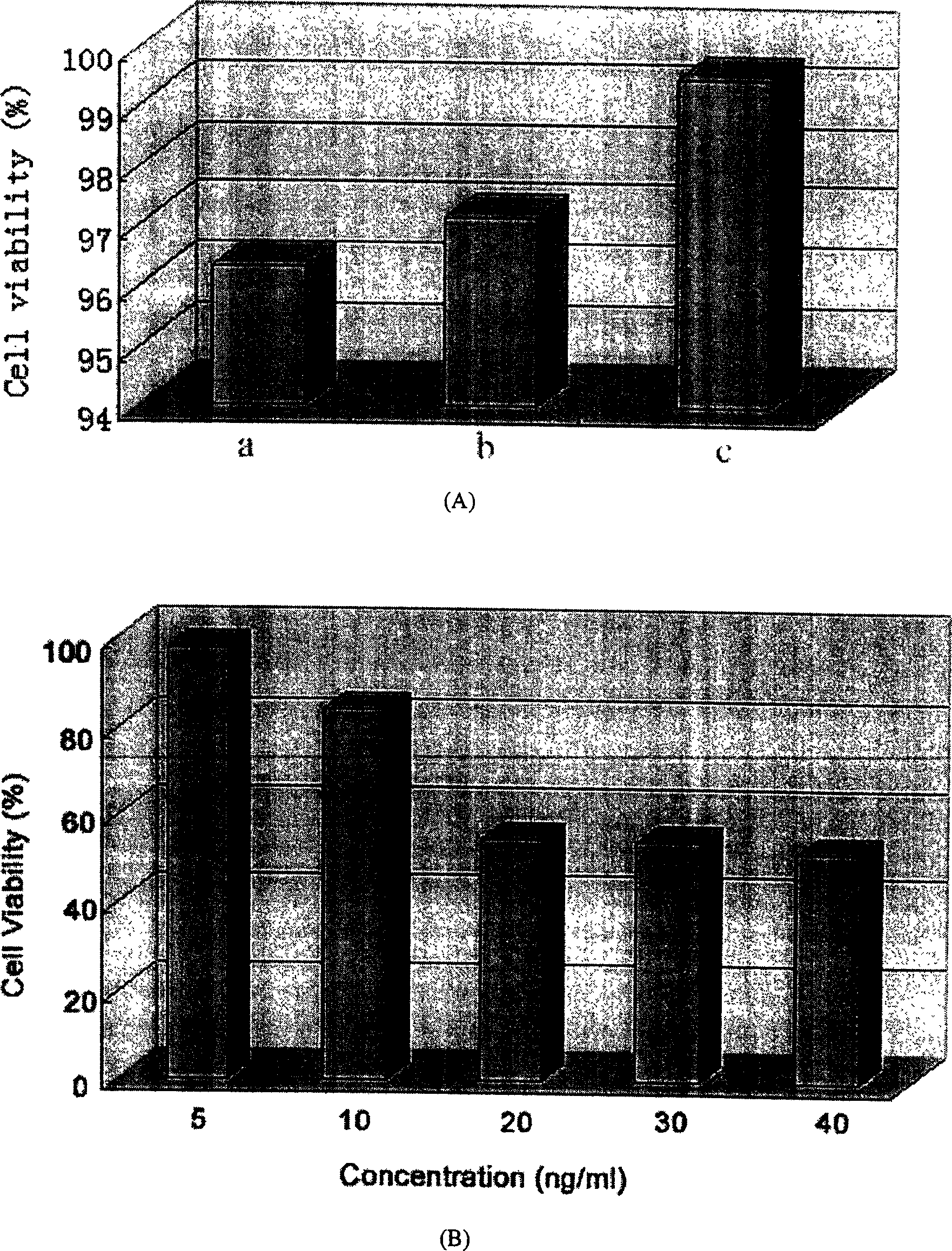
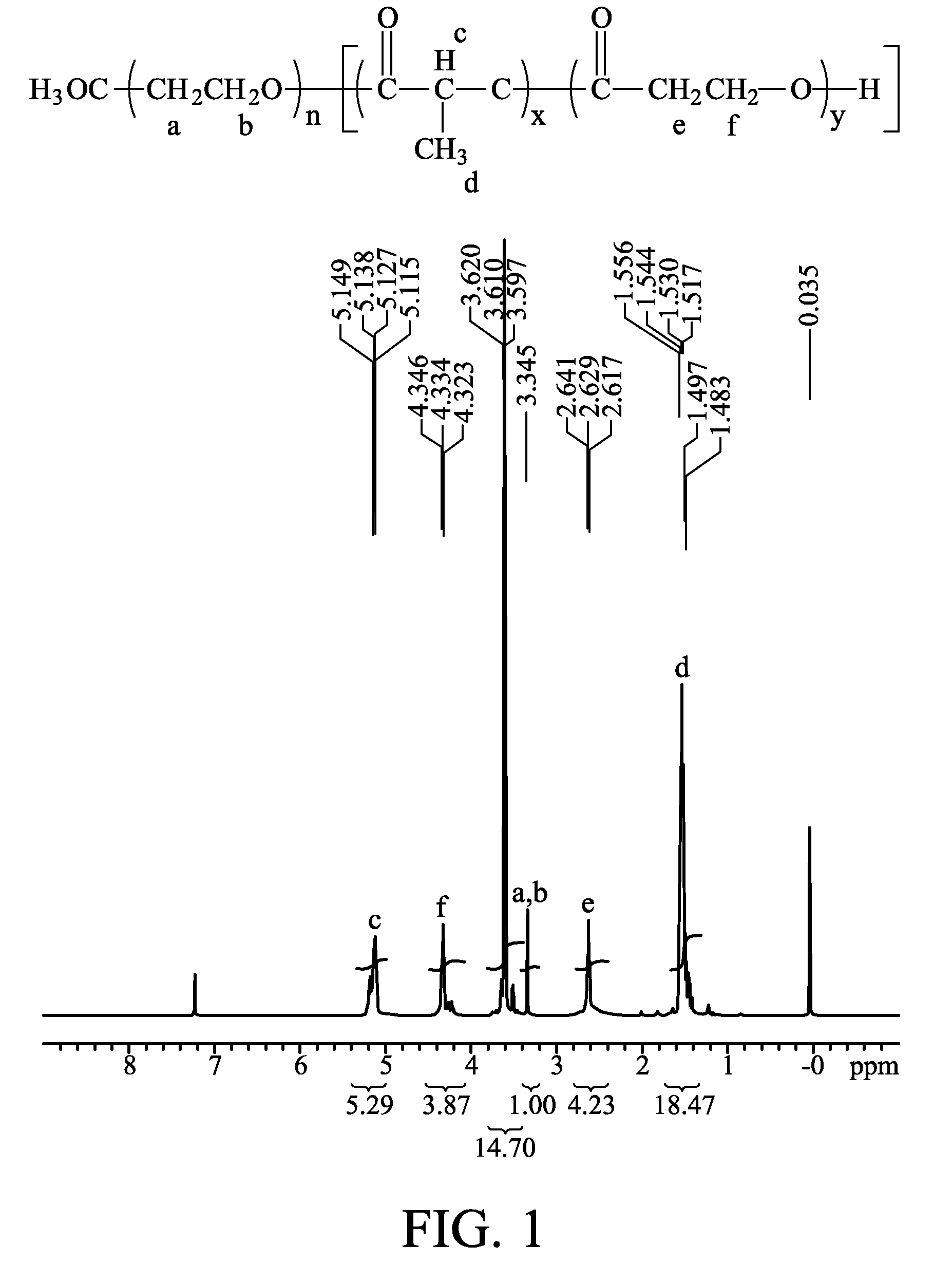
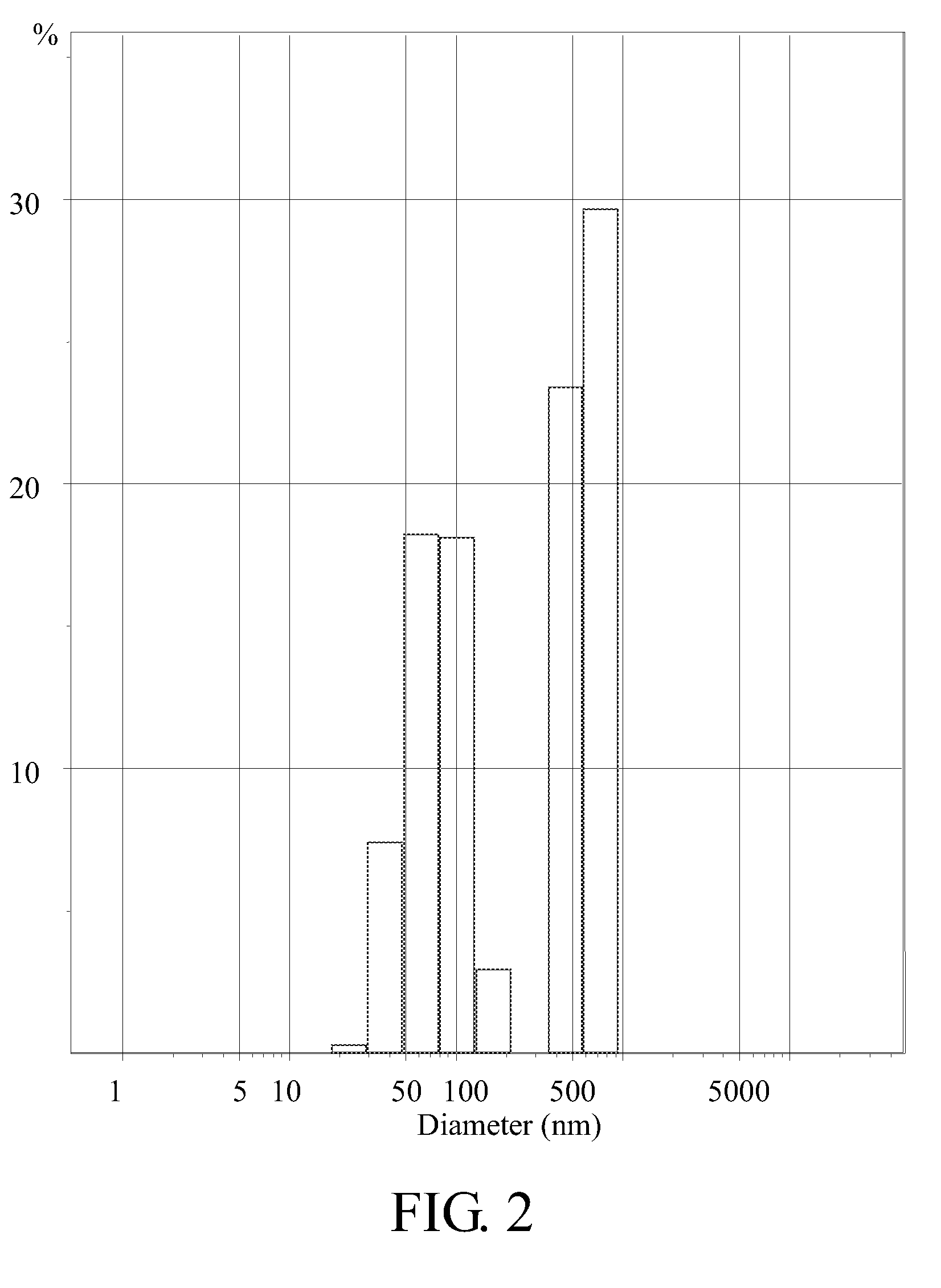
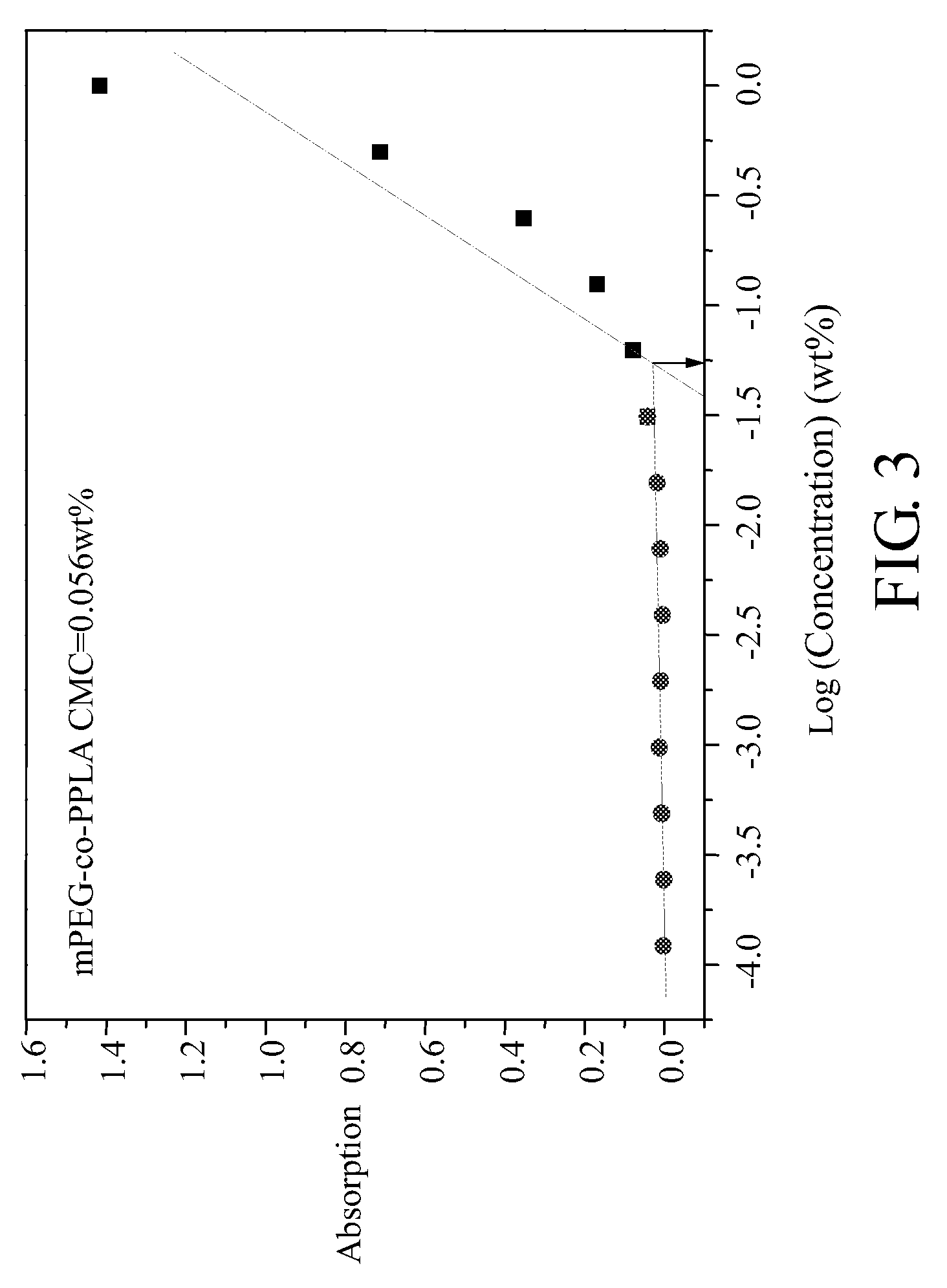
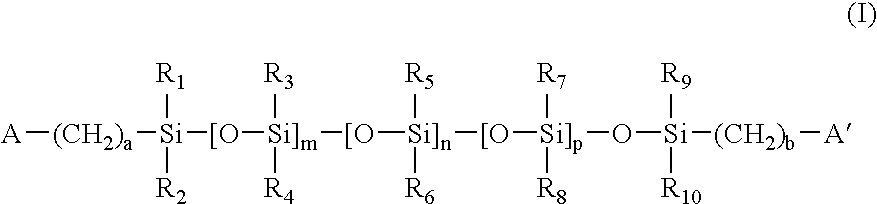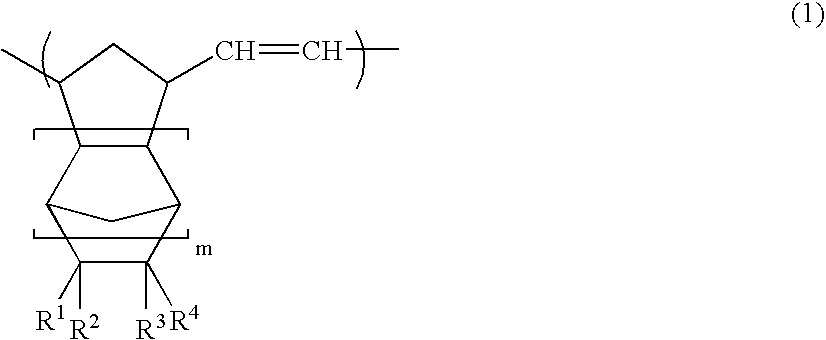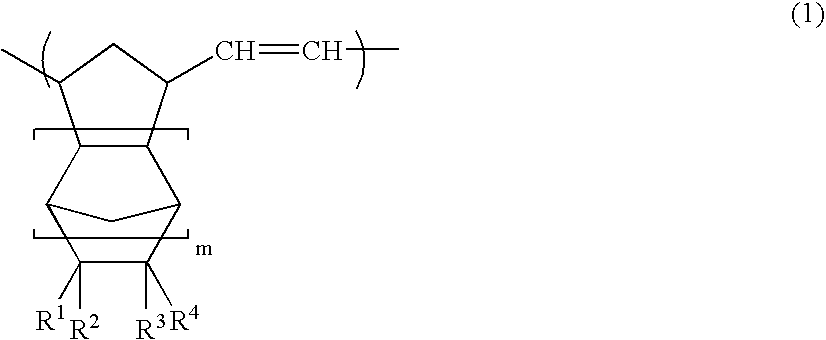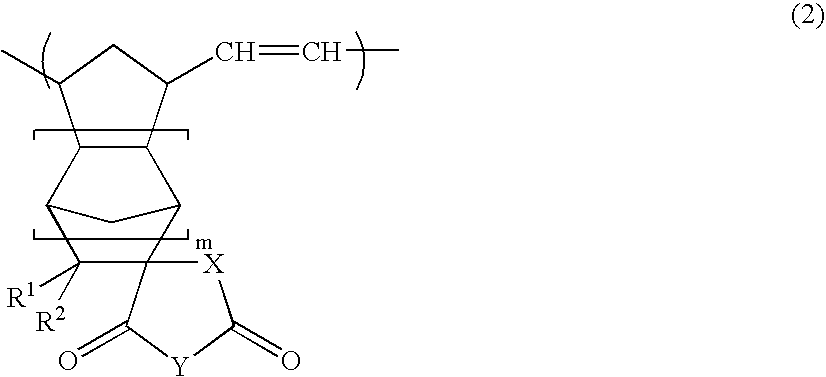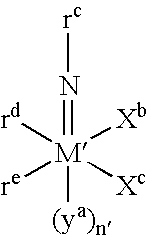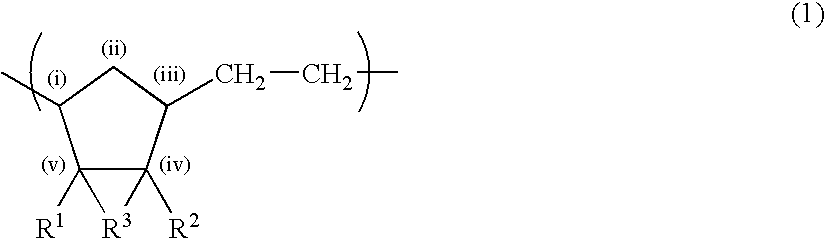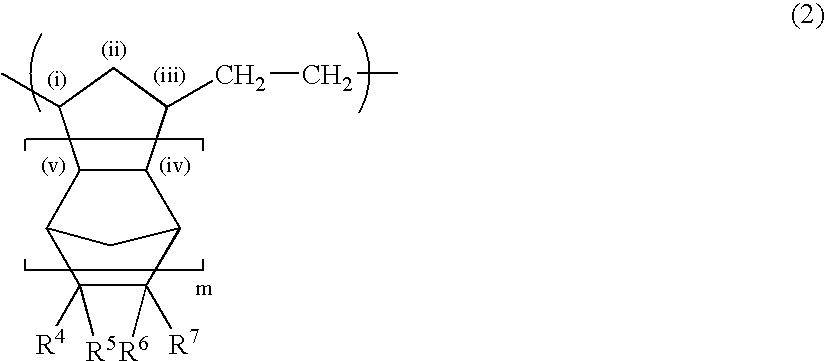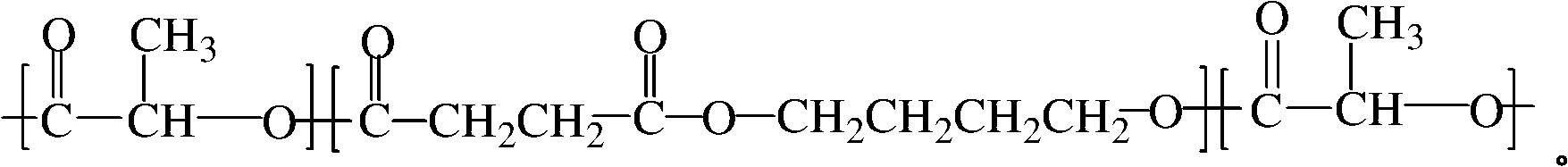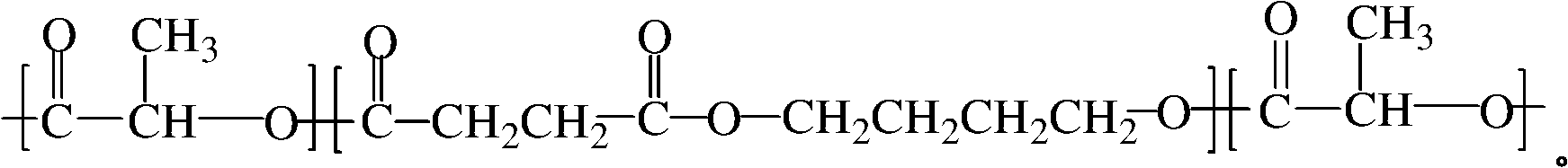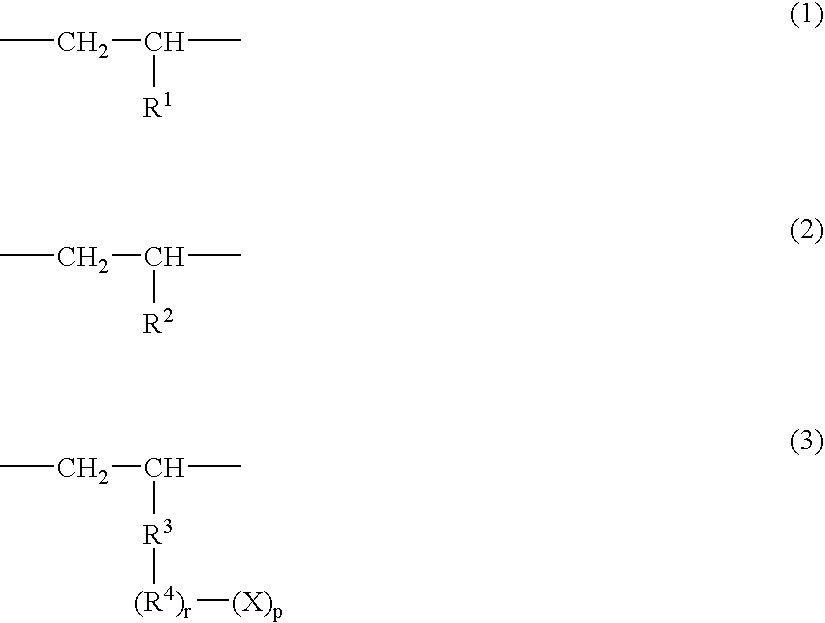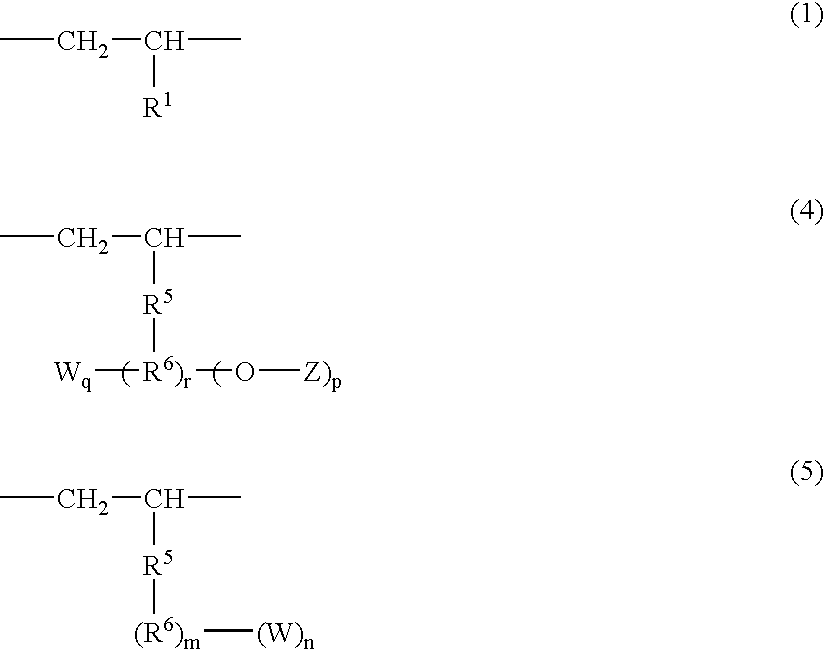Patents
Literature
2496 results about "Ring-opening polymerization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers.
Polymer coating for medical devices
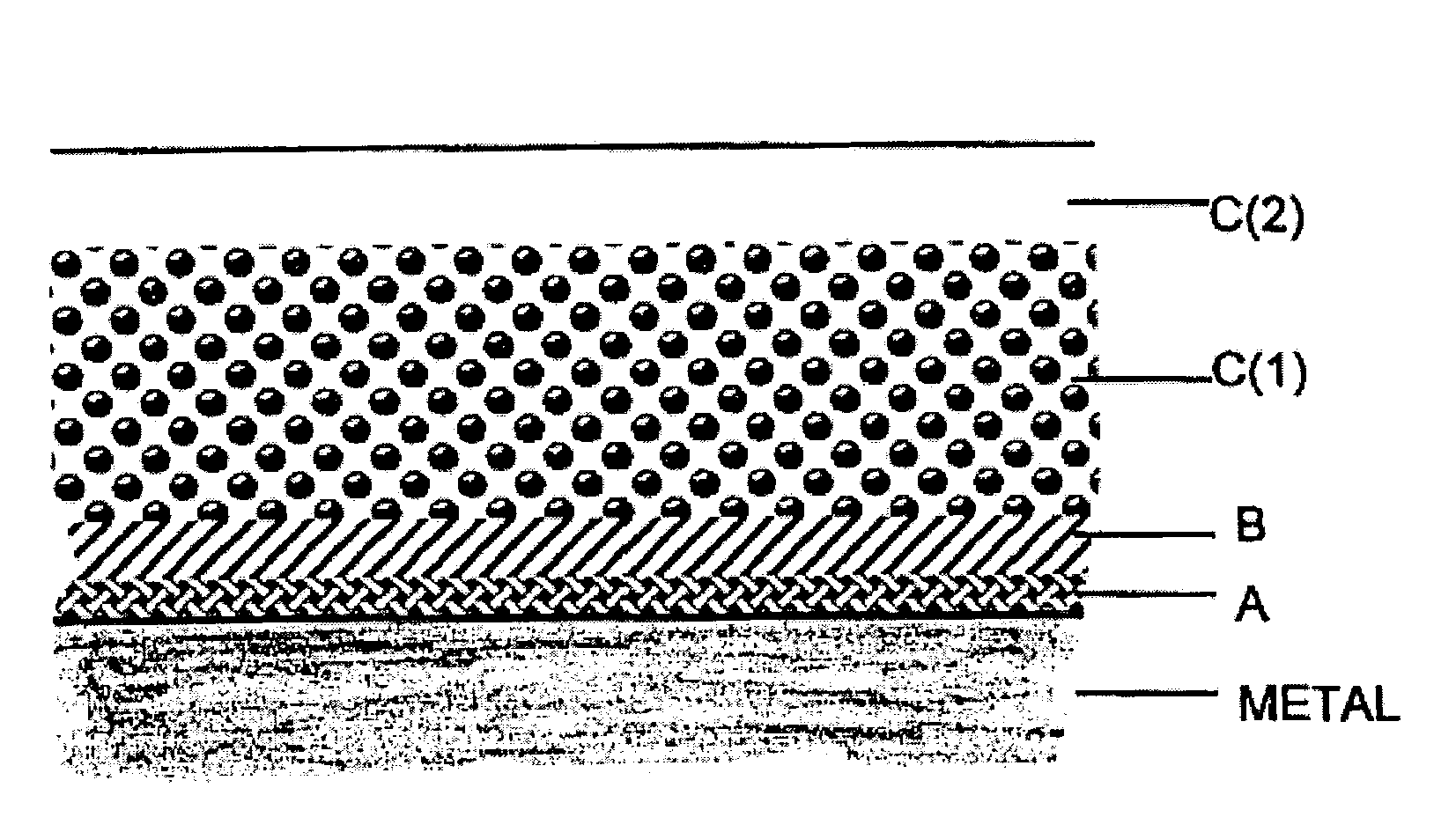
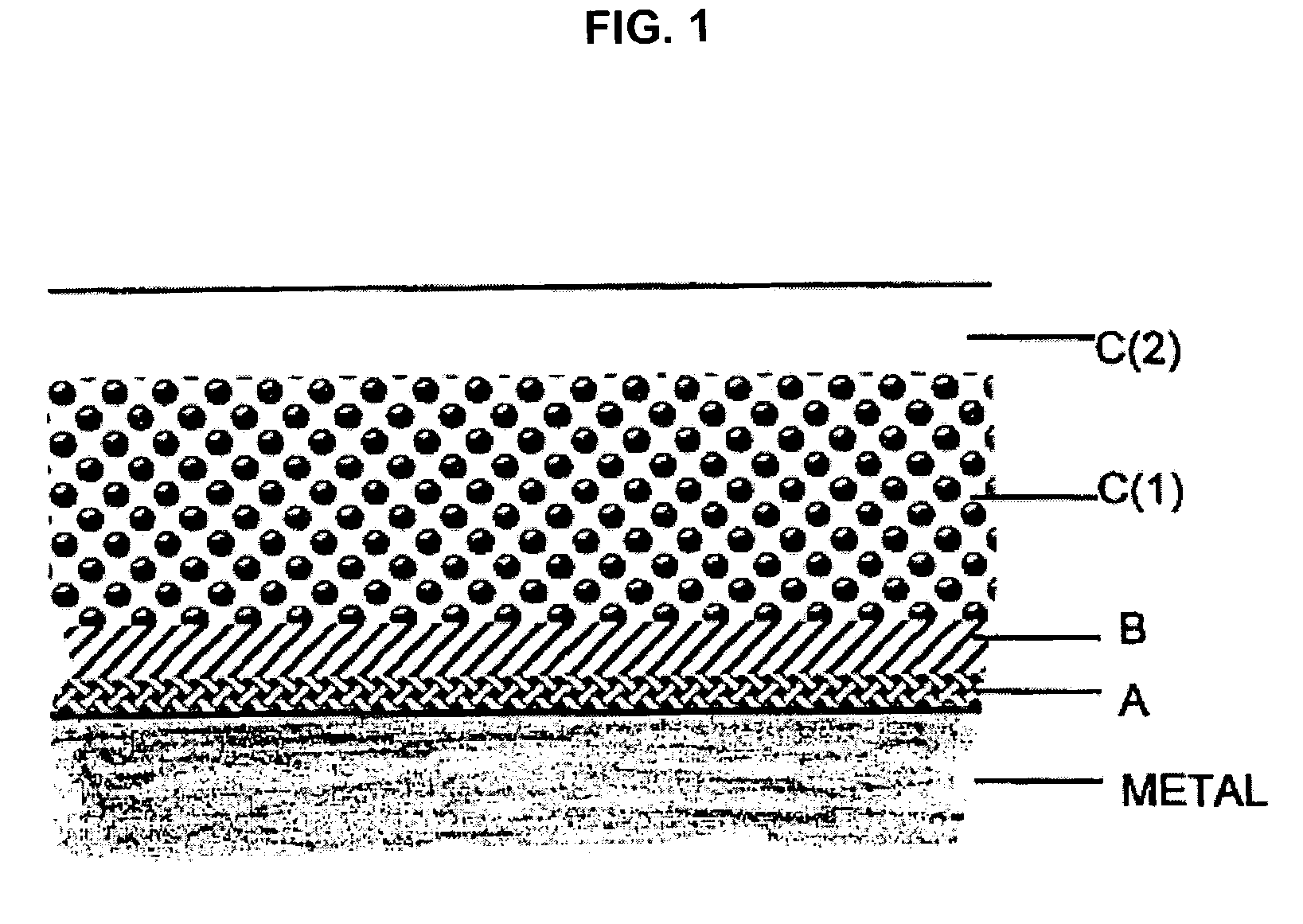
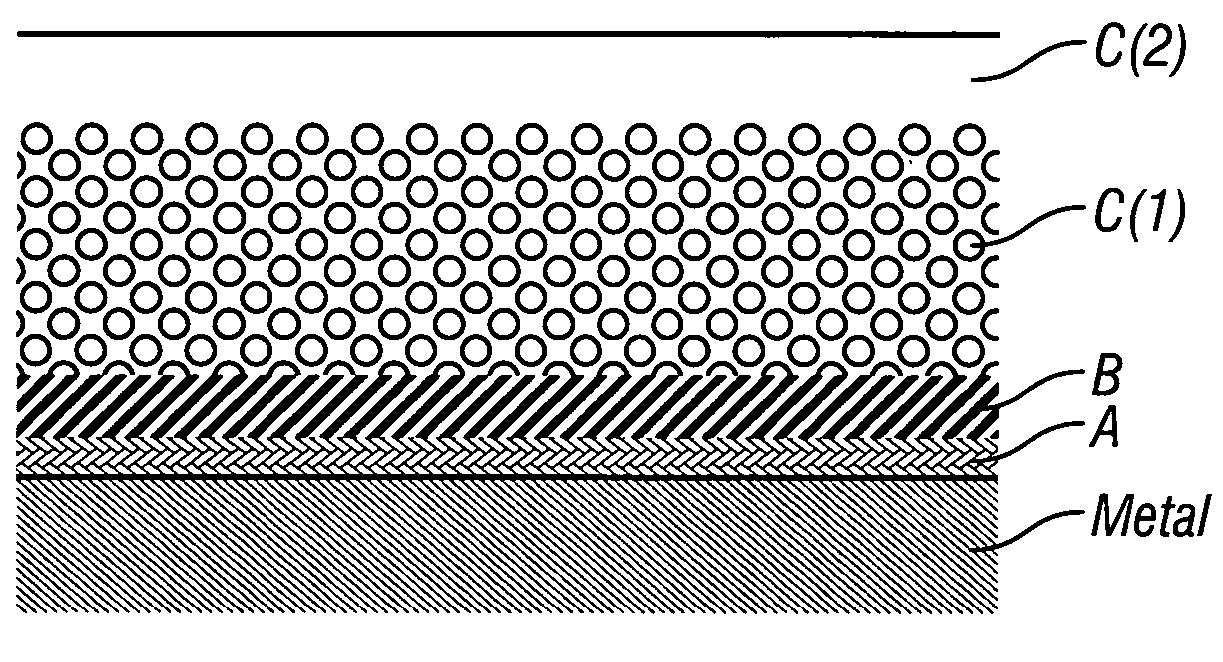
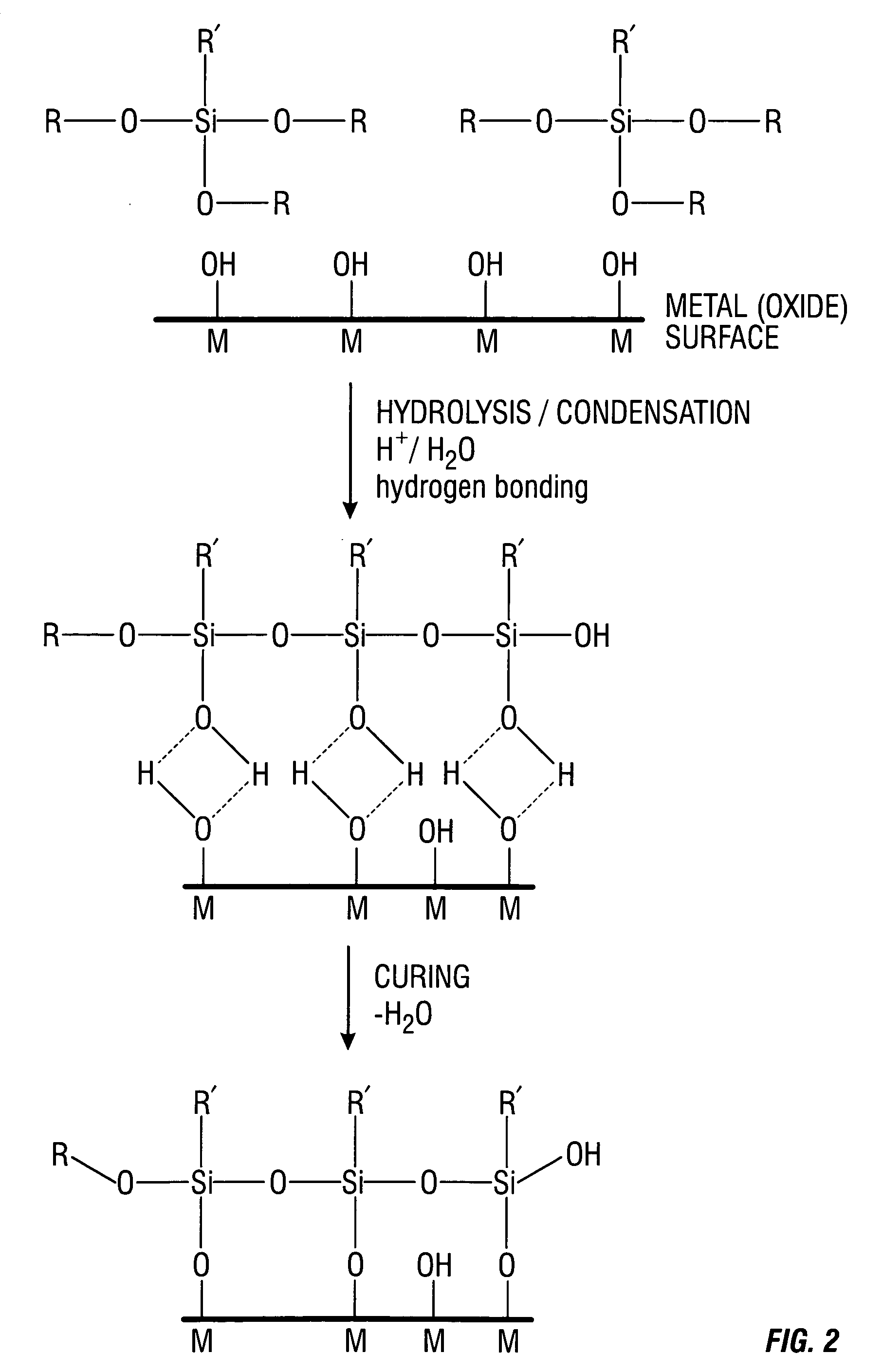
Coatings are provided in which surfaces may be activated by covalently bonding a silane derivative to the metal surface, covalently bonding a lactone polymer to the silane derivative by in situ ring opening polymerization, and depositing at least one layer of a polyester on the bonded lactone. Biologically active agents may be deposited with the polyester layers. Such coated surfaces may be useful in medical devices, in particular stents.
Owner:CV THERAPEUTICS INC
Polymer coating for medical devices
Coatings are provided in which surfaces may be activated by covalently bonding a silane derivative to the metal surface, covalently bonding a lactone polymer to the silane derivative by in situ ring opening polymerization, and depositing at least one layer of poly(lactide-co-caprolactone) copolymer on the bonded lactone. Biologically active agents may be disposed with the poly(lactide-co-caprolactone) copolymer layers. Such coated surfaces may be useful in medical devices, in particular stents.
Owner:CV THERAPEUTICS INC
Thermoplastic dicyclopentadiene-base open-ring polymers, hydrogenated derivatives thereof, and processes for the preparation of both
InactiveUS6511756B1Improve efficiencyGood moldabilityPlastic/resin/waxes insulatorsSynthetic resin layered productsThermoplasticDouble bond
A thermoplastic dicyclopentadiene ring-opening polymer obtained by ring-opening polymerization of a monomer component containing a dicyclopentadiene monomer, characterized in that polycyclic rings which are repeating units of the polymer are bonded in cis-position relative to the carbon-carbon double bond of the main chain of the polymer in 50 mol % or more of the repeating units based on the total repeating units and the content of a low-molecular weight component of 2,000 or less in molecular weight is 10% by weight or less based on the total polymer components, and a hydrogenation product obtained by hydrogenating the carbon-carbon unsaturated bond of the thermoplastic dicyclopentadiene ring-opening polymer.
Owner:ZEON CORP
Particulate drug delivery
InactiveUS20080248126A1Increase stability of particleIncrease loopPowder deliveryBiocideOligomerChemical species
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Cationic ring-opening polymerization of benzoxazines
Cationic polymerization of mono and polyfunctional benzoxazine monomers is described. The chemical structure of the polymers from cationic polymerization of benzoxazine monomers has been identified and distinguished from thermally polymerized products from the same monomers. The controllable microstructure of the polymers from benzoxazines prepared by cationic polymerization offers opportunities to prepare and optimize the polymer structure for specific applications.
Owner:EDISON POLYMER INNOVATION EPIC
Contact lens materials
InactiveUS6921802B2High oxygen permeabilityLow densityOptical partsOptical elementsSingle vesselTrimethylsilyl
A method for reducing the modulus of polymer silicone hydrogel compositions by employing monomeric polysiloxanes endcapped with trimethylsilyl to reduce the crosslinking density of the hydrogel. The synthesis consists of a single vessel acid catalyzed ring opening polymerization and may be employed to produce copolymers useful as hydrogel contact lens materials.
Owner:BARCLAYS BANK PLC AS SUCCESSOR AGENT
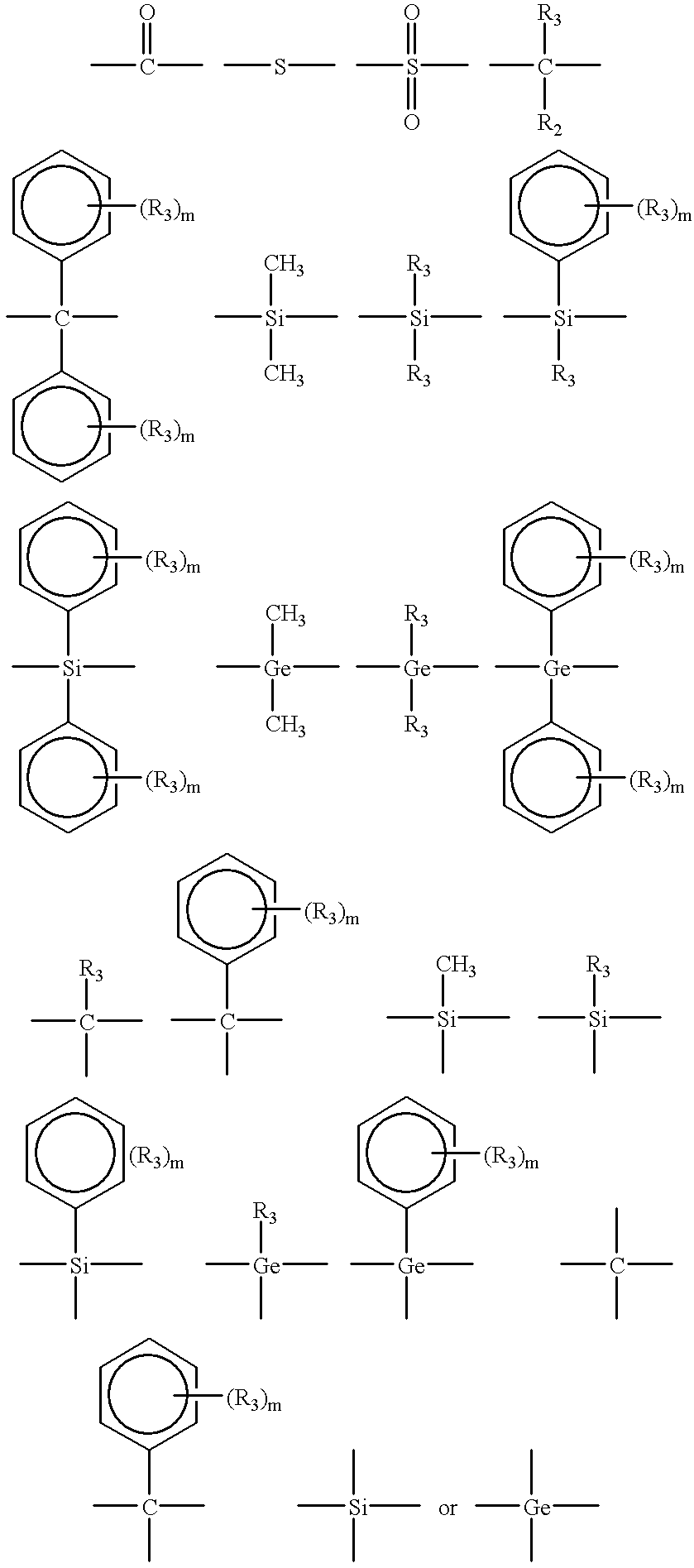
Linear and cross-linked high molecular weight polysilanes, polygermanes, and copolymers thereof, compositions containing the same, and methods of making and using such compounds and compositions
Methods are disclosed of making linear and cross-linked, HMW (high molecular weight) polysilanes and polygermanes, polyperhydrosilanes and polyperhydrogermanes, functional liquids containing the same, and methods of using the liquids in a range of desirable applications. The silane and germane polymers are generally composed of chains of Si and / or Ge substituted with R′ substituents, where each instance of R′ is, for example, independently hydrogen, halogen, alkenyl, alkynyl, hydrocarbyl, aromatic hydrocarbyl, heterocyclic aromatic hydrocarbyl, SiR″3, GeR″3, PR″2, OR″, NR″2, or SR″; where each instance of R″ is independently hydrogen or hydrocarbyl. The cross-linked polymers can be synthesized by dehalogenative coupling or dehydrocoupling. The linear polymers can be synthesized by ring-opening polymerization. The polymers can be further modified by halogenation and / or reaction with the source of hydride to furnish perhydrosilane and perhydrogermane polymers, which are used in liquid ink formulations. The synthesis allows for tuning of the liquid properties (e.g., viscosity, volatility, and surface tension). The liquids can be used for deposition of films and bodies by spincoating, inkjetting, dropcasting, etc., with or without the use of UV irradiation. The deposited films can be converted into amorphous and polycrystalline silicon or germanium, and silicon or germanium oxide or nitride by curing at 400-600 DEG C. and (optionally) laser- or heat-induced crystallization (and / or dopant activation, when dopant is present).
Owner:ENSURGE MICROPOWER ASA
Atom or group transfer radical polymerization
A polymerization process comprising initiating a first polymerization of monomers using an initiator functionalized with an ATRP initiating site, wherein the first polymerization is selected from the group of cationic polymerization, anionic polymerization, conventional free radical polymerization, metathesis, ring opening polymerization, cationic ring opening polymerization, and coordination polymerization to form a macroinitiator comprising an ATRP initiating site and further initiating an ATRP polymerization of radically polymerizable monomers using the macroinitiator comprising an ATRP initiating site. Novel block copolymers may be formed by the disclosed method.
Owner:CARNEGIE MELLON UNIV
Norbornene-based ring-opening polymerization polymer, product of hydrogenation of norbornene-based ring-opening polymerization polymer, and processes for producing these
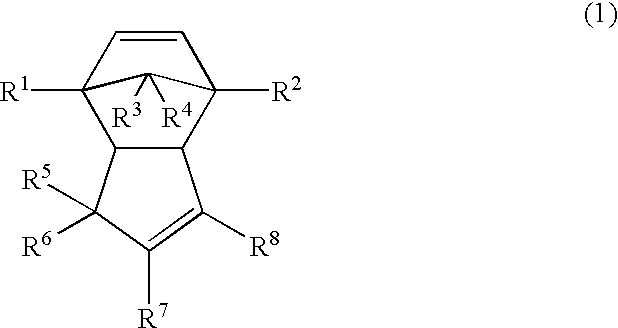
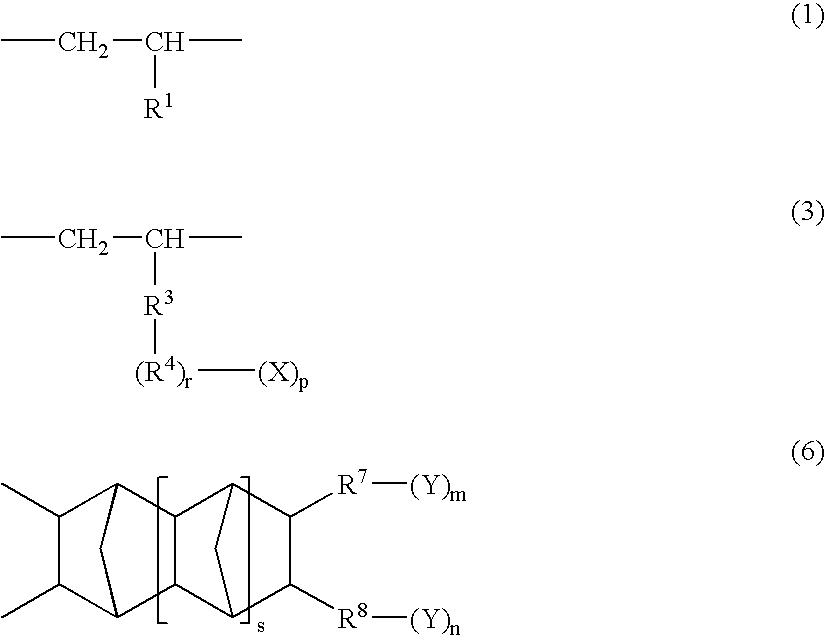
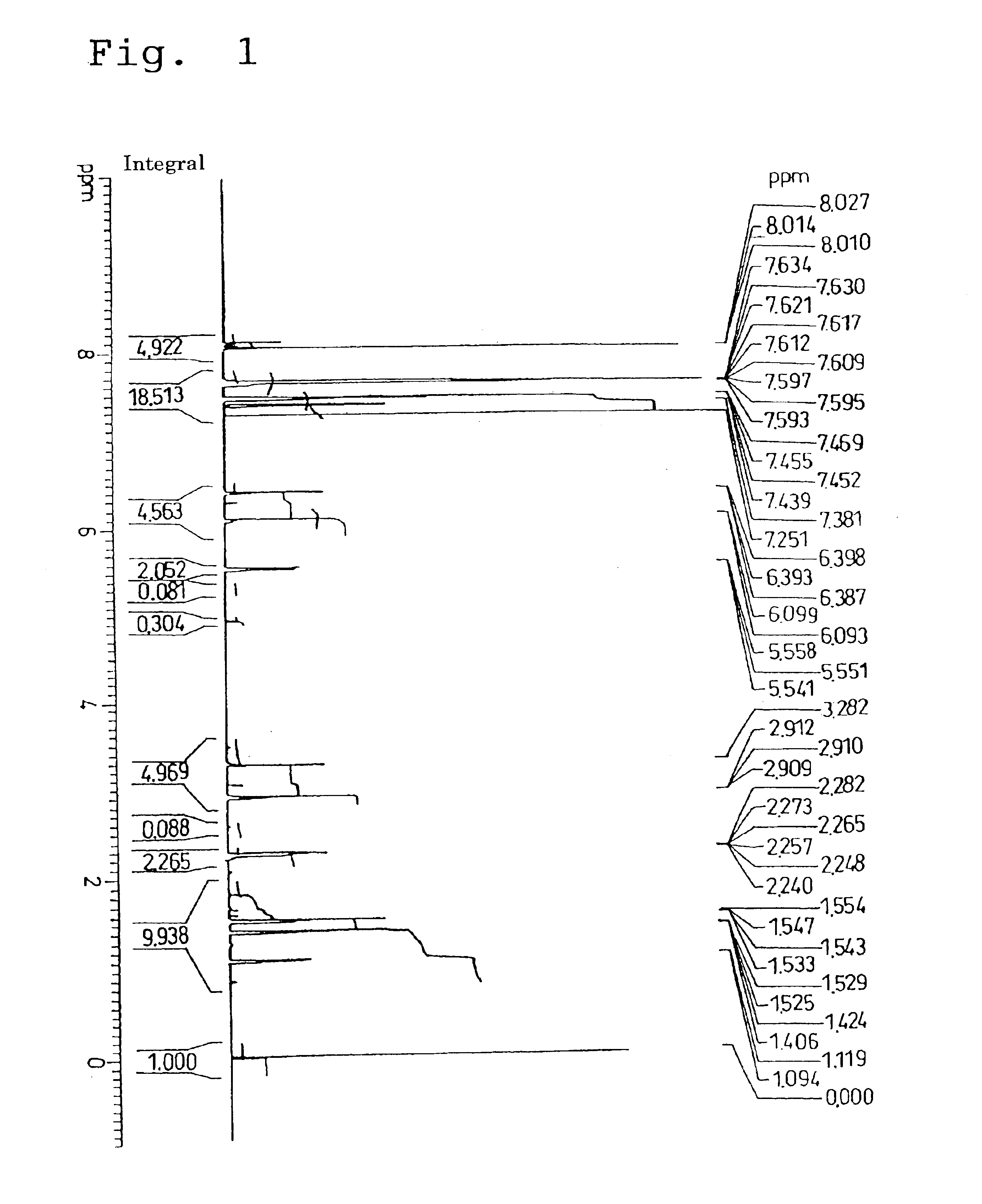
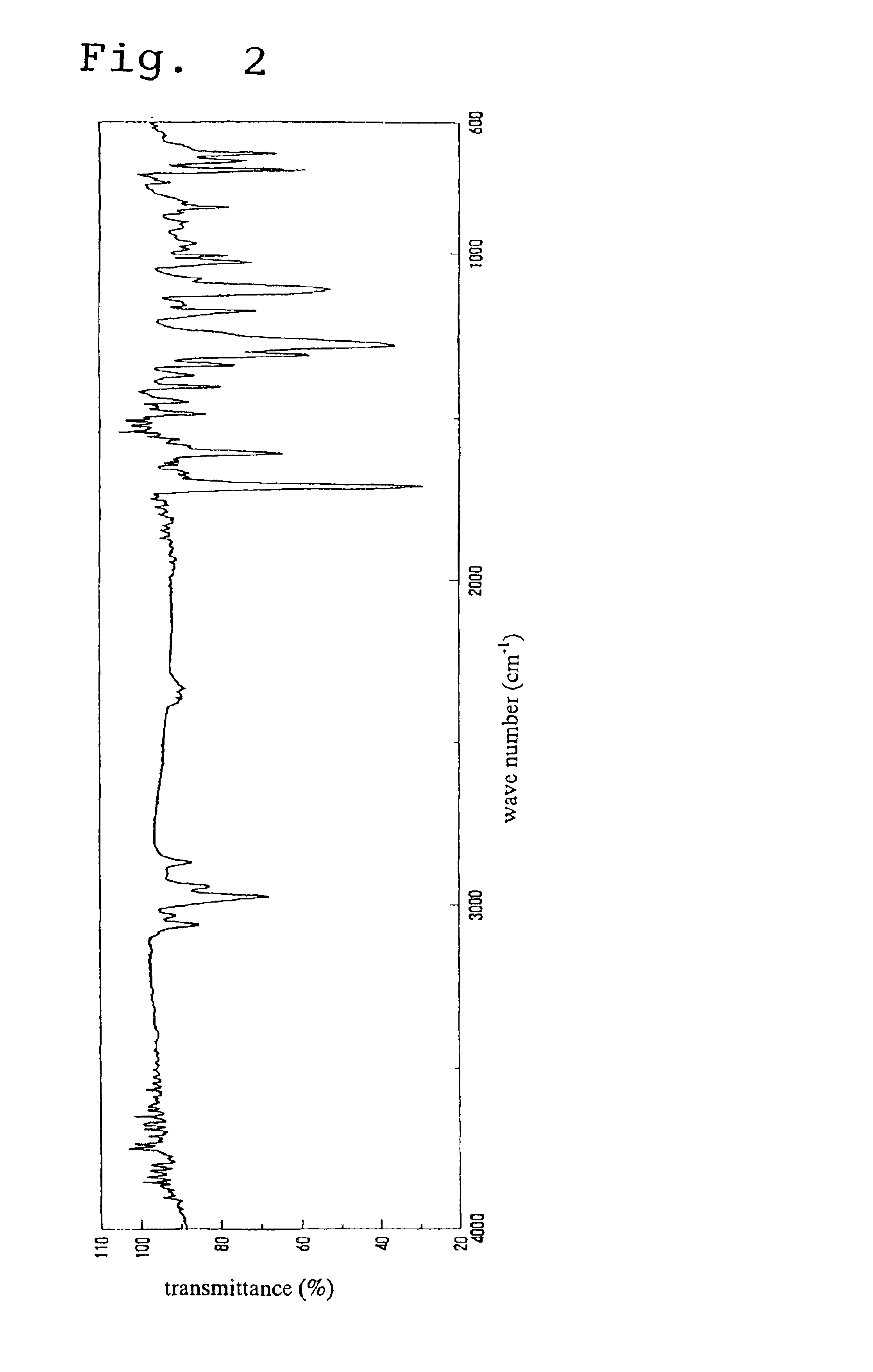
A norbornene ring-opened polymer which in the molecule has repeating units represented by the formula (1): wherein R1 represents Q, R2 represents Q or C(═O)R5, R3 represents Q or C(═O)R6, and R4 represents Q or X—C(═O)R7 (wherein Q represents hydrogen, a C1-10 hydrocarbon group, etc.; R5, R6 and R7 each represents hydroxyl, C1-10 alkoxyl, etc., provided that R6 and R7 may be bonded to each other to constitute oxygen, NH, etc.; X represents methylene, etc., provided that when R2 is Q, then R3 is C(═O)R6 and R4 is X—C(═O)R7 and that when R4 is Q, then R2 is C(═O)R5, R3 is C(═O)R6, and the configuration of R2 and R3 is trans, and m is 0 or 1), the polymer having a weight-average molecular weight as determined by gel permeation chromatography of 1,000 to 1,000,000. Also provided is a hydrogenation product of the norbornene ring-opened polymer. The norbornene ring-opened polymer and the hydrogenation product are excellent in heat resistance, electrical properties, etc.
Owner:ZEON CORP
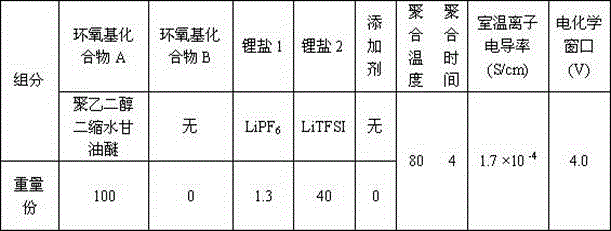
Preparation method of all-solid polymer electrolyte through in-situ ring opening polymerization of epoxy compound, and application of the all-solid polymer electrolyte in all-solid lithium battery
ActiveCN105914405AIncrease contactImprove interface compatibilityFinal product manufactureLi-accumulatorsEpoxySolid state electrolyte
The invention discloses a preparation method of an all-solid polymer electrolyte through in-situ ring opening polymerization of an epoxy compound, and an application of the all-solid polymer electrolyte in an all-solid battery. The preparation method is characterized in that a liquid-state epoxy compound, a lithium salt, a battery additive and the like are employed as precursors and are injected into between a positive pole sheet and a negative pole sheet of the battery, and under a heating condition, in-situ polymerization solidification is carried out to form the all-solid polymer electrolyte, and furthermore, the all-solid battery is produced. The ionic conductivity at room temperature of the all-solid polymer electrolyte can reach from 1*10<-5> S / cm to 9*10<-3> S / cm and electric potential window is 3.5-5 V. The all-solid polymer electrolyte is prepared through the in-situ copolymerization method, so that the all-solid polymer electrolyte has excellent contact with electrodes, thereby greatly improving interface compatibility of the solid-state battery, reducing interface wetting and modification steps of the solid-state battery, reducing production cost of the solid-state battery and improving performances of the solid-state battery. The invention also discloses an all-solid polymer lithium battery assembled from the all-solid polymer electrolyte.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
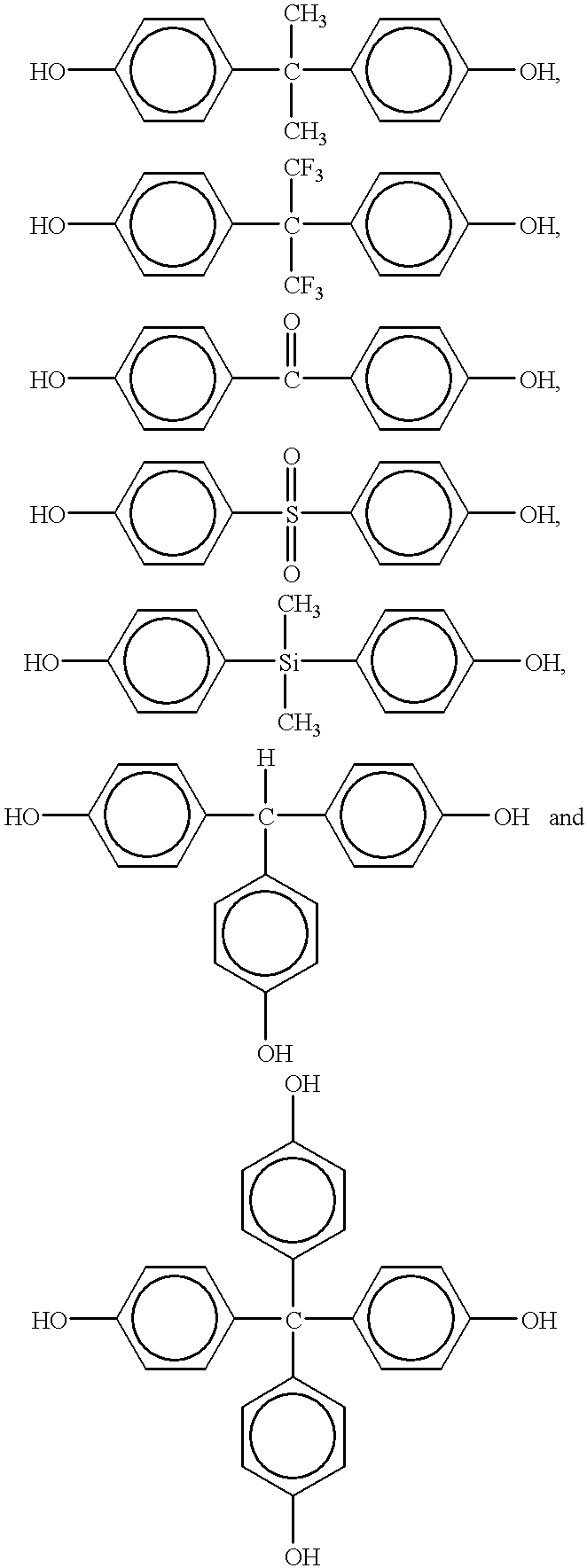
Surfaces containing coupling activator compounds and reinforced composites produced therefrom
InactiveUS20100286343A1Improve interface adhesionEnhanced couplingLamination ancillary operationsLaminationCouplingSilanes
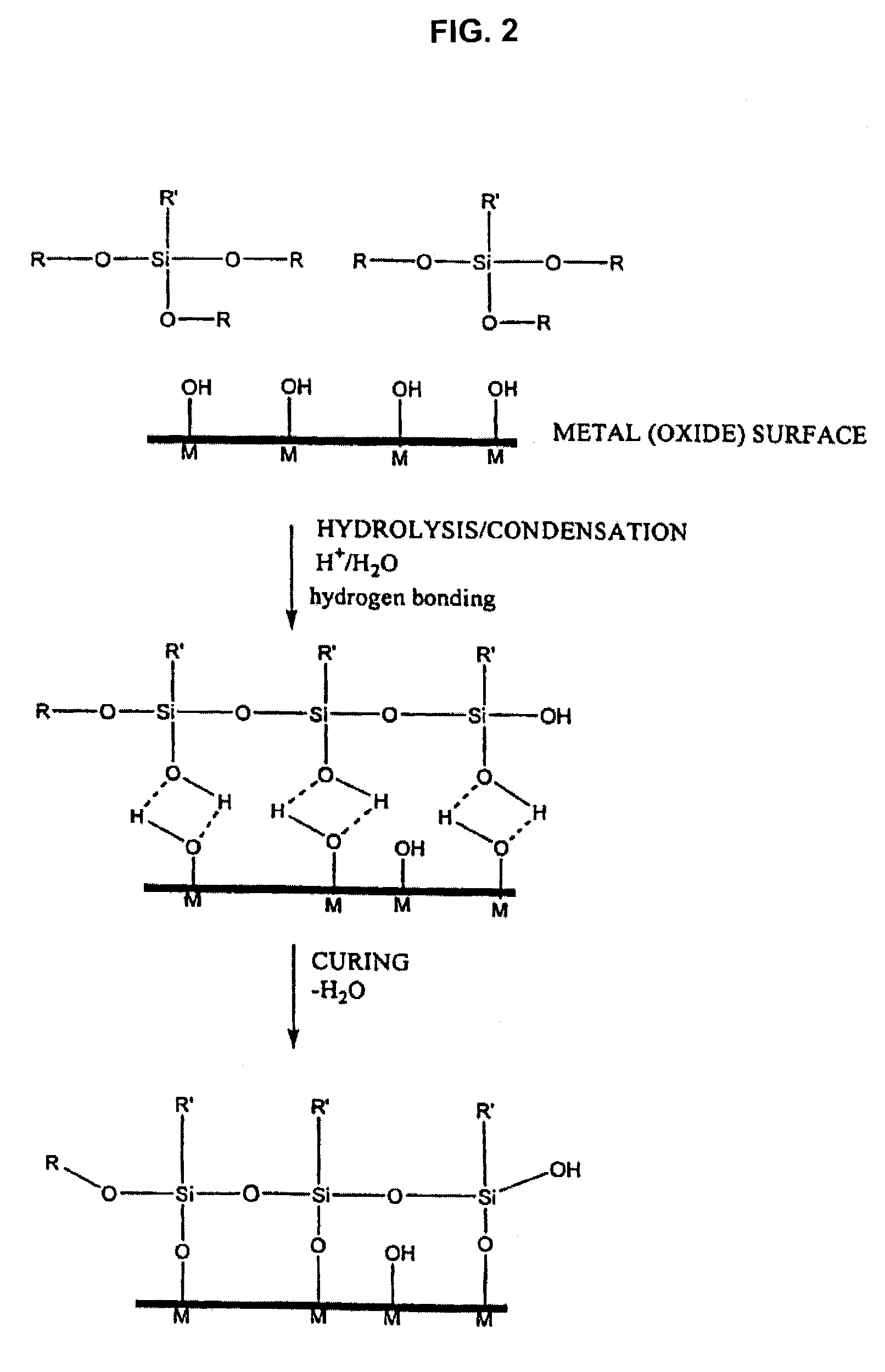
The invention relates to products and processes employing coupling activator compounds represented by the following formula I:S—X-A (I)wherein S represents a silane coupling moiety capable of bonding with the surface of an inorganic substrate, A represents a ring-opening polymerization activator moiety, or blocked precursor thereof, and X represents a linking moiety. Substrates containing the coupling activator compounds are useful in preparing reinforced resins.
Owner:JOHNS MANVILLE CORP
Polyoxyalkylenepolyols and process for producing ring-opened polymer
InactiveUS6531566B1Improve responseImprove adhesionOrganic compound preparationOther chemical processesPolyolHydrogen
A polyoxyalkylene polyol or monool (I) of the general formula (1) below, in which not less than 40% of the terminally located hydroxyl-containing groups, namely -AO-H groups, are primary hydroxyl-containing groups of the general formula (2) below, or;a method of producing ring-opening polymerization products, by subjecting a heterocyclic compound to ring-opening addition polymerization with an active hydrogen-containing compound, using as a catalyst tris(pentafluorophenyl)borane, tris(pentafluorophenyl)aluminum, etc.
Owner:SANYO CHEM IND LTD
Polymer coating for medical devices
InactiveUS20080152929A1Functional densityLayered productsMetal rolling stand detailsPolyesterSilanes
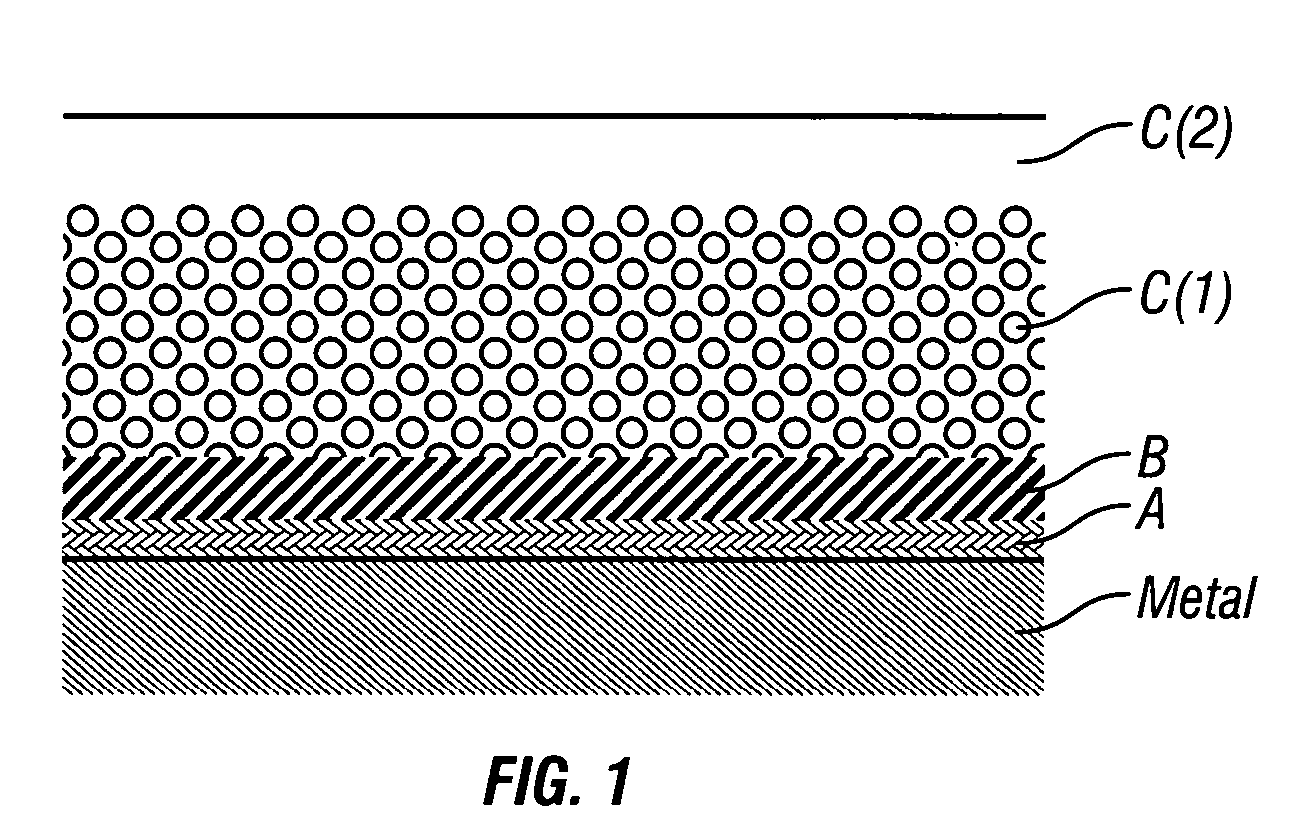
Coatings are provided in which surfaces may be activated by covalently bonding a combination of silane derivatives (A) to the metal surface, covalently bonding a lactone polymer (B) to the silane derivative by in situ ring opening polymerization, and depositing at least one layer of a polyester (C) on the bonded lactone polymer. Biologically active agents or therapeutic compounds may be deposited with any of the polyester layers. Such coated surfaces may be useful in medical devices, in particular stents.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Degradable polymers
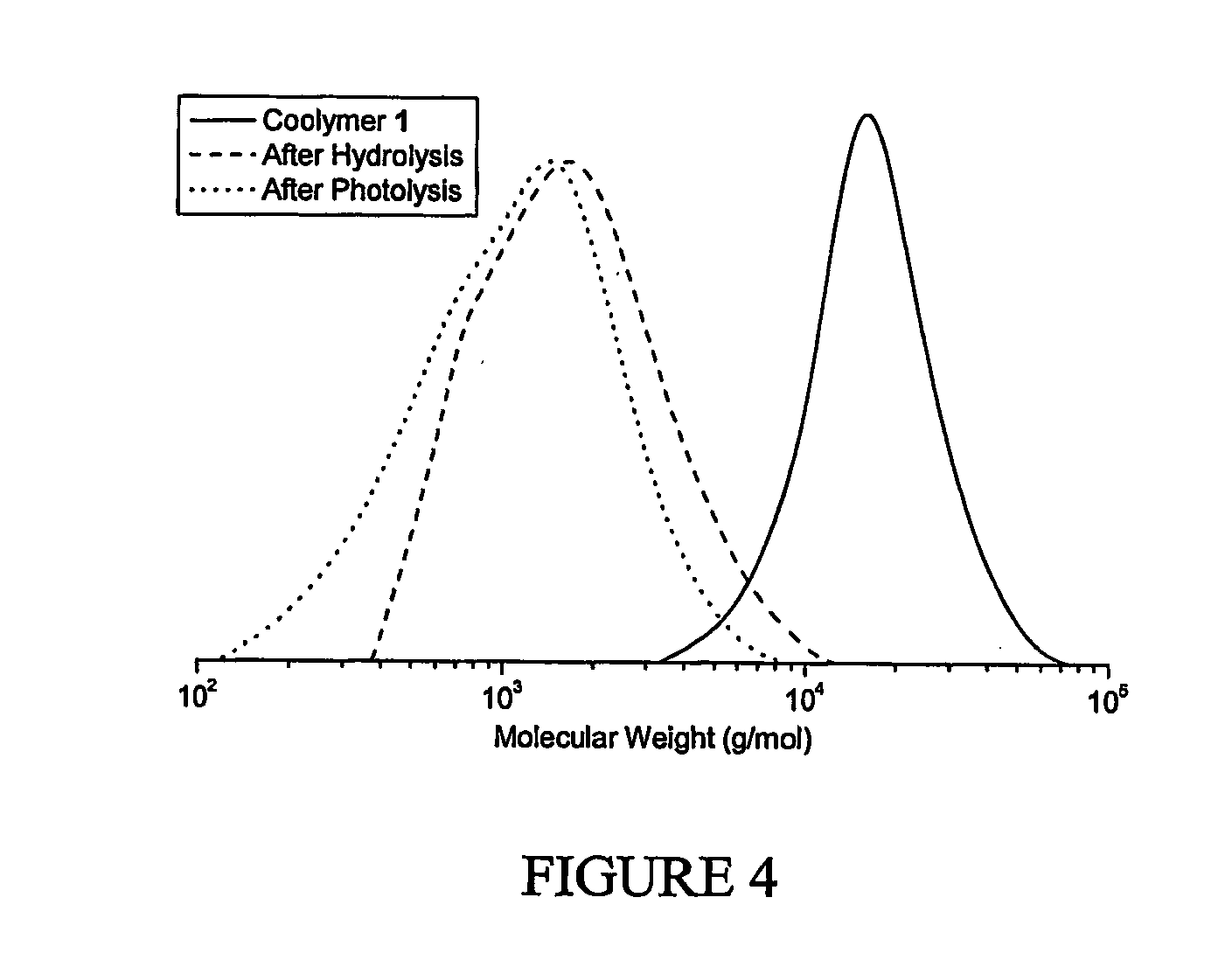
Polymers comprising a polymer backbone comprising one or more degradable units are described. The polymer may additionally comprise two or more polymer segments comprising radically (co)polymerizable vinyl monomer units. The degradable units may be independently selected from, but not limited to, at least one of hydrodegradable, photodegradable and biodegradable units between the polymer segments and dispersed along the polymer backbone. The degradable units may be derived from one or more monomers comprising a heterocyclic ring that is capable of undergoing radical ring opening polymerization, a coupling agent, or from a polymerization initiator, radically polymerizable monomers, as well as other reactive sources. Embodiments of the degradable polymer of claim are capable of degrading by at least one of a hydrodegradation, photodegradation or biodegradation mechanisms to form at least one of telechelic oligomer and telechelic polymeric fragments of the polymer. The degradable polymer may be able to degrade into polymer fragments having a molecular weight distribution of less than 5, or in certain applications it may be preferable for embodiments of the polymer to be capable of forming polymer fragments having a molecular weight distribution of the polymer fragments less than 3.0 or even less than 2.5. Embodiments of the present invention also include methods of producing degradable polymers.
Owner:MATYJASZEWSKI KRZYSZTOF +6
Process for producing hydrogenated product of cyclic olefin polymer prepared through ring-opening polymerization
A process for producing a hydrogenated product of a polymer prepared through ring-opening polymerization which comprises a polymerization step of polymerizing a cyclic olefin through ring-opening polymerization in the presence of a polymerization catalyst comprising an organoruthenium compound or an organoosmium compound to prepare a polymer, and a hydrogenation step of adding a hydrogenation catalyst and hydrogen into a polymerization system resulting from the polymerization step to hydrogenate the carbon-carbon double bonds of the polymer prepared through the ring-opening polymerization. When the organoruthenium compound- or organoosmium compound-containing catalyst further comprises a carbene compound, the catalyst exhibits a higher activity for the ring-opening polymerization.
Owner:ZEON CORP
Method for preparing durable hydrophilic polyether modified amino polysiloxane soft agent
The invention discloses a method for preparing a perdurable hydrophilic polyether modified amino-polysiloxane softener. The method is characterized by comprising the following steps: octamethylcy-clotetrasiloxane and amino silane coupling agents with different structures are used as raw materials; with a bulk polymerization method, under the analysis of an alkali catalyst, firstly, an amino organic polysiloxane softener with high ammonia value is generated through ring-opening polymerization; and secondly, on the basis of the amino organic polysiloxane softener, amidogen is subjected to etherifying modification, thereby synthesizing the hydrophilic polyether modified amino-polysiloxane softener with good hand feeling and hydrophilic property. The prepared softener keeps the hydrophilic property and simultaneously improves the softness of hydrophilic silicon oil so that the hand feeling of textile can reach the hand feeling of the prior linearity amino silicon oil after the textile is treated by the softener and the treated textile can keep the hydrophilic property permanently.
Owner:SHAOXING UNIVERSITY
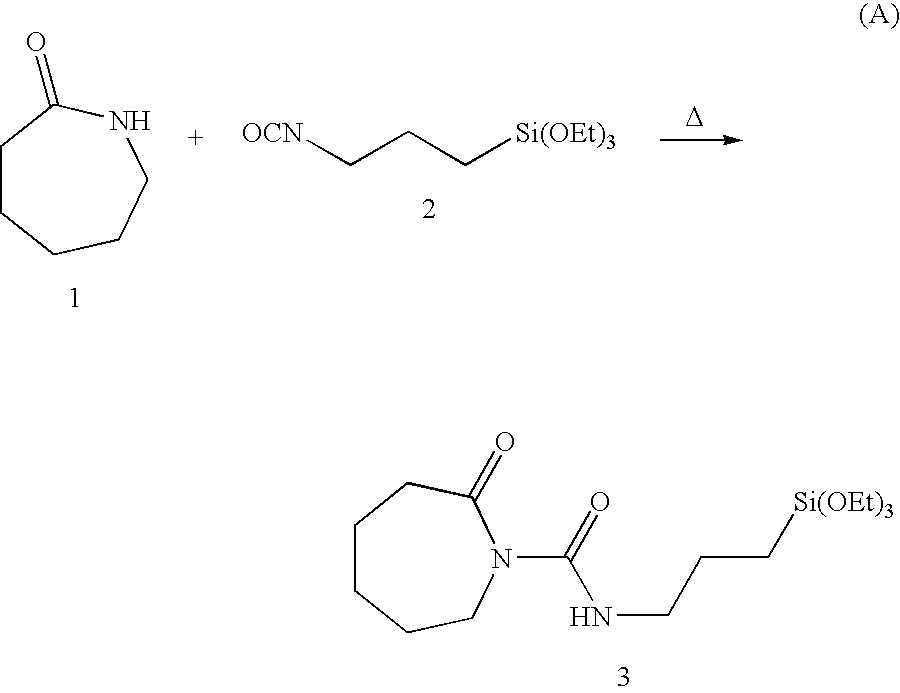
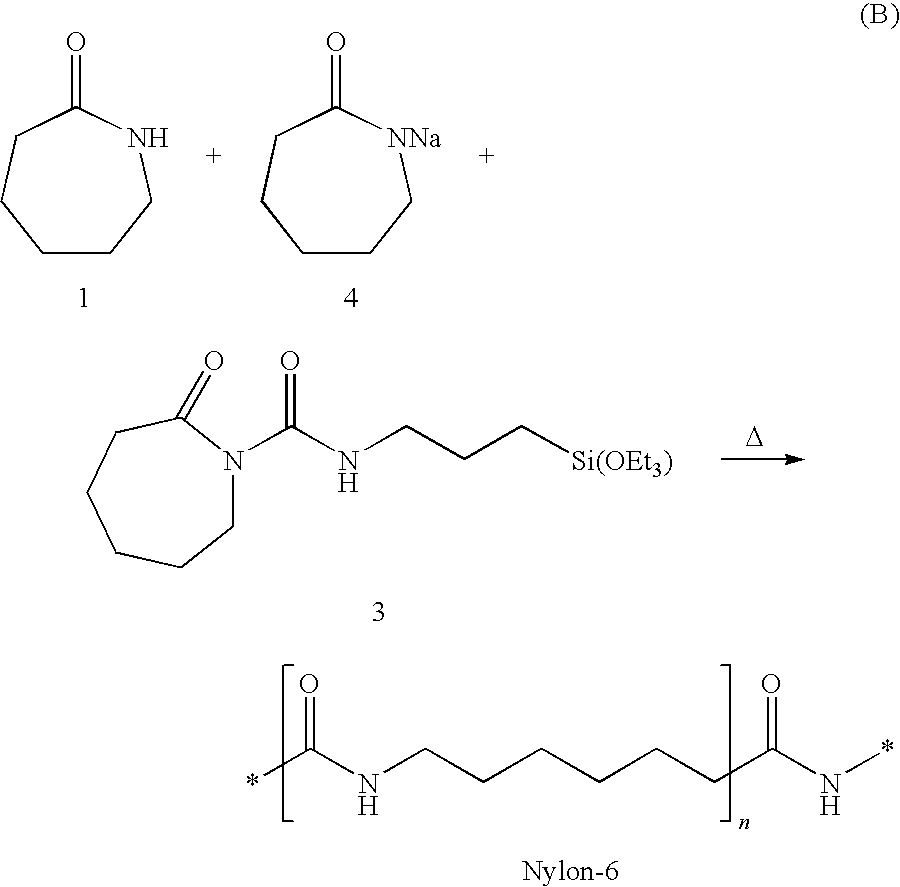
Intercalates, exfoliates and concentrates thereof formed with low molecular weight; nylon intercalants polymerized in-situ via ring-opening polymerization
InactiveUS6906127B2Improve propertiesGood dispersionMaterial nanotechnologyLayered productsSolution polymerizationRing-opening polymerization
Nylon-intercalated (concentrate nylon), layered silicates, in concentrated form, containing at least 6% to about 40% by weight layered silicate, preferably about 8% to about 30% by weight layered silicate, intercalated with a nylon that is polymerized while in contact with the clay, during ring-opening polymerization of the nylon reactants (monomers) and melt processing. The in-situ nylon polymerization reaction to form the concentrate nylon is continued until the number average molecular weight (Mn) of the nylon is about 25,000 or less, preferably 15,000 or less, more preferably about 5,000-10,000, to maintain melt processability of the resulting concentrate.
Owner:AMCOL INTERNATIONAL CORPORATION
Method for preparing polyester-polyester blocked copolyester
The invention discloses a method for preparing polyester-polyester blocked copolyester, which comprises the following steps: (1) synthesizing an aromatic polyester hard segment to obtain a prepolymer P1 with a number-average molecular weight in the range of 500-10,000g / mol; (2) synthesizing an aliphatic polyester soft segment to obtain a prepolymer P2, wherein the prepolymer P2 can also be obtained by ring-opening polymerization of caprolactone monomer, and the number-average molecular weight of the prepolymer P2 is in the range of 500-10,000g / mol; (3) carrying out polycondensation reaction of the aromatic polyester oligomer and the aliphatic polyester oligomer: mixing the esterified product P1 and the esterified product P2, adding antioxidant, catalyst, passsivator and chain expander, allowing reaction at 220-260 DEG C to obtain a polyester-polyester block polyester elastomer. The catalysts used in the steps (1) and (2) are selected from titanium-containing organic substances. The method disclosed by the invention has the following advantages: (1) because the aliphatic polyester soft segment is introduced, the product has both the mechanical properties of the rigid aromatic polyester hard segment and the flexibility of the soft segment; and (2) because a number of assistants are added, the good chain expansion effect is achieved, and the reaction conditions are controlled.
Owner:KINGFA SCI & TECH CO LTD +2
Norbornene ring-opened polymer hydrogenated product and process for producing same
A norbornene ring-opened polymer hydrogenated product having a syndiotactic structure and a process for producing the same are disclosed. The ring-opened polymer hydrogenated product contains a repeating unit originating from a polycyclic norbornene monomer with three or more rings in the polymer repeating units, having a weight average molecular weight of 500 to 1,000,000, and having a racemo diad proportion of 51% or more. The process comprises a step of polymerizing a polycyclic norbornene monomer having three or more rings by solution polymerization using a group 6 transition metal compound with a hydroxyl group-containing aryloxy group or the like bonded thereto as a polymerization catalyst to obtain a ring-opened polymer and a step of hydrogenating double bonds in the main chain of the ring-opened polymer.
Owner:ZEON CORP
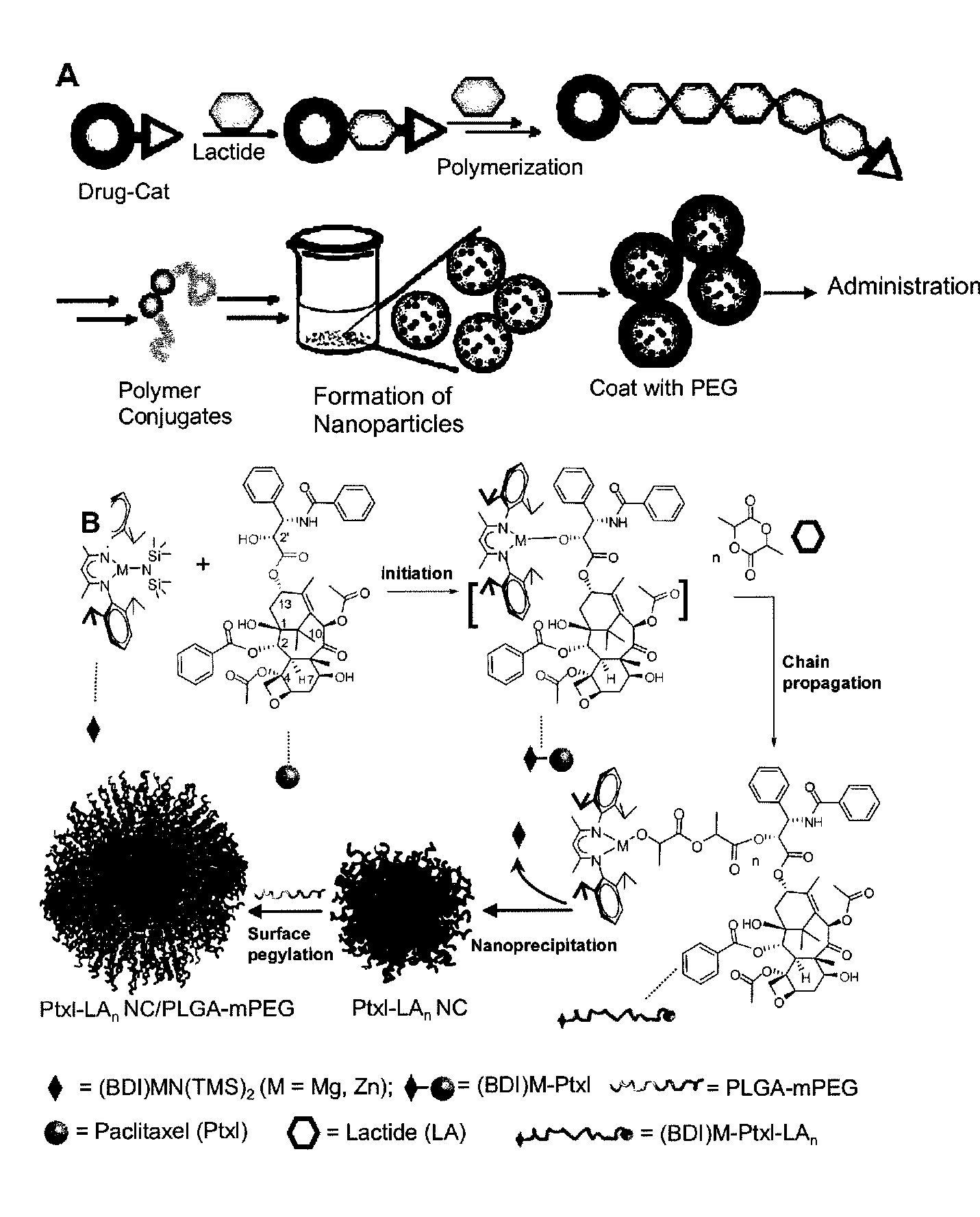
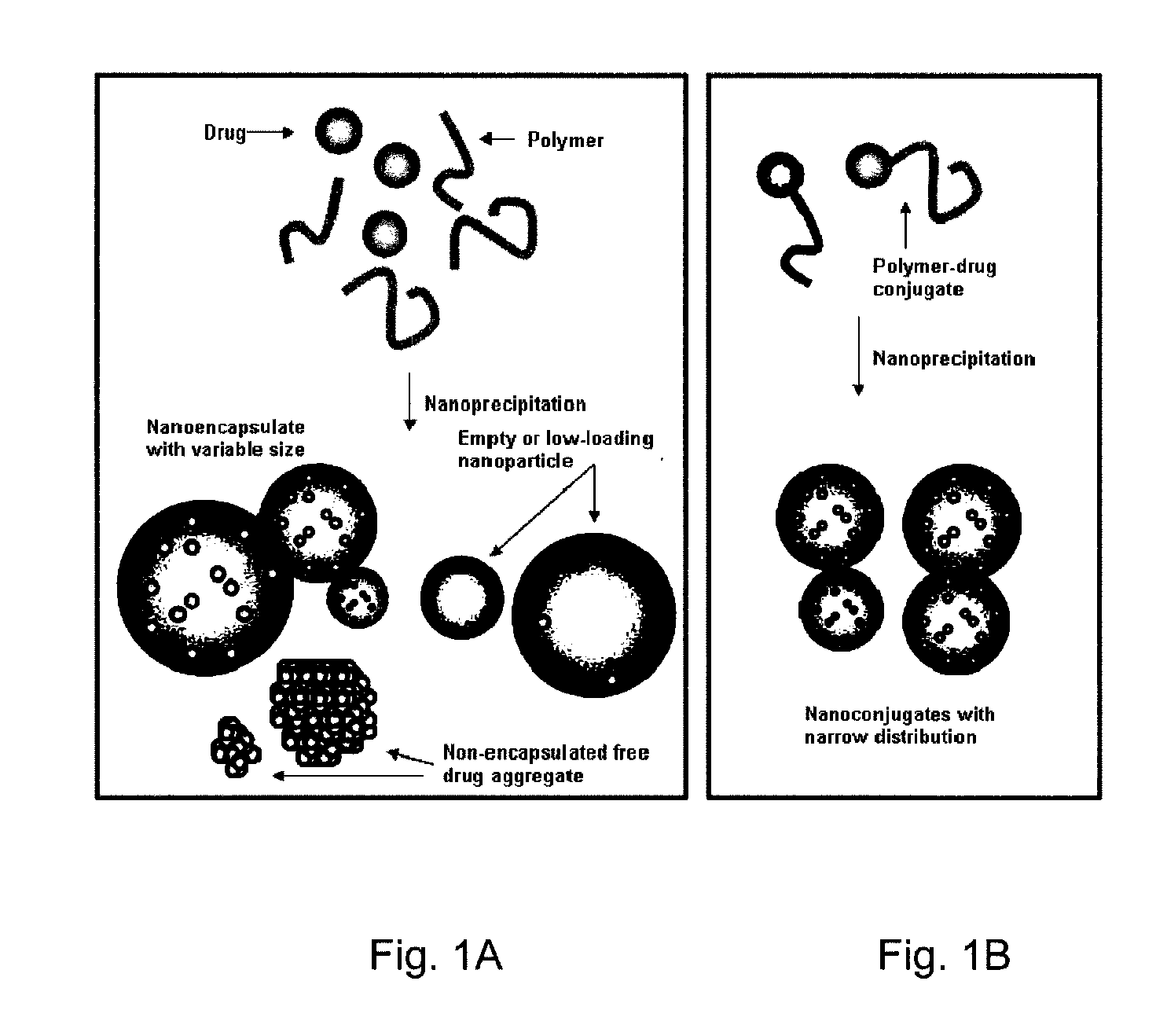
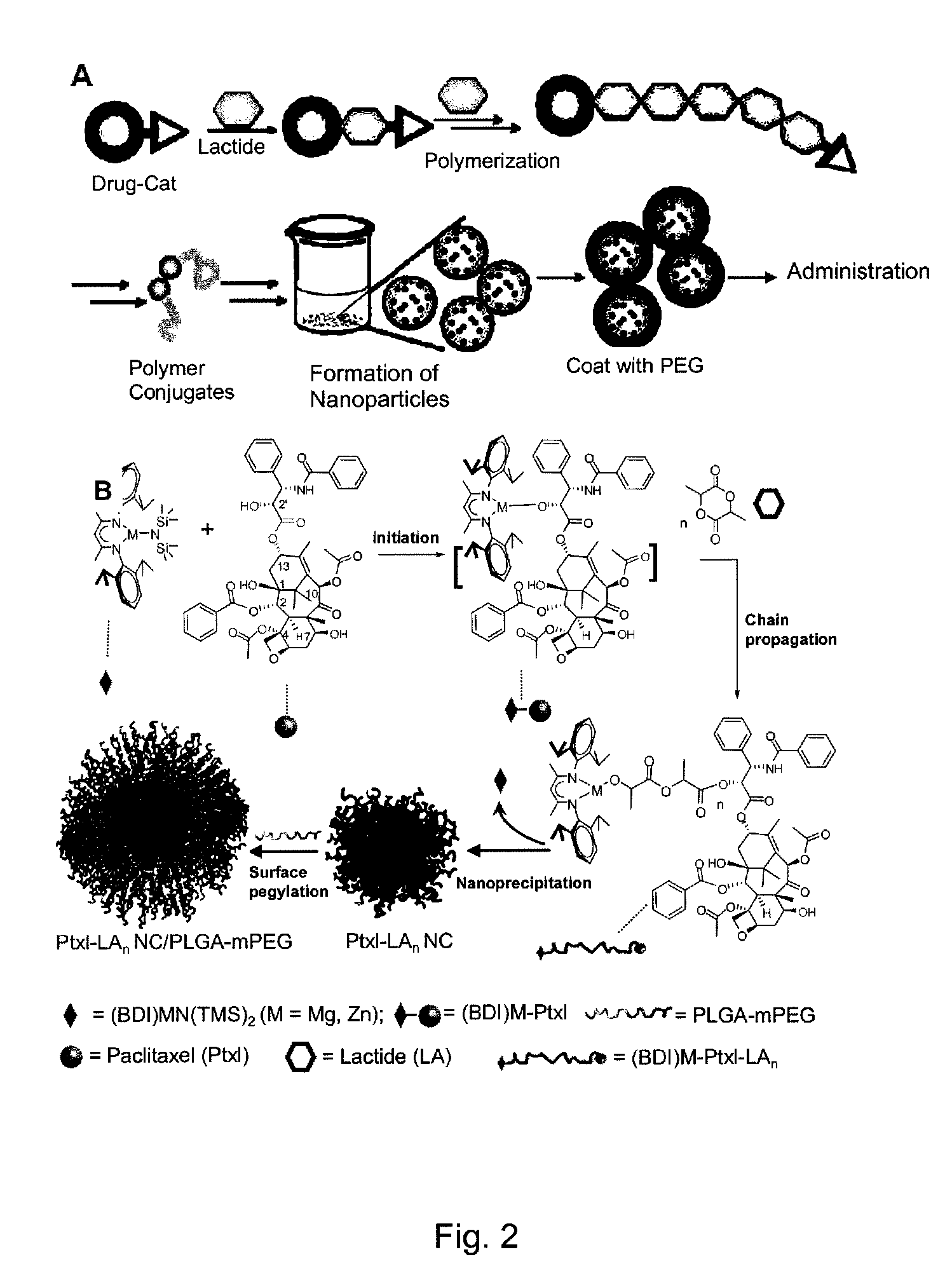
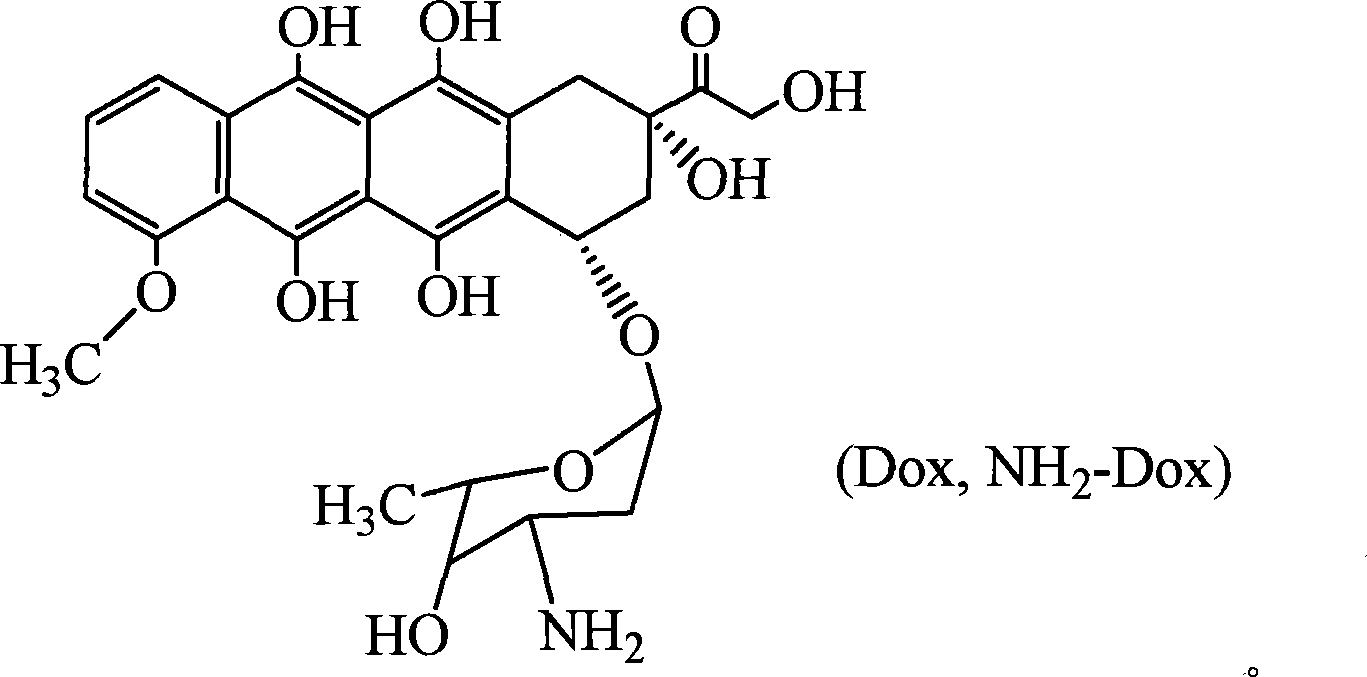
High molecule bonding adriamycin medicine, nano capsule and preparation thereof
InactiveCN101234204AReduce manufacturing costPlay a role in isolationOrganic active ingredientsPharmaceutical non-active ingredientsPolymer scienceEnd-group
The invention provides a macromolecule bonding adriamycin medicine, a nanometer capsule and a preparation method thereof. The bonding medicine is bonded by a block copolymer of poly ethylene glycol-polylactic acid and adriamycin. Firstly, the block copolymer of poly ethylene glycol-polylactic acid is acquired by ring-opening polymerization of aliphatic cyclic esters with poly ethylene glycol, solvent and catalyst being in existence; then a carboxyl end group is transformed by a hydroxyl-terminated; and then with a condensing agent being in existence, the carboxyl end group carries out amidation reaction with the adriamycin; thus acquiring the macromolecule adriamycin bonding medicine. The bonding medicine has amphiphilic property, thus being able to self assembly to prepare the nanometer capsule with a core-shell structure in the water solution. The adriamycin is wrapped inside the capsule to play a role of isolation and protection, which overcomes the deficiencies of short circulation time in vivo, large dosage and severe allergic reaction existing in the current adriamycin preparation. In addition, the nanometer particles are expected to congregate in the blood circulation through the so-called 'enhanced infiltration and retention effect', so as to improve the targeting action of the adriamycin on the tumor location.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Metal catalyst for ring-opening polymerization of heterocyclic compound
InactiveUS20040030093A1Improve responseReduce deflectionGroup 1/11 element organic compoundsGroup 3/13 element organic compoundsMetal catalystRing-opening polymerization
The object of the present invention is to provide a metal catalyst which allows the production of a ring-opened polymer available from a heterocyclic compound, having primary terminal heteroatomic groups (terminal hydroxyl groups, in particular) in a ratio exceeding 75%. The above object can be attained by using a metal catalyst comprising a ligand and a metal atom for ring-opening polymerization of heterocyclic compounds in which, of all maximum angles (D) meeting the following definition, the smallest maximum angle (Dm) is not larger than 60 degrees; The maximum angle (D) is the largest angle between an imaginary line (X) and an imaginary line (Y) of all the angles which can be assumed in a metal catalyst comprising a ligand and a metal atom, said imaginary line (X) means a line perpendicular to an imaginary plane (P) including the respective centers of 3 coordinating atoms among those directly coordinating the metal atom (M) and not substitutable by a reaction substrate (S) and passing through the center of the metal atom, said imaginary line (Y) means a line linking the center of a non-coordinating atom in the ligand and the center of the metal atom, and said maximum angle (D) exists in a number equal to the number of non-coordinating atoms, that is, the number of imaginary lines (Y).
Owner:SANYO CHEM IND LTD
Polyurethane elastomer and method for its production
The present invention provides a method for producing a thermoplastic polyurethane elastomer having excellent heat resistance and mechanical properties, and capable of obtaining one having a low hardness without using a plasticizer. A method for producing a polyurethane elastomer, which comprises reacting a polyol compound and a polyisocyanate compound, characterized by using, as all or part of the above polyol compound, a polyester ether polyol (A) obtainable by ring-opening polymerization of a mixture of an alkylene oxide and a lactone monomer with an initiator.
Owner:ASAHI GLASS CO LTD
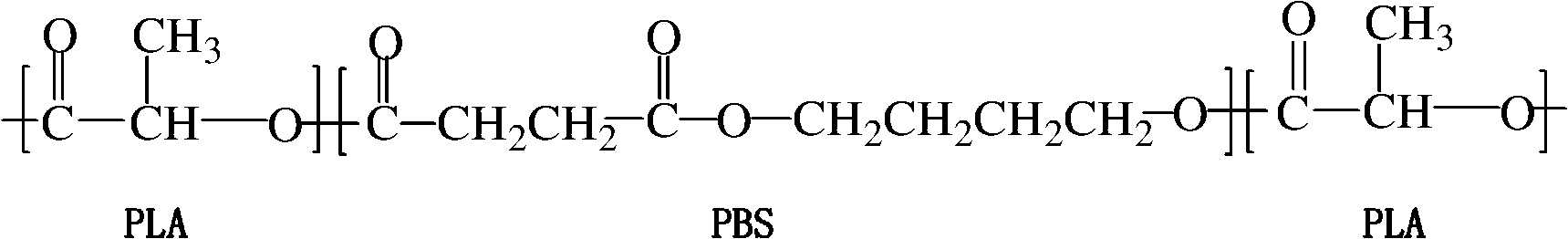
Polylactic acid toughening modifier and preparation method thereof
The invention relates to a polylactic acid toughening modifier in the technical field of high polymer material and a preparation method thereof. Lactide ring-opening polymerization is initiated on the terminal hydroxyl of poly (butylene succinate), and purification is carried out, thus obtaining the polylactic acid toughening modifier, the chemical structural formula is as follows. The modifier of the invention prepared by adopting degradable material can solve transport phenomenon and diosmose in the prior art.
Owner:SHANGHAI JIAO TONG UNIV
Polymers bearing pendant pentafluorophenyl ester groups, and methods of synthesis and functionalization thereof
ActiveUS20100305281A1Efficiently formedEasy to functionalizeOrganic chemistryChemical recyclingSide chainPentafluorophenyl esters
A one pot method of preparing cyclic carbonyl compounds comprising an active pendant pentafluorophenyl ester group is disclosed. The cyclic carbonyl compounds can be polymerized by ring opening methods to form ROP polymers comprising repeat units comprising a side chain pentafluorophenyl ester group. Using a suitable nucleophile, the pendant pentafluorophenyl ester group can be selectively transformed into a variety of other functional groups before or after the ring opening polymerization.
Owner:IBM CORP +1
Crosslinked hydrogel copolymers
The present invention relates to crosslinked polymers, synthesized through ring-opening polymerization of ethylenically unsaturated epoxides, in combination with α-hydroxy acids using a hydrophilic macroinitiator, such as poly(ethylene glycol), to form substituted copolymers having ethylenically unsaturated functionality randomly distributed along the polyester polymer backbone. That copolymer is subsequently crosslinked to form a hydrogel network. More particularly, the present invention relates to the synthesis of biodegradable poly(α-hydroxy acid-co-glycidyl methacrylate)-block-poly(ethylene glycol)-block-poly(α-hydroxy acid-co-glycidyl methacrylate) copolymers, which are subsequently crosslinked to form hydrogel networks. The invention also relates to the use of these hydrogel networks in various applications, in particular, for the controlled release of drugs and proteins.
Owner:ALKERMES INC
Paclitaxol predrug of biodegradable polymer and its synthesis method
The invention provides a paclitaxol predrug of biodegradable polymer and its synthesis method, which is bond from polyoxyalkylene-aliphatic polyester blocked copolymer and paclitaxol, the synthesizing process comprises, carrying out ring-opening polymerization of aliphatic cyclic esters at the presence of polyethylene glycol (PEG), solvent and catalyst, obtaining polyethylene glycol - aliphatic polyester blocked copolymer, then converting its end hydroxyl into end carboxyl, reacting with paclitaxol for esterification at the presence of condensing agent, the obtained paclitaxol predrug has amphiphilic, thus can be prepared into water based preparation.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Biodegradable copolymer and thermosensitive material
The disclosed is a biodegradable copolymer, an amphiphilic diblock copolymer, composed of a hydrophilic segment and a hydrophobic segment. The hydrophilic segment is an endcapped PEG or derivatives thereof. The hydrophilic segment is a random polymer polymerized of lactone or cyclic C3-C6 molecule and lactic acid / glycolic acid. There is no coupling agent between the hydrophilic and hydrophobic segments, and the biodegradable copolymer is formed by one-pot ring-opening polymerization. The biodegradable copolymer can be dissolved in water to form a thermosensitive material having a phase transfer temperature of 25 to 50° C., thereby being applied to biological activity factor delivery, tissue engineering, cell culture and biological glue.
Owner:IND TECH RES INST
Polylactic acid block polymer and preparation method thereof
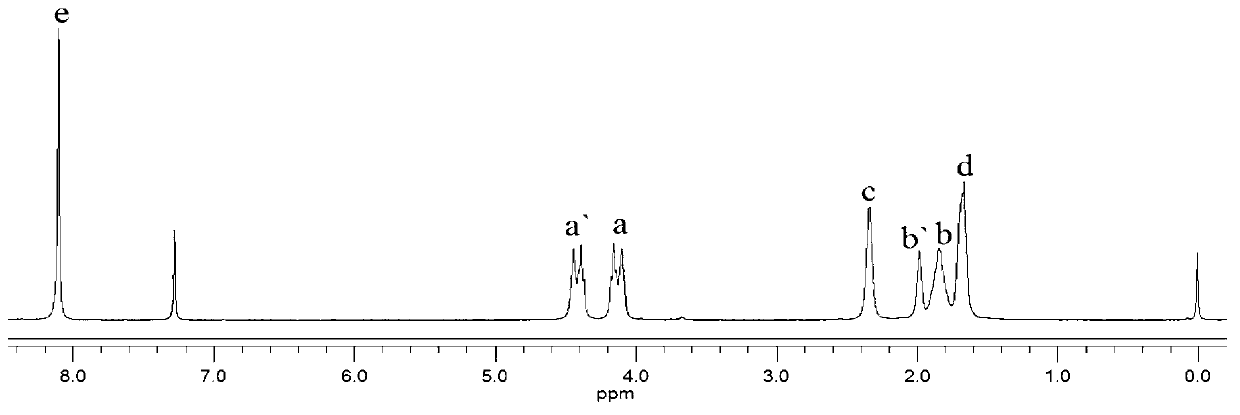
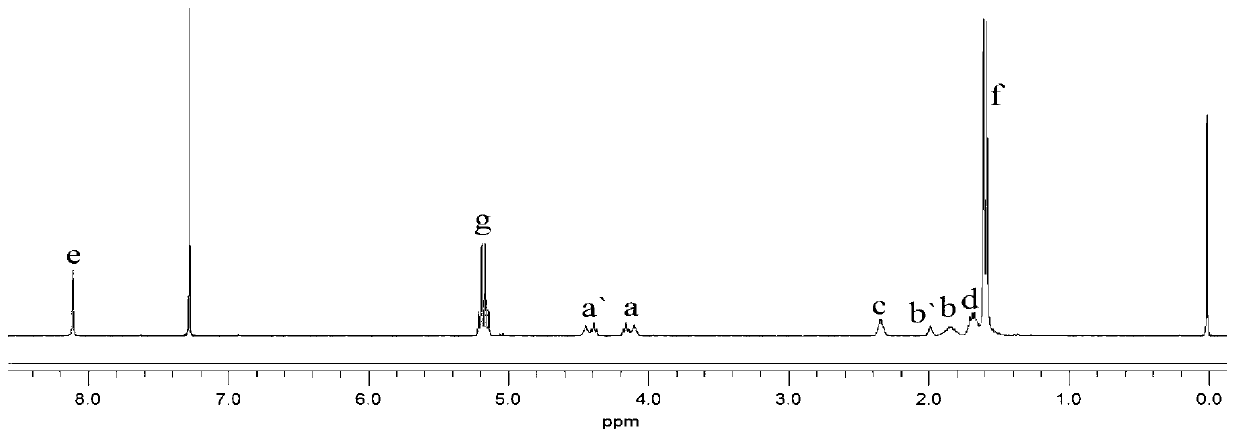
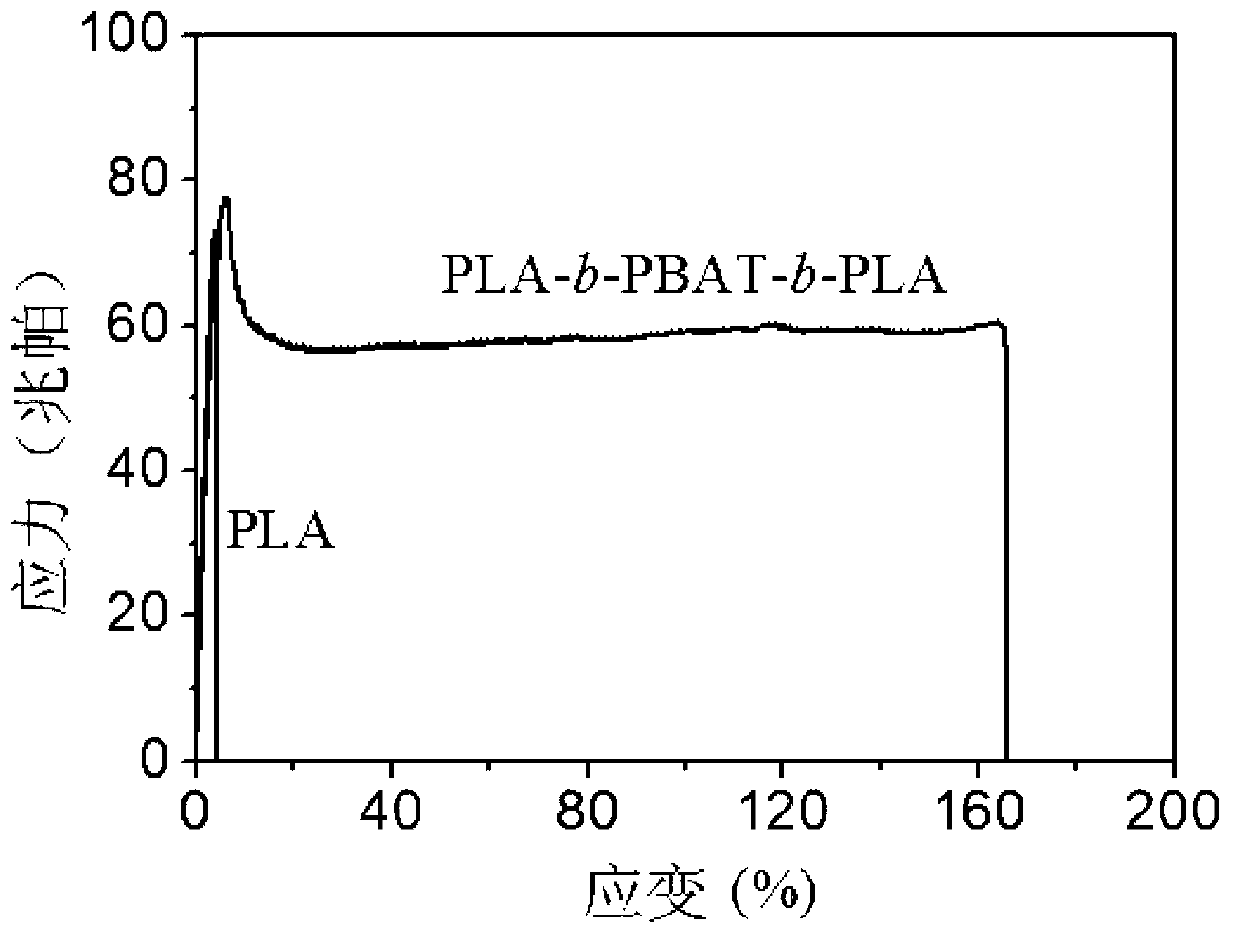
The invention provides a polylactic acid block polymer and a preparation method thereof. The preparation method comprises the following steps: mixing poly(adipic acid-terephthalic acid)butanediol copolyester, lactide and a first catalyst, and heating to a molten state to react, thereby obtaining the polylactic acid block polymer disclosed as Formula (I). Compared with the polymer obtained by the PLA-PBAT chain extension reaction initiated by diisocyanate in the prior art, the invention uses the molten-state poly(adipic acid-terephthalic acid)butanediol copolyester as the reactant to directly initiate the ring-opening polymerization of lactide, and the polymerization method is mass polymerization without adding the chain extender diisocyanate or any organic solvent, thereby avoiding jeopardizing the human body and environment; the raw materials can be mixed and heated to react to obtain the polylactic acid block polymer, so that the preparation method is simpler and lowers the preparation cost; and the polylactic acid block polymer provided by the invention has definite structure and narrow molecular weight distribution.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Polar group-containing olefin copolymer, process for preparing the same, thermoplastic resin composition containing the copolymer, and uses thereof
The present invention is a polar group-containing olefin copolymer having excellent adhesion properties to metals or polar resins and excellent compatibility therewith. A process for preparing the copolymer, a thermoplastic resin composition containing the copolymer, and uses thereof are also described. The polar group-containing olefin copolymer comprises a constituent unit derived from an α-olefin of 2 to 20 carbon atoms, and a constituent unit derived from a straight-chain, branched or cyclic polar group-containing monomer having at the end a polar group such as a hydroxyl group or an epoxy group and / or a constituent unit derived from a macromonomer having at the end a polymer segment obtained by anionic polymerization, ring-opening polymerization or polycondensation. The polar group-containing olefin copolymer and the thermoplastic resin composition containing the copolymer are used for films, sheets, modifiers, building / civil engineering materials, automobile exterior trim, electric / electronic parts, coating bases, compatibilizing agents, etc.
Owner:MITSUI CHEM INC
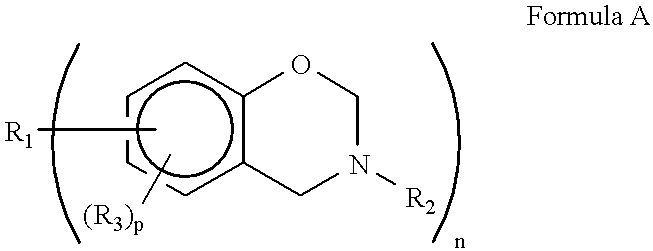
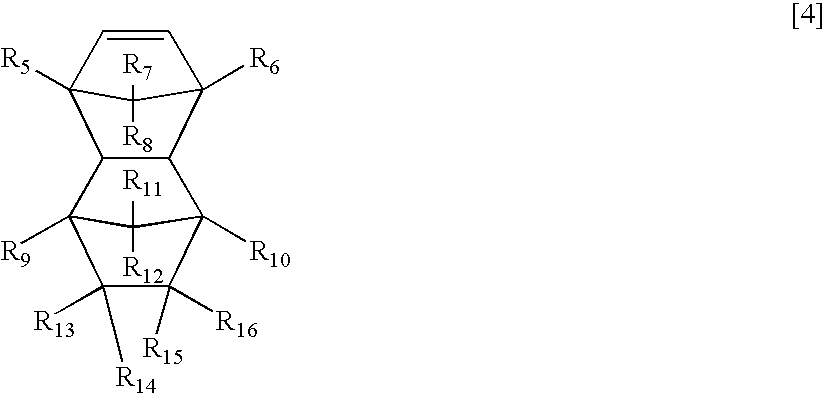
Norbornene derivative and norbornene polymer obtained therefrom through ring opening polymerization
A novel norbornene derivative represented by a general formula (1m) shown below is provided. By conducting a ring opening polymerization of this norbornene derivative, or by performing a subsequent hydrogenation following the ring opening polymerization, a ring opening polymer or a hydrogenated product thereof with an excellent low birefringence can be obtained. [wherein, at least one of R1 to R4 is a group selected from the group consisting of groups represented by a general formula (1-1) shown below and groups represented by a general formula (1-2) shown below][wherein, at least one of RA, RB and Z is a group represented by the formula —C(O)O—].
Owner:JSR CORPORATIOON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com