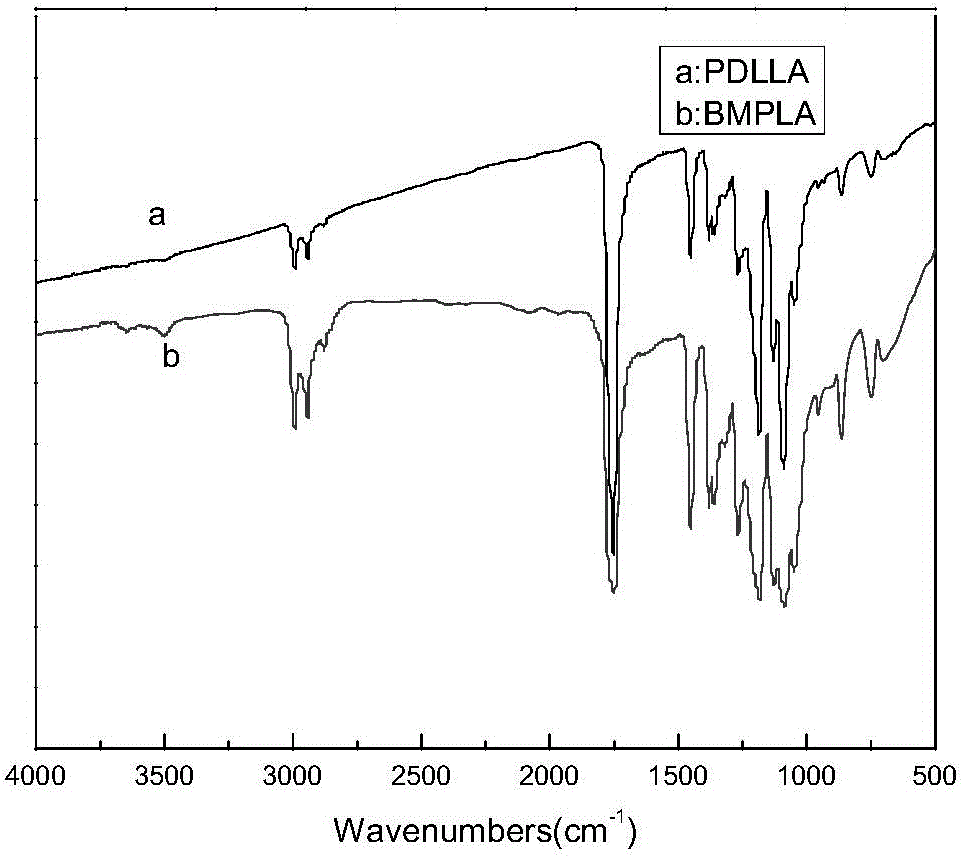
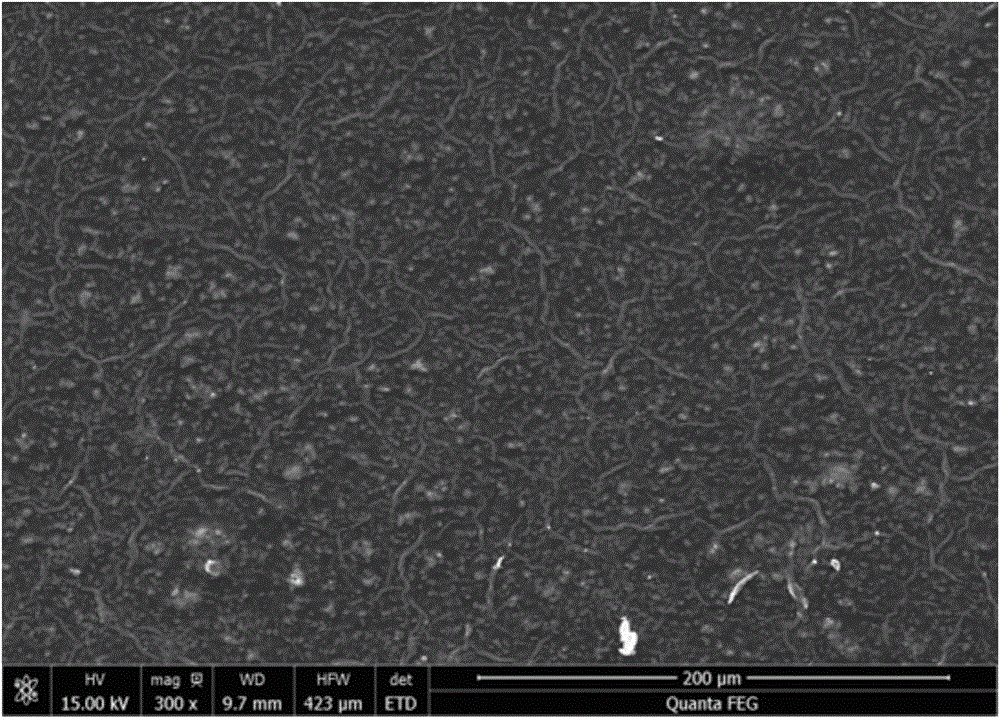
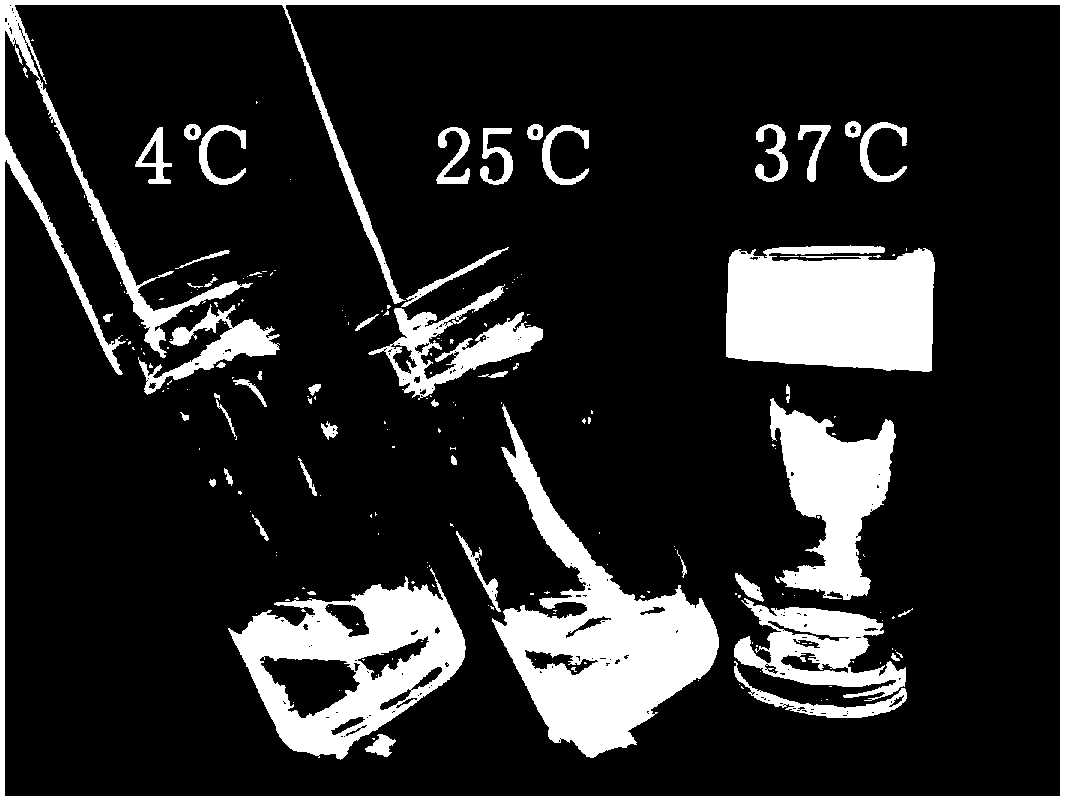
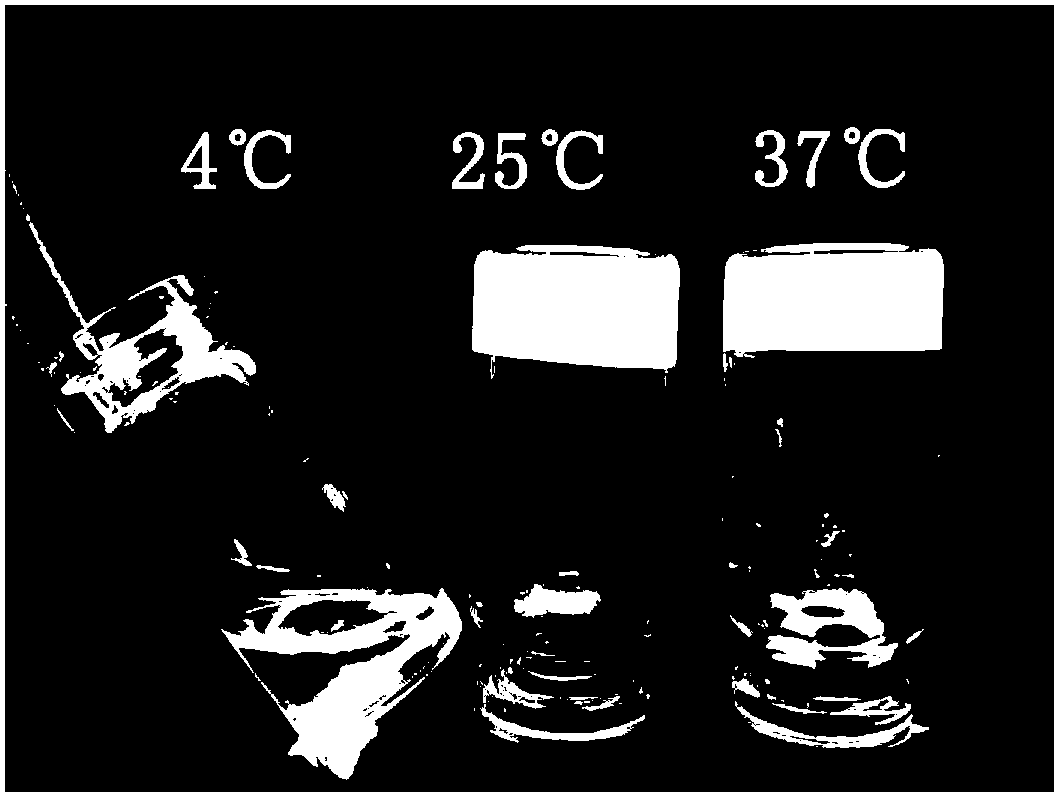
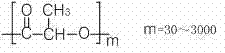Patents
Literature
49 results about "DL-Lactic Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
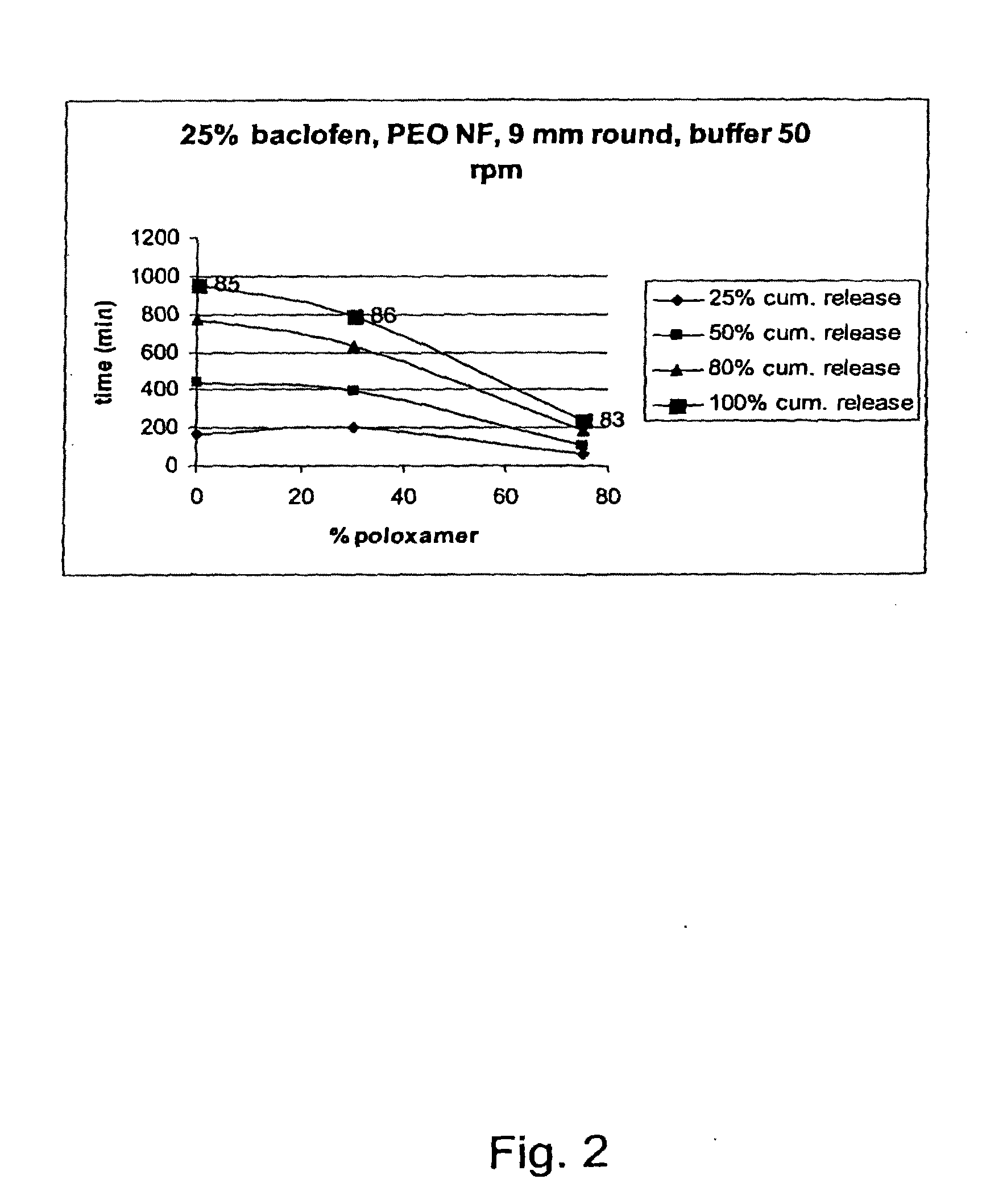
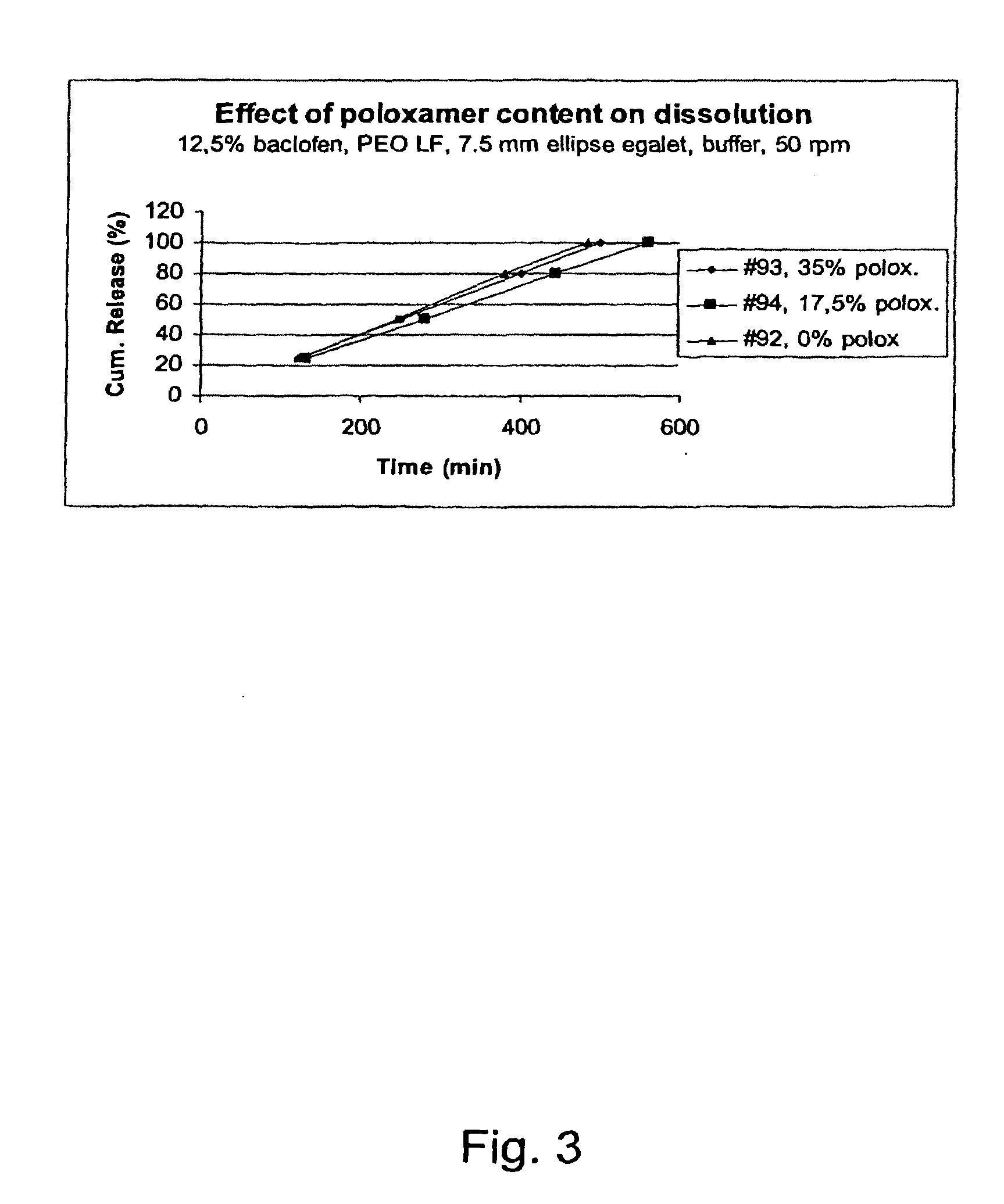
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
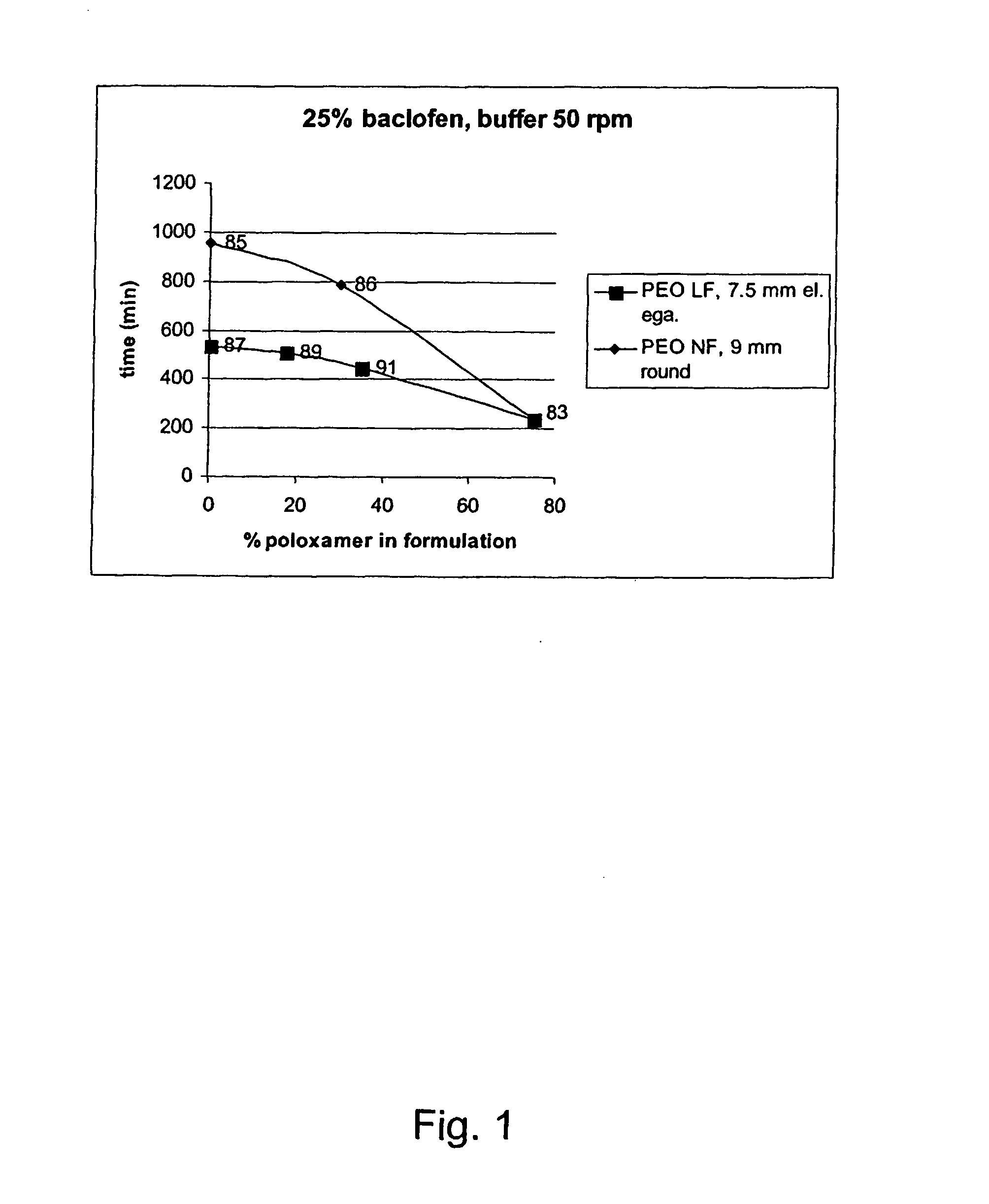
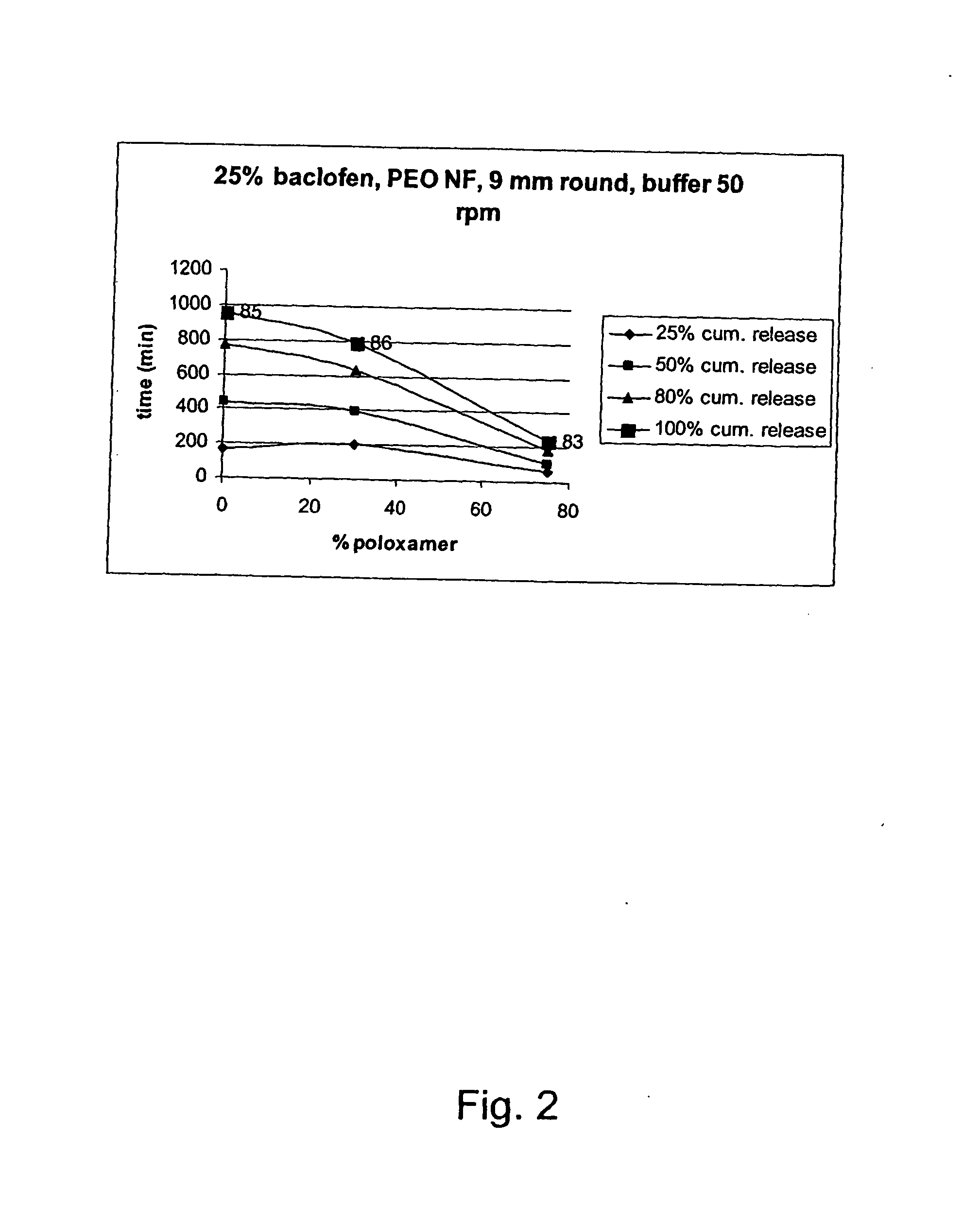
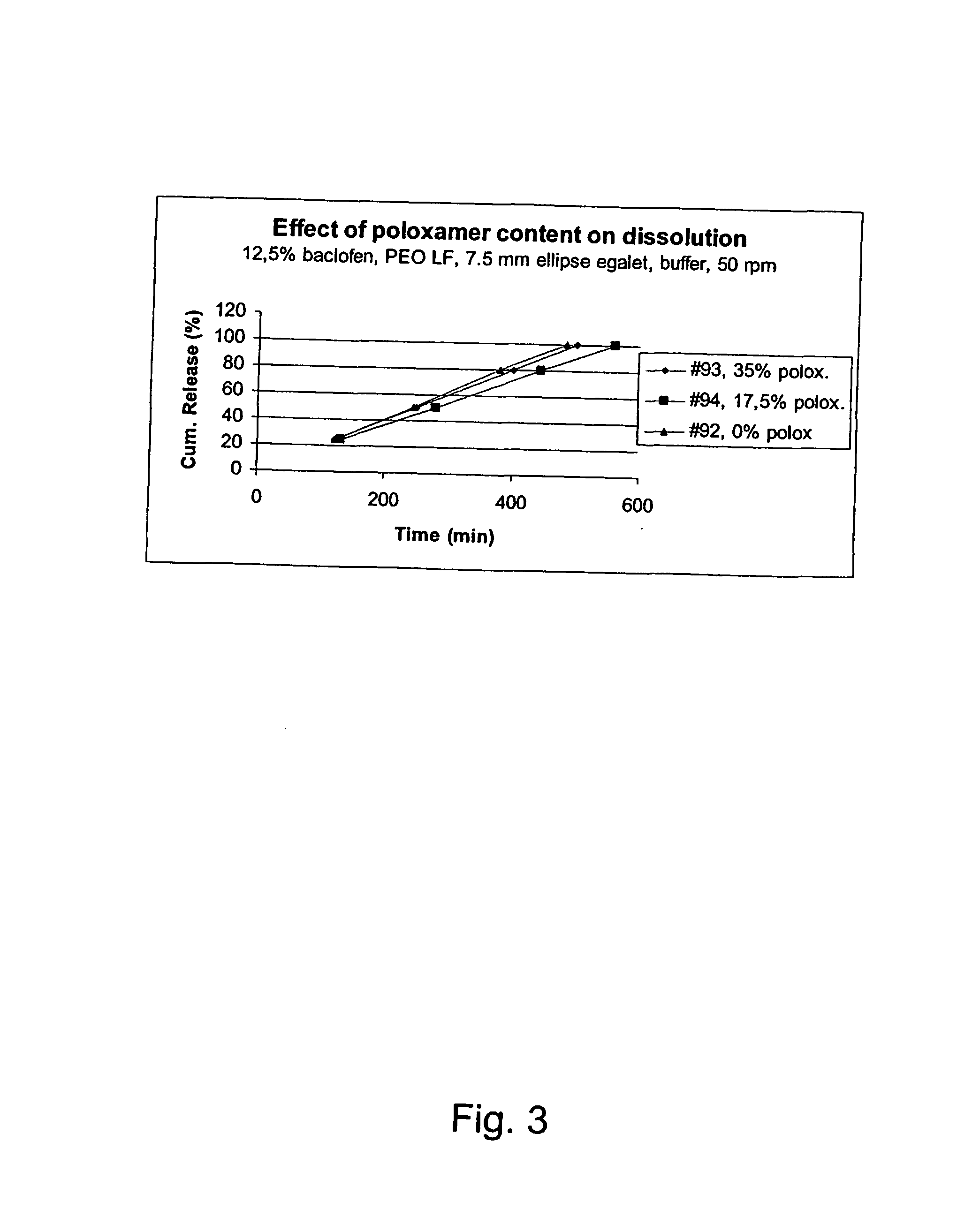
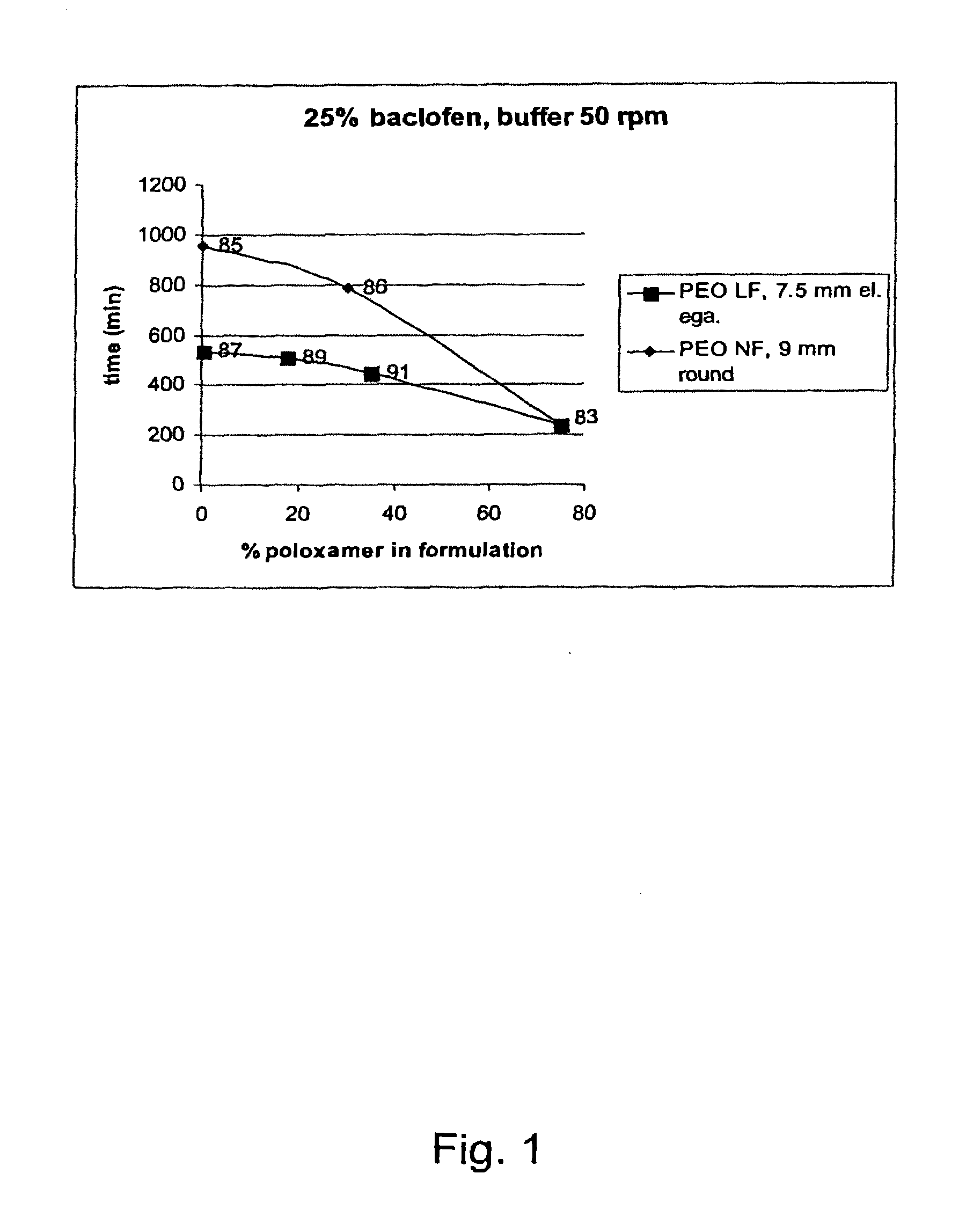
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
Polylactic acid foaming material and preparation method thereof
The invention provides a polylactic acid foaming material and a preparation method thereof. The polylactic acid foaming material is prepared from the following ingredients according to parts by weight: 45-93 parts of polylactic acid, 5-28 parts of toughener, 1-5 parts of nucleating agent and 1-10 parts of foaming agent. The polylactic acid is one of poly-L-lactic acid, poly-D-lactic acid and poly-DL-lactic acid or a mixture or a polymer thereof, and has the weight average molar mass of 0.8-3.5 million, molecular weight distribution of 1.2-2.5, and degree of crystallinity of 15-60 percent. The toughener is one of poly succinic acid butyl ester and poly adipate / butylene terephthalate or a mixture of two with random ratio. Other accessory ingredients can also be added. The preparation method comprises the following steps: evenly mixing the polylactic acid, the toughener, the nucleating agent, the foaming agent, the accessory ingredients and the like in a high mixing machine according to a certain proportion, then milling for 5-25 minutes at the temperature of 100-170 DEG C, and carrying out die pressing foaming on obtained materials at the temperature of 120-210 DEG C on a vulcanizing machine for 2-10 minutes to prepare the polylactic acid foaming material. The polylactic acid foaming material prepared by using the preparation method has the advantages of high resistance to shock, high elongation at break, high tensile strength and complete biodegradation after used.
Owner:南京冠创生物科技有限公司
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
Poly lactic acid composition film and its preparing method and use
The polylactic acid film contains polylactic acid 85-98 wt% and medical plasticizer 2-15 wt%, the polylactic acid may be poly L-lactic acid, poly DL-lactic acid, lactic acid-glycolic acid copolymer or the copolymer of poly L-lactic acid and poly DL-lactic acid with molecular weight of 20-1500 KDa, and the medical plasticizer is tributyl citrate, tributyl acetylcitrate, polyethylene glycol or lactic acid oligomer. The polylactic acid composition of the present invention has excellent flexibility, may be used in introducing the growth of hard and soft tissues in repairing cleft palate or as regenerating film, and introducing the regeneration of bone tissue.
Owner:成都迪康中科生物医学材料有限公司
Interverterbral fusion implement
InactiveCN1436518ANo foreign body reactionPromote bone fusionInternal osteosythesisProsthesisHuman bodyBiocompatibility Testing
The interverterbral fusion implement for cervical vertebra anterior operation is one hollow column with polygonal or circular cross section, holes on side wall and sharp teeth on the upper and the lower surfaces. It is made of polymer material capable of being absorbed by human body, poly(DL-lactic acid), poly(L-lactic acid), polyglycolic acid or their copolymer. It has determined early-stage locking effect, late-stage bone fusion effect, good biocompatibility and proper mechanical structure, and may be self-degraded after being fused with vertebra. It results in no rejection and may be also used in thoracic vertebra and lumbar vertebra operation.
Owner:成都迪康中科生物医学材料有限公司
Hemostatic material in medical use
InactiveCN1727013AGood biocompatibilityImprove ductilitySurgical adhesivesPharmaceutical containersLactideTriol
A medical styptic material able to be fully absorbed and degradated is composed of the basic material prepared by fusion polycondensation reaction of low-molecular poly-DL-lactic acid or poly-L-lactic acid or polyethanol-acid copolymer / mixture, and the auxiliary which may be polyether diol, polyether triol, high-molecular polylactic acid, high-molecular glycolide-lactide copolymer, high-molecular caprolactone-lactide copolymer, or high-molecular caprolactone-glycolide copolymer through fusion and mixing.
Owner:HUIZHOU HUAYANG MEDICAL EQUIP
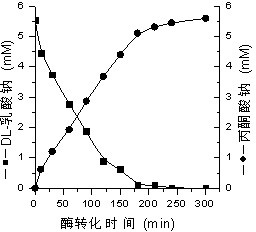
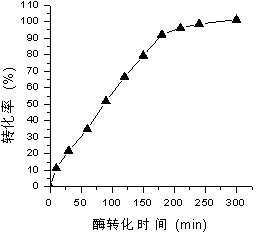
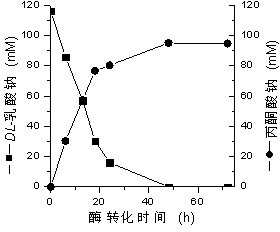
Method for preparing pyruvic acid by converting DL-lactic acid
InactiveCN102199632AImprove conversion rateGuaranteed stabilityMicroorganism based processesFermentationMicroorganismLactate oxidase
The invention belongs to the technical field of pyruvic acid preparation method, and relates to a method for preparing pyruvic acid through a process of converting DL-lactic acid by using lactic acid oxidase (LOD) and catalase (CAT) in Pseudomonas fluorescens. The invention provides a method for preparing pyruvic acid through a process of converting DL-lactic acid by using lactic acid oxidase (LOD) and catalase (CAT) in microbe bacterial strain (ATCC 948)-Pseudomonas fluorescens crude enzymes liquor. According to the invention, the conversion rate of the products can be raised and the products stability can be maintained.
Owner:UNIV OF JINAN
Biodegradable spiral ureter bracket and preparation method thereof
The invention relates to a biodegradable spiral ureter bracket which is characterized by being prepared from biodegradable polymer material, preferably poly-L-lactic acid or a mixture of the poly-L-lactic acid and poly-DL-lactic acid. The invention further comprises a preparation method of the biodegradable spiral ureter bracket, which comprises the step of: forming a film by a solution prepared by a solvent in a die, cutting the film into strips and winding the film strips to shape spirally. By using a biodegradable material which forms tiny fragments in a process of degradation in vitro, the bracket provided by the invention can be excreted from the body with urine and does not block the ureter. Meanwhile, the bracket can be completely degraded without being taken out through a secondary operation after a period of time. Therefore, sufferings of patients are abated.
Owner:GENERAL HOSPITAL OF PLA +1
Preparation method of RGD polypeptide grafted poly(maleic anhydride-hexamethylendiamine-DL-lactic acid)/modified hydroxyapatite porous composite material
The invention relates to a preparation method of an RGD polypeptide grafted poly(maleic anhydride-hexamethylendiamine-DL-lactic acid) / modified hydroxyapatite porous composite material. The preparation method comprises the following steps: (1) introducing maleic anhydride and hexamethylendiamine to a side chain of polylactic acid at first, and then grafting adhesive RGD polypeptide; (2) dissolving the generated RGD polypeptide grafted poly(maleic anhydride-hexamethylendiamine-DL-lactic acid) in dichloromethane, adding modified nano modified hydroxyapatite, performing ultrasonic dispersion, stirring fully to mix uniformly, weighing sodium chloride particles screened by a molecular sieve, adding the sodium chloride particles into the mixed solution and stirring; and (3) when a solvent is volatilized, drying to perform volatilization, and soaking the material in distilled water to separate out the sodium chloride particles, thereby obtaining the RGD polypeptide grafted poly(maleic anhydride-hexamethylendiamine-DL-lactic acid) / modified hydroxyapatite porous composite material at last. The preparation method provided by the invention has the advantages that a manufacturing process is simple and controllable.
Owner:WUHAN UNIV OF TECH
Use of PDLLLA (Poly Dl Lactic Acid)-PEG (Polyethylene Glycol)-PDLLA triblock copolymer in preparing medical anti-adhesion material
The invention relates to the field of medical high polymer materials and in particular relates to a use of a PDLLA (Poly Dl Lactic Acid)-PEG (Polyethylene Glycol)-PDLLA triblock copolymer in a preparing medical anti-adhesion material. The invention aims to provide a novel choice with lower cost and better effect for the medical anti-adhesion material. The technical scheme of the invention is about a novel use of the PDLLA-PEG-PDLLA triblock copolymer in preparing the medical anti-adhesion material. The invention further provides a medical anti-adhesion material. The invention further provides a method for preparing the medical anti-adhesion material. The invention provides the novel choice for the field that needs using the medical anti-adhesion material, and therefore, the application prospect is extensive.
Owner:SICHUAN UNIV
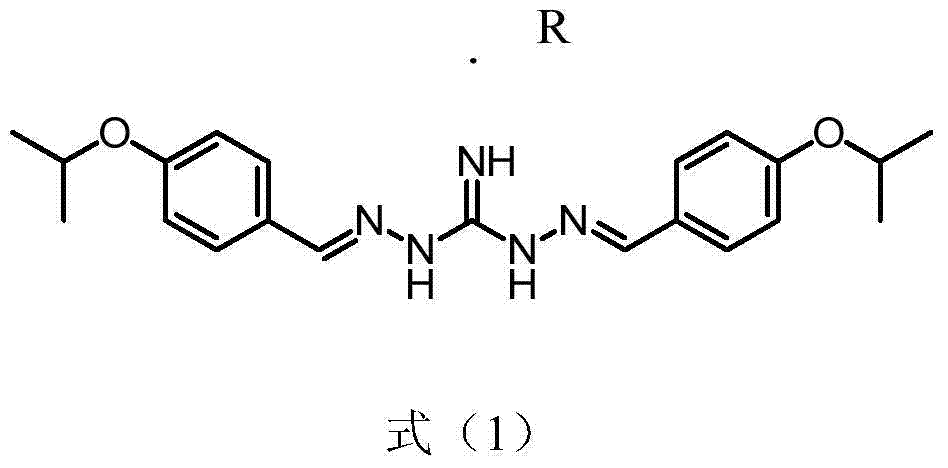
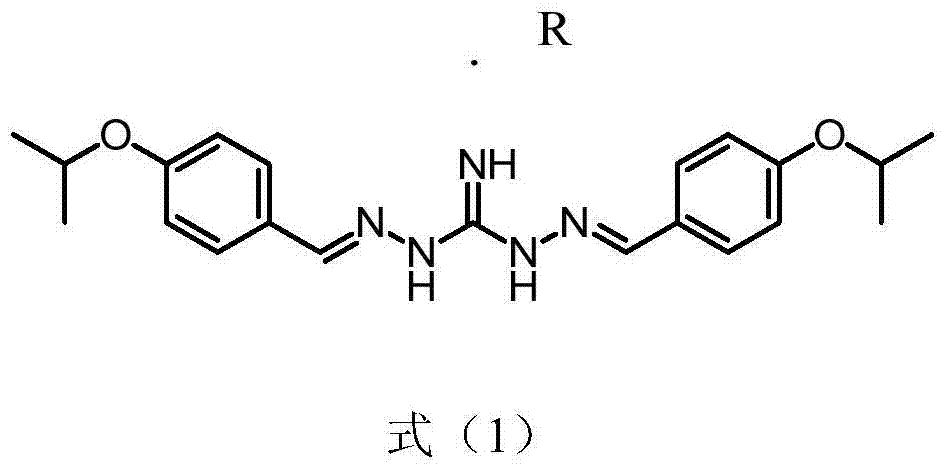
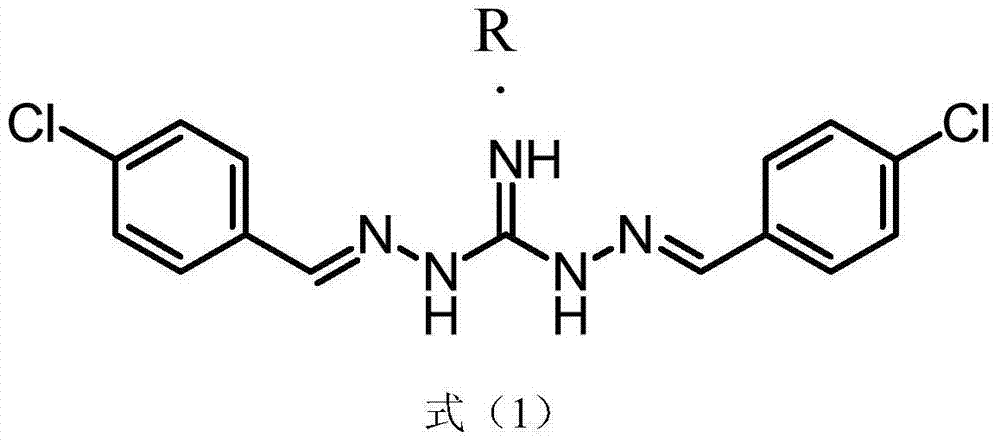
Series salt of isopropoxy phenylguanidine and application thereof in preparing feed growth promoter
InactiveCN104744312AIncrease production capacityOrganic chemistryAnimal feeding stuffBenzoic acidOXALIC ACID DIHYDRATE
The invention discloses series salt of isopropoxy phenylguanidine and application thereof in preparing an animal feed growth promoter. The structural formula of the series salt of the isopropoxy phenylguanidine is as shown in formula (1), wherein R stands for DL-lactic acid, methylsulfonic acid, 2-isethionic acid, citric acid, tartaric acid, benzoic acid, succinic acid, fumaric acid, maleic acid, acetic acid, sulfuric acid, phosphoric acid or oxalic acid. Through feeding experiments, the research firstly discovers that the series salt of the isopropoxy phenylguanidine, applied to ducks, can be used for significantly improving the production performance, and the research, through the feeding experiments, also finds that the isopropoxy phenylguanidine, applied to pigs and chickens, can be used for significantly improving the production performance as well (as shown in Specification).
Owner:GUANGZHOU INSIGHER BIOTECHNOLOGY CO LTD
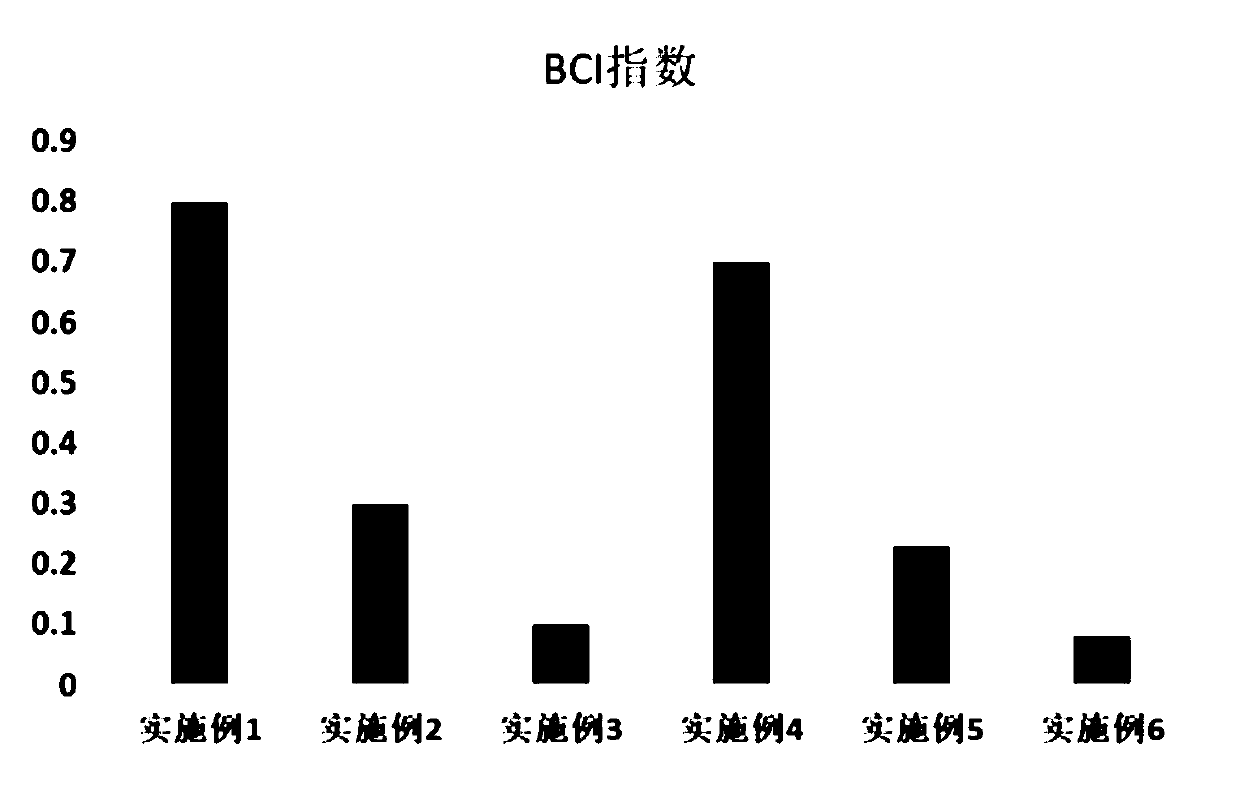
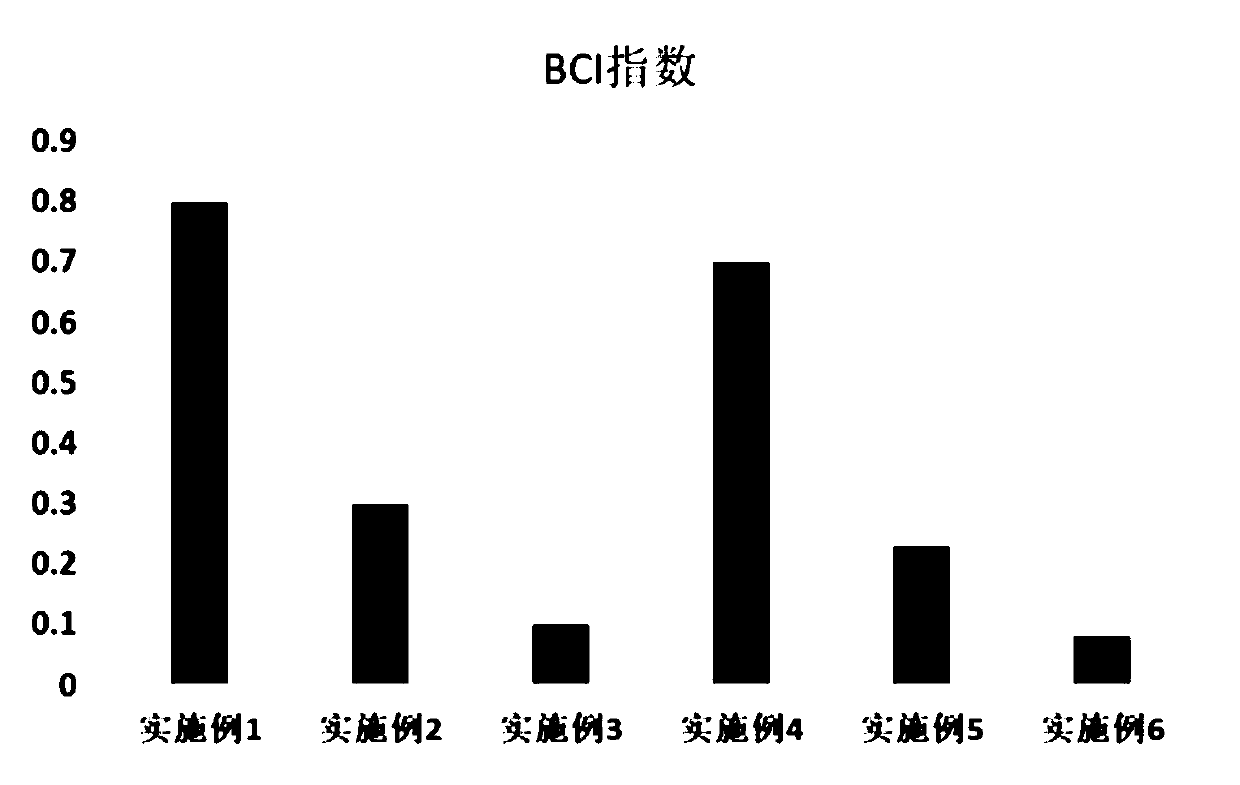
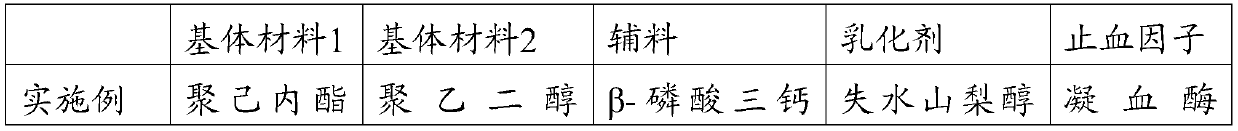
Absorbable hemostasis bone wax and preparation method thereof
The invention discloses absorbable hemostasis bone wax and a preparation method thereof. The bone wax is prepared from a degradable material, can be metabolized or absorbed by the body after being implanted for a period of time and does not stay in the body for a long time, so that probability of foreign matter infection is lowered; by adding of thrombin as a hemostasis factor, an immediate hemostasis effect is improved; due to high adhesion with a bone wound, a continuous hemostasis effect can be achieved. The absorbable hemostasis bone wax comprises a base material, an auxiliary material, anemulsifying agent and the hemostasis factor. The base material is prepared by mixing any two or more of poly-DL-lactic acid, poly-L-lactic acid, polyglycolic acid, polycaprolactone, polyethylene glycol, polyvinyl alcohol, polyether glycol, polyether triol, polylactic acid, a glycolide-lactide copolymer, a caprolactone lactide copolymer and a caprolactone-glycolide copolymer. The auxiliary material is prepared by mixing any one or two of beta-tricalcium phosphate and hydroxyapatite. The emulsifying agent is prepared by mixing any one or two of sorbitan fatty acid ester and water-soluble cellulose. Thrombin serves as the hemostasis factor.
Owner:GUANGZHOU SUN SHING BIOTECH CO LTD
Inter vertebral fusing device
InactiveCN1640368AHigh mechanical strengthGood biocompatibilityInternal osteosythesisSpinal implantsMetallic materialsCervical vertebral body
The present invention discloses a cervical synostosis device for front enter operation of cervical vertebrae. Said device is formed from upper and lower two arms, screws and a hollow column body and is prepared by using absorbable material of poly-DL-lactic acid, poly-L-lactic acid and polyglycollic acid or copolymer of them, biological ceramics or composite of hydroxyapatite and the above-mentioned polymer. The side wal of said column body can have or have not open pore, its upper surface is made into the risen form, and its upper and lower surface can have or have not pointed tooth, and its cross-section is polygonal or circular. Said invention also provides its advantages and application range.
Owner:宋跃明 +2
Preparation of absorbable medical membrane
InactiveCN101274104ASimple processDegradation time controllableAbsorbent padsBandagesSurgical operationBulk polymerization
The invention pertains to the field of preparation of medical supplies, in particular to a preparation method of a medical absorbable membrane. In the method, lactic acid and polyethylene glycol are copolymerized by a method of bulk polymerization so as to obtain the medical absorbable membrane of lactic acid / polyethylene glycol copolymer. The preparation method is characterized in that the lactic acid is poly-DL-lactic acid, the limiting viscosity number of which is 0.2 to 1; the molecular weight of polyethylene glycol is 2000 to 8000 and the proportion of each component is poly-DL-lactic acid of 1 to 99.5 percent and 0.5 to 99 percent of polyethylene glycol; the reaction temperature is 100 to 280 DEG C and the reaction time is 2 to 12 hours. The preparation method of the invention has simple process and low production cost and by adopting the preparation method, the medical absorbable membrane have the advantages of uniform thickness, stable quality, good biocompatibility and reliable degradation in human body and being capable of preventing adhesion caused in the surgical operation.
Owner:上海致远生物科技有限公司
Partially covered stent used for treating bifurcation lesion coronary perforation and provided with biological absorption film
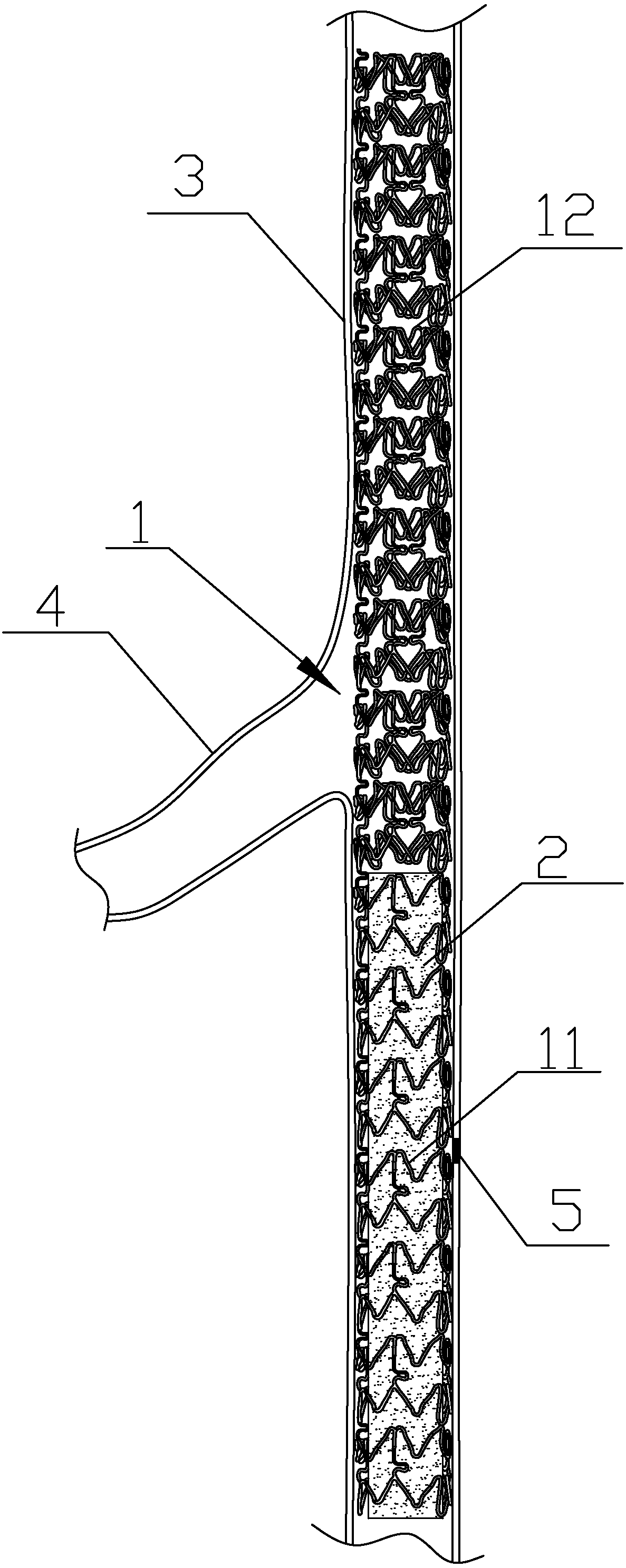
PendingCN108158701AAids in endothelializationAvoid blockageStentsMedical devicesInsertion stentThrombus
The invention relates to a partially covered stent used for treating bifurcation lesion coronary perforation and provided with a biological absorption film. The partially covered stent comprises a stent body composed of a tubular inner layer stent and a tubular outer layer stent, wherein the tubular inner layer stent and the tubular outer layer have identical length concentric line engraved nettedstructures, a tubular covering film is clamped between the inner layer stent and the outer layer stent, and the partially covered stent is characterized in that one end of the covering film is flushwith the stent body, and the length of the covering film accounts for 30-50% that of the stent body; the covering film is the biological absorption film; the biological absorption film is a poly-DL-lactic acid film. The partially covered stent has the advantages of being capable of covering a blood vessel perforation part to form a complete blood flow channel, effectively preventing closing and plugging of side branches, meanwhile easily realizing endothelialization in a stent body, and effectively reducing forming of thrombi.
Owner:TIANJIN CHEST HOSPITAL
Application of isoalantolactone derivative and salt thereof in preparation of medicines for treating lung fibration
ActiveCN106496243AGood treatment effectOrganic active ingredientsOrganic chemistryBenzoic acidPhosphomolybdic acid
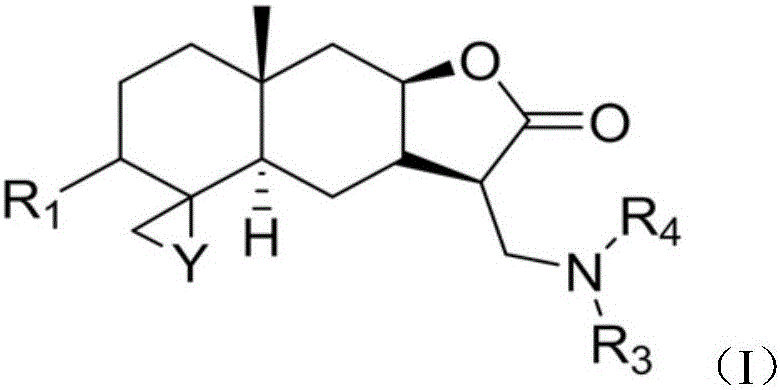
The invention relates to an application of an isoalantolactone derivative and a salt thereof in the preparation of medicines for treating lung fibration, and provides an isoalantolactone derivative represented by formula (I). An acid for forming the salt is an inorganic acid or an organic acid, the inorganic acid is selected from hydrofluoric acid, hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, nitric acid, phosphoric acid, carbonic acid, boric acid, seleninic acid, phosphomolybdic acid, phosphorous acid and sulfurous acid, and the organic acid is selected from citric acid, maleic acid, D-malic acid, L-malic acid, DL-malic acid, L-lactic acid, D-lactic acid, DL-lactic acid, oxalic acid, methanesulfonic acid, pentanoic acid, oleic acid, lauric acid, p-methyl benzenesulfonic acid, 1-naphthalenesulfonic acid, 2-naphthalenesulfonic acid, phthalic acid, tartaric acid, propane diacid, succinic acid, fumaric acid, glycollic acid, thioglycollic acid, glycine, sarcosine, sulfonic acid, nicotinic acid, methylpyridine acid, isonicotinic acid, benzoic acid and substituted benzoic acid.
Owner:NANKAI UNIV
Absorbable bone wax and preparation method thereof
InactiveCN109893677ASolve AbsorbencySolve the defects left in the body that are not conducive to wound healingSurgical adhesivesCelluloseLactide
The invention provides absorbable bone wax. The technical scheme is characterized in that the absorbable bone wax comprises a matrix material, an auxiliary material, an emulsifier and a hemostatic factor, wherein the matrix material is a mixture of any two or more of poly-DL-lactic acid, poly-L-lactic acid, polyglycolic acid, polycaprolactone, polyethylene glycol, polyvinyl alcohol, polyether diol, polyether triol, polylactic acid, poly(L-lactide-co-glycolide), a caprolactone-lactide copolymer and a caprolactone-glycolide copolymer; the auxiliary material is any one or a mixture of two of beta-tricalcium phosphate and hydroxyapatite; the emulsifier is any one or a mixture of two of sorbitan fatty acid ester and water soluble cellulose. On account of weight percentage wt%, the matrix material accounts for 20%-90%, the auxiliary material accounts for 10%-80%, and the emulsifier is 1%-20% of the total mass of the matrix material and the auxiliary material.
Owner:GUANGZHOU SUN SHING BIOTECH CO LTD
Polylactic acid block copolymer
The invention relates to a polylactic acid block copolymer, which solves the problems that current method is hard to prepare the polylactic acid block copolymer with high-melting point polyester. The polylactic acid block copolymer is a two-block copolymer which is shown as A-b-B, wherein A is a single-end hydroxyl aromatic polyester block, and B is a polylactic acid block. The polylactic acid block is one or more of a poly-L-lactic acid block, a poly-D-lactic acid block and a poly-DL lactic acid block. The polylactic acid block copolymer is prepared by the following step of: adopting an organic solvent which can dissolve reactants of single-end hydroxyl aromatic polyester and lactide to be a reaction medium, tin salt as a catalyst, and the single-end hydroxyl aromatic polyester as an initiator to initiate the ring opening polymerization of lactide. With the adoption of the polylactic acid block copolymer, the polymerizing temperature can be controlled to reach a scope in which polylactic acid and lactide cannot be degraded, and the racemization cannot be carried out, so that the generation of transesterification can be effectively inhibited, the regularity of the chain section can be ensured, and the block copolymers of polyester and the polylactic acid can be successfully prepared, including aromatic polyester with the high-melting point.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
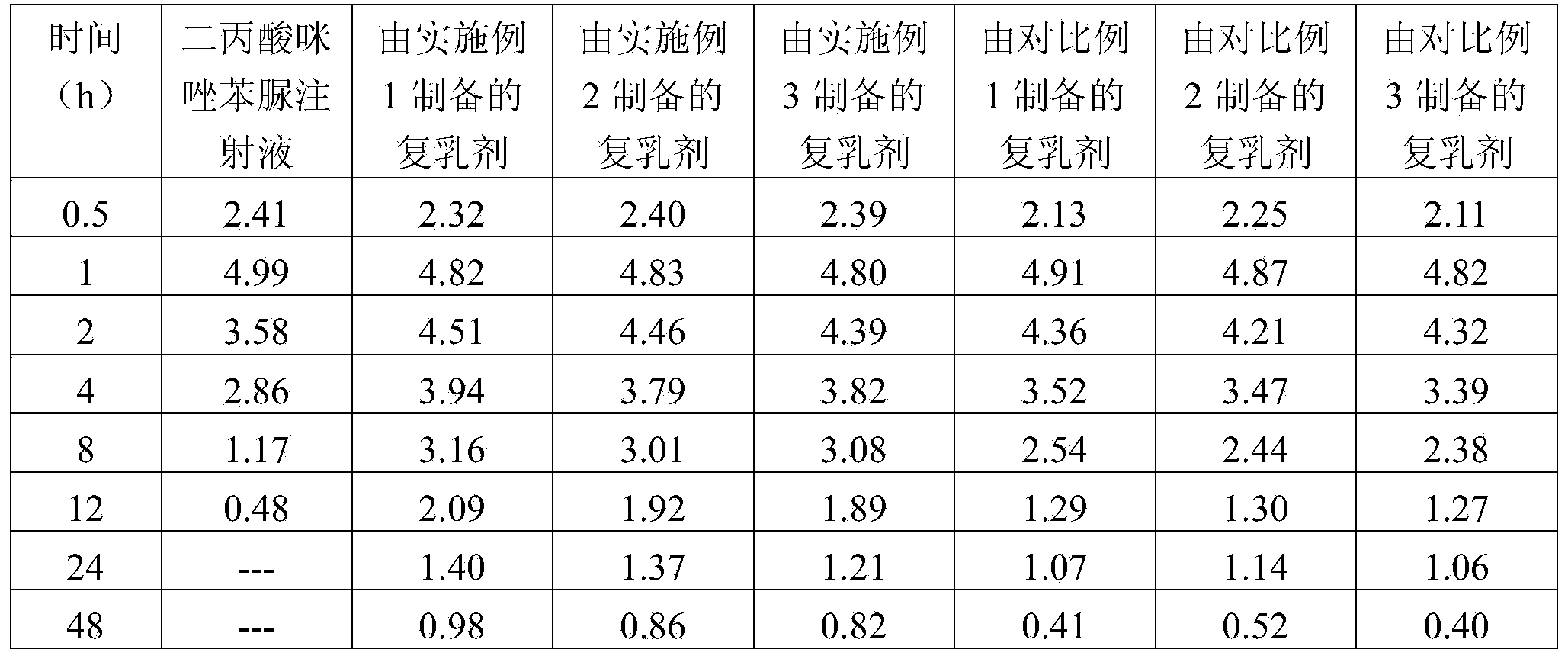
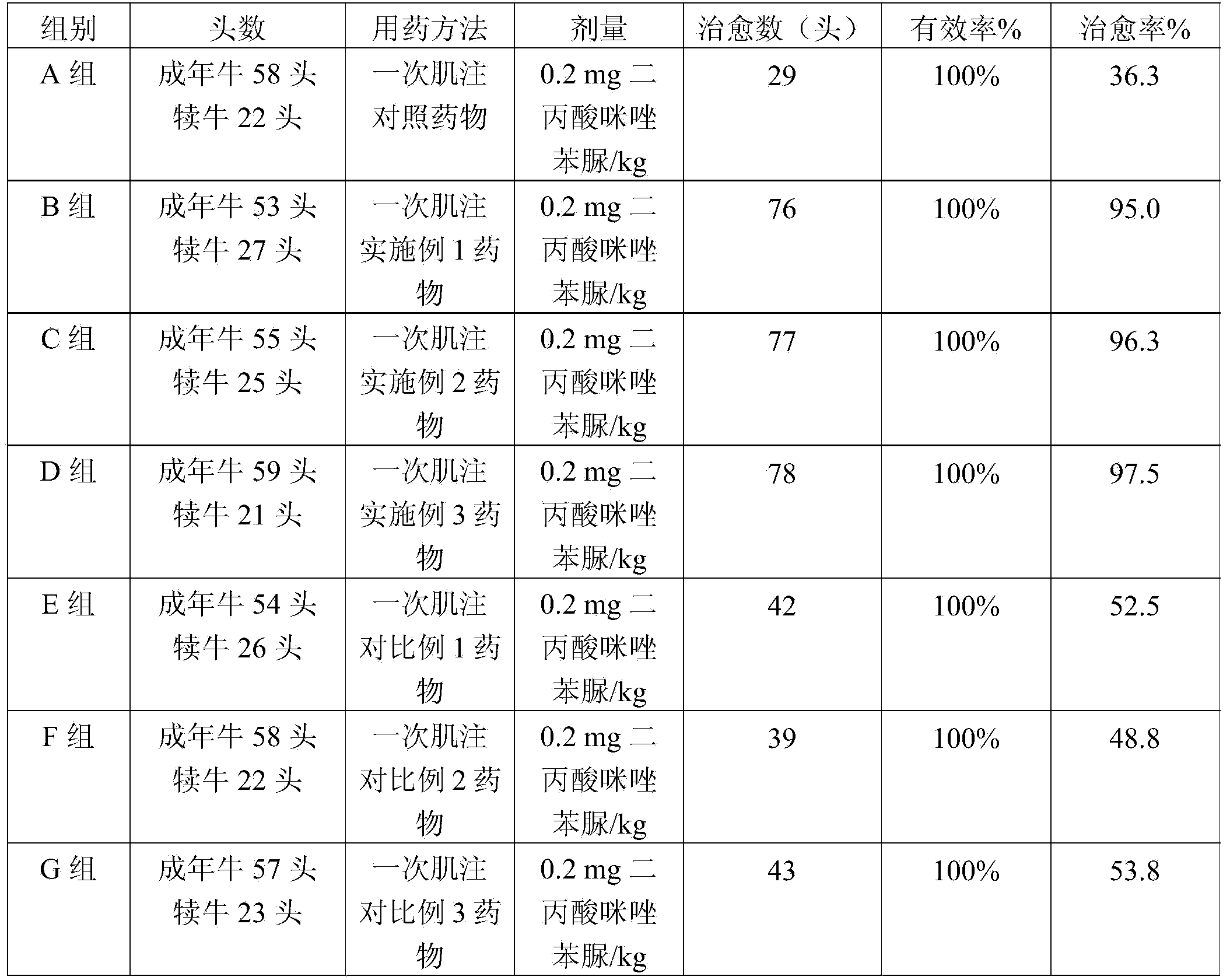
Preparation method of veterinary long-acting preparation of imidocarb dipropionate
ActiveCN103520119AReduce releaseEnsure the effect of epidemic preventionOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereBovine babesiosis
The invention relates to a preparation method of a veterinary long-acting preparation of imidocarb dipropionate. The preparation method comprises the following steps: (1) dissolving imidocarb dipropionate into water for injection to obtain an internal water phase; (2) dissolving a polylactic acid-glycolic acid copolymer into an organic solvent to obtain an organic phase; (3) adding the internal water phase into the organic phase, and emulsifying to obtain primary emulsion; (4) adding the primary emulsion into a sodium chloride solution of polyvinyl alcohol, and emulsifying to obtain multiple emulsion; (5) adding the multiple emulsion into a sodium chloride solution, removing impurities, and drying, thereby obtaining the long-acting preparation. The veterinary long-acting preparation of imidocarb dipropionate is prepared from poly(DL-lactic acid-glycolic acid) copolymer with the specific mole ratio of monomer polylactic acid and glycolic acid as an auxiliary material and imidocarb dipropionate microspheres prepared by the secondary emulsification method. By adopting the preparation, the defects that an existing medicament for treating bovine babesiosis is short in time of duration, is required to be fed repeatedly, and cannot maintain the prevention effect for a long time are solved.
Owner:QILU ANIMAL HEALTH PROD +1
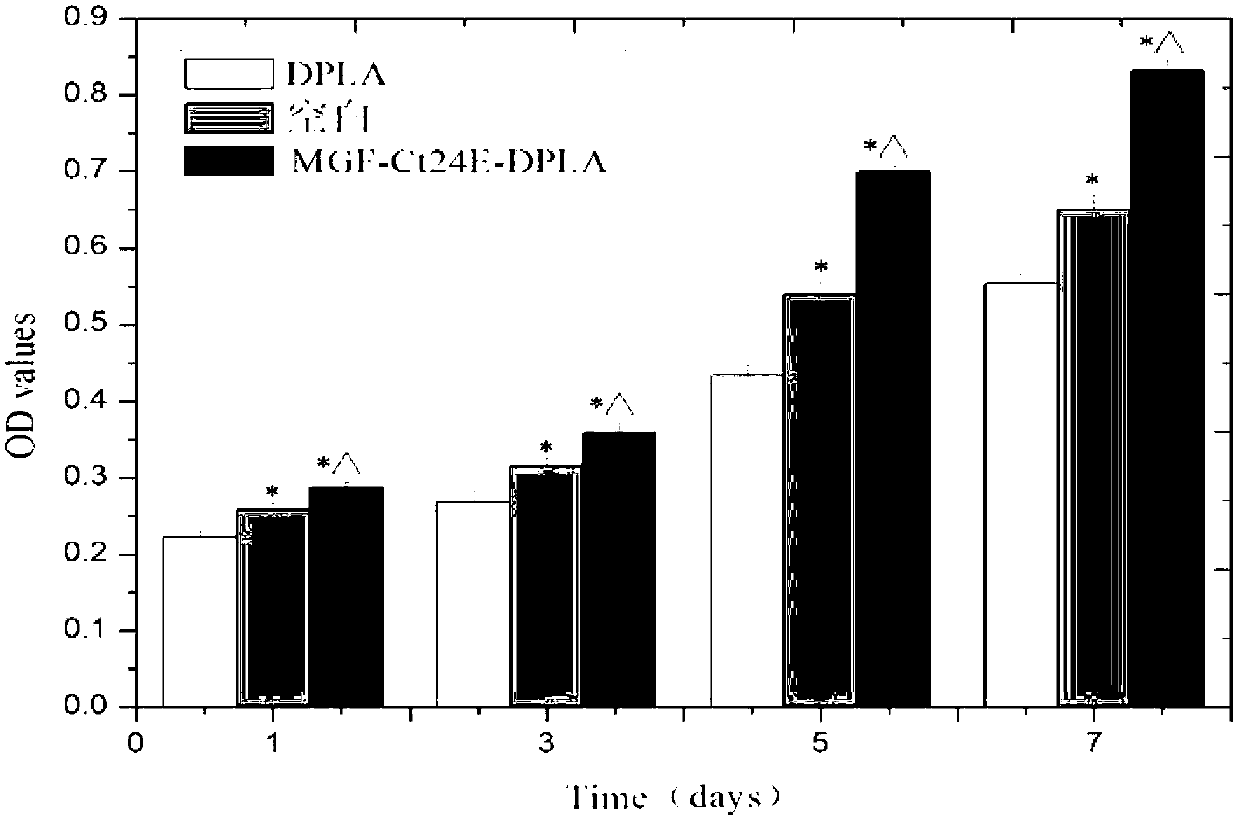
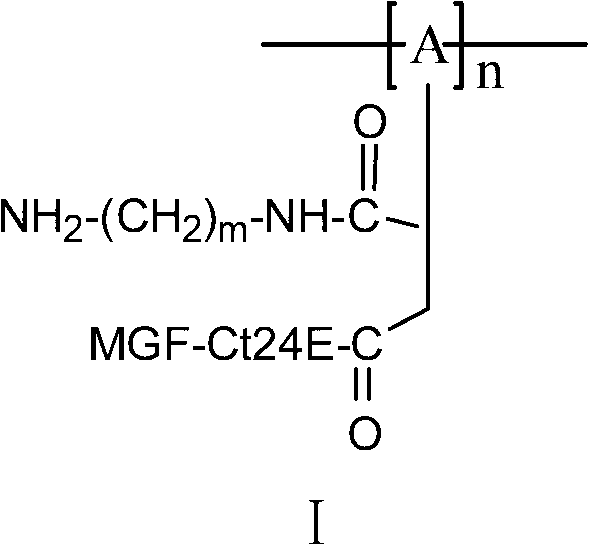
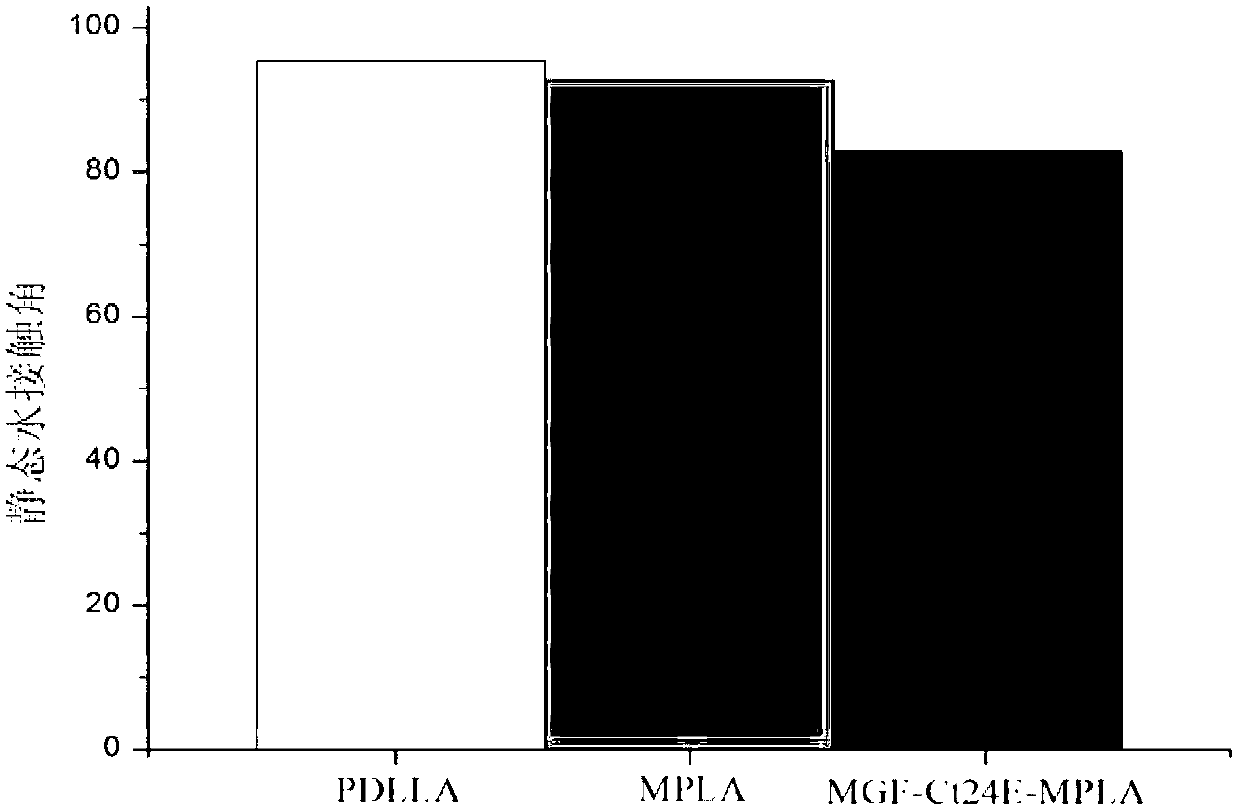
Poly(DL-lactic acid) material modified based on 24 peptides in E domain of mechano-growth factor (MGF) and diamine, and preparation method and application thereof
The invention relates to a poly(DL-lactic acid) material modified based on 24 peptides in E domain of MGF and diamine. The molecular formula of the poly(DL-lactic acid) material is represented by I. A preparation method for the poly(DL-lactic acid) material is as follows: diamine reacts with maleic anhydride modified poly(DL-lactic acid) to produce diamine-maleic anhydride modified poly(DL-lactic acid); diamine-maleic anhydride modified poly(DL-lactic acid) and the 24 peptides in the E domain of MGF are used as raw materials, one selected from the group consisting of DCC, EDC and a mixture is used as a condensing agent, then the raw materials and the condensing agent are subjected to a reaction at a temperature of 0 to 50 DEG C for 8 to 48 h, an obtained reaction solution is added into an excess aqueous medium, a membrane-like precipitate is collected, and the membrane-like precipitate is the poly(DL-lactic acid) material modified based on the 24 peptides in the E domain of MGF and diamine. The invention also discloses application of the poly(DL-lactic acid) material modified based on the 24 peptides in the E domain of MGF and diamine in preparation of bioactive bionic materials. The material provided in the invention has better hydrophilicity, cellular affinity and biodegradability and is expected to mitigate related problems caused by understressing during the process of tissue repair or regeneration treatment.
Owner:CHONGQING UNIV
Bone cement filling balloon structure
ActiveCN102835995AHas bone conduction propertiesOsteogenic activitySurgeryPolyethylene glycolAbsorbent material
The invention discloses a bone cement filling balloon structure, which comprises a pipeline-shaped injector, and a balloon connected onto the lower end of the injector, wherein a preassembled rod extending into the balloon is arranged in an inner cavity of the injector; the balloon is made of absorbable materials, such as poly-L-lactic acid, poly-DL-lactic acid, polyglycolic acid, polycaprolactone, polyethylene glycol, poly-di-chalk alkyl ketone, polytrimethylene carbonate, and a copolymer or a polymer blend thereof; and the molecular weight of the absorbable materials is 5,000-1,000,000. The bone cement filling balloon structure disclosed by the invention has the advantages that not only is the distribution of bone cement in a vertebral body be effectively controlled to prevent the bone cement from leaking in quantity, but also the bone cement can be absorbed in the body; and the bone cement filling balloon structure is simple to operate during an operation.
Owner:ZHEJIANG APELOA JIAYUAN BIOMEDICAL MATERIAL +1
Production method of polylactic acid-trimethylene carbonate nano-fiber film
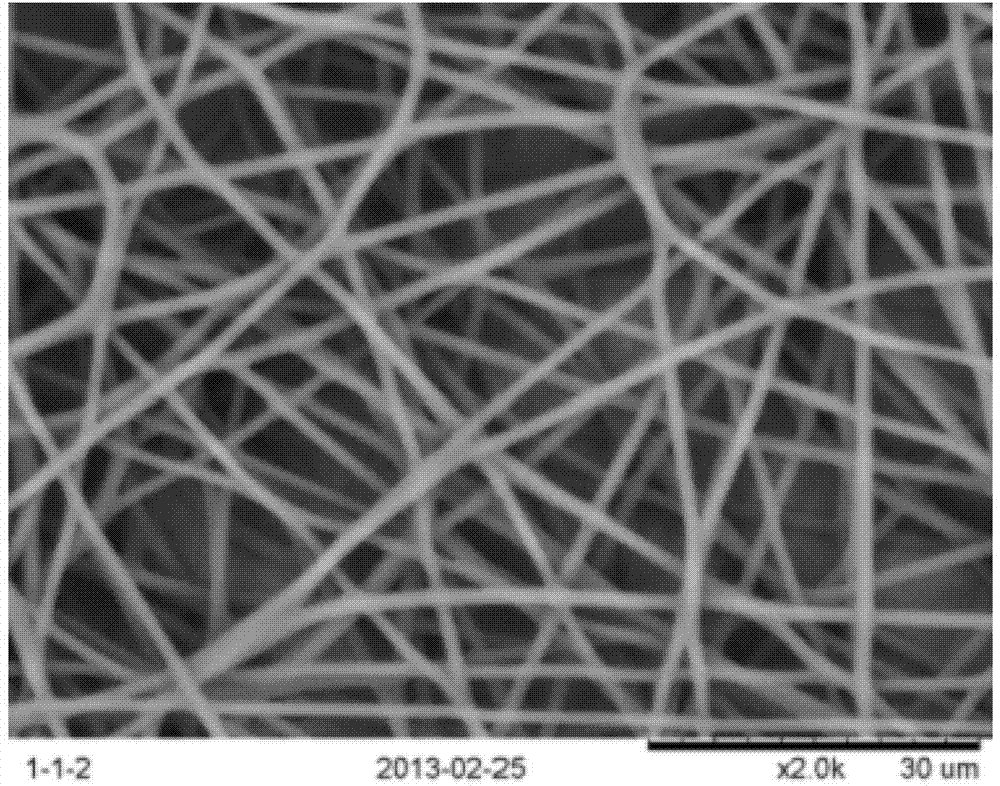
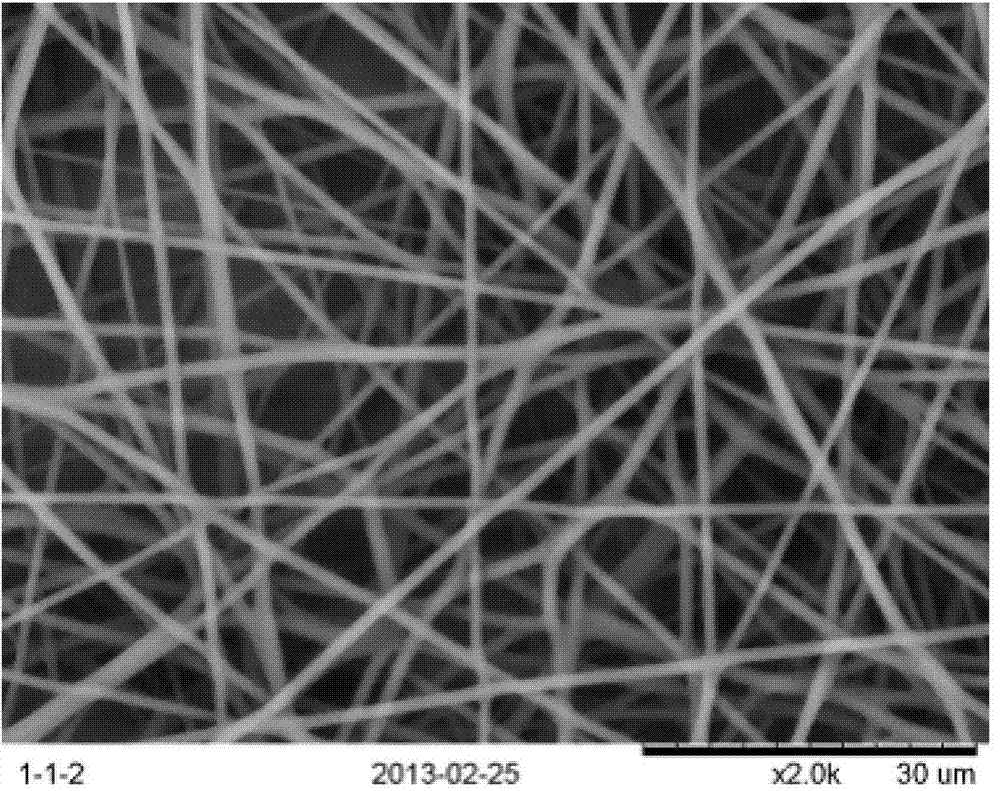
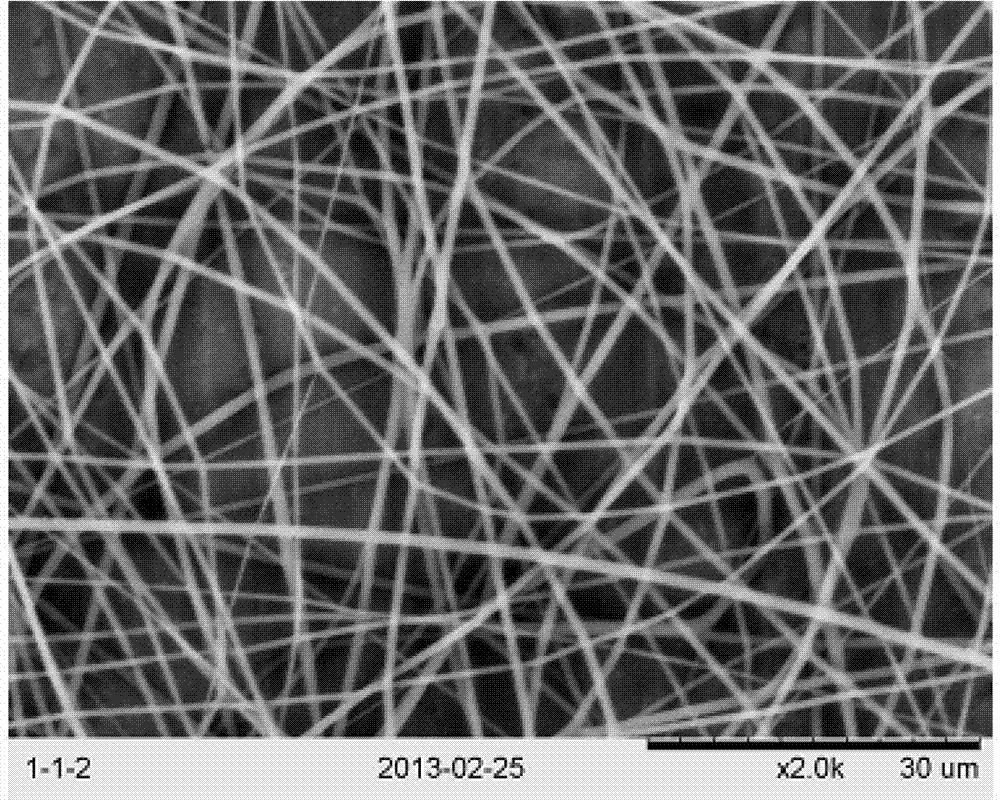
InactiveCN103397477AAchieve minimally invasive implantationSolving the Stress Shading ProblemFilament/thread formingNon-woven fabricsFiberOrganic solvent
The invention relates to a production method of polylactic acid-trimethylene carbonate nano-fiber film. The method includes: dissolving PDLLA-PTMC (poly-DL-lactic acid and poly trimethylene carbonate) into organic solvent to obtain spinning dope, performing electrospinning, collecting fiber film, and vacuum-drying to obtain the polylactic acid and trimethylene carbonate nano-fiber film. The PDLLA-PTMC is 10%-25%w / w in the spinning dope in terms of concentration. The produced nano-fiber film is shape memory film featuring imitated natural extracellular matrix, and can provide cells with an ideal microenvironment for growth, proliferation and differentiation.
Owner:DONGHUA UNIV
Complexus containing polylactic acid and native copper, and its use
InactiveCN101002966AOsteoinductive activityPromote value-addedSurgeryProsthesisPyriteBiocompatibility Testing
A compound able to be used to prepare various medical devices with high biocompatibility, absorptivity and bone inducing function is prepared from the polyacetic acid chosen from poly-L-lactic acid, poly-DL-lactic acid and lactic acid- oxyacetic acid copolymer and the pyrite in weight ratio of (60-99%): (1-40%).
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Hemostatic material in medical use
InactiveCN100344335CGood biocompatibilityHigh mechanical strengthSurgical adhesivesPharmaceutical containersLactideTriol
A medical styptic material able to be fully absorbed and degradated is composed of the basic material prepared by fusion polycondensation reaction of low-molecular poly-DL-lactic acid or poly-L-lactic acid or polyethanol-acid copolymer / mixture, and the auxiliary which may be polyether diol, polyether triol, high-molecular polylactic acid, high-molecular glycolide-lactide copolymer, high-molecular caprolactone-lactide copolymer, or high-molecular caprolactone-glycolide copolymer through fusion and mixing.
Owner:HUIZHOU HUAYANG MEDICAL EQUIP
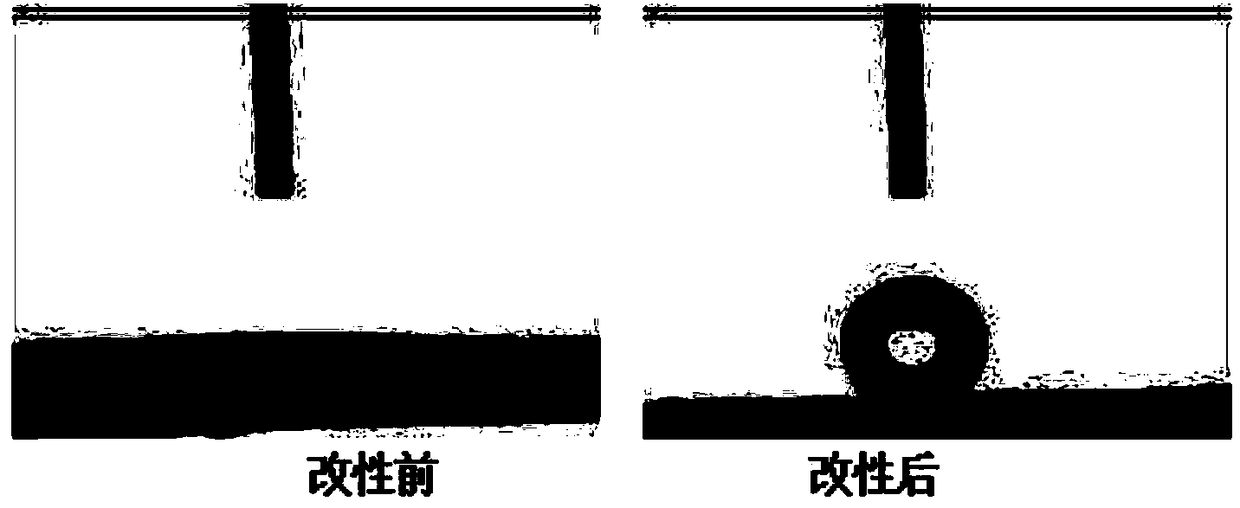
Surface hydrophobic modification method of nano cellulose
InactiveCN108610474ALengthy exchange processProcess environmental protectionOrganic solventDistillation
The invention relates to a surface hydrophobic modification method of nano cellulose. The method comprises: preparing a nano cellulose suspension first, then adding a DL-lactic acid solution, and uniformly dispersing the materials to obtain a mixed solution; then adding a metal oxide catalyst, uniformly stirring the materials, and heating the mixture to 150-200 DEG C for distillation; when 50-100%of water in the mixed solution is distilled out, adding an esterification modifier and a metal oxide catalyst, stirring the materials evenly and raising the temperature to 170-220 DEG C for reaction;and after the reaction, adding the reaction product into ethanol and carrying out centrifugal separation to obtain a solid which is modified nano cellulose. The method provided by the invention solves the problems of complicated and lengthy steps, high organic solvent consumption and the like existing in a modification method in the prior art, has few steps, avoids a lengthy solvent exchange process, can recycle excessive reaction reagents after reaction, and has obvious economic and environmental protection advantages.
Owner:NANJING FORESTRY UNIV
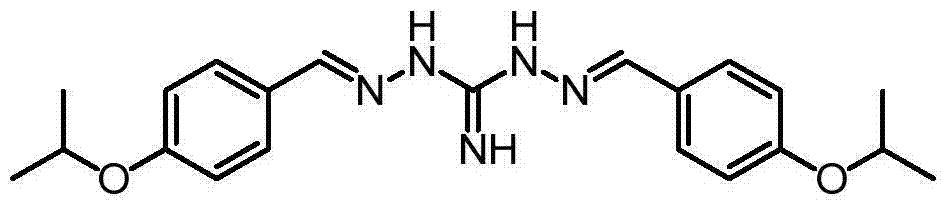
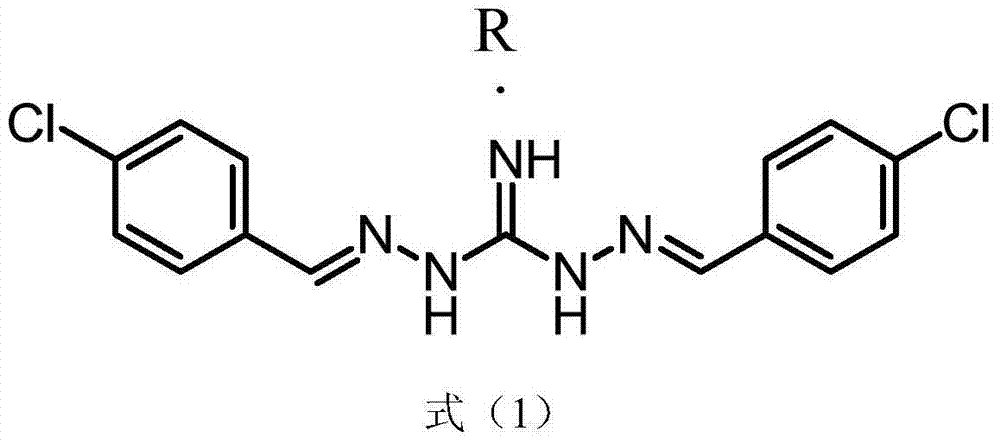
Series salts of robenidine and application thereof in preparation of animal feeding growth promoter
InactiveCN104744313AIncrease production capacityOrganic chemistryAnimal feeding stuffBenzoic acidPhosphoric acid
The invention discloses series salts of robenidine and an application thereof in preparation of an animal feeding growth promoter. The series salts of robenidine have a structural formula shown in the formula (1), wherein R is DL-lactic acid, methanesulfonic acid, 2-isethionic acid, citric acid, tartaric acid, benzoic acid, succinic acid, fumaric acid, maleic acid, acetic acid, sulfuric acid, phosphoric acid or oxalic acid. Through feeding experiments, the research, for the first time, discovers that after application of the series salt compounds of robenidine, the production performance of ducks is improved obviously. Through animal feeding experiments, the research also discovers that after application of the series salt compounds of robenidine, the production performance of sows and hens is improved.
Owner:GUANGZHOU INSIGHER BIOTECHNOLOGY CO LTD
Magnetic polylactic acid tissue engineering bracket
The invention discloses a magnetic polylactic acid tissue engineering bracket. A magnet tissue engineering bracket suitable for targeting of a magnetic targeting medicine feeding system is produced by adding Fe3O4 into a polylactic acid tissue engineering bracket and is a multiporous material containing the Fe3O4; the polylactic acid comprises poly-L-lactic acid, poly-R-lactic acid or poly-dl-lactic acid. The toxic action of medicines to the whole body is reduced, a complicated step of the medicine feeding process is reduced, and the magnetic polylactic acid tissue engineering bracket can be used for other treatment measures taking magnetism as targeting.
Owner:SHANDONG UNIV
Disposable polylactic acid composite material for injector
Owner:WEIGAO HLDG +1
Poly(DL-lactic acid) material modified based on 24 peptides in E domain of mechano-growth factor (MGF), and preparation method and application thereof
The invention relates to a polypeptide modified poly(DL-lactic acid) material. The molecular formula of the poly(DL-lactic acid) material is represented by I or II; and in the formula, A is one selected from the group consisting of D-lactic acid, L-lactic acid and D,L-lactic acid, n is equal to 100 to 20000, and Ct24E-MGF is 24 peptides in the E domain of MGF and has an amino acid sequence as represented by SEQ ID NO. 1. A preparation method for the polypeptide modified poly(DL-lactic acid) material is as follows: maleic anhydride modified poly(DL-lactic acid) having a molecular formula as represented by III and the 24 peptides in the E domain of MGF represented by SEQ ID NO. 1 are used as raw materials, DCC, EDC and NHS are used as condensing agents, then the raw materials and the condensing agents are subjected to a reaction at a temperature of 0 to 50 DEG C for 8 to 48 h, an obtained reaction solution is added into an excess aqueous medium, a membrane-like precipitate is collected, and the membrane-like precipitate is the poly(DL-lactic acid) material modified based on the 24 peptides in the E domain of MGF. Compared to poly(DL-lactic acid) materials which are not modified by polypeptide, the poly(DL-lactic acid) material provided in the invention has better hydrophilicity and cellular affinity and can activate growth of osteoblasts and vascular endothelial cells and promote regeneration of blood vessels and bone tissue.
Owner:CHONGQING UNIV
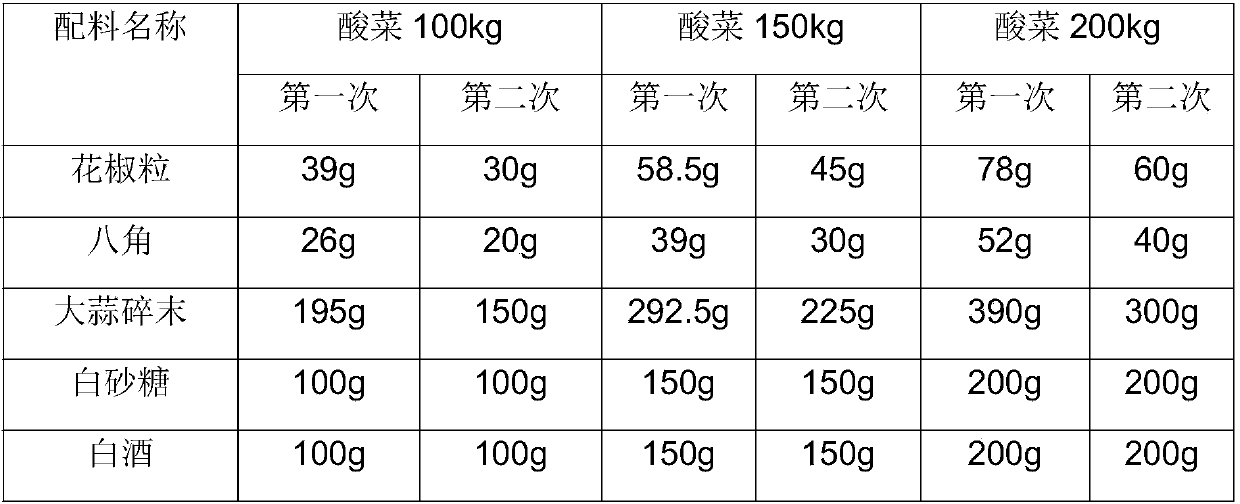
Secondary fermentation technique for pickled Chinese cabbage bag
The invention provides a secondary fermentation technique for a pickled Chinese cabbage bag. The secondary fermentation technique comprises the following process steps of checking and accepting raw materials, treating the checked and accepted raw materials, performing cleaning, detecting salinity, performing loading in a barrel, adding seasoning, adding salt water, performing pressing with stones,and performing fermentation, wherein the seasoning is prepared from Chinese prickly ash granules, star aniseeds, chopped garlic, white granulated sugar and Baijiu, and the Chinese prickly ash granules, the star aniseeds and the chopped garlic are uniformly stirred; the compounding proportion of salt water is that the proportion of boiled fish with pickled Chinese cabbages and chili to the salt water is 100 to 45, the level of the salt water needs to be higher than the level of pickled Chinese cabbages, and the salt water contains 0.2kg of DL-lactic acid, 6-8k of salt and 94-92k of water whichform 100kg. The secondary fermentation technique for a pickled Chinese cabbage bag, provided by the invention, has the beneficial effects that a special fermentation process and a special seasoning formula are used, and the pickled Chinese cabbage bag in the market is subjected to secondary fermentation, so that the mouth feel of the pickled Chinese cabbages is improved, the flavor of the pickledChinese cabbages is improved, and the vacancy of a secondary fermentation process of the pickled Chinese cabbages in the market is filled.
Owner:芜湖市风蝉电竞文化传媒有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com