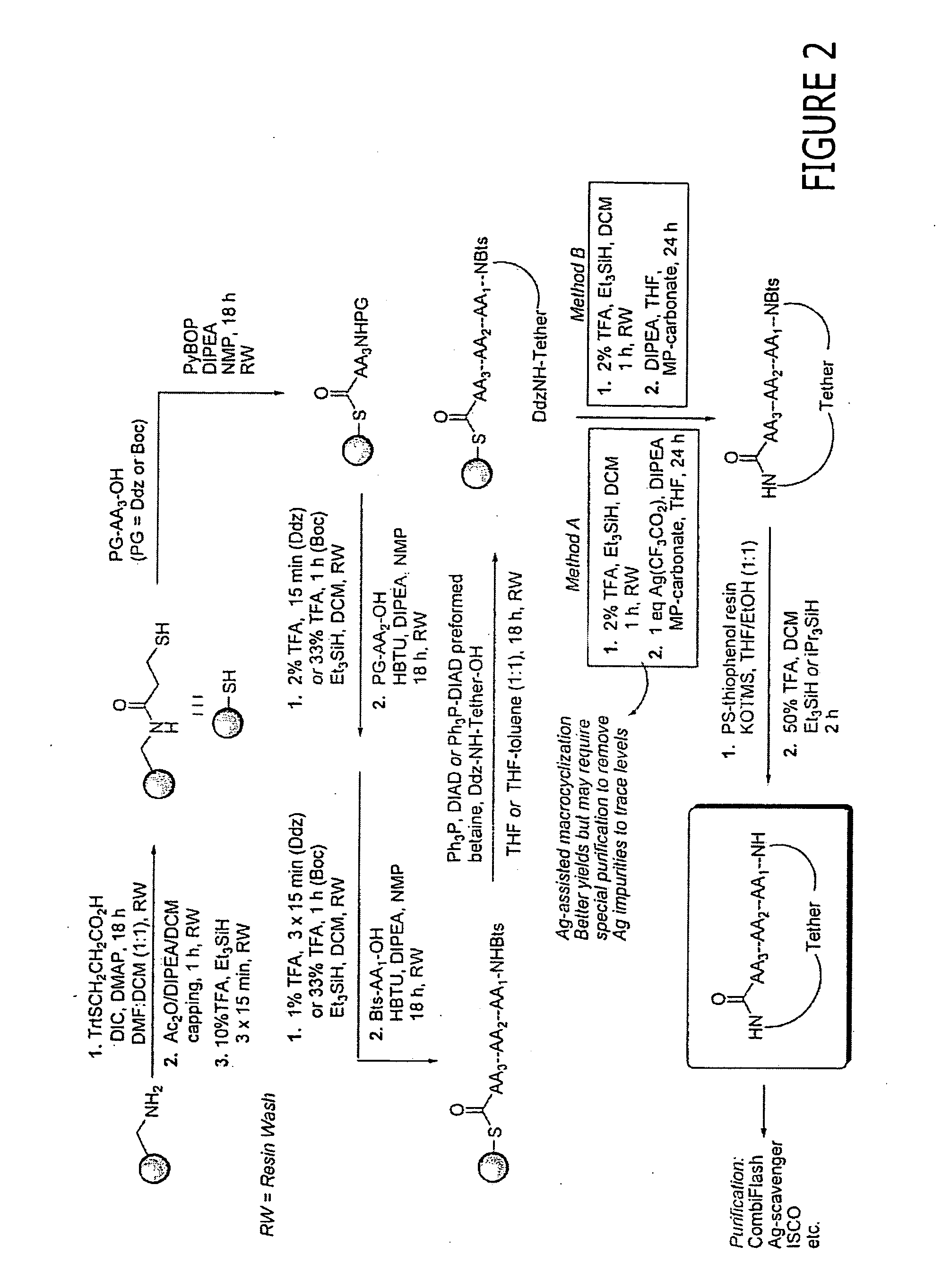
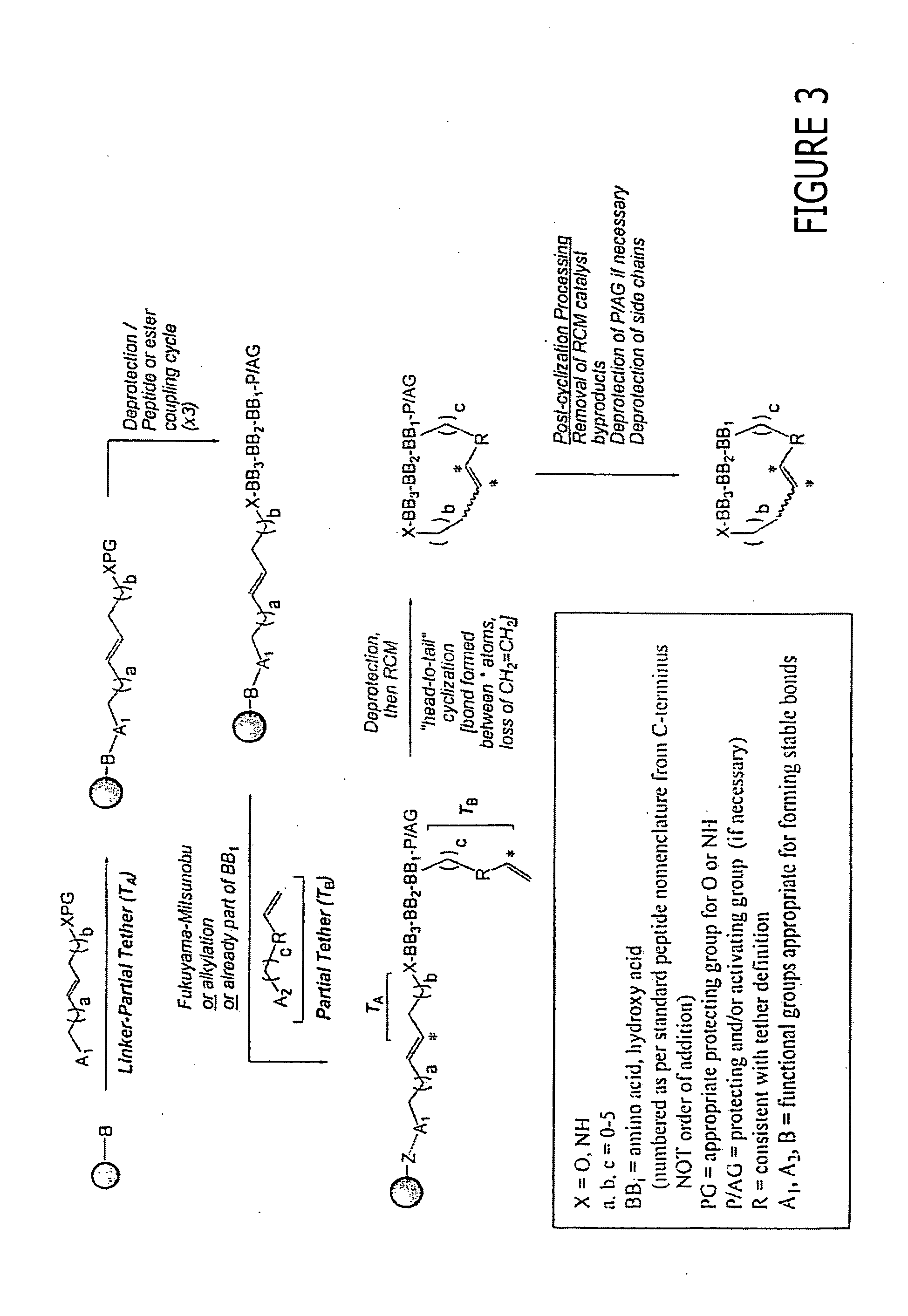
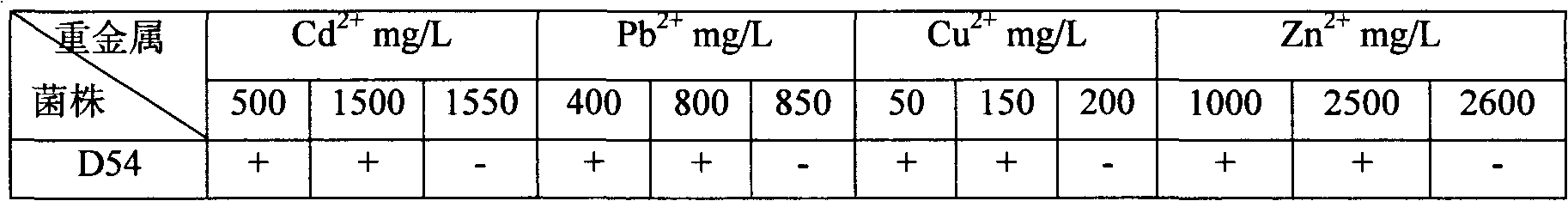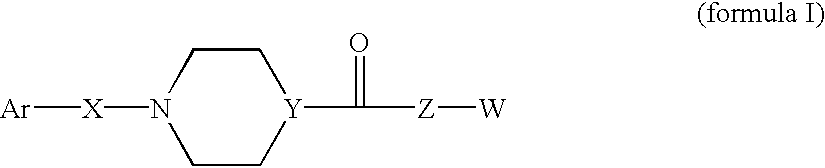Patents
Literature
1026 results about "Growth hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Growth hormone (GH) or somatotropin, also known as human growth hormone (hGH or HGH) in its human form, is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals. It is thus important in human development.GH also stimulates production of IGF-1 and raises the concentration of glucose and free fatty acids.
Cysteine variants of erythropoietin
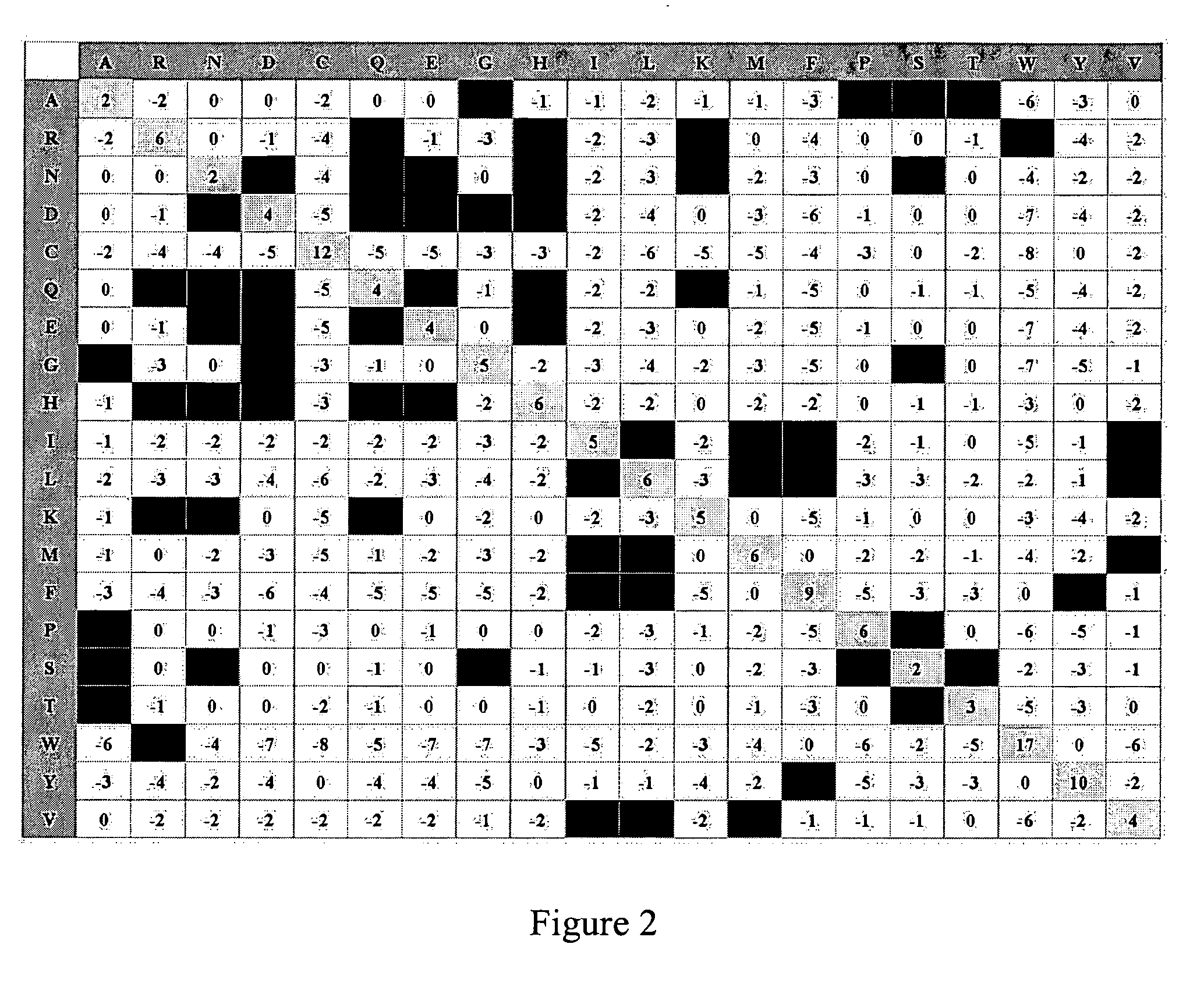
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
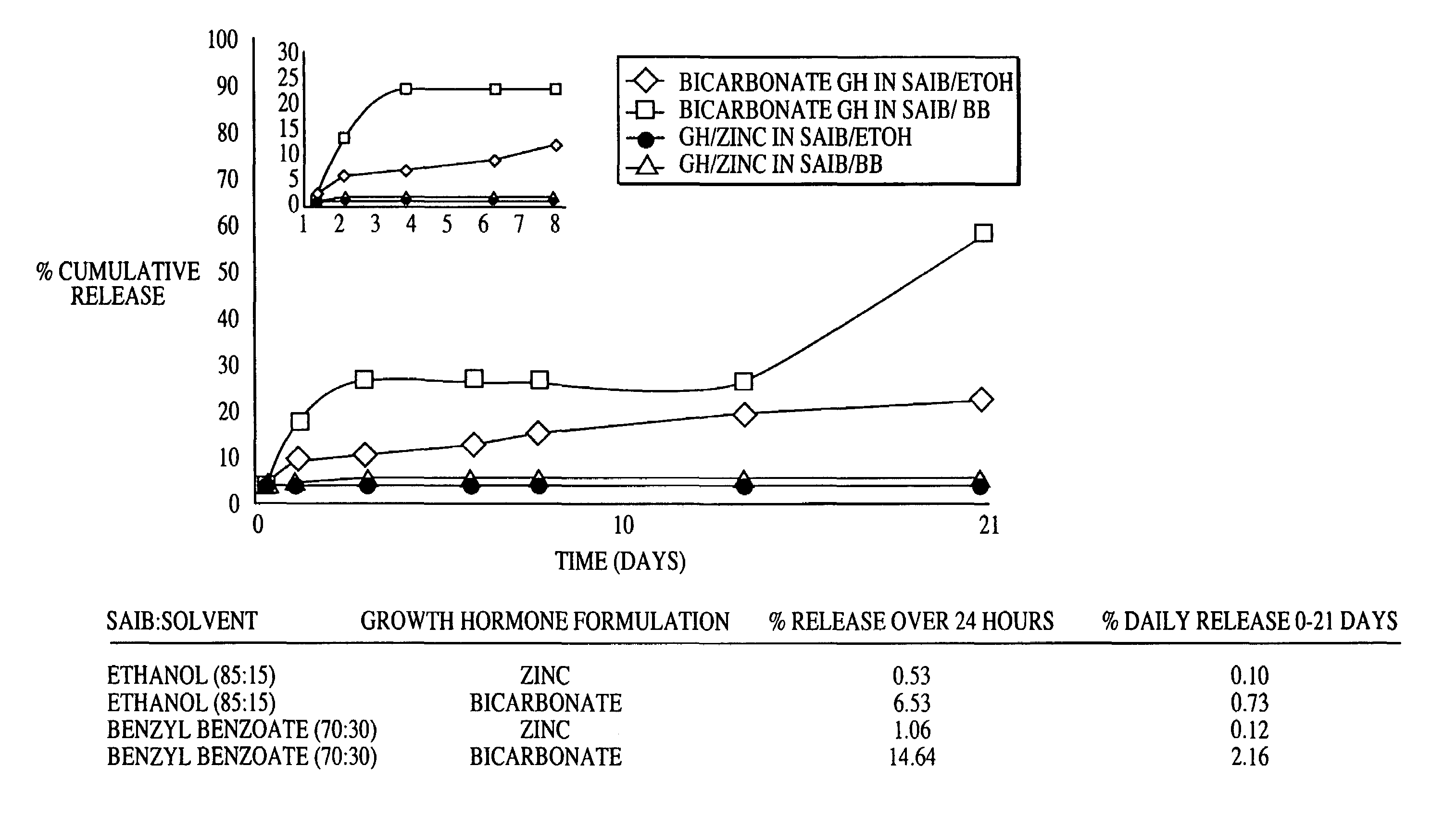
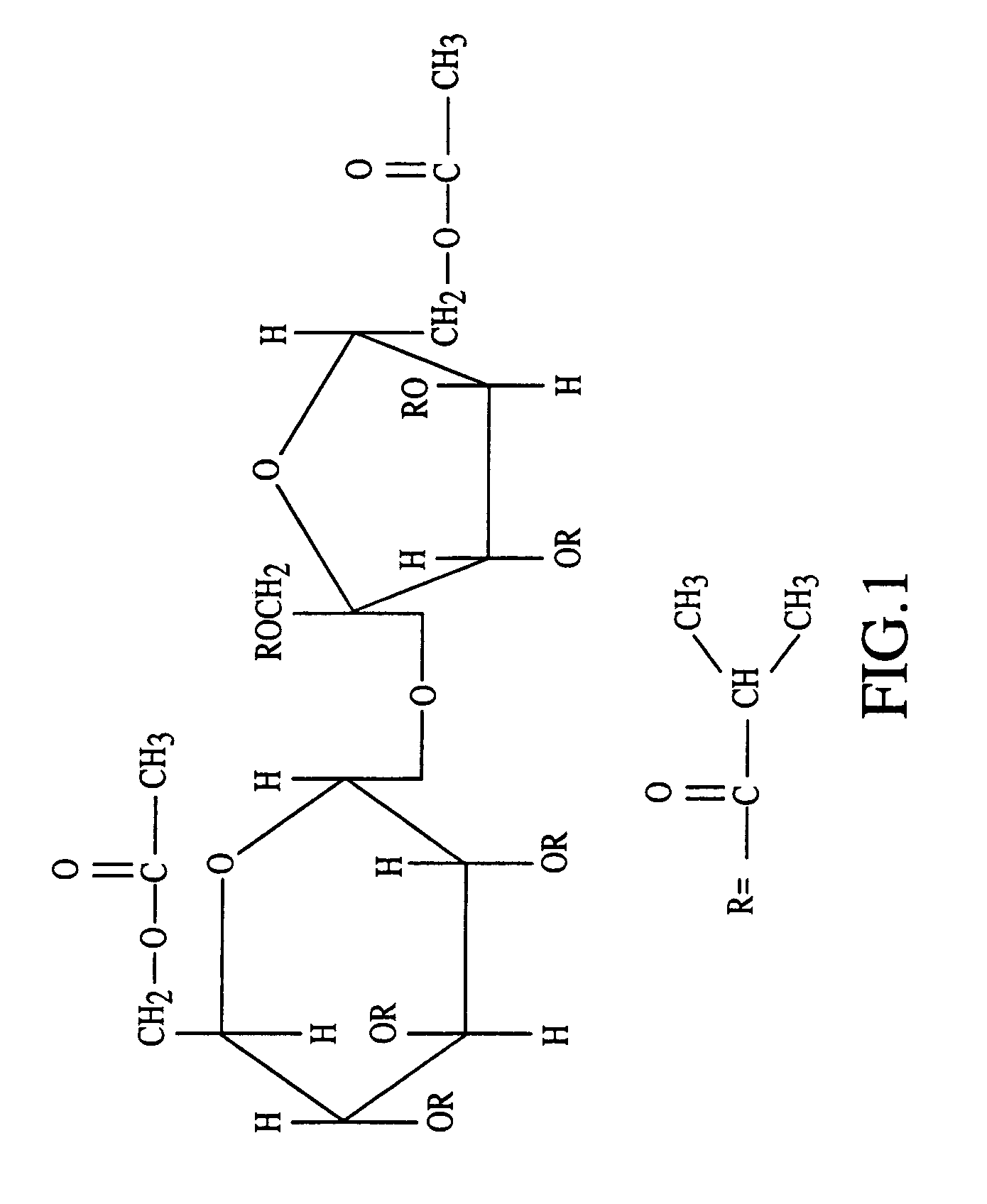
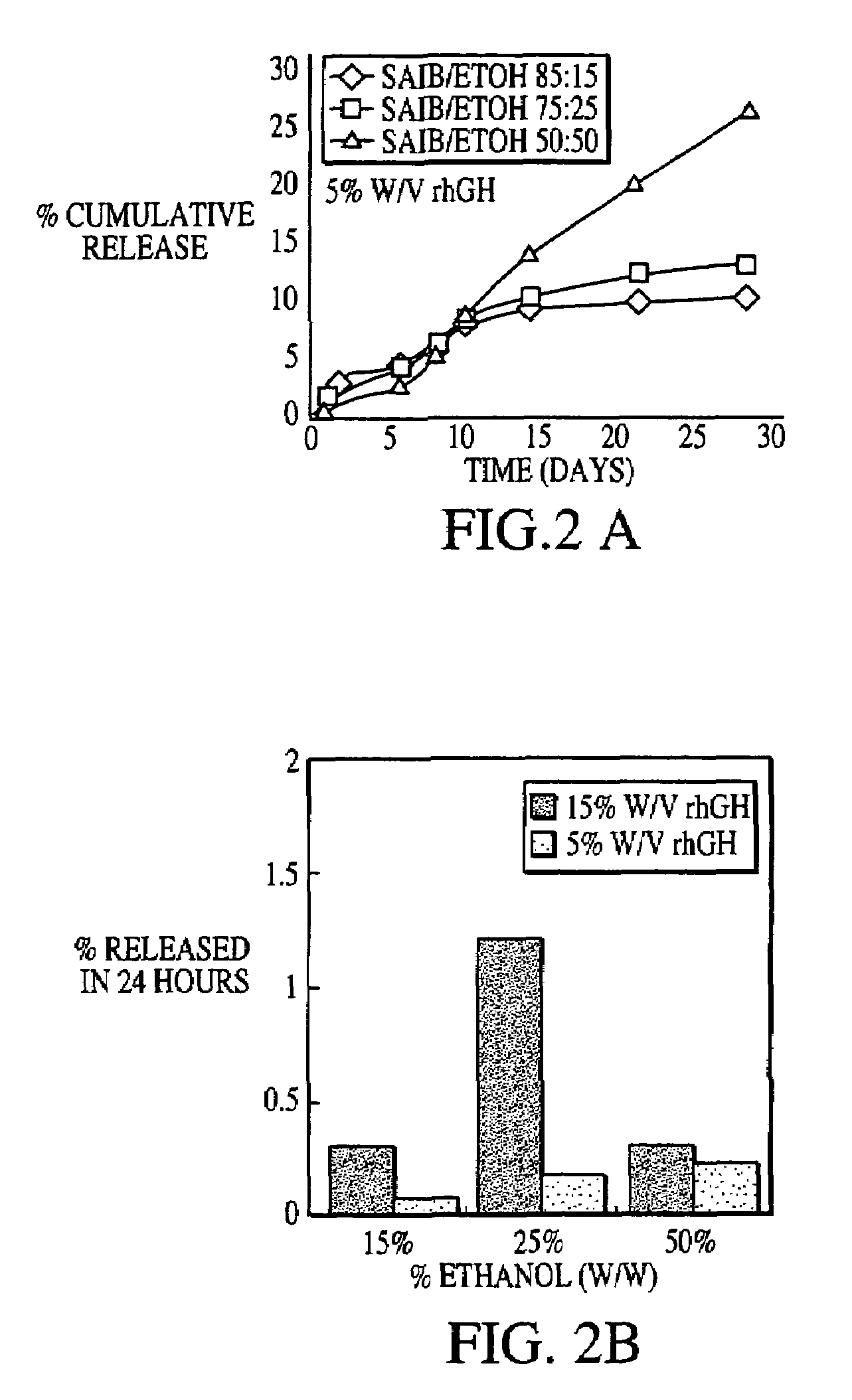
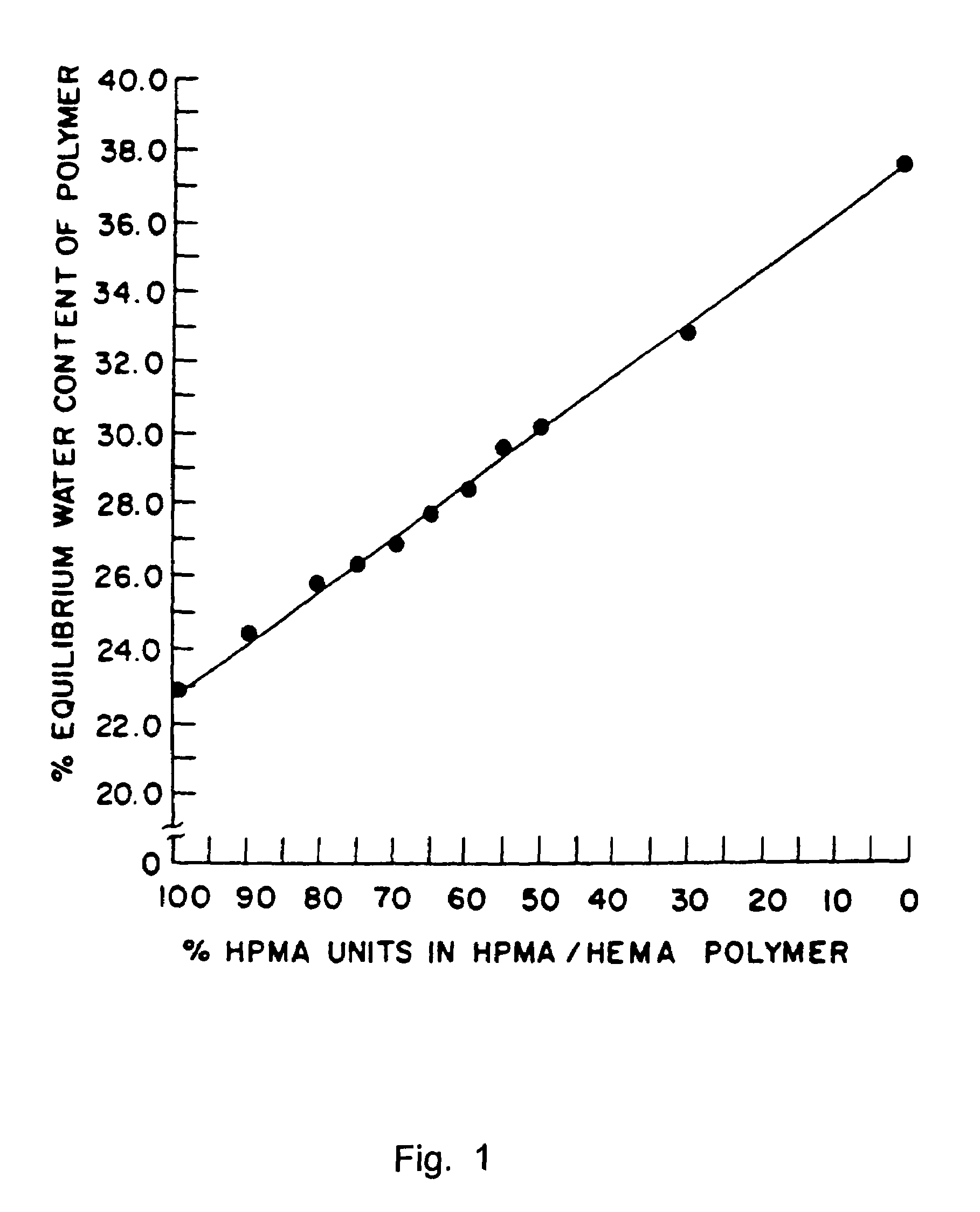
Sustained release formulations
A composition for sustained release comprises a carrier material containing a non-polymeric, non-water soluble liquid material having a viscosity of at least 5,000 cP at 37° C. that does not crytallize neat under ambient physiological conditions, a multivalent metal cation, and growth hormone.
Owner:DURECT CORP
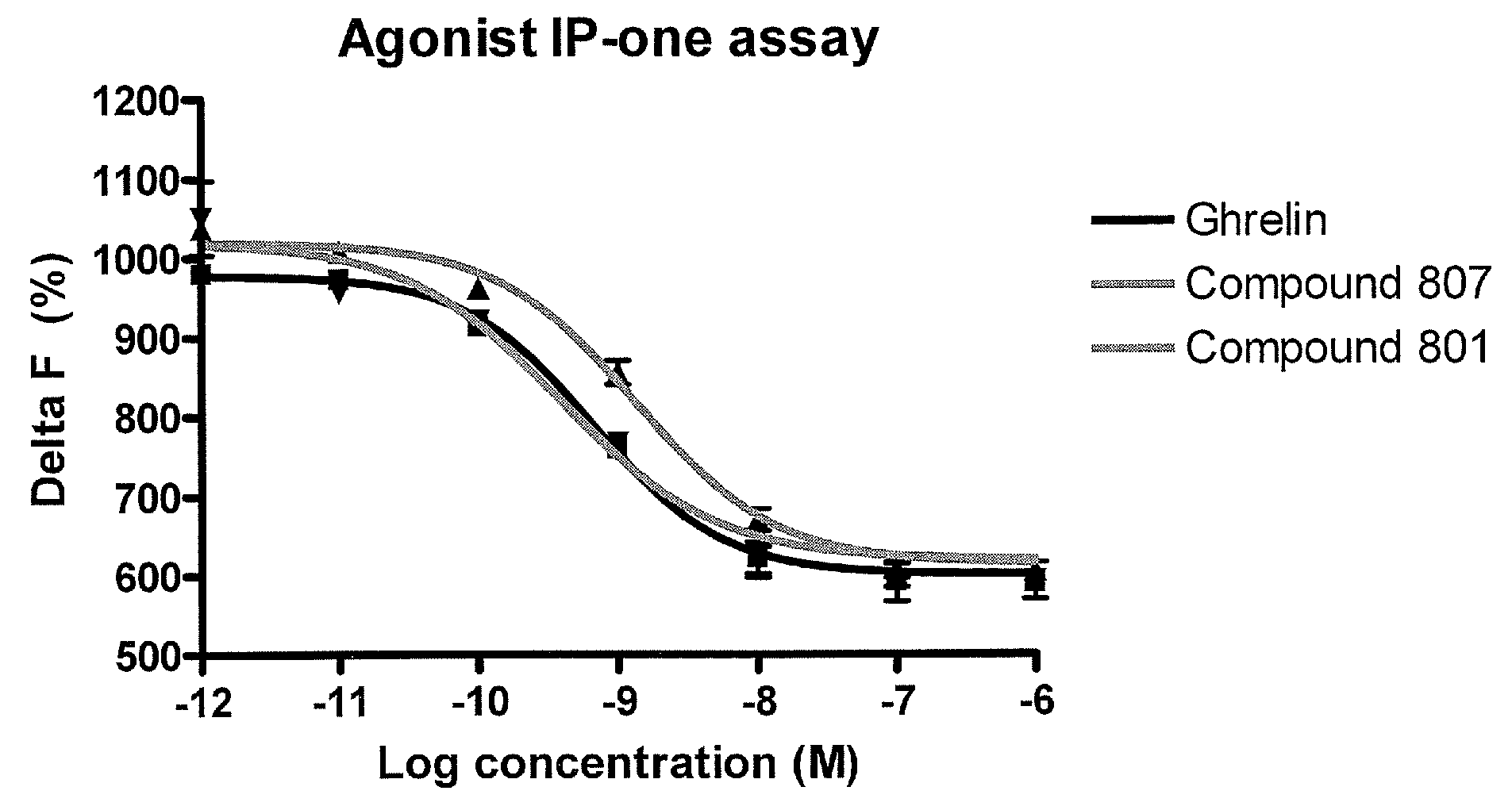
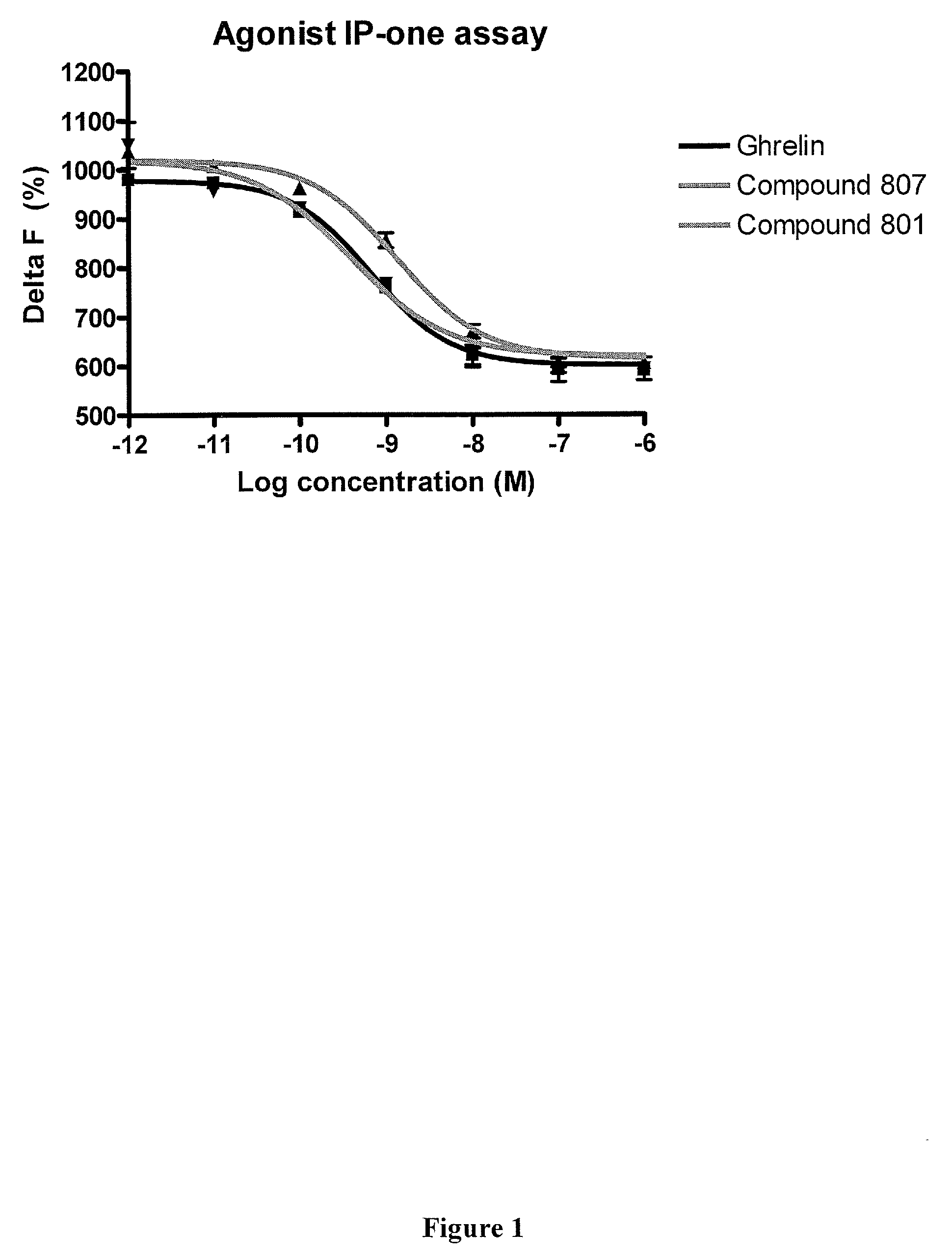
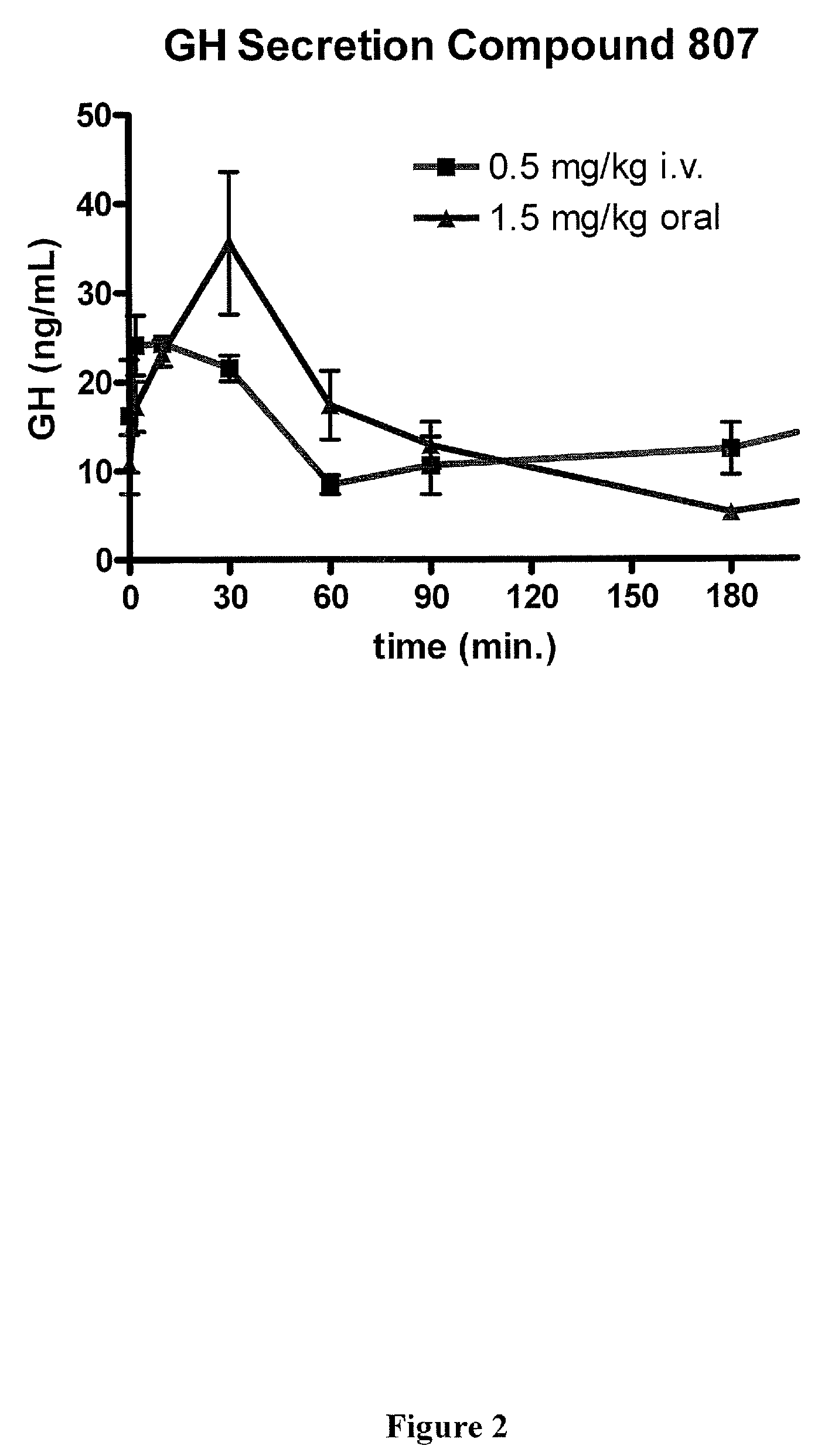
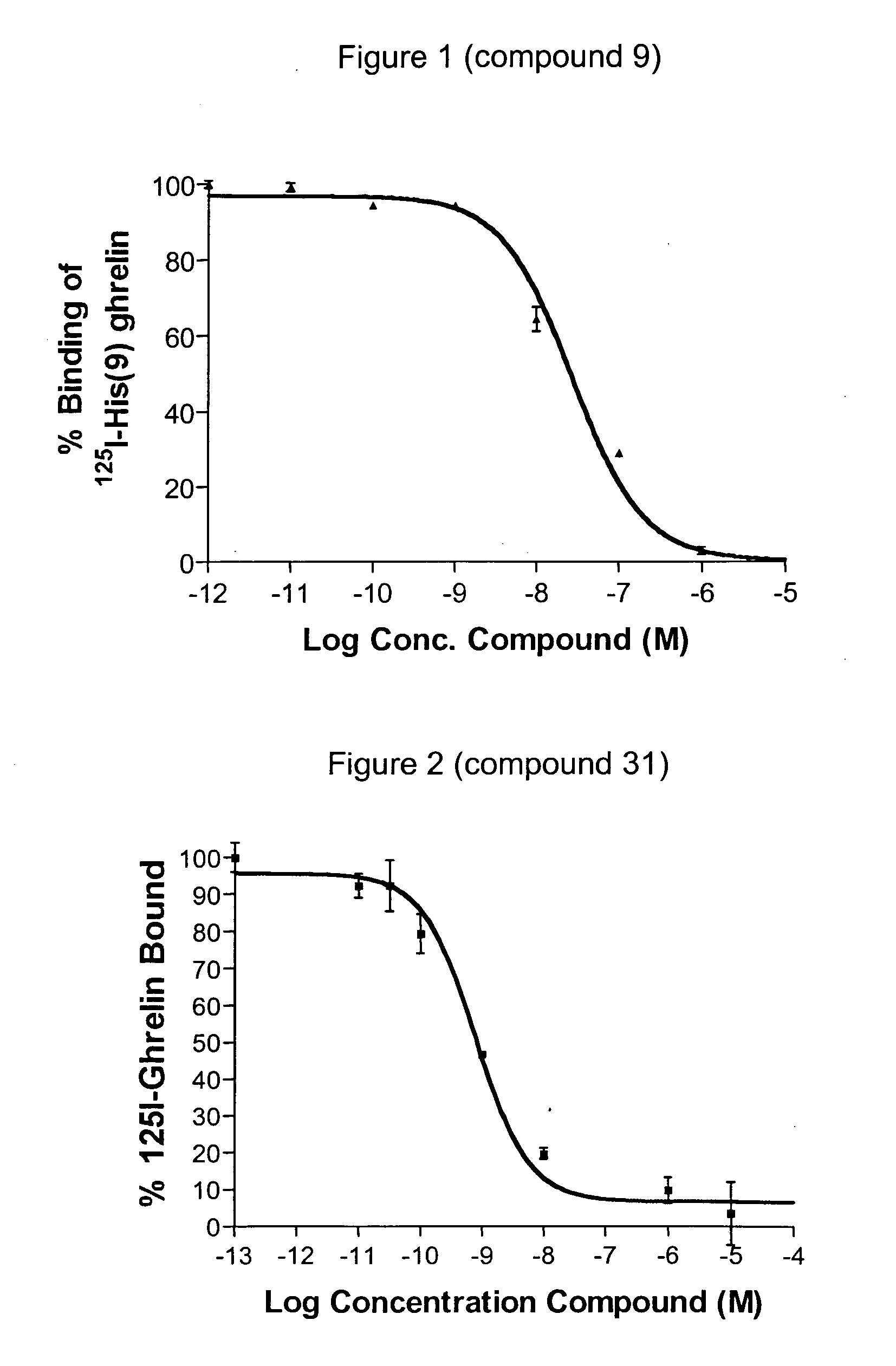
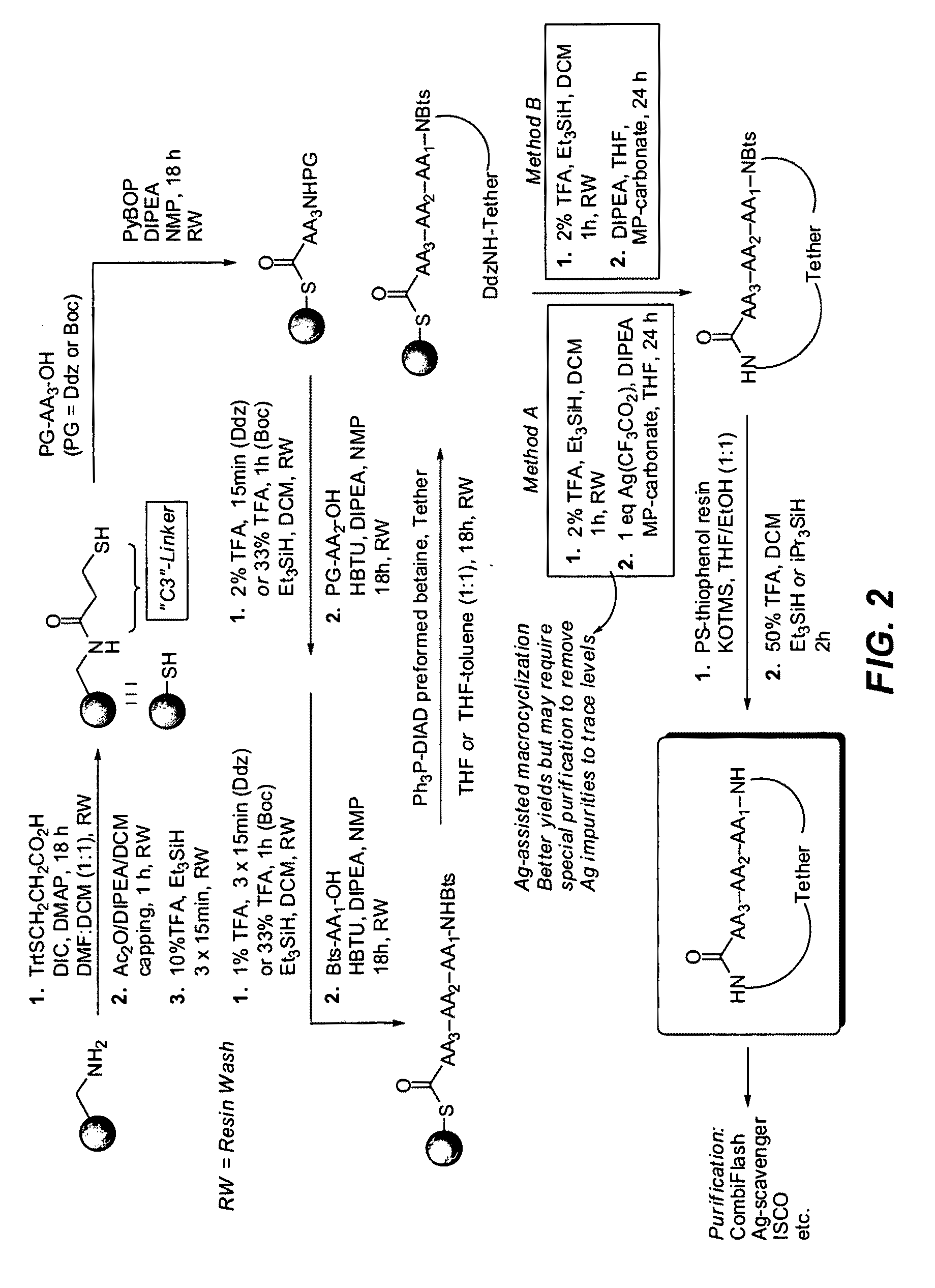
Methods of using macrocyclic modulators of the ghrelin receptor
ActiveUS20070021331A1Promote gastrointestinal motilityModulating activity of receptorBiocideTripeptide ingredientsGrowth hormone-releasing peptideGenetic disorder
The present invention provides novel conformationally-defined macrocyclic compounds that have been demonstrated to be selective modulators of the ghrelin receptor (growth hormone secretagogue receptor, GHS-R1a and subtypes, isoforms and variants thereof). Methods of synthesizing the novel compounds are also described herein. These compounds are useful as agonists of the ghrelin receptor and as medicaments for treatment and prevention of a range of medical conditions including, but not limited to, metabolic and / or endocrine disorders, gastrointestinal disorders, cardiovascular disorders, obesity and obesity-associated disorders, central nervous system disorders, genetic disorders, hyperproliferative disorders and inflammatory disorders.
Owner:OCERA THERAPEUTICS INC
Long-acting growth hormone and methods of producing same
ActiveUS20120035101A1Decreasing body fatReduce weight lossPeptide/protein ingredientsMetabolism disorderSomatotropic hormoneNucleotide
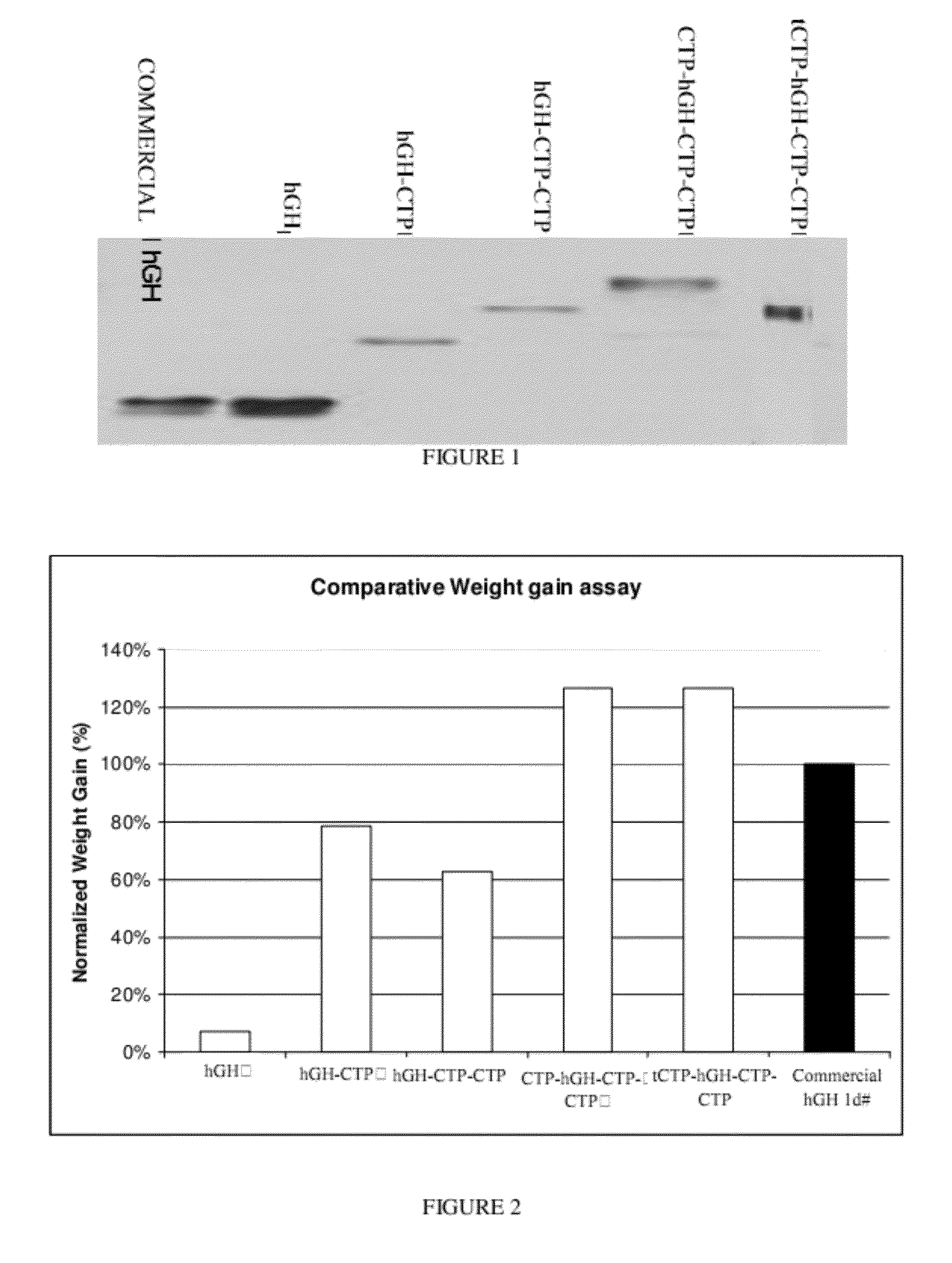
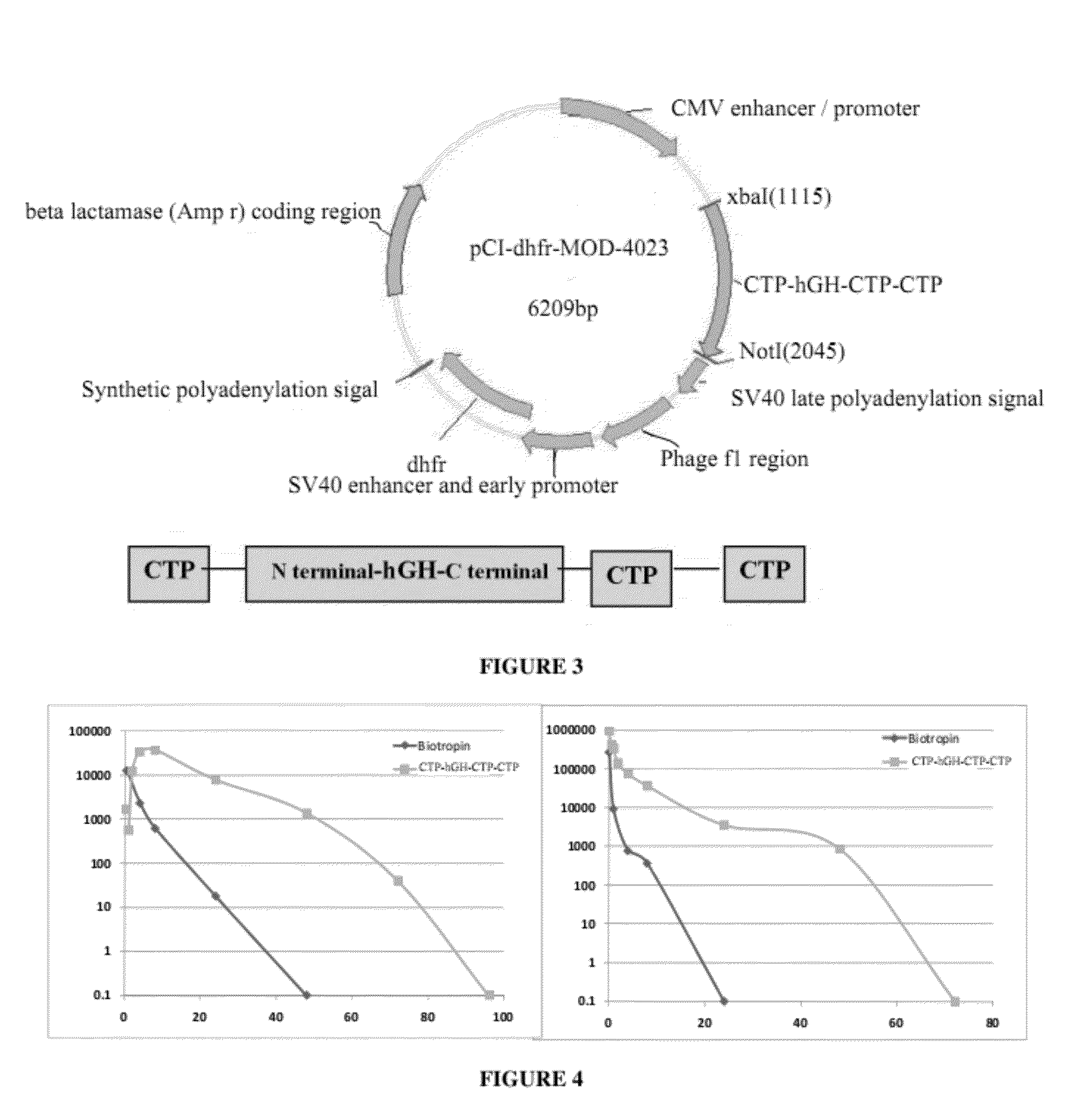
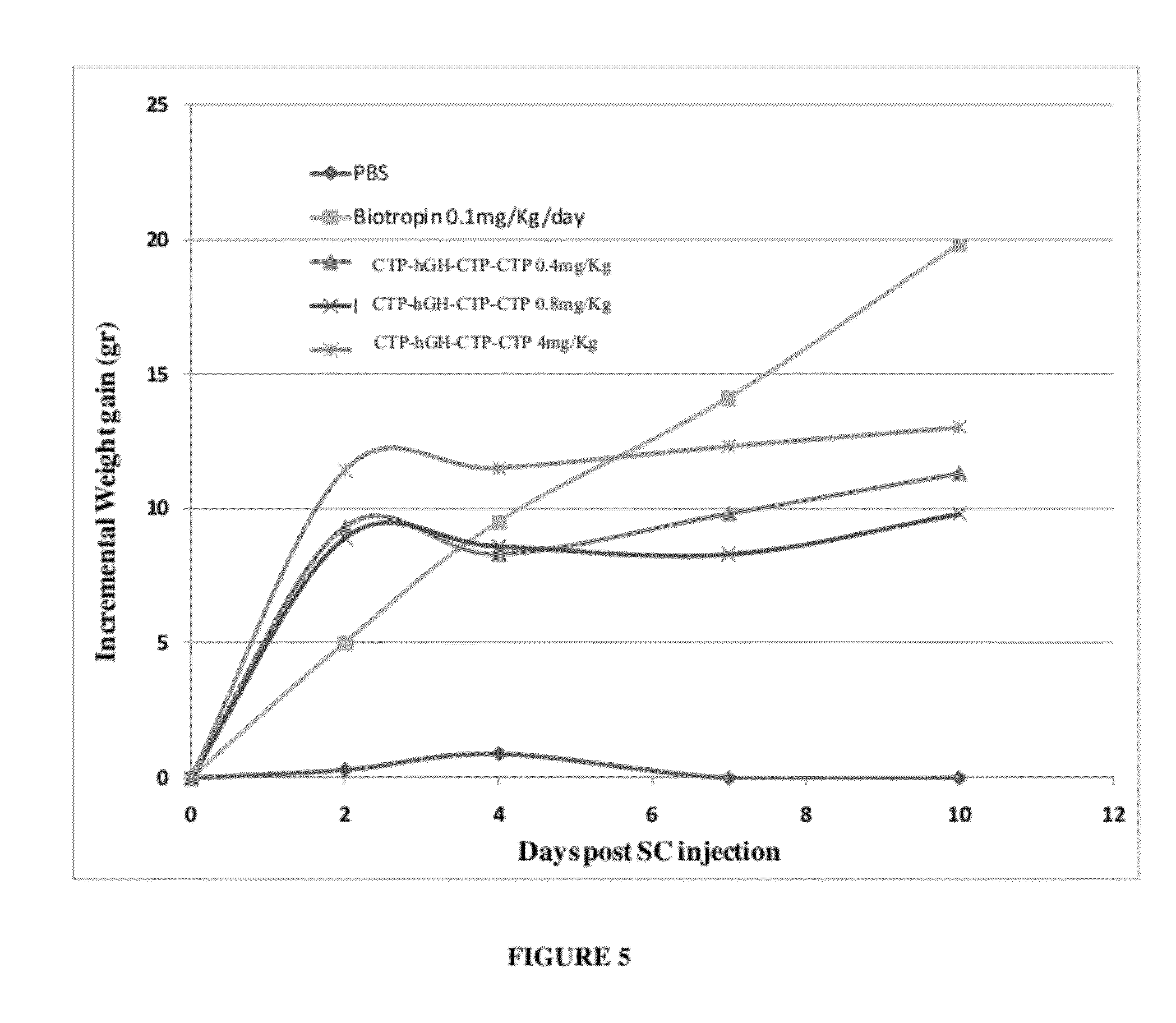
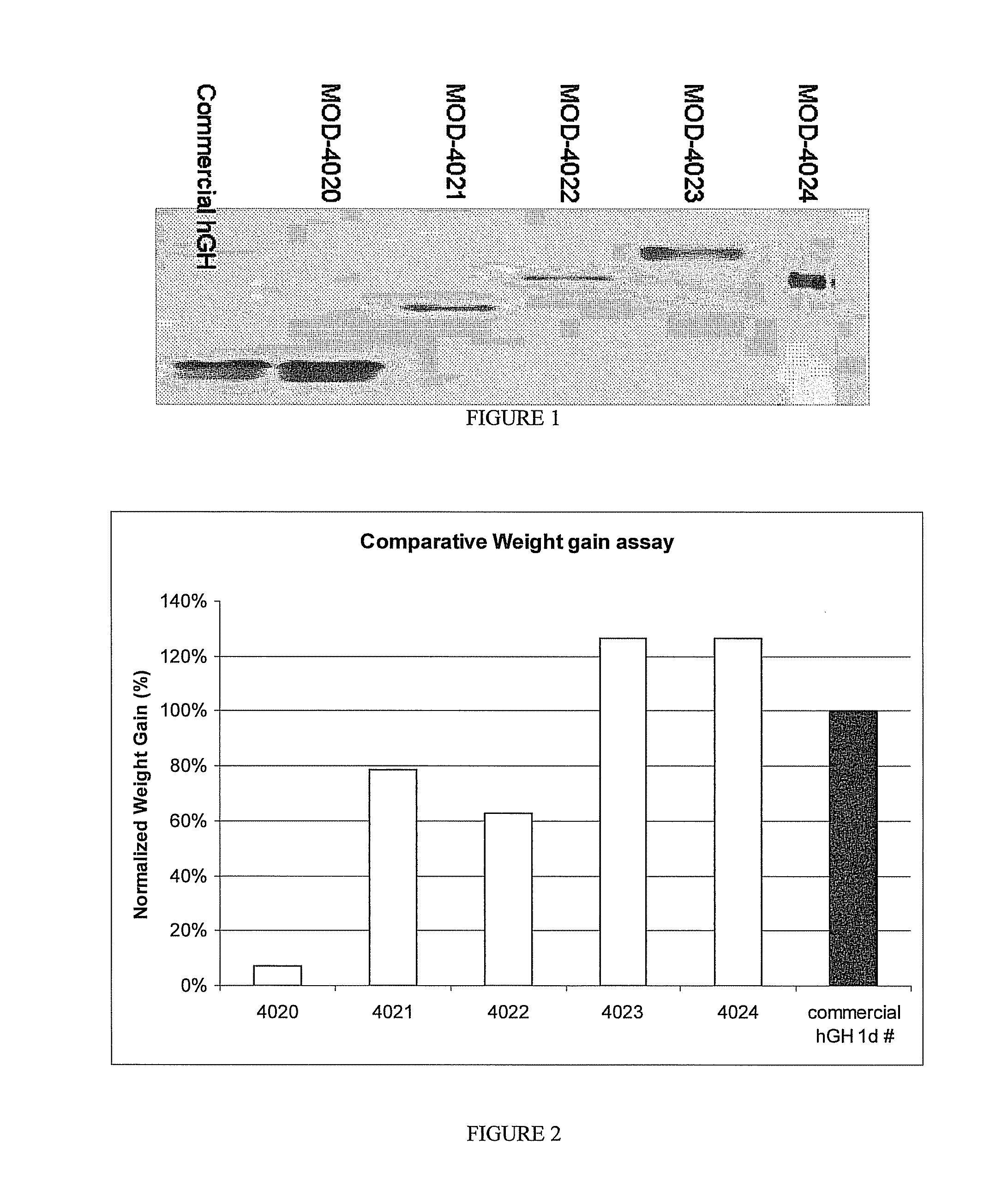
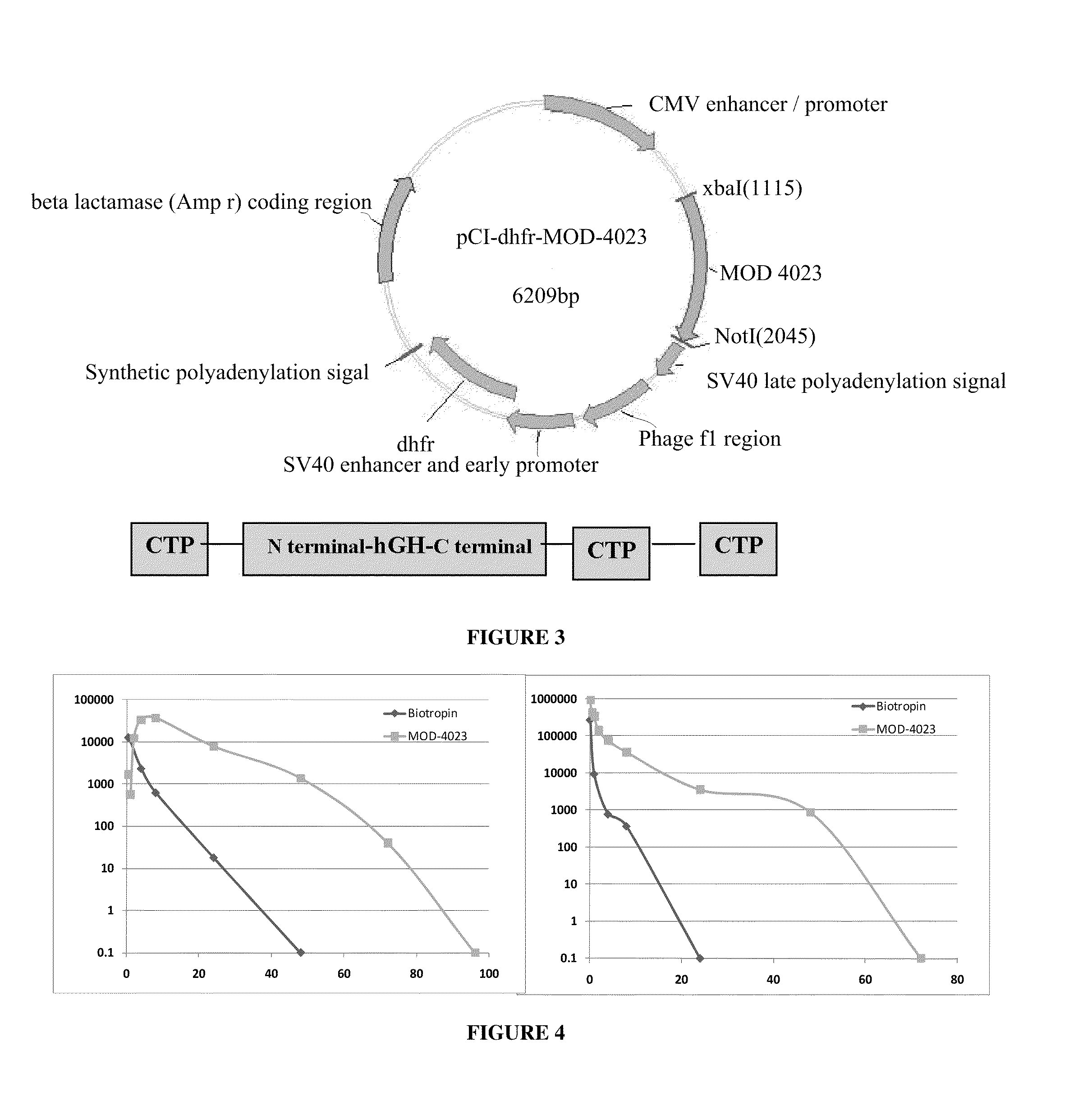
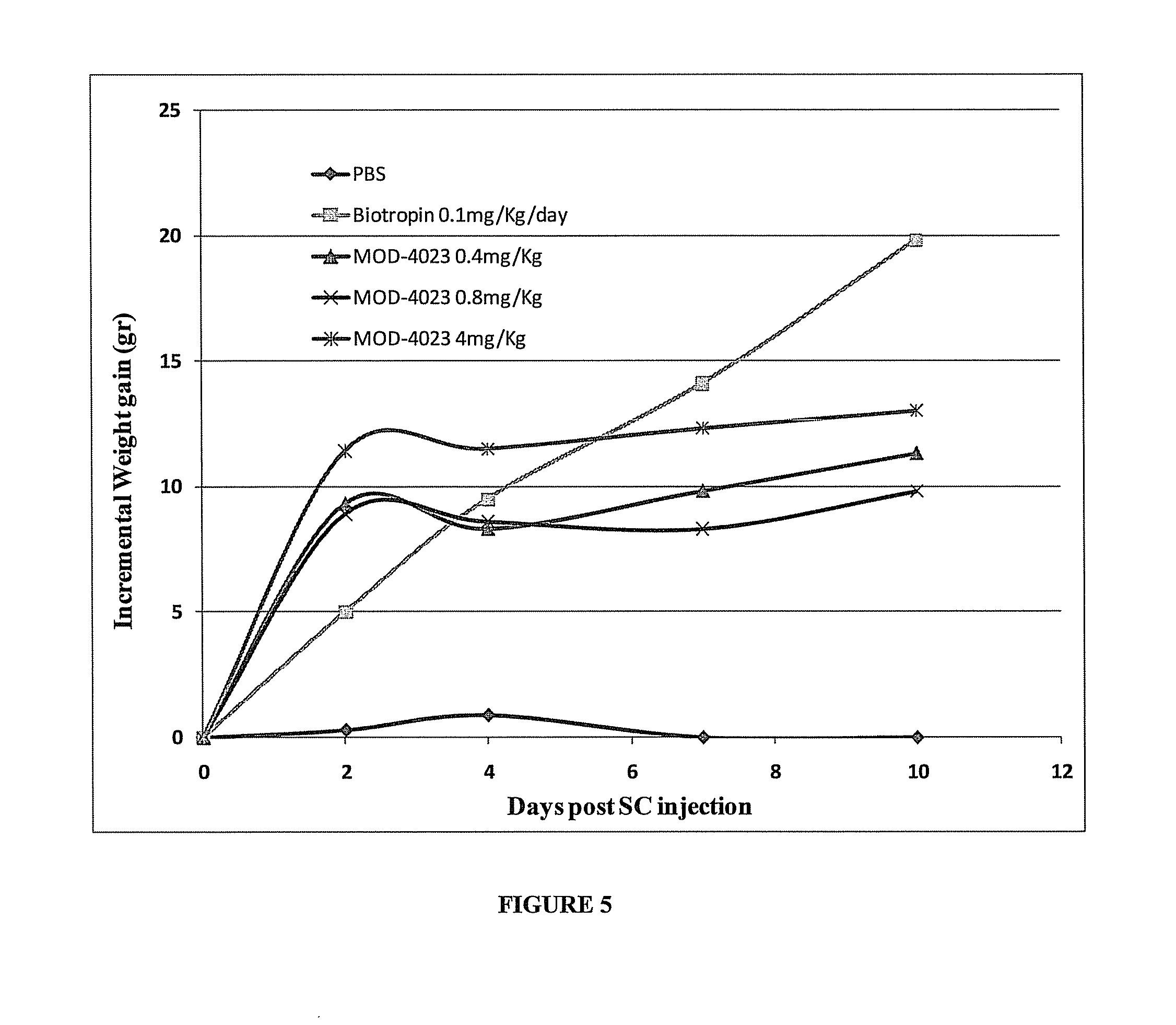
Use of a growth hormone protein and polynucleotides encoding same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing growth or weight gain, method of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
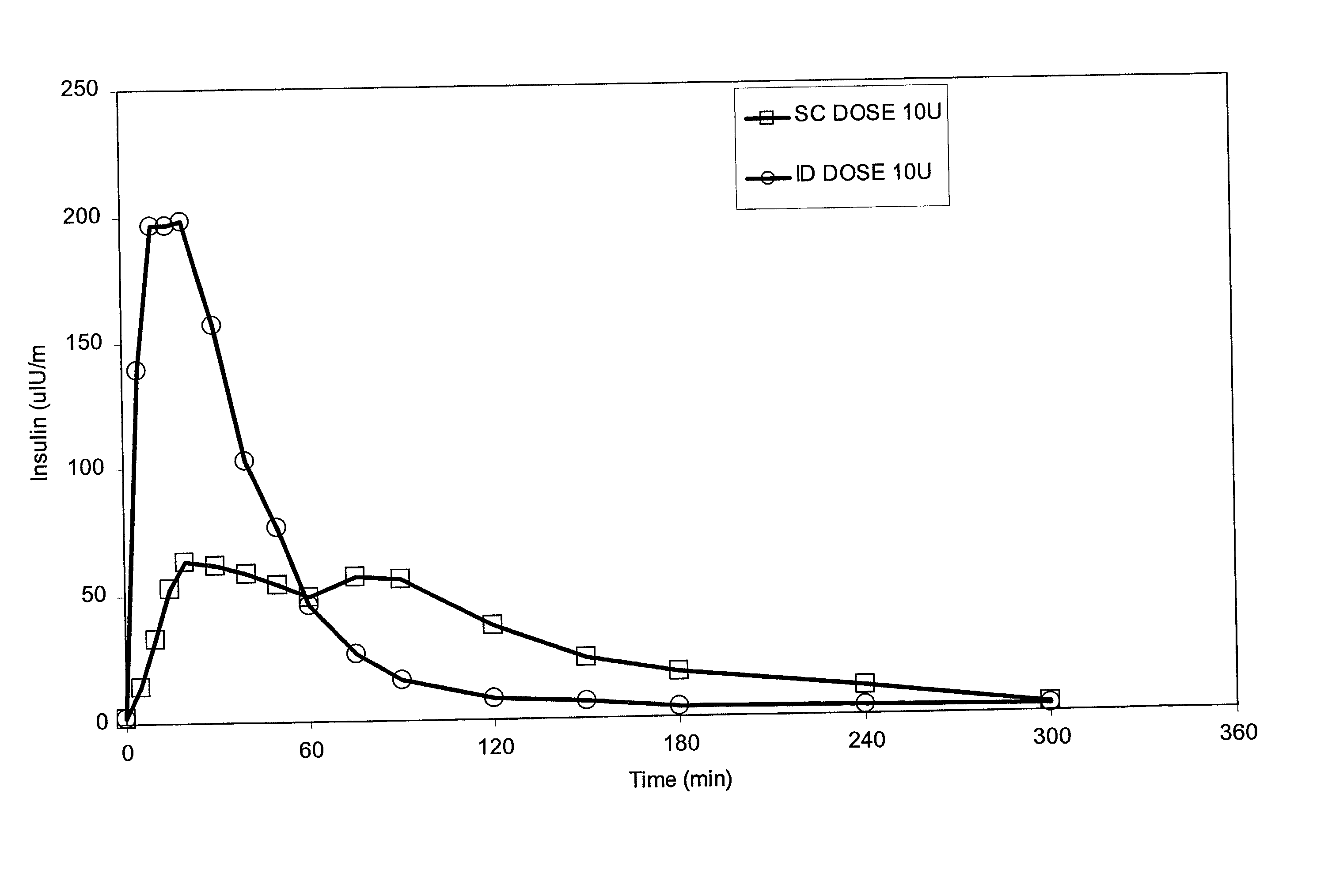
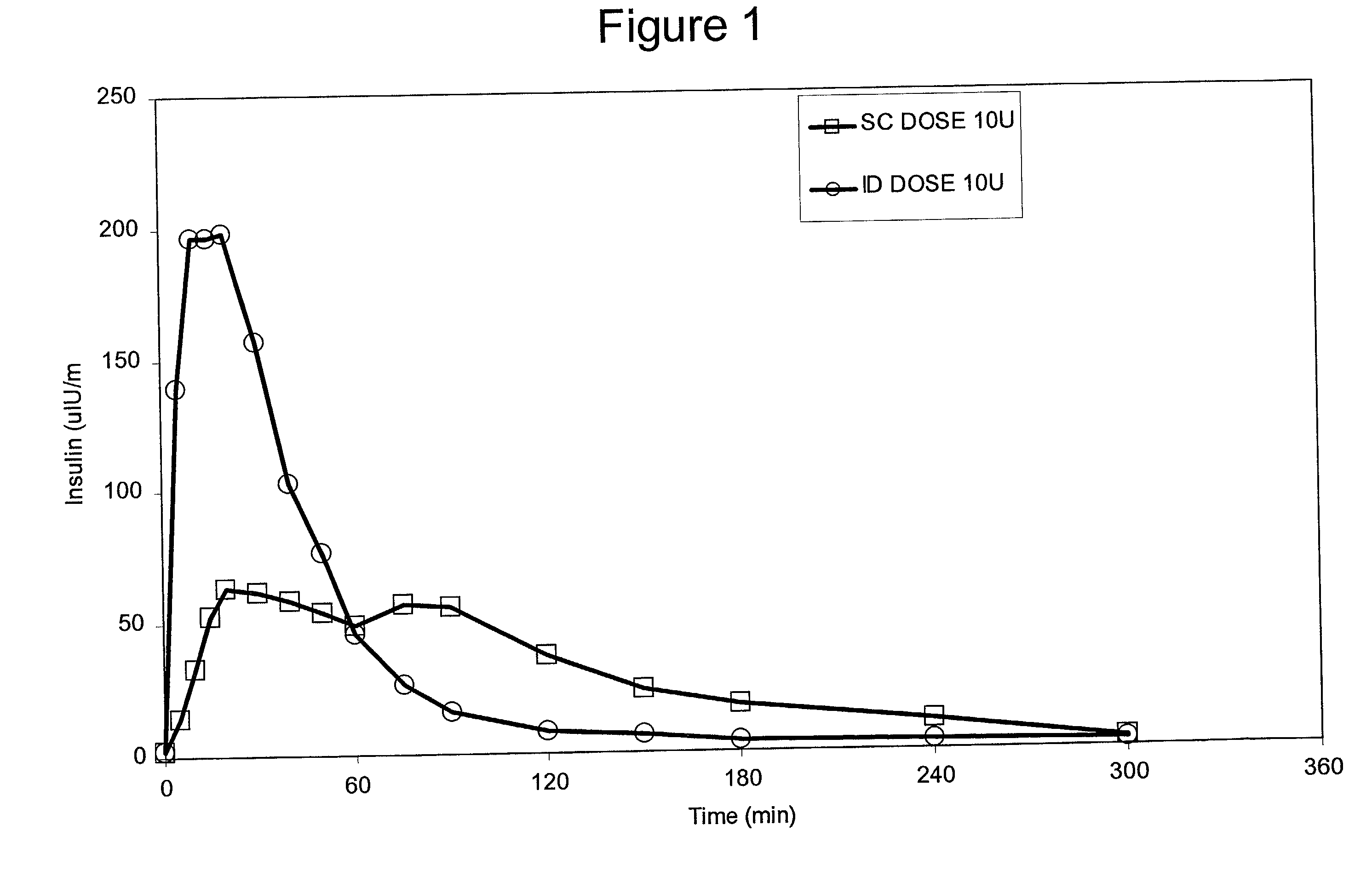
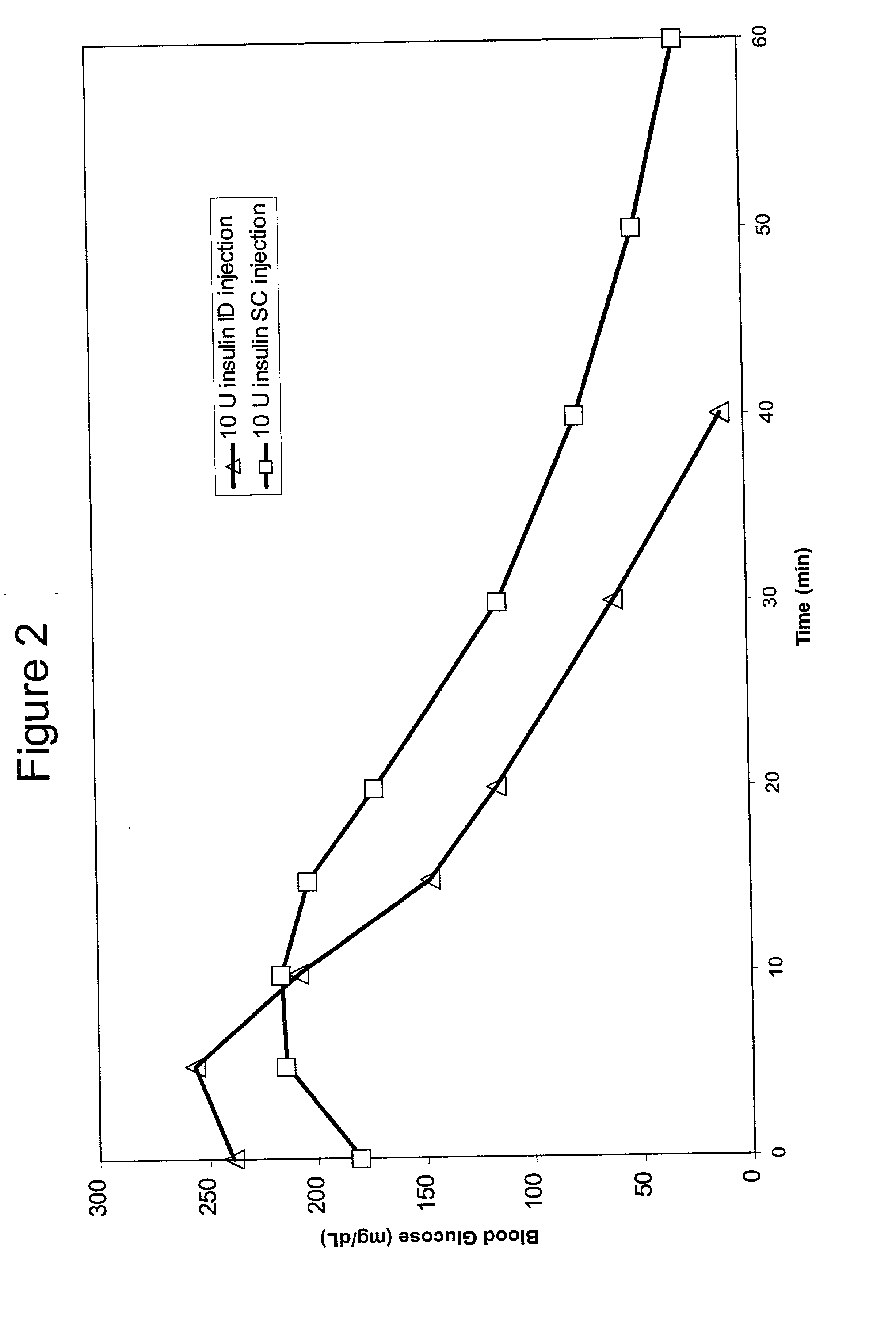
Intradermal delivery of substances
InactiveUS20040073160A1Increase uptakeRapid uptake rateJet injection syringesPeptide/protein ingredientsWhole bodyGrowth hormone
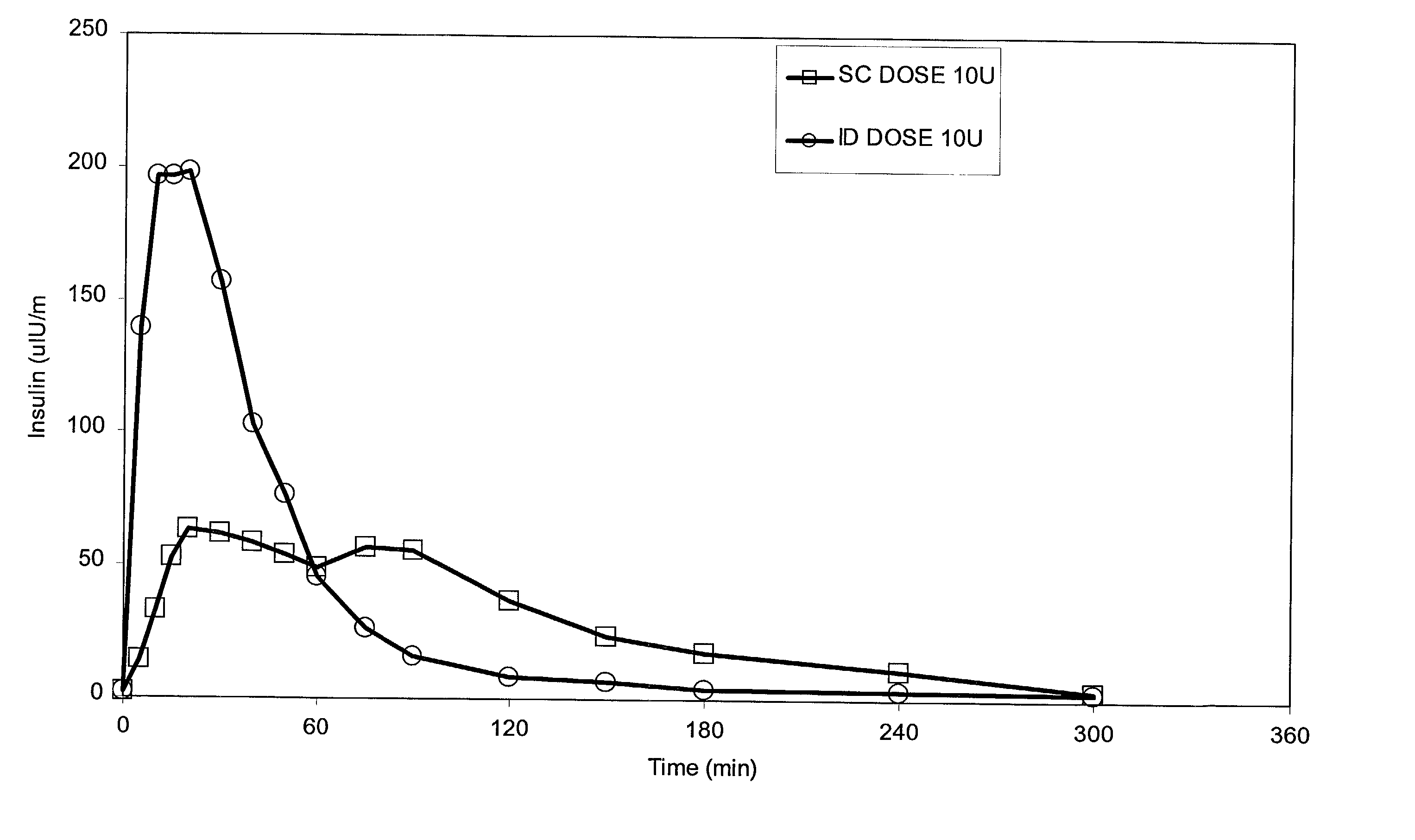
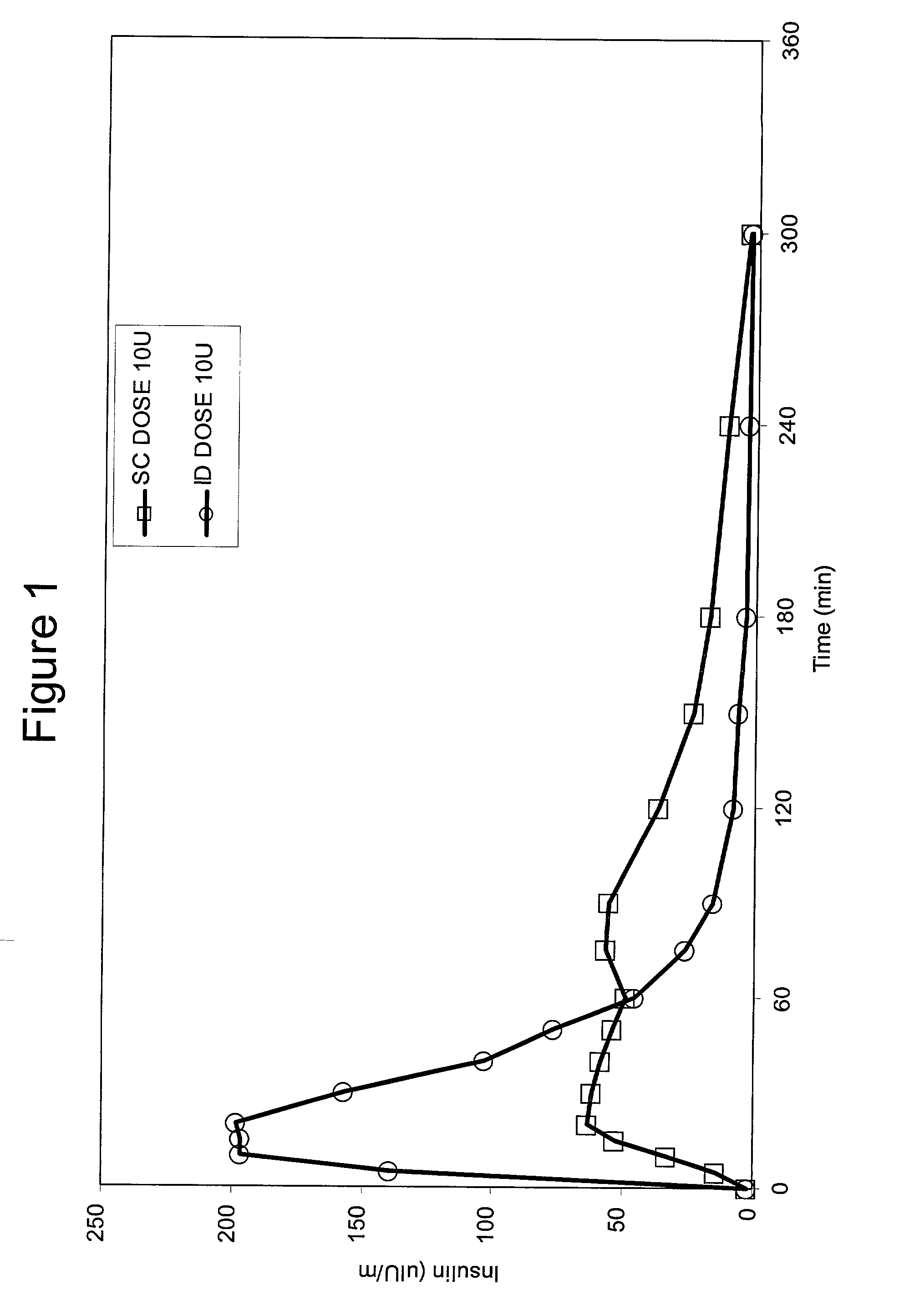
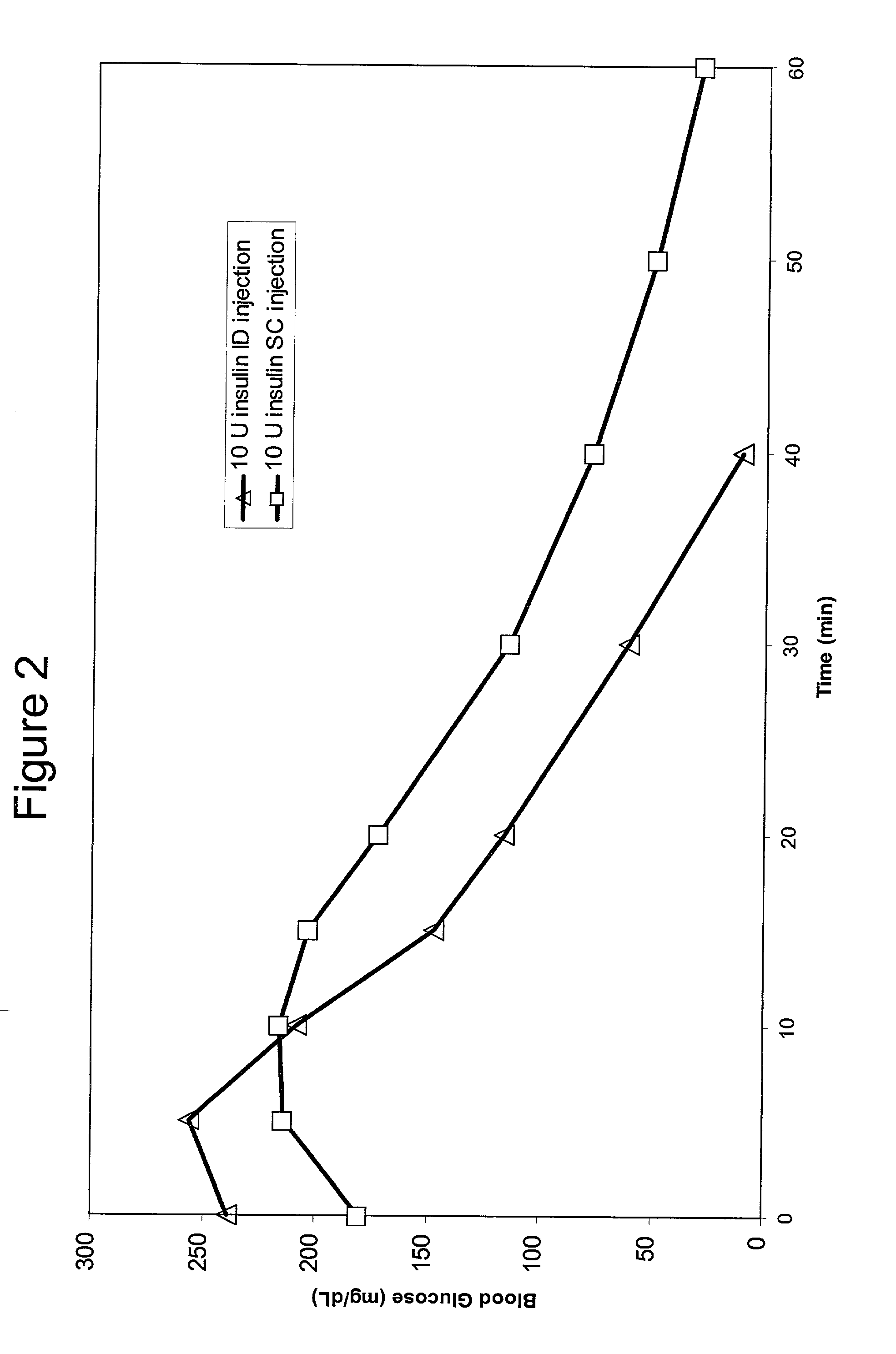
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
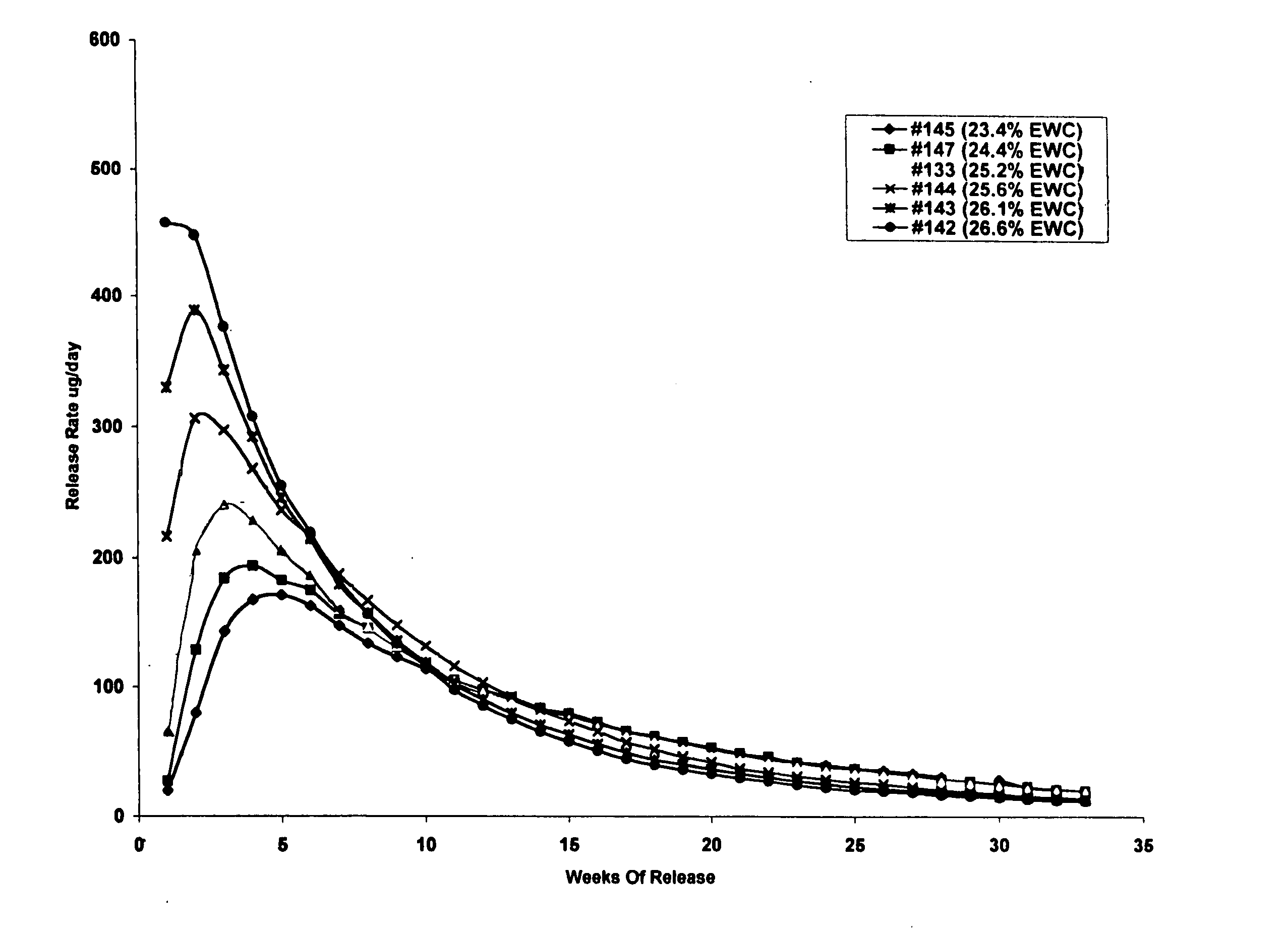
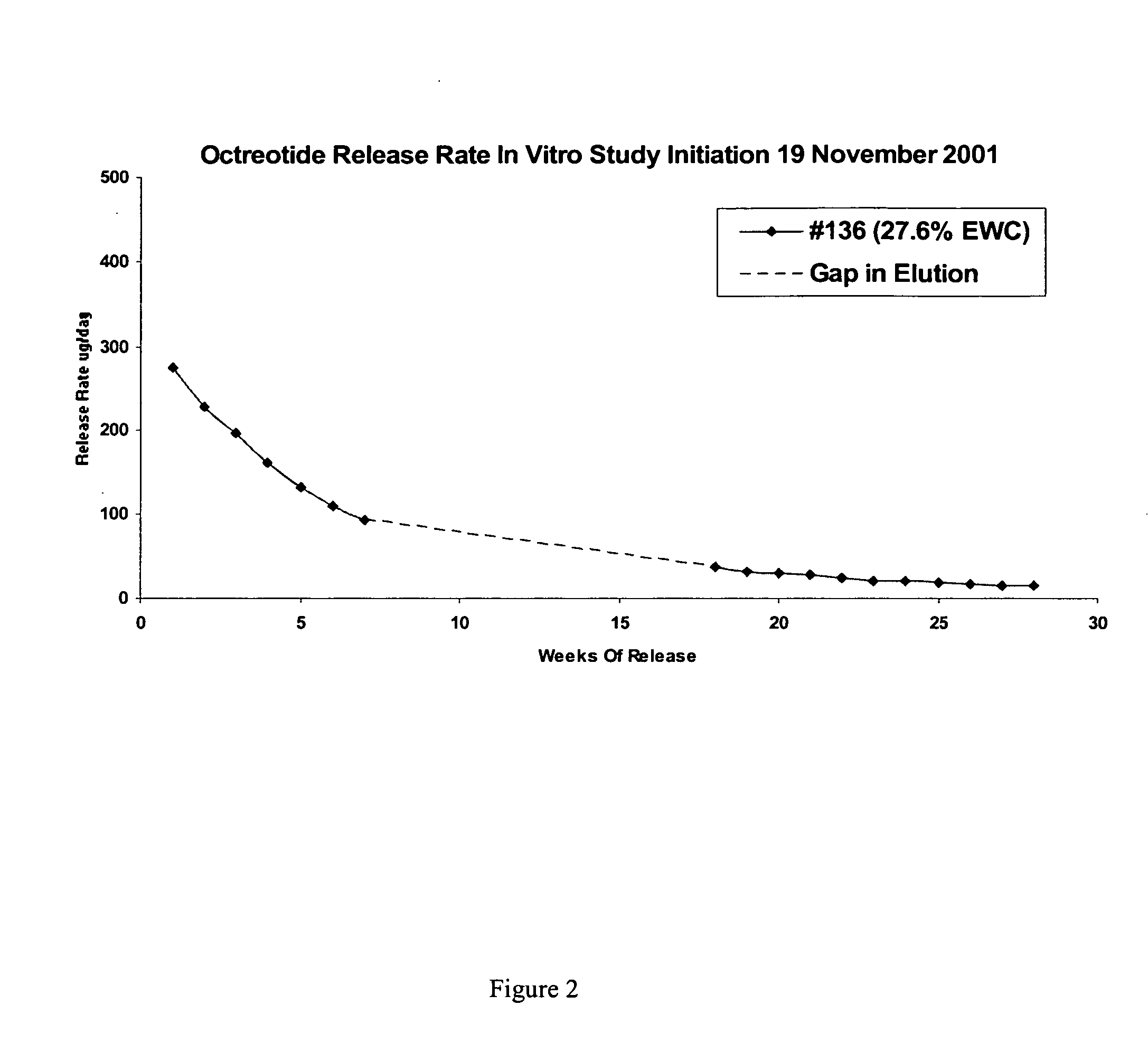
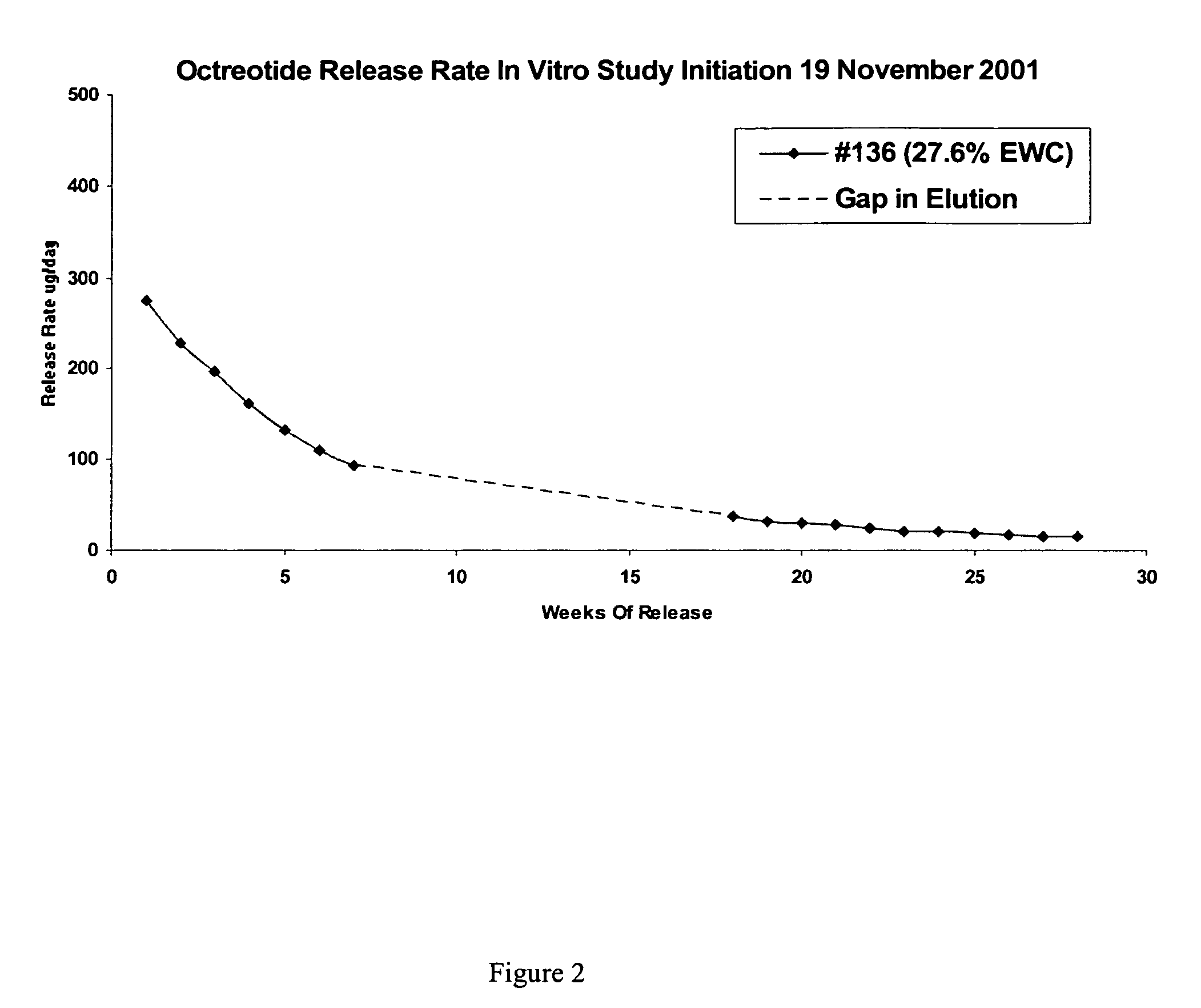
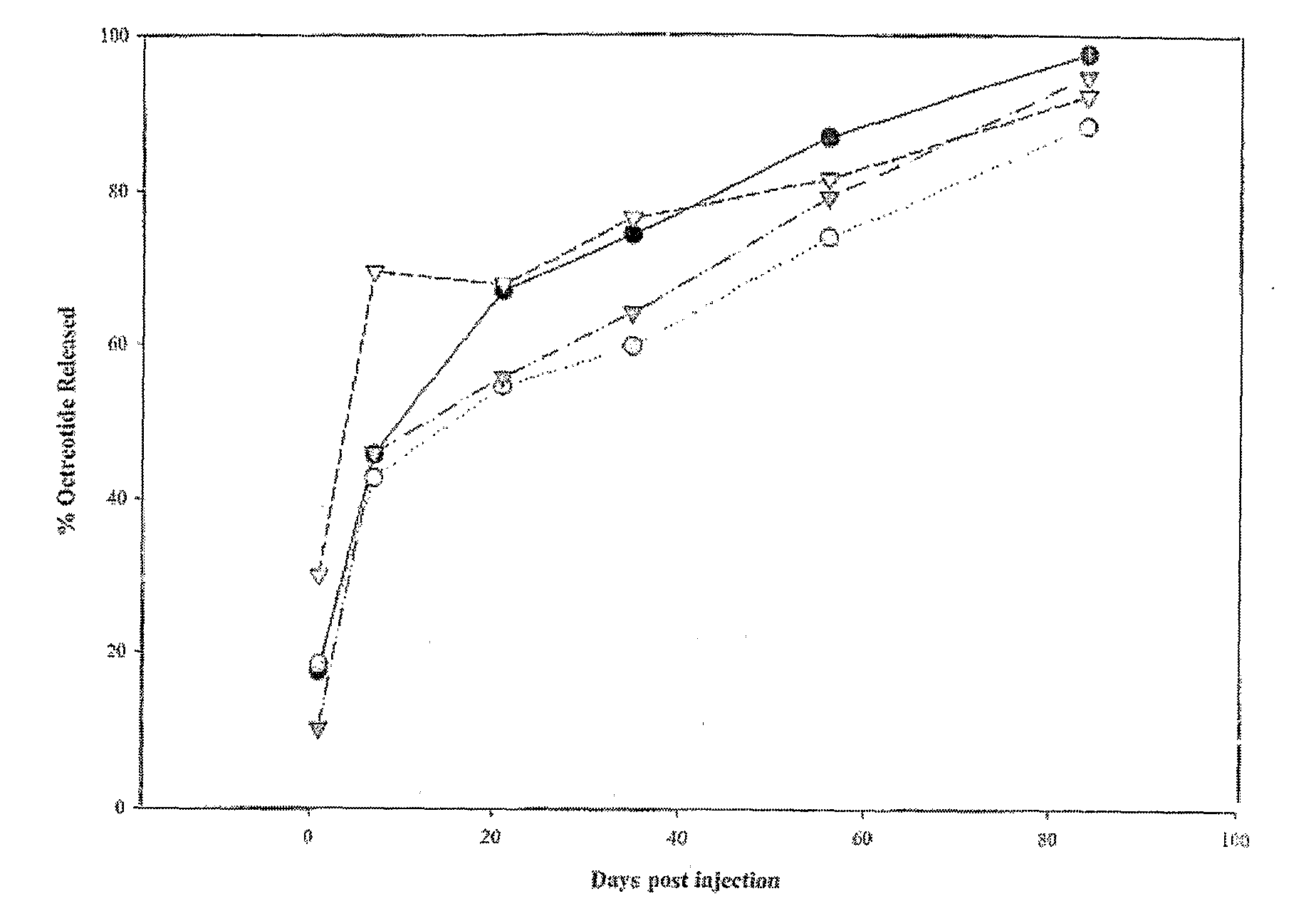
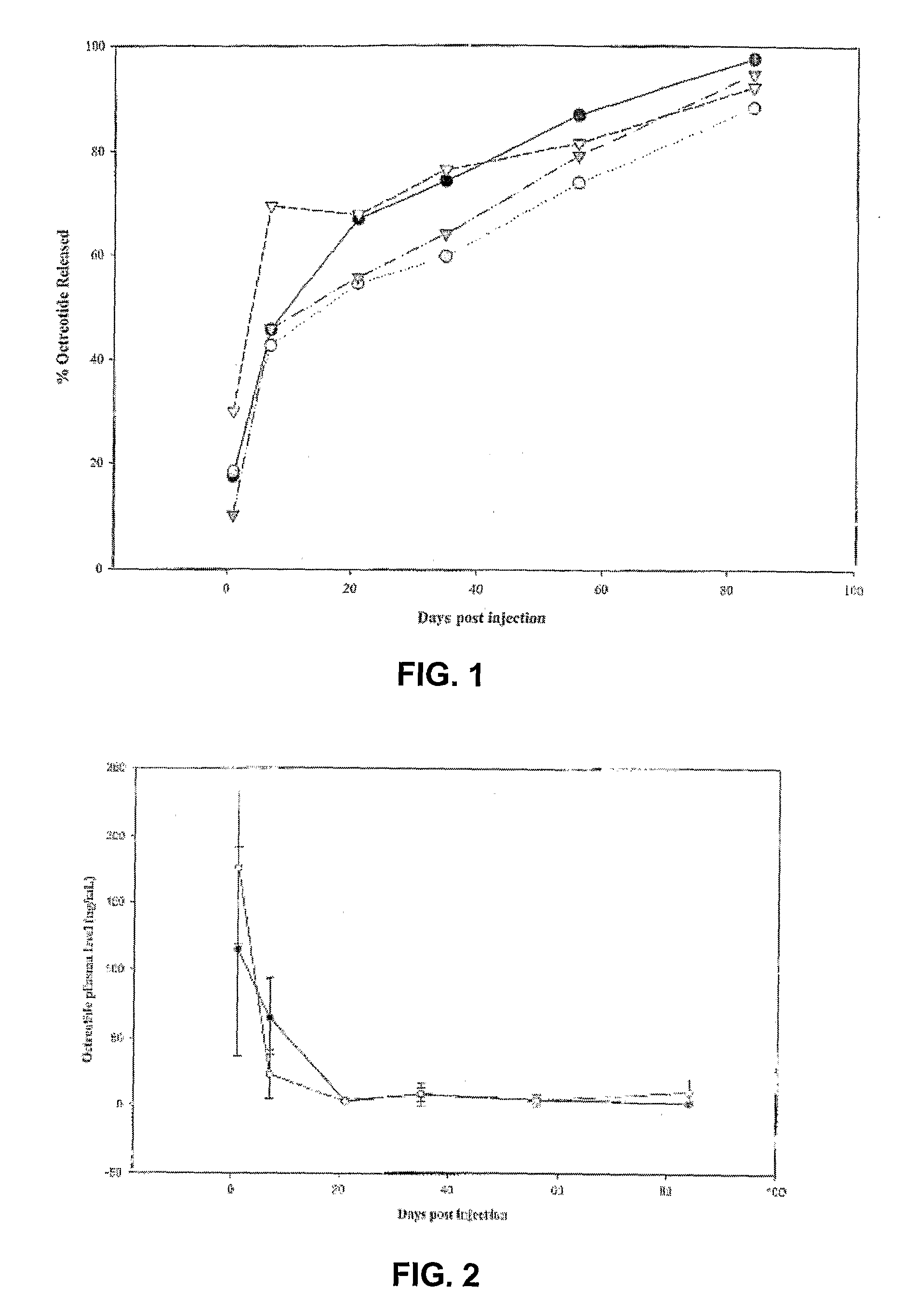
Controlled release formulations of octreotide
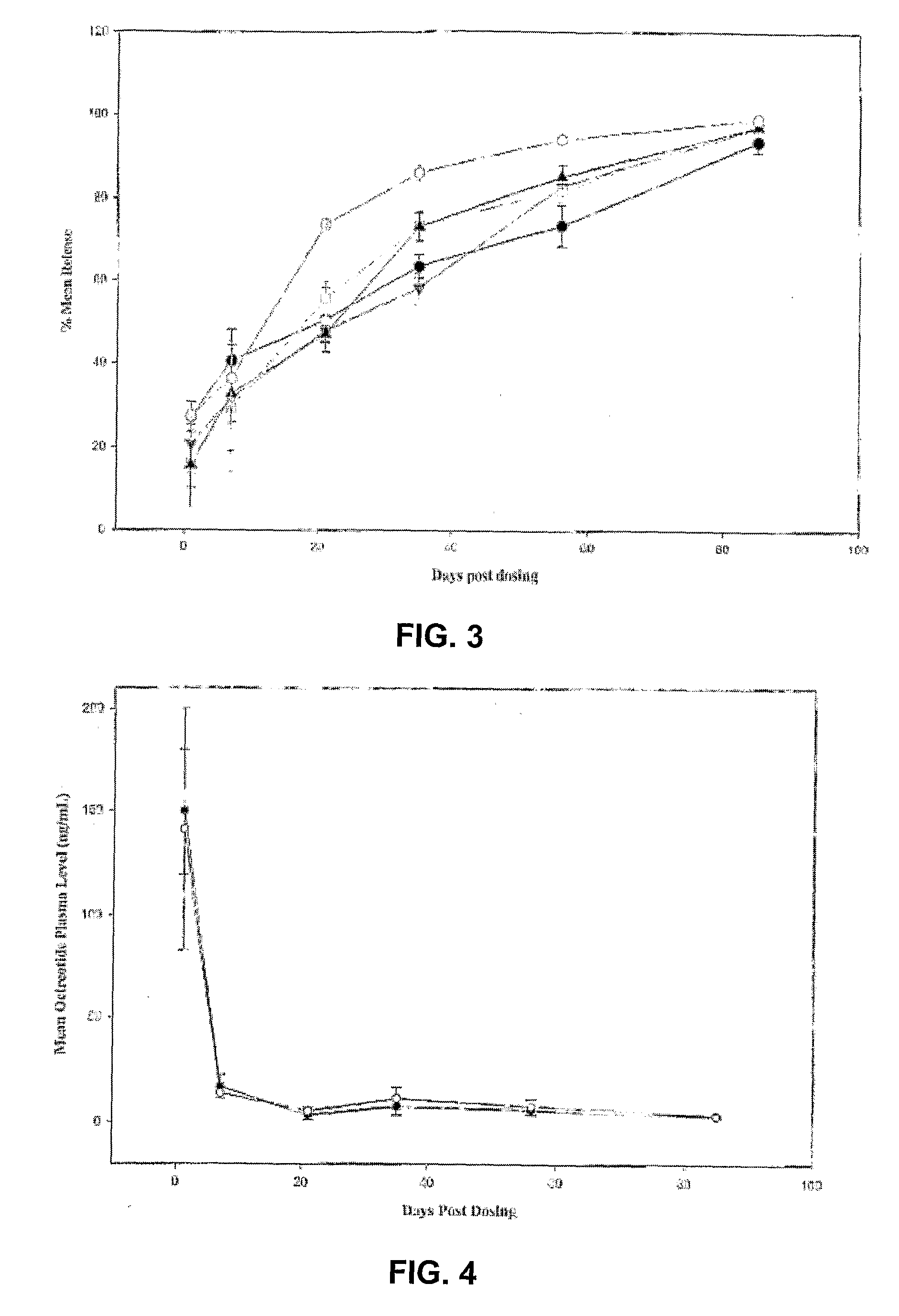
ActiveUS20060204540A1Avoid large peakImprove the level ofPeptide/protein ingredientsMetabolism disorderAcromegalyMalignant carcinoid tumors
A formulation of octreotide or pharmaceutically acceptable salts thereof, which provides controlled release of a therapeutically effective amount of octreotide for a period of at least about two months. Methods of treating acromegaly, decreasing growth hormone, decreasing IGF-1, and treating conditions associated with carcinoid tumors and VIPomas by administering a controlled release formulation of octreotide are provided herein.
Owner:ENDO PHARMA SOLUTIONS
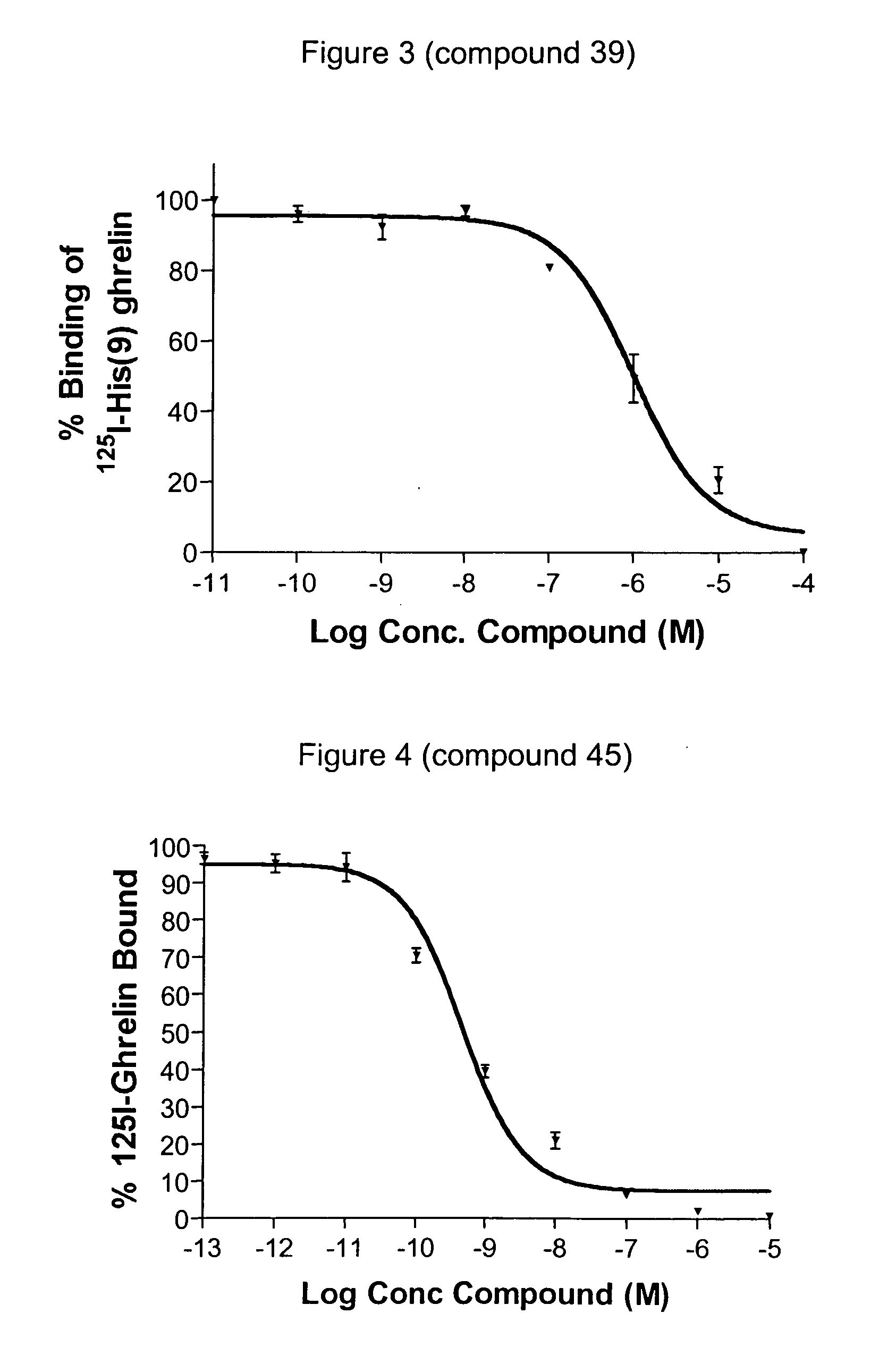
Macrocyclic ghrelin receptor modulators and methods of using the same
ActiveUS20080194672A1Reduced and dysfunctional gastrointestinal motilityInhibit gastrointestinal motilityAntibacterial agentsOrganic active ingredientsSomatotropic hormonePharmaceutical Substances
The present invention provides novel conformationally-defined macrocyclic compounds that can function as selective modulators of the ghrelin receptor (growth hormone secretagogue receptor, GHS-R1a and subtypes, isoforms and variants thereof). Methods of synthesizing the novel compounds are also described herein. These compounds are useful as agonists of the ghrelin receptor and as medicaments for treatment and prevention of a range of medical conditions including, but not limited to, metabolic and / or endocrine disorders, gastrointestinal disorders, cardiovascular disorders, obesity and obesity-associated disorders, central nervous system disorders, bone disorders, genetic disorders, hyperproliferative disorders and inflammatory disorders.
Owner:OCERA THERAPEUTICS INC
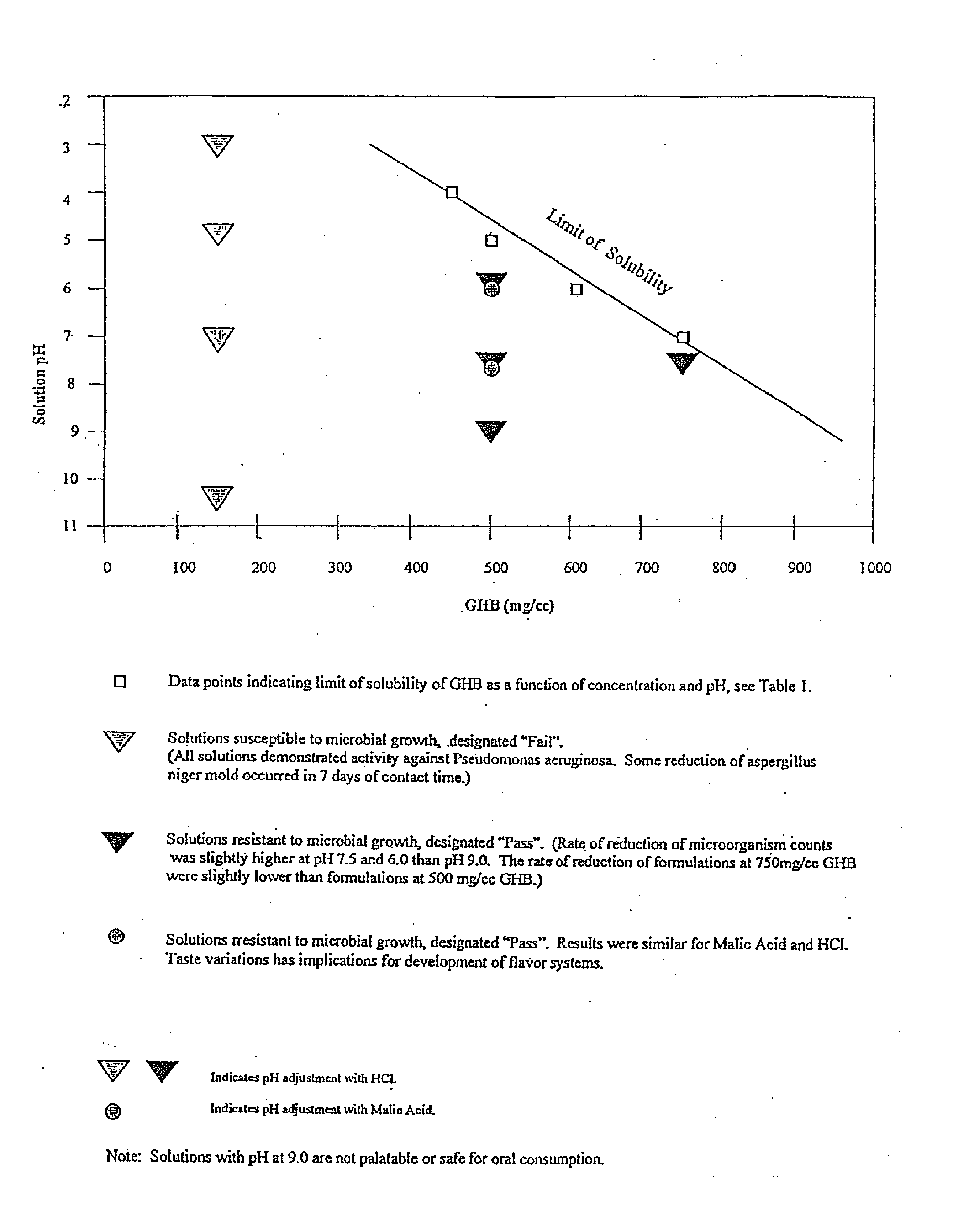
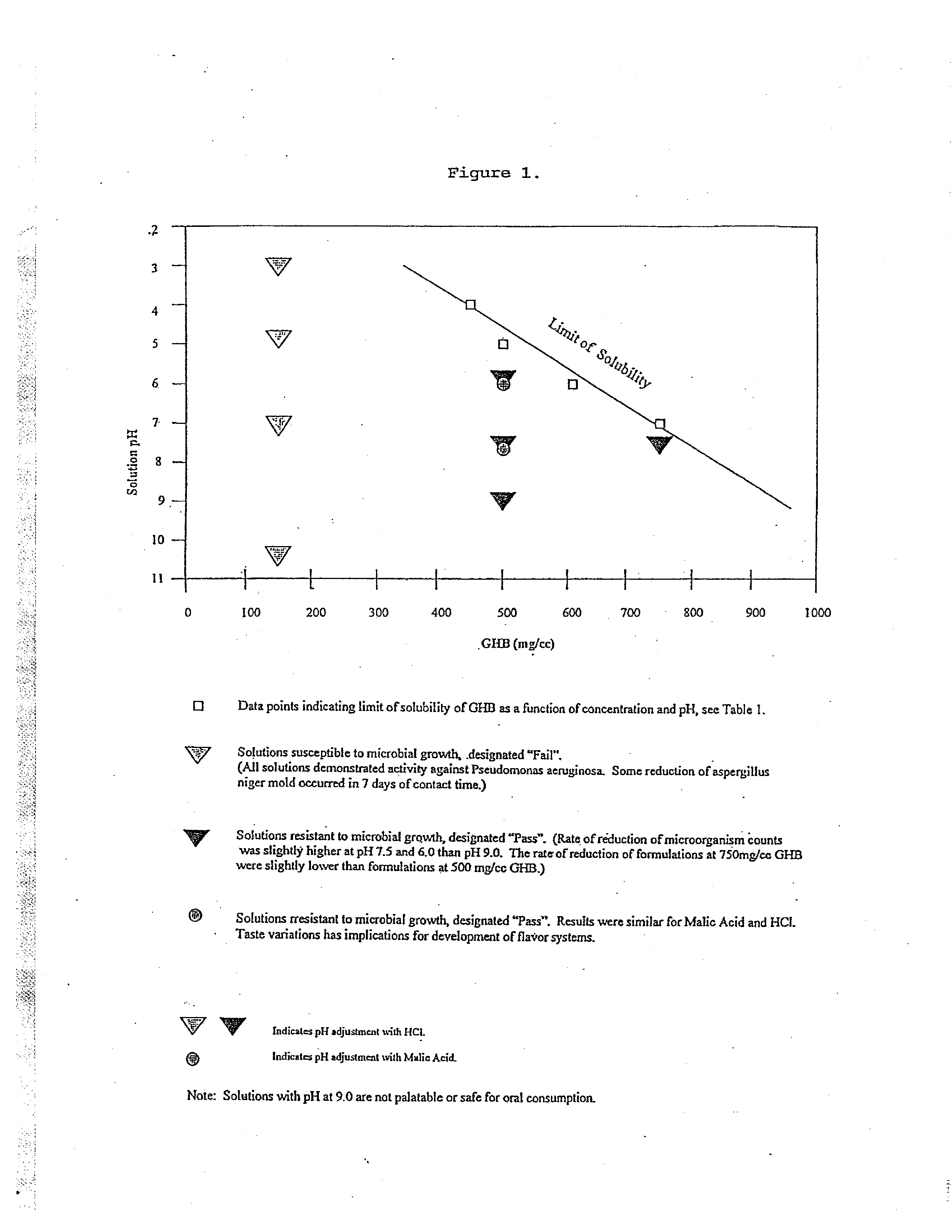
Microbiologically sound and stable solutions of gamma-hydroxybutyrate salt for the treatment of narcolepsy
InactiveUS20070270491A1Lower Level RequirementsImprove the level ofBiocideNervous disorderOpioid withdrawalNarcolepsy
Disclosed are formulations of gamma-hydroxybutyrate in an aqueous medium that are resistant to microbial growth. Also disclosed are formulations of gamma-hydroxybutyrate that are also resistant to the conversion into GBL. Disclosed are methods to treat sleep disorders, including narcolepsy, with these stable formulations of GHB. The present invention also provides methods to treat alcohol and opiate withdrawal, reduced levels of growth hormone, increased intracranial pressure, and physical pain in a patient.
Owner:JAZZ PHARMA INC
Long-acting growth hormone and methods of producing same
ActiveUS20100081614A1Increase the areaReduce dosing frequencyNervous disorderPeptide/protein ingredientsNucleotideGrowth hormone
A polypeptide and polynucleotides encoding same comprising one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to an amino terminus of a growth hormone and two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a growth hormone are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Enhanced pharmacokinetic profile of intradermally delivered substances
InactiveUS20030073609A1Increase uptakeEnhanced iontophoresisPowder deliveryOrganic active ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
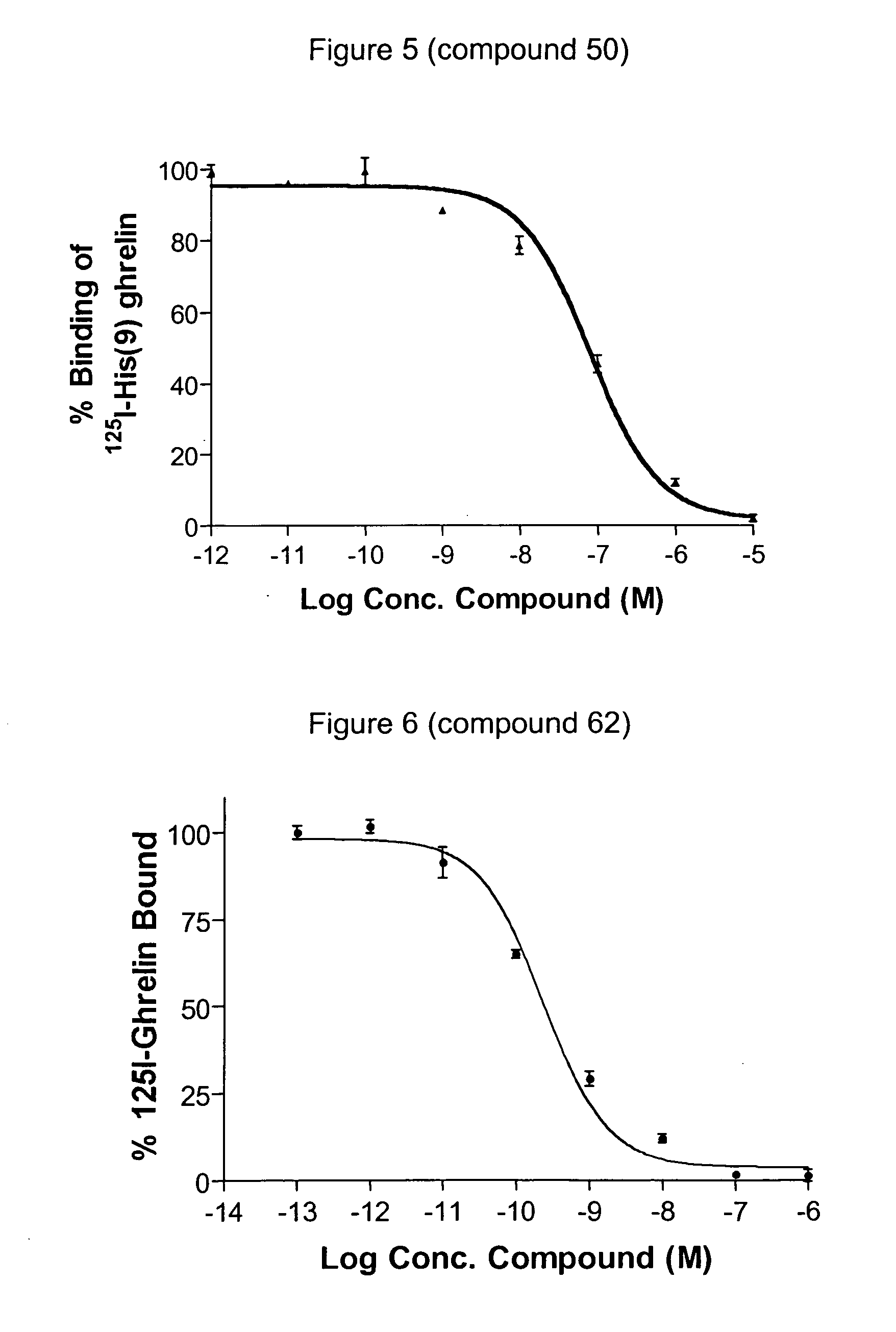
Macrocyclic modulators of the ghrelin receptor
ActiveUS20060025566A1Promote gastrointestinal motilityModulating activity of receptorDigestive systemCyclic peptide ingredientsInflammationCentral nervous system
Owner:OCERA THERAPEUTICS INC
Controlled release formulations of octreotide
ActiveUS7452868B2Reduce needLower Level RequirementsPeptide/protein ingredientsMetabolism disorderAcromegalyMalignant carcinoid tumors
Owner:ENDO PHARMA SOLUTIONS
Methods for treating acromegaly and giantism with growth hormone antagonists
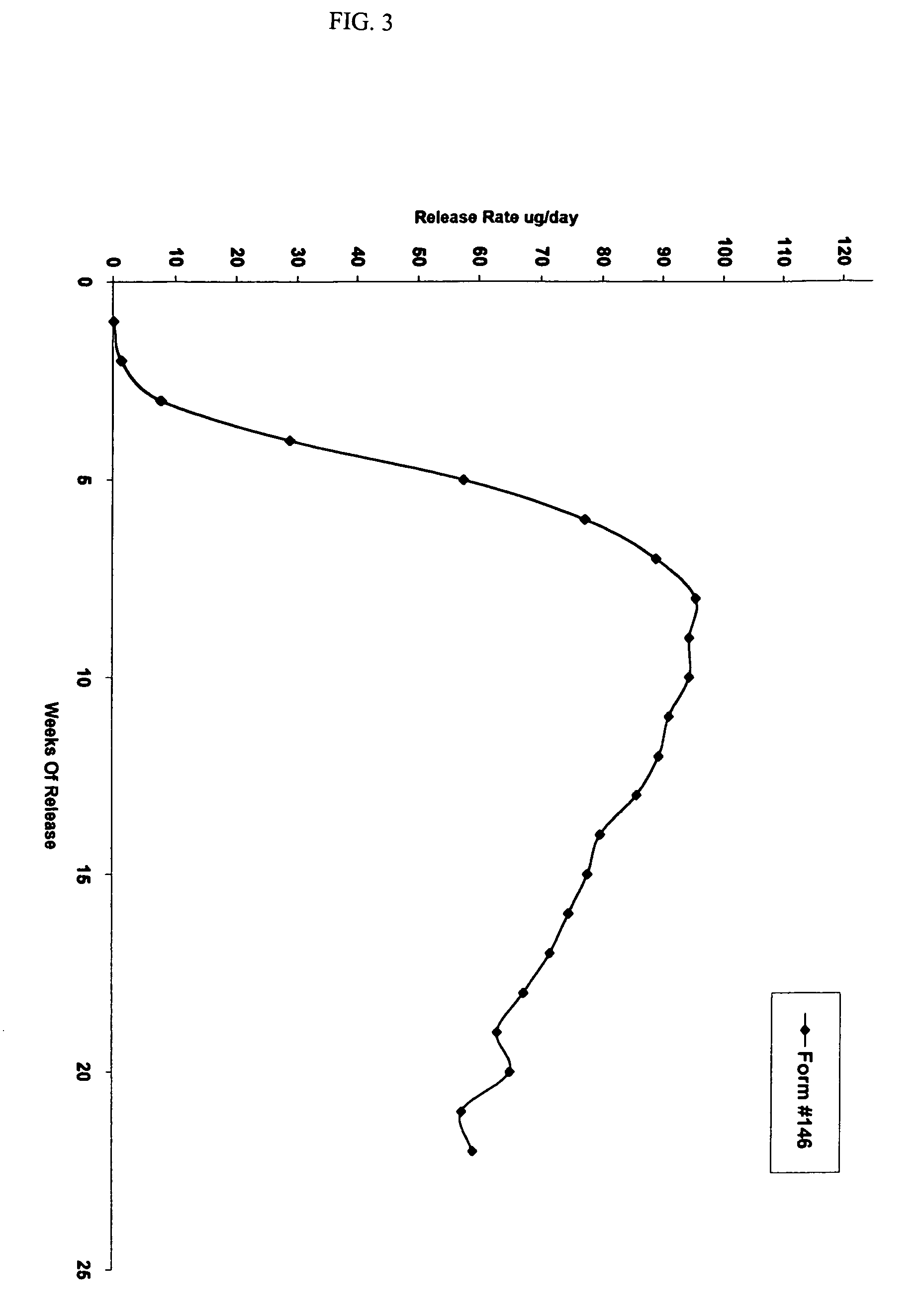
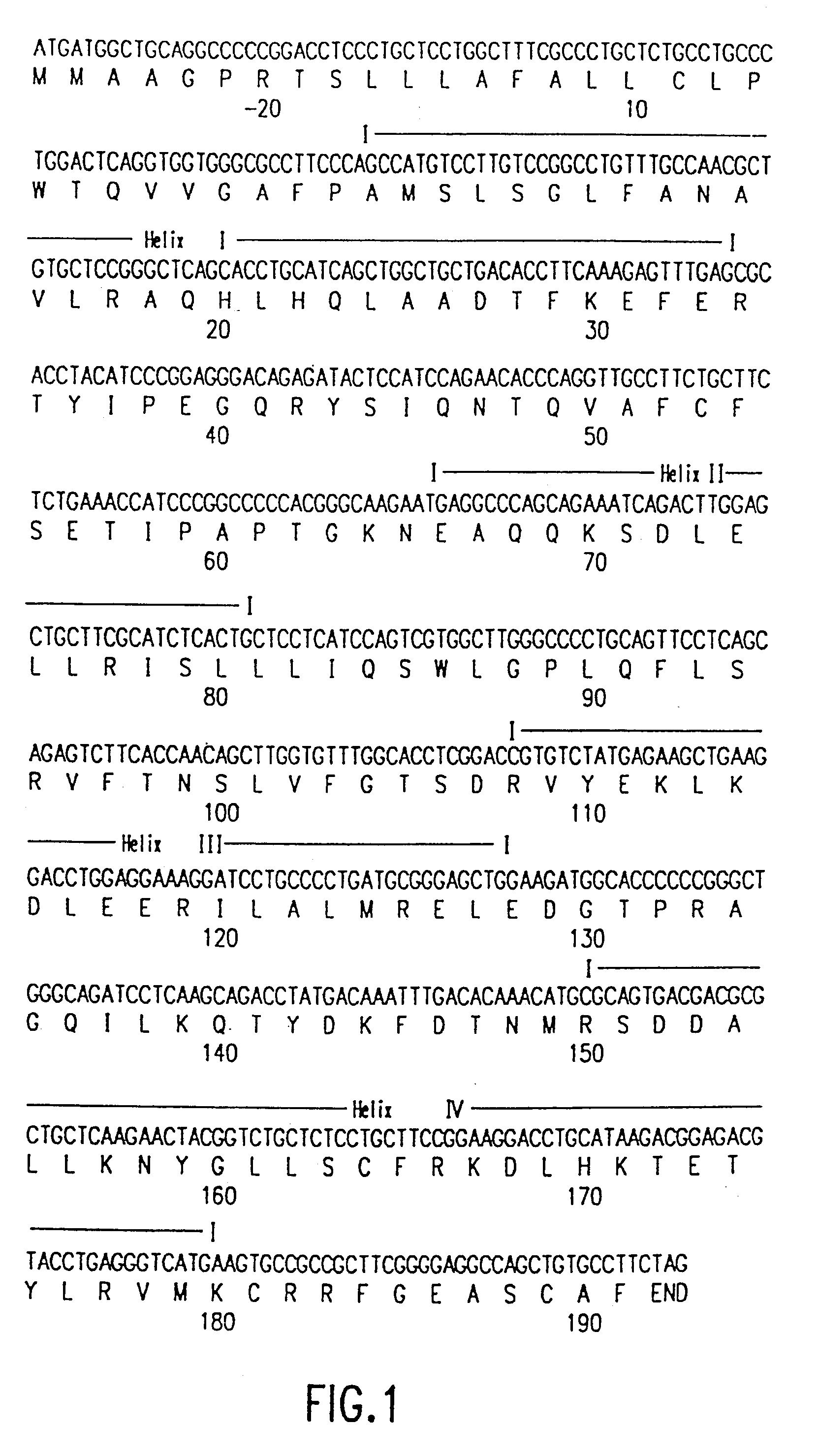
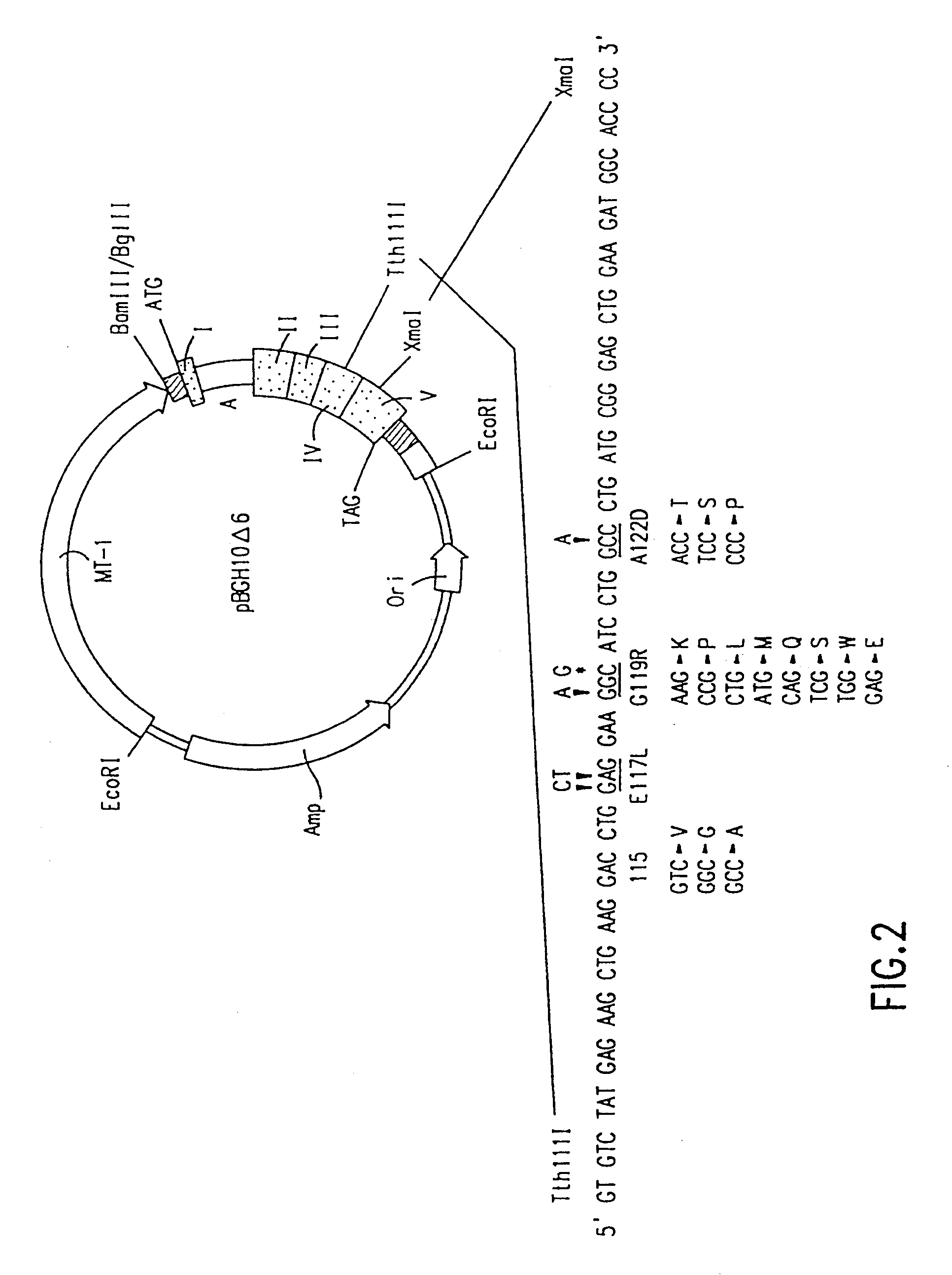
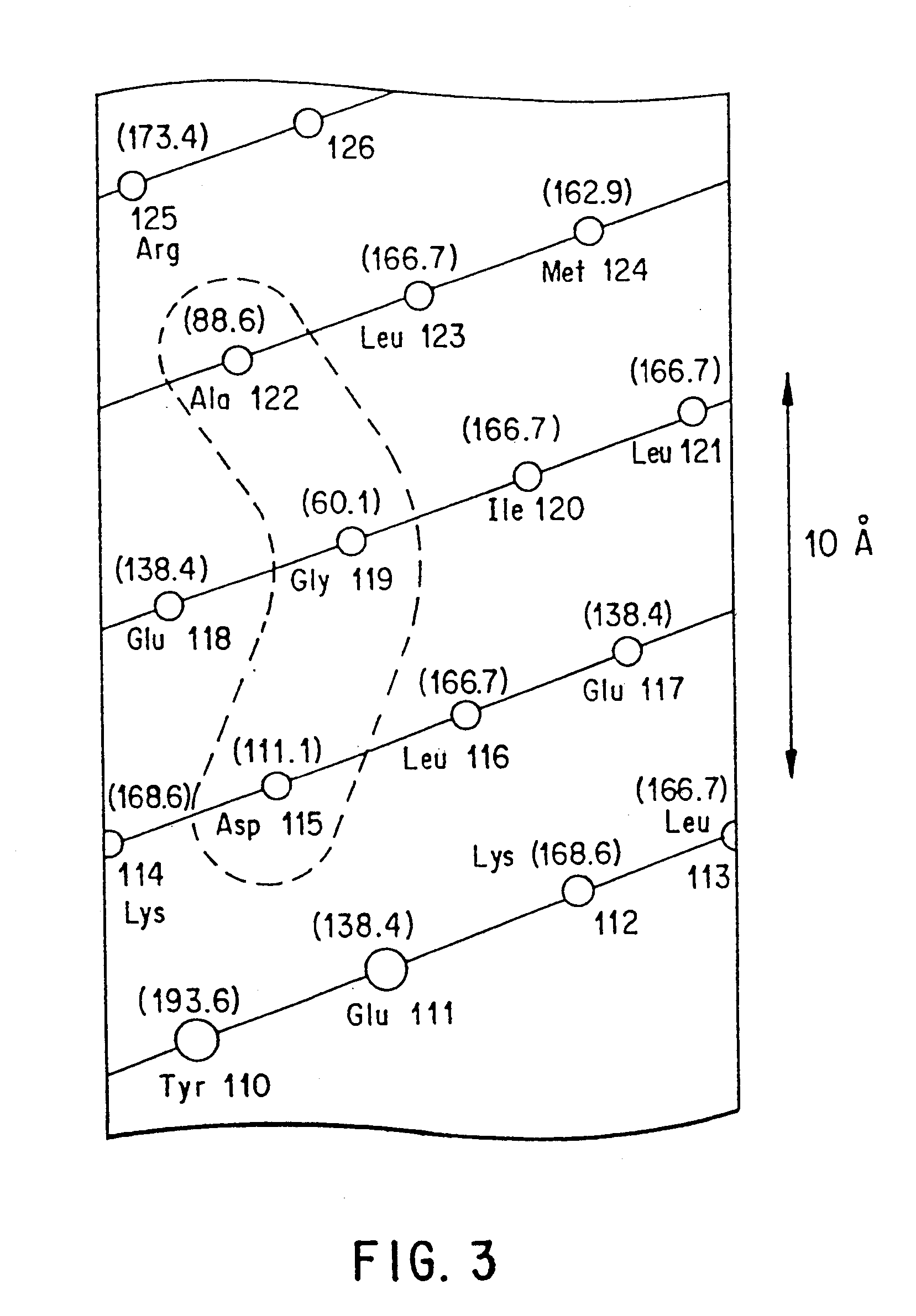
The present invention relates to antagonists of vertebrate growth hormones obtained by mutation of the third alpha helix of such proteins (especially bovine or human GHs). These mutants-have growth-inhibitory or other GH-antagonizing effects. These novel hormones may be administered exogenously to animals, or transgenic animals may be made that express the antagonist. Animals have been made which exhibited a reduced growth phenotype. The invention also describes methods of treating acromegaly, gigantism, cancer, diabetes, vascular eye diseases (diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration, retinopathy of sickle-cell anemia, etc.) as well as nephropathy and other diseases, by administering an effective amount of a growth hormone antagonist. The invention also provides pharmaceutical formulations comprising one or more growth hormone antagonists.
Owner:OHIO UNIV EDISON ANIMAL BIOTECH INST
Method for growing blueberry
InactiveCN101904286AIncrease contentIncrease productionOther chemical processesCultivating equipmentsBetainePropanoic acid
The invention relates to a method for growing blueberry, which comprises soil modification, seedling cultivation, seedling planting and fertilization. In the invention, a soil modifier is applied to soil and the organic materials in the soil modifier increase the content of fatty acids such as acetic acid and propionic acid of the soil, so the rate of mycorrhizal infection, which is capable of promoting the growth of the blueberry, improving blueberry yield, contributing to productivity improvement and soil modification and improving quality and sugar content of fruits, of the blueberry is improved; meanwhile, pine soil and grass carbon are used to regulate the pH value of the soil. In the invention, a rooting medium which contains IAA growth hormone and glycine betaine is used to cultivate the seedlings, so the rooting rate of cuttings and transplanting survival rate are improved, the seedling cultivation time is reduced greatly, and the propagation efficiency is improved obviously. In the invention, a microbial fertilizer, a potassium sulfate type compound fertilizer, ammonium sulfate and diammonium phosphate are used for fertilizing the blueberry, wherein the ammonium sulfate can lower the pH value of the soil, namely the ammonium sulfate provides nutrients for the blueberry for growth and improves the growing environment of the blueberry; and thus, the blueberry yield is more improved effectively.
Owner:董文卓
Sleep quality improvement using a growth hormone secretagogue
InactiveUS6071926AImprove sleep qualityImprove the level ofBiocideAnimal repellantsPhysiologyGrowth hormone
PCT No. PCT / US97 / 09188 Sec. 371 Date Jun. 15, 1999 Sec. 102(e) Date Jun. 15, 1999 PCT Filed May 22, 1997 PCT Pub. No. WO97 / 44042 PCT Pub. Date Nov. 27, 1997A method for sleep quality is disclosed comprising administering an effective amount of N-[1(R)-[(1,2-dihydro-1-methanesulfonylspiro-(3H-indole-3,4'-piperidin)-1'-yl)carbonyl]-2-(phenylmethoxy)-ethyl]-2-amino-2-methylpropanamide methansulfonate.
Owner:ARCH DEVMENT
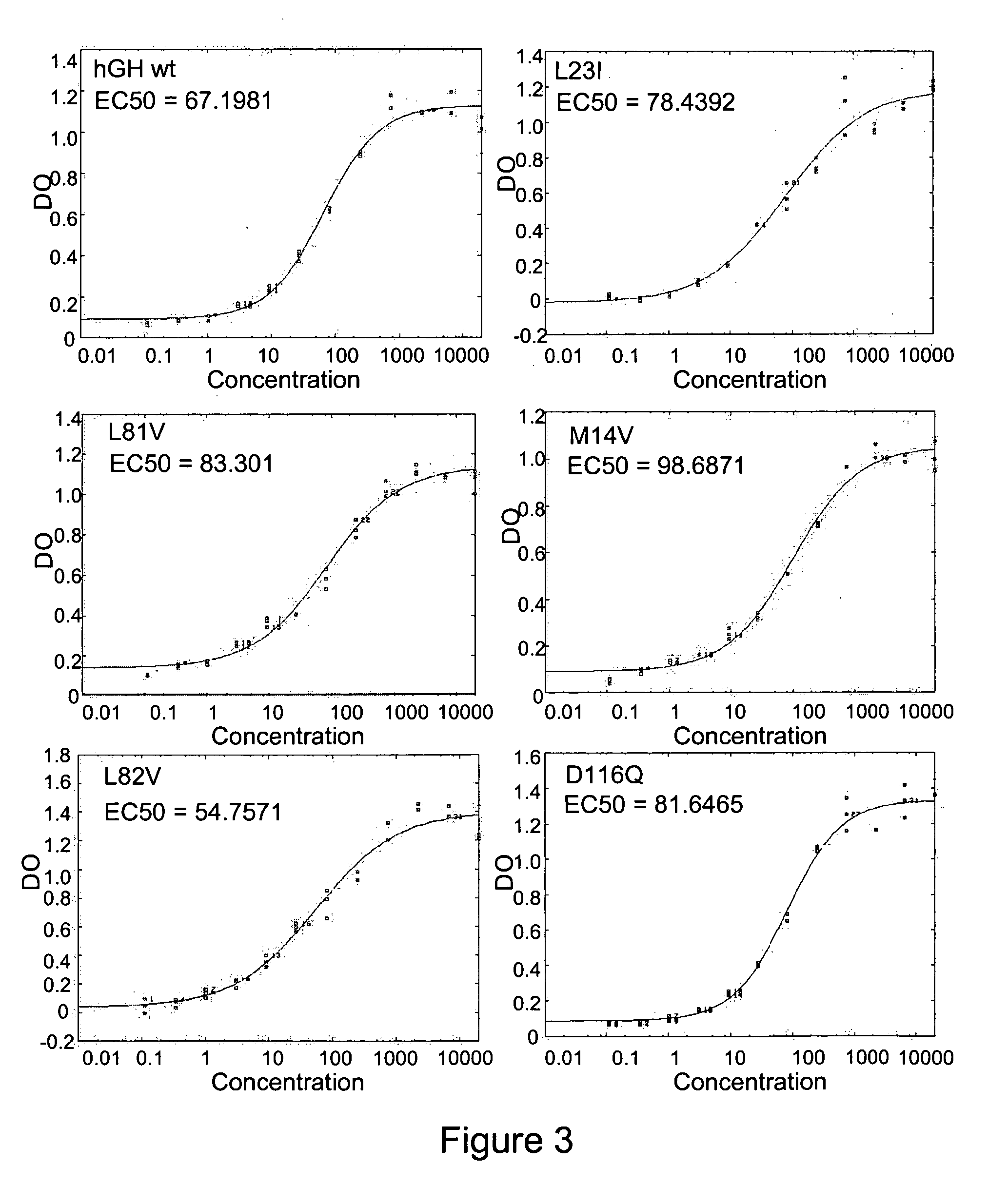
Modified growth hormones
InactiveUS20060094655A1Improved therapeutic propertyImprove protein stabilityPeptide/protein ingredientsAntipyreticGrowth hormoneTreatment use
Provided are modified growth hormone polypeptides, nucleic acid molecules encoding modified growth hormone polypeptides and methods of generating modified growth hormone polypeptides. Also provided are methods of treatment using modified growth hormone polypeptides.
Owner:HANALL PHARMA CO LTD
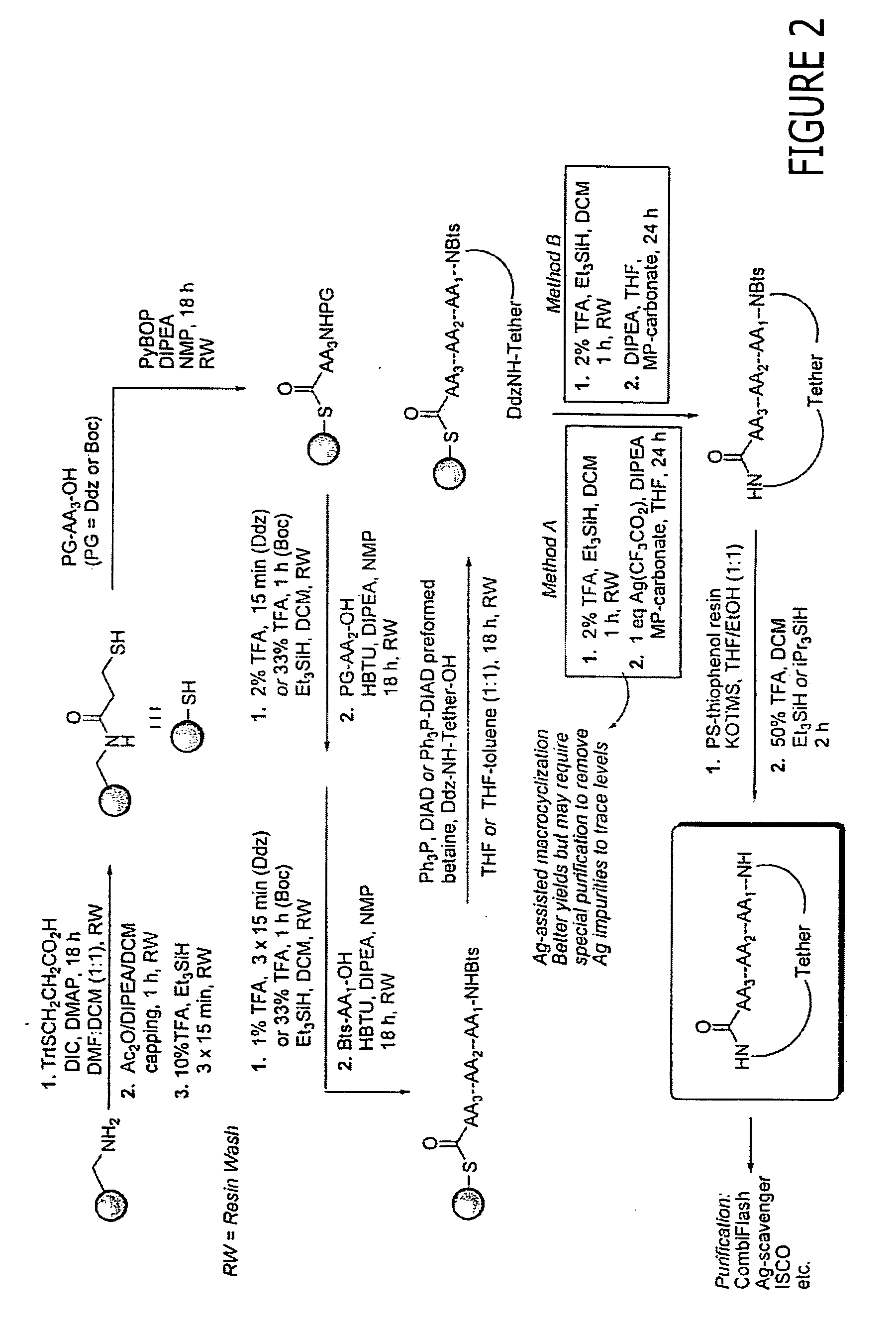
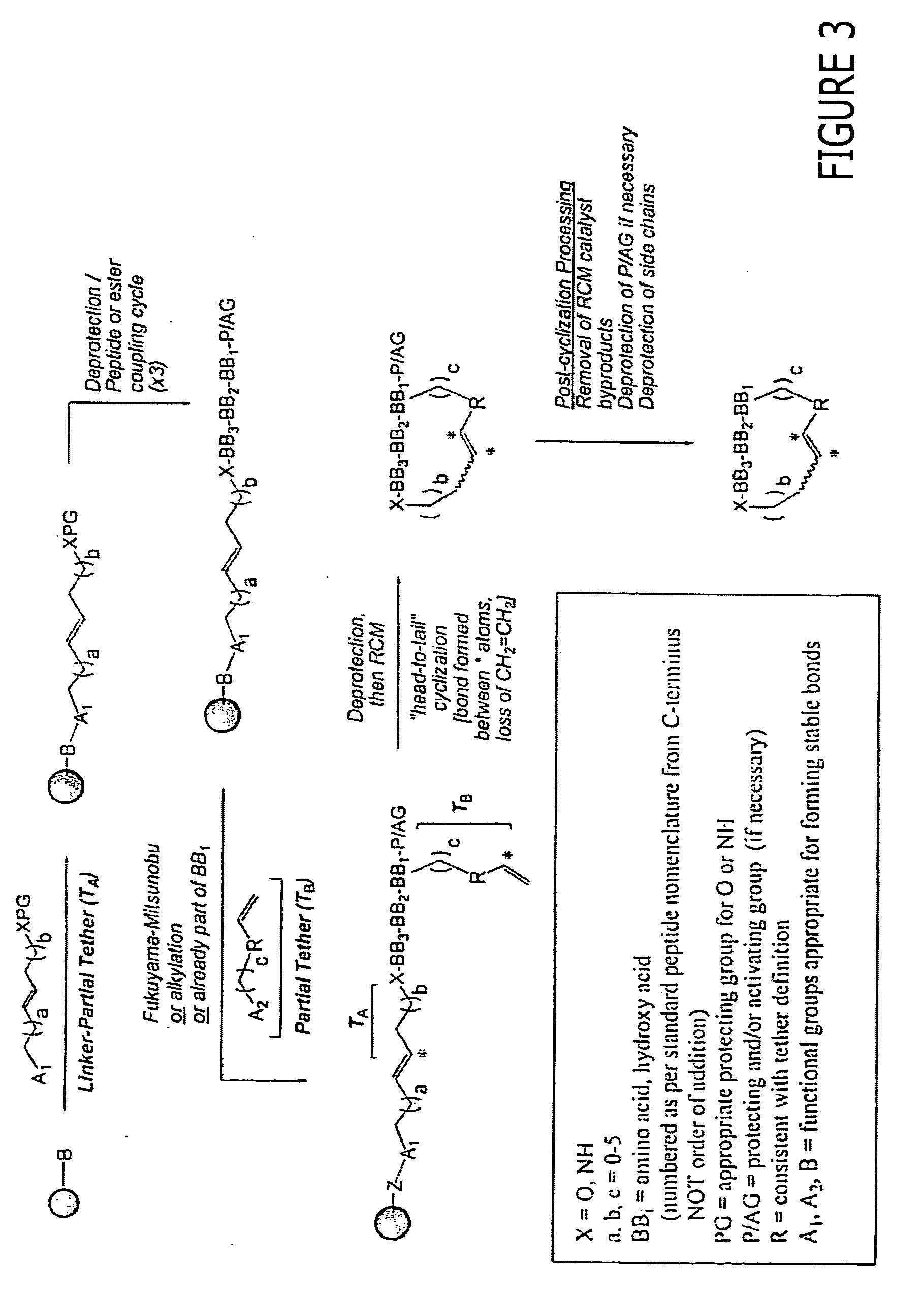
Sustained Delivery Formulations of Octreotide Compounds
InactiveUS20090092650A1Improve bioavailabilityLeast riskSenses disorderPeptide/protein ingredientsMedicineOrganic liquids
The present invention relates to an octreotide sustained release delivery system for treatment of diseases relating to somatotropin and / or somatostatin. The sustained release delivery system of the invention includes a flowable composition containing an octreotide compound, and an implant containing the octreotide compound. The flowable composition may be injected into tissue whereupon it coagulates to become the solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid and an octreotide compound.
Owner:QLT USA INC
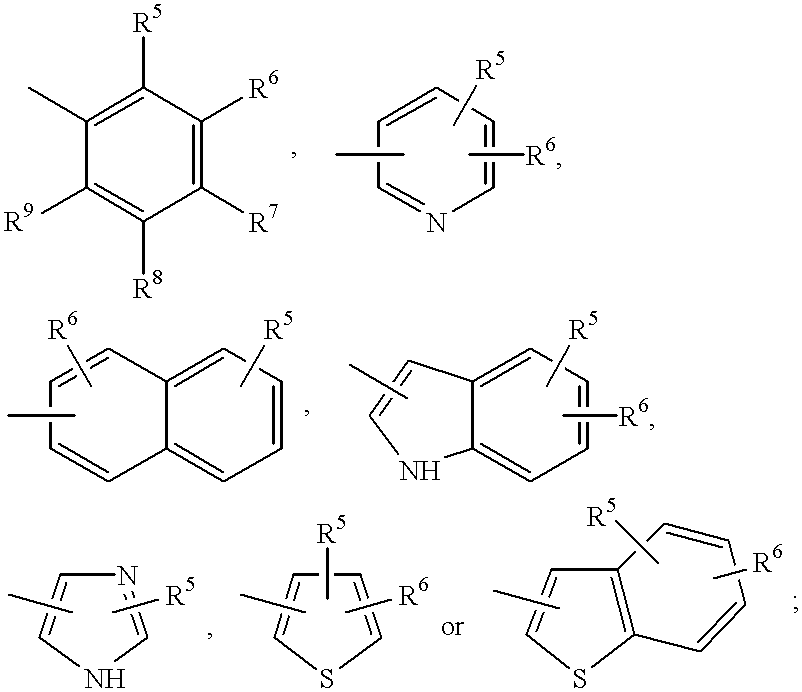
Novel triazole derivatives as ghrelin analogue ligands of growth hormone secretagogue receptors
ActiveUS20070037857A1Good metabolic stabilityImprove bioavailabilityBiocideSenses disorderAdipogenesisTriazole derivatives
The present invention provides novel triazole derivatives as ghrelin analogue ligands of growth hormone secretagogue receptors according to formula (I) that are useful in the treatment or prophylaxis of physiological and / or pathophysiological conditions in mammals, preferably humans, that are mediated by GHS receptors. The present invention further provides GHS receptor antagonists and agonists that can be used for modulation of these receptors and are useful for treating above conditions, in particular growth retardation, cachexia, short-, medium- and / or long term regulation of energy balance; short-, medium- and / or long term regulation (stimulation and / or inhibition) of food intake; adipogenesis, adiposity and / or obesity; body weight gain and / or reduction; diabetes, diabetes type I, diabetes type II, tumor cell proliferation; inflammation, inflammatory effects, gastric postoperative ileus, postoperative ileus and / or gastrectomy (ghrelin replacement therapy).
Owner:ZENTARIS GMBH +3
Compositions and methods for stimulating gastrointestinal mobility
The present invention is directed to methods for stimulating the motility of the gastrointestinal system in a patient which comprises administering a growth hormone secretagogue, a prodrug thereof or a pharmaceutically acceptable salt of said secretagogue or said prodrug. More particularly, the present invention provides methods for stimulating the motility of the gastrointestinal system in a patient which comprises administering a compound of Formula I: a prodrug thereof or a pharmaceutically acceptable salt of said secretagogue or said prodrug.
Owner:RAQUALIA PHARMA INC
Cuttage and breeding method for sabina vulgalis
InactiveCN101699955AImprove survival rateHigh reproductive coefficientCultivating equipmentsHorticultureShootGrowth hormone
A cuttage and breeding method for sabina vulgalis comprises the steps of renovating a cuttage bed, picking branches, processing shoot for cutting, performing cuttage and the like; deeply ploughing a seedling tray, clearing off impurities, applying cottage, leveling, widening to make a lower bed, and paving clean river sand on the seedling bed; sterilizing the sand bed which is 5-10cm thick with 800-1000 times of carbendazol liquid medicine; picking 1-2 years old healthy and strong branches without diseases or pests at dawn, putting the branches cut down in a plastic cask constraining water timely to prevent drying-up due to water loss; processing shoot for cutting by adopting different medicines and plant growing hormone with concentration being 50-150ppm for dipping for 2-6h, so that breeding coefficient is higher. Facts prove that the cuttage and breeding survival rate of sabina vulgalis bred by using the invention can reach up to 75-89%, thus realizing the aim of enhancing breeding coefficient.
Owner:GANSU PROVINCE ACAD OF QILIAN WATER RESOURCE CONSERVATION FORESTS RES INST
Heterocyclic compounds useful as growth hormone secretagogues
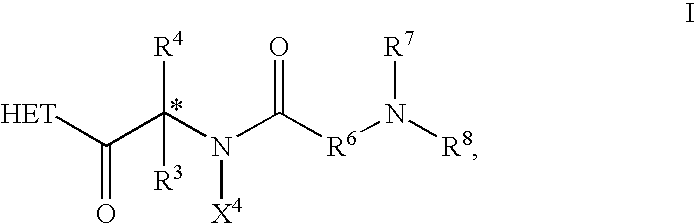
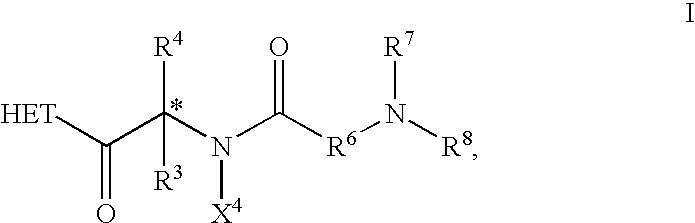
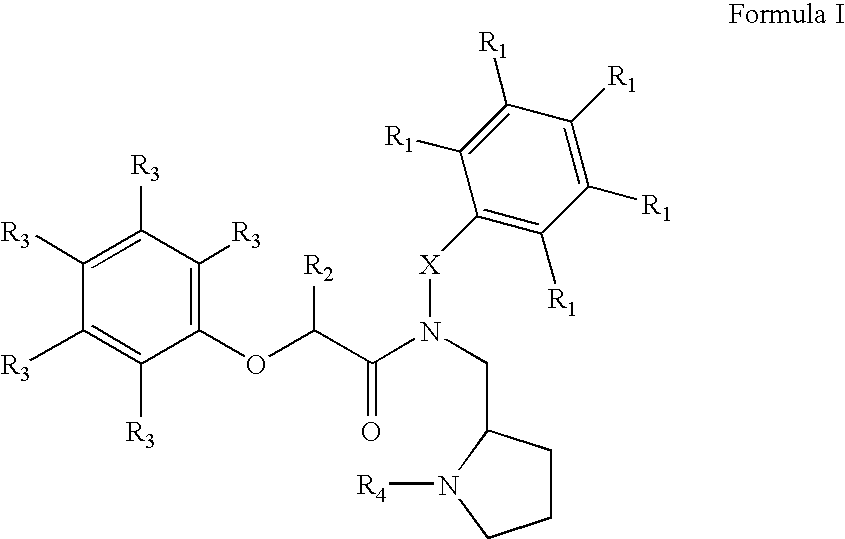
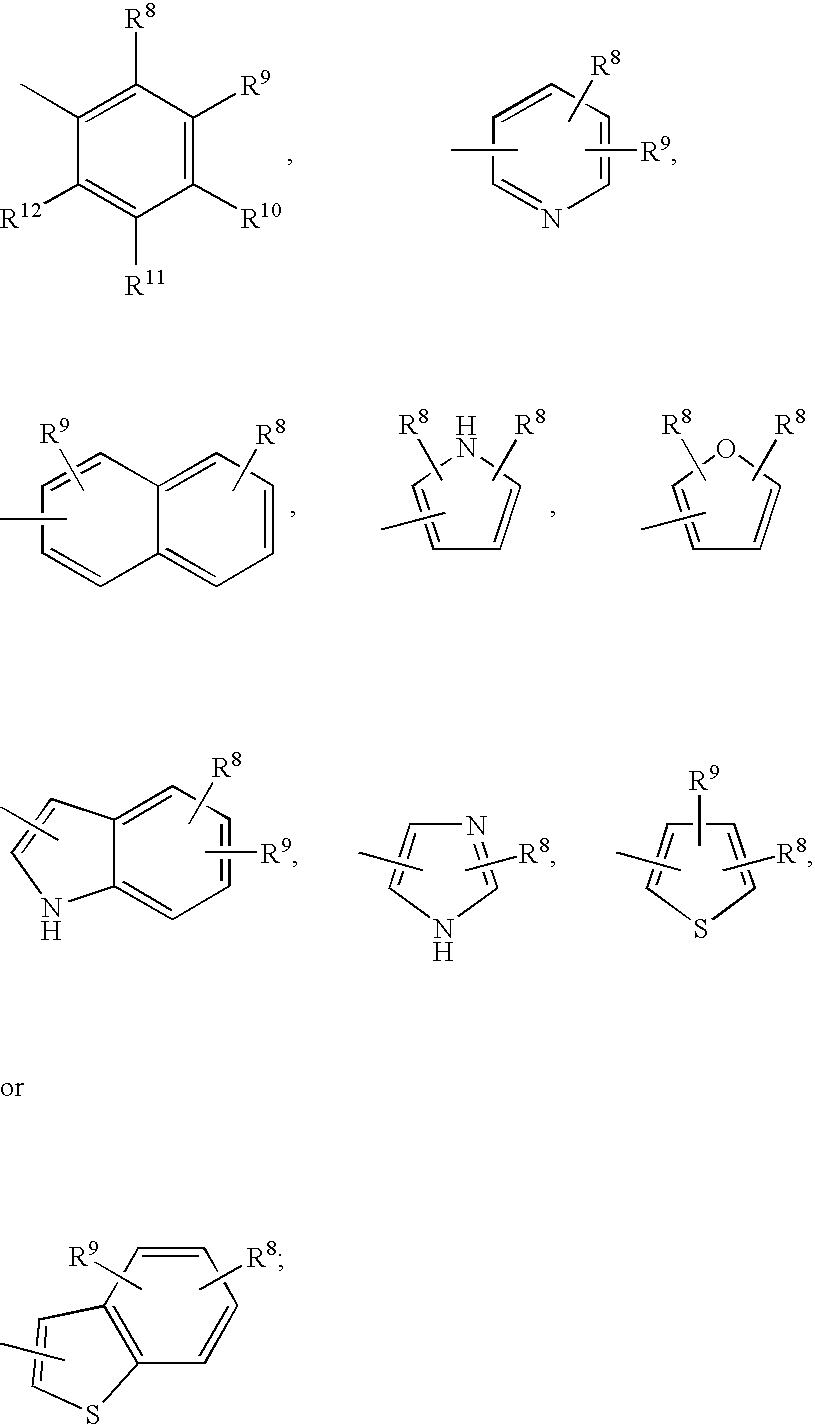
The present invention relates to novel heterocyclic compounds, according to Formula I, that stimulate endogenous production and / or release of growth hormone, wherein R1, R2, R3, R4 and X are defined herein. Further, the present invention relates to methods for using such compounds and to pharmaceutical compositions containing such compounds.
Owner:BRISTOL MYERS SQUIBB CO
Heavy metal resistance plant growth-promoting bacteria preparation and applying method thereof
InactiveCN101671636APromote absorptionPromote growthBiocidePlant growth regulatorsHigh resistanceDisease
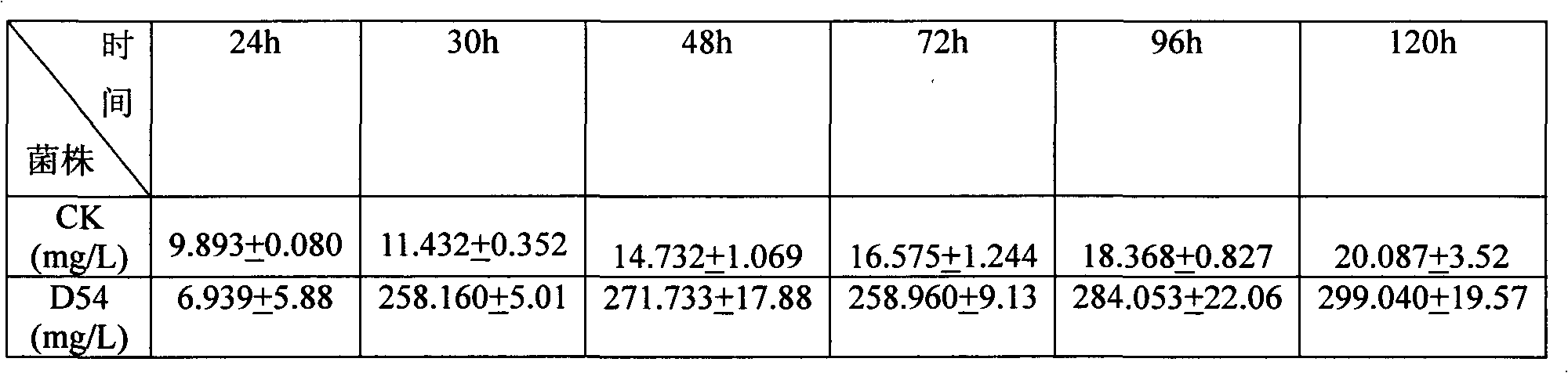
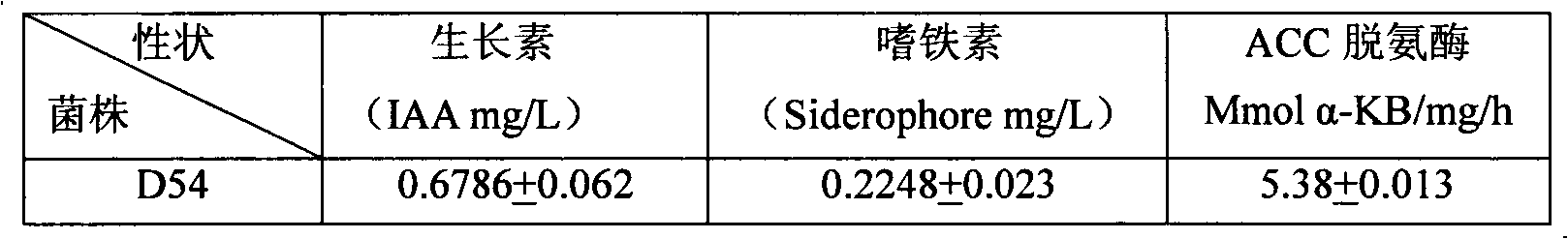
The invention relates to heavy metal resistance plant growth-promoting bacteria and a preparation applying method thereof, which belongs to the bioremediation field of heavy metal polluted environment. A bacteria strain D54 with a preservation number of CGMCC No. 3223 belongs to the Burkholderia sp. and has higher resistance to a plurality of heavy metals, wherein the resistances to Pb <2+>, Cd <2+>, Cu <2+>, Zn <2+> respectively reach 800 mg / L, 1500 mg / L, 150 mg / L and 2500 mg / L. In addition, the bacteria strain D54 has the plant growth-promoting functions of producing plant growth hormone (IAA), producing 1-amino-1-carboxyl cyclopropane (ACC) deaminase, secreting siderophore, dissolving inorganic phosphate, fixing nitrogen and the like, has the biological prevention functions of antagonizing plant pathogenic bacteria inbreak and the like, and can obviously improve the biomass of the plants applied the invention and improve the resistance to diseases and stresses. The number of the effective viable bacteria in liquid preparation reaches 1-2 billion / ml and the number of the effective viable bacteria in solid preparation reaches 1 billion / g. Soaking seeds for 1-2 hours in the liquidpreparation which is diluted 100 times and irrigating the diluted liquid preparation 1-2 times (10ml / kg) after 2-3 weeks of the sprouting of the seeds can effectively improve plant viable bacteria infection probability.
Owner:AGRO ENVIRONMENTAL PROTECTION INST OF MIN OF AGRI
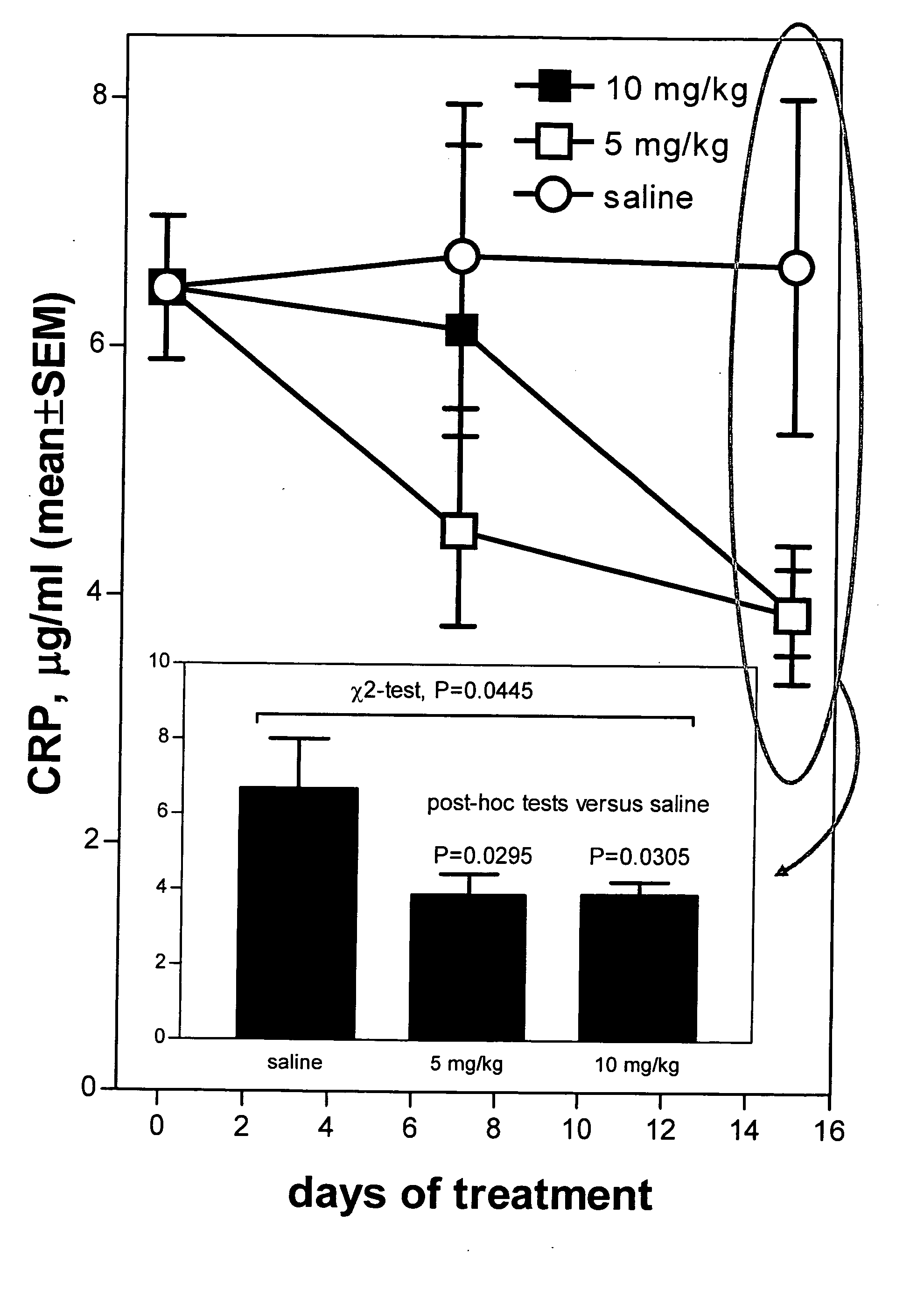
Method of reducing C-reactive protein using growth hormone secretagogues
The present invention relates to a method of reducing C-reactive protein in a subject in need of treatment thereof, wherein the subject is at risk of having or the subject has already had a vascular event or suffering from an inflammatory disease or disorder. In one embodiment, the vascular event is a cardiovascular event (e.g., myocardial infarction). In another embodiment, the vascular event is a cerebrovascular event (e.g., stroke (such as transient ischemic attacks (TIAs)). In yet another embodiment the vascular event is a peripheral vascular event (e.g., intermittent claudication). The method comprises administering a therapeutically effective amount of at least one growth hormone secretagogue compound or a pharmaceutically acceptable salt, hydrate or solvate thereof. The growth hormone secretagogue can be coadministered with a second growth hormone secretagogue, HMG CoA reductase inhibitor, an ACAT inhibitor, a CETP inhibitor, an anti-inflammatory agent, an ACE inhibitor, a Beta blocker, a cholesterol absorption inhibitor, a nicotonic acid, a fibric acid derivative, a bile acid sequestering agent or a combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
Medicinal product and method for treatment of conditions affecting neural stem cells or progenitor cells
InactiveUS20050032702A1Modulate proliferationModulate differentiationNervous disorderPeptide/protein ingredientsOligodendrocytePharmacy medicine
Use of a substance that upon administration will lead to increased concentrations of growth hormone, such as growth hormone, a functionally equivalent analogue thereof or a substance that will increase the release of endogenous growth hormone, for the production of a medicinal product for treatment of abnormal conditions affecting neural stem cells, progenitor cells and / or cells derived from neural stem cells or progenitor cells, especially conditions affecting the oligodendroglia, astroglia, and / or neuronal cells. In vitro and in vivo methods for inducing lineage determination, propagating and / or inducing or maintaining the genesis of neurons, oligodendrocytes, astroglial cells from progenitor cells, stem cells and / or cells derived from said cells by administrating to the cells a substance that increases the concentration of growth hormone. Also a method of reducing the genesis of oligodendrocytes, neurons, astroglial cells from progenitor cells or stem cells, wherein a pharmaceutically effective amount of a substance that will lead to a decreased concentration of growth hormone or a functionally equivalent analogue thereof is administered to said patient.
Owner:ERIKSSON PETER
Methods of using macrocyclic modulators of the ghrelin receptor
ActiveUS20090170757A1Tripeptide ingredientsCyclic peptide ingredientsSomatotropic hormonePharmaceutical Substances
The present invention provides novel conformationally-defined macrocyclic compounds that have been demonstrated to be selective modulators of the ghrelin receptor (growth hormone secretagogue receptor, GHS-R1a and subtypes, isoforms and variants thereof). Methods of synthesizing the novel compounds are also described herein. These compounds are useful as agonists of the ghrelin receptor and as medicaments for treatment and prevention of a range of medical conditions including, but not limited to, metabolic and / or endocrine disorders, gastrointestinal disorders, cardiovascular disorders, obesity and obesity-associated disorders, central nervous system disorders, genetic disorders, hyperproliferative disorders and inflammatory disorders.
Owner:OCERA THERAPEUTICS INC
Green biological seedling raising matrix
InactiveCN102126877APromote differentiationNutritional diversityBio-organic fraction processingOrganic fertiliser preparationDiseaseNicotiana tabacum
The invention discloses a green biological seedling raising matrix, relating to the seedling raising matrix technology. The preparation method comprises the following steps of: mixing straw powder, organic fertilizer and nutrient soil to be uniform, adding water until the water content is 45-55%, adding compound bacteria and then mixing to be uniform, stacking for fermenting, turning when the temperature increases to 50 + / - 2 DEG C, turning and cooling for 1-2 days when the temperature is increased to 50 DEG C again, adding microelements and growth hormone, stirring to be uniform, and packaging. The invention has the beneficial effects that: (1) the matrix is rich in organic matters and beneficial biological germs, has comprehensive nutrition and is loose and soft; (2) the matrix is seed-saving and labour-saving and can shorten seedling raising period; (3) the matrix can be used to ensure uniform and strong seedlings and high sprout rate; (4) the matrix can protect roots and hold water and fertilizer and has good air permeability; (5) the matrix has the functions of promoting flower bud differentiation and improving the yield; and (6) the matrix has the effect of resisting soil diseases. The matrix disclosed by the invention is applicable to seedling raising of vegetable, melon and fruit, flower and plant, tea, tobacco and cotton.
Owner:徐贵阁
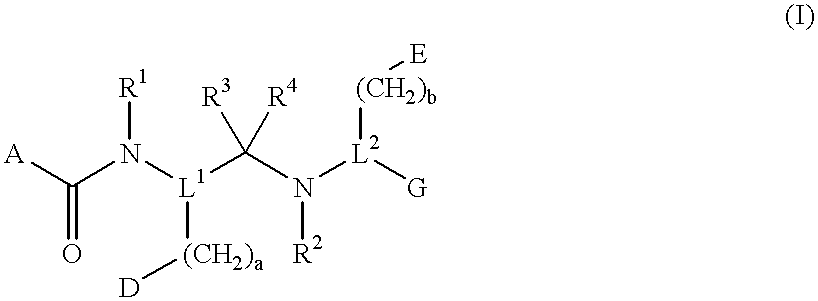
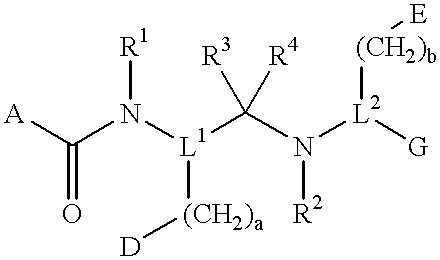
Compounds with growth hormone releasing properties
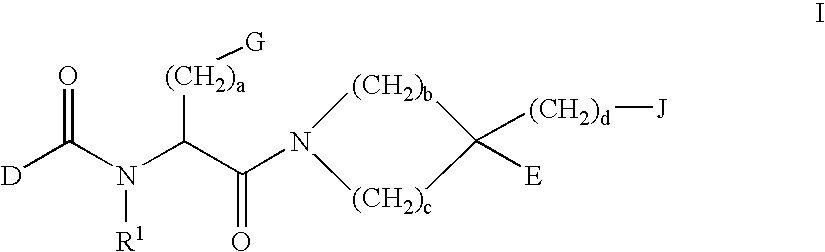
InactiveUS6274584B1Promote growthIncrease ratingsPowder deliveryBiocideMedical disorderBioavailability
Novel peptide derivatives, compositions containing them, and their use for treating medical disorders resulting from a deficiency in growth hormone are disclosed. The peptides have the formula (1):wherein a, b, A, R1, L1, D, R3, R4, R2, L2, E and G are as defined in the specification. These peptides exhibit improved resistance to proteolytic degradation, and hence, improved bioavailability.
Owner:HELSINN THERAPEUTICS (US) INC
Somatostatin antagonists and agonists that act at the sst subtype 2 receptor
InactiveUS20020128206A1Ease of detectabilityEasy to prepareDipeptide ingredientsMetabolism disorderArylMammal
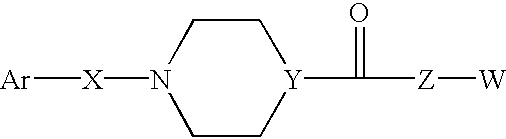
Compounds according to the formula: 1 and pharmaceutically acceptable salts, solvates or hydrates thereof, wherein group Ar is optionally substituted (C.sub.6-C.sub.10)aryl or (C.sub.1-C.sub.9)heteroaryl; X is a direct link, --CH.sub.2 --, --SO.sub.2 --, --CO--, --CHR.sup.1-- where R.sup.1 is(C.sub.1-C.sub.6) alkyl, or --CR.sup.1'R.sup.1"-where both R.sup.1' and R.sup.1" are, independently, (C.sub.1-C.sub.6)alkyl; Y is N or CH; and Z and W are as herein defined, and pharmaceutical compositions thereof, and methods useful to facilitate secretion of growth hormone(GH) in mammals.
Owner:HAY BRUCE A +2
Cysteine variants of alpha interferon-2
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby. Also disclosed are therapeutic methods for using the cysteine variants of the invention.
Owner:BOLDER BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com