Matrix compositions for controlled delivery of drug substances
a technology of matrix composition and controlled delivery, applied in the field of matrix composition, can solve the problems of reducing the aqueous solubility of compound, unable to achieve zero order release rate, and unable to achieve continuous release of active substance with tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0177]Test on different excipients on the release time of an active ingredient with a solubility of 4.3 mg / mL, 23° C., water at pH 7.6 in a matrix composition consisting of poloxamer 188 tested in dissolution medium of pH 1.0 and buffer 6.8 in order to evaluate the effect of such excipients in a matrix composition according to the invention. The matrix composition is mixed heated and finally moulded into cylindrical plugs which are inserted into cylindrical shells before dissolution.
[0178]The release rate of baclofen from Poloxamer 188 matrix increased in buffer 6.8 when any of the organic acids were included. The most profound effect was observed when citric acid was used. This could be correlated to the different pKa-values and solubilities of the acids.
Baclofen content: 12.5% (w / w); Poloxamer 188 as Carrier.DissolutionOrganic acids:mediumDissolution rateCitric acid (4.5%)5 mm handmade plugsIDpHRelease (mm / min)08022004(k1_k3)1 0.08309022004(k1_k3)6.80.042Citric acid (4.5%) PVP (2...
example 2
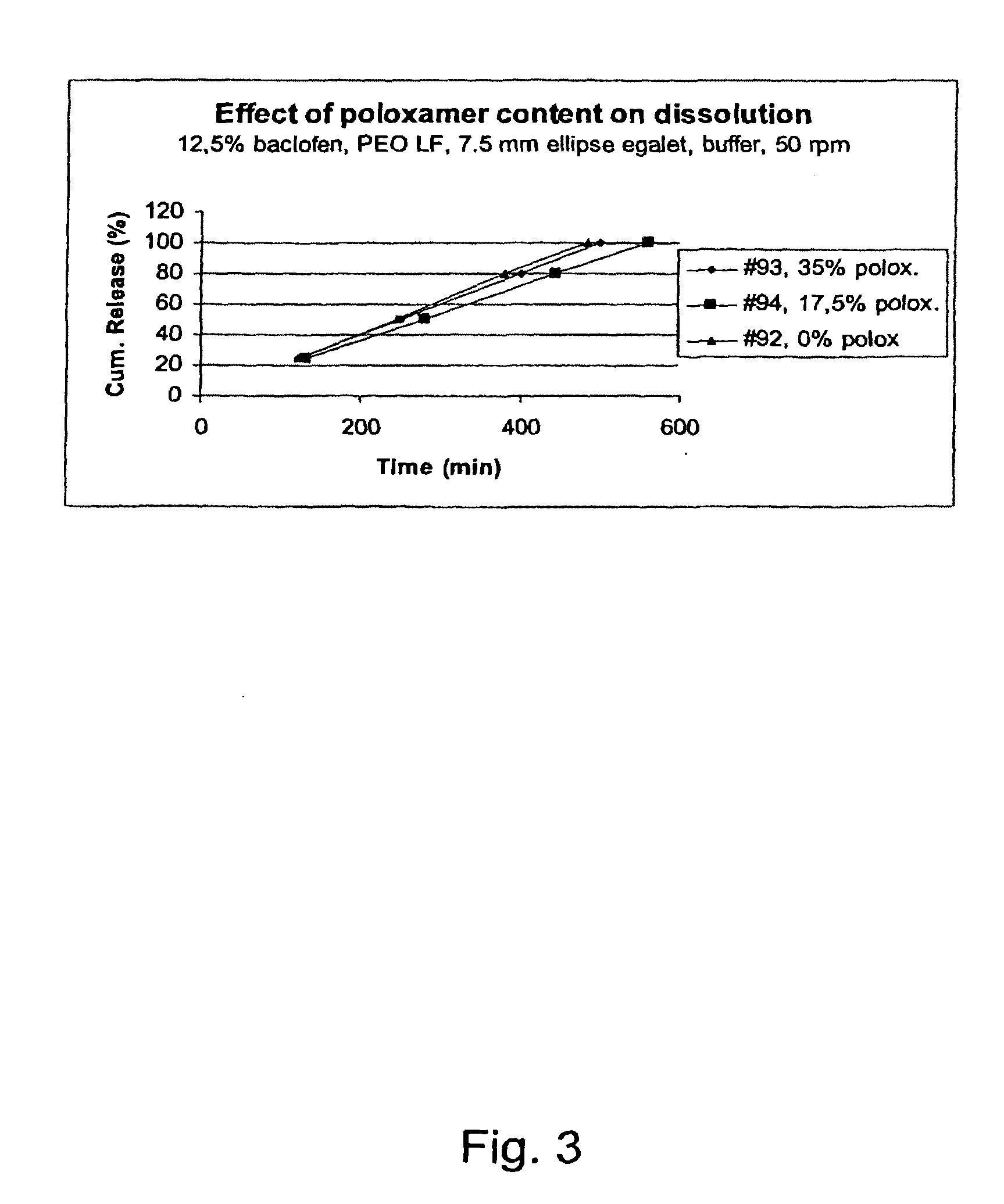
[0179]Compositions with active ingredient having a solubility of 4.3 mg / mL, 23° C., water at pH 7.6 and the corresponding dissolution time and release rates in different matrix compostions according to the invention. The results demonstrated the possibility of controlling the release rate by use of different ratios of the PEO and block copolymer according to the invention.
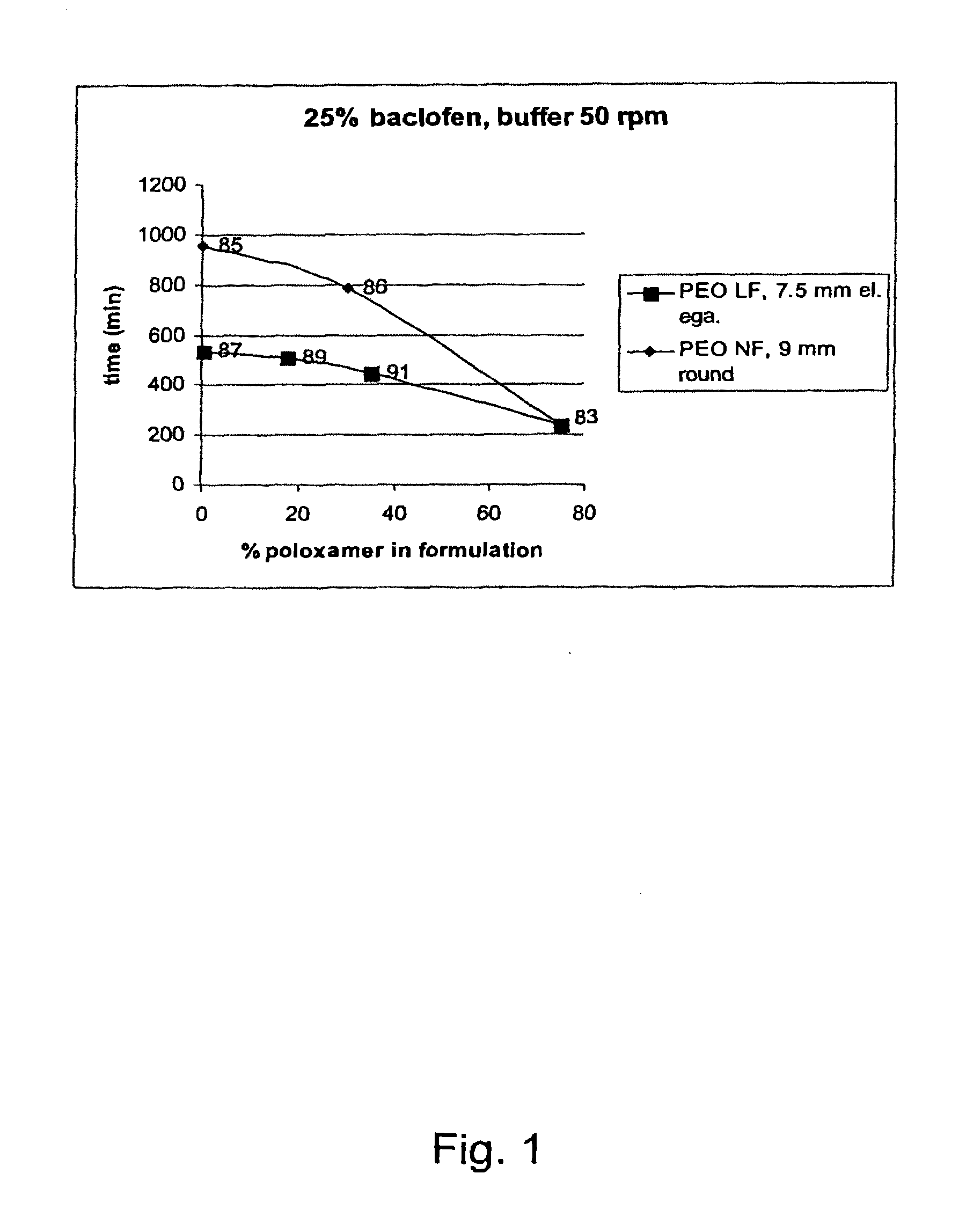
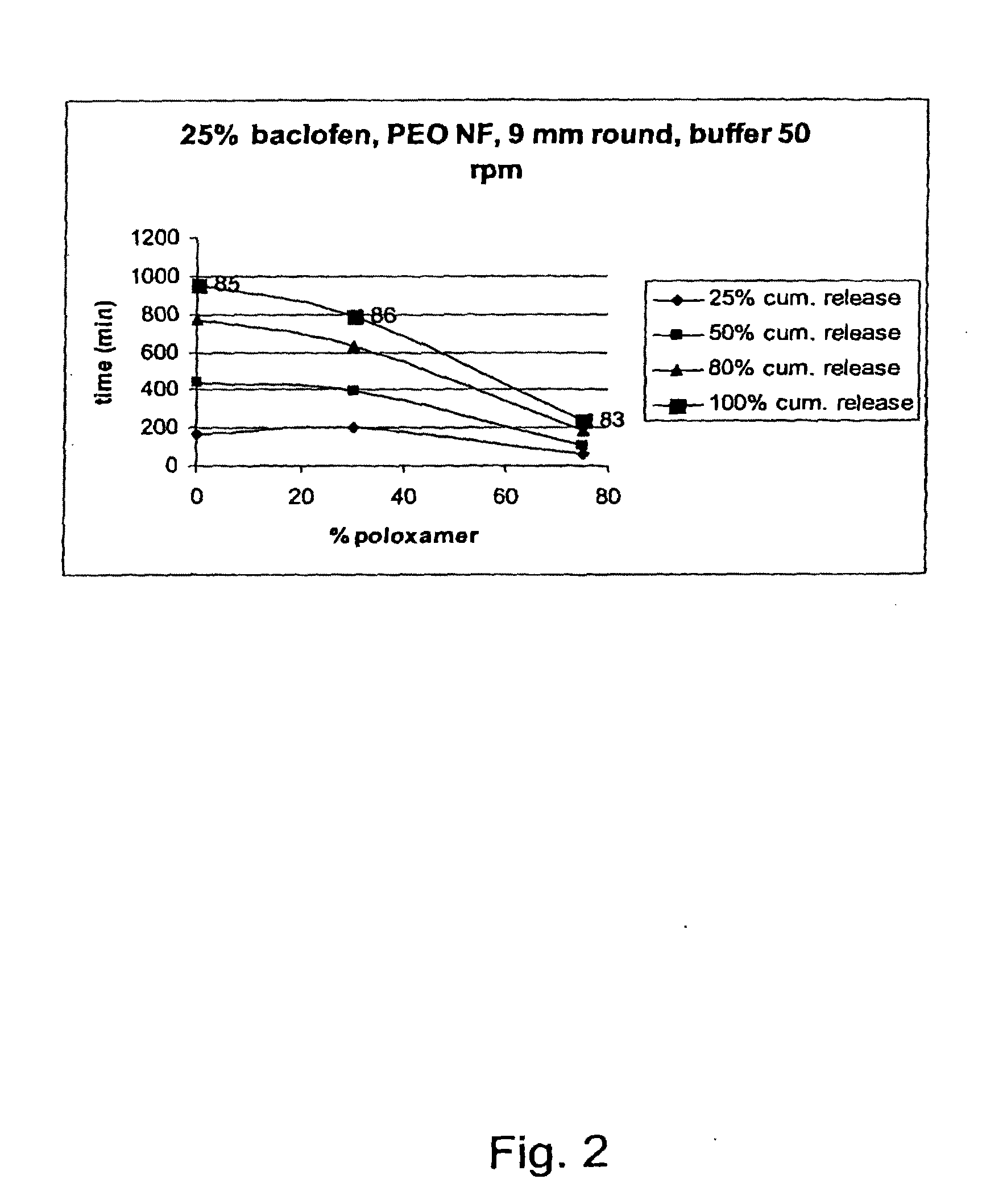
ReleaseBatchDissolutionrateNo.APIExcipientsAppearanceCondition25%50%80%100%(min / mm)03-25% W / W9 mmBuffer60105180240270083-75% w / wroundpH105Poloxamerplug6.8 50 rpm18803-25% W / W9 mmBuffer1704407559551060085-75% w / w PEOroundpH105200.000 NFplug6.8 50 rpm03-25% W / W9 mmBuffer198395630786870086-45% w / w PEOroundpH105200.000 NFplug6.8 50 rpm30% w / wPoloxamer188 (60:40)03-75% w / w PEO7.5 mmBuffer115255420535710087-200.000 LFellipsepH105Egalet6.8 50 rpm03-25% W / W7.5 mm0089-60% w / w PEOellipse105200.000 LFEgalet15% w / wPoloxamer188 (80:20)03-25% W / W7.5 mmBuffer120225360445590091-45% w / w PEOellipsepH105200.000 LFEgalet6.8 50 rpm30% ...
example 3
[0183]A composition (batch No. 02-0121-042) according to the invention was prepared from the following ingredients.
Matrix:% w / wPEO 200.000 LF70.68%PolyXamer [Lutrol F127]16.97%Carvedilol11.67%PM0.19%BHT0.49%
[0184]One doses form contains 22 mg Carvedilol. The composition was 6 mm long and had an oval cross sectional shape.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com