Green preparation technology of 2-thiopheneacetic acid
A technology of thiophene acetic acid and thiophene acetonitrile, applied in the direction of organic chemistry and the like, can solve the problems of difficult industrial production, low product yield and the like, and achieve the effects of mild reaction conditions, high safety performance and favorable industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
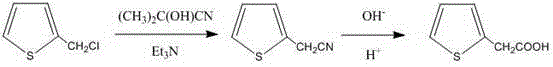
[0016] (1) Synthesis of 2-thiophene acetonitrile: Put 102.1g of acetone cyanohydrin, 150mL of ethanol, and 8.2g of triethylamine into a flask, heat up to 60-65°C, add 133.2g of 2-chloromethylthiophene, methyl iso Add 200mL of butyl ketone mixture slowly, keep it warm for 6 hours, then add 250mL of purified water, let stand to separate the organic phase after cooling slightly; the organic phase is distilled under reduced pressure to remove the organic solvent, and then further distilled to obtain 112.6 g of 2-thiophene acetonitrile, yield 91.5%.
[0017] (2) Synthesis of 2-thiopheneacetic acid: Heat 150g of 30% sodium hydroxide aqueous solution to reflux, then slowly add 112g of 2-thiopheneacetonitrile dropwise, and then reflux for 3 hours (there is a large amount of ammonia gas during the reaction produce). After the reaction is complete, lower the temperature to 20-30°C, and then use 200mL of ethyl acetate to extract impurities three times. After the extraction is complete,...
Embodiment 2
[0019] (1) Synthesis of 2-thiophene acetonitrile: put 102.6g of acetone cyanohydrin, 150mL of ethanol, and 11.5g of potassium carbonate into a flask, heat up to 60-65°C, and mix 133.3g of 2-chloromethylthiophene, methyl isobutyl methyl Add 200mL of the ketone mixture slowly, and keep it warm for 7 hours after the dropwise addition, then add 250mL of purified water, let it stand to separate the organic phase after cooling slightly; the organic phase is distilled under reduced pressure to remove the organic solvent, and then further distilled to obtain 2 - Thiophene acetonitrile 112.1g, yield 91.2%.
[0020] (2) Synthesis of 2-thiopheneacetic acid: Heat 150g of 30% sodium hydroxide aqueous solution to reflux, then slowly add 113g of 2-thiopheneacetonitrile dropwise, and then reflux for 2 hours after the drop (a large amount of ammonia gas is produced during the reaction process) ). After the reaction is complete, lower the temperature to 20-30°C, and then use 200mL of ethyl ace...
Embodiment 3
[0022] (1) Synthesis of 2-thiophene acetonitrile: Put 102.6g of acetone cyanohydrin, 150mL of ethanol, and 10.1g of triethylamine into a flask, raise the temperature to 60-65°C, add 133.3g of 2-chloromethylthiophene, methyl iso Add 200mL of butyl ketone mixture slowly, keep it warm for 10 hours after the addition, then add 250mL of purified water, let stand to separate the organic phase after cooling slightly; the organic phase is distilled under reduced pressure to remove the organic solvent, and further distilled to obtain 113.3 g of 2-thiophene acetonitrile, yield 92.1%.
[0023] (2) Synthesis of 2-thiopheneacetic acid: Heat 150g of 30% sodium hydroxide aqueous solution to reflux, then slowly add 113g of 2-thiopheneacetonitrile dropwise, and then reflux for 5 hours after the drop (a large amount of ammonia gas is produced during the reaction process) ). After the reaction is complete, lower the temperature to 20-30°C, and then use 200mL of ethyl acetate to extract impuriti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com