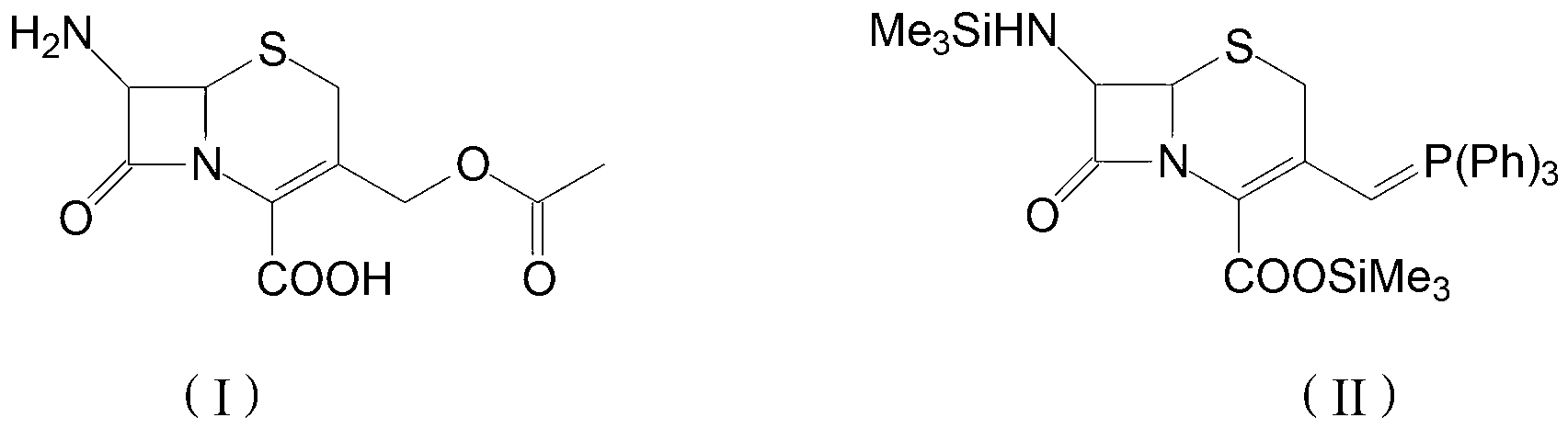Patents
Literature
69 results about "Cefprozil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
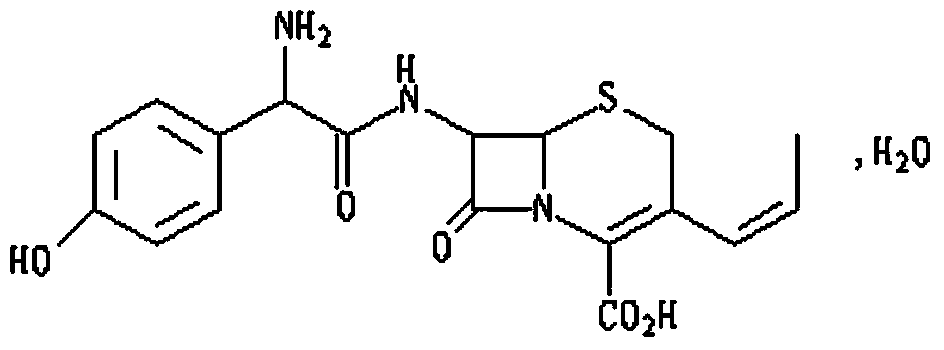
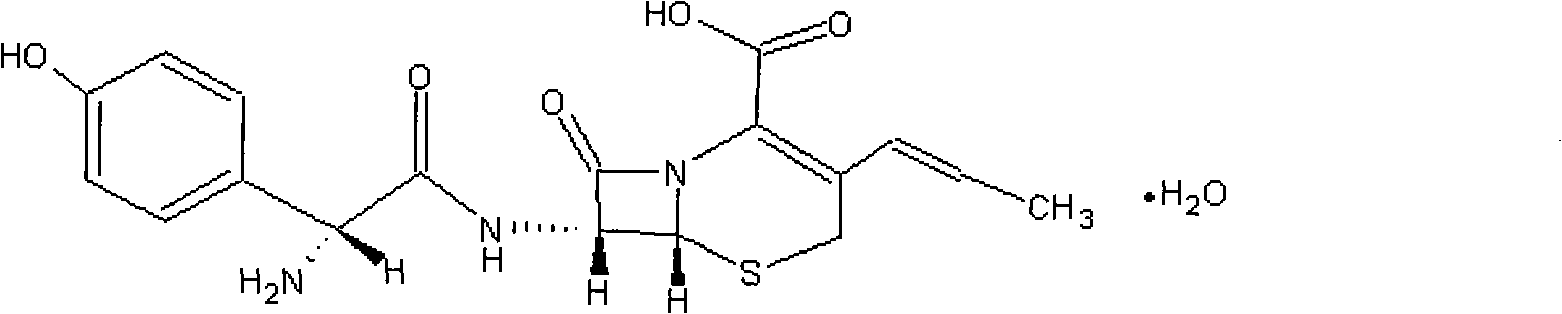
Cefprozil is used to treat a wide variety of bacterial infections.
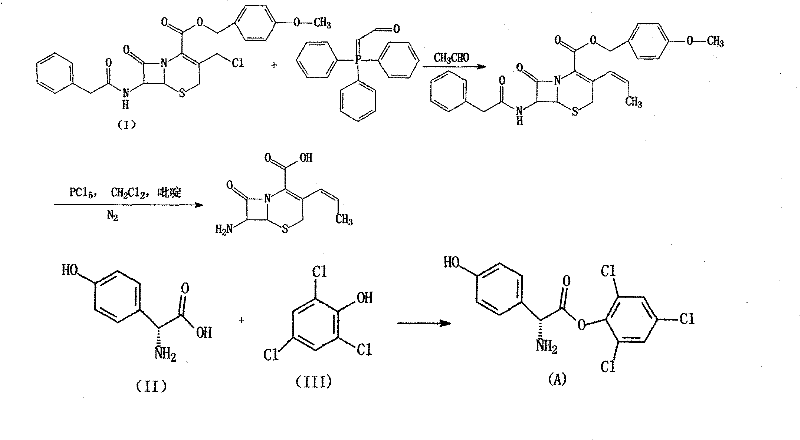
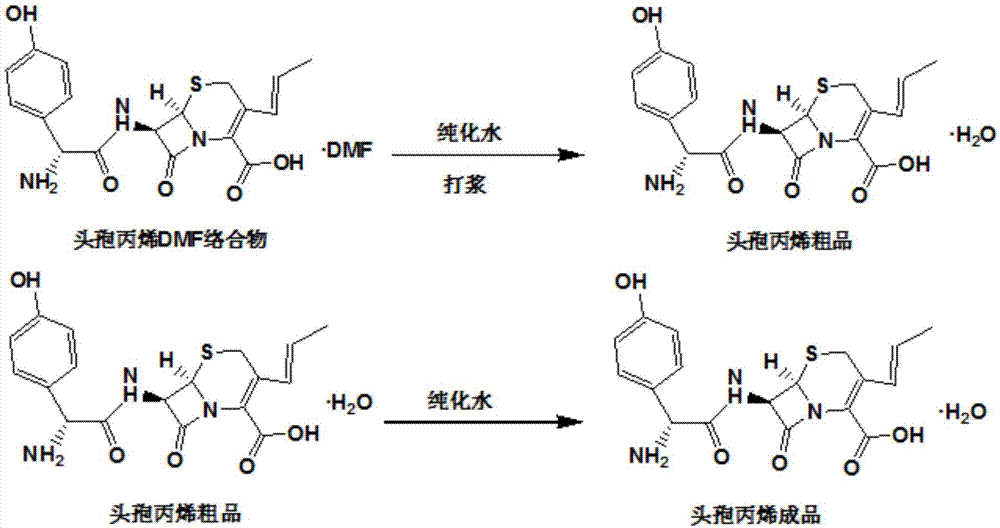
Method for preparing cephalosporin propylene
The invention discloses a preparation method of cefprozil, which comprises: 7-amin cethalosporanic acid (7-ACA) reacts with triphenyl phosphine to get 7- trimethylsilyl amino-3-triphenyl phosphate methylene-4-cethalosporanic acid trimethylsilyl ester through silanization protection and iodination reagent replacing under the condition of catalyst existing; WITTIG reaction is made for the product and acetaldehyde to get 7-trimethylsilyl amino -3-(propenyl-1-alkenyl)-4-cethalosporanic acidtrimethylsilyl ester; then the compound reacts with D-para hydroxybenzene glycine dane potassium salt to geta compound (6R, 7R)-7-[(2R)-2- ethoxycarbonyl-1-methyl - ethylene amino (4-trimethylsilyl oxyphenyl) acetamido group(amide)]-8-oxo-3- (1- propenyl)-5-thio-1- heterobicycle [4.2.0] octylene-2-ene-2-carboxylic acid trimethylsilyl ester; hydrolytic treatment is then used to get the cefprozil. The invent adopts the method of one pot and can participate in next reaction without separating intermediateproducts. The preparation method of cefprozil has the advantages of low cost, convenient operation and high overall yield, adapting to demands of industrial production.
Owner:南通康鑫药业有限公司
Cefprozil medicinal composition
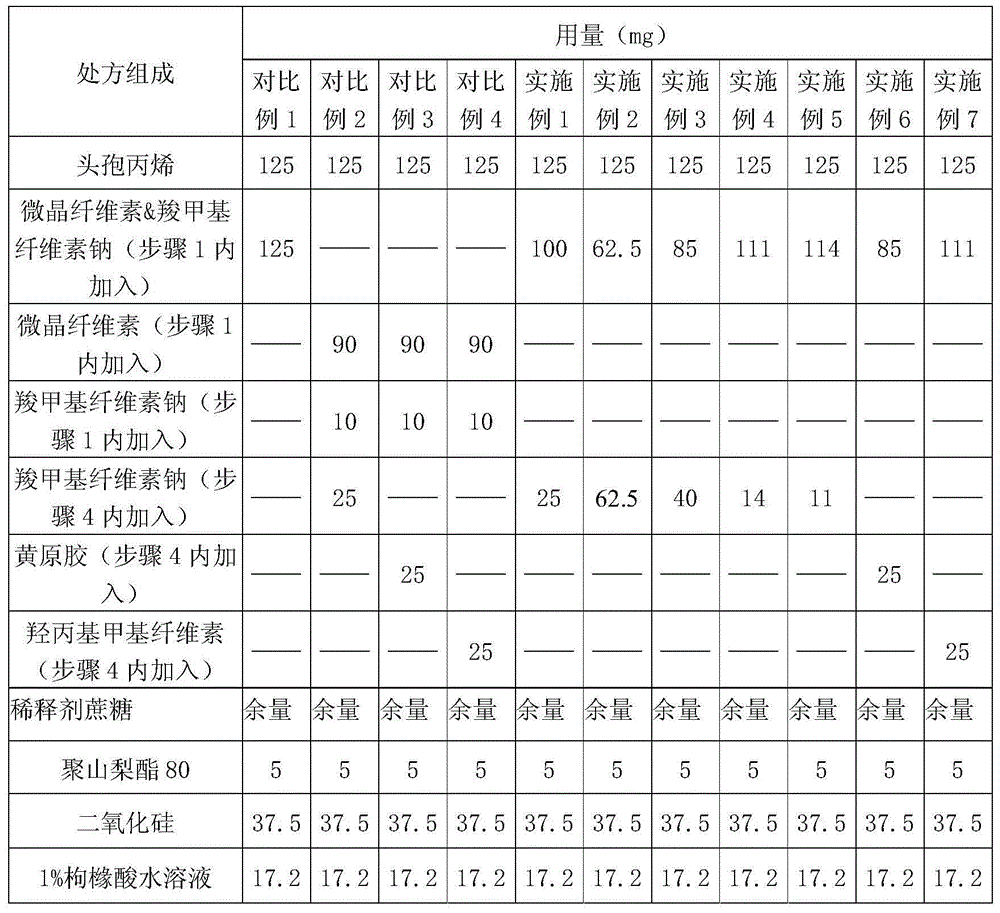
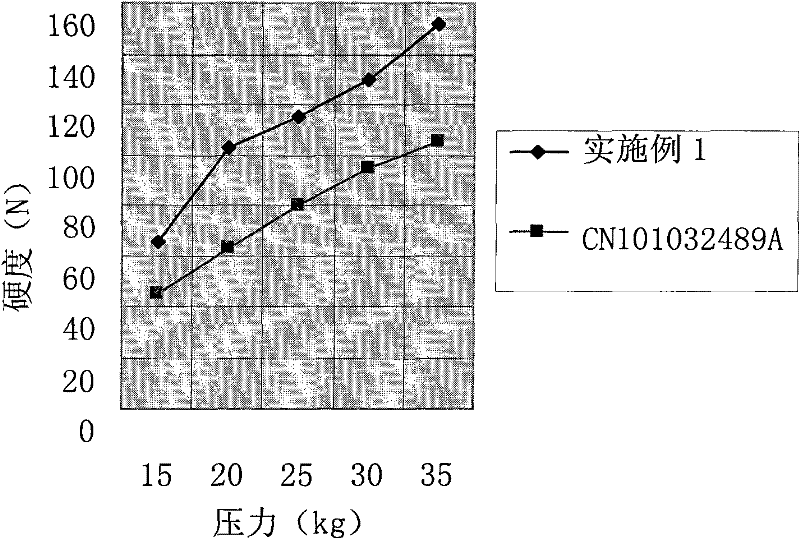
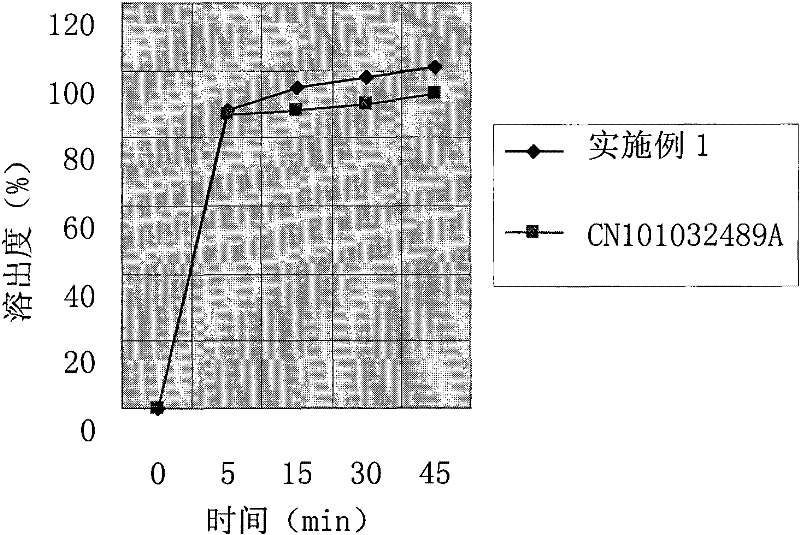
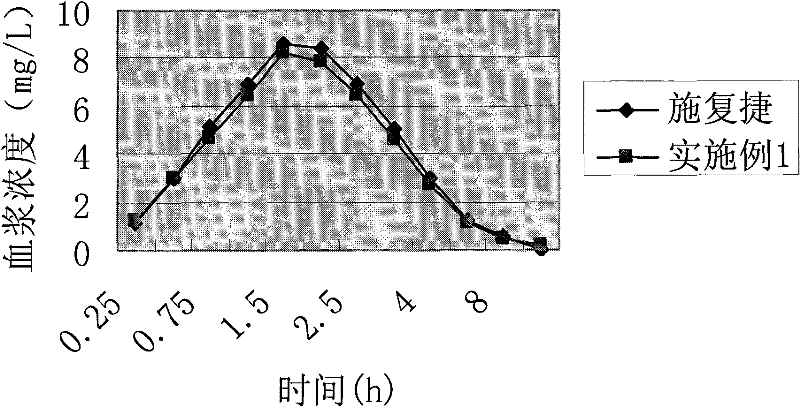
The invention relates to a cefprozil medicinal composition, in particular to a medicinal composition containing cefprozil and cellulose derivatives, which is prepared by a dry granulation method. The cefprozil and the cellulose derivatives are uniformly mixed, and the mixture is pressed into plates under the pressure of 0.1 to 25MPa so as to form tight combination; and then, according to requirements, the plates are prepared into tablets, powder, granules or capsules. The medicinal composition can further contain other conventional auxiliary materials; the obtained plates are ground, mixed with a diluent, a disintegrating agent, a lubricating agent, an adhesive and / or other auxiliary materials; and the mixture is prepared into tablets, powder, granules, suspension or capsules according to requirements. The dry granulation process is adopted; and after the cefprozil and the cellulose derivatives are tightly combined through proper pressure, the beta-lactam ring of the cefprozil is effectively prevented from ring-opening degradation, the stability of the medicinal composition is improved, and the effectiveness and safety of clinical medication are further guaranteed.
Owner:SINOPHARM SHANTOU JINSHI PHARMA CO LTD
Process for the preparation of 3-propenyl cephalosporin DMF solvate
InactiveUS6903211B2High purityHigh yieldAntibacterial agentsOrganic chemistryEnzymatic hydrolysisBis(trimethylsilyl)amine
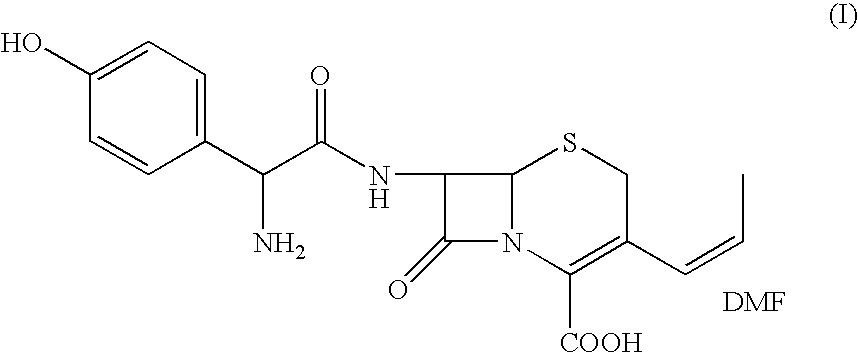
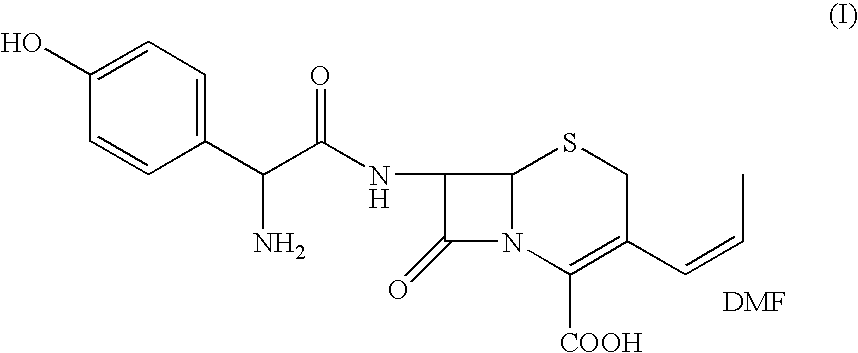
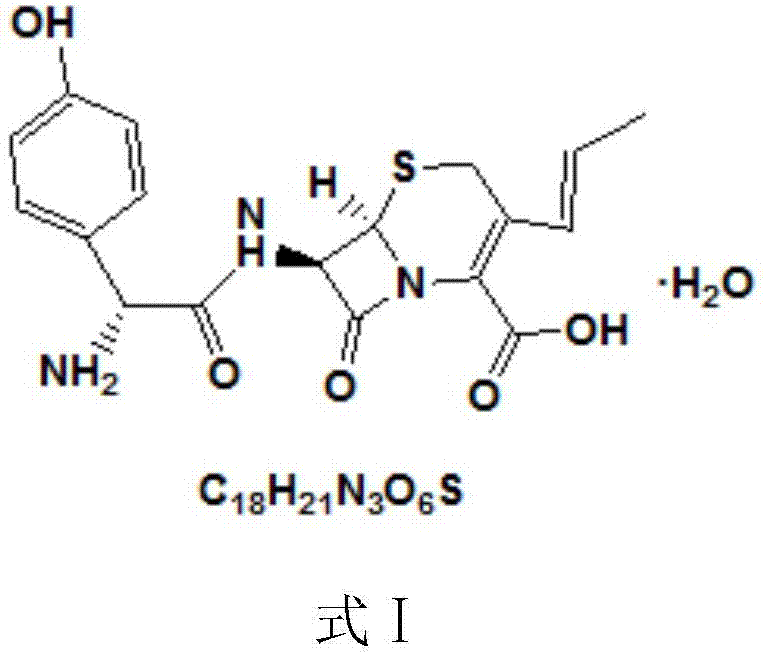
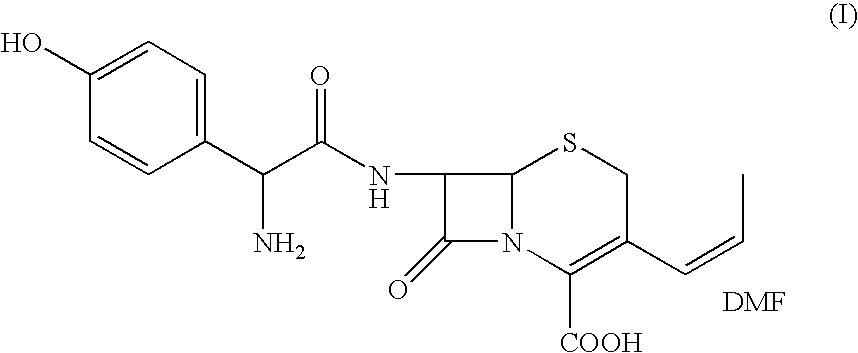
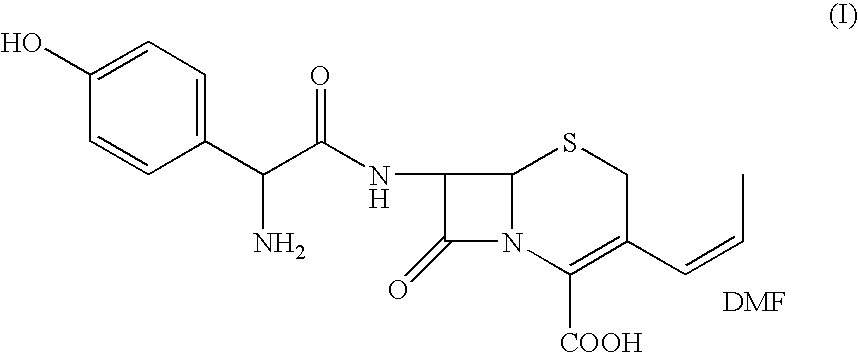
The present invention relates to an improved process for the preparation of cefprozil DMF solvate of formula (I), which is useful for the preparation of cefprozil, comprising:i) reacting a compound of formula (VIII) with acetaldehyde to produce a compound of formula (IX),ii) deesterifying the carboxy protecting group of the compound of formula (IX) using an acid to yield a compound of formula (X),iii) converting the compound of formula (X) to a compound of formula (XI),iv) neutralizing the compound of formula (XI) followed by enzymatic hydrolysis to produce an APCA of formula (V),v) silylating the APCA using a mixture of trimethylsilylchloride and hexamethyldisilazane to produce silylated APCA of formula (XII), andvi) condensing the silylated APCA with a mixed anhydride to produce the DMF solvate compound of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Cefprozil submicron emulsion solid preparation and new application thereof
InactiveCN101700232AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsYolkEmulsion
The invention relates to a cefprozil submicron emulsion solid preparation and new application thereof in the preparation of medicine for treating acute plasma cell mastitis. The cefprozil submicron emulsion granule comprises cefprozil, yolk lecithin, poloxamer 188 and deoxysodium cholate with the weight ratio of 1:2.3-14:1.2-8:0.8-10.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefprozil dispersible tablet and preparation method thereof
ActiveCN103110596ASimple production processDissolution rate is fastAntibacterial agentsOrganic active ingredientsAdditive ingredientDissolution
The invention relates to a preparation method of a medicinal preparation taking cefprozil as a main ingredient, and in particular relates to a preparation method of a cefprozil dispersible tablet with higher bioavailability. The method comprises the following steps of: taking the cefprozil in a therapeutic dose as a main active medicine ingredient; micronizing the cefprozil; adding an additive in proper quantity into the cefprozil to be pelletized by using a dry method; and pressing a pellet into the tablet. The cefprozil dispersible tablet comprises the following ingredients in parts by weight: 250+ / -5 parts of the cefprozil, 180+ / -20 parts of microcrystalline cellulose, 100+ / -10 parts of micronized lactose or pregelatinized starch, 30+ / -3 parts of hydroxypropyl cellulose; 5+ / -2 parts of lauryl sodium sulfate, 2.5+ / -1 parts of magnesium stearat and 2.5+ / -1 parts of stevioside, wherein the micronized cefprozil is in the shape of powder with the particle diameter of 60 to 150 mu m. The method provided by the invention has the advantages that the production process is simple; and the prepared cefprozil dispersible tablet is high in dissolution rate and stable in quality.
Owner:GUANGDONG BOZHOU PHARMA
Cefprozil suspension pharmaceutical composition
InactiveCN102144975AGood redispersibilityImprove stabilityAntibacterial agentsPowder deliveryPreservativeDeodorant
The invention relates to a cefprozil suspension pharmaceutical composition, in particular to a suspension pharmaceutical composition containing cefprozil and xanthan gum. The cefprozil and the xanthan gum are uniformly mixed according to a certain proportion, pharmaceutic adjuvants, such as appropriate amount of disintegrating agent, diluent, flavoring agent, deodorant or lubricant and the like, are added in the mixture, then, the mixture is prepared into standard suspension in a manner of dry granulation or directly filling dry powder. The xanthan gum used as a suspending aid is less in dosage and excellent in adding suspension, the stability of the basic remedy is excellent, and simultaneously, the composition is free from solubilizer and preservative, so that the problem that the existing cefprozil suspension is long in dispersion time in water and low in safety, and has various accessories; and the compliance for taking the composition by a patient is further improved while the effectiveness and the safety of clinical application are ensured.
Owner:SHANDONG INST OF PHARMA IND
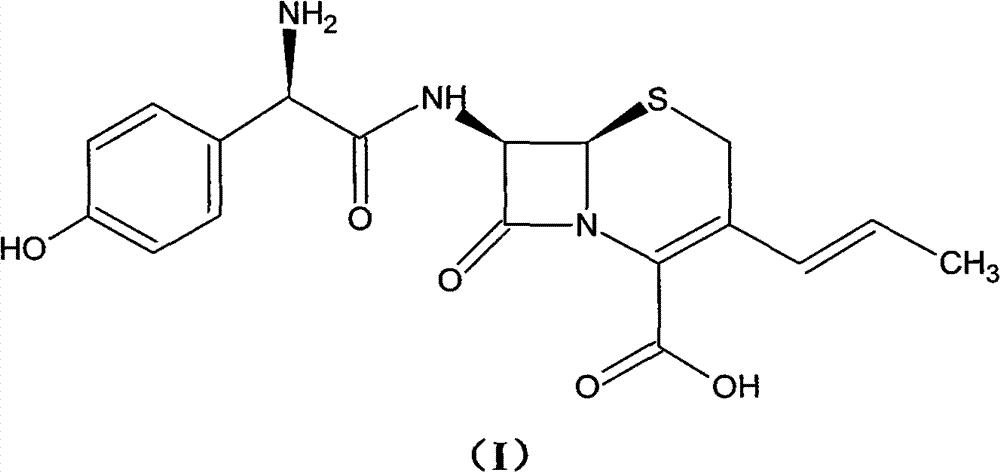
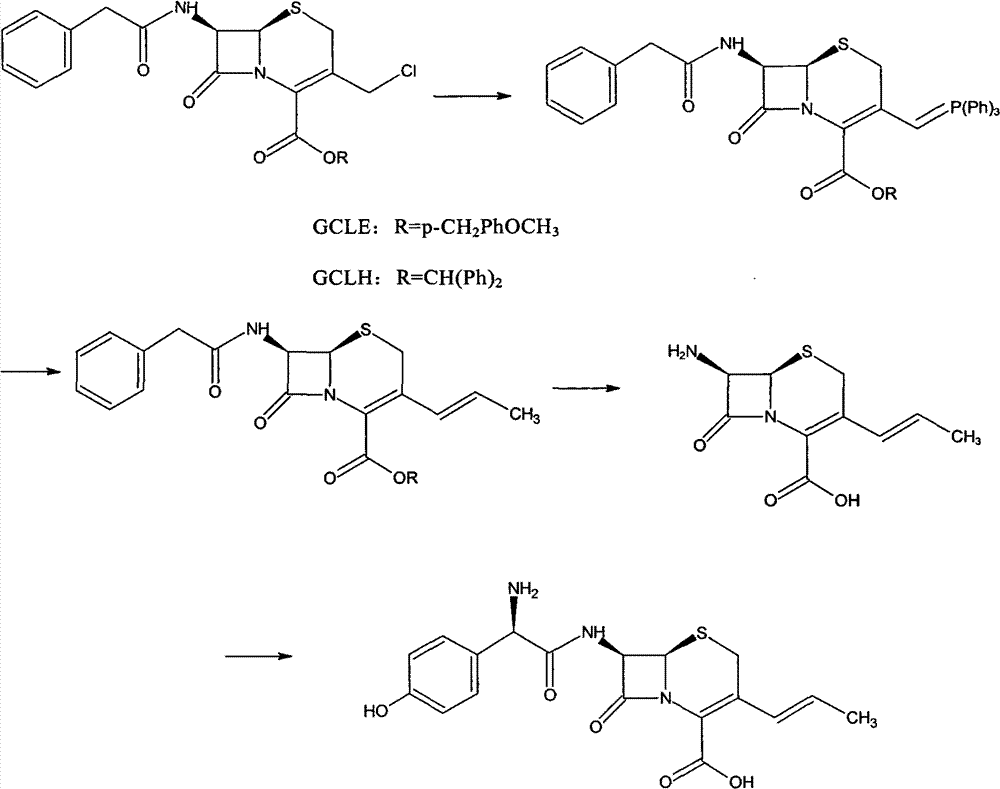
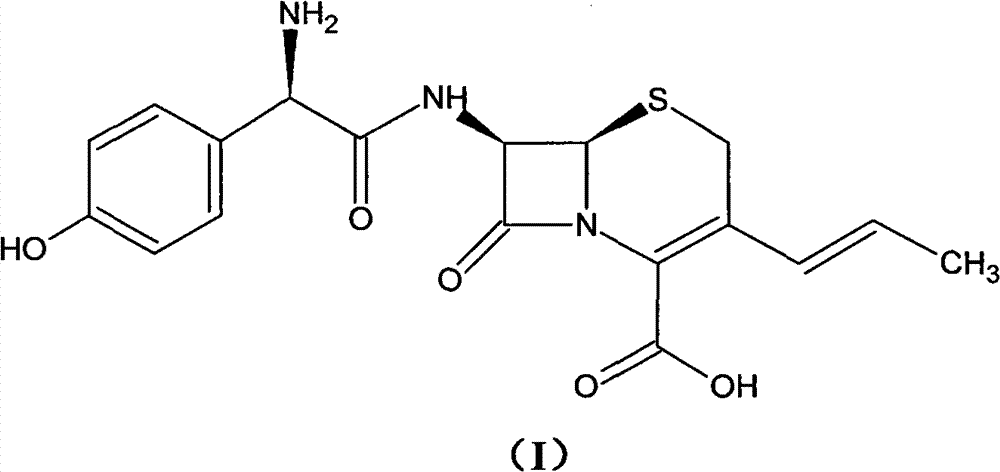
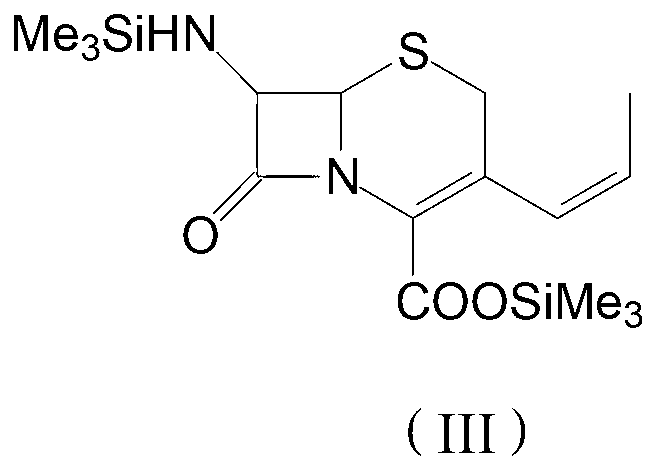
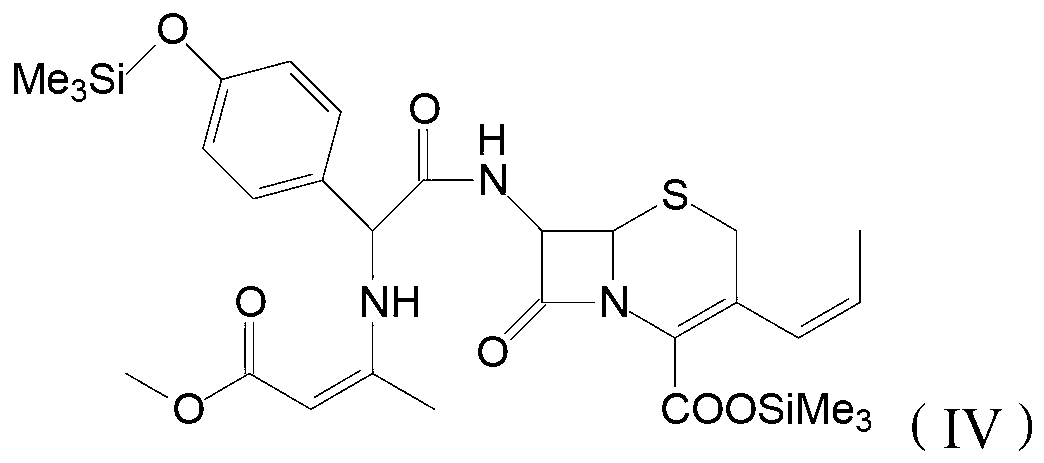
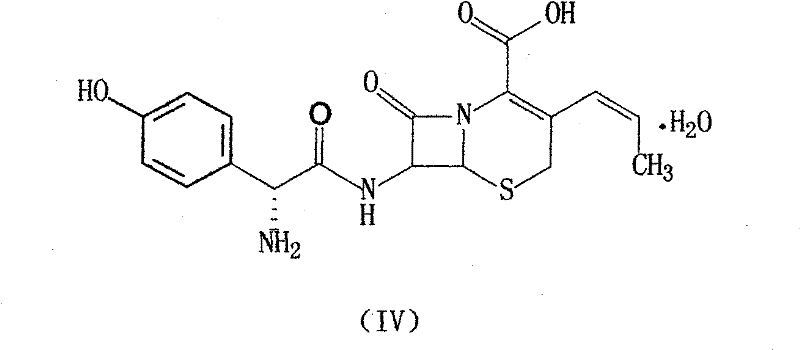
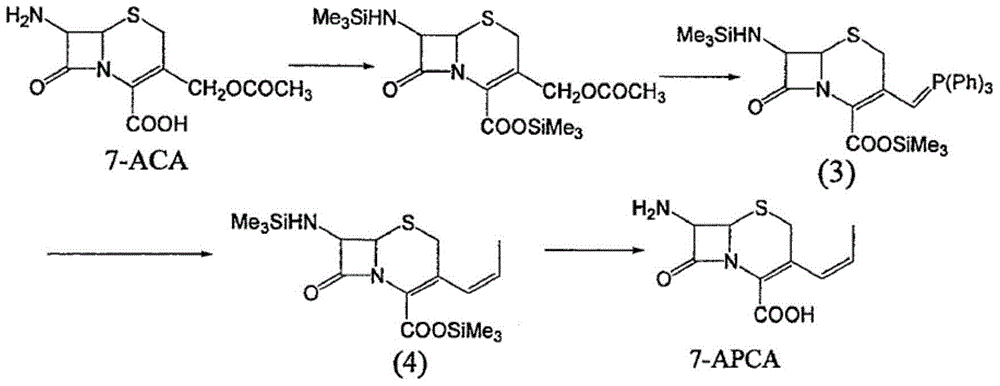
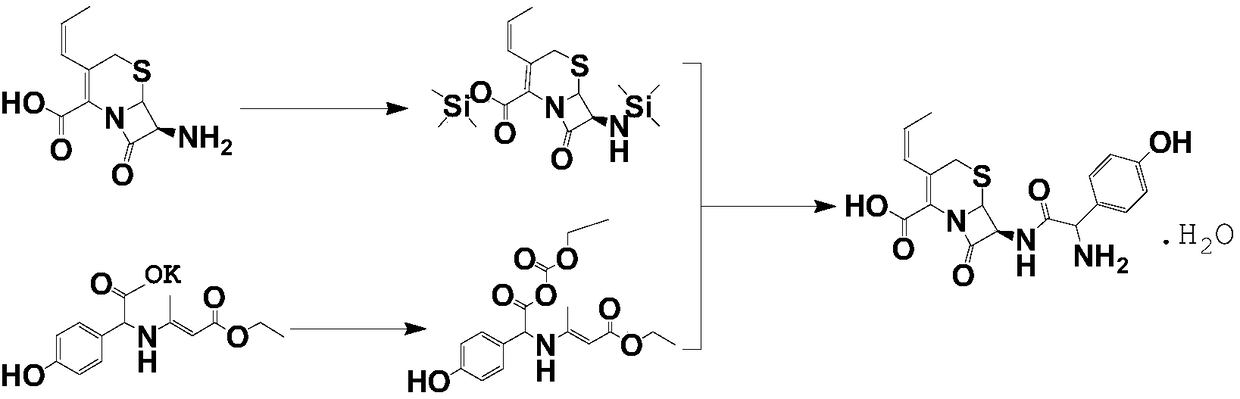
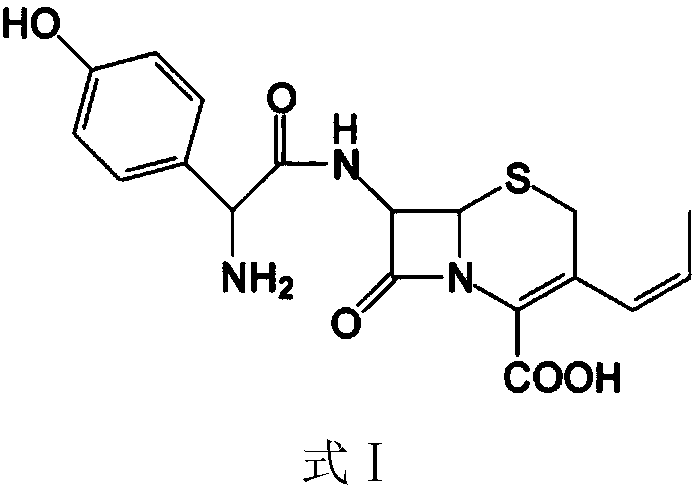
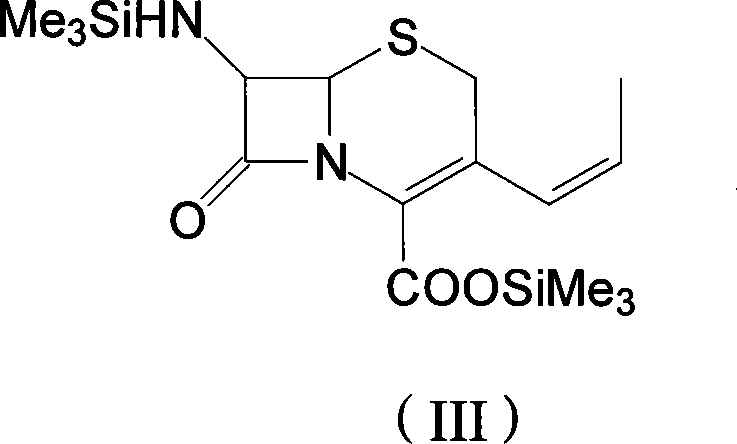
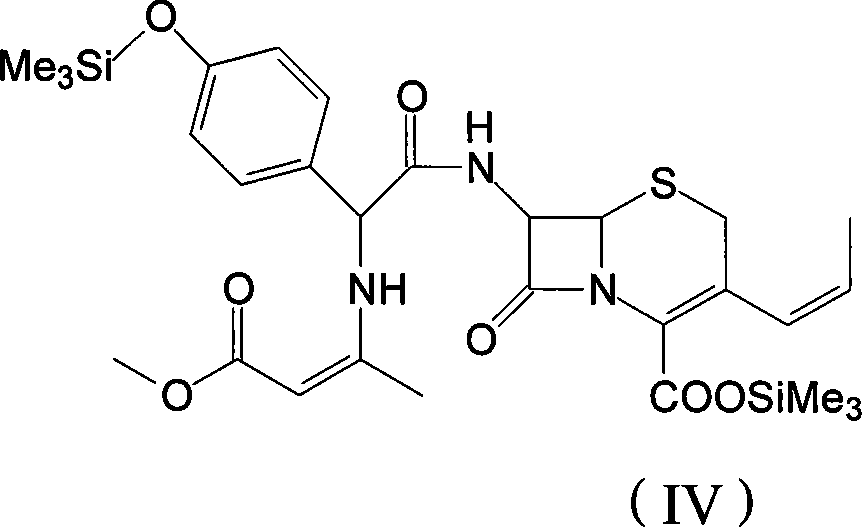
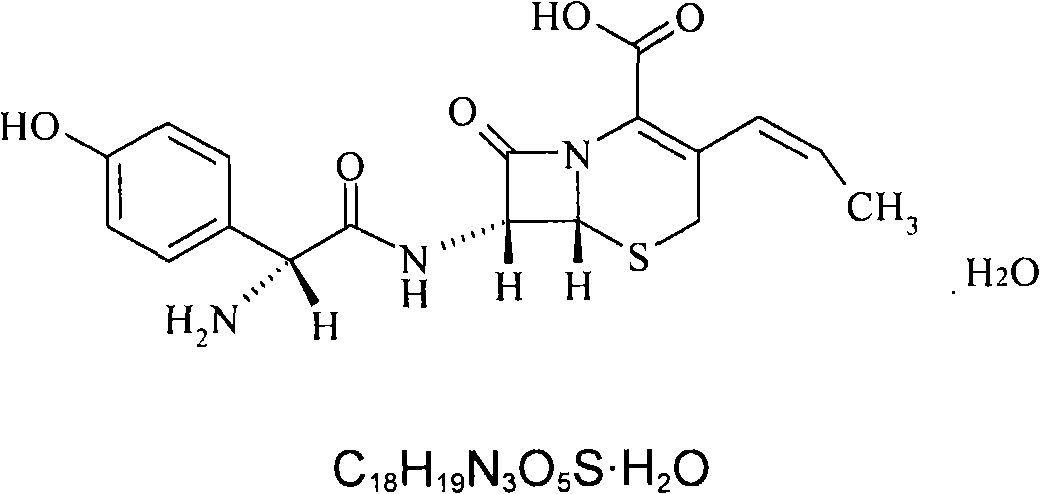
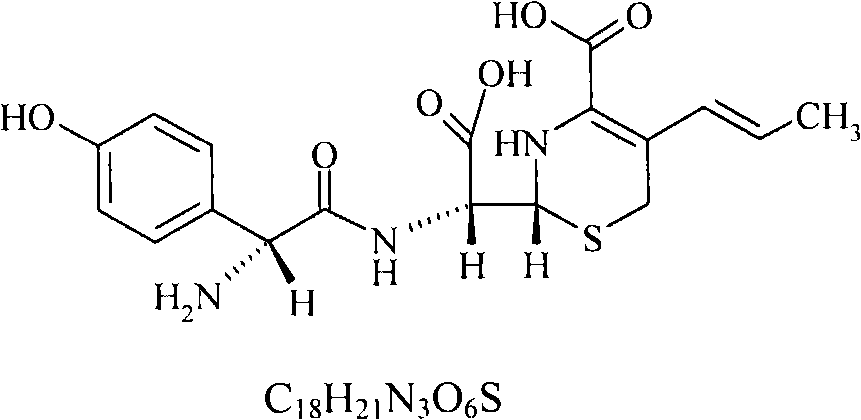
Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid
InactiveUS20060149096A1High purityHigh yieldGroup 4/14 element organic compoundsOrganic compound preparationCarboxylic acidHydrolysis
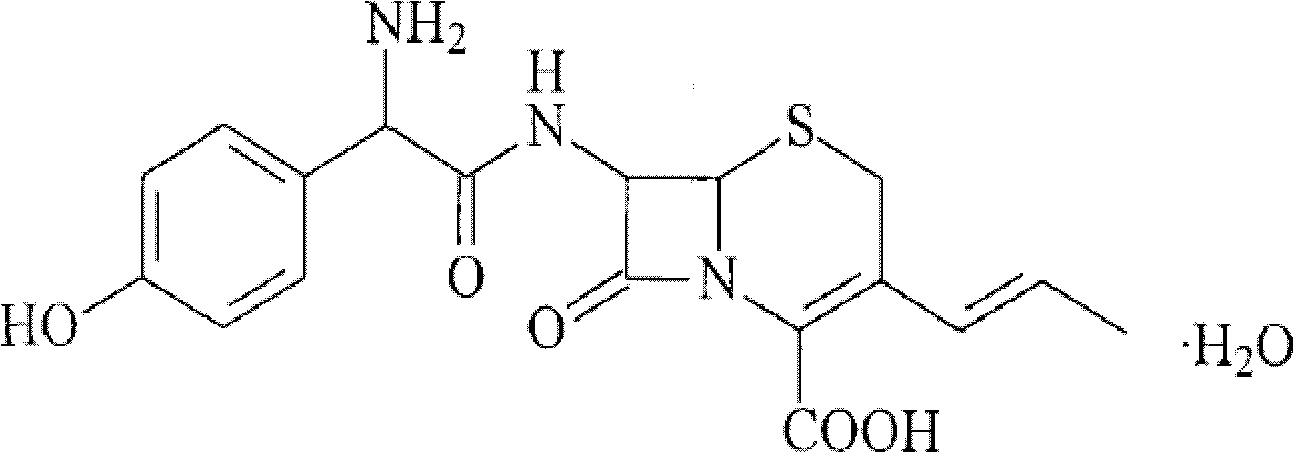
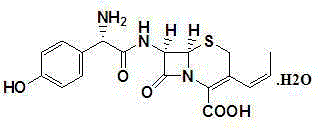
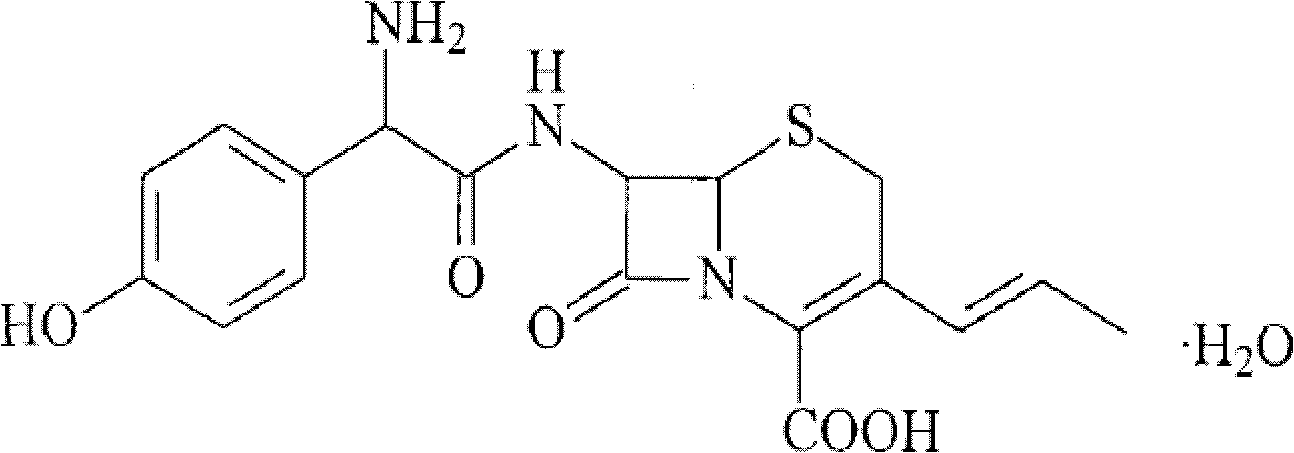
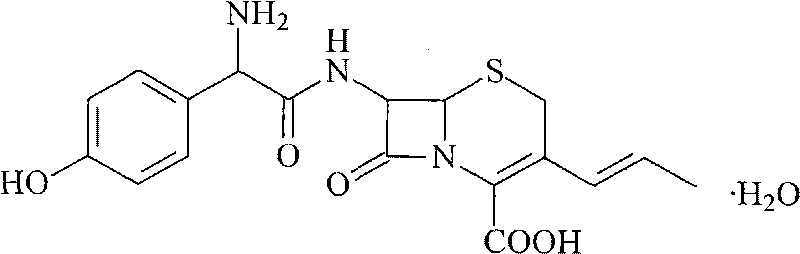
A process for preparation of 7-[D-α-amino-α-(4-hydroxyphenyl)acetamido]-3-(1-propen-1-yl)-3-cepham-4-carboxylic acid viz. Cefprozil of formula (I) igh purity, substantially free of impurities, which comprises preparation of mixed acid anhydride by selecting the sequence and temperature of addition of the reagents and its subsequent condensation with a protected 7-APCA; followed by hydrolysis, isolation and purification to give Cefprozil of formula (I) in the form of a monohydrate.
Owner:LUPIN LTD
Preparation method of cefprozil
ActiveCN102030762BHigh yieldEasy to purifyOrganic chemistryFermentationPurification methodsCefprozil
Owner:国药集团致君(苏州)制药有限公司
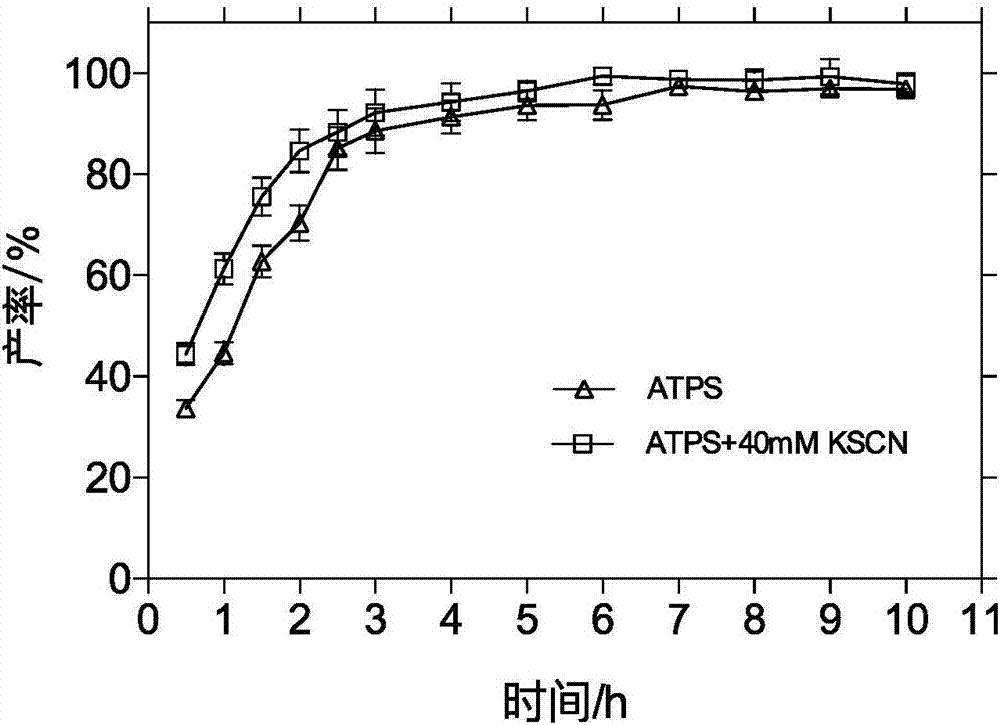
Method for enzymatic synthesis of cefprozil in recyclable aqueous two-phase system by using immobilized penicillin acylase
The invention discloses a method for the enzymatic synthesis of cefprozil in a recyclable aqueous two-phase system by using immobilized penicillin acylase. The method comprises the following steps: dissolving a cefprozil parent nucleus and an acylation reagent in an aqueous two-phase polymer solution, wherein the molar ratio of the parent nucleus to the acylation reagent is 1: (1-4); and adding immobilized penicillin G acylase, then adding a two-phase distribution conditioning agent, adjusting the pH value to 4.5-7, and reacting for 1 to 80 hours at the temperature of 5-30 DEG C, wherein the concentration of enzyme in a reaction system is 50-150U / ml. By adopting the method, the hydrolytic activity of the penicillin acylase can be effectively inhibited, thus the hydrolysis degrees of the acylation reagent and the cefprozil product can be reduced. Compared with a method using water as a medium, the method disclosed by the invention has the advantages that the product and the enzyme are distributed in two different water phases, a reactant is fully in contact with the enzyme, so that a synthesis / hydrolysis specific value is greatly increased, the yield of the cefprozil can be increased by 20%, and the highest yield can reach 78%; the adopted novel recyclable aqueous two-phase system can be recycled, so that the production cost is reduced.
Owner:南通康鑫药业有限公司 +1
Cefprozil compound, and dispersible tablets, dry suspension and preparation method thereof
ActiveCN103524533AHigh puritySuitable for clinical applicationAntibacterial agentsOrganic active ingredientsCefprozilPowder diffraction
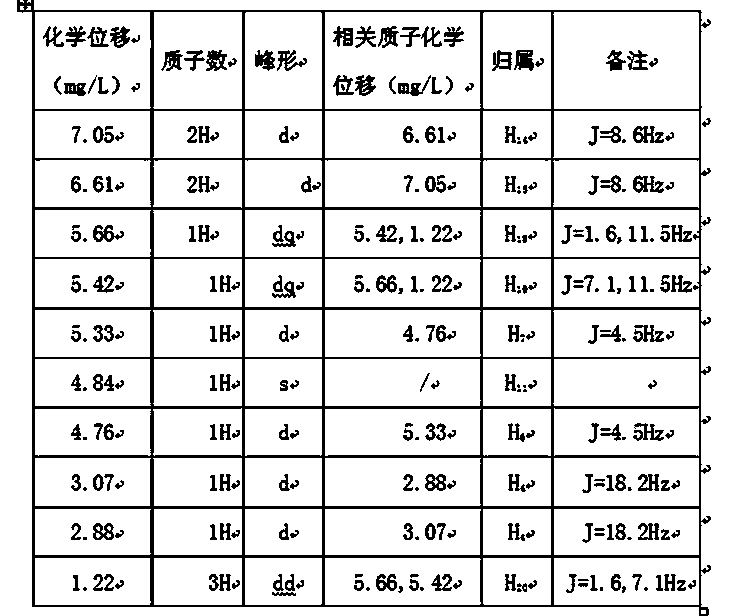
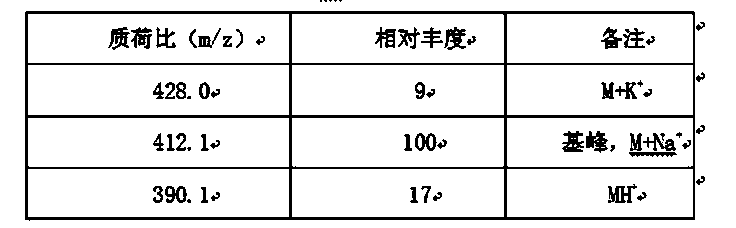
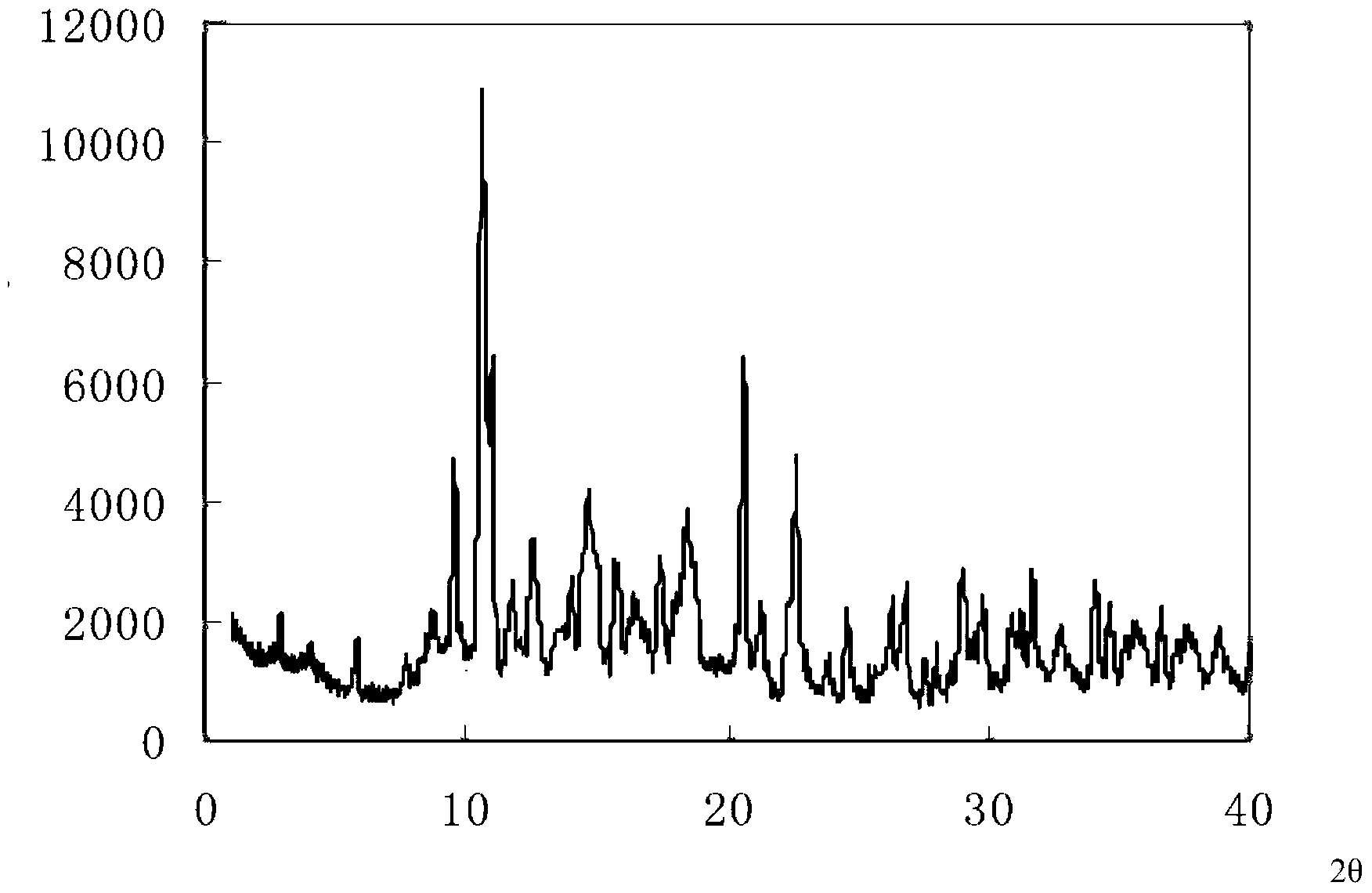
The invention relates to the field of medicine, and in particular relates to a cefprozil compound, and dispersible tablets, a dry suspension and a preparation method thereof. The cefprozil compound is a crystal, and an X-ray powder diffraction diagram obtained by Cu-K alpha ray measurement is shown in figure 1. Through experimental detection, the cefprozil dispersible tablets and the dry suspension have the advantages of superior stability and quite good smell and taste, and are quite suitable for clinical application.
Owner:金鸿药业股份有限公司
Recovery method of cefprozil
The invention discloses a recovery method of cefprozil. The method comprises the steps of dripping dimethylformamide and acetone in a cefprozil refined mother solution to obtain the crude cefprozil; adding the crude cefprozil into a mixed solvent, adjusting the pH value of the solution with hydrochloric acid, activated carbon fading, and adjusting the pH value of stronger ammonia water; and finally cooling crystallization, suction filtration and drying to obtain the end product of cefprozil. The recovery method has the advantages that 95% of cefprozil can be extracted from the mother solution, so that the wastewater treatment pressure is greatly reduced, and the end product meets the pharmacopeia standards after recovery and treatment; and in addition, the recovery method is simple and convenient to operate, and dimethylformamide, acetone and isopropanol can be recycled and utilized.
Owner:南通康鑫药业有限公司
Process for the preparation of 3-propenyl cephalosporin DMF solvate
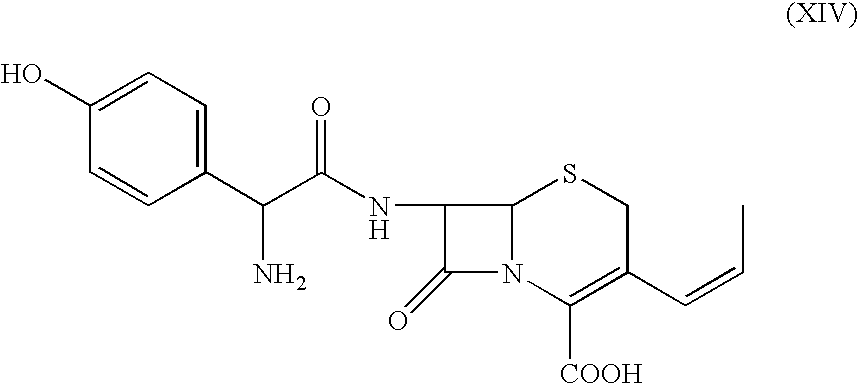
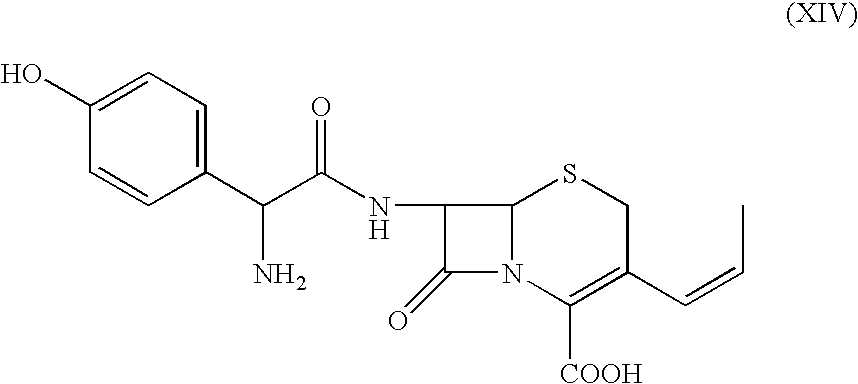
The present invention relates to an improved process for the preparation of 3-propenyl cephalosporin DMF solvate, more particularly, the present invention relates to an improved process for the preparation of cefprozil DMF solvate of the formula (I), which is useful for the preparation of cefprozil of the formula (XIV).
Owner:ORCHID CHEM & PHARM LTD
Dry suspension containing cefprozil liposome and preparation method thereof
InactiveCN101953789AStable storageImprove bioavailabilityAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefprozil liposome, a preparation method thereof and a dry suspension containing the cefprozil liposome. The cefprozil liposome comprises cefprozil, hydrogenated soya bean lecithin, hydrogenated yolk lecithin, cholesterol and vitamin E in the weight ratio of 1:(1.25-5):(1.25-5):(2.5-10):(0.1-3). The dry suspension containing the cefprozil liposome not only conforms to the requirements of Chinese pharmacopoeia, but also has the advantages of more stable storage at normal temperature, rapider effect taking and obviously increased bioavailability compared with common cefprozil medicine compositions.
Owner:王丽燕
Method for synthesizing cefprozil through green enzymatic method
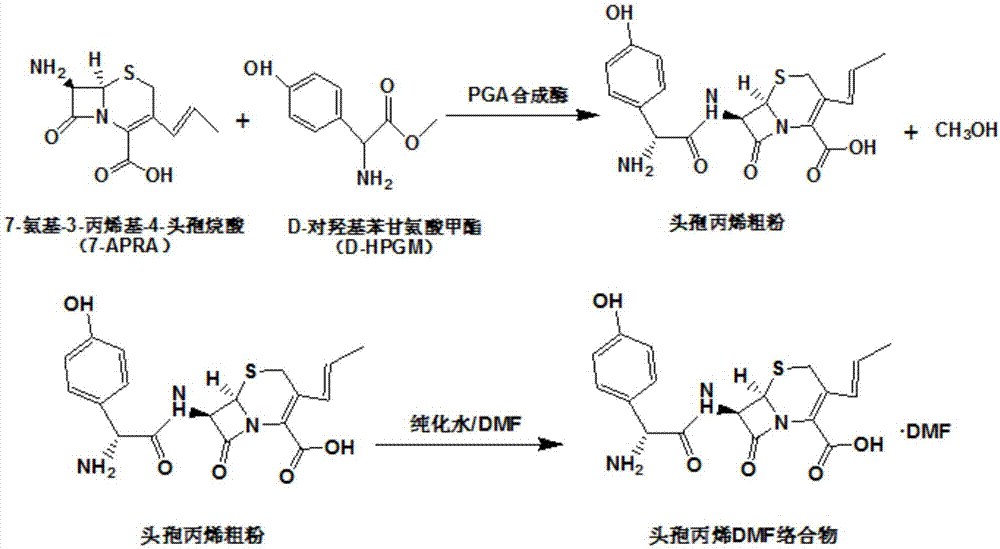
The invention relates to a method for synthesizing cefprozil through a green enzymatic method. The method includes following steps: S1, adding parent nucleus 7-APRA or hydrochloride thereof into a buffer solution, and adding D-para hydroxybenzene glycinate derivative and / or D-para hydroxybenzene glycine amide under the condition that pH is 5-8; S2, adding cefprozil synthetase into the S1, reacting at temperature of 15-30 DEG C and pH of 6.5-7.8 for 1-3h, separating out separation liquid after reaction finishes, and immobilizing cefprozil synthetase to obtain a cefprozil crude product; S3, subjecting the crude product obtained in the S2 to acidolysis dissolved clarification, filtering and re-crystallizing to obtain cefprozil. Raw materials are cheap and easy to obtain, reaction is simple, hydrolysis activity of penicillin acylase can be effectively inhibited, and production cost is low; compared with conventional chemical synthesis methods, the method is simple and convenient to operate and low in cost, synthesis period is shortened, production efficiency is improved, total yield is high, controllability is high, and needs on industrial production are met.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Method for preparing cefprozil in pH responsive regenerative double aqueous phase system
PendingCN106939327AIncreased molar yieldEasy to separateFermentationChemical synthesisCentrifugation
The invention relates to a method for preparing cefprozil in a pH responsive regenerative double aqueous phase system. The method comprises (1) preparing two double-aqueous phase systems, (2) orderly adding 7-APRA, D-p-hydroxyphenylglycine methyl ester hydrochloride into the systems, adjusting solution pH to 5.00-6.50 and controlling a solution temperature in a range of 10-30 DEG C, (3) adding immobilized penicillin acylase into the solution obtained by the step (2), and (4) standing the mixed solution, removing the immobilized penicillin acylase, adjusting the pH of the reaction solution, recovering P<ADBA> / <PMDB> and P<ADB> / P<MDB> polymers of the double-aqueous phase systems, feeding the supernatant to a crystallization section, carrying out crystallization, and carrying out centrifugation, washing and drying to obtain a product. The method reduces a chemical synthesis cost, improves a low product conversion rate of the monohydrolase catalytic reaction, effectively improves a yield, simplifies the operation, reduces a cost and realizes easy recovery of the double-aqueous phase systems.
Owner:EAST CHINA UNIV OF SCI & TECH
Synthesis method of cefprozil
The invention relates to a synthesis method of cefprozil. The synthesis method comprises the following steps: under the existence of an enzyme, enabling parent nucleus 7-APRA and a D-4-hydroxyphenylglycine ester derivative to react with each other, and before the precipitation of cefprozil, adding cefprozil, with the purity greater than 99%, taken as a seed crystal, into a reaction system. According to the invention, the reaction efficiency is improved, the synthesis period is shortened, the conversion rate of 7-APRA is increased, and the purity of the obtained cefprozil is improved; the method is an enzymatic method, and the enzymatic catalysis is only required, water is taken as a solvent, and any organic solvent is not required during the reaction process, so that the environment friendliness is achieved, the route is simple, the product yield is high, and the purity is high.
Owner:ZHEJIANG DONGYING PHARMA
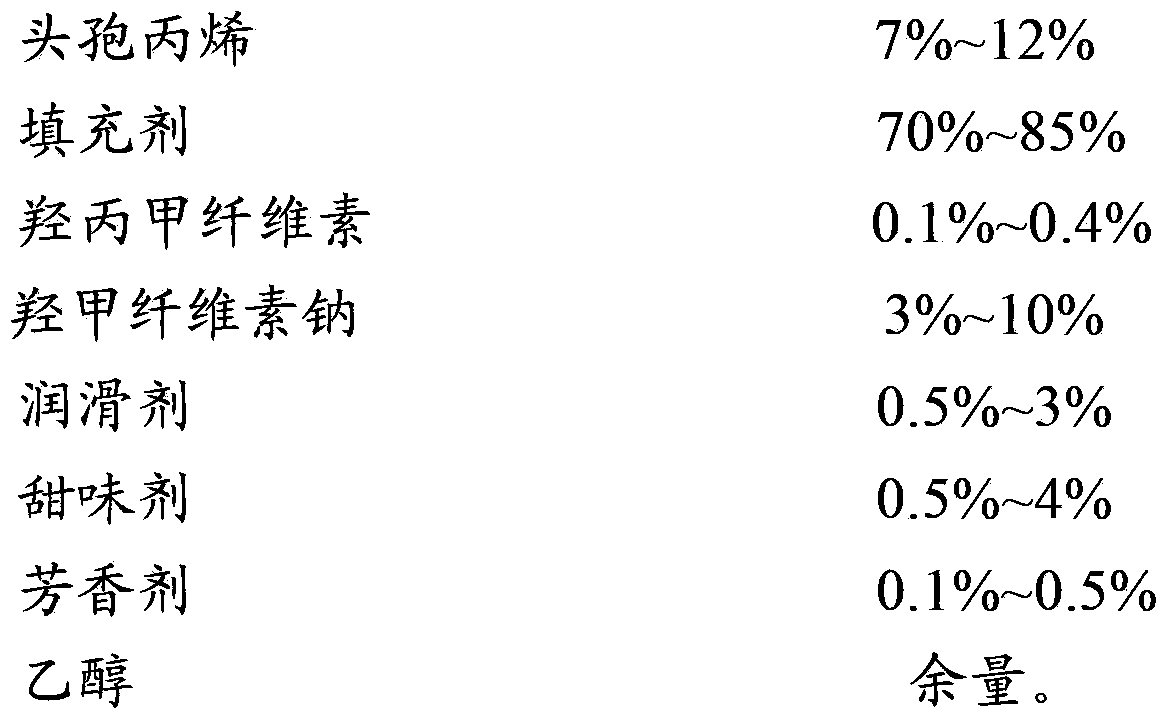
Cefprozil dry suspension and preparation method thereof
ActiveCN103432076ASedimentation volume ratio is goodGood redispersibilityAntibacterial agentsPowder deliveryCarboxymethylcellulose SodiumCefprozil
The invention discloses a cefprozil dry suspension which is composed of the following raw materials by weight: 1.0%-5.0% of cefprozil, 10%-60% of a filler, 1.0%-4.0% of hydroxypropyl methylcellulose K4M, 0.2%-0.5% of carboxymethylcellulose sodium, 0.2%-0.4% of a lubricant, 0.2%-0.5% of a sweetener, 0.3%-0.8% of an aromatic and proper amount of ethanol. The invention also discloses a preparation method of the cefprozil dry suspension. The preparation method comprises: firstly preparing blank particles, then mixing uniformly with main drugs, performing sub-packaging, and combining with specific auxiliary materials to improve product mouthfeel, sedimentation volume ratio and redispersibility. The cefprozil dry suspension is avoided in the phenomenon that the content of correlated materials is raised because the main drugs are heated; and the preparation method is simple, no special equipment is needed, and the preparation method is applicable to industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
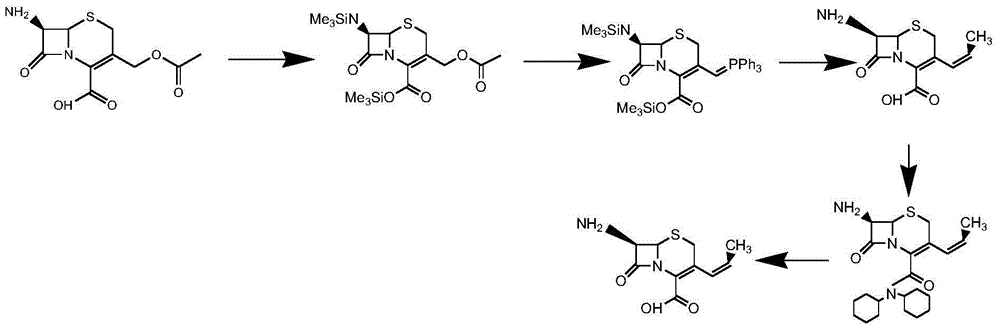
Method for preparing cephalosporin propylene
The invention discloses a preparation method of cefprozil, which comprises: 7-amin cethalosporanic acid (7-ACA) reacts with triphenyl phosphine to get 7- trimethylsilyl amino-3-triphenyl phosphate methylene-4-cethalosporanic acid trimethylsilyl ester through silanization protection and iodination reagent replacing under the condition of catalyst existing; WITTIG reaction is made for the product and acetaldehyde to get 7-trimethylsilyl amino -3-(propenyl-1-alkenyl)-4-cethalosporanic acidtrimethylsilyl ester; then the compound reacts with D-para hydroxybenzene glycine dane potassium salt to geta compound (6R, 7R)-7-[(2R)-2- ethoxycarbonyl-1-methyl - ethylene amino (4-trimethylsilyl oxyphenyl) acetamido group(amide)]-8-oxo-3- (1- propenyl)-5-thio-1- heterobicycle [4.2.0] octylene-2-ene-2-carboxylic acid trimethylsilyl ester; hydrolytic treatment is then used to get the cefprozil. The invent adopts the method of one pot and can participate in next reaction without separating intermediateproducts. The preparation method of cefprozil has the advantages of low cost, convenient operation and high overall yield, adapting to demands of industrial production.
Owner:南通康鑫药业有限公司
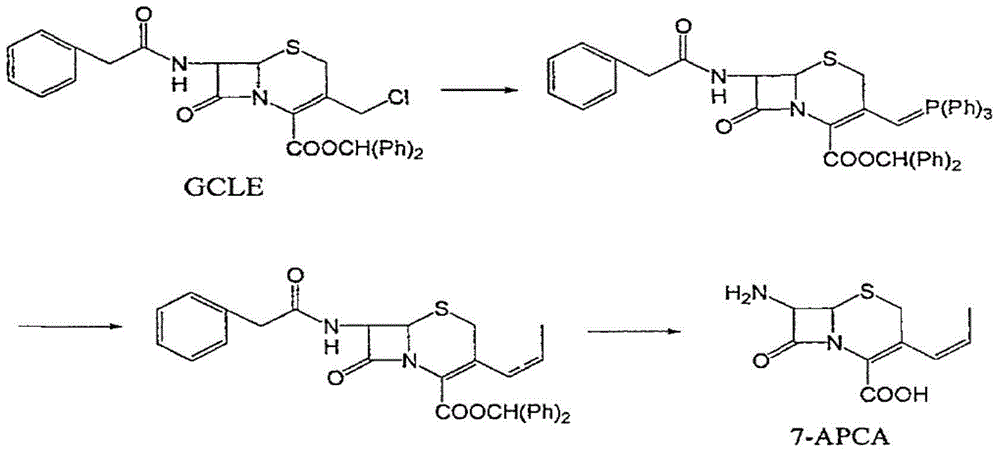
Method for preparing cefprozil compound
InactiveCN101798312BSimple processHigh purityOrganic compound preparationAmino-carboxyl compound preparationChlorobenzeneAklanonic acid
Owner:HAINAN MEIDA PHARMA
Method for preparing cefprozil mother nucleus 7-amino-3-acryl cephalosporanic acid
ActiveCN105001238AEasy to separateNot easy to patinaOrganic chemistryWittig reactionCephalosporanic Acids
The invention relates to a method for preparing cefprozil mother nucleus 7-amino-3-acryl cephalosporanic acid. According to the method, 7-amino cephalosporanic acid is adopted as a starting raw material, silylation protection, phosphorusylide formation, wittig reaction and silylation protection group removing are sequentially performed to obtain a cefprozil mother nucleus crude product, and a cefprozil mother nucleus crude product refining post-treatment process is added, wherein the cefprozil mother nucleus crude product refining post-treatment process comprises: a, amine salt forming, b, decolorization treatment, and c, cefprozil mother nucleus refined product preparing. According to the present invention, the prepared cefprozil mother nucleus has characteristics of high purity, good crystalline form, good color and high yield, the ratio of the E isomer content to the Z type isomer content is optimal so as to ensure the improved quality of the cefprozil prepared at the latter stage, the cefprozil mother nucleus purity can achieve 99.7%, the 7-ADCA is less than or equal to 0.15%, the crystalline form is hexagonal columnar, separation is easy, the patina is not easily generated, the color is less than or equal to Y-4 and is basically bright white, the yield is high, the quality is good, and the mass yield is more than 65%.
Owner:HEBEI JIUTIAN BIOLOGICAL PROD CO LTD
Cefprozil suspension and preparation method thereof
ActiveCN104688743AImprove uniformityReduce contentAntibacterial agentsPowder deliveryDissolutionSuspending Agents
The invention discloses cefprozil suspension. The cefprozil suspension is prepared through the following steps that 1, a diluent and microcrystalline cellulose & sodium carboxymethylcellulose are placed in a wet type granulator to be premixed; 2, cosolvent and wetting agents are added to conduct wet granulation; 3, whole grains are dried and marked as the material A; 4, cefprozil, suspending agents and wetting agents are mixed with the material A in an equivalent progressively-increasing mode, and the mixture is subpackaged after being evenly mixed, and the cefprozil suspension is obtained; the suspending agents are selected from one of xanthan gum or sodium carboxymethylcellulose or hydroxypropyl methyl cellulose; the weight proportion of the microcrystalline cellulose & sodium carboxymethylcellulose and suspending agents ranges from 2:1 to 8:1; the wetting agents are folic acid solutions with the concentration being 1 wt%. The cefprozil suspension is low in impurity content, high in dissolution rate, good in stability, even in main medicine spreading and simple in preparing process, no special equipment is needed, and the cefprozil suspension is capable of being suitable for industrialized mass production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefprozil medicinal composition
ActiveCN102526059ASimple wet granulationHigh hardnessOrganic active ingredientsPill deliveryFiller ExcipientBULK ACTIVE INGREDIENT
The invention relates to an anti-infection oral medicinal preparation and discloses a cefprozil medicinal composition. The cefprozil medicinal composition comprises cefprozil serving as an active ingredient, at least one filling agent, at least one disintegrating agent, at least one lubricating agent, 1 to 10 weight percent of pregelatinized starch and 0.5 to 2 weight percent of hydroxypropyl methylcellulose. The invention also relates to a preparation method for the preparation and application of the preparation to treatment for upper respiratory tract infection, lower respiratory tract infection and skin and skin soft tissue infection.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Cefprozil tablet and preparation method thereof
ActiveCN103417509AReduced ring opening degradationReduced loop-opening degradationAntibacterial agentsOrganic active ingredientsCross-linkMagnesium stearate
The invention provides a cefprozil tablet which comprises a tablet core and a thin-film coating. The cefprozil tablet is characterized in that the tablet core is composed of cefprozil and medicinal excipients, and the medicinal excipients include microcrystalline cellulose, vertical-compression lactose, cross-linked polyvinylpolypyrrolidone, magnesium stearate and silicon dioxide. The invention further provides a preparation method of the cefprozil tablet. The preparation method includes under the conditions that the temperature is 18 DEG C-26 DEG C and relative humidity is 45-65%, sieving, premixing, tableting and coating. The cefprozil tablet prepared by the method can maintain high stability and dissolubility in the process of preparation and long-term storage.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA +1
Recovery method of cefprozil
The invention discloses a recovery method of cefprozil. The method comprises the steps of dripping dimethylformamide and acetone in a cefprozil refined mother solution to obtain the crude cefprozil; adding the crude cefprozil into a mixed solvent, adjusting the pH value of the solution with hydrochloric acid, activated carbon fading, and adjusting the pH value of stronger ammonia water; and finally cooling crystallization, suction filtration and drying to obtain the end product of cefprozil. The recovery method has the advantages that 95% of cefprozil can be extracted from the mother solution, so that the wastewater treatment pressure is greatly reduced, and the end product meets the pharmacopeia standards after recovery and treatment; and in addition, the recovery method is simple and convenient to operate, and dimethylformamide, acetone and isopropanol can be recycled and utilized.
Owner:南通康鑫药业有限公司
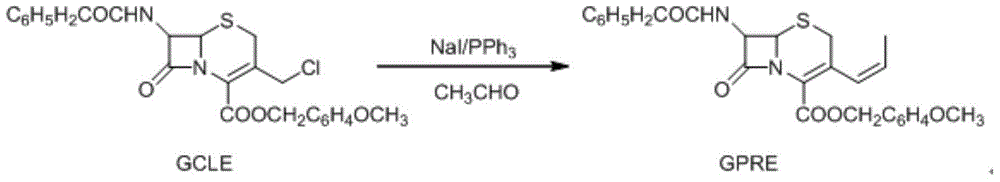
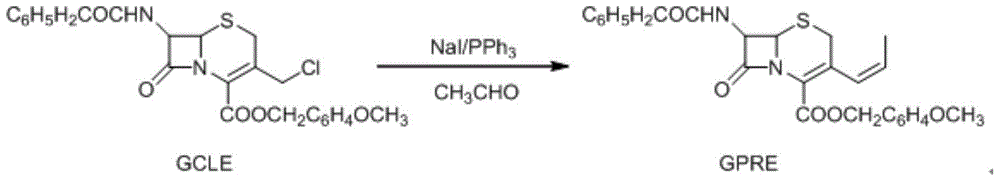
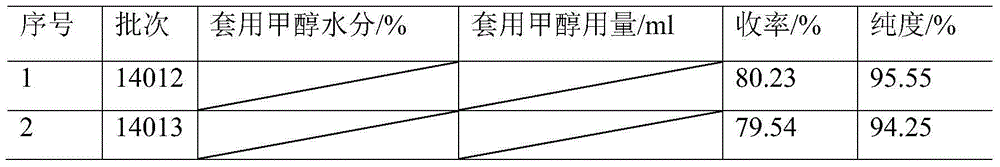
Method for recycling methanol in preparation of cefprozil intermediate
InactiveCN104370940AReduce manufacturing costSolve pollutionHydroxy compound separation/purificationDistillationSodium iodide
The invention relates to a method for recycling methanol in the preparation of a cefprozil intermediate. The method comprises the following steps: (1) reacting GCLE used as an initial raw material with sodium iodide and triphenylphosphine to generate intermediate phosphorus onium salt, and carrying out Witing reaction on phosphorus onium salt and acetaldehyde to generate GPRE; (2) after the Witing reaction is finished, carrying out reduced pressure distillation to remove dichloromethane, and then dropwise adding methanol and water to precipitate the GPRE; (3) carrying out suction filtration on a reaction liquid, wherein a filter cake is the GPRE, filter liquor is mother liquor and contains methanol and the water; (4) transferring the mother liquor to an evaporator, and evaporating and recovering methanol (which contains the water); (5) measuring the water ratio of recovered methanol; and (6) adding recovered methanol to the step (2) in proportion to continue a subsequent step. The method disclosed by the invention can be used for mainly solving the problem of recycling of methanol, obtaining preferable yield and product quality, solving the problem of pollution of a large amount of organic waste liquor, reducing the cefprozil production cost and realizing the maximization of economic benefits.
Owner:TIANJIN PHARMA GROUP GENCOM PHARMA
Cefprozil mother liquor application technique
The invention discloses a cefprozil mother liquor application technique, comprising the steps of (1) adding saturated FeCl3 solution into cefprozil mother liquor to adjust pH; (2) after cefprozil synthetic reaction is over, adding the cefprozil mother liquor treated in step (1) into the reaction liquid, raising the temperature, stirring at a controlled temperature, allowing standing, and dividingthe liquid; (3) cooling an aqueous layer divided from step (2) to below -5 DEG C, adding dimethylformamide at a controlled temperature, adding acetone washing liquid, stirring, filtering, and collecting filtrate; (4) cooling the filtrate, dropwise adding liquid ammonia to adjust pH, cooling, stirring, filtering, collecting filter cake, washing with dimethylformamide, washing with acetone, and drying in the vacuum to obtain crude cefprozil; and (5) refining the crude cefprozil. The cefprozil mother liquor application technique is simple and convenient to perform, usage of dimethylformamide andacetone is greatly reduced, and the cefprozil mother liquor application technique has significant economic and environmental benefits.
Owner:TOPFOND PHARMA CO LTD
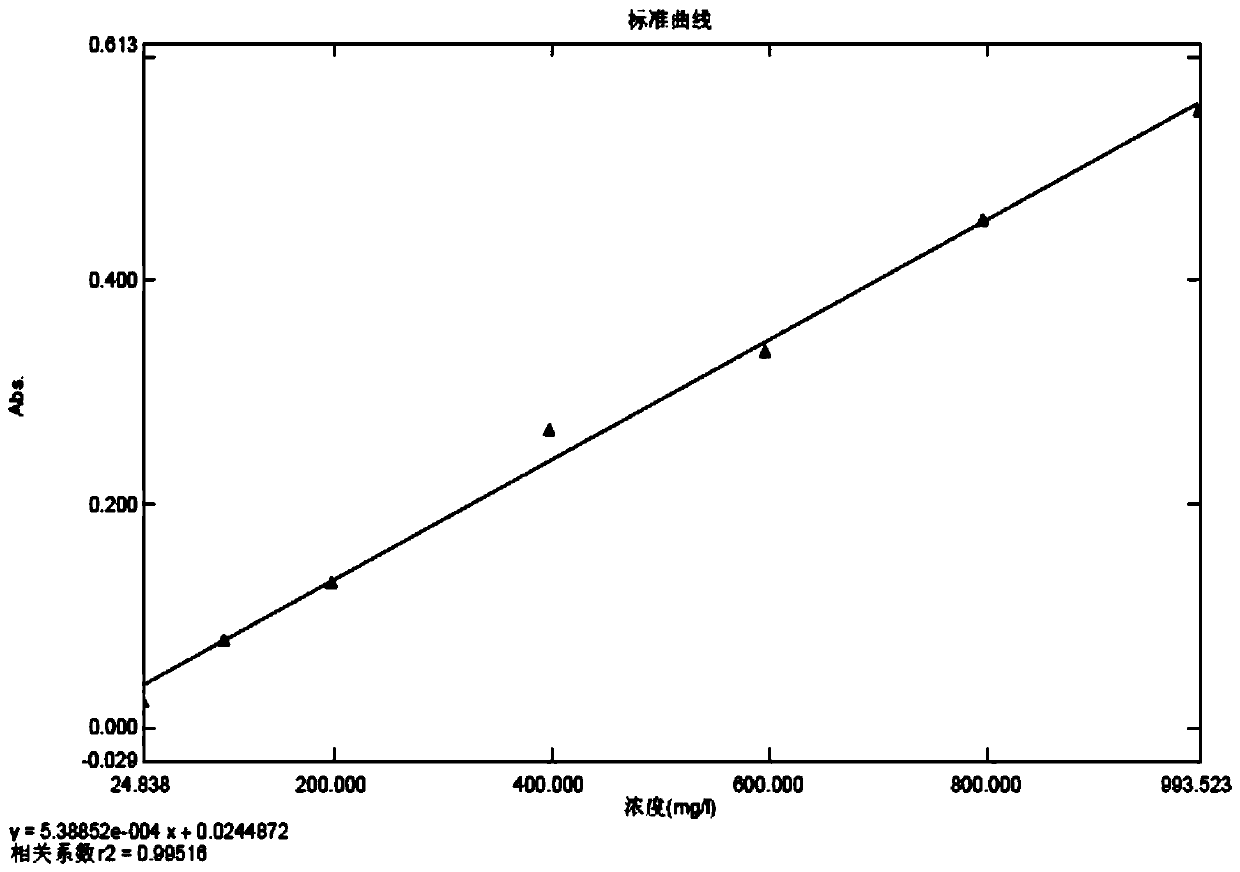
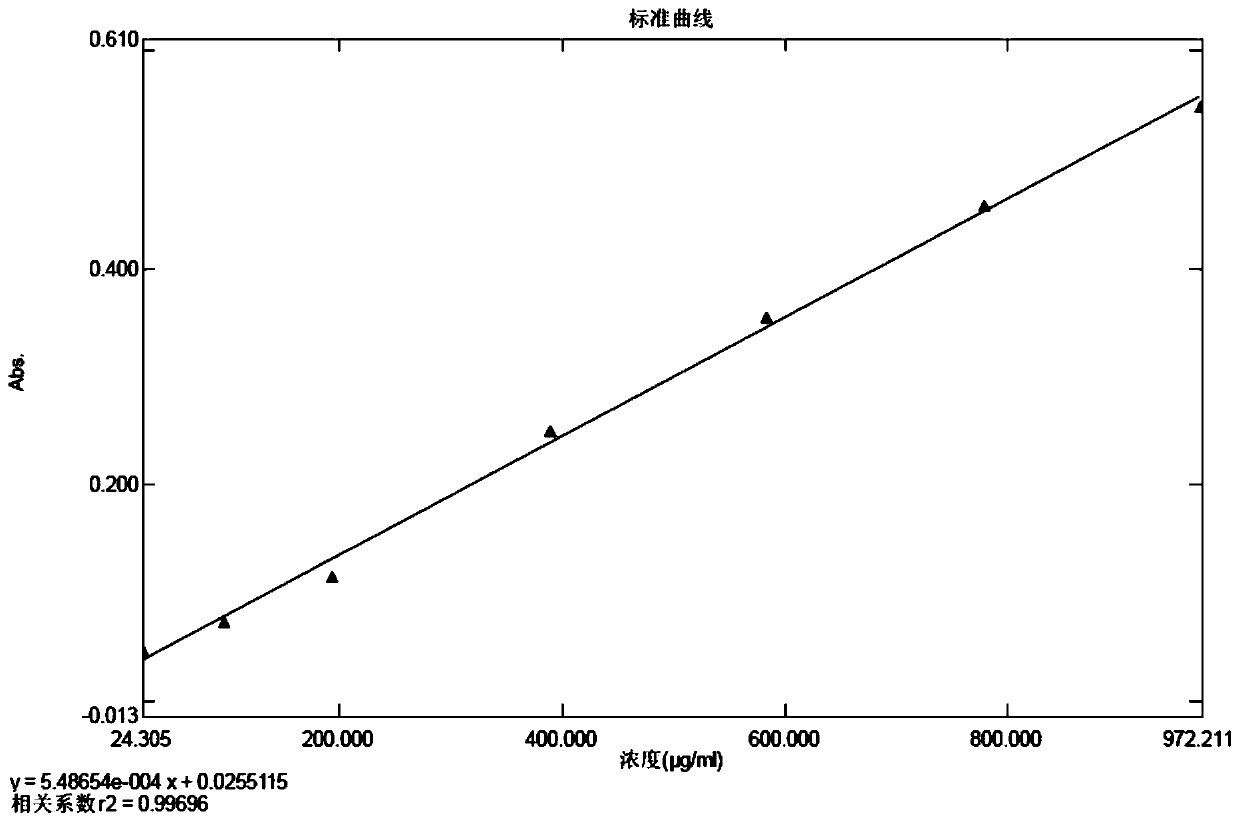
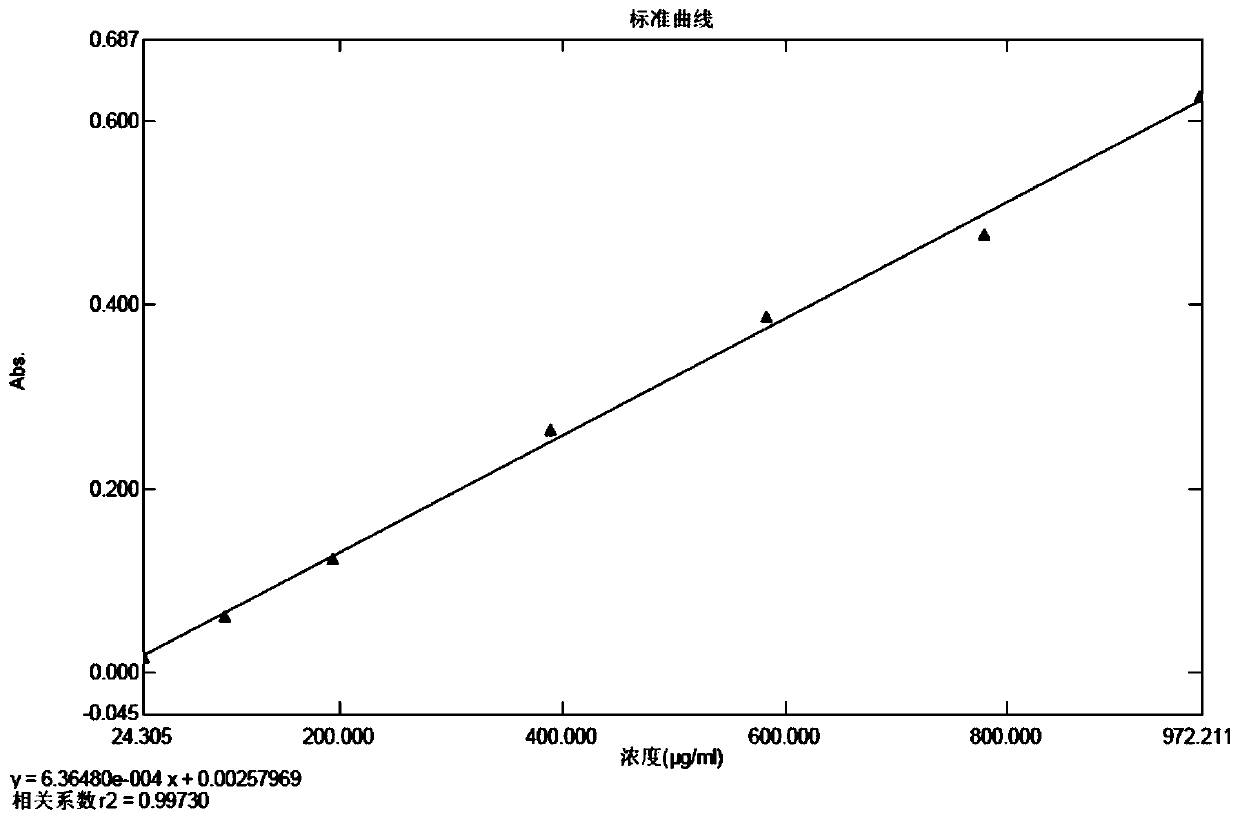
Method for detecting zymoprotein residues in cefprozil prepared by enzymatic method
PendingCN110954392AThe detection method is reasonableEasy to operatePreparing sample for investigationColor/spectral properties measurementsSodium bicarbonateTest sample
The invention provides a method for detecting zymoprotein residues in cefprozil prepared by an enzymatic method. The method is characterized by comprising the following steps: preparing a Coomassie brilliant blue G-250 dye solution and a standard protein stock solution; preparing a standard curve solution by taking a sodium bicarbonate solution as a solvent; preparing a blank solution by taking asodium bicarbonate solution as a solvent; preparing a test sample determination solution by taking a sodium bicarbonate solution as a solvent; drawing an absorbance-protein concentration standard curve through blank solution correction by using an ultraviolet spectrophotometer; and calculating the residual content of zymoprotein contained in the test sample according to the absorbance value read by the test sample determination solution. Sodium bicarbonate is ingeniously selected as a solvent, cefprozil can be dissolved, zymoprotein can be fully dissolved, the residual quantity of zymoproteinin a cefprozil product prepared through an enzymatic method can be effectively measured, the medication safety of medicine is improved, and the detection method is easy to operate, high in accuracy and good in reproducibility and reliability.
Owner:广药白云山化学制药(珠海)有限公司 +1
Method for synthesizing cefprozil by enzymic method
The invention relates to a method for synthesizing cefprozil by an enzymic method. The method is characterized in that 7-APRA and a D-hydroxy phenylglycine derivative are added in water for being stirred and mixed, PGA synthetase is added for a reaction, after the reaction is finished, enzyme, coarse powder and clear liquid are separated, coarse powder is added in clear liquid, a pH value is adjusted, N,N-dimethyl formamide is added, temperature is controlled at the temperature of 15-20 DEG C, the pH value is adjusted to 5.0-6.5 and crystallization is carried out, the materials are stirred, then cooled to 5-10 DEG C and crystal growing is carried out, filtering is performed, the material is dried at the temperature of 40-45 DEG C to obtain a cefprozil DMF complex, the cefprozil DMF complexis added in water, the temperature is controlled at 10-20 DEG C, the material is stirred for crystal transformation, and then filtered, wet powder is added in water, the temperature is controlled at10-20 DEG C, the pH value is adjusted to 4.5-5.5 and crystallization is carried out, and the material is filtered, washed, and dried to obtain a cefprozil finished product. The method has the advantages that the reaction time is short, the generated impurities are less, the method is benefit for separating and purifying, usage of a solvent is reduced, safety and environmental protection pressure can be reduced, product yield is high, and purity is high.
Owner:福安药业集团重庆博圣制药有限公司
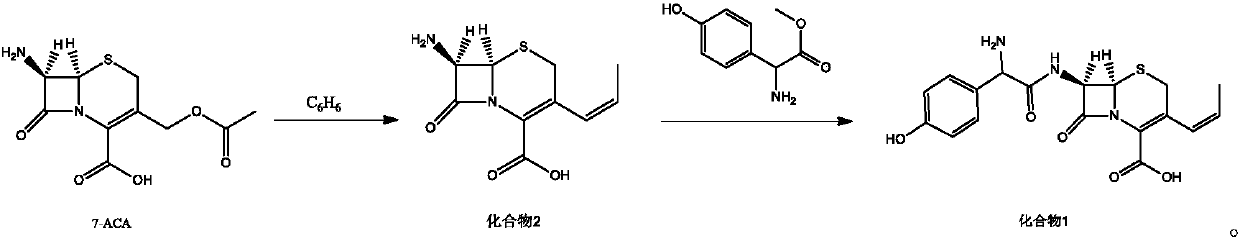
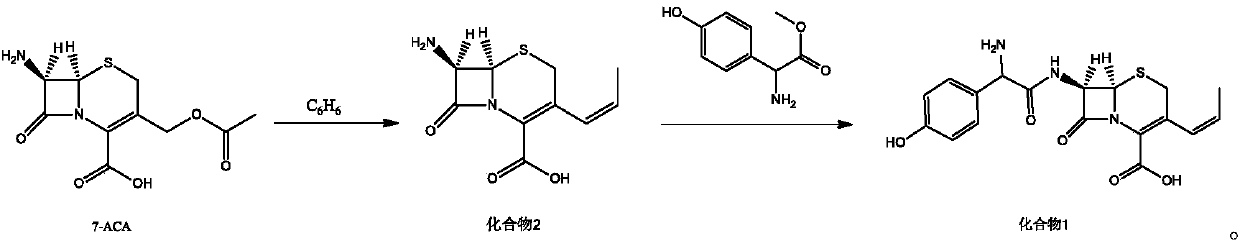
Preparation method of cefprozil
The invention relates to a preparation method of cefprozil. A synthesis method comprises the following steps: taking 7-ACA (7-aminocephalosporanic acid) as a starting raw material; after carrying outsilylation protection, carrying out a series of reactions including iodination and the like to generate a compound 2; taking the compound 2 to react with methyl D-(-)-4-hydroxy-phenylglycinate under the catalysis of immobilized penicillin acylase to generate a compound 1, so as to obtain a target product cefprozil. The preparation method provided by the invention has the advantages of moderate reaction conditions, environmental friendliness, high conversion ratio, simple technology and high cis-isomer content.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Capsule containing cefprozil liposome and preparation method thereof
InactiveCN101953791AHigh dissolution rateThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefprozil liposome and a preparation method thereof as well as a capsule containing the cefprozil liposome. The cefprozil liposome comprises cefprozil, hydrogenated yolk lecithin, soyabean lecithin, cholesterol and vitamin E, wherein the weight ratio of the cefprozil to the hydrogenated yolk lecithin to the soyabean lecithin to the cholesterol and to the vitamin E is 1:(1.25-5):(1.25-5):(2.5-10):(0.1-3). The cefprozil capsule not only meets the Chinese pharmacopoeia requirement but also has the advantages of higher dissolution rate, faster action of medicament effect and obvious improvement of bioavailability compared with those of a common cefprozil pharmaceutical composition.
Owner:王丽燕
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
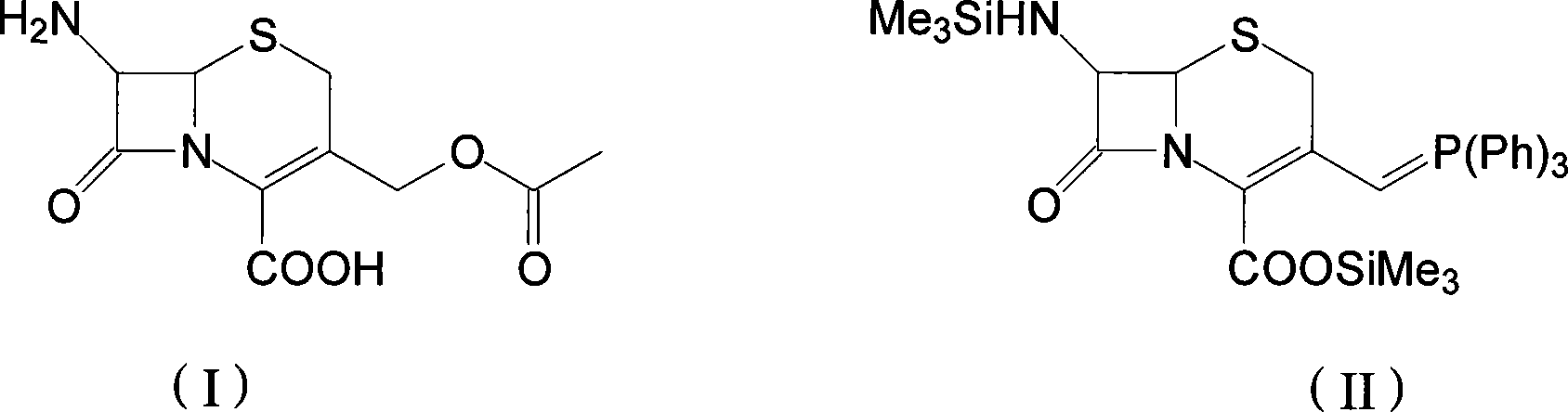


![Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid](https://images-eureka.patsnap.com/patent_img/4c95adda-5677-4886-a0e7-3e1580e61e75/US20060149096A1-20060706-C00001.png)
![Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid](https://images-eureka.patsnap.com/patent_img/4c95adda-5677-4886-a0e7-3e1580e61e75/US20060149096A1-20060706-C00002.png)
![Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid](https://images-eureka.patsnap.com/patent_img/4c95adda-5677-4886-a0e7-3e1580e61e75/US20060149096A1-20060706-C00003.png)