Patents
Literature
862 results about "Intravascular stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
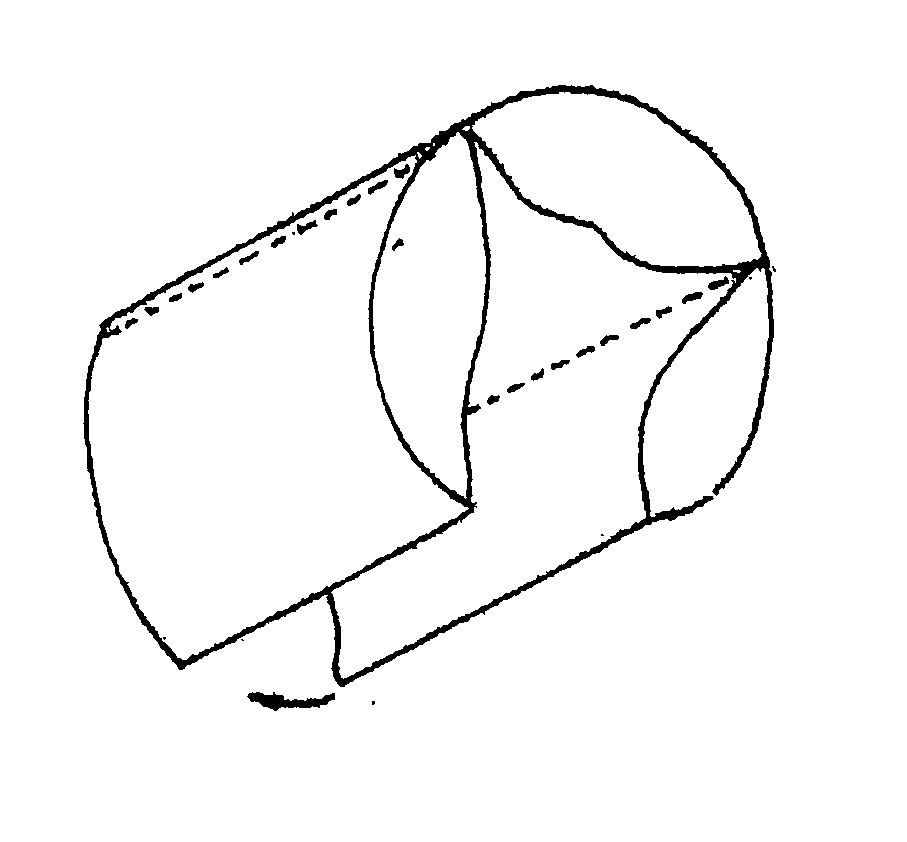
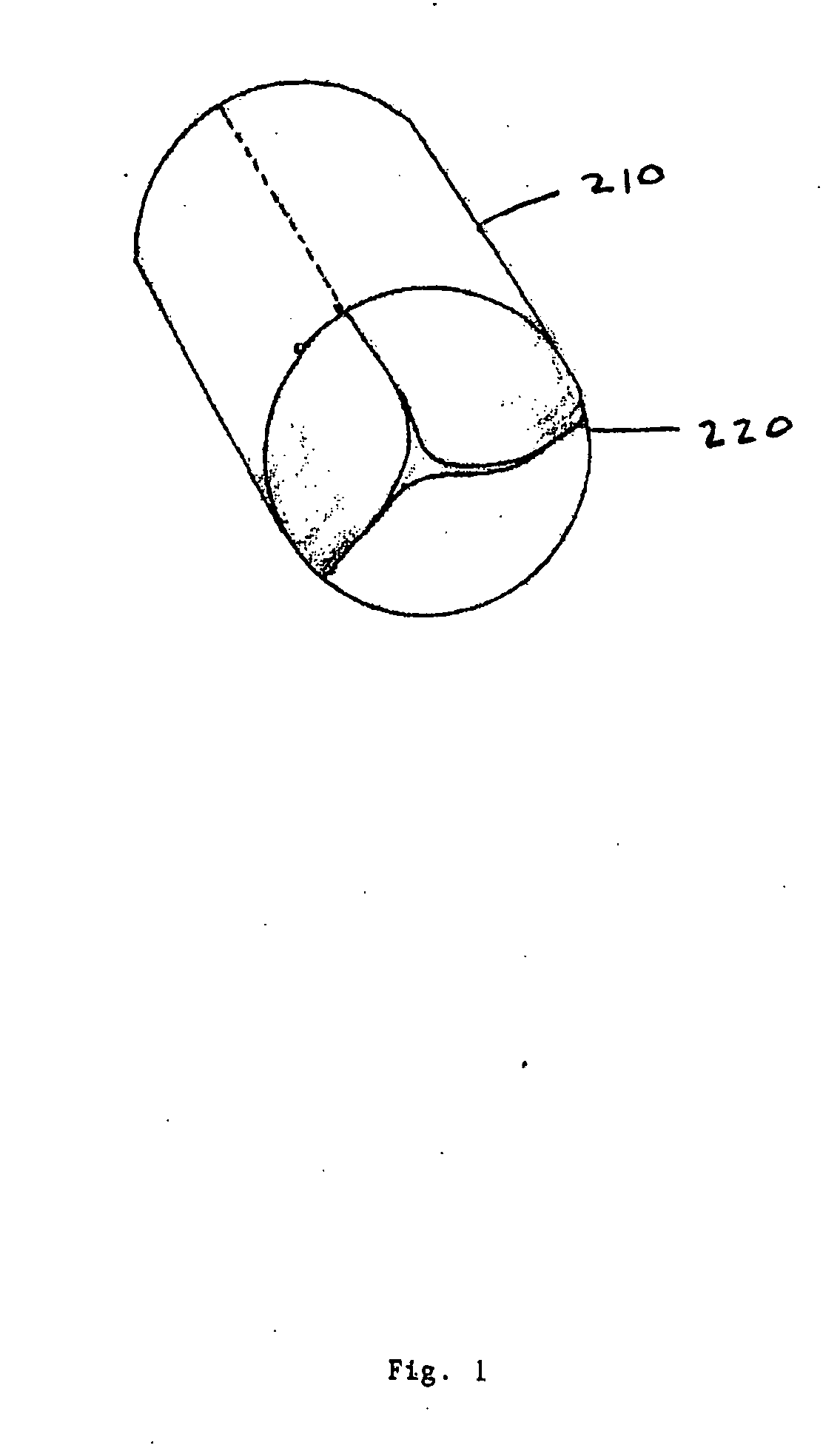
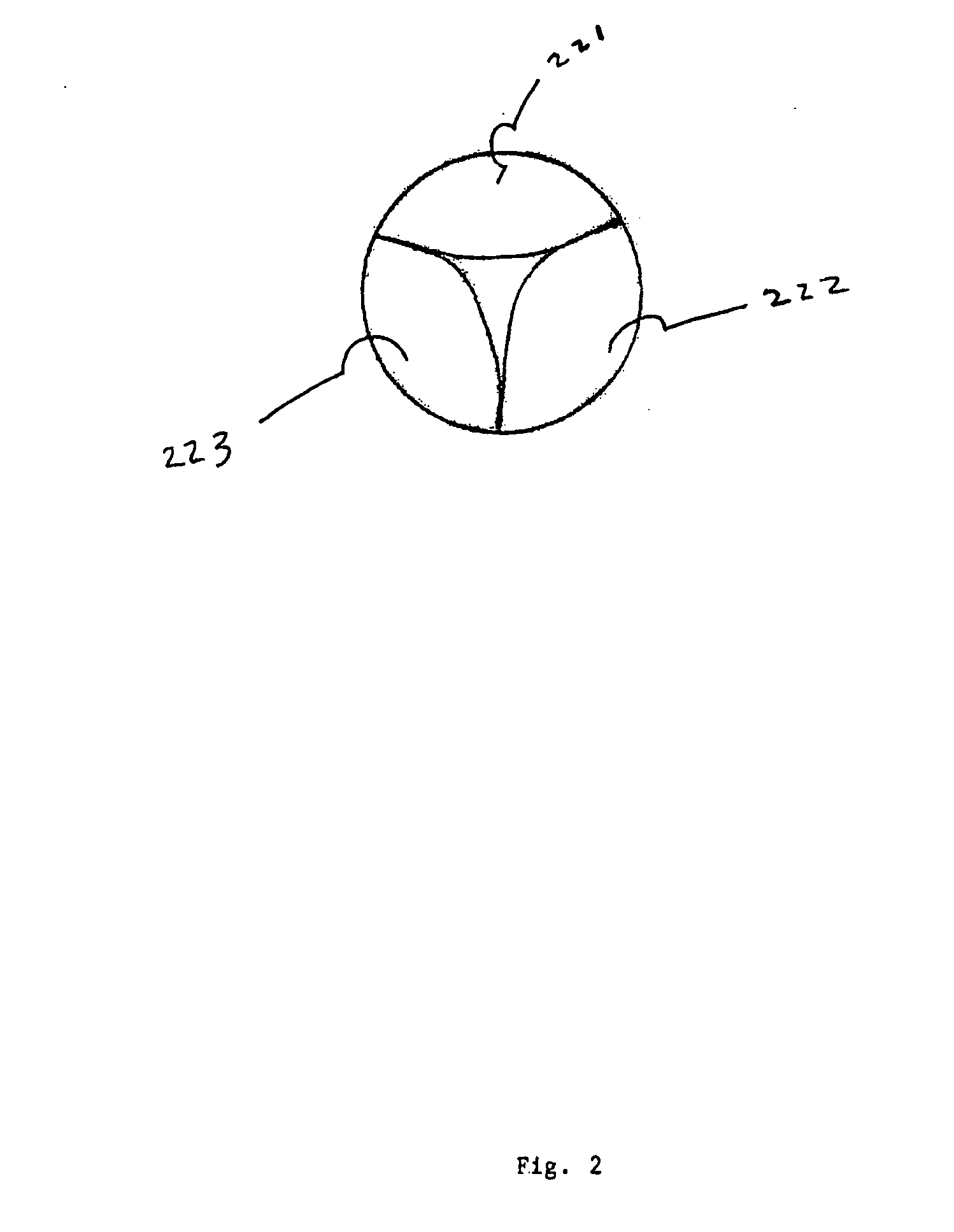
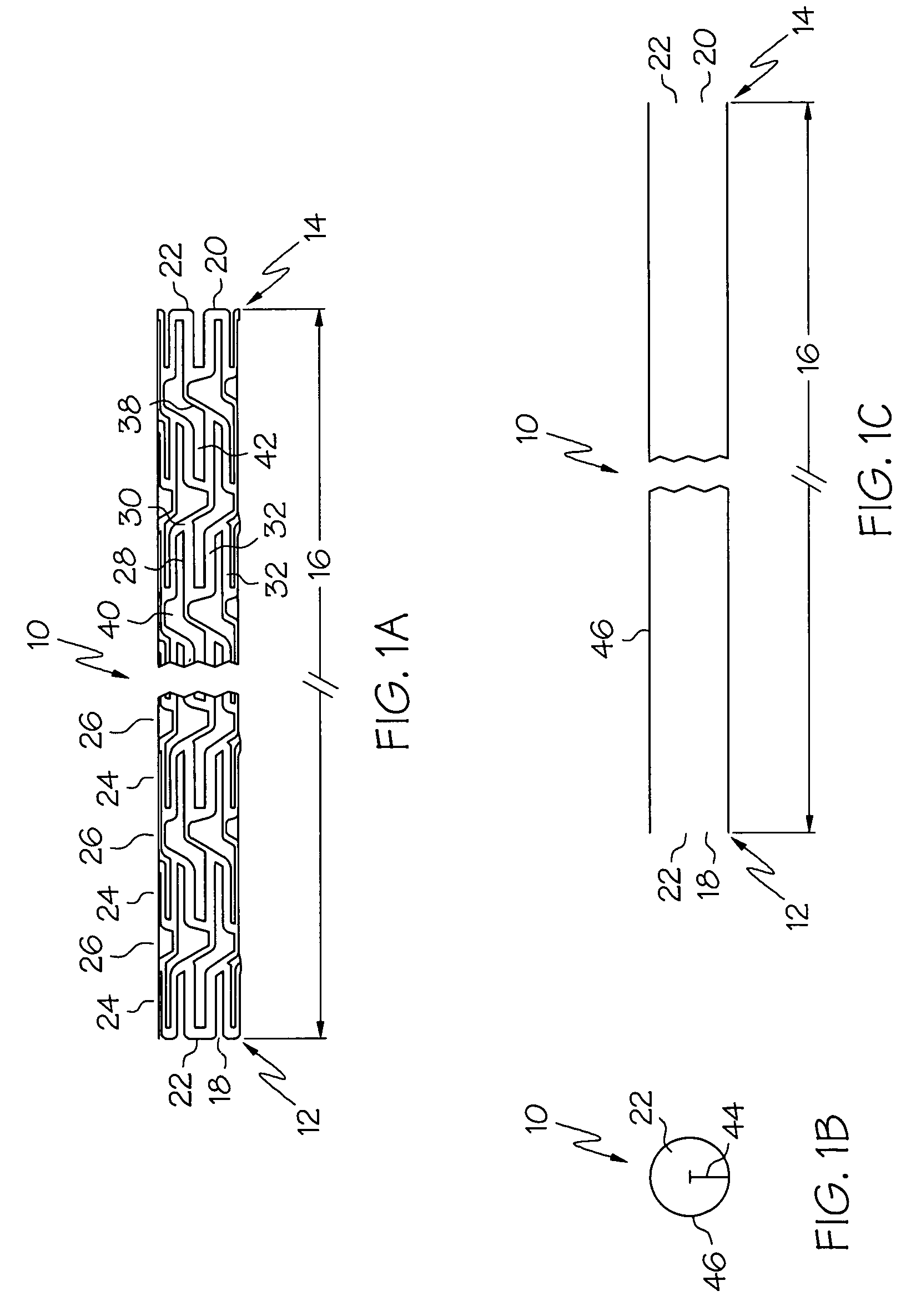
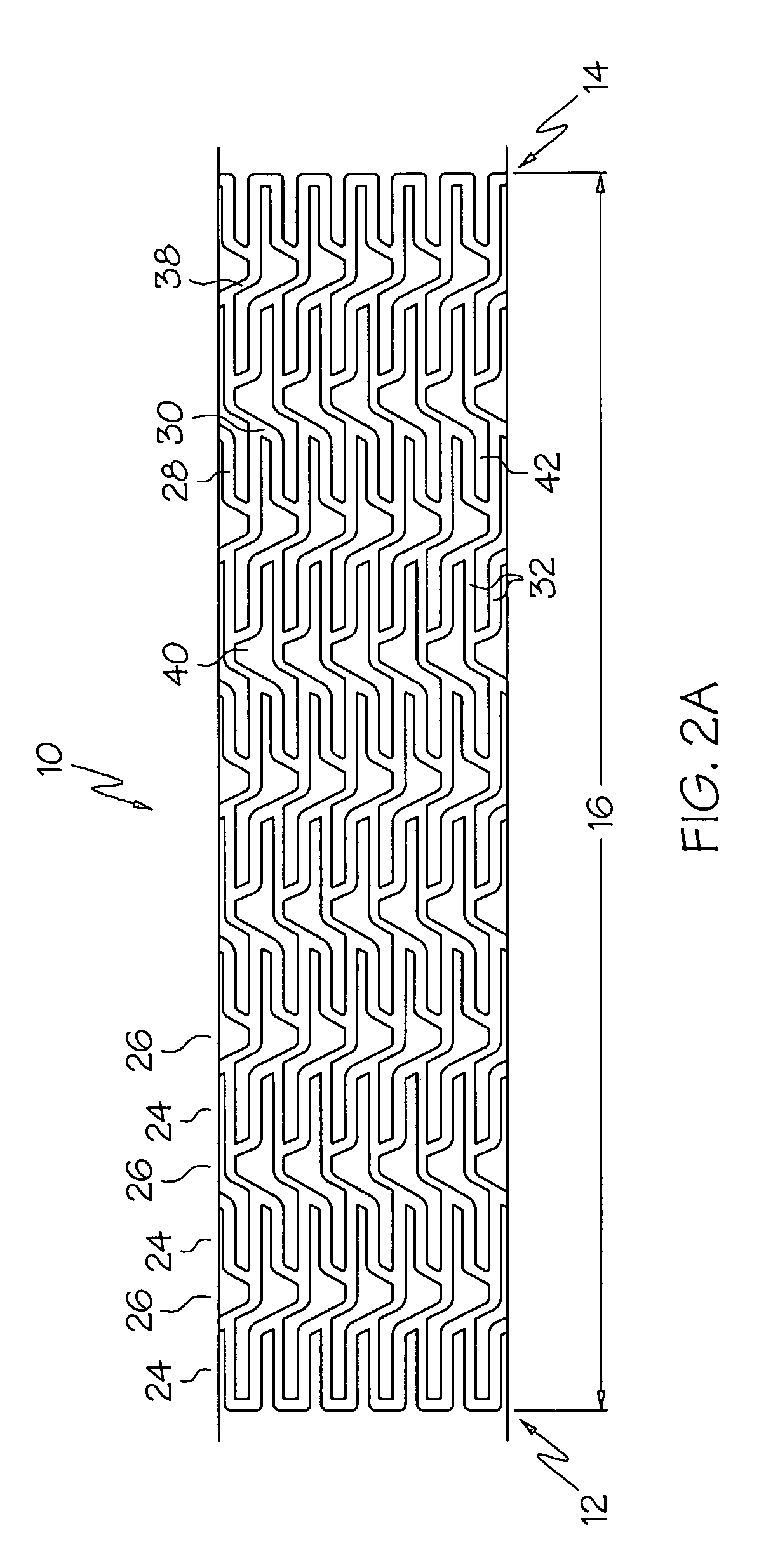
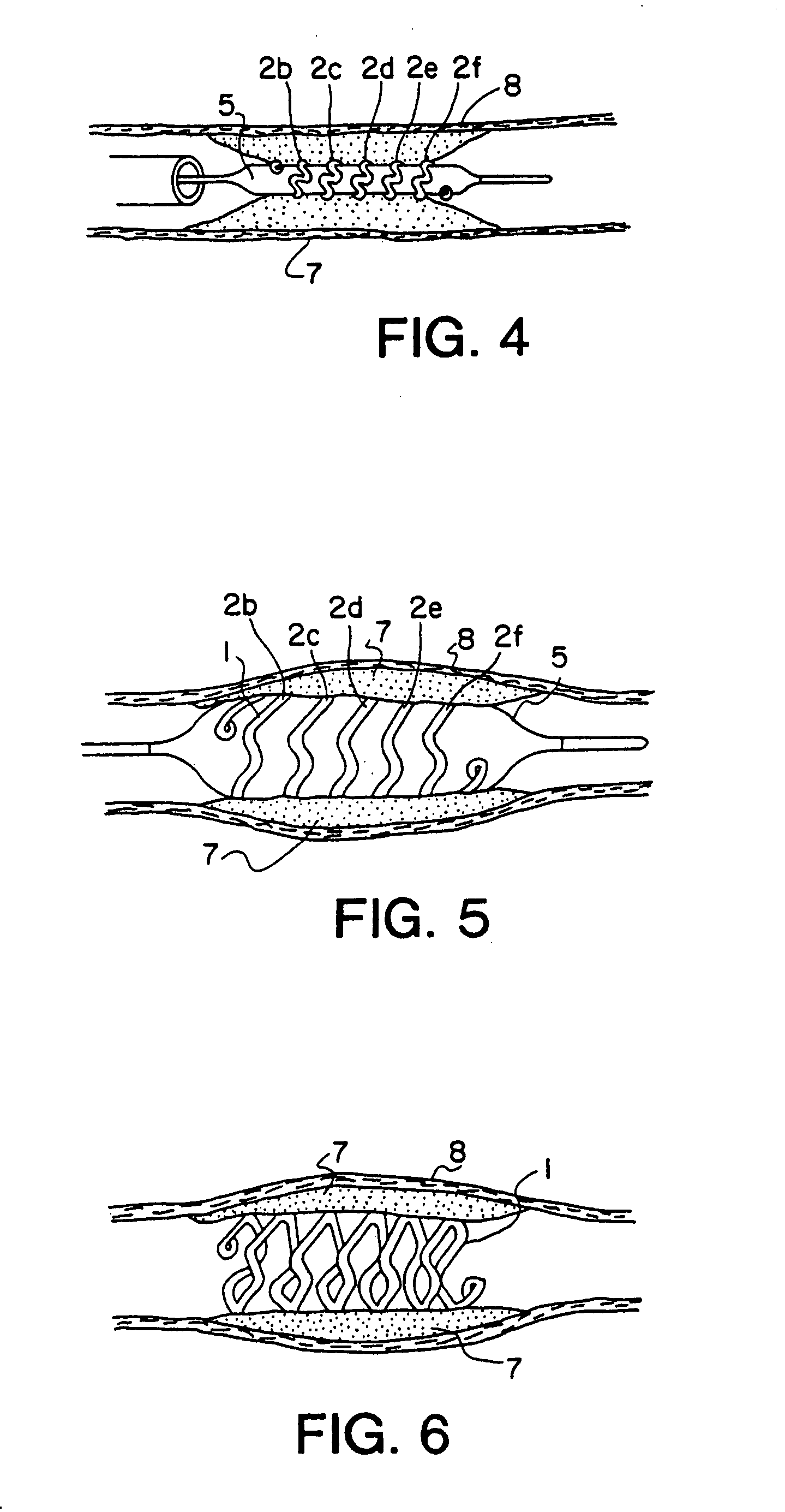
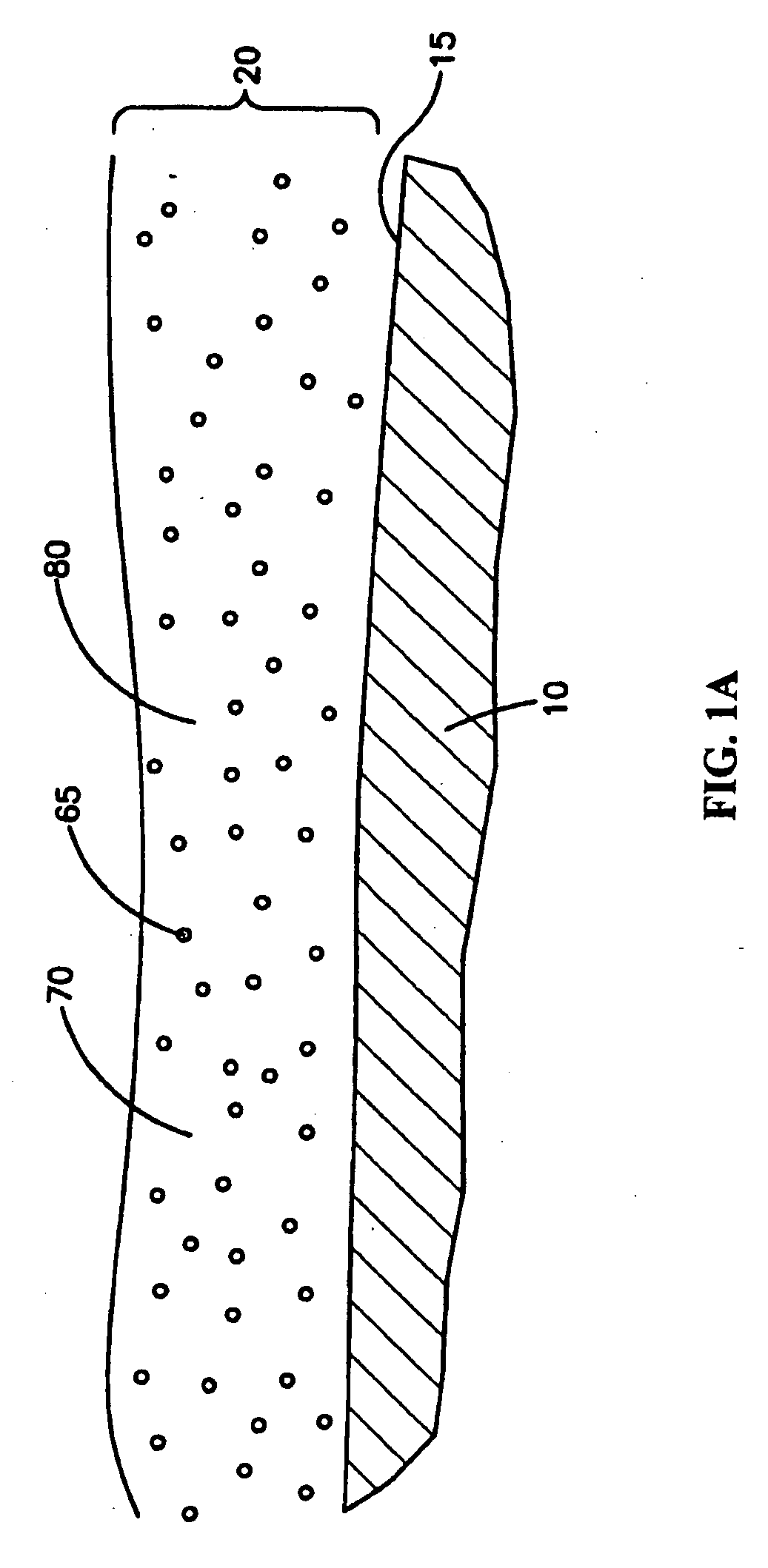
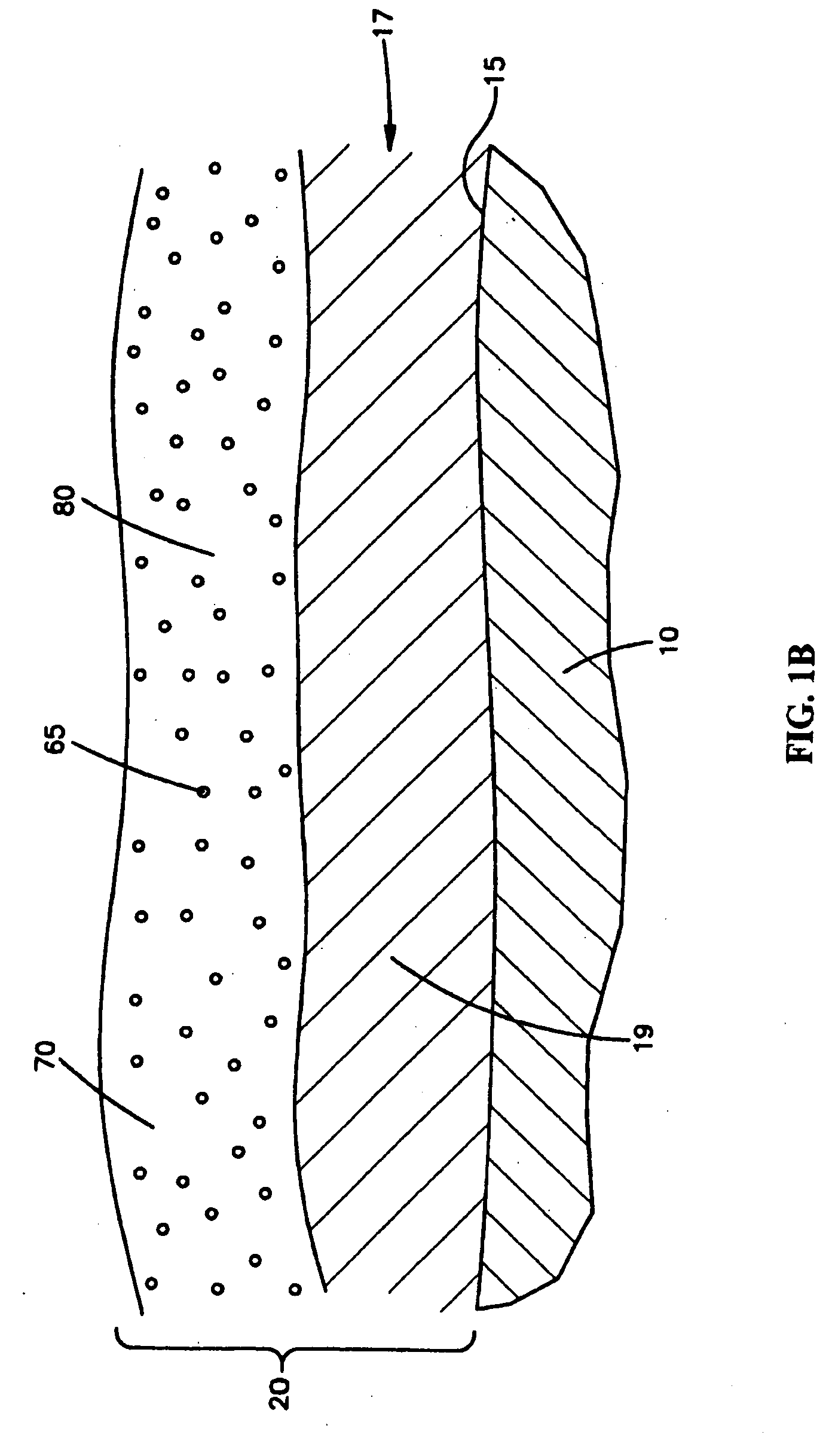
An intravascular stent is a synthetic tubular structure intended for permanent implant in native or graft vasculature. The stent is designed to provide mechanical radial support after deployment; this support is meant to enhance vessel patency over the life of the device.
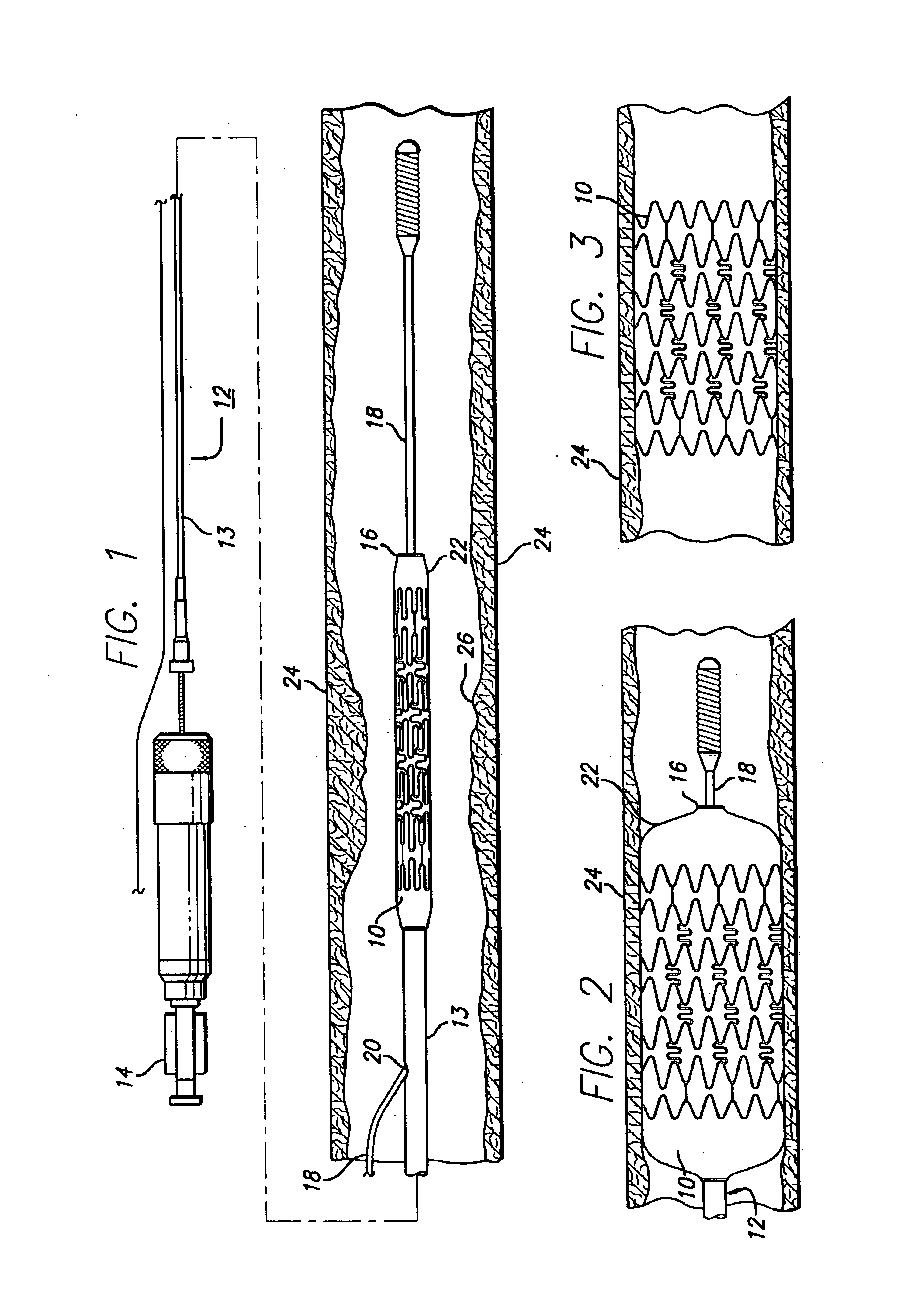
Percutaneously implantable replacement heart valve device and method of making same
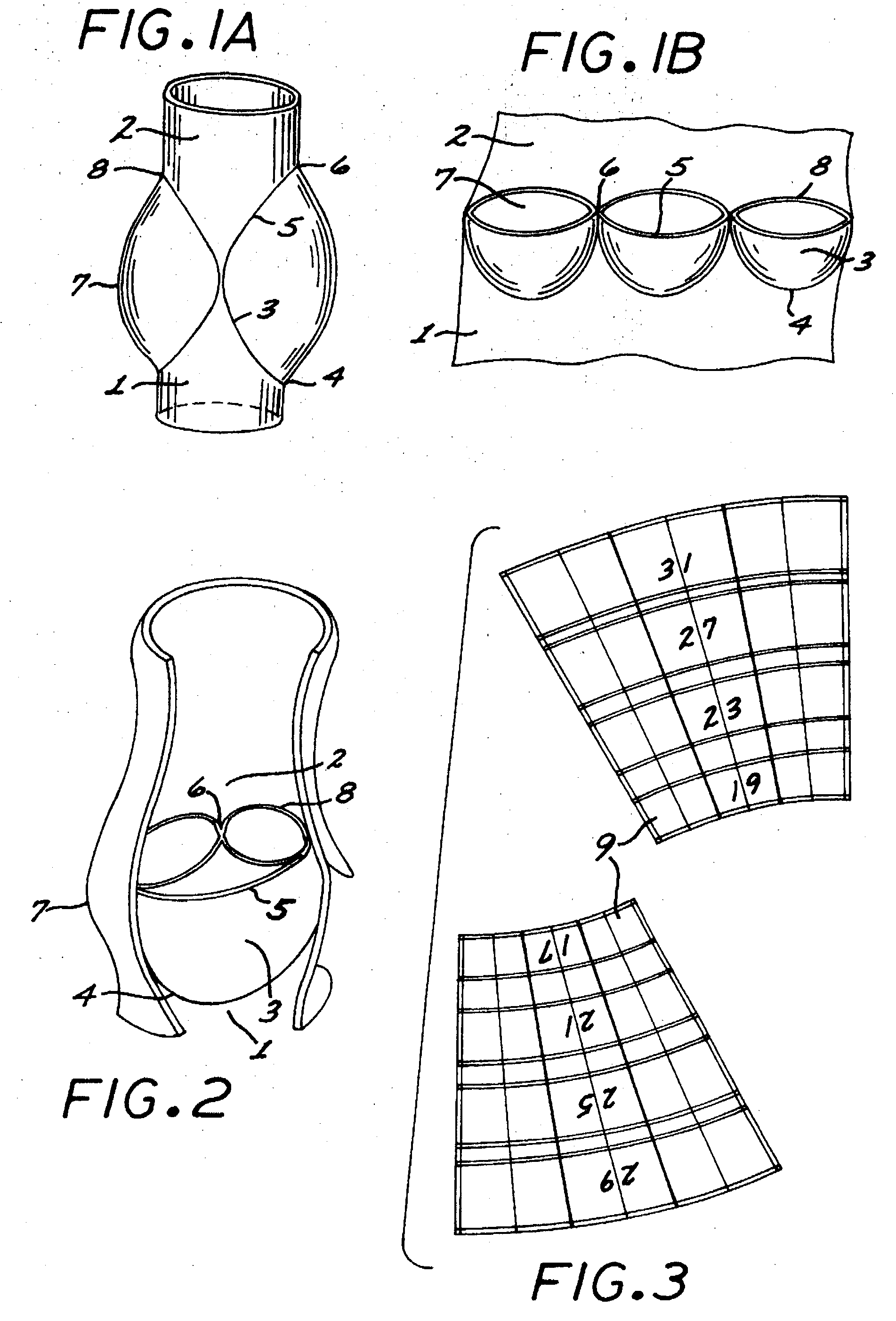
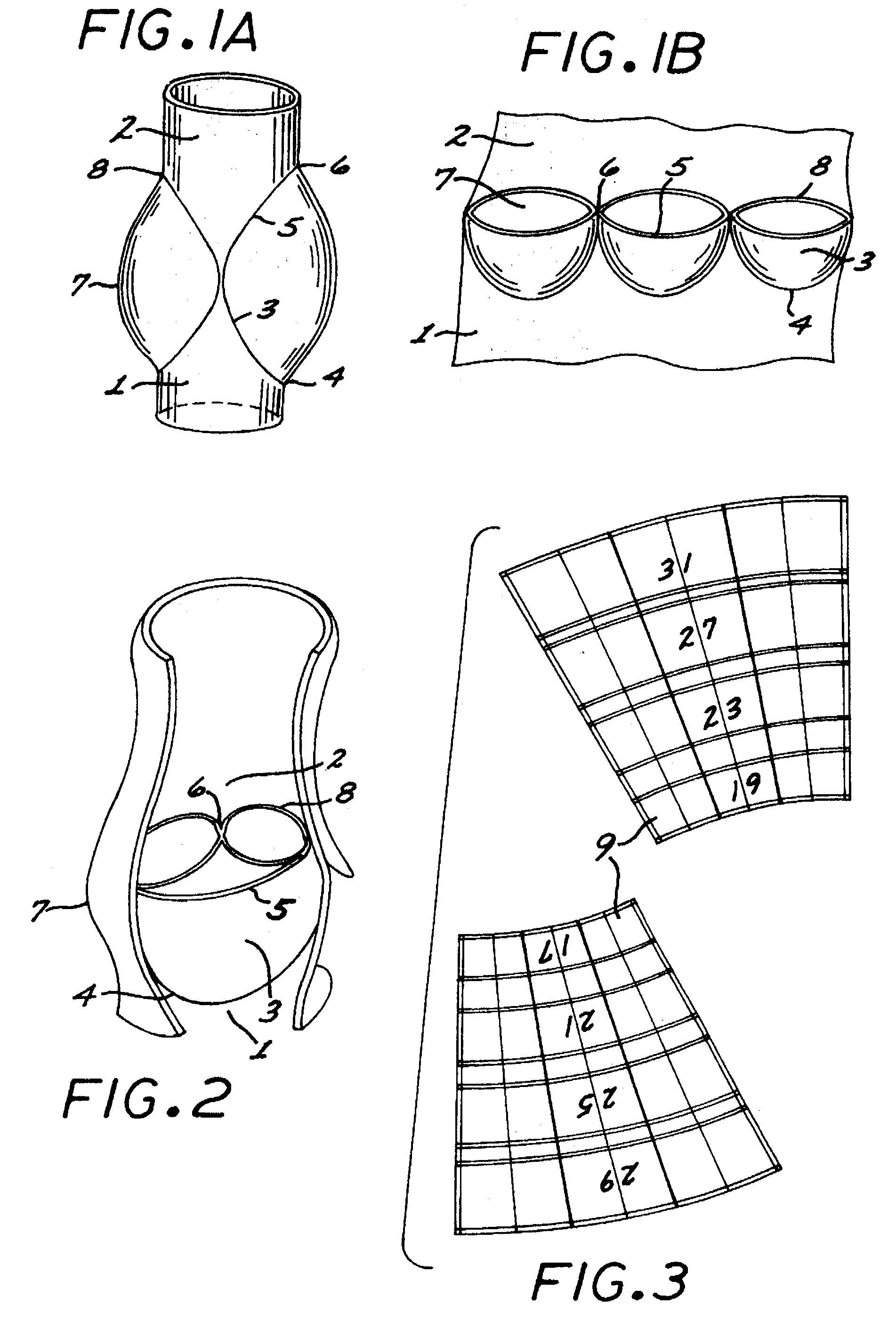
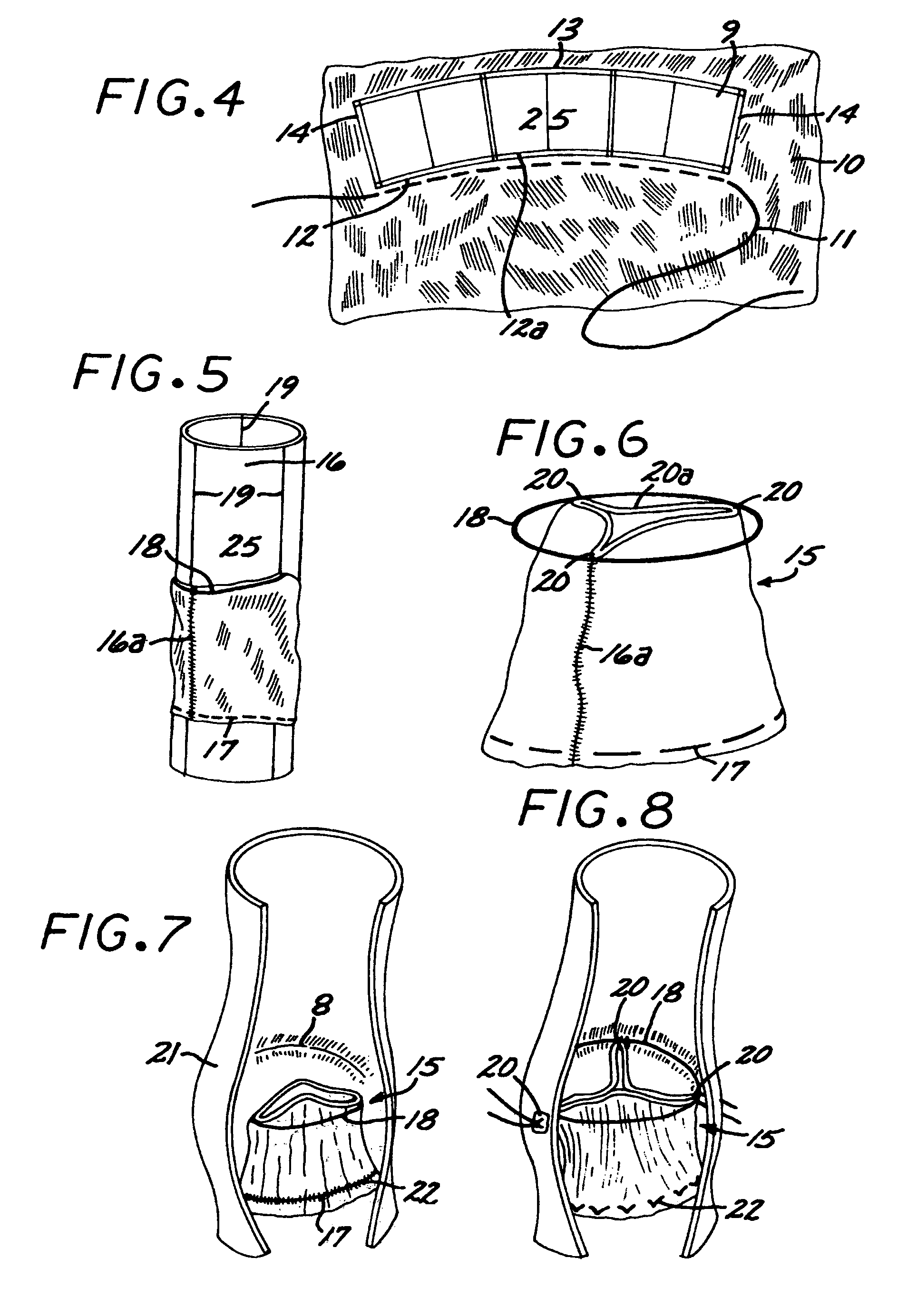
The present invention comprises a percutaneously implantable replacement heart valve device and a method of making same. The replacement heart valve device comprises a stent member made of stainless steel or self-expanding nitinol, a biological tissue artificial valve means disposed within the inner space of the stent member. An implantation and delivery system having a central part which consists of a flexible hollow tube catheter that allows a metallic wire guide to be advanced inside it. The endovascular stented-valve is created from a glutaraldehyde fixed biocompatible tissue material which has two or three cusps that open distally to permit unidirectional blood flow. The present invention also comprises a novel method of making a replacement heart valve by taking a fragment of biocompatible tissue material and treating, drying, folding and rehydrating it in such a way that forms a two- or three-leaflet / cusp valve with the leaflets / cusps formed by folding, thereby eliminating the extent of suturing required, providing improved durability and function.
Owner:COLIBRI HEART VALVE
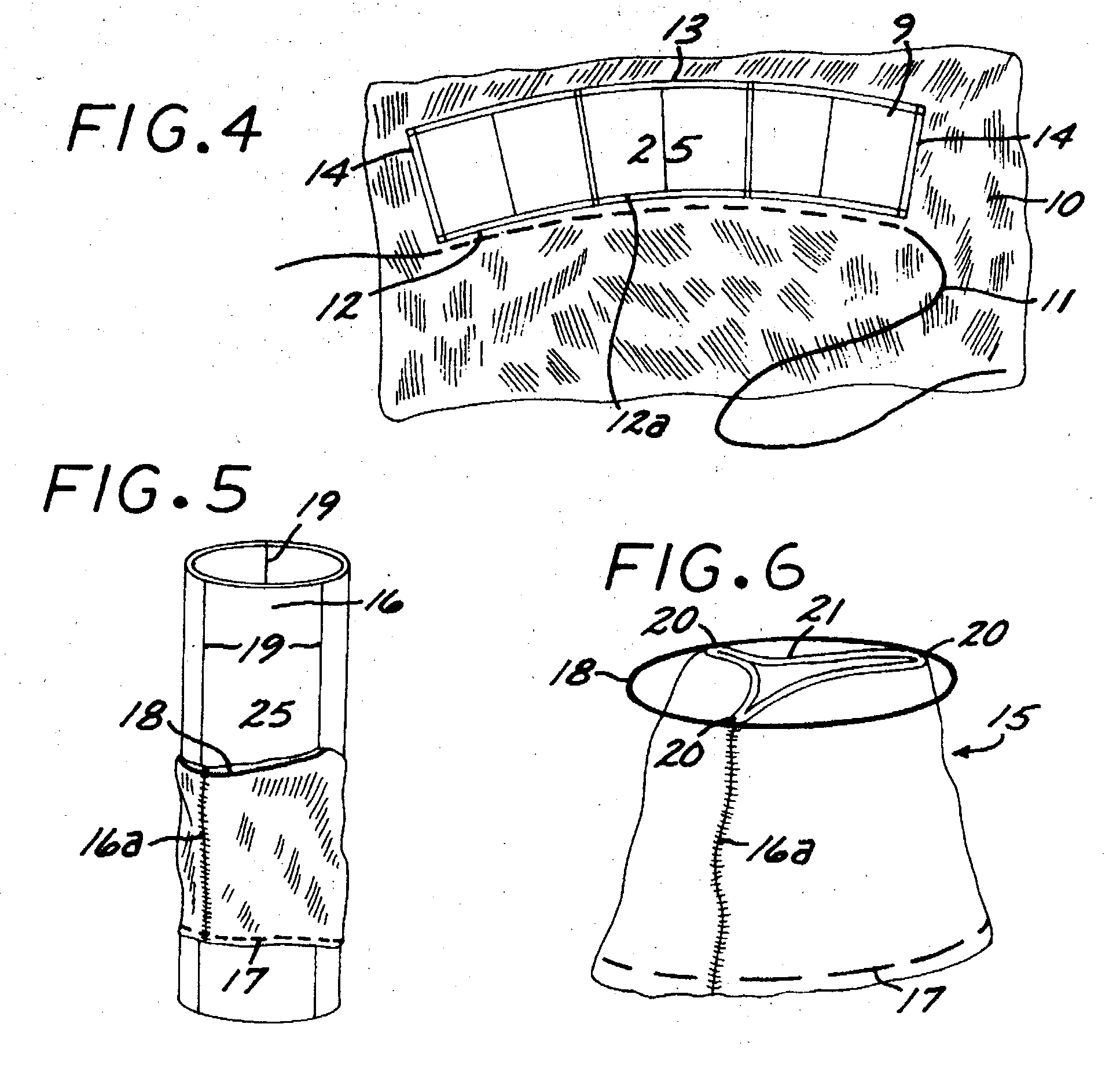
Sigmoid valve and method for its percutaneous implantation
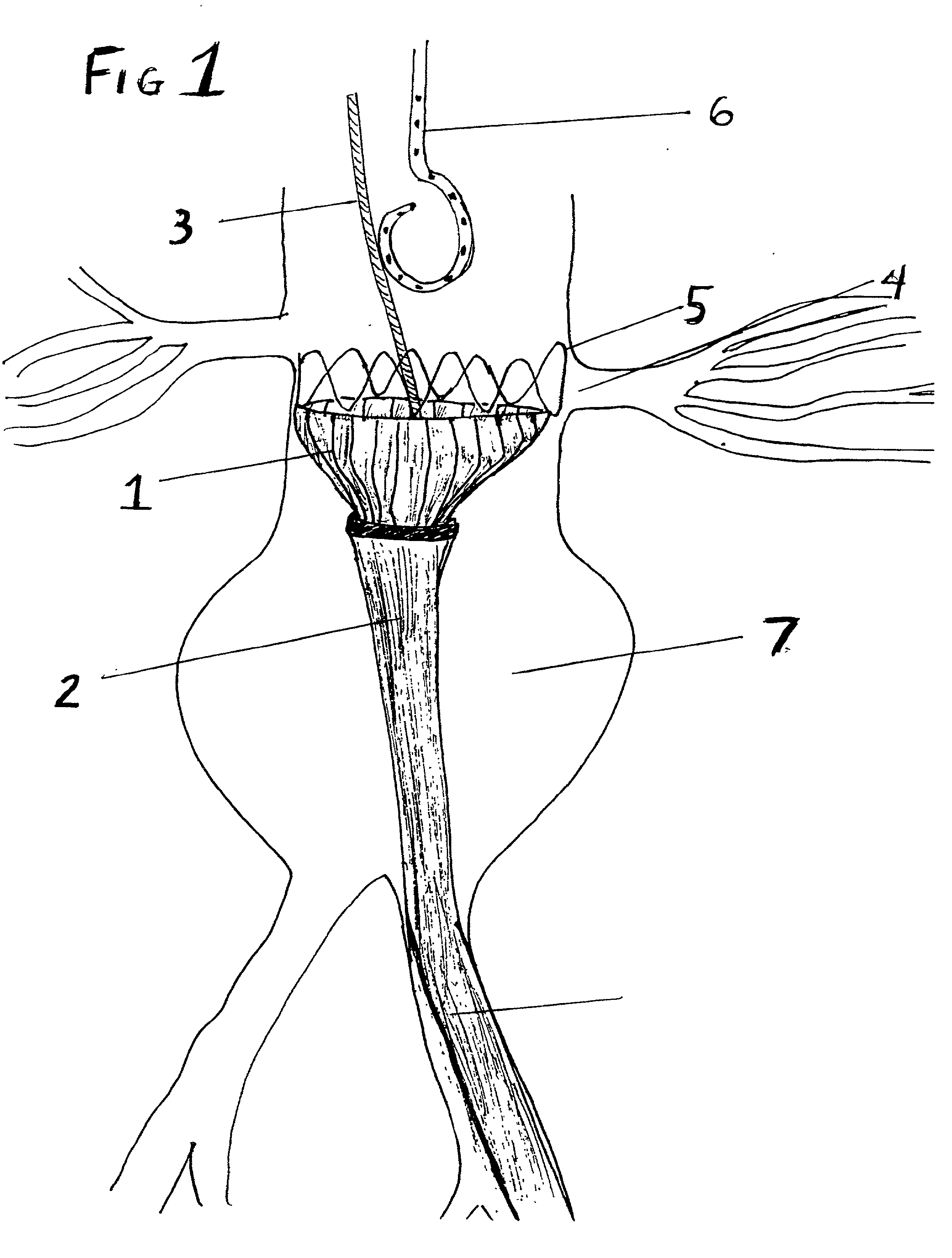
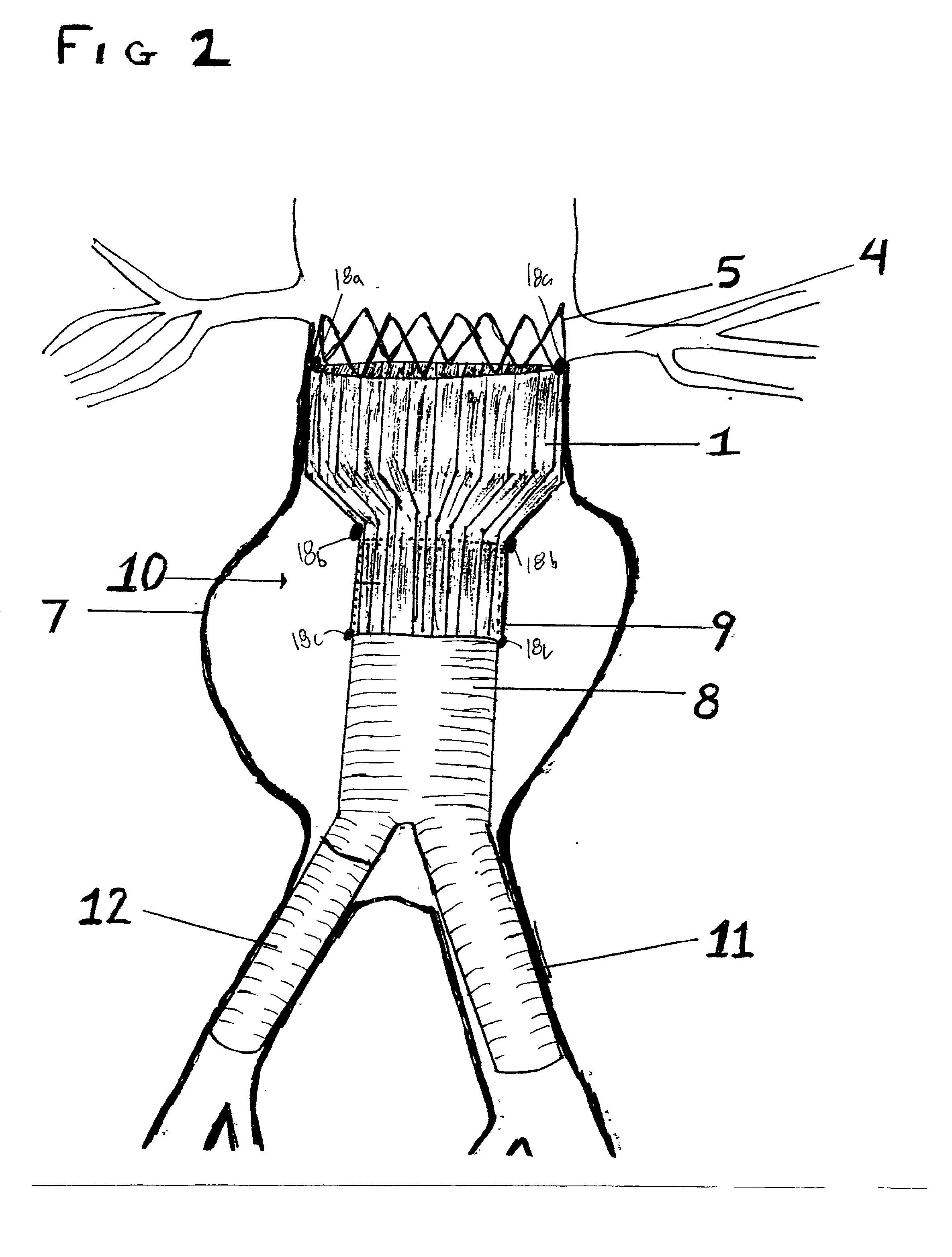
A multi-leaflet valve adapted to serve as a prosthesis for diseased native valve of a mammal is incorporated in self-expandable or inflatable endovascular stents or stents to form a combination which is introduced on a catheter with a guide wire into the circulatory system of the mammal to replace the diseased native valve. Once the combination is at the desired location the stent is caused to expand and affix itself to the patient's vessel wall. The prosthetic valve has the shape of a truncated cone that has an inflow and an outflow orifice with leaflets forming the outflow orifice and forming a plurality of commissures. A first flexible circular support is affixed in a substantially circular fashion around the truncated cone in proximity of the inflow orifice, and a second flexible circular support is affixed at the location of the commissures to form a circle around the truncated cone in proximity of the outflow orifice. The circular supports maintain the shape of the valve during the surgical implantation procedure and thereafter.
Owner:THE INT HEART INST OF MONTANA FOUND
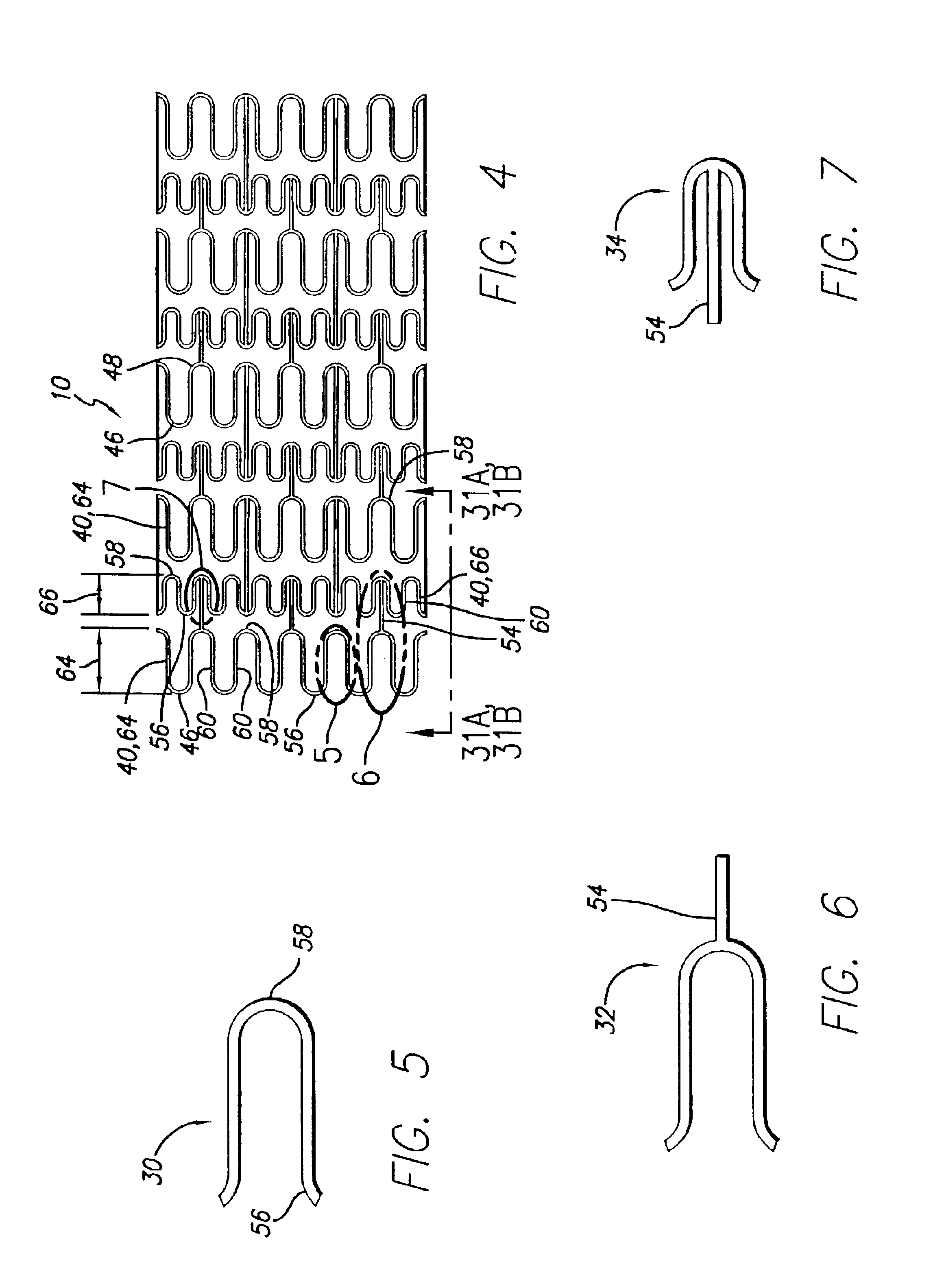
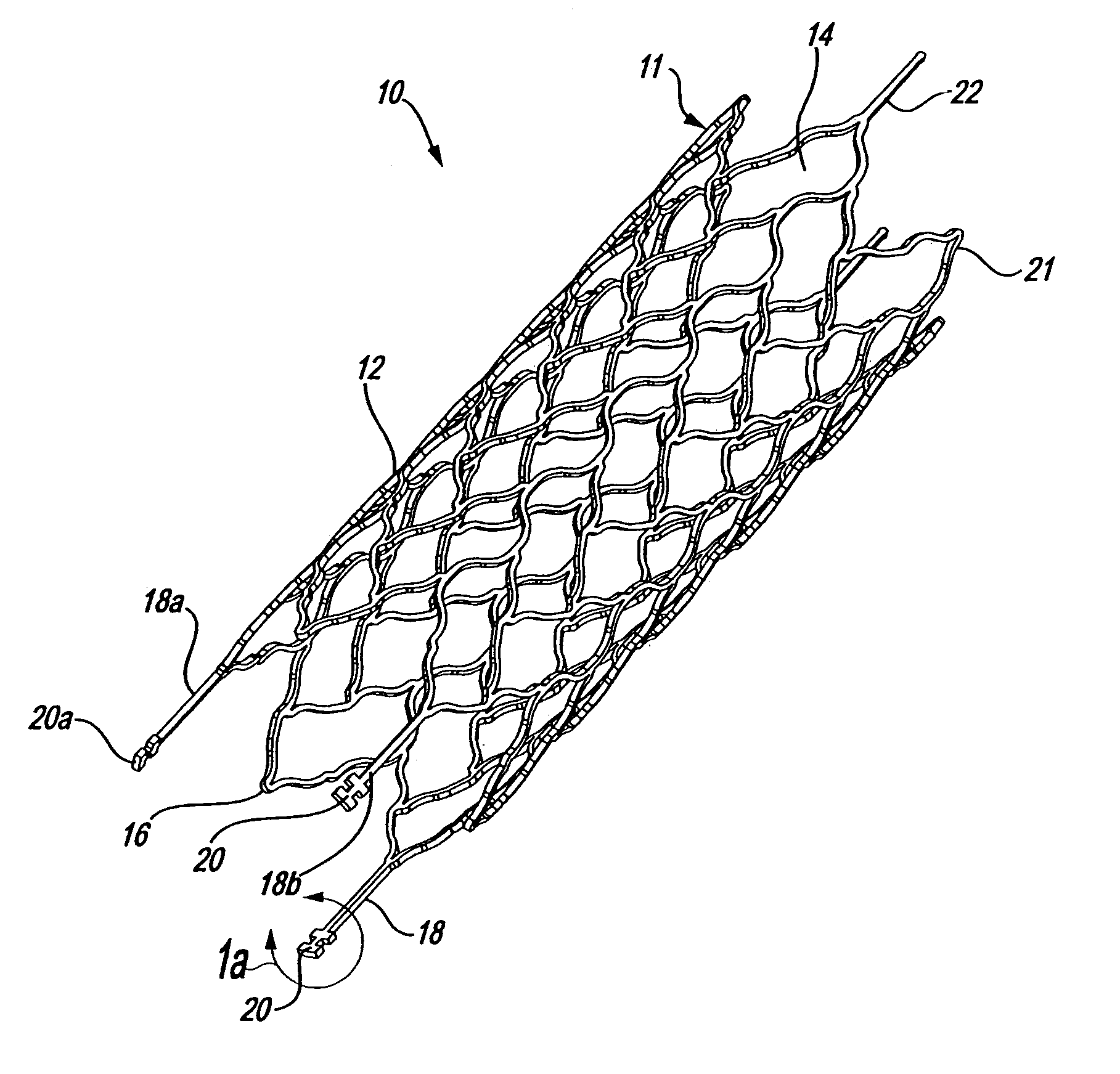
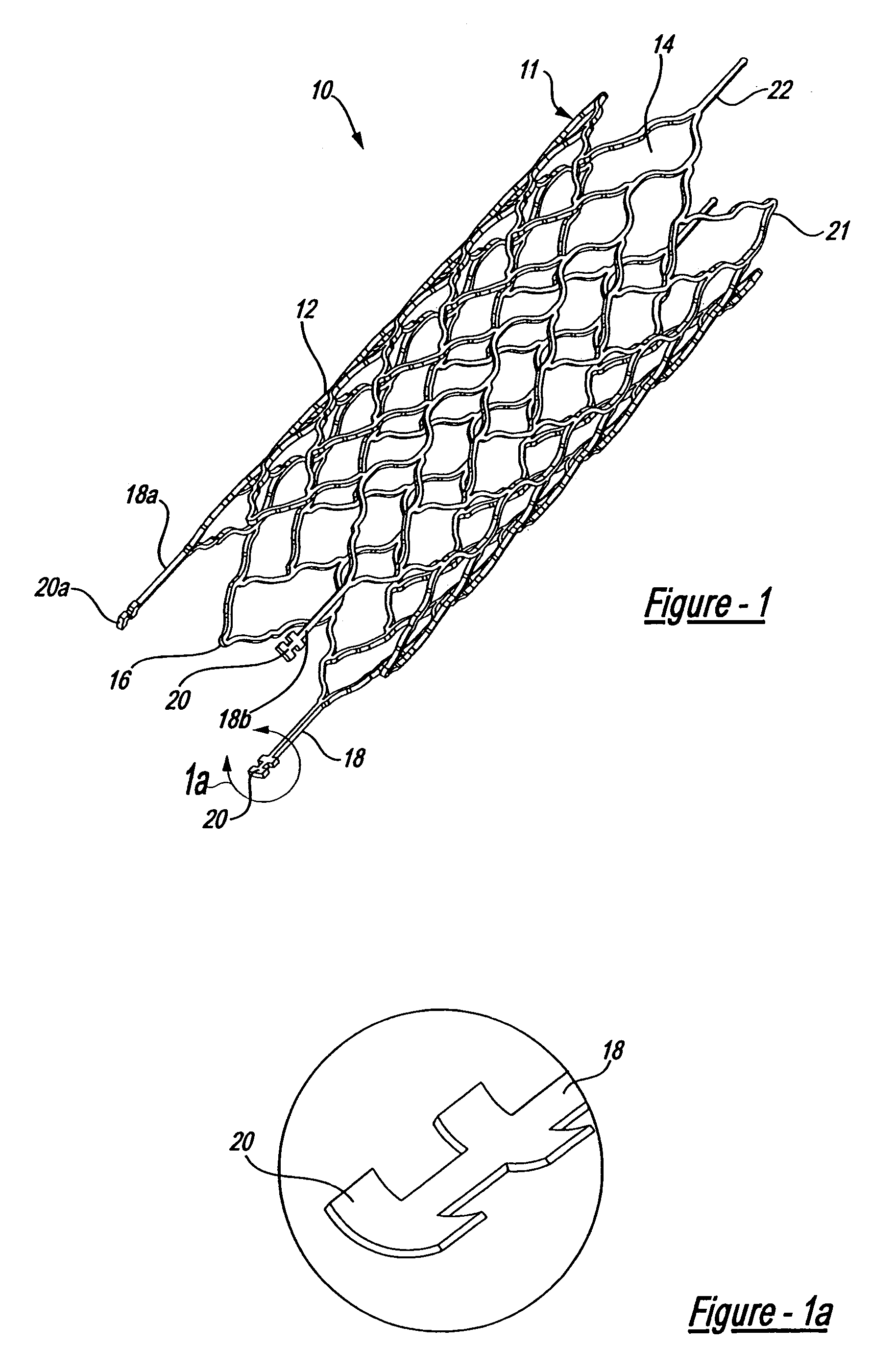
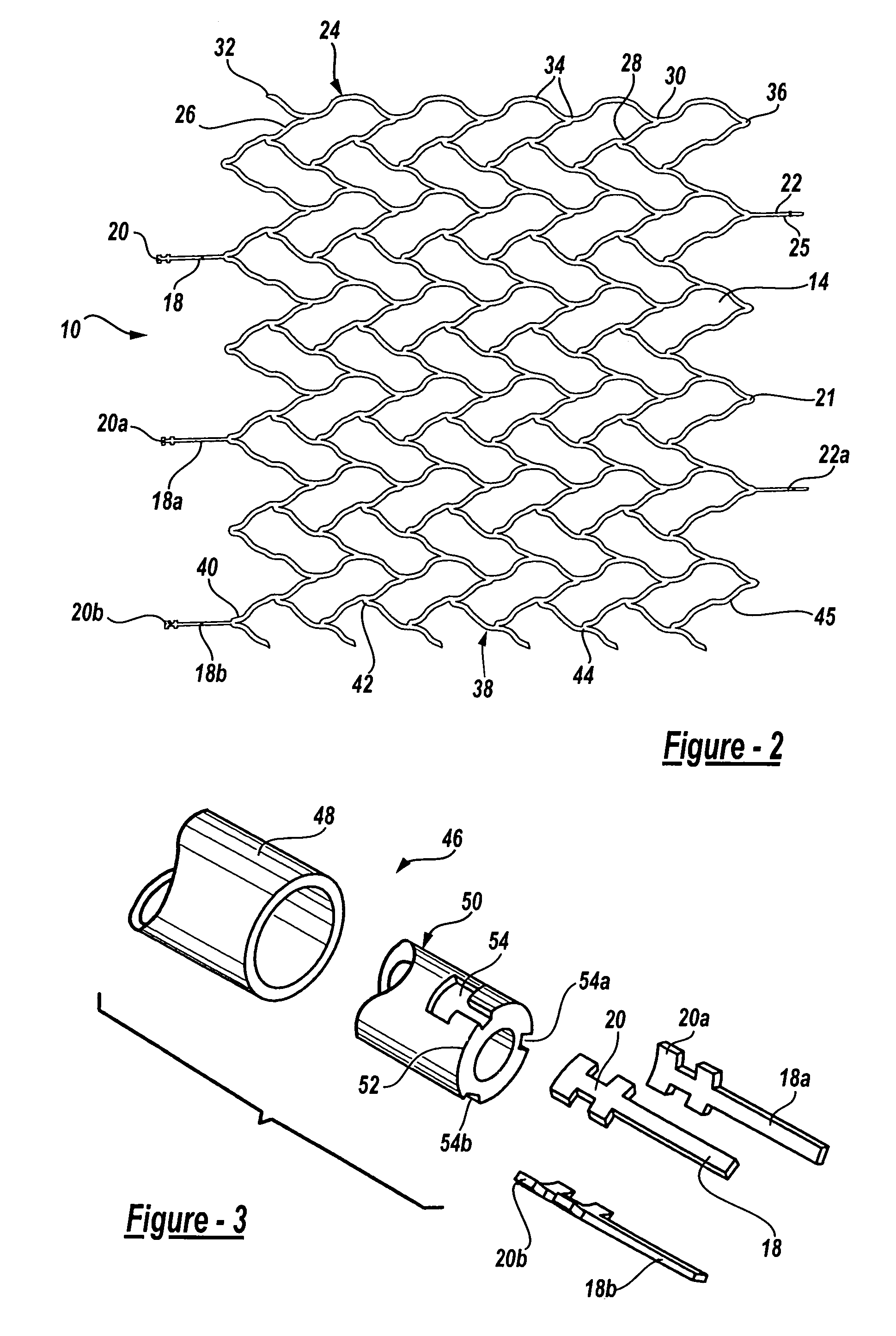
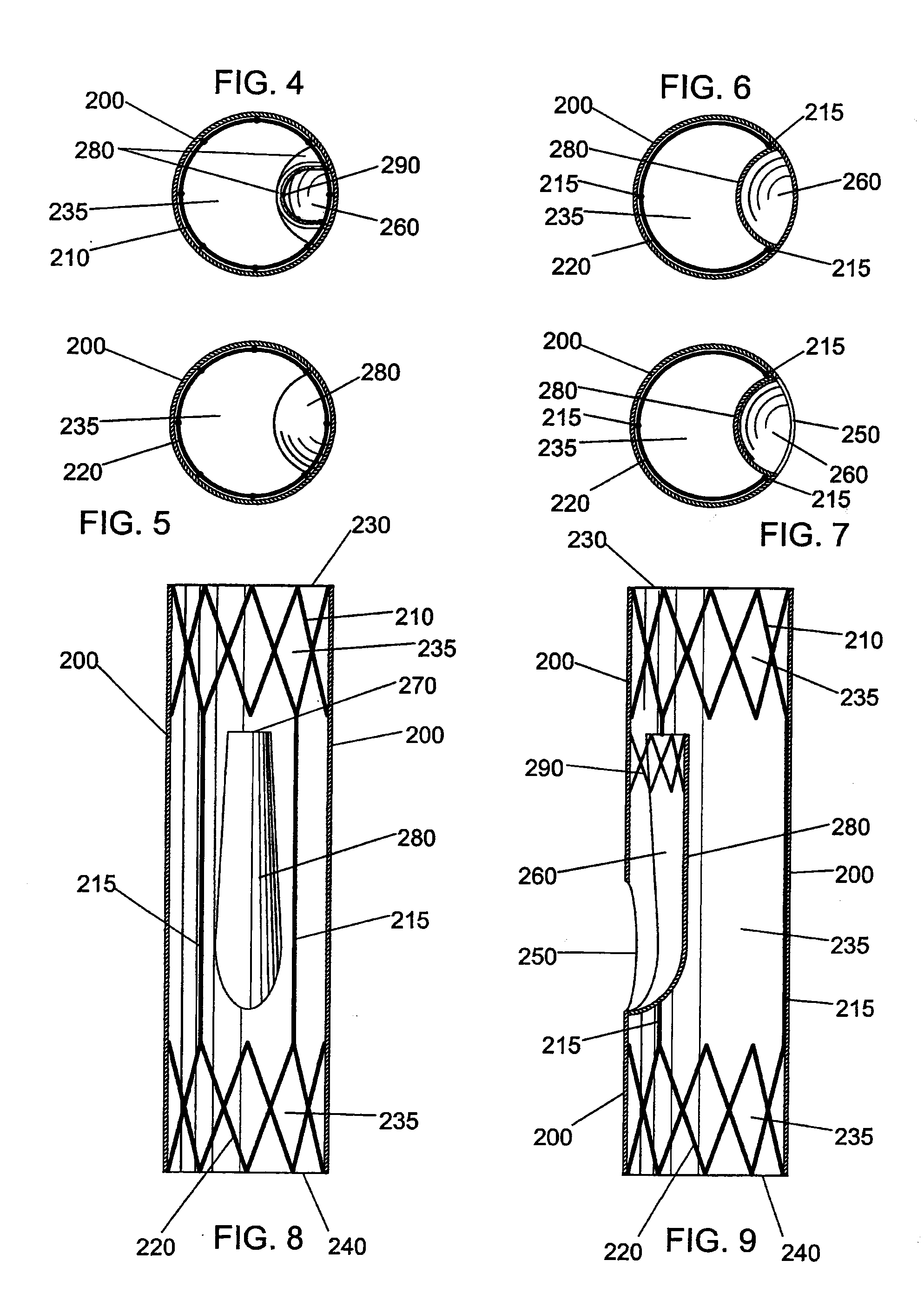
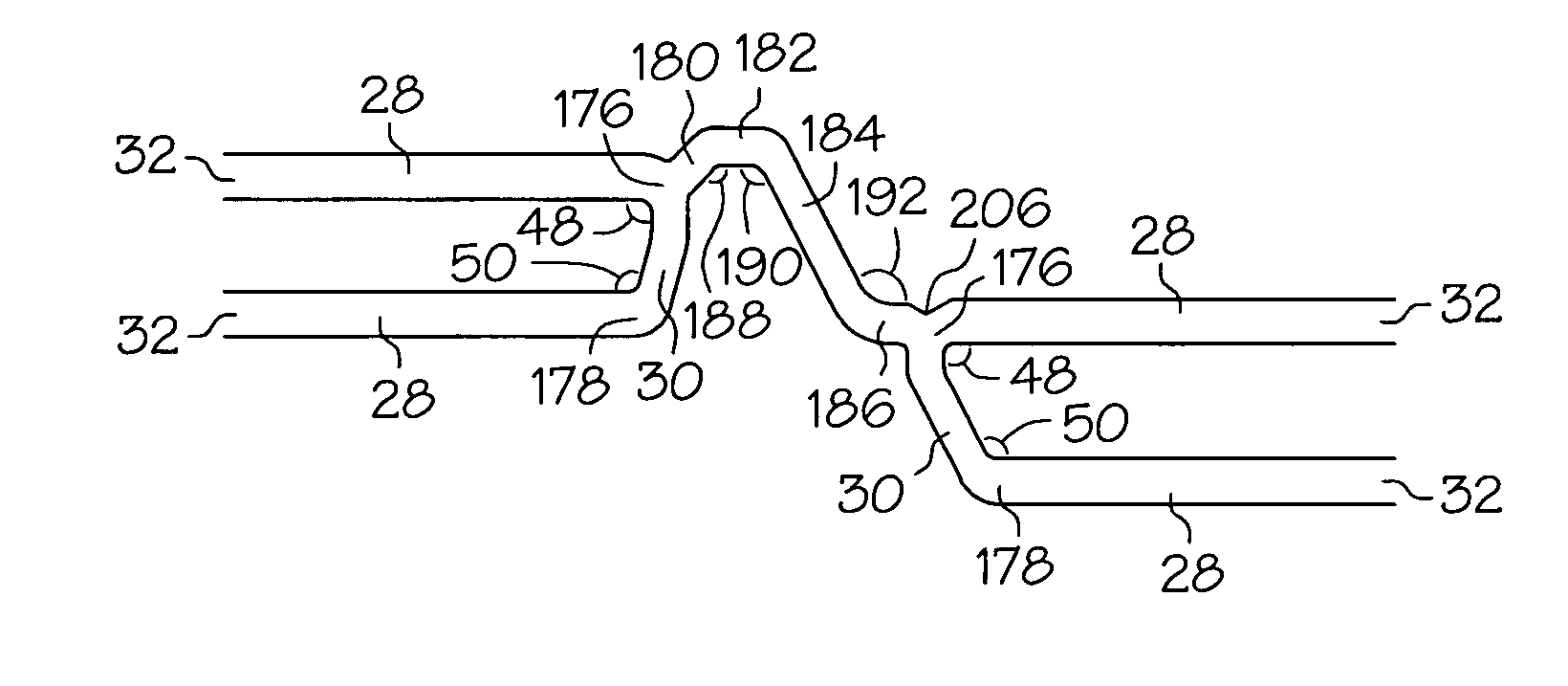
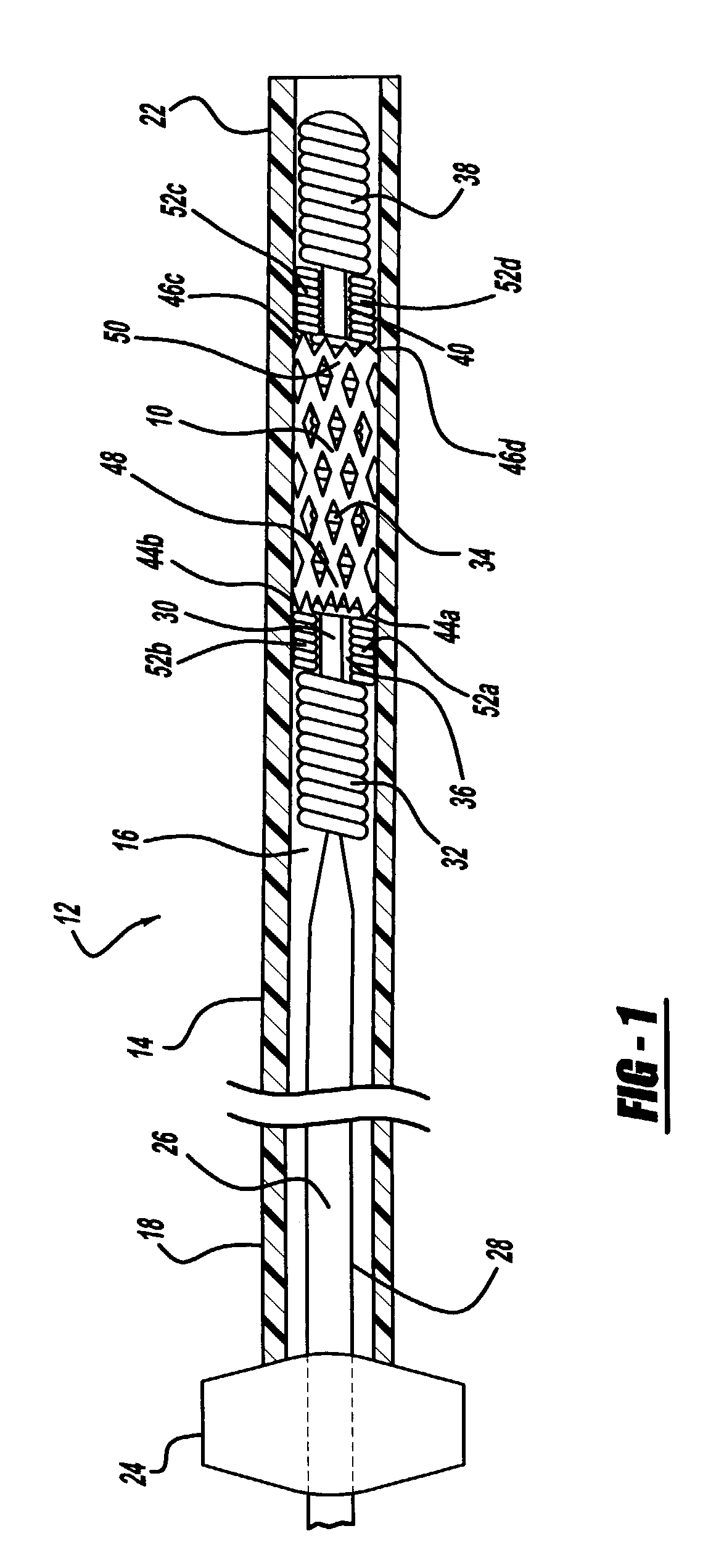
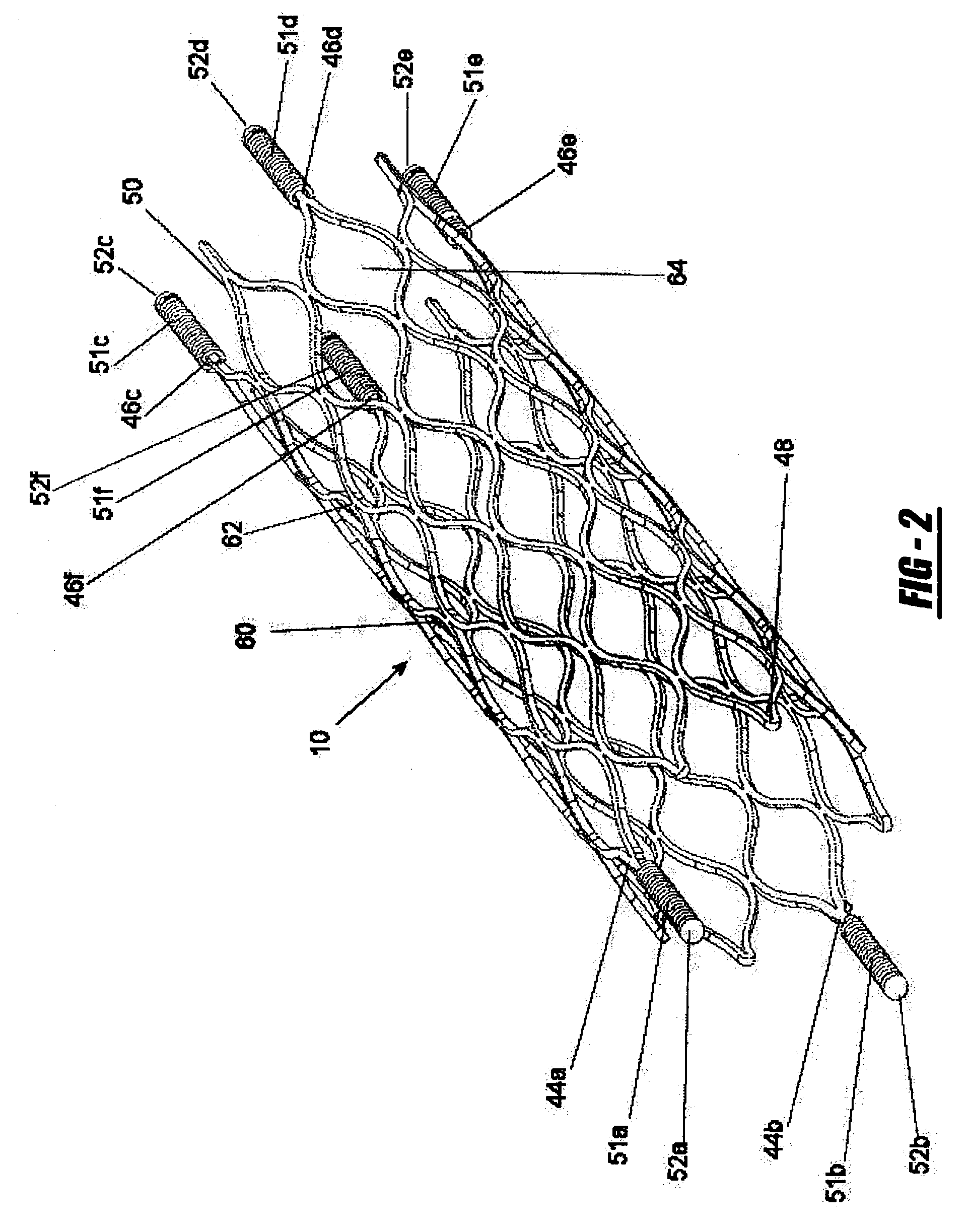
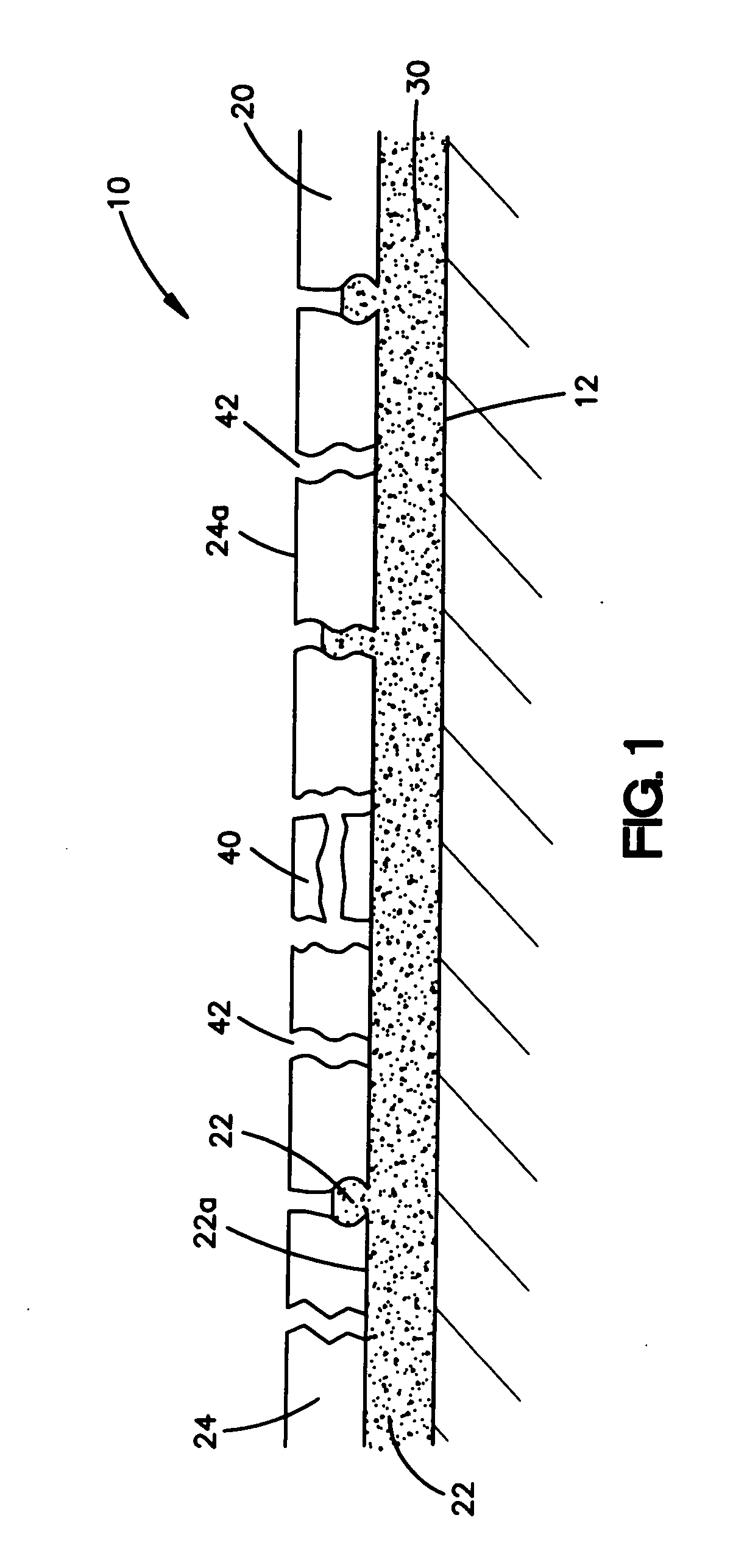
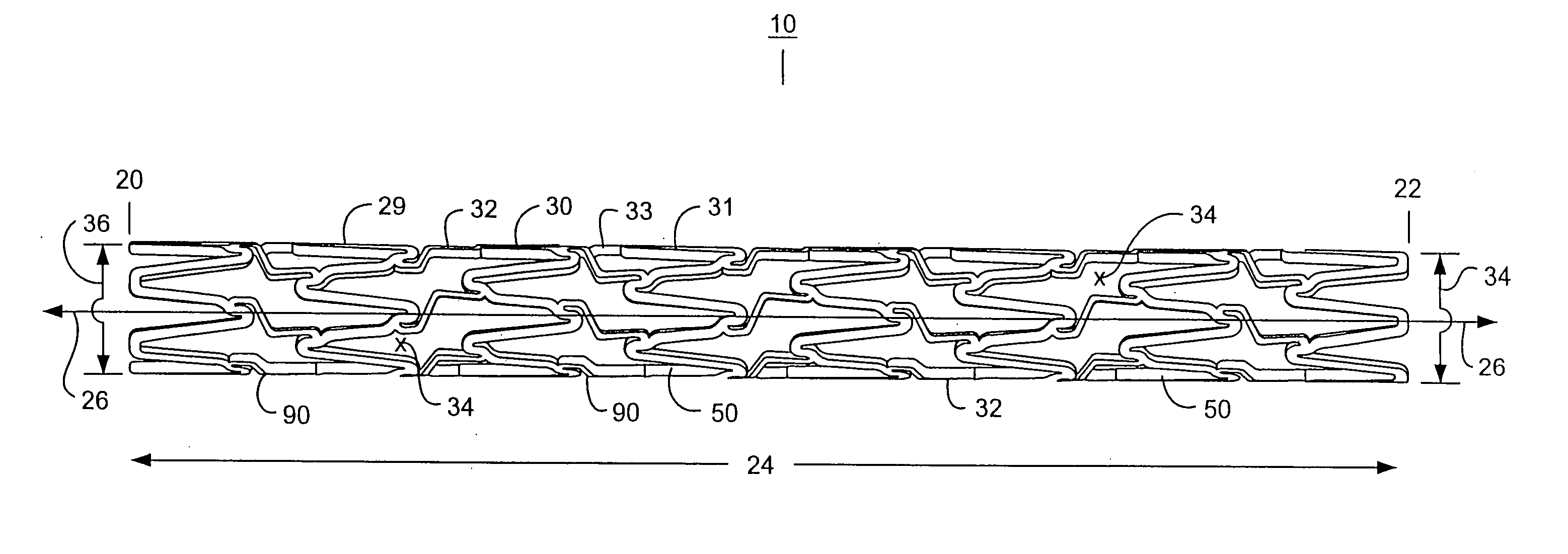
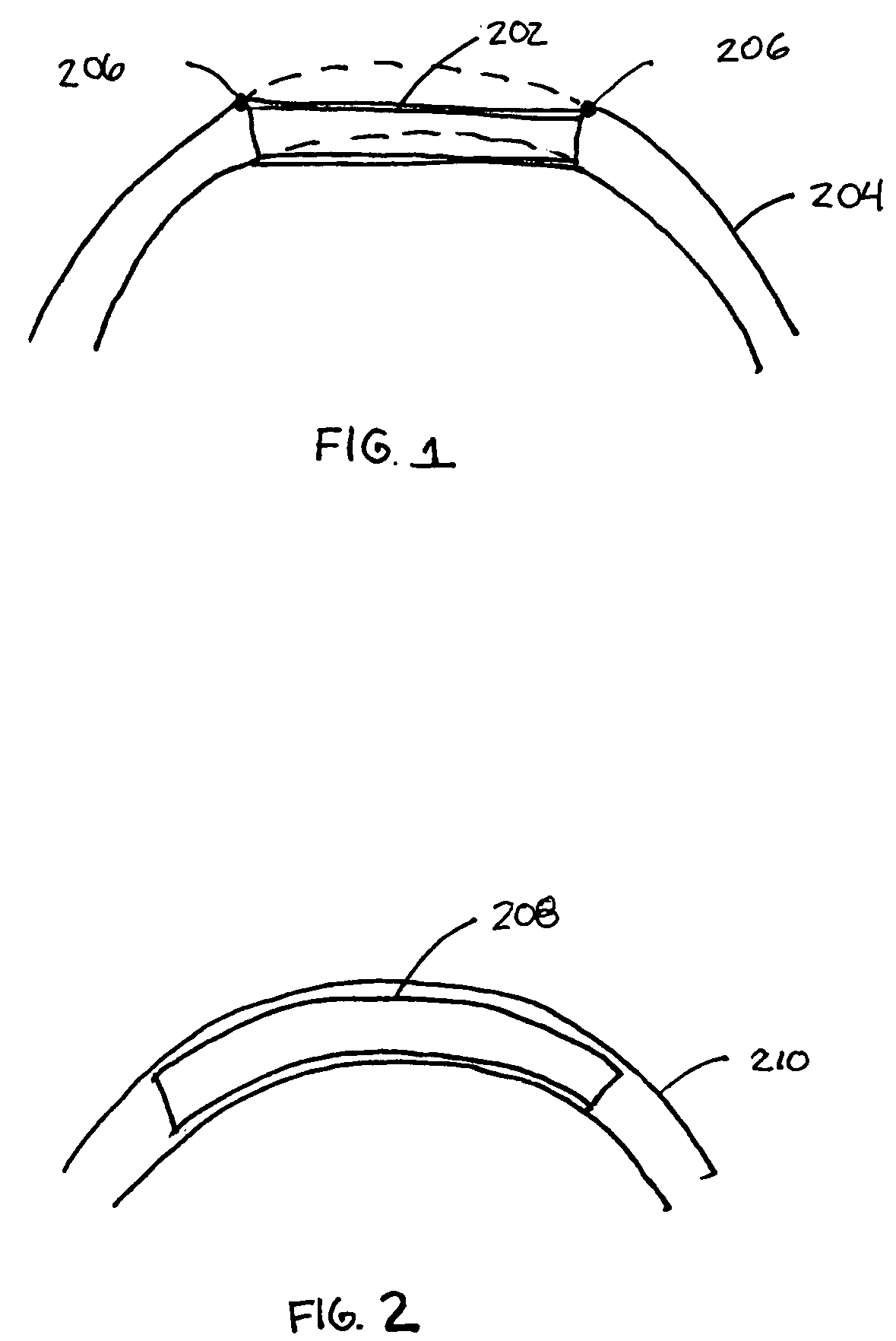
Intravascular stent
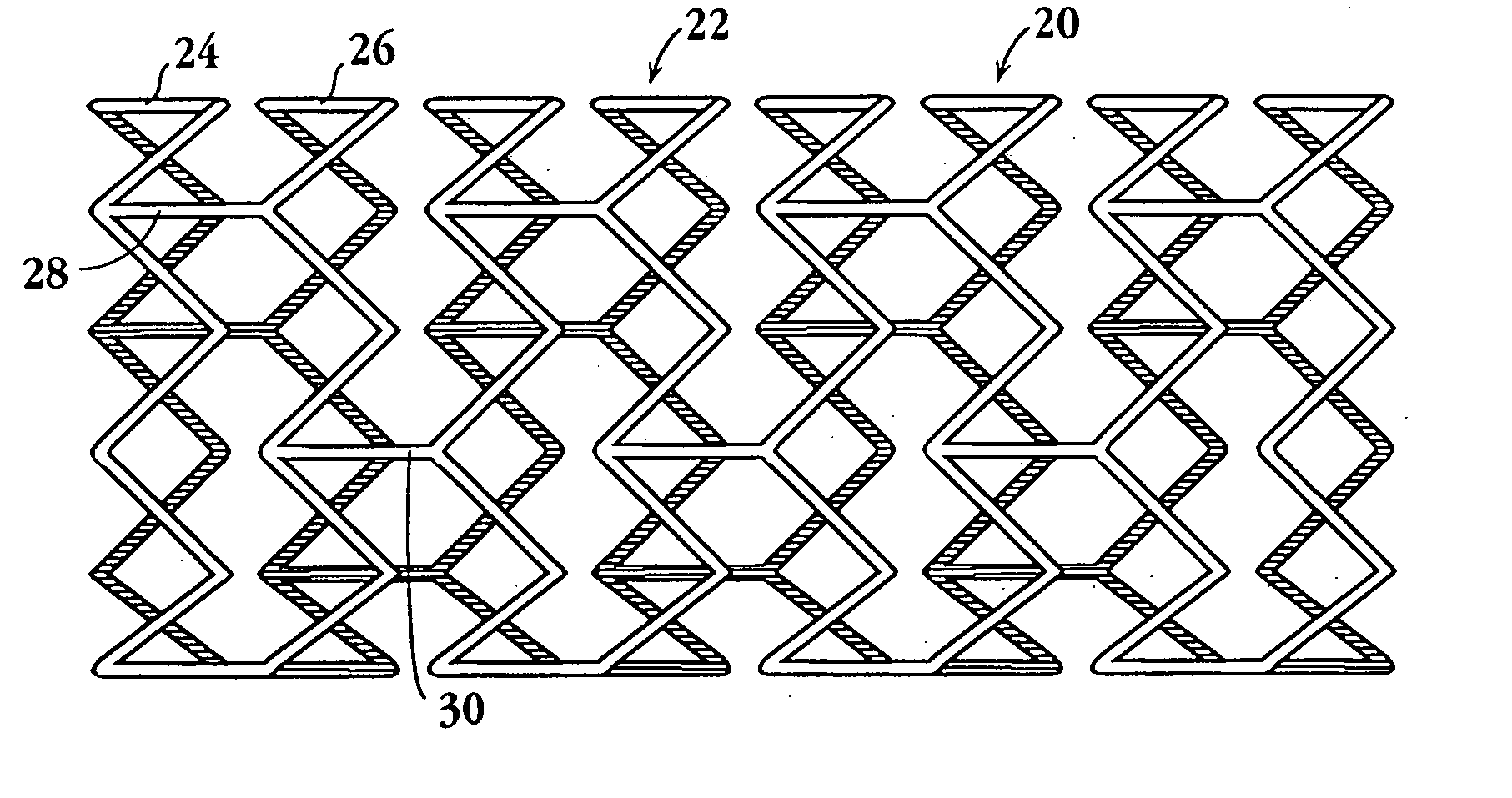
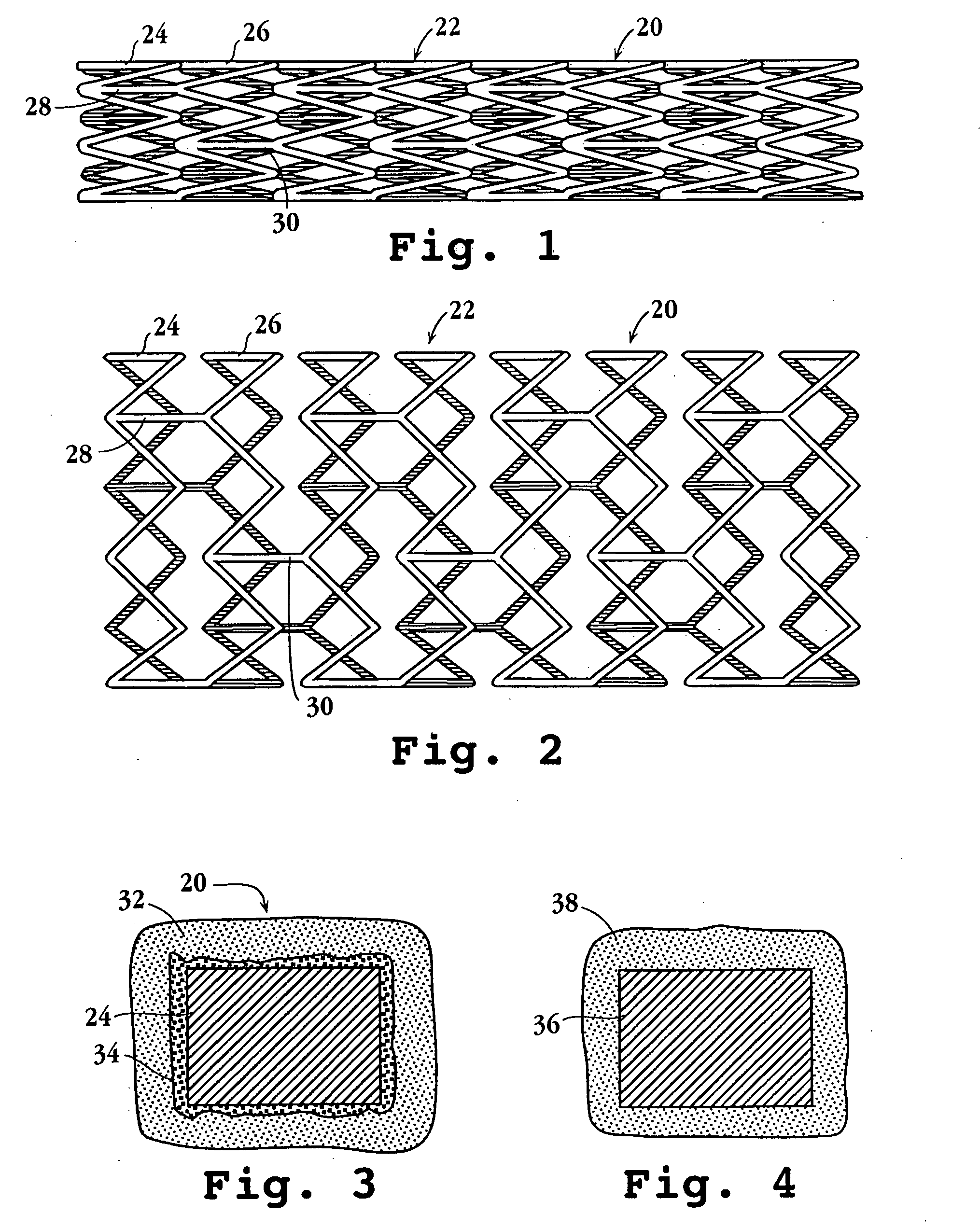
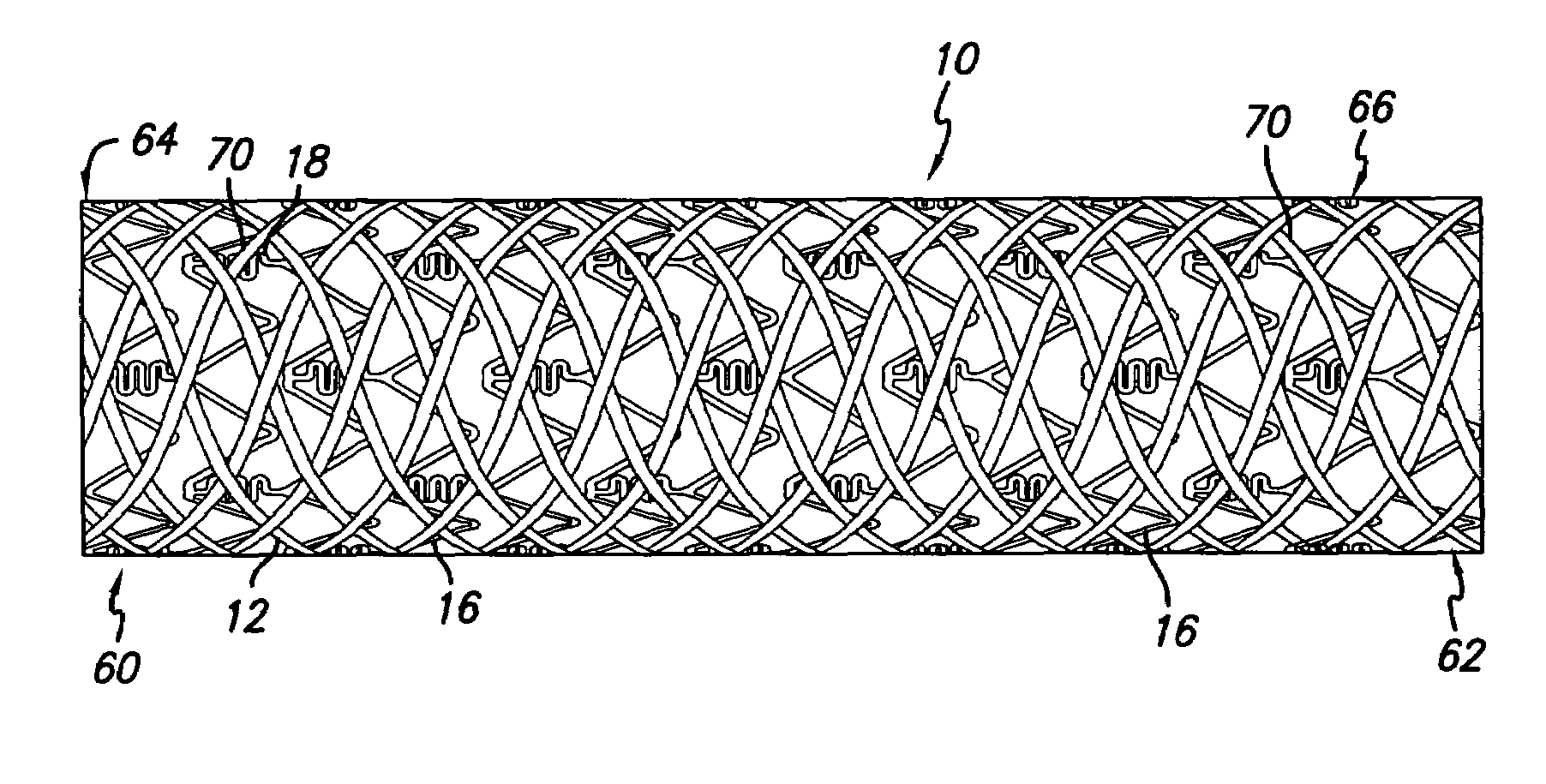
An intravascular stent assembly for implantation in a body vessel, such as a coronary artery, includes undulating circumferential rings having peaks on the proximal end and valleys on the distal end. Adjacent rings are coupled together by links. The rings and links are arranged so that the stent has good conformability as it traverses through, or is deployed in, a tortuous body lumen. The stent is also configured such that the likelihood of peaks and valleys on adjacent rings which point directly at each other to overlap in tortuous body vessels is reduced.
Owner:ABBOTT CARDIOVASCULAR
Methods of using an intravascular balloon catheter in combination with an angioscope
A method of performing a medical procedure is disclosed which involves the use of a balloon catheter in combination with an angioscope to monitor an intravascular stent before, during or after deploying the stent. Specifically, the method may include the steps of (1) delivering an intravascular stent into the vasculature of a patient, (2) positioning a balloon catheter in the vasculature such that the inflatable balloon is adjacent the stent, (3) inserting an angioscope into the balloon catheter such that the distal end of the angioscope is adjacent an optically-transparent tube traversing the interior of the balloon, and (4) visually monitoring the stent before, during or after deploying the intravascular stent. The optically-transparent tube may define a guide wire lumen for use with a guide wire and the angioscope may be inserted into the guide wire lumen. The intravascular stent may be a balloon-expandable stent, a self-expanding stent, or a stent made of a photo-responsive polymer.
Owner:BOSTON SCI SCIMED INC
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
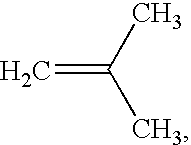
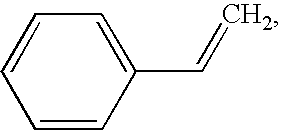
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
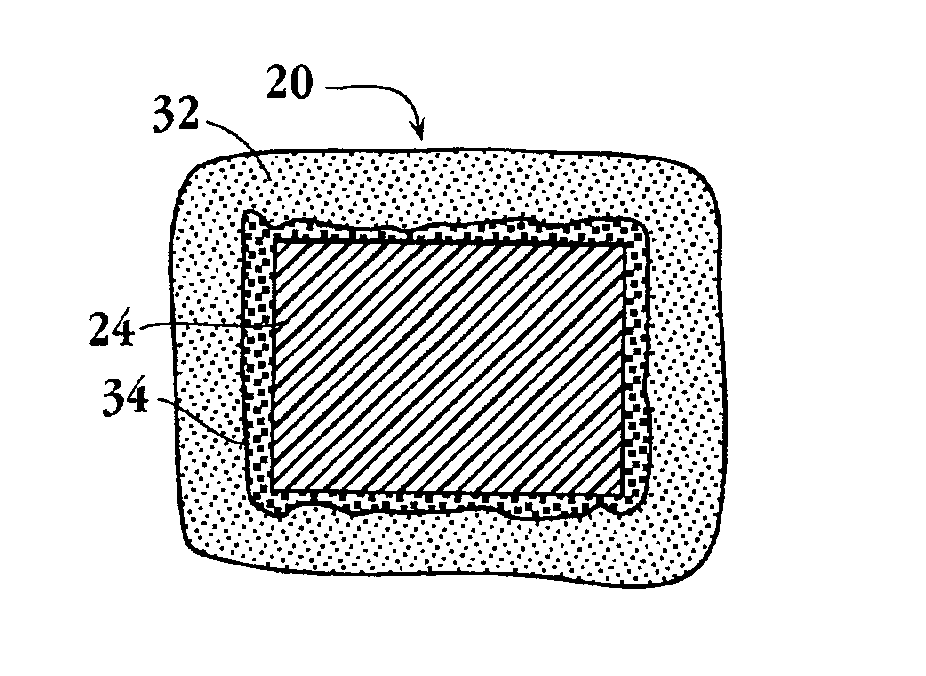
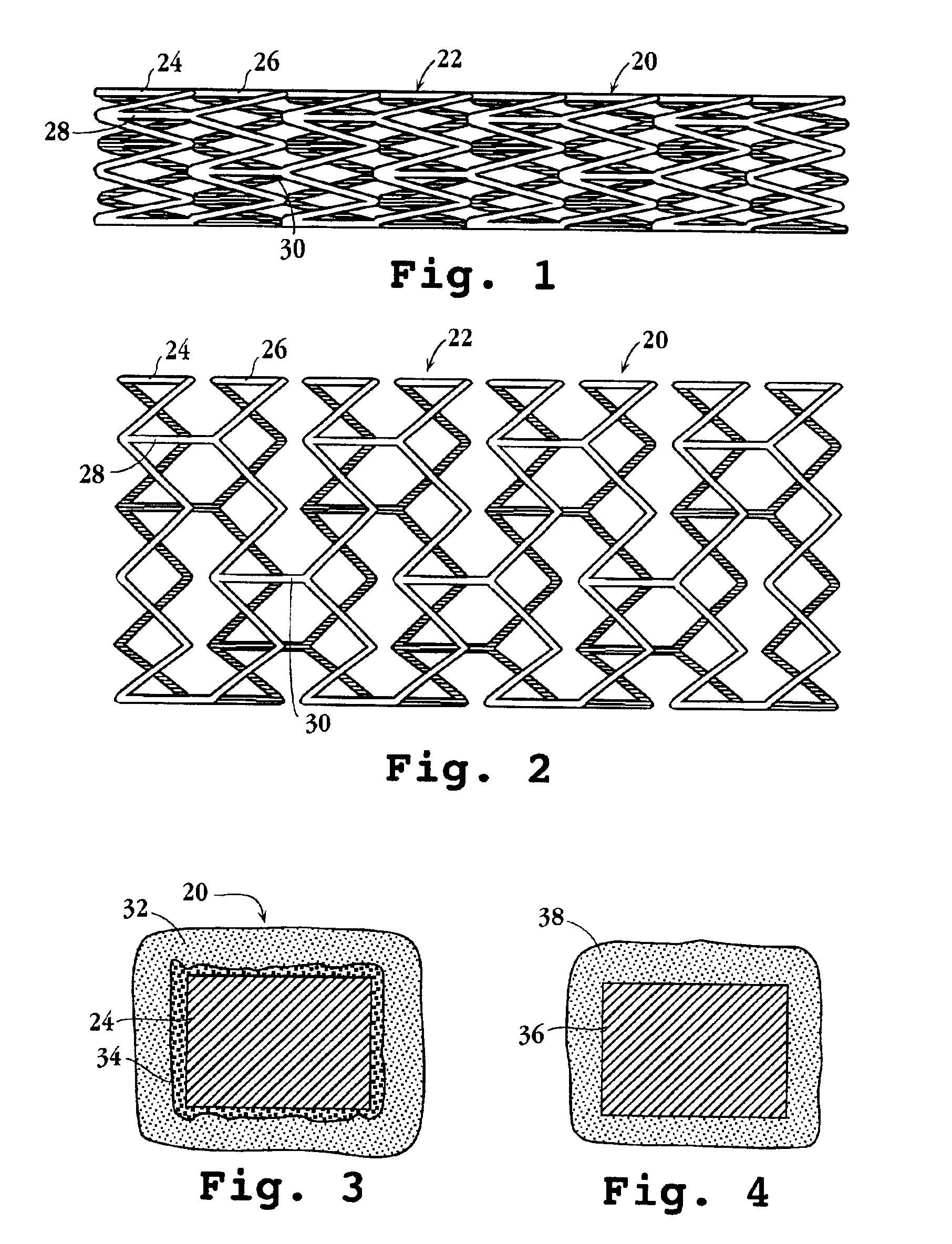
Intravascular stent device
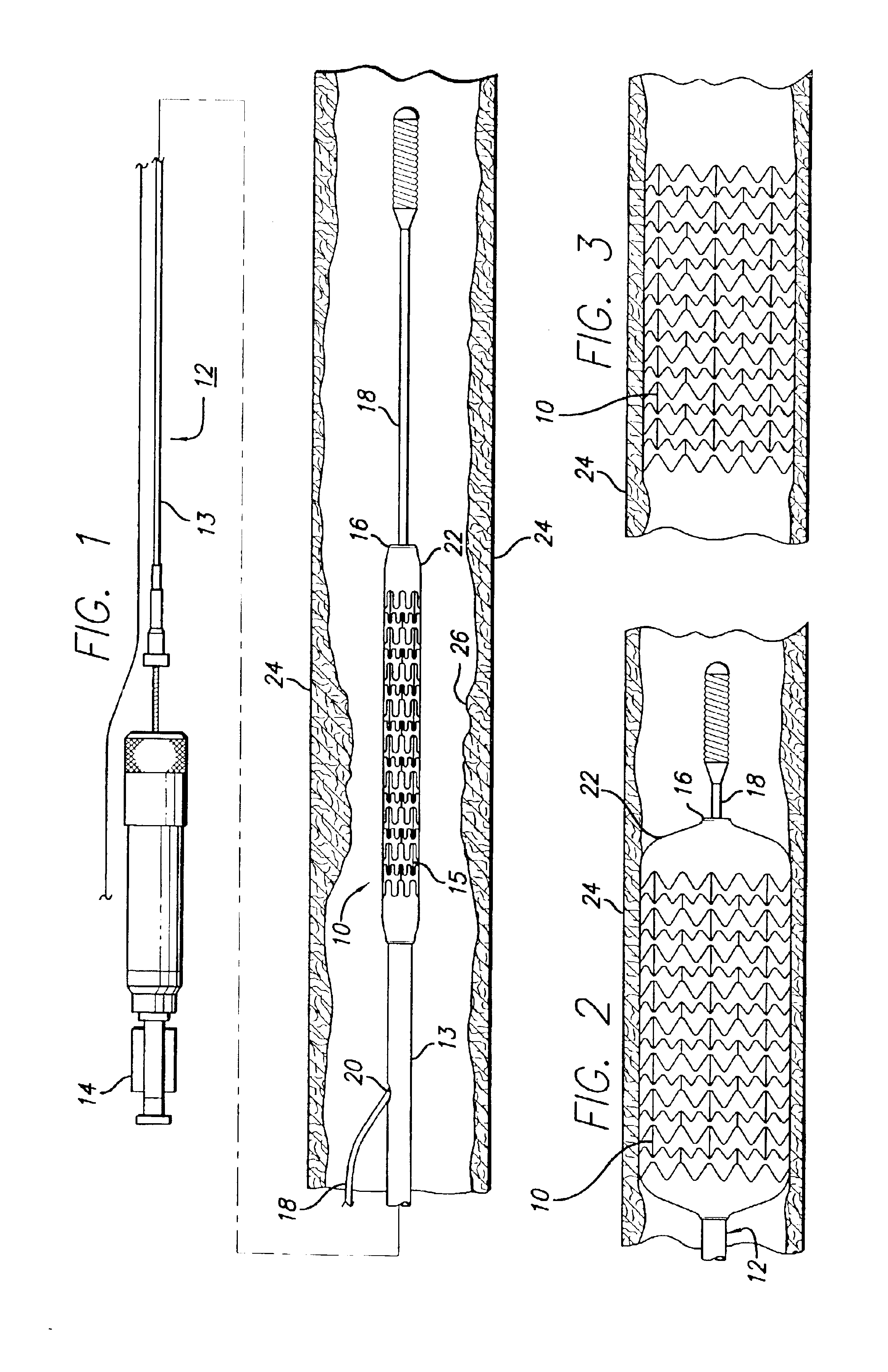
A very small diameter intravascular stent device which may be used to occlude or partially occlude an aneurysm in the human brain which is comprised of a thin-walled skeletal cylindrical tube formed of undulating or sinusoidal elements which, when compressed, nest tightly with each other.
Owner:CODMAN & SHURTLEFF INC
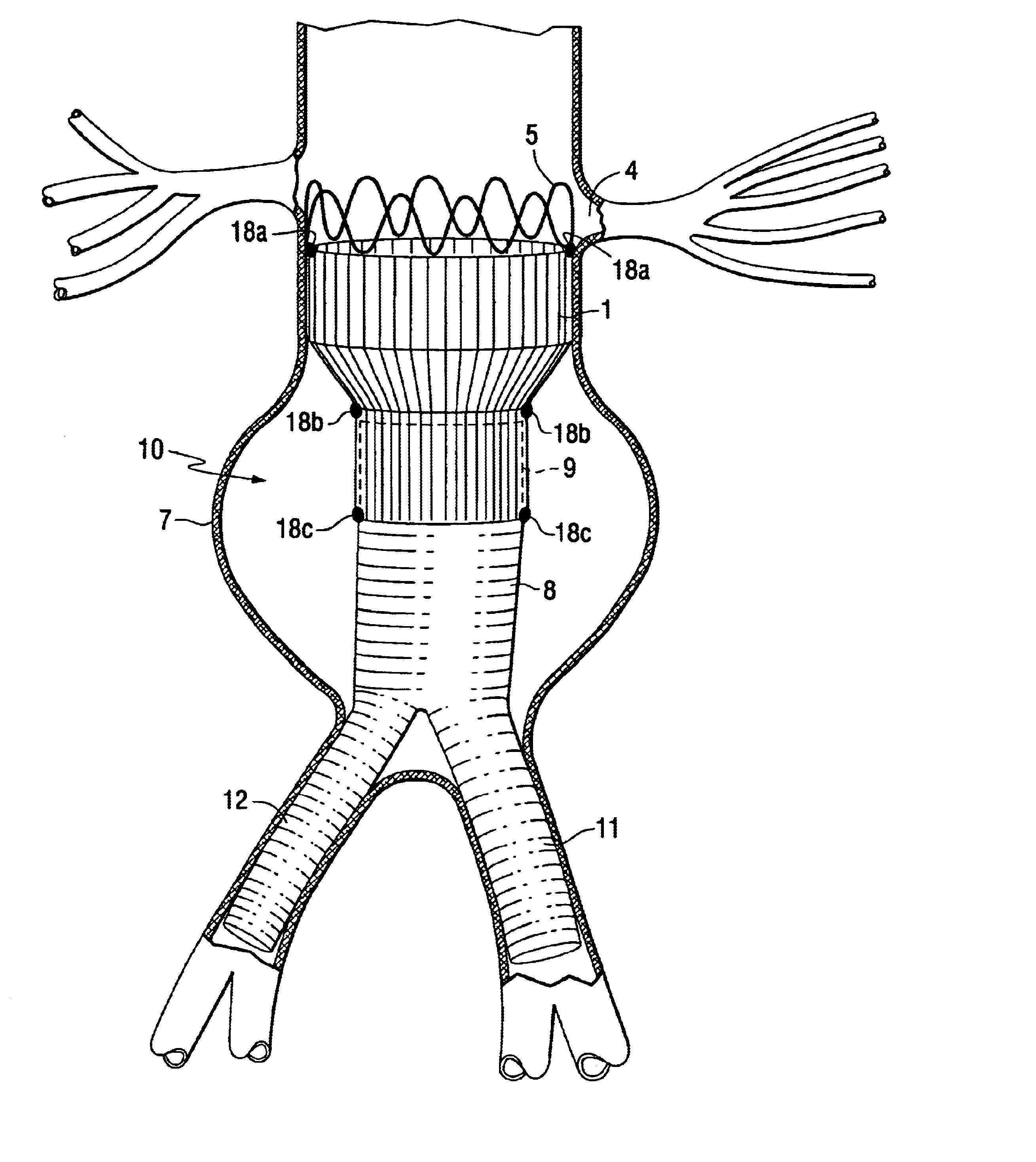
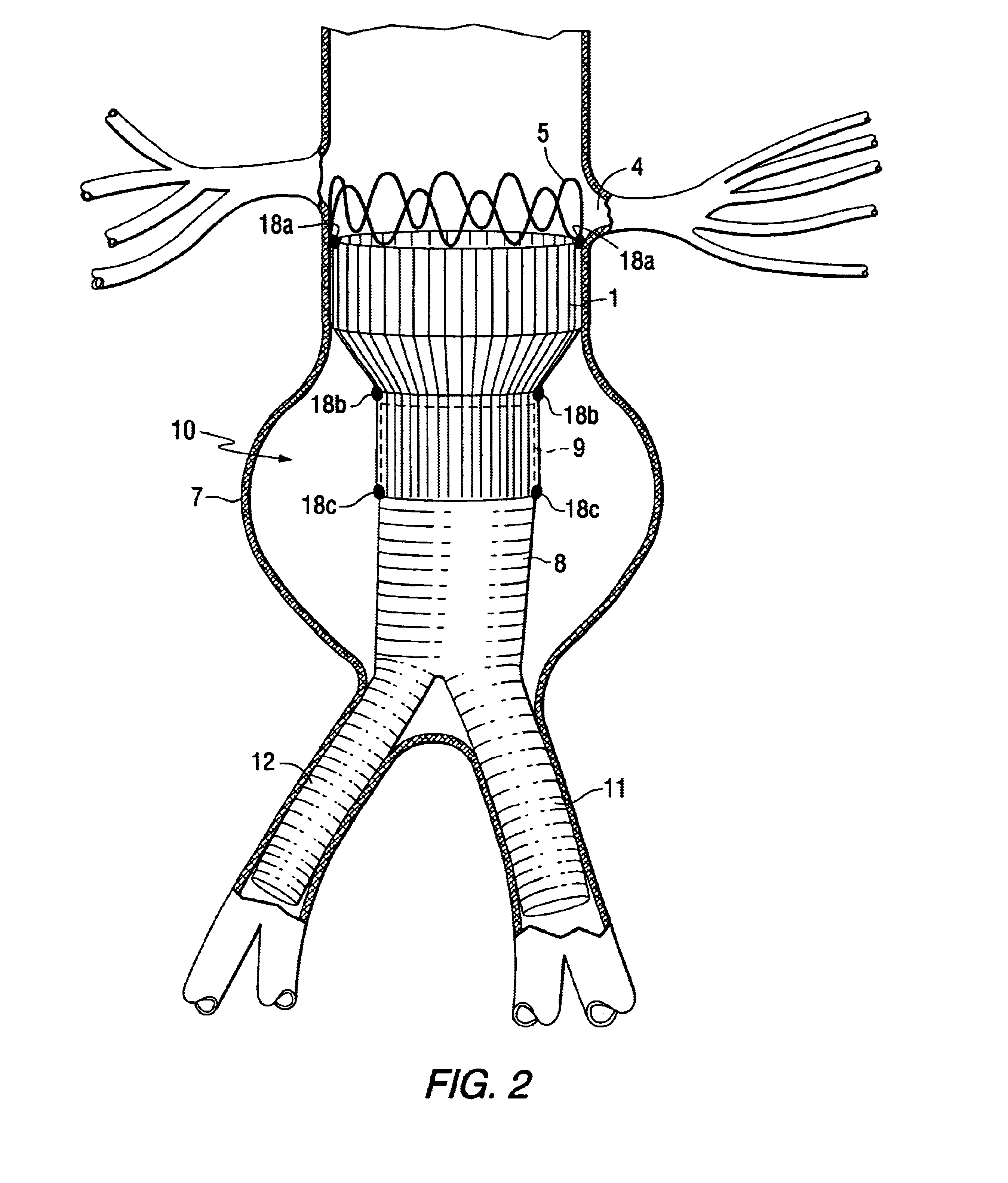
Tapered endovascular stent graft and method of treating abdominal aortic aneurysms and distal iliac aneurysms
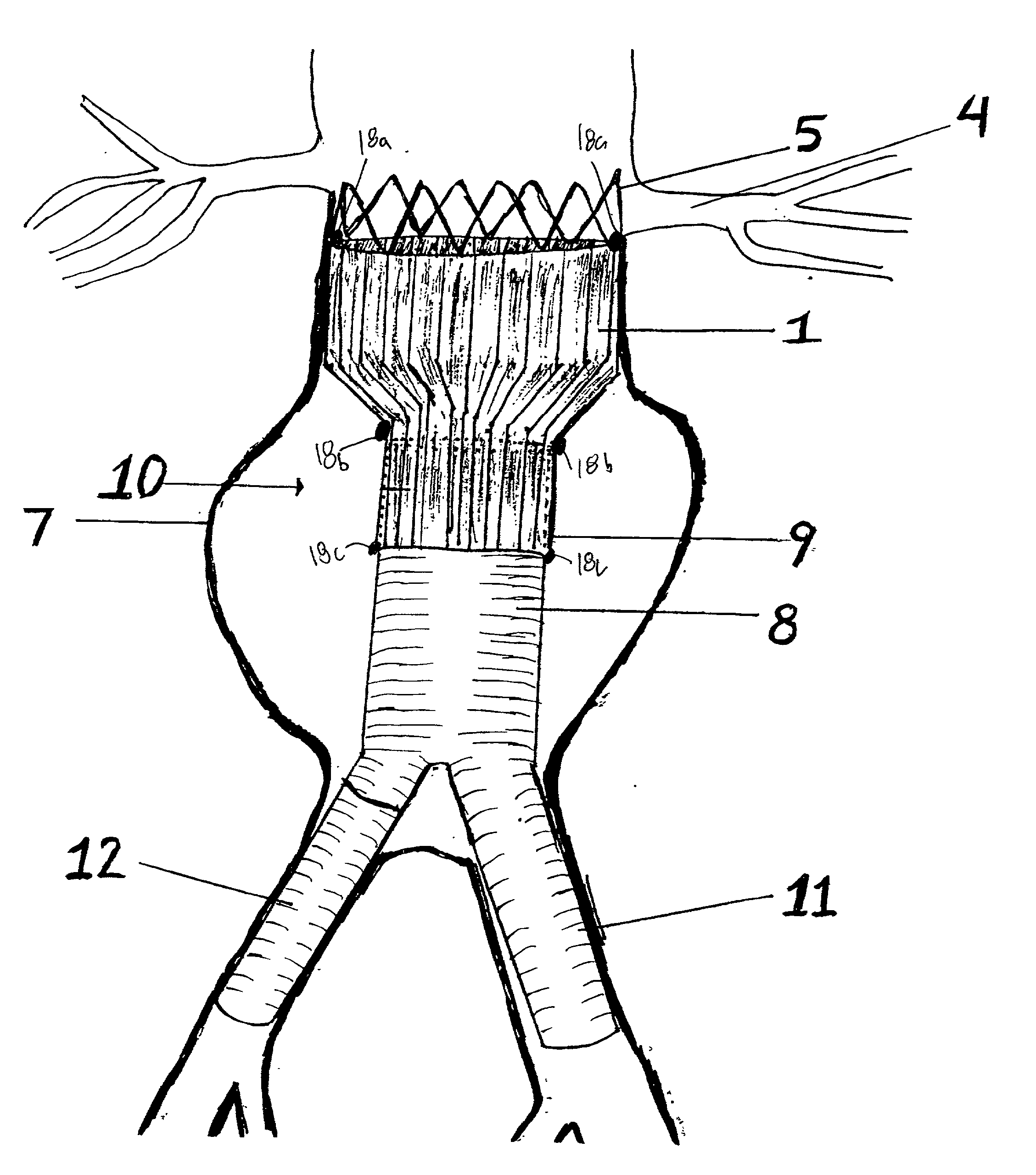
An endovascular stent graft is provided for use in treating abdominal aortic aneurysms. The endovascular stent has a tapered section which allows it to accommodate markedly large aortas such as the abdominal aorta and still connects to standard modular aortic stent grafts. Methods of utilizing the stent to treat abdominal aortic aneurysms and distal iliac aneurysms are also provided.
Owner:COOK MEDICAL TECH LLC
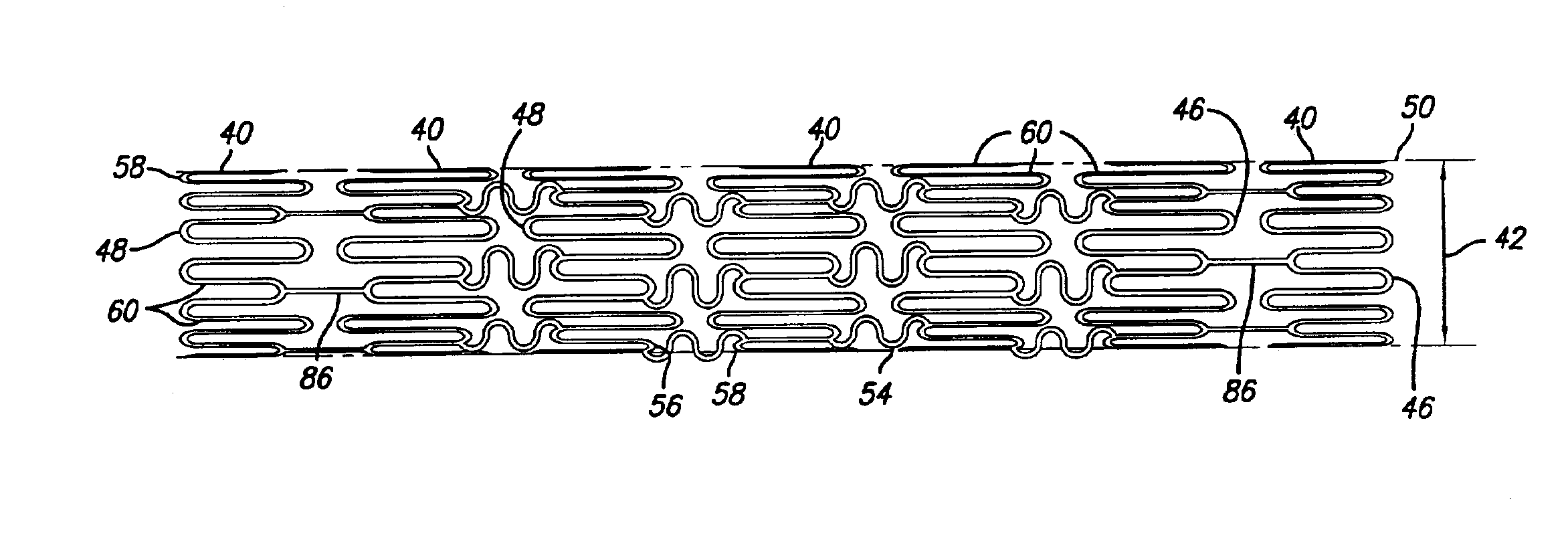
Hybrid intravascular stent
InactiveUS6866805B2Increase flexibilityImprove radial strengthStentsSurgeryCoronary arteriesIntravascular stent
A hybrid stent is formed which exhibits both high flexibility and high radial strength. The expandable hybrid stent for implantation in a body lumen, such as a coronary artery, consists of radially expandable cylindrical rings generally aligned on a common longitudinal axis and interconnected by one or more links. In one embodiment, a dip-coated covered stent is formed by encapsulating cylindrical rings within a polymer material. In other embodiments, at least some of the rings and links are formed of a polymer material which provides longitudinal and flexural flexibility to the stent. These polymer rings and links are alternated with metallic rings and links in various configurations to attain sufficient column strength along with the requisite flexibility in holding open the target site within the body lumen. Alternatively, a laminated, linkless hybrid stent is formed by encapsulating cylindrical rings within a polymer tube.
Owner:ABBOTT CARDIOVASCULAR
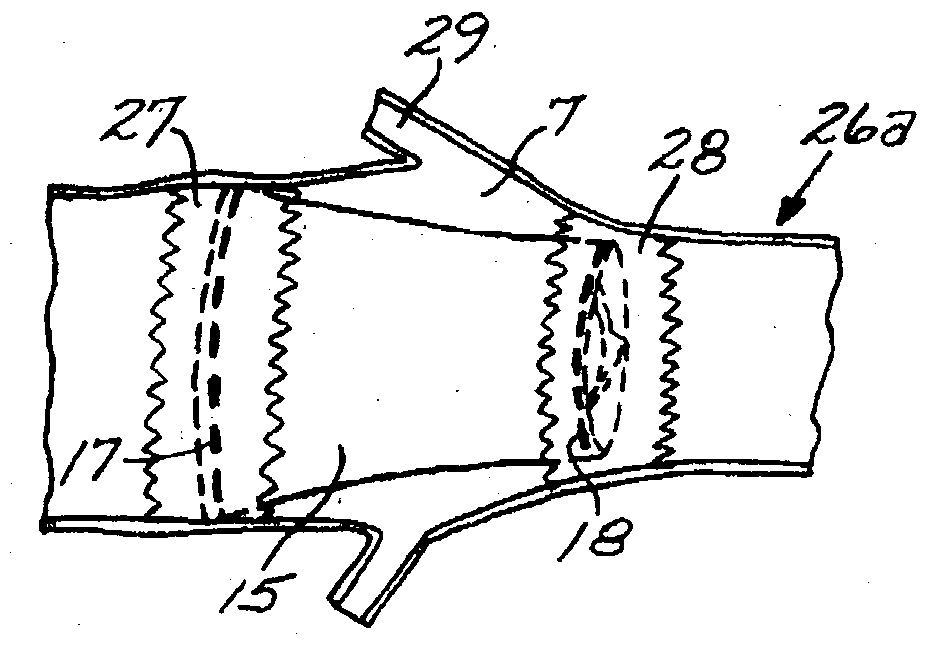
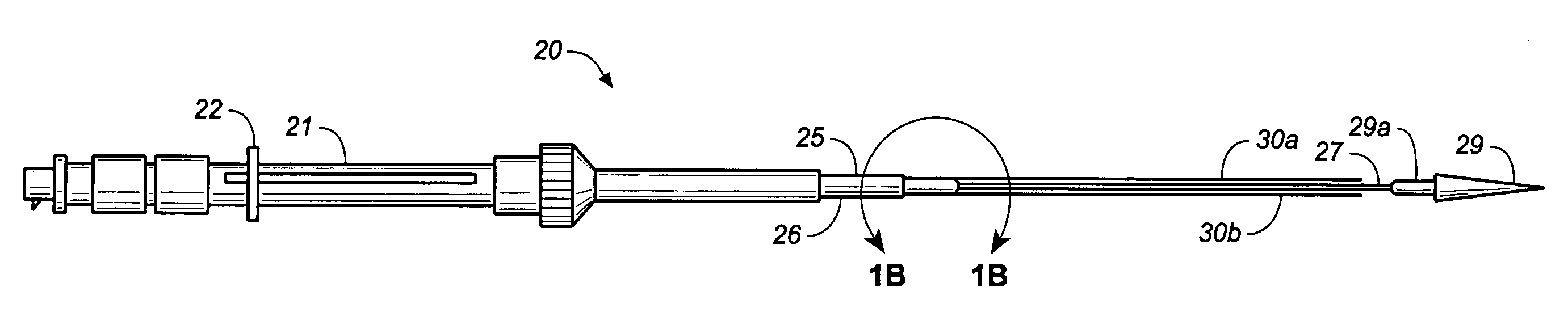
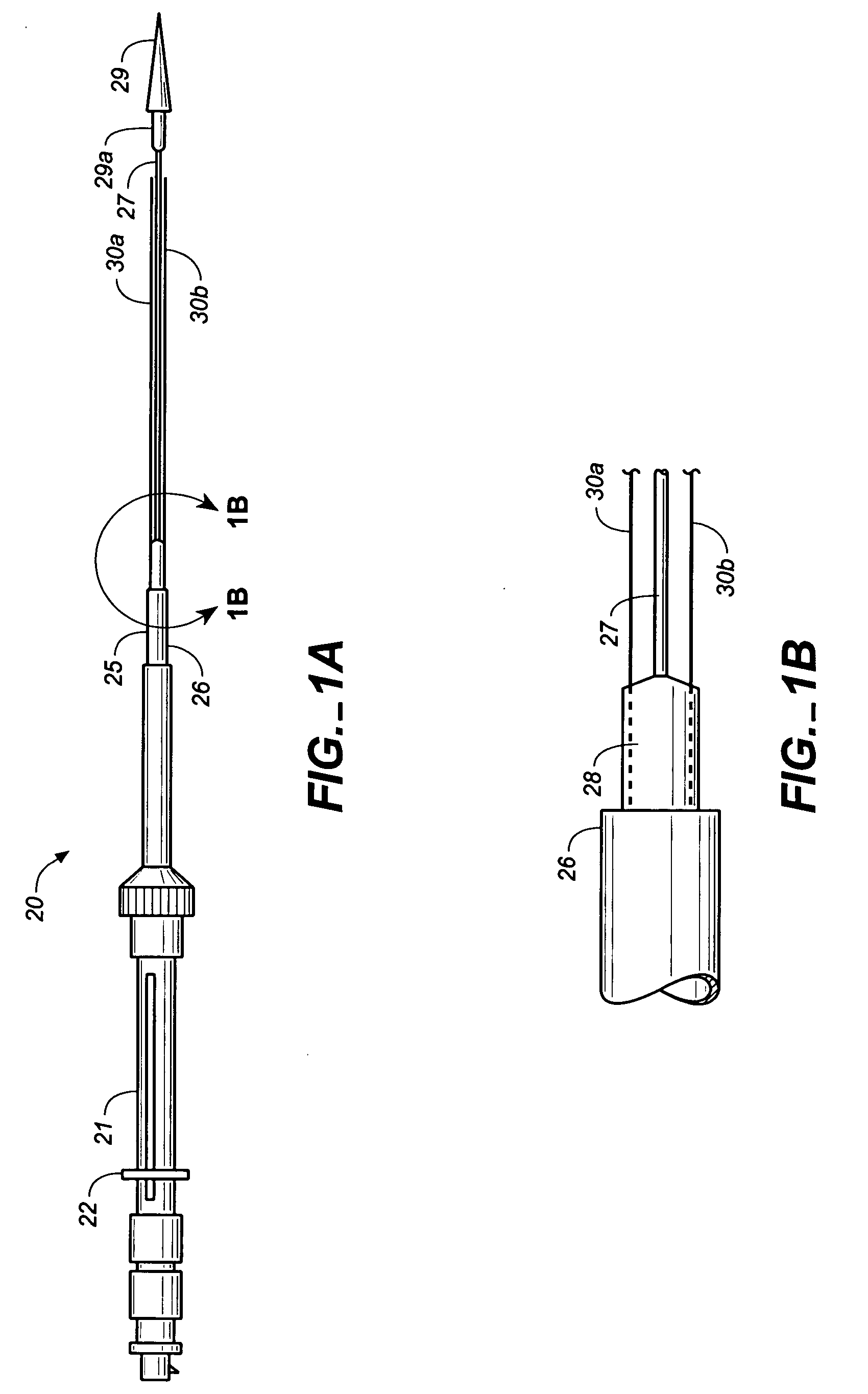
Hydraulic stent deployment system
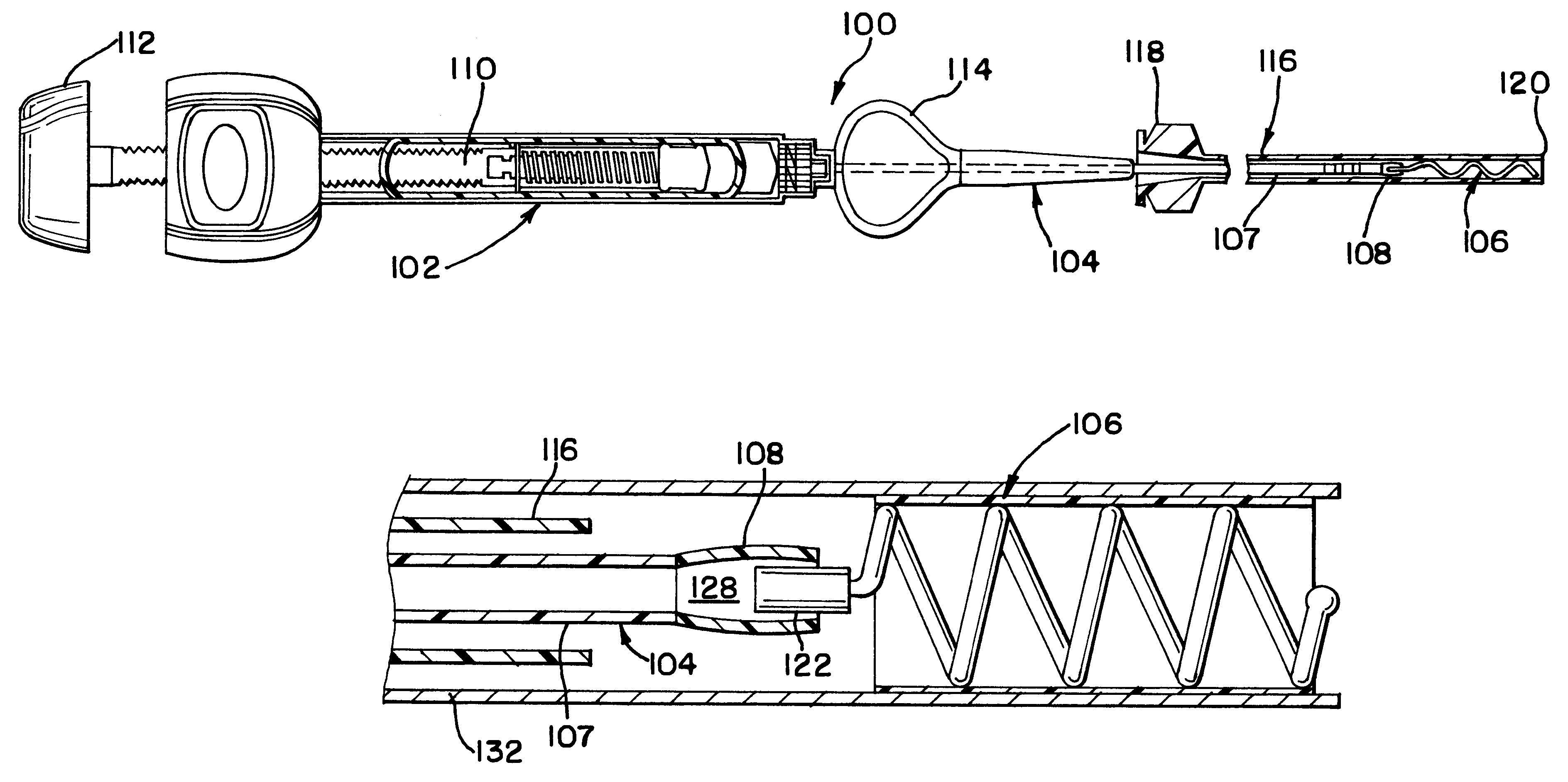
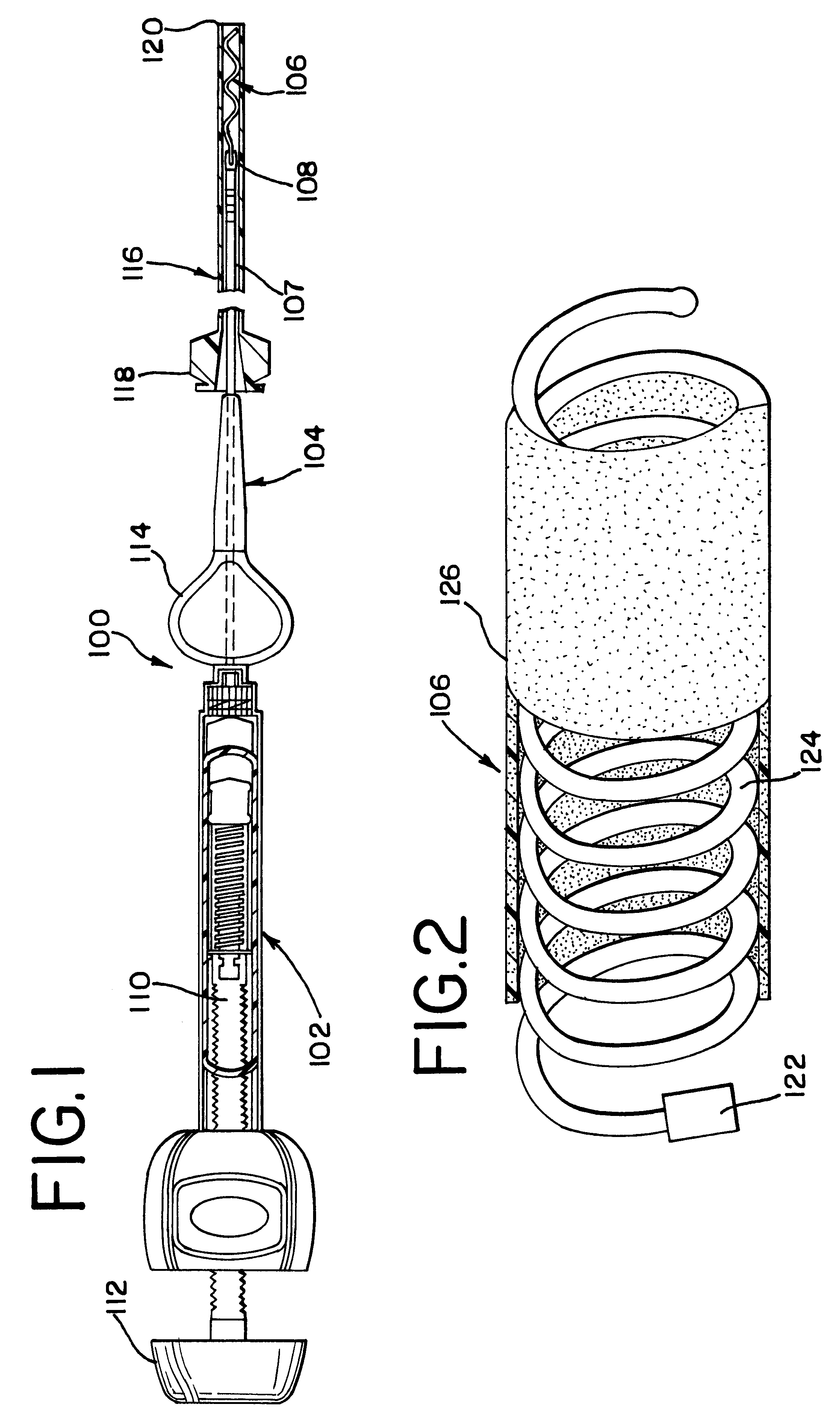
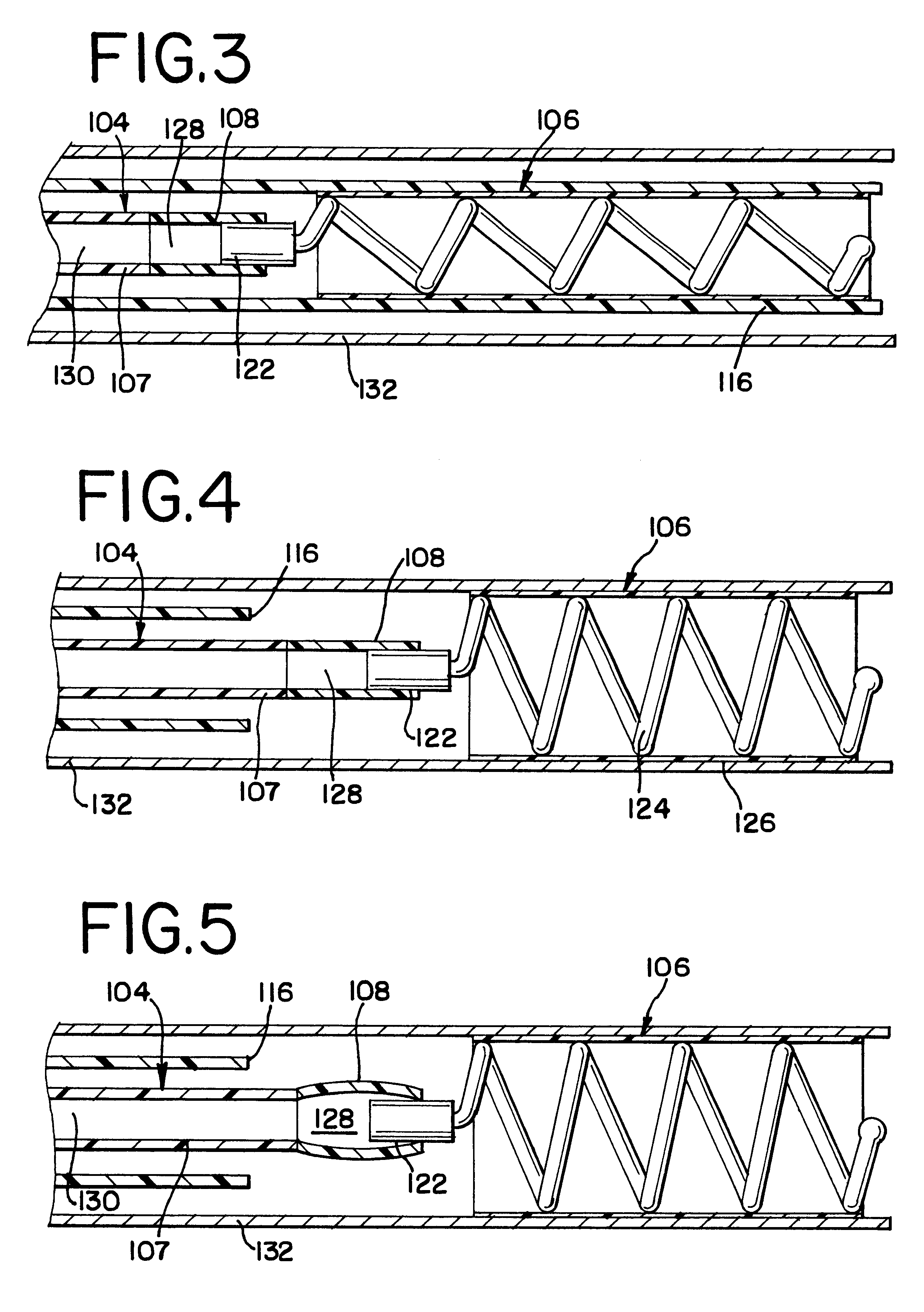
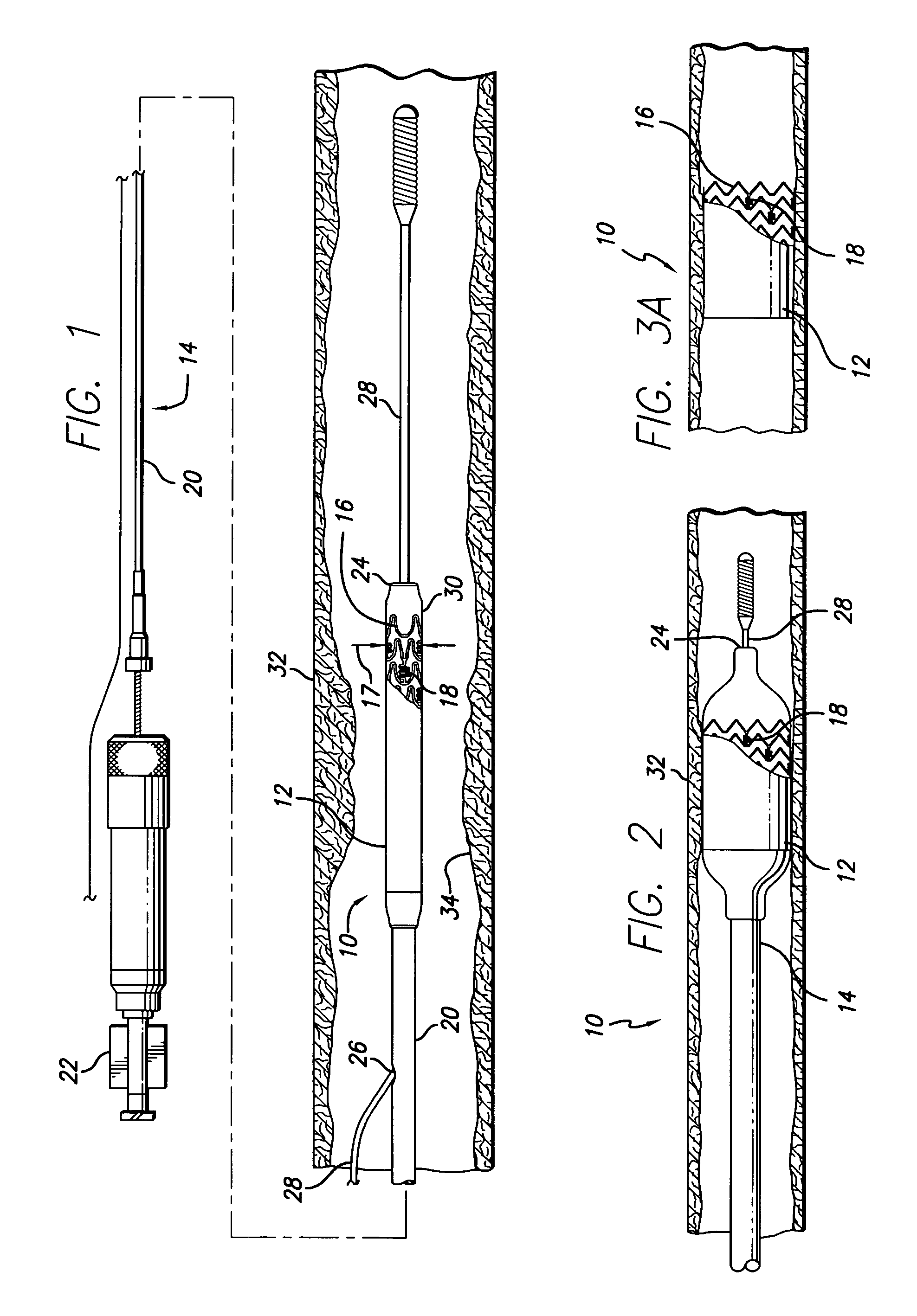
The present invention relates to a medical device for placing an intravascular stent at a preselected location within a vessel of the human body, and more particularly, relates to a catheter having a distal tip for retaining the stent in order to transport the stent to a preselected position within the vessel and a control mechanism for releasing the stent at the preselected position.
Owner:CODMAN & SHURTLEFF INC
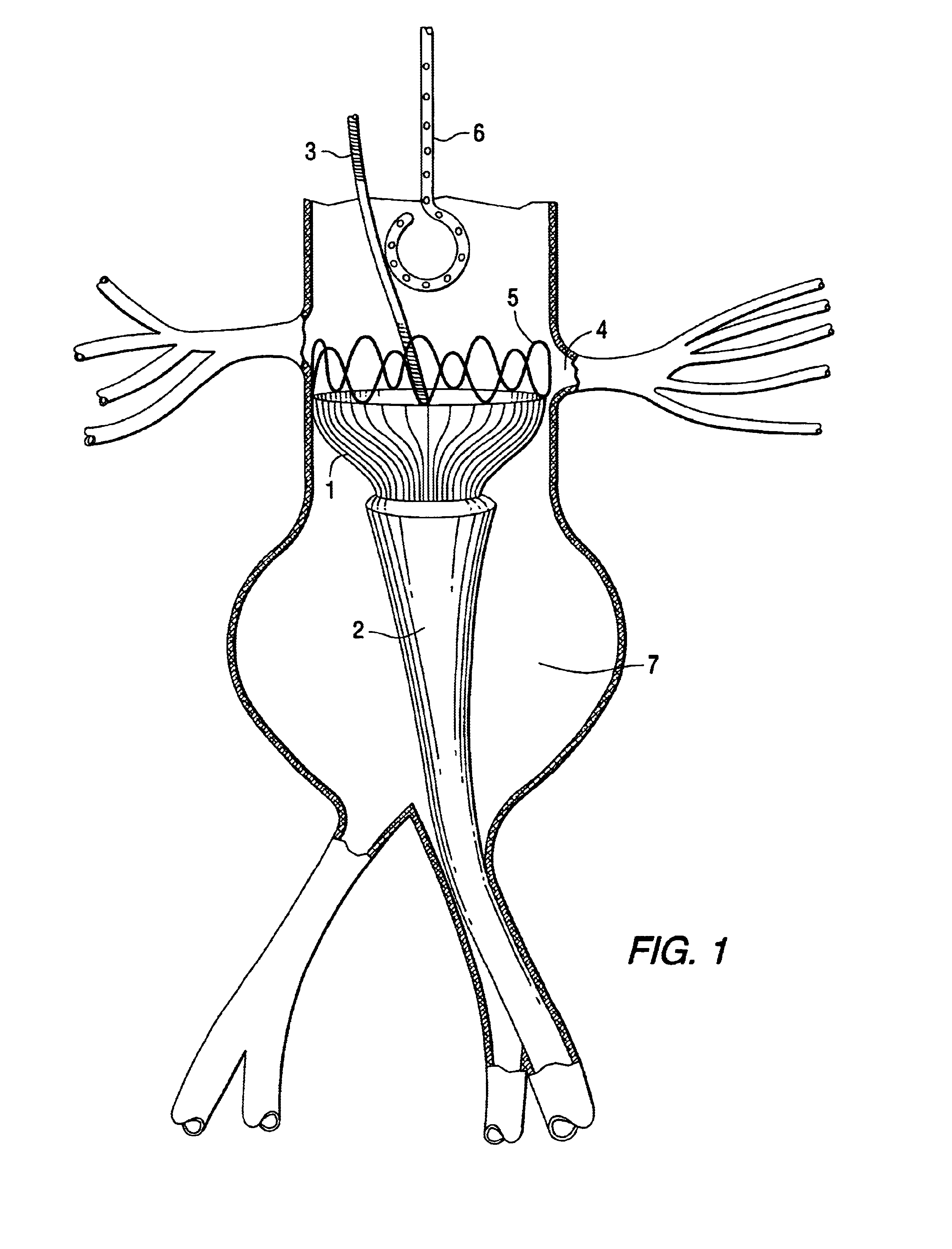
Tapered endovascular stent graft and method of treating abdominal aortic aneurysms and distal iliac aneurysms
The present invention relates to an improved endovascular stent. The endovascular stent is to be used in treating abdominal aortic aneurysms. The endovascular stent has a tapered section which allows it to accommodate markedly large aortas such as the abdominal aorta and still connect to standard modular aortic stent grafts. Methods of utilizing the stent to treat abdominal aortic aneurysms and distal iliac aneurysms are also provided.
Owner:COOK MEDICAL TECH LLC
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
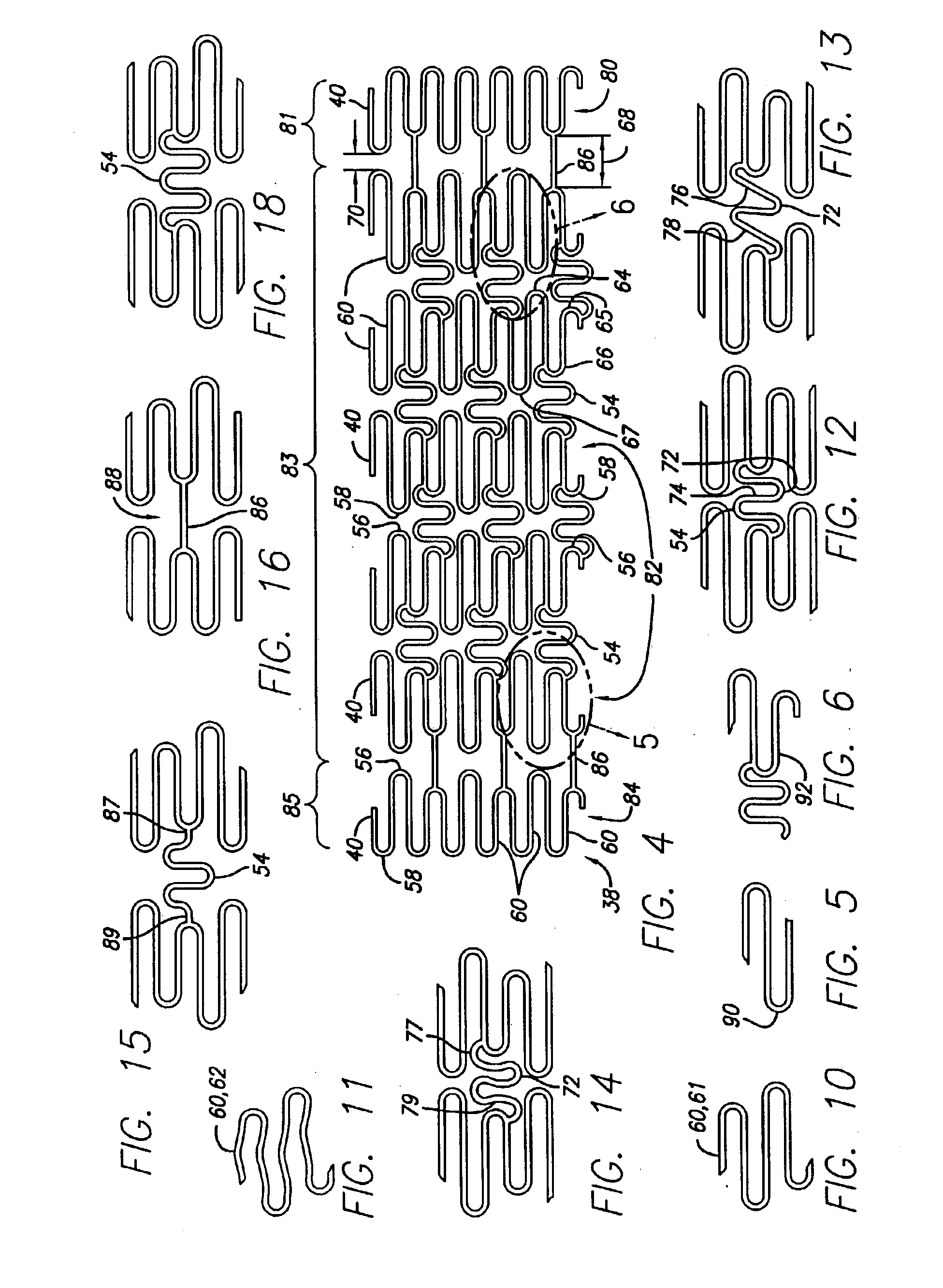
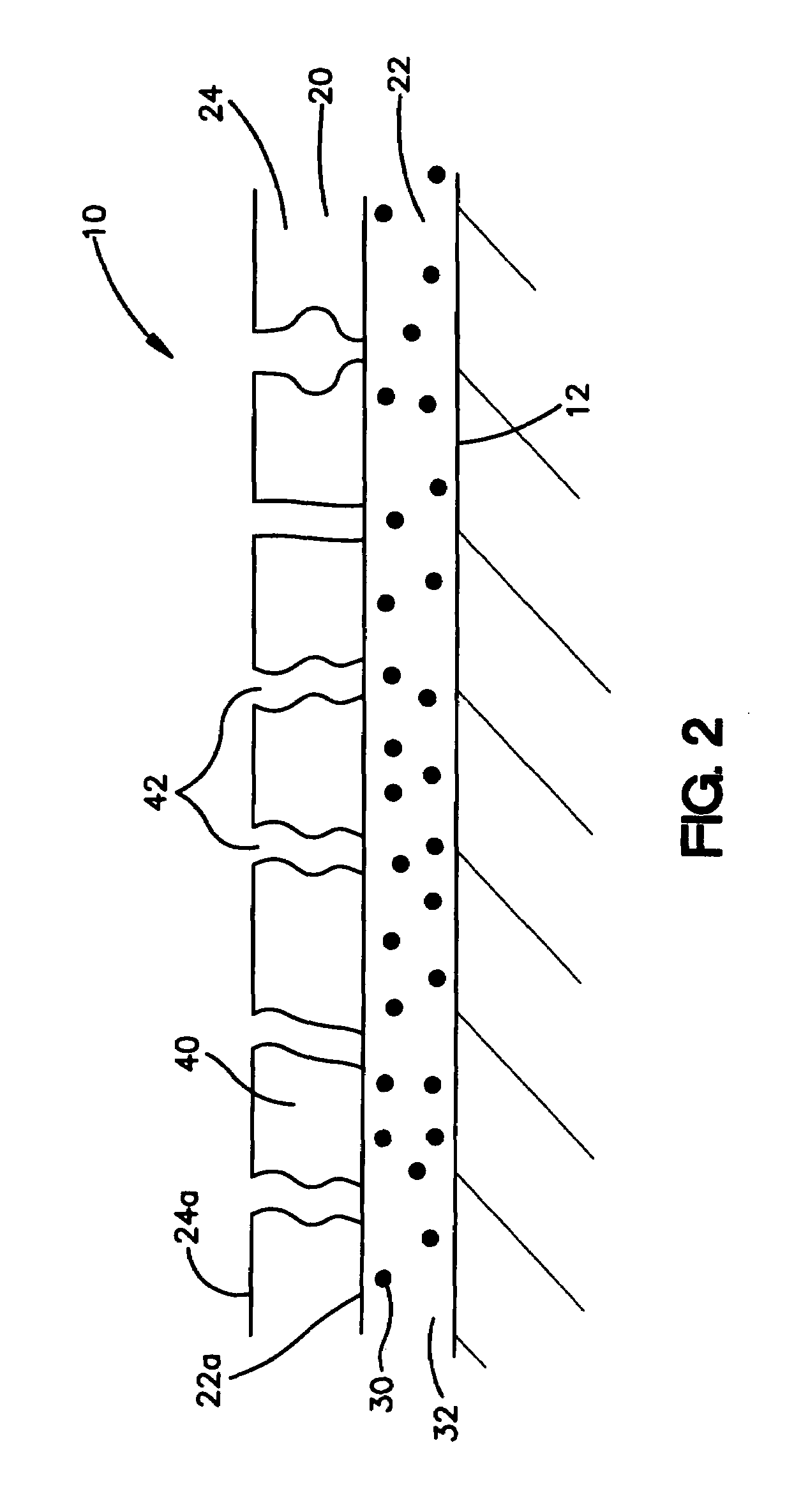
Intravascular stent
ActiveUS6896697B1Minimize alterationMaintain complianceStentsBlood vesselsCoronary arteriesLoop of Henle
An intravascular stent assembly for implantation in a body vessel, such as a coronary artery, includes undulating circumferential rings having peaks on the proximal end and valleys on the distal end. Adjacent rings are coupled together between peaks on one ring and valleys on the proximally adjacent ring. To increase flexibility of the stent, the links do not couple all of the peaks on a ring to the proximally adjacent ring.
Owner:ABBOTT CARDIOVASCULAR
Method for reducing restenosis in the presence of an intravascular stent
InactiveUS6939345B2Reduce generationLot of damageDilatorsDiagnostic recording/measuringCoronary arteriesProximate
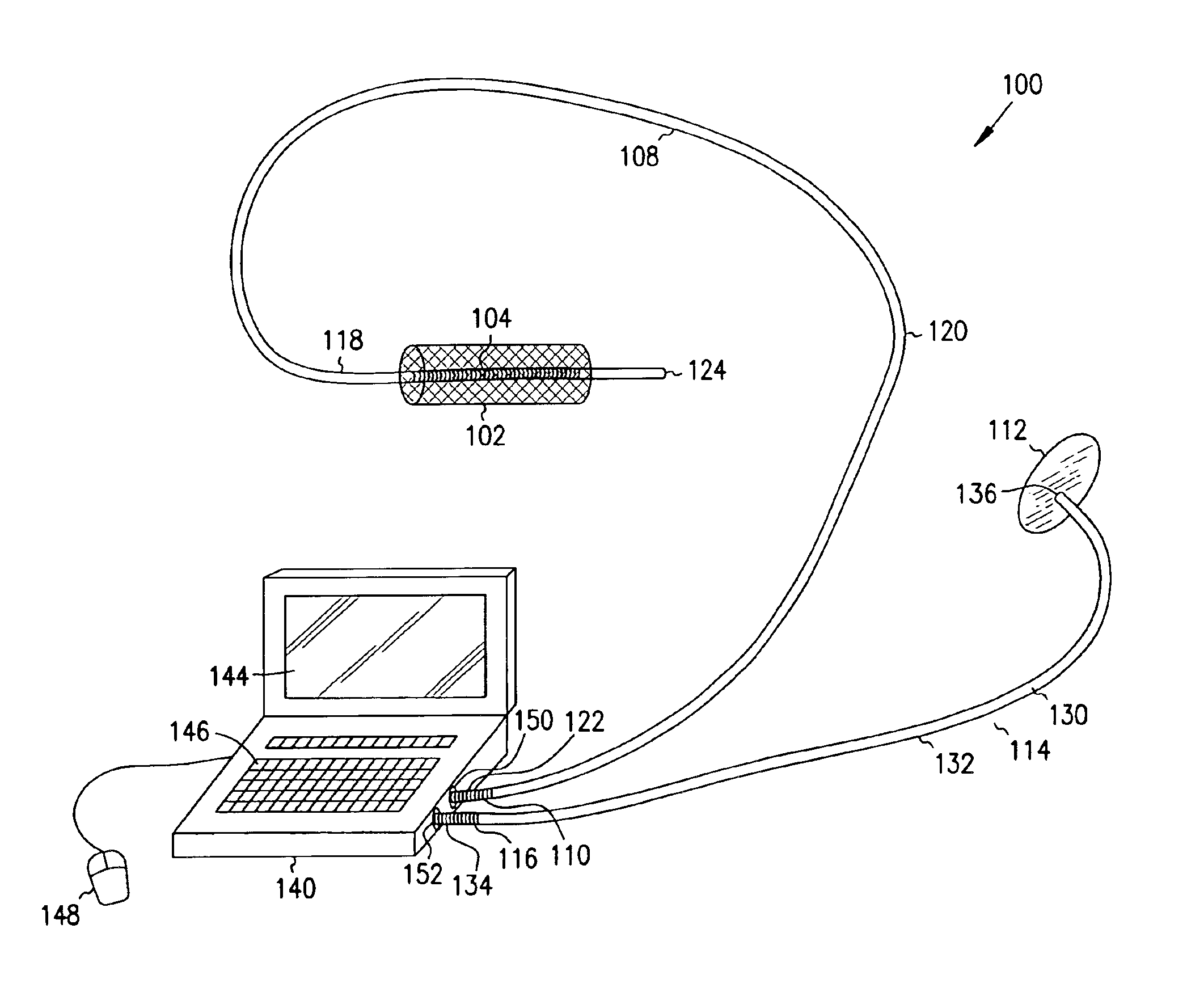
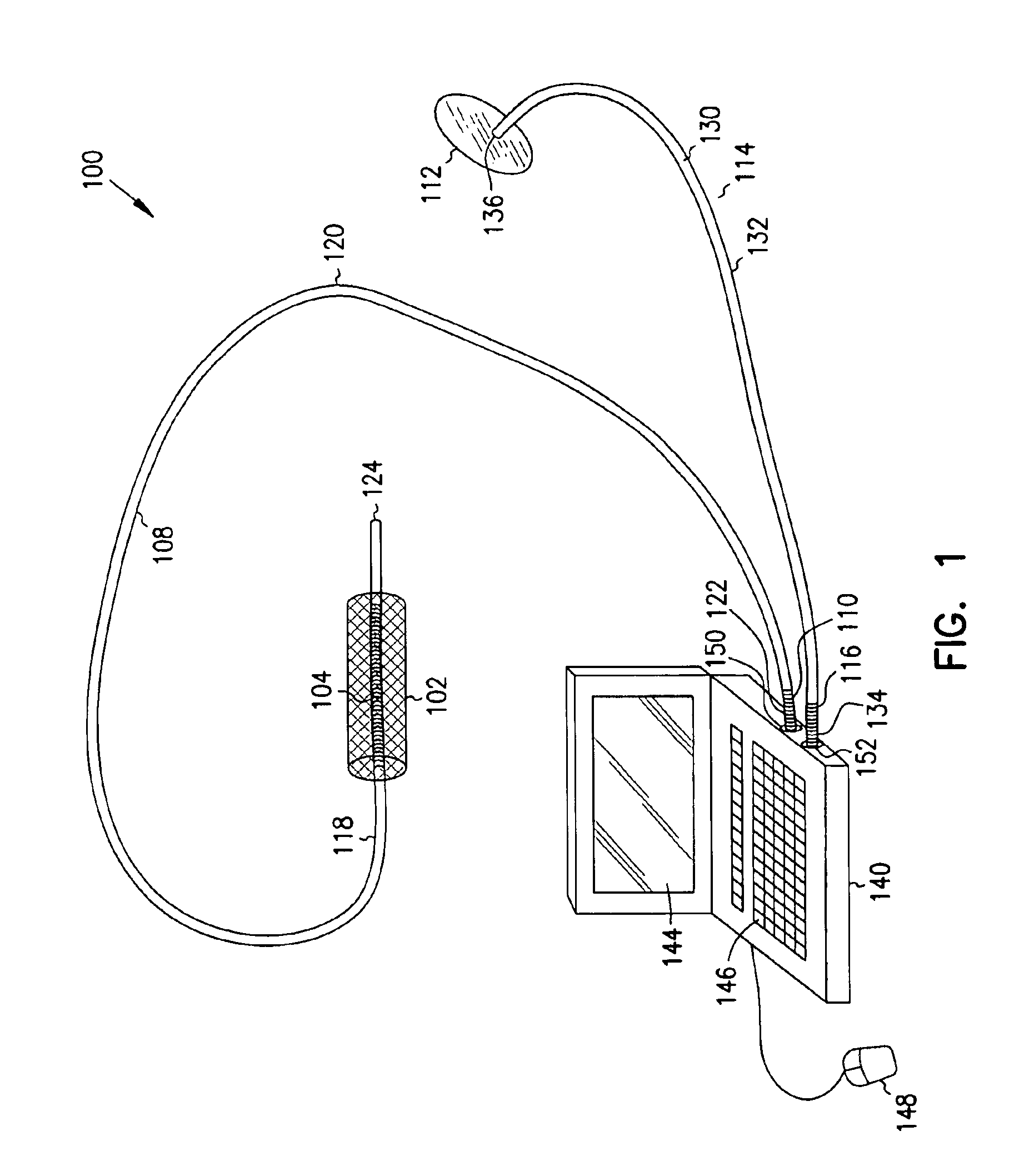
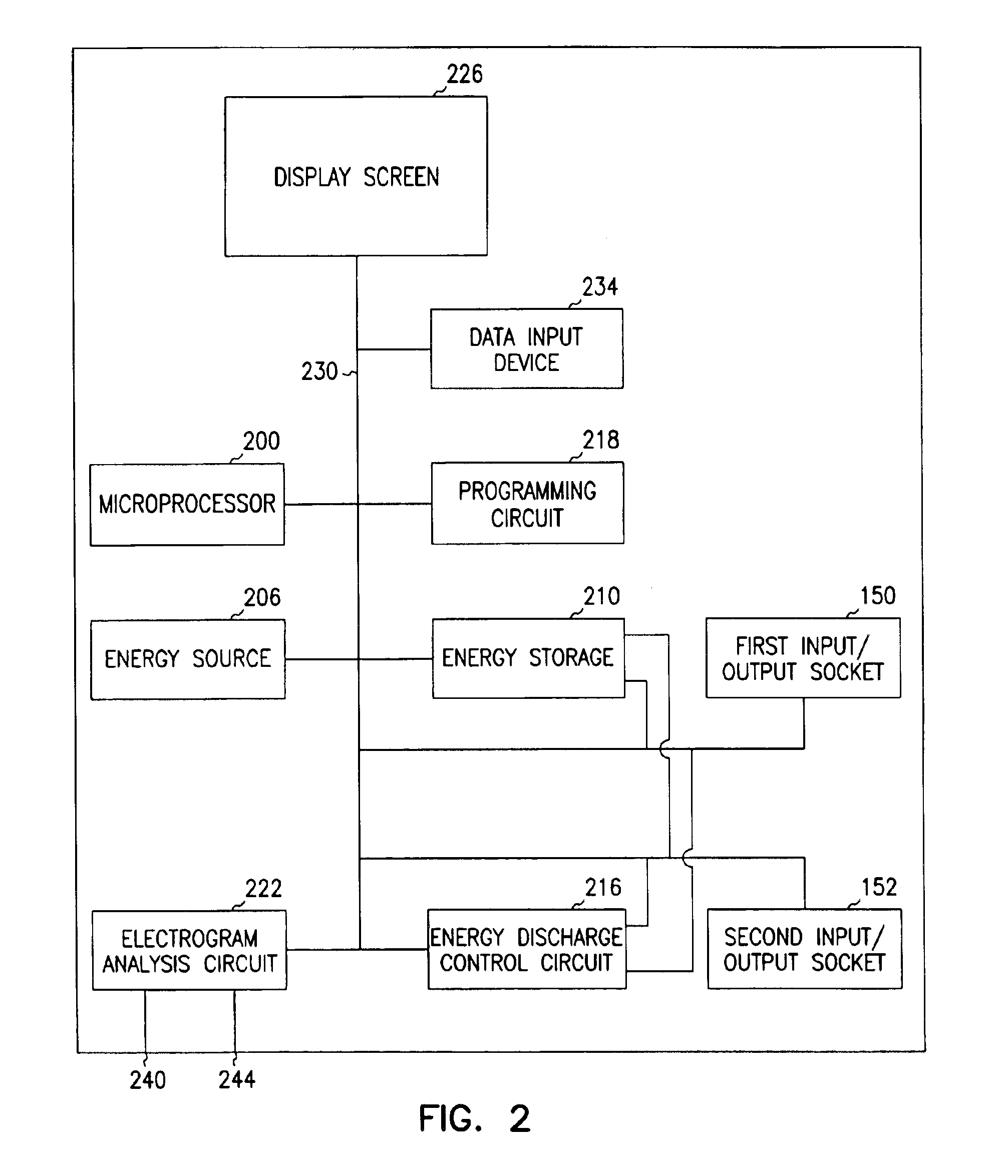
A first electrode is positioned within an artery proximate an implanted intravascular stent. A second electrode is positioned at a separate location relative the position of the first electrode. Electrical energy is then delivered between the first and the second electrodes to produce an electrical field adjacent the implanted intravascular stent. When a intravascular stent is implanted in a coronary artery, the delivery of the electrical energy is coordinated to cardiac cycles detected in sensed cardiac signals, where the delivery of the electrical energy between the first electrode and the second electrode occurs during a predetermined portion of the cardiac cycle.
Owner:CARDIAC PACEMAKERS INC
Sigmoid valve and method for its percutaneous implantation
Owner:THE INT HEART INST OF MONTANA FOUND
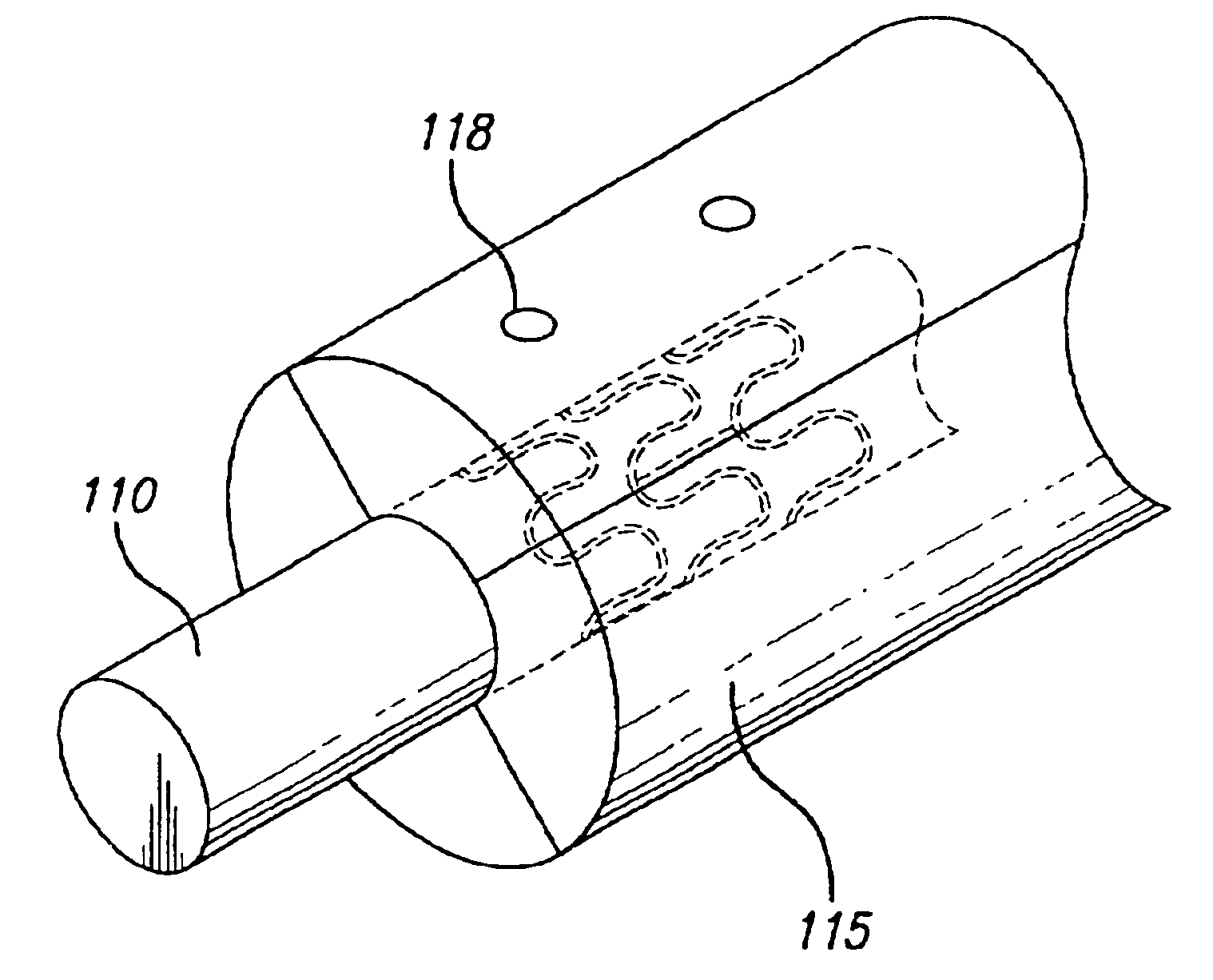
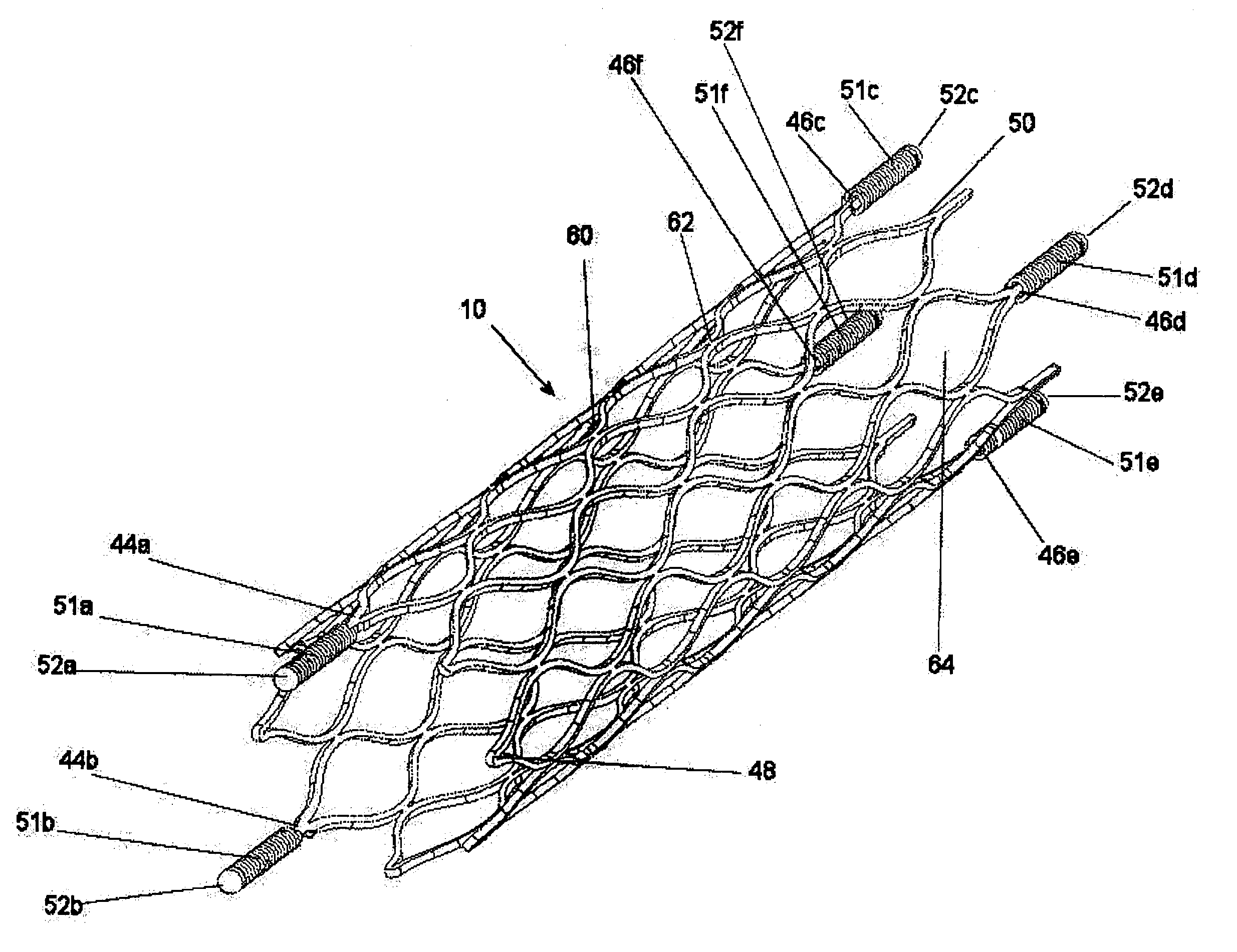
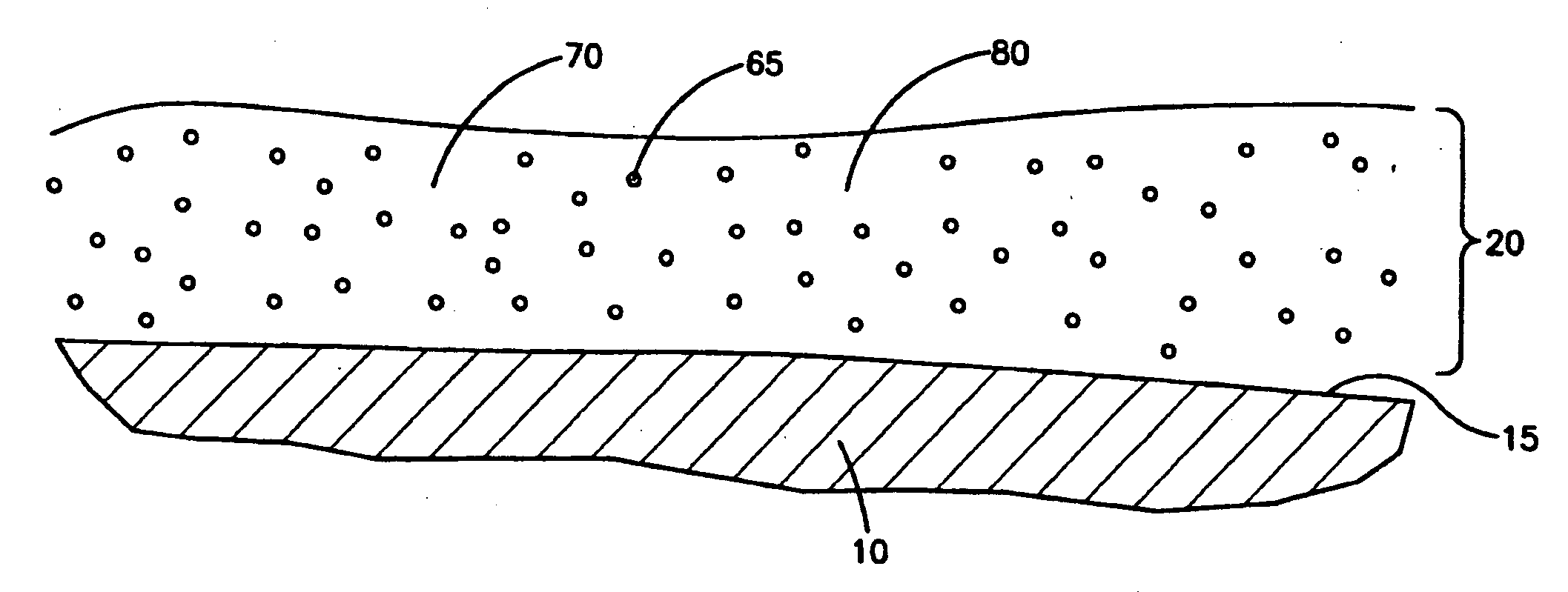
Drug-eluting stent cover and method of use
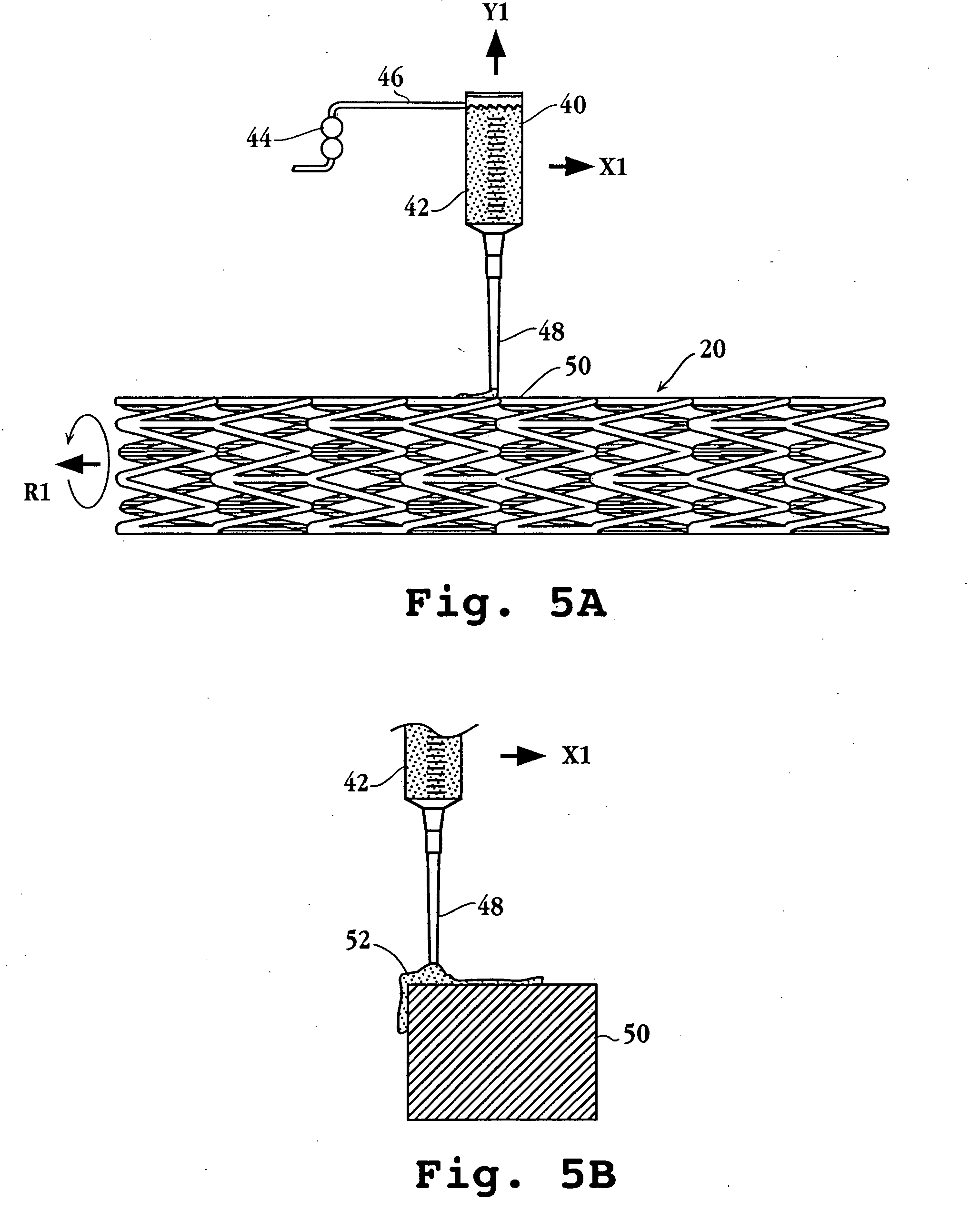
An intravascular stent includes an eluting sheath fabricated from a mesh for controlled release of therapeutic drugs and for delivery of the therapeutic drugs in localized drug therapy in a blood vessel. The eluting sheath is attached to at least a portion of an outside surface area of the stent structure and is fabricated from a mesh designed to neck down in response to a radially outward directed force resulting in the uniform expansion of the stent. The eluting sheath can be loaded with at least one therapeutic drug for the release thereof at a treatment site to facilitate repair of a damaged vessel. The stent has a high degree of flexibility in the longitudinal direction, yet has adequate vessel wall coverage and radial strength sufficient to hold open an artery or other body lumen.
Owner:ABBOTT CARDIOVASCULAR
Method of coating an intravascular stent with an endothelial cell adhesive five amino acid peptide
InactiveUS6140127APromoting cell attachmentRestore patencyBiocidePeptide/protein ingredientsCell specificArginine
Endothelial cell attachment to an intravascular stent is promoted by coating the stent with an endothelial cell specific adhesion peptide. Coating is preferably carried out by activating the intravascular stent using plasma glow discharge, applying on the stent a layer or plurality of layers of a polymer such as poly(2-hydroxyethylmethacrylate), applying a tresylation solution containing pyridine and tresyl chloride, and applying a five amino acid peptide having the sequence glycine-arginine-glutamic acid-aspartic acid-valine.
Owner:CORDIS CORP
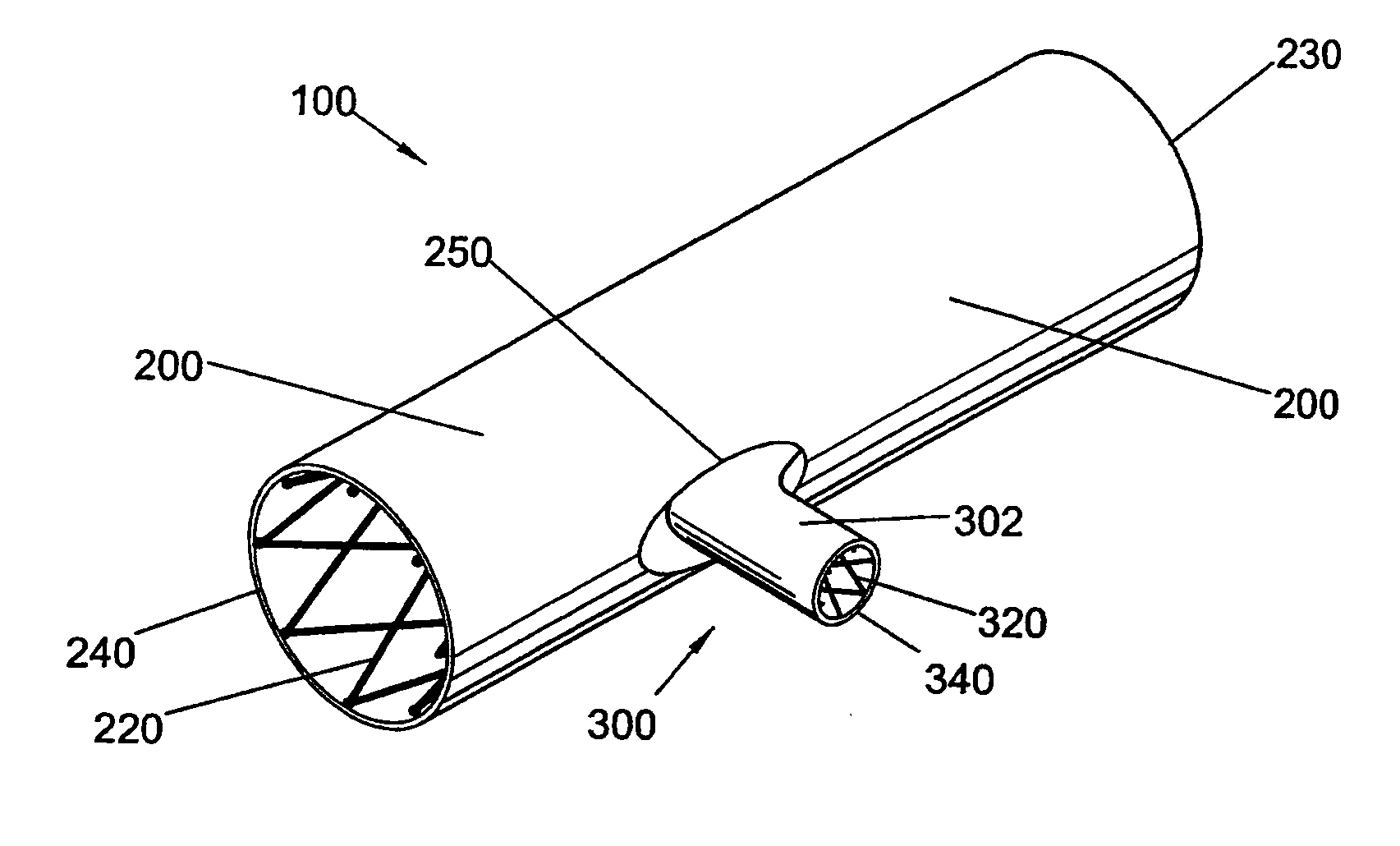
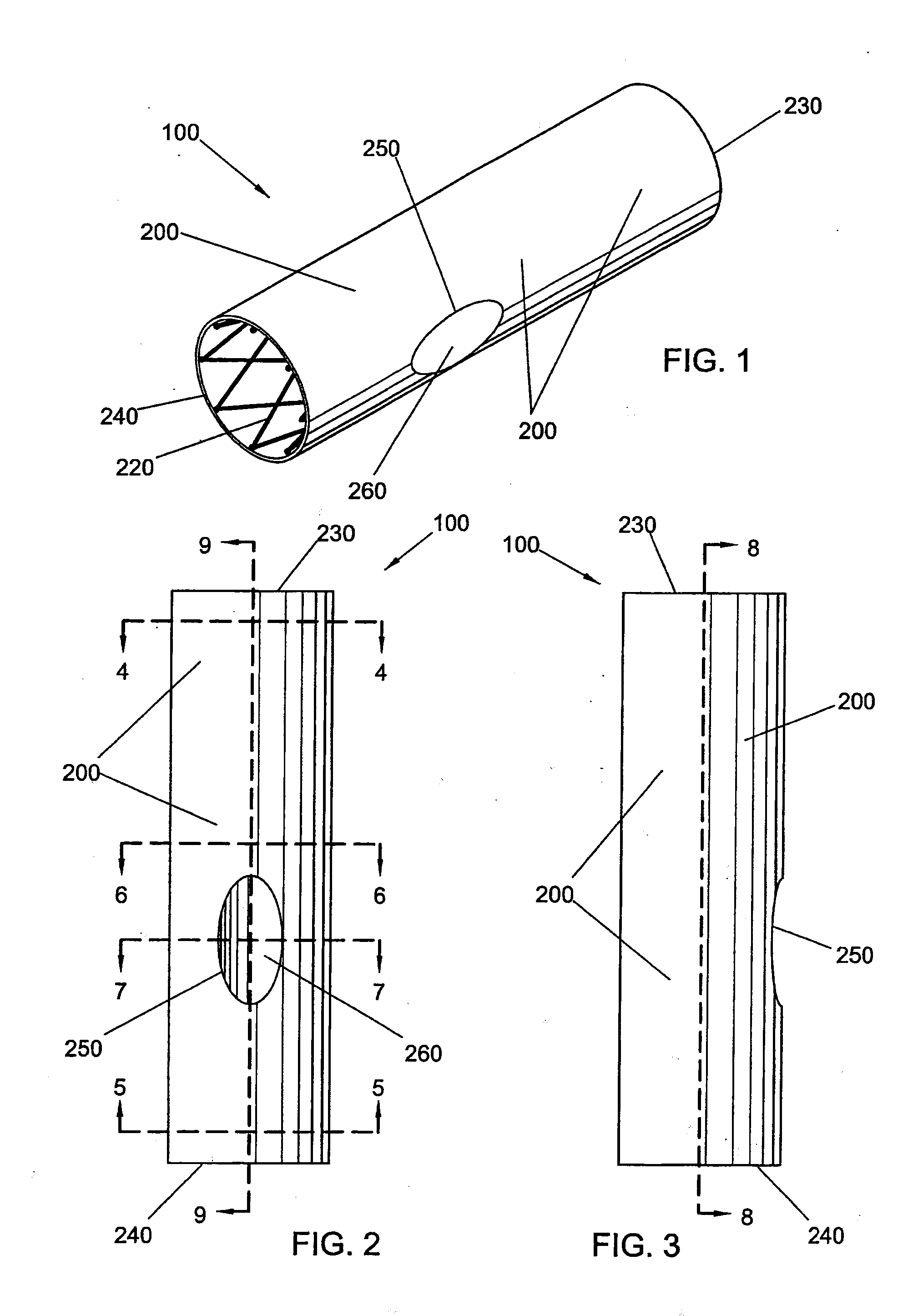
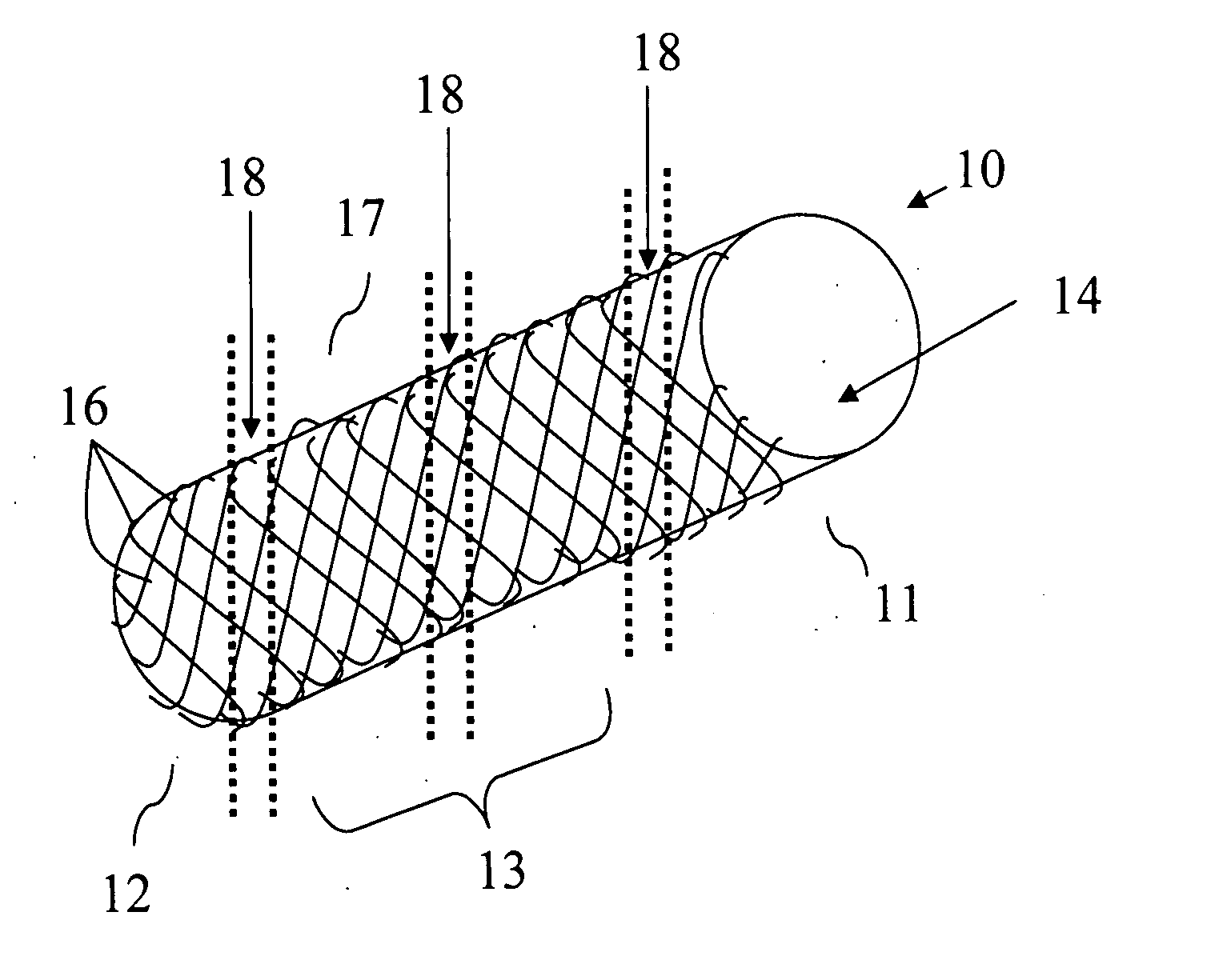
Bifurcated side-access intravascular stent graft
InactiveUS20080109066A1Readily and accurately positionedStentsBlood vesselsStent graftingMain channel
A bifurcated intravascular stent graft comprises primary stent segments and a primary graft sleeve, forming a main fluid channel and having a side opening therethrough. An external graft channel formed on the primary graft sleeve has a first end communicating with the side opening and an open second end outside the primary graft sleeve, thereby providing a branch flow channel from the main channel out through the side opening and external graft channel. The primary stent segments and graft sleeve engage an endoluminal surface of a main vessel and form substantially fluid-tight seals. The stent graft further comprises a secondary stent graft, which may be positioned partially within the external graft channel, through the open second end thereof, and partially within a branch vessel. The secondary stent graft engages the inner surface of the external graft channel and the endoluminal surface of the branch vessel, thereby forming substantially fluid-tight seals.
Owner:WL GORE & ASSOC INC
Device and method for delivering an endovascular stent-graft having a longitudinally unsupported portion
InactiveUS20060020319A1Reduce forceAvoid graft kinking and wrinklingStentsEar treatmentStent graftingInsertion stent
An endoluminal prosthesis having an unsupported or flexible region and a delivery system for delivering the endoluminal prosthesis is provided. The delivery system includes a prosthesis delivery catheter with stiffening elements that provide longitudinally rigid support to a flexible or unsupported portion of an endoluminal prosthesis as the prosthesis is being deployed. A prosthesis is removably coupled to the stiffening elements. The endoluminal prosthesis can be a stent or a stent graft or graft.
Owner:MEDTRONIC VASCULAR INC
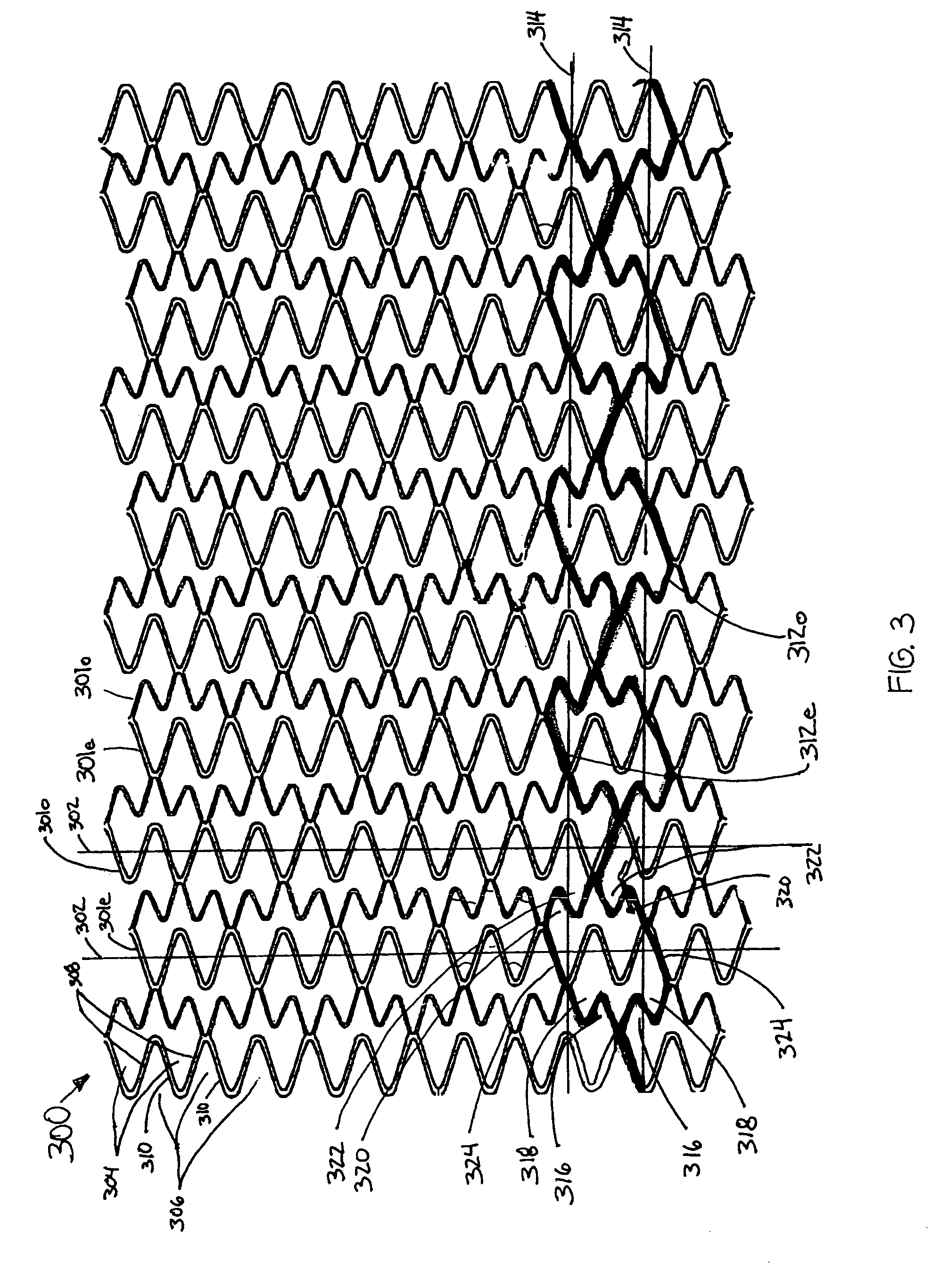
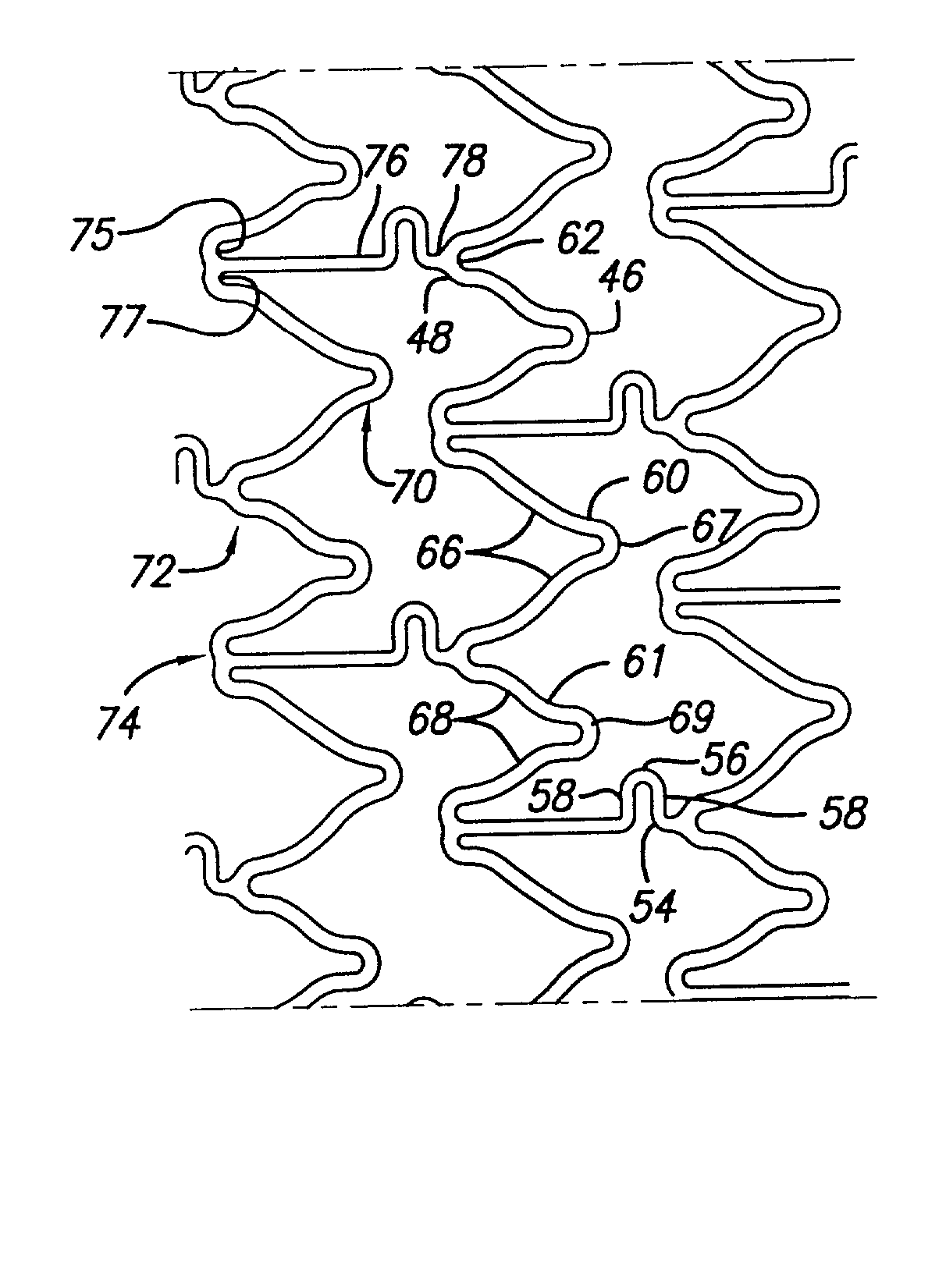
Intravascular stent
InactiveUS7326241B2Reduces vessel “ hang up ”Increase radialStentsSurgeryEngineeringIntravascular stent
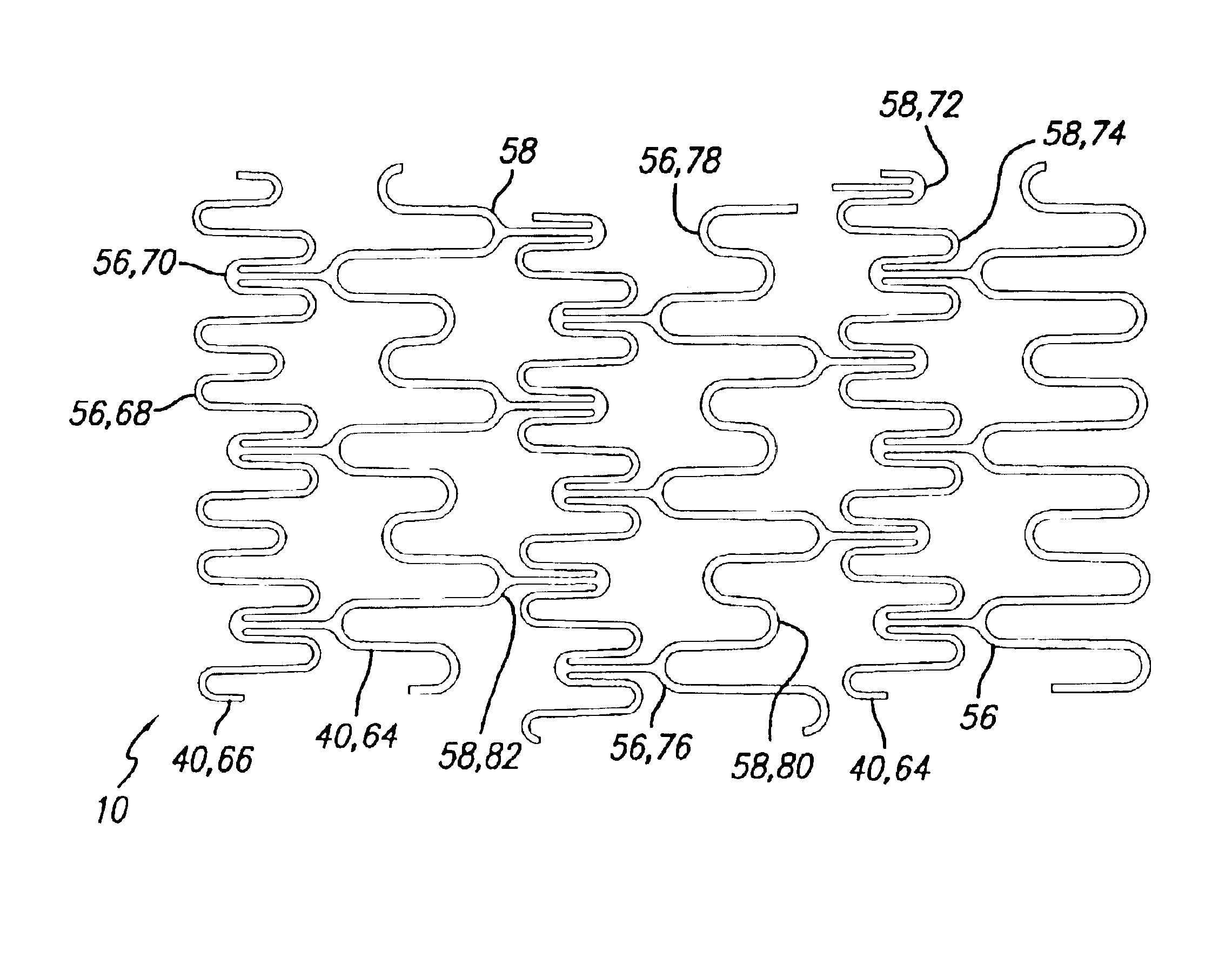
A stent in a non-expanded state has a first and second expansion column, each consisting of a plurality of expansion strut pairs. An expansion strut pair includes a first expansion strut, a second expansion strut and a joining strut that couples the first and second expansion struts at one end. Expansion strut pairs include expansion strut pair first and second corners formed where the joining strut couples the first and second expansion struts. A connecting strut column, formed of a plurality of connecting struts couples the first and second expansion columns. Connecting struts include a proximal section, a distal section and an intermediate section. The proximal section is coupled to the corner of an expansion strut pair of the first expansion column, and the distal section is coupled to the joining strut of an expansion strut pair of the second expansion column intermediate the expansion strut pair first corner and the expansion strut pair second corner.
Owner:BOSTON SCI SCIMED INC
Intravascular stent device
Disclosed is a very small diameter intravascular stent, or aneurysm cover, which may be used to occlude, or partially occlude, an aneurysm in the human brain. The stent is comprised of a thin-walled skeletal tubular member formed of interconnected sinusoidal members to thereby form a pattern of cells along the skeletal tubular member. The stent also includes anchor members, which are comprised of a longitudinal leg member having a radiopaque coil disposed about the leg member, which serve to retain the stent during delivery of the stent.
Owner:CODMAN & SHURTLEFF INC
Intravascular stent
InactiveUS6923828B1Small frontal areaLow profileStentsWire articlesIntravascular stentBalloon catheter
A medical device for use in the interior of a body lumen includes a balloon catheter and a radially expandable stent. The stent includes a plurality of zig-zags of a low memory metal formed into a hollow, open-ended cylindrical shape. The individual zig-zags have a curved portion forming a reversing bend which allows the zig-zags to expand and deform as the balloon radially expands the stent. The curved portions of the zig-zags are aligned along the length of the stent in a spaced-apart arrangement with some curved portions attached and others unattached to adjacent zig-zags. The resulting stent is longitudinally flexible throughout its length when unexpanded and is also capable of conforming to a bend in the body lumen when expanded.
Owner:MEDTRONIC INC
Medical devices to treat or inhibit restenosis
Implantable medical devices having anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of certain antiproliferative agents, particularly a certain endothelin receptor inhibitor, are disclosed. The anti-restenotic endothelin receptor inhibitor is atrasentan, and pharmaceutically acceptable derivatives thereof. The anti-restenotic medical devices include stents, catheters, micro-particles, probes and vascular grafts. Intravascular stents are preferred medical devices. The medical devices can be coated using any method known in the art including compounding the endothelin receptor inhibitor with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-endothelin receptor inhibitor blends are disclosed. Additionally, medical devices having a coating comprising at least one endothelin receptor inhibitor in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the anti-restenotic implantable devices are also disclosed.
Owner:MEDTRONIC VASCULAR INC
Coatings for medical devices comprising a therapeutic agent and a metallic material
InactiveUS20080003251A1Suitable release rateReduce the amount requiredBiocideOrganic active ingredientsMetallic materialsIntravascular stent
The invention relates generally to an implantable medical device for delivering a therapeutic agent to the body tissue of a patient, and a method for making such a medical device. In particular, the invention pertains to an implantable medical device, such as an intravascular stent, having a coating comprising a first coating composition comprising a therapeutic agent and, optionally, a polymer; and a second coating composition comprising a metallic material.
Owner:BOSTON SCI SCIMED INC
Medical devices with coatings for delivery of a therapeutic agent
Described herein are implantable coated medical devices, such as intravascular stents, for delivering therapeutic agents to the body tissue of a patient, and methods for making such medical devices. In particular, described herein are implantable coated medical devices comprising a substrate having a surface, and a coating disposed upon the surface that comprises a coating composition that includes a releasable metal oxide. The coating is free of polymer or a particular type of polymer that is not a part of any releasable metal oxide.
Owner:BOSTON SCI SCIMED INC
Intravascular stent and assembly
InactiveUS20040133271A1Maximum possible flexibility and conformabilityOptimal metal fractionStentsSurgeryVascular lumenIntravascular stent
Various intravascular stents, such as intracoronary stents, include improved expansion and connecting strut designs. Such stents can be both very flexible and fully cover vessel surface inside the vascular lumen, and be well designed for both the delivery phase and the deployed phase of the stent life cycle.
Owner:BOSTON SCI SCIMED INC
Coating for medical devices comprising an inorganic or ceramic oxide and a therapeutic agent
InactiveUS20070264303A1Improve coating adhesionGood biocompatibilityStentsHeavy metal active ingredientsIntravascular stentMedical device
The invention relates generally to an implantable medical device for delivering a therapeutic agent to the body tissue of a patient, and a method for making such a medical device. In particular, the invention pertains to an implantable medical device, such as an intravascular stent, having a coating comprising an inorganic or ceramic oxide, such as titanium oxide, and a therapeutic agent.
Owner:BOSTON SCI SCIMED INC
Drug-delivery endovascular stent and method of forming the same
ActiveUS7727275B2Efficient releaseOrganic active ingredientsOrganic chemistryPolymer substratePercent Diameter Stenosis
Owner:BIOSENSORS INT GROUP
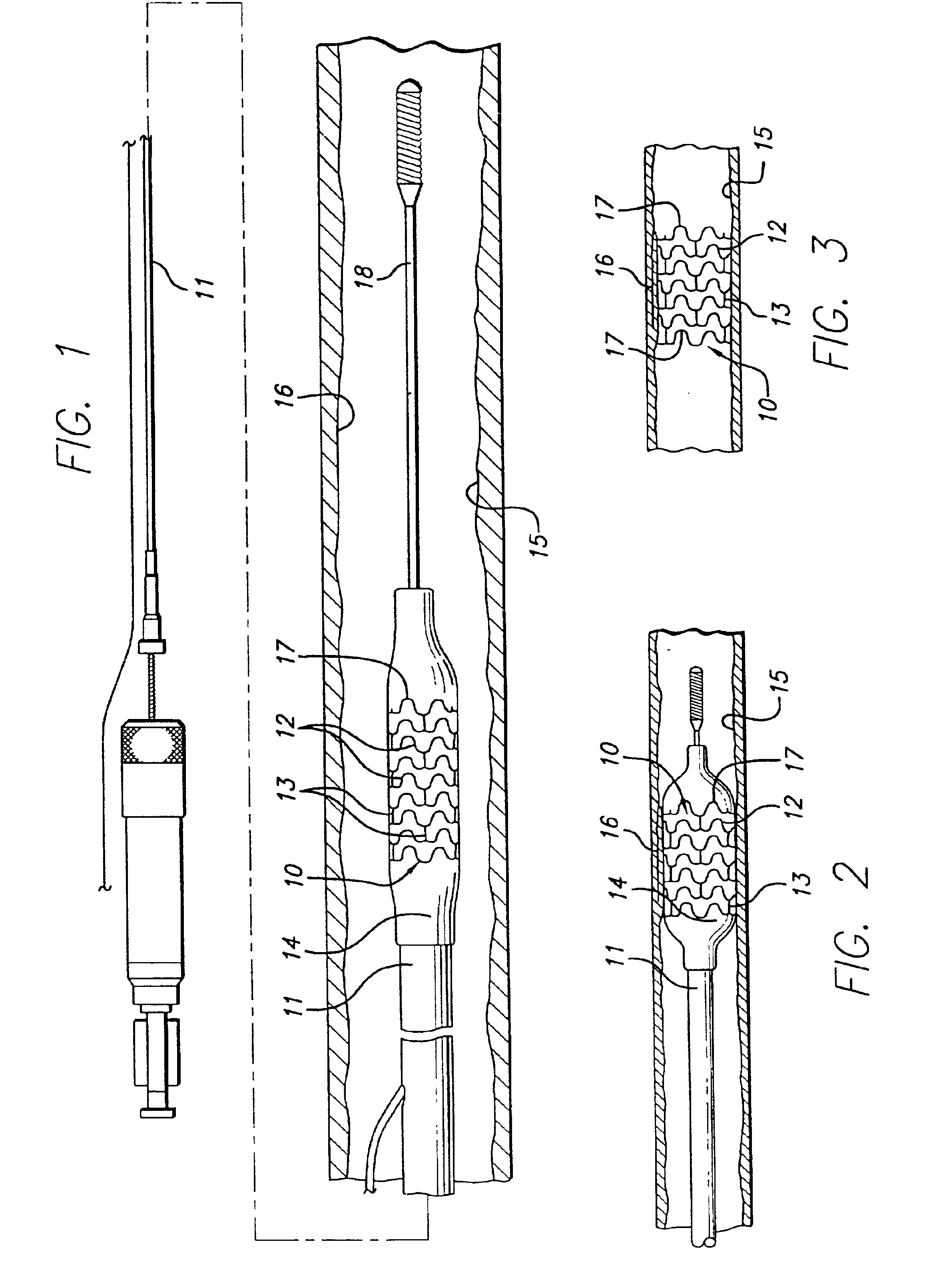
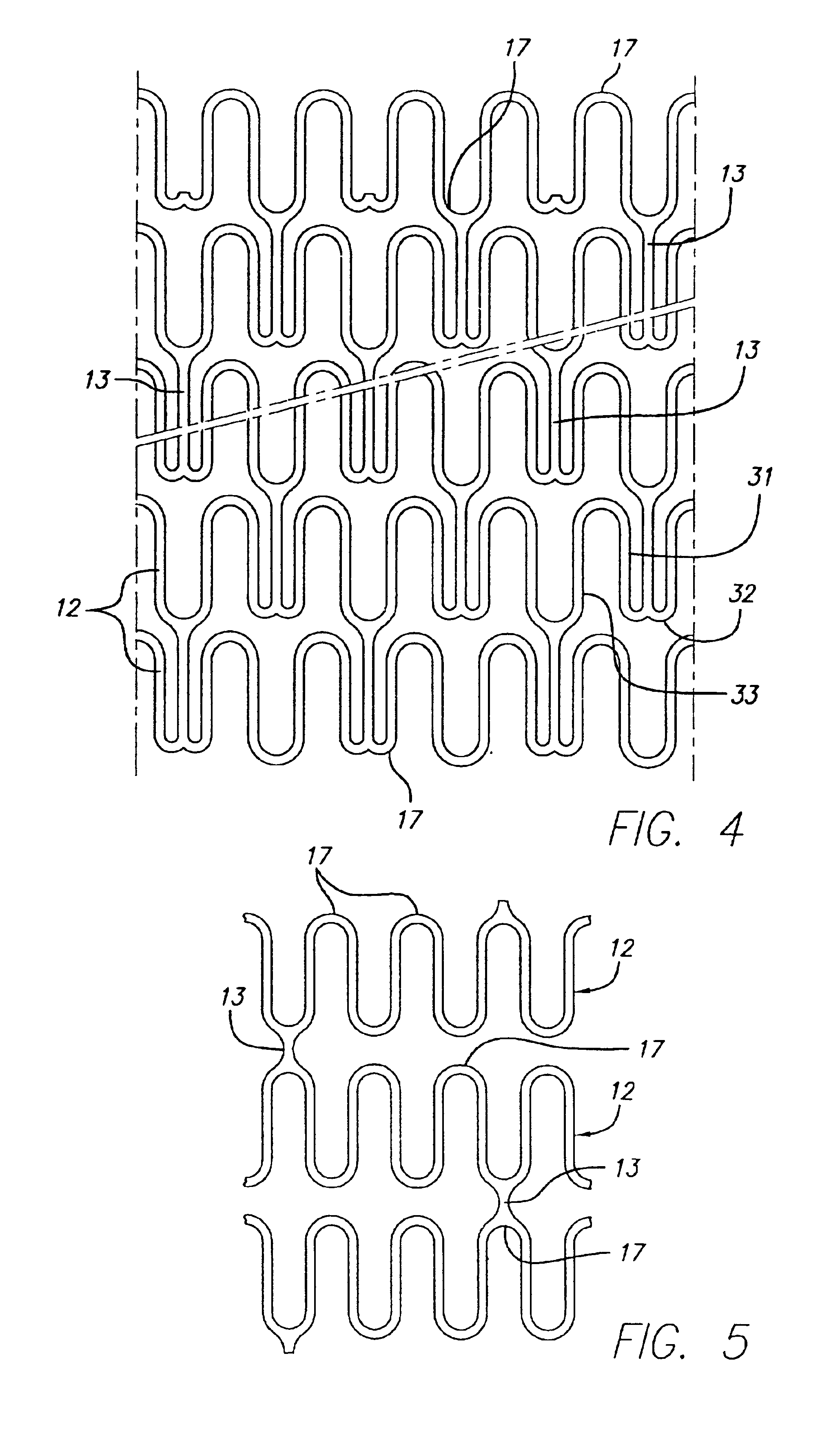
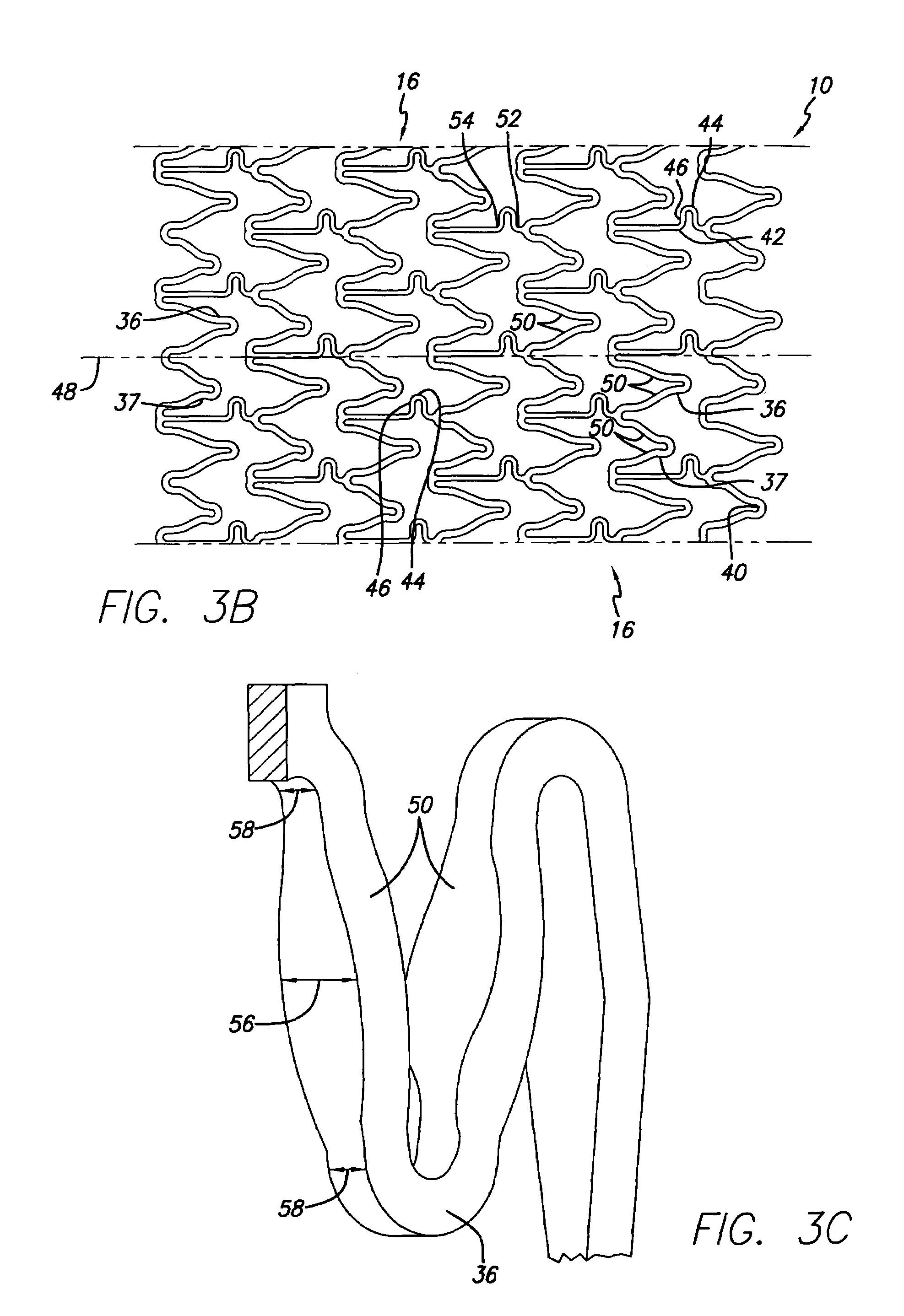
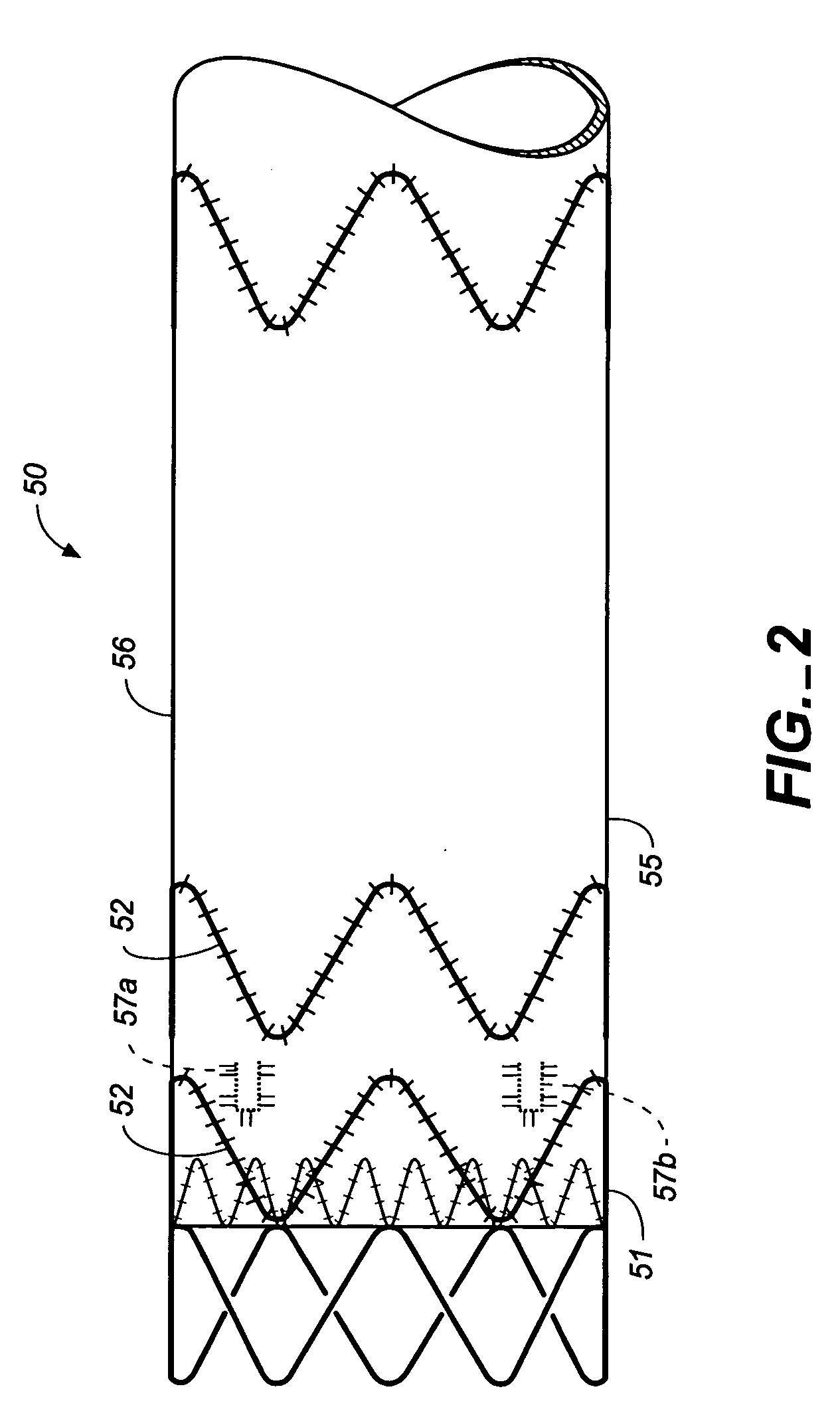
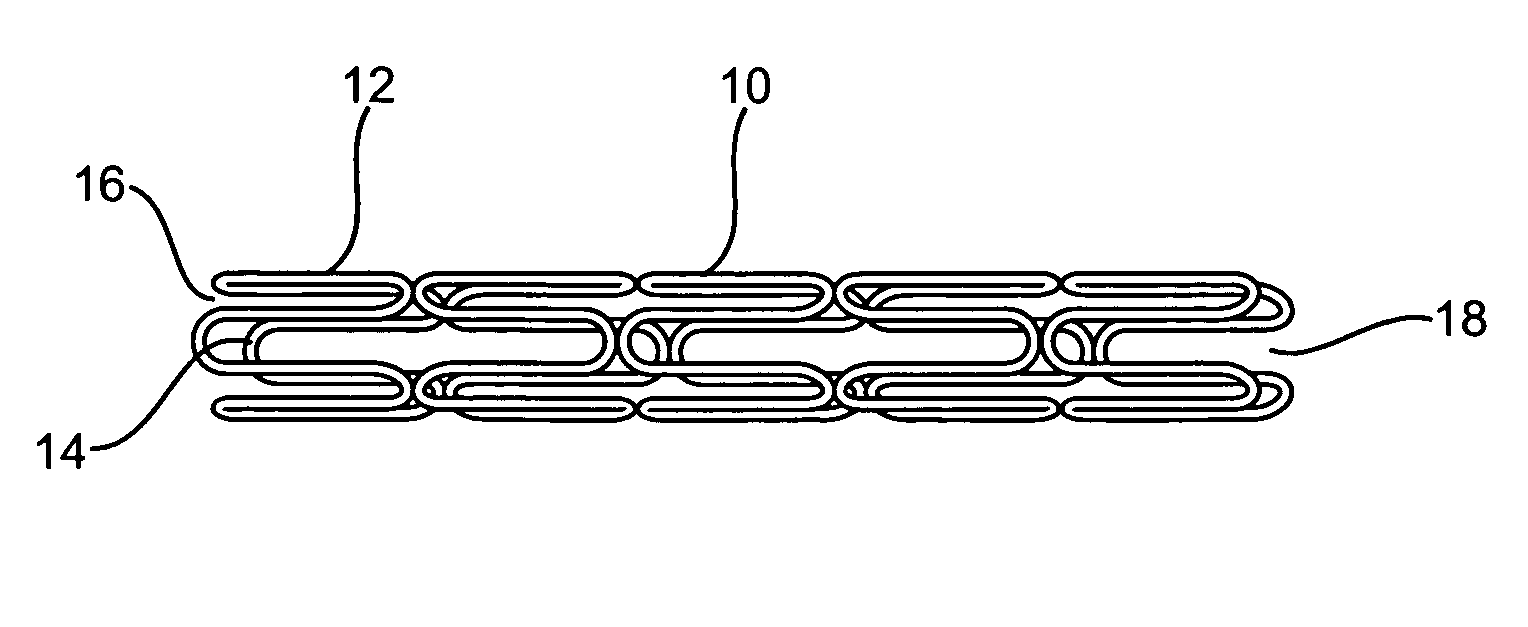
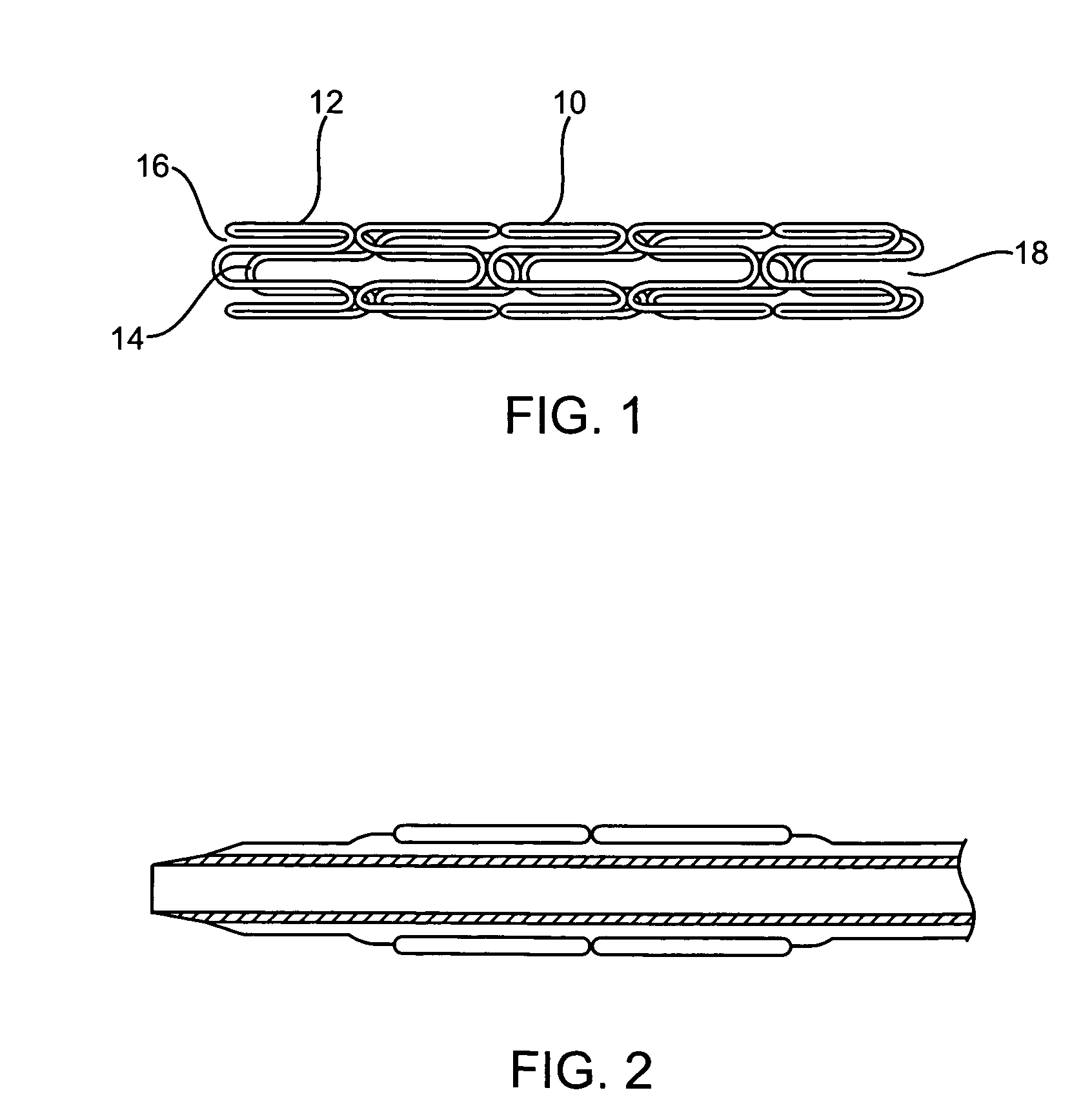
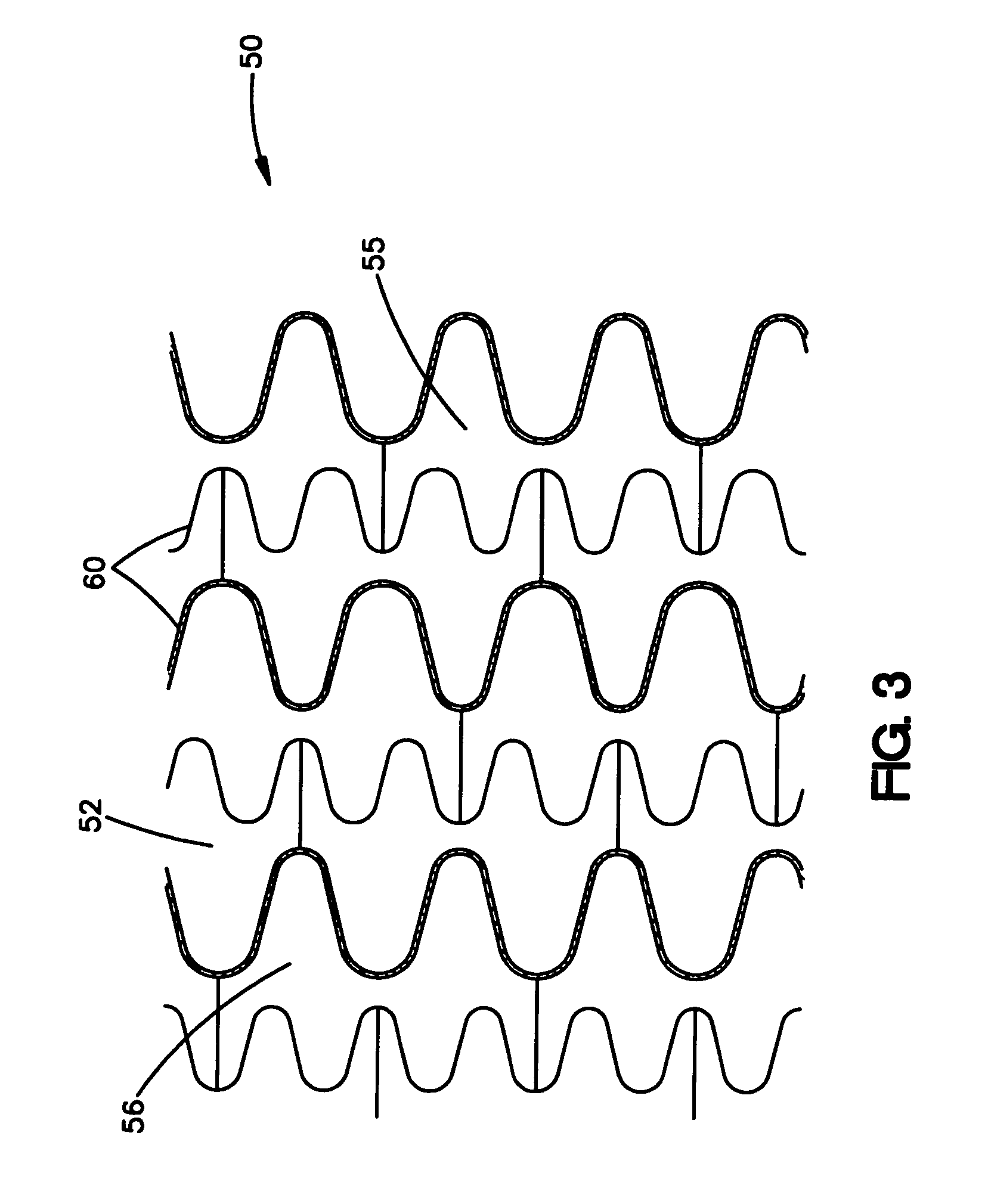
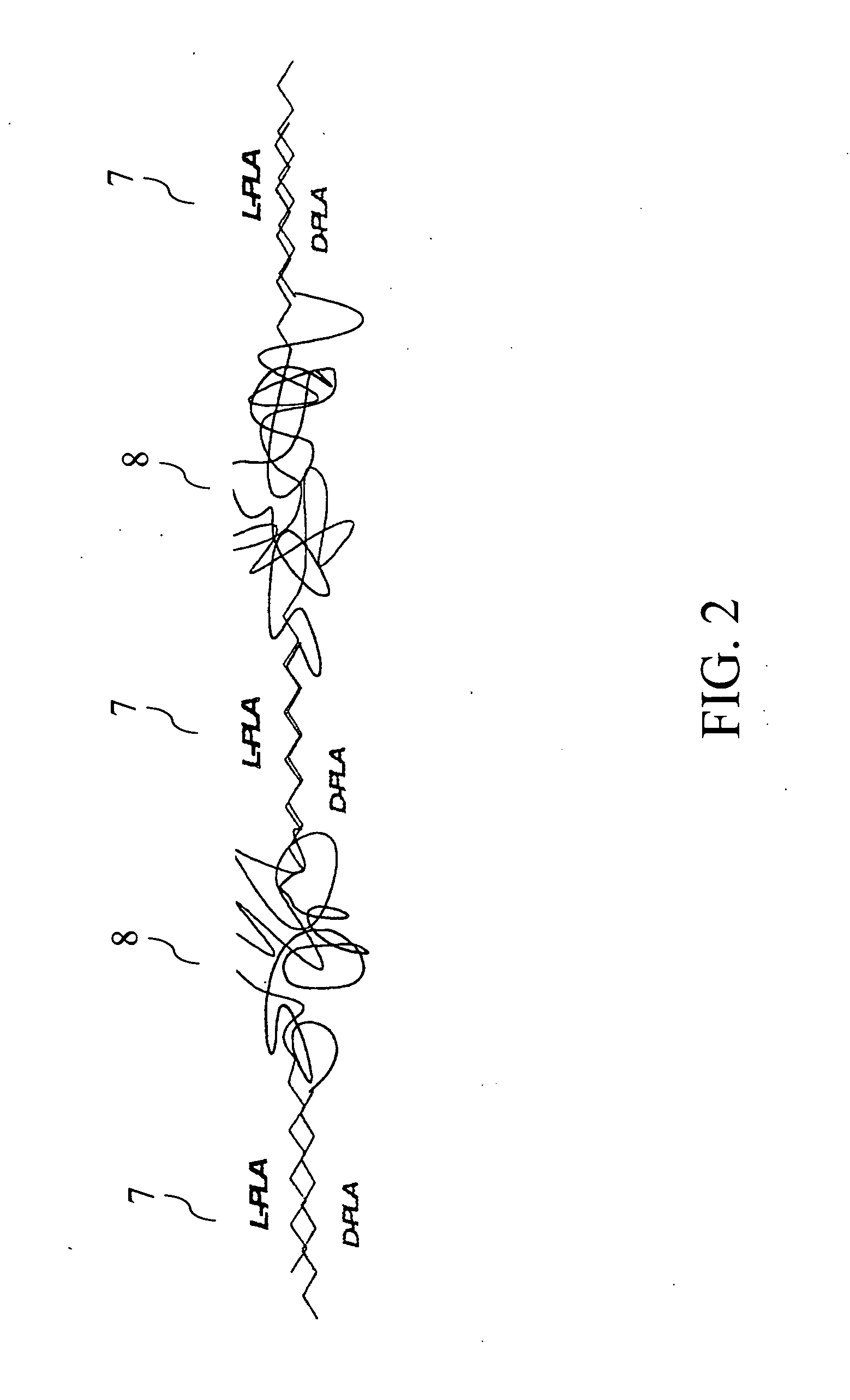
Longitudinally flexible stent
An intravascular stent especially suited for implanting in curved arterial portions. The stent retains longitudinal flexibility after expansion. The stent is formed of intertwined meander patterns forming triangular cells. The triangular cells are adapted to provide radial support, and also to provide longitudinal flexibility after expansion. The triangular cells provide increased coverage of a vessel wall. The stent can have different portions adapted to optimize radial support or to optimize longitudinal flexibility. The stent can be adapted to prevent flaring of portions of the stent during insertion.
Owner:MEDINOL LTD
Biodegradable endovascular stent using stereocomplexation of polymers
A biodegradable stent is comprised of a stereocomplex of polylactide enantiomers. The unique characteristics of stereocomplex polymers allows for the stent to have variable material characteristics along its length, while retaining uniform geometry. Thus, the stent is designed to be flexible upon insertion through the blood vessels while still retaining its rigidity and support upon expansion within the vessel. Furthermore, the material characteristics can be manipulated such that high strengths are possible even with small thicknesses of struts within the stent.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Intravascular stent
The invention is directed to an expandable stent for implanting in a body lumen, such as a coronary artery, peripheral artery, or other body lumen. The invention provides for an intravascular stent having a plurality of cylindrical rings connected by undulating links. The stent has a high degree of flexibility in the longitudinal direction, yet has adequate vessel wall coverage and radial strength sufficient to hold open an artery or other body lumen. The stent can be compressed or crimped onto a catheter to a very low profile since the peaks that are adjacent the curved portion of the undulating link are shorter than other peaks in the same cylindrical ring to prevent overlap yet still achieve a very low profile, tightly crimped stent onto a catheter.
Owner:ABBOTT CARDIOVASCULAR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com