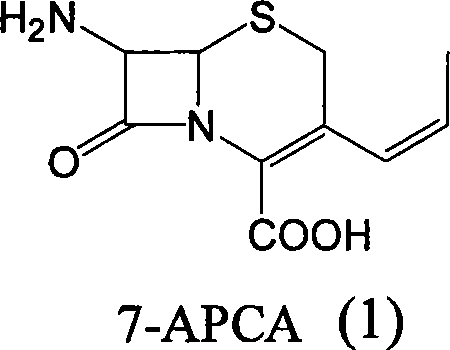
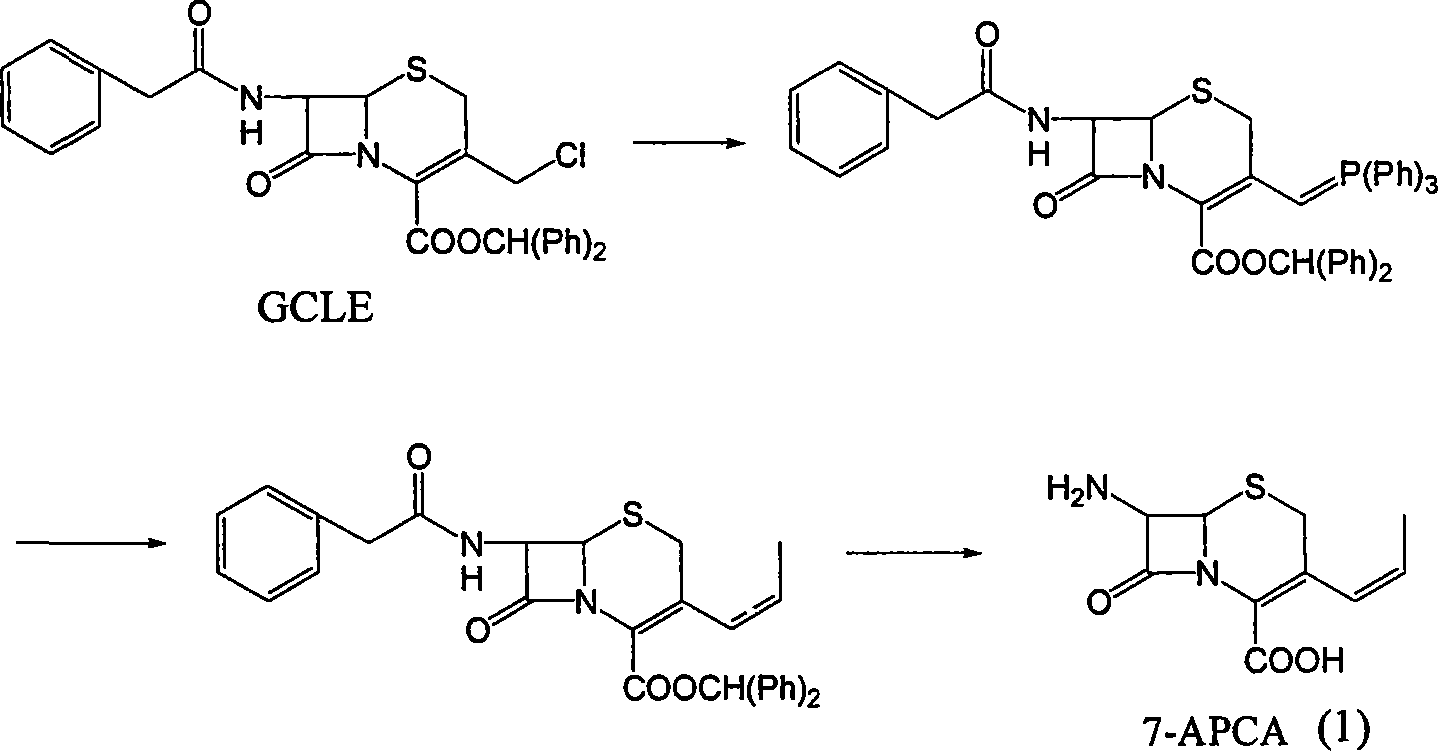
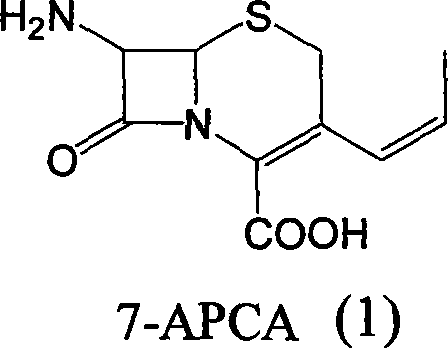
Method of preparing cefprozil parent nucleus 7-amino-3-propenylcephalosporanic acid
A technology of propenyl cephalosporanic acid and cefprozil parent nucleus, applied in the direction of organic chemistry and the like, can solve the problems of low yield, high cost, unstable quality and the like, and achieve the effects of high yield, low cost and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1 At room temperature, add 12g of 7-ACA, 100ml of dichloromethane, 10g of hexamethyldisilazane (HMDS), 1ml of trimethylchlorosilane into a dry reaction flask, and raise the temperature to 40-55°C under the protection of nitrogen , reflux for 7-8 hours, and when it is detected that there is almost no ammonia escape in the reflux tail gas, the reflux is stopped and the temperature is lowered to 0-5° C. to obtain a silylated solution of 7-ACA. 13.5 g of triphenylphosphine was added to the reaction liquid, and 11.5 g of iodotrimethylsilane was added in about 10 minutes, and the temperature should not be higher than 5°C. After the addition is complete, keep the reaction at 5-10°C for 3-5 hours, take a sample to detect that the 7-ACA residue is less than 0.5g / L (HPLC), after the reaction is completed, cool down to -5-0°C to obtain a mixture of compound (3) .
[0029] Step 2 Add 87ml of DMF to the mixed solution of compound (3) obtained in Step 1, control the temperature...
Embodiment 2
[0032] Step 1 At room temperature, add 12g of 7-ACA and 100ml of dichloromethane into the dry reaction flask, cool down to 0-5°C, add 13.5g of triphenylphosphine, and add 33g of iodotrimethylsilane within about 10 minutes, The temperature shall not be higher than 5°C. After the addition is complete, keep the reaction at 5-10°C for 3-5 hours, take a sample to detect that the residual 7-ACA is less than 0.5g / L (HPLC), after the reaction is completed, lower the temperature to -5-0°C to obtain a mixture of compound (3) .
[0033] Subsequent operation is the same as embodiment one, and the result is the same.
Embodiment 3
[0035] Step 1, step 2 are the same as embodiment 1.
[0036] Step 3 The mixture of compound (4) obtained in step 2 was vacuum distilled to remove dichloromethane. The distillation temperature was controlled below 50°C. After the distillation was completed, it was lowered to room temperature, 5g of activated carbon was added, stirred for 30 minutes, and filtered. Add 400ml of water to the filtrate, heat up to 35-40°C, keep stirring for 30 minutes, cool down to 0-5°C, stir for 1 hour, filter, and wash with 50ml of water and 100ml of acetone. Vacuum drying at 35°C for 7-8 hours gave 10.2 g of 7-APCA with a purity of 95.5% (HPLC cis / trans = 92.5 / 7.5). Yield 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com