Patents
Literature
5703results about "Metal rolling stand details" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
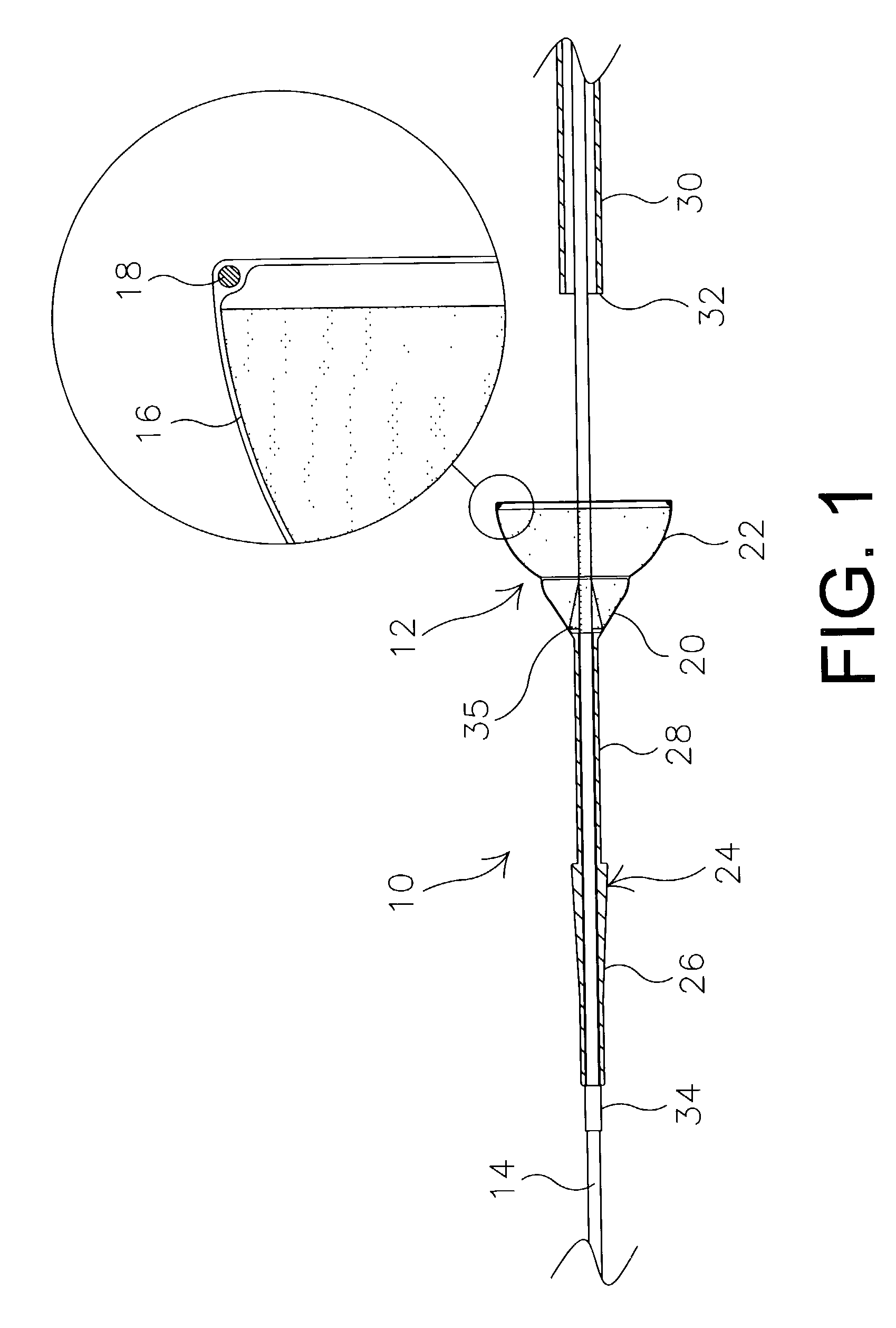
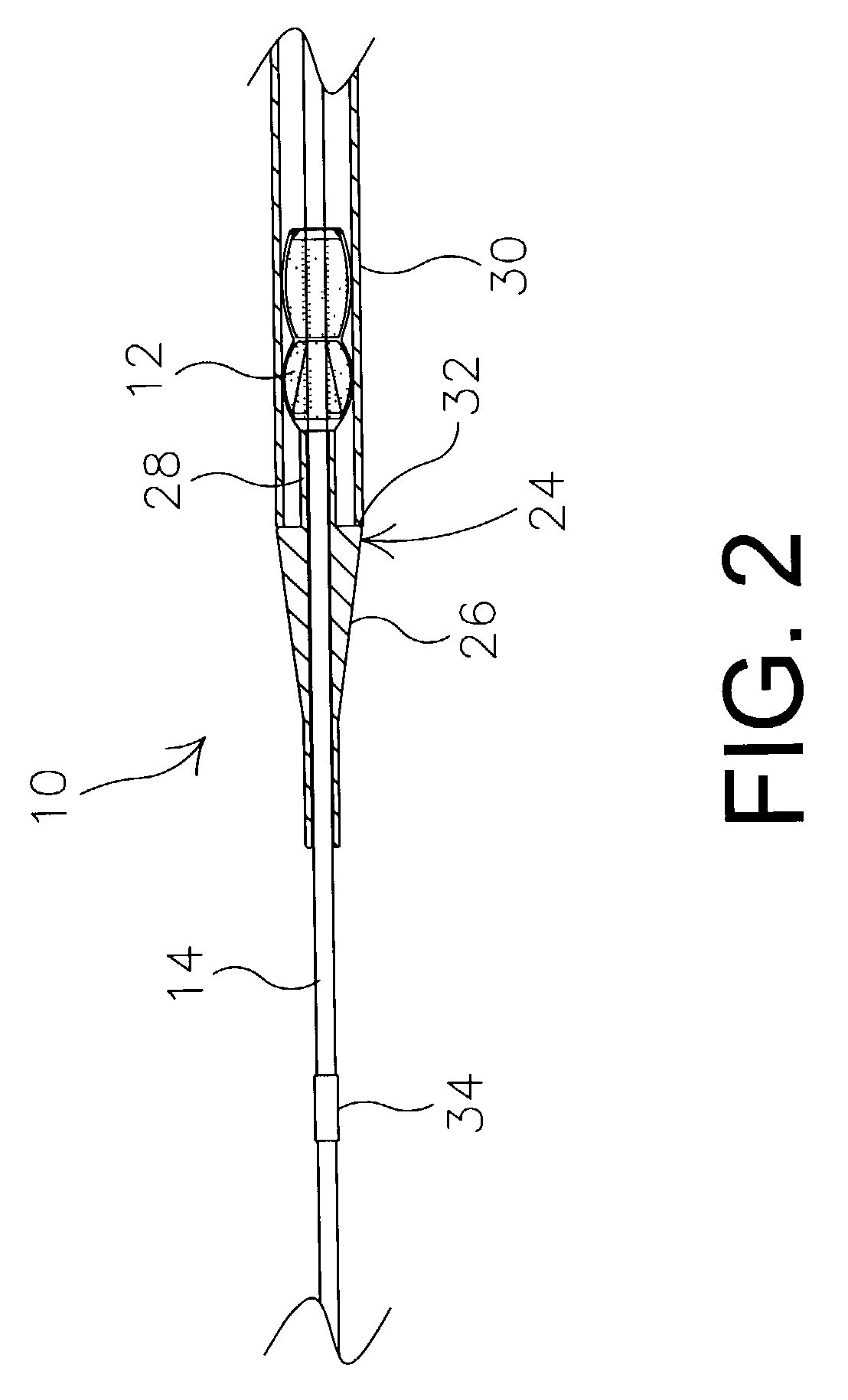
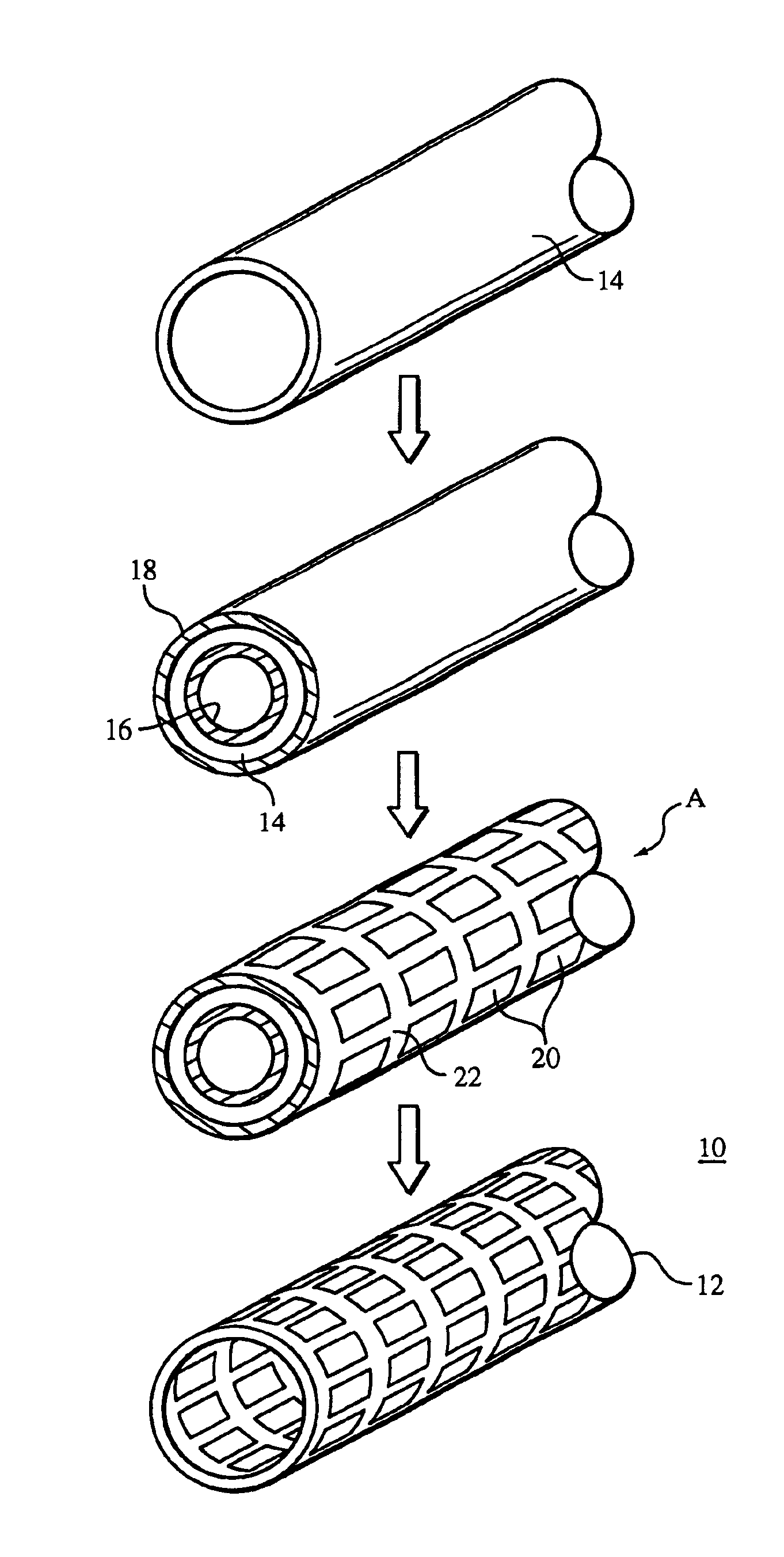
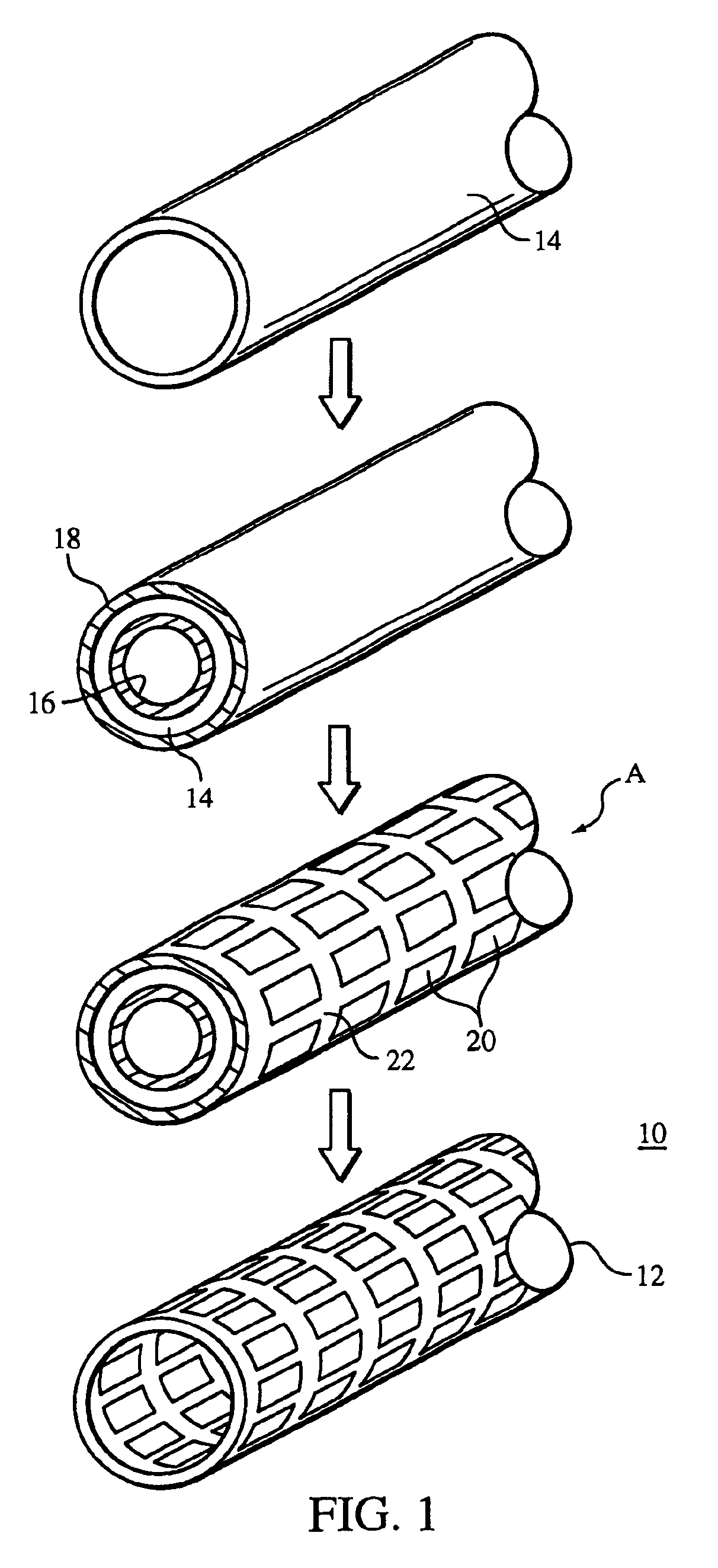
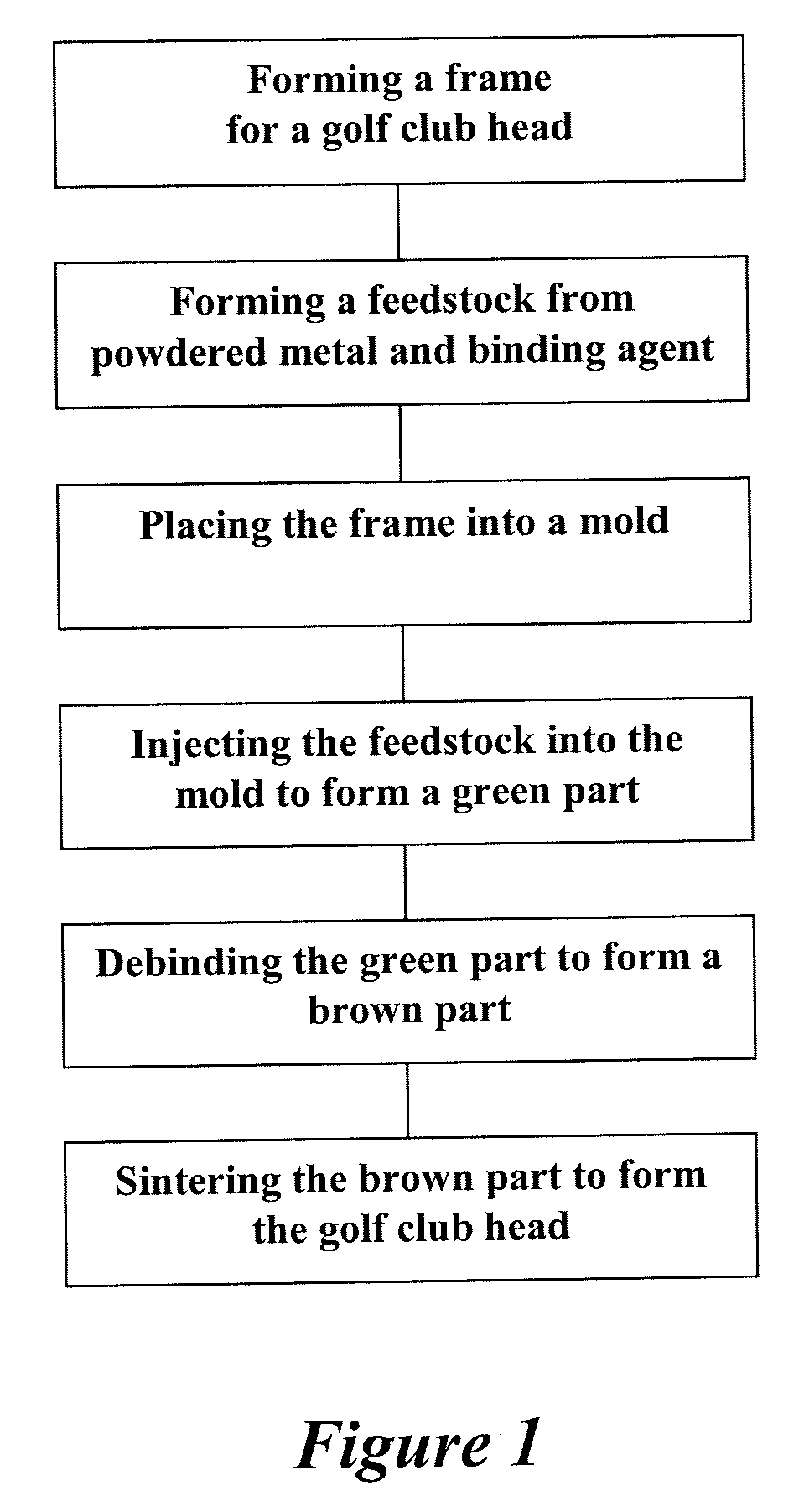
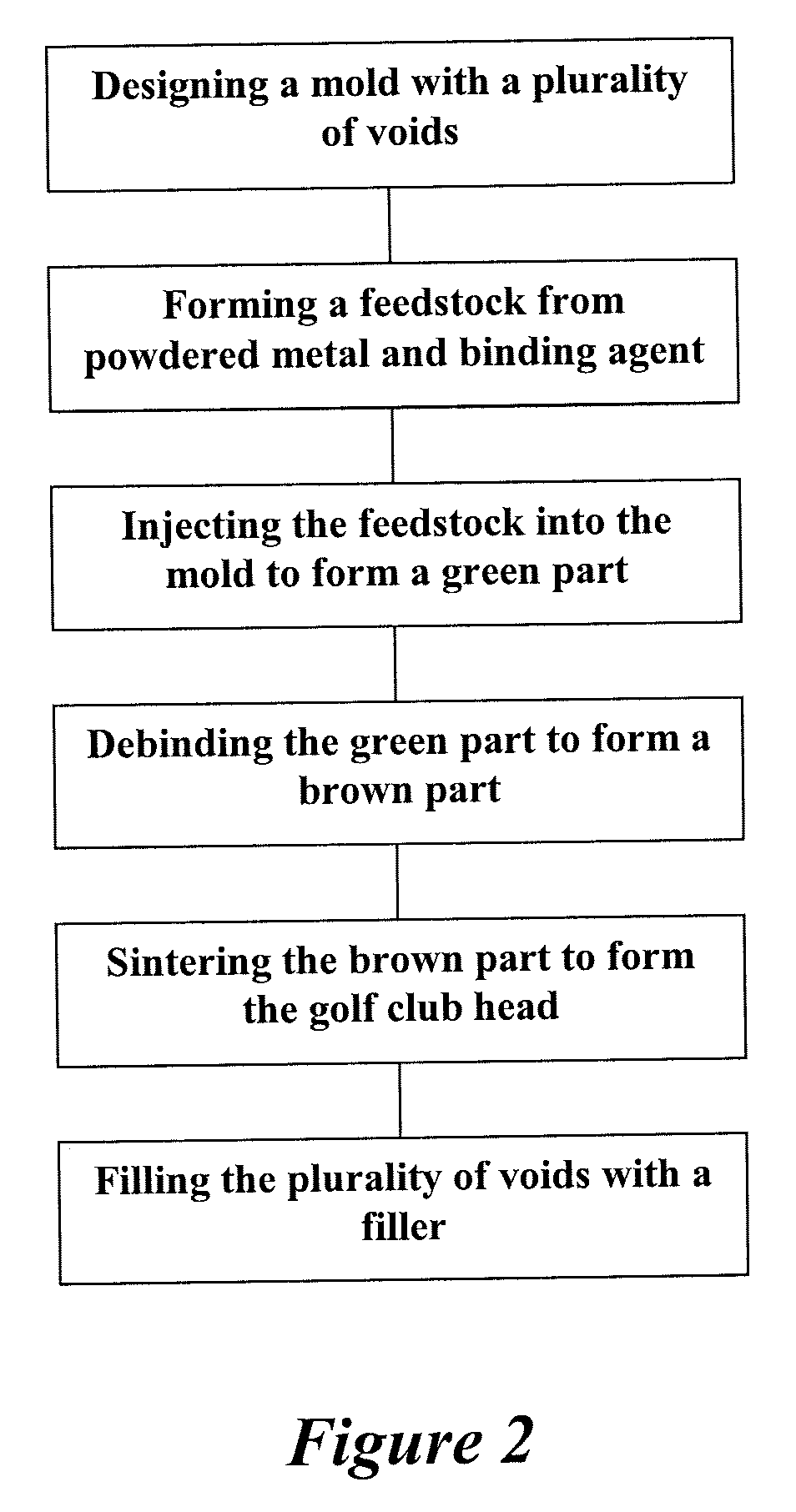
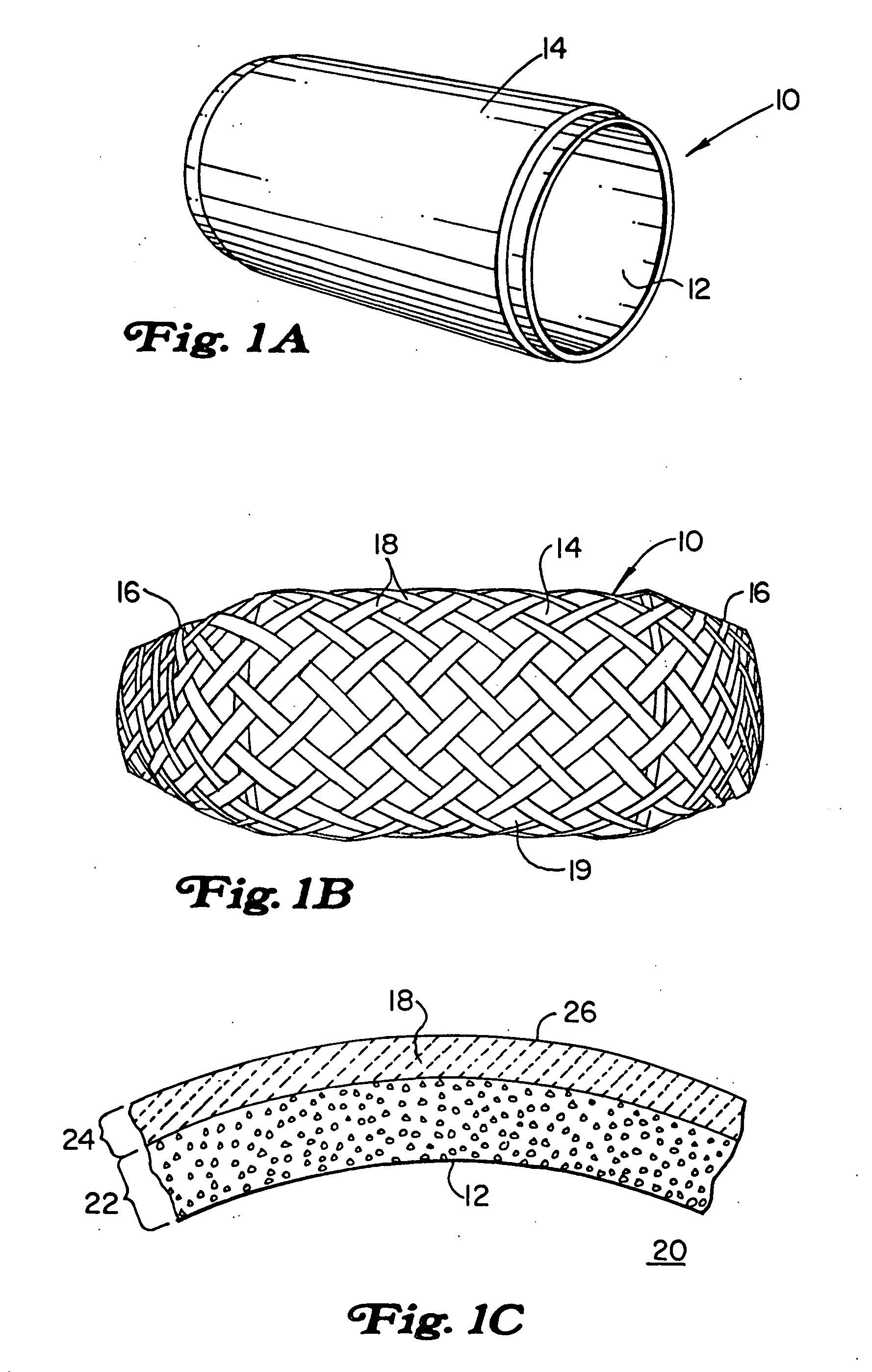
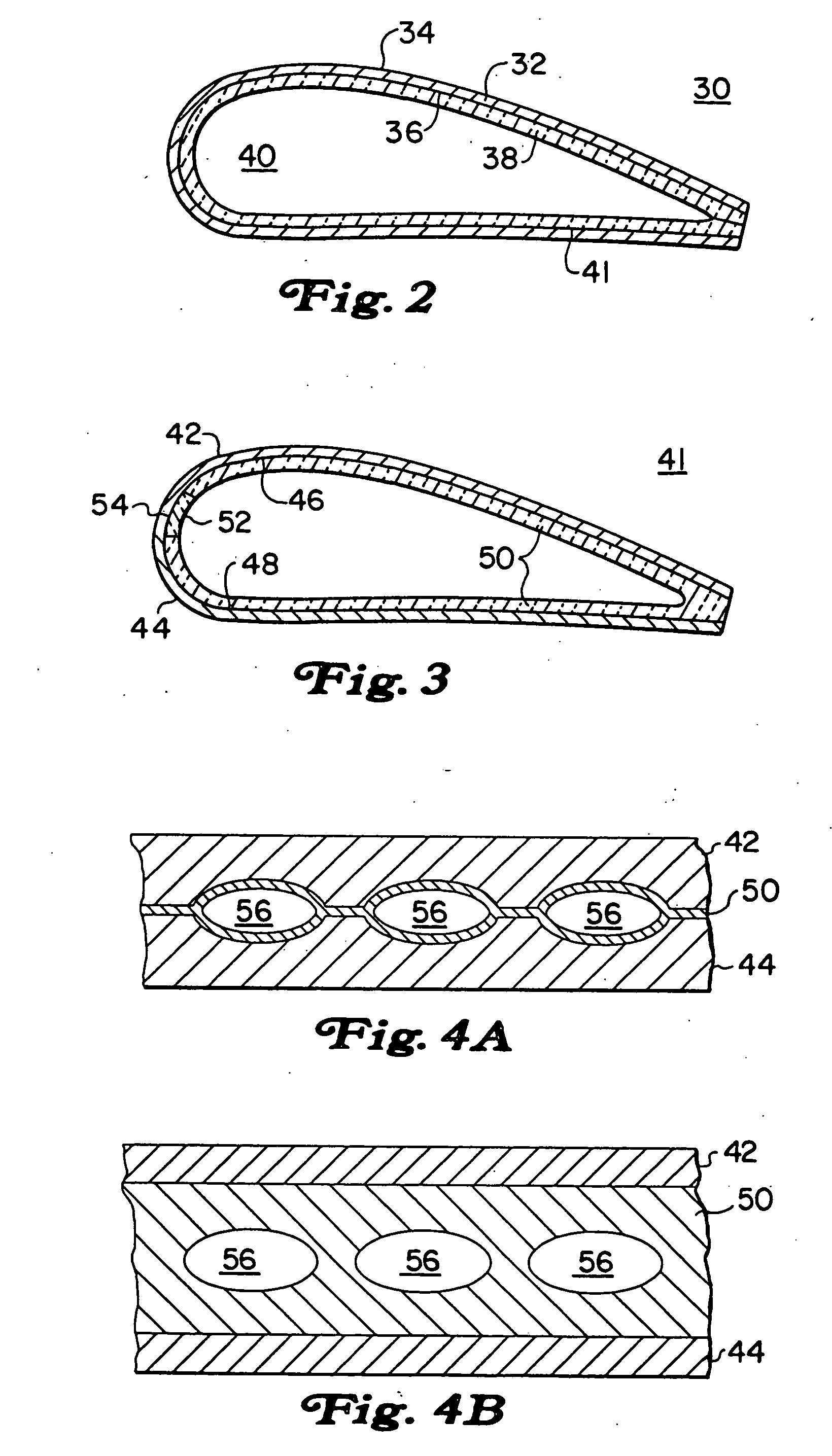
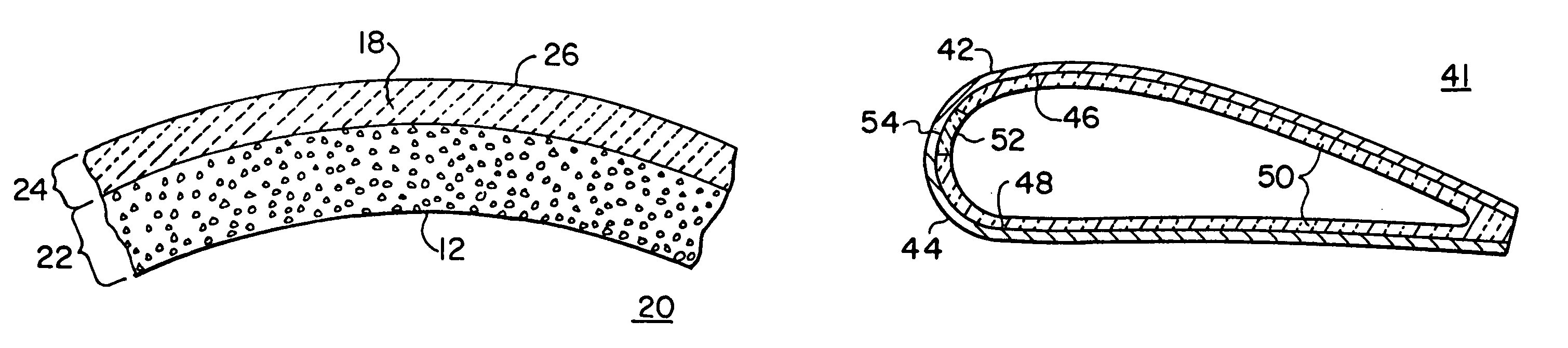
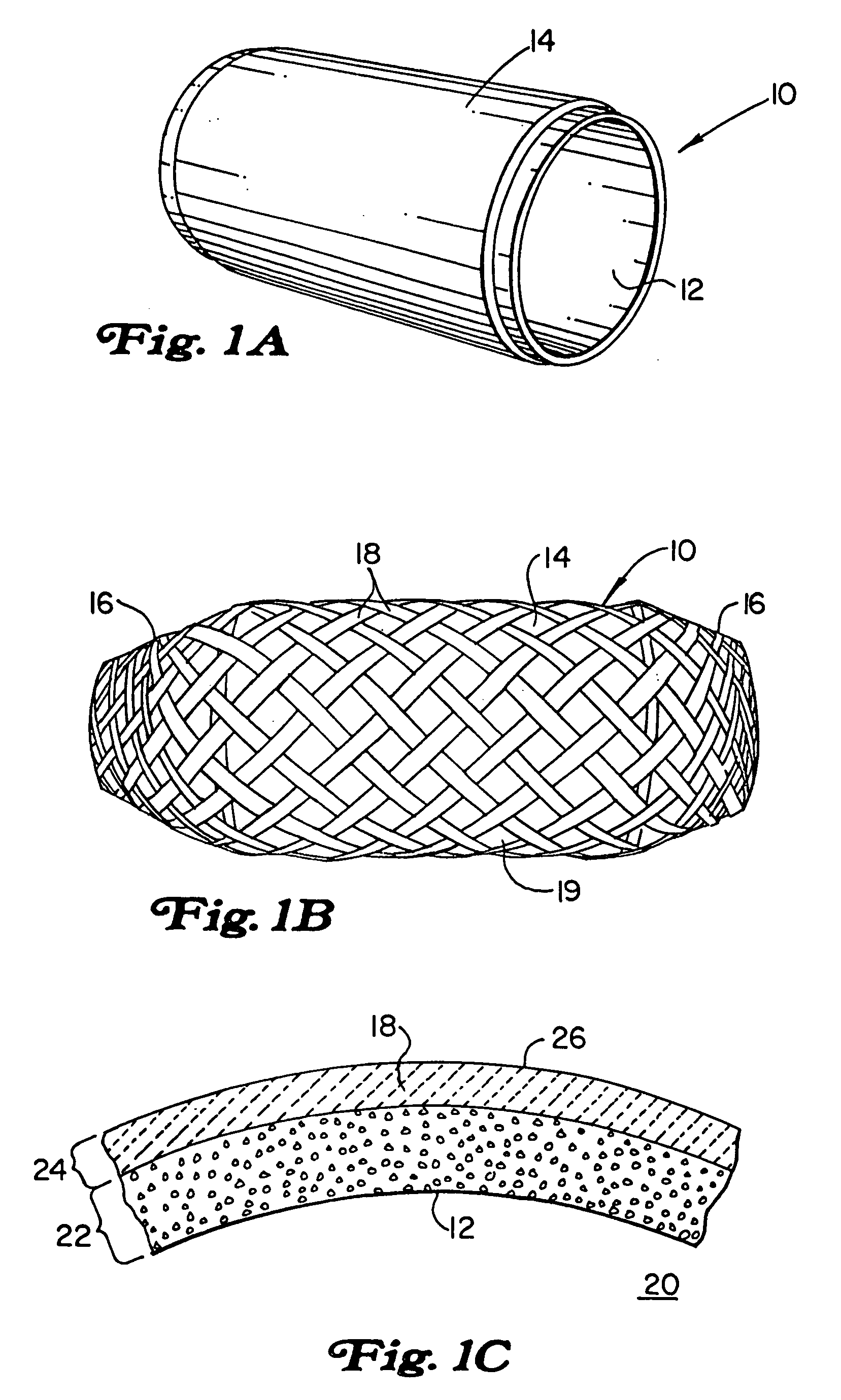
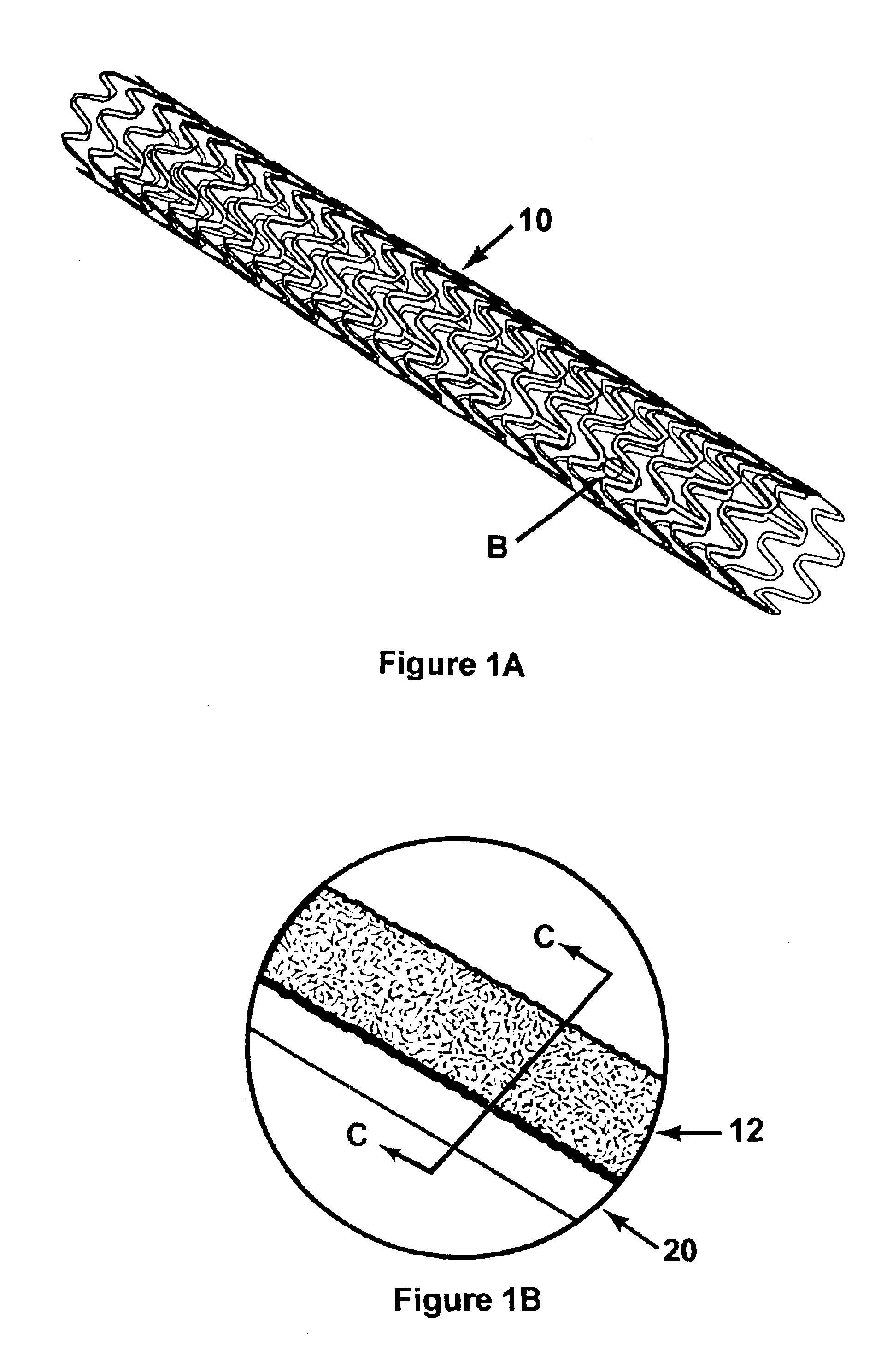
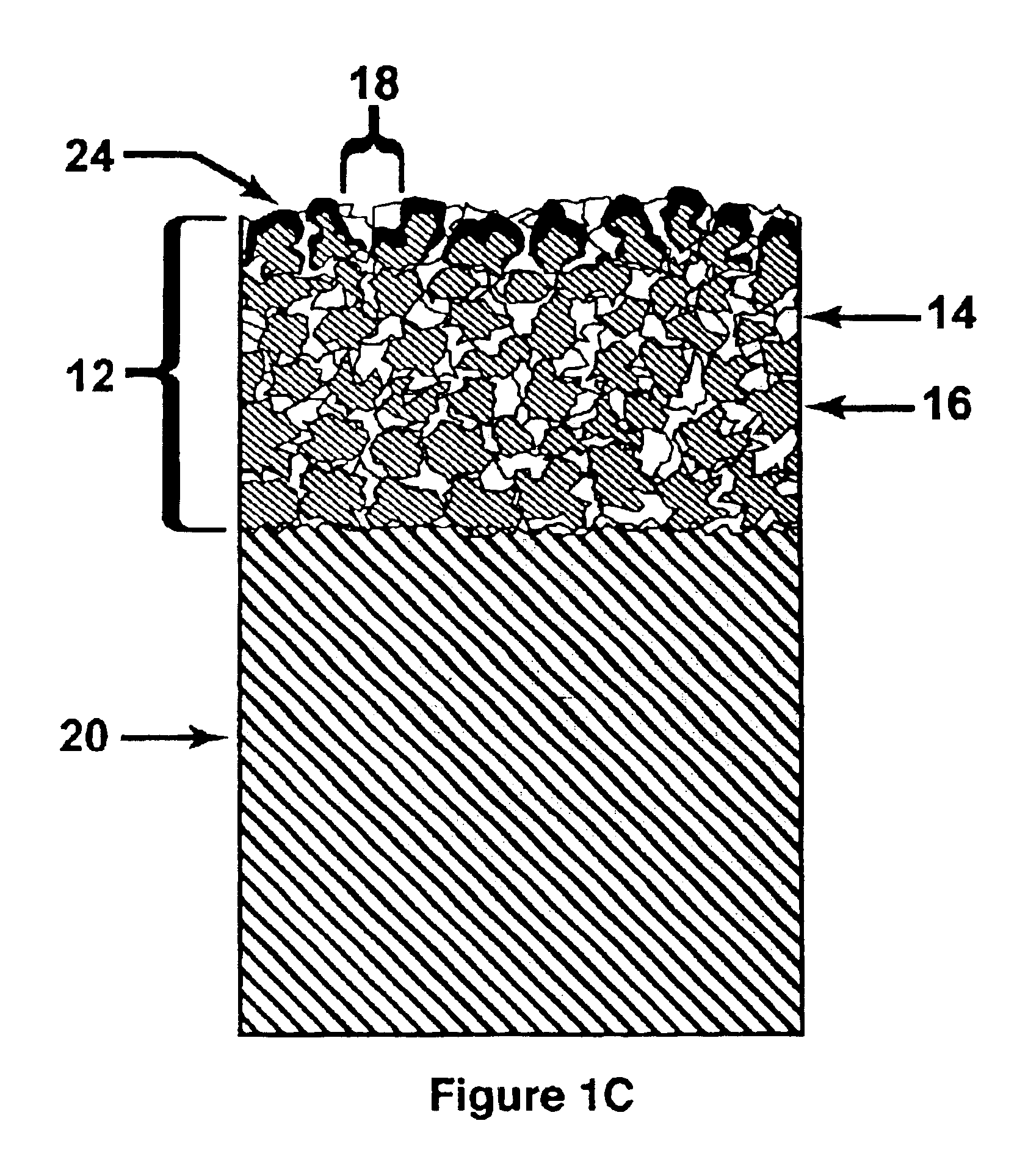
Method of making an embolic filter
Embolic protection filters and methods of making and using such devices are disclosed. An illustrative method of making a device for filtering embolic debris from a body may include the steps of molding a filter assembly that includes a distal tip and a filter portion, forming a plurality of apertures within the filter portion, and coupling a support member to the filter assembly that is adapted to shift the filter portion between a collapsed configuration and an expanded configuration.
Owner:BOSTON SCI SCIMED INC
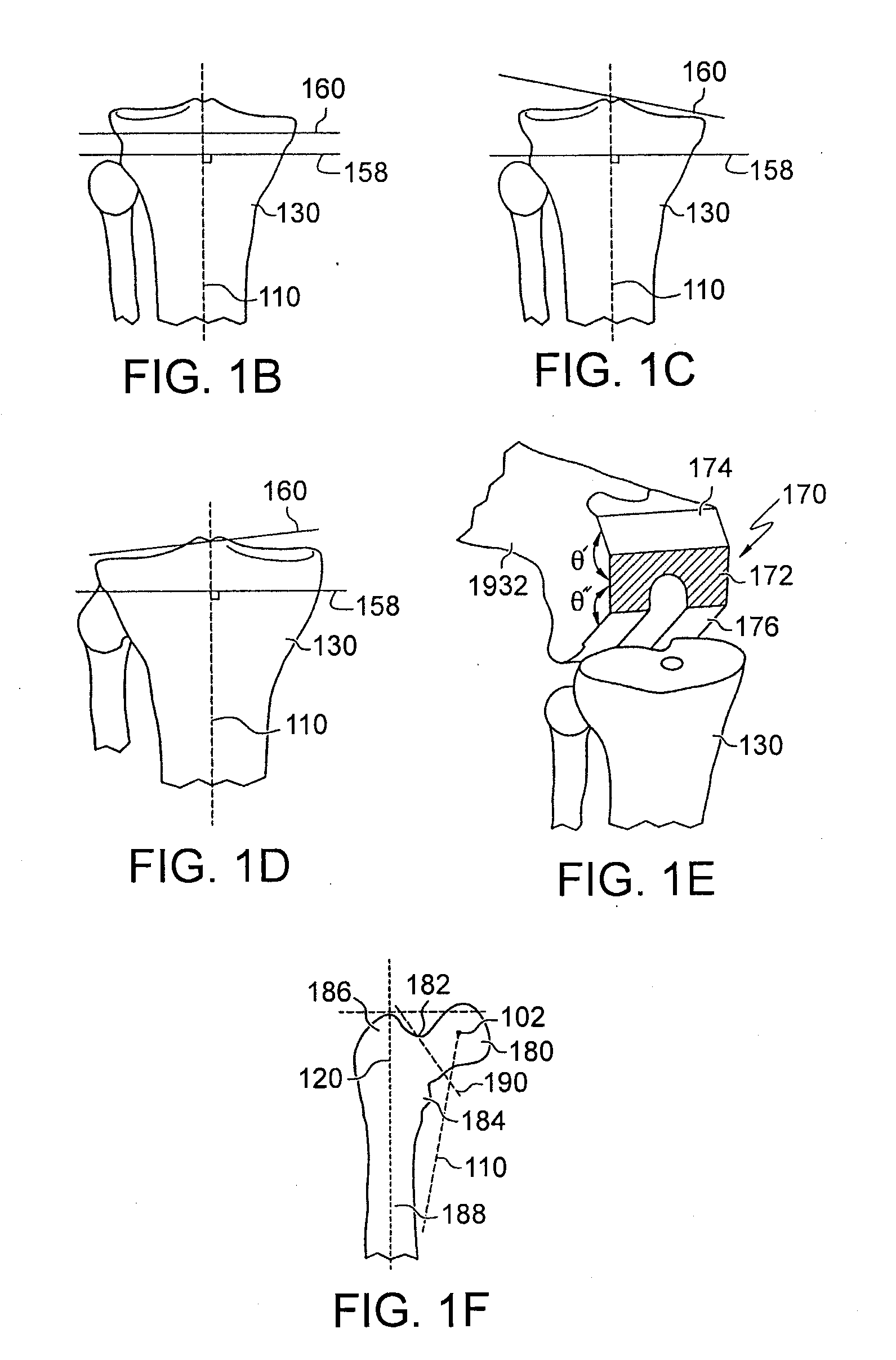
Surgical Tools Facilitating Increased Accuracy, Speed and Simplicity in Performing Joint Arthroplasty
InactiveUS20090307893A1Metal rolling stand detailsDiagnostic recording/measuringArticular surfacesArticular surface
Disclosed herein are tools for repairing articular surfaces repair materials and for repairing an articular surface. The surgical tools are designed to be customizable or highly selectable by patient to increase the speed, accuracy and simplicity of performing total or partial arthroplasty.
Owner:CONFORMIS
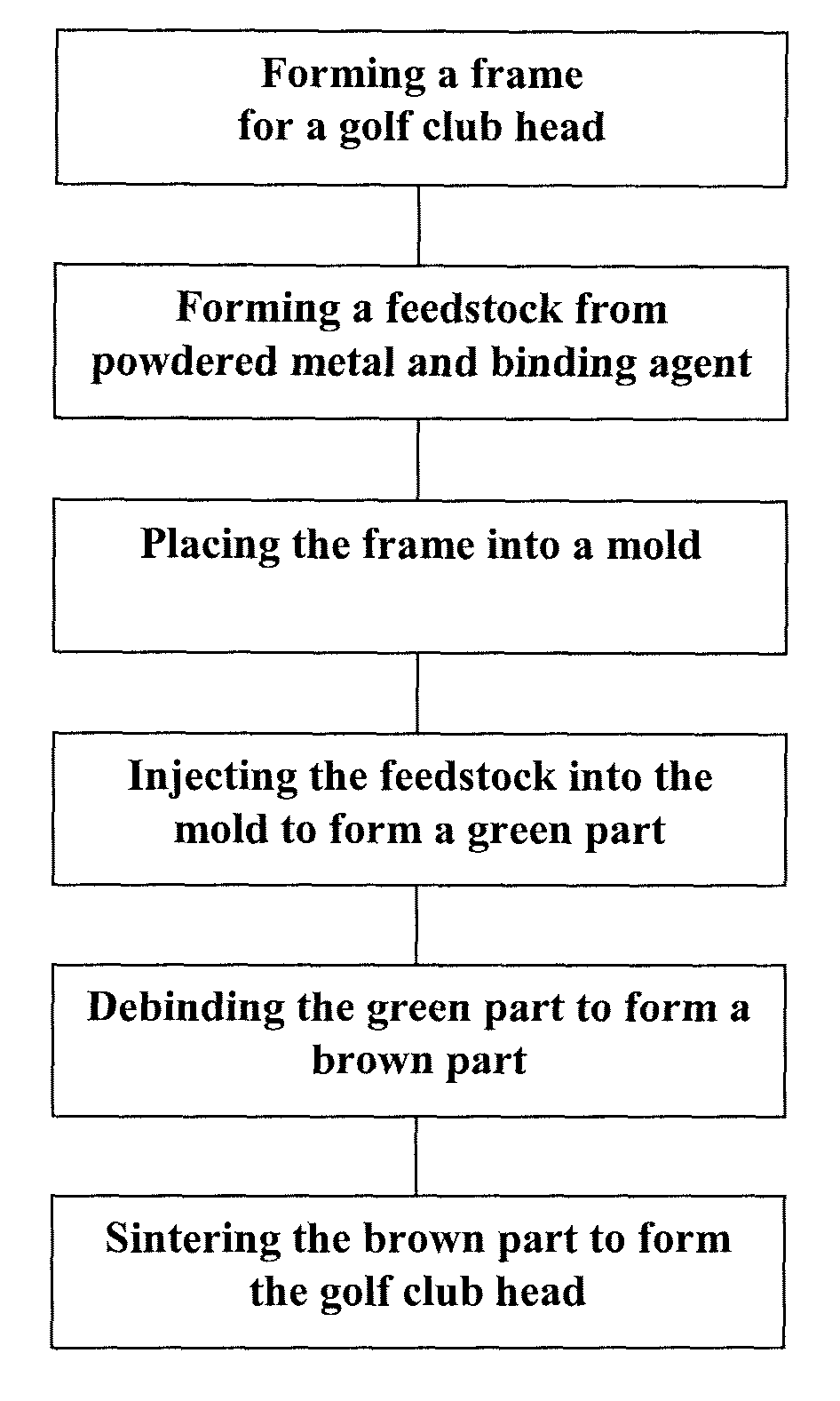
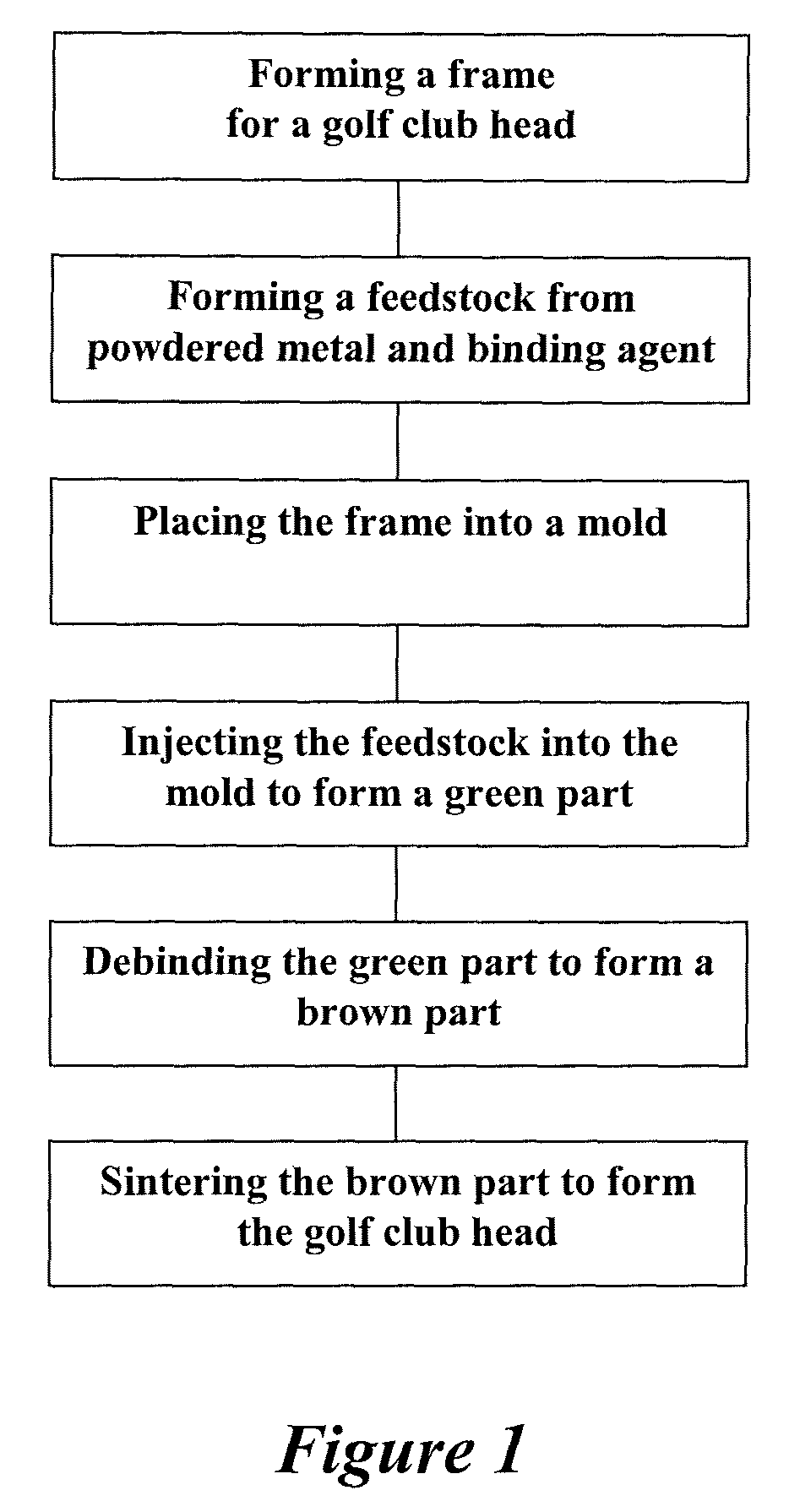
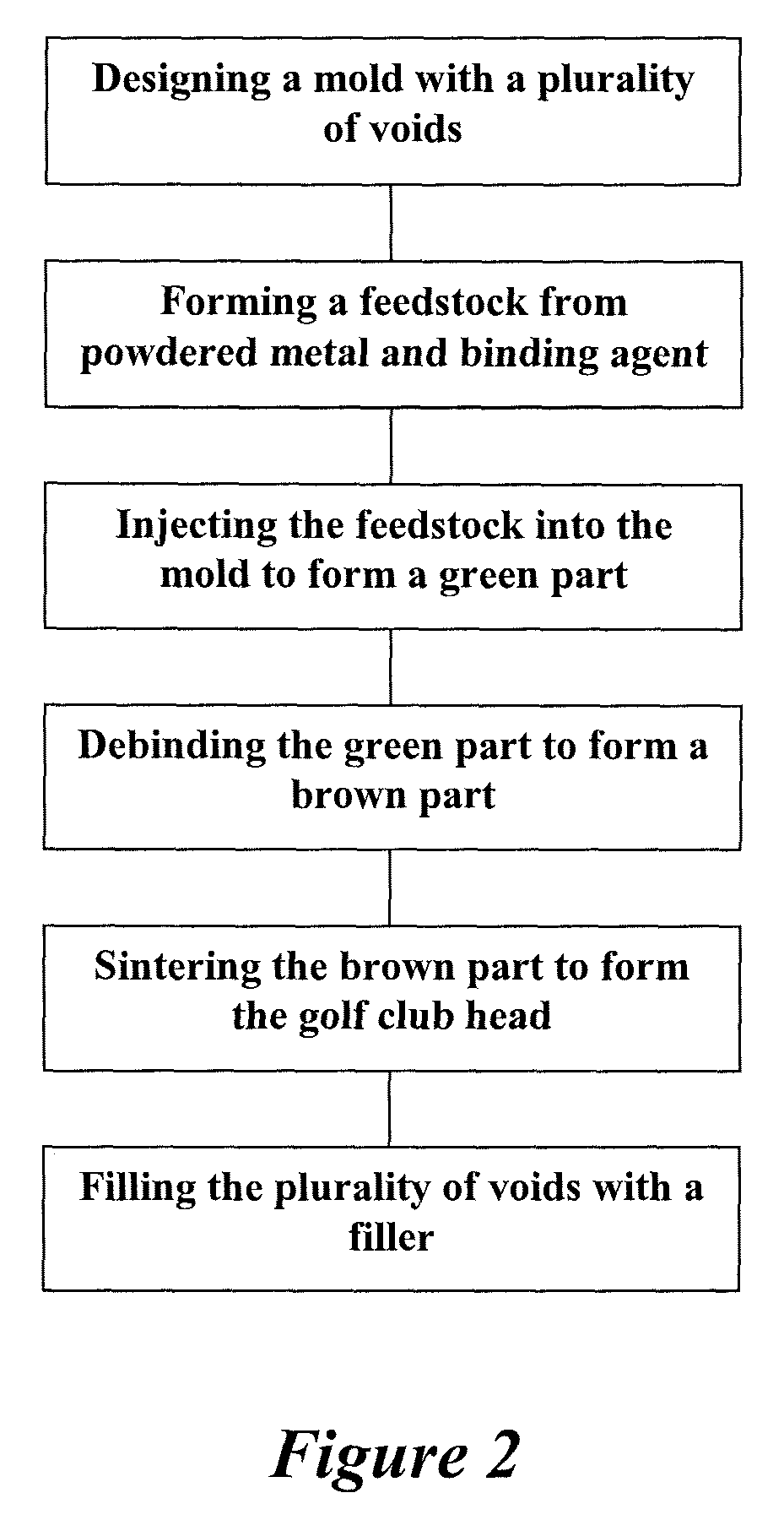
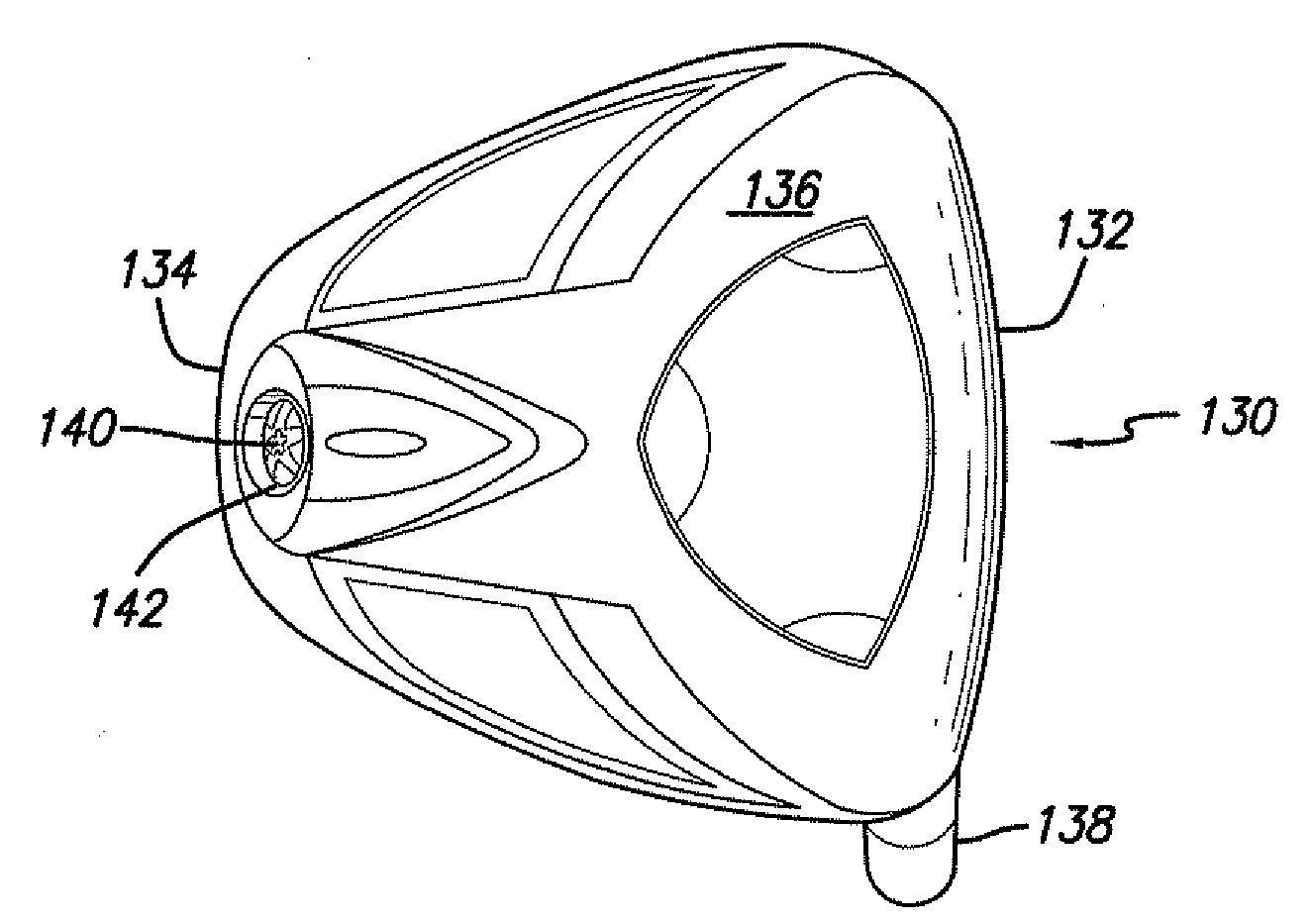
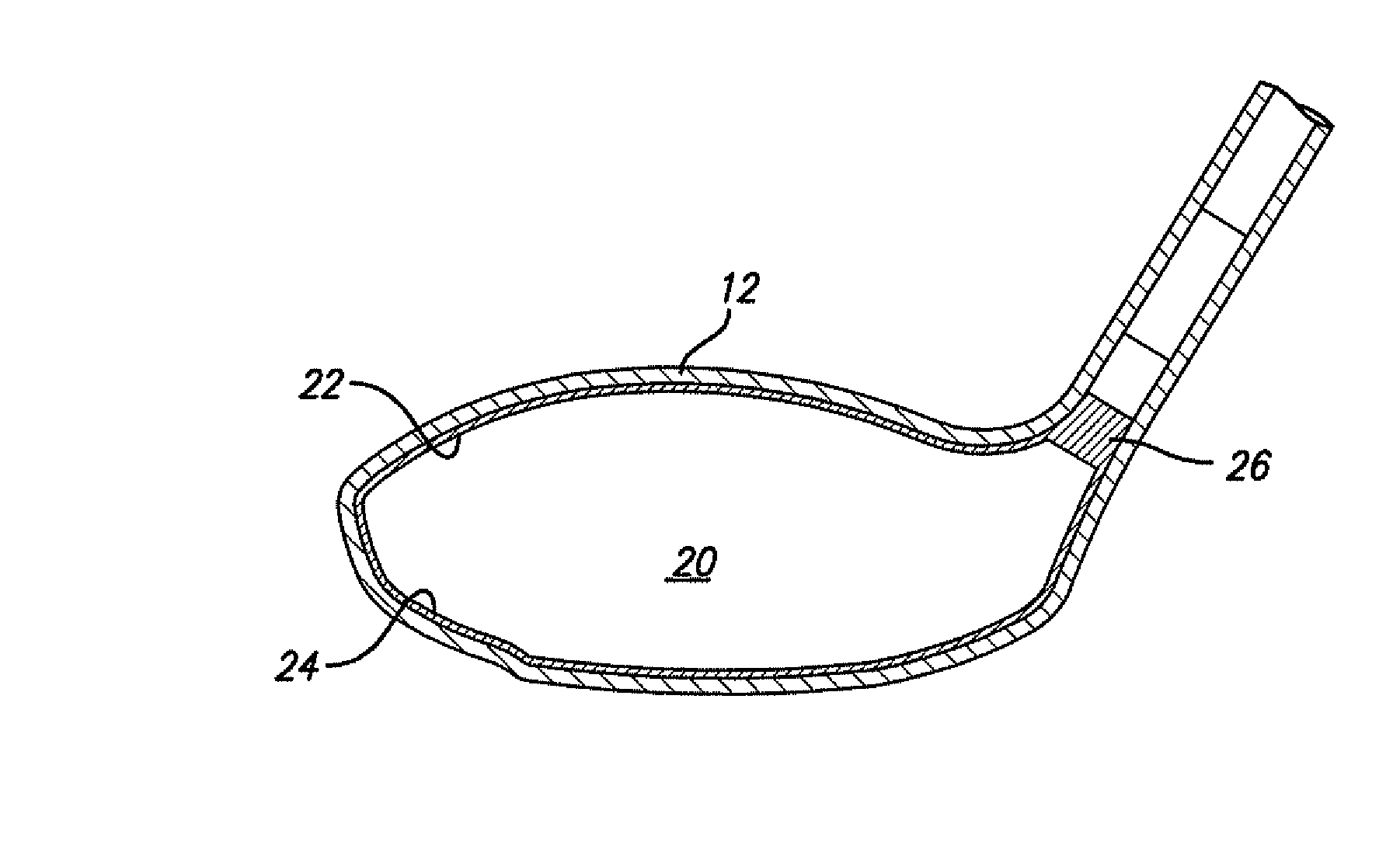
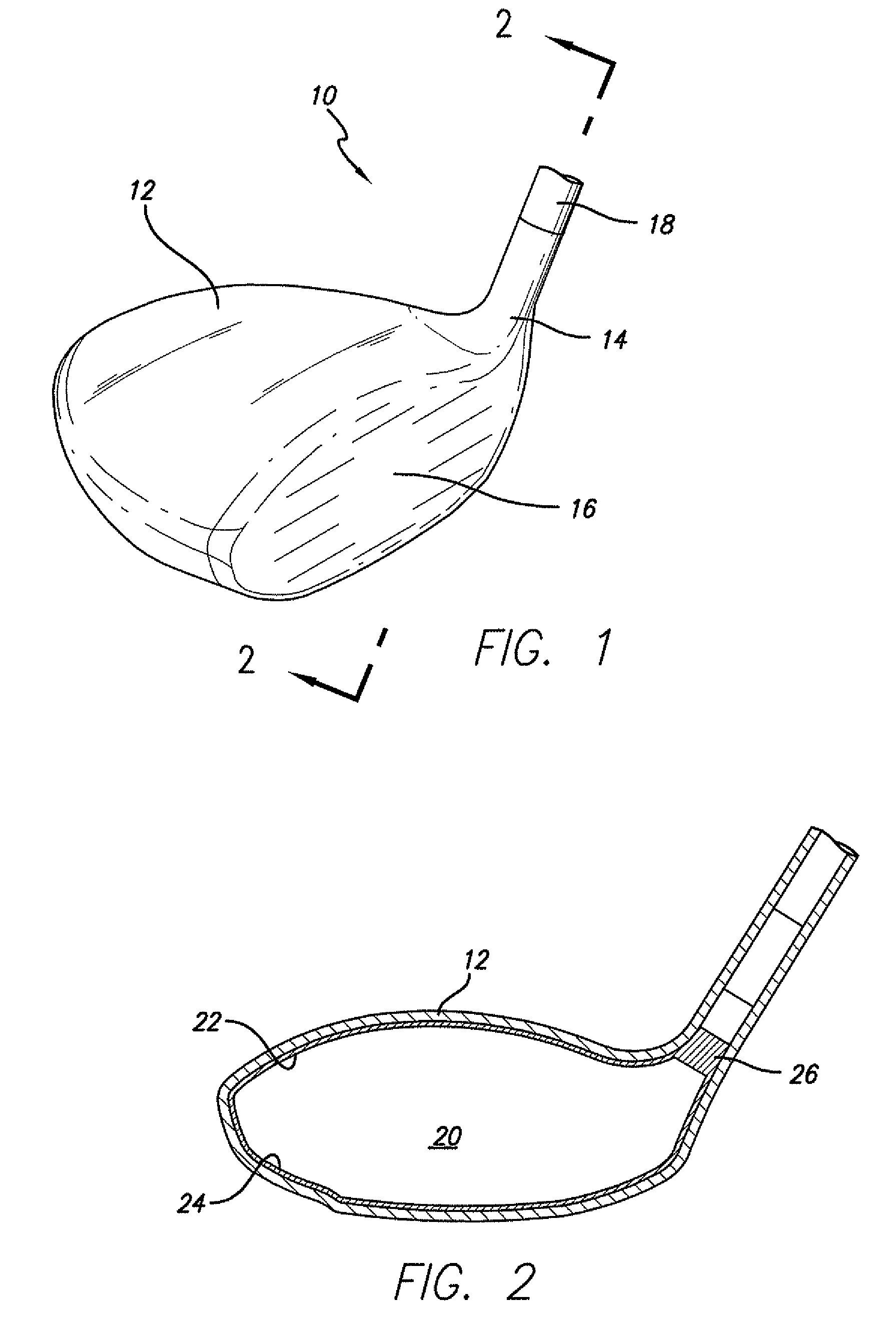
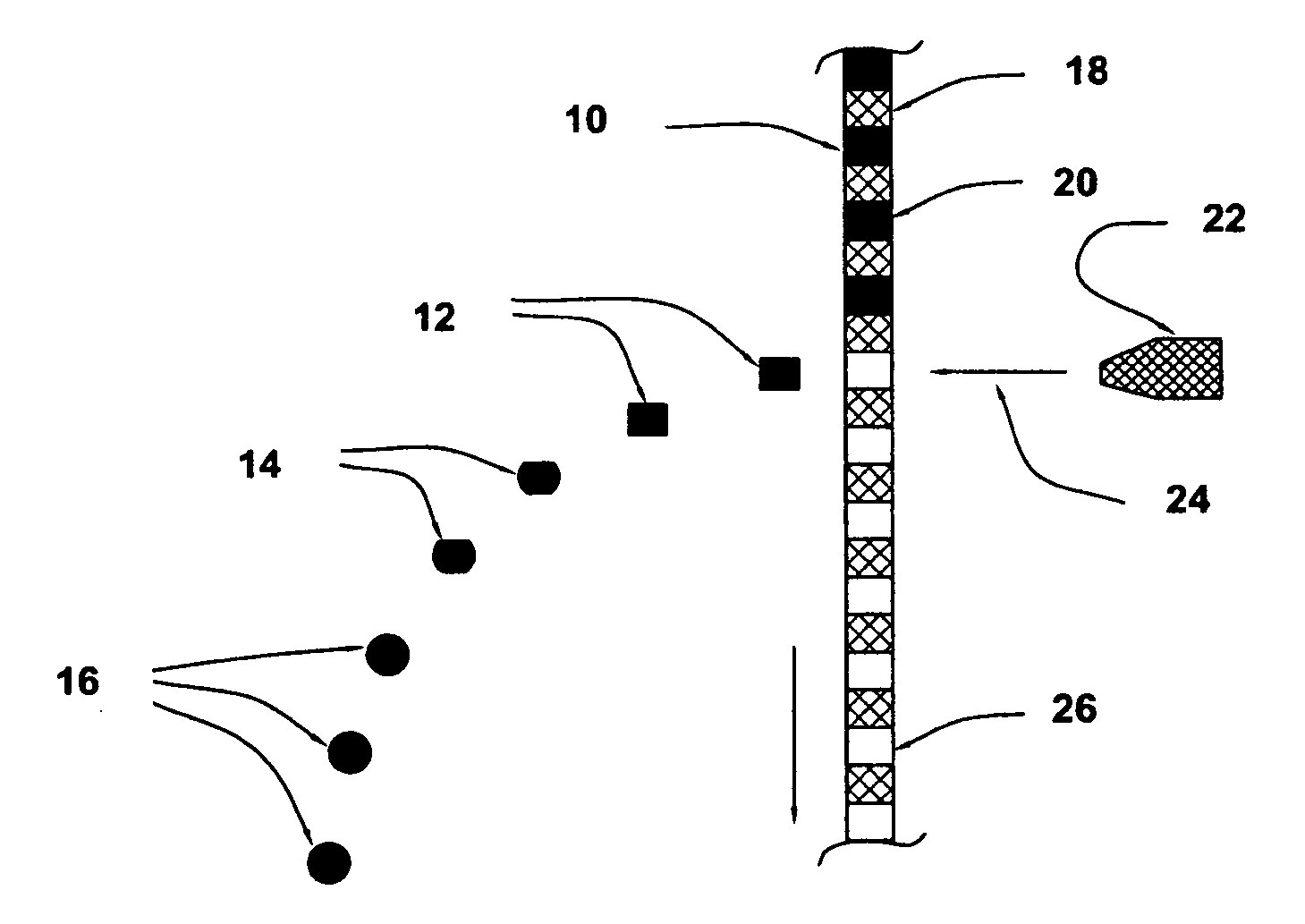
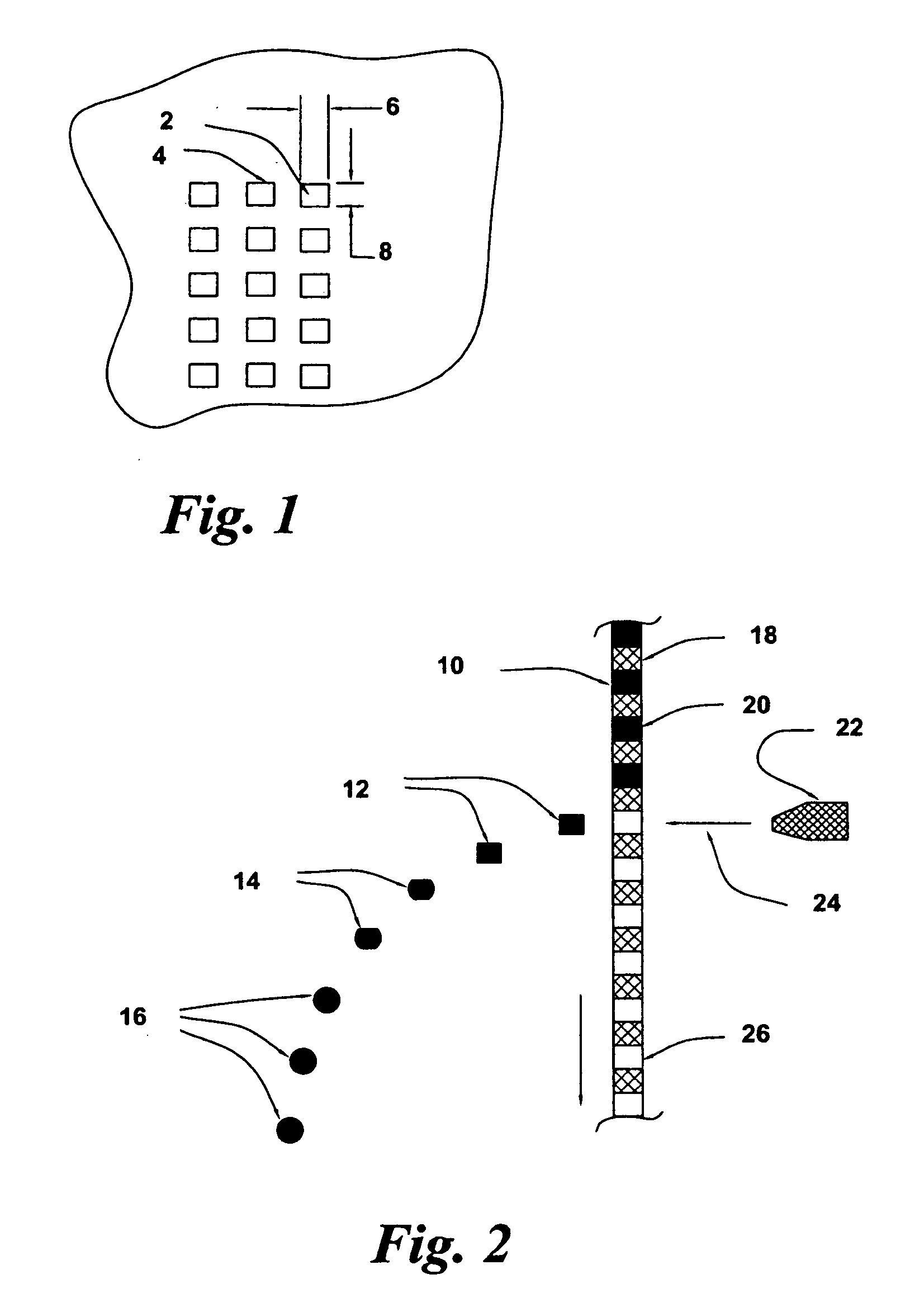
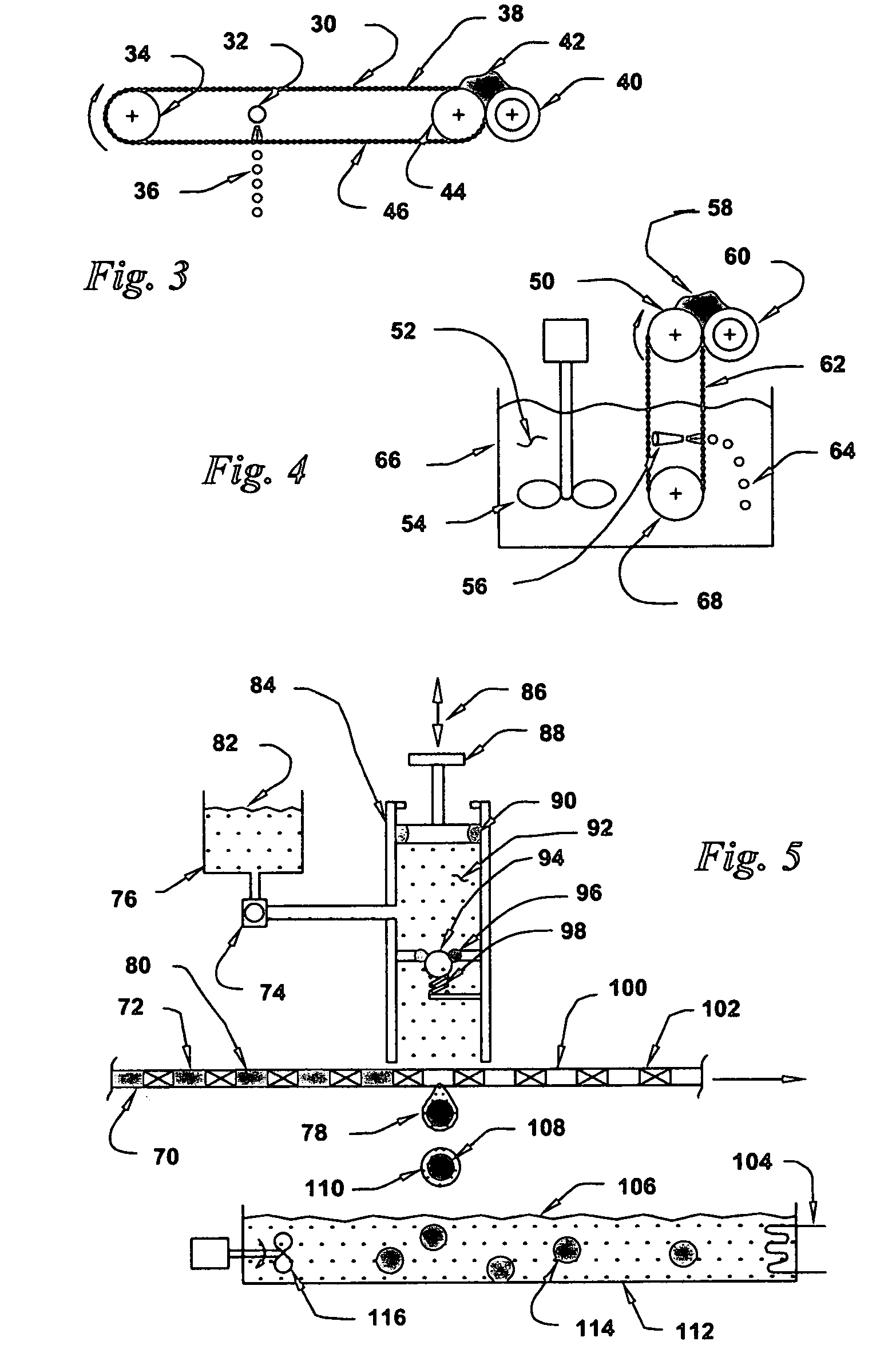
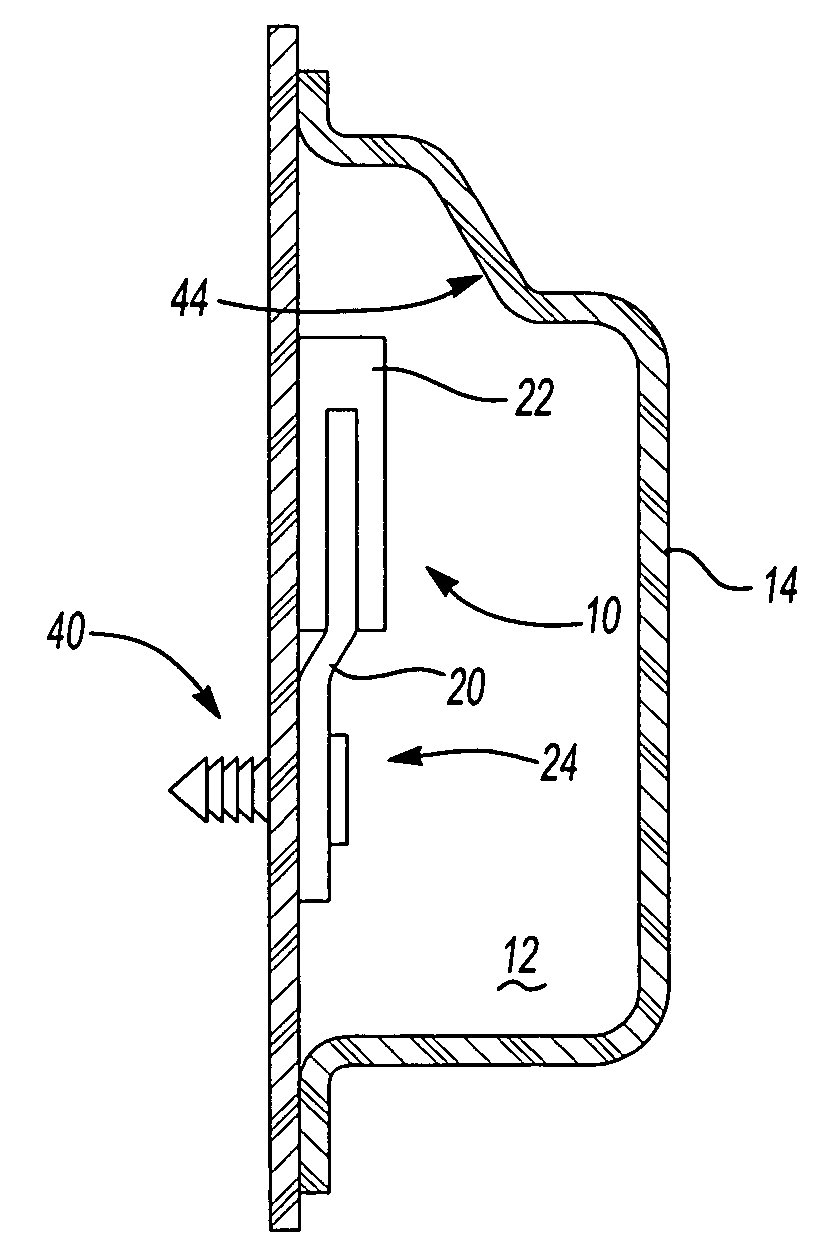
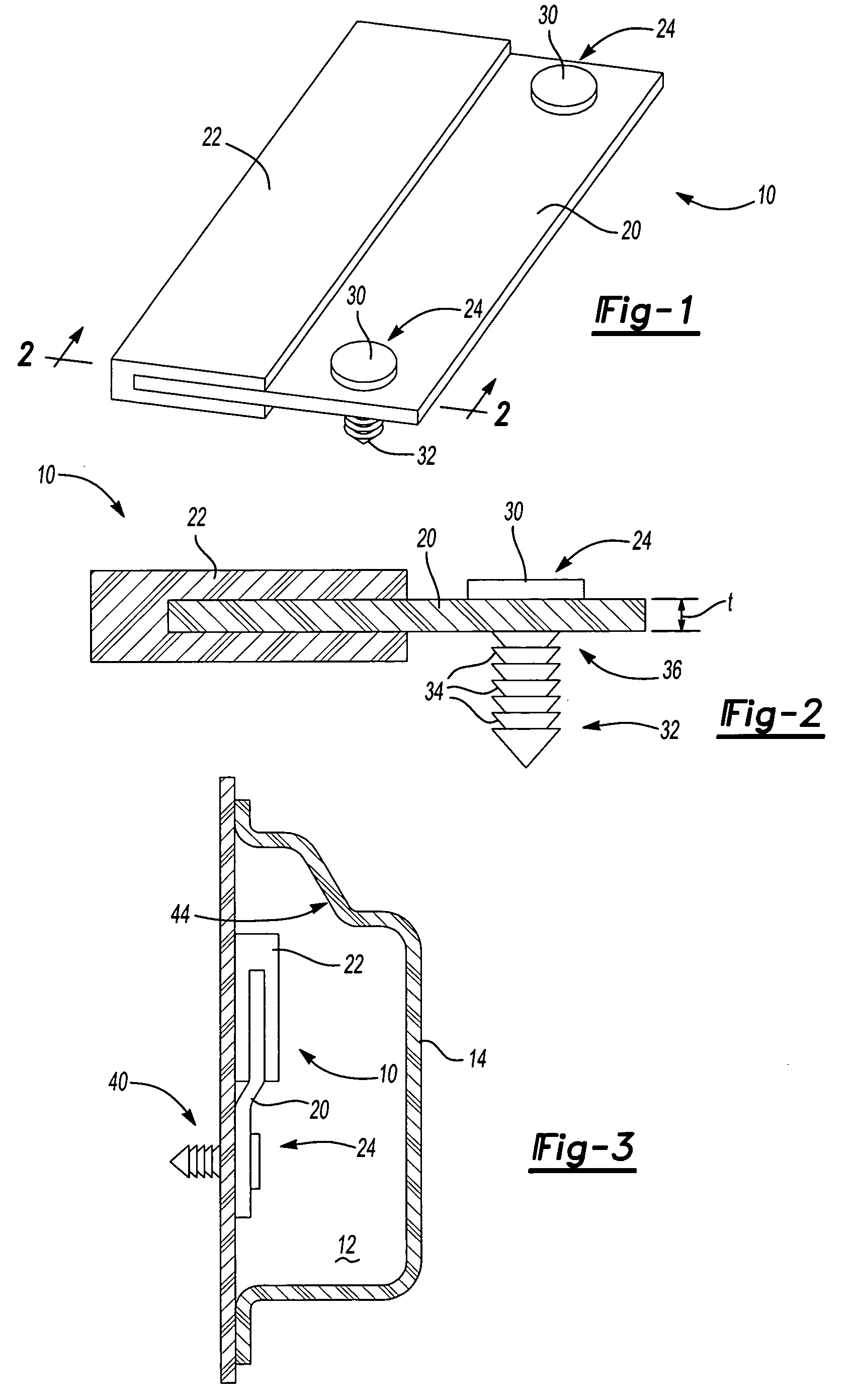
Golf club having a hollow pressurized metal head
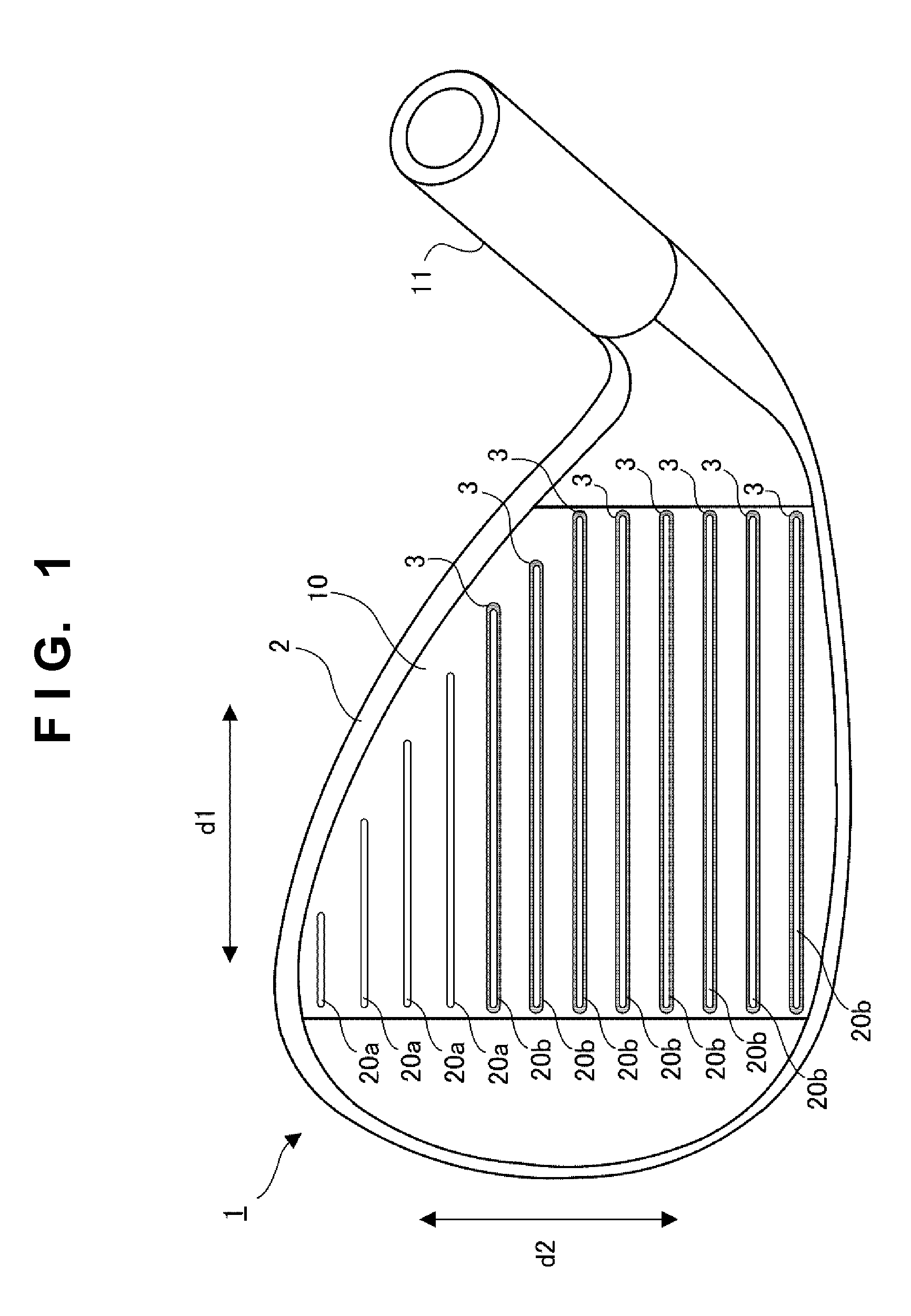
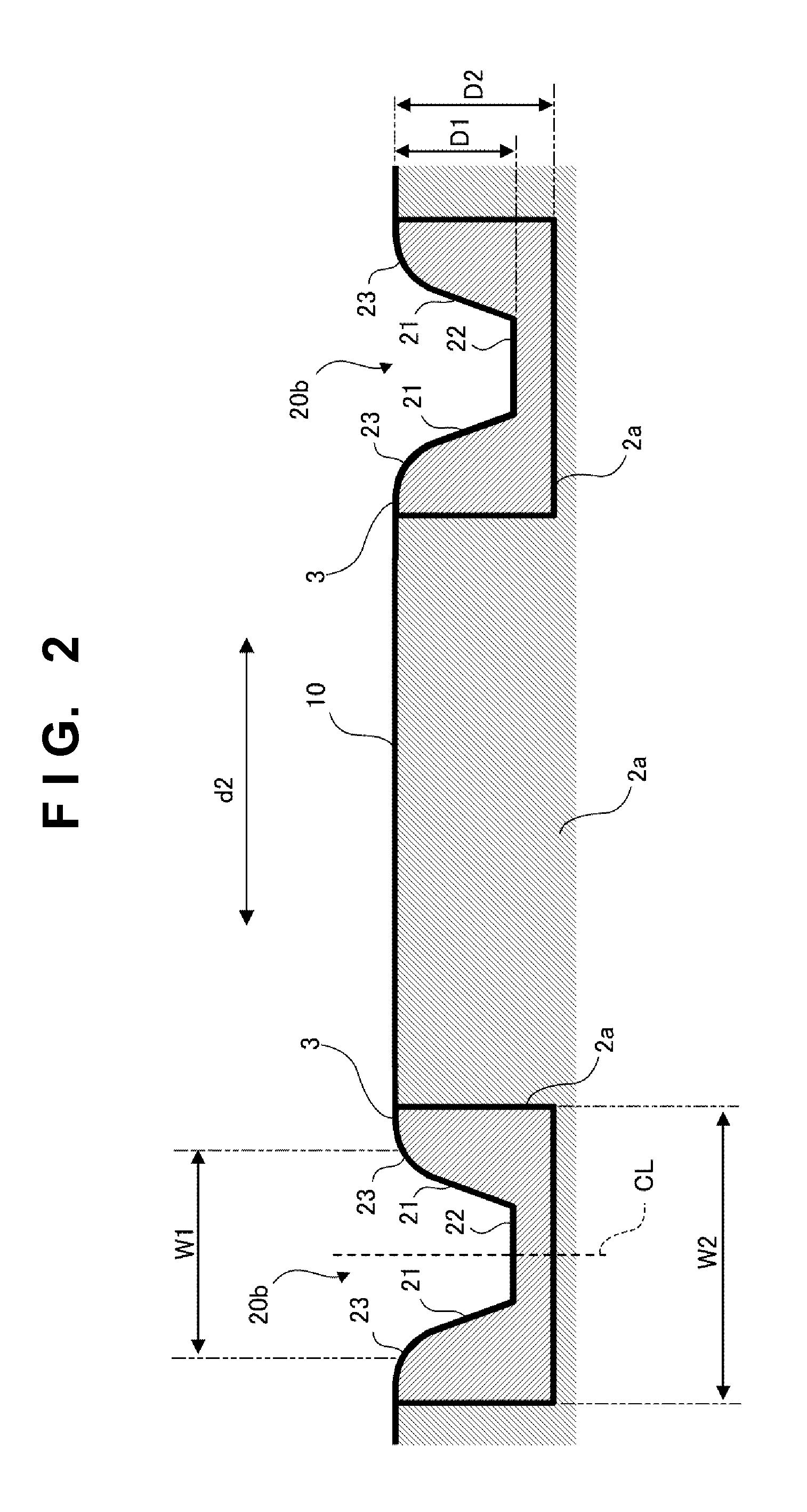
ActiveUS20080188322A1Avoid passingPrevent escapeFilling using counterpressureMetal rolling stand detailsShell moldingPlastic materials
A golf club having a hollow golf club head which is filled with a gas under pressure. The interior surface of the golf club head is coated with a solidified layer of plastic material. The pressurized gas permits the use of thinner face plates by compensating for forces generated when the face plate strikes a golf ball. The plastic layer is preferably applied through the process of rotational molding using a thermoplastic material.
Owner:BLOWERS ALDEN J
Golf club having a hollow pressurized metal head
ActiveUS8663026B2Prevent escapeMetal rolling stand detailsPackaging under special atmospheric conditionsPlastic materialsEngineering
A golf club having a hollow golf club head which is filled with a gas under pressure. The interior surface of the golf club head is coated with a solidified layer of plastic material. The pressurized gas permits the use of thinner face plates by compensating for forces generated when the face plate strikes a golf ball. The plastic layer is preferably applied through the process of rotational molding using a thermoplastic material.
Owner:BLOWERS ALDEN J
Circumferential ablation device assembly and methods of use and manufacture providing an ablative circumferential band along an expandable member
InactiveUS6954977B2Strong connectionConsistent positionElectrotherapyDiagnosticsFluoropolymerBalloon catheter
A medical balloon catheter assembly includes a balloon having a permeable region and a non-permeable region. The balloon is constructed at least in part from a fluid permeable tube such that the permeable region is formed from a porous material which allows a volume of pressurized fluid to pass from within a chamber formed by the balloon and into the permeable region sufficiently such that the fluid may be ablatively coupled to tissue engaged by the permeable region. The non-permeable region is adapted to substantially block the pressurized fluid from passing from within the chamber and outwardly from the balloon. The porous material may be a porous fluoropolymer, such as porous polytetrafluoroethylene, and the pores may be created by voids that are inherently formed between an interlocking node-fibril network that makes up the fluoropolymer. Such voids may be created according to one mode by expanding the fluoropolymer. The balloon may be formed such that the porous material extends along both the permeable and non-permeable regions. In one mode of this construction, the porous material is porous along the permeable region but is non-porous along the non-permeable region, such as for example by expanding only the permeable region in order to render sufficient voids in the node-fibril network to provide permeable pores in that section. The voids or pores in the porous material may also be provided along both permeable and non-permeable sections but are substantially blocked with an insulator material along the non-permeable section in order to prevent fluid from passing therethrough. The insulator material may be dip coated, deposited, or extruded with the porous material in order to fill the voids. The insulator material may in one mode be provided along the entire working length of the balloon and then selectively removed along the permeable section, or may be selectively exposed to only the non-permeable sections in order to fill the voids or pores there.
Owner:MAGUIRE MARK A +1
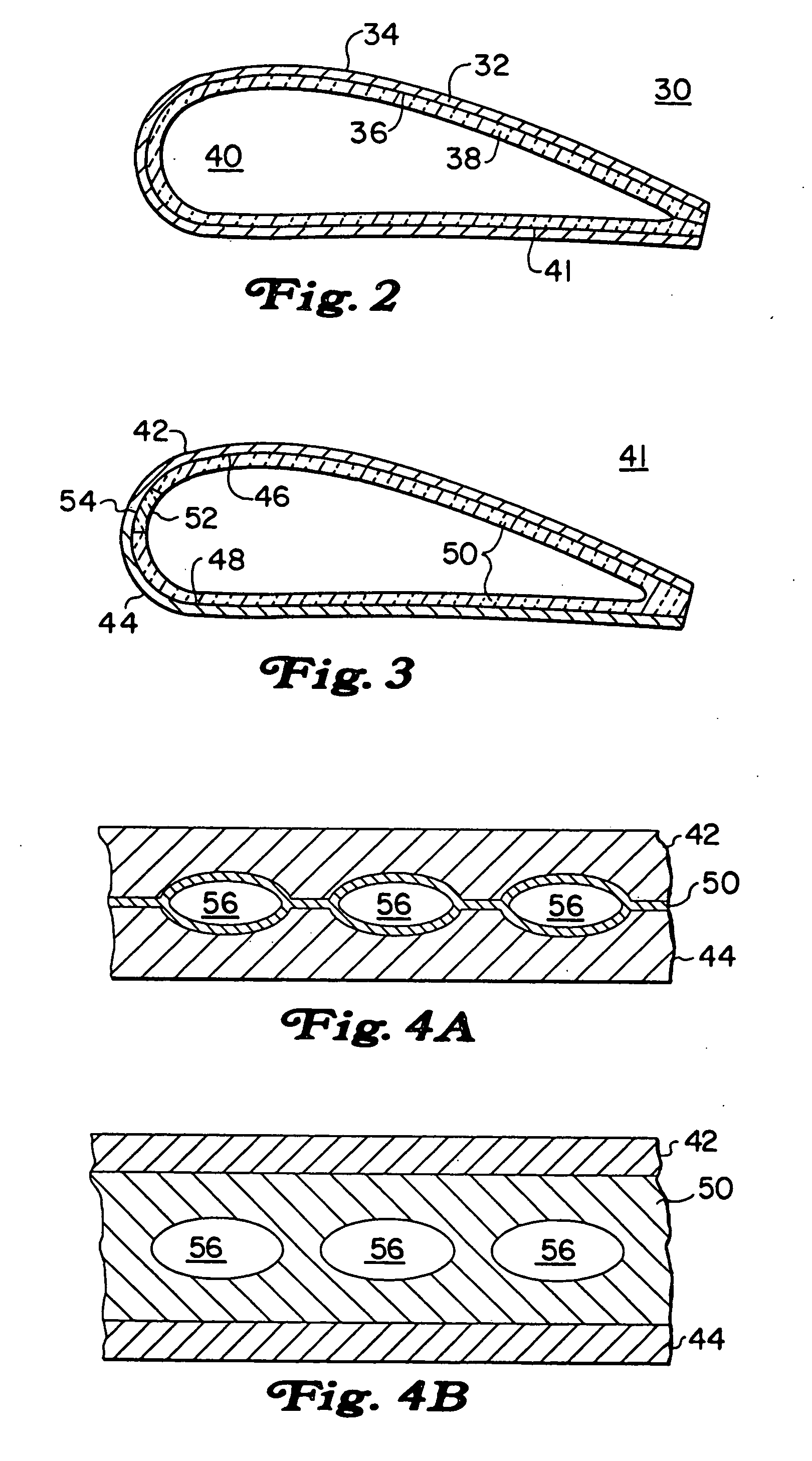
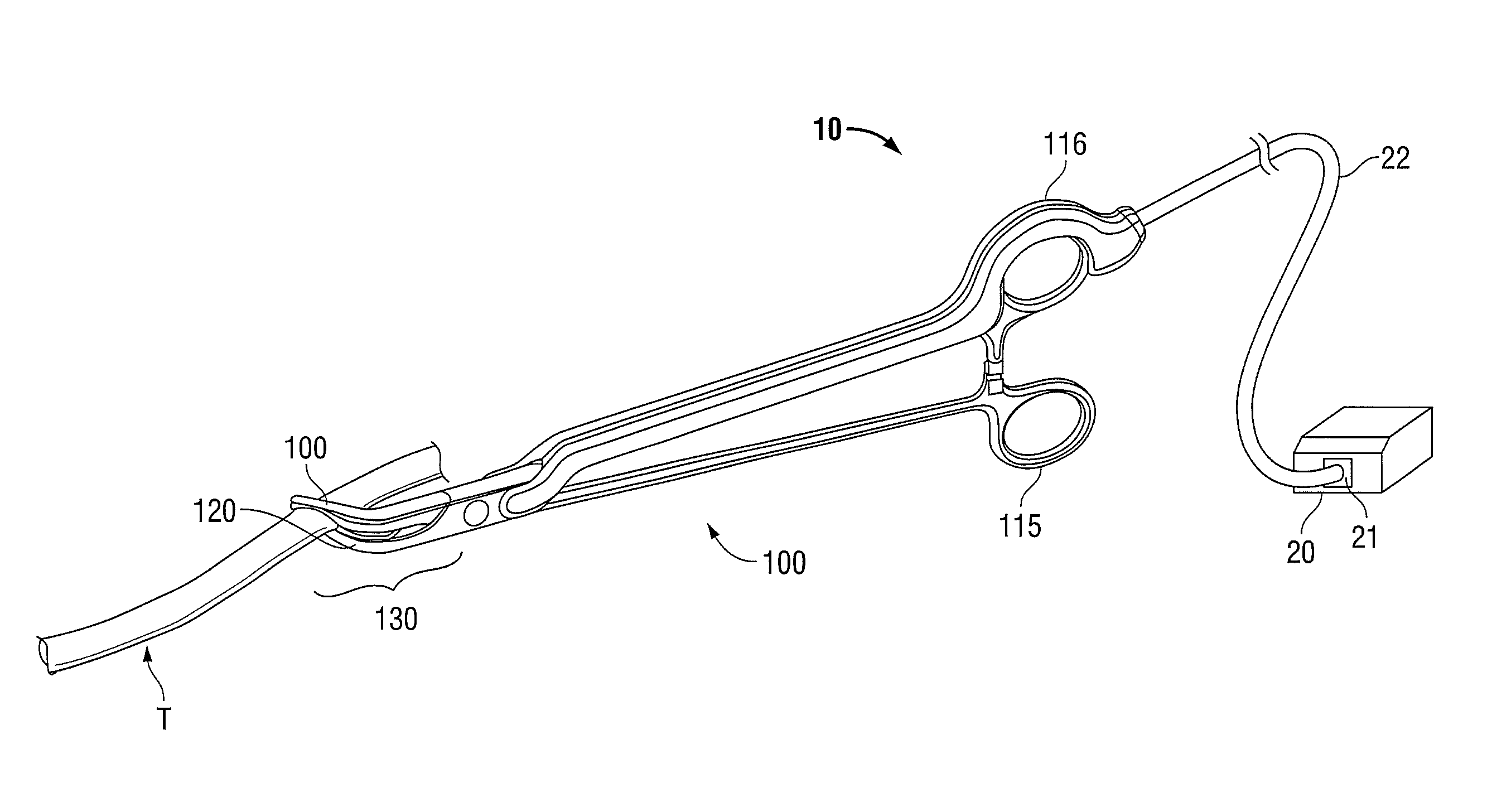
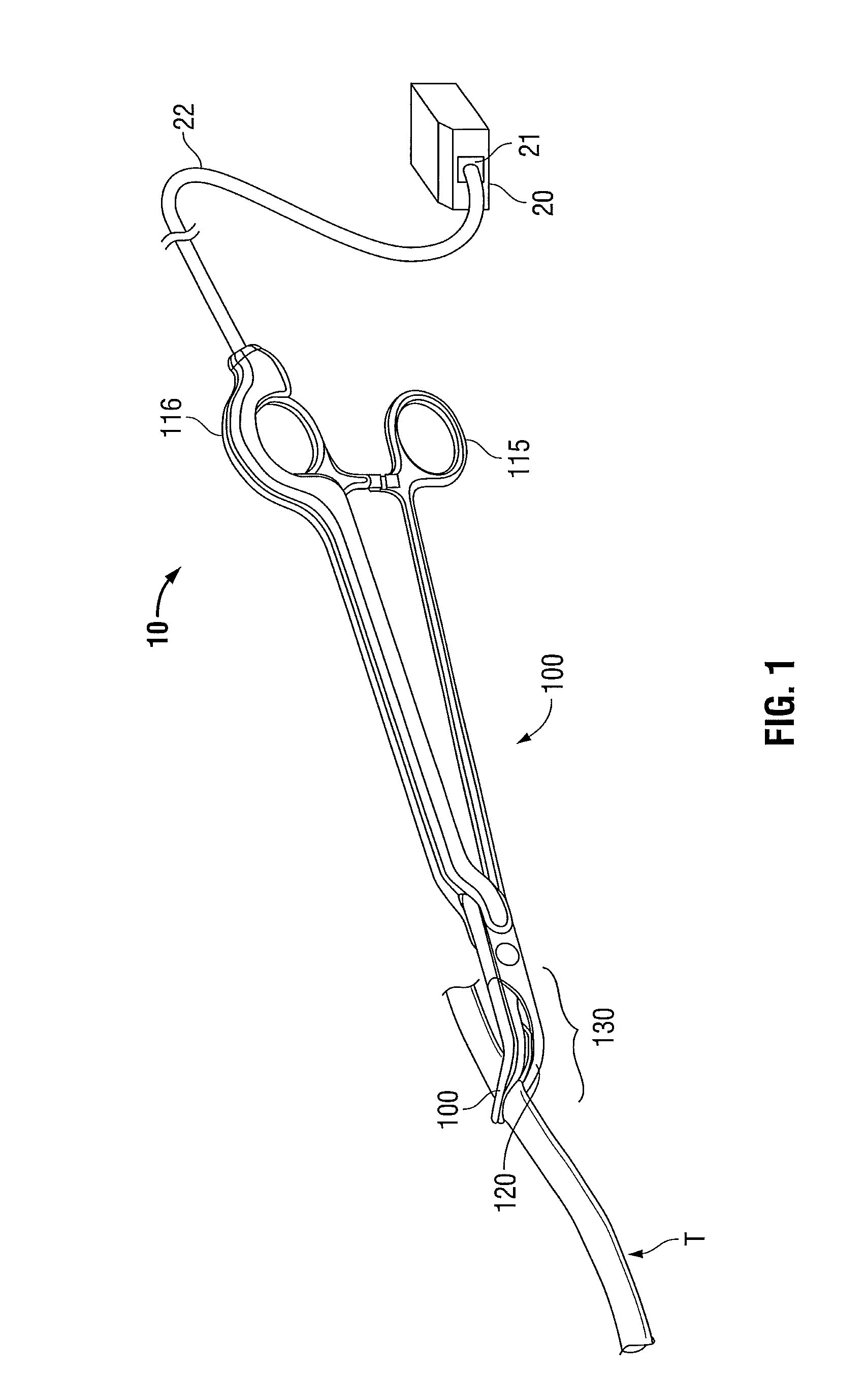
Molded insulating hinge for bipolar instruments
An electrosurgical instrument includes a pair of first and second elongated shafts each having an end effector attached to a distal end thereof and a handle. The handle is movable from a first position wherein the end effectors are disposed in spaced relation relative to one another to a second position wherein the end effectors are closer relative to one another. Each of the elongated shafts includes a hinge plate which mounts atop a pivot assembly for effecting movement of the end effectors relative to one another. The instrument also includes a hinge assembly made from an overmold composition which encapsulates and secures the hinge plates and the pivot assembly. The overmold composition is made from an electrically insulating material which insulates the end effectors from one another.
Owner:COVIDIEN AG
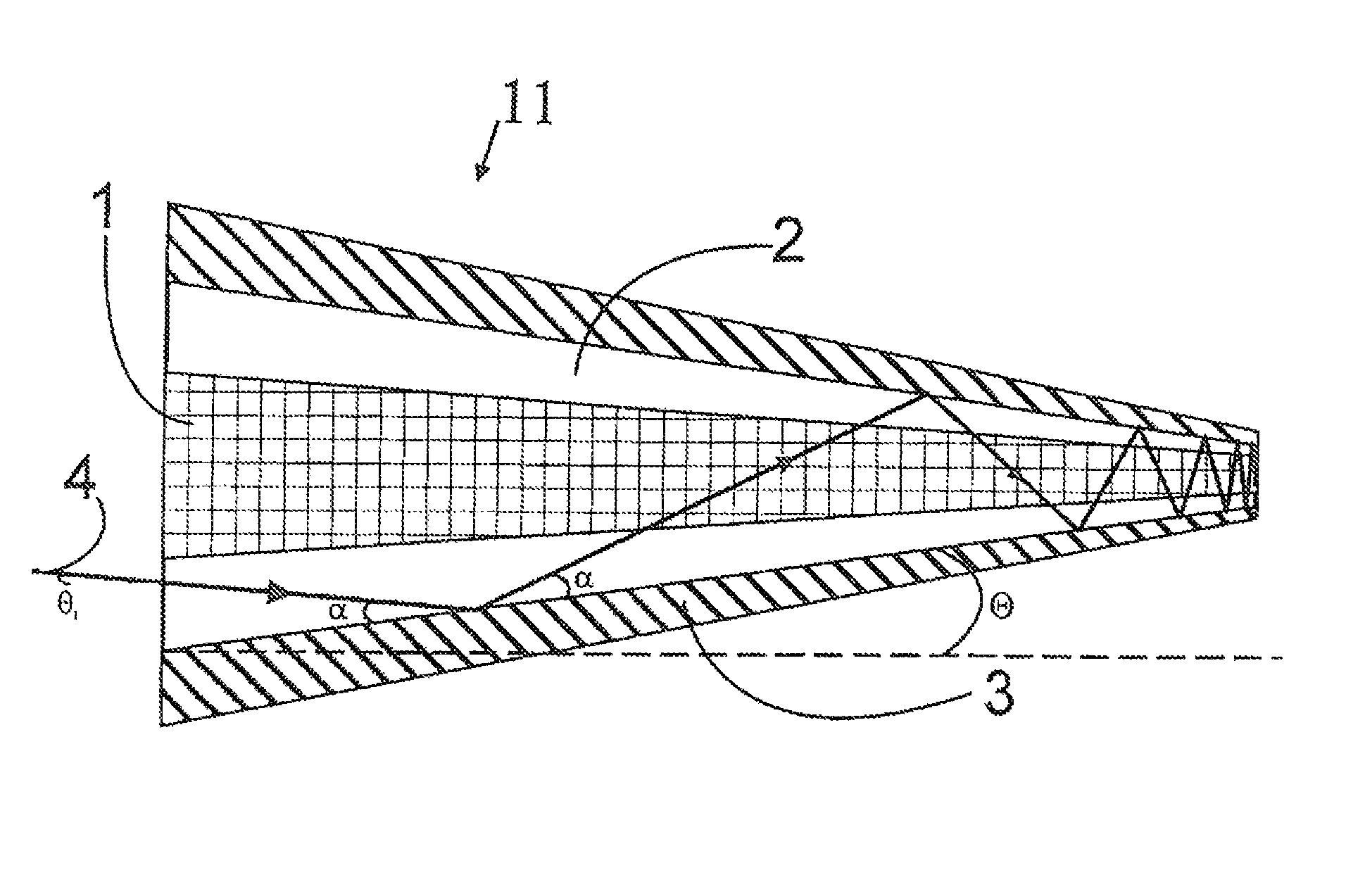
Active optical fiber and method for fabricating an active optical fiber
ActiveUS8433168B2Reduce the overall diameterLarge volumeLaser detailsMetal rolling stand detailsFiberActive core
A section of active optical fiber (11) which comprises an active core (1), an inner cladding layer (2) and an outer cladding layer (3). The diameter of said core 1) and the thickness of said inner cladding (2) change gradually along the length of said section of active optical fiber (11). This forms tapered longitudinal profile enabling a continuous mode conversion process along the length of the section of fiber (11). The method for fabricating a section of tapered active optical fiber comprises the steps of fabricating a preform for drawing active optical fiber from said preform, installing said preform into a drawing tower, drawing optical fiber in said drawing tower and altering at least one of the two parameters including the take-off preform speed and the take-up fiber speed during drawing of the optical fiber.
Owner:AMPLICONYX OY
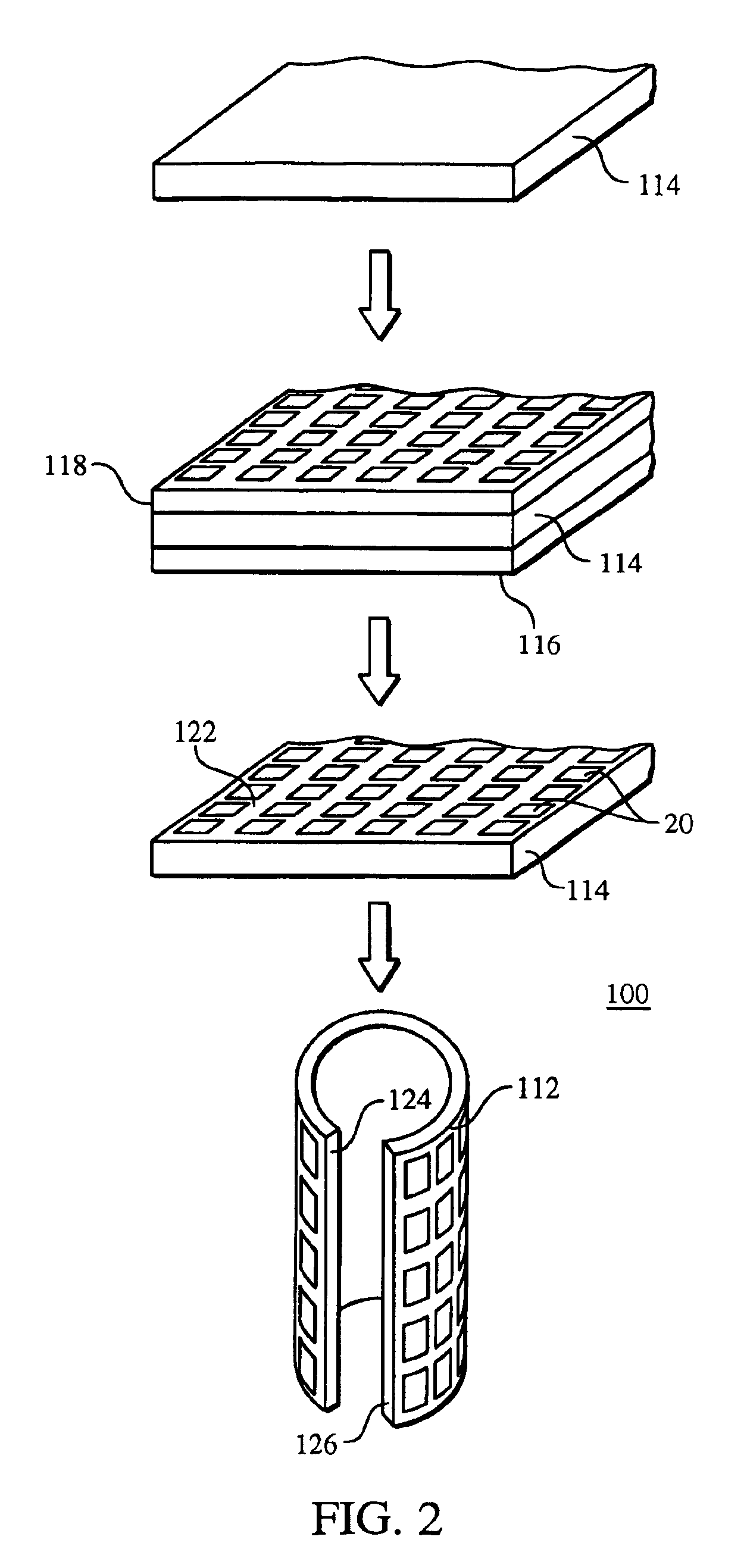
Methods of making medical devices
InactiveUS6865810B2Reduce the amount requiredEasy to disassemblePretreated surfacesAntithrombogenic treatmentInsertion stentMedical device
A method of making a stent includes providing a tubular member having a first layer, the first layer and the tubular member having different compositions, removing a portion of the tubular member, and removing a portion of the first layer from the tubular member.
Owner:BOSTON SCI SCIMED INC
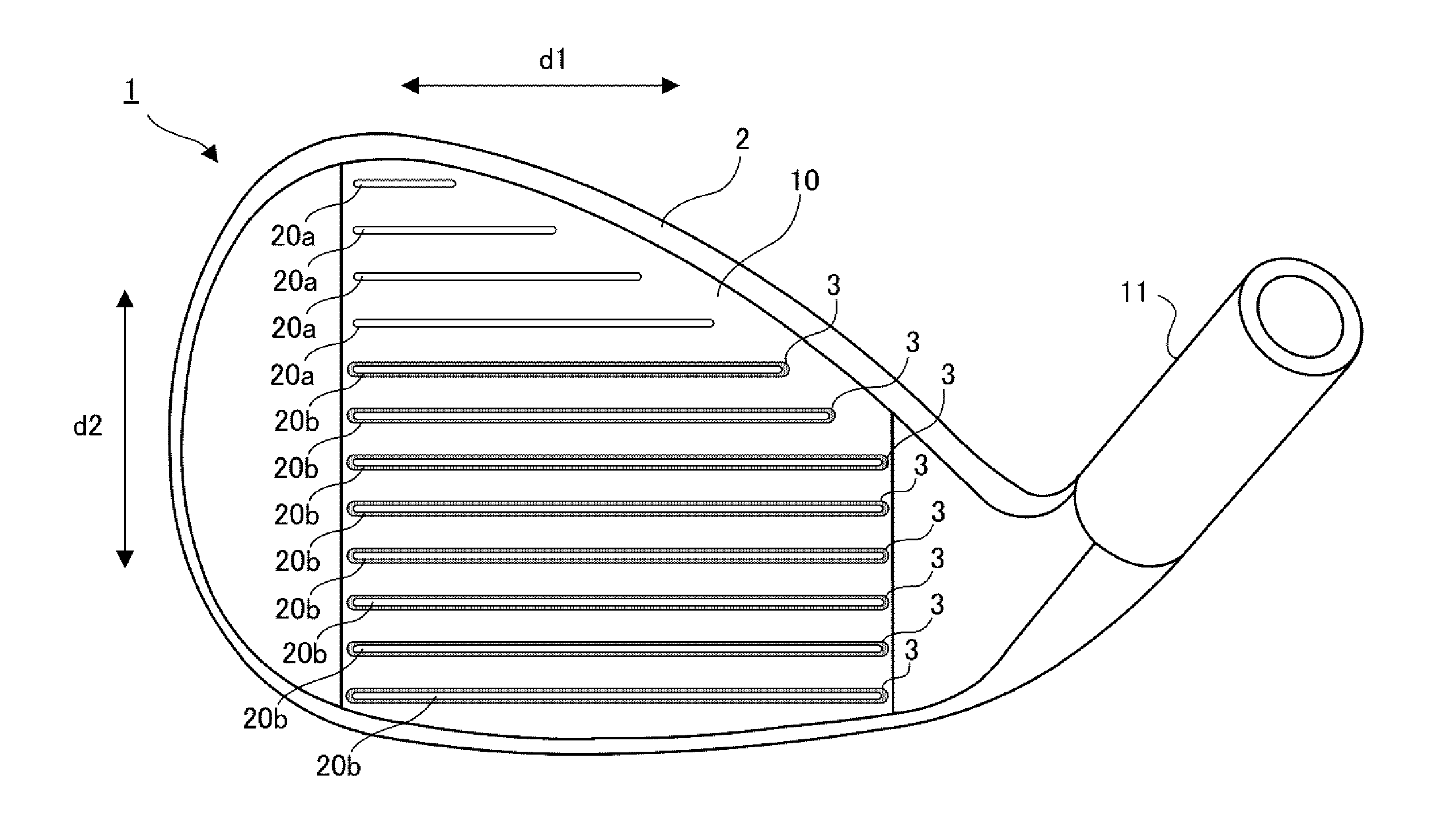
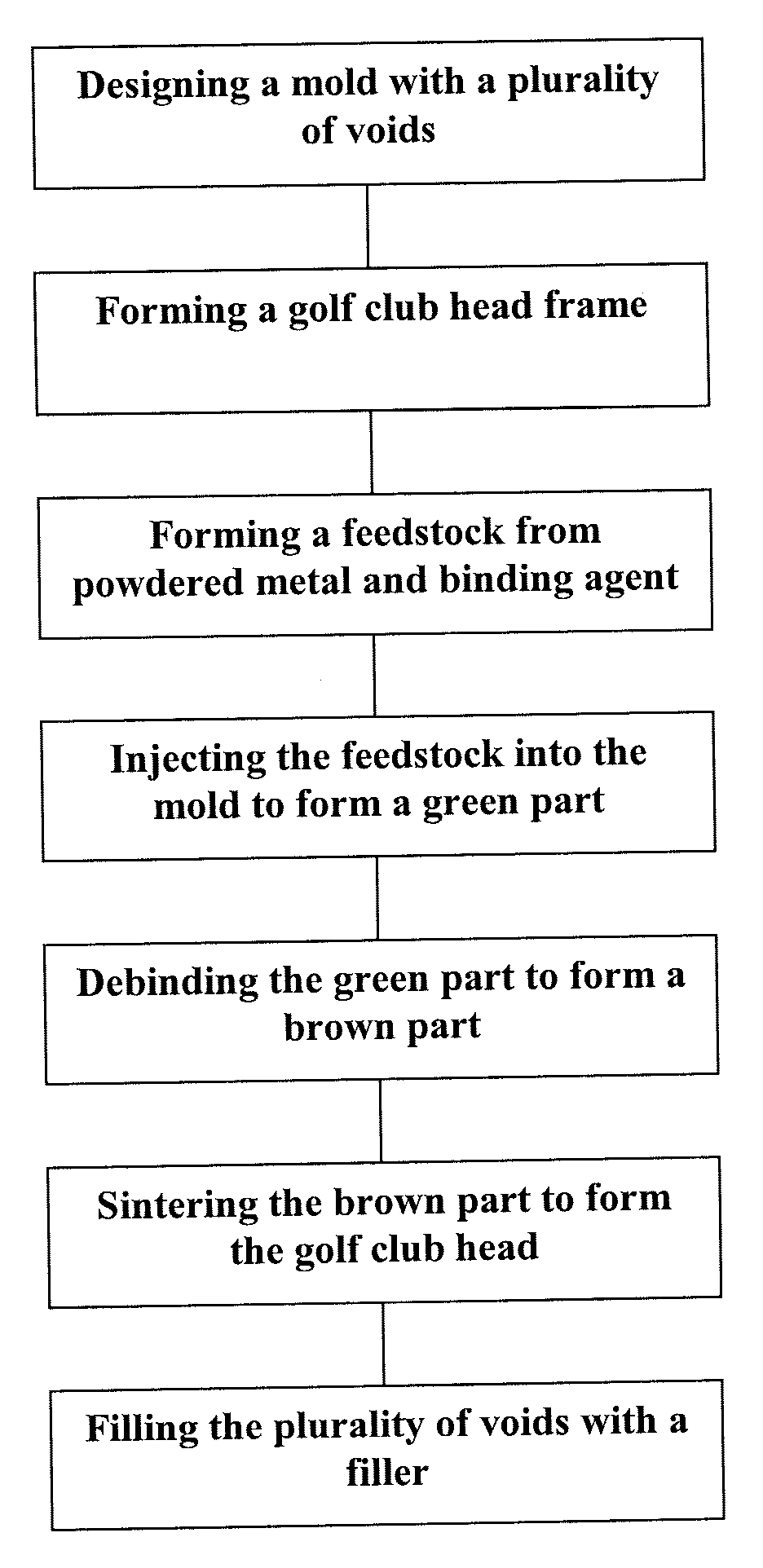
Manufacturing method and golf club head
ActiveUS20130303303A1Excellent abrasion resistanceImprove impact feelMetal rolling stand detailsMetal working apparatusEngineeringGolf Ball
This invention provides a method of manufacturing a golf club head including a face surface. This manufacturing method includes a step of forming a plurality of recessed portions in the face surface, included in a first member, to extend in the toe-to-heel direction, a fixing step of fixing, to the recessed portions, second members which are formed by a material different from that of the first member, and fill the recessed portions, and after the fixing step, a scoreline forming step of forming at least one scoreline in each of the second members, while not forming the scoreline in a portion, between the recessed portions, of the face surface of the first member.
Owner:BRIDGESTONE SPORTS
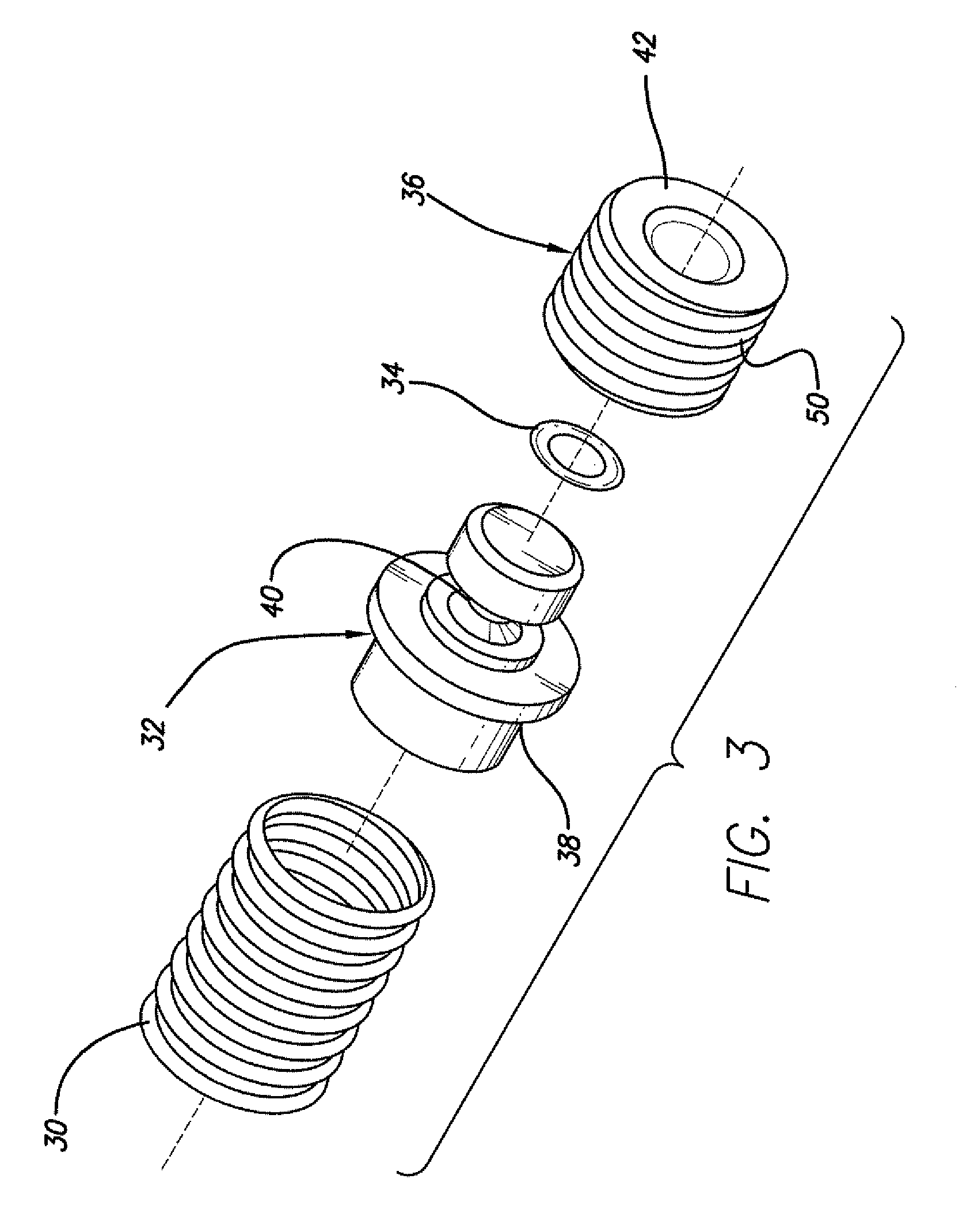
Multiple segment catheter and method of fabrication
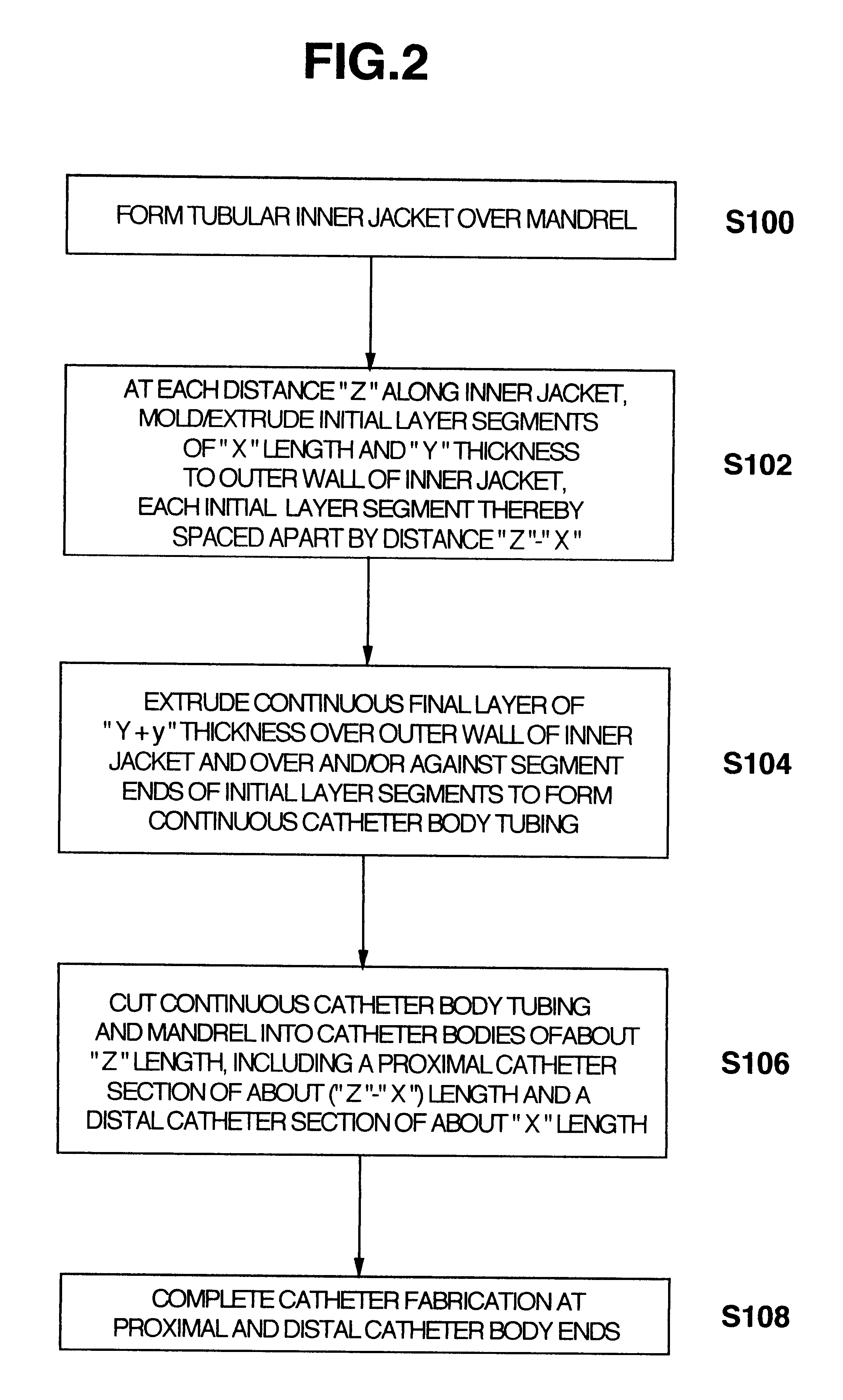
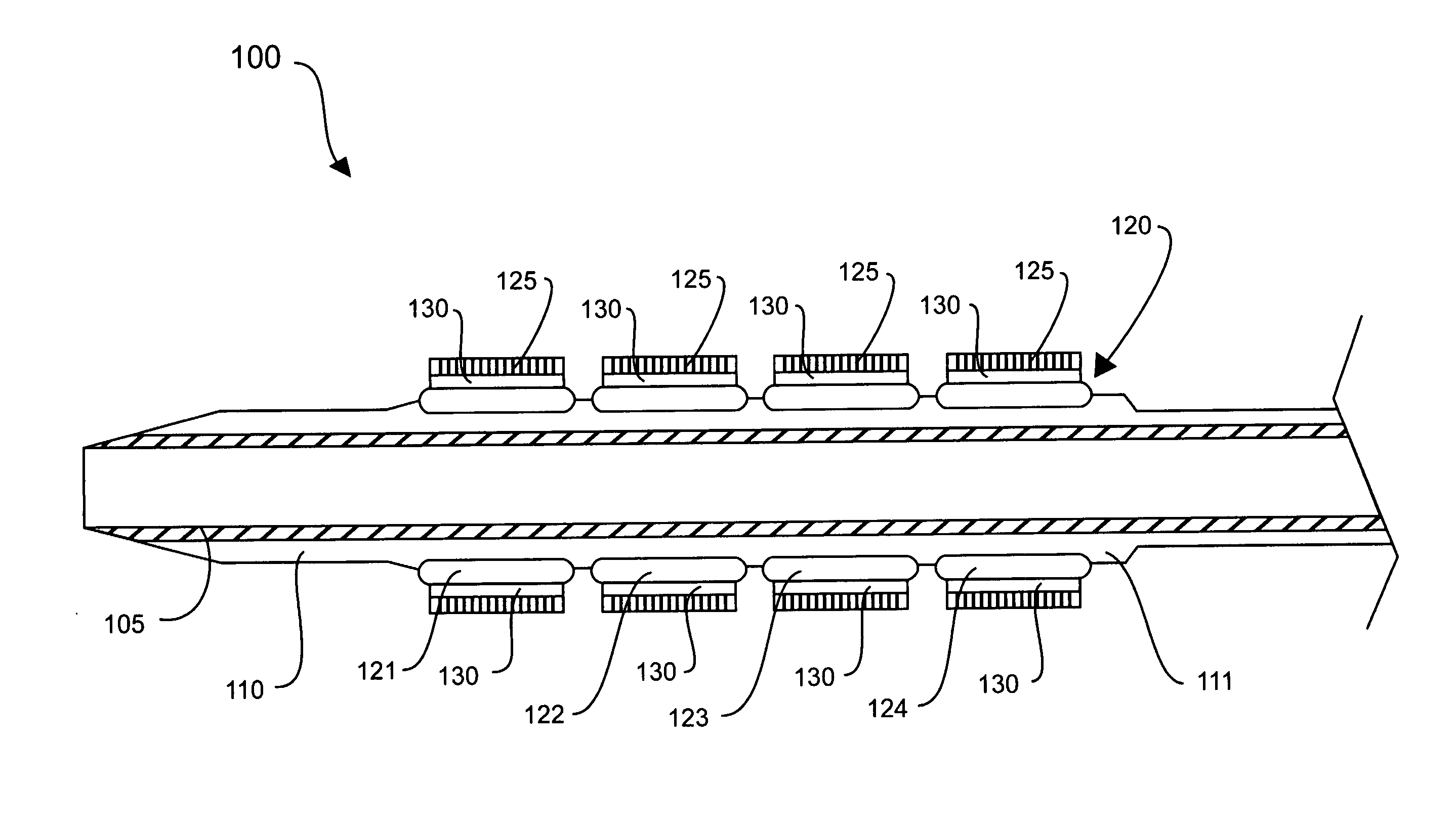
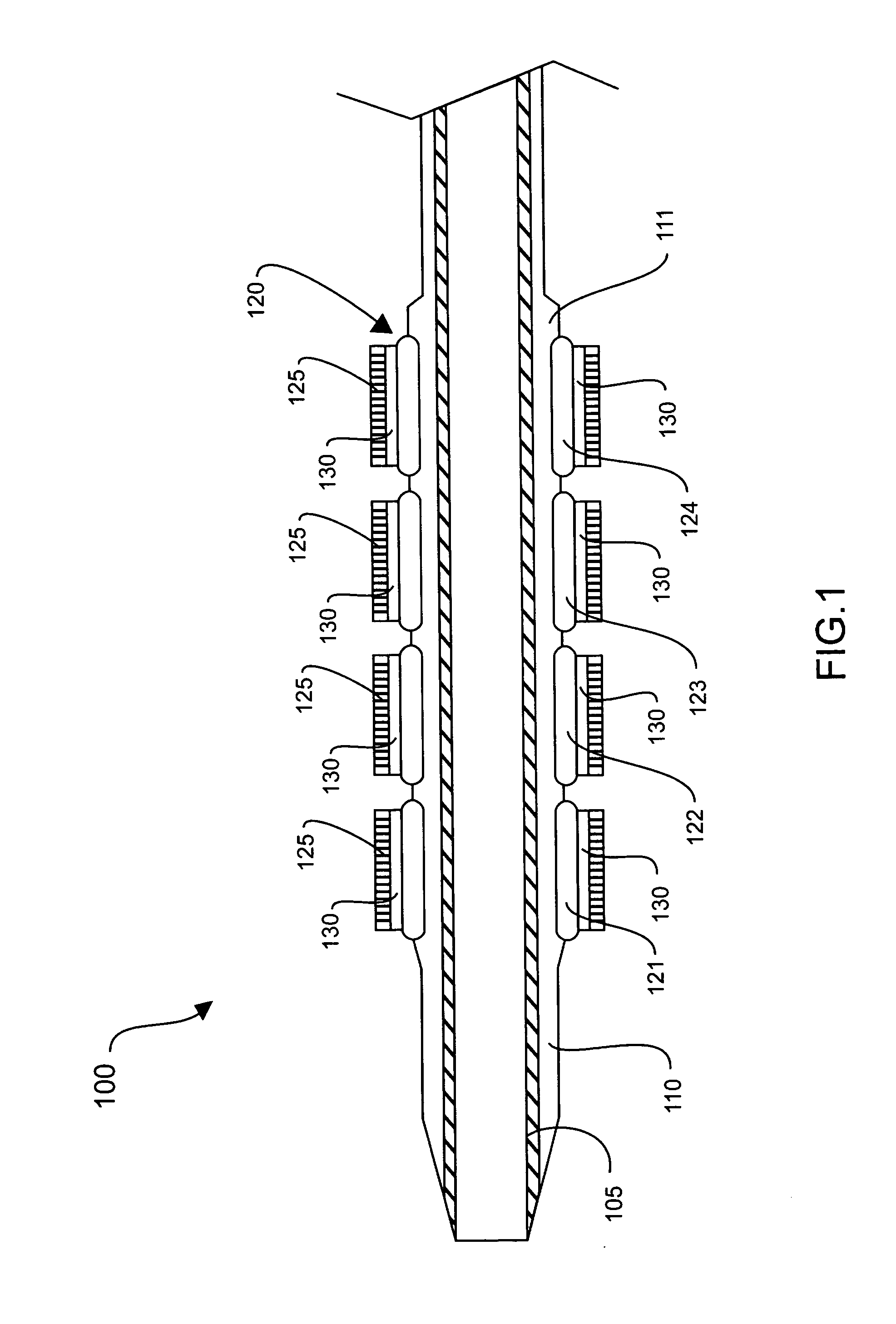
Methods of fabricating medical vascular catheters adapted to be inserted into a blood vessel from an incision through the skin of a patient for introducing other devices or fluids for diagnostic or therapeutic purposes and particularly methods for fabricating such catheters with catheter bodies having catheter sections of differing flexibility are disclosed. Such catheter bodies having a proximal catheter body end and a distal catheter body end and formed of a proximal section and at least one distal section that have differing flexibilities are formed in a process comprising the steps of: (1) forming a continuous tubular inner jacket preferably of an inner liner and a reinforcement layer; (2) forming initial layer segments having an initial layer thickness along the length of the inner jacket from a material of first durometer hardness, whereby each initial layer segment is separated by a separation distance: (3) forming a final layer of a material of second durometer hardness with a second layer thickness over the tubular inner jacket along the separation distances and over and / or against the proximal and distal initial layer ends of the initial layer segments to form a continuous catheter body tubing; (4) severing the continuous catheter body tubing into catheter body lengths including a proximal catheter section formed of the material of second hardness and a distal catheter section of the material of first hardness; and (5) completing the catheter fabrication at the proximal catheter body end and the distal catheter body end. Centerless grinding of the catheter body or body tubing, formation of Intermediate catheter body sections, distal soft tips, and discontinuities in the reinforcement layer formed prior to step (2) are also disclosed.
Owner:MEDTRONIC INC
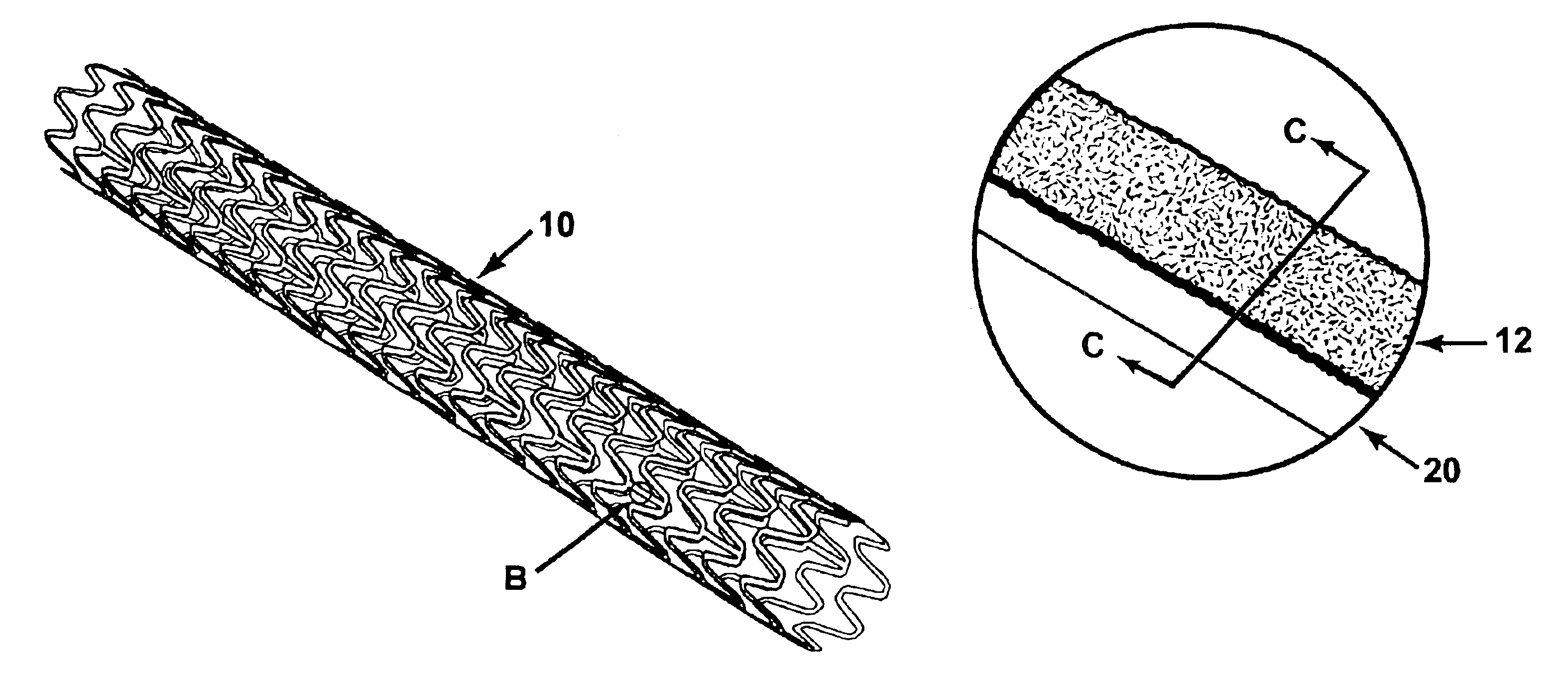
Stent with improved drug loading capacity
The present invention provides a method of forming a drug eluting stent, the method comprising coupling a stent framework to a mandrel, inserting the mandrel with stent framework into an open die, the die including a forming surface including a plurality of raised indention forming portions; closing the die against the stent framework; pressing the raised indention portions into the stent framework to form indentions in the stent framework; and inserting at least one drug polymer into the indentions formed in the stent framework.
Owner:MEDTRONIC VASCULAR INC
Monocoque jaw design
Owner:TYCO HEALTHCARE GRP LP
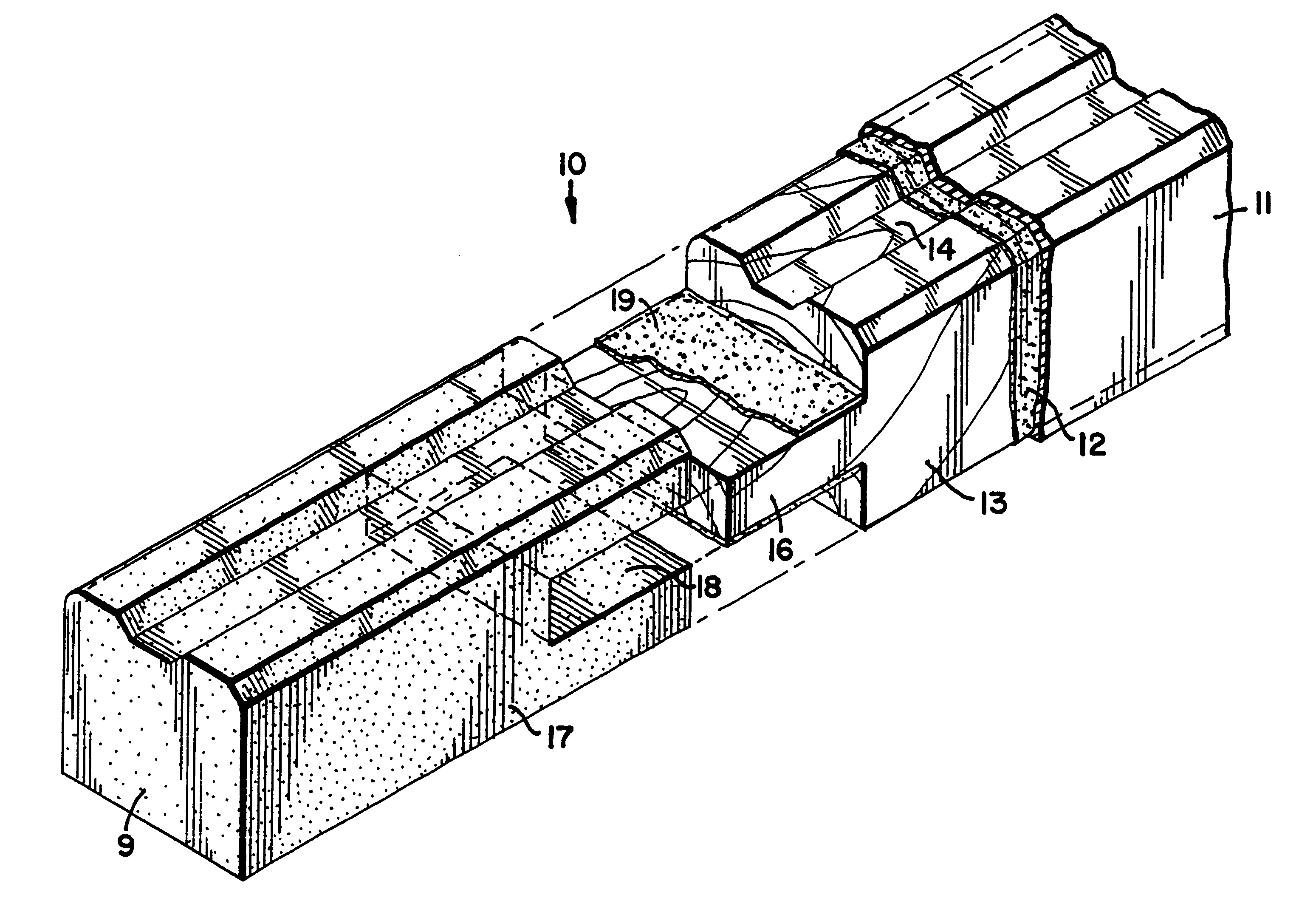
Polymer covered advanced polymer/wood composite structural member
InactiveUS6357197B1Corner/edge jointsMetal rolling stand detailsComposite constructionMoisture absorption
A composite structural member of the invention comprises a linear member having a first end and a second end attached to each end of the linear member as an end piece or end cap structure. Covering the composite member is a thermoplastic envelope preferably adherently bonded to the composite member. The end caps or end pieces are preferably thermoplastic materials typically thermoplastic composites comprising a thermoplastic resin and a fiber. Such a member is environmentally stable, resists moisture absorption, forms strong mitered joints and can be used in the assembly of fenestration products for commercial and residential real estate.
Owner:ANDERSEN CORPORATION
Method and apparatus for indirect bonding of orthodontic appliances
ActiveUS7020963B2Broaden applicationEasy to disengageBracketsAdditive manufacturing apparatusEngineeringAnodic bonding
Owner:3M INNOVATIVE PROPERTIES CO
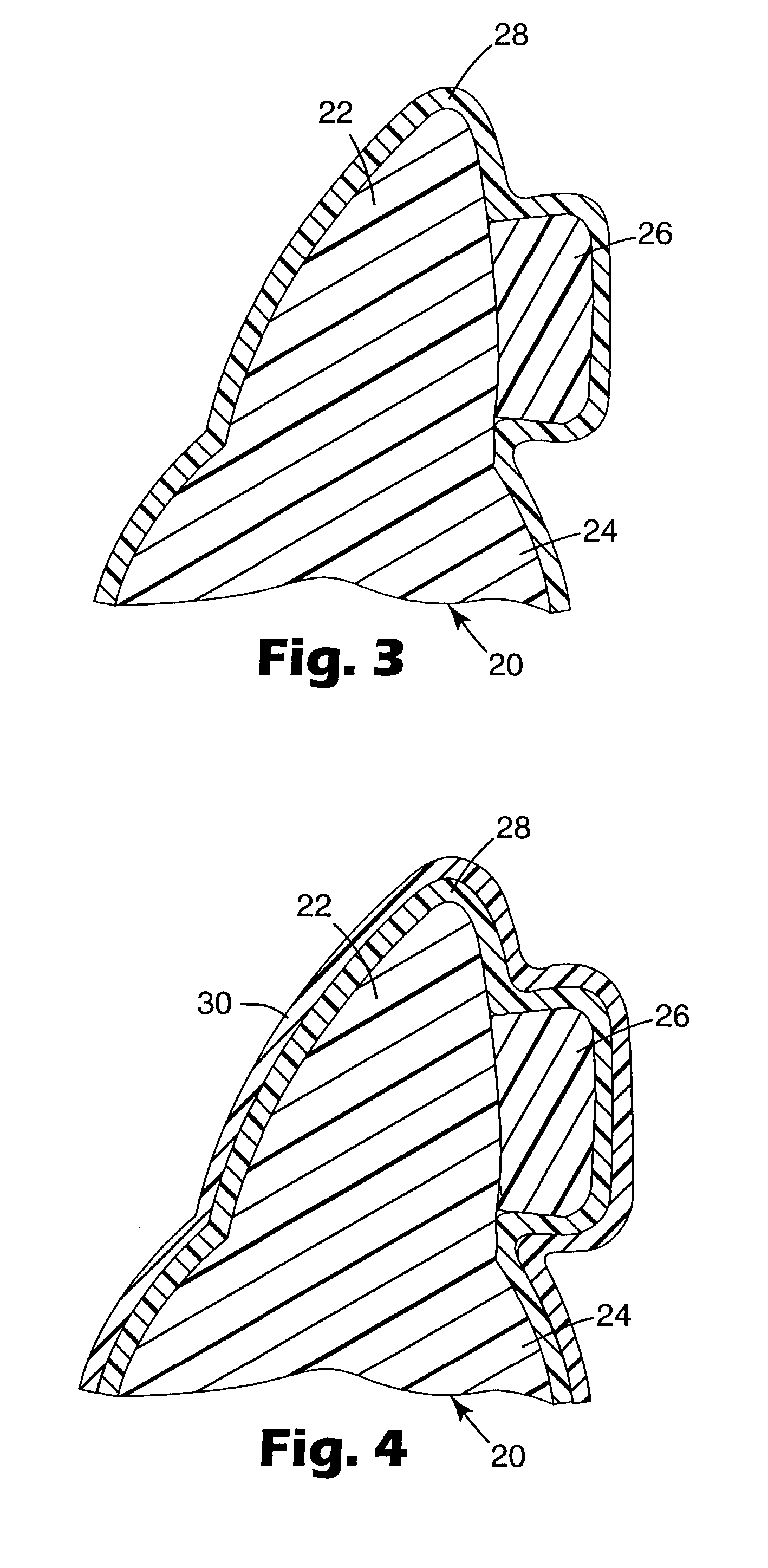
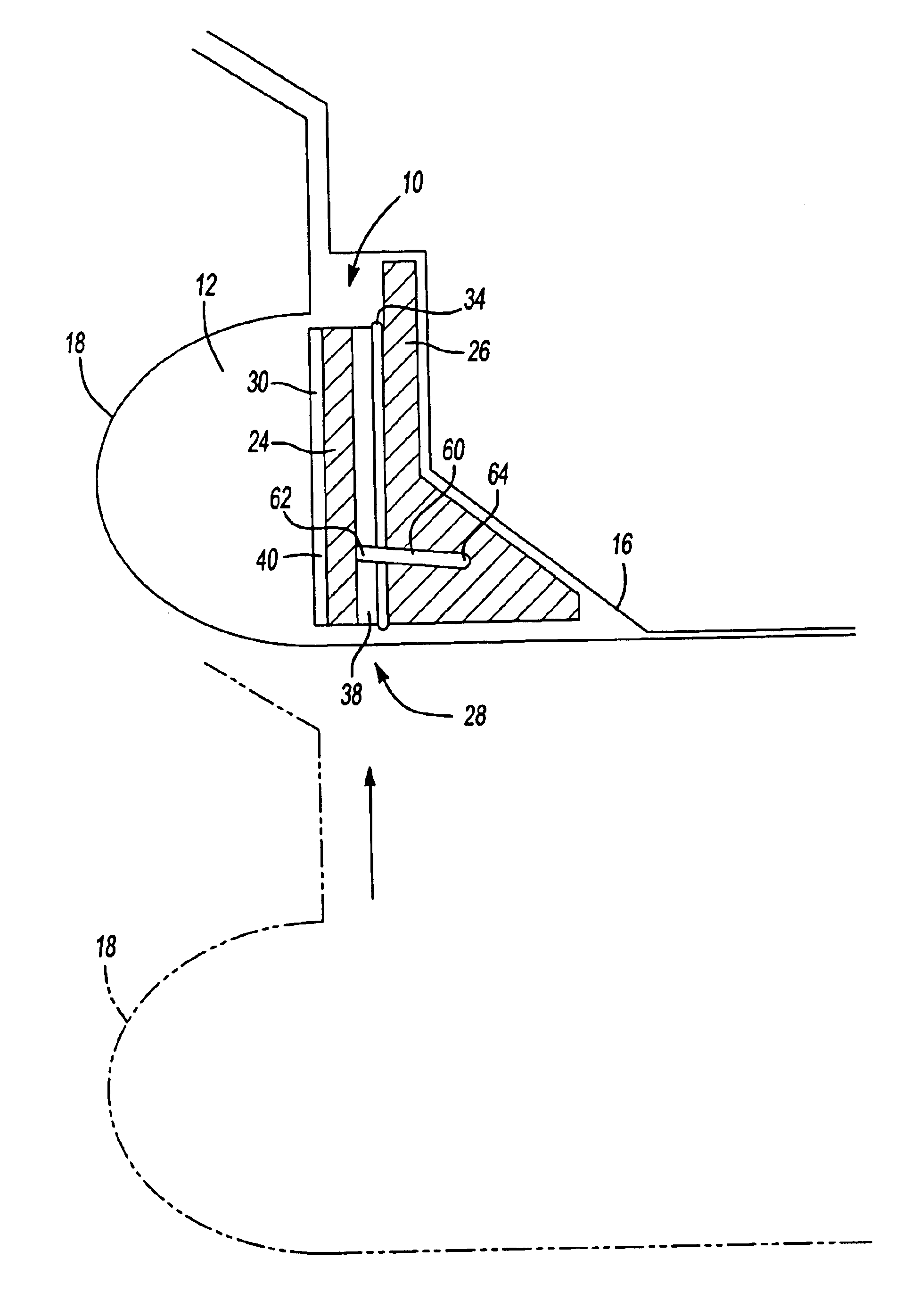
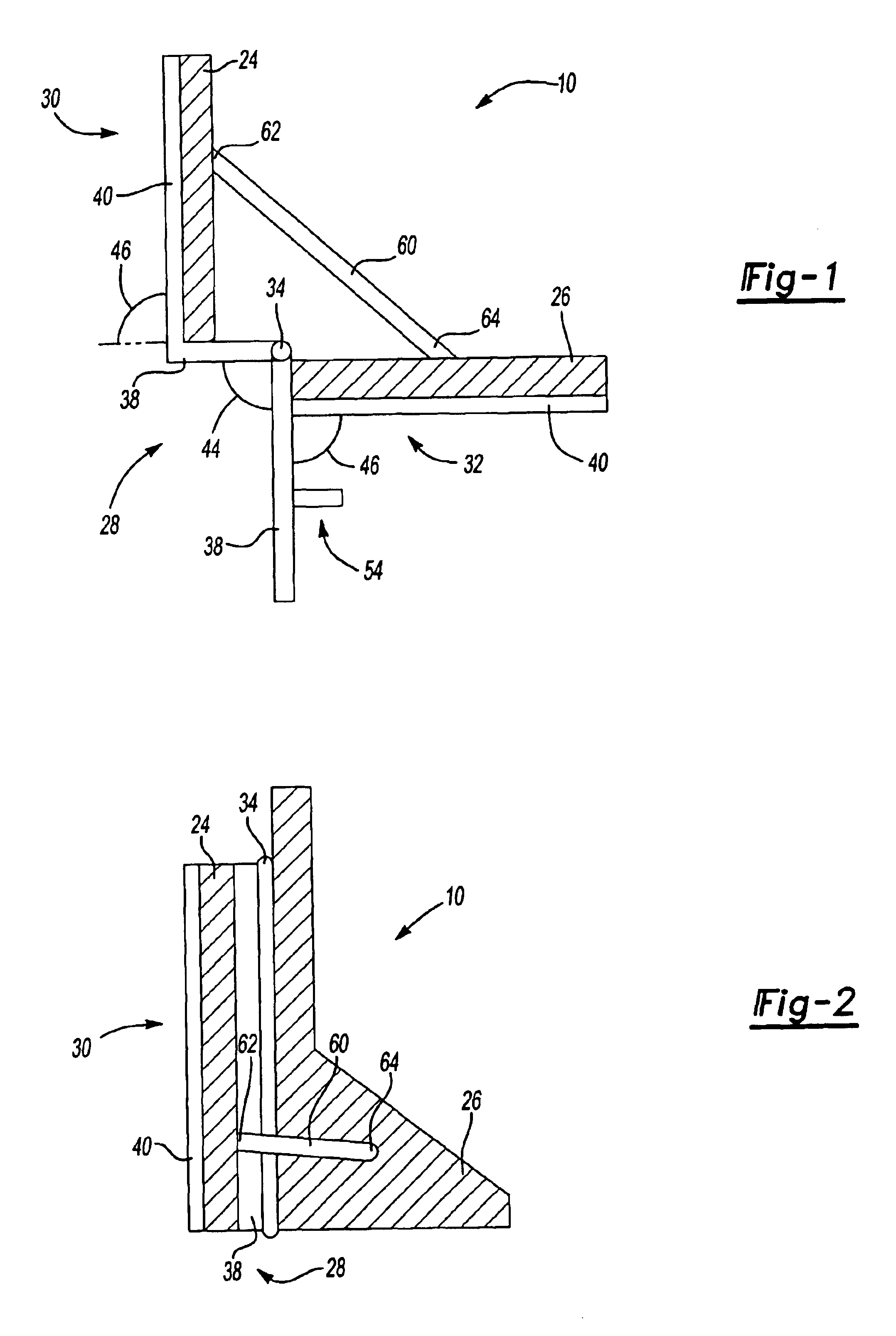
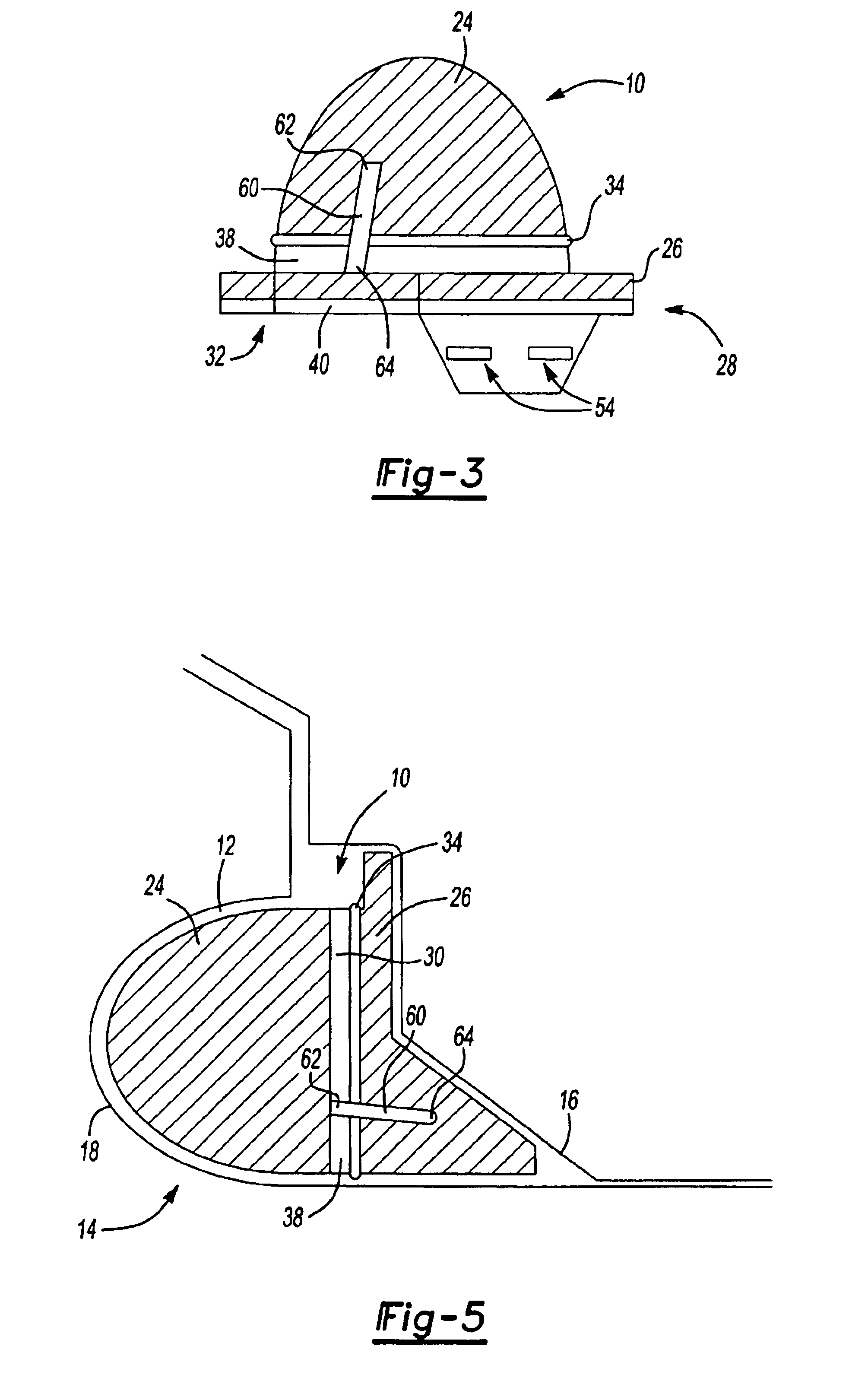
Dynamic self-adjusting assembly for sealing, baffling or structural reinforcement
There is disclosed a reinforced structural assembly and a method of using the assembly for sealing, baffling and / or reinforcing components of an automotive vehicle. The assembly generally includes at least a first mass of expandable material. Preferably, the first mass is movably (e.g., rotatably) connected to a second mass of material and / or another member. According to the method, the assembly is placed in a cavity of an automotive vehicle and the at least one mass is activated to expand. Preferably, upon activation, the first mass self adjusts by moving (e.g., rotating) relative to the second mass and / or the other member.
Owner:ZEPHYROS INC
Equal sized spherical beads
InactiveUS20080299875A1Other chemical processesMetal rolling stand detailsSpherical shapedMaterials science
A method of producing equal-sized spherical shaped beads of a wide range of materials is described. These beads are produced by forming the parent bead material into a liquid solution and by filling equal volume cells in a sheet with the liquid solution. The sheet cells establish the volumes of each of the cell mixture volumes which are then ejected from the cells by an impinging fluid. Surface tension forces acting on the ejected equal sized solution entities form them into spherical beads. The ejected beads are then subjected to a solidification environment which solidifies the spherical beads. The beads can be solid or porous or hollow and can also have bead coatings of multiple material layers.
Owner:DUESCHER WAYNE O
Method for shielding an electronic component
Owner:PPG IND OHIO INC
Smart contact lenses for augmented reality and methods of manufacturing and operating the same
ActiveUS20160091737A1Widen perspectiveSpectales/gogglesWave amplification devicesEngineeringContact lens
Example embodiments disclose a smart contact lens for augmented reality and methods of manufacturing and operating the smart contact lens. The smart contact lens includes a first contact lens, a display unit in a center region of the first contact lens, a peripheral device on the first contact lens and around the display unit, the peripheral device being connected to the display unit, and a passivation layer covering the display unit and the peripheral device. The method of manufacturing the smart contact lens includes forming a display unit; mounting the display unit in a center region of a first contact lens, forming a peripheral device on the first contact lens, around the display unit and in connection with the display unit, and forming a passivation layer to cover the display unit and the peripheral device.
Owner:SAMSUNG ELECTRONICS CO LTD
Composite structure formed by CMC-on-insulation process
ActiveUS20050076504A1Improved ceramic composite structureWood working apparatusCeramic layered productsCombustorEngineering
A method of manufacturing a composite structure uses a layer of an insulating material (22) as a mold for forming a substrate of a ceramic matrix composite (CMC) material (24). The insulating material may be formed in the shape of a cylinder (10) with the CMC material wound on an outer surface (14) of the cylinder to form a gas turbine combustor liner (20). Alternatively, the insulating material may be formed in the shape of an airfoil section (32) with the CMC material formed on an inside surface (36) of the insulating material. The airfoil section may be formed of a plurality of halves (42, 44) to facilitate the lay-up of the CMC material onto an easily accessible surface, with the halves then joined together to form the complete composite airfoil. In another embodiment, a box structure (102) defining a hot gas flow passage (98) is manufactured by forming insulating material in the shape of opposed airfoil halves (104) joined at respective opposed ends by platform members (109). A layer of CMC material (107) is then formed on an outside surface of the insulating material. A number of such composite material box structures are then joined together to form a vane ring (100) for a gas turbine engine.
Owner:SIEMENS ENERGY INC
Composite structure formed by CMC-on-insulation process
A method of manufacturing a composite structure uses a layer of an insulating material (22) as a mold for forming a substrate of a ceramic matrix composite (CMC) material (24). The insulating material may be formed in the shape of a cylinder (10) with the CMC material wound on an outer surface (14) of the cylinder to form a gas turbine combustor liner (20). Alternatively, the insulating material may be formed in the shape of an airfoil section (32) with the CMC material formed on an inside surface (36) of the insulating material. The airfoil section may be formed of a plurality of halves (42, 44) to facilitate the lay-up of the CMC material onto an easily accessible surface, with the halves then joined together to form the complete composite airfoil. In another embodiment, a box structure (102) defining a hot gas flow passage (98) is manufactured by forming insulating material in the shape of opposed airfoil halves (104) joined at respective opposed ends by platform members (109). A layer of CMC material (107) is then formed on an outside surface of the insulating material. A number of such composite material box structures are then joined together to form a vane ring (100) for a gas turbine engine.
Owner:SIEMENS ENERGY INC
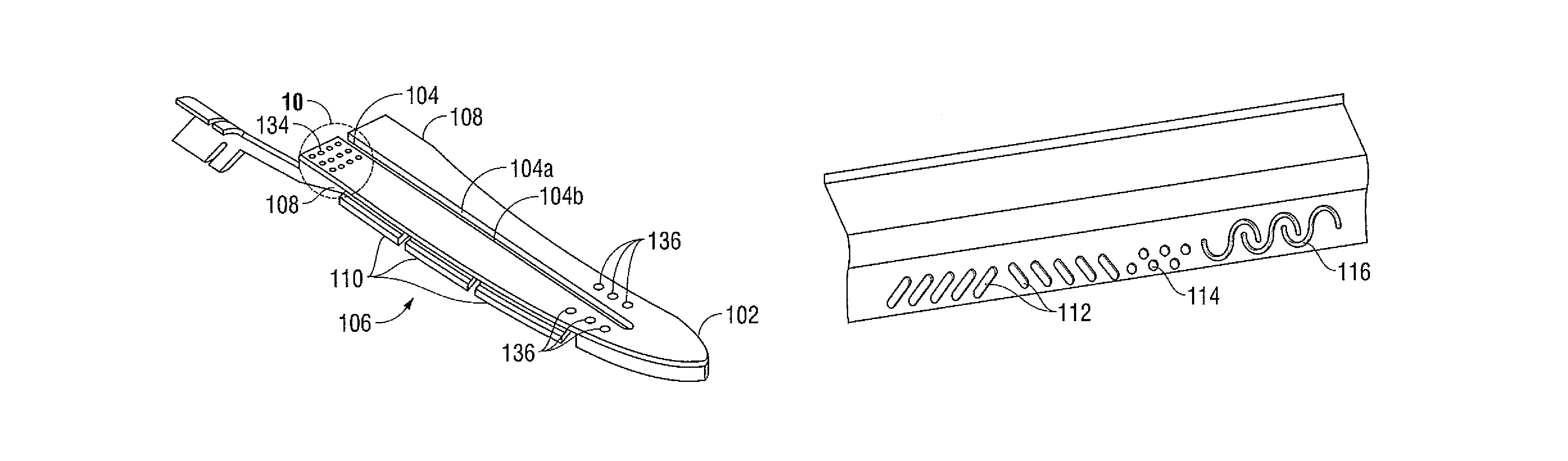
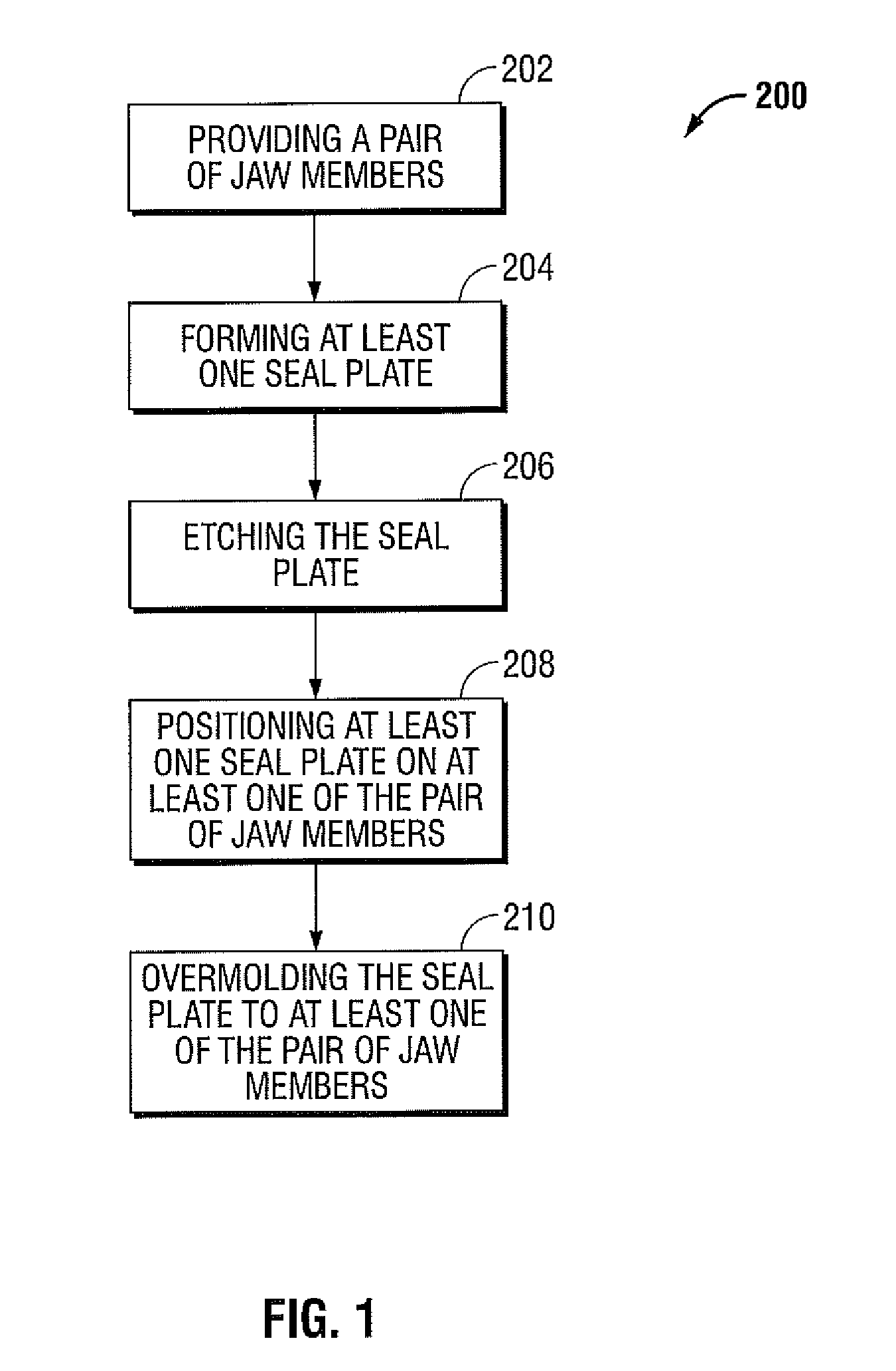
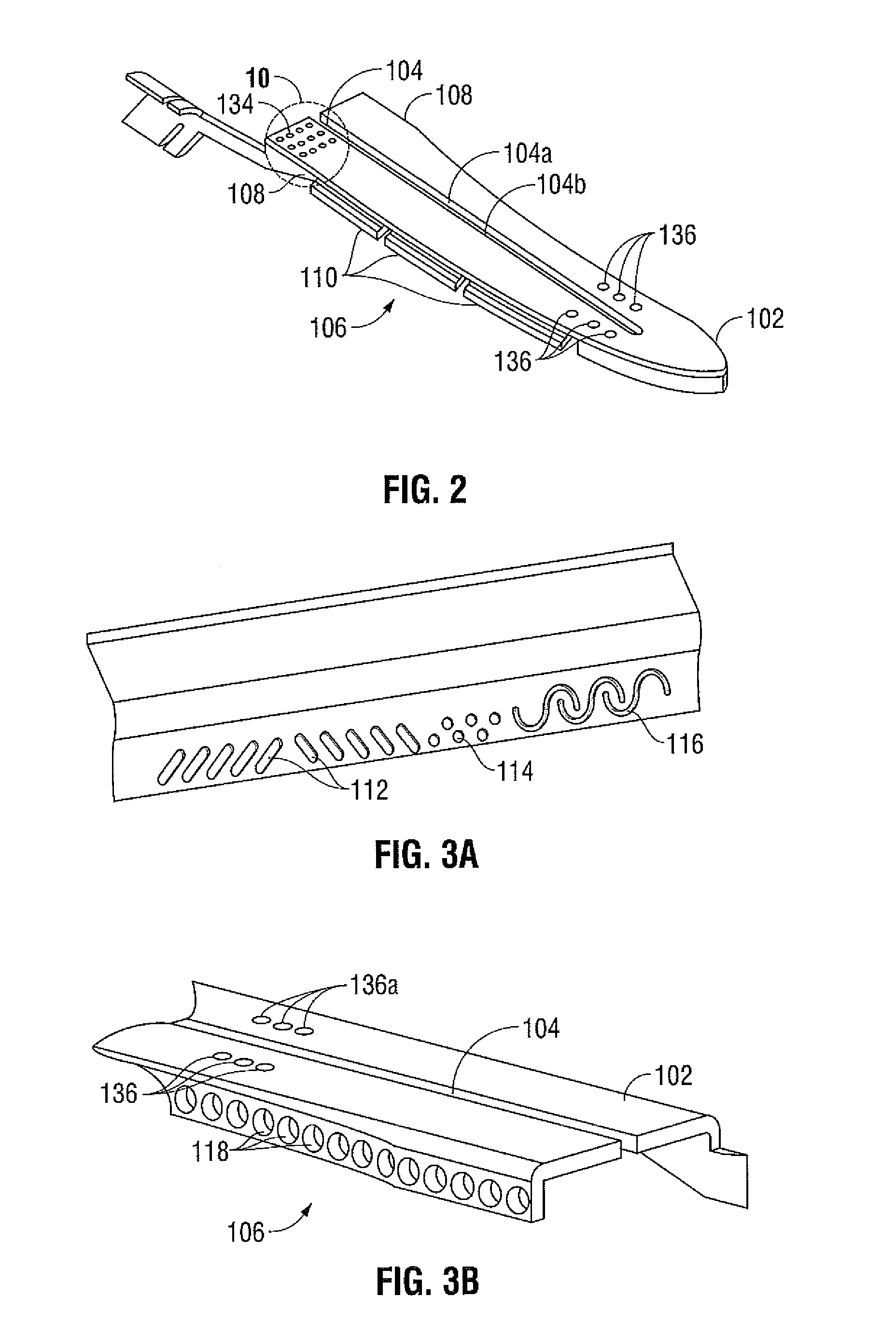
Method and system for manufacturing electrosurgical seal plates
ActiveUS8266783B2Avoid flowMeet the height limit requirementsPrinted circuit assemblingLine/current collector detailsEngineeringActuator
A method of manufacture for an end effector assembly is provided. The method includes providing a pair of jaw members. A step of the method includes forming one or more seal plates positionable on one of the pair of jaw members. Etching a dam along a side of the one or more seal plates is a step of the method, wherein the etched dam inhibits the flow of a plastic on the one or more seal plate such that a height of the plastic with respect to the at least one seal plate during an overmolding process may be controlled. The method includes positioning the one or more seal plates on the one of the pair of jaw members; and overmolding the seal plate to one or more of the pair of jaw members.
Owner:TYCO HEALTHCARE GRP LP
Method for producing a part and device for carrying out this method
InactiveUS7003864B2Faster machinabilityReduced tool wearMouldsWood working apparatusEngineeringRapid prototyping
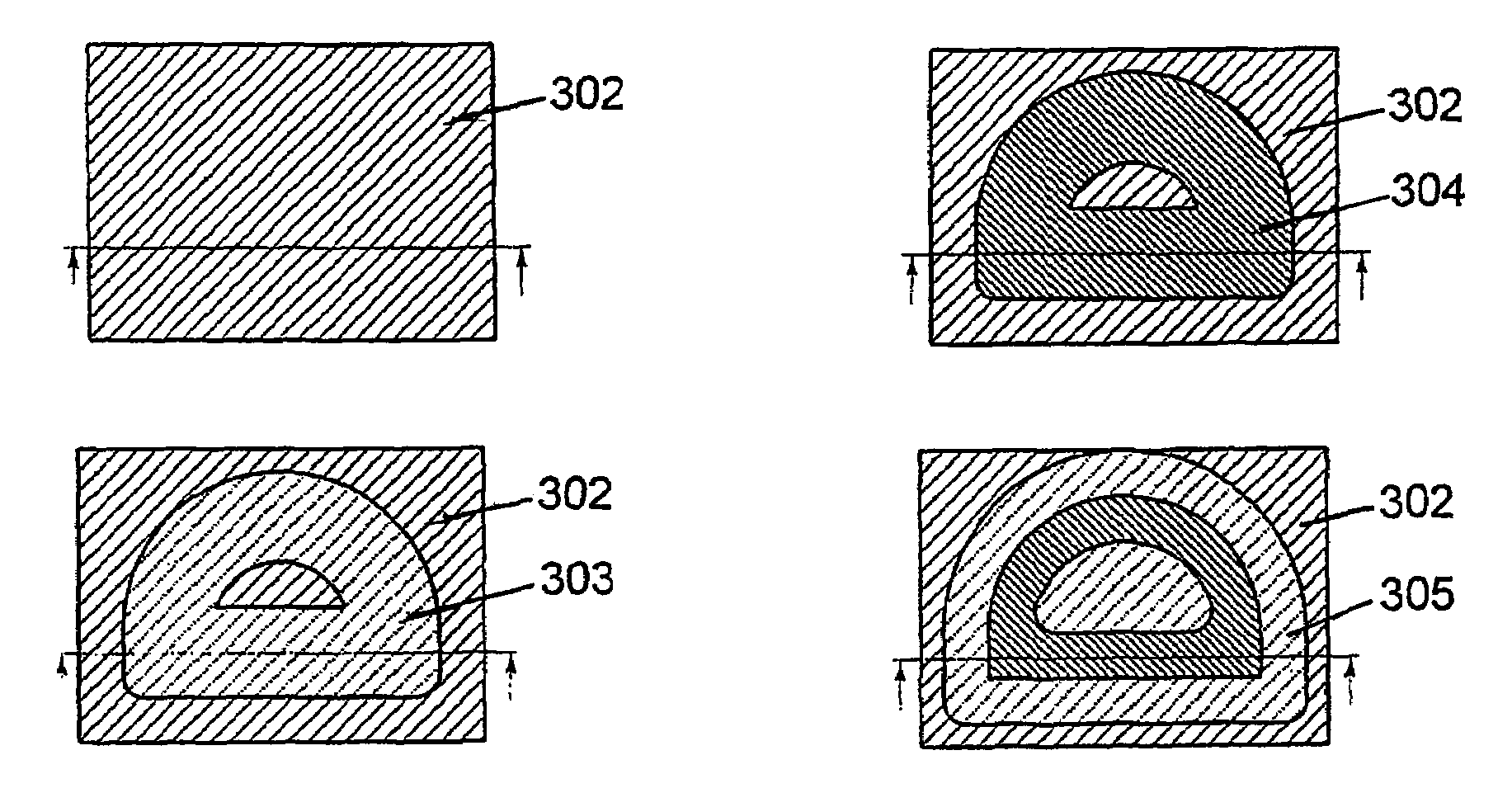
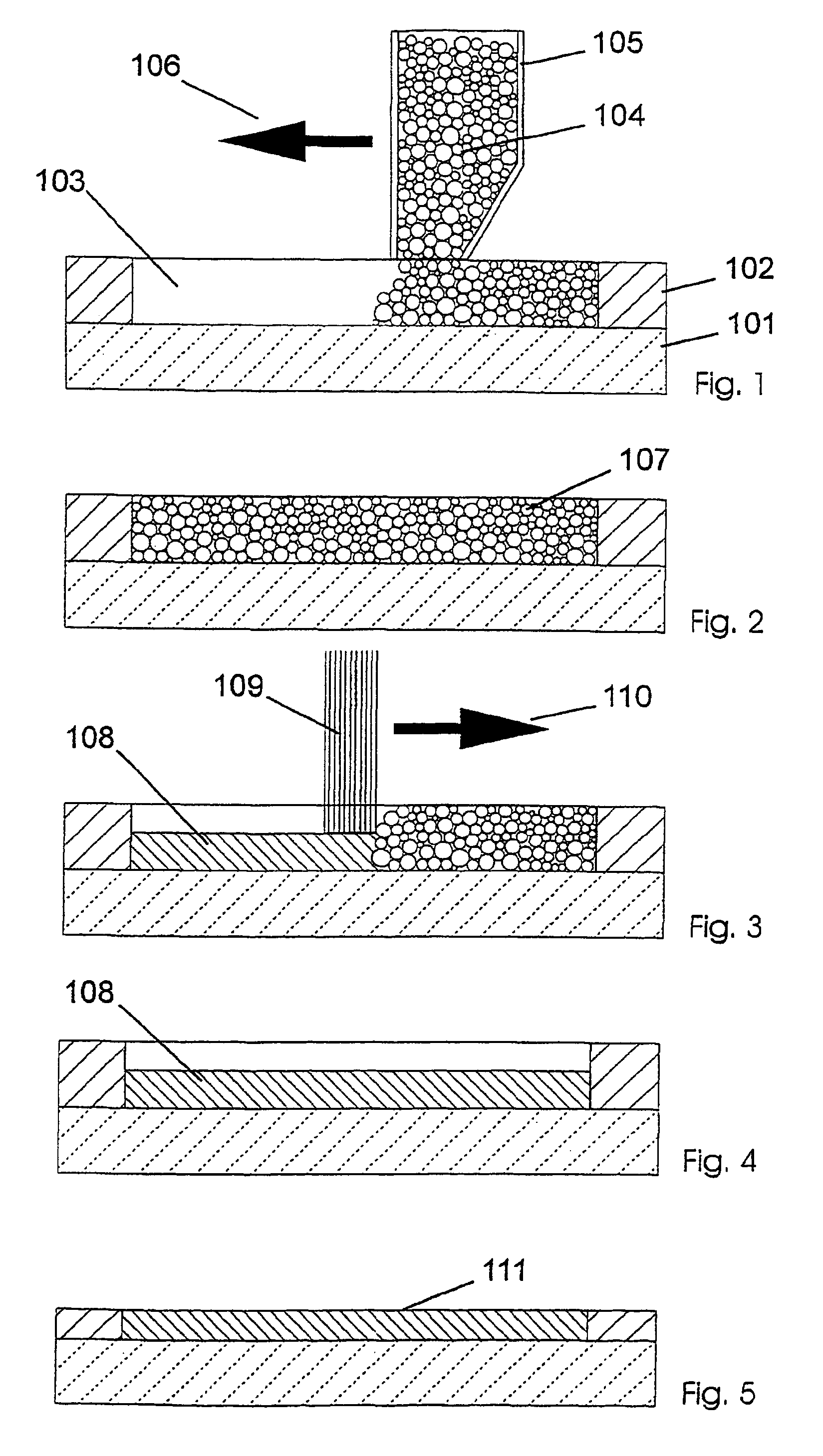
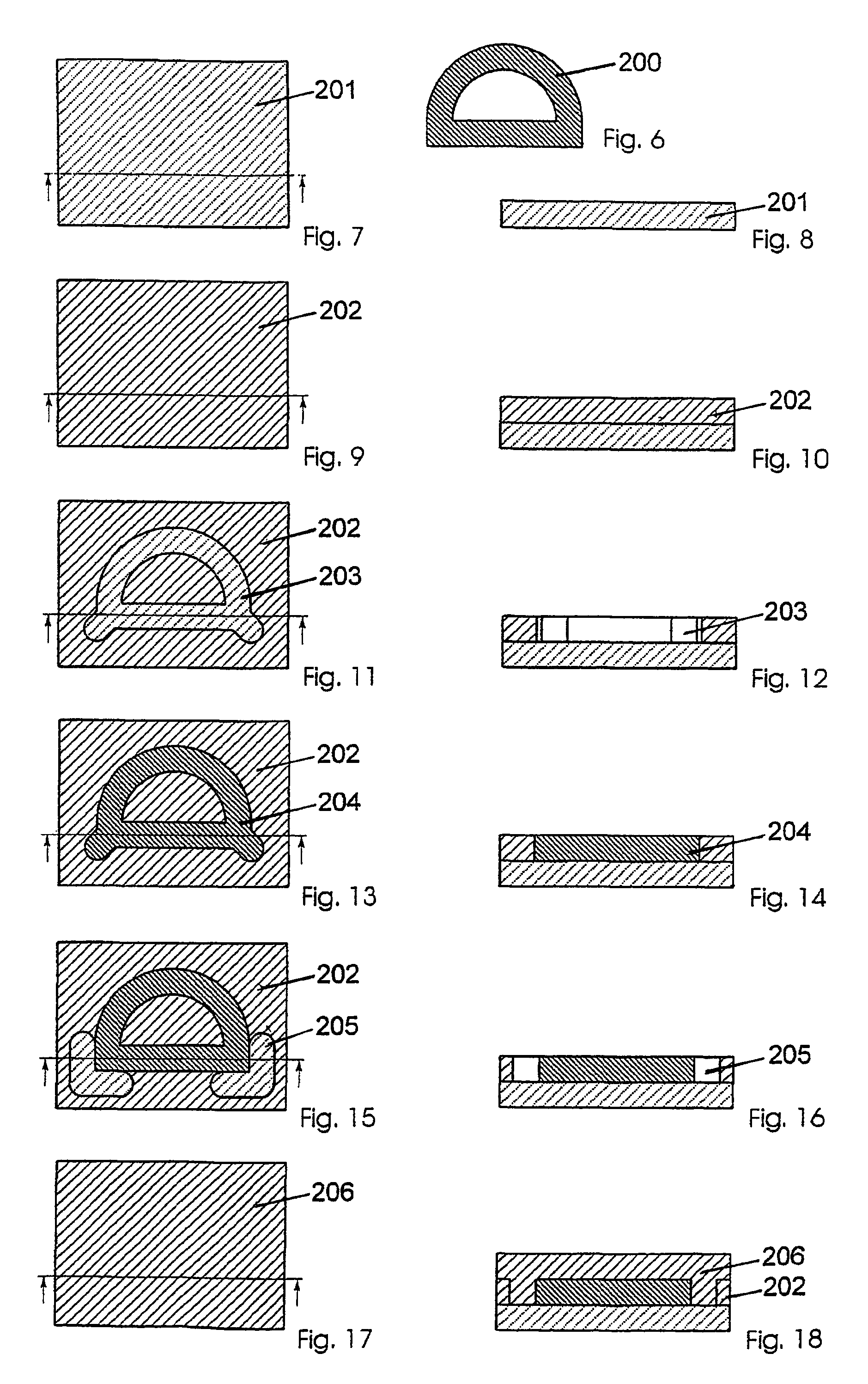
The present invention relates to a method for producing a part (108, 200, 300), comprising the following steps:a) applying a first flat layer consisting of a support material (102, 202, 302), to a construction platform (101, 201, 301),b) introducing at least one recess (103, 203, 303) into the support material (102, 202, 302),c) filling the recess (103, 203, 303) with a construction material (104, 204, 304),d) applying a further layer of support material (102, 202, 302),e) repeating steps b) through d) until completion of the part (108, 200, 300), andf) removing the support material (102, 202, 302).This method is to provide a manufacturing method and a device that combine the advantages of the layerwise construction (rapid prototyping) with the advantages of machining (e.g. high-speed cutting) and particularly permit the production of sharp-edged contours.Furthermore, the present invention relates to a device for carrying out the method.
Owner:HERMLE MASCHENBAU
Integrated Hinged Cartridge Housings for Sample Analysis
ActiveUS20110150705A1Reduce the cross-sectional areaLow variabilityAnalysis material containersMetal rolling stand detailsAnalyteEngineering
The invention relates to a cartridge housing for forming a cartridge capable of measuring an analyte or property of a liquid sample. The housing comprising a first substantially rigid zone, a second substantially flexible zone, a hinge region, and at least one sensor recess containing a sensor. The housing is foldable about said hinge region to form a cartridge having a conduit over at least a portion of said sensor. The invention also relates to methods for forming such cartridges and to various features of such cartridges.
Owner:ABBOTT POINT CARE
Control housing and method of manufacturing same
InactiveUS7146701B2Easy to cleanFlat surfaceContact surface shape/structureEmergency casingsEngineering
Owner:NEECO TRON
Devices and methods for abluminally coating medical devices
A stent crimping and coating apparatus is disclosed. The apparatus includes a plurality of crimping blades positioned in a radial array and collectively forming a central crimping lumen, wherein the plurality of crimping blades radially movable to alter the diameter of the central crimping lumen. Each of the crimping blades includes a first surface configured to at least in part define the central crimping lumen. One or more of the crimping blades includes a fluid channel extending therein and a plurality of openings in fluid communication with the fluid channel. The plurality of openings are located at the first surface of the one or more crimping blades and adapted to discharge a fluid into the central crimping lumen.
Owner:BOSTON SCI SCIMED INC
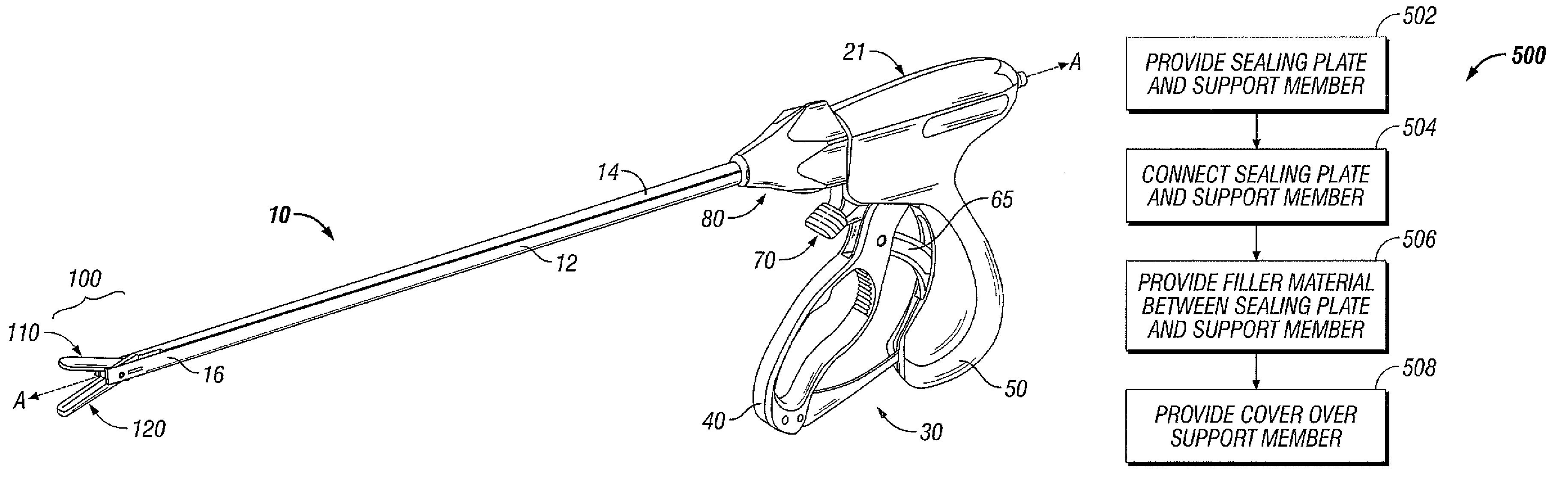
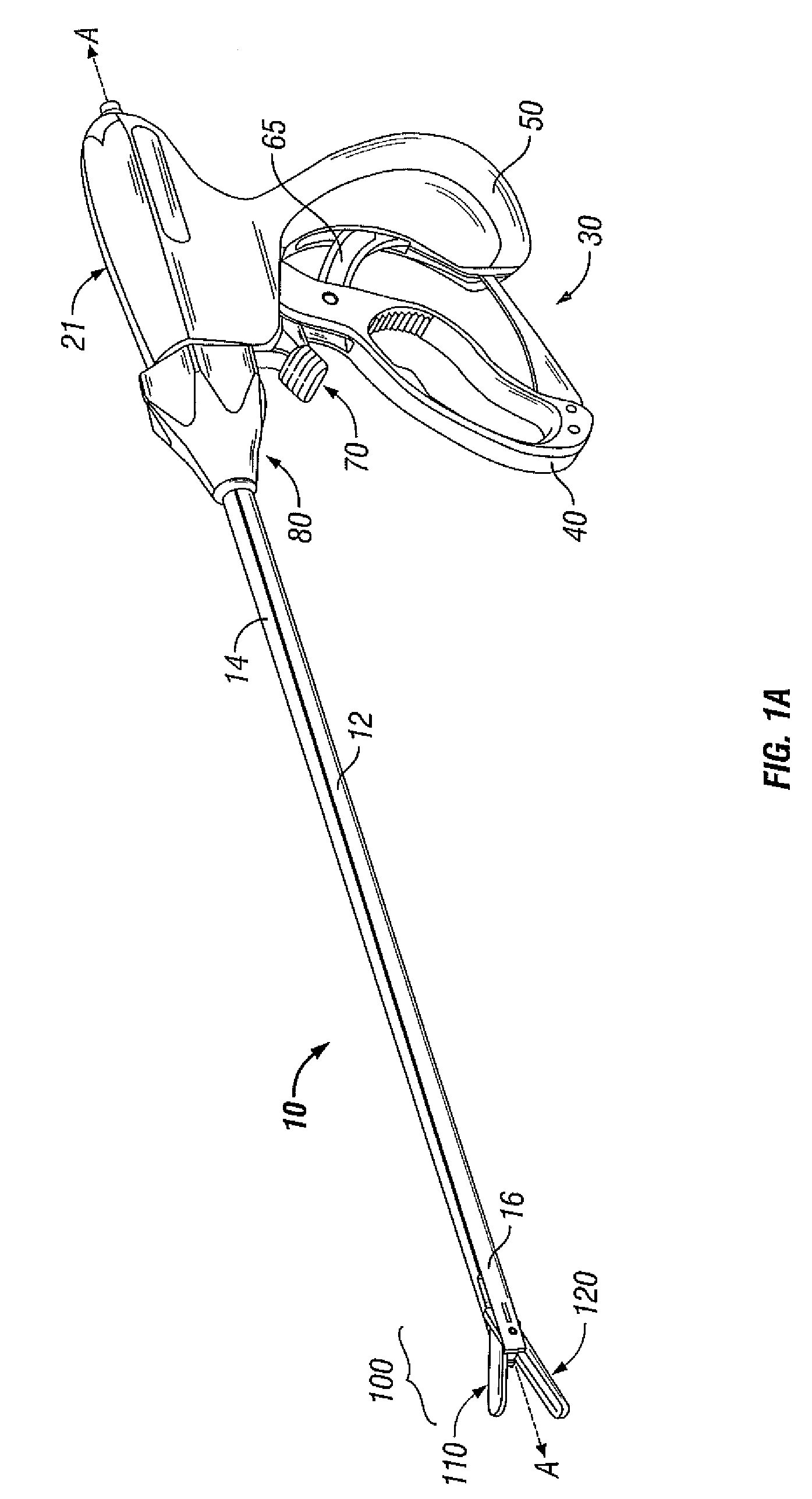
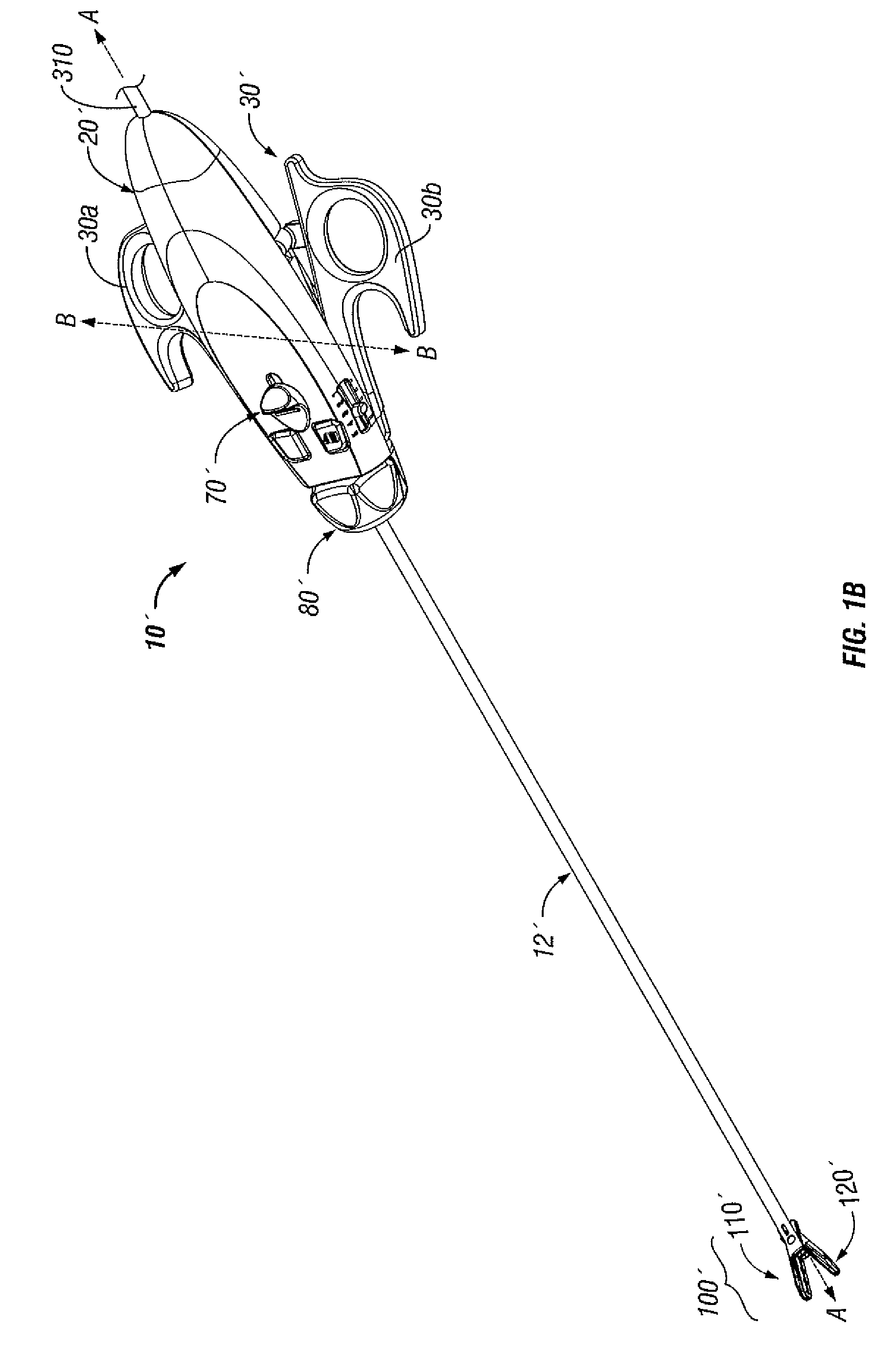
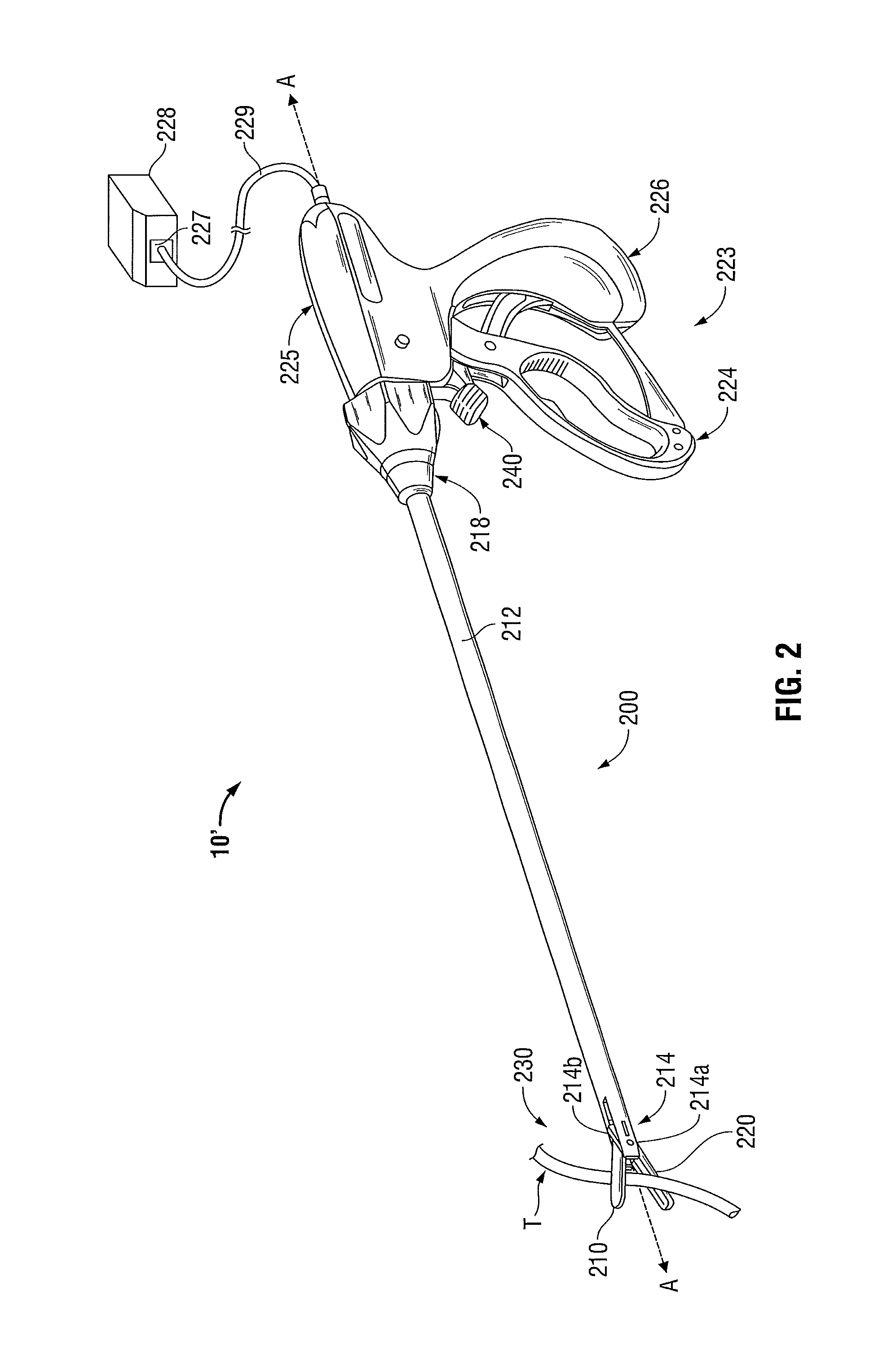
Vessel sealing instrument with reduced thermal spread and method of manufacture therefor
ActiveUS8887373B2Easy to installImprove adhesionMetal rolling stand detailsSurgical instrument detailsVessel sealingEngineering
An electrosurgical vessel sealing instrument having a first and a second opposing jaw member at a distal end thereof, wherein each jaw member includes a jaw housing, a seal plate having a tissue contacting surface and a side wall, and an insulating region disposed on the side wall of the seal plate. The instrument includes the ability to move the jaw members relative to one another from a first position wherein the jaw members are disposed in spaced relation relative to one another to a second position wherein the jaw members cooperate to grasp tissue. The insulating region enables precision overmolding of the jaw housing to the seal plate, while advantageously reducing thermal spread and edge cutting during vessel sealing procedures.
Owner:COVIDIEN LP
Process for forming a porous drug delivery layer
InactiveUS6904658B2Increase drug retention timeImprove adhesionStentsMetal rolling stand detailsPorous layerVolumetric Mass Density
The present invention is directed to a process for forming a drug delivery device by electroplating onto a substrate a porous layer having pores of a controlled size and density and loading a drug into the pores. Preferably the drug delivery device is a gold stent plated with a gold porous layer.
Owner:ELECTROFORMED STENTS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com