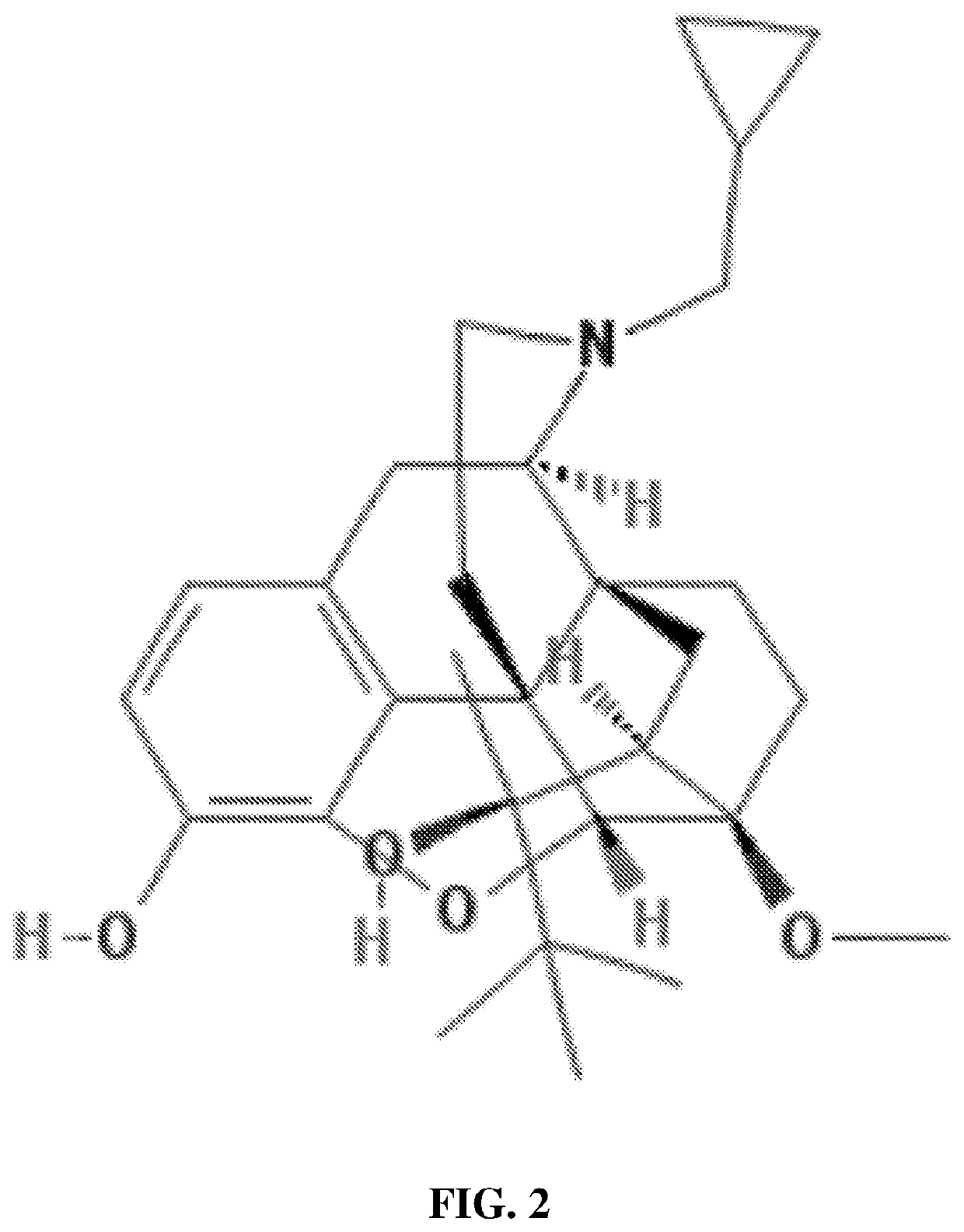
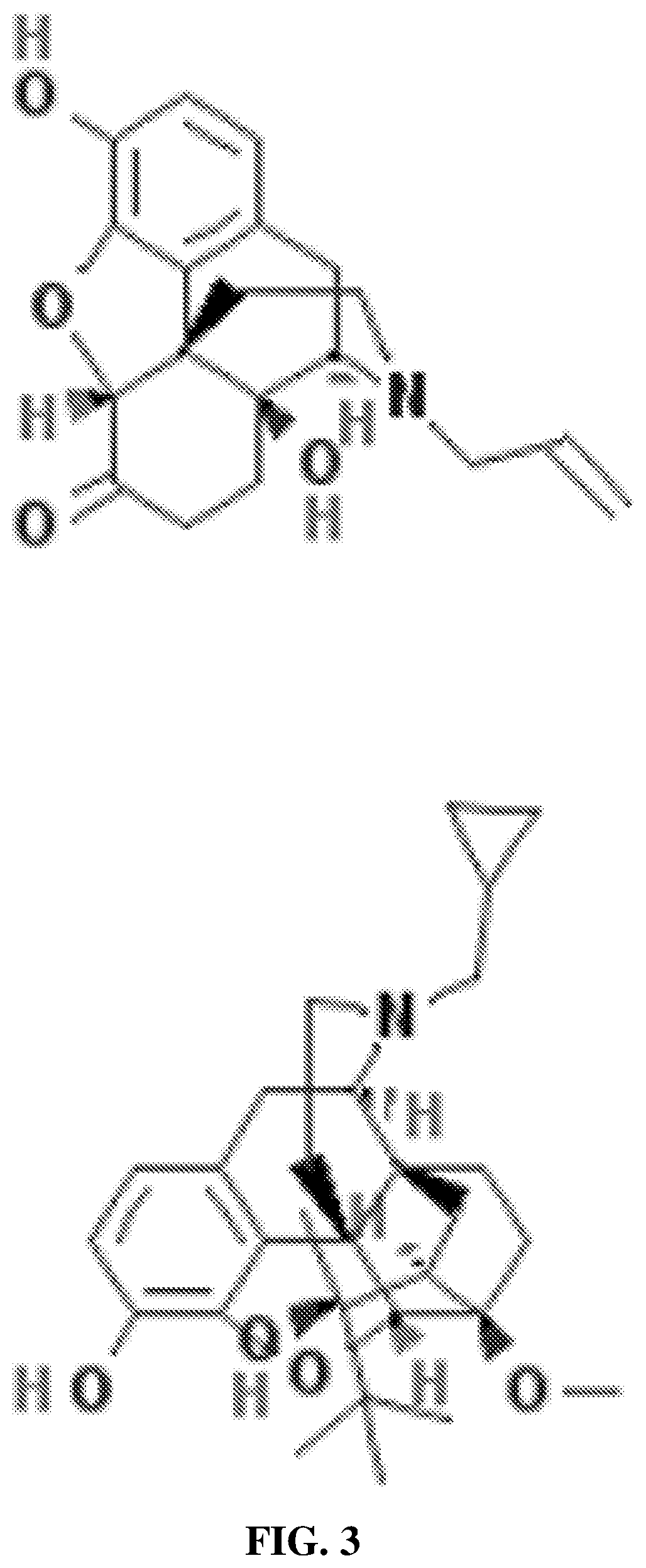
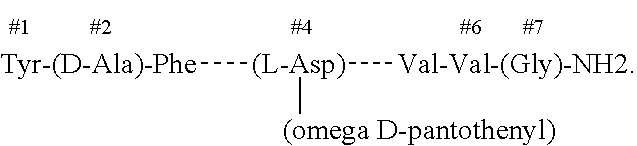Patents
Literature
32 results about "Opioid withdrawal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
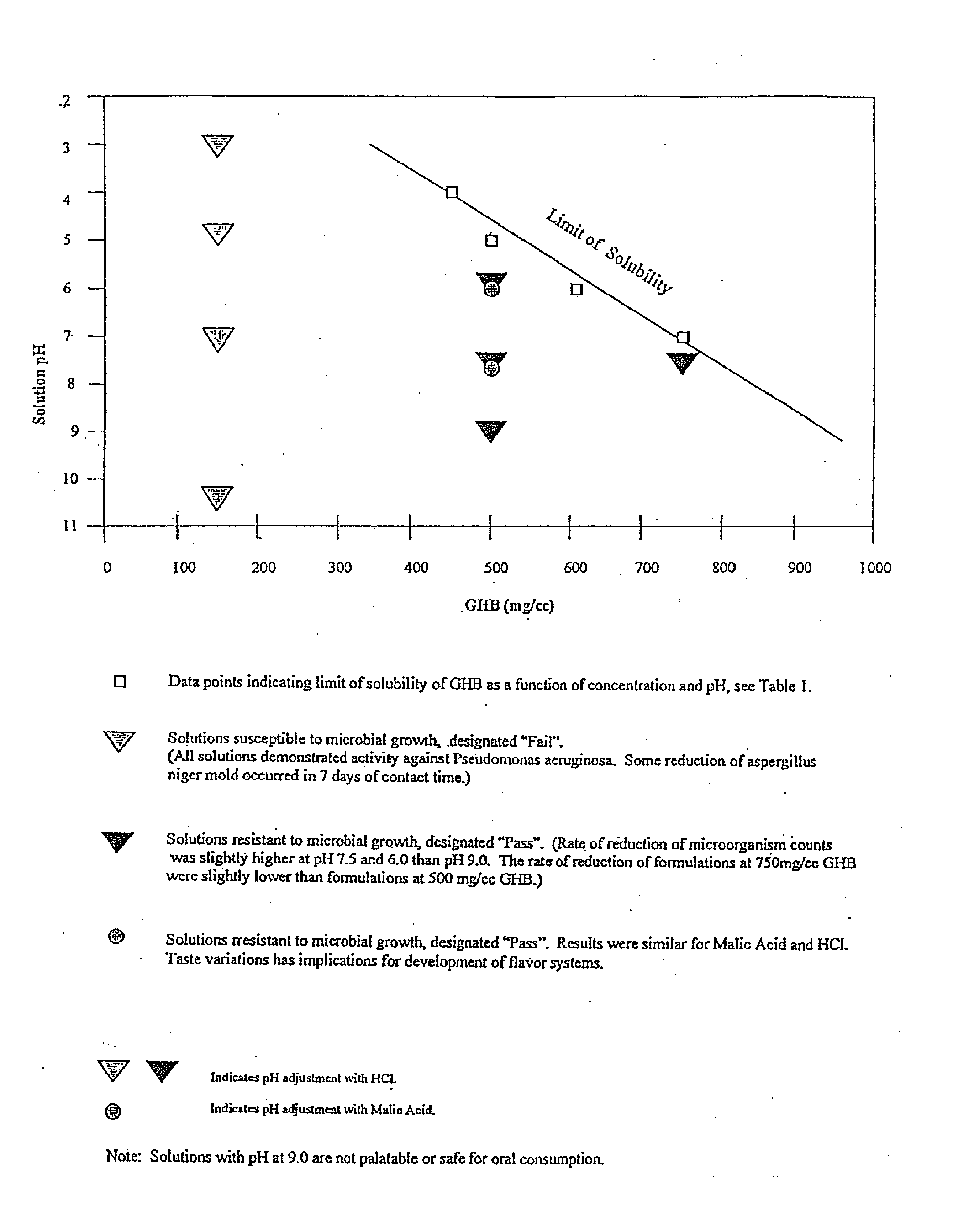
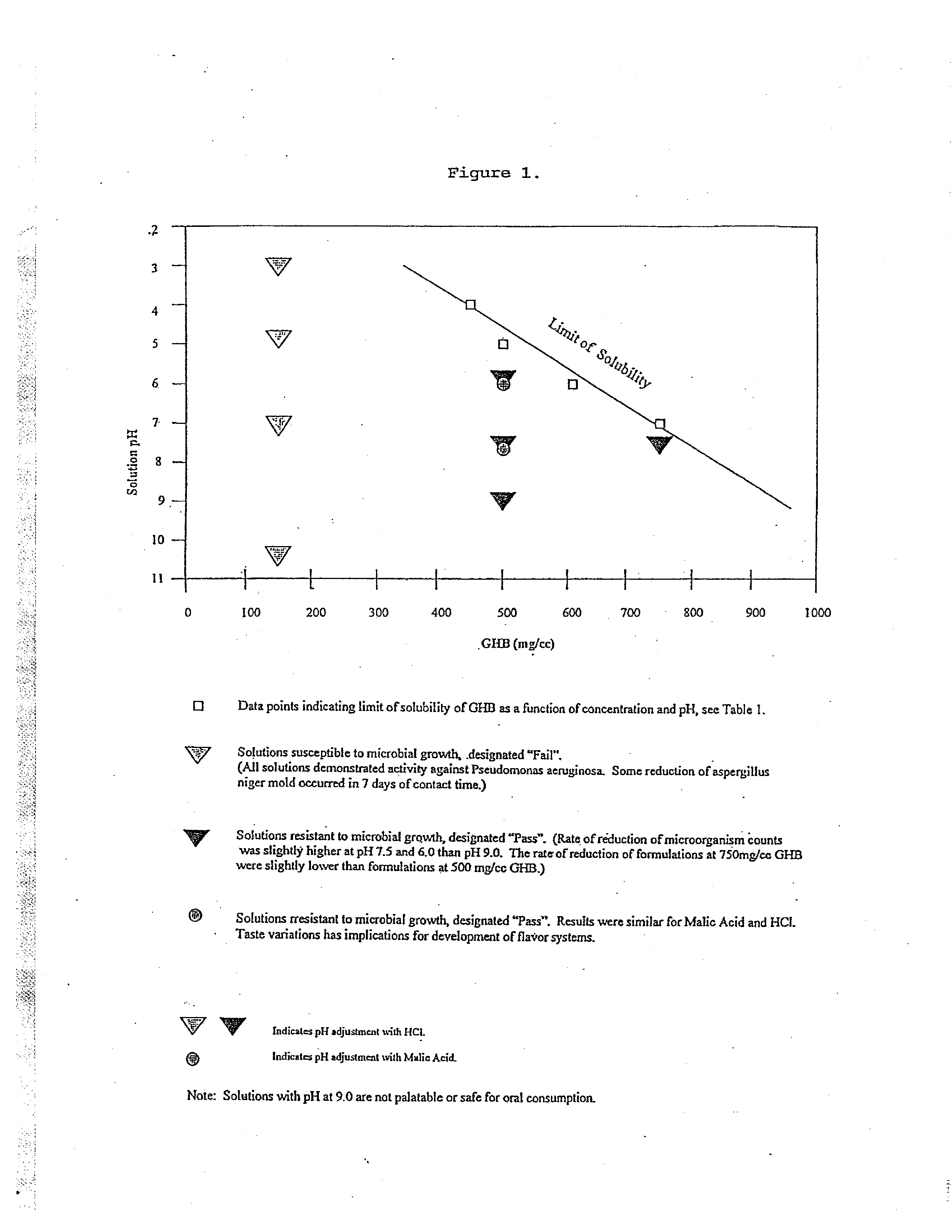
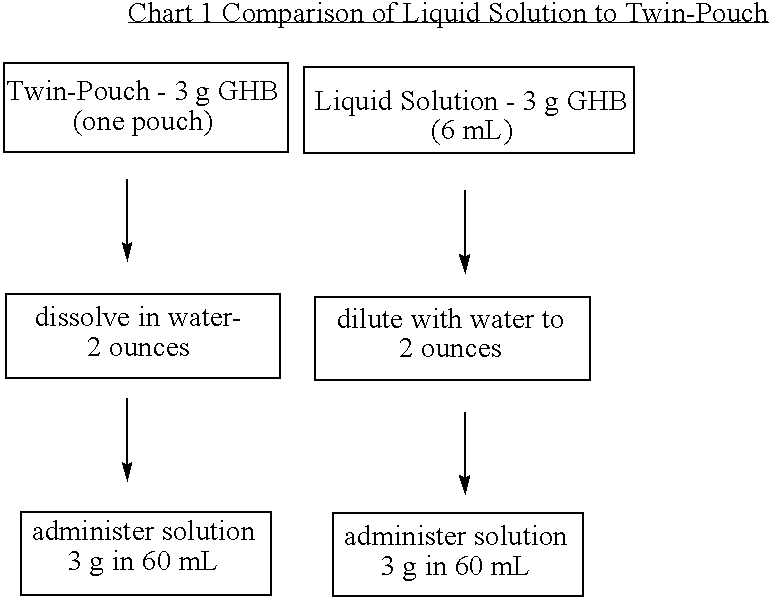
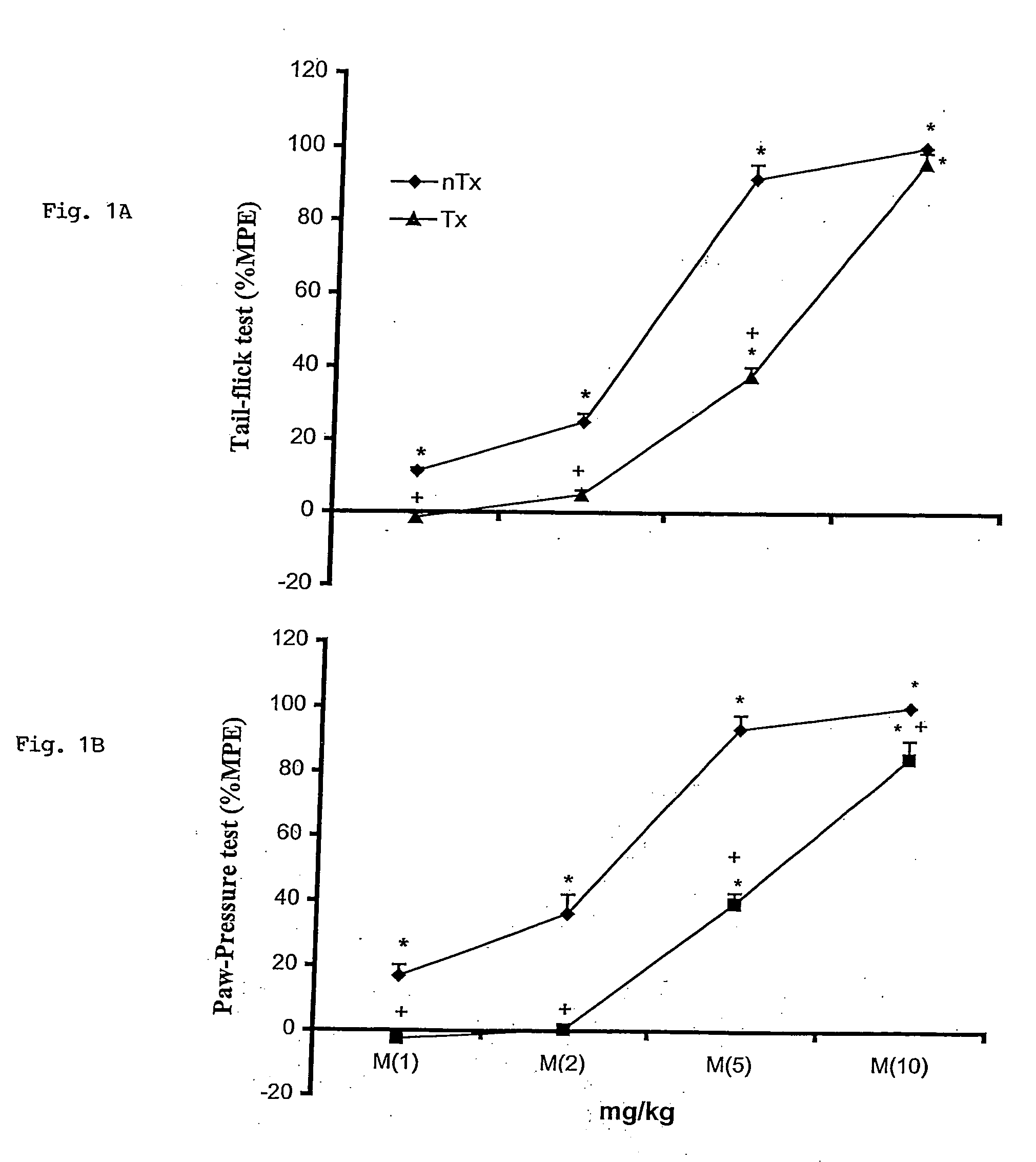
Microbiologically sound and stable solutions of gamma-hydroxybutyrate salt for the treatment of narcolepsy
InactiveUS20070270491A1Lower Level RequirementsImprove the level ofBiocideNervous disorderOpioid withdrawalNarcolepsy
Disclosed are formulations of gamma-hydroxybutyrate in an aqueous medium that are resistant to microbial growth. Also disclosed are formulations of gamma-hydroxybutyrate that are also resistant to the conversion into GBL. Disclosed are methods to treat sleep disorders, including narcolepsy, with these stable formulations of GHB. The present invention also provides methods to treat alcohol and opiate withdrawal, reduced levels of growth hormone, increased intracranial pressure, and physical pain in a patient.
Owner:JAZZ PHARMA INC
Compositions and method for enhancing the therapeutic activity of opioids in treatment of pain
Compositions and methods for inhibiting opioid tolerance and opioid withdrawal-induced hyperalgesia are provided.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
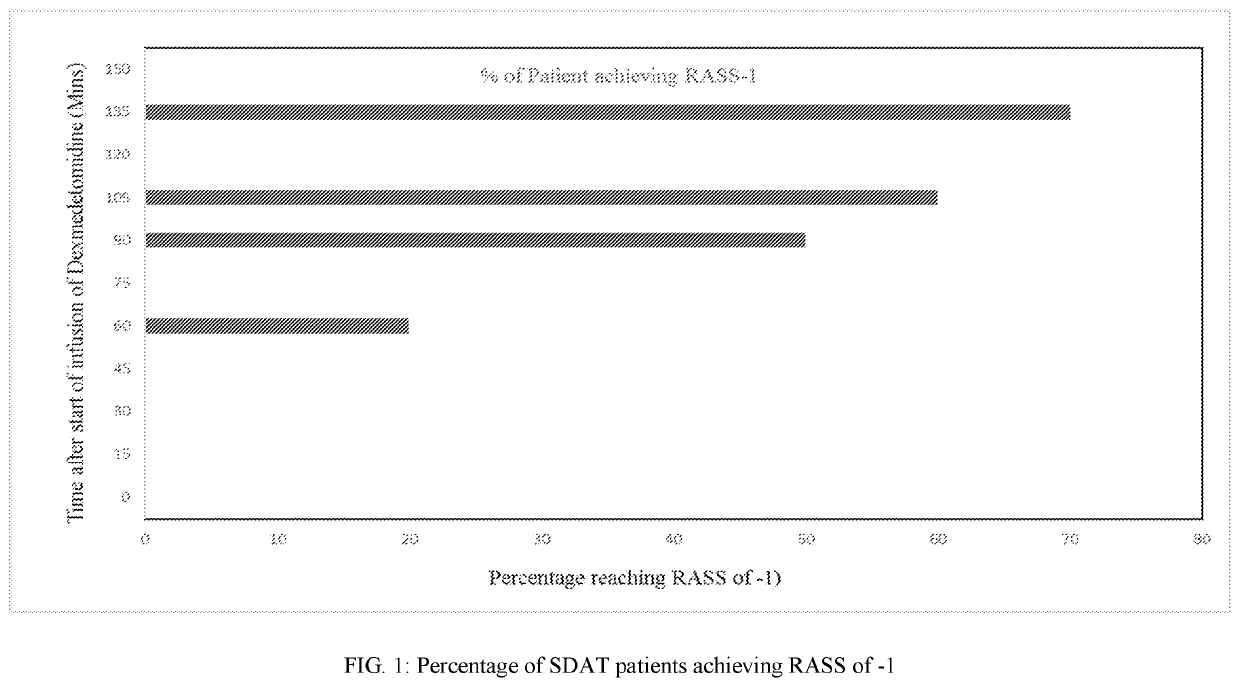
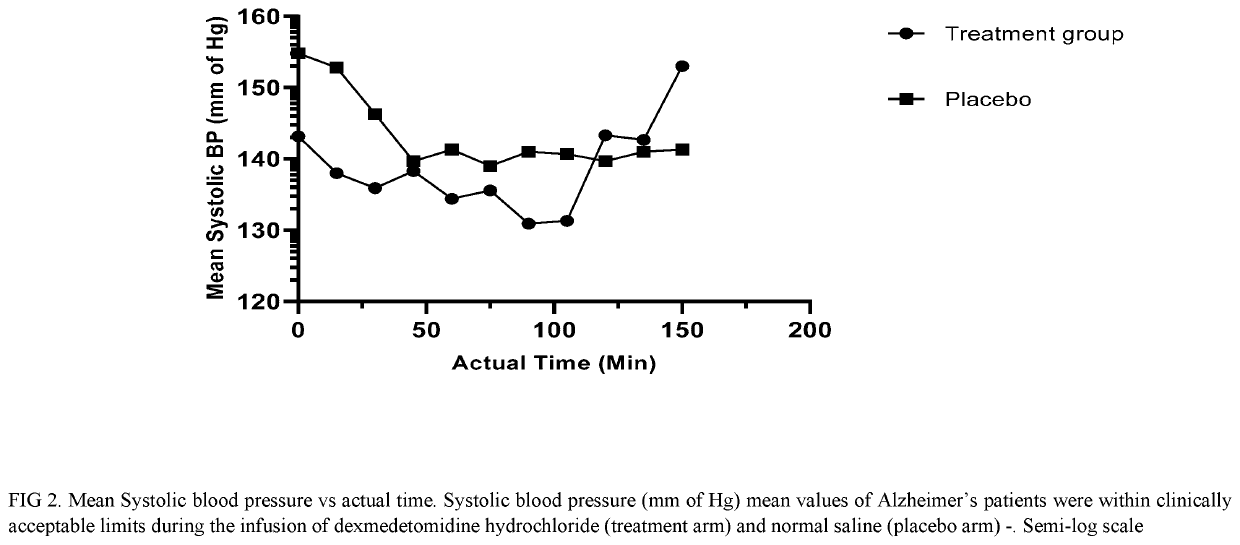
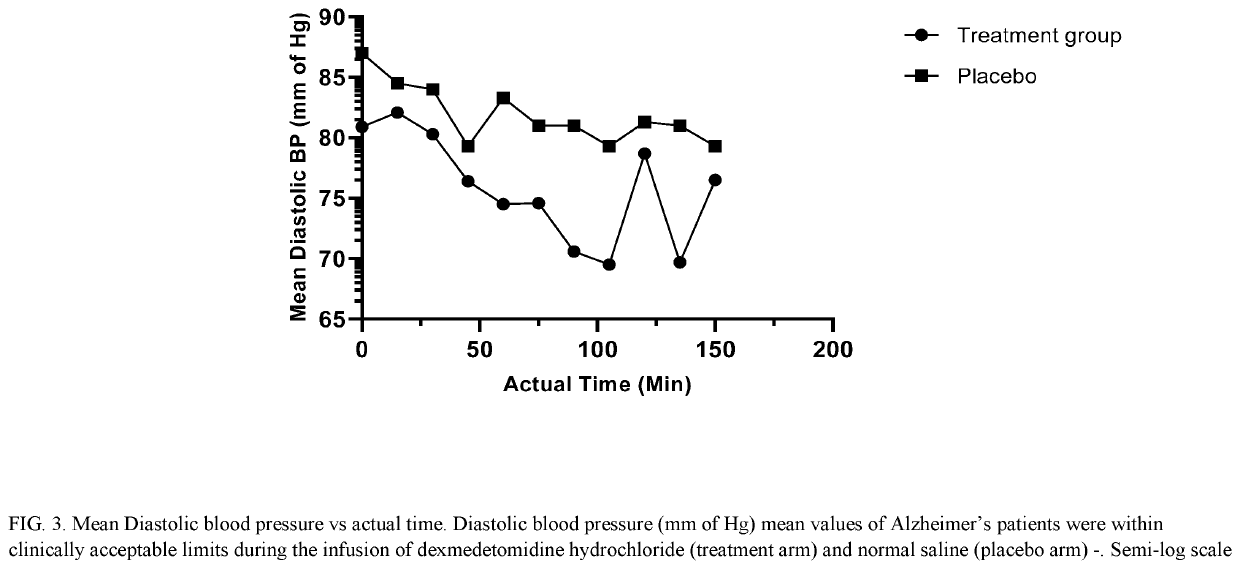
Methods for treating agitation using dexmedetomidine hydrochloride
PendingUS20210267944A1Reduce agitationReduce signOrganic active ingredientsNervous disorderIntravenous routePharmaceutical drug
The present disclosure relates to the treatment of agitation or signs of agitation in certain human subjects, including subjects with a neurodegenerative, neuropsychiatric or opioid withdrawal disorder, by administering dexmedetomidine hydrochloride by the intravenous route.
Owner:BIOXCEL THERAPEUTICS INC
Carboxylic diarythiazepineamines as mixed mu-and delta-opioid receptor agonists
PendingUS20200079745A1Organic chemistryHeterocyclic compound active ingredientsPharmaceutical drugPharmaceutical medicine
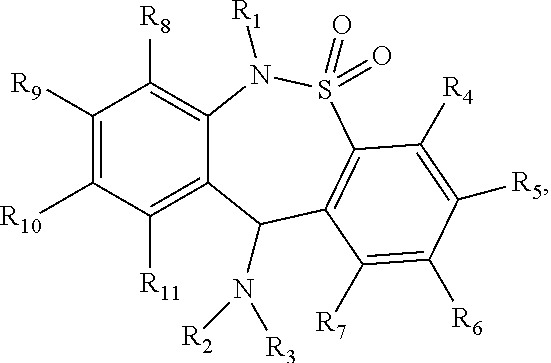
The present invention provides a compound having the structure:or a pharmaceutically acceptable salt or ester thereof, and a method of treating a subject afflicted with a pain, a depressive disorder, a mood disorder, an anxiety disorder, borderline personality disorder, opioid addiction, or opioid withdrawal symptoms by administering the compound to the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Use of opioid receptor antagonist for gastrointestinal tract disorders
The disclosure relates to a method of treating or preventing a condition in a subject associated with the activation of an opioid receptor in the periphery by administering an effective amount of 5-(2-methoxy-4-{[2-(tetrahydro-pyran-4-yl)-ethylamino]-methyl}-phenoxy)-pyrazine-2-carboxamide (Compound I). In particular, the disclosure relates to a method of treating or preventing opioid- induced constipation or opioid-induced bowel dysfunction in a human without reducing centrally-mediated opioid analgesia or producing central opioid withdrawal symptoms by administering an effective amount of Compound (I). The disclosure further relates to the use of Compound (I) for the preparation of a medicament for the treatment or prevention of a condition in a subject associated with the activation of an opioid receptor in the periphery.
Owner:APOLOR CORP
Pharmaceutical formulations comprising opioid receptor agonist as active ingredients, methods of manufacture and therapeutic uses thereof
ActiveUS20190117556A1Formula SafetyAdequate shelf lifeOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTDrug withdrawal syndrome
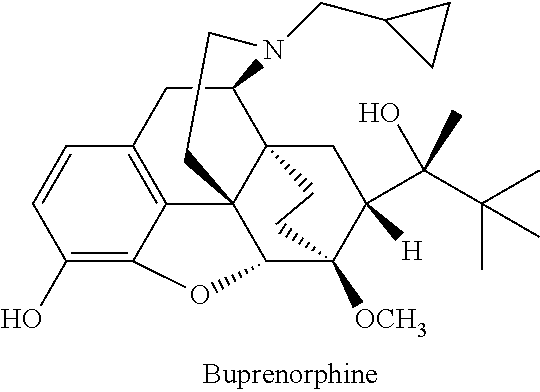
Formulation comprising buprenorphine or a pharmaceutically acceptable salt thereof as the sole active ingredient, a viscosity enhancer, and a buffering agent in an amount to provide a pH of from 5.0 to 7.0 are useful for treating opioid withdrawal syndrome.
Owner:CHIESI FARM SPA
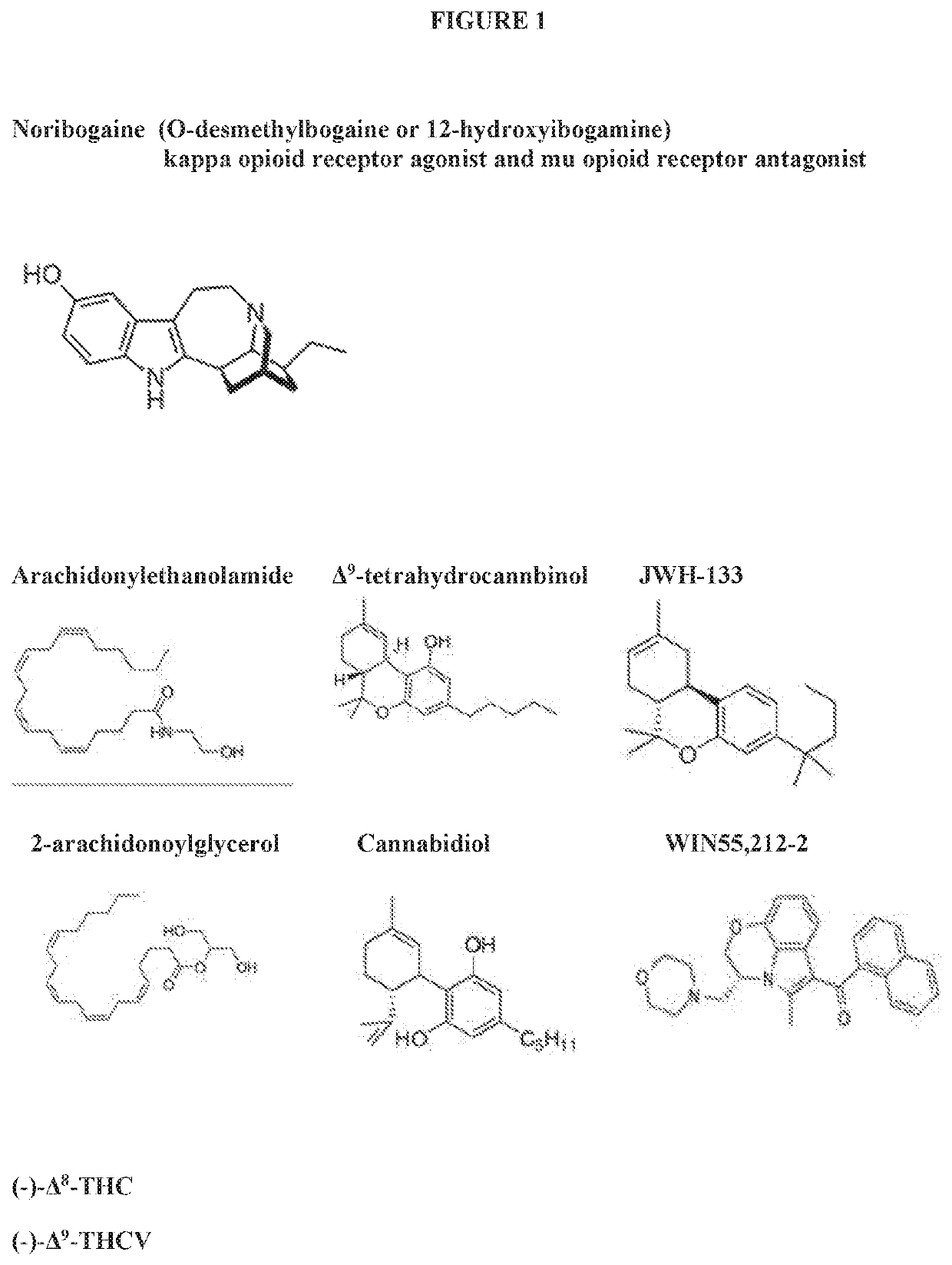
Methods for the non-toxic treatment for opioid drug withdrawal combining noribogaine and cannabinoids
PendingUS20220265675A1Without leading to any significant deleterious clinical consequenceImprove efficacyNervous disorderHydroxy compound active ingredientsSubstance abuserDrug withdrawal
The present invention is directed to the treatment of chemical substance abuse and withdrawal symptoms, especially withdrawal symptoms associated with opioids, utilizing a combination of an effective amount of a cannabinoid, in particular, cannabidiol (CBD) and / or A9-tetrahydrocannabinol (THC) in combination with low dosage noribogaine or a derivative salt, solvate or ansolvate thereof to treat withdrawal symptoms, both acute and longer term. It has unexpectedly been discovered that the cannabinoids as described herein work in conjunction with noribogaine to synergistically ameliorate and / or attenuate chemical substance abuse, especially including opioid withdrawal symptoms, both acute and longer term symptoms, such that low dose noribogaine (often in daily dosages of 30 milligrams or less) may be favorably co-administered to a patient suffering from withdrawal symptoms, substantially shortening the QT interval of the patient and providing for a particularly safe and effective method to treat opioid withdrawal symptoms.
Owner:DEMERX
Method for treating neonatal opiod withdrawal syndrome
InactiveUS20200330455A1Stable and fastReduce usageOrganic active ingredientsNervous disorderMedicineDrug withdrawal syndrome
Owner:CHIESI FARM SPA
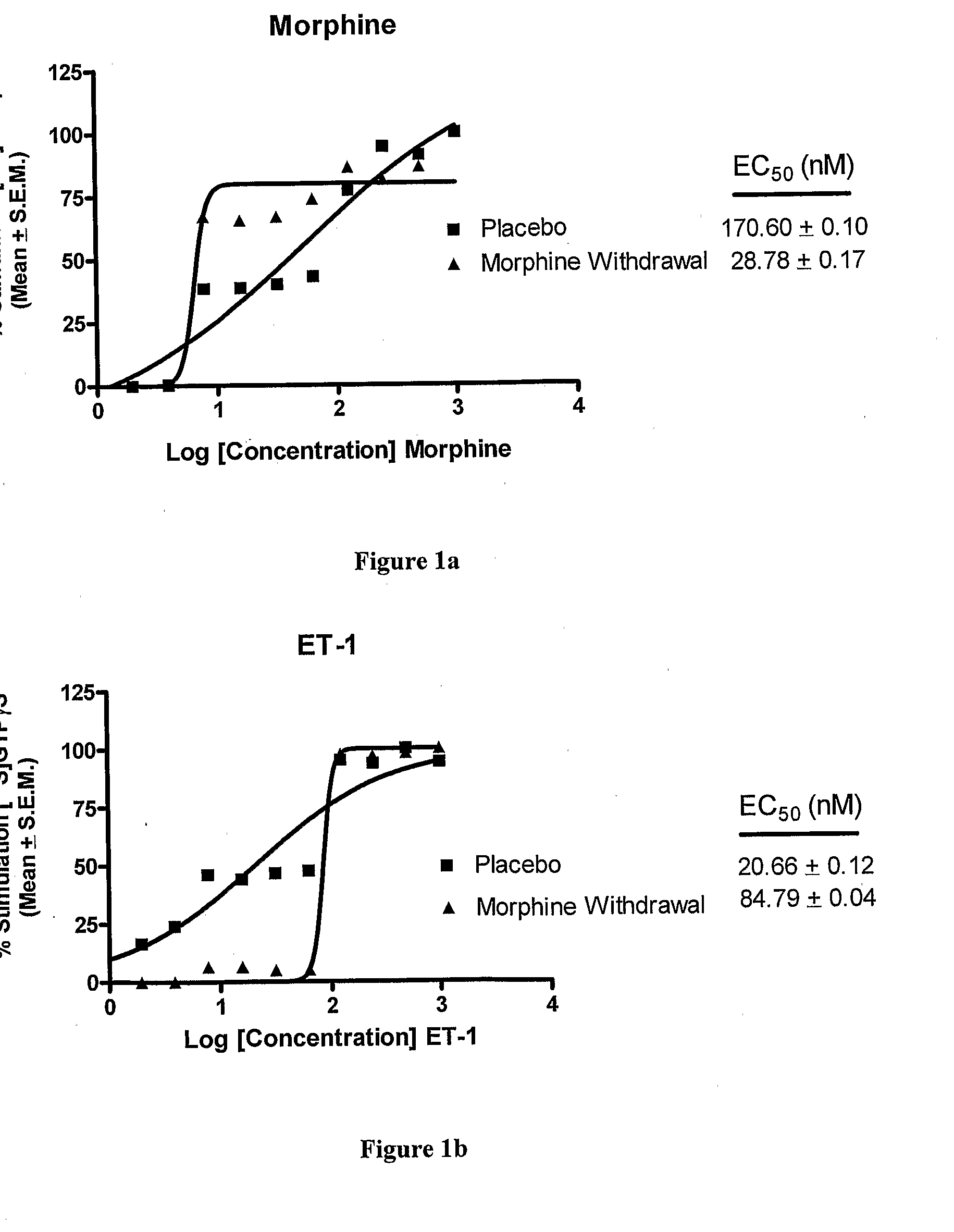
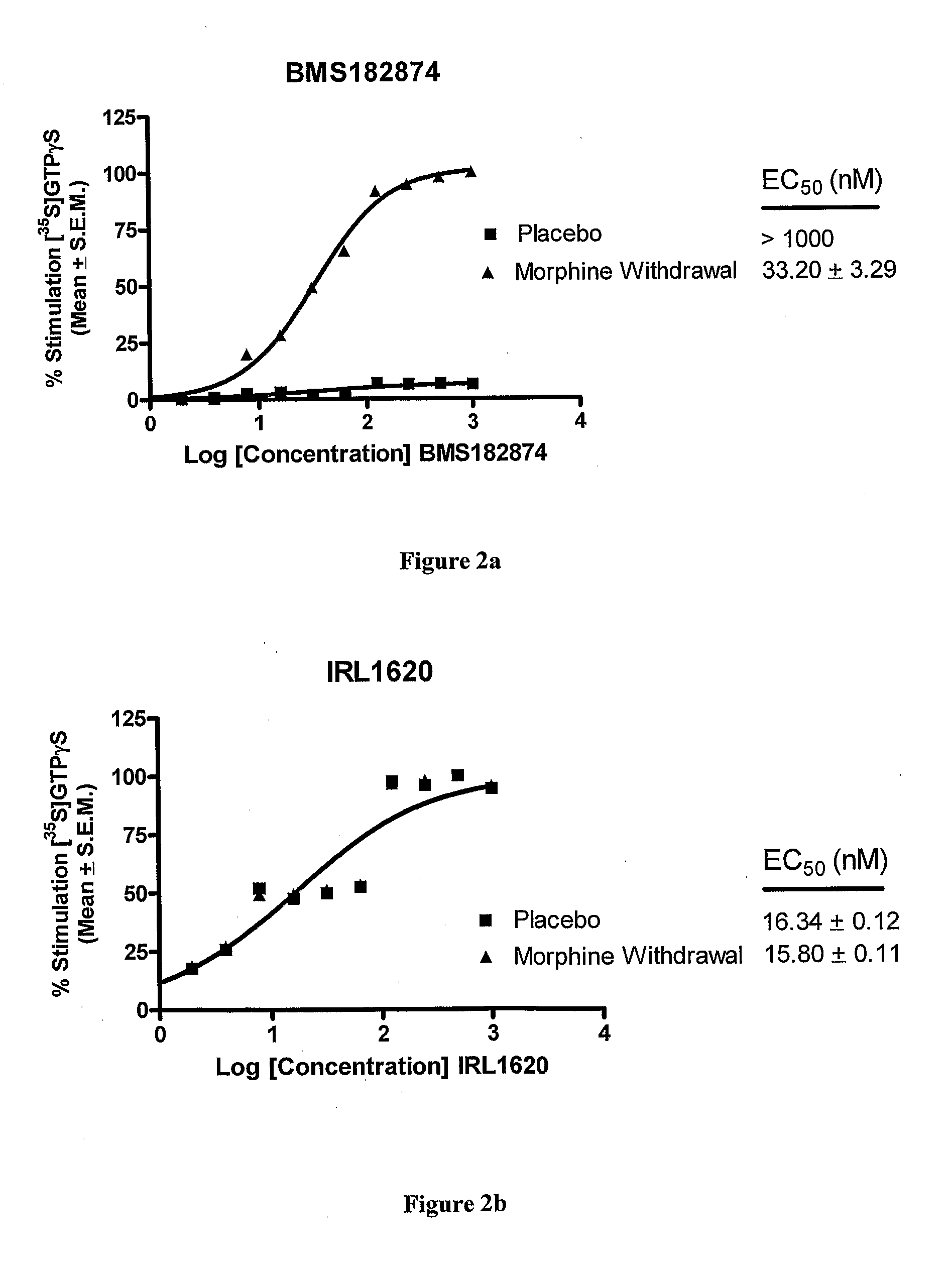
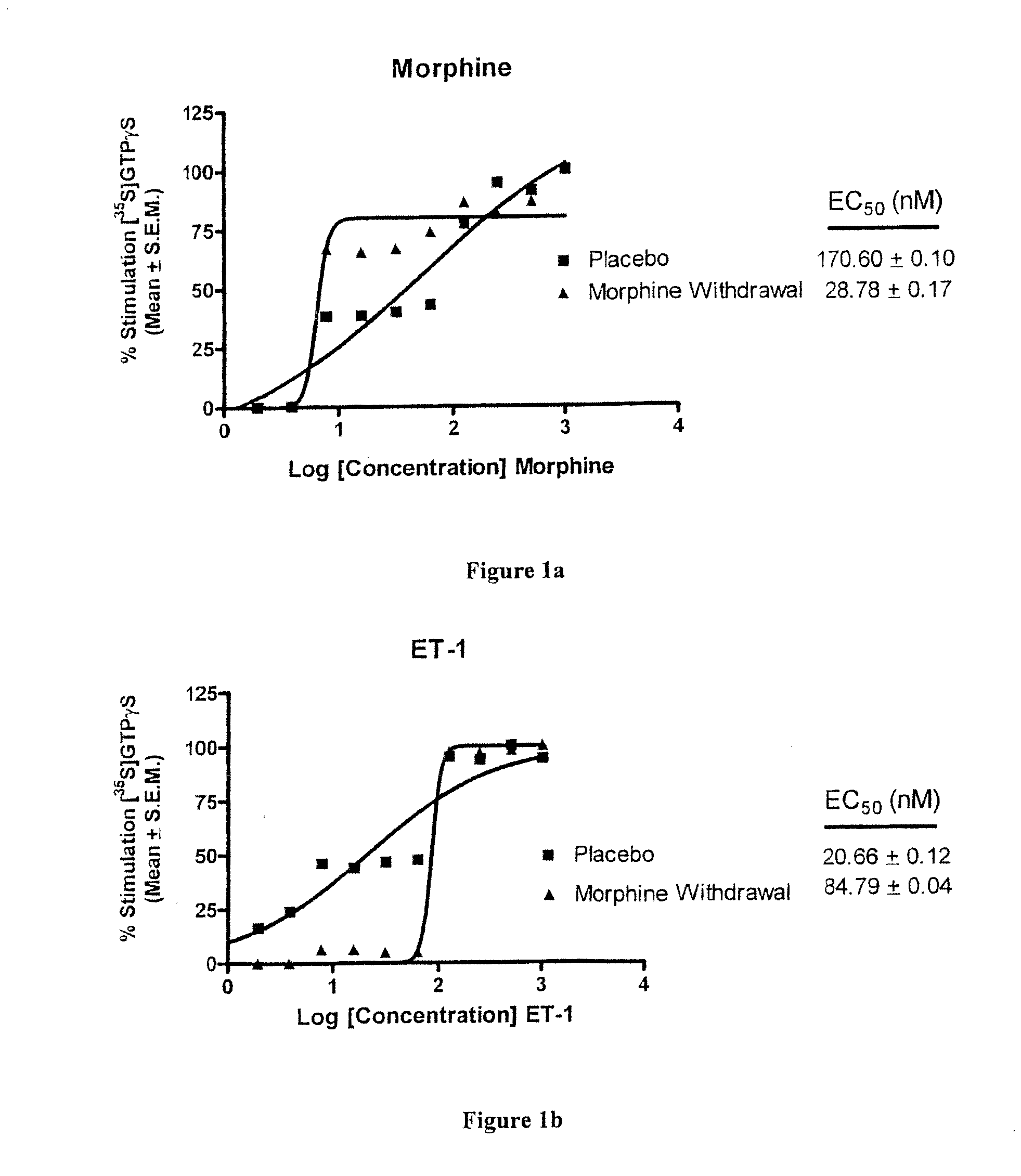
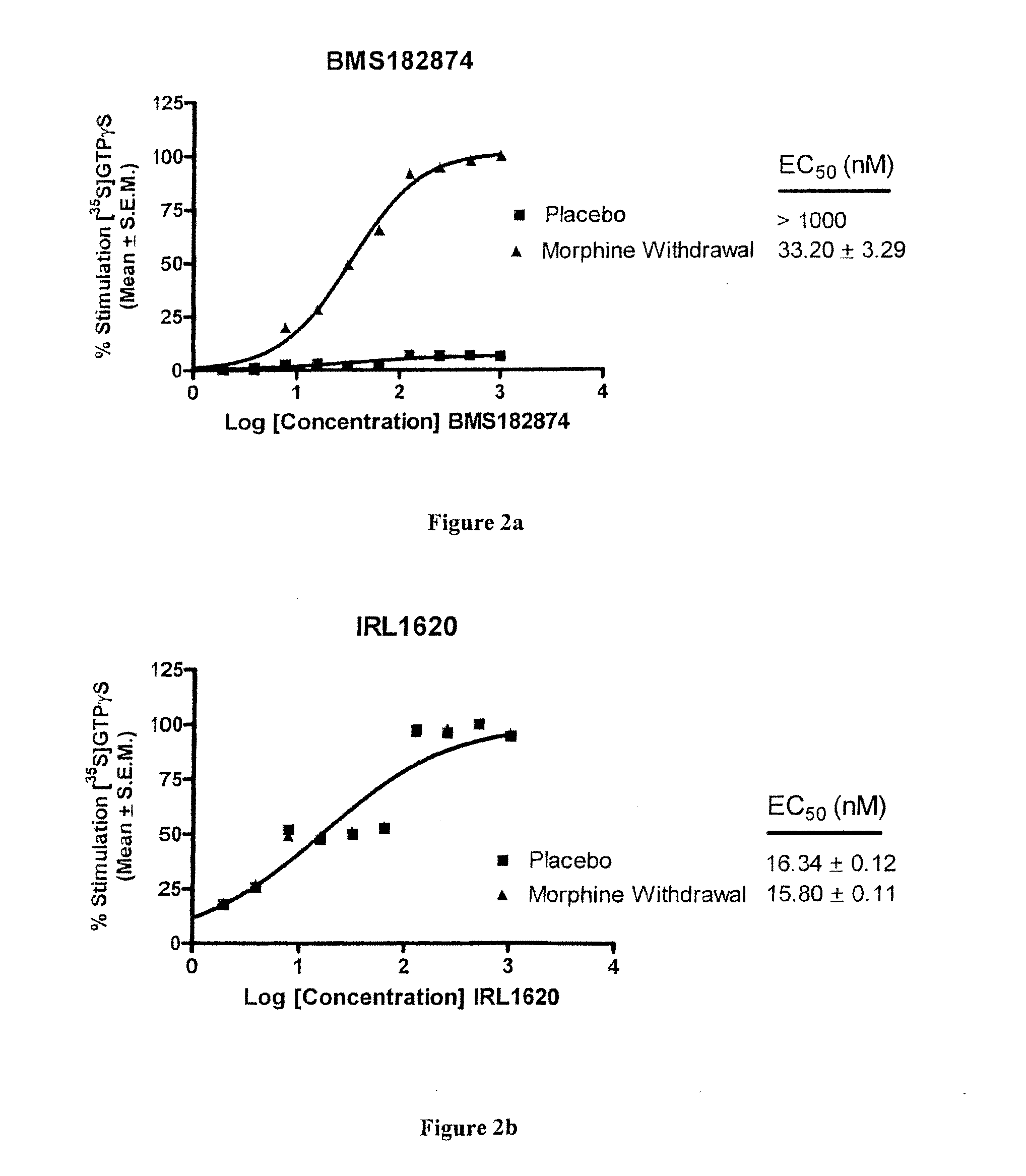
Endothelin Receptors In Morphine Withdrawal
InactiveUS20090221490A1Improve toleranceEasy to manageOrganic active ingredientsNervous disorderTolerabilityMedicine
The present invention relates to compositions and methods for managing opioid tolerance and reducing opioid withdrawal. More specifically, the present invention provides for endothelin, endothelin receptors, and endothelin receptor antagonists and agonists as a means for managing G-protein activity in the context of opioid tolerance and withdrawal.
Owner:GULATI ANIL +1
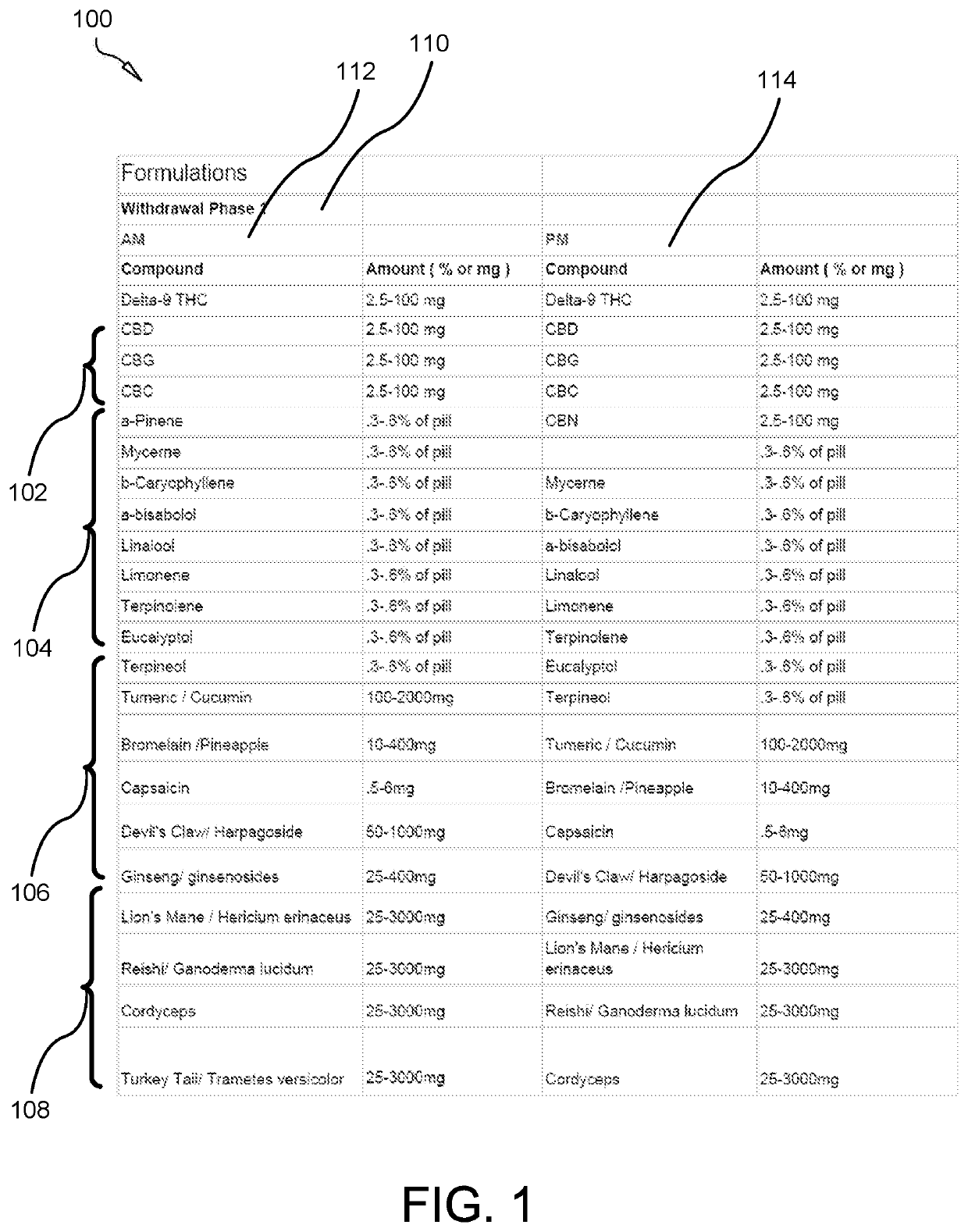
Composition for treating opioid withdrawal and method of manufacture
PendingUS20220218649A1Improve bioavailabilityAvoid symptomsHydrocarbon active ingredientsHydroxy compound active ingredientsTherapeutic effectPsychiatry
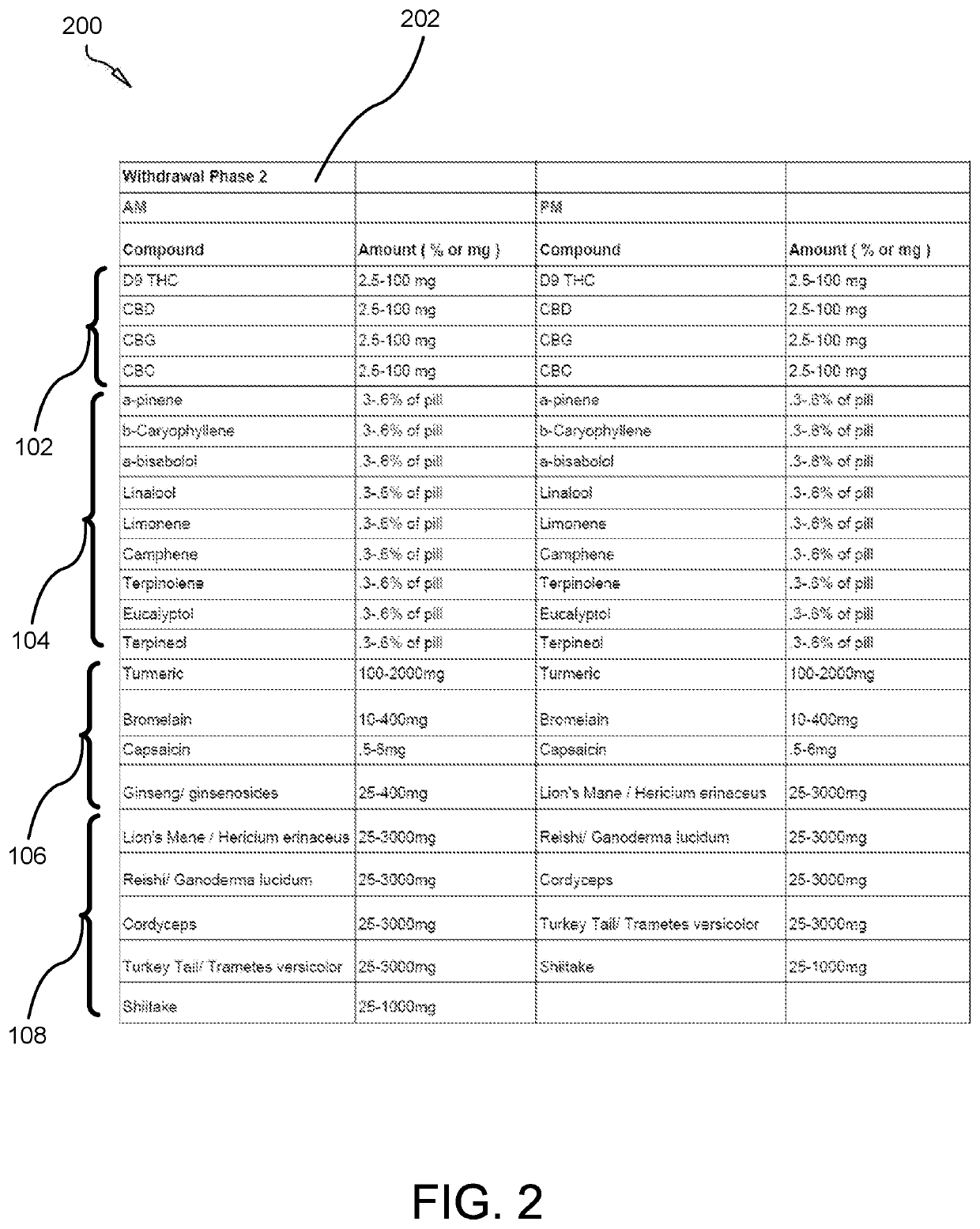
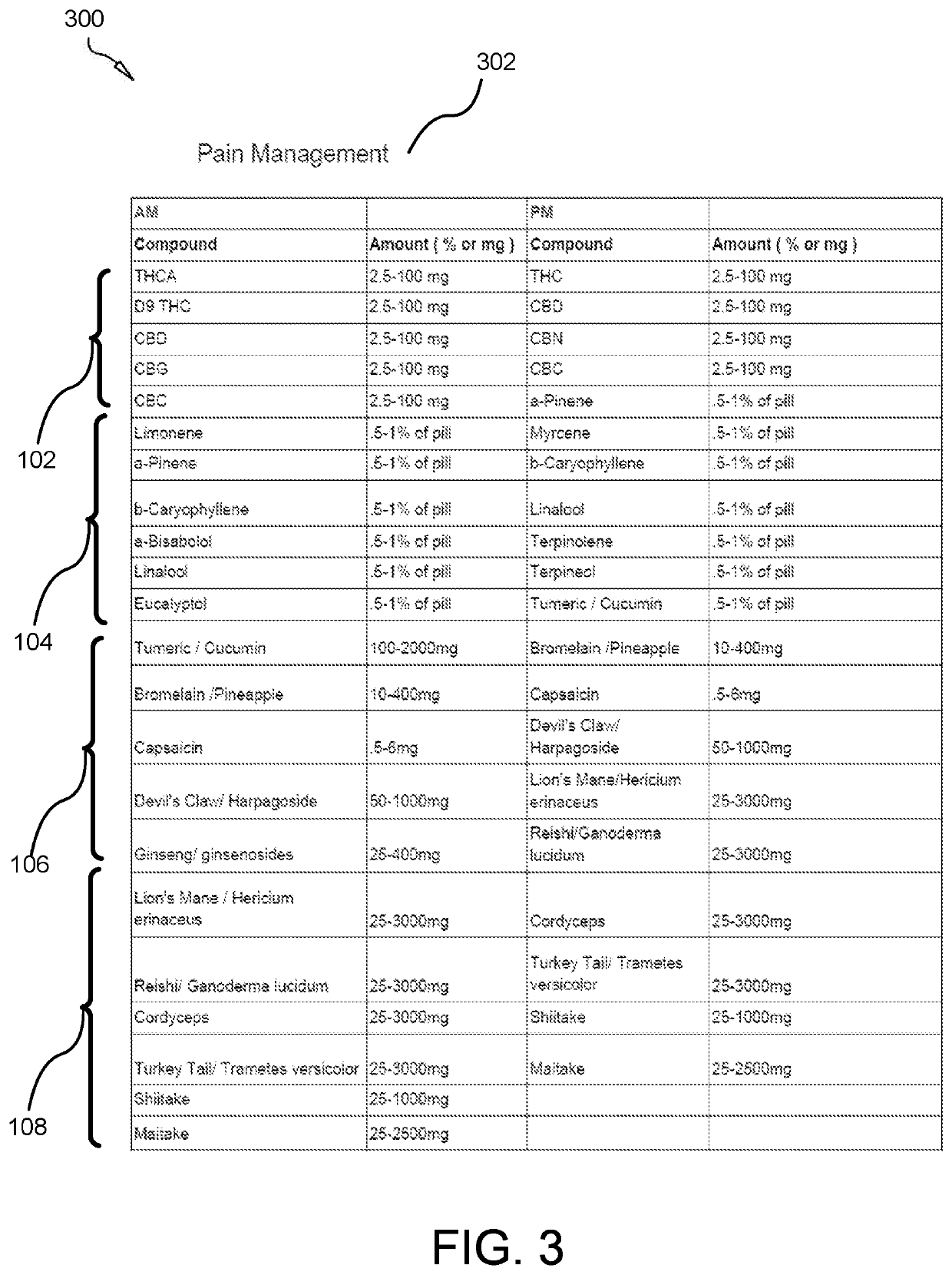
A composition for treating opioid withdrawal and method of manufacture and administration of the composition creates a synergistic combination of cannabinoids, terpenoids, herbs, and medicinal mushrooms that help to eliminate the symptoms of opioid withdrawal by creating a medicinal entourage. The composition combines: between about 2.5 to 100 milligrams of a cannabinoid, between about 0.084 to 33.6 milligrams of a terpenoid, between about 0.5 to 3000 milligrams of an herb, and between about 25 to 2500 milligrams of a medicinal mushroom. The composition is orally administered in a lipid-enclosed capsule form. The terpenoid has a lipophilic nature that enhances the bioavailability of the cannabinoid. The herb works with the body's endocannabinoid system to enhance pain relief capacity of the cannabinoid, so as to help inhibit symptoms of opioid withdrawal. The medicinal mushroom comprises a psychedelic drug that creates a therapeutic effect with the cannabinoid to inhibit symptoms of opioid withdrawal.
Owner:NGUYEN MAI
Methods and compositions for modulating opioid withdrawal symptoms
ActiveCN109641030AImprove severityPromote activationCompounds screening/testingOrganic active ingredientsPlaceboPharmaceutical drug
A method of screening a compound or a composition for use in modulating opioid withdrawal symptoms in a mammal. The method comprises the steps of: selecting a group of test animals; separating the group into two subgroups; inducing pannexin -1 activation or expression in both subgroups; dosing a first subgroup with a candidate compound; dosing a second subgroup with a placebo; measuring ATP released in spinal microglia of test animals from both subgroups; quantifying the difference in ATP released in spinal microglia of test animals from the first subgroup and the second subgroup; if the difference in the ATP released in the first subgroup and the ATP released in the second subgroup is greater than 25%, then formulating the candidate compound into a pharmaceutical composition. Also disclosed, pharmaceutical compositions for modulating opioid withdrawal symptoms in a subject comprising a pannexin- 1 inhibitor. Said inhibitor may be lOpanx peptide with amino acid sequence WRQAAFVDS Y, mefloquine or probenecid.
Owner:UTI LLP (CA)
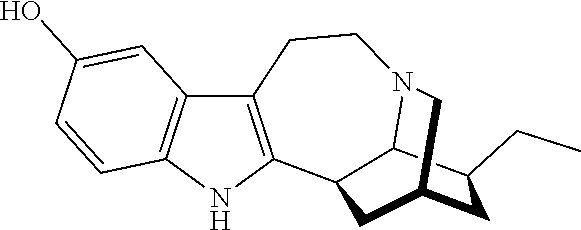
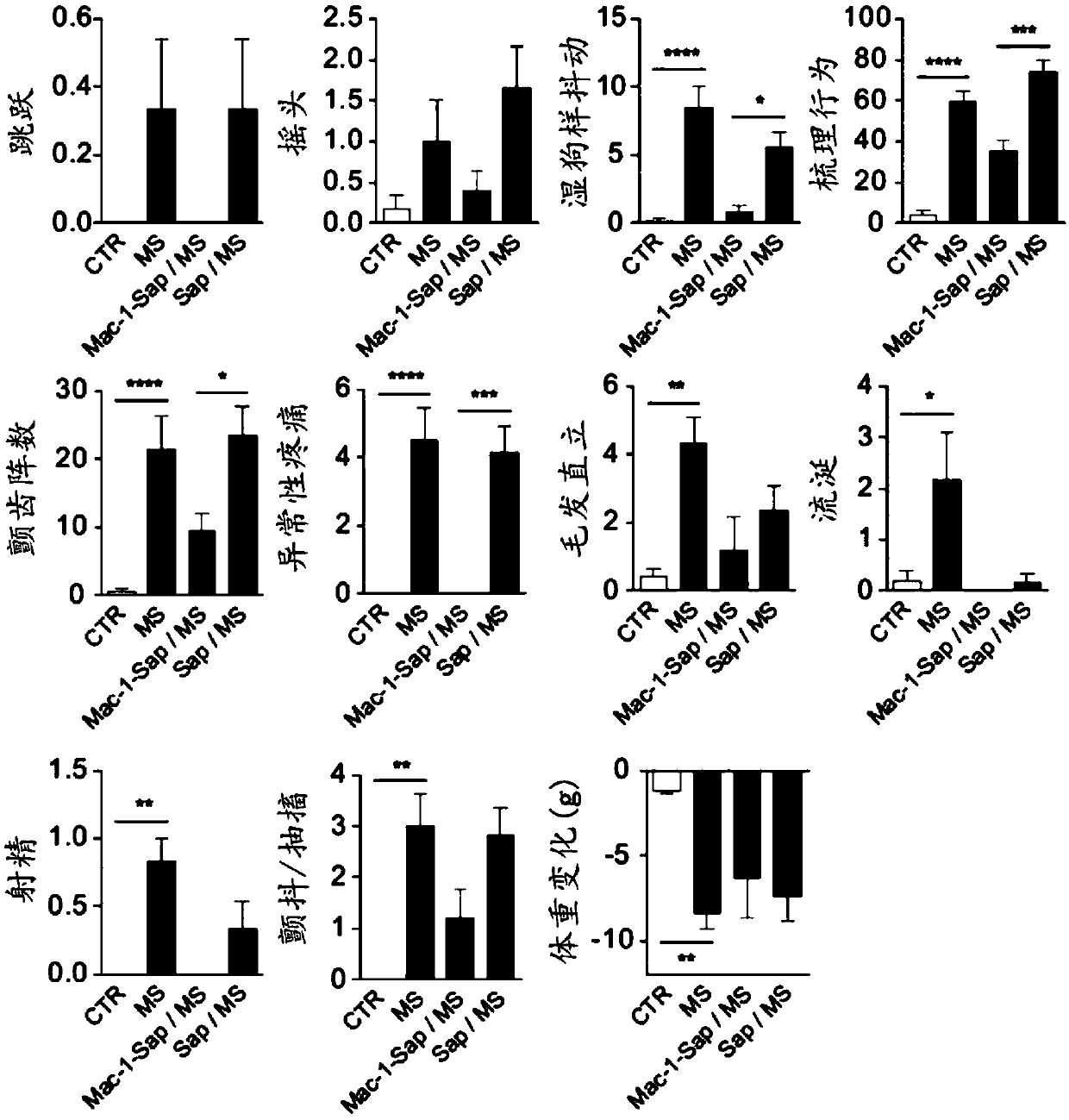
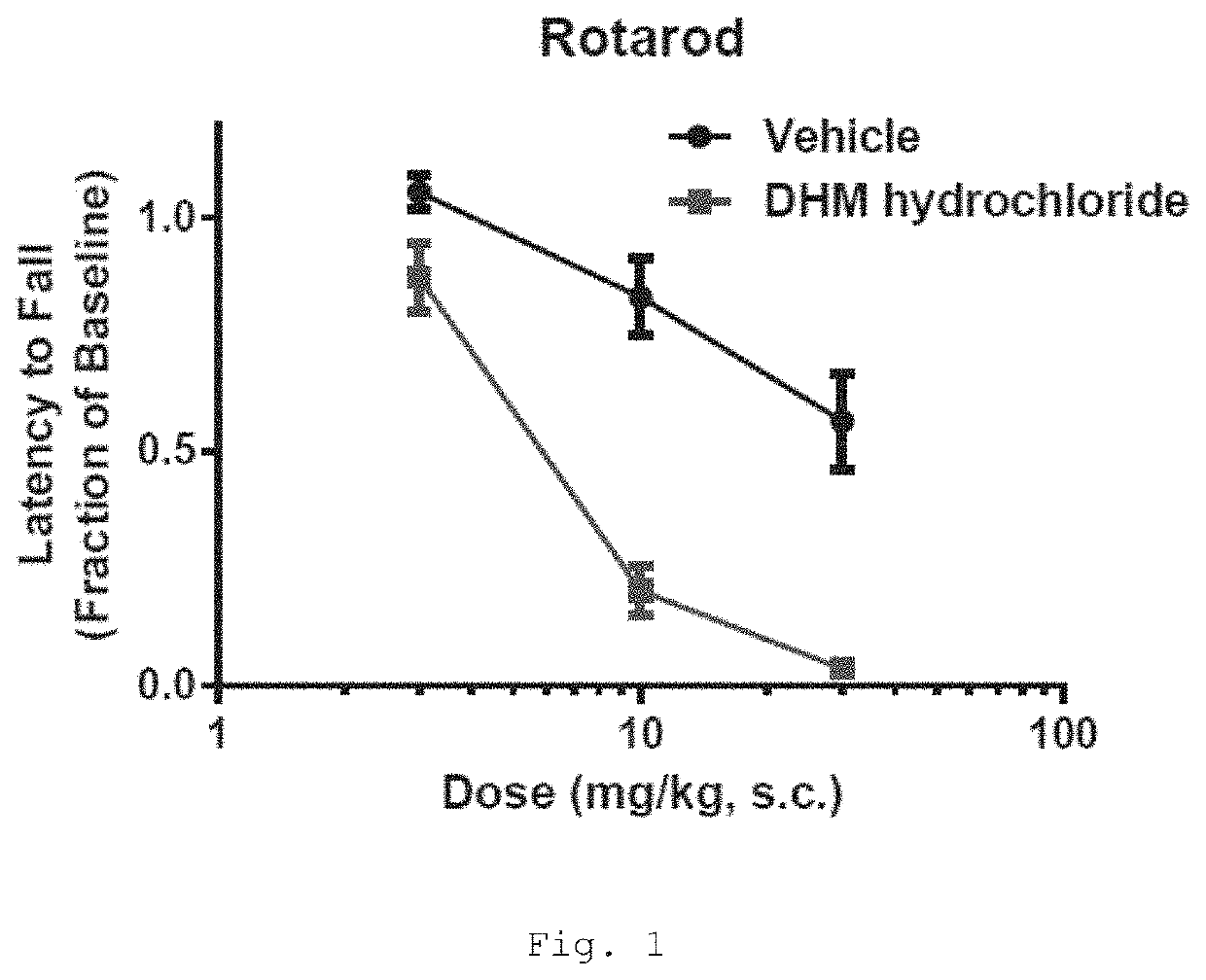
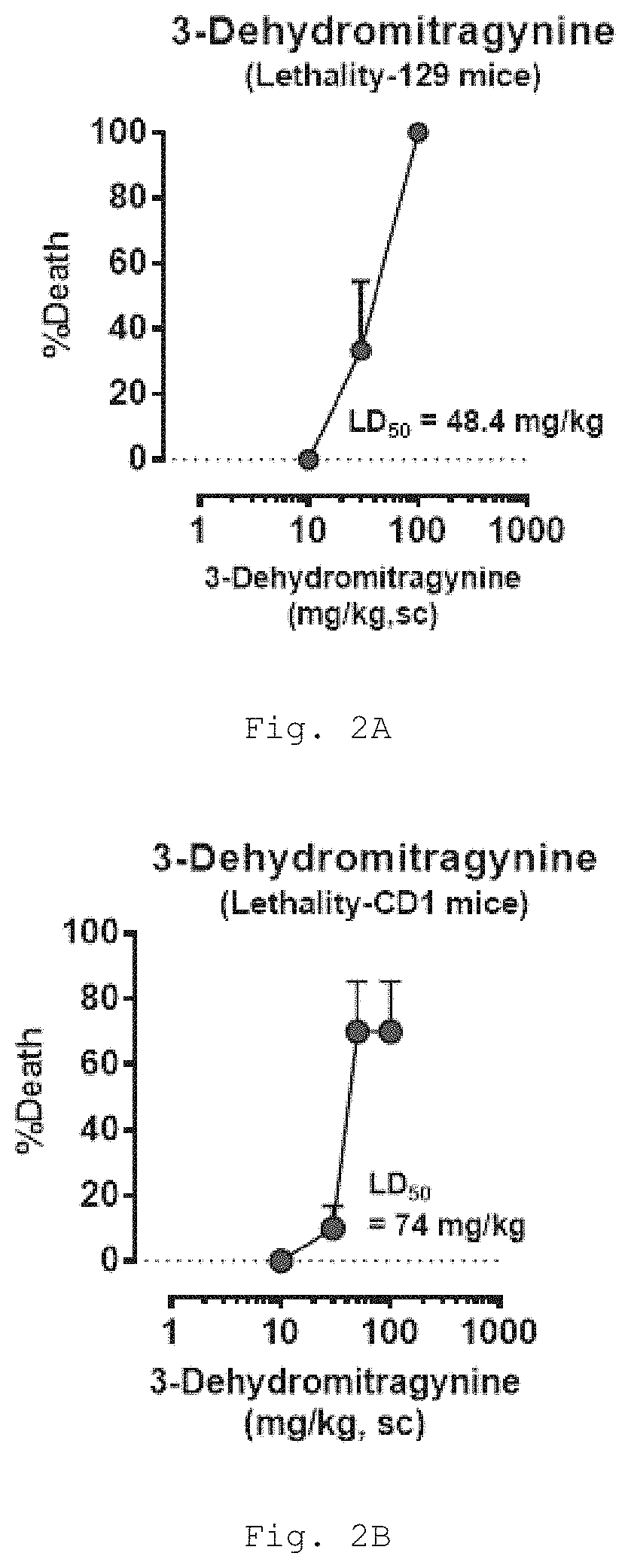
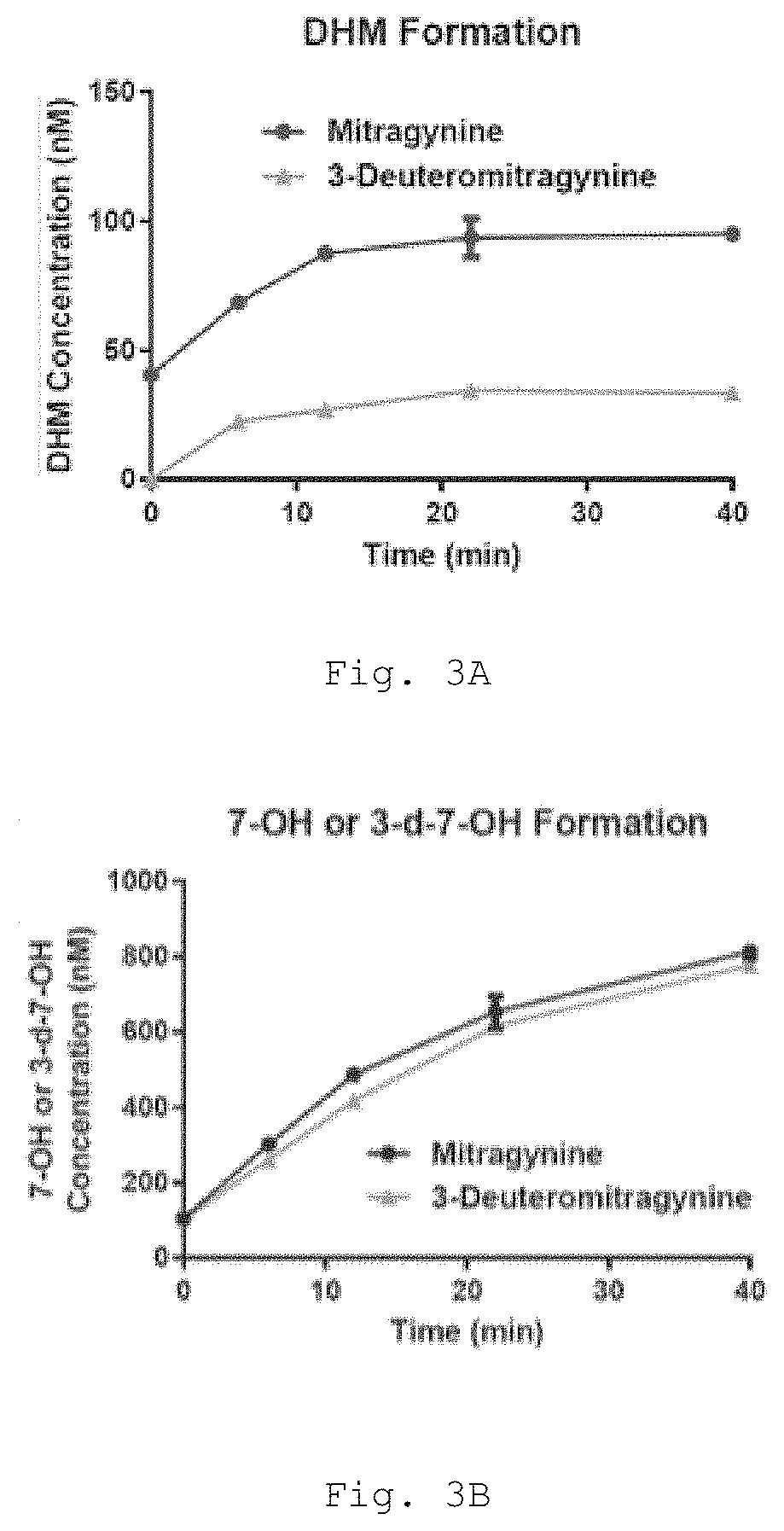
Deuterated mitragynine analogs as safer opioid modulators in the mitragynine class
PendingUS20220135564A1Lower performance requirementsUseful measurementNervous disorderOrganic chemistryMitragynineOpioid use disorder
Owner:SLOAN KETTERING INST FOR CANCER RES +2
A digital therapeutic component to optimize induction of buprenorphine-containing products
PendingCN113811950AMedical communicationOrganic active ingredientsOpioid use disorderEmergency medicine
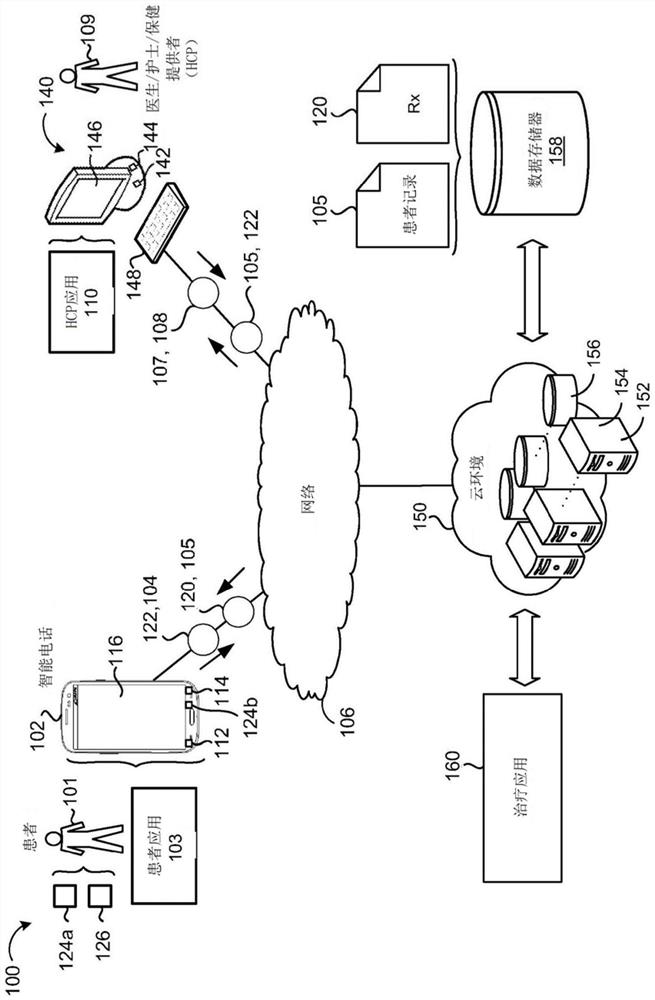
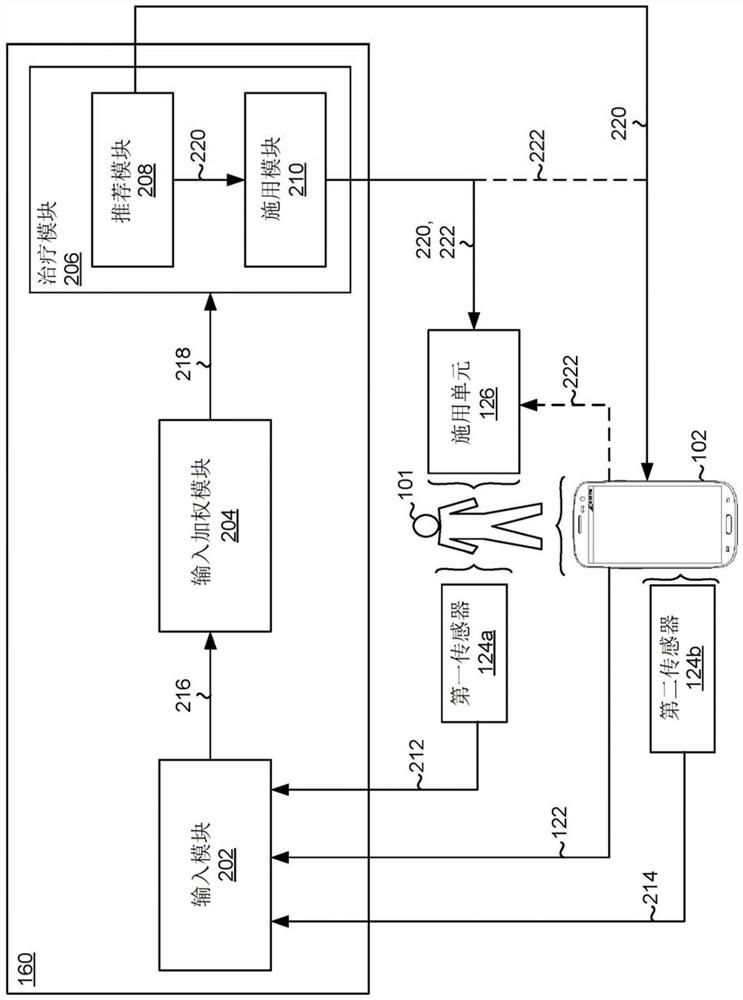
A system comprises data processing hardware and memory hardware in communication with the data processing hardware, the memory hardware storing instructions that when executed on the data processing hardware cause the data processing hardware to perform operations comprising executing a prescription digital therapeutic configured to treat symptoms associated with opioid use disorder in a patient, wherein executing the prescription digital therapeutic comprises receiving a plurality of inputs associated with the patient from one or more of (i) first sensors associated directly with the patient and (ii) second sensors associated with a patient electronic device, wherein the plurality of inputs represent a level of opioid withdrawal associated with the patient, weighting the plurality of inputs associated with the patient to provide a plurality of weighted inputs, determining a recommended dosage of a buprenorphine-containing product for the patient based on the plurality of weighted inputs, and instructing an administration unit to administer the recommended dosage of the buprenorphine-containing product to the patient.
Owner:ペア セラピューティクス(ユーエス)インコーポレイテッド
Treatment of Opioid Withdrawal
PendingUS20220288060A1Prevent opioid withdrawalOrganic active ingredientsNervous disorderOpioid withdrawalPharmaceutical Substances
Owner:KINOXIS THERAPEUTICS PTY LTD
Compositions and method for enhancing the therapeutic activity of opiods in treatment of pain
Compositions and methods for inhibiting opioid tolerance and opioid withdrawal-induced hyperalgesia are provided.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Therapeutic prodrugs for neurologic maladies
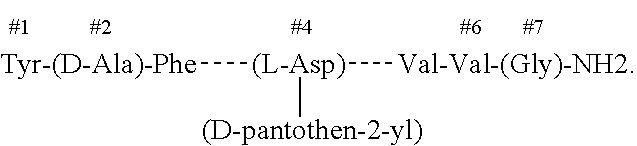
InactiveUS20200000928A1Peptide/protein ingredientsPharmaceutical non-active ingredientsNervous systemPantothenic acid
Owner:BOWSHER DENNIS JAMES
Pharmaceutical formulations comprising opioid receptor agonist as active ingredients, methods of manufacture and therapeutic uses thereof
ActiveUS10660849B2Formula SafetyAdequate shelf lifeOrganic active ingredientsNervous disorderPharmaceutical medicineAgonist
Formulation comprising buprenorphine or a pharmaceutically acceptable salt thereof as the sole active ingredient, a viscosity enhancer, and a buffering agent in an amount to provide a pH of from 5.0 to 7.0 are useful for treating opioid withdrawal syndrome.
Owner:CHIESI FARM SPA
Endothelin Receptors in Morphine Withdrawal
InactiveUS20100323456A1Easy to manageEasy to understandOrganic active ingredientsMaterial analysisTolerabilityMorphine withdrawal
The present invention relates to compositions and methods for managing opioid tolerance and reducing opioid withdrawal. More specifically, the present invention provides for endothelin, endothelin receptors, and endothelin receptor antagonists and agonists as a means for managing G-protein activity in the context of opioid tolerance and withdrawal.
Owner:GULATI ANIL +1
Methods and compositions for the treatment of opioid dependence and for the treatment of pain
PendingUS20220031798A1Prevent relapseReduce the possibilityNervous disorderOrganic chemistrySubstance dependencePharmaceutical drug
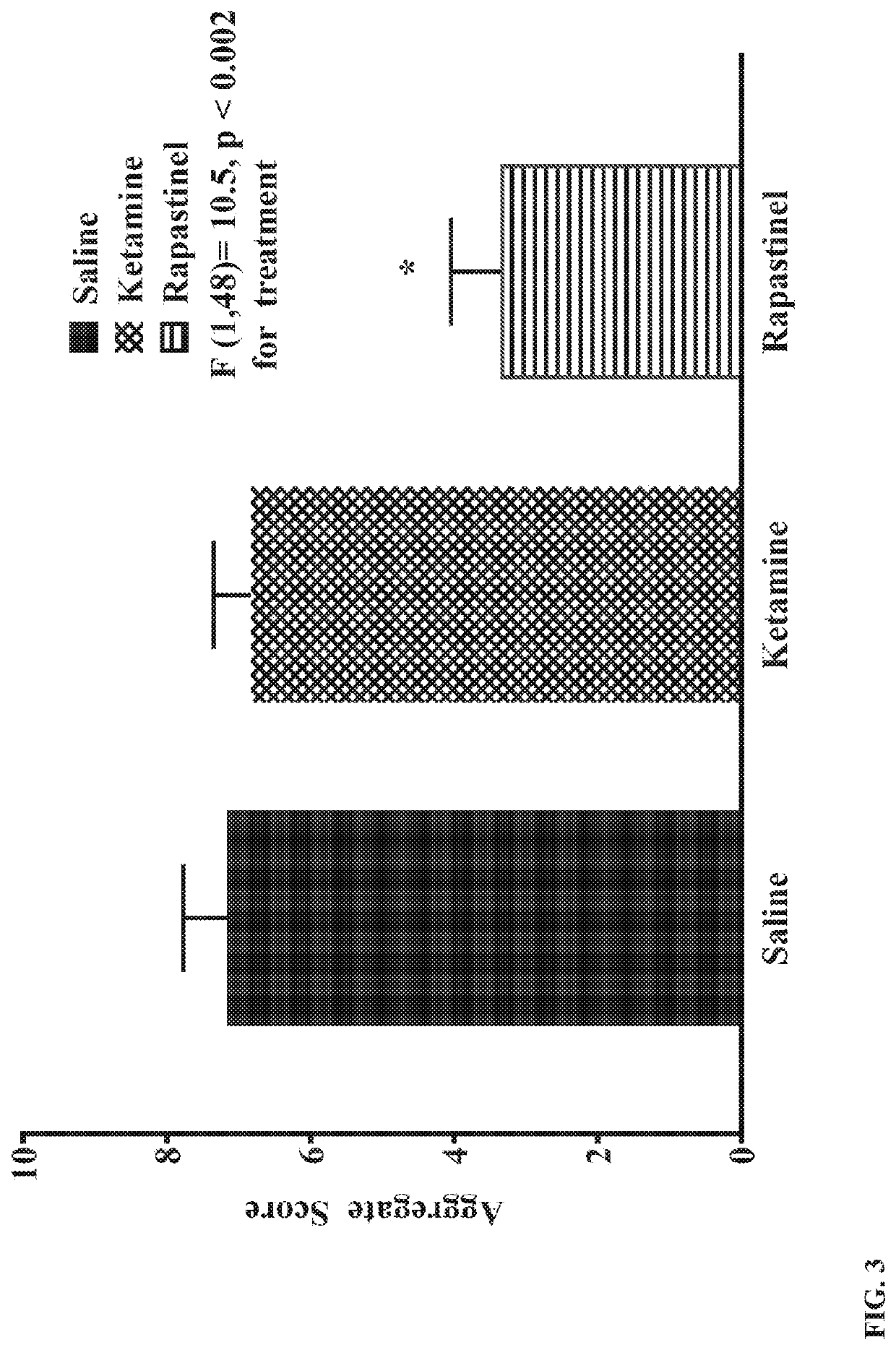
The present invention relates to methods of treating opioid dependence, enhancing the treatment of opioid dependence, treating opioid withdrawal, or alleviating one or more opioid withdrawal symptoms in a subject, preventing or reducing the likelihood of opioid dependence relapse in a subject treated for opioid dependence, reducing the vulnerability of a subject to develop opioid dependence in adulthood following opioid exposure during adolescence, or treating pain in a subject, comprising administering a therapeutically effective amount of a N-methyl-D-aspartate receptor (NMDA) partial agonist to the subject. The present invention further relates to compositions comprising an NMDA partial agonist for use with the aforementioned methods.
Owner:DUKE UNIV
Benzoic Acid, Benzoic Acid Derivatives and Heteroaryl Carboxylic Acid Conjugates of Hydrocodone, Prodrugs, Methods of Making and Use Thereof
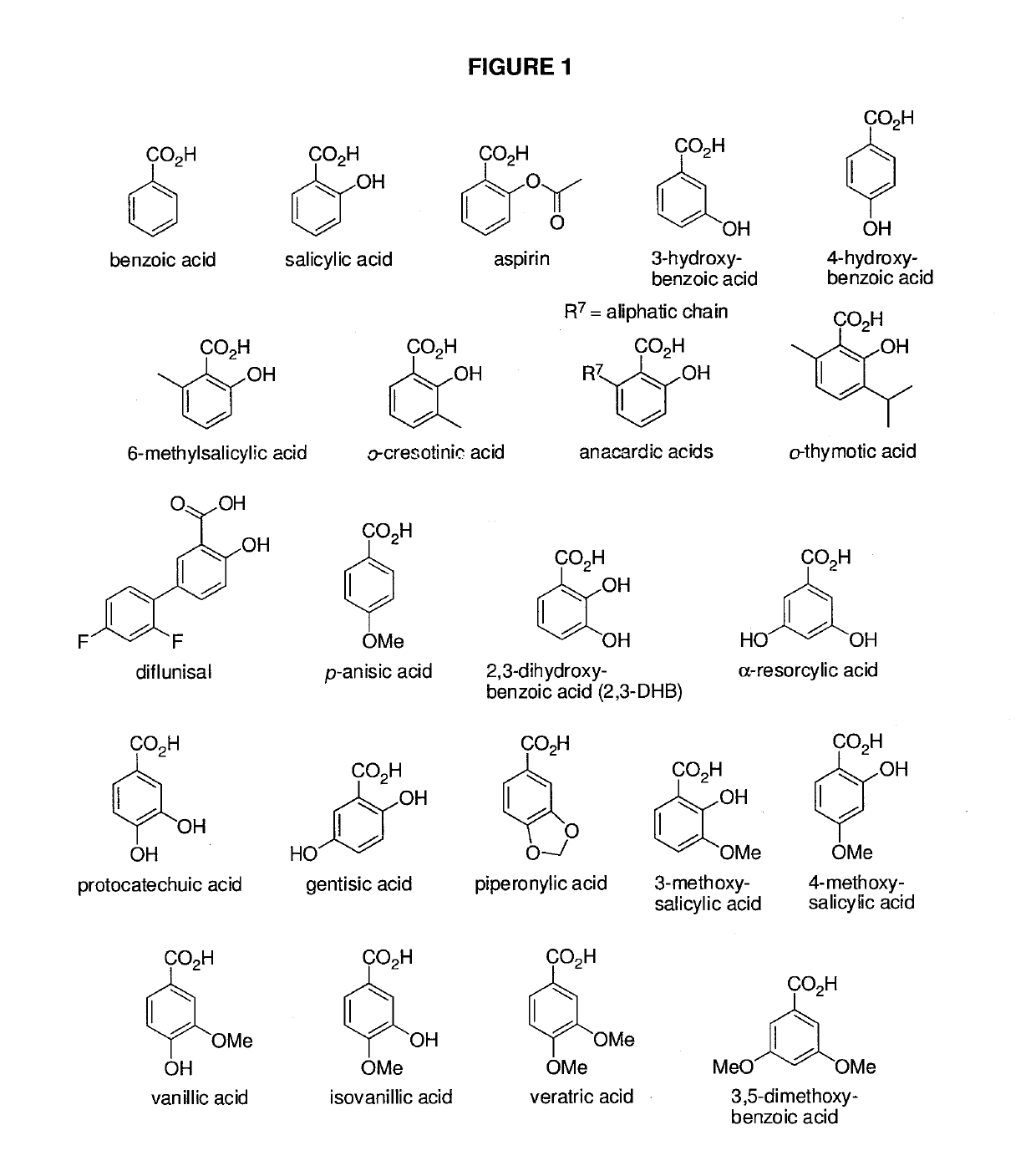
ActiveUS20190160175A1Lower potentialDecrease its potential for causing overdose or abuseOrganic active ingredientsOrganic chemistryBenzoic acidSide effect
The presently described technology provides methods of treating a patient having moderate to severe pain, narcotic or opioid abuse or narcotic or opioid withdrawal. The presently described methods are carried out by comprising administering to the patient a pharmaceutically effective amount of a composition comprising acetaminophen and benzoate-hydrocodone hydrochloride. The composition has reduced side effects when compared with unconjugated hydrocodone.
Owner:KEMPHARM INC
Compositions and methods for the treatment of restless leg syndrome and fibromyalgia
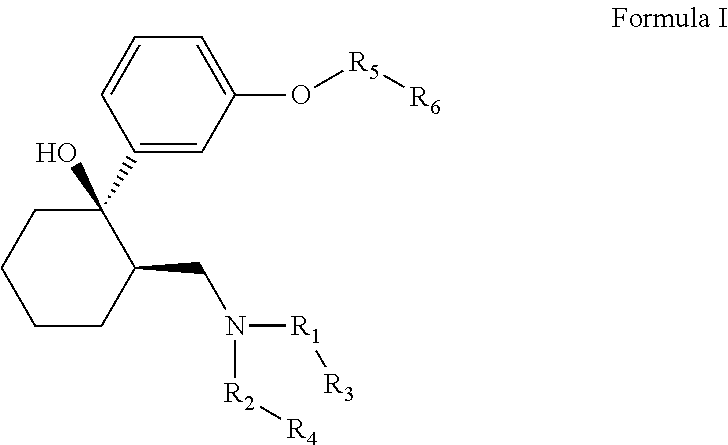
The invention relates to the compounds of formula I or its pharmaceutical acceptable salts, as well as polymorphs, solvates, enantiomers, stereoisomers and hydrates thereof. The pharmaceutical compositions comprising an effective amount of compounds of formula I, and methods for the treatment of fibromyalgia, restless leg syndrome may be formulated for oral, buccal, rectal, topical, transdermal, transmucosal, intravenous, parenteral administration, syrup, or injection. Such compositions may be used to treatment of motor neurone disease, diabetic neuropathy, postherpetic neuralgia, acute opioid withdrawal management, obsessive-compulsive disorder, premature ejaculation, PTSD, injury, post-operative pain, osteoarthritis, rheumatoid arthritis, multiple sclerosis, spinal cord injury, migraine, HIV related neuropathic pain, bipolar depression, depression, stress, cancer pain and lower back pain.
Owner:CELLIXBIO PTE LTD
Pharmaceutical formulations comprising opioid receptor agonist as active ingredients, methods of manufacture and therapeutic uses thereof
InactiveUS20200237650A1Formula SafetyAdequate shelf lifeOrganic active ingredientsNervous disorderPharmaceutical medicineAgonist
Formulation comprising buprenorphine or a pharmaceutically acceptable salt thereof as the sole active ingredient, a viscosity enhancer, and a buffering agent in an amount to provide a pH of from 5.0 to 7.0 are useful for treating opioid withdrawal syndrome.
Owner:CHIESI FARM SPA
A kind of pharmaceutical composition and application for alleviating or eliminating opioid withdrawal syndrome
ActiveCN112494486BImprove Medication AdherenceImprove experienceOrganic active ingredientsNervous disorderWithdrawal syndromeUse medication
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Treatment of opioid withdrawal
PendingCN114502170AOrganic active ingredientsNervous disorderOpioid withdrawalPharmaceutical Substances
Owner:KINOXIS THERAPEUTICS PTY LTD
Deuterated mitragynine analogs as safer opioid modulators in the mitragynine class
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +2
Injectable buprenorphine formulation
InactiveUS20180015031A1Improved release profileHigh viscosityOrganic active ingredientsNervous disorderMaintenance therapySubstance dependence
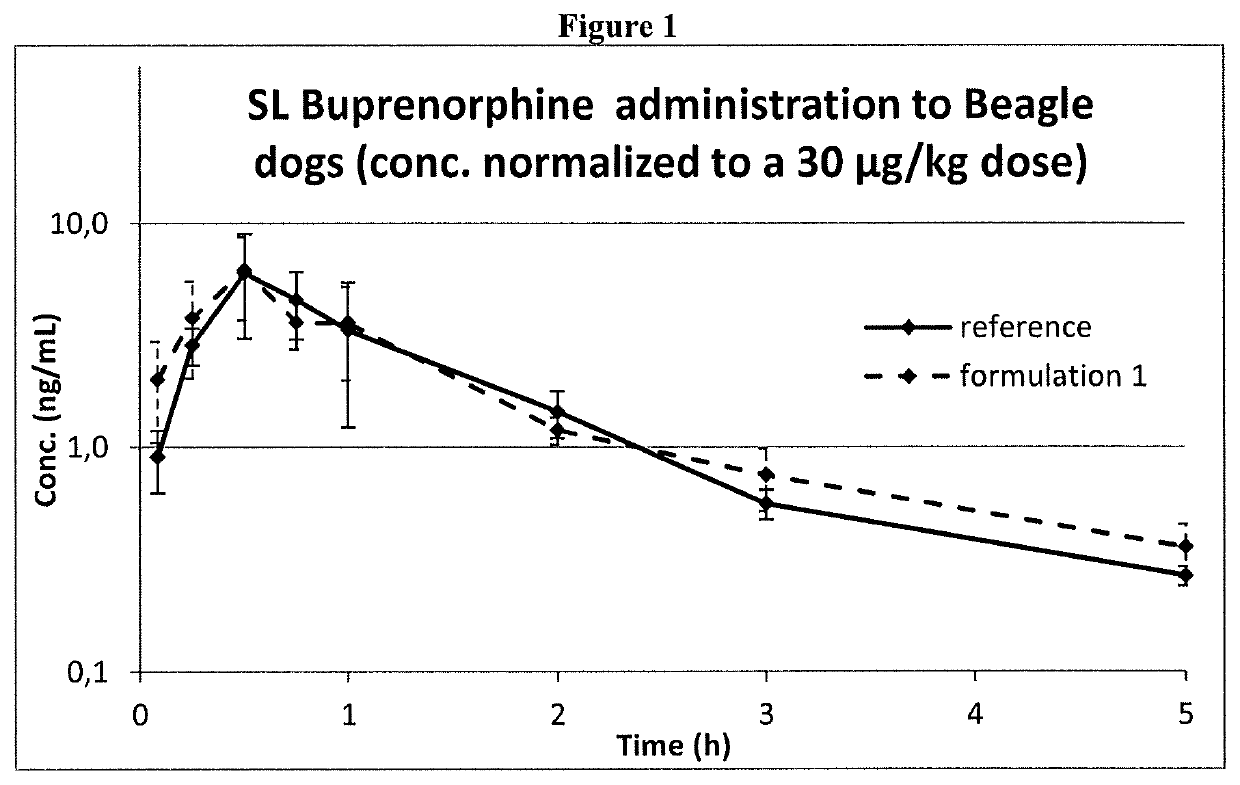
The present invention provides an injectable liquid formulation with controlled release comprising: a) a lipid controlled-release matrix comprising at least 50% triacyl lipids; b) at least one oxygen containing organic solvent; c) at least 16% by weight of at least one active agent selected from buprenorphine and salts thereof, calculated as buprenorphine free base. The invention also provides a method for the treatment of pain, for opioid maintenance therapy or for the treatment of opioid dependence by detoxification and / or maintenance or for the treatment or prophylaxis of the symptoms of opioid withdrawal and / or cocaine withdrawal by injecting such a liquid composition.
Owner:CAMURUS AB
Compositions and methods for the treatment of restless leg syndrome and fibromyalgia
ActiveUS20150133408A1Treat and prevent and ameliorate effectBiocideNervous disorderEnantiomerMigraine
The invention relates to the compounds of formula I or its pharmaceutical acceptable salts, as well as polymorphs, solvates, enantiomers, stereoisomers and hydrates thereof. The pharmaceutical compositions comprising an effective amount of compounds of formula I, and methods for the treatment of fibromyalgia, restless leg syndrome may be formulated for oral, buccal, rectal, topical, transdermal, transmucosal, intravenous, parenteral administration, syrup, or injection. Such compositions may be used to treatment of motor neurone disease, diabetic neuropathy, postherpetic neuralgia, acute opioid withdrawal management, obsessive-compulsive disorder, premature ejaculation, PTSD, injury, post-operative pain, osteoarthritis, rheumatoid arthritis, multiple sclerosis, spinal cord injury, migraine, HIV related neuropathic pain, bipolar depression, depression, stress, cancer pain and lower back pain.
Owner:CELLIXBIO PTE LTD
Use of an opioid receptor antagonist for the treatment or prevention of gastrointestinal tract disorders
The disclosure relates to a method of treating or preventing a condition in a subject associated with the activation of an opioid receptor in the periphery by administering an effective amount of 5-(2-methoxy-4-{[2-(tetrahydro-pyran-4-yl)-ethylamino]-methyl}-phenoxy)-pyrazine-2-carboxamide (Compound I). In particular, the disclosure relates to a method of treating or preventing opioid-induced constipation or opioid-induced bowel dysfunction in a human without reducing centrally-mediated opioid analgesia or producing central opioid withdrawal symptoms by administering an effective amount of Compound I. The disclosure further relates to the use of Compound I for the preparation of a medicament for the treatment or prevention of a condition in a subject associated with the activation of an opioid receptor in the periphery.
Owner:MERCK & CO INC
Injectable buprenorphine formulation
PendingUS20210308041A1High viscosityImprove experienceOrganic active ingredientsNervous disorderMaintenance therapySubstance dependence
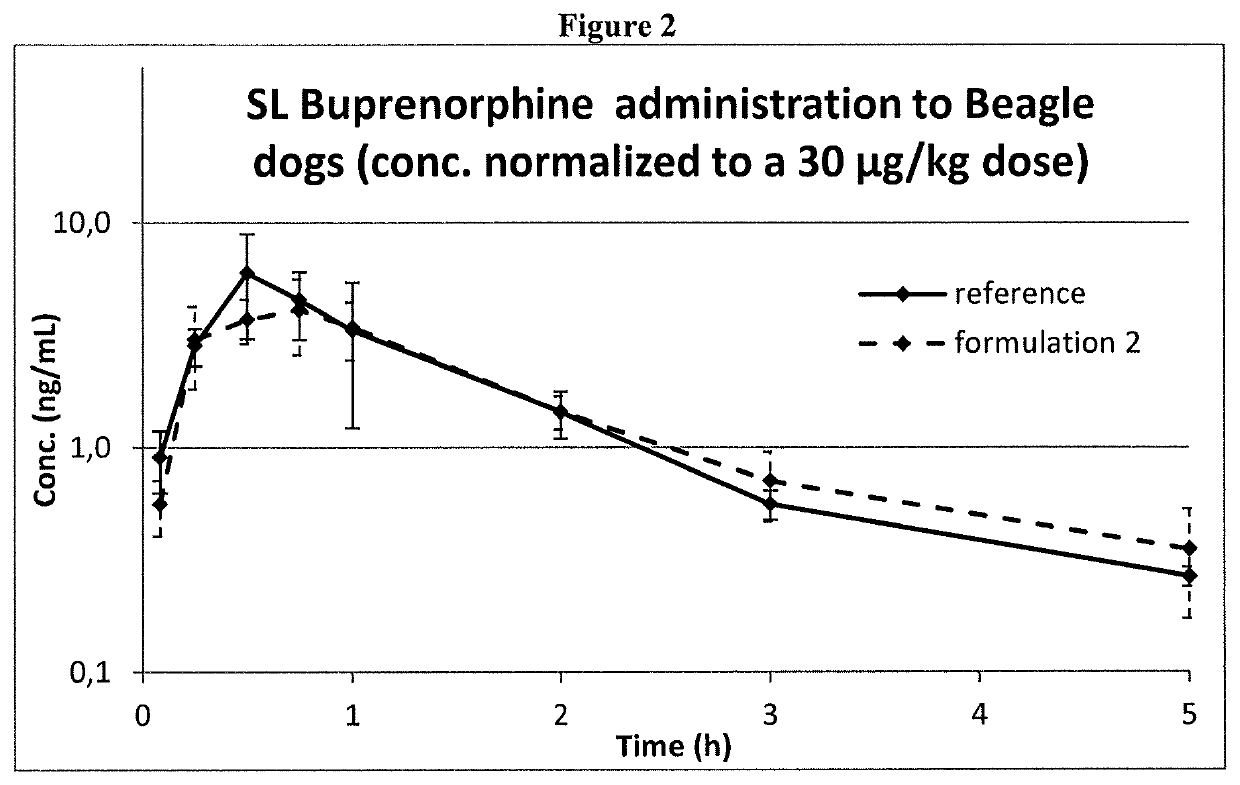
The present invention provides an injectable liquid formulation comprising: a) a lipid controlled-release matrix comprising at least 50% triacyl lipids; b) at least one oxygen containing organic solvent; c) at least 16% by weight of at least one active agent selected from buprenorphine and salts thereof, calculated as buprenorphine free base. The invention also provides a method for the treatment of pain, for opioid maintenance therapy or for the treatment of opioid dependence by detoxification and / or maintenance or for the treatment or prophylaxis of the symptoms of opioid withdrawal and / or cocaine withdrawal by injecting such a liquid composition.
Owner:CAMURUS AB
Treatment of opioid use disorder, opioid withdrawal symptoms, and chronic pain
PendingUS20200345655A1Safe and effectiveNervous disorderHydroxy compound active ingredientsOpioid use disorderOpioidergic
The current methods and compositions provide for a novel and effective therapeutic method for treating opioid use disorder, opioid withdrawal symptoms and / or chronic pain. Accordingly, certain aspects of the disclosure relate to a method for treating opioid use disorder, opioid withdrawal symptoms and / or chronic pain in a subject, the method comprising administering at least one purified cannabinoid compound and a partial opioid agonist. In some embodiments, the method comprises administering a composition comprising cannabidiol and buprenorphine.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com