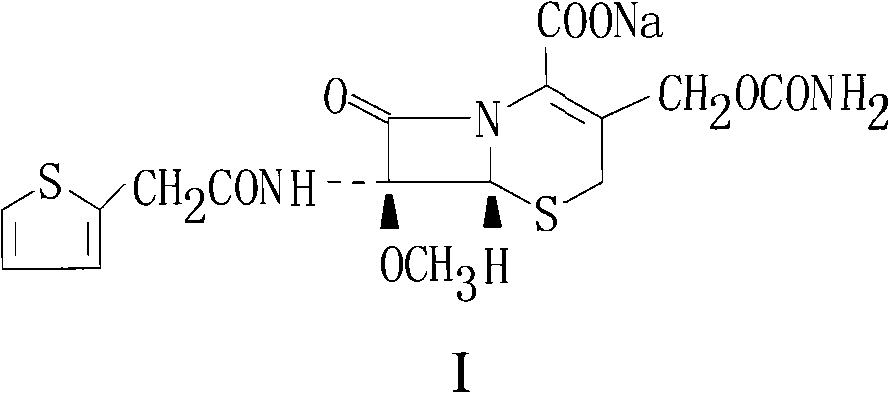
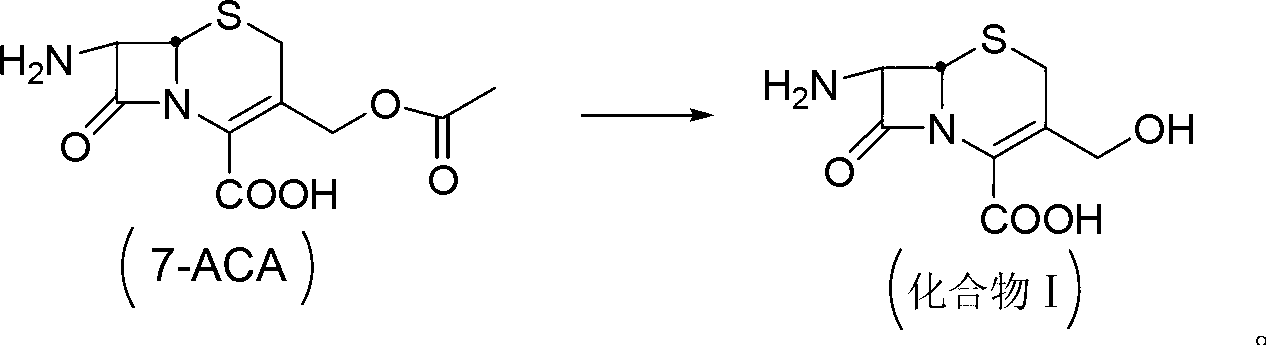
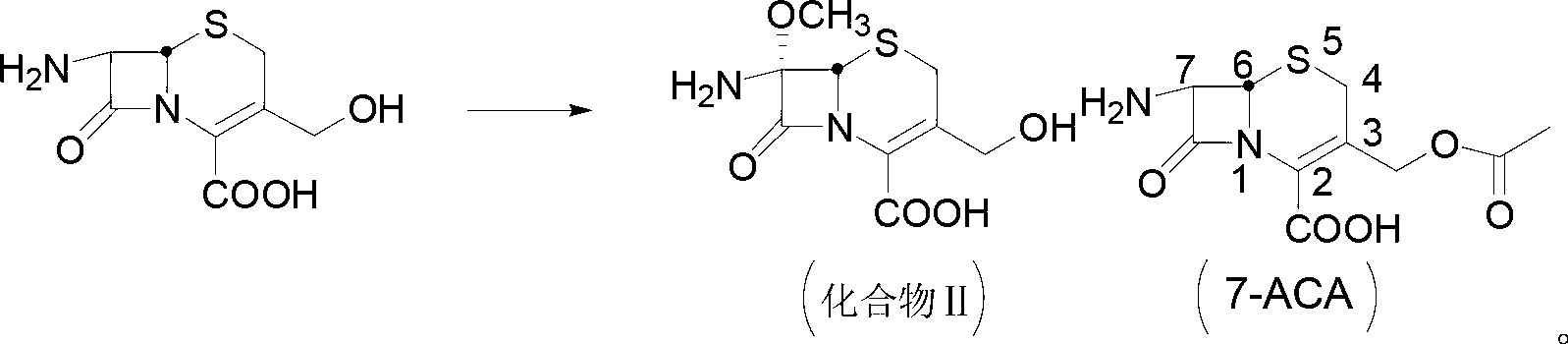
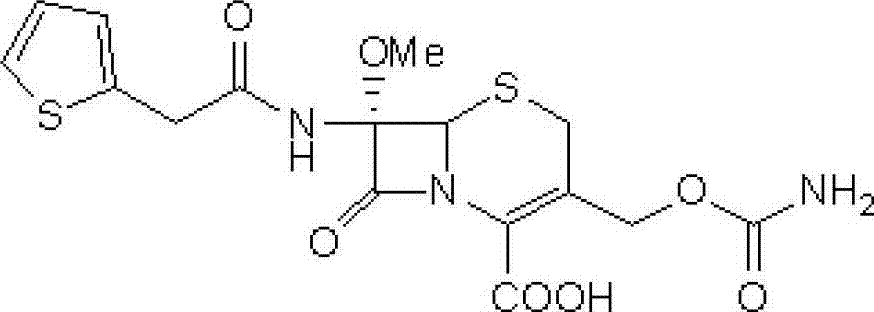
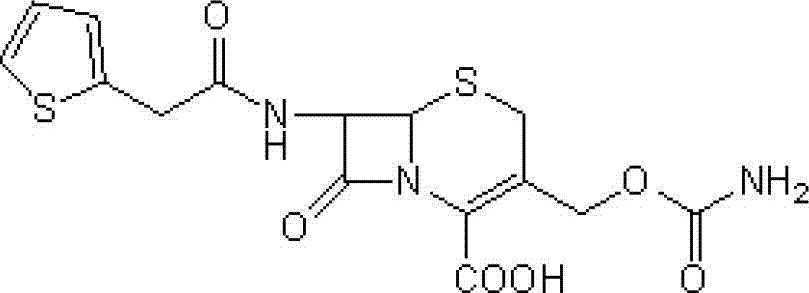
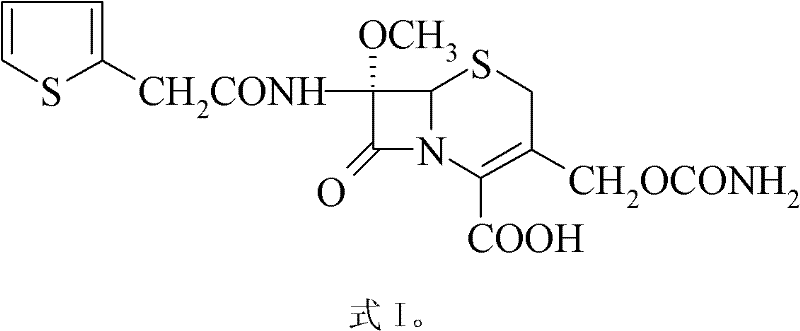
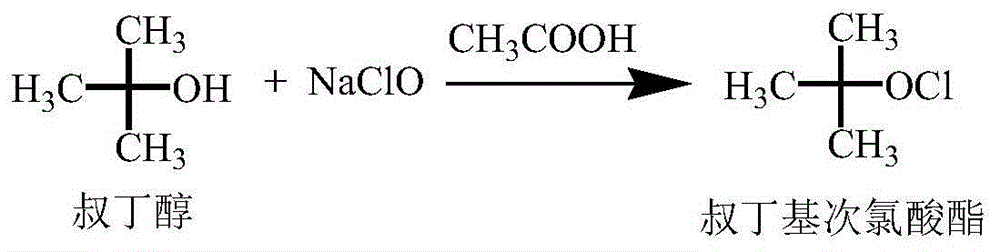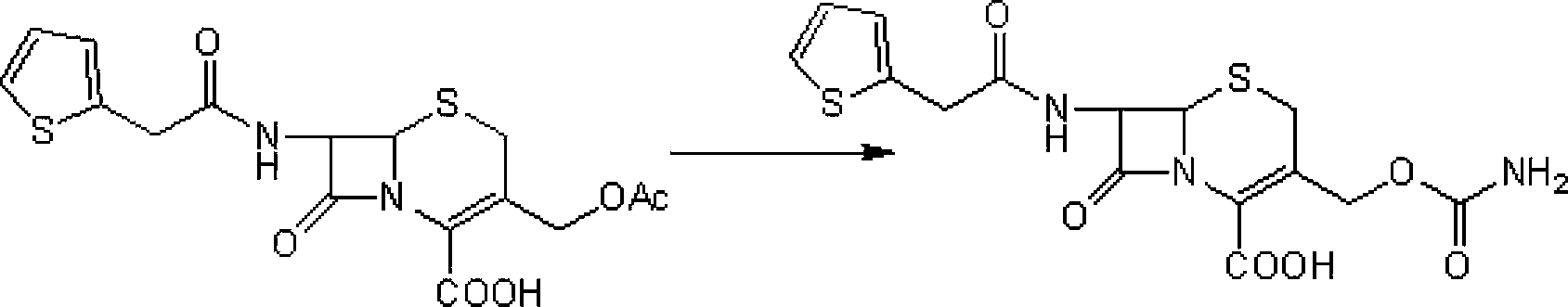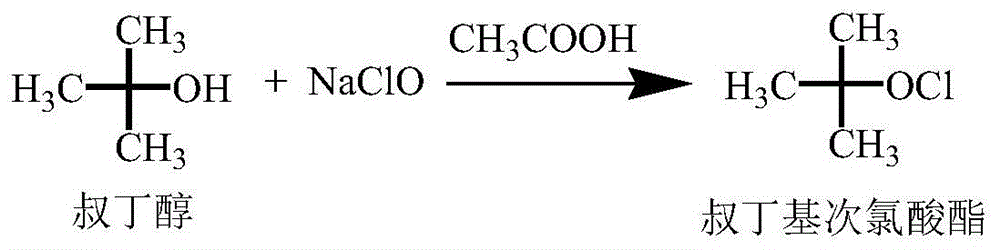Patents
Literature
48 results about "Cefoxitin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
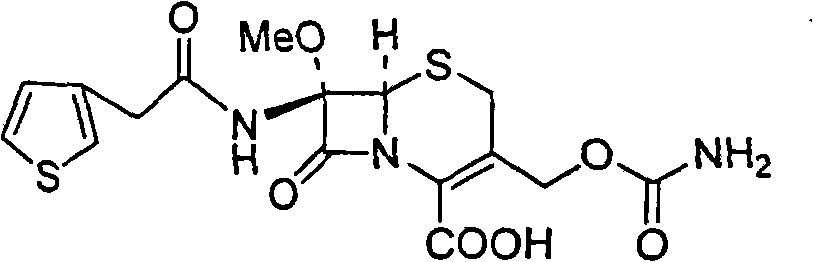
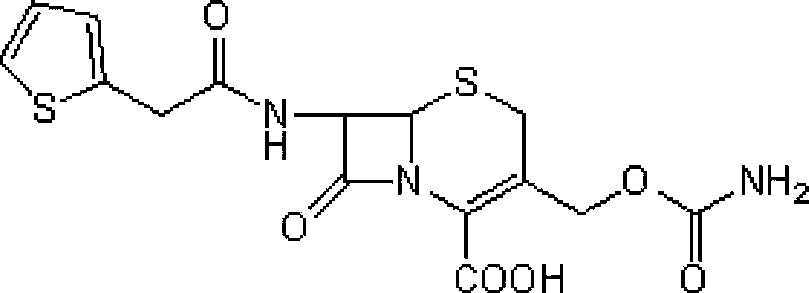
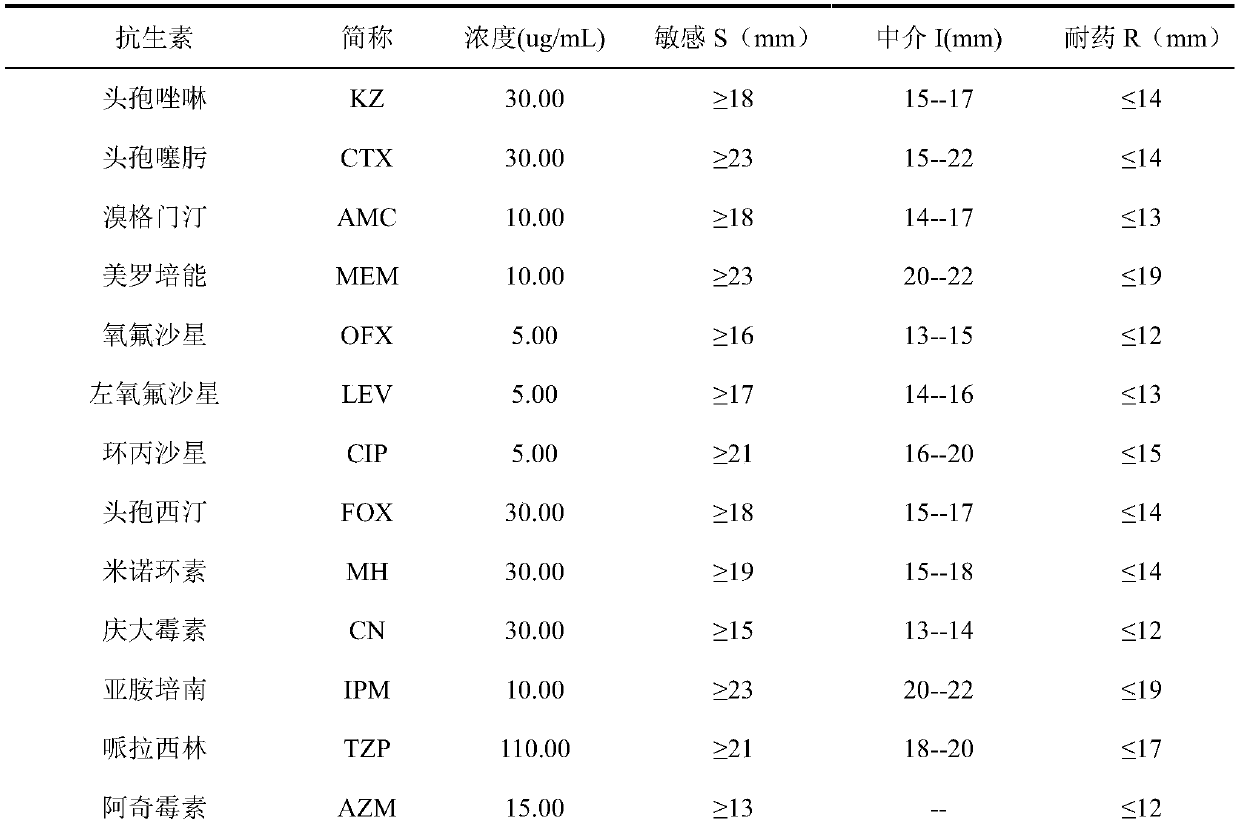
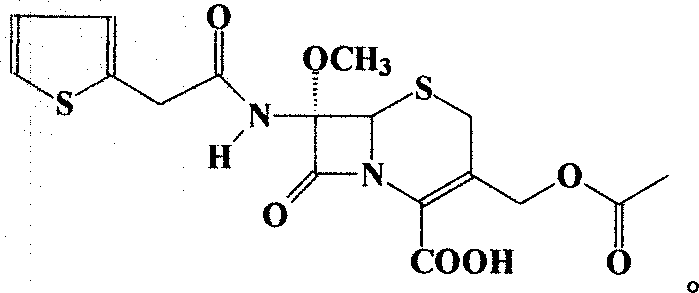
Cefoxitin is an antibiotic used to treat a wide variety of bacterial infections. It may also be used before and during certain surgeries to help prevent infection.
Synthetic method of antibiotic cefoxitin
ActiveCN101555252AEmission reductionEliminate emissionsAntibacterial agentsOrganic chemistryCefoxitinAlkaloid
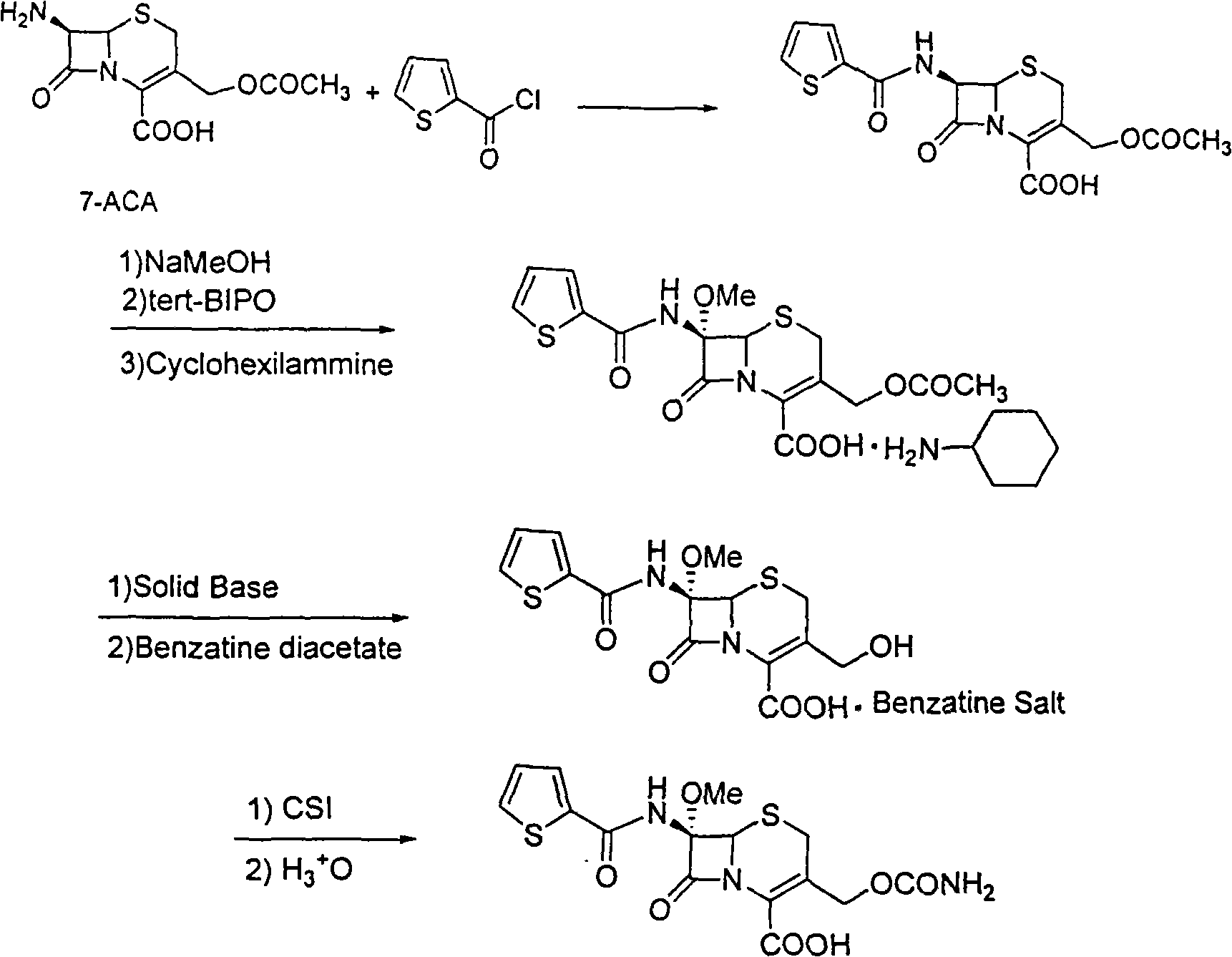
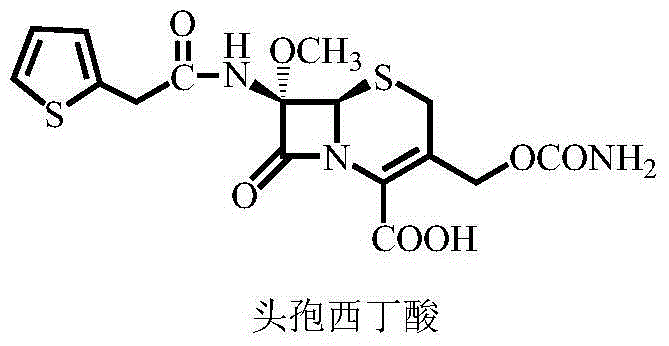
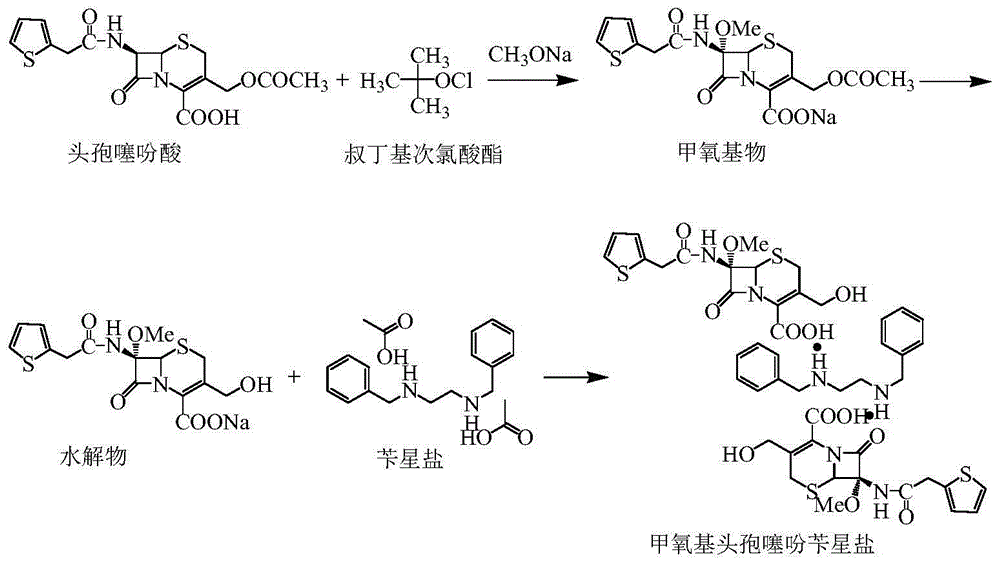
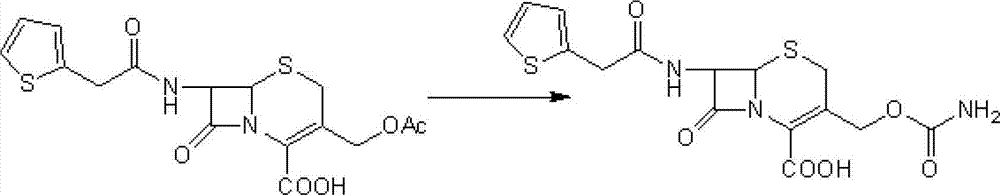
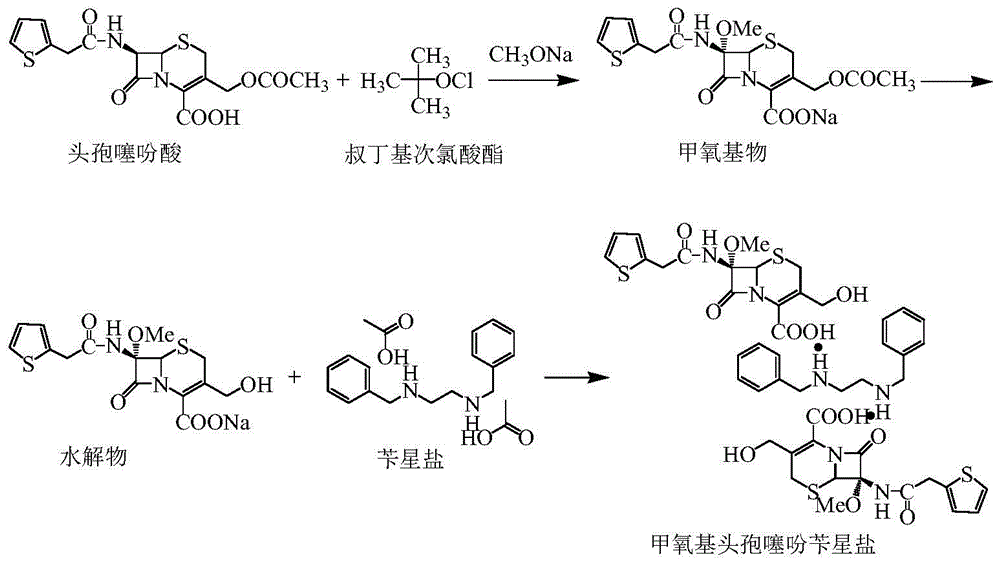
The invention relates to a synthetic method of antibiotic cefoxitin. 7-ACA is taken as a starting material and firstly reacts with thiopheneacetyl chloride, cephalothin acid is obtained by separation; the cephalothin acid is not purified, and the methoxylation is directly carried out on the cephalothin acid to obtain an intermediate A which introduces methoxy on the position of 7, the intermediate A obtains 7-alpha-methoxy cephalothin cyclohexylamine salt under the action of cyclohexylamine; the 7-alpha-methoxy cephalothin cyclohexylamine salt reacts with benzathine diacetate under the catalysation of solid alkaloid to obtain 7-alpha-methoxy-3-deacetoxy cephalothin benzathine salt; and the carbamylation is carried out under the action of chlorosulfonyl isocyanate to obtain the cefoxitin. The synthetic method has the advantages that compared with the prior art, the synthetic method simplifies the operation process, has high product yield, reduces the production cycle, eliminates the discharge of waste water containing organic solvent and reduces the production cost. The product quality is stable, and the synthetic method is applicable to the large-scale industrial production.
Owner:国药集团致君(苏州)制药有限公司
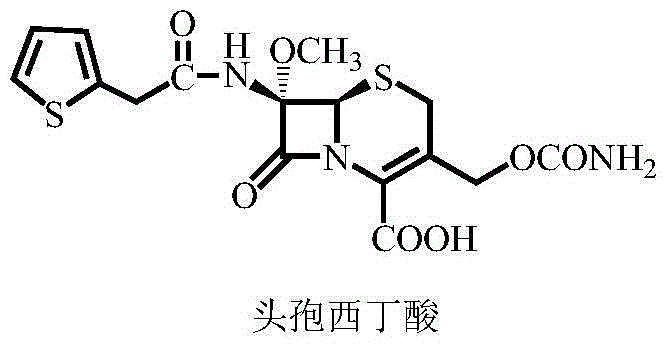
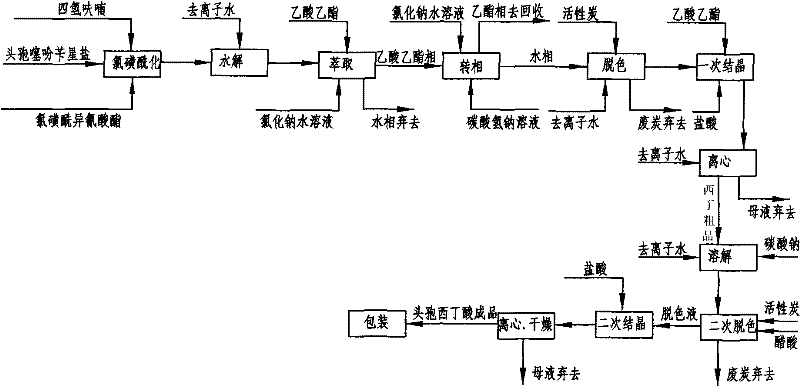
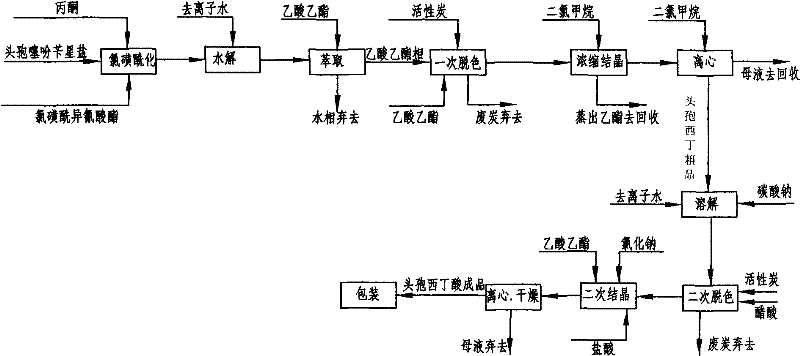
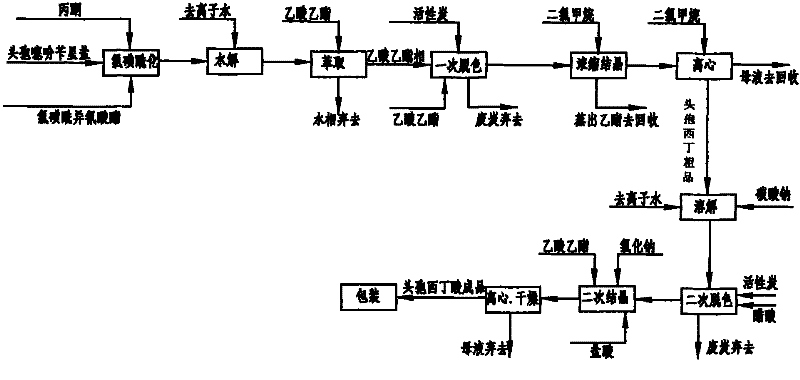
Cefoxitin acid preparation method
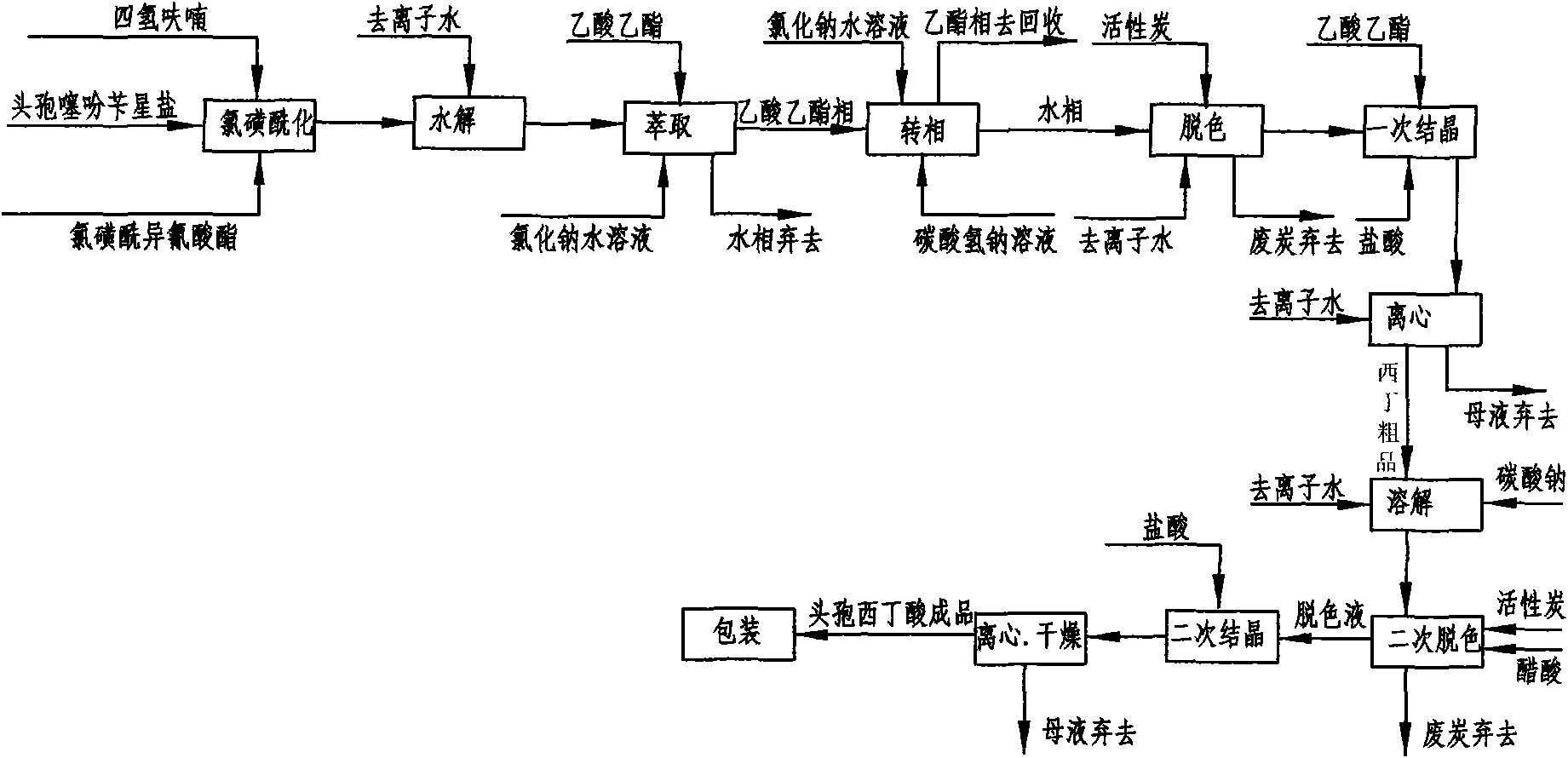
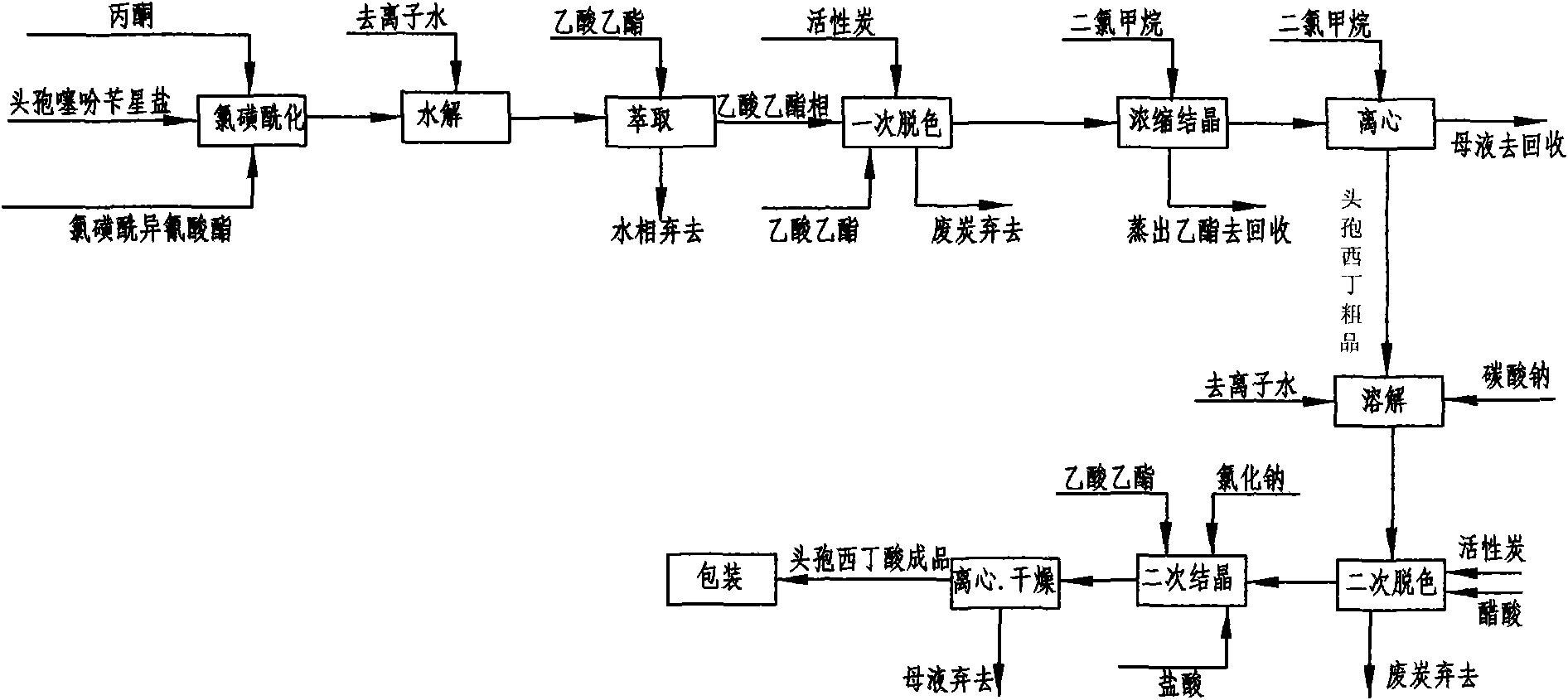
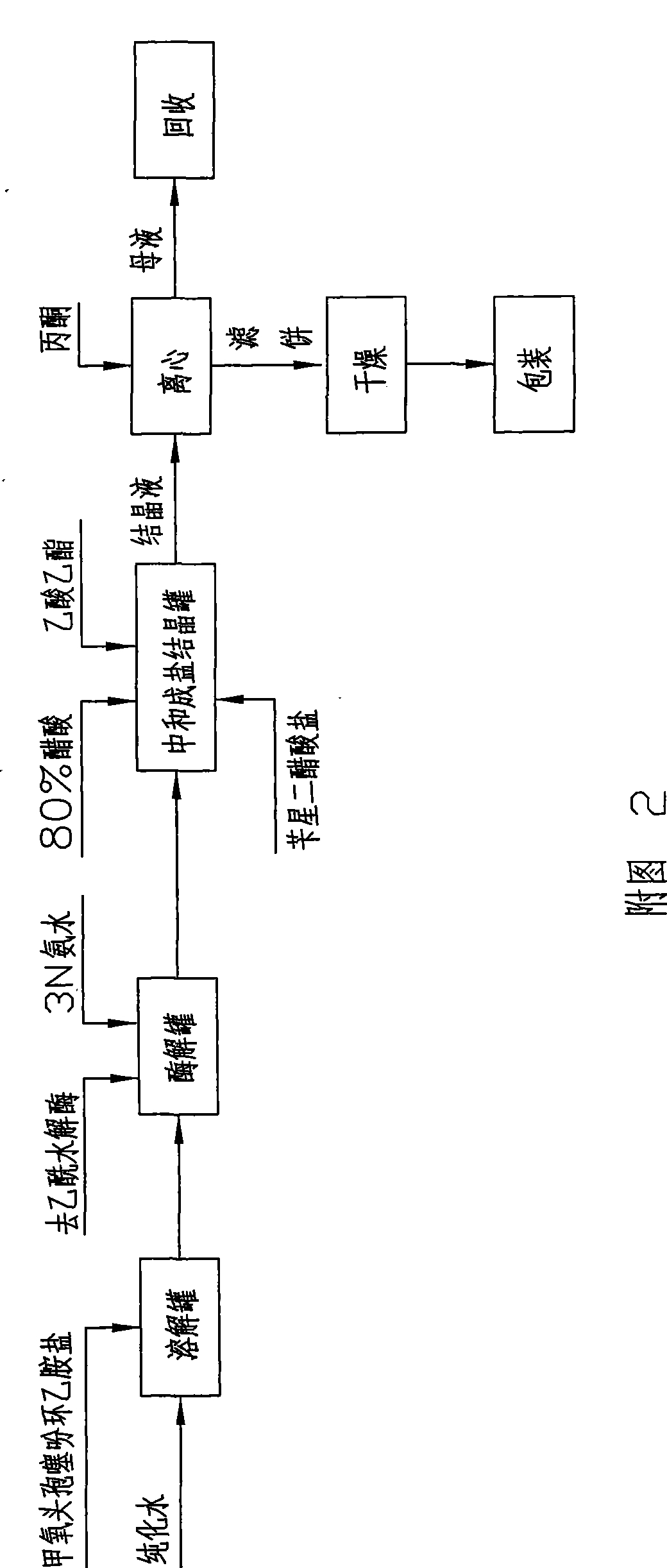
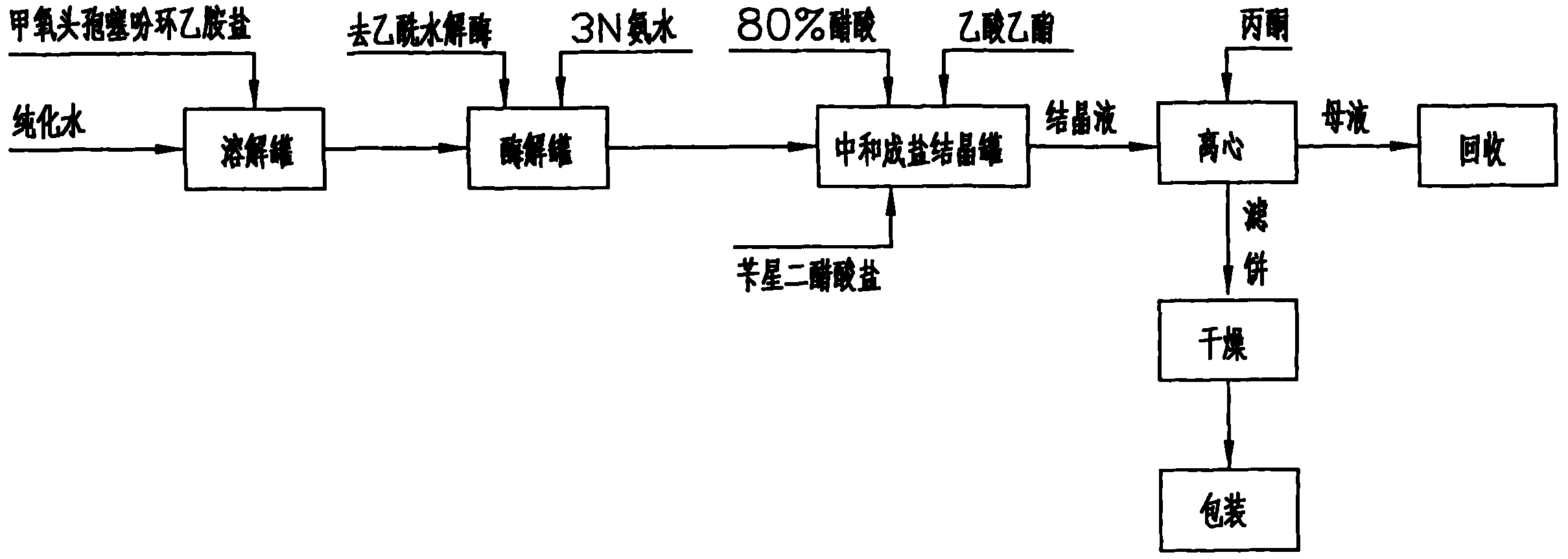
The invention discloses a cefoxitin acid preparation method, comprising the following steps: adding 7-alpha-methoxyl-3-deacetylcefalotin benzathine salt in acetone at room temperature, cooling the solution and adding chlorosulfonyl isocyanate to react and then producing the finished product by hydrolysis, extraction, decoloration, concentrated crystallization, filtration, dissolution and secondary crystallization. In the invention, acetone with a consumption of 2.6kg / kg which is easy to recycle is used instead of tetrahydrofuran which is used in the prior art with a consumption of 13kg / kg and the production cost is reduced by 120 Yuan / Kg. In the invention water phase decarburization is changed to organic phase decarburization, thus eliminating the step of phase inversion in the original process and avoiding the yield loss in phase inversion process; water phase crystallization is changed to the crystallization method which adopts organic phase for concentrating and adds dichloromethane for crystallizing so that the crystallization is realized fully, the yield is higher and the total yield of cefoxitin is increased from 58% to 67%. The material cost of the cefoxitin acid can be reduced by 240 Yuan / Kg, the total reduced cost can be about 360 Yuan / Kg and the product prepared by the method has stronger market competitiveness and remarkable economic benefit.
Owner:河北九派制药股份有限公司
Preparation method of cefoxitin
InactiveCN102633819AOvercoming the drawbacks of 101613361Avoid condensation side reactionsOrganic chemistrySodium methoxideHypochlorite
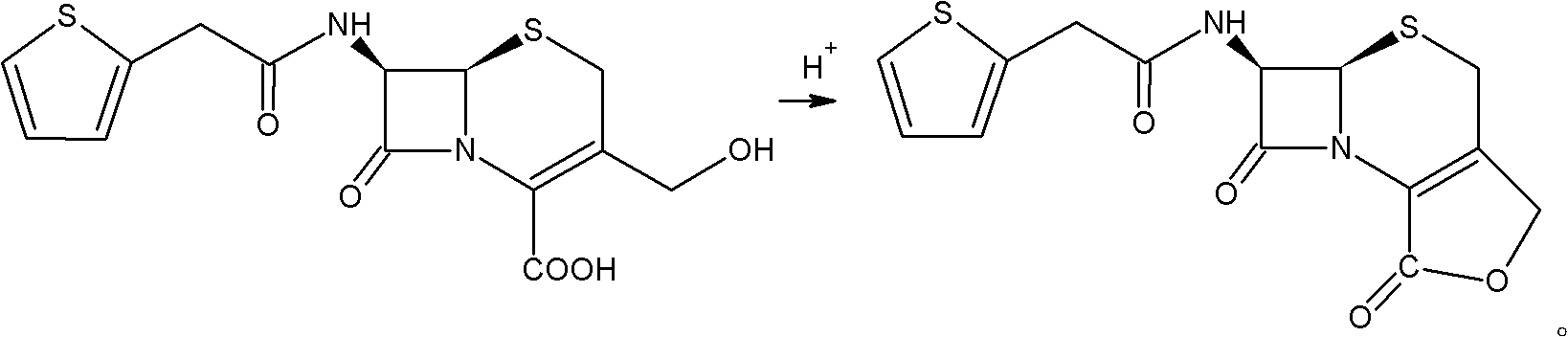
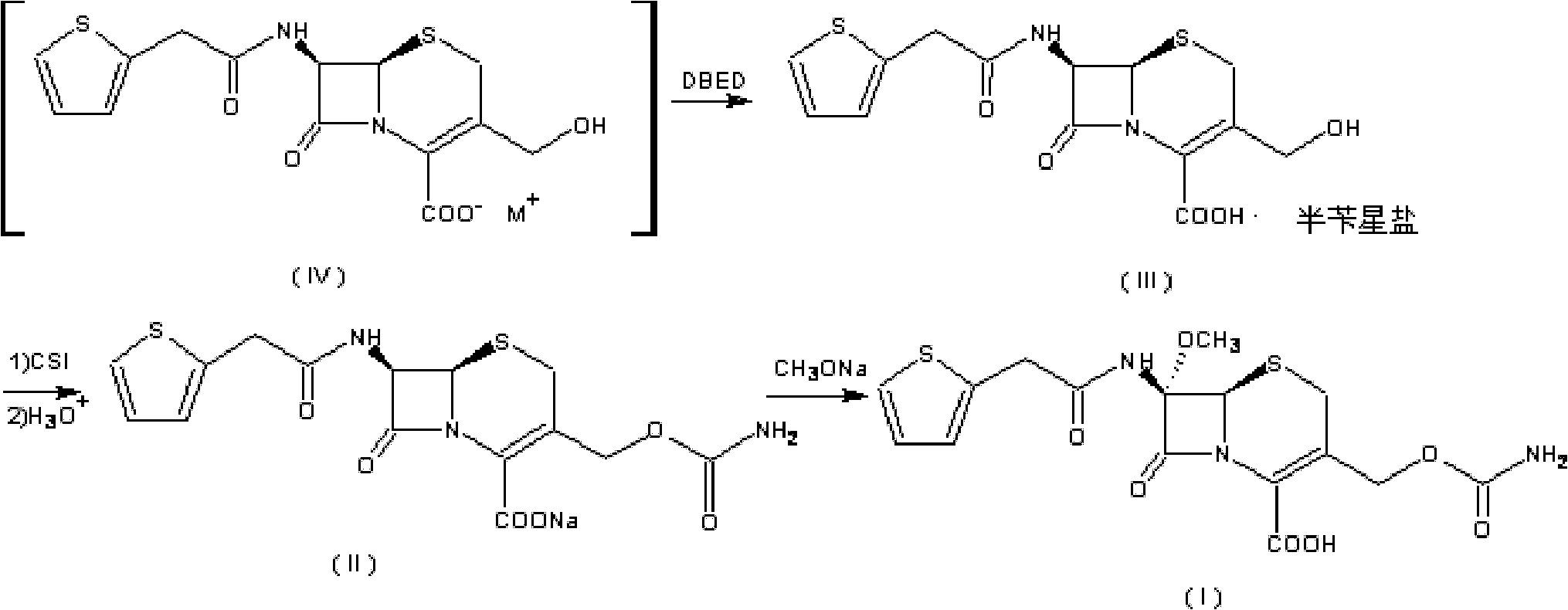
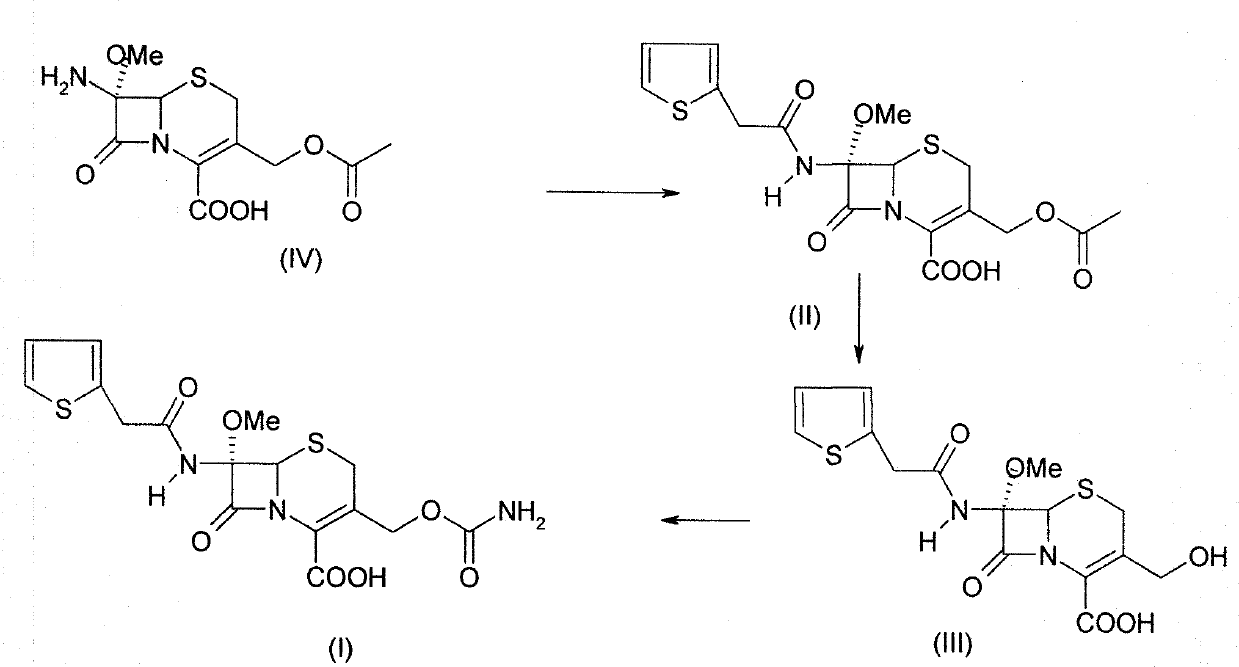
The invention relates to a preparation method of cefoxitin. The method comprises the following steps: adding a solvent A into a compound aqueous solution with the structure shown in the formula (IV), adding N,N'-dibenzylethylenediamine diacetate or aqueous solution thereof, filtering to obtain a compound with an intermediate structure shown in the formula (III); adding the compound with the intermediate structure shown in the formula (III) into acetone or tetrahydrofuran, adding chloriosulfonyl isocyanate to react, and hydrolyzing; adding acetic ether, filtering, extracting, decoloring, salting out, and filtering to obtain a compound with an intermediate structure shown in the formula (II); adding the compound with the intermediate structure shown in the formula (II) into an organic solvent B, adding organic acid to dissolve, adding a methanol solution with sodium methoxide and tert-butyl hypochlorite to perform methylation reaction, adding sodium pyrosulfite and acetic acid for neutralizing, adding water for extracting, acidifying water phase, filtering to obtain cefoxitin. According to the preparation method, deacetyled cefoxitin always exists in a mode of sodium salt or amine salt, so that the condensation side reaction easily caused by low pH can be avoided, and the product quality and yield can be well controlled.
Owner:山东安弘制药有限公司
Synthetic method of cefoxitin acid
The invention discloses a synthetic method of cefoxitin acid. Cephalotin acid used as a raw material reacts with prepared tert-butyl hypochlorite in the presence of sodium methylate to obtain a methoxy substance; the methoxy substance is subjected to a hydrolysis reaction and salt formation is carried out in the presence of benzathine to obtain methoxy cefalotin benzathine; methoxy cefalotin benzathine reacts with CSI, and hydrolysis is carried out to obtain a cefoxitin acid crude product; and the cefoxitin acid crude product undergoes the step of recrystallization to finally prepare cefoxitin acid. The synthetic method is simple to operate and is low-cost. By the synthetic method, quality and total yield reach 95.8%. The produced cefoxitin acid is a white solid powder, and purity of the product reaches more than 99.0%. The product has good quality and is suitable for industrial production.
Owner:YANCHENG KAIYUAN MEDICINE CHEM
Method for detecting impurities in cefoxitin product
ActiveCN101556262AEnsure safetySimple methodOrganic chemistryComponent separationDipotassium hydrogen phosphateO-Phosphoric Acid
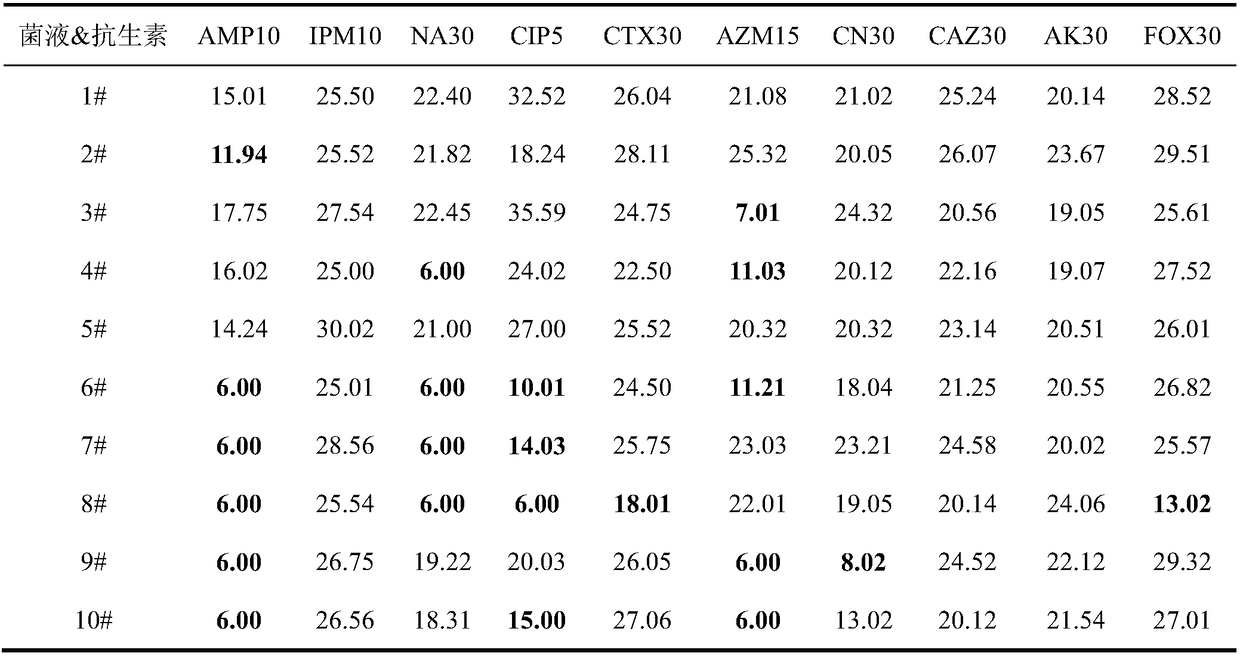
The invention provides a method for detecting impurities in a cefoxitin product. The method adopts high performance liquid chromatography and comprises the steps that an inverted octadecylsilyl bonded phase silica gel chromatographic column is adopted; 0.7 percent (weight / volume) of dipotassium hydrogen phosphate aqueous solution with a pH of 3.5 adjusted by phosphoric acid serves as a mobile phase A and acetonitrile as a mobile phase B; the mobile phase A and the mobile phase B are combined into a mixed mobile phase which goes through gradient elution under the following conditions: during 0 to 5 minutes, the volume ratio of the mobile phase A and the mobile phase B is 95:5; during 5 to 15 minutes, the volume ratio of the mobile phase A and the mobile phase B is uniformly changed from 95:5 to 70:30; during 15 to 35 minutes, the volume ratio of the mobile phase A and the mobile phase B is uniformly changed from 70:30 to 30:70; during 35 to 45 minutes, the volume ratio of the mobile phase A and the mobile phase B is uniformly changed from 30:70 to 15:85; and during 45 to 60 minutes, the volume ratio of the mobile phase A and the mobile phase B is uniformly changed from 15:85 to 95:5. The method can be used for respectively conducting quantitative detection on four impurities in cefoxitin, which is simple and convenient and has strong specificity.
Owner:HAIKOU PHARMA FACTORY
Compositions and Methods for Diagnosing and Treating Community-Acquired Methicillin-Resistant Staphylococcus Aureus
ActiveUS20100197649A1Suitable for topical applicationSugar derivativesMicrobiological testing/measurementCefoxitinMRSA infection
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Process for the preparation of cefoxitin
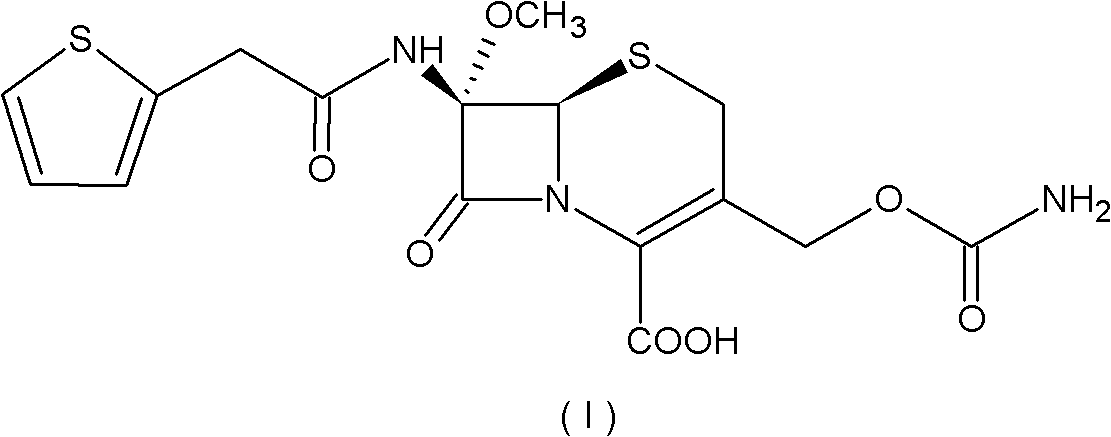
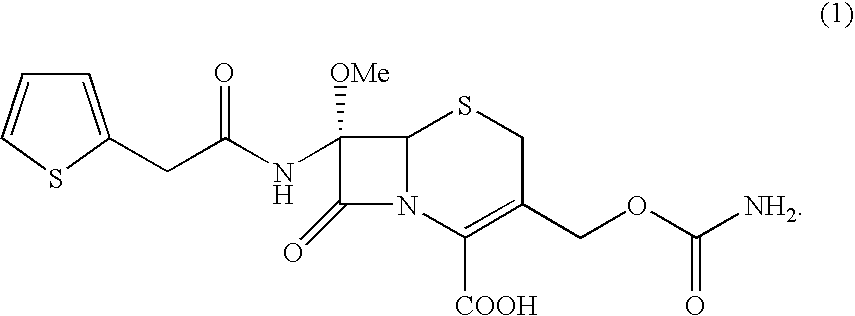
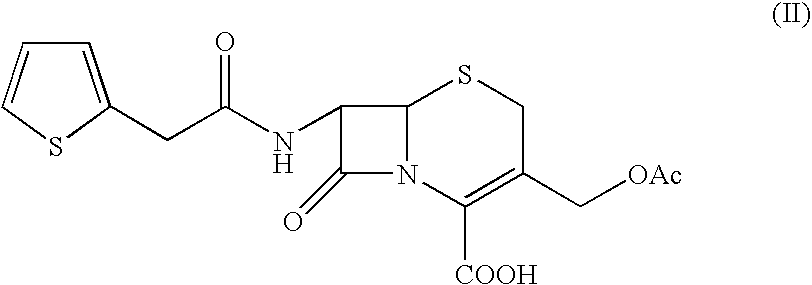
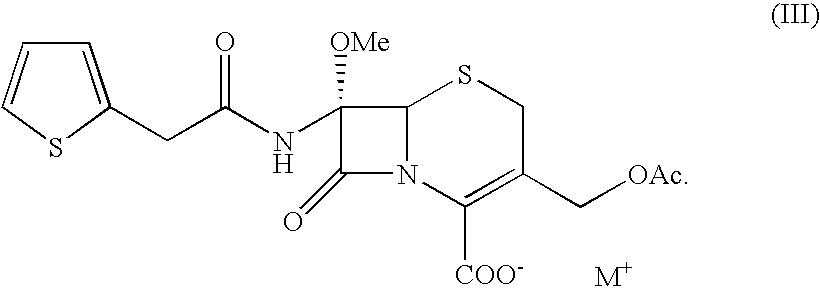
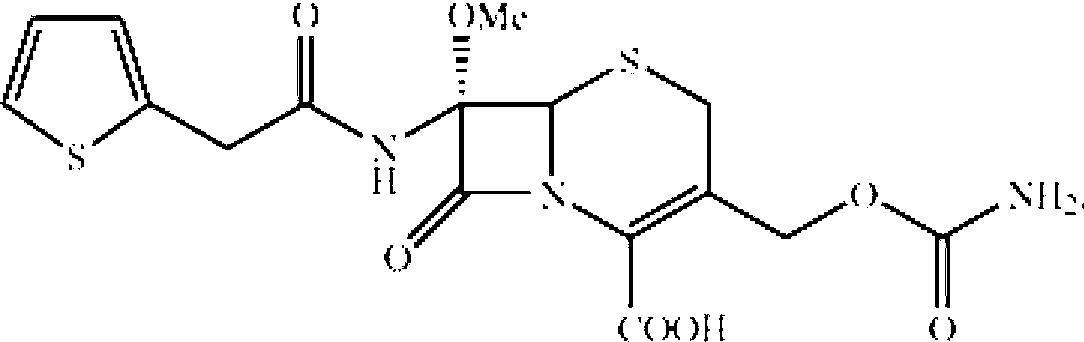
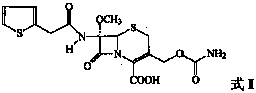
A process for the preparation of cefoxitin of formula (I)The process includes treating the compound of formula (II)with a halogenating agent in an organic solvent, followed by treatment with alkali / alkaline earth metal methoxide at a temperature in the range of −100° C. to 0° C. The product formed is then isolated as an organic amine salt of the formula (III),The salt of formula (III) is treated with a base in the presence of solvent at a temperature in the range of −75 to 10° C., the product formed is isolated as an organic amine salt of the formula (IV)The compound of formula (IV) is carbamoylated with isocyanate of formula (V)RNCO (V)in the presence of a solvent at a temperature in the range of −60° C. to 10° C., and isolating to get cefoxitin of the formula (I).
Owner:ORCHID CHEM & PHARM LTD
Compound injection with probenecid, potassium and beta-lactam antibiotic and its use
InactiveCN1853724AIncrease the total amount of drugReduce dosageAntibacterial agentsOrganic active ingredientsCefotaximePenicillin
A compound powder injection or freeze-dried powder injection is prepared from the sodium (or potassium) salt of probenecid and the beta-lactam kind of antibiotics including penicillin G, ampicillin, ancef, cefuroxime, cefotaxime and cefoxitin. It can elongate the half life of antibiotic, increase the AUC and plasma level and prevent the generation of drug-resistant bacteria.
Owner:吴晓辉
Process for producing 7-alpha-methoxy-3-deacetyled cefoxitin benzathine
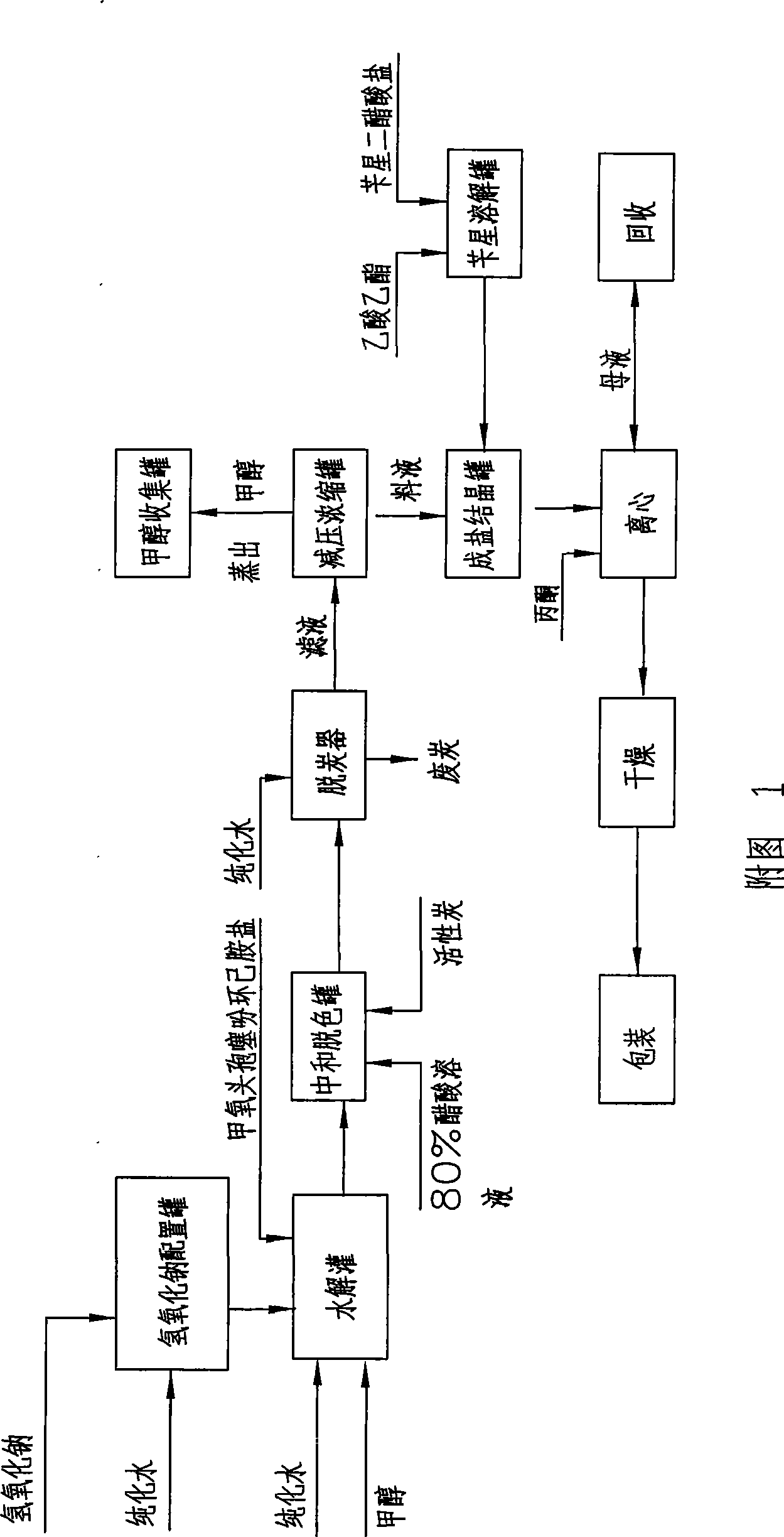
The invention discloses a method for preparing 7-alpha-methoxyl-3-deacetyl cefoxitin benzathine salt by reaction of immobilized deacetylases, which comprises the following steps: adding 7-alpha-methoxyl cefoxitin cyclohexylamine salt into purified water, dissolving the salt, adjusting pH, then carrying out enzymolysis, and adjusting pH of enzymolysis liquor after the enzymolysis is finished; adding ethyl acetate into the neutralized enzymolysis liquor, and adding benzathine diacetate into the solution under stirring; and crystallizing the solution, and then throw-drying and drying the crystal to obtain the product. The method reduces links of decoloring and reduced pressure condensing in the prior art, simplifies operation, reduces consumption, reduces production cost, is convenient for operation, avoids using methanol and sodium hydrate, reduces usage amount of acetic acid, reduces pollution, avoids using liquid nitrogen cold source, and does not need subsequent decoloring process. The material cost of the 7-alpha-methoxyl-3-deacetyl cefoxitin benzathine salt can be reduced by 150 Yuan per kilogram, the production period is shortened to one fourth, and the yield of the product can be improved to 73 percent, so the salt has remarkable social and economic effects, and popularization and application prospects.
Owner:河北九派制药股份有限公司
Process for producing 7-methoxy-3-desacetylcefalotin
ActiveUS20090093032A1Increase production capacityConvenience to workOrganic chemistryBacteriaCefoxitinHydrolysis
Process for producing 7-methoxy-3-desacetylcefalotin by a hydrolysis process which takes place in water and is catalyzed by an enzyme. Cefoxitin can be obtained from this compound by known methods.
Owner:ACS DOBFAR SPA
Method for preparing cefoxitin acid as antibacterial medicament
The invention discloses a method for preparing cefoxitin acid as an antibacterial medicament, comprising the following steps: (1) dissolving 7-aminocephalosporanic acid as a raw material into an alkaline solution, stirring after the alkaline solution becomes clear, adding ethyl acetate, adjusting the pH value, and separating out a crystal to obtain a compound I; (2) carrying out a reaction on the compound I, NBS (N-bromosuccinimide) and sodium methoxide, and introducing a methoxyl to the site 7 of the compound 1 to obtain a mixed solution containing a compound II; (3) carrying out a reaction on the compound II and a 2-thienyl acetylation reagent, introducing thienyl acetyl, and separating out a crystal to obtain a compound III; and (4) enabling the compound III to act with an ammonia methoxyl acylation reagent, and introducing a carbamoyl-methoxyl to the site 3 of the compound III to obtain the cefoxitin acid. The method is simple in preparation process and low in cost, is easy to implement, is suitable for large-scale production, adopts the low-cost raw materials which are easy to purchase and improves the yield of the cefoxitin acid significantly.
Owner:YIYUAN XINQUAN CHEM
Method for producing cefoxitin acid
ActiveCN102925525AEasy to separate and purifySimple stepsOrganic chemistryFermentationSodium methoxideCefoxitin
The invention discloses a method for producing cefoxitin acid. The method comprises the following steps: (1) cephalothin is taken as a raw material to be reacted with a carbamylation reagent in organic solvent, carbamoyloxymethyl is introduced to 3-position, so that an intermediate is produced; and (2) the intermediate is reacted with a methoxylation reagent, and a methoxy group is introduced to 7-position, so that cefoxitin acid is obtained, wherein the methoxylation reagent is one of solid sodium methoxide, methanol-sodium methoxide solution or asolute methanol-beta lactam immobilized enzyme, and the beta lactam immobilized enzyme is a catalyst which consists of beta lactamase and organic or inorganic carrier. The method for producing cefoxitin acid has simple steps and less side reactions, the reagents are easily obtained and can be recycled and reused, reaction conditions are moderate, and the method is suitable for industrial production.
Owner:ZHEJIANG XIANFENG TECH
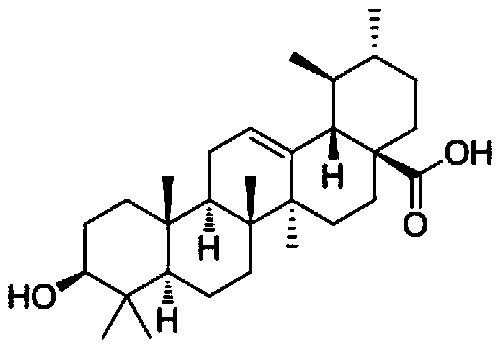
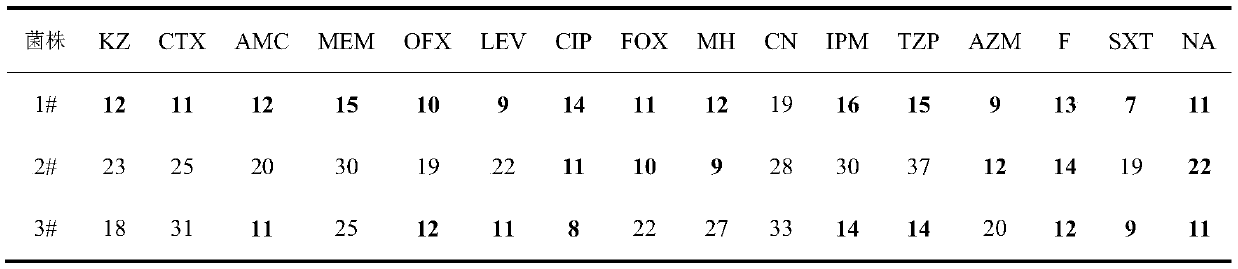
Application of ursolic acid in inhibiting growth of multi-drug-resistance enterobacter cloacae
InactiveCN110279701AMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsOrganic active ingredientsInfections problemsCase fatality rate
The invention discloses application of ursolic acid in inhibiting growth of multi-drug-resistance enterobacter cloacae. According to a good in-vitro killing function of ursolic acid upon anthropogenic multi-drug-resistance enterobacter cloacae which resists to cefazolin, cefotaxime, aupmentn, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocycline, imipenem, piperacillin, azithromycin and macrodantin, growth of multi-drug-resistance enterobacter cloacae can be inhibited, the lowest sterilization concentration is 0.6mg / mL, and the lowest antibacterial concentration is 0.3mg / mL. The invention discloses an inhibition function of the ursolic acid upon the multi-drug-resistance enterobacter cloacae, and the ursolic acid is capable of effectively alleviating or solving the drug-resistance infection problem of the multi-drug-resistance enterobacter cloacae and reducing the case fatality rate, provides new ideas for inhibiting anthropogenic multi-drug-resistance enterobacter cloacae, and has great practical significances.
Owner:SHAANXI UNIV OF SCI & TECH
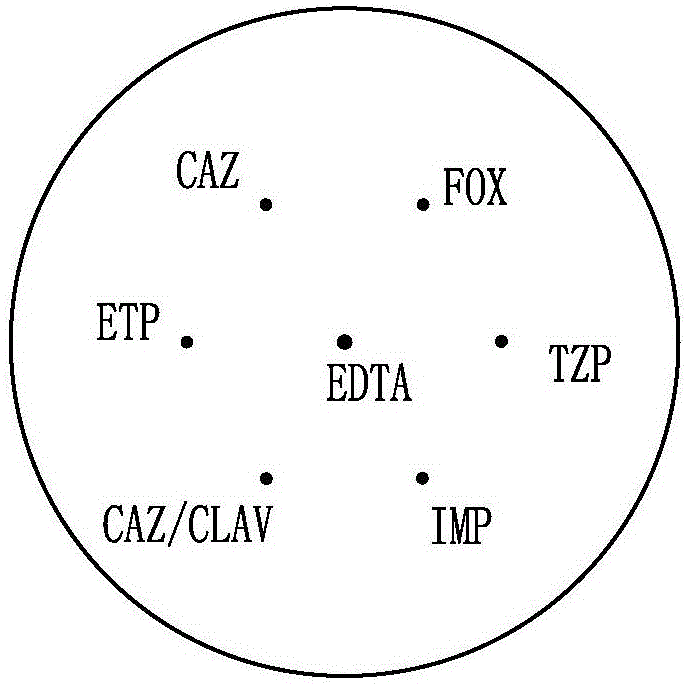
Method for simultaneously classifying and detecting carbapenemase, AmpC enzyme and extended-spectrum beta-lactamase
InactiveCN106047986AAvoid missing detectionIncrease positive rateMicrobiological testing/measurementErtapenemCefoxitin
The invention relates to the technical field of clinical microorganism bacterial detection, in particular to a method for simultaneously classifying and detecting carbapenemase, AmpC enzyme and extended-spectrum beta-lactamase. The method includes the steps that antibiotic test paper and EDTA test paper are selected for use, detected drug-resistant to-be-detected bacteria are prepared into a 0.5 McFarland unit bacterium suspension and evenly smeared to the surface of an M-H plate, the test paper containing EDTA is placed in the center of the surface of the M-H plate coated with the to-be-detected bacteria, ceftazidime (CAZ) antibiotic test paper, ertapenem (ETP) antibiotic test paper, piperacillin / tazobactam (TZP) antibiotic test paper, ceftazidime / clavulanic acid (CAZ / CLAV) antibiotic test paper, imipenem (IMP) antibiotic test paper, and cefoxitin (FOX) antibiotic test paper are attached around the EDTA test paper, gaps are reserved between the EDTA test paper and other antibiotic test paper and between every two pieces of antibiotic test paper, incubation is conducted for 16-18 h at 35 DEG C, and antibacterial zone diameters of the EDTA test paper and other antibiotic test paper are observed and recorded respectively. Cost is low, the detection rate is high, and application and popularization in a clinical laboratory are easy.
Owner:明德松
Method for producing cefoxitin acid
ActiveCN102925525BEasy to separate and purifySimple stepsOrganic chemistryFermentationSodium methoxideCefoxitin
The invention discloses a method for producing cefoxitin acid. The method comprises the following steps: (1) cephalothin is taken as a raw material to be reacted with a carbamylation reagent in organic solvent, carbamoyloxymethyl is introduced to 3-position, so that an intermediate is produced; and (2) the intermediate is reacted with a methoxylation reagent, and a methoxy group is introduced to 7-position, so that cefoxitin acid is obtained, wherein the methoxylation reagent is one of solid sodium methoxide, methanol-sodium methoxide solution or asolute methanol-beta lactam immobilized enzyme, and the beta lactam immobilized enzyme is a catalyst which consists of beta lactamase and organic or inorganic carrier. The method for producing cefoxitin acid has simple steps and less side reactions, the reagents are easily obtained and can be recycled and reused, reaction conditions are moderate, and the method is suitable for industrial production.
Owner:ZHEJIANG XIANFENG TECH
Application of citral in inhibiting growth of multi-drug resistant enterobacter cloacae
ActiveCN110279679AAvoid drug resistanceMitigate or resolve drug-resistant infectionsAntibacterial agentsAldehyde active ingredientsNalidixic acidCefotaxime
The invention discloses an application of citral in inhibiting growth of multi-drug resistant enterobacter cloacae. The citral can inhibit growth of multi-drug resistant enterobacter cloacae as the citral has relatively good in vitro killing action to multi-drug resistant enterobacter cloacae resisting cefazolin, cefotaxime, augmentin, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocyline, imipenem, piperacillin, azithromycin, macrodantin, sulfamethoxazole and nalidixic acid. The minimum bactericidal concentration is 1.6 mg / mL and the minimal inhibitory concentration is 1.0 mg / mL. The invention provides inhibiting action of citral to multi-drug resistant enterobacter cloacae and the citral has wide application value in the field of medicine.
Owner:SHAANXI UNIV OF SCI & TECH
A kind of synthetic method of cefoxitin acid
The invention discloses a synthetic method of cefoxitin acid. Cephalotin acid used as a raw material reacts with prepared tert-butyl hypochlorite in the presence of sodium methylate to obtain a methoxy substance; the methoxy substance is subjected to a hydrolysis reaction and salt formation is carried out in the presence of benzathine to obtain methoxy cefalotin benzathine; methoxy cefalotin benzathine reacts with CSI, and hydrolysis is carried out to obtain a cefoxitin acid crude product; and the cefoxitin acid crude product undergoes the step of recrystallization to finally prepare cefoxitin acid. The synthetic method is simple to operate and is low-cost. By the synthetic method, quality and total yield reach 95.8%. The produced cefoxitin acid is a white solid powder, and purity of the product reaches more than 99.0%. The product has good quality and is suitable for industrial production.
Owner:YANCHENG KAIYUAN MEDICINE CHEM
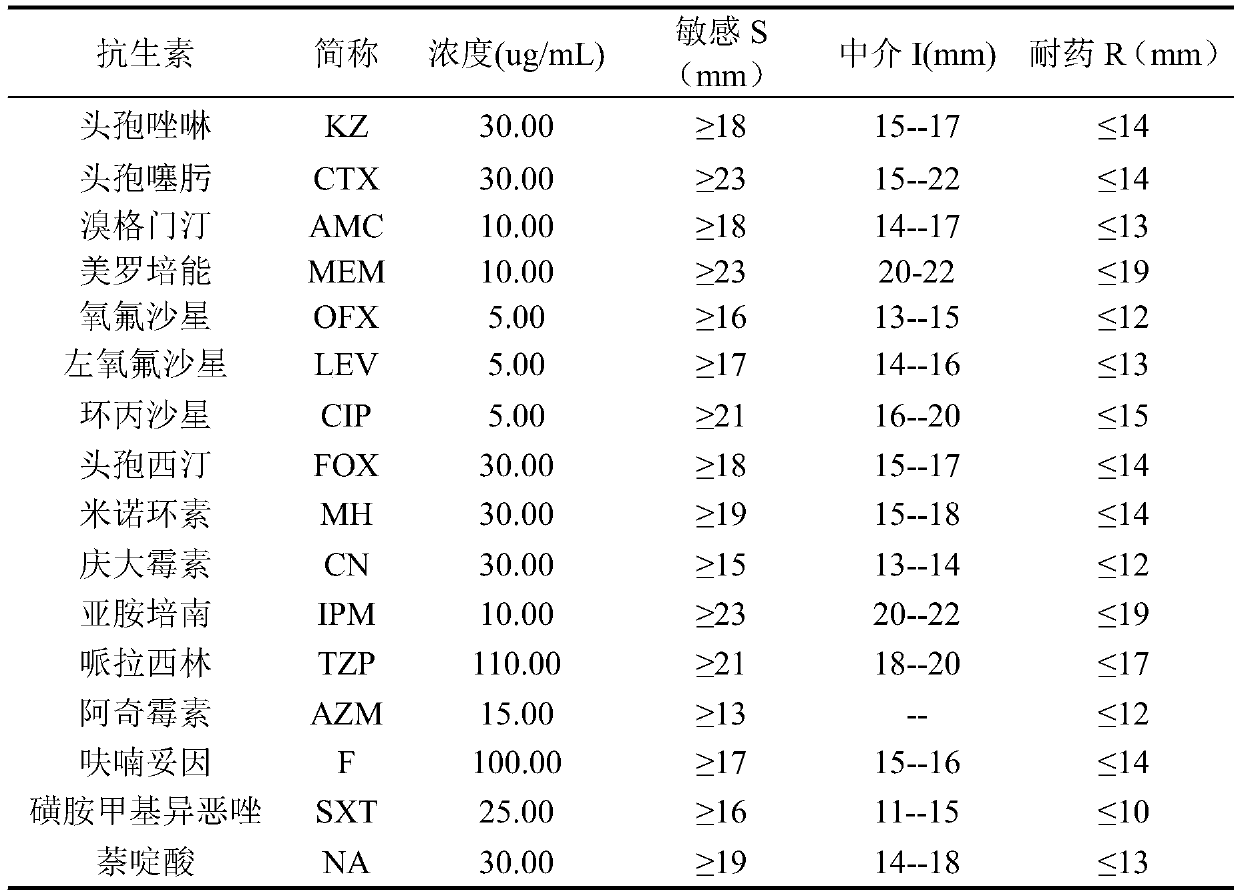
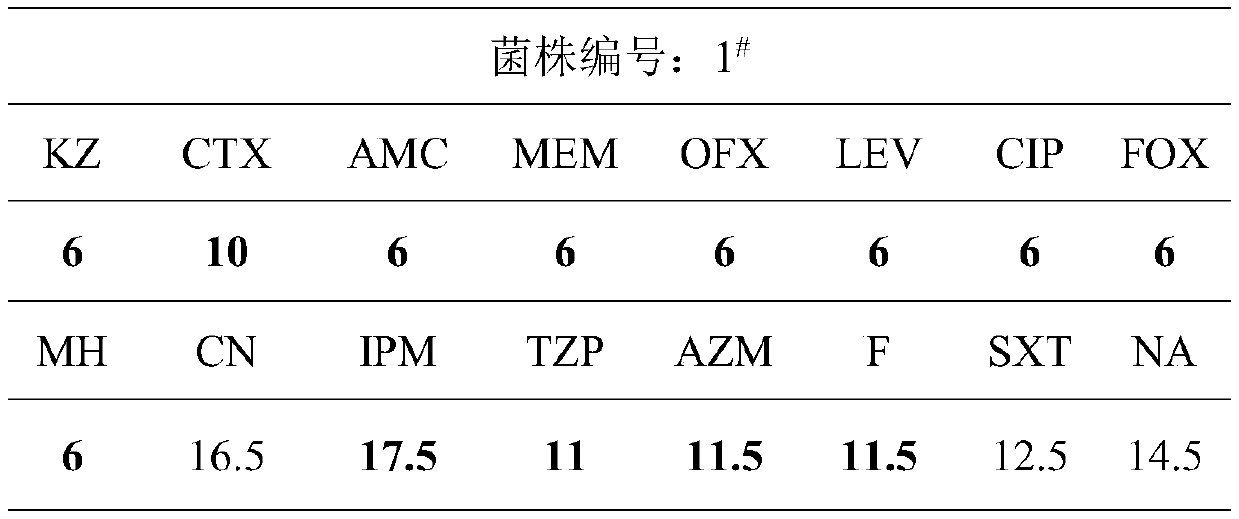
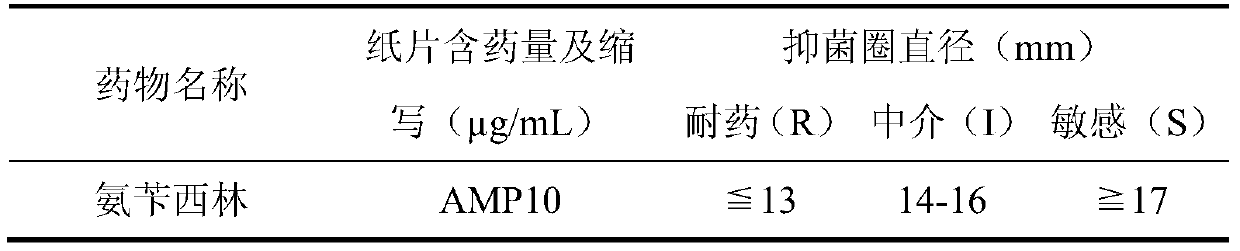
Staphylococcus drug sensitive strip and preparation method thereof
InactiveCN106148179AProvide accuratelyReduce breedingBioreactor/fermenter combinationsBiological substance pretreatmentsAmpicillinPenicillin
The invention discloses a staphylococcus drug sensitive strip which is prepared by the following steps: 1) preparing carnosol water solution; 2) sub-packaging; 3) preparing antibiotic stock solution; 4) sub-packaging liquid materials; 5) adding samples and sub-packaging a strip; 6) drying the strip; 7) performing vacuum packaging; 8) storing the strip. 19 antibiotics are combined on the same detection plate, and MIC (minimal inhibitory concentration) values of the antibiotics are more accurately provided. The antibiotics include gentamicin, rifampicin, erythrocin, clindamycin, compound sulfonamides, vancomycin, penicillin, oxacillin, ampicillin, minocycline, linezolid, gatifloxacin, levofloxacin, cefoxitin, daptomycin, tigecycline, macrodantin, streptomycin and erythrocin / clindamycin. Breeding of drug-resistant strains can be decreased, and drug use of patients is more effectively instructed. The staphylococcus drug sensitive strip functions in monitoring special drug-resistant strain variation and disease prevention and treatment and has an important clinical guiding significance and a great application value.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD +1
A kind of cefoxitin compound and composition thereof
ActiveCN102295654AImprove solubilitySolubility stabilityAntibacterial agentsOrganic active ingredientsCefoxitinChemical compound
The invention relates to a cefoxitin compound. The cefoxitin compound is determined by the method of powder X-ray diffractometry, and characteristic diffraction peaks appear at 5.8 degrees, 7.4 degrees, 8.6 degrees, 13.0 degrees, 14.8 degrees, 15.2 degrees, 16.5 degrees, 18.7 degrees, 23.1 degrees and 25.2 degrees in an X-ray powder diffraction spectrum which is represented by the angle of diffraction 2theta plus or minus 0.2 degree. The invention also relates to a composition of the cefoxitin compound, and the composition comprises 90 to 100 parts of the cefoxitin compound and 0 to 2000 parts of a pharmaceutically acceptable carrier, preferably 0.1 part to 1000 parts of the pharmaceutically acceptable carrier, more preferably 1 part to 500 parts, and most preferably 1 part to 5 parts.
Owner:江西新先锋医药有限公司
Application of preclinical drug metabolism and pharmacokinetics key technology and research system to cefoxitin
The invention provides an application of the preclinical drug metabolism and pharmacokinetics key technology and a research system to cefoxitin. The application includes the steps that 1, after cefoxitin in a certain concentration is fed to an experimental animal for a certain time, one or more biological samples are collected from blood, urine and feces; 2, the biological samples obtained in the step 1 are treated with the liquid-liquid extraction technology, the protein precipitation technology and the solid phase extraction technology, and corresponding solutions are prepared; 3, the solutions prepared in the step 2 are analyzed with liquid chromatogram-mass spectrum (LC-MS) and liquid chromatogram-tandem mass spectrum (LC-MS / MS). According to the application, the membrane permeation performance and the cell absorbing capacity of the cefoxitin can be further detected through Caco-2 cells, or detection of the in-vitro metabolism stability and identification of metabolite of the cefoxitin are achieved through the whole animal, S9, human intestine microsome and monoclonal purifying enzymes.
Owner:刘晓东 +1
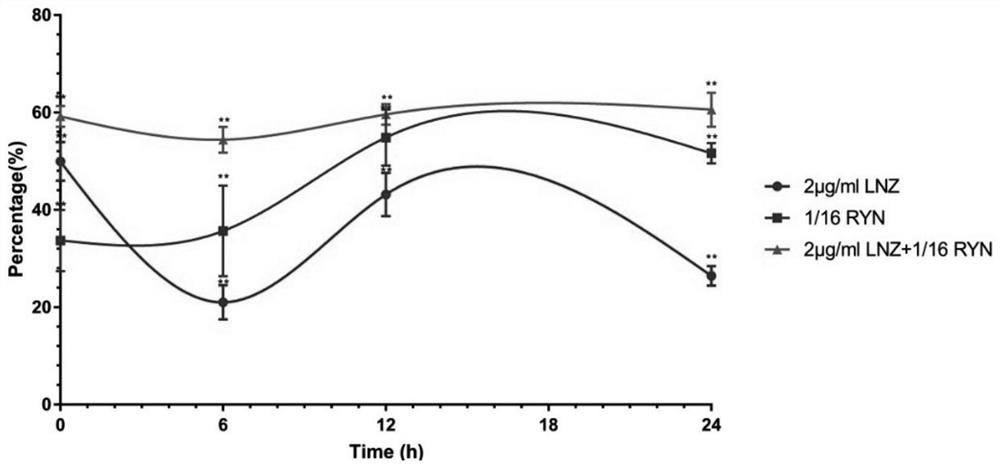
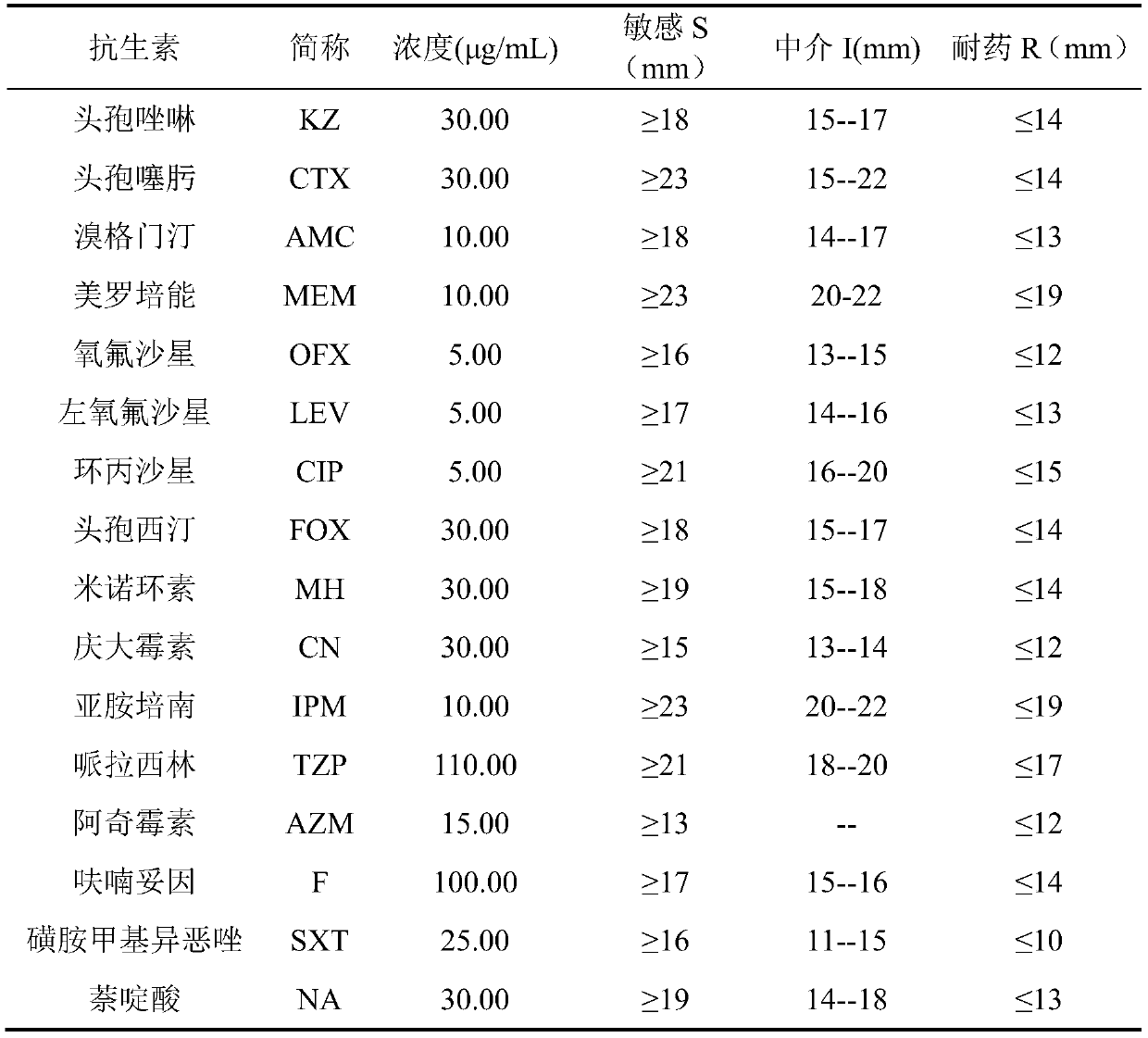
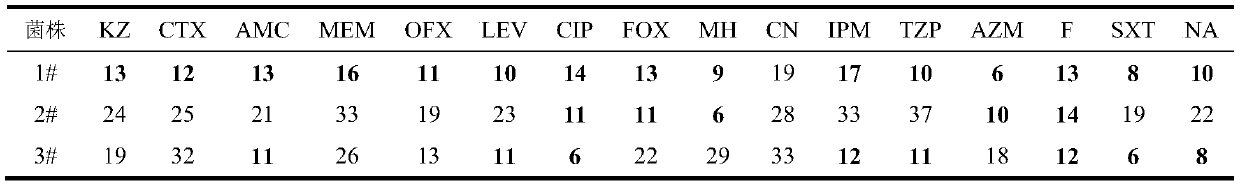
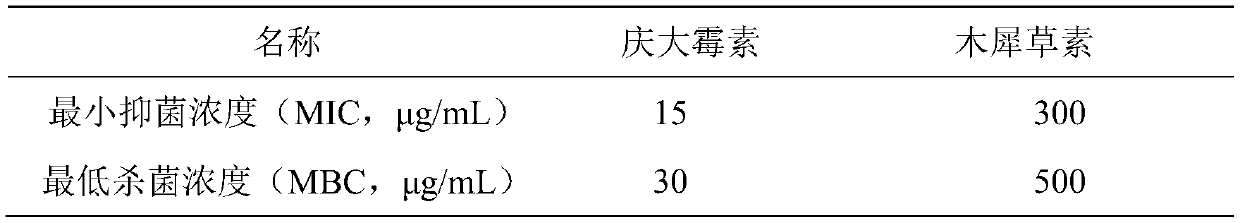
Application of Reyanning combined with antibiotics in interfering antibacterial effect against pathogenic bacteria
ActiveCN111084836BOptimal combined dosageAntibacterial agentsOrganic active ingredientsDiseaseCefoxitin
Owner:TSING HUA DE REN XIAN HAPPINESS PHARMA
Application of luteolin in inhibiting growth of multi-drug resistant enterobacter cloacae
PendingCN111000841AGrowth inhibitionDefinite inhibitory effectAntibacterial agentsOrganic active ingredientsNalidixic acidCefotaxime
The invention discloses application of luteolin in inhibiting the growth of multi-drug resistant enterobacter cloacae. Luteolin has a good in-vitro killing effect on multi-drug resistant enterobactercloacae with resistance to cefazolin, cefotaxime, augmentin, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocycline, imipenem, piperacillin, azithromycin, furantoin, sulfamethoxazole and nalidixic acid, can inhibit the growth of the multi-drug resistant enterobacter cloacae, and has a minimum bactericidal concentration of 0.5mg / mL and a minimum inhibitory concentration of 0.3mg / mL. The invention provides the inhibition effect of luteolin on the multi-drug resistant enterobacter cloacae, and has wide application value in the fields of medicine and the like.
Owner:SHAANXI UNIV OF SCI & TECH
Compositions and methods for diagnosing and treating community-acquired methicillin-resistant Staphylococcus aureus
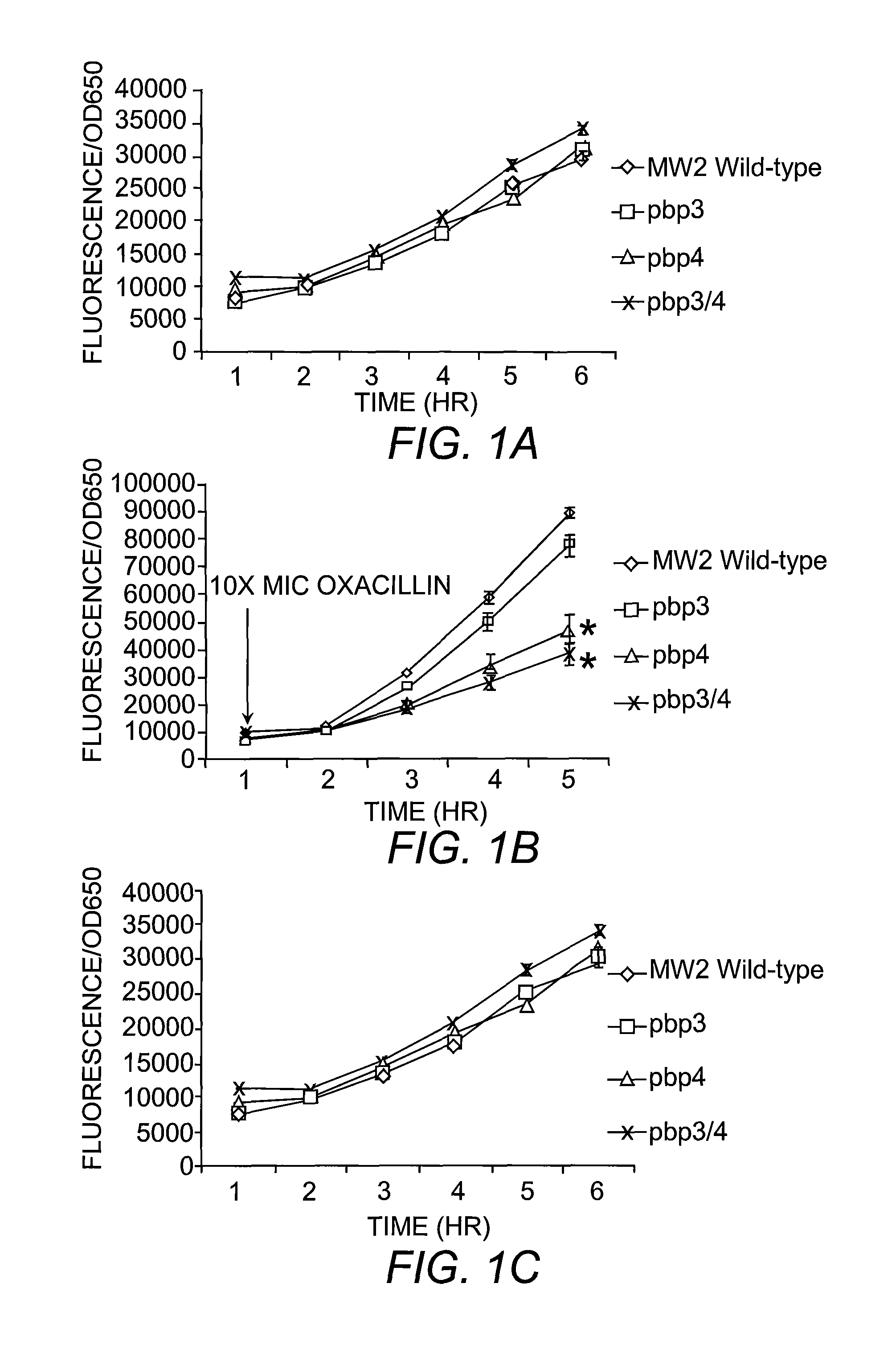
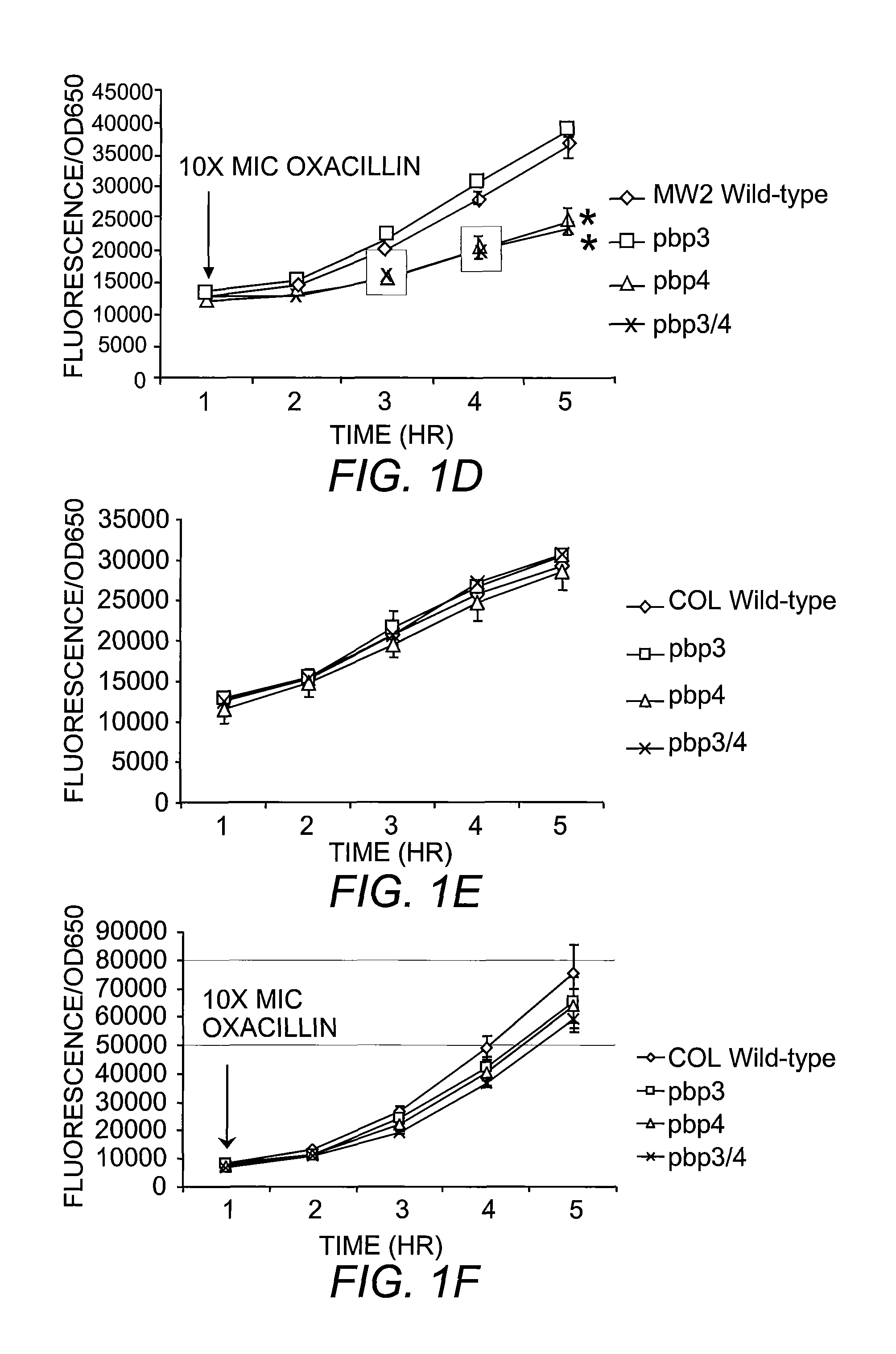
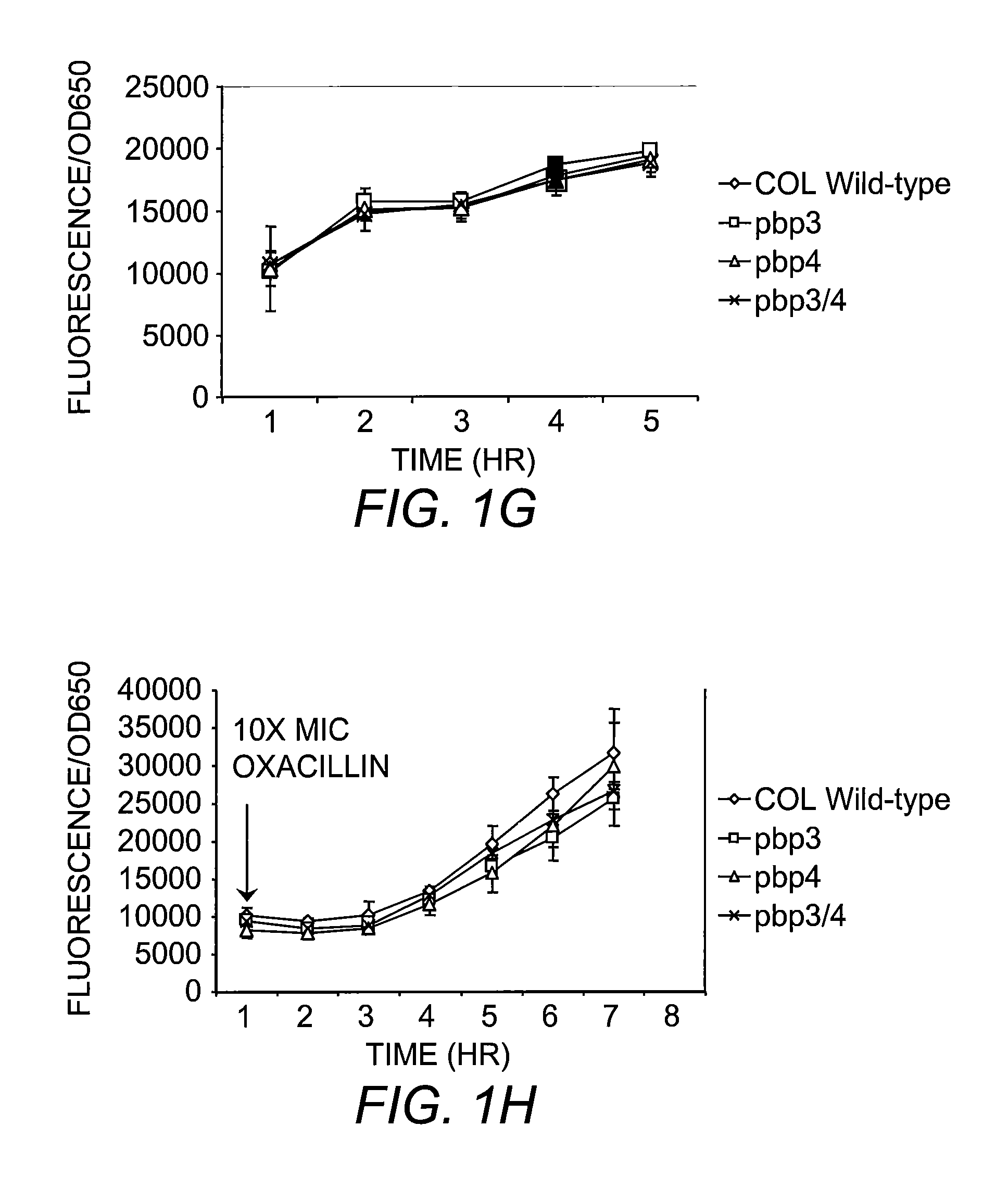
The present invention includes compositions and methods for diagnosing and treating CA-MRSA infections in patients. The methods are based on the finding that combining cefoxitin and a synthetic penicillin in a treatment regimen results in a synergistic effect of the two drugs, an effect that is related to PBP4 activity in CA-MRSA isolates. Also provided is a CA-MRSA-specific biomarker which can be used to detect the presence of a CA-MRSA infection in a patient.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Application of schisandrin in inhibiting the growth of multi-drug resistant Escherichia coli
ActiveCN108113980BMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsEther/acetal active ingredientsEscherichia coliResistance infection
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
Applications of deoxyschizandrin in inhibiting growth of multi-drug-resistance escherichia coli
ActiveCN108113980AMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsEther/acetal active ingredientsEscherichia coliInfections problems
The invention discloses applications of deoxyschizandrin in inhibiting growth of multi-drug-resistance escherichia coli. Deoxyschizandrin has good in-vitro killing action on human multi-drug-resistance Escherichia coli resisting ampicillin, nalidixic acid, cefotaxime, ciprofloxacin, cefoxitin and the like, the deoxyschizandrin can inhibit the growth of multi-drug-resistance escherichia coli at theminimum bactericidal concentration of 25.57 microg / mL and minimum inhibitory concentration of 3.13 microg / mL. The invention provides the inhibition of deoxyschizandrin on human multi-drug-resistanceescherichia coli. The deoxyschizandrin can effectively relieve or solve the drug-resistance infection problem of the multi-drug-resistance escherichia coli, reduce the case fatality rate, thus havingimportant practical significance on providing a new concept for inhibiting the human multi-drug-resistance Escherichia coli.
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
A preparation method of silver-containing carbon dots and the application of the carbon dots in the preparation of antibacterial agents
ActiveCN107926979BGood light stabilitySuitable for mass productionBiocideFungicidesCefoxitinAntimicrobial drug
The invention discloses a preparation method for a silver-containing carbon dot and application of the silver-containing carbon dot to preparation of an antibacterial agent. The preparation method comprises the following steps: with polyethyleneimine (PEI), citric acid and silver nitrate as raw materials, synthesizing a silver-doped functionalized carbon dot solution (a PEI-Ag carbon dot solution)by using a hydrothermal process or microwave process; and then carrying out centrifugation and drying so as to obtain the solid PEI-Ag carbon dot. The preparation method provided by the invention isenvironment-friendly in preparation and uses cheap and easily available raw materials; and the prepared PEI-Ag carbon dot has good biocompatibility and high quantum yield, can be individually used orcooperatively used with antibacterial agents like tetracycline, gentamycin and cefoxitin, and show inhibitory effect on Staphylococcus aureus, Escherichia coli, Candida albicans and the like.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Cefoxitin acid preparation method
The invention discloses a cefoxitin acid preparation method, comprising the following steps: adding 7-alpha-methoxyl-3-deacetylcefalotin benzathine salt in acetone at room temperature, cooling the solution and adding chlorosulfonyl isocyanate to react and then producing the finished product by hydrolysis, extraction, decoloration, concentrated crystallization, filtration, dissolution and secondary crystallization. In the invention, acetone with a consumption of 2.6kg / kg which is easy to recycle is used instead of tetrahydrofuran which is used in the prior art with a consumption of 13kg / kg andthe production cost is reduced by 120 Yuan / Kg. In the invention water phase decarburization is changed to organic phase decarburization, thus eliminating the step of phase inversion in the original process and avoiding the yield loss in phase inversion process; water phase crystallization is changed to the crystallization method which adopts organic phase for concentrating and adds dichloromethane for crystallizing so that the crystallization is realized fully, the yield is higher and the total yield of cefoxitin is increased from 58% to 67%. The material cost of the cefoxitin acid can be reduced by 240 Yuan / Kg, the total reduced cost can be about 360 Yuan / Kg and the product prepared by the method has stronger market competitiveness and remarkable economic benefit.
Owner:河北九派制药股份有限公司
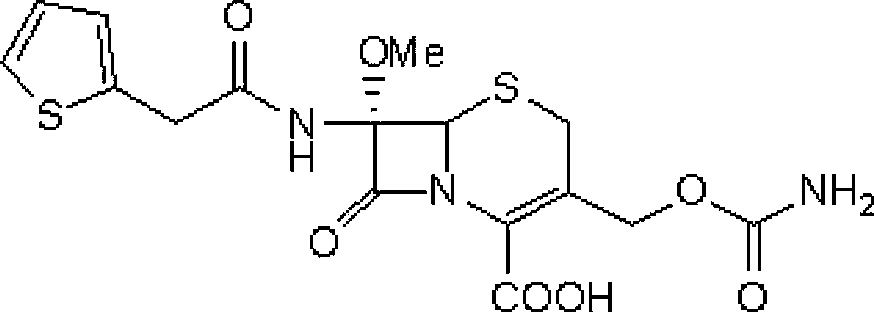
Cefoxitin esterified prodrug compound and oral preparations
InactiveCN102633817ACertain curative effectImprove stabilityOrganic active ingredientsOrganic chemistryCefoxitinDrug compound
The invention discloses an esterified prodrug compound and salt forming compounds and oral preparations thereof. The prodrug compound is shown in the structural formula (IV) in the specification, takes cefoxitin as an active ingredient and can exist in the form of salts. The prodrug compound has the following advantages that a lipophilic group acetoxy-1-halothane is introduced on the basis of cefoxitin to esterify carboxyl to become the esterified prodrug, thus providing an active pharmaceutical ingredient changing the original dosage forms; orally taken, the esterified prodrug not only relieves pains suffered by the patients during injection but also is not only used in professional organizations, thus saving time and widening the application range of the drug; and the esterified prodrug has a confirmed curative effect.
Owner:李莎
Process for producing 7-alpha-methoxy-3-deacetyled cefoxitin benzathine
The invention discloses a method for preparing 7-alpha-methoxyl-3-deacetyl cefoxitin benzathine salt by reaction of immobilized deacetylases, which comprises the following steps: adding 7-alpha-methoxyl cefoxitin cyclohexylamine salt into purified water, dissolving the salt, adjusting pH, then carrying out enzymolysis, and adjusting pH of enzymolysis liquor after the enzymolysis is finished; adding ethyl acetate into the neutralized enzymolysis liquor, and adding benzathine diacetate into the solution under stirring; and crystallizing the solution, and then throw-drying and drying the crystalto obtain the product. The method reduces links of decoloring and reduced pressure condensing in the prior art, simplifies operation, reduces consumption, reduces production cost, is convenient for operation, avoids using methanol and sodium hydrate, reduces usage amount of acetic acid, reduces pollution, avoids using liquid nitrogen cold source, and does not need subsequent decoloring process. The material cost of the 7-alpha-methoxyl-3-deacetyl cefoxitin benzathine salt can be reduced by 150 Yuan per kilogram, the production period is shortened to one fourth, and the yield of the product can be improved to 73 percent, so the salt has remarkable social and economic effects, and popularization and application prospects.
Owner:河北九派制药股份有限公司
Antibacterial drugs cefoxitin preparation process
Owner:SHANDONG SALUBRIS PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com