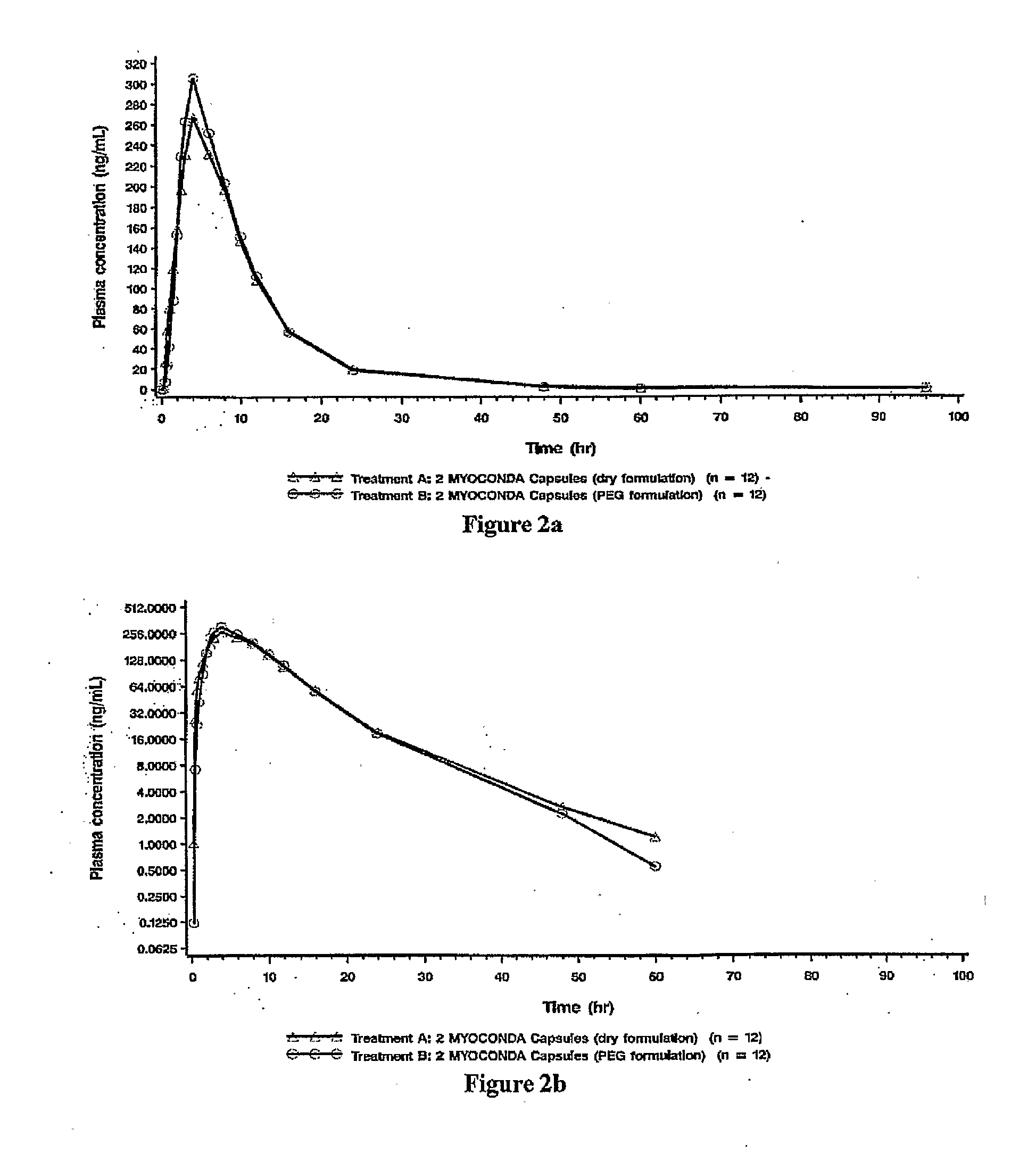
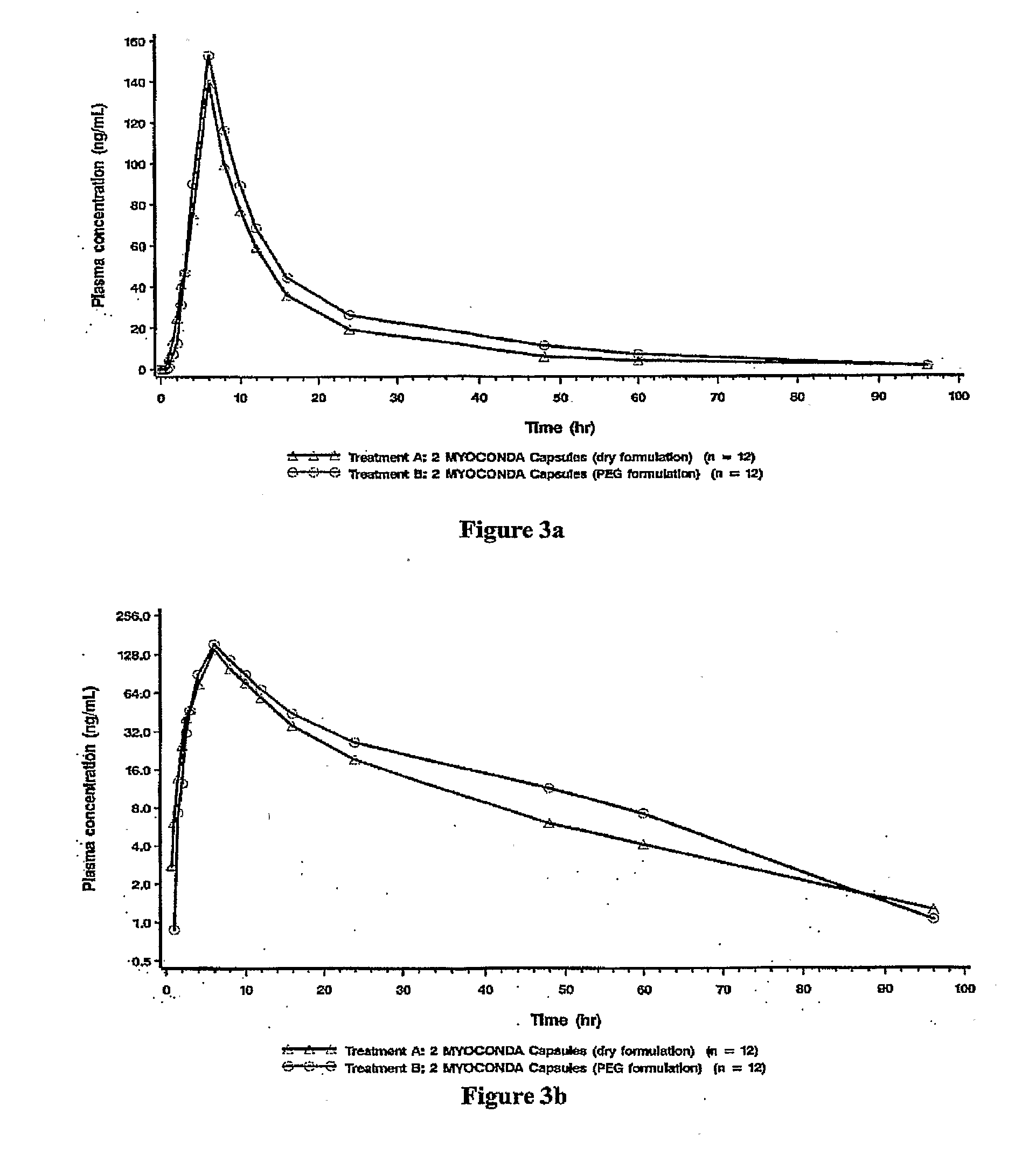
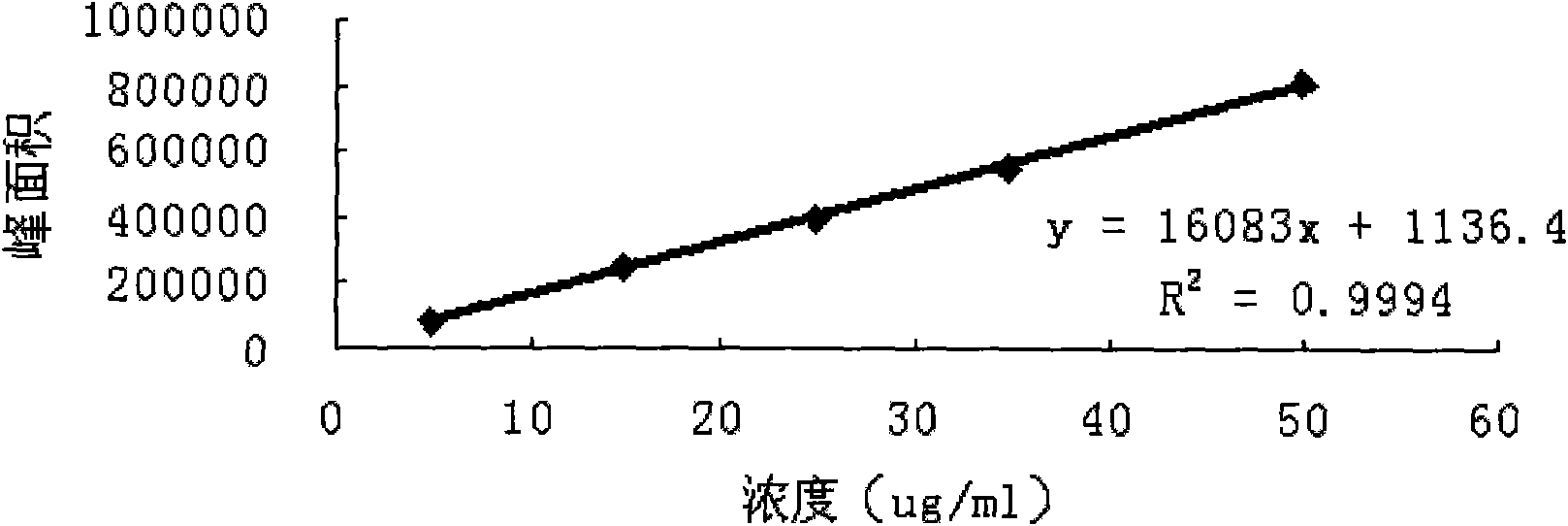
Patents
Literature
238 results about "Caplet Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
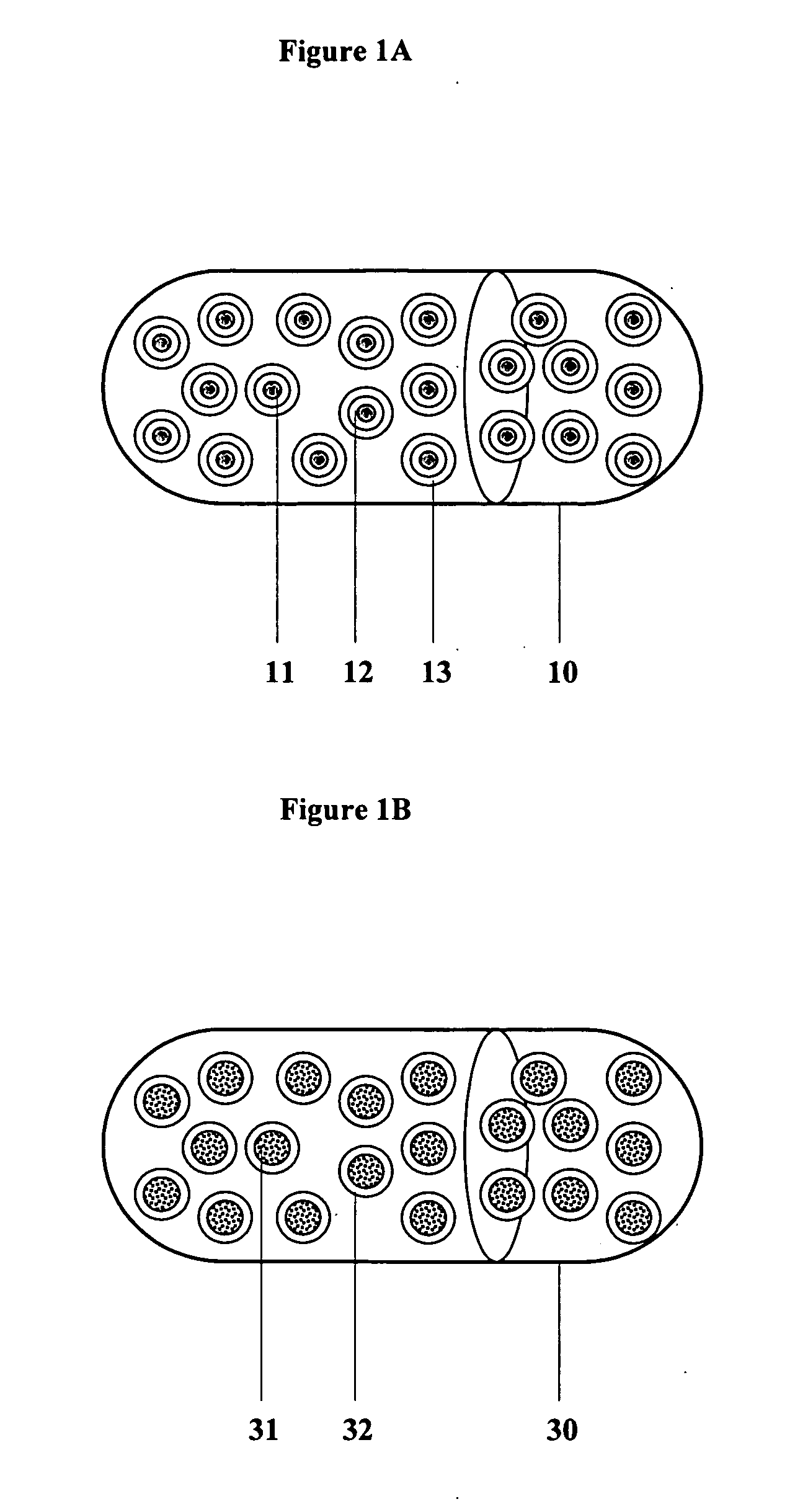
A solid dosage form in which a tablet has been compacted into capsule shape.
Controlled regional oral delivery
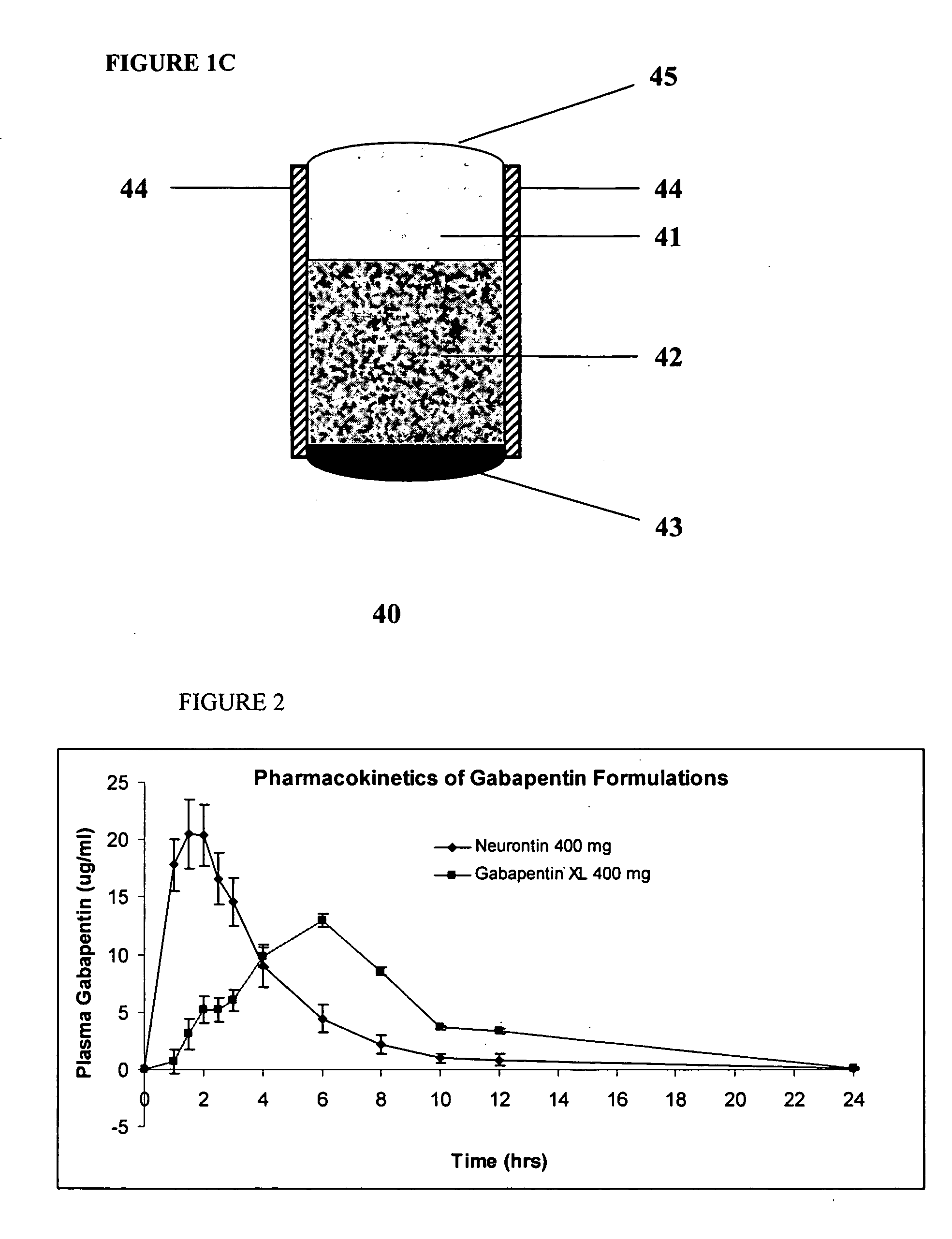
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
Oral liposomal delivery system
A liposome-capsule dosage unit system for the delivery of a biologically active material is formed by encapsulating a biologically active materials in liposomes and then placing the liposome encapsulated material into a capsule. The capsule is typically a soft gel capsule or a two piece capsule capable of tolerating a certain amount of water. A less water tolerant capsule can be employed if the liposomes are dehydrated prior to placement within the capsule. Biologically active material include drugs, nutritional supplements, vitamins, minerals, enzymes, hormones, proteins and polypeptides. The system is especially suited for the delivery of materials with poor oral solubility, materials that are not absorbed or are poorly absorbed from the gastrointestinal tract, and materials that have conventionally been given by an invasive route. The system can be administered orally, intra-occularly, intranasally, rectally, or vaginally.
Owner:BIOZONE LAB
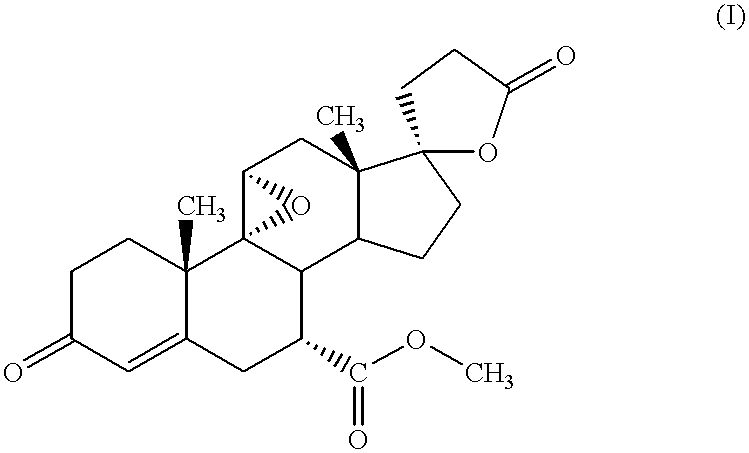
Nanoparticulate eplerenone compositions
InactiveUS20020006919A1Good physical propertiesImprove bioavailabilityPowder deliveryOrganic active ingredientsOral medicationCaplet Dosage Form
There is provided a pharmaceutical composition comprising eplerenone in solid particulate form, wherein at least 90% of the eplerenone particles are smaller than about 15 .mu.m, for example about 0.01 .mu.m to about 1 .mu.m, in diameter. The composition can be adapted for oral administration, for example as a tablet or capsule comprising eplerenone in a unit dosage amount of about 10 mg to about 1000 mg and one or more excipients.
Owner:THOSAR SHILPA S +3
Compositions comprising weakly basic drugs and controlled-release dosage forms
InactiveUS20090258066A1Antibacterial agentsNervous disorderOrally disintegrating tabletMicroparticle
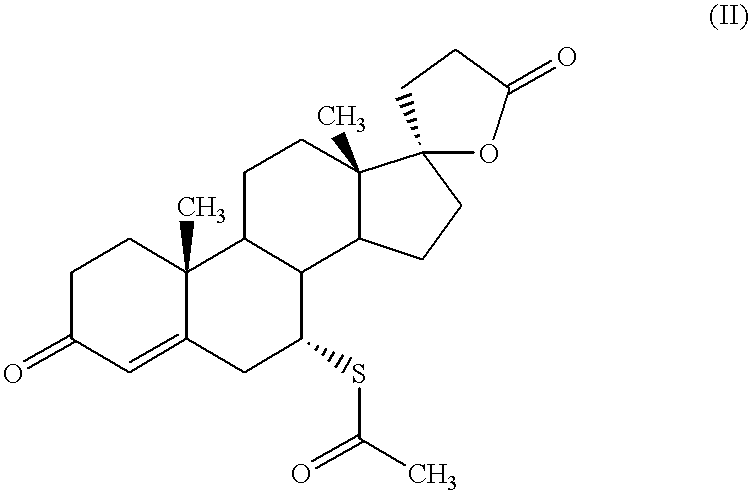
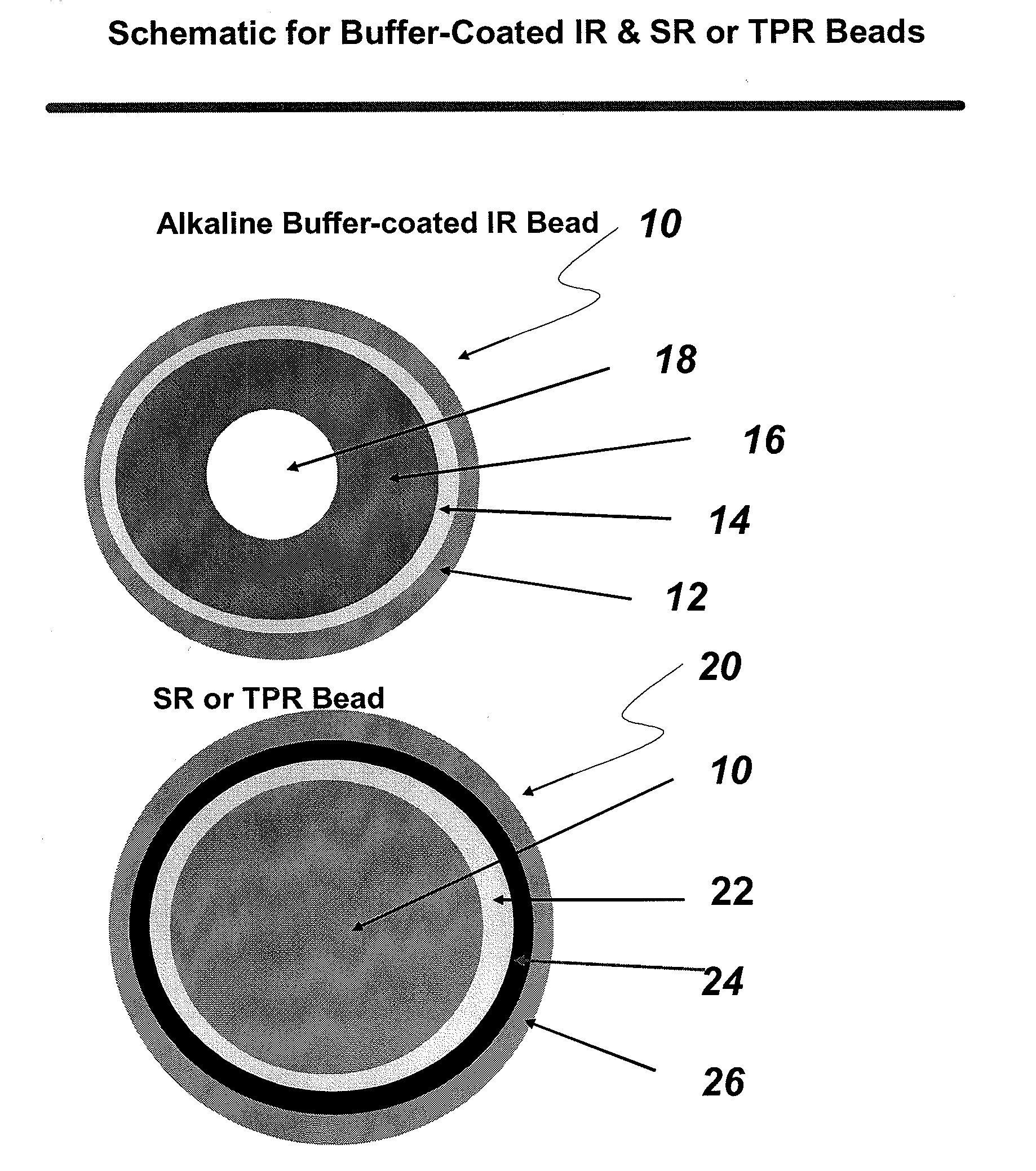
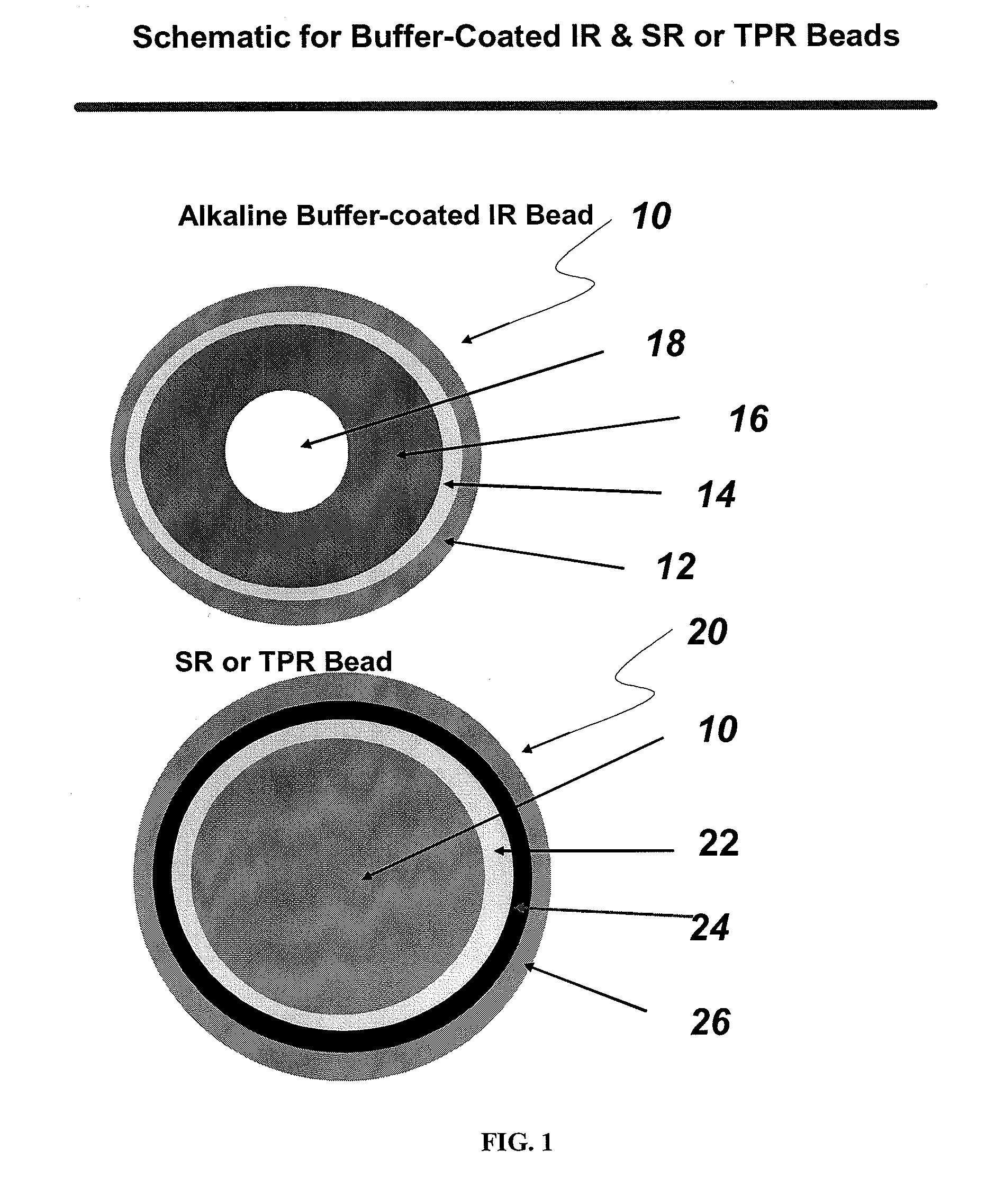
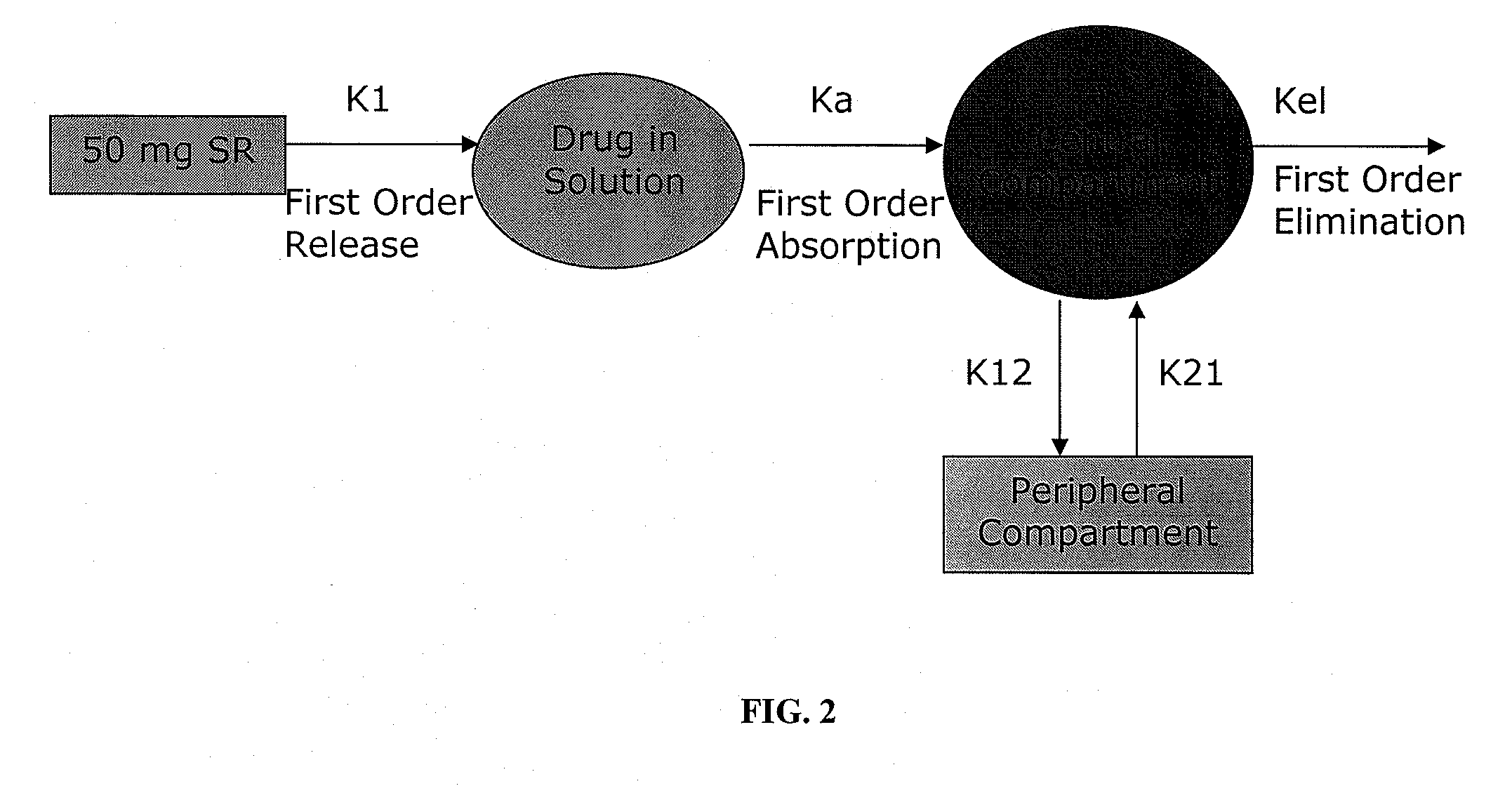
The present invention is directed to pharmaceutical compositions, and methods of making such compositions, comprising microparticles containing a weakly basic drug core, a layer of alkaline buffer, and a controlled-release coating. The present invention is also directed to pharmaceutical dosage forms, including orally disintegrating tablets, conventional tablets, and capsules, and methods for their preparation.
Owner:ADARE PHARM INC
Porous paclitaxel matrices and methods of manufacture thereof
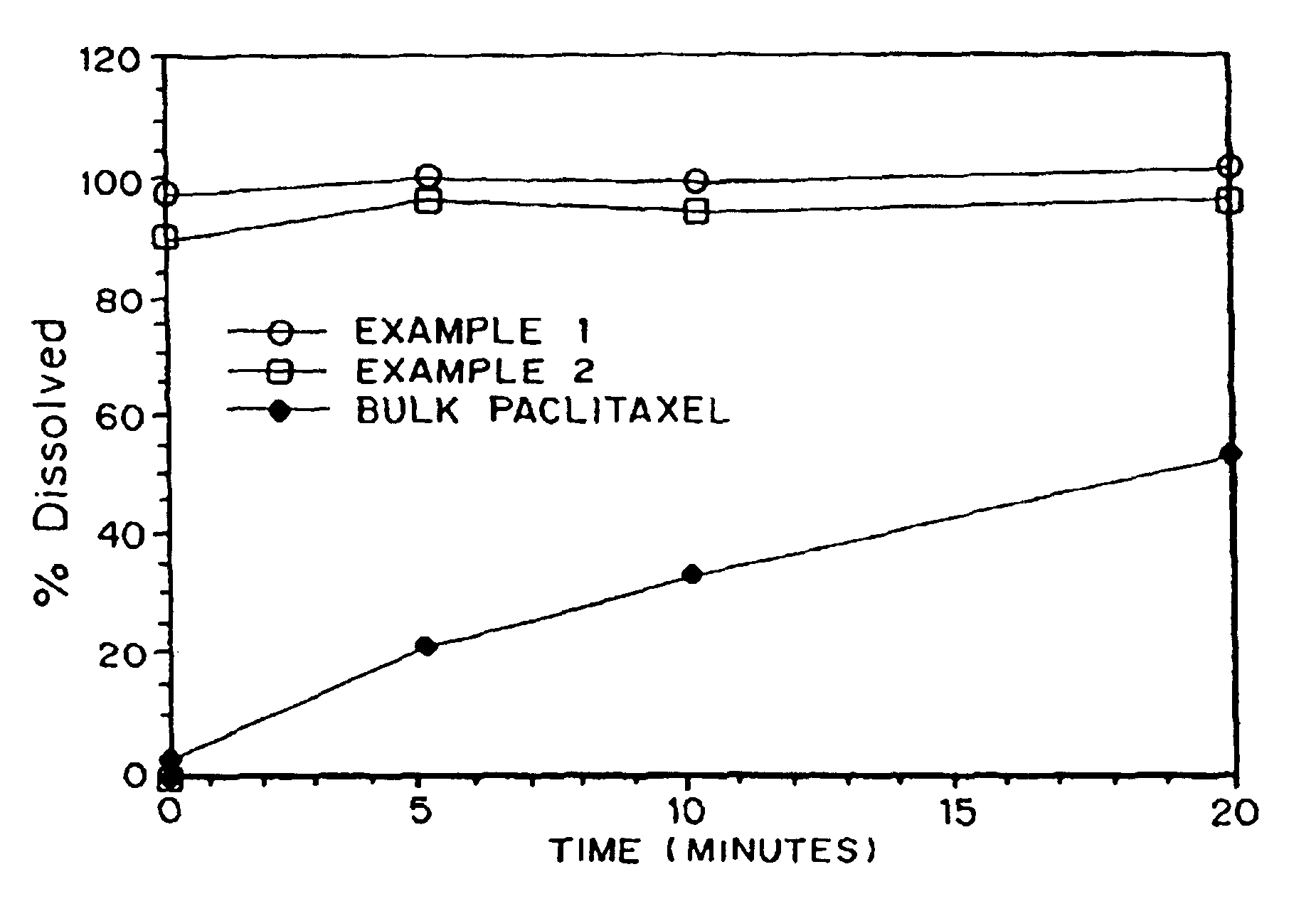
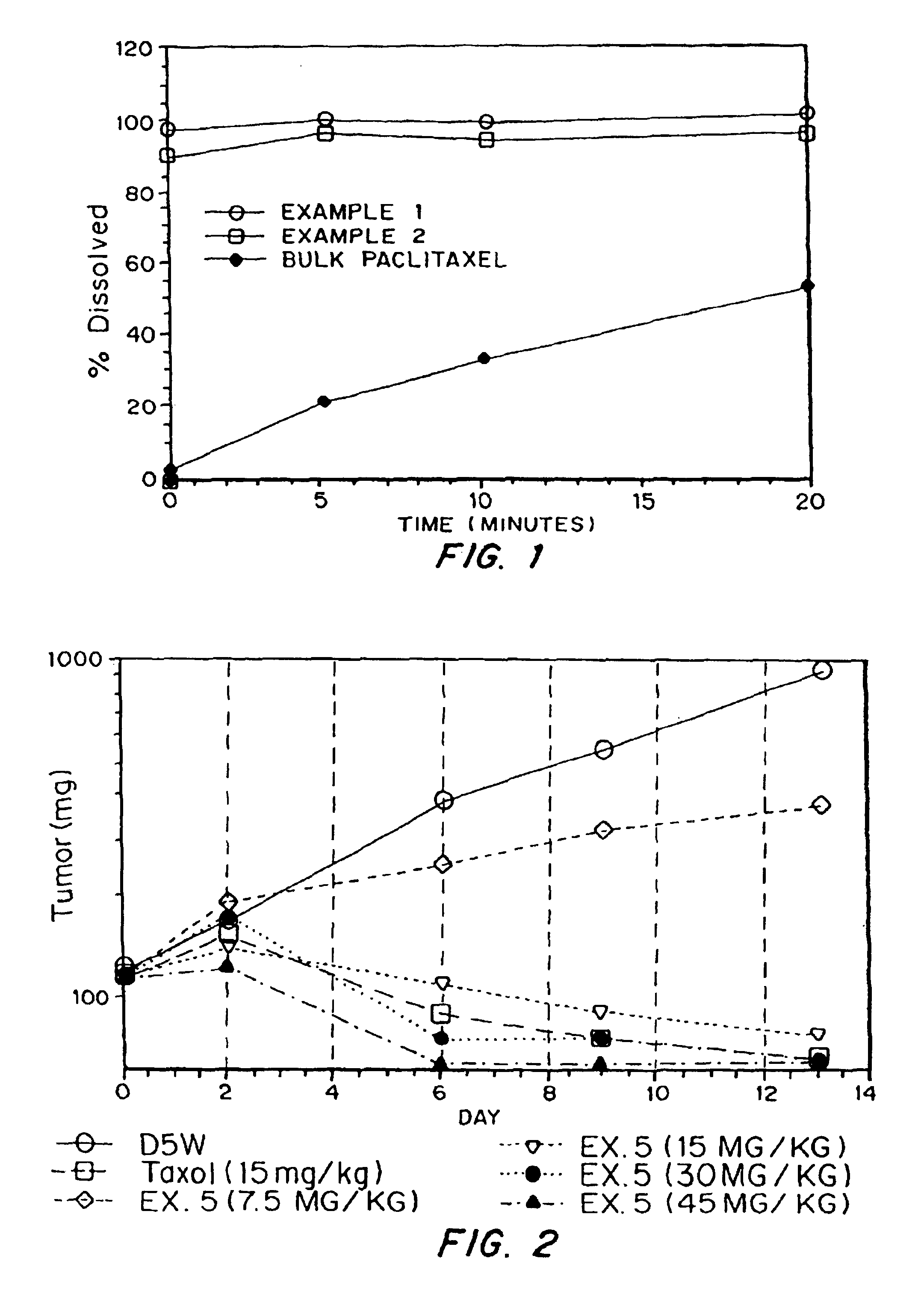
InactiveUSRE40493E1Fast dissolutionLower the volumeBiocideOrganic active ingredientsCaplet Dosage FormEngineering
Paclitaxel is provided in a porous matrix form, which allows the drug to be formulated without Cremophor and administered as a bolus. The paclitaxel matrices preferably are made using a process that includes (i) dissolving paclitaxel in a volatile solvent to form a paclitaxel solution, (ii) combining at least one pore forming agent with the paclitaxel solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of paclitaxel. The pore forming agent can be either a volatile liquid that is immiscible with the paclitaxel solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. In a preferred embodiment, microparticles of the porous paclitaxel matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
Oral pulsed dose drug delivery system
InactiveUSRE42096E1Control erosionDelayed release timePowder deliveryOrganic active ingredientsCaplet Dosage FormImmediate release
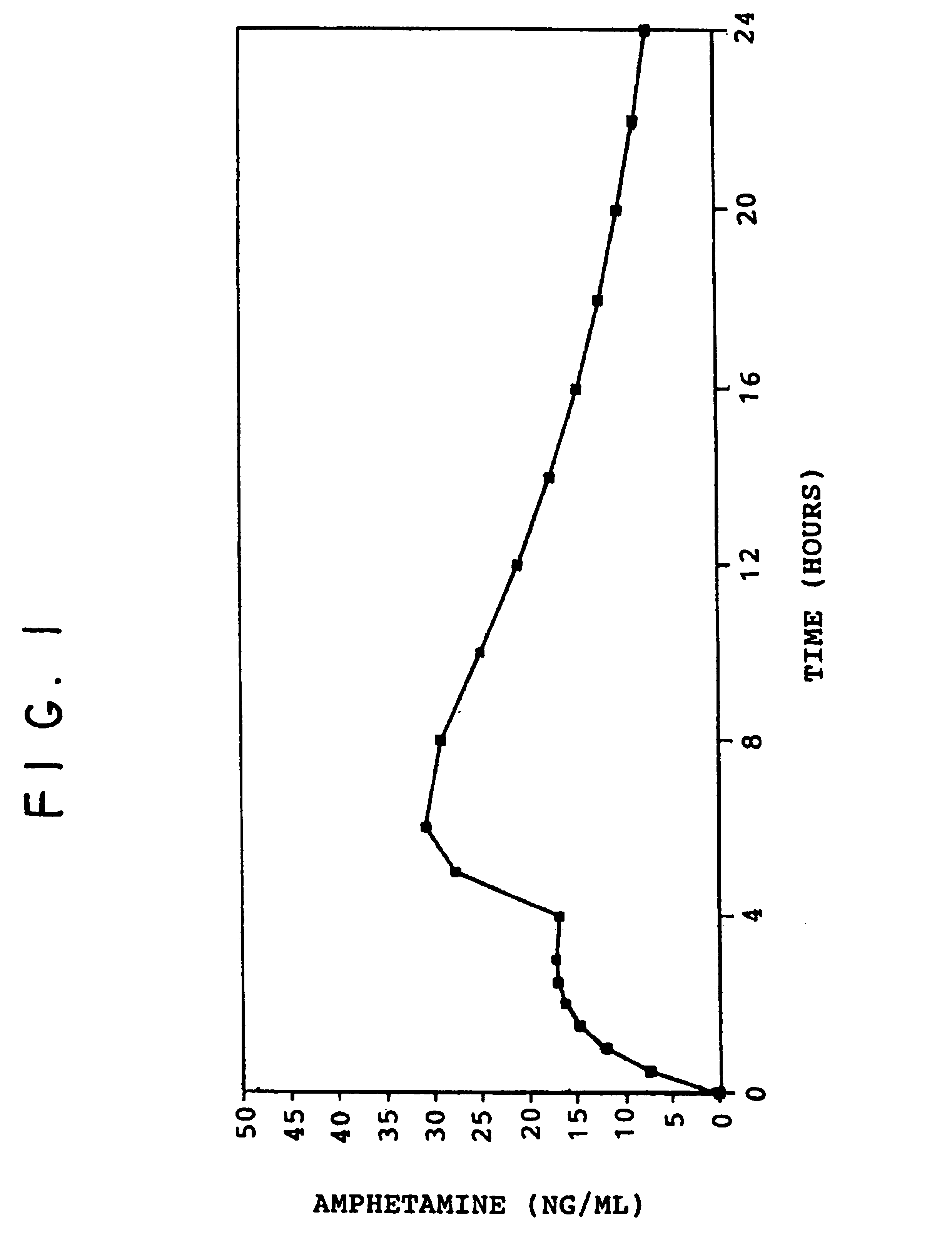
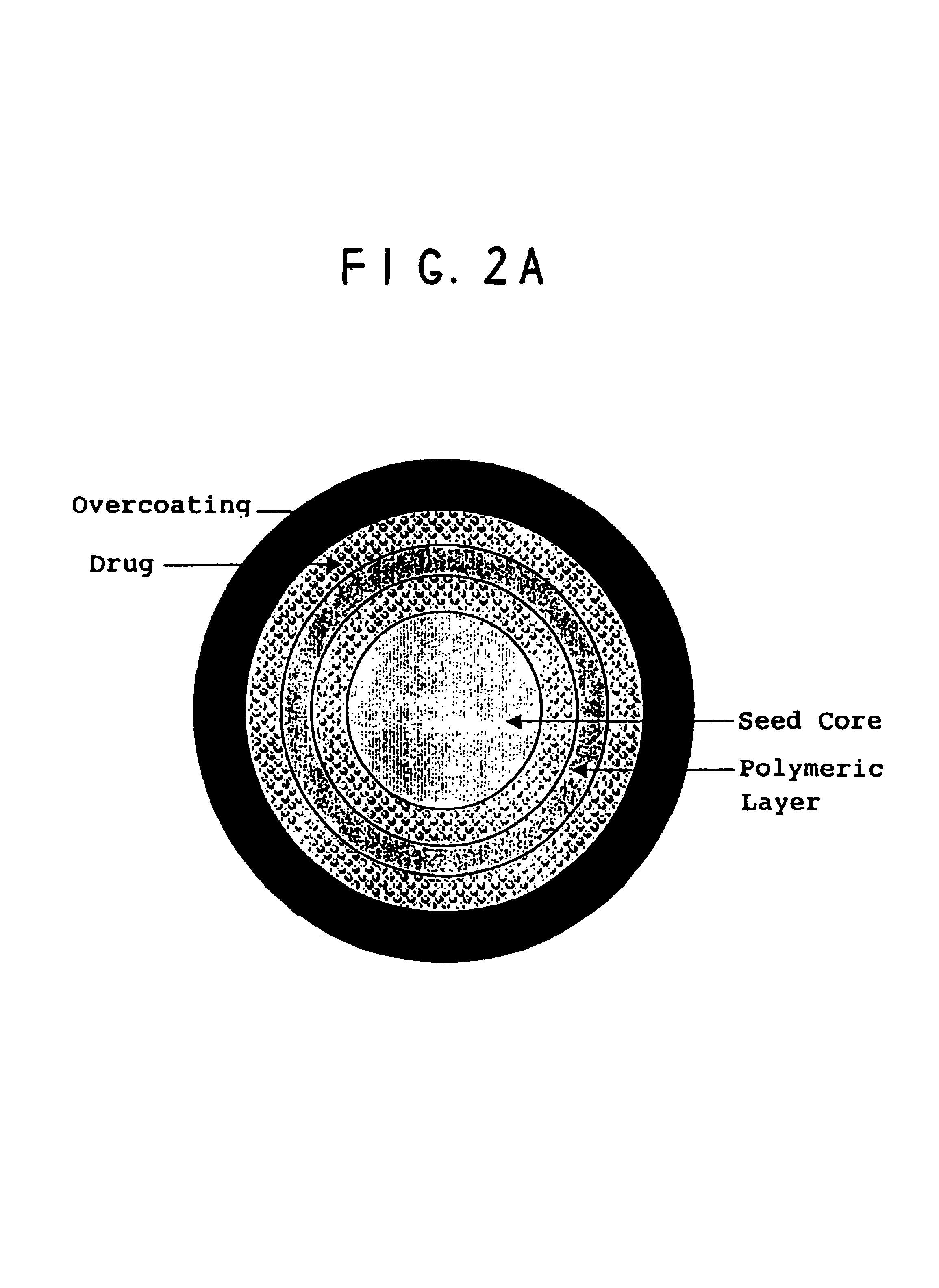
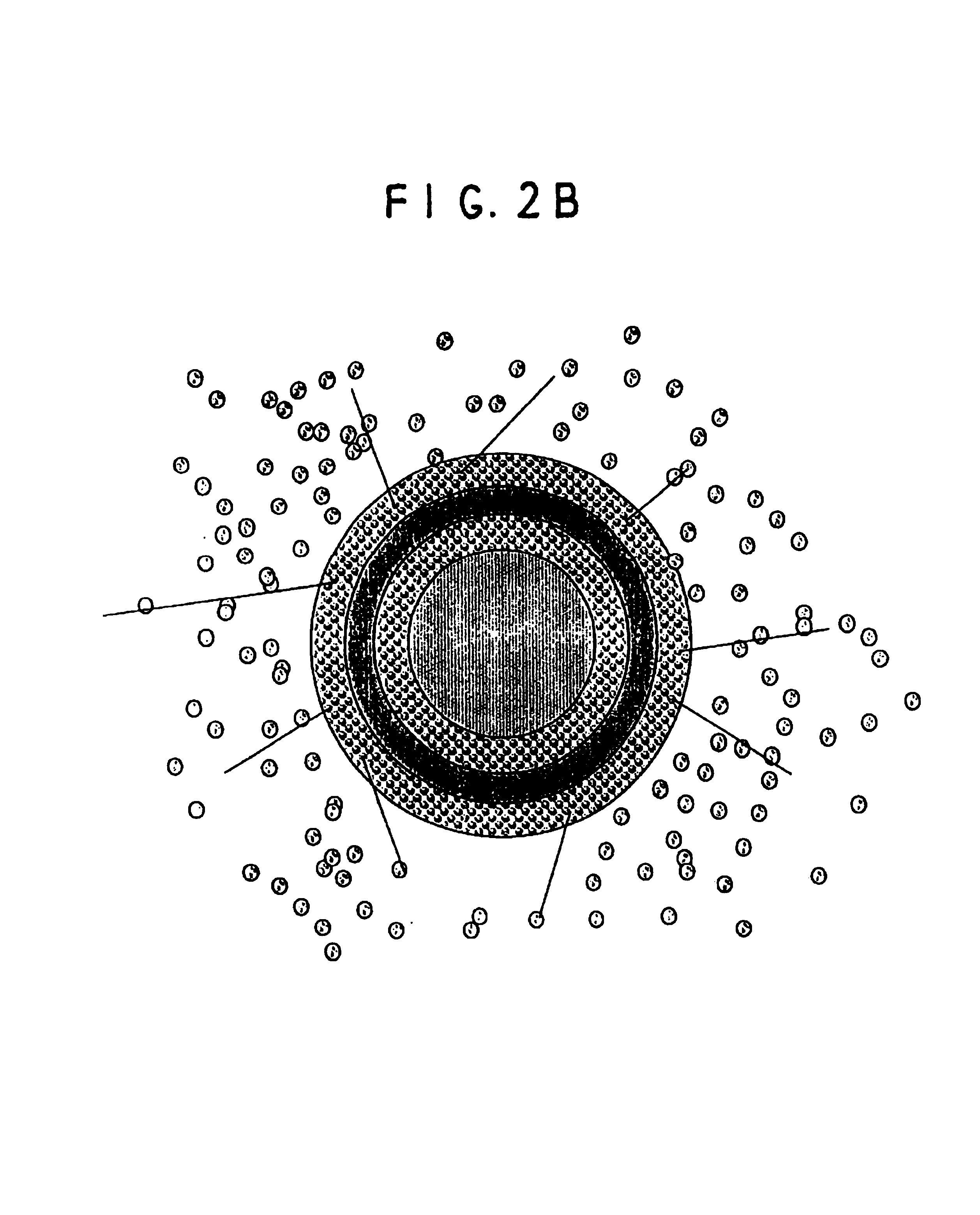
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising an immediate-release component and an enteric delayed-release component wherein (1) the enteric release coating has a defined minimum thickness and / or (2) there is a protective layer between the pharmaceutically active amphetamine salt and the enteric release coating and / or (3) there is a protective layer over the enteric release coating. The product can be composed of either one of a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:SHIRE PLC
Coated pharmaceutical capsule dosage form
InactiveUS20100291201A1Improve oral bioavailabilityImprove solubilityBiocideNervous disorderAdditive ingredientCapsule Dosage Form
Owner:CEROVENE
Effervescent tablet containing imatinib mesylate and preparation method thereof
InactiveCN101401797AEasy to storeEasy to carryOrganic active ingredientsPharmaceutical delivery mechanismEffervescent tabletPharmacy
The invention relates to the technical field of pharmaceutical preparation, in particular to an effervescent tablet containing imatinib mesylate and a method for preparing the same. The effervescent tablet comprises 25 to 500 milligrams of the imatinib mesylate and an acid base pair which is acceptable in pharmacy; furthermore, a filling agent, an adhesive, a disintegrating agent, a lubricating agent, a sweetening agent and a flavour modifying agent which are acceptable in pharmacy can be added in the effervescent tablet. The effervescent tablet containing the imatinib mesylate has the advantages of faster action speed compared with the tablets and capsulated drugs in the market, has convenient use and good taste in taking, and is more suitable for children, the elderly, and patients who can not swallow solid medicines.
Owner:BEIJING TRADE STAR MEDICAL TECH
Drug delivery systems comprising weakly basic drugs and organic acids
Owner:ADARE PHARM INC
Oral liquid-crystal sustained-release composition and preparation
InactiveCN101347622ASimple preparation processSuitable for industrial productionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsLiquid crystalHard Capsule
The invention relates to an oral liquid crystal sustained-released composition and a preparation method thereof. Active ingredients are distributed in glyceryl monooleate and lipid material in the composition to achieve the aim of slowly releasing the medicine. The preparation method of the pharmaceutical composition is as follows: the active ingredients, glyceryl monooleate and lipid material are prepared in a moderate method; the mixture is separately loaded in a hard capsule shell or soft capsule, and the sustained-released capsule is prepared. The content of the sustained-released capsule forms liquid crystal phase after encountering water to inhibit the release of the medicine. The pharmaceutical composition of the invention has simple preparing process, the active ingredients can be sustained-release, and the parameters influencing the release are few. Therefore, the composition of the invention is more suitable for industrial production with better safety.
Owner:CHINA PHARM UNIV
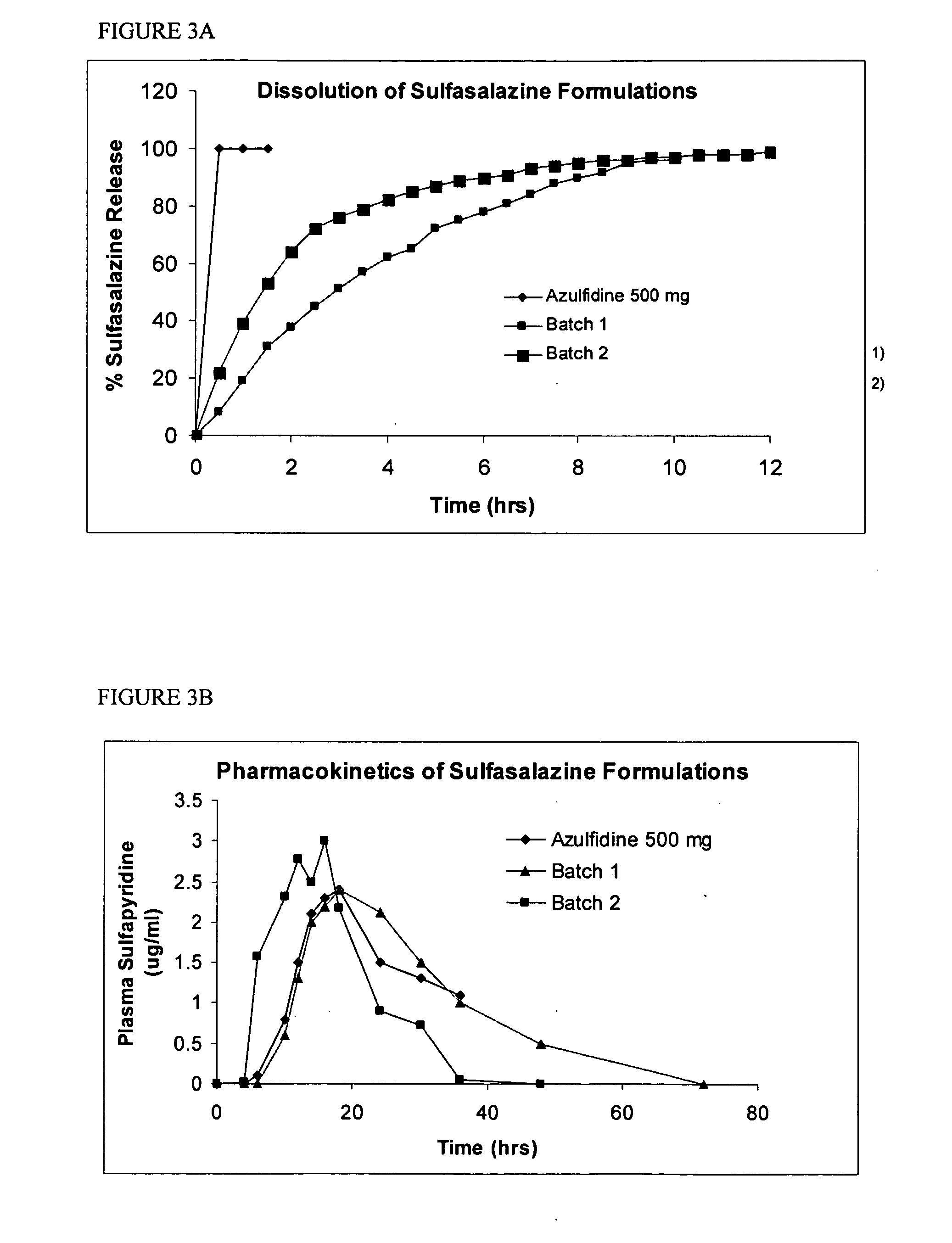
Methods and Compositions for Treating Inflammatory Bowel Disease
ActiveUS20110059136A1Increasing reduced metabolismReducing increased metabolismAntibacterial agentsBiocideRifabutinCaplet Dosage Form
The present disclosure provides improved compositions comprising rifabutin, clarithromycin, and clofazimine for use in the treatment of Inflammatory Bowel Diseases. In one instance, the compositions may comprise a formulation of rifabutin, clarithromycin, and clofazimine in a single dosage form, such as a capsule, tablet, etc., with one or more specific excipients.
Owner:REDHILL BIOPHARMA +1
Pharmaceutical compositions of mesalamine
The present invention relates to pharmaceutical compositions of mesalamine. The composition of the invention is a capsule dosage form filled with a tablet. The invention also relates to process for preparing such compositions. The invention specifically relates to a composition comprising an effective amount of mesalamine having higher bulk density.
Owner:CADILA HEALTHCARE LTD
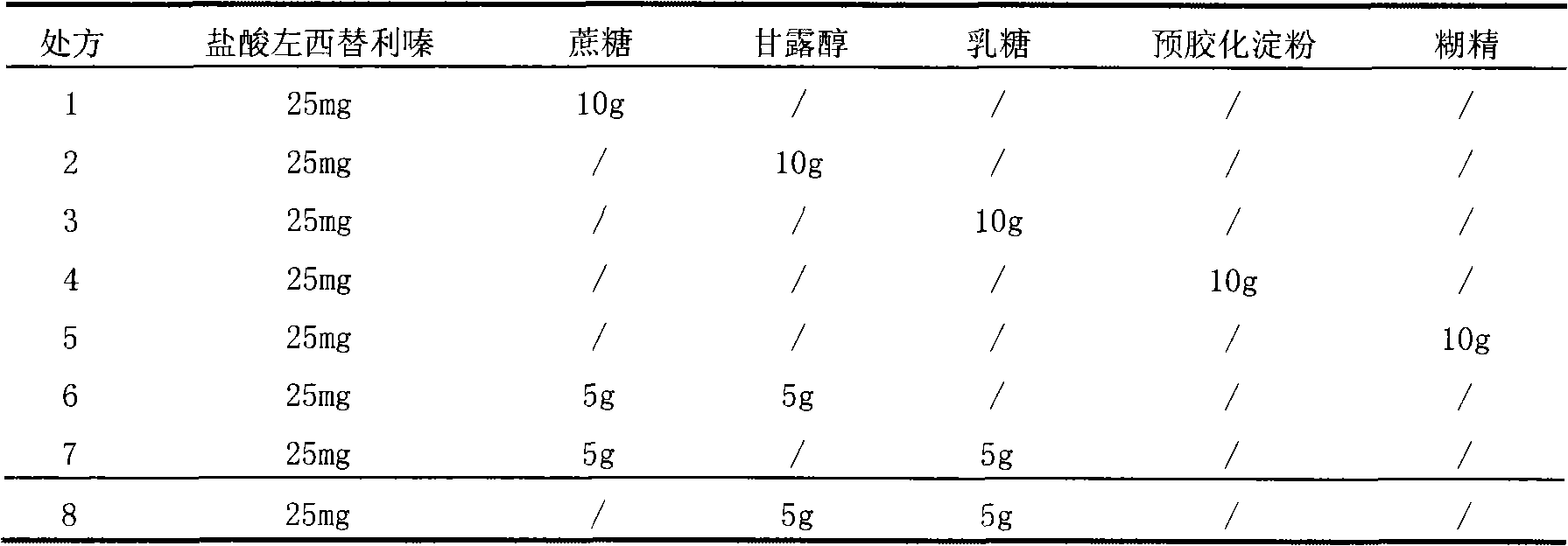
Levocetirizine dihydrochloride granule and preparation and detection methods thereof
ActiveCN101669913ALower control costsSimple processOrganic active ingredientsPharmaceutical non-active ingredientsCurative effectLactose
The invention discloses a levocetirizine dihydrochloride granule and preparation and detection methods thereof. The specification of the levocetirizine dihydrochloride particle is 2.5 mg, and milk sugar is used as filling agent. The levocetirizine dihydrochloride granule dispenses with the process of disintegration of tablets and capsules in a human body, the degree of dispersion in the human bodyis superior to the tablets and the capsules, and the absorption is faster than the tablets, the capsules and dispersible tablets; and the flowability, the dispersibility and the adhesiveness are better than the dispersible tablets, the granule is convenient to take, the mouthfeel is easier to adjust, and the curative effect of the medicine can be guaranteed to be better played.
Owner:HAINAN HONZ PHARMA
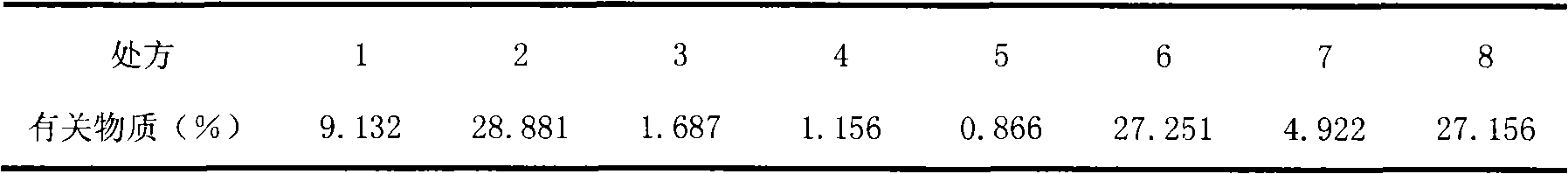
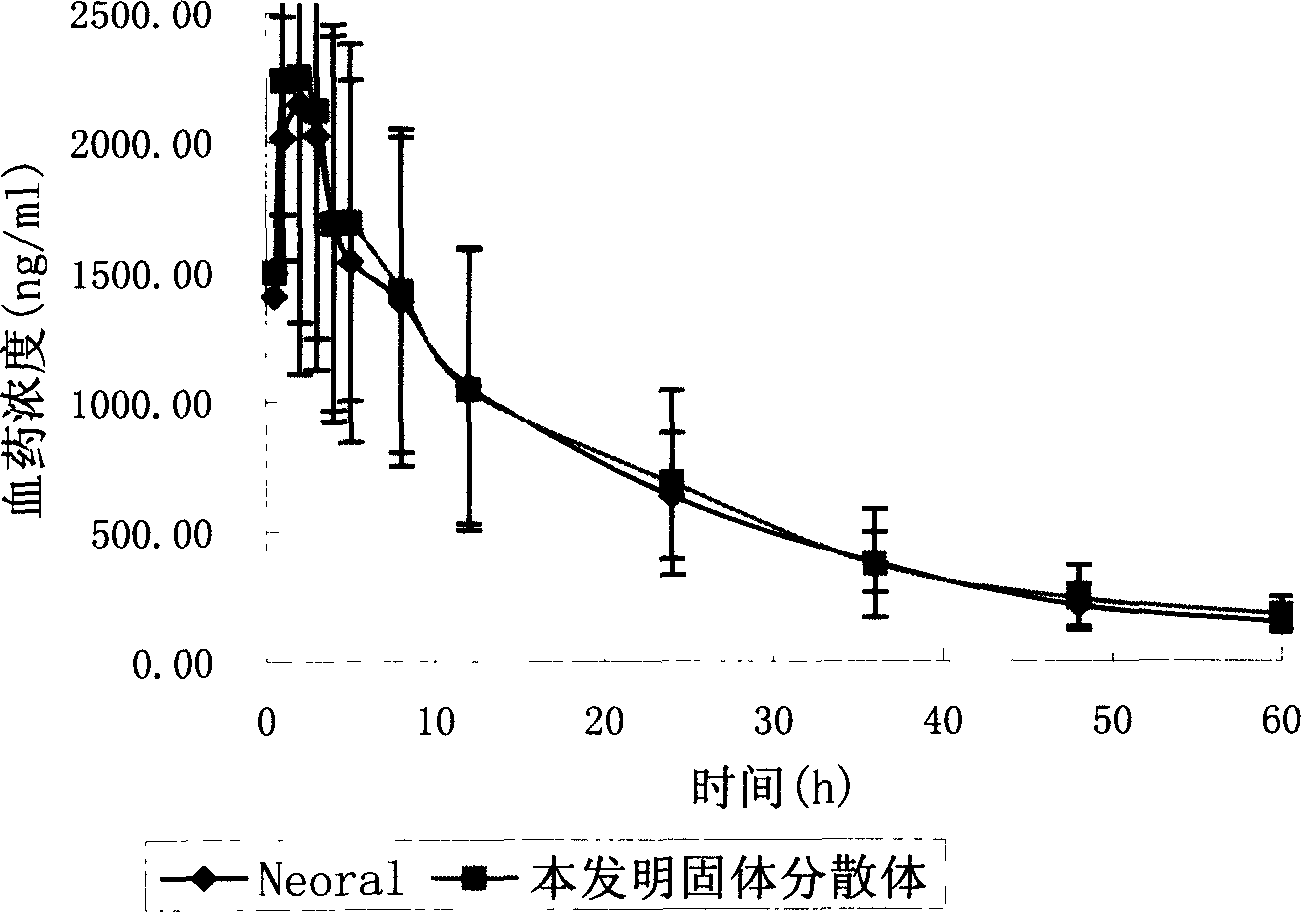
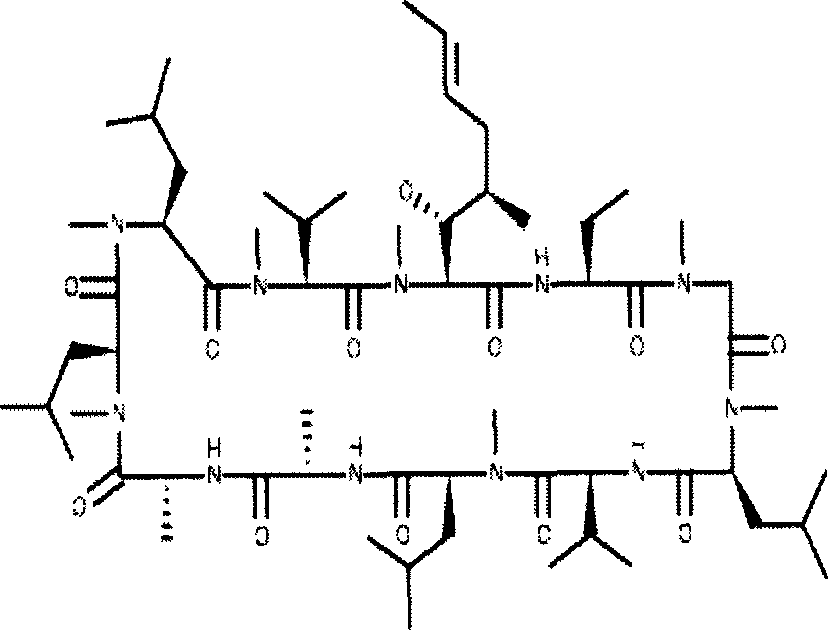
Cyclosporin A dispersion solid and its preparation method
InactiveCN1559606AIncrease dissolution ratePowder deliverySuppositories deliverySolubilityCaplet Dosage Form
A dispersing solid (capsule, tablet, particle, suppository and dripping pill) of cyclosporin A is prepared from cyclosporin A and carrier by solvent method, solvent fusion method, etc. It has high solubility of easy absorption.
Owner:FUDAN UNIV +1
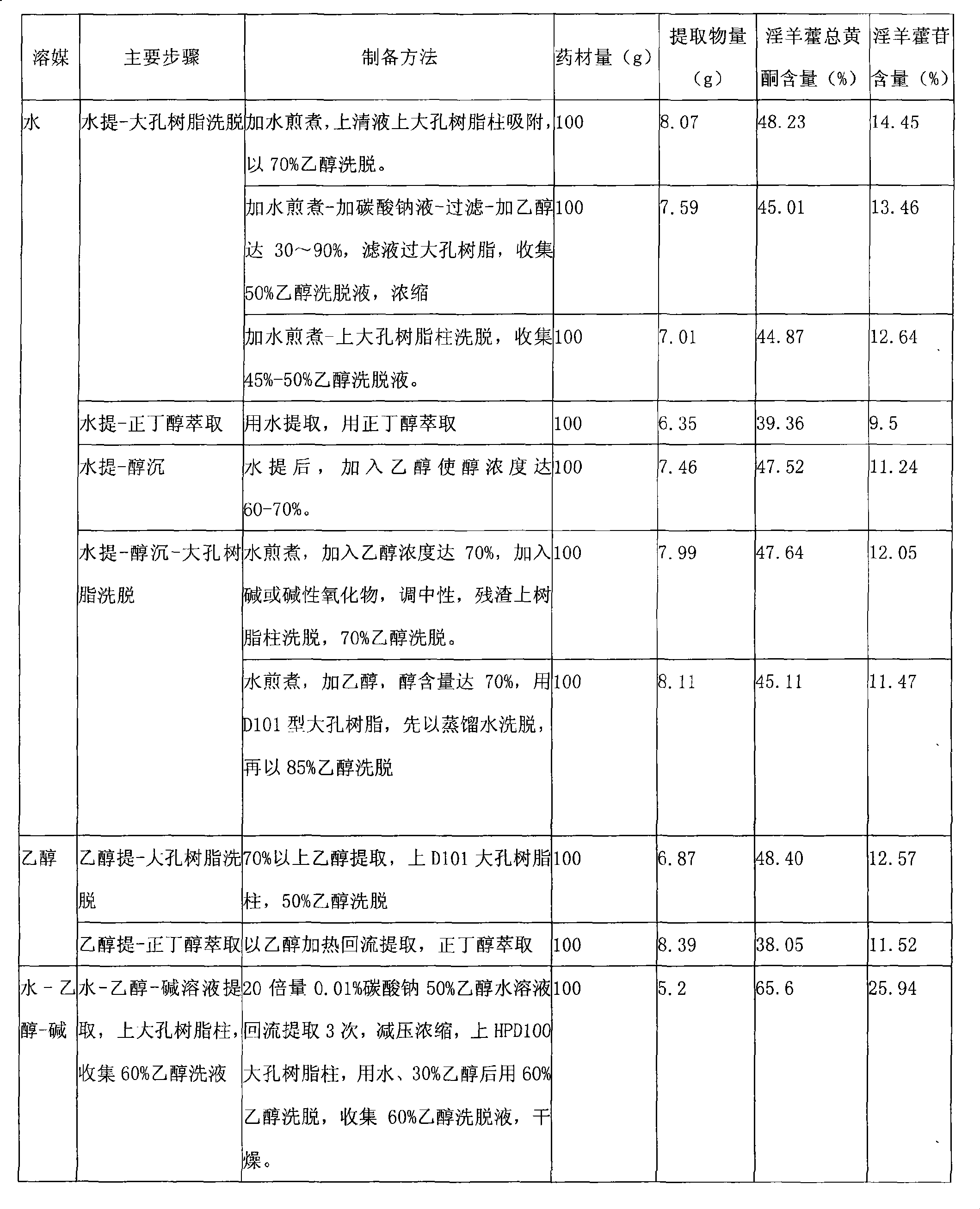
Epimedium extract and preparation method, preparation and use thereof
The invention provides a herba epimedii extract, in particular to a high-content herba epimedii total flavonoids extract, and the preparation method thereof mainly comprises the following steps: (1) alkali-ethanol-water is taken as a menstruum for refluxing extraction; (2) decompression and concentration are carried out; (3) pH value is adjusted; (4) macroporous resin column elution is carried out; and (5) an eluant is dried and the content of the obtained total flavonoids is over 65 percent and the content of icariin is over 20 percent. The extract can be prepared into various oral preparations according to the requirements in pharmacy, which can be hard capsules, soft capsules, conventional tablets, orally disintegrating tablets, buccal tablets, dispersible tablets, sustained-release preparations, controlled release preparations, granules, water-paste pills, dripping pills, honeyed pills, and the like. The extract and effective medicines containing the extract can be applied to preparing brain tissue protective agents, especially to preparing medicines that prevent and treat cerebral infarction, cerebral infarction sequela and other ischemic cerebrovascular diseases.
Owner:南京宇道科技开发有限公司
Traditional Tibetan medicine Ruyizhenbao composite preparation and preparation method thereof
ActiveCN102430090AFast absorptionStable active ingredientsOrganic active ingredientsAntipyreticMedicinal herbsCaplet Dosage Form
The invention discloses a Ruyizhenbao medicine composite, which is prepared by adopting the following steps of essential oil extraction and inclusion, ethanol extraction, water extraction, fluid extract drying, medicinal material fine powder crushing, expensive and fine medicine fine powder preparation and the like. Pharmaceutically conventional auxiliary materials are added according to a conventional process to be prepared into clinically acceptable dosage forms such as: powders, capsules, tablets, oral liquid, condensed pills, granular formulations, pills, pellets and slow release preparations. The invention is characterized in that the Ruyizhenbao composite is prepared by taking phytochemical components as the material base and the pharmacodynamics activity as the guidance by adoptinga modern extraction preparation method. The medicinal preparation prepared by the method disclosed by the method has the advantages of high absorption rate, stable pharmacodynamic components, high bioavailability and the like under the condition that the pesticide effects of the raw preparation are remained.
Owner:JINHE TIBETAN MEDICINE
Brand-new oral solid medicinal composition and preparation method thereof
ActiveCN102335176AGuaranteed curative effectHigh content of the main drugOrganic chemistryCapsule deliveryCross-linkValsartan
The invention discloses a brand-new oral solid medicinal composition. The medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine, valsartan and pharmaceutically acceptable auxiliaries, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of the hydrochlorothiazide, 2.5-5 parts of the levamlodipine, 80-160 parts of the valsartan, 40-120 parts of microcrystalline cellulose, 30-90 parts of compressible starch, 5-25 parts of cross-linked sodium carboxymethylcellulose, 3-8 parts of silicon dioxide and 1-2 parts of stearic acid. The medicinal composition disclosed by the invention has the advantages of scientific and reasonable prescription, low auxiliary content and high bioavailability, and is a first choice of medicine for treating hypertension.
Owner:HAINAN JINRUI PHARMA
Compound medicine compounds containing adefovir dipivoxil, preparing method and uses thereof
The invention relates to a medical composition with active components of adefovir dipivoxil and another rnucleotide (acid) anti-virus medicine and the preparation method and usage thereof. The adefovir dipivoxil and anther rnucleotide (acid) anti-virus medicine are taken as the active components, and mixed with a plurality of pharmaceutically acceptable supplements to prepare the medical composition; and the invention can be applied in treatment of viral hepatitis B. The adefovir dipivoxil and anther rnucleotide (acid) anti-virus medicine are taken as raw materials of the invention content, a plurality of supplements with special type and proportion are added, and various kinds of oral preparations such as tablet, capsule, dispersion, chewable tablet, oral disintegrating tablet, buccal tablet, dropping pill and soft capsule are prepared and developed according to the technical method described by the invention.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Oral Drug Compliance Monitoring Using Sound Detection
InactiveUS20100135907A1Pharmaceutical delivery mechanismAuscultation instrumentsSound detectionSound wave
A tablet, pill or capsule containing a material which produces sound waves when the tablet, pill or capsule is exposed to the gastrointestinal system. A two step method for oral drug compliance monitoring. The first step is to ingest a tablet, pill or capsule containing a material which produces sound waves when the tablet, pill or capsule is exposed to the gastrointestinal system of a person. The second step is to detect the sound waves produced when the tablet, pill or capsule is exposed to the gastrointestinal system to confirm that the person has ingested the tablet, pill or capsule.
Owner:DOW GLOBAL TECH LLC
Composition and preparation method and preparation thereof
InactiveCN110812365AImprove stabilityControlled release rateOrganic active ingredientsSenses disorderOral suspensionsCaplet Dosage Form
The invention discloses a composition as well as a preparation method and a preparation thereof. The composition of the present invention includes nicotinamide mononucleotide, an ion exchange resin, acoating material, and a plasticizer. The preparation method comprises the following steps: preparing the drug-loaded resin particles, and coating the drug-loaded resin particles. The coated drug-loaded resin particles are mixed with other auxiliary materials to prepare corresponding dosage forms, such as oral suspension, tablets, capsules, granules, cream, ointment, facial masks and the like. Thecomposition is simple in production process, easy to amplify and produce, capable of effectively improving the stability of nicotinamide mononucleotide, accurate in dosage, lasting in effect and stable in curative effect.
Owner:明特奇点医疗科技(北京)有限公司
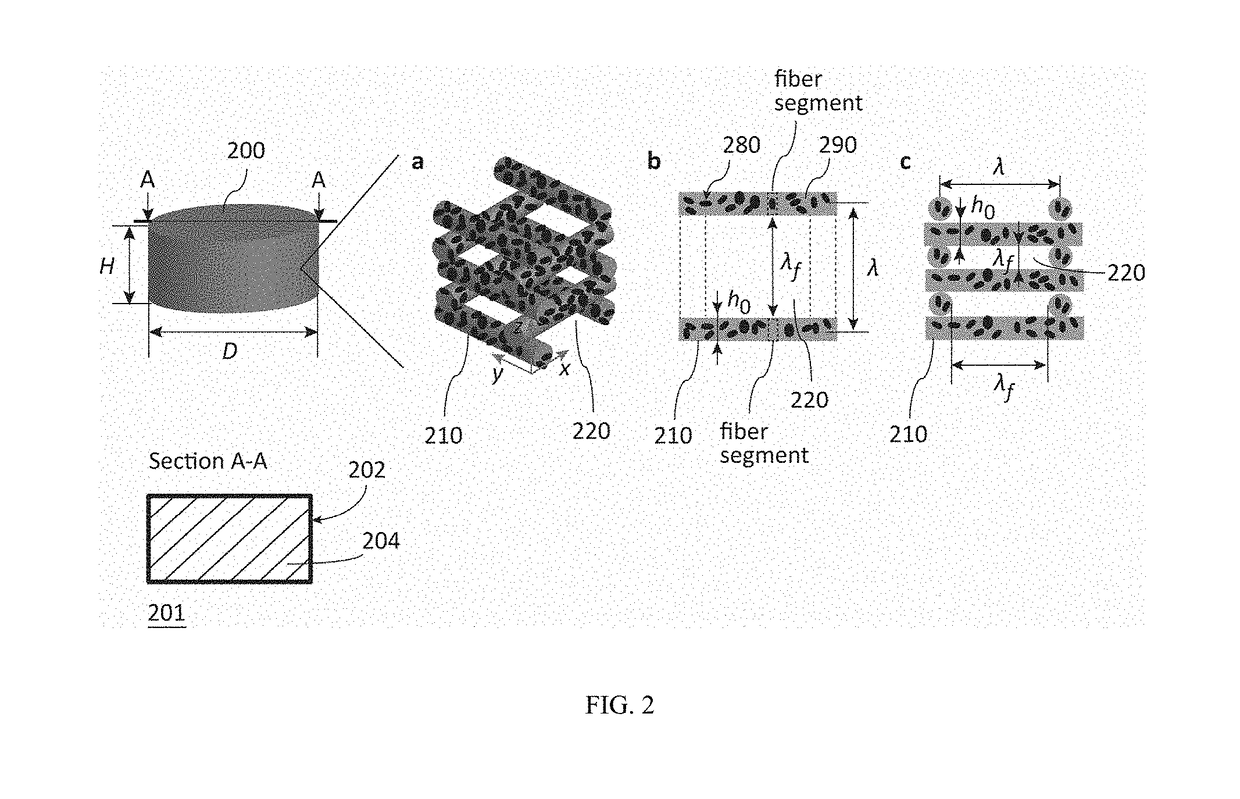
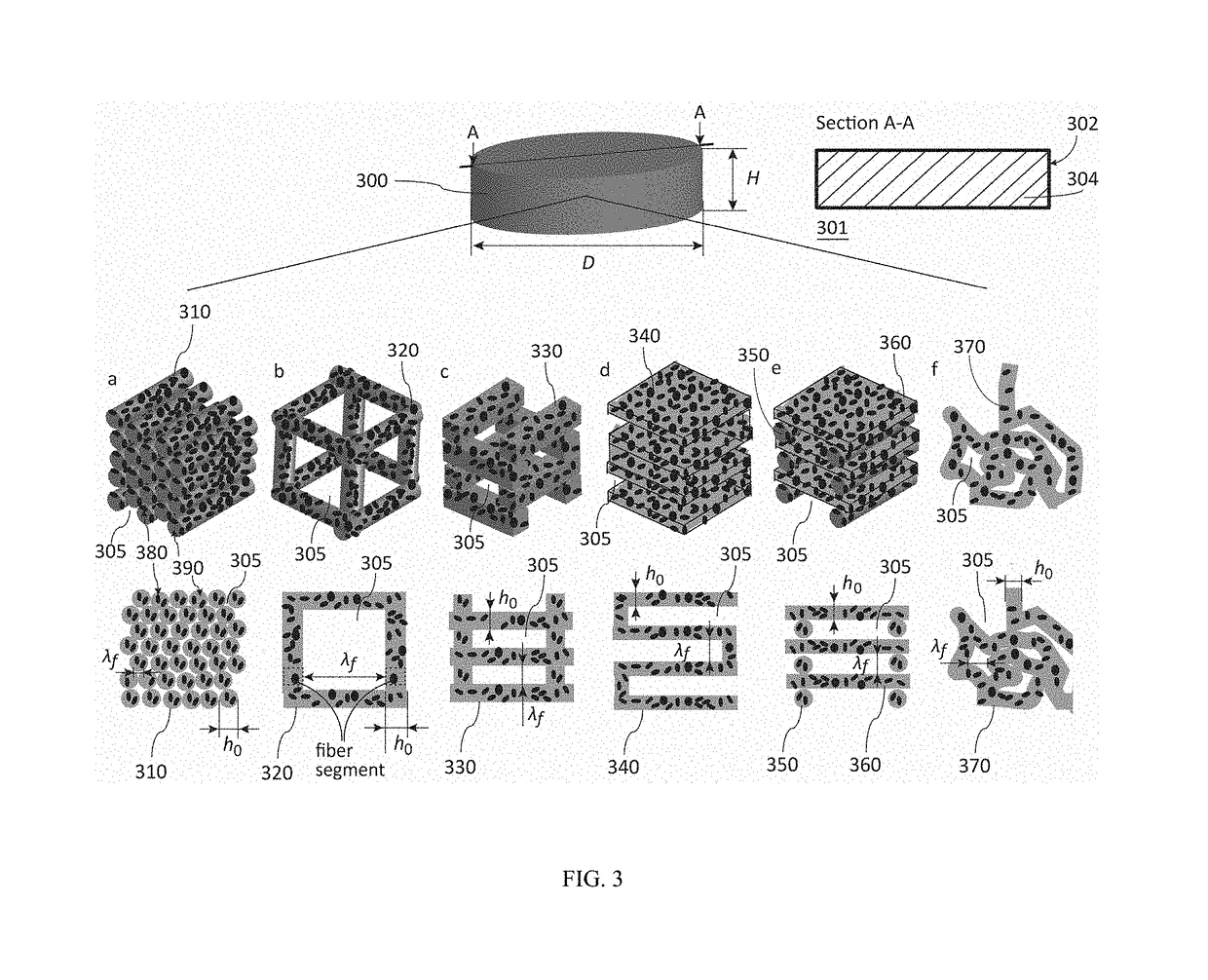
Fibrous dosage form
ActiveUS20180049993A1Organic active ingredientsAdditive manufacturing apparatusDrug release rateCaplet Dosage Form
At present, the most prevalent pharmaceutical dosage forms, the oral immediate-release tablets and capsules, are granular solids. The problem of such solids is that their microstructure and properties are not predictable from physical models. As a consequence, product development and manufacture are resource-intensive and time-consuming, and quality control is statistical by testing instead of by design. Furthermore, the range of the drug release rate, and the variety of active ingredients that can be processed to a functional product, are limited in such dosage forms. Presented herein, accordingly, is a fibrous dosage form suitable for immediate-release applications prepared by a predictable liquid-based process. The fibrous dosage form includes a drug-containing solid comprising a three dimensional structural network of one or more drug-containing fibers.
Owner:BLAESI ARON H DR
Self-emulsifying capsule of docetaxel and its preparation method
InactiveCN101011376AHigh drug loadingImprove bioavailabilityOrganic active ingredientsCapsule deliveryEmulsionDocetaxel
The invention relates to a method for preparing doxitasa self-emulsion capsule agent, which comprises doxitasa, oil, emulsifier, and auxiliary emulsifying agent. And the preparation comprises that dissolving the materials into oil via mixing or ultrasonic wave, adding emulsifier and auxiliary emulsifying agent, mixing or using ultrasonic wave to obtain uniform transparent solution; filling into capsule to obtain soft or hard capsule. The invention has simple process, high stability and high utilization.
Owner:黄成安
Pharmaceutical composition containing butylphthalide and novel solubilizer
InactiveCN105688220AImprove securityLow hemolytic activityOrganic active ingredientsPowder deliverySolubilityButylphthalide
The invention relates to the field of medicine, in particular to a pharmaceutical composition containing butylphthalide and a novel solubilizer to improve the water solubility of butylphthalide by means of the novel solubilizer, clinically required solid form, or semisolid form or liquid form of butylphthalide is developed, so that the treatment effect of butylphthalide can be better realized. The composition can be used for preparing various drug forms such as tablets, capsules, particles, powder, ointment, cream, gel, infusion, squirt cut, powder filling and oral liquid. Compared with the prior art, the pharmaceutical composition is better in safety performance and water solubility of butylphthalide.
Owner:SICHUAN MANSAISI MEDICINE TECH CO LTD
Colon-targeted capsule for treating ulcerative colitis and preparation technology thereof
ActiveCN108888670ARegulatory immunityRegulate physiqueAntipyreticDigestive systemPositive controlTreatment effect
The invention relates to the technical field of medicines, in particular to a colon-targeted capsule for treating ulcerative colitis and a preparation technology thereof. Capsule contents of the colon-targeted capsule are prepared from 5-10 parts of gastric-dissolved pellets and 10-20 parts of enteric-coated pellets, the gastric-dissolved pellets are prepared from rhizoma atractylodis macrocephalae volatile oil, beta-cyclodextrin, a pill accelerator and the like, the enteric-coated pellets are prepared from coptidis rhizoma, saposhnicovia divaricata, fructus mume extracts, a pill accelerator and a colon-targeted coating material. Accordingly, the colon-targeted capsule is characterized in that the symptoms and the causes are treated, bioavailability is high, different ingredients are released in the stomach and the colon, and the capsule has the advantages that oral administration is safe and convenient, the coating prescription is simple, and industrial production can be conducted. Animal experiments show that the colon-targeted capsule has the obvious therapeutic effect on the ulcerative colitis, the treatment effect of the medium dosage is close to and even superior to the positive control drug SASP, and a brand new preparation is provided for clinically treating the ulcerative colitis.
Owner:苏州玉森新药开发有限公司
Gastrodine compound and pharmaceutical composition thereof
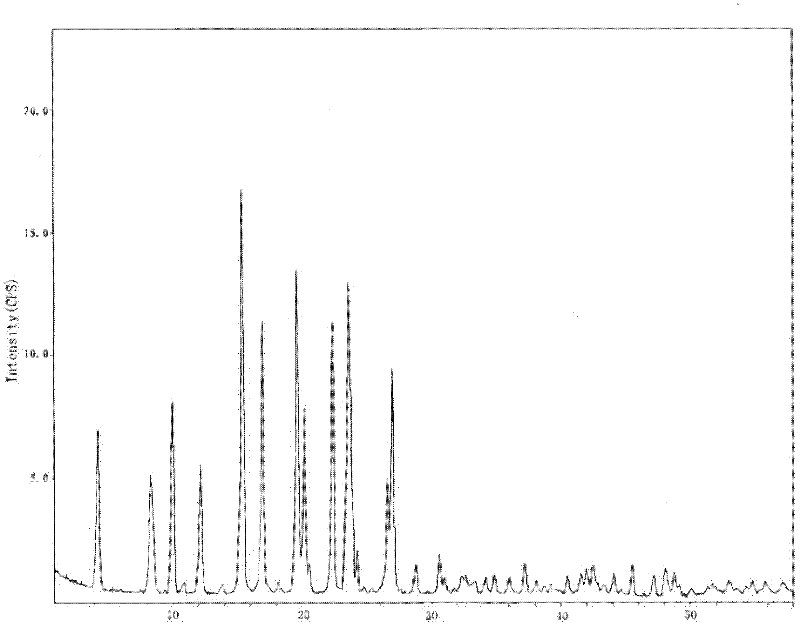
ActiveCN103224539AThe prescription process is simpleImprove stabilityOrganic active ingredientsNervous disorderCaplet Dosage FormPharmaceutical drug
The invention relates to a gastrodine compound and a pharmaceutical composition thereof. The gastrodine compound is a crystal. The X-ray powder diffraction determines that the characteristic peaks are displayed when 2theta+ / -0.2 degree is 4.5 degrees, 5.7 degrees, 7.9 degrees, 10.3 degrees, 11.2 degrees, 13.0 degrees, 16.4 degrees, 19.6 degrees, 21.2 degrees, 22.7 degrees, 24.8 degrees, 26.7 degrees, 29.6 degrees, 34.1 degrees and 35.3 degrees. The gastrodine compound has very high stability, thereby greatly enhancing the medicine safety. The invention also relates to a pharmaceutical composition preparation containing the gastrodine compound. The composition preparation can be a freeze-dried powder injection, injection, tablet or capsule. The gastrodine freeze-dried powder injection, injection, tablet or capsule preparation provided by the invention has the advantages of simple prescription technique, obviously higher stability, and enhances the medicine safety and effectiveness.
Owner:湖北美林药业有限公司
Sildenafil citrate sublingual tablet and its preparation method
InactiveCN101057850ARich blood vesselsImprove permeabilityPill deliverySexual disorderCITRATE ESTERMedicine
The invention discloses an acidum citricum hypoglossal tablet and the preparing method, relating to a tablet and the preparation method. The invention means to solve problems: current acidum citricum are all oral tablets and capsule, the content is large and increases liver injury, and it is easy to generate adverse effect. The comprised component and their weight proportion are as follows: acidum citricum 20 -30 units, manna sugar 45-65 units, lactin 65-105 units, sweetener 0. 5-1. 5 units, ethanol solution with concentration being 5%PVPK30 30 -40 units and dolomol 4-8 units. The preparation method comprises following steps: sifting sot material with nylon screen of 18-20 order to produce particular, drying at 55-65 Deg. C; sifting granular with screen of 14-16 order and placing for 5-7 hours for sheeting. The tablet is characterized by small content, increased biological utilization rate, low adverse effect rate, and the method is characterized by short and simple process.
Owner:HEILONGJIANG UNIV
Moxifloxacin capsules, and preparation method thereof
InactiveCN103860520ADissolution behavior did not changeAntibacterial agentsOrganic active ingredientsPharmaceutical industryMoxifloxacin hydrochloride
The invention belongs to the field of pharmaceutical industry, and relates to moxifloxacin capsules. A content of the moxifloxacin capsules comprises moxifloxacin hydrochloride, microcrystalline cellulose, sodium carboxymethyl starch, and magnesium stearate; the ingredients above are mixed, an obtained mixture is made into particles, and the particles are packaged by gelatin capsules so as to obtain the moxifloxacin capsules. Novel prescription and novel technology adopted by a preparation method of the moxifloxacin capsules are capable of avoiding reduction of dissolubility in storage processes, and ensuring f2 equivalence of the moxifloxacin capsules in four dissolution mediums with original grinded capsules.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Lansoprazole compound and pharmaceutical composition thereof
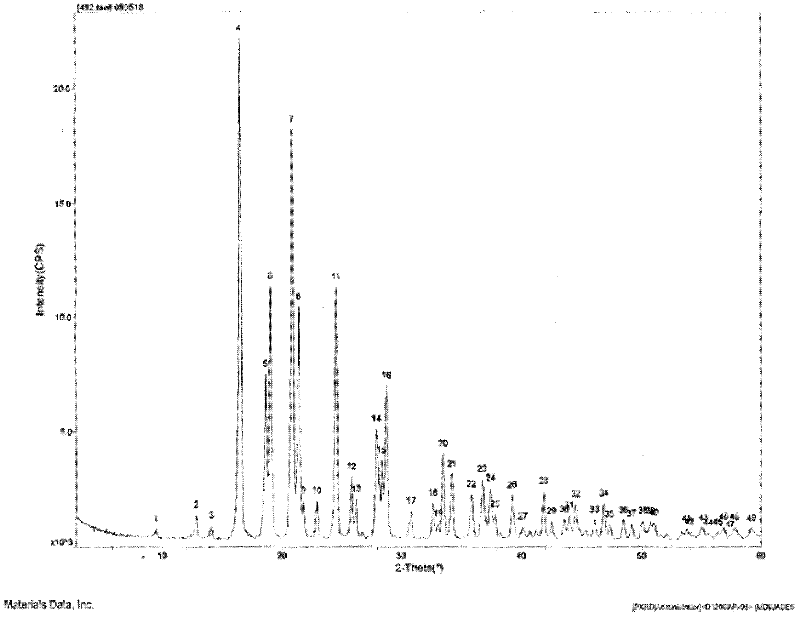
ActiveCN103254174AImprove stabilityLow impurity contentAntibacterial agentsOrganic active ingredientsLansoprazoleCoated tablets
The invention relates to a lansoprazole compound and a pharmaceutical composition thereof. The lansoprazole compound is a crystal. The characteristic peaks measured by X-ray powder diffraction are shown as 3.6, 4.8, 10.9, 14.0, 15.4, 16.9, 22.6, 24.7, 28.5, 32.1, 35.2, 36.7 and 39.3 at 2theta+ / -0.2 degrees. The medical composition preparation comprises the lansoprazole compound. The preparation is in form of freeze-dried powder injections, troches, capsules, enteric-coated tablets or enteric-coated capsules. The lansoprazole compound prepared by the invention has better solubleness and stability. The lansoprazole pharmaceutical composition prepared by using the lansoprazole compound provided by the invention as the effective component is good in stability.
Owner:湖北美林药业有限公司
Sustained releasing formulation of compound glucosamine, preparation process and application thereof
InactiveCN1961887ASafe releaseSmooth releaseOrganic active ingredientsAntipyreticSide effectVolumetric Mass Density
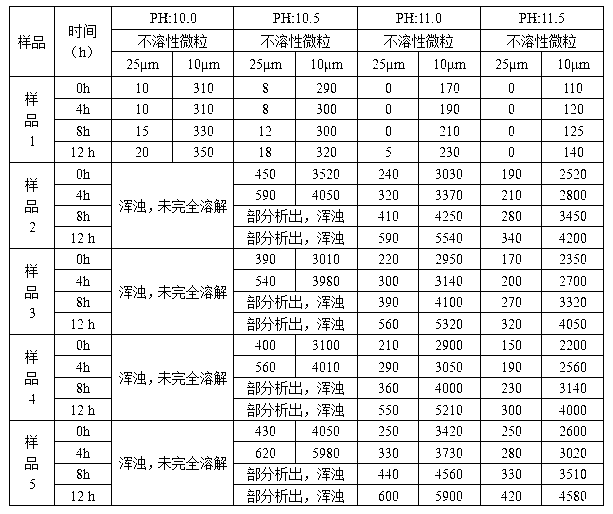
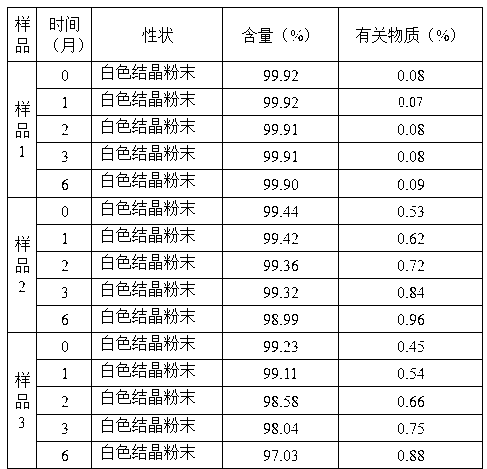
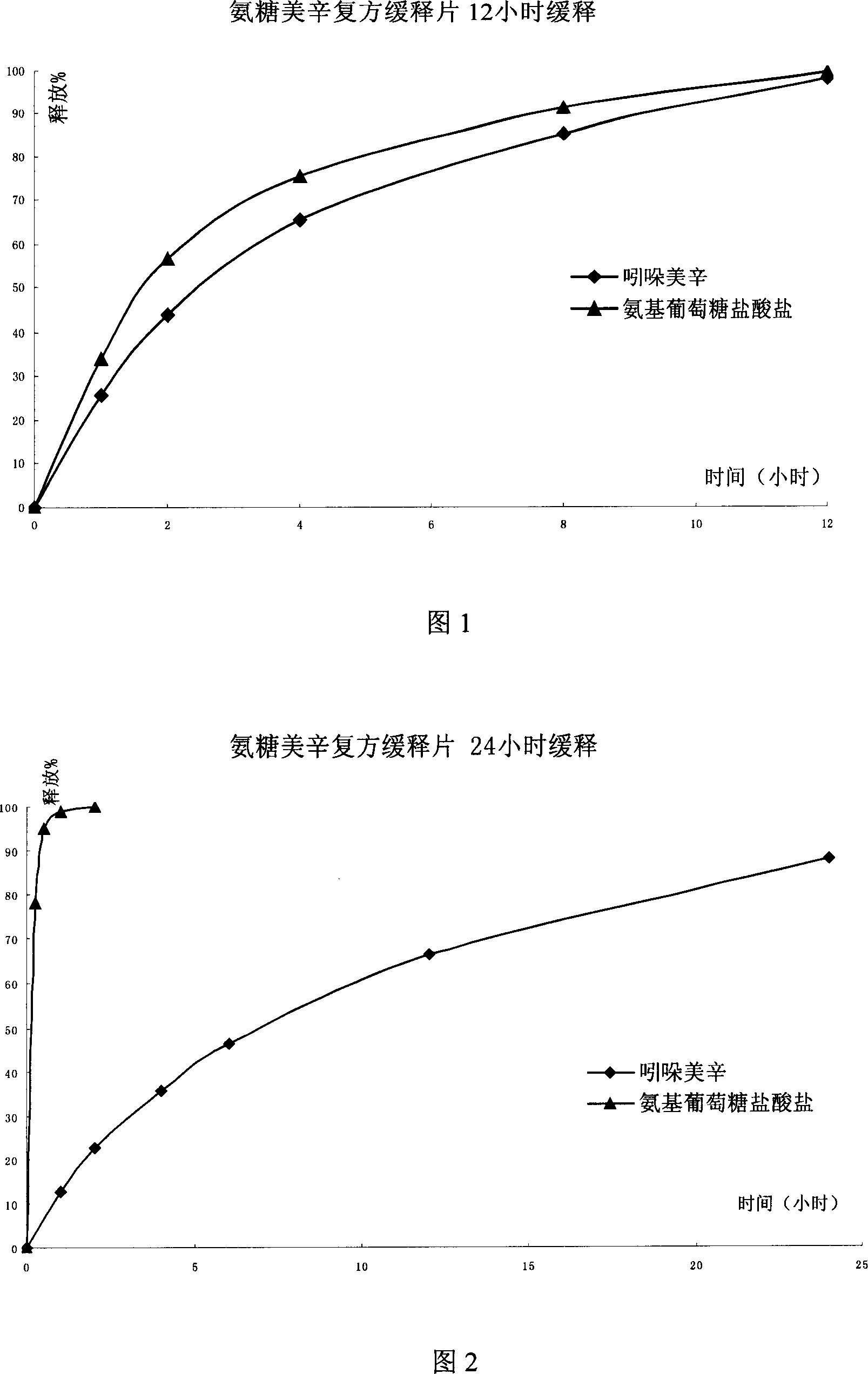
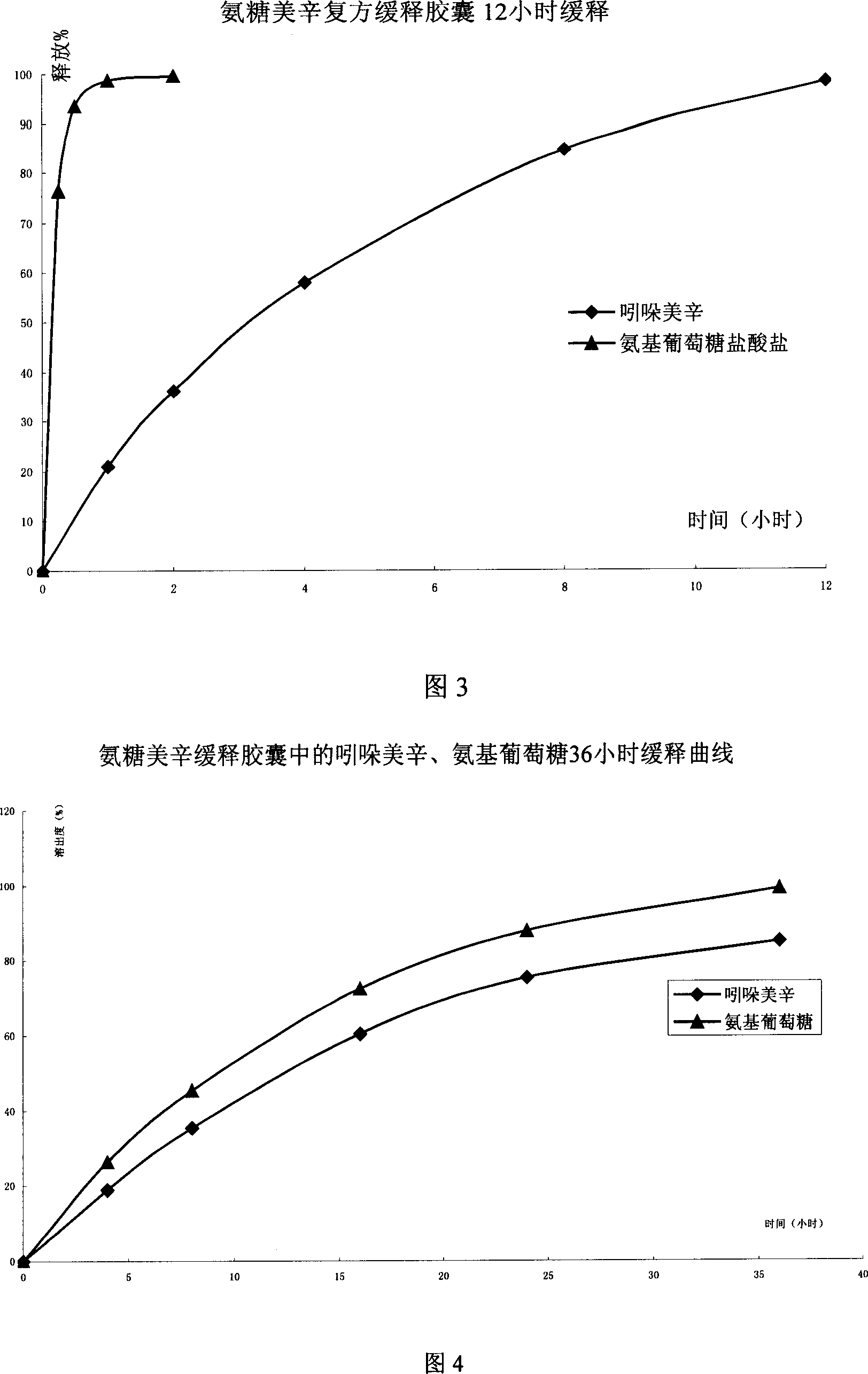
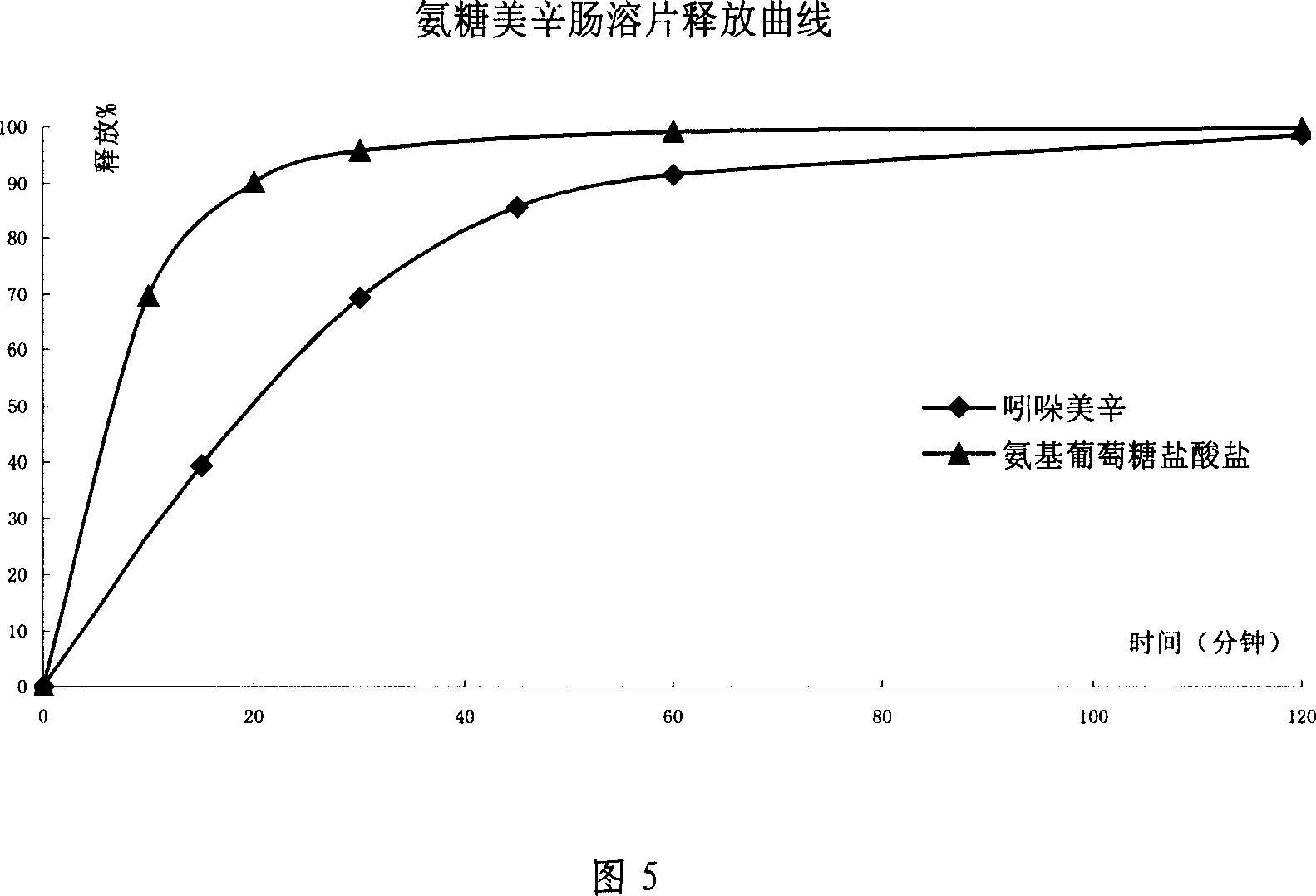
The invention relates to a composite slow-release agent, wherein it contains indomethacin, aminoglucose salt, slow-release material and other findings, while their ratios are 5-45%, 20-80%, 1-75%, and the left is findings. Its preparation comprises that mixing indomethacin and / or aminoglucose salt, and slow-release material and / or packs, then adding other findings, using general slow-release technique to obtain one or several skeleton or mould-contoro slow-release agent. The invention can be made into particle, oral, tablet or capsule. The invention can keep stable blood drug density in 8-36h, reduce side effect.
Owner:ZHEJIANG HAILISHENG PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com