Patents
Literature
18795 results about "Dissolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
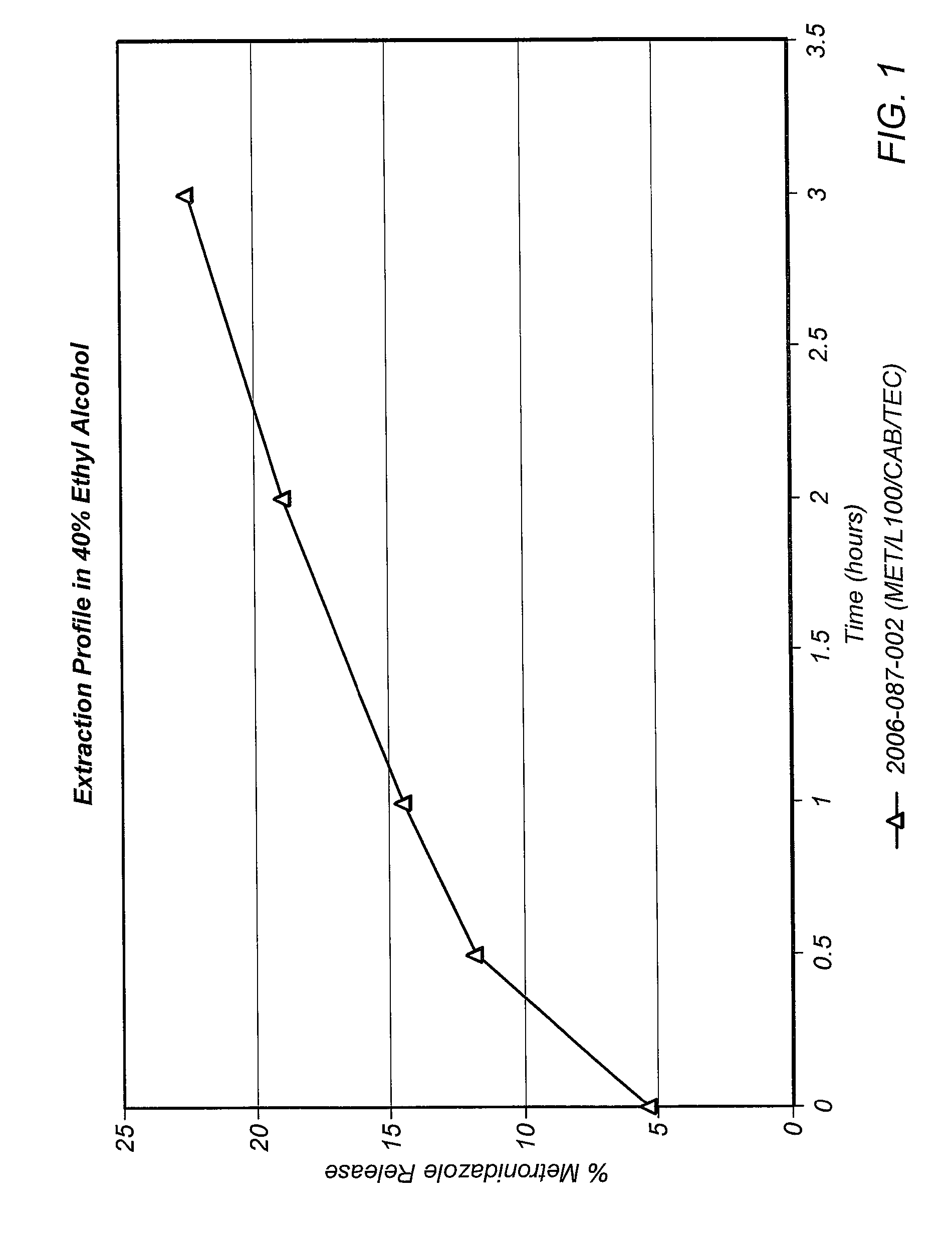
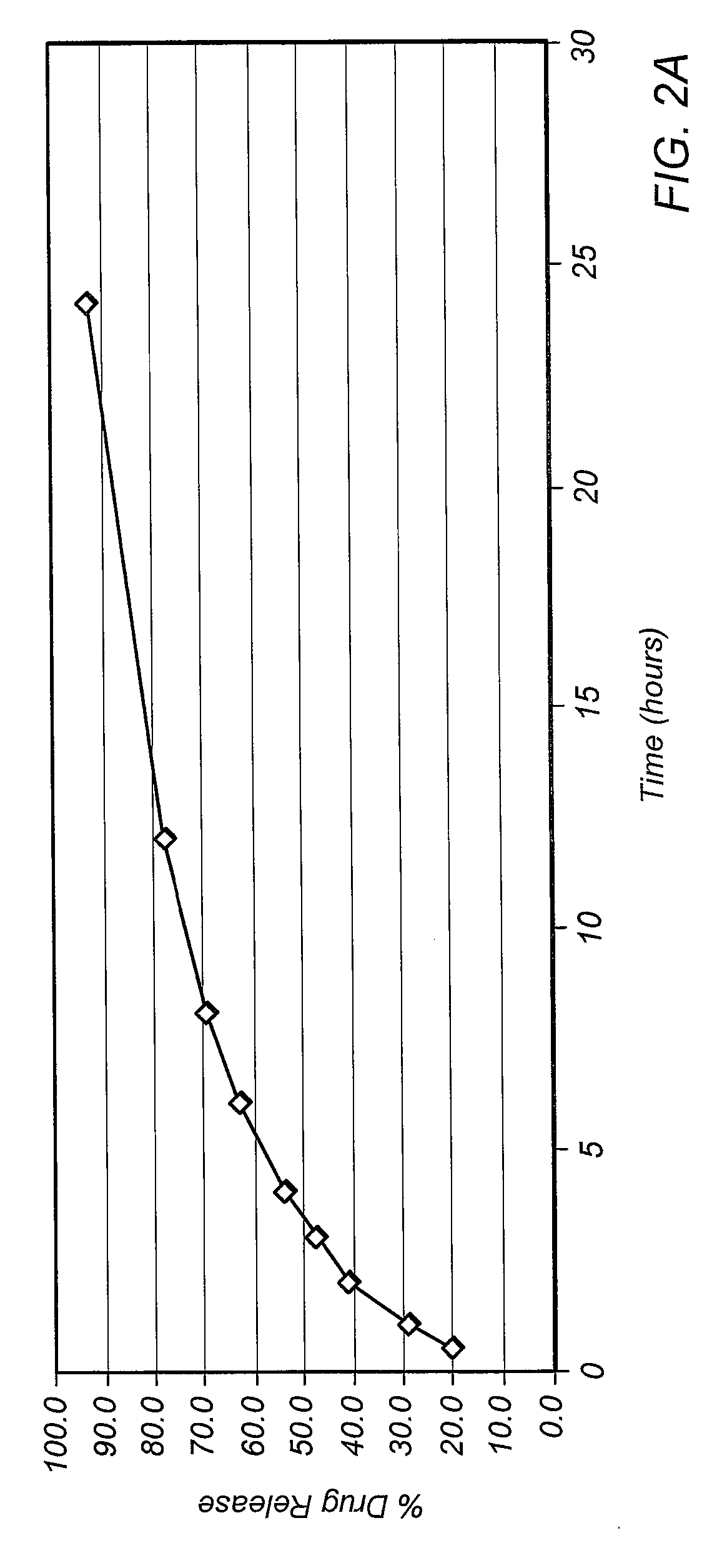
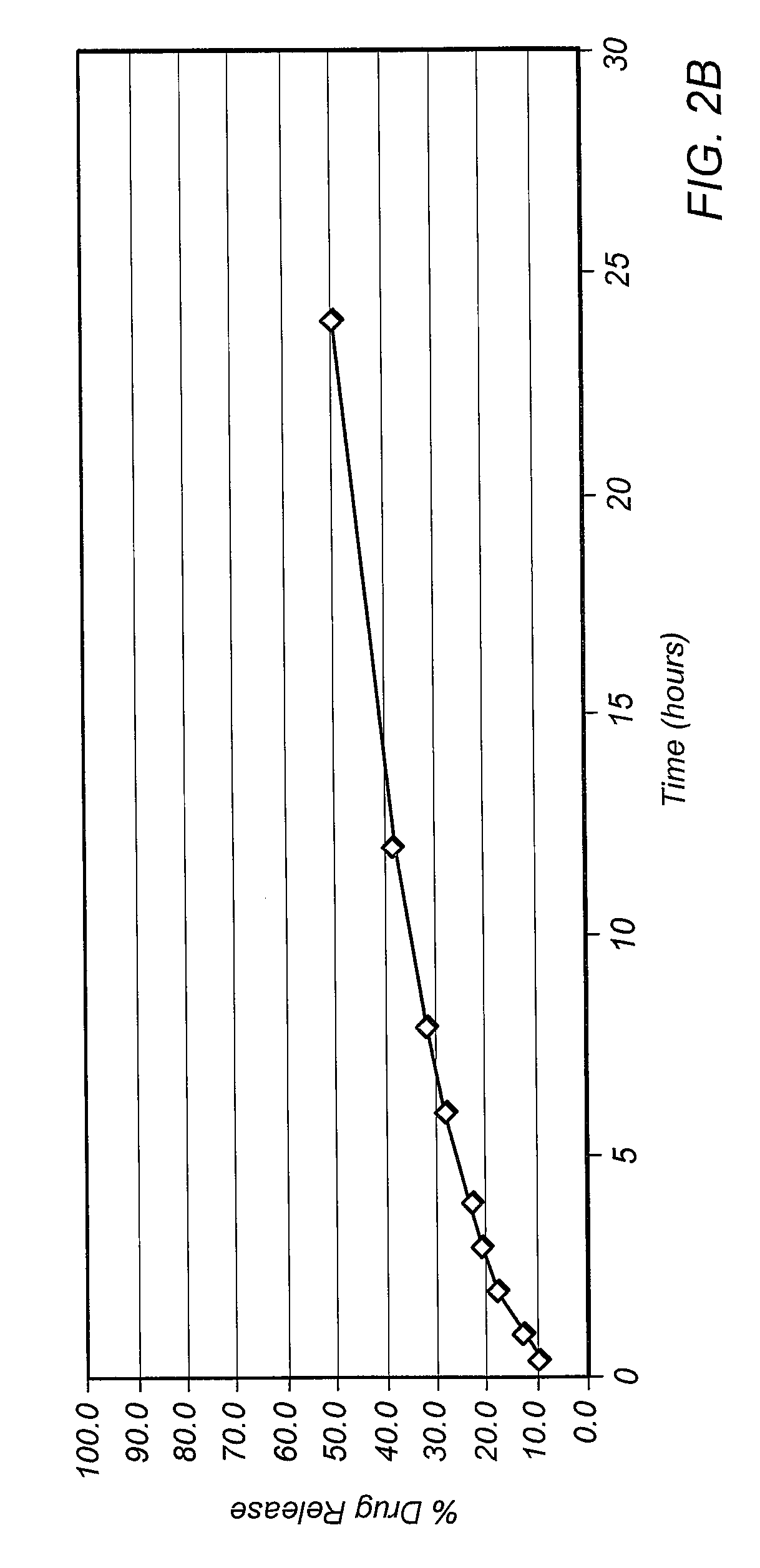
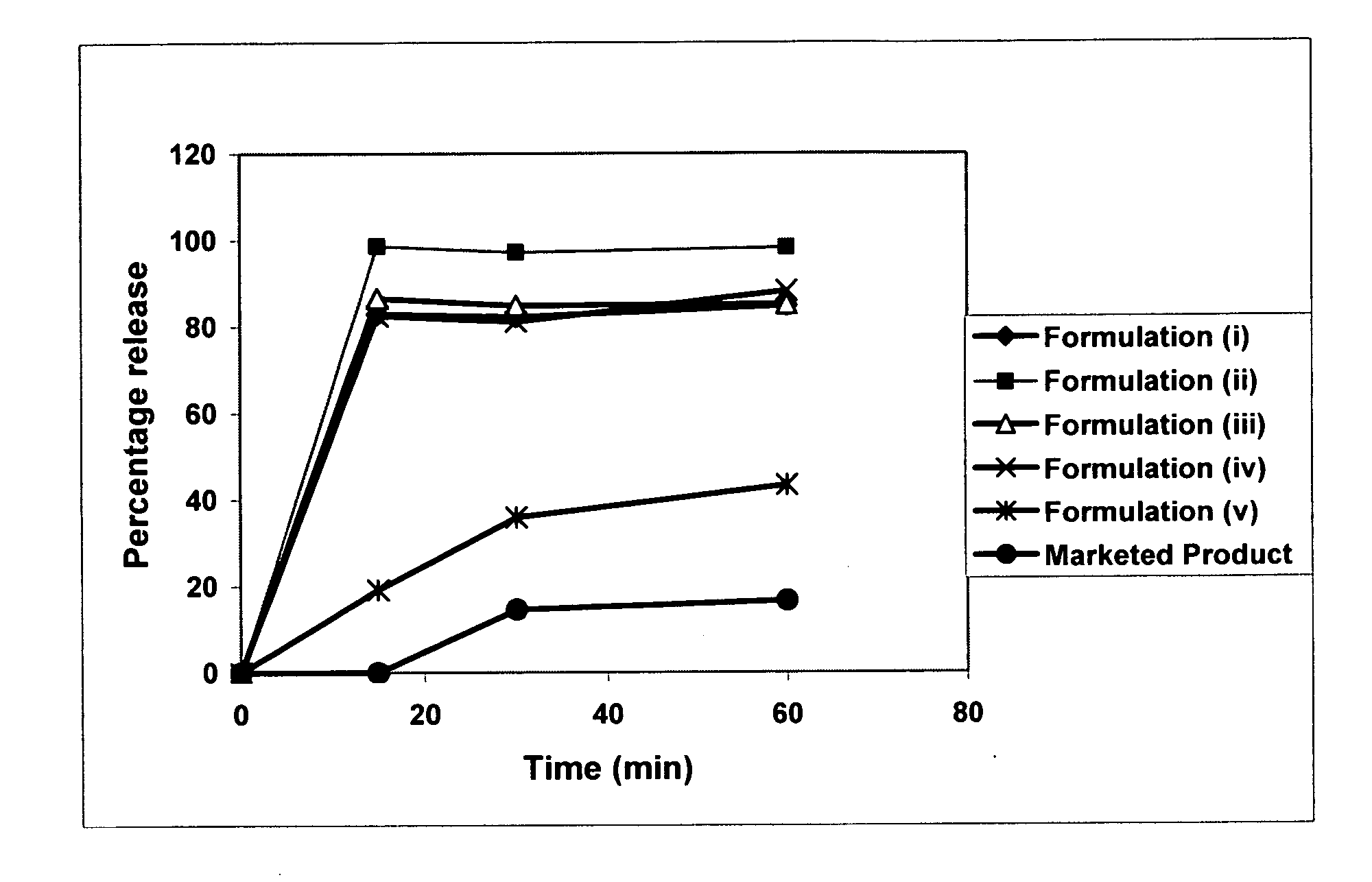
Dissolution is the process by which a solute forms a solution in a solvent. The solute, in the case of solids, has its crystalline structure disintegrated as separate ions, atoms, and molecules form. For liquids and gases, the molecules must be adaptable with those of the solvent for a solution to form. The outcome of the process of dissolution is governed by the thermodynamic energies involved, such as the heat of solution and entropy of solution, but the dissolution itself is not. Overall the free energy must be negative for net dissolution to occur. In turn, those energies are controlled by the way in which different chemical bond types interact with those in the solvent. Solid solutions occur in metal alloys and their formation and description is governed by the relevant phase diagram. Dissolution process is of fundamental importance to the description of numerous natural processes on earth, and it is commonly utilized by humans. Dissolution testing is widely used in the pharmaceutical industry for optimization of formulation and quality control.
Controlling the dissolution of dissolvable polymer components in plural component fibers
The dissolution of dissolvable components in plural component polymer fibers is achieved by providing a polymer fiber including at least two sections, where at least one fiber section includes a dissolvable component. The rate at which at least part of the fiber dissolves is controlled by at least one of a fiber section having a non-round cross-sectional geometry, and at least two fiber sections including two different dissolvable components. In an exemplary embodiment, island-in-the-sea fibers are formed with non-round and elongated cross-sectional geometries. In another embodiment, sheath-core fibers are formed in which the sheath and core include different dissolvable components.
Owner:HILLS CO
Chewable soft capsules and method for preparing same
ActiveCN101810336ADissolution stabilityRapid dissolutionFood shapingFood preparationFlavorPlasticizer
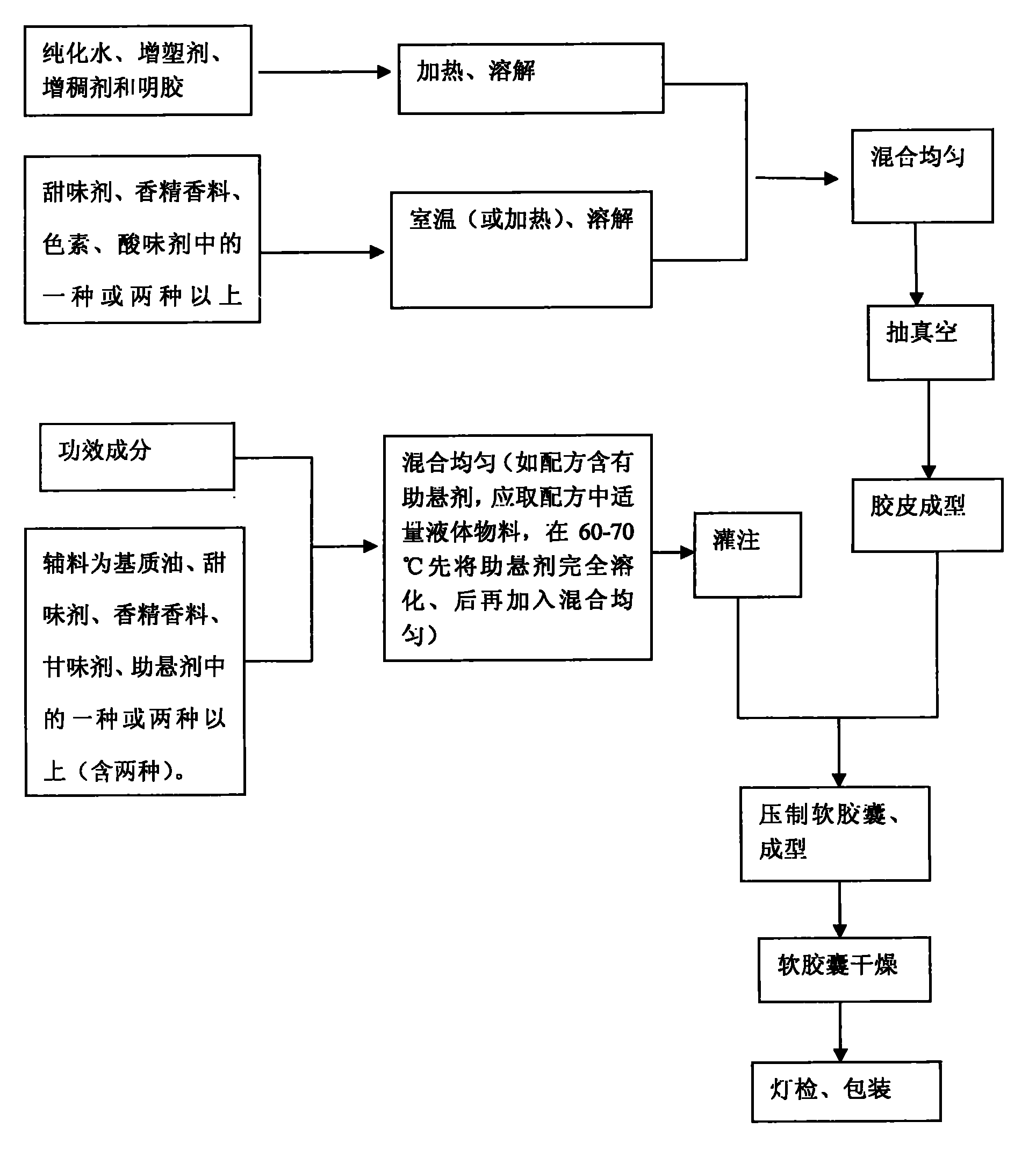
The invention discloses chewable soft capsules, which comprise capsule shells and contents sealed in the capsule shells. The chewable soft capsules are characterized by comprising the following raw materials in part by weight: 25 to 65 parts of glutin, 1 to 25 percent of thickening agent, 18 to 65 parts of plasticizer, 4 to 16 parts of water and a mixture containing one or more of 0.005 to 20 parts of sweetening agent, 0.005 to 2 parts of essence, 0.0001 to 10 parts of pigment and 0.0001 to 5 parts of acid additive. The invention also discloses a method for preparing the novel chewable soft capsules. The chewable soft capsules have the advantages of reasonable blending ratio, proper hardness, good temperature resistance, no adhesion, no deformation, stable storage, quick dissolution of effectively components and high chewiness; and when the chewing soft capsules are chewed, tastes of the capsule shells and the contents easily and uniformly spread in the mouth so as to cover up bad taste of raw materials.
Owner:SIRIO PHARMA CO LTD
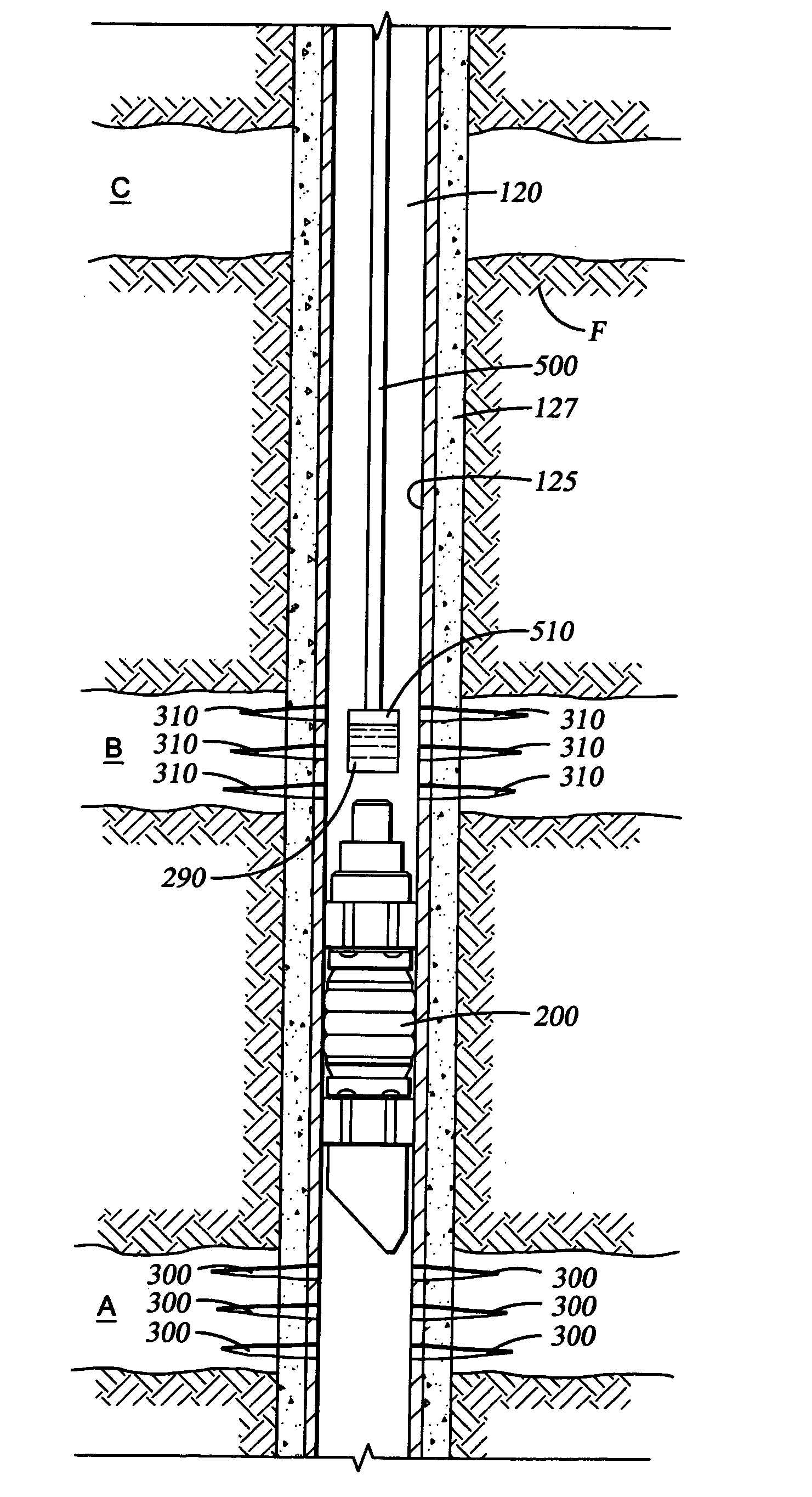
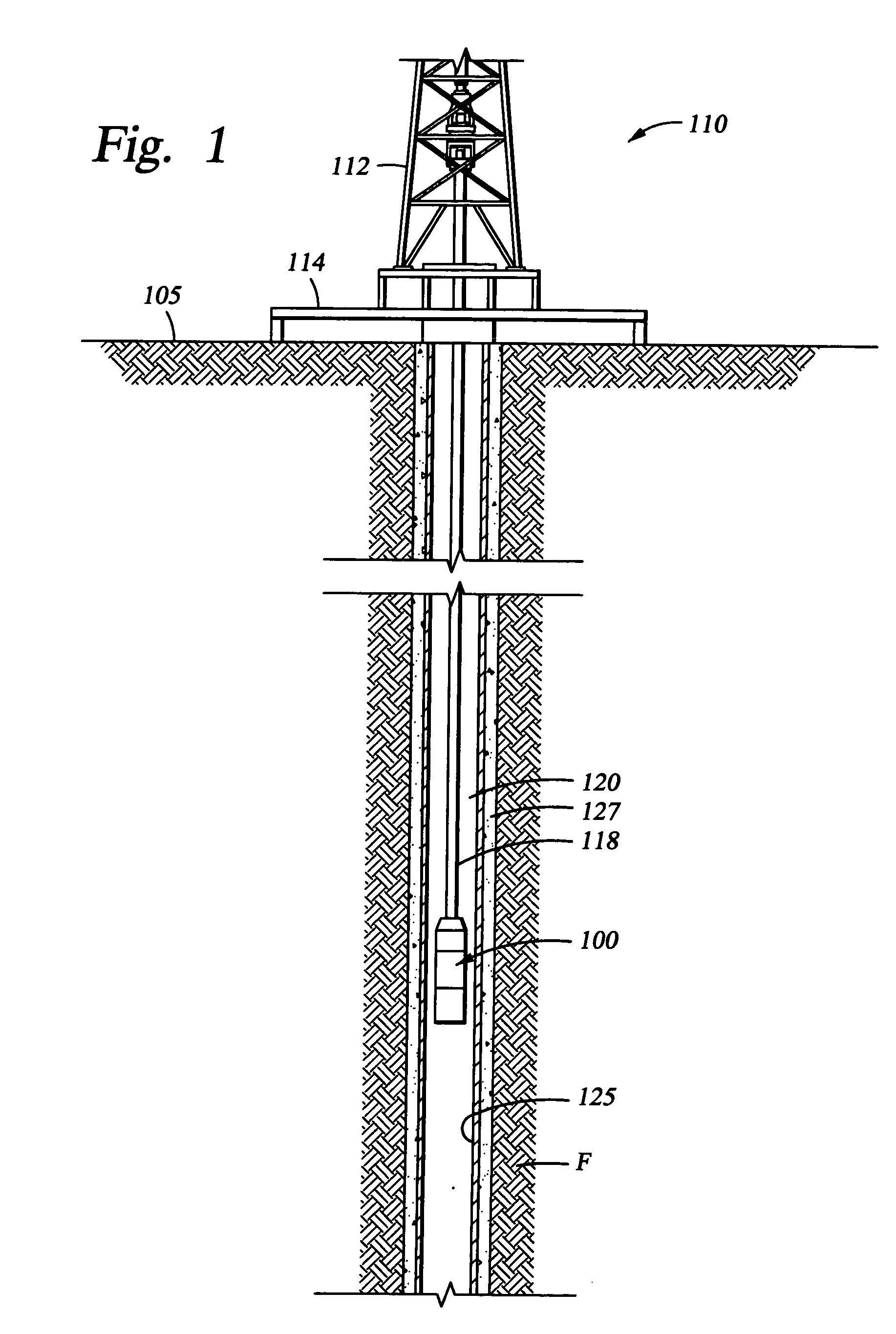
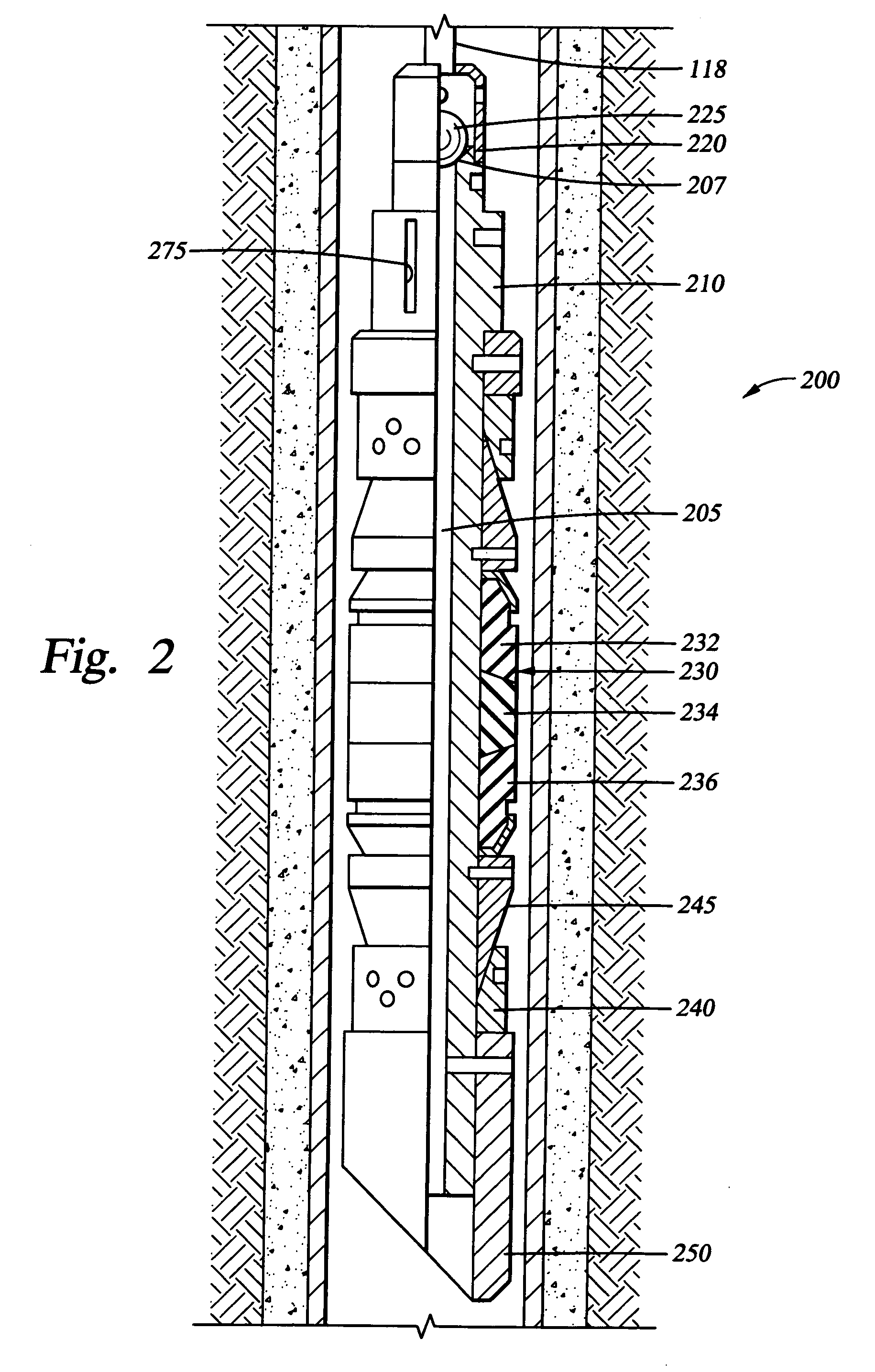
Dissolvable downhole tools
A disposable downhole tool comprises a material that dissolves when exposed to a chemical solution, an ultraviolet light, a nuclear source, or a combination thereof. In an embodiment, the material comprises an epoxy resin, a fiberglass, or a combination thereof. In another embodiment, the material comprises a fiberglass and a binding agent. The material may also be customized to achieve a desired dissolution rate of the tool. In an embodiment, the disposable downhole tool further comprises an enclosure for storing the chemical solution. The tool may also comprise an activation mechanism for releasing the chemical solution from the enclosure. In an embodiment, the disposable downhole tool is a frac plug. In another embodiment, the tool is a bridge plug. In yet another embodiment, the tool is a packer.
Owner:HALLIBURTON ENERGY SERVICES INC
Sustained release opioid formulations and method of use
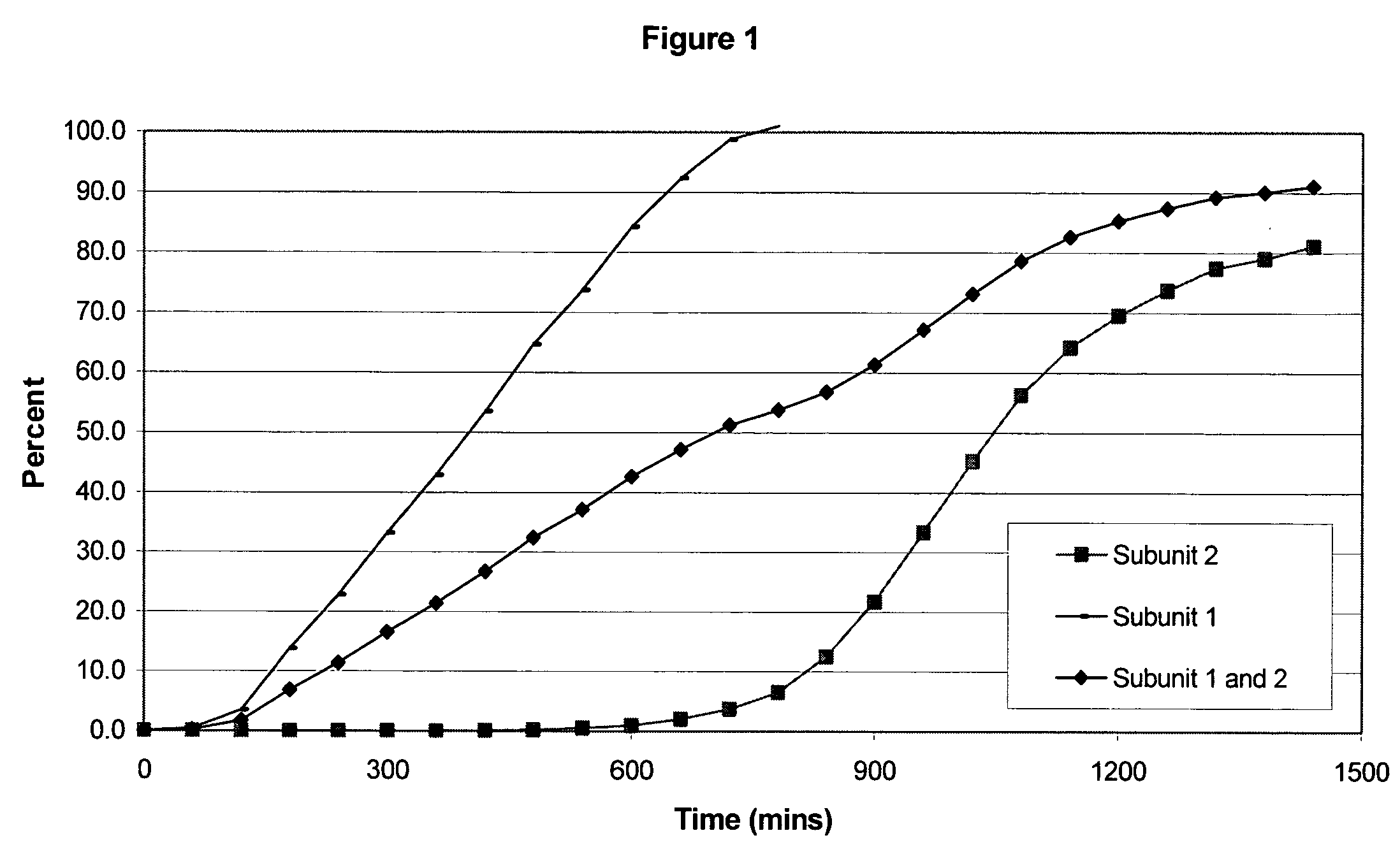
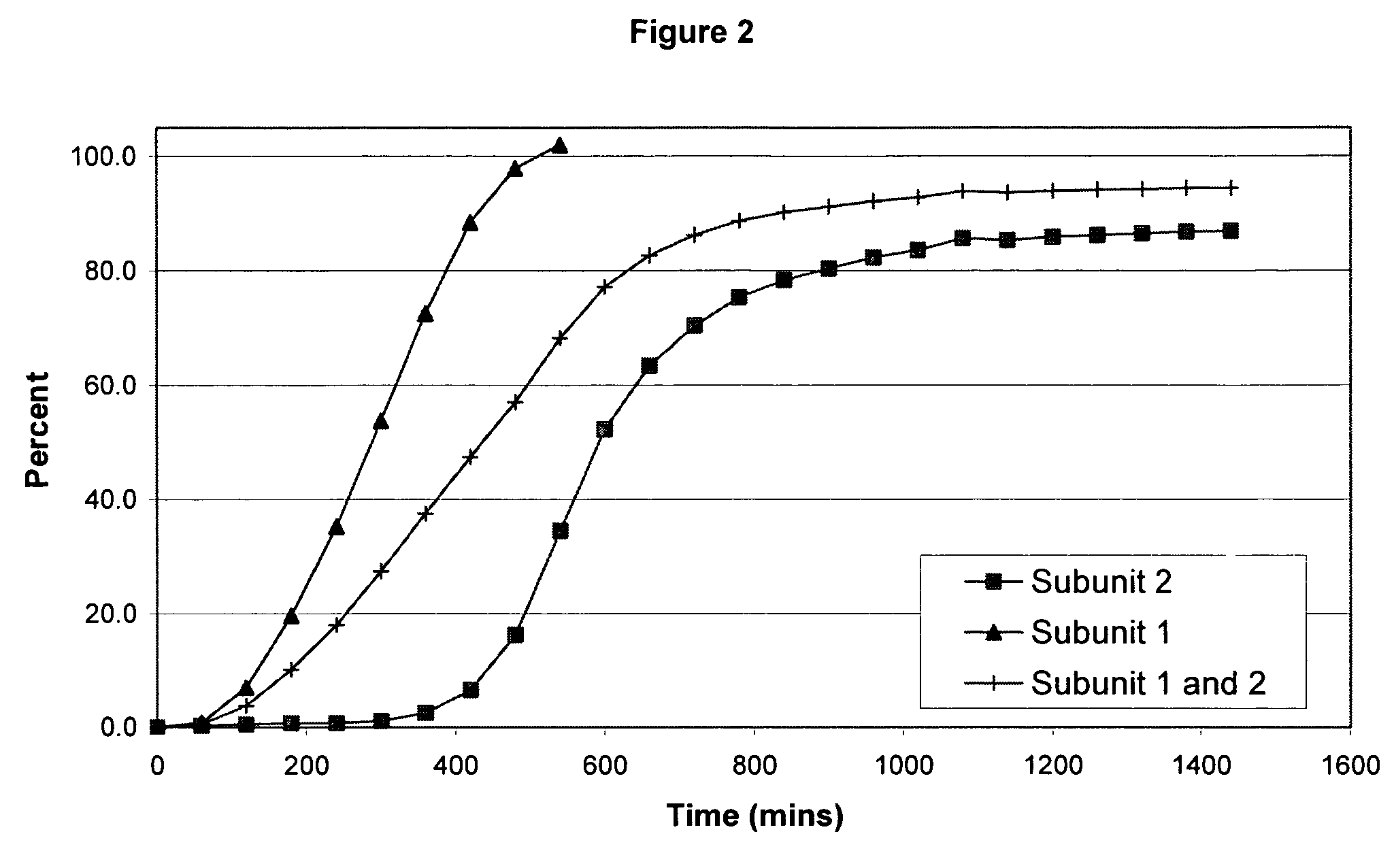
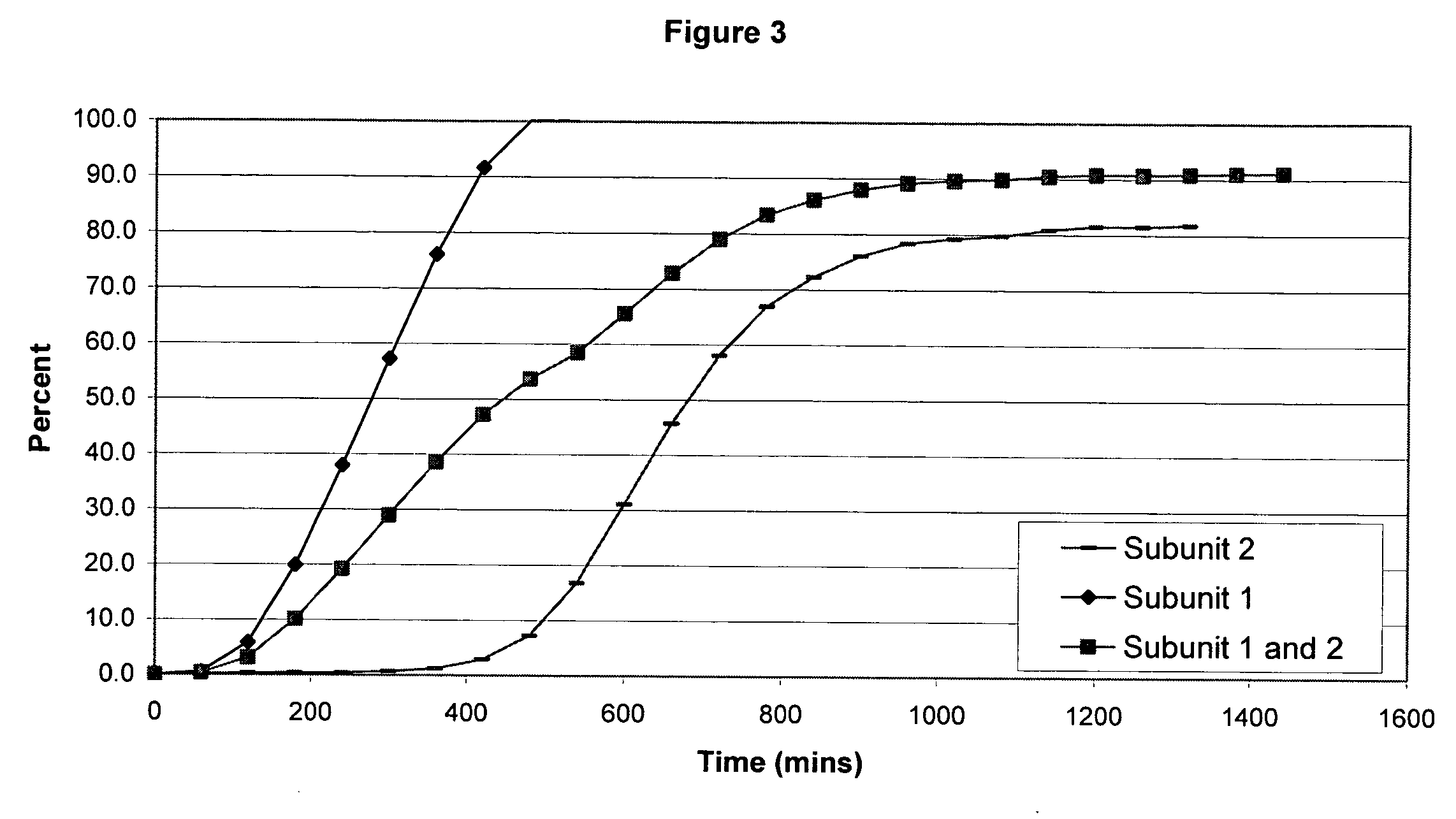
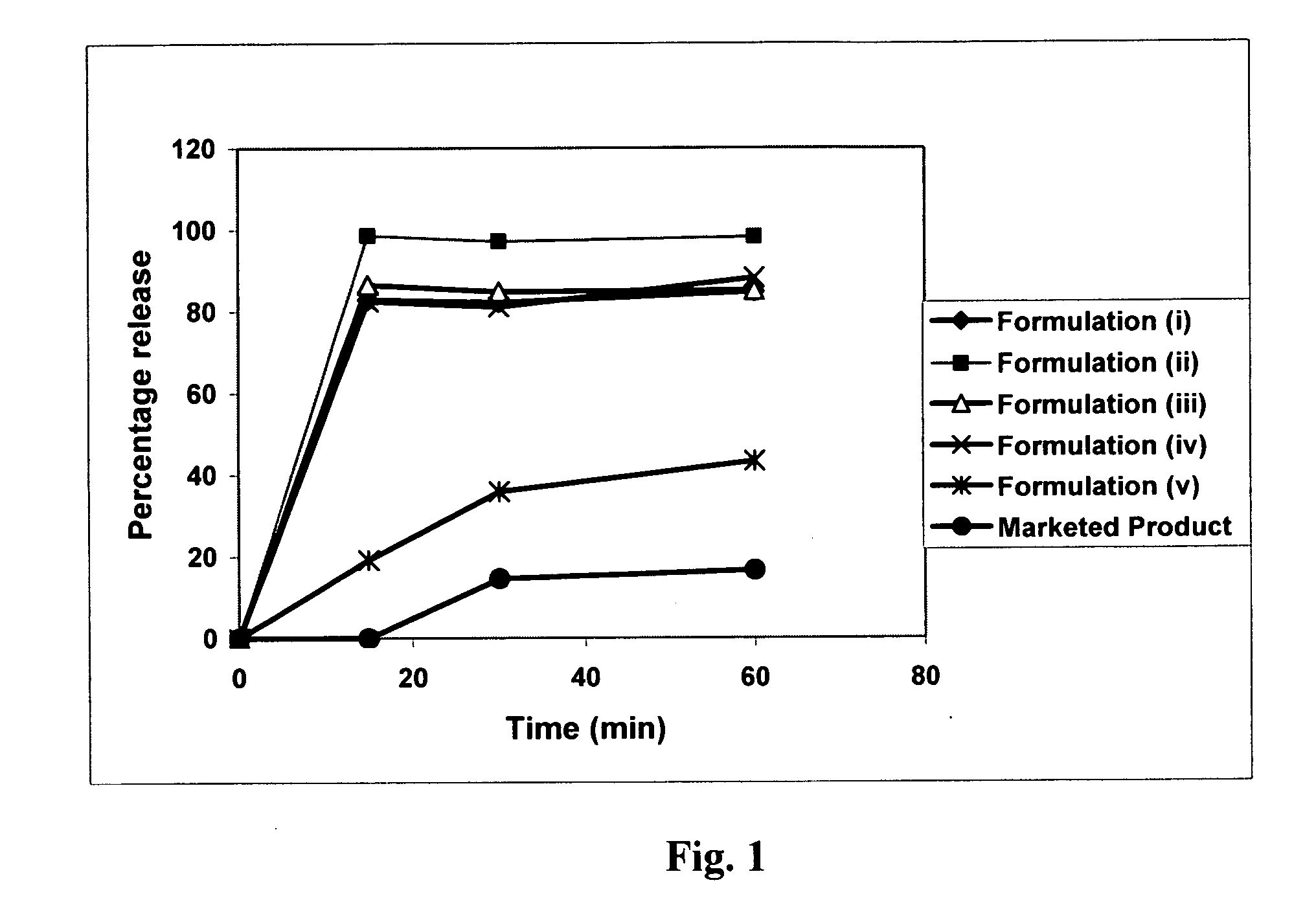
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
Orally disintegrable tablets
InactiveUS6328994B1Sufficient oral disintegrabilityHigh strengthOrganic active ingredientsPowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Dissolvable downhole tools
A disposable downhole tool comprises a material that dissolves when exposed to a chemical solution, an ultraviolet light, a nuclear source, or a combination thereof. In an embodiment, the material comprises an epoxy resin, a fiberglass, or a combination thereof. In another embodiment, the material comprises a fiberglass and a binding agent. The material may also be customized to achieve a desired dissolution rate of the tool. In an embodiment, the disposable downhole tool further comprises an enclosure for storing the chemical solution. The tool may also comprise an activation mechanism for releasing the chemical solution from the enclosure. In an embodiment, the disposable downhole tool is a frac plug. In another embodiment, the tool is a bridge plug. In yet another embodiment, the tool is a packer.
Owner:HALLIBURTON ENERGY SERVICES INC
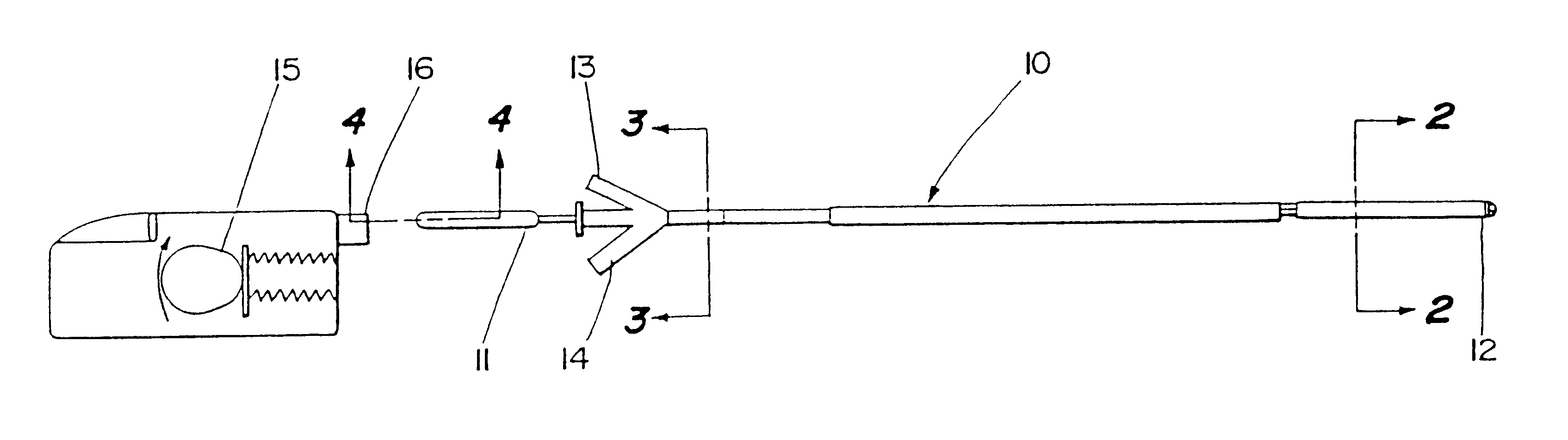
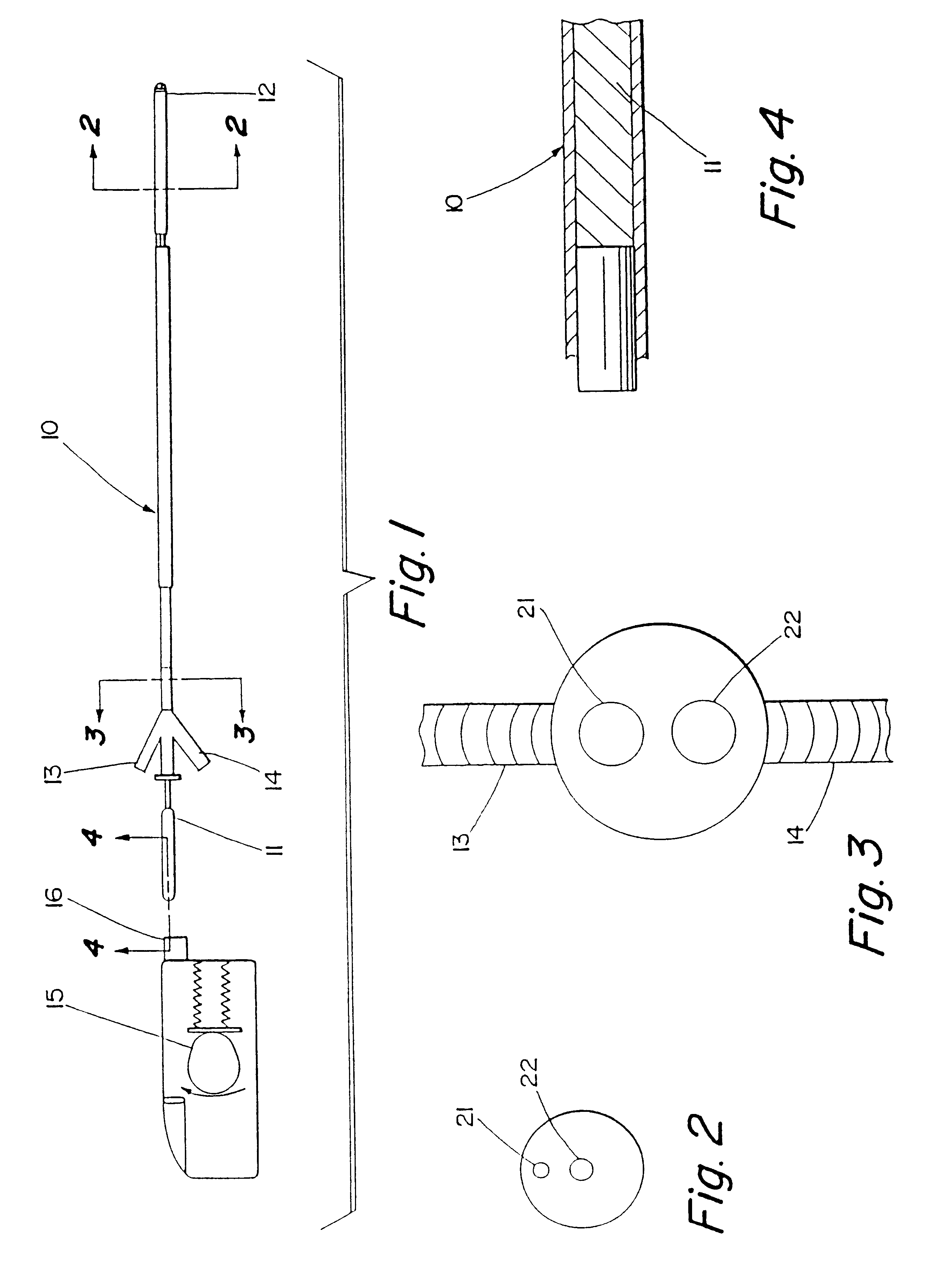
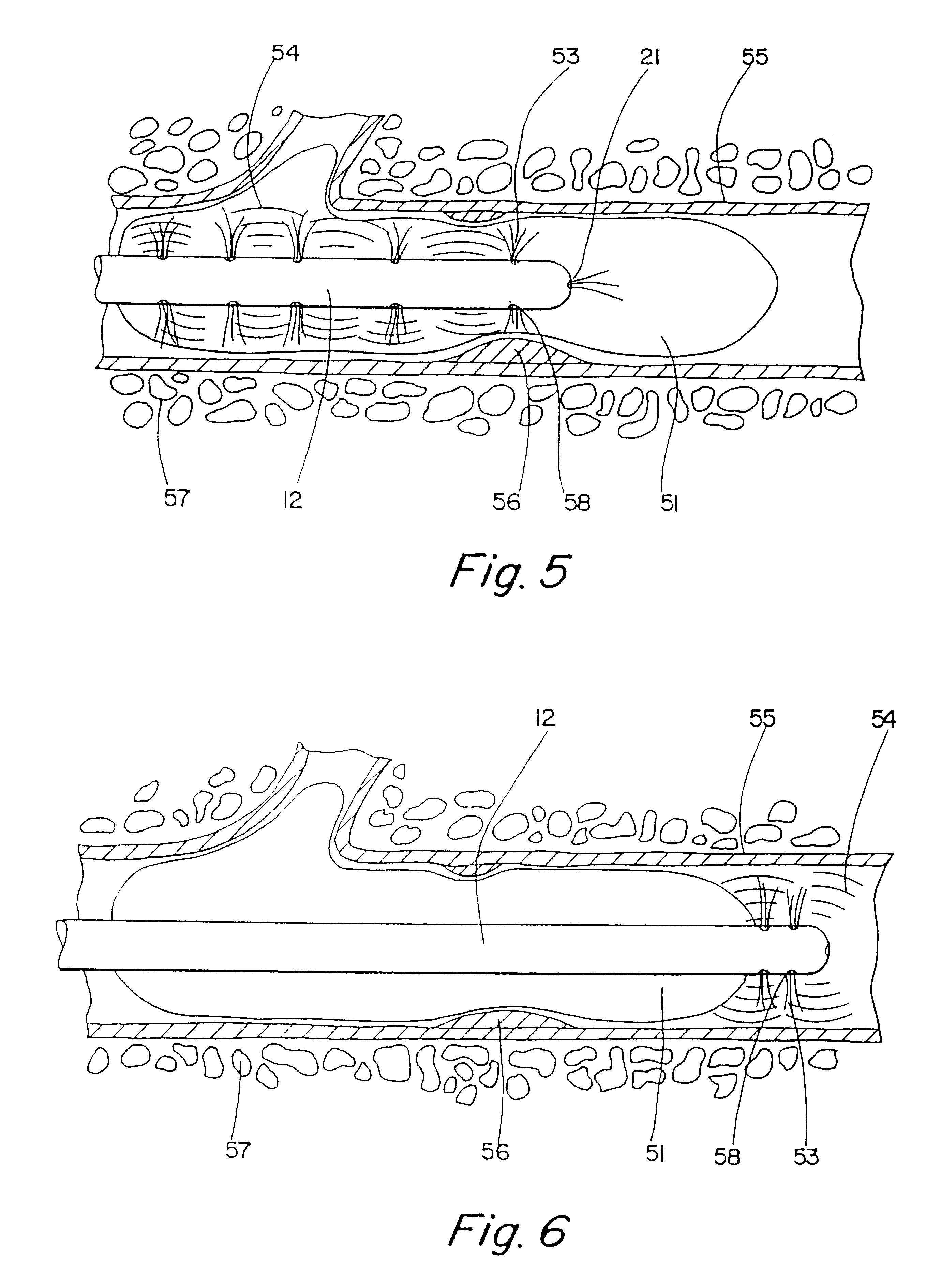
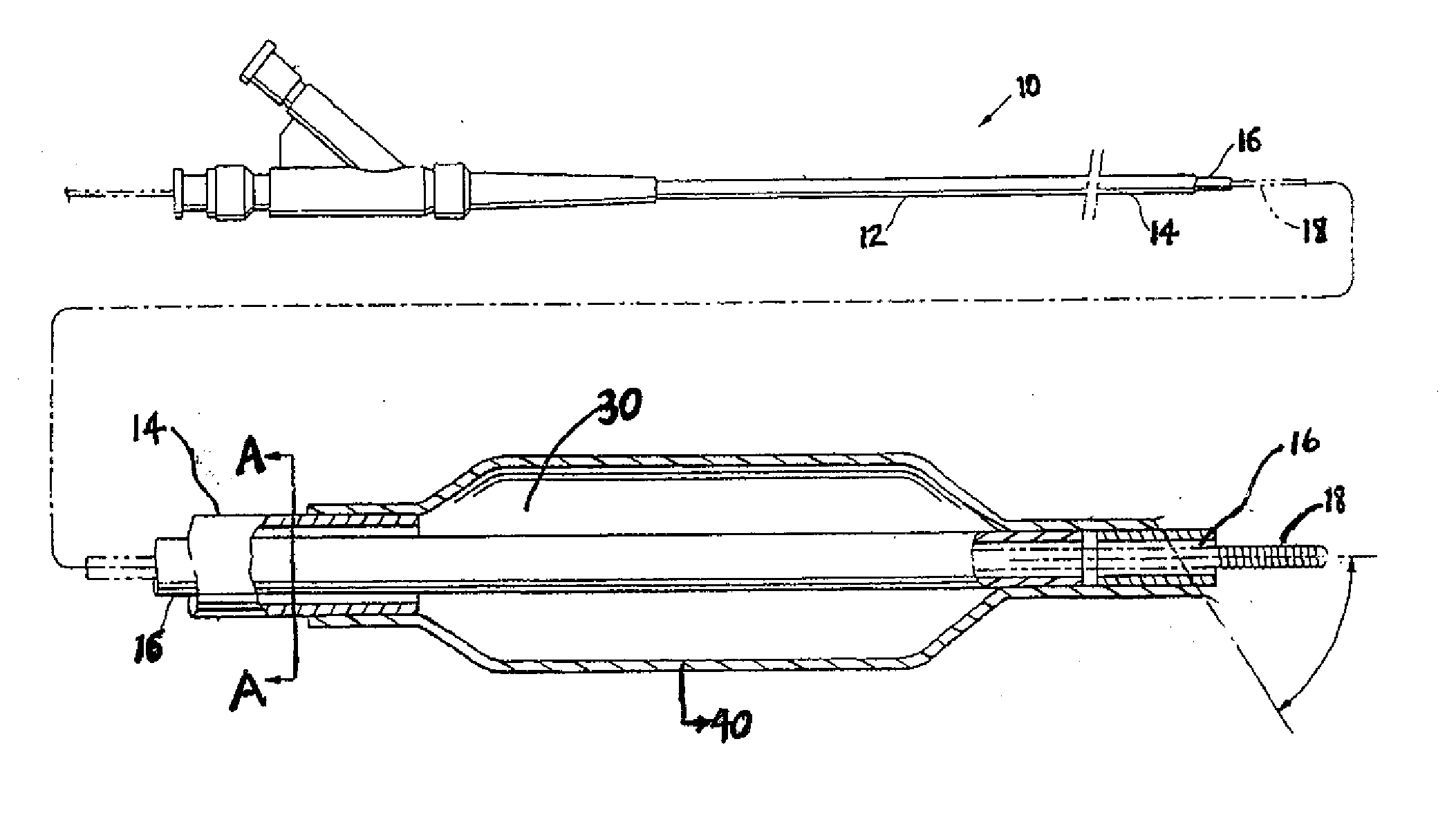
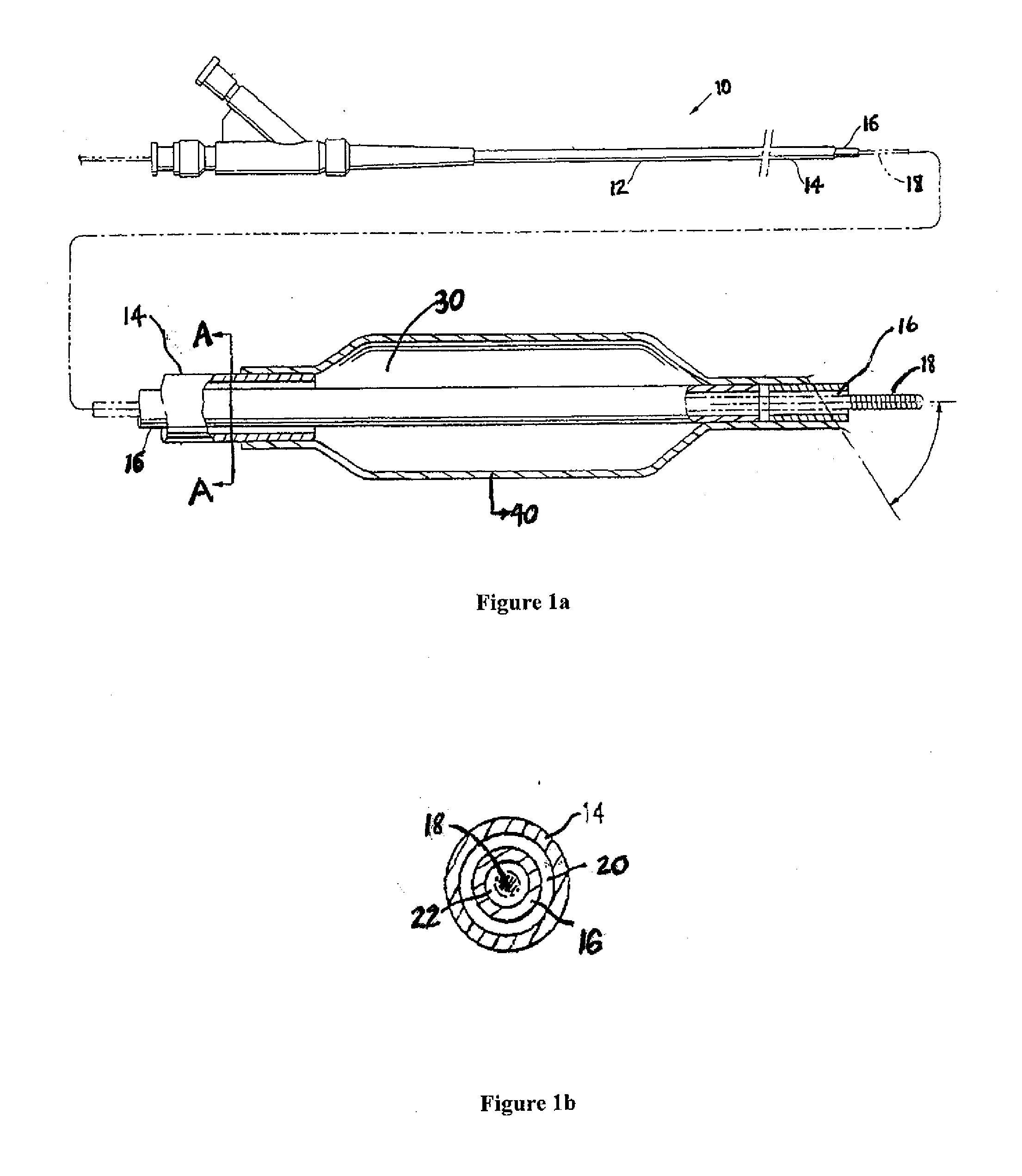
Motion catheter
The present invention describes a catheter suitable for introduction into a tubular tissue for dissolving blockages in such tissue. The catheter is particularly useful for removing thrombi within blood vessels. In accordance with the preferred embodiments, a combination of vibrating motion and injection of a lysing agent is utilized to break up blockages in vessels. The vessels may be veins, arteries, ducts, intestines, or any lumen within the body that may become blocked from the material that flows through it. As a particular example, dissolution of vascular thrombi is facilitated by advancing a catheter through the occluded vessel, the catheter causing a vibrating, stirring action in and around the thrombus usually in combination with the dispensing of a thrombolytic agent such as urokinase into the thrombus. The catheter has an inflatable or expandable member near the distal tip which, when inflated or expanded, prevents the passage of dislodged thrombus around the catheter. The dislodged portions of thrombus are directed through a perfusion channel in the catheter, where they are removed by filtration means housed within the perfusion channel before the blood exits the tip of the catheter. Catheters that allow both frequency (1-1000 Hz) vibratory motion and delivery of such agents to a blockage and a method for using such catheters are disclosed.
Owner:TYCO HEALTHCARE GRP LP
Dissolving Filter Cake
InactiveUS20040094300A1Needed propertyWider fractureCleaning apparatusFluid removalSolid baseCarrier fluid
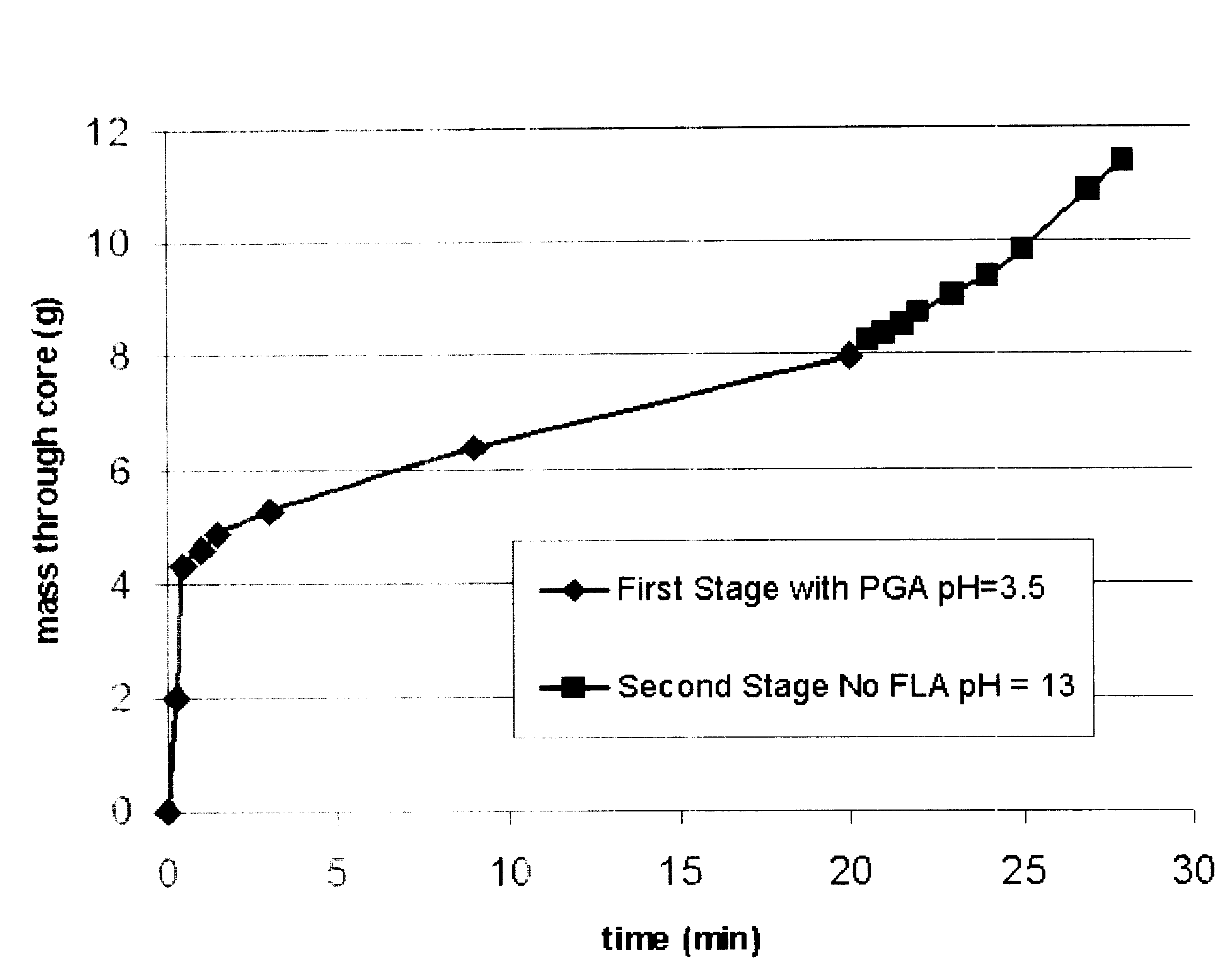
Methods are presented to induce a screenout during a subterranean formation fracturing or combined fracturing and gravel packing treatment having a viscoelastic surfactant-based carrier fluid by laying down a filter cake at least a portion of which is a base-soluble material, injecting proppant slurry, and causing hydrolysis and dissolution of the solid base-soluble material by adding base so that leak-off increases, the concentration of proppant in the fracture increases, and the proppant screens out. A method of gravel packing with a viscoelastic surfactant-based carrier fluid by laying down a filter cake at least a portion of which is a base-soluble material, injecting gravel, and causing hydrolysis and dissolution of the solid base-soluble material by adding base. Methods of slowing or accelerating the hydrolysis and dissolution are given, and addition of bridging-promoting materials is included.
Owner:SCHLUMBERGER TECH CORP
Viscosity reduction of viscoelastic surfactant based fluids
InactiveUS6881709B2Improve efficiencyImproves and optimizes conditionOther chemical processesFluid removalAlcoholSolid particle
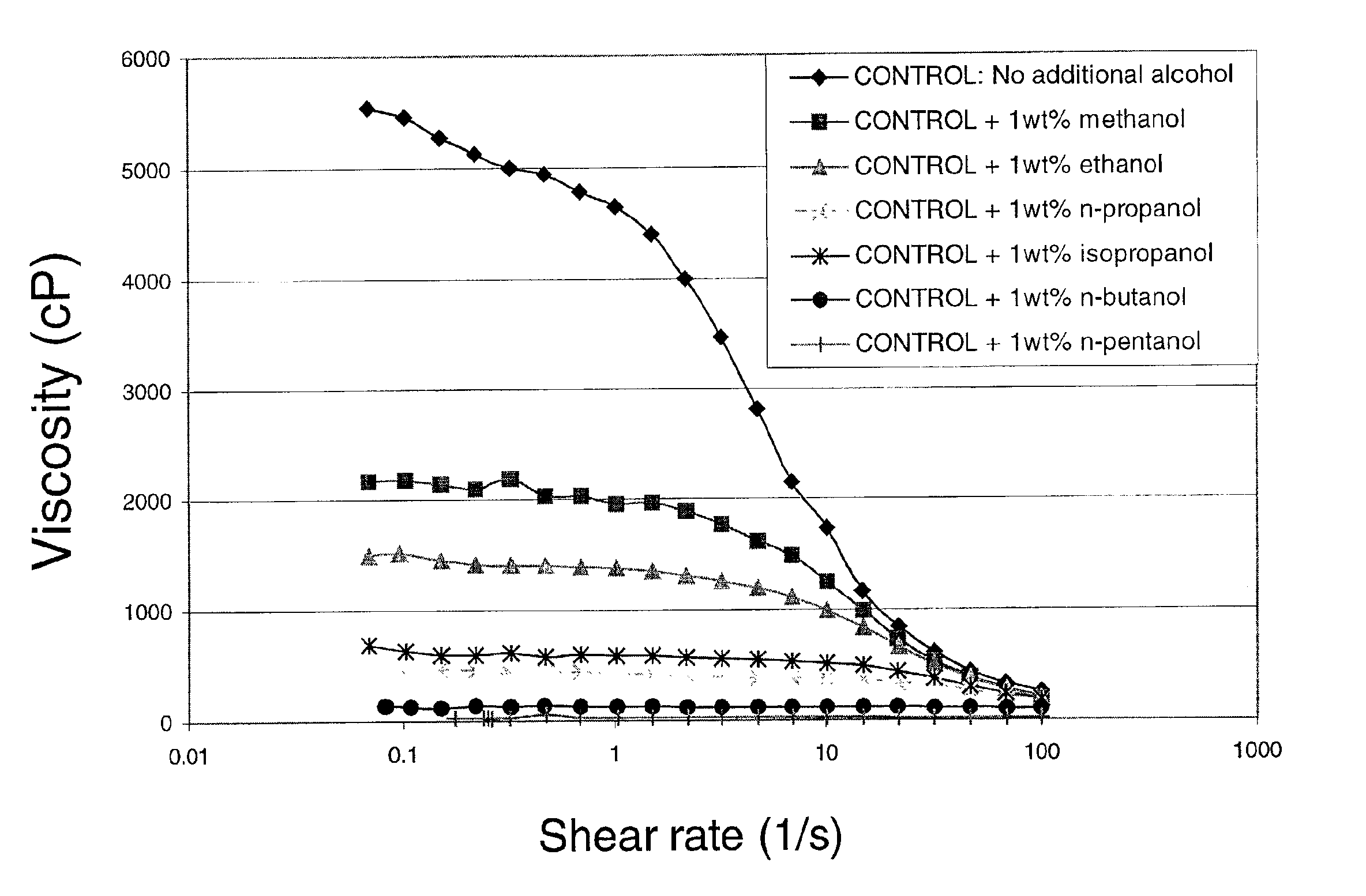
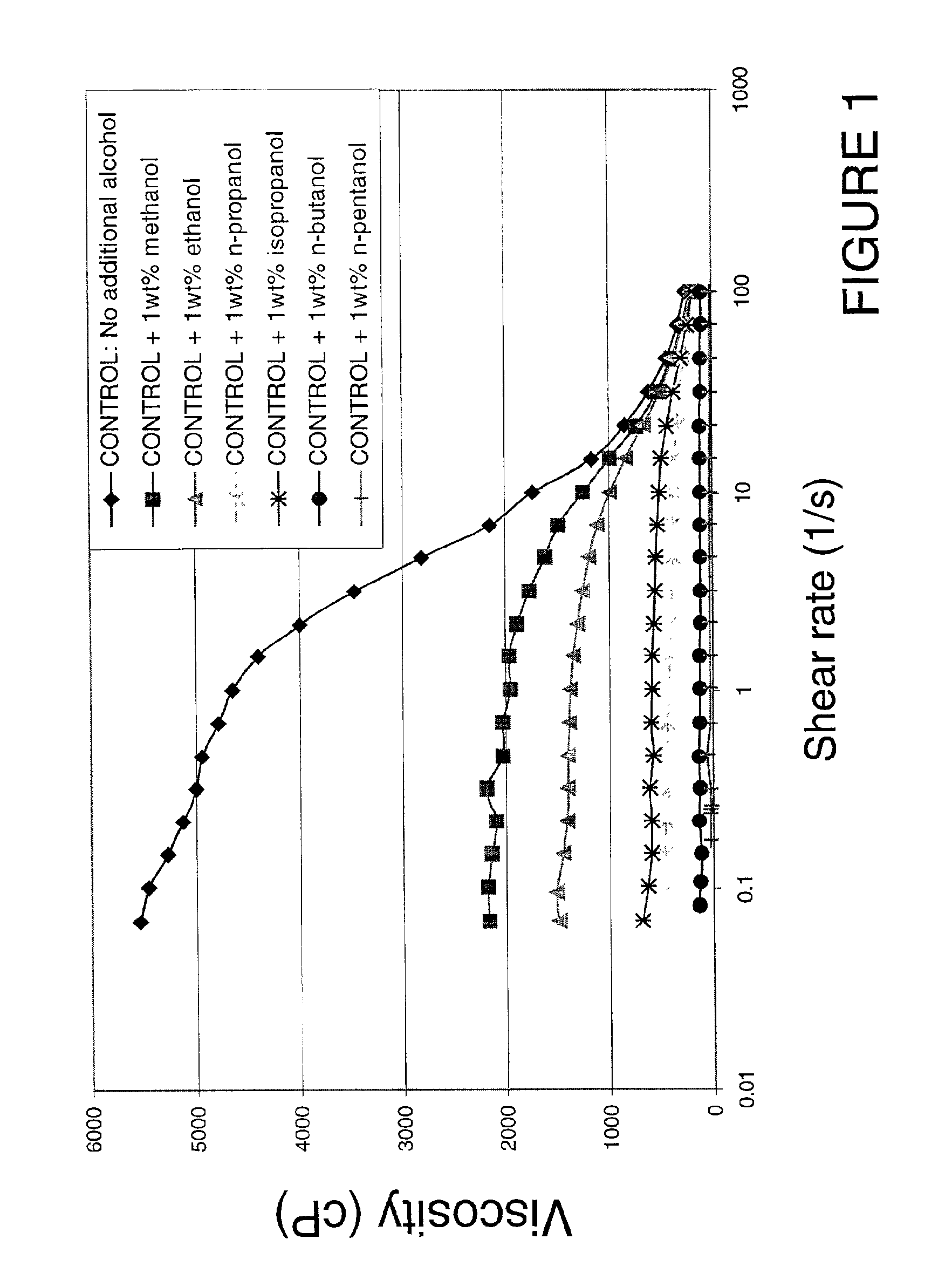
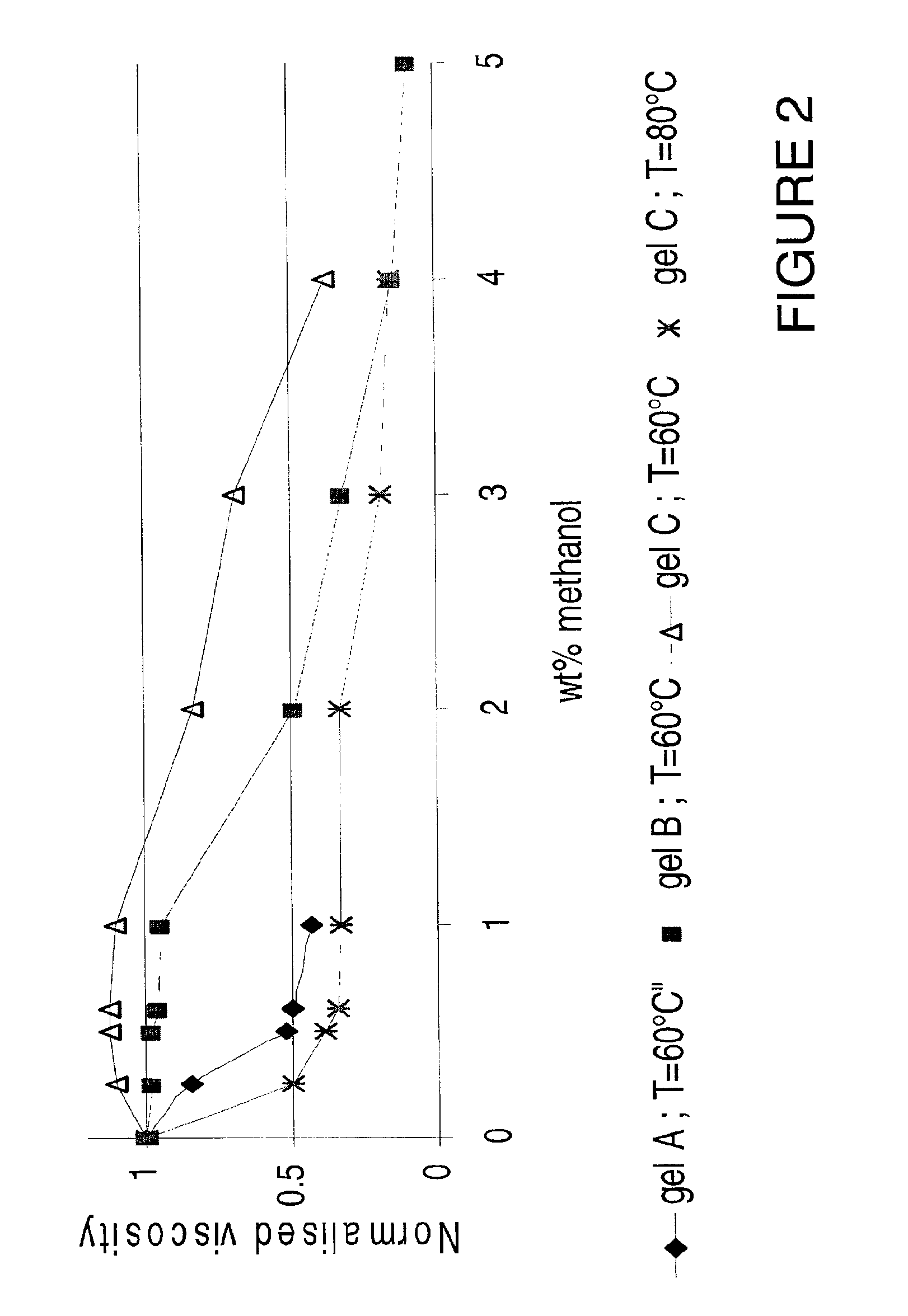
Methods and compositions are disclosed for controlled addition of components that decrease the viscosity of the viscoelastic surfactant fluids or for controlled changes in the electrolyte concentration or composition of the viscoelastic surfactant fluids. One aspect of the invention relates to the use of internal breakers with a delayed activation. Another aspect of the invention relates to the use of precursors that release a breaking system such as alcohol by a process such as melting, slow dissolution, reaction with a compound present in the fluid or added to the fluid during or after the step of injecting, rupture of an encapsulating coating and de-adsorption of a breaking agent absorbed into solid particles. In another aspect of the invention, alcohols are included in a pad to reduce the low-shear viscosity and reduce the resistance to flow of the treatment fluids during a desired phase of the treatment.
Owner:SCHLUMBERGER TECH CORP
Controlled release formulations coated with aqueous dispersions of acrylic polymers
InactiveUS6143353ADissolution stabilityIncrease weight gainPretreated surfacesMedical devicesWater insolubleActive agent
A stable solid controlled release formulation having a coating derived from an aqueous dispersion of a hydrophobic acrylic polymer includes a substrate including an active agent selected from the group consisting of a systemically active therapeutic agent, a locally active therapeutic agent, a disinfecting and sanitizing agent, a cleansing agent, a fragrance agent and a fertilizing agent, overcoated with an aqueous dispersion of the plasticized water-insoluble acrylic polymer. The formulation provides a stable dissolution of the active agent which is unchanged after exposure to accelerated storage conditions.
Owner:PURDUE PHARMA LP
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer
InactiveUS6129933AReduce reunionLiquid surface applicatorsGranular deliveryHydrophobic polymerDissolution
A stabilized solid controlled release dosage form having a coating derived from an aqueous dispersion of ethylcellulose is obtained by overcoating a substrate including a therapeutically active with an aqueous dispersion of ethylcellulose and then curing the coated substrate at a temperature and relative humidity elevated to a suitable level above ambient conditions until the coated dosage form attains a stabilized dissolution profile substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:PURDUE PHARMA LP
Reversible electrochemical mirror (REM) state monitoring
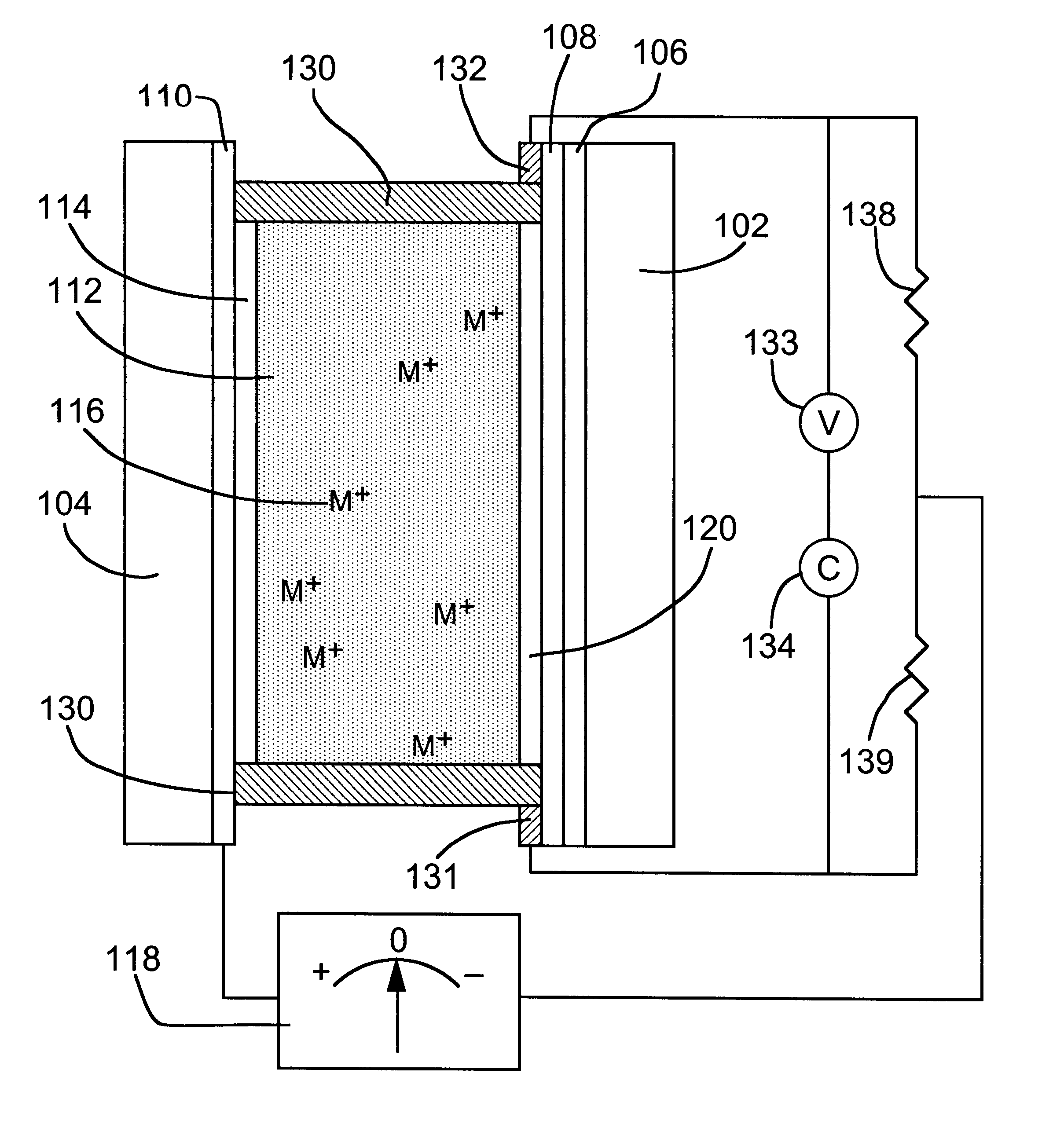
Reversible electrochemical mirror (REM) devices typically comprise a conductive oxide mirror electrode that is substantially transparent to radiation of some wavelengths, a counter electrode that may also be substantially transparent, and an electrolyte that contains ions of an electrodepositable metal. A voltage applied between the two electrodes causes electrodeposition of a mirror deposit on the mirror electrode and dissolution of the mirror deposit on the counter electrode, and these processes are reversed when the polarity of the applied voltage is changed. Such REM devices provide precise control over the reflection and transmission of radiation and can be used for a variety of applications, including smart windows and automatically adjusting automotive mirrors. According to the present invention, measurements of the sheet resistance of the mirror electrode in a REM device are correlated with the thickness of electrodeposited mirror metal and can be used to monitor the reflectance of the device. Sheet resistance measurements can be performed while the mirror state of the device is being switched if adequate isolation between the measurement and switching circuits is provided. This can be accomplished by use of external resistors or more sophisticated circuitry, or by taking advantage of the relatively high sheet resistance of the mirror electrode itself. Monitoring the reflectance of REM devices according to this invention provides significant cost and performance advantages.
Owner:TELEDYNE SCI & IMAGING
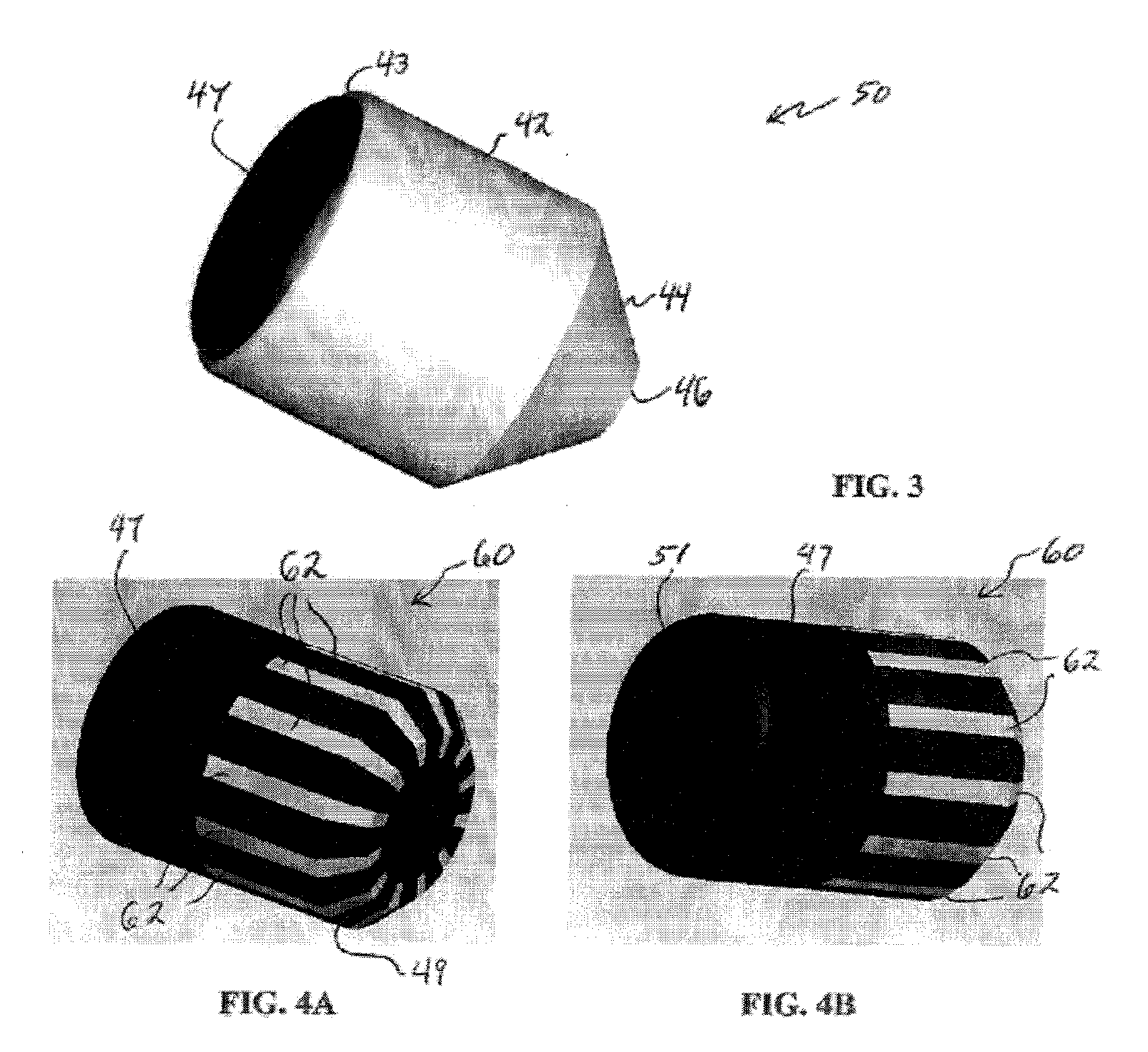
Well Operating Elements Comprising a Soluble Component and Methods of Use
Well operating elements are described, one embodiment comprising a first component that is substantially non-dissolvable when exposed to a selected wellbore environment and a second component that is soluble in the selected wellbore environment and whose rate and / or location of dissolution is at least partially controlled by structure of the first component. A second embodiment includes the component that is soluble in the selected wellbore environment, and one or more exposure holes or passages in the soluble component to control its solubility. The second embodiment may or may not include a substantially non-dissolvable component. Methods of using the well operating elements in oilfield operations are also described. This abstract allows a searcher or other reader to quickly ascertain the subject matter of the disclosure. It will not be used to interpret or limit the scope or meaning of the claims.
Owner:SCHLUMBERGER TECH CORP
Microbial pesticidal composition
Owner:KUMIAI CHEM IND CO LTD
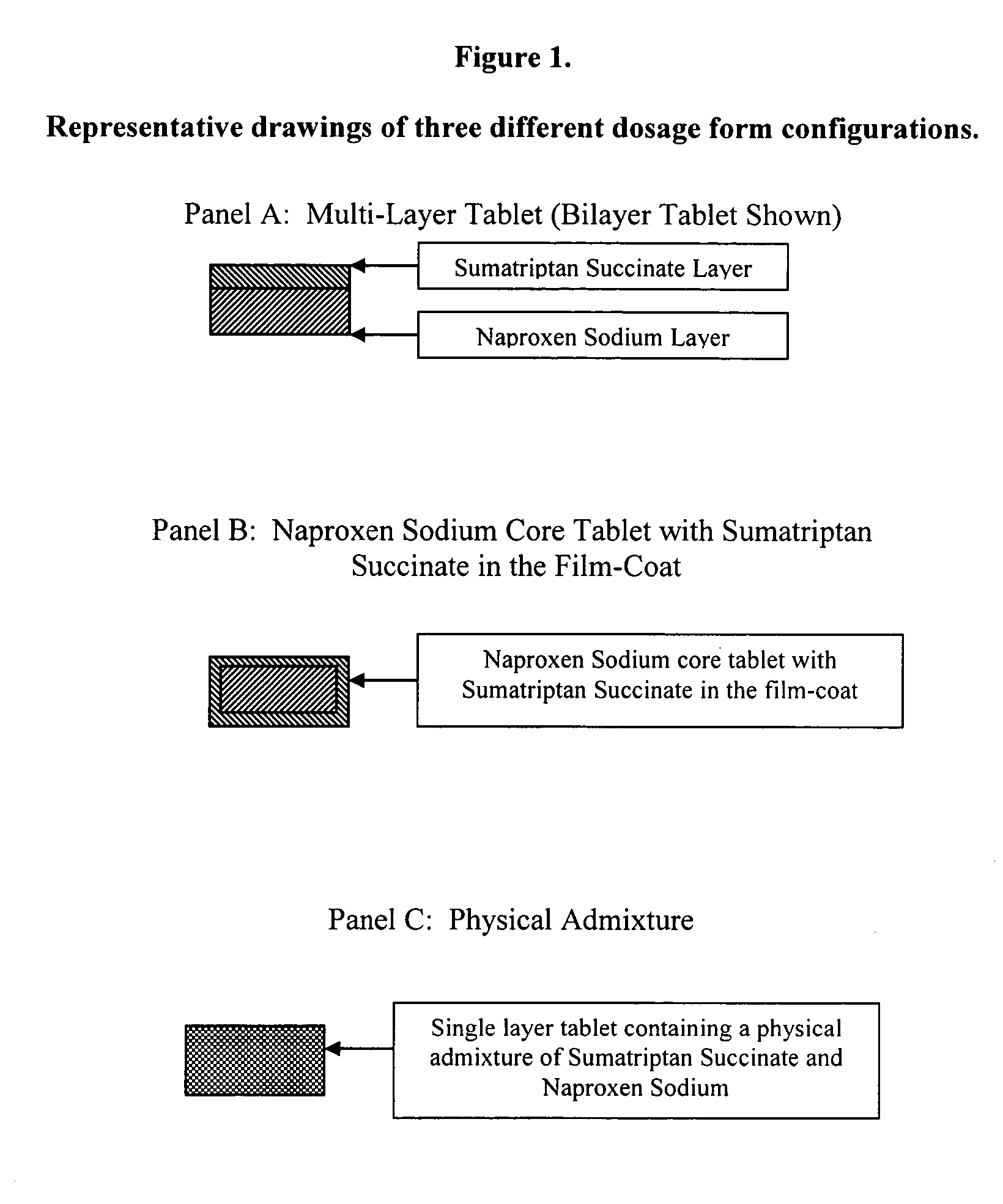
Multilayer dosage forms containing NSAIDs and triptans
The present invention is directed to multilayer pharmaceutical tablets in which an NSAID and a triptan are present in separate and distinct layers. The layers are in a side-by-side configuration, which allows the dissolution of triptan and NSAID to occur independently and immediately.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO
Beverage cartridge and method for beverage formation using filter aid
InactiveUS20110076361A1Speed up circulationReduce cloggingTea extractionBeverage vesselsParticulatesCellulose
A method and apparatus for forming a beverage involves the use of a filter aid, e.g., that is provided in a beverage cartridge with a beverage medium in a dry state. In one embodiment, a cartridge including a dried fruit material may also include a filter aid, such as perlite, diatomaceous earth or cellulose, that is mixed together with the fruit material. The filter aid may assist in flow through the beverage medium or through a filter, e.g., by helping prevent the clogging of pores of a filter used to remove particulate from a beverage formed by interaction of the beverage medium with water introduced into the cartridge. Thus, the filter aid may permit the use of some beverage media that would otherwise clog a filter and / or prevent proper dissolution of materials in the beverage media without the filter aid.
Owner:GREEN MOUNTAIN COFFEE ROASTERS INC
Catheter devices and methods for their use in the treatment of calcified vascular occlusions
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Hydrophobic abuse deterrent delivery system
Disclosed herein are oral dosage forms of therapeutic agents that are resistant to abuse and methods of their formulation. In particular, oral dosage forms that are resistant to dissolution in aqueous solutions of ethanol are described.
Owner:LAB INT
Delivery of tetrahydrocannabinol
InactiveUS20070104741A1Avoiding hepatic first-pass metabolismGood choiceBiocideNervous disorderChylomicronTG - Triglyceride
A self-emulsifying drug delivery system to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron delivery and optimal bioavailability to a mammalian intestinal lumen. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY PHARMA
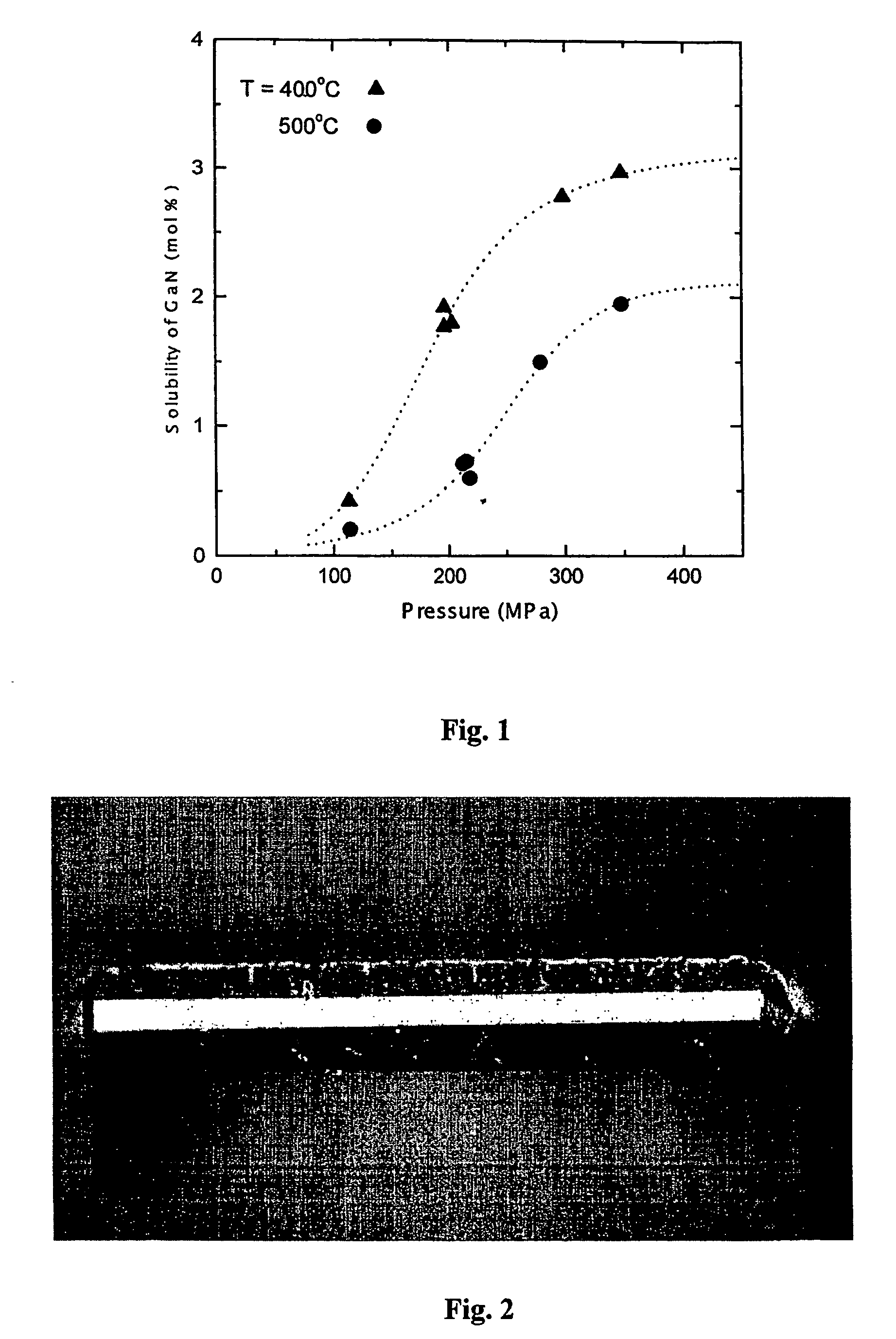
Bulk nitride mono-crystal including substrate for epitaxy
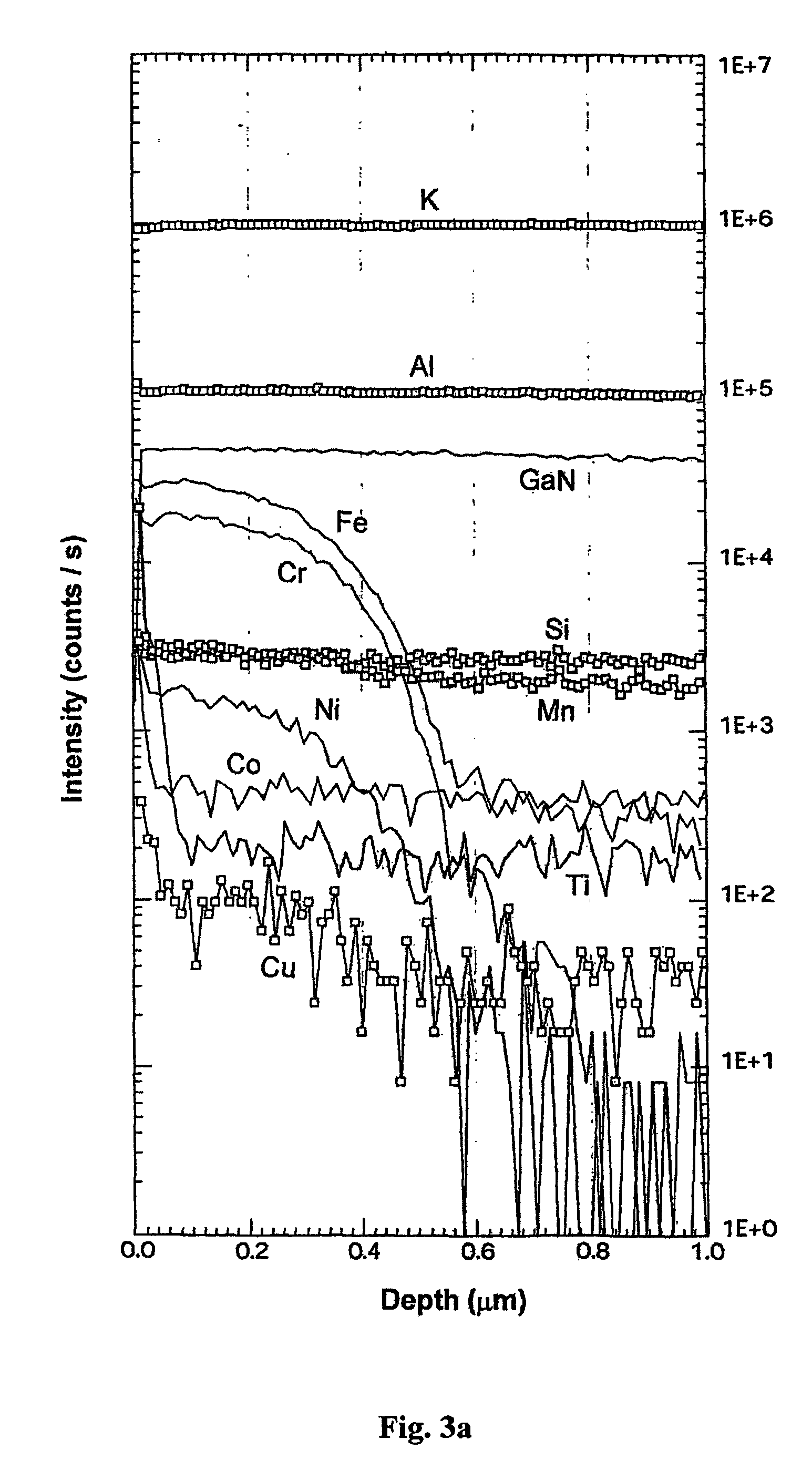
InactiveUS7132730B2Reduced life-timeLaser detailsFrom normal temperature solutionsDissolutionSolvent
The invention relates to a substrate for epitaxy, especially for preparation of nitride semiconductor layers. Invention covers a bulk nitride mono-crystal characterized in that it is a mono-crystal of gallium nitride and its cross-section in a plane perpendicular to c-axis of hexagonal lattice of gallium nitride has a surface area greater than 100 mm2, it is more than 1.0 μm thick and its C-plane surface dislocation density is less than 106 / cm2, while its volume is sufficient to produce at least one further-processable non-polar A-plane or M-plane plate having a surface area at least 100 mm2. More generally, the present invention covers a bulk nitride mono-crystal which is characterized in that it is a mono-crystal of gallium-containing nitride and its cross-section in a plane perpendicular to c-axis of hexagonal lattice of gallium-containing nitride has a surface area greater than 100 mm2, it is more 1.0 μm thick and its surface dislocation density is less than 106 / cm2. Mono-crystals according to the present invention are suitable for epitaxial growth of nitride semiconductor layers. Due to their good crystalline quality they are suitable for use in opto-electronics for manufacturing opto-electronic semiconductor devices based on nitrides, in particular for manufacturing semiconductor laser diodes and laser devices. The a.m bulk mono-crystals of gallium-containing nitride are crystallized on seed crystals. Various seed crystals may be used. The bulk mono-crystals of gallium-containing nitride are crystallized by a method involving dissolution of a gallium-containing feedstock in a supercritical solvent and crystallization of a gallium nitride on a surface of seed crystal, at temperature higher and / or pressure lower than in the dissolution process.
Owner:AMMONO SP Z O O (PL) +1
Well treatment with dissolvable polymer
InactiveUS20060157248A1Ease and flexibility for subsequent completion processEasy to processInsulationLiquid/gas jet drillingPolyvinyl alcoholDissolution
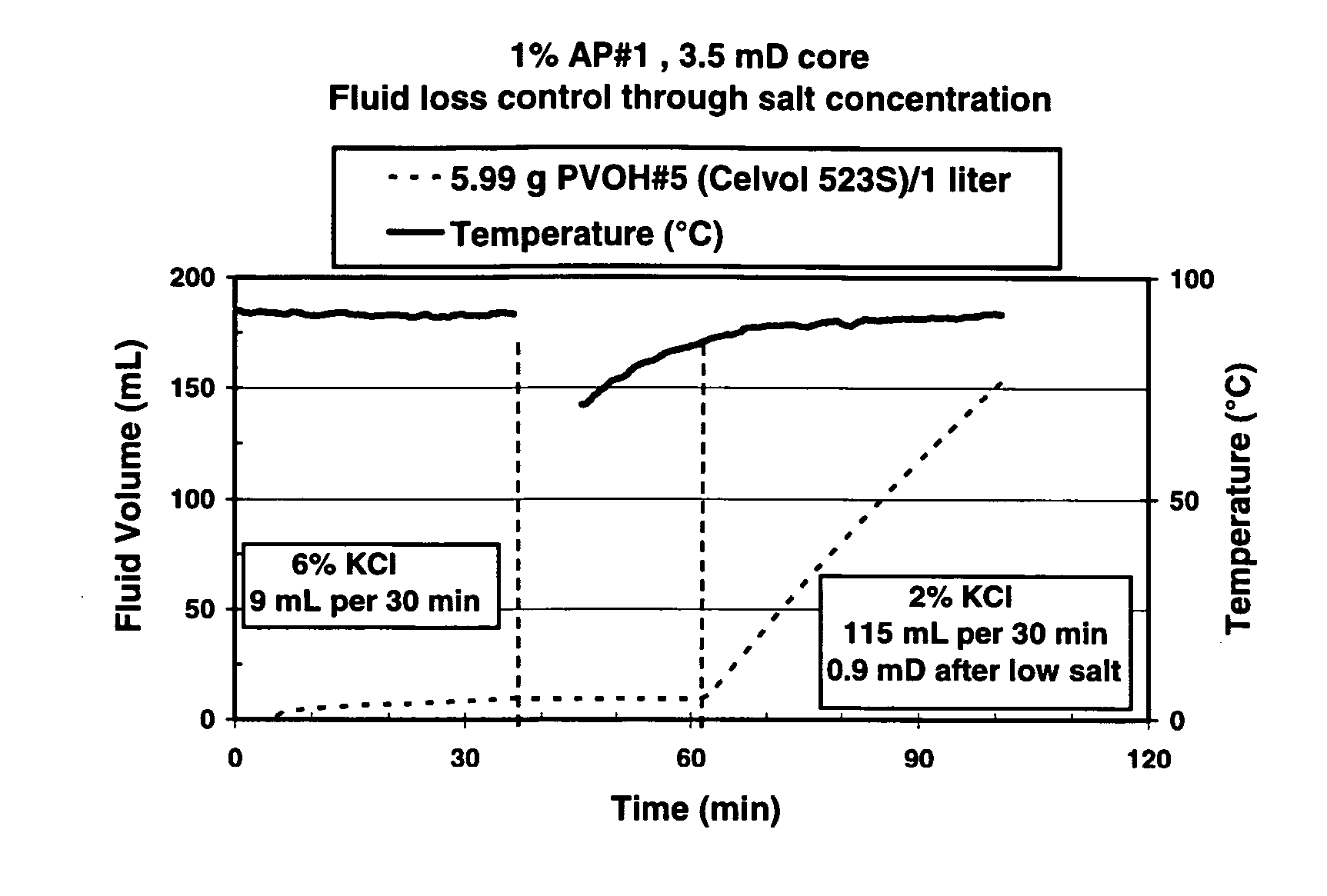
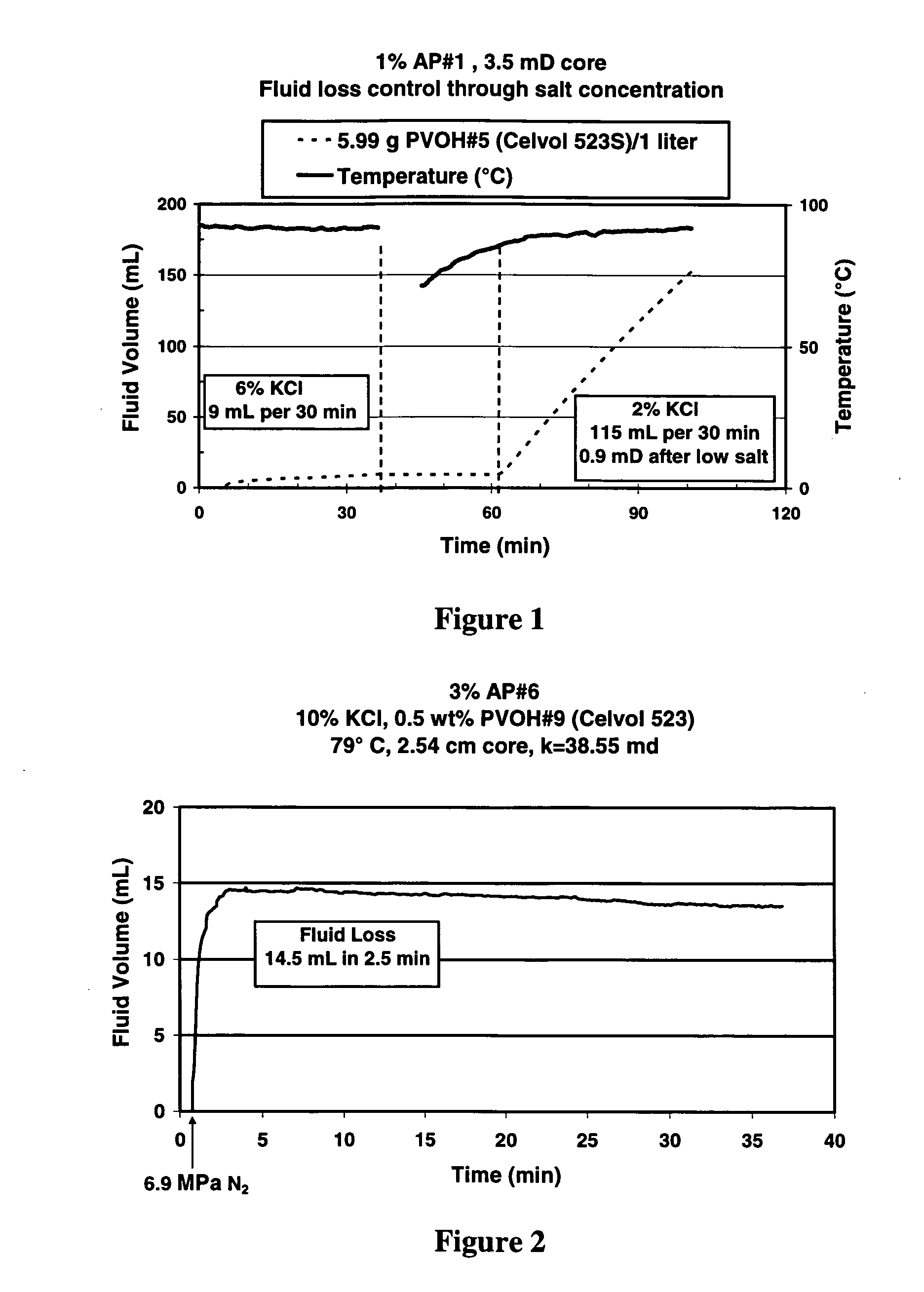
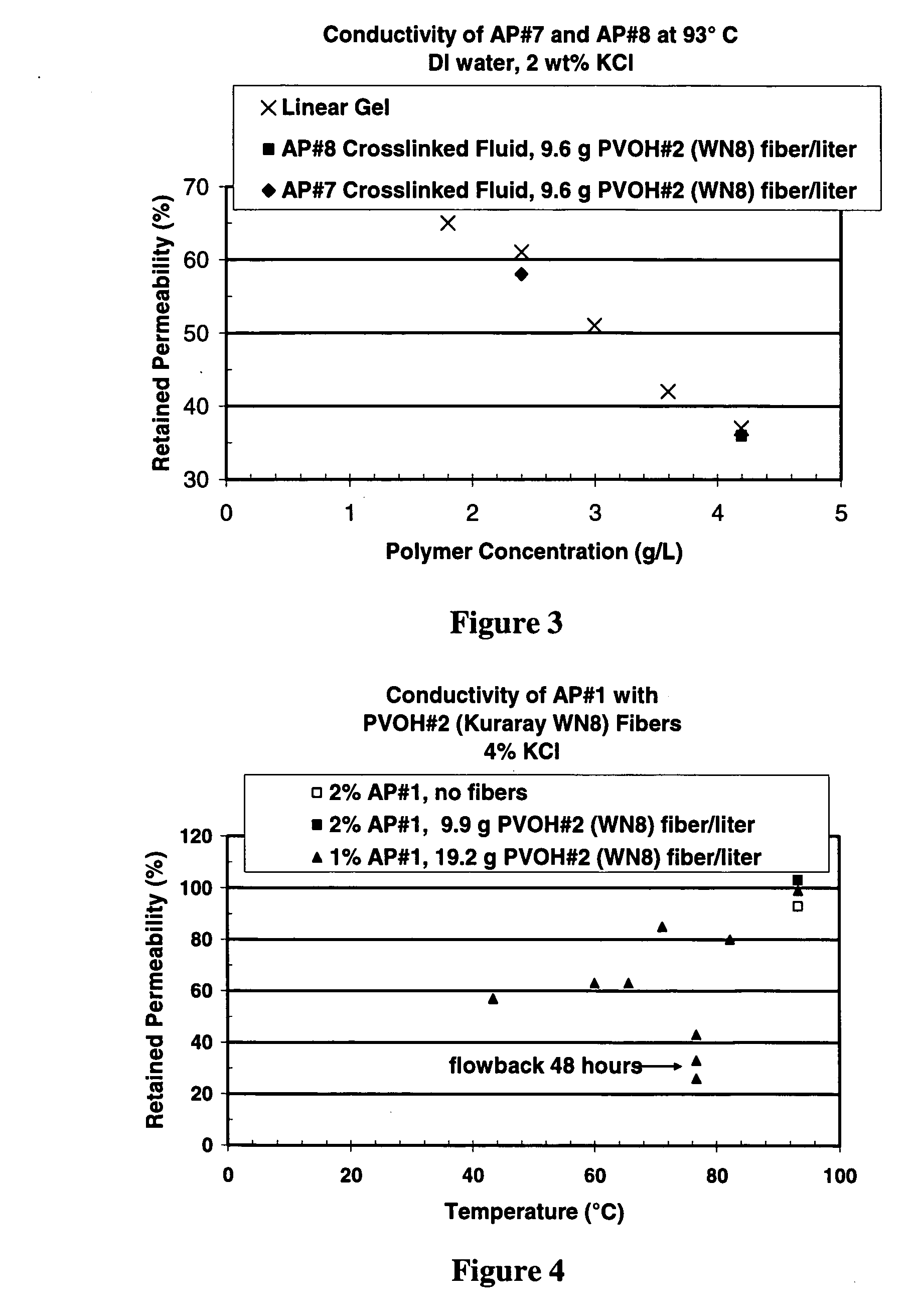
Well treatment is disclosed that includes injecting a well treatment fluid with insoluble polyol polymer such as polyvinyl alcohol (PVOH) dispersed therein, depositing the insoluble polymer in the wellbore or an adjacent formation, and thereafter dissolving the polymer by reducing salinity and / or increasing temperature conditions in the environment of the polymer deposit. The method is disclosed for filter cake formation, fluid loss control, drilling, hydraulic fracturing and fiber assisted transport, where removal of the polyol at the end of treatment or after treatment is desired. The method is also disclosed for providing dissolved polyol as a delayed breaker in crosslinked polymer viscosified systems and viscoelastic surfactant systems. Also disclosed are well treatment fluids containing insoluble amorphous or at least partially crystalline polyol, and a PVOH fiber composition wherein the fibers are stabilized from dissolution by salinity.
Owner:SCHLUMBERGER TECH CORP
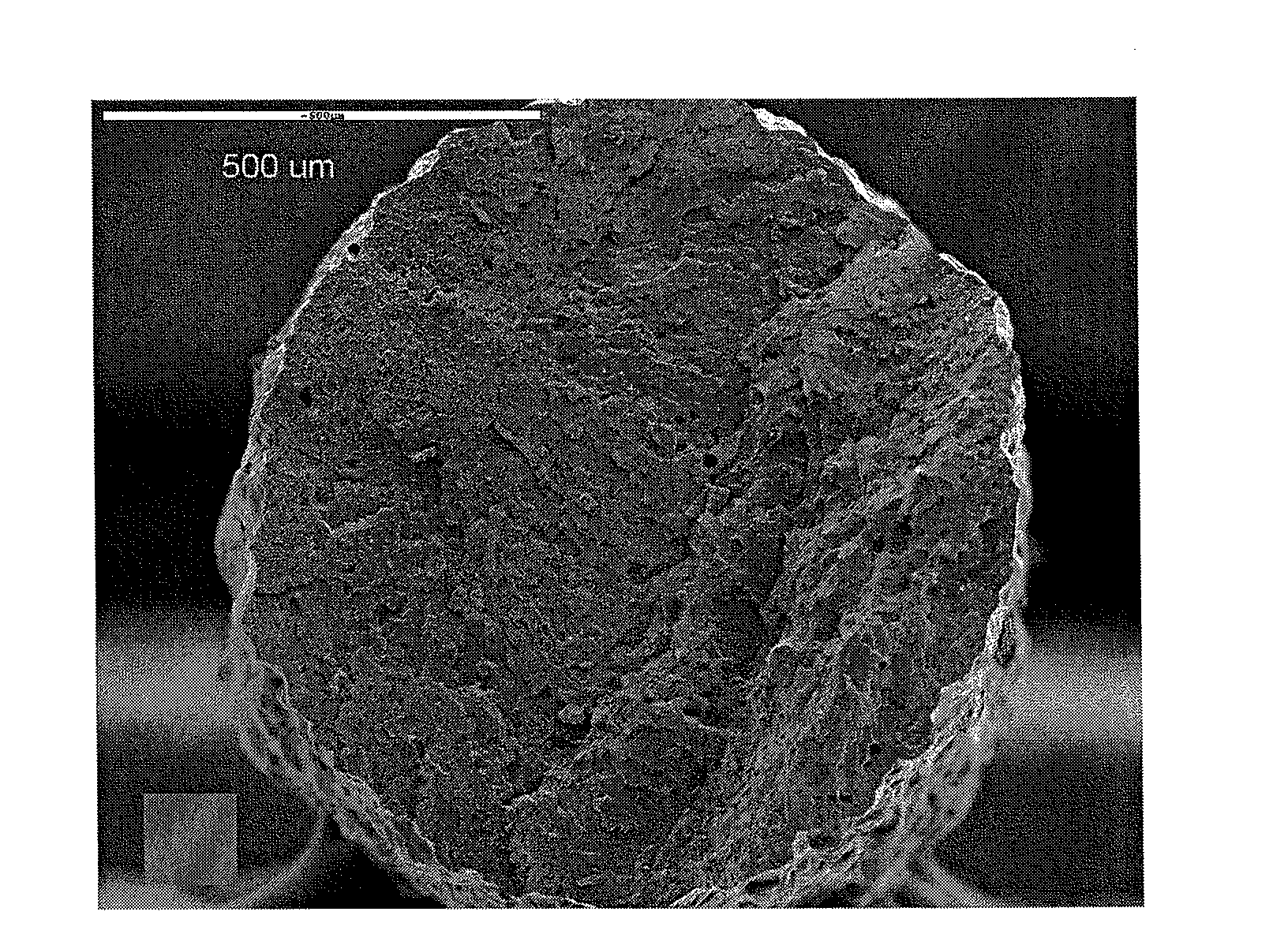
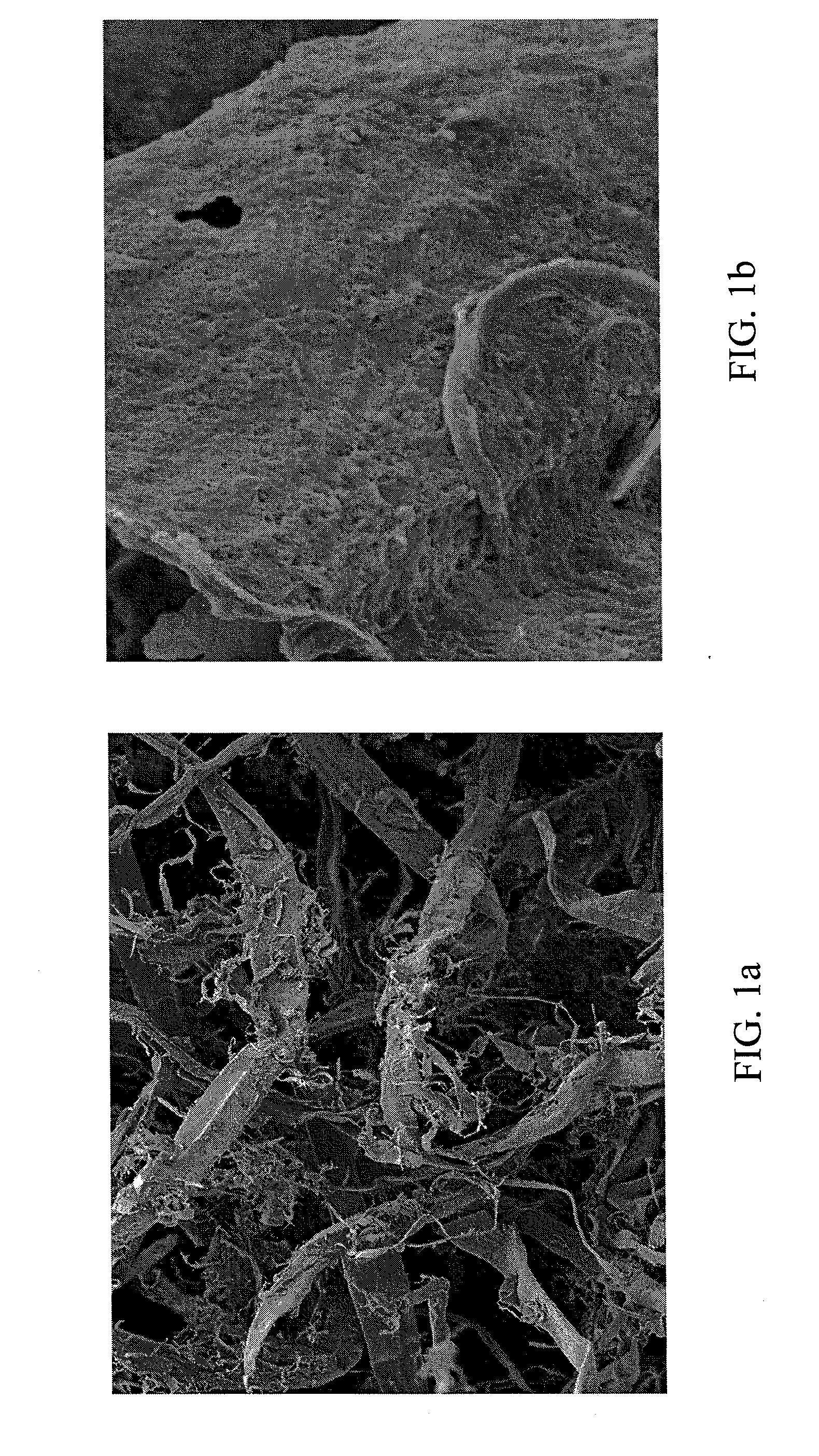
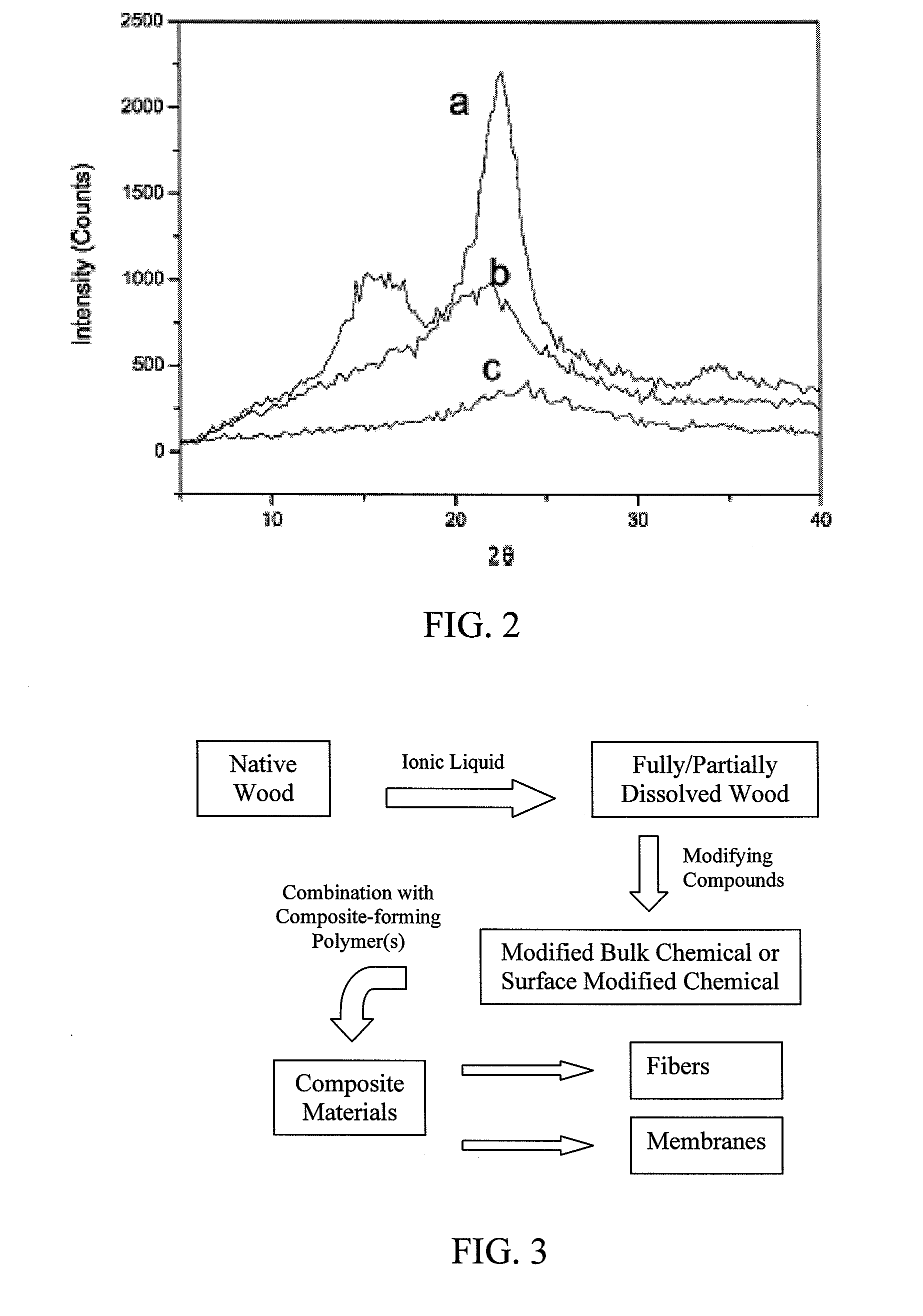
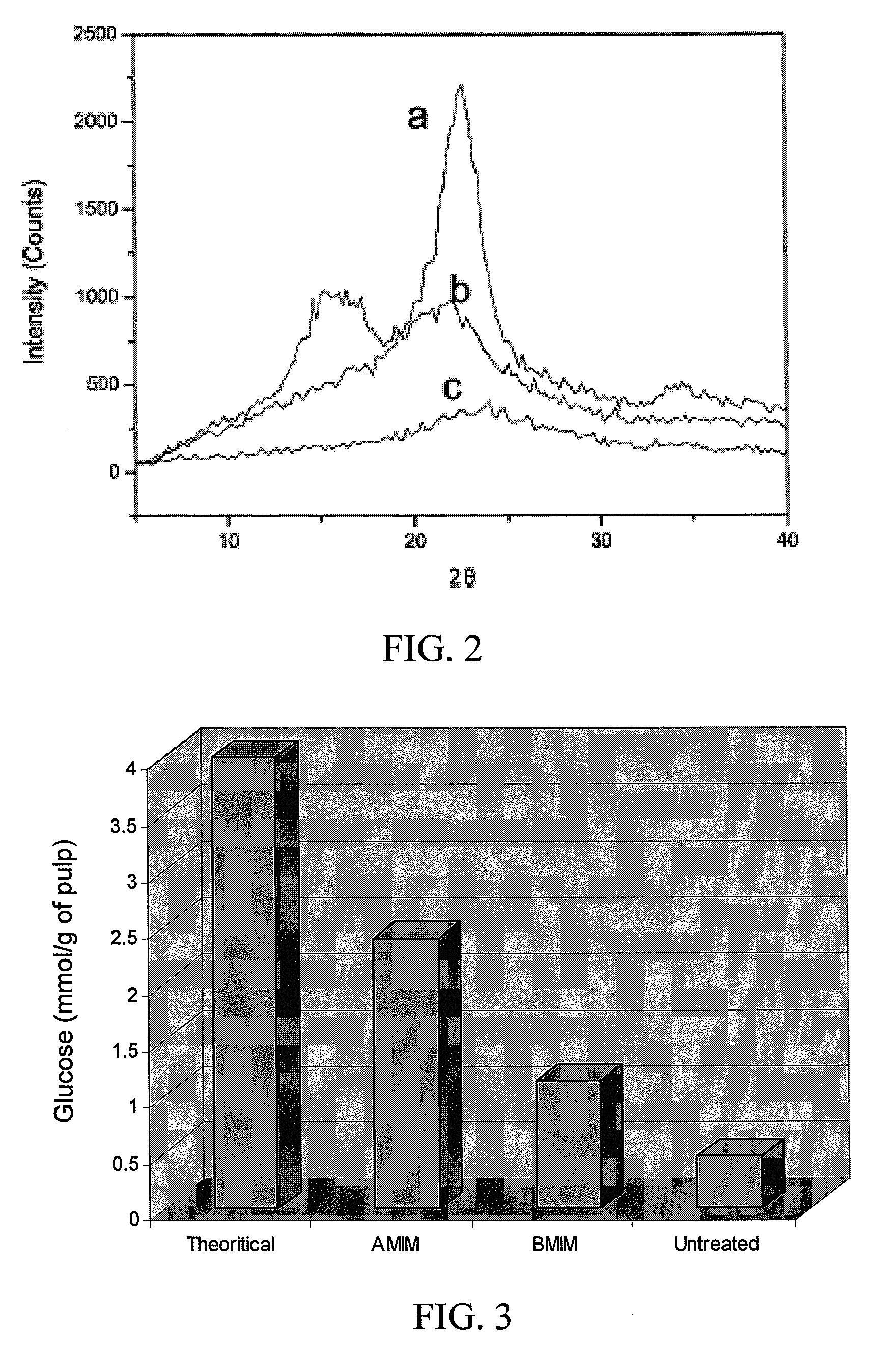
Polymer derivatives and composites from the dissolution of lignocellulosics in ionic liquids
The present invention provides wood derivatives and composite materials prepared by first solvating a lignocellulosic material using an ionic liquid. The solvated lignocellulosic material can be derivatized to incorporate functional groups, particularly groups that facilitate later combination with polymer materials, including non-polymer polymers. The polymeric materials can be combined with the derivatized lignocellulosic material in solution, or the derivatized lignocellulosic material can be isolated and later combined with the polymeric material in a melt. The invention encompasses a variety of wood derivatives, composites, and nanocomposites useful for preparing multiple types of products, including membranes, fibers, and formed parts.
Owner:NORTH CAROLINA STATE UNIV
Coatings with tunable molecular architecture for drug-coated balloon
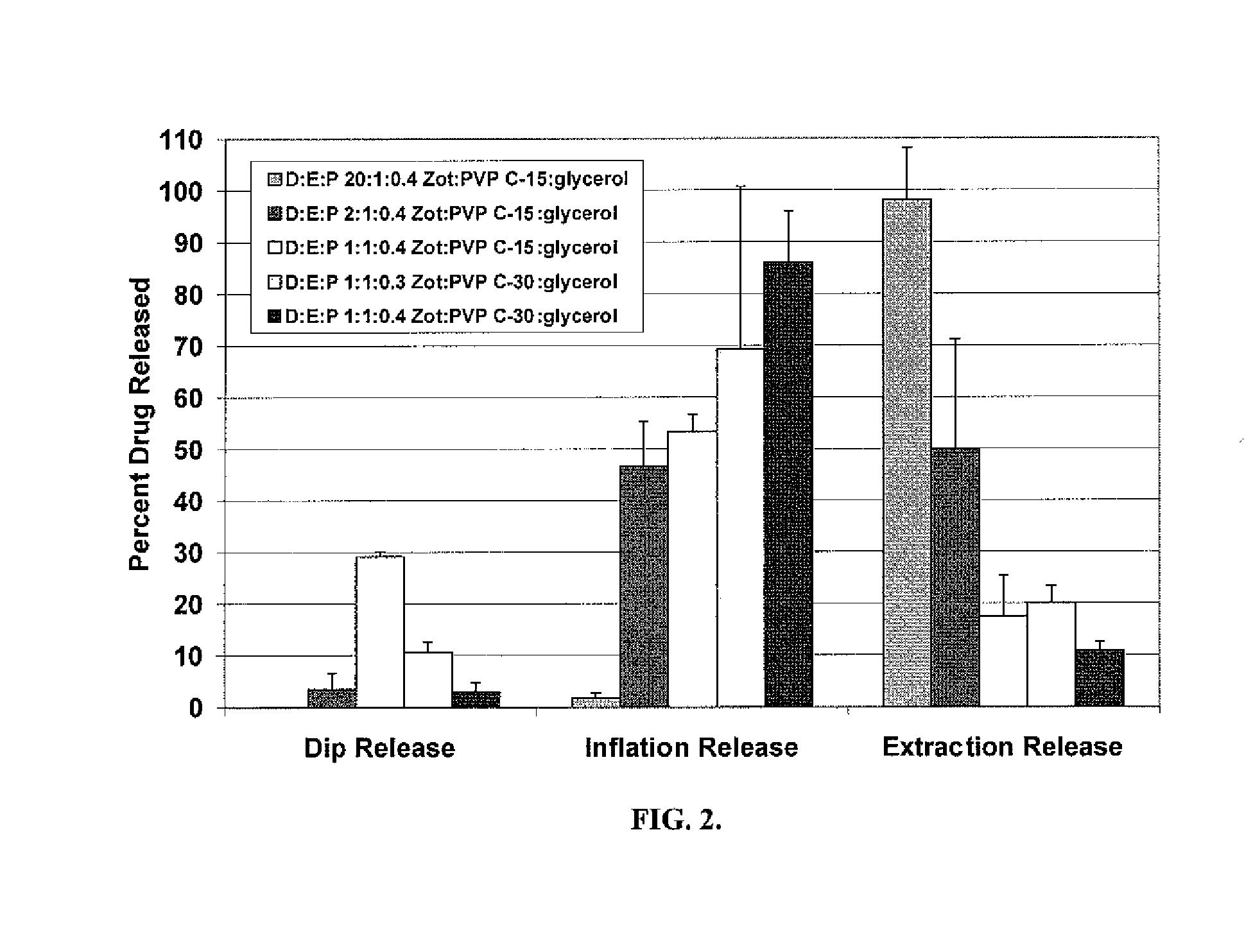
InactiveUS20110143014A1Low elastic modulusLow release rateStentsBalloon catheterDissolutionNuclear medicine
A drug delivery balloon is provided, the a balloon having an outer surface, and a tunable coating disposed on at least a length of the balloon surface. The tunable coating includes a first therapeutic agent and a first excipient, and can include a second therapeutic agent and a second excipient. The first and second therapeutic agents have different dissolution rates during balloon inflation and therefore provide a coating that is tunable.
Owner:ABBOTT CARDIOVASCULAR
Use of lignocellulosics solvated in ionic liquids for production of biofuels
InactiveUS20080190013A1Guaranteed economic efficiencyReduced availabilityCellulosic pulp after-treatmentSugar productsBiofuelEvaporation
The present invention provides a method for converting lignocellulosic material into biofuel. In particular embodiments, the method comprises pre-treating lignocellulosic material by dissolving the material in ionic liquids. The pretreated lignocellulosic material can be isolated, such as by precipitation with a regenerating solvent (e.g., water), and be used directly in the formation of biofuel, including undergoing hydrolysis to form sugar and fermentation to form fuel, such as bioethanol. The ionic liquid can be recycled for further use, such as by evaporation of the water introduced during precipitation, and the recycling provides a route to a hemicellulose rich fraction and an ionic liquid of consistent quality and wood dissolution characteristics. The recovered hemicelluloses are of significant utilization potential toward commodity and specialty applications.
Owner:NORTH CAROLINA STATE UNIV
Dissolving filter cake
Owner:SCHLUMBERGER TECH CORP
Polypropylene block-copolymer resin and process for producing it
Polypropylene block-copolymer resin exhibiting high melt tension and improved moldability with balanced stiffness and impact resistance may be molded at high speed into large-sized articles, including, stretched films, with good appearance and resistance to deformation. The block copolymer includes a higher molecular weight polypropylene segment, a lower molecular weight polypropylene segment and an ethylene alpha-olefin copolymer segment. When subjected to dissolution fractionation m paraxylene, a large proportion is insoluble at 23° C. but soluble at 135° C., and a smaller portion is soluble at 23° C. The block copolymer has a melt flow rate of 0.01 to 5 g / 10 min at 230 C. (2.16 kg) and a molecular weight distribution Mw / Mn of 6-20 and Mz / Mw of at least 3.5. A continuous multistage polymerization may be used to form the block copolymer.
Owner:PRIME POLYMER CO LTD
Rapid acting drug delivery compositions
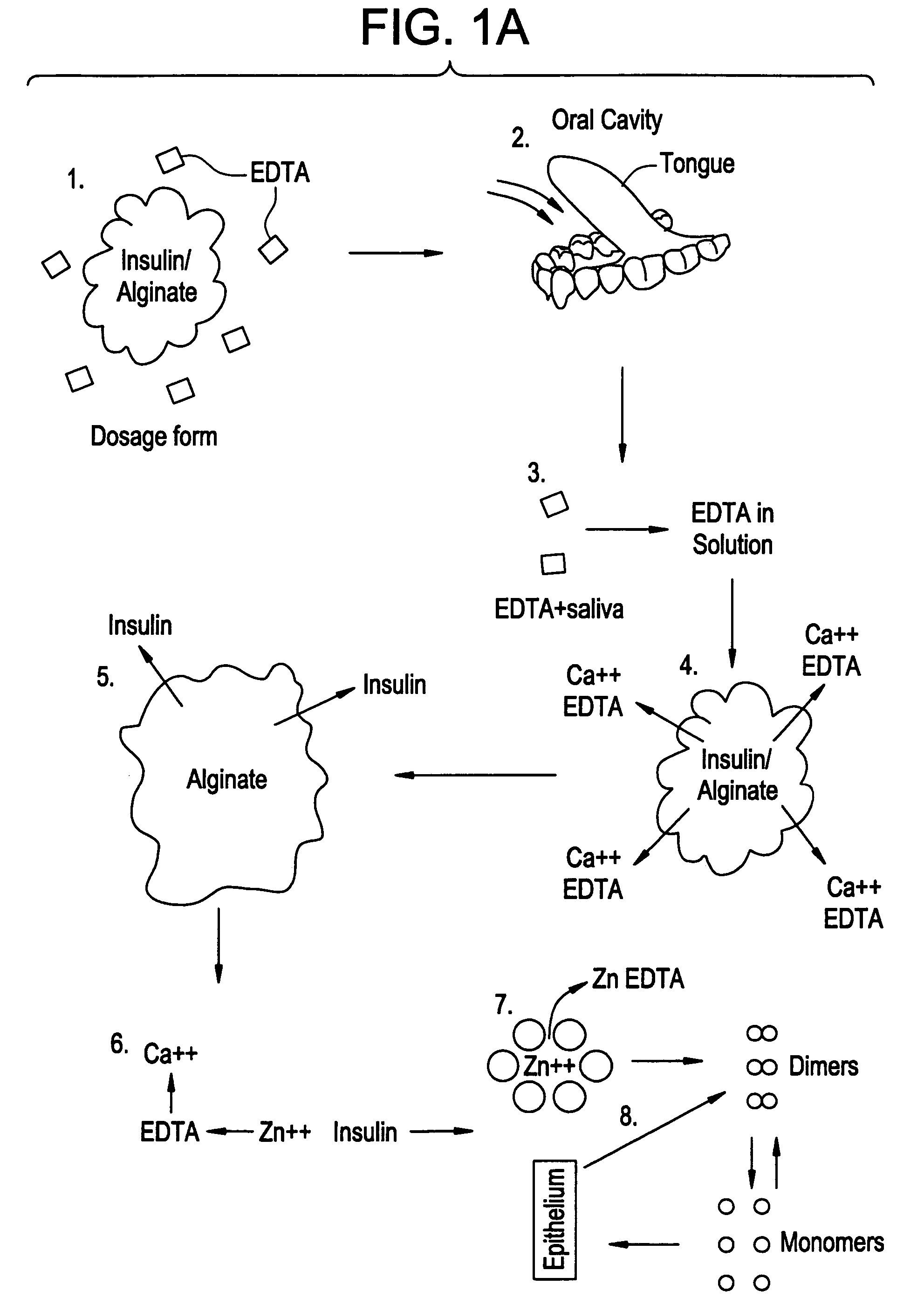
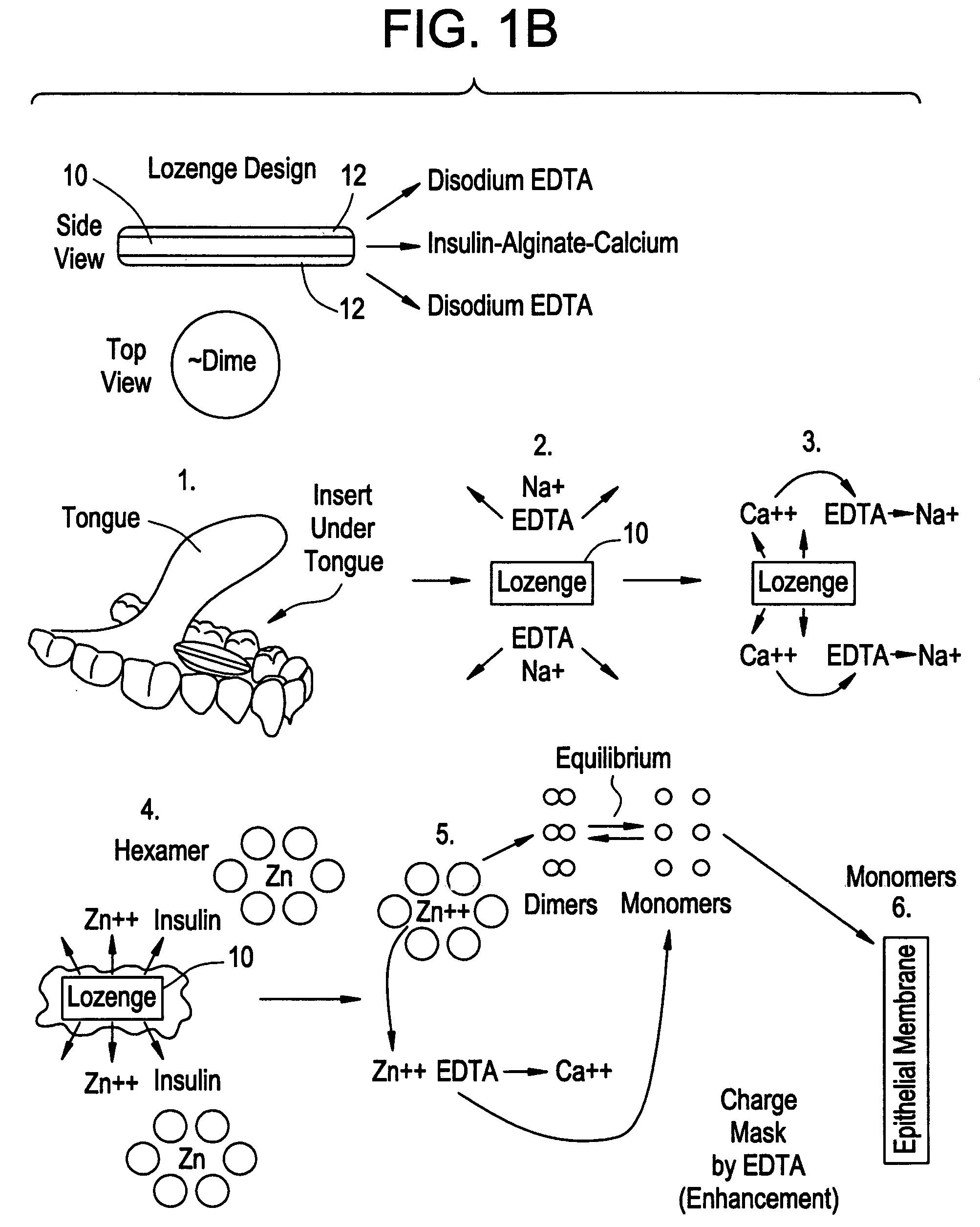
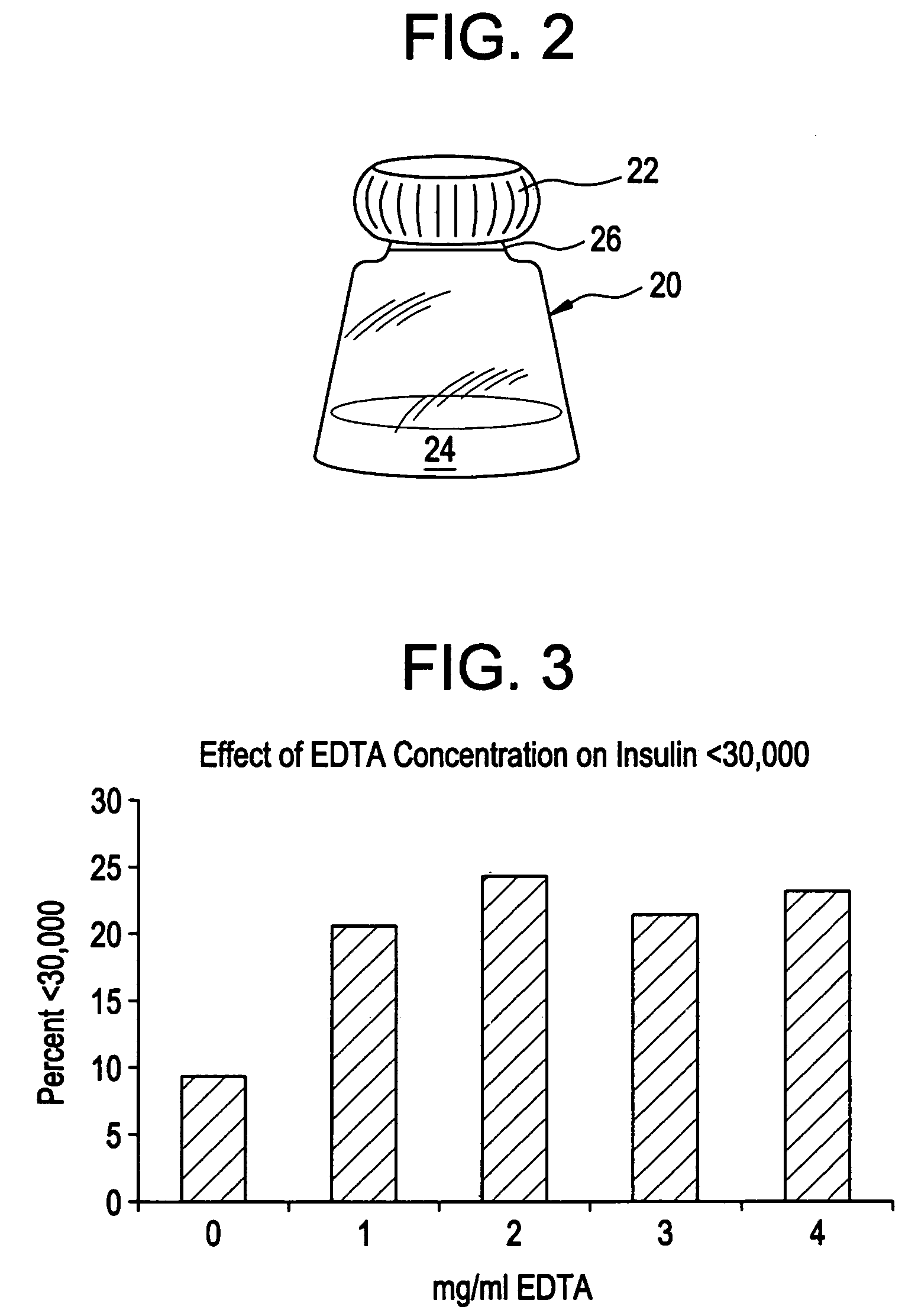
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
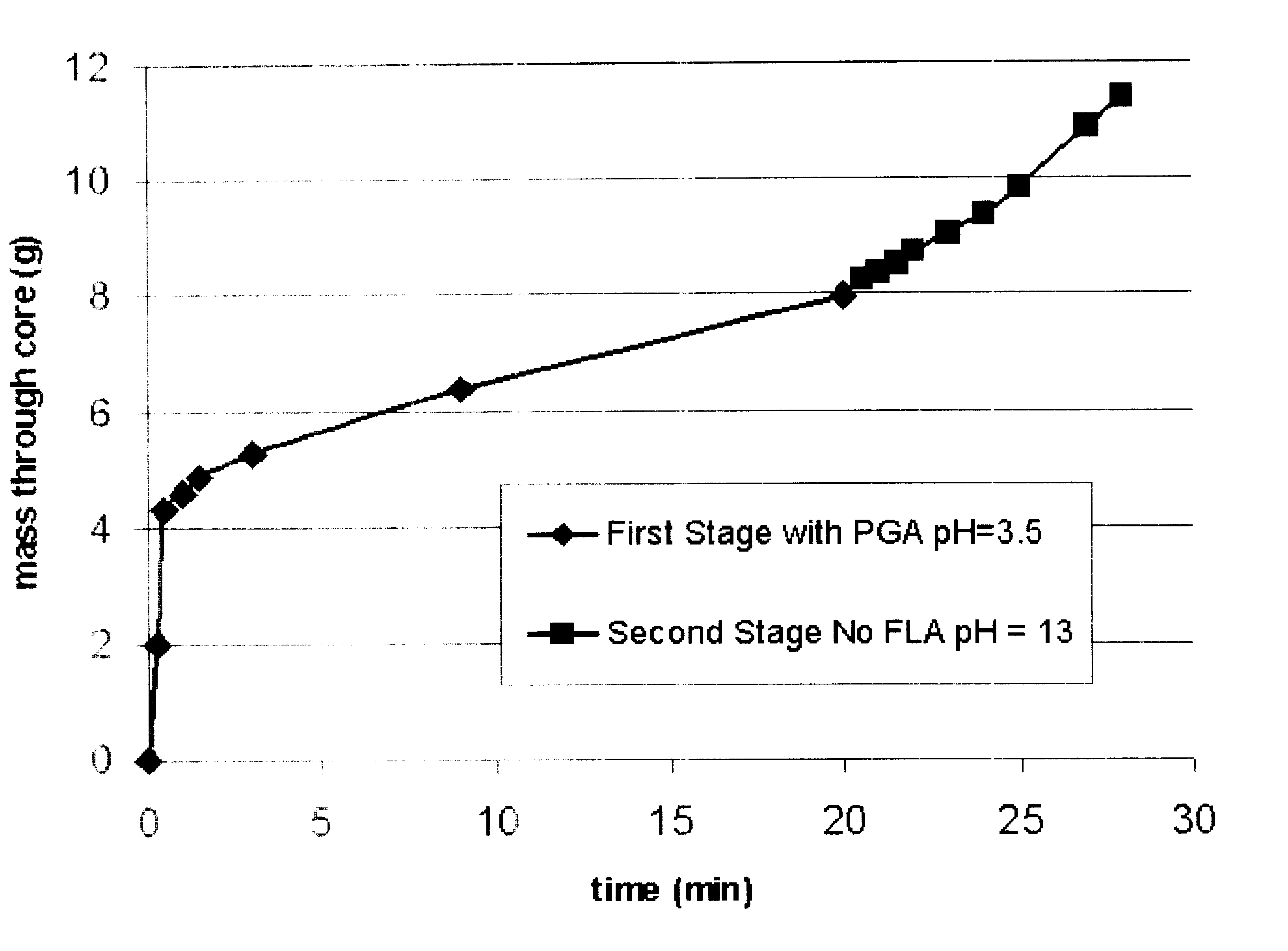
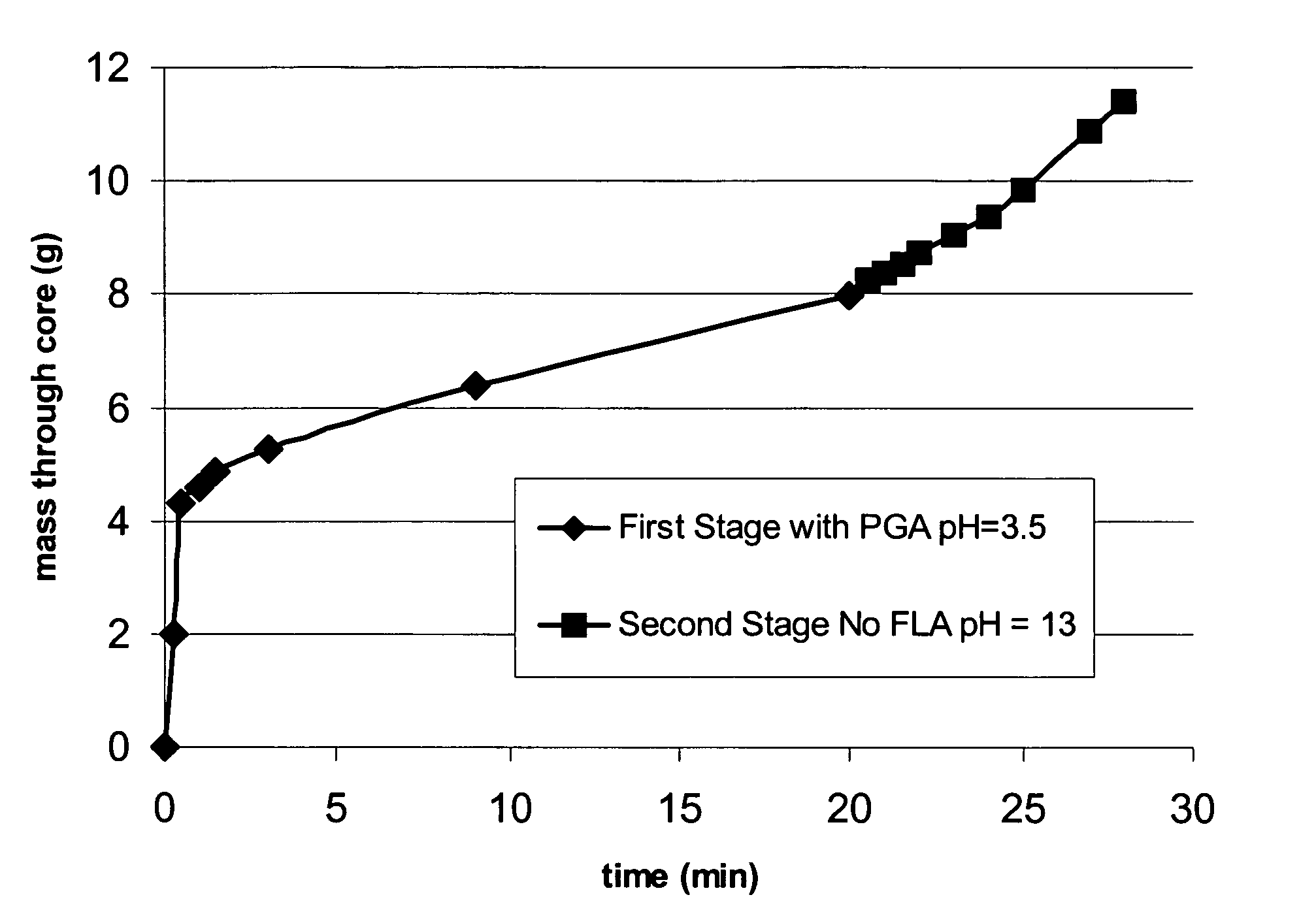
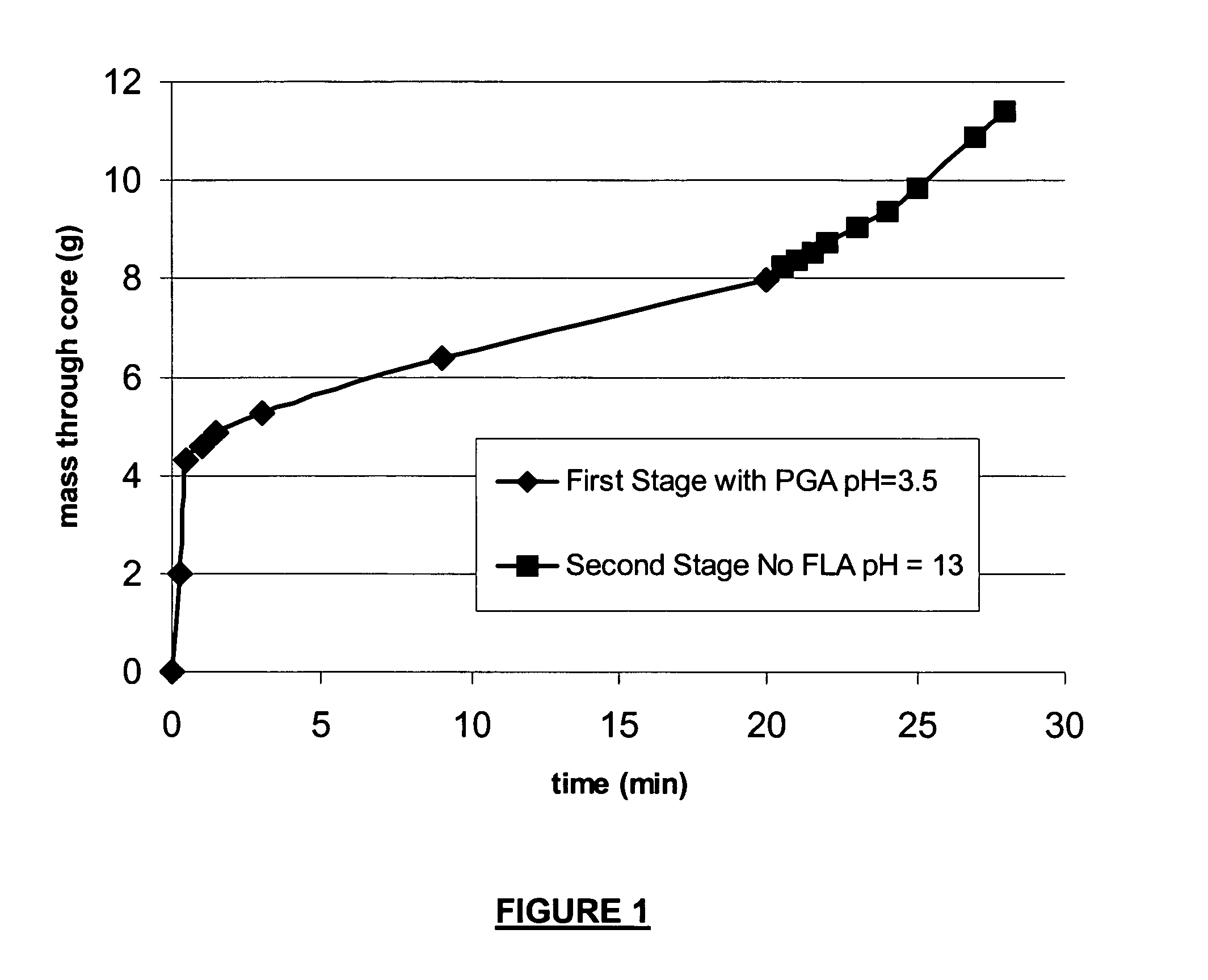
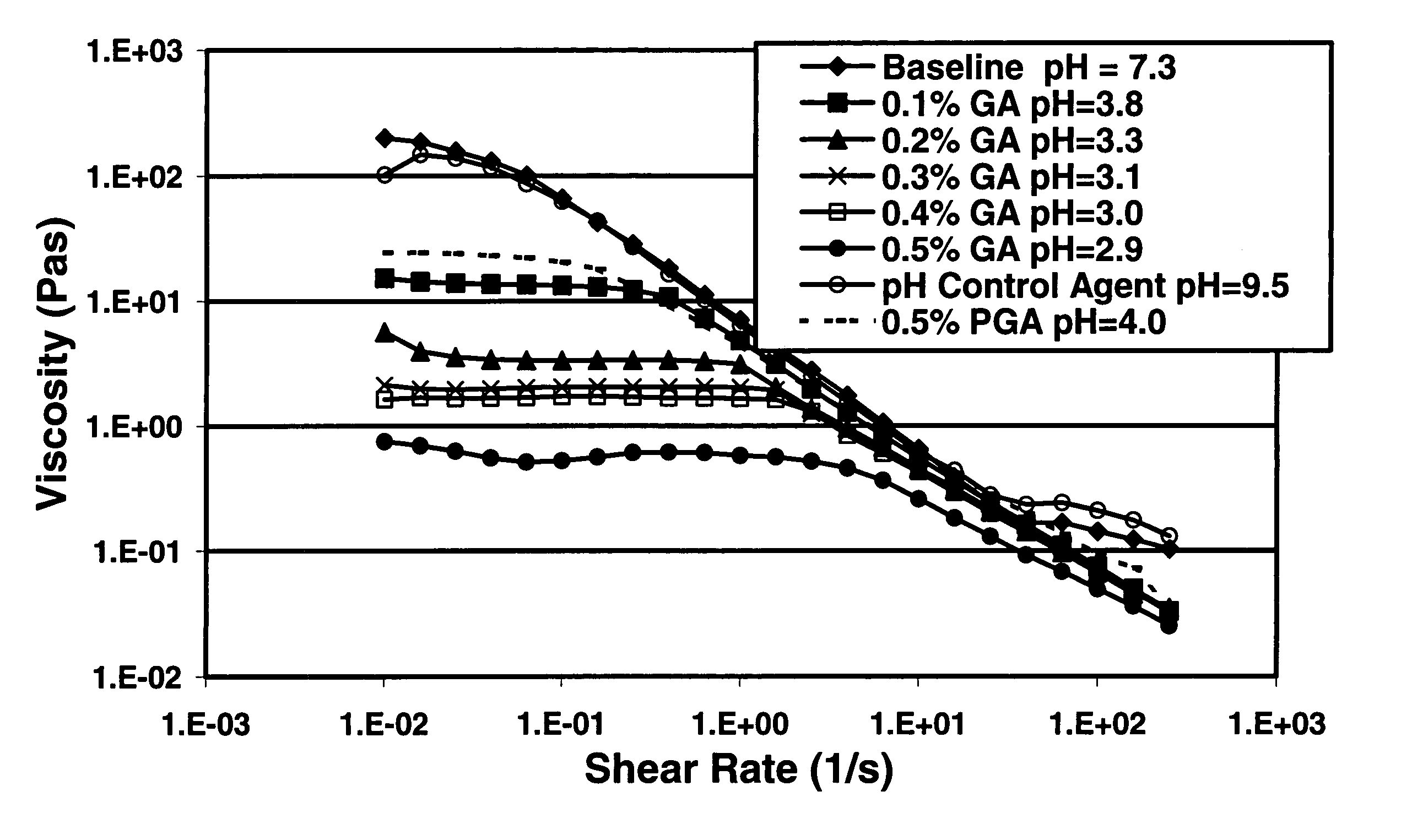
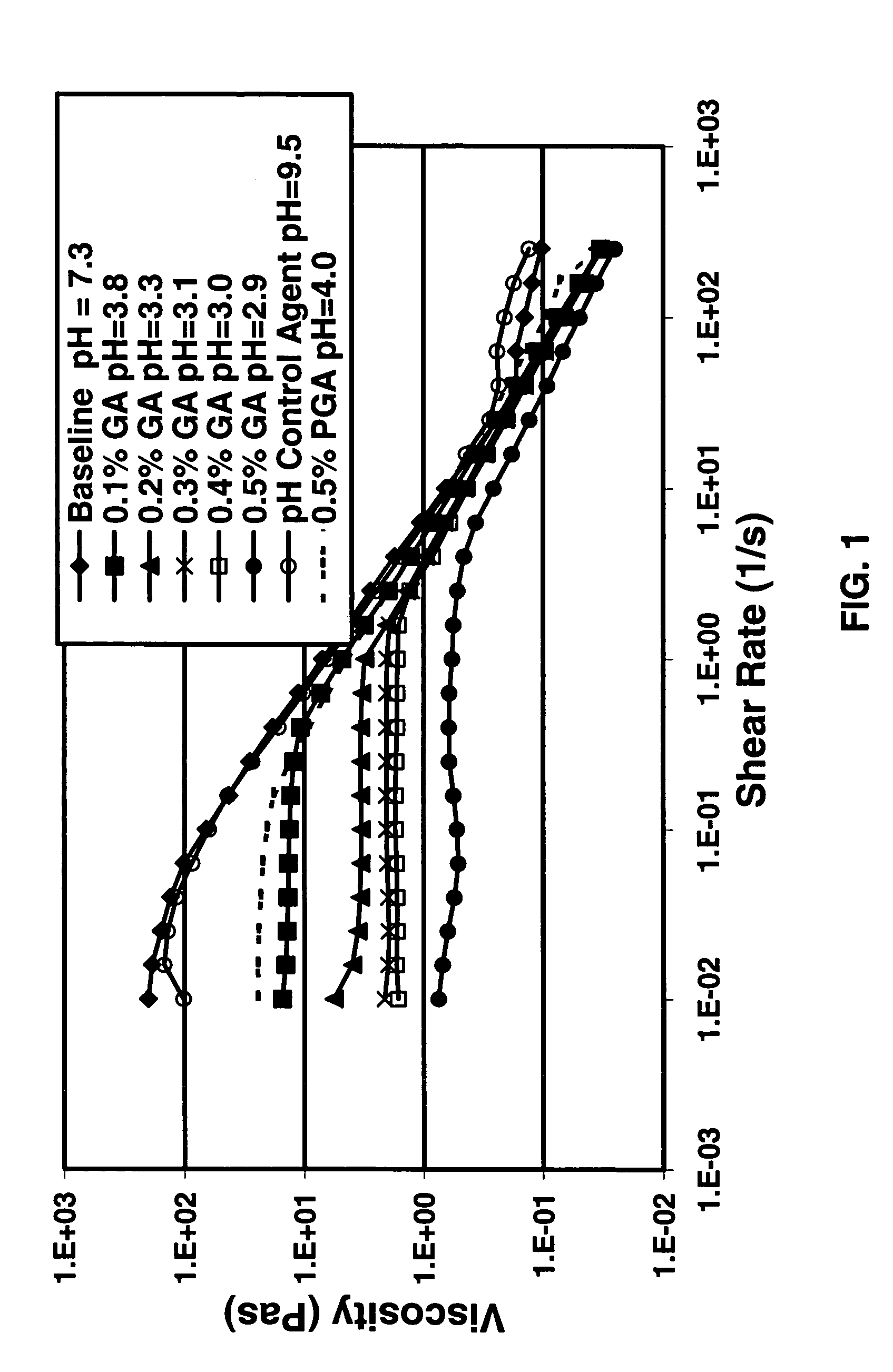
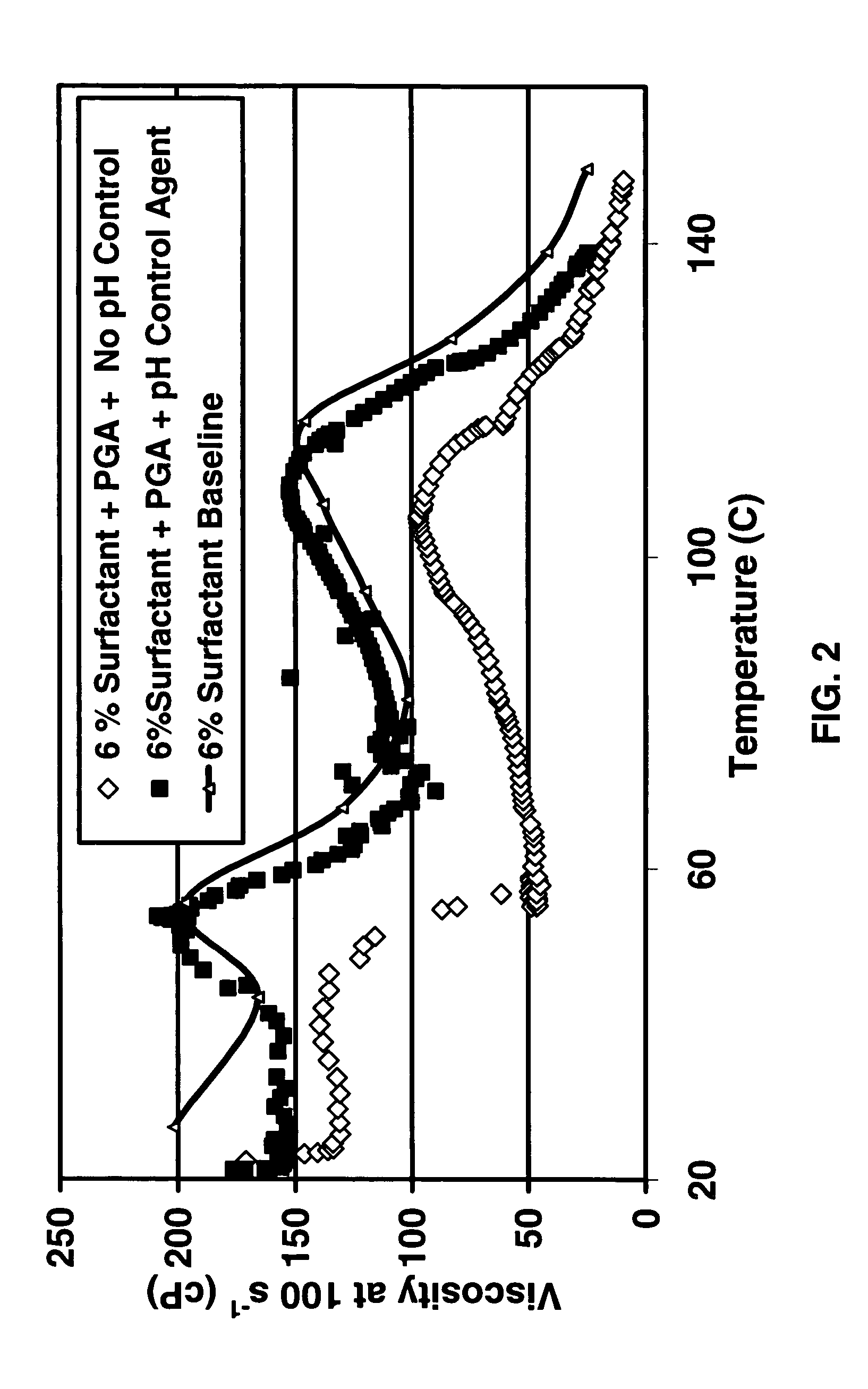
Degradable additive for viscoelastic surfactant based fluid systems
A method is given for treating a subterranean formation penetrated by a wellbore with a viscosified fluid. The fluid contains a solid hydrolysable polyacid that upon dissolution and hydrolysis releases an acid that is a breaker for the viscosifying system. Suitable solid hydrolysable polyacids include polylactic acid and polyglycolic acid. The fluid also contains a pH control agent, present in an amount sufficient to neutralize any acid present in the solid hydrolysable polyacid before the injection and to neutralize any acid generated by the solid hydrolysable polyacid during the injection, so that the acid breaker is not available to break the fluid during the injection. In one embodiment the viscosifier is a viscoelastic surfactant fluid system and the solid hydrolysable polyacid is of a size selected to be a fluid loss additive, for example in fracturing or gravel packing. In another embodiment, the solid hydrolysable polyacid is used in particles sufficiently small that they enter the pores of the formation. In either case, the viscosifier is broken after the solid releases more acid than can be neutralized by the pH control agent.
Owner:SCHLUMBERGER TECH CORP
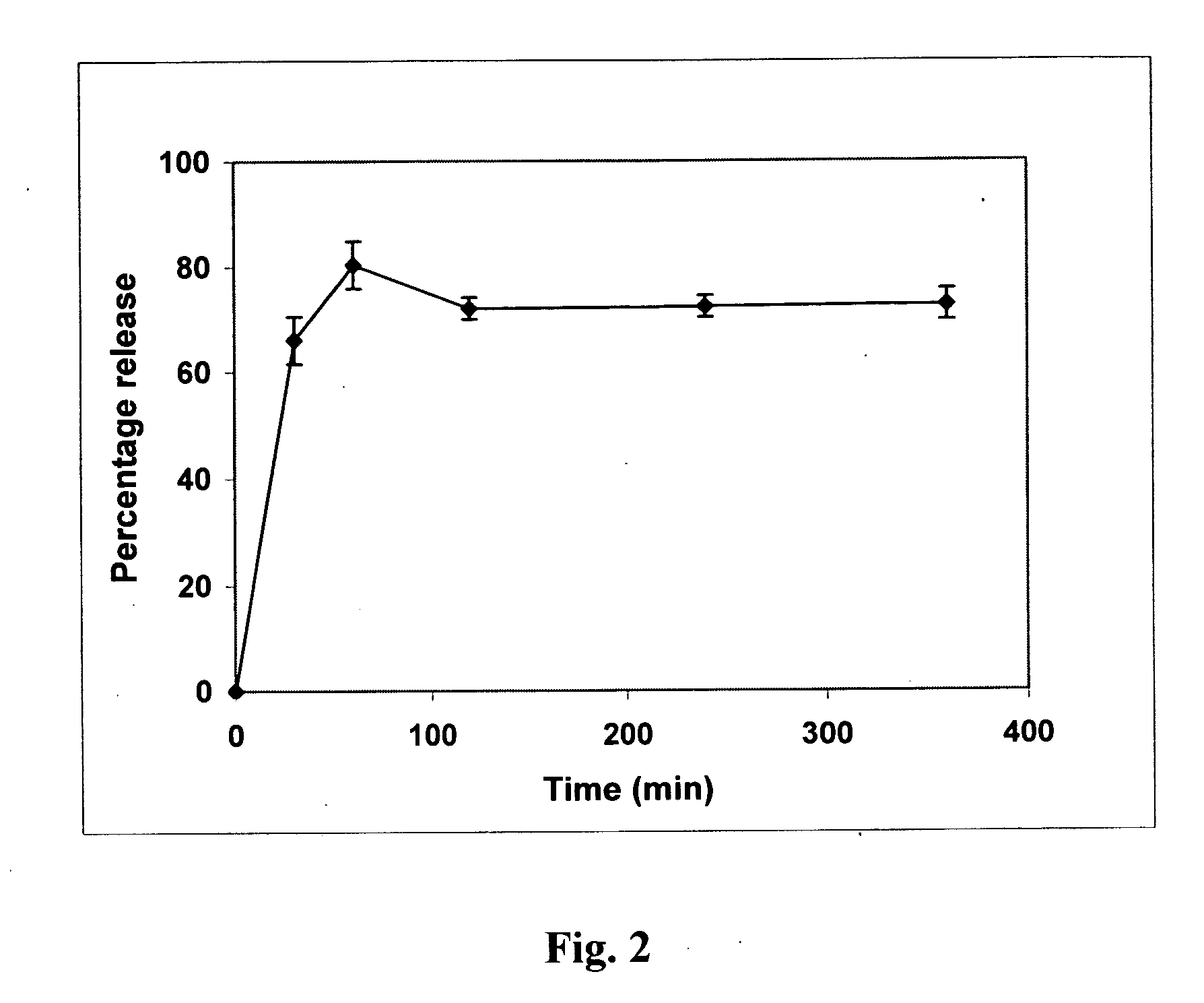
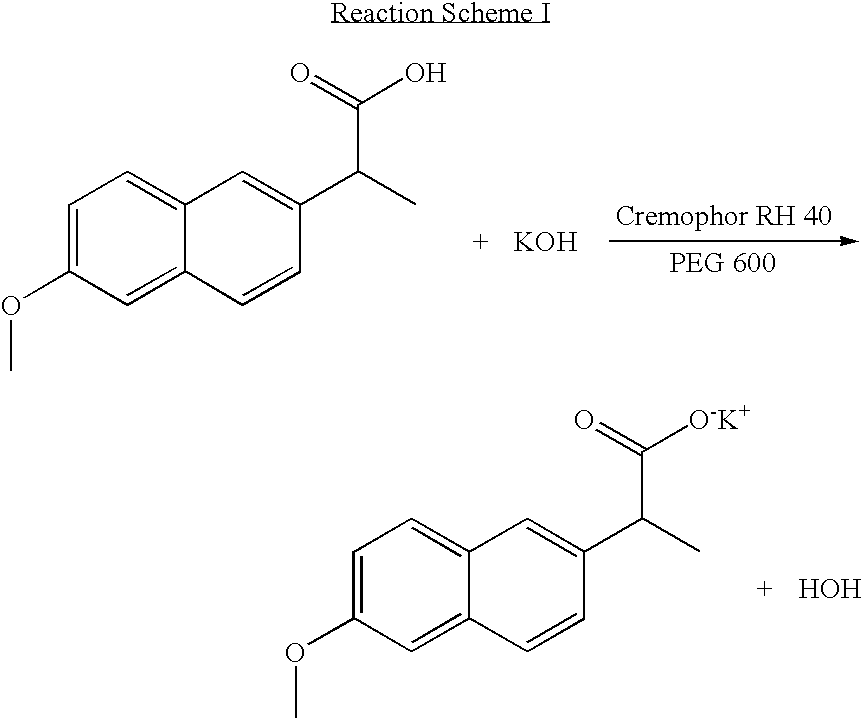
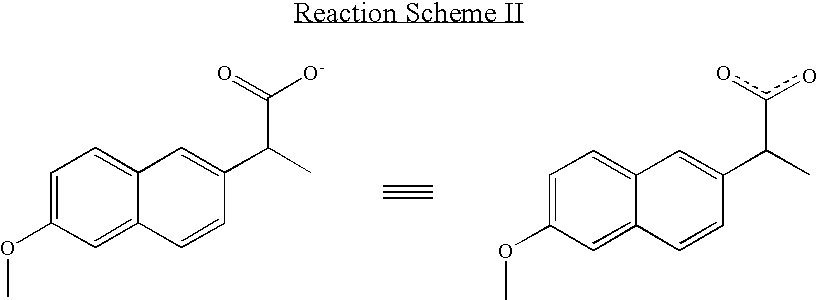
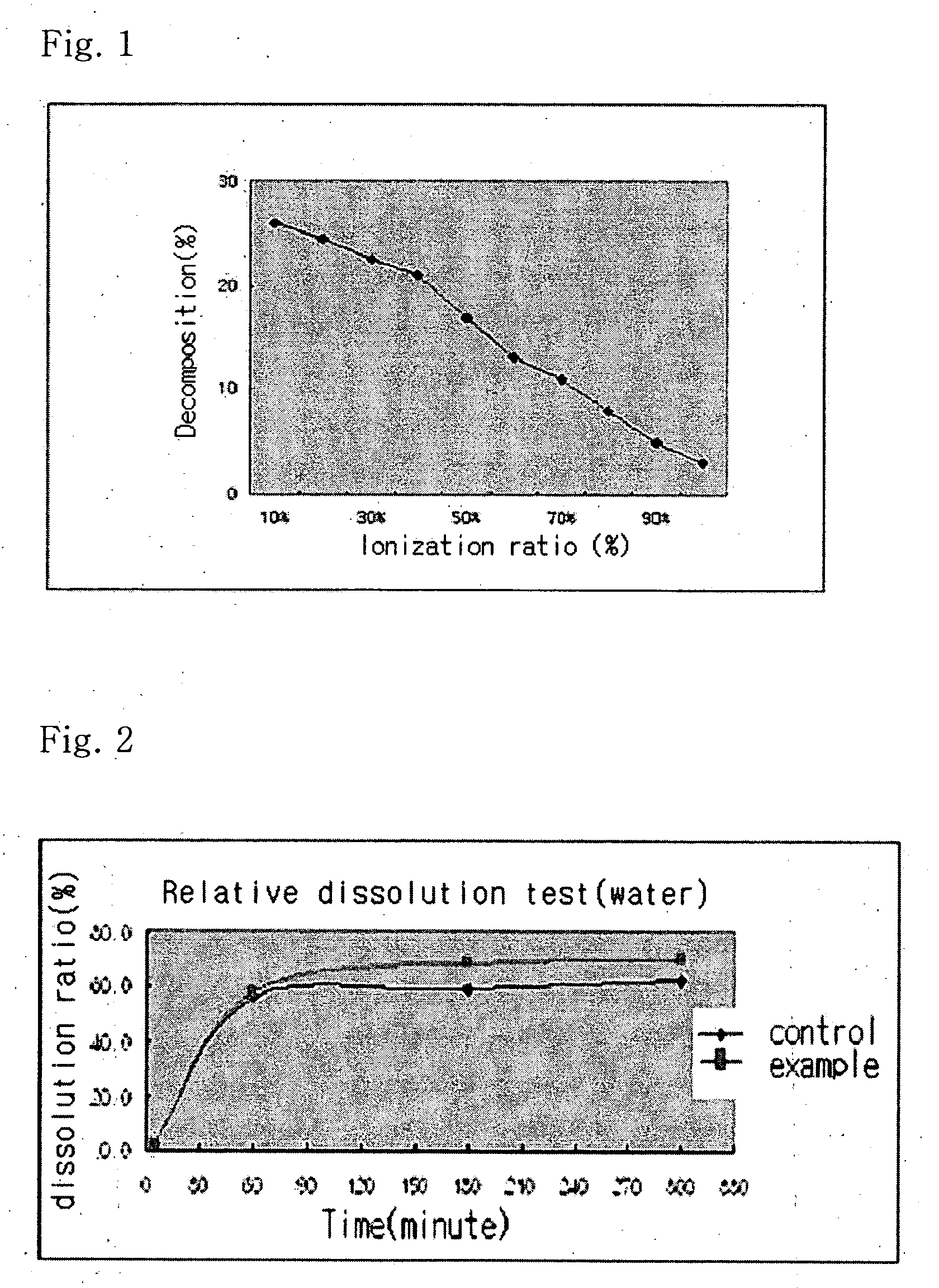
Solvent system of hardly soluble drug with improved dissolution rate
InactiveUS20040157928A1Good disintegrationPromote dissolutionBiocideAntipyreticDissolutionIonization
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptance, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com