Cefprozil tablet and preparation method thereof
A technology for cefprozil and propylene tablets, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of low dissolution rate, difficulty in meeting quality requirements, poor stability, etc. , to achieve the effect of simple steps, reduced ring-opening degradation, good stability and dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
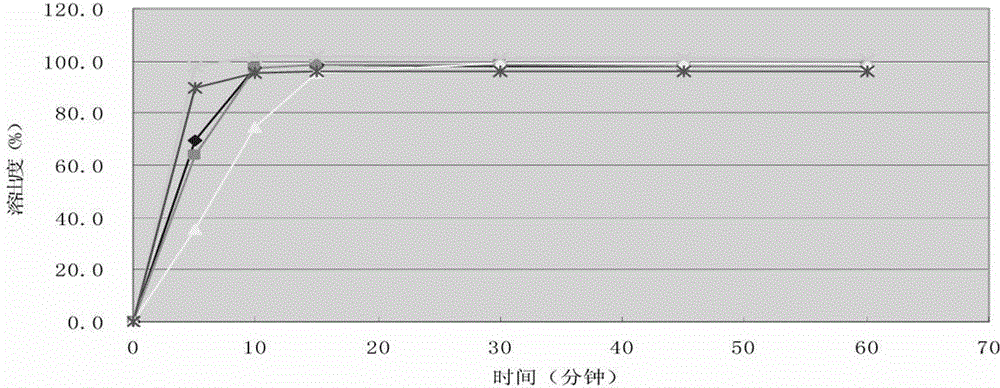
Embodiment 1
[0036] According to the following formula, prepare cefprozil tablet, batch number is 142111102.
[0037]
[0038]
[0039] The preparation steps are as follows:
[0040] (1) Sieve
[0041] Cefprozil, crospovidone, and microcrystalline cellulose are passed through 80 mesh sieves respectively, and lactose is directly pressed through a 60 mesh sieve to collect the powder under the sieve; wherein, the purity of cefprozil is 96.3%; the model of microcrystalline cellulose is Microcrystalline Cellulose 101.
[0042] (2) premixed
[0043] Weigh the cefprozil, microcrystalline cellulose, direct-pressed lactose, and crospovidone that have been sieved in step (1), add them into the mixer, and pre-mix for 10 minutes;
[0044] (3) total mix
[0045] Weigh magnesium stearate and silicon dioxide respectively, add them into the mixer described in step (2), and mix for 10 minutes.
[0046] After mixing, insert the sampling probe into the upper, middle and lower parts of the mixer bu...
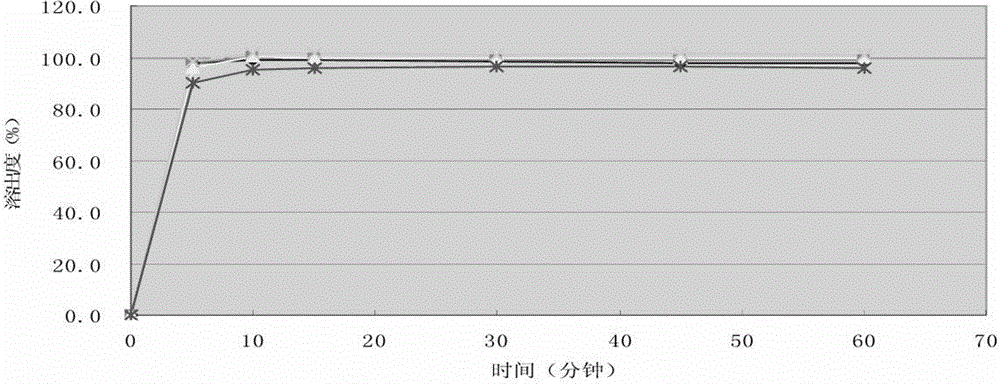
Embodiment 2
[0063] According to the following formula, prepare cefprozil tablet, batch number is 142111103.
[0064]
[0065] The preparation steps are as follows:
[0066] (1) Sieve
[0067] Cefprozil, crospovidone, and microcrystalline cellulose are passed through 80 mesh sieves respectively, and lactose is directly pressed through a 60 mesh sieve to collect the powder under the sieve; wherein, the purity of cefprozil is 96.3%; the model of microcrystalline cellulose is Microcrystalline Cellulose 101.
[0068] (2) premixed
[0069] Weigh the cefprozil, microcrystalline cellulose, direct-pressed lactose, and crospovidone that have been sieved in step (1), add them into the mixer, and pre-mix for 10 minutes;
[0070] (3) total mix
[0071] Weigh magnesium stearate and silicon dioxide respectively, add them into the mixer described in step (2), and mix for 10 minutes.
[0072] After mixing, insert the sampling probe into the upper, middle and lower parts of the mixer bucket, and take...
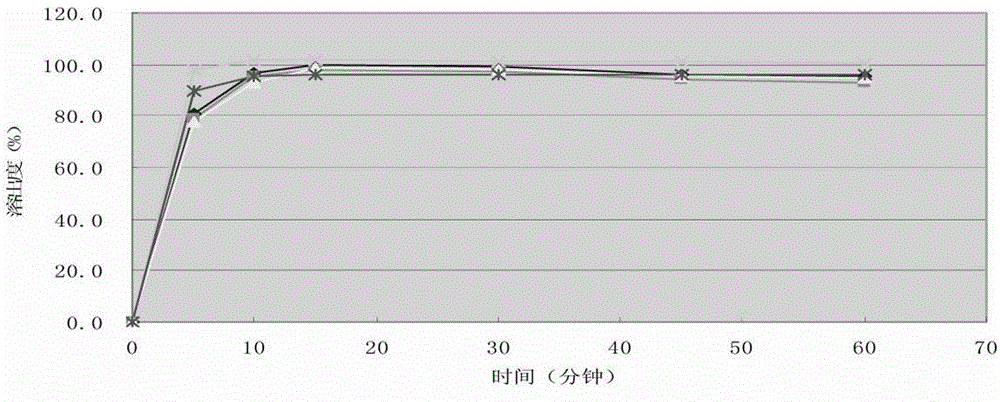
Embodiment 3
[0085] According to the following formula, prepare cefprozil tablet, batch number is 142111104.
[0086]
[0087] The preparation steps are as follows:
[0088] (1) Sieve
[0089] Cefprozil, crospovidone, and microcrystalline cellulose are passed through 80 mesh sieves respectively, and lactose is directly pressed through a 60 mesh sieve to collect the powder under the sieve; wherein, the purity of cefprozil is 96.3%; the model of microcrystalline cellulose is Microcrystalline Cellulose 101.
[0090] (2) premixed
[0091] Weigh the cefprozil, microcrystalline cellulose, direct-pressed lactose, and crospovidone that have been sieved in step (1), add them into the mixer, and pre-mix for 10 minutes;
[0092] (3) total mix
[0093] Weigh magnesium stearate and silicon dioxide respectively, add them into the mixer described in step (2), and mix for 10 minutes.
[0094] After mixing, insert the sampling probe into the upper, middle and lower parts of the mixer bucket, and ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com