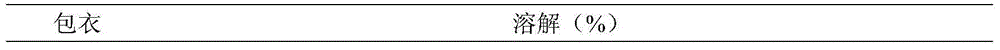Patents
Literature
188 results about "Antiepileptic drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
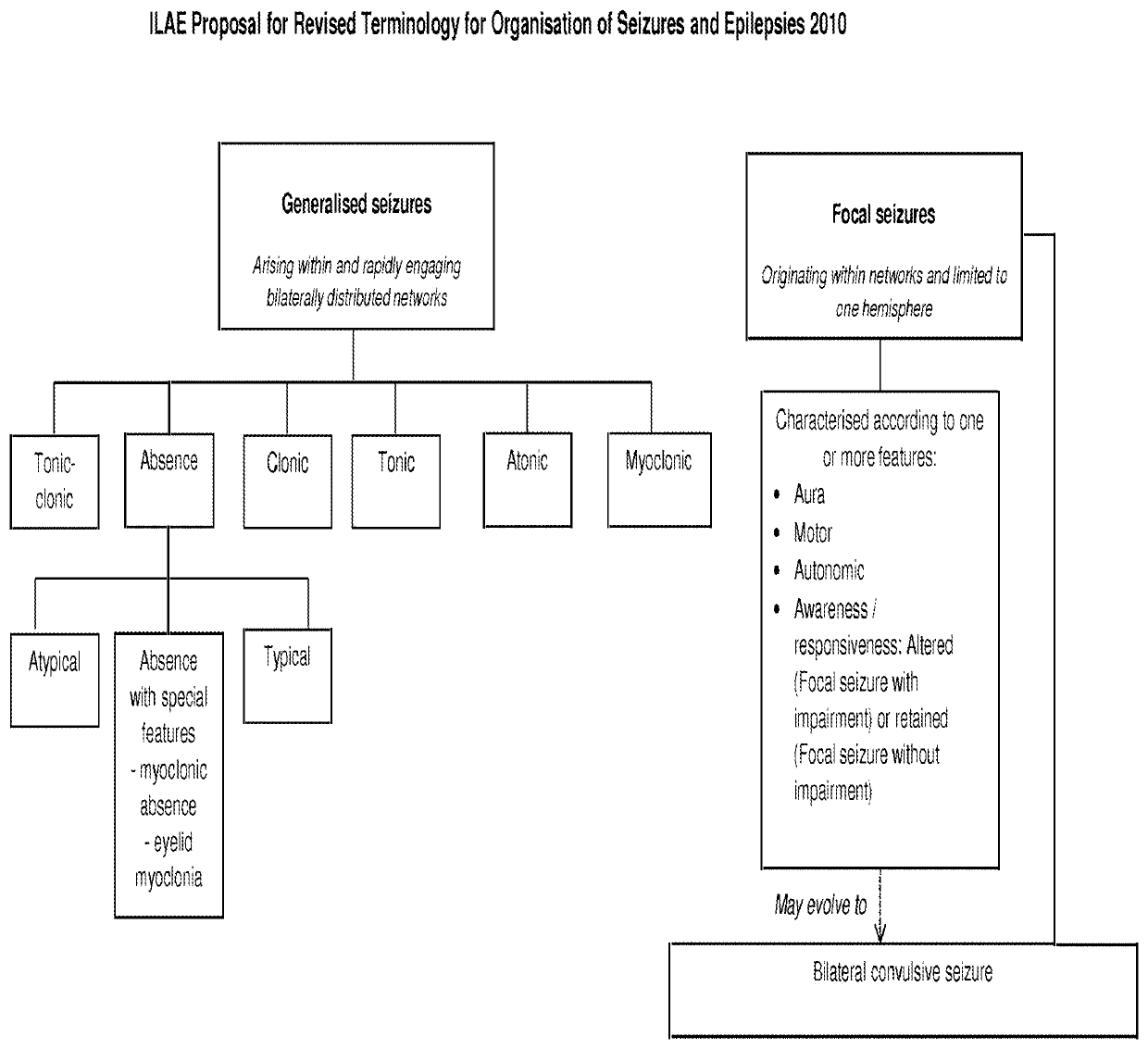
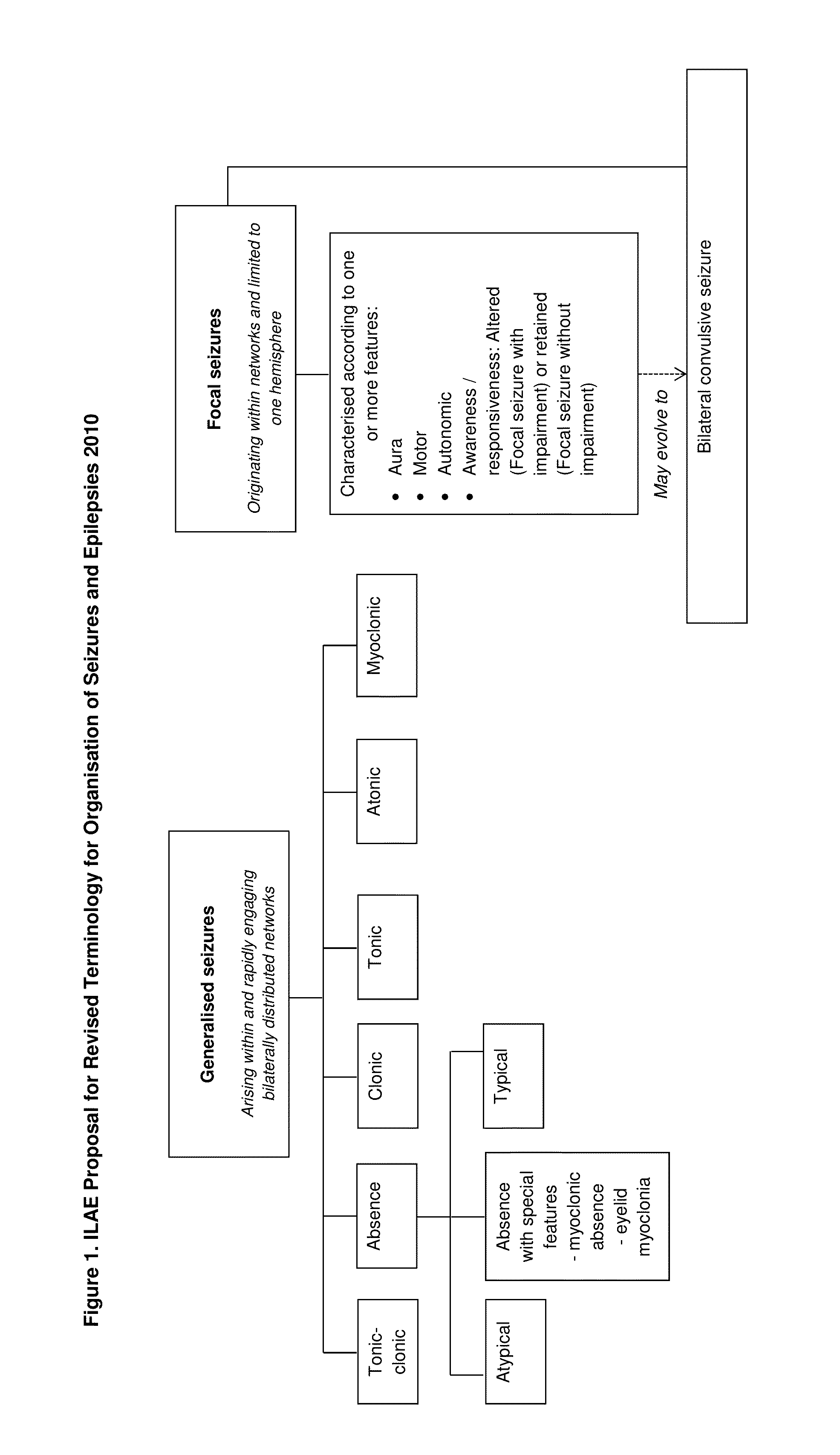
Antiepileptic drug, any drug that is effective in the treatment of epilepsy, a chronic disorder of the central nervous system that is characterized by sudden and recurrent seizures.
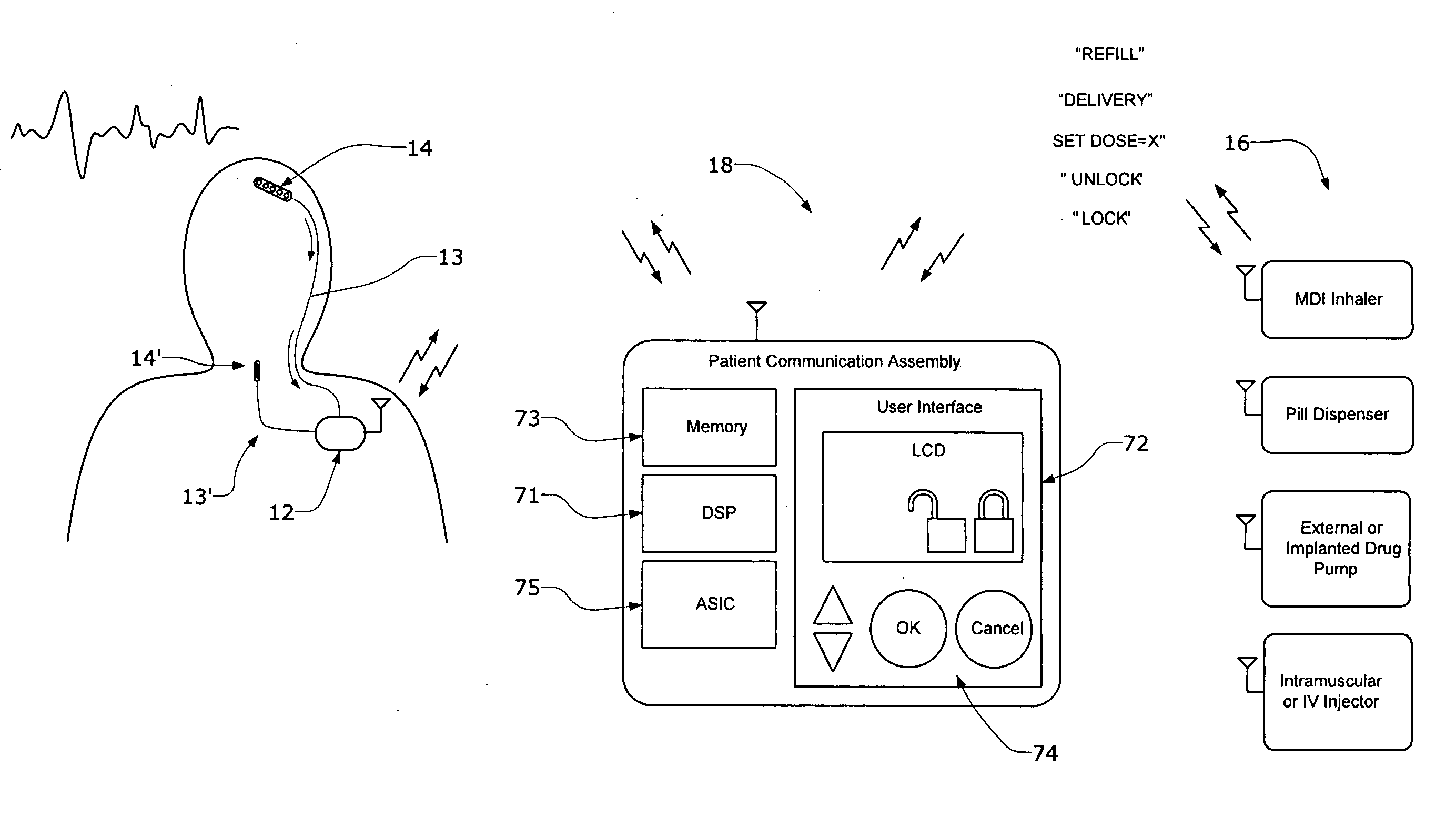
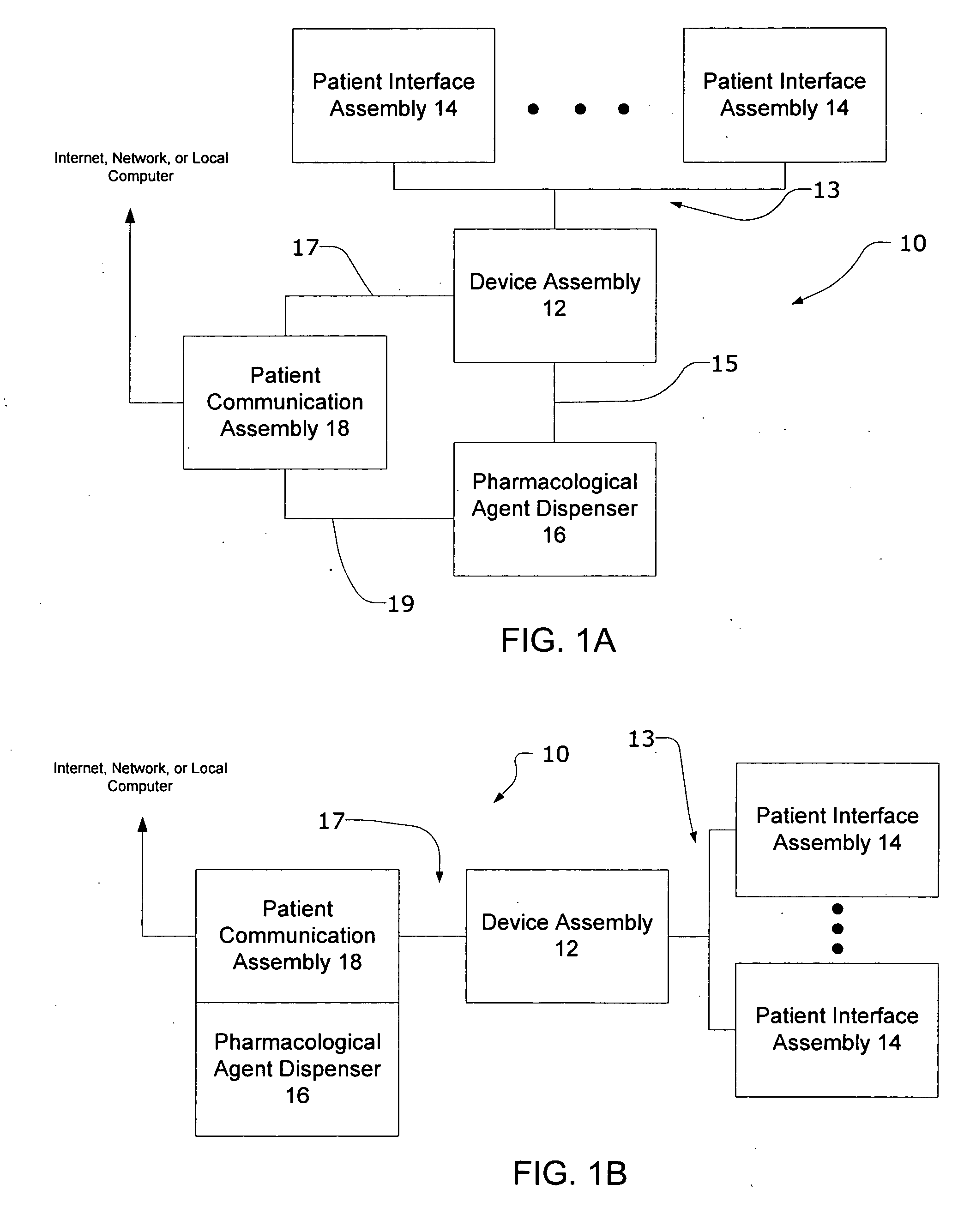
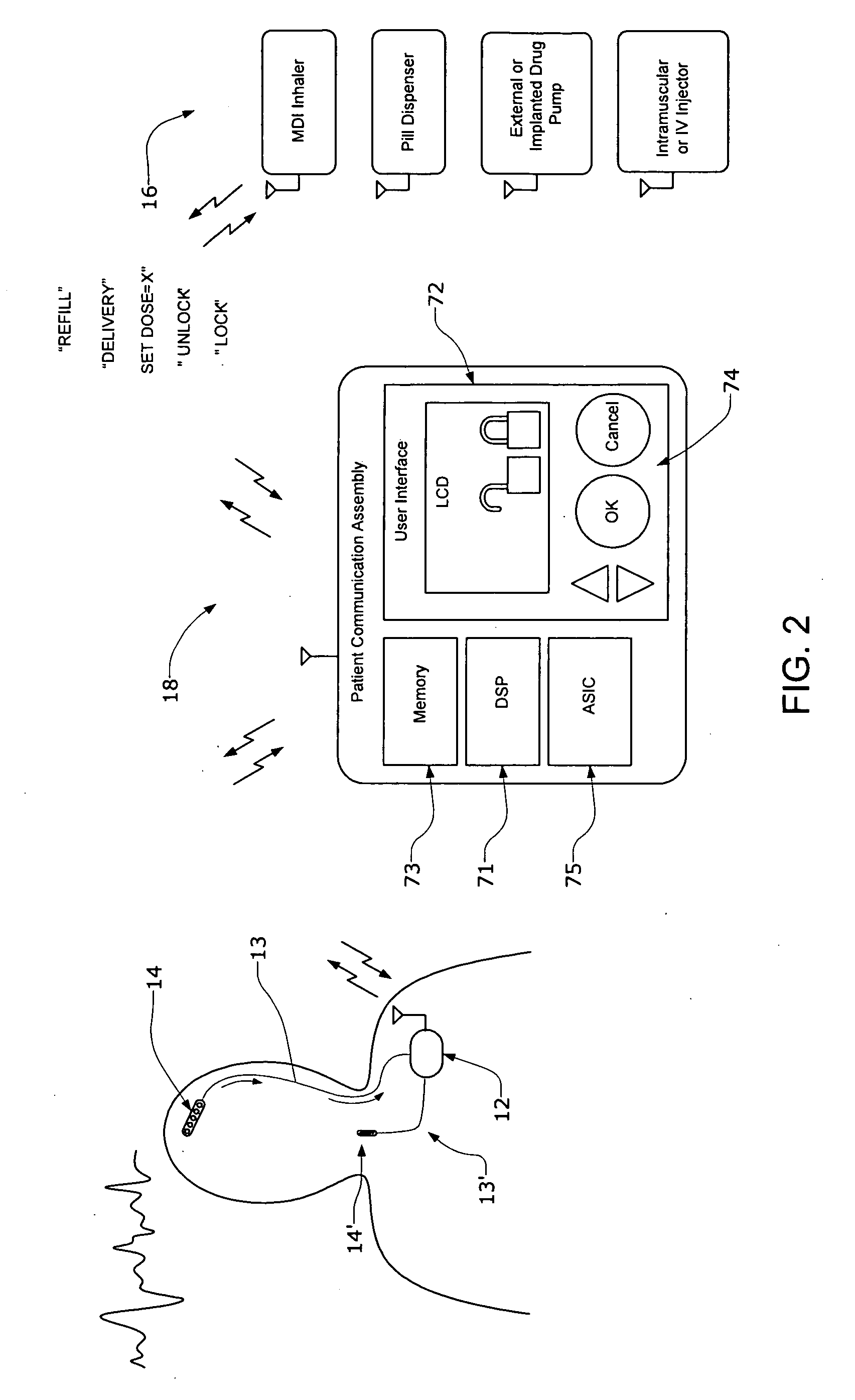
Systems and methods for characterizing a patient's propensity for a neurological event and for communicating with a pharmacological agent dispenser
InactiveUS20070149952A1Selectively limit accessSelectively limit to administrationDrug and medicationsTelemedicinePharmacometricsAntiepileptic drug
The present invention provides systems and methods for managing intake of a pharmacological agent. In one method of the present invention, the systems and methods are for controlling intake of an anti-epileptic drug. In such embodiments, one or more signals from a patient are processed to predict an onset of a seizure. Upon the prediction of the seizure, the patient is allowed to access the pharmacological agent in a pharmacological agent dispenser.
Owner:CYBERONICS INC
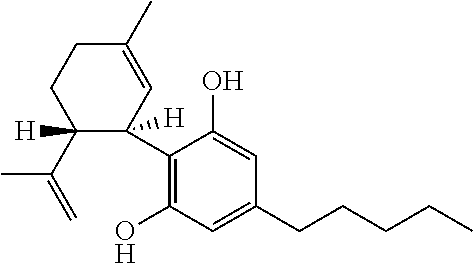
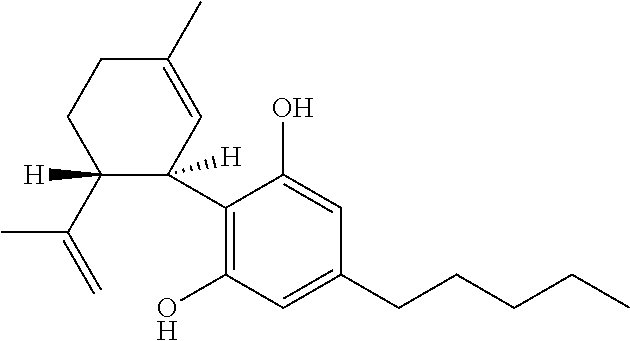
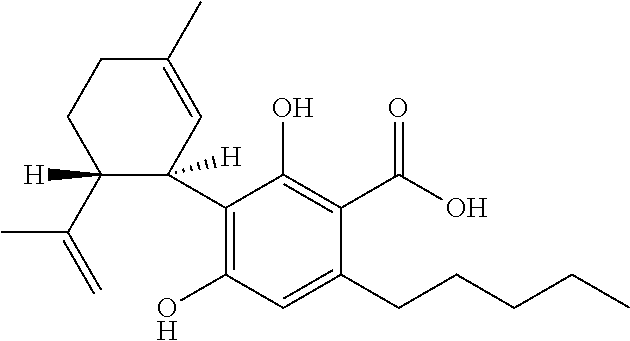
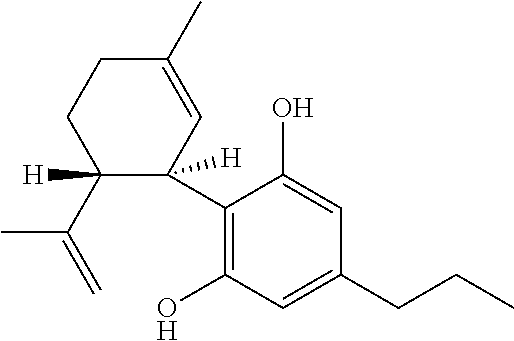
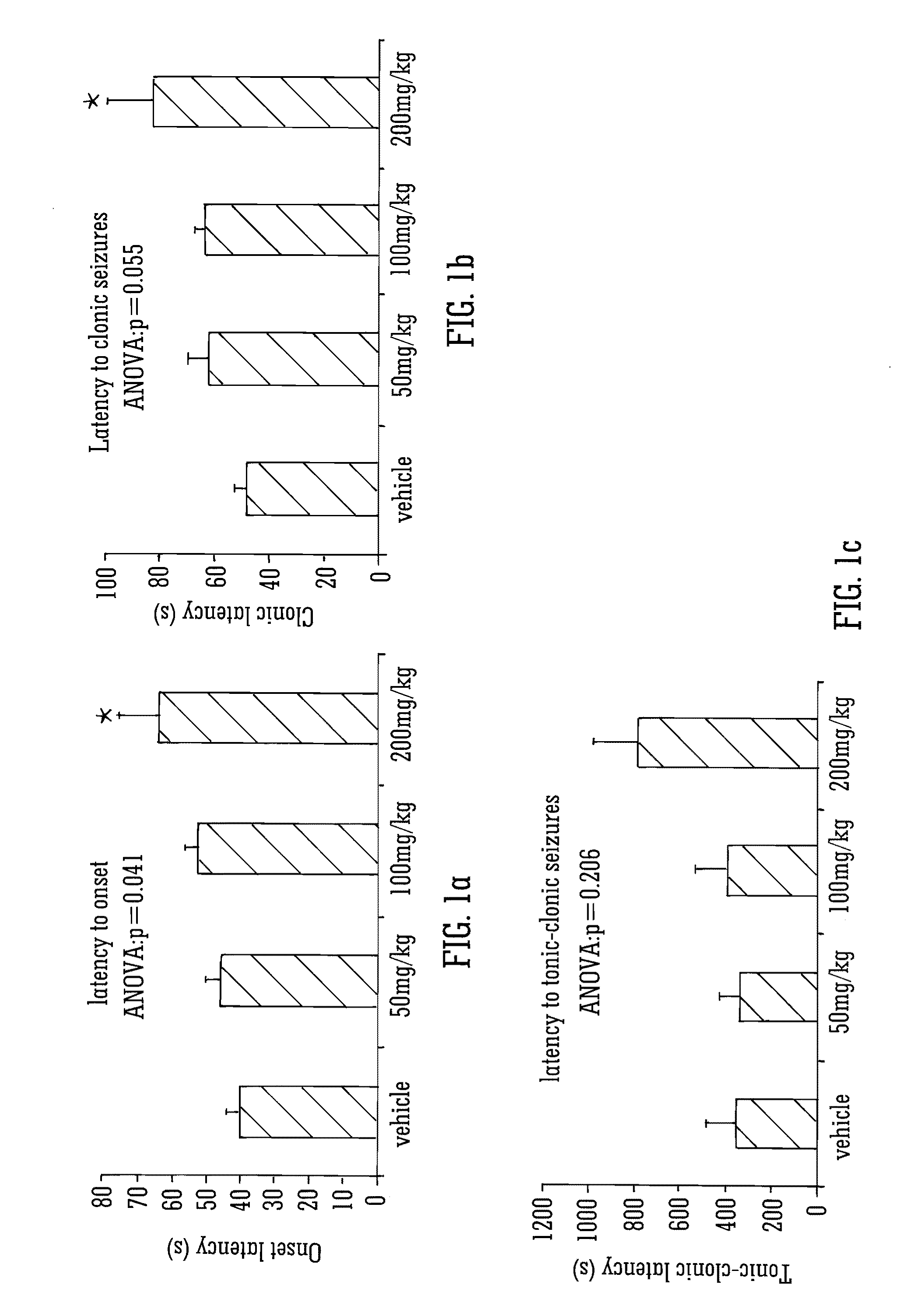
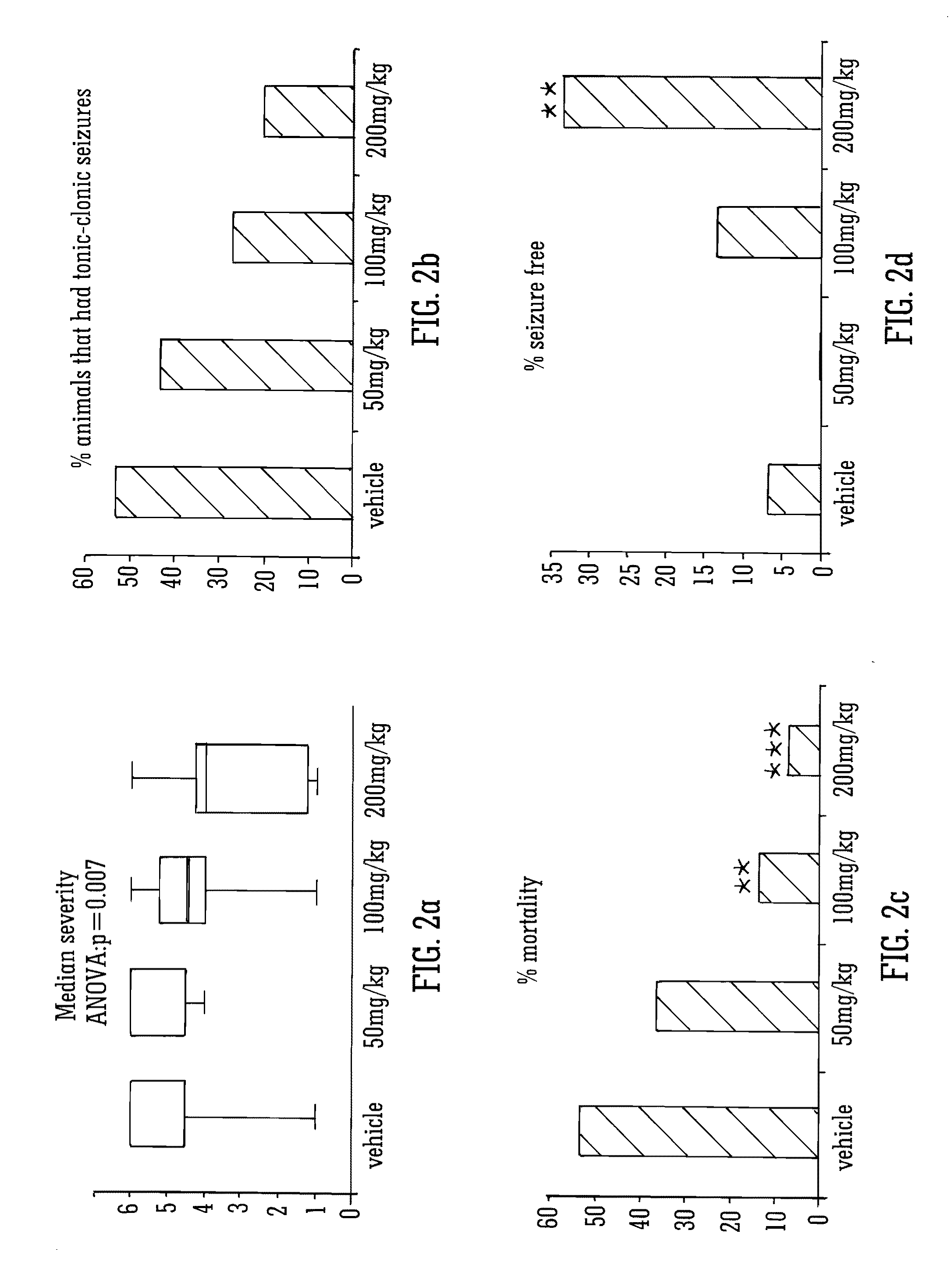
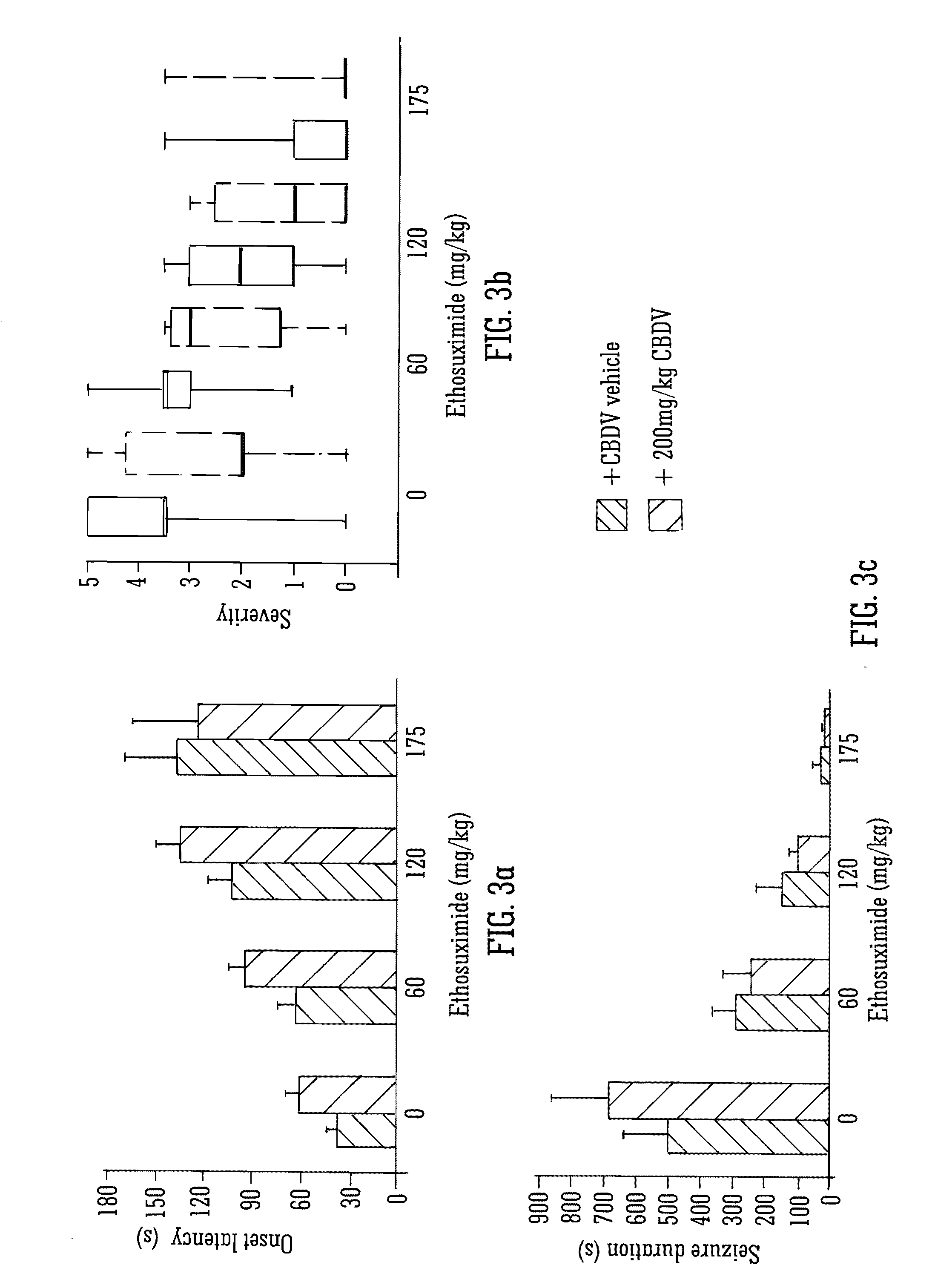
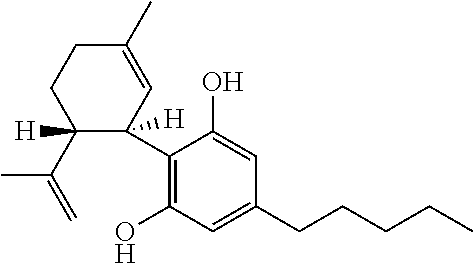
Use of the phytocannabinoid cannabidivarin (CBDV) in the treatment of epilepsy
ActiveUS20120004251A1Well side effect profileGrowth inhibitionBiocideNervous disorderPhenobarbitalDrug
This invention relates to the use of the phytocannabinoid cannabidivarin (CBDV) and combinations of the phytocannabinoid CBDV with tetrahydrocannabivarin (THCV) and cannabidiol (CBD) in the treatment of epilepsy. The invention further relates to the use of the phytocannabinoid CBDV in combination with standard anti-epileptic drugs (SAEDs). Preferably the SAED is one of ethosuximide, valproate or phenobarbital.
Owner:GW PHARMA LTD +1
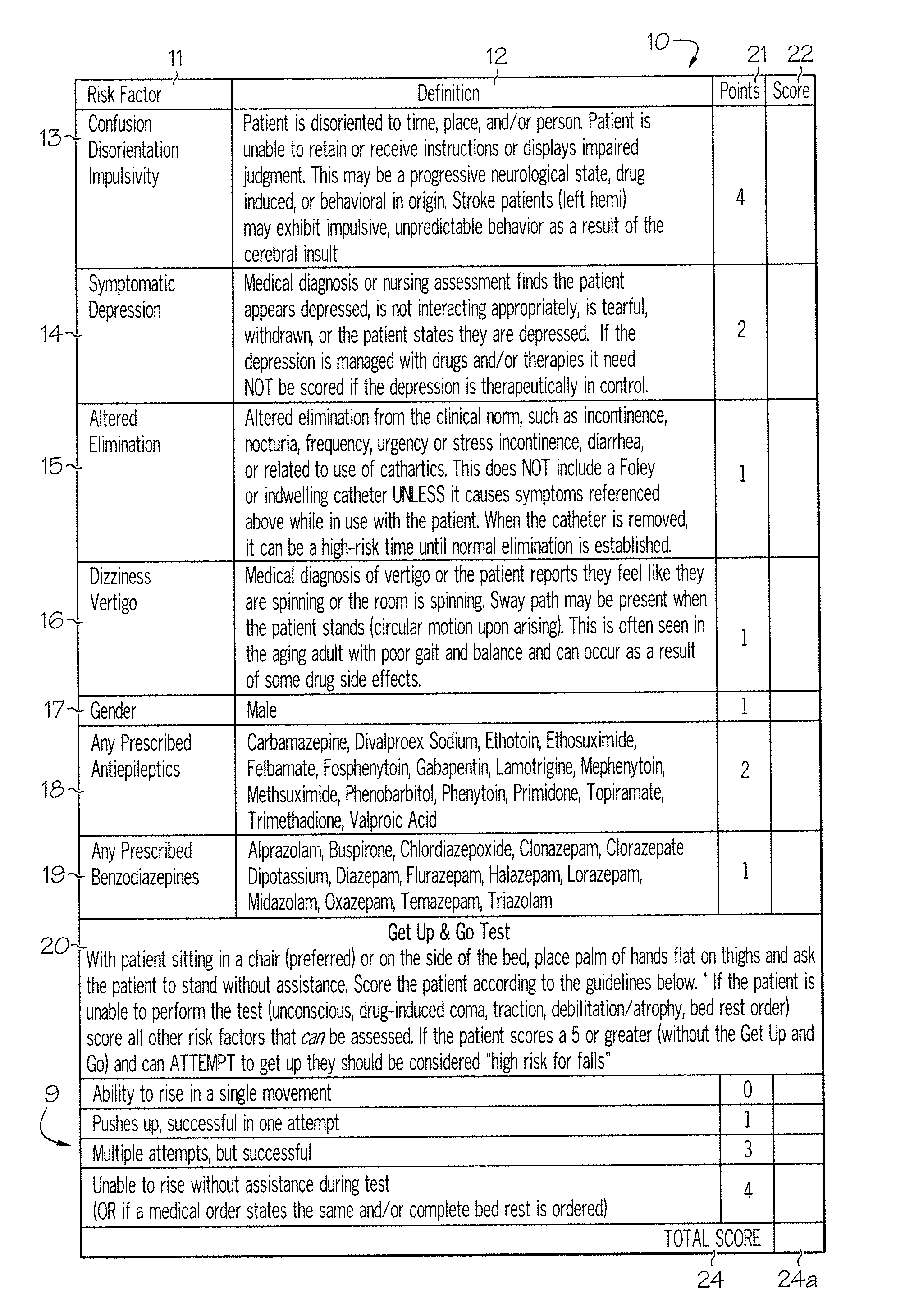
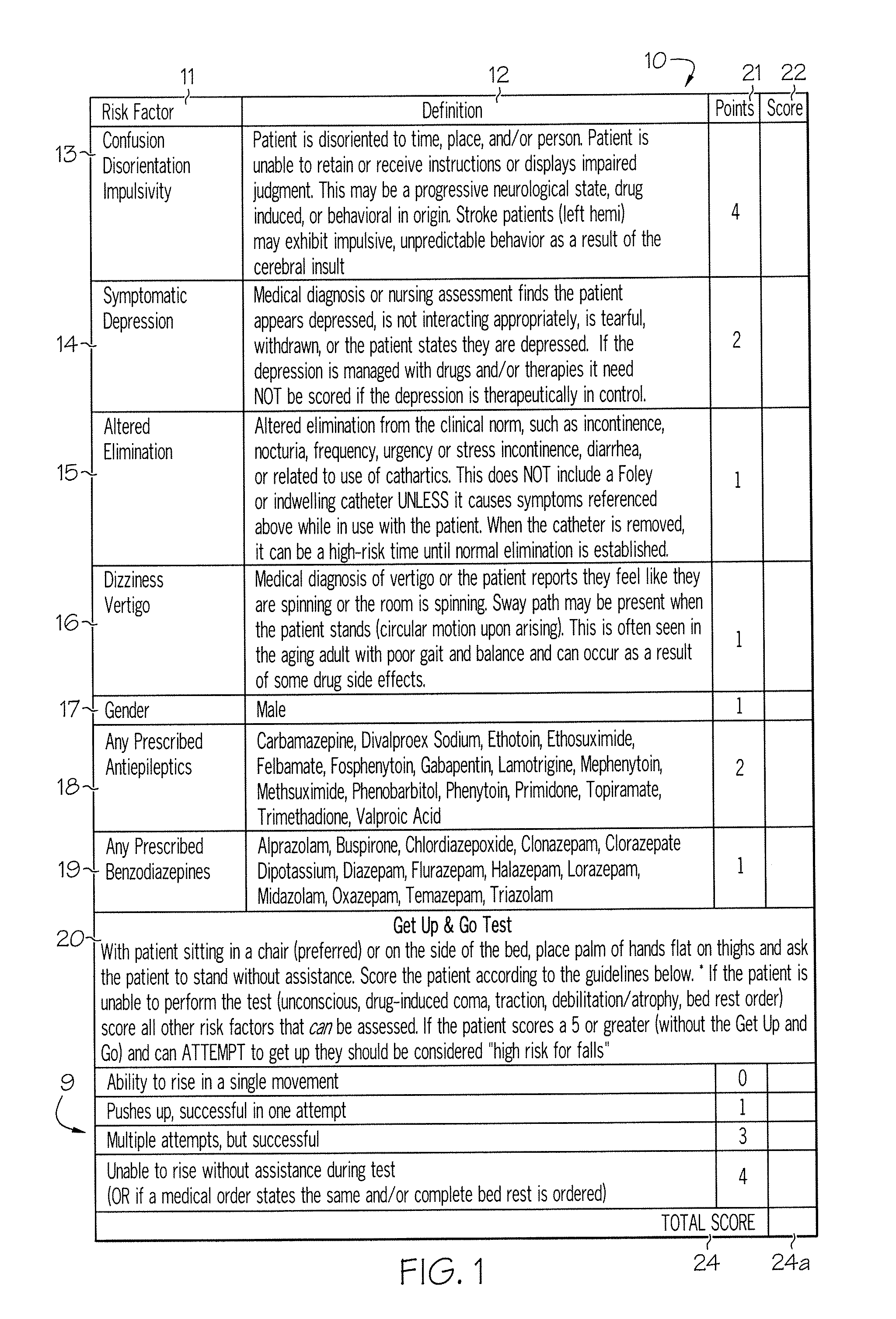
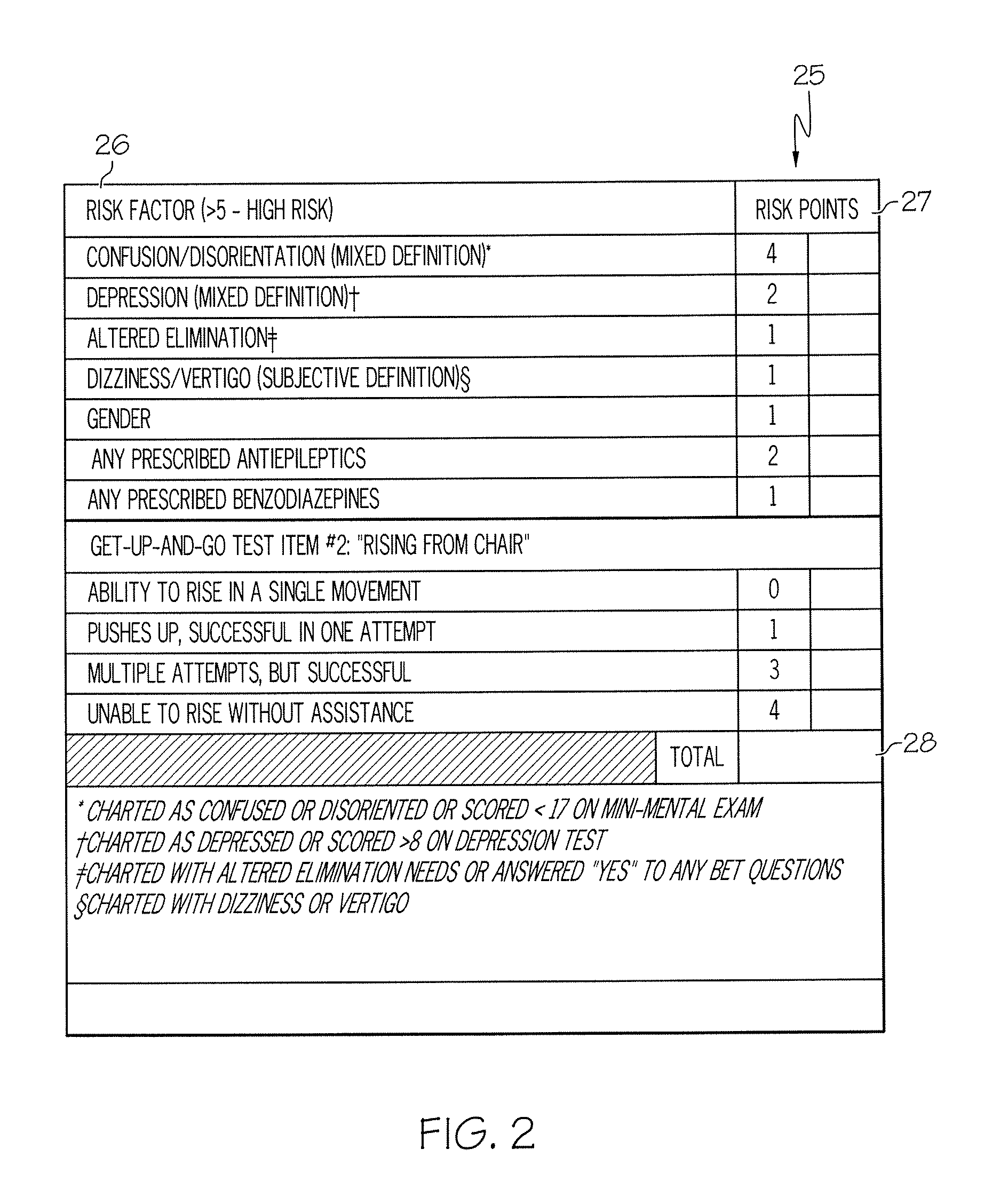
Method and system for assessing fall risk
ActiveUS20080009686A1Easy to addAccurate predictionData processing applicationsTherapiesBenzodiazepineTotal risk
A method and system for determining the fall risk of a patient is provided. The method includes the evaluation of a patient to determine whether the patient exhibits one or more intrinsic fall risk factors selected from a group consisting of confusion, depression, altered elimination, dizziness, male gender, antiepileptic / anticonvulsant prescriptions and benzodiazepine prescriptions. A specific point value is assigned to each of the intrinsic risk factors found to be exhibited by the patient. A mobility test is also performed on the patient to evaluate the patient's ability to rise from a seated position, and a specific mobility test point value is assigned to the patient based upon the patient's performance of the mobility test. Each intrinsic risk factor's specific point value is then summed together with the specific mobility test point value to achieve a total risk score, and the patient's fall risk is determined based on the total risk score. An intervention process may be developed for the patient based on the patient's fall risk.
Owner:AHI OF INDIANA
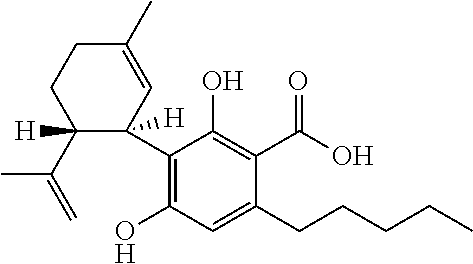
Use of cannabinoids in the treatment of epilepsy
The present disclosure relates to the use of cannabidiol (CBD) in the treatment of absence seizures. In particular, the disclosure relates to the use of CBD for reducing absence seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; CDKL5; Dup15q; Jeavons syndrome; Myoclonic Absence Epilepsy; Neuronal ceroid lipofuscinoses (NCL) and brain abnormalities. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Compositions and methods for treating obesity and related disorders
InactiveUS20080255093A1Reduces unwanted side effectGood for weight lossBiocideNervous disorderSYMPATHOMIMETIC AGENTSSerotonin receptor agonist
The present invention is drawn to combinations of pharmaceutical agents having similar chemical and / or pharmacological properties, wherein the combinations maximize the therapeutic effect of the drug while minimizing their adverse effects. The methods and compositions of the invention are particularly useful in the treatment of obesity and related conditions which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) or bupropion in combination with an anti-epileptic agent (e.g., topiramate, zonisamide), CB1 antagonists (e.g., rimonabant), or a 5HT2C-selective serotonin receptor agonist, (e.g., lorcaserin) for the treatment of obesity and related conditions. The invention also features kits for use in the practice of these novel therapies.
Owner:VIVUS
Use of cannabinoids in the treatment of epilepsy
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of atonic seizures. In particular the CBD appears particularly effective in reducing atonic seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; Aicardi syndrome; CDKL5 and Dup15q in comparison to other seizure types. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
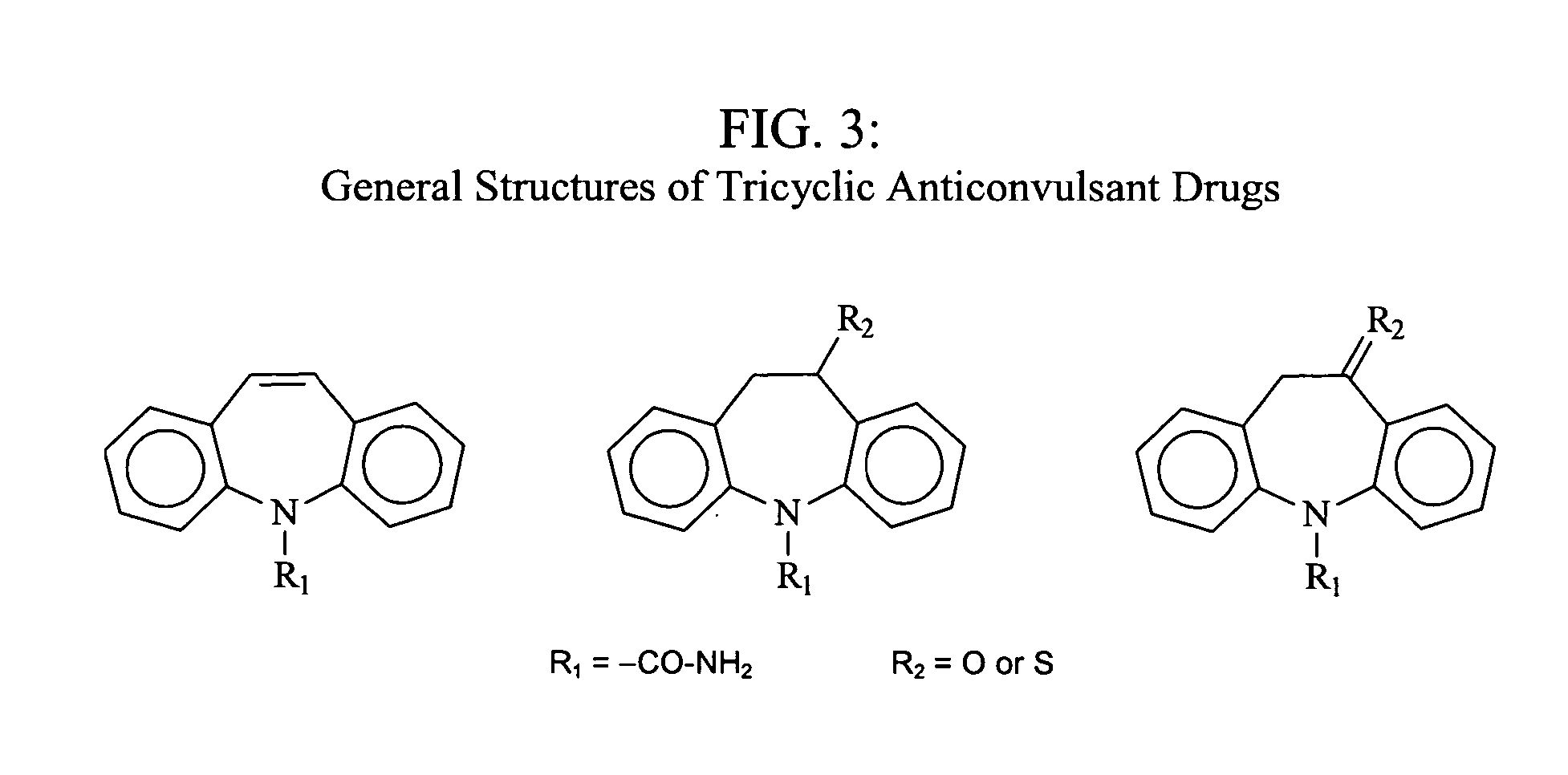
Topical anesthetic formulation
The topical medicament gel formulation of the present invention includes an anesthetic, an anti-microbial, an oxidant, a nutrient, a diuretic, an opioid, an anti-emetic, an anti-seizure drug, and a non-steroidal anti-inflammatory drug (NSAID), USP in a molecular, as opposed to a salt form, as the active ingredient. Additional constituents illustratively include a skin penetration enhancer and a gelling agent. This invention deals with problems commonly associated with topical application of local medicaments such as: slow onset of action; need for occlusion; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the medicament and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:WEPFER SCOTT
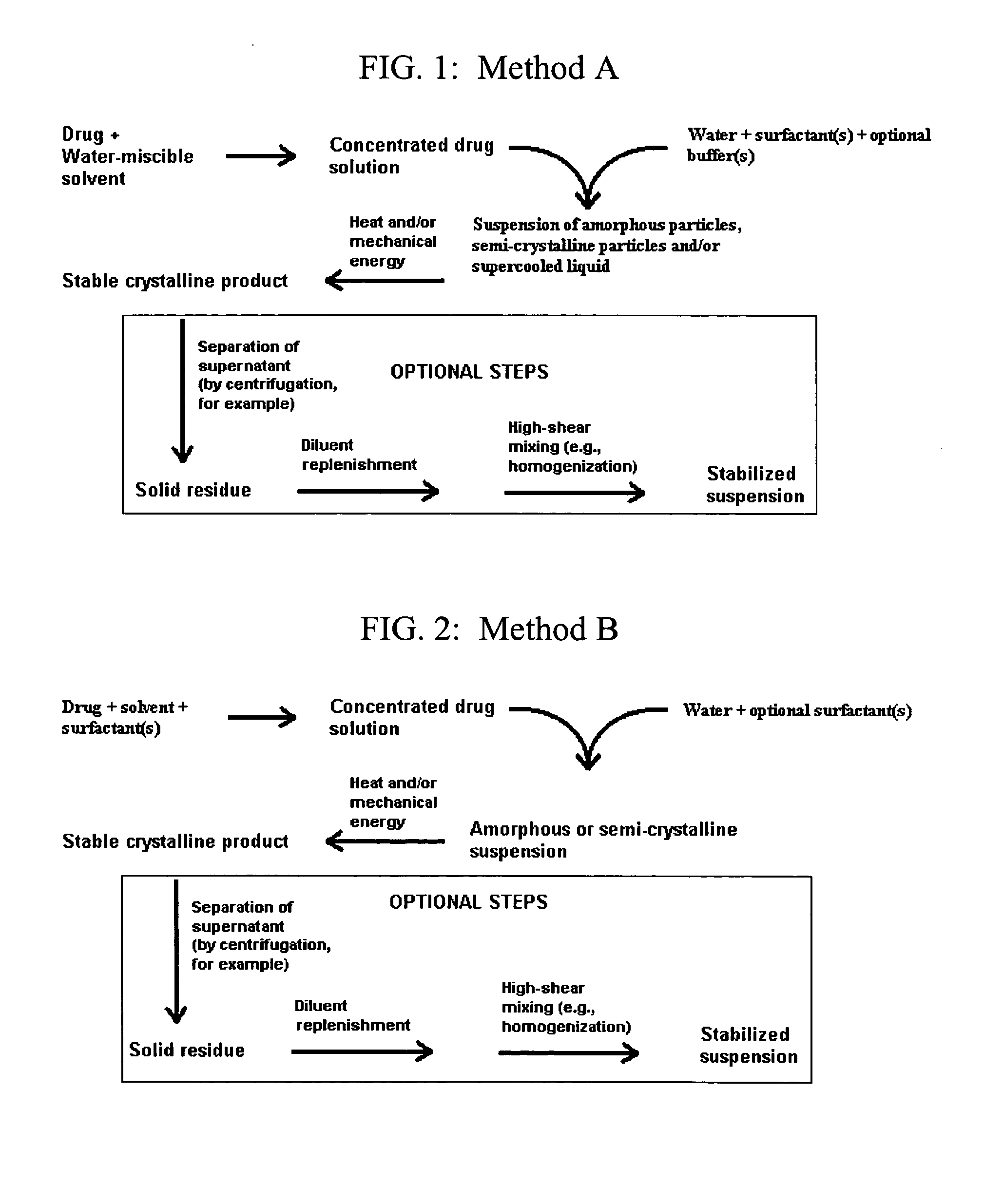
Small-particle pharmaceutical formulations of antiseizure and antidementia agents and immunosuppressive agents
InactiveUS20050244503A1High drug loadingMinimize side effectsPowder deliveryCyclic peptide ingredientsMedicineCyclosporins
This invention pertains to the formulation of small-particle suspensions of anticonvulsants and antidementia, particularly carbamazepine, for pharmaceutical use. This invention also pertains to the formulation of small-particle suspensions of immunosuppressive agents, particularly cyclosporin, for pharmaceutical use.
Owner:BAXTER INT INC +1
Co-therapy for the treatment of epilepsy and related disorders
The present invention is directed to co-therapy for the treatment of epilepsy and related disorders comprising administering to a subject in need thereof, co-therapy with a therapeutically effective amount of a benzo-fused heterocycle sulfamide derivative and a therapeutically effective amount of one or more anticonvulsant and / or anti-epileptic agents.
Owner:JANSSEN PHARMA NV
Co-therapy for the treatment of epilepsy and related disorders
The present invention is directed to a method for the treatment of epilepsy and related disorders comprising administering to a subject in need thereof, co-therapy with a therapeutically effective amount of a benzo-heteroaryl sulfamide derivative as described herein and a therapeutically effective amount of one or more anticonvulsant and / or anti-epileptic agents.
Owner:SMITH SWINTOSKY VIRGINIA L +3
Methods and compositions for the treatment of amyloid-and epileptogenesis-associated diseases
InactiveUS20050038000A1Neurodegeneration and reduced and inhibitedToxicity reduced and inhibitedBiocideOrganic chemistryConvulsionDisease
Methods of treating or preventing an amyloid-related disease in a subject by administering to a subject a therapeutic amount of a compound of the invention are described. Also included are methods for inhibiting epileptogenesis in a subject, by administering to a subject an effective amount of an anti-epileptogenic agent. Methods for treating a subject suffering from an epileptogenesis-associated condition, by administering to the subject an effective amount of an anti-epileptogenic agent are also included. Methods for treating convulsions in a subject by administering to the subject an effective amount of a therapeutic amount of a compound of the invention are also described.
Owner:BELLUS HEALTH (INT) LTD (CH)
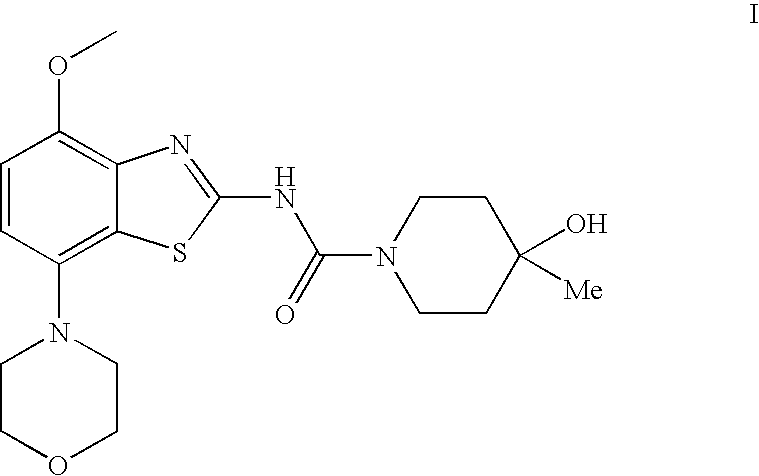
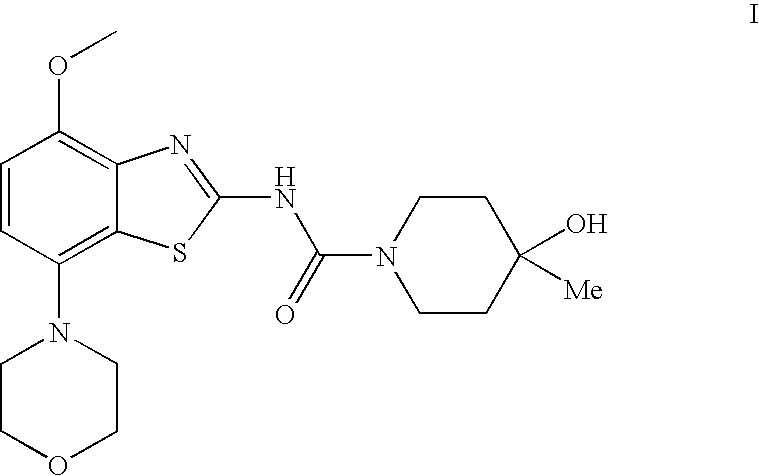
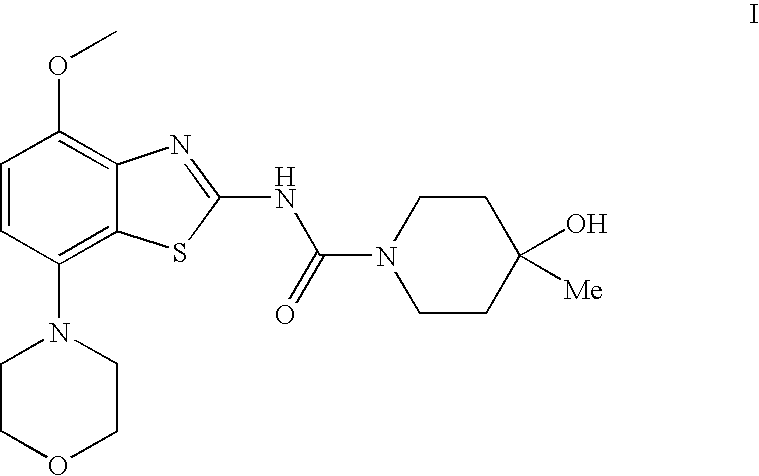
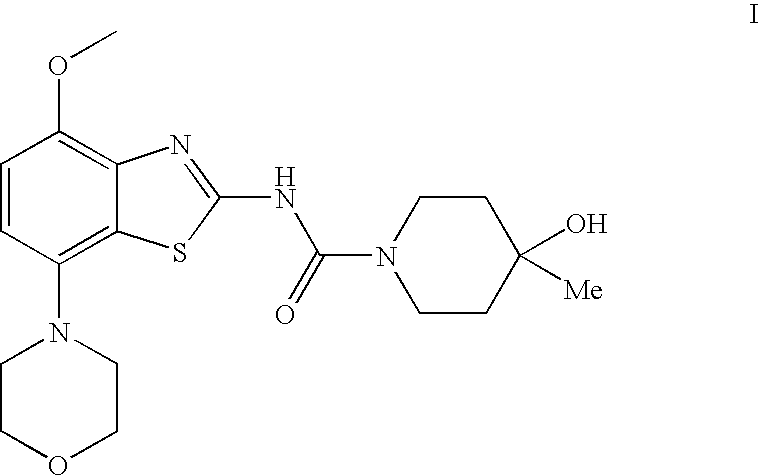
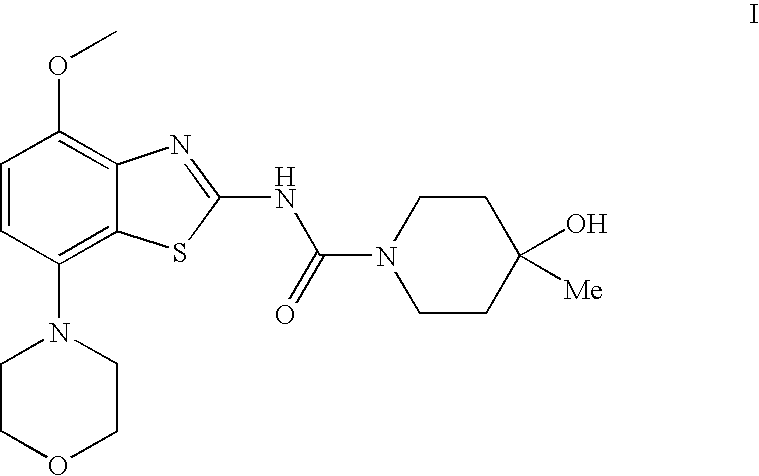
4-Hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide
ActiveUS20050261289A1High affinityOrganic active ingredientsNervous disorderSubstance abuserDisease cause
The present invention relates to the compound of formula which is 4-hydroxy-4-methyl-piperidine-1-carboxylic acid(4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide, and to pharmaceutically acceptable acid addition salts thereof. It has been found that the compound is useful for the treatment or prevention of Alzheimer's disease, Parkinson's disease, Huntington's disease, neuroprotection, schizophrenia, anxiety, pain, respiration deficits, depression, ADHD (attention deficit hyper-activity disorder), drug addiction to amphetamines, cocaine, opioids, ethanol, nicotine, or cannabinoids, or for the treatment of asthma, allergic responses, hypoxia, ischemia, seizure, substance abuse, or for use as muscle relaxants, antipsychotics, antiepileptics, anticonvulsants and cardioprotective agents.
Owner:F HOFFMANN LA ROCHE INC
Effective therapy for epilepsies
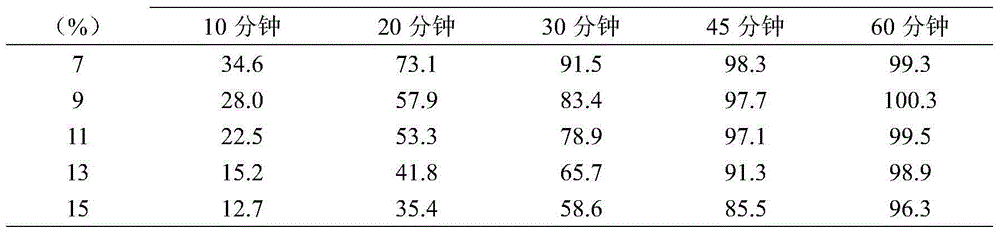
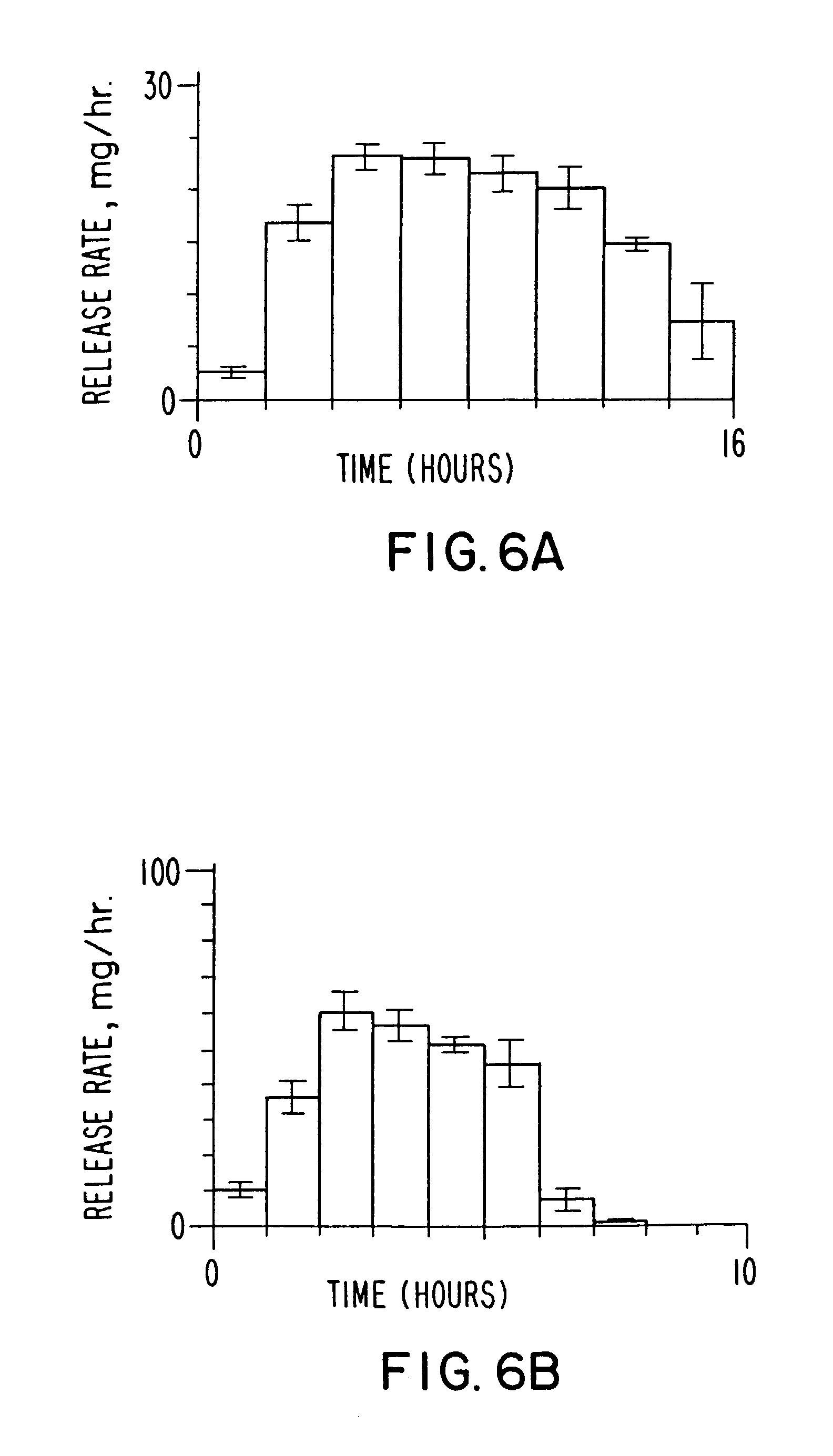
InactiveUS20030056896A1Simultaneously-avoiding toxic rangeExclude influenceLayered productsInorganic adhesivesAntiepileptic drugDosage form
A dosage form is disclosed for delivering an antiepileptic drug, which dosage form comprises for maintaining the integrity of the dosage form and of the antiepileptic drug.
Owner:JAO FRANK +4
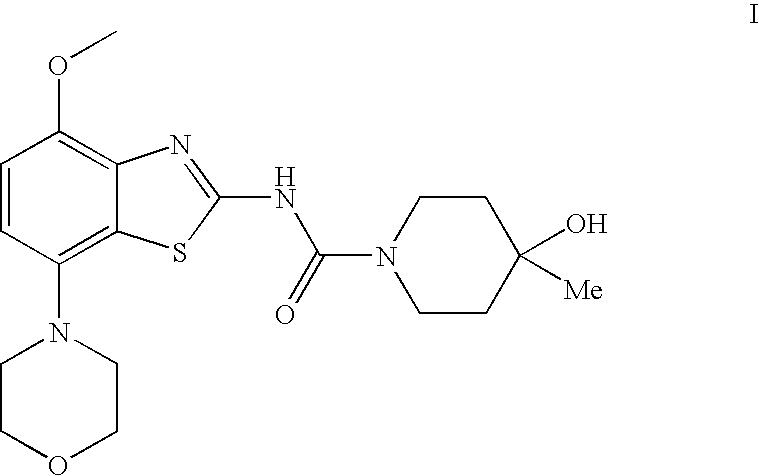
4-hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide
The present invention relates to the compound of formulawhich is 4-hydroxy-4-methyl-piperidine-1-carboxylic acid(4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide, and to pharmaceutically acceptable acid addition salts thereof. It has been found that the compound is useful for the treatment or prevention of Alzheimer's disease, Parkinson's disease, Huntington's disease, neuroprotection, schizophrenia, anxiety, pain, respiration deficits, depression, ADHD (attention deficit hyper-activity disorder), drug addiction to amphetamines, cocaine, opioids, ethanol, nicotine, or cannabinoids, or for the treatment of asthma, allergic responses, hypoxia, ischemia, seizure, substance abuse, or for use as muscle relaxants, antipsychotics, antiepileptics, anticonvulsants and cardioprotective agents.
Owner:F HOFFMANN LA ROCHE INC
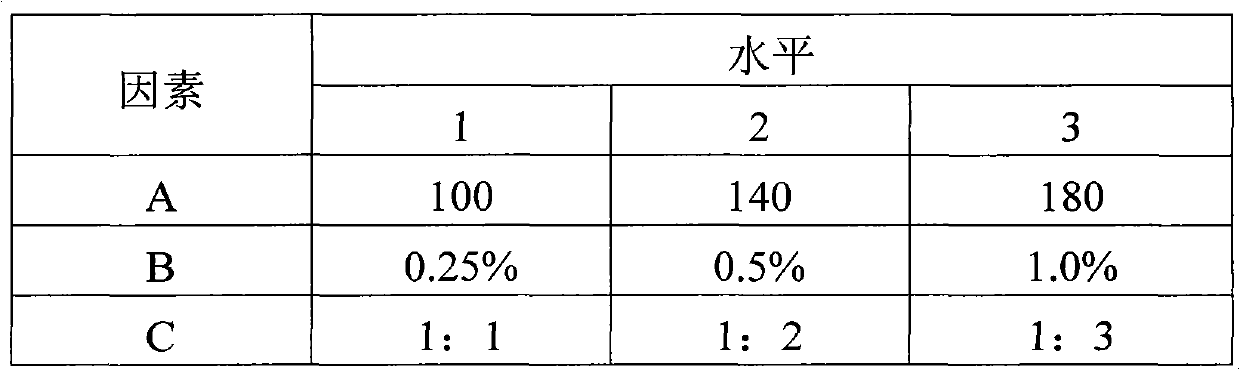
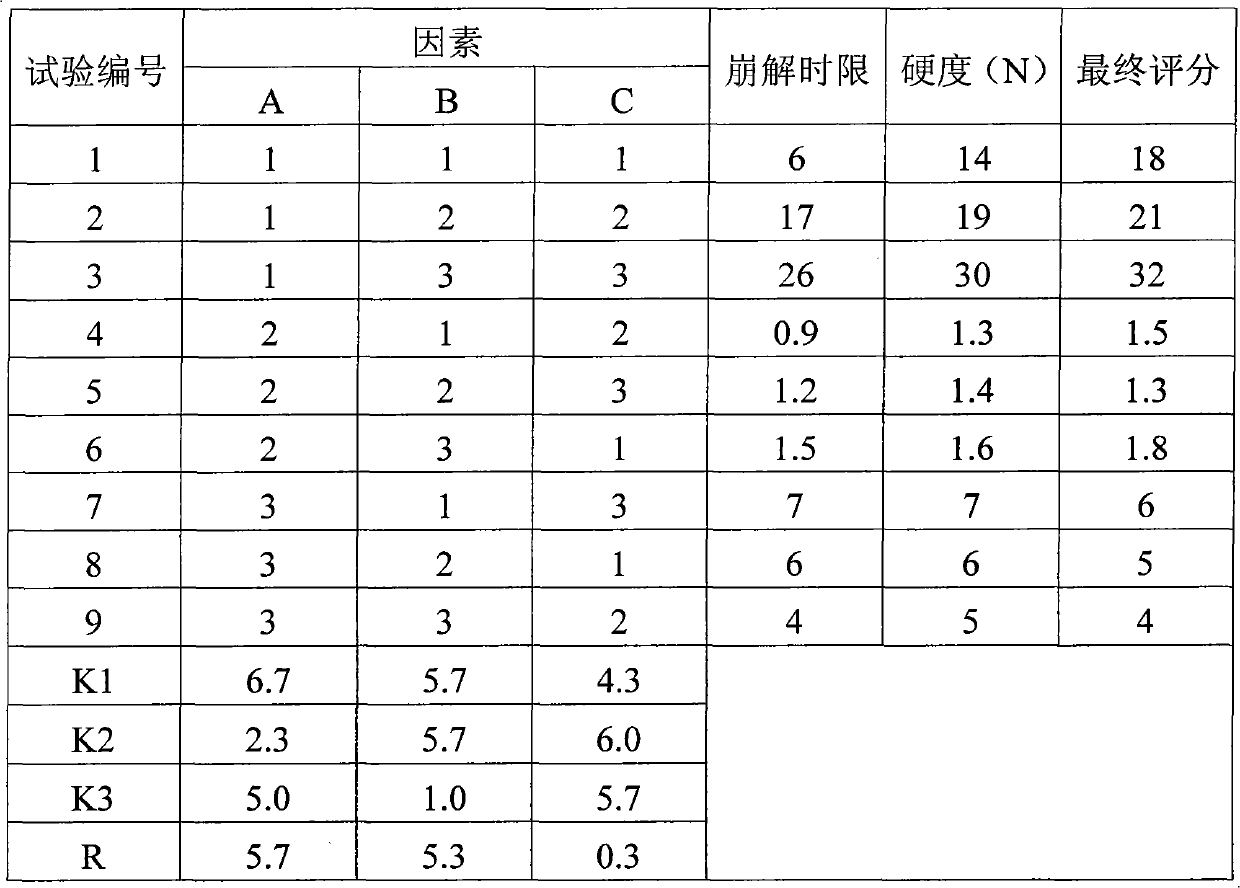
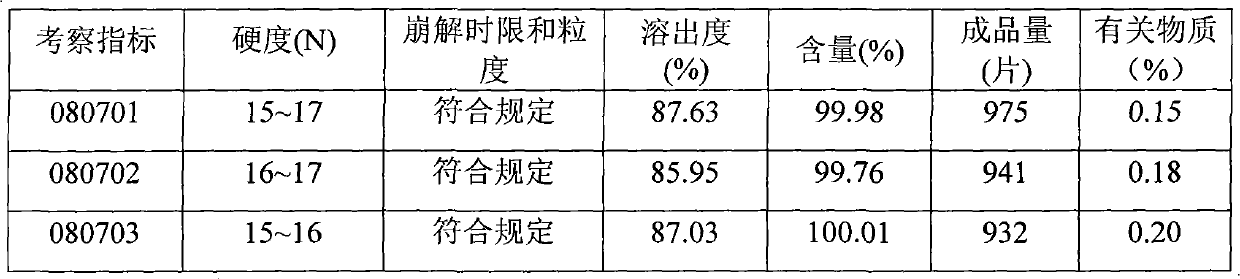
Levetiracetam oral disintegrating tablet and preparation method thereof
ActiveCN102085194AOrganic active ingredientsNervous disorderOrally disintegrating tabletAdditive ingredient
The invention provides a levetiracetam oral disintegrating tablet and a preparation method thereof. The levetiracetam oral disintegrating tablet is prepared by adopting a freeze drying process and contains levetiracetam as an active constituent, polyethylene glycol 6000 as a solid dispersing agent, maltodextrin as a freeze-drying protective agent and hydrolyzed gelatin as an adhesive. The levetiracetam is a novel antiepileptic drug, and the levetiracetam oral disintegrating tablet provided by the invention has the advantage of improving the medication compliance of patients and the curative effect.
Owner:BEIJING YILING BIOENG
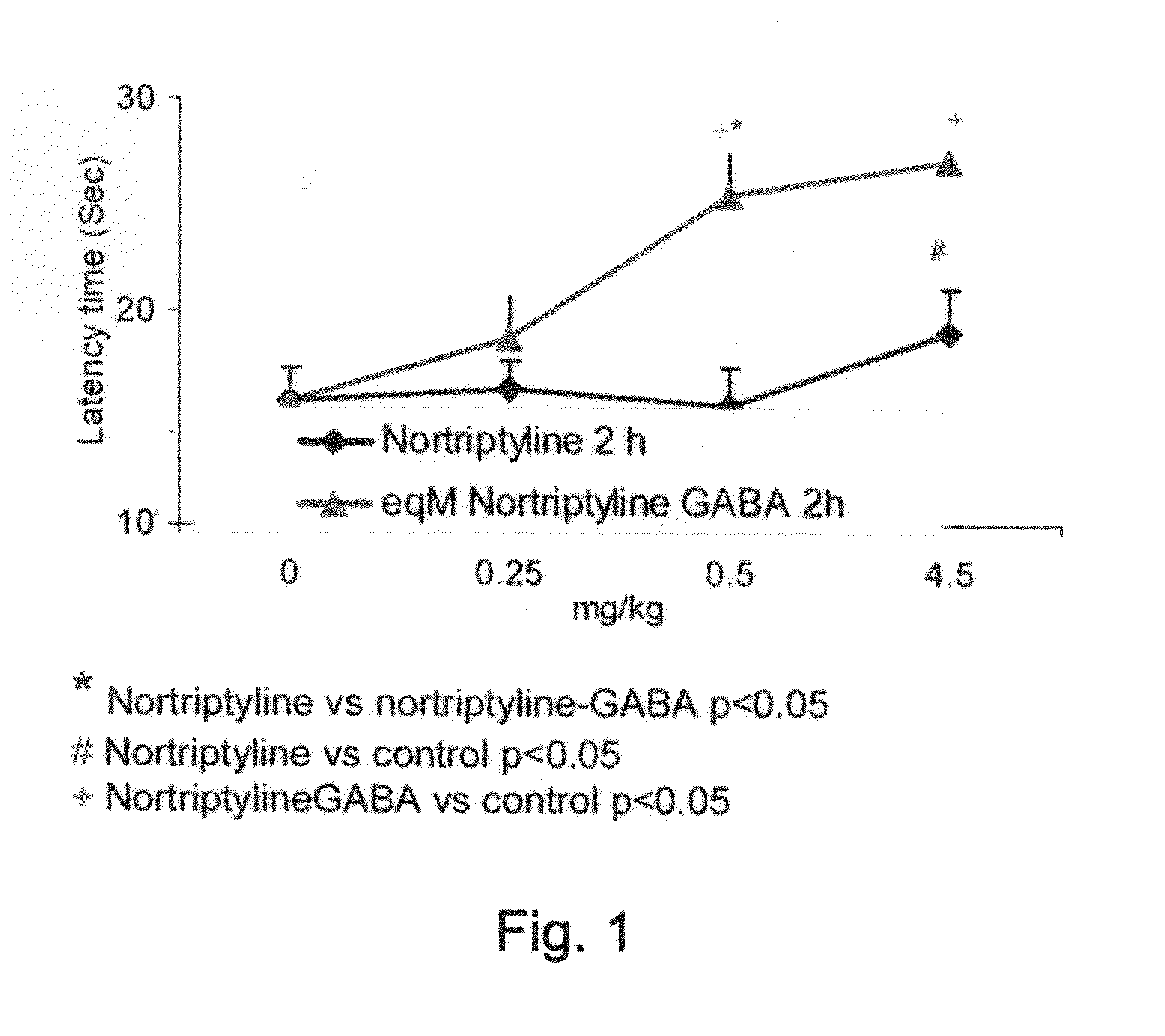
Conjugates Comprising a gaba-or glycine compound, pharmaceutical compositions and combinations thereof as well as their use in treating cns disorders
InactiveUS20100144869A1Strong therapeutic activityAccelerated onset of their protective effectBiocideNervous disorderDiseaseOrganic acid
Owner:RAMOT AT TEL AVIV UNIV LTD +1
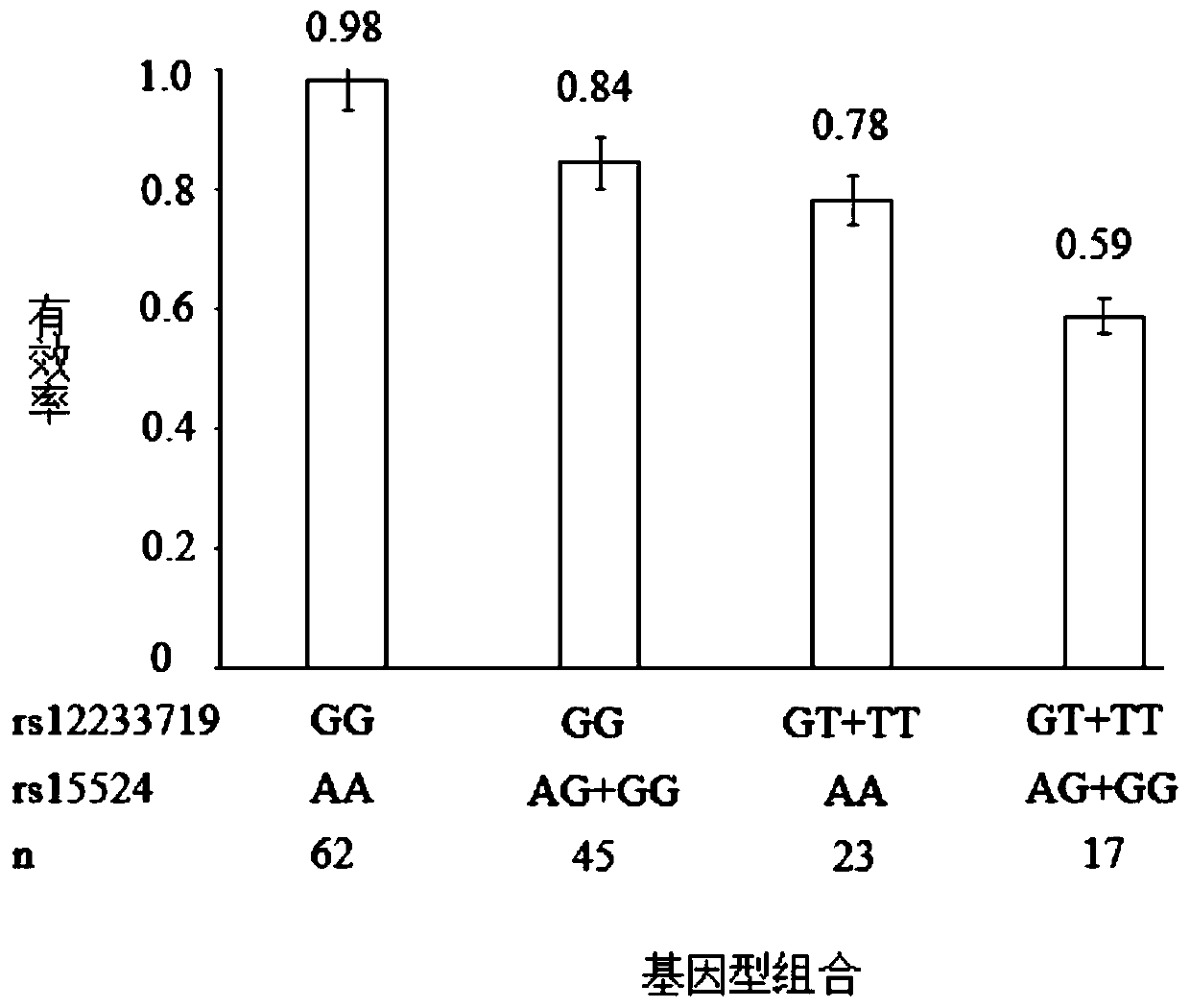
Genetic predictor for clinical use of drugs used in the treatment of neurological conditions
InactiveUS20060269935A1Affects proportionNervous disorderMicrobiological testing/measurementDiseaseNervous system
A method for determining the dosage regime of a drug suitable for use in the treatment of a neurological condition in a subject, which method comprises typing the SCN1A gene of the subject. The method may be used to determine the dosage regime of an anti-epileptic drug (AED) in a subject. A subject may be treated in accordance with the dosage regime determined using such a method.
Owner:UCL BUSINESS PLC
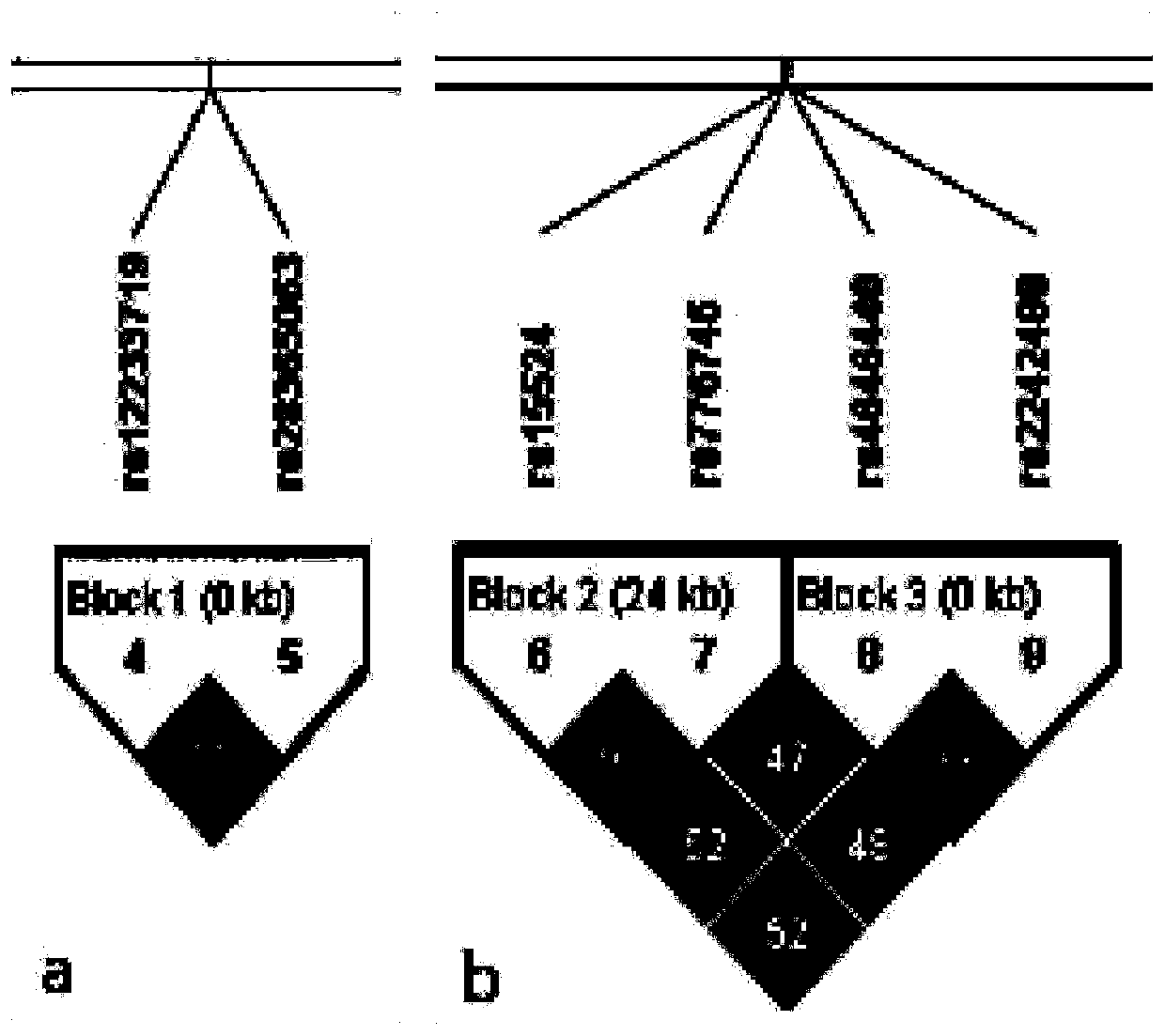
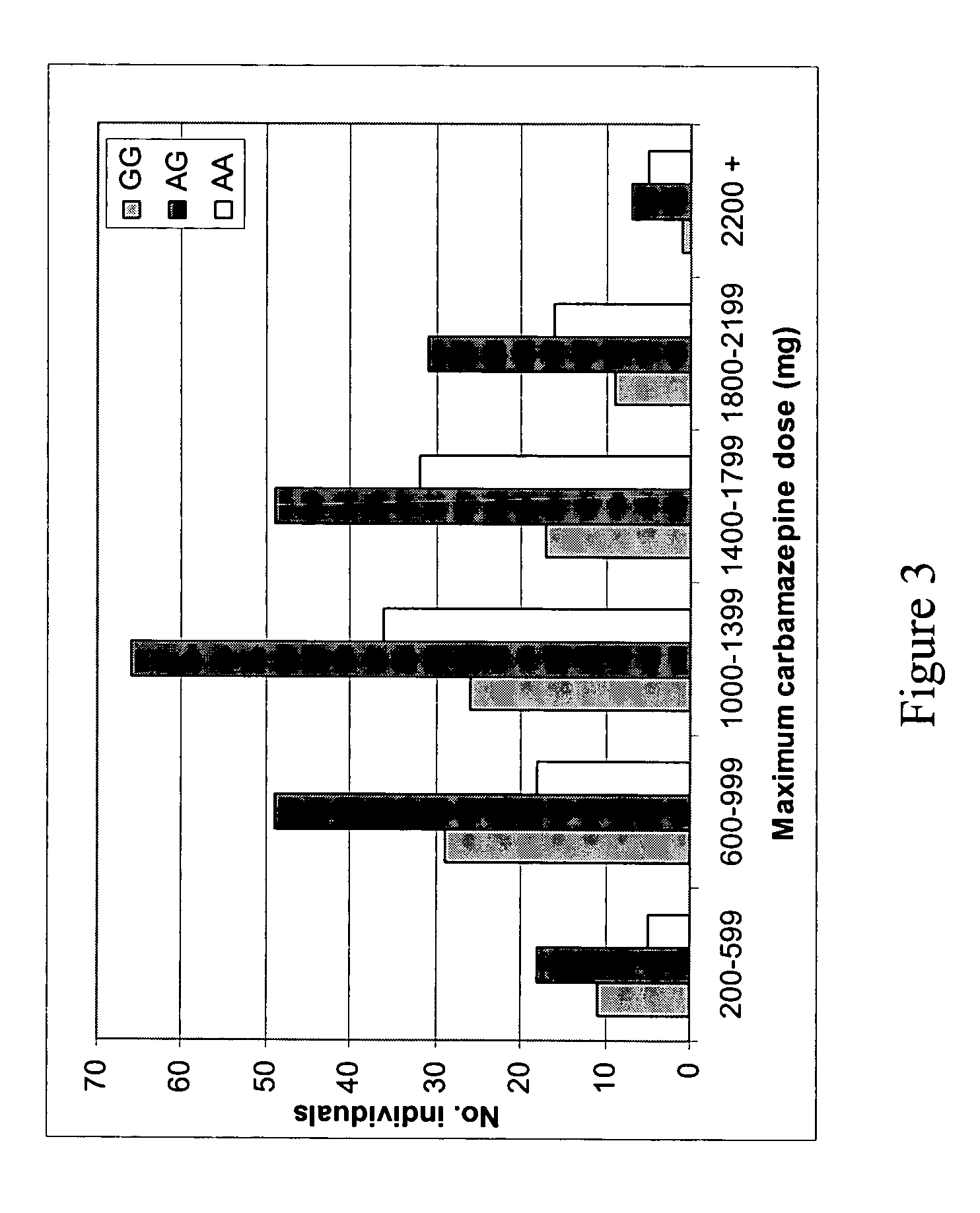
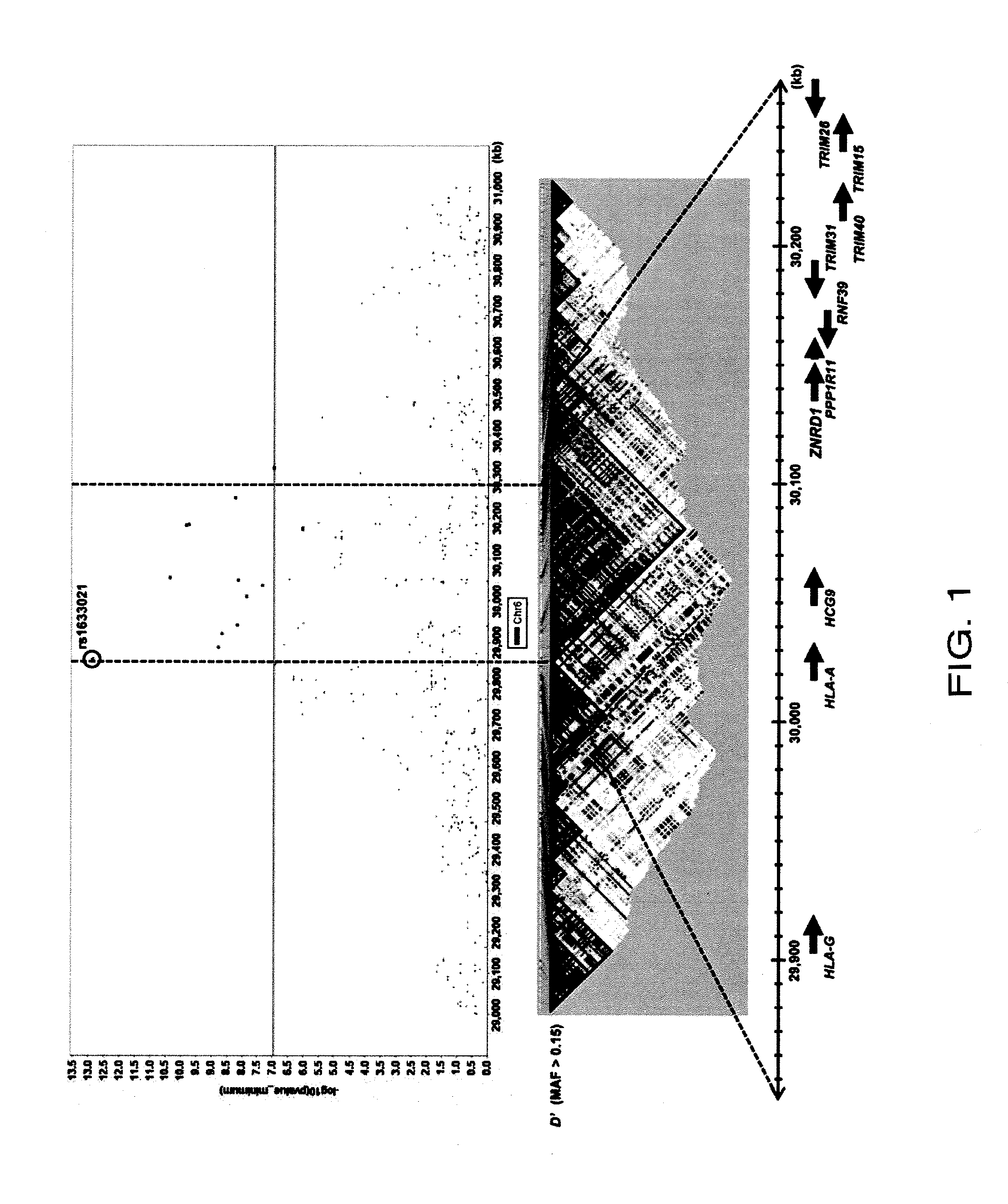
Method for diagnosing drug eruption risk induced by antiepileptic drug based on single nucleotide polymorphism in 21.33 region of short arm of chromosome 6
InactiveUS20120238455A1Efficient managementNervous disorderSugar derivativesAntiepileptic drugDrug eruption
The genotype of a single nucleotide polymorphism (SNP) existing in the 21.33 region of the short arm of chromosome 6, such as an SNP at the HLA-A locus, is analyzed, and, based on the results, drug eruption risk induced by an antiepileptic drug is diagnosed.
Owner:RIKEN
Use of cannabidiol in the treatment of epilepsy
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of Tuberous Sclerosis Complex (TSC). In particular the TSC is treatment resistant and is characterised by generalised seizures or focal seizures with impairment. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
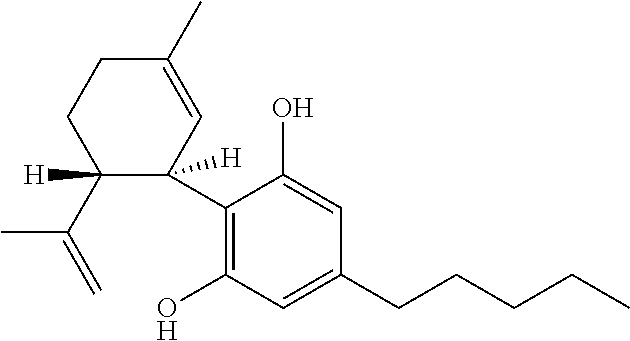
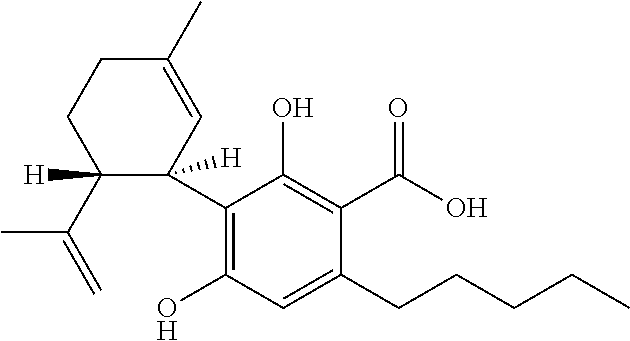
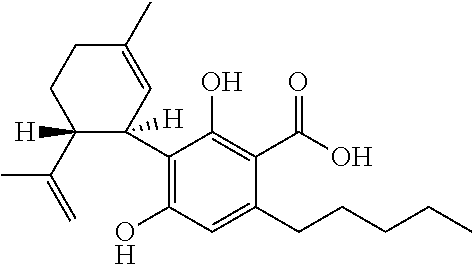
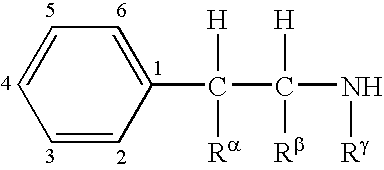
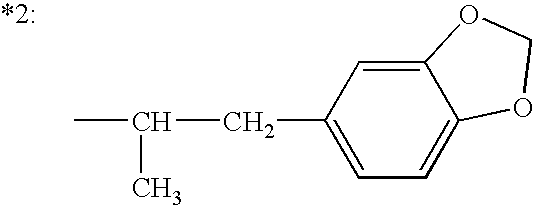
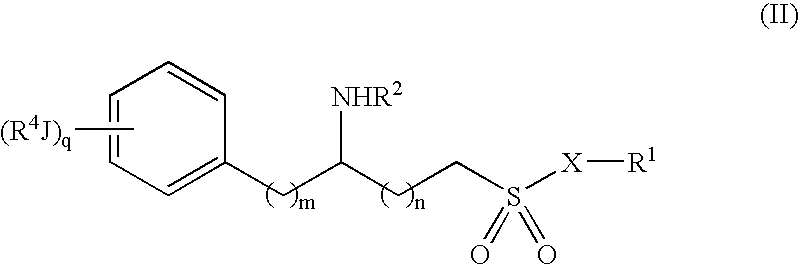
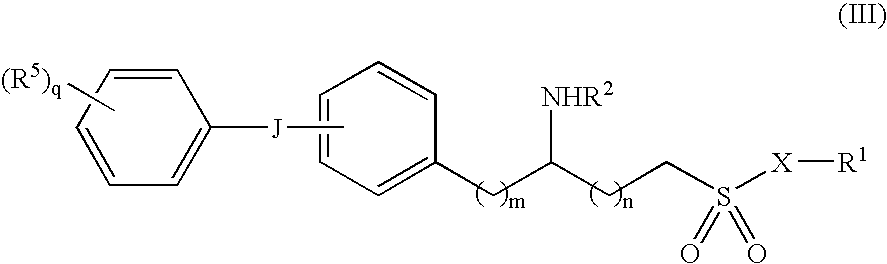
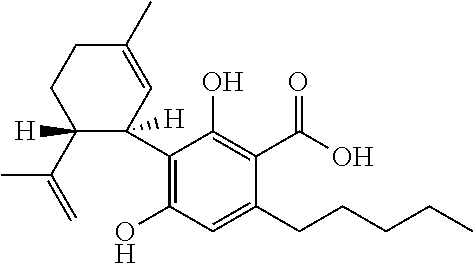
6-(substituted phenoxy)-tetrazolo[5,1-a] phthalazine derivatives as antuepileptics and their pharmaceutically acceptable salts
ActiveCN101676287ALow toxicityOrganic active ingredientsNervous disorderMedicinal chemistryAddition reaction
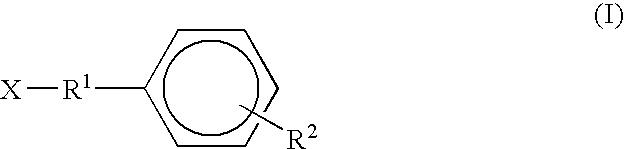
A compound represented by a general formula I, and salts formed by the addition reaction of the same and the pharmaceutically acceptable acids, wherein, R is selected from various halogenated alkanes.The compound represented by the general formula I and salts formed by the addition reaction of the same and the pharmaceutically acceptable acids, provided by the invention, can be used for treatingepilepsy.
Owner:JILIN YINGLIAN SHANGDE SCI & TECH DEV
Solid preparation for treating pediatric epilepsy and preparation method thereof
InactiveCN104922075AMask bad tasteImprove stabilityNervous disorderUnknown materialsActive agentMedicine
The invention discloses a solid preparation for treating pediatric epilepsy and a preparation method thereof. The solid preparation is a dispersed preparation and comprises active agent core particles, wherein the core particles just comprise medicines for treating epilepsy or comprise the medicines for treating epilepsy and one or several excipients. Through the adoption of a taste masking mixture coating comprises the core particles of anti-epileptic medicines, coating particles are formed. The solid preparation is capable of masking the uncomfortable taste, has the function of improving the stability of the medicines, and can be used for solving the problem that children have difficulty in swallowing the bitter medicines.
Owner:黑龙江童医生儿童生物制药有限公司
Use of cannabidiol in the treatment of epilepsy
ActiveUS10765643B2Nervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of seizures associated with Aicardi Syndrome. In one embodiment the seizures associated with Aicardi Syndrome are convulsive seizures; focal seizures with impairment or infantile spasm. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
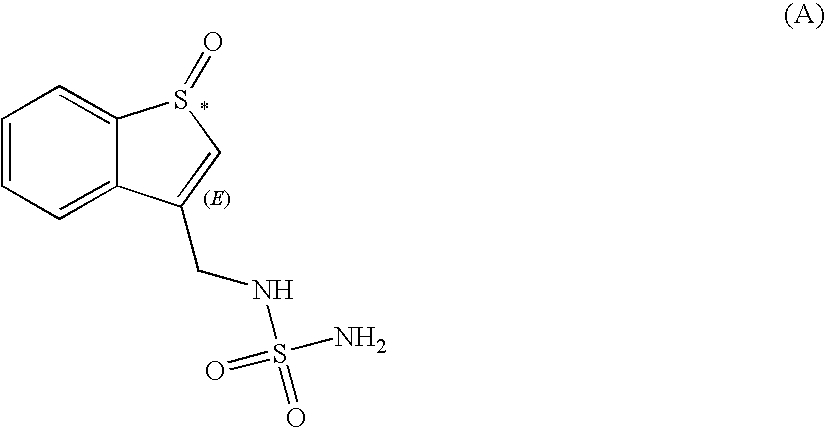
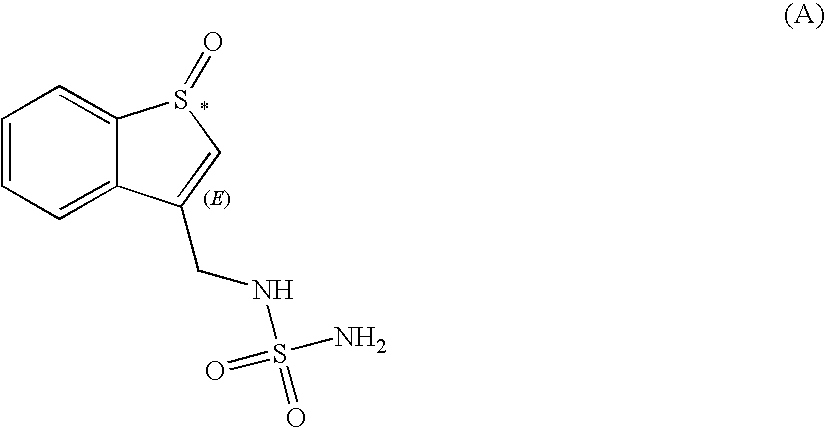
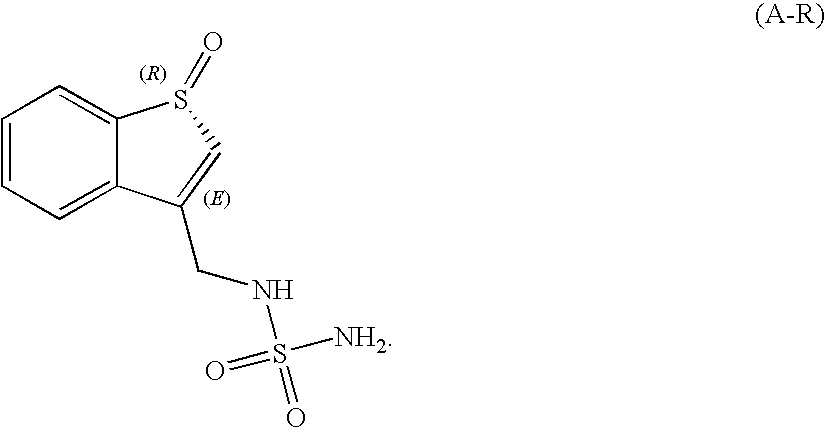
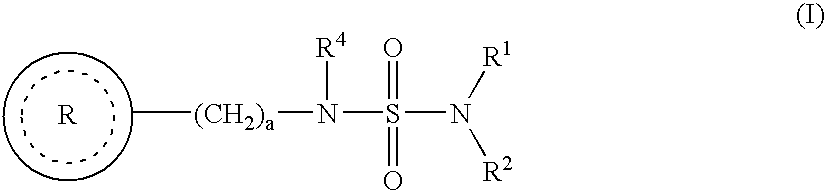
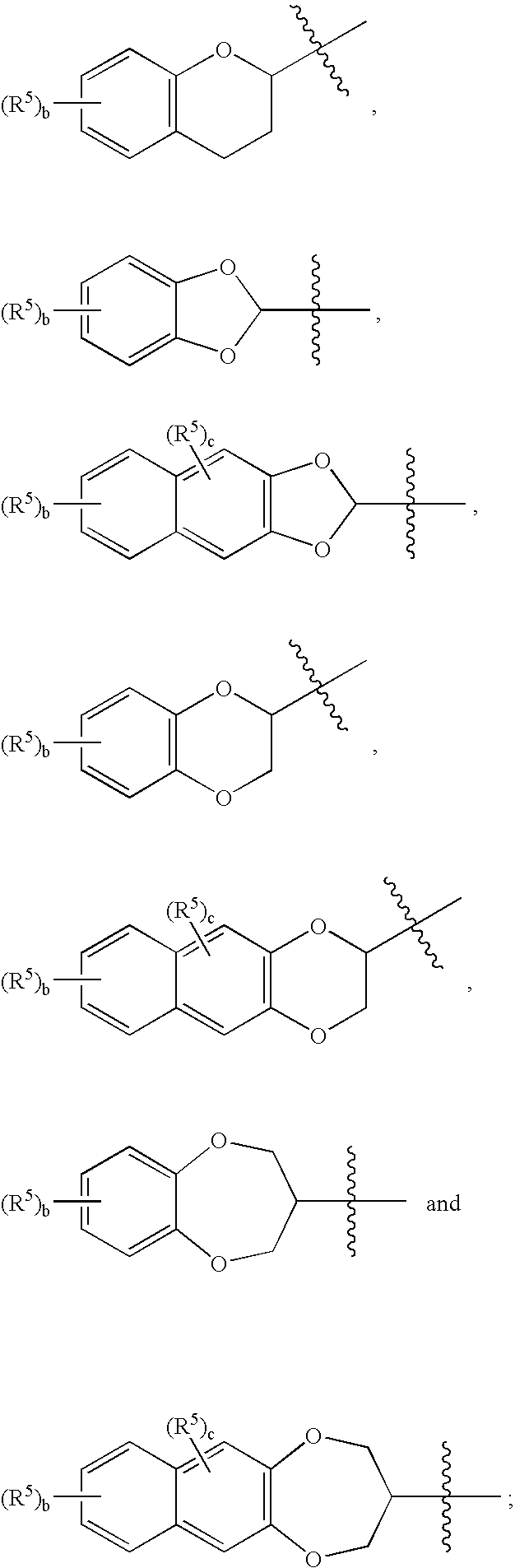
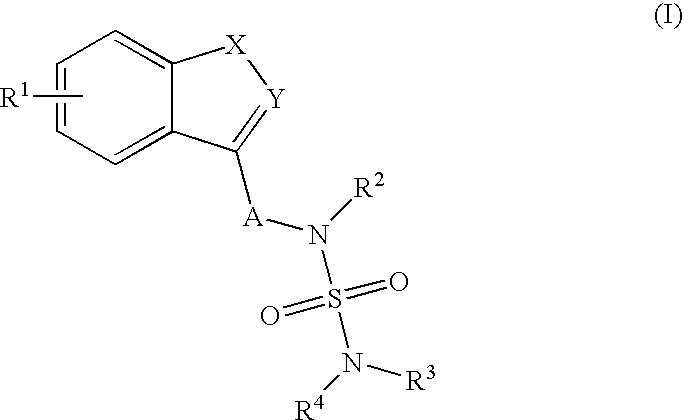
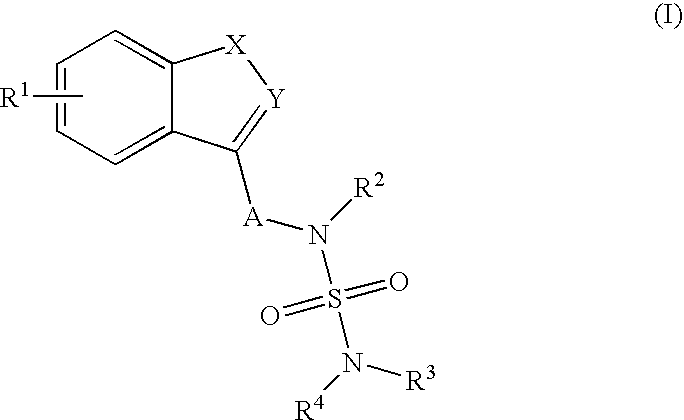
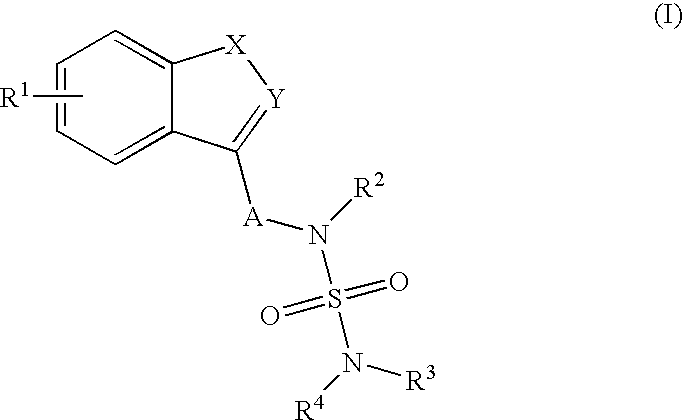
Sulfamide derivative useful for the treatment of epilepsy
The present invention is directed to novel sulfamide derivatives, pharmaceutical compositions comprising said compounds and methods for the treatment of epilepsy and related disorders comprising administering to a subject in need thereof, said compounds, either alone or as co-therapy with one or more anticonvulsant and / or anti-epileptic agents.
Owner:JANSSEN PHARMA NV
Use of cannabidiol in the treatment of epilepsy
ActiveUS11065209B2Reduce in quantityReduce doseNervous disorderDispersion deliveryFocal EpilepsiesAntiepileptic drug
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of Tuberous Sclerosis Complex (TSC). In particular the TSC is treatment resistant and is characterised by generalised seizures or focal seizures with impairment. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
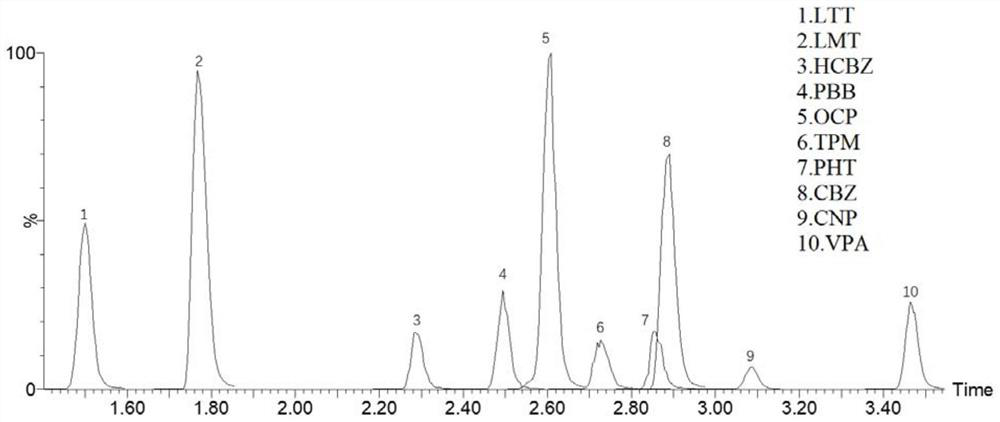
Method for detecting concentration of antiepileptic drug in serum
PendingCN111812216AHigh sensitivityStrong specificityComponent separationAntiepileptic drugPharmaceutical drug
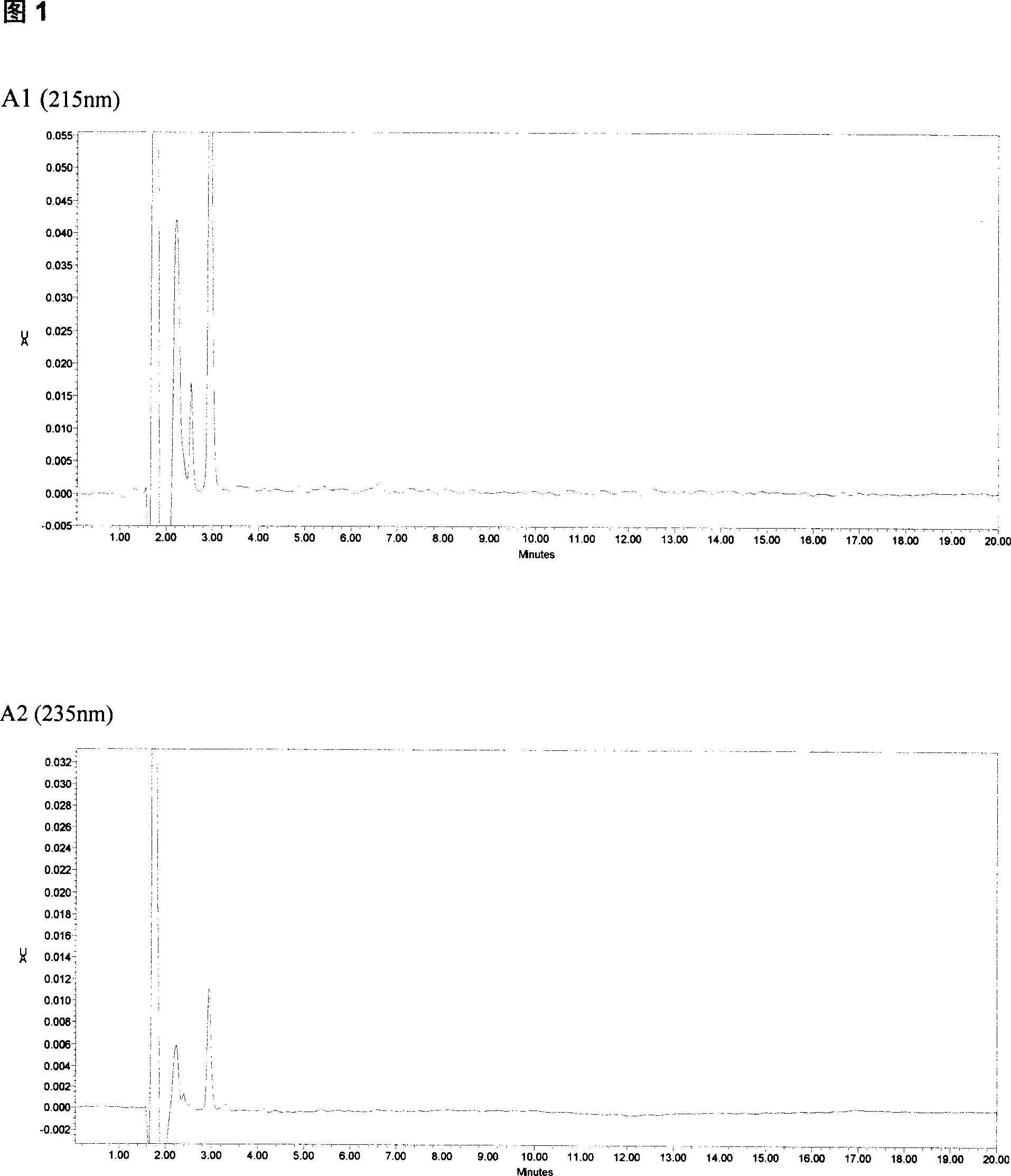
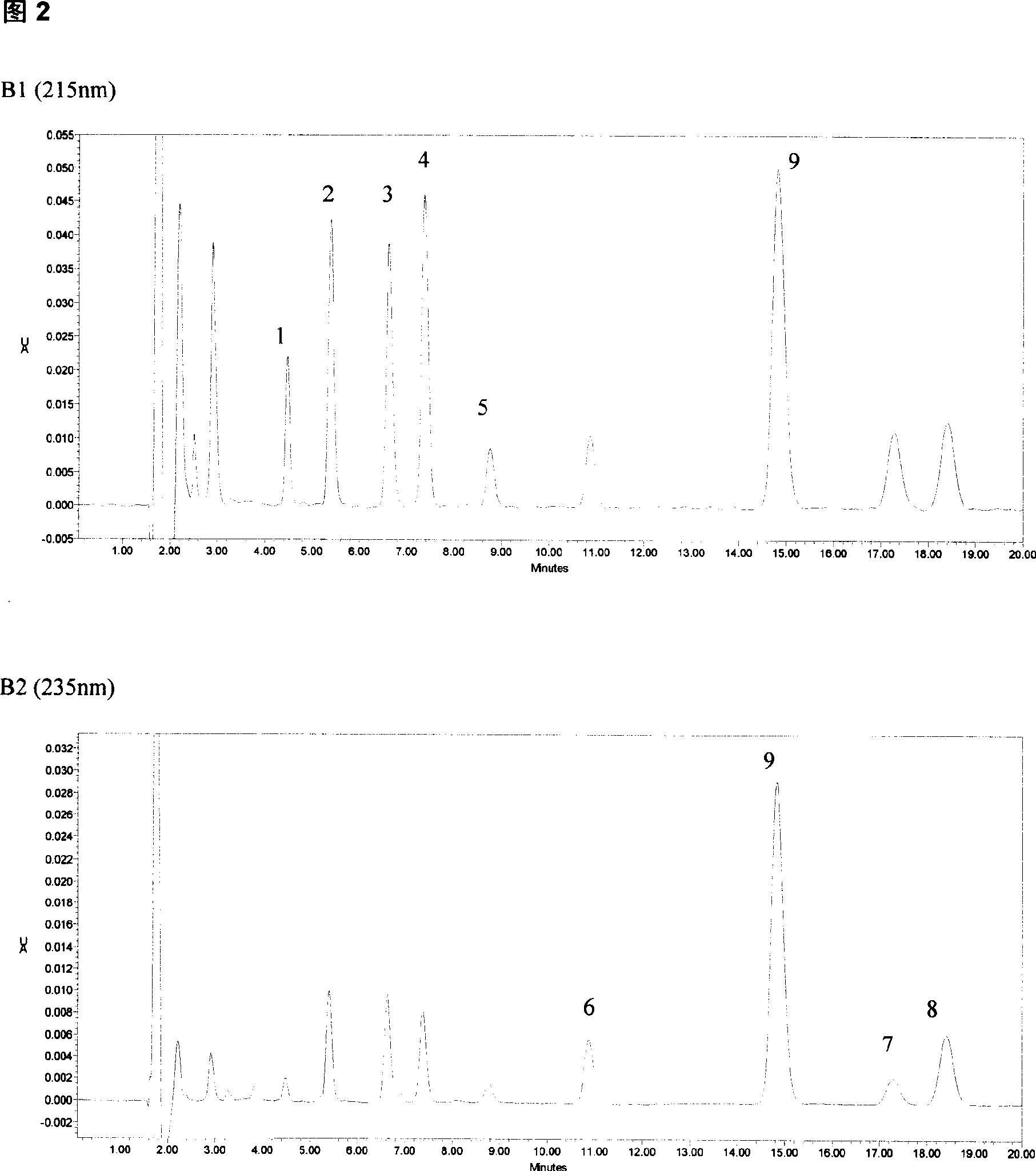
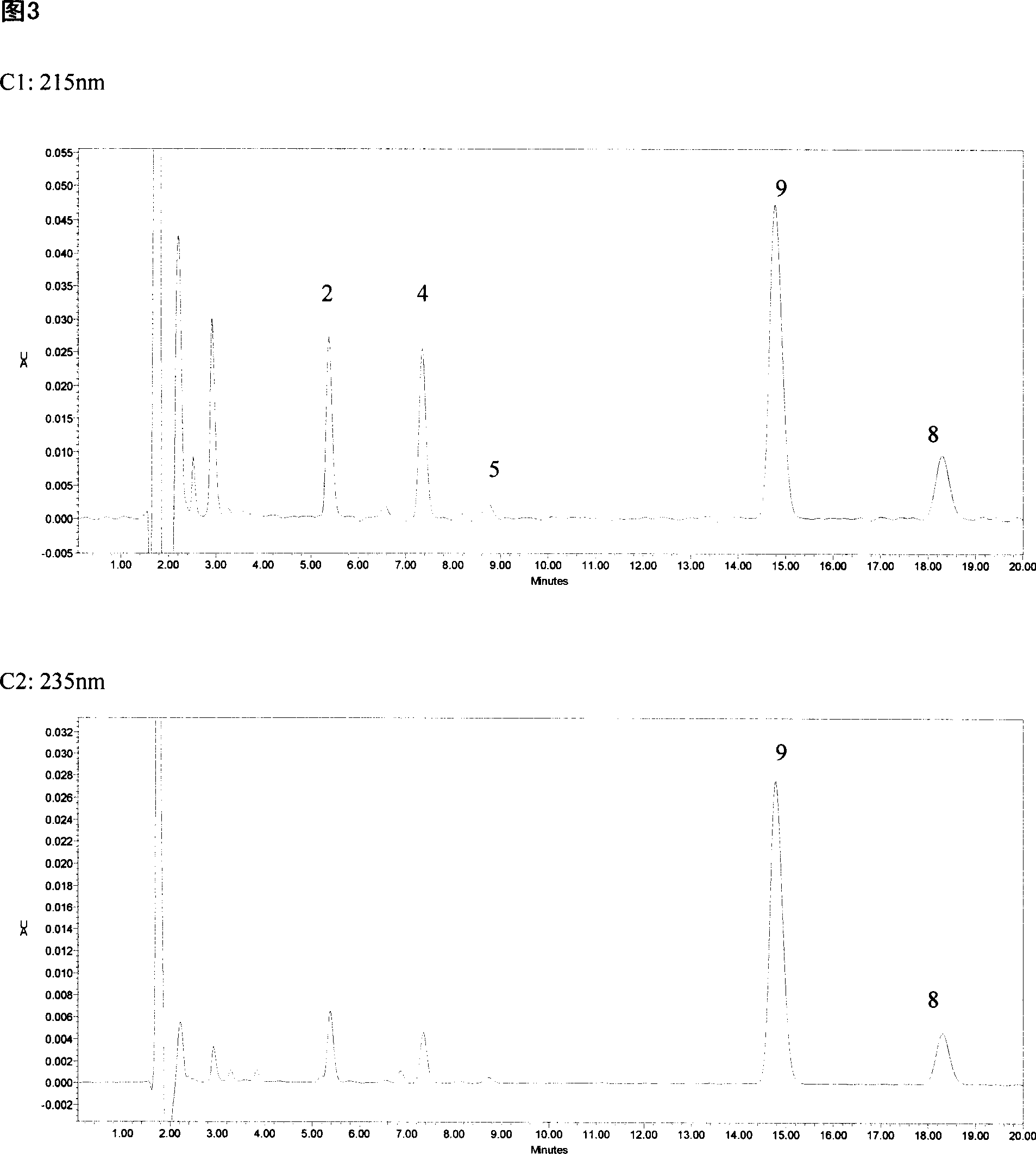
The invention relates to a method for detecting the concentration of an antiepileptic drug in serum. The method is high in sensitivity, high in specificity, good in accuracy and simple in pretreatmentprocess, separation and detection of the antiepileptic drug in serum are completed within 6.0 min, the accuracy and precision of the method basically meet requirements, the method can be used for quantitative analysis of the antiepileptic drugs in the serum clinically, and a simple and rapid detection method is provided for concentration monitoring of the antiepileptic drugs clinically.
Owner:南京品生医学检验实验室有限公司
Pharmaceutical compositions containing alpha3beta4 nicotinic receptor antagonists and methods of their use
InactiveUS20030199496A1Relief the painIncreased dopamineBiocidePeptide/protein ingredientsAntiepileptic drugAmphetamine use
A pharmaceutical composition comprising an .alpha.3.beta.4 nicotinic receptor antagonist effective to diminish the brain-derived feeling of pleasure due to increased dopamine in the pleasure-reward center of the brain typically associated with administration of an opioid agonist analgesic, a muscle relaxant, an anti-seizure medication, an anxiolytic drug, an amphetamine, a central nervous system stimulant, a tetrahydrocannabinol or that associated with an otherwise pleasurable or self-reinforcing behavior.
Owner:SIMON DAVID LEW
Controlled release formulation comprising Anti-epileptic drugs
InactiveUS20090196923A1Reduces fluctuation of blood levelControl releaseNervous disorderPill deliveryImmediate releaseAntiepileptic drug
The present invention relates to pharmaceutical formulation of antiepileptic drug preferably oxcarbazepine. The formulation comprises multiple tablets or pellets of immediate release or controlled release nature, which are filled, inside the capsule to provides drug effect for 24 hours and is suitable for once a day administration. The patent also provides process of preparation of the dosage form.
Owner:ASTRON RES LTD
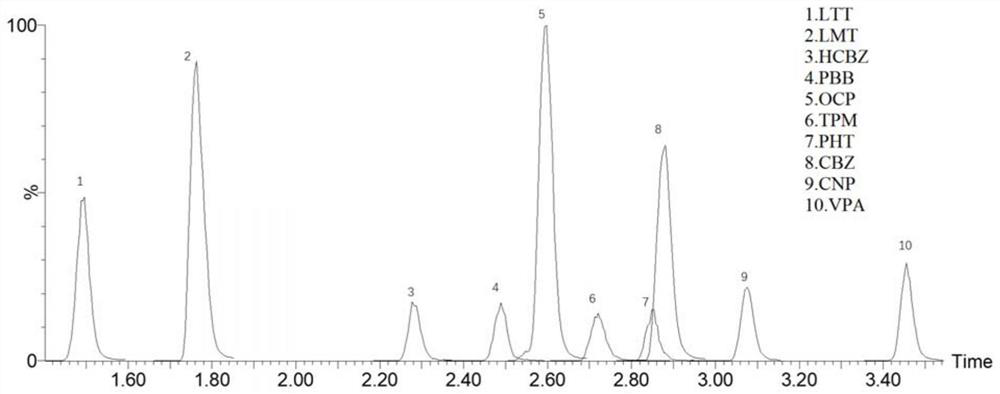
Method for detecting blood concentration of multiple antiepileptic drugs simultaneously
InactiveCN1971270ASuitable for routine testingDo not interfere with determinationTesting dairy productsTesting medicinal preparationsEpoxyPretreatment method
The invention belongs to field of medical examination and relates to assay determination method of internal medicine, specially a detecting method of six antiepileptics medicines of primidone, phenylethylmalonylurea, diphenylhydantoin, carbamazepine, Larmortriazine, okazepine and the active product monohydroxy okazepine of okazepine and the active product epoxy carbamazepine of carbamazepine in human plasma. The sample to be measured is eluted equicontinuously in condition of acidic mobile phase via the pretreatment of protein precipitation, and separated by chromatographic column, and detected by ultraviolet detector. The quantity of sample in the invention is small; the pretreatment method is simple, fast and sensitive, free of expensive apparatus and agents, applicability is wide, cost is low, it fits for the detection of normal blood concentration in clinic.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Epilepsy medication recommendation method and system
PendingCN111462921AHigh control rateShorten the timeBiostatisticsProteomicsAntiepileptic drugMetabolic enzymes
The invention provides an epilepsy medication recommendation method and system, and belongs to the technical field of biomedicine and data processing. According to the invention, deep sequencing is carried out on an antiepileptic drug metabolic enzyme gene, a transporter gene and a drug action target gene, related coding gene polymorphic sites are screened, a Bayesian accumulation regression treemodel and random forest regression are utilized to obtain a personalized medication scheme depending on individual genes, and a subset with high treatment effect is identified, so that clinical medication is guided in a targeted manner, the epilepsy control rate is improved, adverse reactions are reduced, the time for doctors and patients to explore the optimal treatment scheme is shortened, the diagnosis and treatment efficiency is improved, and the method has good practical application value.
Owner:SHANDONG UNIV
Use of cannabinoids in the treatment of seizures as associated with lennox-gastaut syndrome
InactiveUS20210169824A1Nervous disorderHydroxy compound active ingredientsAntiepileptic drugSeizure frequency
The present invention relates to the use of cannabidiol (CBD) in the treatment of patients with Lennox-Gastaut syndrome (LGS) who are deemed to be treatment failures on their existing medication. In particular the use of CBD was found to provide a statistically significant reduction in both drop seizures and total seizure frequency in patients who have tried and failed anti-epileptic drugs (AEDs) or those who were currently taking AEDs but have uncontrolled seizures. Preferably the AEDs which have been shown to be treatment failures are one or more of rufinamide, lamotrigine, topiramate and / or felbamate. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
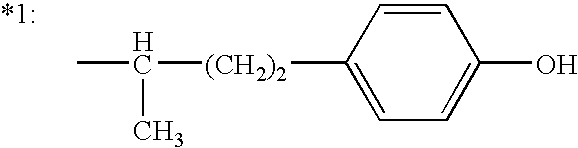
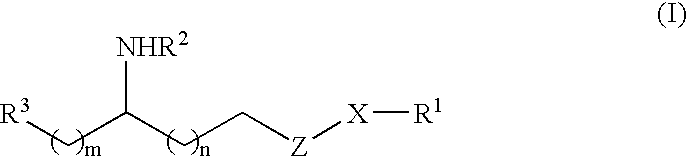
![6-(substituted phenoxy)-tetrazolo[5,1-a] phthalazine derivatives as antuepileptics and their pharmaceutically acceptable salts 6-(substituted phenoxy)-tetrazolo[5,1-a] phthalazine derivatives as antuepileptics and their pharmaceutically acceptable salts](https://images-eureka.patsnap.com/patent_img/46d5db3d-7b45-4d9e-bf28-3b8cc5c72628/A2008101657340002C1.PNG)
![6-(substituted phenoxy)-tetrazolo[5,1-a] phthalazine derivatives as antuepileptics and their pharmaceutically acceptable salts 6-(substituted phenoxy)-tetrazolo[5,1-a] phthalazine derivatives as antuepileptics and their pharmaceutically acceptable salts](https://images-eureka.patsnap.com/patent_img/46d5db3d-7b45-4d9e-bf28-3b8cc5c72628/A2008101657340003C1.PNG)
![6-(substituted phenoxy)-tetrazolo[5,1-a] phthalazine derivatives as antuepileptics and their pharmaceutically acceptable salts 6-(substituted phenoxy)-tetrazolo[5,1-a] phthalazine derivatives as antuepileptics and their pharmaceutically acceptable salts](https://images-eureka.patsnap.com/patent_img/46d5db3d-7b45-4d9e-bf28-3b8cc5c72628/A20081016573400041.PNG)