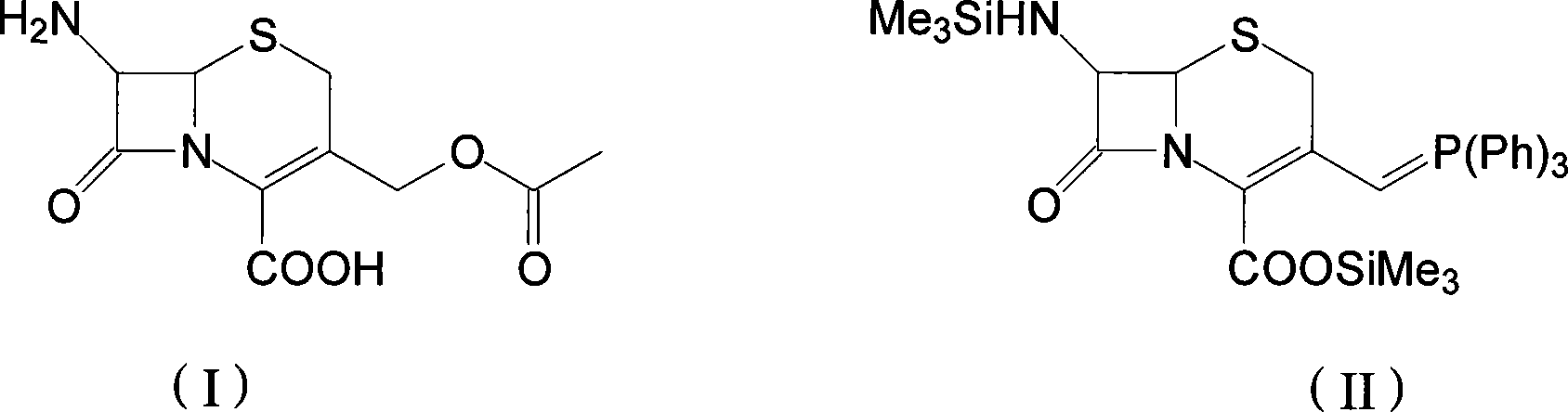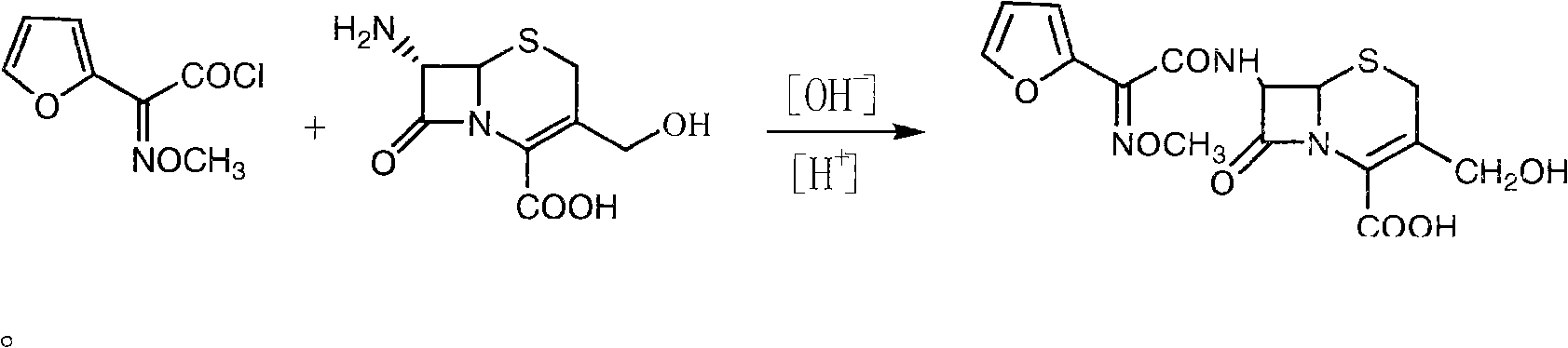Patents
Literature
153 results about "7-ACA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
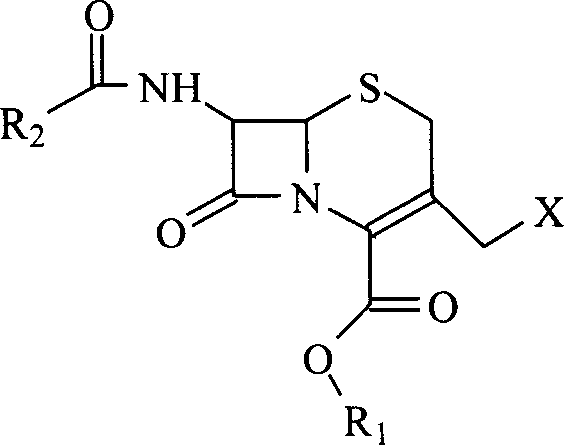
7-ACA (7-aminocephalosporanic acid) is the core chemical structure for the synthesis of cephalosporin antibiotics and intermediates. It can be obtained by chemoenzymatic hydrolysis of cephalosporin C.
Method for preparing cephalosporin propylene
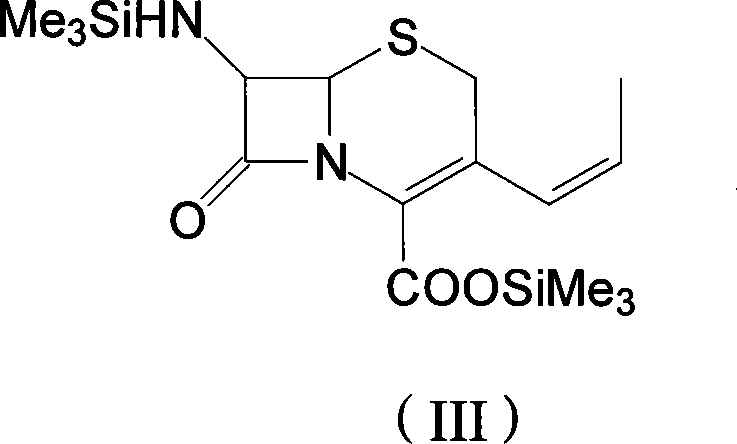
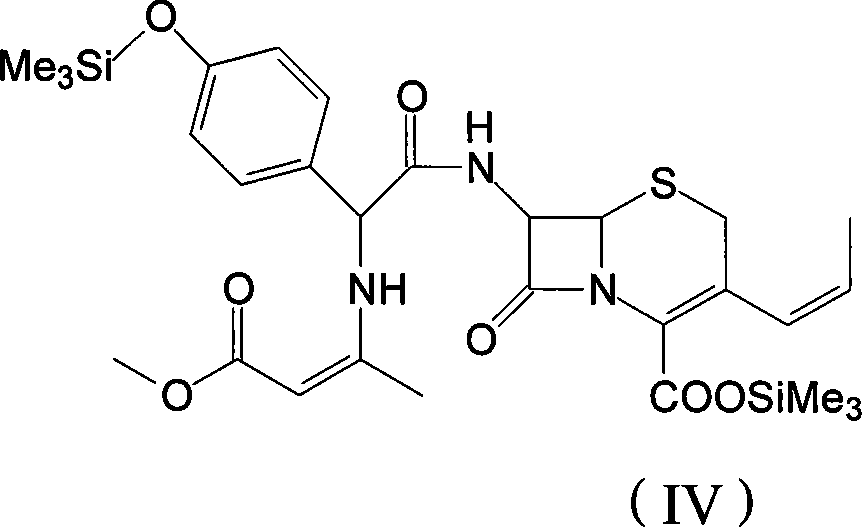
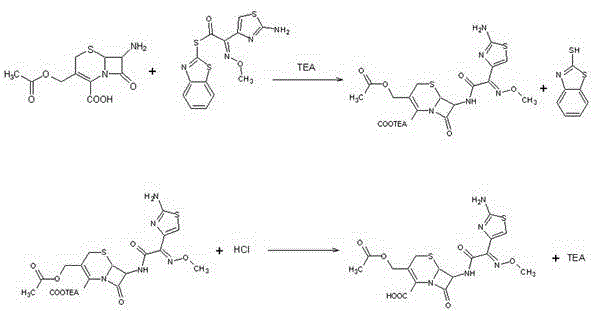
The invention discloses a preparation method of cefprozil, which comprises: 7-amin cethalosporanic acid (7-ACA) reacts with triphenyl phosphine to get 7- trimethylsilyl amino-3-triphenyl phosphate methylene-4-cethalosporanic acid trimethylsilyl ester through silanization protection and iodination reagent replacing under the condition of catalyst existing; WITTIG reaction is made for the product and acetaldehyde to get 7-trimethylsilyl amino -3-(propenyl-1-alkenyl)-4-cethalosporanic acidtrimethylsilyl ester; then the compound reacts with D-para hydroxybenzene glycine dane potassium salt to geta compound (6R, 7R)-7-[(2R)-2- ethoxycarbonyl-1-methyl - ethylene amino (4-trimethylsilyl oxyphenyl) acetamido group(amide)]-8-oxo-3- (1- propenyl)-5-thio-1- heterobicycle [4.2.0] octylene-2-ene-2-carboxylic acid trimethylsilyl ester; hydrolytic treatment is then used to get the cefprozil. The invent adopts the method of one pot and can participate in next reaction without separating intermediateproducts. The preparation method of cefprozil has the advantages of low cost, convenient operation and high overall yield, adapting to demands of industrial production.
Owner:南通康鑫药业有限公司
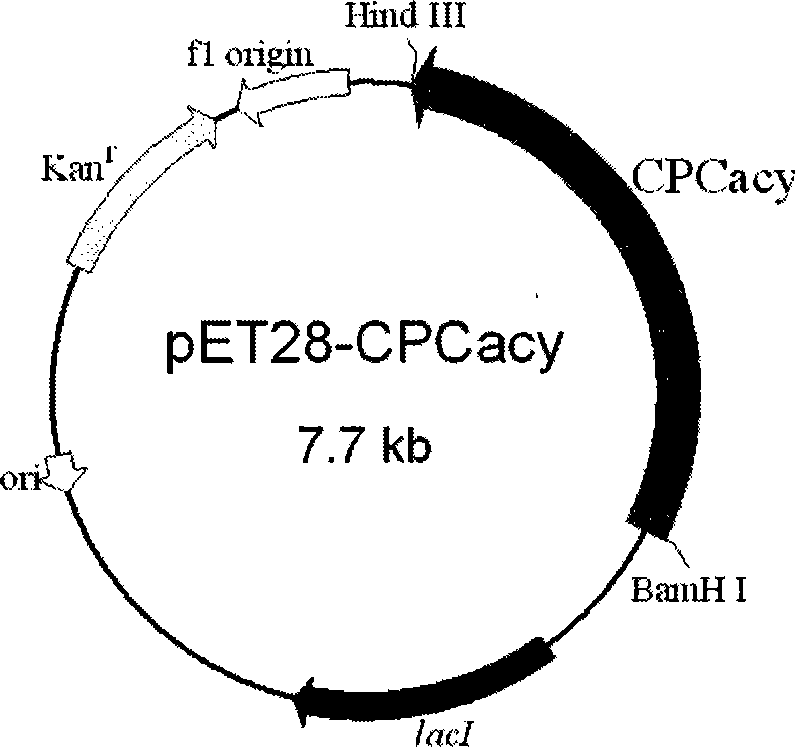
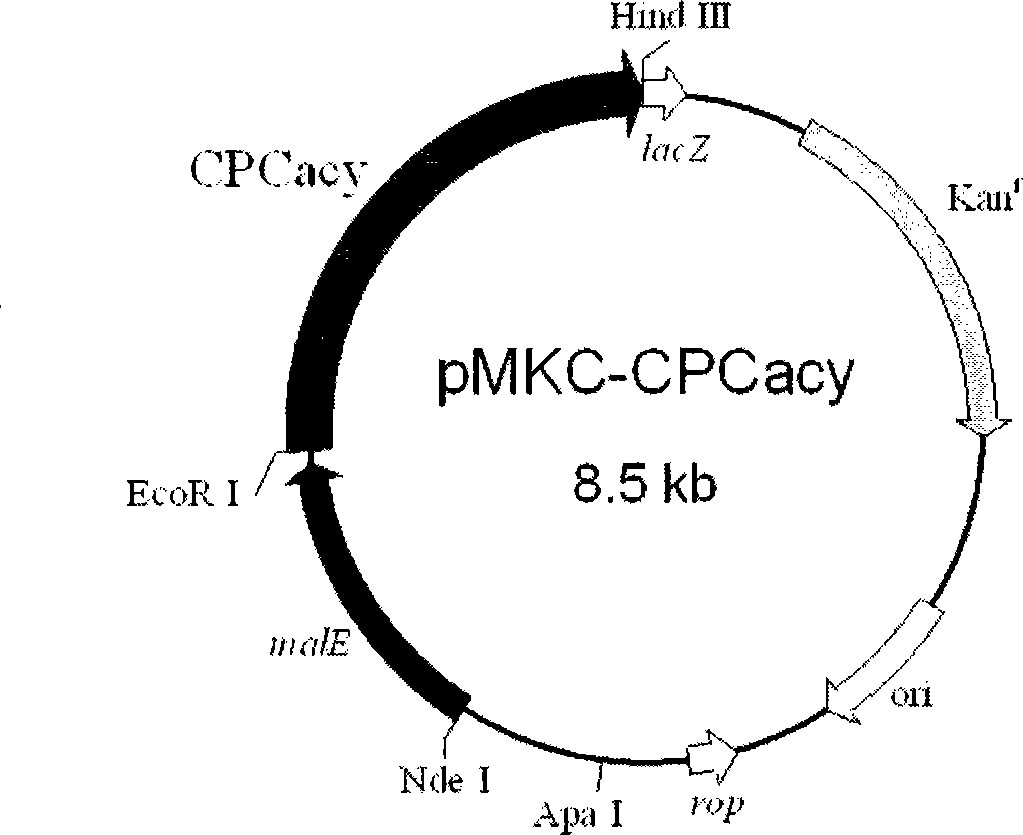
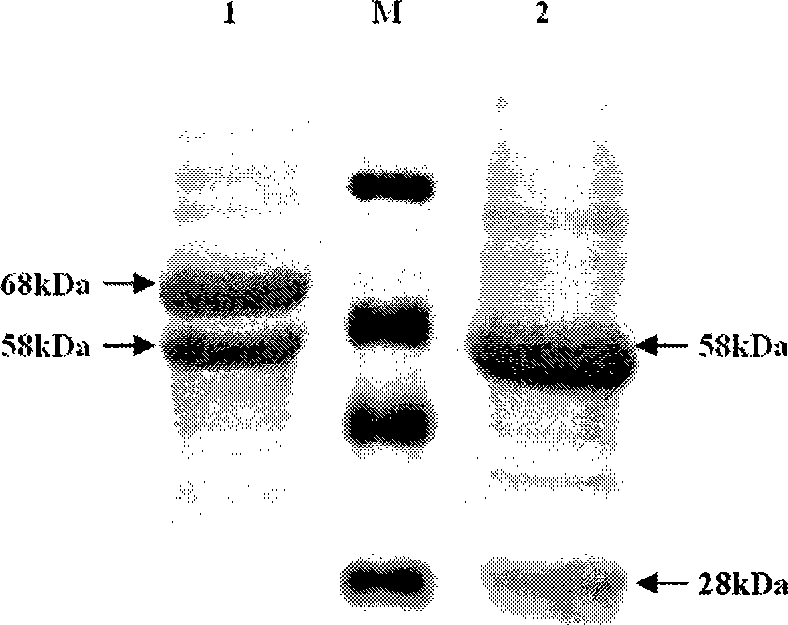
Cephalosporin C acrylase and its vector and application
InactiveCN101240285AHigh gene expression activityHigh expression activityBacteriaMicroorganism based processes7-ACAMicrobiology
The invention discloses a CPC acylation enzyme whose encode gene is DNA sequence shown as SEQ IN NO: 4 and DNA sequence having at least 80154000mology with the above DNA sequence. The invention also discloses carriers and transformants containing coding CPC acylation enzyme and the use of the enzyme. The enzyme has high expressing activity, high catalytic activity and high outcome tolerance to catalysis bottom object in high efficiency to prepare outcome 7-ACA.
Owner:TSINGHUA UNIV
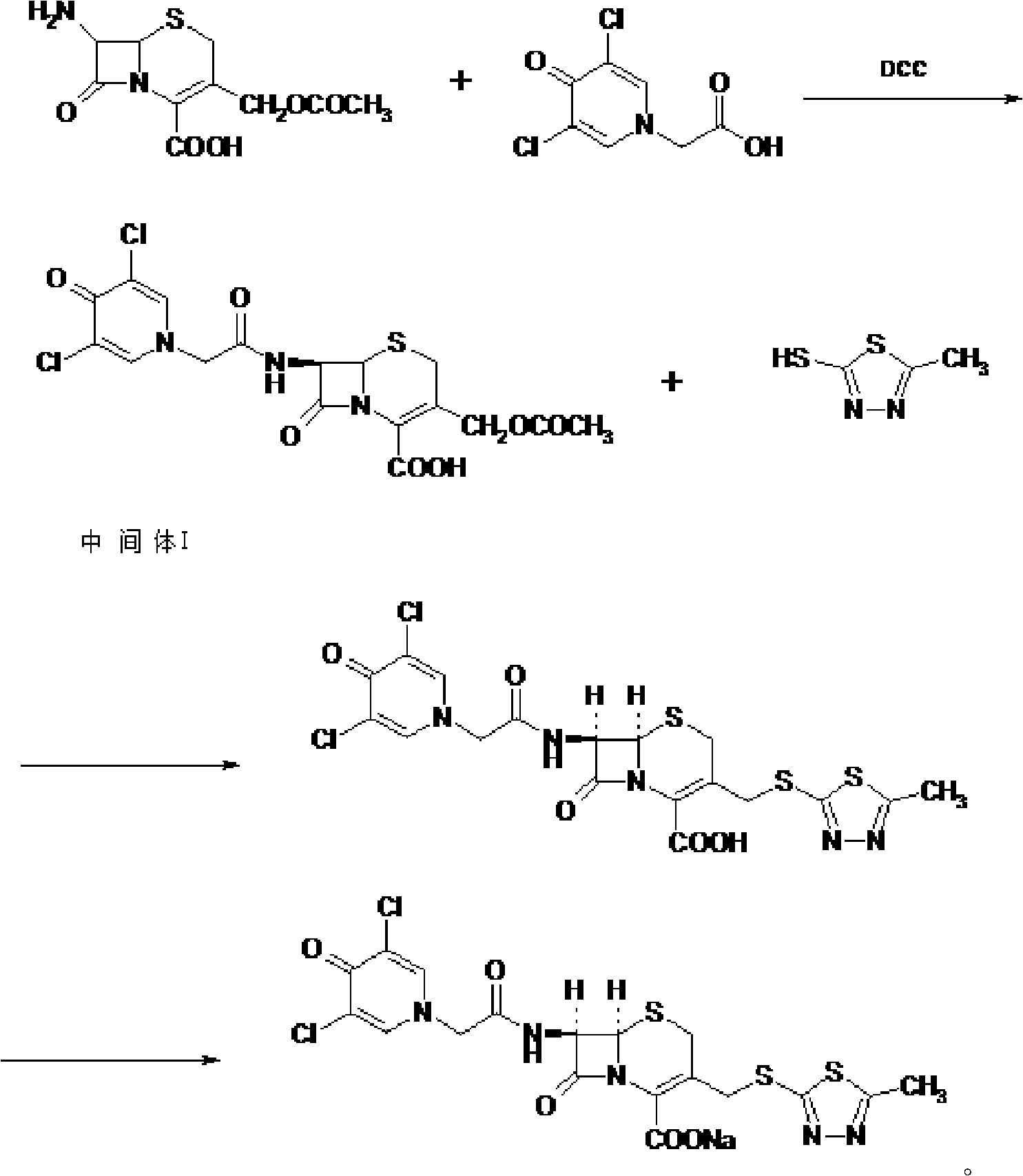
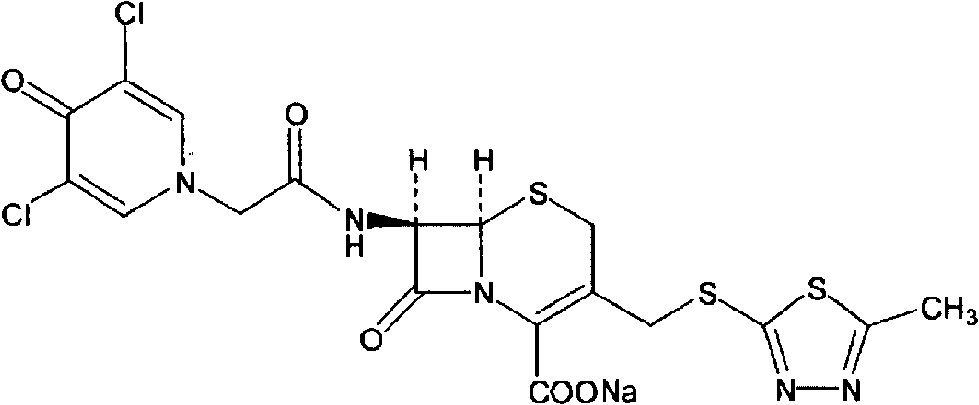
Cefazedone sodium medicament powder injection and method for synthesizing raw medicine of Cefazedone sodium
ActiveCN101584671ASingle componentImprove solubilityAntibacterial agentsPowder deliverySodium bicarbonateNitrogen gas
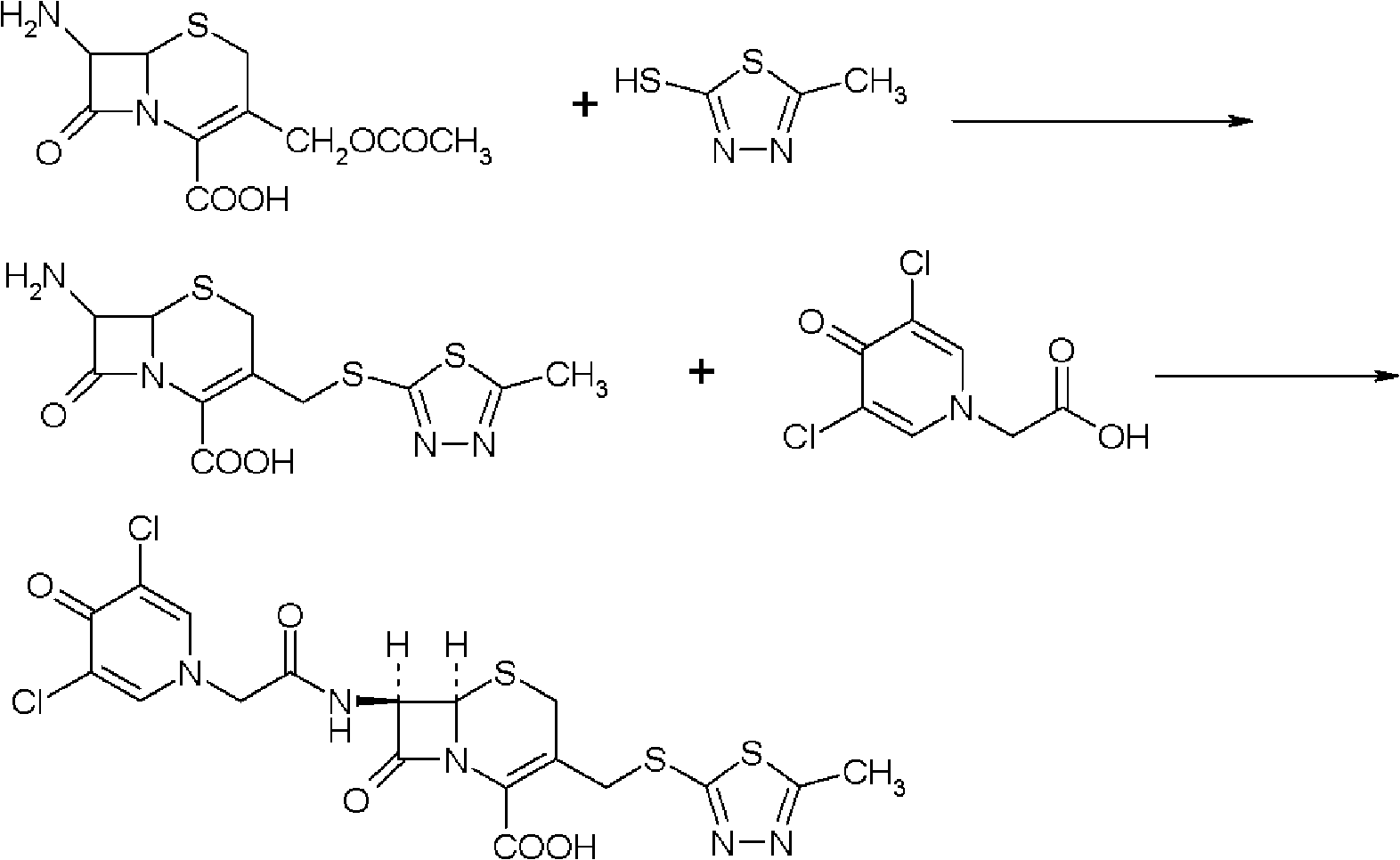
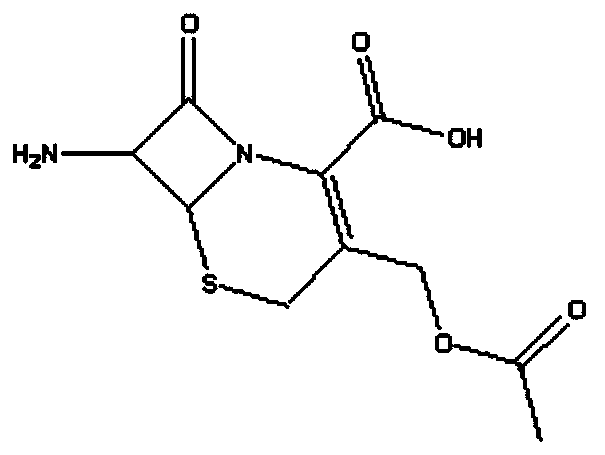
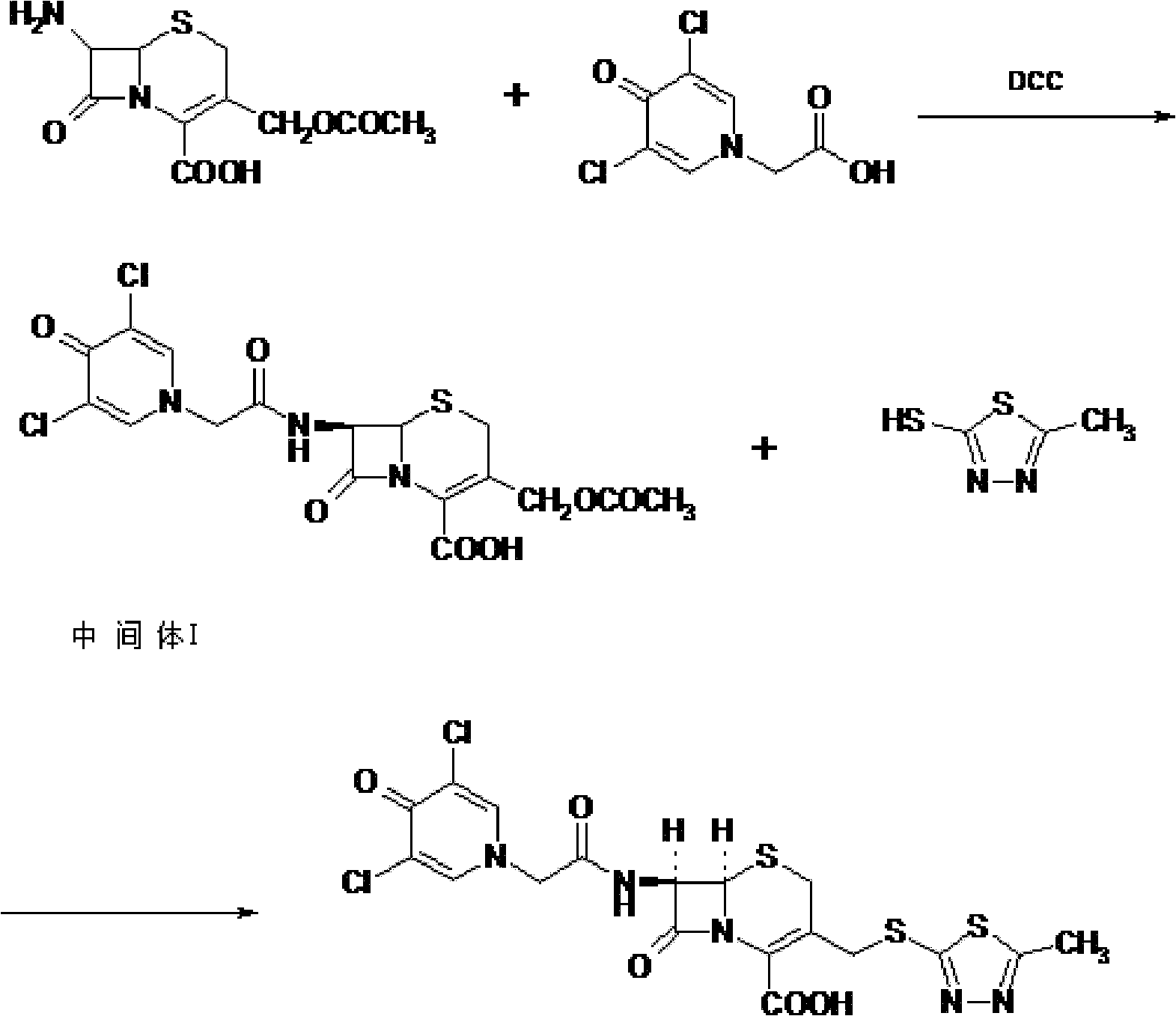
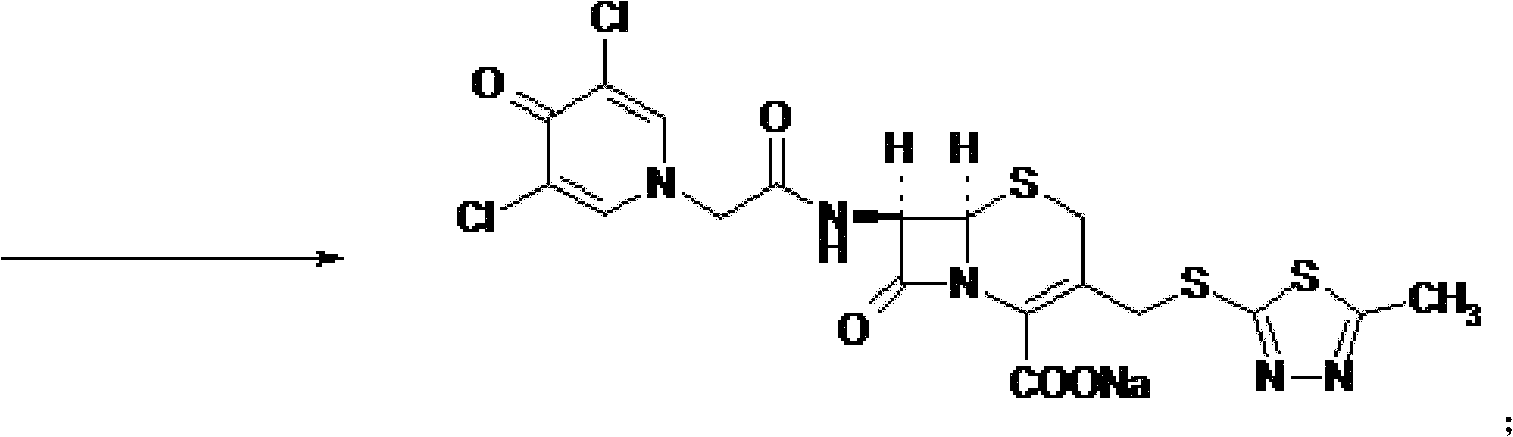
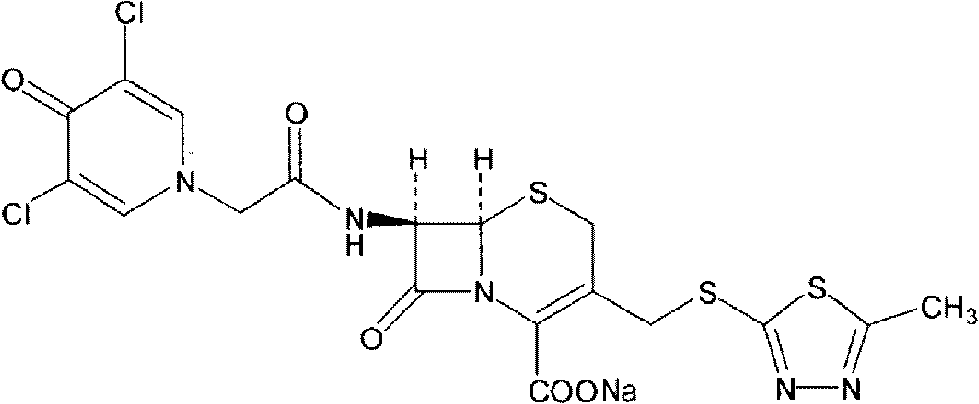
The present invention provides a cefazedone sodium medicament powder injection composed of 100% of cefazedone sodium. The cefazedone sodium is prepared by a method as follows: (1) 7-ACA and 3, 5-dichloro pyridine acetic acid react with each other with the action of an anhydrating agent, a mixture after the reaction is post-processed to obtain an intermediate product I; (2) the intermediate product I and 2-mercapto-5-methyl-1, 3, 4-thiadiazoles react with each other with the protection of nitrogen at a temperature of 50 to 90 DEG C, a mixture after the reaction is purified to obtain a water solution which is added with an inorganic acid to regulate pH value to be equal to 1 to 3, a precipitation is extracted from the water solution and is post-processed to obtain cefazedone; (3) the cefazedone and sodium hydrogen carbonate react with each other in water to obtain a cefazedone sodium solid body after an aftertreatment. The powder injection has single component and perfect dissolution performance, the raw medicine has a short synthetic route, the aftertreatment of the intermediate product or final product are all simple, and the yield and purity of the whole reaction process are all high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Process for preparing cephalosporins with salified intermediate
InactiveUS20050119244A1Promote crystallizationOrganic active ingredientsOrganic chemistry7-ACASilanes
Cephalosporins may be conveniently prepared by a process in which 7-ACA is silylated, acylated, desilylated and then salified to give an intermediate which is eventually cyclized with thiourea.
Owner:ACS DOBFAR SPA
Synthetic method of antibiotic cefoxitin
ActiveCN101555252AEmission reductionEliminate emissionsAntibacterial agentsOrganic chemistryCefoxitinAlkaloid
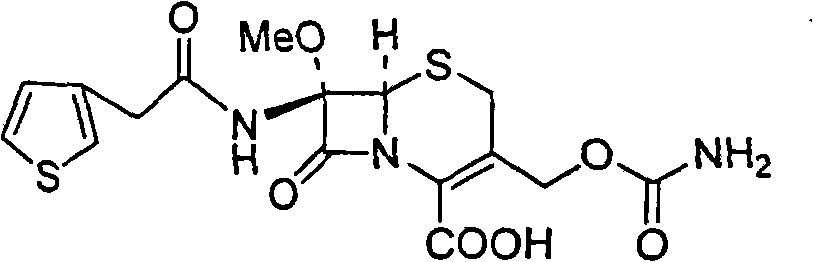
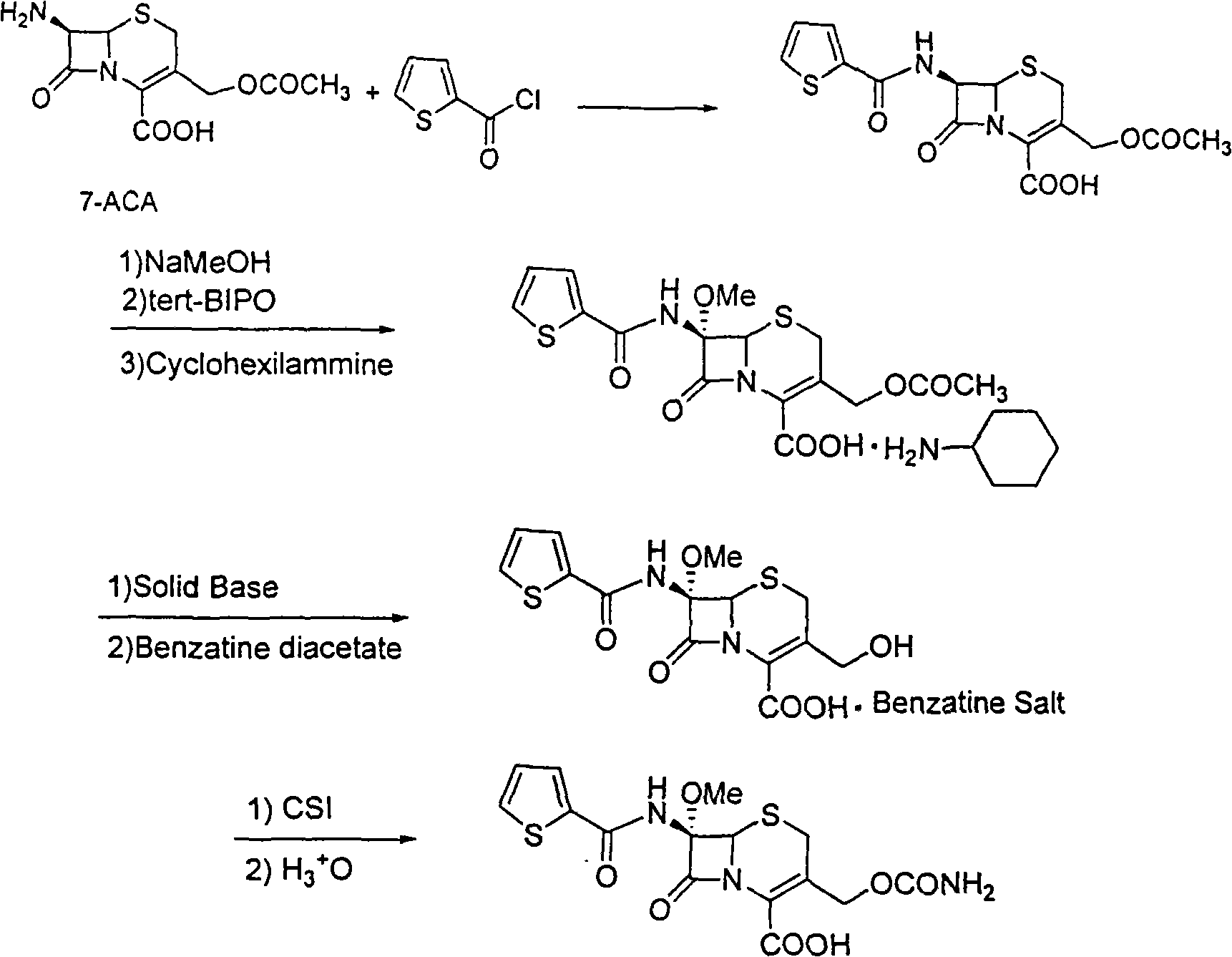
The invention relates to a synthetic method of antibiotic cefoxitin. 7-ACA is taken as a starting material and firstly reacts with thiopheneacetyl chloride, cephalothin acid is obtained by separation; the cephalothin acid is not purified, and the methoxylation is directly carried out on the cephalothin acid to obtain an intermediate A which introduces methoxy on the position of 7, the intermediate A obtains 7-alpha-methoxy cephalothin cyclohexylamine salt under the action of cyclohexylamine; the 7-alpha-methoxy cephalothin cyclohexylamine salt reacts with benzathine diacetate under the catalysation of solid alkaloid to obtain 7-alpha-methoxy-3-deacetoxy cephalothin benzathine salt; and the carbamylation is carried out under the action of chlorosulfonyl isocyanate to obtain the cefoxitin. The synthetic method has the advantages that compared with the prior art, the synthetic method simplifies the operation process, has high product yield, reduces the production cycle, eliminates the discharge of waste water containing organic solvent and reduces the production cost. The product quality is stable, and the synthetic method is applicable to the large-scale industrial production.
Owner:国药集团致君(苏州)制药有限公司
Novel method for synthesizing cefoperazone sodium compound
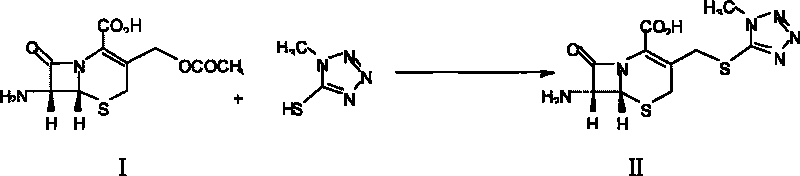
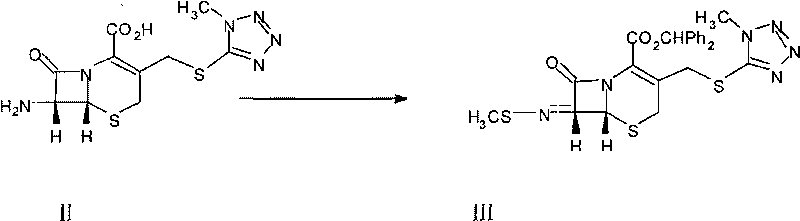
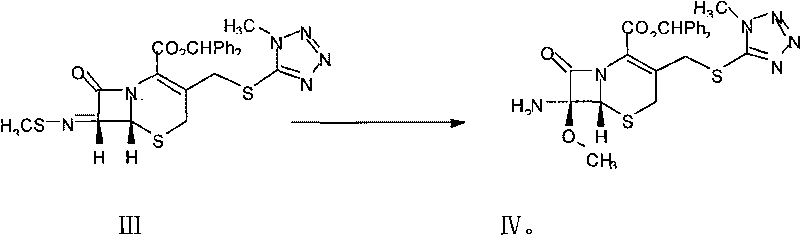
The invention relates to a novel preparation method for (6R,7R)-3-[[(1-methyl-1H-tetrazole-5 radial)sulphur]methyl]-7-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazine carbon amido)-2-p-hydroxyl phenyl-acetamido]-8-keto-5-thia-1-polyaza[4.2.0]octane-2-alkene-2-sodiumformate(cefoperazone sodium) shown as a formula (I). The formula (I) is shown in the specifications. The invention aims to provide a novel method for synthesizing a cefoperazone sodium compound. The method has the advantages of synthesis of TZA from 7-ACA (Acetic Acid) and 1-methyl-5-sulfydryl tetrazole, mild reaction condition, easiness for operating, one-step use of recyclable dimethyl carbonate serving as an environmentally-friendly solvent, great saving in the cost, high yields of products prepared in each step, good quality, low cost, high product purity and suitability for industrial production.
Owner:哈药集团股份有限公司 +1
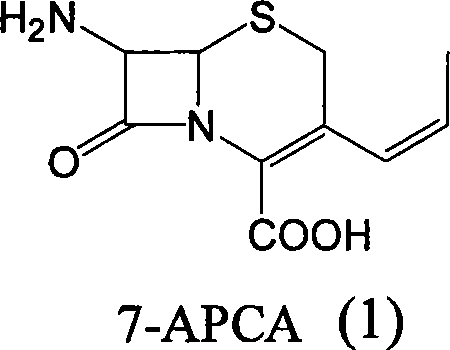
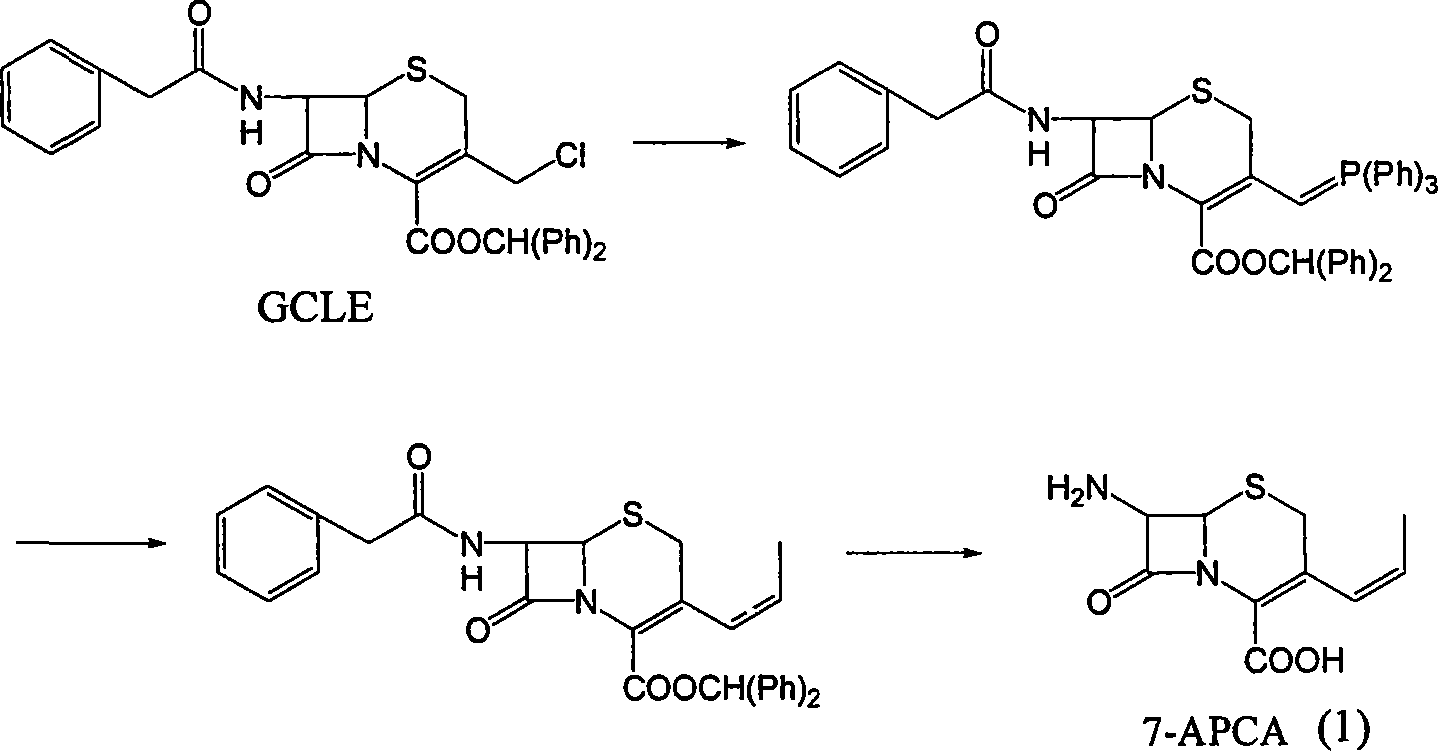
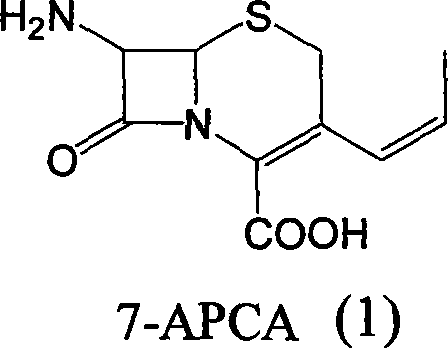
Method of preparing cefprozil parent nucleus 7-amino-3-propenylcephalosporanic acid
The invention discloses a new making method of cepham propone parent nucleus 7-amino-3-propenyl cepham alkyl acid (7-APCA), which comprises the following steps: reacting 7-ACA(2) protected by silicane and substituted by iodine with triphenylphosphor in the solvent during -10-120 deg. c; obtaining the compound (3); reserving the compound (3) in the alkaline metal salt; reacting the compound (3) and acetaldehyde to obtain the compound (4); crystallizing the compound (4) through removing the protector; obtaining the product to synthesize the cepham propone.
Owner:SHANGHAI JUNJIE BIOCHEM TECH
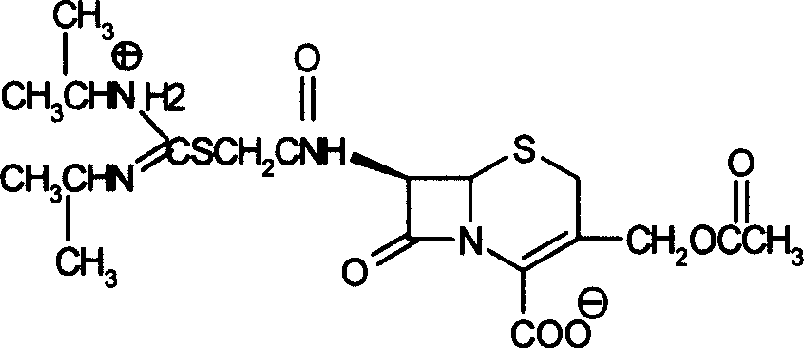
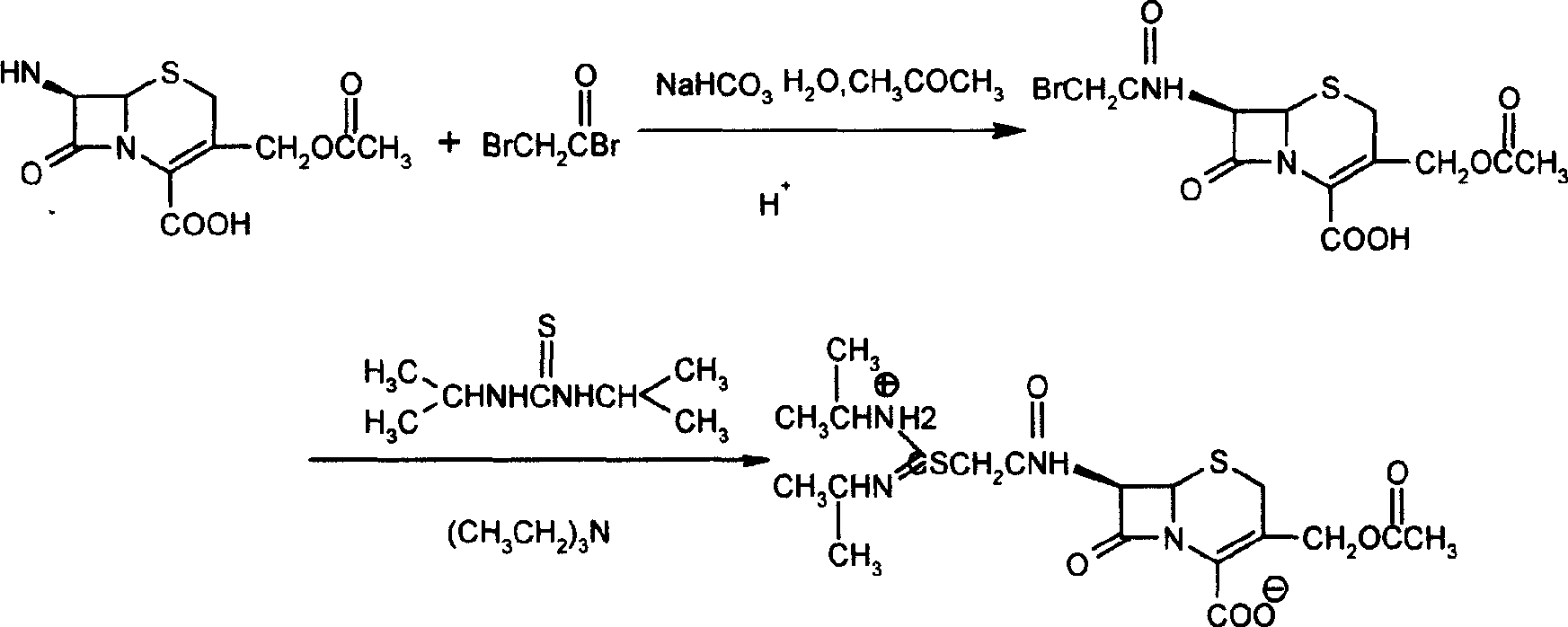
Method for preparing cefathiamidide
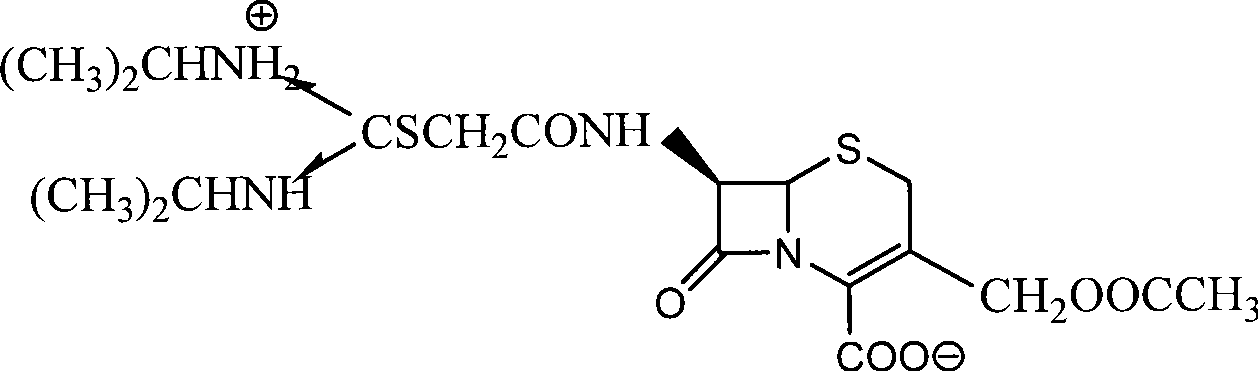
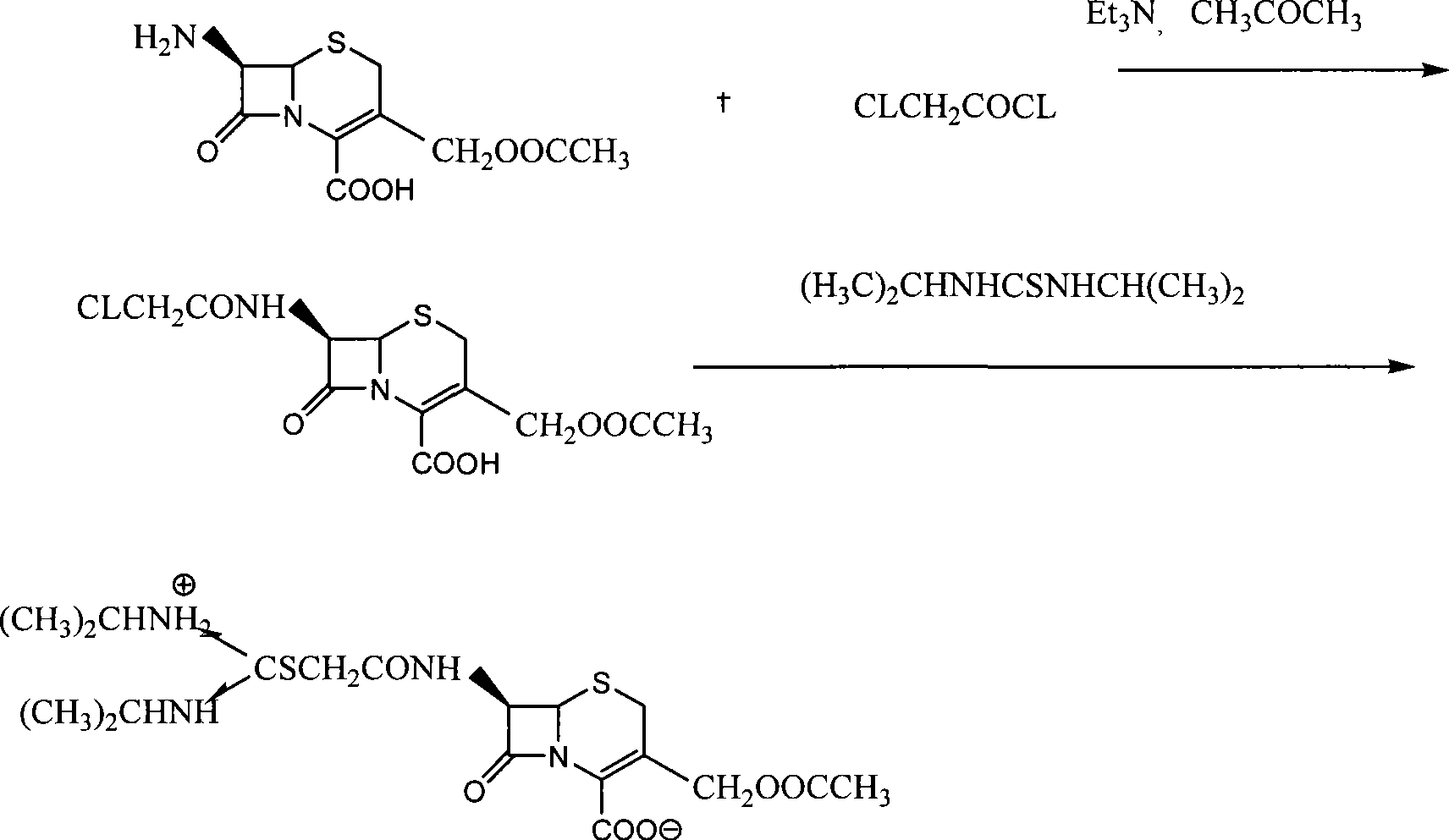
The preparation process of cefathiamidine includes the following two synthesis steps: 1) preparing cefathiamidine acid through the reaction between 7-ACA and bromoacetyl bromide, acidifying, solvent extraction, concentration and drying; and 2) preparing cefathiamidine product through adding alkali reagent to dissolve cefathiamidine acid, reaction with diisopropyl thiourea, and adding semi-soluble solvent to separate out cefathiamidine product. The said preparation process has less steps, and is simple and feasible.
Owner:托新权 +1
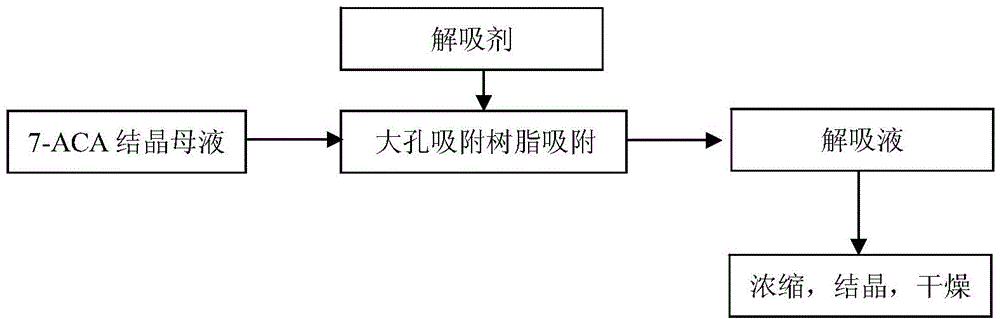
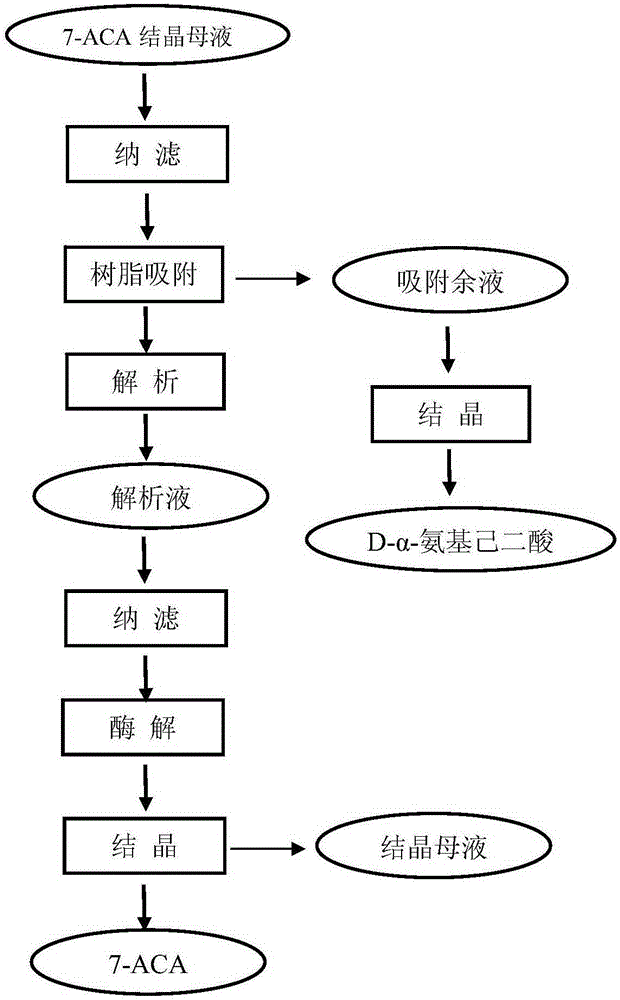
Process for recovering 7-ACA (aminocephalosporanic acid) crystallization mother liquor
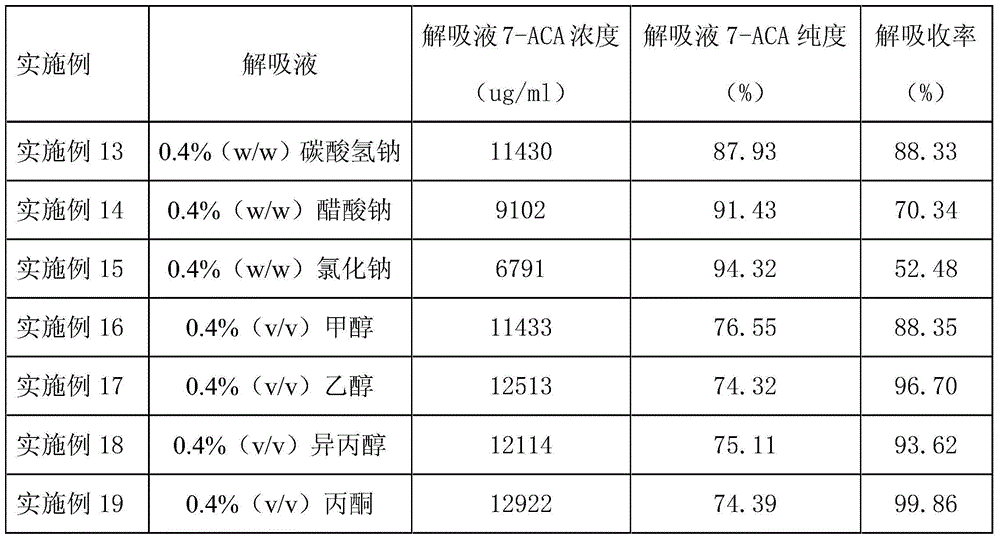
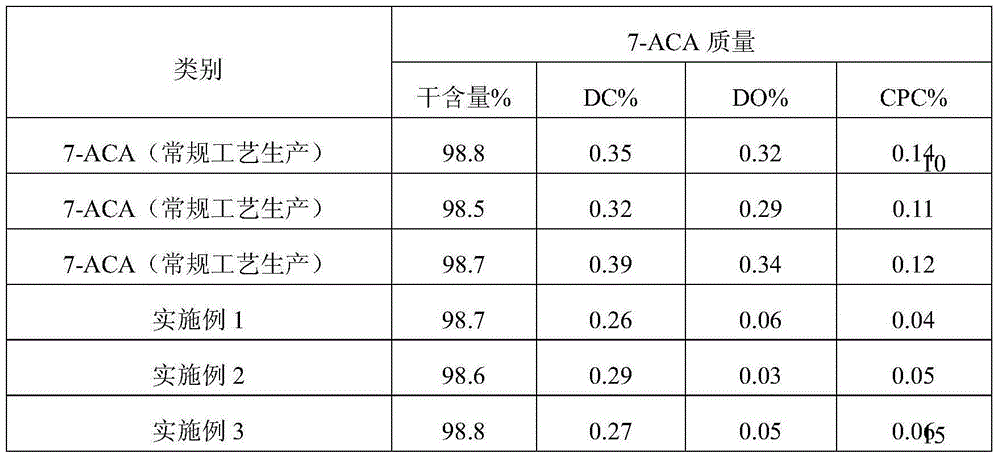
The invention provides a process for recovering a 7-ACA (aminocephalosporanic acid) product from mother liquor through macroporous adsorption resin. The process mainly comprises the following steps: performing resin adsorption, performing resin desorption, concentrating and the like, wherein in the resin absorption step, the pH value of 7-ACA crystallization mother liquor is adjusted, the crystallization mother liquor passes through a macroporous adsorption resin layer from top to bottom at a certain flow speed, and when the valence of 7-ACA contained in effluent of a resin column is 1% of that of the crystallization mother liquor at an inlet, resin adsorption is stopped. According to the method, the qualified 7-ACA product with the purity of greater than or equal to 98% and the color grade of greater than 4 can be obtained; the extraction yield of the resin is greater than or equal to 90% and the total process yield is greater than or equal to 40%; based on estimation of 200t of the mother liquor produced each day, the economic benefit of a year can reach more than ten million yuan.
Owner:SUNRESIN NEW METERIALS CO LTD XIAN
Method for synthesizing cefepime hydrochloride
InactiveCN101735251AHigh densityHigh reactivityAntibacterial agentsOrganic chemistryCefepime hydrochlorideVolumetric Mass Density
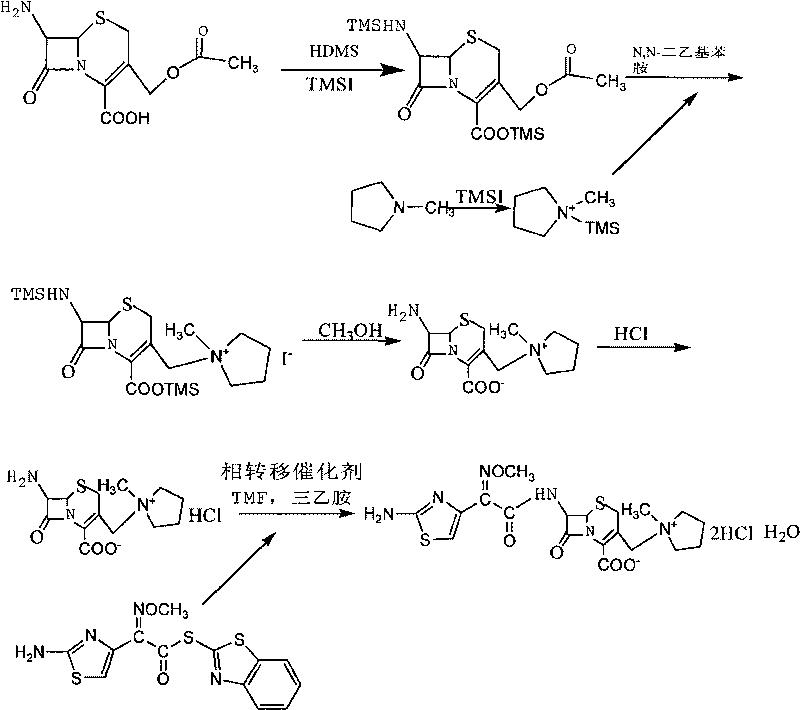
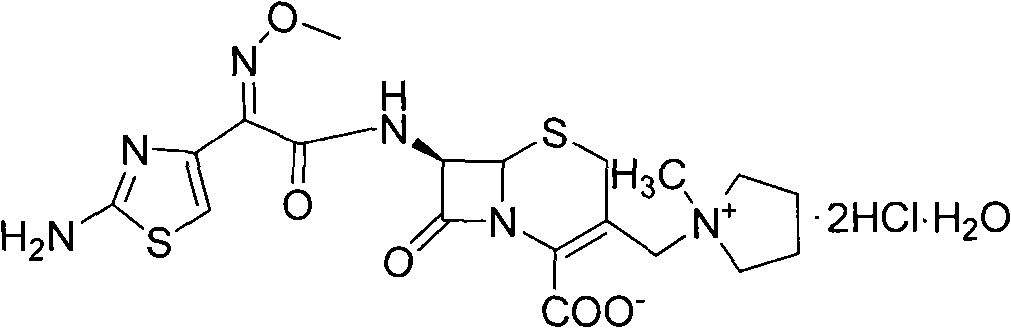
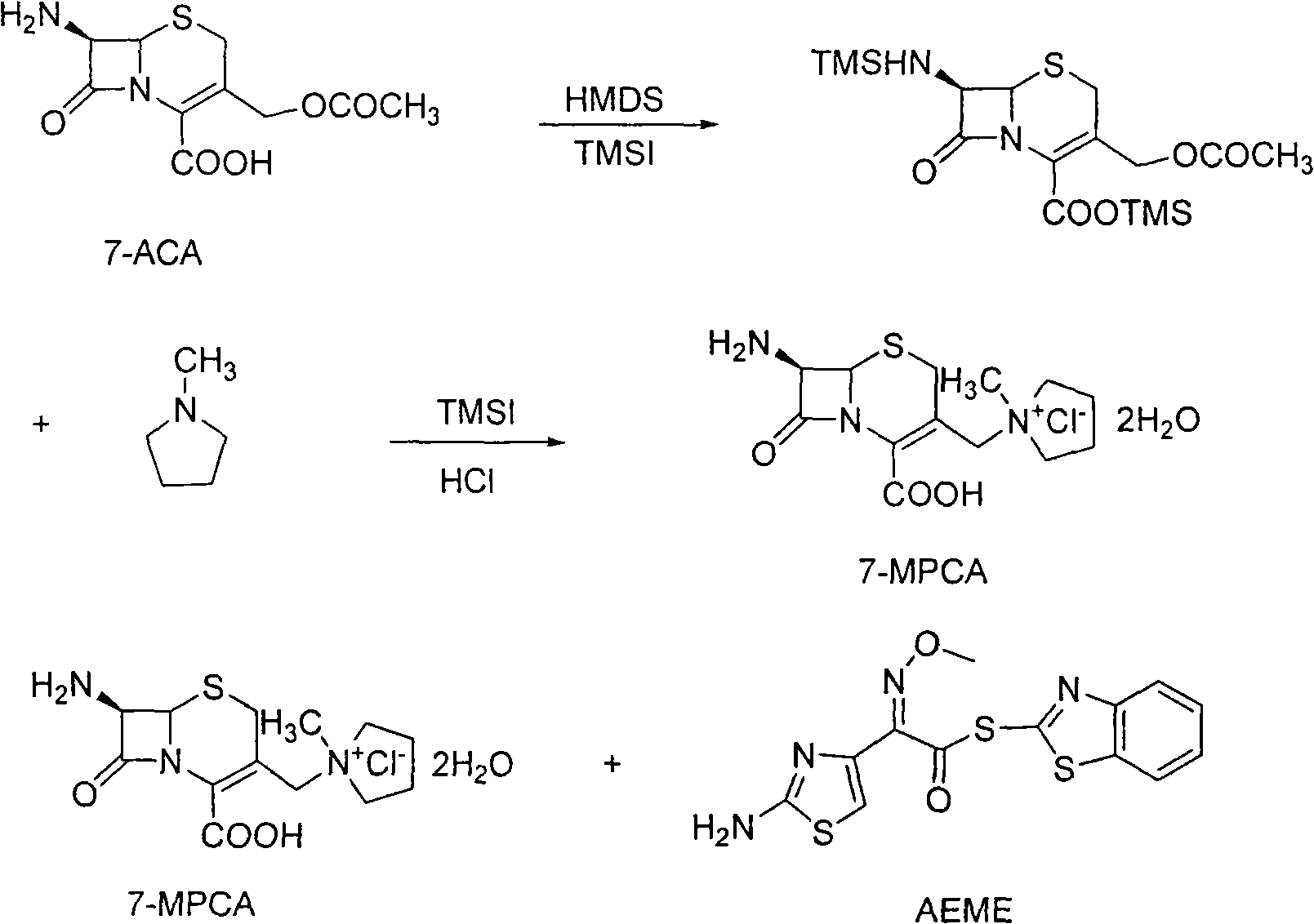
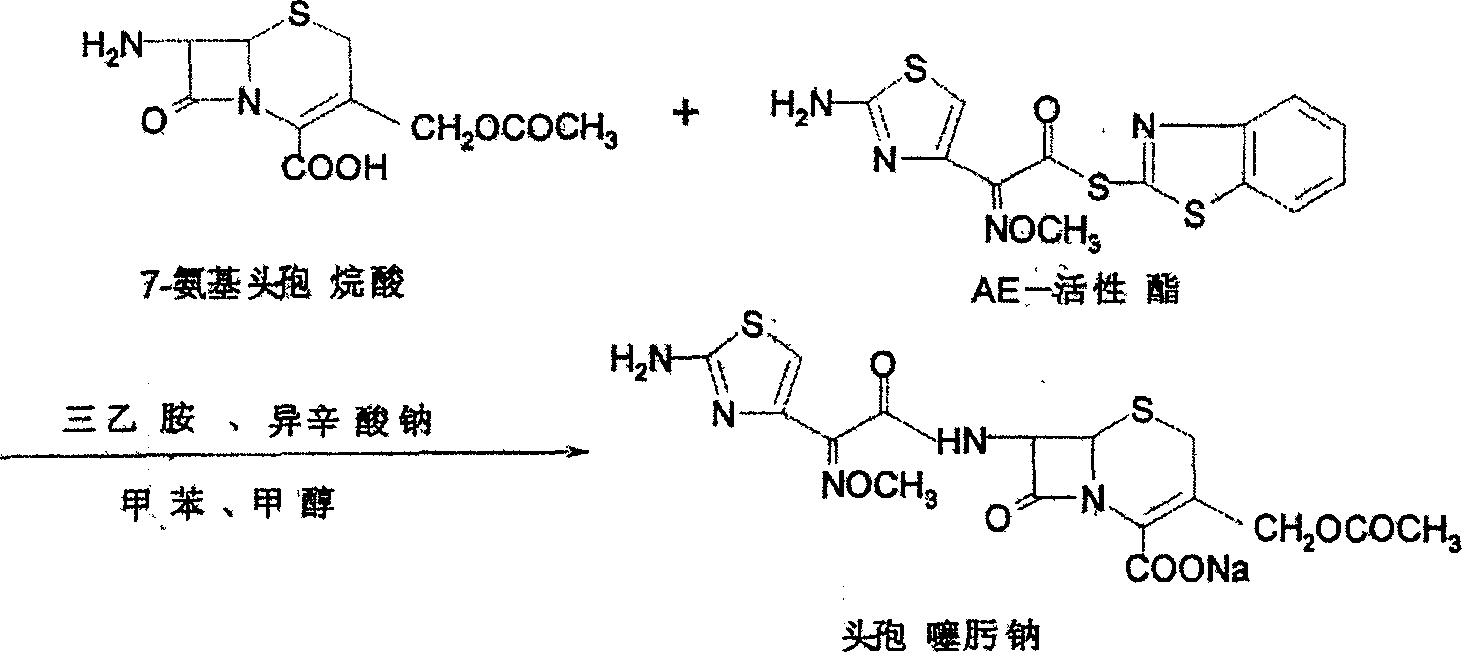
The invention relates to a method for synthesizing cefepime hydrochloride. The method comprises the following steps: taking 7-aminoce-phalosporanic acid (7-ACA) and N-methylpyrrolidine as raw materials, firstly, carrying out carboxylic and amino protection on the 7-ACA by HMDS, then preparing the N-methylpyrrolidine and iodotrimethylsilane into a quaternary ammonium salt intermediate, finally, adding the intermediate into the protected 7-ACA solution and reacting to prepare 7-MPCA; taking the 7-MPCA and AE-active ester, adding a phase transfer catalyst into an organic phase for carrying out an N-acidylating reaction, salifying and reacting to obtain the cefepime hydrochloride. The invention has the main characteristics that the quaternary ammonium salt intermediate is prepared in the step (1), the defects of high electron cloud density, strong reactivity and many side reactions of the N atom of N-methyl pyrrole are overcome, the yield is enhanced by 7%, and the product purity is enhanced. During the N-acidylating reaction in the organic phase in the step (2), the phase transfer catalyst is added, so that the conversion rate of the reaction is enhanced by 5%, and the product yield is enhanced.
Owner:YIYUAN XINQUAN CHEM
Method for preparing cefuroxime acid
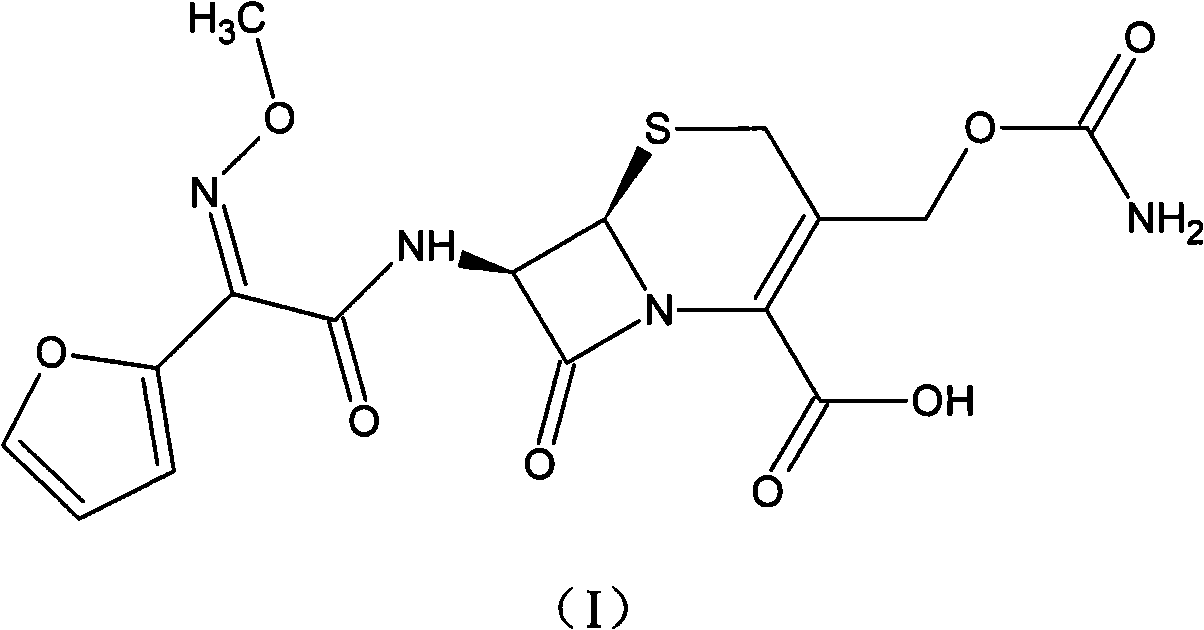
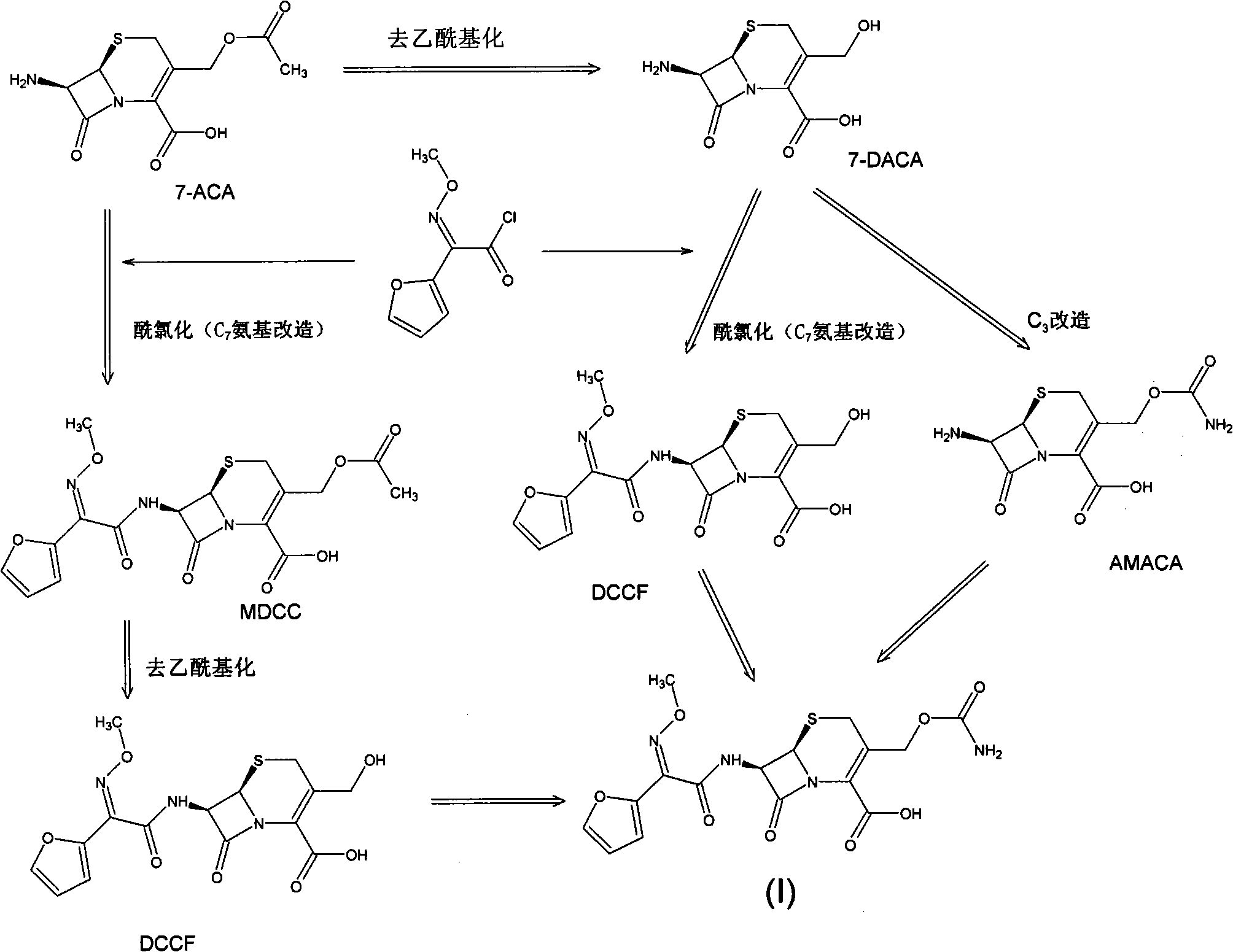
The invention discloses a method for preparing cefuroxime acid. The method comprises the following steps of: performing a chloride acylation reaction on 7-aminocephalosporanic acid (7-ACA) and methoxyiminofuran acetate serving as raw materials; performing deacetylation to synthesize DCCF; performing nucleophilic addition on the DCCF and chlorosulfonyl isocyanate (CSI) serving as a strong carbamoyl reagent to obtain chlorosulfonyl cefuroxime; and hydrolyzing the chlorosulfonyl cefuroxime to obtain cefuroxime acid. In the preparation method, the preparation process is simple, and the cefuroximeacid is crystallized by adopting aqueous solution, so that the loss of organic solvents is reduced; simultaneously, aids are added selectively in the reaction process to improve the quality of products, so that finished products with high purity and yield and good colors are obtained. The purity of the cefuroxime acid prepared by the method is more than 98.5 percent, and the weight yield is approximately 100 percent.
Owner:国药集团致君(苏州)制药有限公司
Synthetic method of 7-MAC intermediate
The invention discloses a synthetic method of a 7-MAC intermediate, which comprises the following steps: reacting 7-ACA as raw material with MMTZ to obtain 7-TMAC; enabling the 7-TMAC and methyl sulfur bromide to undergo imidization, and then, reacting the imidization product with diphenyl diazomethane to obtain the intermediate; and enabling the intermediate and a methoxylation reagent to undergo methoxylation reaction to obtain a finished product. In the synthetic method, the 7-ACA which can be obtained easily is used as original material to synthesize the 7-TMAC, thereby reducing the production cost. In the imidization reagent of the synthetic method, methyl sulfur chloride is replaced by the methyl sulfur bromide, thereby increasing the reaction activity, improving the yield, simplifying the operation and reducing the cost.
Owner:CANGZHOU SENARY CHEM SCI TEC
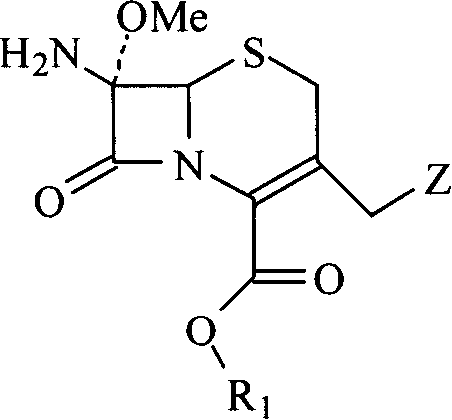
Methoxy cephalosporin intermediate
The invention relates to a synthesis method of an intermediate of methoxyl cephalosporin. C3 site methyl halide of a cephems compound with C4,7 site being protected, instead of the ordinary 7-ACA, is used as a material, functionalized at C3 site, and then methoxylated at C7 site, finally de-protected, and the intermediate of methoxyl cephalosporin is prepared. The compound can be conveniently used in the synthesis of various methoxyl cephalosporin medicines. The invention has apparent advantages of clear synthesis route, less steps, less and inexpensive reagents, comparatively single solvent, convenience for recovery and recycling, low material cost and operation expenses.
Owner:北京金源化学集团有限公司
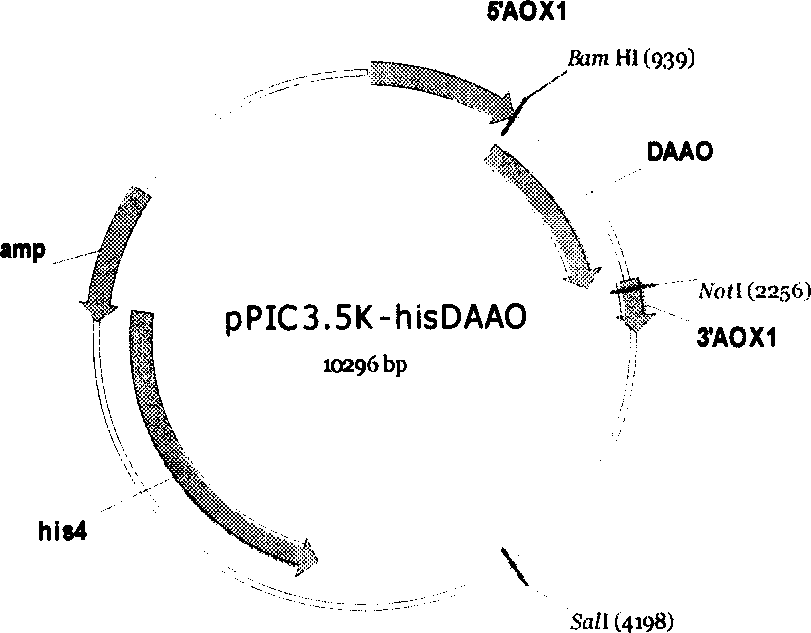
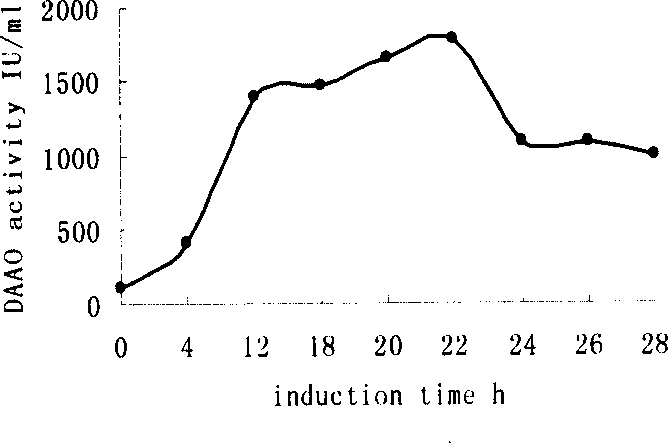
Process of preparing D-amino acid oxydase
The invention is a method of preparing D-amino acid oxidase, relating to a method of preparing flavo-enzyme D-amino acid oxidase. Concretely, it relates to a method of highly efficiently producing mutational D-amino acid oxidase by constructing recombinant engineering strains. It reconstructs wild D-amino acid oxidase coming from Trigonopsis variablilis by means of gene engineering, fuses a segment of Histag at N end and C end of the wild enzyme, and further implements high-efficiency fast separation of mutational enzyme by affinity chromatography. The expression level of the enzyme in fermentation liquor is 4000IU / mL, higher than that of the wild enzyme, and the recovery ratio of Ni-column affinity chromatography is 50%. The purified enzyme can be used in making high-efficient oxidation conversion of cephalosporin C- to produce glutaryl-7-ACA and be used together with GL-7-ACA to produce 7-ACA.
Owner:FUDAN UNIV
Preparation technology of cefotaxime
The invention relates to a preparation technology of cefotaxime, belonging to the preparation technology of medical compounds. The technology comprises the following steps: adding 7-ACA and AE-active resin reagents in solvent, and then adding an organic base and an alcohol catalyst to carry out a synthesis reaction. The preparation technology is characterized in that an acidifier is directly added for acidification after the reaction is finished so as to devitrified. The invention has simple and convenient operation, short production period, high product yield and low solvent consumption and can obtain products with uniform crystallized grains, good colors, high purity, low cost and stable quality.
Owner:REYOUNG PHARMA
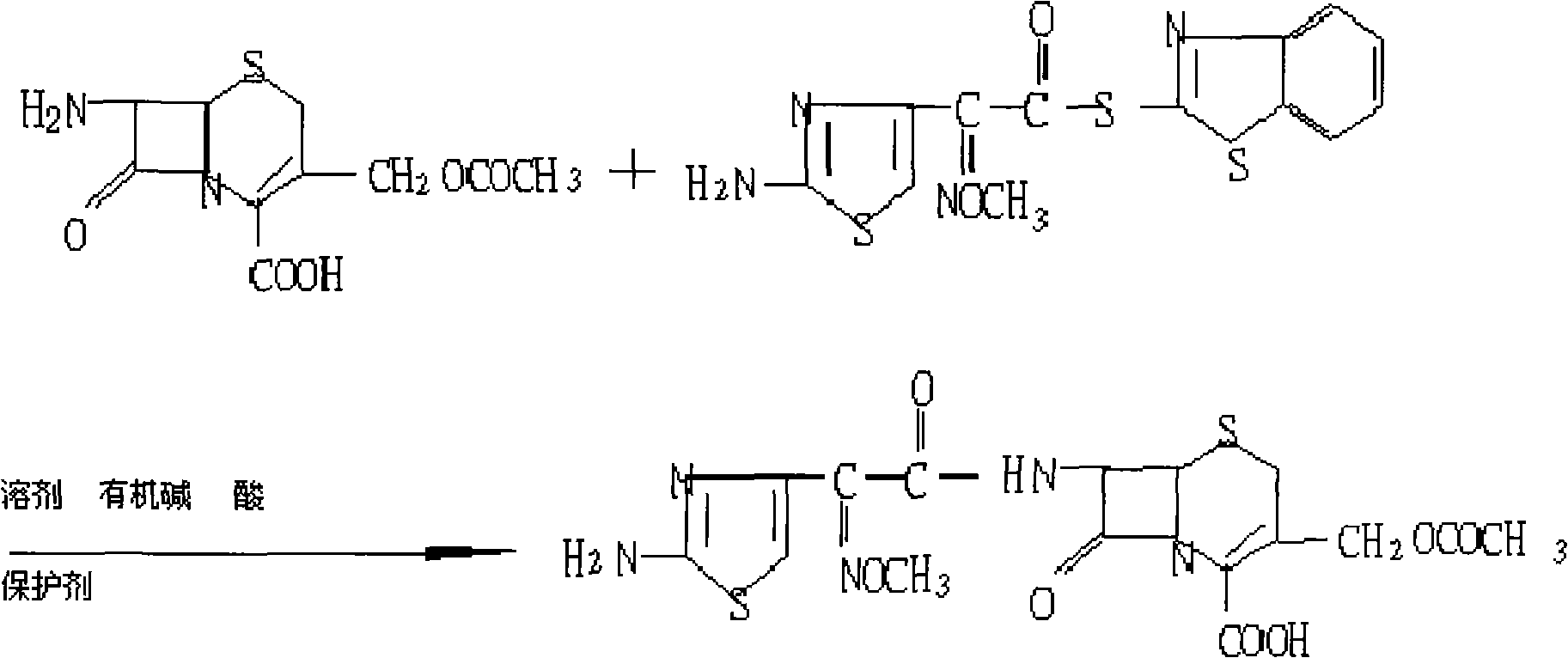
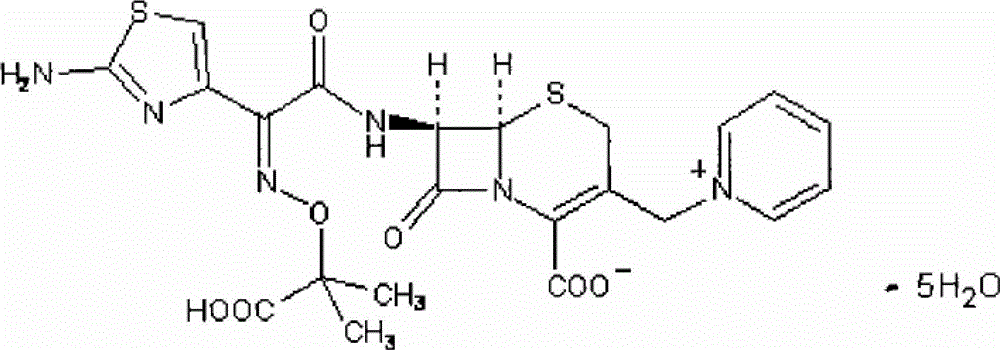
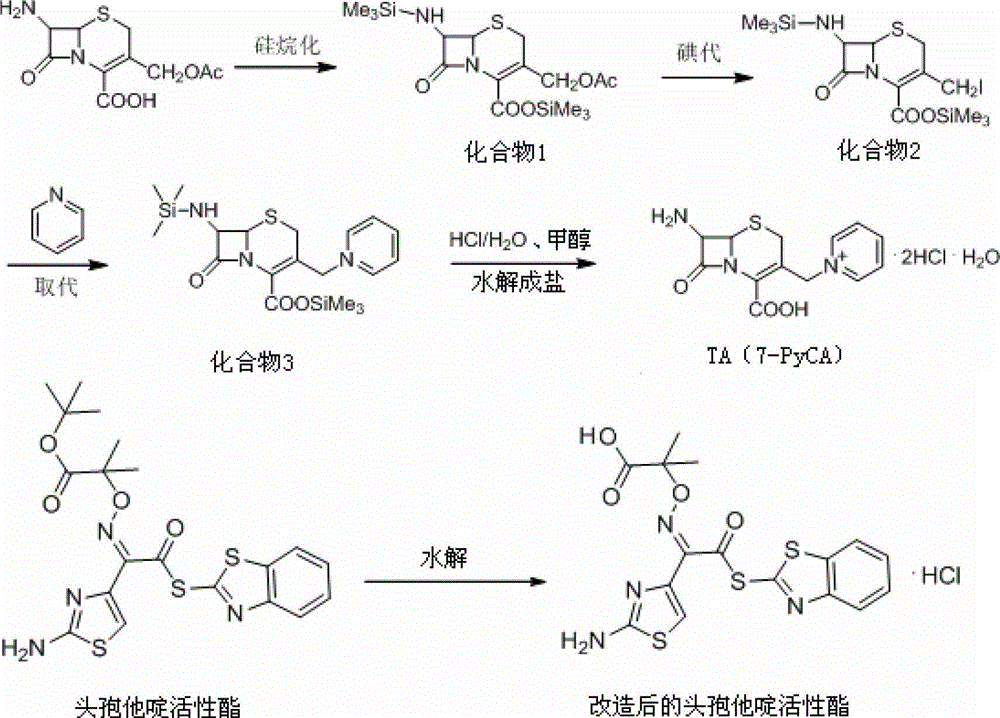
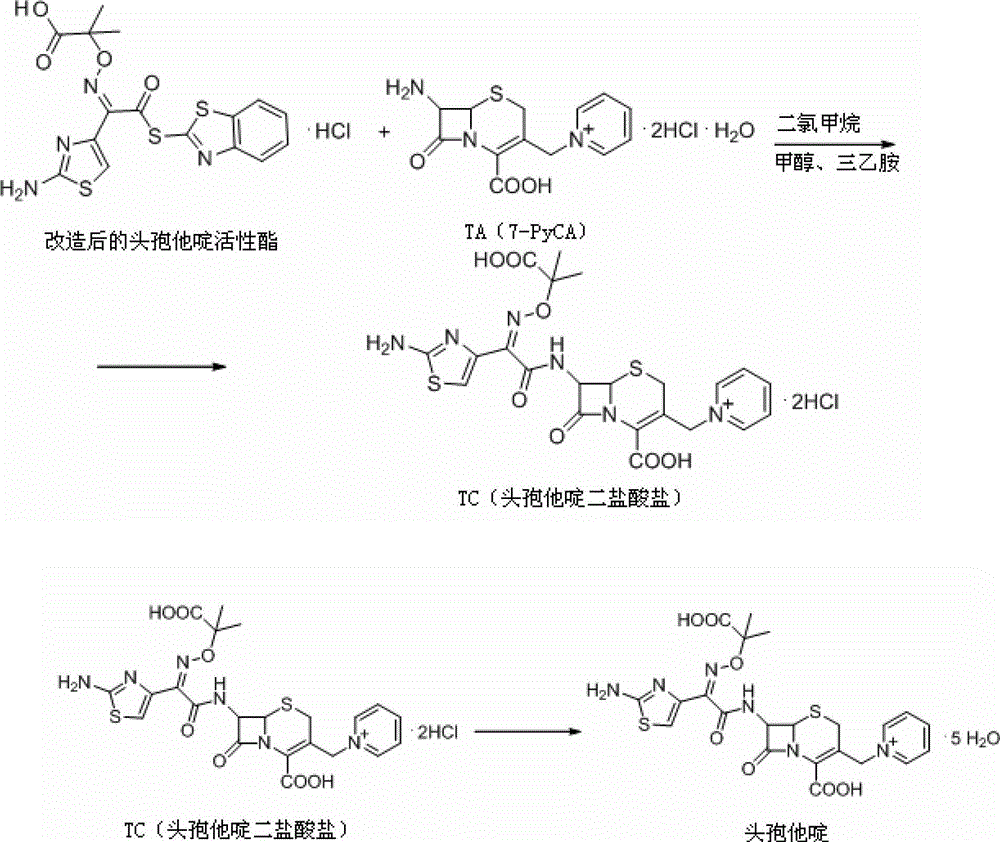
Synthesis of antibiotic ceftazidime, ceftazidime for injection and preparation method of ceftazidime
InactiveCN102875576ASimple operation processImprove single step yieldAntibacterial agentsOrganic active ingredientsDissolutionSolvent
The invention discloses synthesis of antibiotic ceftazidime, ceftazidime for injection and a preparation method of ceftazidime. 7-ACA is served as a raw material, silanization and iodo reaction are carried out, and pyridine, hydrochloric acid and water are added to obtain TA (7-PyCA); ceftazidime active ester is added in an organic solvent and an inorganic solvent to obtain new modified ceftazidime active ester; TA is added in new modified ceftazidime active ester, organic mixed solvent and triethylamine are added, then acid water and an acetone solution are added to obtain TC (ceftazidime dihydrochloride), and ceftazidime is obtained through the reaction. The synthesis of ceftazidime provided by the invention has the advantages that ceftazidime is high in yield, operation processes are simplified, production cost is low, ceftazidime is applicable to industrial production, obtained products can be stored for a long time, a structure is stable, and impurity content is low. Ceftazidime for injection has the advantages of being moderate in grain size, good in clarity, less in impurity content, excellent in quality, stable and the like, and is high in split charging efficiency in production, good in content uniformity, high in medicine dissolution rate in clinical application and good in dissolubility.
Owner:国药集团致君(苏州)制药有限公司 +1
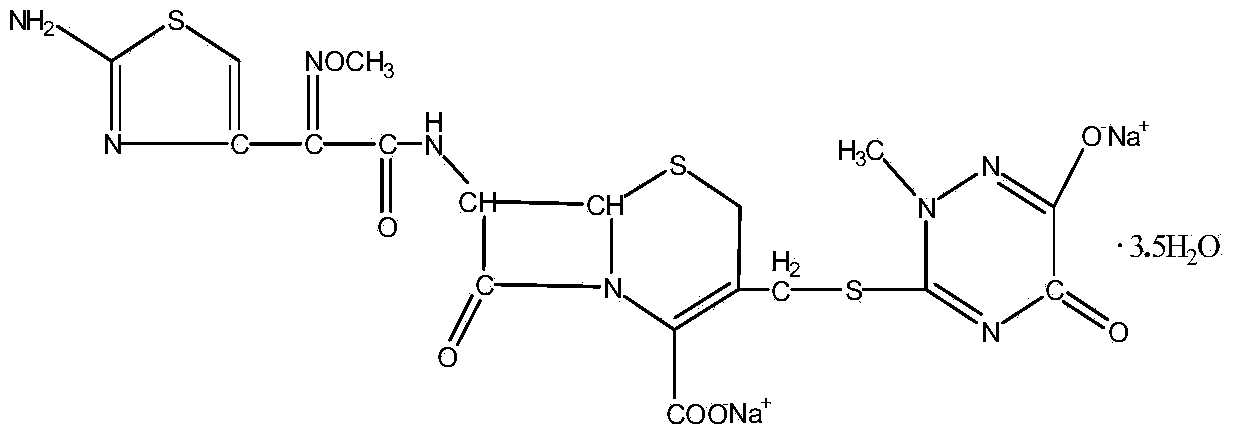
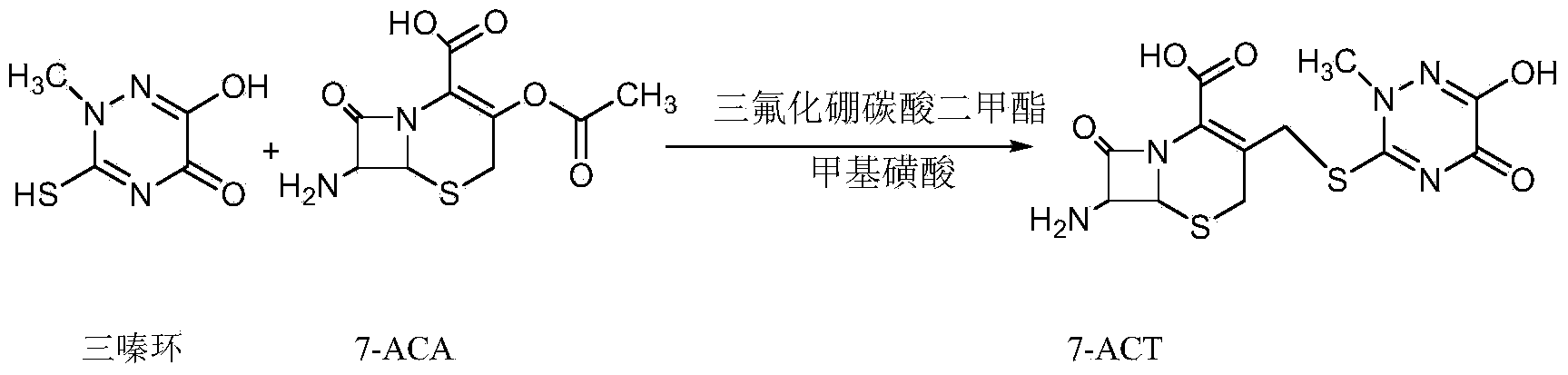
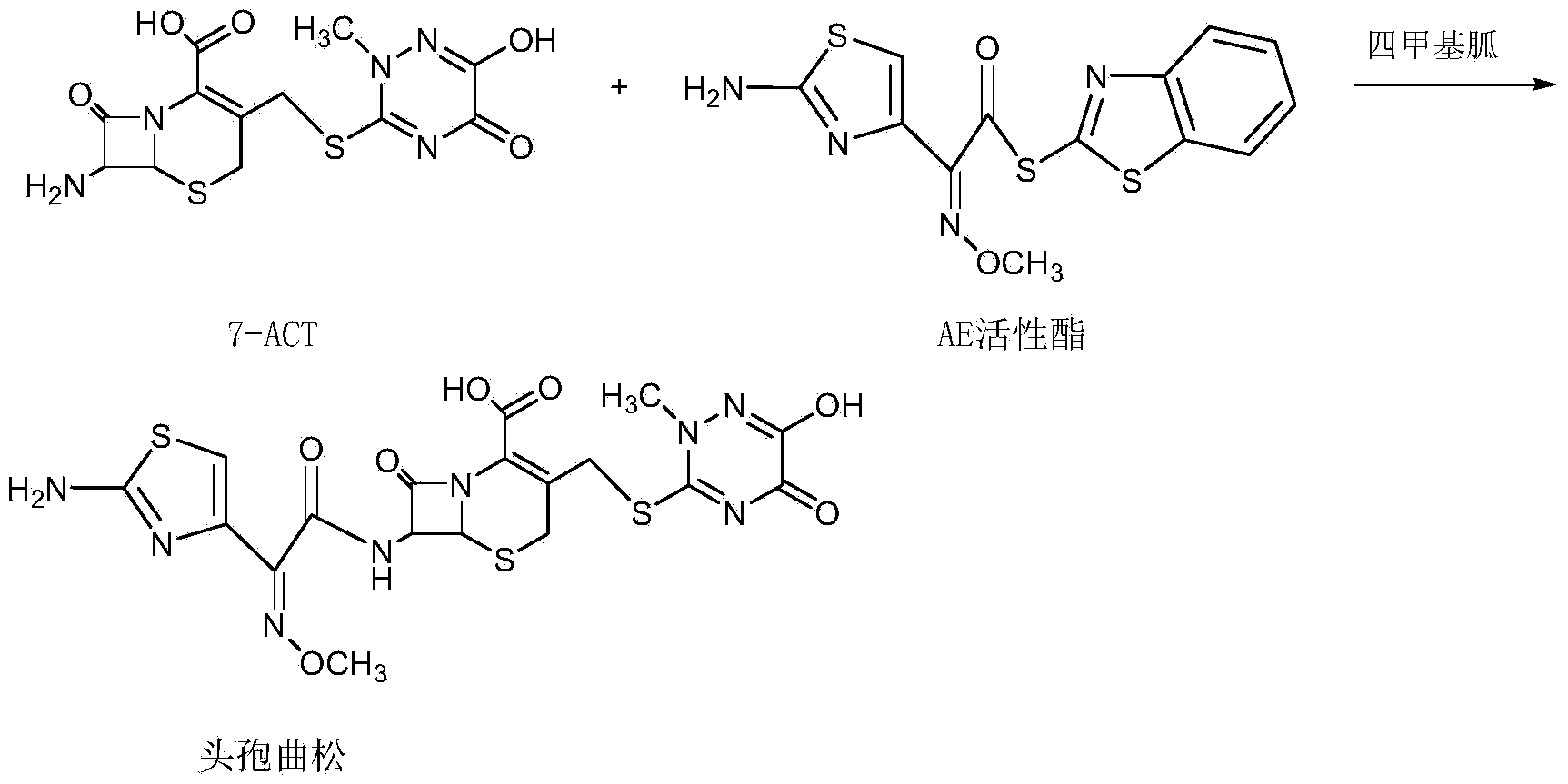
Method for synthesizing ceftriaxone sodium
The invention discloses a method for synthesizing ceftriaxone sodium. The method comprises the steps of firstly carrying out condensation on 7-ACA and triazine ring by taking dimethyl carbonate and organic acid as a mixed solvent and taking boron trifluoride dimethyl carbonate as a catalyst, so as to produce 7-ACT, then, enabling 7-ACT and AE (Active Ester) to react by taking tetramethyl guanidine as a cosolvent, so as to produce ceftriaxone, and finally, adding sodium acetate, thereby synthesizing the sodium salt. The method has the advantages that the operating method is simple, the conditions are mild, the raw materials are easily available, the pollution is little, the cost is very low, the purity of the finally obtained ceftriaxone sodium product is over 99.6%, and the molar yield is over 94%.
Owner:哈药集团股份有限公司 +1
Process for preparing cefathiamidine
The invention relates to the field of the synthesis of chemical medicaments and discloses a preparation method of cefathiamidine; the method takes chloracetyl chloride as a raw material and comprises the following steps: (1) on the condition of the presence of a solvent, alkali is added so as to cause 7-ACA to be dissolved, and then the chloracetyl chloride is added for a condensation reaction; after the condensation reaction is finished, chloracetyl 7-ACA crystals are separated out with an acid; and (2) on the condition of the presence of both the solvent and a catalyst of a catalyzing amount, the chloracetyl 7-ACA reacts with N, N-di-isopropyl thiourea to produce the cefathiamidine. Besides the advantages of bromoacetyl-bromide preparation method of cefathiamidine, the technology of adopting chloracetyl chloride as the raw material to produce the cefathiamidine also has the advantages that: as no alkali is added into the reaction between the chloracetyl 7-ACA and the N, N-di-isopropyl thiourea, the produced cefathiamidine has lighter color, and better and more stable quality, is more beneficial to store and transport, improves the overall yield, lowers the cost and has broader prospects; and the price of the chloracetyl chloride is one sixth of that of the bromoacetyl bromide, which significantly reduce the cost.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Novel process for synthesizing 3-deacetyl cefuroxime sodium (DCCF)
InactiveCN101289457AReduce typesEasy to recycleAntibacterial agentsOrganic chemistry7-ACAChemical reaction
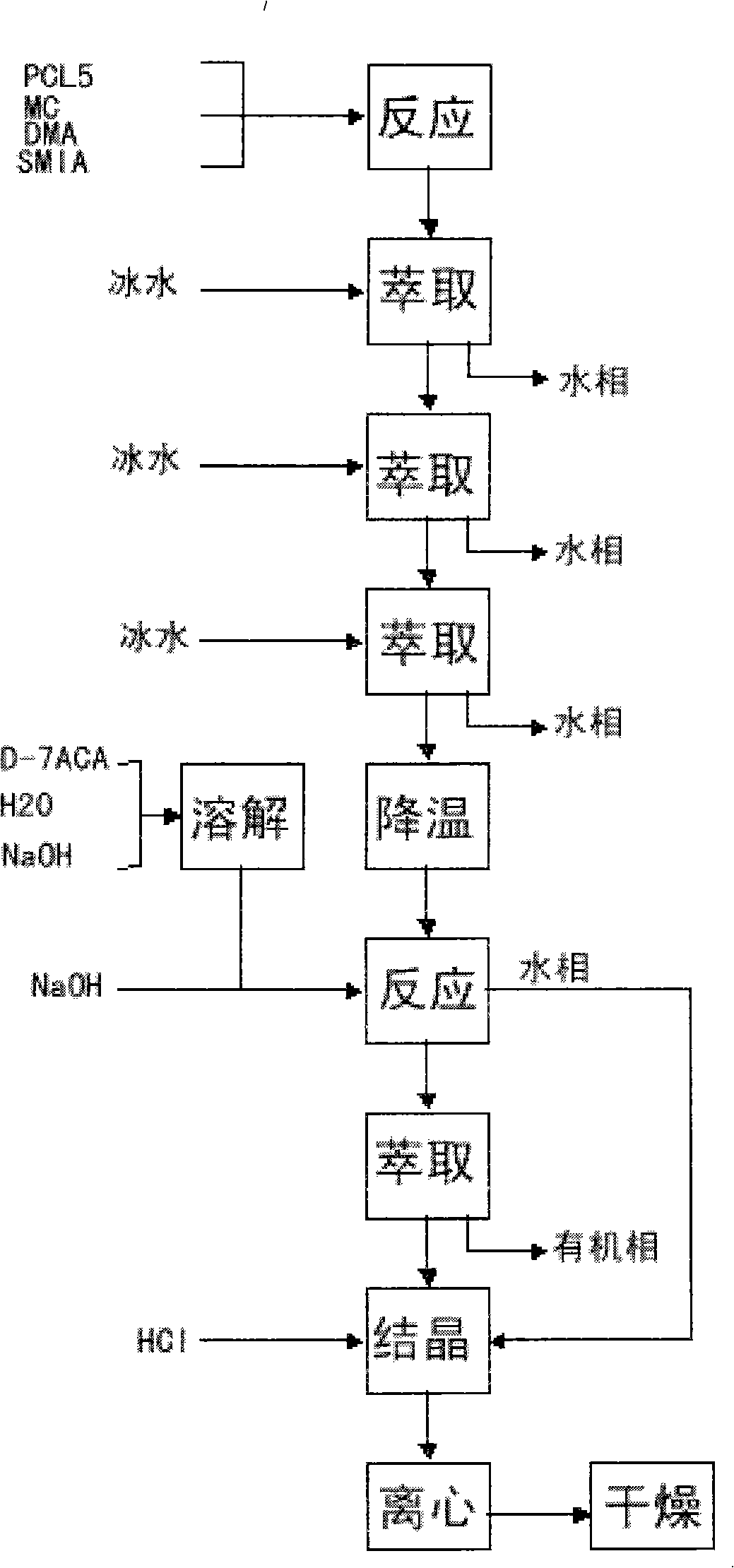
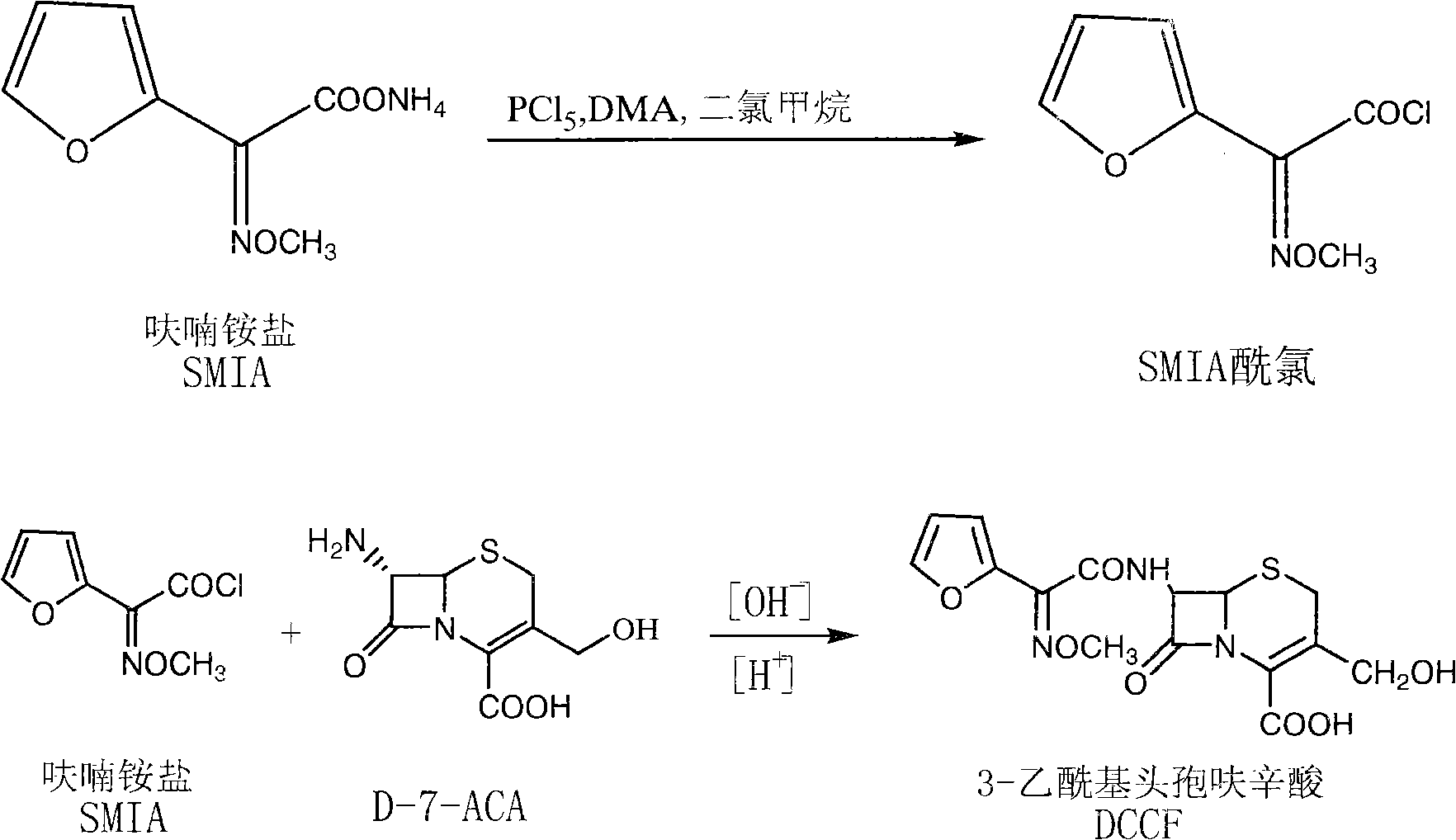
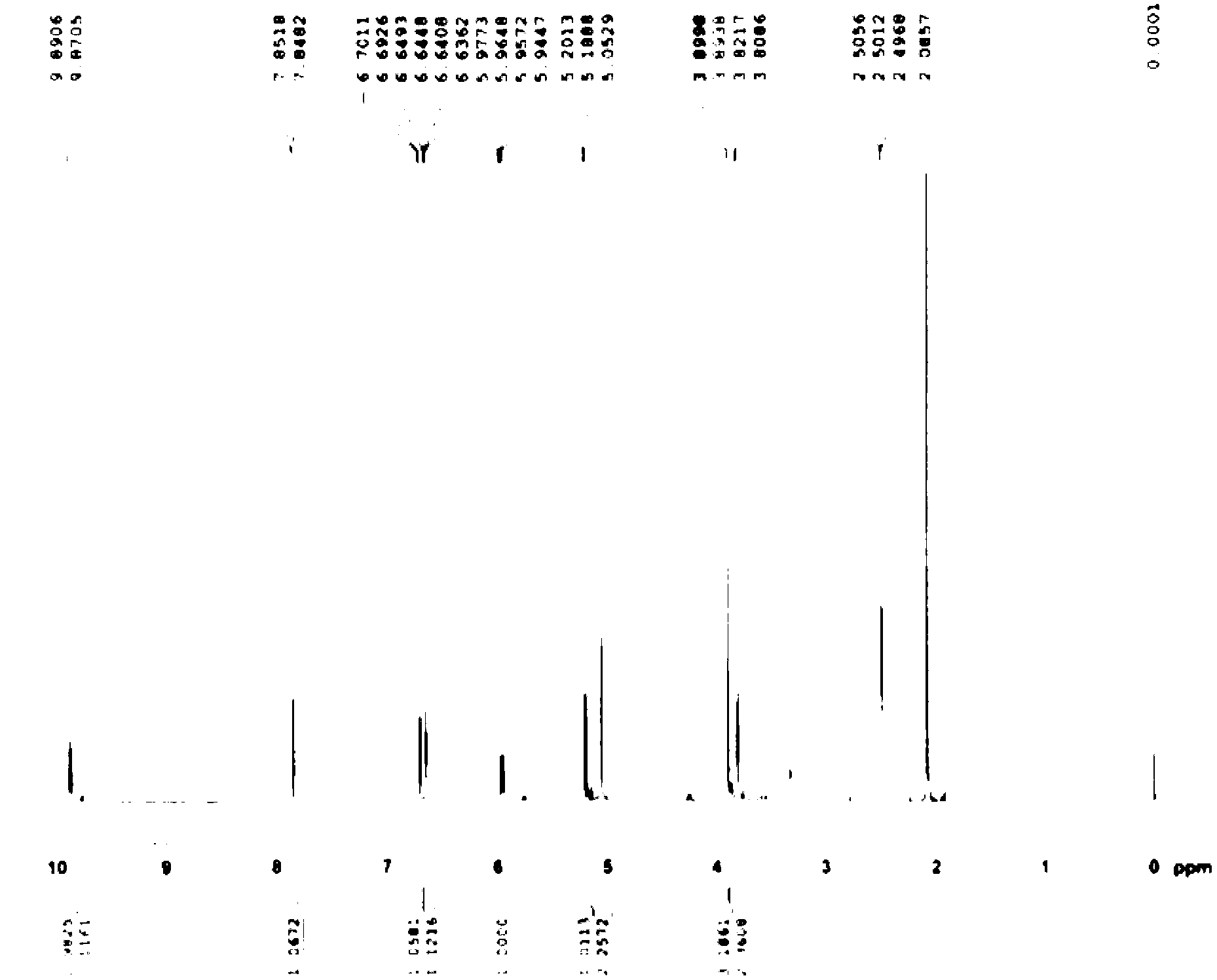
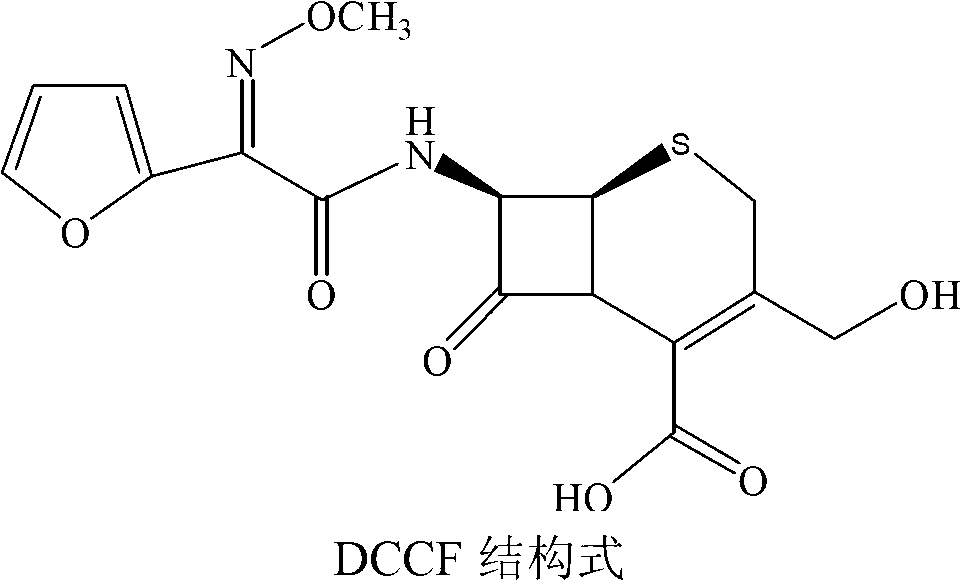
The invention relates to a synthesis art of a chemical product, in particular to a new synthesis art for 3-deacetyl cefuroxime acid (DCCF). The art of the invention uses SMIA acyl chloride and 3-deacetyl cephalosporin acid (D-7-ACA) as the main synthesis ingredients to replace the SMIA acyl chloride and the 7-ACA, which reduces the chemical reaction process needed in the synthesis, shortens the production cycle, reduces the using solvent species, facilitates the solvent recovery and improves the product synthesis rate.
Owner:河源市制药工程技术研究开发中心
Preparation method and application of polyvinyl alcohol immobilized acylase
The invention discloses a preparation method of polyvinyl alcohol immobilized acylase. The preparation method comprises the steps: preparing a mixed aqueous solution from polyvinyl alcohol and a cross-linking agent, adding acylase into the mixed aqueous solution of the polyvinyl alcohol and the cross-linking agent, uniformly mixing to obtain an acylase polyvinyl alcohol gel aqueous solution, and carrying out formation and solidification process treatment on the acylase polyvinyl alcohol gel aqueous solution to obtain the polyvinyl alcohol immobilized acylase. The polyvinyl alcohol immobilized acylase is used for synthesizing a cephalosporin antibiotics midbody 7-ACA (Aminocephalosporanic Acid) or 7-ADCA (Aminodesacetoxycephalosporanic Acid). According to the preparation method disclosed by the invention, as PVA (Polyvinyl Alcohol) is used for immobilizing biological enzyme, the enzyme activity recovery rate can reach above 80 percent, immobilized acylase is also more stable and can be reused for above 50 times, so that the application cost of the acylase in the production of the 7-ACA and the 7-ADCA is greatly lowered, and the production efficiency and the product quality are improved.
Owner:JIANGSU HUITENG BIOMEDICAL TECH
Method for preparing 3-descarbamoyl-cefuroxime acid
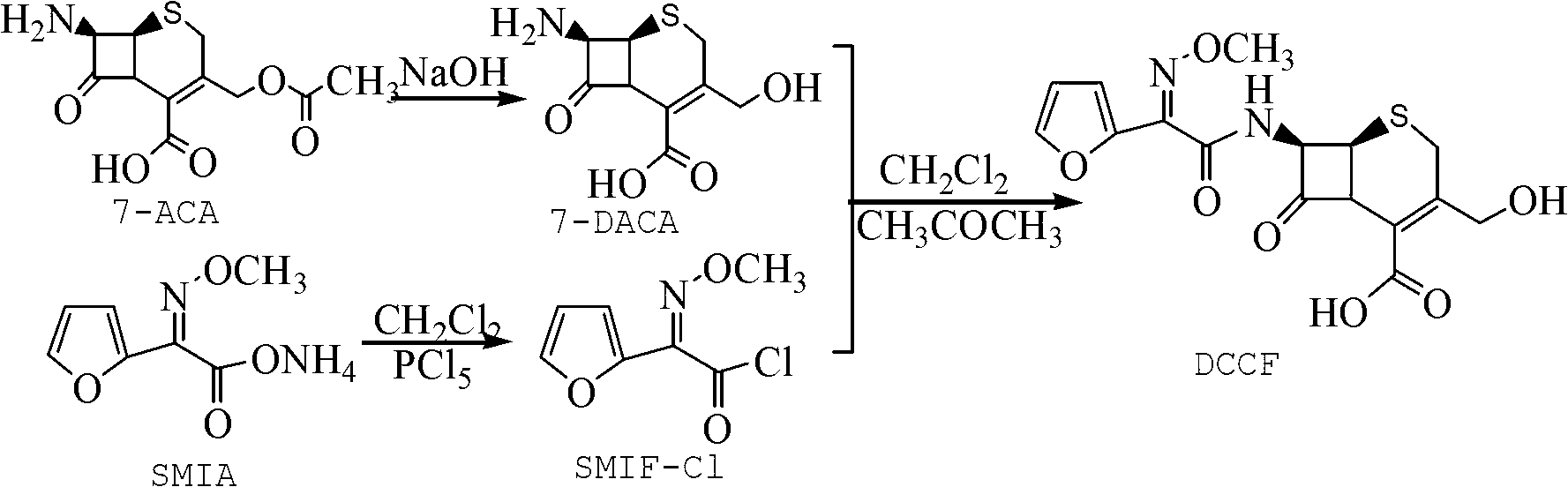
The invention relates to a method for preparing 3-descarbamoyl-cefuroxime acid. The method comprises the following steps of: (1) dissolving 7-aminocephalosporanic acid (7-ACA) in water or a methanol solution to prepare a 7-ACA solution, and performing hydrolysis reaction to obtain a 3-deacetyl-7-aminocephalosporanic acid (7-DACA) solution; (2) dissolving an acylchlorination reagent in a solvent, adding a cosolvent, adding (Z)-2-methoxyimino-2-(furyl-2-yl)acetic acid ammonium salt (SIMA), reacting, filtering, adding water or performing rotary evaporation to remove the excessive acylchlorination reagent, performing vacuum rotary evaporation, adding a homogenization reagent, and dissolving to obtain a (Z)-2-(furyl-2-yl)-2-(methoxyimino)-acetyl chloride (SMIF-Cl) solution; (3) adding the homogenization reagent into the 7-DACA solution, dripping the SMIF-Cl solution, regulating the pH value, preserving heat and reacting to obtain a reaction solution; and (4) decolorizing the reaction solution, regulating the pH value, adding purified water, growing a crystal, filtering, and performing vacuum drying. Reactants form a homogeneous system by adding the homogenization reagent, so that the contact area of the reactants is expanded, the reaction rate is increased, and reaction time is shortened.
Owner:SHANDONG UNIV
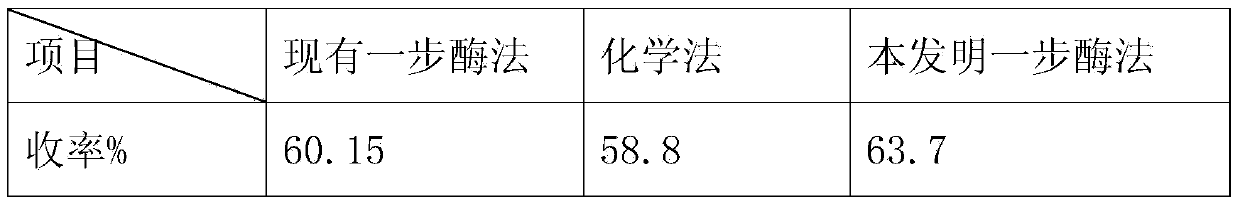
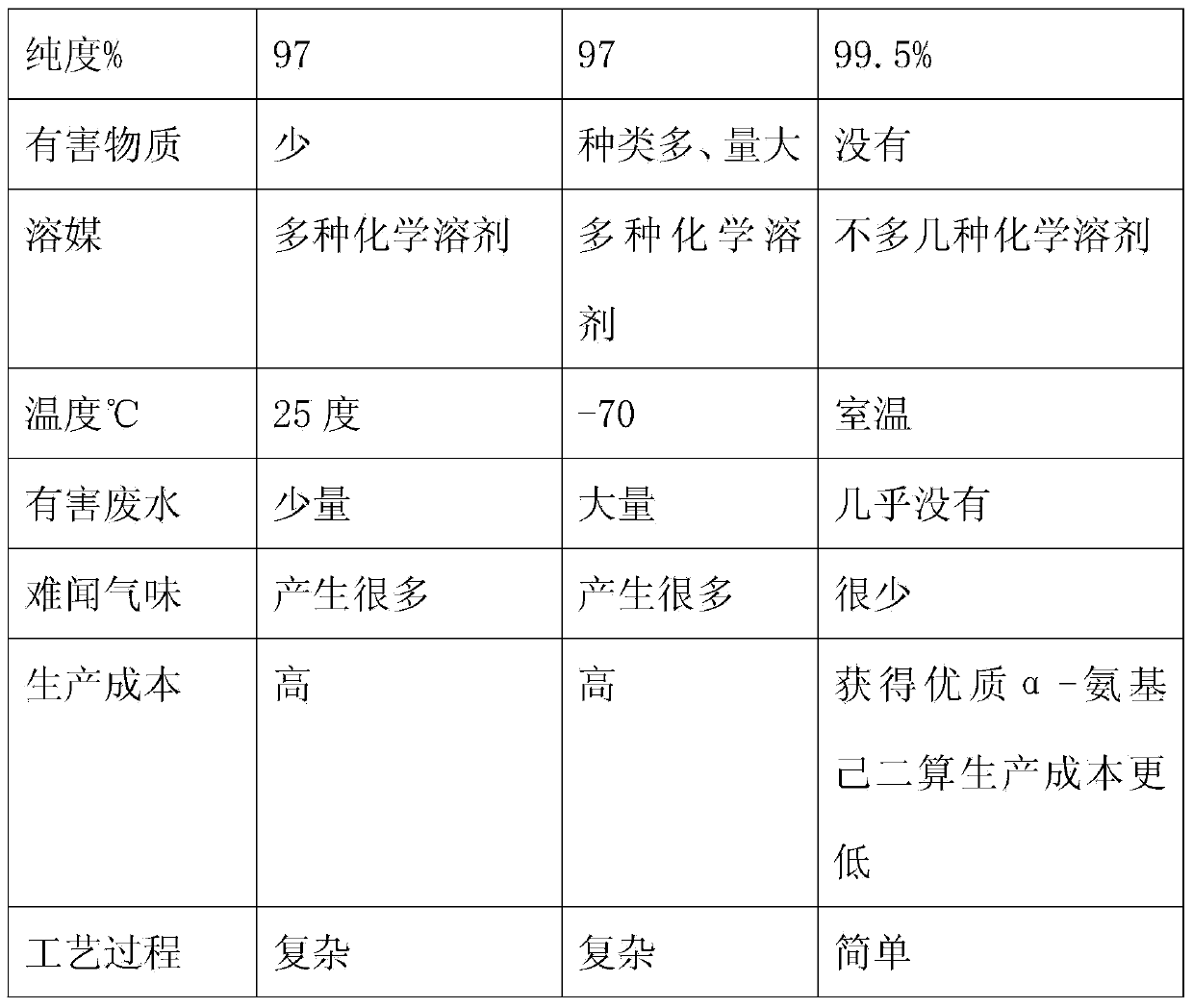
Method for preparing 7-ACA (aminocephalosporanic acid) and obtaining alpha-aminoadipic acid by one-step enzymatic reaction
The invention relates to a method for preparing 7-ACA (aminocephalosporanic acid) and obtaining alpha-aminoadipic acid. The method comprises the following steps of (1) treating cephalosporin C fermentation liquid; (2) performing an enzymolysis reaction of cephalosporin C under the effect of cephalosporin C acyltransferase to obtain 7-ACA; (3) treating the 7-ACA lysate after the enzymolysis; (4) separating out the 7-ACA; (5) separating alpha-aminoadipic acid from the separated 7-ACA lysate.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Cefazedone sodium medicament powder injection and method for synthesizing raw medicine of Cefazedone sodium
ActiveCN101584671BSingle componentImprove solubilityAntibacterial agentsOrganic active ingredientsSodium bicarbonateNitrogen gas
The present invention provides a cefazedone sodium medicament powder injection composed of 100% of cefazedone sodium. The cefazedone sodium is prepared by a method as follows: (1) 7-ACA and 3, 5-dichloro pyridine acetic acid react with each other with the action of an anhydrating agent, a mixture after the reaction is post-processed to obtain an intermediate product I; (2) the intermediate productI and 2-mercapto-5-methyl-1, 3, 4-thiadiazoles react with each other with the protection of nitrogen at a temperature of 50 to 90 DEG C, a mixture after the reaction is purified to obtain a water solution which is added with an inorganic acid to regulate pH value to be equal to 1 to 3, a precipitation is extracted from the water solution and is post-processed to obtain cefazedone; (3) the cefazedone and sodium hydrogen carbonate react with each other in water to obtain a cefazedone sodium solid body after an aftertreatment. The powder injection has single component and perfect dissolution performance, the raw medicine has a short synthetic route, the aftertreatment of the intermediate product or final product are all simple, and the yield and purity of the whole reaction process are all high.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Method for synthesizing antibiotic cefepime hydrochloride
ActiveCN101337971ASimple process conditionsEasy to operateAntibacterial agentsOrganic chemistryCefepime hydrochlorideHexamethyldisilane
The invention relates to a synthesis method of cefepime dihydrochloride that is a bacteriophage. 7-amin cethalosporanic acid (7-ACA) is used as starting material and reacts with hexamethyldisilane amine (HMDS) and iodotrimethylsilane (TMSI) first to obtain 7-ACA for protecting amino and carboxyl; then 7-ACA, amino and carboxyl of which are protected, reacts with iodotrimethylsilane and N-methylpyrrolidine to synthesize (6R, 7R)-7-amino-3-((1-methyl-1-pyrrolidine) methyl) cephalosporin-3-alkene-4-carboxylic acid hydrochloride (7-MPCA) through a one-pot method; 7-MPCA reacts with AE active ester to obtain a product of cefepime dihydrochloride through acidylation reaction and salifying reaction. Compared with the existing technical route, the synthesis method has the advantages that the process conditions are simple, the operation is convenient, the product yield is high, the product quality is stable, the method is suitable for the large-scale industrialized production, etc.
Owner:国药集团致君(苏州)制药有限公司
Preparation method of glutaryl-7-aminocefaphytanic acid acyltransferase
The method for preparing glutaryl-7-aminocephaphylanic acid acylase is characterized by that it utilizes colibacillus gene engineering bacterium to make shake-flask strain culture in culture medium, fermentation strain culture and fermentation culture, then utilizes centrifuge to collect thallus, soakes to extract enzyme liquor, makes the enzyme liquor undergo the processes of plate-frame filtration, ultrafiltration concentration, purification and fixation so as to obtain the invented product GL-7-ACA ACY.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
Preparation method for cephalosporin anti-infective drug
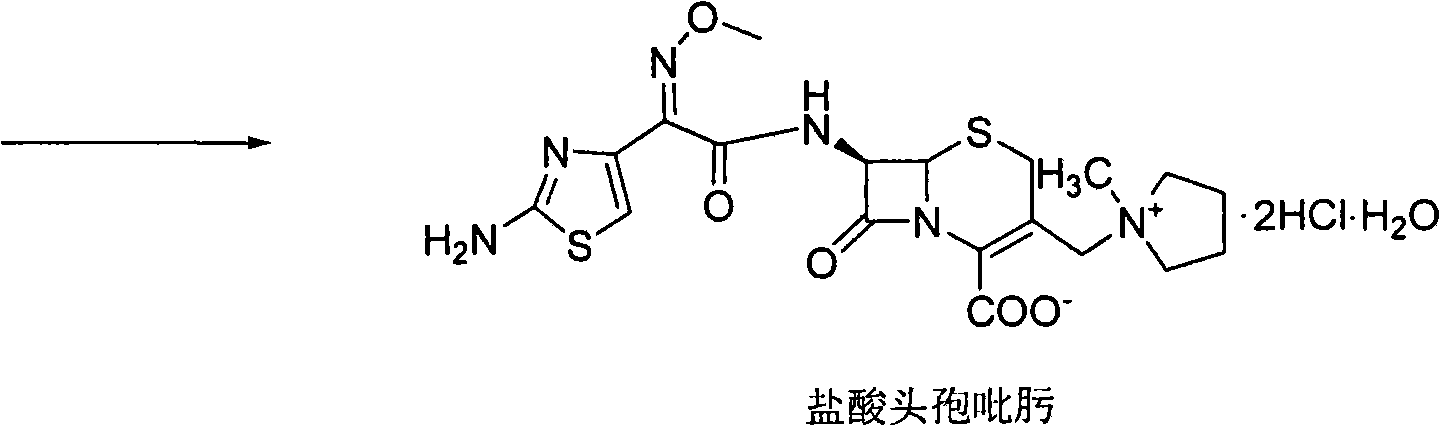
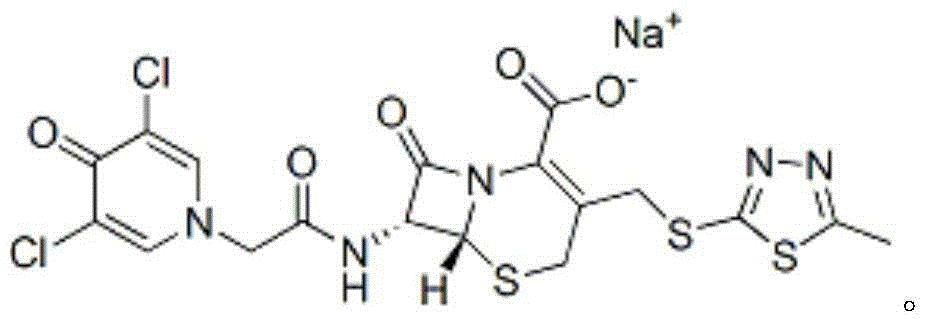
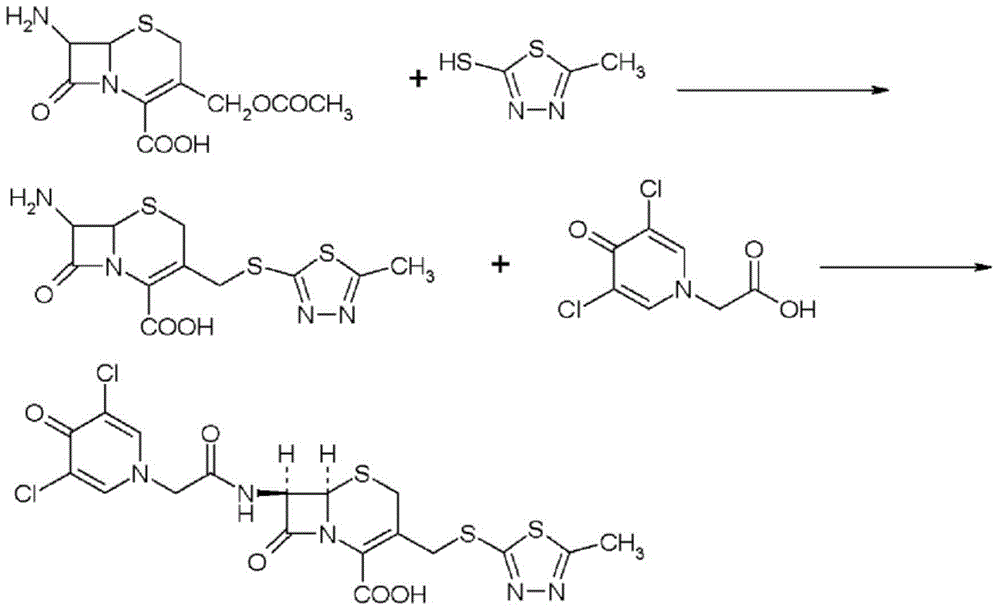
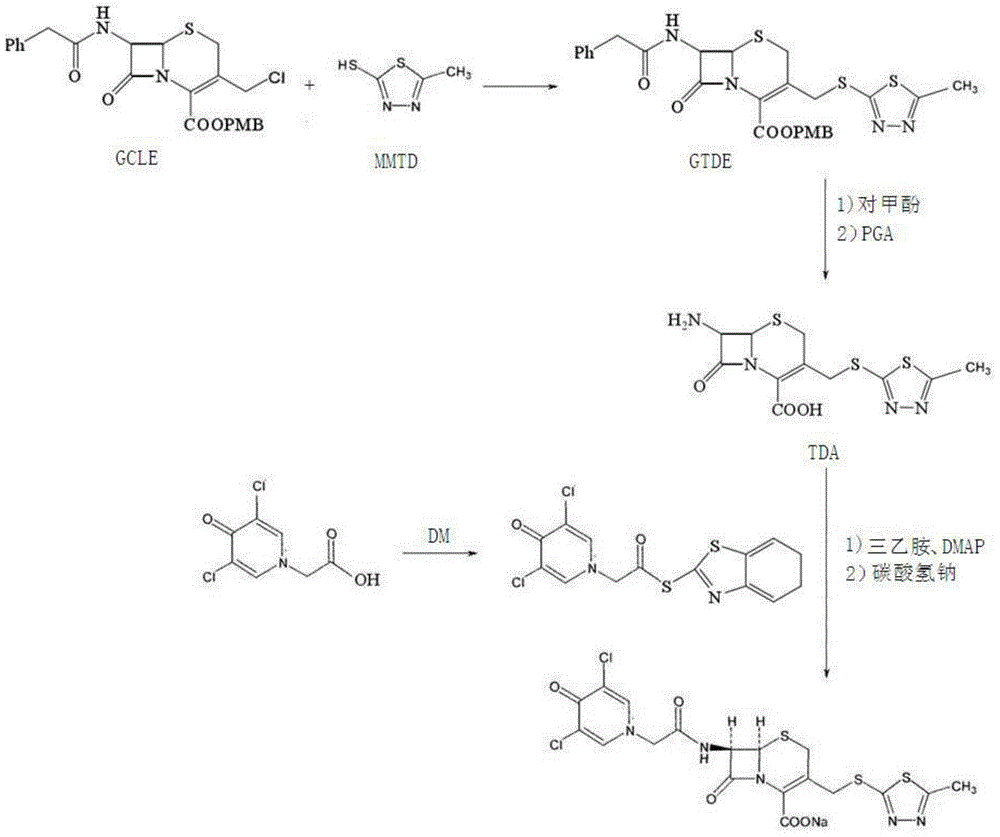
ActiveCN105017286ASimple preparation processReduce generationOrganic chemistry7-ACACefazedone sodium
The invention relates to a preparation method for a cephalosporin anti-infective drug-cefazedone sodium, belonging to the field of pharmaceutical synthesis. According to the invention, the method uses GCLE as a raw material to substitute 7-ACA and overcomes the defects of low yield, high pollution and the like in prior art; the preparation method with mild reaction conditions, little side reaction and simple process is provided; meanwhile, the method has the advantages of cheap and easily-available raw materials, low cost, high product yield, high product purity and applicability to industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Process for preparing cefotaxime sodium
Disclosed is a process for preparing cefotaxime sodium belonging to compound preparation technical field. In a solvent, 7-ACA reacts with AE-active ester under the action of amine intermediate reactant, then a sodium salt forming agent is added in, and the cefotaxime sodium is obtained after reaction and seedout. The invention is characterized in that the solvent is a mixed solvent composed of benzene organic solvent, ethyl acetate, or acetone and alcohol organic solvent. The technological process is simplified, and the product yield is high.
Owner:REYOUNG PHARMA
Ceftezole sodium compound with novel route
InactiveCN101735250AAvoid reactionAvoid processing powerOrganic chemistryAcetic acidCeftezole Sodium
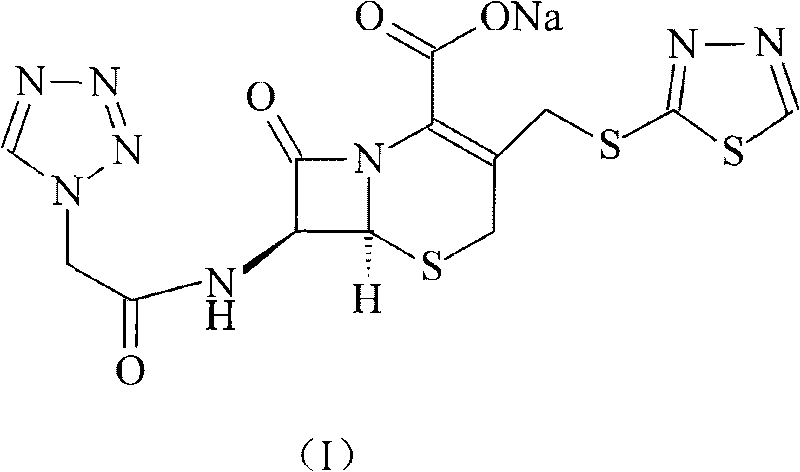
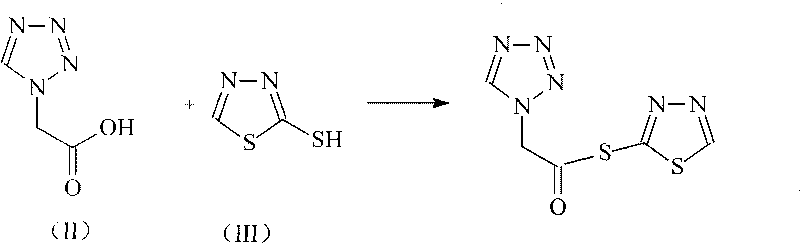
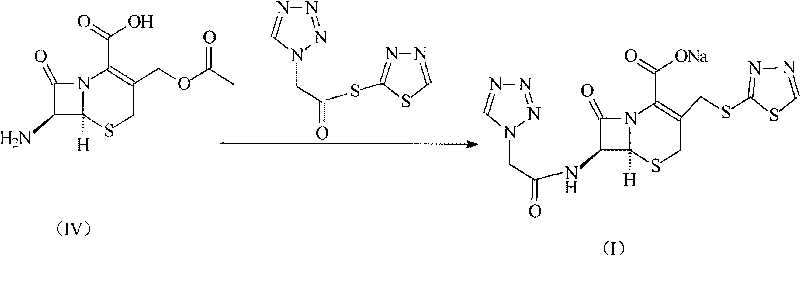
The invention relates to a cephalo class compound, in particular to a Ceftezole sodium compound which belongs to the technical field of medicine. Tetrazolyl acetic acid is adopted to condense with 2-mercapto-1,3,4-thiadiazole to generate tetrazolyl acetic acid 2-mercapto-1,3,4-thiadiazole ester, and the tetrazolyl acetic acid 2-mercapto-1,3,4-thiadiazole ester and 7-aminoce-phalosporanic acid (7-ACA) react to directly obtain a target compound of Ceftezole sodium. The multi-step reaction and the complicated postprocessing of the prior art are avoided, the problems existing in the prior art are solved, the steps are simplified, the cost is lowered, and the yield is enhanced greatly.
Owner:HAINAN LINGKANG PHARMA CO LTD
Comprehensive recovery method for effective ingredients in 7-amidogen cephalosporins alkanes acid crystallization mother liquor produced through enzymatic hydrolysis method
ActiveCN105198762AImprove qualityGuaranteed yieldOrganic compound preparationAmino-carboxyl compound preparationRecovery methodHigh concentration
The invention belongs to the technical field of pharmacy, and relates to a comprehensive recovery method for effective ingredients in 7-amidogen cephalosporins alkanes acid crystallization mother liquor produced through an enzymatic hydrolysis method. The method comprises the following steps that 1, the 7-ACA mother liquor is prepared; 2, 7-ACA and CPC are adsorbed and decomposed; 3, D-alpha-aminoadipicacid is prepared; 4, 7-ACA is prepared. 7-ACA and D-alpha-aminoadipicacid in the 7-ACA crystallization mother liquor are separated through the specific adsorption effect of macroreticular resin on 7-ACA, appropriate decomposition agents is adopted for decomposing 7-ACA and CPC adsorbed to the resin, then D-alpha-aminoadipicacid and 7-ACA are recovered from adsorption raffinate and decomposition liquid respectively, the quality of the recovered 7-ACA products is superior to that of 7-ACA products obtained through the enzymatic hydrolysis method, and D-alpha-aminoadipicacid can be used for preparing a fermentation medium of CPC. The effective ingredients in the mother liquor are successfully recovered through the brand-new process, the emission of high-concentration waste water is greatly reduced, the cost for treating sewage is reduced, and environment-friendly, clean and green production is achieved.
Owner:SHANXI WEIQIDA PHARMA IND
Preparation method for cefotaxime acid
InactiveCN105503904AImprove effectivenessLarge granularityOrganic chemistrySodium bicarbonateCefotaxime
The invention discloses a preparation method for cefotaxime acid, and belongs to the technical field of medicine. The preparation method comprises the following steps: firstly mixing dichloromethane with ethanol, purified water and isopropyl alcohol, and then adding 7-ACA (aminocephalosporanic acid), AE-active ester and antioxygen to obtain mixed liquor; dropwise adding triethylamine into the mixed liquor in 2 to 3 hours for a reaction, and when HPLC (high performance liquid chromatography) is adopted to detect that 7-ACA residual amount is less than 1 percent, regarding as a complete reaction; adding sodium bicarbonate aqueous solution of which the mass concentration is 1 percent to 5 percent for extraction, after reduced pressure suction filtration on an aqueous phase, adding acetone and mixing, dropwise adding hydrochloric acid of which the mass concentration is 10 percent to 25 percent under temperature of 10 to 15 DEG C until a pH (potential of hydrogen)value is 2.5 to 3.0, and cultivating crystals for 1 to 3 hours; centrifuging, spin-drying, leaching, and drying after spin-drying to obtain the cefotaxime acid. The cefotaxime acid prepared by the invention has uniform particle size distribution and stable performance; mass yield reaches more than 170 percent, and product purity can reach more than 99 percent.
Owner:HENAN KANGDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com