene polymorphism detection kit. The human HLA-B*5801 gene polymorphism detection kit comprises PCR damping liquid, a specific primer, a specific probe, an interior label system, a Taq enzyme, a UNG enzyme, a weakly-positive control group and a blank control group, and also comprises a blood treating agent; a blood sample is simply treated and can be directly subjected to PCR amplification, the DNA extracting process is omitted, and operating time is saved. According to the human HLA-B*5801 gene polymorphism detection kit, the SNP probe is used in cooperation with the technology of the ARMS primer, and it is achieved that two different gene types are detected in one pipe; meanwhile, the interior label system is designed and used for monitoring the quality of the sample, and the weakly-positive control group and the blank control group are designed and used for monitoring the quality of the kit. The human HLA-B*5801 gene polymorphism detection kit for detecting HLA-B*5801 alleles has the advantages of being high in specificity and sensitivity, rapid and easy to operate, safe, objective in result interpretation and the like when being used for detecting HLA-B*5801 alleles.
Owner:WUHAN YZY MEDICAL SCI & TECH
Molecular-determinant based typing of kir alleles and kir-ligands
The present invention relates to an assay to perform a molecular determinant-based functional killer immunoglobulin-like receptors (KIR) allele typing and ligand typing. In particular the present invention provides methods, compositions, and kits for a single nucleotide polymorphism (SNP) assay to type various allele groups of KIR2DL1 and KIR ligand with distinct functional properties based on polymorphism at position 245 in KIR2DL1, position 77 in HLA-C, and position 83 in HLA-B and HLA-A. The assays are suitable for use in predicting NK cell activity in health, disease, and transplantation.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
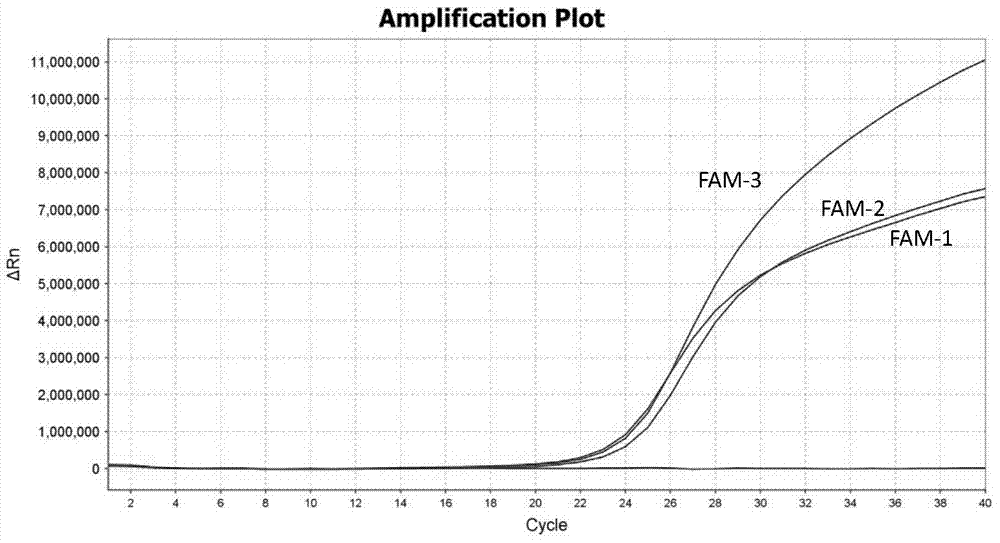
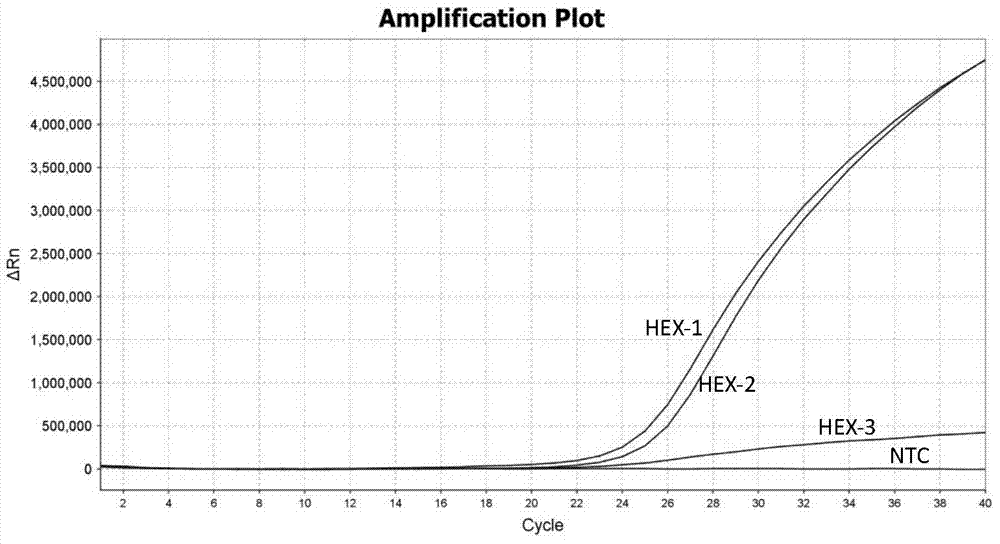
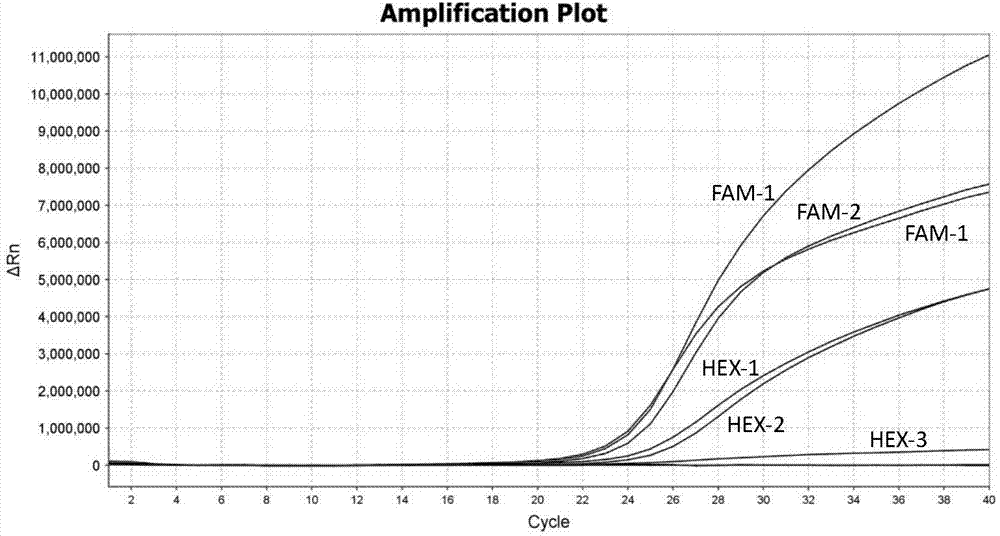
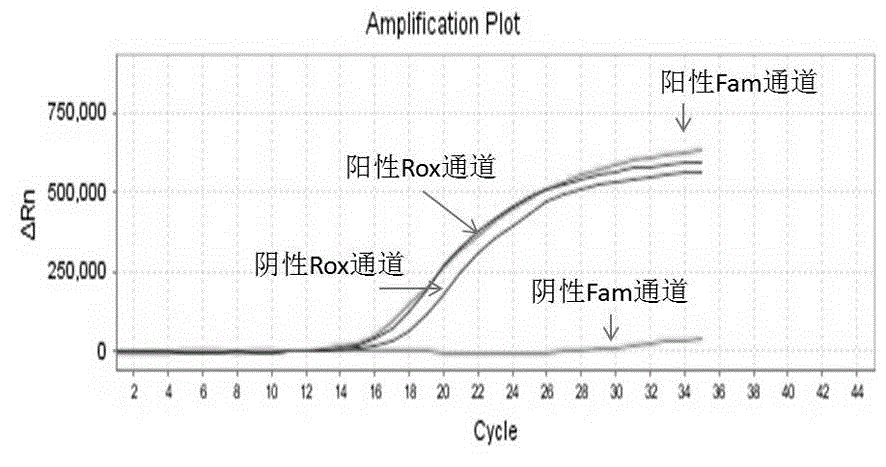
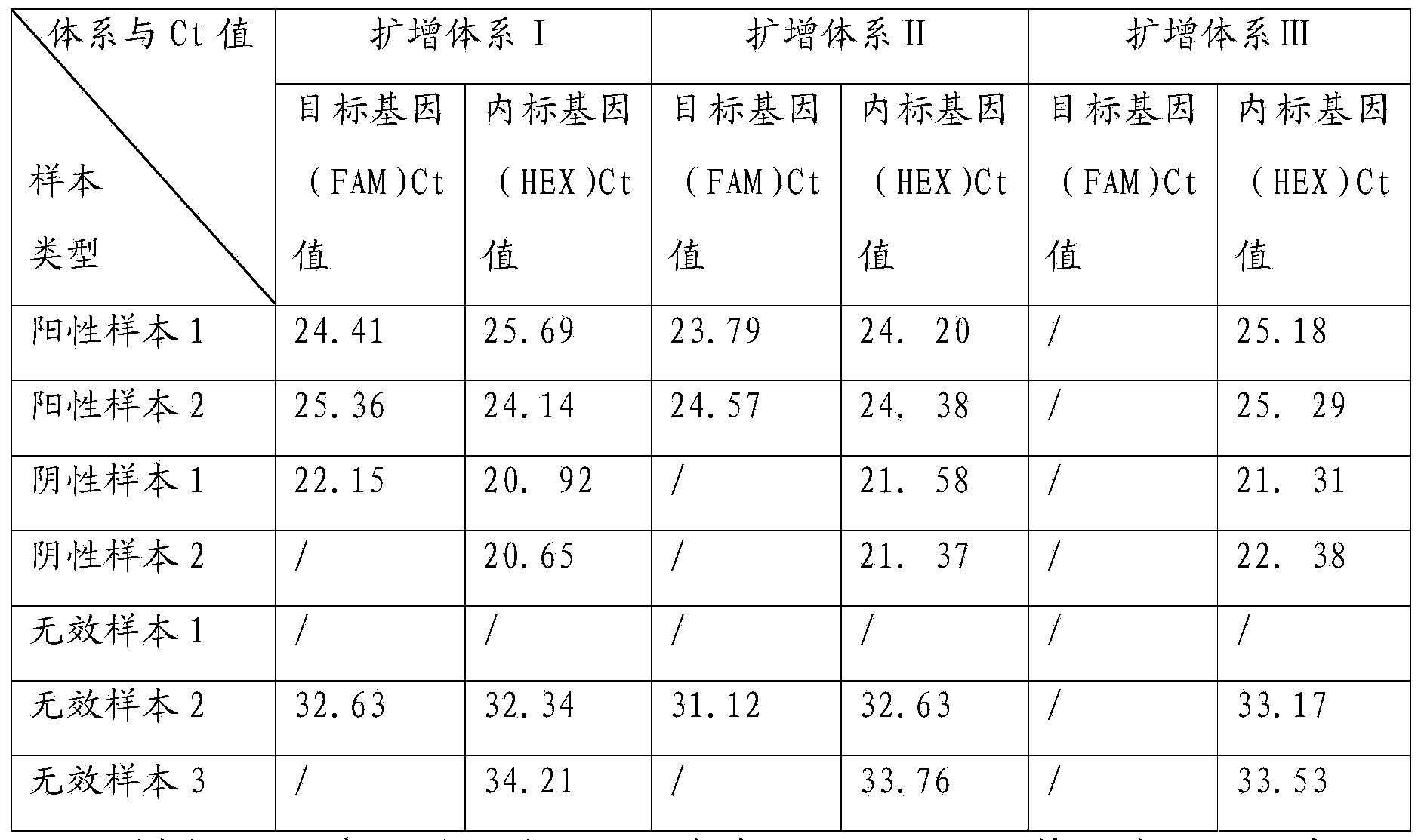
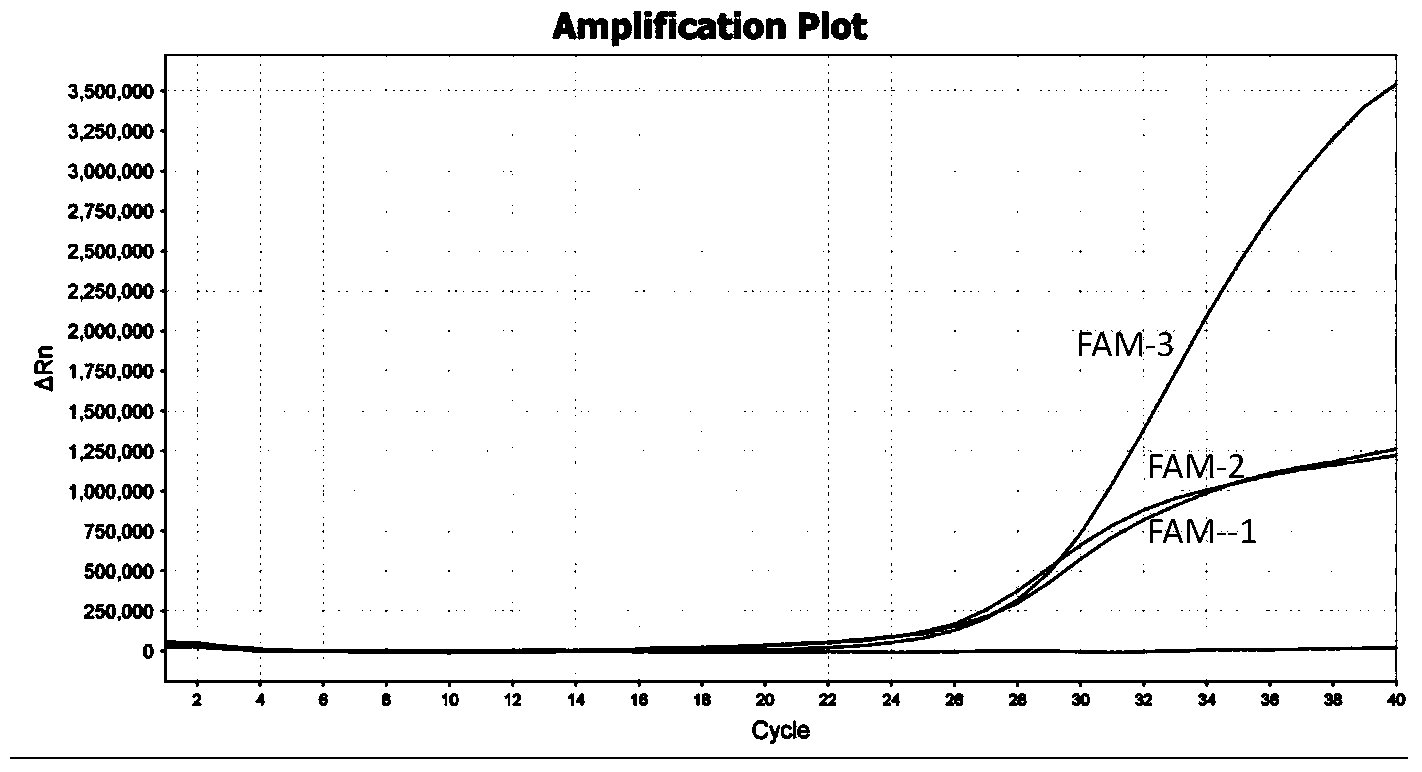
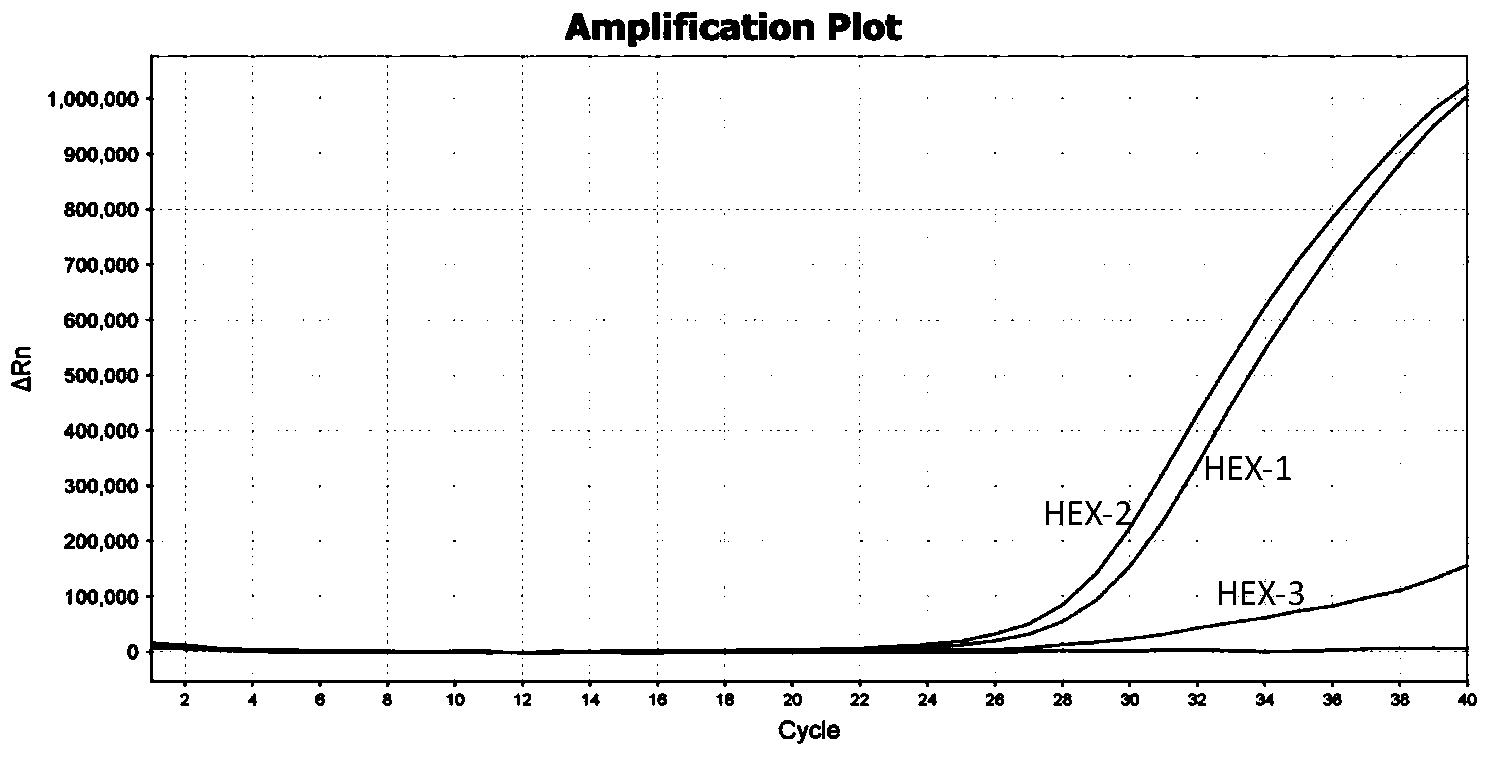
TaqMan probe real-time fluorescence PCR (Polymerase Chain Reaction) method for detecting HLA (Human Leukocyte Antigen)-B*5801 alleles
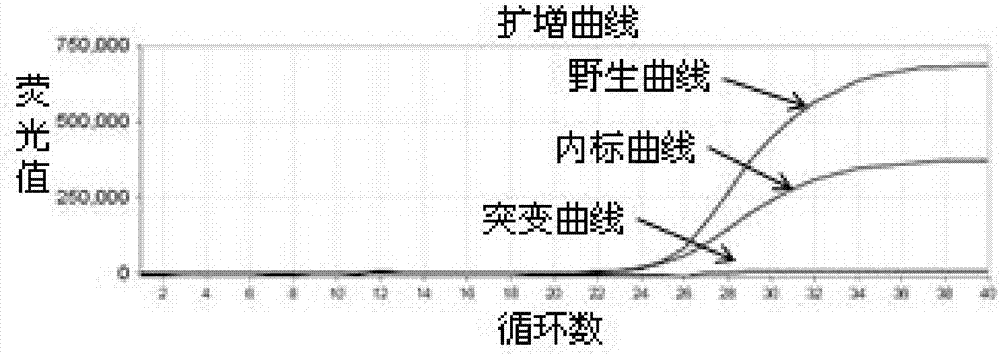
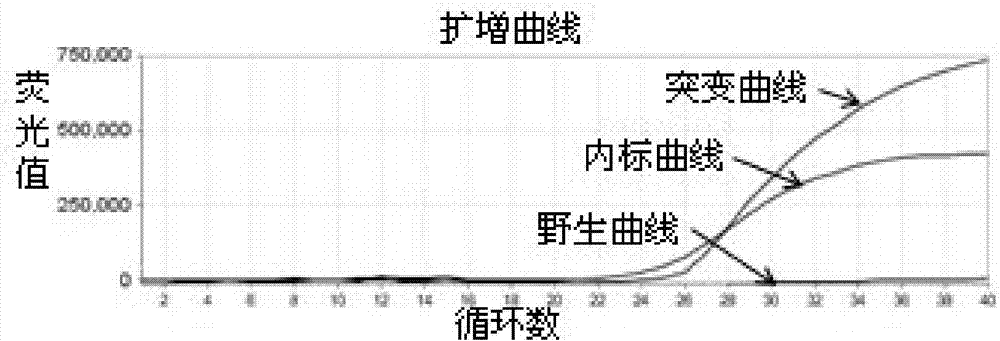
ActiveCN104232781ASave consumablesShorten the timeMicrobiological testing/measurementDNA/RNA fragmentationReference genesFluorescence
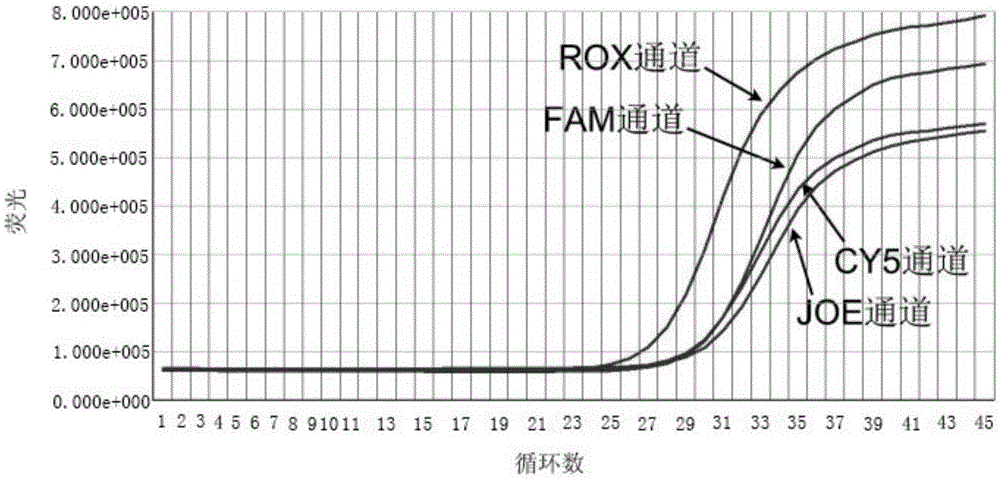
The invention discloses a primer probe assembly for high-specificity amplification HLA (Human Leukocyte Antigen)-B*5801 alleles on the basis of a TaqMan probe detection method. The primer probe assembly comprises an upstream primer Fp: 5'-AGGGGCCGGAGTATTGGGATG-3', a downstream primer Rp: 5'-TTGGCCTCAACTGAAAATGAAAC-3' and a probe: 5'-HEX-TCAGGGAGGCGGATCTCGGAC-BHQ2-3'. Primers and probes of reference genes ACTB are used simultaneously, target genes and the reference genes are added into a tube for dual-channel fluorescent quantitative PCR reaction, and a result is analyzed through an amplification curve. The method has the characteristics of simplicity, convenience, flexibility, quickness, high specificity, high flux, zero pollution, high resolution and the like, and is applicable to detection of the HLA-B*5801 alleles of a whole-genome DNA (Deoxyribonucleic Acid) sample in the peripheral blood and the saliva of a human body.
Owner:SHANXI LIFEGEN
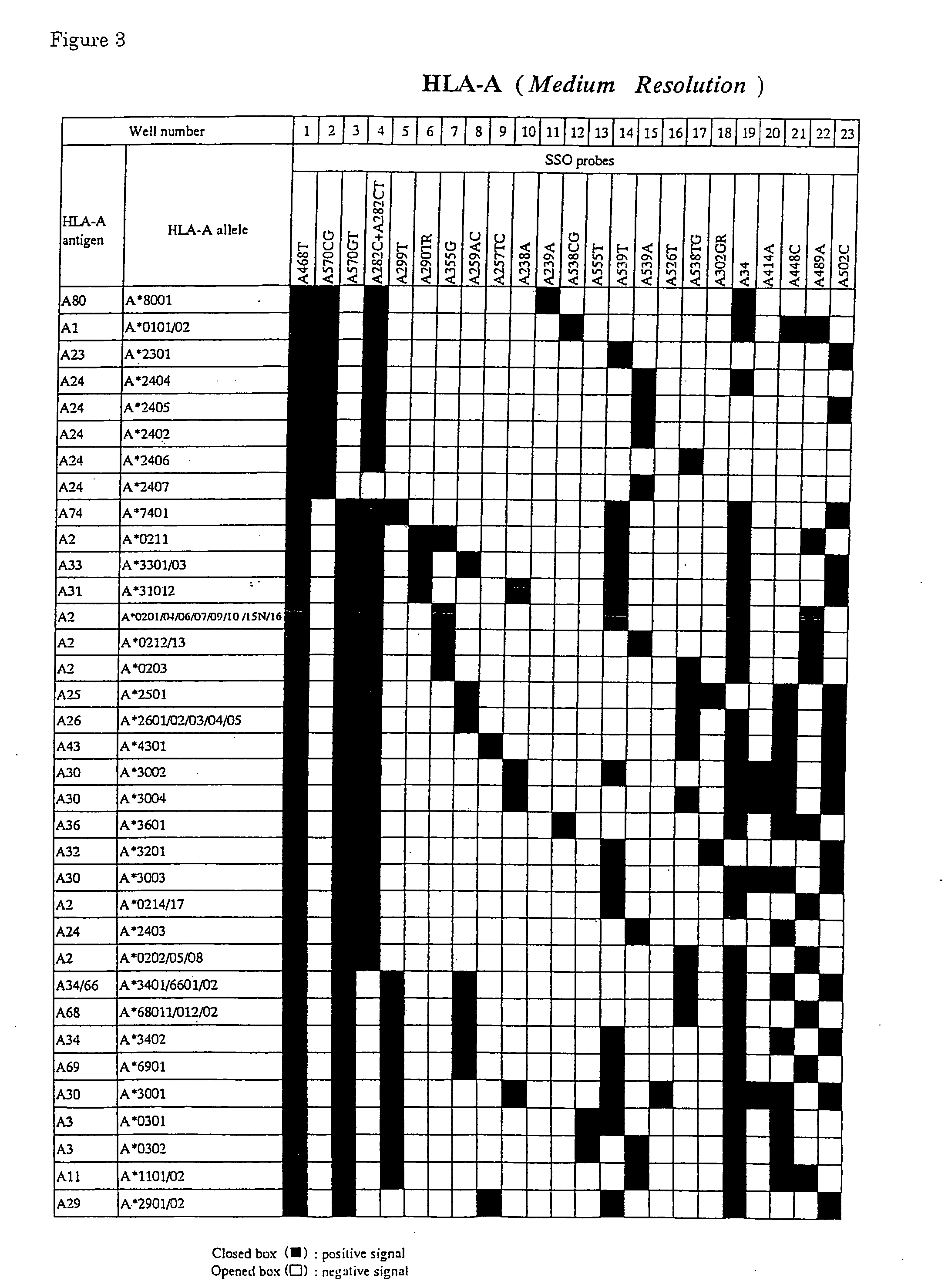
Methods and Compositions for Very High Resolution Genotyping of HLA
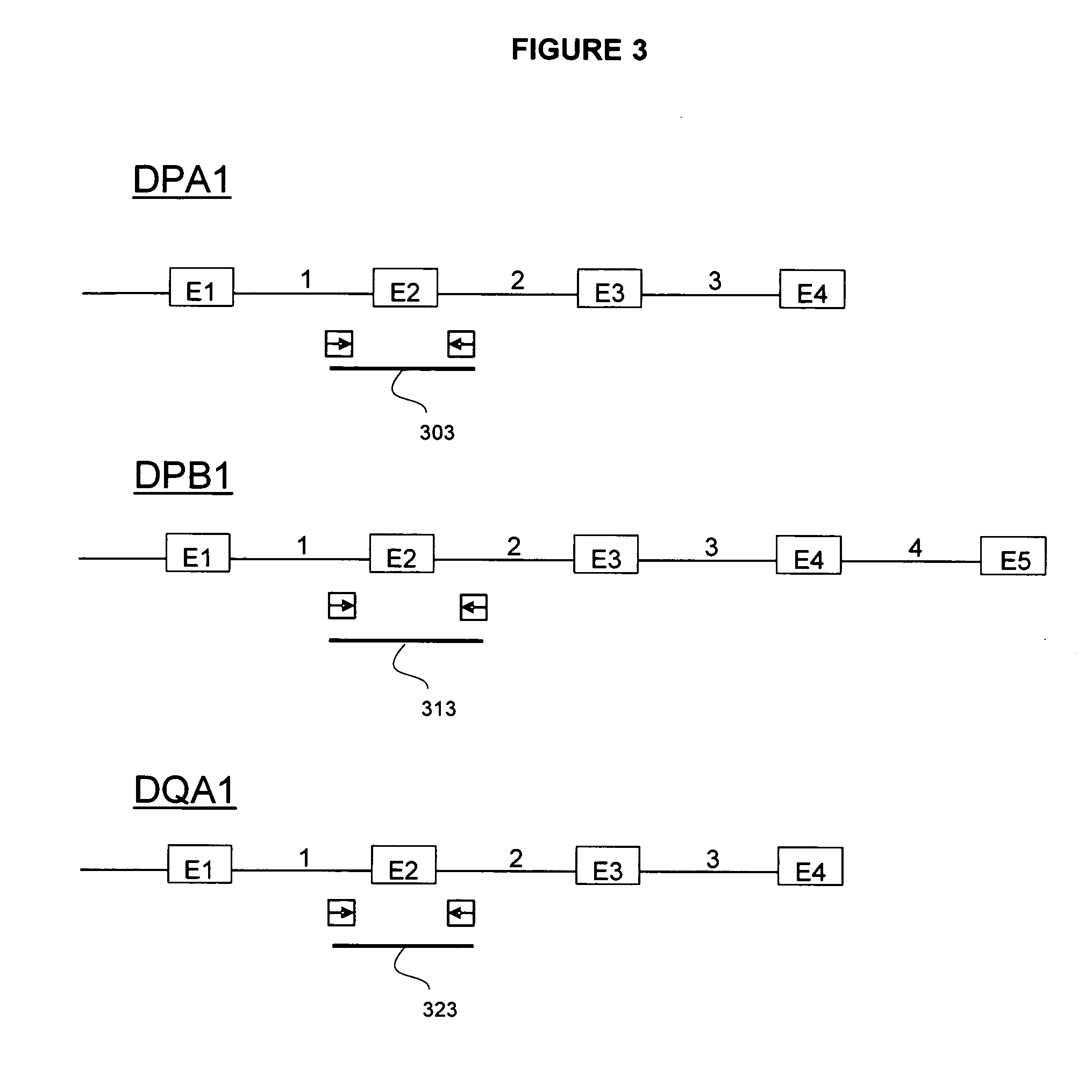
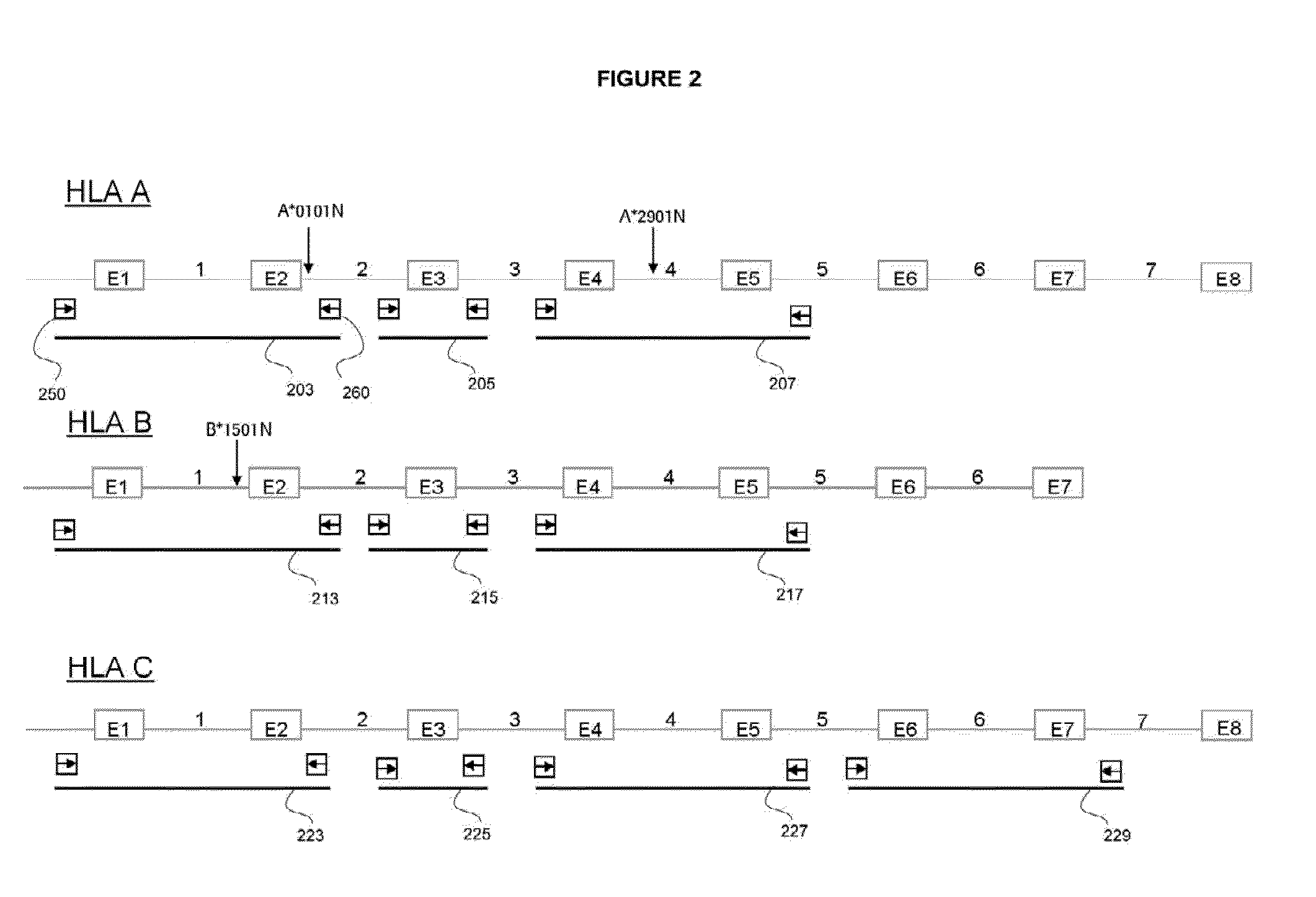
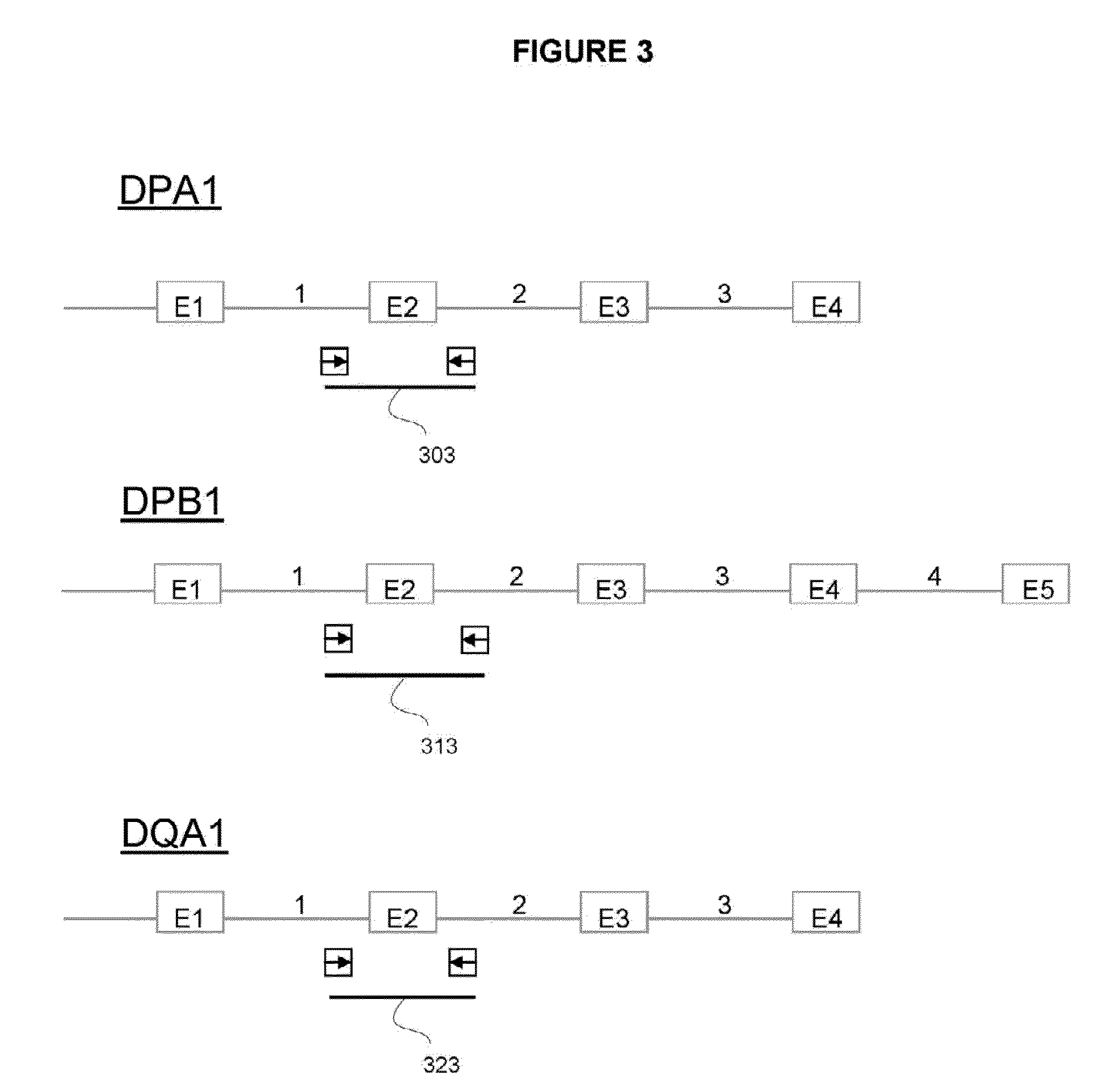
The invention is a method of determining HLA genotype for HLA-A, HLA-B, HLA-C, DQB1, DRB1, DRB3, DRB4, DRB5, DPA1 and DPB1. Reagents and kits are also disclosed.
Owner:ROCHE MOLECULAR SYST INC
Primers, probe, fluorescent PCR kit and method for detecting human HLA-B*1301 gene
InactiveCN104450912ALow efficiencyLow costMicrobiological testing/measurementDNA/RNA fragmentationHuman leucocyte antigenHLA-B
The invention provides primers, a probe, a fluorescent PCR kit and a detection method for detecting a human HLA-B*1301 allele. According to the theory of 'allele specific PCR', a human leucocyte antigen HLA-B*1301 allele can be detected on a real-time fluorescent quantitative PCR technical platform.
Owner:SUZHOU KUANGYUAN MOLECULAR BIOTECH
sybr Green I detection method of hla-b*1502 gene and its special primers and kits
InactiveCN102296109AStrong specificityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceNucleic acid detectionHLA-B
The invention provides a SYBR Green I detection method for detecting HLA-B*1502 gene and its special primers and kit. The primers are designed according to the 2nd and 3rd exon regions of the HLA-B*1502 gene, and are used for qualitative detection of the HLA-B*1502 gene in the sample to be tested. The upstream primer of the primers has the nucleotide sequence of SEQ ID NO: 1 in the sequence listing, and the downstream primer has the nucleotide sequence of SEQ ID NO: 2 in the sequence listing. The invention can quickly and accurately detect the HLA-B*1502 gene, and is of great significance for ensuring human health and the drug safety of carbamazepine drugs. The detection method and the kit of the present invention can be used for nucleic acid detection of the HLA-B*1502 gene in various clinical samples (bone marrow, peripheral blood or tissue), and have broad application prospects.
Owner:BEIJING SEARCH BIOTECH
Human leukocyte antigen gene detection kit and application thereof
PendingCN106282328AContribute to the establishment of a method for screening drug eruptions caused by metronidazoleTo establish a method for screening metronidazole-induced drug eruptionMicrobiological testing/measurementMetaboliteWhite blood cell
The invention belongs to the fields of biomedicine and reagent detection, and relates to a human leukocyte antigen gene detection kit and application thereof in screening metronidazole-induced adverse drug reactions of skin. The kit contains a reagent for detecting human leukocyte antigen gene HLA-B*39:01 nucleic acid or protein, and amplification primers or a labeled probe of the human leukocyte antigen gene or a specific antibody containing the human leukocyte antigen. The HLA-B*39:01 gene can be used as a labeled gene for predicting metronidazole-induced epispasis. The detection kit can be used for further preparing or evaluating a metronidazole-induced epispasis screening kit, thereby providing valuable reference data for instructing clinical medication, and reducing the possibility of metronidazole-induced epispasis; or the detection kit can also be used for preparing or evaluating therapeutic drugs and especially targeted drugs for metronidazole-induced epispasis, thereby preventing the interactions between the drugs and metronidazole or metabolites thereof in disease development.
Owner:FUDAN UNIV +1
Detection method and kit of HLA (Human Leukocyte Antigen)-B*58:01 allele
The invention relates to a detection method of HLA (Human Leukocyte Antigen)-B*58:01 allele. The detection method comprises the following steps: providing a mixture of a sample to be tested, a nucleic acid amplification system and a fluorescence detection system; circularly amplifying target polynucleotide through an amplified reaction; indirectly combining a fluorescence generating group and an amplified target polynucleotide sequence; and detecting the fluorescent amount generated by the fluorescence generating group so as to determine existence of the target polynucleotide and the relative amount thereof. The invention further discloses a kit for detecting the allele. The kit comprises a plurality of sealed centrifuge tubes which are respectively filled with a reaction liquid 1, a reaction liquid 2, a reaction liquid 3, an EZ Taq enzyme mixed liquid, a standard liquid 1 and a standard liquid 2 as well as the kit which separates and packages the centrifuge tubes in a centralized manner. The kit and the detection method provided by the invention are simple and convenient to operate, short in time consumed, strong in specificity and high in sensitivity. The kit and the detection device can be widely applied to detecting the HLA-B*58:01 allele and clinically avoiding severe untoward effects of skins of the HLA-B*58:01 allele patients caused by using allopurinol.
Owner:GUANGDONG UNITY BIOTECH
Method for the amplification of HLA class I alleles
InactiveUS20020197613A1Simple methodImprove methodMicrobiological testing/measurementFermentationForward primerIntein
The present invention relates to a method and to specific primers for the locus-specific, separate amplification of exon 2, exon 3 and / or exon 4 of HLA-A, HLA-B or HLA-C alleles, making use of at least one primer set wherein: for the amplification of exon 2, the reverse primer specifically hybridizes to a locus-specific target sequence in intron 2 of respectively HLA-A, HLA-B or HLA-C; for the amplification of exon 3, the forward primer specifically hybridizes to a locus-specific target sequence in intron 2 of respectively HLA-A, HLA-B or HLA-C and / or the reverse primer specifically hybridizes to a locus-specific target sequence in intron 3 of respectively HLA-A, HLA-B or HLA-C; for the amplification of exon 4, the forward primer specifically hybridizes to a locus-specific target sequence in intron 3 of respectively HLA-A, HLA-B or HLA-C. In accordance, the present invention provides an improved method for the typing or subtyping of HLA Class I alleles making use of the amplification method of the invention.
Owner:INNOGENETICS NV
Primer, kit and method for quickly detecting HLA-B*5801 allele
ActiveCN104017898AImprove bindingAvoid it happening againMicrobiological testing/measurementDNA/RNA fragmentationHLA-BBiology
The invention belongs to the technical field of gene engineering, and discloses a primer for quickly detecting HLA-B*5801 allele, a kit containing the primers and a method for quickly detecting HLA-B*5801 allele by using the primers and kit. By using the HLA-B*5801 gene specific primer and adopting the multiplex primer combination design, the SNP (single-nucleotide polymorphism) site of the HLA-B*5801 gene is completely covered, the specific combination is enhanced, and the generation of the false positive is prevented more effectively. Besides, the specific primer, dNTPs (deoxyribonucleotide triphosphates), PCR (polymerase chain reaction) buffer solution and dyes are mixed previously, thereby greatly saving the operation time and workload; and the method is quick, simple, accurate and visual, and can the screening and typing experiment of the whole gene within 3 hours, thereby solving the problem of safe application instructions of the HLA-B*5801 gene in drugs for treating gout and the like.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
Multicolor fluorescence PCR kit and method for detecting HLA-B*5801 allele
InactiveCN105624293AShorten the timeSave consumablesMicrobiological testing/measurementForward primerFluorescence
The invention provides a multicolor fluorescence PCR kit and method for detecting HLA-B*5801 allele. The multicolor fluorescence PCR kit is characterized by comprising a detection primer-probe combination of two forward primers, two reverse primers and three detection probes for detecting the gene HLA-B*5801 and an internal-control primer probe comprising a forward primer, a reverse primer and an internal-control probe for detecting a reference gene. Based on the primer design and PCR reaction characteristics, the time and consumables can be saved to a great degree, the detection process only needs 1-1.5 hours, the operation is simple and easy to implement, and the whole experiment can be finished within 2.5 hours.
Owner:SHANGHAI TONEKER BIOTECH
Reagent for detecting antiepileptic drug allergic reaction associated antigen genetype and clinical application method
InactiveCN101353698AReduce riskImprove accuracyMicrobiological testing/measurementAntigenAntiepileptic drug
The invention relates to a reagent used for detecting antigen genotypes relevant to the anaphylactic reaction of antiepileptic drugs, and a clinical application method thereof. The reagent is characterized in that: 1) the reagent comprises Taq enzyme, a chain of seven tubes and a PCR primer; 2) the PCR primer is shown in the sequence list SEQ ID No.1. The clinical application method is characterized in that: 1) four pairs of the sequence specific primers and two pairs of internal reference primers are designed by utilizing a sequence specific primer PCR-SSP according to an HLA-B sequence, and PCR amplification is carried out by taking gDNA extracted from human peripheral blood or other issues as a template; 2) gel electrophoresis is adopted for the detection so as to confirm that the genotype of the sample is HLA-B multiplied by 1502. The reagent and the method of the invention have the advantages of simple operation, high accuracy and low cost, and are especially suitable for determining that whether the antiepileptic drugs such as carbamazepine, etc. can be taken by the HLA-B multiplied by 1502 genotype detection before patients in China or Asia take antiepileptic drugs such as the carbamazepine, etc.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Universal chimeric antigen receptor T cell preparation technology
InactiveCN109456943AHydrolasesStable introduction of DNAHLA-BChimeric Antigen Receptor T-Cell Therapy
The invention relates to a universal chimeric antigen receptor T cell, a preparation method thereof and an application of the cell, and particularly provides a CAR (chimeric antigen receptor)-T cell.Binding of the CAR and cells HLA-A / HLA-B and TCR is inhibited, and TCR (T cell receptor) gene expression of the cell is silenced. The universal CAR-T cell can be used for treating allogenic tumors, GVHD (graft-versus-host disease) and HVG (host versus graft) reaction are avoided in allogenic infusion, and survival and anti-tumor effects of the allogenic CAR-T cell in a receptor are improved.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
Kit and method for detecting human leucocyte antigen HLA-B*1502 genetype
InactiveCN103114130AEasy to operateAccurate operationMicrobiological testing/measurementHuman leucocyte antigenForward primer
The invention discloses a kit and a method for detecting human leucocyte antigen HLA-B*1502 genetype. The kit disclosed by the invention is characterized by comprising the following primer sequences: a forward primer FP: 5'-CGACGCCGCGAGTCCCAGG-3'; and a reverse primer RP: 5'-CGTCGTAGGCGGACTGGTCATA-3'. The kit further comprises the following probe sequence: a probe PR: 5'-Cy5-AACACACAGATCTCCAAGACCAACACAC-p-3'. The kit disclosed by the invention has good specificity and sensitivity for carrying out PCR (Polymerase Chain Reaction) detection of the human leucocyte antigen HLA-B*1502. The PCR detection method disclosed by the invention has the advantages of being simple for operation (closed pipe), rapid, accurate and the like and is applied to clinical molecular diagnosis.
Owner:瀚吉康生物科技(北京)有限公司
Detection kit for human leucocyte antigen genes
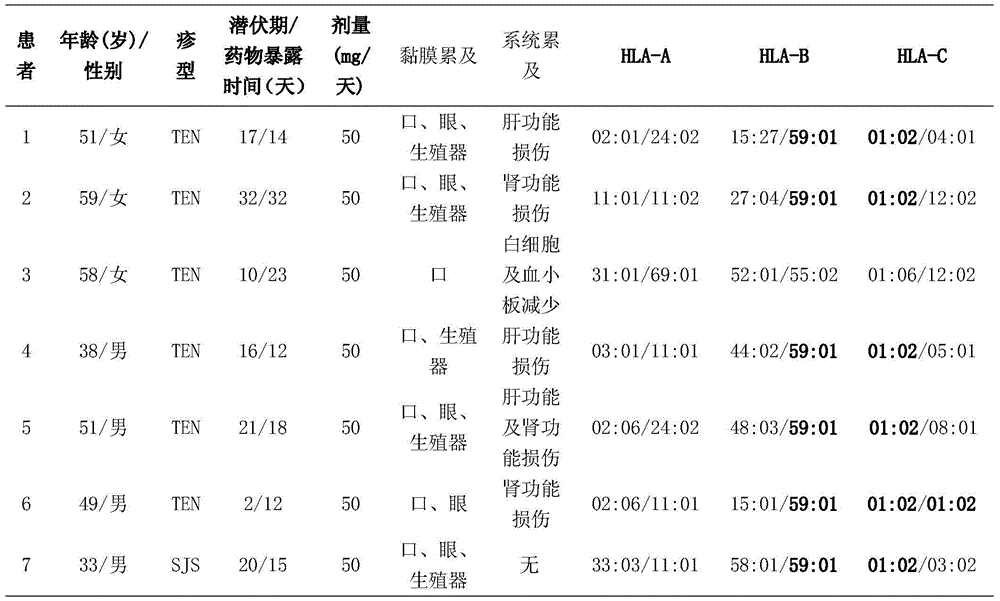
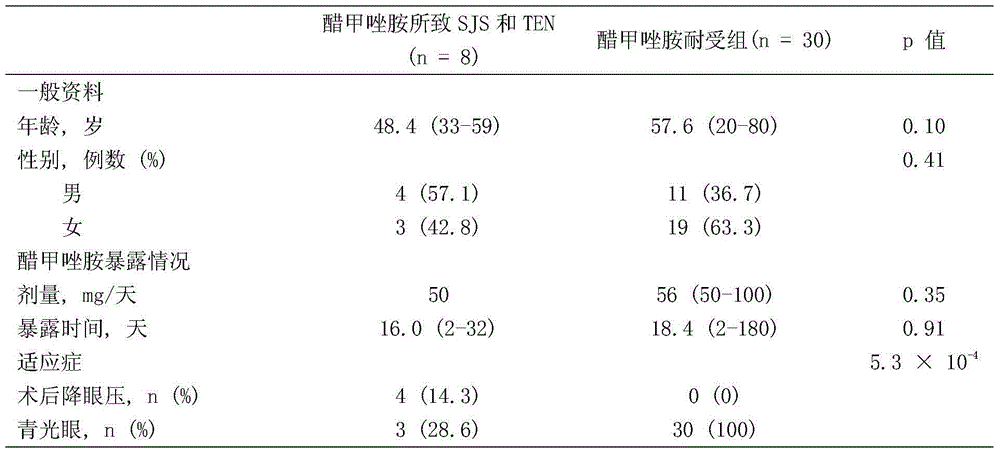
PendingCN104862379ABlocking interactionMicrobiological testing/measurementBiological testingMetaboliteHLA - Human leukocyte antigen gene
The invention belongs to the technical field of biological medicines and relates to a detection kit for human leucocyte antigen (HLA) genes--HLA-B*59: 01 genes, and the HLA-B*59: 01 genes can serve as marker genes for forecasting methazolamide caused SJS (Stevens-Johnson syndrome) and TEN (Toxic epidermal necrolysis). According to the detection kit, after DNA extracted from the peripheral blood of a patient, the PCR-SSO method is adopted to detect the HLA-B*59 :01 genes. The detection kit for HLA-B*59: 01 genes can be used for examining before application of methazolamide and screening targeted medicines for treating methazolamide caused SJS and TEN; and examination can guide clinical medication to reduce incidence of SJS and TEN caused by methazolamide, and the screening mainly acts on HLA-B*59: 01 molecules in a targeted manner, so that interaction between the HLA-B*59: 01 molecules and methazolamide or other metabolic products during attack.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Method for typing of HLA class I alleles
InactiveUS20050112624A1EasilySimple processMicrobiological testing/measurementBiological testingTypingHLA-B
This invention provides a method, a kit and a reagent for typing of the HLA class I alleles. Explaining concretely, a single HLA class I antigen or allele is determined by combining PCR amplification using a primer pair which can amplify all HLA-A alleles, all HLA-B alleles or all HLA-C alleles, or which is specific to the common sequence to alleles of the specific group consisting of the specific HLA-A alleles or the specific HLA-B alleles, with reverse hybridization analysis using DNA probes capable of specifically hybridizing with the sequence of al least a specific HLA-A allele, at least a specific HLA-B allele or at least a specific HLA-C allele, which are covalently immobilized on wells of microtiter plates.
Owner:SHIONOGI & CO LTD
Method for detecting HLA-B * 5801 allele based on real-time fluorescence PCR
ActiveCN104293932AEasy to operateReliable resultsMicrobiological testing/measurementDNA/RNA fragmentationReference genesFluorescence
The invention designs a primer-probe combination capable of amplifying HLA-B * 5801 allele with high specificity based on a TaqMan probe detecting method: Fp: 5'-AGGGGCCGGAGTATTGGGATG-3', Rp:5'-TTGGCCTCAACTGAAAATGAAAC-3', MGB probe: 5'-HEX-VI-TCAGGGAGGCGGATCTCGGAC-MGB3'; meanwhile, by virtue of primers and probes for a reference gene ACTB, a target gene and the reference gene are added into a tube to have a dual-channel fluorescence quantitative PCR reaction, and the result is analyzed by an amplification curve. The method provided by the invention is simple and convenient, flexible, fast, high in specificity, high in throughput, pollution-free, and high in resolution, and is applicable to detection of the HLA-B * 5801 allele of whole genome DNA samples in human peripheral blood and saliva.
Owner:SHANXI LIFEGEN
Multiplex real-time fluorescent PCR (polymerase chain reaction) method for detecting HLA-B*15:02 alleles
ActiveCN104830852AShorten the timeAvoid secondary pollutionMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceHLA-B
The invention discloses a primer-probe combination of two pairs of high specific amplification HLA-B*15:02 alleles designed on the basis of using a TaqMan probe detection method. On the basis, by using a primer and probe of a reference gene beta-Actin, specific primers and probes of two pairs of target genes and the primer and probe of the reference gene are added into a same pipe to carry out a multiplex fluorescent PCR, and then results are analyzed by using a fluorescent amplification curve. The method disclosed by the invention has the characteristics of high specificity, flexibility, rapidness, high flux, no pollution, high resolution, capability of carrying out real-time monitoring on a reaction process, and the like, and can be applied to the detection of HLA-B*15:02 alleles of whole-genome DNA samples in human peripheral blood and saliva.
Owner:SHANXI LIFEGEN
Human leukocyte antigen gene detection kit for screening skin drug adverse reactions caused by salazosulfapyridine
ActiveCN104818316ABlocking interactionMicrobiological testing/measurementBiological testingMetaboliteWhite blood cell
The invention provides a human leukocyte antigen gene detection kit for screening skin drug adverse reaction caused by salazosulfapyridine, belongs to the technical field of biomedicine, and relates to a detection kit of human leukocyte antigen gene HLA-B*13:01, wherein the HLA-B*13:01 gene can be adopted as the marker gene of salazosulfapyridine-induced DRESS. According to the present invention, DNA is extracted from the peripheral blood of a patient, and the HLA-B*13:01 gene is detected by using the current method, such as PCR-SSO (sequence-specific oligonucleotide probe) method; and the HLA-B*13:01 gene detection kit of the present invention can be used for screening before the salazosulfapyridine use and screening the target drug for treatment of salazosulfapyridine-induced DRESS, wherein the screening before the salazosulfapyridine use can guide the clinical medication so as to reduce the occurrence of the salazosulfapyridine-induced DRESS, and the drug screening mainly act on the HLA-B*13:01 molecules in a targeting manner so as to block the interaction between the HLA-B*13:01 molecules and the salazosulfapyridine or the metabolites thereof in the pathogenesis.
Owner:FUDAN UNIV +1
Use of hla-b*1301 allele
The present invention discloses uses of a HLA-B*1301 allele, comprising: 1) a use of a substance for detecting whether a person has the HLA-B*1301 allele in preparation of a product for evaluating a risk of adverse drug reactions in response to dapsone in the person; 2) a method for detecting or evaluating a risk of adverse drug reaction in response to dapsone in a person, comprising detecting whether the person has the HLA-B*1301 allele, wherein, a person with LA-B*1301 allele suffers a higher risk of adverse drug reaction upon being administered dapsone, as compared with a person without HLA-B*1301 allele, and a person with LA-B*1301 alleles at both chromosomes of a pair of homologous chromosomes suffers a higher risk of adverse drug reaction upon being administered dapsone, as compared with a person with HLA-B*1301 allele at only one of a pair of homologous chromosomes.
Owner:SHANDONG PROVINCIAL INST OF DERMATOLOGY & VENEREOLOGY
Fluorescent PCR kit for qualitative detection of HLA-B*1502 gene subtypes
ActiveCN102660635AStrong specificityQualitatively accurateMicrobiological testing/measurementFluorescence/phosphorescenceNucleic acid detectionFluorescence
The invention provides a fluorescent PCR kit for qualitative detection of HLA-B*1502 gene subtypes, and belongs to the field of in-vitro nucleic acid detection. The kit comprises uracil DNA glycosylase, Taq polymerase, PCR genotyping primers and a fluorescence probe; the PCR genotyping primer sequence is as shown in SEQ ID NO: 3-4 and / or SEQ ID NO: 6-7. The kit provided in the invention has high sensitivity and good specificity, and can monitor reaction process in real time and the reaction time is short; in addition, closed tube operation is performed, and subsequent treatment is not needed, which can maximally avoid the pollution of a reaction product, thus being capable of replacing traditional cell detection.
Owner:CHANGSHA 3G BIOTECH
Risk assessment for adverse drug reactions
ActiveUS20080227109A1Microbiological testing/measurementBiological testingFixed drug eruptionsMicrosatellite
The present invention provides a method of predicting the risk of a patient for developing adverse drug reactions, particularly SJS or TEN. It was discovered that an HLA-B allele, HLA-B* 1502, is associated with SJS / TEN that is induced by a variety of drugs. The correlation with HLA-B* 1502 is most significant for carbamazepine-induced SJS / TEN, wherein all the patients tested have the HLA-B* 1502 allele. In addition, another HLA-B allele, HLA-B*5801, is particularly associated with SJS / TEN induced by allopurinol. Milder cutaneous reactions, such as maculopapular rash, erythema multiforme (EM), urticaria, and fixed drug eruption, are particularly associated with a third allele, HLA-B *4601. For any of the alleles, genetic markers (e.g., HLA markers, microsatellite, or single nucleotide polymorphism markers) located between DRB 1 and HLA-A region of the specific HLA-B haplotype can also be used for the test.
Owner:ACAD SINIC
HLA-B*1502 detection kit
InactiveCN105296615AStrong specificityReduce the possibility of non-specific amplificationMicrobiological testing/measurementDNA/RNA fragmentationHLA-BCarbamazepine
The invention provides an HLA-B*1502 detection kit and belongs to the technical field of carbamazepine use detection. The HLA-B*1502 detection kit is characterized in that the Allele-Specific LAMP technology is used for designing specific primers aiming at the C allele of rs10484555, the relevance between the C allele of rs10484555 and HLA-B*1502 is used for judging whether a patient carries HLA-B*1502 allele or not, so that personalized carbamazepine use can be guided, and problems caused by direct testing for HLA-B*1502 are avoided. The HLA-B*1502 detection kit is low in cost, short in detecting time, high in accuracy and easy to popularize.
Owner:BEIJING JINQI BIOLOGICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com