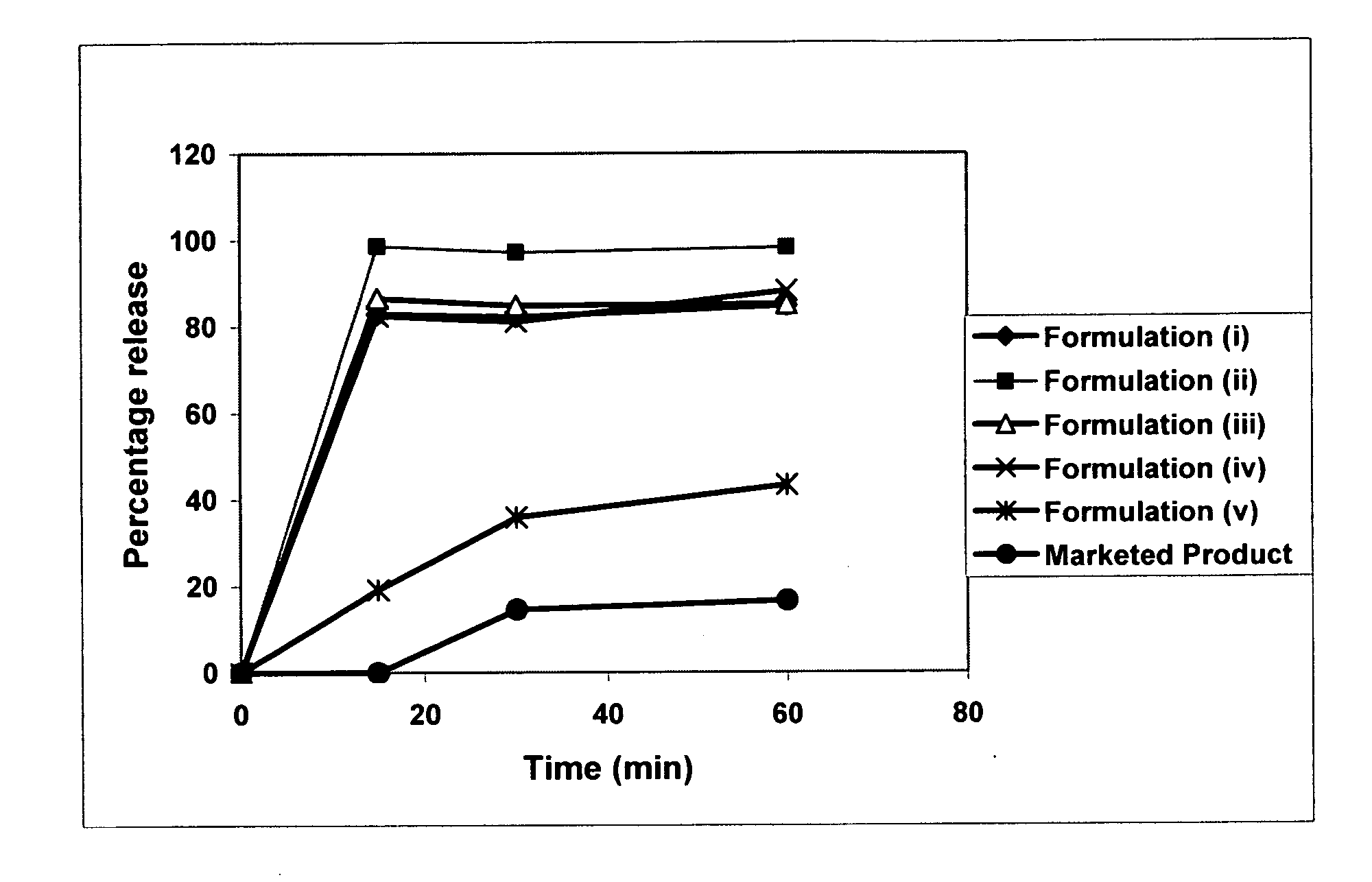
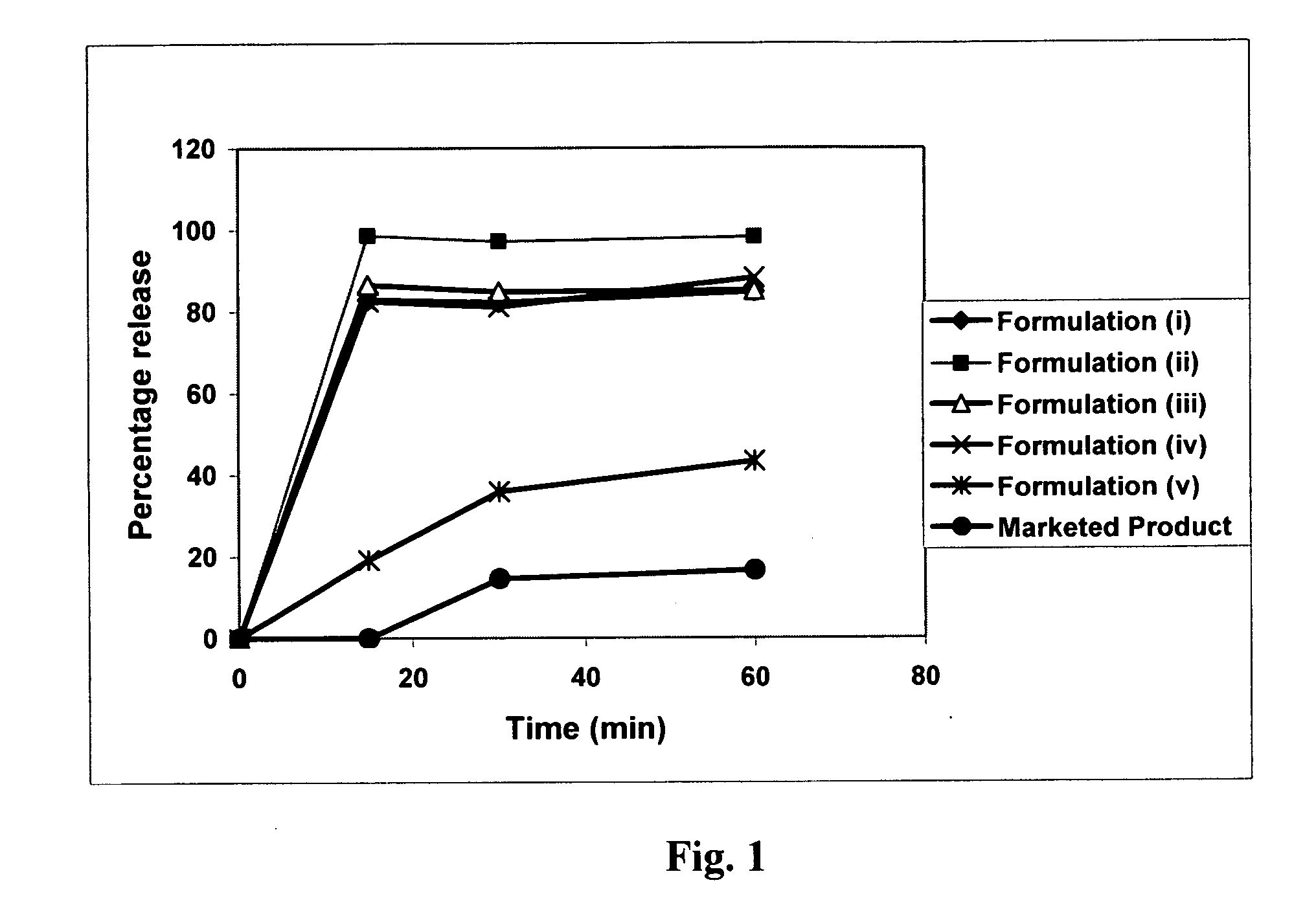
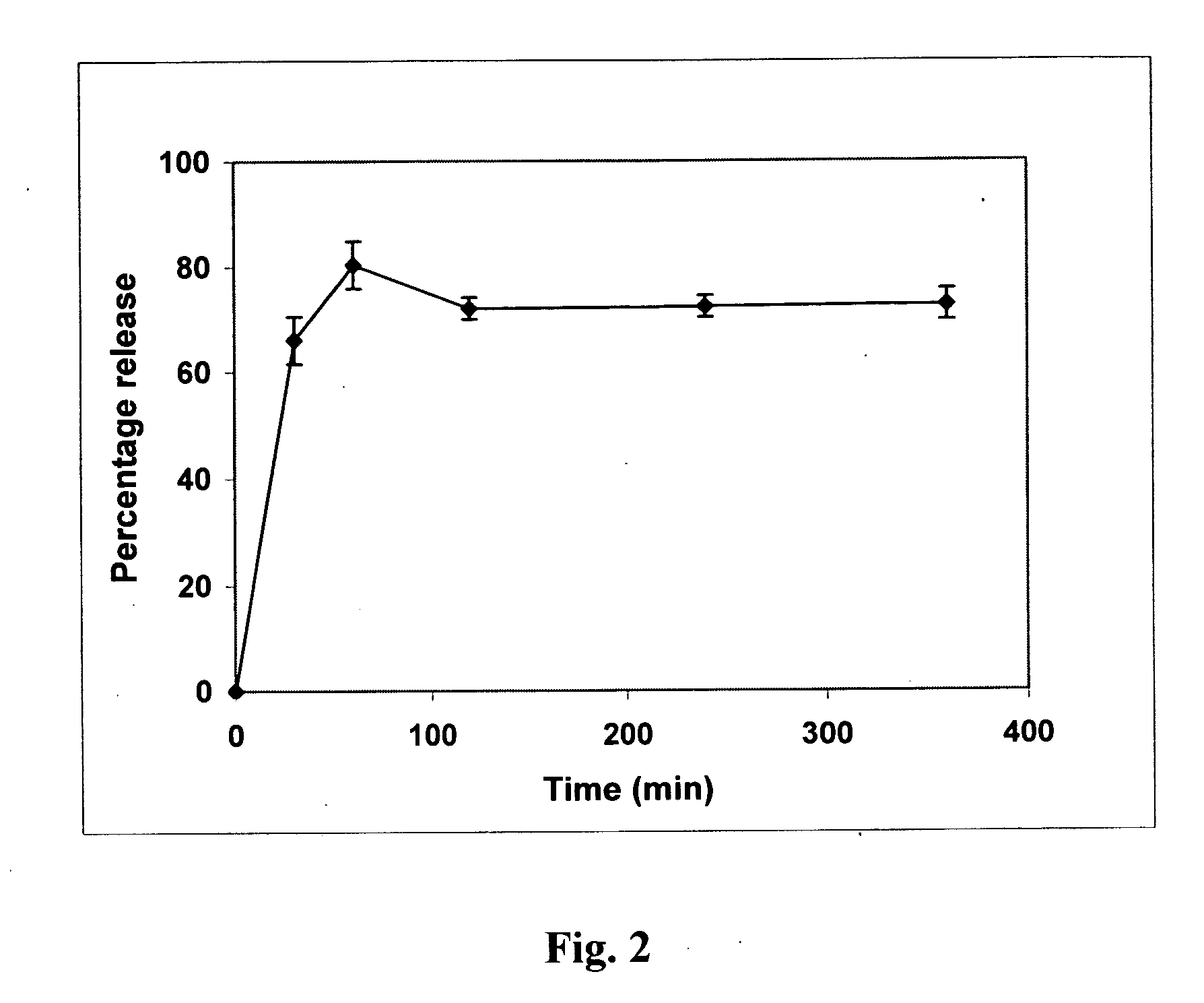
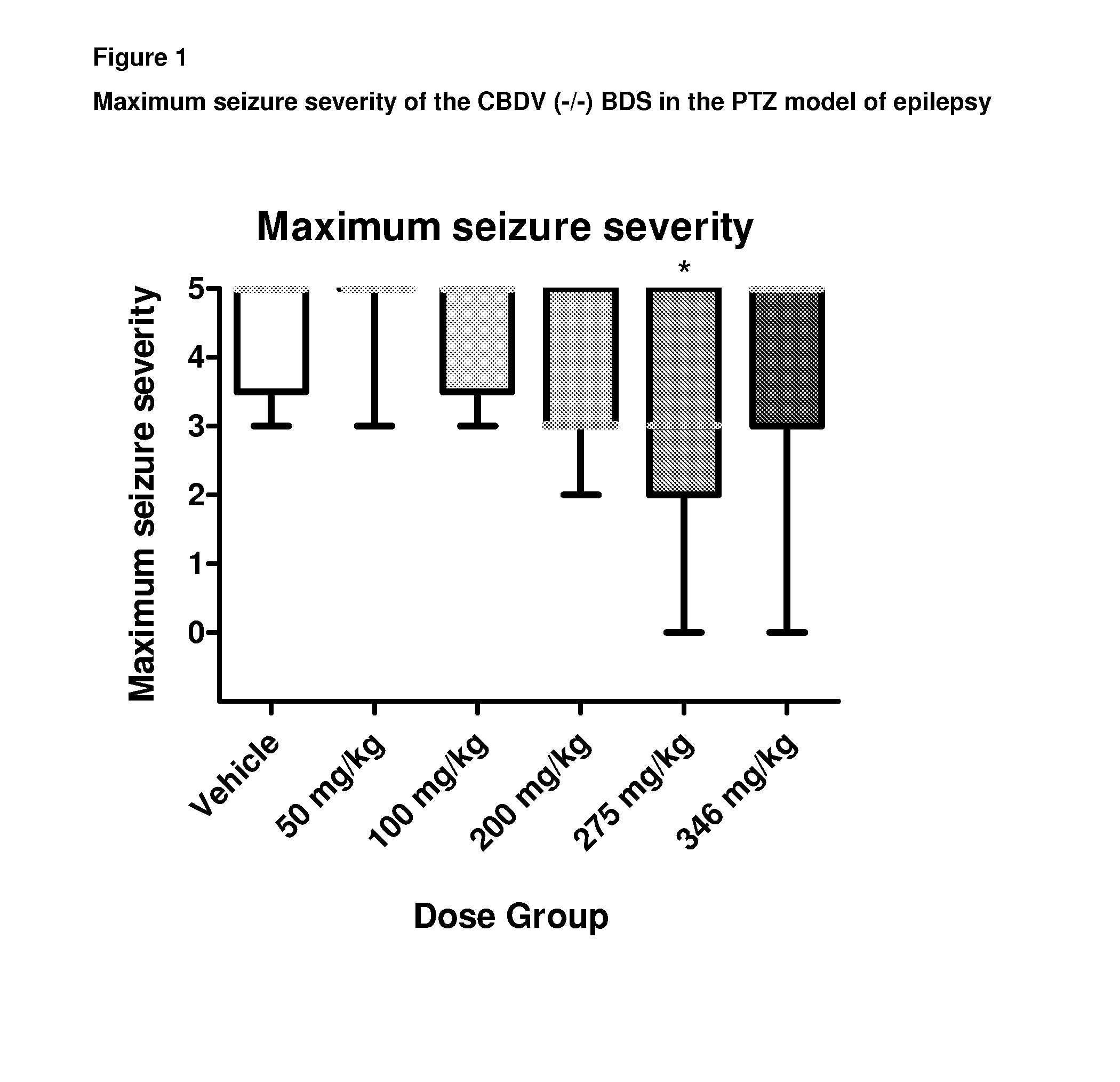
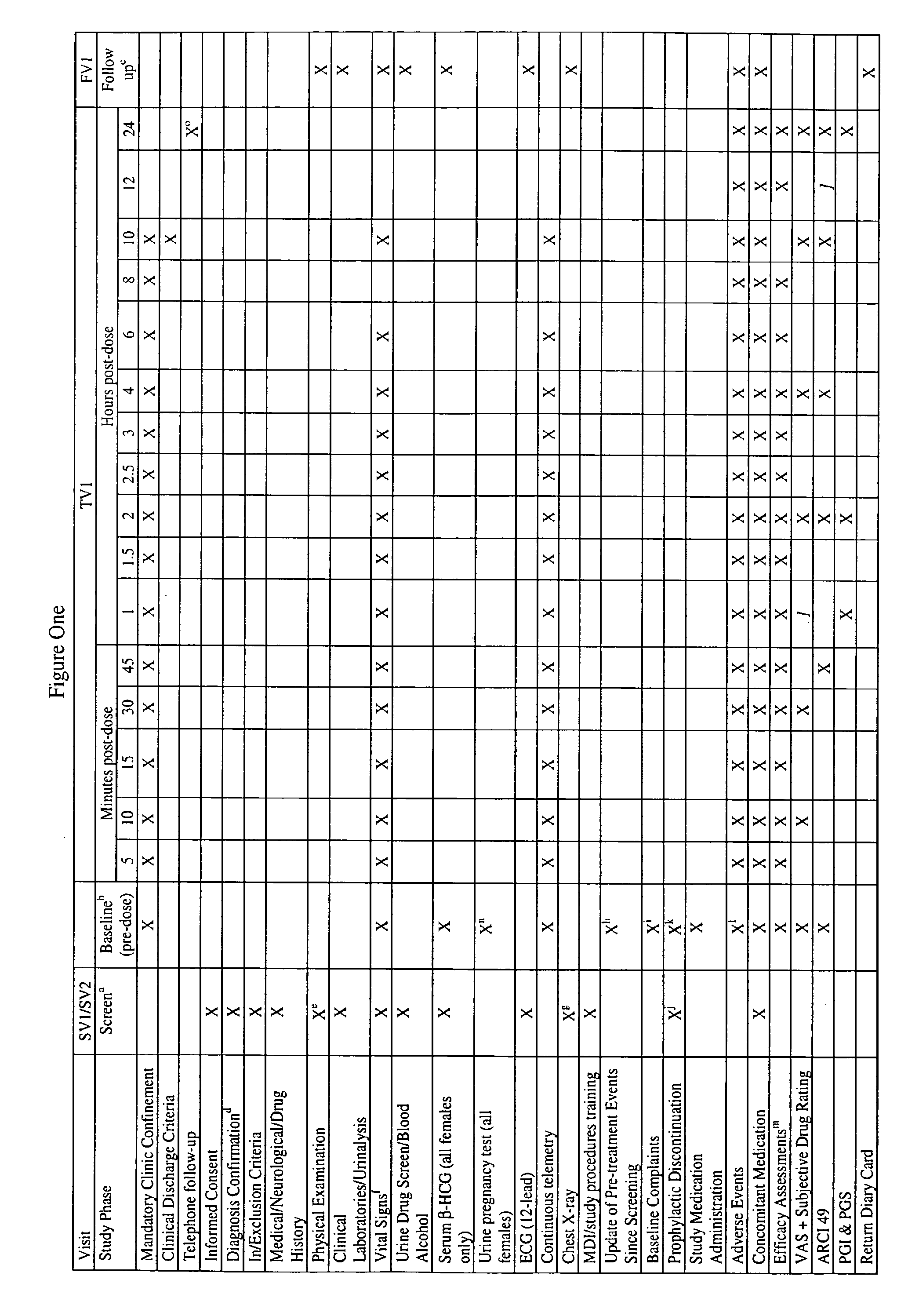
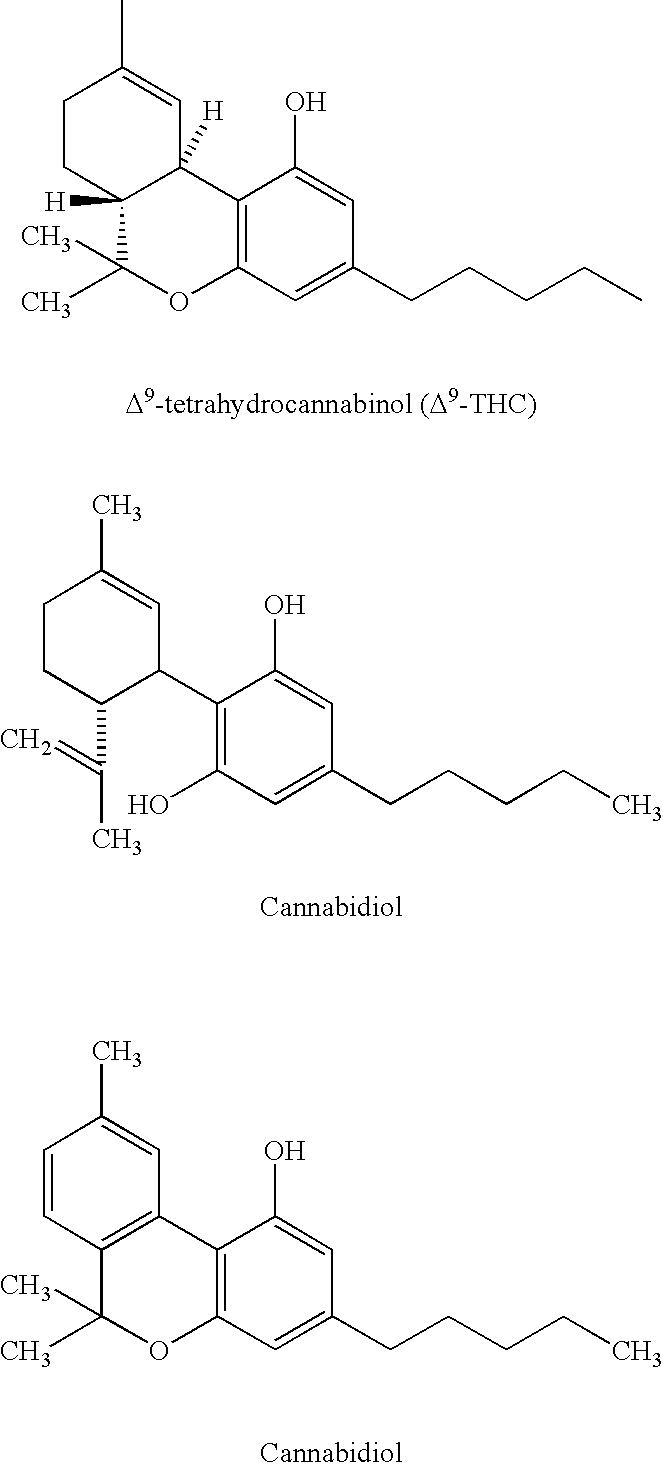
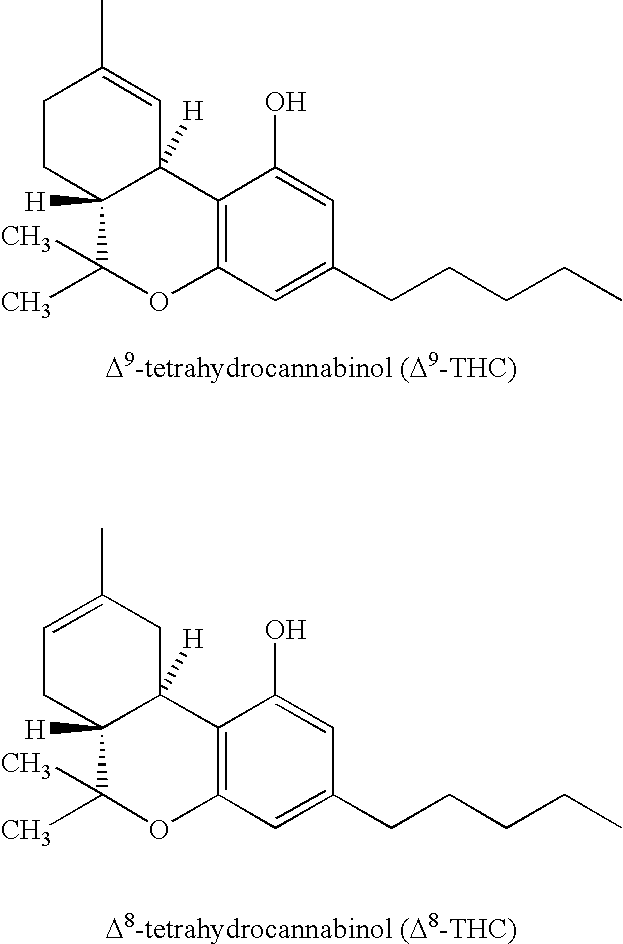
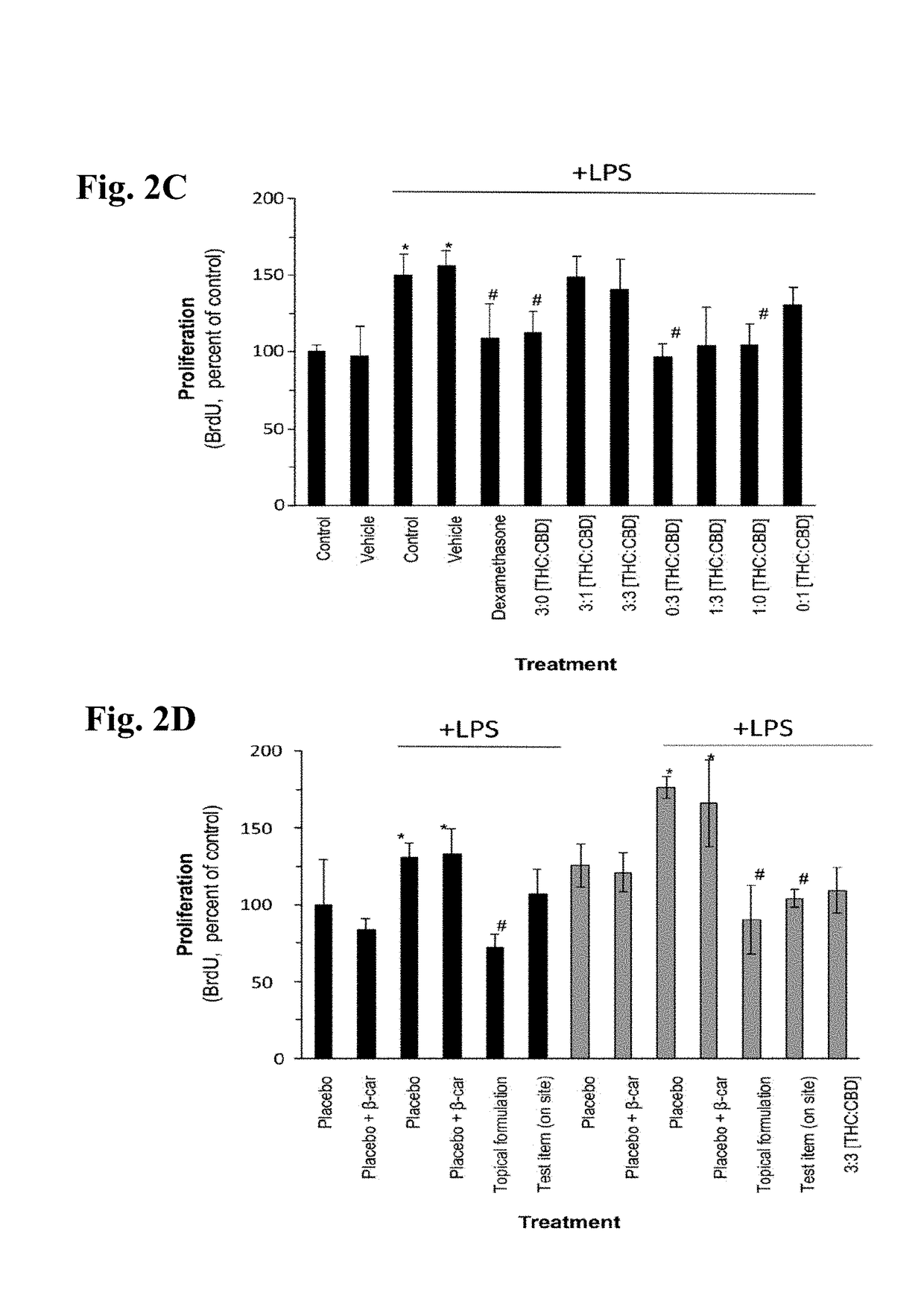
Patents
Literature
295 results about "Tetrahydrocannabinol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
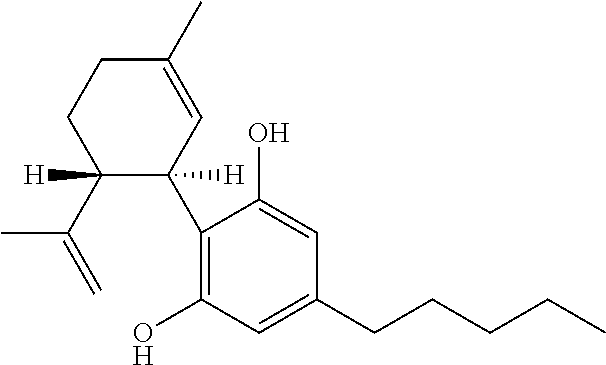
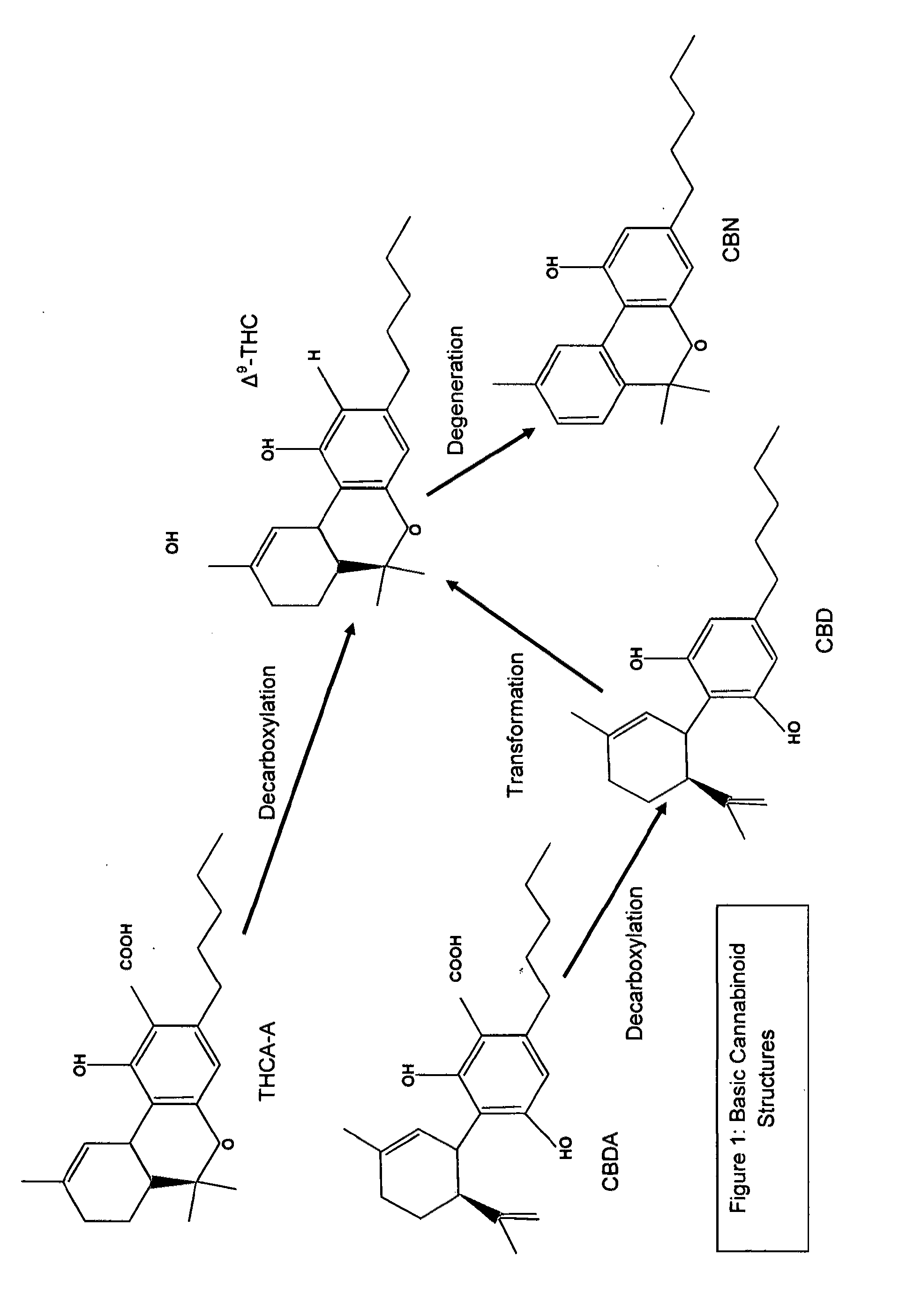
Tetrahydrocannabinol (THC) is one of at least 113 cannabinoids identified in cannabis. THC is the principal psychoactive constituent of cannabis. With chemical name (−)-trans-Δ⁹-tetrahydrocannabinol, the term THC also refers to cannabinoid isomers.
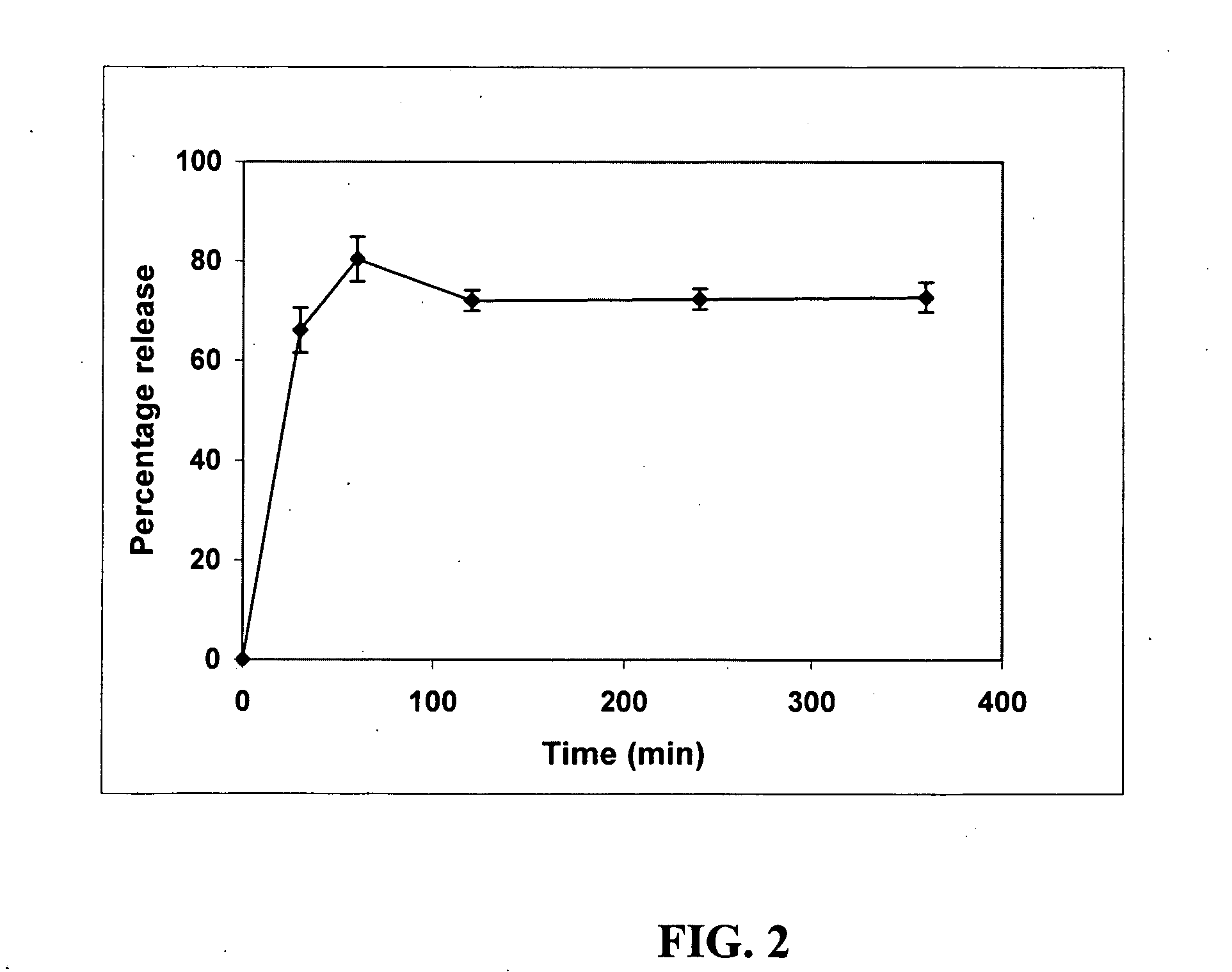
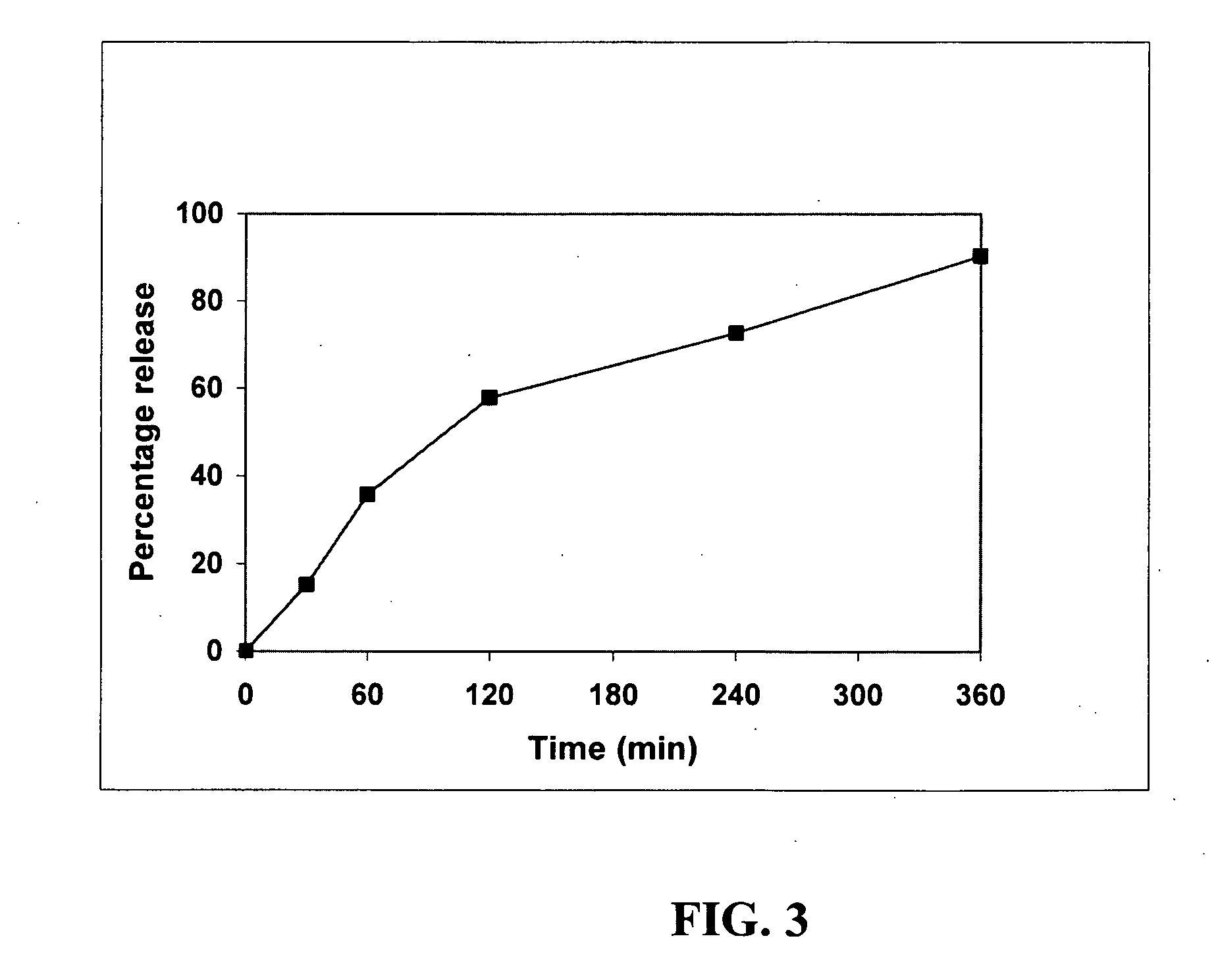
Delivery of tetrahydrocannabinol
InactiveUS20070104741A1Avoiding hepatic first-pass metabolismGood choiceBiocideNervous disorderChylomicronTG - Triglyceride
A self-emulsifying drug delivery system to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron delivery and optimal bioavailability to a mammalian intestinal lumen. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY PHARMA
Method of relieving analgesia and reducing inflamation using a cannabinoid delivery topical liniment
InactiveUS6949582B1Good effectSafe and effectiveBiocideHydroxy compound active ingredientsSide effectCannabinoid receptor
A method of relieving analgesia and reducing inflammation using a cannabinoid delivery topical liniment composition containing from about 97.5% to about 99.5% by weight a 70% monohydric alcohol solution, and from about 0.5% to about 2.5% by weight of a synergistic cannabinoid mixture extracted from the female plant Cannabis sativa L, including in combination: 9-Tetrahydrocannabinol (delta-9-THC), 9-THC Propyl Analogue (THC-V), Cannabidiol (CBD), Cannabidiol Propyl Analogue (CBD-V), Cannabinol (CBN), Cannabichromene (CBC), Cannabichromene Propyl Analogue (CBC-V), Cannabigerol (CBG), terpenoids, and flavonoids. The liniment is applied topically, preferably by spraying, and the constituents of the mixture are absorbed through the skin and interact with cannabinoid receptors in the body and tissues of a human patient to produce therapeutic analgesic and anti-inflammatory effects without undesirable psychotropic side effects.
Owner:WALLACE WALTER H
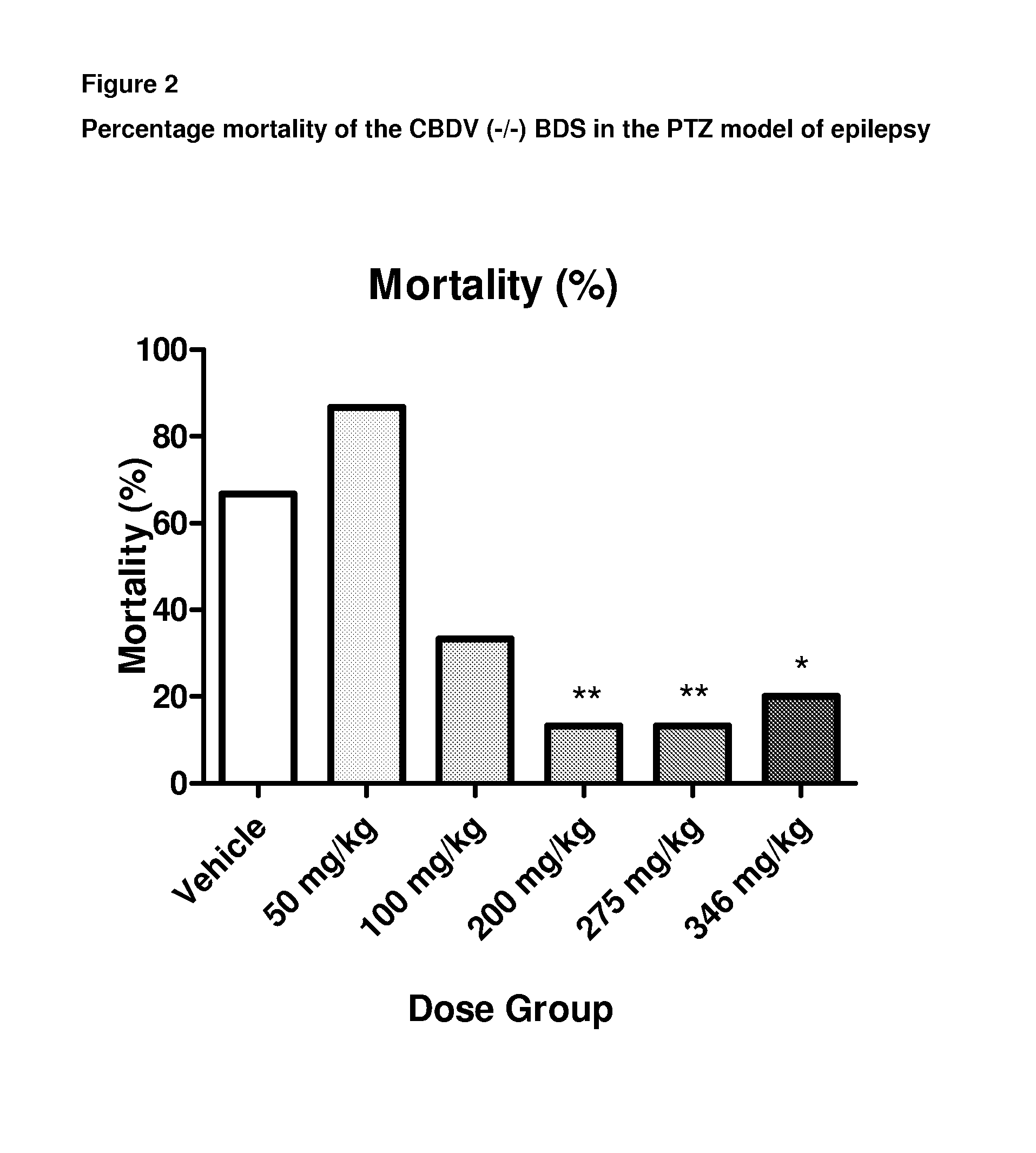
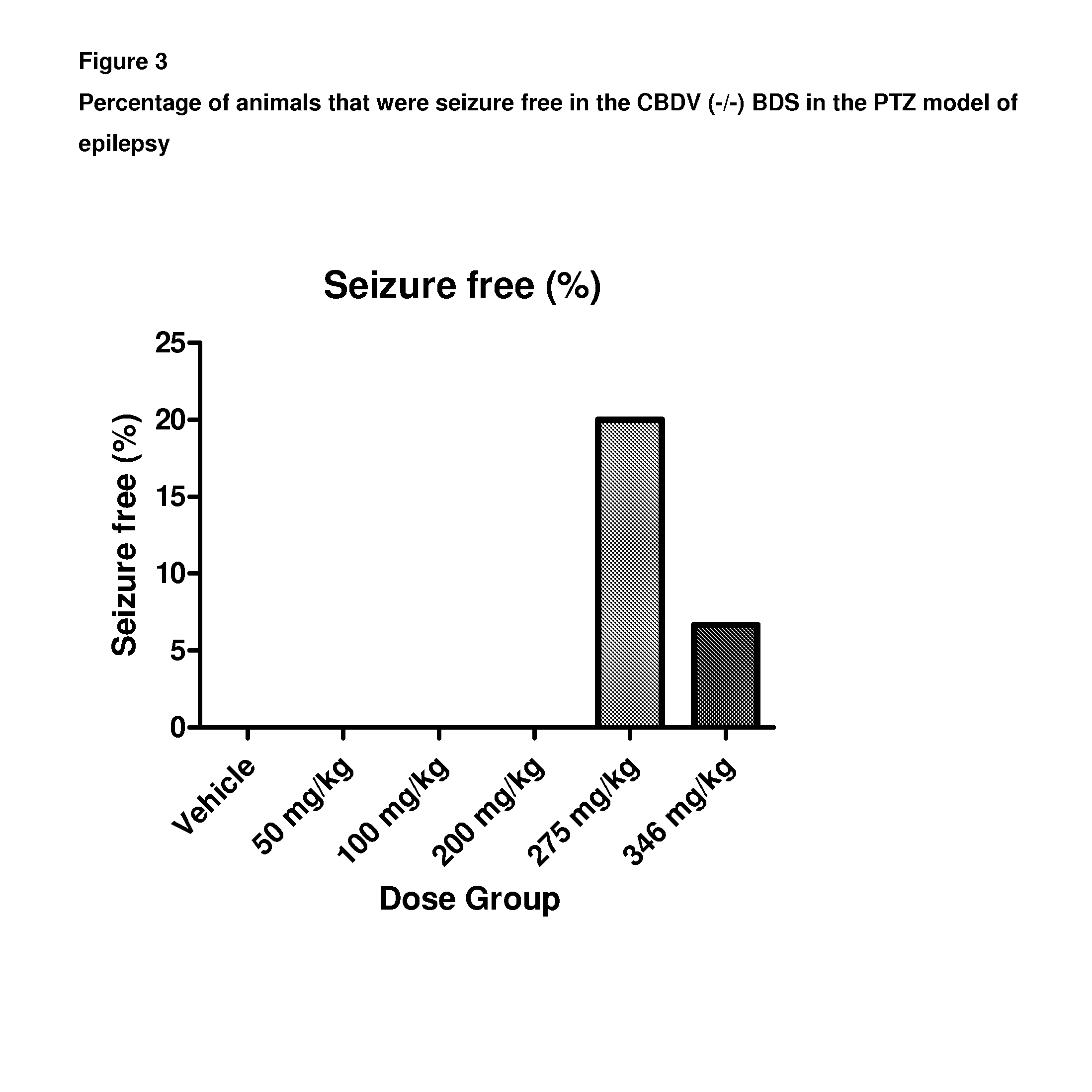
A pharmaceutical composition comprising the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD)
This invention relates to a pharmaceutical composition comprising or consisting essentially of the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD). The composition is particularly safe and efficacious for use in the treatment of neurological conditions, characterized by hyper-excitability of the central nervous system, convulsions or seizures such as occur in epilepsy. Preferably the CBDV and the CBD are present with at least one non-cannabinoid component of cannabis such as one or more terpenes or a terpene fraction. More particularly the composition further comprises one or more cannabichromene type compounds. Particularly cannabichromene propyl variant (CBCV) and / or cannabichromene (CBC). More particularly still the composition is absent or substantially absent of other cannabinoids, including in particular tetrahydrocannabinol (THC) and tetrahydrocannabivarin (THCV), which would normally be present in significant amounts in cannabis chemotypes bred to contain a significant amount of CBDV and / or CBD.
Owner:GW PHARMA LTD
Enhanced Delivery of Nicotine, THC, Tobacco, Cannabidiol or Base Alkaloid from an Electronic Cigarette or Other Vapor or Smoke Producing Device Through Use of an Absorption Conditioning Unit
A method for the administration of nicotine, THC, tobacco, cannabidiol or a base alkaloid includes administering in the oral or nasal cavity an absorption conditioning unit having at least two agents selected from the group consisting of (a) a buffer agent, (b) a capturing agent, (c) a penetration agent, and (d) a thermal agent, to the mammal, and then administering by inhalation a bioactive agent selected from the group consisting of nicotine, THC, cannabidiol and a base alkaloid. The absorption conditioning unit may be in a dosage form not containing a drug. The absorption conditioning unit may create a pH in the oral cavity or nasal cavity of 7.8-10 for a period of ten minutes or more after administration, the dosage form not containing an acid and not containing a drug.
Owner:FUISZ RICHARD C +1
Method for extracting cannabidiol from cannabis
ActiveCN106831353AHigh yieldHigh purityOrganic chemistryOrganic compound preparationAlcoholExtraction Fraction
The invention discloses a method for extracting cannabidiol from cannabis. The method comprises the following steps: smashing and drying extraction fractions of cannabis to obtain medicine material powder; extracting the medicine material powder with 30% to 100% (V / V) of ethanol to obtain an extracting solution; concentrating the extracting solution to obtain an extract; carrying out water precipitation on the extract, and removing impurities to obtain a water precipitation solution; centrifuging the water precipitation solution and adding 10% to 100% (V / V) of ethanol into an obtained precipitate, and dissolving the precipitate to obtain an alcoholic solution of the precipitate; carrying out column chromatography on the alcoholic solution of the precipitate; eluting an obtained eluant after the concentration column chromatography, and adding ethanol for supersaturated dissolution to obtain a crystal substance; adding the crystal substance into purified water or ethanol for washing to obtain a primary product; blending the primary product with purified water, and drying to obtain the cannabidiol. Only highly safe ethanol is adopted as a solvent, the purity of the cannabidiol in a finished product is improved, and the psychotoxic component of tetrahydrocannabinol is also removed, so that the product safety is improved, and the method is applicable for industrial production.
Owner:YUNNAN HANSU BIO TECH CO LTD
Method for extracting cannabidiol from industrial hemp floral leaves
InactiveCN106278828AHigh purityReduce the impactOrganic chemistryOrganic compound preparationAlcoholSolvent
The invention discloses a method for extracting cannabidiol from industrial hemp floral leaves. The method comprises the following steps: grinding and drying floral leaves of industrial hemp, thereby obtaining medicinal powder; extracting the medicinal powder by adopting 30-100v% of ethanol, thereby obtaining extracting solution; concentrating the extracting solution to obtain extract; performing water precipitation on the extract, removing the impurities, thereby obtaining water precipitation solution; centrifuging the water precipitation solution, adding 10-100v% of ethanol into the obtained precipitate, dissolving to obtain an alcoholic solution of the precipitate; performing column chromatography on the alcoholic solution of the precipitate; concentrating the eluent obtained by eluting after column chromatography, adding ethanol to perform supersaturation dissolving, thereby obtaining a crystal; adding the crystal into purified water or ethanol for washing, thereby obtaining a primary product; uniformly mixing the primary product by using the purified water, drying, thereby obtaining the cannabidiol. According to the method disclosed by the invention, the highly safe ethanol serves as a solvent, so that the purity of the cannabidiol in the finished product is improved, a psychotoxic component, namely tetrahydrocannabinol, is removed, the product safety is improved, and the method is suitable for industrial production.
Owner:YUNNAN HANSU BIO TECH CO LTD
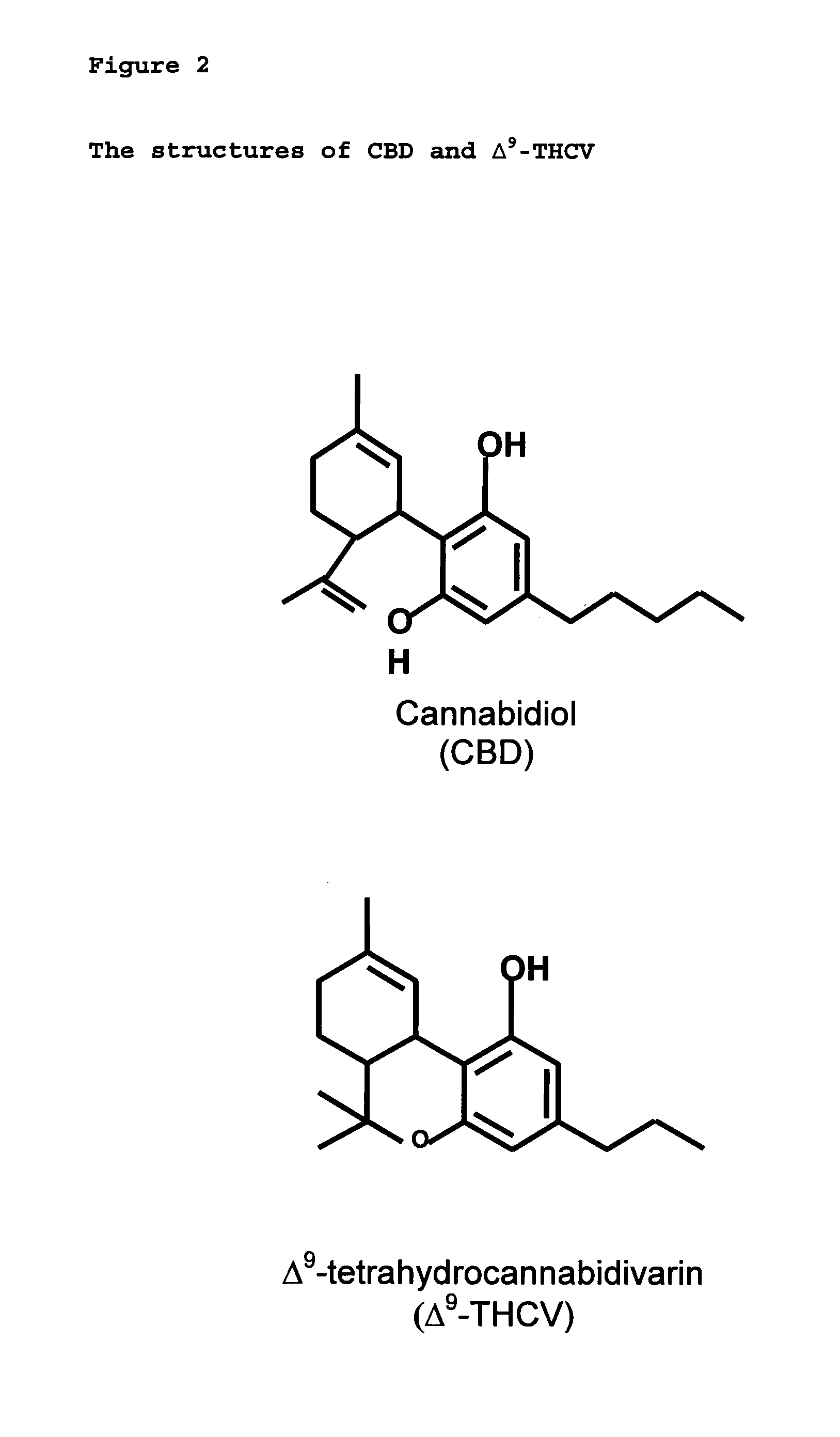
New pharmaceutical formulation comprising cannabidiol and tetrahydrocannabidivarin
InactiveUS20100317729A1Enhanced treatment optionGood for weight lossBiocideNervous disorderCannabinoidPharmaceutical formulation
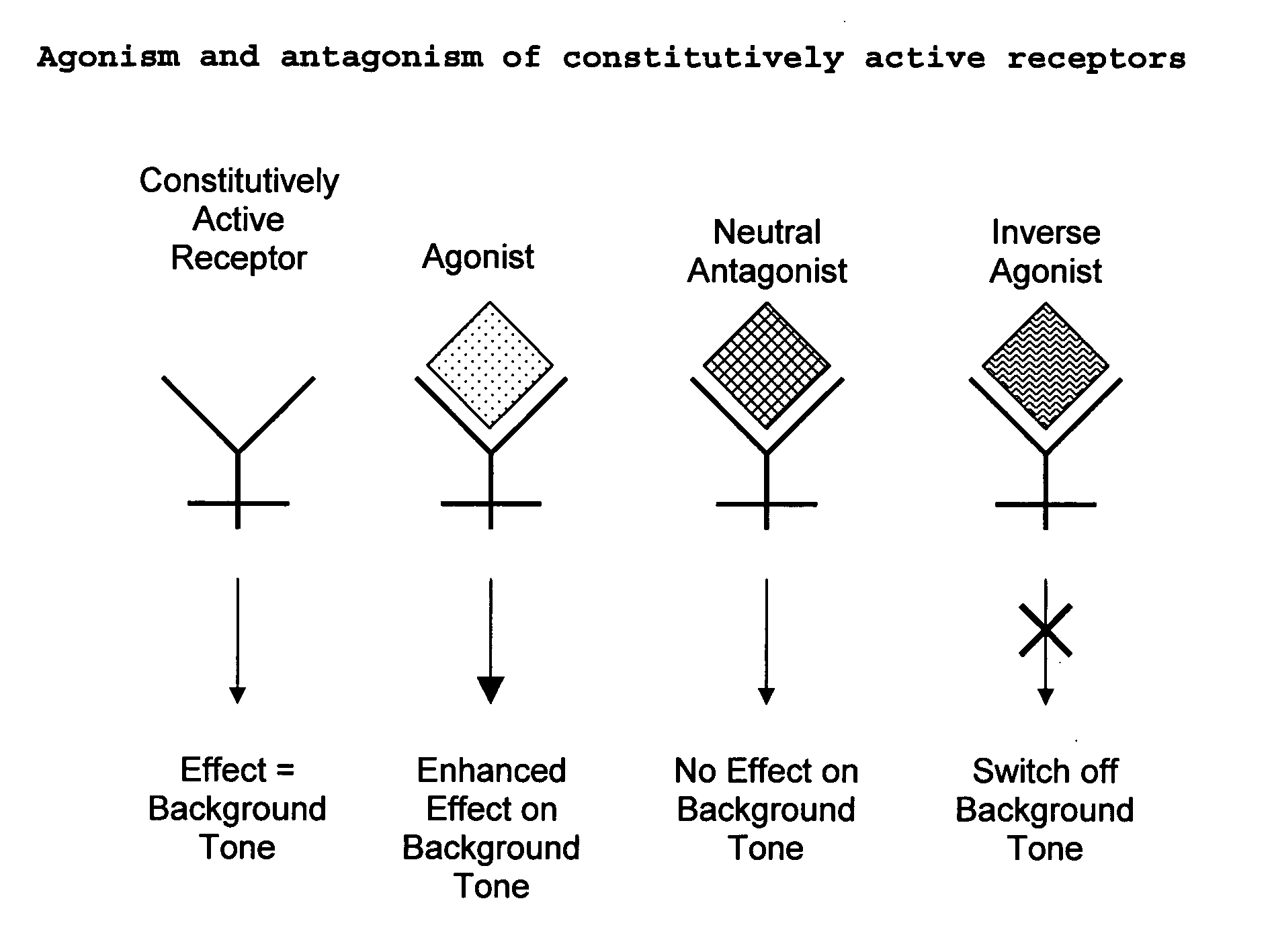
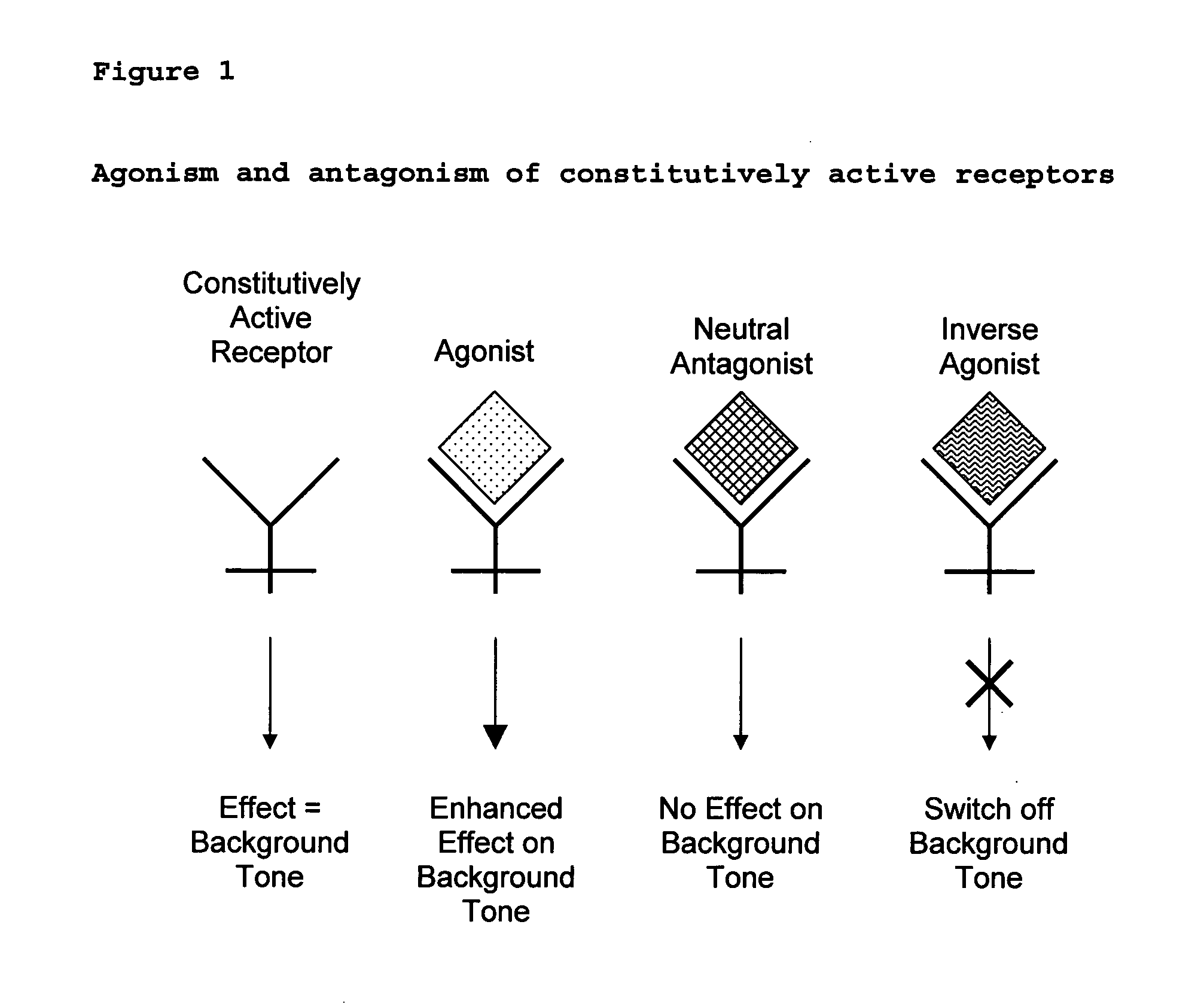
The present invention relates to a novel pharmaceutical formulation comprising a ratioed mix of: (i) one or more compounds that acts as an inverse agonist of the CB1 and / or CB2 receptor; and (ii) one or more compounds that acts as a neutral antagonist of the CB1 and / or CB2 receptor. Preferably both the inverse agonist of the CB1 and / or CB2 receptor and the neutral antagonist of the CB1 and / or CB2 receptor are cannabinoids. Preferably the cannabinoids are tetrahydrocannabidivarin (THCV) and cannabidiol (CBD).
Owner:GW PHARMA LTD
Oral Dosage Form Of Tetrahydrocannabinol And A Method Of Avoiding And/Or Suppressing Hepatic First Pass Metabolism Via Targeted Chylomicron/Lipoprotein Delivery
ActiveUS20110092583A1Easy to transportPromote lymphatic transportBiocideSenses disorderChylomicronCytochrome P450
Self-emulsifying drug delivery systems are provided to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron / lipoprotein delivery and optimal bioavailability through the mammalian intestinal tract. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY RAM B +1
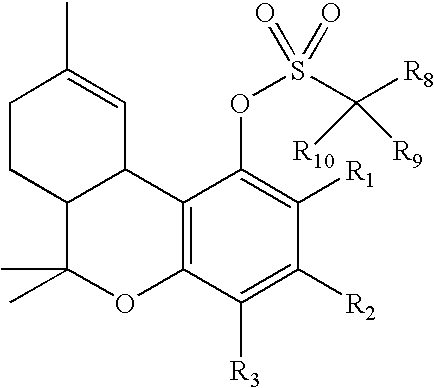
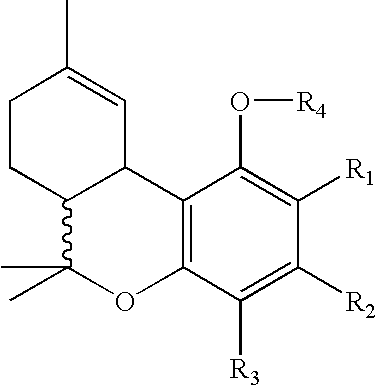
Process for production of delta-9-tetrahydrocannabinol
InactiveUS7674922B2High selectivityLower overall renovationOrganic compound preparationCarboxylic acid esters preparationArylPtru catalyst
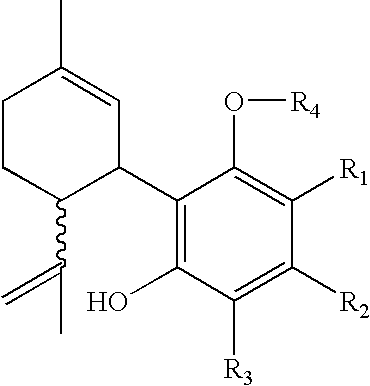
The present invention relates to a process for preparation of a delta-9-tetrahydrocannabinol compound or derivative thereof involving treating a first intermediate compound with an organoaluminum-based Lewis acid catalyst, under conditions effective to produce the delta-9-tetrahydrocannabinol compound or derivative thereof. Another aspect of the present invention relates to a process for preparation of a cannabidiol or cannabidiolate compound involving reacting a first starting compound with a second starting compound in the presence of a metal triflate catalyst, under conditions effective to form the cannabidiol or cannabidiolate compound. The present invention also relates to a compound of the formula:where R8, R9, and R10 are the same or different and independently selected from the group consisting of H, substituted or unsubstituted alkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or halo, with R1, R2, and R3 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Method for extracting and purifying cannabidiol from China-hemp
ActiveCN107337586AImprove solubilityHigh extraction rateOrganic chemistryOrganic compound preparationOrganic solventElution
The invention belongs to the technical field of extraction and purification of plant ingredients and particularly relates to a method for extracting and purifying cannabidiol from China-hemp. In the method, an ingredient of cannabidiol is extracted from floral leaves in a mature period of the China-hemp according to a supercritical CO2 extraction technique, a cannabidiol extract is further purified by macroporous adsorbent resin and a rapid purification system, a cannabidiol content of an obtained product is 98% or higher, and the cannabidiol product does not contain a psychotoxic ingredient tetrahydrocannabinol. According to the method, a chemical organic solvent is not used in an extraction process, so that the method is environment-friendly and is low in toxicity and high in efficiency; the loss of cannabidiol in an adsorption elution process is reduced in a purification process, so that the impurity ingredients such as tetrahydrocannabinol and the like are effectively removed, and the use of an organic regent is decreased, so that the purification cost is lowered, and the environmental pollution is reduced. The product is high in yield and purity, and the requirement for development of a value-added product of the China-hemp, in particular to the market requirement of the active ingredient of the cannabidiol product in the China-hemp, can be met, and especially, the cannabidiol product has the great application advantage in the pharmaceutical field.
Owner:DAQING BRANCH OF HEILONGJIANG ACAD OF SCI
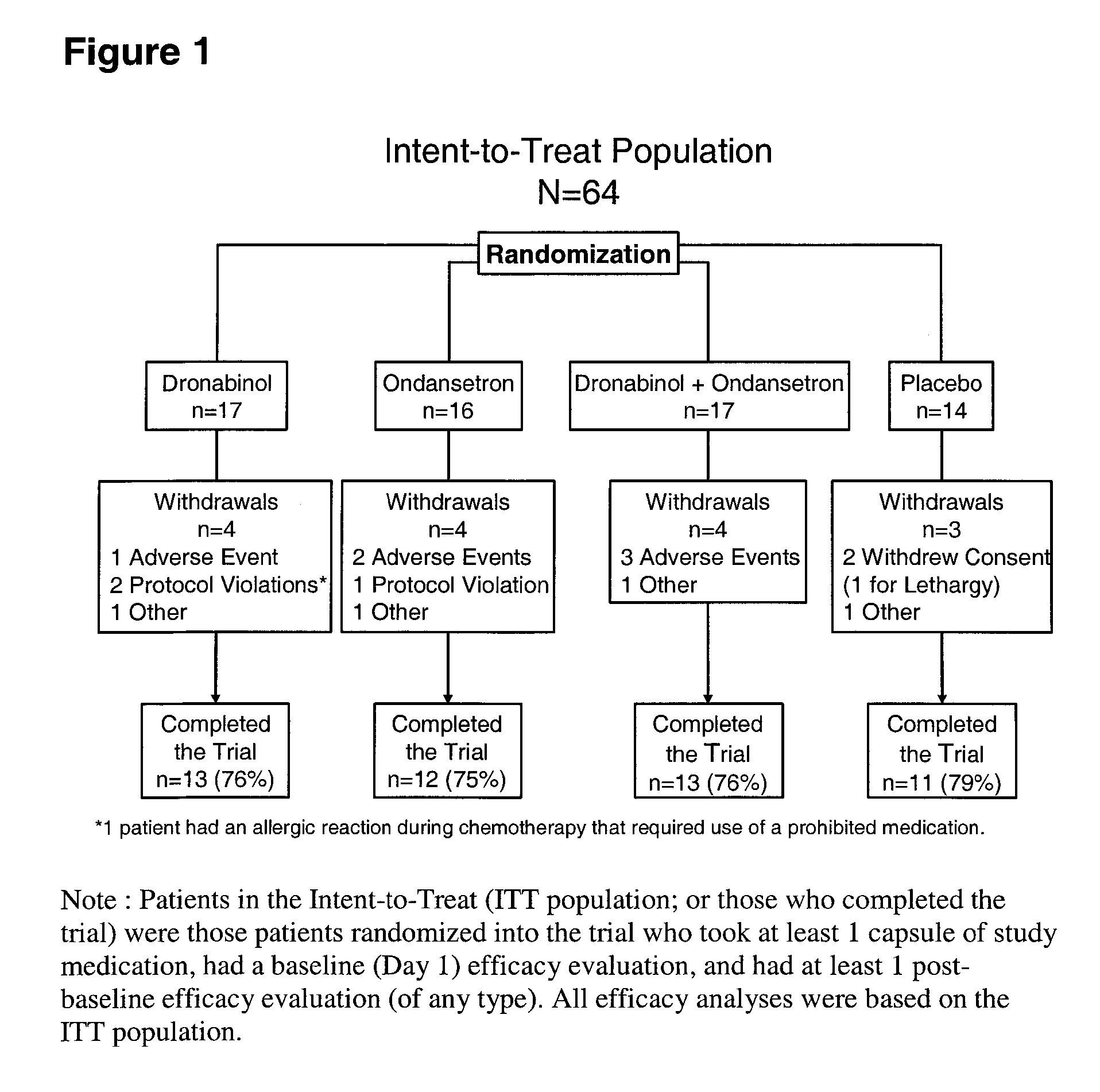
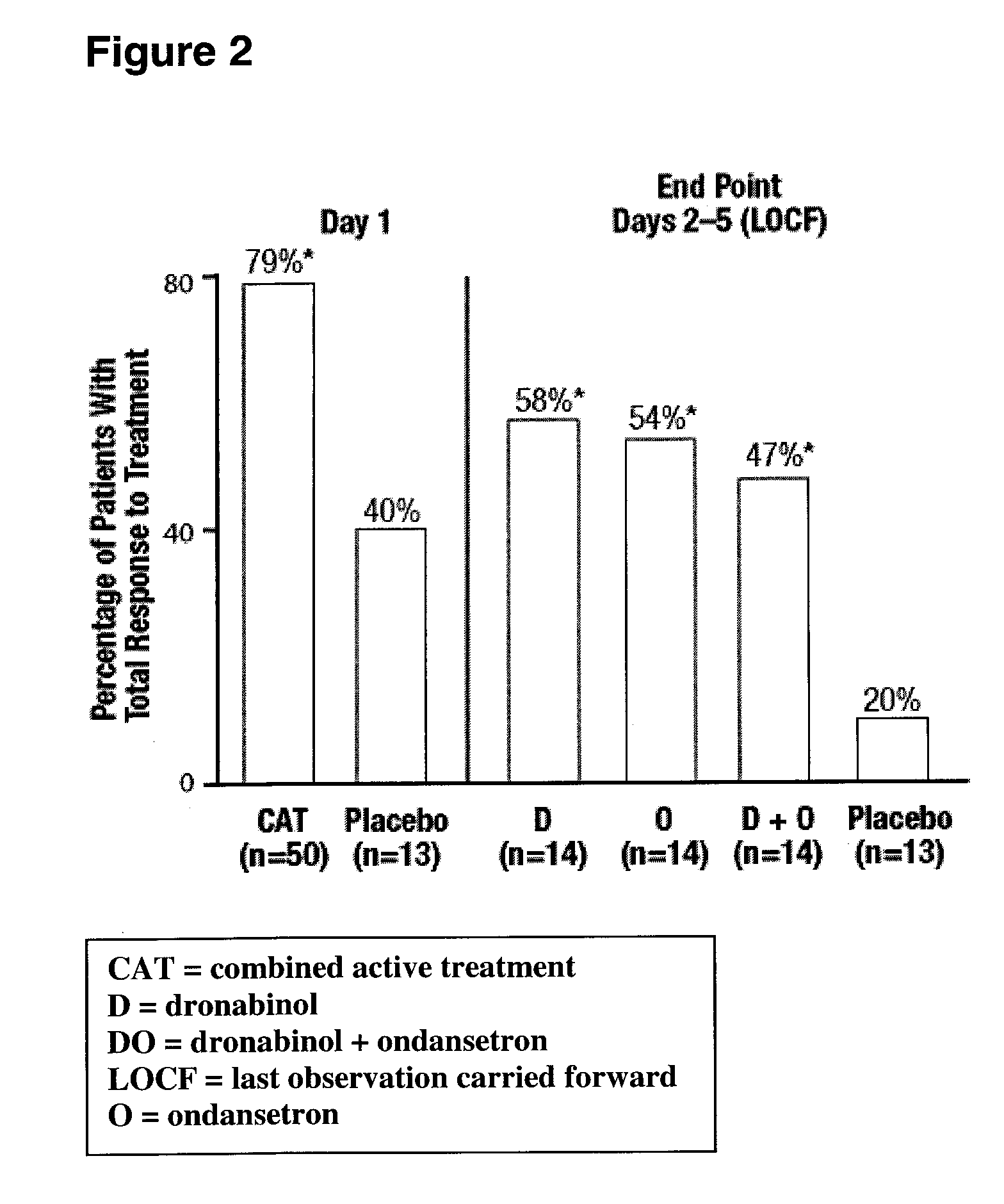
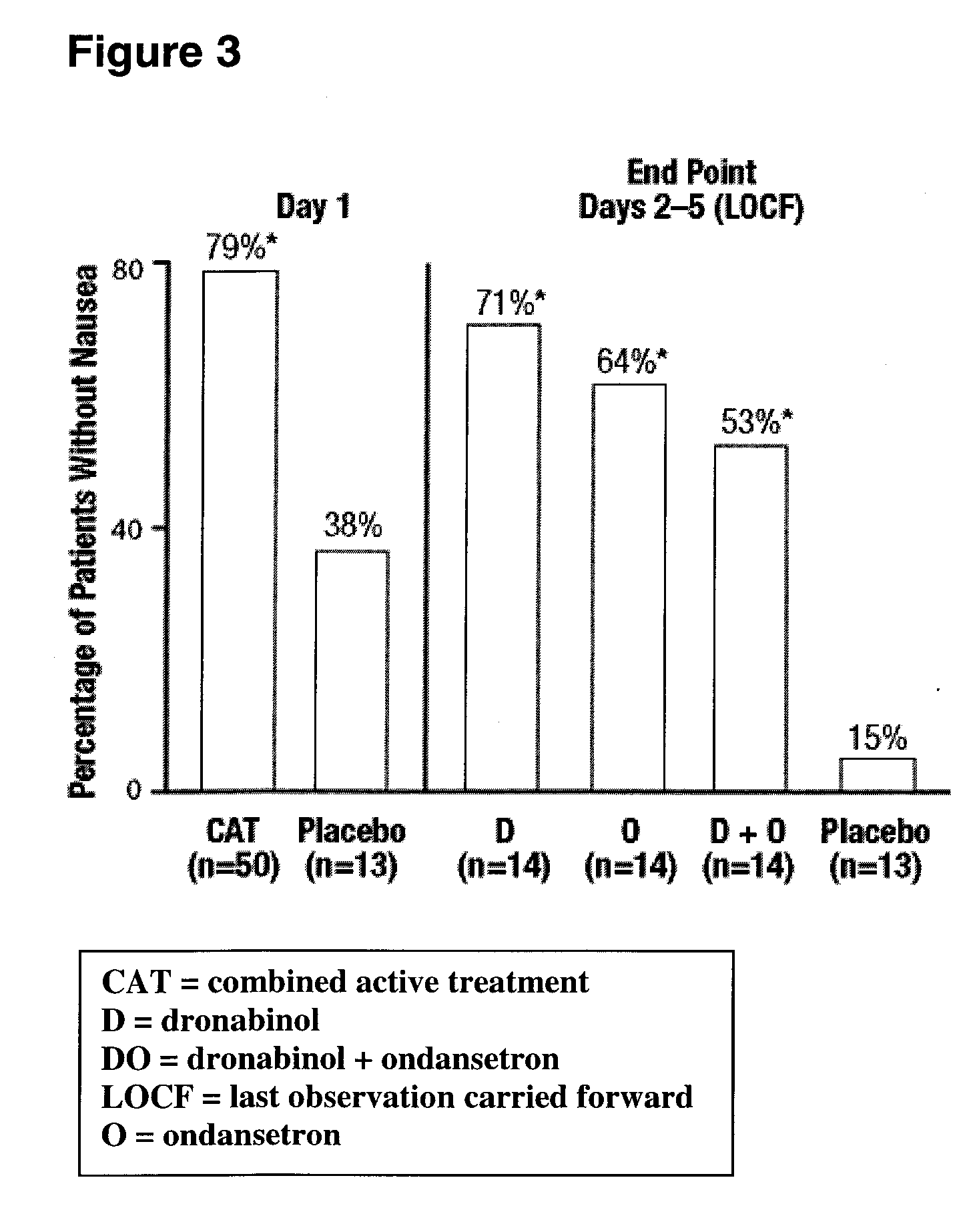
Dronabinol treatment of delayed chemotherapy-induced nausea and vomiting
InactiveUS20070072938A1Reduce developmentPrevent reduce developmentBiocideDigestive systemDelta-9-tetrahydrocannabinolChemotherapy-induced nausea and vomiting
In various embodiments, the present invention provides pharmaceutical compositions comprising delta-9-tetrahydrocannabinol and methods of administering such compositions prior to the administration of chemotherapy to prevent or reduce the development of delayed chemotherapy-induced nausea and vomiting.
Owner:UNIMED PHARMA LLC
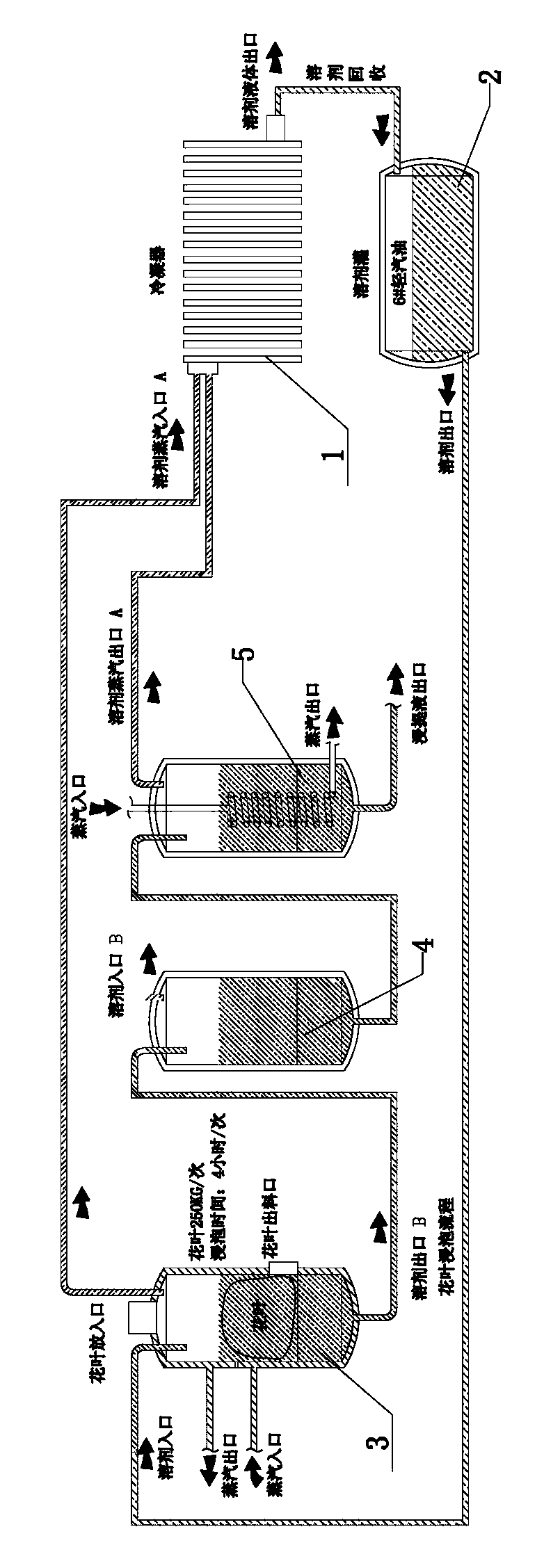
Extraction method and extraction device for cannabidiol-enriched industrial hemp essential oil
ActiveCN104277917AEnsure safetyEnsure normativeEssential-oils/perfumesRotary evaporatorProcess engineering
The invention discloses an extraction method and an extraction device for cannabidiol-enriched industrial hemp essential oil. By adopting devices such as a screening machine, an oven, a soaking tank, a rotary evaporator, an ultrasonic agitation tank, a butterfly centrifuge, a first climbing-film evaporator, a proportioning tank, a pressurized chromatography silica gel column, an eluate tank, a second climbing-film evaporator, a rotary evaporator, a finished product tank and a solvent recovery tank which are sequentially connected by virtue of pipelines, industrial hemps are stepwise subjected to the operation steps comprising screening, baking, extracting, re-treating, filtering, monitoring, sampling, chromatography and concentrating to obtain the finished product and the effective component cannabidiol-enriched industrial hemp essential oil is finally extracted. By virtue of the extraction device disclosed by the invention, the defects of low efficiency, large consumption and inconvenience in scale production of the conventional process are overcome, the semi-finished product containing tetrahydrocannabinol in the processing does not artificially contact and flow, is legal, safe and efficient, the process method is simple and can be easily promoted and the product has high purity, complete active ingredients and low cost.
Owner:HANKANG YUNNAN BIOTECH
Method for enriching cannabidiol
ActiveCN107011125AIncrease contentOrganic chemistryOrganic compound preparationChemical industryGradient elution
The invention belongs to the technical field of chemical industry, and particularly relates to a method for enriching cannabidiol. The method includes steps of S1, drying Cannabis sativa flower and leaf for 0.5-4 hours at 120 DEG C, and smashing until the particle size of the smashed material meets the requirement of over 50 meshes; performing carbon dioxide supercritical extraction for 1-9 hours under the conditions of 30-55 DEG C and 13-30 MPa, and recycling carbon dioxide to obtain initial extractive of Cannabis sativa flower and leaf; S2, dissolving the initial extractive of Cannabis sativa flower and leaf in ethanol or methanol, refining, filtering and removing impurities, distilling and drying the solvent for future use; S3, filling a chromatographic column with the pretreated filler, sampling by a wet method or a dry method, wherein the sampling amount is 3-15 wt%; S4, performing gradient elution or isocratic elution by one or mixture of water, methanol, ethanol, n-butyl alcohol, acetone or chloroform; collecting the dilution section with enriched cannabidiol. The method is simple in technique; content of cannabidiol is improved, and tetrahydrocannabinol content is reduced.
Owner:YUNNAN HEMPSON BIO-TECH CO LTD
Pharmaceutical compositions for the treatment of pain
The present invention relates to treatment of cancer related pain and constipation. Preferably the subject in need is administered a combination of the cannabinoids cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC). More preferably the cannabinoids are in a predefined ratio by weight of approximately 1:1 of CBD to THC.
Owner:GW RES LTD
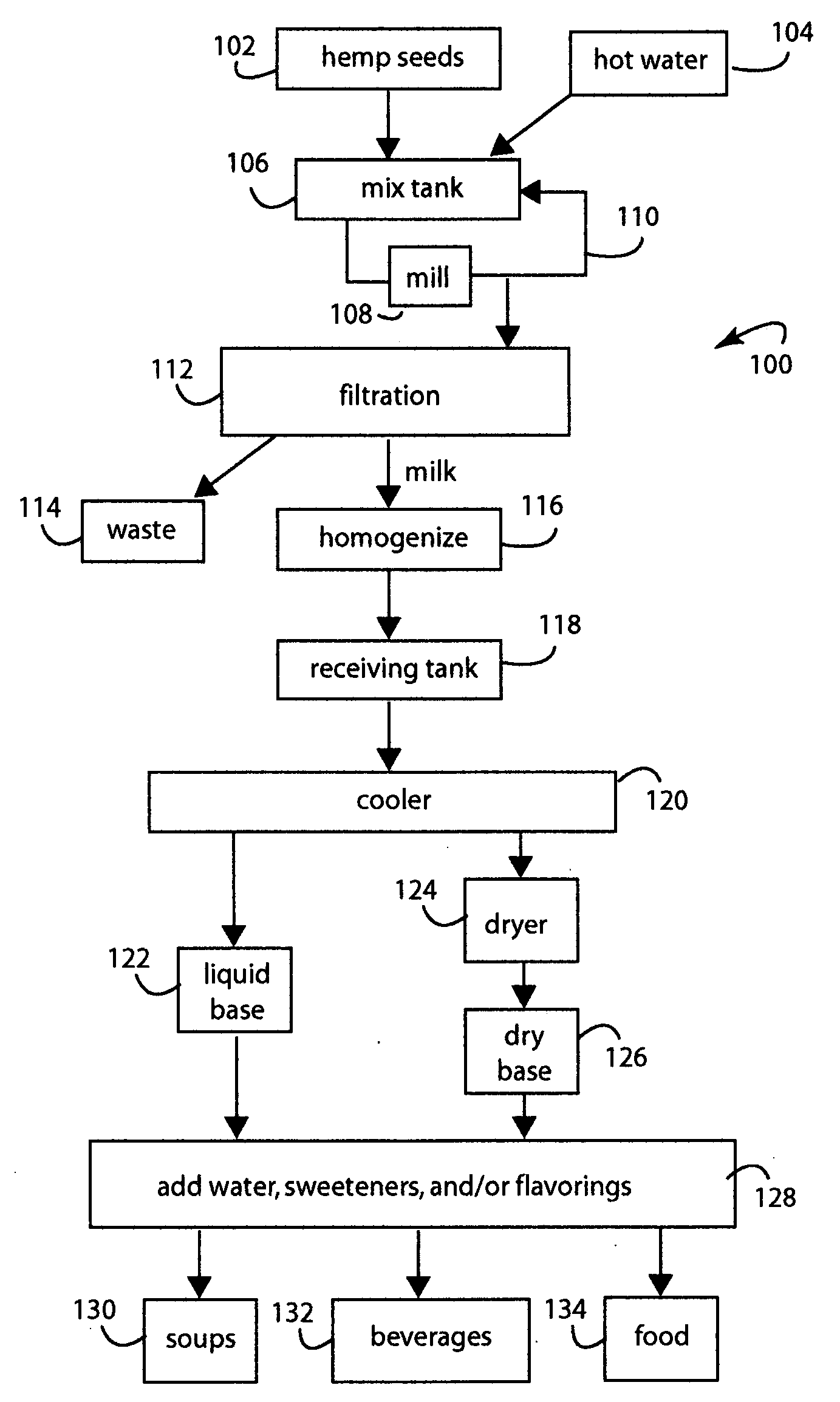
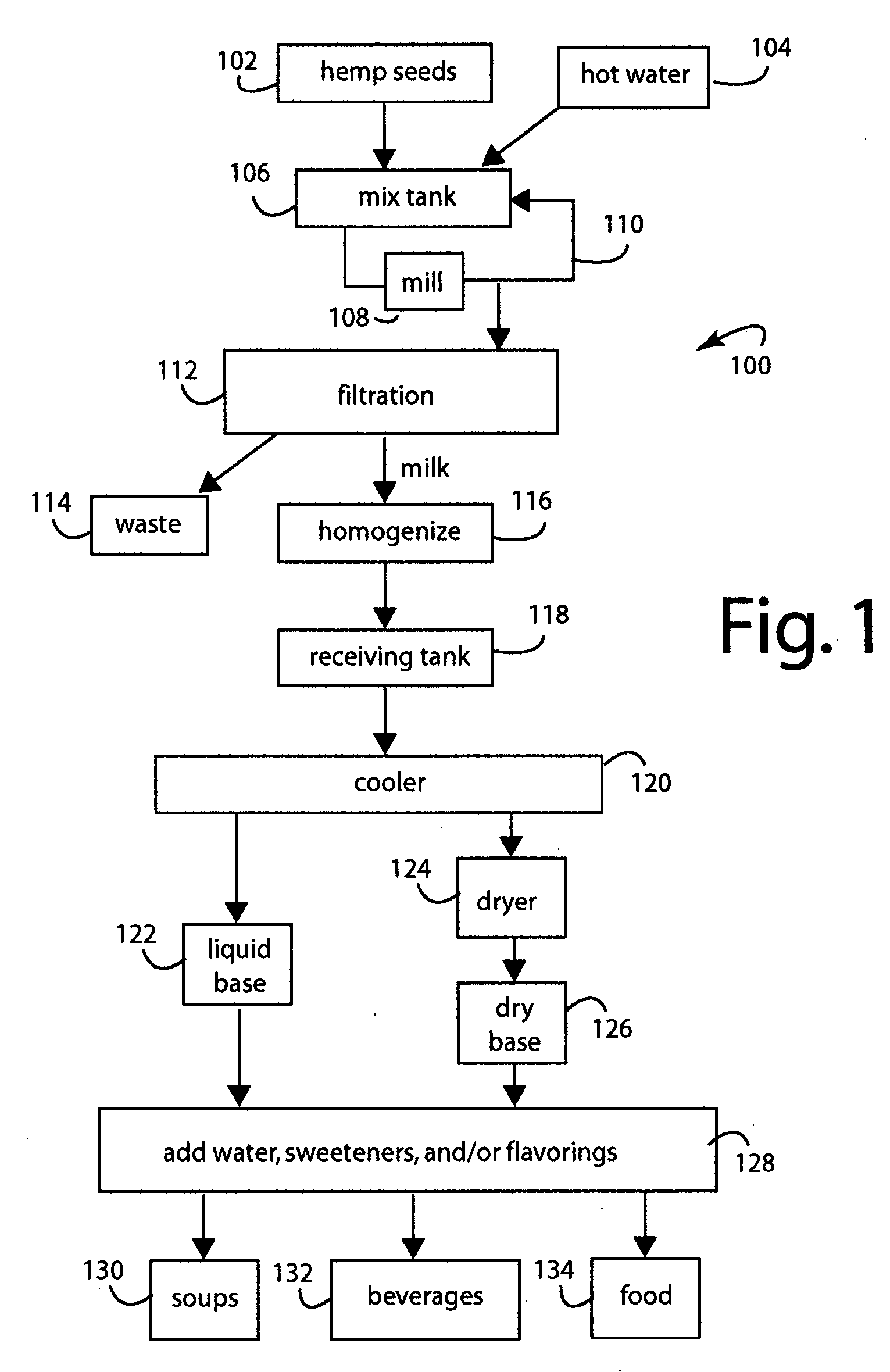
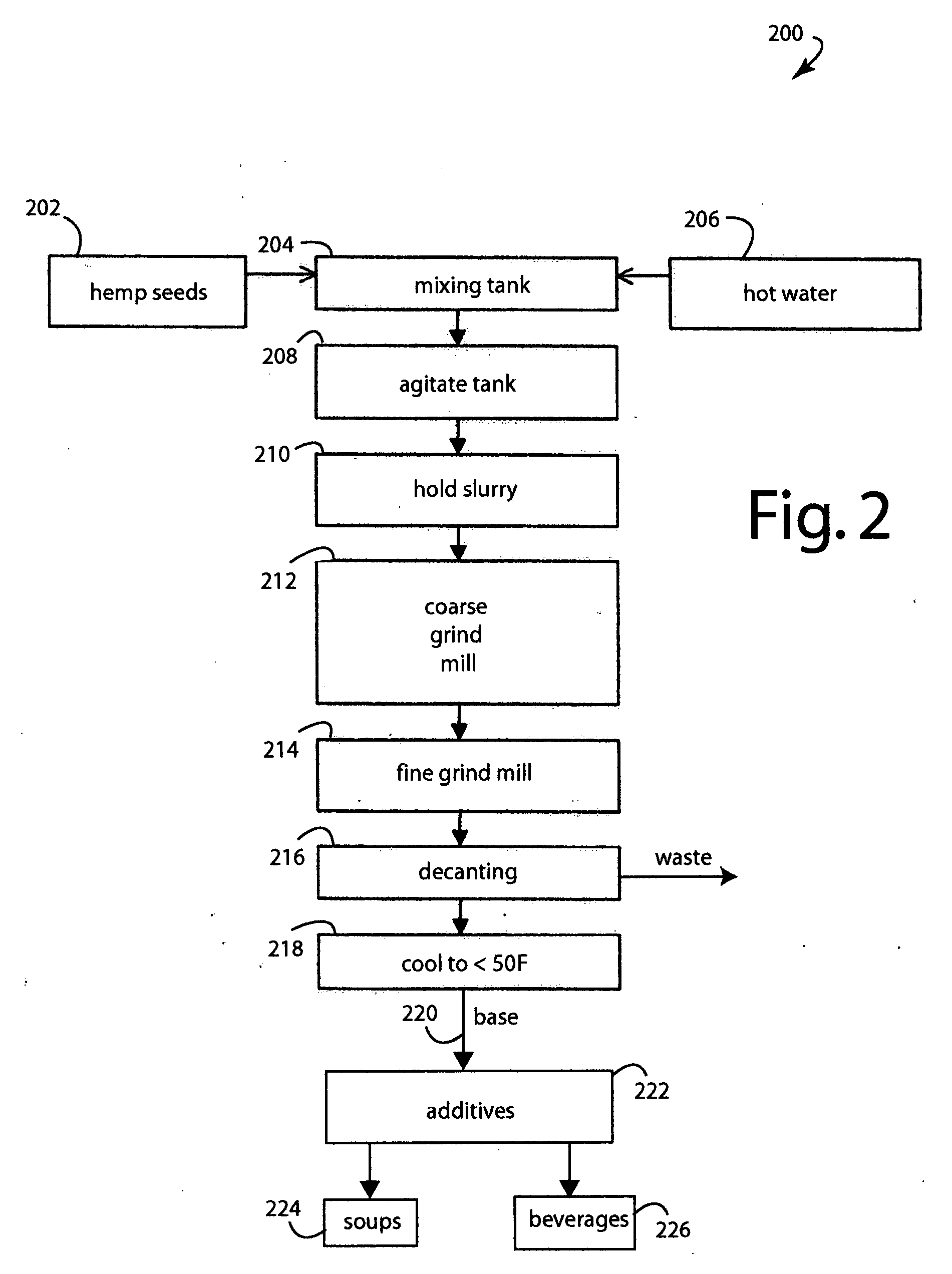
Hemp food product base and processes
InactiveUS20080241339A1Pleasant flavorLow carbohydrateFood preparationGrain millingFlavorProduct base
A food process comprises starting with hemp (Cannabis sativa) seeds that have been cultivated to have low levels of tetrahydrocannabinol (THC) alkaloid, e.g., less than 0.3%. The hemp seeds are dehulled to produce split seed kernels, mixing the split seed kernels with hot water to hydrate them into a slurry, grinding the slurry to blend and smooth it into a product base, cooking the product base to achieve a particular flavor and aroma consistent with a target food product, cooling the product base to stop cooking, and further processing the product base into a target food product like soups and beverages. The products produced have high levels of protein, vitamins, and other nutritional values.
Owner:CALIFORNIA NATURAL PRODS
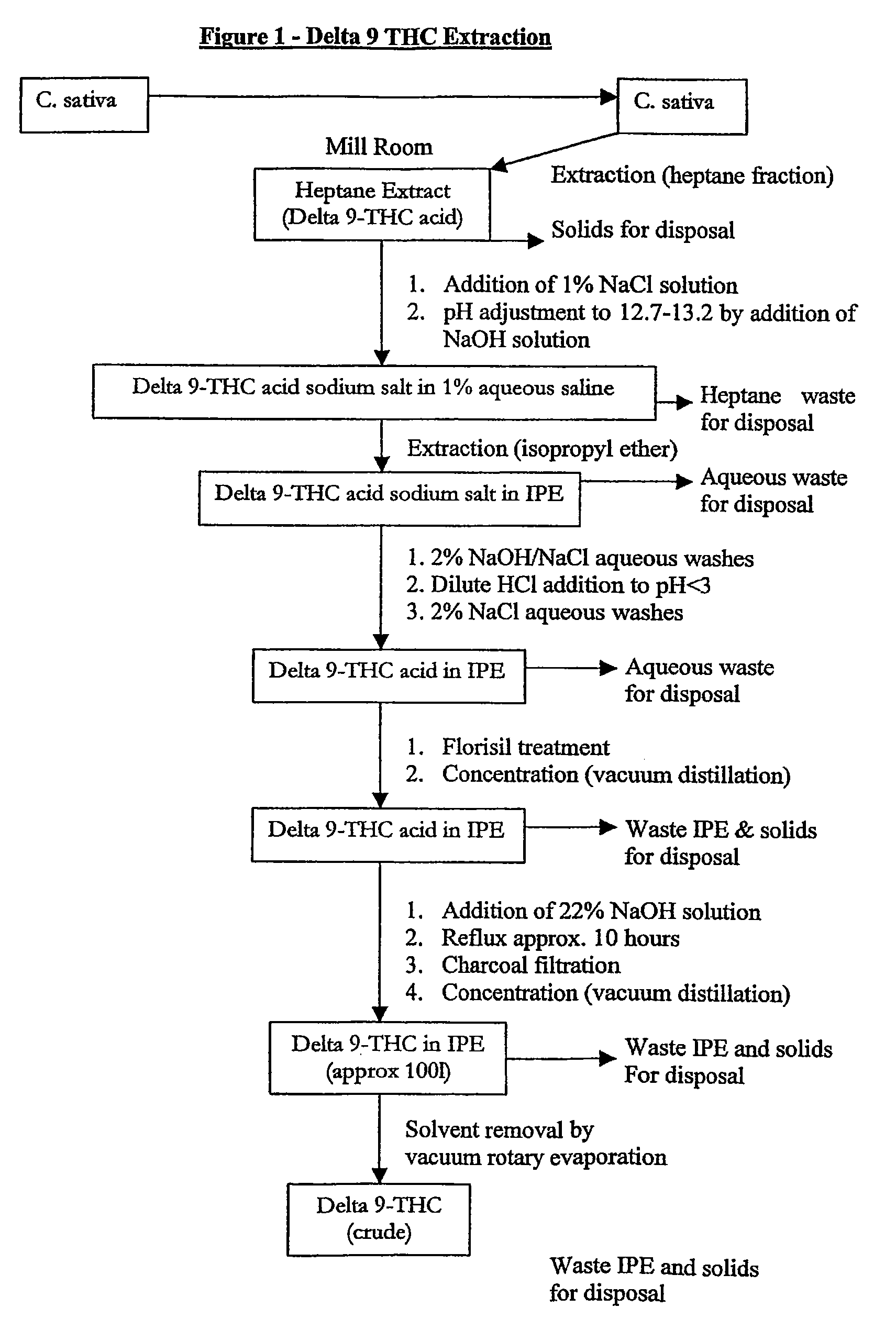
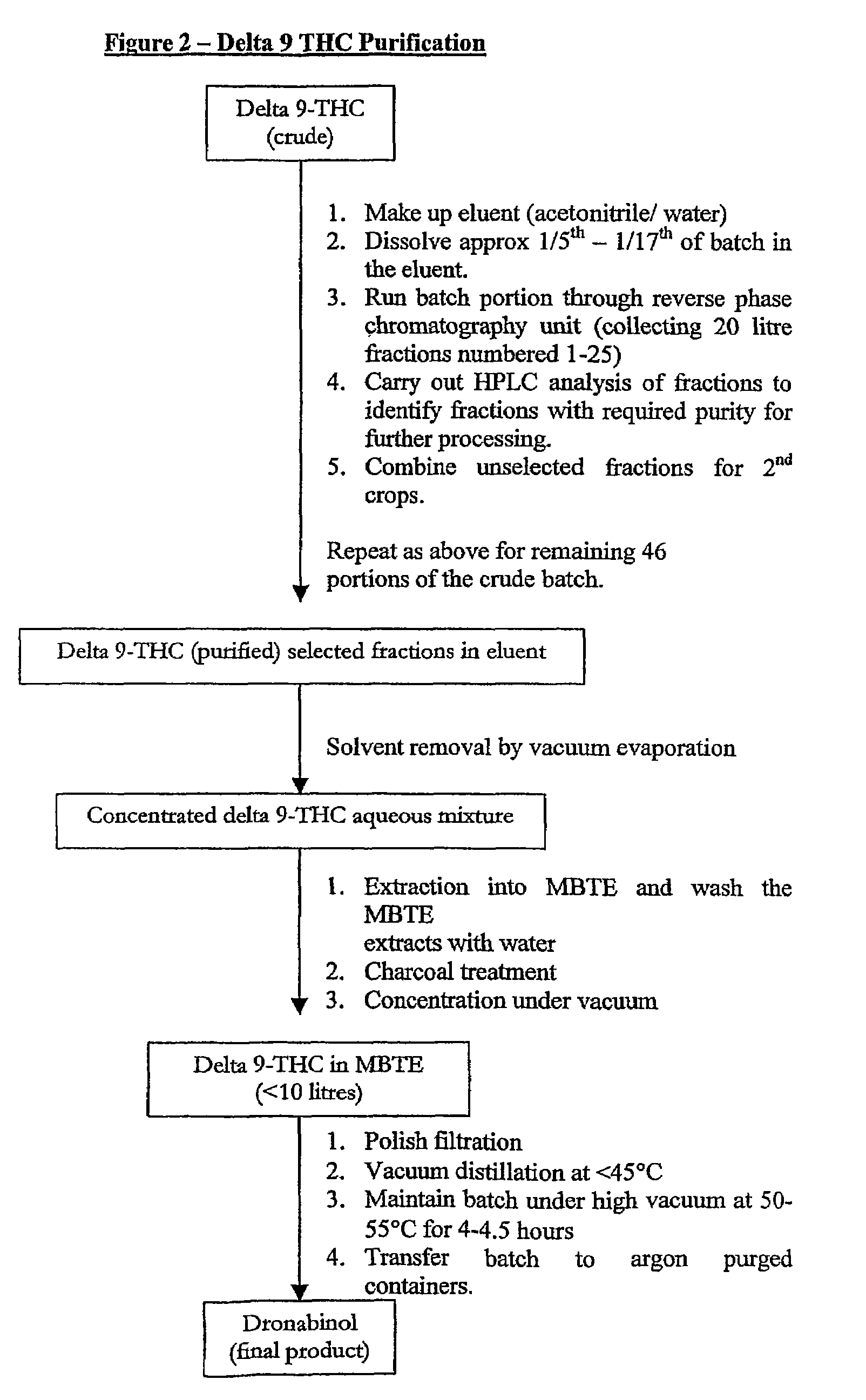
Production of Δ 9 tetrahydrocannabinol
Δ9 THC acid is obtained from plant material and extracted into an aqueous solvent under conditions of pH control. The acid is converted to a salt and the salt extracted into a polar solvent, yielding acid of high purity. The Δ9 THC acid is then converted to Δ9 THC which is further purified and combined with a carrier for pharmaceutical use.
Owner:RESOLUTION CHEM
Use of dronabinol for treatment of side effects of Hepatitis C therapy
InactiveUS20060258738A1Avoid gastrointestinal side effectsBiocidePeptide/protein ingredientsSide effectDelta-9-tetrahydrocannabinol
The present invention provides methods for treating and preventing the gastrointestinal side effects associated with anti-HCV therapy comprising administering delta-9-tetrahydrocannabinol.
Owner:MT SINAI SCHOOL OF MEDICINE
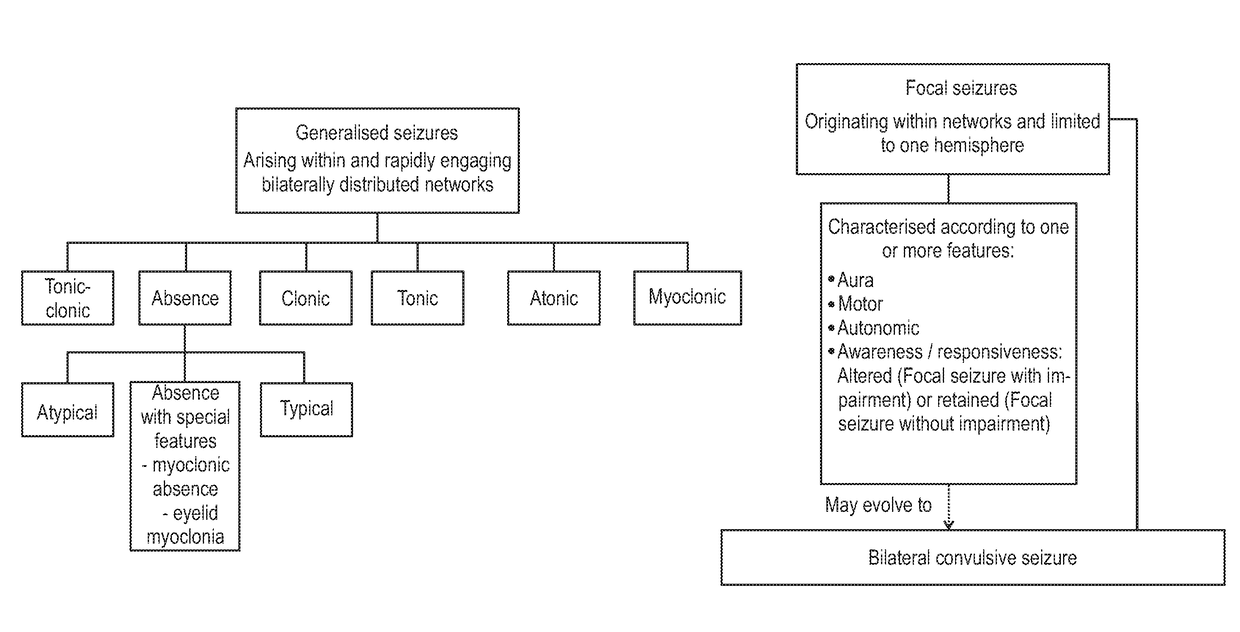
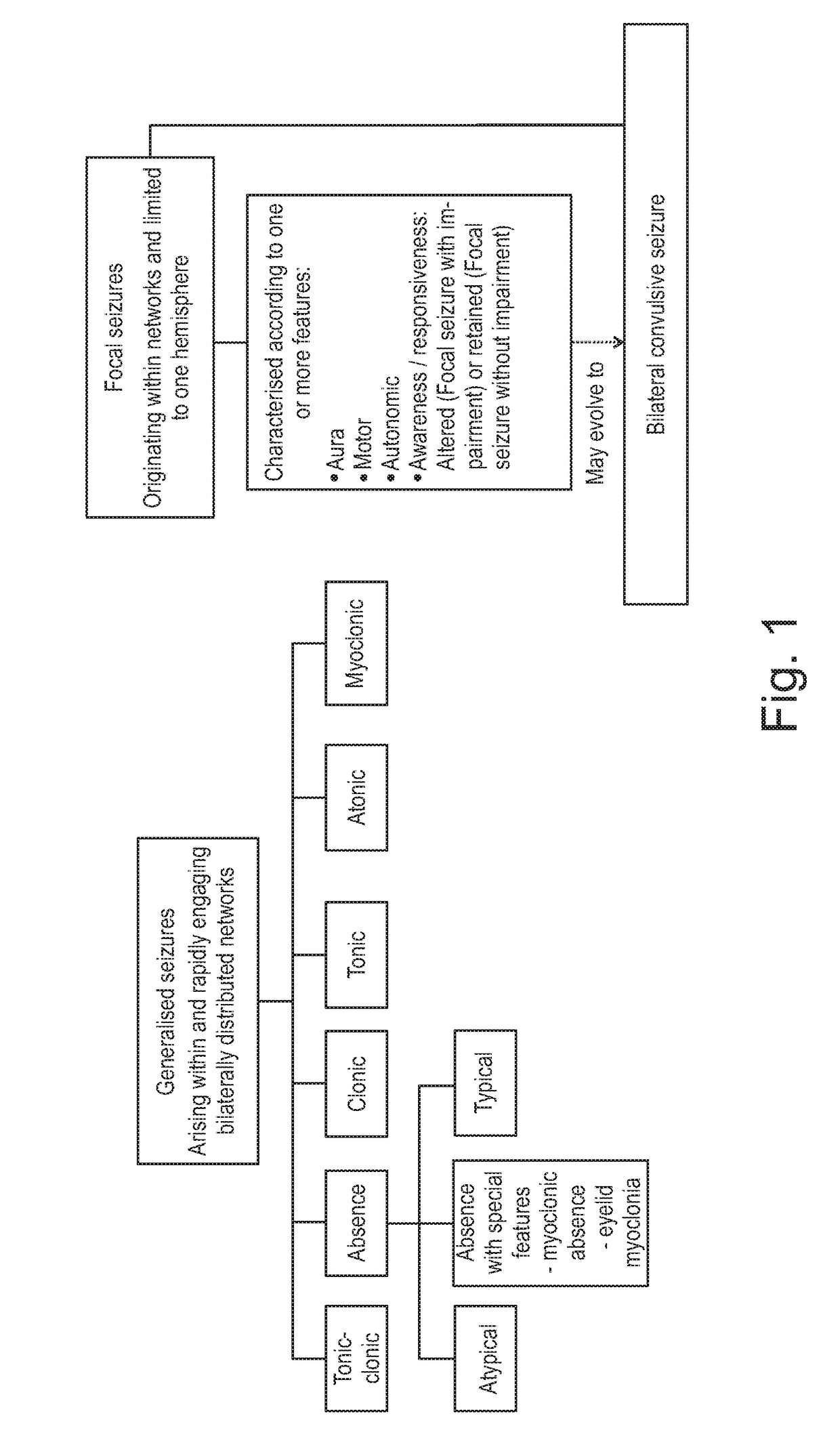
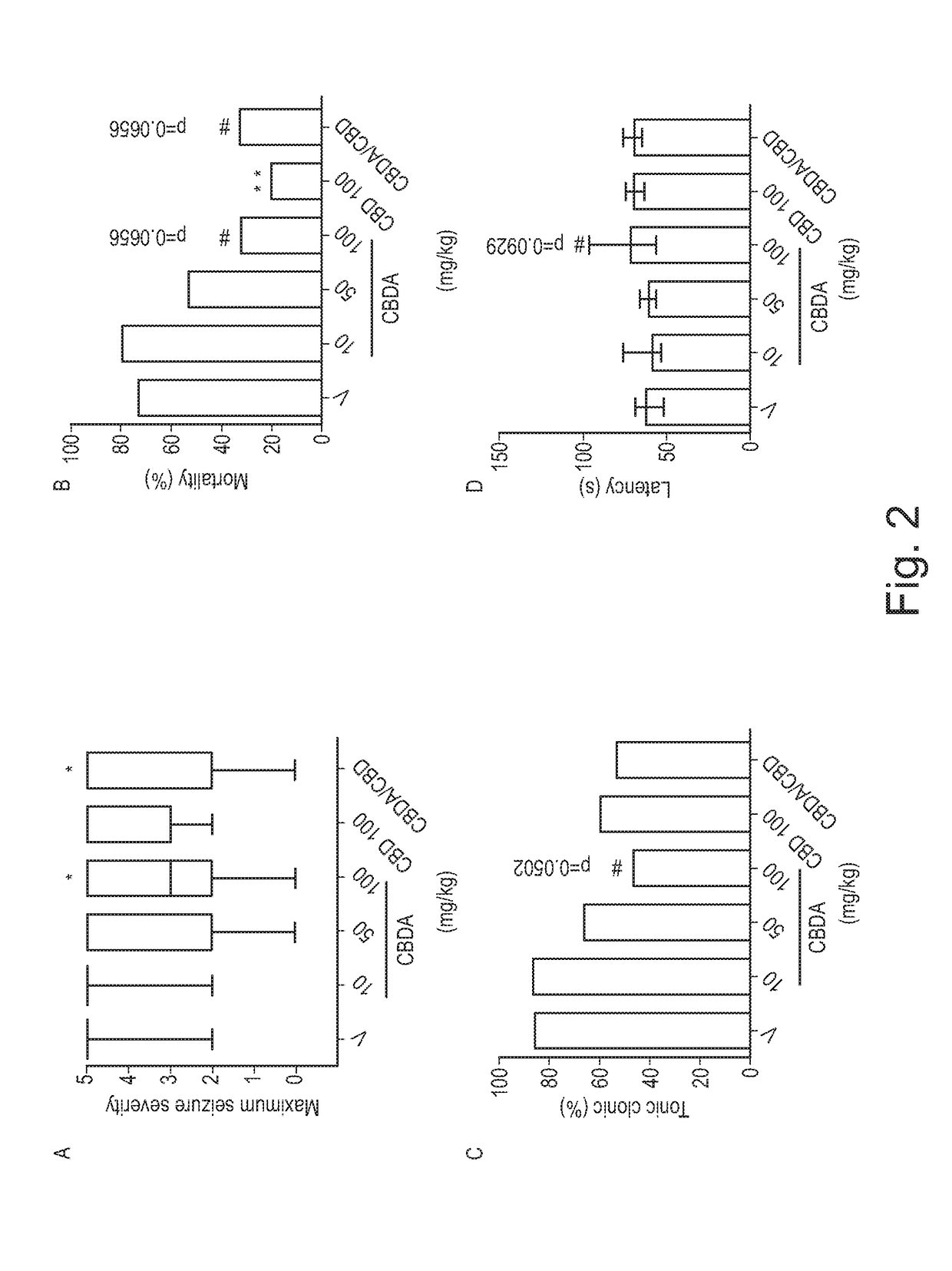
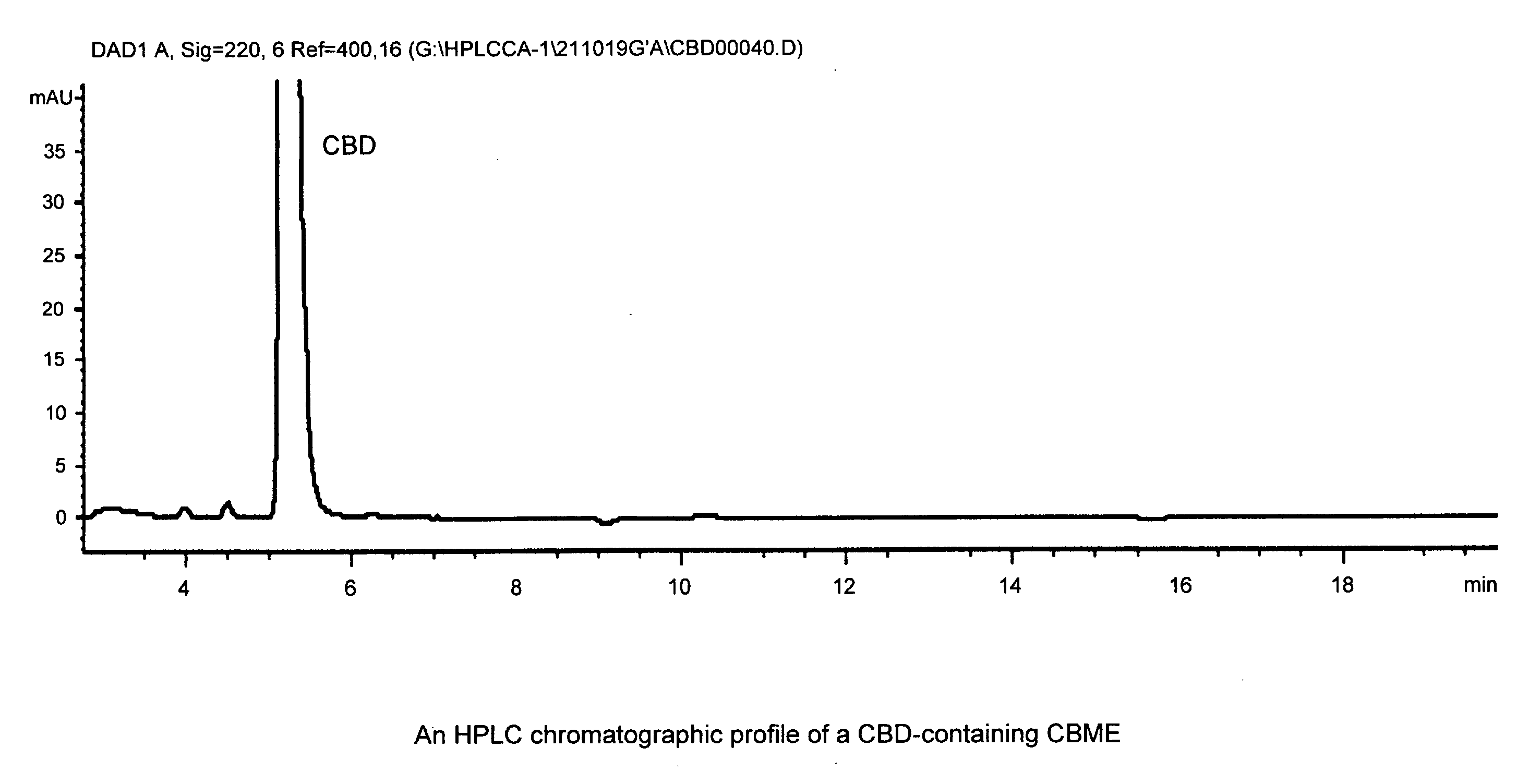
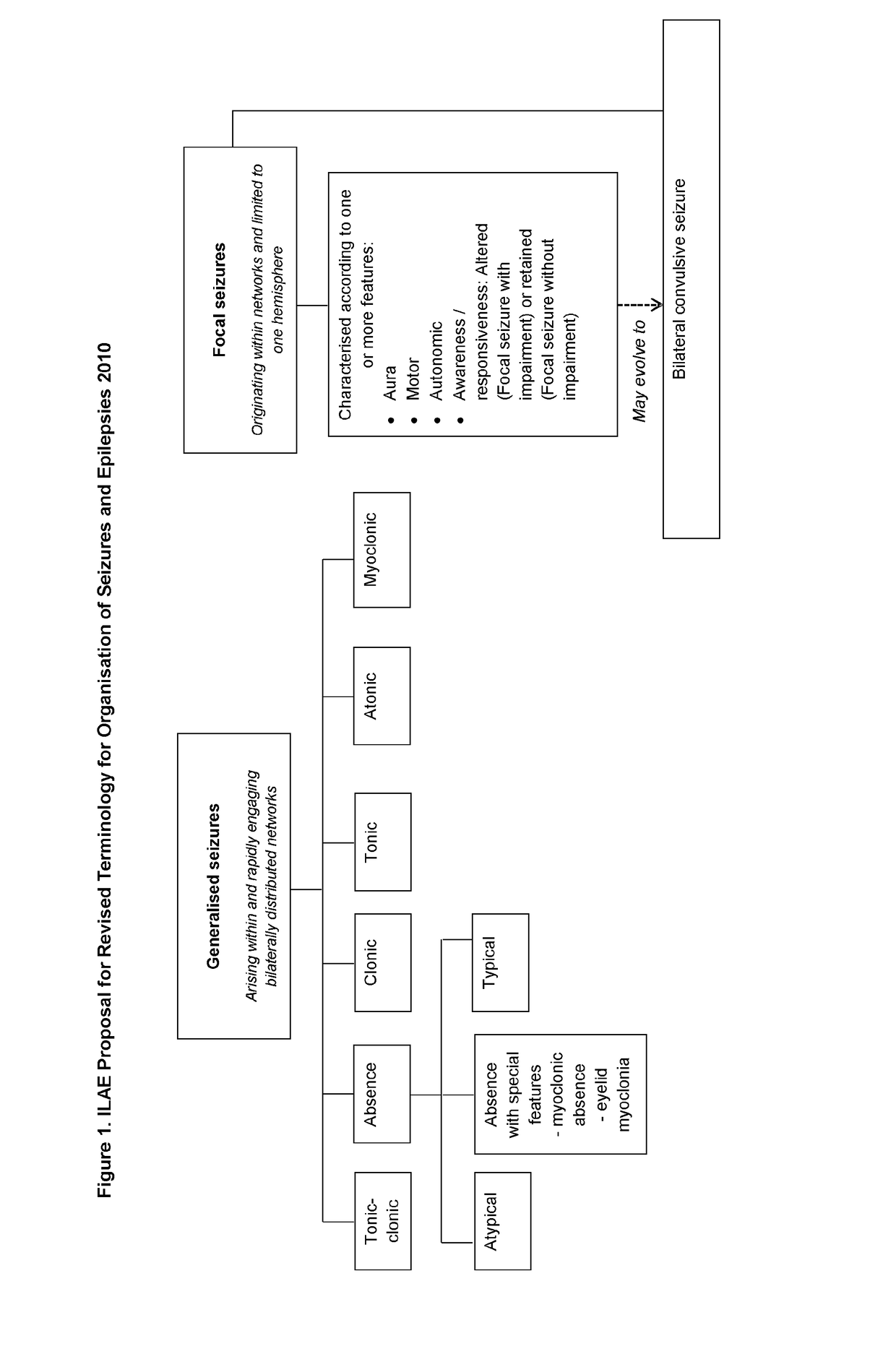
Use of cannabinoids in the treatment of epilepsy
ActiveUS20180228751A1Reduce doseReduced dosNervous disorderHydroxy compound active ingredientsTonic-clonic seizuresEpileptic seizure
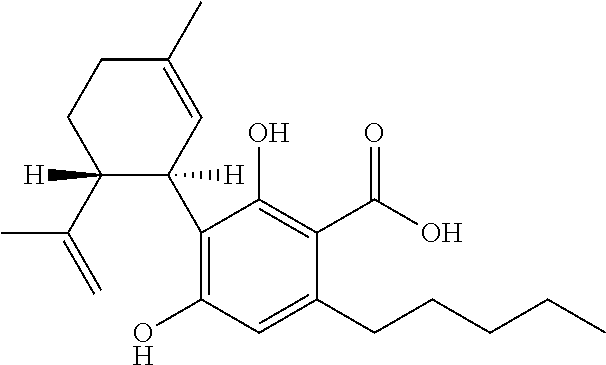
The present invention relates to the use of a therapeutically effective amount of cannabidiolic acid (CBDA) in the treatment of epilepsy. In one embodiment the CBDA is used in the treatment of generalised seizures, preferably tonic-clonic seizures. Preferably the CBDA used is in the form of a botanical drug substance in which the CBDA content is greater than 60%, and most preferably, it is a highly purified extract of cannabis such that the CBDA is present at greater than 95%, through 96% and 97% to most preferably, greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoids tetrahydrocannabinol (THC) or tetrahydrocannabinol acid (THCA) have been substantially removed. Alternatively, the CBDA may be synthetically produced.
Owner:GW RES LTD
Pharmaceutical Compositions For the Treatment of Disease and/or Symptoms in Arthritis
InactiveUS20080139667A1Increase flexibilityLess side effectsPowder deliveryBiocideDrugDisease modification
The invention relates to the use of a combination of cannabinoids for the treatment of pain, inflammation and / or disease modification in arthritis. Preferably the cannabinoids are selected from cannabidiol (CBD) or cannabidivarin (CBDV) and delta-9-tetrahydrocannabinol (THC) or tetrahydrocannabinovarin (THCV). More preferably the cannabinoids are in a predefined ratio by weight of less than or equal to 19:1 of CBD or CBDV to THC or THCV.
Owner:GW PHARMA LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS20190083418A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsSturge–Weber syndromeMedicine
The present invention relates to the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Sturge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Dronabinol treatment for migraines
Owner:BARBATO LOU
Aerosol formulations of delta tetrahydrocannabinol
The application discloses an aerosol formulation comprising Δ8 Tetrahydrocannabinol for use as a medicine, and the use of Δ8 Tetrahydrocannabinol to treat a condition selected from pain, appetite loss, multiple sclerosis and asthma.
Owner:NORTON HEALTHCARE
Pharmaceutical compositions for the treatment of pain
ActiveUS20060247304A1Small doseBiocideNervous disorderCancer-Related PainDelta-9-tetrahydrocannabinol
The present invention relates to treatment of cancer related pain and constipation. Preferably the subject in need is administered a combination of the cannabinoids cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC). More preferably the cannabinoids are in a predefined ratio by weight of approximately 1:1 of CBD to THC.
Owner:GW RES LTD
Novel natural cyclodextrin complexes
The present invention is directed to the novel use of complexes of cyclodextrin. In particular the invention is directed to a complex of a cyclodextrin selected from the group consisting of α-CD, β-CD and γ-CD and a cannabinoid selected from the classical cannabinoid-group consisting of cannabinol, tetrahydrocannabinol and cannabidiol.
Owner:PEDIPHARM
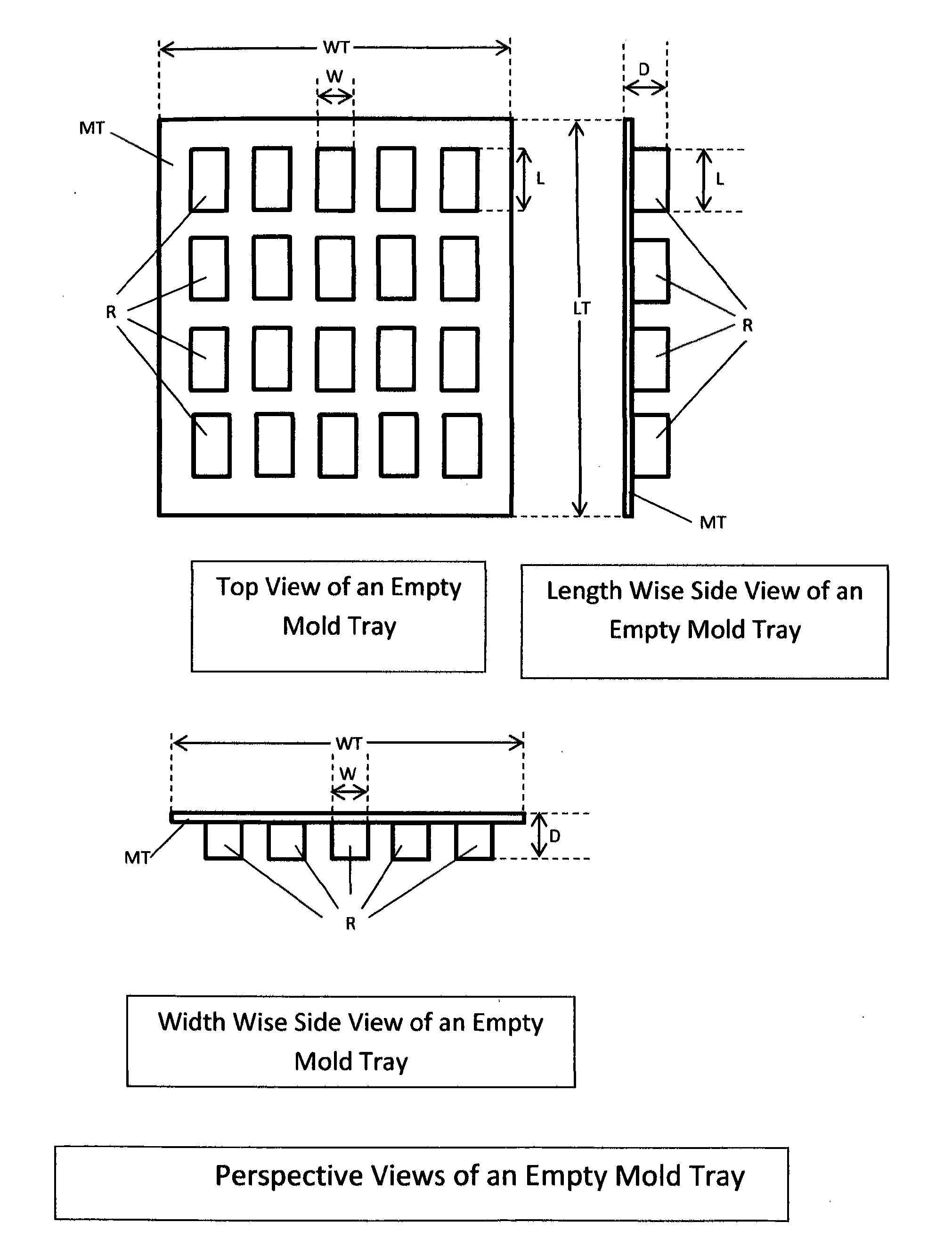
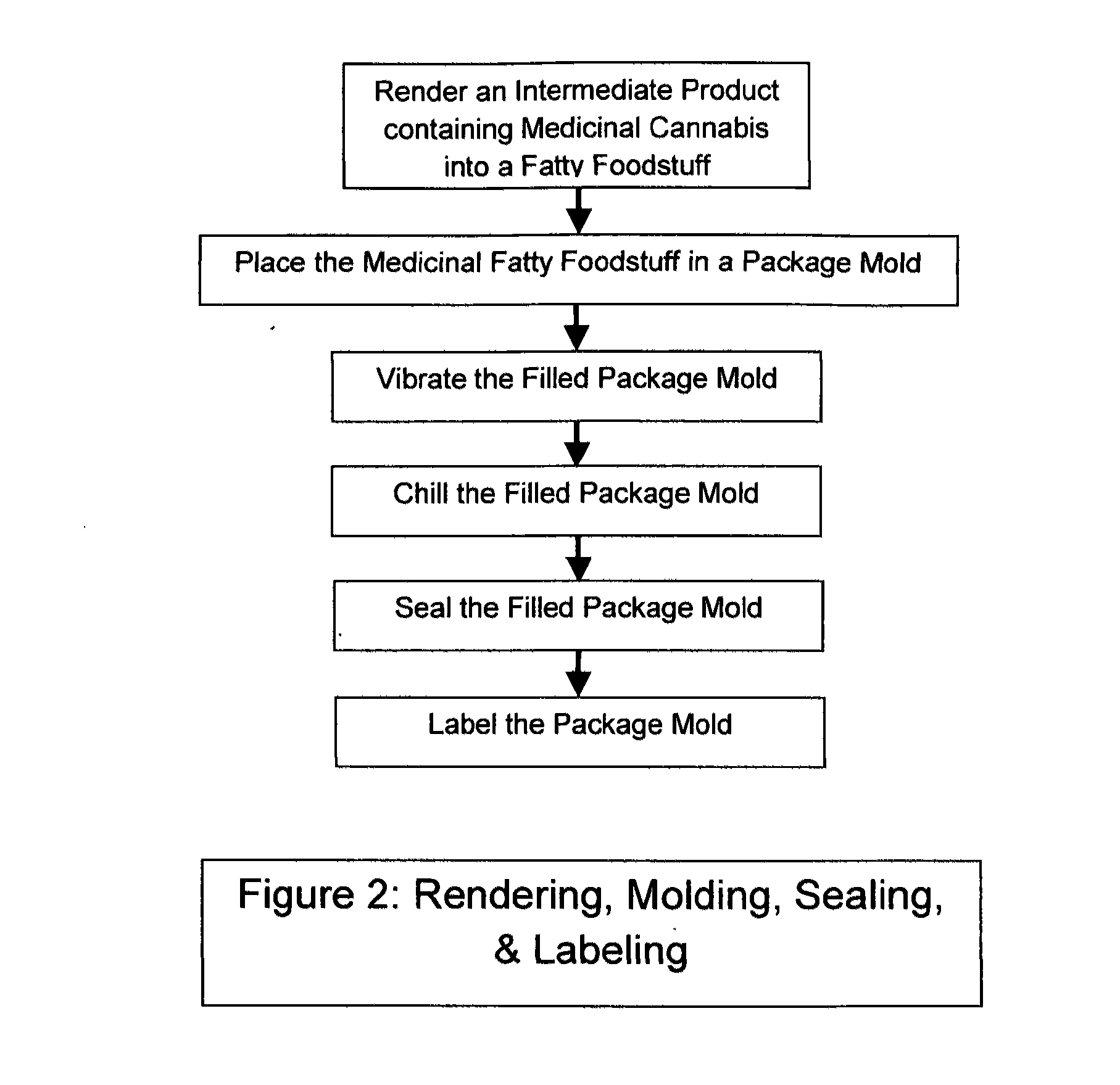
Medicinal cannabis fatty foodstuff
InactiveUS20120043242A1Organic active ingredientsNervous disorderDelta-9-tetrahydrocannabinolMedicine
The invention is a product and a process wherein Medicinal Delta-9 tetrahydrocannabinol (Δ9-THC) and potentially other cannabinoids (medicinal cannabis substances) associated with decarboxylated cannabis, including yet not necessarily limited to cannbidiols, and cannabigerol are rendered into a fatty foodstuff and then molded into a mold that also acts as a package. The best mode of the invention is a blister pack containing a plurality of voids or receptacles of desired sizes. A product that is characterized by a controlled amount of medicinal cannabis per unit volume of a fatty foodstuff base material is inserted into the mold, then cooled, and finally sealed. Each void or receptacle contains a known amount of medicinal cannabis that are independently dispensable.
Owner:HOSPODOR ANDREW DAVID
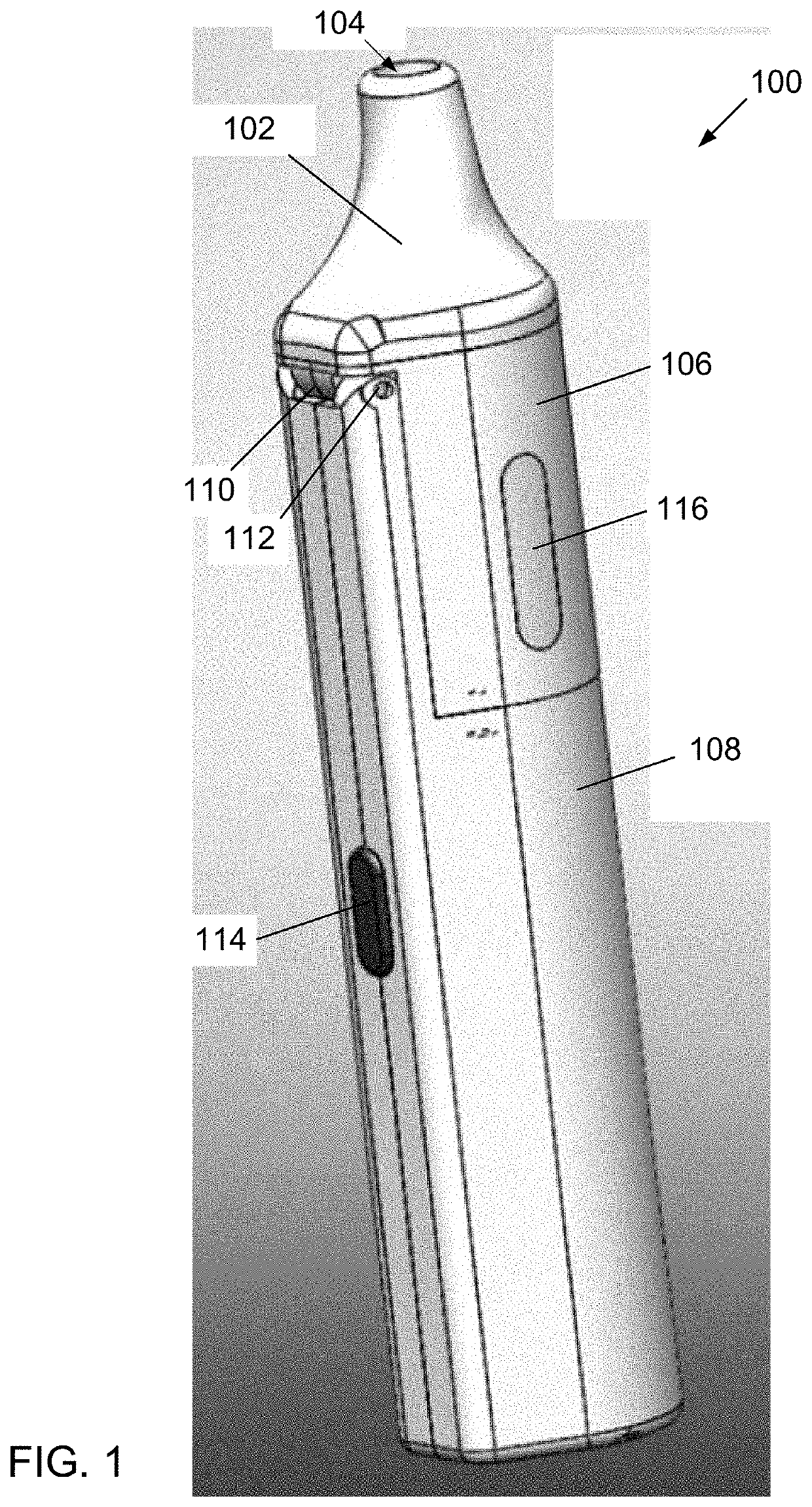
Liquid-filled cartridge for electronic device that produces an aerosol for inhalation by a person
A disposable cartridge is disclosed for use with an electronic device for producing an aerosol for inhalation by a person. The cartridge comprises a liquid container containing a liquid which when aerosolized is intended for inhalation by a person; and a transducer that, when actuated, causes the liquid from the container to be aerosolized such that the aerosol may be inhaled by a person. In various embodiments, the liquid comprises: a liposomal carrier; a liquid mixture having an active agent entrapped by liposomes; and a liquid mixture comprising a nanodispersion. The liquid may include nicotine, tetrahydrocannabinol, cannabidiol, or nanoparticles formed by an oil droplet covered by a monolayer of phosphatidylcholine. The transducer preferably comprises a mesh material with the transducer and the mesh material forming a piezo mesh disk for aerosolizing the liquid.
Owner:QNOVIA INC
Cannabis-based extracts and topical formulations for use in skin disorders
The present invention discloses a pharmaceutical topical composition comprising cannabidiol (CBD) or a derivative thereof and Tetrahydrocannabinol (THC) or a derivative thereof in an about 1:1 ratio, useful for treatment or prevention of inflammatory skin disorders, and treatment methods thereof.
Owner:ONE WORLD CANNABIS LTD
Methods for treatment of inflammatory diseases using CT-3 or analogs thereof
The present invention relates to non-psychoactive derivatives of tetrahydro-cannabinol, which exhibit anti-inflammatory and analgesic activities. In particular, the present invention relates to methods of administering the derivatives and pharmaceutical compositions as therapeutic agents in the treatment of pain and tissue inflammation.
Owner:INDEVUS PHARMACEUTICALS INC
Cannabis Drug Detection Device
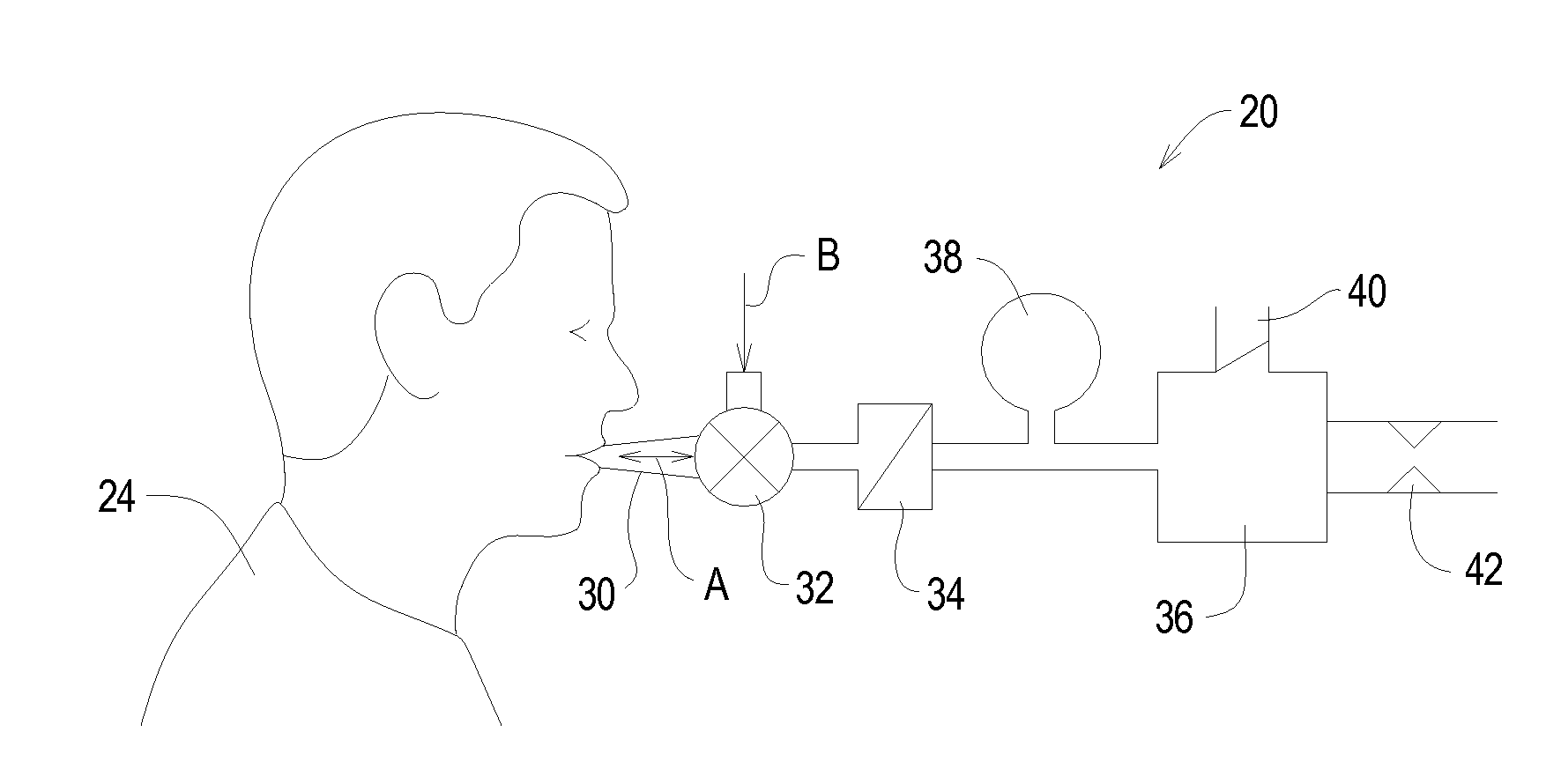
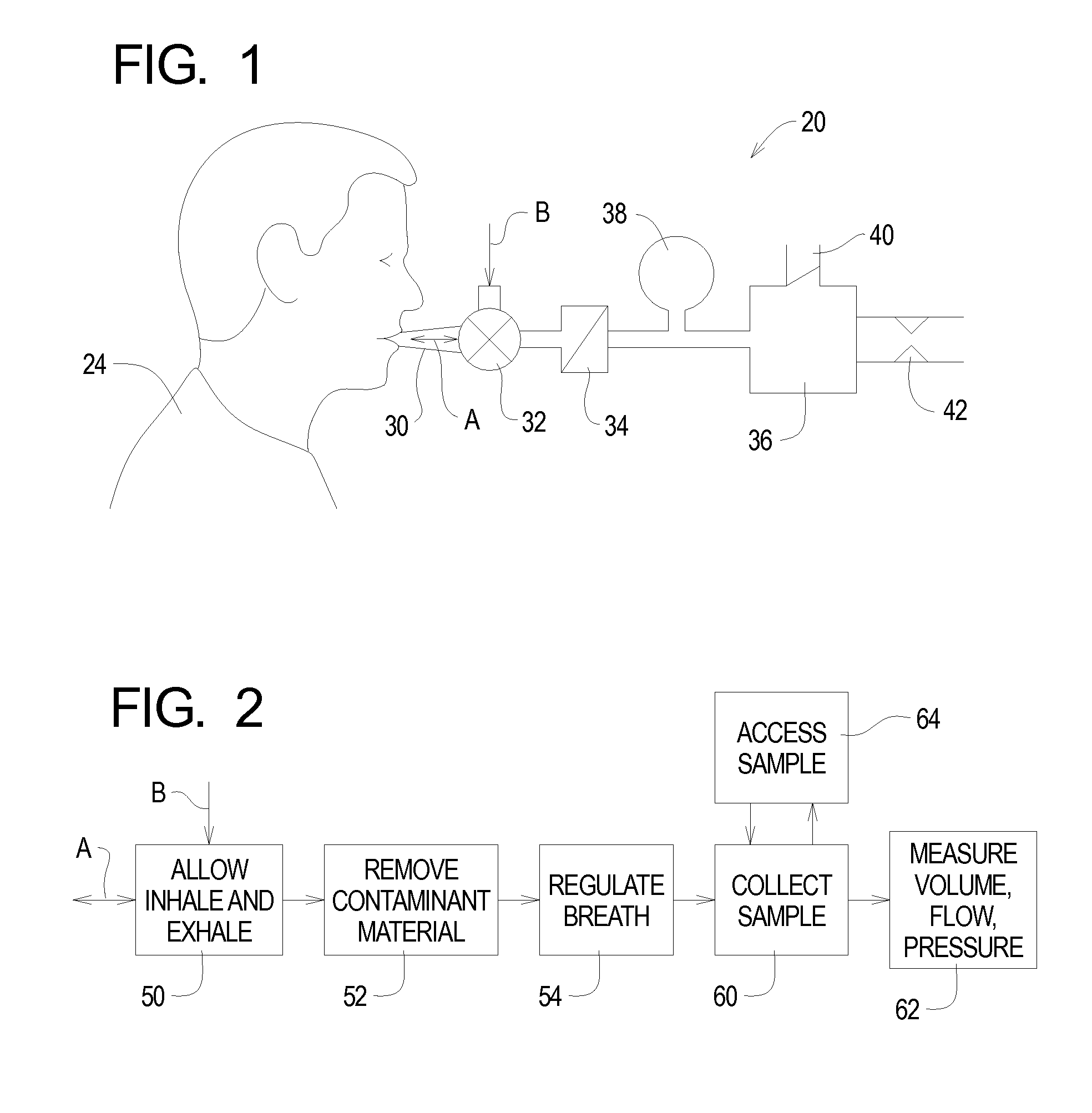
A system for collecting cannabis and the psychoactive component tetrahydrocannabinol from a sample of exhaled breath is disclosed. Single or multiple exhaled breaths are conditioned by removing contaminants, and regulating flow rate and / or pressure to collect a sample of tetrahydrocannabinol for timely local or remote analysis. The cannabis detection system comprises a containment trap for removing interfering materials from the breath of the subject and a collection component for sampling components of breath introduced into the system through the containment trap for analysis to determine a presence of THC in the breath.
Owner:CANNABIX TECH INC
Detection of cannabis use
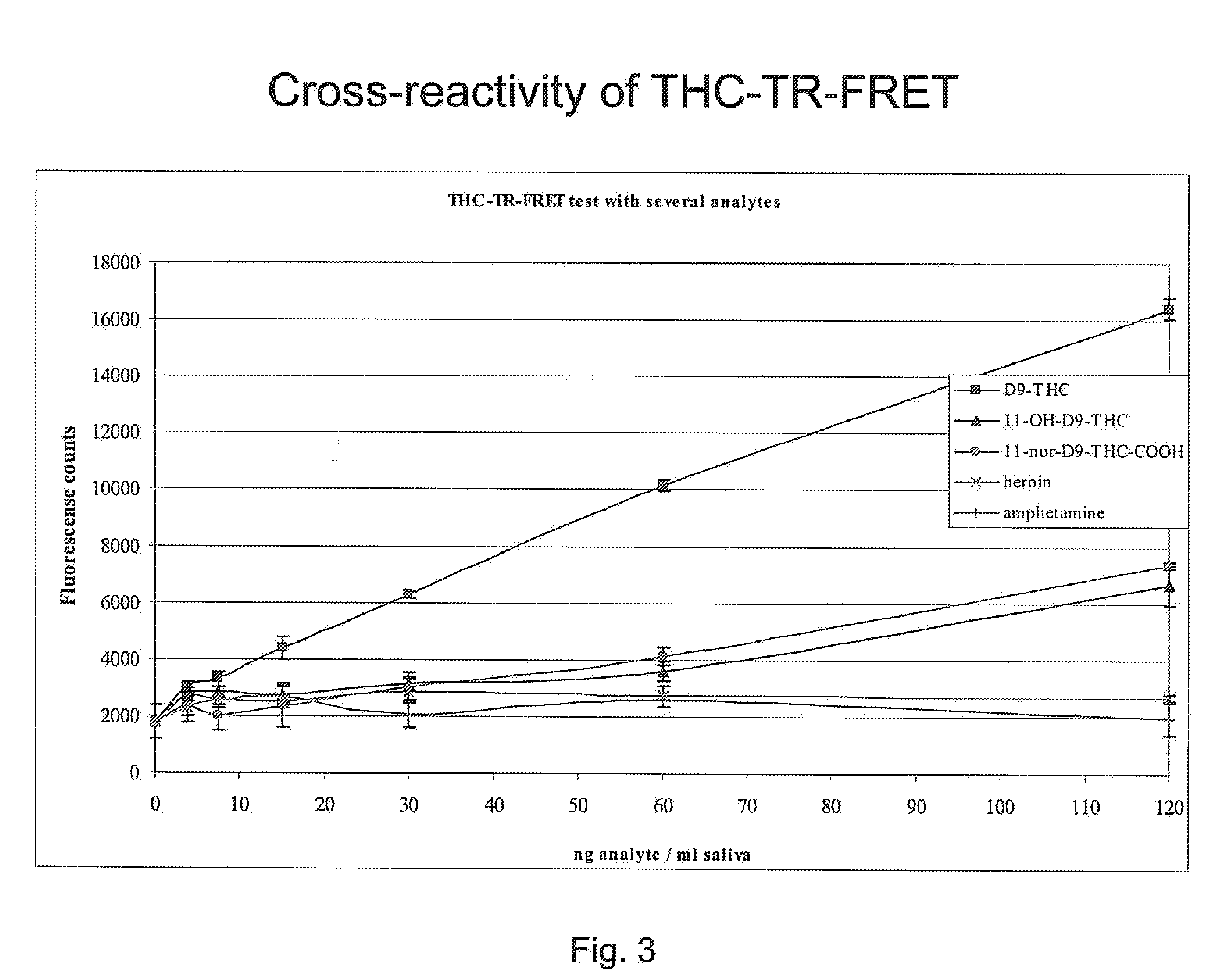
ActiveUS20110086364A1Facilitates rapidFacilitates reliable detectionSugar derivativesChemiluminescene/bioluminescenceImmune complex depositionAntibody fragments
A binding partner, especially an antibody fragment that specifically recognizes an antigen-antibody immune complex between anti-THC and THC (tetrahydrocannabinol), is disclosed. The binding partner facilitates a non-competitive homogenous immunoassay for detection of cannabis use. A test kit comprising the binding partner is also described. Preferably the immunoassay is applied for roadside testing of saliva from suspected drivers.
Owner:TEKNOLOGIAN TUTKIMUSKESKUS VTT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com