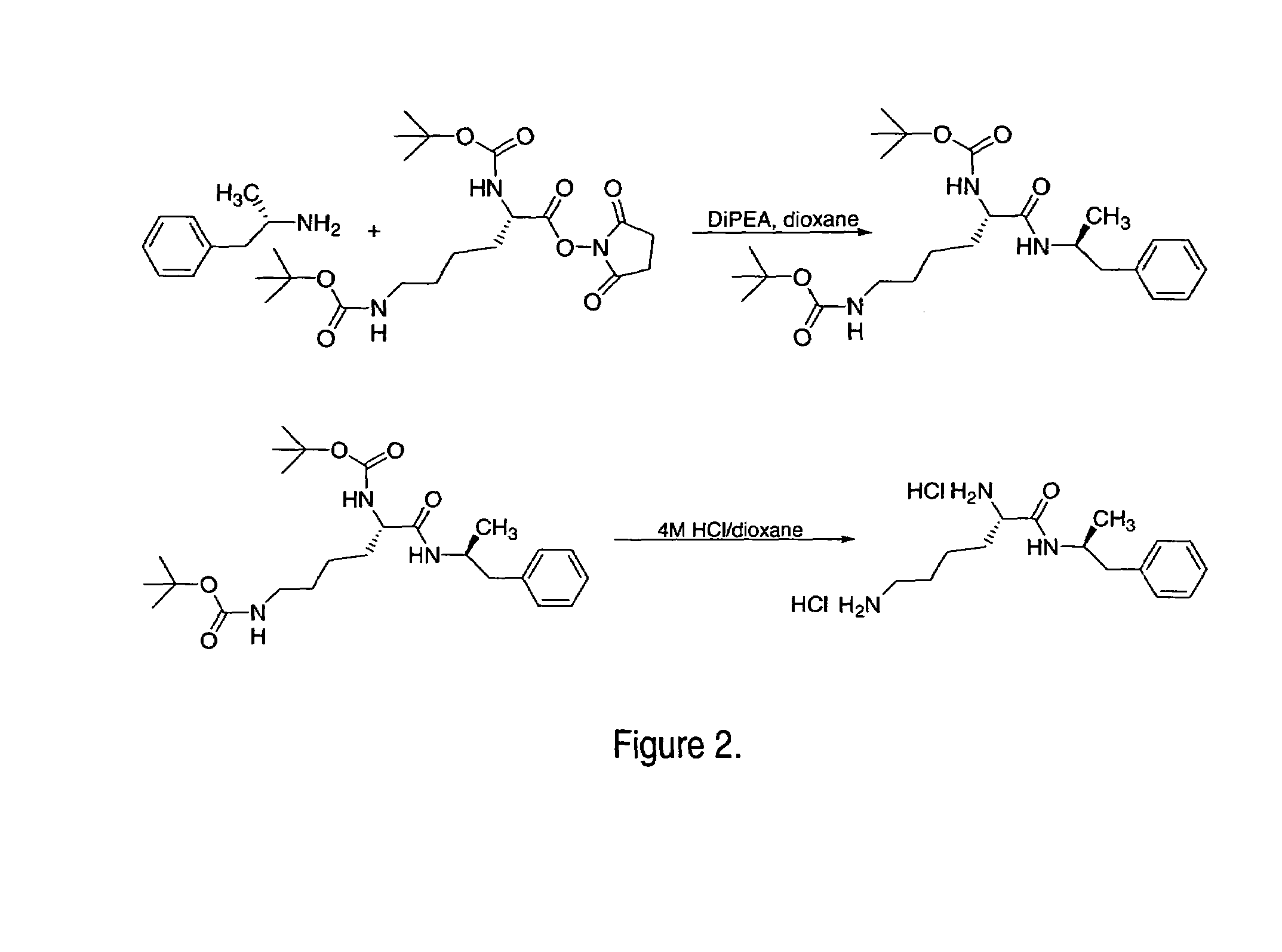
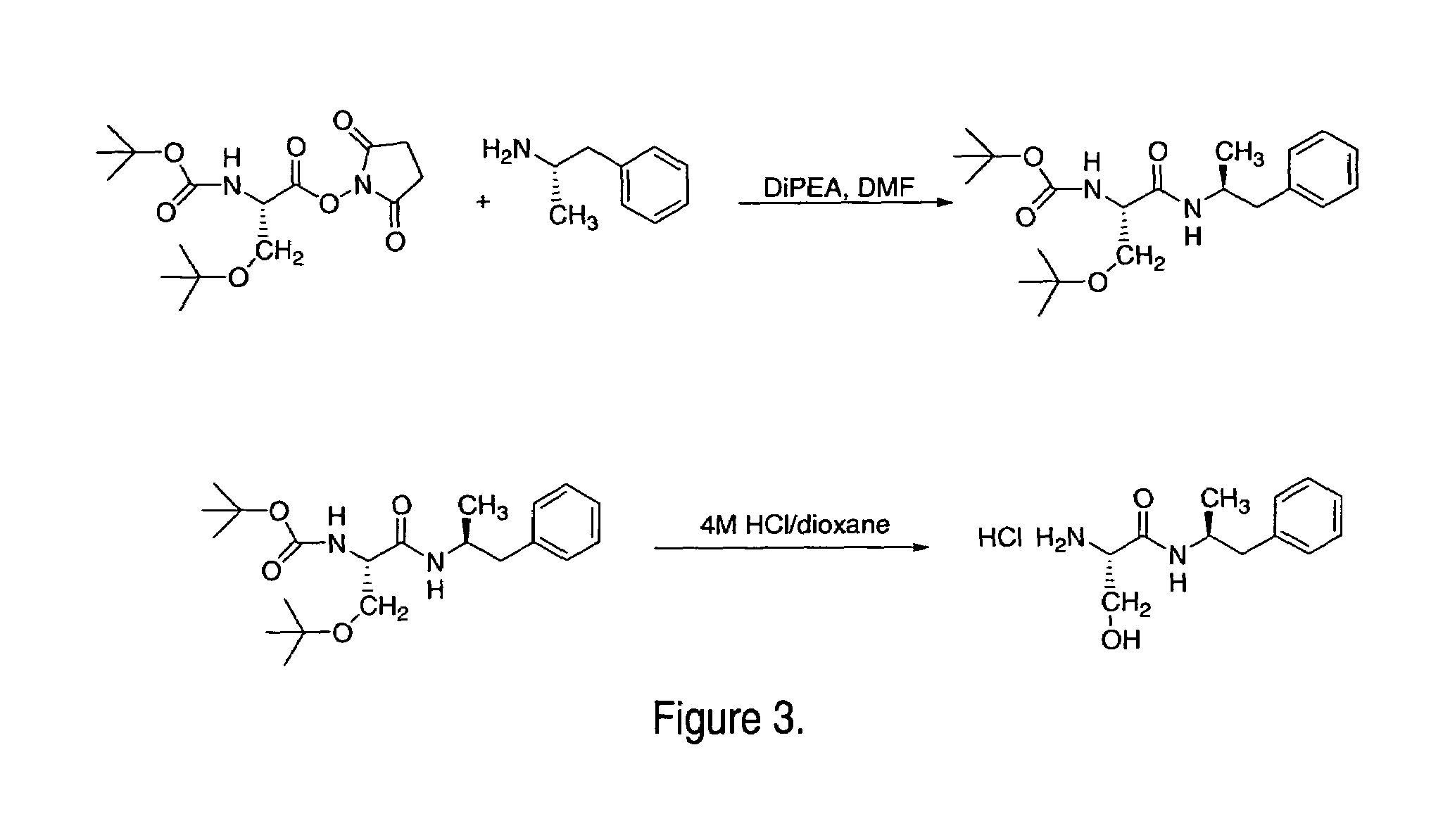
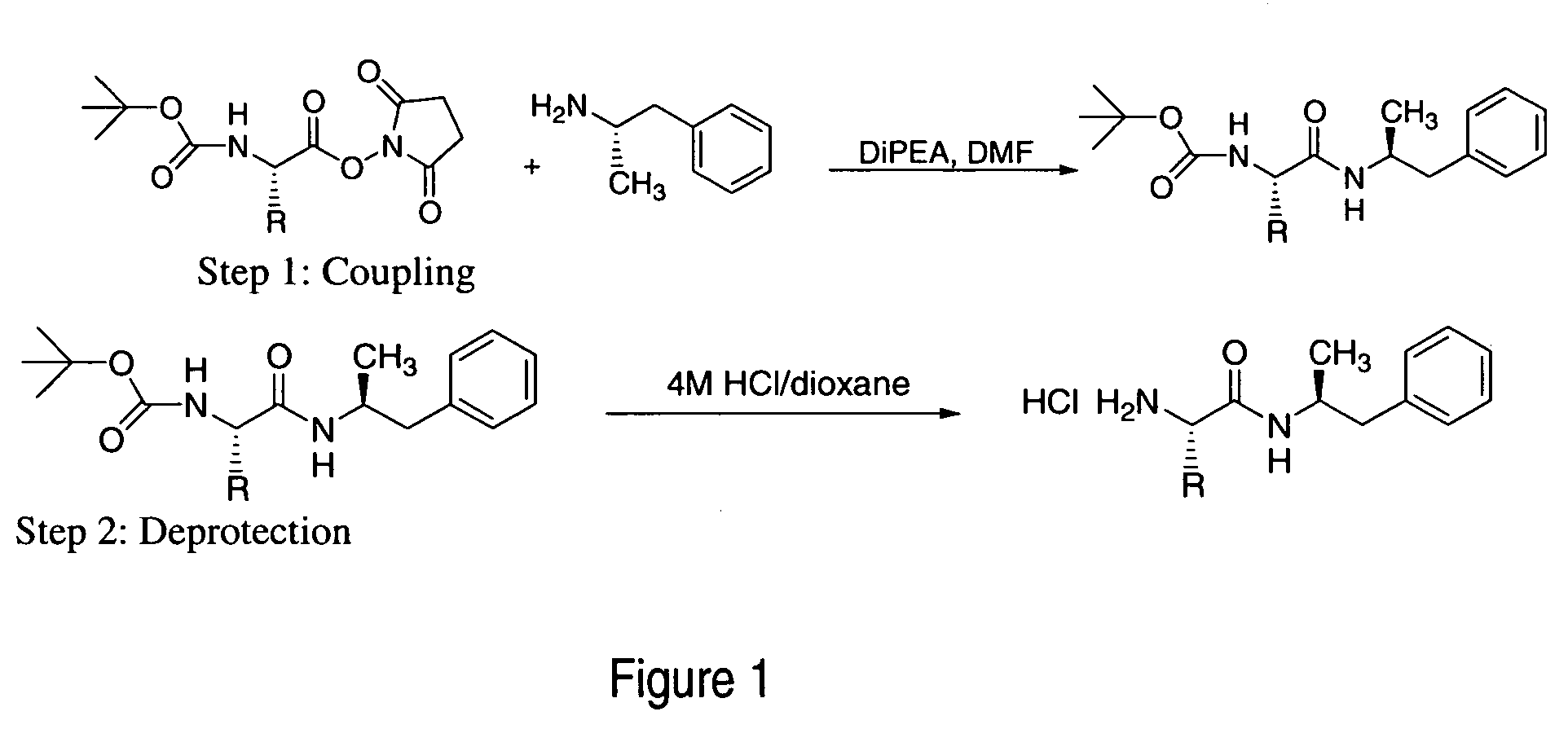
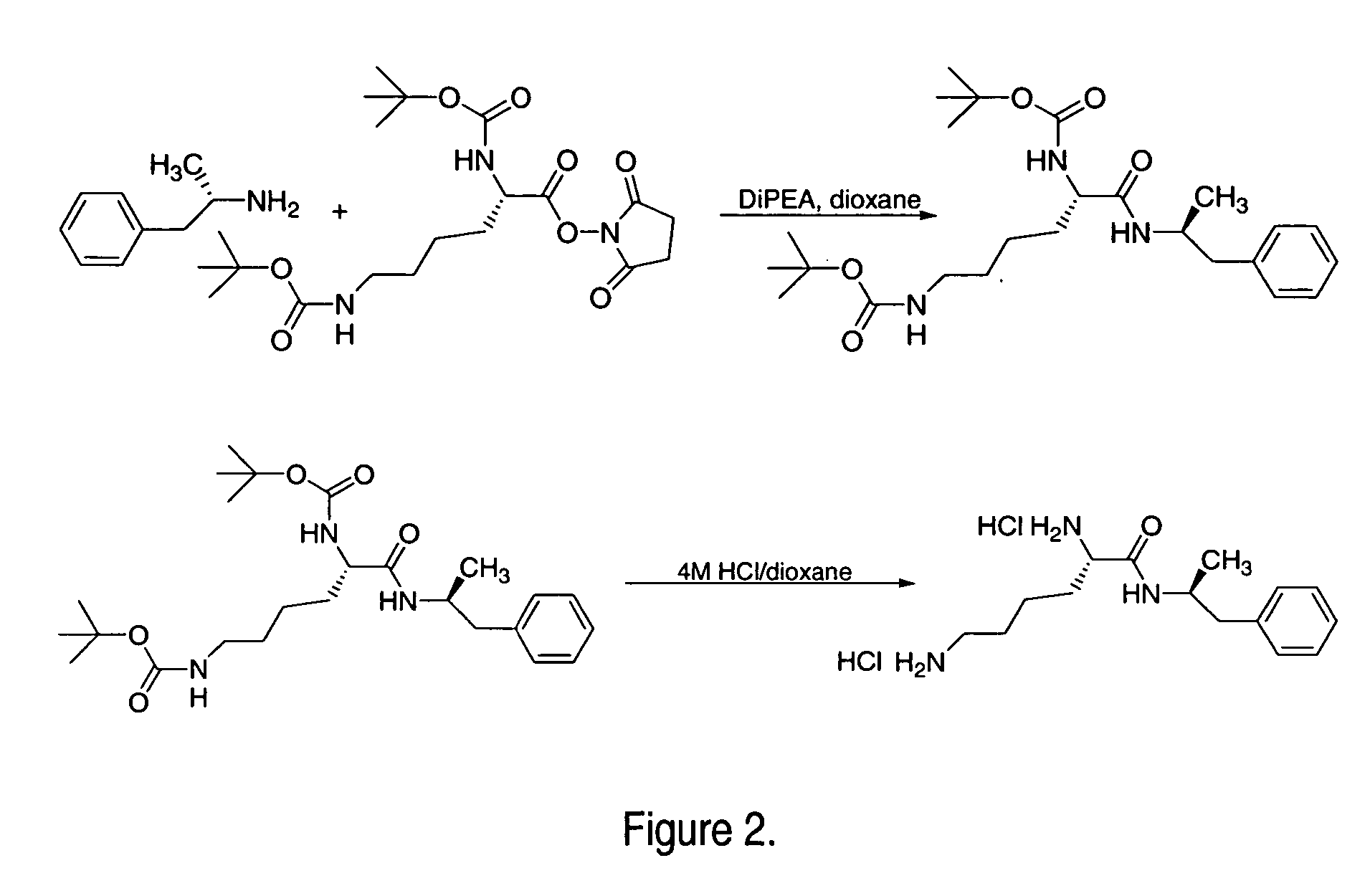
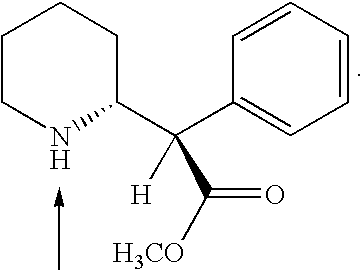
Patents
Literature
212 results about "Amphetamine use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amphetamine is used to treat attention deficit hyperactivity disorder (ADHD), narcolepsy (a sleep disorder), and obesity, and is sometimes prescribed off-label for its past medical indications, particularly for depression and chronic pain.
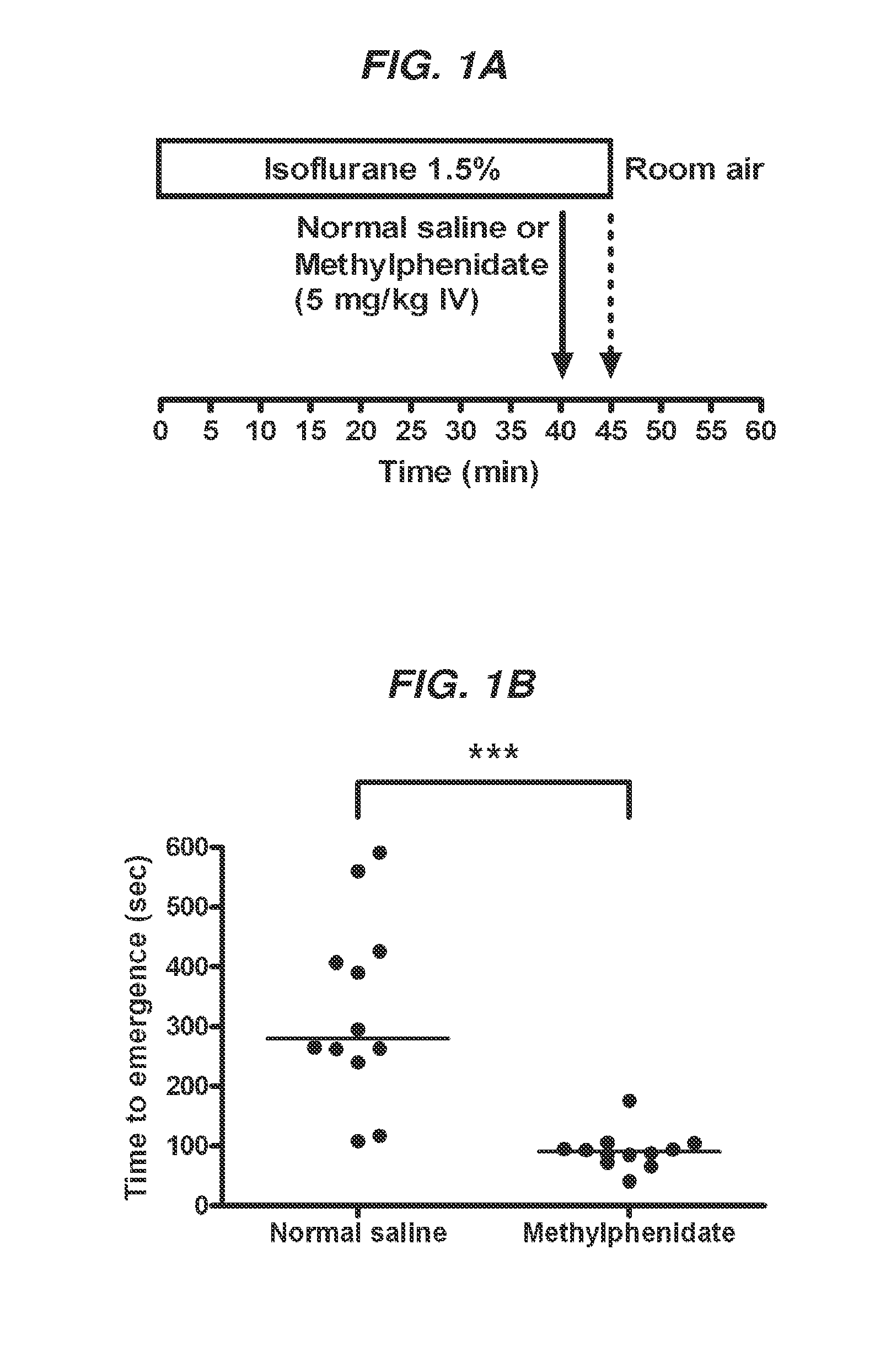
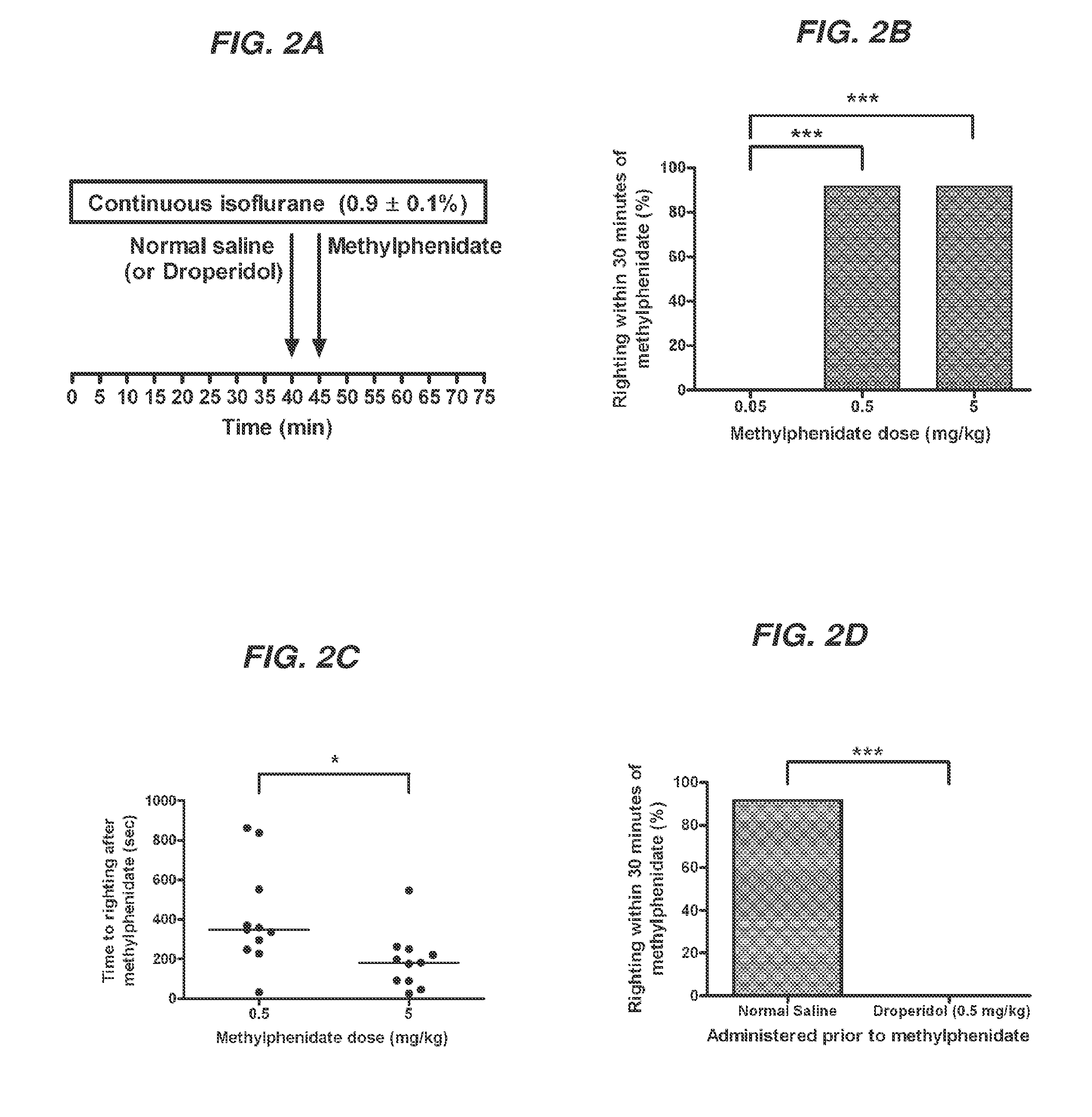
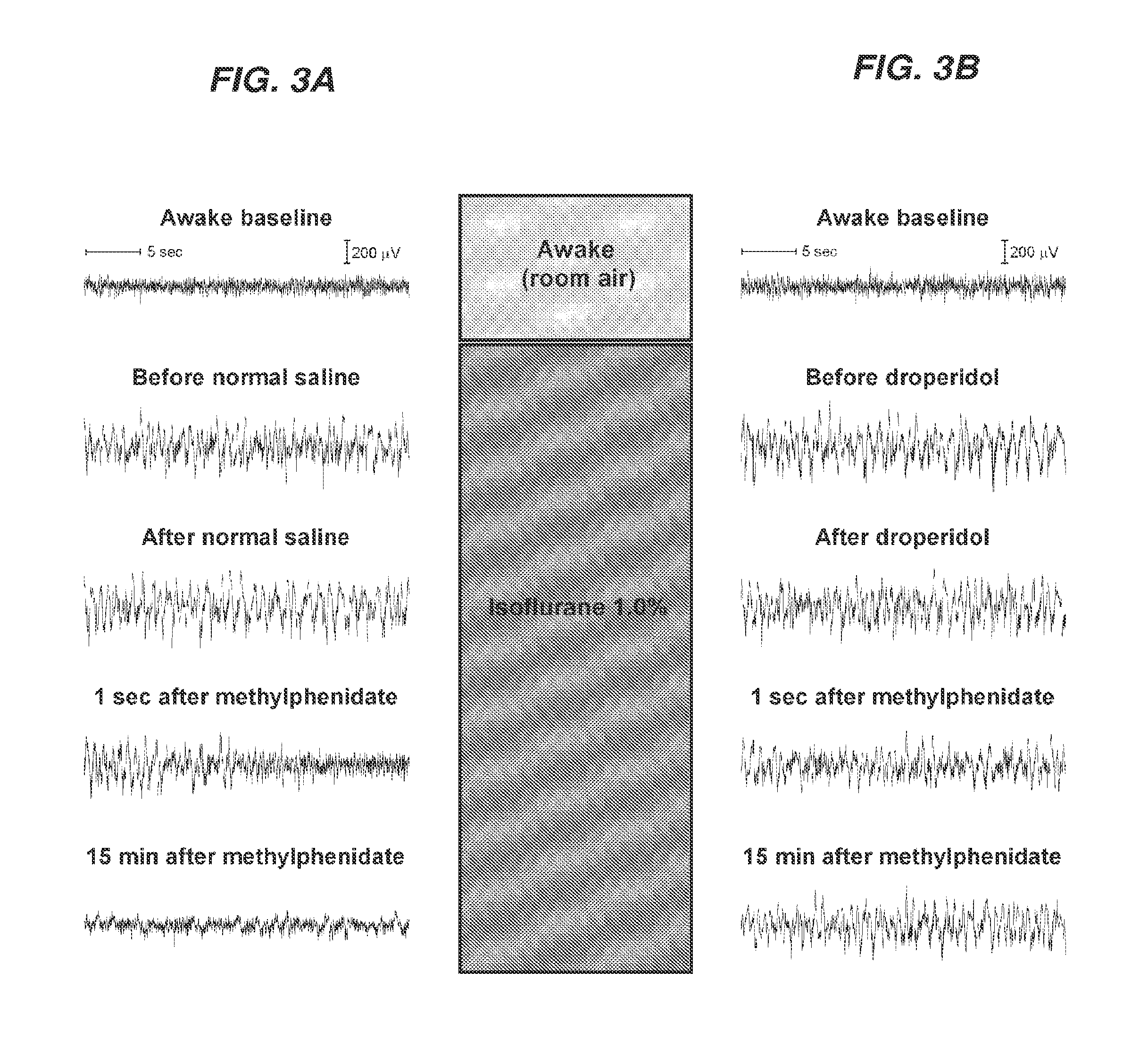
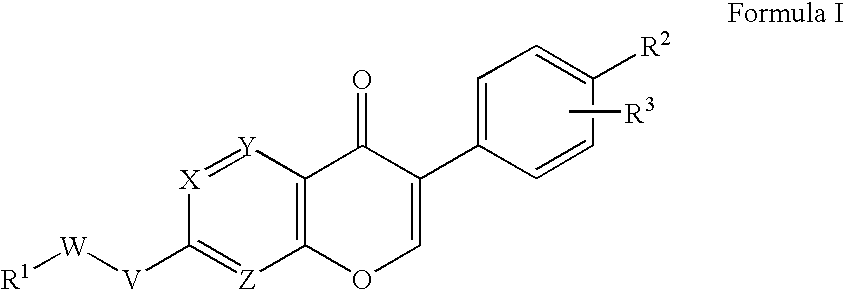
Reversal of General Anesthesia by Administration of Methylphenidate, Amphetamine, Modafinil, Amantadine, and/or Caffeine
ActiveUS20150196249A1High speedReduces and eliminates effectElectroencephalographyPharmaceutical delivery mechanismUnconsciousnessWhole body
Owner:THE GENERAL HOSPITAL CORP
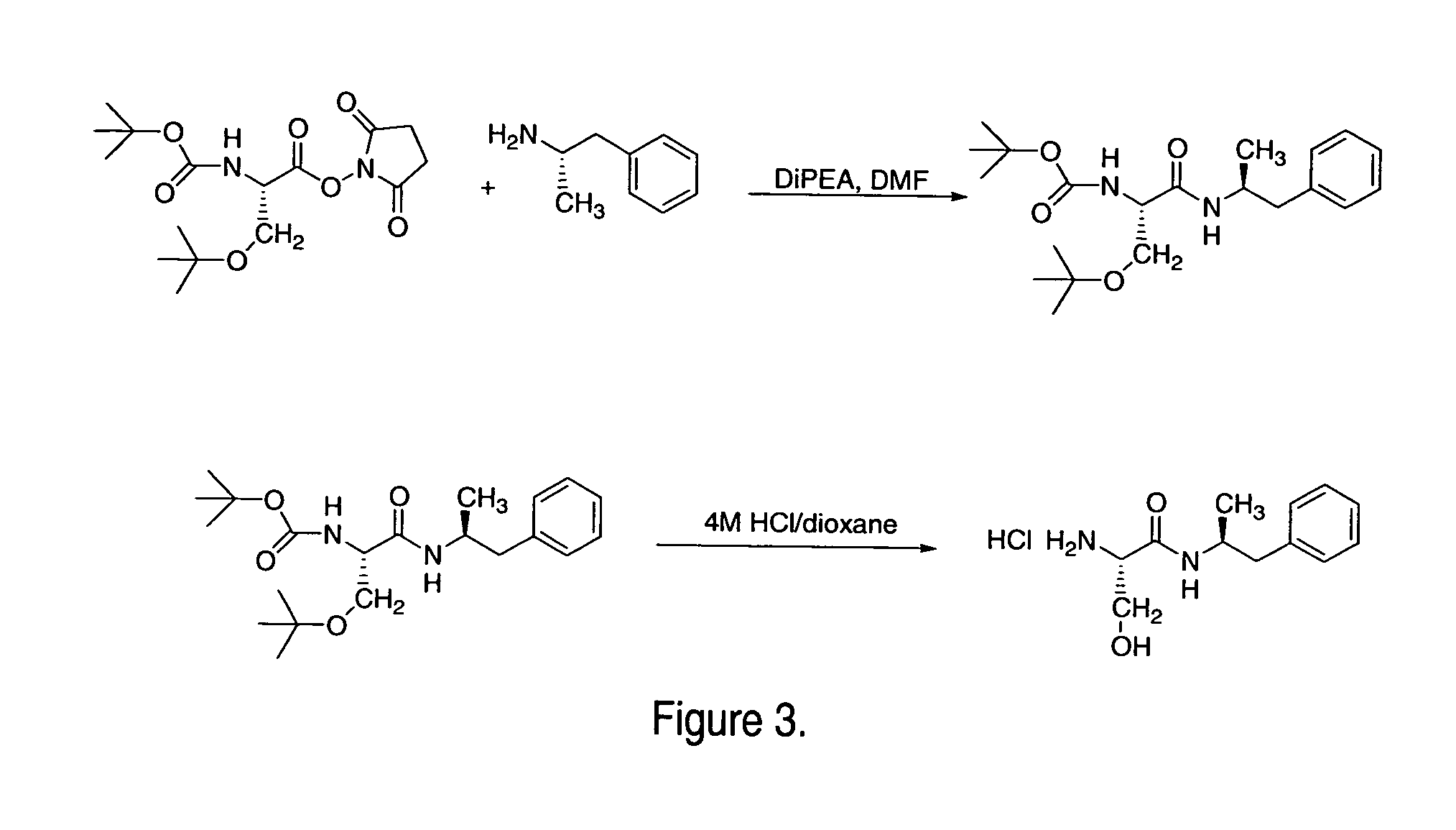
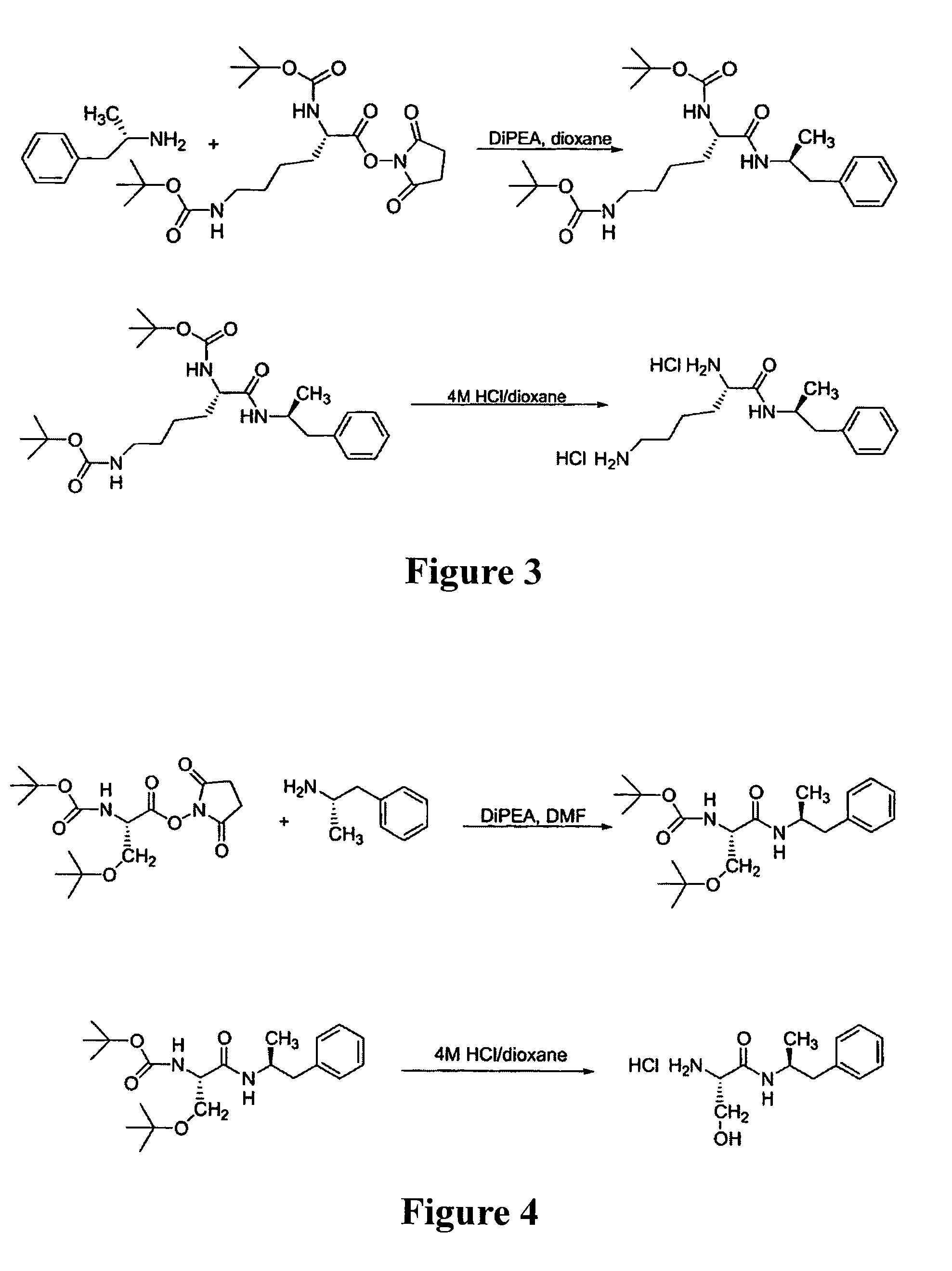
Abuse-resistant amphetamine compounds
InactiveUS7105486B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsPeptide/protein ingredientsChemical MoietyDisease
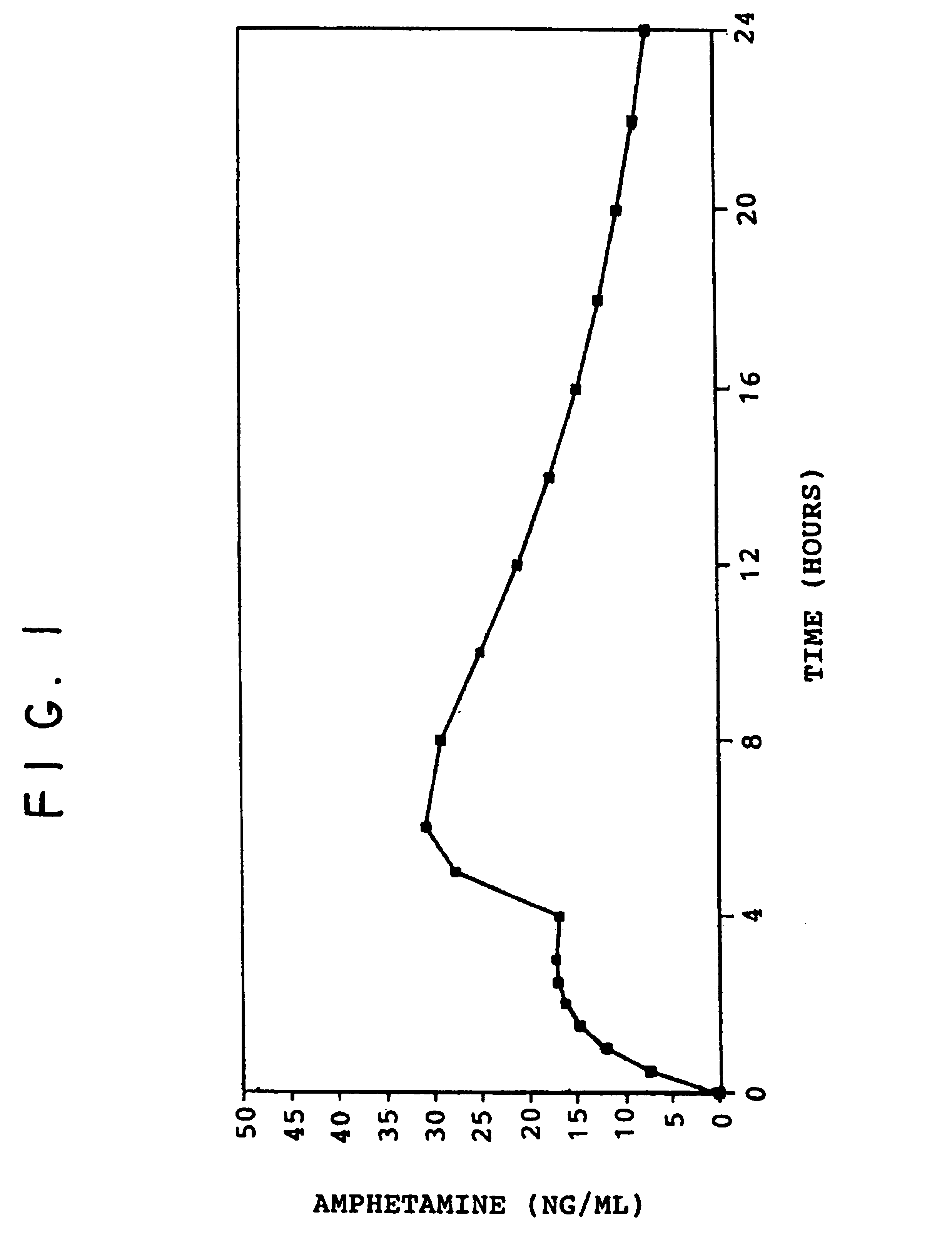
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
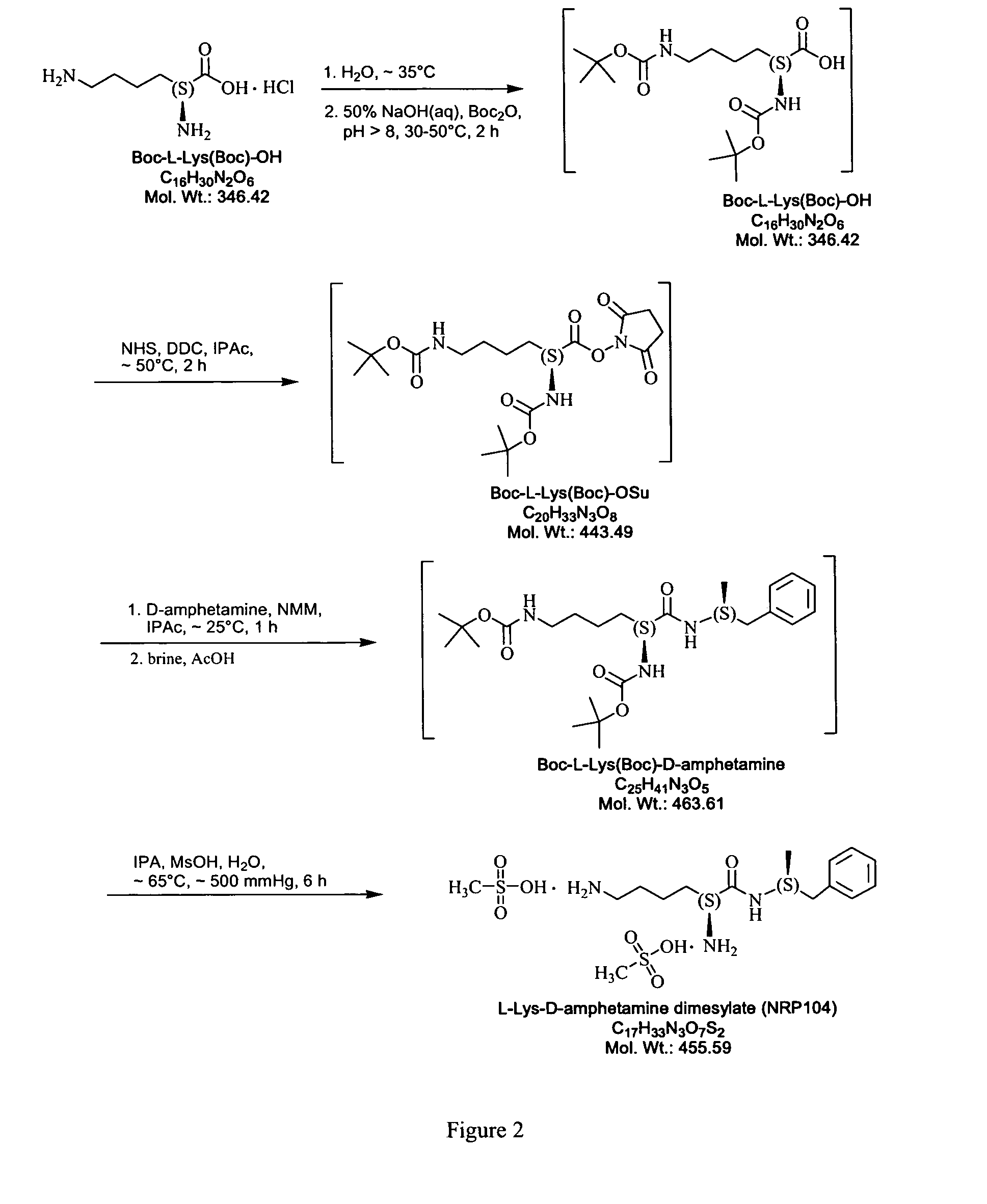
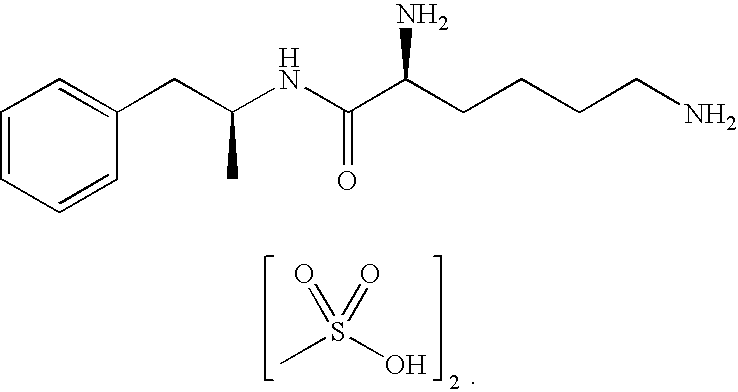
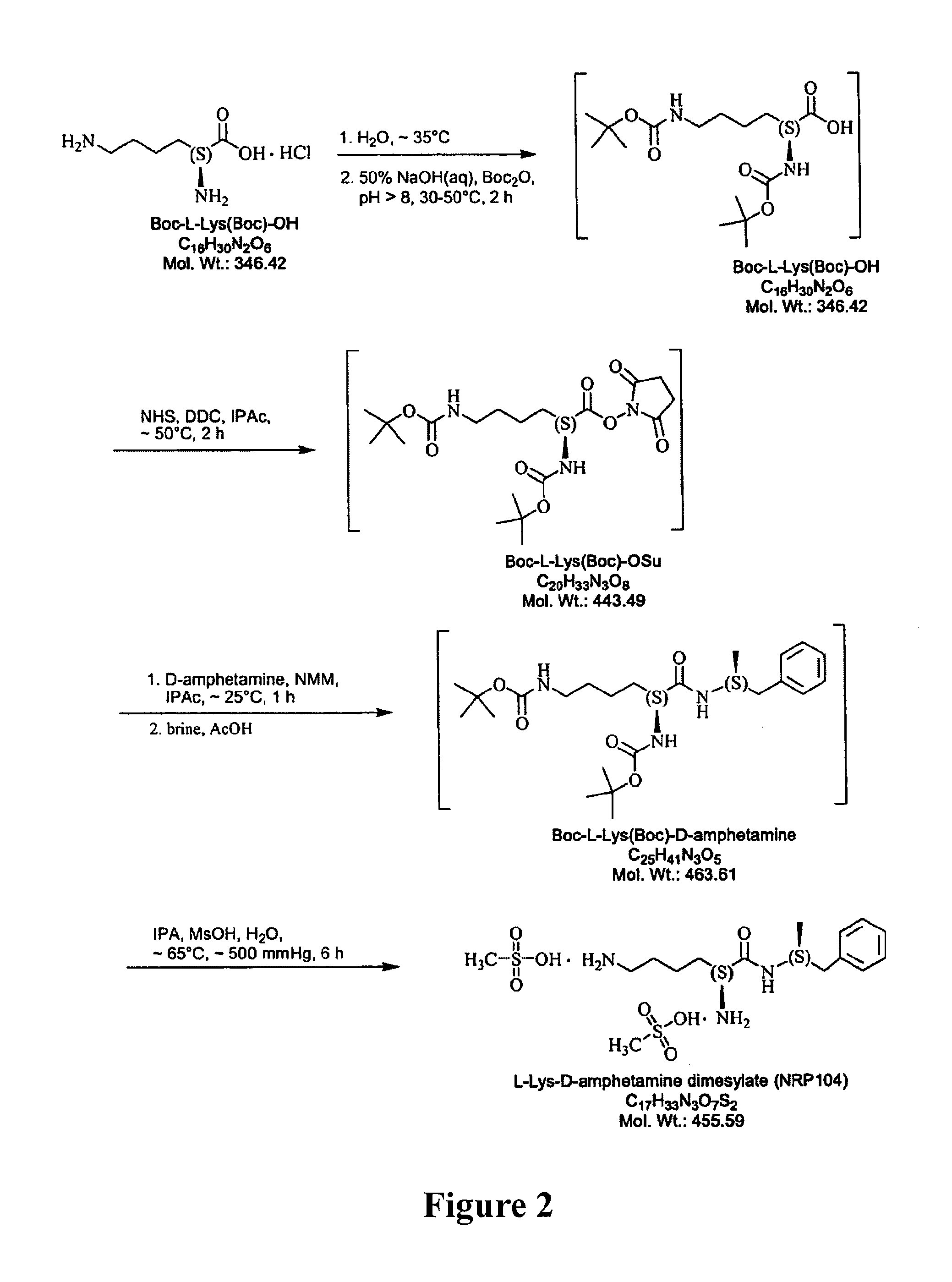
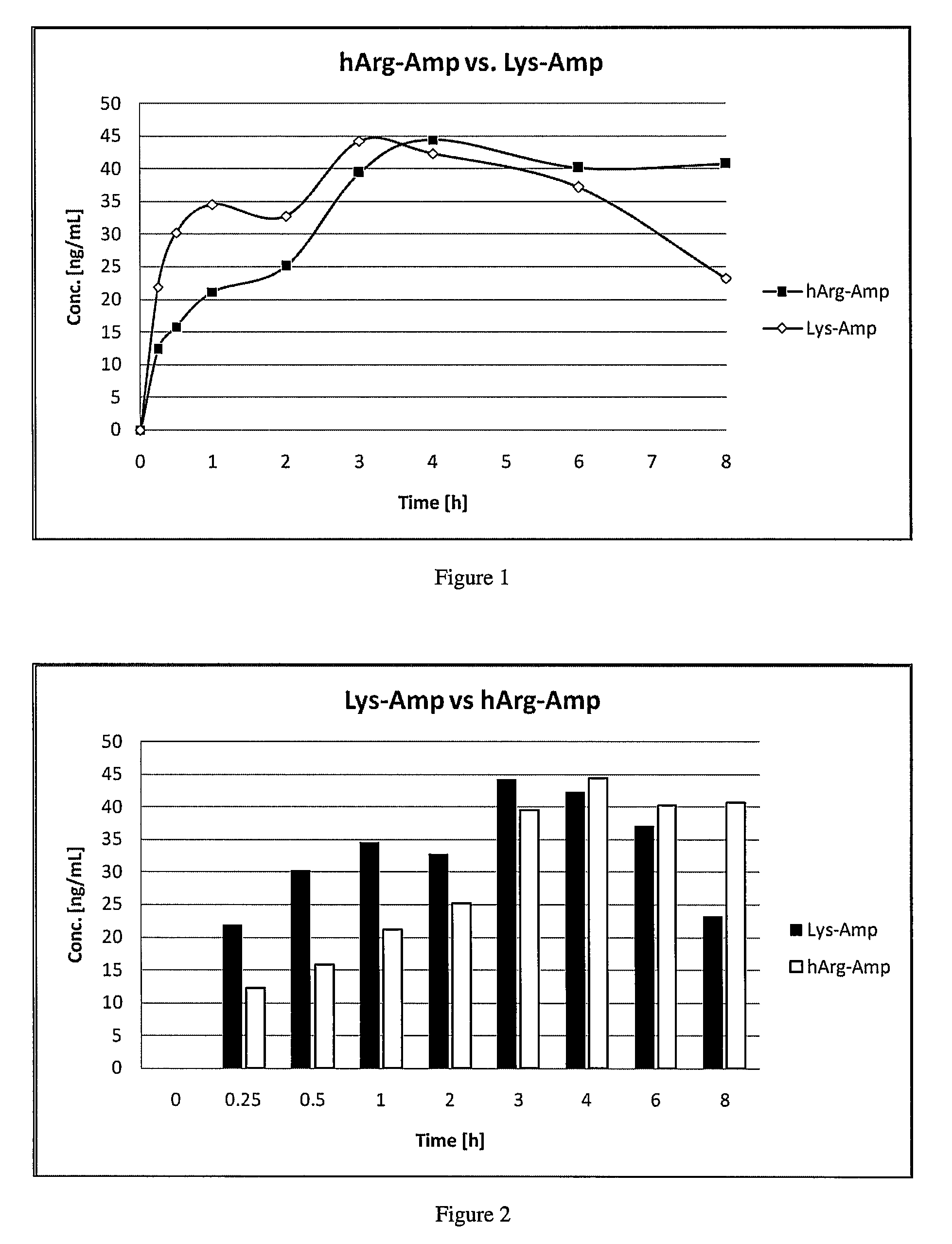
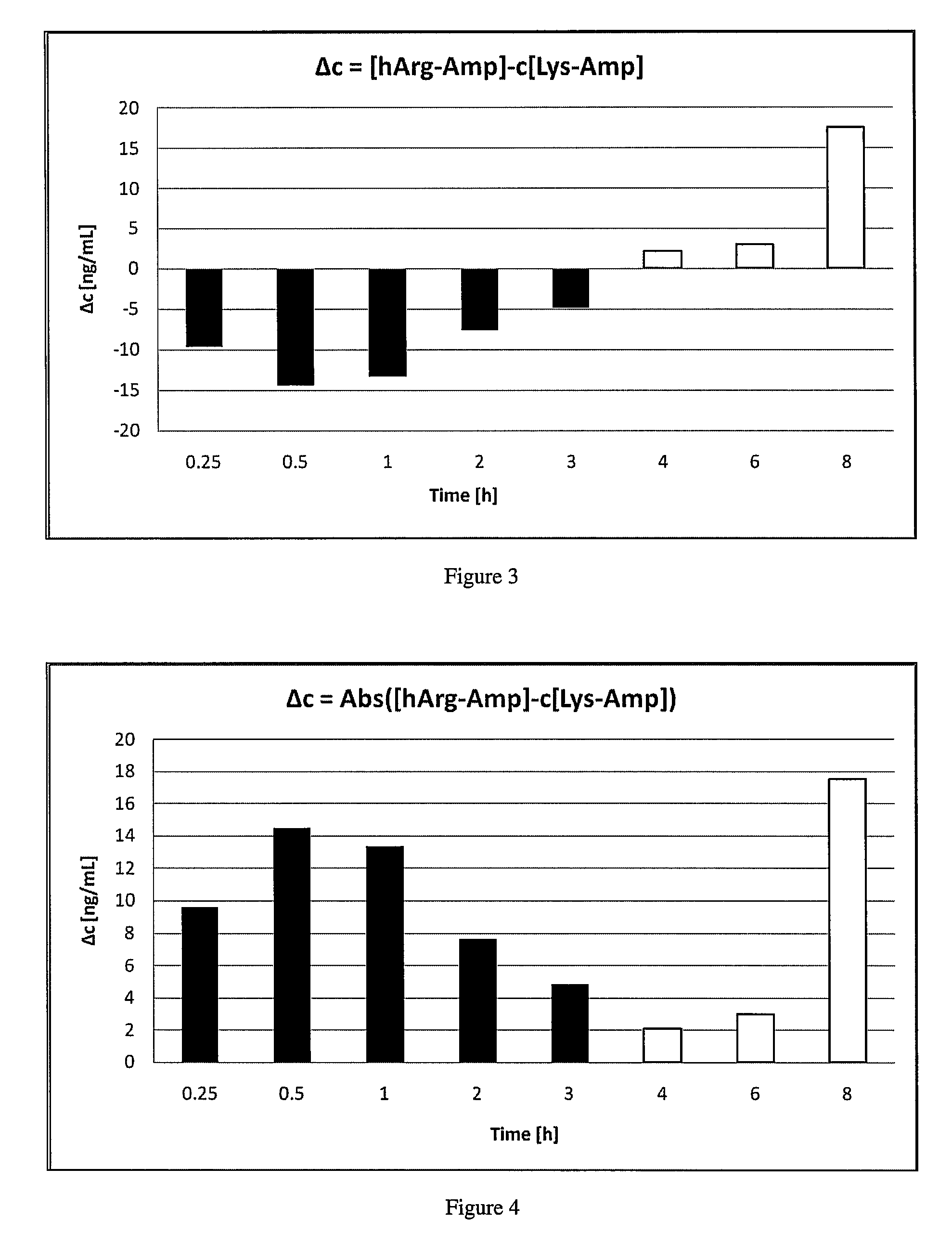
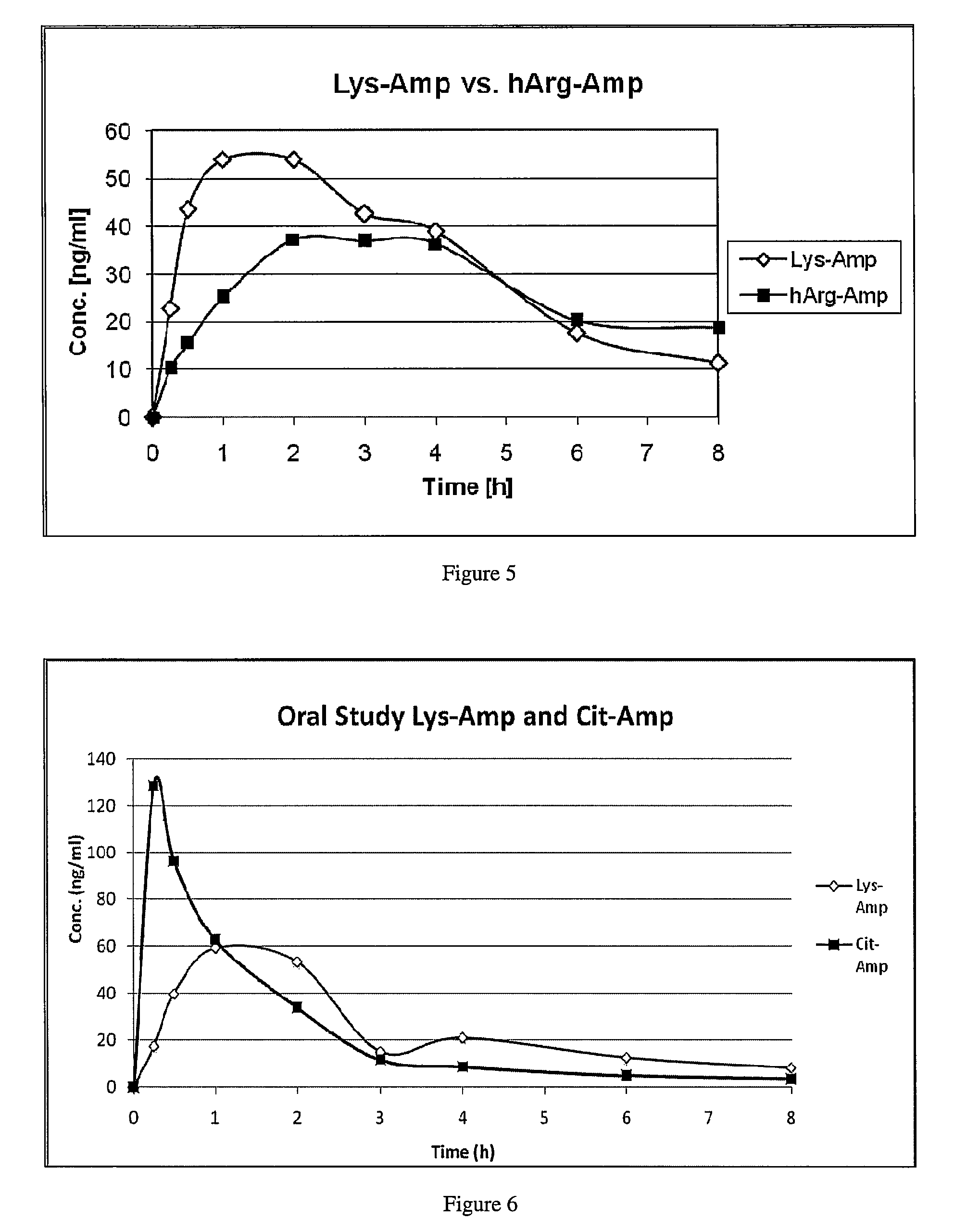
Abuse resistant lysine amphetamine compounds
ActiveUS20050038121A1Prevents euphoriaLower potentialOrganic active ingredientsBiocideDiseaseAlternative treatment
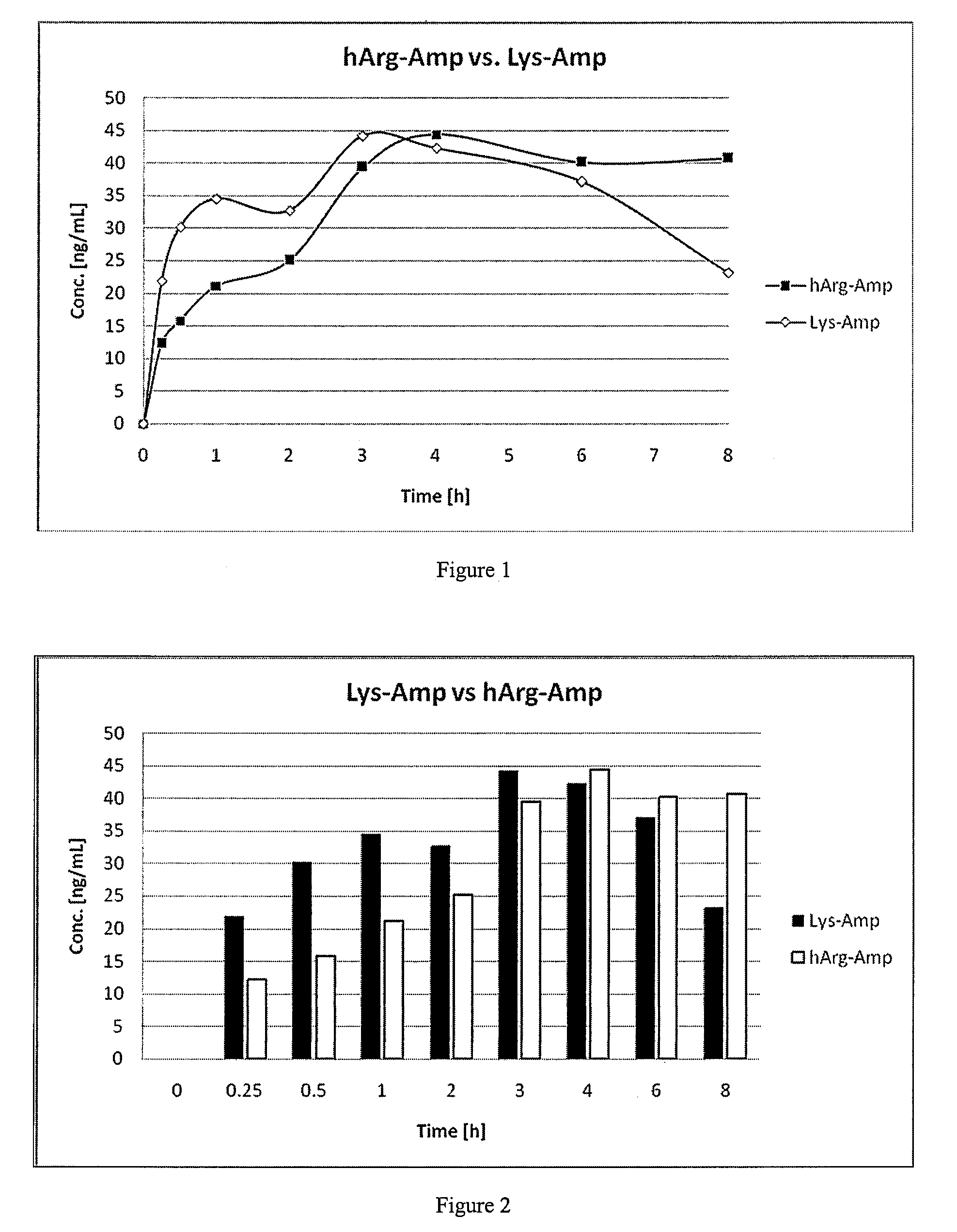
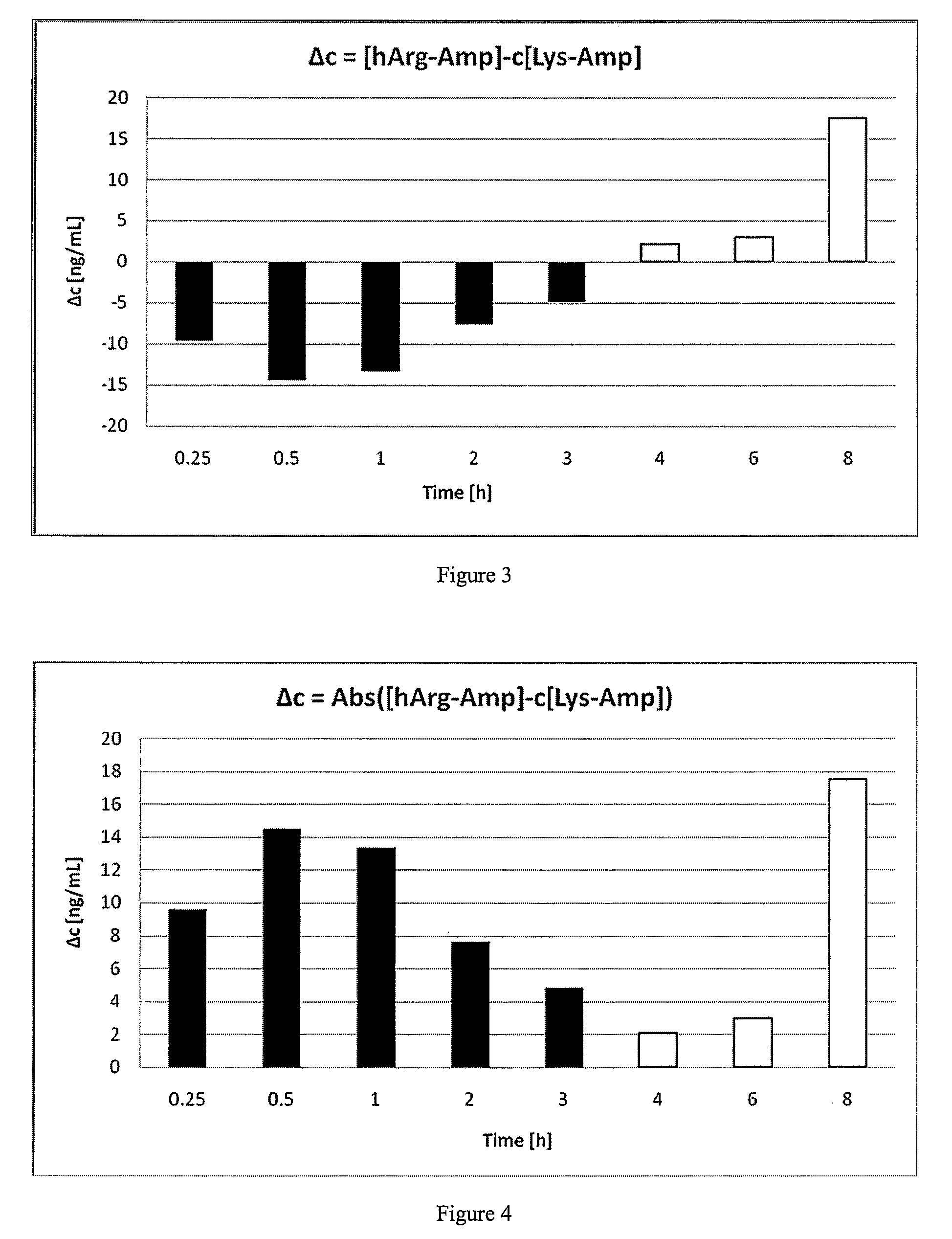
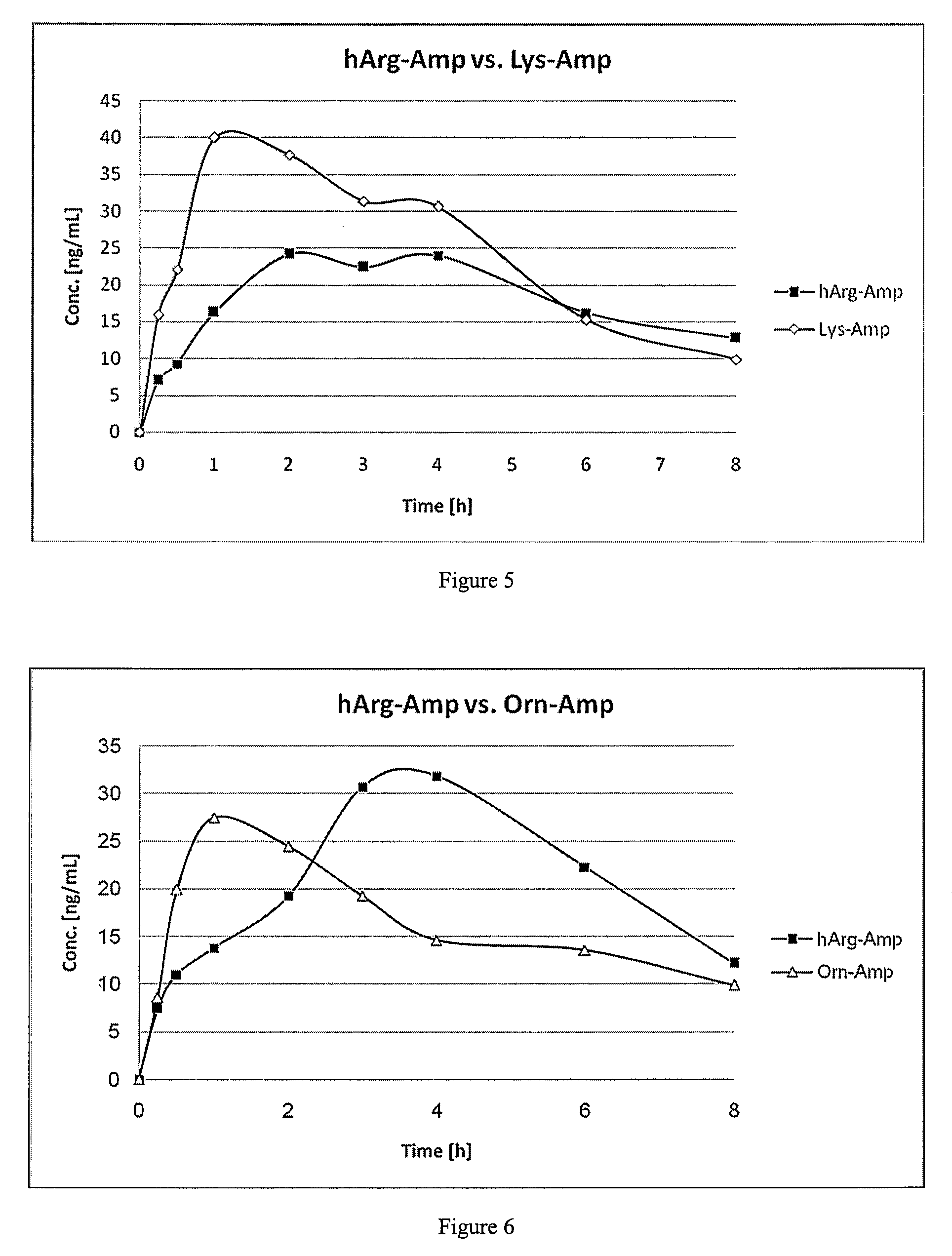
The present invention describes compounds, compositions and methods of using the same comprising lysine covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
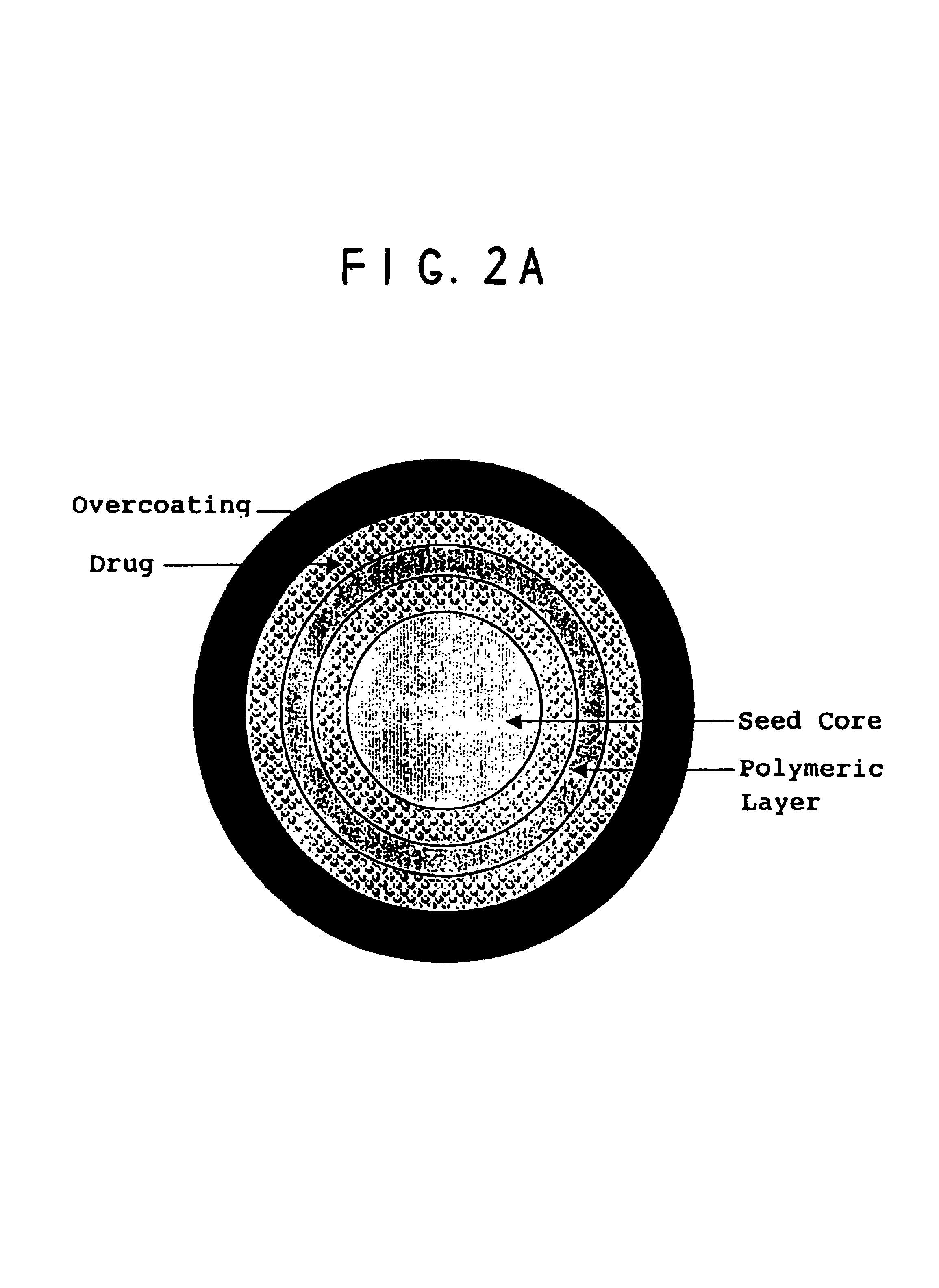
Controlled dose drug delivery system
ActiveUS20070264323A1Meet needsReduce the amount requiredOrganic active ingredientsBiocideImmediate releaseDose delivery
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising a pharmaceutically active amphetamine salt covered with an immediate-release coating and a pharmaceutically active amphetamine salt covered with an enteric coating wherein the immediate release coating and the enteric coating provide for multiple pulsed dose delivery of the pharmaceutically active amphetamine salt. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:TAKEDA PHARMA CO LTD
Abuse-resistant amphetamine compounds
InactiveUS20050054561A1Prevents euphoriaLower potentialBiocidePeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Method and synergistic composition for treating attention deficit/hyperactivity disorder
InactiveUS6541043B2Minimize side effectsBiocideHydroxy compound active ingredientsBeta-CaroteneBetaine
A composition and method for treating Attention Deficit / Hyperactivity Disorder (ADHD) is provided which can be used both with and without ethical drugs now used to treat ADHD. The composition contains dimethylaminoethanol (DMAE), omega 3-fatty acids, betaine, oligomeric proanthocyanidins (OPC), folic acid, vitamins C, E, B12, B6, B5 and beta-carotene and minerals (calcium, magnesium, zinc and selenium). Ethical drugs such as amphetamines, methylphenidate HCl and pemoline are known to control ADHD, but each has significant side effects when used in their therapeutic dose. When combining the composition with such ethical drugs, the amount of the ethical drug can be lowered below a level which causes undesirable side effects which is an important feature. Preferred compositions contain one or more of lecithin, choline, 5-hydroxytryptophan, tyrosine, Reishi Extract, Kava Extract, Gingko, Ginseng and St. John's Wort.
Owner:PHILIP C LANG
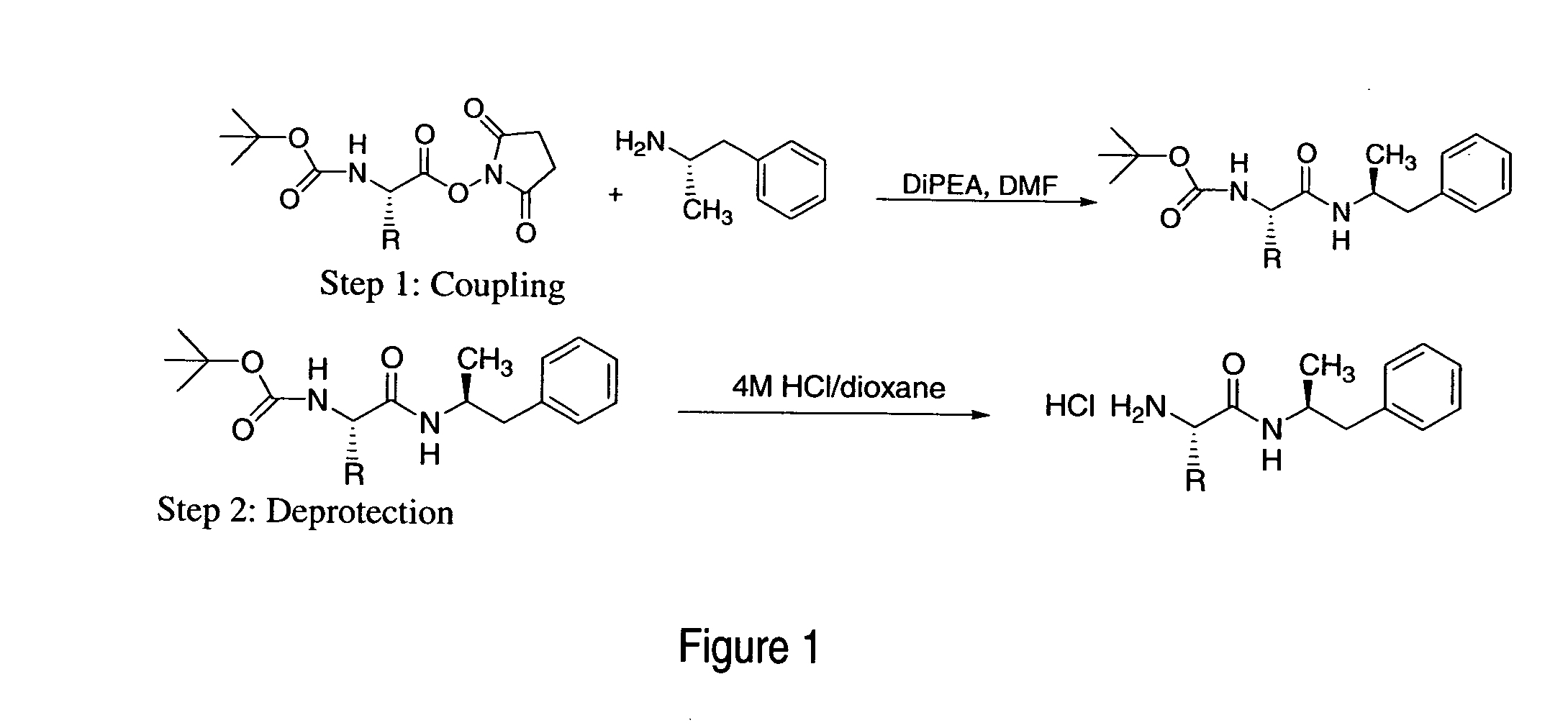
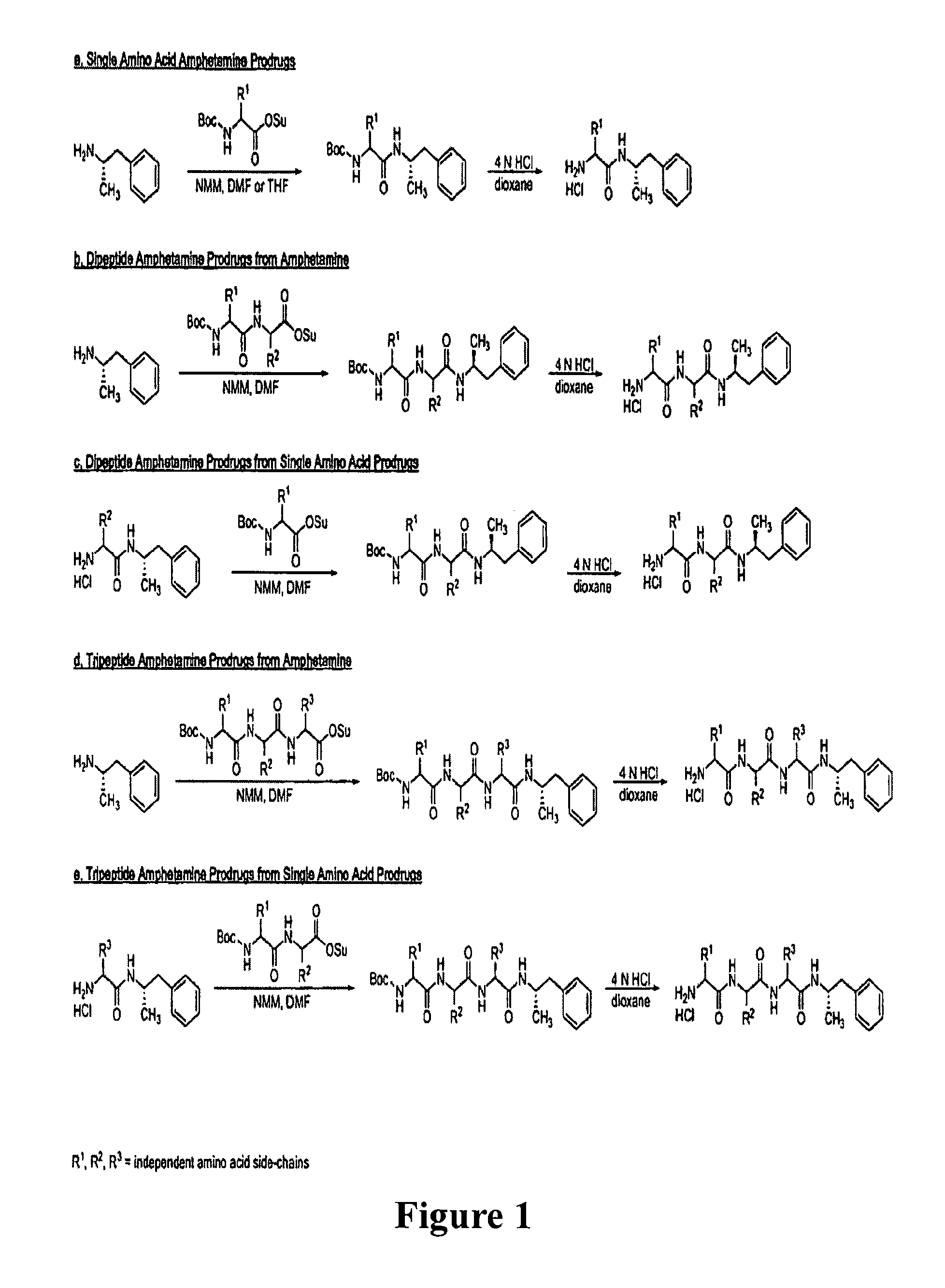
Abuse-resistant amphetamine prodrugs
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Method of treating depressive disorders
The invention provides methods of treating depressive disorders, in particular major depression but other depressive orders also, with prodrug stimulants or analogs including amphetamine prodrugs, methylphenidate prodrugs, and methylphenidate analogs, Such methods of treatment may utilize the prodrug stimulant or analog as monotherapy or, more commonly, as an adjunct to antidepressant medication treatment to augment their effect. The invention includes combination methods of treatment in which an amphetamine prodrug, methylphenidate prodrug, or methylphenidate analog is administered to an individual in need with one or more other active agents, either in separate forms or as a single pharmaceutical formulation. Packaged pharmaceutical compositions containing an amphetamine or methylphenidate prodrug, instructions for using the prodrug to treat certain disorders, and optionally one or more other active agents are provided by the invention.
Owner:LUCERNE BIOSCI
Method for qualitatively screening 242 kinds of compounds by liquid phase chromatography-mass spectra at the same times
ActiveCN101398414AFast wayStrong detection specificityComponent separationMaterial analysis by electric/magnetic meansBenzodiazepineCarbamate
The invention discloses a method which can carry out qualitative screening to 242 compounds (drugs or toxicants) simultaneously. A mode of liquid chromatogram-mass spectrometry (LC-MS / MS) multi-reaction monitoring (MRM) is adopted to carry out determinedness to objects by two pairs of parent ion-daughter ion pair and retention time. The 242 drugs or toxicants comprise toxic products such as opioids, amphetamine and cocaines and the like, bromazepam such as benzodiazepines and barbiturates, and common drugs, alkaloid, pesticide (including organophosphates, carbamates, pyrethroid pesticide residues and organochlorine pesticide), weed killer, raticide and the like.
Owner:上海市公安局刑事侦查总队
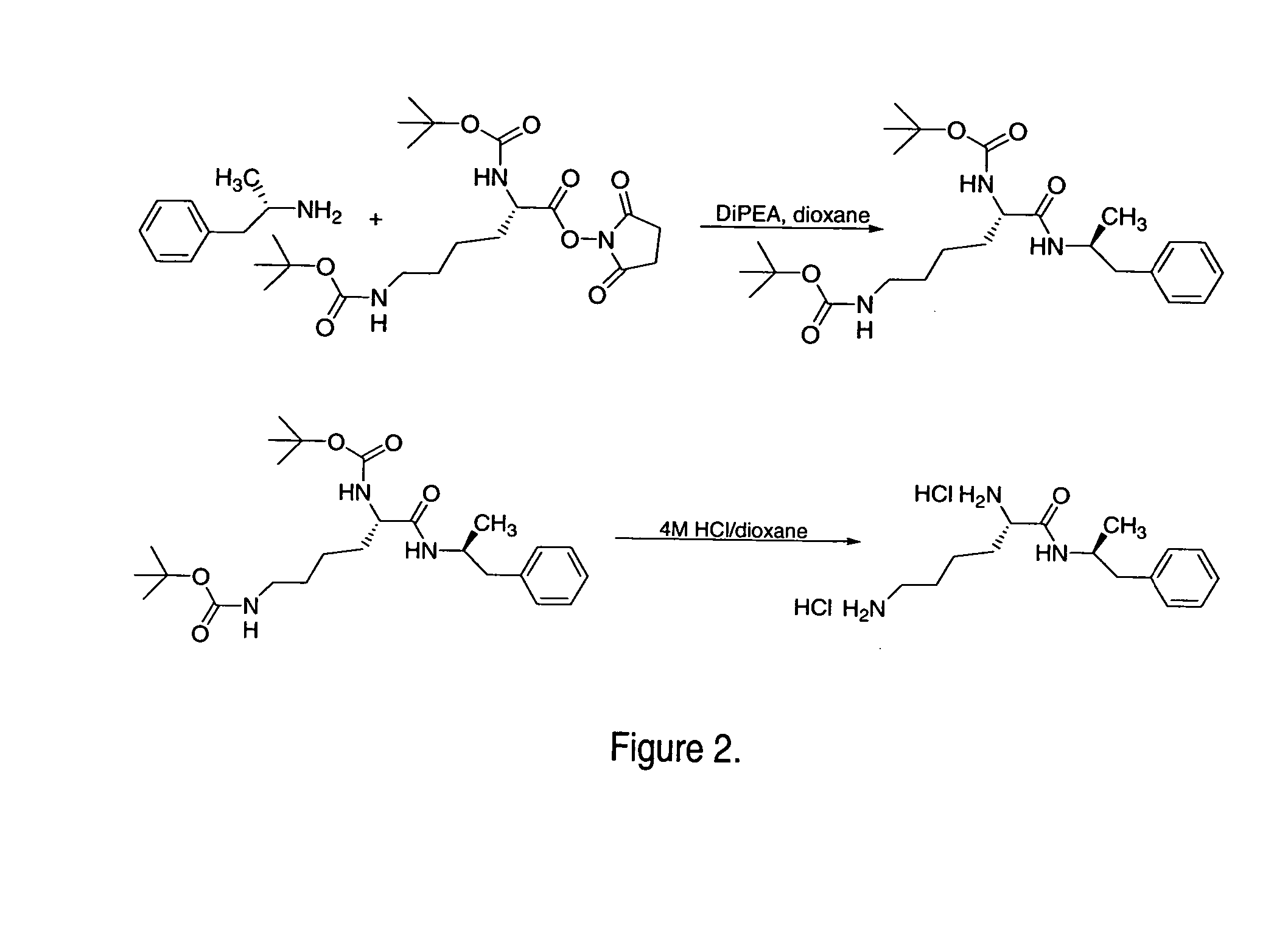
Abuse resistant lysine amphetamine compounds
InactiveUS7223735B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsNervous disorderDiseaseAlternative treatment
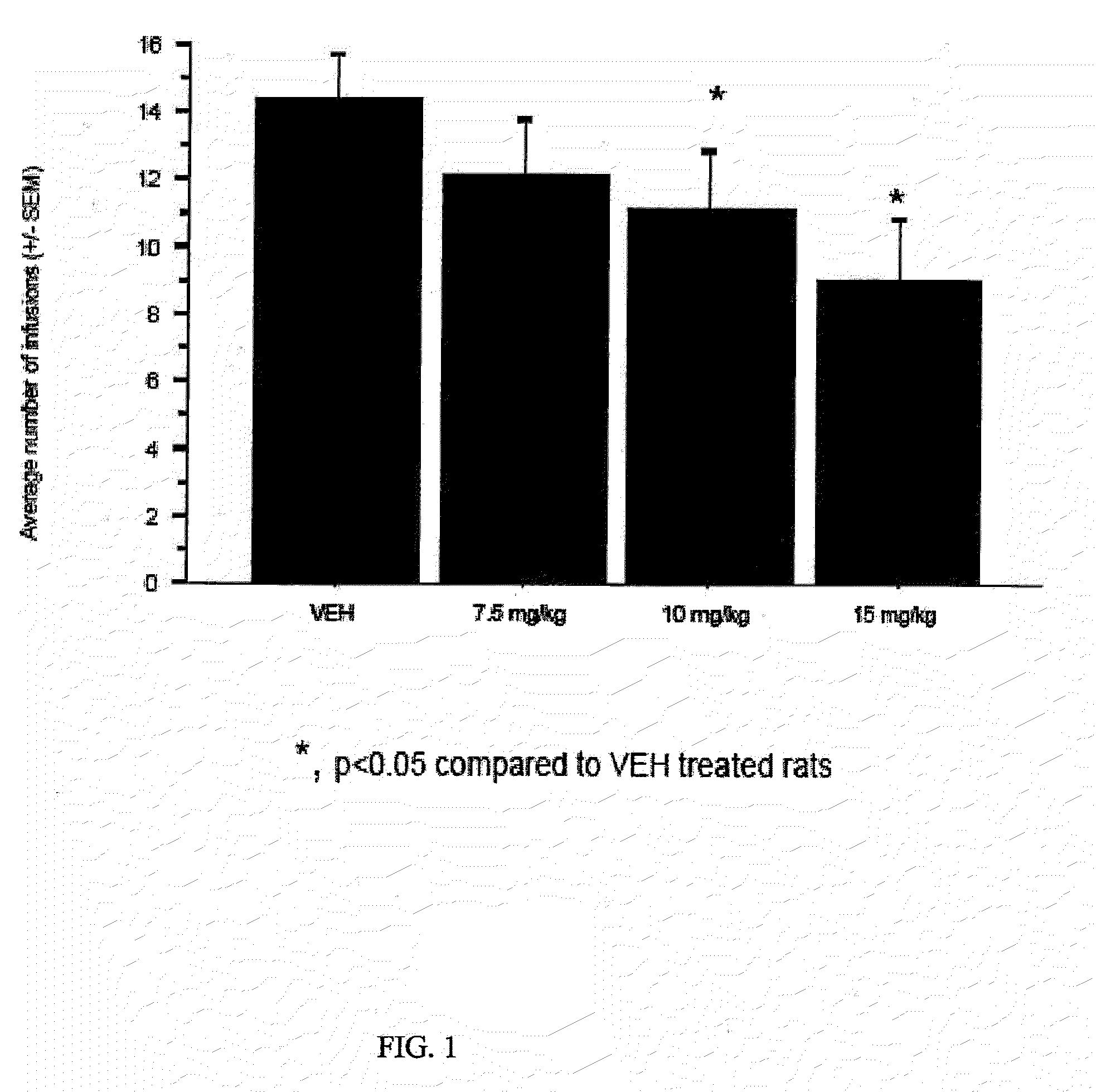
The present invention describes compounds, compositions and methods of using the same comprising lysine covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
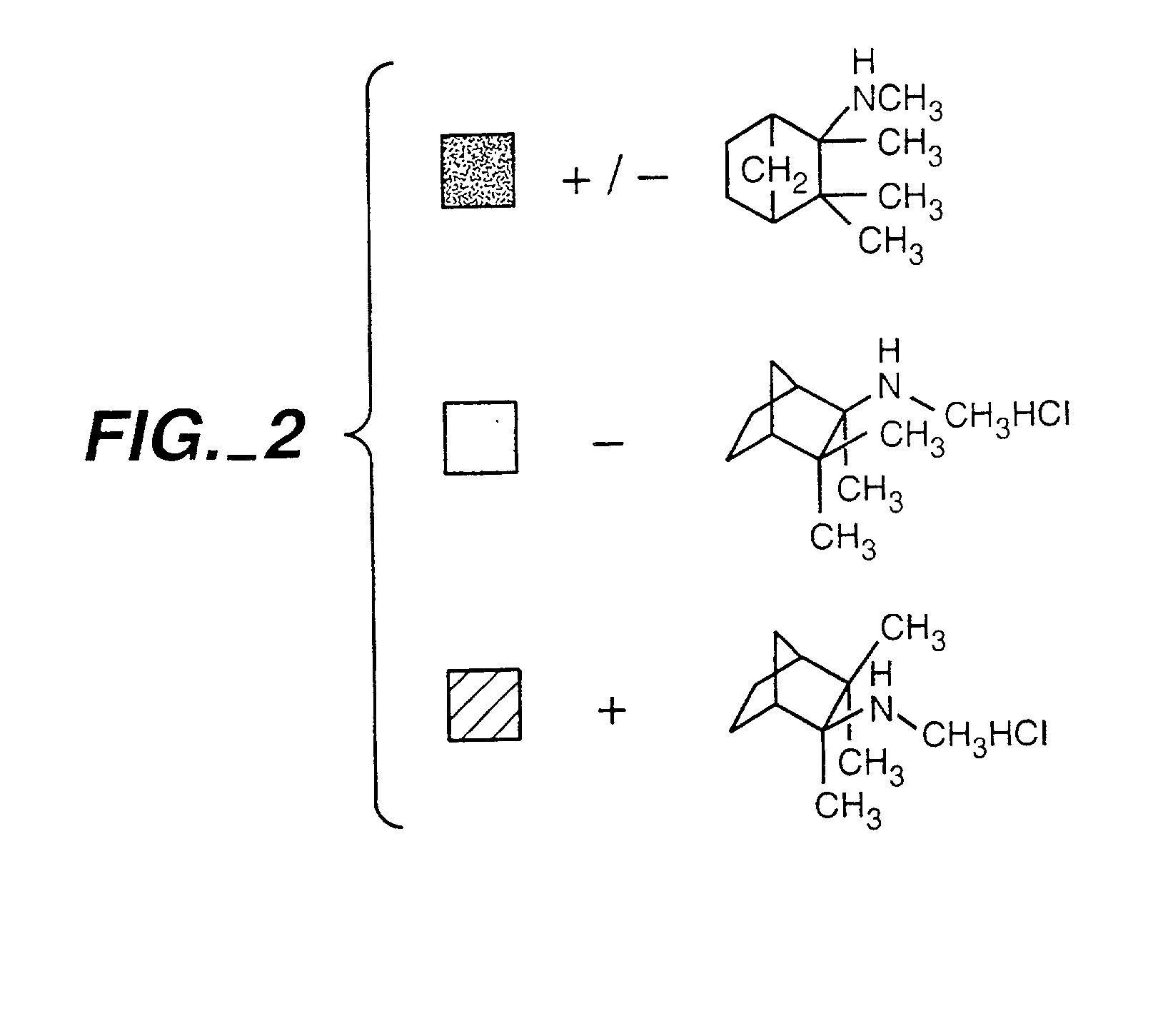
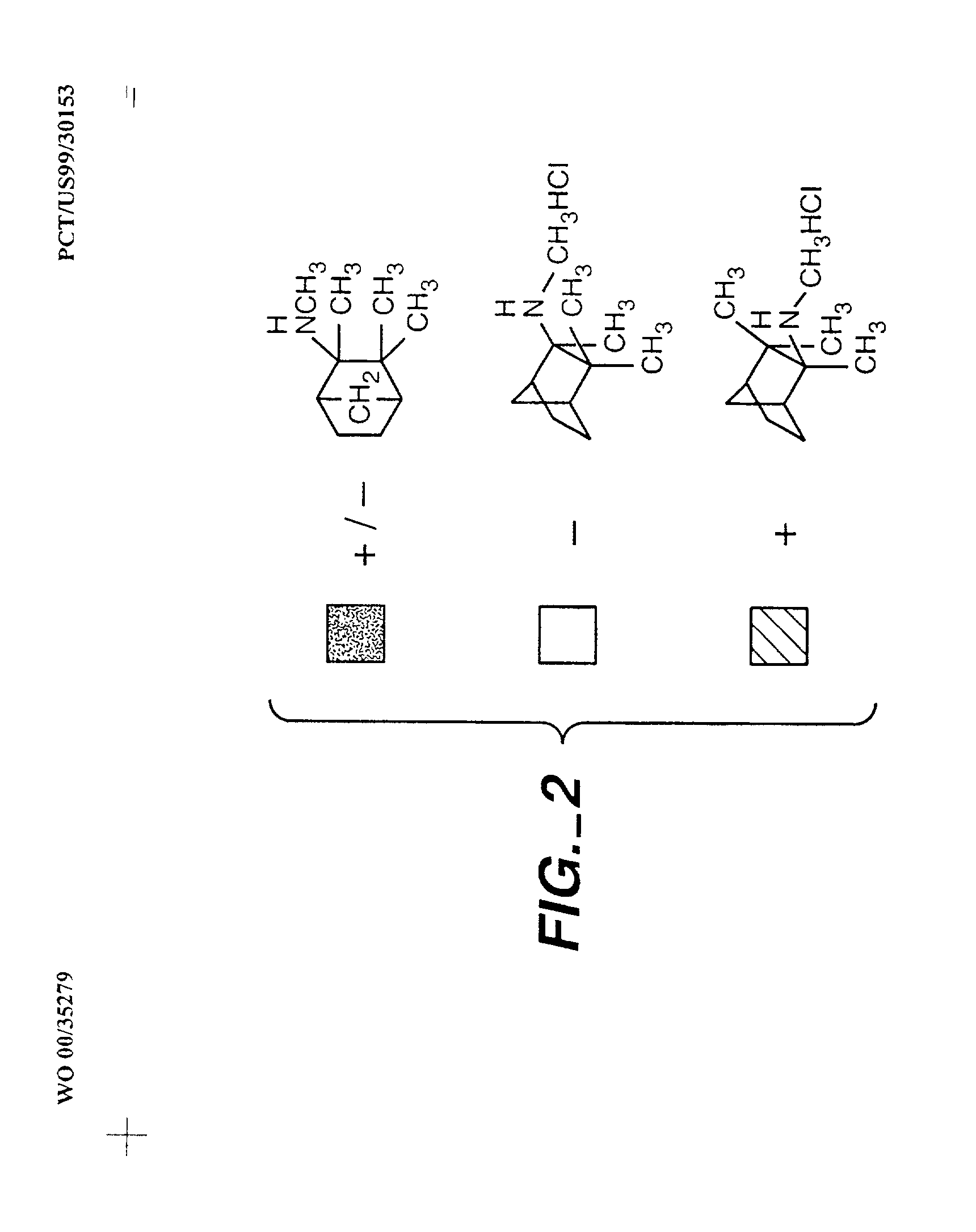
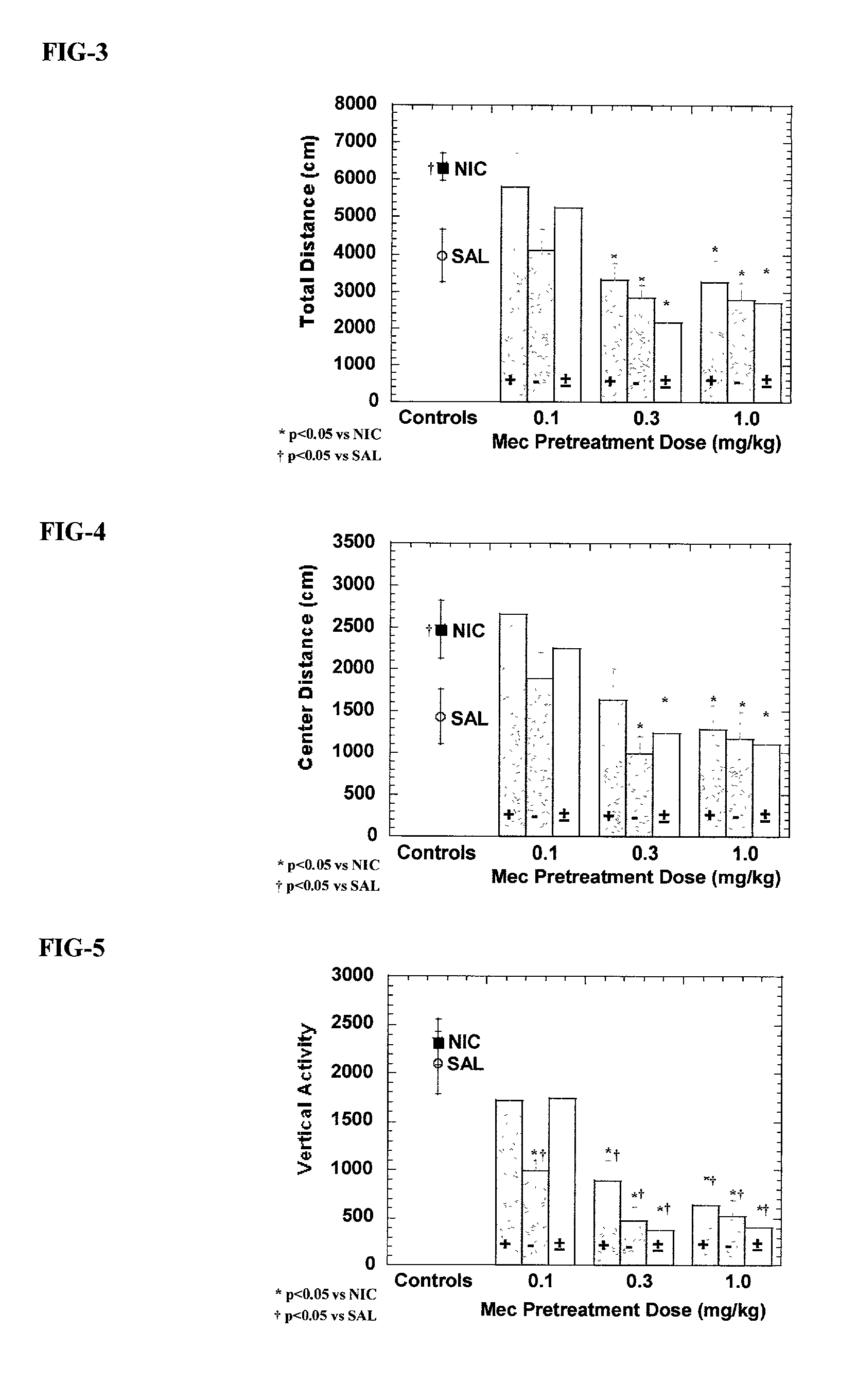
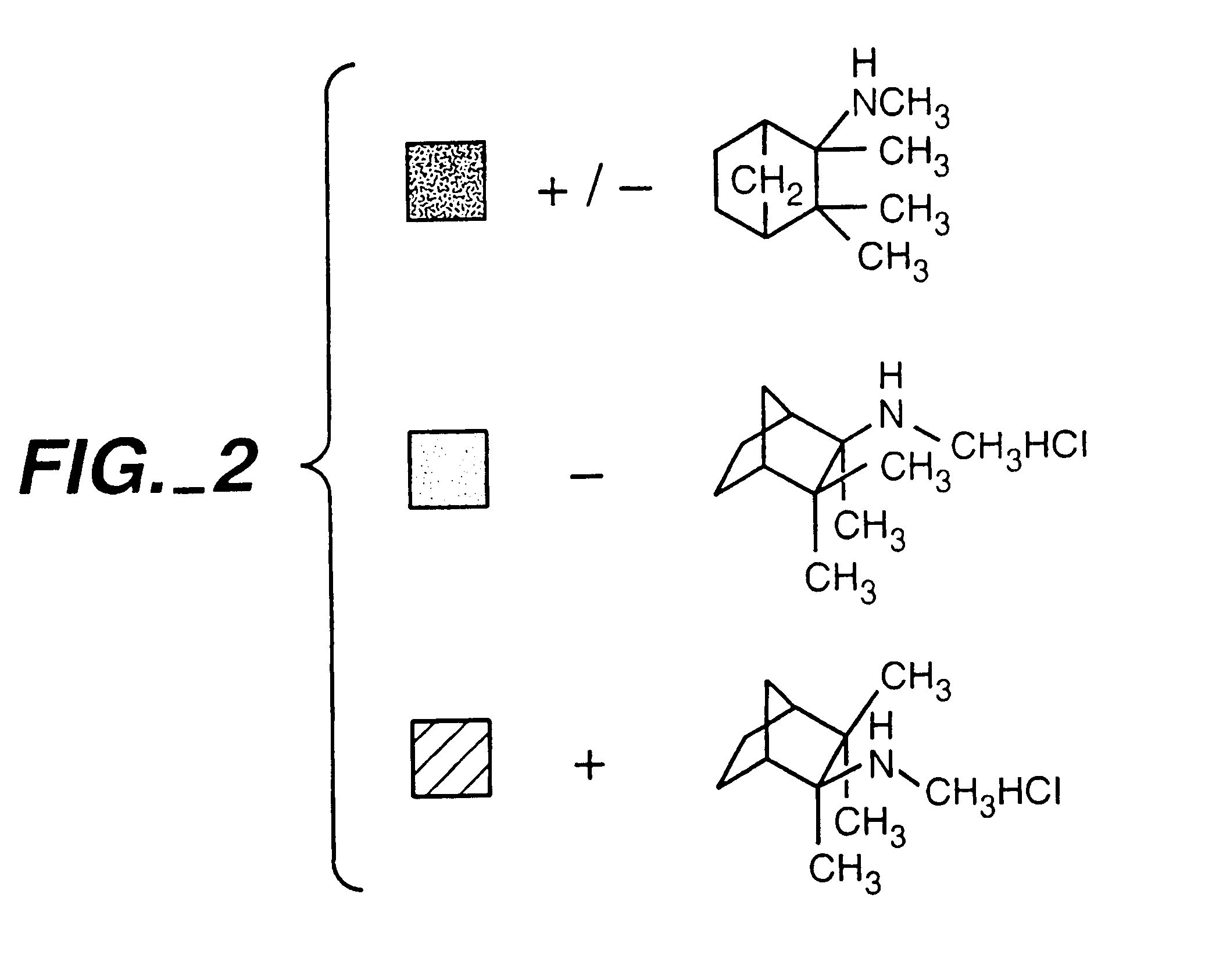
Exo-S-mecamylamine formulation and use in treatment
InactiveUS20020016371A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationWeight gainingSmoking cessation
A pharmaceutical composition includes a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-R-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-R-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
A2a adenosine receptor antagonists
The present invention relates to novel compounds that are A2A adenosine receptor antagonists, and to their use in treating mammals for various disease states, such as obesity, CNS disorders, including the “movement disorders” (Parkinson's disease, Huntington's Chorea, and catelepsy), and cerebral ischemia, excitotoxicity, cognitive and physiological disorders, depression, ADHD, and drug addiction (alcohol, amphetamine, cannabinoids, cocaine, nicotine, and opioids) and to their use in the enhancement of immune response. The invention also relates to methods for the preparation of such compounds, and to pharmaceutical compositions containing them.
Owner:GILEAD SCI INC
Exo-R-mecamylamine formulation and use in treatment
InactiveUS20020016370A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationStimulantS syndrome
A pharmaceutical composition includes a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-S-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-S-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
Abuse-resistant amphetamine prodrugs
InactiveUS7659253B2Reducing patient to patient variabilityRisk minimizationBiocideOrganic chemistryDiseaseChemical Moiety
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Oral pulsed dose drug delivery system
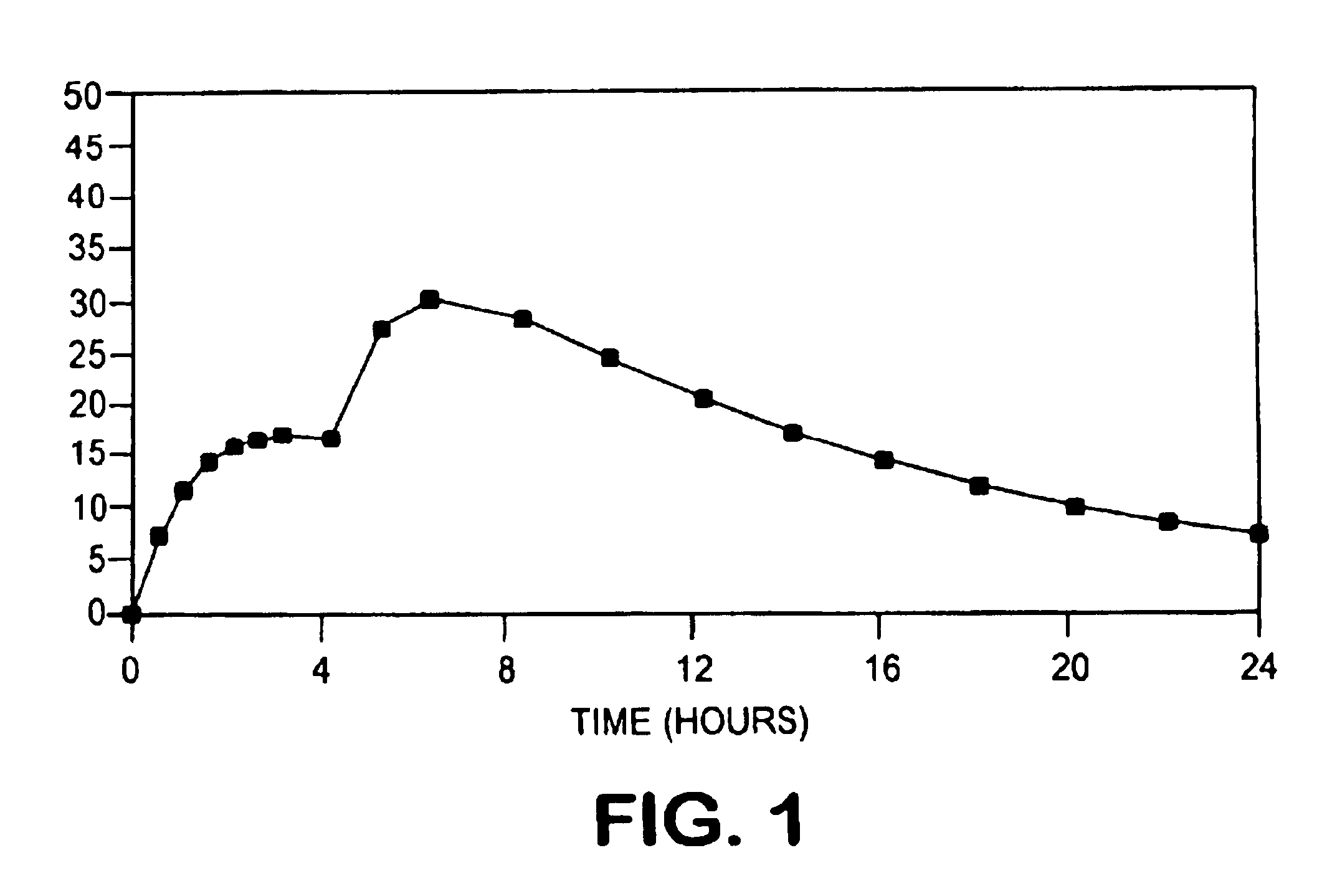
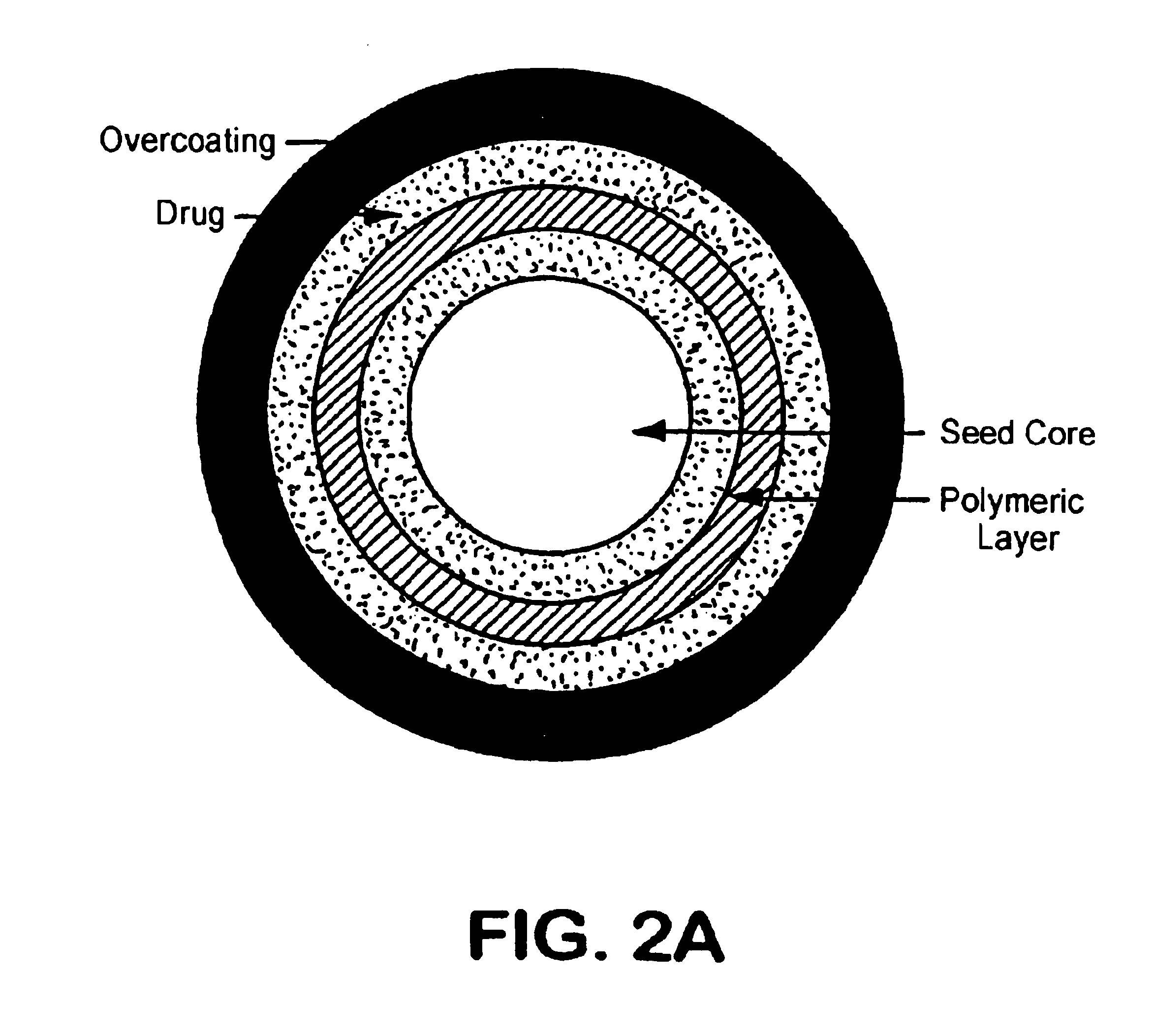
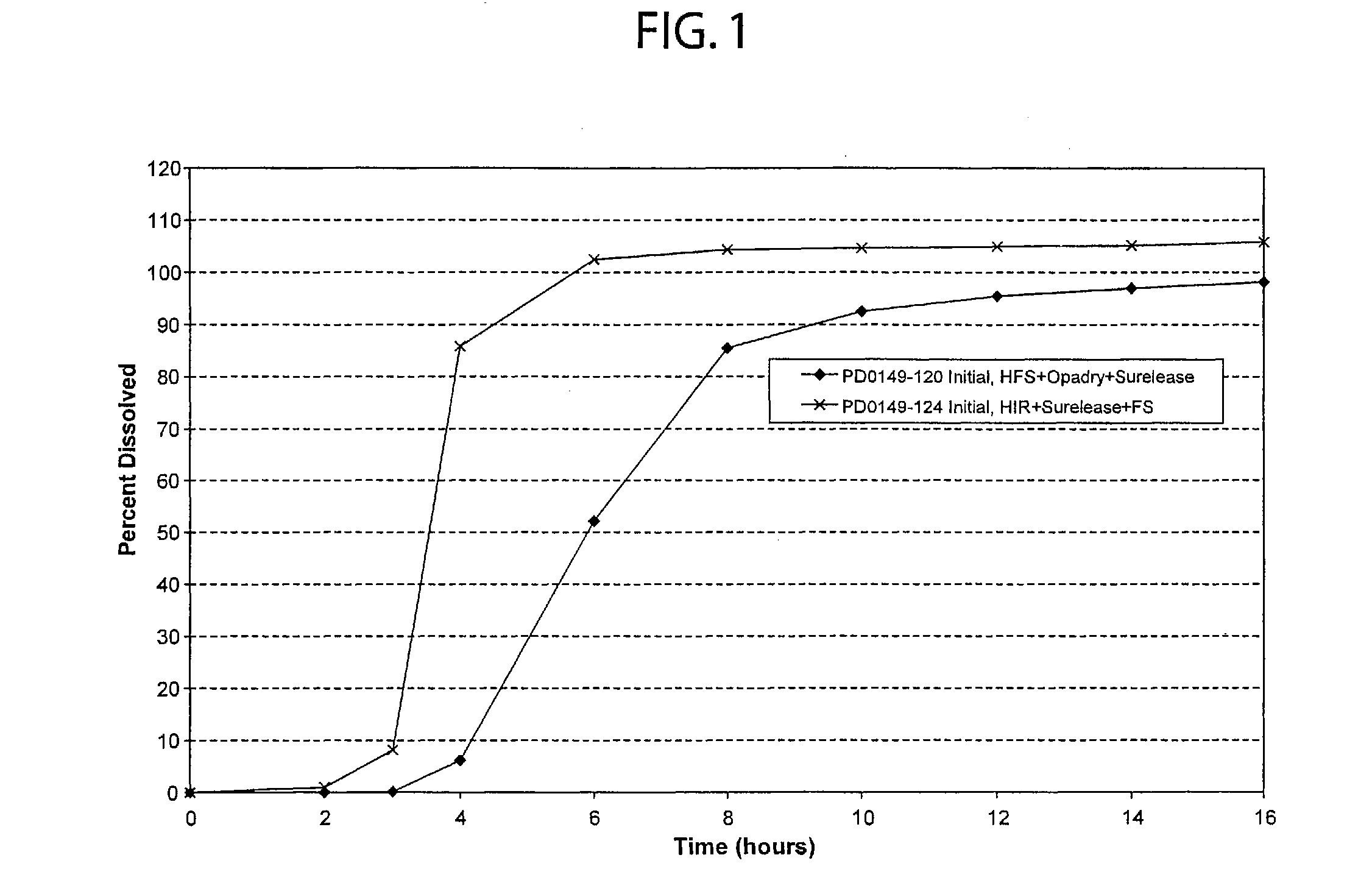
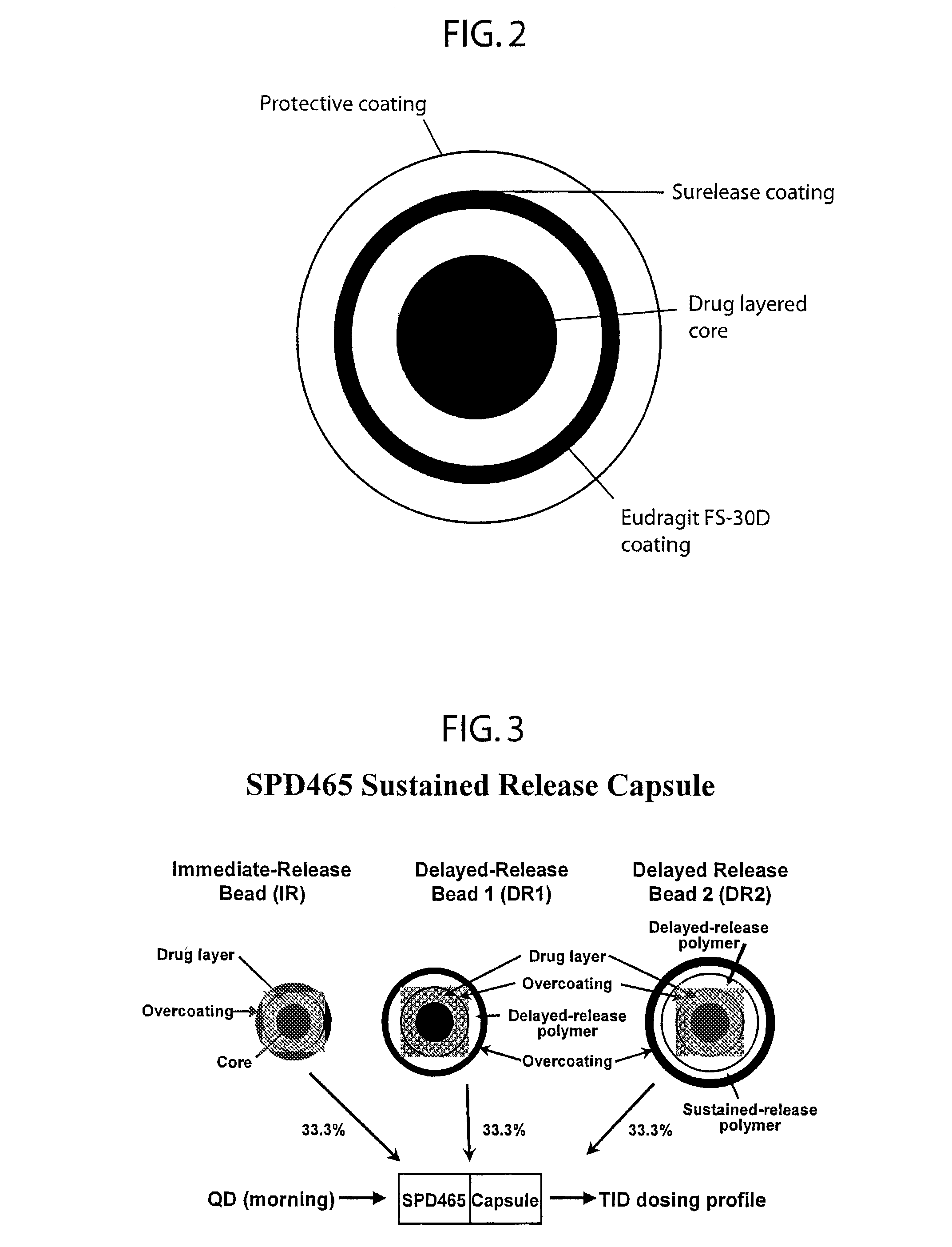
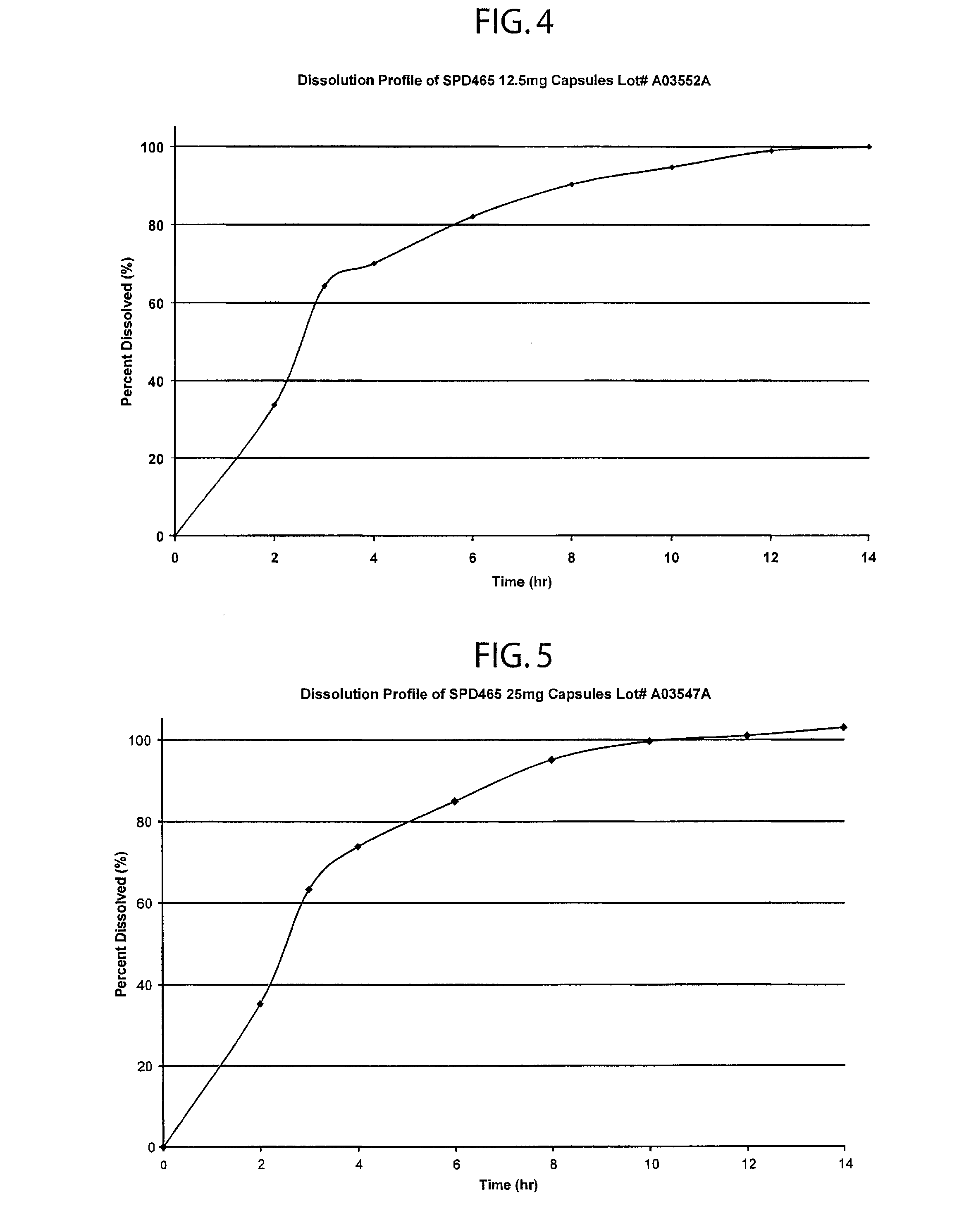
InactiveUSRE41148E1Prolonged delayed release timeDelayed release timeBiocideOrganic active ingredientsImmediate releaseDosing drugs
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising an immediate-release component and an enteric delayed-release component wherein (1) the enteric release coating has a defined minimum thickness and / or (2) there is a protective layer between the pharmaceutically active amphetamine salt and the enteric release coating and / or (3) there is a protective layer over the enteric release coating. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:SHIRE PLC
Monoclonal antibodies that selectively recognize methamphetamine and methamphetamine like compounds
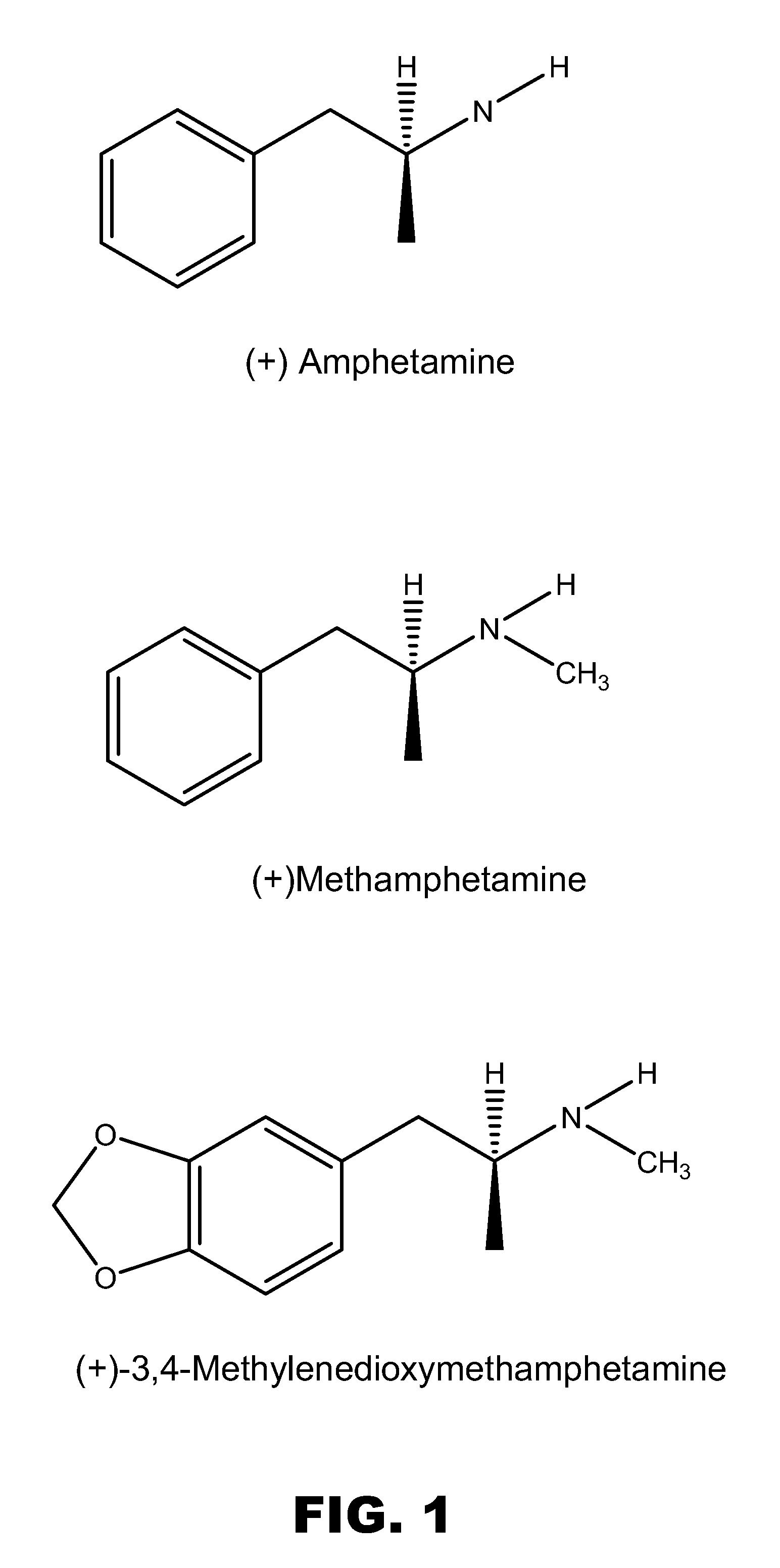
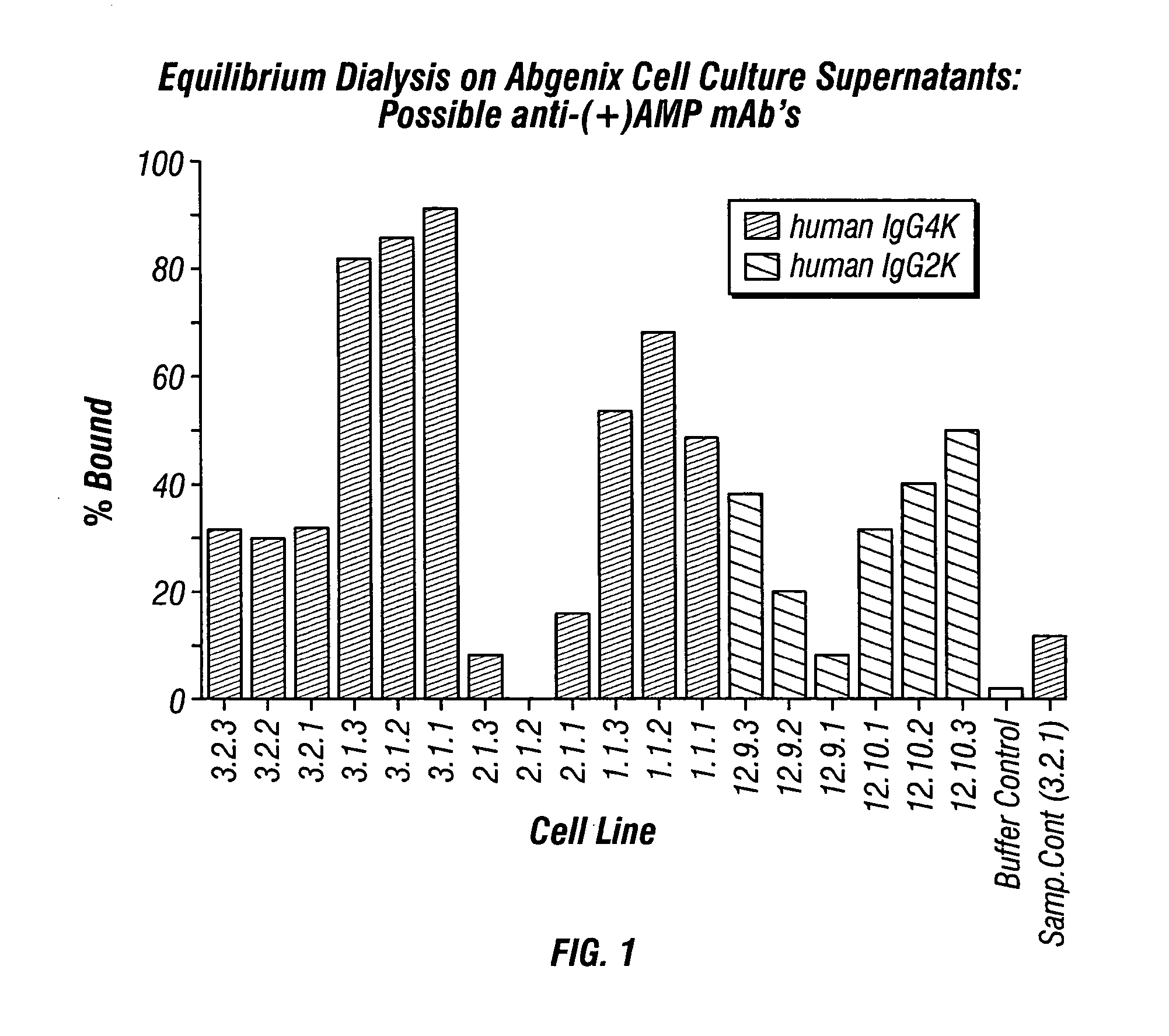
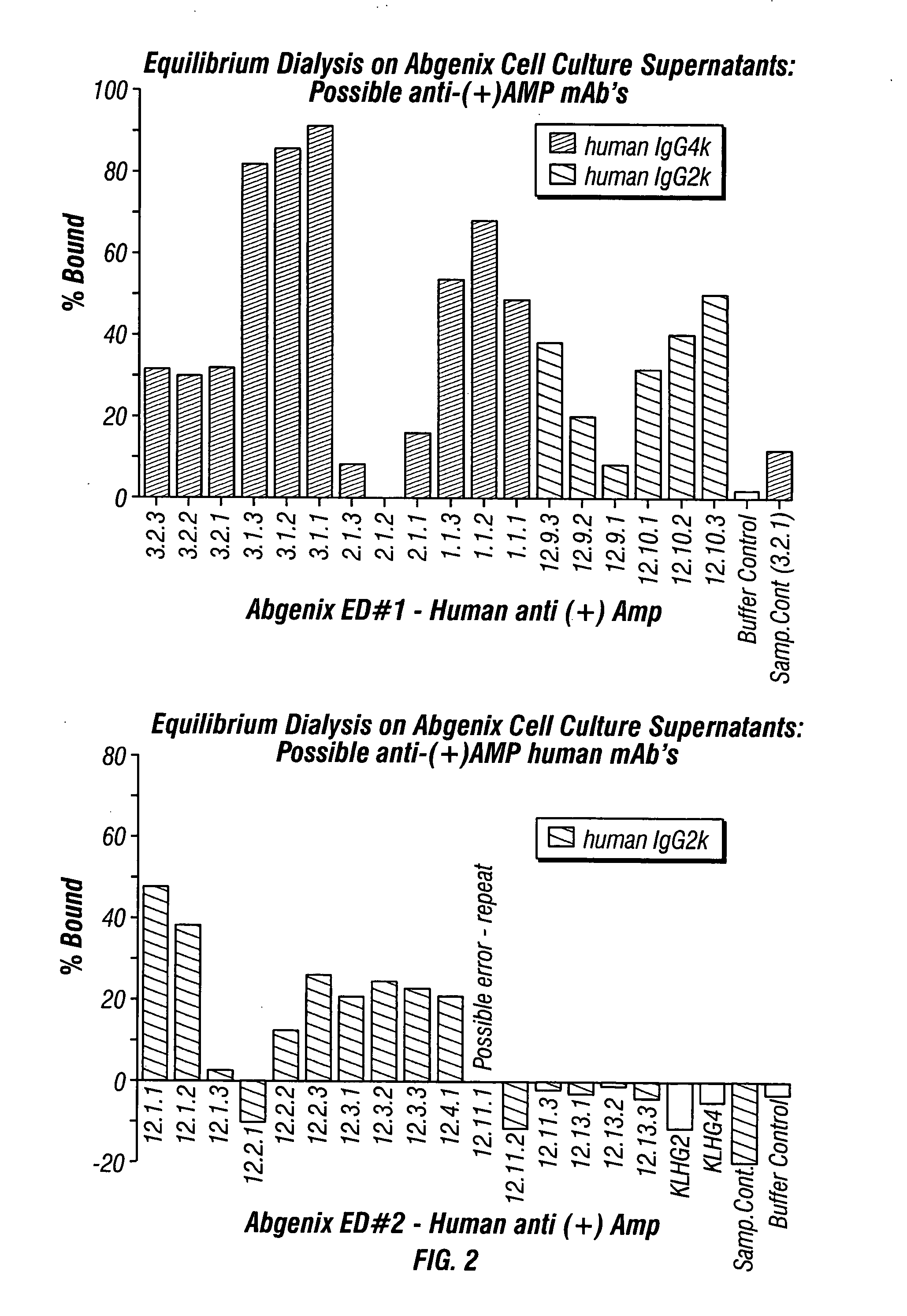
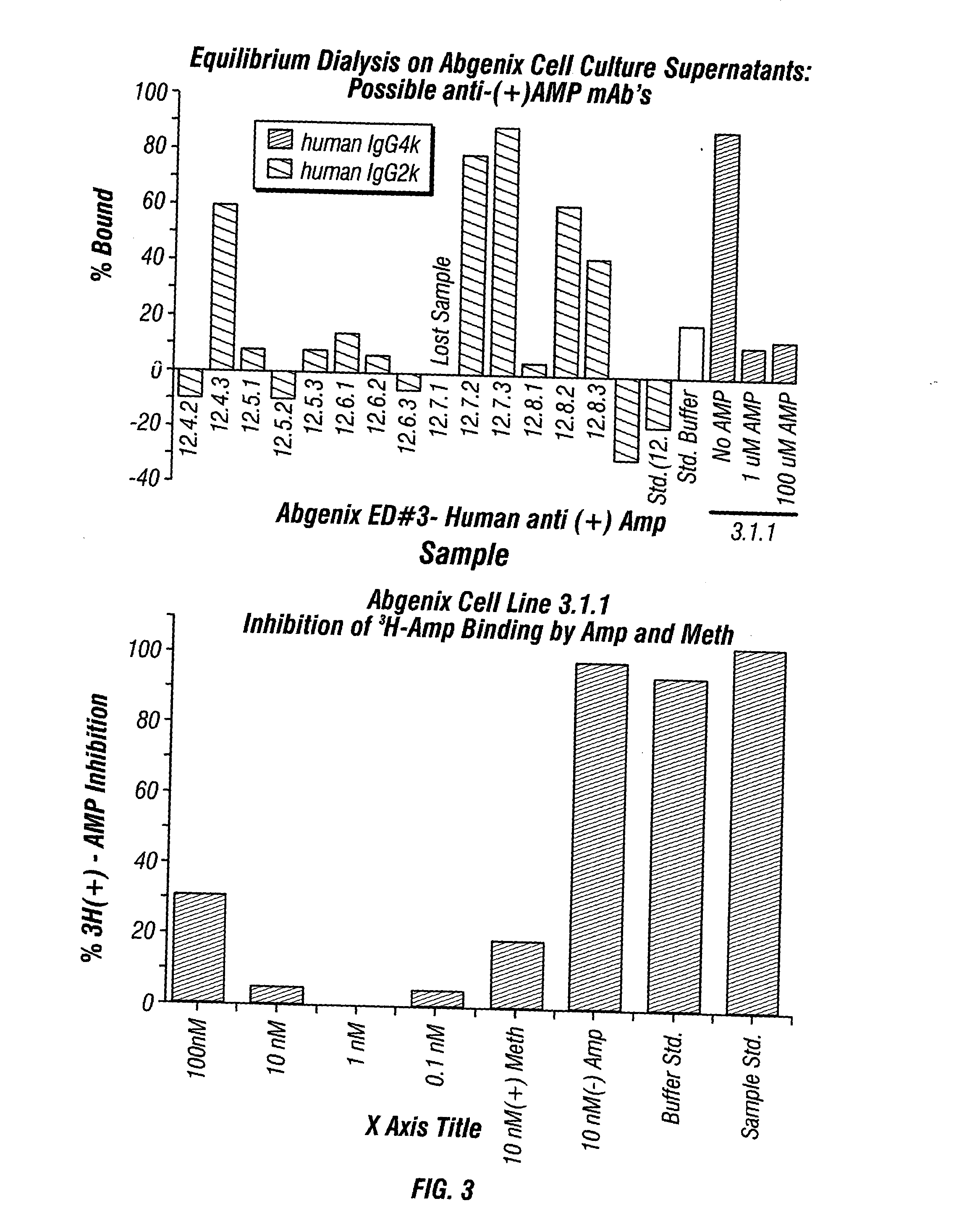
ActiveUS20080125579A1Reduce concentrationImmunoglobulins against animals/humansAntibody ingredientsMonoclonal antibodyMDMA
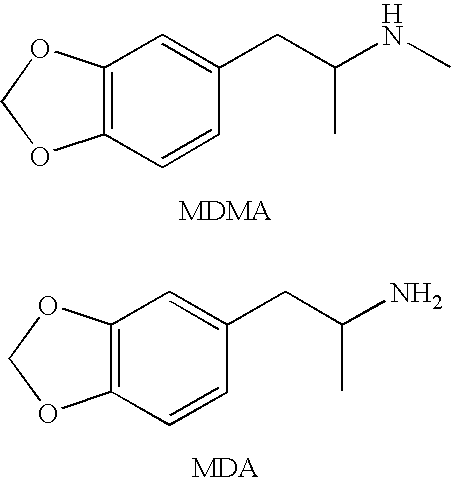
The invention generally relates to monoclonal antibodies that recognize at least one compound from the group consisting of (+) methamphetamine, (+) amphetamine, and (+) 3,4-methylenedioxymethamphetamine ((+) MDMA). Generally speaking, the monoclonal antibodies do not recognize (−) methamphetamine, (−) amphetamine, or (−) MDMA.
Owner:BIOVENTURES LLC
Controlled dose drug delivery system
ActiveUS8846100B2Meet needsReduce the amount requiredOrganic active ingredientsBiocideDose deliveryImmediate release
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising a pharmaceutically active amphetamine salt covered with an immediate-release coating and a pharmaceutically active amphetamine salt covered with an enteric coating wherein the immediate release coating and the enteric coating provide for multiple pulsed dose delivery of the pharmaceutically active amphetamine salt. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:TAKEDA PHARMA CO LTD
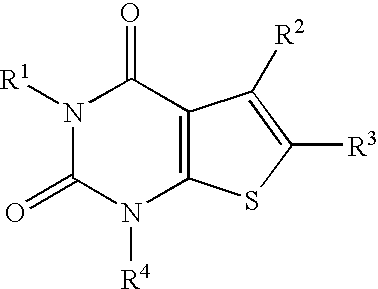
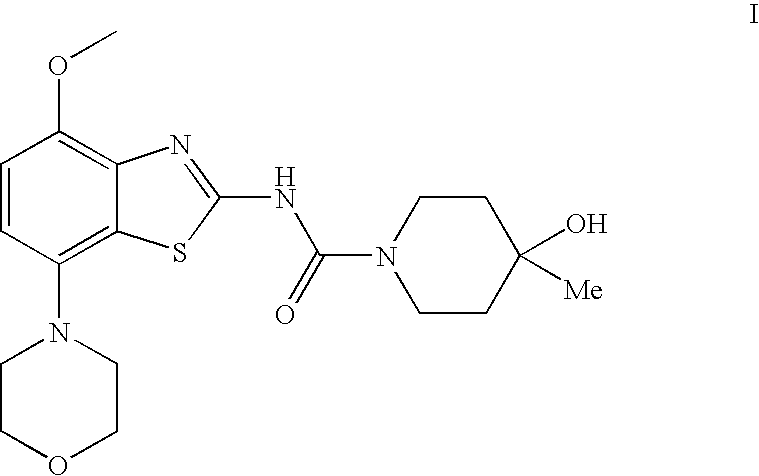
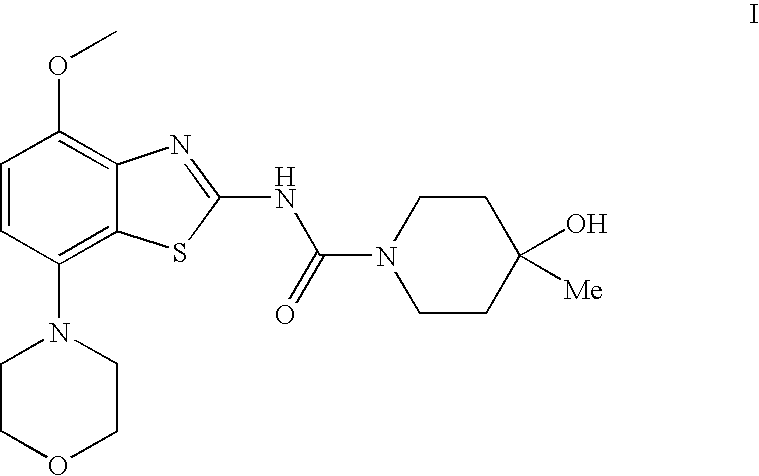
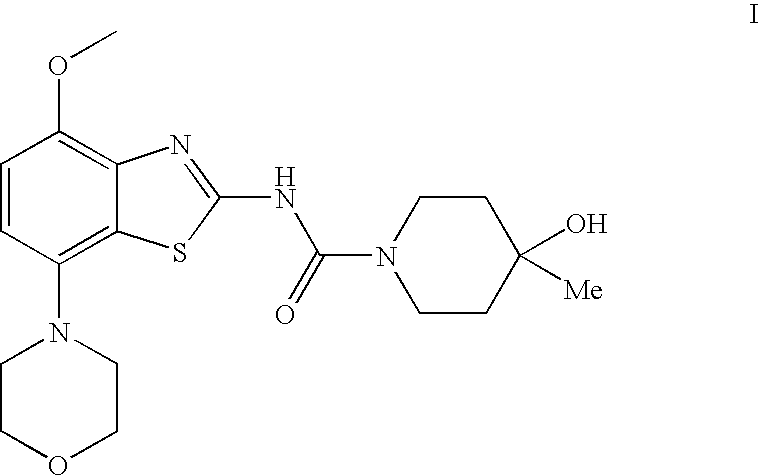
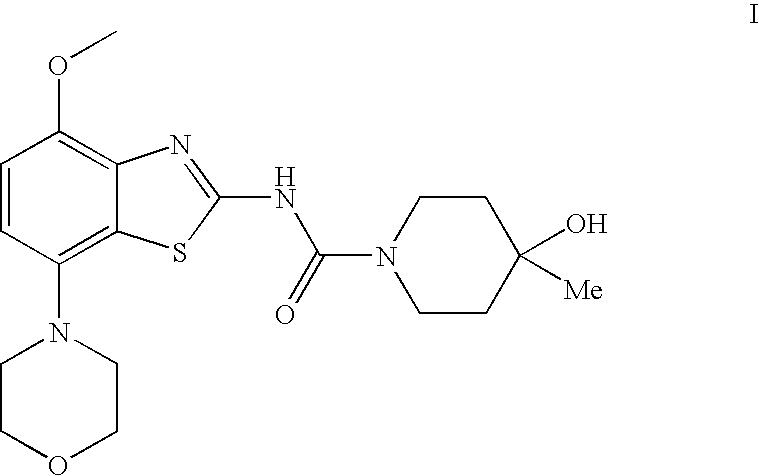
4-Hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide
ActiveUS20050261289A1High affinityOrganic active ingredientsNervous disorderSubstance abuserDisease cause
The present invention relates to the compound of formula which is 4-hydroxy-4-methyl-piperidine-1-carboxylic acid(4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide, and to pharmaceutically acceptable acid addition salts thereof. It has been found that the compound is useful for the treatment or prevention of Alzheimer's disease, Parkinson's disease, Huntington's disease, neuroprotection, schizophrenia, anxiety, pain, respiration deficits, depression, ADHD (attention deficit hyper-activity disorder), drug addiction to amphetamines, cocaine, opioids, ethanol, nicotine, or cannabinoids, or for the treatment of asthma, allergic responses, hypoxia, ischemia, seizure, substance abuse, or for use as muscle relaxants, antipsychotics, antiepileptics, anticonvulsants and cardioprotective agents.
Owner:F HOFFMANN LA ROCHE INC
Exo-S-mecamylamine formulation and use in treatment
InactiveUS6734215B2Improve Medication AdherenceImprove the quality of lifeBiocideNervous disorderStimulantOpiate
Medical conditions are treated by administering a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-R-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), Tourette's Syndrome, and neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder).
Owner:UNIV OF SOUTH FLORIDA
Oral pulsed dose drug delivery system
InactiveUSRE42096E1Control erosionDelayed release timePowder deliveryOrganic active ingredientsCaplet Dosage FormImmediate release
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising an immediate-release component and an enteric delayed-release component wherein (1) the enteric release coating has a defined minimum thickness and / or (2) there is a protective layer between the pharmaceutically active amphetamine salt and the enteric release coating and / or (3) there is a protective layer over the enteric release coating. The product can be composed of either one of a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:SHIRE PLC
Antibodies against drugs of abuse
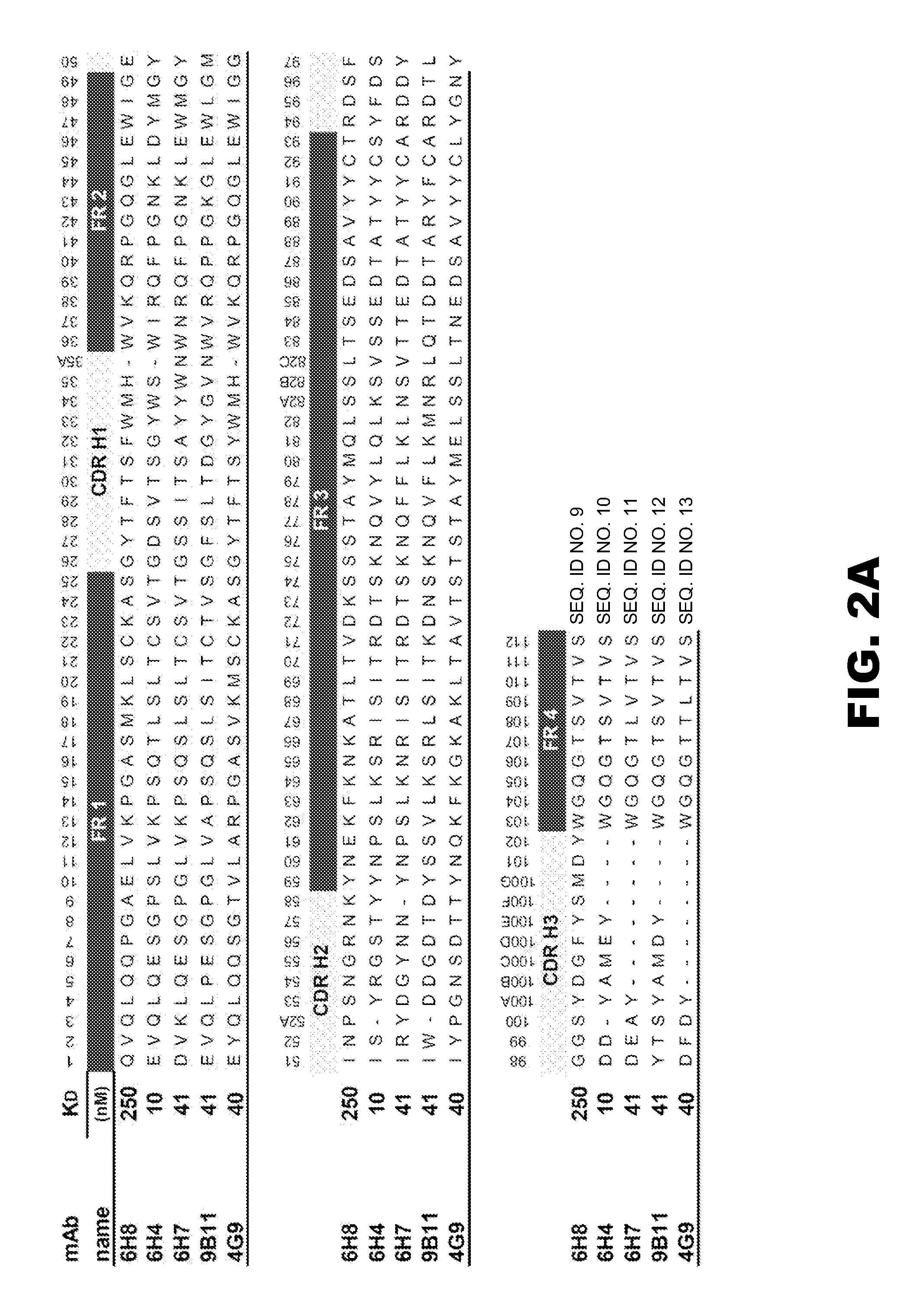
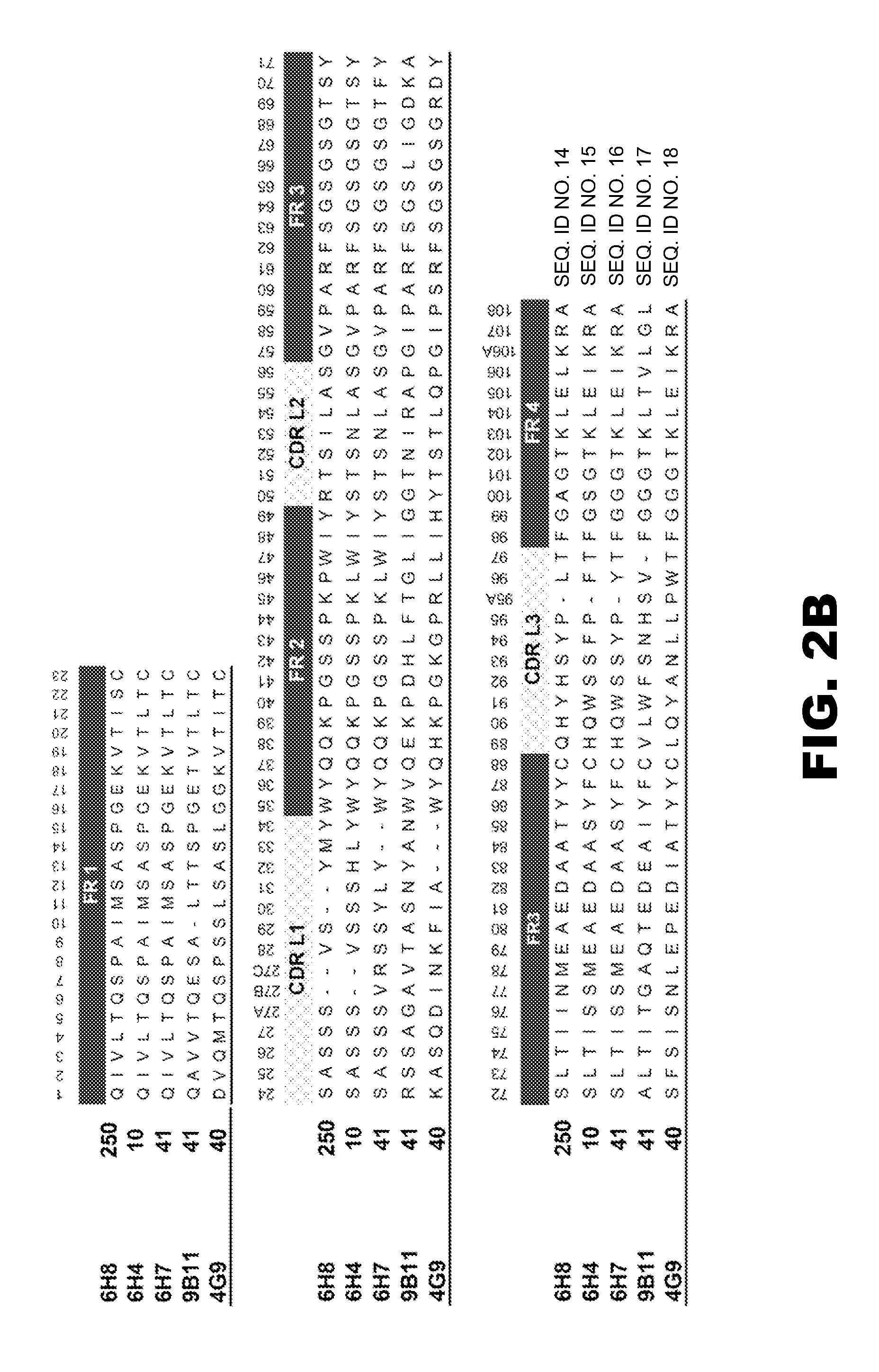
InactiveUS20050013809A1Reduce exposureAnimal cellsImmunoglobulins against animals/humansComplementarity determining regionHeavy chain
The present invention is related to antibodies directed to various drugs of abuse and uses of such antibodies. In preferred embodiments, the drugs of abuse are amphetamine, methamphetamine, or phencyclidine (PCP). In particular, in accordance with the present invention, there are provided fully man monoclonal antibodies directed to drugs of abuse. Nucelotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDR's), specifically from FR1 through FR3 or CDR1 through CDR3, are provided. Hybridomas or other cell lines expressing such immunoglobulin molecules and monoclonal antibodies are also provided.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
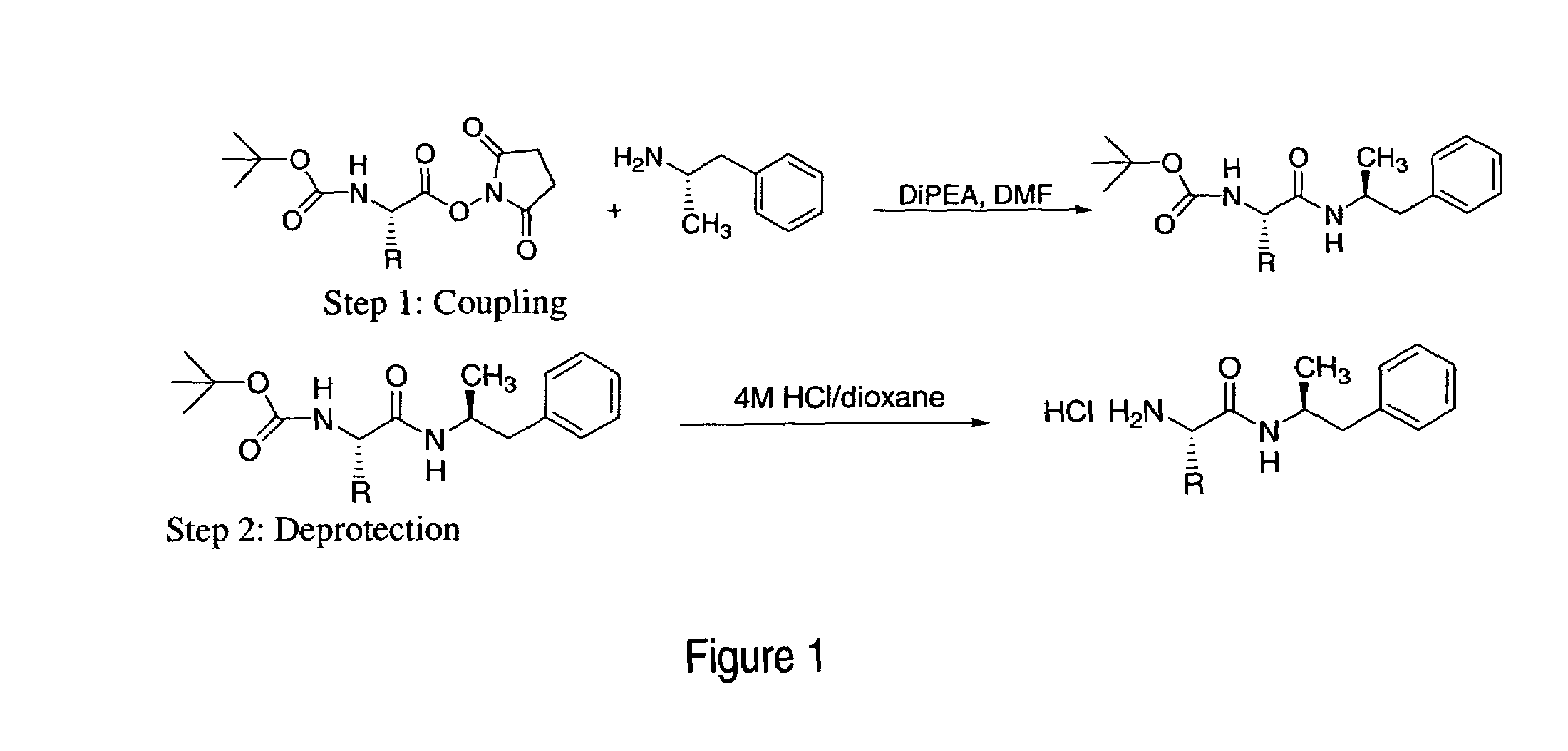
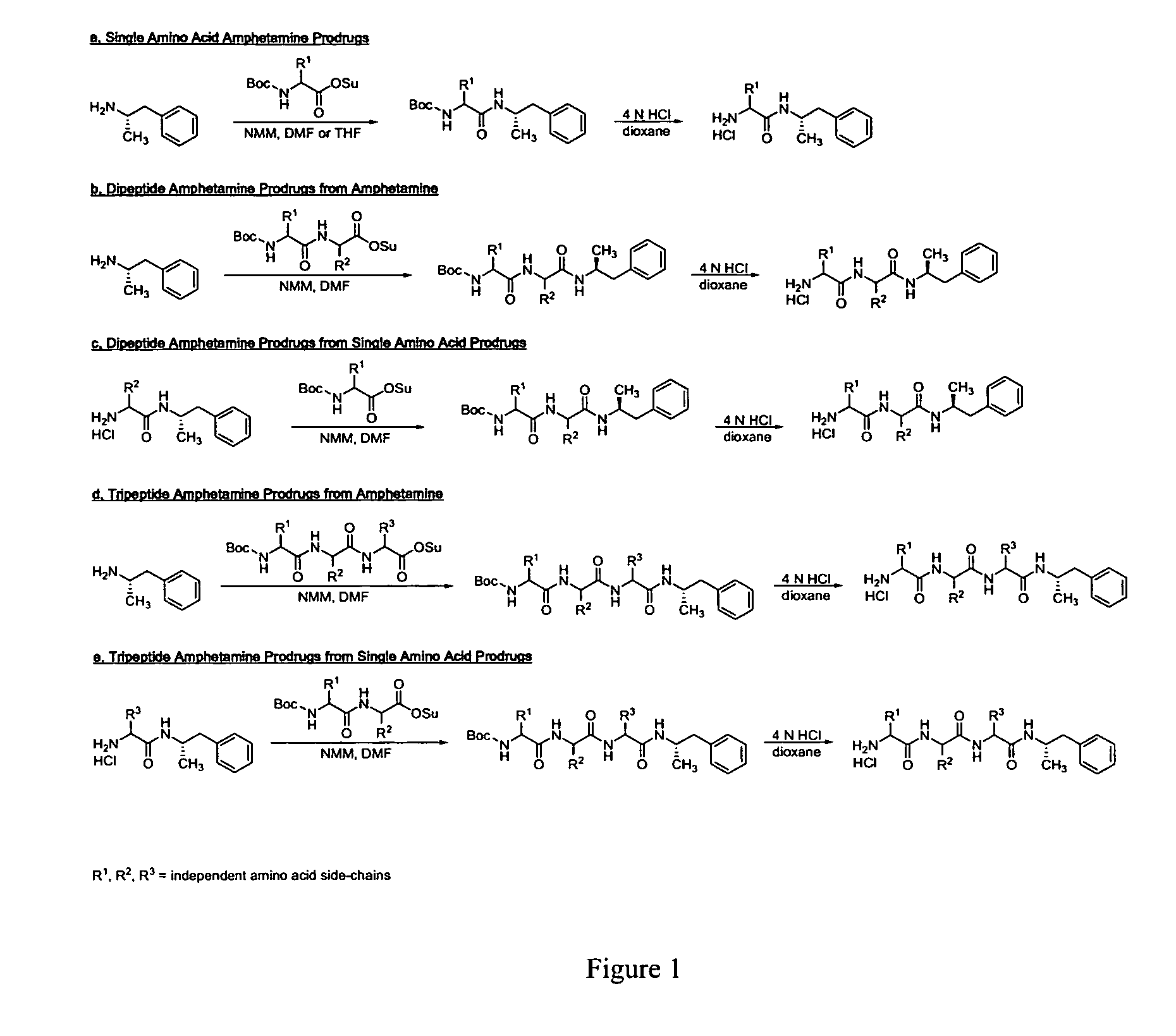
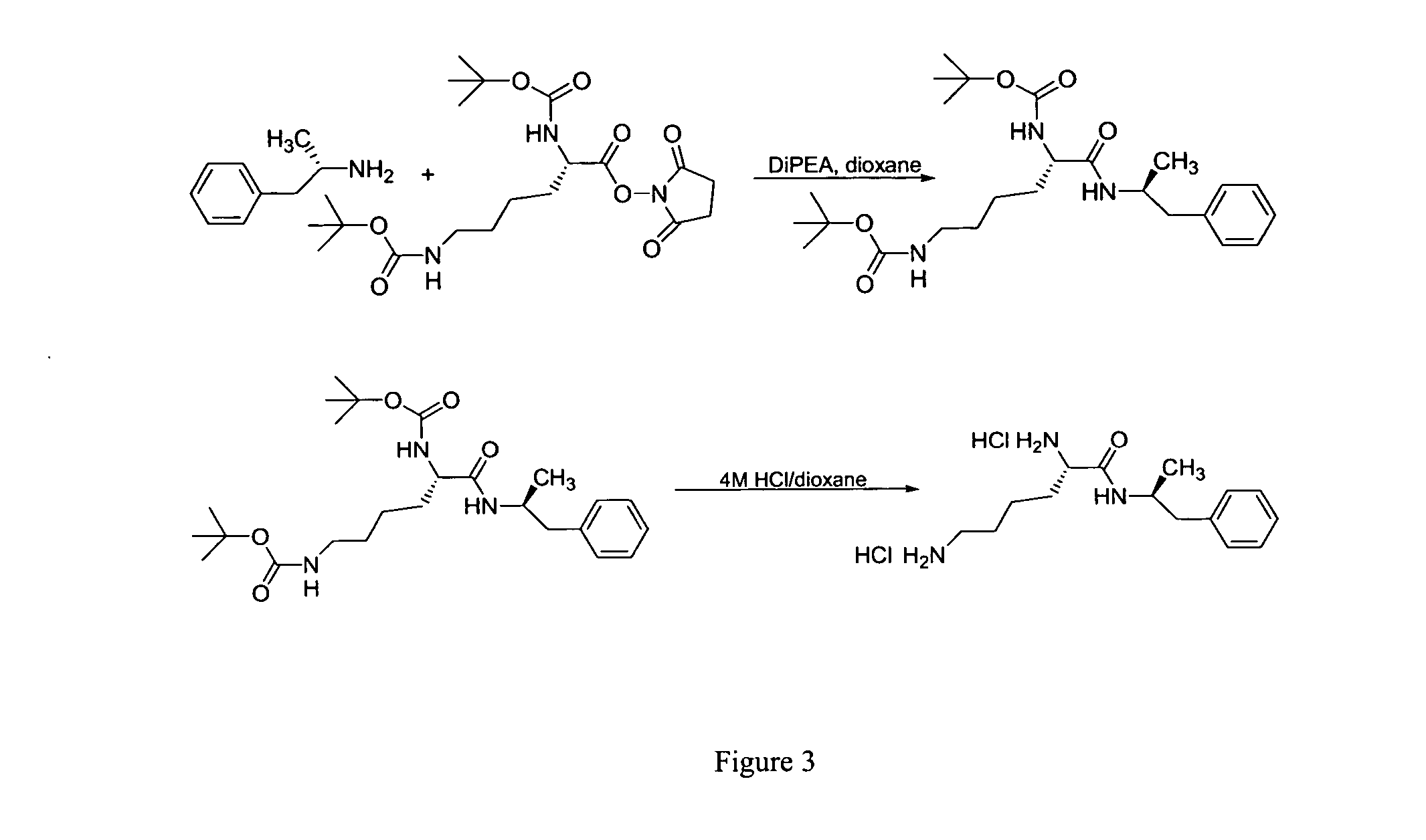
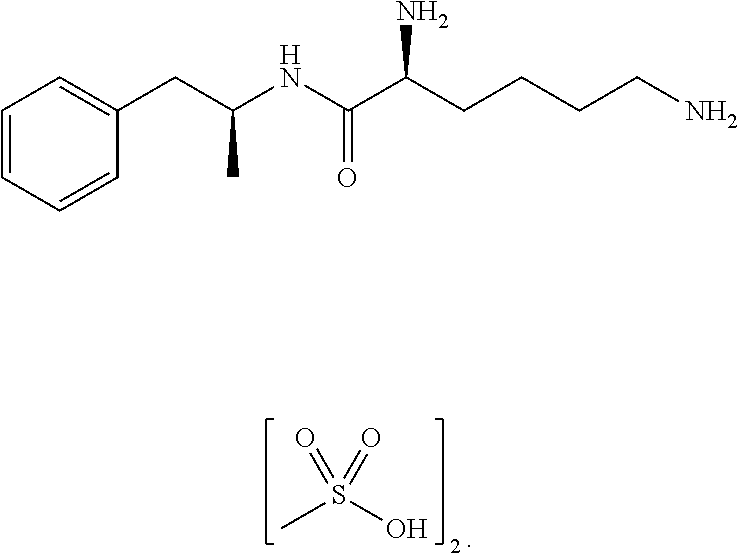
Non-standard amino acid conjugates of amphetamine and processes for making and using the same
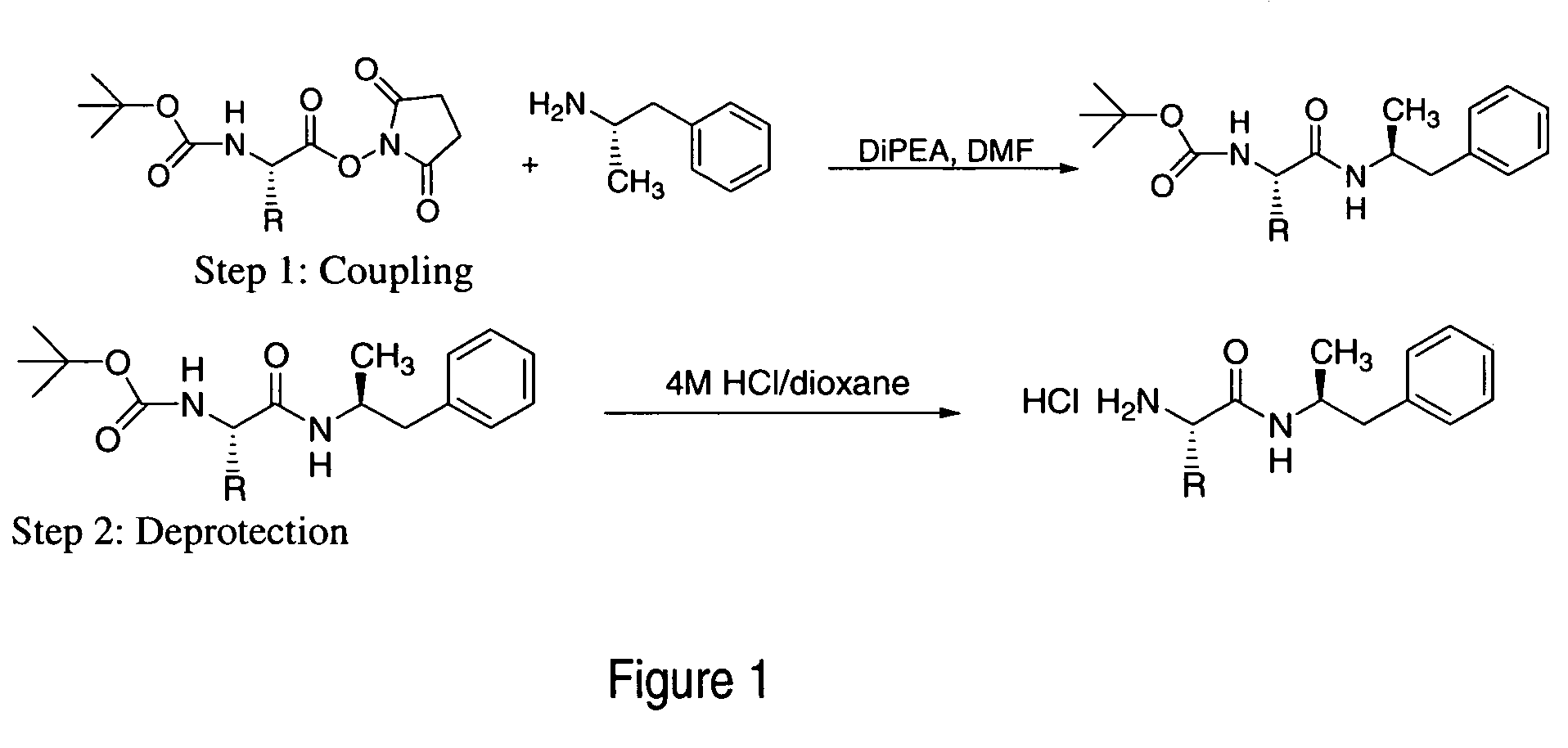
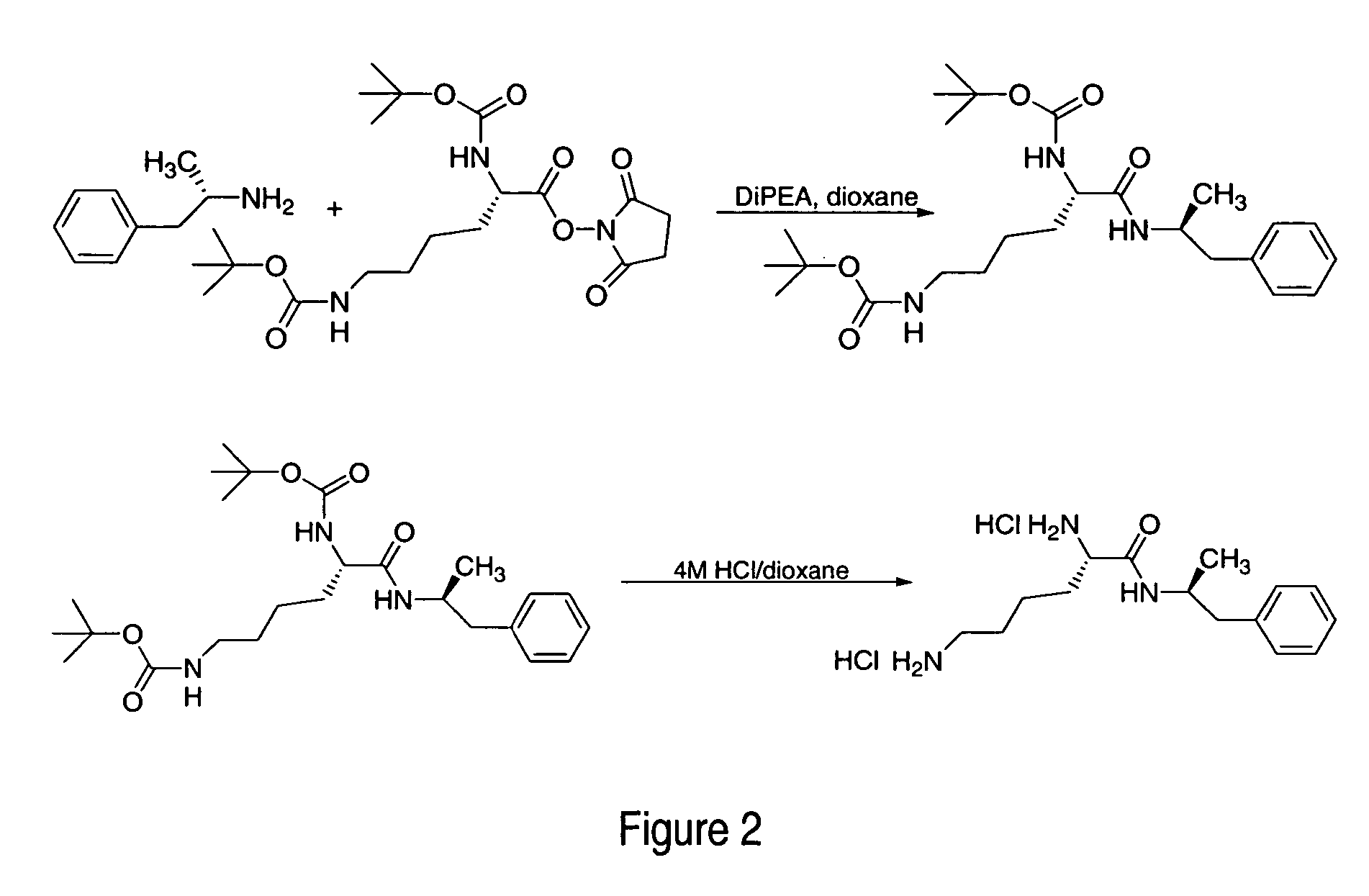
ActiveUS7776917B2Diminish and eliminate pharmacological activityInhibition effectBiocideNervous disorderAmphetamine useAmino acid
Disclosed are amphetamine prodrug compositions comprising at least one non-standard amino acid conjugate of amphetamine, a salt thereof, a derivative thereof, or a combination thereof. Methods of making and using the same are also disclosed.
Owner:TAKEDA PHARMA CO LTD
Method of treating binge eating disorder and obesity resulting from binge eating behavior
Owner:LUCERNE BIOSCI
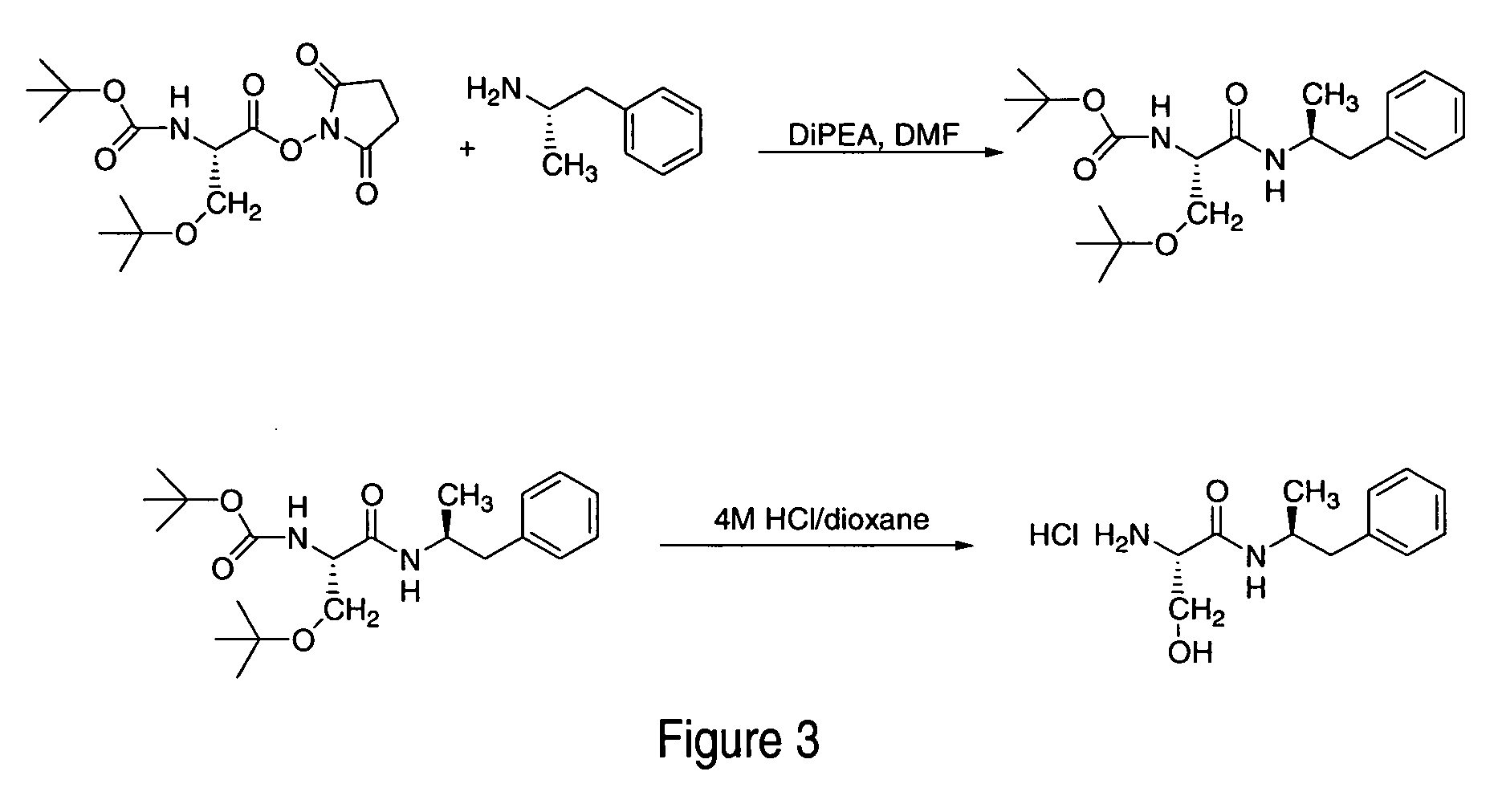
Polar hydrophilic prodrugs of amphetamine and other stimulants and processes for making and using the same
ActiveUS7772222B2Eliminate the effects ofReduce stressBiocideNervous disorderChemical ligationStimulant
Owner:TAKEDA PHARMA CO LTD
Reversal of general anesthesia by administration of methylphenidate, amphetamine, modafinil, amantadine, and/or caffeine
InactiveUS20130310422A1High speedReduces and eliminates effectBiocidePharmaceutical delivery mechanismUnconsciousnessWhole body
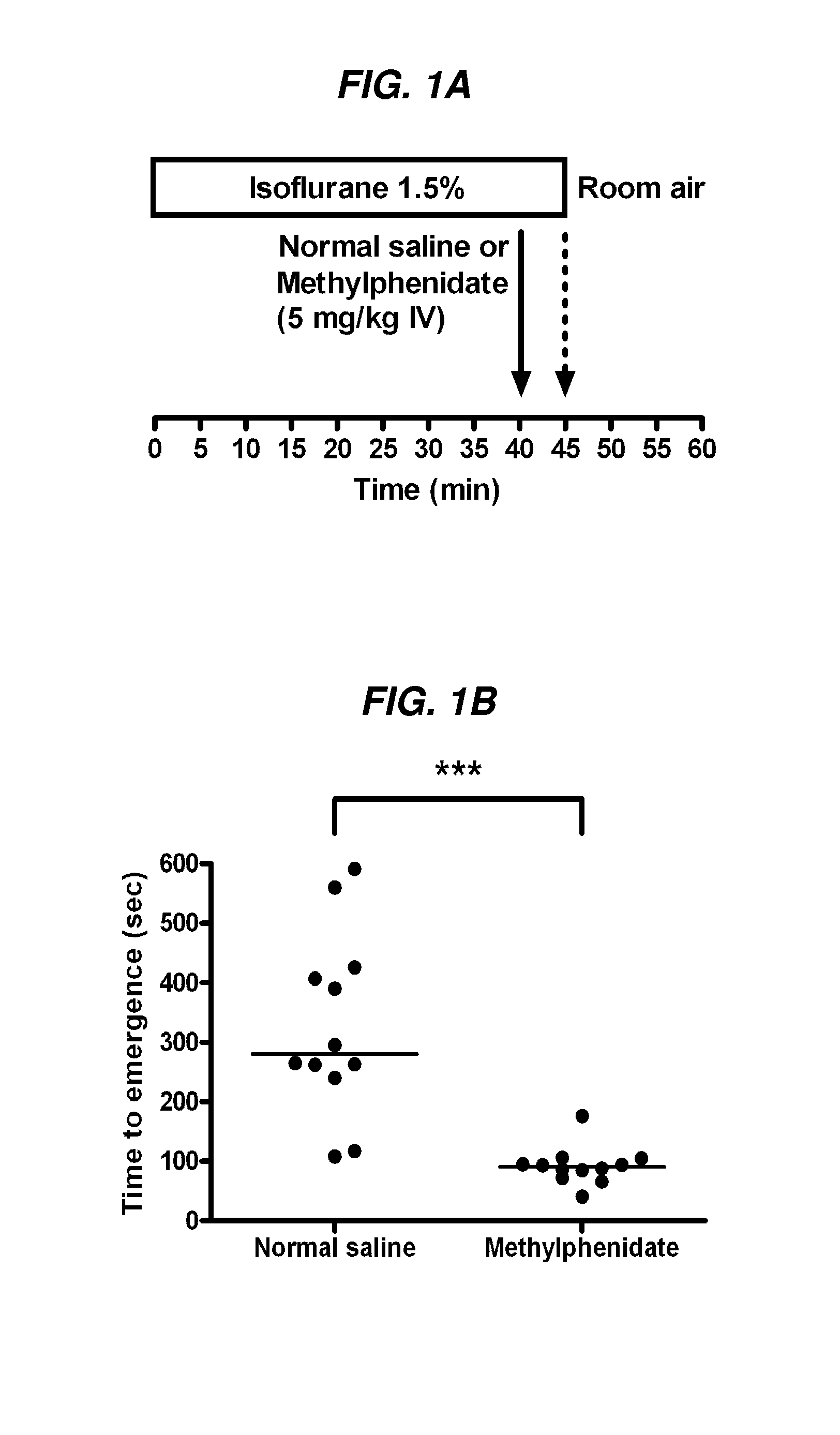
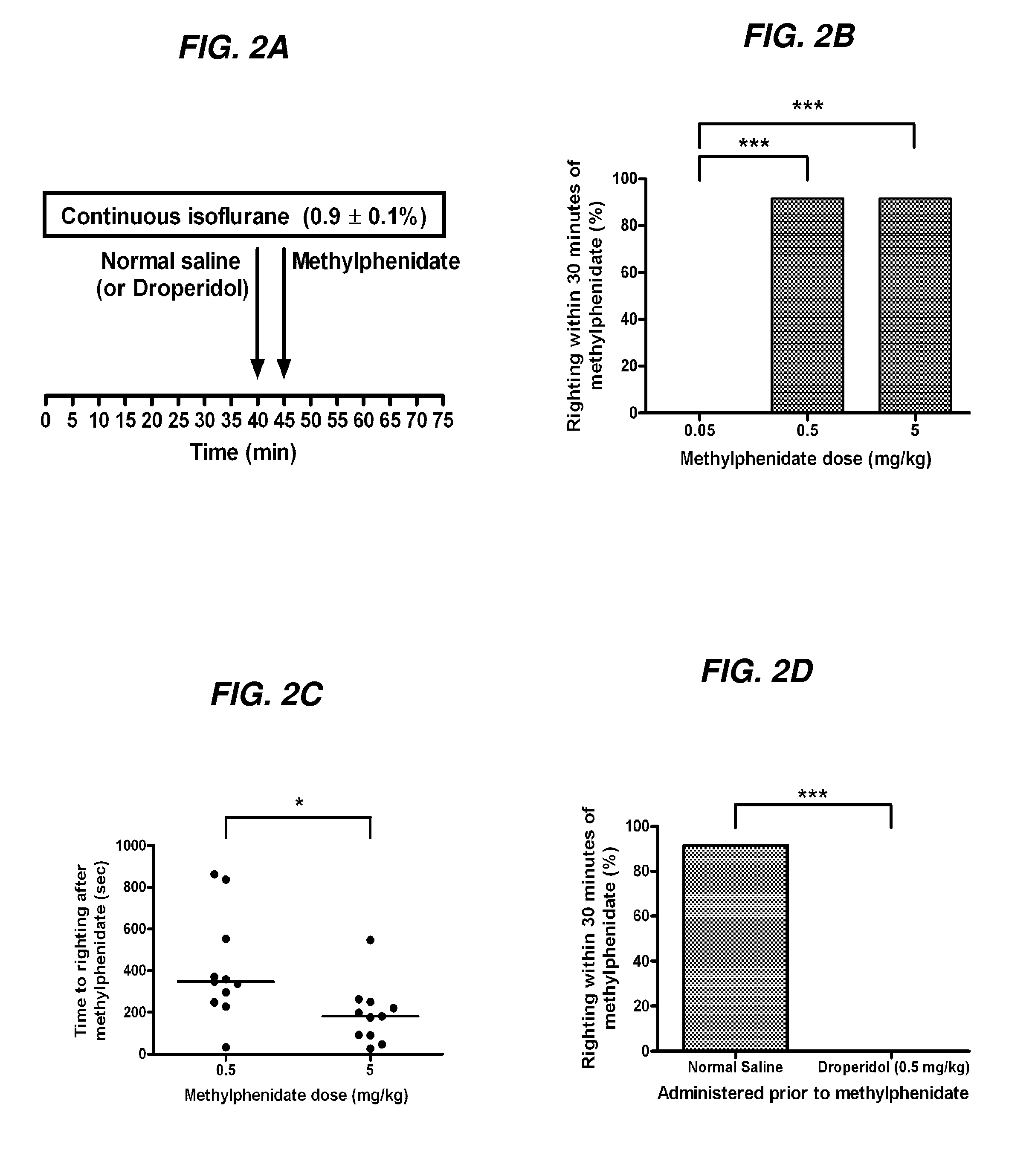
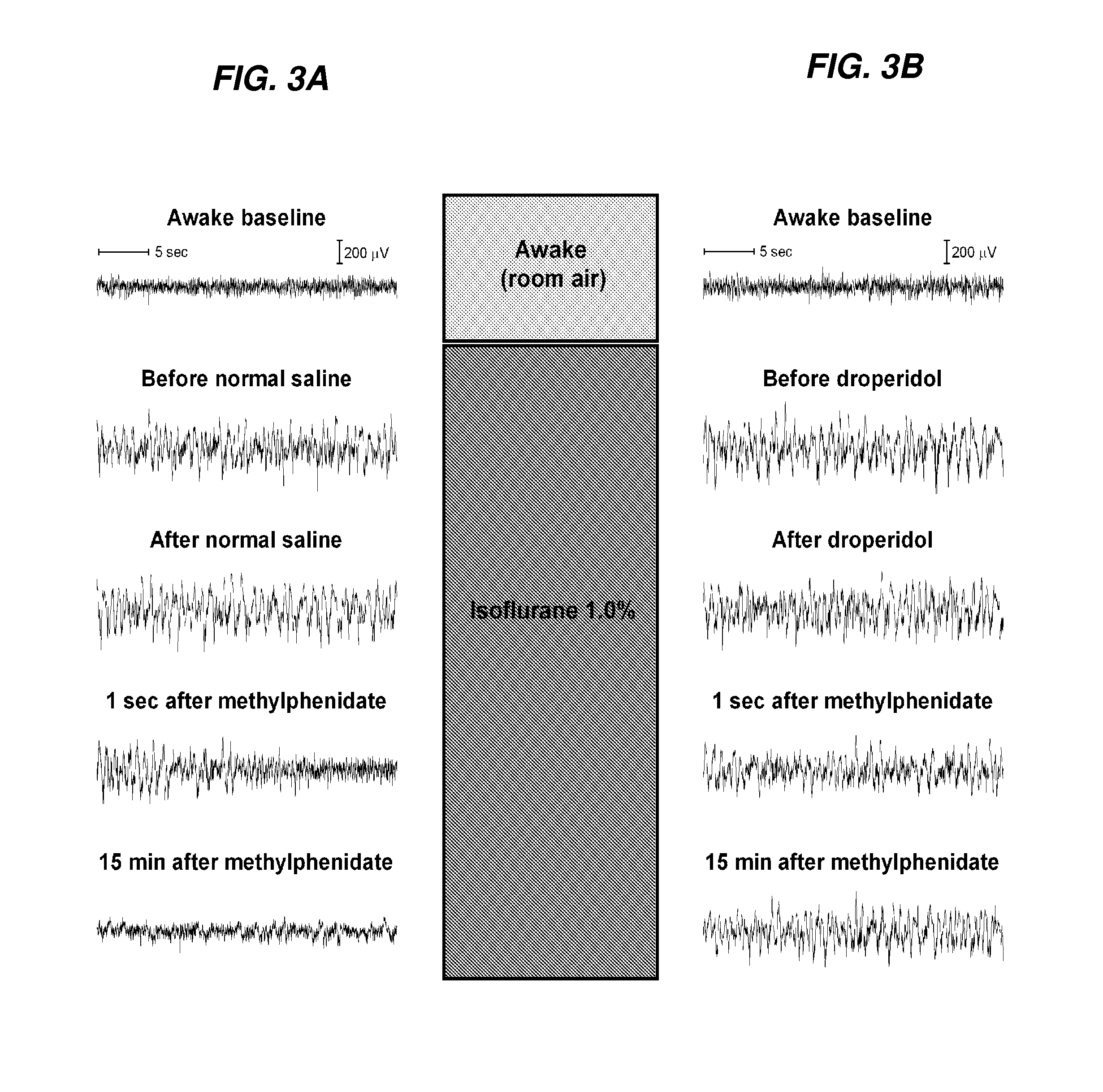
The present invention generally relates to compositions comprising anesthesia-reversing agents which facilitate or increase the time of awakening or reverse the effects of general anesthesia-induced unconsciousness. In some embodiments, the anesthesia reversing agent can be selected from any or a combination of methylphenidate (MPH), amphetamine, modafinil, amantadine, caffeine, or analogues or derivatives thereof. In some embodiments, compositions comprising at least one or more anesthesia-reversing agents can be used to facilitate awakening from anesthesia without or decreasing occurrence of delirium, and can be used in methods to treat or prevent the symptoms associated with emergence delirium, as well as treat a subject oversedated with general an esthesia. The invention also relates to methods for administering these compositions comprising anesthesia-reversing agents to subjects and for use.
Owner:THE GENERAL HOSPITAL CORP
Compositions for control of drug abuse
InactiveUS20130295170A1Challenge can be overcomePrevent degradationBiocidePowder deliveryBenzodiazepinePersulfate
Opiates, amphetamines, barbiturates and other drugs such as benzodiazepines are extensively abused or misused and are frequently the cause of death by overdosing. These drugs are also prone to oxidation and the final degradation products depend on the reactants and the reaction conditions. This invention describes the use of inactivating agents such as permanganates, peroxides, persulfates, bismuthates, periodates or other oxidants in a dosage form as an approach to minimize abuse and overdose. The product is designed such that the inactivating agent is released if there is an attempt to extract the drug from the formulation or in cases of overdose. Once released, the inactivating agent quickly degrades the drug and converts it into inactive compounds. Since the reactants (drug and inactivating agent) are incompatible in situations of normal drug usage, they are kept separated within the vehicle of the invention, but released for interaction in case of misuse. A catalyst may be included in the formulation to facilitate the reaction.
Owner:KYDES PHARMA
Methods of providing neuroprotection
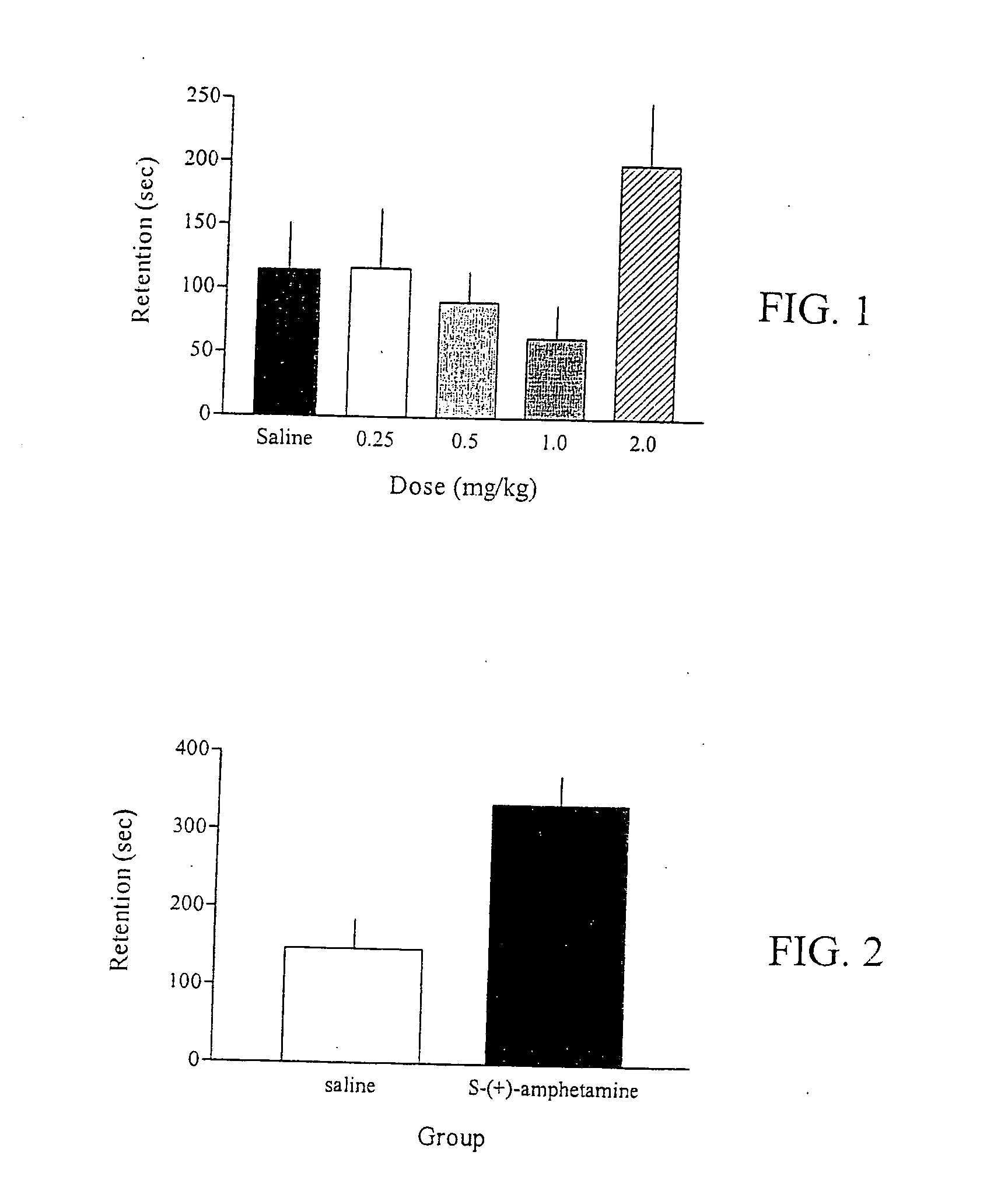
Cognitive impairments are treated and cognition is improved with an amphetamine compound. In one embodiment, the method includes administering an 1-amphetamine compound. In another embodiment, the method includes administering an 1-methamphetamine compound.
Owner:EPSTEIN MEL H +1
Preparation and utility of substituted amphetamines
Provided herein are substituted amphetamines, processes of preparation and pharmaceutical compositions thereof. Also provided are methods of their use for the treatment and / or management of trauma associated with a terminal disease, a post-traumatic-stress-disorder, or a psychological disorder.
Owner:AUSPEX PHARMA INC
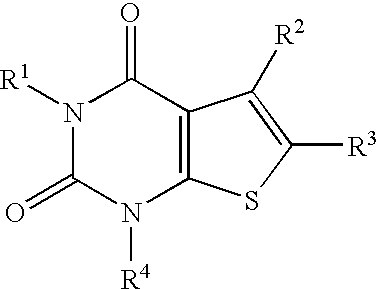
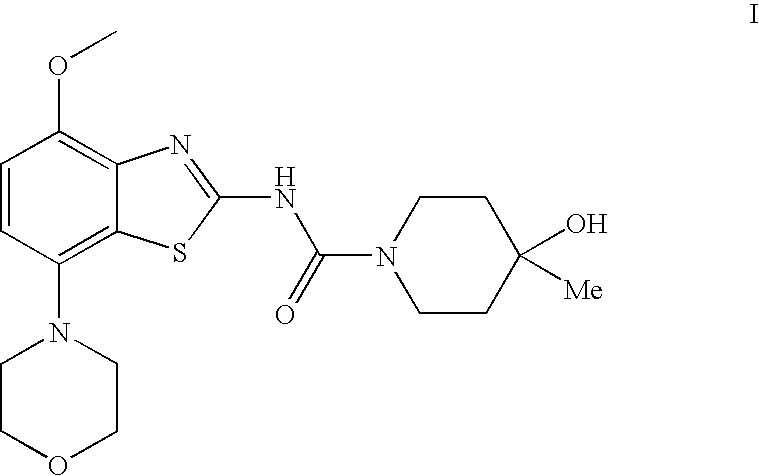
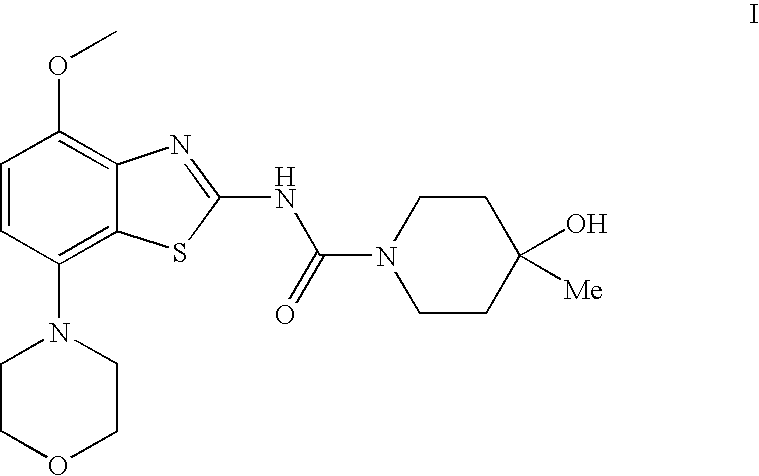
4-hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide
The present invention relates to the compound of formulawhich is 4-hydroxy-4-methyl-piperidine-1-carboxylic acid(4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide, and to pharmaceutically acceptable acid addition salts thereof. It has been found that the compound is useful for the treatment or prevention of Alzheimer's disease, Parkinson's disease, Huntington's disease, neuroprotection, schizophrenia, anxiety, pain, respiration deficits, depression, ADHD (attention deficit hyper-activity disorder), drug addiction to amphetamines, cocaine, opioids, ethanol, nicotine, or cannabinoids, or for the treatment of asthma, allergic responses, hypoxia, ischemia, seizure, substance abuse, or for use as muscle relaxants, antipsychotics, antiepileptics, anticonvulsants and cardioprotective agents.
Owner:F HOFFMANN LA ROCHE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com