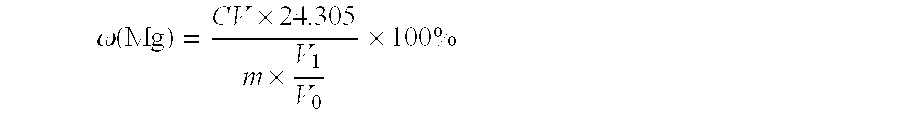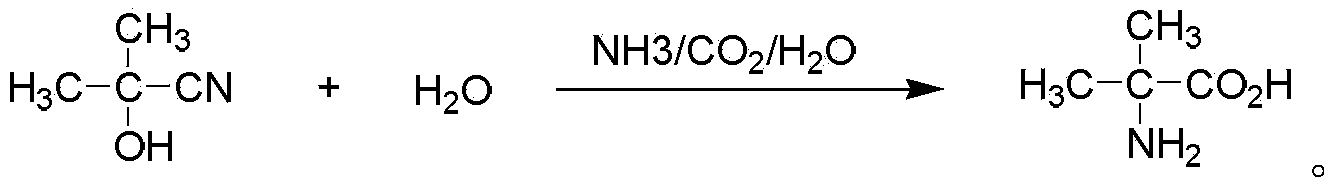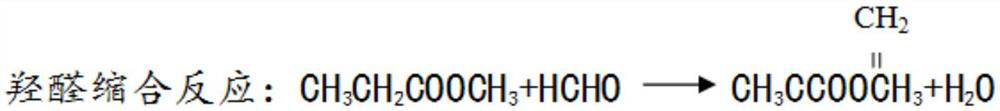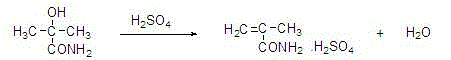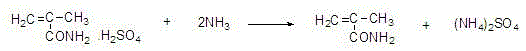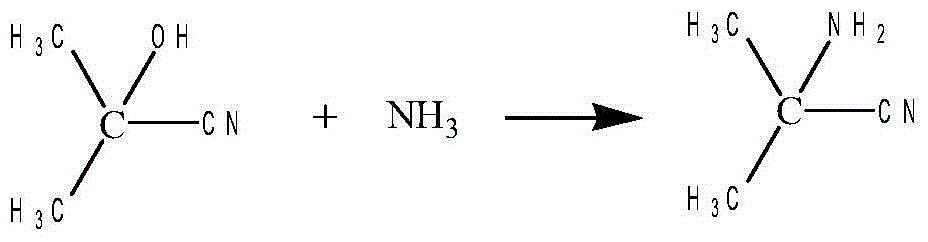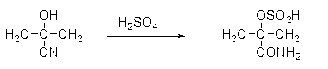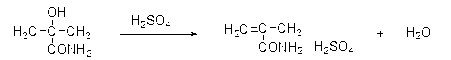Patents
Literature
104 results about "Acetone cyanohydrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic.
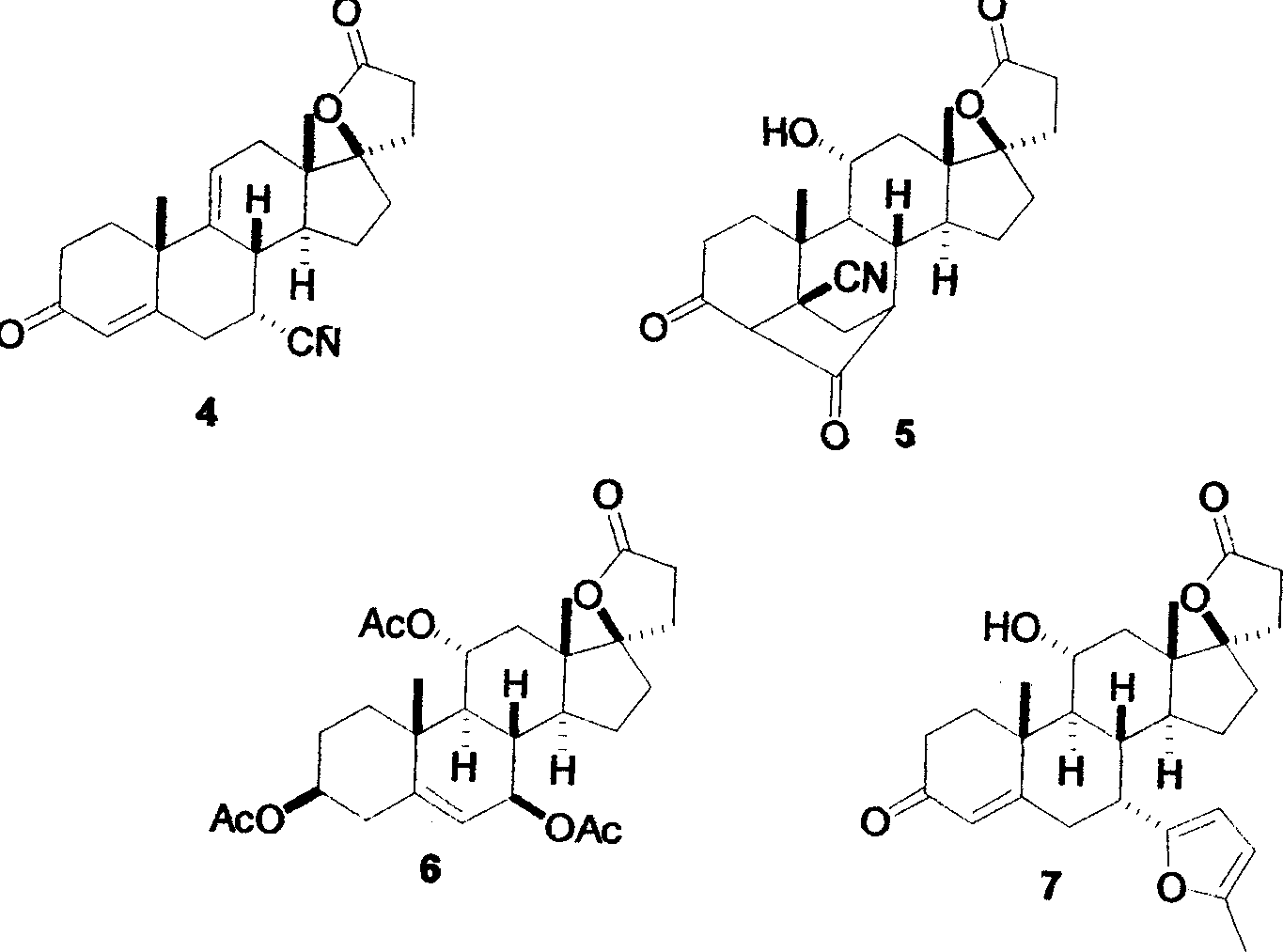
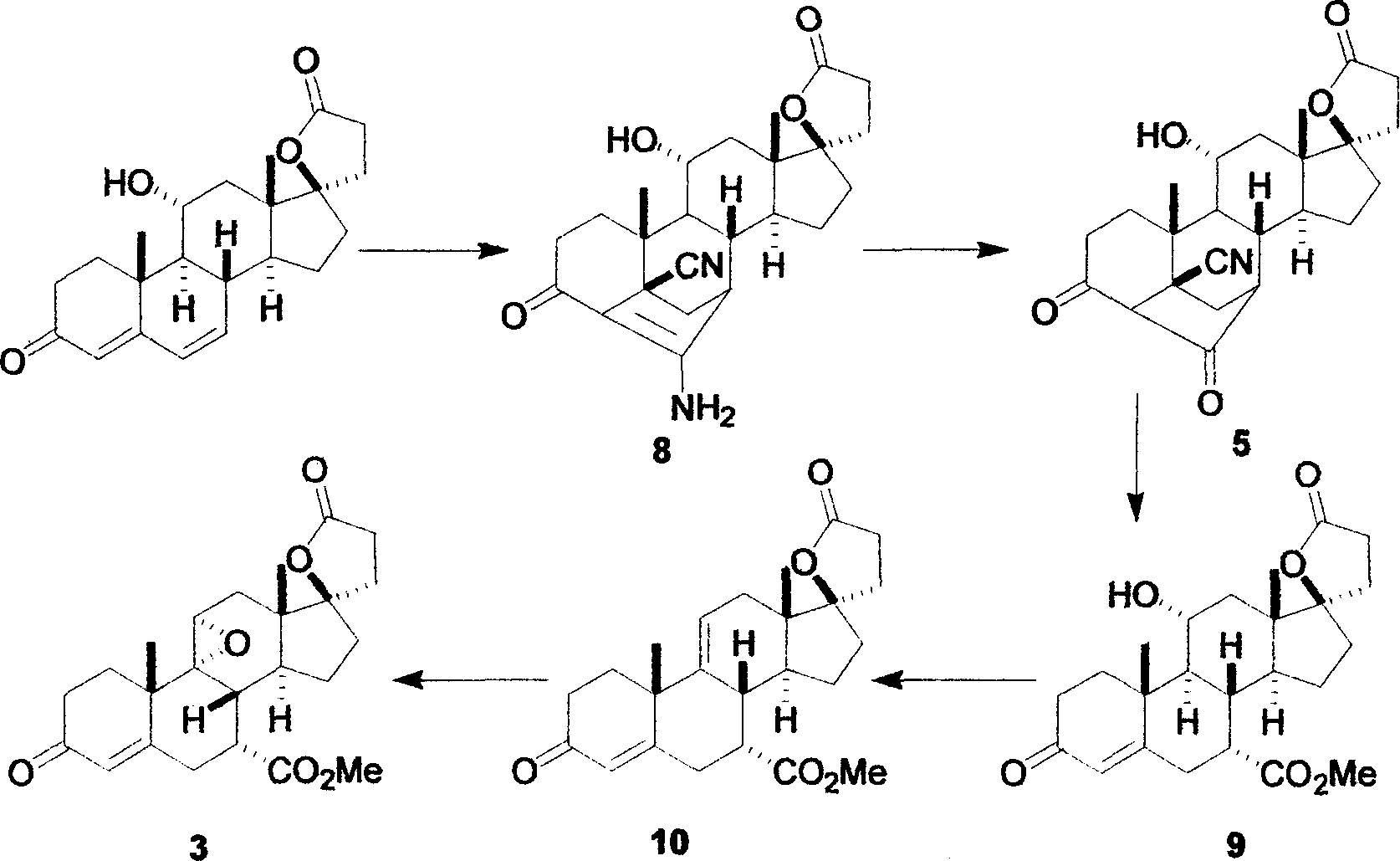
Synthesis method of 17alpha-hydroxyl progesterone
The invention discloses a synthesis method of 17alpha-hydroxyl progesterone, which comprises the steps of by taking 4-androstene-diketone as a starting raw material, carrying out vyanation via acetone cyanohydrin, protecting 3carbonyl by using triethyl orthoformate and ethyl alcohol, protecting 17hydroxy by using butyl vinyl ether, and carrying out hydrolysis after Grignard reaction to generate the 17alpha-hydroxyl progesterone. According to the synthesis method, the cost is reduced, the environment pollution is decreased, the reaction time is shortened, the aftertreatment process of the industrial production is simplified, the production time and cost are greatly saved, the productivity is improved and convenience is brought to the industrial implementation. Compared with the traditional process, the synthesis method has the characteristics of low raw material cost, simple and convenient method, high yield, good selectivity, mild reaction condition, small pollution and applicability to industrial production; and the method is stable and easy to realize.
Owner:ZHEJIANG PURUI PHARMA
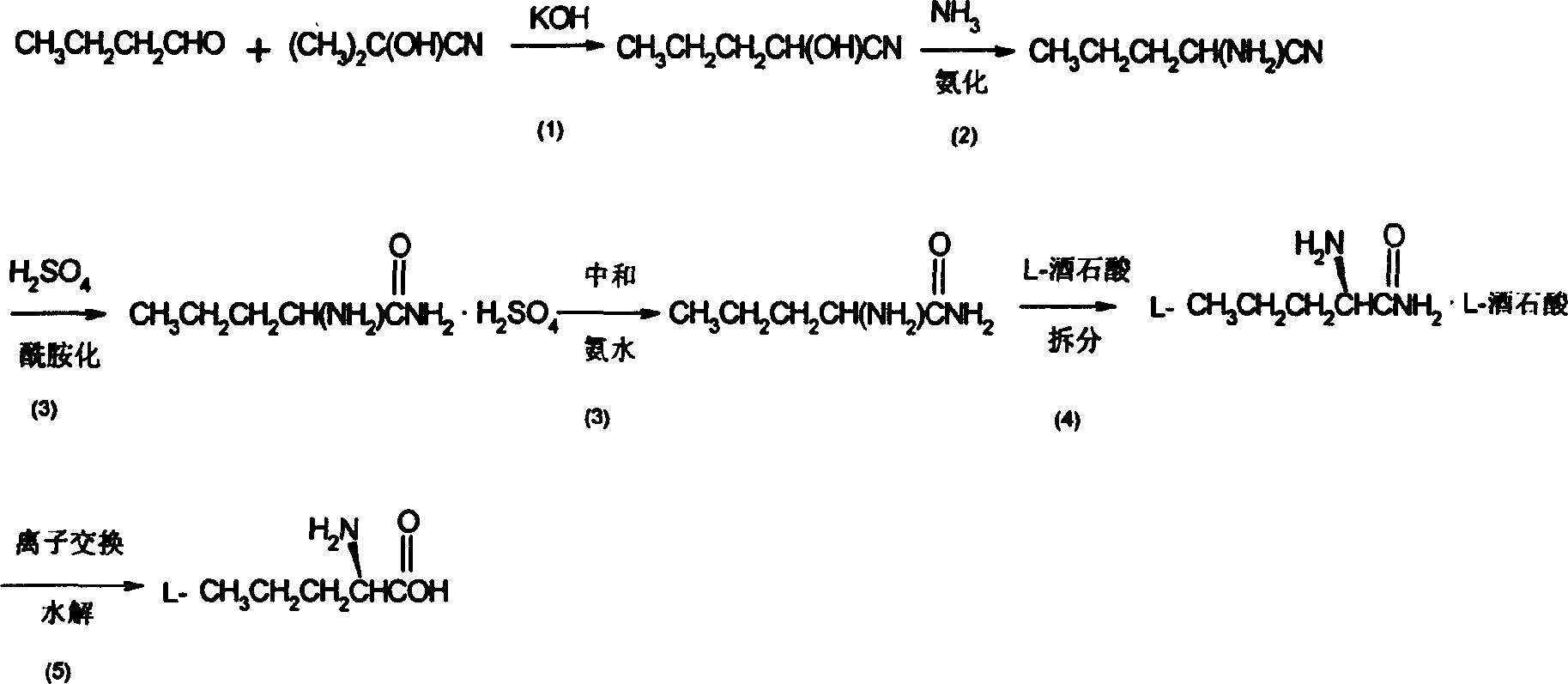
Synthesis method of L-n-valaine
InactiveCN1651400ALess investmentLow costOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsIon exchange
A process for synthesizing L-valine from n-butanol and acetone cyanohydrin includes cyanation reacting under action of alkaline catalyst to obtain butanol cyanohydrin, ammonifying reacting on liquid ammonia, deammonifying, dewatering to obtain aminopentylonitrile, hydrolyzing in concentrated sulfuric acid, neutralizing, extracting, concentrating to obtain dl-aminopentyl amide, splitting, recrystallizing, dissolving in water, ion exchanging by cationic exchange resin, eluting by ammonia water, decoloring, dewatering, rinsing and drying.
Owner:王旭
Synthetic method for eplerenone
The synthesis process of eplerenone includes the following steps: double serial Michael addition / Aldo condensation reaction of 11alpha-hydroxyl curry ketone and acetone cyanohydrin to produce enamine, partially hydrolyzing the enamine to produce intermediate compound, opening ring of the intermediate compound to obtain the compound IX; eliminating reaction produced olefine ester from the compound IX, reaction with phosphorous oxychloride at room temperature for 12-24 hr, extracting with methane dichloride solvent, merging organic phase, washing and drying to obtain compound X; performing 9alpha, 11-double bond selective expoxidation, extracting the water layer with methane dichloride and merging the organic phase; washing with NaHSO3 solution, saturated Na2SO3 solution, dilute hydrochloric acid solution and saturated hydrochloric acid solution successively, drying, distillation at normal pressure and concentration to obtain eplerenone.
Owner:NANJING UNIV
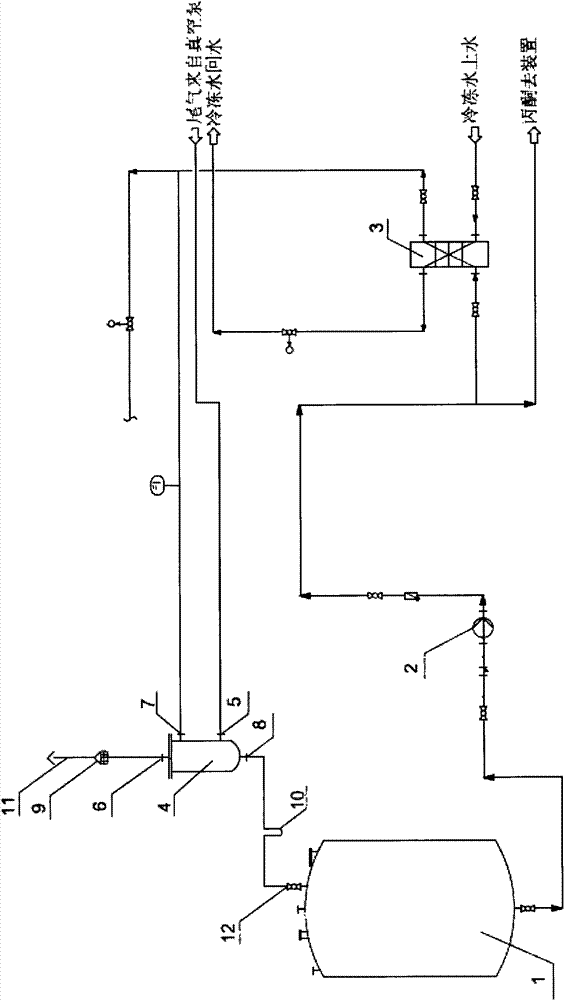
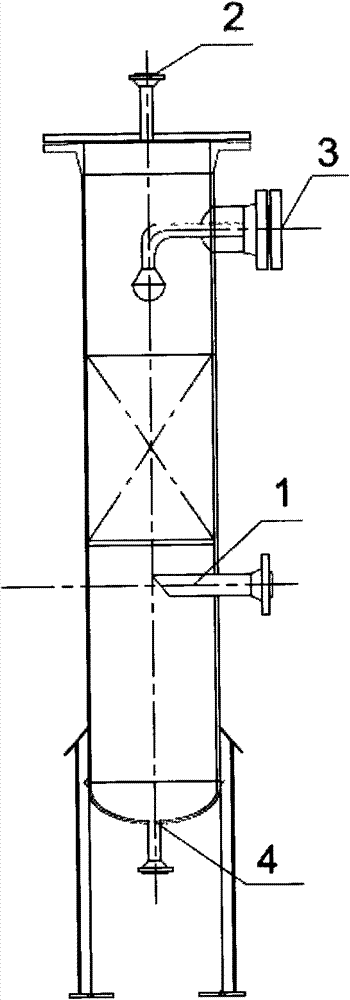
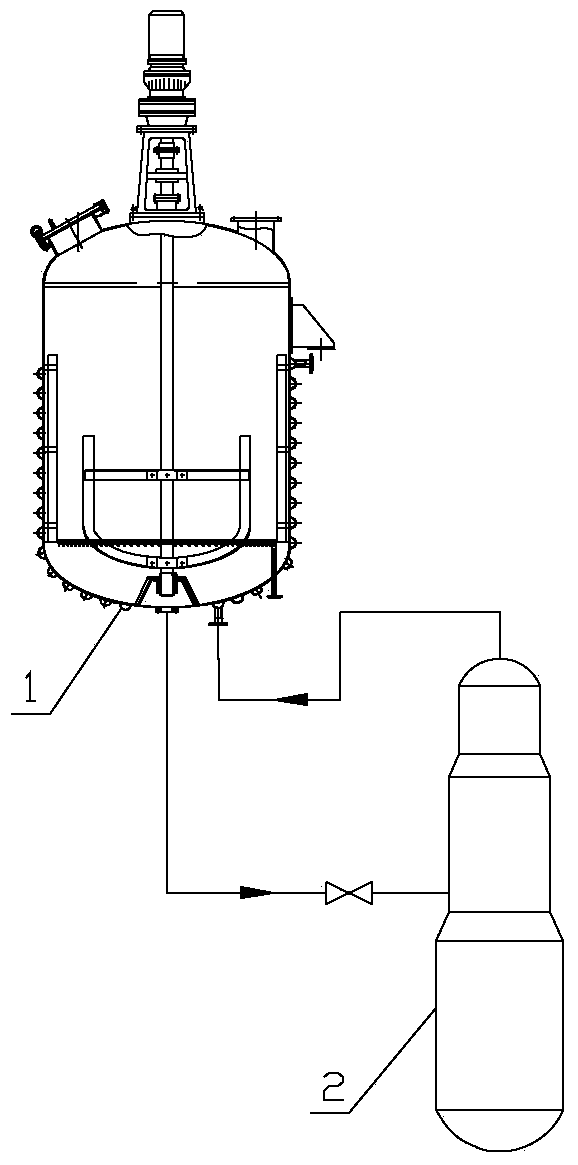
Tail gas absorbing device in acetone cyanohydrin production
InactiveCN102728185ASolve the emission problemEnsure safetyDispersed particle separationSocial benefitsWastewater
The invention relates to an organic chemical substance production device, and concretely relates to a tail gas absorbing device in acetone cyanohydrin production. The device is characterized in that an acetone pump is arranged, fresh acetone is cooled by an acetone cooler and is sent to a tail gas absorbing tower, and a waste tail gas is discharged to air. The tail gas absorbing device has the following beneficial effects: the tail gas absorbing tower is arranged to treat the tail gas of the acetone cyanohydrin device on the basis of an acetone tank, the acetone pump and the acetone cooler which have been arranged in the acetone cyanohydrin device, so the tail gas discharge problem of the acetone cyanohydrin device is solved, tail gas discharge standards reach national standards, wastewater amount reduction, energy saving, emission reduction and good economic benefits are realized, cyanogen harms are released, the safeties of onsite operators are guaranteed, the environment is protected, and country and human benefiting is realized. The device allows the cost for burning cyanogen-containing wastewater to be saved by above 2 billion yuan each year, so the device has substantial economic and social benefits.
Owner:JILIN DESIGNING INST OF CNPC NORTHEAST REFINING & CHEM ENG
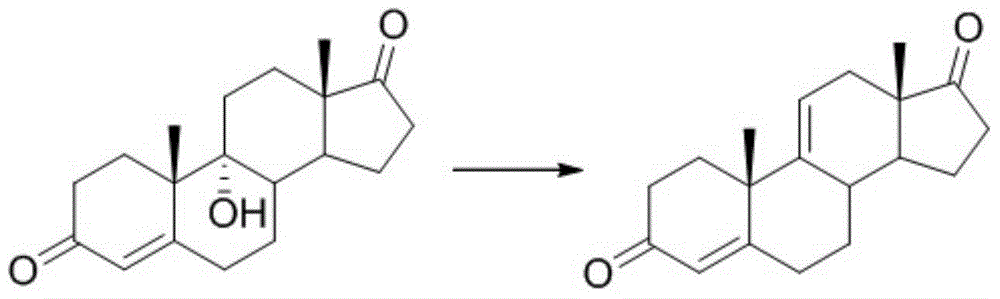
Synthesis method and intermediates of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone
ActiveCN104628808AReduce usageRaw materials are cheap and easy to getSteroids preparationBulk chemical productionSynthesis methodsSide chain
The invention relates to a synthesis method and main intermediates of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. The synthesis method sequentially comprises the following steps of reacting a second intermediate and tosylmethyl isocyanide in an organic solvent at the temperature of lower than 35 DEG C below zero to generate a third intermediate; reacting the third intermediate and a methylated reagent in an organic solvent at the temperature of 70-90 DEG C, and then, removing methyl ether protecting groups and tosylmethyl isocyanide under the action of an acid to obtain a fourth intermediate; and reacting the fourth intermreidate under the action of 3-ketosteroid-1-dehydrogenase to generate pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. Raw materials of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone are cheap and available; the yield of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone is relatively high; a C17-position side chain is introduced by using tosylmethyl isocyanide, so that acetone cyanohydrin serving as a highly-toxic reagent is prevented from being used; and the synthesis method is safe, environment-friendly and suitable for industrial production.
Owner:山东国九堂制药集团股份有限公司
Determination method of magnesium content in aluminium alloy
ActiveUS20110027895A1Rapidly and accurately determinedEfficiently maskedAnalysis using chemical indicatorsChemical analysis using titrationMethylthymol blueEthylene diamine
The present invention discloses a method for determination of magnesium content in aluminum alloy, including: dissolving an aluminum alloy sample, using one or more compounds selected from the group consisting of mercapto-containing compound, acetone cyanohydrin, β-aminoethyl mercaptan, triethanolamine, tetraethylenepentamine, ethylene diamine and oxydol as masking agent, using eriochrome black T or methyl thymol blue as indicator, and using EDTA or CDTA to titrate the sample.
Owner:SOUTHWEST ALUMINUM GRP
Preparation method of alpha-hydroxypyridine by acetone cyanohydrin
ActiveCN107417570AControl concentrationImprove conversion rateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidRoom temperature
The invention relates to a preparation method of alpha-hydroxypyridine by acetone cyanohydrin, and relates to a preparation method of alpha-hydroxypyridine. The method solves the problems that the existing preparation method of the alpha-hydroxypyridine is low in yield, big in catalyst toxicity, high in price, complex in preparation method, and long in reaction time. The method includes steps of firstly, immersing cation exchange resin in 20% by weight of organic amine methanol solution; filtering, washing and drying the solution to obtain catalyst; secondly, dissolving acetone cyanohydrin and aromatic aldehyde in the methanol, and adding catalyst to react and obtain the alpha-hydroxypyridine; filtering the catalyst, depressurizing and evaporating methanol and acetone; extracting and separating the methanol and acetone; after drying, rotationally evaporating and removing ethyl acetate to obtain the alpha-hydroxypyridine. The method applies the simple and easily-prepared catalyst, and the reaction can be completed under room temperature condition; the reaction yield is up to over 95%. The operation method is simple and easy to practice, and the catalyst can be repeatedly used and is more applicable to the industrial production. The preparation method is applied to prepare alpha-hydroxypyridine.
Owner:HARBIN UNIV OF SCI & TECH
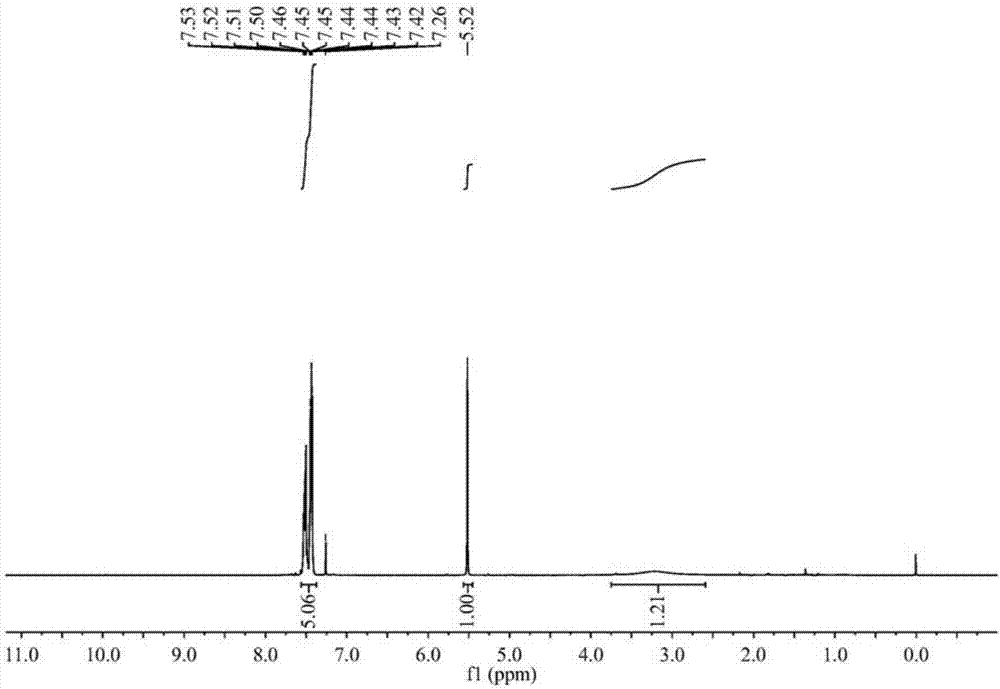
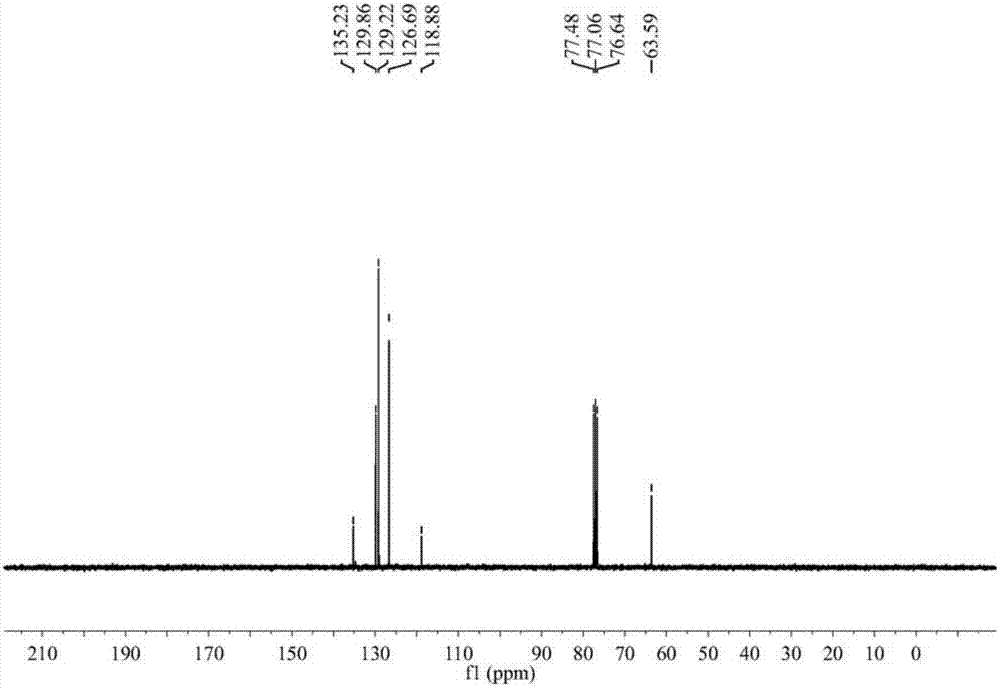
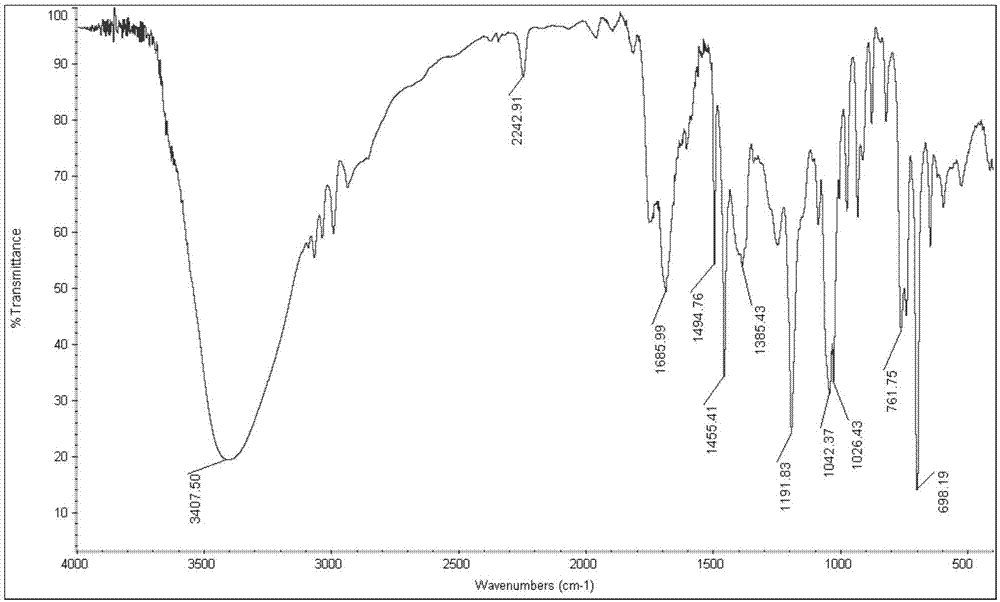
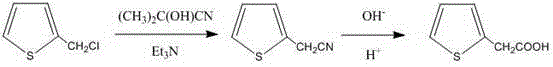
Green preparation technology of 2-thiopheneacetic acid
The invention discloses a synthesis technology of 2-thiopheneacetic acid. The technology comprises the steps that 2-chloromethyl thiophene is taken as a raw material and reacts with acetone cyanohydrin in the presence of an organic solvent under the condition of a catalyst triethylamine to generate 2-thiopheneacetonitrile; 2-thiopheneacetonitrile is subjected to a series of reactions to generate 2-thiopheneacetic acid. The synthesis technology has the advantages that by selecting acetone cyanohydrin as a cyaniding reagent, usage of an extremely toxic substance sodium cyanide is avoided, the reaction yield is increased, the product quality is improved, the safety performance is relatively high, and industrialized production is promoted; the reaction conditions are mild, a little quantity of the catalyst is needed, and the processes are simple. Compared with an existing synthesis technology, the synthesis technology achieves the obvious economic benefits and environmental benefits.
Owner:LUDONG UNIVERSITY
Preparation method of azodiisobutyronitrile
The present invention relates to method for preparing azobisisobutyronitrile. It is an improvement on two-step reaction process including the procedures of utilizing acetone cyanohydrin and hydrazine hydrate to form diisobutyronitrile, using mixed waste water in the reaction and introducing chlorine gas to make oxidation and form azobisisobutyronitrile. Said improvement includes the following several contents: controlling pH value of condensed water used in first-step reaction and making said pH value be 4-5; in second-step reaction adopting two sets of oxidation devices to alternatively make chlorine gas introduction reaction and negative pressure absorption of released residual chlorine gas and its circular comprehensive utilization; and using 10% hypobromite solution to control end point of oxidation reaction. Said method can raise total yield to above 82%, and the utilization rate of chlorine gas can be raised to about 81-86%.
Owner:SHANGHAI NO 4 REAGENT & H V CHEM
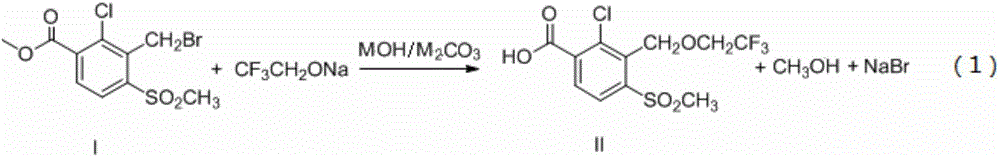
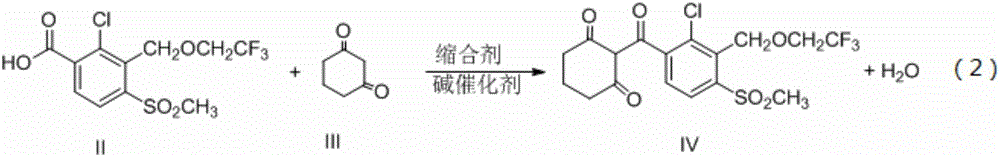
Synthetic process of herbicide tembotrione
PendingCN109678767AReduce security risksReduce manufacturing costOrganic chemistryOrganic compound preparationSolventAcetone cyanohydrin
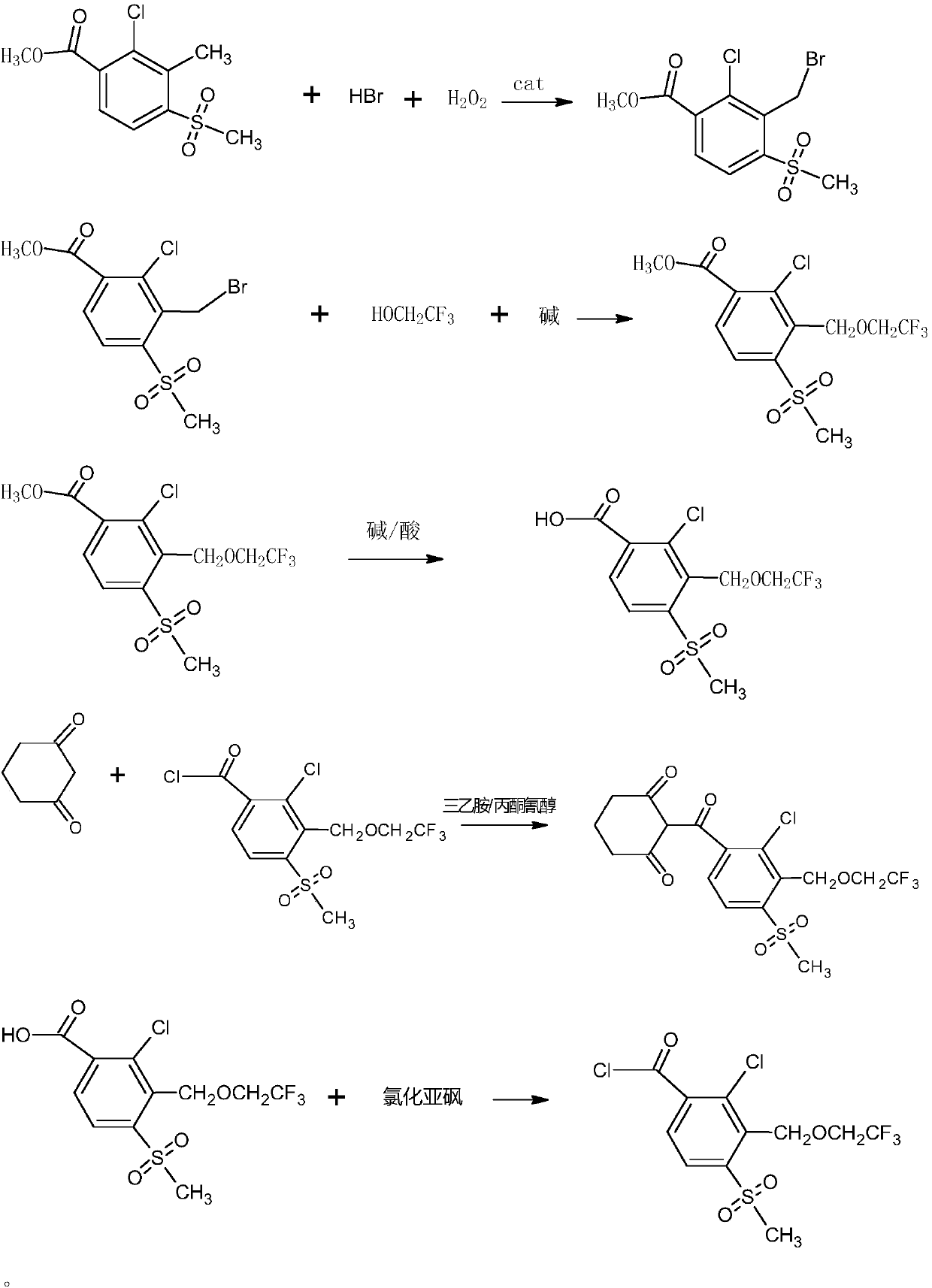
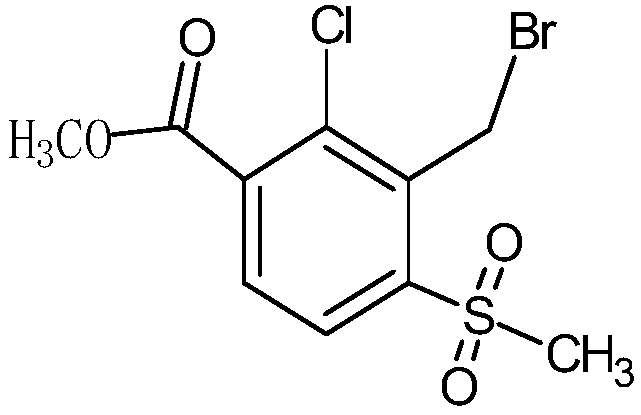
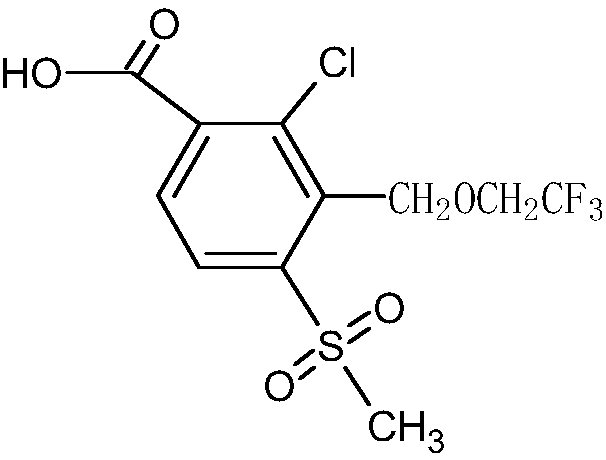
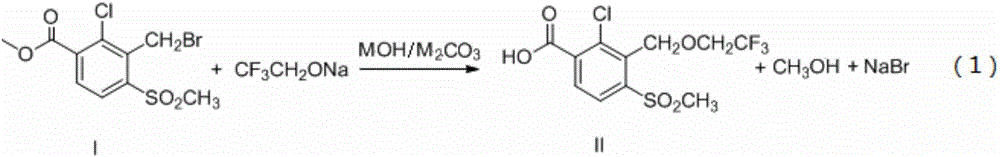
The invention discloses a synthetic process of herbicide tembotrione. The synthetic process comprises the following steps: step 1) adding methyl 2-chloro-3-methyl-4-methylsulfonylbenzoate, a solvent,a catalyst and hydrobromic acid at the first, then dropwise adding hydrogen peroxide, washing with water, concentrating and recrystallizing after reaction to obtain bromine methyl 2-chloro-3-bromomethyl-4-methylsulfonylbenzoate; step 2) enabling the bromide, an alkali 1, the catalyst, the solvent and 2,2,2-trifluoroethanol to react, filtering, washing with water and concentrating after reaction; adding an alkali 2 and water to react, acidifying, filtering, washing and drying to obtain an etherate 2-chloro-3-(2,2,2-trifluoroethoxy)methyl-4-methanesulfonylbenzoic acid; and step 3) removing the solvent after reacting the etherate, the catalyst, thionyl chloride and the solvent; adding 1,3-cyclohexanedione and the solvent, dropwise adding triethylamine; adding acetone cyanohydrin after reaction, washing with water, layering, removing the solvent from an oil layer, adding the solvent for recrystallization, filtering and drying to obtain a beige solid, namely tembotrione. The synthetic process provided by the invention increases the yield of an intermediate, is more environmentally-friendly and safer, and reduces production costs.
Owner:ZHEJIANG ZHONGSHAN CHEM IND GRP
Process for preparing cyanohydrins and their use in the preparation of alkyl esters of methacrylic acid
ActiveUS20100076214A1Avoid decompositionImprove cooling effectCarboxylic acid nitrile preparationOrganic compound preparationCyanohydrinAcetone cyanohydrin
The invention relates to a process for preparing acetone cyanohydrin and to a process for preparing alkyl methacrylates, in which an acetone cyanohydrin as can be prepared in accordance with the present invention is used.
Owner:ROHM GMBH
Method and system for feeding acetone cyanohydrin during preparation of methyl methacrylate
ActiveCN106588650AImprove conversion rateReduce consumptionOrganic compound preparationCarboxylic acid esters preparationDecompositionCirculating pump
The invention discloses a method for feeding acetone cyanohydrin during preparation of methyl methacrylate. The method comprises a step of jetting acetone cyanohydrin into an amide circulation loop, wherein the amide circulation loop is composed of an amide cooler, a gas separator and an amide circulation pump which is located between the outlet of the gas separator and the inlet of the amide cooler. According to the invention, through jetting of acetone cyanohydrin into the amide circulation loop at a plurality of positions, smaller acetone cyanohydrin drops are formed, so material mixing is more sufficient and faster, the decomposition degree of acetone cyanohydrin is reduced, the conversion rate of amide is increased, and unnecessary consumption of acetone cyanohydrin is lowered; thus, the conversion rate of an acylation reaction is increased.
Owner:中石油吉林化工工程有限公司
Benzoyl parazole compound as well as synthesis method thereof and application of same as herbicide
The invention discloses a benzoyl parazole compound as well as a synthesis method of the benzoyl parazole compound and an application of the benzoyl parazole compound as herbicide, relates to the benzoyl parazole compound as the herbicide and a synthesis method of the benzoyl parazole compound, and mainly solves the problem that the conventional benzoyl parazole compound as the herbicide is low in herbicide activity. According to the formula of the benzoyl parazole compound, the synthesis method comprises the following steps of: firstly, mixing a pyrazolone compound, acetonitrile and triethylamine, further dropping an acetonitrile-dissolved benzoyl chloride compound, thus obtaining a benzoyl pyrazol ester compound through reduced pressure distillation; and secondly, mixing the acetonitrile, acetone cyanogens alcohol and the benzoyl pyrazol ester compound, adding triethylamine, raising the reaction temperature, thus obtaining the benzoyl parazole compound through liquid separation and solvent evaporation. The seven benzoyl parazole compounds disclosed by the invention are excellent in herbicide activity and are mainly applied to herbicide as active components.
Owner:HEILONGJIANG SHENGNONG SCI & TECH DEV CO LTD
Method for preparing alpha-aminoisobutyric acid
InactiveCN103864633AAvoid pollutionAvoid askingOrganic compound preparationAmino-carboxyl compound preparationChemical industryAcetone cyanohydrin
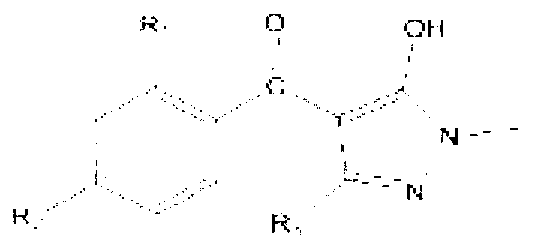
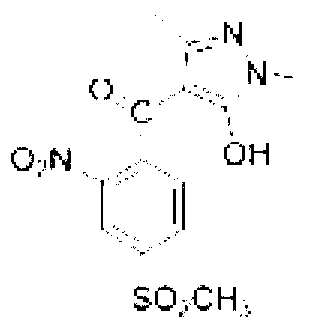
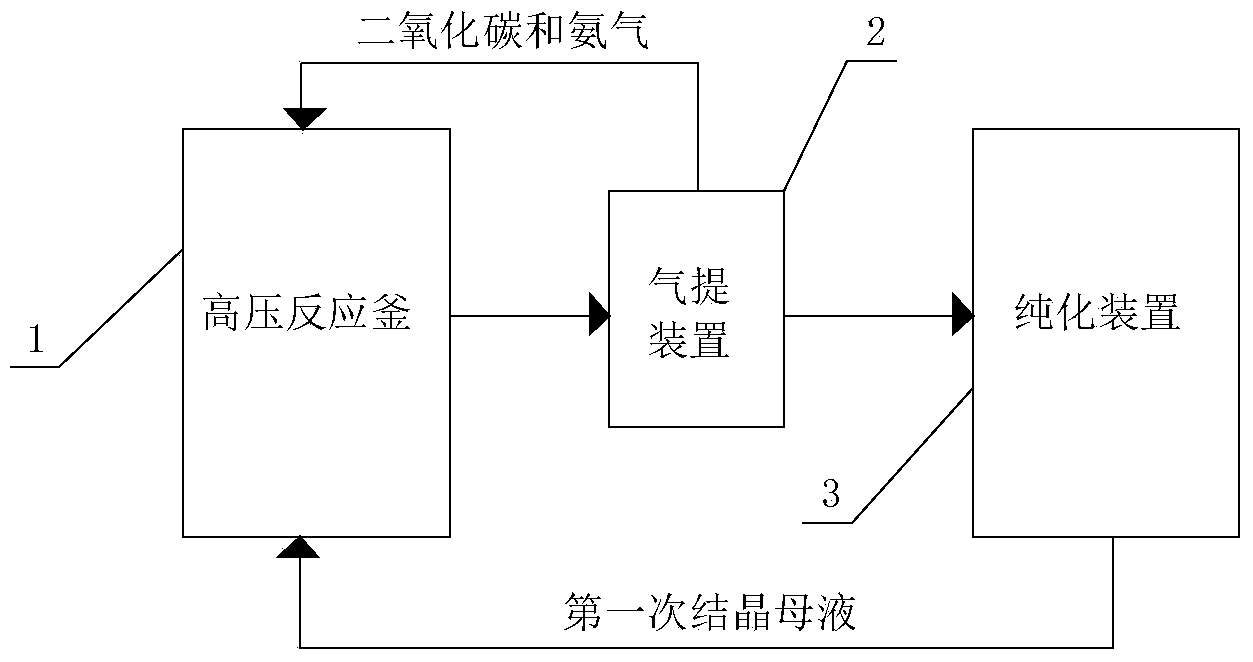
The invention aims at the field of chemical industry and relates to a method for preparing alpha-aminoisobutyric acid. The method comprises the step of enabling acetone cyanohydrin and ammonium carbonate to be subjected to pressurized and heated reaction in an aqueous medium, thereby synthesizing alpha-aminoisobutyric acid, wherein ammonium carbonate can be replaced with ammonium bicarbonate or ammonia and carbon dioxide. The invention further relates to preparation using an alpha-aminoisobutyric acid production device, wherein a gas stripping device is especially used for recovering carbon dioxide and ammonia gas which are generated during synthesis reaction and are recycled for the synthesis of alpha-aminoisobutyric acid. According to the method disclosed by the invention, the yield is high, the purity of a product is high, and the equipment investment is small; in a whole process, carbon dioxide and ammonia are hardly consumed, no byproduct inorganic salt is produced, and inorganic base is not consumed, so that a very positive effect on the full and comprehensive utilization of resources and environmental protection is exerted; recovered carbon dioxide and ammonia and crystallized mother liquor are sufficiently utilized, so that the problem of pollution to environment caused by waste gases, waste water and waste residues can be excellently solved, and the environmental-friendly and clean production is really achieved.
Owner:CHONGQING UNISPLENDOUR INT CHEM
Method of preparing azodiisobutyronitrile
InactiveCN103896808AEasy to produceReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationEthyl ChlorideAcetone cyanohydrin
The invention relates to a method of preparing azodiisobutyronitrile. The method comprises the following steps: a, carrying out a reaction on acetone cyanohydrin and hydrazine hydrate to generate diisobutyronitrile hydrazine; and b, introducing chlorine to diisobutyronitrile hydrazine to react to generate azodiisobutyronitrile, wherein hydrazine hydrate used in the step a is prepared by a urea method. The hydrazine hydrate is specifically prepared by the following steps: (1) introducing chlorine to a caustic soda solution to generate sodium hypochlorite; (2) adding solid urea to prepare a urea saturated solution and carrying out a reaction with sodium hypochlorite to obtain a coarse solution containing hydrazine hydrate; and (3) freezing and crystallizing the coarse solution containing hydrazine hydrate to separate sodium carbonate decahydrate so as to obtain a refined solution of hydrazine hydrate, adding a neutralizer to neutralize the pH to 2-3, removing sodium hydroxide and sodium carbonate in the solution, and then adding the refined solution of hydrazine hydrate to reversely neutralize to PH to 10-10.5. According to the method provided by the invention, the purification process of the hydrazine hydrate solution is canceled and the production procedure is simplified, so that the production cost is greatly lowered.
Owner:唐山晨虹实业有限公司
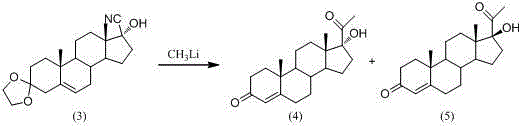
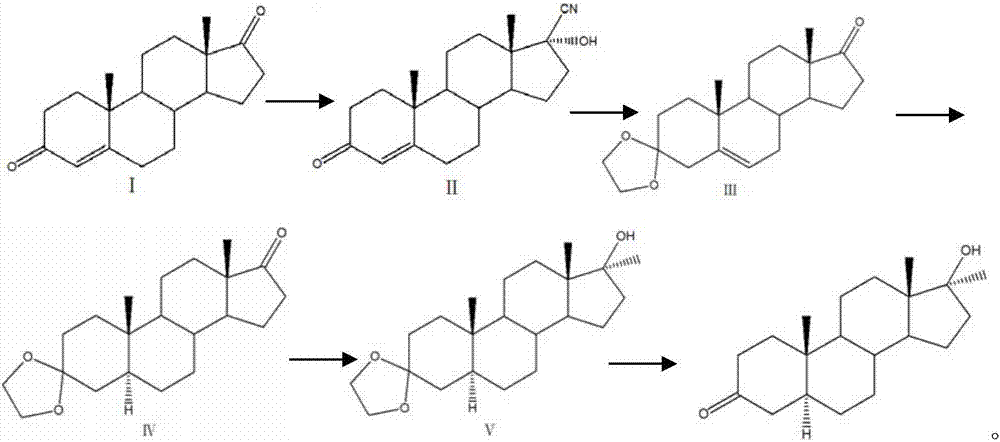
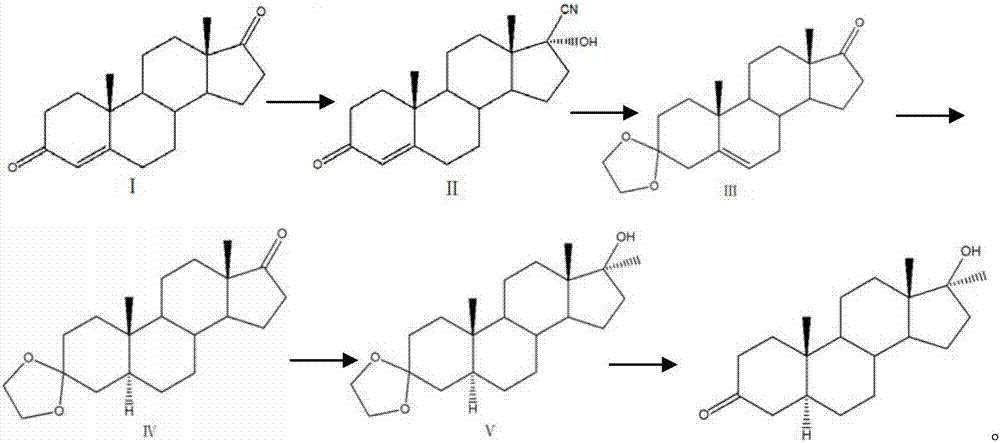
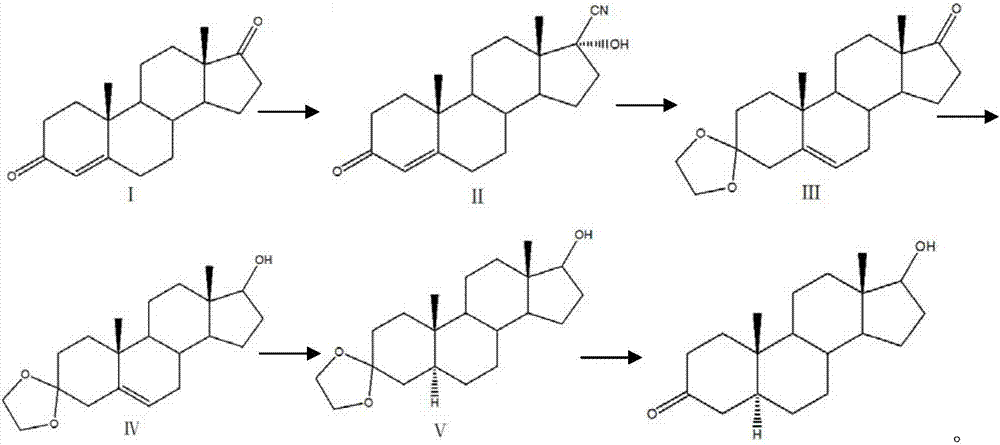
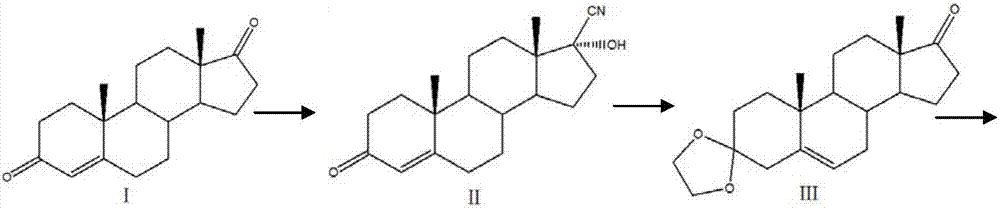
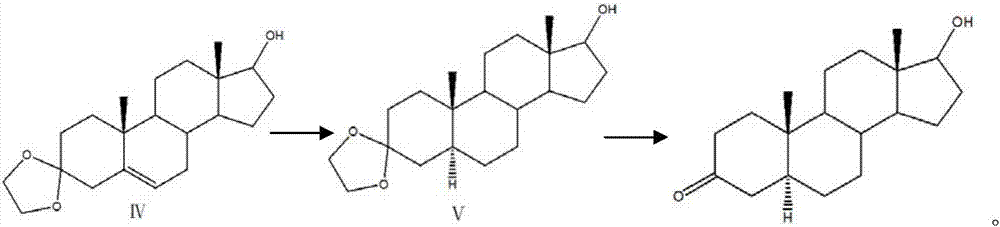
Preparation method of mestanolone
The invention discloses a preparation method of mestanolone. The structural formula of the mestanolone is as shown in the specification. The specific preparation method comprises the following steps of (1) generating a compound II with cyan from a compound I and acetone cyanohydrin or sodium cyanide; (2) adjusting a solution of the compound II into acidity, tracking ethylene glycol and triethyl orthoformate into ketal, adjusting the solution into strong basicity to form a compound III; (3) adding a catalyst and hydrogen to the solution of the compound III to generate a compound IV; (4) putting the compound IV into a container and dissolving by using tetrahydrofuran, dropping a Grignard reagent of methylmagnesium chloride or methyl magnesium bromide to obtain a compound V; and (5) elutriating the compound V in the same container under an acid condition to obtain 3-ketal, namely mestanolone. Chemical production of the mestanolone is achieved by adopting the simplest process, the required raw materials are few, the pollutants are few, meanwhile, the produced mestanolone is high in purity and the relative yield is increased.
Owner:ZHEJIANG PURUI PHARMA
Method for preparing 5,5-dimethylhydantion
The disclosed preparation method for 5, 5-dimethylhydantoin comprises: adding ammonia water and acetone cyanohydrin into the kettle for reaction; while starting the gas input valve of kettle and the connected gas output valve of condenser to lead NH4HCO3 converted gas into the kettle till there is no CN- in reaction material. This invention reduces device cost and CO2 waste, has some protection to environment, and improves production and economic benefit.
Owner:JIANGSU JIUJIUJIU TECH
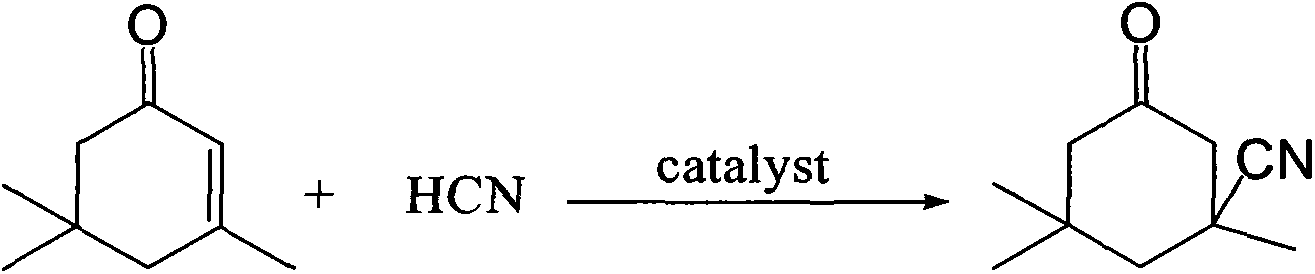
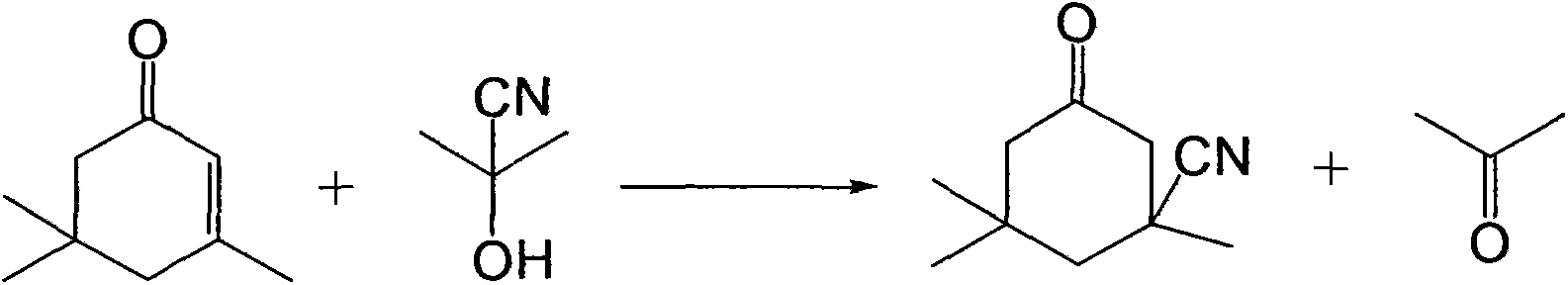
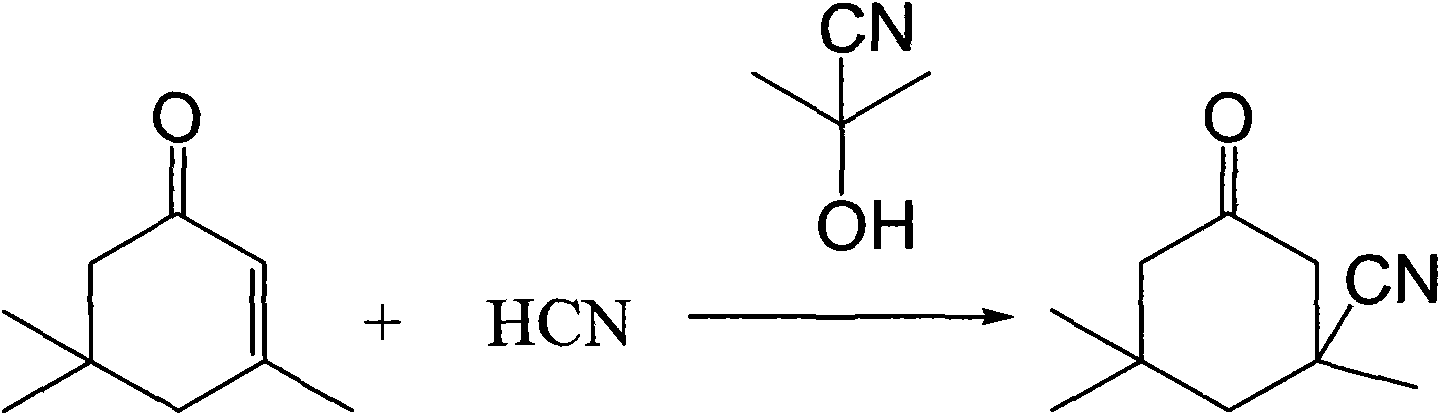
Industrial production method of 3-cyano-3,5,5-trimethylcyclohexanone
ActiveCN103408462ASave the production linkSimple processCarboxylic acid nitrile preparationOrganic compound preparationIsophoroneAcetone cyanohydrin
The invention relates to an industrial production method of 3-cyano-3,5,5-trimethylcyclohexanone, which comprises the following steps: reacting raw materials isophorone and gas hydrocyanic acid by using acetone cyanohydrin as a catalyst; and after the reaction is finished, cooling, and filtering to obtain the 3-cyano-3,5,5-trimethylcyclohexanone. The raw material hydrocyanic acid is introduced into the reaction system in a gas mode, thereby saving the step of production of liquid hydrocyanic acid; no alkaline catalyst is needed in the reaction process, so that neutralizing treatment is not needed after the reaction is finished, thereby avoiding generation of salt and reducing the operation; and thus, the method has the advantages of simpler procedure, lower production cost and higher operation safety.
Owner:HEBEI CHENGXIN
Method for producing 5,5-dimethyl hydantoin
InactiveCN102002000AProcess response is simpleLow reaction temperatureOrganic chemistryFiltrationReaction temperature
The invention discloses a method for producing 5,5-dimethyl hydantoin which is prepared from the following substances in parts by weight according to the steps as follows: adding water to ammonium bicarbonate and acetone cyanohydrin, stirring until the ammonium bicarbonate and the acetone cyanohydrin are fully dissolved, and sealing up while heating up to 10 DEG C; introducing ammonia to the mixture, heating slowly to 50-65 DEG C, stirring, heating up to 75-85 DEG C after keeping temperature constant for 1-2 hours, introducing air for 30-50 minutes, and filtering after cooling to a room temperature, thus obtaining crude dimethyl hydantoin and a mother liquid; and putting the crude dimethyl hydantoin into the mixture of water and the mother liquid while stirring, adding active carbon after heating up to 80-98 DEG C, decolouring, removing the active carbon by filtration, recooling, crystallizing, filtering centrifugally, and drying, thus obtaining the finished product of dimethyl hydantoin. The method has the advantages of simple technological reaction, low reaction temperature, short reaction time, less side reaction and high yield reaching 85%, and is easy to control; the raw material of ammonium bicarbonate applicable to the method belongs to chemical fertilizers, and is easy to obtain; and the filtrate produced by the technology can be used repeatedly, and no three wastes are discharged.
Owner:祝莉宁
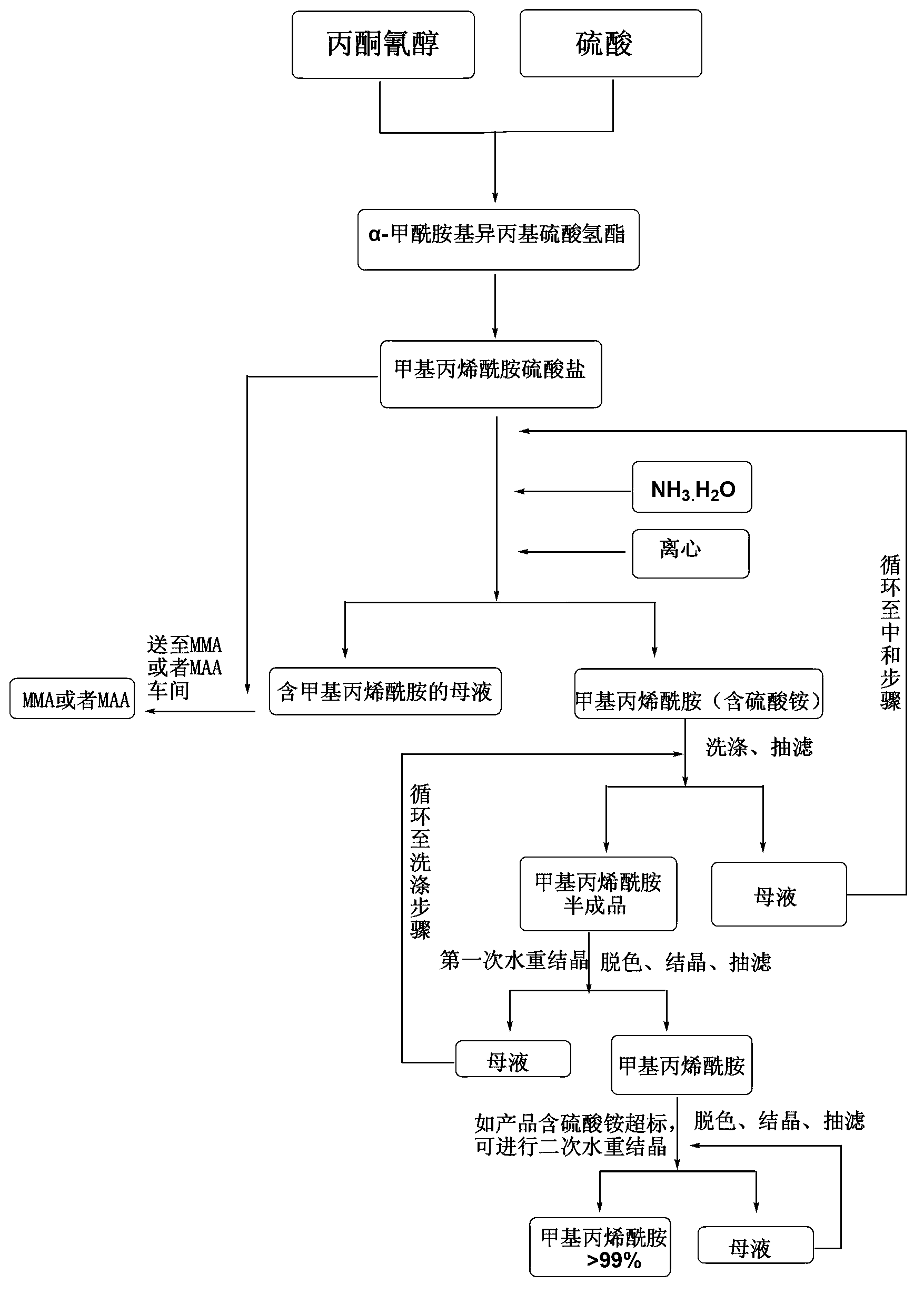
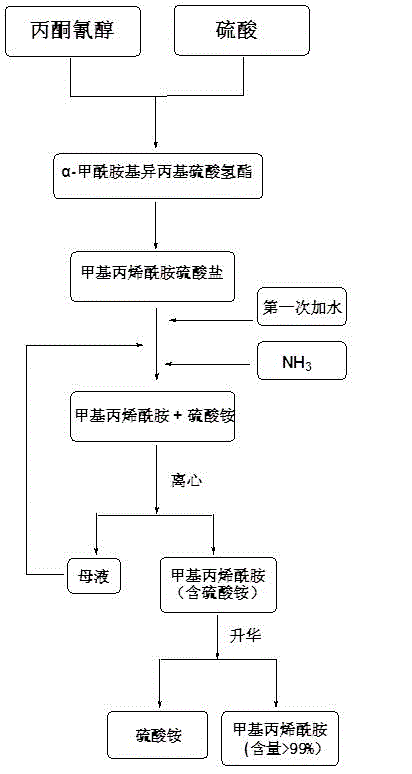
Method for joint production of methacrylamide, methyl methacrylate and methacrylic acid
ActiveCN103254091AReduce lossesHigh yieldOrganic compound preparationCarboxylic acid esters preparationAcetone cyanohydrinHydrolysis
The invention discloses a method for joint production of methacrylamide, methyl methacrylate and methacrylic acid. Methacrylamide sulfate is prepared from acetone cyanohydrin which is taken as a major raw material through concentrated sulfuric acid hydrolysis, dehydration and other effects; the methacrylamide sulfate is neutralized through ammonia gas or ammonia water, and at least one part of neutralized mother liquor circulates to an esterification step of producing methyl methacrylate and a hydrolysis step of producing methacrylic acid, so as to produce methyl methacrylate and methacrylic acid. The joint production method can realize the complete reuse of the methacrylamide in the mother liquor and convert the methacrylamide into the methyl methacrylate and the methacrylic acid, so as to reduce the loss of the methacrylamide, improve the yields of the methyl methacrylate and the methacrylic acid, and reduce the discharge of wastewater; and besides, the neutralized mother liquor can be extracted to avoid the accumulation of impurities in a process of purifying the methacrylamide.
Owner:CHONGQING UNISPLENDOUR CHEM
Process for production of methacrylic acid esters
ActiveCN105324172AImprove stabilityOrganic compound preparationCarboxylic acid esters preparationMethacrylateBoron
The invention discloses a method for producing alpha-, beta-unsaturated carboxylic acid esters in high yield from acetone cyanohydrin and sulfuric acid through the separation and concurrent catalytic conversion of reaction side products to additional alpha-, beta- unsaturated carboxylic acid ester product. The catalyst comprises at least one Group IA element, and boron as a promoter, on a porous support.
Owner:ROHM & HAAS CO
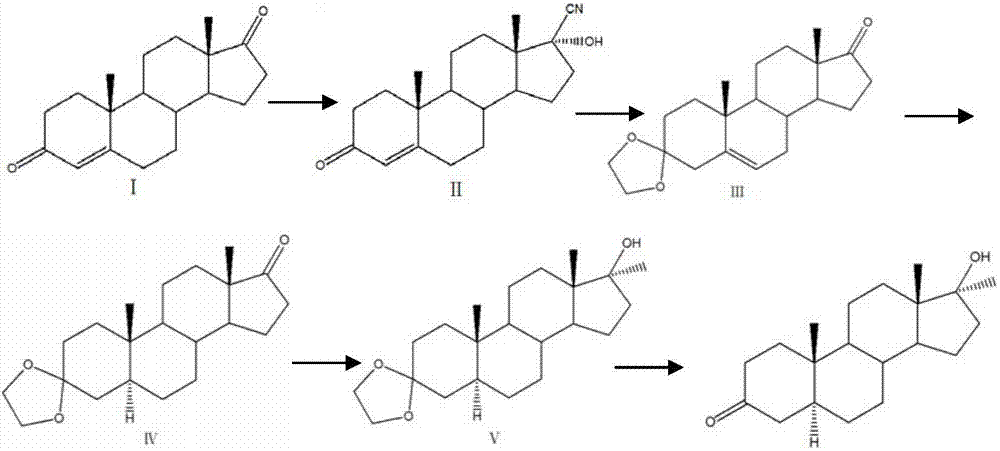
Preparation method of Stanolone
The invention discloses a preparation method of Stanolone, and the structure formula of the Stanolone is as shown in the specification. The preparation method is as follows: (1) addition reaction of a compound I with acetone cyanohydrin or sodium cyanide to obtain a compound II with a cyanogroup; (2) regulation of the pH of a solution of the compound II to acid, ketalation of the solution of the compound II with ethylene glycol and triethyl orthoformate, regulation of the pH of the solution to strong alkaline to remove the cyanogroup to finally produce a compound III; (3) reduction of 17-site keto group to hydroxy in the compound III by use of sodium borohydride or potassium borohydride for formation a compound IV; (4) addition of a catalyst to a solution of the compound IV, and hydrogenation to obtain a compound V; and (5) hydrolysis ketalation of the compound V in acetone under acidic conditions to obtain the target product stanolone. The process route of the method is simple, the stanolone is finally prepared based on the addition reaction, less pollution is produced, production efficiency is high, and the prepared stanolone has high purity.
Owner:ZHEJIANG PURUI PHARMA
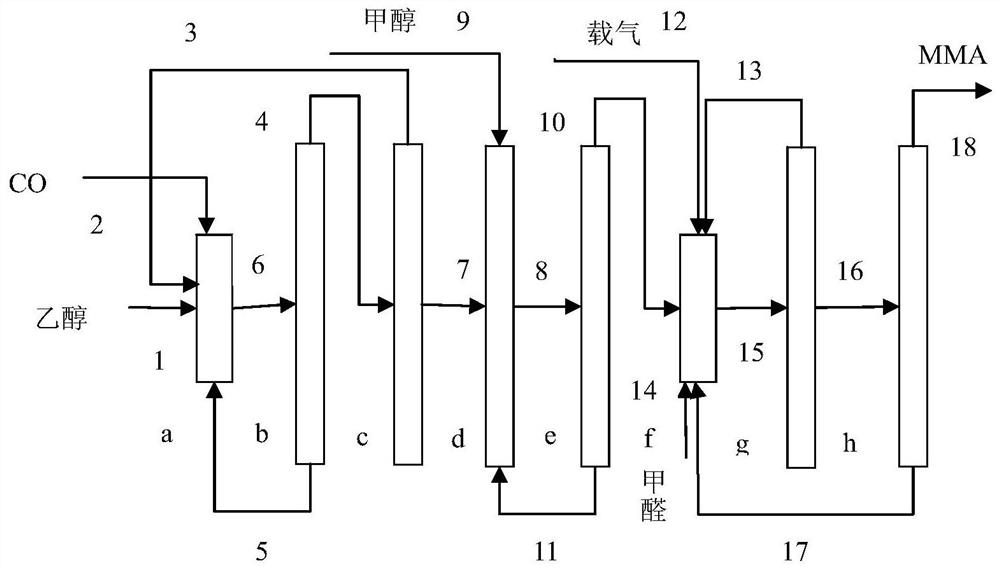
Process for synthesizing methyl methacrylate from ethanol
InactiveCN112851507APerfect rationalityClean and comprehensiveOrganic compound preparationCarboxylic acid esters preparationPropanoic acidPtru catalyst
The invention provides a process for synthesizing methyl methacrylate from ethanol, and belongs to the technical field of methyl methacrylate synthesis. The process comprises the steps of taking ethanol and CO as raw materials, carrying out carbonylation reaction under the action of a catalyst to synthesize propionic acid, carrying out esterification reaction on propionic acid and methanol under the action of a strong acid catalyst to generate methyl propionate, and carrying out gas-phase aldol condensation reaction on methyl propionate and formaldehyde under the action of an acid-base catalyst to obtain a methyl methacrylate product. The existing MMA production technology such as an acetone cyanohydrin method (ACH), an isobutene method (C4 route) and an ethylene method belongs to production process technology of the petroleum route, the process provided by the inventiontakes ethanol as a raw material and is an MMA production technology of the coal-based route, and the development of the MMA production technology of the coal-based route has important practical significance for the current situation of less-oil and more-coal energy.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
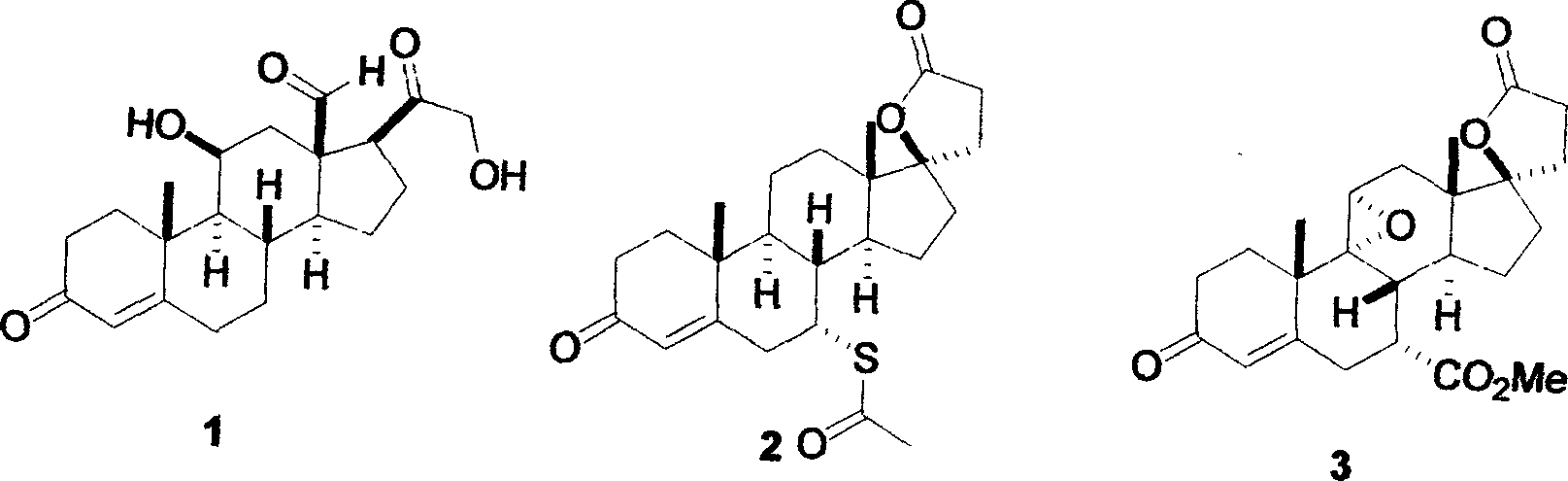
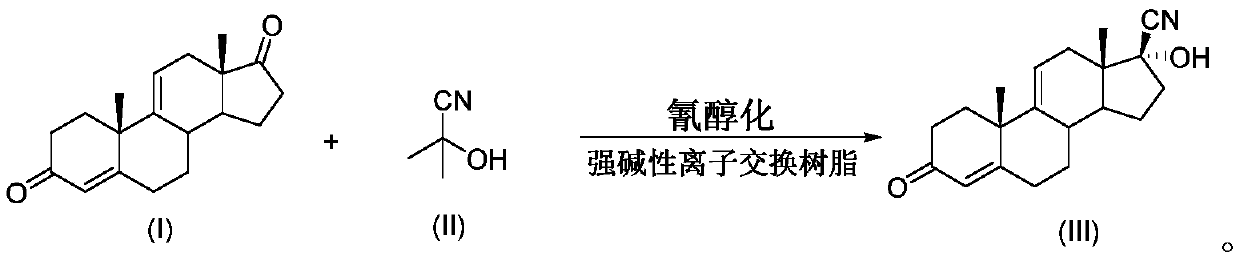
Method for preparing 17beta-cyano-17alpha-hydroxy-9-dehydroandrostenedione
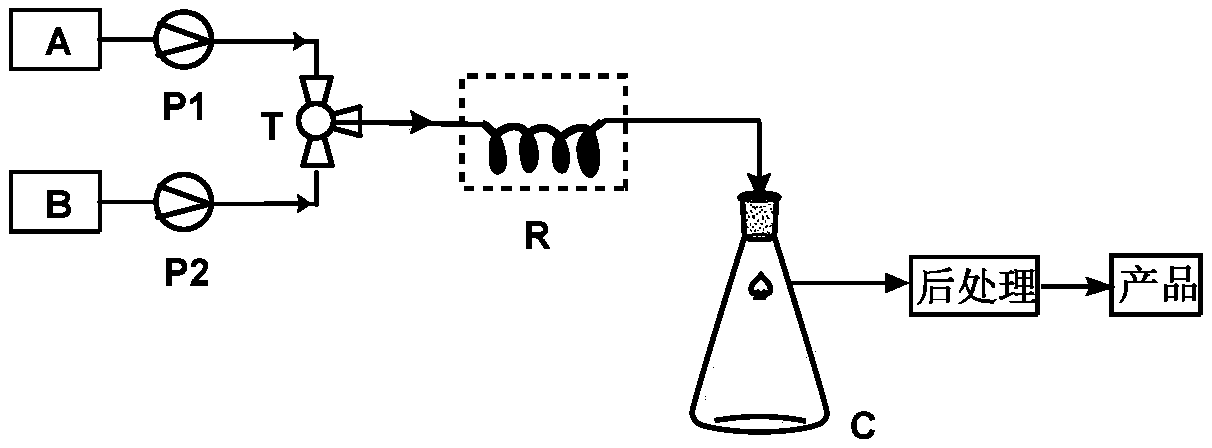
InactiveCN111320665ASmall amount of online responseReduce backmixingSteroidsChemical/physical processesOrganic solventIon exchange
The invention discloses a method for preparing 17 beta-cyano-17 alpha-hydroxy-9-dehydroandrostenedione. The method comprises the following steps: (1) dispersing 9-dehydroandrostenedione (I) and acetone cyanohydrin (II) into an organic solvent; (2) carrying out a cyanohydrination reaction on the obtained mixed raw material liquid in a tubular reactor filled with strongly basic ion exchange resin, and (3) carrying out post-treatment on the material liquid after the cyanohydrination reaction to obtain 17beta-cyano-17alpha-hydroxy-9-dehydroandrostenedione (III), the reaction formula of which is shown in the specification.
Owner:台州仙琚药业有限公司
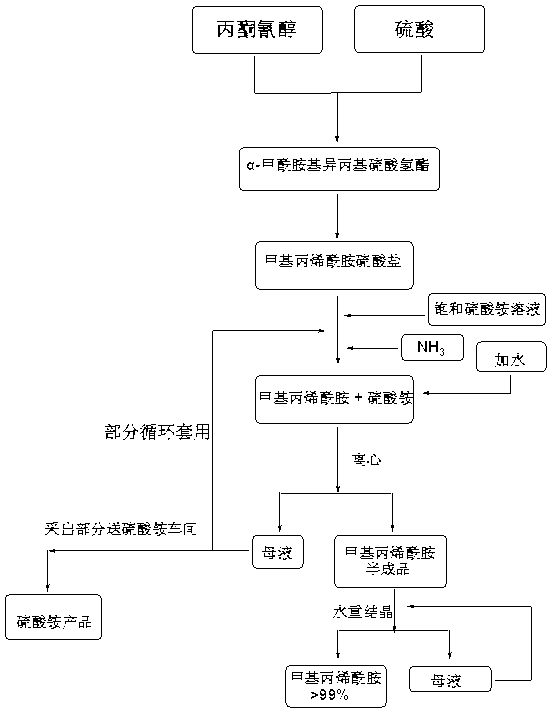
Preparation and purification method of methacrylamide
ActiveCN103145578AReduce solubilityHigh yieldOrganic compound preparationCarboxylic acid amides preparationCyanohydrinAcetone cyanohydrin
The invention discloses a preparation and purification method of methacrylamide. The method comprises the following steps: hydrolyzing and dehydrating by concentrated sulfuric acid to generate methacrylamide sulfate based on acetone cyanohydrin as a main raw material; neutralizing the methacrylamide sulfate by ammonia gas or ammonia water; circularly applying the neutralized centrifuged mother liquor; and purifying the methacrylamide crude product containing ammonium sulfate by sublimating, thereby obtaining the pure methacrylamide. According to the process, the mother liquor can be circularly applied so as to greatly reduce the discharge of salt-containing acid-containing sewage and avoid the environment pollution, reduce the dissolving of the methacrylamide in the mother liquor and improve the yield of the methacrylamide; and the purity of the obtained pure methacrylamide can be more than 99%, and the yield is more than 90%.
Owner:CHONGQING UNISPLENDOUR CHEM
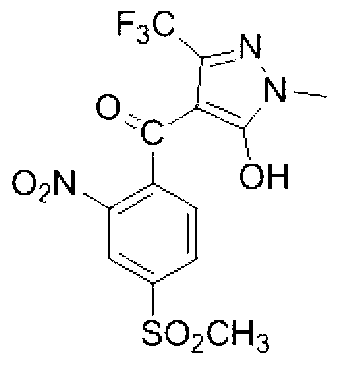
Method for preparing tembotrions
InactiveCN106008290ALow yieldOvercomes the disadvantage of requiring a highly toxic catalyst, acetone cyanohydrinOrganic chemistryOrganic compound preparationBenzoic acidMethyl benzoate
The invention discloses a method for preparing tembotrions, and belongs to the technical field of organic chemical industry. The method comprises the steps that sodium 2,2,2-trifluoroethanolate and 2-chloro-3-brooethyl-4-methylsulfonylpropyl methyl benzoate react, and generated 2-chloro-3-trifluoro-ethoxy methyl-4-methyl sulfone chloride benzoic acid and 1,3-cyclohexanedione are condensed and rearranged to prepare tembotrions. According to the method for preparing tembotrions, the defects that in a traditional method, 2-chloro-3-brooethyl-4-methyl sulfone chloride methyl benzoate, trifluoroethanol and potassium tert-butoxide are used for preparing 2-chloro-3-trifluoro-ethoxy methyl-4-methyl sulfone chloride benzoic acid, the yield is low, and a potassium tert-butoxide reagent is expensive are overcome, meanwhile, the defect that after carboxyl is acylate chlorinated into ester, a toxic catalyst acetone cyanohydrins is needed in a rearrangement method is overcome, the advantages of being high in yield, low in cost, easy to operate, low in pollution, safe, environmentally friendly and the like are achieved, and the method is suitable for large-scale industrial production.
Owner:HEFEI JIUYI AGRI DEV
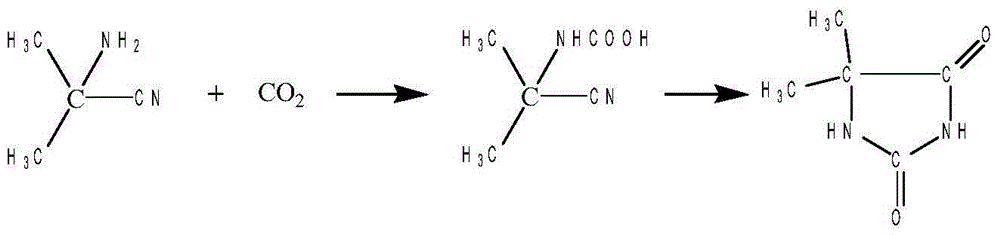
Process for preparing 5,5-dimethyl-2,4-imidazolinedione by intermittent presurizing method
InactiveCN1356321AEasy to makeProcess conditions are easy to controlOrganic chemistryReaction temperatureSolvent
A process for preapring 5,5-dimethyl-2,4-imidazolinedione by intermittent pressurizing method includes dissolving the acetone cyanohydrin and ammonium dicarbonate as raw material in water or mother liquid, heating, cyclizing reaction at 50-90 deg.C and 0.8-1.8 MPa for 0.5-20 hrs, cooling-crystallizing filtering and drying. Its advantages include simple process, easy control, and high purity and output rate of product.
Owner:TIANJIN UNIV
Method for synthesizing bis-isobutyronitrile hydrazine
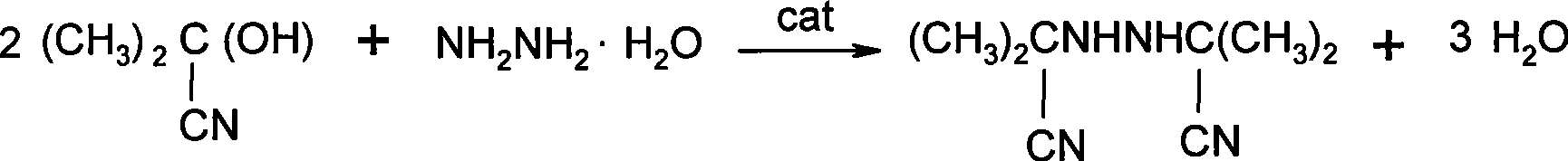
ActiveCN101445471APromote generationReduce the amount addedCarboxylic acid nitrile preparationOrganic compound preparationAmmonium compoundsHydrazine compound
The invention relates to a method for synthesizing bis-isobutyronitrile hydrazine. Acetone cyanohydrins and hydrazine hydrate are used as raw materials to carry out condensation reaction by the action of phase transfer catalyst quaternary ammonium compound. The phase transfer catalyst not only promotes the reaction speed and enhances the synthesis yield to 98.23 percent to 98.60 percent, but also reduces the addition of water in the reaction, thus improving the productivity greatly and reducing the production cost as the phase transfer catalyst also takes effects of dispersing. A product has stable quality, and the purity of the product is more than 98 percent. Therefore, the method for synthesizing the bis-isobutyronitrile hydrazine is a method fit for industrialized production.
Owner:SHANGHAI NO 4 REAGENT & H V CHEM
Method for producing 5,5-dimethylhydantoin
ActiveCN104557721AGenerate high added valueReduce pollutionProductsOrganic chemistryAmmoniaSodium cyanate
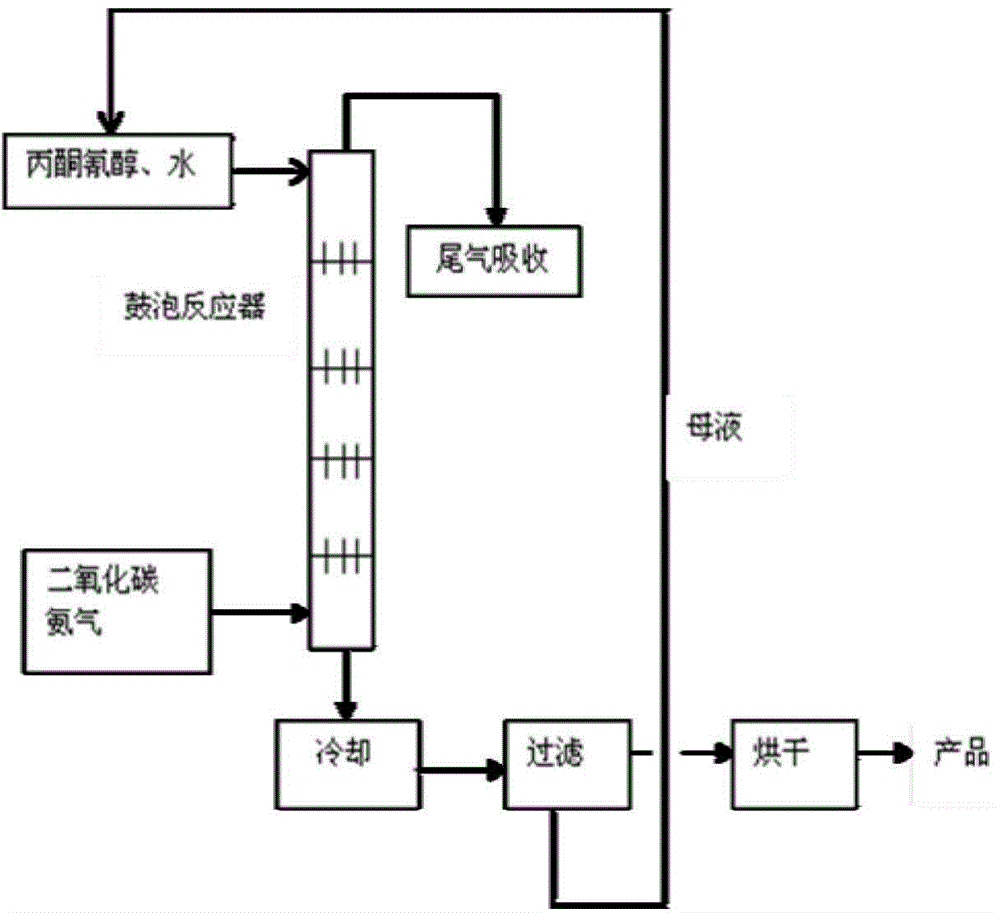
The invention provides a method for preparing 5,5-dimethylhydantoin from tail gas which contains ammonia and carbon dioxide and is produced during industrial production of melamine and sodium cyanate. The tail gas which contains the ammonia and the carbon dioxide is fed into a bubbling reactor to react with an acetone cyanohydrin aqueous solution at the temperature of 95-100 DEG C for 20-30 min, the yield is 99.6%, and the product content is 99.7% (HPLC). With the adoption of the method, the tail gas which contains the ammonia and the carbon dioxide can be combined with fine chemicals, the 5,5-dimethylhydantoin with the high added value can be produced, environmental pollution can be reduced, and harmful gases are turned into useful things.
Owner:SHENYANG RES INST OF CHEM IND
Preparation and purification method of clean methacrylamide
InactiveCN103130671AReduce solubilityHigh yieldOrganic compound preparationCarboxylic acid amide separation/purificationSulfateCyanohydrin
The invention discloses a preparation and purification method of clean methacrylamide. The preparation and purification method disclosed by the invention comprises the following steps: taking acetone cyanohydrin as a main raw material, generating methacrylamide sulfate by hydrolysis with concentrated sulfuric acid, dehydration and other actions, neutralizing the methacrylamide sulfate with ammonia or ammonia water, then supplementing a certain amount of water to dissolve generated ammonium sulfate, getting a methacrylamide semi-finished product, indiscriminately using part of mother liquor after neutralization in a circulating manner, and performing re-crystallization and purification on the methacrylamide semi-finished product to get a methacrylamide pure product, wherein the recrystallization mother liquor can also be indiscriminately used in the circulating manner. According to the preparation and purification method disclosed by the invention, a new process route is adopted, and the mother liquor can be used indiscriminately in the circulating manner, so that the emission of wastewater containing salts and acids is greatly reduced, the environmental pollution caused by the wastewater is avoided, the dissolution of the methacrylamide in the mother liquor is further reduced, the yield of the methacrylamide is improved, the methacrylamide with the content of above 98.5% is obtained, the content of the ammonium sulfate is within 1.5%, and the yield is above 90%.
Owner:CHONGQING UNISPLENDOUR CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com