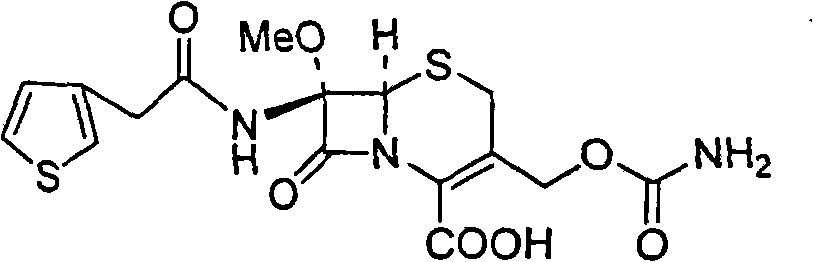
Synthetic method of antibiotic cefoxitin
A cefoxitin and synthesis method technology, applied in antibacterial drugs, organic chemistry, etc., can solve the problems of cefoxitin acid synthesis technology, such as difficulty, no application value, and difficult industrial production, so as to shorten the production cycle and product yield The effect of high efficiency and elimination of wastewater discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
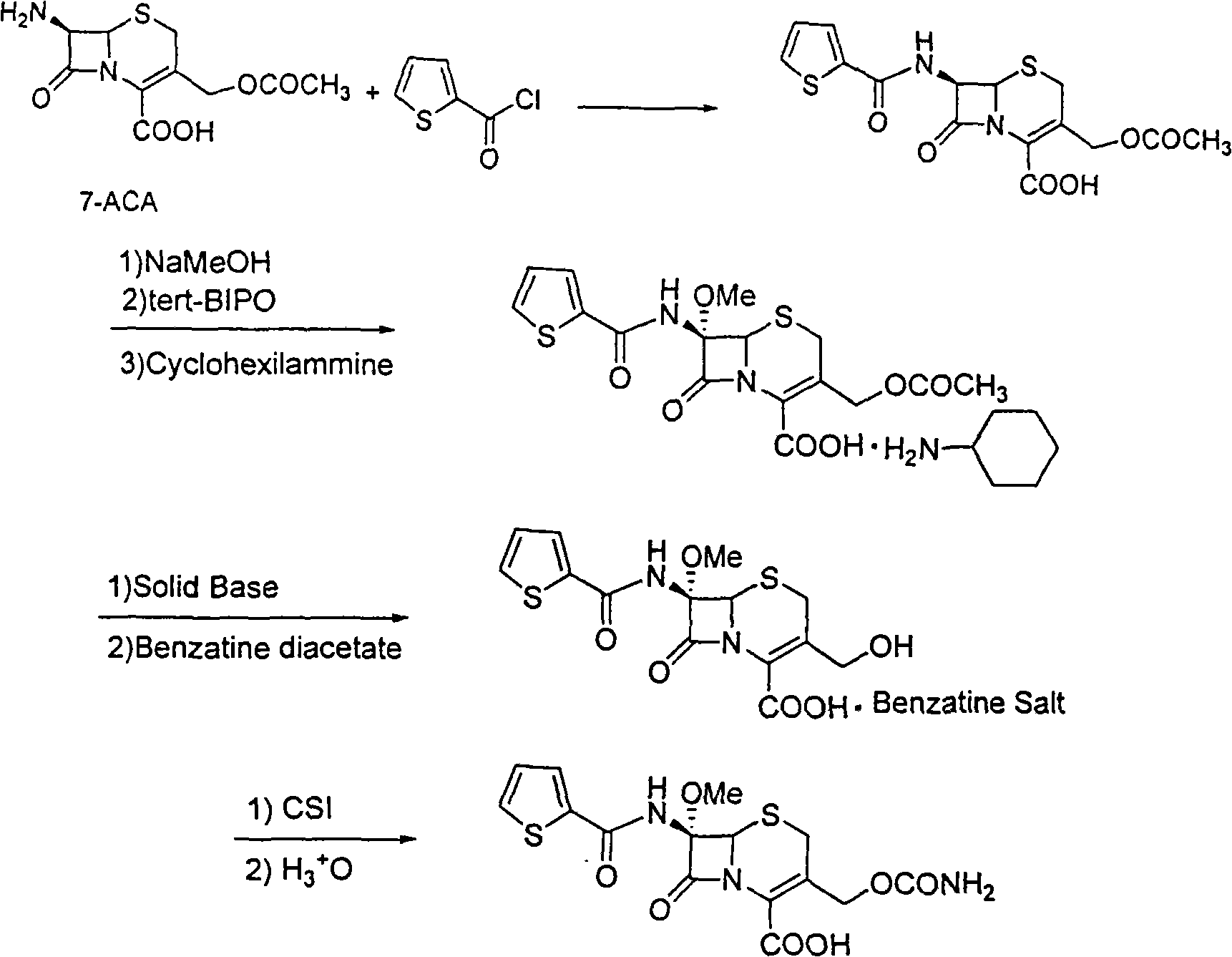
[0014] The synthetic method of antibiotic cefoxitin comprises following processing steps:
[0015] (1) Dissolve 7-ACA in an organic solvent, add alkali, drop thiopheneacetyl chloride for acylation to obtain cephalothinic acid, the temperature of the acylation reaction is -20 to 50°C, and the optimum temperature is -10 ~25°C;
[0016] (2), without purification, the cephalotinic acid obtained in step (1) is directly methoxylated under the action of tert-butyl hypochlorite and sodium methylate to obtain the methoxyl group introduced at the 7-position Intermediate A, under the action of cyclohexylamine, intermediate A can obtain 7-α-methoxycefalotin cyclohexylamine salt; the reaction temperature of intermediate A and cyclohexylamine is 20-50°C, and the optimum temperature is 35°C ~45°C;
[0017] (3), the 7-alpha-methoxycefalotin cyclohexylamine salt obtained in step (2) is reacted with benzathine diacetate under solid alkaloid catalysis to obtain 7-alpha-methoxy -3-Desacetoxy c...
Embodiment 1
[0027] The synthesis of embodiment 1 cephalothin acid
[0028] In the flask, add 40g of 7-aminocephalosporanic acid (7-ACA), 17.2g of triethylamine, and 240mL of dichloromethane, control the temperature at 0-10°C, add 27.2g of thiopheneacetyl chloride dropwise under stirring, and dropwise add complete.
[0029] Then keep the temperature and react for 5 hours. After the reaction is finished, filter to remove the solid, and the filter cake is washed with 100 mL of dichloromethane. Combine the filtrate and lotion, and evaporate to dryness to obtain 56 g of cephalothinic acid, with a yield of 1.4%. In actual production, the combined filtrate and washings can be directly used in the next reaction.
[0030] Synthesis of 7-α-Methoxycefalotin Cyclohexylamine Salt
[0031] In the flask, add 60 g of cephalothinic acid, 200 mL of tetrahydrofuran, and 500 mL of dichloromethane, and stir for 10 min to completely dissolve the cephalothinic acid. Cool to -85°C with liquid nitrogen, and a...
Embodiment 2
[0038] The synthesis of embodiment 2 cephalothinic acid
[0039] In the flask, add 40g of 7-aminocephalosporanic acid (7-ACA), 30.2g of N,N-dimethylaminopyridine, 250mL of ethyl acetate, control the temperature at 0-10°C, and add 27.2g of thiopheneacetyl chloride dropwise under stirring (0.17mol), the dropwise addition was completed in three hours.
[0040] Then keep the temperature and react for 5 hours. After the reaction is finished, filter to remove the solid, and the filter cake is washed with 100 mL of ethyl acetate. Combine the filtrate and lotion, and evaporate to dryness to obtain 52 g of cephalothinic acid, with a yield of 1.3%. In actual production, the combined filtrate and washings can be directly used in the next reaction.
[0041] Synthesis of 7-α-Methoxycefalotin Cyclohexylamine Salt
[0042] In the flask, add 60 g of cephalotinic acid, 200 mL of DMF, and 500 mL of dichloromethane, and stir for 10 min to completely dissolve the cephalothinic acid. Cool to -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com