Implantable medical devices
a medical device and implantable technology, applied in the direction of prosthesis, catheter, blood vessel, etc., can solve the problems of prostate hyperplasia, difficult or impossible urination, and dangerous conditions,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
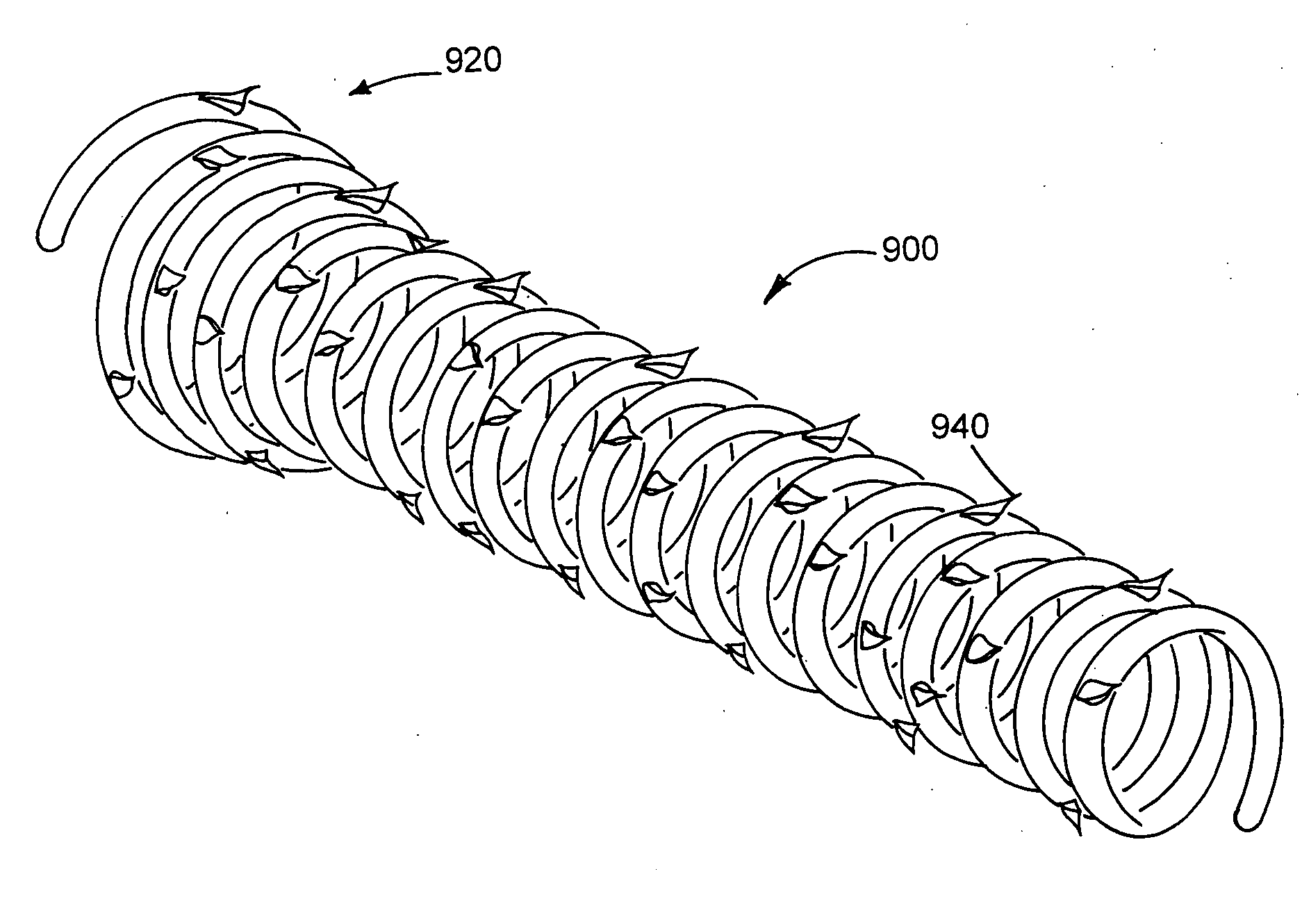
A 56:24:20 mixture of PVAc / PVDF / PMMA is dry bended and loaded into the hopper of an extruder. The PVAc is grade B-100, the PVDF is Solvay SOLEF® 1010 and the PMMA is Atofina PLEXIGLAS® V045. The mixture is melt processed to produce 1.27 mm (0.05 inch) monofilament. The rod is made into a coil by winding it around wrapping fixture 200. The fixture and the rod are immersed into a 50° C. water bath. At this temperature, the rod becomes malleable enough to wind easily around the mandrel and secured in place to prohibit the uncoiling of the helical shape. The mandrel is removed from the fixture with the stent locked in place and placed into an oven at 110° C. for one hour to anneal the stent. This annealing process locks the permanent shape of the coil. The mandrel and coil are cooled to room temperature, and the stent is removed from the mandrel. The stent had on overall length of approximately 73 mm and a flared end portion length of approximately 7 mm. The diameter d1 of the body is ...
example 2
A 70:30 mixture of PVAc / PVDF is dry bended and loaded into the hopper of an extruder. The mixture is melt processed to produce 1.27 mm (0.05 inch) monofilament. The rod is made into a coil by winding it around wrapping fixture 200. The fixture and the rod are immersed into a 50° C. water bath. At this temperature, the rod becomes malleable enough to wind easily around the mandrel and secured in place to prohibit the uncoiling of the helical shape. The mandrel is removed from the fixture with the stent locked in place and placed into an oven at 110° C. for one hour to anneal the stent. This annealing process locks the permanent shape of the coil. The mandrel and coil are cooled to room temperature, and the stent is removed from the mandrel. The stent had on overall length of approximately 73 mm and a flared end portion length of approximately 7 mm. The diameter d1 of the body is approximately 6 mm and the maximum diameter d2 of the flare on the open end is approximately 11 mm. Befor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt temperature | aaaaa | aaaaa |
| melt temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com