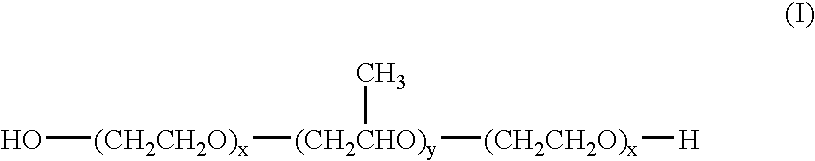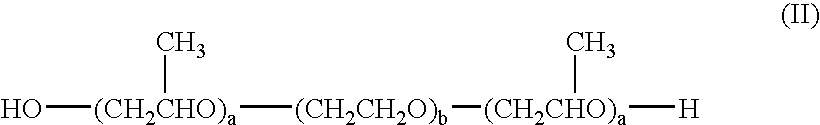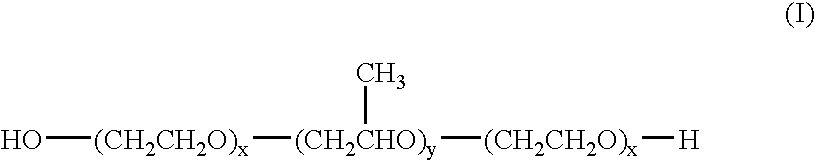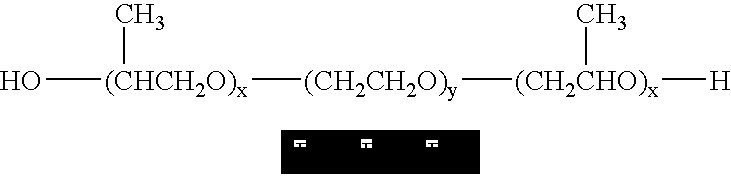Patents
Literature
1721 results about "Poloxamer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
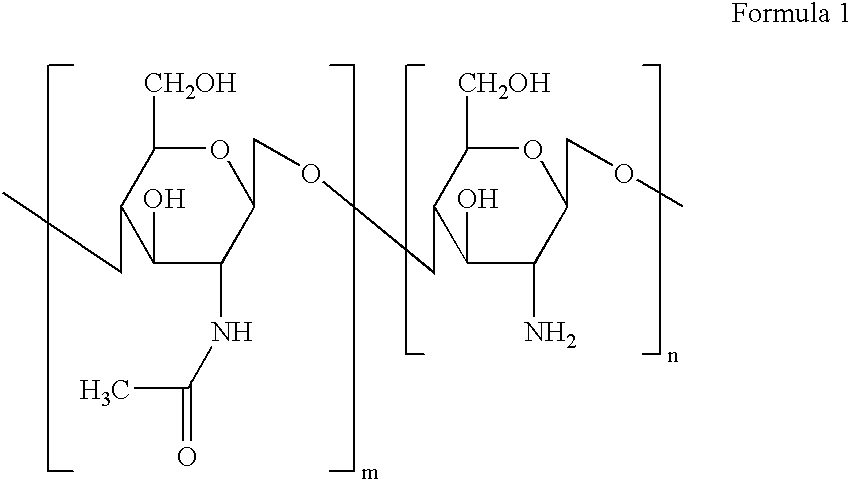
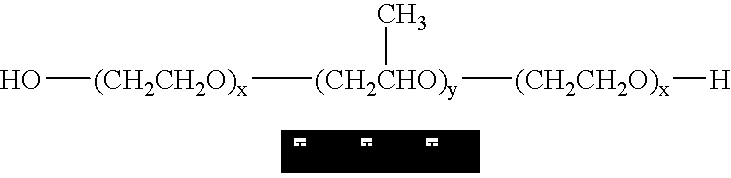
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene (poly(propylene oxide)) flanked by two hydrophilic chains of polyoxyethylene (poly(ethylene oxide)). The word poloxamer was coined by the inventor, Irving Schmolka, who received the patent for these materials in 1973. Poloxamers are also known by the trade names Synperonics, Pluronics, and Kolliphor.
Antimicrobial Compositions and Methods for Locking Catheters
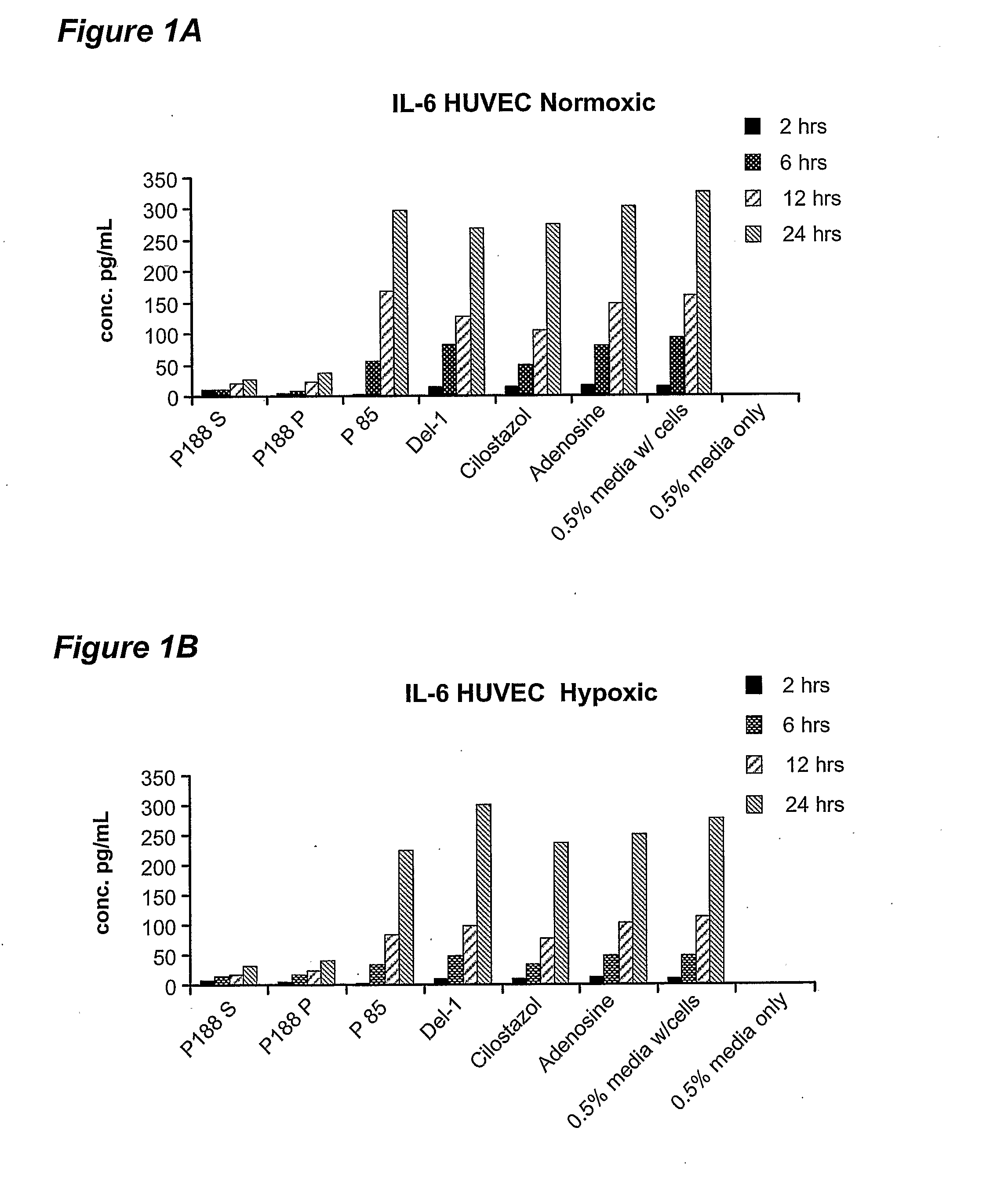
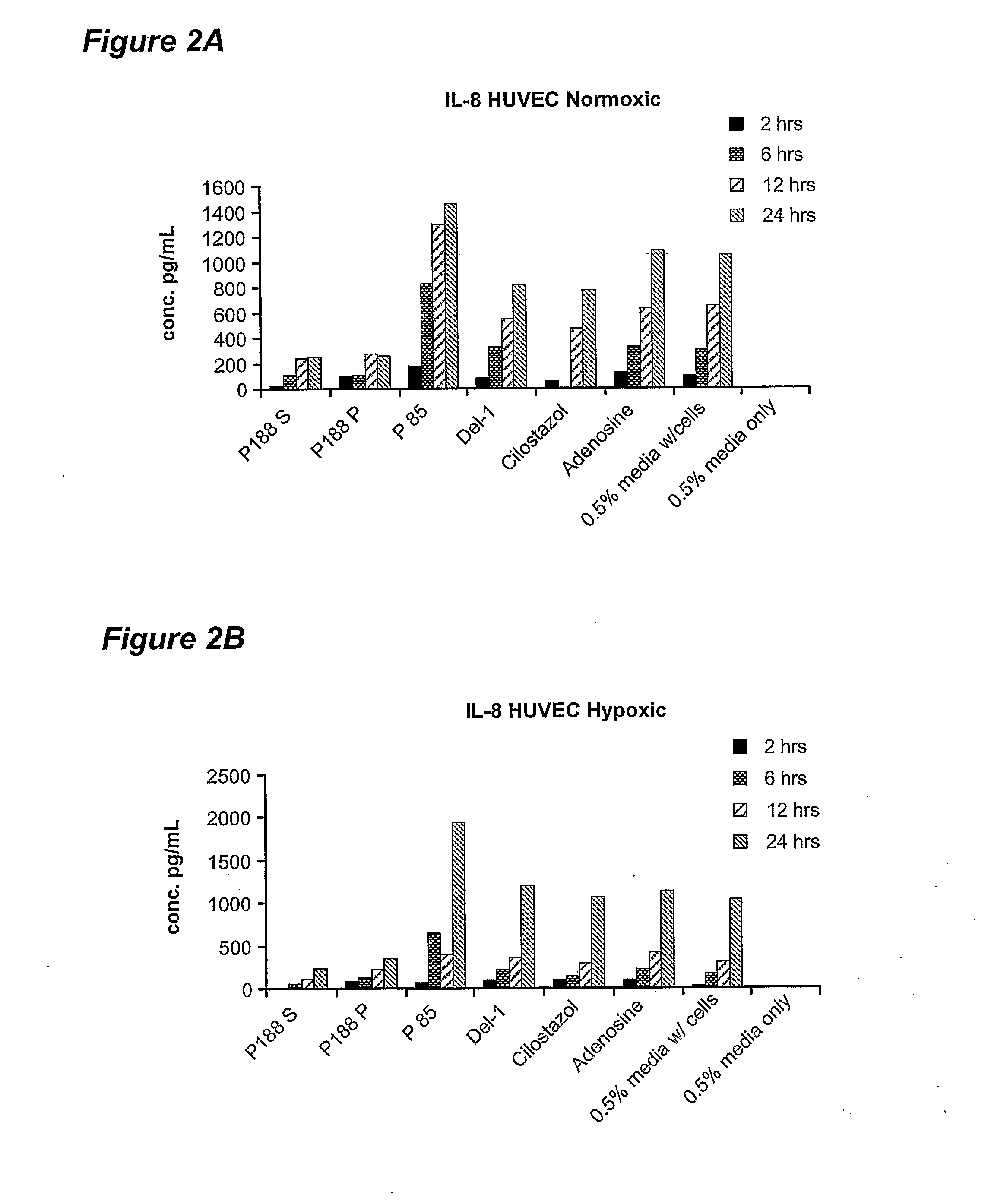
Antimicrobial compositions for use in locking catheters and other devices are provided. In some embodiments, the composition includes at least one alcohol, at least one biocidal agent which is not an alcohol, and one or more poloxamers; in other embodiments, the composition comprises at least one poloxamer and at least one alcohol. The composition can provide long-lasting antimicrobial activity. Methods of using the composition are also provided.
Owner:BECTON DICKINSON & CO
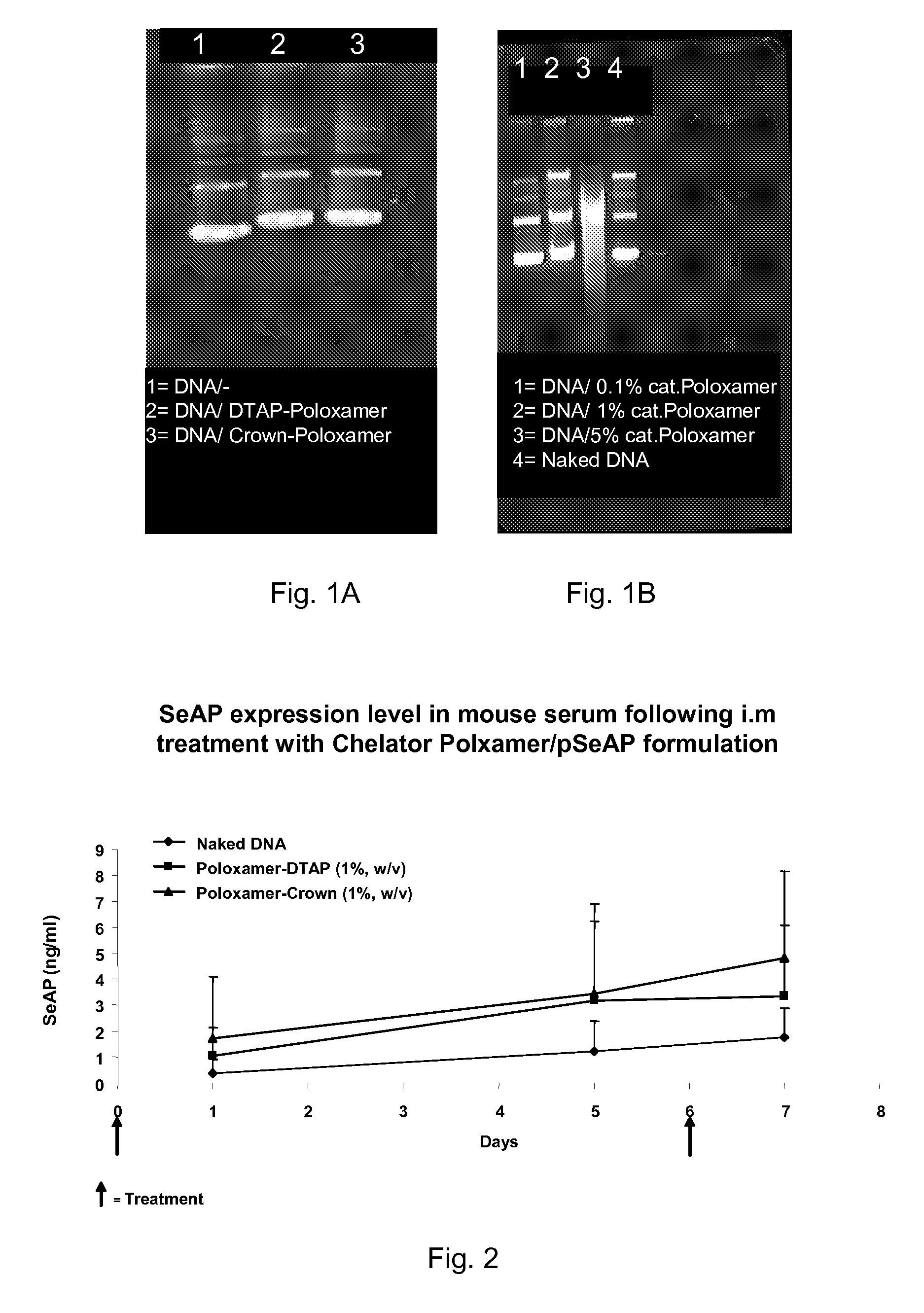
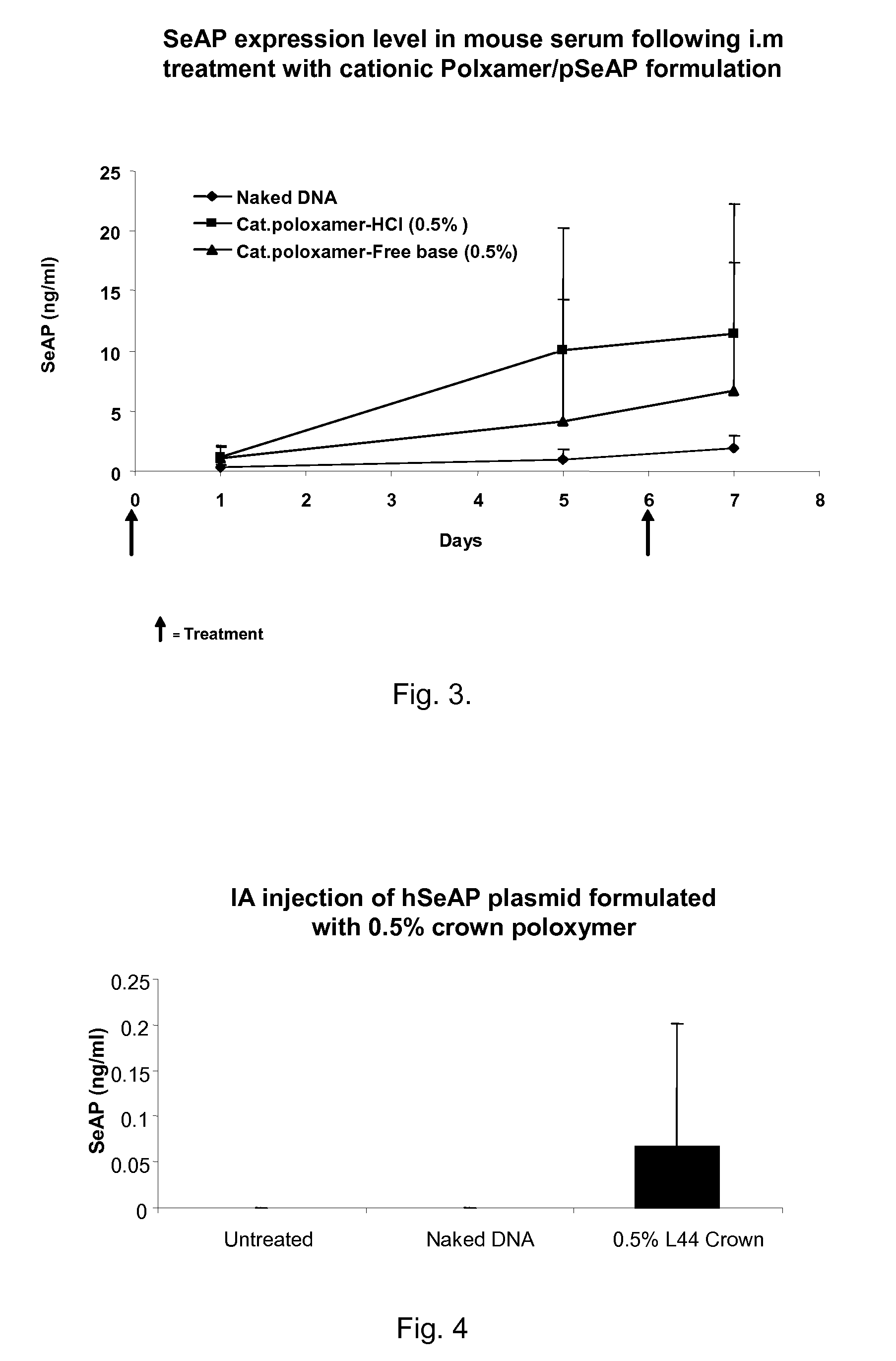
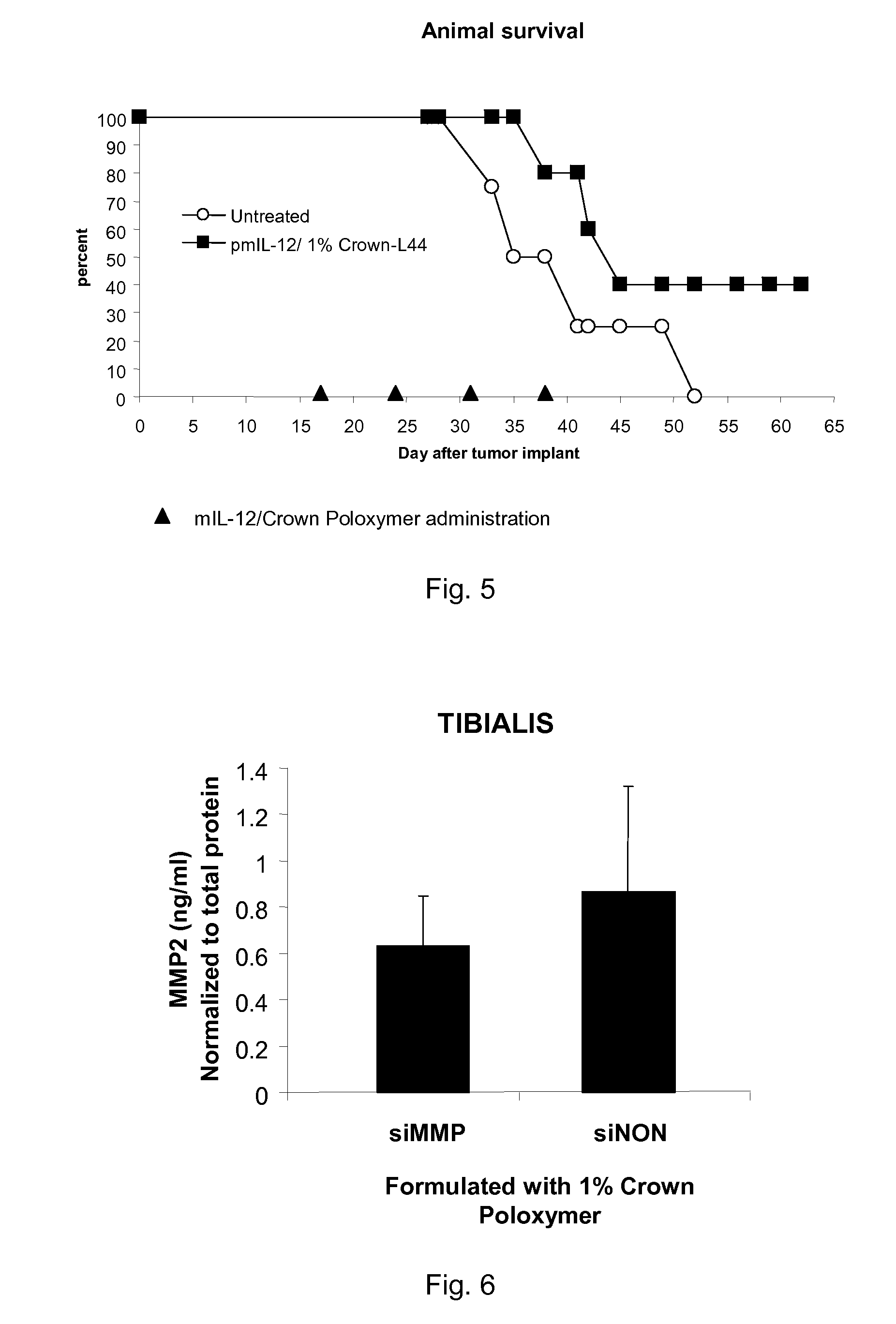
Modified Poloxamers for Gene Expression and Associated Methods
InactiveUS20100004313A1Inhibit expressionReduce deliveryGenetic material ingredientsPharmaceutical delivery mechanismGene deliverySolid tissue
Nucleotide delivery polymers, compositions, and associated methods for the enhancement of gene delivery and expression in solid tissues are provided. In one aspect, for example, a nucleotide delivery polymer may include a poloxamer backbone having a metal chelator covalently coupled to at least one terminal end of the poloxamer backbone. In another aspect, the nucleotide expression polymer has a metal chelator coupled to at least two terminal ends of the poloxamer backbone.
Owner:CLSN LAB
Suspension formulations of nepafenac and other ophthalmic drugs for topical treatment of ophthalmic disorders
Topical aqueous suspension compositions of sparingly soluble ophthalmic drugs are disclosed. The compositions comprise a combination of a poloxamer or meroxapol surfactant and a glycol tonicity-adjusting agent such as propylene glycol.
Owner:NOVARTIS AG
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
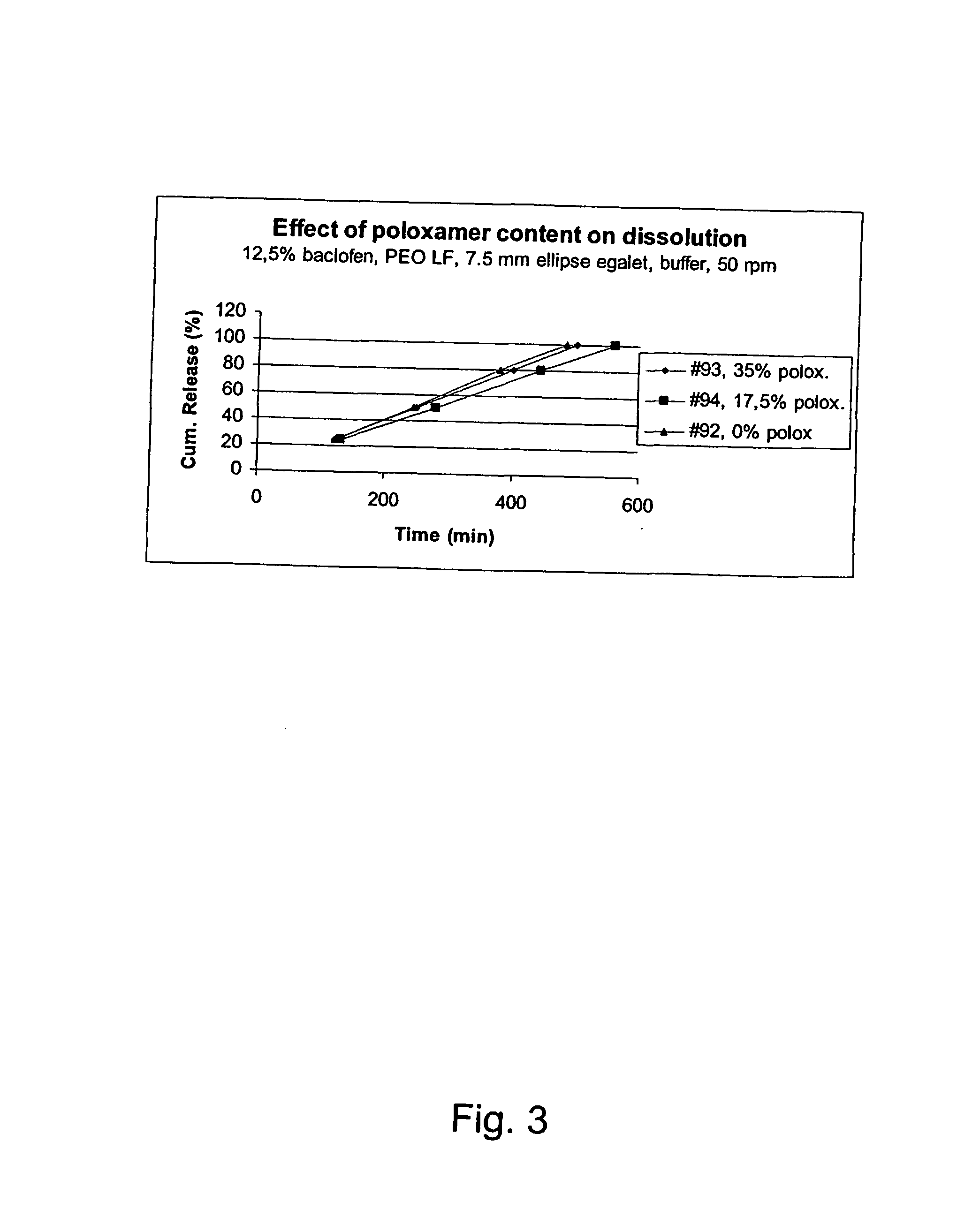
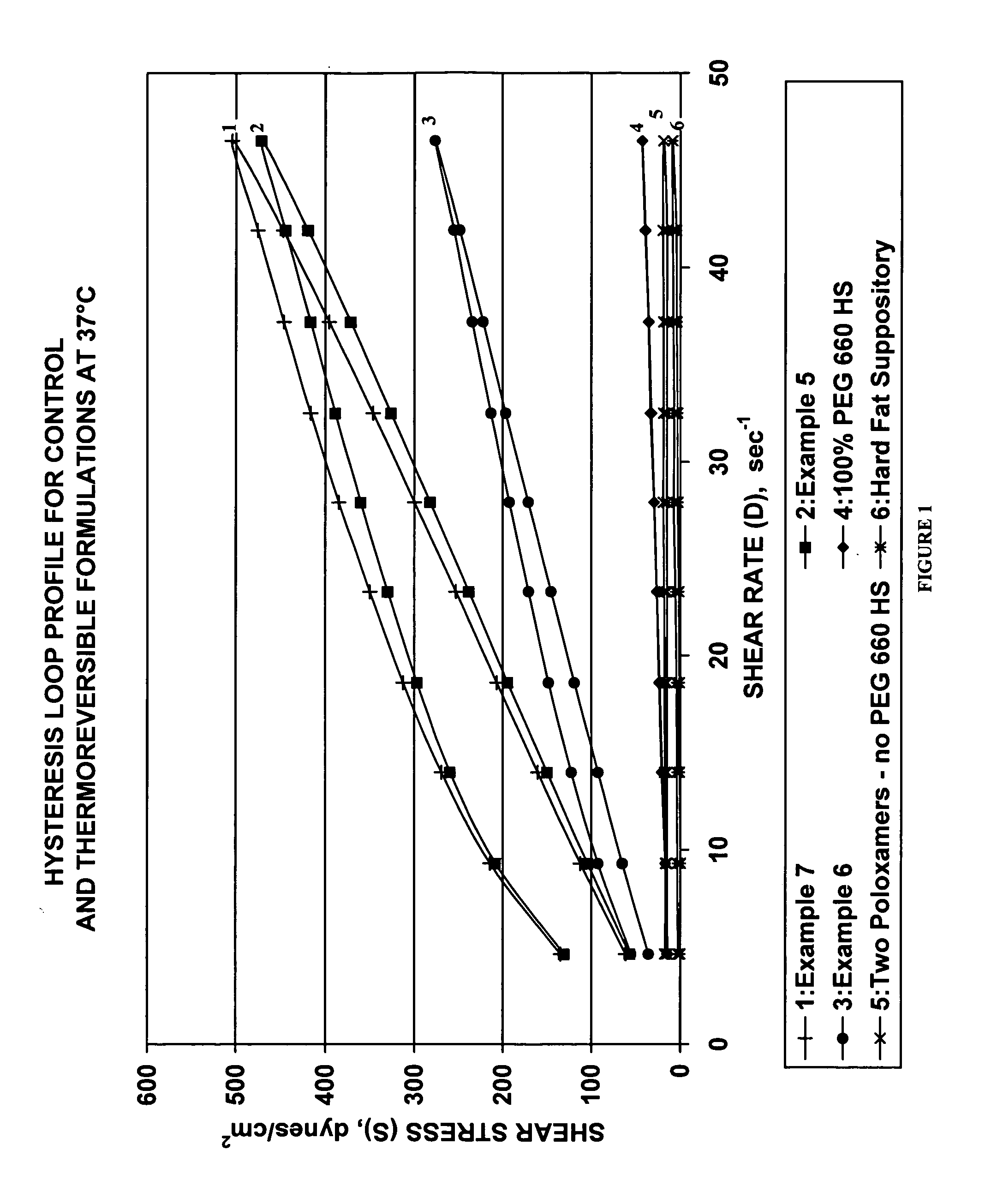
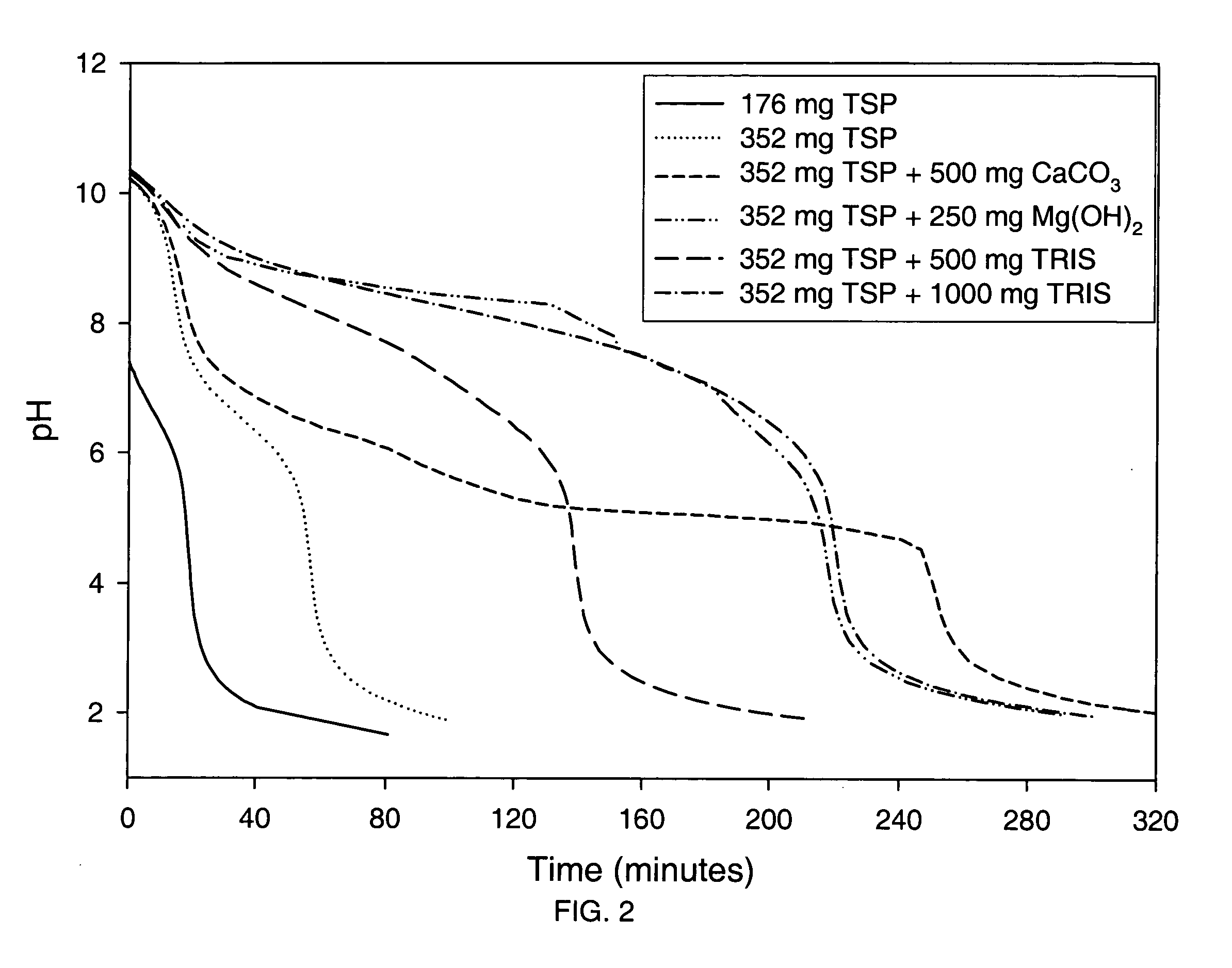
Thermoreversible pharmaceutical formulation for anti-microbial agents comprising poloxamer polymers and hydroxy fatty acid ester of polyethylene glycol
The present invention provides a pharmaceutical formulation having thermoreversible properties, comprising: a) an anti-microbial agent; b) a poloxamer mixture containing at least two poloxamer polymers; and c) a hydroxy fatty acid ester of polyethylene glycol, wherein the formulation is a solid at room temperature and is a liquid-gel at body temperature. The thermoreversible pharmaceutical formulation has a viscosity of about 8,500 cP to about 400,000 cP at room temperature, and a viscosity of about 1,000 cP to about 8,000 cP at body temperature and exhibits a hysteresis loop behavior. The present invention further provides a process of preparing as well as a method of treating a microbial infection in a mammal using the same.
Owner:TARO PHARMA INDS
Azithromycin dosage forms with reduced side effects
ActiveUS6984403B2Reduce gastrointestinal side effectsAntibacterial agentsPowder deliveryOral suspensionsGLYCERYL MONOBEHENATE
The present invention is related to an oral dosage form comprising an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a glyceride which comprises glyceryl monobehenate, glyceryl dibehenate, glyceryl tribehenate, or a mixture thereof and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule.
Owner:PFIZER INC
Stabilized Hme Composition With Small Drug Particles
ActiveUS20080274194A1Facilitated releaseReduce releasePowder deliveryCyclic peptide ingredientsAntioxidantCarrier system
A hot-melt extruded composition having finely divided drug-containing particles dispersed within a polymeric and / or lipophyllic carrier matrix is provided. The carrier softens or melts during hot-melt extrusion but it does not dissolve the drug-containing particles during extrusion. As a result, a majority or at least 90% wt. of the drug-containing particles in the extrudate are deaggregated during extrusion into essentially primary crystalline and / or amorphous particles. PEO is a suitable carrier material for drugs insoluble in the solid state in this carrier. Various functional excipients can be included in the carrier system to stabilize the particle size and physical state of the drug substance in either a crystalline and / or amorphous state. The carrier system is comprised of at least one thermal binder, and may also contain various functional excipients, such as: super-disintegrants, antioxidants, surfactants, wetting agents, stabilizing agents, retardants, or similar functional excipients. A hydrophilic polymer, such as hydroxypropyl methylcellulose (HPMC E15), polyvinyl alcohol (PVA), or poloxamer, and / or a surfactant, such as sodium lauryl sulfate (SLS), can be included in the composition. A process for preparing the extrudate is conducted at a temperature approximating or above the softening or melting temperature of the matrix and below the point of solubilization of drug-containing particles in the carrier system, and below the recrystallization point in the case of amorphous fine drug particles.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Solid compositions of low-solubility drugs and poloxamers
InactiveUS20070141143A1Reduce solubilityImprove concentrationPill deliveryGranular deliverySolubilityPoloxamer
Solid compositions of low-solubility drugs and poloxamers that provide concentration enhancement when administered to an aqueous environment of use are disclosed.
Owner:BEND RES
Formulations and Methods for Treatment of Inflammatory Diseases
InactiveUS20070237740A1Half-life effectiveEffective presenceBronchoscopesSenses disorderVascular diseaseDisease cause
The present inventors have developed a novel composition and method for inhibiting inflammation and treating of symptoms of tissue ischemia, including that associated with peripheral and cardiac vascular disease by local administration of a pharmaceutical composition including an effective amount of a poloxamer.
Owner:VALENTIS INCORPORATED
Polymer blends that swell in an acidic environment and deswell in a basic environment
InactiveUS6537584B1Improve propertiesControlling the swelling and/or deswelling behaviorBiocidePowder deliveryChemical structurePolymer dissolution
A polymer blend is prepared by dissolving chitosan and a second polymer in an acidic aqueous solution to form an aqueous polymer blend, dehydrating said aqueous polymer blend, and recovering said polymer blend. The second polymer may be selected from the group consisting of polyether glycols including polyethylene glycols; cellulose esters including cellulose acetate; poloxamers; polysaccharides including dextran and guar; polyvinylpyrrolidones; polyvinyl alcohols; and mixtures or copolymers thereof. These polymer blends swell in an acidic environment and deswell in a more neutral or basic environment. This technology is valuable for the dispensing of biologically active material or drugs into a surrounding environment, especially the environment as is found in the gastrointestinal tract. Since the various polymer blends of the present invention are not covalently or ionically crosslinked, but are physically combined, each polymer in the physical blend maintains its original chemical structure, and therefore, is safe for oral administration.
Owner:BTG INT LTD
Recipe of breviscapine drop pills and preparing method
InactiveCN1408392AReduce the number of medicationsSmooth releaseUnknown materialsPill deliveryParaffin waxIce water
The breviscaping drop pill prepared via a solid dispersing process has the recipe comprising breviscapine 20-30 wt%, polyglycol-6000 40-60 wt%, stearic acid 10-30 wt% and poloxamer-188 5-25 wt%. The preparation process includes heating to smelt polyglycol-6000, adding stearic acid and poloxamer-188, adding breviscaping powder while stirring, transferring to dropping pipe while maintaining the temperature, dropping the molten into liquid paraffin in ice water bath to solidify into pill and washing out the liquid paraffin. The present invention has high biological utilization of medicine, delays the medicine release time and reduces medicine taking times.
Owner:HUAZHONG UNIV OF SCI & TECH +2
Hybrid hydrogel scaffold compositions and methods of use
ActiveUS20130115196A1Reduce usageReduce scarsBiocidePeptide/protein ingredientsHybrid materialMixed materials
The present invention includes new hybrid hydrogel scaffolds comprised of a polyoxyethylene-polyoxypropylene (block) copolymer (a “poloxamer”) and a self-assembling peptide, which maintain the mechanical and bioactive properties of its individual constituents (as compared to when the individual constituents are scaffolds or hydrogels by themselves). The hydrogels of the invention can include a combination of materials from different origins or with different properties that provides a hybrid material that meets the multiple needs of a scaffold for tissue engineering.
Owner:SAMSUNG DISPLAY CO LTD +1
Semi-synthetic platelet gel and method for the preparation thereof
A semi-synthetic platelet gel comprising a platelet-rich plasma, at least one platelet activator, and a biocompatible polymer selected from the group comprising carbomers, polyalkylene glycols, poloxamers, polyesters, polyethers, polyanhydrides, polyacrylates, polyvinyl acetates, polyvinyl pyrrolidones, polysaccharides, and derivatives thereof. A method for preparing a semi-synthetic platelet gel comprising the steps of (a) mixing a platelet-rich plasma with at least one platelet activator, and, before the start of clot formation, (b) adding the mixture thus obtained to a biocompatible polymer selected from the group comprising carbomers, polyalkylene glycols, poloxamers, polyesters, polyethers, polyanhydrides, polyacrylates, polyvinyl acetates, polyvinyl pyrrolidones, polysaccharides, and derivatives thereof.
Owner:LECTIO PHARMAENTWICKLUNGS UND VERW
Novel formulations which mitigate agitation-induced aggregation of immunogenic compositions
InactiveUS20130273098A1Stabilize immunogenic compositionInhibit aggregationAntibacterial agentsBiocideStreptococcus pneumoniaeCarrier protein
The present invention provides novel formulations which mitigate agitation-induced aggregation of immunogenic compositions particularly those having polysaccharide-protein conjugates. Specifically, the novel formulations comprise a poloxamer within a molecular weight range of 1100 to 17,400 which provides significant advantages over previously used surfactants including polysorbate 80. In one embodiment, the present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates and a poloxamer. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197.
Owner:MERCK SHARP & DOHME CORP
Topical Poloxamer Formulations for Enhancing Microvascular Flow: Compositions and Uses Thereof
InactiveUS20100183519A1Decreased blood flowIncrease blood flowAntibacterial agentsOrganic active ingredientsDiseaseDecreased blood flow
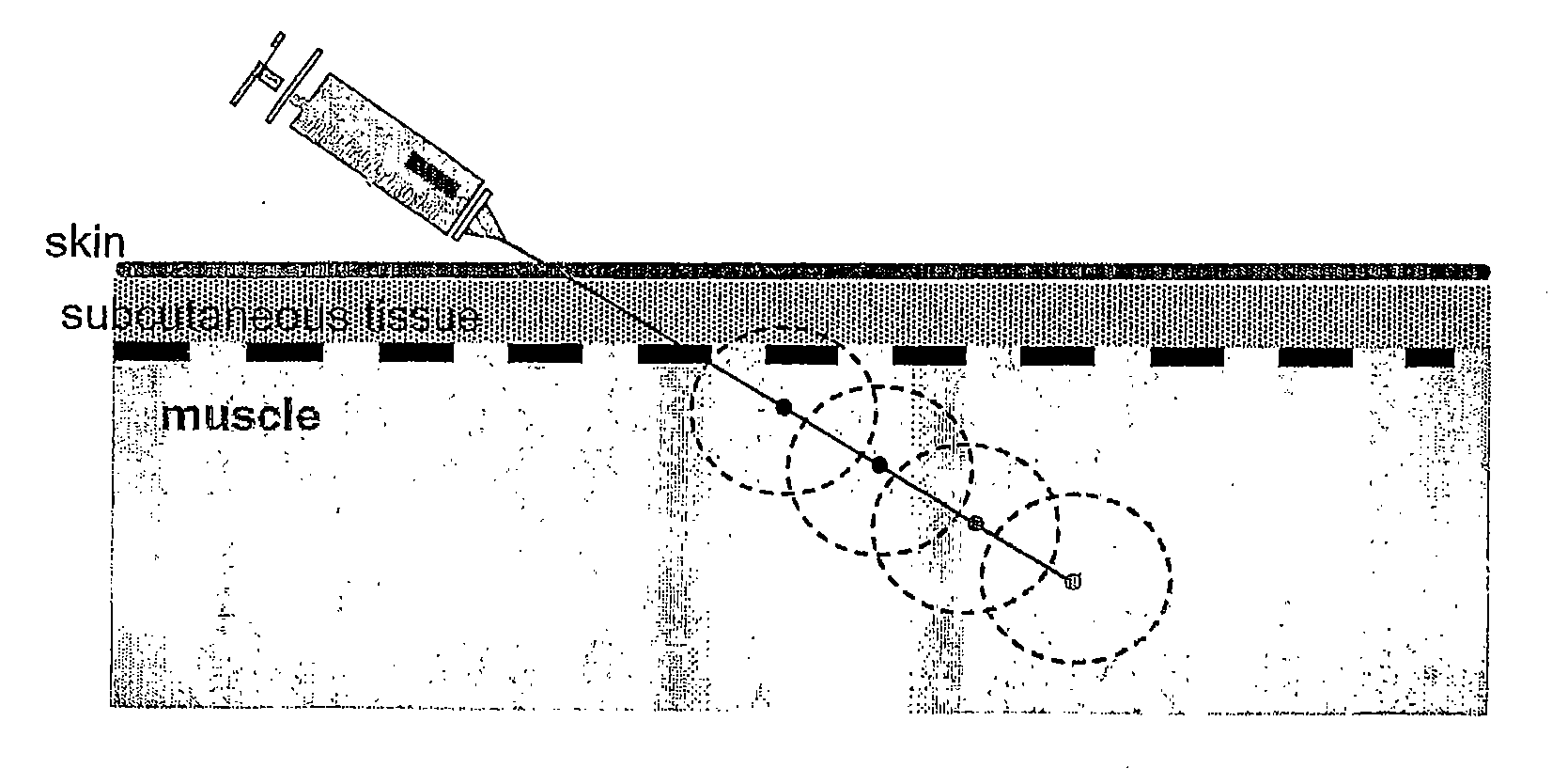
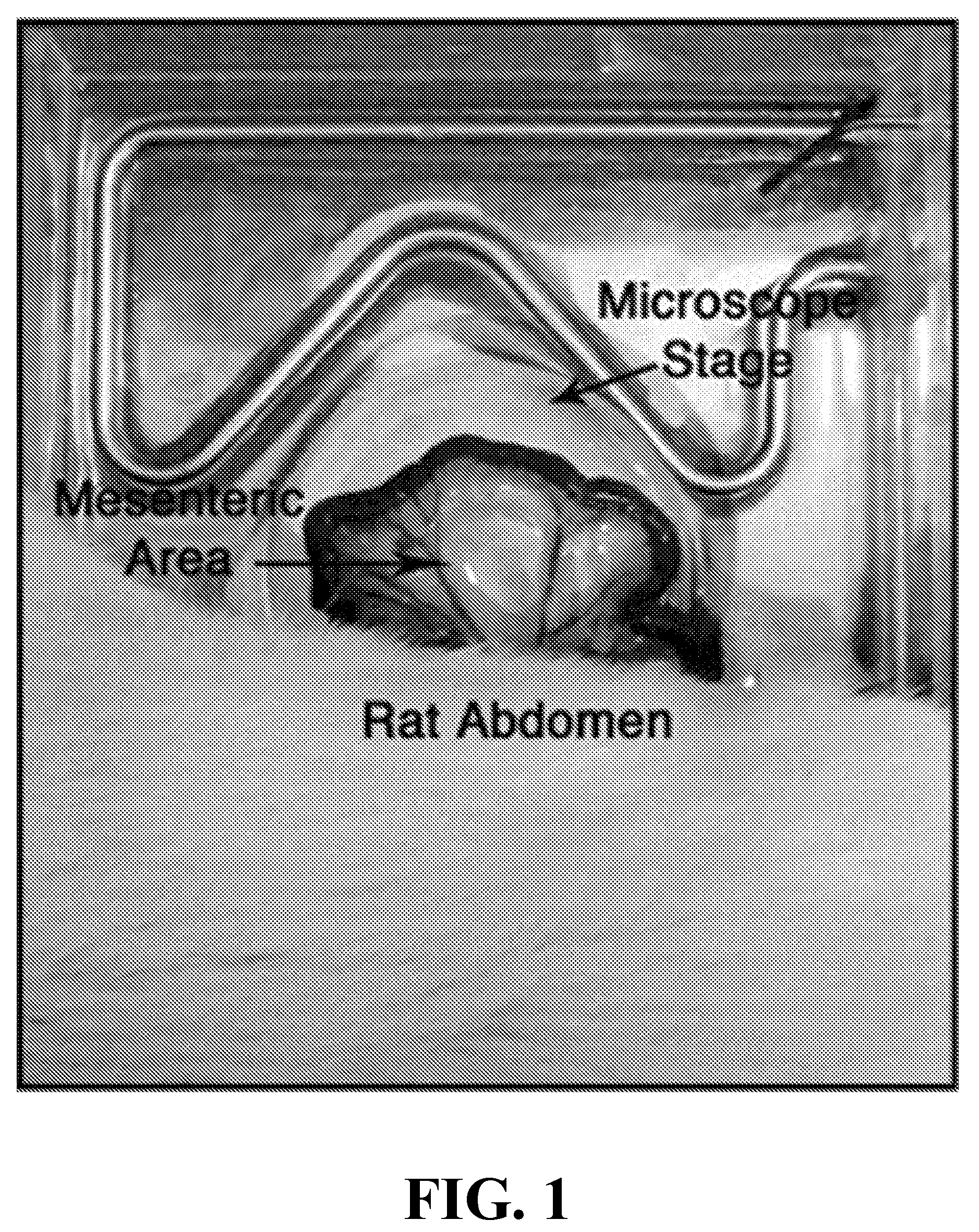
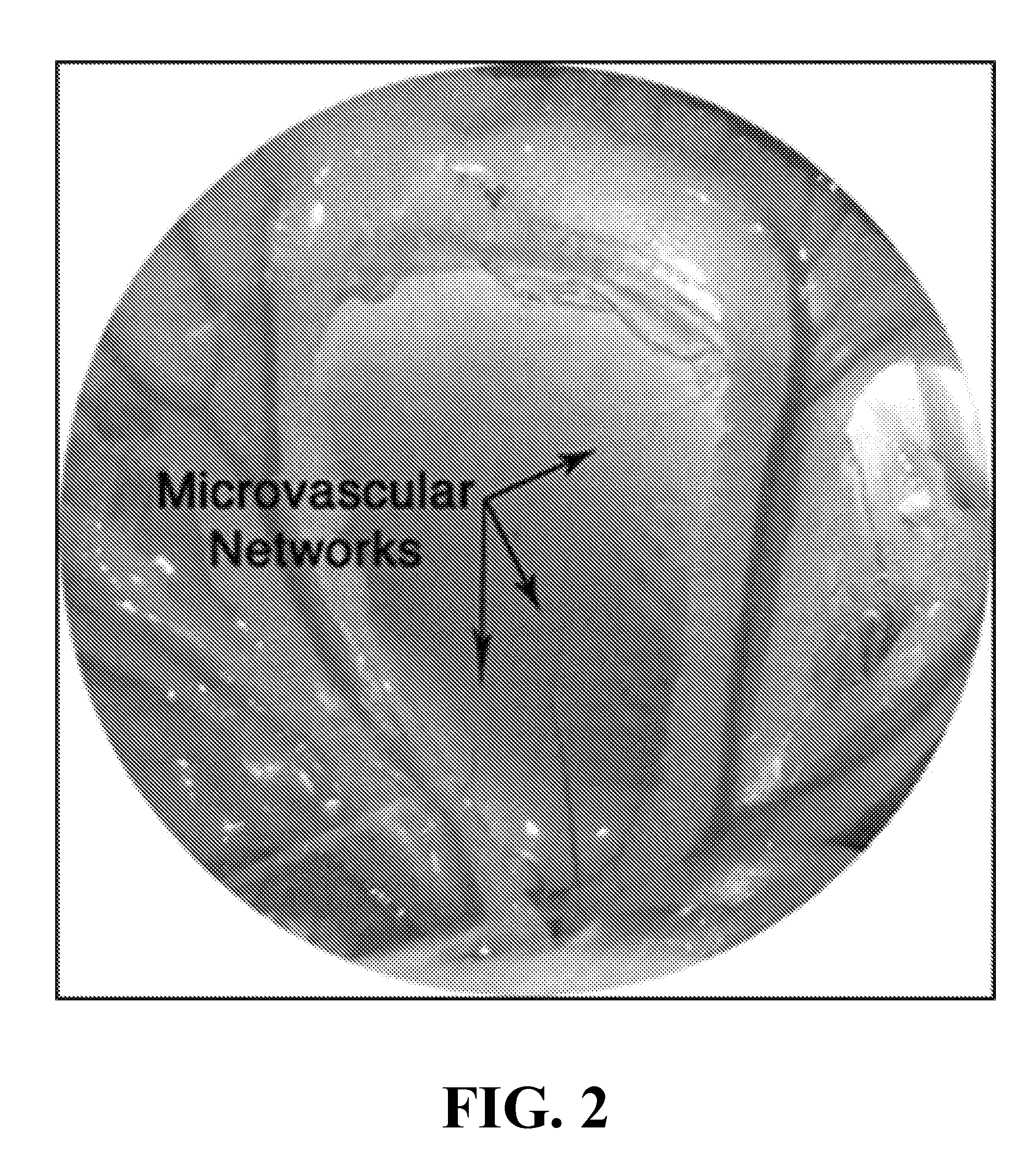
This invention relates to therapeutic compositions comprising a surface active copolymer, such as poloxamer-188, in an amount effective to enhance microvascular blood flow and / or inflammation in injured skin or other tissue, and methods of using the therapeutic compositions of the invention to inhibit decreased blood flow associated with an injury, disease, or disorder.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
Multiparticulate crystalline drug compositions having controlled release profiles
ActiveUS20050181062A1High drug loadingLow viscosityPowder deliveryBiocideControlled releaseCrystallization
A multiparticulate for controlled release of a drug comprises crystalline drug, a glyceride having at least one alkylate substituent of at least 16 carbon atoms, and a poloxamer, wherein at least 70 wt % of the drug in the multiparticulate is crystalline.
Owner:PFIZER INC
End modified thermal responsive hydrogels
InactiveUS7008628B2Improve flow characteristicsIncreasing the thicknessCosmetic preparationsImpression capsOligomerMedicine
A pharmaceutic composition includes a pharmaceutically acceptable carrier, comprising a reverse thermally viscosifying polymer. The polymer includes a linear block copolymer, wherein at least one block comprises a poloxamer; and at least one block comprises a biocompatible polymer or oligomer, in an aqueous medium. The composition also includes an active agent which imparts a pharmaceutic or cosmetic effect. The composition viscosifies in response to an environmental stimulus. The composition is suitable for administration of the pharmaceutical agent across dermal, otic, rectal, vaginal, ophthalmic, esophageal and nasal mucosal membranes.
Owner:MADASH
Novel dosage formulation
InactiveUS20070071813A1Sufficient availabilityPractice is disadvantageousBiocideNervous disorderImmediate releasePharmaceutical medicine
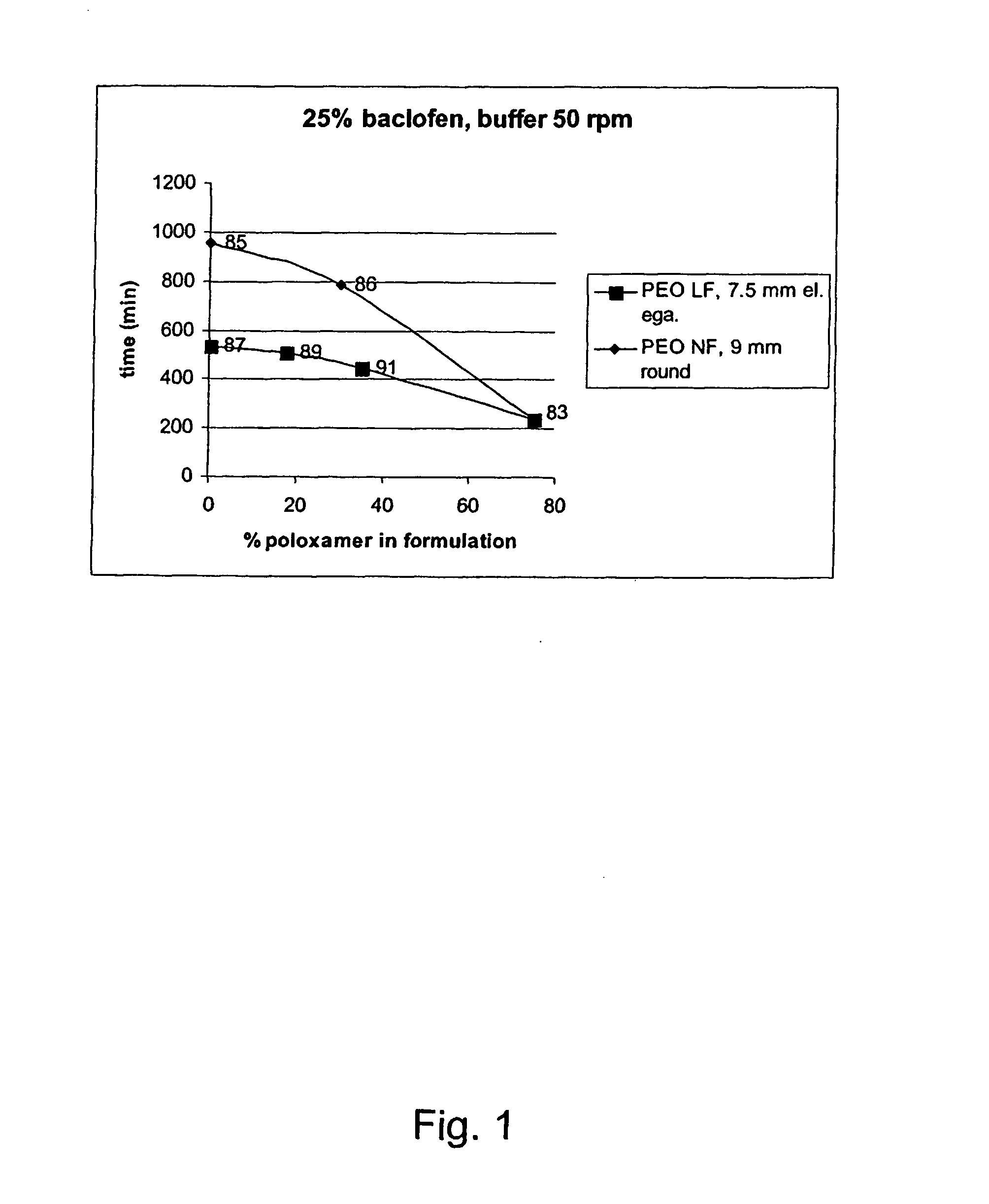
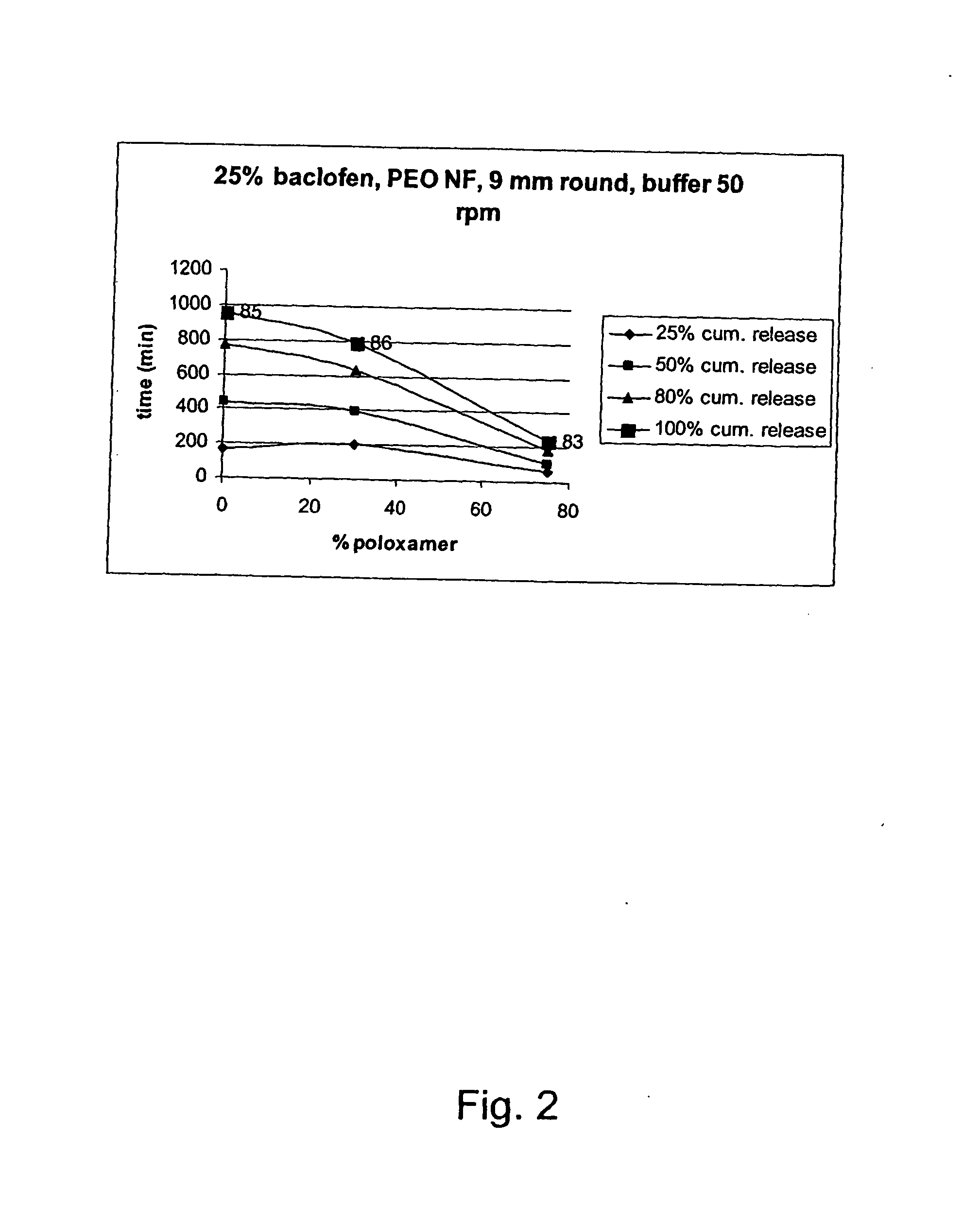
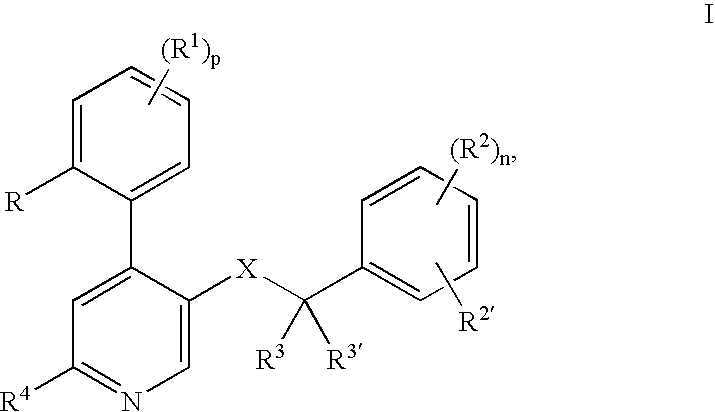
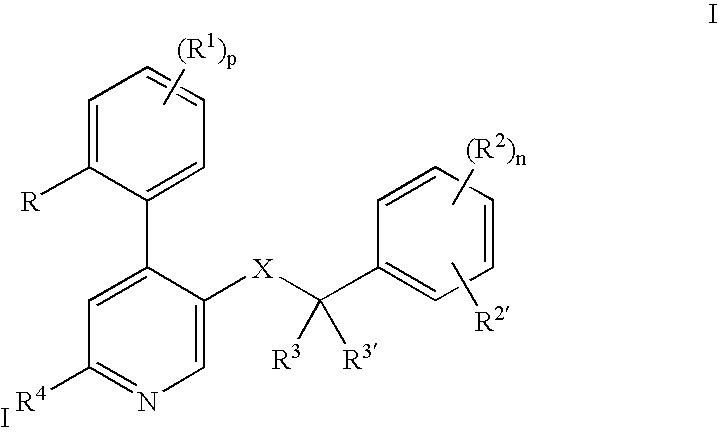
The invention relates to a process for preparing a pharmaceutical tablet composition which comprises an active pharmaceutical ingredient of formula I wherein the definitions are described in claim 1, or pharmaceutically acceptable acid addition salts thereof and a water soluble poloxamer in which the compound of formula I and the water soluble poloxamer are processed by hot melt extrusion, and then the hot melt extrudate is mixed with other ingredients to form a tablet, that is optionally coated with a composition comprising an immediate release film coating system and purified water. The invention also relates to such pharmaceutical compositions and hot melt extrudates.
Owner:AHMED HASHIM A +2
Sustained-release blood vessel embolic gel used for treating tumor, and preparation method thereof
ActiveCN102988274AEasy to injectNot easy to refluxPeptide/protein ingredientsDigestive systemEndovascular treatmentBlood vessel
The invention provides a sustained-release temperature-sensitive blood vessel embolic gel used for treating tumor. The sustained-release blood vessel embolic gel is prepared by entrapping a medicine by using a pharmaceutically acceptable carrier. The medicine is an anti-tumor medicine, and the pharmaceutically acceptable carrier comprises a gel prepared from poloxamer polymer, polyvinyl pyrrolidone, and the like or a composition thereof. The polymer material accounts for 5-65% of a gel mass. The particle size of the gel is in a range of 10nm to 150mum. The embolic agent is liquid gel under normal temperature, and can be used for direct injection through catheter. After injection into body, with the increase of temperature, the liquid gel is rapidly solidified into gel. Also, according to requirements, different medicines can be entrapped, and embolism and medication dual effect can be achieved through local sustained-release. Therefore, the gel provided by the invention can be used as an embolic agent for endovascular treatment, and can be used in various benign and malignant tumor transcatheter arterial chemoembolizations. The preparation method provided by the invention is simple, and is suitable for industrialized productions.
Owner:江苏申命医疗科技有限公司
Stabilized liquid protein formulations in pharmaceutical containers
ActiveUS20070092487A1Reduce surface tensionArea minimizationSmall article dispensingPeptide/protein ingredientsMedicineMethionine biosynthesis
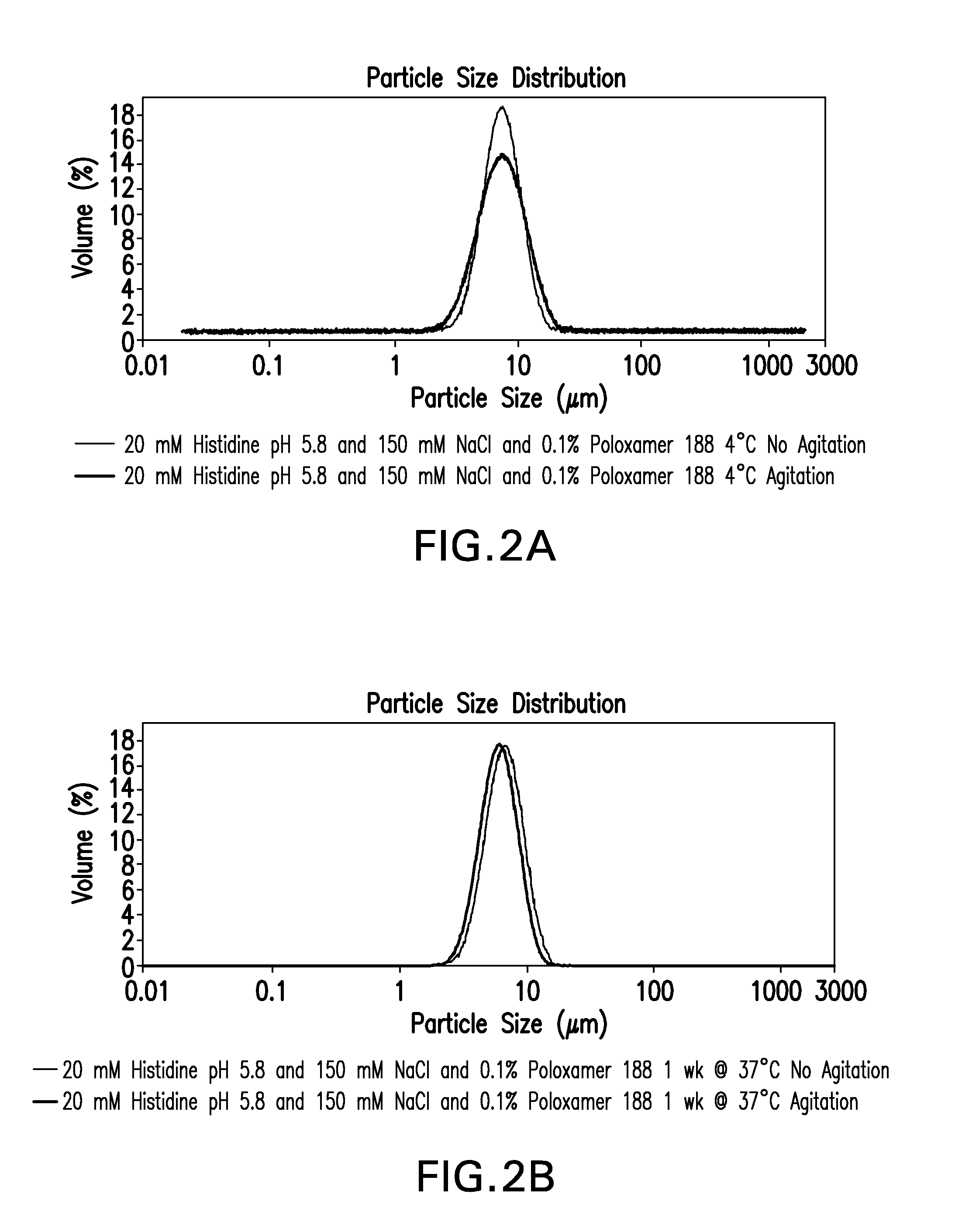
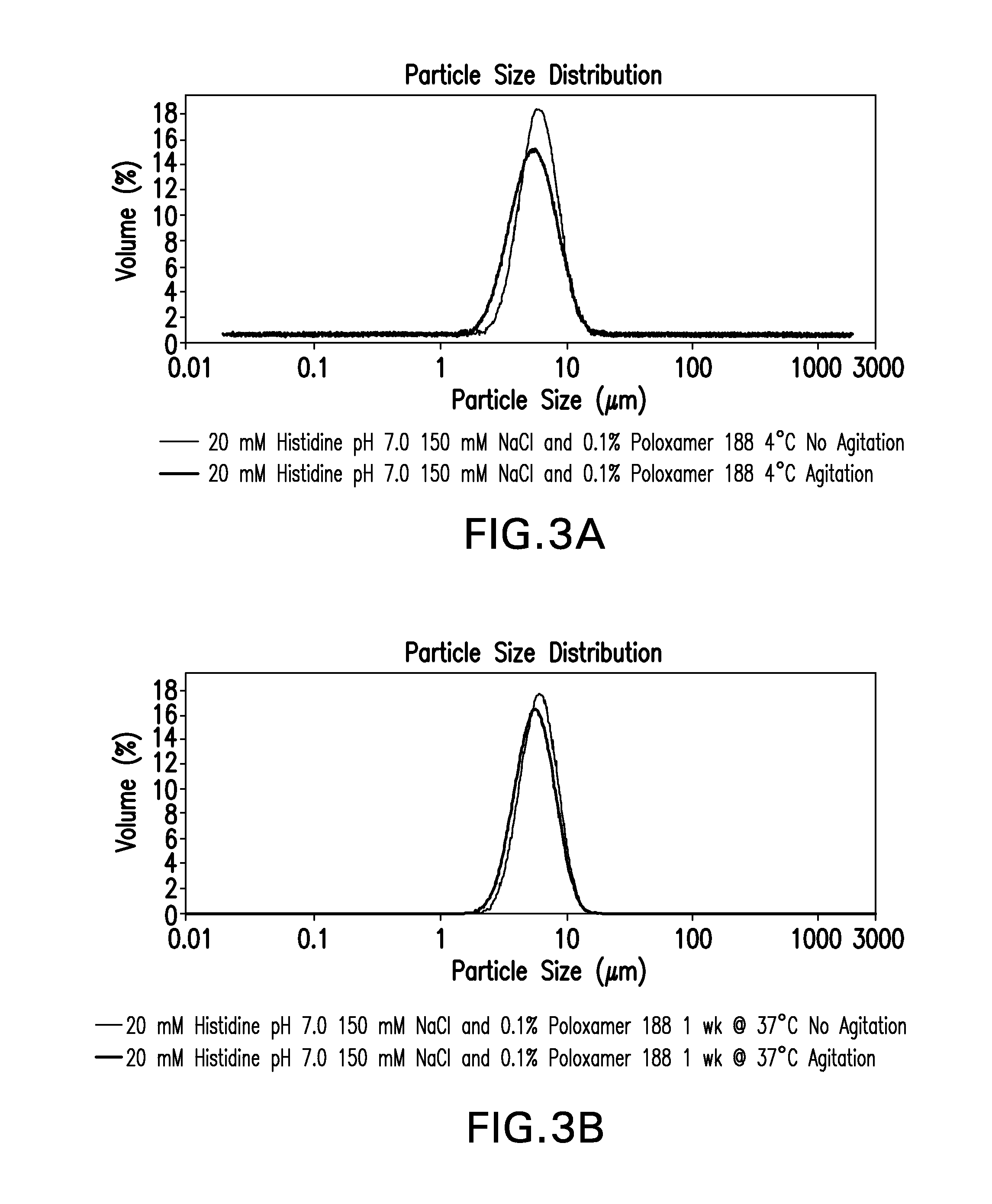
A container comprising a closure means coated by an inert fluorinated material and containing a liquid pharmaceutical composition. In particular, the container comprises a closure means coated by TEFLON and contains a HSA-free Interferon-β formulation having the following composition: 30 to 100 μg / ml of interferon-β, an isotonicity agent, 0.1 to 2 mg / ml of Poloxamer 188, at least 0.12 mg / ml of L-Methionine and a buffer solution capable of maintaining the pH of the liquid formulation at a value between 3.0 and 4.0.
Owner:ARES TRADING SA
Temperature sensitive gel formulation for in situ vagina uses
InactiveCN1593386AMedication convenienceImprove compliancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineTherapeutic effect
The invention relates to a temperature sensitive gel formulation for in situ vagina uses which is prepared from effective dosage of medicament for treating female vagina infection and macromolecular findings through dissolving into cushioning solution or pure water, and charging poloxamer.
Owner:SHANGHAI PHARMCO RES +1
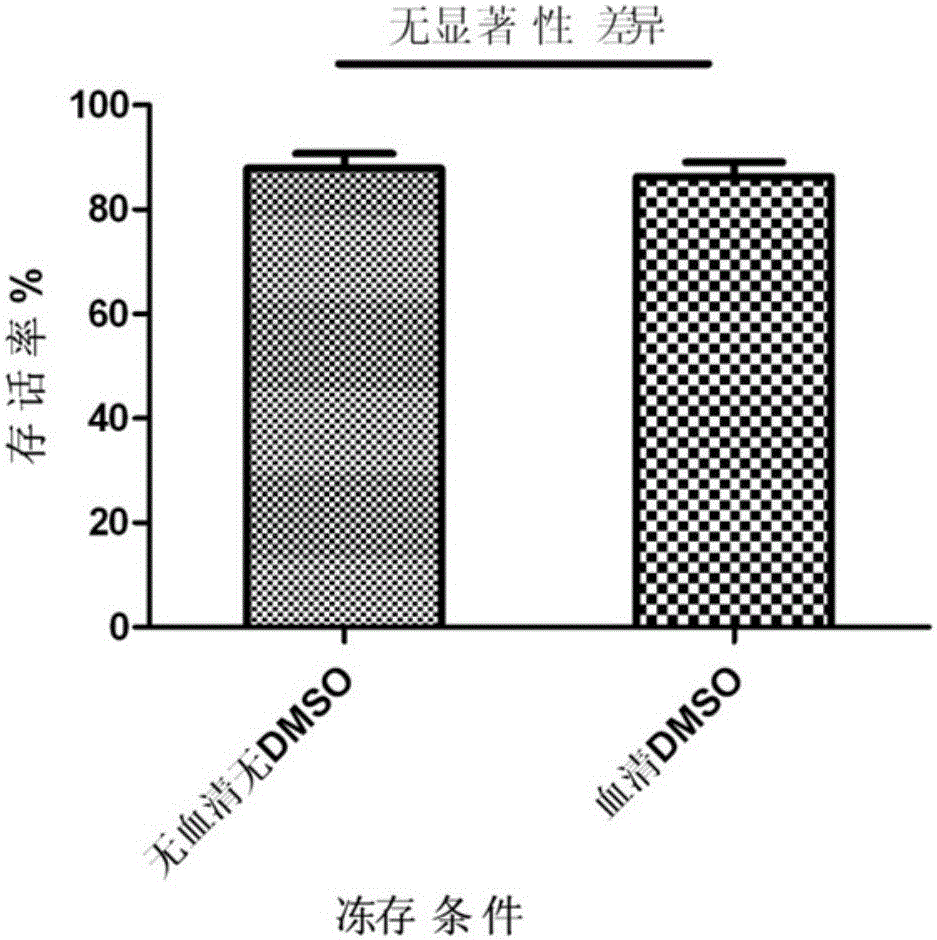
Cell cryopreservation fluid
InactiveCN105961374AImprove freezing effectClear ingredientsDead animal preservationAdditive ingredientPolyethylene glycol
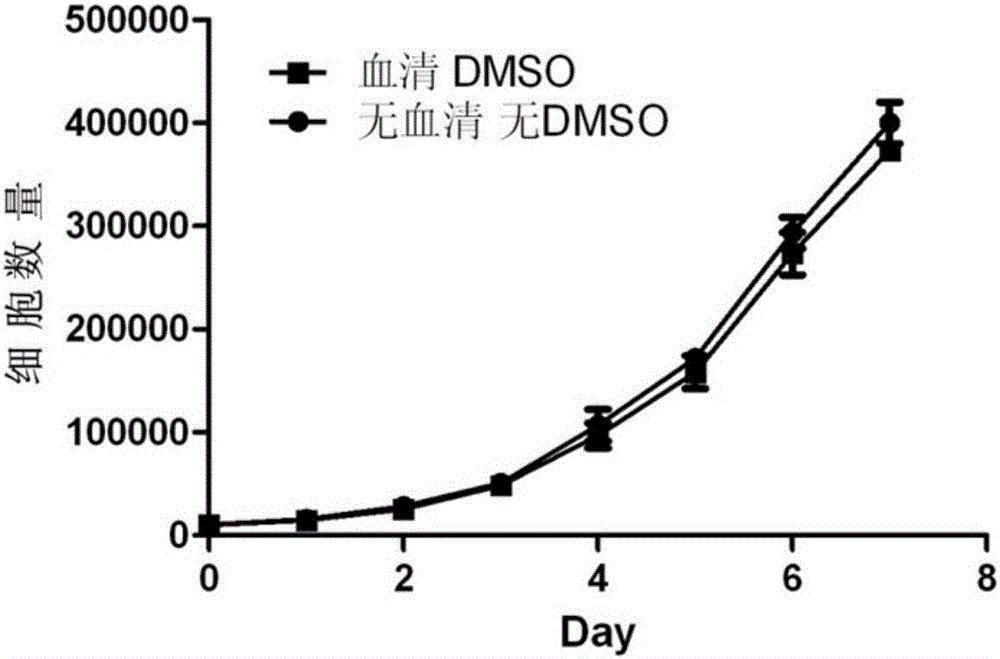
The invention discloses a cell cryopreservation fluid which can be used for performing cryopreservation on mesenchymal stem cells or other cells. The cell cryopreservation fluid is prepared from the following ingredients: PBS or normal saline or a basal culture medium serving as a main ingredient, as well as one or more of polyethylene glycol, propanediol, Ectoin, albumin, trehalose, proline and poloxamer 188 which serve as additive ingredients. The cell cryopreservation fluid disclosed by the invention does not contain serums and DMSO, have the definite additive ingredients, and is controllable in quality, high in batch stability and high in safety. By virtue of preserving cells with the cell cryopreservation fluid, revived cells are high in survival rate, and the cellular morphology, the multiplication capacity and the differentiation potential are not influenced; the cell cryopreservation fluid can be used for replacing cryopreservation fluids containing the serums and the DMSO.
Owner:SHENZHEN HORNETCORN BIOTECH
Apitoxin liposome preparation and preparation method thereof
InactiveCN101391098AReduce releaseImprove bioavailabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectPhospholipid
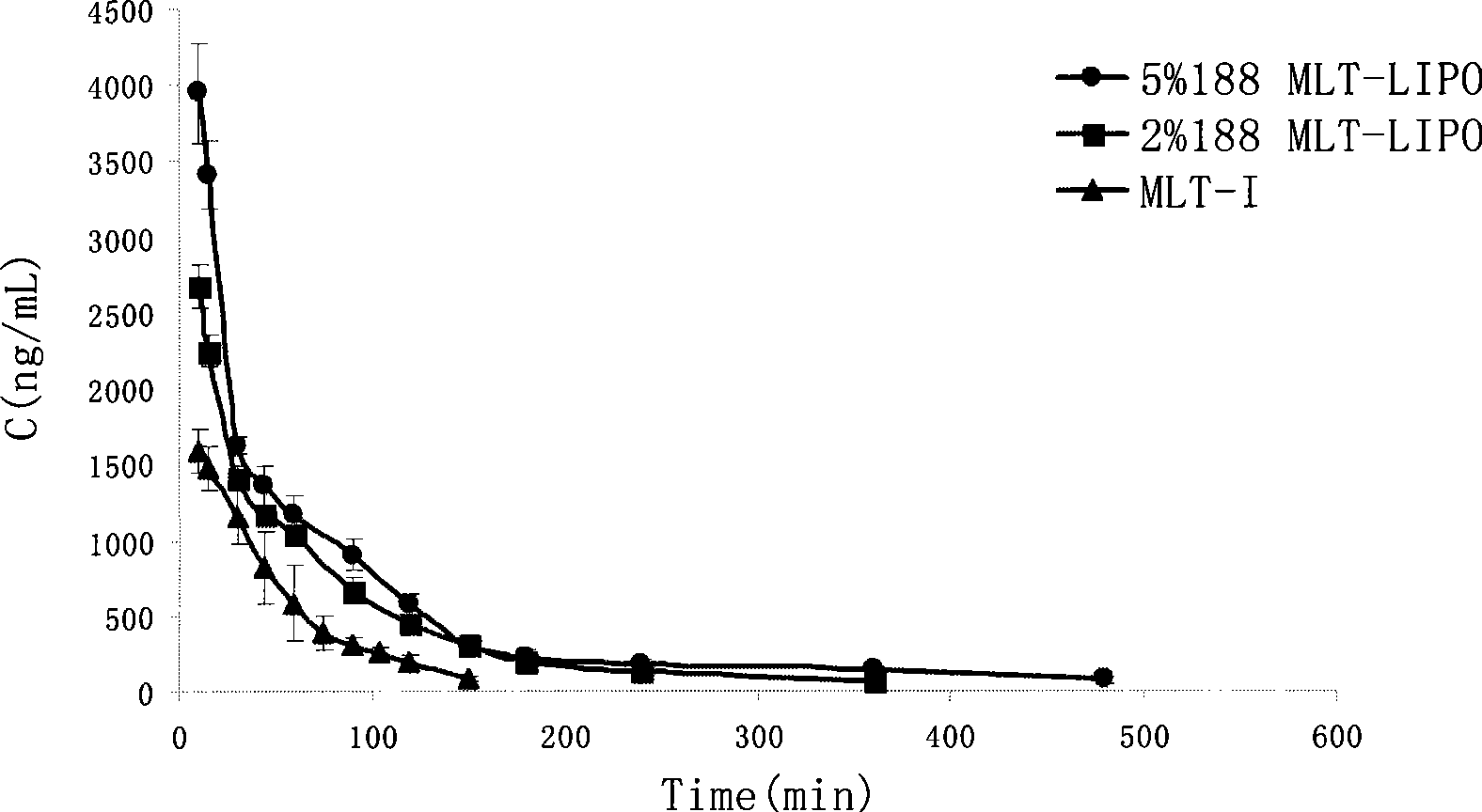
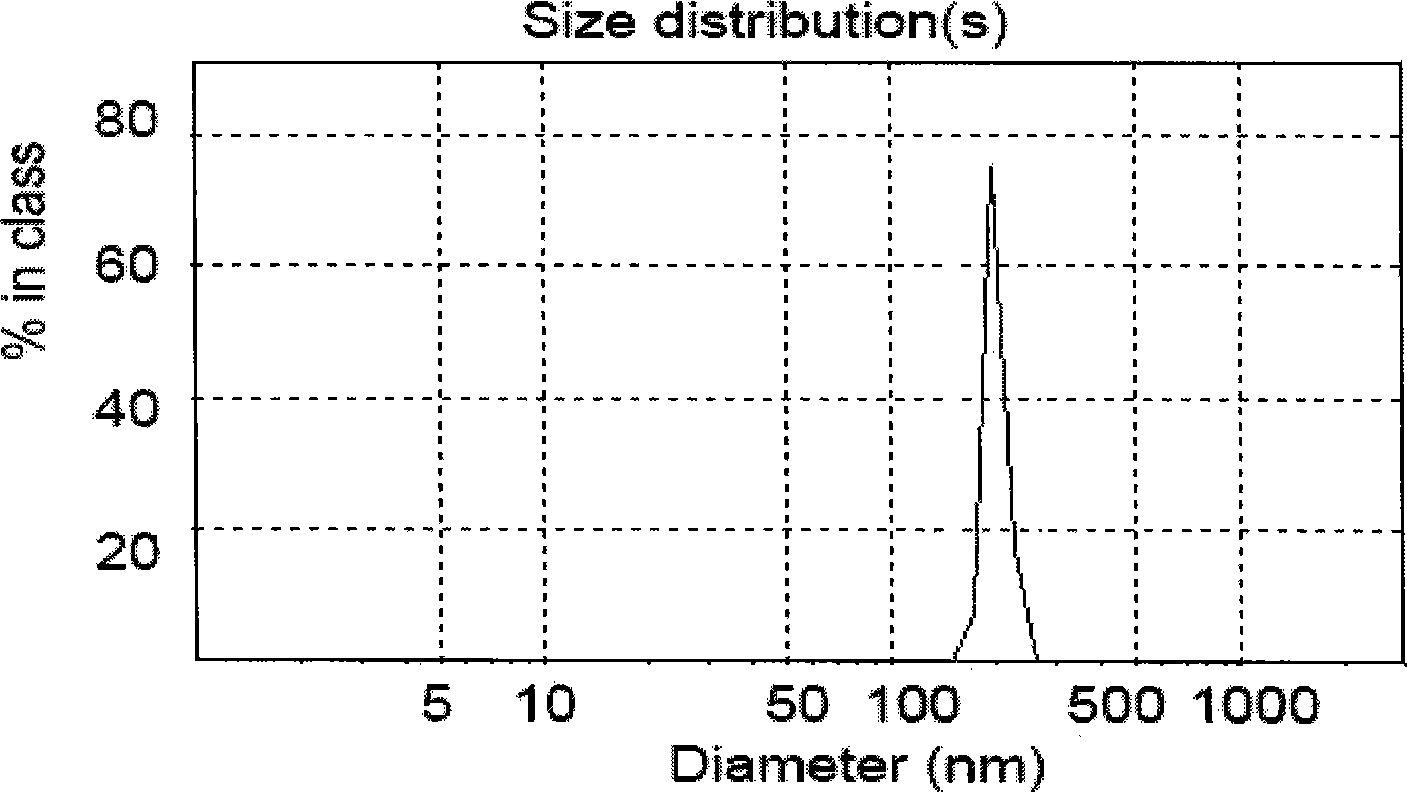
The invention relates to the field of medicine preparation, in particular to a melittin lipidosome preparation. The lipidosome preparation is composed of one part of melittin, 5-40 parts of phospholipid, 1.3-10 parts of cholesterin and 10-140 parts of poloxamer. The invention also discloses the preparing method of the melittin lipidosome preparation. The melittin lipidosome preparation not only can delay the medicine release in the lipidosome, prong the circulating time in the blood and improve the bioavailability of the medicine, but also can obviously reduce the side effect of the medicine and improve the adaptability of a patient.
Owner:安徽省百春制药有限公司 +1
Gene delivery formulations and methods for treatment of ischemic conditions
InactiveUS20040009940A1Avoid toxicityEfficient deliveryPowder deliveryPeptide/protein ingredientsGene deliveryAngiogenesis Factor
The present inventors have developed a novel approach for efficient delivery of angiogenic factors to the cardiac and peripheral vasculature that avoids problems with toxicity inherent to existing delivery technologies. Vectors carrying coding sequences for angiogenic agents including Del-1 or VEGF, or both, can be formulated with poloxamers or other polymers for delivery into ischemic tissue and delivered to areas of peripheral ischemia in a flow to no-flow pattern and to the heart by retrograde venous perfusion.
Owner:VICAL INC
Fat emulsion containing docetaxel and its preparing method
InactiveCN1709236ASolve the existing technology deficiencies of low solubilityImprove securityOrganic active ingredientsDiseaseVegetable oil
The present invention relates to an infatmul containing docetaxel and its preparation method. Said preparation can be directly used for intravenous injection. At the same time of that it is used as medicine for curing tumor disease said preparation also can provide nutrients for patient. The weight volume concentration range of docetaxel contained by said preparation is 0.1-1.0 mg / ml, and its specification is that 1-500 mg of active component docetaxel is contained. Said infatmul composition includes docetaxel, vegetable oil, phospholipids and injection water, at the same time the components of glycerin and group emulsion, etc. can be added, in which the optimum group emulsion is poloxamer 188. Besides, said invention also provides the concrete steps of its preparation method and its application in preparation of medicine for resisting tumor.
Owner:CISEN PHARMA
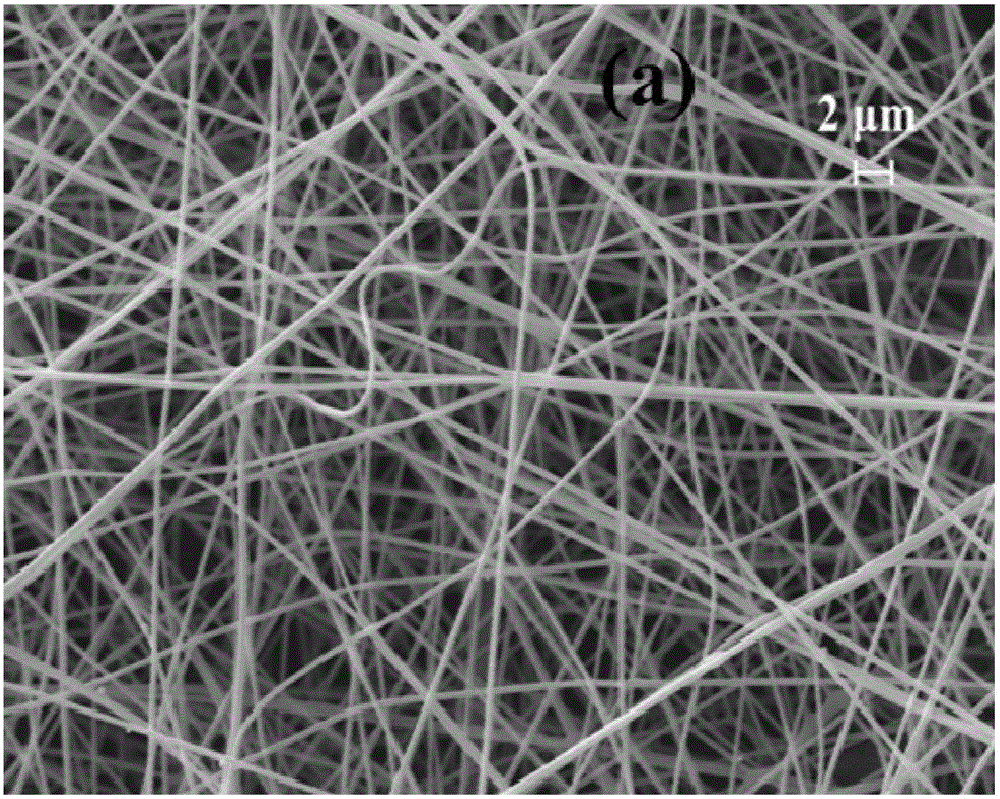
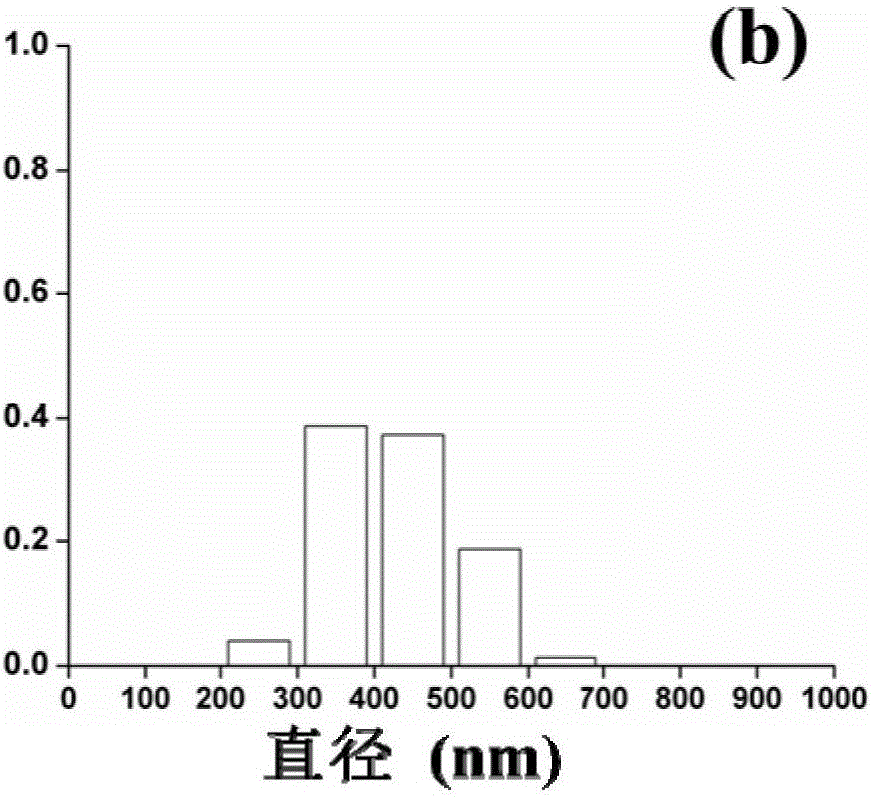
Kernel-shell structural nanofiber membrane, preparation method thereof and application
ActiveCN106702597AIncrease release rateLow release ratePeptide/protein ingredientsPharmaceutical delivery mechanismChitosan nanoparticlesPolyvinyl alcohol
The invention discloses a kernel-shell structural nanofiber membrane, a preparation method thereof and an application; the preparation method includes steps of (1), preparation of spinning; dissolving sodium alginate, polyoxyethylene and poloxamer F127 in distilled water, stirring evenly and acquiring shell layer spinning solution; mixing the chitosan nanoparticle solution loaded with active matters with polyving akohol solution evenly, and acquiring the kernel layer spinning solution; (2), coaxial electrostatic spinning: respectively injecting the shell layer spinning fluid and kernel layer spinning fluid in a static spinning device containing a coaxial needle, performing the coaxial electrostatic spinning under room temperature, performing solvent evaporation in the spinning process, and acquiring the kernel-shell structural nanofiber membrane loaded with the nanoparticle. The preparation method is simple in operation and gentle in reaction condition and can well control the slow release of the active matter; moreover, the prepared colon targeting controlled release system has obvious colon targeting property, thereby improving the utilization degree of the active matter. The colon targeting controlled release system can be applied to functional food domain.
Owner:SOUTH CHINA UNIV OF TECH
Pharmaceutical composition
InactiveUS20050220881A1High dissolution rateImproves dissolution and dissolution rateBiocidePowder deliveryPolyethylene-polypropylene glycolPharmacology
The present invention provides a composition including an association complex of a pharmaceutical composition and one or more polyethylene-polypropylene glycol block co-polymers (poloxamers). The pharmaceutical composition may include a member selected from the group consisting of (a) an association complex of an eprosartan composition including eprosartan or a pharmaceutically acceptable salt of eprosartan and (b) the non-zwitterionic compound 2-(7-chloro-5-methyl-4-oxo-3-phenyl-4,5 dihydro-3H-pyridazino (4,5-b)indol-1-yl)-N,N-dimethylacetamide (NZ).
Owner:BVM HLDG
Stable formulations of polypeptides and uses thereof
InactiveUS20120244158A1Improve solubilityImprove stabilityInorganic non-active ingredientsPharmaceutical delivery mechanismSolubilityPharmaceutical formulation
Owner:ABLYNX NV
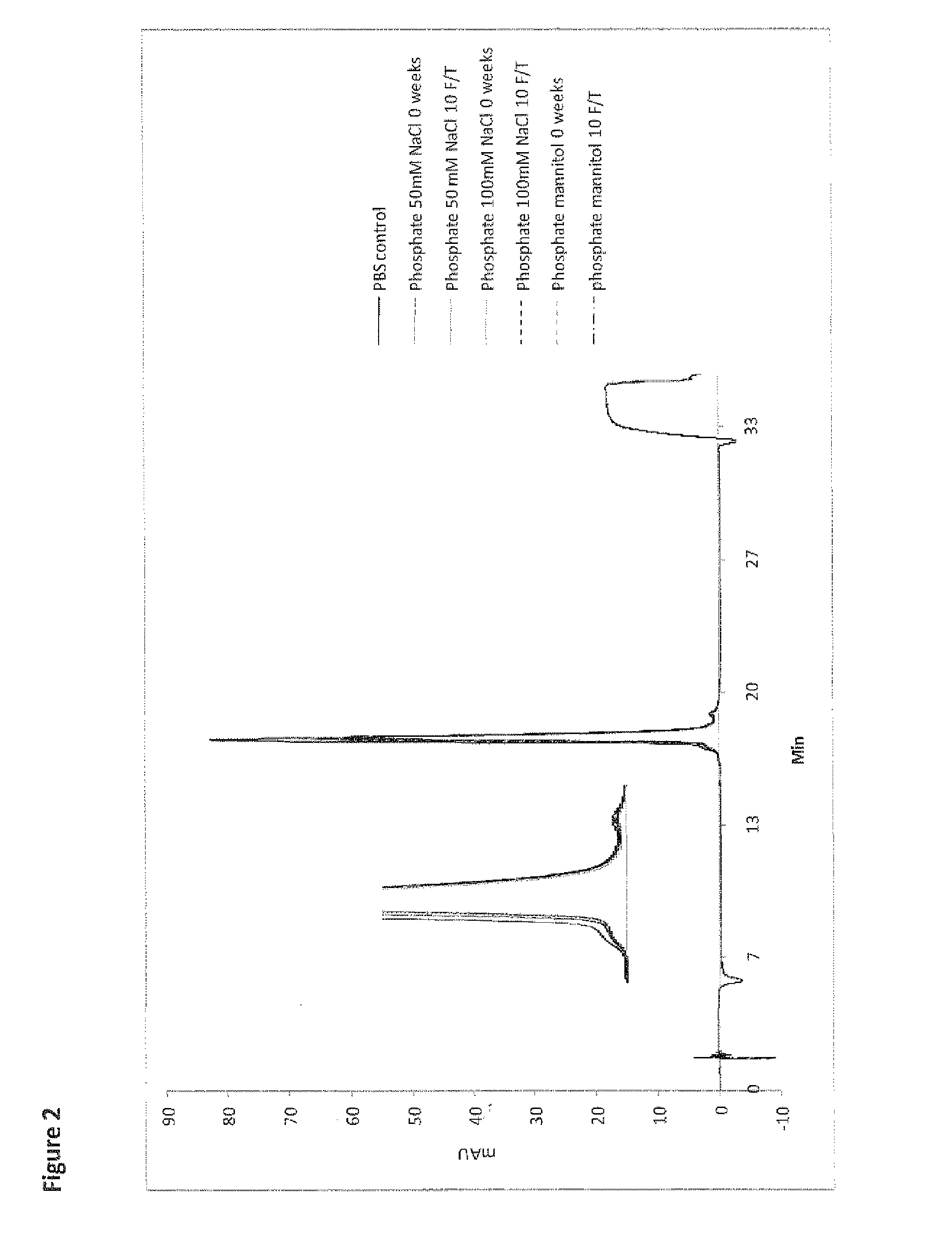
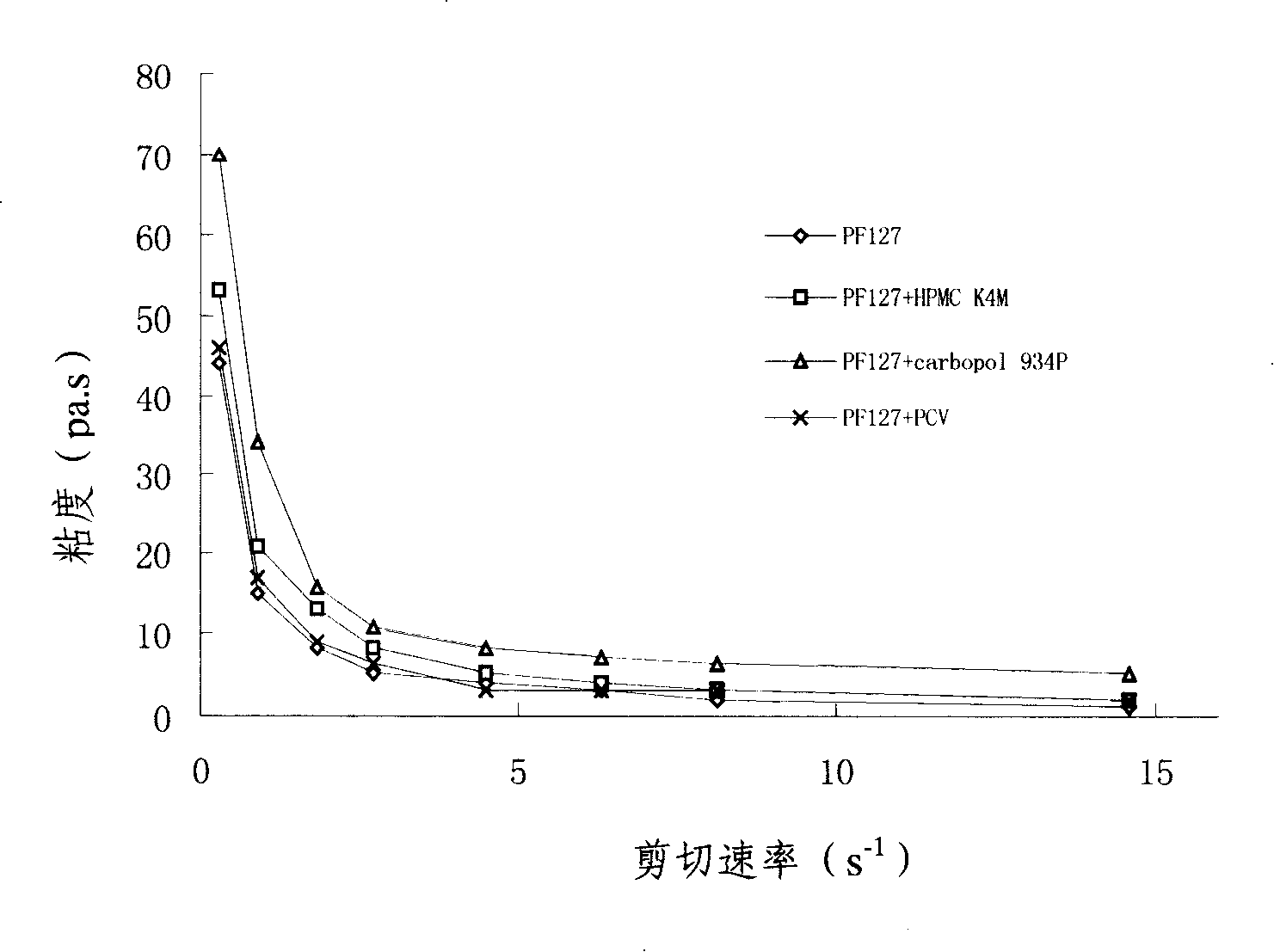
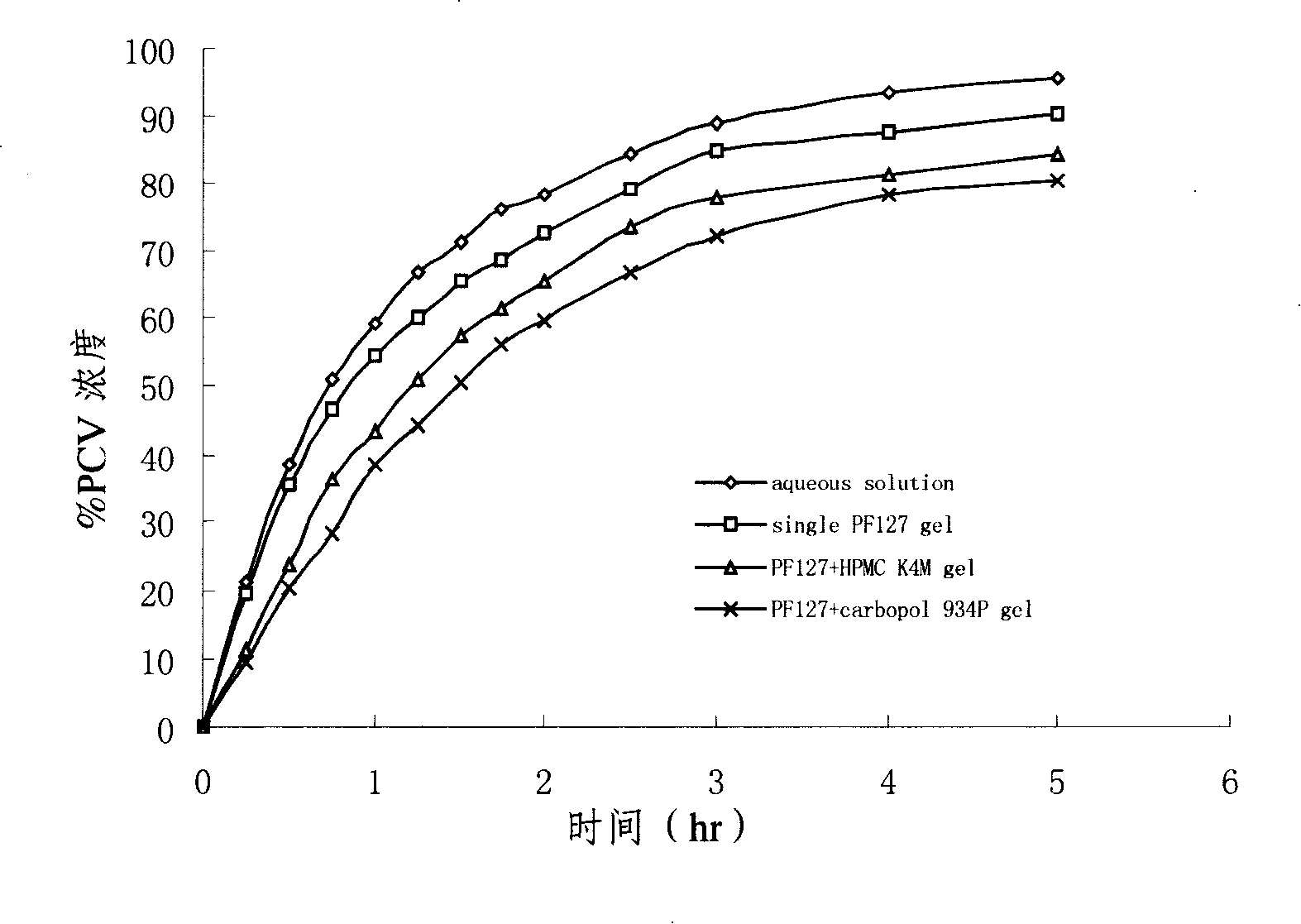
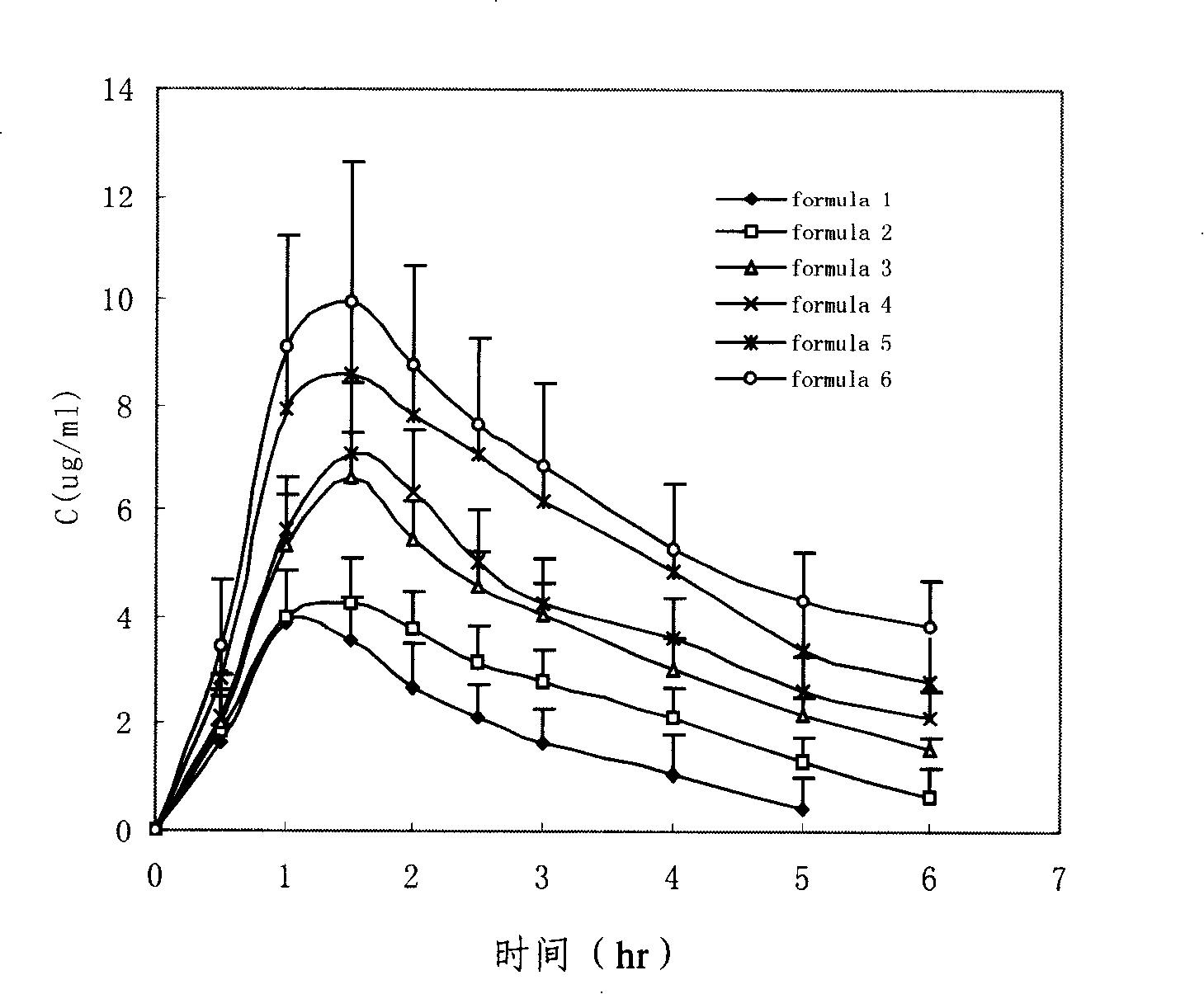
Penciclovir ophthalmic temperature sensitivity in situ gel preparation and preparation method thereof
InactiveCN101185650AGood biocompatibilityMedication convenienceSenses disorderPharmaceutical delivery mechanismRetention timeBiocompatibility Testing
The invention provides an in situ eye gel preparation of penciclovir. The compositions and weight percentages thereof are 0.01-2.0wt% of penciclovir, 5-40wt% of poloxamer, 0.1-5wt% of tackifier high polymer base, 0.8-10wt% of osmotic regulation agent, 0.001-0.5wt% of antiseptics and the rest is distilled water. Meanwhile, the preparation method is also provided. The in situ gel preparation has good biocompatibility and glutinousness; compared with a liquid preparation, the invention can increase retention time of drug in the eyes and delay elimination, thus improving the bioavailability of the eyes and reducing the times of administration; meanwhile, the invention avoids weaknesses that operation is inconvenient during gel administration, the preparation is not easy to spread in eyes, the dosage can not be controlled accurately, etc.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com