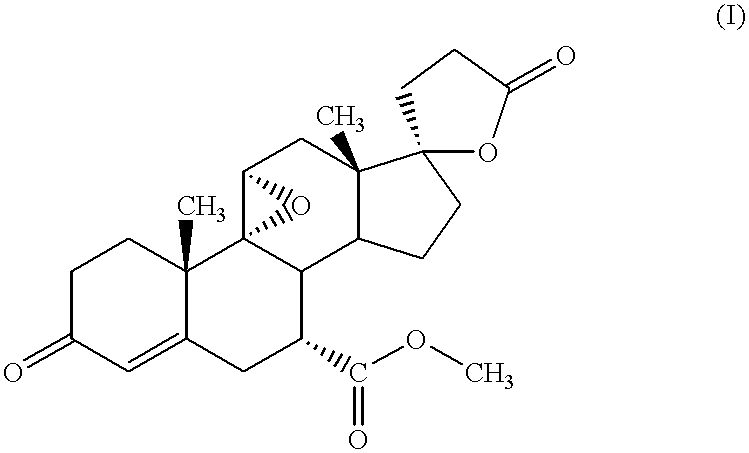
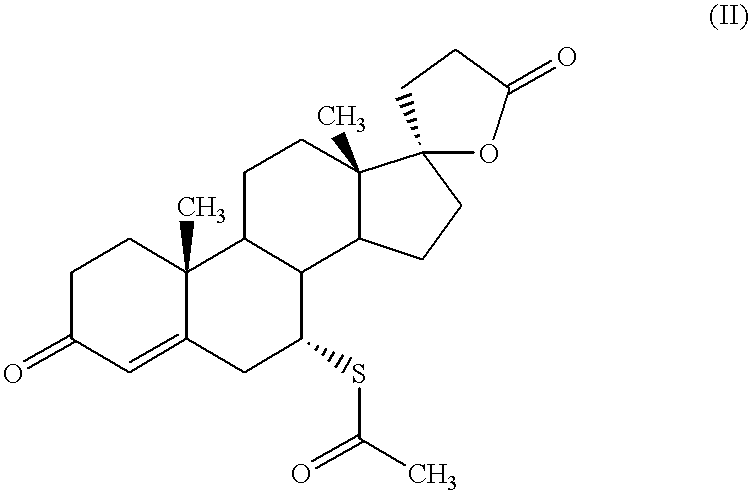
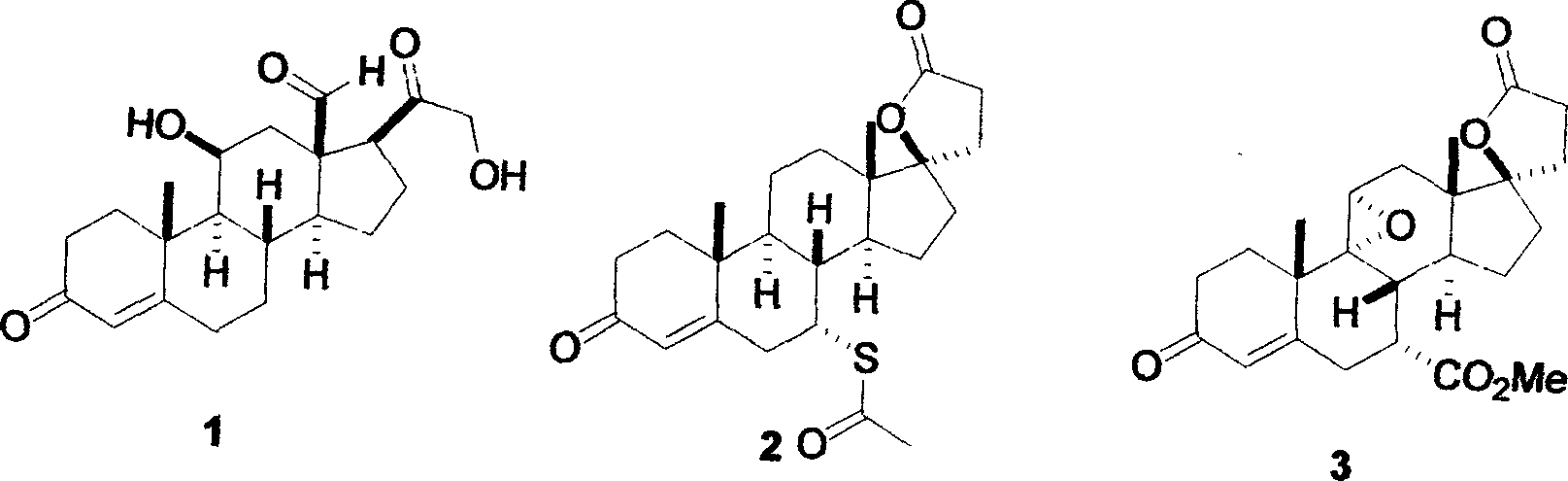
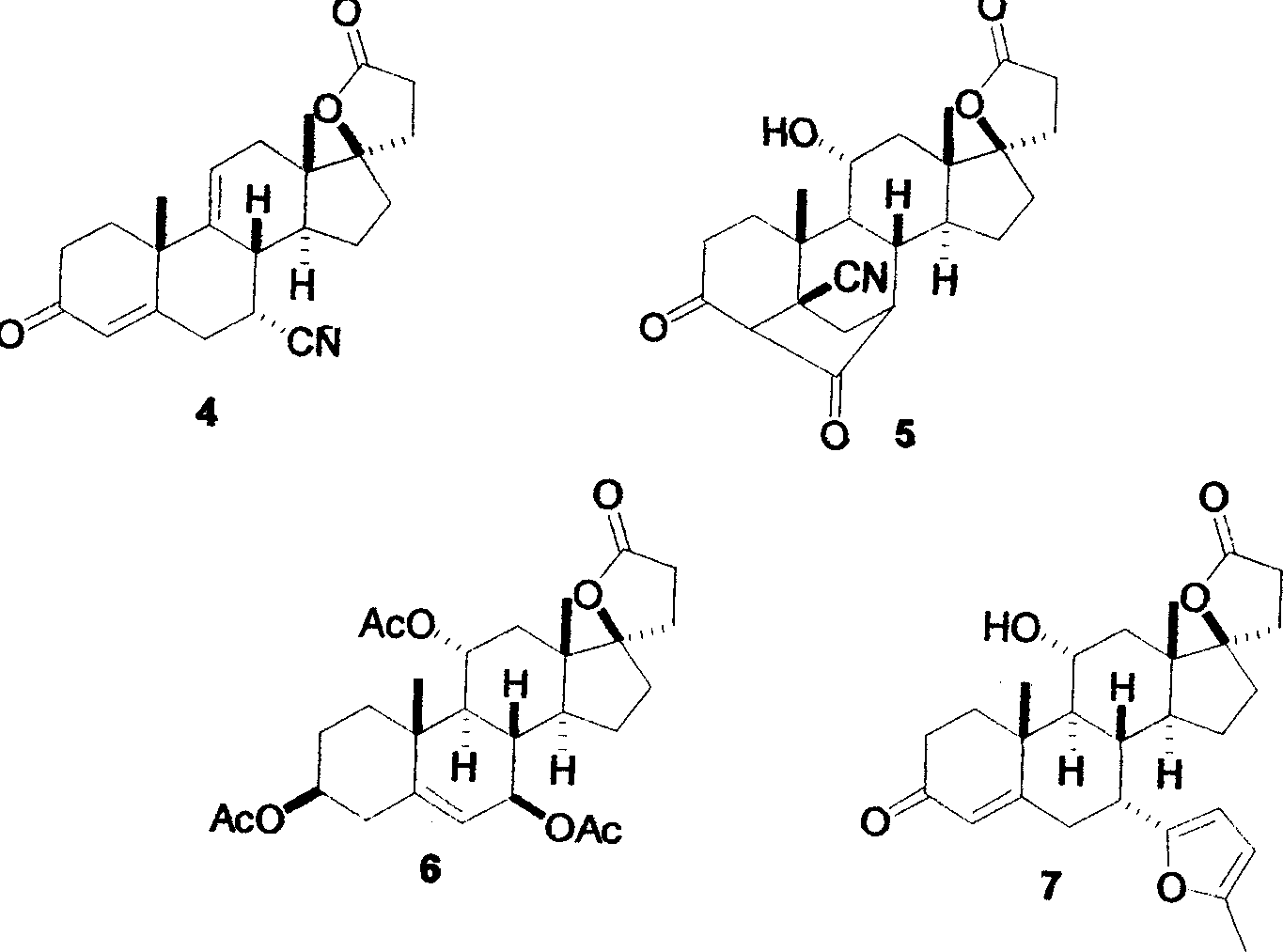
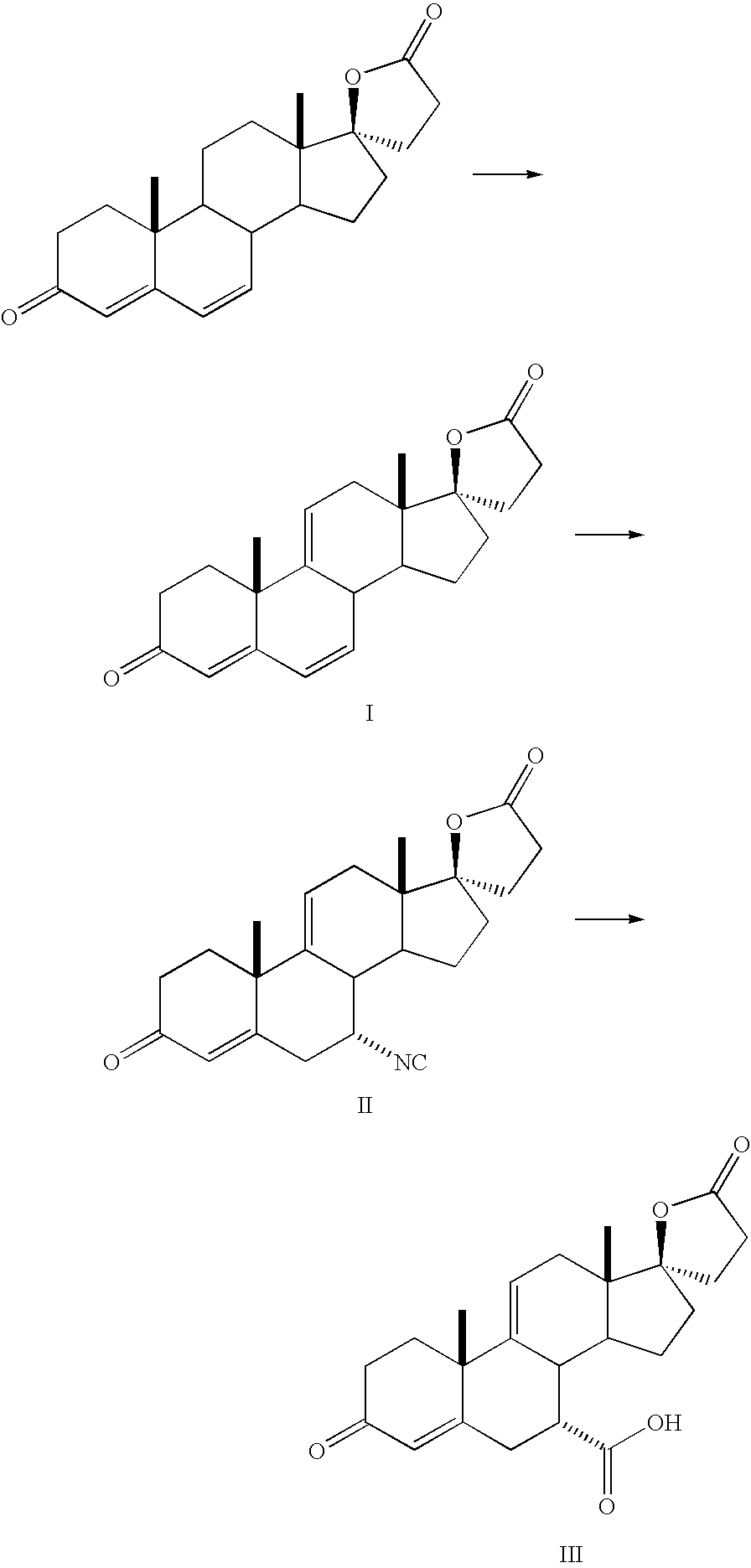
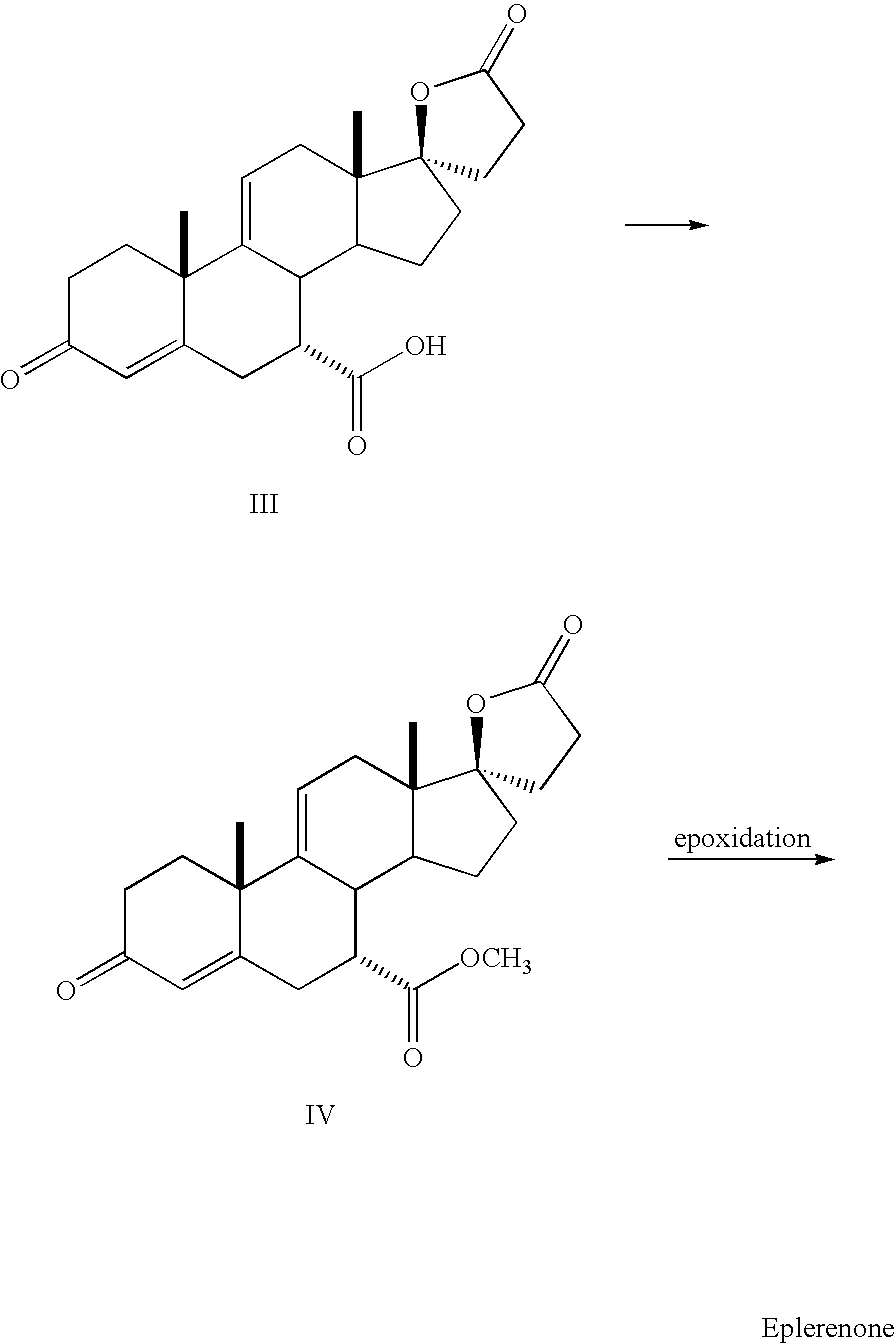
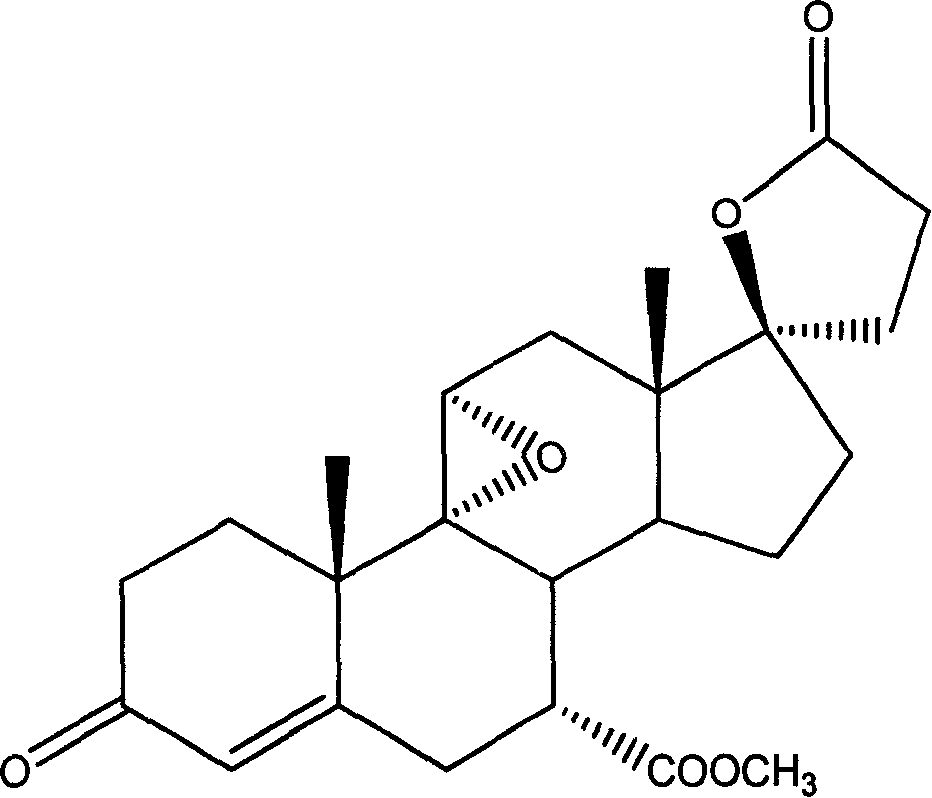
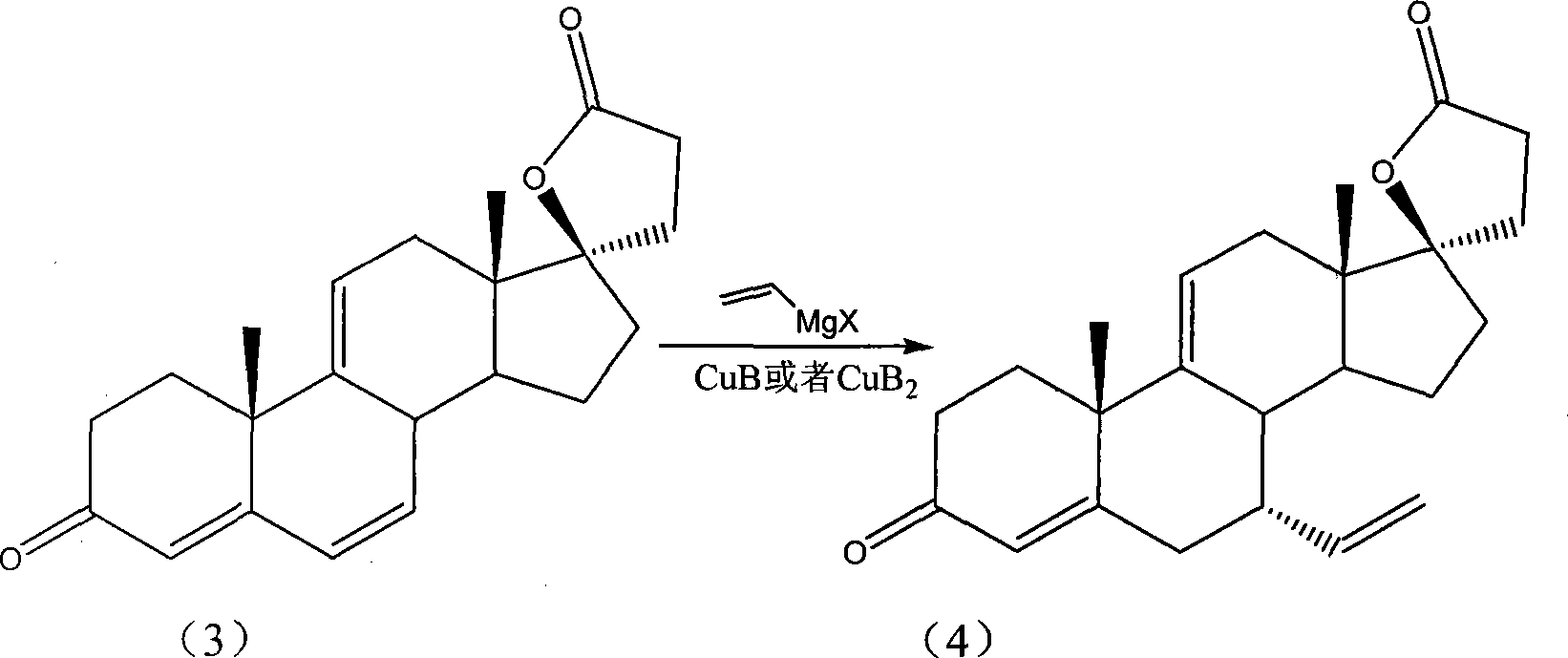
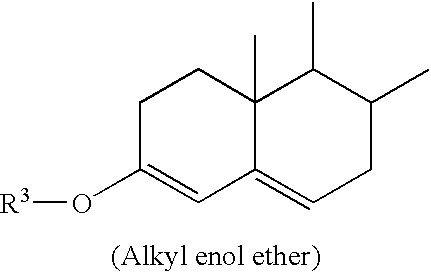
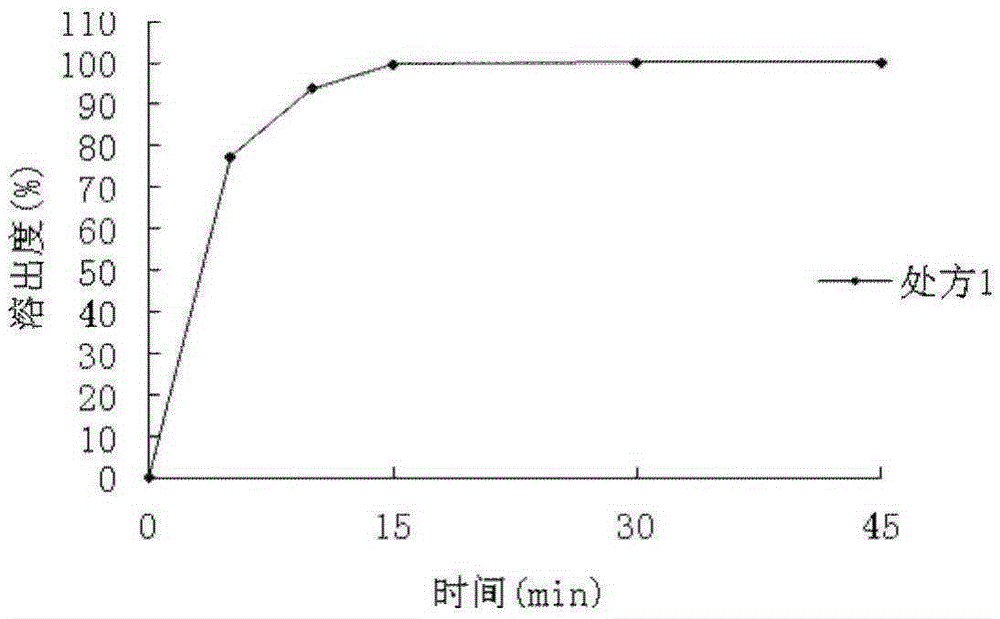
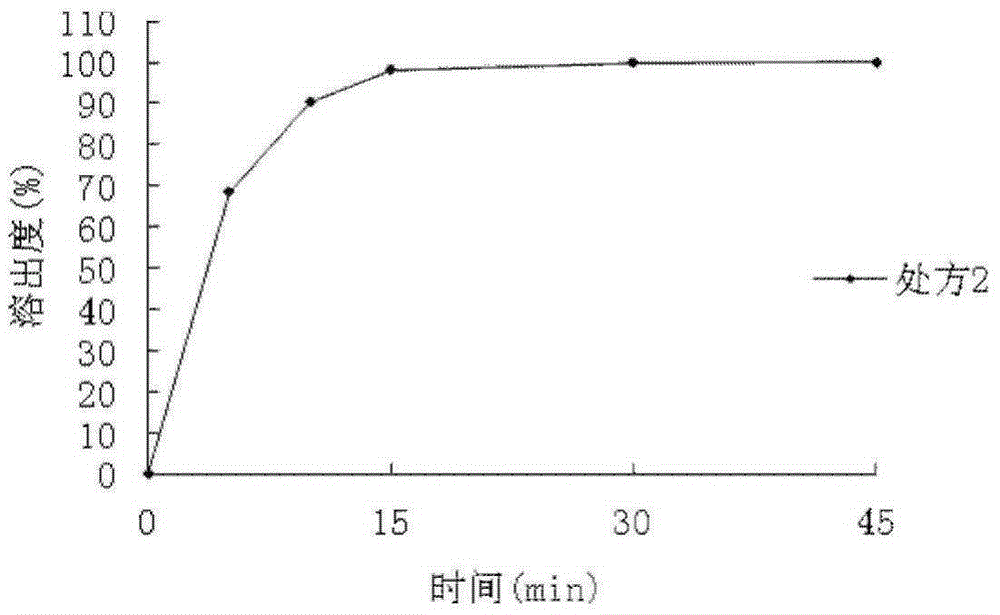
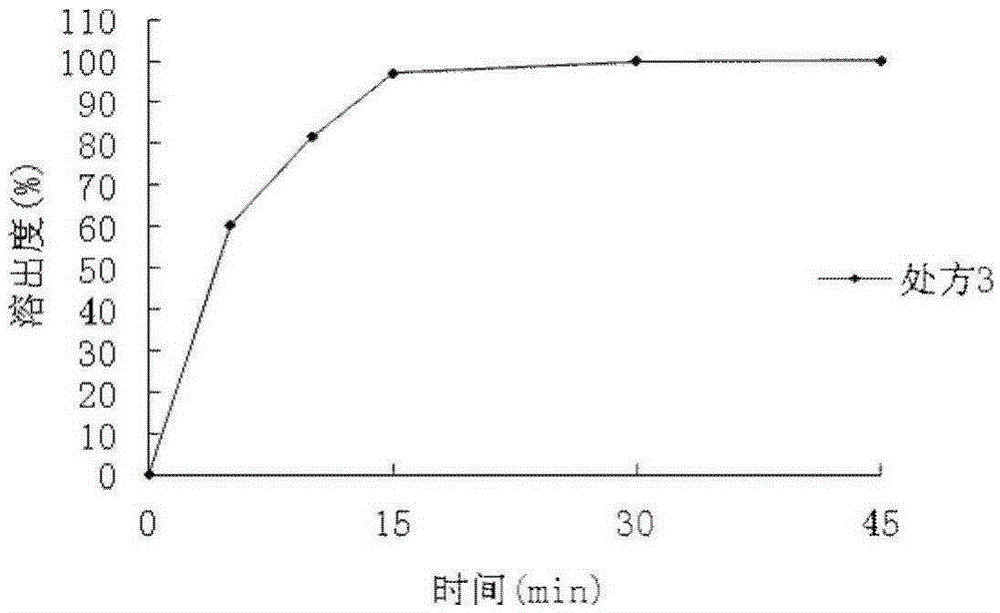
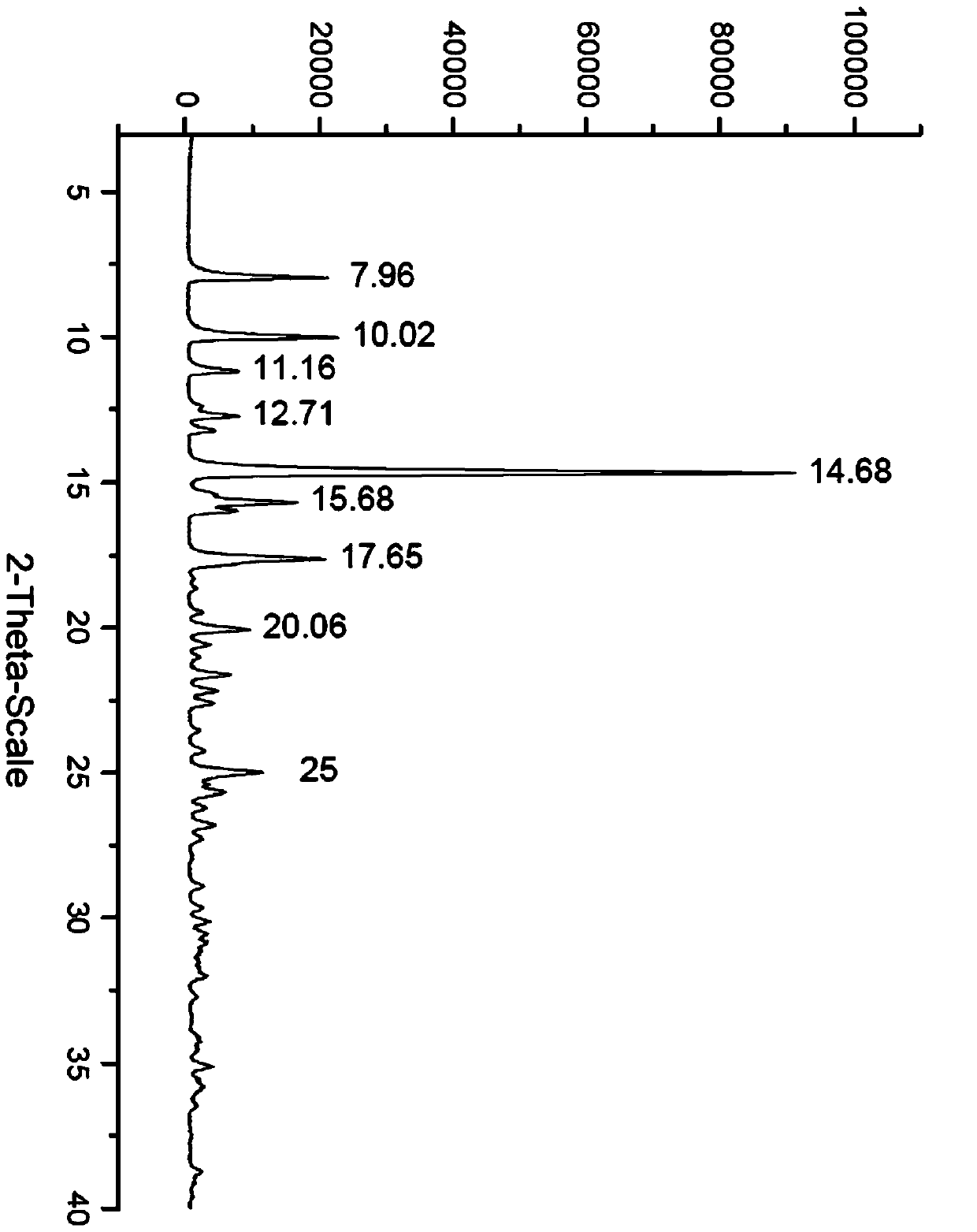
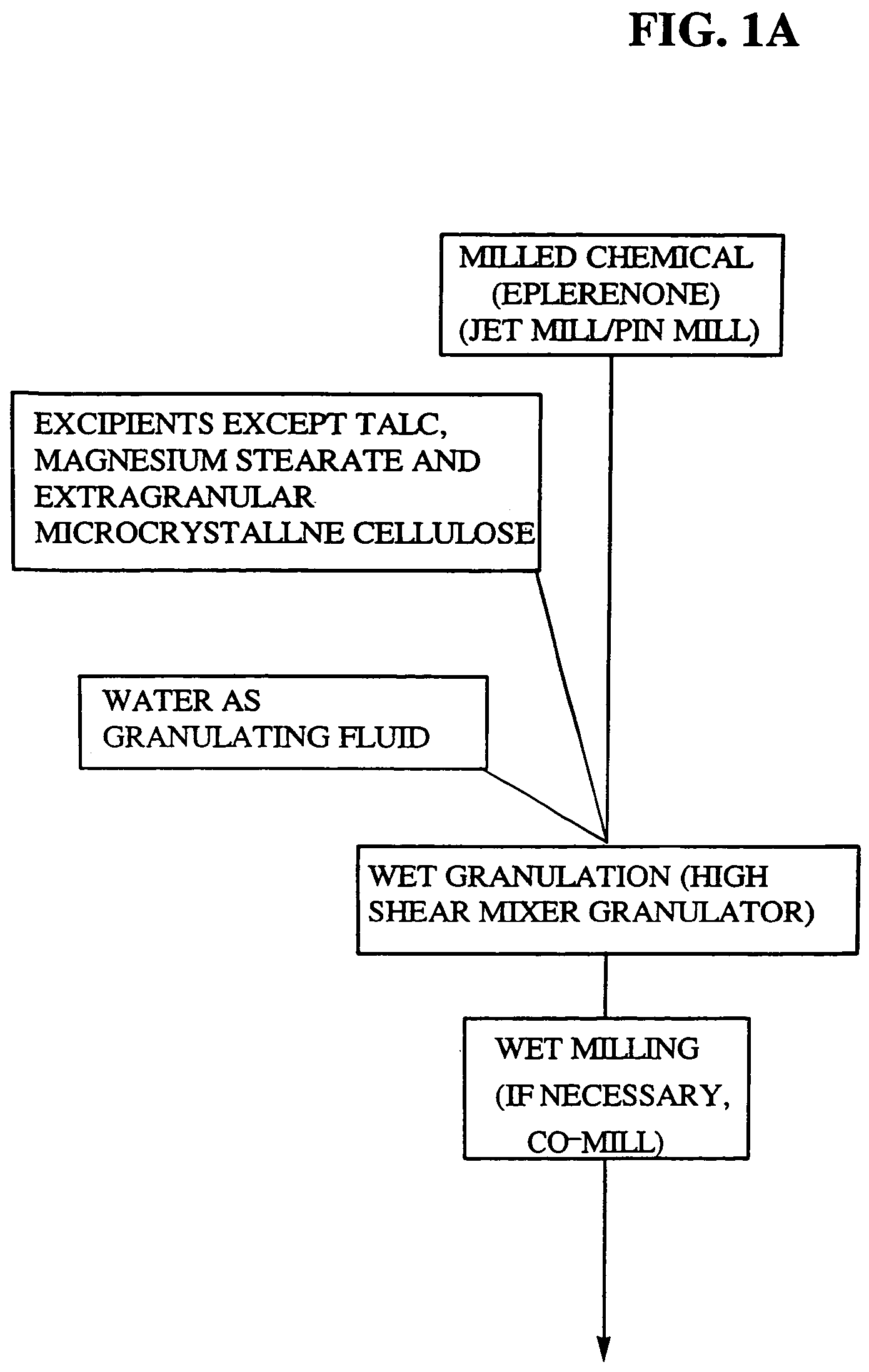
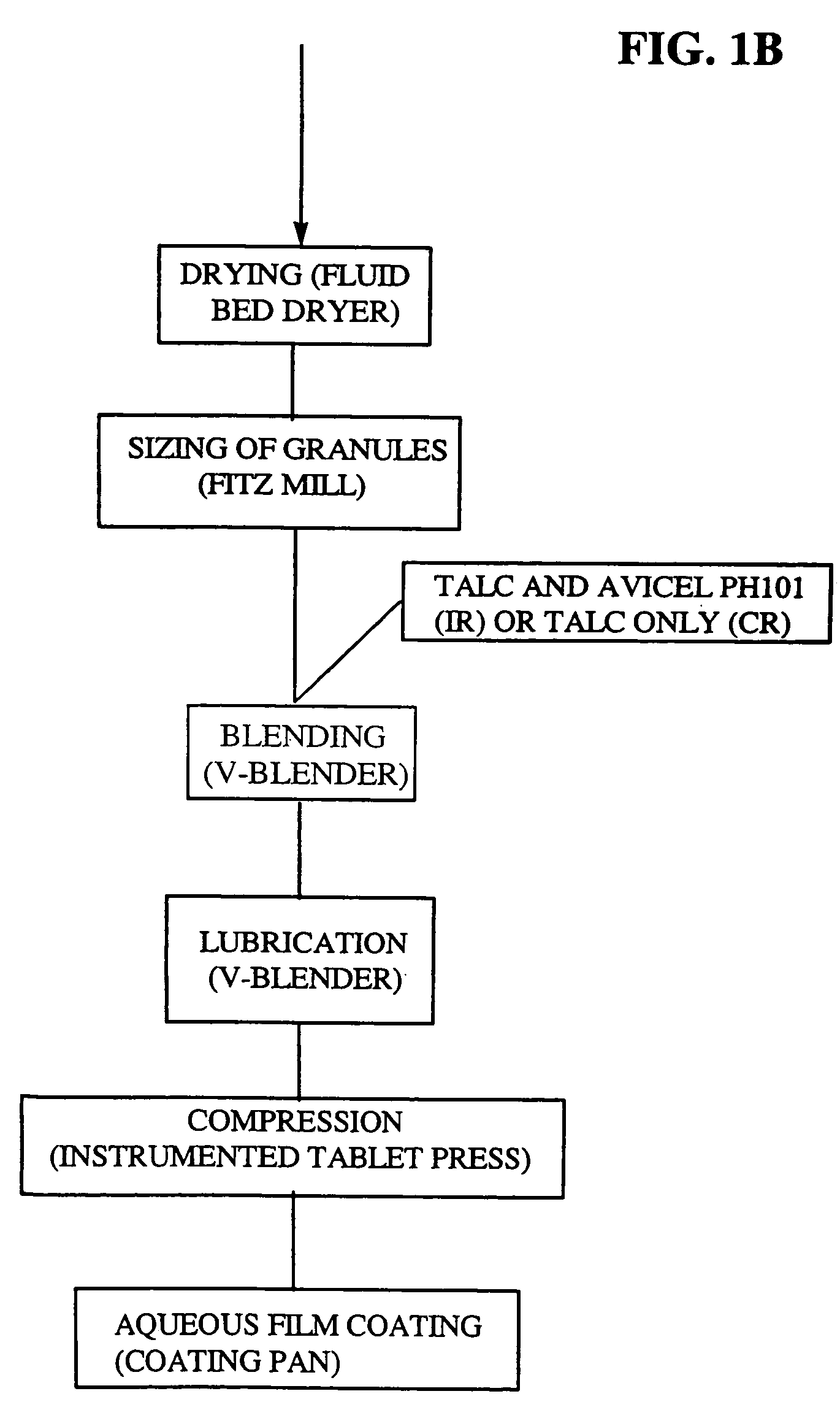
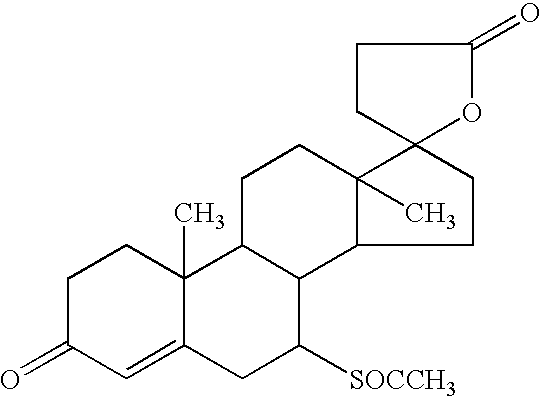
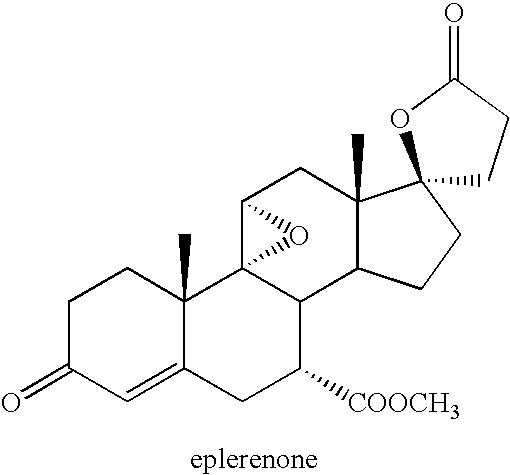
Patents
Literature
63 results about "Eplerenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
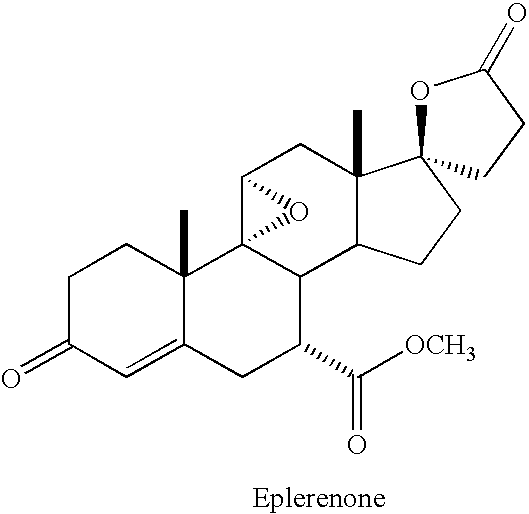
This medication is used alone or in combination with other medicines to treat high blood pressure.
Molecular dispersions of drospirenone
InactiveUS20050220825A1Improve bioavailabilityGood chemical stabilityPowder deliveryPill deliveryParticulatesDrospirenone
Described are pharmaceutical compositions comprising at least one steroidal drug such as a progestin (e.g. drospirenone, progesterone, eplerenone, etonogestrel) and / or an estrogen (estradiol and esters thereof) in molecularly dispersed form. The composition comprises a steroidal drug, preferably drospirenone, which is present in the composition in a non-particulate form. Preferably, the drug is present in a dissolved state in the excipient. The molecularly dispersed drug will be released very fast as dissolution takes place instantly when the dosage unit has disintegrated. Also described are methods for preparing the pharmaceutical compositions and methods of using the compositions.
Owner:BAYER SCHERING PHARMA AG
Nanoparticulate eplerenone compositions
InactiveUS20020006919A1Good physical propertiesImprove bioavailabilityPowder deliveryOrganic active ingredientsOral medicationCaplet Dosage Form
There is provided a pharmaceutical composition comprising eplerenone in solid particulate form, wherein at least 90% of the eplerenone particles are smaller than about 15 .mu.m, for example about 0.01 .mu.m to about 1 .mu.m, in diameter. The composition can be adapted for oral administration, for example as a tablet or capsule comprising eplerenone in a unit dosage amount of about 10 mg to about 1000 mg and one or more excipients.
Owner:THOSAR SHILPA S +3
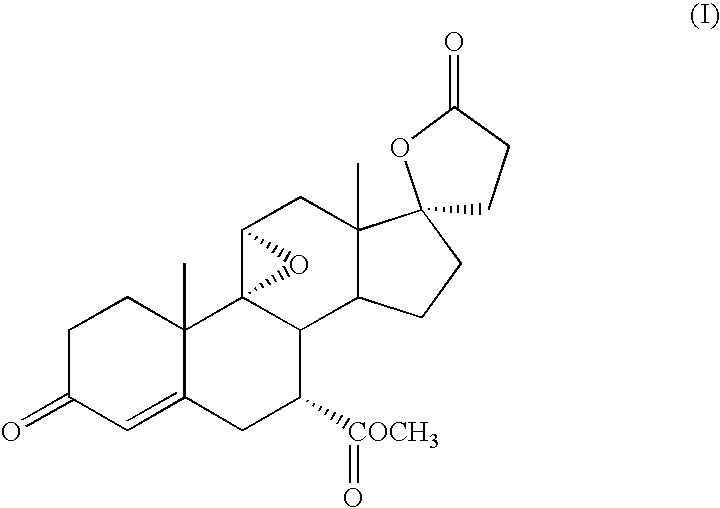
Synthetic method for eplerenone
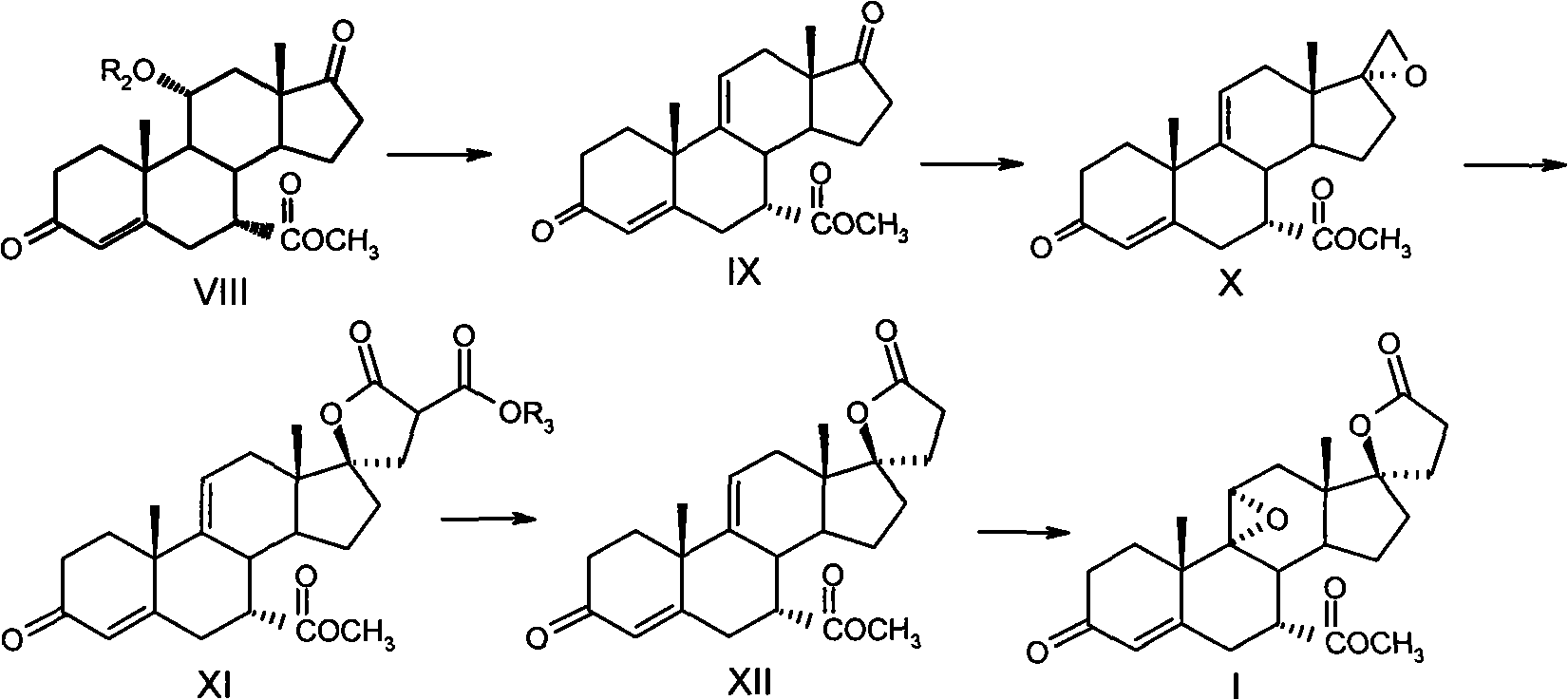
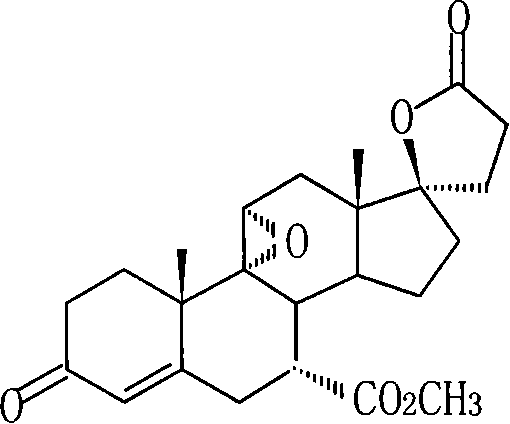
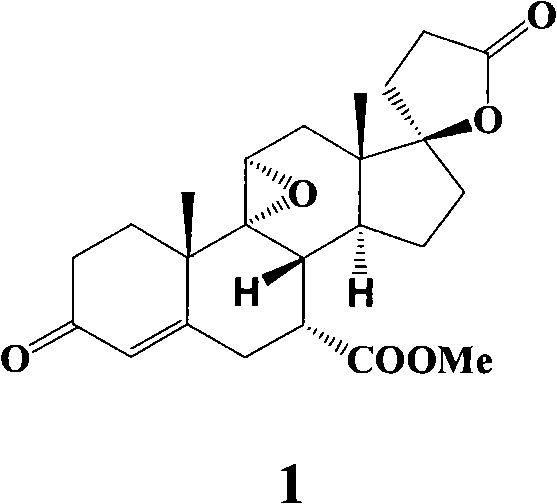
The synthesis process of eplerenone includes the following steps: double serial Michael addition / Aldo condensation reaction of 11alpha-hydroxyl curry ketone and acetone cyanohydrin to produce enamine, partially hydrolyzing the enamine to produce intermediate compound, opening ring of the intermediate compound to obtain the compound IX; eliminating reaction produced olefine ester from the compound IX, reaction with phosphorous oxychloride at room temperature for 12-24 hr, extracting with methane dichloride solvent, merging organic phase, washing and drying to obtain compound X; performing 9alpha, 11-double bond selective expoxidation, extracting the water layer with methane dichloride and merging the organic phase; washing with NaHSO3 solution, saturated Na2SO3 solution, dilute hydrochloric acid solution and saturated hydrochloric acid solution successively, drying, distillation at normal pressure and concentration to obtain eplerenone.
Owner:NANJING UNIV
Canrenone derivative steroid compound, preparation method and application in eplerenone preparation thereof
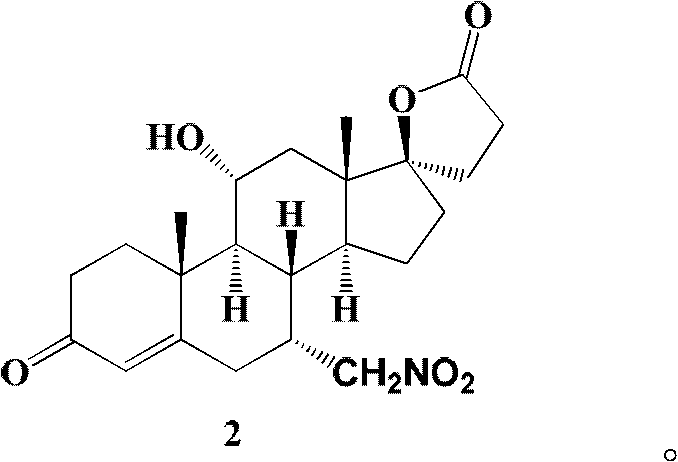
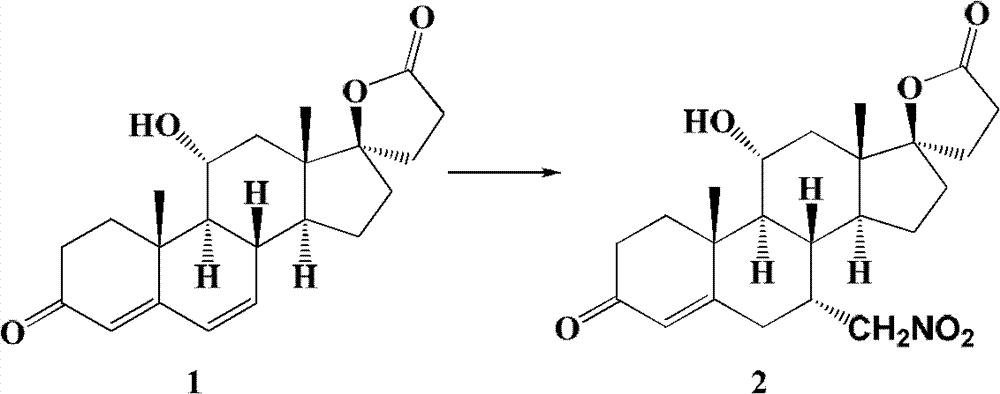
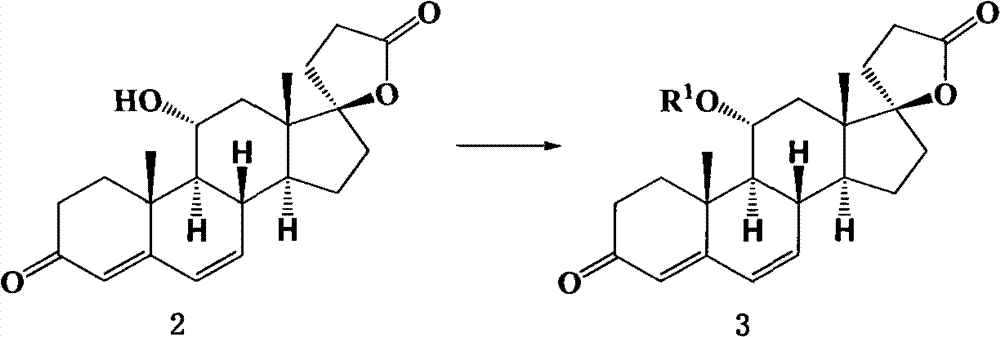
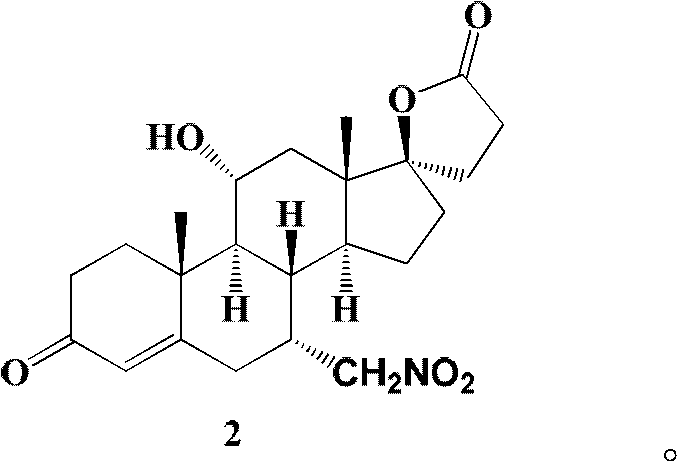
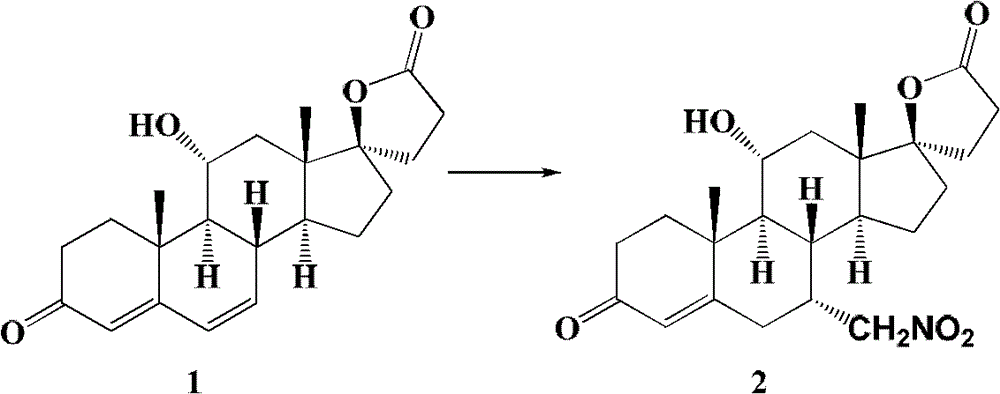
The invention relates to a canrenone derivative steroid compound, a preparation method and an application in the medicine field, and particularly relates to 7alpha-nitro methyl-11alpha,17beta-dihydroxy-3-oxo-17alpha-pregna-4-ene-21-carboxylic acid-gamma-lactone (a compound shown in formula 2), a preparation method and an application in eplerenone preparation. The key steps of the invention are that nitromethane is used as a nucleophilic reagent; the alpha-nitro methyl group is introduced to the C-7 position stereoselectively so as to further construct a carboxylic acid methyl ester structure with a C-7alpha position configuration of eplerenone; the method of the invention has the characteristics of short steps, mild conditions, and low cost.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
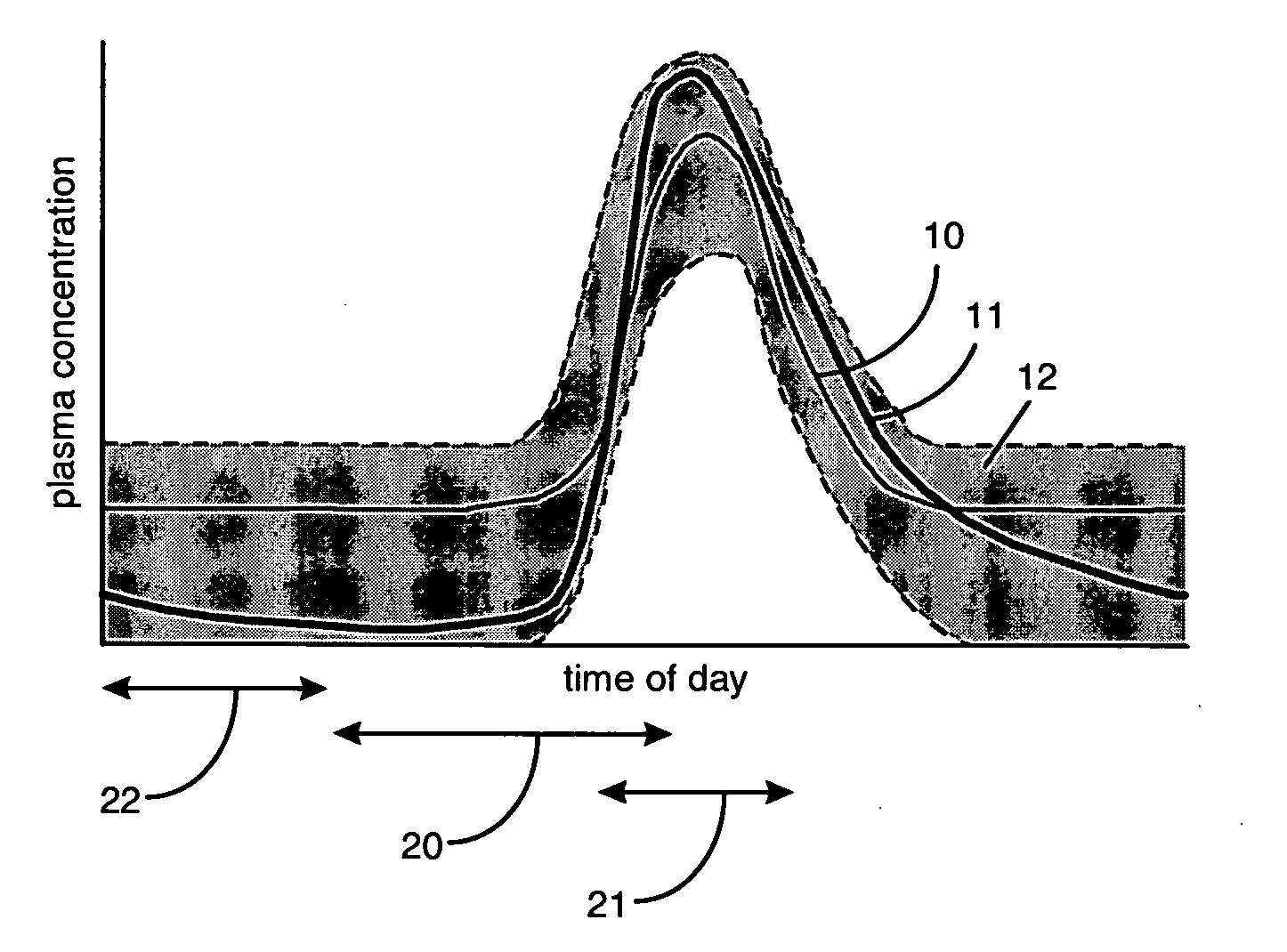
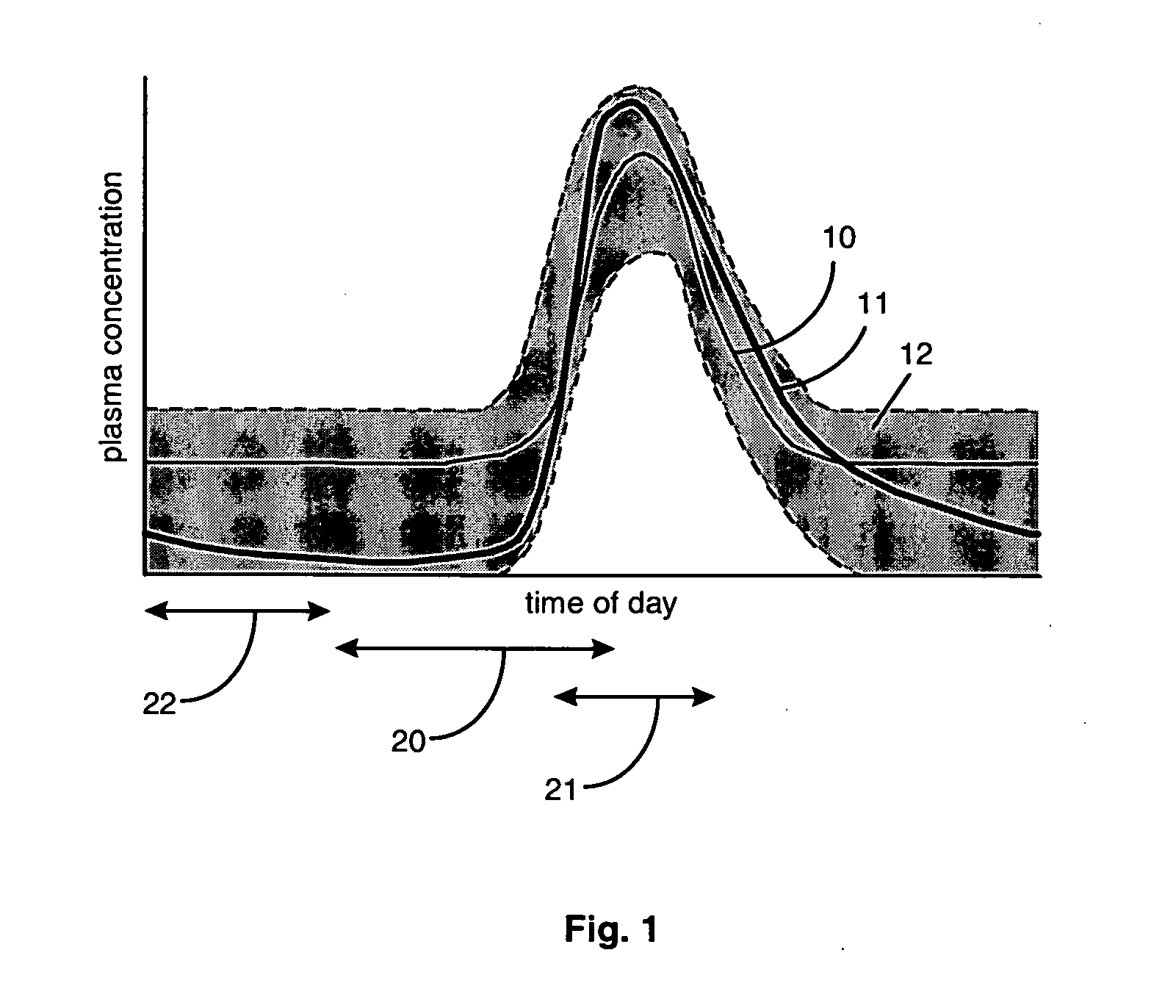
Aldosterone antagonist compositions for release during aldosterone acrophase
InactiveUS20050192259A1Reduce the possibilityGood blood pressureBiocideOrganic active ingredientsDrugEplerenone
A pharmaceutical composition is provided for administration to a subject mammal such as a human exhibiting a diurnal cycle of plasma aldosterone concentration, the composition comprising a delayed-release formulation of an aldosterone antagonist drug, e.g., eplerenone, in a therapeutically effective amount. The delayed-release formulation, when administered about 6 hours to about 12 hours prior to the acrophase, results in a profile of plasma drug concentration that corresponds substantially to the diurnal cycle of plasma aldosterone concentration.
Owner:PHARMACIA CORP
Method for synthesizing eplerenone
InactiveCN101318986ASources are cheap and readily availableProcess operation is easy to controlSteroidsSide chainHydrocortisone
The invention discloses a new method for synthesizing steroid drug eplerenone. A surface cortisol as a byproduct of hydrocortisone is used as a raw material to oxide 17-side chain into ketone; 5, 6-double bond is introduced by 3-one protection; 7-ester group is introduced by cyaniding; 11-hydroxy is eliminated and 9,11-double bond is introduced; 17-ketone group is subjected to epoxidation, condensation and decarboxylation; and finally, the 9,11-double bond is epoxidated to produce the eplerenone. The method has raw materials with cheap prices and available sources, the easy control of process operation, high yield and low cost and is suitable for industrialized production.
Owner:JIANGSU CHUANGUO PHARMA CO LTD
Synthesizing method of eplerenone
The invention provides a novel synthesizing method of a steroid drug eplerenone.According to the synthesizing method, 11-alpha-hydroxycarvenone serves as the starting material, and eplerenone is generated through a series of reactions such as addition, substitution, elimination, oxidation, esterification and epoxidation.According to the synthesizing method of eplerenone, the starting material is low in price and easy to purchase, operation is simple and easy to control, the yield is high, the cost is low, and the method is suitable for industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
Process for the preparation and purification of eplerenone
InactiveUS20080234478A1Robust and amenable to to levelsProcess robust and amenableSteroidsPurification methodsEplerenone
A process for the preparation and purification of Eplerenone is described wherein hydroxylated impurities are removed using a novel derivatization procedure.
Owner:APOTEX PHARMACHEN INC
Eplerenone pharmaceutical composition
InactiveCN101152187AGood curative effectDisintegrates quicklyOrganic active ingredientsMetabolism disorderSurface-active agentsBioavailability
The invention discloses a drug combination containing eplerenone. The combination also contains nonionic surface active agent, has high bioavailability, and is used to treat cardiovascular diseases including hypertension.
Owner:BEIJING D VENTUREPHARM TECH DEV
Preparation method of intermediate for preparing eplerenone
ActiveCN101121652AAvoid problems such as serious pollutionHigh yieldCarboxylic preparation by ozone oxidationCarboxylic acidSolvent
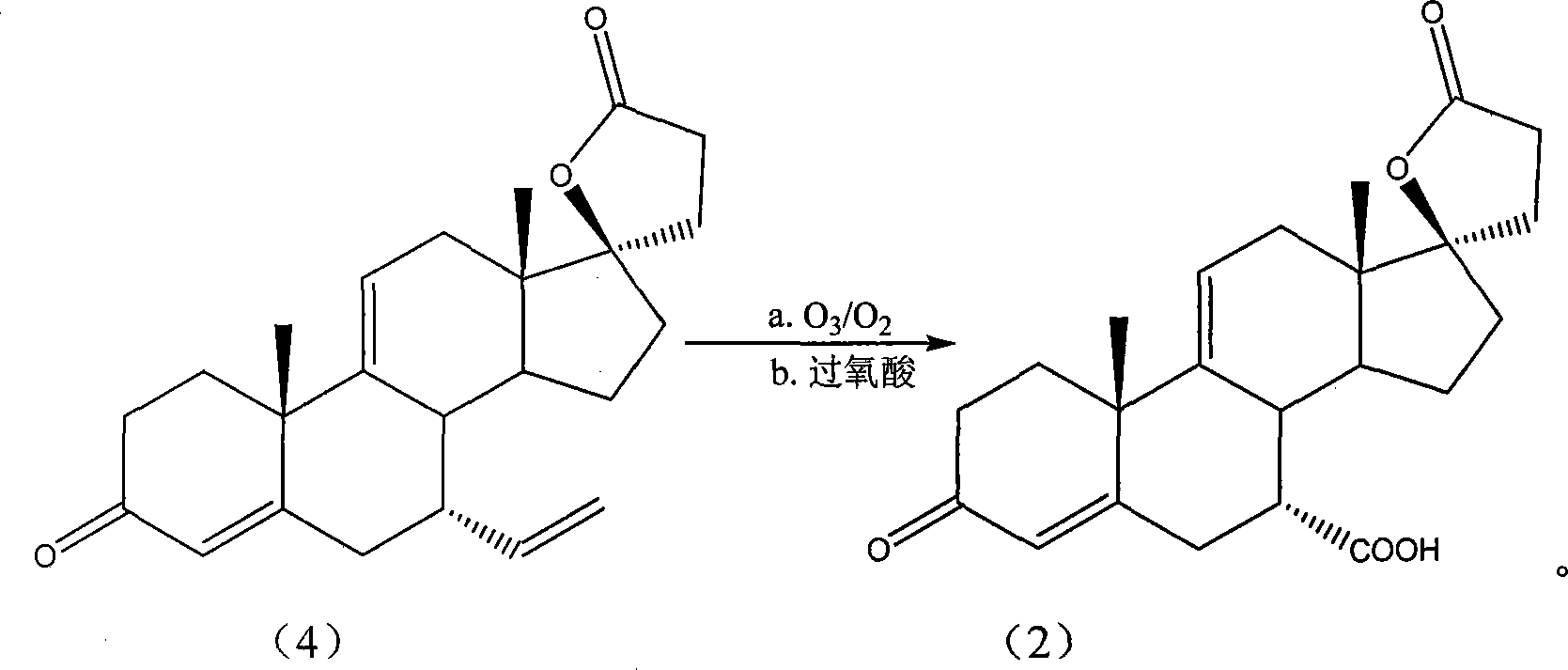
The invention provides a preparation method of the 17 beta- hydroxy-7alpha-carboxyl-3- oxo-17alpha-pregna-4, 9(11)-diene-21-carboxylic acid-gamma-lactone; the starting material of the invention is delta9 (11)- risperidone; with the use of the CuB or CuB2, the starting material reacts with the alkenyl halogenated magnesium; then with the solvent, the mixture of the ozone and oxygen takes part in the reaction; the peracid is added in, then the target product can be collected from the reaction products. The invention can get the product of the higher purity with a higher collection rate and a higher selectivity; the method is simple and safe; the purification is convenient; the invention greatly lowers the production cost. The environmental pollution is little and thus the invention facilitates the implementation of the industrialization.
Owner:湖南玉新药业有限公司
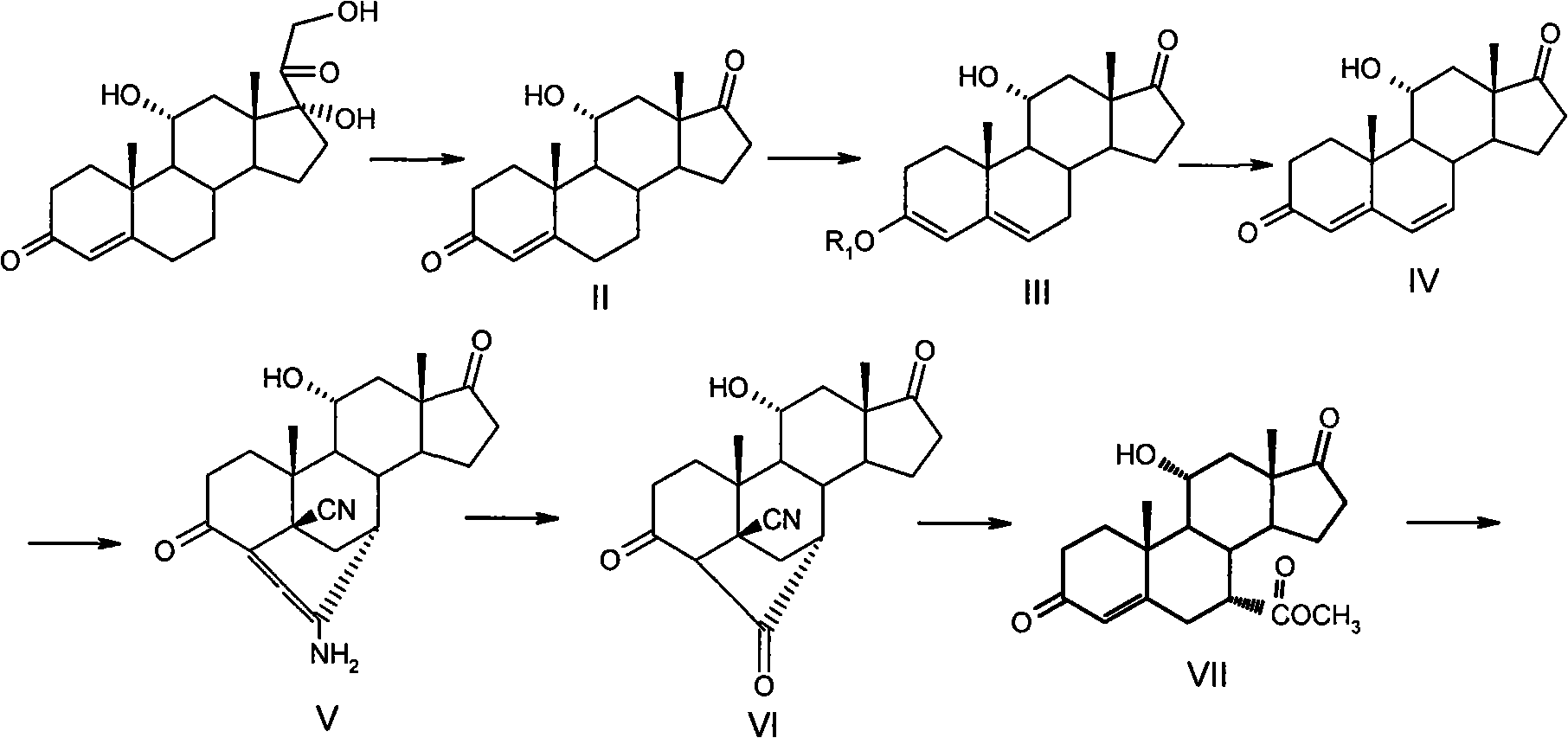
Process to prepare eplerenone
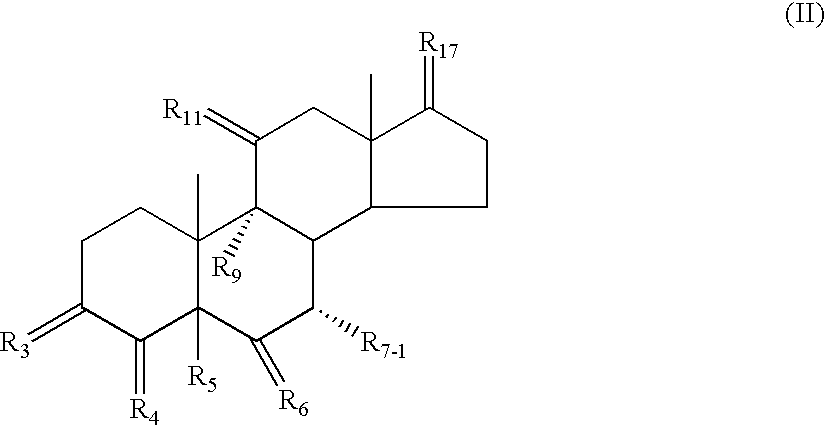
The present invention involves intermediates, including a 7alpha-substituted steroid (II), and processes which are used to prepare eplerenone, a useful pharmaceutical agent.
Owner:PHARMACIA & UPJOHN CO
Eplerenone dispersible tablet
ActiveCN105362242ALarge distribution areaIncreased absorption pointsOrganic active ingredientsDispersion deliveryAdjuvantAdditive ingredient
The invention discloses an eplerenone dispersible tablet, which takes eplerenone as an active drug ingredient and uses a disintegrating agent, a cosolvent and an auxiliary additive as non-active ingredients, wherein the disintegrating agent, the cosolvent and the auxiliary additive are common medicinal dispersible tablet dosage adjuvant materials for preparing the dosage form of the dispersible tablet. The eplerenone dispersible tablet disclosed by the invention can overcome the shortcomings of a conventional eplerenone tablet which, due to slow disintegration, is slow in dissolution, insufficient in absorption, not high in bioavailability and the like; the dispersible tablet can rapidly disintegrate in water so as to form a uniform suspension, and the dispersible tablet can rapidly disintegrate and disperse in gastrointestinal tract; a drug distribution area is enlarged, absorption points are increased and absorption is accelerated, and bioavailability is significantly higher than that of the conventional tablet; and the preparation is stable, more convenient to take, low in adverse reactions and is more suitable for patients of hypertension at various ages.
Owner:HEFEI JIUNUO MEDICAL TECH
Method for preparing eplerenone
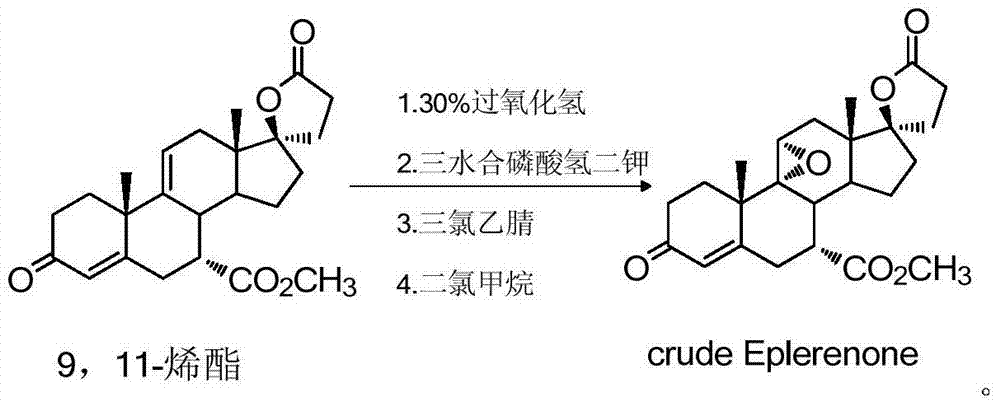
The invention provides a method for preparing eplerenone, which comprises the following steps of: (1) in a solvent, in the presence of a secondary reaction inhibitor, in a buffer system of trichlormethyl eyanide, an oxidizing agent and phosphate, performing double bond selective epoxidation on 17 alpha-hydroxy-3-keto-gamma-lactone-pregna-4,9(11)-diene-7 alpha,21-dicarboxylicacid methyl ester IV to prepare crude eplerenone; and (2) recrystallizing the crude eplerenone to obtain quality eplerenone. The high-purity eplerenone can be prepared by the method, the purity reaches 99.5 percent, the yield reaches 87 percent, and the method is suitable for large-scale industrial production.
Owner:AURISCO PHARMACEUTICAL CO LTD
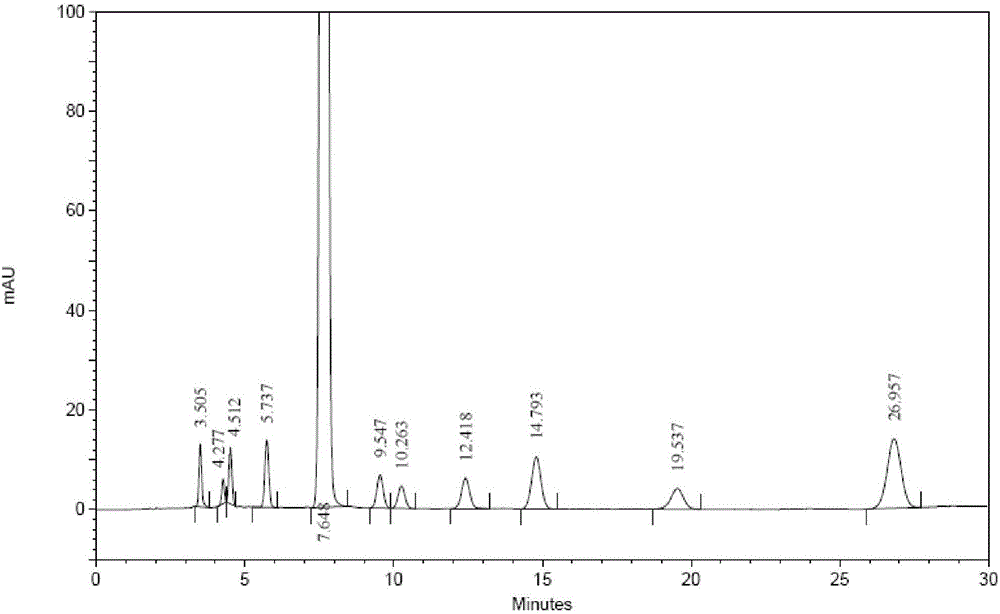
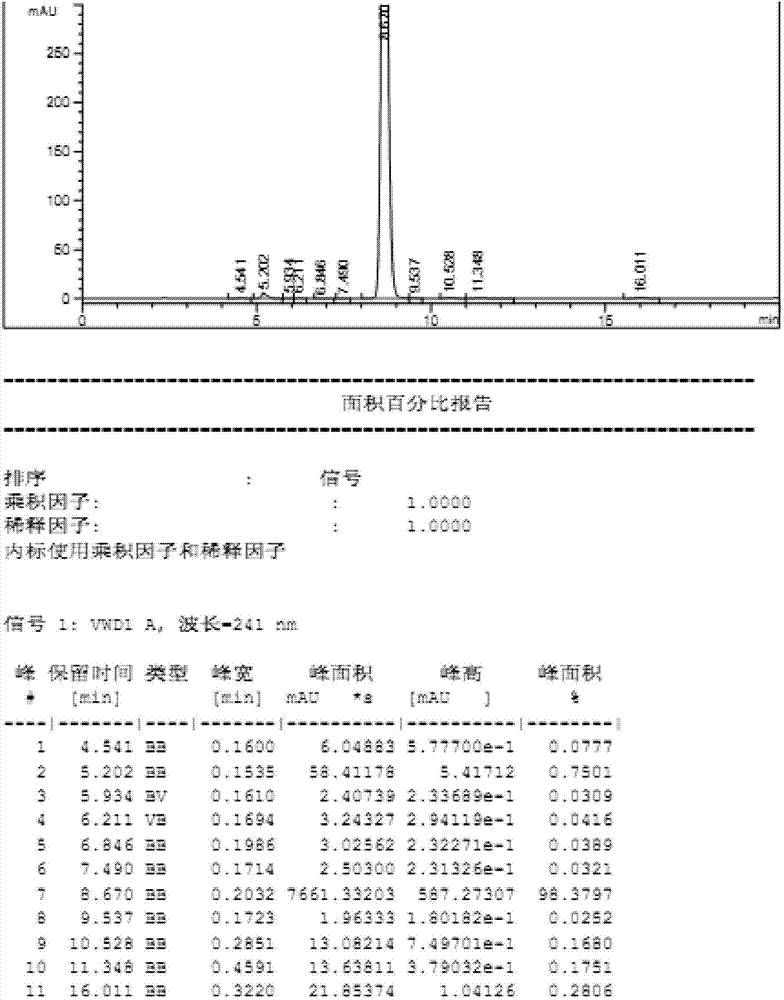
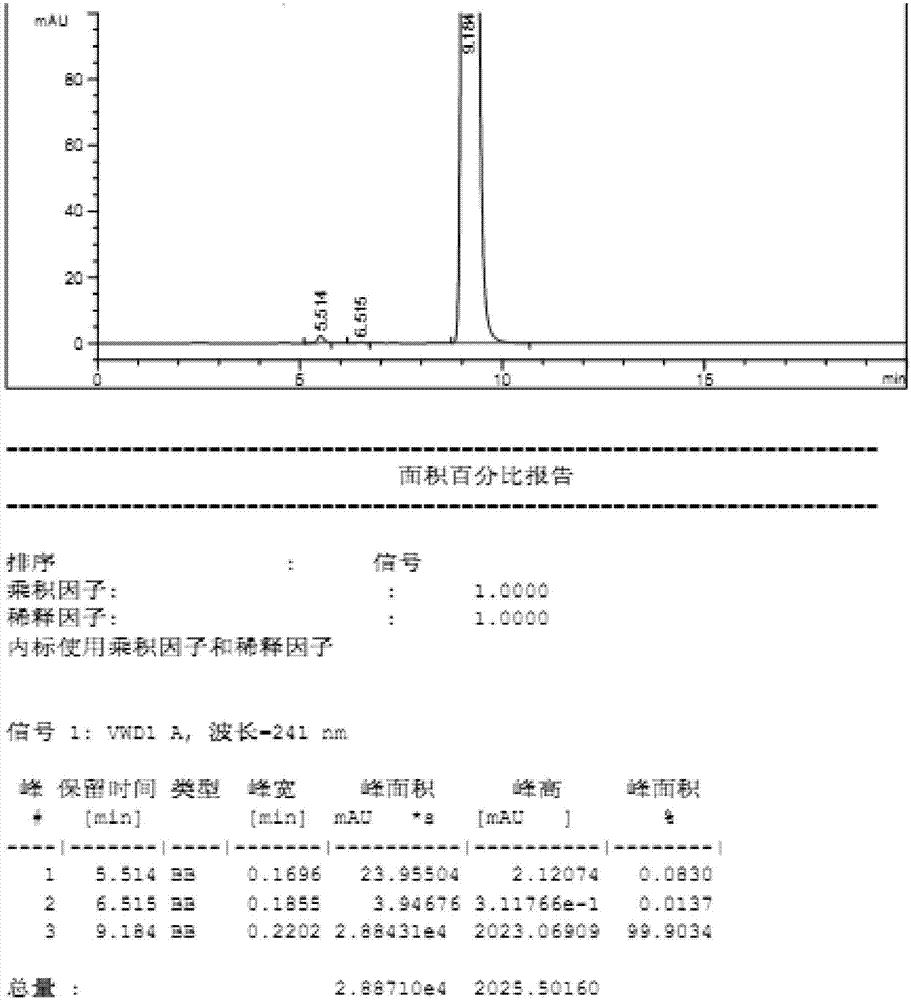
High performance liquid chromatography analyzing method of eplerenone related substances
The invention discloses a high performance liquid chromatography analyzing method of eplerenone related substances. The high performance liquid chromatography analyzing method is characterized in that a reversed-phase chromatographic column and an ultraviolet detector are used and an acetonitrile-methanol-phosphate buffer solution is used as the flowing phase to perform isocratic elution. The high performance liquid chromatography analyzing method has the advantages that all the known impurities in eplerenone raw materials and eplerenone preparations can be analyzed at the same time, the content of the known impurities can be effectively controlled through the self-contrasted method of the main component added with correction factors, the separation degree between a main peak and an impurity peak adjacent to the main peak and between every two impurity peaks is larger than 1.5, and the purity of the main peak and the impurity peaks is 1.0. By the high performance liquid chromatography analyzing method, a simple and reliable analyzing method is provided for the quality control analyzing of the eplerenone raw materials and the eplerenone preparations.
Owner:HEFEI JIUNUO MEDICAL TECH
L-crystal form eplerenone refining method
The invention discloses an L-crystal form eplerenone refining method which is as follows: an eplerenone raw product is added into 1, 2-dimethoxy ethane solvent system, heated to dissolve, and filtered, the filtrate is cooled for crystallization, filtration, and drying to obtain L-crystal form eplerenone. The volume percentage of 1, 2-dimethoxy ethane in the 1, 2-dimethoxy ethane solvent system is greater than or equal to 50%. The method is simple in operation, high in yield, low in drying temperature, good in effect of removing impurity, and the purity of the L-crystal form eplerenone is above 99.9% (detected by HPLC), and the single impurity peak is less than 0.1%.
Owner:HEFEI JIUNUO MEDICAL TECH
Inhalation medicinal composition prepared from eplerenone and glucocorticoid serving as active ingredients
ActiveCN102370984AInhibit compensatoryLittle side effectsOrganic active ingredientsRespiratory disorderFlunisolideGlucocorticoid
The invention discloses an inhalation medicinal composition prepared from eplerenone and glucocorticoid serving as active ingredients. The inhalation medicinal composition consists of eplerenone, glucocorticoid and one or more carriers suitable for inhalation administration. The glucocorticoid is one or more of ciclesonide, desonide, fluticasone propopnate, mometasone furoate, beclomethasone dipropionate, flunisolide, and triamcinolone acetonide and medicinal salts or esters thereof; the inhalation medicinal composition is preferably prepared into aerosol powder which consists of eplerenone, glucocorticoid and carrier micro powder; the average grain diameter of the eplerenone and glucocorticoid micro powder is 0.5 to 1.0 mu m; and the average grain diameter of the carrier micro powder is 20 to 45 mu m.
Owner:TIANJIN JINYAO GRP
Micronized eplerenone compositions
InactiveUS7157101B2Efficient deliveryReduce solubilityPowder deliveryOrganic active ingredientsActive agentIncreased aldosterone
The invention relates to oral pharmaceutical compositions useful as aldosterone receptor blockers comprising the active agent micronized eplerenone in an amount of about 10 mg to about 1000 mg and one or more carrier materials.
Owner:GD SEARLE & CO
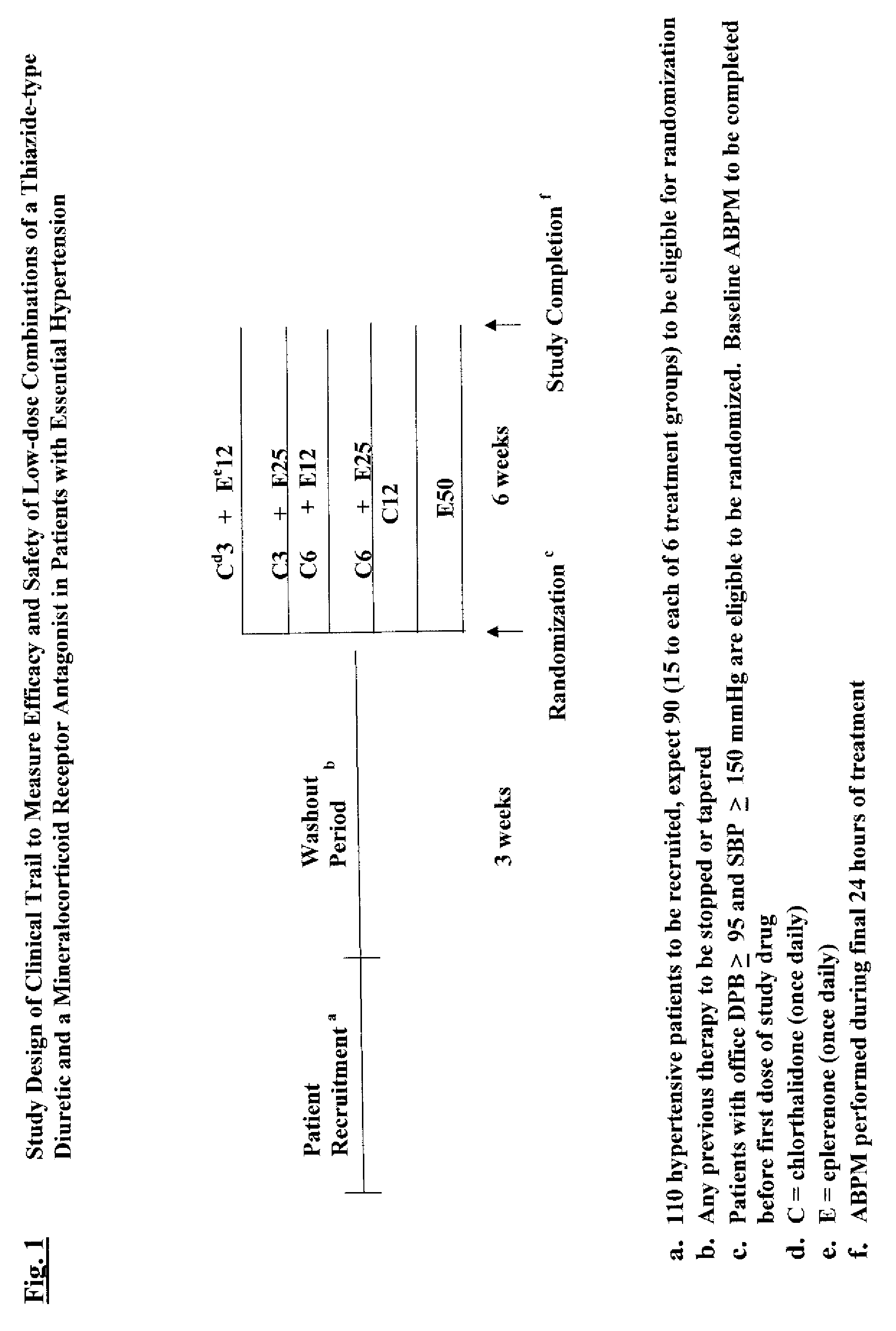
Compositions and methods comprising a thiazide diuretic and a mineralocorticoid inhibitor
InactiveUS20070287690A1Minimizes and completely avoids adverse effectEffective treatmentBiocideAnimal repellantsDiseaseMedicine
The invention relates to low dose combinations of a thiazide diuretic and a mineralocorticoid inhibitor and methods for the treatment of hypertension and related disorders. Methods and compositions for administering a thiazide diuretic, such as chlorthalidone, and an mineralocorticoid inhibitor such as spironolactone or eplerenone in a dose range of between 3 mg and 12 mg or eplerenone in a dose range of between 3 mg and 25 mg each,
Owner:SOLOMON LAWRENCE +1
Method for preparing and refining eplerenone
InactiveCN104262450AShort reaction timeReduce manufacturing costSteroidsTrichloroacetonitrileHydrogen phosphate
The invention discloses a method for preparing and refining eplerenone. The method for preparing and refining eplerenone comprises the following steps: taking 9,11-alkenyl ester to react at 0-30 DEG C in the presence of potassium hydrogen phosphate trihydrate, trichloroacetamide and hydrogen peroxide so as to prepare an eplerenone crude product; carrying out re-crystallization by 2-butanone to obtain an eplerenone fine product with a purity of greater than 99.9%. The method is simple to operate; poisonous and harmful trichloroacetonitrile is not used; the use amount of hydrogen peroxide is greatly reduced; the production period is greatly shortened; the yield and purity of products are greatly improved; the method is suitable for industrial application.
Owner:JIANGSU SINOBIOPHARMA
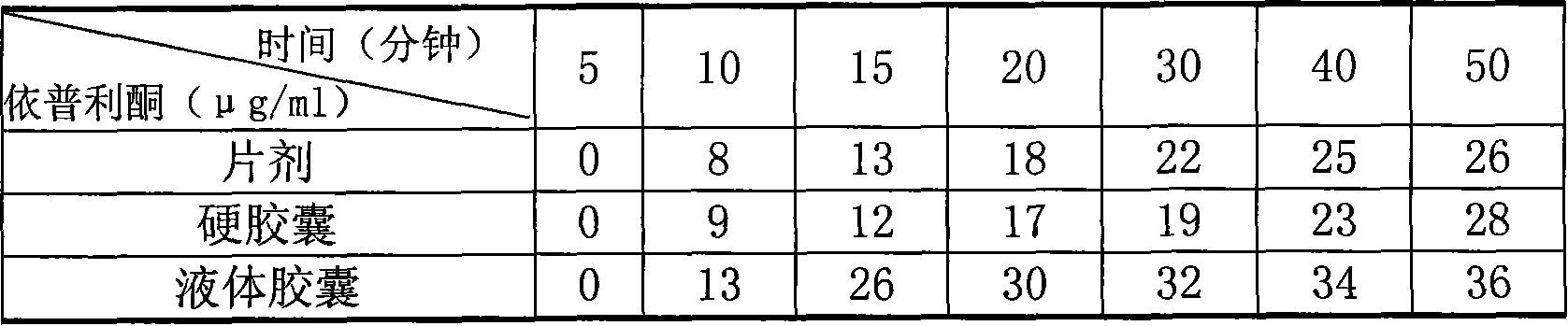
Eplerenone liquid capsule preparations and method for preparing the same
InactiveCN101116658AOmit the step of preparing particlesReduce drynessOrganic active ingredientsCapsule deliveryOrganic solventMedicine
The invention relates to eplerenone liquid capsule preparation and the preparation method thereof, which solves the problems of poor stability and low bioavailability of the prior eplerenone preparation. The contents liquid medicine of each hard capsule of the eplerenone liquid capsule preparation comprises raw material of following weight account: 25 mg to 100 mg eplerenone, 250 mg to 1000 mg organic solvent and 0 mg to 0.005 mg preservative. The eplerenone liquid capsule preparation has high bioavailability, good sealing performance, accurate content, crystal-clear and aesthetic appearance, perfect heat and wet resisting property, high toughness and ideal stability. The preparation method abandons a plurality of preparation procedures before filling ordinary capsules as well as necessary equipment and process adopting during soft capsule production such as glue digestion, pressing and drying, thereby reducing the production cost.
Owner:李林
Hydroxy removing process for eplerenone intermediate
The invention discloses a hydroxy removing process for an eplerenone intermediate 5beta-cyan-11alpha,17beta-dihydroxy-3,5'-oxo-4,7-methylene-21-carboxylic acid-17alpha pregna-gamma-lactone compound in a formula I. The process comprises the following steps of: reacting an eplerenone intermediate and an acid hydroxy removing agent in a solvent at a certain temperature, separating out ice water after the reaction is finished, adjusting the solution to be neutral by using alkali solution, filtering the solution, and drying the filtrate to obtain an eplerenone intermediate hydroxy removed 5beta-cyan-9(11)-alkene-17beta-hydroxy-3,5'-oxo-4,7-methylene-21-carboxylic acid-17alpha pregna-gamma-lactone compound in a formula II.
Owner:YUEYANG HUANYU PHARMA
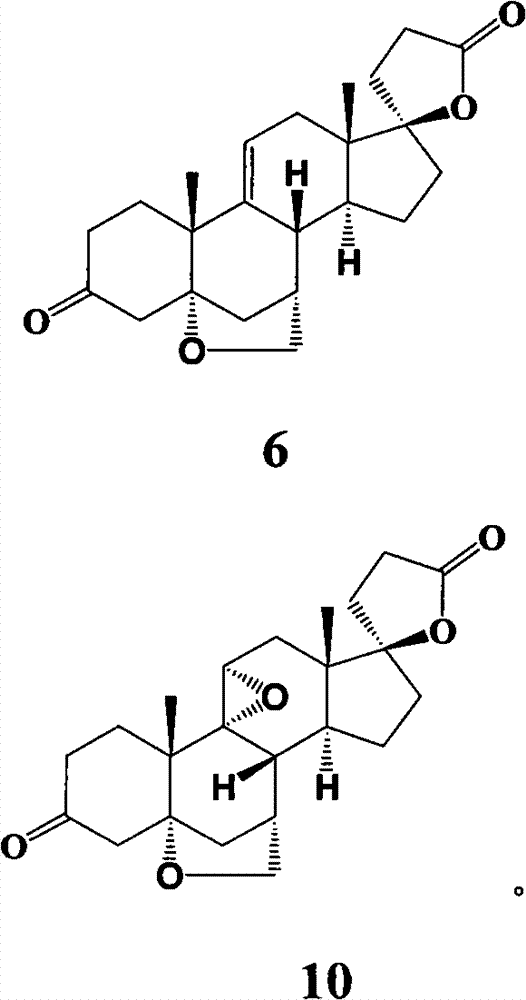
Preparation method of eplerenone and intermediate thereof
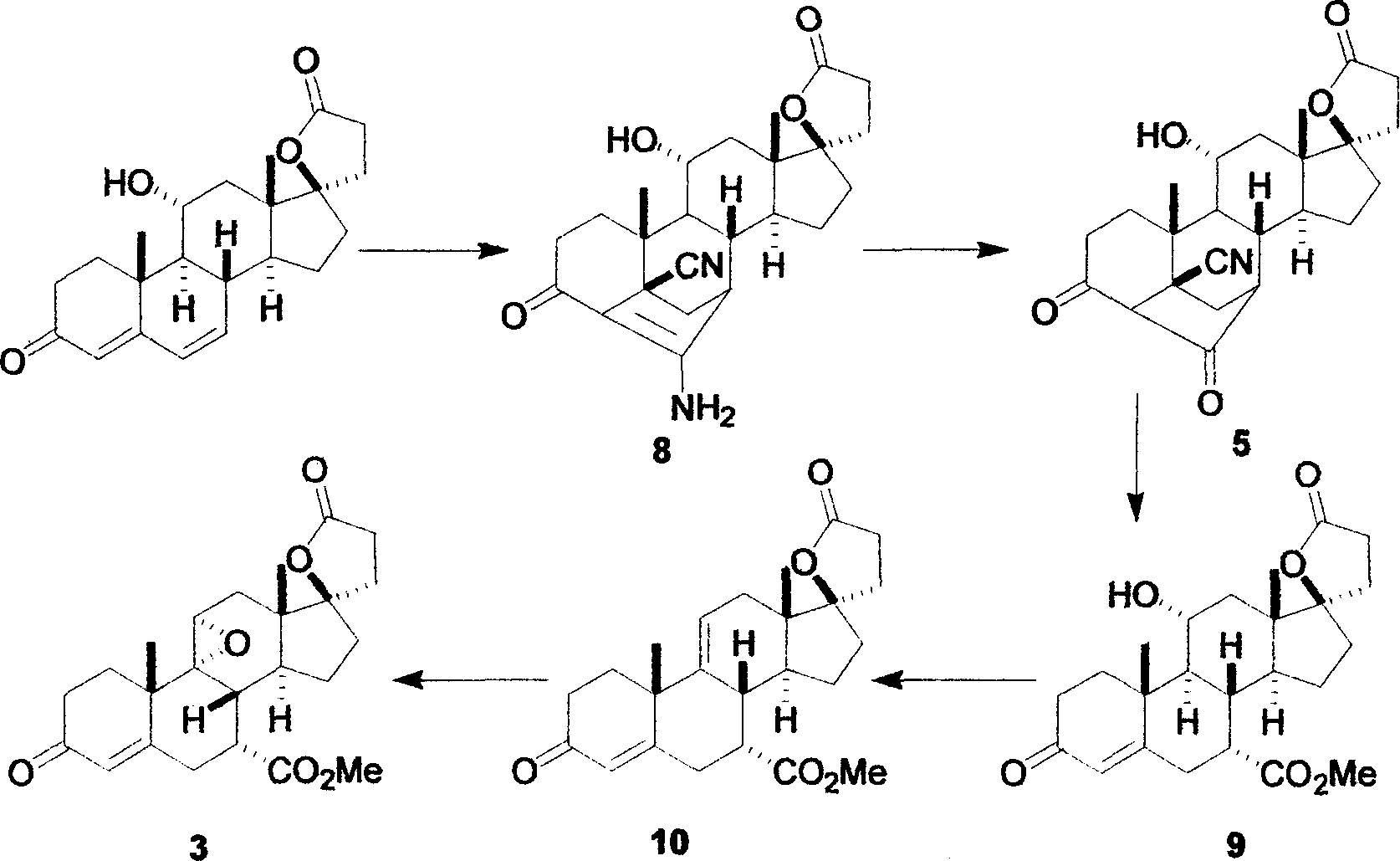
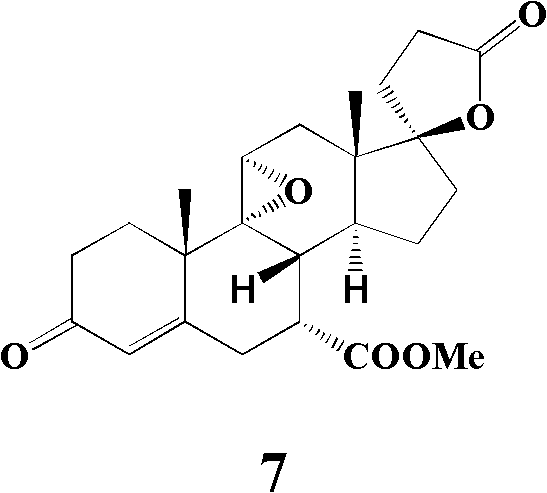
The invention provides an important intermediate compound used for preparing eplerenone: 5alpha-oxygen-7alpha-methylene-17beta-hydroxy-3-oxo-17alpha-pregn-9(11)-alkene-21-carboxylic acid-gamma-lactone (shown in a formula 6) and 9alpha, 11alpha-epoxy-5alpha-oxygen-7alpha-methylene-17beta-hydroxy-3-oxo-17alpha-pregn-21-carboxylic acid-gamma-lactone (shown in a formula 10), a method for preparing the intermediate compound and an application.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
A kind of canrenone derivative steroid compound, its preparation method and its application in the preparation of eplerenone
The invention relates to a canrenone derivative steroid compound, a preparation method and an application in the medicine field, and particularly relates to 7alpha-nitro methyl-11alpha,17beta-dihydroxy-3-oxo-17alpha-pregna-4-ene-21-carboxylic acid-gamma-lactone (a compound shown in formula 2), a preparation method and an application in eplerenone preparation. The key steps of the invention are that nitromethane is used as a nucleophilic reagent; the alpha-nitro methyl group is introduced to the C-7 position stereoselectively so as to further construct a carboxylic acid methyl ester structure with a C-7alpha position configuration of eplerenone; the method of the invention has the characteristics of short steps, mild conditions, and low cost.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Compositions comprising co-precipitate of eplerenone and a water-soluble excipient
Solid compositions for oral administration comprising a co-precipitate of eplerenone and a water-soluble excipient.
Owner:SHERMAN
Method for preparing 17 alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7 alpha, 9 alpha)-dicarboxylic acid lactone
The invention discloses a method for preparing 17alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7alpha,9alpha)-dicarboxylic acid lactone. Firstly, a compound I dissolved in a solvent and methanesulfonyl chloride are subjected to 11-hydroxyl sulfonylation reaction under the action of an acid-binding agent, and a compound II is obtained; then the compound II and inorganic alkali are added into the solvent and react to obtain the target compound 17alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7alpha,9alpha)-dicarboxylic acid lactone; finally, the target product high in purity is obtained through re-crystallization and column chromatography. The 17alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7alpha,9alpha)-dicarboxylic acid lactone can be prepared to serve as a reference substance, and accordingly the quality of eplerenone can be better controlled.
Owner:ZHEJIANG XIANJU PHARMA
Eplerenone oral solid preparation and preparation method therefor
InactiveCN107456445AChange physical and chemical propertiesImprove solubilityOrganic active ingredientsPill deliveryAlcoholFluidized bed
The invention discloses an eplerenone oral solid preparation. The eplerenone oral solid preparation comprises an eplerenone-polyvinylpyrrolidone compound, wherein the eplerenone-polyvinylpyrrolidone compound is prepared by the steps of a, dissolving polyvinylpyrrolidone into ethyl alcohol or acetone, adding eplerenone, and performing heating and dissolving to obtain a clear solution; and b, performing spraying and drying on the clear solution obtained in the step a by adopting a fluidized bed, and then performing one-step palletizing to obtain the oral solid preparation. In addition, the invention also discloses a preparation method for the oral solid preparation. The dissolution rate of the eplerenone oral solid preparation is greater than 90% in 10min.
Owner:NANJING CAVENDISH BIO ENG TECH +1
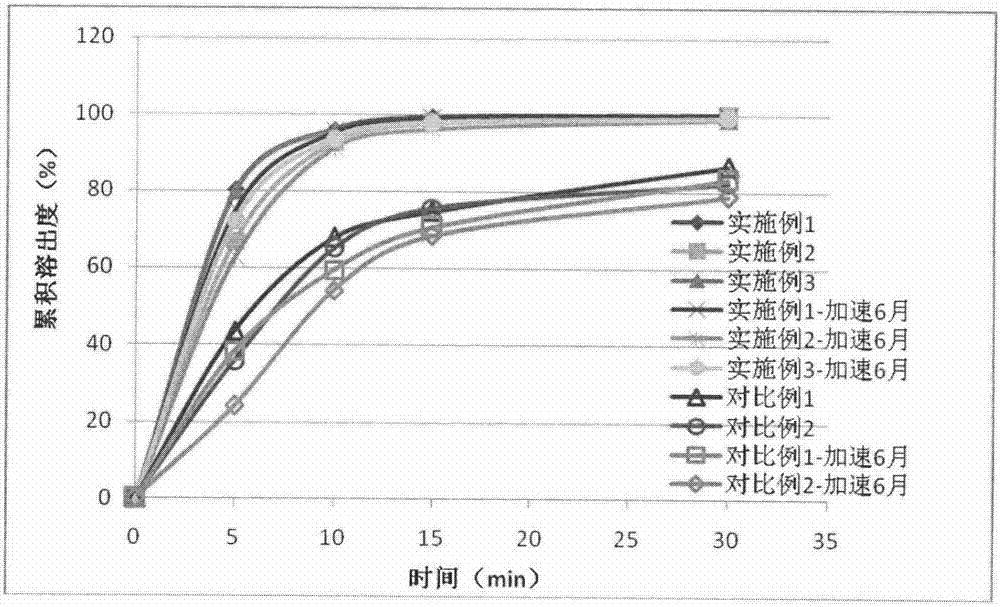
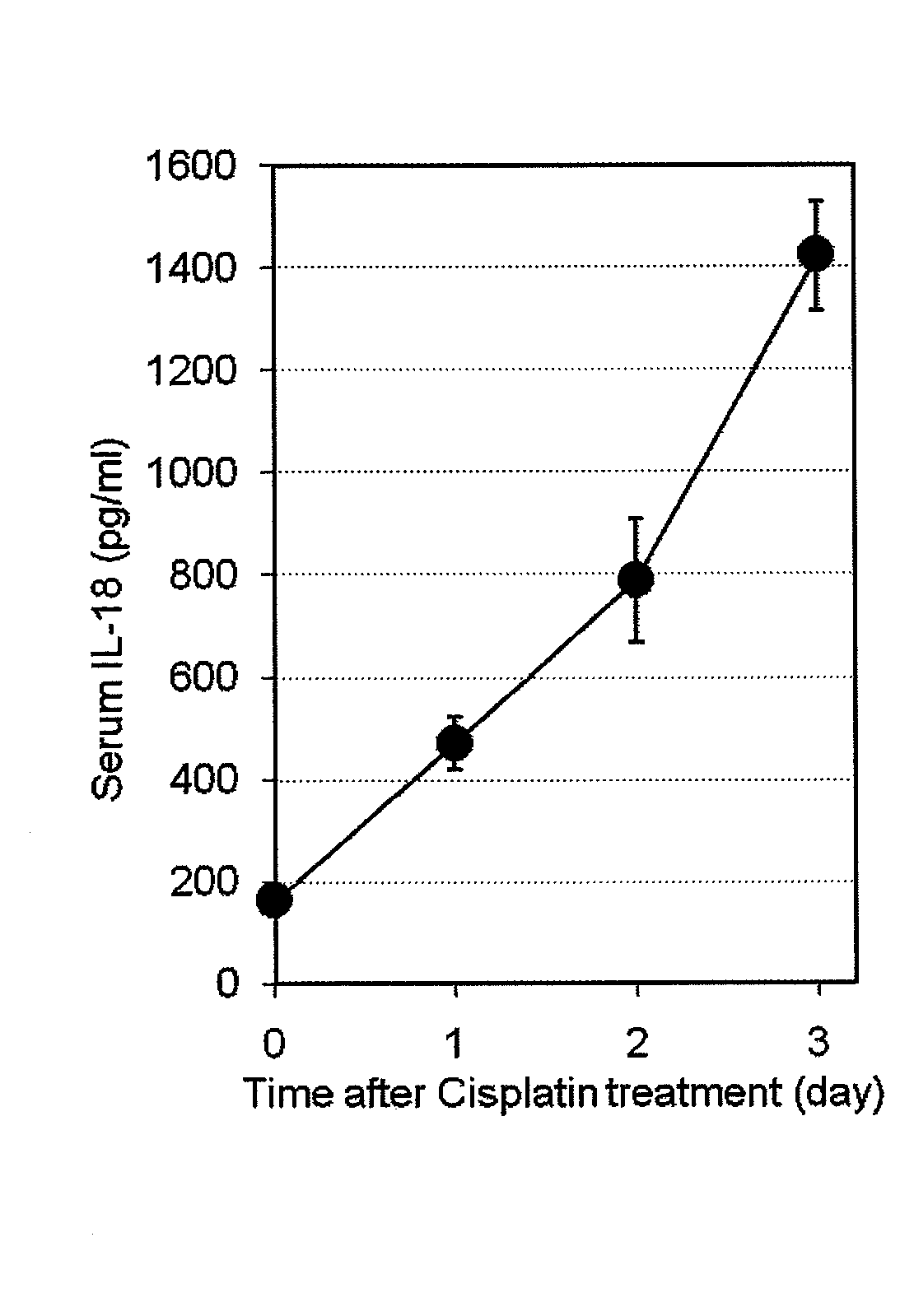
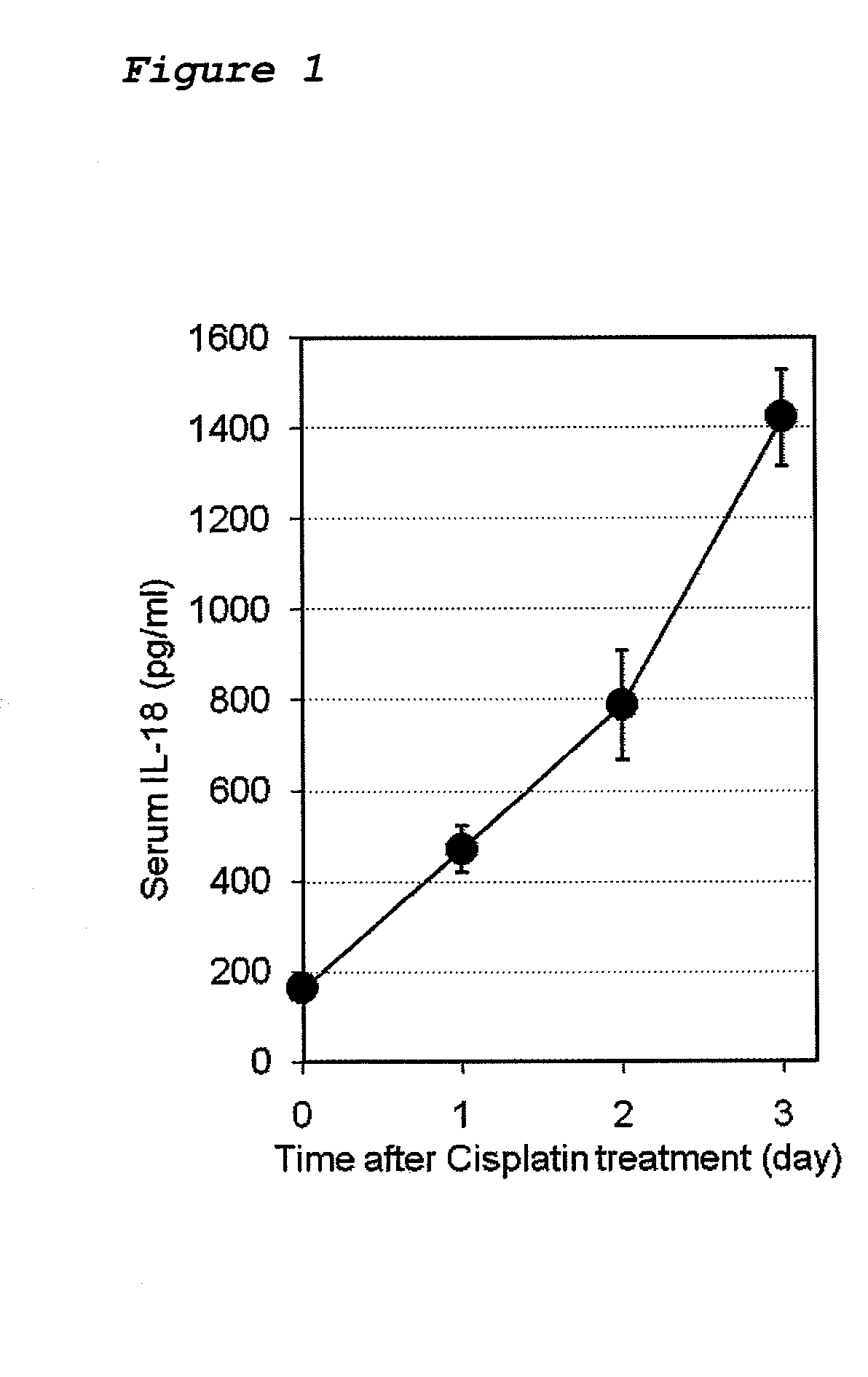
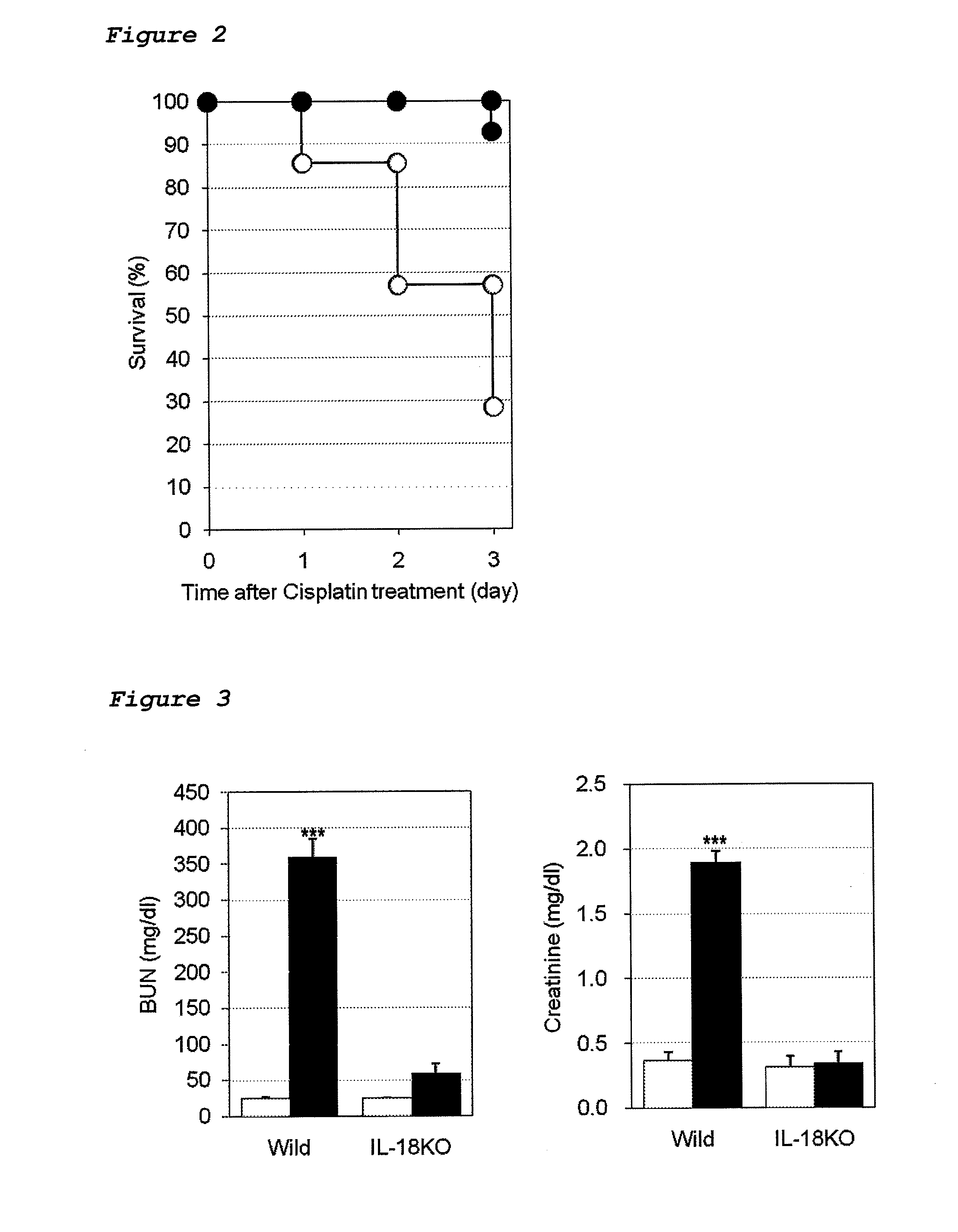
Method for preventing or treating cisplatin-induced nephrotoxicity
InactiveUS20100035853A1Avoid accumulationPrevent nephrotoxicityOrganic active ingredientsUrinary disorderEplerenoneKidney
Provided is a method for preventing or treating cisplatin induced nephrotoxicity, which comprises administering a patient who is receiving cisplatin a therapeutically effective amount of an aldosterone blocker such as eplerenone or spironolactone.
Owner:HYOGO COLLEGE OF MEDICINE
Micronized Eplerenone Compositions
InactiveUS20100087412A1Efficient deliveryReduce solubilityPowder deliveryDispersion deliveryActive agentEplerenone
The invention relates to oral pharmaceutical compositions useful as aldosterone receptor blockers comprising the active agent micronized eplerenone in an amount of about 10 mg to about 1000 mg and one or more carrier materials.
Owner:PFIZER INC
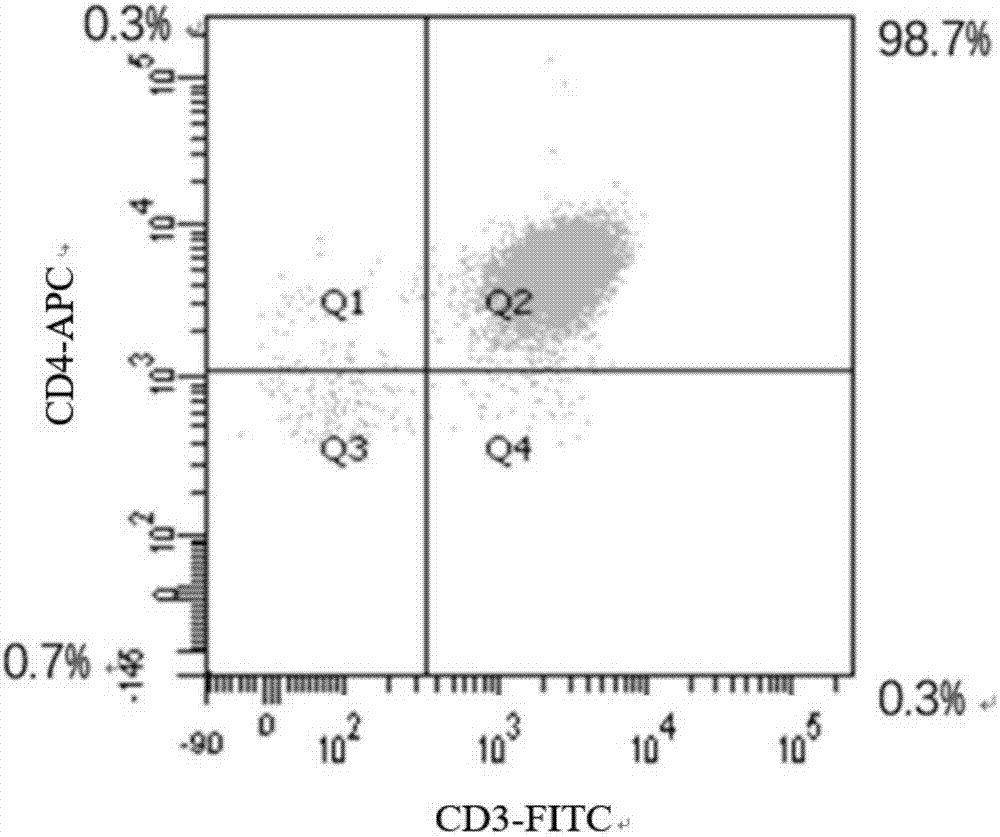
Inhibiting effect of eplerenone for activation/proliferation of helper T cells of chronic heart failure patients
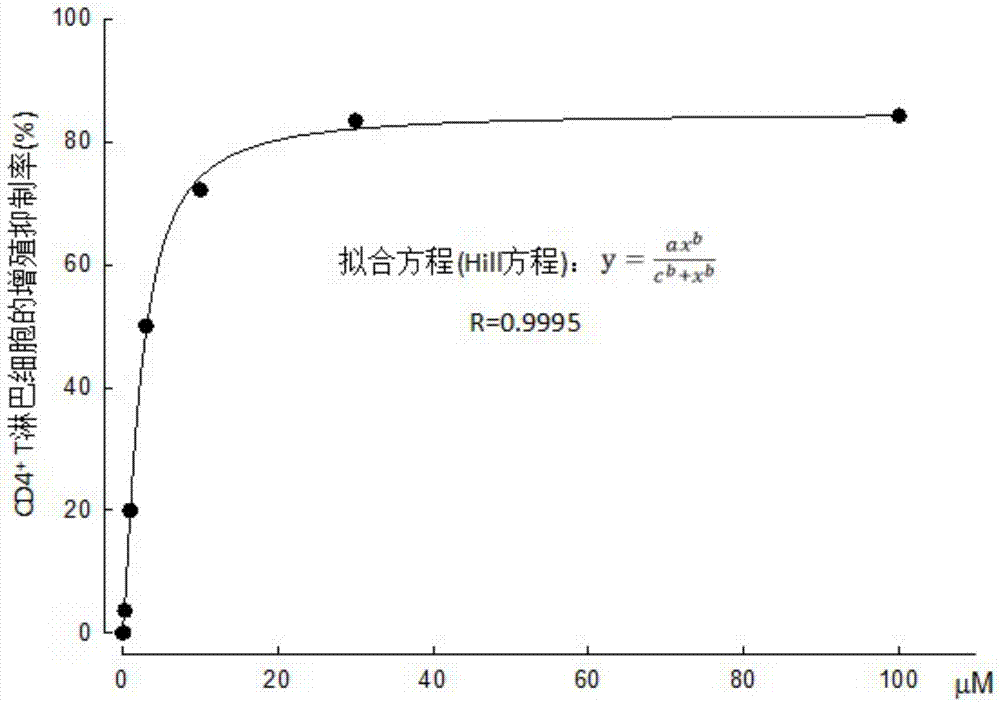
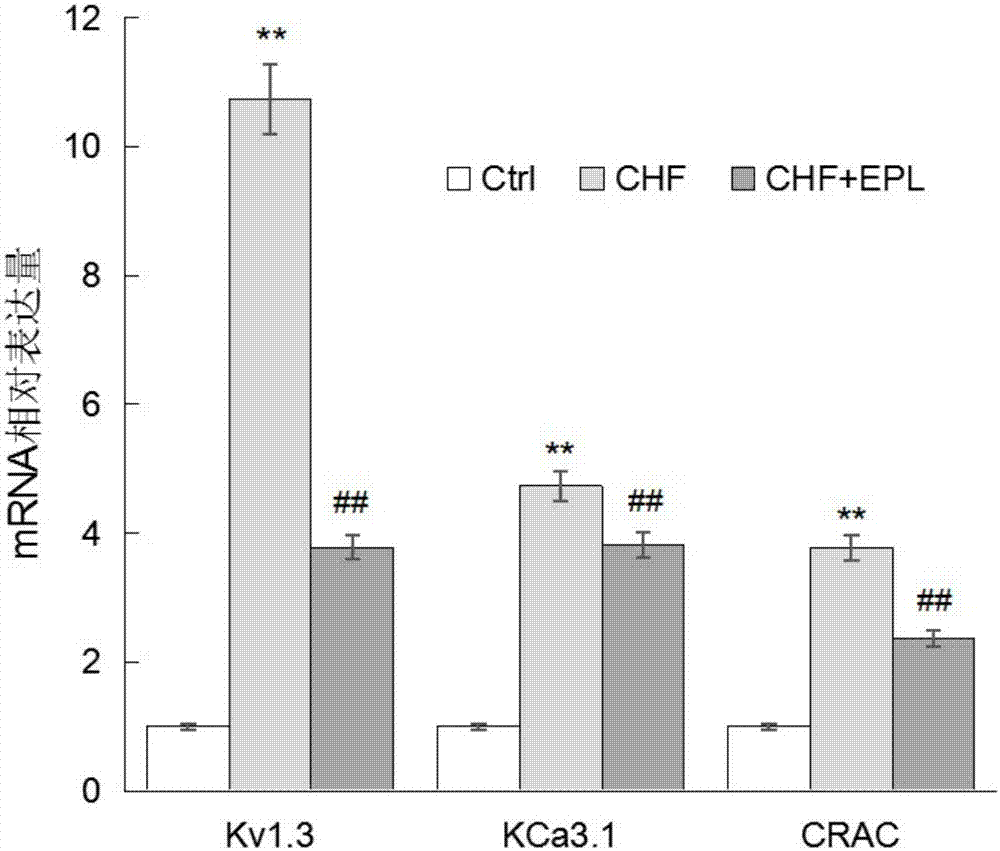
InactiveCN107569495AOrganic active ingredientsImmunological disordersSmallerThanBiological activation
The invention discloses influences of eplerenone (EPL) for expression of mRNA and protein from the channels including Kv1.3 and the like of CD4+T lymphocytes of chronic heart failure (CHF) patients, and the result shows that eplerenone has an inhibiting effect for the activation / proliferation of the CD4+T lymphocytes of the chronic heart failure patients. The research result proves that the expression of mRNA from the channels including Kv1.3, KCa3.1 and CRAC of the CD4+T lymphocytes of the chronic heart failure patients is obviously increased compared with that of the normal group, after 30 [mu] M of eplerenone is administrated, the expression of mRNA is obviously inhibited, wherein the inhibiting for the Kv1.3 channel is the most obvious, the inhibiting rate is about 64.8% (P is smallerthan 0.01), therefore, eplerenone has good significance for detecting the protein of the Kv1.3 channel. The protein of Kv1.3 of the CHF group is increased by 120.8%, and 39.6% (P is smaller than 0.01)is inhibited after 30 [mu] M of eplerenone is added.
Owner:XINJIANG MEDICAL UNIV
Synthetic method for eplerenone
The synthesis process of eplerenone includes the following steps: double serial Michael addition / Aldo condensation reaction of 11alpha-hydroxyl curry ketone and acetone cyanohydrin to produce enamine, partially hydrolyzing the enamine to produce intermediate compound, opening ring of the intermediate compound to obtain the compound IX; eliminating reaction produced olefine ester from the compound IX, reaction with phosphorous oxychloride at room temperature for 12-24 hr, extracting with methane dichloride solvent, merging organic phase, washing and drying to obtain compound X; performing 9alpha, 11-double bond selective expoxidation, extracting the water layer with methane dichloride and merging the organic phase; washing with NaHSO3 solution, saturated Na2SO3 solution, dilute hydrochloric acid solution and saturated hydrochloric acid solution successively, drying, distillation at normal pressure and concentration to obtain eplerenone.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com