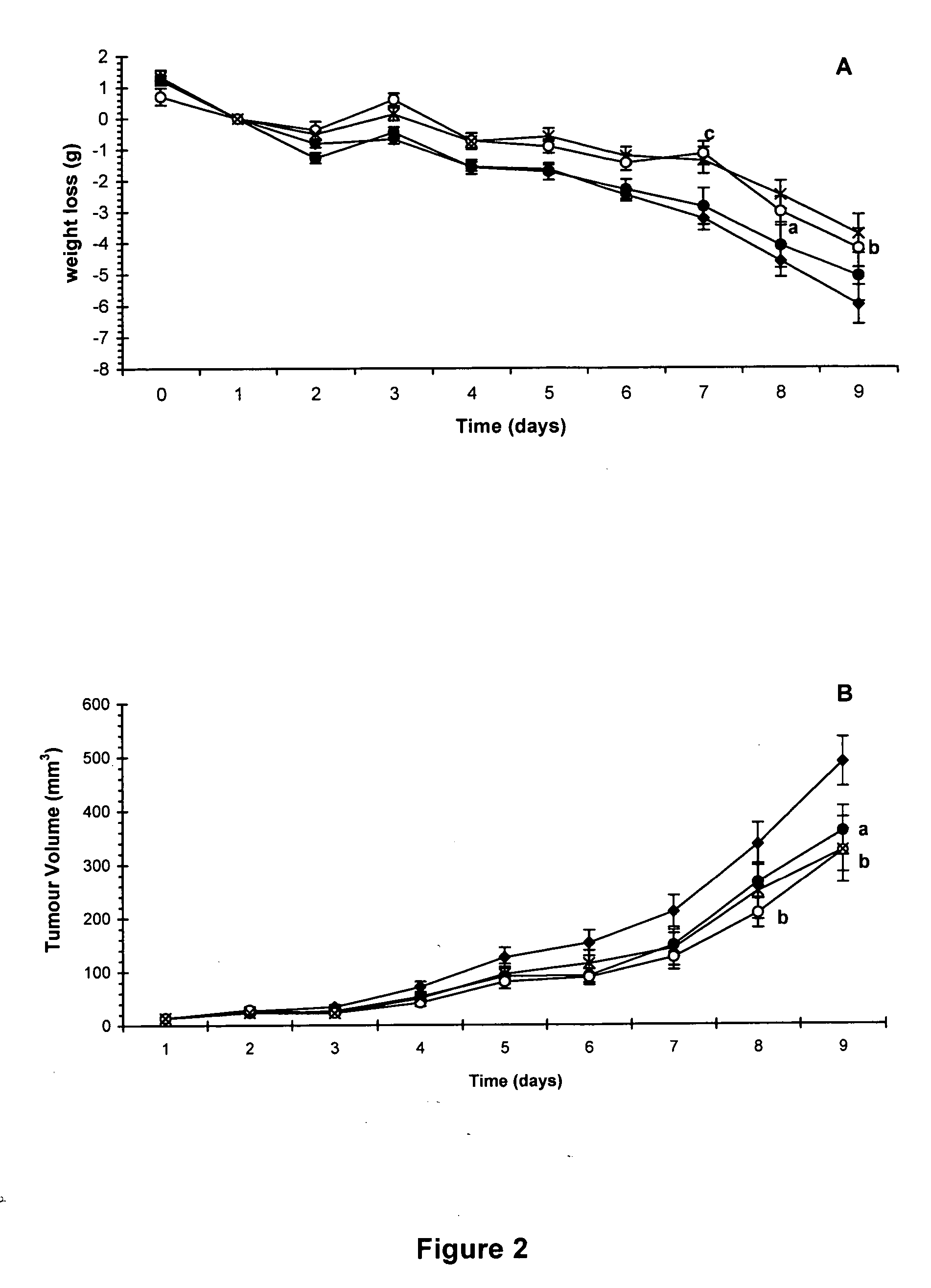
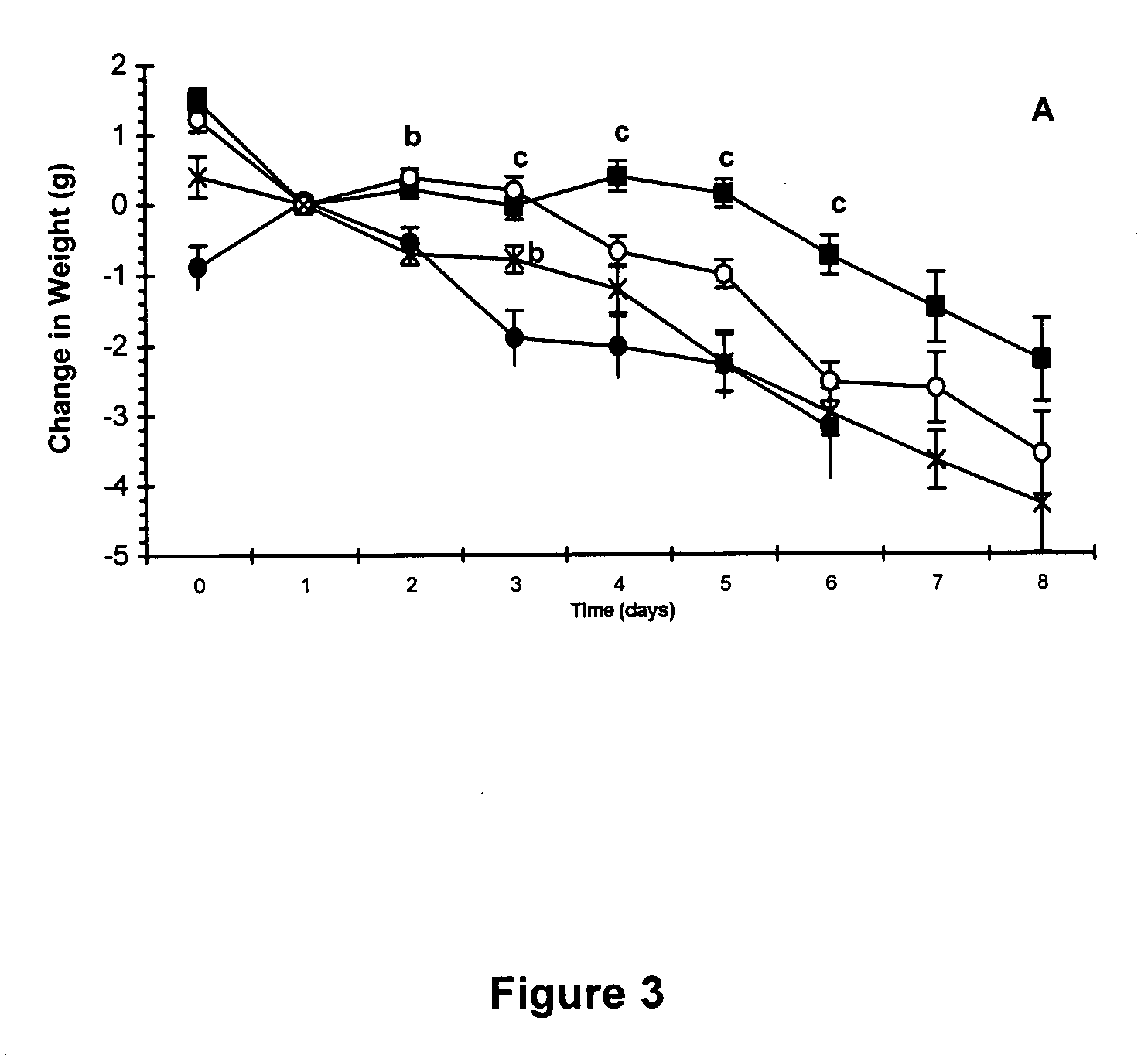
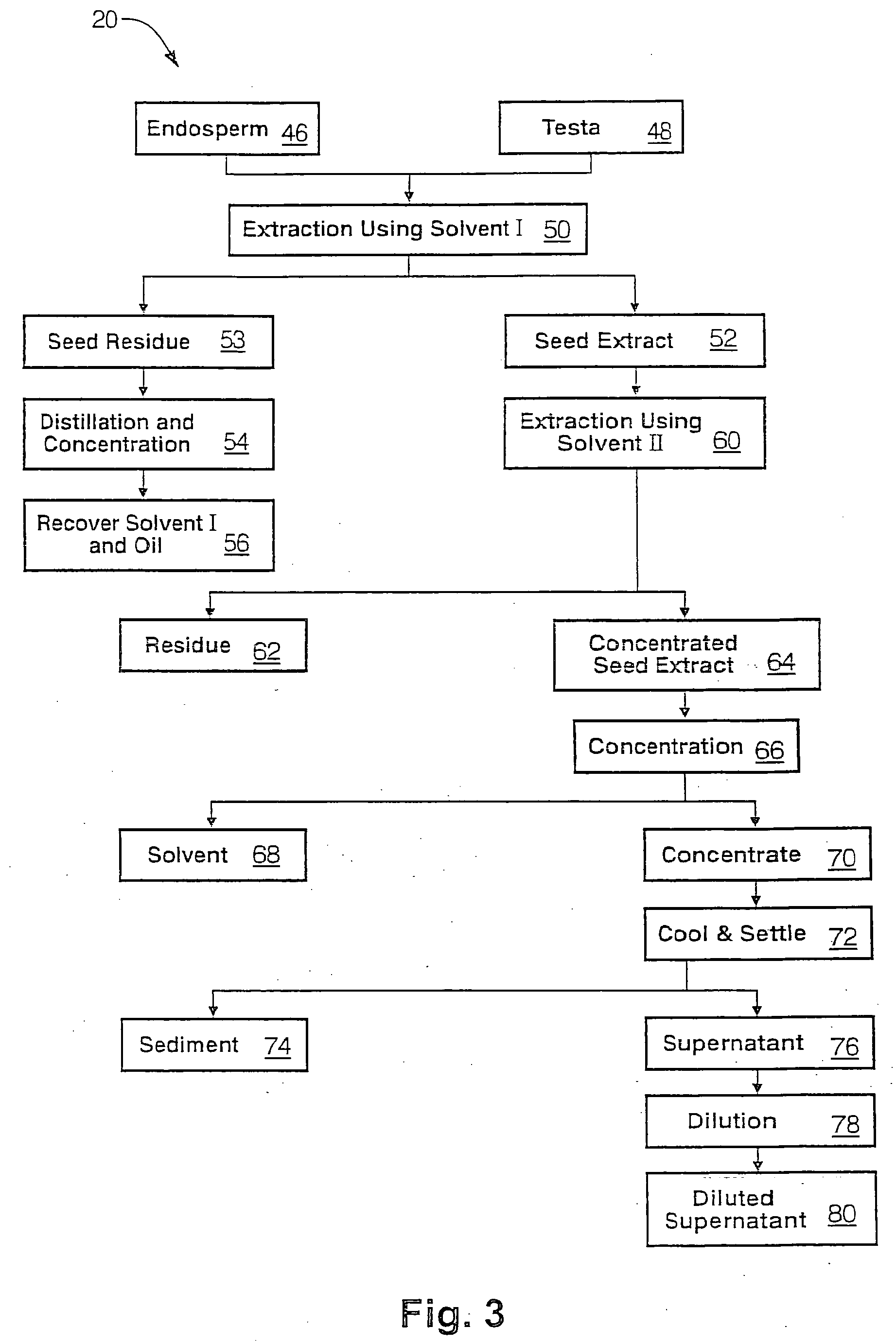
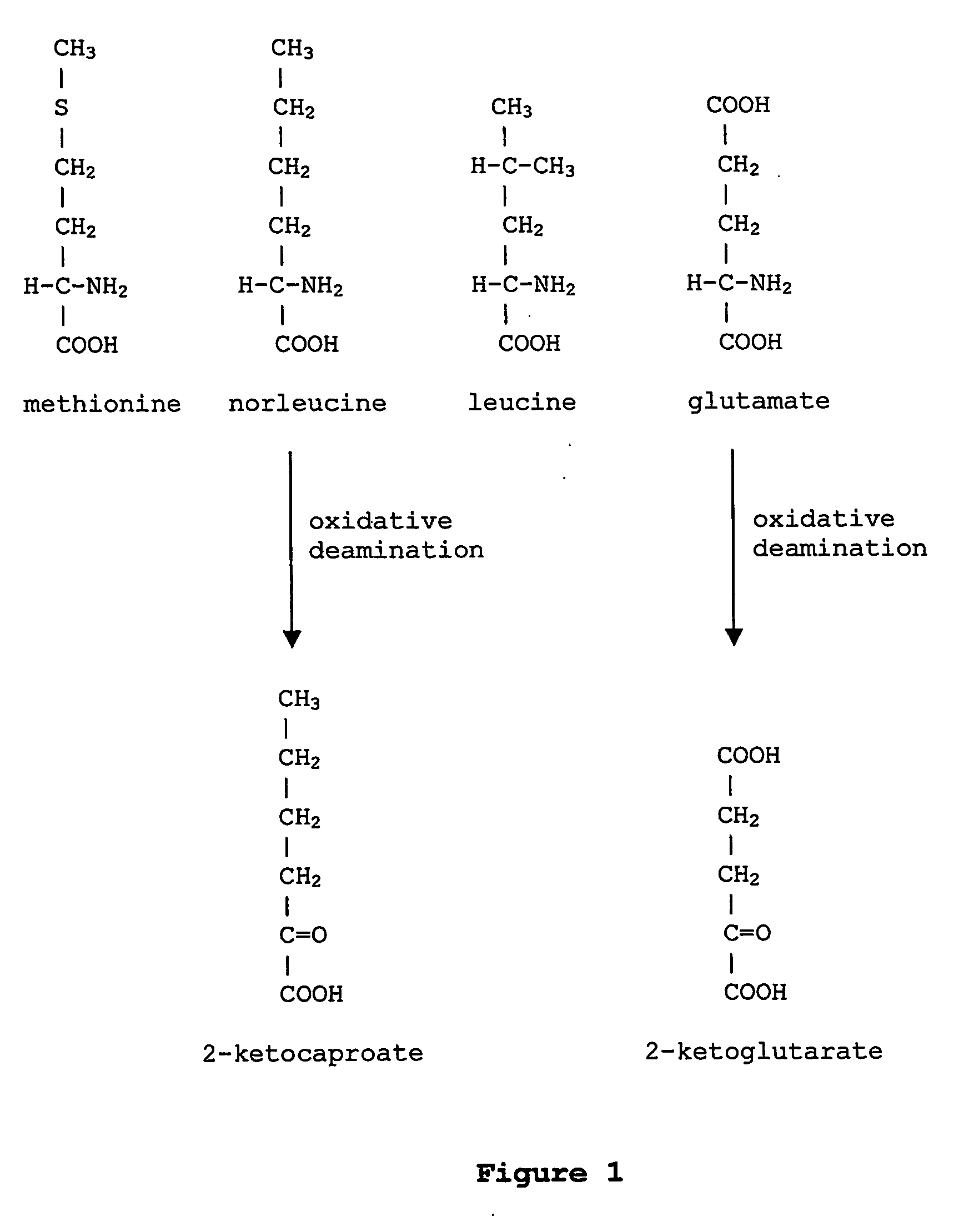
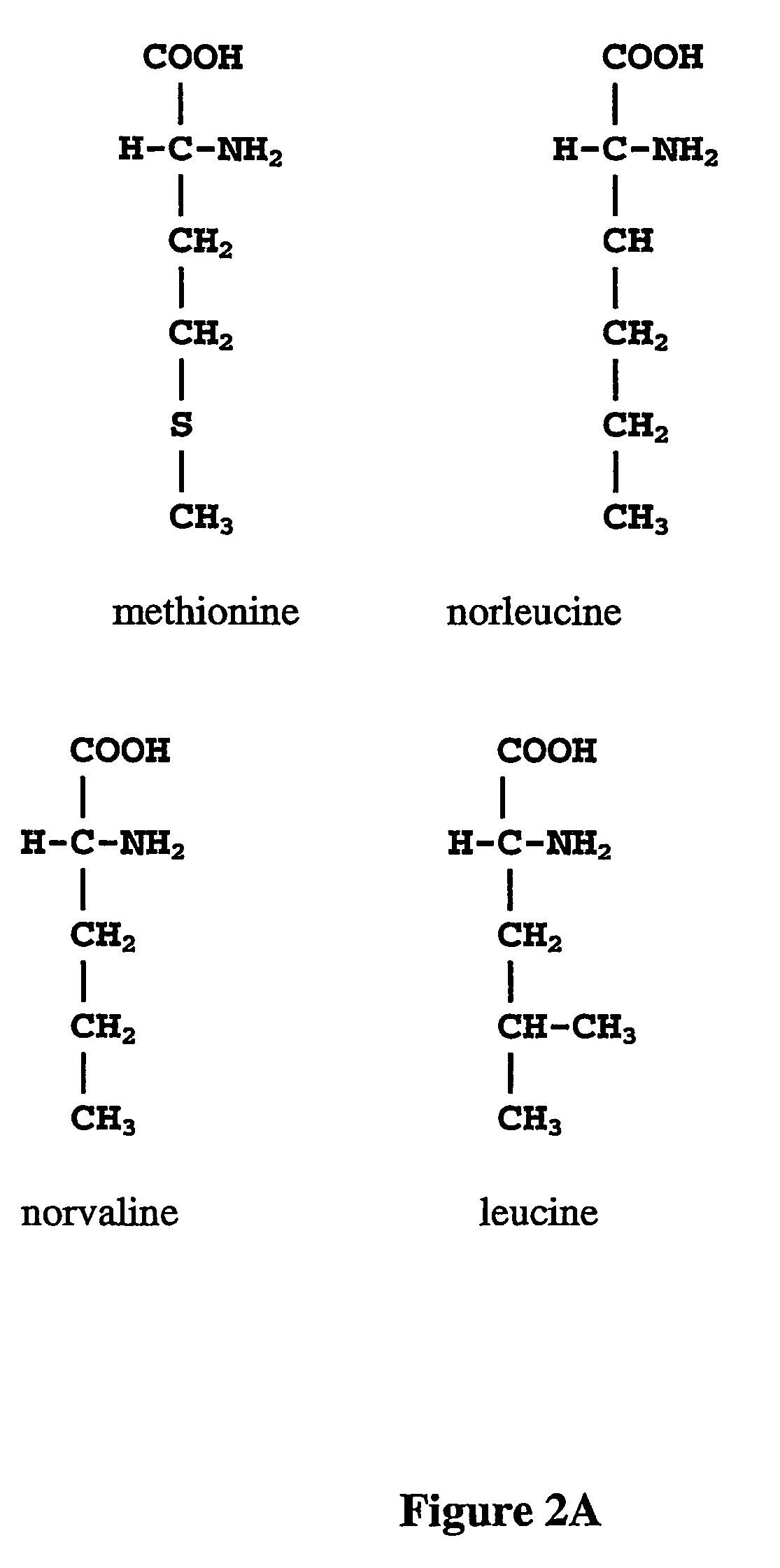
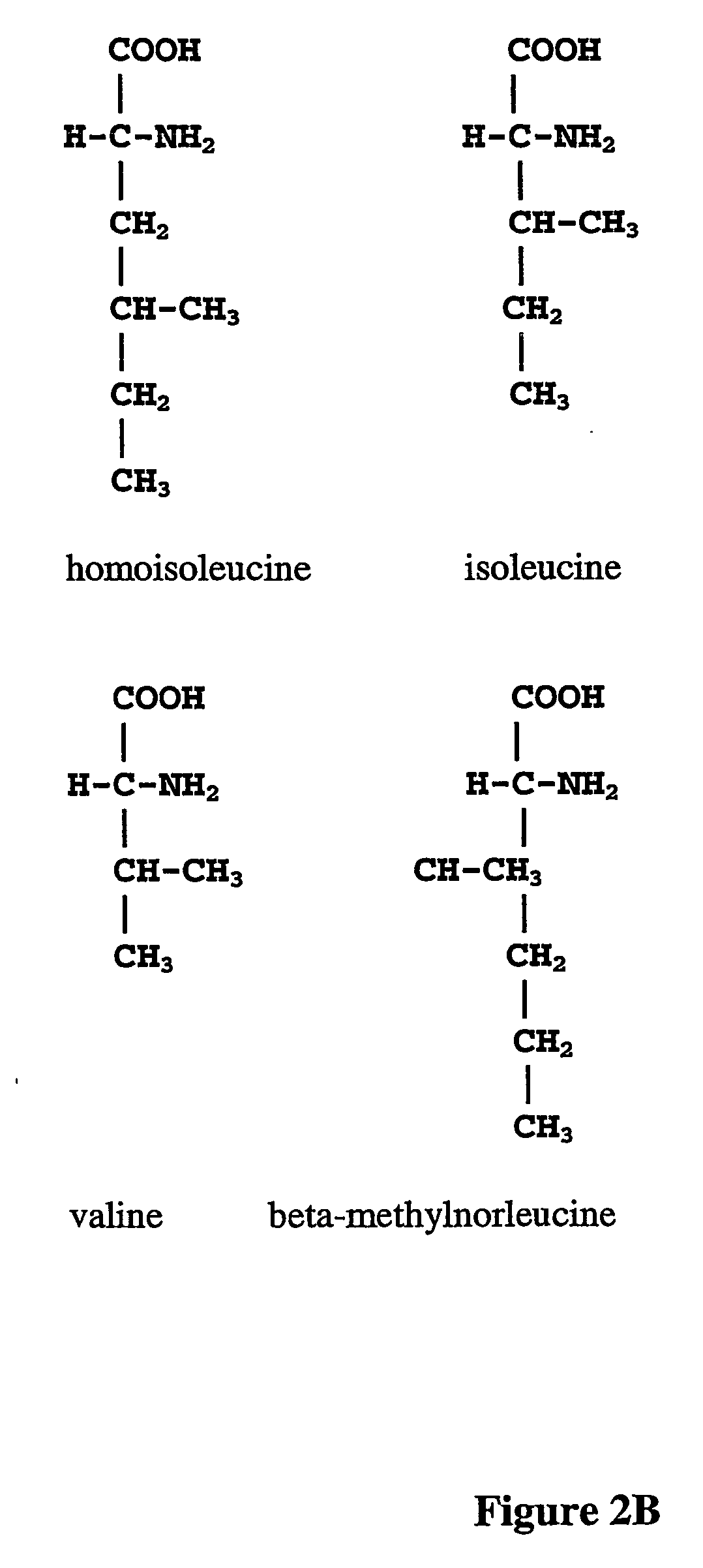
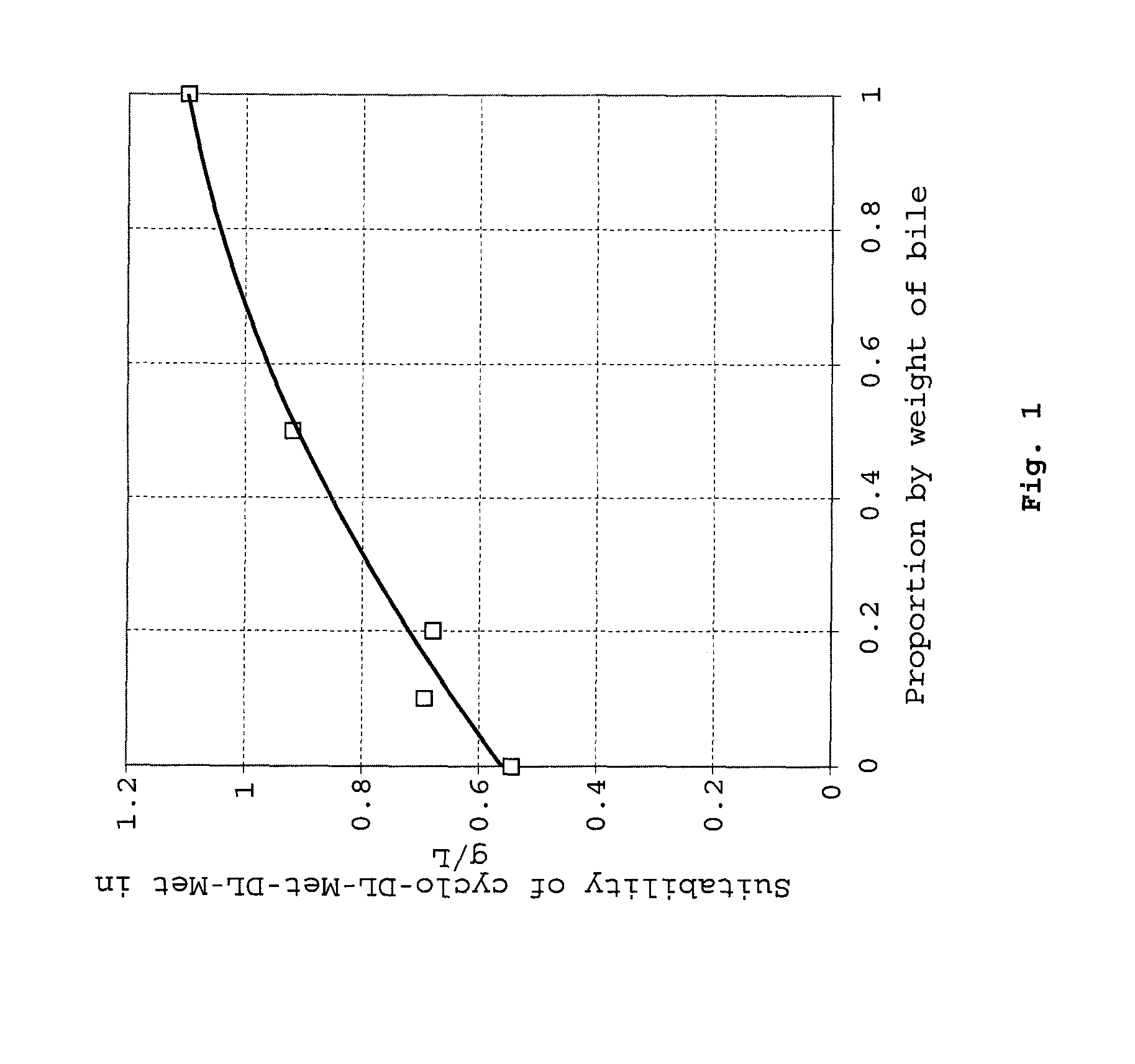
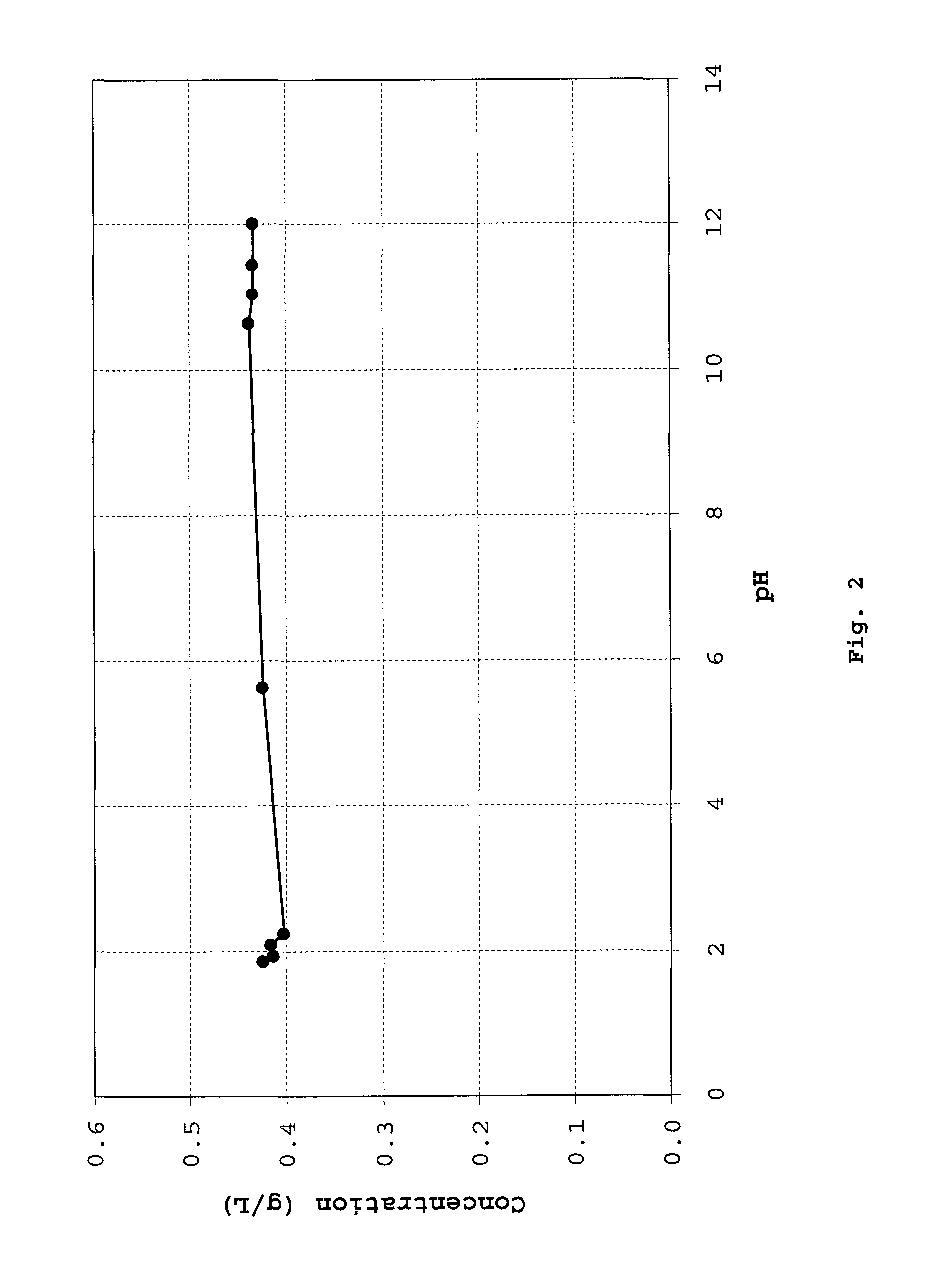
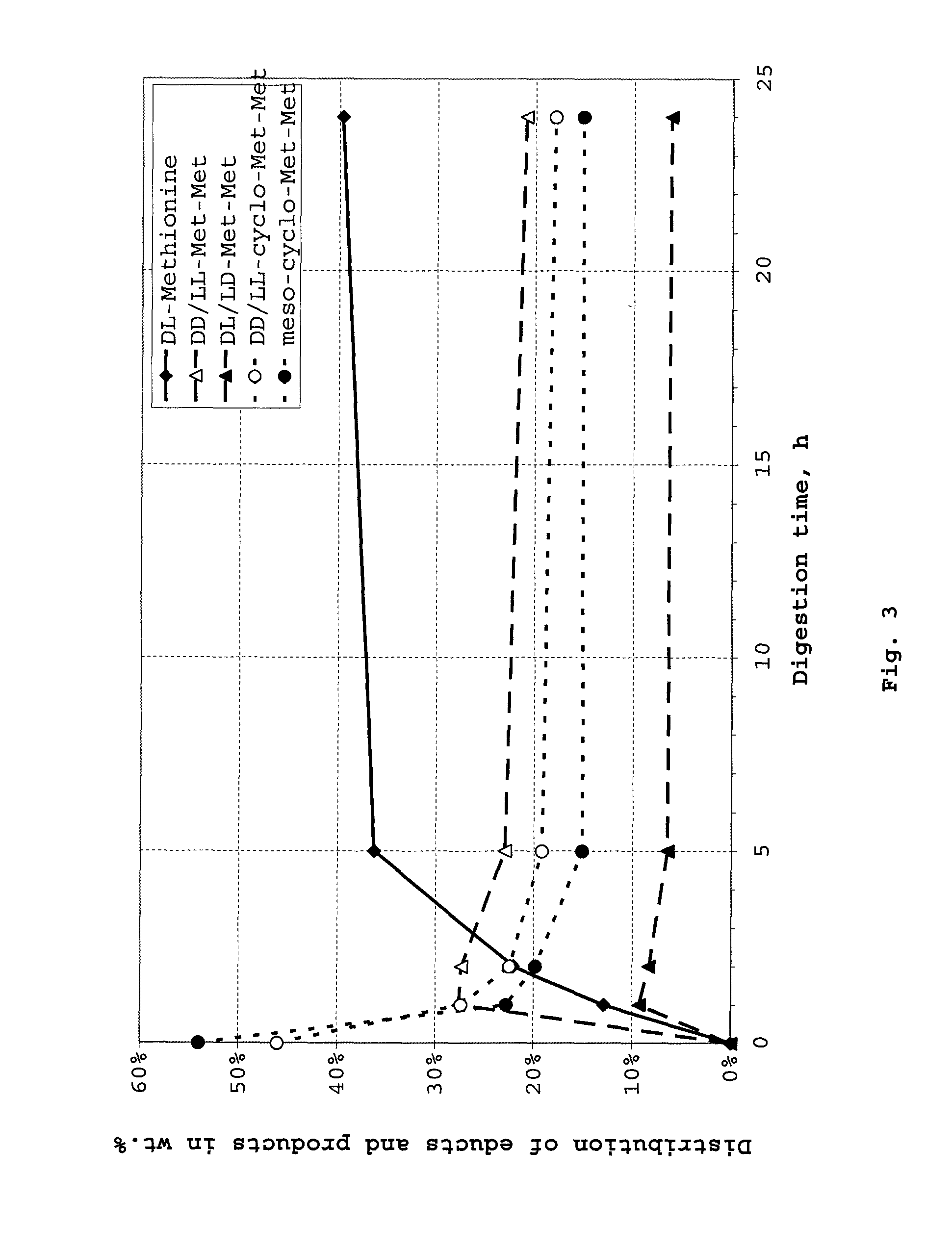
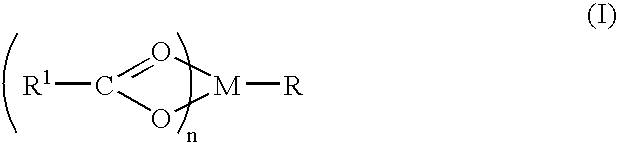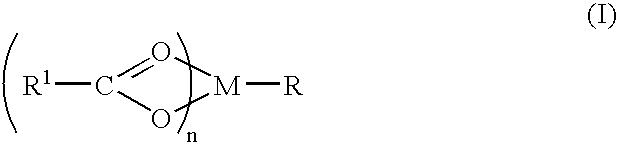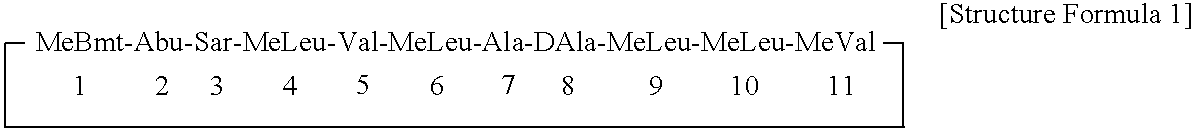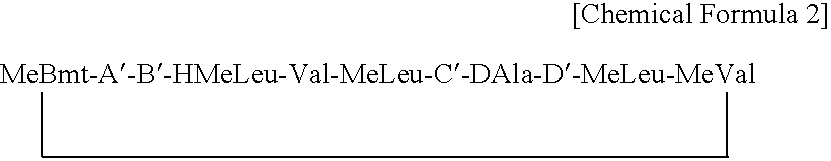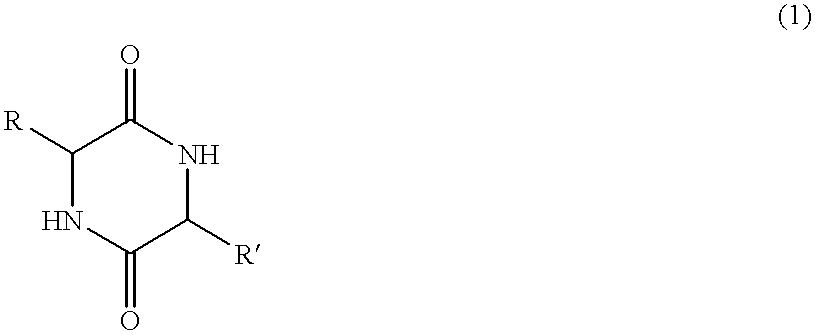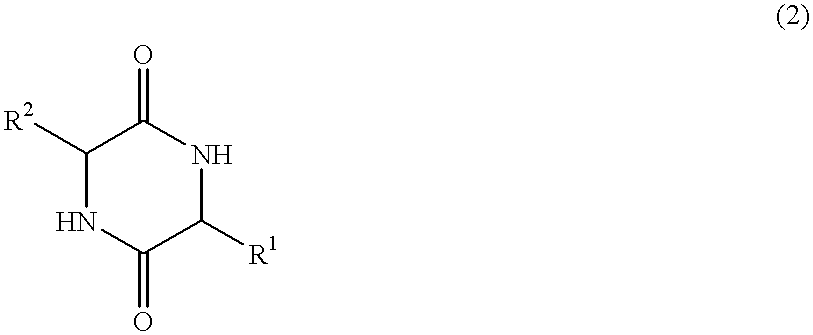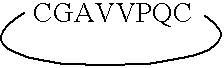Patents
Literature
883 results about "Tert-leucine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The leucines are primarily the four isomeric amino acids: leucine, isoleucine, tert-leucine and norleucine. Being compared with the four butanols, they could be classified as butyl-substituted glycines; they represent all four possible variations. Leucine and isoleucine belong to the proteinogenic amino acids; the others are non-natural.
HMB compositions and uses thereof
InactiveUS20050215640A1Reduce tumor growth rateReduce rateBiocideNervous disorderInvoluntary weight lossNeutral Amino Acids
The present invention relates to methods for the prevention and treatment of chronic inflammatory diseases, cancer, and involuntary weight loss. In the practice of the present invention patients are enterally administered HMB alone or alternatively in combination with eicosapentaenoic (20:5 ω-3), FOS, carnitine and mixtures thereof. HMB may be added to food products comprising a source of amino-nitrogen enriched with large neutral amino acids such as leucine, isoleucine, valine, tyrosine, threonine and phenylalanine and subtantially lacking in free amino acids.
Owner:ABBOTT LAB INC
Engineered transgene integration platform (ETIP) for gene targeting and trait stacking
An Engineered Transgene Integration Platform (ETIP) is described that can be inserted randomly or at targeted locations in plant genomes to facilitate rapid selection and detection of a GOI that is perfectly targeted (both the 5′ and 3′ ends) at the ETIP genomic location. One element in the subject disclosure is the introduction of specific double stranded breaks within the ETIP. In some embodiments, an ETIP is described using zinc finger nuclease binding sites, but may utilize other targeting technologies such as meganucleases, CRISPRs, TALs, or leucine zippers. Also described are compositions of, and methods for producing, transgenic plants wherein the donor or payload DNA expresses one or more products of an exogenous nucleic acid sequence (e.g. protein or RNA) that has been stably-integrated into an ETIP in a plant cell. In embodiments, the ETIP facilitates testing of gene candidates and plant expression vectors from ideation through Development phases.
Owner:CORTEVA AGRISCIENCE LLC
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Gene products of bacillus licheniformis which form odorous substances and improved biotechnological production methods based thereon
InactiveUS20070190605A1Reduce formationImprove filtering effectBacteriaHydrolasesBacillus licheniformisPropanoic acid
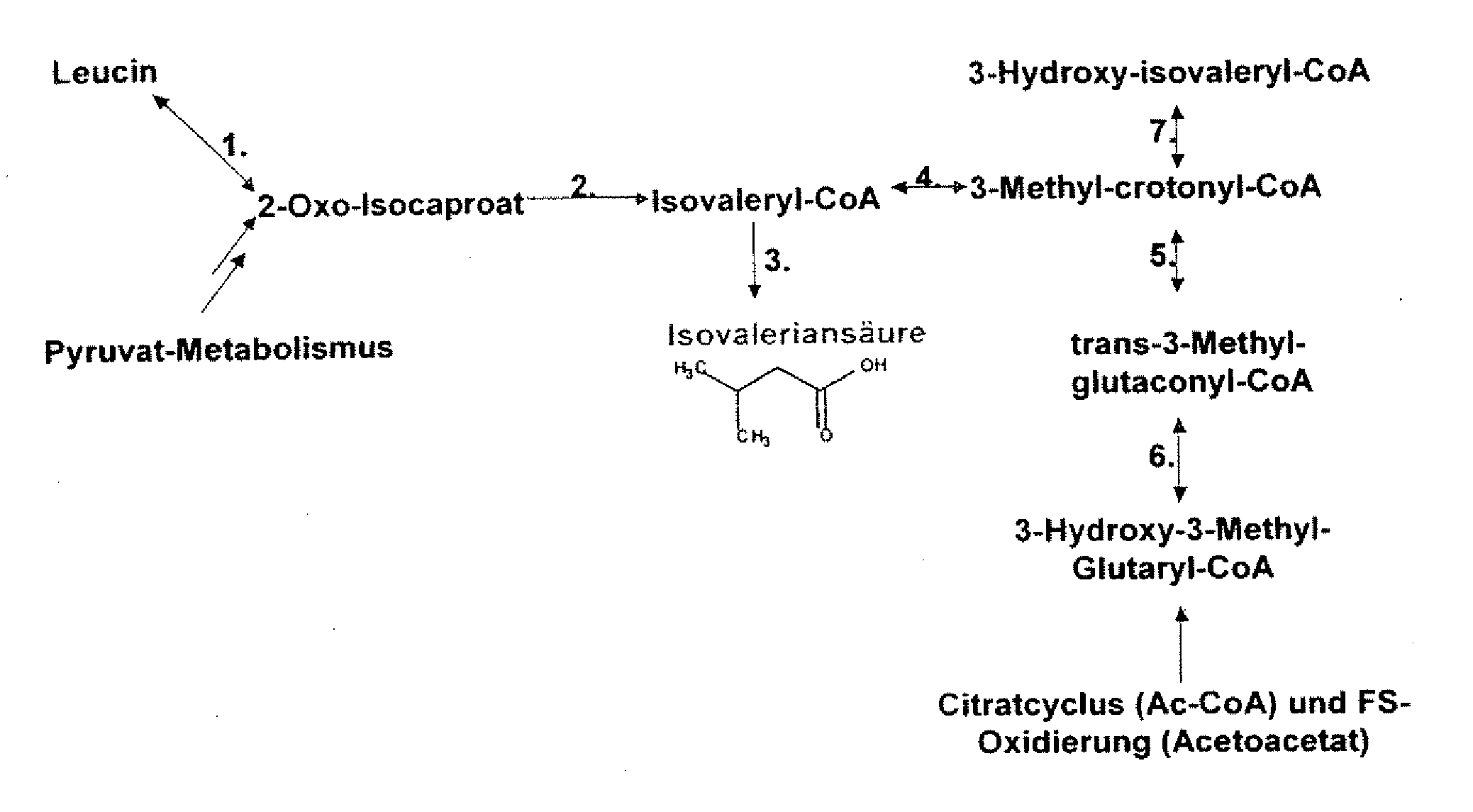
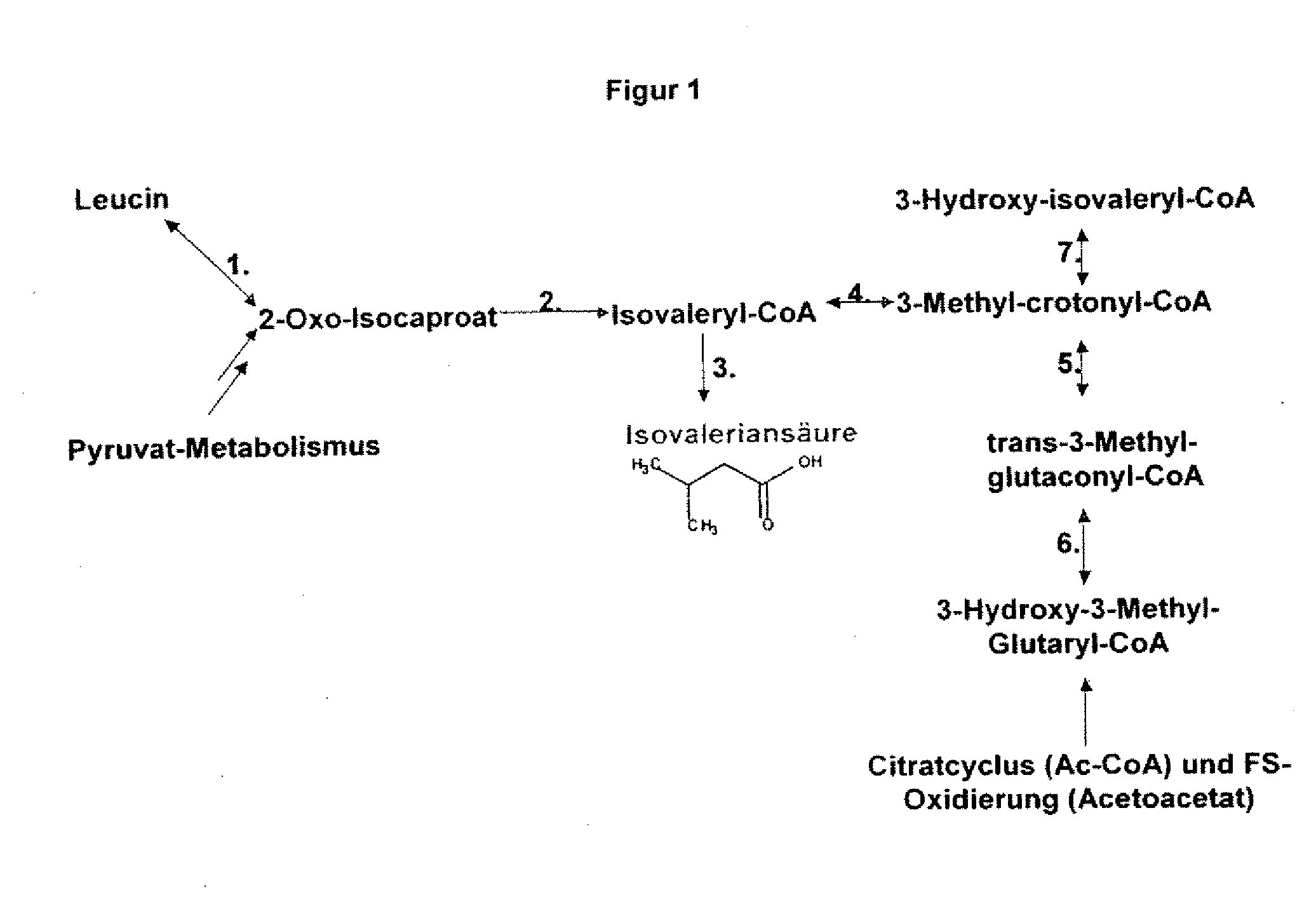
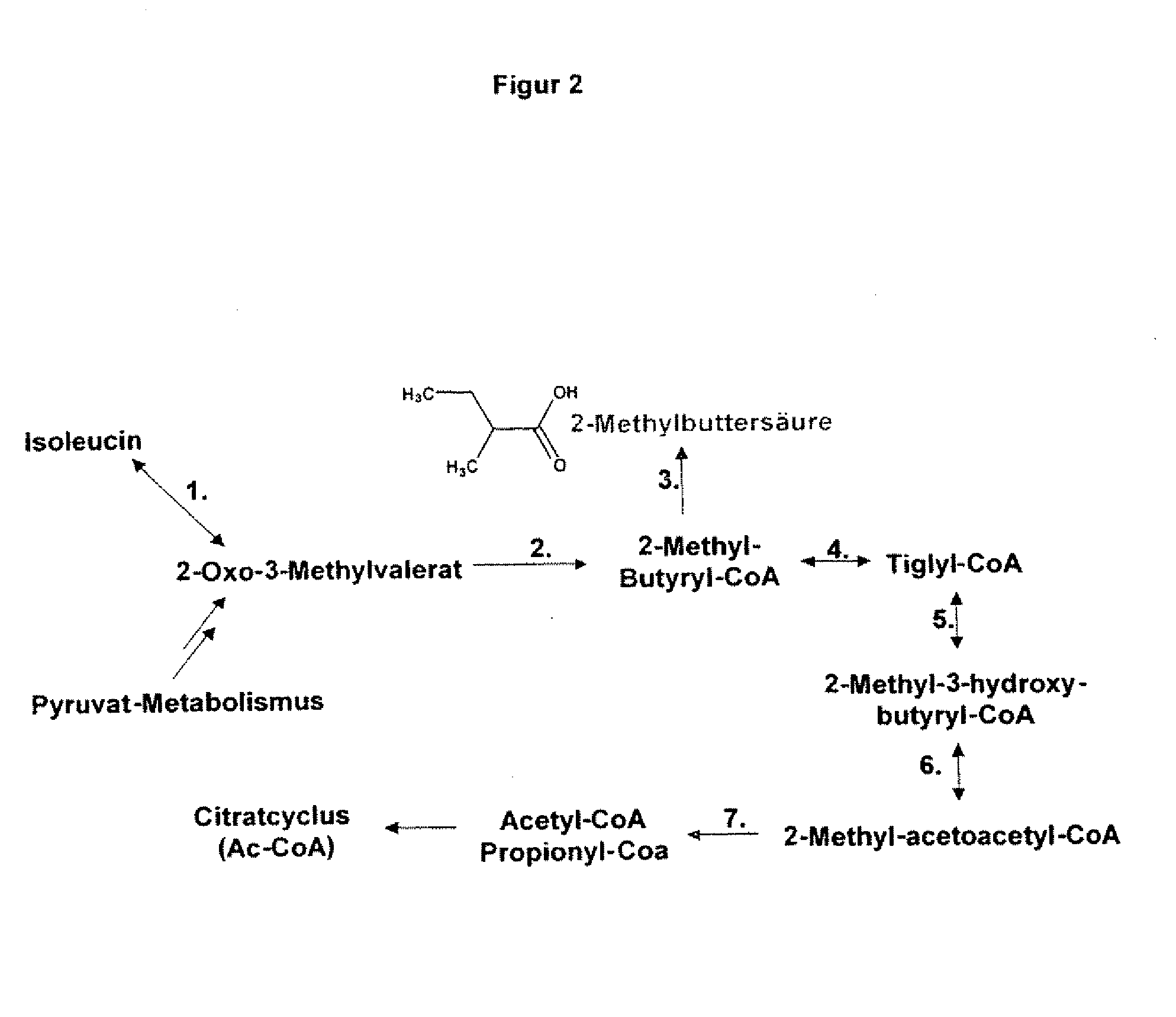
The present invention relates to 25 hitherto undescribed genes of B. licheniformis and gene products derived therefrom and all sufficiently homologous nucleic acids and proteins thereof. They occur in five different metabolic pathways for the formation of odorous substances. The metabolic pathways in question are for the synthesis of: 1) isovalerian acid (as part of the catabolism of leucine), 2) 2-methylbutyric acid and / or isobutyric acid (as part of the catabolism of valine and / or isoleucine), 3) butanol and / or butyric acid (as part of the metabolism of butyric acid), 4) propyl acid (as part of the metabolism of propionate) and / or 5) cadaverine and / or putrescine (as parts of the catabolism of lysine and / or arginine). The identification of these genes allows biotechnological production methods to be developed that are improved to the extent that, to assist these nucleic acids, the formation of the odorous substances synthesized via these metabolic pathways can be reduced by deactivating the corresponding genes in the micro-organism used for the biotechnological production. In addition, these gene products are thus available for preparing reactions or for methods according to their respective biochemical properties.
Owner:BASF AG
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
Screening assay for TLR7, TLR8 and TLR9 agonists and antagonists
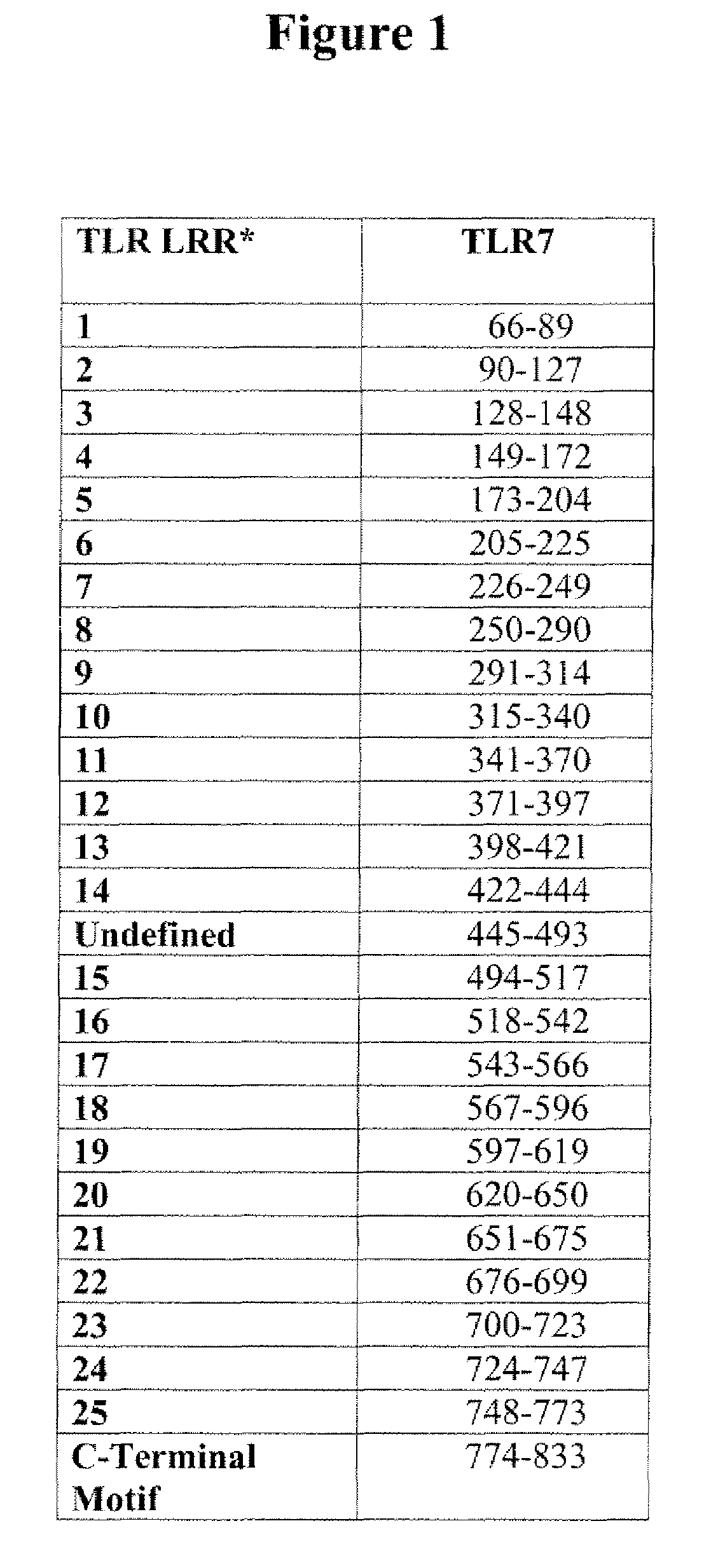
InactiveUS7498409B2Efficient methodCell receptors/surface-antigens/surface-determinantsLibrary screeningTLR8Screening method
The present invention relates to novel screening methods for identifying agonists and antagonists of toll-like receptor (TLR) 7, TLR8 or TLR9. Methods are disclosed for identifying agonists and antagonists of TLR7, TLR8 or TLR9 using mutant TLR proteins containing deletions in one or more extracellular leucine rich regions (LRRs). Such agonists and antagonists have utility in the prevention, treatment and / or cure of various diseases and conditions, including cancer, virus infection, allergy, asthma, and chronic obstructive pulmonary disease (COPD).
Owner:SCHERING CORP
Materials and methods for controlling pests
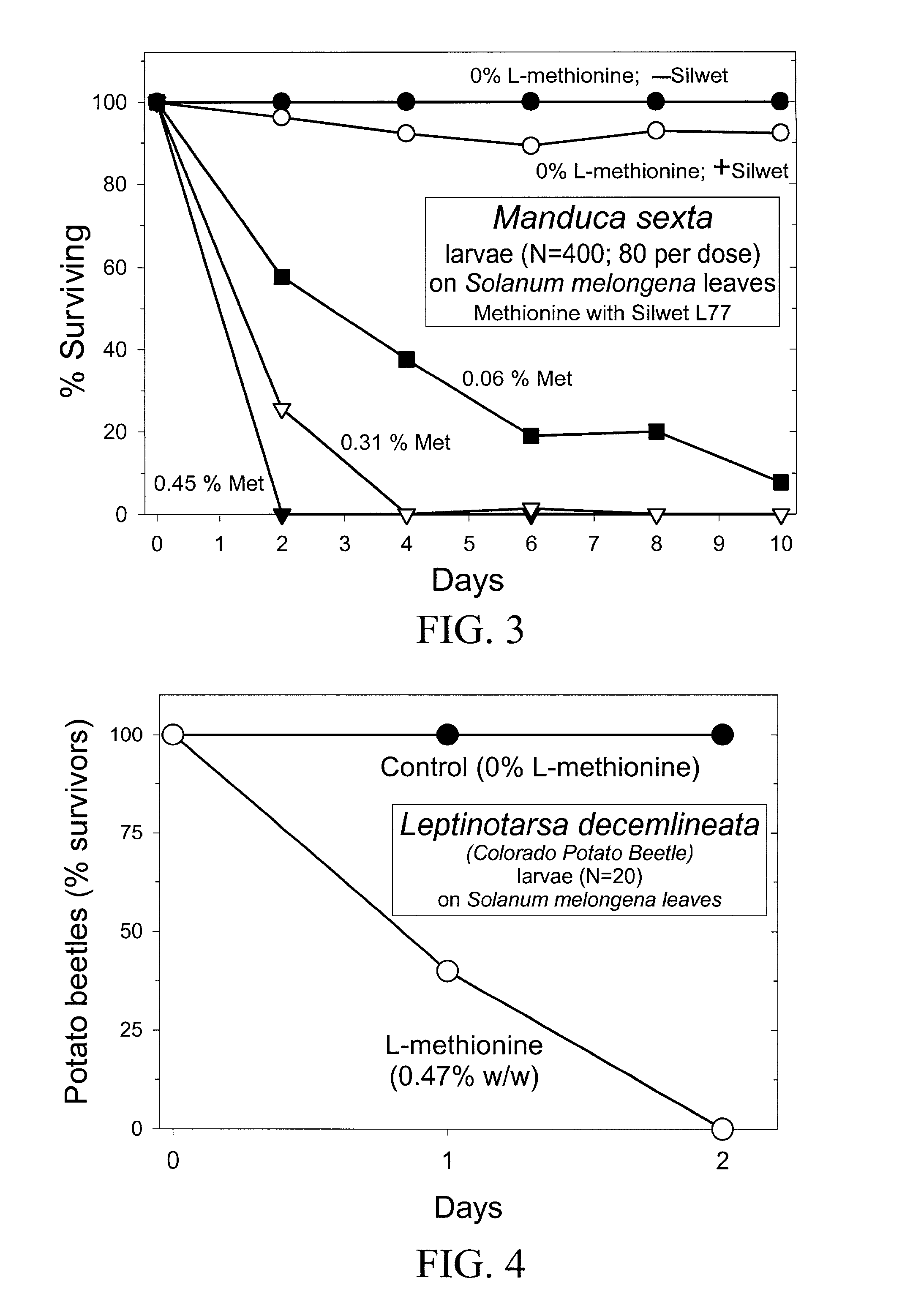
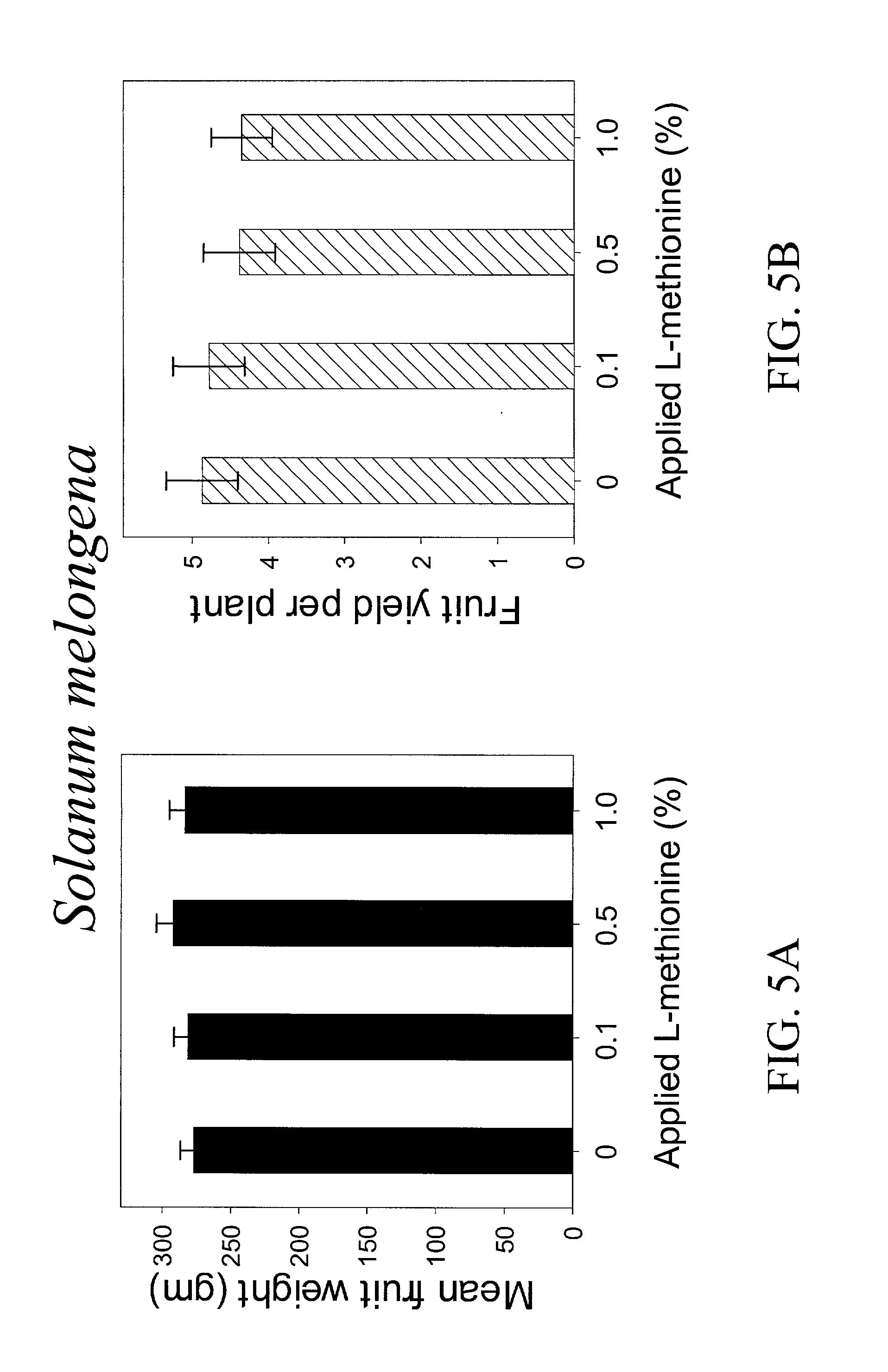
InactiveUS20030154508A1Low toxicitySustainable, pesticide-free food supplyOrganic active ingredientsBiocideSolute transportersMethionine biosynthesis
Owner:FLORIDA UNIV OF A FLORIDA +1
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Surfactant peptide nanostructures, and uses thereof
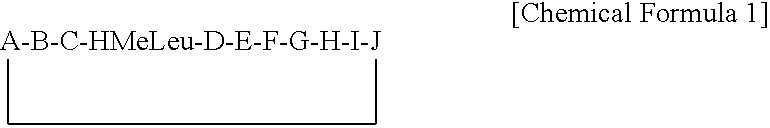
ActiveUS7179784B2Improve efficiencyIncrease flexibilityMaterial nanotechnologyBiocideActive agentTert-leucine
This work describes a new class of short polypeptides that can self-assemble to form regular nanotubes with an average diameters of about 50 nm. These peptides (7 to 8 amino acids) have a structure very similar to those observed in surfactant molecules with a defined hydrophilic head group constituting of charged amino acids and a lipophilic tail made out of hydrophobic amino acids such as alanine, valine or leucine. Cryo-TEM micrographs show numerous three-fold junctions connecting the self-assembling nanostructures and thus leading to the formation of a rather dense network of entangled nanotubes. Additionally, the observation of clear openings at the end of the supramolecular structures confirms the presence of tubular organization.
Owner:MASSACHUSETTS INST OF TECH
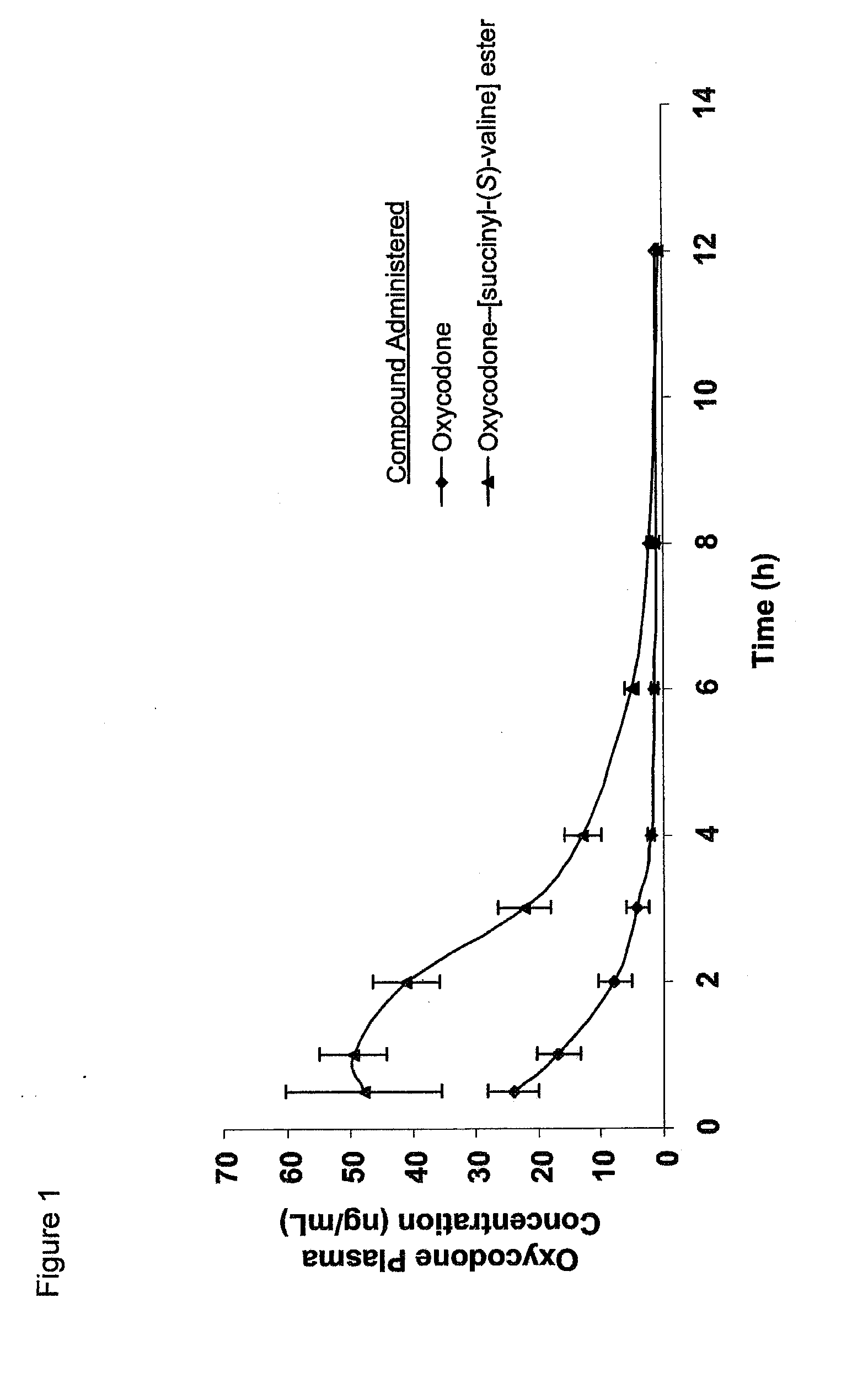
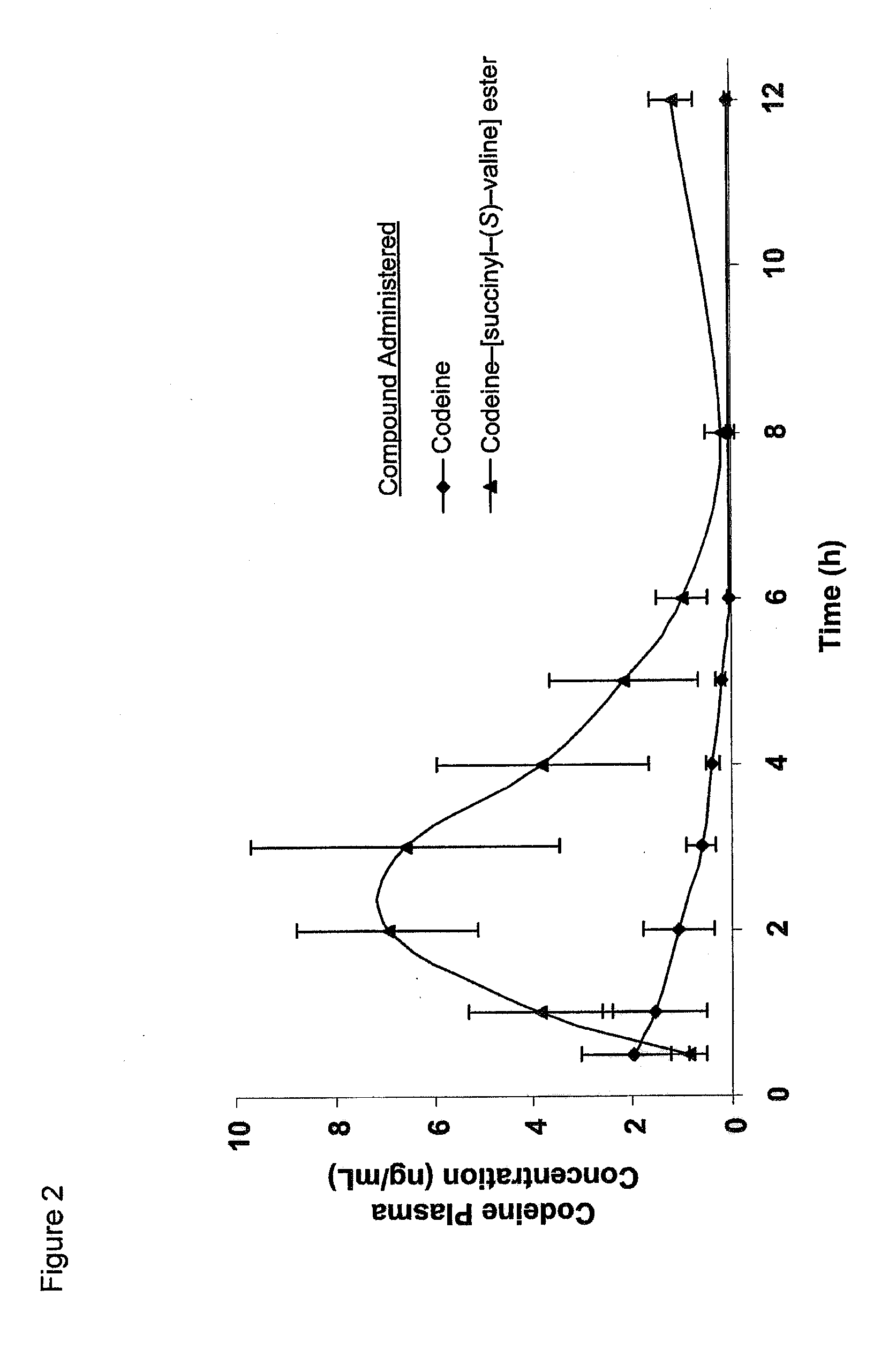
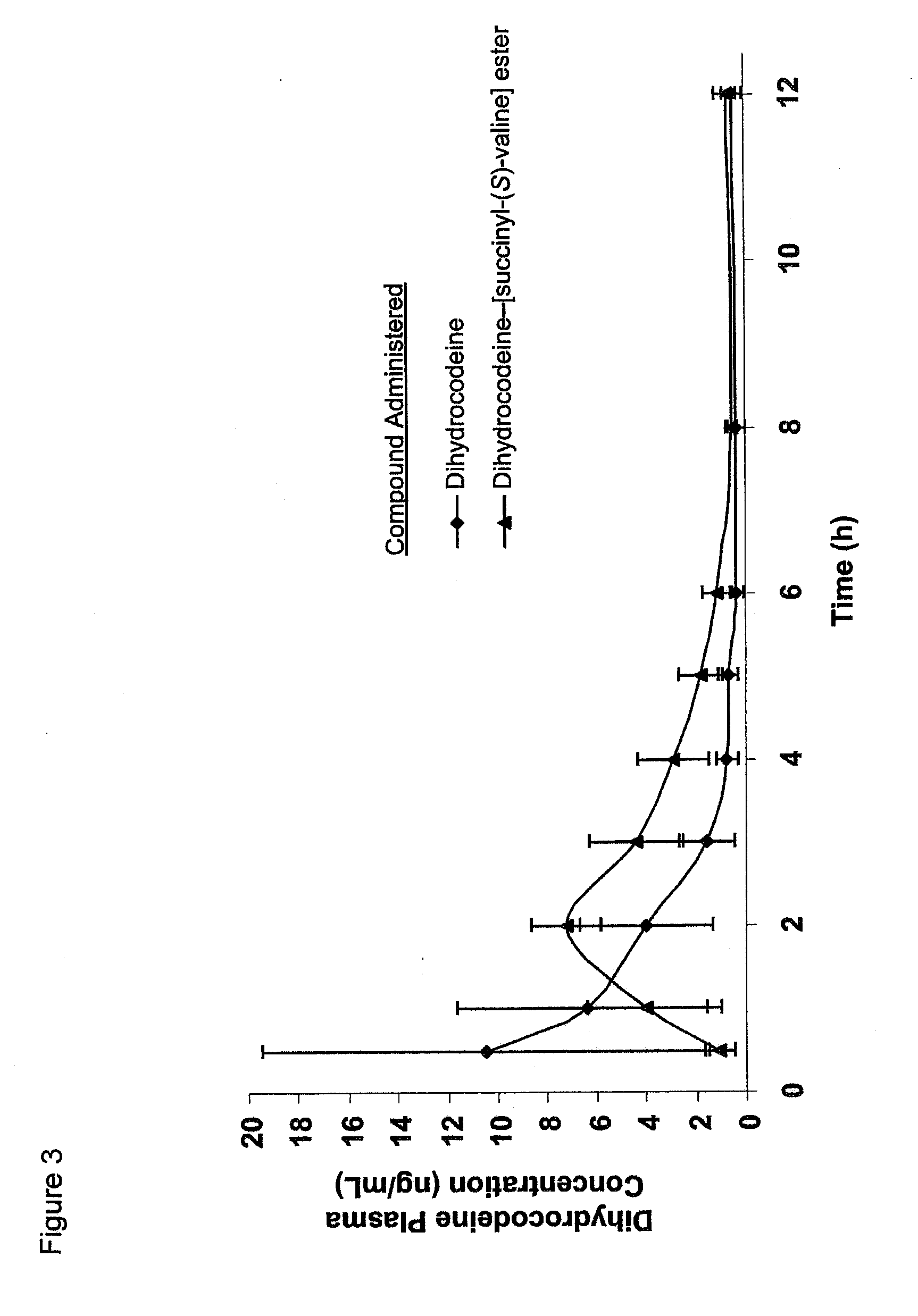
Novel dicarboxylic acid linked amino acid and peptide prodrugs of opioids and uses thereof
InactiveUS20100286186A1Low variabilityReduction and elimination of painBiocideNervous disorderSide effectAmino acid side chain
The present invention concerns dicarboxylic acid linked amino acid and peptide prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing pain relief, decreasing the adverse GI side effects of the opioid analgesic and increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are also provided. In one embodiment, prodrugs having the amino acid side chains of valine, leucine, isoleucine and glycine; and mono-, di- and tripeptides thereof are provided.
Owner:SHIRE PLC
TGF-B inhibitors and methods
A family of small peptides have been found to be inhibitory to TGF-beta activity, and preferably have the primary structure of Formula I:wherein AA1 is leucine, phenylalanine, alpha-aminoisobutric acid, N-methylalanine, N-methylisoleucine, or isoleucine; AA2 is the same or a different amino acid residue as in AA1; and AA3 is alanine or N-methylalanine.
Owner:RGT UNIV OF CALIFORNIA
Topical compositions containing nonimmunosuppressive cyclosporin derivatives for treating hair loss
InactiveUS20050074468A1Good hair growthGood effectCosmetic preparationsHair cosmeticsCyclosporinsTert-leucine
The present invention discloses a topical scalp and transdermal preparation with excellent penetration to the skin and follicle, containing a [γ-hydroxy-N-methyl-L-leucine4] cylosporin derivative which is a non-immunosuppressive component with hair growth stimulating ability. The topical scalp and transdermal preparation is prepared by incorporating the cyclosporin derivative into a liposome, microcapsule, micro-sphere, composite particle or emulsion, capable of being employed as a hair growth stimulating agent and applied for the prevention of hair loss.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
HMB compositions and uses thereof
The present invention relates to methods for the prevention and treatment of chronic inflammatory diseases, cancer, and involuntary weight loss. In the practice of the present invention patients are enterally administered HMB alone or alternatively in combination with eicosapentaenoic (20:5 ω-3), FOS, carnitine and mixtures thereof. HMB may be added to food products comprising a source of amino-nitrogen enriched with large neutral amino acids such as leucine, isoleucine, valine, tyrosine, threonine and phenylalanine and substantially lacking in free amino acids.
Owner:ABBOTT LAB INC
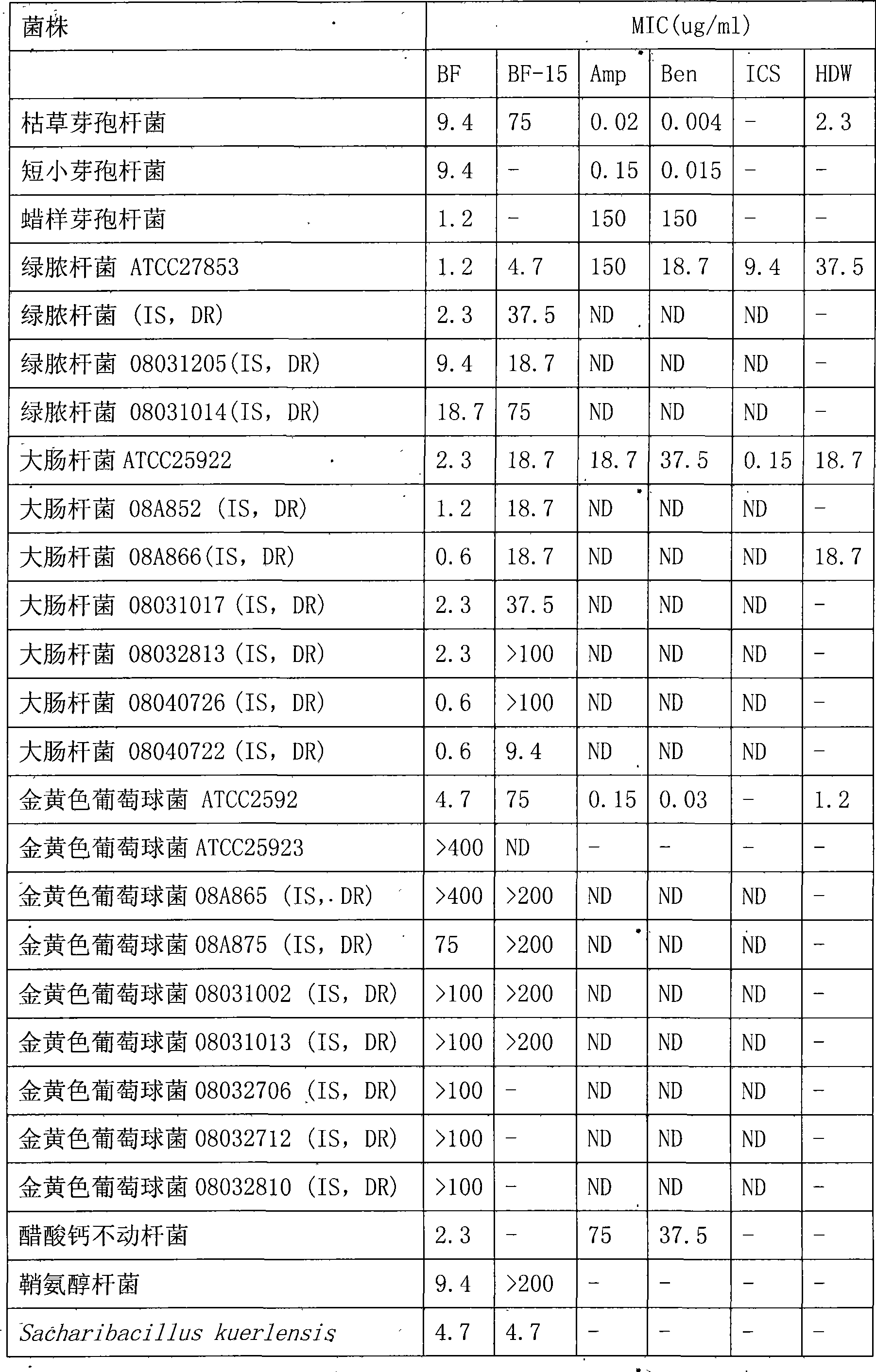
Bungarus fasciatus antibacterial peptide cathelicidin-BF, and genes and uses thereof
InactiveCN101412753ASmall molecular weightImprove the bactericidal effectFermentationAnimals/human peptidesArginineAntibiotic Y
The invention discloses cathelicidin-BF and a gene and application thereof, which belong to the field of biomedicine. The cathelicidin-BF is straight chain polypeptide and contains thirty amino acid residues, the molecular weight is 3,637.54Da, and the isoelectric point is 11.79. The complete sequence of the cathelicidin-BF is lysine-phenyl alanine-phenyl alanine-arginine-lysine-leucine- lysine-lysine-serine-valine-lysine-lysine-arginine-lactamine-lysine-glutamic acid-phenyl alanine- phenyl alanine-lysine-lysine-proline-arginine-valine-isoleucine-glycin-valine-serine-isoleucine- praline-phenyl alanine. The gene for encoding the cathelicidin-BF consists of 750 ribonucleotides, wherein 484th to 573rd ribonucleotides are used for encoding a mature peptide part. The cathelicidin-BF has small molecular weight, strong sterilization effect, and quick action time, and has quite strong killing function to a plurality of kinds of clinical drug-fast bacteria. In addition, the cathelicidin-BF also has the advantages of broad-spectrum antibiotics, salt independence and so on.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
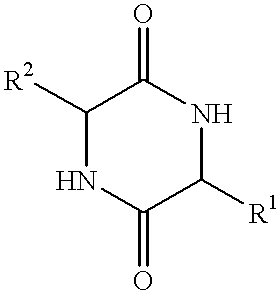
Method of synthesizing diketopiperazines
InactiveUS6967202B2Prevent unwanted side reactionInhibit side effectsOrganic active ingredientsDipeptide ingredientsSide chainTyrosine
The invention provides a method of synthesizing a diketopiperazine of the formula: wherein:R1 is —CH2COR3, or —CH2CH2COR3;R2 is the side chain of an amino acid selected from the group consisting of glycine, alanine, valine, leucine, isoleucine, serine, threonine, aspartic acid, asparagine, glutamic acid, glutamine, lysine, hydroxylysine, histidine, arginine, phenylalanine, tyrosine, tryptophan, thyroxine, cysteine, methionine, norvaline and ornithine;R3 is —OH, —NH2, —OR4, —NHR4, or —NR4R4; andeach R4 is independently an alkyl, aryl, alkylaryl, or arylalkyl.
Owner:AMPIO PHARMA
Orally administered small peptides synergize statin activity
InactiveUS7148197B2Readily taken up and deliveredMany symptomOrganic active ingredientsPeptide/protein ingredientsThreonineTyrosine
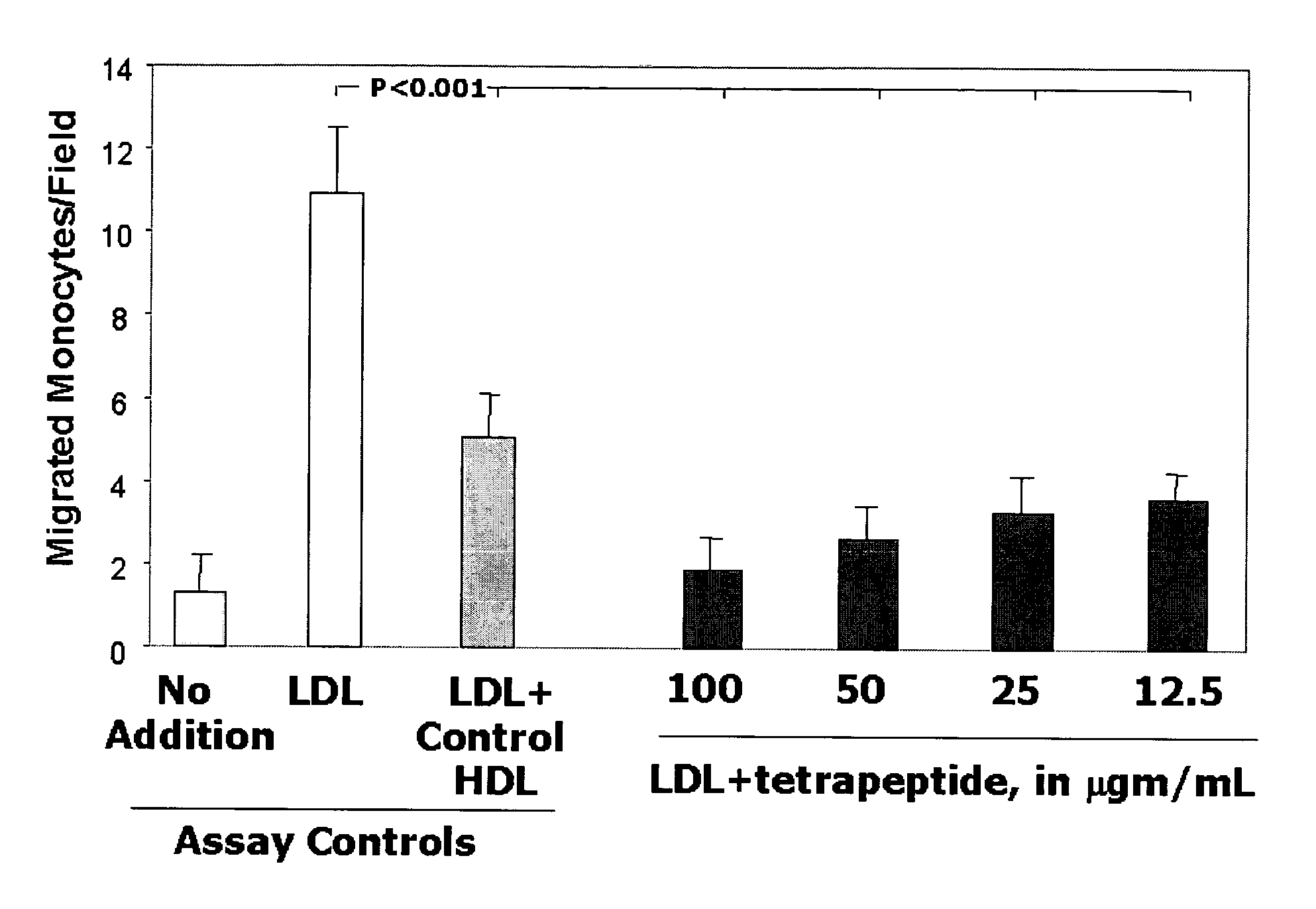
This invention provides novel peptides for the treatment of atherosclerosis. In certain embodiments the peptide is X1-X2-X3-X4 where X1 and X4 are independently selected from the group consisting of alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), tryptophan (Trp), methionine (Met), serine (Ser) bearing a hydrophobic protecting group, beta-naphthyl alanine, alpha-naphthyl alanine, norleucine, cyclohexylalanine, threonine (Thr) bearing a hydrophobic protecting group, tyrosine (Tyr) bearing a hydrophobic protecting group, lysine (Lys) bearing a hydrophobic protecting group, arginine (Arg) bearing a hydrophobic protecting group, ornithine (Orn) bearing a hydrophobic protecting group, aspartic acid (Asp) bearing a hydrophobic protecting group, cysteine (Cys) bearing a hydrophobic protecting group, and glutamic acid (Glu) bearing a hydrophobic protecting group; X2 and X3 are independently selected from the group consisting of Asp, Arg, and Glu; and the peptide converts pro-inflammatory HDL to anti-inflammatory HDL or makes anti-inflammatory HDL more anti-inflammatory.
Owner:RGT UNIV OF CALIFORNIA +1
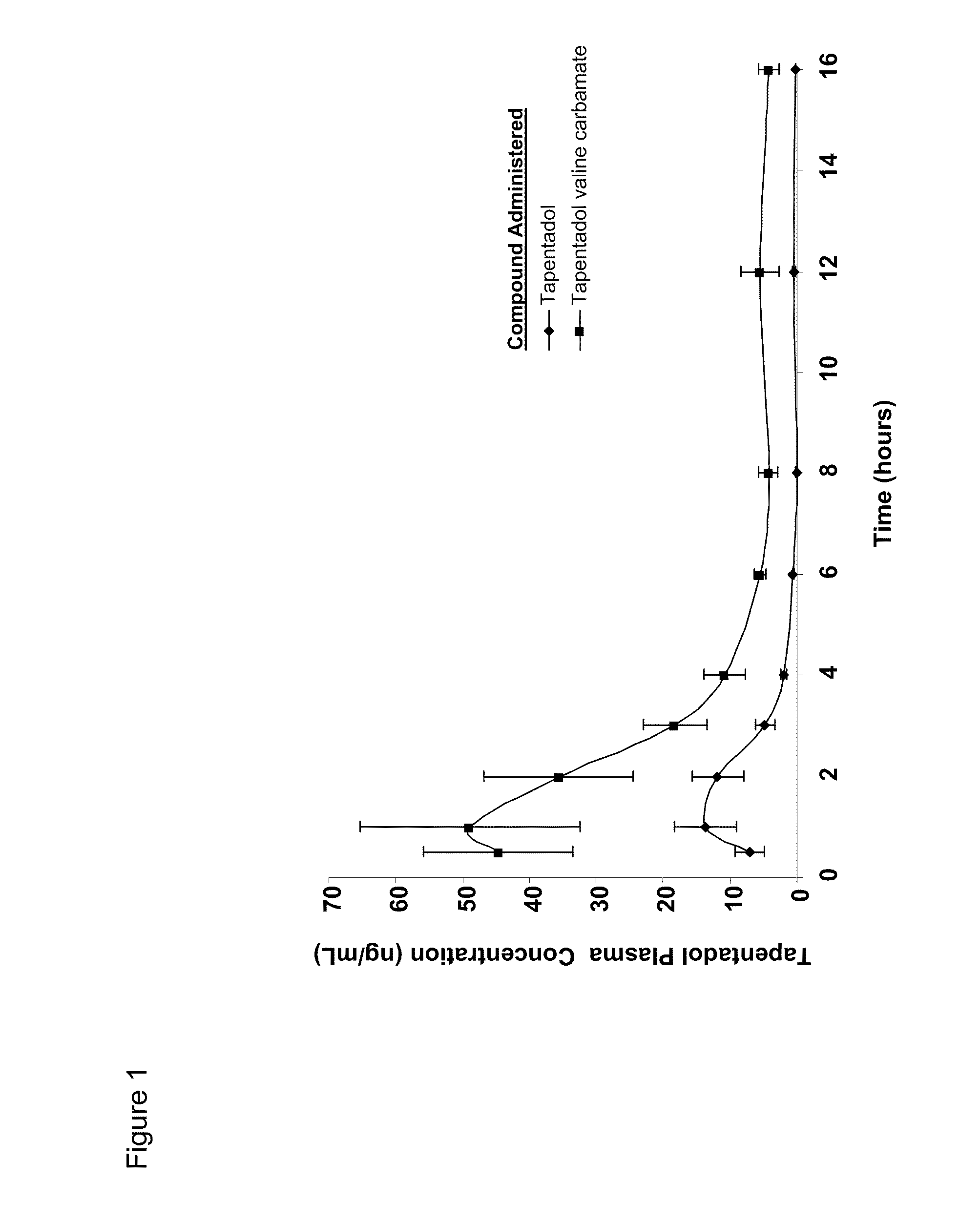
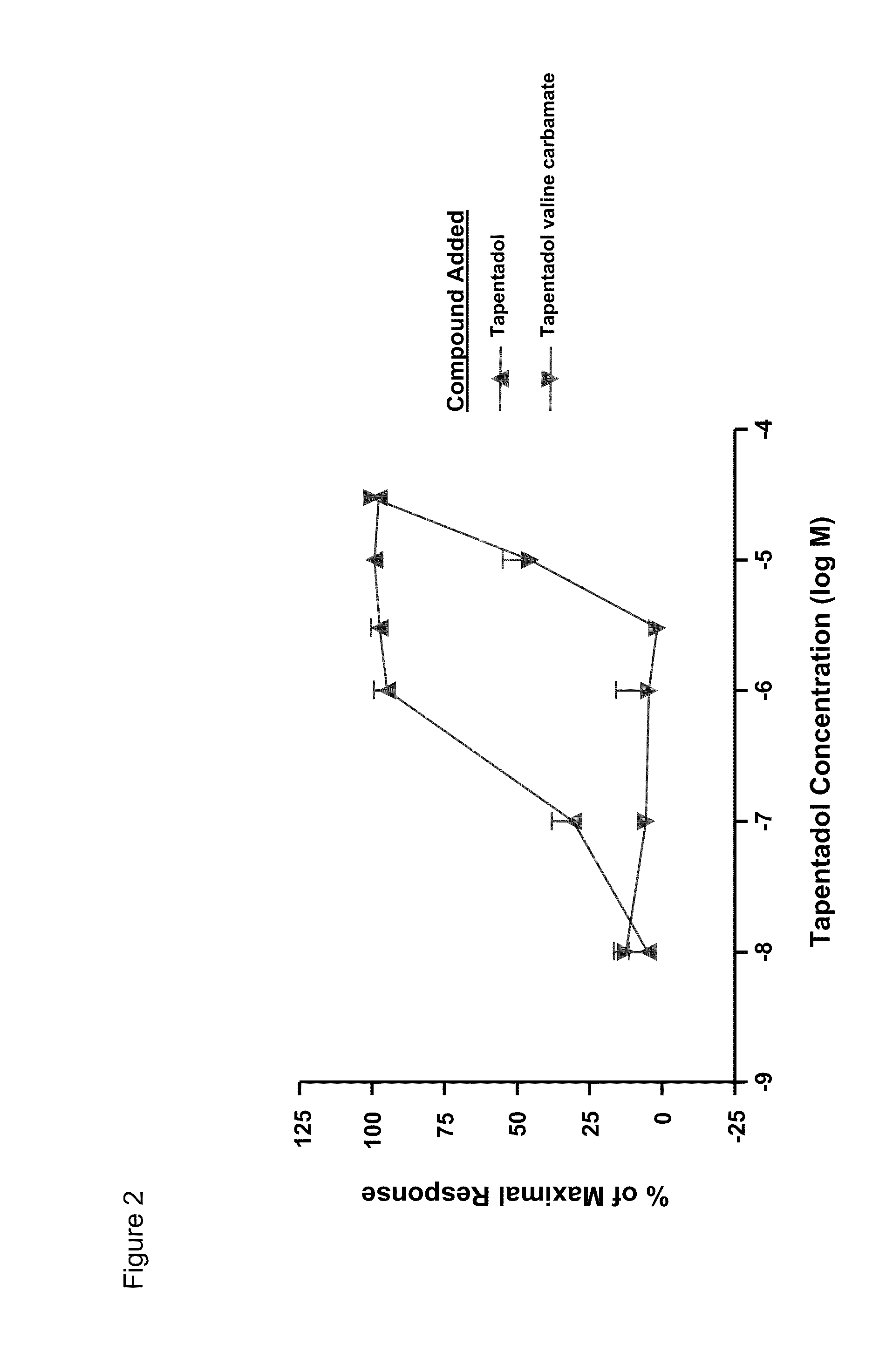
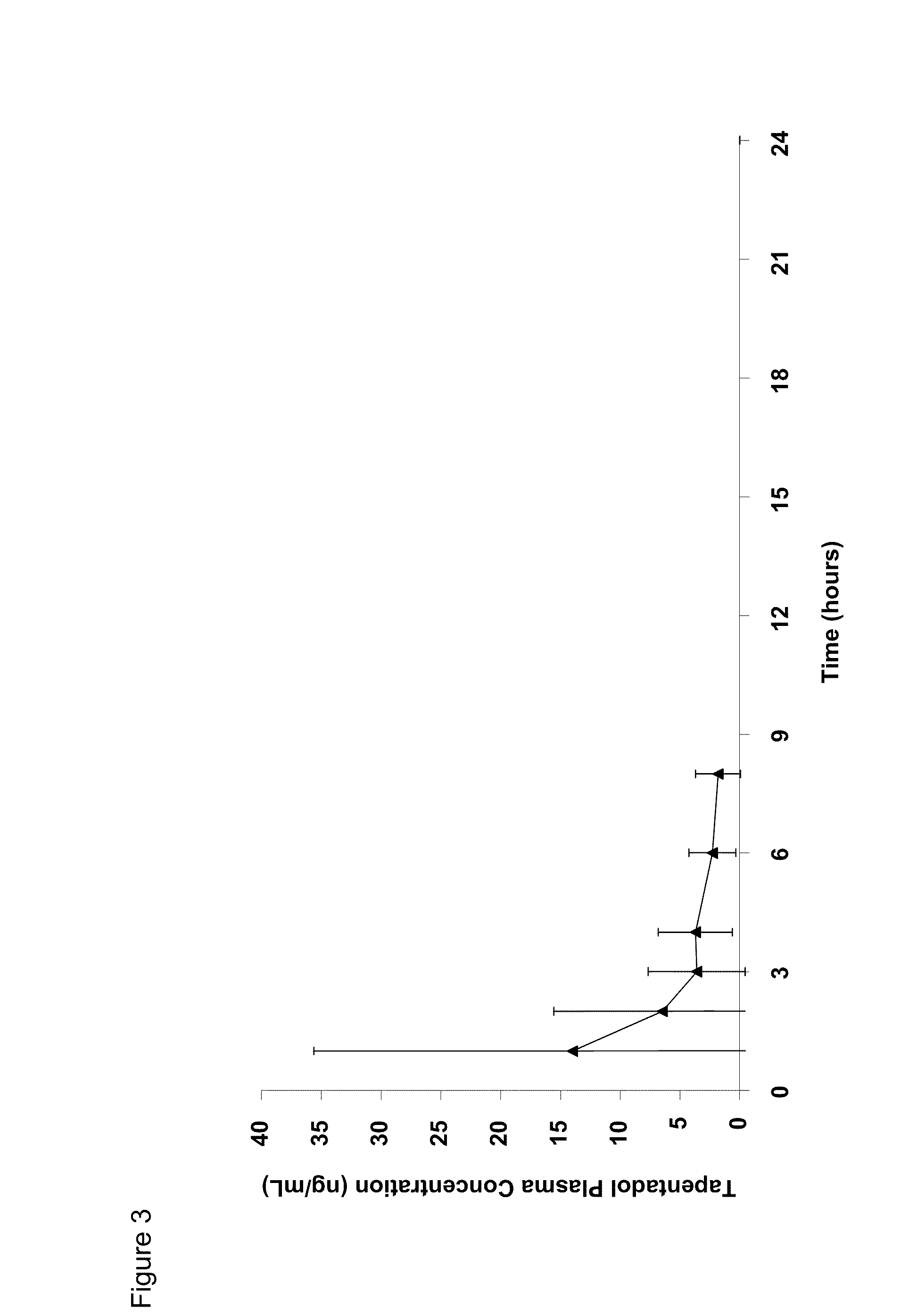
Amino acid and peptide carbamate prodrugs of tapentadol and uses thereof
InactiveUS20100227921A1Sufficient amountMinimizing the gastrointestinal (GI) side effectsBiocideNervous disorderCarbamateSide effect
Prodrugs of tapentadol with amino acids or short peptides, pharmaceutical compositions containing such prodrugs and a method for providing pain relief with the tapentadol prodrugs are provided herein. Prodrugs having side chains of valine, leucine, isoleucine and glycine amino acids and mono-, di- and tripeptides thereof are preferred. Additionally, methods for avoiding or minimizing the adverse gastrointestinal side effects associated with tapentadol administration, as well as increasing the oral bioavailability of tapentadol are provided herein.
Owner:SHIRE PLC
Methods for enhancing the transport of glucose into muscle
InactiveUS20050226948A1Effective quantityLow production costBiocideUnknown materialsCysteine thiolateTryptophan
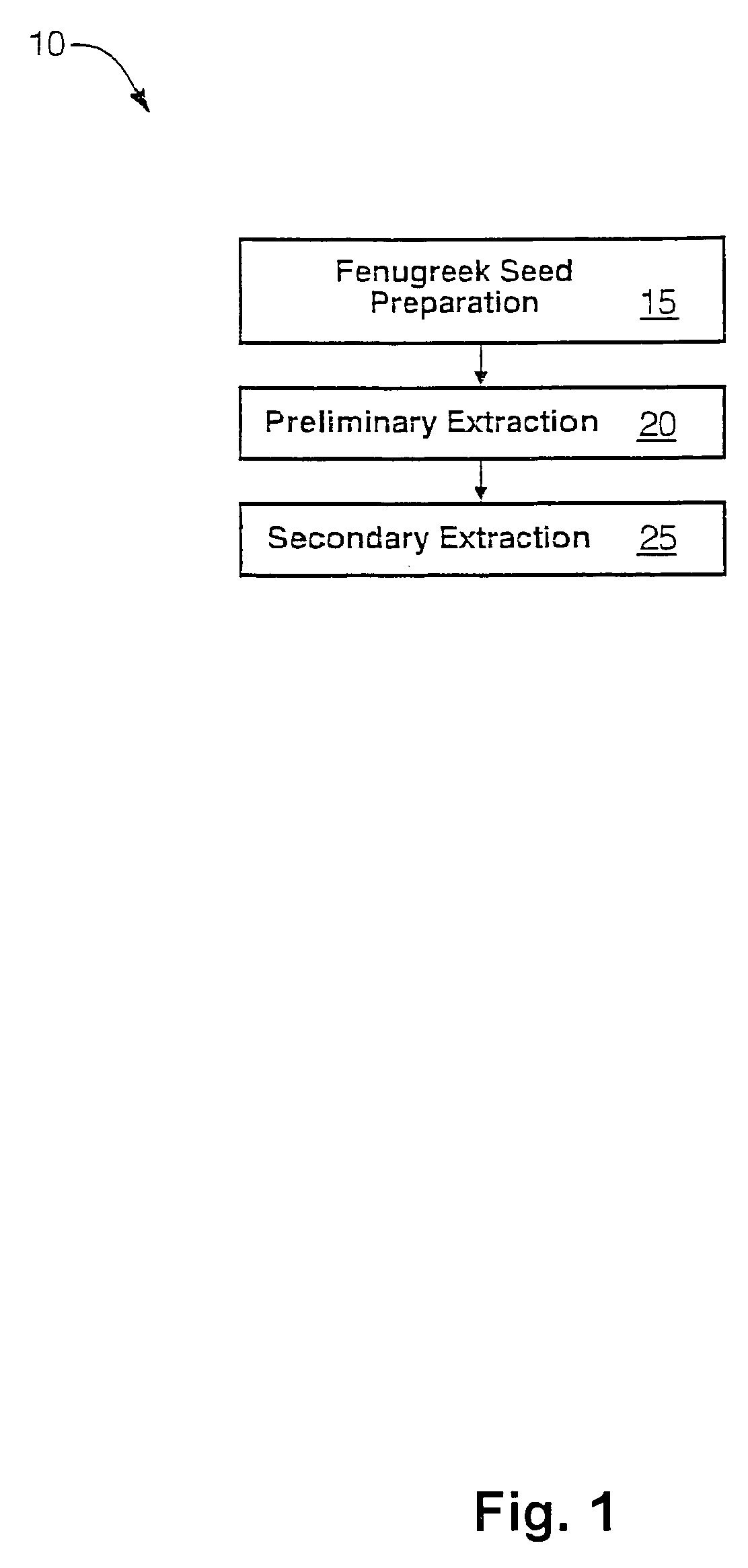
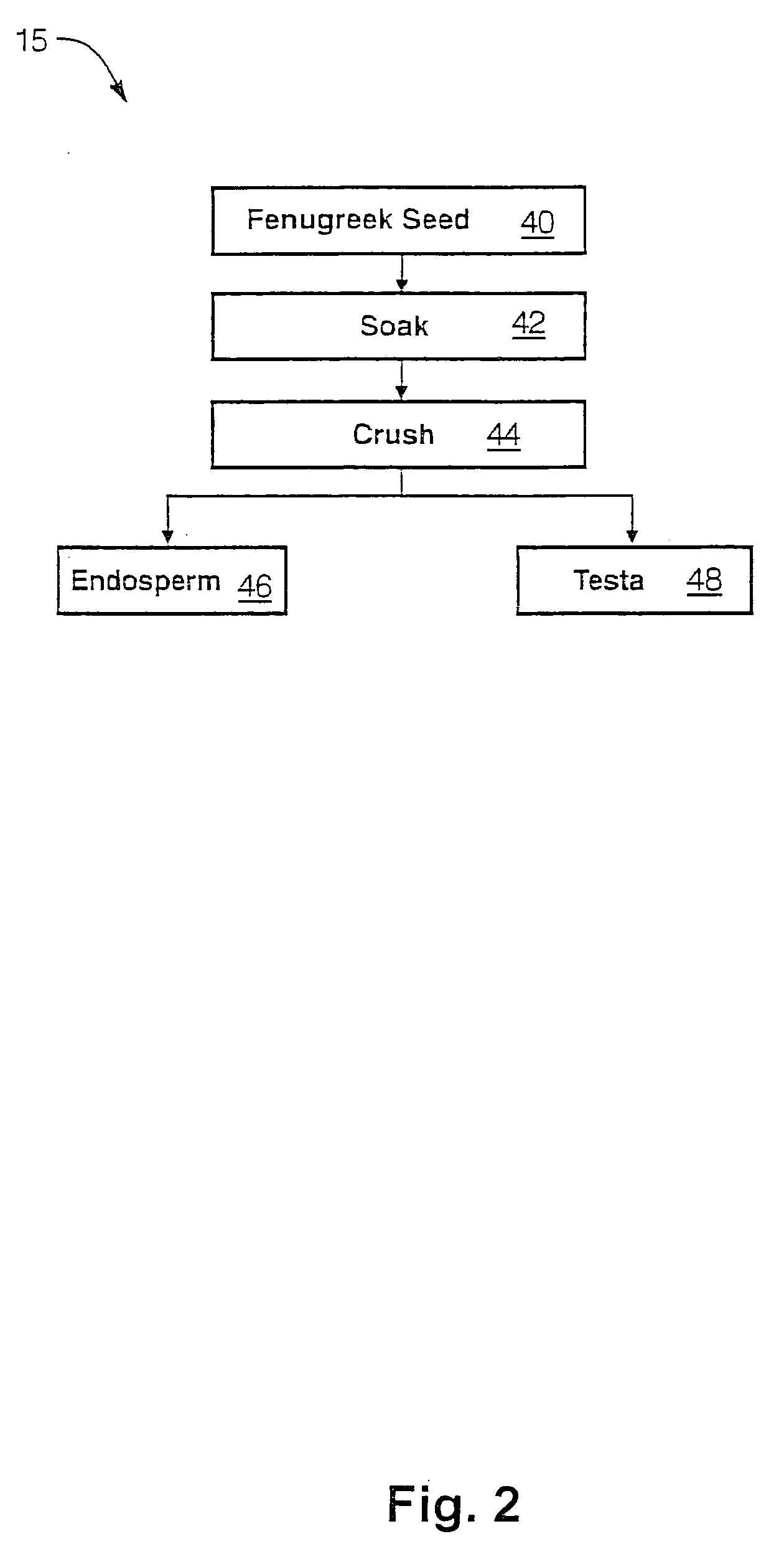
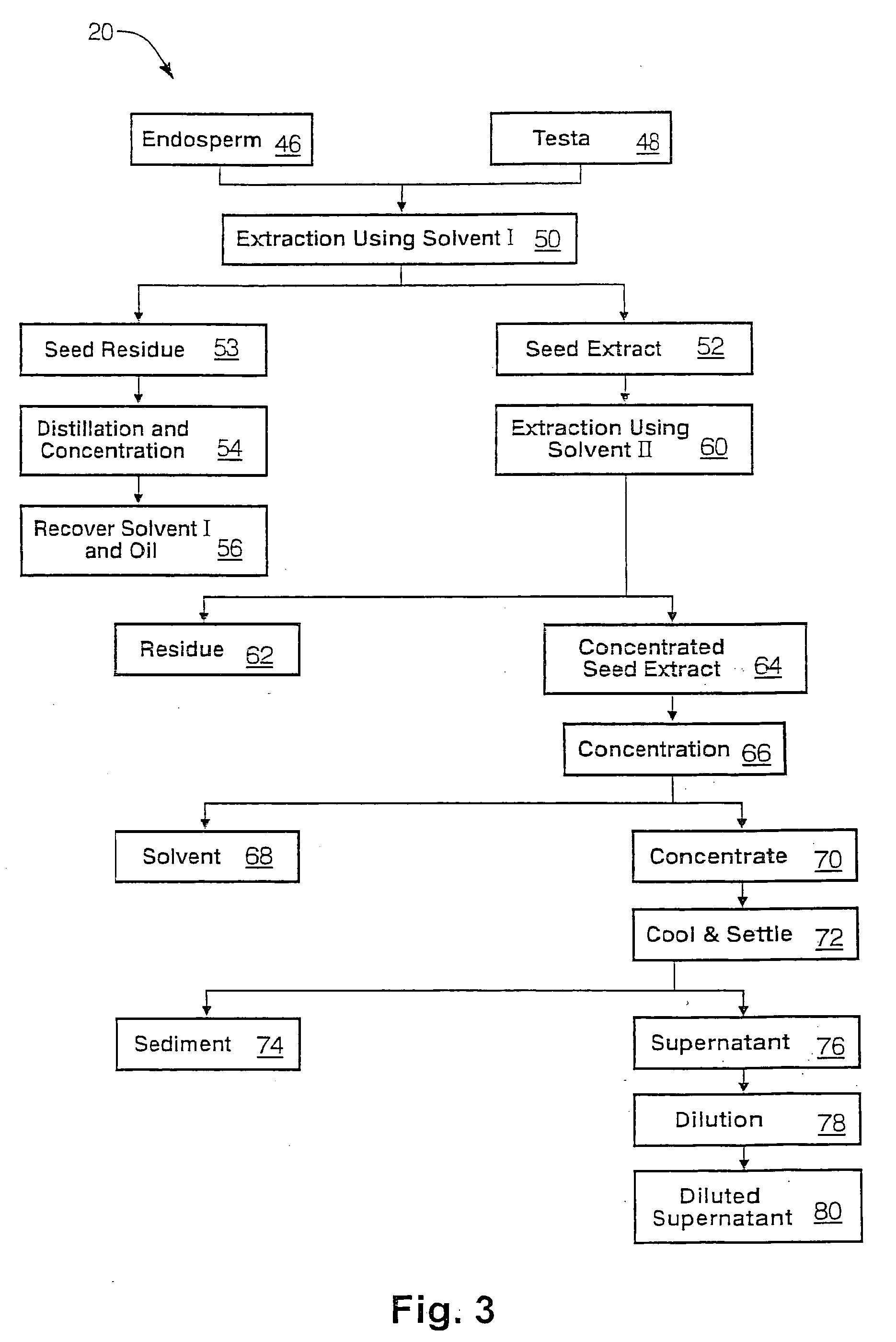
The present invention is directed to novel compositions of bio-active compounds comprising 4-hydroxyisoleucine and one or more compounds selected from the group of amino acids, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Preferably, the novel compositions of bio-active compounds include 4-hydroxyisoleucine and one or more amino acids selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, proline, lysine, histidine, and gamma-aminobutyrate. The composition of bio-active compounds preferably include between about ten percent and about seventy percent of 4-hydroxyisoleucine and between about twenty percent and about forty percent of other amino acids. The bio-active compounds of the novel composition of the present invention may be derived, isolated, and / or extracted from Fenugreek seeds. A preferred method for extracting the bio-active compounds from Fenugreek seeds includes the steps of: (1) providing a plurality of Fenugreek seeds; (2) preparing the Fenugreek seeds; and (3) extracting a novel composition of bio-active compounds from the Fenugreek seeds, which include a preliminary extraction step and a secondary extraction step. The compositions of bio-active compounds have been found to be helpful in restoring healthy energy balance in humans and animals, aiding in weight management efforts, and for balancing blood sugar levels by way of assisting the body to make more efficient use of existing (i.e., endogenous) insulin.
Owner:TSI INC
Prevention of incorporation of non-standard amino acids into protein
ActiveUS20070009995A1Cellular level is reducedReducing, or substantially eliminating, endogenous cellular levels of norleucineBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
Medicinal compositions & therapeutic methods
The compositions of this invention comprise a mixture of (1) phenylalanine and a dietary food supplement, (2) leucine and a dietary food supplement, and (3) hydrocinnamic acid and a dietary food supplement. The compositions are used for medicinal purposes to alleviate a variety of maladies.
Owner:WELLER HEALTH
Peptide, a method for its preparation and a pharmaceutical composition containing the peptide
A peptide of the formula Iwherein X is hydrogen, glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophan, gamma-aminobutyric acid or ζ-aminocaproic acid; A is D-gluptamic acid or D-y-glutamic acid; and Y is glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophn, gamma-aminobutyric acid, ζ-aminocaproic acid, hydroxyl, or an amide group.
Owner:IMMUNOTECH DEV
Sport drink containing amino acids and carbohydrates
InactiveUS20070270355A1Quick changeSpeed recovery timeBiocidePeptide/protein ingredientsArginineTryptophan
A composition includes a plurality of amino acids. The plurality of amino acids includes at least one essential amino acid and at least one non-essential amino acid. The plurality of amino acids also includes at least one branch-chain amino acid. The composition also includes a source of carbohydrates. The compositions also includes purified water. The plurality of amino acids comprises about 1 wt % of the composition. A composition includes a plurality of amino acids, sodium citrate, sodium chloride, potassium phosphate, flavoring, a source of carbohydrates, and purified water. In some embodiments of the composition, the plurality of amino acids includes alanine, arginine, aspartate, cystine, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonin, tryptophan, tyrosine, and valine.
Owner:GARCIA RAMON D +3
Methods for affecting homeostasis and metabolism in a mammalian body
InactiveUS20050233014A1Effective quantityLow production costOrganic active ingredientsBiocideCysteine thiolateTryptophan
The present invention is directed to novel compositions of bio-active compounds comprising 4-hydroxyisoleucine and one or more compounds selected from the group of amino acids, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Preferably, the novel compositions of bio-active compounds include 4-hydroxyisoleucine and one or more amino acids selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, proline, lysine, histidine, and gamma-aminobutyrate. The composition of bio-active compounds preferably include between about ten percent and about seventy percent of 4-hydroxyisoleucine and between about twenty percent and about forty percent of other amino acids. The bio-active compounds of the novel composition of the present invention may be derived, isolated, and / or extracted from Fenugreek seeds. A preferred method for extracting the bio-active compounds from Fenugreek seeds includes the steps of: (1) providing a plurality of Fenugreek seeds; (2) preparing the Fenugreek seeds; and (3) extracting a novel composition of bio-active compounds from the Fenugreek seeds, which include a preliminary extraction step and a secondary extraction step. The compositions of bio-active compounds have been found to be helpful in restoring healthy energy balance in humans and animals, aiding in weight management efforts, and for balancing blood sugar levels by way of assisting the body to make more efficient use of existing (i.e., endogenous) insulin.
Owner:TSI INC
Elastin peptide analogs and methods of using same
InactiveUS6506731B1Increase elasticityImprove functionalityCosmetic preparationsDipeptide ingredientsGlycineElastin peptides
The present invention is directed to a composition which is used to enhance the elasticity and / or appearance of tissue. Specifically, the present invention is directed to a composition formulated from peptides having low molecular weights and which substantially correspond to sequences found in elastin More preferably, the present invention corresponds to the general formula R1-Leucine-Glycine-Alanine-Glycine-Glycine-Alanine-Glycine-R2.
Owner:CONNECTIVE TISSUE IMAGINEERING
Pharmaceutical composition containing 18 kinds of amino acid
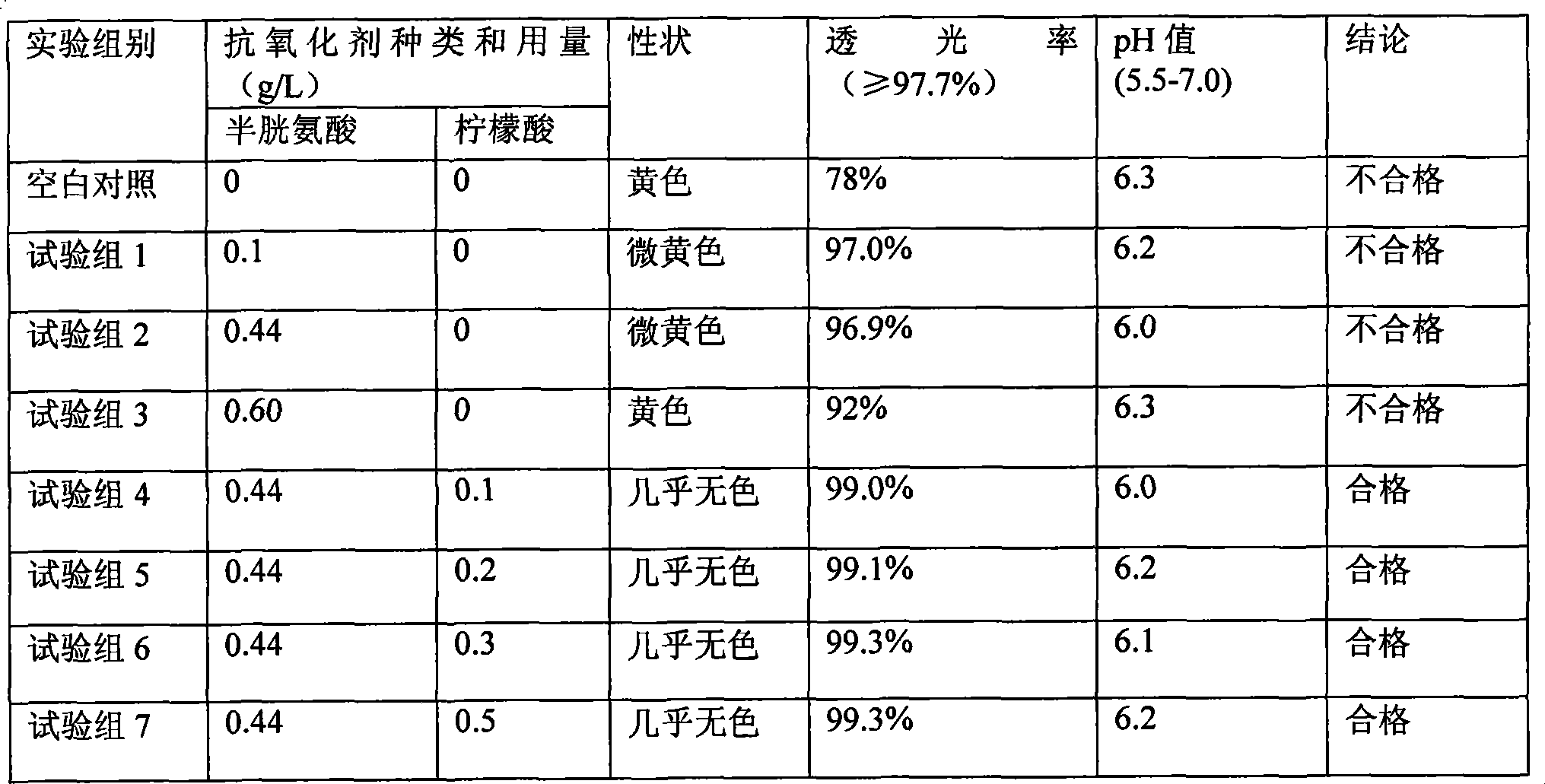
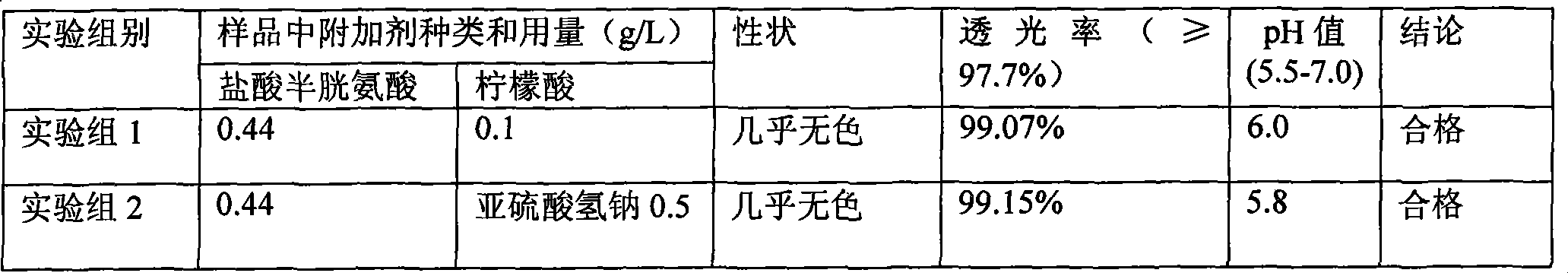
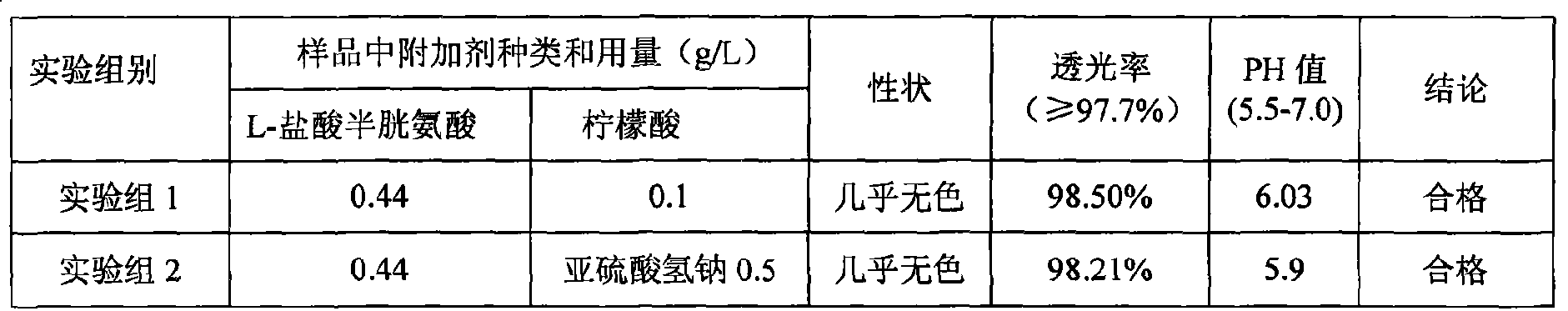
ActiveCN101439036AInhibition of oxidative decomposition reactionsQuality assuranceOrganic active ingredientsMetabolism disorderAntioxidantTryptophan
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-V) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 2.89 of arginine hydrochloride, 2.46 of histidine hydrochloride, 3.79 of leucine, 1.70 of isoleucine, 3.33 of lysine hydrochloride, 2.83 of phenylalanine, 1.97 of threonine, 1.36 of valine, 1.06 of methionine, 0.39 of tryptophan, 3.24 of glycine, 1.88 of alanine, 1.00 of proline, 0.11 of tyrosine, 0.67 of serine, 0.44 of cysteine hydrochloride, 1.15 of aspartic acid, 1.97 of glutamic acid, 50 of xylitol, 0.10 to 0.30 of citric acid and injection water with proper amount. The pharmaceutical composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test and a quality test, results show that the pharmaceutical composition is as stable as or more stable than like products (18AA-V) which are sold in the markets and contain sulfites.
Owner:福州凯瑞医药咨询有限公司
Cyclic dipeptides as feed additives
Feed additives containing essential amino acids which are diketopiperazines of formulas (IV) or (V) or salts thereof are provided:In formulas (IV) and (V), R1 and R2 may be an amino acid residue such as methionine, lysine, threonine, tryptophan, histidine, valine, leucine, isoleucine, phenylalanine, arginine, and cysteine, and may optionally be the same residue.Additionally provided are the diketopiperazines of formulas (IV) and (V) and a method to for their production.
Owner:EVONIK DEGUSSA GMBH
Skin external preparation
InactiveUS20080058400A1Good moisturizing effectMaintain abilityCosmetic preparationsBiocideHydroxyprolineTyrosine
A skin external preparation includes at least arginine, aspartic acid, isoleucine, leucine, lysine, threonine, glycine, histidine, serine, valine, tyrosine, cysteine, phenylalanine, hydroxyproline and acylglutamine among amino acids, or salts thereof, and a skin external preparation includes: at least arginine, aspartic acid, isoleucine, leucine, lysine and threonine among amino acids, or salts thereof, and a hydrolyzed silk.
Owner:FUJIFILM CORP
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Compositions, methods and kits for reducing lipid levels
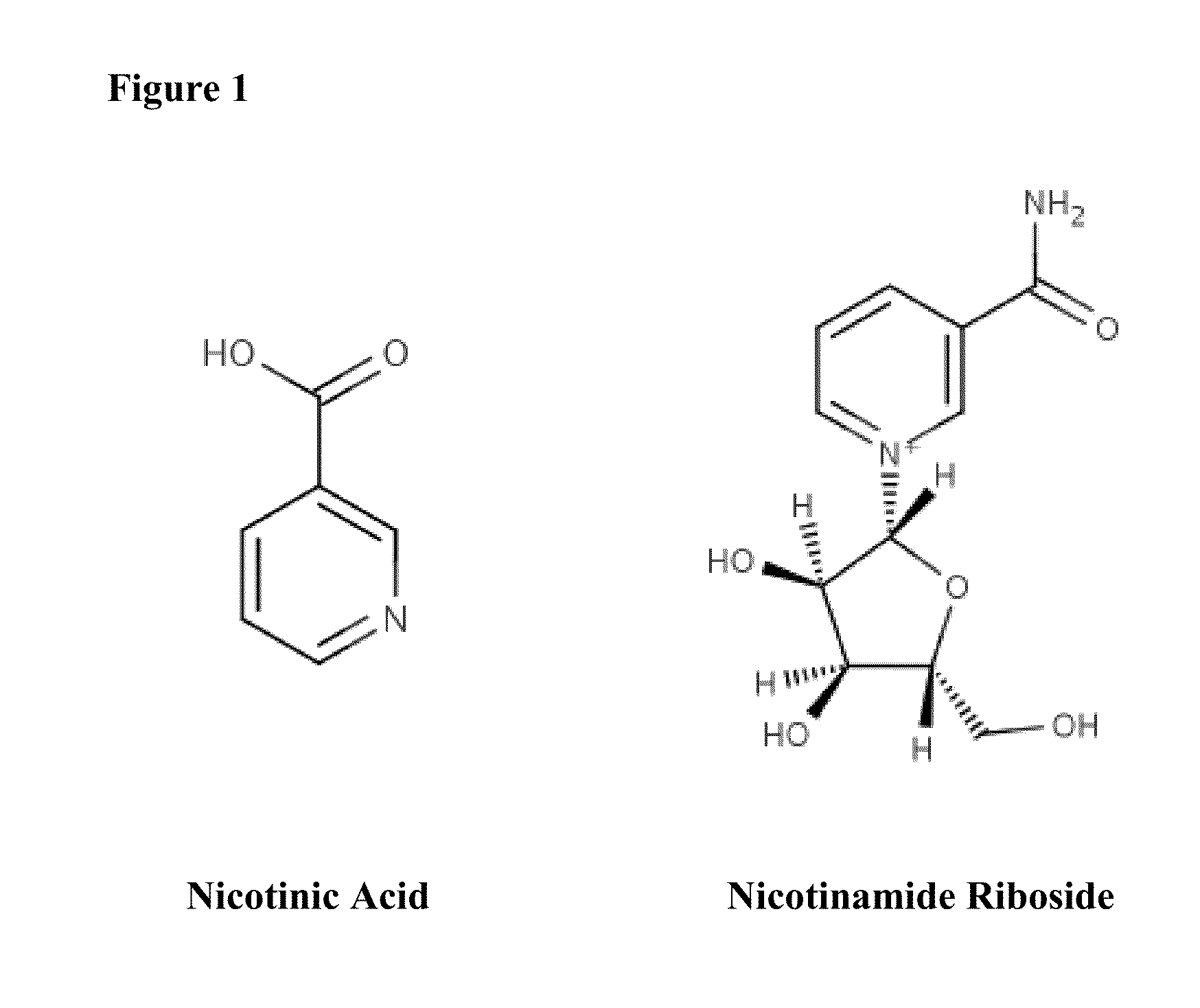
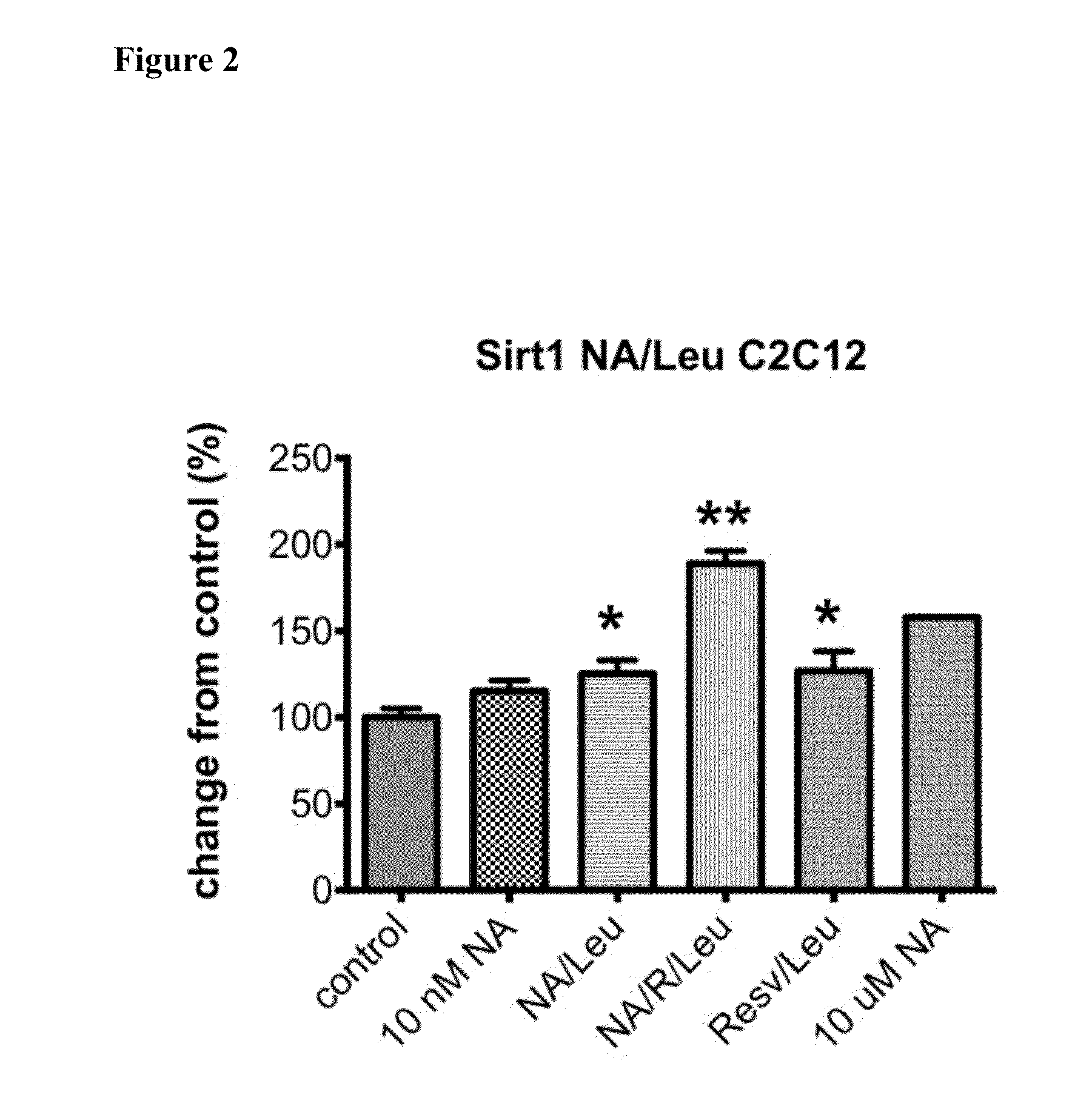
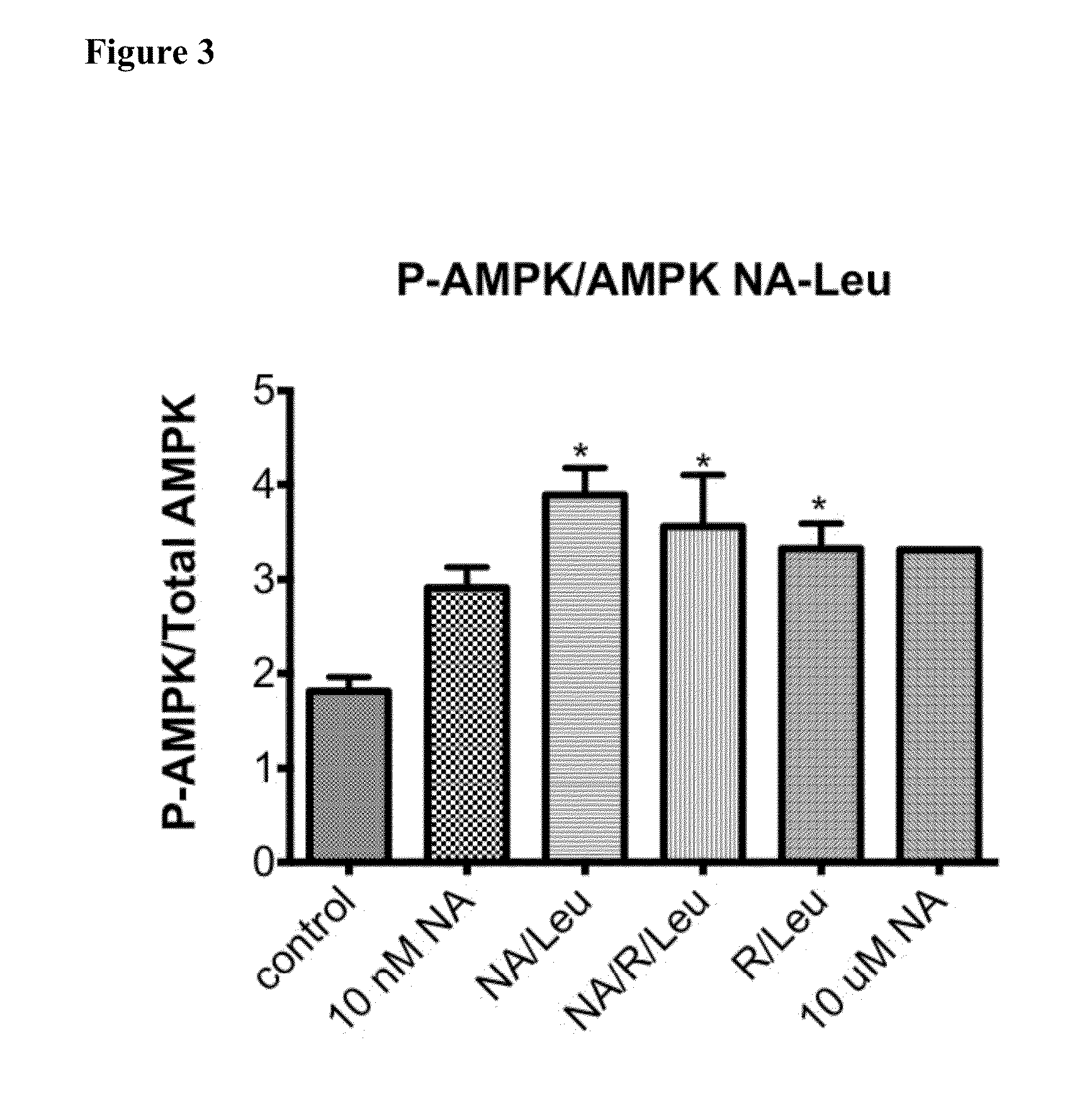
ActiveUS20150056274A1Lower cholesterol levelsLow in lipidsBiocideHydroxy compound active ingredientsMetaboliteNicotinamide riboside
Compositions, methods, and kits useful for treating hyperlipidemic conditions are provided herein. Such compositions can contain synergizing amounts of nicotinic acid, nicotinamide riboside and / or nicotinic acid metabolites in combination with leucine and / or a leucine metabolite, with or without resveratrol.
Owner:NUSIRT SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com