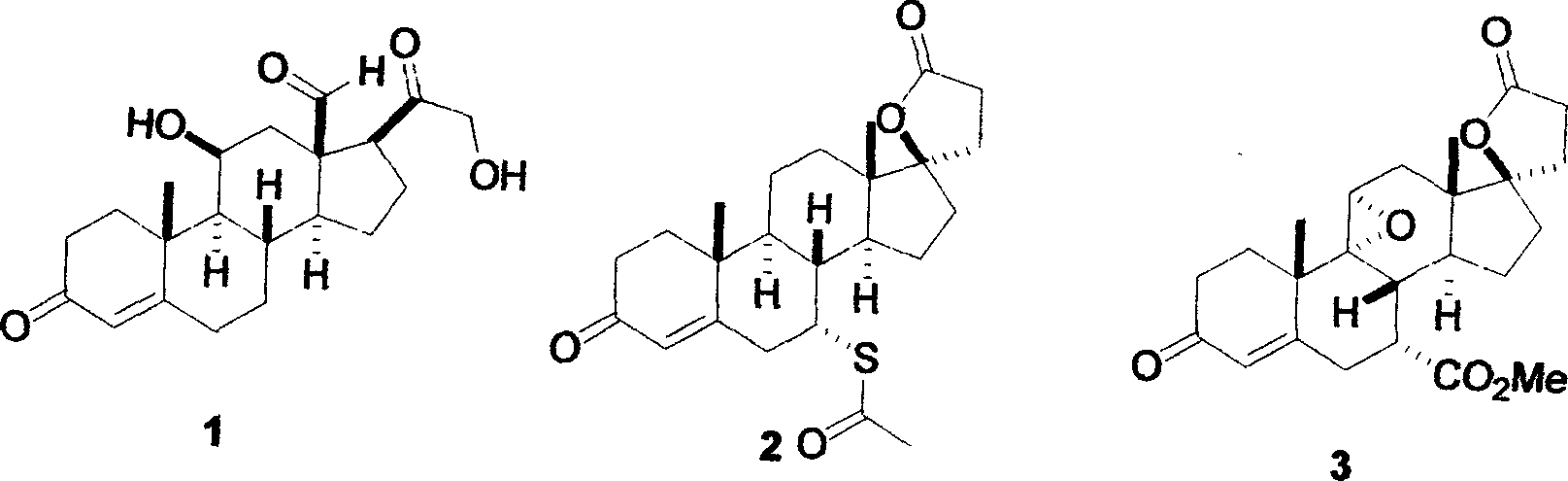
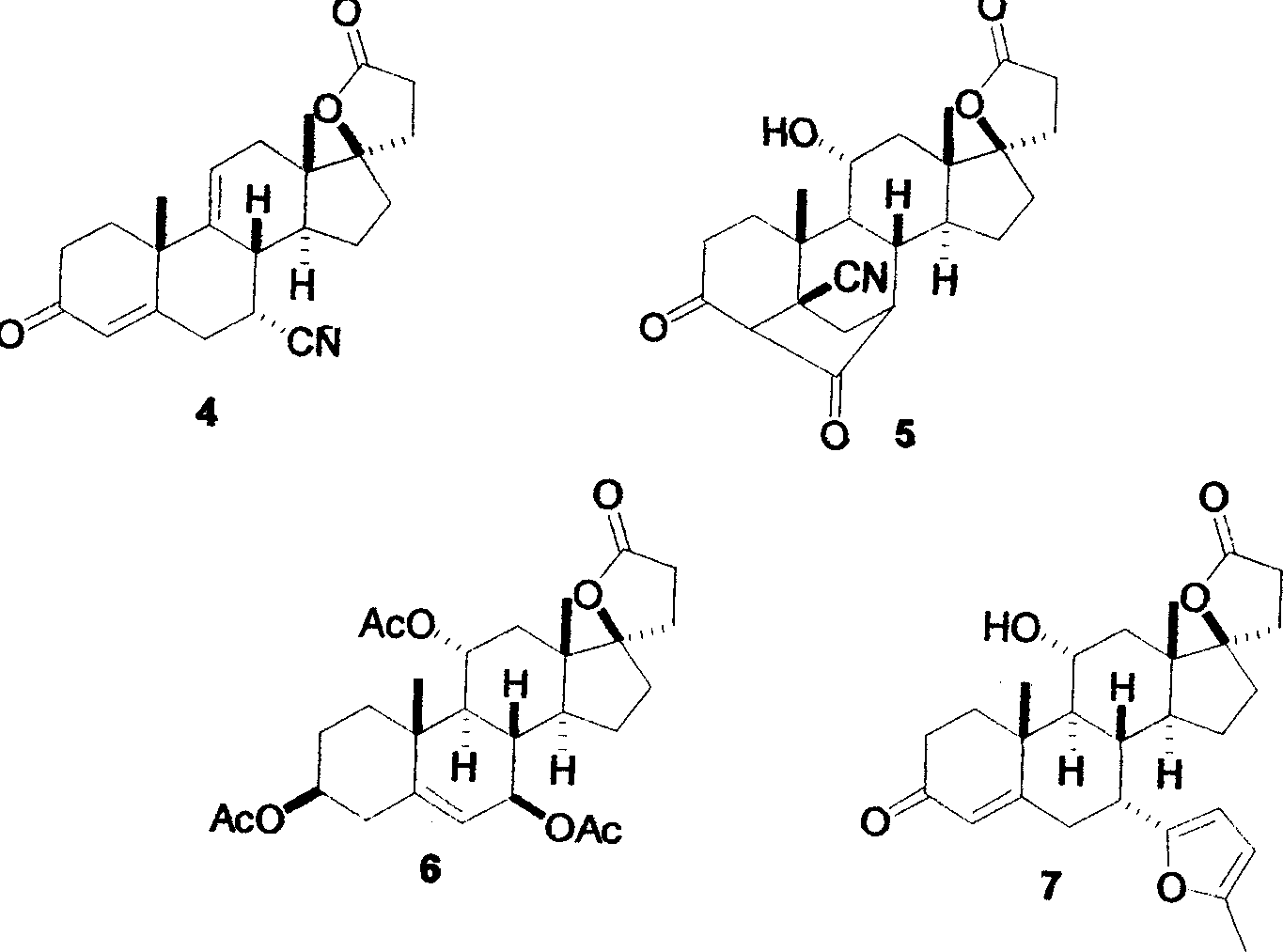
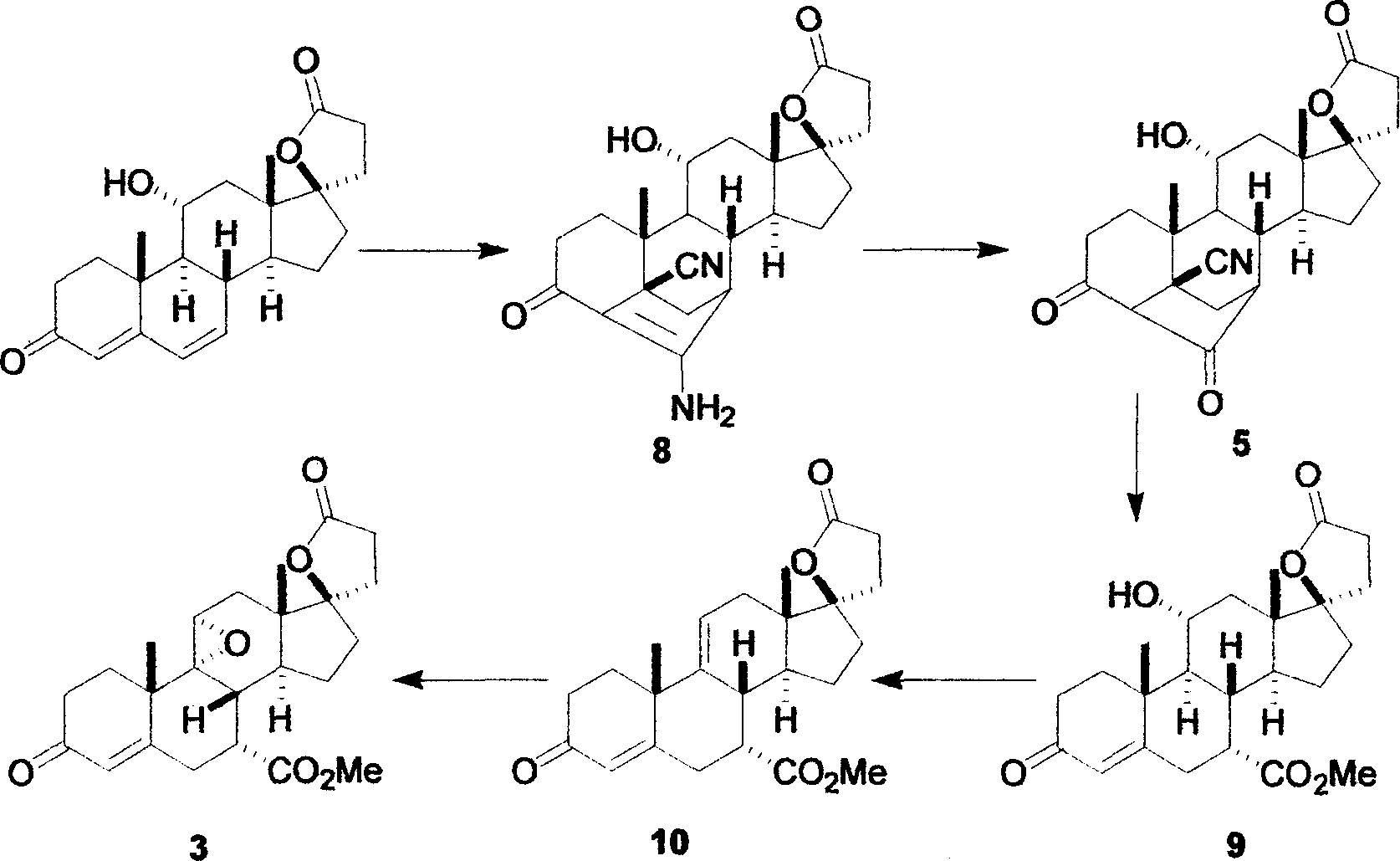
Synthetic method for eplerenone
A synthesis method and technology of eplerenone, applied in the direction of steroids, organic chemistry, etc., can solve problems such as lack of large-scale synthesis advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] Embodiment: As above-mentioned experimental procedure: Under the situation that substrate 11α-curry ketone, acetone cyanohydrin and solvent consumption are the same, use other quaternary ammonium bases such as 130.0mL25% tetraethylammonium hydroxide aqueous solution, 180.0mL25% tetrapropylhydrogen Ammonium oxide, 228.0mL 25% tetrabutylammonium hydroxide or 147.0mL 25% benzyltrimethylammonium hydroxide had no significant effect on the yield of enamine 8, but the amount of quaternary ammonium bases with different structures changed.
[0023] Embodiment: Adopt propanol, isopropanol or butanol and ethanol solvent to have no obvious difference, but reflux temperature is different, and reaction time is 0.5 hour productive rate is low, and the time is long to 10 hours efficiency low; Reaction temperature has no obvious requirement, can be in The reaction is at room temperature, and the temperature does not exceed 70-100°C of the reflux temperature of the solvent when the temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com