Patents
Literature
1857 results about "Valine" patented technology
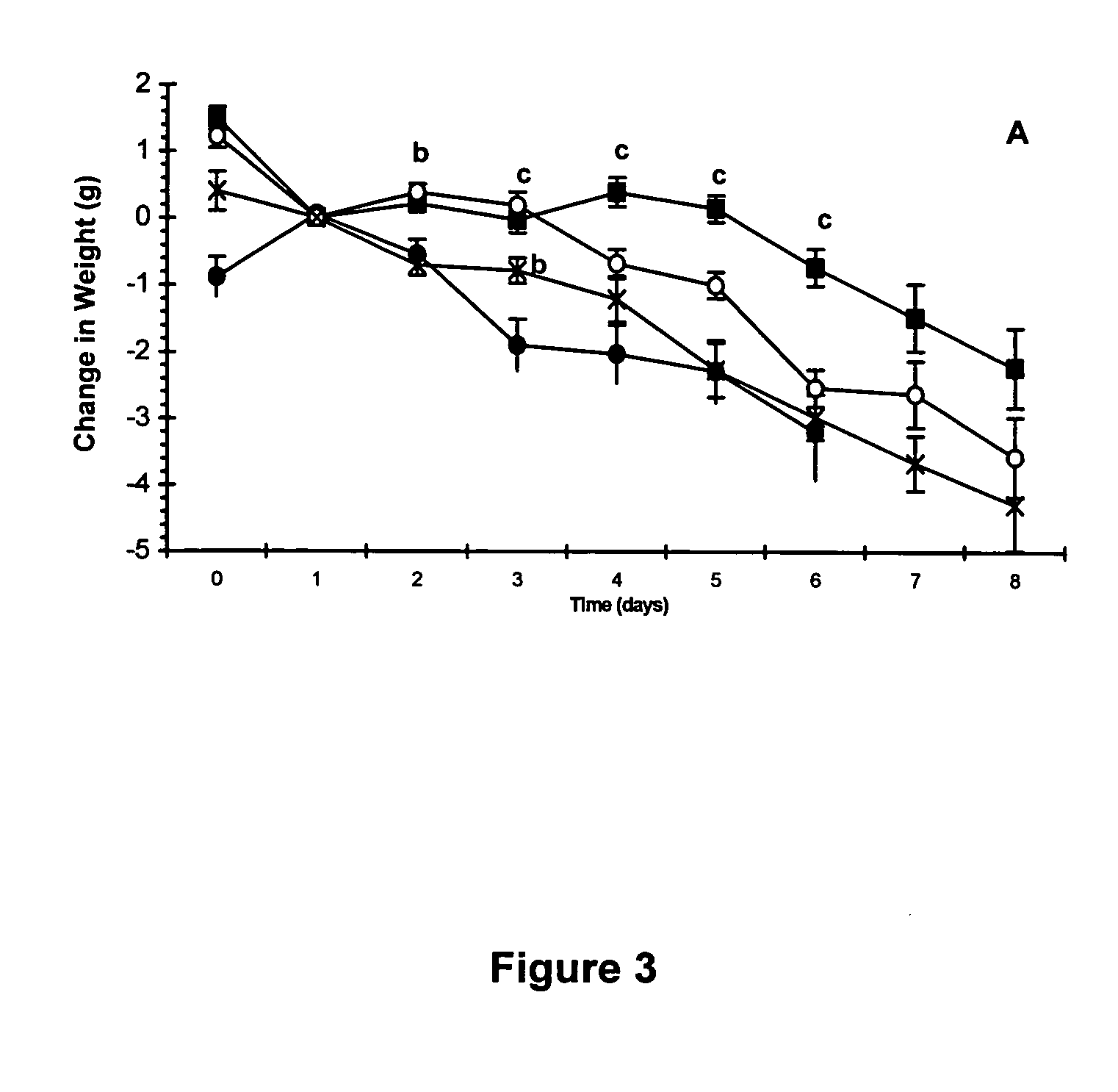
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH₃⁺ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO⁻ form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG).
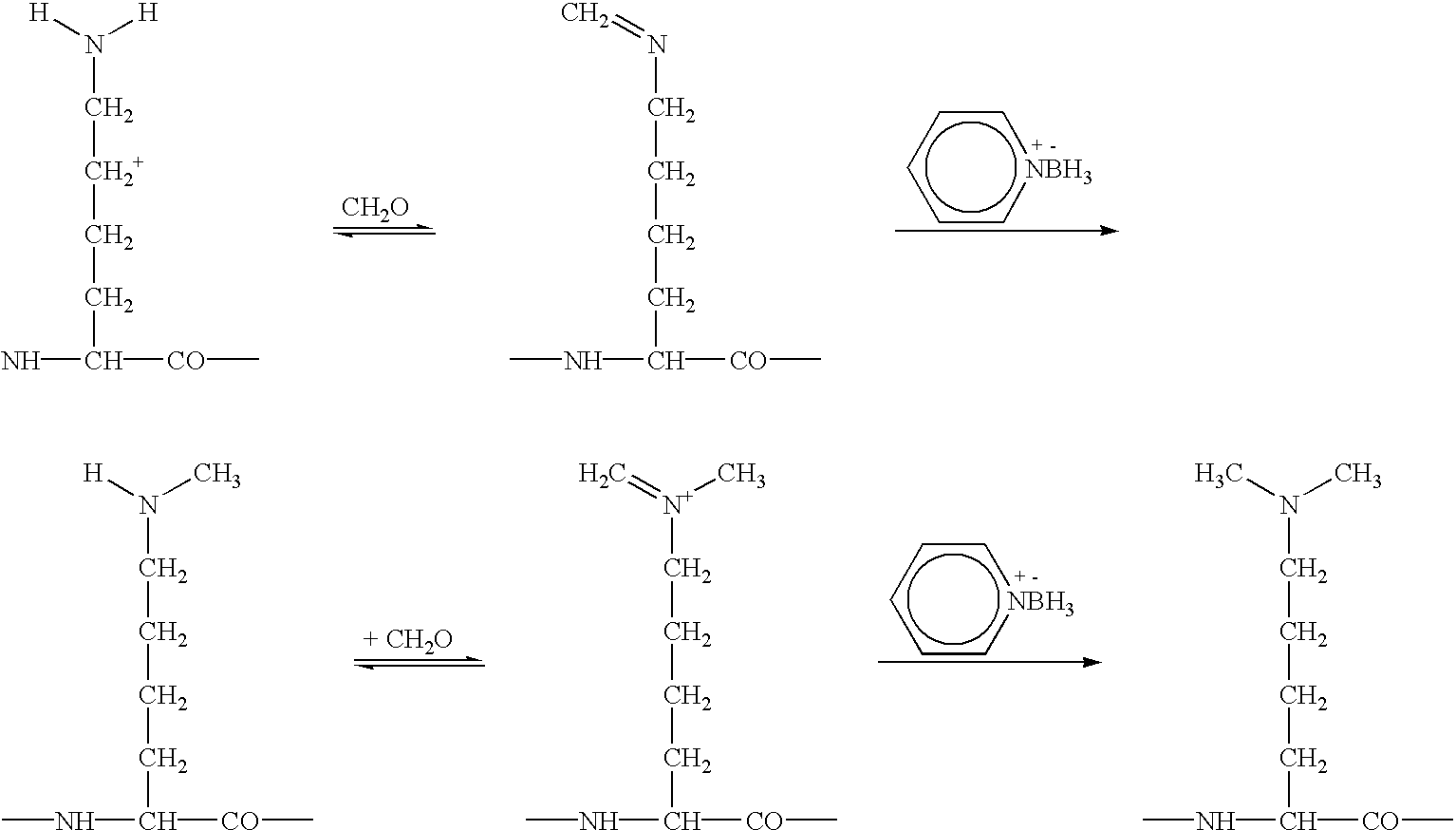
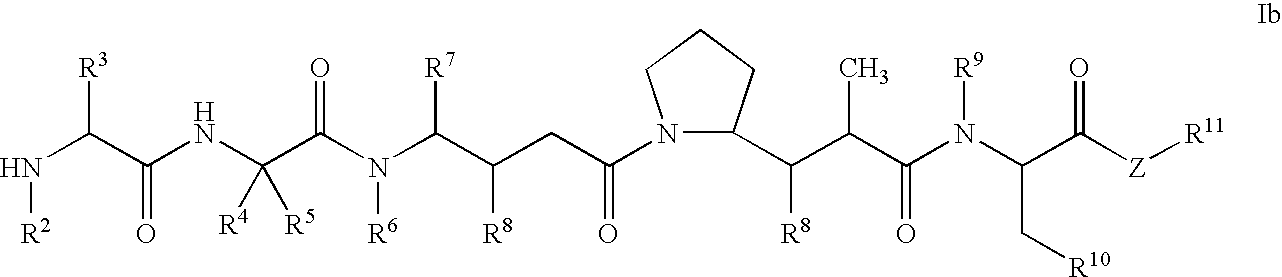
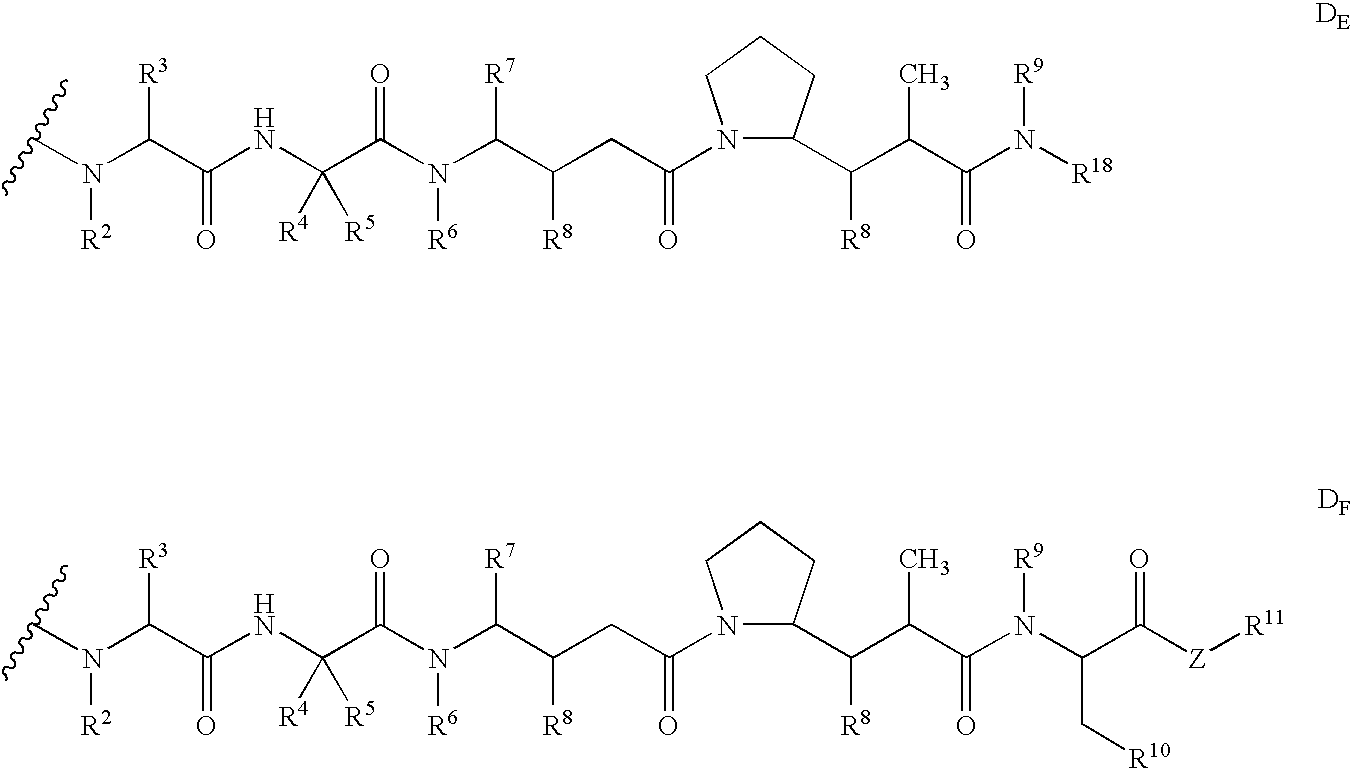
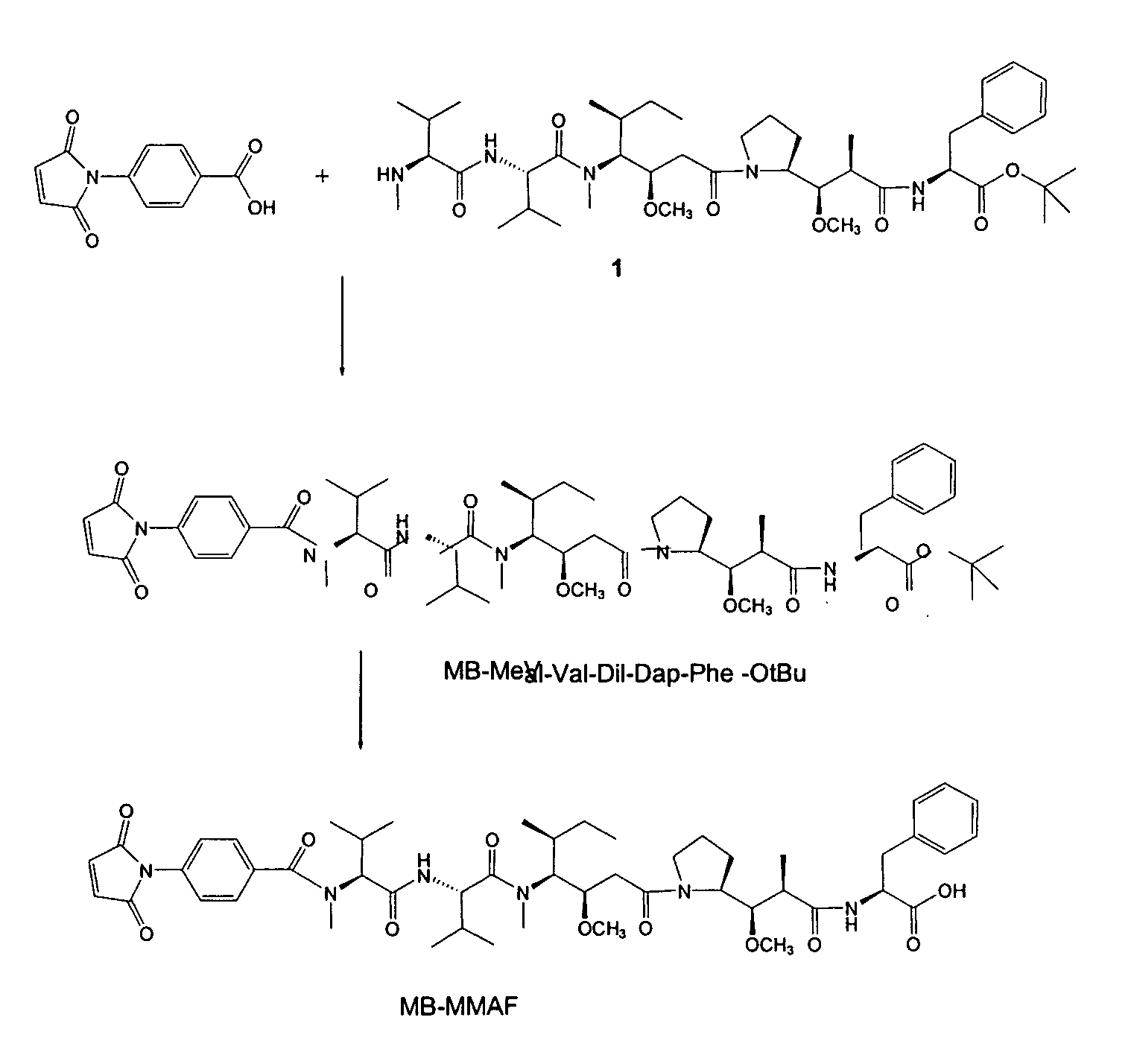
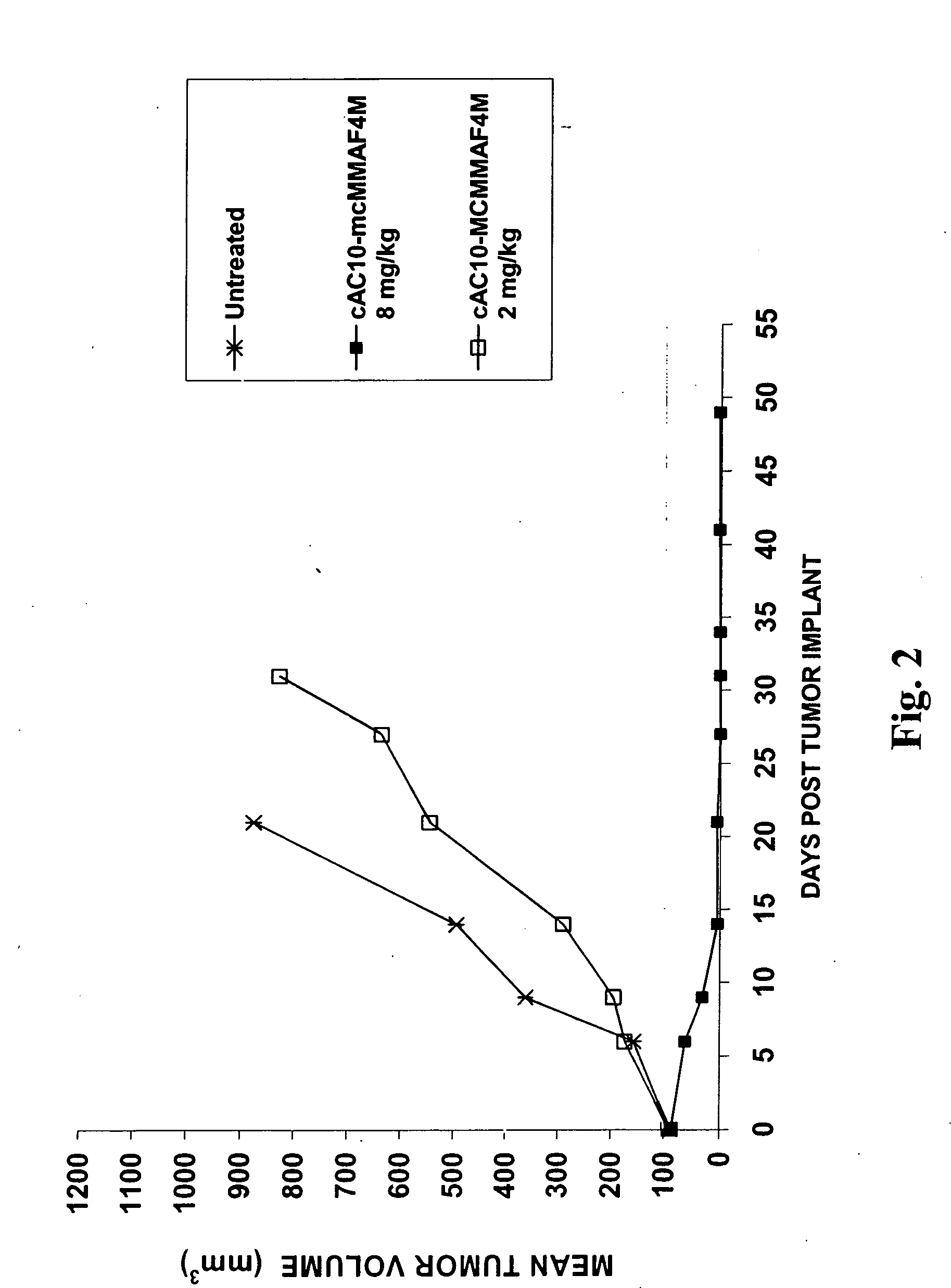
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
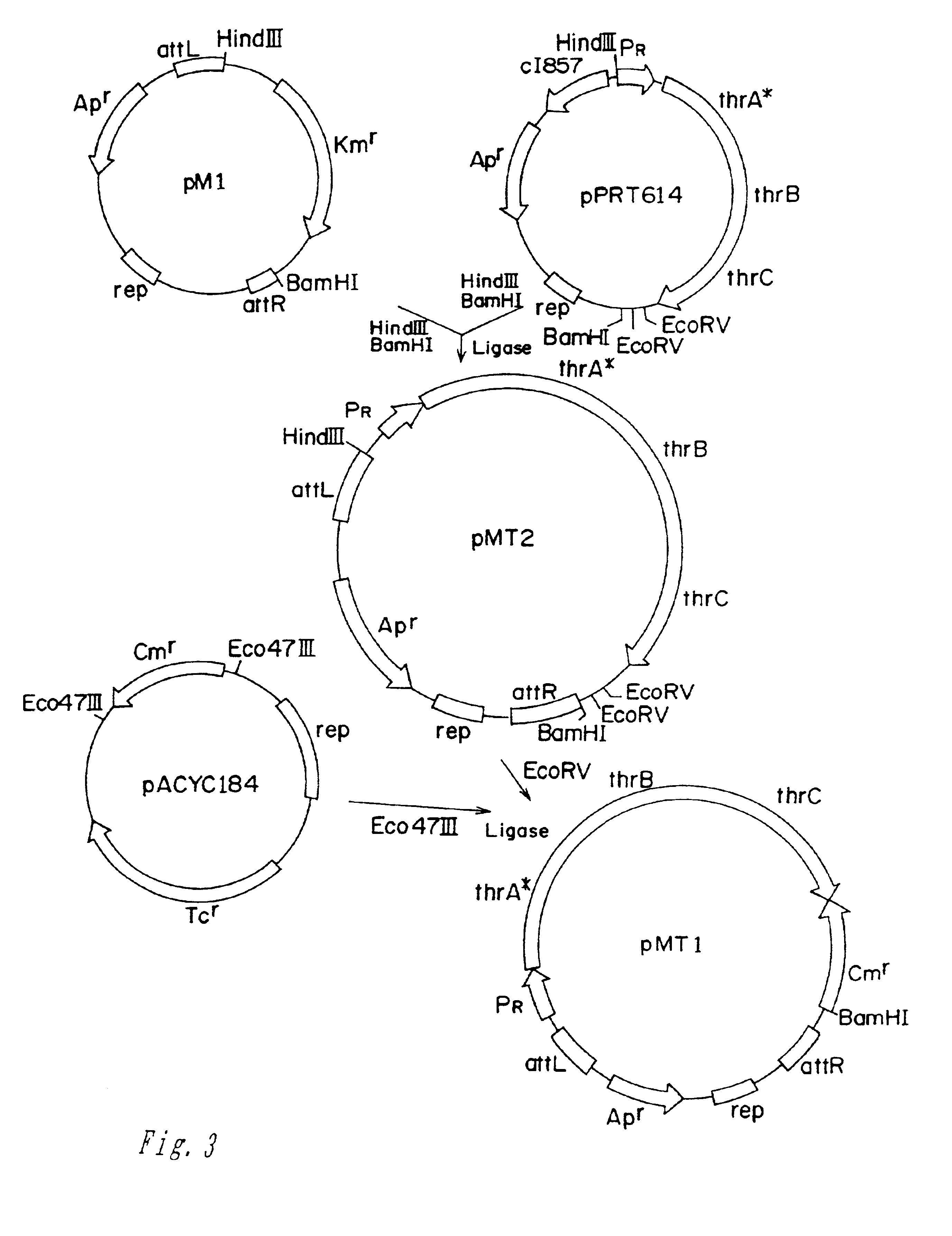
Methods of making amino acids using E. coli transformed with csc genes
An amino acid such as threonine, homoserine, isoleucine, lysine, valine and tryptophan is produced using a bacterium belonging to the genus Escherichia which has been constructed from sucorse non-assimilative strain belonging to the genus Escherichia and which harbors sucrose non-PTS (phosphoenol pyruvate-dependent sucrose-6-phosphotransferase system) genes and has an ability to produce the amino acid.
Owner:AJINOMOTO CO INC
Method of coating an intravascular stent with an endothelial cell adhesive five amino acid peptide
InactiveUS6140127APromoting cell attachmentRestore patencyBiocidePeptide/protein ingredientsCell specificArginine
Endothelial cell attachment to an intravascular stent is promoted by coating the stent with an endothelial cell specific adhesion peptide. Coating is preferably carried out by activating the intravascular stent using plasma glow discharge, applying on the stent a layer or plurality of layers of a polymer such as poly(2-hydroxyethylmethacrylate), applying a tresylation solution containing pyridine and tresyl chloride, and applying a five amino acid peptide having the sequence glycine-arginine-glutamic acid-aspartic acid-valine.
Owner:CORDIS CORP
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
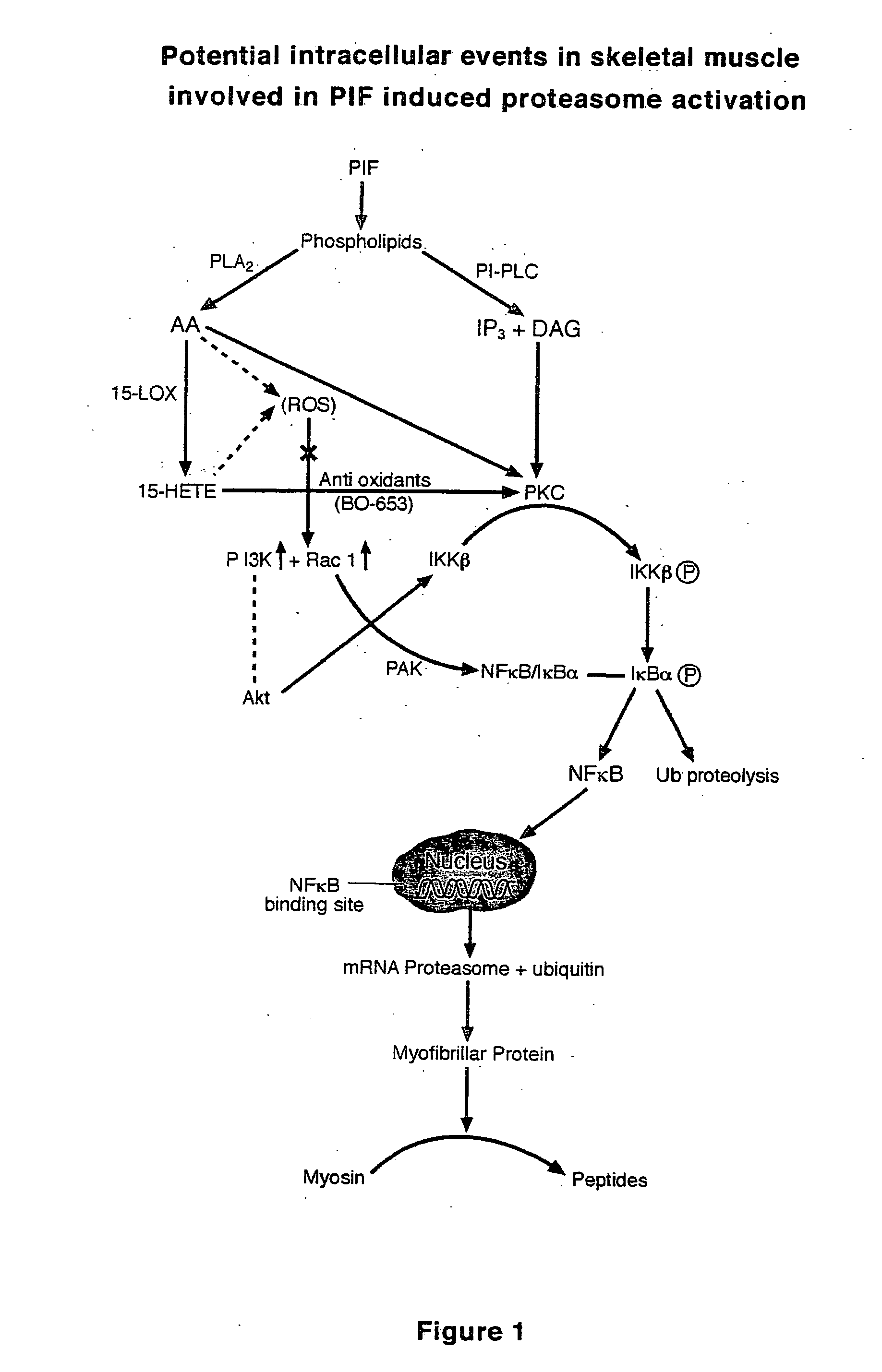
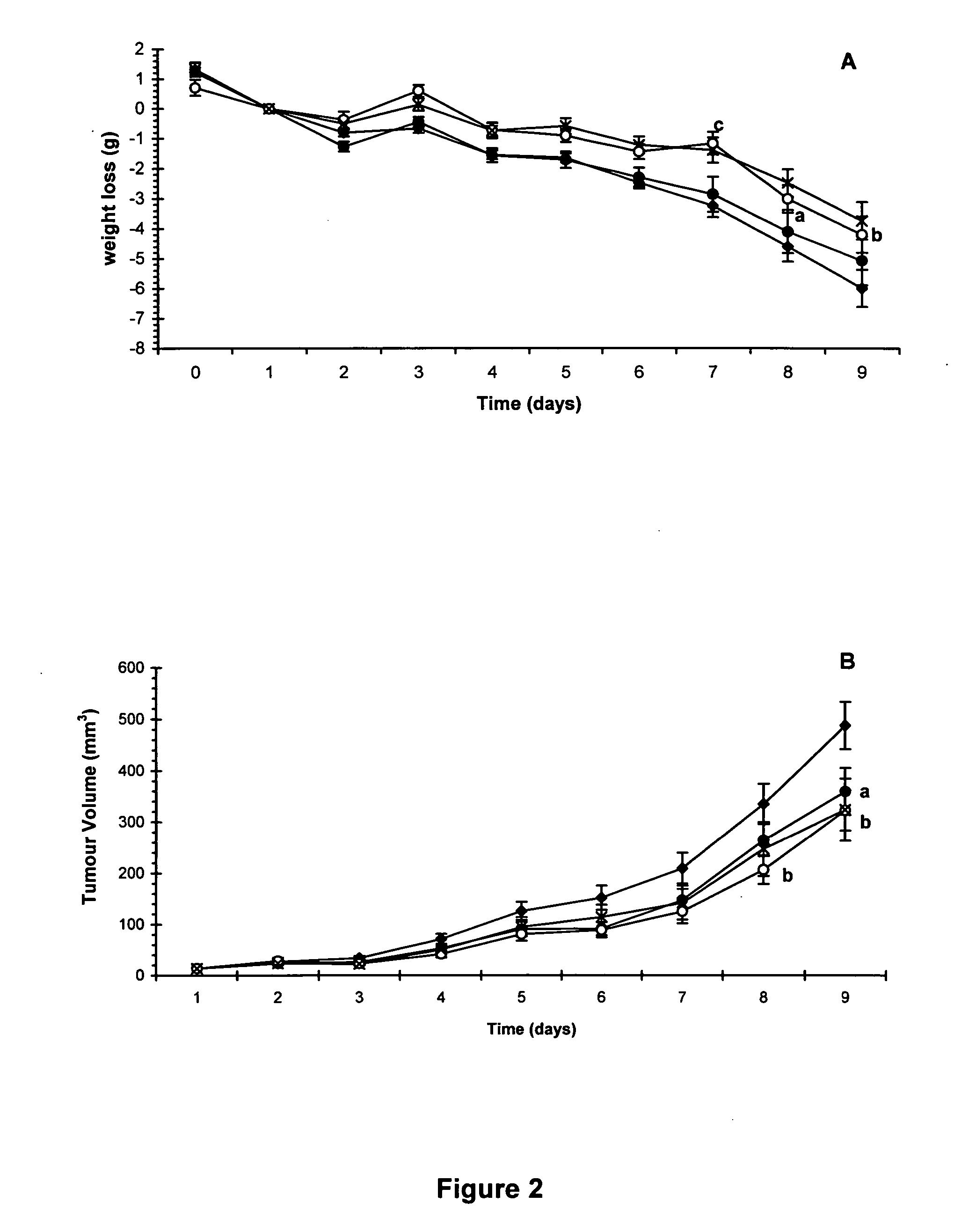
HMB compositions and uses thereof
InactiveUS20050215640A1Reduce tumor growth rateReduce rateBiocideNervous disorderInvoluntary weight lossNeutral Amino Acids
The present invention relates to methods for the prevention and treatment of chronic inflammatory diseases, cancer, and involuntary weight loss. In the practice of the present invention patients are enterally administered HMB alone or alternatively in combination with eicosapentaenoic (20:5 ω-3), FOS, carnitine and mixtures thereof. HMB may be added to food products comprising a source of amino-nitrogen enriched with large neutral amino acids such as leucine, isoleucine, valine, tyrosine, threonine and phenylalanine and subtantially lacking in free amino acids.
Owner:ABBOTT LAB INC
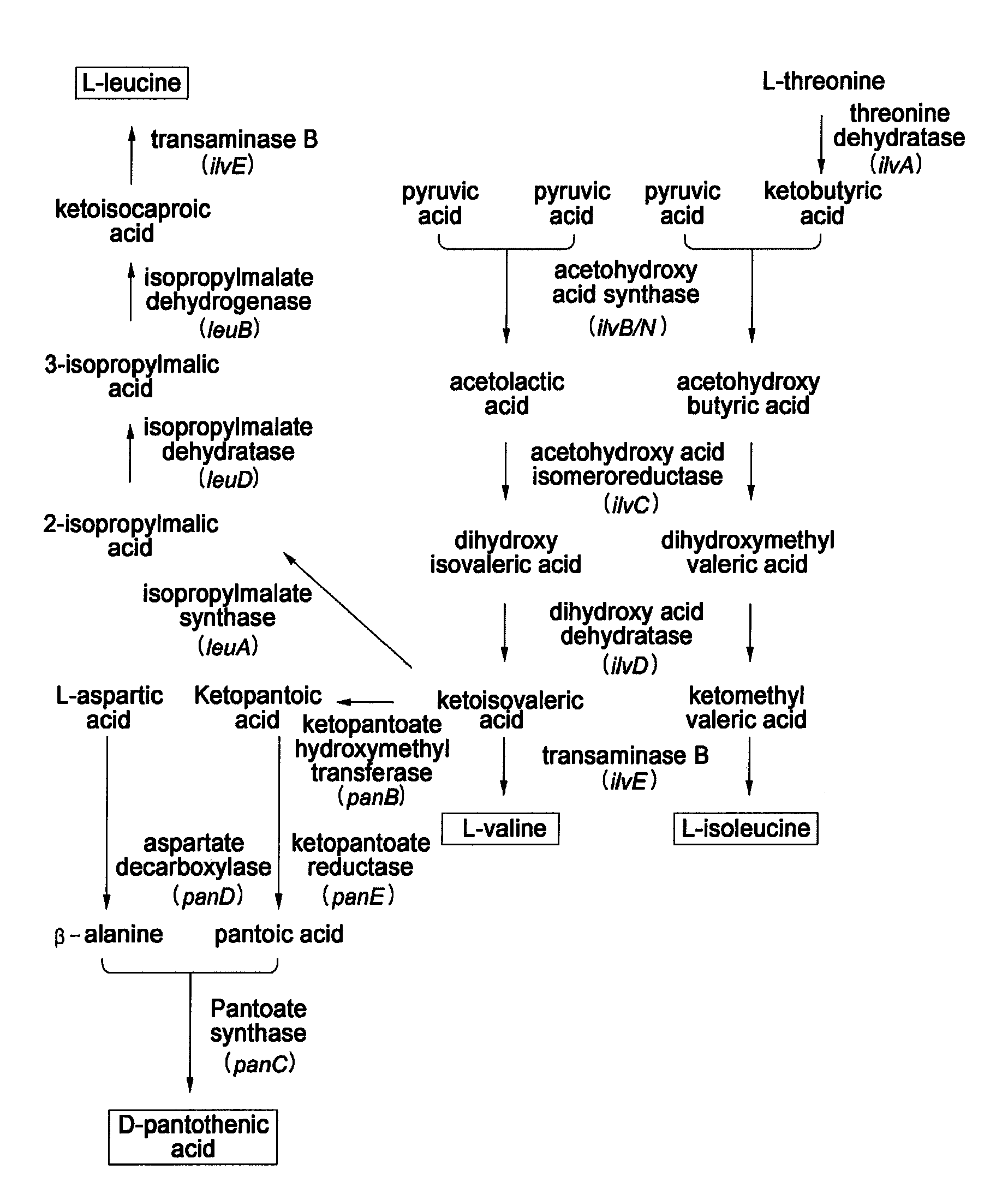
Microorganism having enhanced L-valine productivity and method for producing L-valine using the same
The present invention relates to a microorganism having an enhanced L-valine productivity and a method for producing L-valine using the same. More particularly, the present invention relates to a Corynebacterium glutamicum mutant strain that has resistance to L-valine and derivatives thereof so as to have an enhanced L-valine productivity, and a method for producing L-valine using the same.
Owner:CJ CHEILJEDANG CORP
Thermal treatment process for tobacco materials
ActiveUS8434496B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Gene products of bacillus licheniformis which form odorous substances and improved biotechnological production methods based thereon
InactiveUS20070190605A1Reduce formationImprove filtering effectBacteriaHydrolasesBacillus licheniformisPropanoic acid
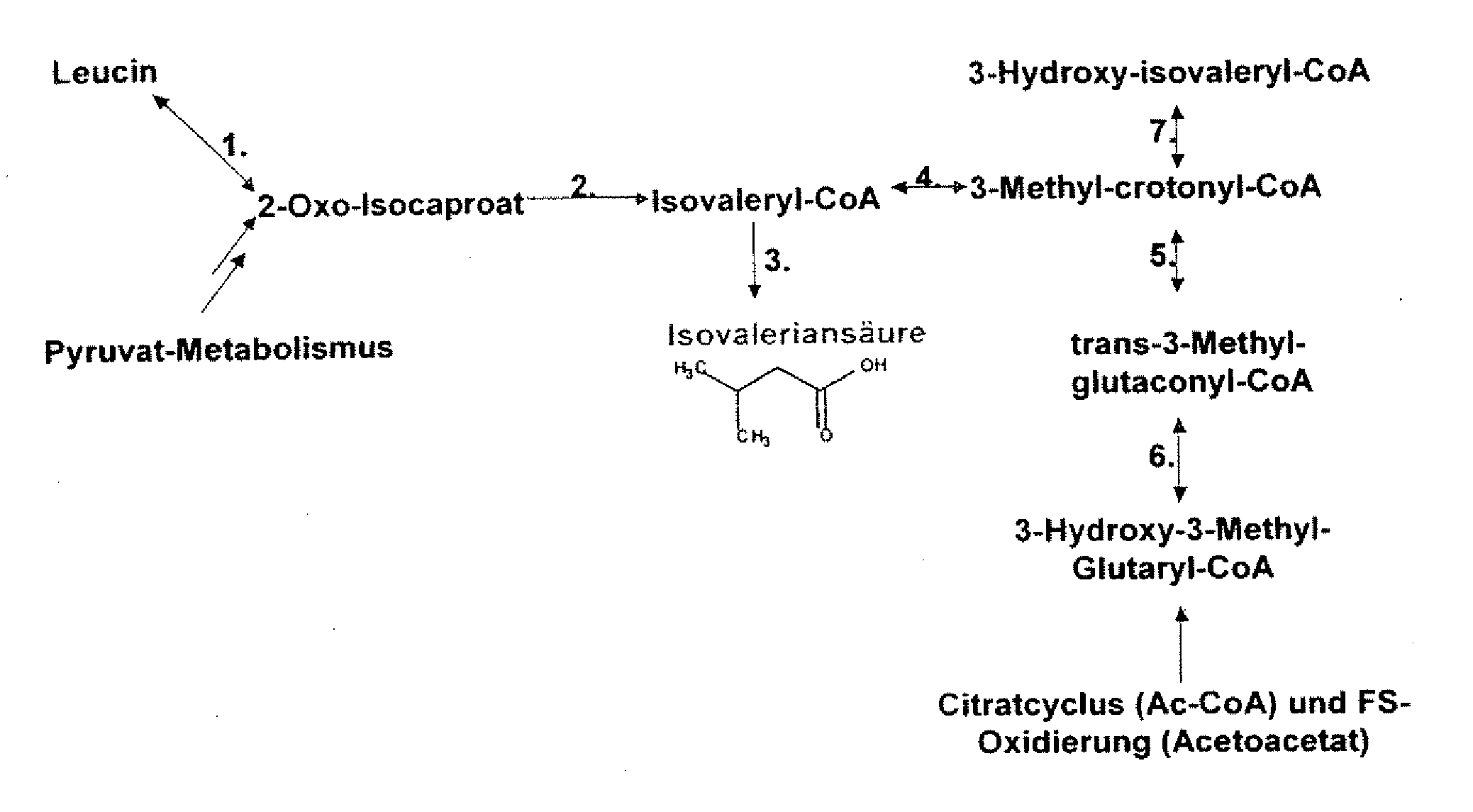
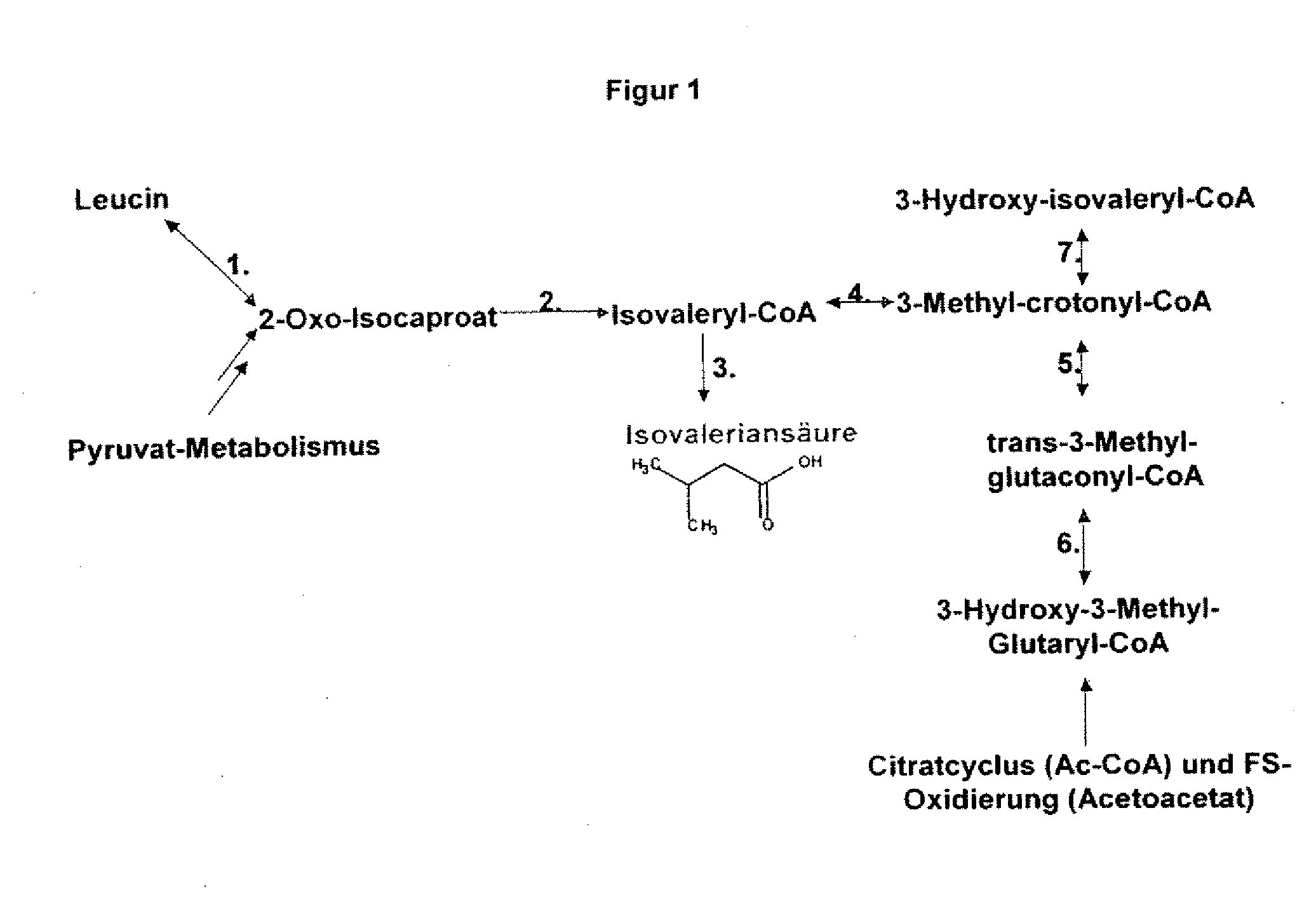
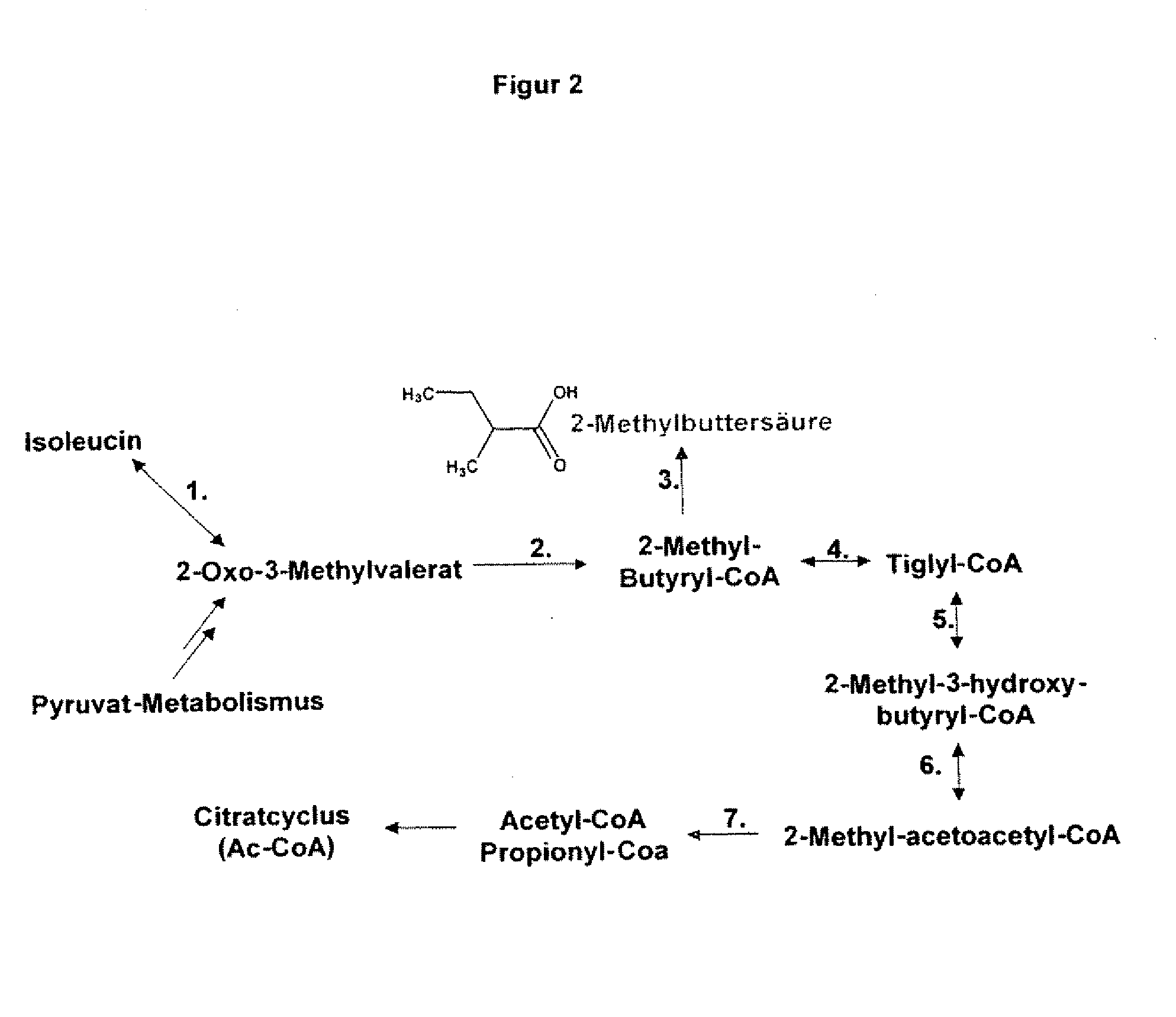
The present invention relates to 25 hitherto undescribed genes of B. licheniformis and gene products derived therefrom and all sufficiently homologous nucleic acids and proteins thereof. They occur in five different metabolic pathways for the formation of odorous substances. The metabolic pathways in question are for the synthesis of: 1) isovalerian acid (as part of the catabolism of leucine), 2) 2-methylbutyric acid and / or isobutyric acid (as part of the catabolism of valine and / or isoleucine), 3) butanol and / or butyric acid (as part of the metabolism of butyric acid), 4) propyl acid (as part of the metabolism of propionate) and / or 5) cadaverine and / or putrescine (as parts of the catabolism of lysine and / or arginine). The identification of these genes allows biotechnological production methods to be developed that are improved to the extent that, to assist these nucleic acids, the formation of the odorous substances synthesized via these metabolic pathways can be reduced by deactivating the corresponding genes in the micro-organism used for the biotechnological production. In addition, these gene products are thus available for preparing reactions or for methods according to their respective biochemical properties.
Owner:BASF AG
Thermal treatment process for tobacco materials
ActiveUS8991403B2Alter natureAlter characterTobacco preparationTobacco treatmentArginineTobacco product
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
2'-C-methyl-3'-O-L-valine ester ribofuranosyl cytidine for treatment of flaviviridae infections
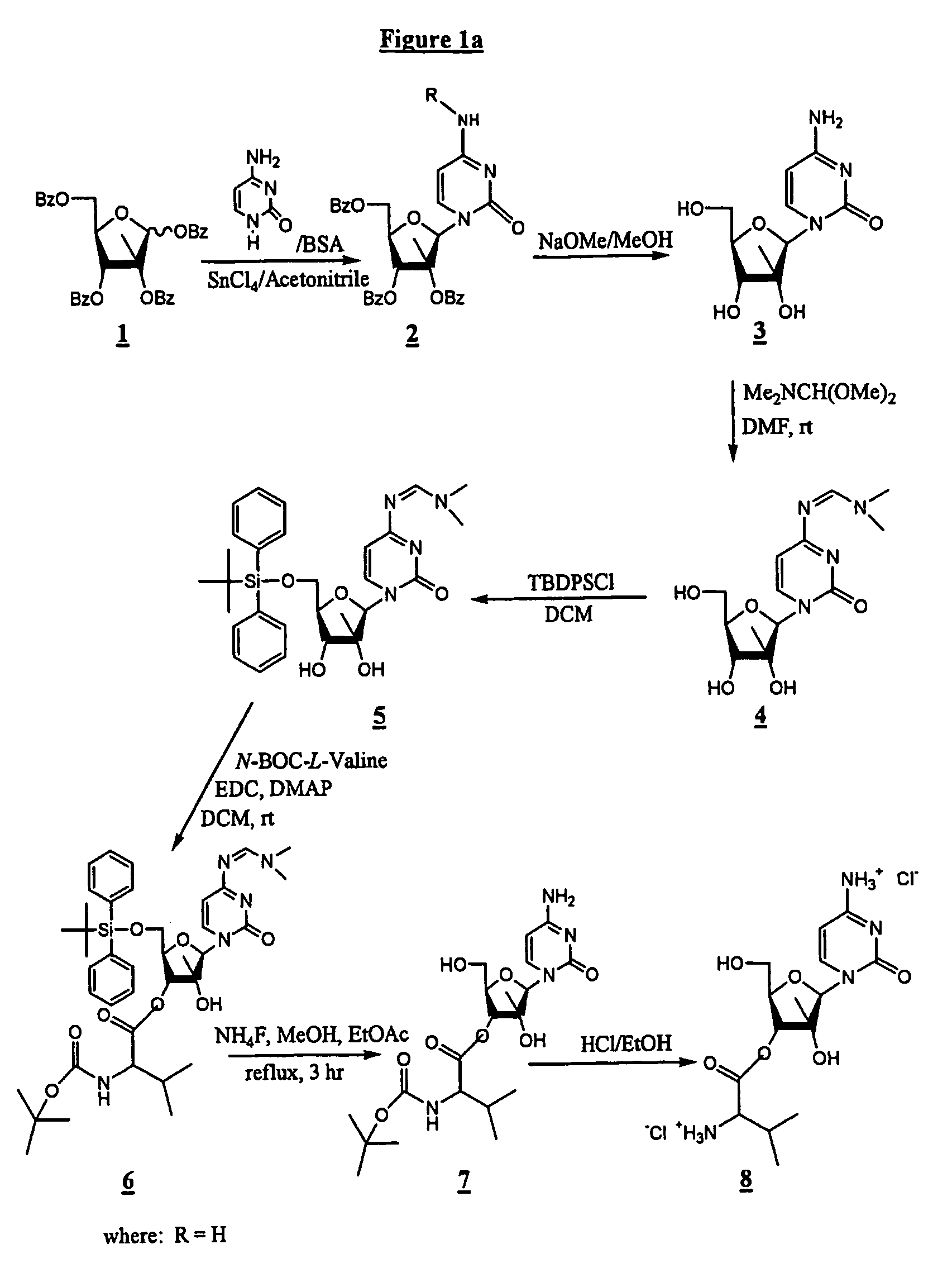
The 3′-L-valine ester of β-D-2′-C-methyl-ribofuranosyl cytidine provides superior results against flaviviruses and pestiviruses, including hepatitis C virus. Based on this discovery, compounds, compositions, methods and uses are provided for the treatment of flaviviridae, including HCV, that include the administration of an effective amount of val-mCyd or its salt, ester, prodrug or derivative, optionally in a pharmaceutically acceptable carrier. In an alternative embodiment, val-mCyd is used to treat any virus that replicates through an RNA-dependent RNA polymerase.
Owner:INDENIX PHARM LLC +3
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20080248053A1Improve bioavailabilityImprove compoundAntibacterial agentsTripeptide ingredientsD-norephedrineBiochemistry
Owner:SEAGEN INC
Thermal treatment process for tobacco materials
ActiveUS8944072B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of preparing a tobacco material for use in a smoking article is provided, including (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof; (ii) heating the mixture; and (iii) incorporating the heat-treated mixture into a smoking article as a smokable material. A smoking article in the form of a cigarette is also provided that includes a tobacco material pre-treated to inhibit reaction of asparagine to form acrylamide in mainstream smoke. Upon smoking, the smoking article is characterized by an acrylamide content of mainstream smoke that is reduced relative to an untreated control smoking article.
Owner:R J REYNOLDS TOBACCO COMPANY
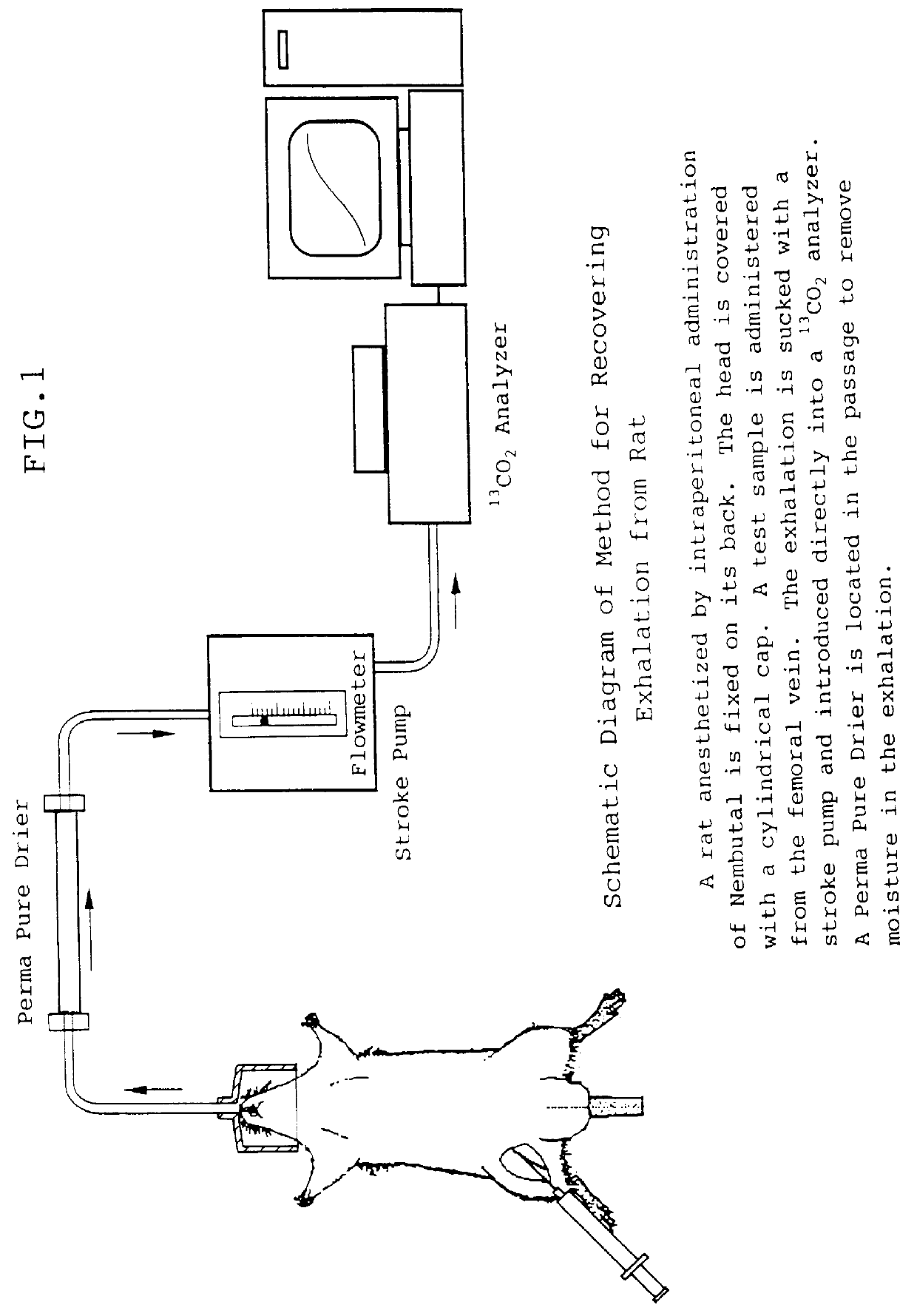
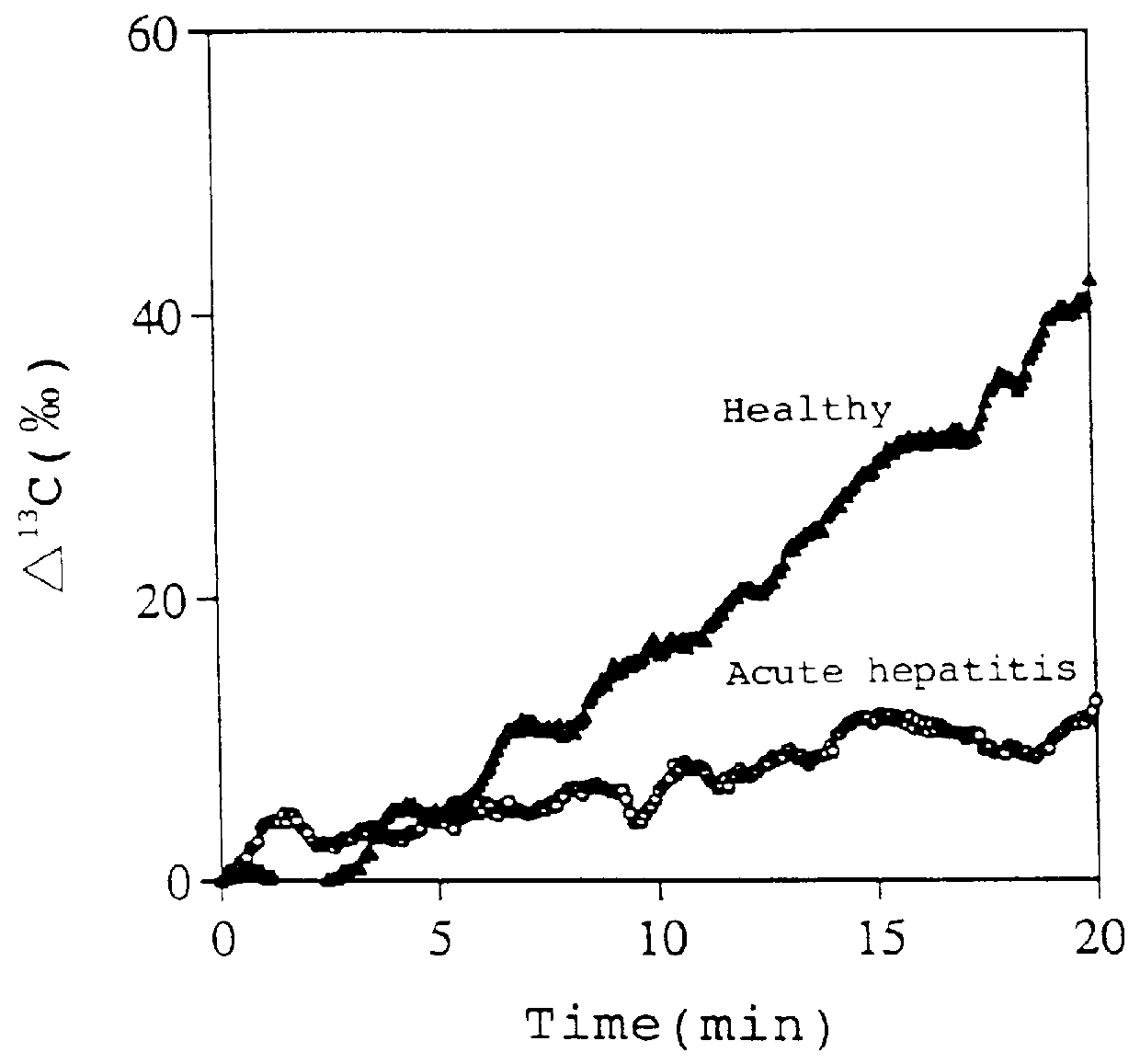
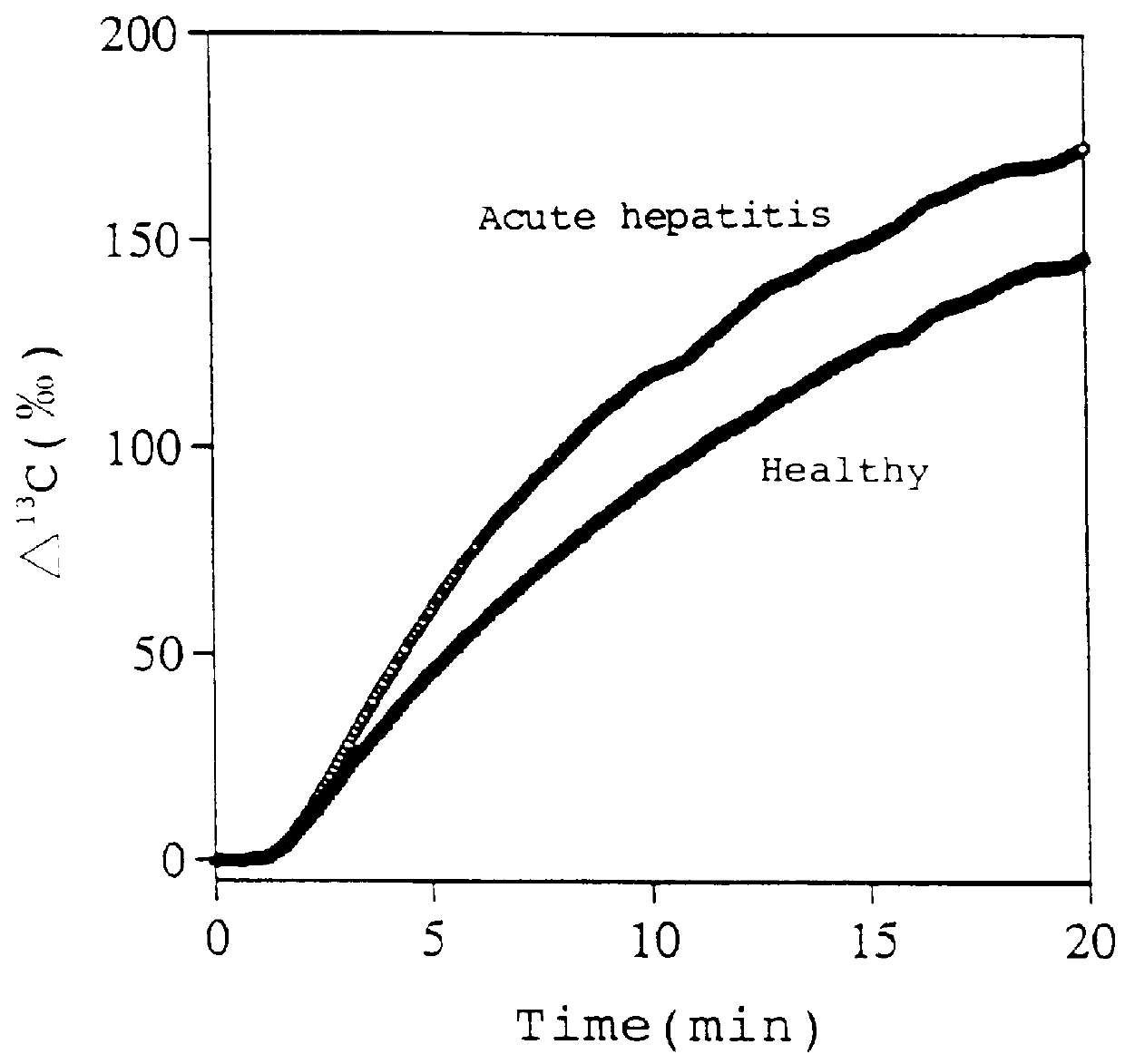
Diagnostic agent for liver function
InactiveUS6071245ALessen the burden on the bodyEasy to useRadioactive preparation carriersRespiratory organ evaluationSide effectGlycerol
The present invention relates to a diagnostic agent for liver function, comprising a compound labelled with 13C at least at one specific position selected from the group consisting of the following (a) to (f): (a) galactose, glucose or xylose labelled with 13C at least at one specific position or a starch composed of glucose units labelled with 13C at least at one specific position; (b) a polar amino acid, heterocyclic amino acid, isoleucine or valine labelled with 13C at least at one specific position; (c) a carboxylic acid constituting the glycolytic pathway or the citric acid cycle, labelled with 13C at least at one specific position; (d) a fatty acid labelled with 13C at least at one specific position; (e) a glyceride labelled with 13C at least at one specific position; and (f) glycerol labelled with 13C at least at one specific position. According to the present invention, a diagnostic agent for liver function which imposes less physical burden on a subject, can give accurate test result immediately, and can be used safely without side effects is provided. The diagnostic agent of the invention is useful for evaluating the liver function of a subject at the time when the test is carried out.
Owner:TOKYO GAS CO LTD
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
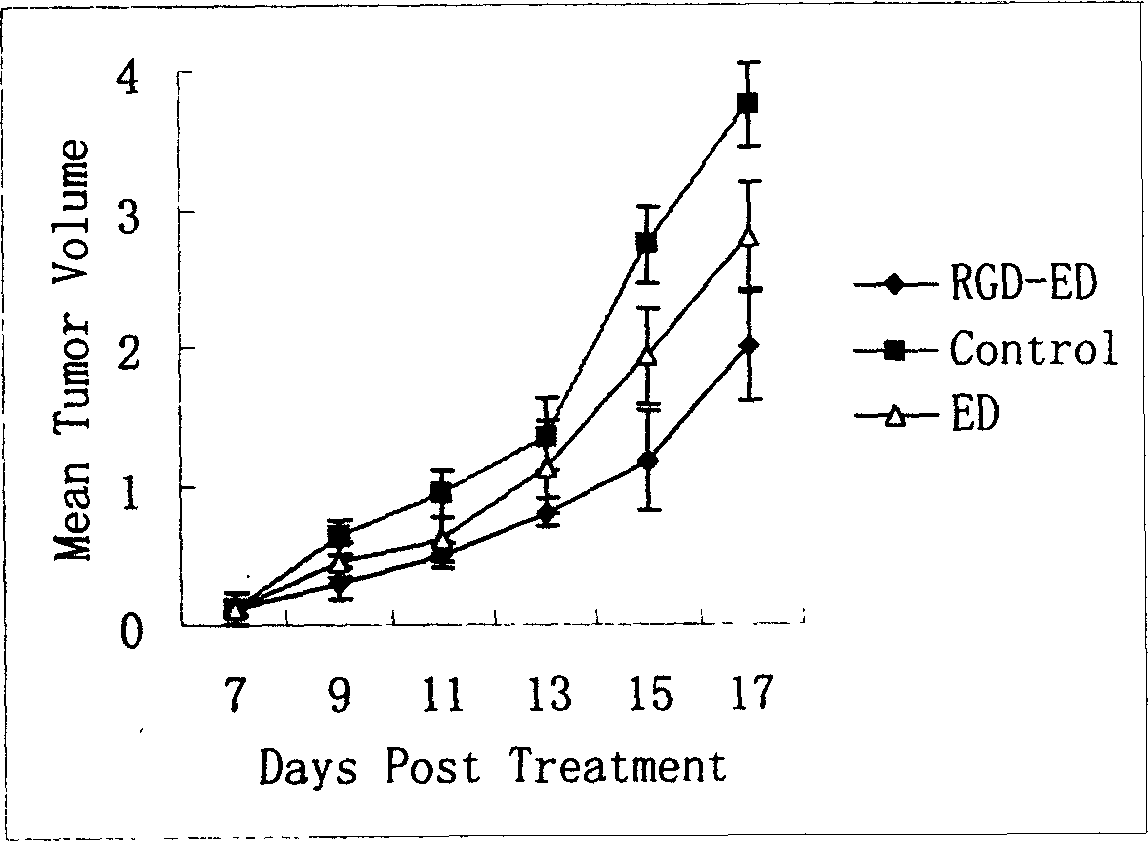
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
Six percent-premix for lactating sows and wheat-type daily ration prepared from six percent-premix
ActiveCN103053852AImprove reproductive performanceIncrease feed intakeAnimal feeding stuffComposite electrolytePhytase
The invention belongs to the field of feeds, and particularly relates to a six percent-premix for lactating sows and a wheat-type daily ration prepared from the six percent-premix. The premix comprises multivitamins, composite trace elements, DL-methionine, lysine, threonine, L-arginine, valine, composite electrolyte, compound enzyme, 5000 IU of phytase, functional additives, feed attractants, calcium hydrogen phosphate, stone powder, salt, choline, antioxidants and defatted rice bran. On the whole, the daily ration for the lactating sows is capable of protecting the development of mammary glands of the sows, ensuring plentiful milk with high quality in the lactation period of the sows and increasing the survival rate of suckling piglets, and enables the piglets to have higher weaning weight.
Owner:河南宏展生物科技有限公司
Surfactant peptide nanostructures, and uses thereof
ActiveUS7179784B2Improve efficiencyIncrease flexibilityMaterial nanotechnologyBiocideActive agentTert-leucine
This work describes a new class of short polypeptides that can self-assemble to form regular nanotubes with an average diameters of about 50 nm. These peptides (7 to 8 amino acids) have a structure very similar to those observed in surfactant molecules with a defined hydrophilic head group constituting of charged amino acids and a lipophilic tail made out of hydrophobic amino acids such as alanine, valine or leucine. Cryo-TEM micrographs show numerous three-fold junctions connecting the self-assembling nanostructures and thus leading to the formation of a rather dense network of entangled nanotubes. Additionally, the observation of clear openings at the end of the supramolecular structures confirms the presence of tubular organization.
Owner:MASSACHUSETTS INST OF TECH
Compositions and methods for glycogen synthesis
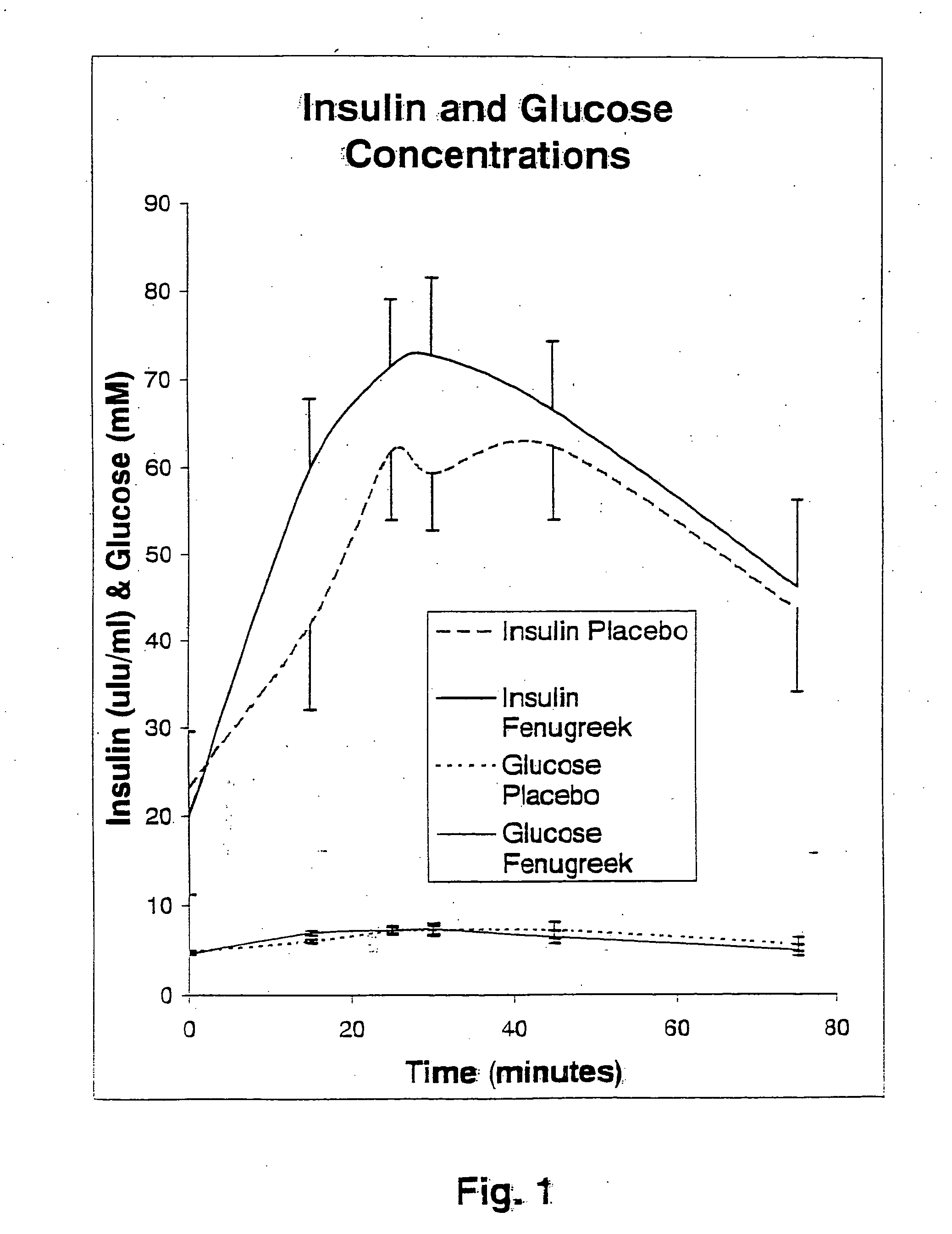
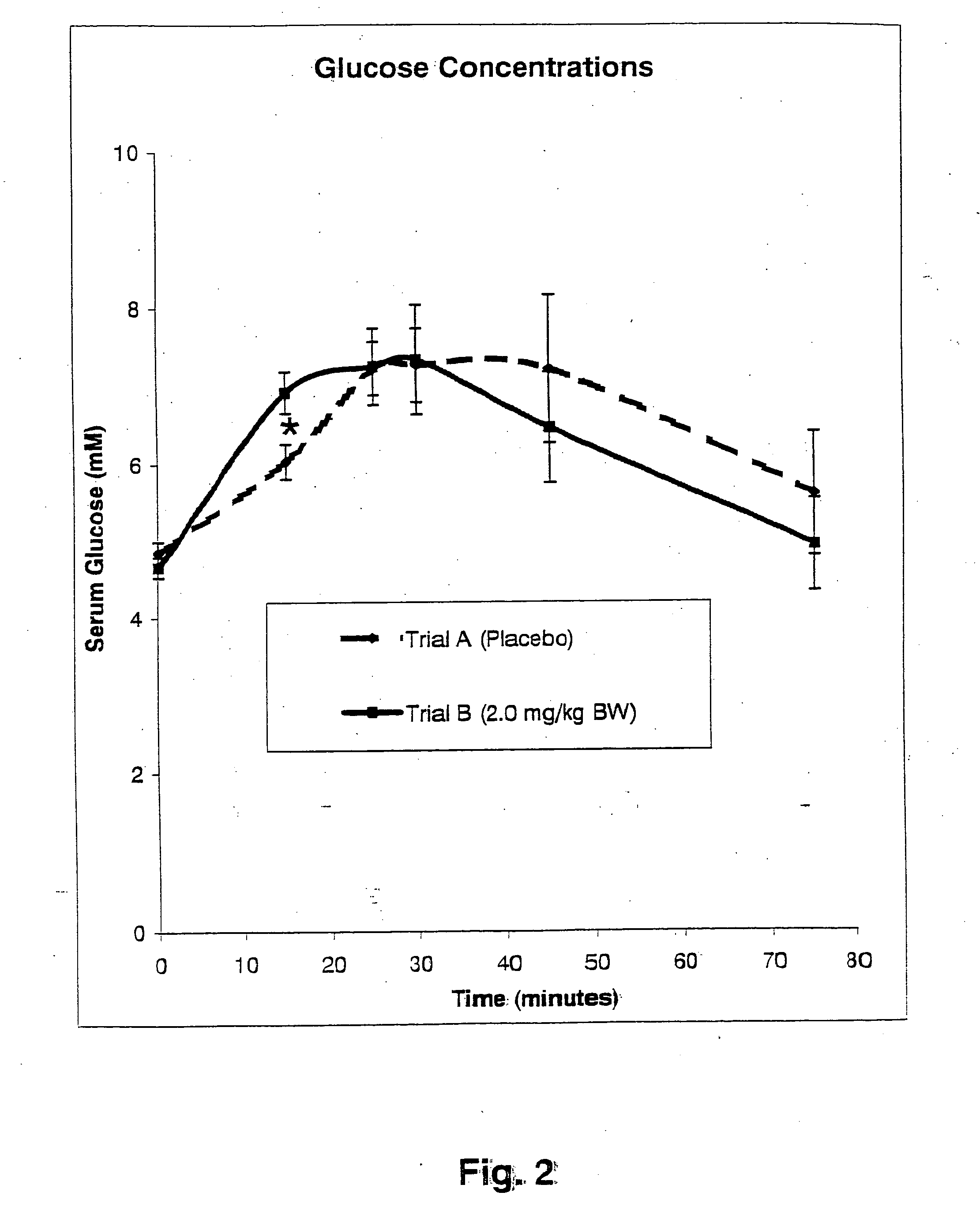
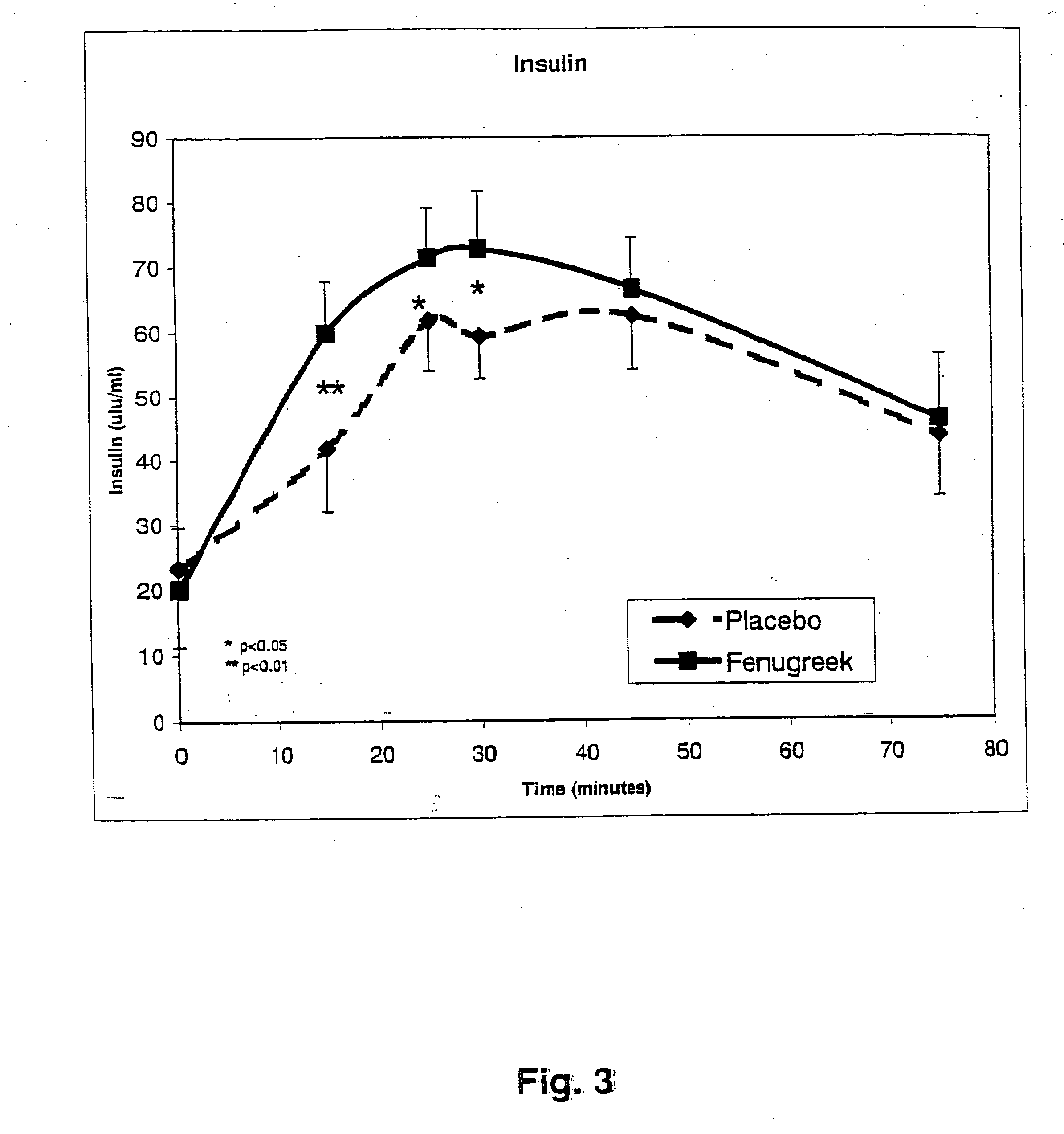
InactiveUS20050176827A1Function increaseIncrease insulin sensitivityBiocideOrganic active ingredientsCysteine thiolateTryptophan
A composition of bio-active compounds and methods for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning. Preferably, the composition of bio-active compounds includes a combination of 4-hydroxyisoleucine with at least one amino acid selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, lysine, histidine, gamma-amino butyrate and tyrosine. In one presently preferred embodiment of the present invention, the combination is derived, isolated, and / or extracted from fenugreek seeds. Methods for using a novel composition of bio-active compounds from fenugreek seed for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning are also disclosed, wherein methods comprise the steps of: (1) providing an effective amount of a composition of bio-active compounds derived, isolated, and / or extracted from fenugreek seeds; and (2) administering the composition to a human or animal.
Owner:TSI INC
Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations
A compound of an angiotensin receptor antagonist (ARB), a neutral endopeptidase inhibitor (NEPi) and one or more monovalent cations are useful for the treatment of hypertension and / or heart failure. ARB includes S—N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine in the anion form, NEPi includes (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester in the anion form and cation includes monovalent cations such as Na+. The compound includes trisodium [3-((1S,3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3′-methyl-2′-(pentanoyl{2″-(tetrazol-5-ylate)biphenyl-4′-ylmethyl}amino)butyrate] hemipentahydrate.
Owner:NOVARTIS PHARM CORP
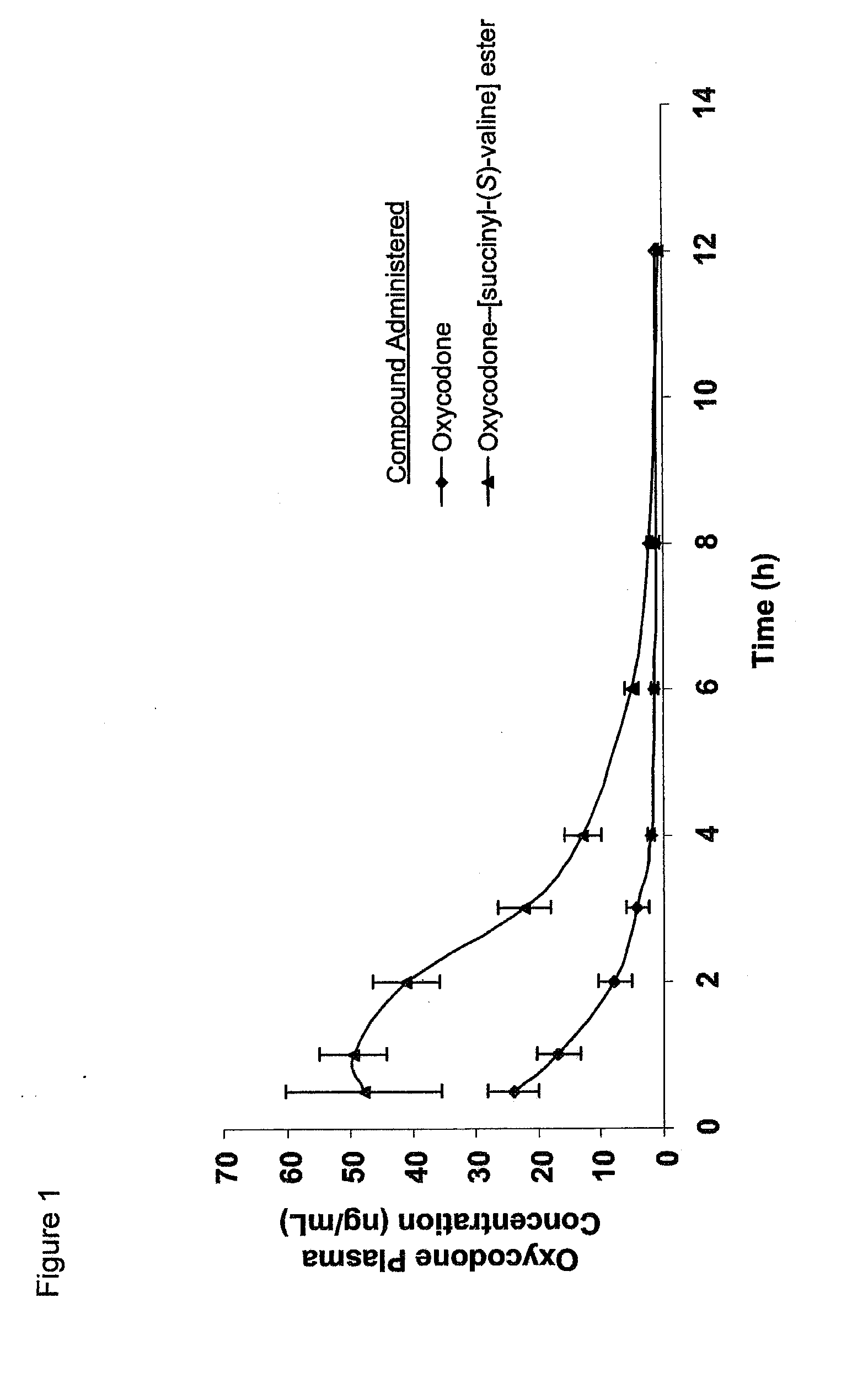
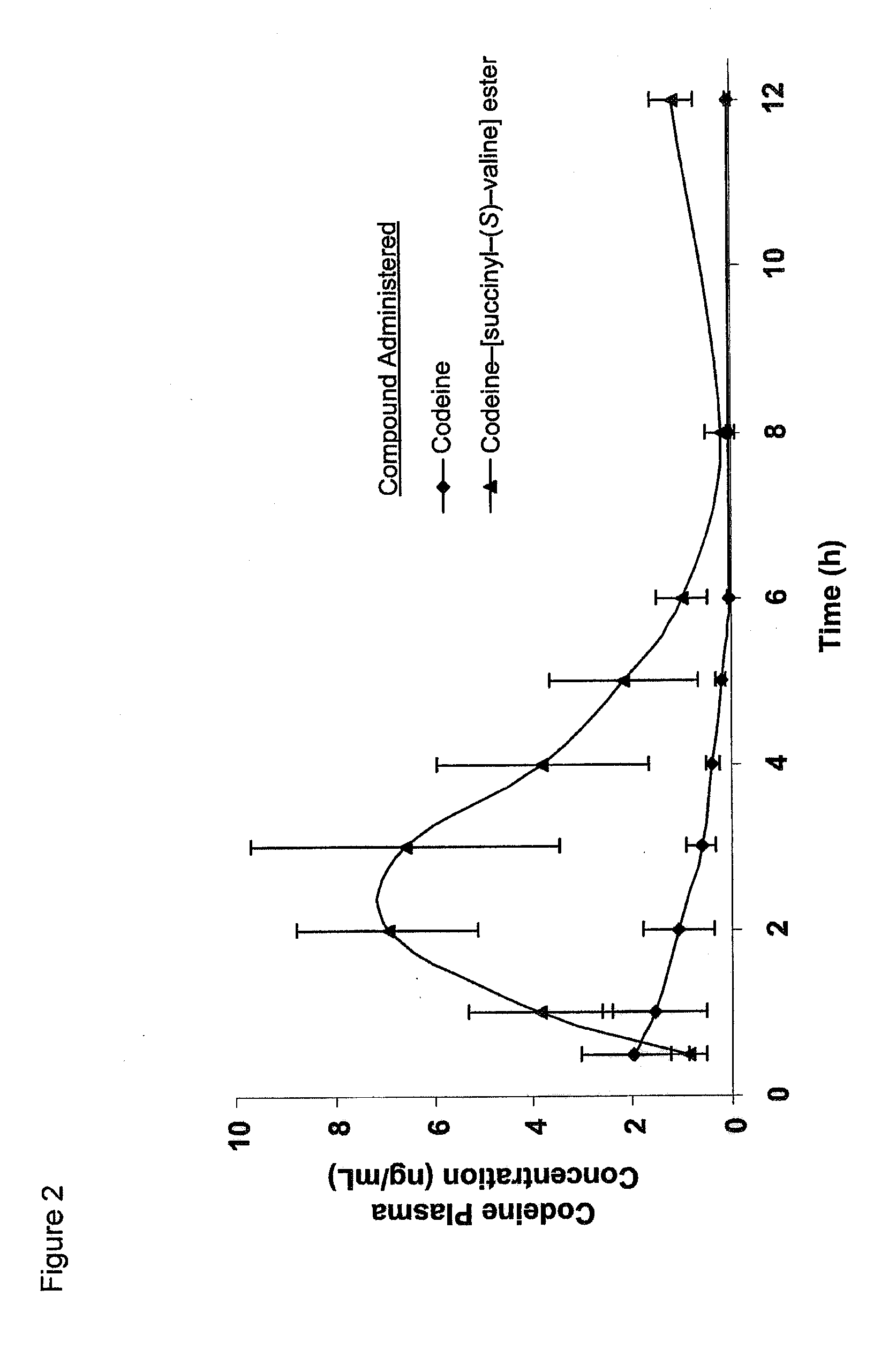
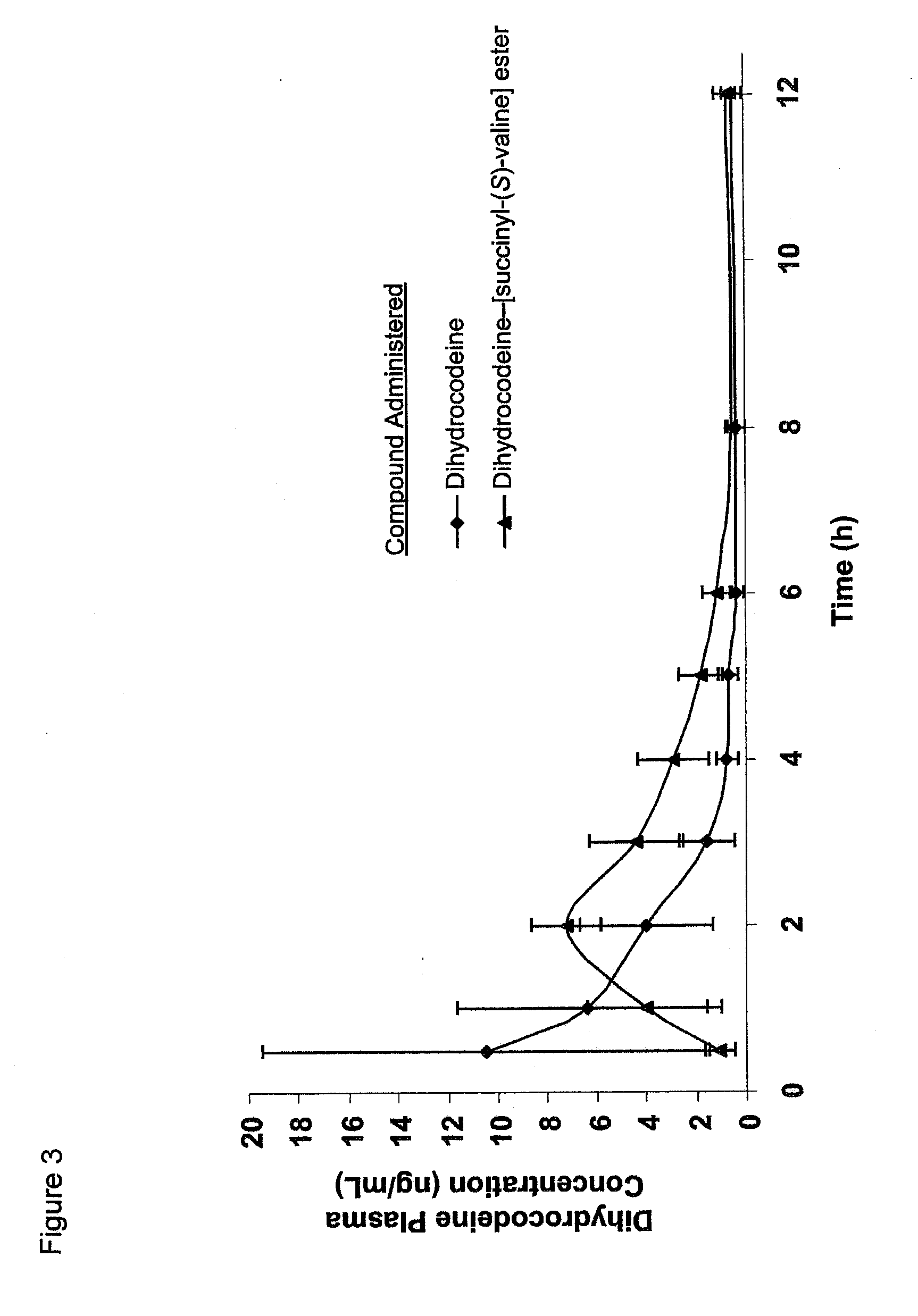
Novel dicarboxylic acid linked amino acid and peptide prodrugs of opioids and uses thereof
InactiveUS20100286186A1Low variabilityReduction and elimination of painBiocideNervous disorderSide effectAmino acid side chain
The present invention concerns dicarboxylic acid linked amino acid and peptide prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing pain relief, decreasing the adverse GI side effects of the opioid analgesic and increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are also provided. In one embodiment, prodrugs having the amino acid side chains of valine, leucine, isoleucine and glycine; and mono-, di- and tripeptides thereof are provided.
Owner:SHIRE PLC
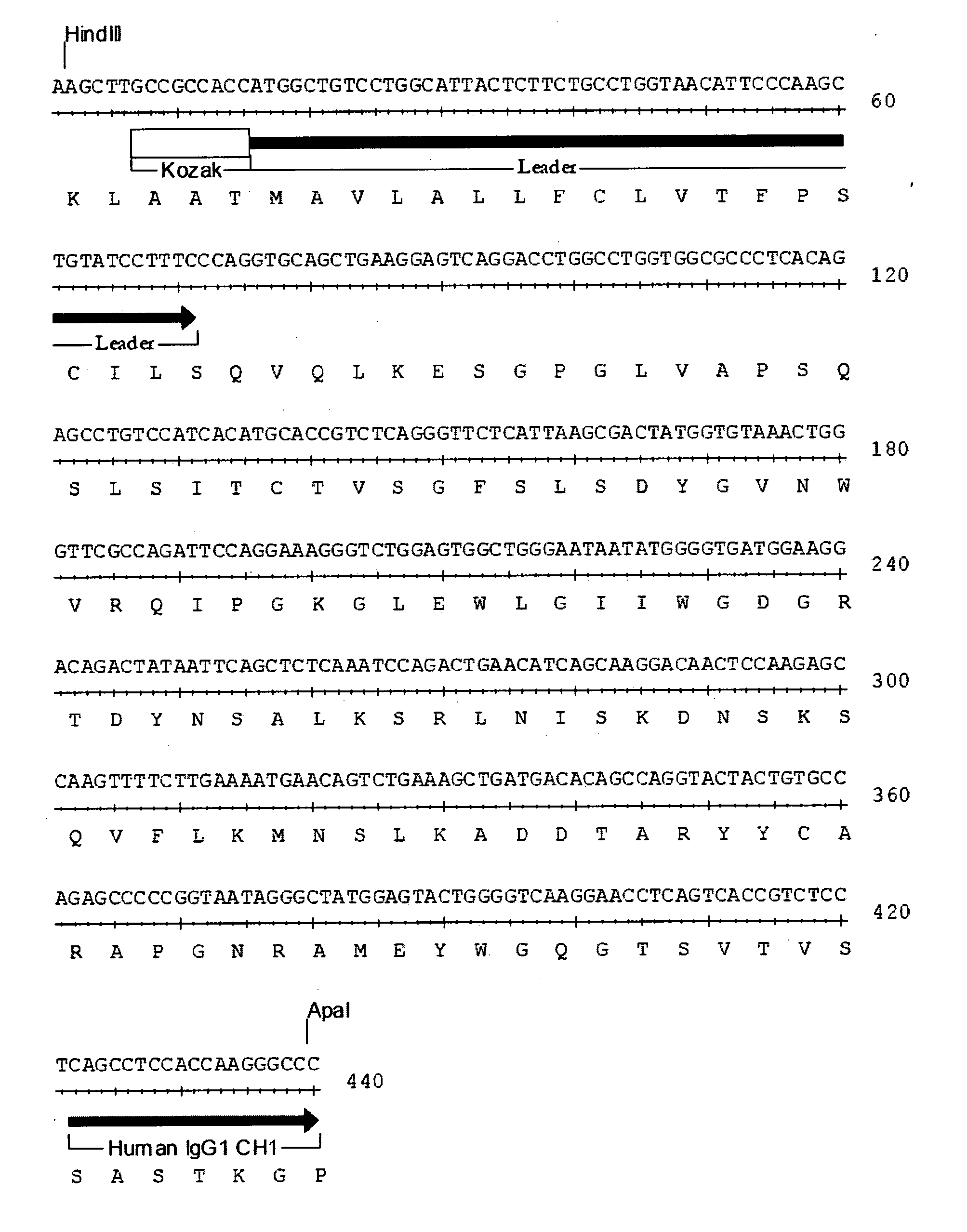
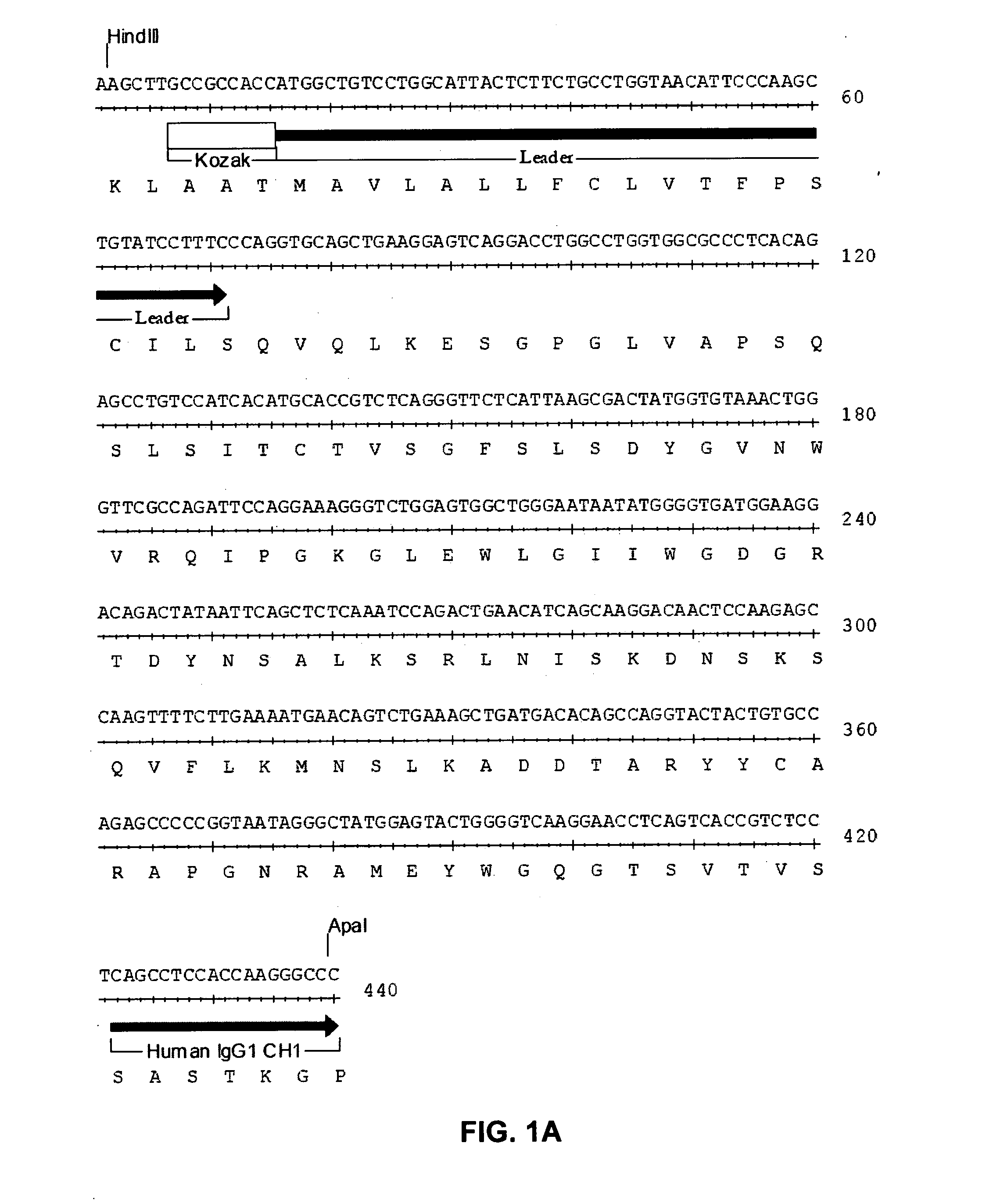
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Compositions containing delta-9-THC-amino acid esters and process of preparation
ActiveUS8809261B2Improve formulation characteristic and bioavailabilityExcellent thermalBiocideSenses disorderDiseaseTyrosine
Compositions of the formulae (I), (II) and (III); where R1, R2 and R3 are residues of amino acids such as, but not limited to, valine, sarcosine, leucine, glutamine, tryptophan, tyrosine, alanine and 4(4-aminophenyl)butyric acid or combination thereof, and salts thereof. Methods of preparation of these compositions and methods of treating any disease condition responsive to THC comprising administration of at least one these compositions in a pharmaceutically acceptable carrier using a pharmaceutically acceptable formulation.
Owner:UNIVERSITY OF MISSISSIPPI
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
HMB compositions and uses thereof
The present invention relates to methods for the prevention and treatment of chronic inflammatory diseases, cancer, and involuntary weight loss. In the practice of the present invention patients are enterally administered HMB alone or alternatively in combination with eicosapentaenoic (20:5 ω-3), FOS, carnitine and mixtures thereof. HMB may be added to food products comprising a source of amino-nitrogen enriched with large neutral amino acids such as leucine, isoleucine, valine, tyrosine, threonine and phenylalanine and substantially lacking in free amino acids.
Owner:ABBOTT LAB INC
Detergent composition containing amino acid component for washing fruits, vegetables and dishes
InactiveCN102492571APromote degradationMaintain stain releaseNon-ionic surface-active compoundsOrganic detergent compounding agentsAntioxidantArginine
The present invention relates to a detergent composition containing an amino acid component for washing fruits, vegetables and dishes, which comprises the following components of an anionic surfactant, a non-ionic surfactant, an amphoteric surfactant, amino acid, a viscosity modifier, a chelating agent, an antiseptic, a light stabilizer, an antioxidant, a flavor and deionized water. The amino acid composition is one or a mixture with more than two of alanine, arginine, asparagine, aspartic acid, cystine, glutamine, glutamic acid, leucine, lysine, methionine, phenylalanine, prolinol acid, serine, threonine, glycine, histidine, isoleucine, tryptophan, tyrosine and valine. The detergent of the invention can maintain good decontamination capability, improve the feeling when the detergent composition is used, reduce the hands skin irritation and enhance the hand skin moisturizing ability to improve dry feeling.
Owner:GUANGZHOU LIBY
Amino acid composition
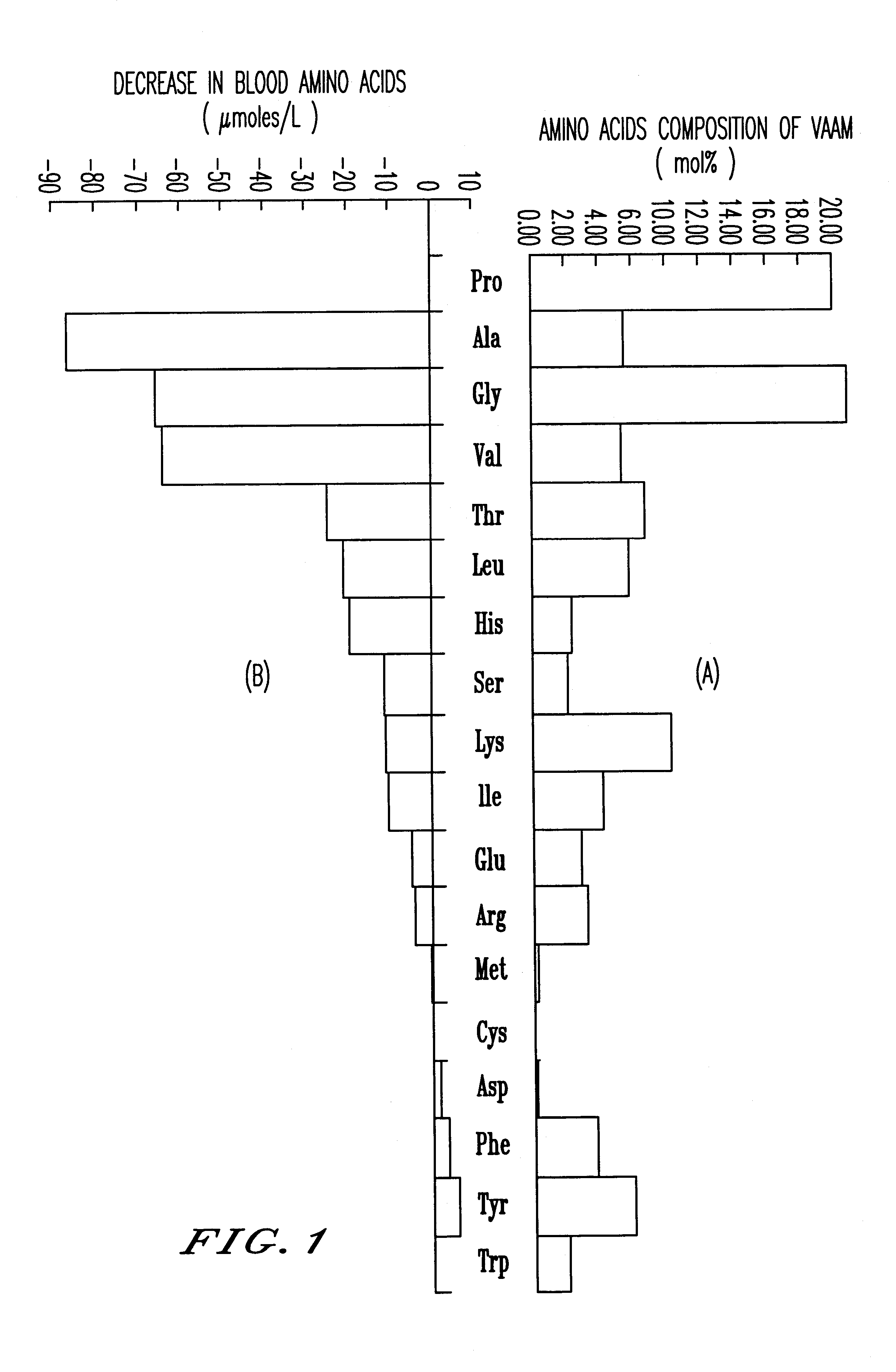
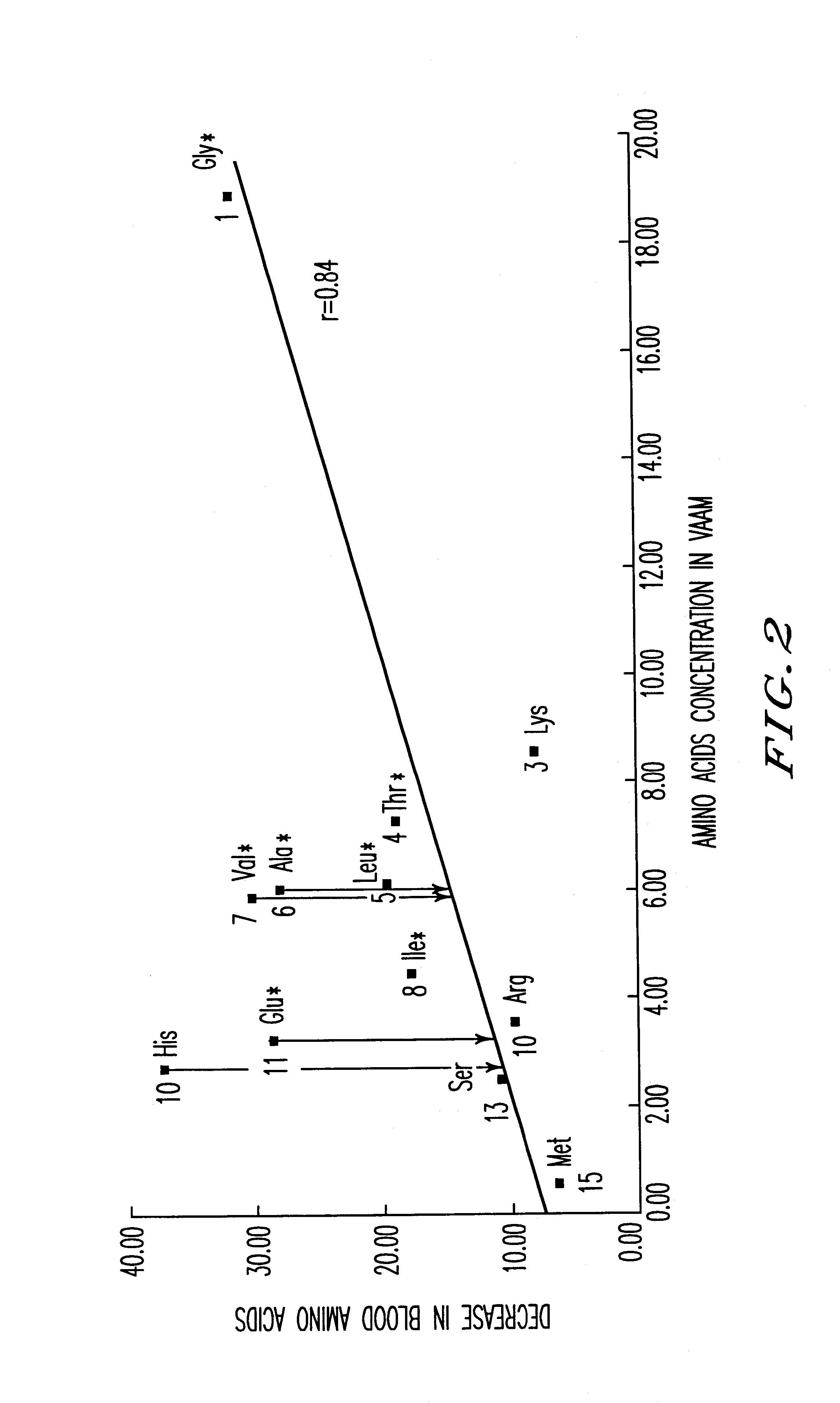
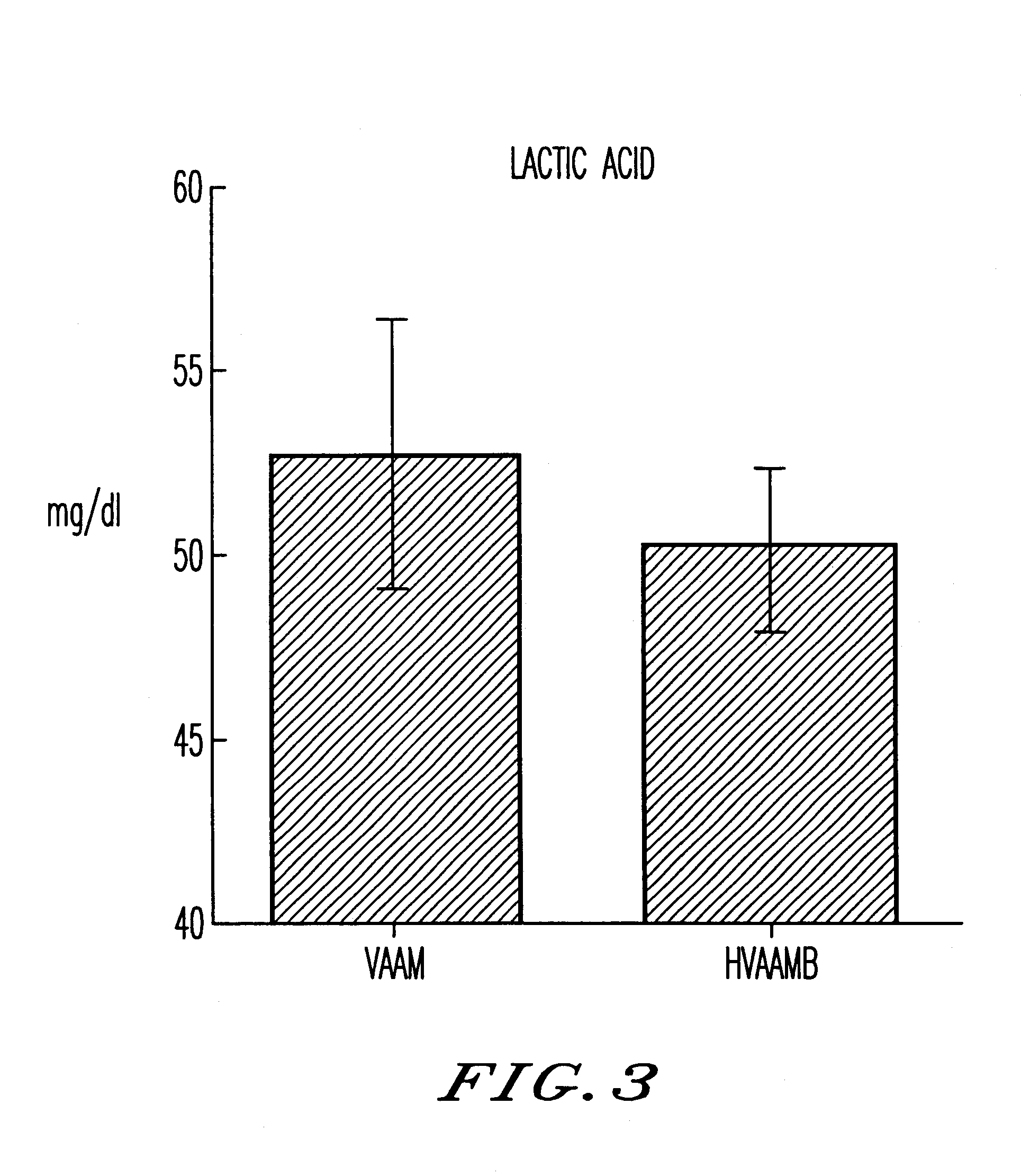
An amino acid composition comprising the following amino acids at the following molar ratio: proline (12.6 to 23.4), alanine (8.4 to 15.6), glycine (13.3 to 24.9), valine (8.2 to 15.4), threonine (5.0 to 9.4), eucine (4.3 to 8.1), histidine (1.8 to 11.9), serine (1.7 to 3.3), lysine (6.0 to 11.2), isoleucine (3.1 to 5.9), glutamic acid (2.2 to 10.4), arginine (2.4 to 4.6), phenylalanine (2.6 to 5.0), tyrosine (4.2 to 7.8) and trypsin (1.5 to 2.9). The composition supplements blood amino acids reduced during hard exercise and shows effects to improve motor function, to reduce fatigue after exercise and to help recovery from the fatigue.
Owner:THE INST OF PHYSICAL & CHEM RES WAKO +1
Orally administered small peptides synergize statin activity
InactiveUS7148197B2Readily taken up and deliveredMany symptomOrganic active ingredientsPeptide/protein ingredientsThreonineTyrosine
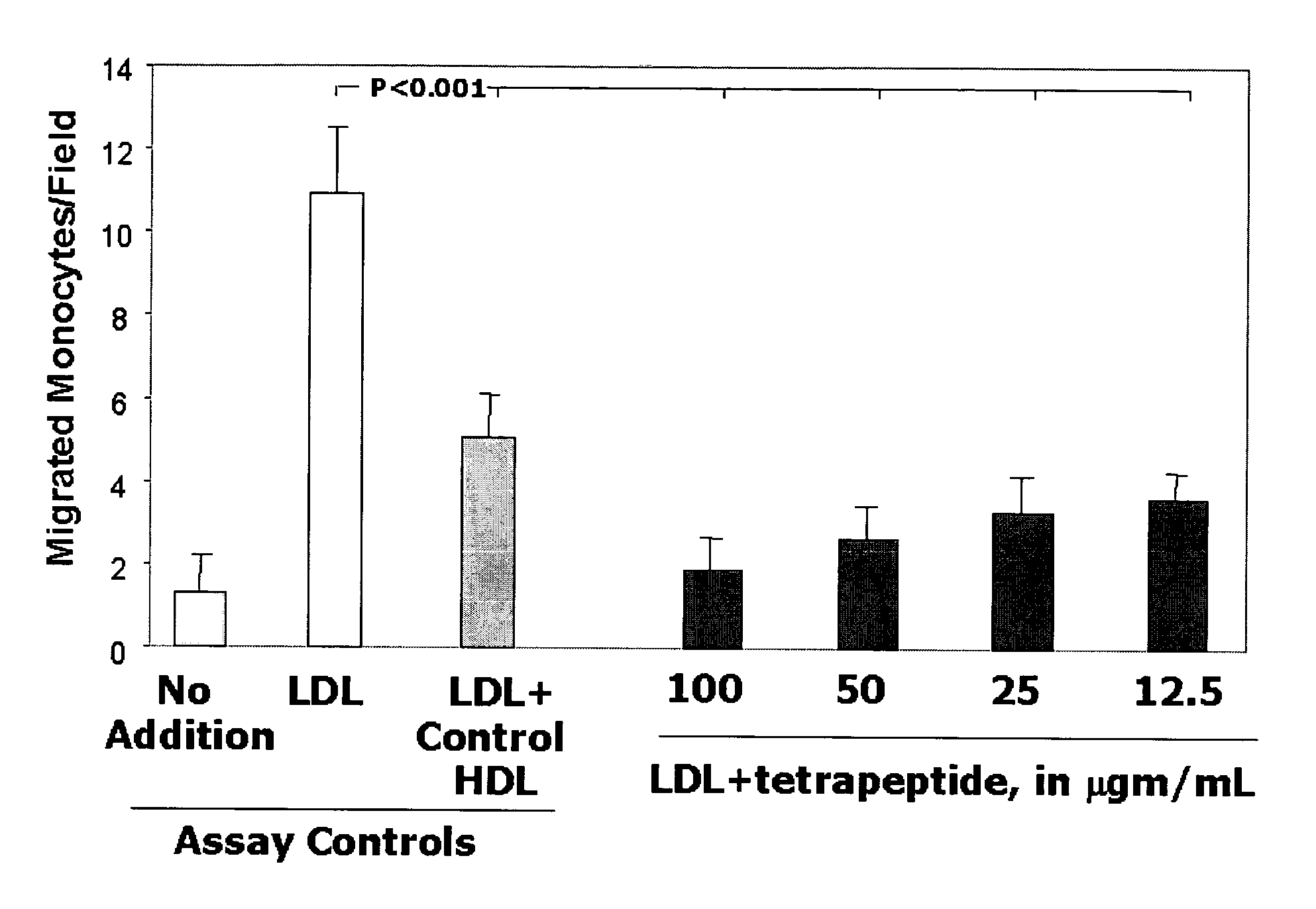
This invention provides novel peptides for the treatment of atherosclerosis. In certain embodiments the peptide is X1-X2-X3-X4 where X1 and X4 are independently selected from the group consisting of alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), tryptophan (Trp), methionine (Met), serine (Ser) bearing a hydrophobic protecting group, beta-naphthyl alanine, alpha-naphthyl alanine, norleucine, cyclohexylalanine, threonine (Thr) bearing a hydrophobic protecting group, tyrosine (Tyr) bearing a hydrophobic protecting group, lysine (Lys) bearing a hydrophobic protecting group, arginine (Arg) bearing a hydrophobic protecting group, ornithine (Orn) bearing a hydrophobic protecting group, aspartic acid (Asp) bearing a hydrophobic protecting group, cysteine (Cys) bearing a hydrophobic protecting group, and glutamic acid (Glu) bearing a hydrophobic protecting group; X2 and X3 are independently selected from the group consisting of Asp, Arg, and Glu; and the peptide converts pro-inflammatory HDL to anti-inflammatory HDL or makes anti-inflammatory HDL more anti-inflammatory.
Owner:RGT UNIV OF CALIFORNIA +1
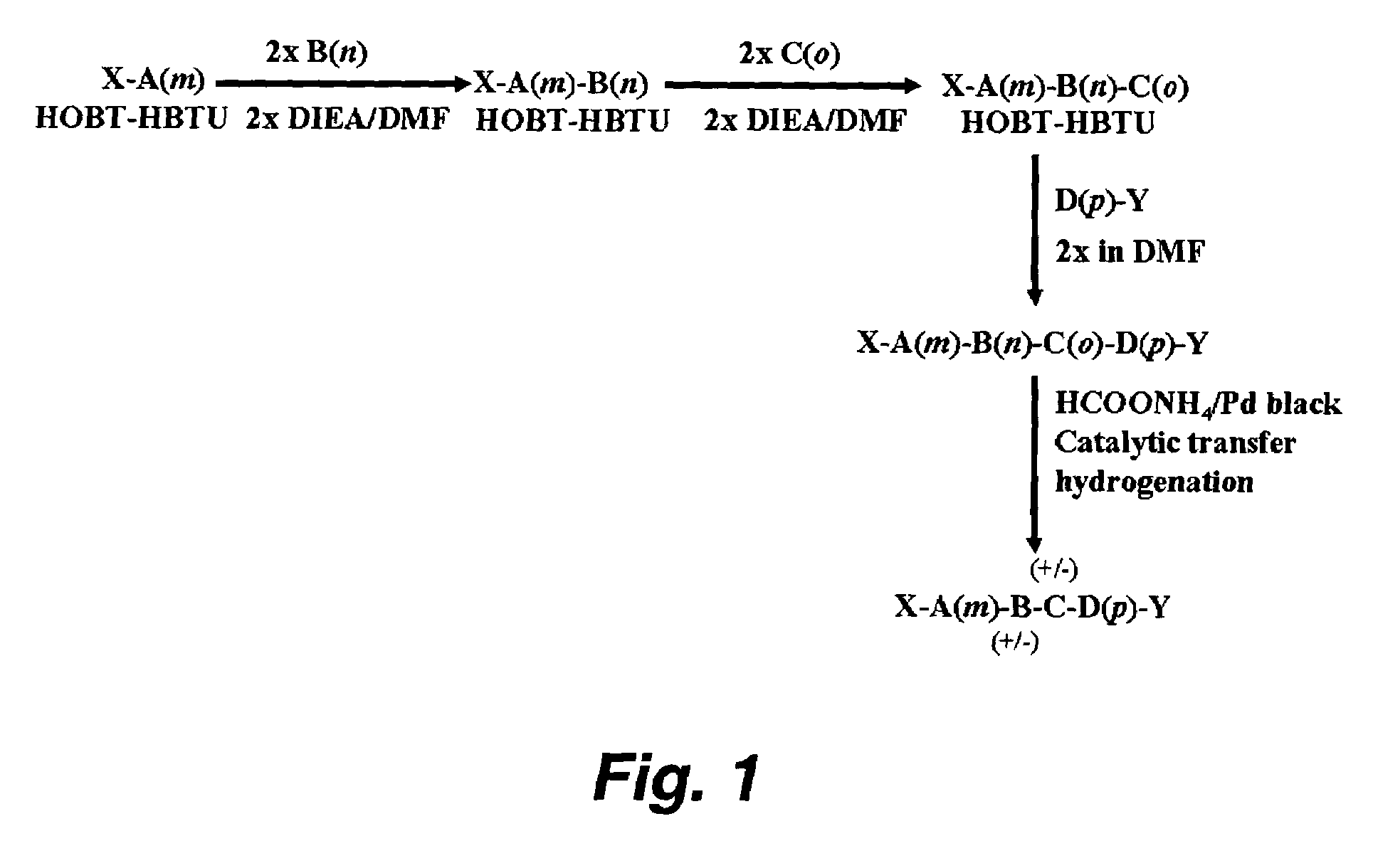
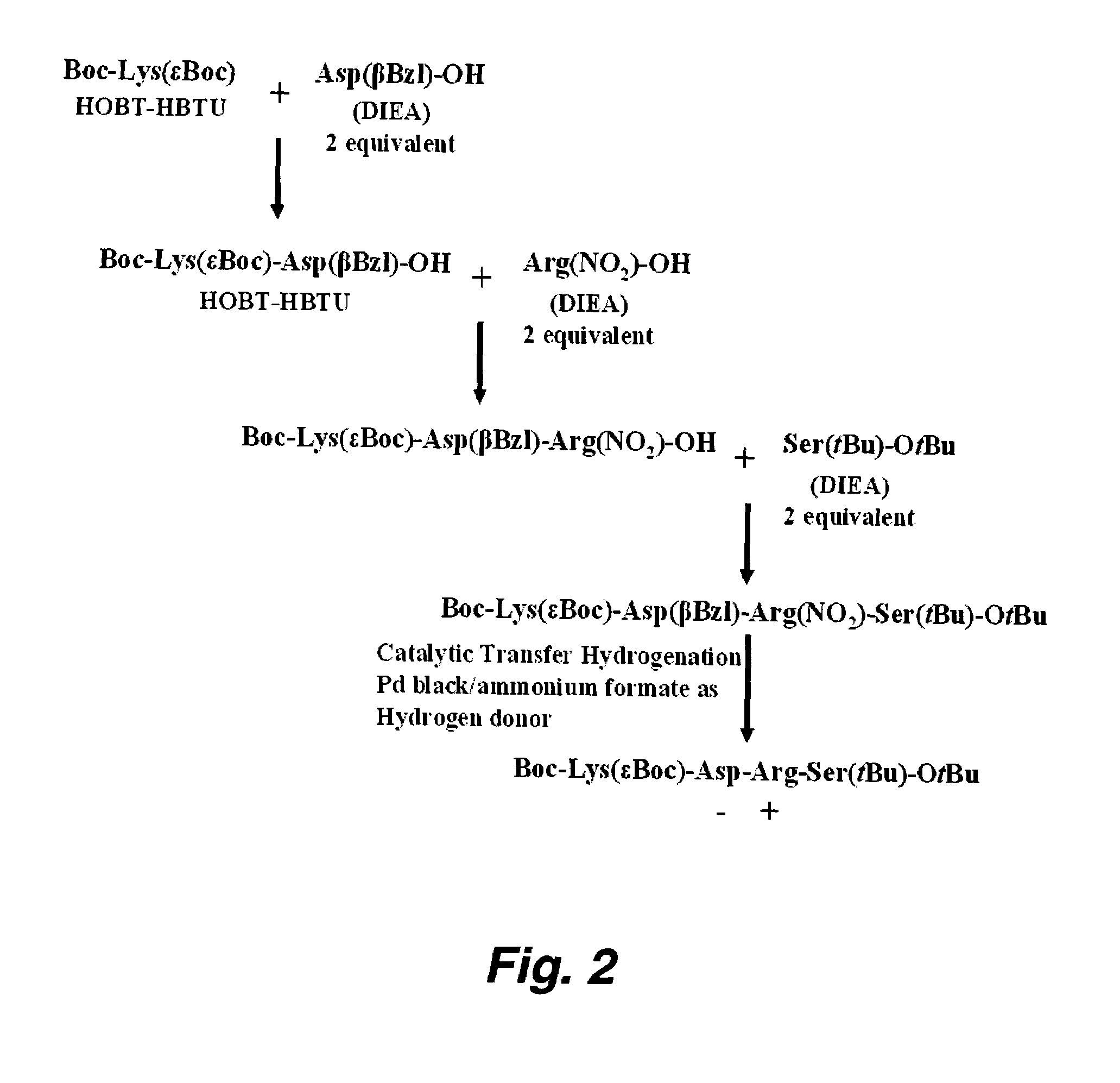
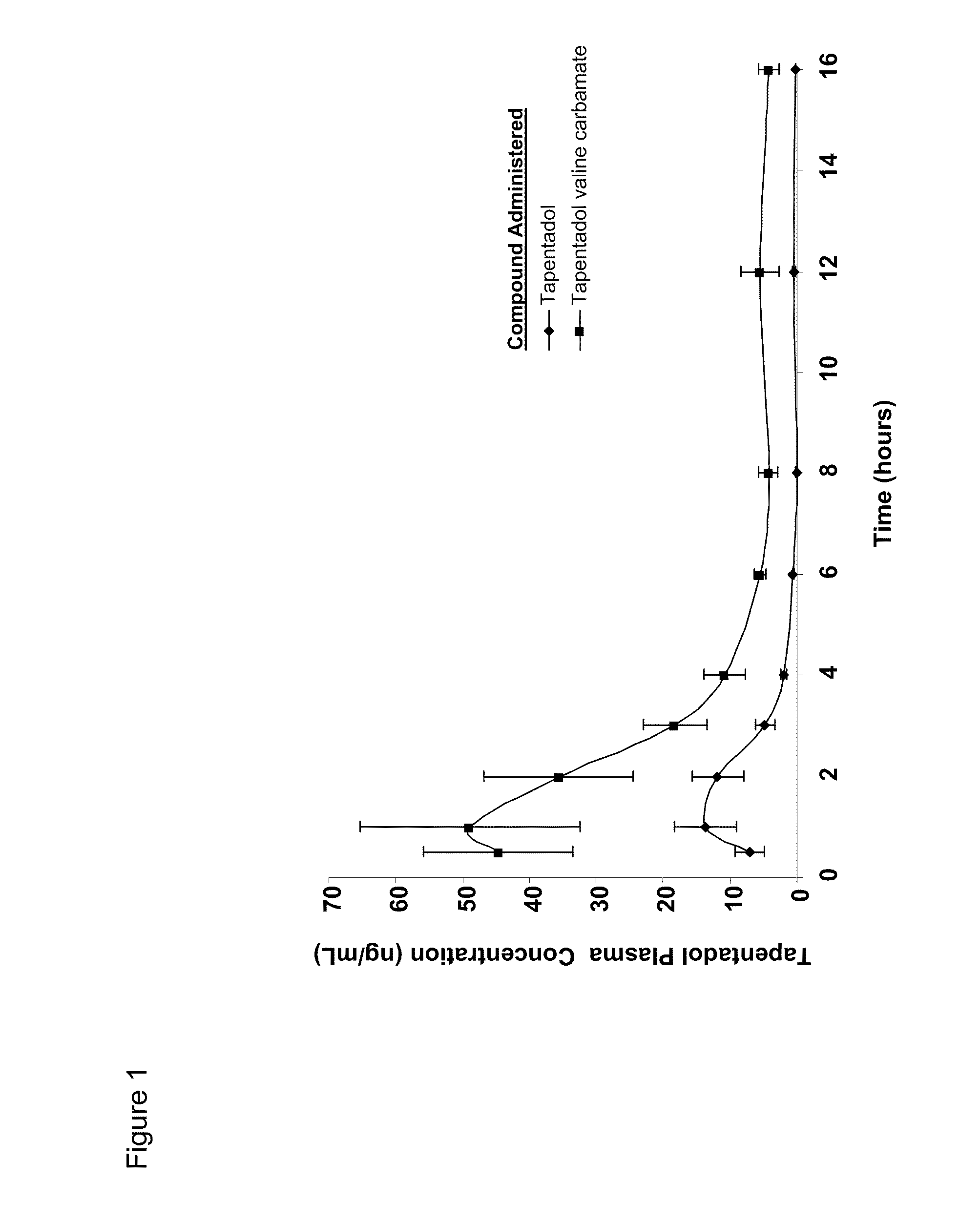
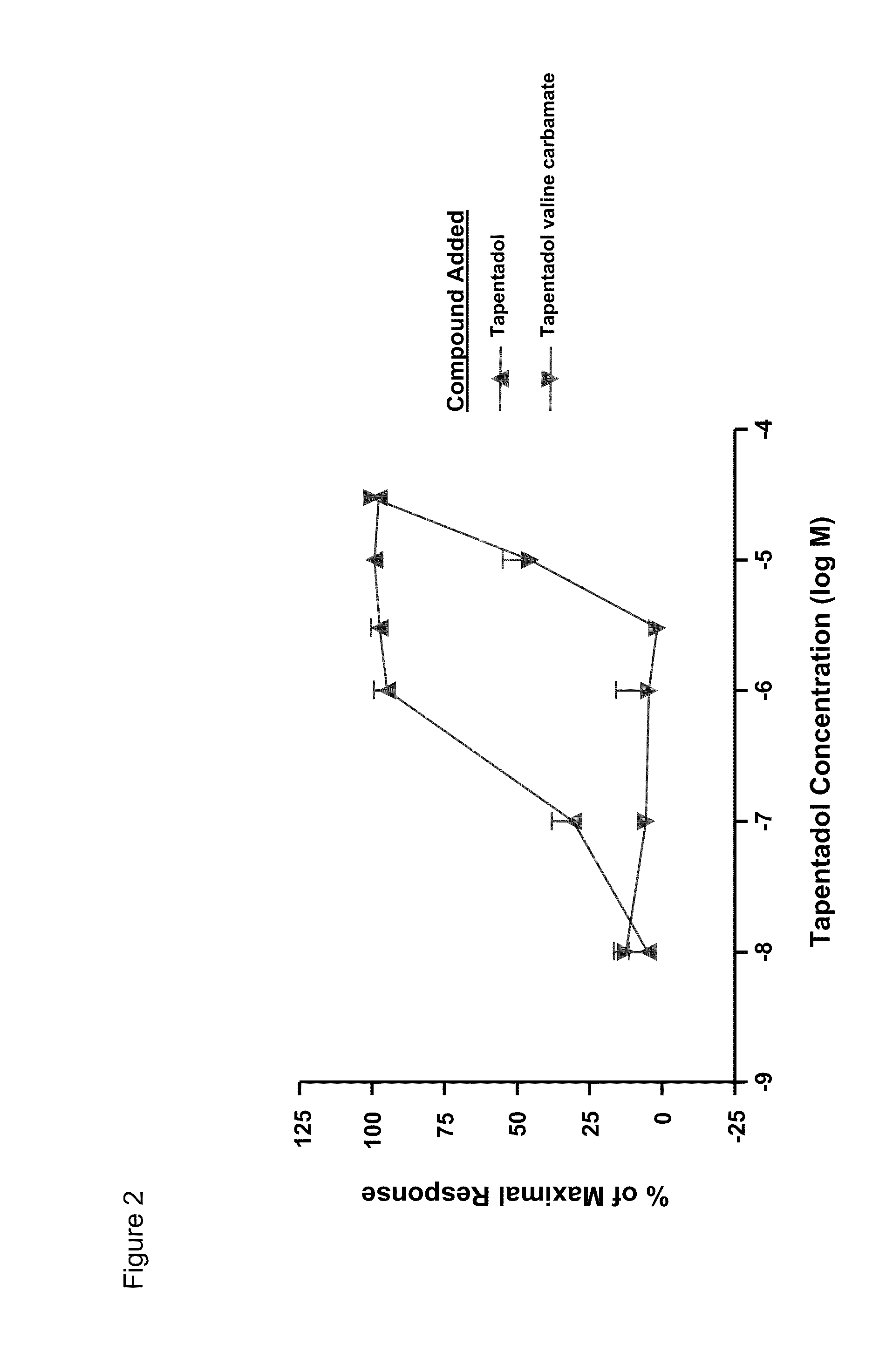
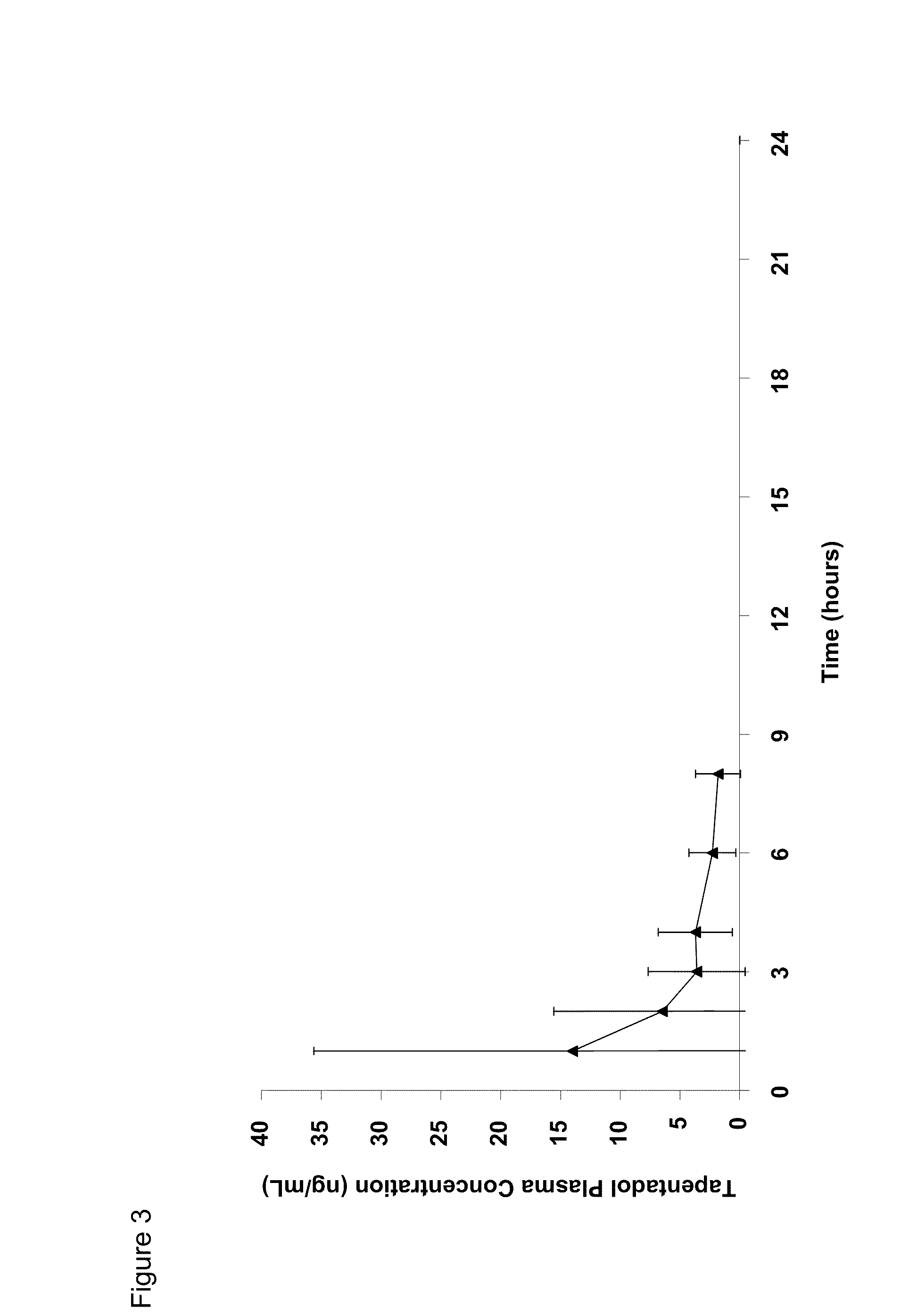
Amino acid and peptide carbamate prodrugs of tapentadol and uses thereof
InactiveUS20100227921A1Sufficient amountMinimizing the gastrointestinal (GI) side effectsBiocideNervous disorderCarbamateSide effect
Prodrugs of tapentadol with amino acids or short peptides, pharmaceutical compositions containing such prodrugs and a method for providing pain relief with the tapentadol prodrugs are provided herein. Prodrugs having side chains of valine, leucine, isoleucine and glycine amino acids and mono-, di- and tripeptides thereof are preferred. Additionally, methods for avoiding or minimizing the adverse gastrointestinal side effects associated with tapentadol administration, as well as increasing the oral bioavailability of tapentadol are provided herein.
Owner:SHIRE PLC
Non-naturally occurring synthetic lytic peptides
InactiveUS6559281B1Improve the immunityImprove stabilityPeptide-nucleic acidsPeptide/protein ingredientsLytic peptideCrystallography
Non-naturally occurring lytic peptides which contain a phenylalanine residue and one or more alanine, valine and lysine residues, and optionally contain chemically masked cysteine or serine residues possess an amphipathic structure which allows them to promote cell lysis in certain pathologic organisms, and particularly in prokaryotes. Peptides having a beta-pleated sheet secondary structure and lacking cysteine residues form one embodiment of these lytic peptides.
Owner:DEMEGEN
Methods for enhancing the transport of glucose into muscle
InactiveUS20050226948A1Effective quantityLow production costBiocideUnknown materialsCysteine thiolateTryptophan
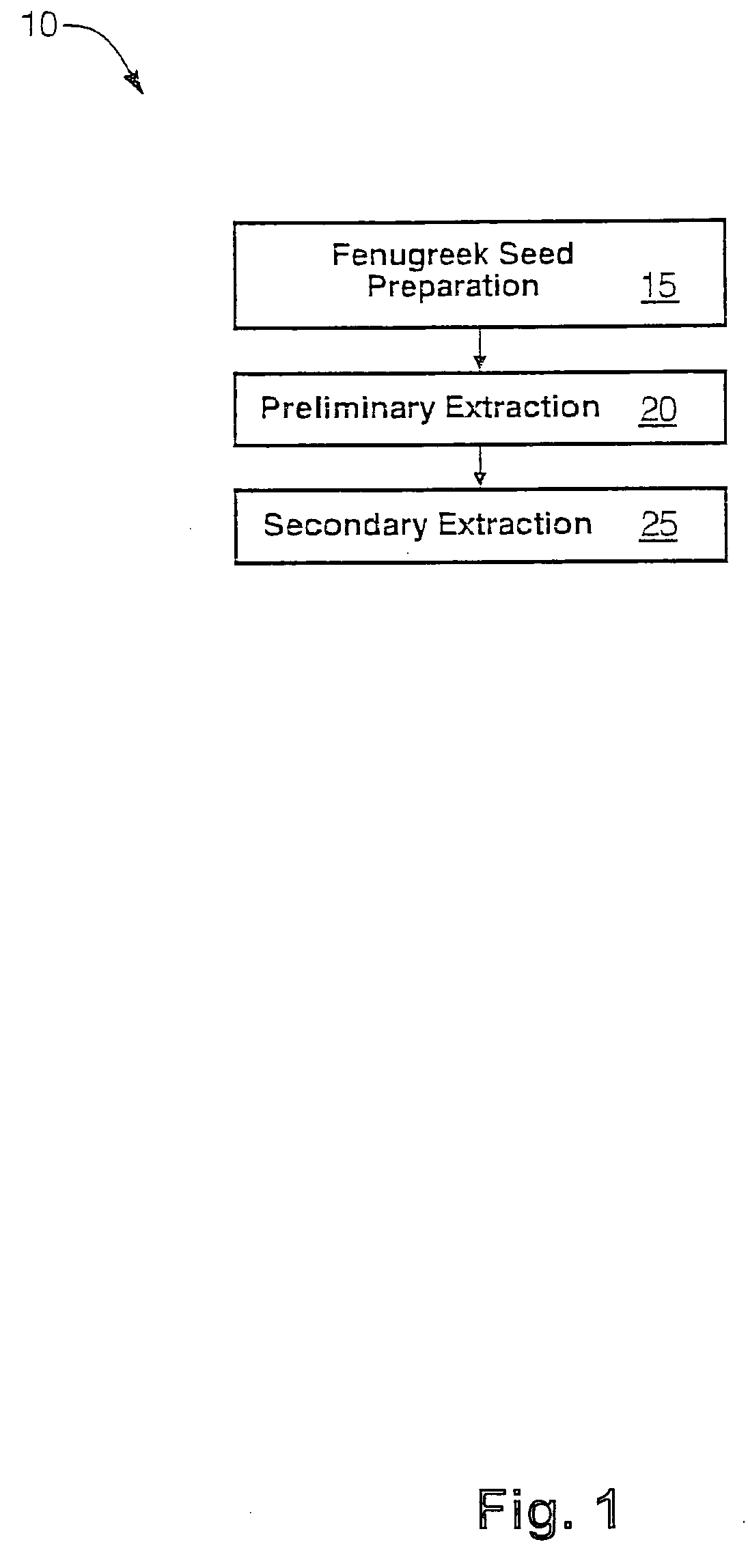
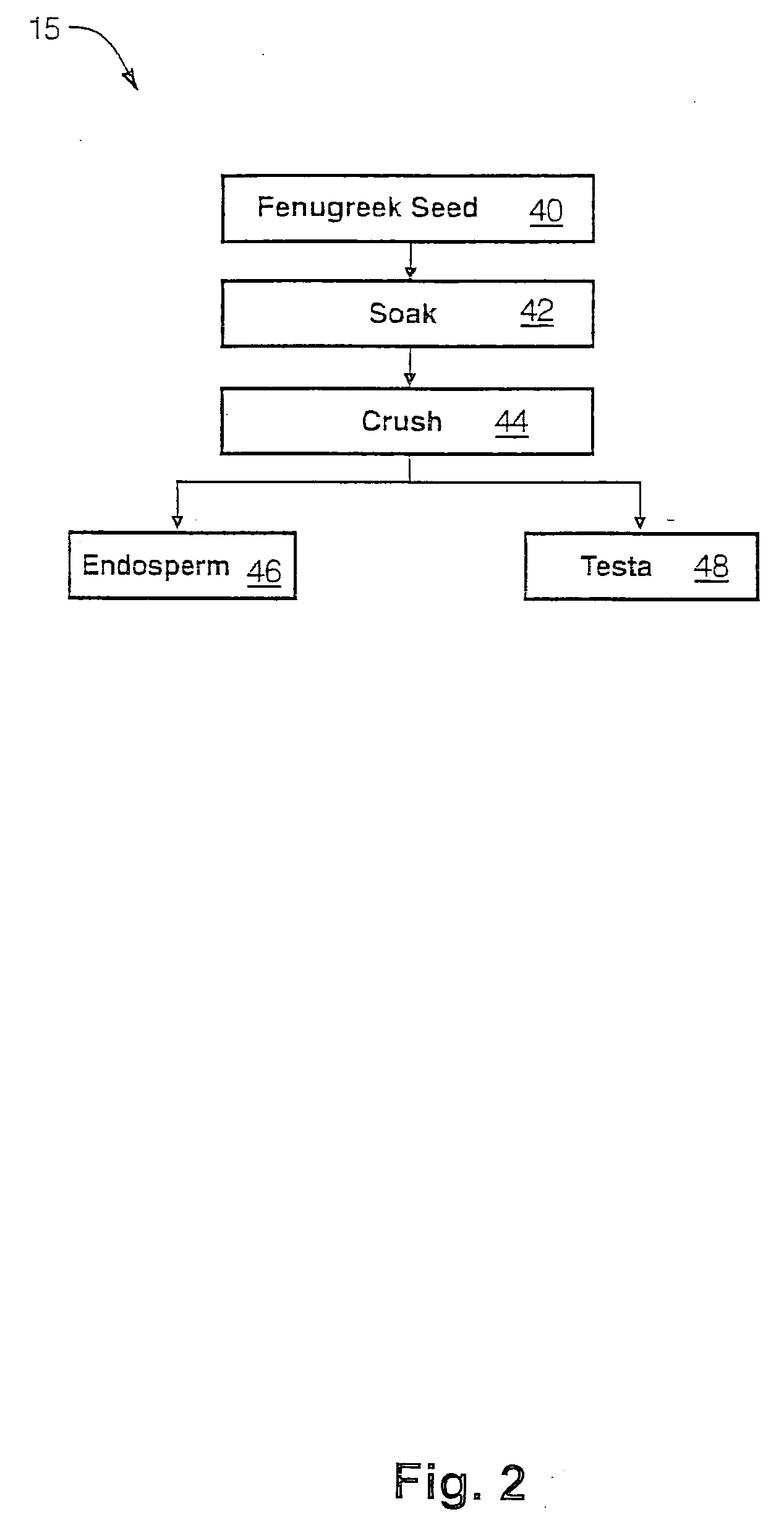
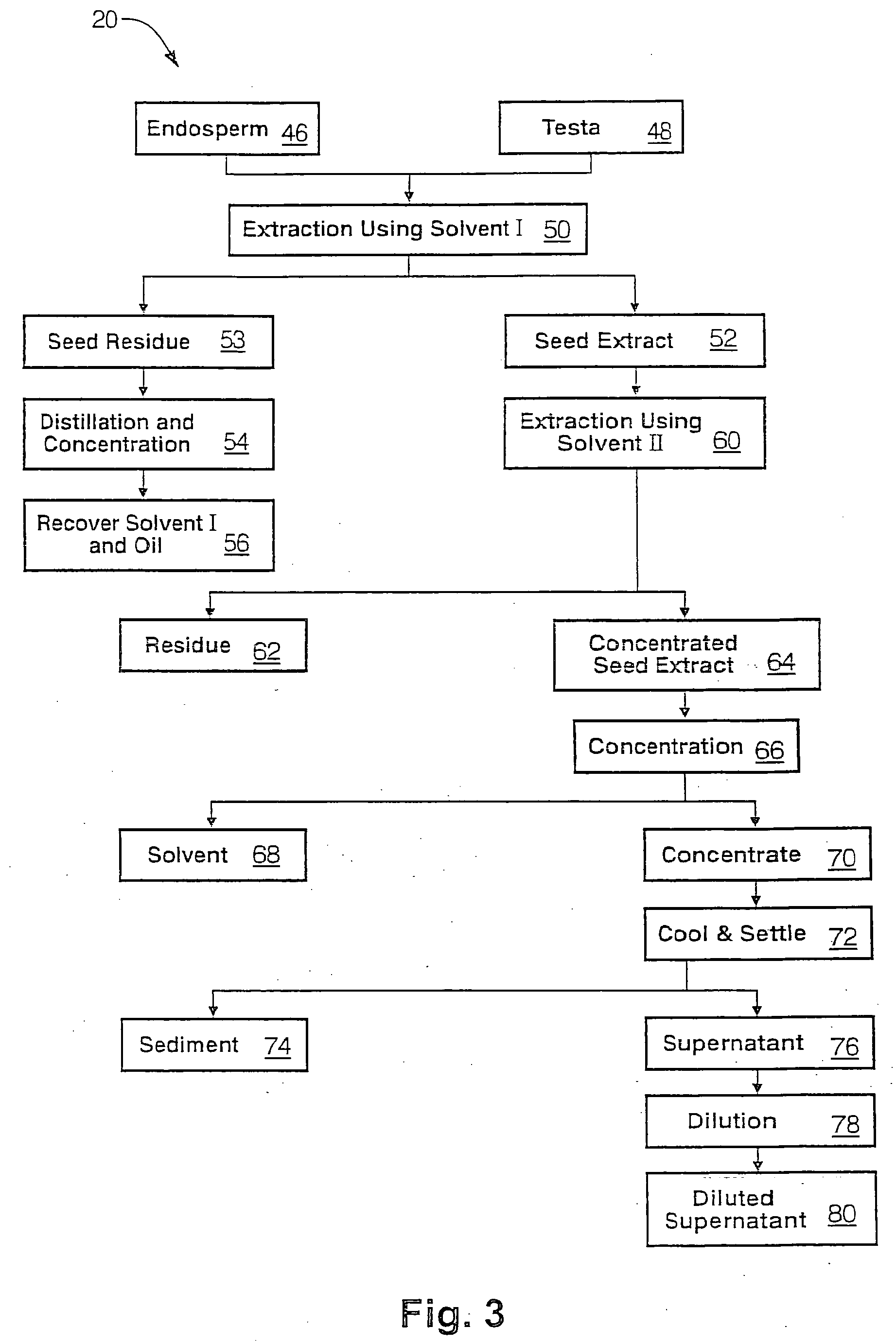
The present invention is directed to novel compositions of bio-active compounds comprising 4-hydroxyisoleucine and one or more compounds selected from the group of amino acids, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Preferably, the novel compositions of bio-active compounds include 4-hydroxyisoleucine and one or more amino acids selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, proline, lysine, histidine, and gamma-aminobutyrate. The composition of bio-active compounds preferably include between about ten percent and about seventy percent of 4-hydroxyisoleucine and between about twenty percent and about forty percent of other amino acids. The bio-active compounds of the novel composition of the present invention may be derived, isolated, and / or extracted from Fenugreek seeds. A preferred method for extracting the bio-active compounds from Fenugreek seeds includes the steps of: (1) providing a plurality of Fenugreek seeds; (2) preparing the Fenugreek seeds; and (3) extracting a novel composition of bio-active compounds from the Fenugreek seeds, which include a preliminary extraction step and a secondary extraction step. The compositions of bio-active compounds have been found to be helpful in restoring healthy energy balance in humans and animals, aiding in weight management efforts, and for balancing blood sugar levels by way of assisting the body to make more efficient use of existing (i.e., endogenous) insulin.
Owner:TSI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

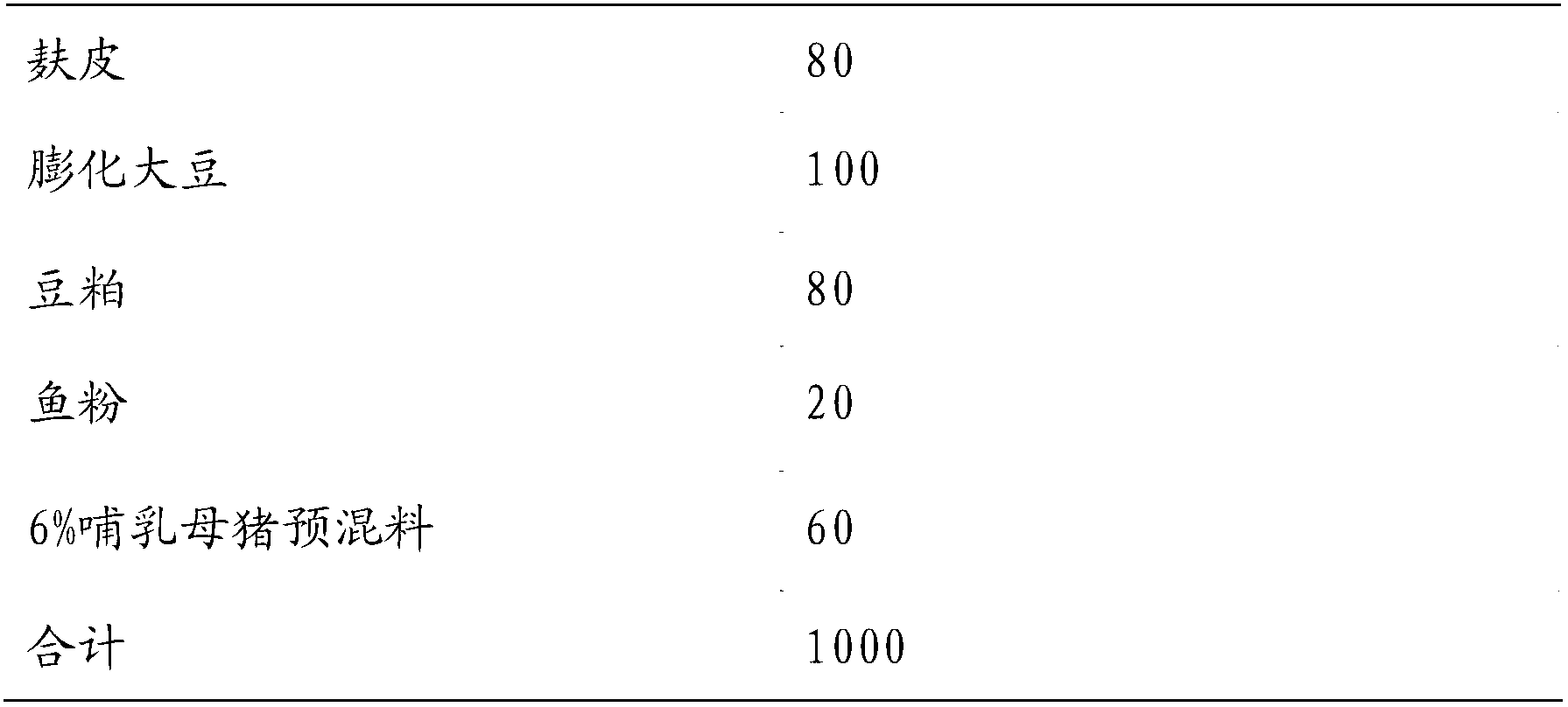
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00000.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00001.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-C00001.png)