Cefprozil tablet and preparation method thereof
A technology of cefprozil and propylene tablets, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as difficult to meet quality requirements, low dissolution rate, poor stability, etc. , to achieve the effect of reducing ring-opening degradation, simple steps, good stability and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
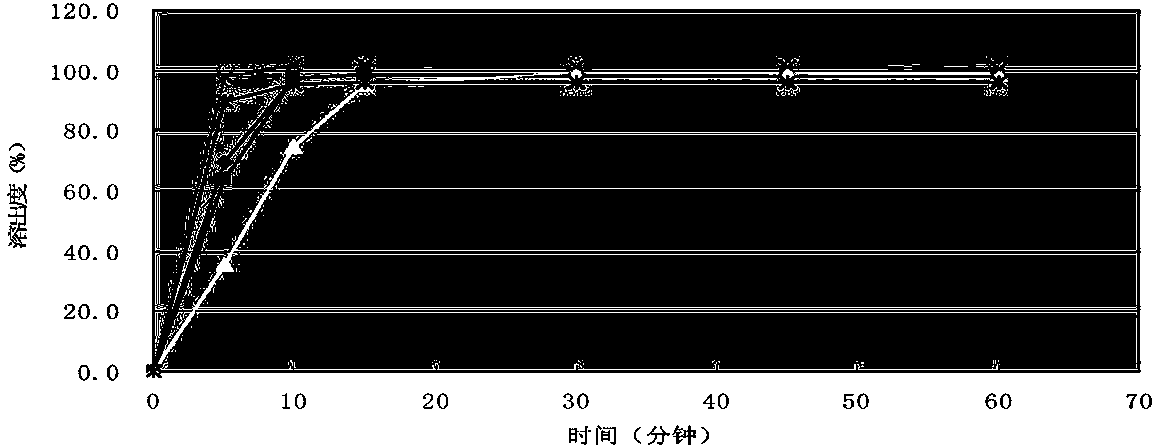
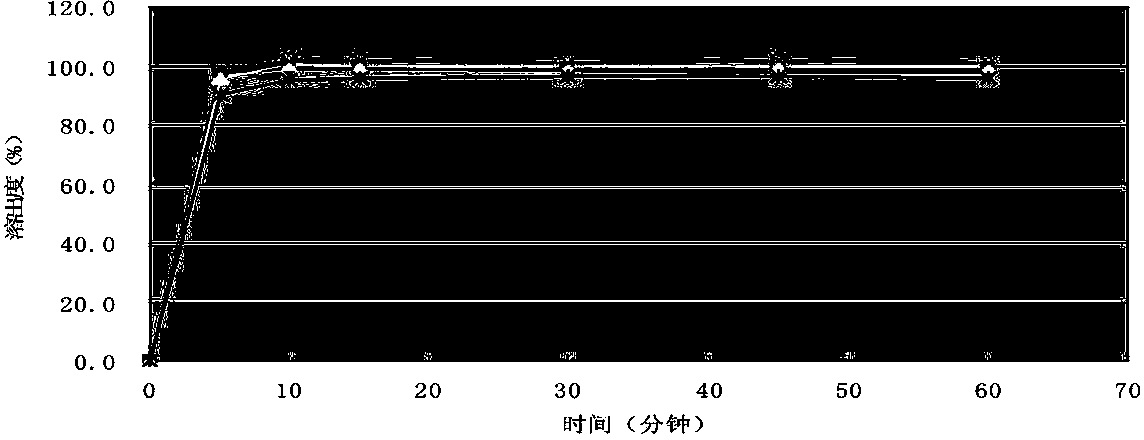
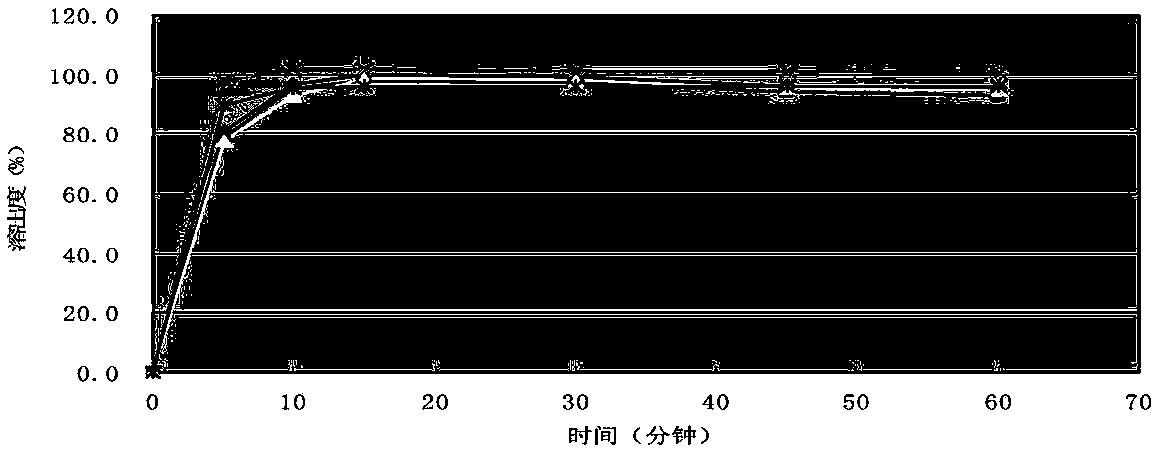
Image
Examples
Embodiment 1
[0036] According to the following formula, cefprozil tablets were prepared with batch number 142111102.
[0037]
[0038]
[0039] The preparation steps are as follows:
[0040] (1) Sieving
[0041] Pass the cefprozil, crospovidone, and microcrystalline cellulose through an 80-mesh sieve, and directly press the lactose through a 60-mesh sieve, and collect the powder under the sieve; the purity of the cefprozil is 96.3%; the model of the microcrystalline cellulose is Microcrystalline cellulose 101.
[0042] (2) Premix
[0043] Weigh the cefprozil, microcrystalline cellulose, direct-pressed lactose, and crospovidone that have been sieved in step (1), add them to the mixer, and premix for 10 minutes;
[0044] (3) Total mixing
[0045] Weigh magnesium stearate and silicon dioxide respectively, add them to the mixer described in step (2), and mix for 10 minutes.
[0046] After mixing, insert a sampling probe into the upper, middle, and lower parts of the mixer barrel, and take samples respecti...
Embodiment 2
[0063] According to the following formula, cefprozil tablets were prepared with batch number 142111103.
[0064]
[0065] The preparation steps are as follows:
[0066] (1) Sieving
[0067] Pass the cefprozil, crospovidone, and microcrystalline cellulose through an 80-mesh sieve, and directly press the lactose through a 60-mesh sieve, and collect the powder under the sieve; the purity of the cefprozil is 96.3%; the model of the microcrystalline cellulose is Microcrystalline cellulose 101.
[0068] (2) Premix
[0069] Weigh the cefprozil, microcrystalline cellulose, direct-pressed lactose, and crospovidone that have been sieved in step (1), add them to the mixer, and premix for 10 minutes;
[0070] (3) Total mixing
[0071] Weigh magnesium stearate and silicon dioxide respectively, add them to the mixer described in step (2), and mix for 10 minutes.
[0072] After mixing, insert a sampling probe into the upper, middle, and lower parts of the mixer barrel, and take samples respectively, of ...
Embodiment 3
[0085] According to the following formula, cefprozil tablets were prepared with batch number 142111104.
[0086]
[0087] The preparation steps are as follows:
[0088] (1) Sieving
[0089] Pass the cefprozil, crospovidone, and microcrystalline cellulose through an 80-mesh sieve, and directly press the lactose through a 60-mesh sieve, and collect the powder under the sieve; the purity of the cefprozil is 96.3%; the model of the microcrystalline cellulose is Microcrystalline cellulose 101.
[0090] (2) Premix
[0091] Weigh the cefprozil, microcrystalline cellulose, direct-pressed lactose, and crospovidone that have been sieved in step (1), add them to the mixer, and premix for 10 minutes;
[0092] (3) Total mixing
[0093] Weigh magnesium stearate and silicon dioxide respectively, add them to the mixer described in step (2), and mix for 10 minutes.
[0094] After mixing, insert a sampling probe into the upper, middle, and lower parts of the mixer barrel, and take samples respectively, of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com