Patents
Literature
2333 results about "Balloon catheter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
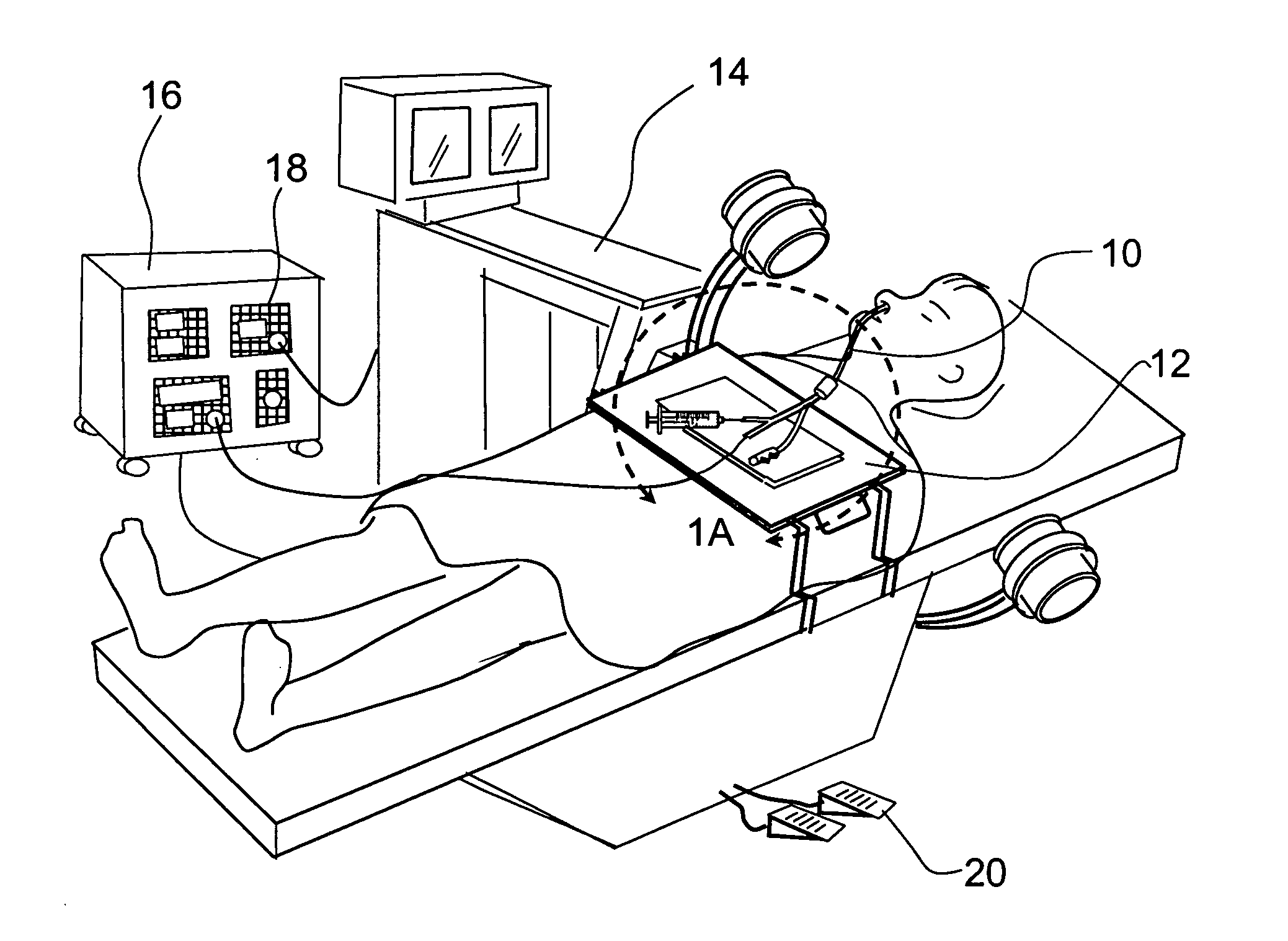
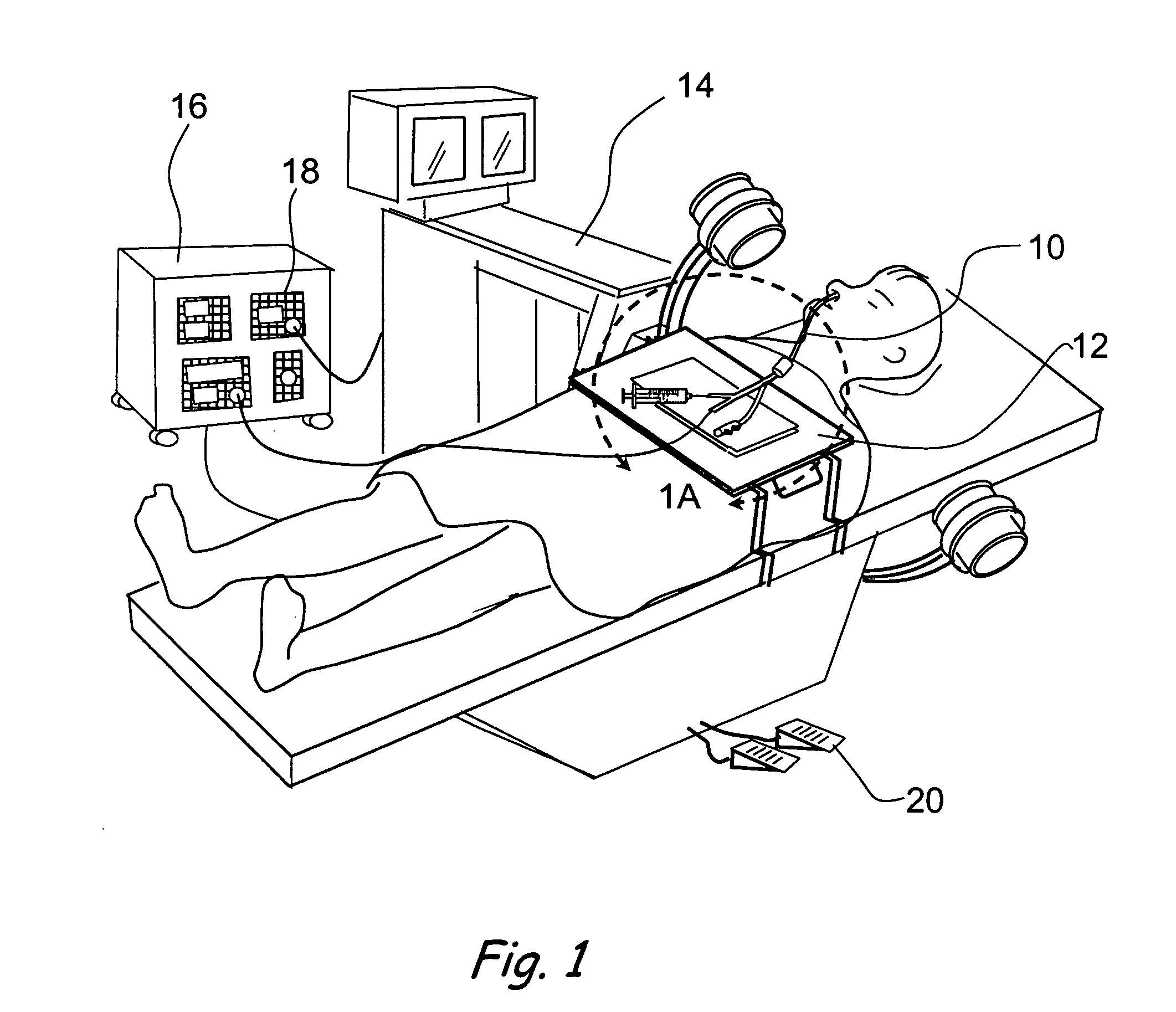
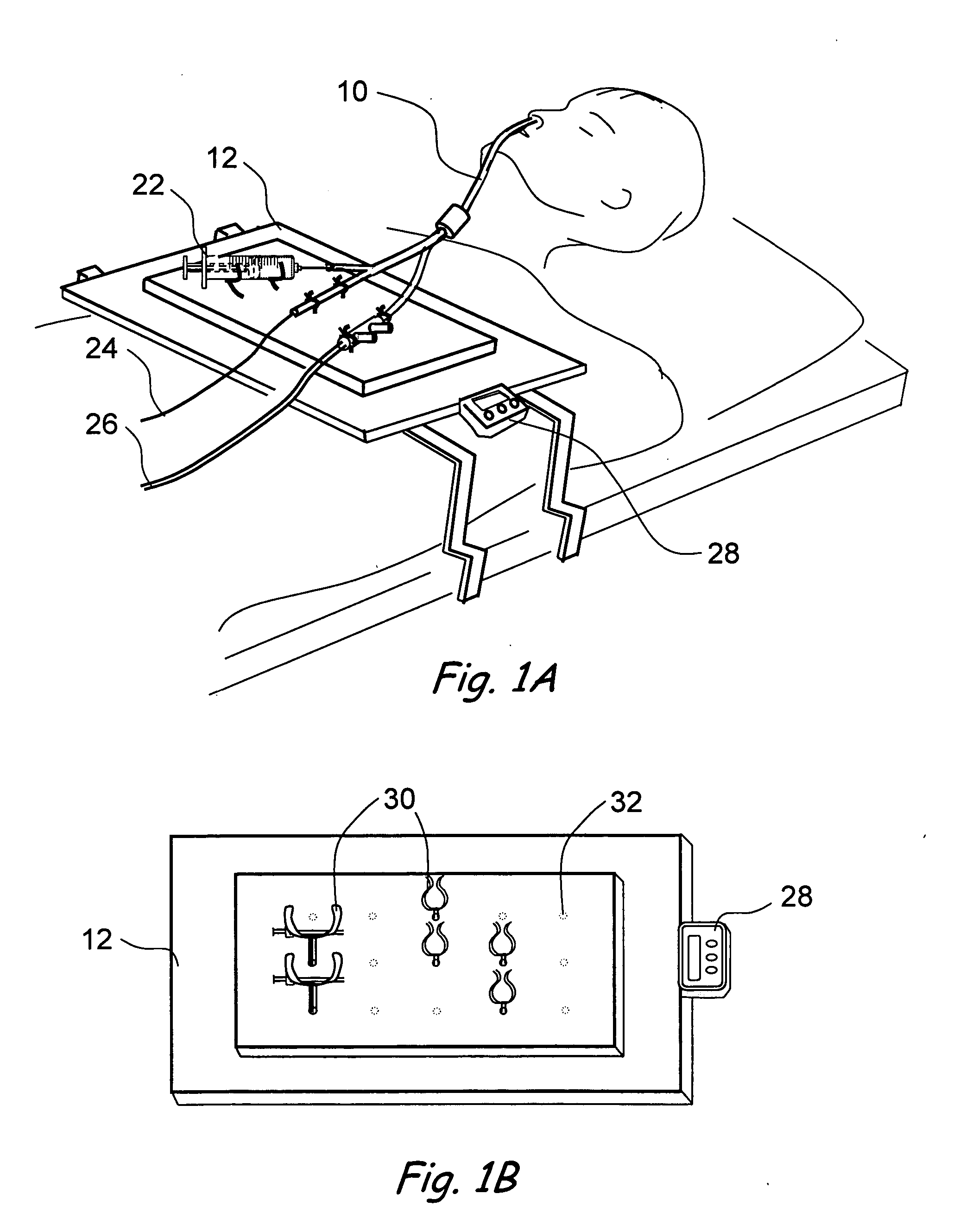
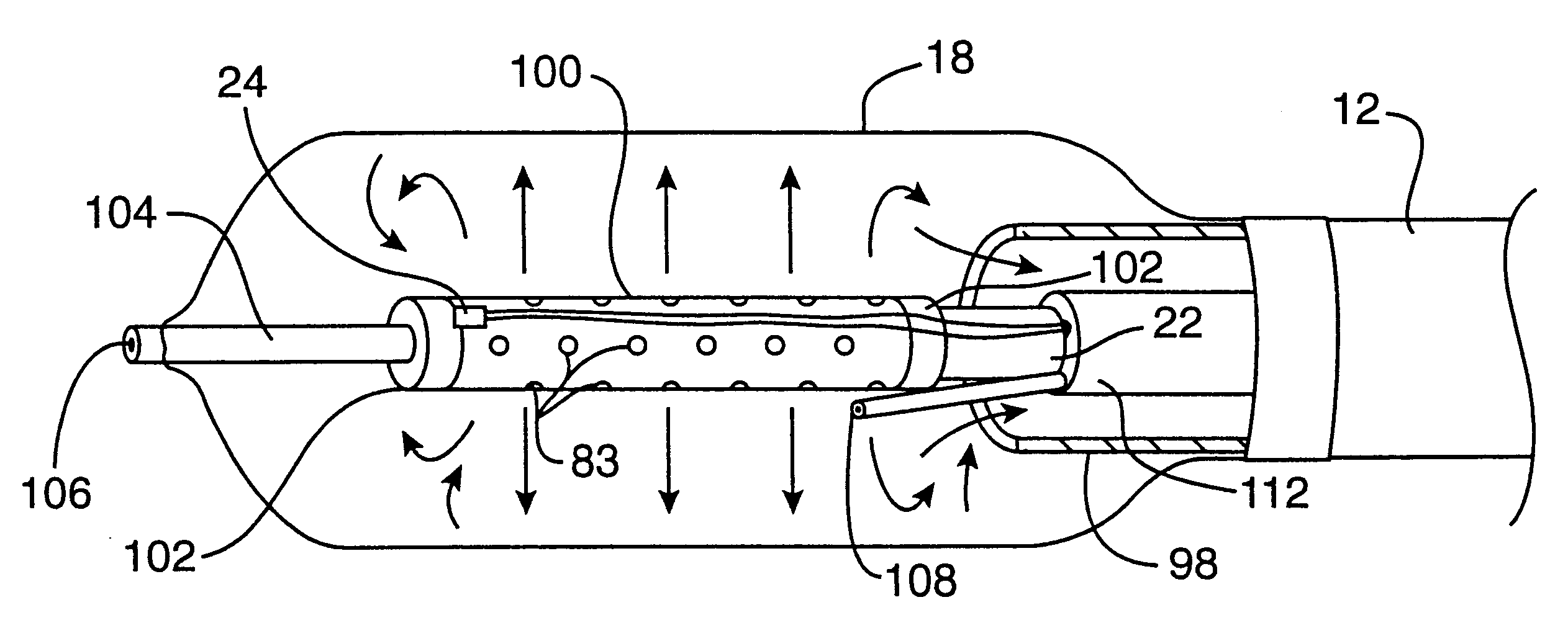
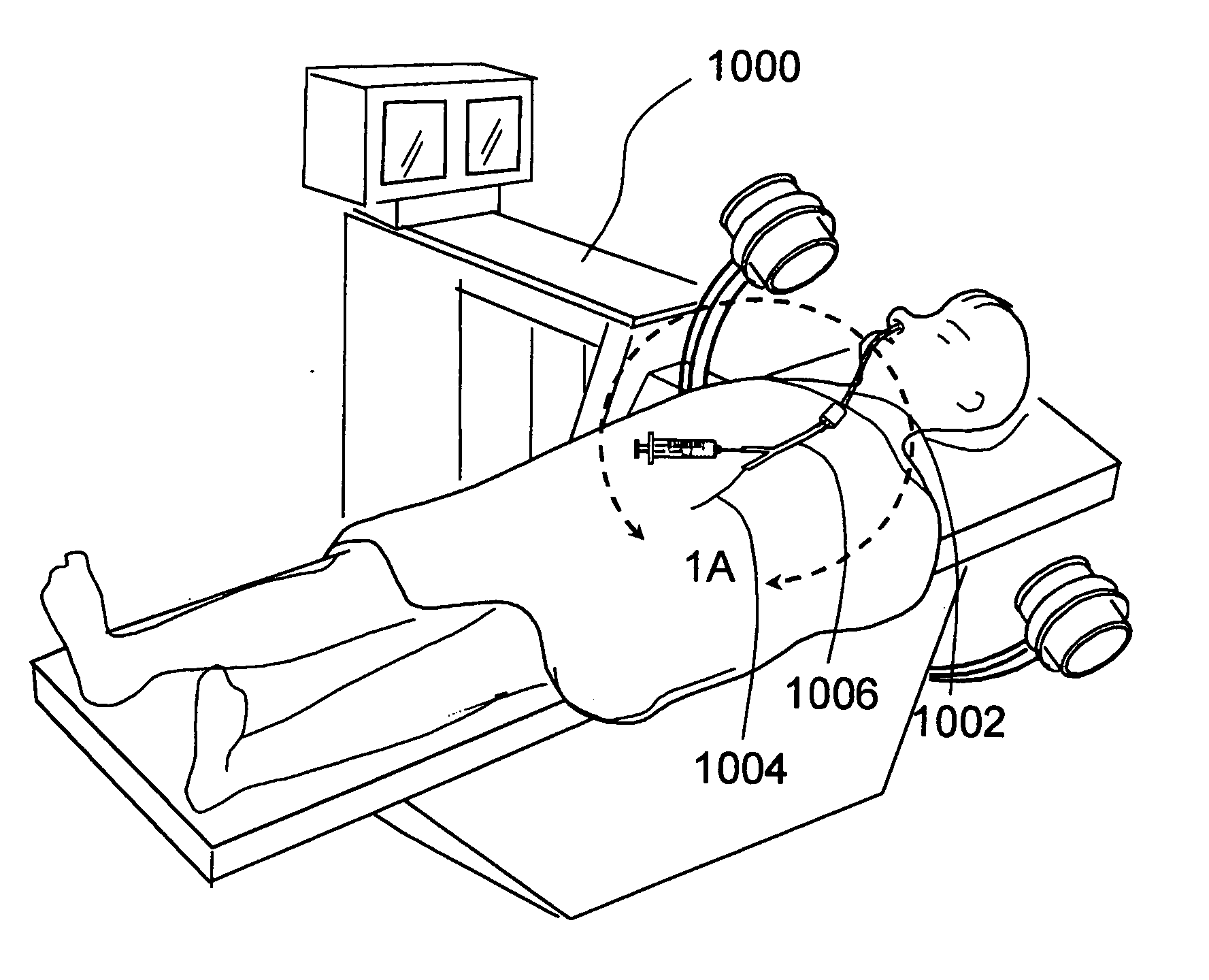
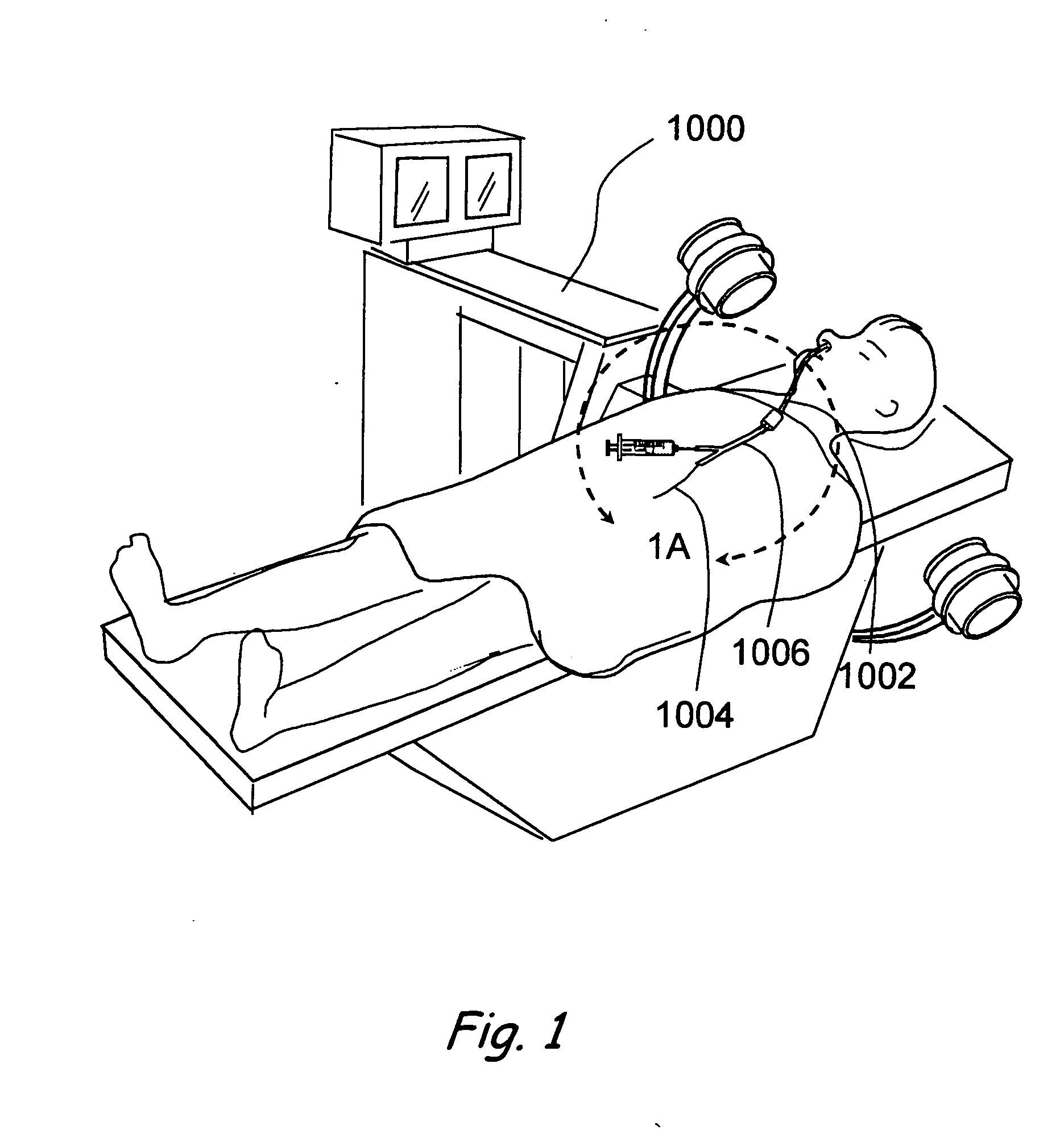
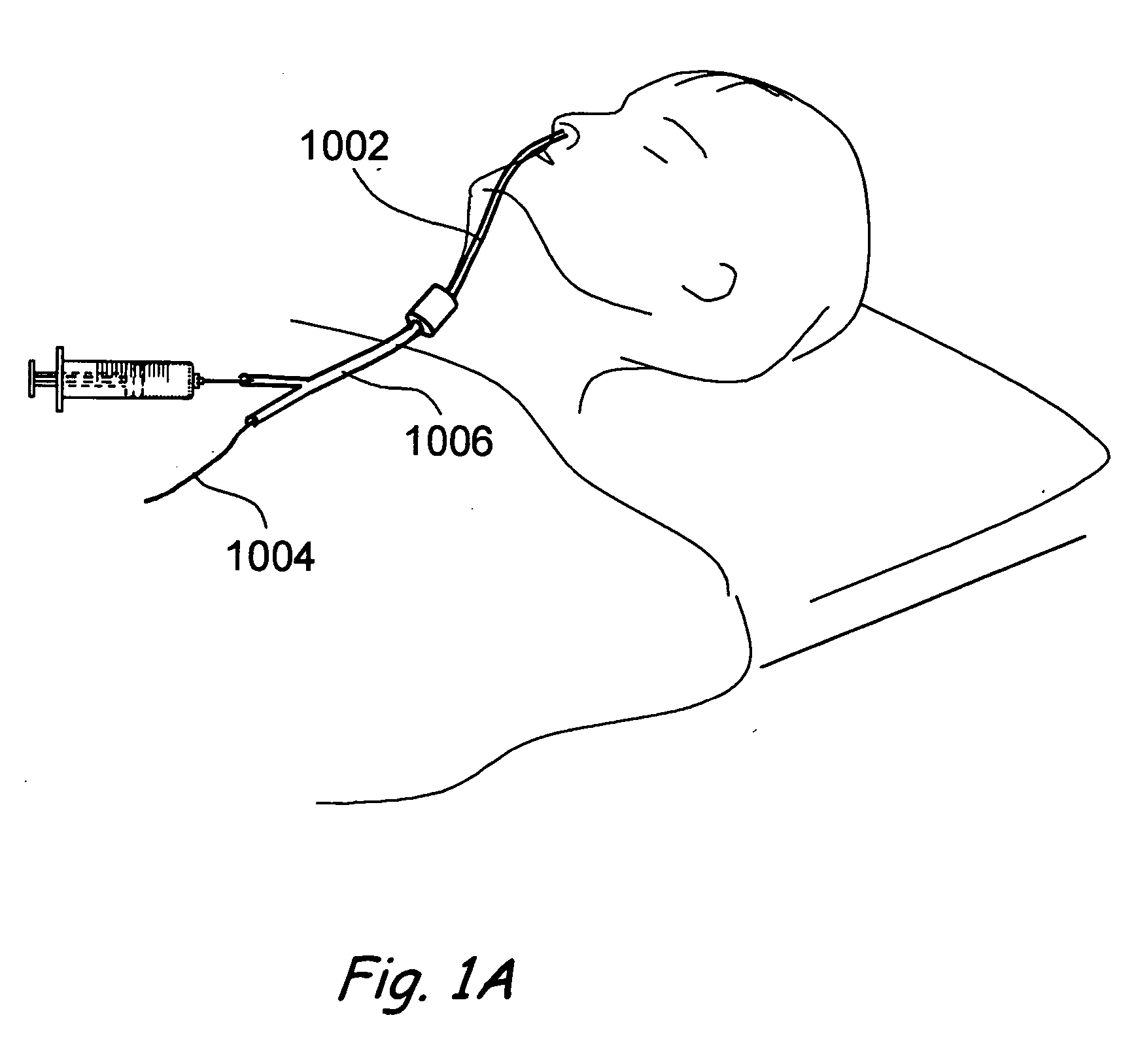
A balloon catheter is a type of soft catheter with an inflatable balloon at its tip which is used during a catheterization procedure to enlarge a narrow opening or passage within the body.
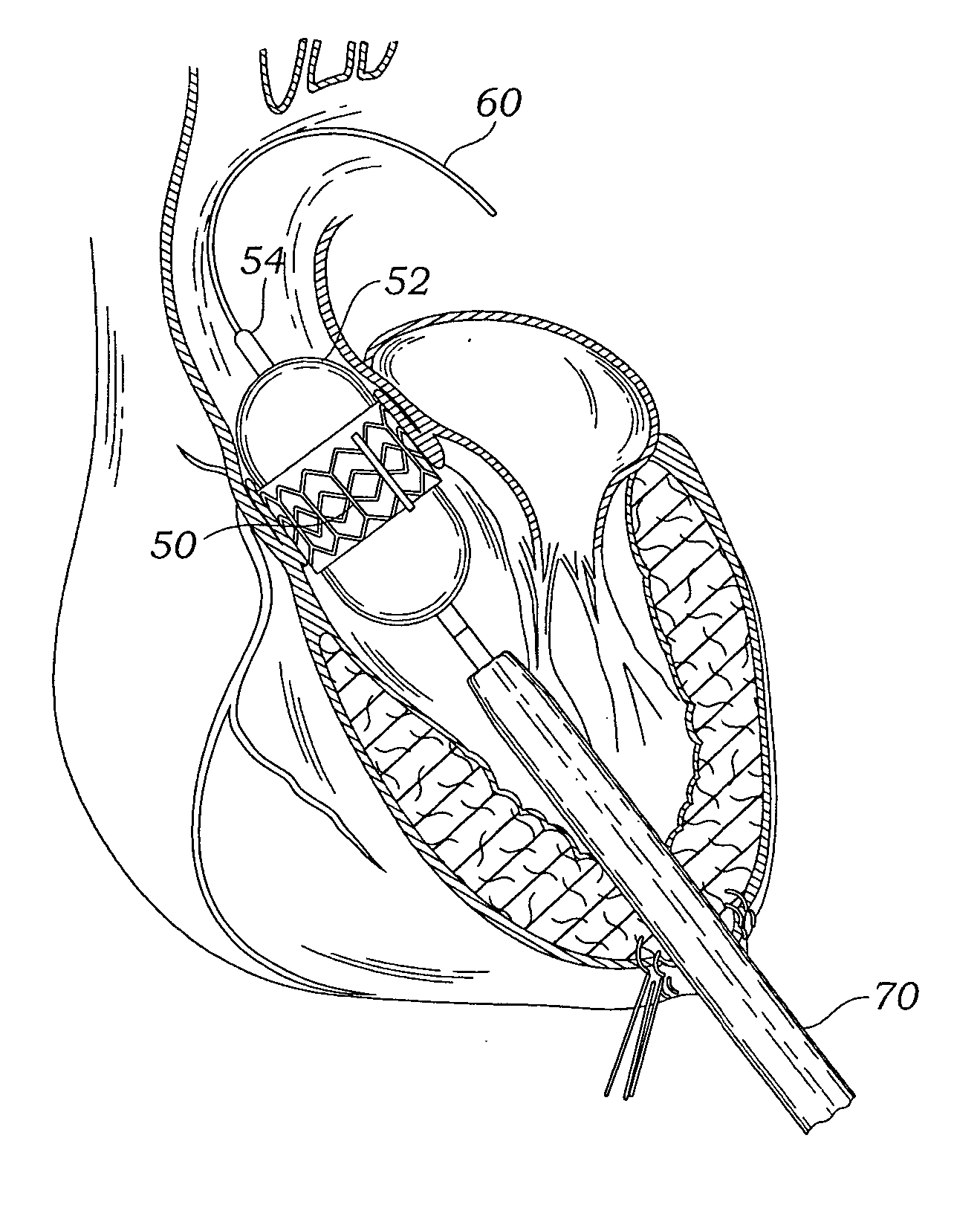
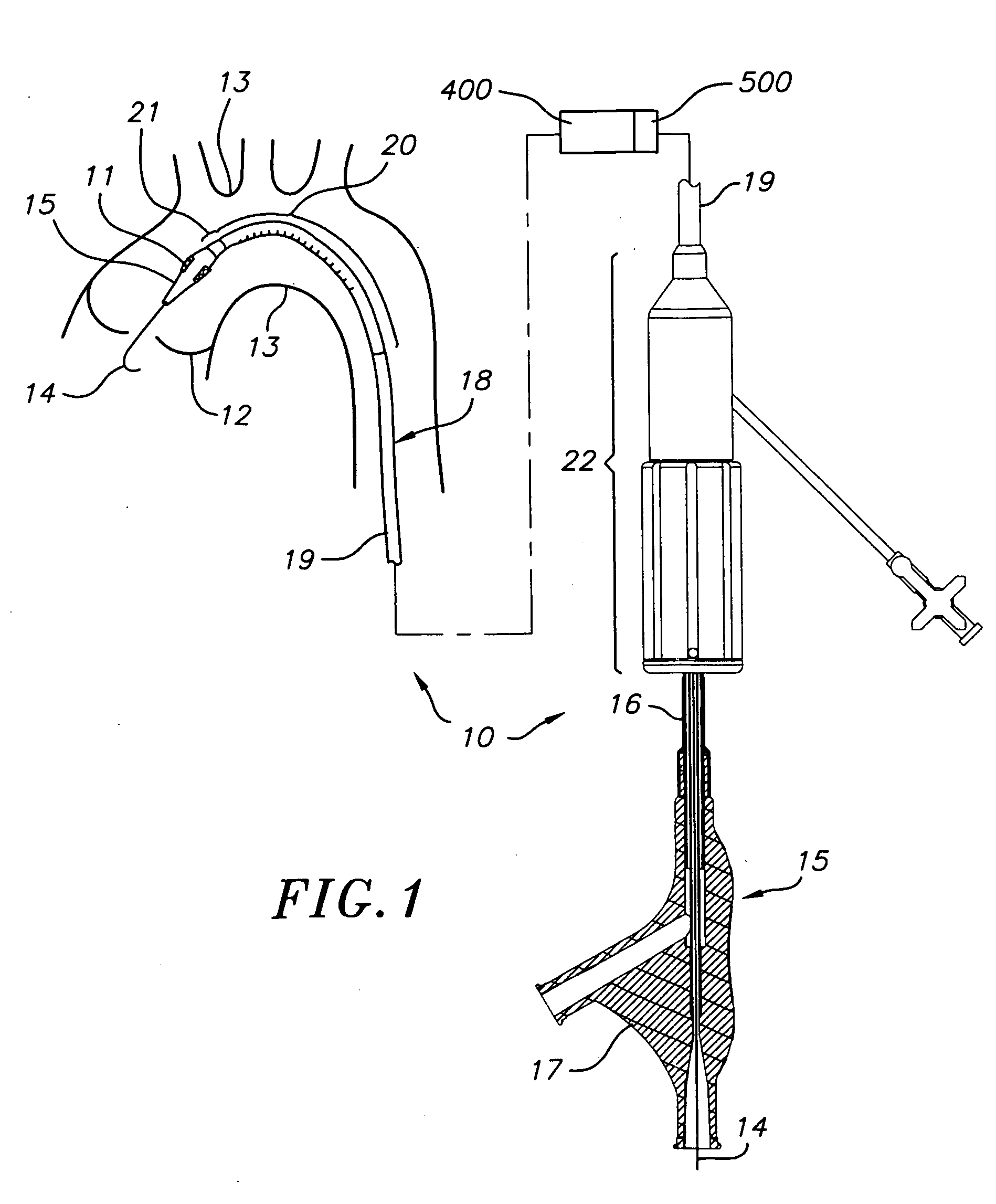
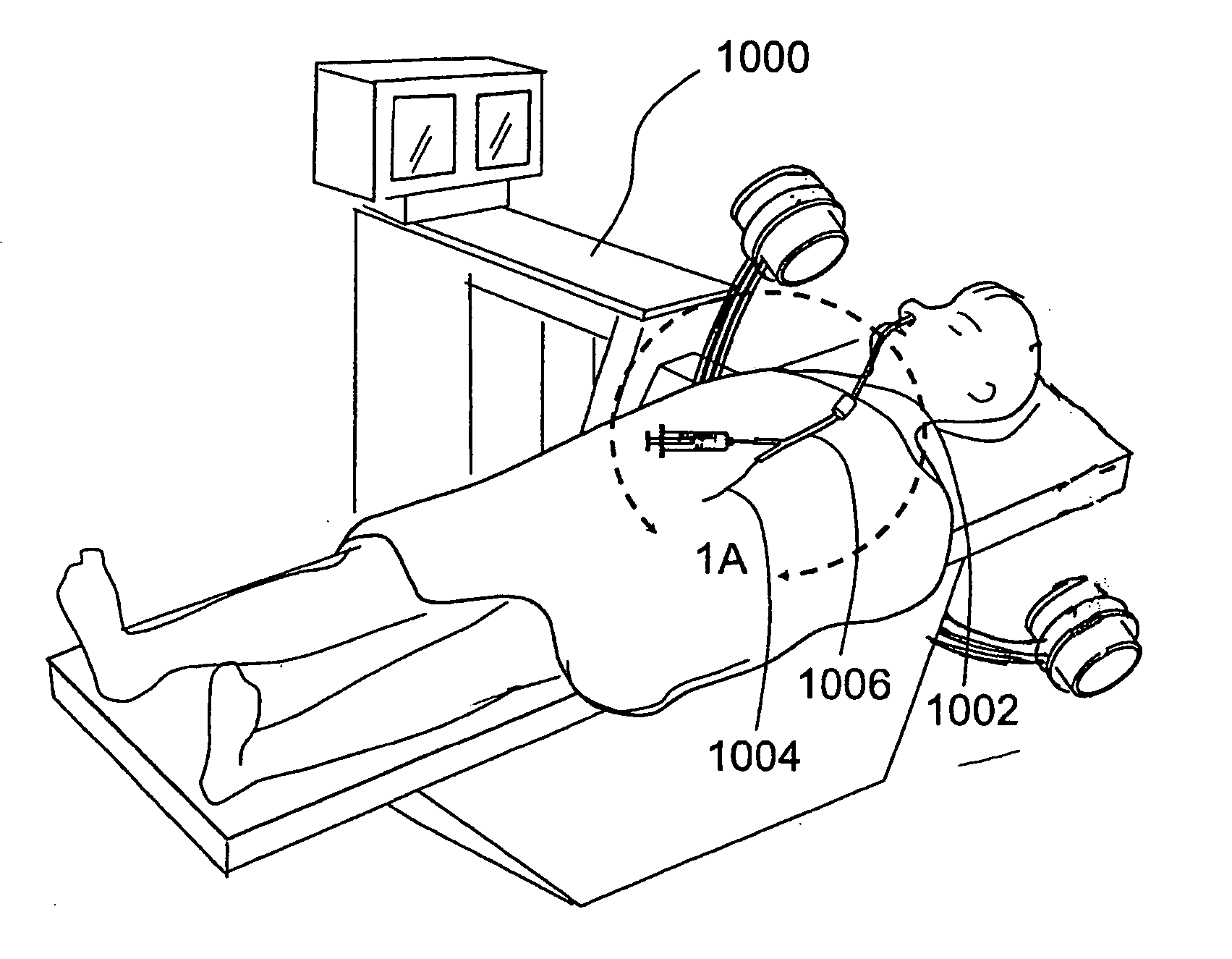
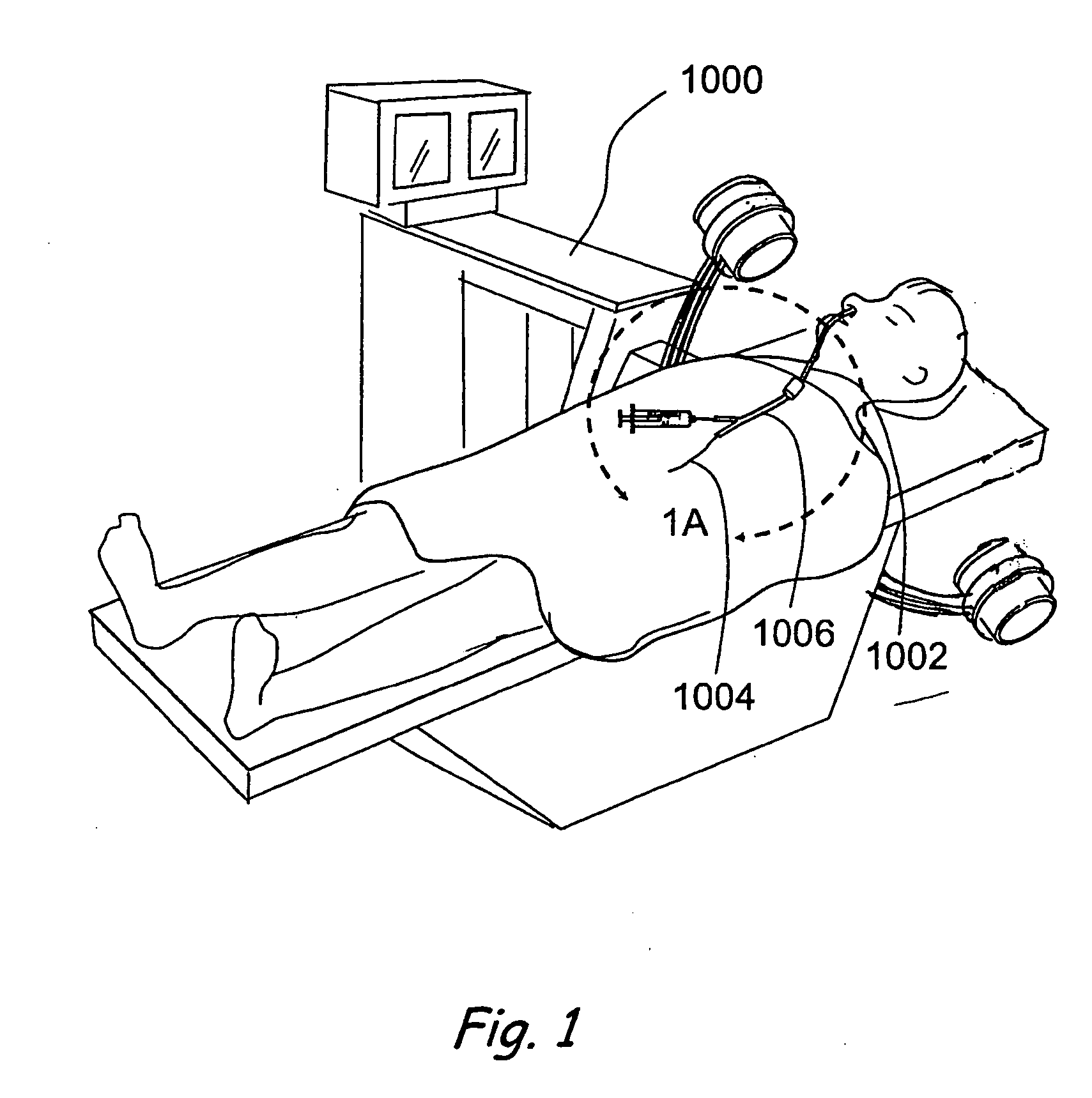
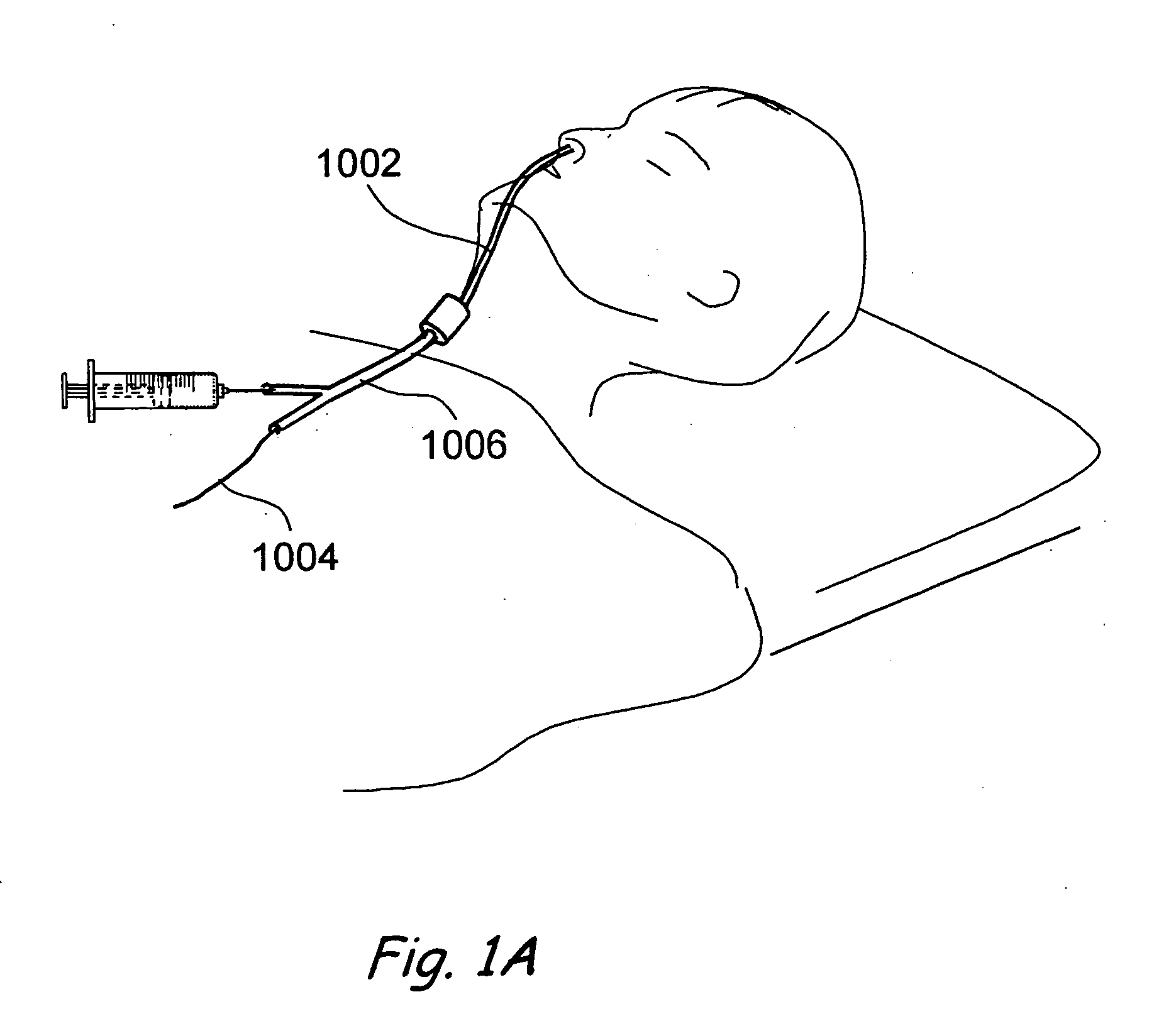
Transapical heart valve delivery system and method
ActiveUS20070112422A1Facilitate positioning of valveHelp positioningStentsBalloon catheterProsthetic heartLeft ventricular apex
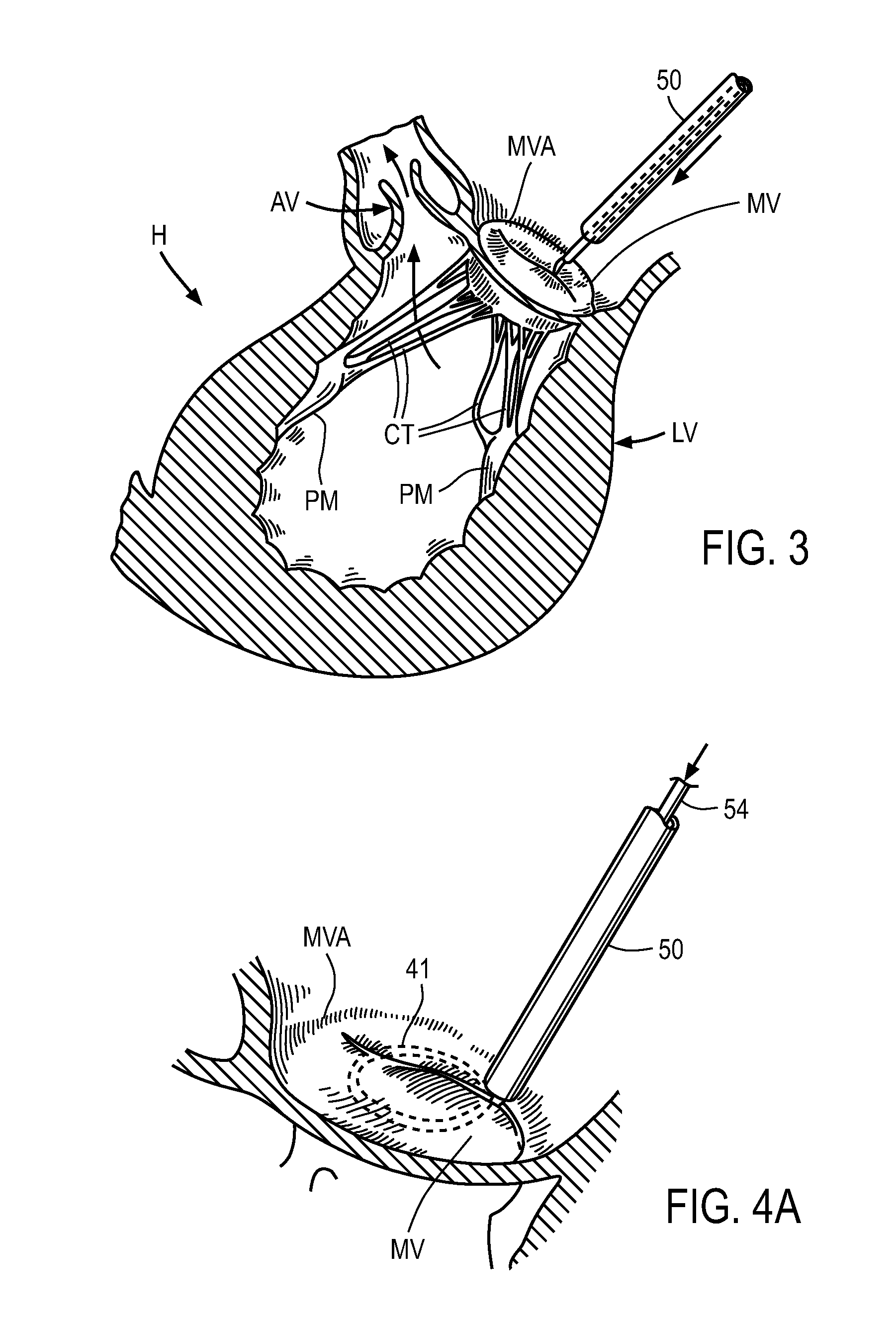
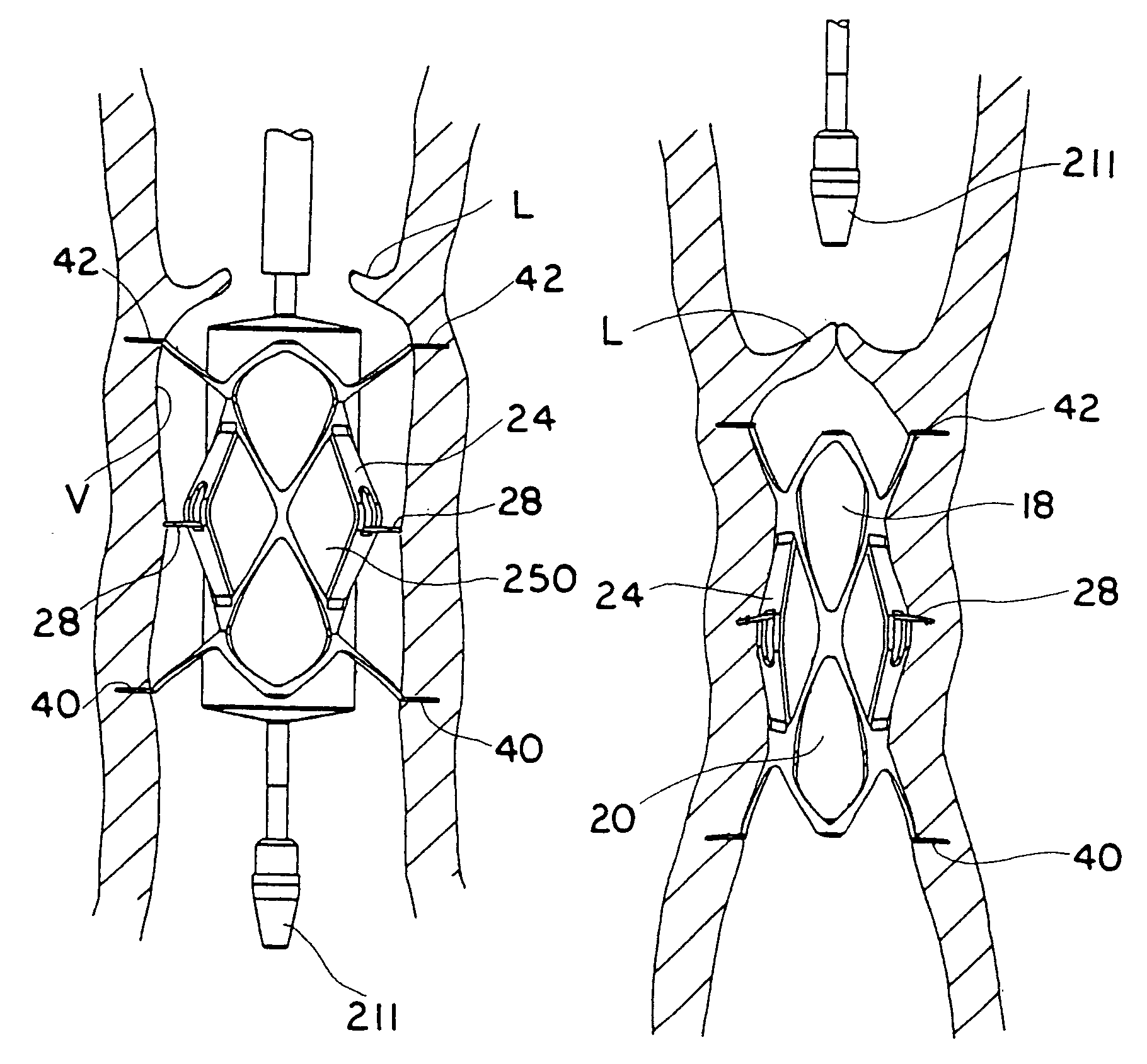
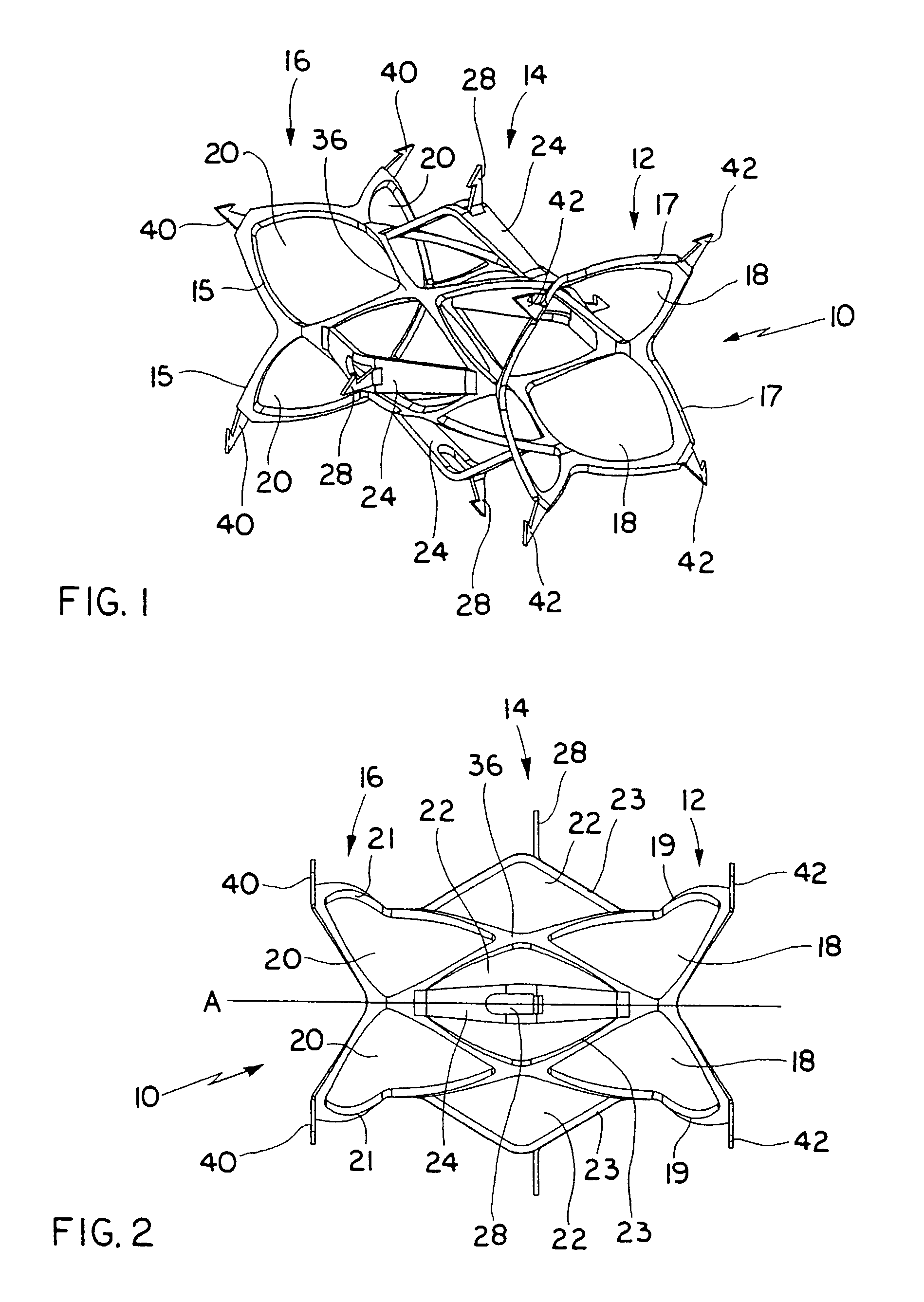
A delivery system and method for delivering a prosthetic heart valve to the aortic valve annulus. The system includes a balloon catheter having a steering mechanism thereon for delivering a balloon-expandable prosthetic heart valve through an introducer in an antegrade fashion to the aortic annulus. The balloon catheter passes through an introducer that accesses the left ventricle through its apex and a small incision in the chest wall. The balloon catheter includes a deflecting segment just proximal to the distal balloon to facilitate positioning of the prosthetic heart valve in the proper orientation within the aortic annulus. A slider in a deflection handle may be coupled to a deflection wire that actuates the deflecting segment. The method includes using two concentric rings of purse-string sutures around the puncture in the left ventricular apex to maintain a good seal around instruments passed therethrough. The prosthetic heart valve may be installed over the existing calcified leaflets, and a pre-dilation valvuloplasty procedure may also be utilized.
Owner:EDWARDS LIFESCIENCES CORP
Heart valve delivery system
ActiveUS20070005131A1Easy to navigateIncrease thrustBalloon catheterHeart valvesProsthetic heartBalloon catheter
A delivery system for delivering a prosthetic heart valve to a native valve site within the human vasculature. The prosthetic valve is disposed on a balloon at the end of a balloon catheter. The balloon catheter passes through a delivery sleeve assembly and handle. A pull wire travels from the handle to a distal end of the delivery sleeve assembly. Actuation of the handle pulls on the pull wire, which causes openings in a slotted tube of the delivery sleeve assembly to close, thus causing the delivery sleeve assembly to bend. A stretchable cover is placed over the slotted tube for biasing the steerable catheter toward a straight position. Once advanced to the native valve site, the prosthetic valve is deployed by inflating the balloon.
Owner:EDWARDS LIFESCIENCES CORP
Kit enabling a prosthetic valve to be placed in a body enabling a prosthetic valve to be put into place in a duct in the body
InactiveUS20050043790A1Destruction damageRisk of destructionHeart valvesBlood vesselsProsthetic valveInsertion stent
The present invention is an assembly comprising a prosthetic valve to be implanted; a radially expandable stent comprising at least one zone intended to be expanded to allow the stent, in the expanded state, to bear against the wall of the body duct to be fitted with the valve, this bearing making it possible to immobilize this stent with respect to this wall; and means for mounting the valve with respect to the stent, making it possible to connect the valve to the stent in such a way that the placement of the stent allows the valve to be mounted in the body duct, and expansion means such as a balloon catheter being provided to trigger expansion of the stent at the implantation site. According to the invention, the valve and the stent are designed in such a way that, at the moment when the stent is expanded, the valve is situated outside the zone or zones of the stent that are subjected to said expansion means. The invention thus consists in separating the valve and said zone or zones to be expanded, so that the expansion of the stent can be effected with an expansion force suitable for perfect anchoring of this stent in the wall of the body duct to be fitted with the valve, and without any risk of destruction or damage of the valve.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
System and method for implanting a two-part prosthetic heart valve
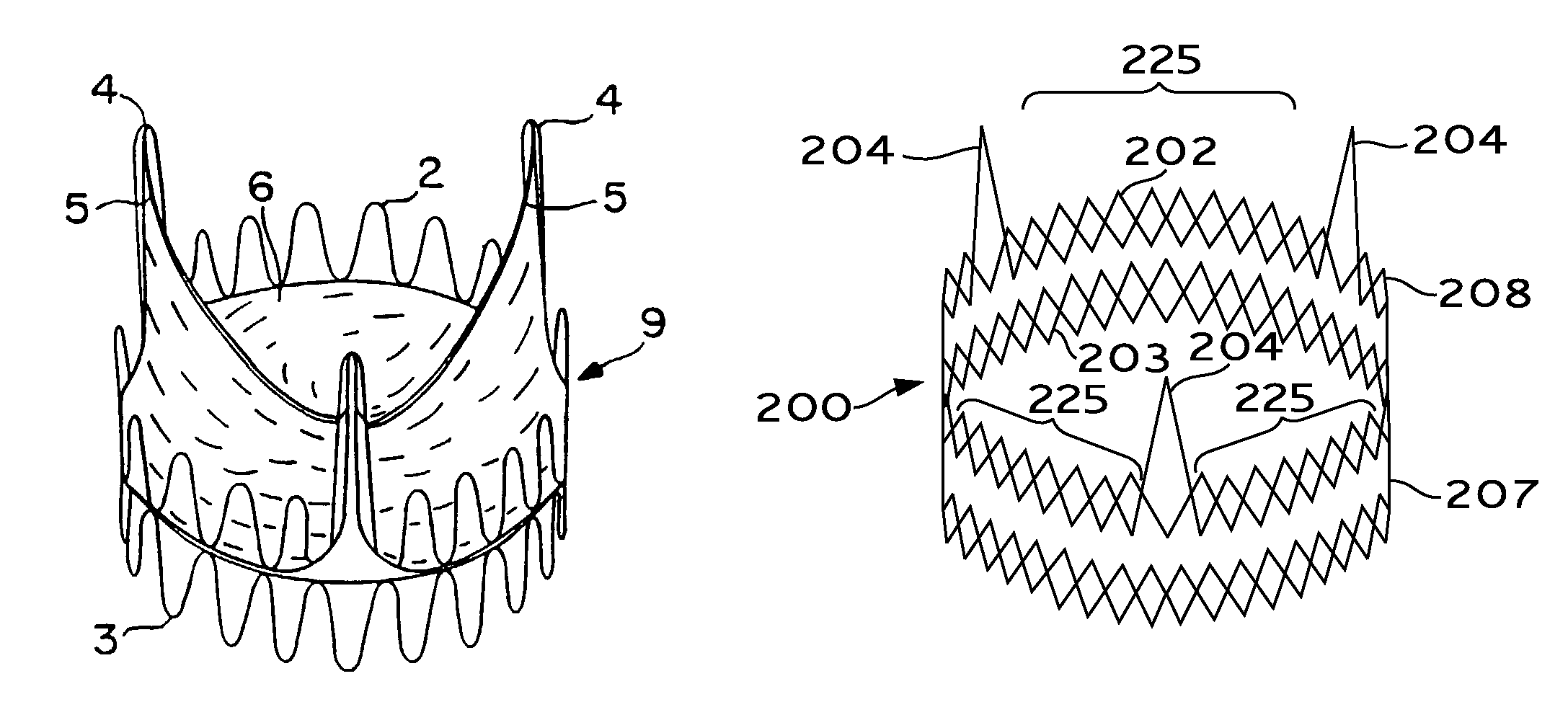
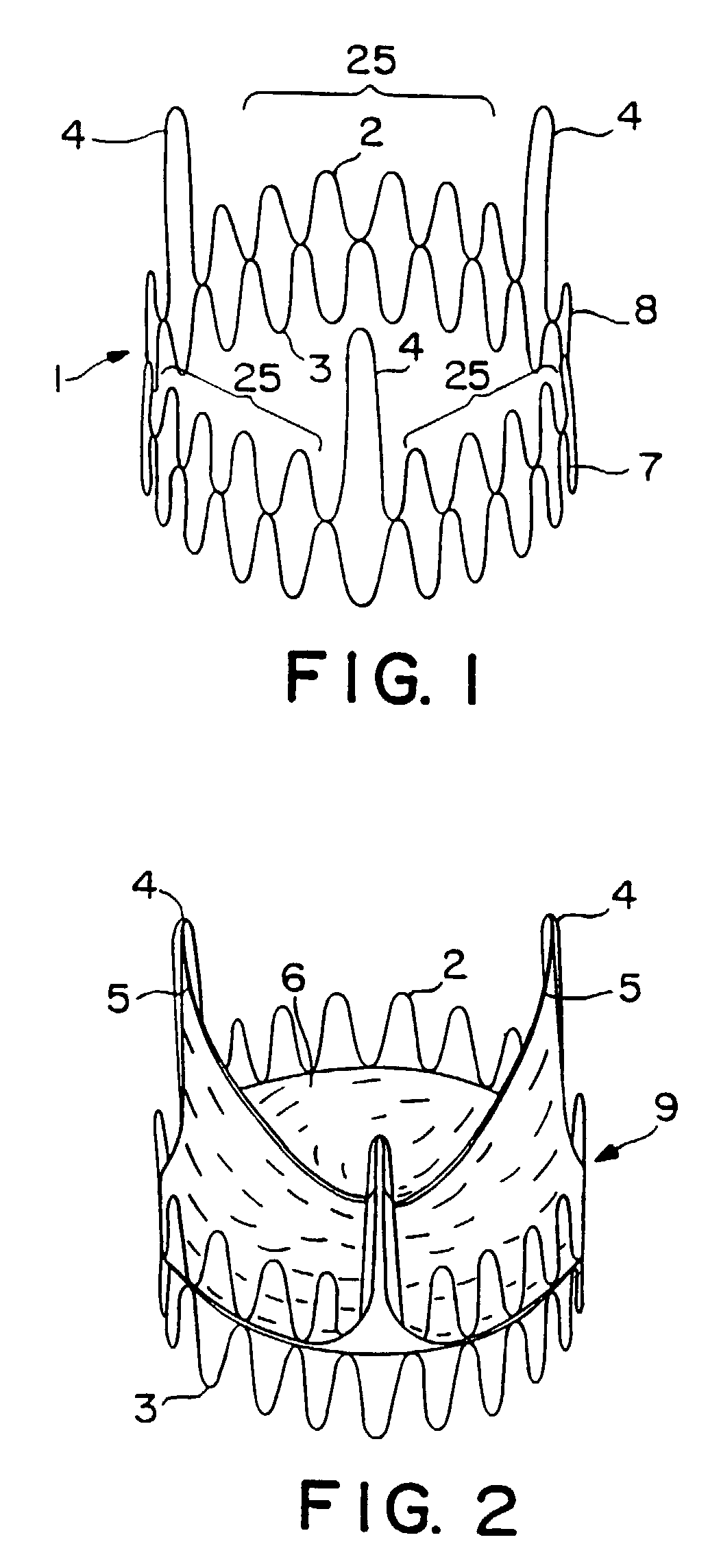
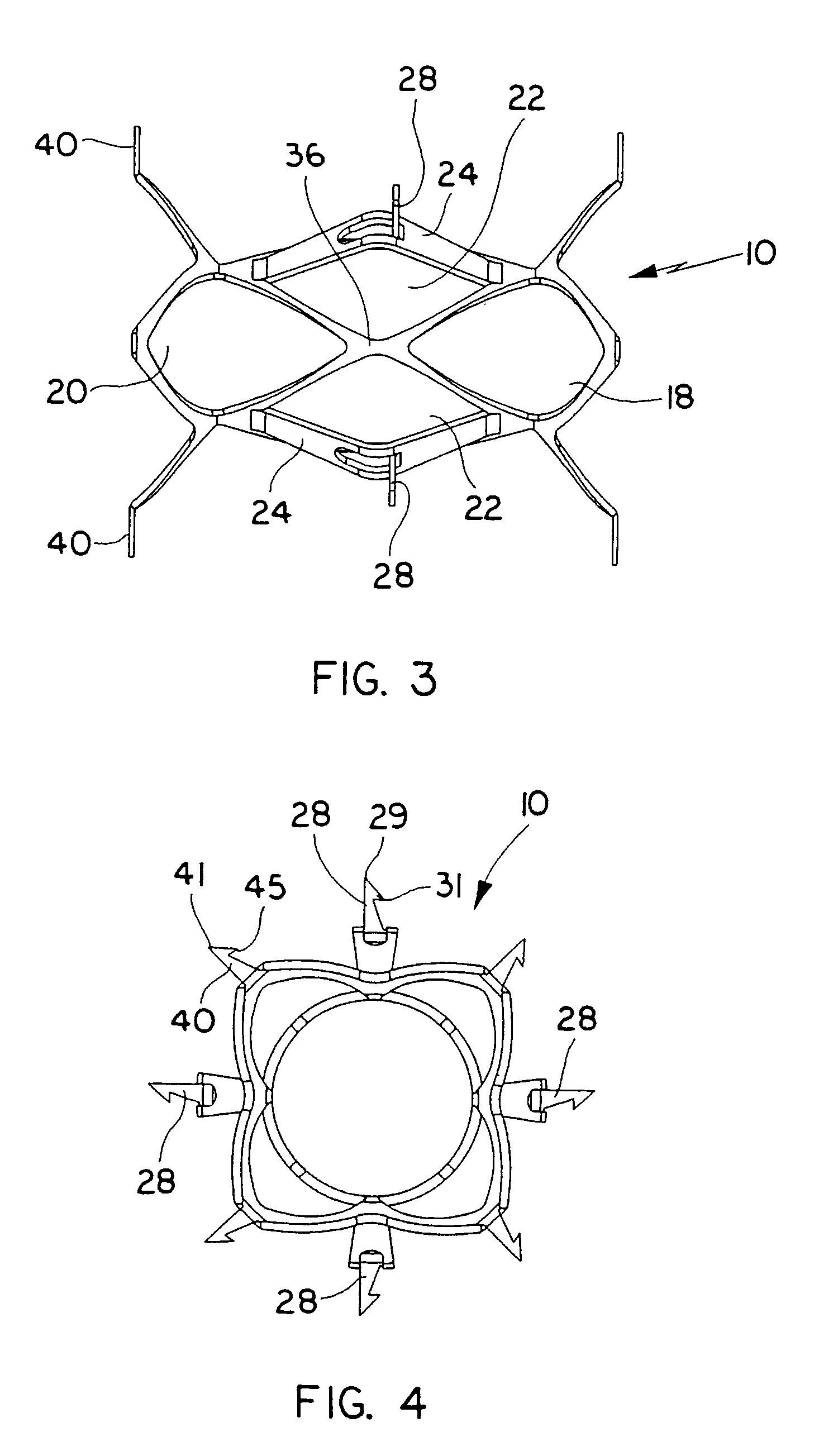
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. In a first embodiment, an expandable pre-assembled heart valve includes a plastically-expandable annular base having plurality of upstanding commissure posts. A tubular flexible member including a prosthetic section and a fabric section is provided, with the prosthetic section being connected to the commissure posts and defining leaflets therebetween, and the fabric section being attached to the annular base. In a second embodiment, an expandable heart valve includes an annular tissue-engaging base and a subassembly having an elastic wireform and a plurality of leaflets connected thereto. The annular base and subassembly are separately stored and connected just prior to delivery to the host annulus. Preferably, the leaflet subassembly is stored in its relaxed configuration to avoid deformation of the leaflets. The expandable heart valves maybe implanted using a balloon catheter. Preferably, the leaflets of the heart valves are secured to the commissure regions of the expandable stents using a clamping arrangement to reduce stress.
Owner:EDWARDS LIFESCIENCES CORP
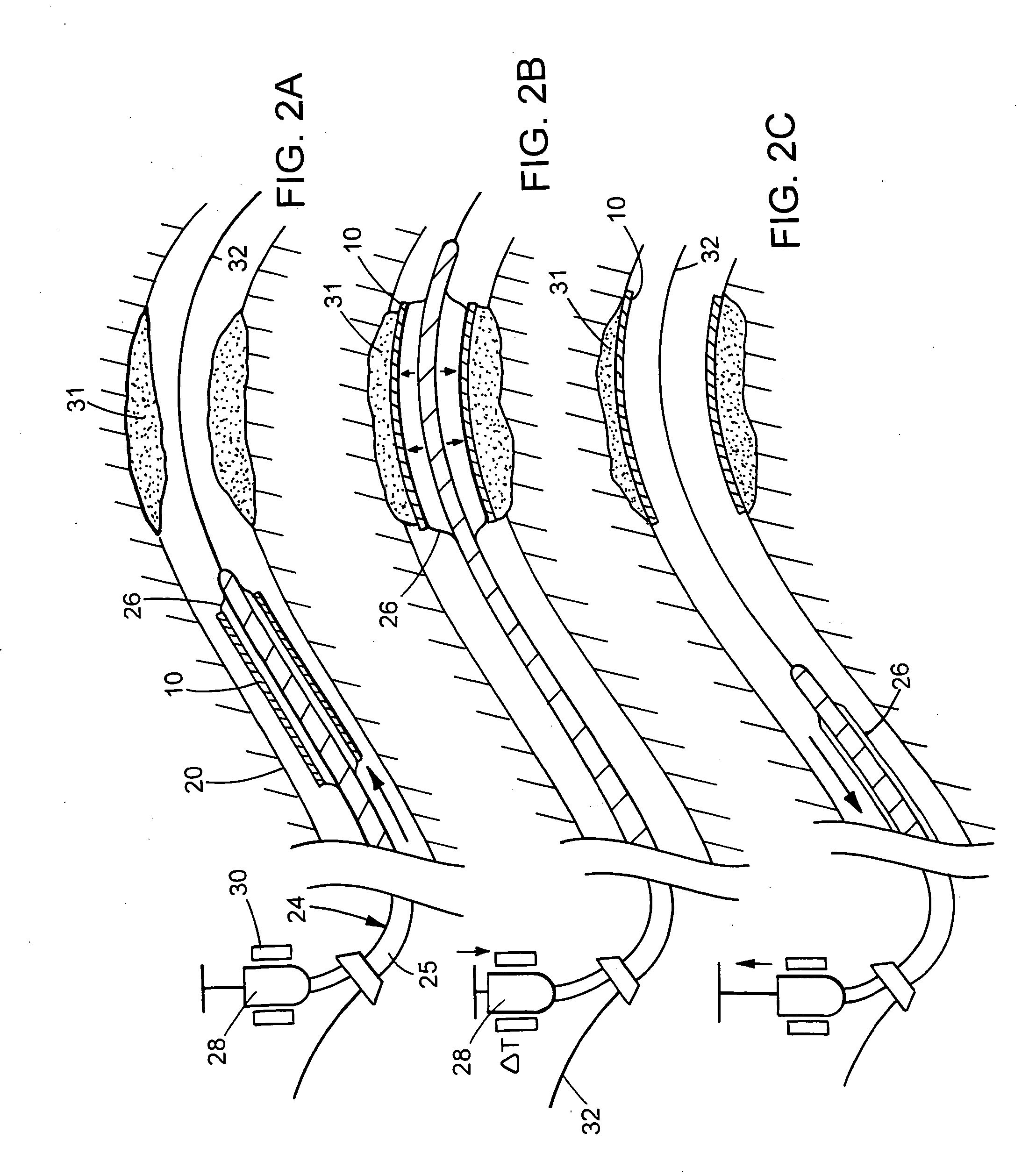
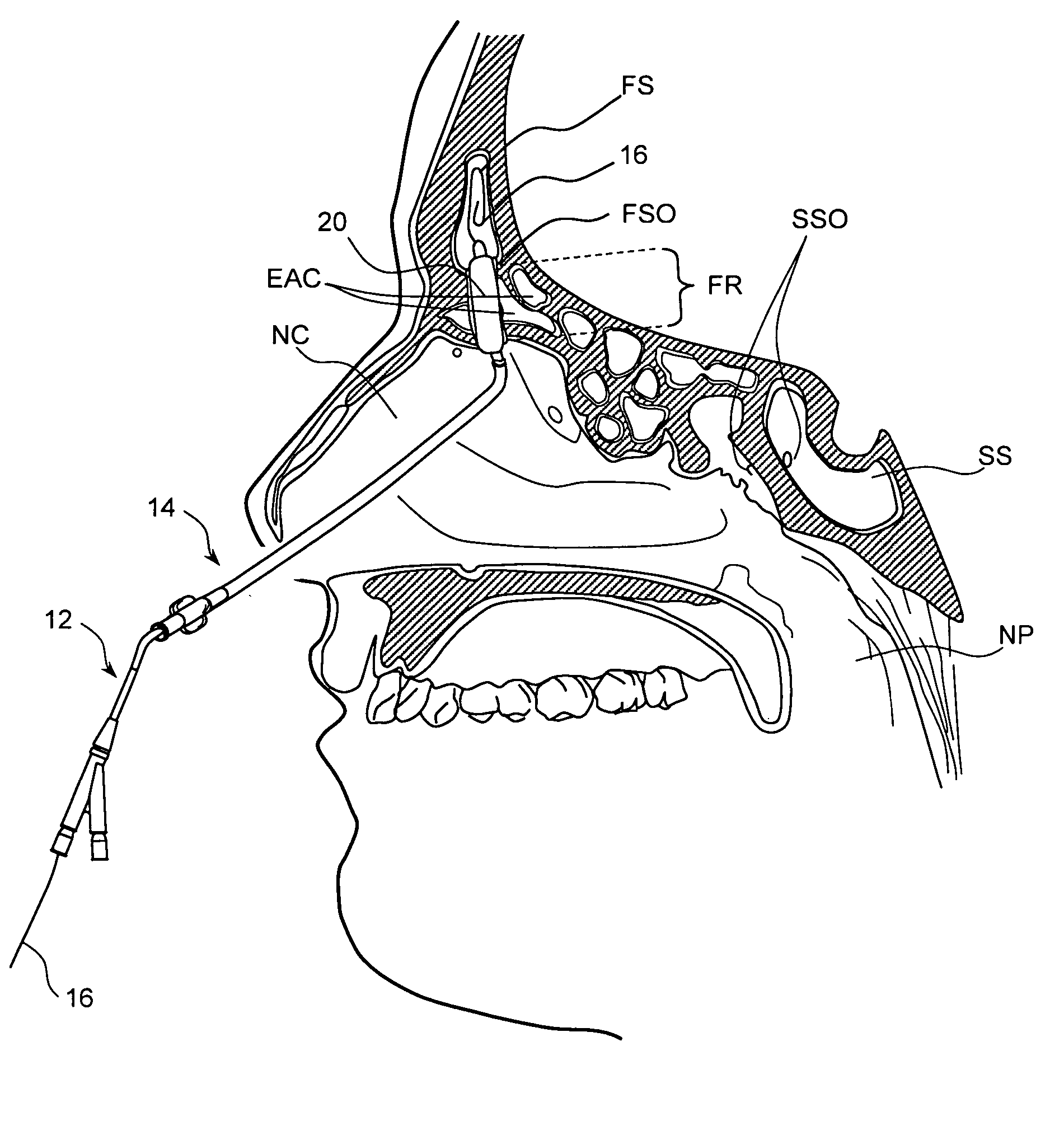
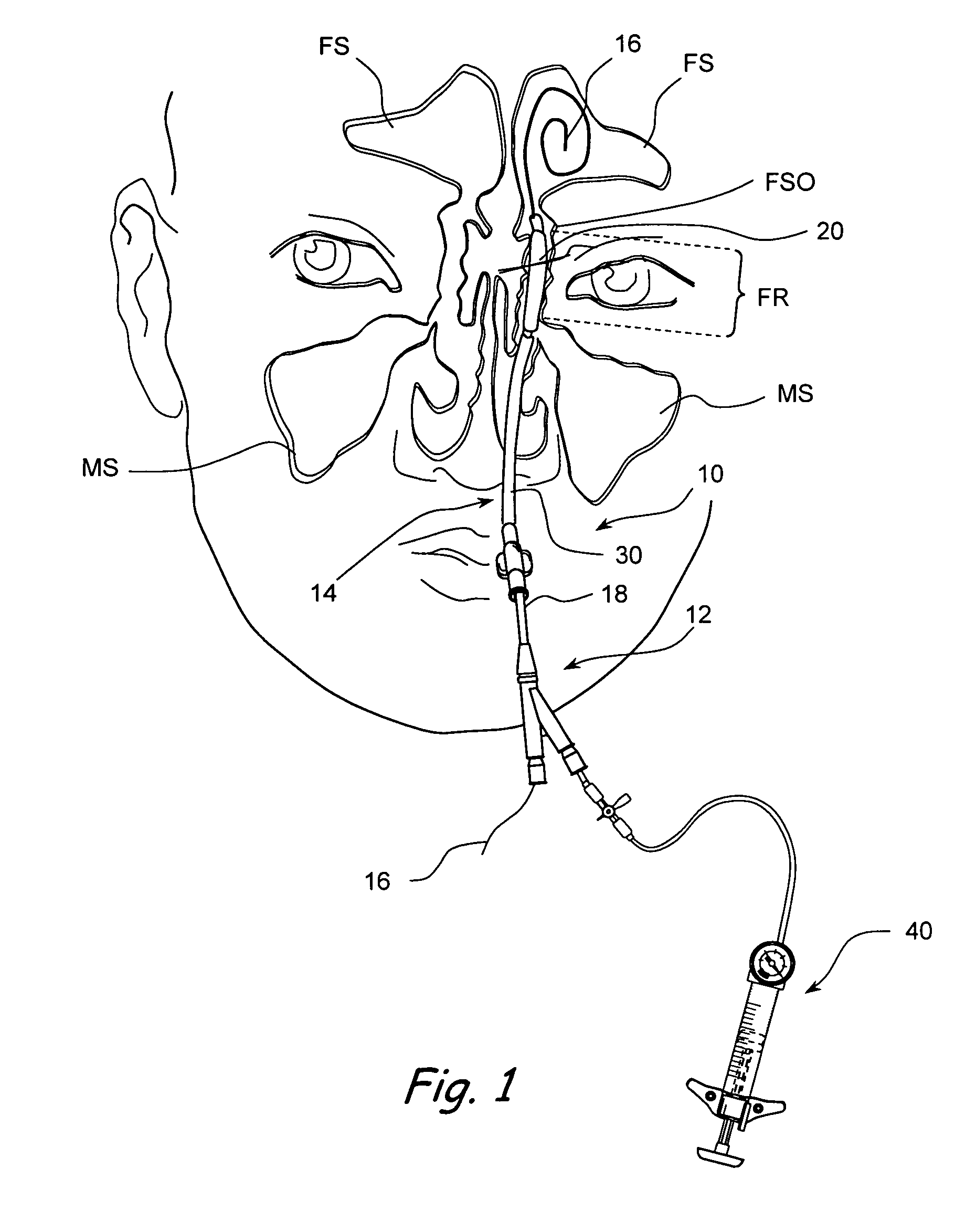
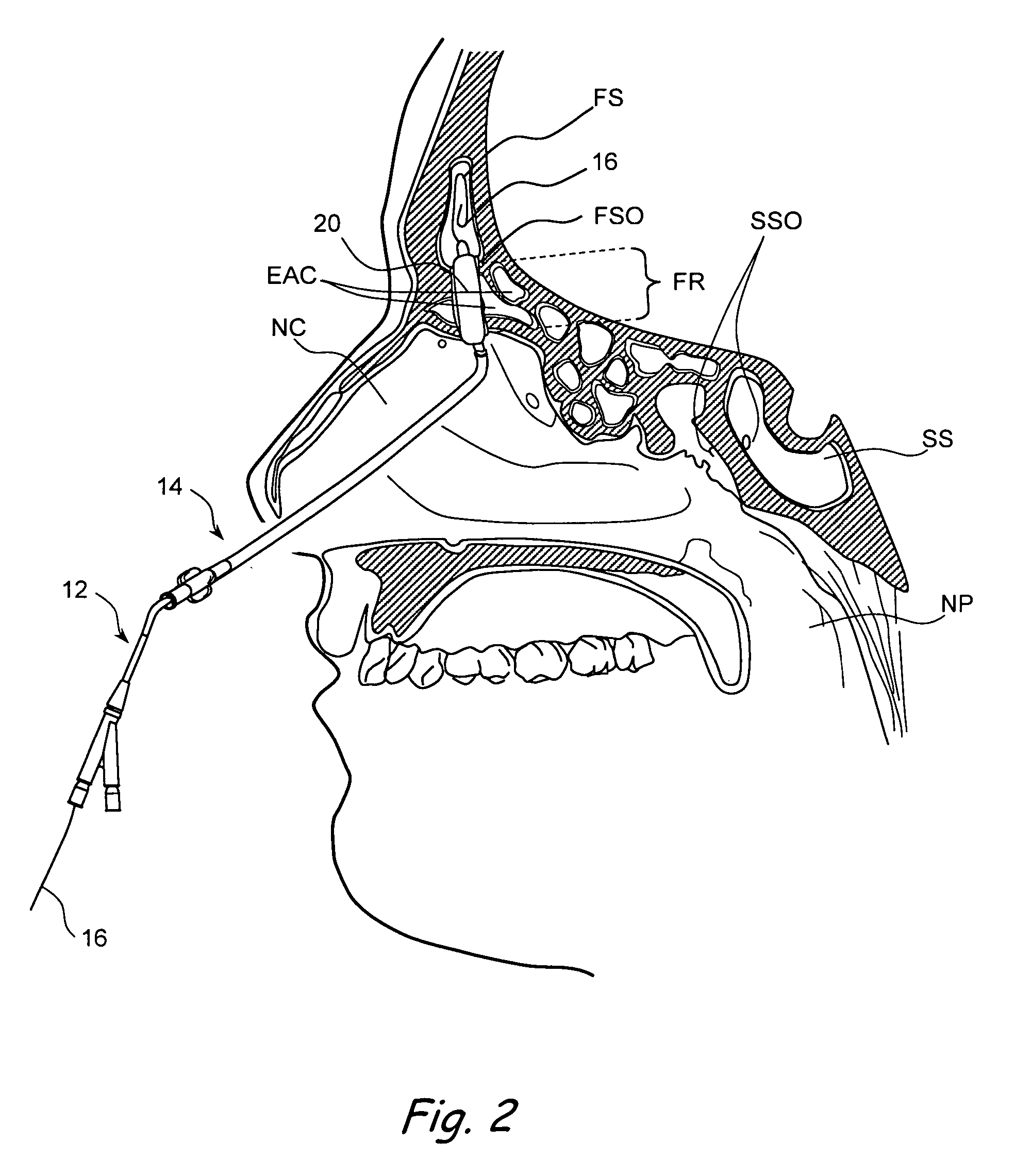
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
Kit enabling a prosthetic valve to be placed in a body enabling a prosthetic valve to be put into place in a duct in the body
The present invention is an assembly comprising a prosthetic valve to be implanted; a radially expandable stent comprising at least one zone intended to be expanded to allow the stent, in the expanded state, to bear against the wall of the body duct to be fitted with the valve, this bearing making it possible to immobilize this stent with respect to this wall; and means for mounting the valve with respect to the stent, making it possible to connect the valve to the stent in such a way that the placement of the stent allows the valve to be mounted in the body duct, and expansion means such as a balloon catheter being provided to trigger expansion of the stent at the implantation site. According to the invention, the valve and the stent are designed in such a way that, at the moment when the stent is expanded, the valve is situated outside the zone or zones of the stent that are subjected to said expansion means. The invention thus consists in separating the valve and said zone or zones to be expanded, so that the expansion of the stent can be effected with an expansion force suitable for perfect anchoring of this stent in the wall of the body duct to be fitted with the valve, and without any risk of destruction or damage of the valve.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Valve prosthesis for implantation in the body and a catheter for implanting such valve prosthesis
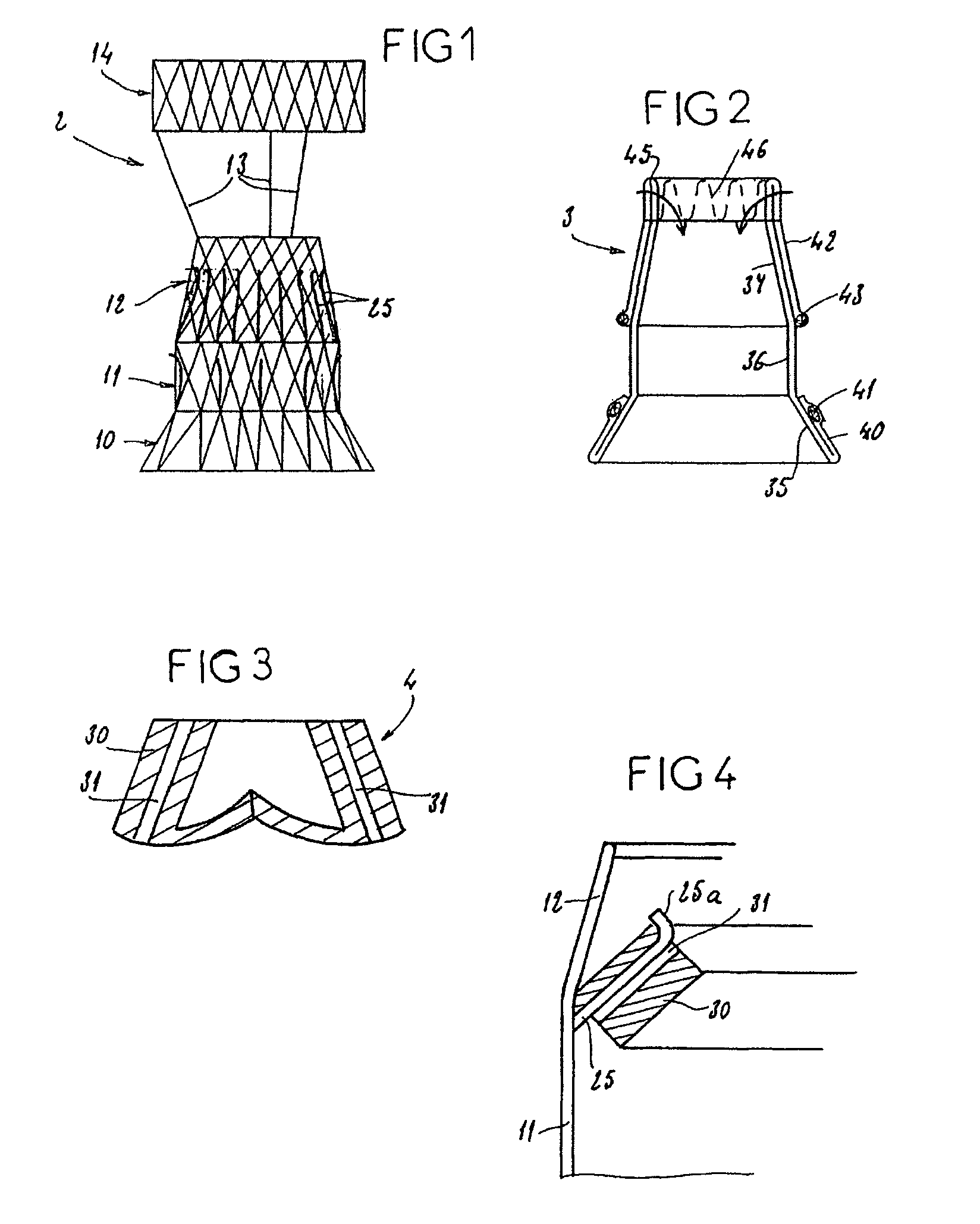
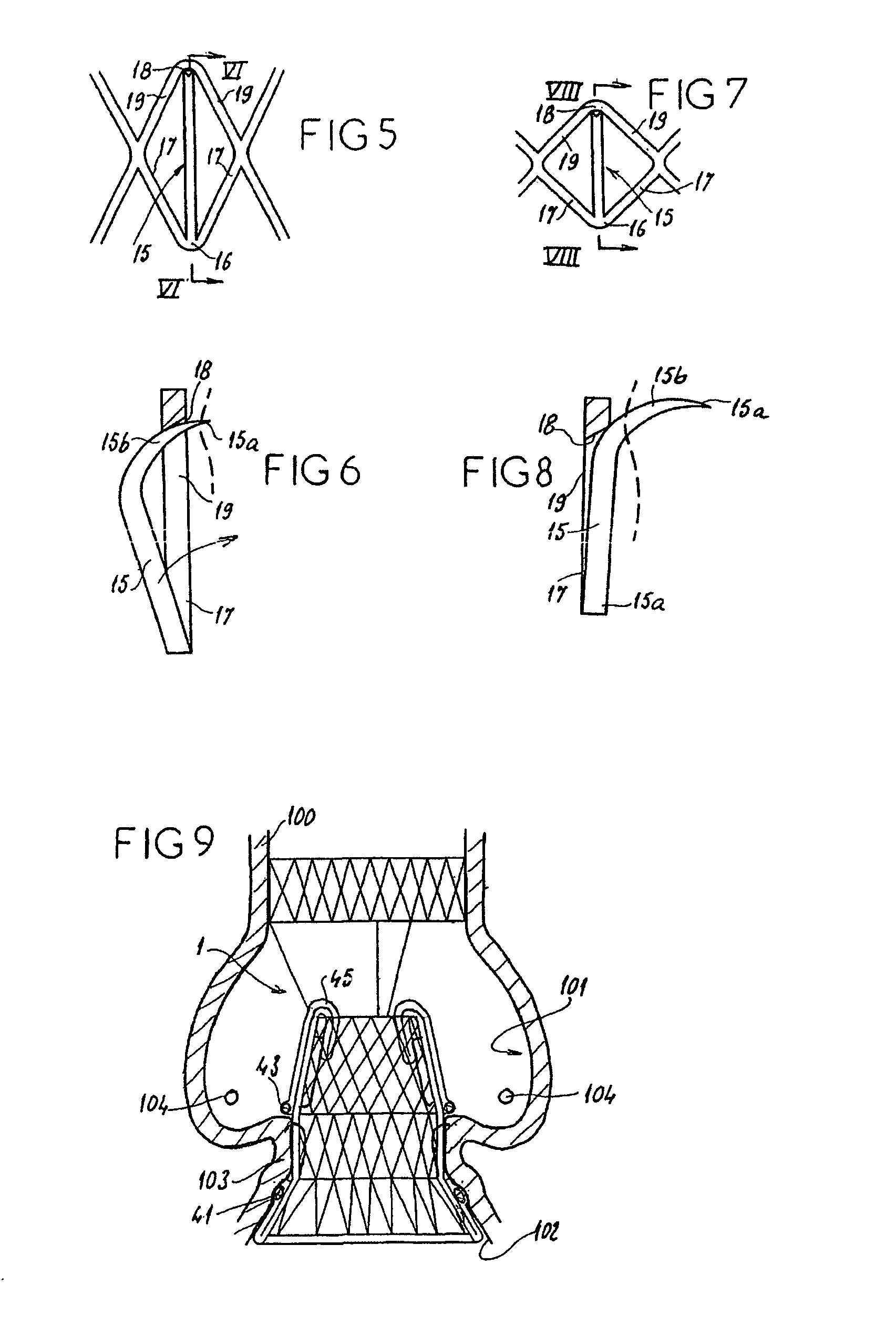
InactiveUS7618446B2Inhibition of contractionEasy to shapeStentsBalloon catheterSurgical operationThoracic structure
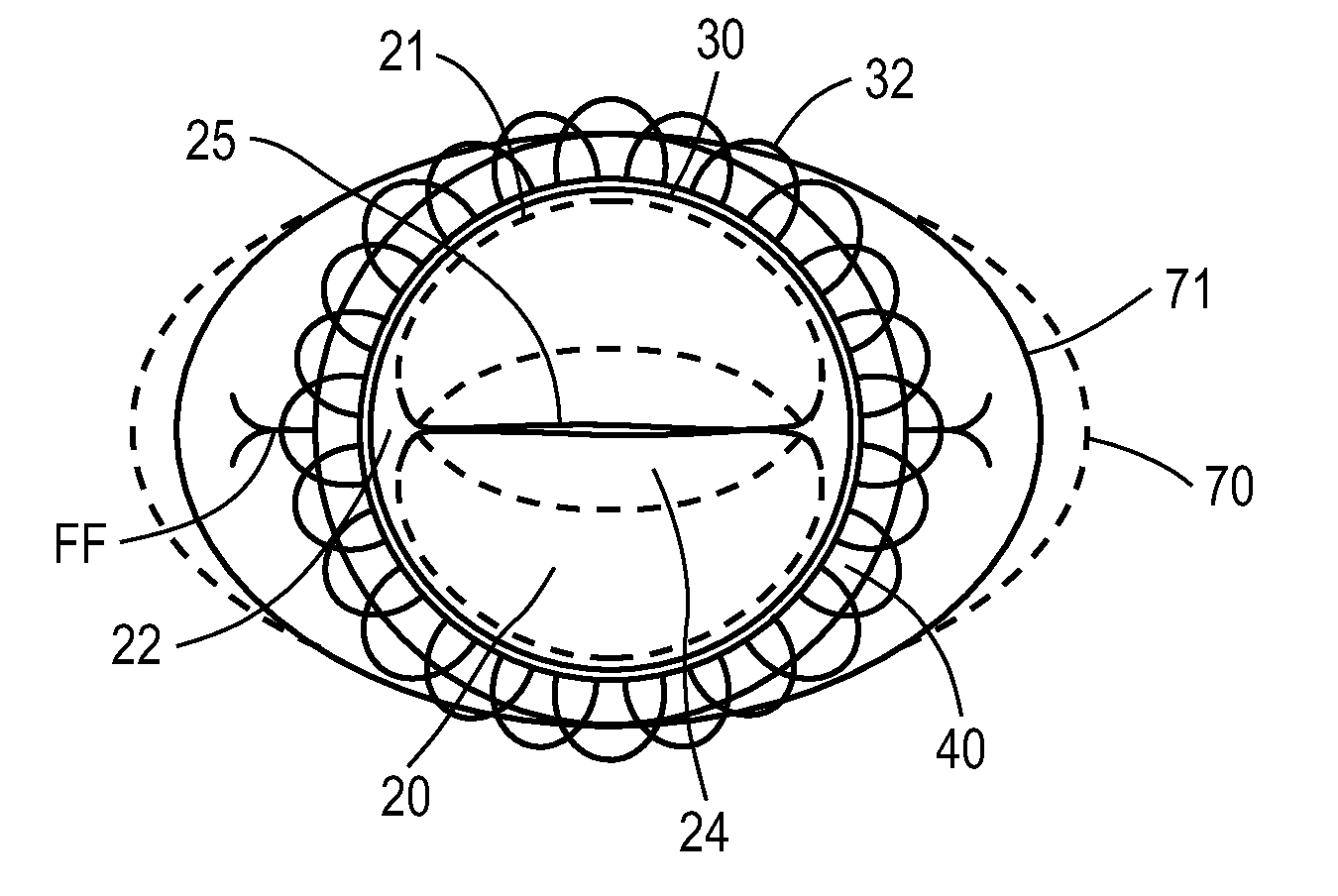
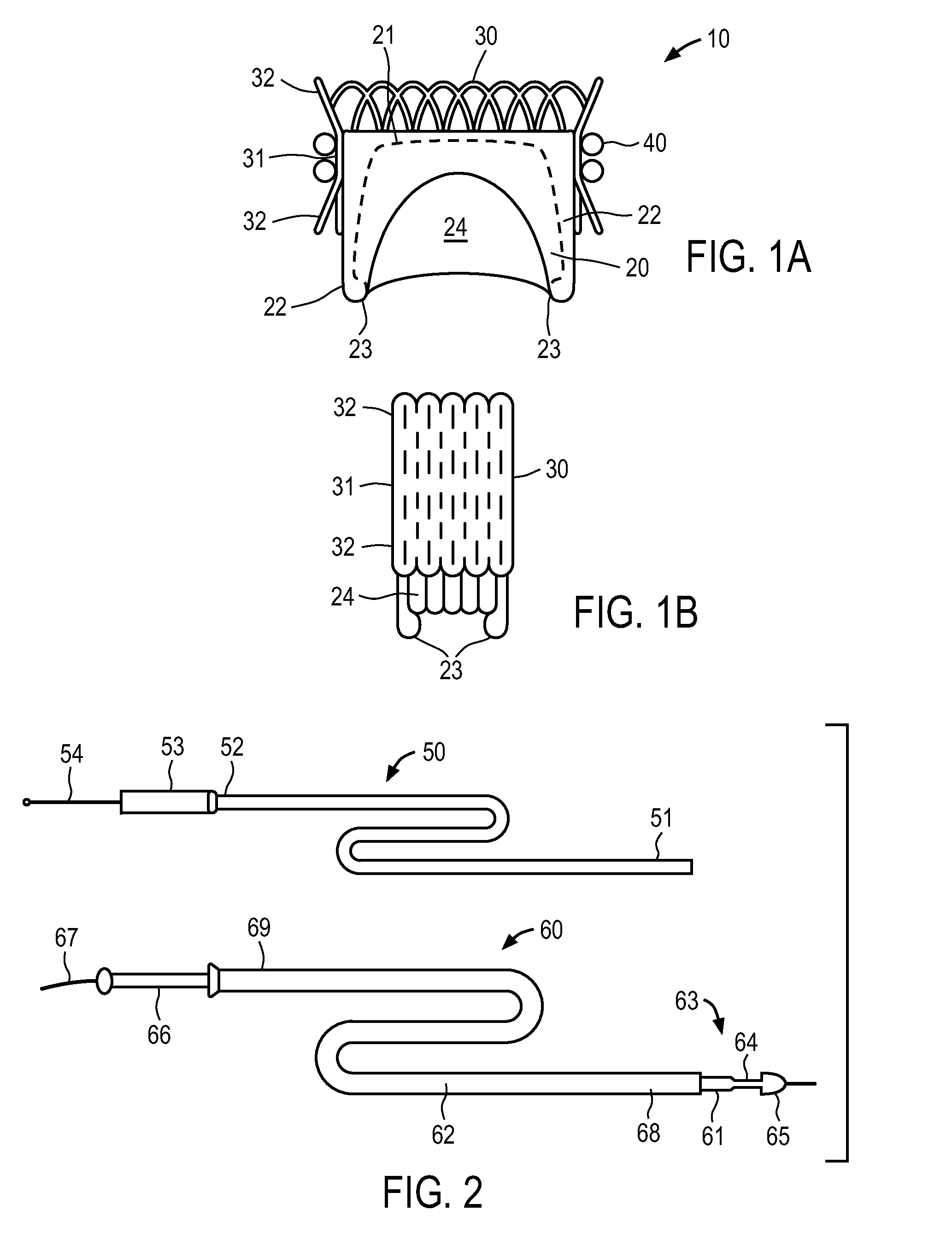
A valve prosthesis for implantation in the body by use of a catheter includes a stent made from an expandable cylinder-shaped thread structure including several spaced apices. The elastically collapsible valve is mounted on the stent as the commissural points of the valve are secured to the projecting apices. The valve prosthesis can be compressed around balloons of the balloon catheter and inserted in a channel, for instance, in the aorta. When the valve prosthesis is placed correctly, the balloons are inflated to expand the stent and wedge it against the wall of the aorta. The balloons are provided with beads to ensure a steady fastening of the valve prosthesis on the balloons during insertion and expansion. The valve prosthesis and the balloon catheter make it possible to insert a cardiac valve prosthesis without a surgical operation involving opening the thoracic cavity.
Owner:EDWARDS LIFESCIENCES AG
Catheter balloon with ultrasonic microscalpel blades
InactiveUS7153315B2Much sharper and cleaner incisionsCutting precision can be improvedCannulasSurgical instrument detailsBalloon catheterMedical treatment
The present invention provides a catheter balloon, and balloon catheter incorporating the catheter balloon, useful in medical dilation procedures. The catheter balloon includes at least one microscalpel operatively disposed on an outer surface thereof. The microscalpel may advantageously be operatively disposed relative to a power source so as to be controllably activatable. Also provided are methods of making the inventive balloon and / or catheter as well as methods of using the inventive catheter in a dilation / incising treatment.
Owner:BOSTON SCI SCIMED INC
Devices, systems and methods for treating disorders of the ear, nose and throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Apparatus and method for cryogenic inhibition of hyperplasia
InactiveUS6355029B1Enhanced inhibitory effectEasy to controlCatheterSurgical instruments for coolingPercutaneous angioplastyBalloon catheter
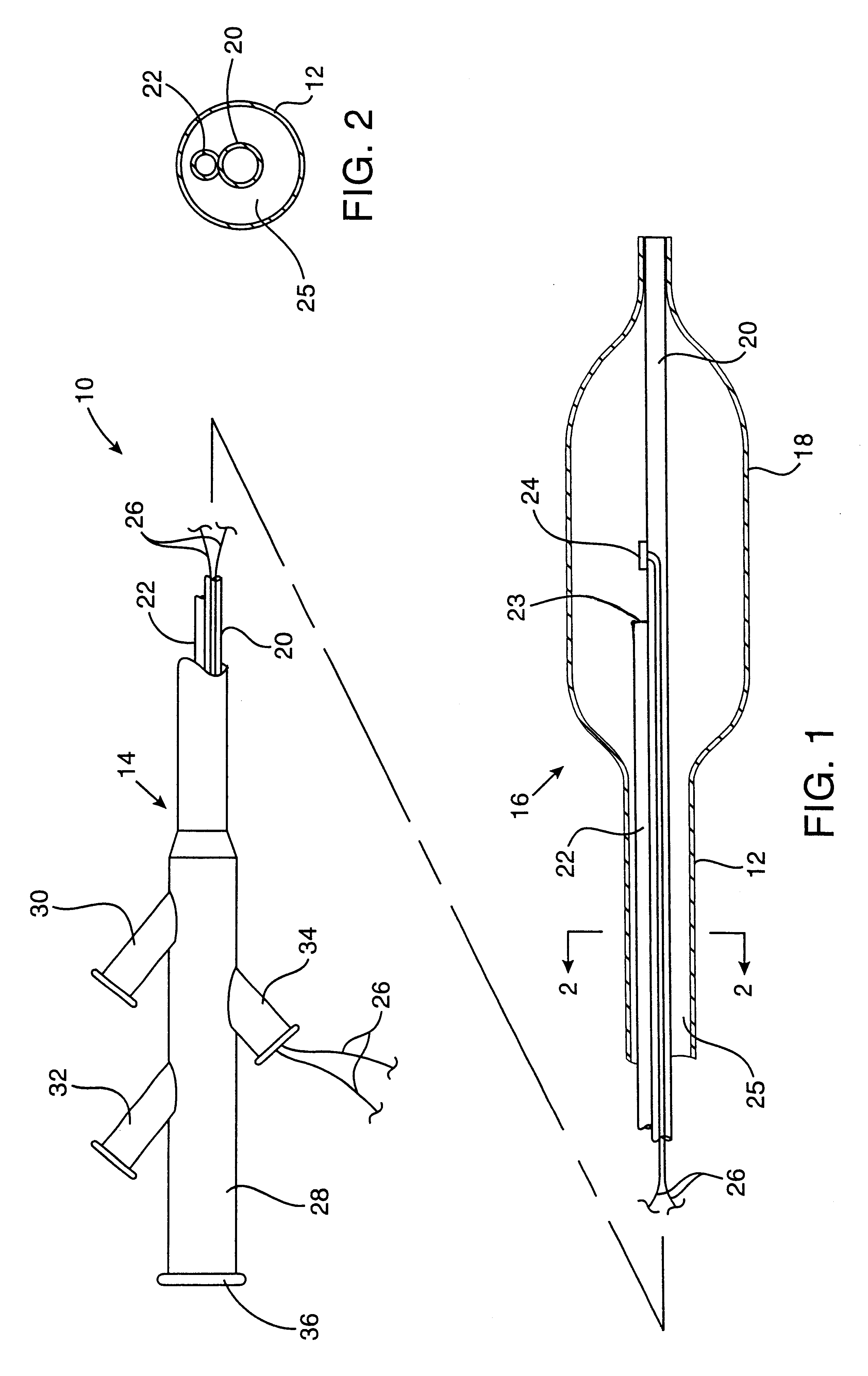
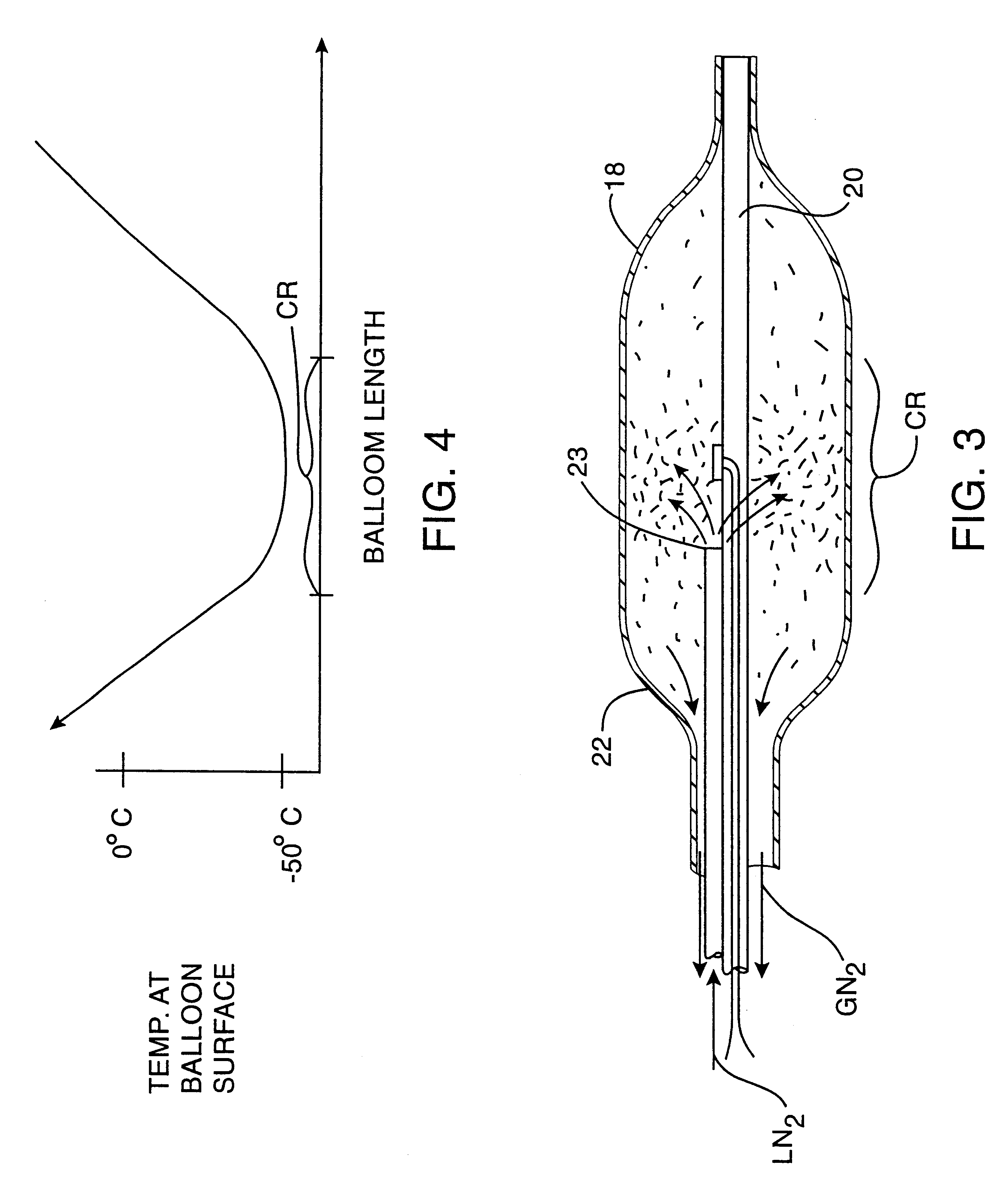
Post-angioplasty hyperplasia in blood vessels is treated using a cryosurgical balloon catheter. The balloon catheter is positioned at a target region within the blood vessel, and the balloon inflated by expanding a cryogenic fluid, such as liquid nitrogen, across an expansion orifice into a balloon. The balloon will be constructed so that cooling is achieved primarily in the central regions of the balloon, with the proximal and distal regions being less cold and acting to insulate adjacent regions of the blood vessel from excessive cooling.
Owner:BOSTON SCI SCIMED INC
Electrically induced vessel vasodilation
InactiveUS6865416B2High strengthSufficient durationNanotechElectrotherapyElectricityDouble balloon catheter
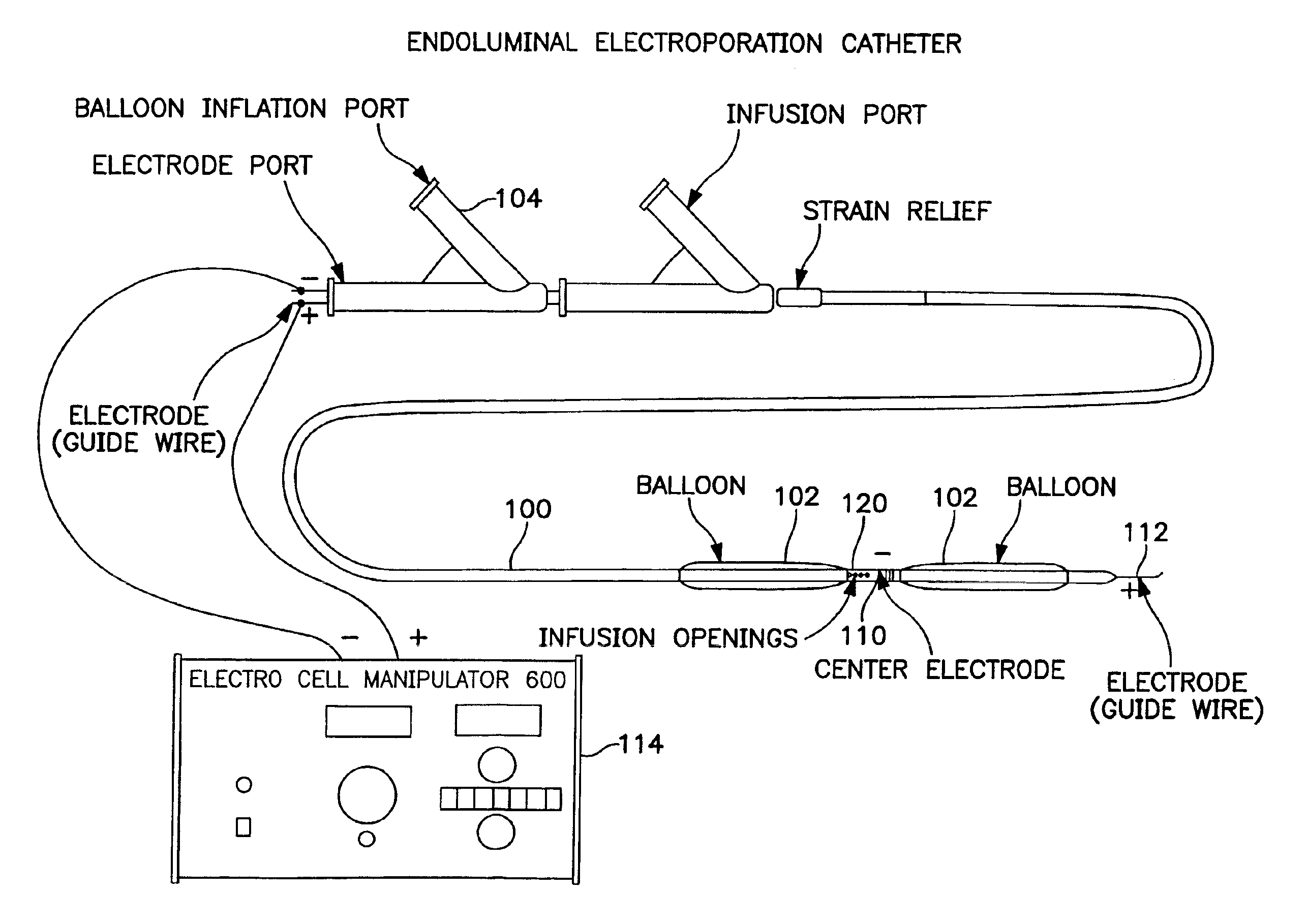
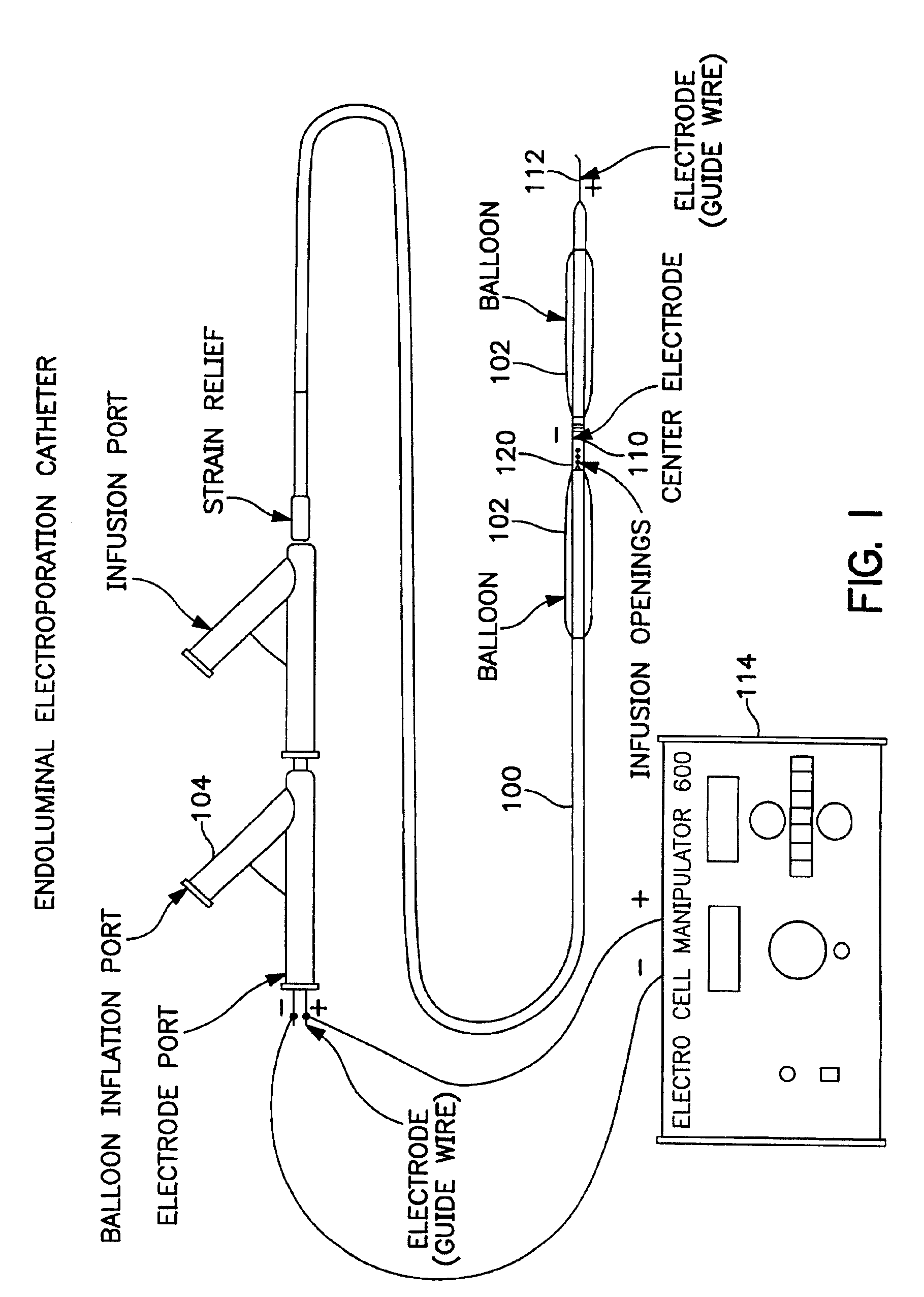
The invention provides methods for inducing or increasing the vasodilation of a vessel. The invention further provides methods for inducing or increasing the flow of fluid through a vessel. An electrical impulse is applied to the vessel in order to induce or increase vessel vasodilation or to induce or increase the flow of fluid through the vessel. In a particular embodiment, a novel double-balloon catheter system incorporating electroporation technology has been designed and is used to apply the electrical impulse endoluminally.
Owner:ONCOSEC MEDICAL
Methods and systems for treating ischemia
InactiveUS6295990B1Promote dissolution and removalMinimize and prevent ischemiaStentsBalloon catheterControl mannerDecreased mean arterial pressure
Methods for treating total and partial occlusions employ a perfusion conduit which is penetrated through the occlusive material. Oxygenated blood or other medium is then perfused through the conduit in a controlled manner, preferably at a controlled pressure below the arterial pressure, to maintain oxygenation and relieve ischemia in tissue distal to the occlusion. In another aspect, interventional devices, such as stents or balloon catheters, are passed through the perfusion catheter to remove obstructions. Optionally, the occlusion may be treated while perfusion is maintained, typically by introducing a thrombolytic or other agent into the occlusive material using the perfusion conduit or by employing mechanical means to remove the obstruction. Such methods are particularly suitable for treating acute stroke to prevent damage to the cerebral tissue.
Owner:SALIENT INTERVENTIONAL SYST
Devices, systems and methods useable for treating sinusitis
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Controlled deployment of a medical device
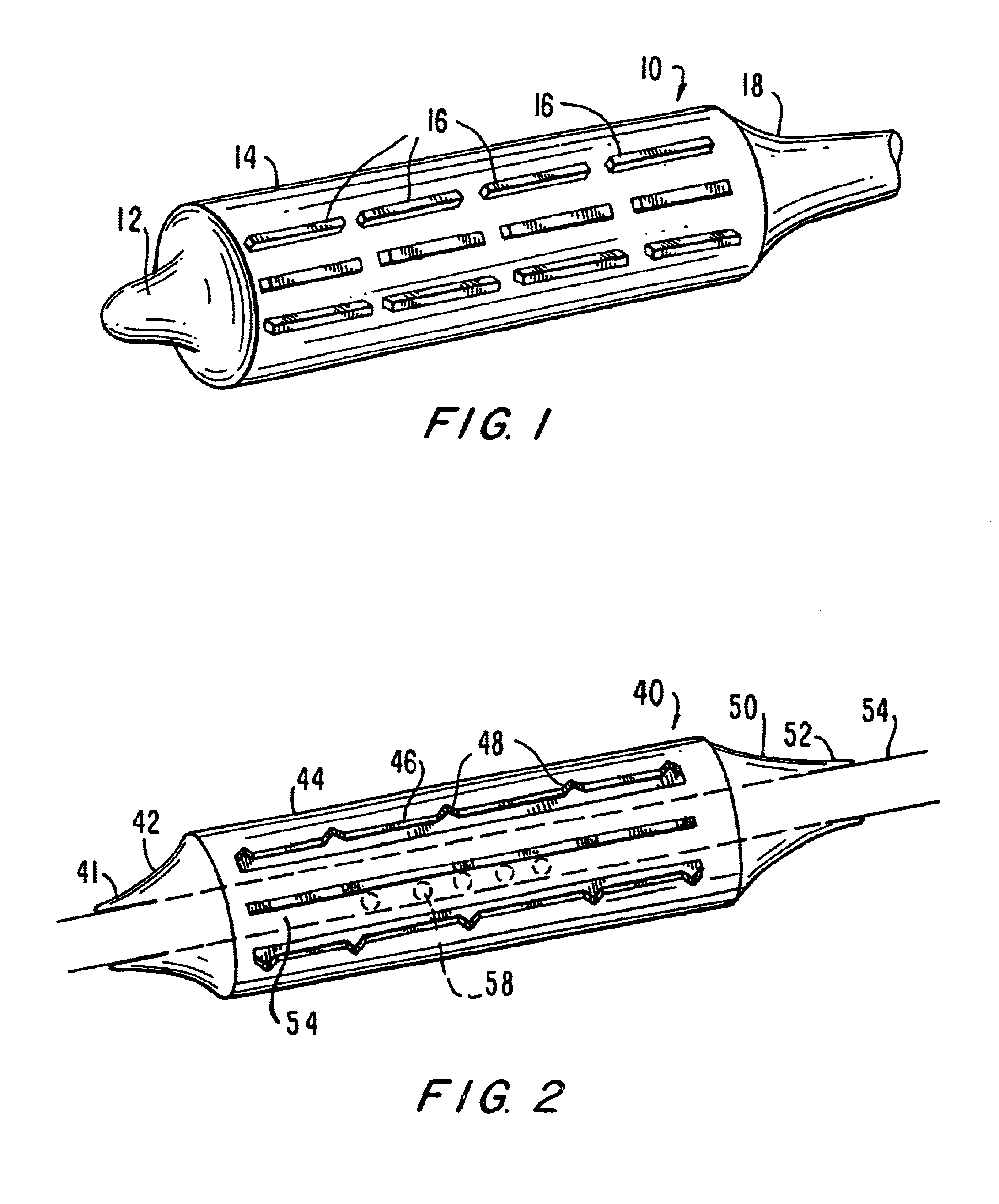
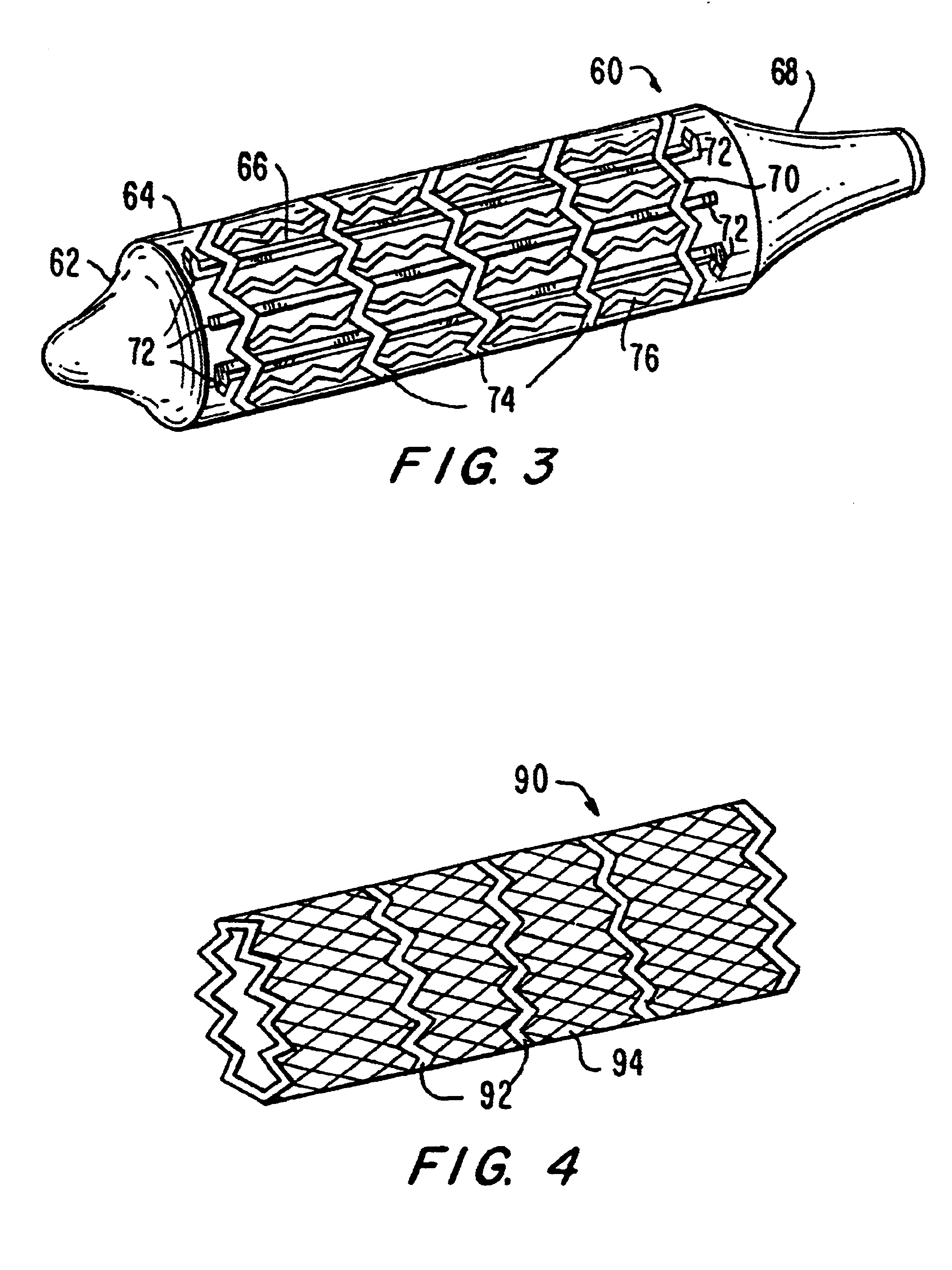
A prosthesis delivery system having a balloon catheter with an inflatable balloon on its exterior. The balloon is inflatable by injection of fluid through a lumen in the catheter and the balloon is initially partially constrained against inflation by a constraint. A tubular prosthesis is disposed on the catheter over at least a portion of the balloon and a portion of the constraint. The tubular prosthesis has a contracted condition and an expanded condition. The tubular prosthesis is initially disposed on the catheter in the contracted condition. Further, a balloon catheter includes a constraint so that the balloon may be sequentially inflated for dilatation purposes such as in a valvuloplasty operation.
Owner:BOSTON SCI SCIMED INC
Implantable medical devices
InactiveUS20050010275A1Improve abilitiesIncrease resistanceStentsWound drainsInsertion stentBalloon catheter
A medical device includes a balloon catheter having an expandable member, e.g., an inflatable balloon, at its distal end and a stent or other endoprosthesis. The stent is, for example, an apertured tubular member formed of a polymer and is assembled about the balloon. The stent has an initial diameter for delivery into the body and can be expanded to a larger diameter by inflating the balloon.
Owner:UNIV OF CONNECTICUT +2
Devices, systems and methods useable for treating frontal sinusitis
Devices, systems and methods wherein a dilator, such as a balloon or other expandable member, is positionable within the frontal sinus ostium and adjacent frontal recess and useable to dilate the frontal sinus ostium and substantially all of the frontal sinus recess without requiring repositioning and repeated re-expansion of the dilator. One balloon catheter device of the invention comprises a catheter body that is less than about 50 cm in length (and in some embodiments less than 25 cm in length and a semi-compliant or non-compliant balloon on the catheter body. The balloon may have a working length of about 12 mm to about 30 mm and a width at its widest point when fully inflated of about 2 mm to about 7 mm. Such balloon may be constructed to withstand inflation pressures of about 12 atmospheres. In some embodiments, the dilator is advanced through or over a guide (e.g., guidewire or guide catheter) that has a preformed shape.
Owner:ACCLARENT INC
Methods of using an intravascular balloon catheter in combination with an angioscope
A method of performing a medical procedure is disclosed which involves the use of a balloon catheter in combination with an angioscope to monitor an intravascular stent before, during or after deploying the stent. Specifically, the method may include the steps of (1) delivering an intravascular stent into the vasculature of a patient, (2) positioning a balloon catheter in the vasculature such that the inflatable balloon is adjacent the stent, (3) inserting an angioscope into the balloon catheter such that the distal end of the angioscope is adjacent an optically-transparent tube traversing the interior of the balloon, and (4) visually monitoring the stent before, during or after deploying the intravascular stent. The optically-transparent tube may define a guide wire lumen for use with a guide wire and the angioscope may be inserted into the guide wire lumen. The intravascular stent may be a balloon-expandable stent, a self-expanding stent, or a stent made of a photo-responsive polymer.
Owner:BOSTON SCI SCIMED INC
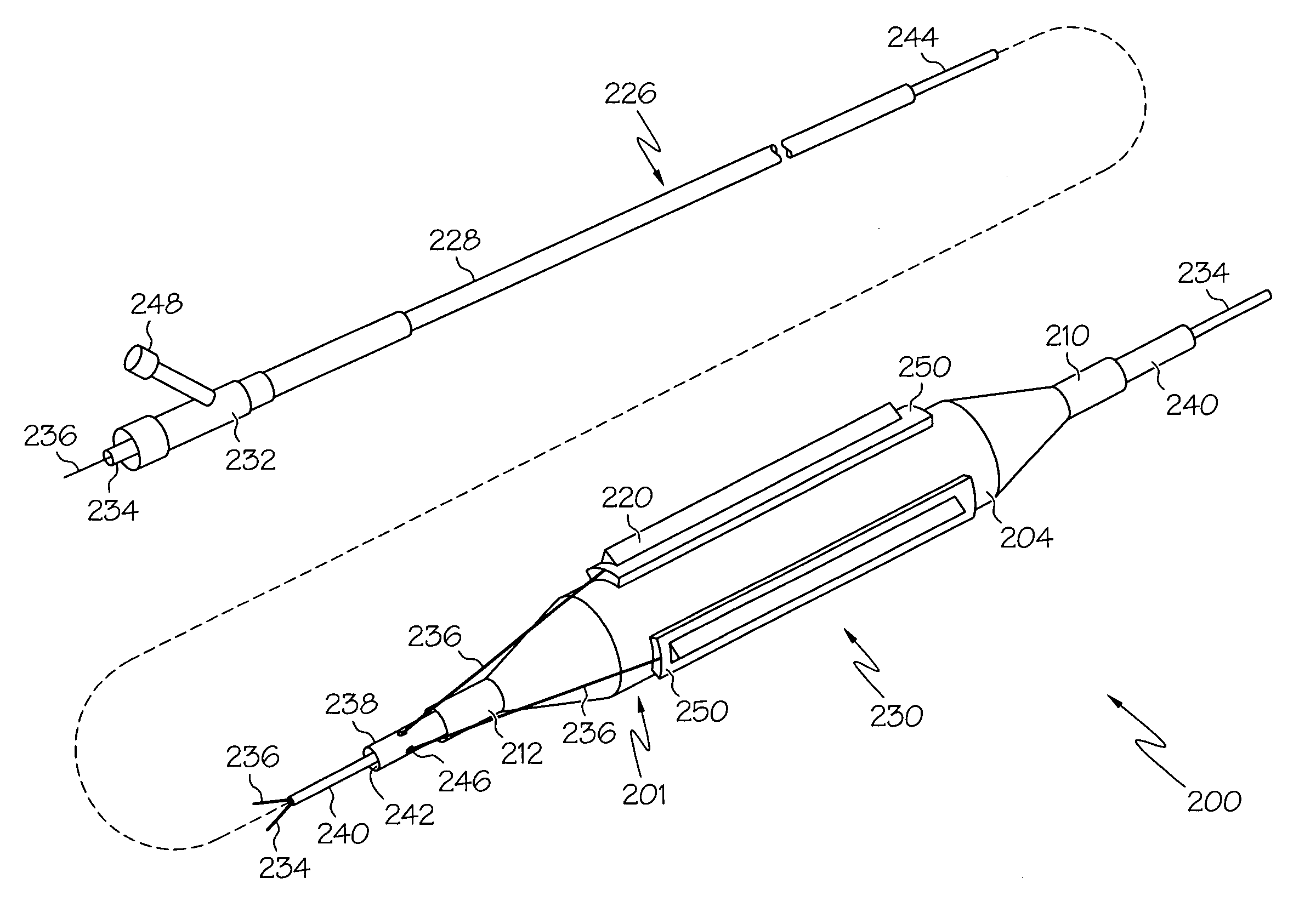
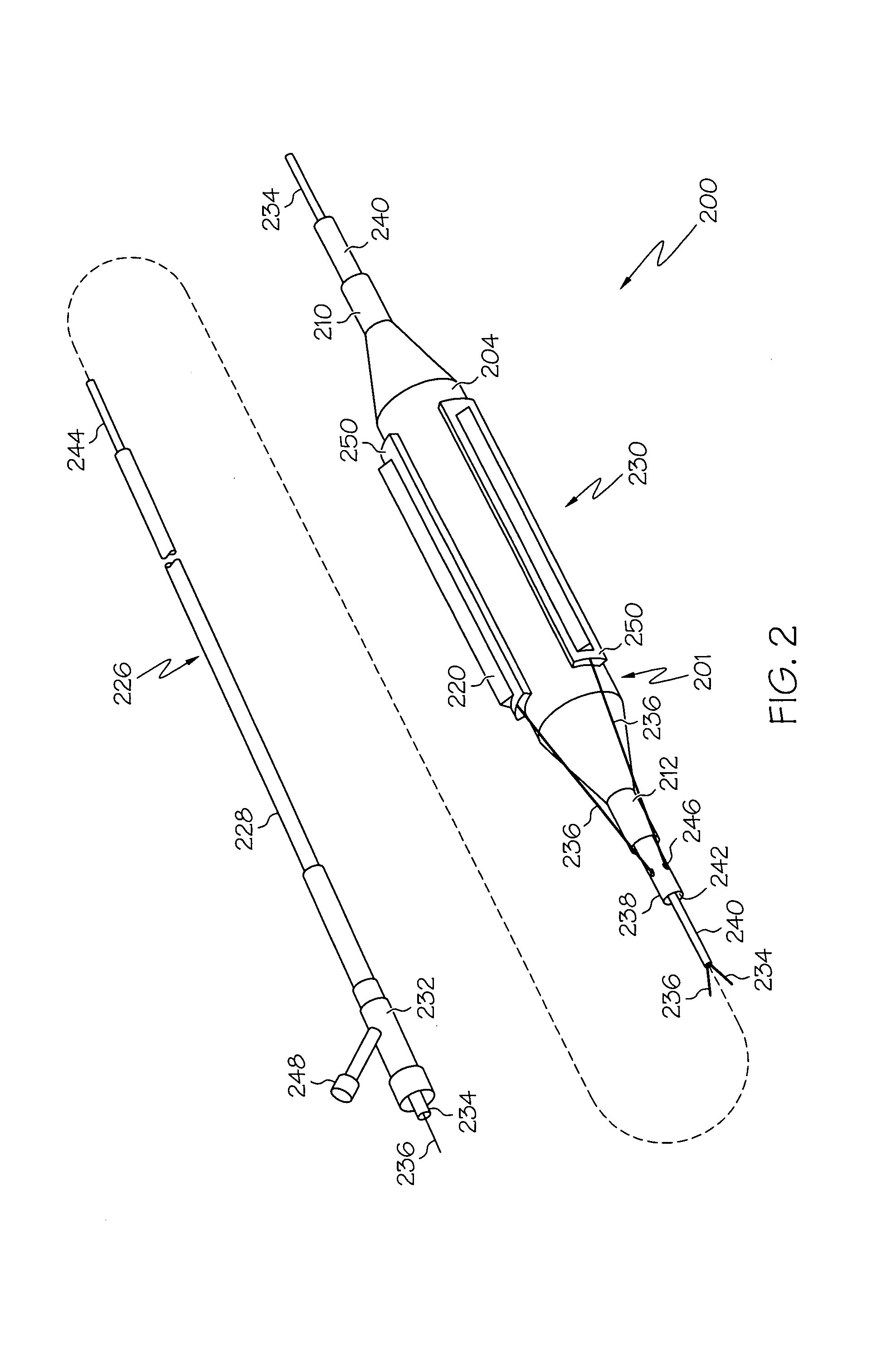
Prosthetic Valve Delivery System
A prosthetic valve delivery system for percutaneously delivering and deploying a prosthetic valve within an existing valve is disclosed. The delivery system includes a stented prosthetic valve having a balloon-expandable stent portion with a prosthetic valve disposed therein and at least one self-expanding stent portion. The delivery system further includes a dual balloon catheter having a first balloon on which the stented prosthetic valve is disposed during delivery and a second balloon. Upon delivery within the existing valve, the self-expanding stent portion contacts the existing valve and the first balloon expands the balloon-expandable stent portion to a first diameter such that the stented prosthetic valve is in a first stage deployment configuration. The second balloon then expands the balloon-expandable stent portion to a second diameter, greater than the first diameter, such that the stented prosthetic valve is in a second stage deployment configuration being fully deployed within the existing valve.
Owner:MEDTRONIC VASCULAR INC
System for percutaneous delivery and removal of a prosthetic valve
A valve-retrieval device permits a non-deployed valve mounted on a balloon catheter to be retracted back into an introducer sheath for removal from a patient's body. In particular embodiments, the valve-retrieval device is adapted to be placed on a balloon catheter shaft and then advanced over the shaft into the blood vessel via the introducer sheath. The valve-retrieval device has an expandable distal end portion that assumes an expanded shape when advanced out of the introducer sheath. The valve is positioned within or adjacent the distal end portion of the retrieval device, and the retrieval device and the balloon catheter are retracted together back into the introducer sheath. The distal end portion of the retrieval device, rather than the outer surface portion of the valve covered thereby, contacts the distal end and inner surface of the introducer sheath to facilitate retraction of the valve into the introducer sheath.
Owner:EDWARDS LIFESCIENCES CORP
Power generating and control apparatus for the treatment of tissue
ActiveUS20120095461A1Calculate system impedanceExact impedanceUltrasound therapyElectrotherapyElectrode ContactBalloon catheter
Apparatus, systems, and methods are provided for the generation and control of energy delivery in a dosage to elicit a therapeutic response in diseased tissue. A balloon catheter can have electrodes attached to a power generator and controller such that the balloon and electrodes contact tissue during energy treatment. Energy selectively may be applied to tissue based on measured impedance to achieve gentle heating. Calibration of the apparatus and identification of attached accessories by computing the circuit impedance prior to energy dosage facilitate regulation of power delivery about a set point. Energy delivery can be controlled to achieve substantially uniform bulk tissue temperature distribution. Energy delivery may beneficially affect nerve activity.
Owner:BOSTON SCI SCIMED INC
Cardiac valve repair system and methods of use
ActiveUS8657872B2Reduce the overall diameterReduce exerciseBalloon catheterAnnuloplasty ringsInsertion stentHeart valve repair
Methods and apparatus are provided for repairing or replacing a defective cardiac valve including an anchor having a double helix configured to engage the cardiac valve leaflets of a diseased or defective cardiac valve, and a replacement valve body disposed in an expandable stent configured to be disposed within the anchor so that the anchor limits expansion of the expandable stent. The expandable stent of the replacement valve body may be self-expanding or mechanically expanded, e.g., using a balloon catheter or catheter-based mandrel and the valve body may be formed of animal tissue or a synthetic fabric.
Owner:BMEYE
Apparatus and methods for treating congestive heart disease
InactiveUS7335192B2Increase blood flowImprove kidney functionBalloon catheterOther blood circulation devicesInsertion stentPeri-aortic
Methods and apparatus are provided for treating congestive heart by actively or passively enhancing perfusion to the renal arteries. A first embodiment comprises a specially configured balloon catheter and extracorporeal pump, wherein the pump operates in a “once-through” fashion or alternating volume displacement mode. In another embodiment the catheter includes a pair of balloons to isolate a region of the aorta, and a third balloon that directs flow into the renal arteries. In still further embodiments, a stent or cuff having a constricted region is deployed in or around the aorta, respectively, to create a backpressure upstream of the stent or cuff. Methods of enhancing renal perfusion also are provided.
Owner:ANGIODYNAMICS INC
Circumferential ablation device assembly and methods of use and manufacture providing an ablative circumferential band along an expandable member
InactiveUS6954977B2Strong connectionConsistent positionElectrotherapyDiagnosticsFluoropolymerBalloon catheter
A medical balloon catheter assembly includes a balloon having a permeable region and a non-permeable region. The balloon is constructed at least in part from a fluid permeable tube such that the permeable region is formed from a porous material which allows a volume of pressurized fluid to pass from within a chamber formed by the balloon and into the permeable region sufficiently such that the fluid may be ablatively coupled to tissue engaged by the permeable region. The non-permeable region is adapted to substantially block the pressurized fluid from passing from within the chamber and outwardly from the balloon. The porous material may be a porous fluoropolymer, such as porous polytetrafluoroethylene, and the pores may be created by voids that are inherently formed between an interlocking node-fibril network that makes up the fluoropolymer. Such voids may be created according to one mode by expanding the fluoropolymer. The balloon may be formed such that the porous material extends along both the permeable and non-permeable regions. In one mode of this construction, the porous material is porous along the permeable region but is non-porous along the non-permeable region, such as for example by expanding only the permeable region in order to render sufficient voids in the node-fibril network to provide permeable pores in that section. The voids or pores in the porous material may also be provided along both permeable and non-permeable sections but are substantially blocked with an insulator material along the non-permeable section in order to prevent fluid from passing therethrough. The insulator material may be dip coated, deposited, or extruded with the porous material in order to fill the voids. The insulator material may in one mode be provided along the entire working length of the balloon and then selectively removed along the permeable section, or may be selectively exposed to only the non-permeable sections in order to fill the voids or pores there.
Owner:MAGUIRE MARK A +1
Endovascular devices and methods of use
ActiveUS20090177090A1StethoscopeHeart/pulse rate measurement devicesBalloon catheterIntravascular device
Owner:TELEFLEX LIFE SCI LTD
Methods and apparatuses for coating balloon catheters
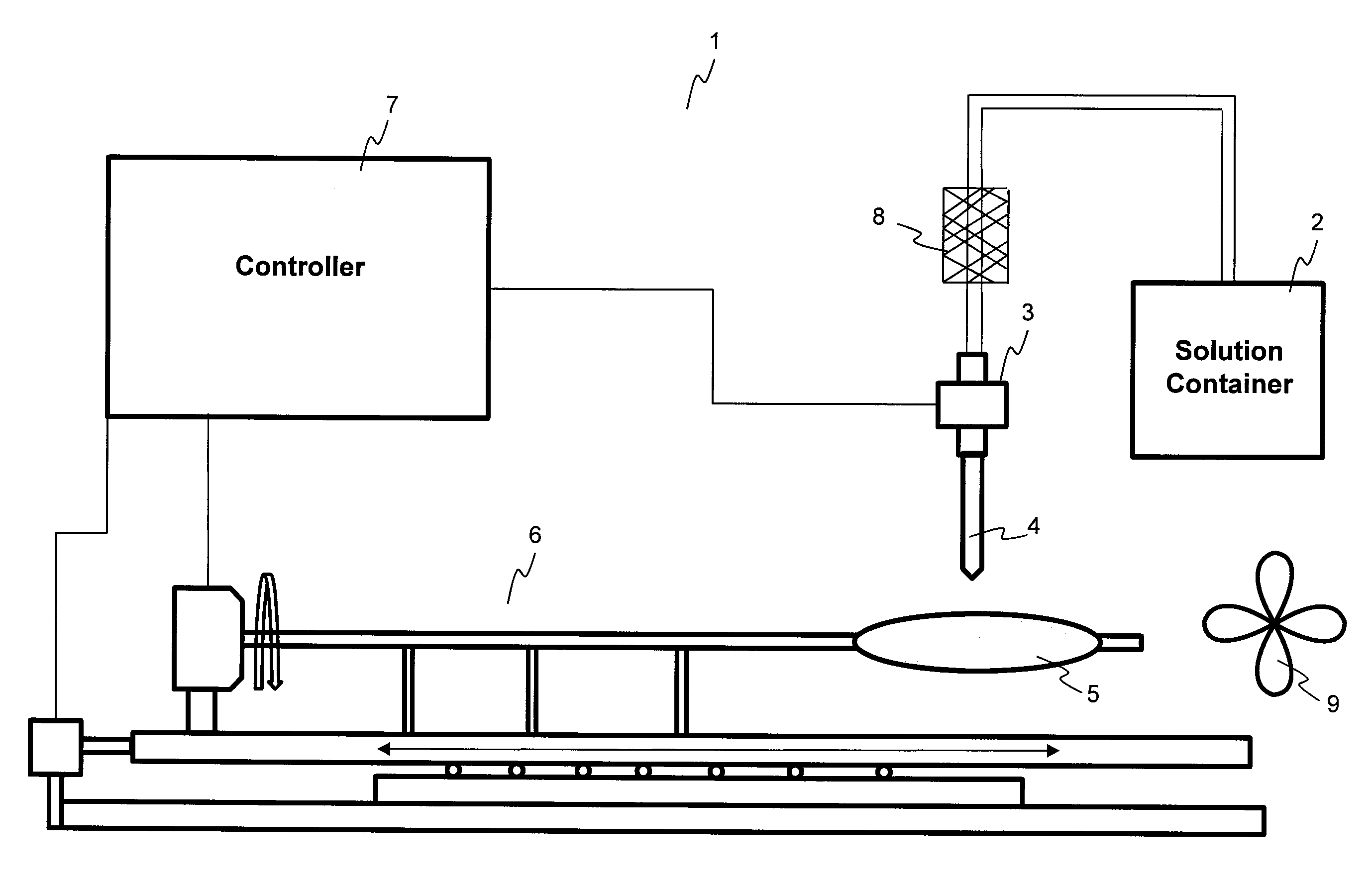
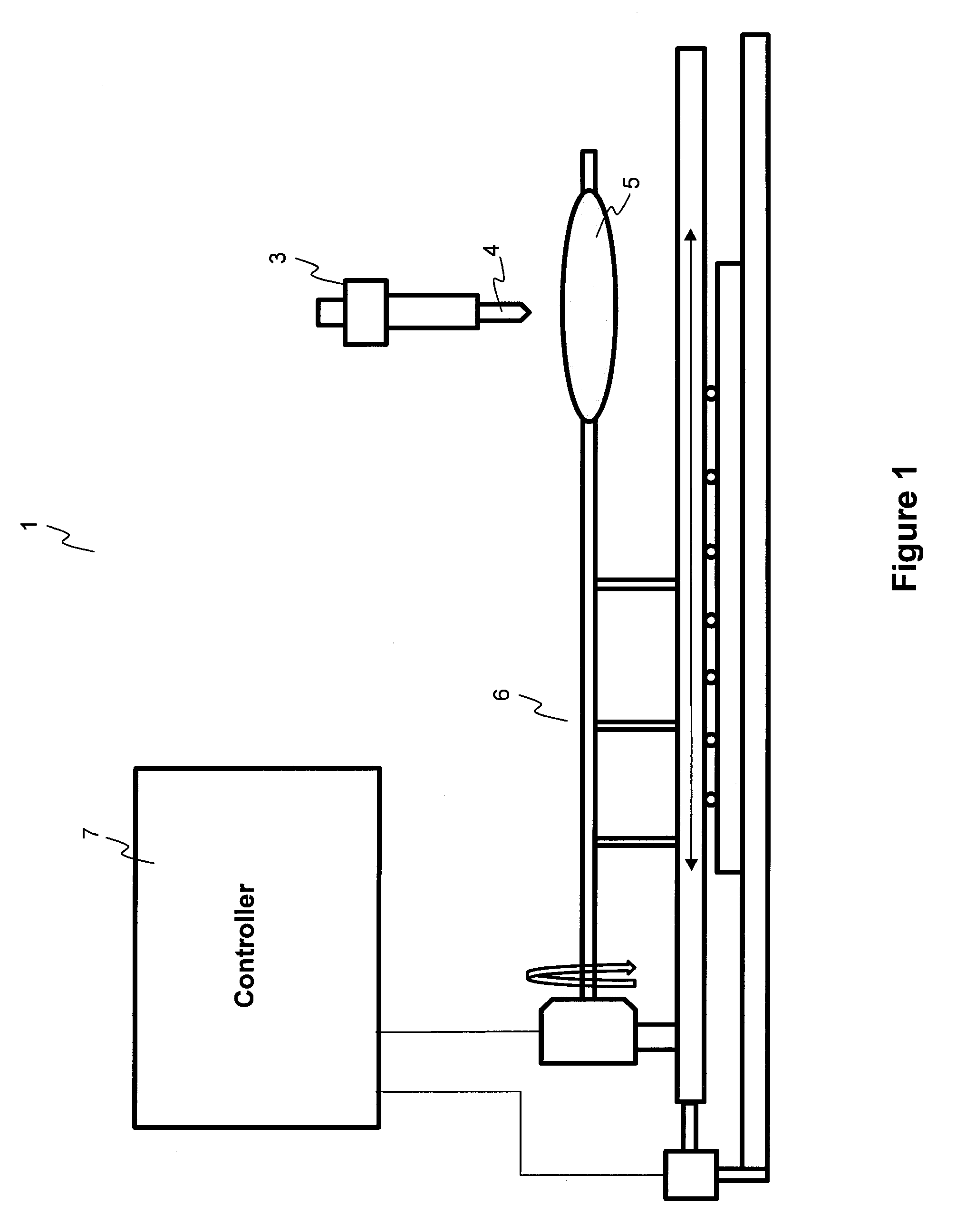
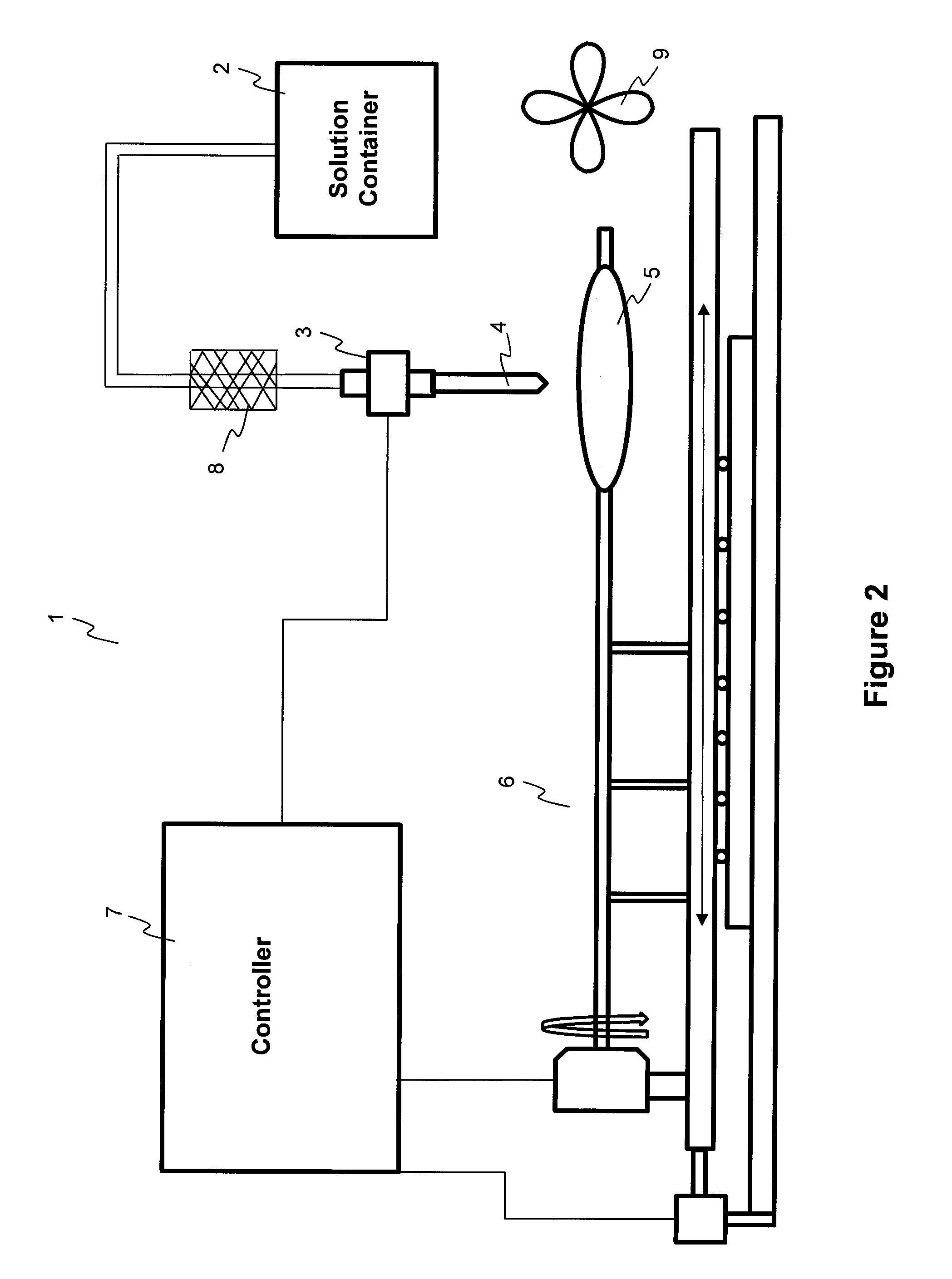
ActiveUS20100055294A1Destroy surface tensionUniform coatingBalloon catheterGlovesTransverse axisSolvent
Embodiments of the invention relate to a method and apparatus for coating a medical device. In one embodiment, the method for preparing a substantially uniform coated medical device includes (1) preparing a coating solution comprising a solvent, a therapeutic agent, and an additive; (2) loading a metering dispenser with the coating solution; (3) rotating the medical device about the longitudinal axis of the device and / or moving the medical device along the longitudinal or transverse axis of the device; (4) dispensing the coating solution from the metering dispenser onto a surface of the medical device and flowing the coating solution on the surface of the medical device while the medical device is rotating and / or linearly moving; and (5) evaporating the solvent, forming a substantially uniform coating layer on the medical device.
Owner:LUTONIX INC
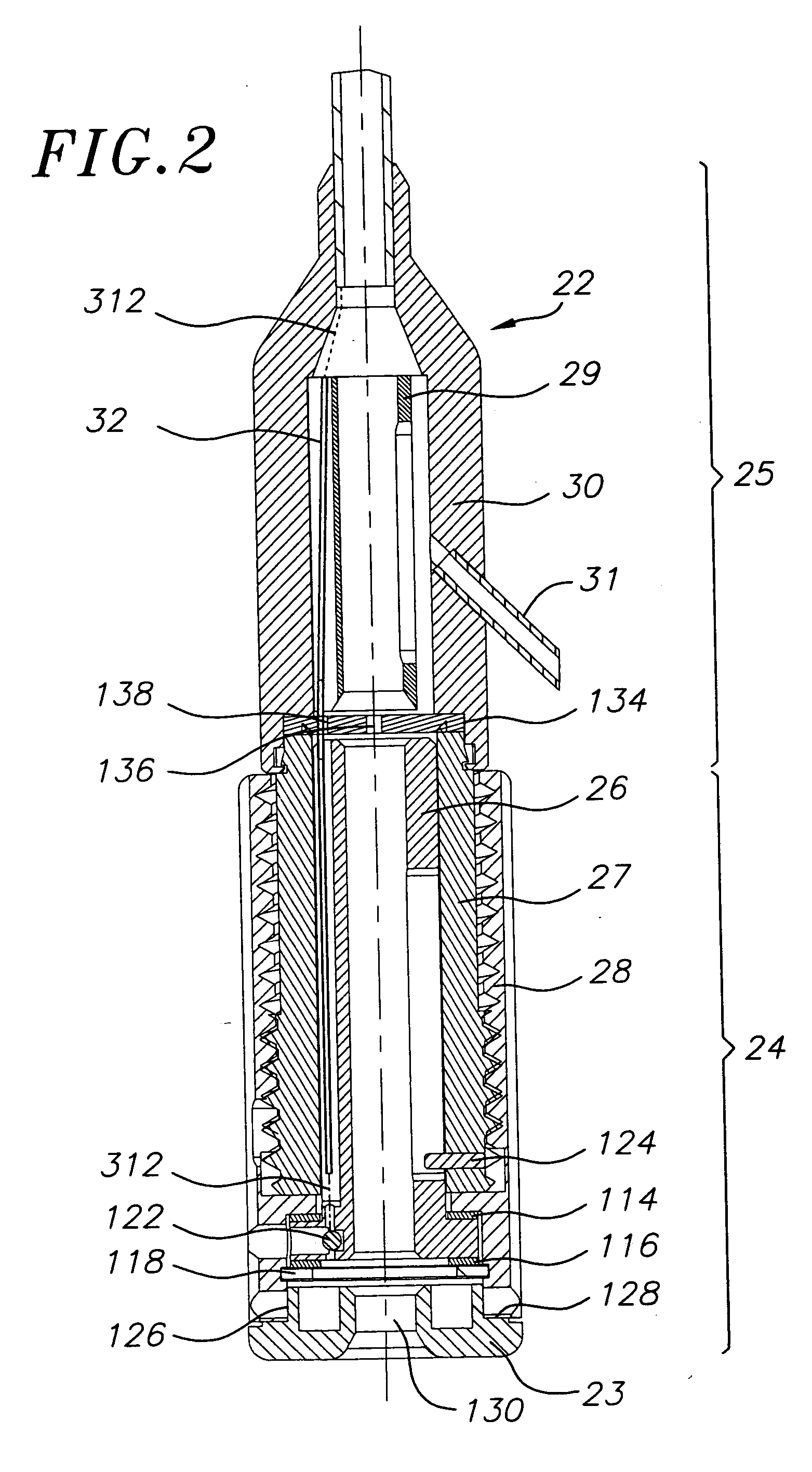
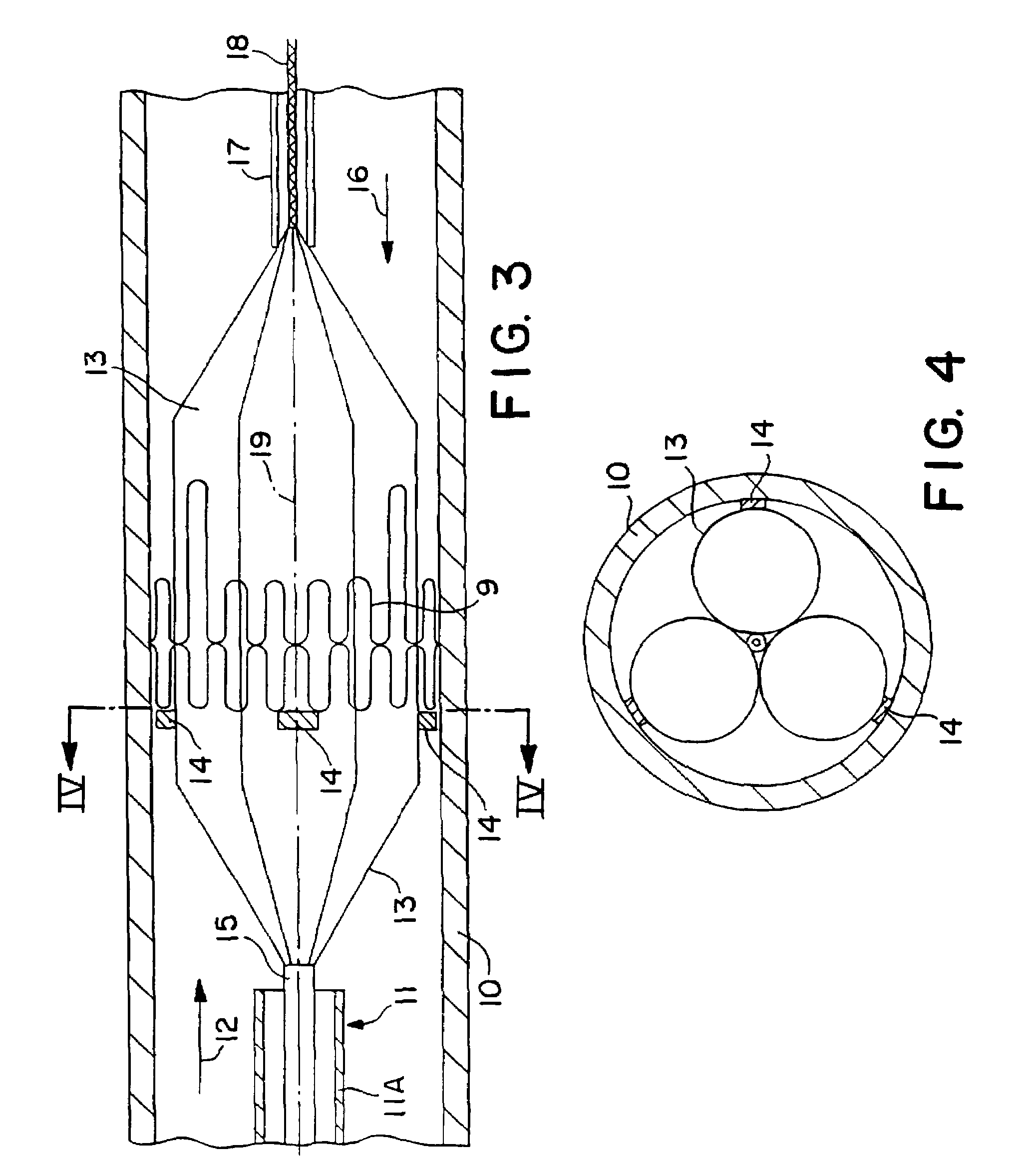
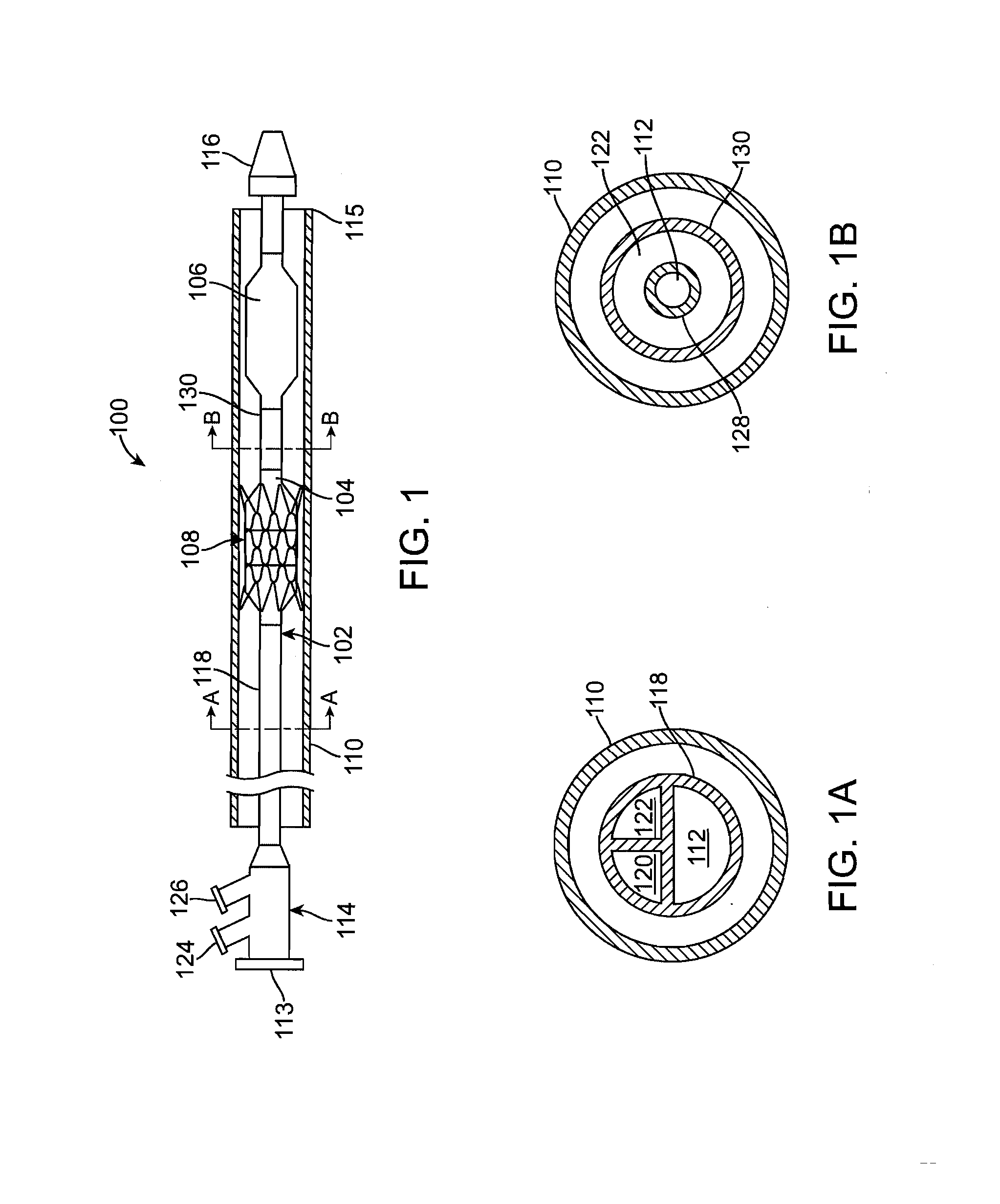
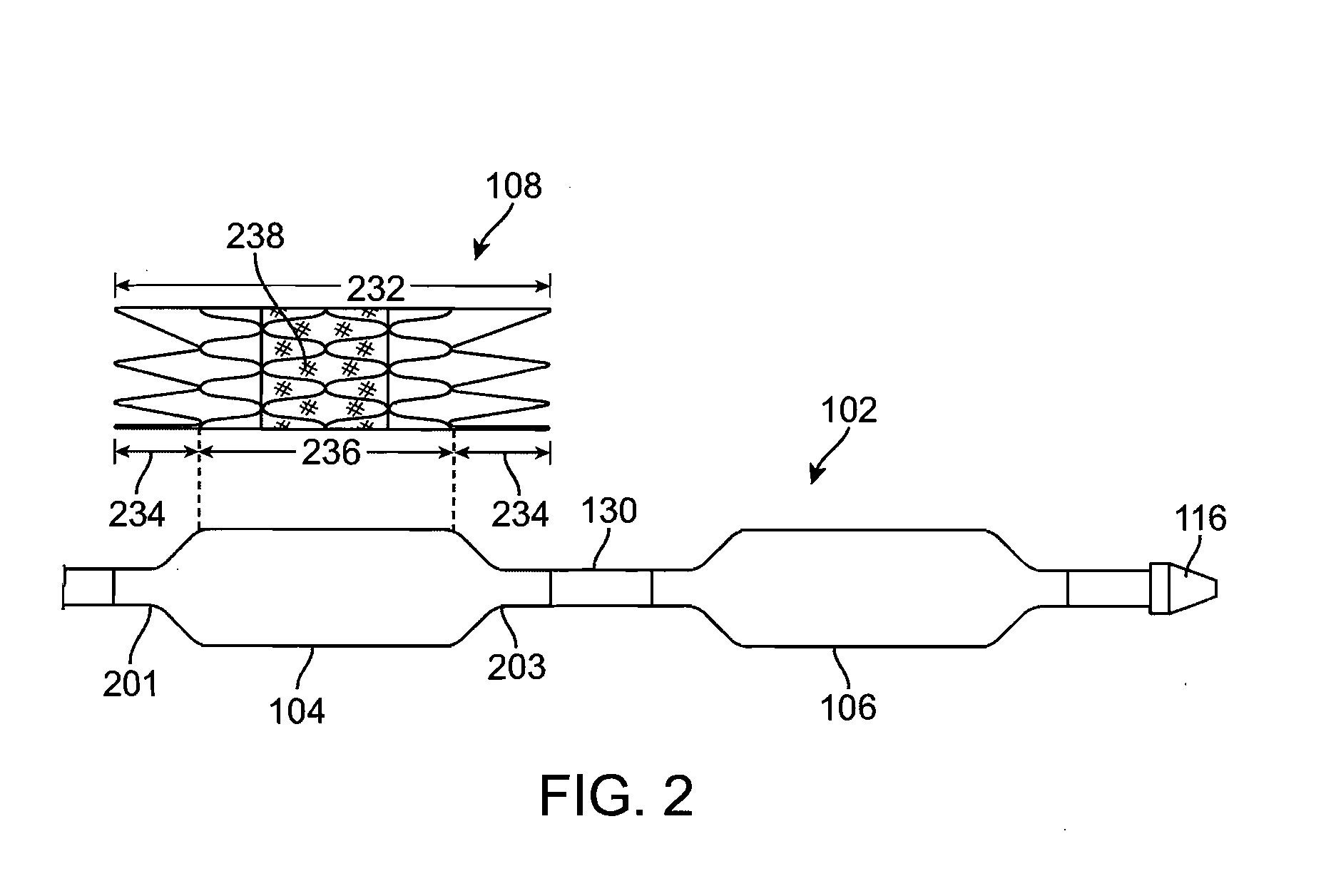
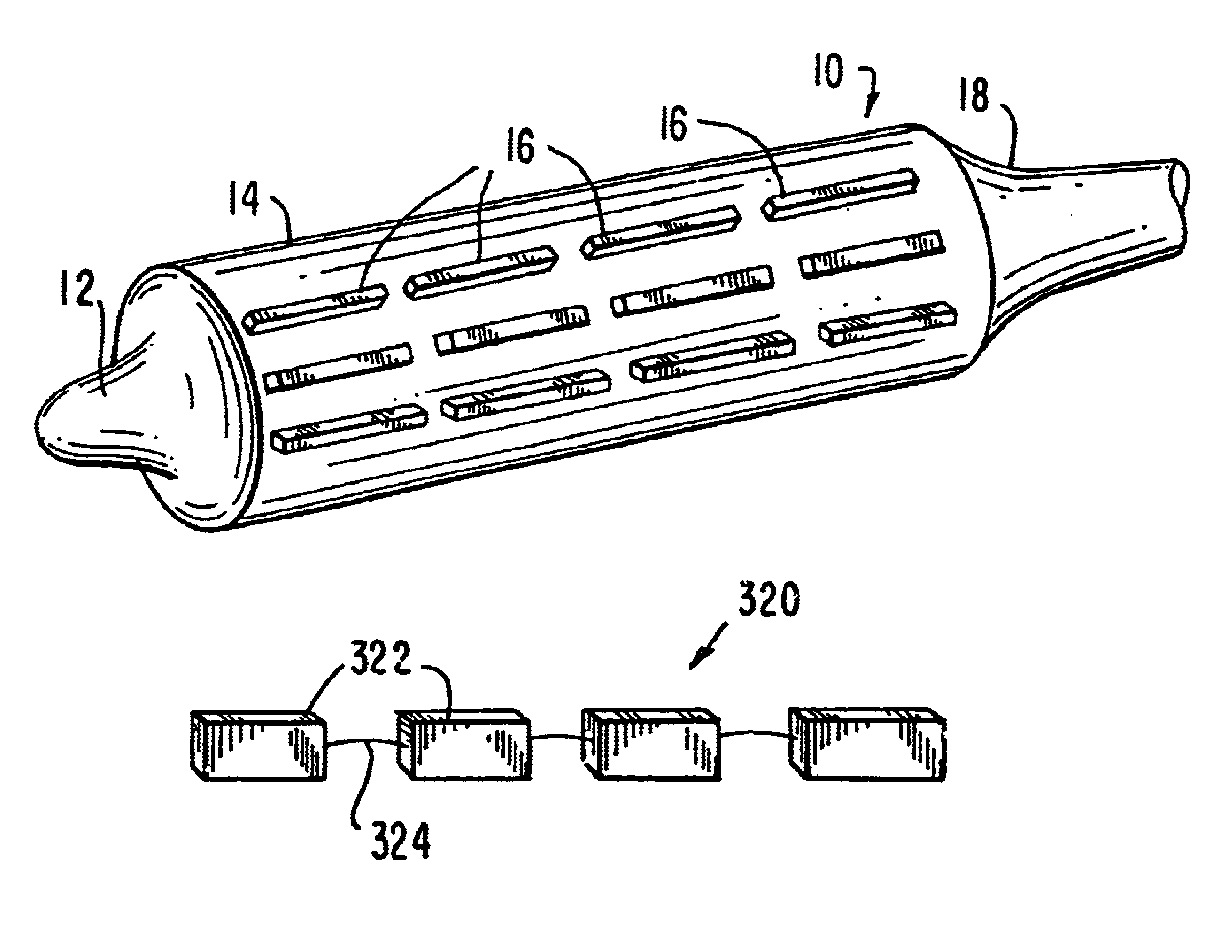
Stiffened balloon catheter for dilatation and stenting
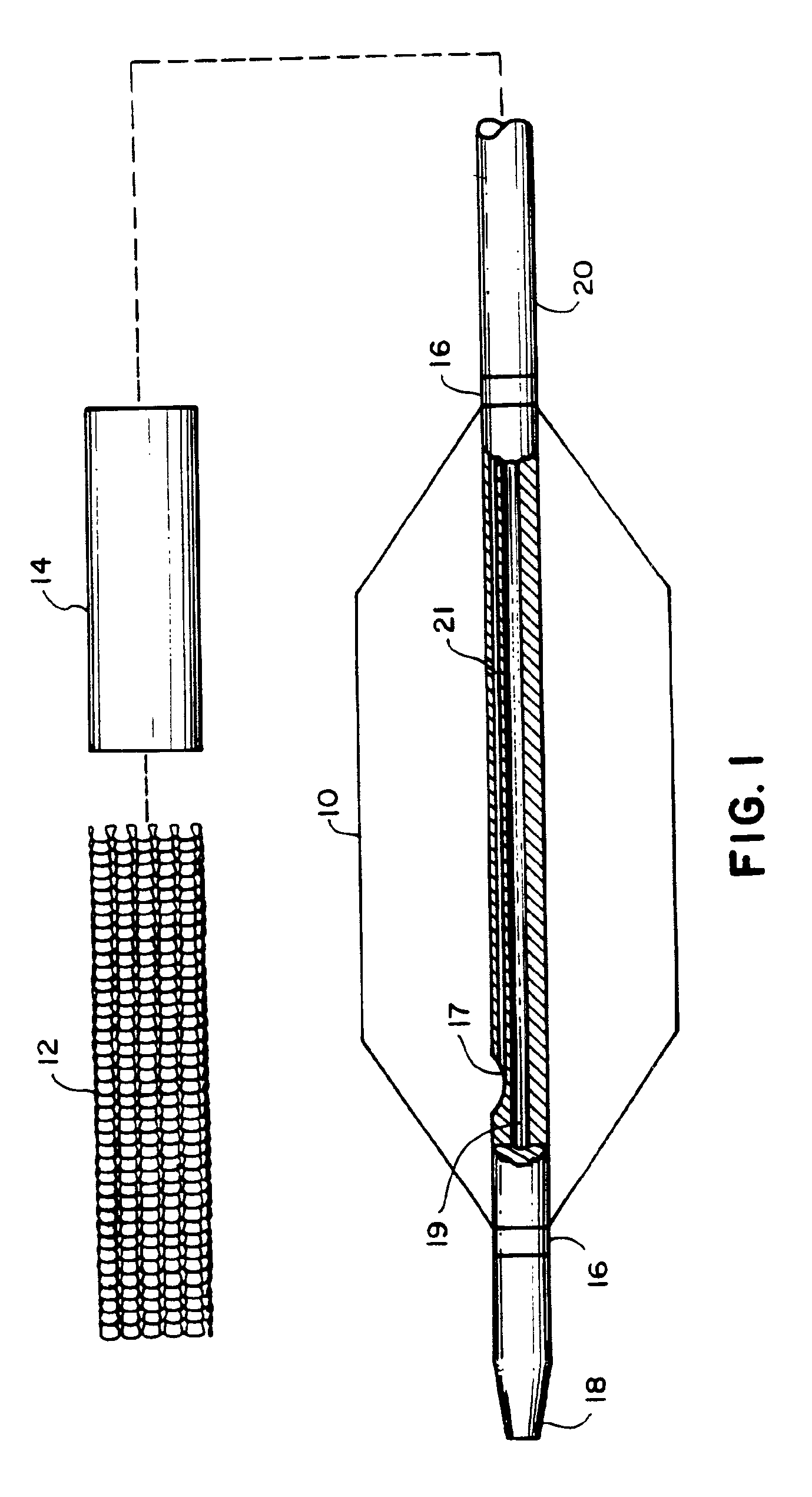
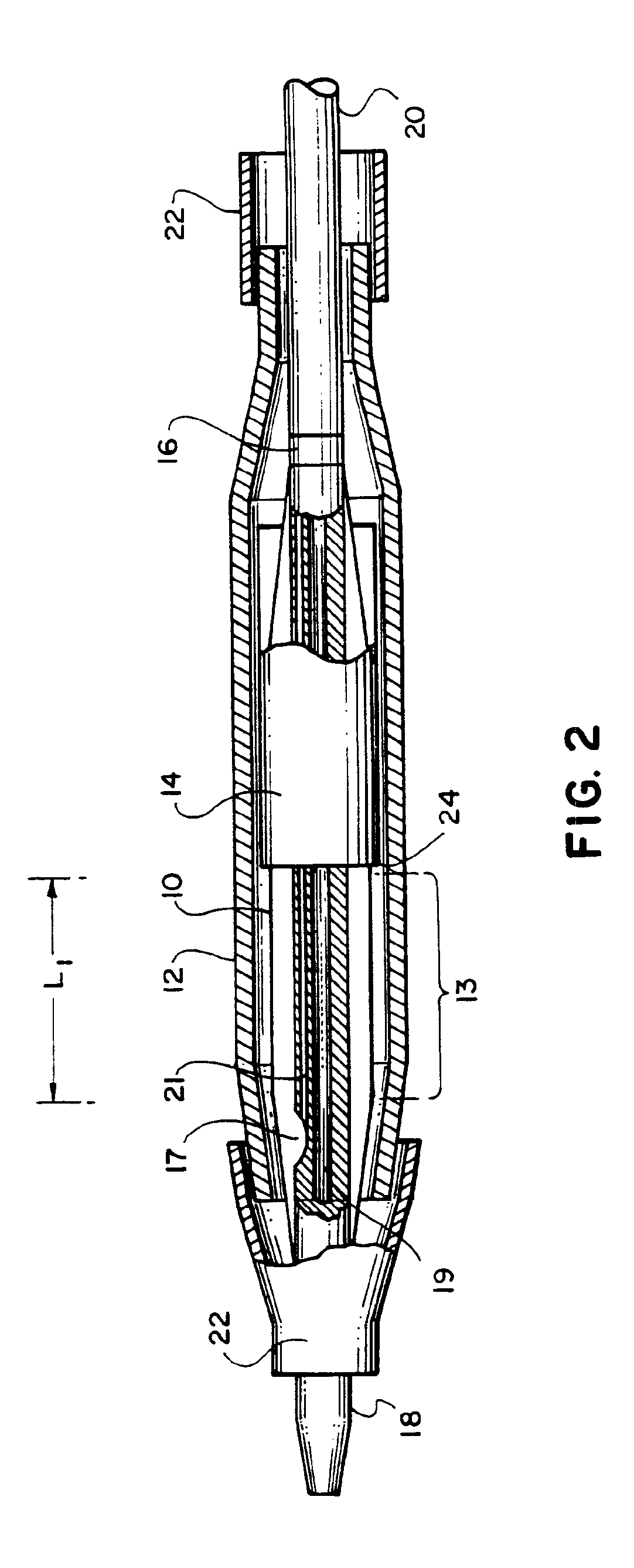
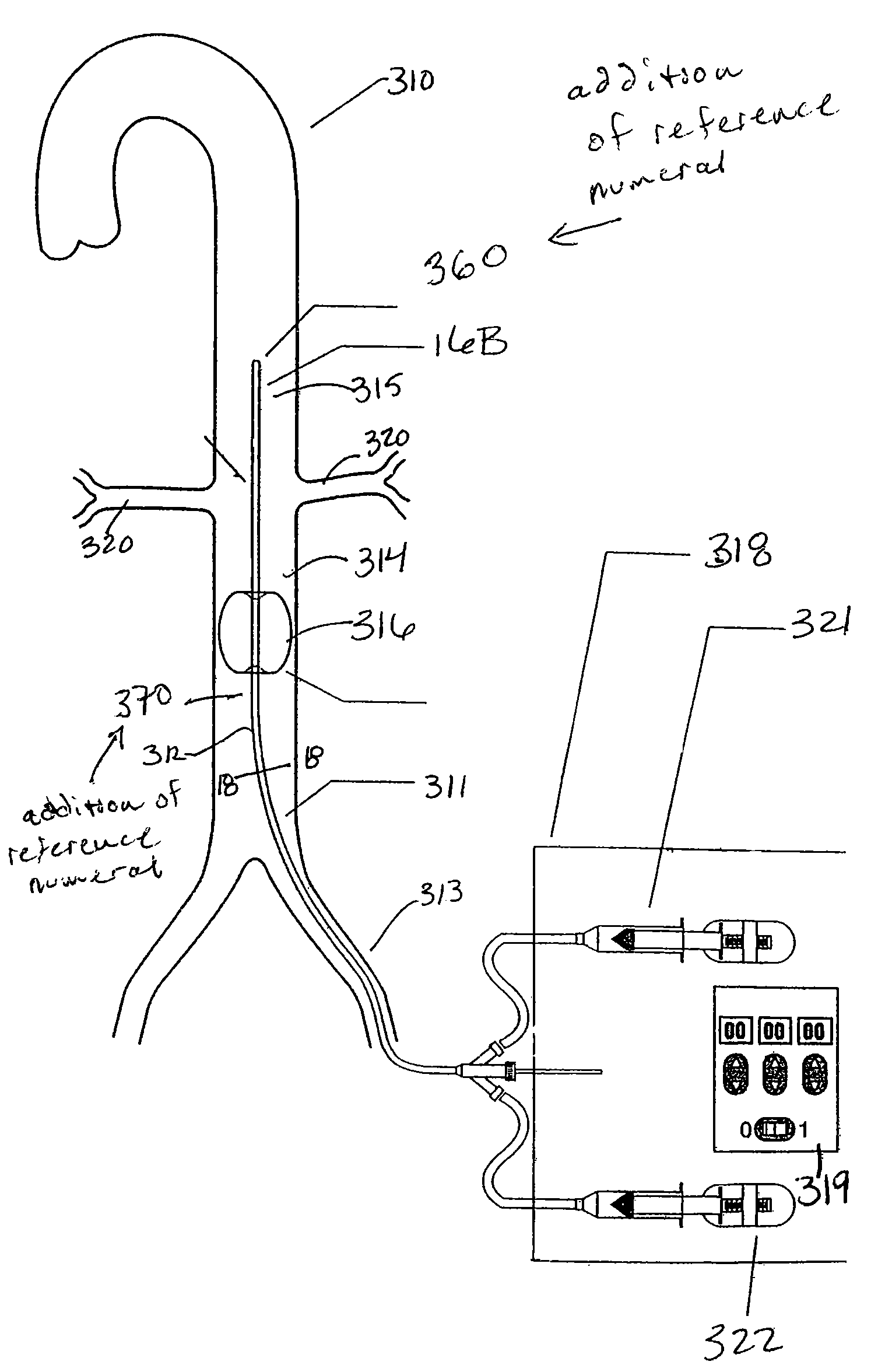
The present invention pertains to a stiffened balloon which can be used in angioplasty, endovascular, and valvuloplasty procedures or as a delivery balloon to deliver a stent or a stent-graft. Longitudinally discontinuous stiffening members connected to the expandable balloon stiffen the balloon but allow it to be navigated through curved passages. Projections on the stiffening members may engage, incise, crush, fracture, or pierce occlusions or retain a stent or stent-graft. Radio-opaque portions may be referenced to determine orientation. Longitudinally continuous stiffening members bearing projections and connected to the balloon perform in a similar manner.
Owner:GRAYZEL JEFFREY +1
Vascular device for valve leaflet apposition
InactiveUS7041128B2Enhance retention (securement)StentsBalloon catheterVascular deviceBalloon catheter
A vascular system comprising a balloon catheter having an elongated shaft and an expandable balloon. A vascular device is mounted over the expandable balloon and composed of shape memory material and has a collapsed position and a memorized position. The vascular device is expandable to an expanded position, to engage the vessel walls and returnable substantially to the memorized position to bring the walls radially inwardly.
Owner:REX MEDICAL LP
Use of mechanical dilator devices to enlarge ostia of paranasal sinuses and other passages in the ear, nose, throat and paranasal sinuses
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Transnasal method and catheter for lacrimal system
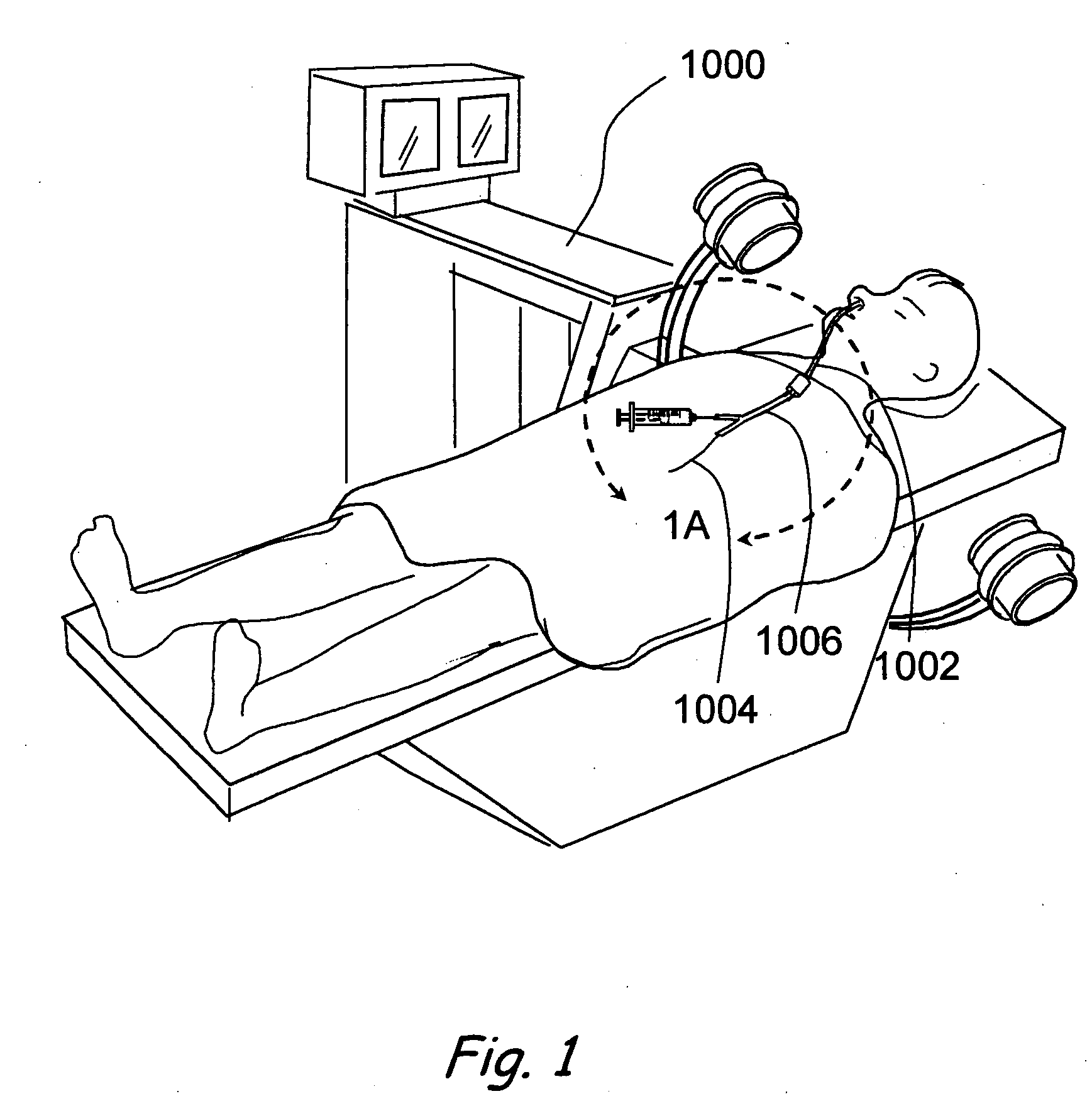
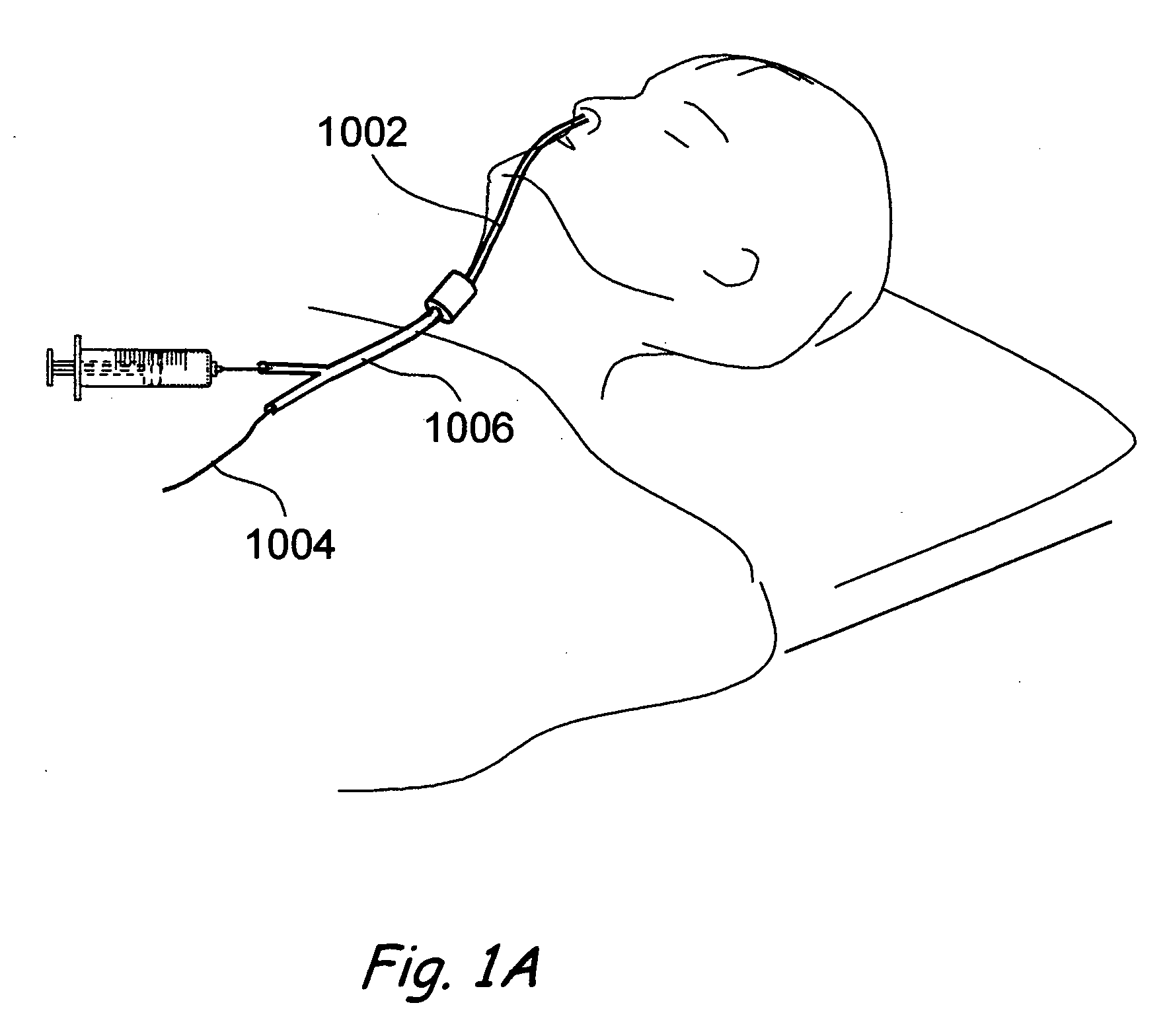
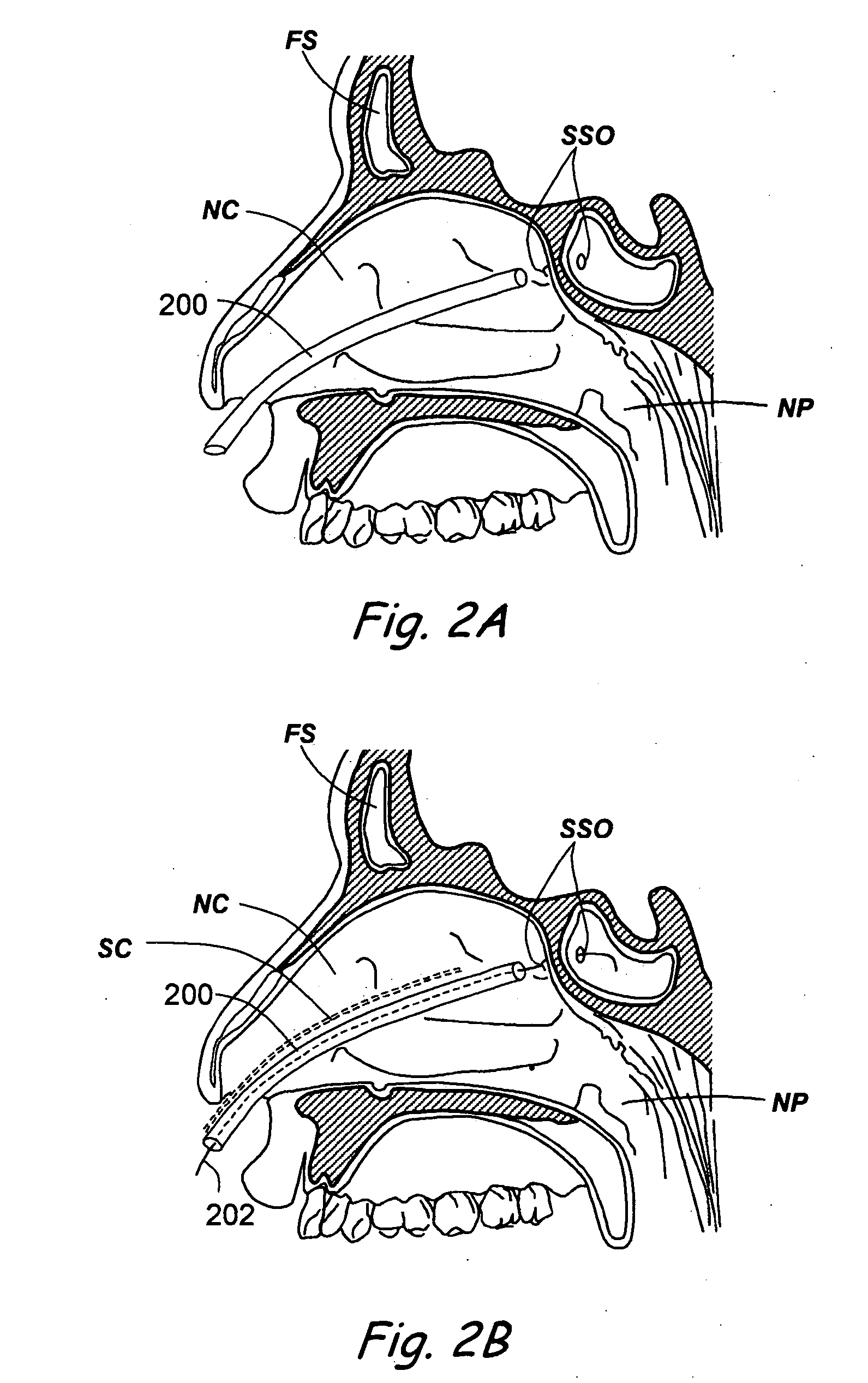
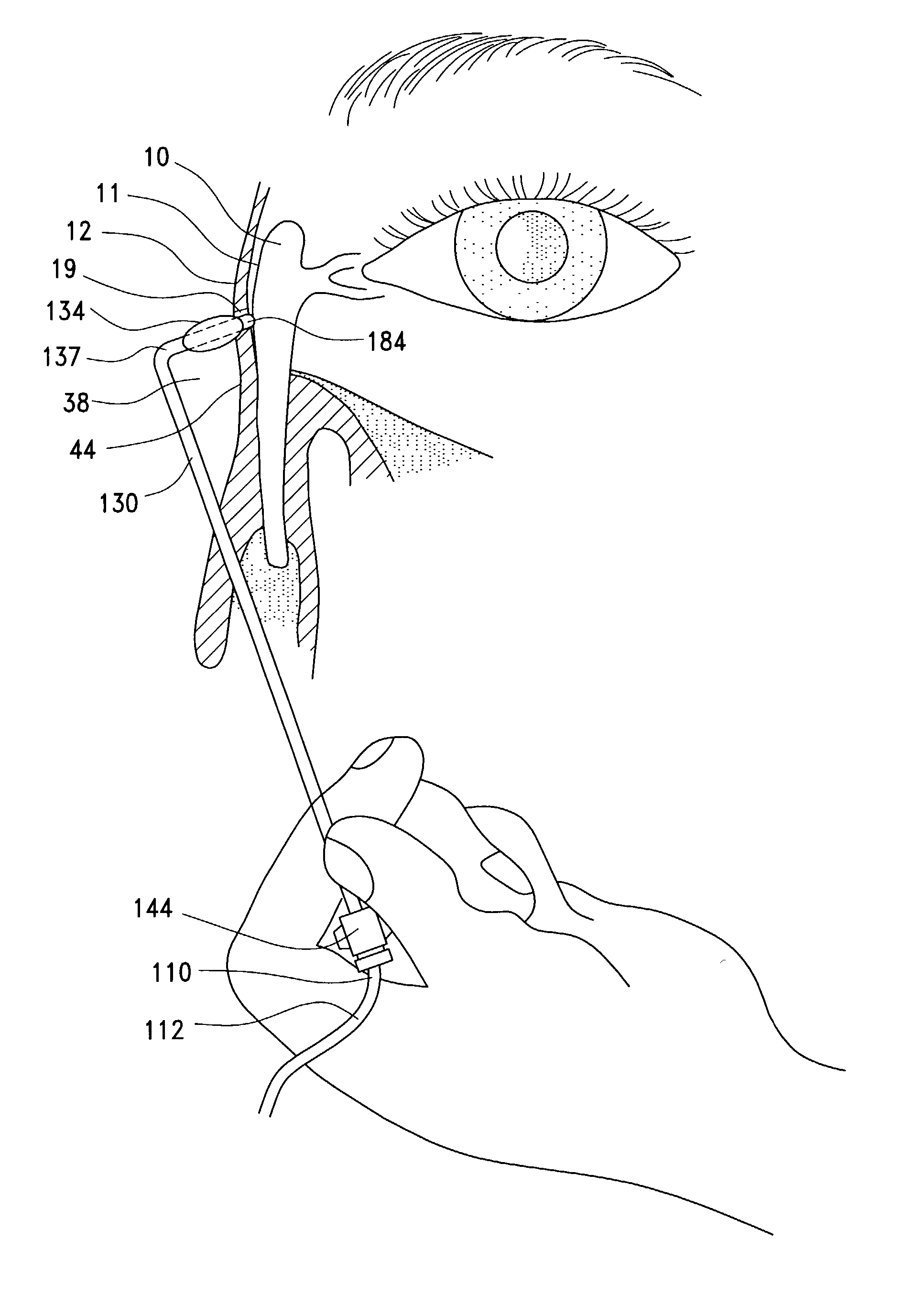
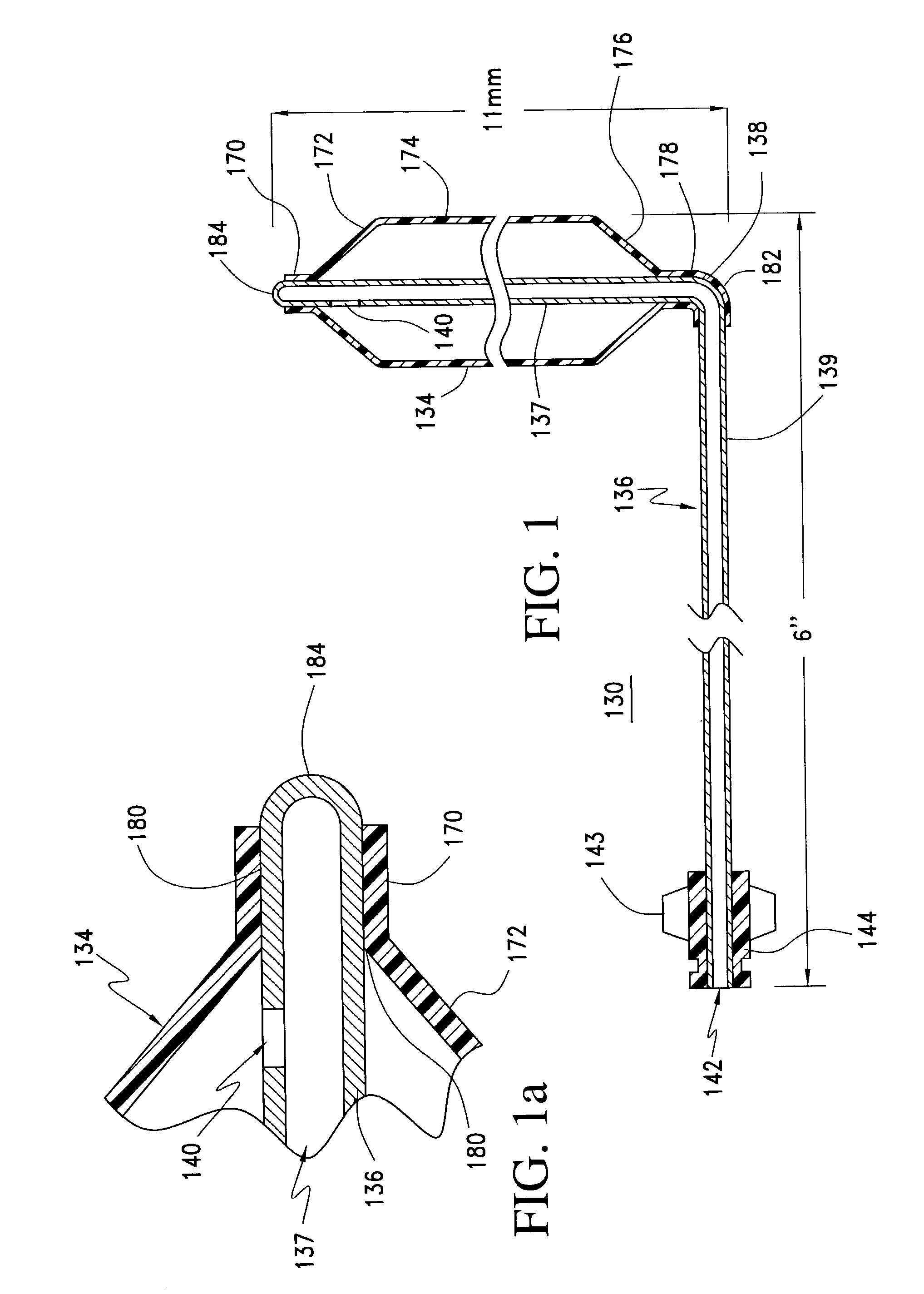
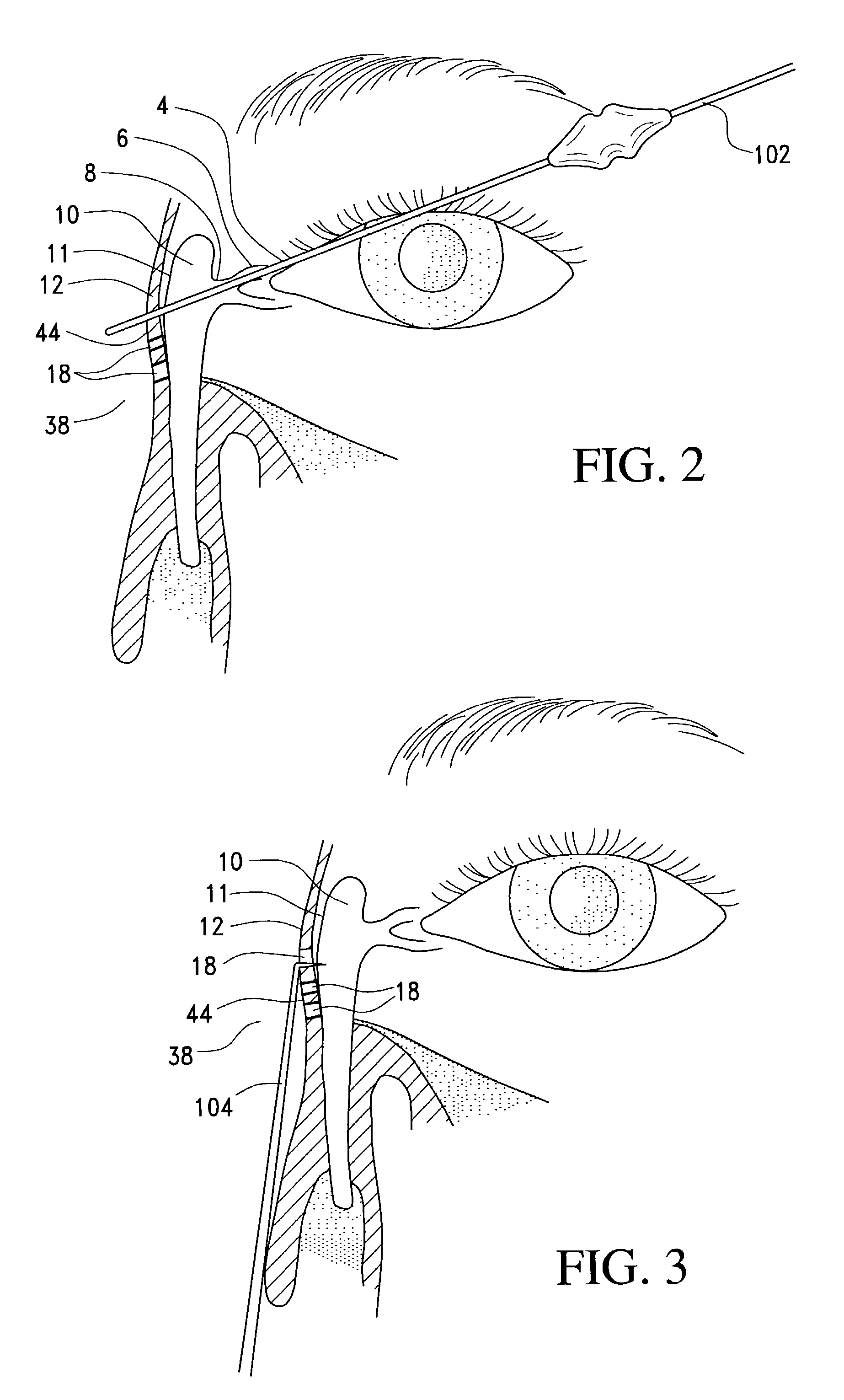
A balloon catheter for treatment of a patient's lacrimal system is applied transnasally without the use of a guide wire or a curve retention member. The catheter uses a stainless steel hypotube of sufficient stiffness and column strength to be pushed from the patent's nasal cavity through an opening-formed through the lateral nasal wall and lacrimal fossa, into the lacrimal sac. The catheter has an inflatable member mounted about a rigid bent distal segment. The opening is first formed by pushing small holes through the medial sac, lacrimal fossa, and lateral nasal wall with an instrument and coalescing the holes. The catheter is then introduced into the nasal cavity and pushed laterally through the opening by manipulating its proximal end. Pressurized fluid is then applied to the catheter to inflate the inflatable member and dilate the opening.
Owner:BECKER BRUCE
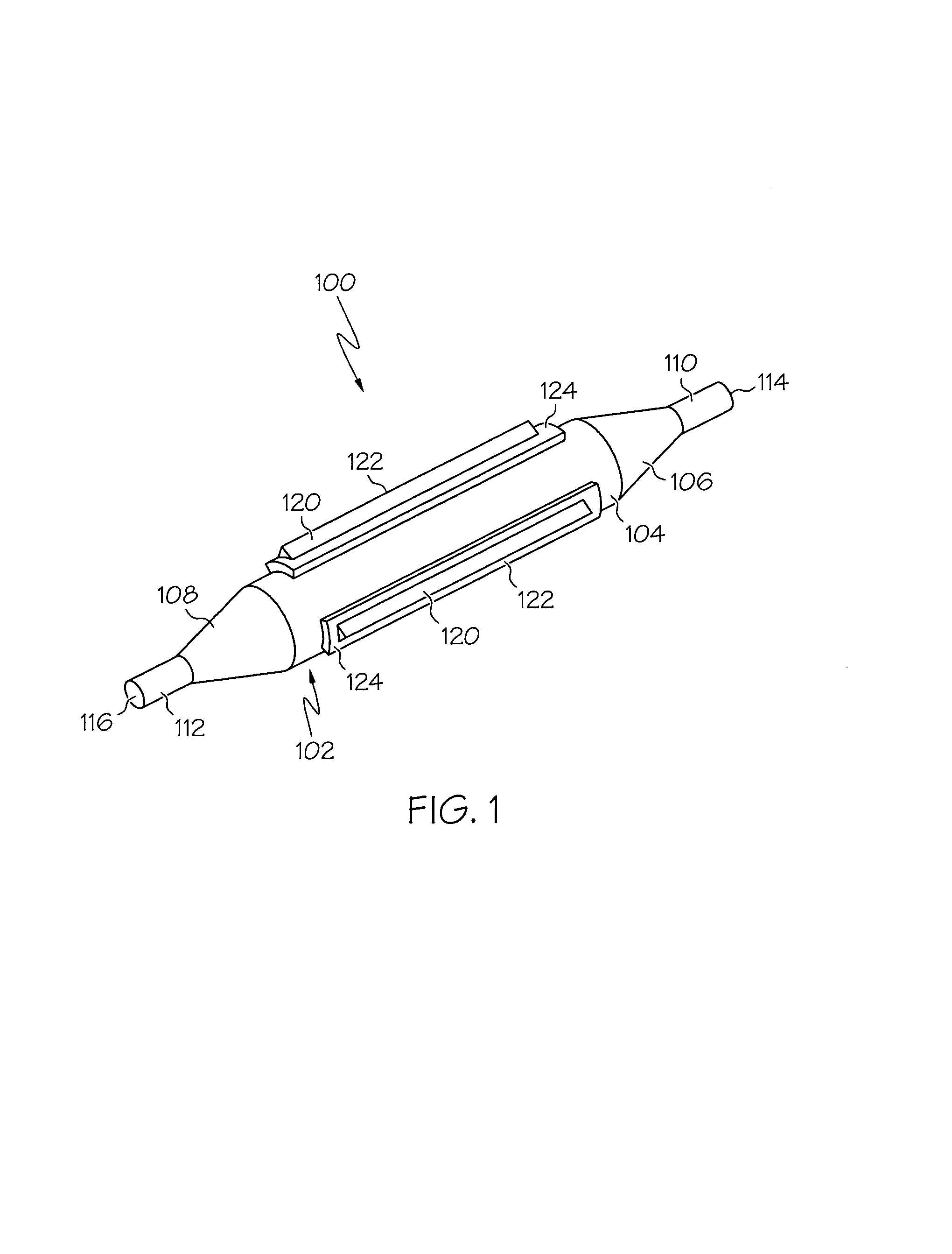
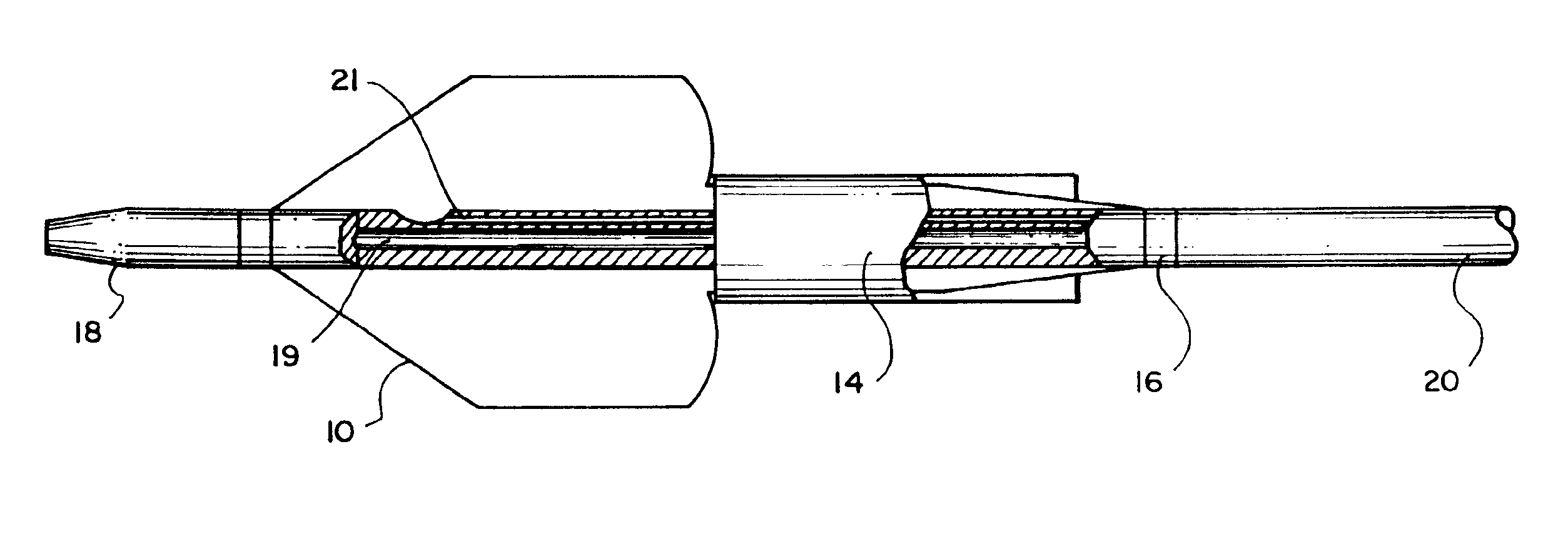
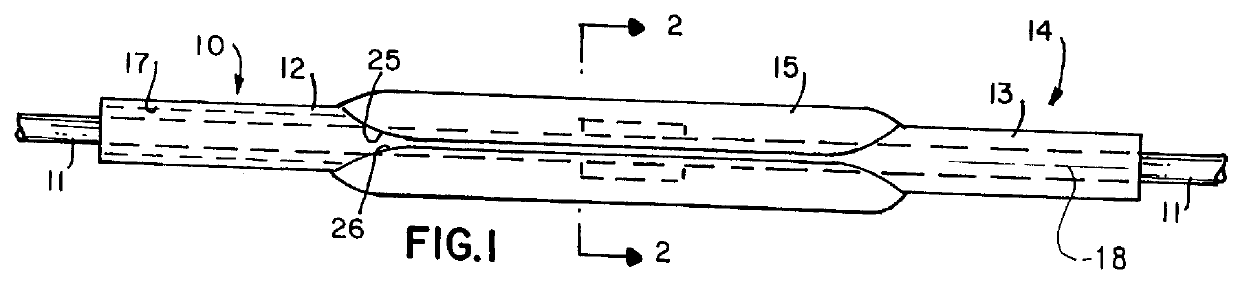
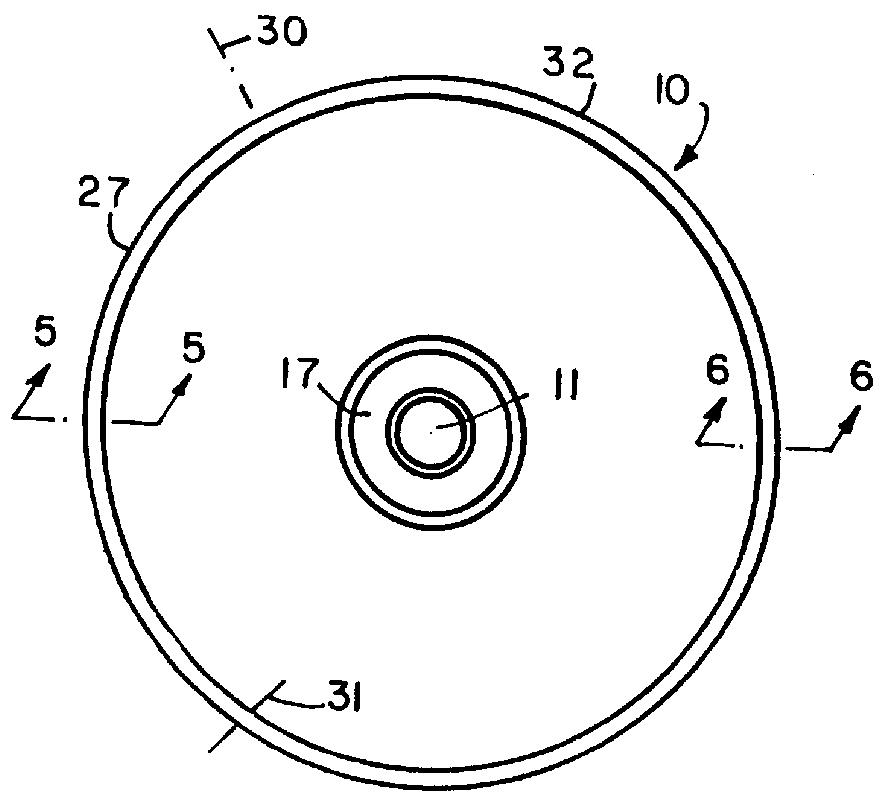
Balloon catheter
InactiveUS6010480AImprovement factorReduce coefficient of frictionStentsBalloon catheterBalloon catheterLesion
An expansible balloon catheter has at least a first exterior surface with a given coefficient of friction and a second exterior surface with a greater coefficient of friction. In a compact form only the first exterior surface is exposed to produce one coefficient of friction during transfer of the collapsed or uninflated balloon to and across a lesion. When inflated, the second surface dominates the first surface and produces a second coefficient of friction.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com