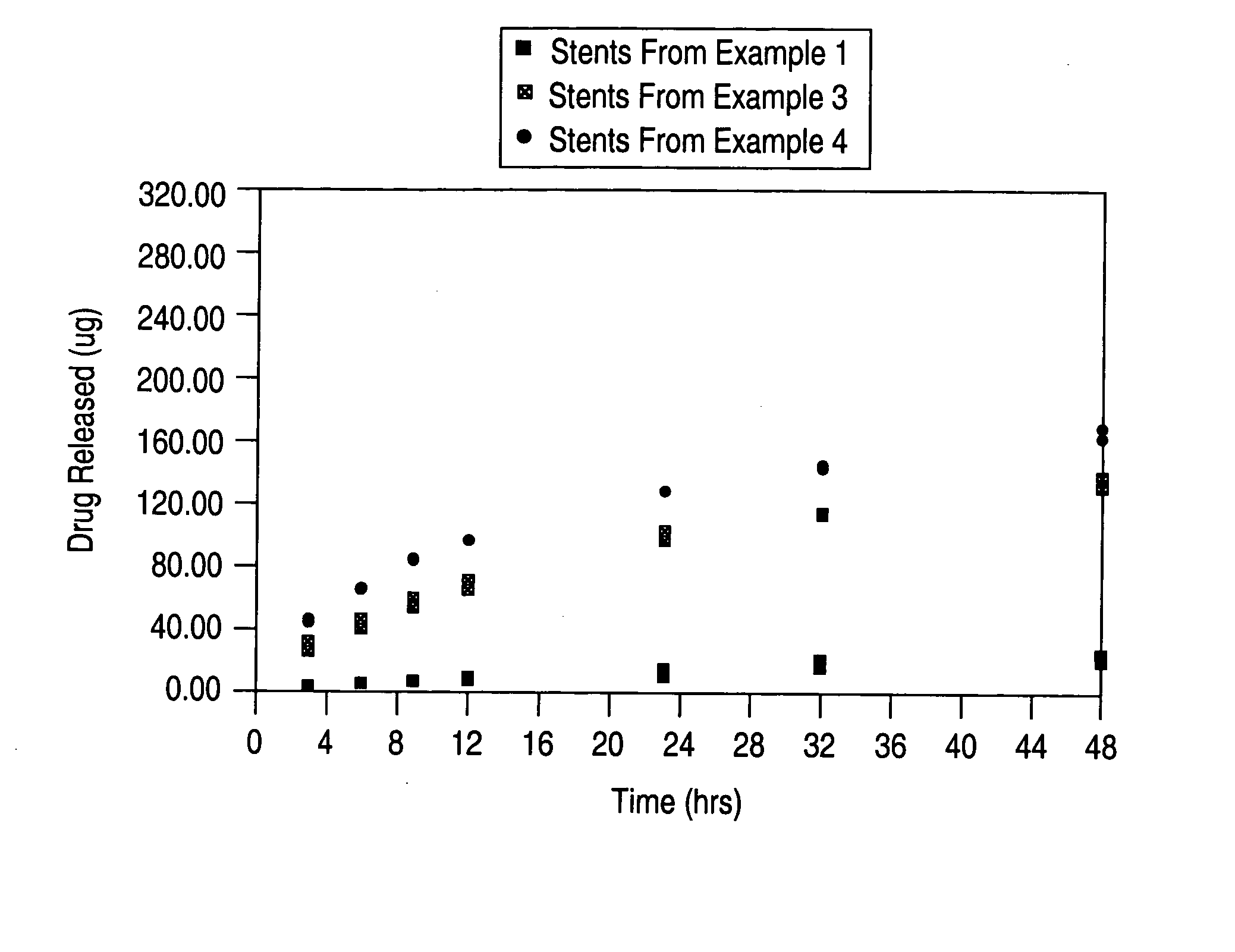
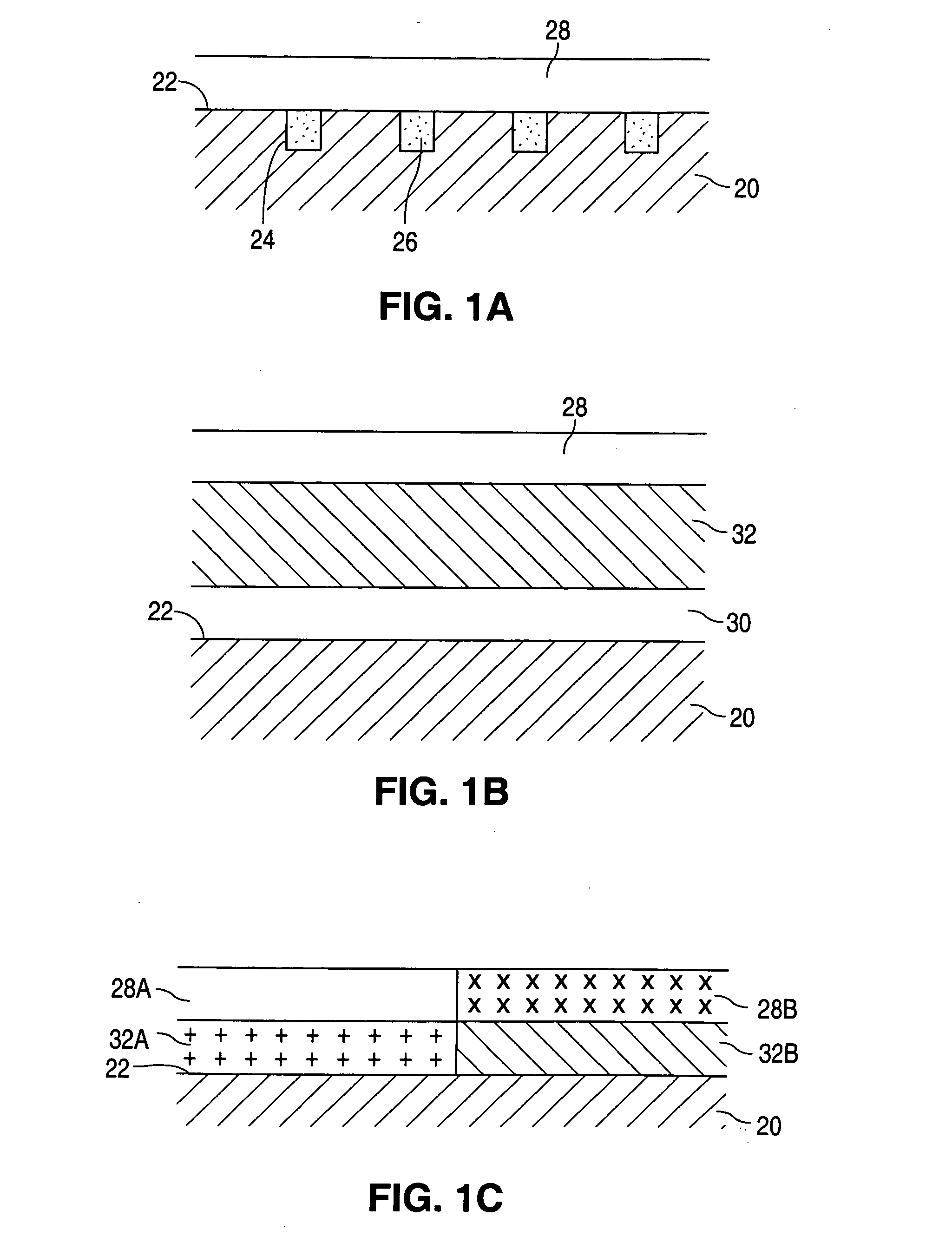
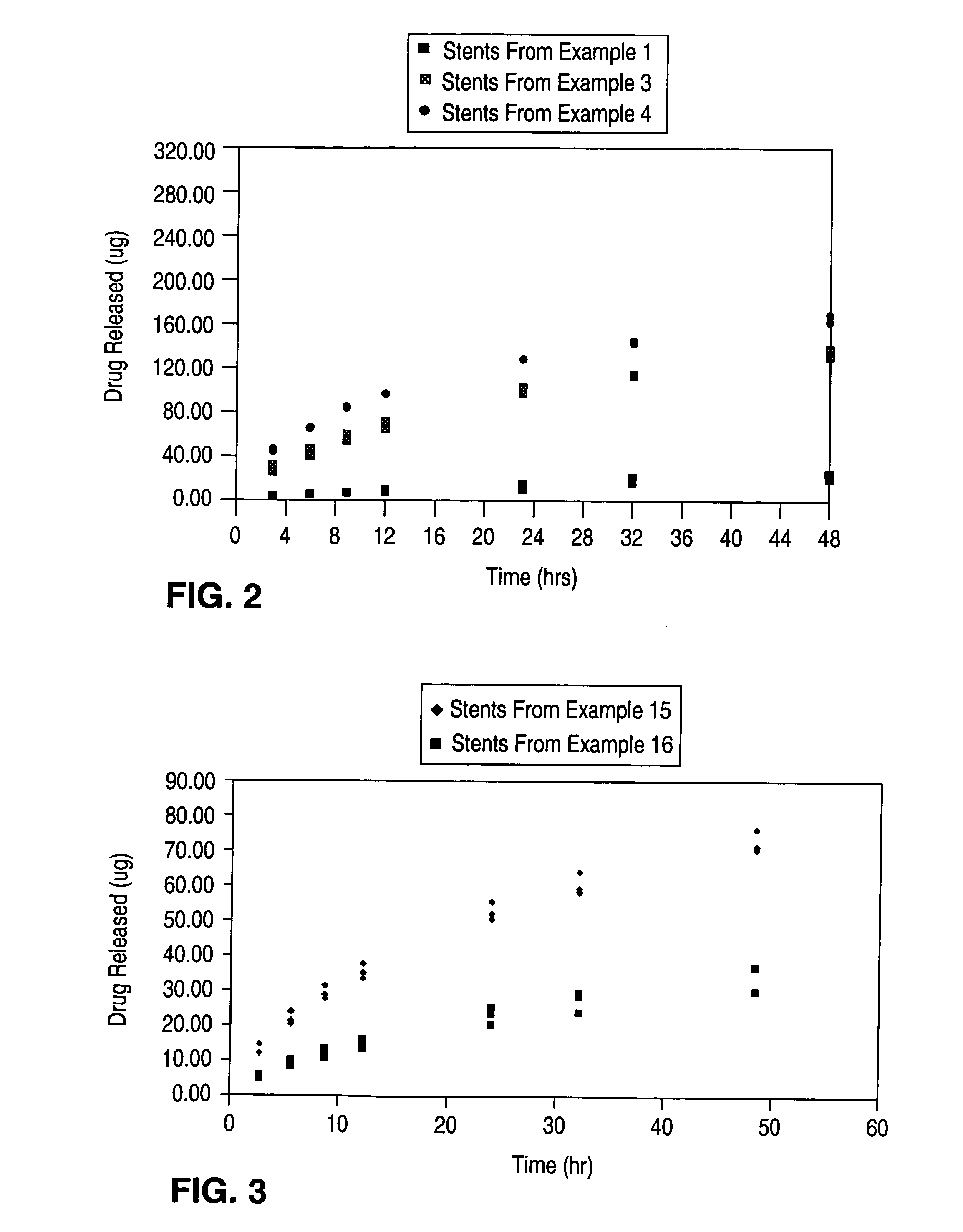
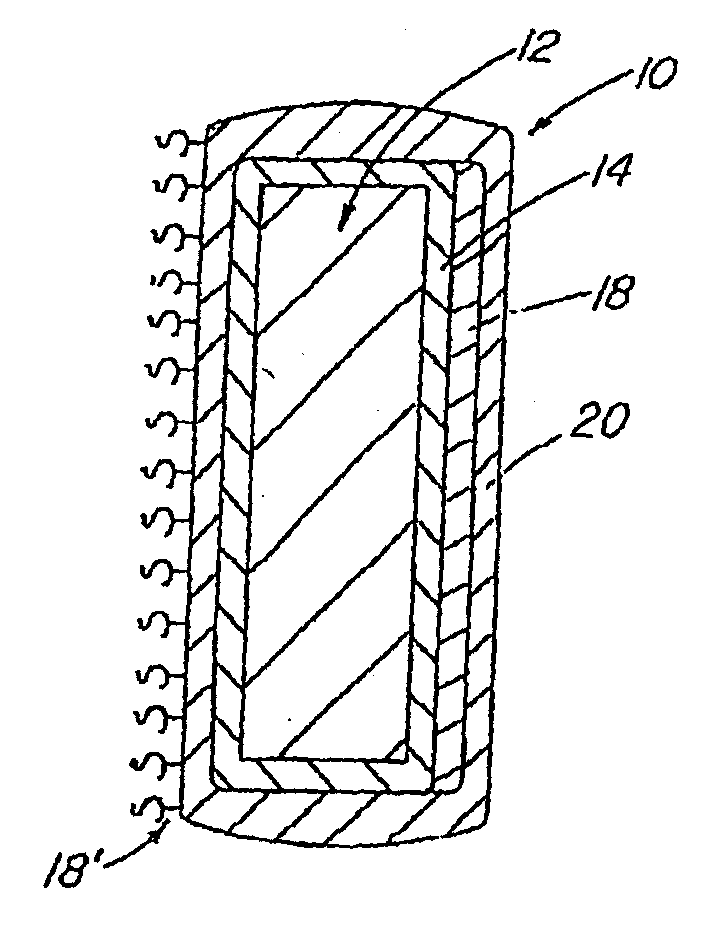
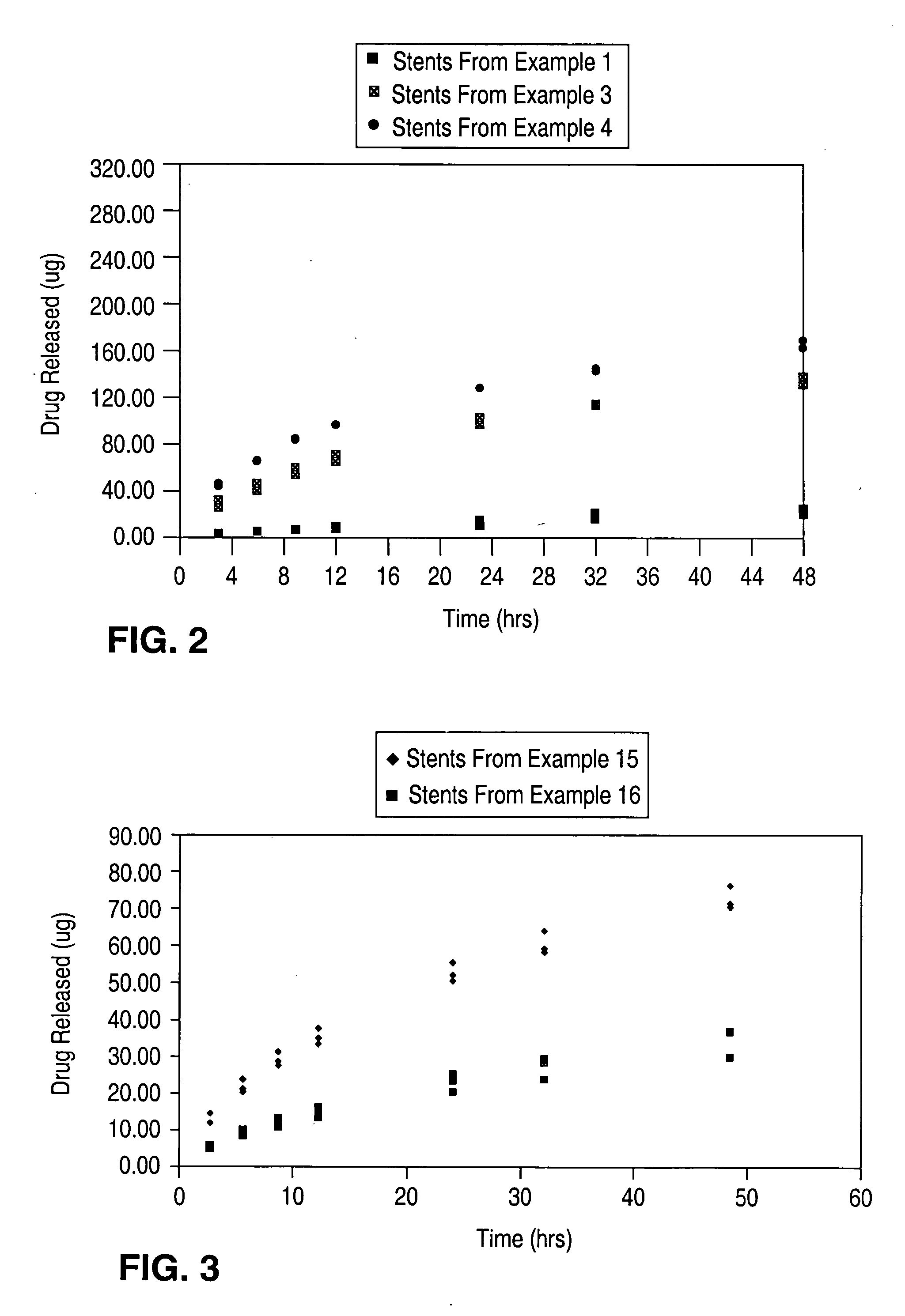
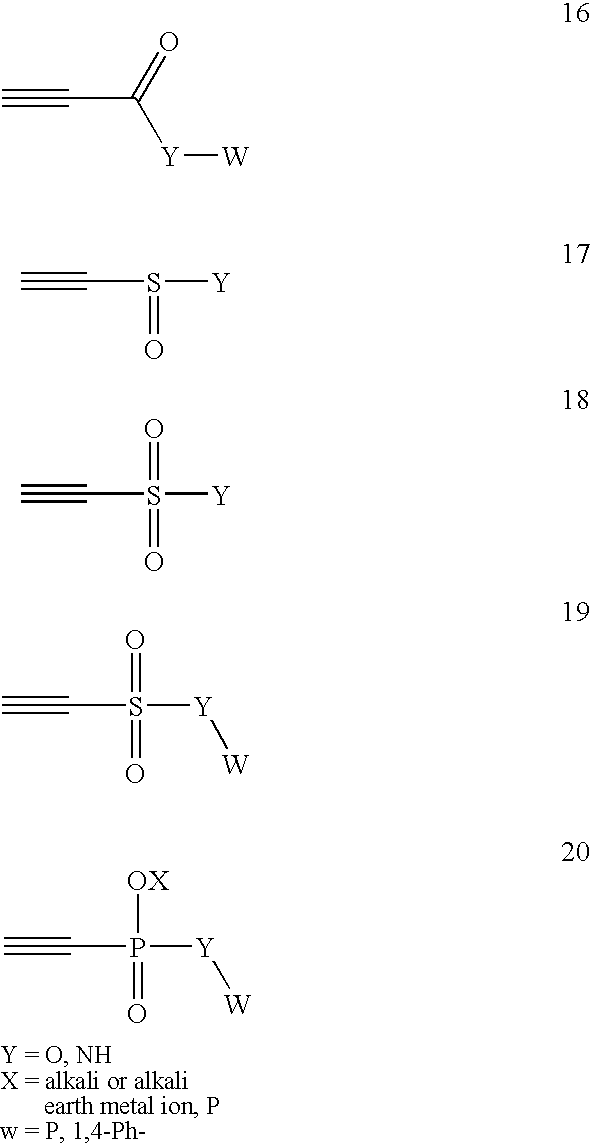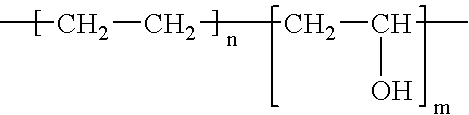Patents
Literature
171results about How to "Low release rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
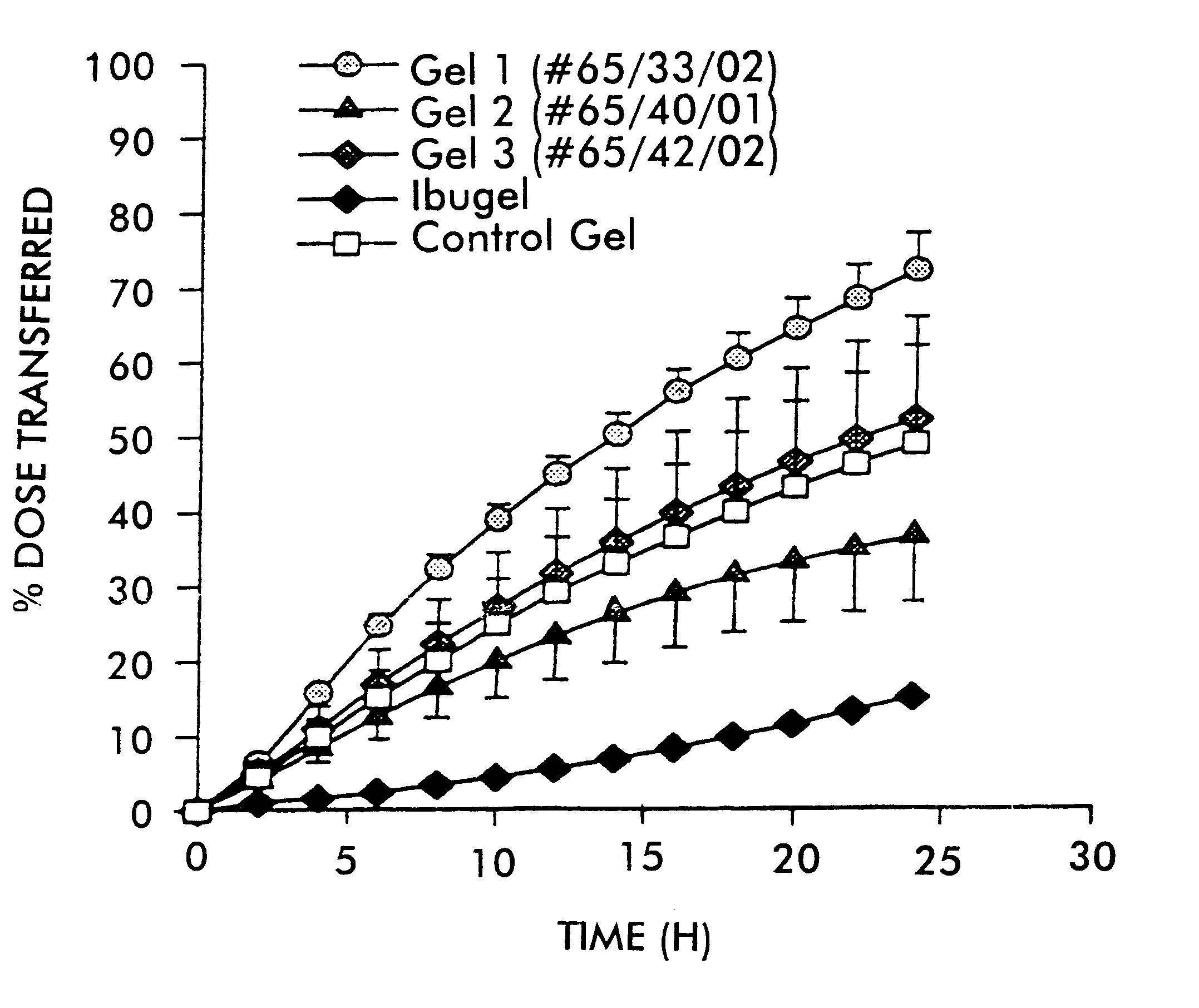
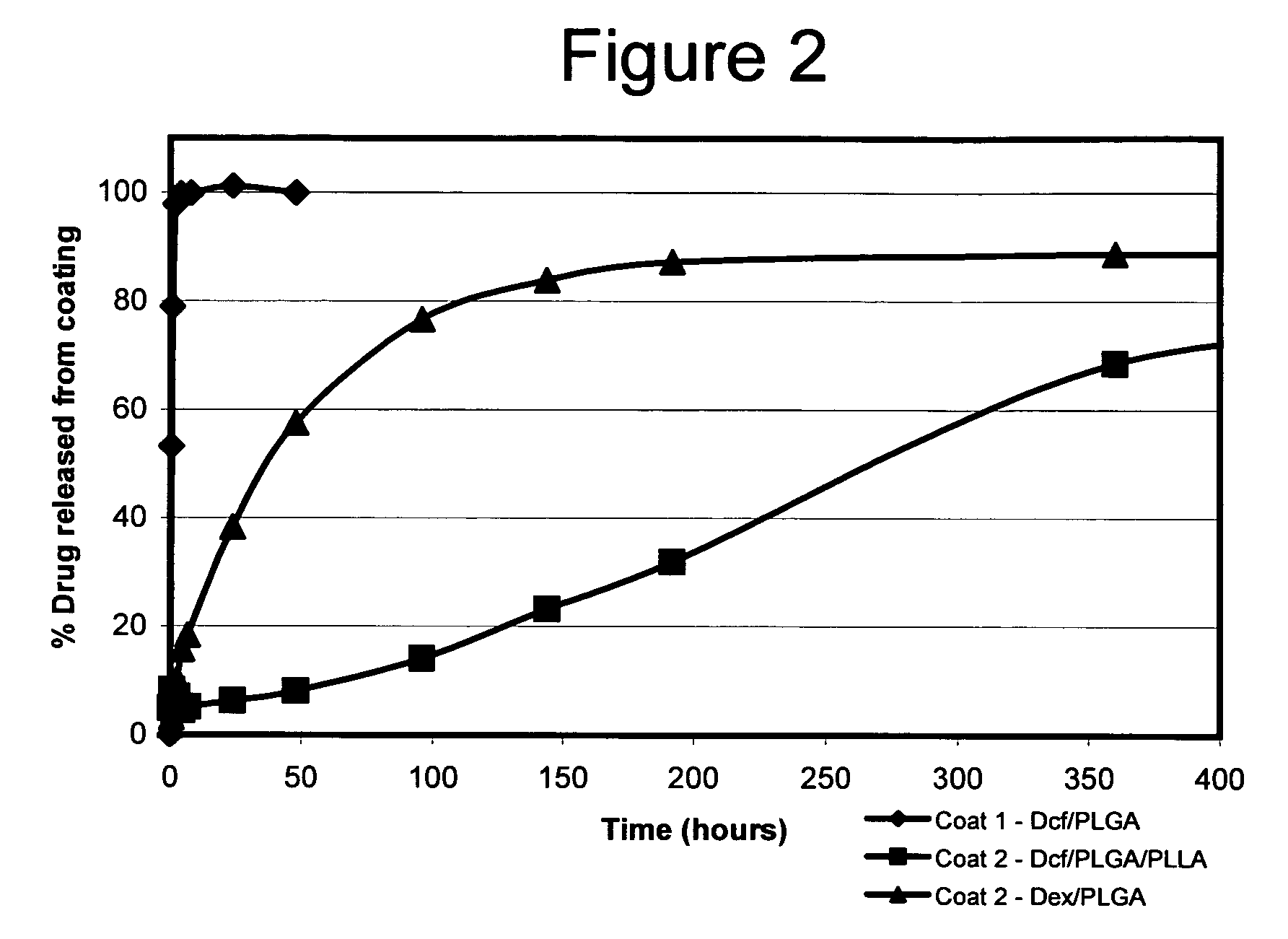
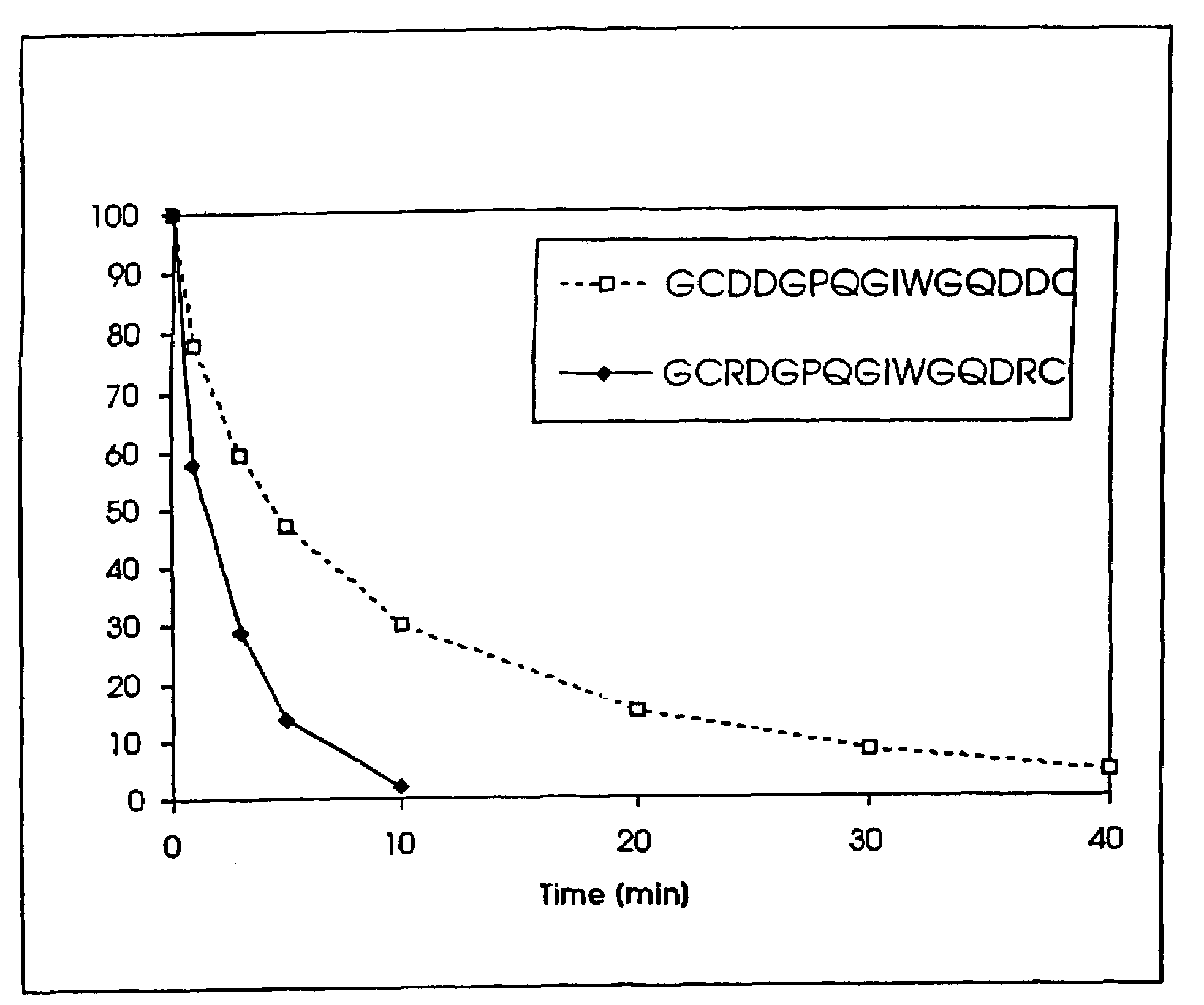
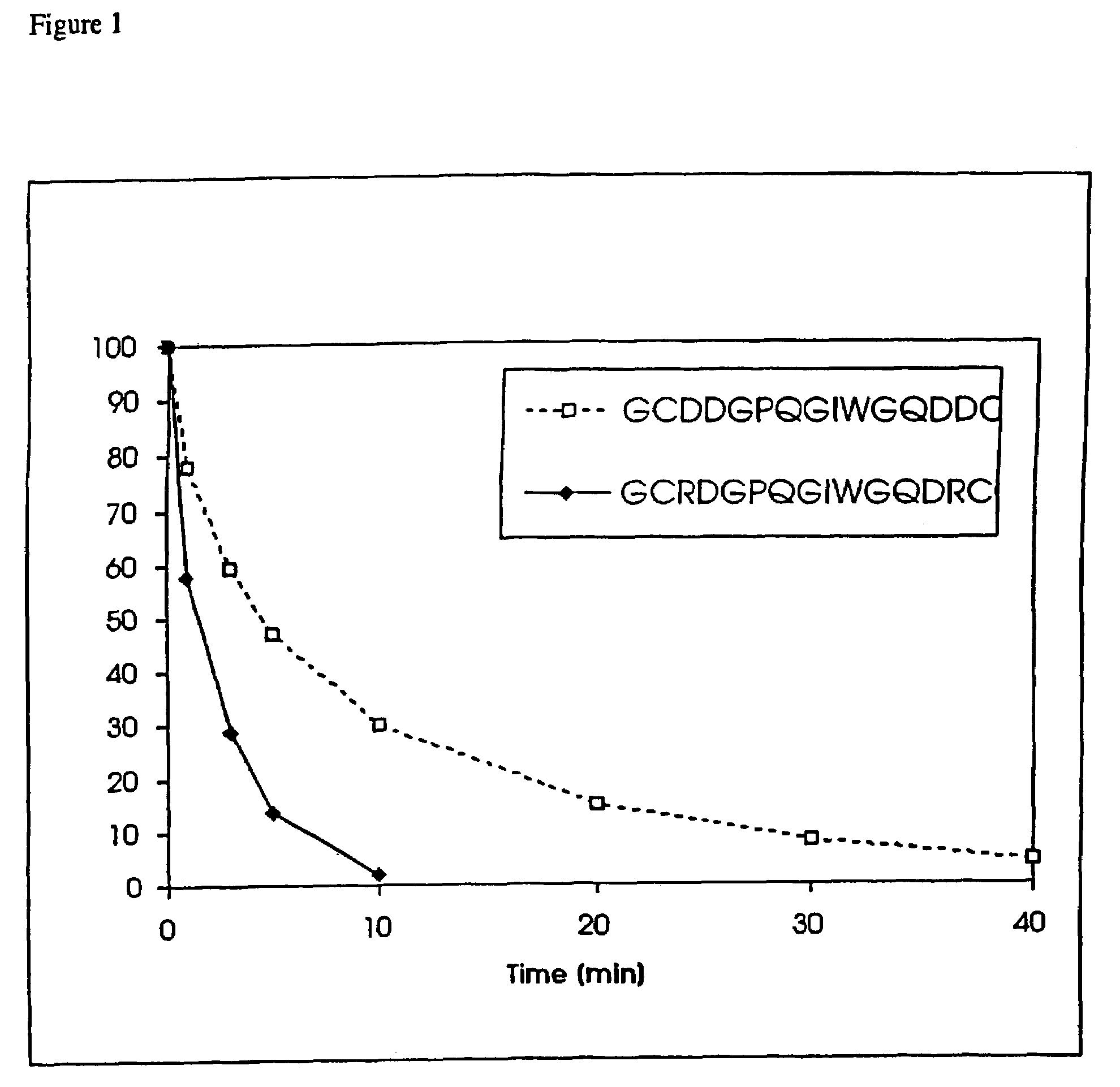
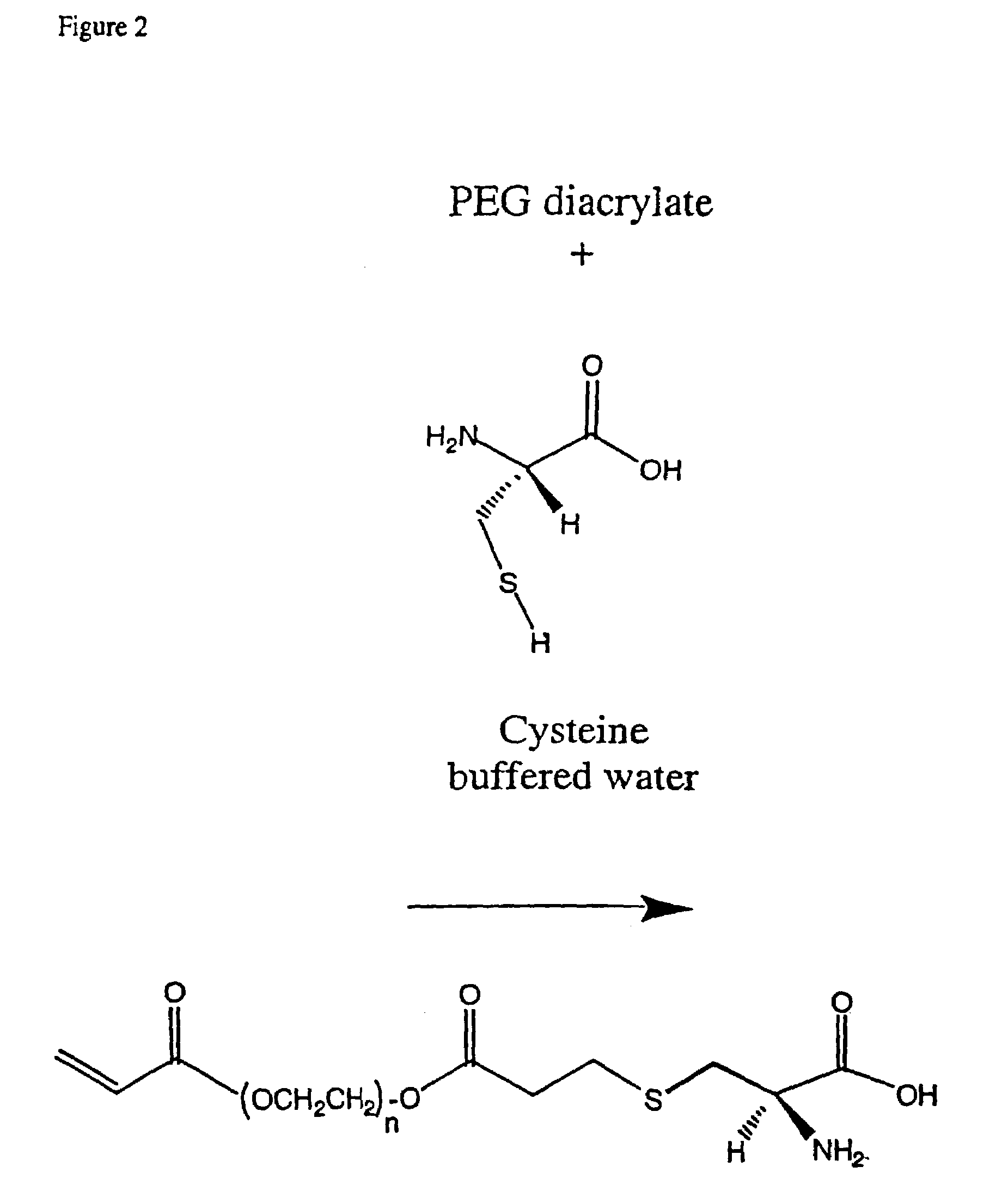
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS6958212B1Reducing and delaying onsetGood water solubilitySugar derivativesPeptide/protein ingredientsBiological materialsPolymer
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:ETH ZZURICH +1
Percutaneous delivery system
InactiveUS6211250B1Good substantivityAppropriate thicknessAntibacterial agentsAntimycoticsActive agentPharmacology
The invention relates to a substantially homogenous liquid composition capable of percutaneous delivery of one or more physiologically active agents, the composition including a rate modulating polymer, a volatile solvent and at least one physiologically active agent, said rate modulating polymer being selected to enable modulation of the rate of delivery of said physiologically active agent. Methods of percutaneous delivery of active agents and of prophylactic or therapeutic antimicrobial, antifungal or antiviral treatment using the compositions of the invention are also described.
Owner:STIEFEL RESEARCH AUSTRALIA PTY LTD
Coatings with tunable molecular architecture for drug-coated balloon
InactiveUS20110143014A1Low elastic modulusLow release rateStentsBalloon catheterDissolutionNuclear medicine
A drug delivery balloon is provided, the a balloon having an outer surface, and a tunable coating disposed on at least a length of the balloon surface. The tunable coating includes a first therapeutic agent and a first excipient, and can include a second therapeutic agent and a second excipient. The first and second therapeutic agents have different dissolution rates during balloon inflation and therefore provide a coating that is tunable.
Owner:ABBOTT CARDIOVASCULAR
Barriers for polymer-coated implantable medical devices and methods for making the same
InactiveUS6953560B1Reduce and prevent and inflammationReduce and prevent proliferationStentsSurgeryHafniumPt element
An implantable medical device and methods for making the implantable medical device are disclosed. The implantable medical device includes a substrate. At least a portion of the substrate is coated with a first layer including a polymer containing a drug. A barrier overlies the first layer. The barrier significantly reduces the rate of release of the drug from the polymer, thereby sustaining release of the drug from the medical device for a longer time.The barrier may be a homogeneous layer overlying the first layer, or a number of discrete deposits over the first layer. Alternatively, the barrier may be intermixed with an outer portion of the first layer. The barrier material is biocompatible, and typically has a thickness ranging from about 50 angstroms to about 20,000 microns. Suitable materials for the barrier include, but are not limited to, inorganic compounds, such as inorganic silicides, oxides, nitrides, carbides, as well as pure metals such as aluminum, chromium, gold, hafnium, iridium, niobium, palladium, platinum, tantalum, titanium, tungsten, zirconium, and alloys of these metals. The barriers disclosed may be applied to the first layer by several techniques, depending on the material being applied. Exemplary deposition techniques include physical vapor deposition, alkoxide hydrolysis, and electroless plating.The implantable device may be a stent or a graft, among other possibilities.
Owner:ABBOTT CARDIOVASCULAR
Fuel filter including slow release additive
This invention relates to a fuel filter for use with internal combustion engines. The fuel filter includes a fuel additive that can be released into fuel. The rate of release for the fuel can be controlled. In one form the fuel additive can be mixed with a matrix material and released at a substantially constant rate to maintain a uniform level of fuel additive in the fuel. Use of the present invention provides a fuel filter having an extended life span that is longer than fuel filters typically used with combustion engines.
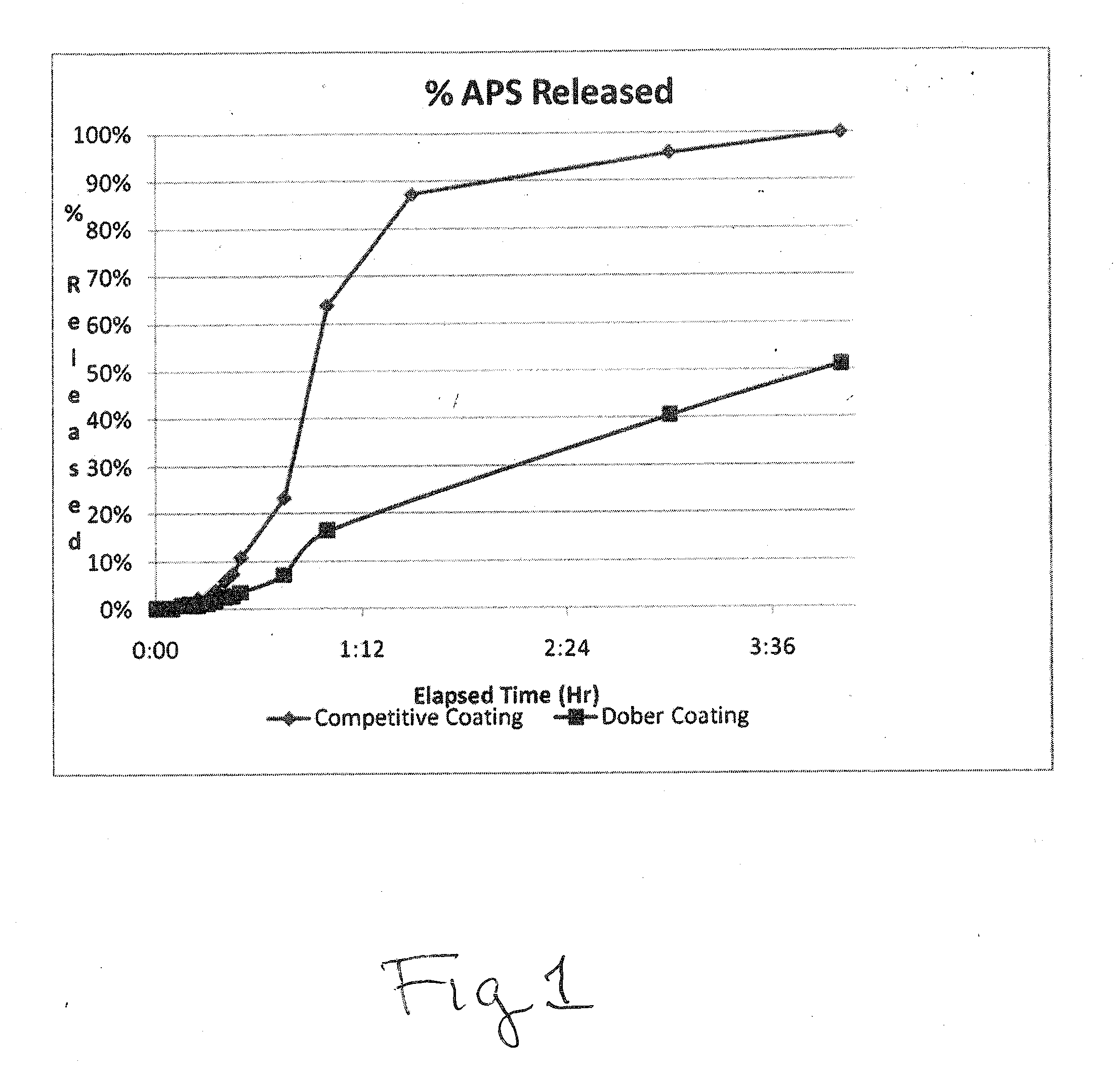
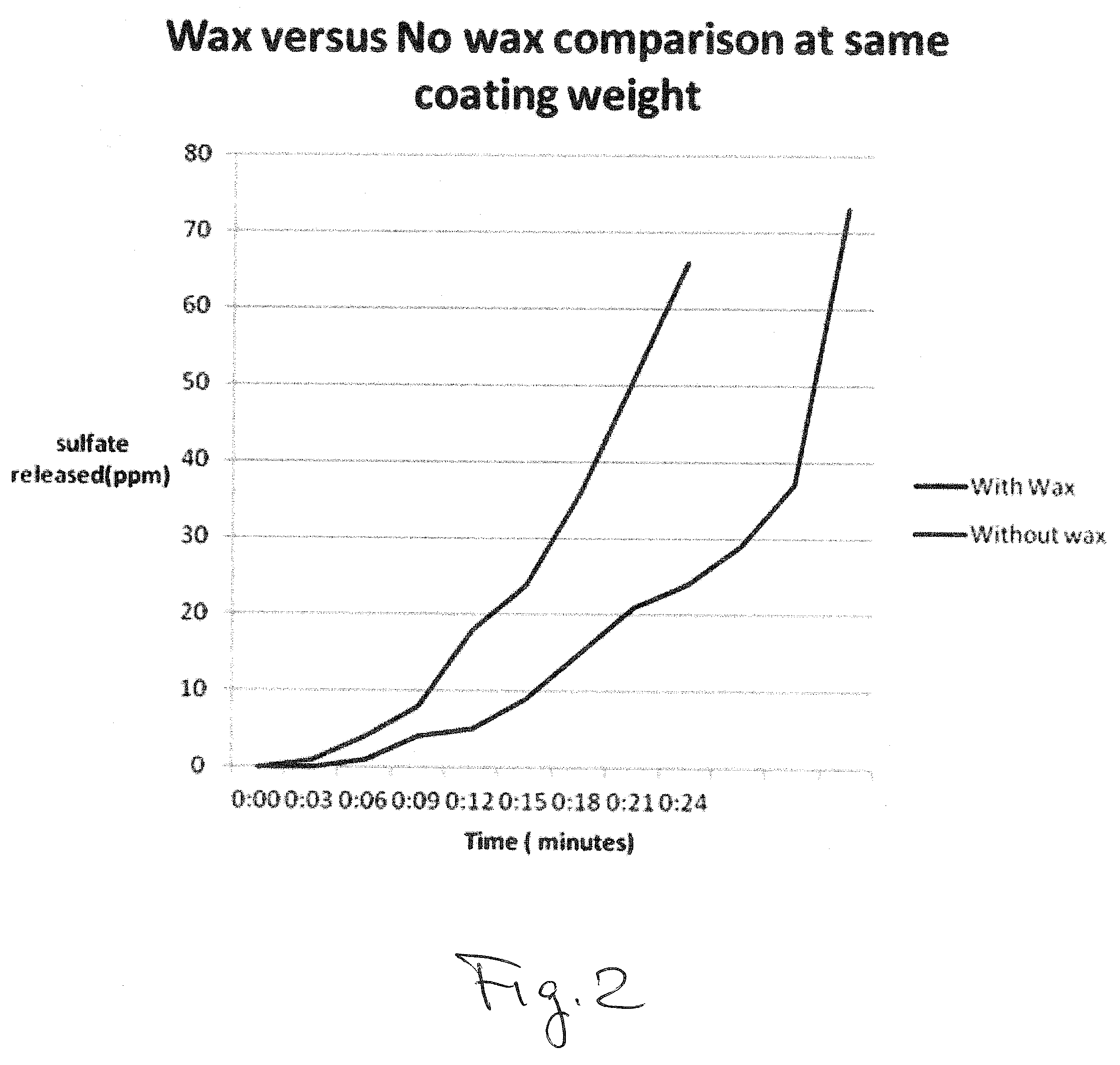
Owner:DOBER CHEMICAL CORPORATION +1
Microparticle containing matrices for drug delivery
ActiveUS8663674B2Low release ratePremature depletionSenses disorderAntimycoticsActive agentPharmaceutical Substances
Polymeric matrices including microparticles that provide sustained release of a hydrophilic bioactive agent are described. The matrices can be in the form of a coating on the surface of an implantable medical article or an in situ formed matrix. The microparticles of the matrix include a first polymer and a second polymer that modulates release of the hydrophilic bioactive agent from the matrix.
Owner:SURMODICS INC
Immediate release composition resistant to abuse by intake of alcohol
The present invention provides immediate release pharmaceutical compositions for oral administration that are resistant to abuse by intake of alcohol.
Owner:EGALET LTD
Methods and compositions for the treatment and diagnosis of vascular inflammatory disorders or endothelial cell disorders
InactiveUS20080199426A1Inhibition effectLow release rateOrganic active ingredientsPeptide/protein ingredientsVasculitisMedicine
Disclosed herein are methods for treating a vascular inflammatory disorder or endothelial cell disorder using inhibitor compounds that inhibit the expression or biological activity of Tie-1, Tie-1 endodomain, thrombin, VEGFR2, VEGFR2 endodomain, EphA2, and any of the cytokines or kinases that are upregulated by activation of Tie-1 or thrombin, as provided herein. Also disclosed are the use of combinations of inhibitor compounds or the use of an eNOS activator compound in combination with any one or more of the inhibitor compounds. Also disclosed are methods for inhibiting the pro-coagulant activity of thrombin using a Tie-1 or Tie-1 endodomain inhibitor compound or an EphA2 inhibitor compound. Methods for diagnosing and monitoring vascular inflammatory disorders or endothelial cell disorders that include the measurement of any of the polypeptides or nucleic acid molecules of the invention are also disclosed.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Coated implantable medical device
InactiveUS20070168012A1Prevent degradationIncrease release rateHeart valvesSurgeryBiomedical engineeringPorous layer
Methods of making coated implantable medical devices are provided. The methods include positioning a first layer comprising a bioactive on at least a portion of a structure, and positioning at least one porous layer over the first layer. The at least one porous layer has a thickness adequate to provide a controlled release of the bioactive.
Owner:COOK MEDICAL TECH LLC
Coated implantable medical device
InactiveUS20070050010A1Prevent degradationIncrease release rateStentsIn-vivo radioactive preparationsParyleneGas phase
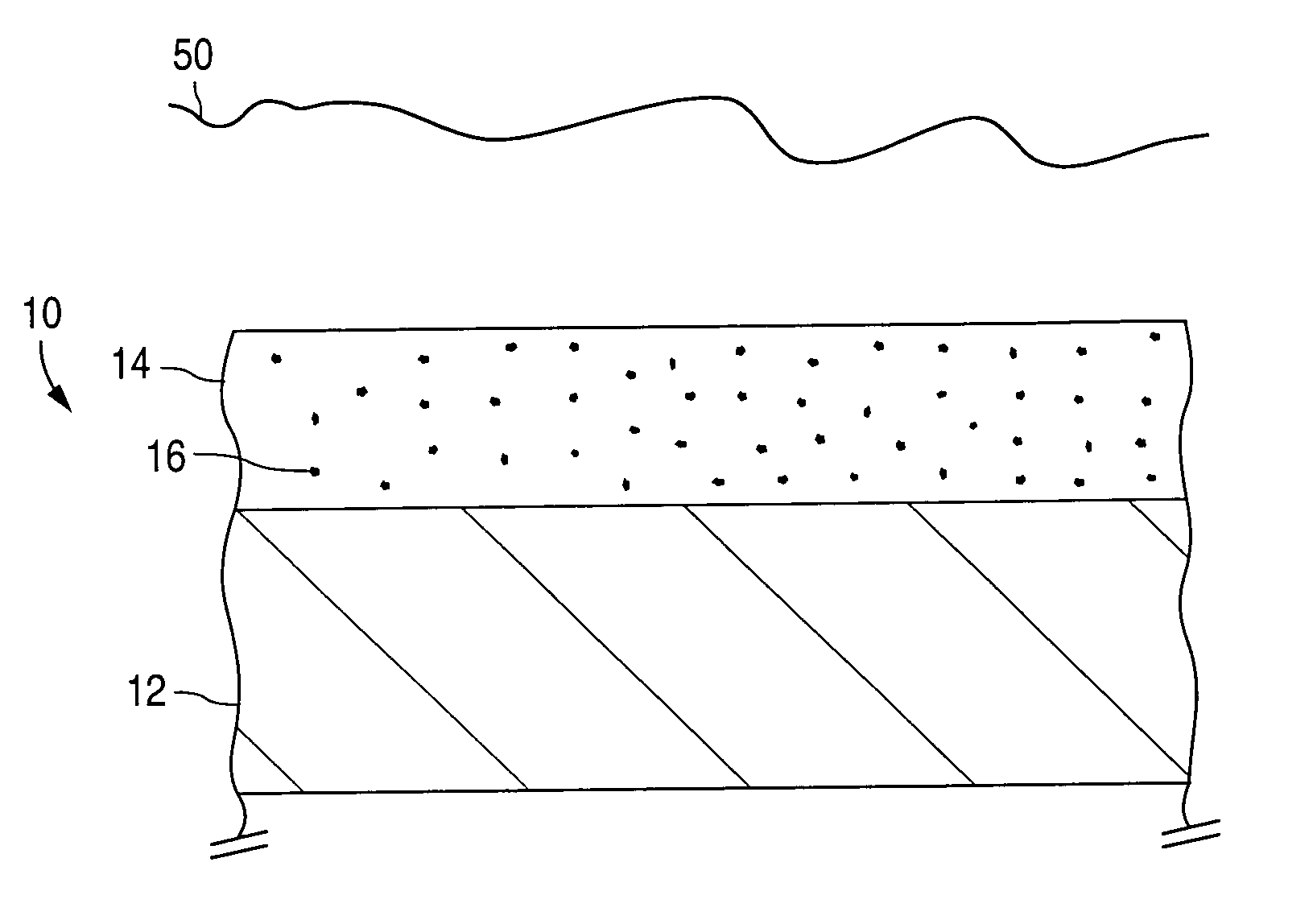
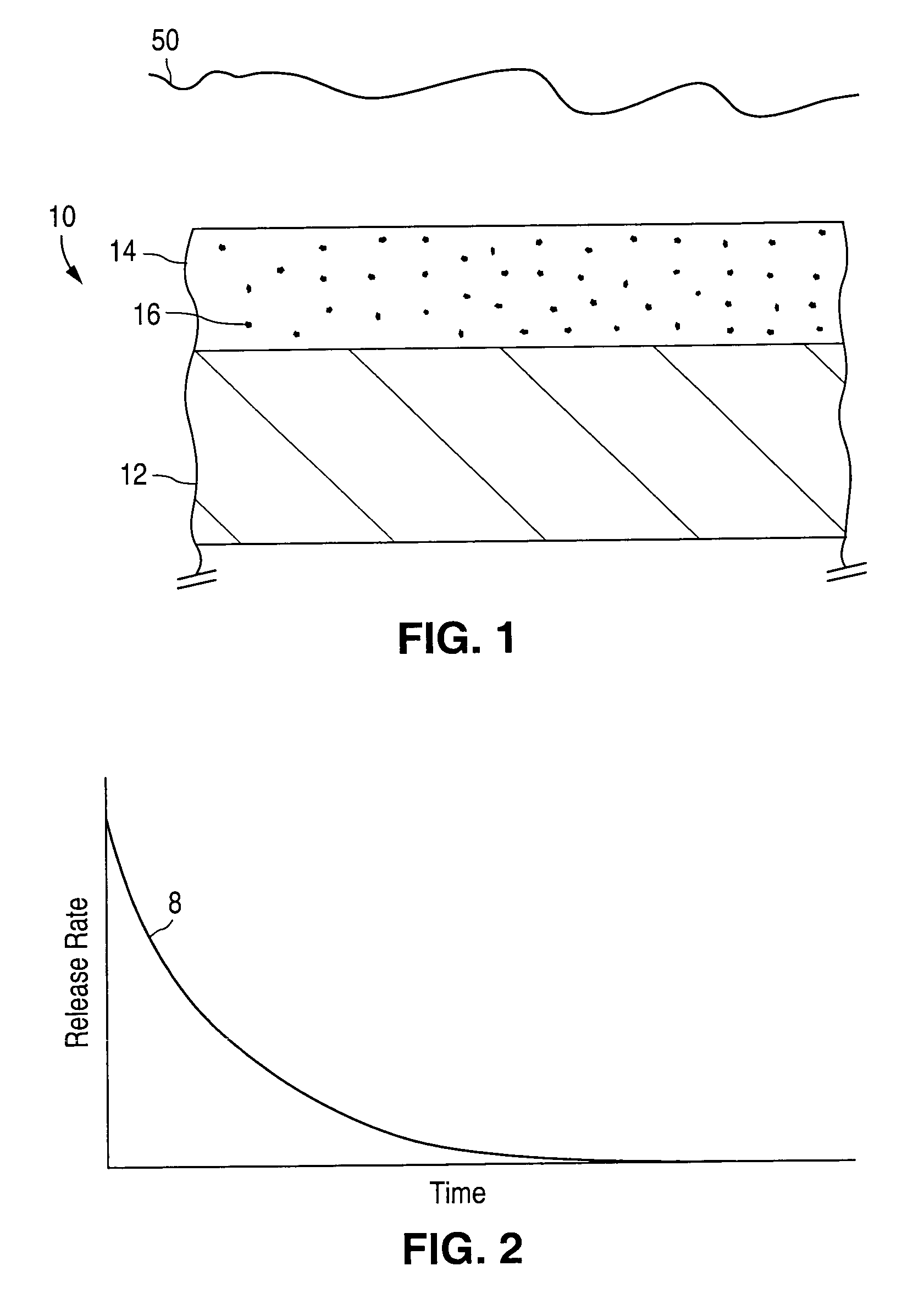
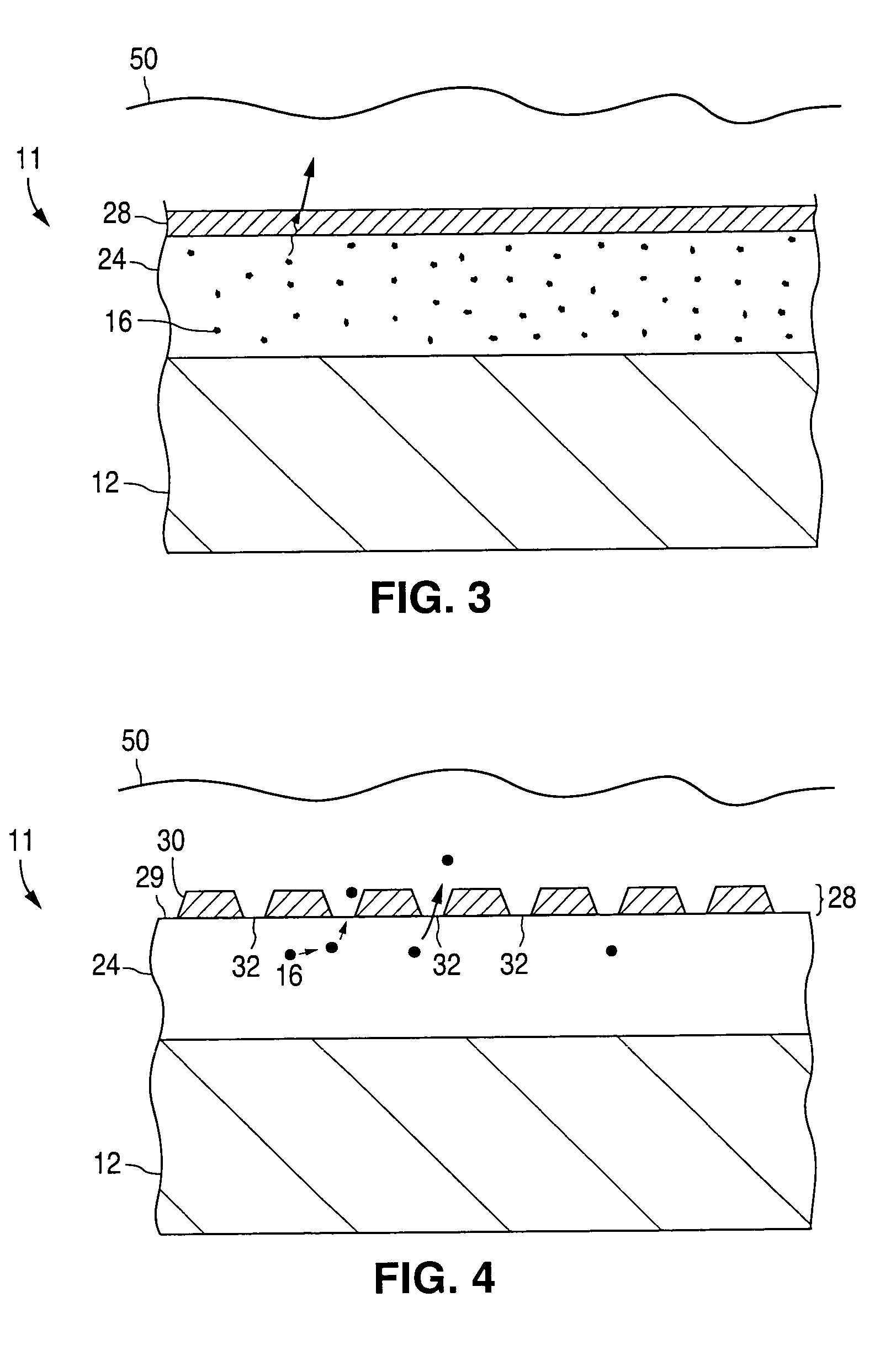
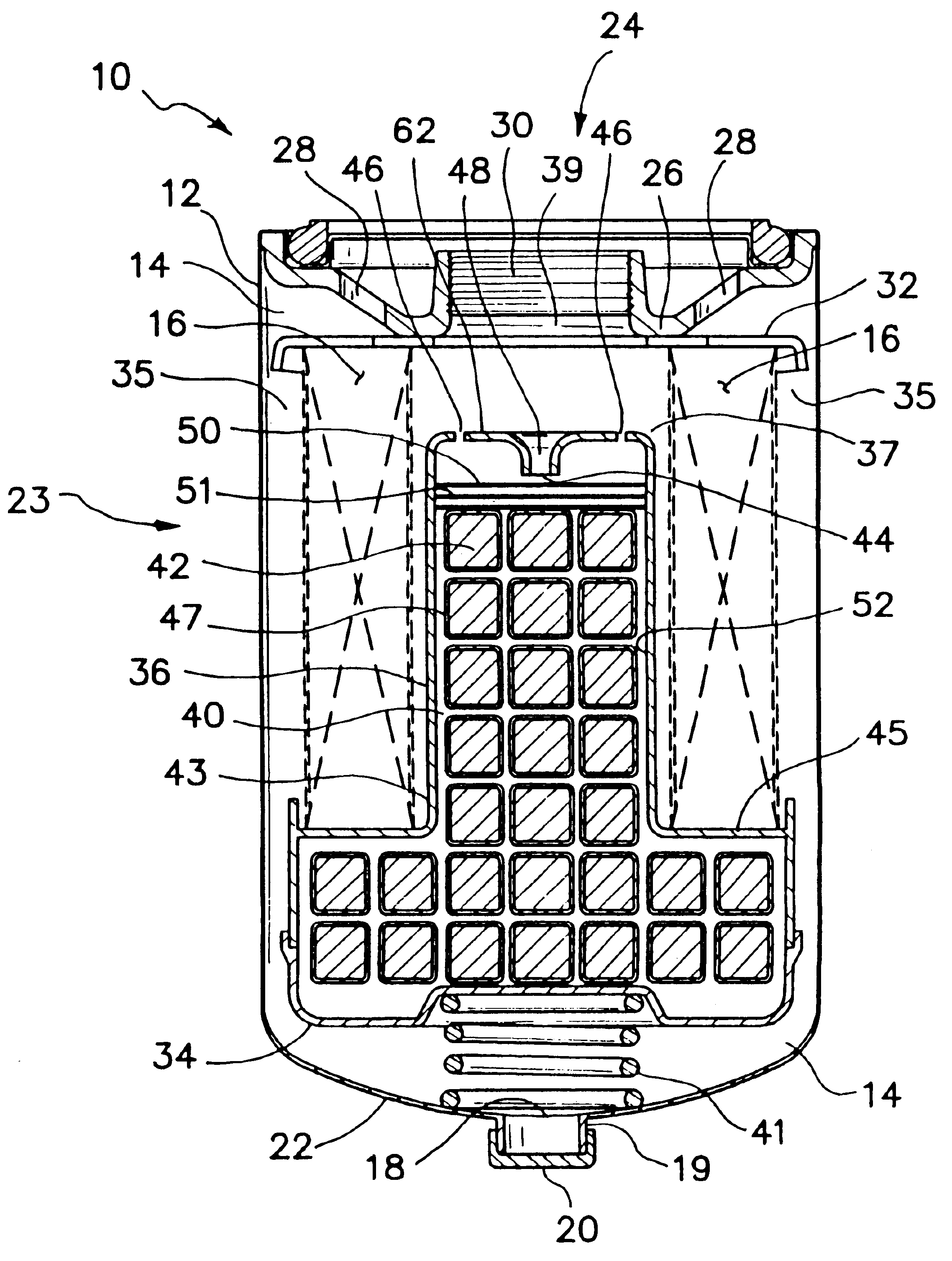
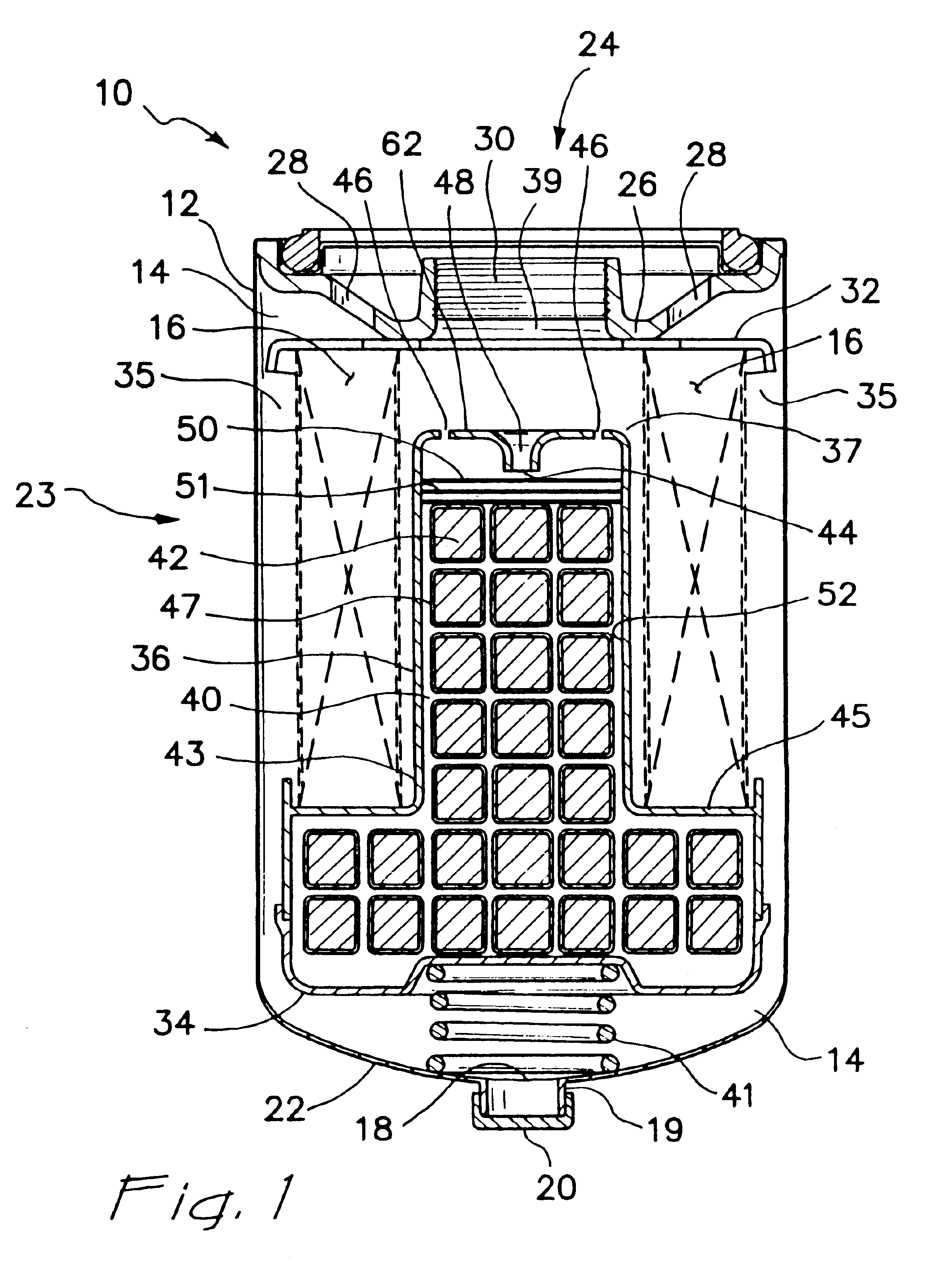
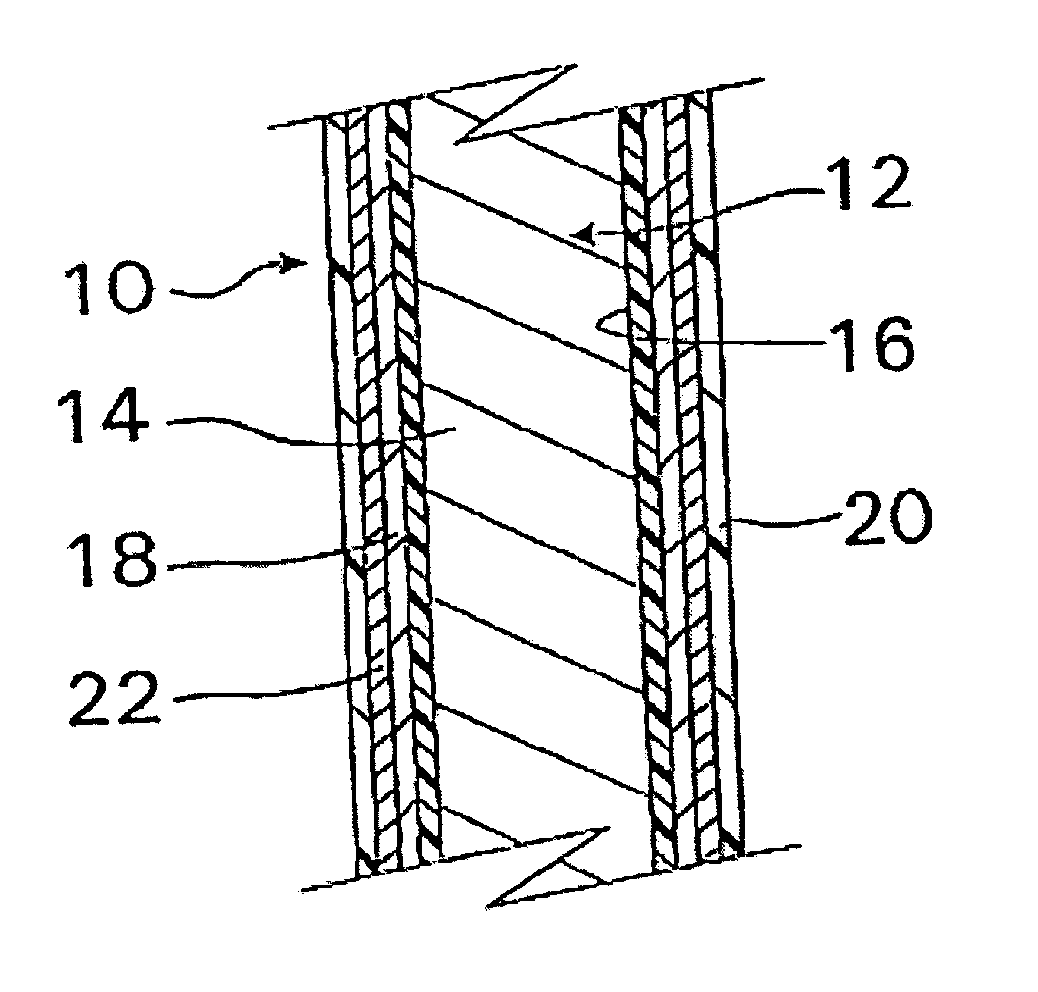
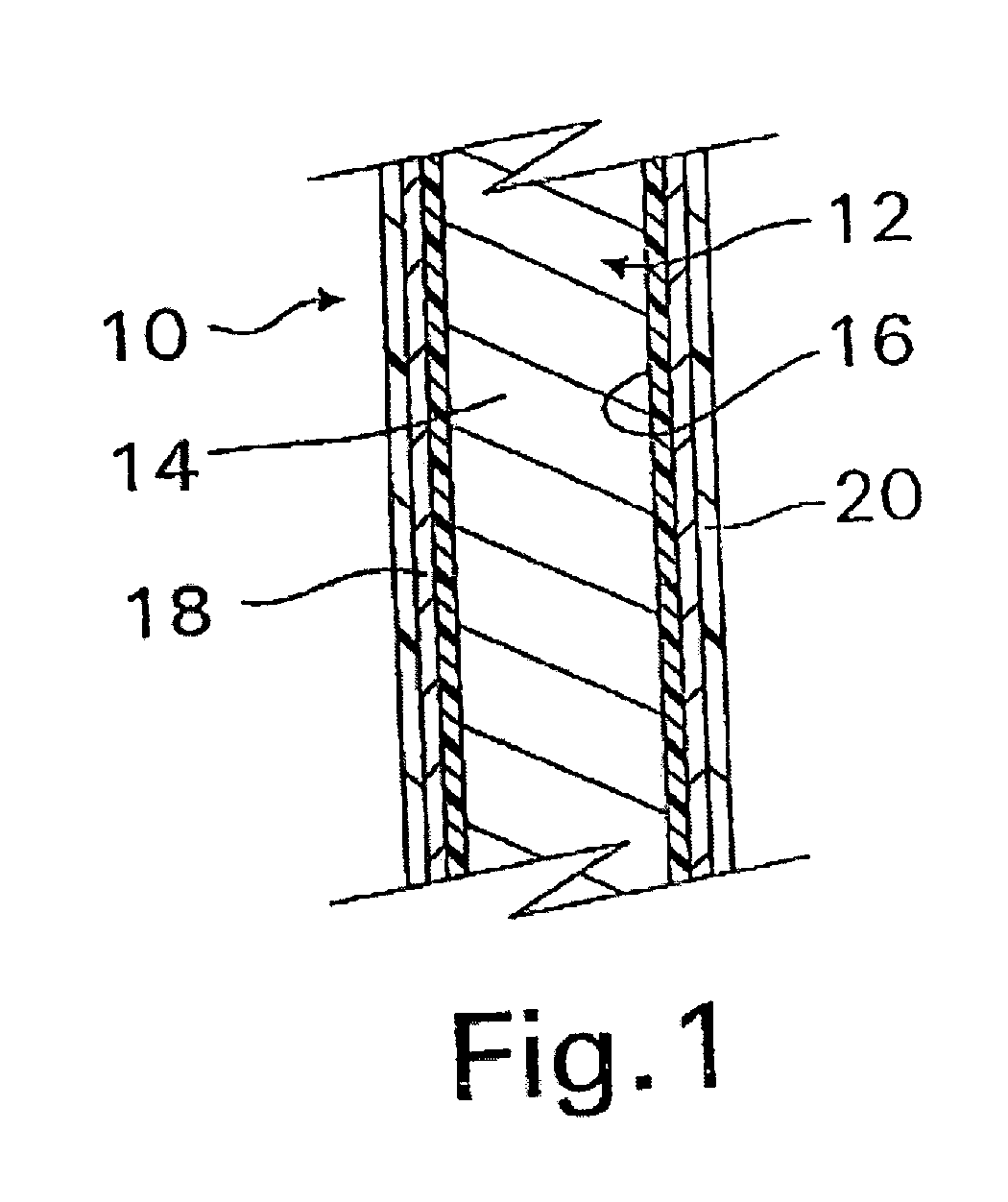
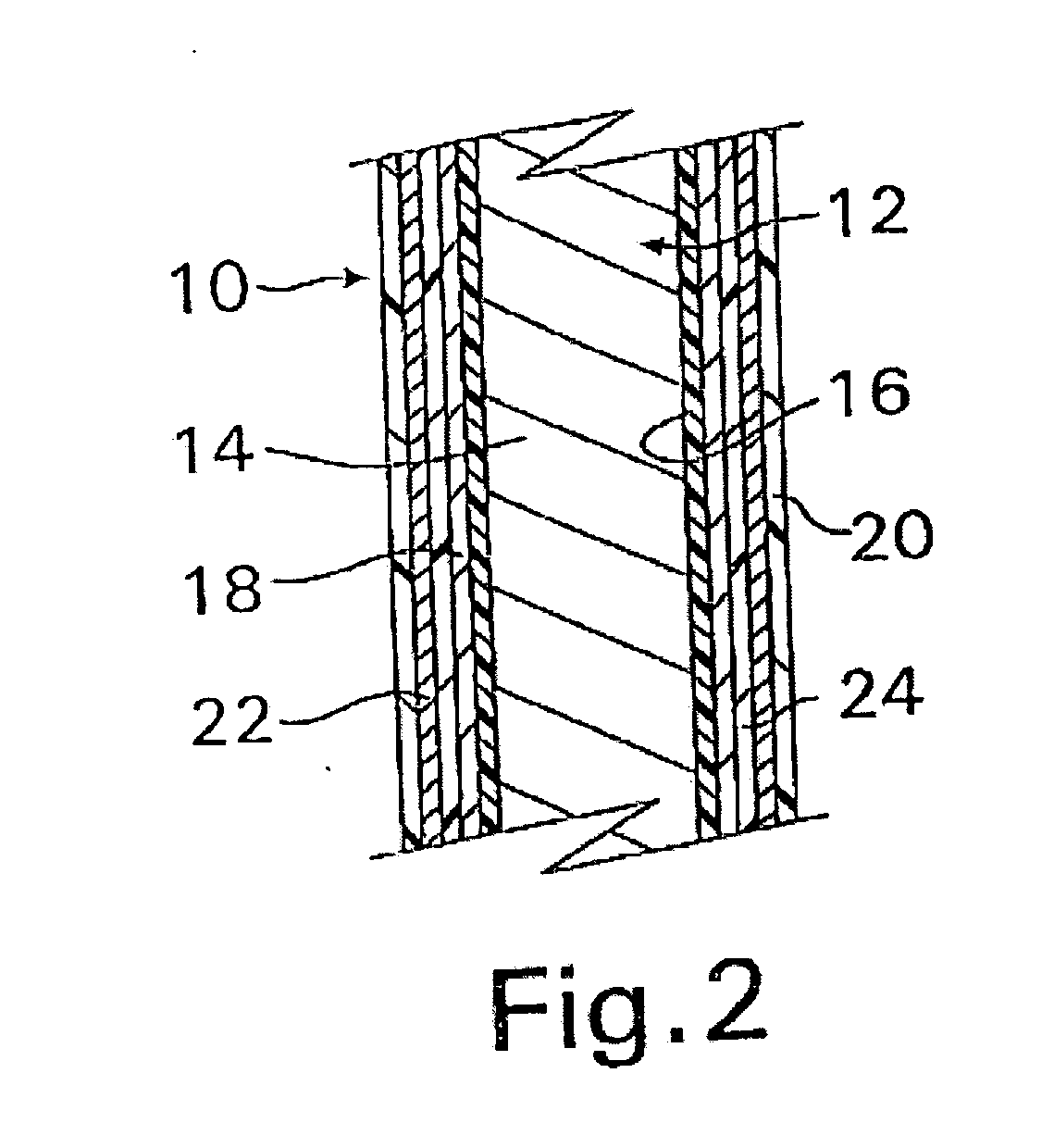
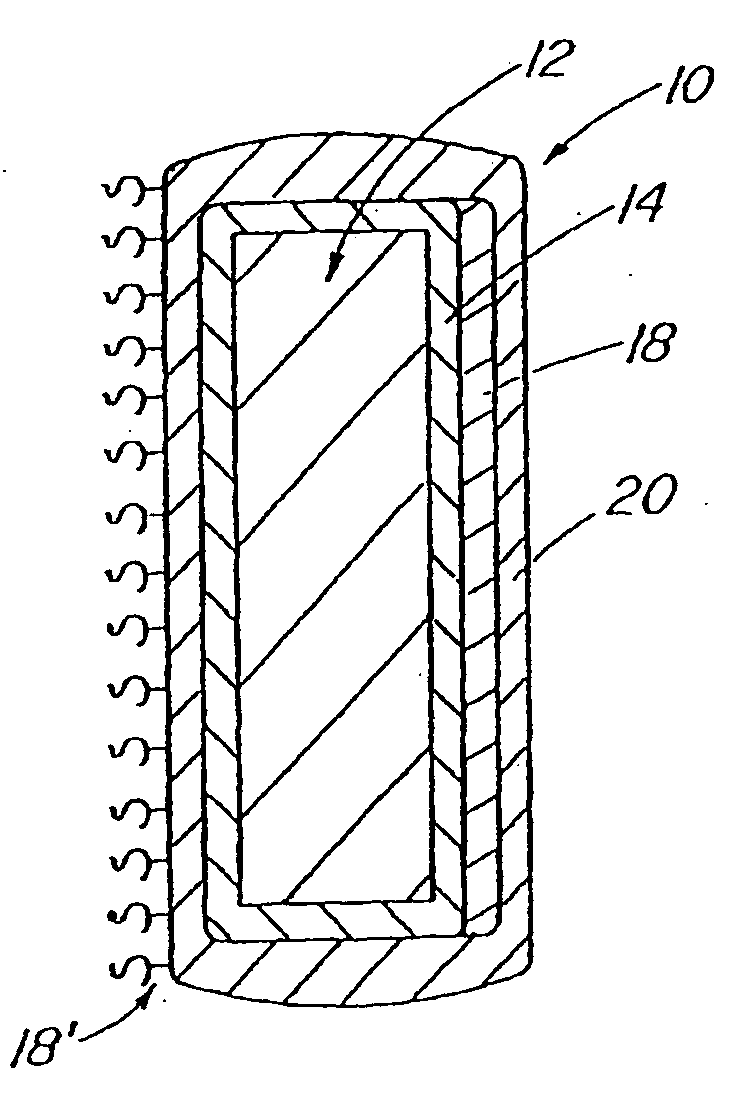
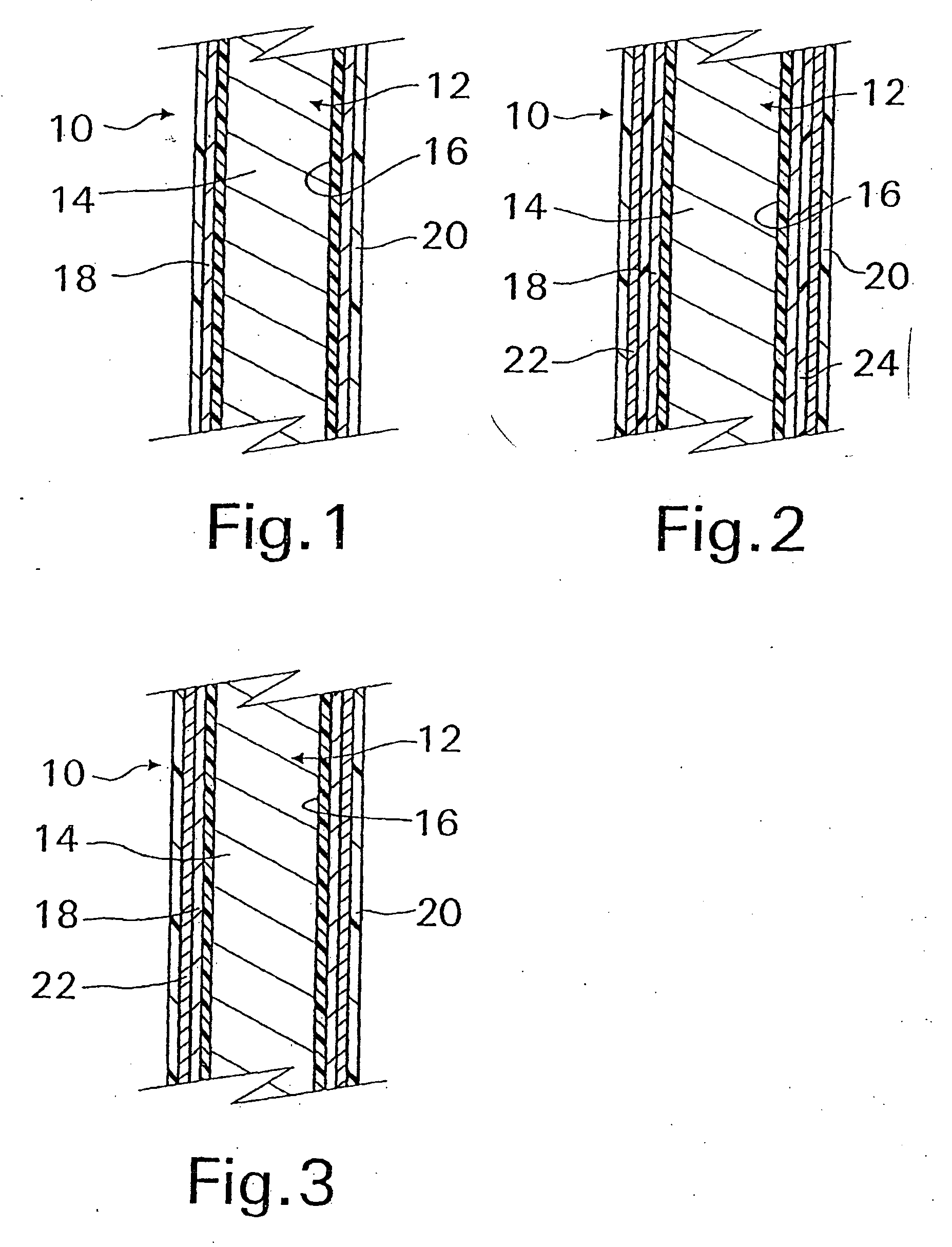
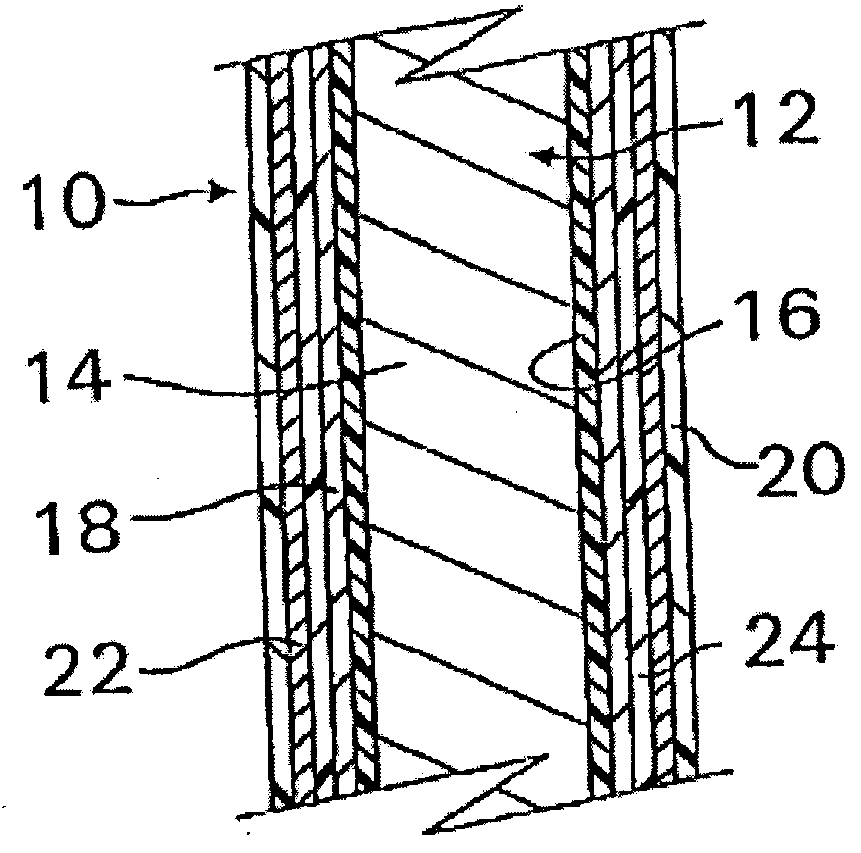
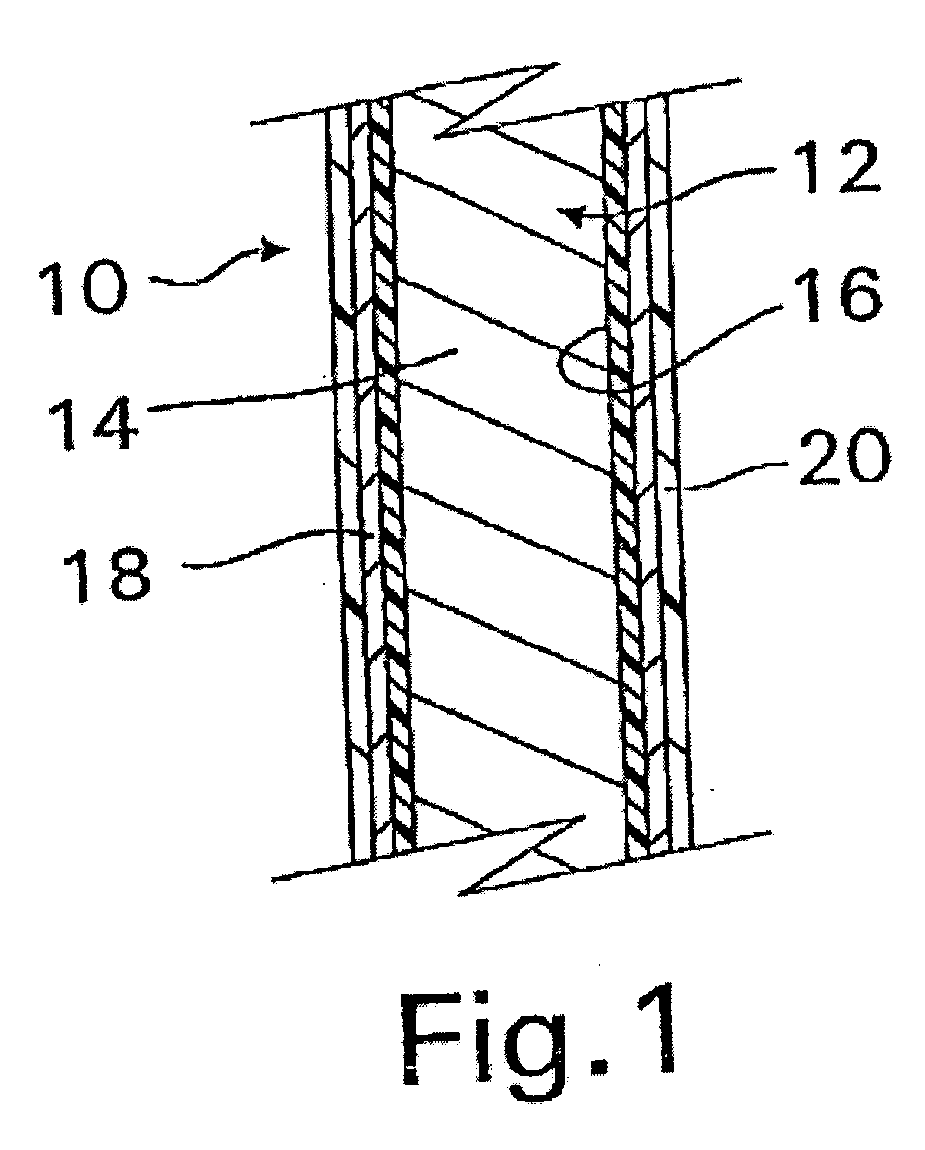
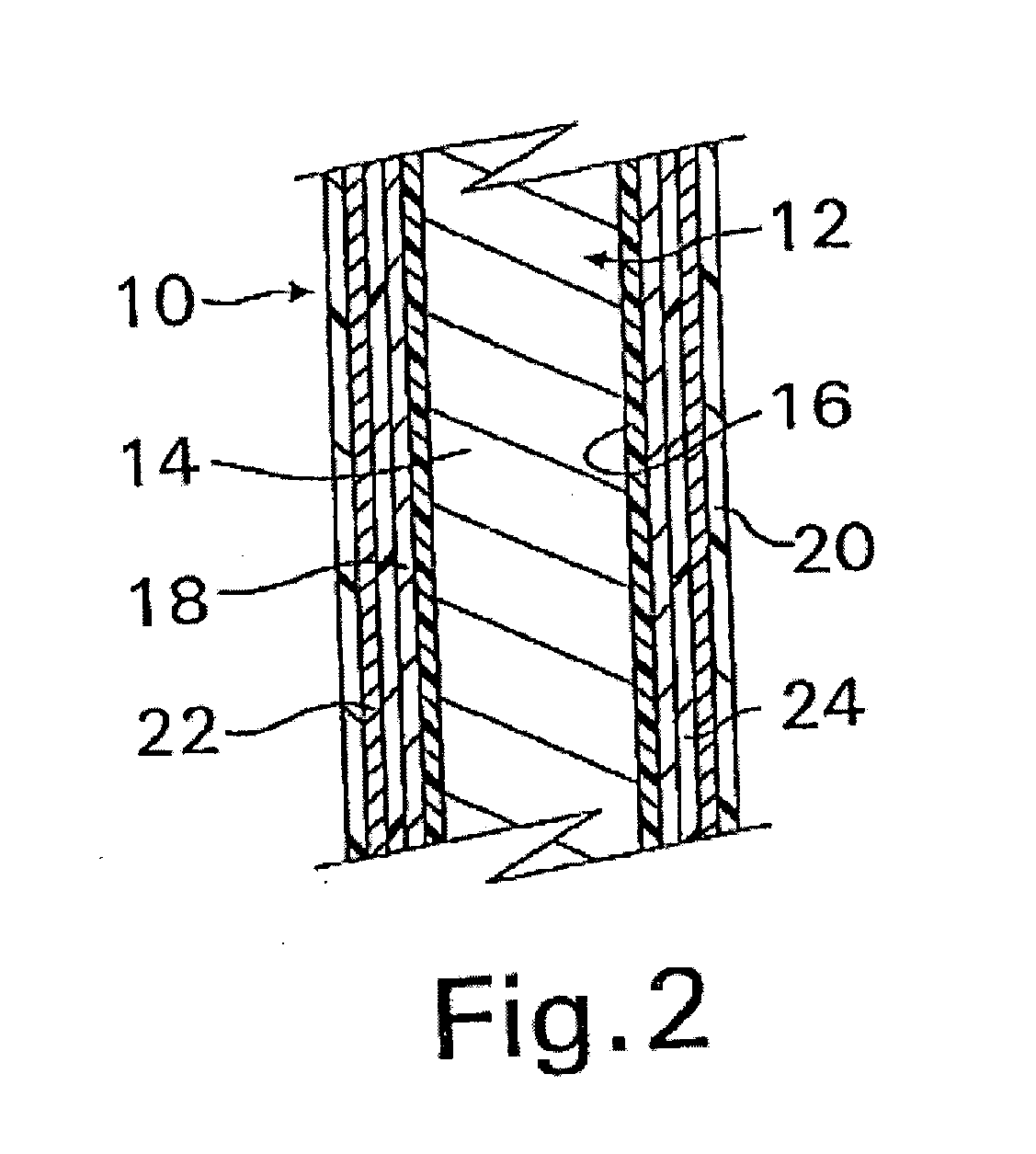
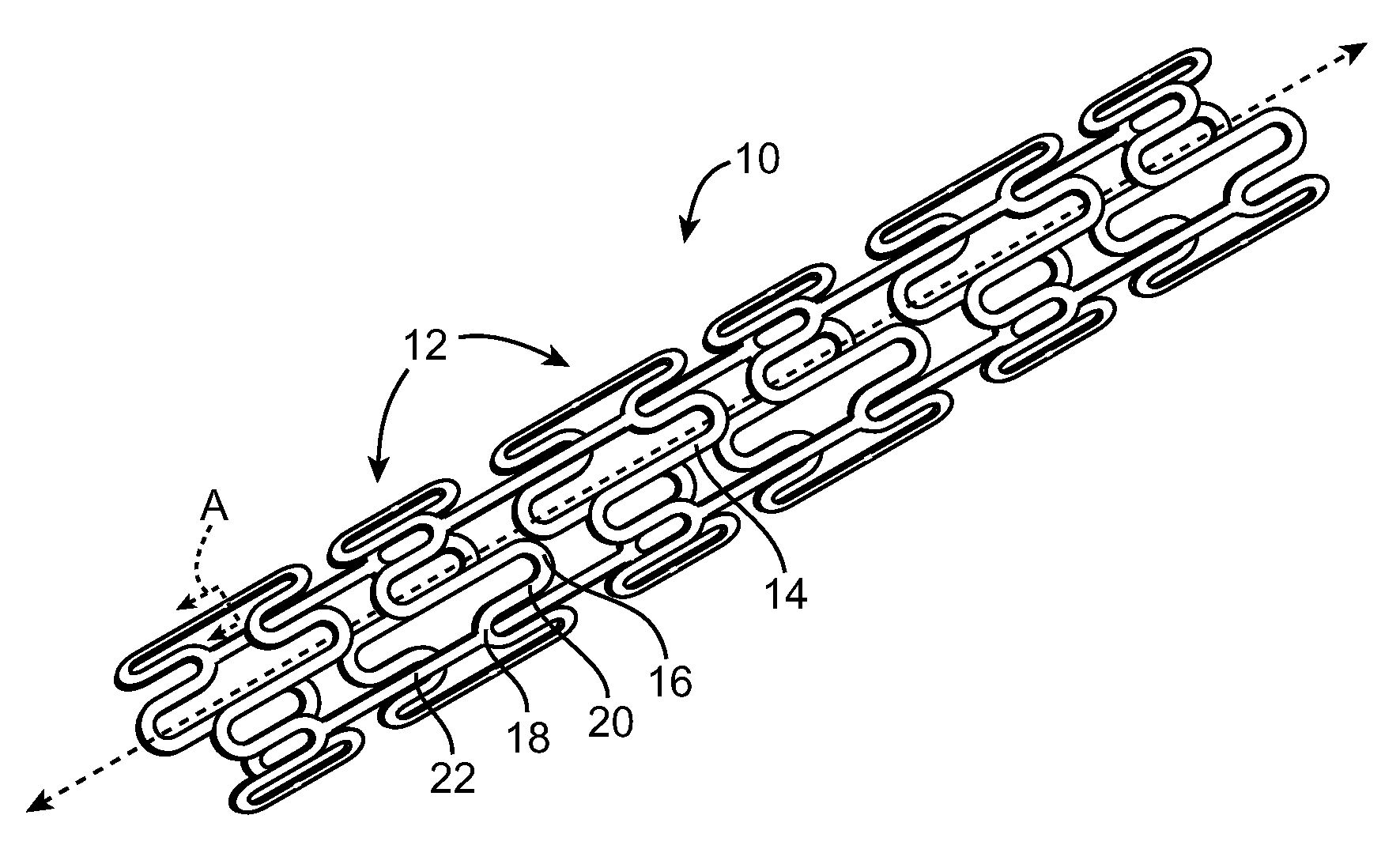
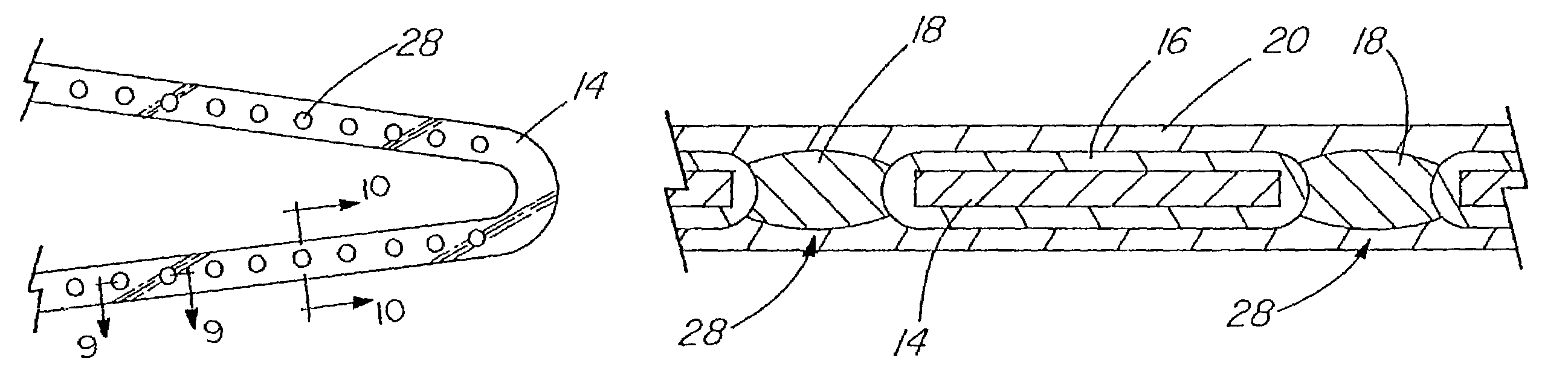
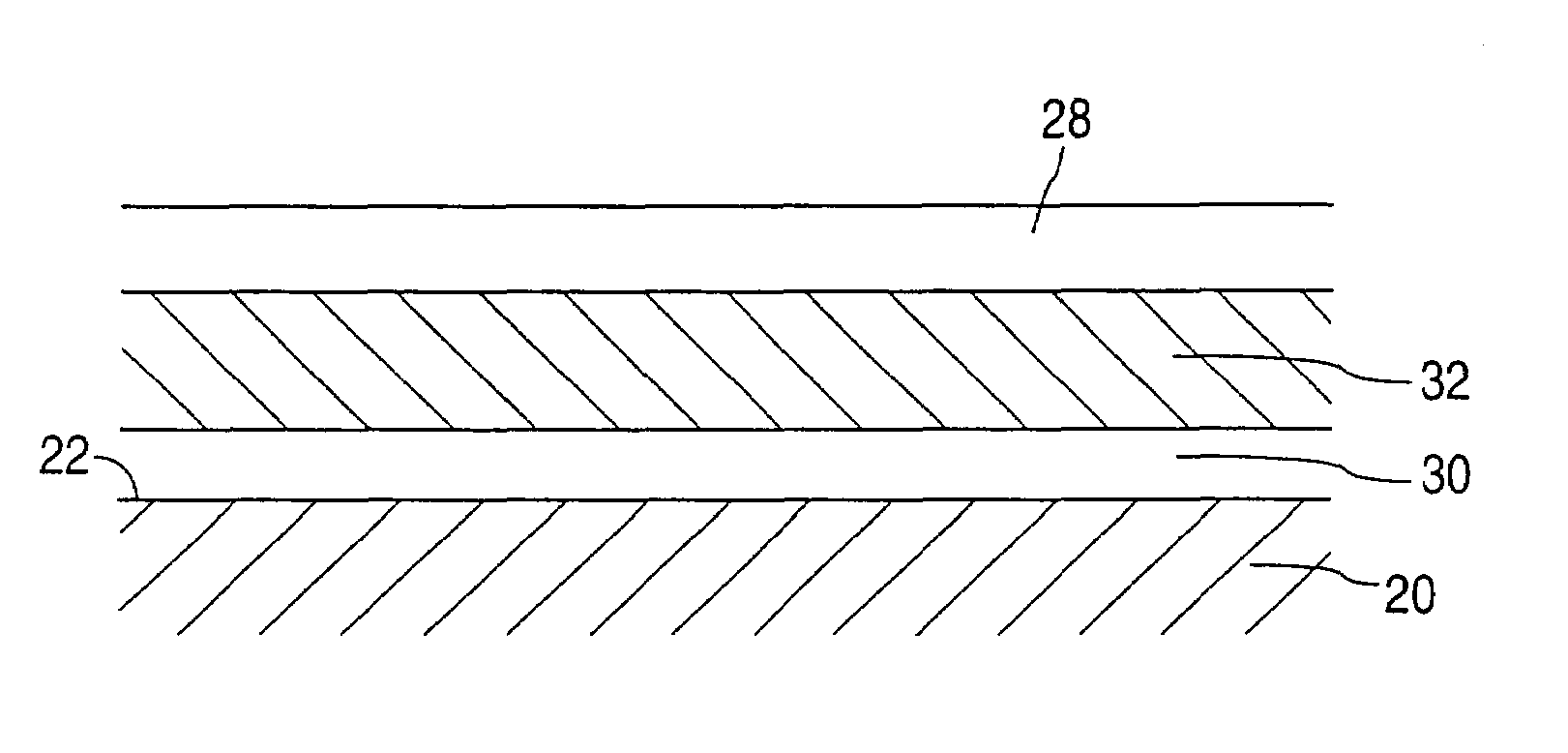
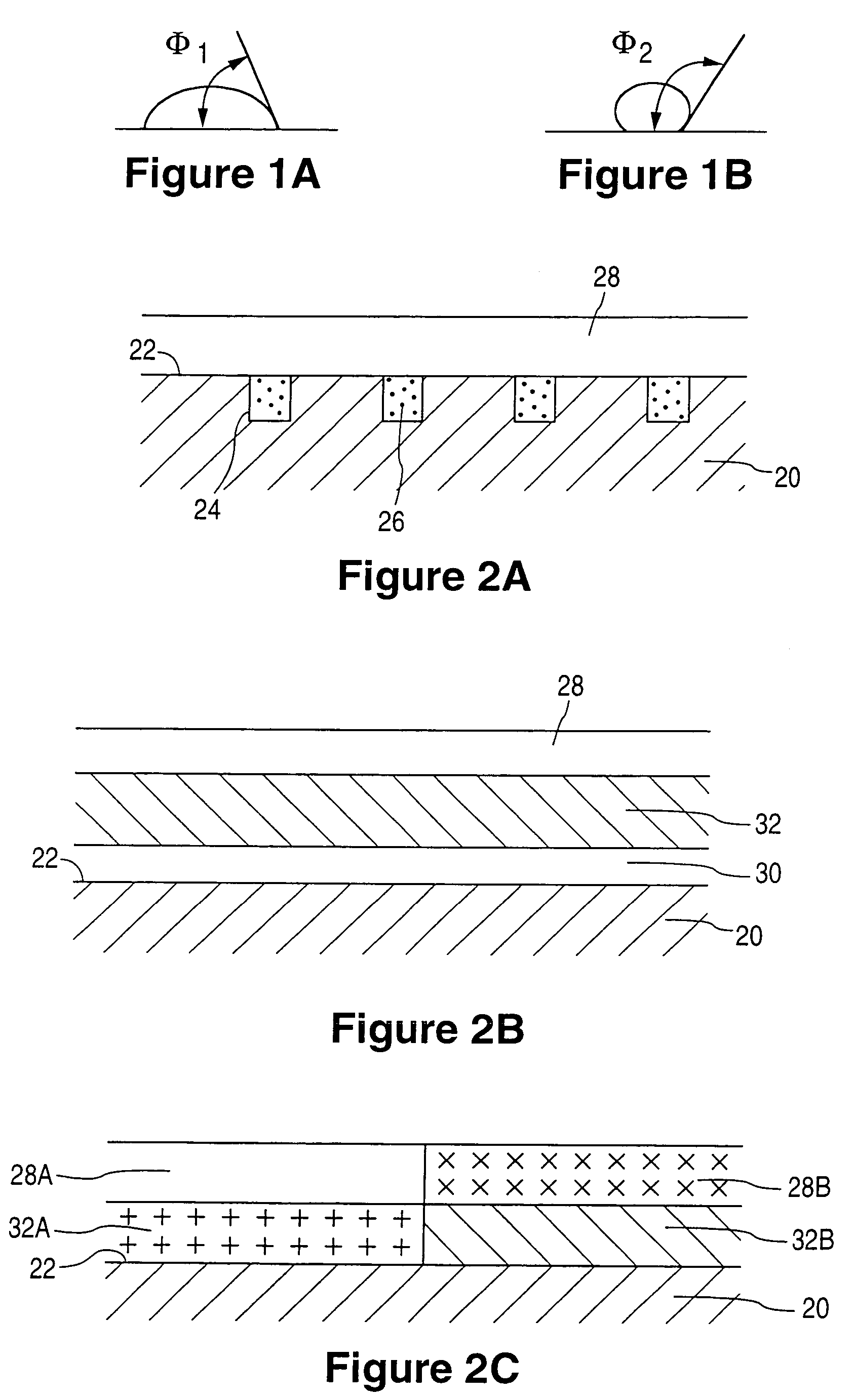
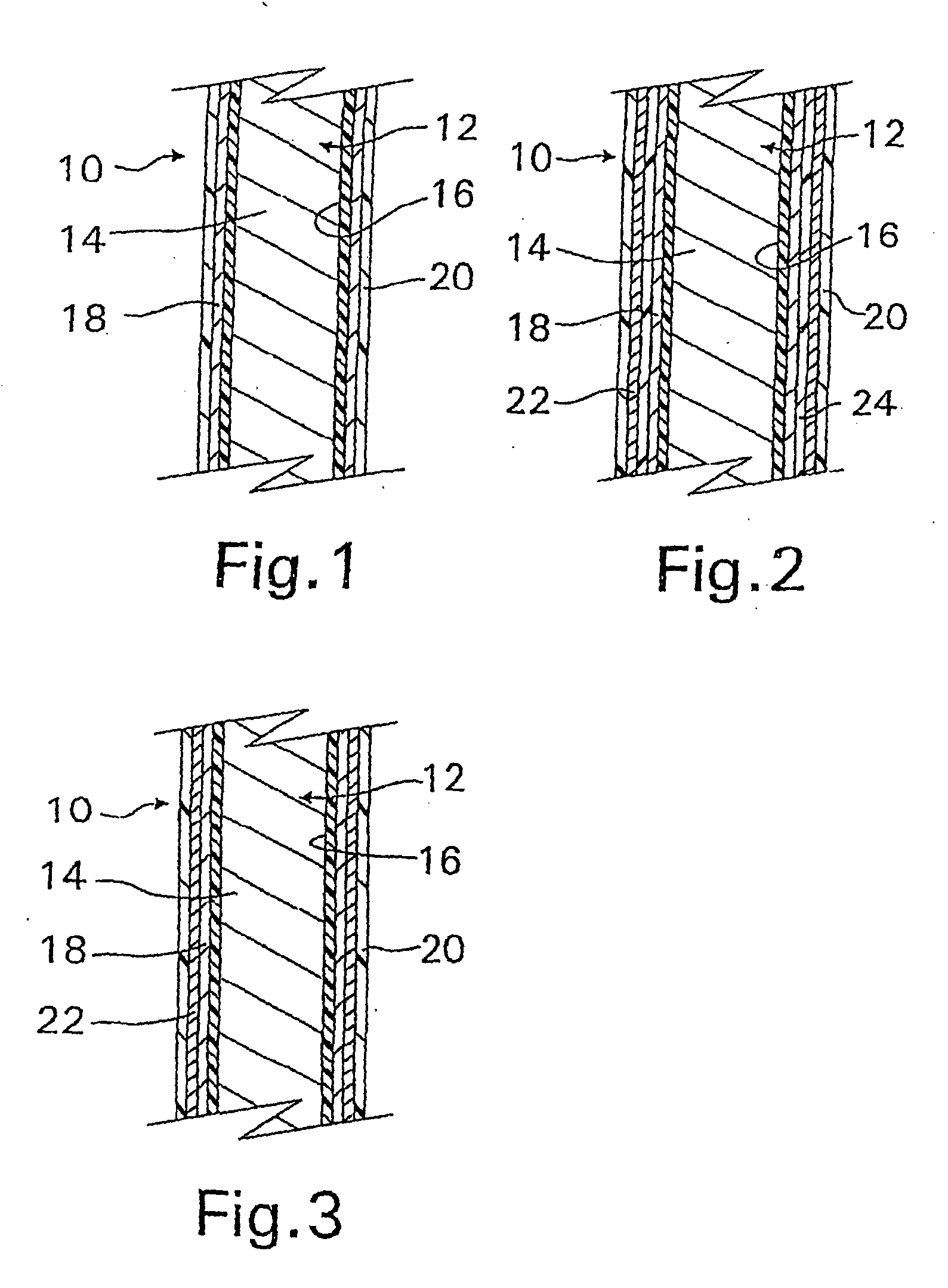
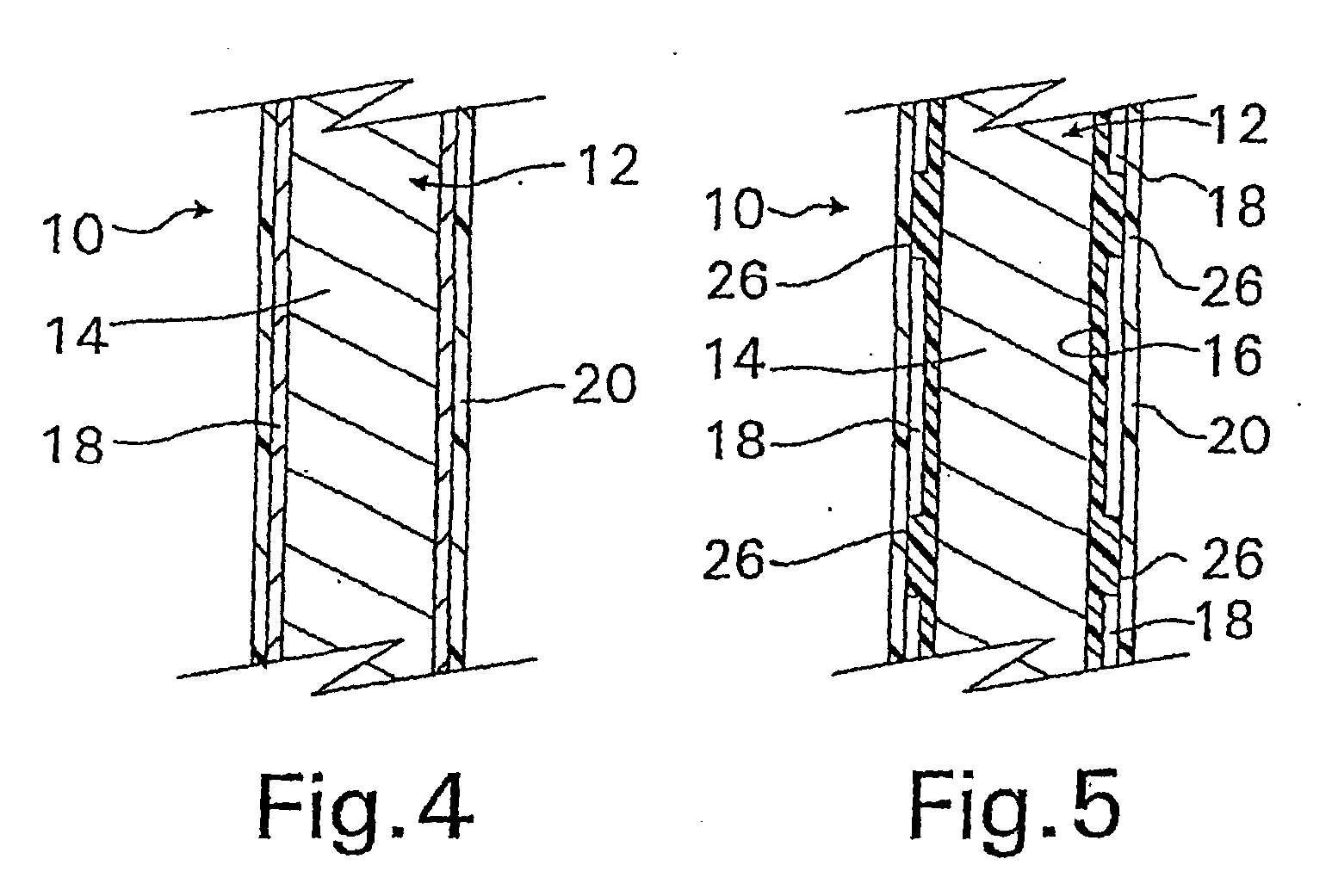
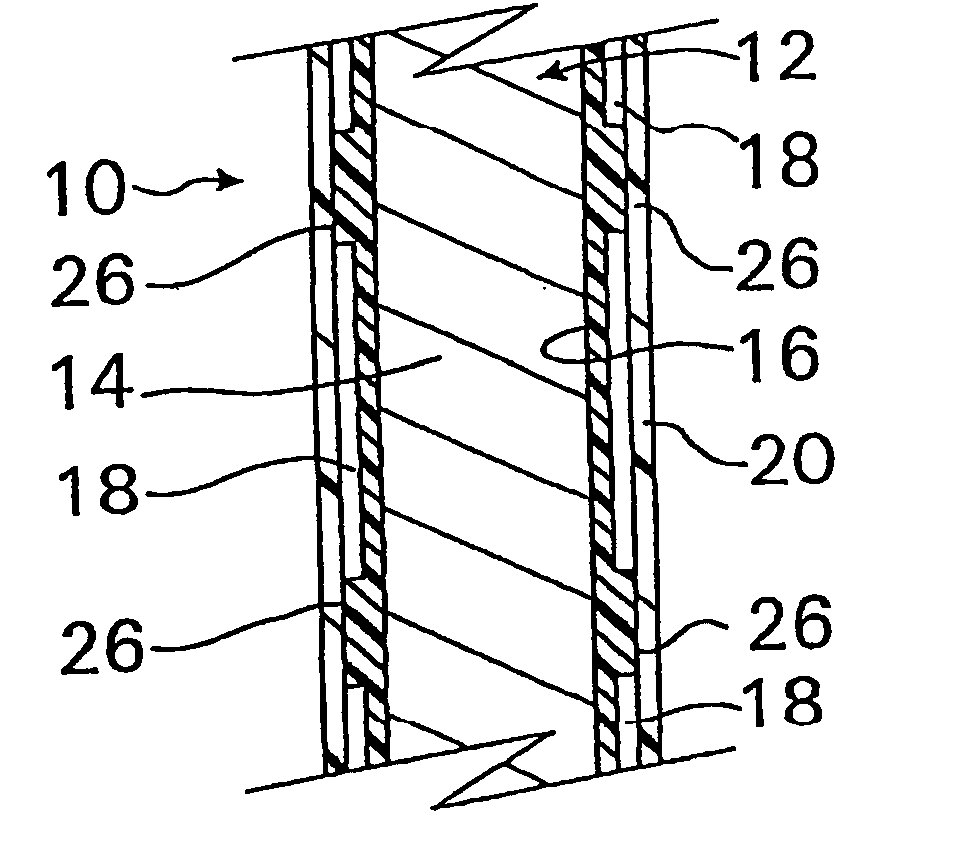
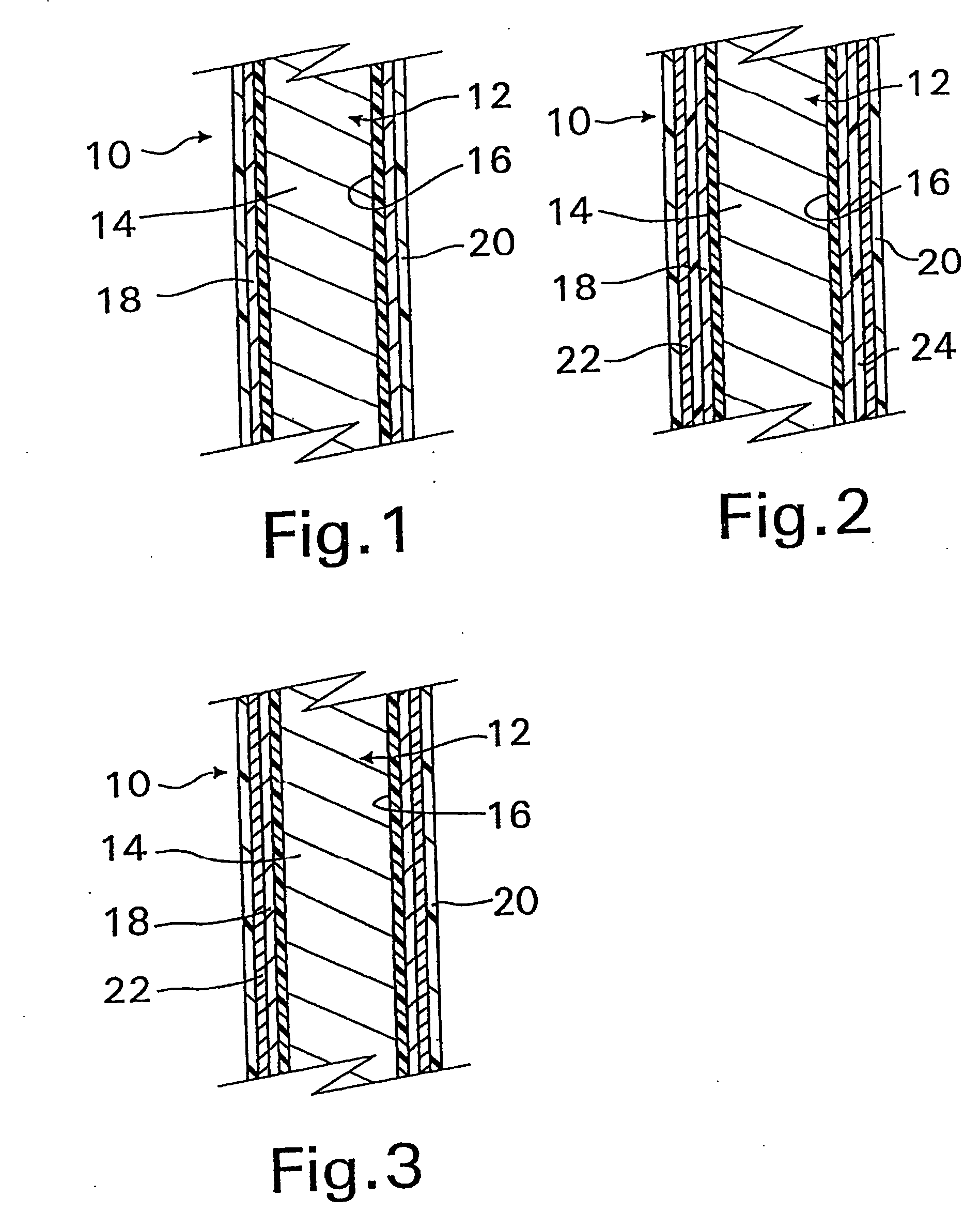
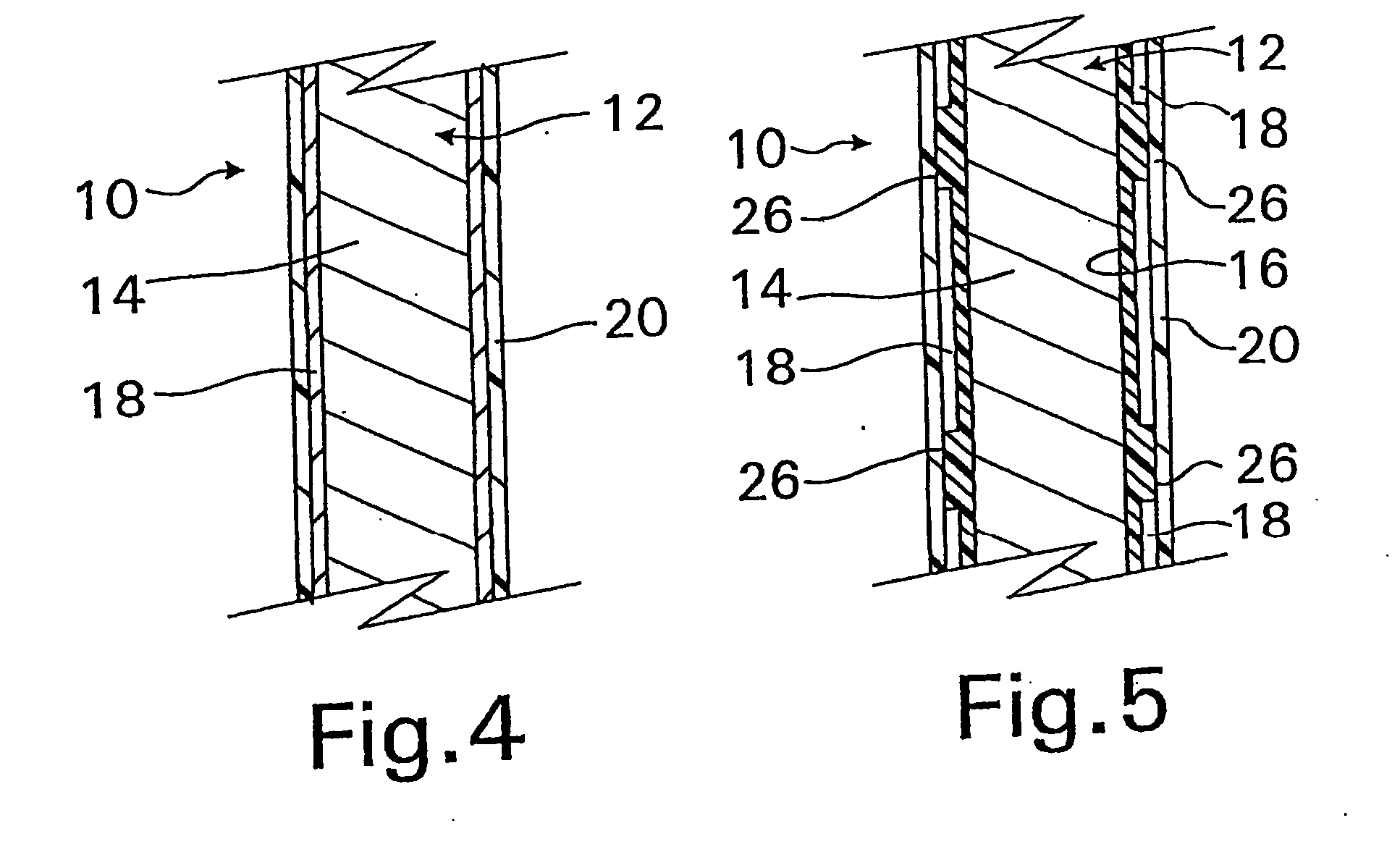
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Barriers for polymer-coated implantable medical devices and methods for making the same
InactiveUS20050208098A1Reduce and prevent and inflammationReduce and prevent proliferationStentsSurgeryBiomedical engineeringMedical treatment
A drug eluting medical device is disclosed. The device includes a barrier layer for reducing the rate of release of a drug.
Owner:ABBOTT CARDIOVASCULAR
Implantable medical device with bioabsorbable coating
InactiveUS20070150047A1Prevent degradationIncrease release rateStentsSurgeryControlled releasePorous layer
Methods of making coated implantable medical devices are provided. The methods include positioning a first layer comprising a bioactive on at least a portion of a structure, and positioning at least one porous layer over the first layer. The at least one porous layer has a thickness adequate to provide a controlled release of the bioactive.
Owner:MED INST INC +1
Biodegradable Metal Barrier Layer for a Drug-Eluting Stent
InactiveUS20080243240A1Reduce and prevent cell proliferationReduce and prevent and inflammationStentsSurgeryAnti mitoticMedical treatment
An implantable medical device includes a substrate, a drug-impregnated layer deposited over the substrate, and a barrier layer at least partially covering the drug-impregnated layer. The barrier layer may be a biodegradable metal, biodegradable metal oxide, or biodegradable metal alloy, such as, magnesium, a magnesium oxide or a magnesium alloy. The drug-impregnated layer includes a therapeutic substance, such as, antineoplastic, anti-inflammatory, antiplatelet, anticoagulant, fibrinolytic, thrombin inhibitor, antimitotic, antiallergic, and antiproliferative substances.
Owner:MEDTRONIC VASCULAR INC
Microparticle containing matrices for drug delivery
ActiveUS20070275027A1Low release ratePremature depletionSenses disorderAntimycoticsActive agentPharmaceutical Substances
Polymeric matrices including microparticles that provide sustained release of a hydrophilic bioactive agent are described. The matrices can be in the form of a coating on the surface of an implantable medical article or an in situ formed matrix. The microparticles of the matrix include a first polymer and a second polymer that modulates release of the hydrophilic bioactive agent from the matrix.
Owner:SURMODICS INC
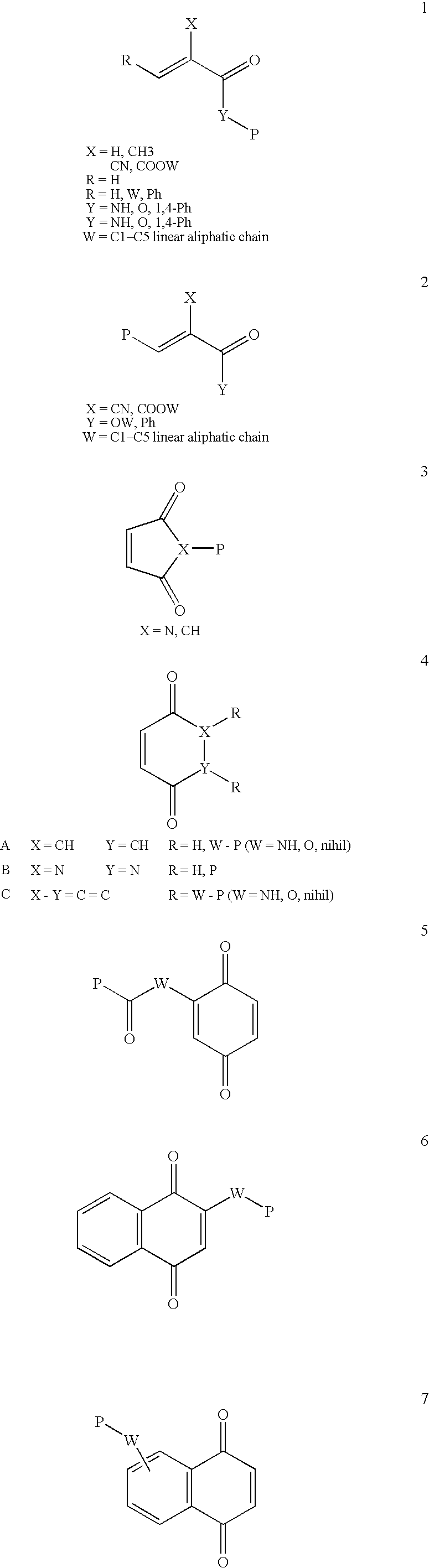
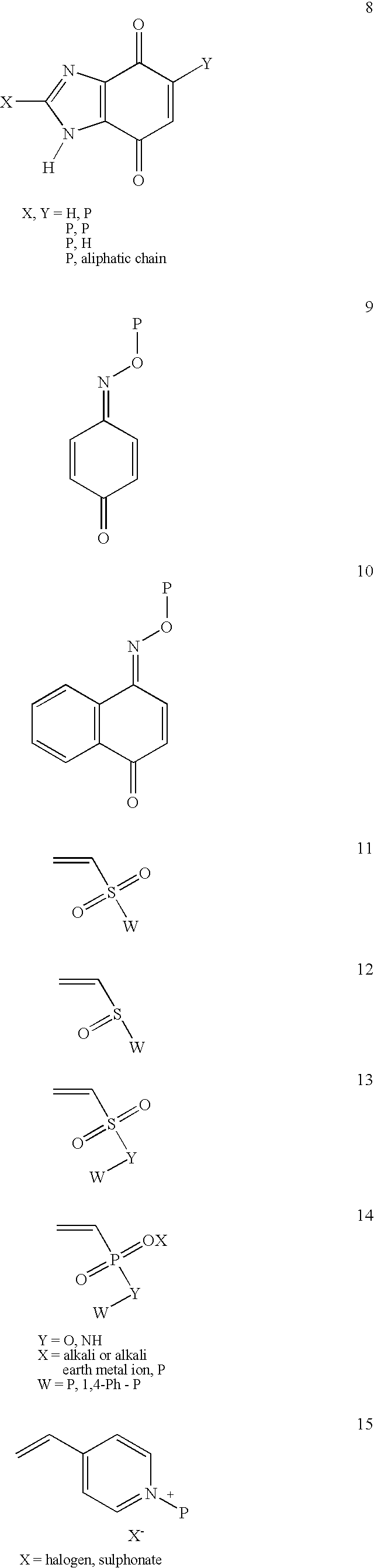
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS7291673B2Reducing and delaying onsetGood water solubilityBiocideSurgical adhesivesPolymerControlled delivery
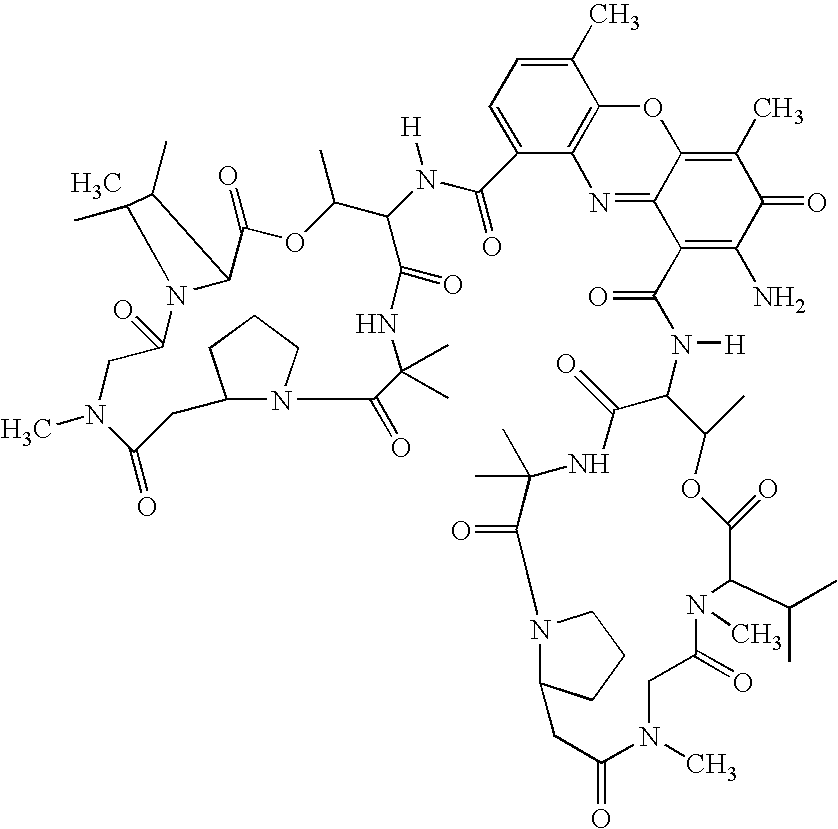
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:UNIV ZURICH +1
Coated implantable medical device
InactiveUS7550005B2Prevent degradationIncrease release rateStentsHeart valvesParylenePlasma deposition
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Rate-reducing membrane for release of an agent
InactiveUS7014913B2Reduces rate of releaseLow release rateGlovesSurgeryBiomedical engineeringMedical treatment
A membrane that reduces the rate at which a therapeutic substance is released from an implantable medical device, such as a stent, is disclosed.
Owner:ABBOTT CARDIOVASCULAR
40-O-(2-hydroxy)ethyl-rapamycin coated stent
A method and coating for reducing the release rate of an active agent from an implantable device, such as a stent, is disclosed.
Owner:ABBOTT CARDIOVASCULAR
Coated implantable medical device
InactiveUS20060195176A1Prevent degradationIncrease release rateHeart valvesSurgeryParylenePorous layer
Bioactive-coated medical devices are provided, including coated vascular stents. The medical device coating can include a coating layer posited over at least a portion of the medical device surface, and can include a butyl methacrylate polymer or an ethylene-vinyl acetate copolymer in combination with a bioactive material that can function as both an immunosuppressive agent and an antiproliferative agent. Optionally, multilayer coatings can further include an adhesion promoting layer comprising parylene positioned between the coating layer and the medical device surface, a porous layer comprising butyl methacrylate positioned over at least a portion of the coating layer, or both. The coating layer preferably comprises between about 0.5 and 2.0 μg / mm2 of the bioactive material on the outer surface of the medical device. The bioactive material can be absorbed into the coating layer, which can have a thickness of between about 0.5 μm to about 5 μm.
Owner:COOK MEDICAL TECH LLC
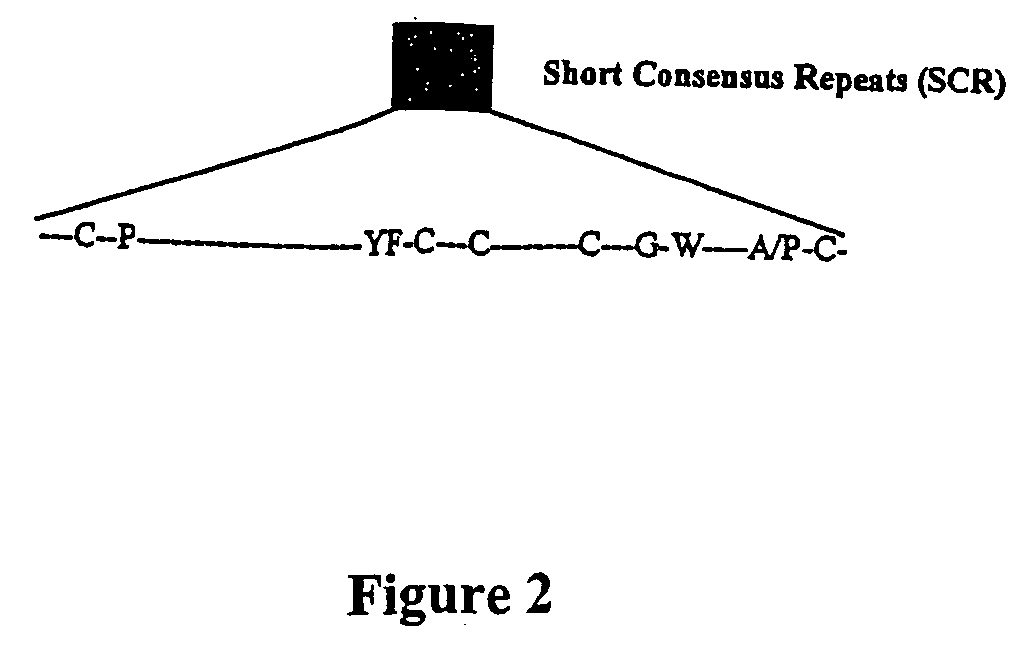
Viral complement control proteins for eye disorders
InactiveUS20060142191A1Inhibit expressionImprove in vivo stabilityBiocideSenses disorderDiseaseCOMPLEMENT REGULATORS
The present invention provides compositions and methods for treating and / or preventing age related macular degeneration and other conditions involving macular degeneration or choroidal neovascularization. Certain of the compositions comprise a poxvirus complement control protein or a complement binding fragment or variant thereof. Other compositions comprise a poxvirus complement control protein linked to a moiety that binds to a component present on or at the surface of cell or noncellular molecular entity, e.g., a component present in the eye of a subject at risk of or suffering from age related macular degeneration or a related condition or choroidal neovascularization. Certain of the methods comprise administering a poxvirus complement control protein or complement binding fragment or variant thereof to a subject.
Owner:POTENTIA PHARMA INC
Coated implantable medical device
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
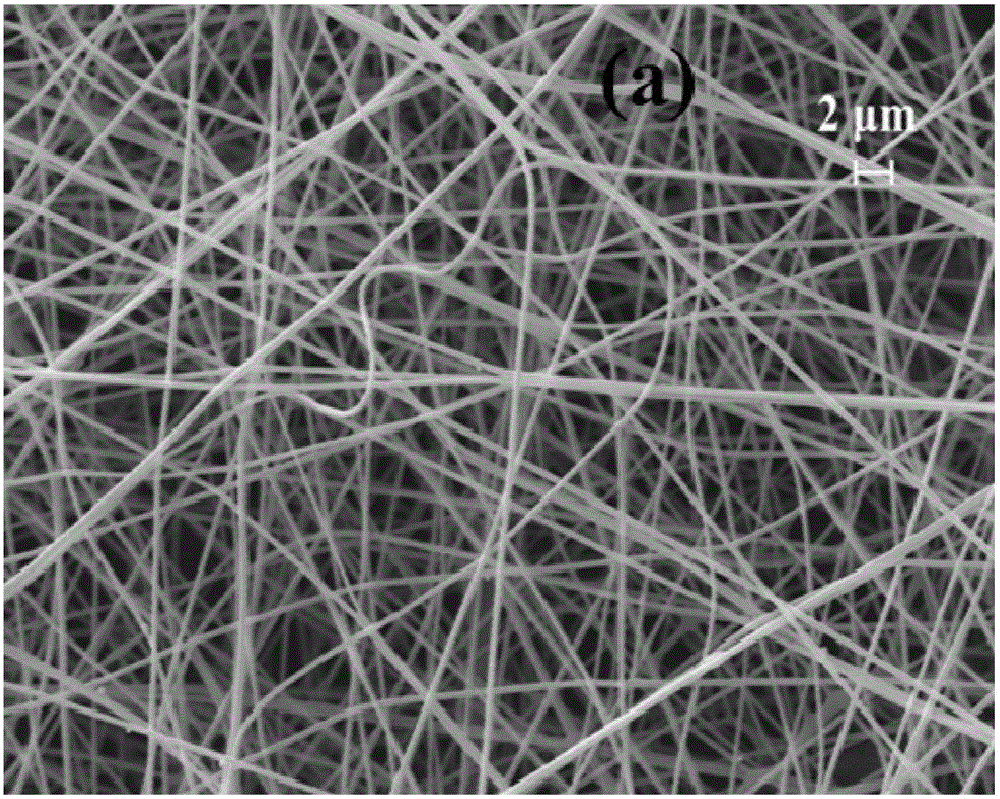
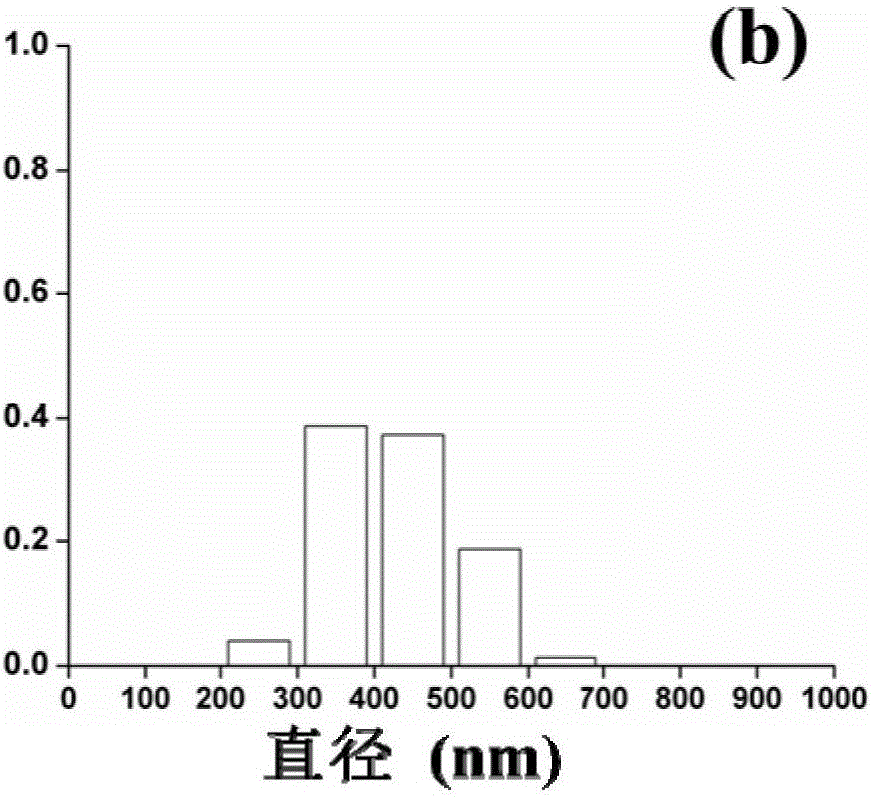
Kernel-shell structural nanofiber membrane, preparation method thereof and application
ActiveCN106702597AIncrease release rateLow release ratePeptide/protein ingredientsPharmaceutical delivery mechanismChitosan nanoparticlesPolyvinyl alcohol
The invention discloses a kernel-shell structural nanofiber membrane, a preparation method thereof and an application; the preparation method includes steps of (1), preparation of spinning; dissolving sodium alginate, polyoxyethylene and poloxamer F127 in distilled water, stirring evenly and acquiring shell layer spinning solution; mixing the chitosan nanoparticle solution loaded with active matters with polyving akohol solution evenly, and acquiring the kernel layer spinning solution; (2), coaxial electrostatic spinning: respectively injecting the shell layer spinning fluid and kernel layer spinning fluid in a static spinning device containing a coaxial needle, performing the coaxial electrostatic spinning under room temperature, performing solvent evaporation in the spinning process, and acquiring the kernel-shell structural nanofiber membrane loaded with the nanoparticle. The preparation method is simple in operation and gentle in reaction condition and can well control the slow release of the active matter; moreover, the prepared colon targeting controlled release system has obvious colon targeting property, thereby improving the utilization degree of the active matter. The colon targeting controlled release system can be applied to functional food domain.
Owner:SOUTH CHINA UNIV OF TECH
40-O-(2-hydroxy)ethyl-rapamycin coated stent
A method and coating for reducing the release rate of an active agent from an implantable device, such as a stent, is disclosed.
Owner:ABBOTT CARDIOVASCULAR
Viral Complement Control Proteins for Eye Disorders
InactiveUS20080075755A1Improve in vivo stabilityLow failure rateBiocideSenses disorderComplement control proteinOcular inflammation
The present invention provides compositions and methods for treating and / or preventing age related macular degeneration and other conditions involving macular degeneration or choroidal neovascularization, ocular inflammation, or any combination of these. Certain of the compositions comprise a poxvirus complement control protein or a complement binding fragment or variant thereof. Other compositions comprise a poxvirus complement control protein linked to a moiety that binds to a component present on or at the surface of cell or noncellular molecular entity, e.g., a component present in the eye of a subject at risk of or suffering from age related macular degeneration or a related condition or choroidal neovascularization, ocular inflammation, or any combination of these. Certain of the methods comprise administering a poxvirus complement control protein or complement binding fragment or variant thereof to a subject.
Owner:POTENTIA PHARMA INC
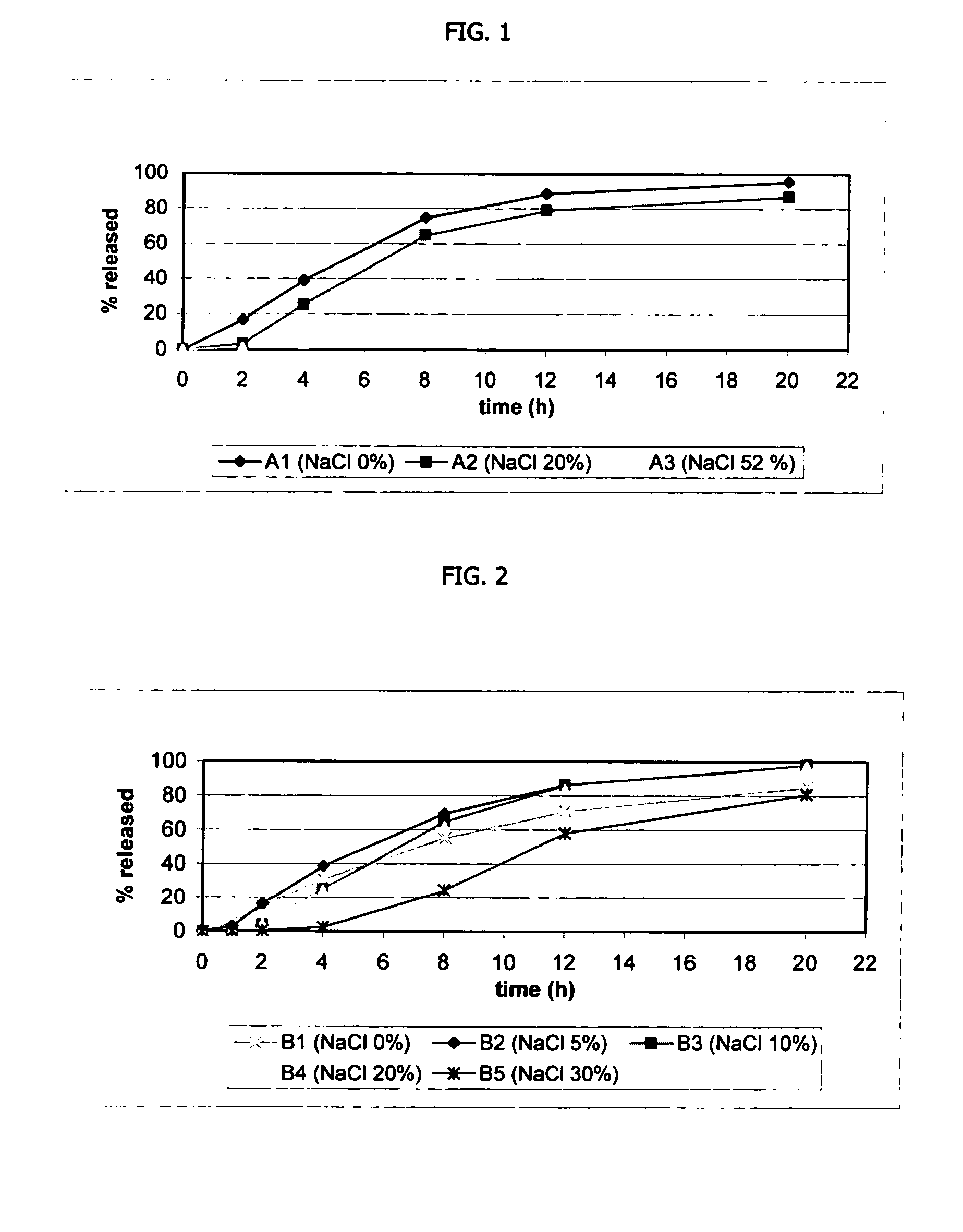
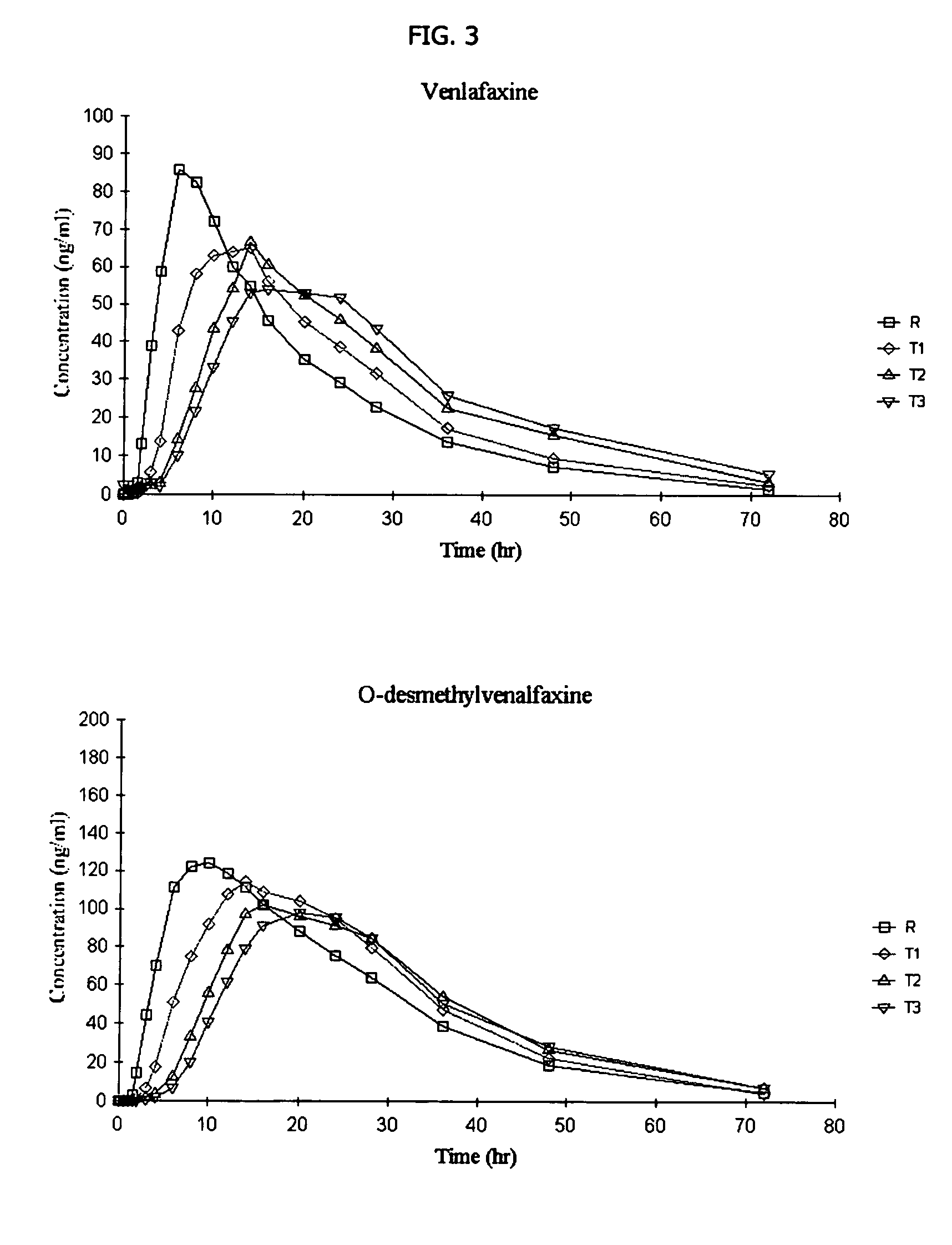
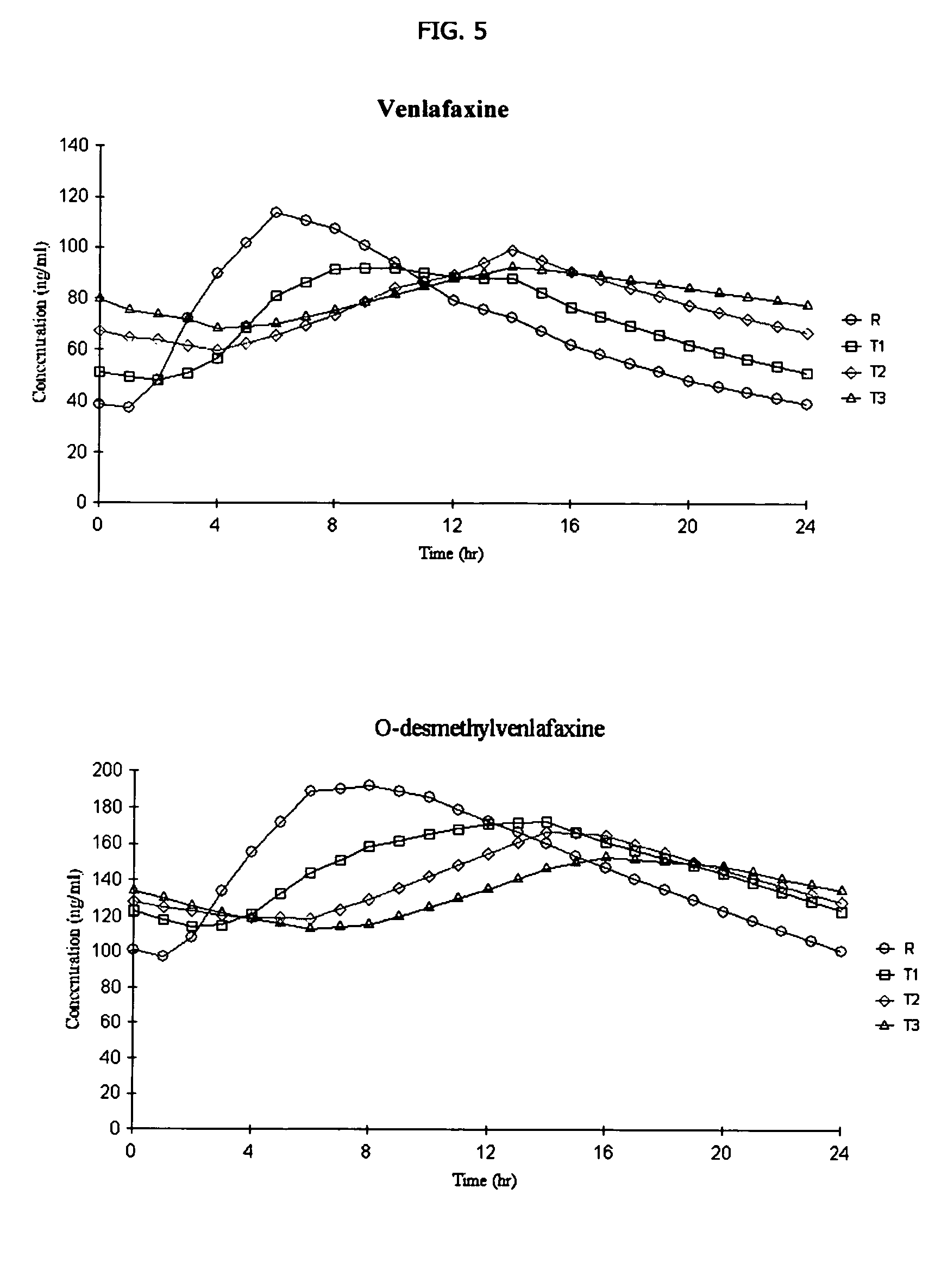
Osmotic device containing a venlafaxine salt and a salt having an ion in common
The osmotic devices of the present invention include a single core comprising a salt of a drug and an osmotic salt, wherein the drug salt and the osmotic salt have a common ion. The release rate of the active drug is reduced, and the release profile of the active drug is modified, from a first order release profile to a zero order, pseudo-zero order, or sigmoidal release profile, by increasing the amount of the sodium chloride in the core of the device. In one embodiment the sodium chloride is used to modify a controlled release profile to a delayed and controlled release profile.
Owner:ACELLA HLDG LLC +1
Pharmaceutical compositions and methods for fabrication of solid masses comprising polypeptides and/or proteins
ActiveUS20170051051A1Increase productionImprove biological activityPowder deliveryPeptide/protein ingredientsIntestinal wallsMedicine
Embodiments of the invention provide shaped masses (SM) comprising one or more drugs such as proteins or polypeptides and methods for forming and delivering such SM's. One embodiment provides a SM comprising a drug e.g., a protein or polypeptide having a biological activity in the body of a mammal. The SM is formed by compression of a precursor material (PM) comprising the drug wherein an amount of biologically active drug in the SM is a minimum level to that in the PM. Drugs which may be incorporated into the SM include insulin, incretins and immunoglobulins e.g., interleukin neutralizing antibodies or TNF-α-inhibiting antibodies. Embodiments of the invention are particularly useful for the oral delivery of drugs which would be degraded within the GI tract, wherein the SM containing the drug is formed as or incorporated into a tissue penetrating member which is inserted into the intestinal wall after oral ingestion.
Owner:RANI THERAPEUTICS
Polymeric coating for reducing the rate of release of a therapeutic substance from a stent
A stent for delivery of a therapeutic agent is disclosed. The stent includes a polymer coating for reducing the rate of release of the therapeutic agent. The polymer has a crystalline structure wherein the polymer is capable of significantly maintaining the crystalline lattice structure while the therapeutic agent is released from the stent such that the aqueous environment to which the stent is exposed subsequent to the implantation of the stent does not significantly convert the crystalline lattice structure of the polymer to an amorphous structure.
Owner:ABBOTT CARDIOVASCULAR
Compositions, Systems and Methods for Releasing Additive Components
InactiveUS20130255951A1Low viscosityReduce releaseLiquid surface applicatorsFluid removalHydraulic fracturingAqueous medium
Compositions, systems and methods for the controlled and / or delayed release of chemical additive components into an aqueous fluid used in hydraulic fracturing of oil and / or gas wells. The chemical additive components may include a viscosity-reducing composition, an oxidizer composition, a pH modulating composition, a lubricant composition, a cross-linking composition, an anti-corrosion composition, an biocide composition, a crosslink-enhancing composition, and / or a combination of two or more of these compositions. Further embodiments include additives and methods of delivering a particle comprising an additive component to a desired site in an aqueous medium prior to release of the additive component into the aqueous medium. The coating is permeable, but insoluble in an aqueous medium, whereupon the additive components are released into the medium.
Owner:DOBER CHEMICAL CORPORATION
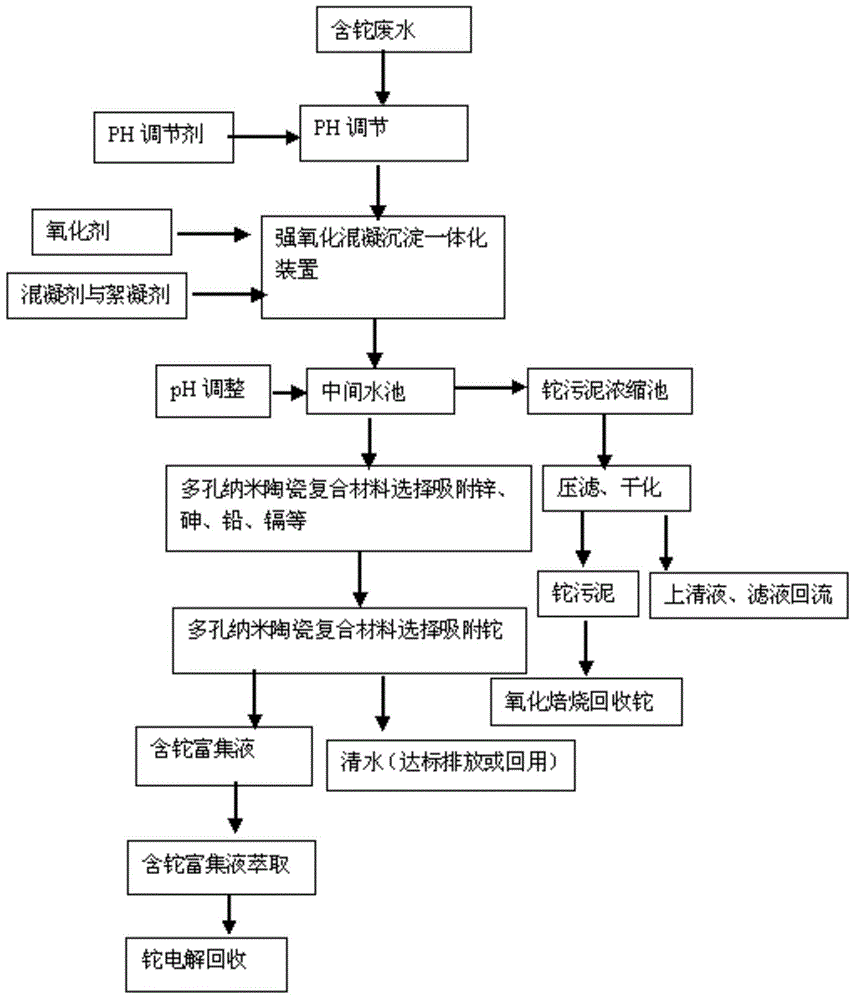
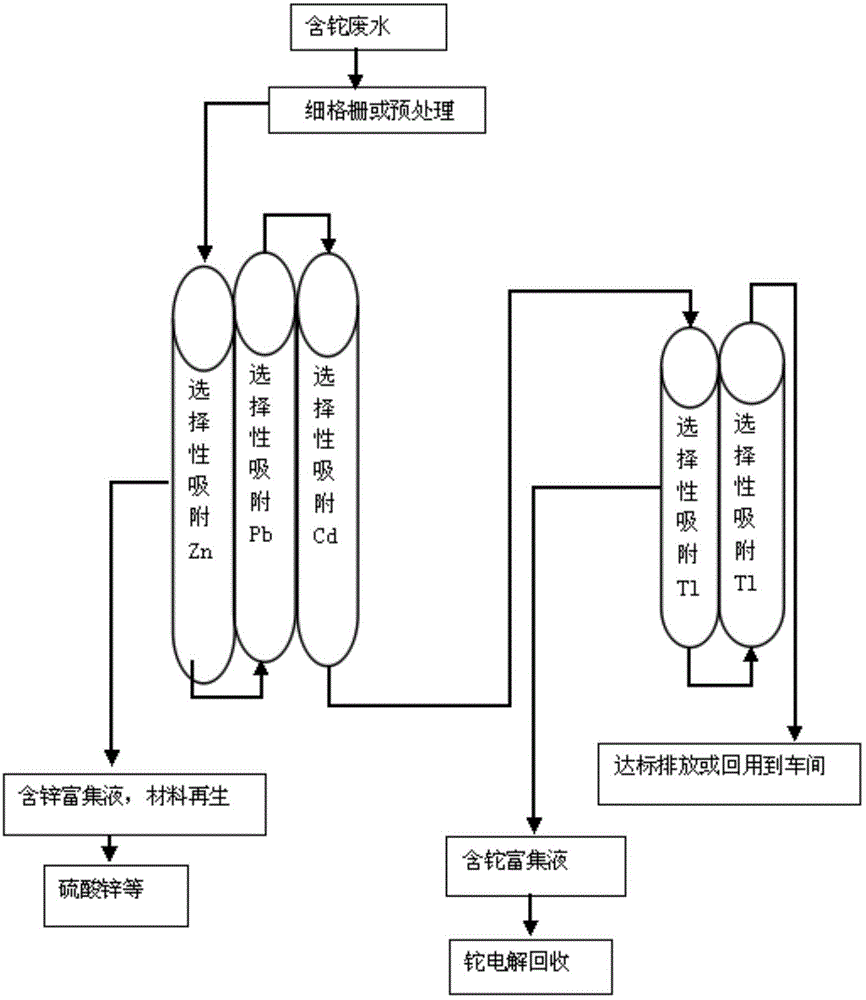
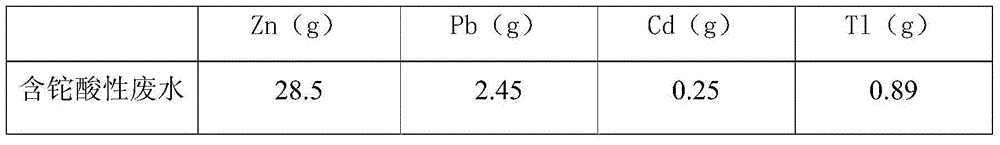
Thallium-containing wastewater strongly oxidizing, coagulating, adsorbing and recovering process
ActiveCN104310672AHighly selective adsorptionLarge specific surface areaWater treatment parameter controlSludge treatmentFiltrationSludge
The invention provides a thallium-containing wastewater strongly oxidizing, coagulating, adsorbing and recovering process. The process includes the following procedures: thallium-containing wastewater concentration, pH regulation, strong oxidization, coagulation, flocculation, precipitated sludge treatment, pH regulation, solid impurity filtration, removal of Zn, Pb, Cd and Tl, and the like. The process has the beneficial effects that the process has the advantages of advanced technology, maturity, good effluent quality, stability in operation, conciseness in process, strong practicability, easiness in start and stop, convenience in maintenance and management, small investment, low operating cost, small floor area for construction, short construction period and large application ranges of projects; treatment of heavy metal ion polluted sewage is not limited by temperatures; the limitation that a biological method can not be used in cold regions in the north can be overcome.
Owner:HUNAN JINGYUAN ENVIRONMENTAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com