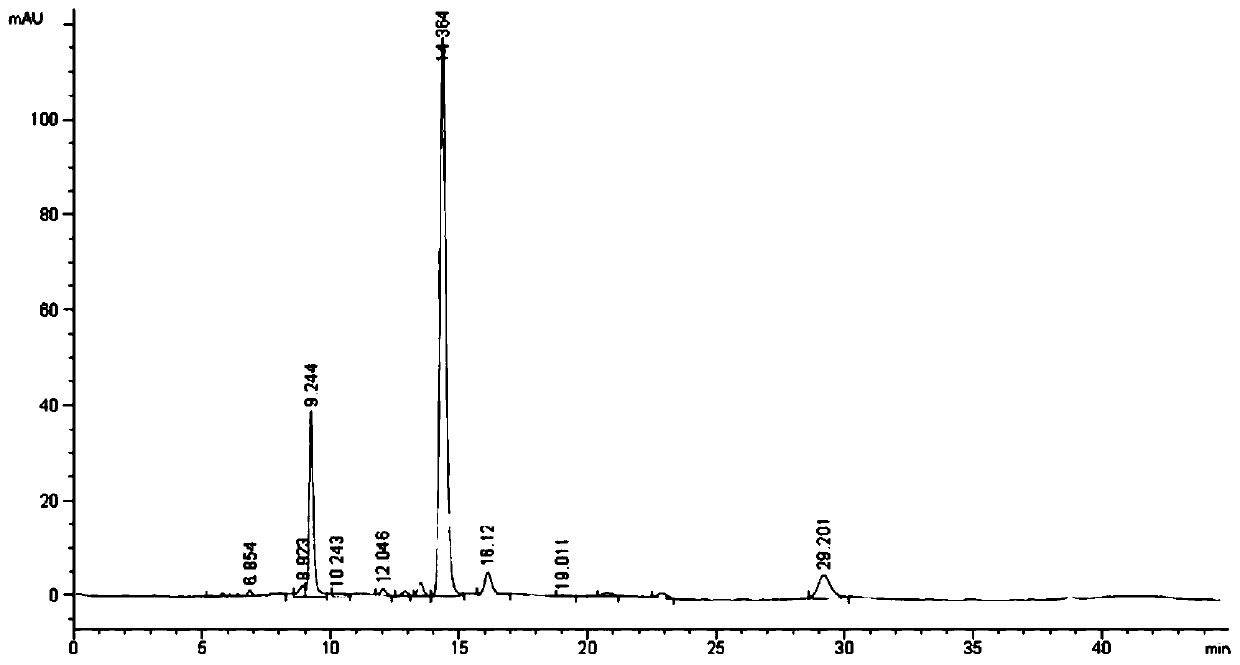
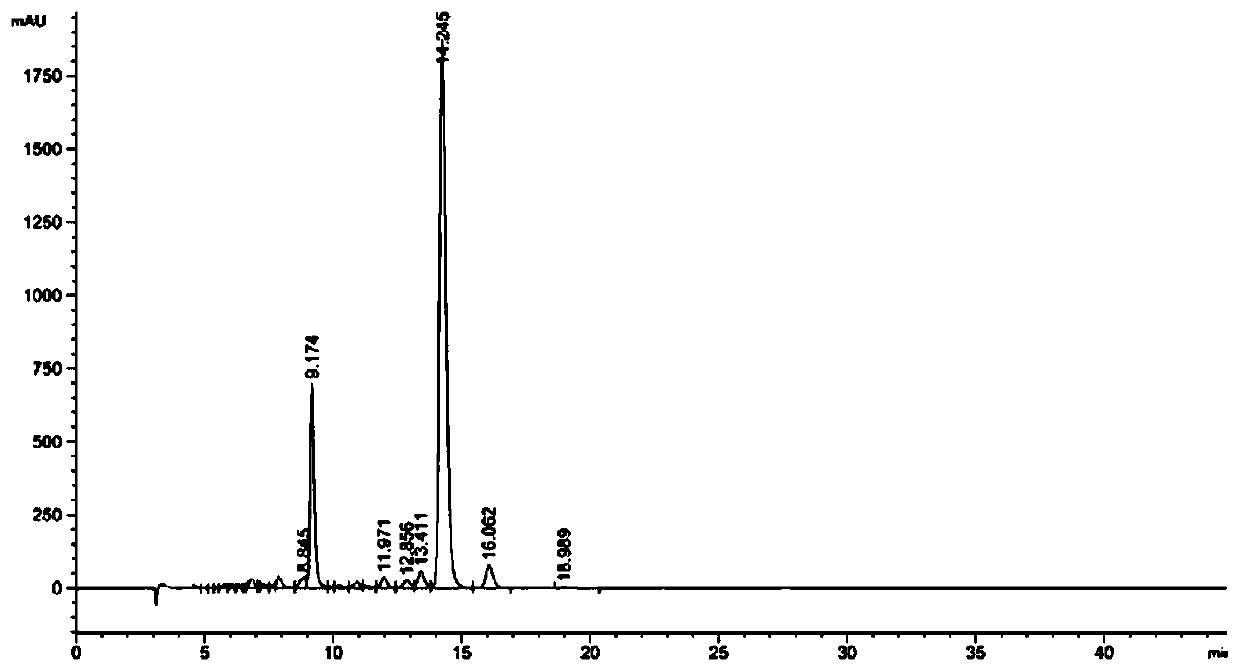
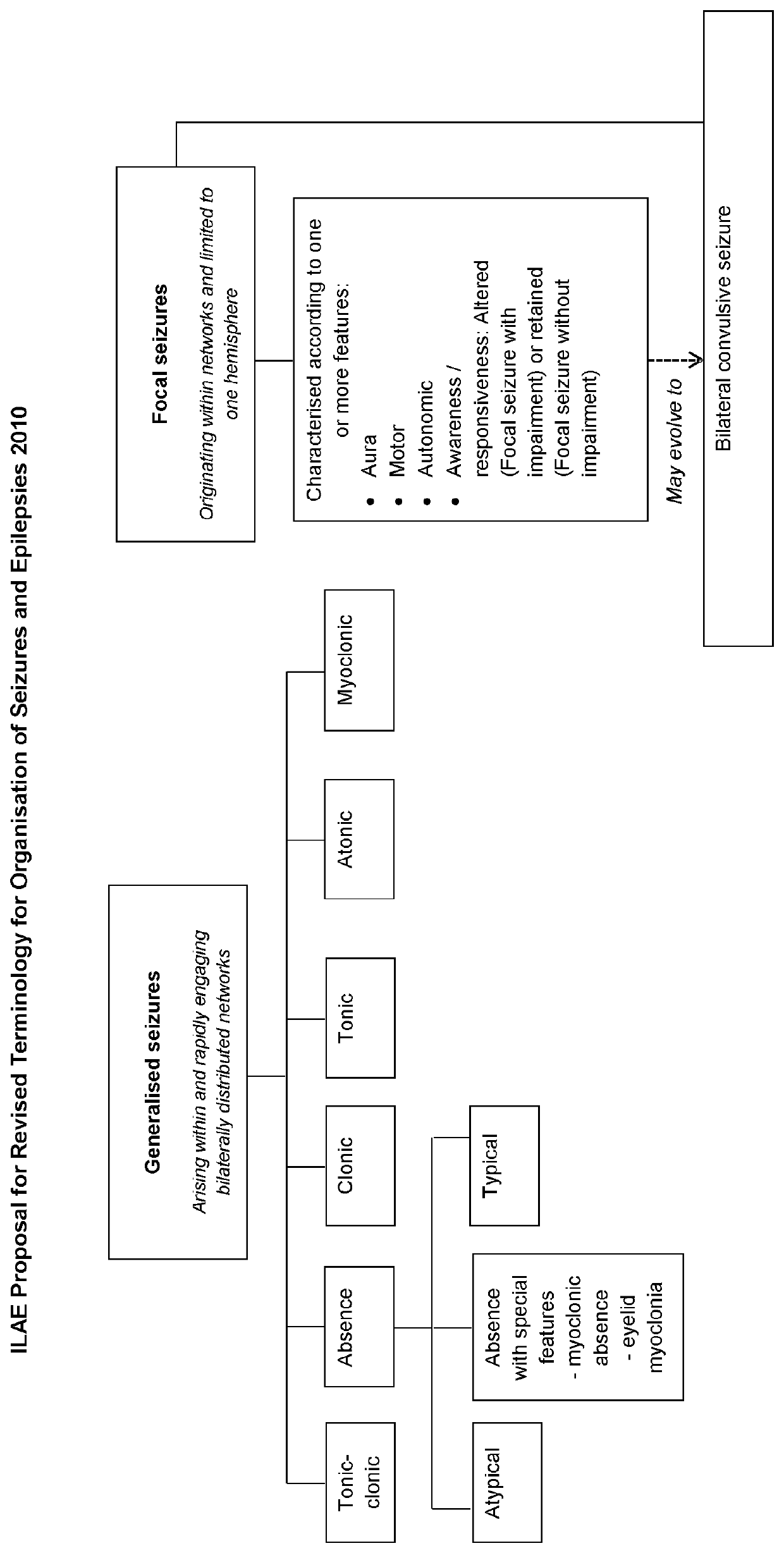
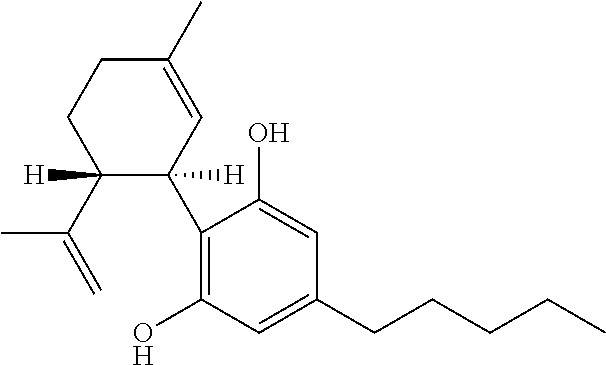
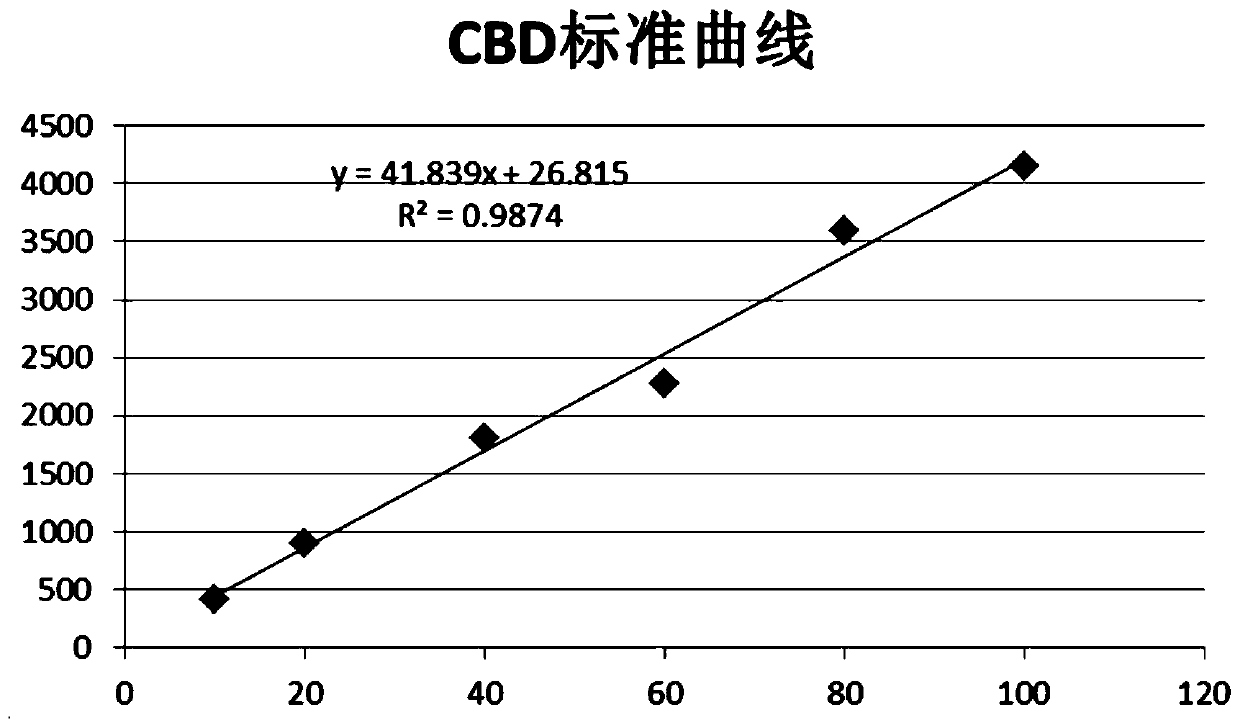
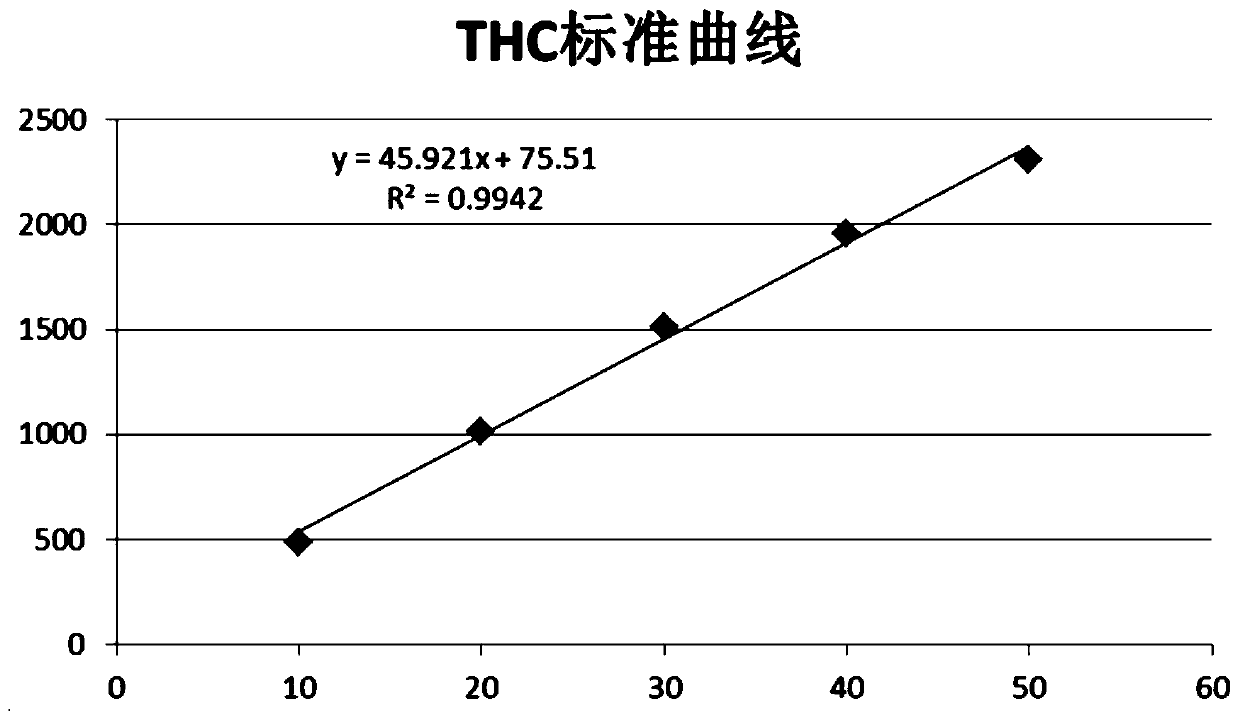
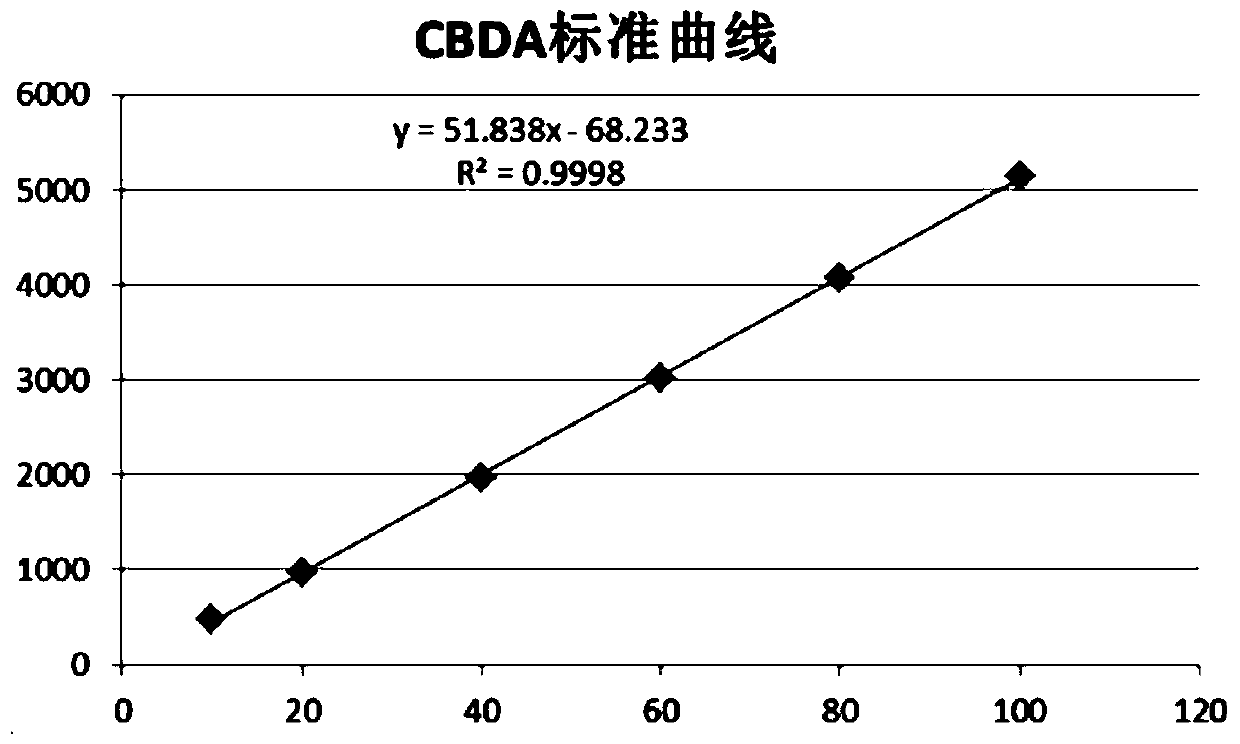
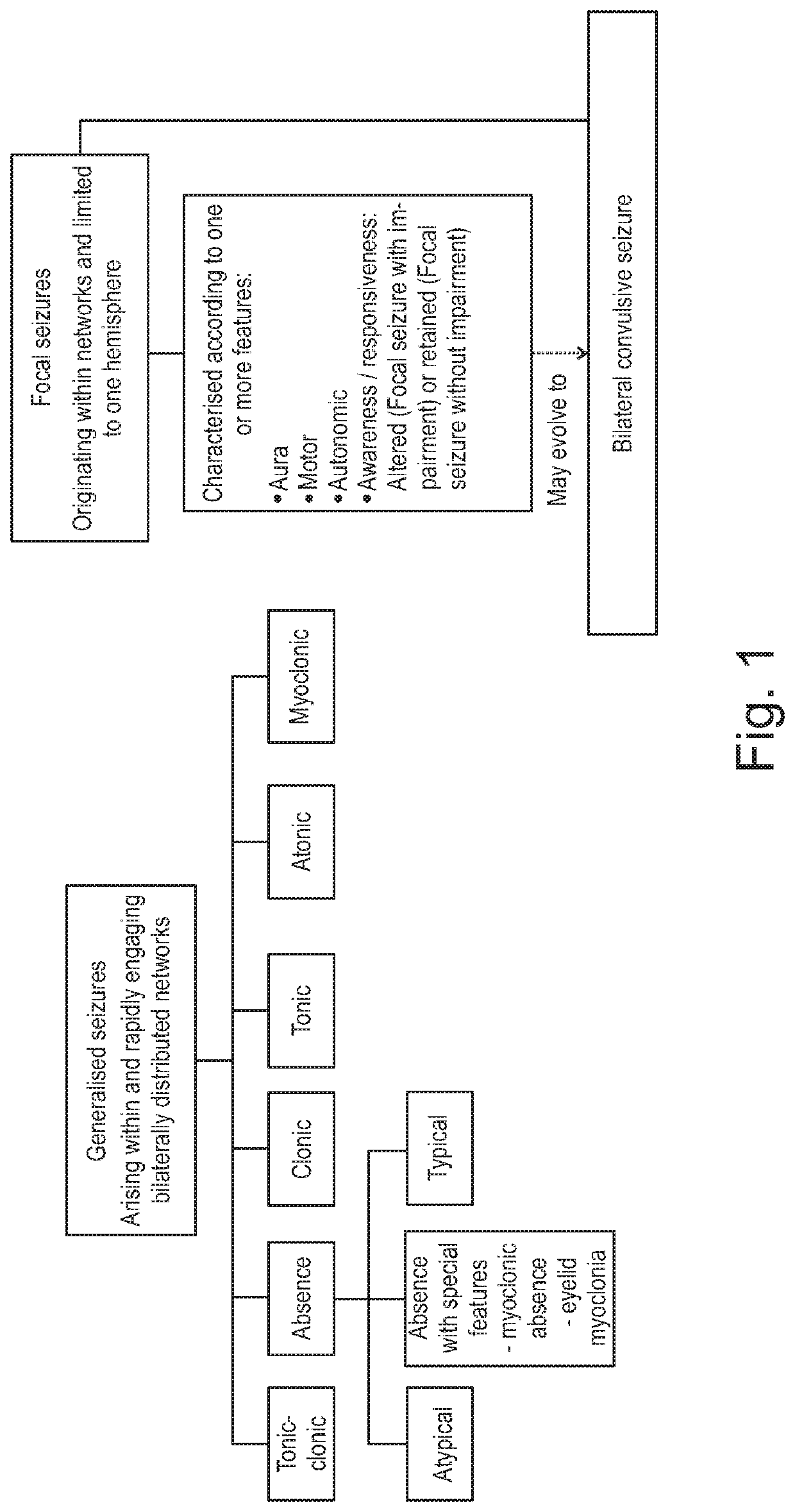
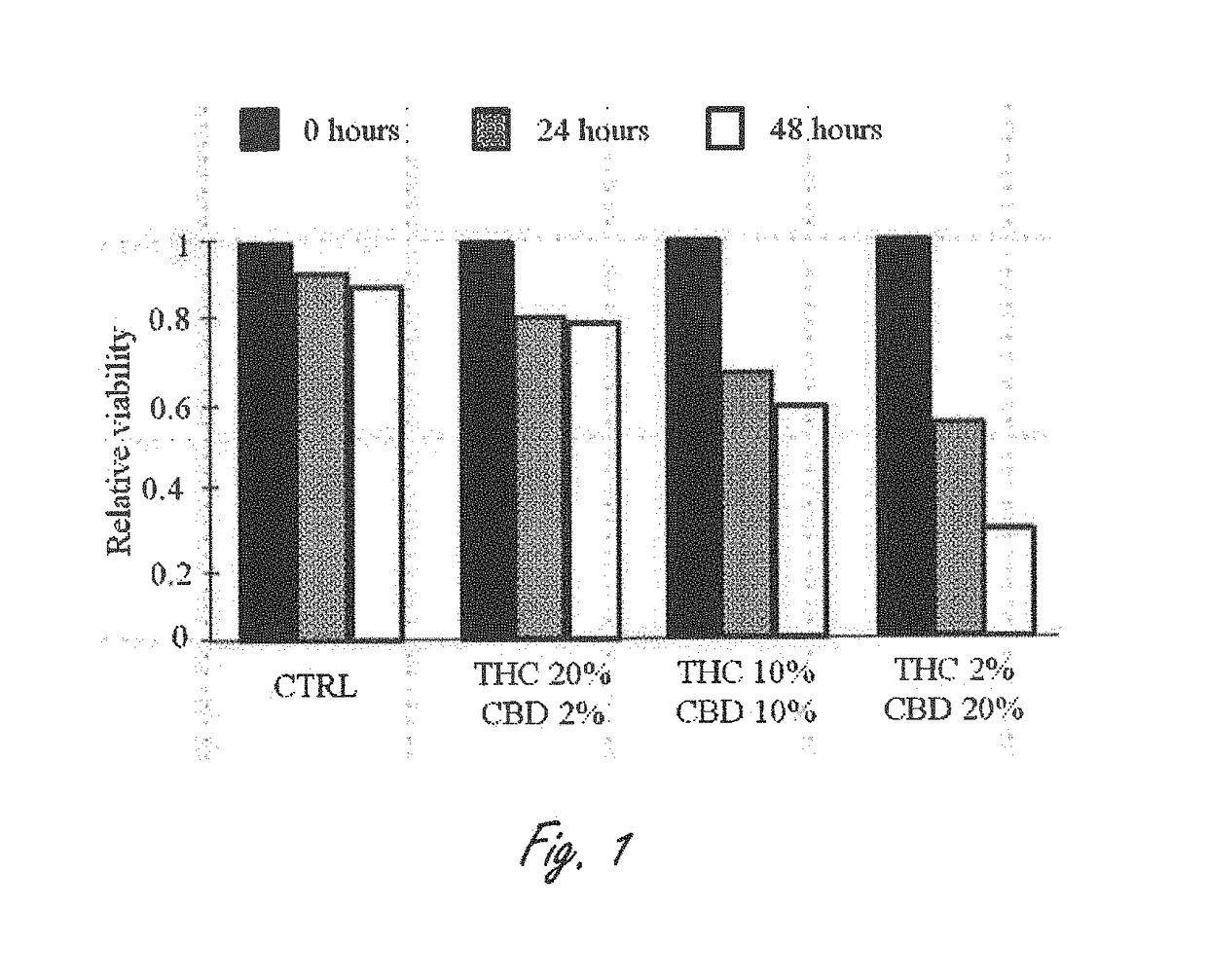
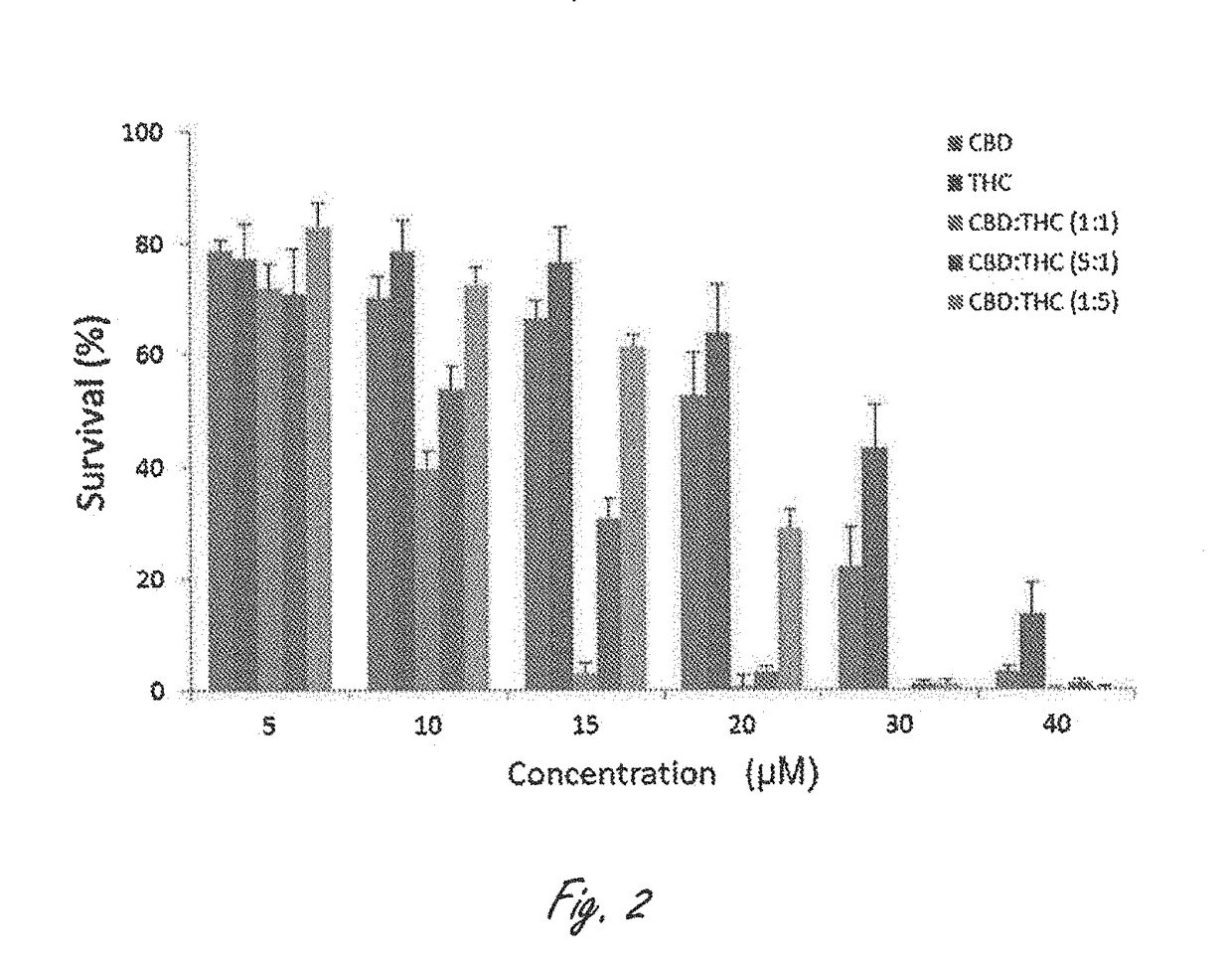
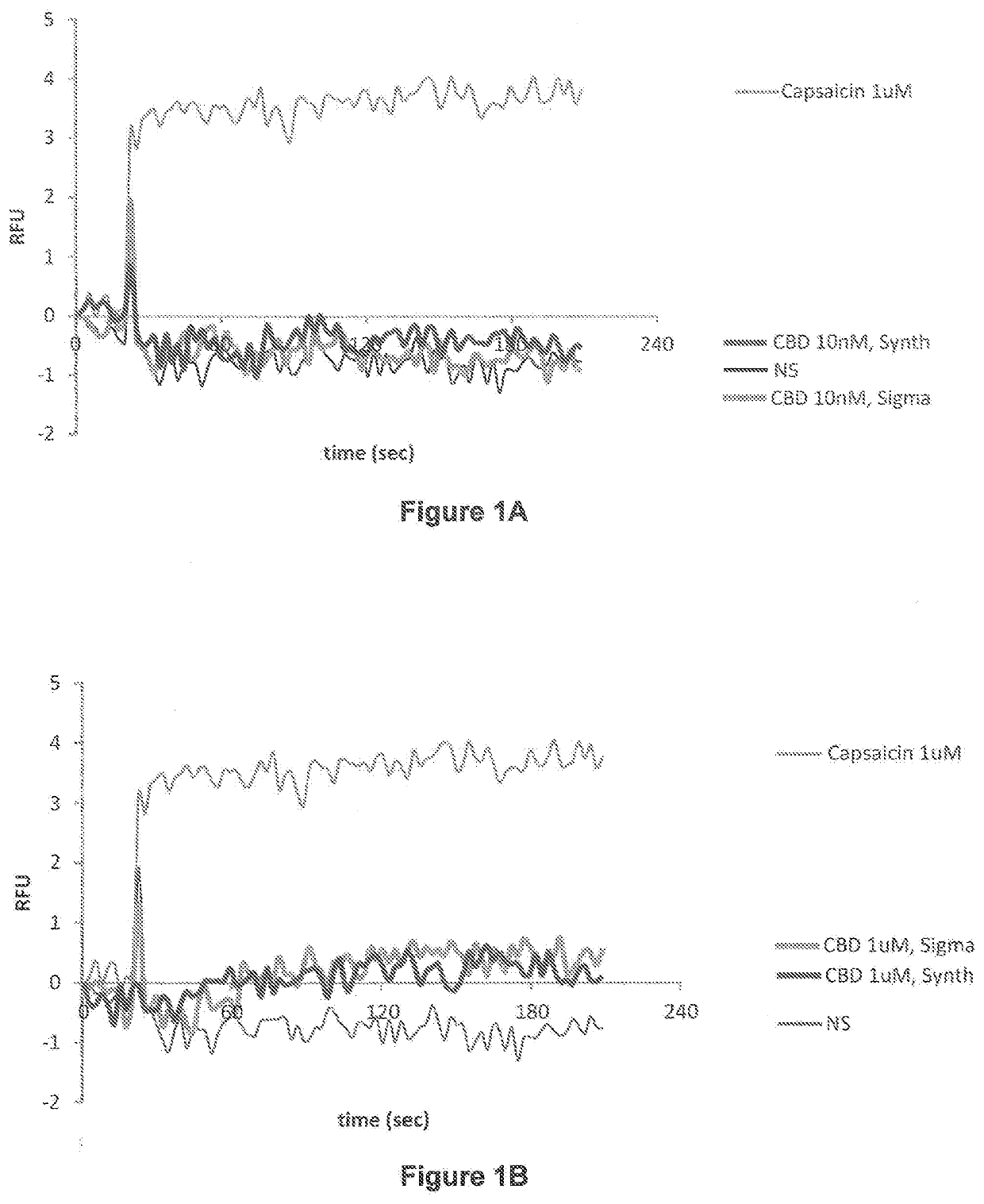
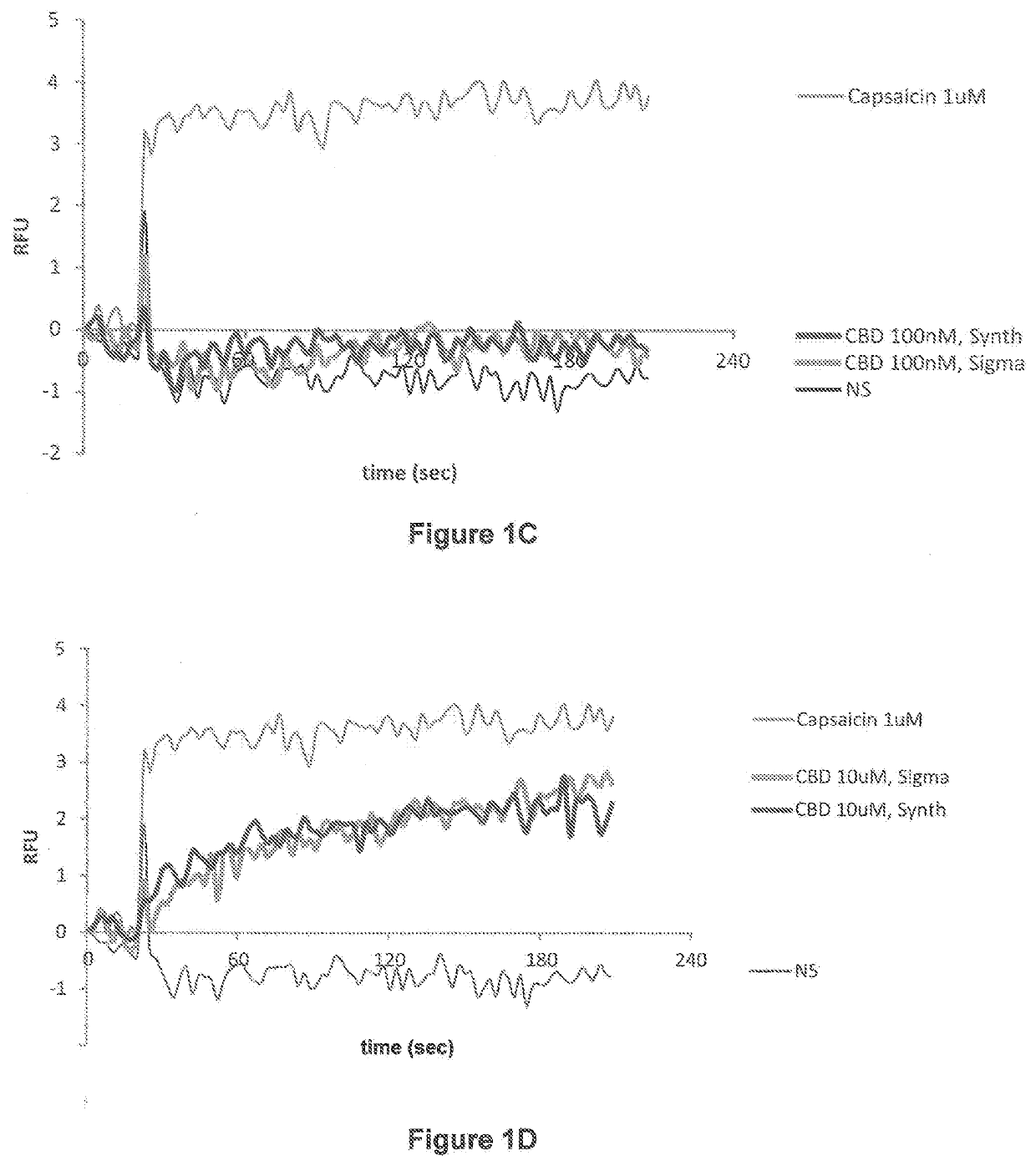
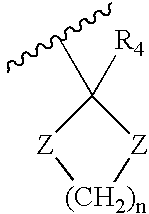Patents
Literature
71 results about "Cannabielsoin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Use of cannabinoids in combination with an Anti-psychotic medicament
ActiveUS20110038958A1High activityLess degree of activityBiocideSenses disorderPsychosis drugTypical antipsychotic
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
Enhanced Delivery of Nicotine, THC, Tobacco, Cannabidiol or Base Alkaloid from an Electronic Cigarette or Other Vapor or Smoke Producing Device Through Use of an Absorption Conditioning Unit
A method for the administration of nicotine, THC, tobacco, cannabidiol or a base alkaloid includes administering in the oral or nasal cavity an absorption conditioning unit having at least two agents selected from the group consisting of (a) a buffer agent, (b) a capturing agent, (c) a penetration agent, and (d) a thermal agent, to the mammal, and then administering by inhalation a bioactive agent selected from the group consisting of nicotine, THC, cannabidiol and a base alkaloid. The absorption conditioning unit may be in a dosage form not containing a drug. The absorption conditioning unit may create a pH in the oral cavity or nasal cavity of 7.8-10 for a period of ten minutes or more after administration, the dosage form not containing an acid and not containing a drug.
Owner:FUISZ RICHARD C +1
Process for production of delta-9-tetrahydrocannabinol
InactiveUS7674922B2High selectivityLower overall renovationOrganic compound preparationCarboxylic acid esters preparationArylPtru catalyst
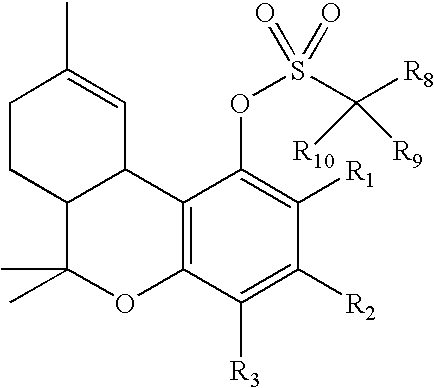
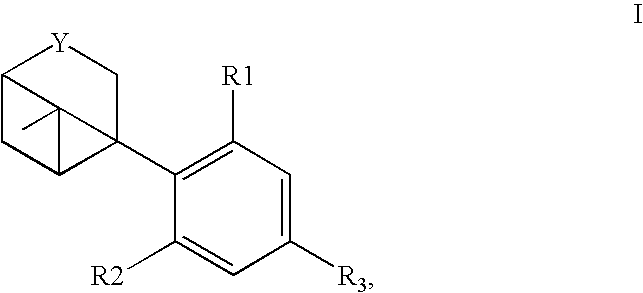
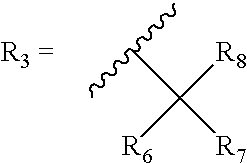
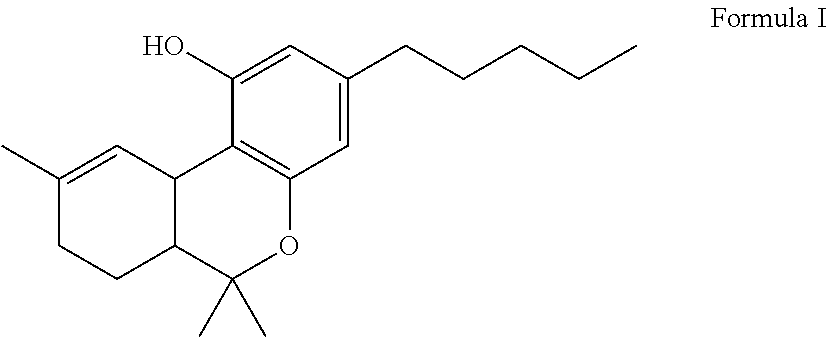
The present invention relates to a process for preparation of a delta-9-tetrahydrocannabinol compound or derivative thereof involving treating a first intermediate compound with an organoaluminum-based Lewis acid catalyst, under conditions effective to produce the delta-9-tetrahydrocannabinol compound or derivative thereof. Another aspect of the present invention relates to a process for preparation of a cannabidiol or cannabidiolate compound involving reacting a first starting compound with a second starting compound in the presence of a metal triflate catalyst, under conditions effective to form the cannabidiol or cannabidiolate compound. The present invention also relates to a compound of the formula:where R8, R9, and R10 are the same or different and independently selected from the group consisting of H, substituted or unsubstituted alkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or halo, with R1, R2, and R3 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Enhanced Delivery of Nicotine, THC, Tobacco, Cannabidiol or Base Alkaloid from an Electronic Cigarette or Other Vapor Producing Device Through Use of an Absorption Conditioning Unit
A method for the administration of nicotine, THC, tobacco, cannabidiol or a base alkaloid includes administering an absorption conditioning unit having at least two agents selected from the group consisting of (a) a buffer agent, (b) a capturing agent, (c) a penetration agent, and (d) a thermal agent, to the mammal, and then administering by inhalation a bioactive agent selected from the group consisting of nicotine, THC, cannabidiol and a base alkaloid. The absorption conditioning unit may be in a dosage form not containing a drug. The absorption conditioning unit may create a pH in the oral cavity of 7.8-10 for a period of ten minutes or more after administration, the dosage form not containing an acid and not containing a drug.
Owner:FUISZ RICHARD C +1
Use of cannabinoids in the treatment of epilepsy
ActiveUS20190083418A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsSturge–Weber syndromeMedicine
The present invention relates to the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Sturge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
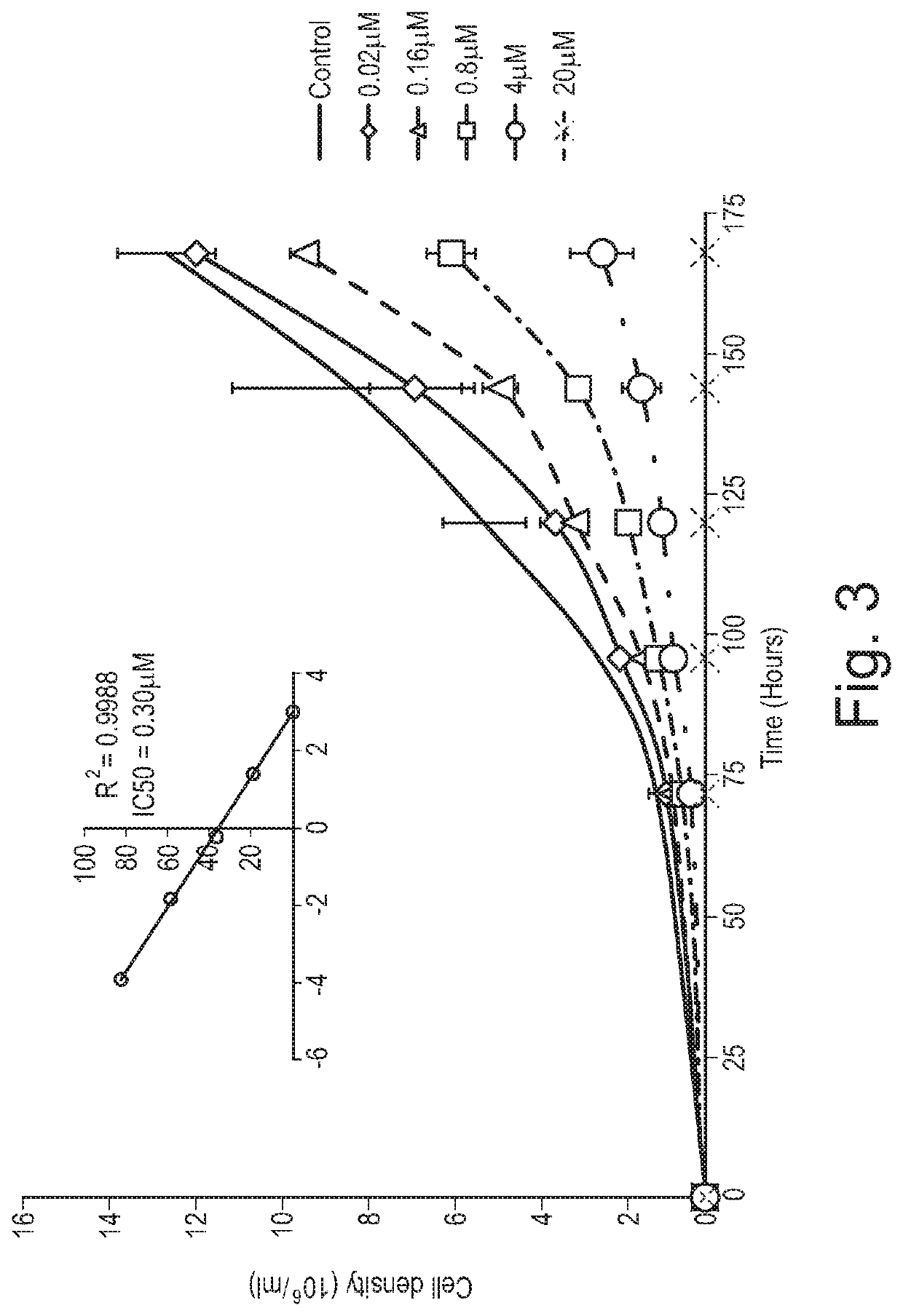
Apparatus and methods for the simultaneous production of compounds
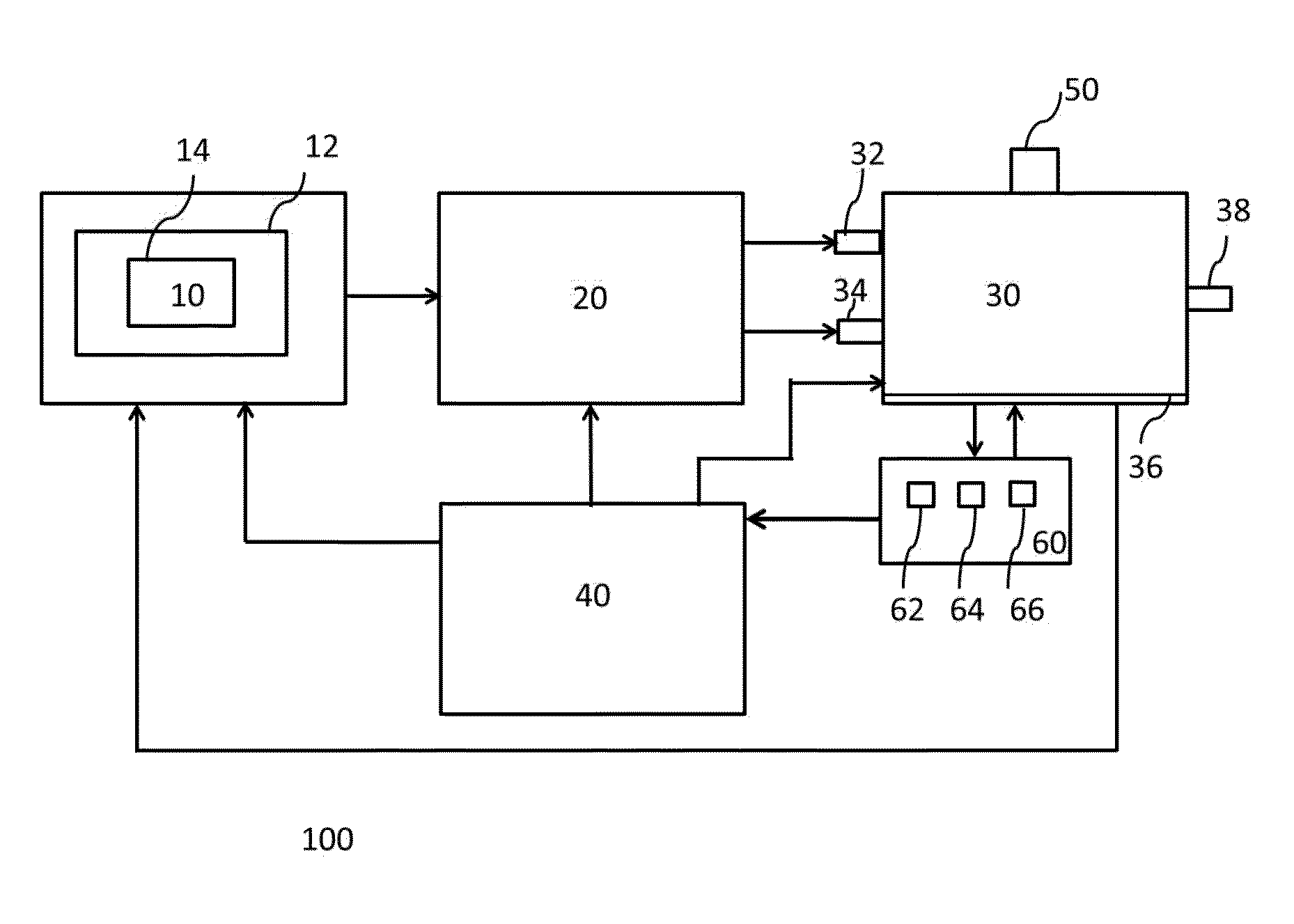
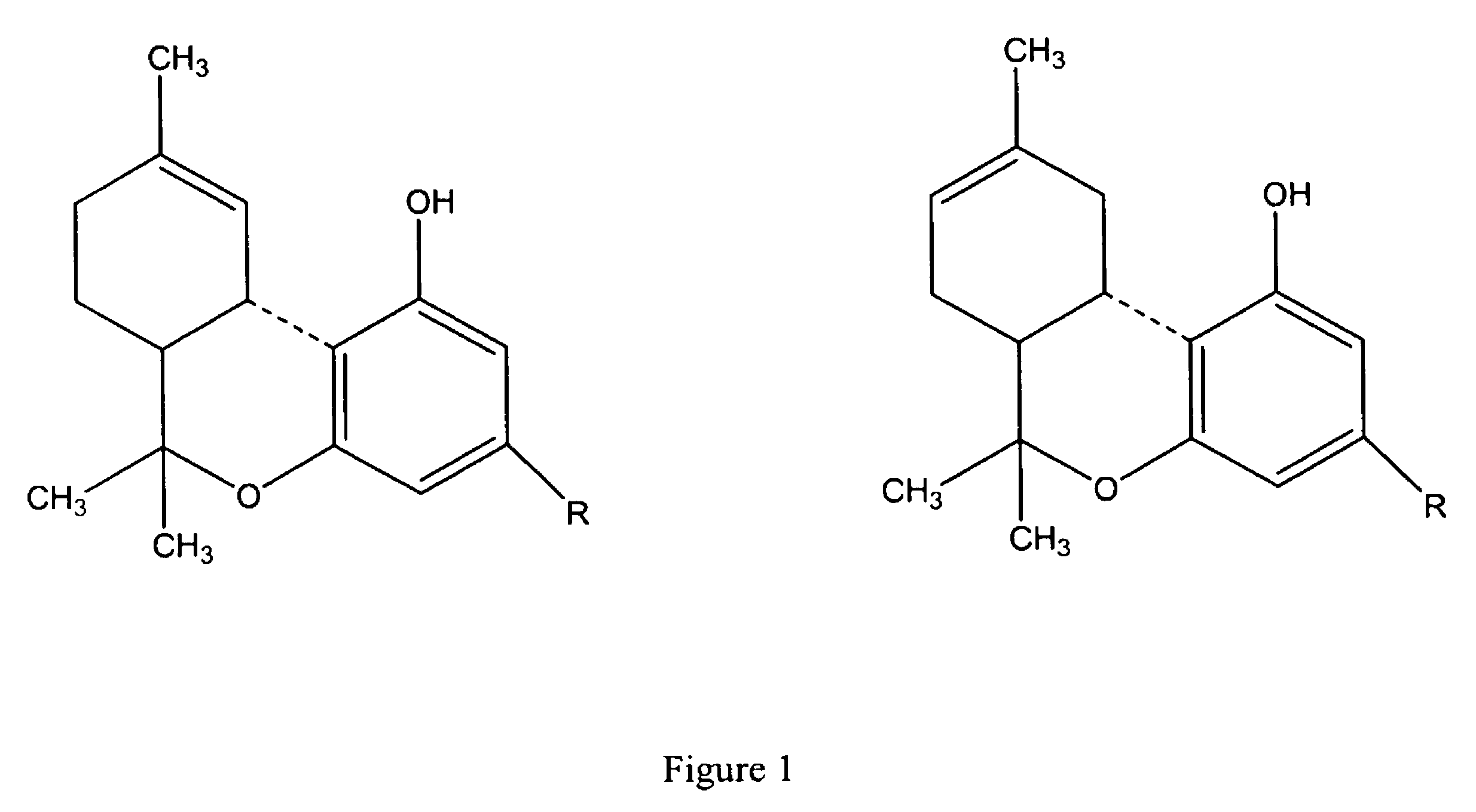
ActiveUS9394510B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCannabielsoinEngineering
The present invention provides an apparatus for simultaneously producing different cannabinoids in different ratios. Specifically, the apparatus according to the invention produces tetrahydrocannabinolic acid (THCA) and cannabichormenic acid (CBCA) or cannabidiolic acid (CBDA) in different ratios, and it comprises a bioreactor that comprises a first automated supply system configured to deliver a substrate and a cannabinoid acid synthase, and a second automated system to cease the reaction; an extractor configured to recover the cannabionoids so produced; and a controller configured to modify at least on property of the reaction.
Owner:TEEWINOT TECH LTD
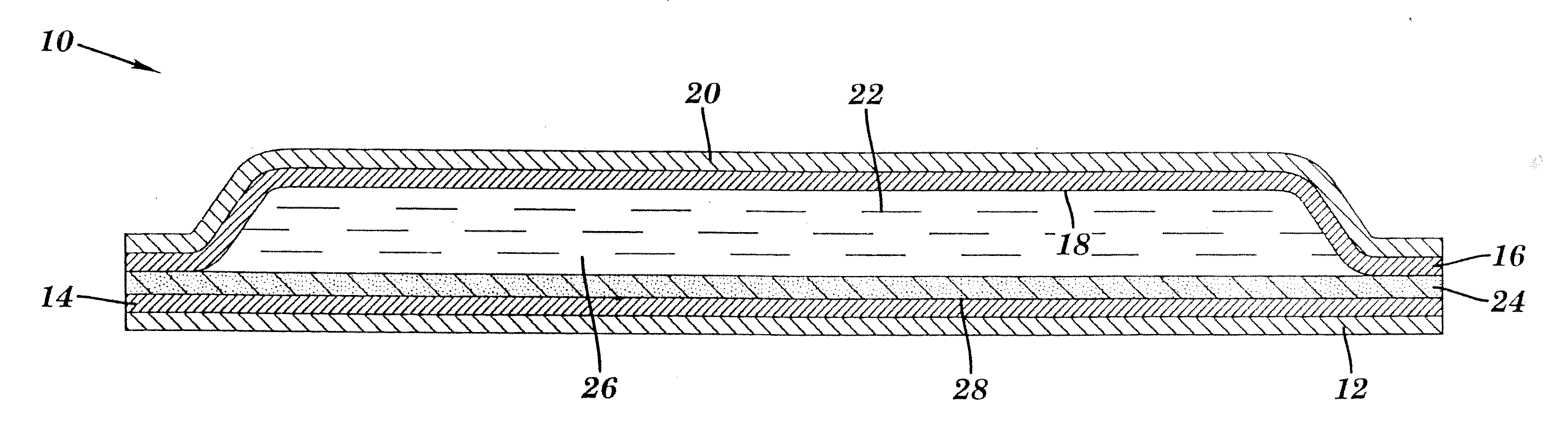
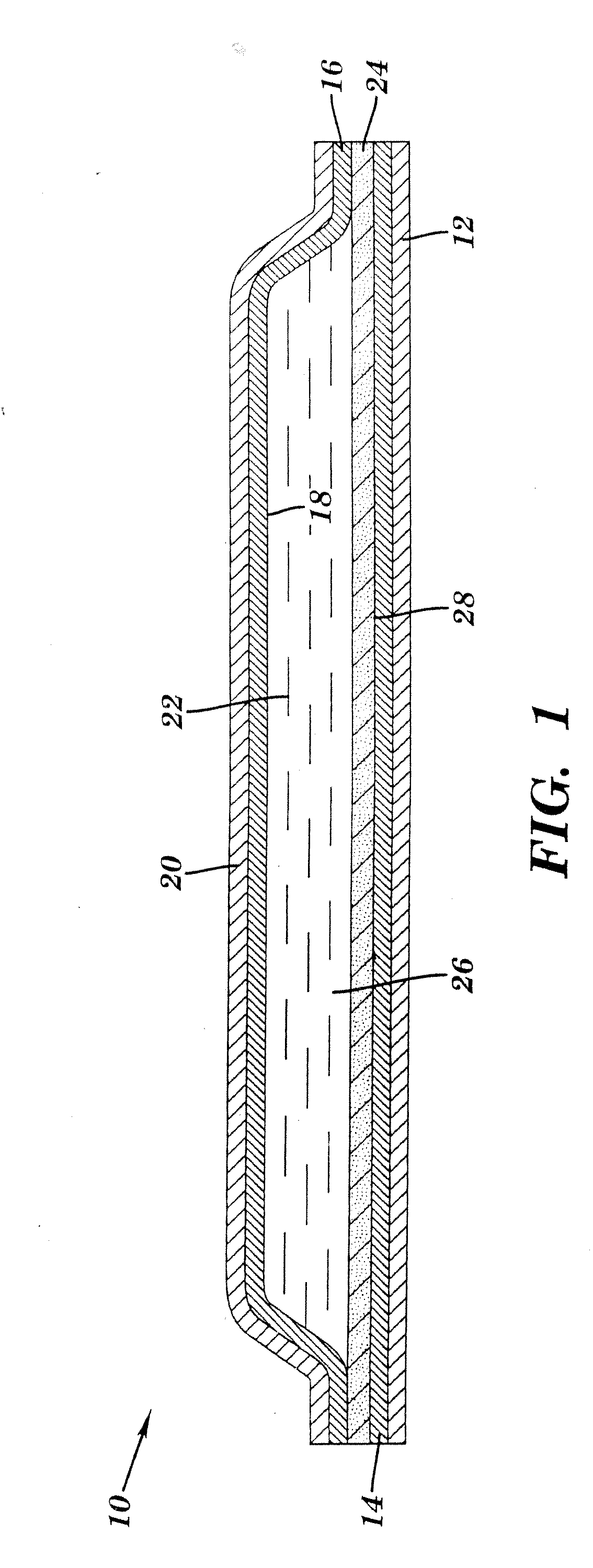
Liquid-filled cartridge for electronic device that produces an aerosol for inhalation by a person
A disposable cartridge is disclosed for use with an electronic device for producing an aerosol for inhalation by a person. The cartridge comprises a liquid container containing a liquid which when aerosolized is intended for inhalation by a person; and a transducer that, when actuated, causes the liquid from the container to be aerosolized such that the aerosol may be inhaled by a person. In various embodiments, the liquid comprises: a liposomal carrier; a liquid mixture having an active agent entrapped by liposomes; and a liquid mixture comprising a nanodispersion. The liquid may include nicotine, tetrahydrocannabinol, cannabidiol, or nanoparticles formed by an oil droplet covered by a monolayer of phosphatidylcholine. The transducer preferably comprises a mesh material with the transducer and the mesh material forming a piezo mesh disk for aerosolizing the liquid.
Owner:QNOVIA INC
Bicyclic and tricyclic cannabinoids
Owner:UNIV OF CONNECTICUT
Preparation method of industrial hemp extract
ActiveCN110330409ASave production cost and quality control costReagent environmental protectionCosmetic preparationsNervous disorderSolventChemistry
The invention discloses a preparation method of an industrial hemp extract. A cannabinol compound is enriched by salting and centrifugation, and water-soluble impurities can be completely removed in one step, thereby avoiding solvent residues such as toluene, xylene and the like introduced when the water-soluble impurities are removed by applying a conventional water-sinking process or macroporousresin, and greatly saving the production cost and the quality control cost; and a psychoactive ingredient THC can be completely excluded. In the above method, only pure water and three kinds of organic reagents with low toxicity are used, therefore, the reagents are environmentally-friendly, the process operation is simple, the instruments and equipment are readily available, the cost can be reduced, the pollution is reduced, and the industrial production is facilitated. The industrial hemp extract obtained by the above method is rich in the cannabinol compounds such as CBDV, CBG, CBD and thelike; the total content of the above cannabinoid compounds can be up to 80 percent or above, and the transfer rate can be up to 99 percent or above.
Owner:HANYI BIO TECH CO LTD
Cannabinol derivatives
Owner:TRAVIS CRAIG R
Method for extracting and separating high-purity cannabidiol from low-content industrial hemp floral leaves
ActiveCN111470953AIncrease contentHigh purityOrganic chemistryOrganic compound preparationCannabielsoinDigestion
The invention discloses a method for extracting and separating high-purity cannabidiol (CBD) from low-content industrial hemp floral leaves. The method adopts the modes of digestion, macroporous resinadsorption and desorption, reverse chromatography and crystallization to finally obtain a cannabidiol pure product with the purity of 99% or above. In order to separate other cannabinoids, extractionand decoloration processes can be added between the digestion process and the macroporous resin adsorption and desorption process, and a polymer reversed-phase filler is used for reversed-phase chromatography, so that cannabidivarin (CBDV), cannabinol (CBN) and tetrahydrocannabinol (THC) in the floral leaves can be separated. The method disclosed by the invention is high in digestion, extractionand decoloration process yield and high in impurity removal rate; the macroporous resin and reversed-phase column chromatography have the advantages of high loading capacity and favorable separation effect. Requirements of development of a plurality of industrial hemp products are met, and the method has great application advantages in the pharmaceutical field and is suitable for industrial popularization.
Owner:SUNRESIN NEW METERIALS CO LTD XIAN
Use of cannabinoids in the treatment of epilepsy
ActiveUS10583096B2Nervous disorderHydroxy compound active ingredientsSturge–Weber syndromeCannabielsoin
The present invention relates to the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Sturge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
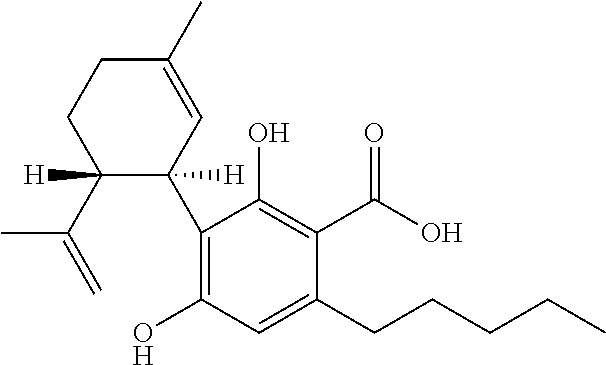
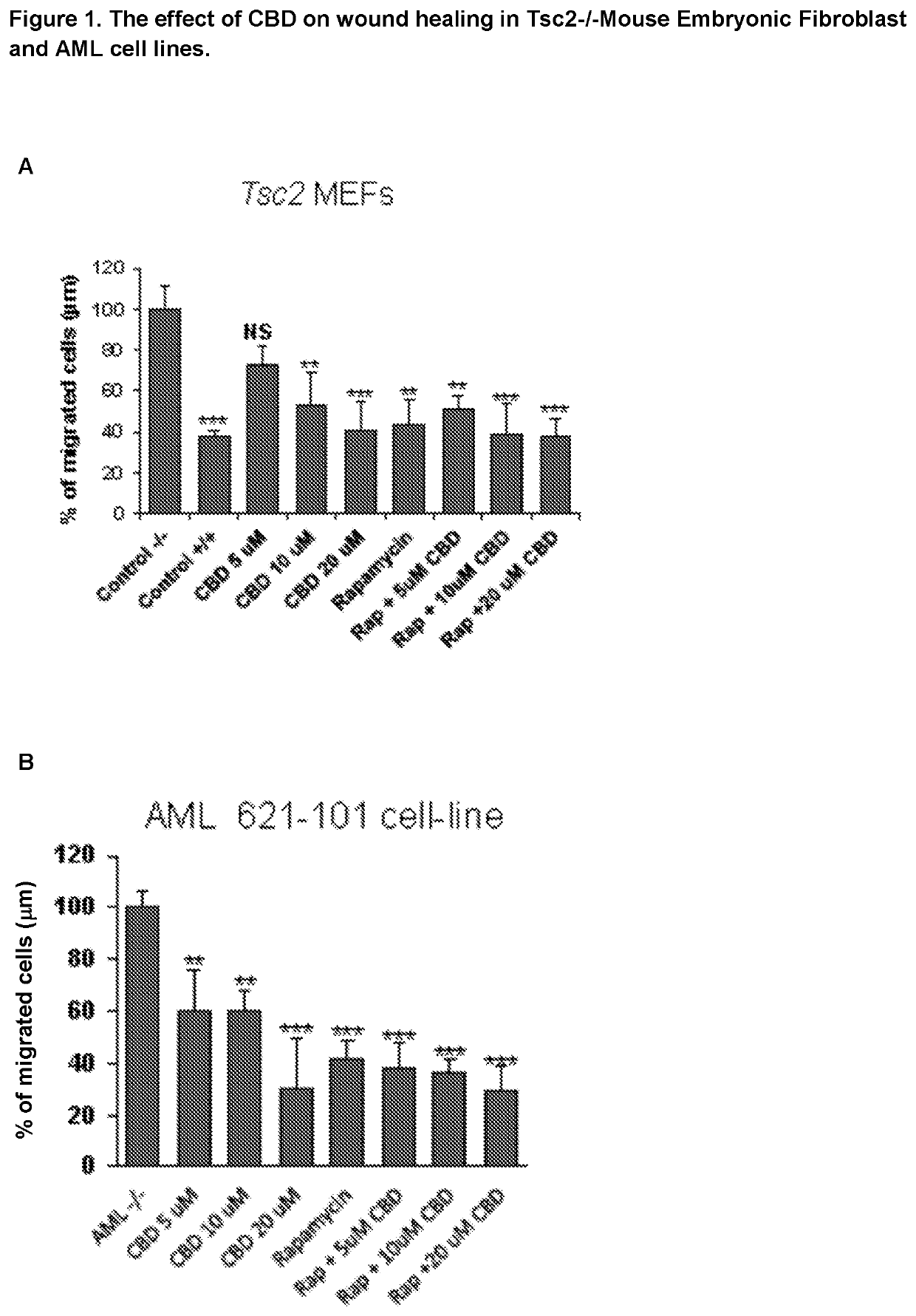
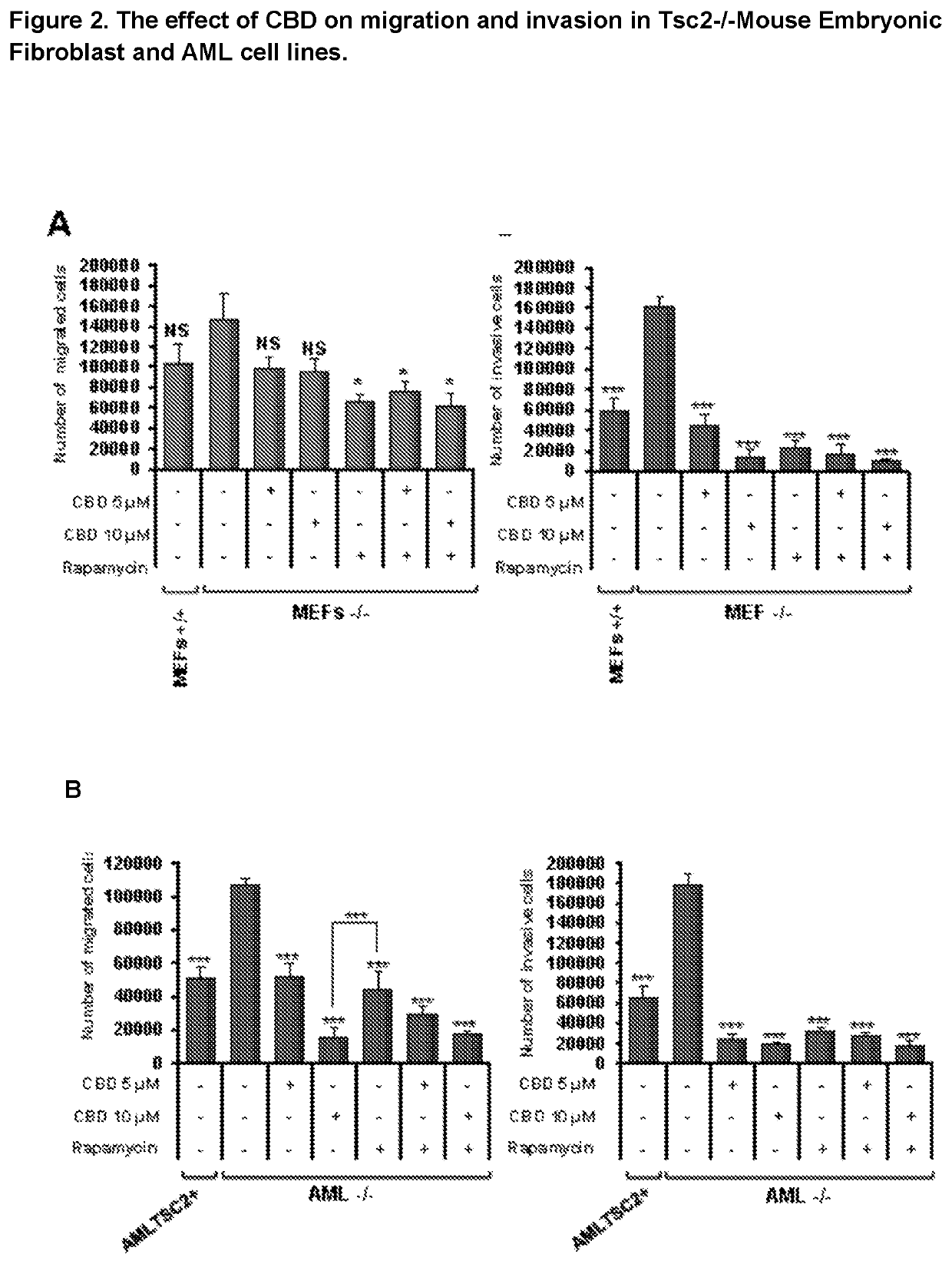
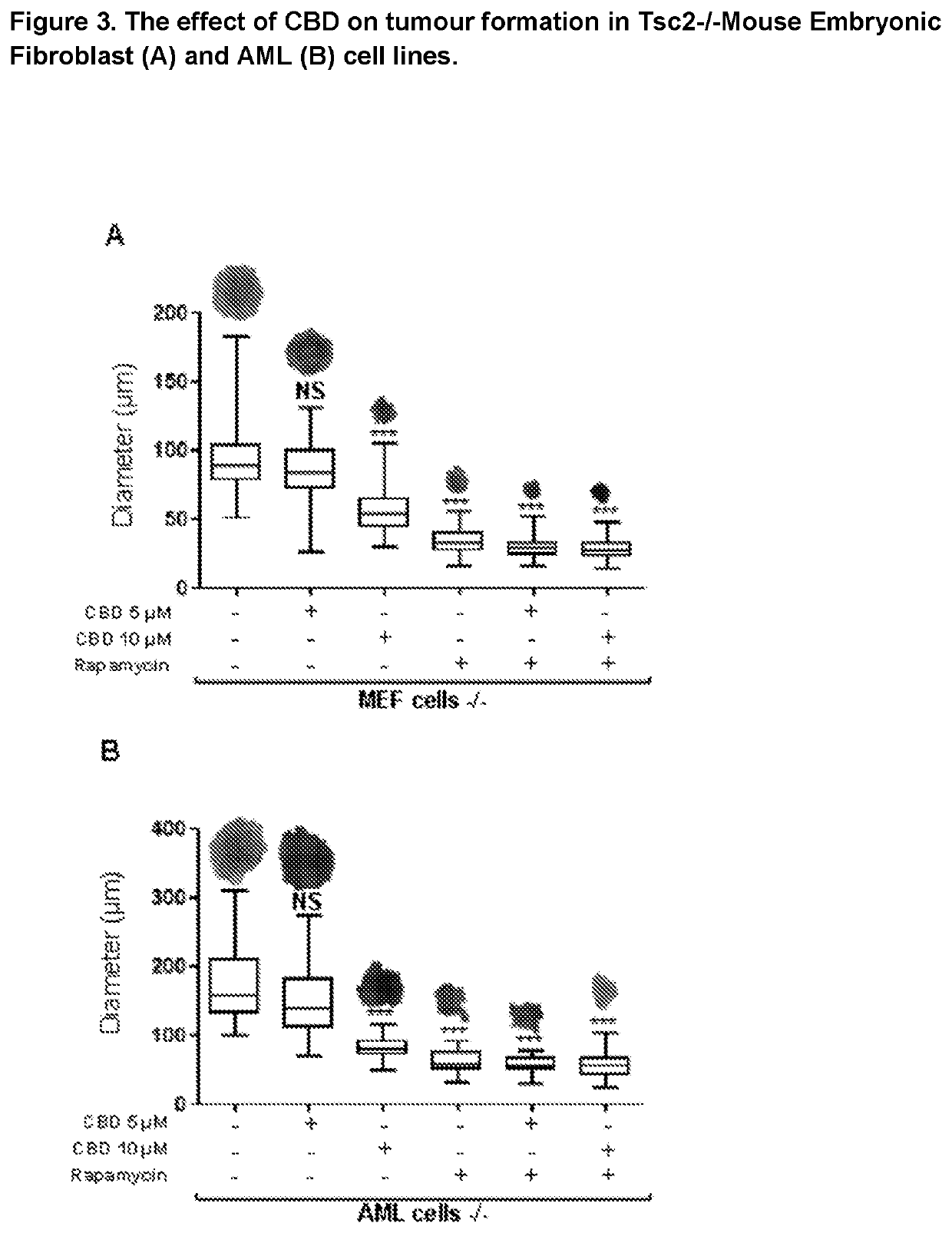
Use of cannabidiol in the treatment of tuberous sclerosis complex
InactiveUS20200206153A1Prevent and reduce tumourReduce doseHydroxy compound active ingredientsAntineoplastic agentsBenign tumoursDisease
The present invention relates to the use of cannabidiol (CBD) for the treatment of tumours associated with Tuberous Sclerosis Complex (TSC). In particular the CBD was able to decrease the number and size of marker cells, pS6, in a zebrafish model of TSC. This is5 suggestive of a disease modifying effect whereby treatment with CBD could result in the reduction or prevention of the benign tumours that occur in TSC patients. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially 10 removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD. In use the CBD is given concomitantly with one or more other drugs used in the treatment of TSC. Such drugs may include rapamycin and / or everolimus.
Owner:GW RES LTD
Psilocybin and/or psilocin in combination with cannabinoids and/or terpenes
Owner:PROCARE BEHEER BV
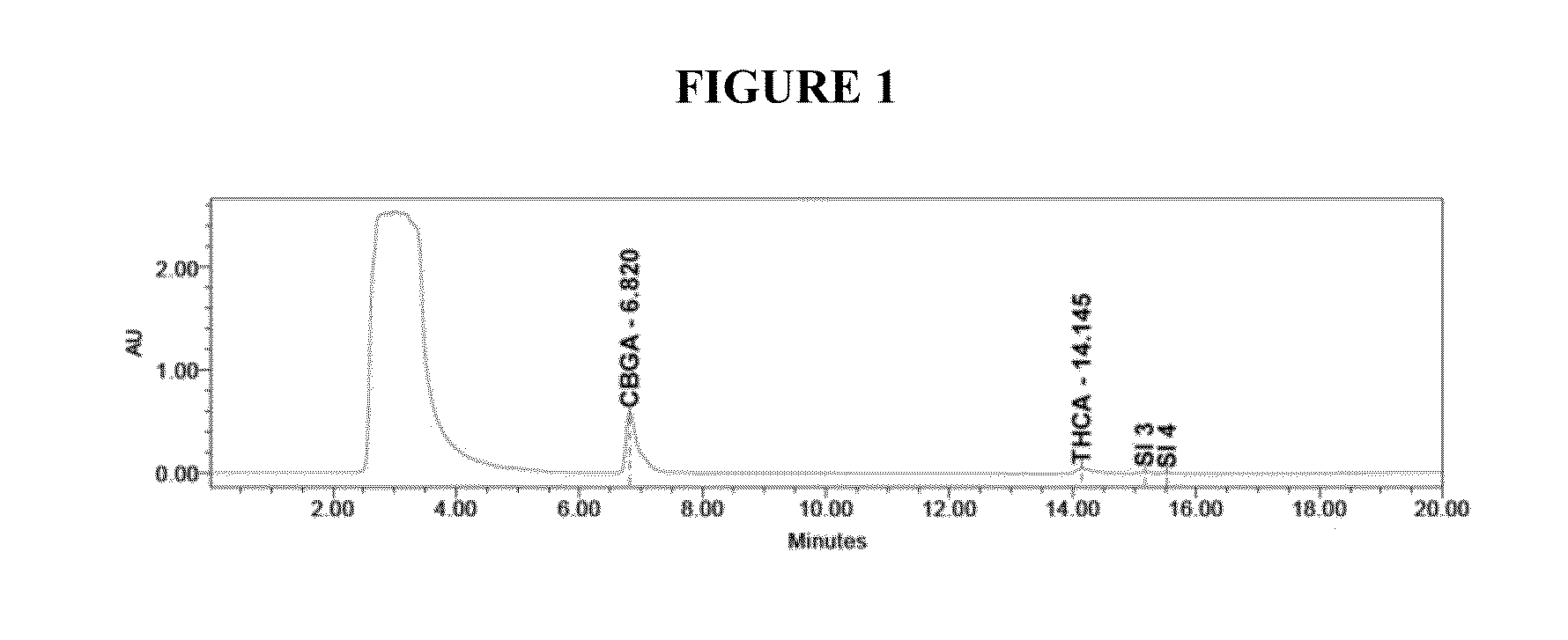
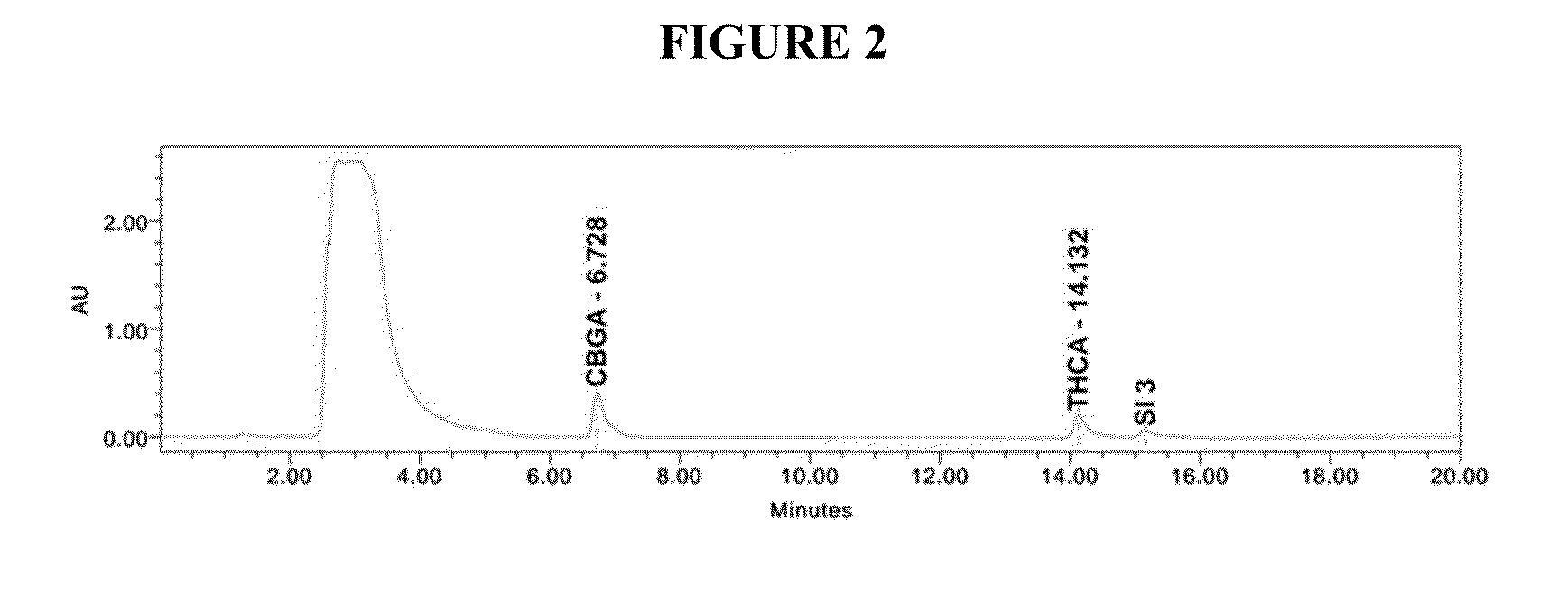
Qualitative and quantitative detection method of one or more of cannabidiol, cannabidiolic acid and tetrahydrocannabinol
InactiveCN109725080AAccurate detectionHigh sensitivityComponent separationTetrahydrocannabinolHplc mass spectrometry
The invention relates to the technical field of detection, in particular to a qualitative and quantitative detection method of one or more of cannabidiol, cannabidiolic acid and tetrahydrocannabinol.The qualitative and quantitative detection method of one or more of the cannabidiol, the cannabidiolic acid and the tetrahydrocannabinol comprises the following steps that: ultrasound extraction is conducted on smashed hemp flower leaves through methyl alcohol, and a test solution is obtained; the test solution is separated and detected through high performance liquid chromatography, a moving phase of the high performance liquid chromatography is a mixture of acetic acid water solution and acetonitrile, and isocratic elution is conducted; and the content of one or more of the cannabidiol, thecannabidiolic acid and the tetrahydrocannabinol are detected through an external standard method. According to the qualitative and quantitative detection method of one or more of the cannabidiol, thecannabidiolic acid and the tetrahydrocannabinol, specific wave length and a moving phase are selected, the peak pattern is good, sensitivity is high, research is conveniently conducted, and qualitative and quantitative treatment of the cannabidiol, the cannabidiolic acid and the tetrahydrocannabinol can be detected quickly and accurately under treatment of the same sample.
Owner:INST OF BAST FIBER CROPS CHINESE ACADEMY OF AGRI SCI +1
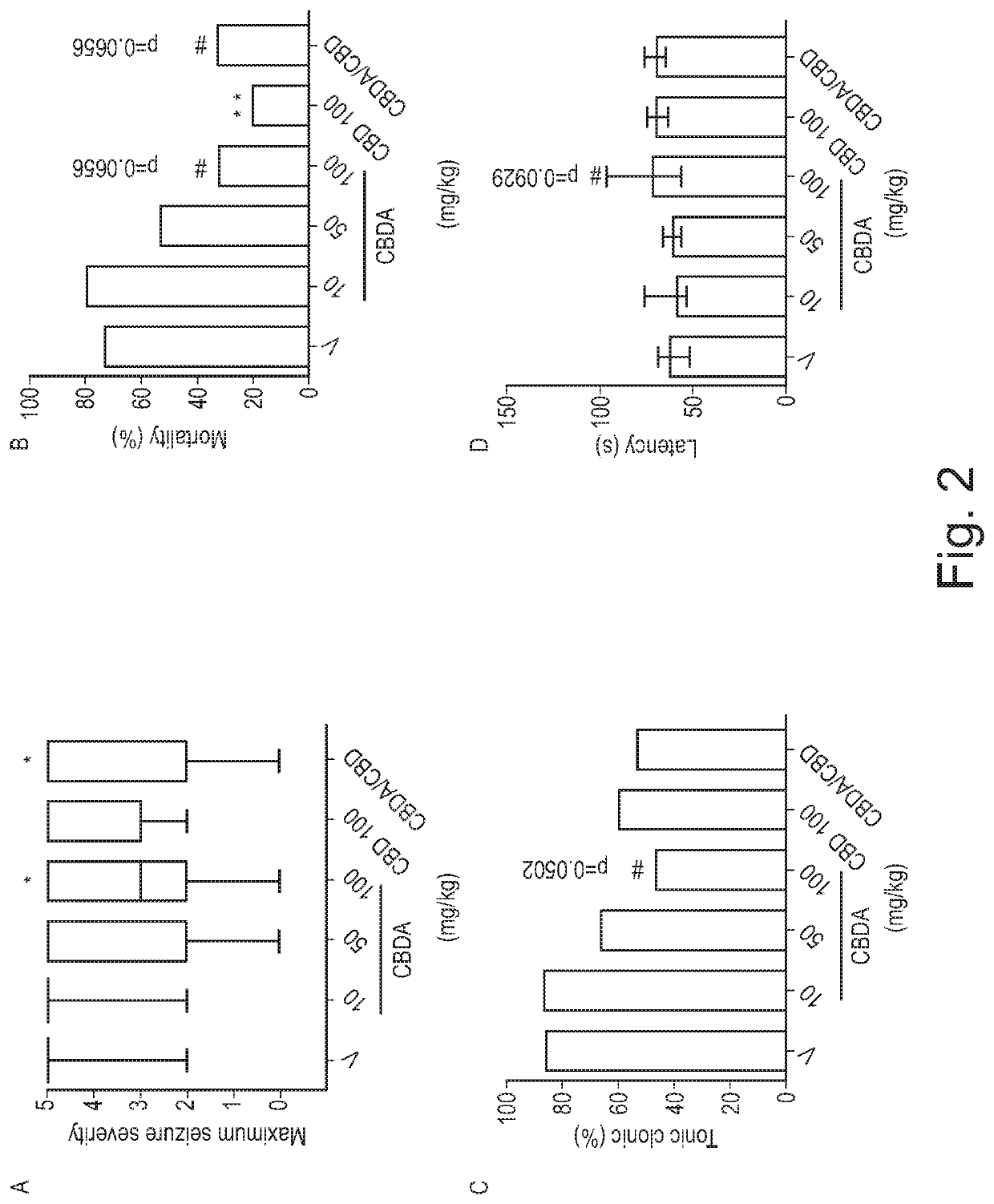
Use of cannabinoids in the treatment of epilepsy
ActiveUS11147783B2Improve bioavailabilityAct more quicklyNervous disorderHydroxy compound active ingredientsClonic seizureCannabielsoin
The present invention relates to the use of a therapeutically effective amount of cannabidiolic acid (CBDA) in the treatment of epilepsy. In one embodiment the CBDA is used in the treatment of generalised seizures, preferably tonic-clonic seizures. Preferably the CBDA used is in the form of a botanical drug substance in which the CBDA content is greater than 60%, and most preferably, it is a highly purified extract of cannabis such that the CBDA is present at greater than 95%, through 96% and 97% to most preferably, greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoids tetrahydrocannabinol (THC) or tetrahydrocannabinol acid (THCA) have been substantially removed. Alternatively, the CBDA may be synthetically produced.
Owner:GW RES LTD
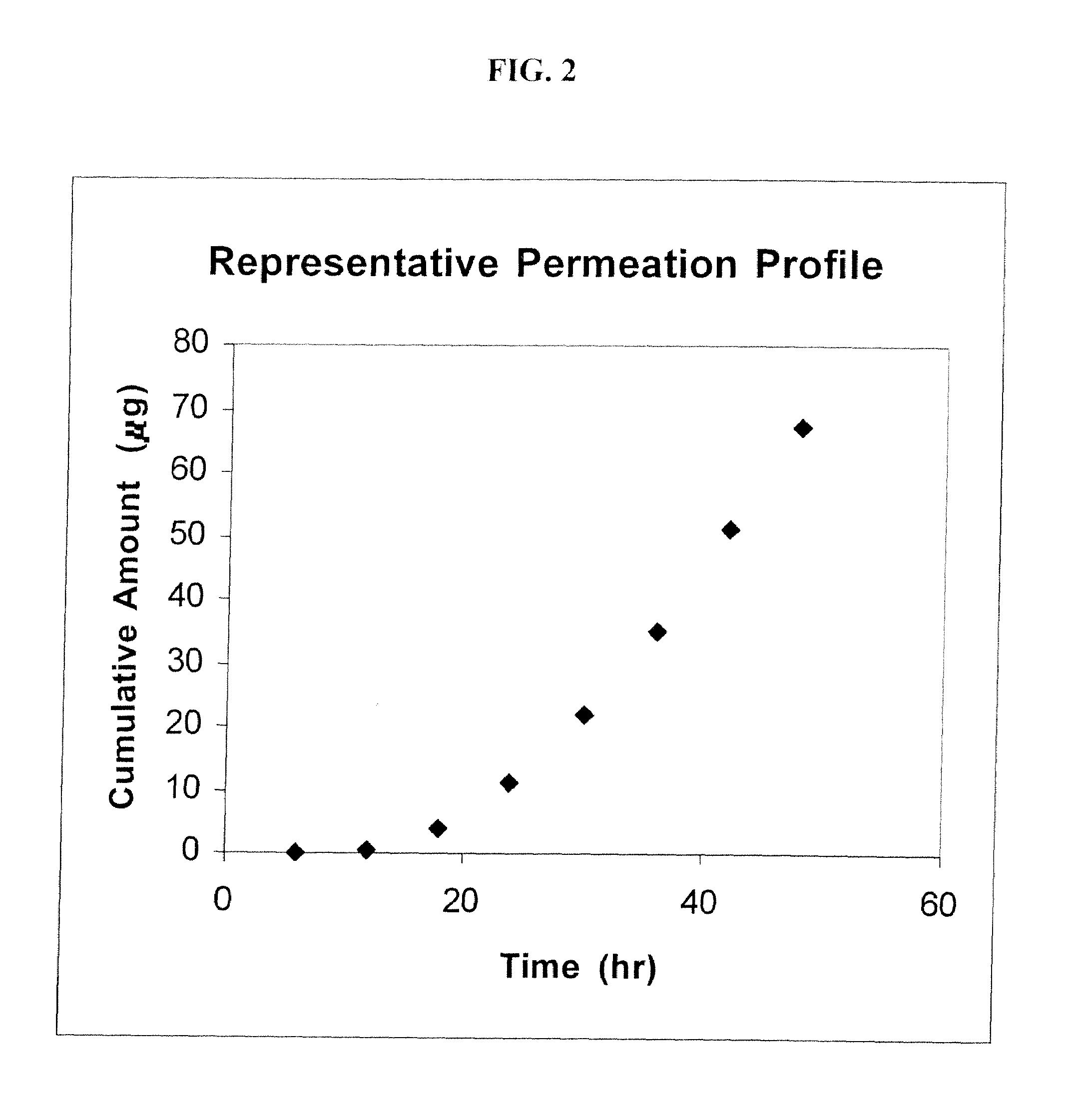
Transdermal delivery of cannabinoids
ActiveUS20090291128A1Avoid gastrointestinal (first-pass) metabolismEliminate side effectsBiocideNervous disorderDiseaseSide effect
The present invention overcomes the problems associated with existing drug delivery systems by delivering cannabinoids transdermally. Preferably, the cannabinoids are delivered via an occlusive body (i.e., a patch) to alleviate harmful side effects and avoid gastrointestinal (first-pass) metabolism of the drug by the patient. A first aspect of the invention provides a method for relieving symptoms associated with illness or associated with the treatment of illness in a mammalian subject, comprising the steps of selecting at least one cannabinoid from the group consisting of cannabinol, cannabidiol, nabilone, levonantradol, (−)-HU-210, (+)-HU-210, 11-hydroxy-Δ9-THC, Δ8-THC-11-oic acid, CP 55,940, and R(+)-WIN 55,212-2, selecting at least one permeation enhancer from the group consisting of propylene glycol monolaurate, diethylene glycol monoethyl ether, an oleoyl macrogolglyceride, a caprylocaproyl macrogolglyceride, and an oleyl alcohol, and delivering the selected cannabinoid and permeation enhancer transdermally to treat an illness.
Owner:KENTUCKY ECONOMIC DEV FINANCE AUTHORITY
Clathrate containing non-psychoactive cannabinoid and preparation method of clathrate
InactiveCN110123876AConducive to subsequent preparation moldingEasy to operateCosmetic preparationsHydroxy compound active ingredientsOrganic solventCannabielsoin
The invention discloses a clathrate containing non-psychoactive cannabinoid and a preparation method of the clathrate. The preparation method includes sequentially subjecting hemp extract to dissolution by organic solvents, clathration by clathration agents, decomposition and impurity removal by water-organic solvents, and vacuum drying and precipitation. The clathrate can completely remove psychoactive components such as tetrahydrocannabinol (THC) and delta 9-tetrahydrocannabivarin (THCV), and effectively enrich non-psychoactive components with the total content of more than 50%, such as cannabidiol (CBD), cannabigerol (CBG) and cannabichromene (CBC). The preparation method has the advantages of simple process and easiness in industrialization.
Owner:HANYI BIO TECH CO LTD
Composition comprising (−)-Δ9-trans-tetrahydrocannabinol
A composition comprising a tetrahydrocannabinol compound, a solvent and an acid, wherein the tetrahydrocannabinol compound may be Δ8 tetrahydrocannabinol, (−)-Δ9-trans-tetrahydrocannabinol or a side chain alkyl derivative of either compound, the solvent may be an oil or C1-C4 alcohol (e.g. sesame oil or ethanol), and the acid may be an organic acid or a mineral acid.
Owner:MACFARLAN SMITH
Apparatus for producing an aerosol for inhalation by a person
A disposable cartridge is disclosed for use with an electronic device for producing an aerosol for inhalation by a person. The cartridge comprises a liquid container containing a liquid which when aerosolized is intended for inhalation by a person; and a transducer that, when actuated, causes the liquid from the container to be aerosolized such that the aerosol may be inhaled by a person. In various embodiments, the liquid comprises: a liposomal carrier; a liquid mixture having an active agent entrapped by liposomes; and a liquid mixture comprising a nanodispersion. The liquid may include nicotine, tetrahydrocannabinol, cannabidiol, or nanoparticles formed by an oil droplet covered by a monolayer of phosphatidylcholine. The transducer preferably comprises a mesh material with the transducer and the mesh material forming a piezo mesh disk for aerosolizing the liquid.
Owner:QNOVIA INC
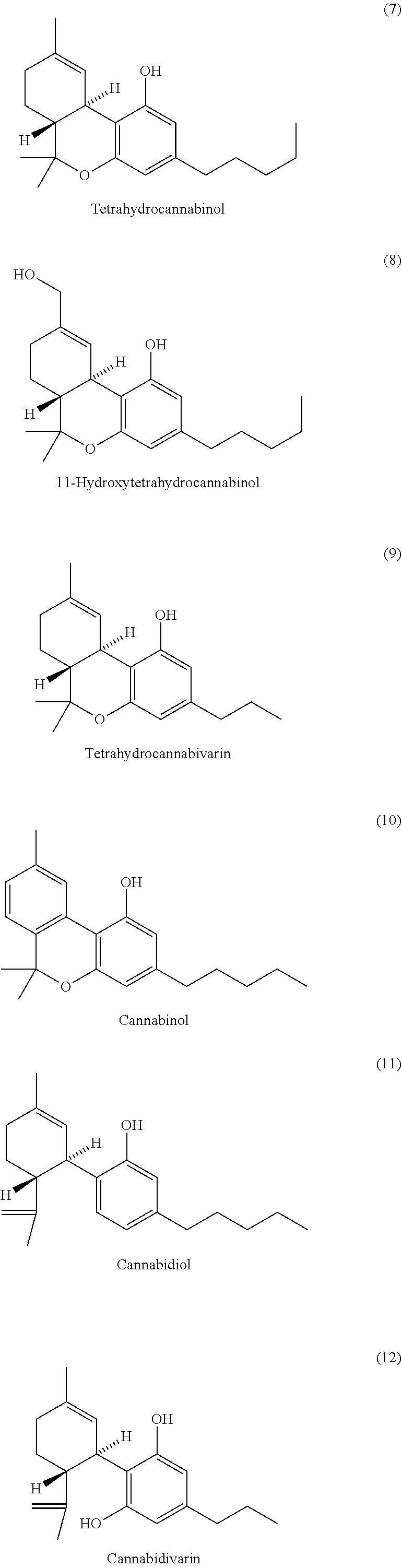
Compositions comprising cannabidiol, tetrahydrocannabinol, terpenes, and flavonoids and use thereof in the treatment of insomnia
InactiveUS20200281890A1Sleep longerMinimized wakefulnessPowder deliveryHydrocarbon active ingredientsXanthonoidCannabielsoin
Compositions comprising two or more cannabinoids, a terpene, and a flavonoid suitable for use in treating insomnia are disclosed. In preferred embodiments, the cannabinoids are cannabidiol and tetrahydrocannabinol, while the terpenes and flavonoids are selected from compounds that naturally occur in Cannabis extracts. Methods of treating insomnia by administering such compositions by a variety of routes are also disclosed.
Owner:TWEED INC
Topical formulations of cannabinoids and use thereof in the treatment of pain
PendingUS20200289458A1Increase mechanical thresholdReduce and eliminate hyperalgesiaNervous disorderHydrocarbon active ingredientsCannabielsoinPharmaceutical Substances
Pharmaceutical compositions comprising one or more cannabinoids and a pharmaceutically acceptable carrier are disclosed. The compositions are in a form suitable for topical administration, and are useful in the treatment of pain. The cannabinoids suitable for use include cannabidiols, cannabinols, and various other naturally occurring and synthetic cannabinoids. The compositions may also further include anti-inflammatory agents, steroids, or terpenoids. In preferred embodiments, the cannabinoids incorporated in the compositions are cannabidiol (CBD) and cannabinol (CBN) while the carriers are selected from the group consisting of Labrasol™, Transcutol™, lecithin, lysolecithin, isopropyl palmitate, and isopropyl myristate.
Owner:INMED PHARMA INC
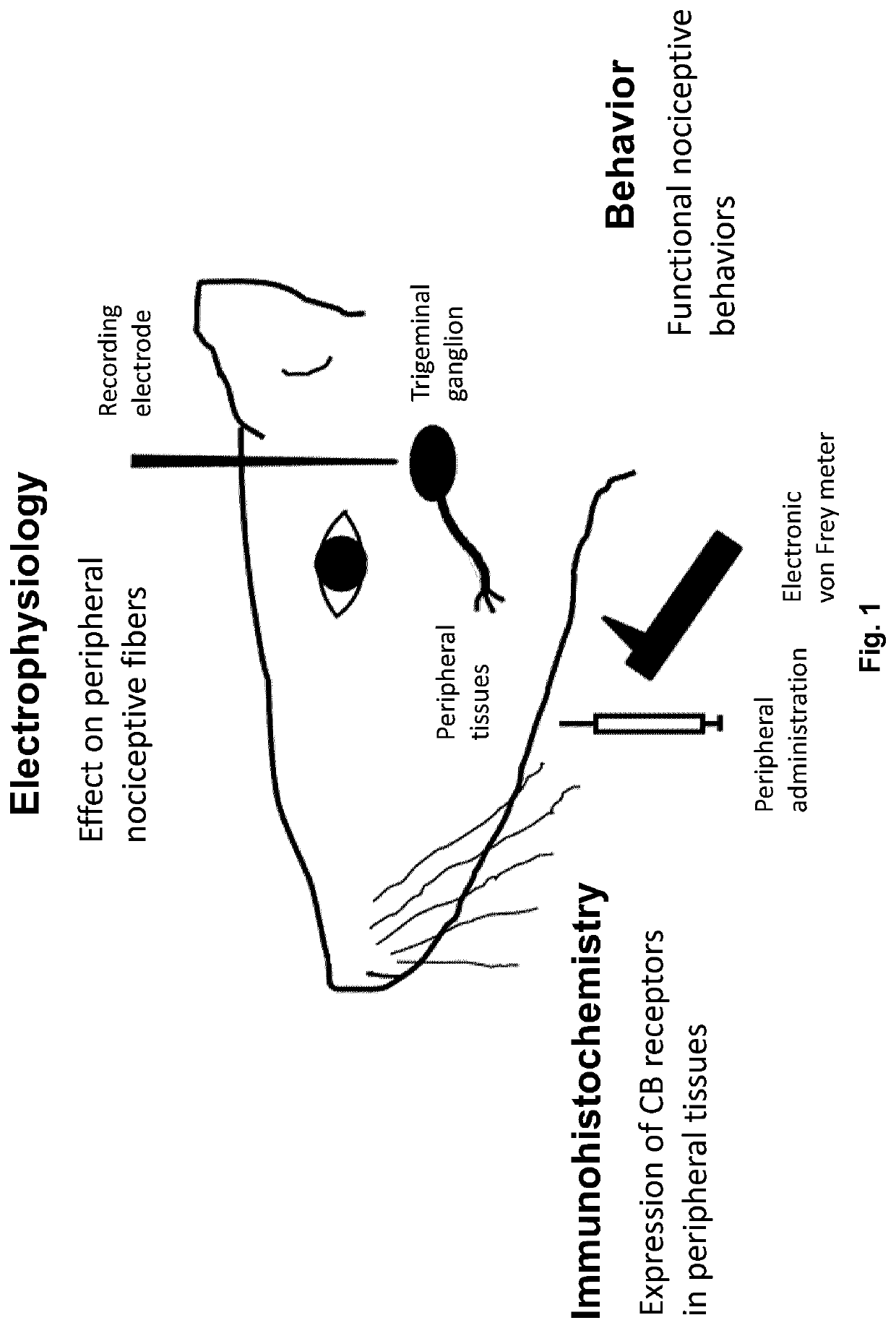
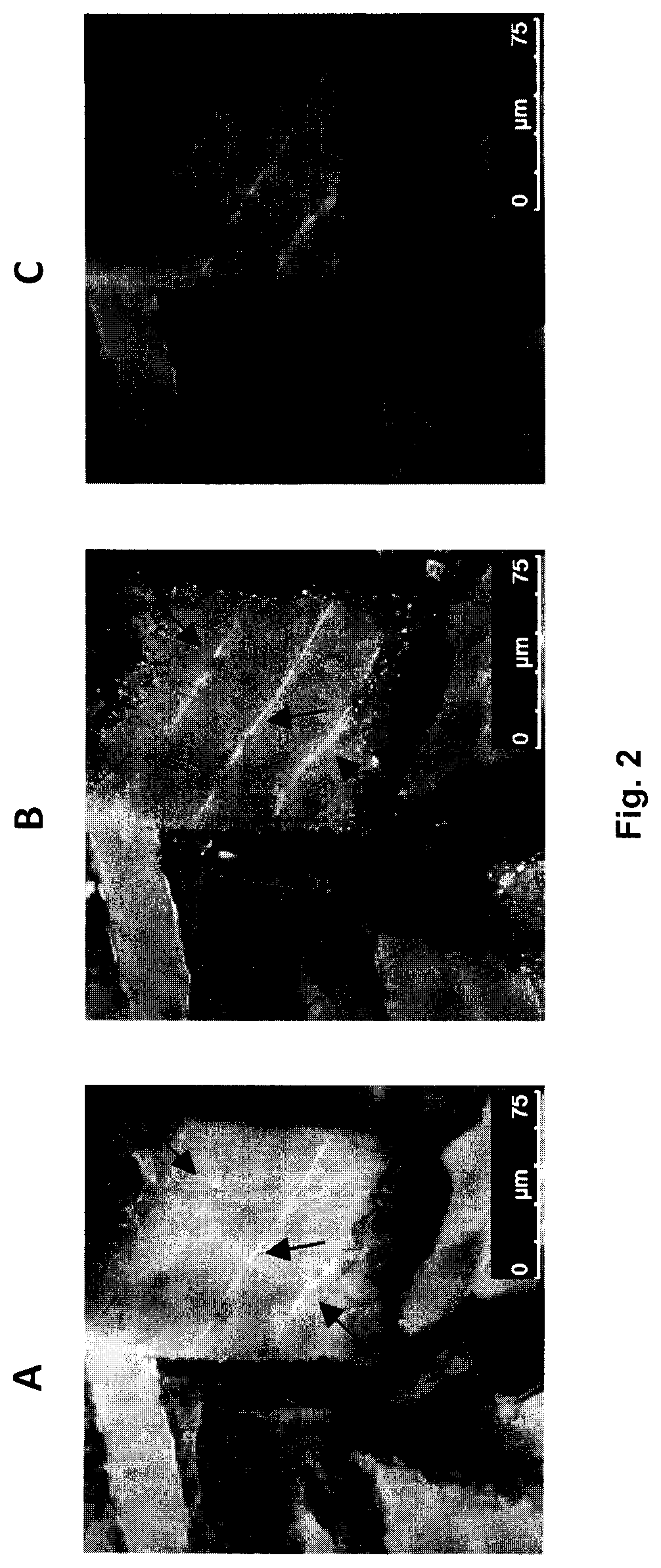
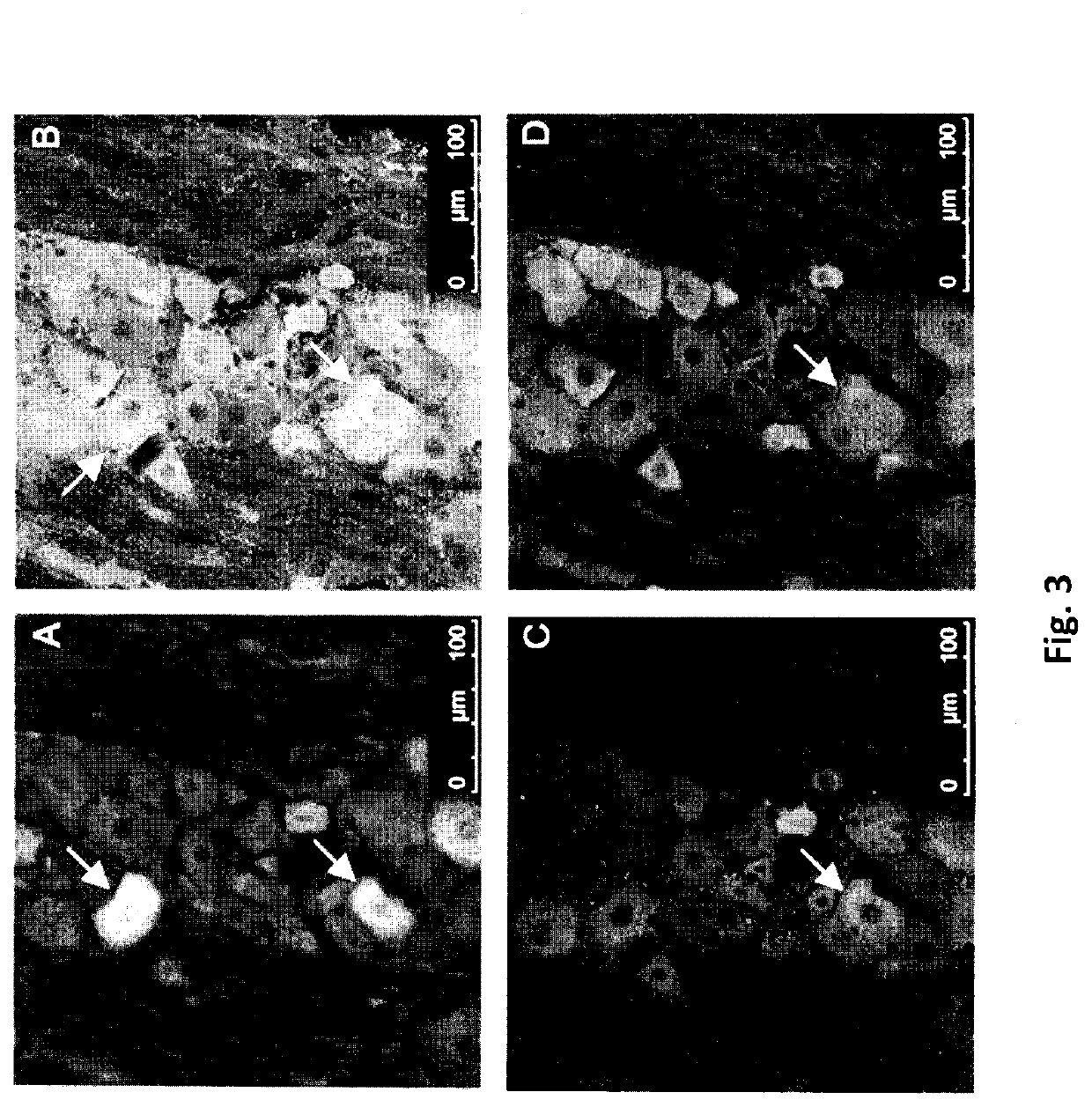
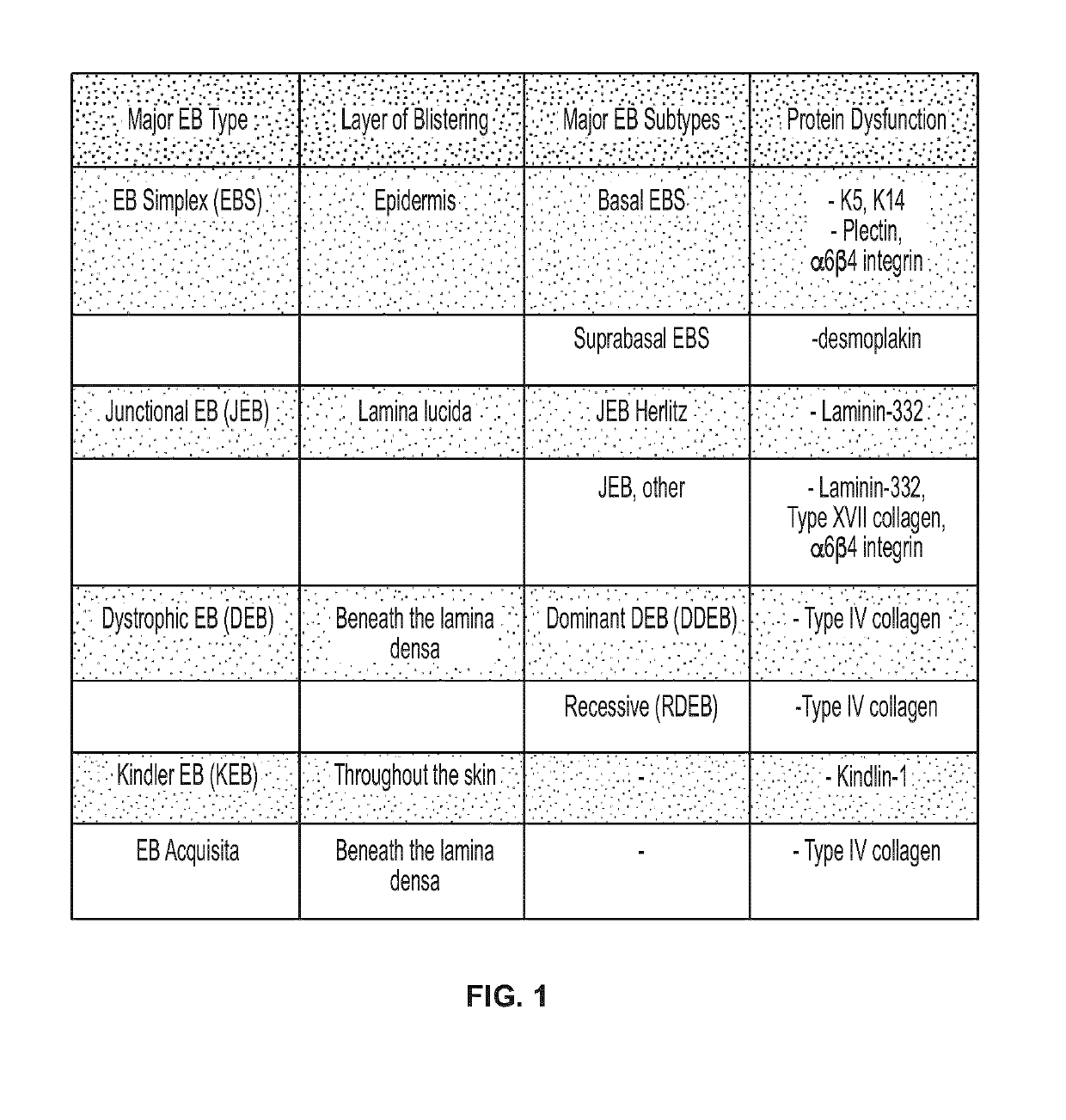
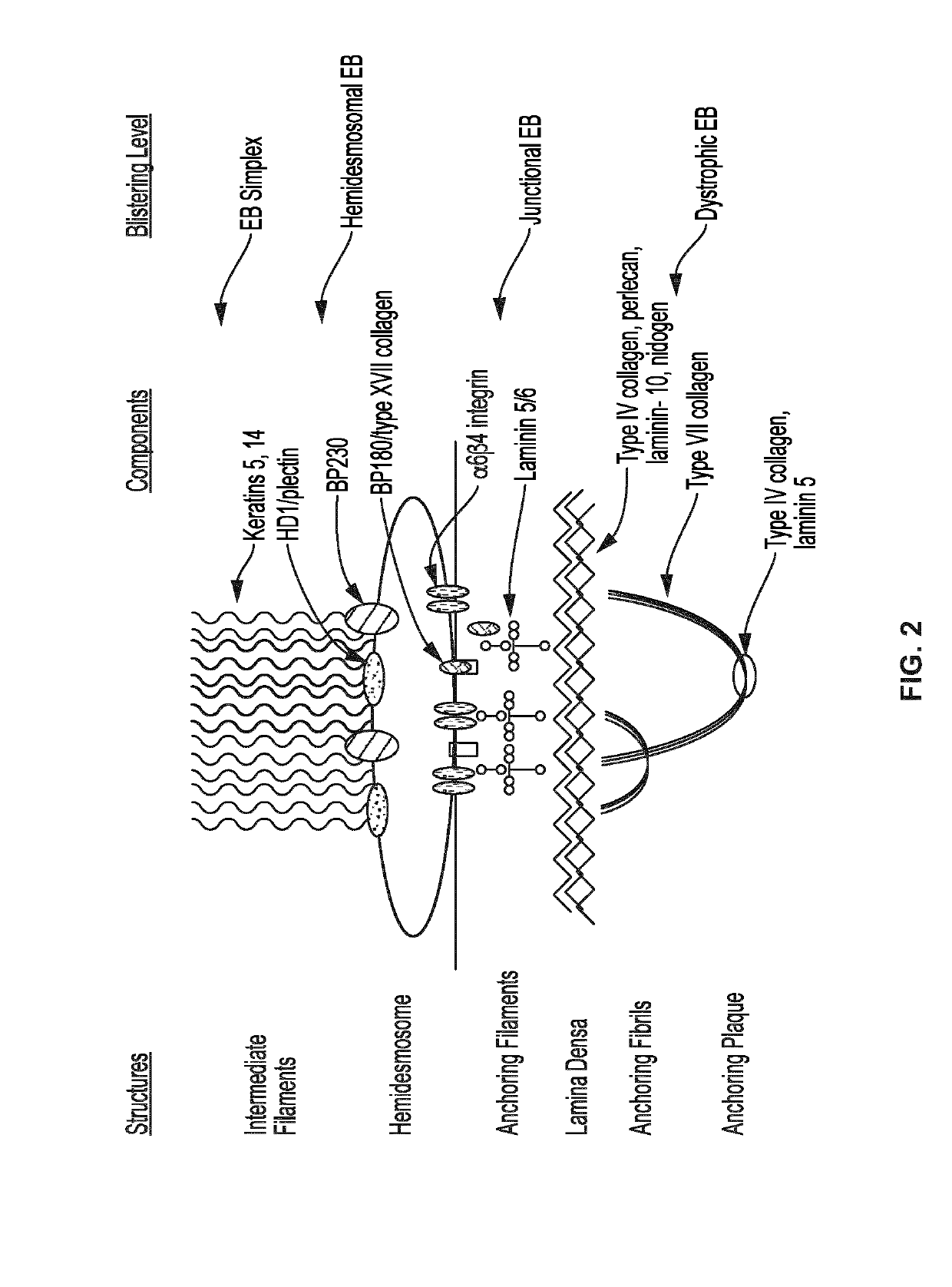
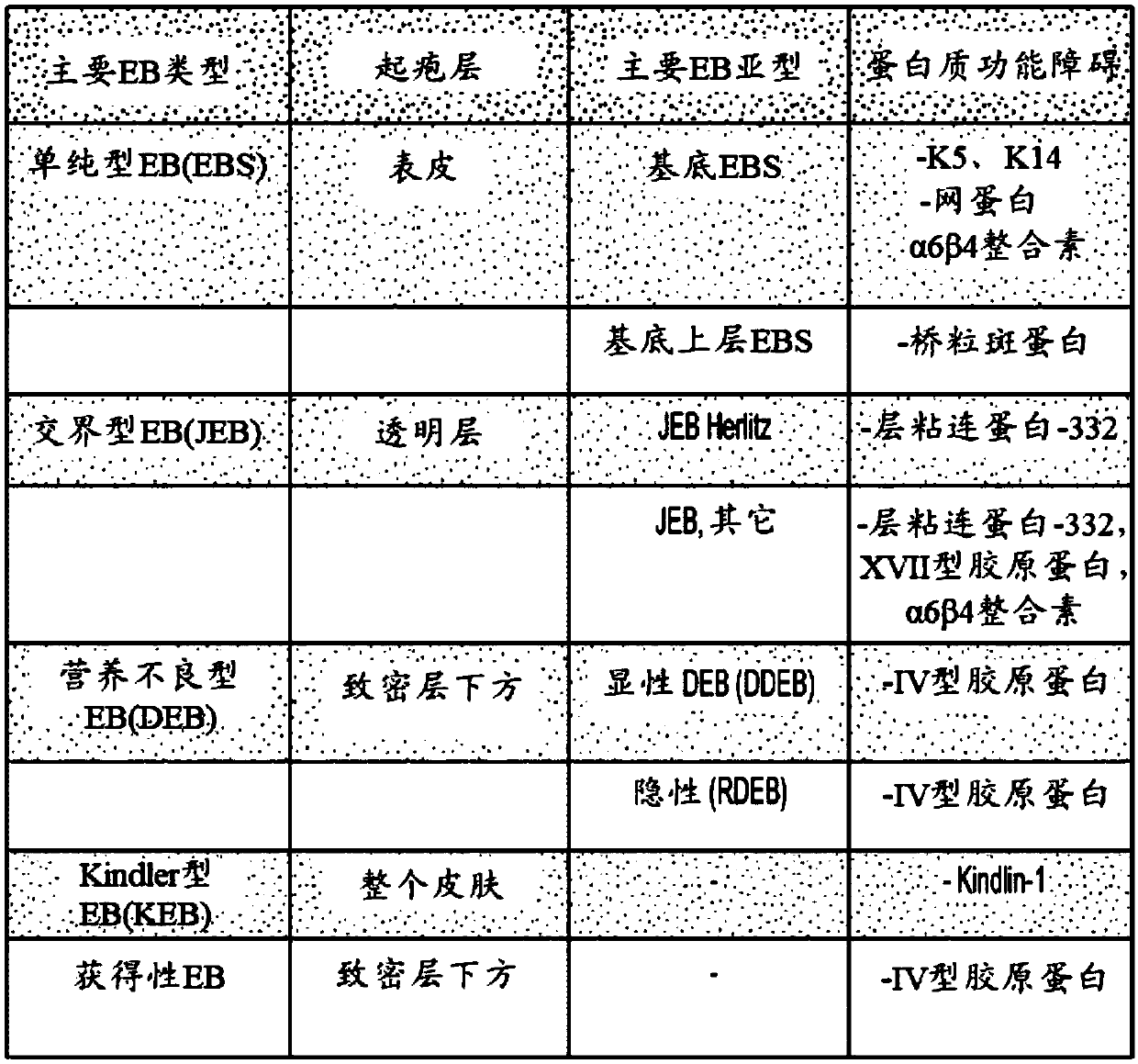
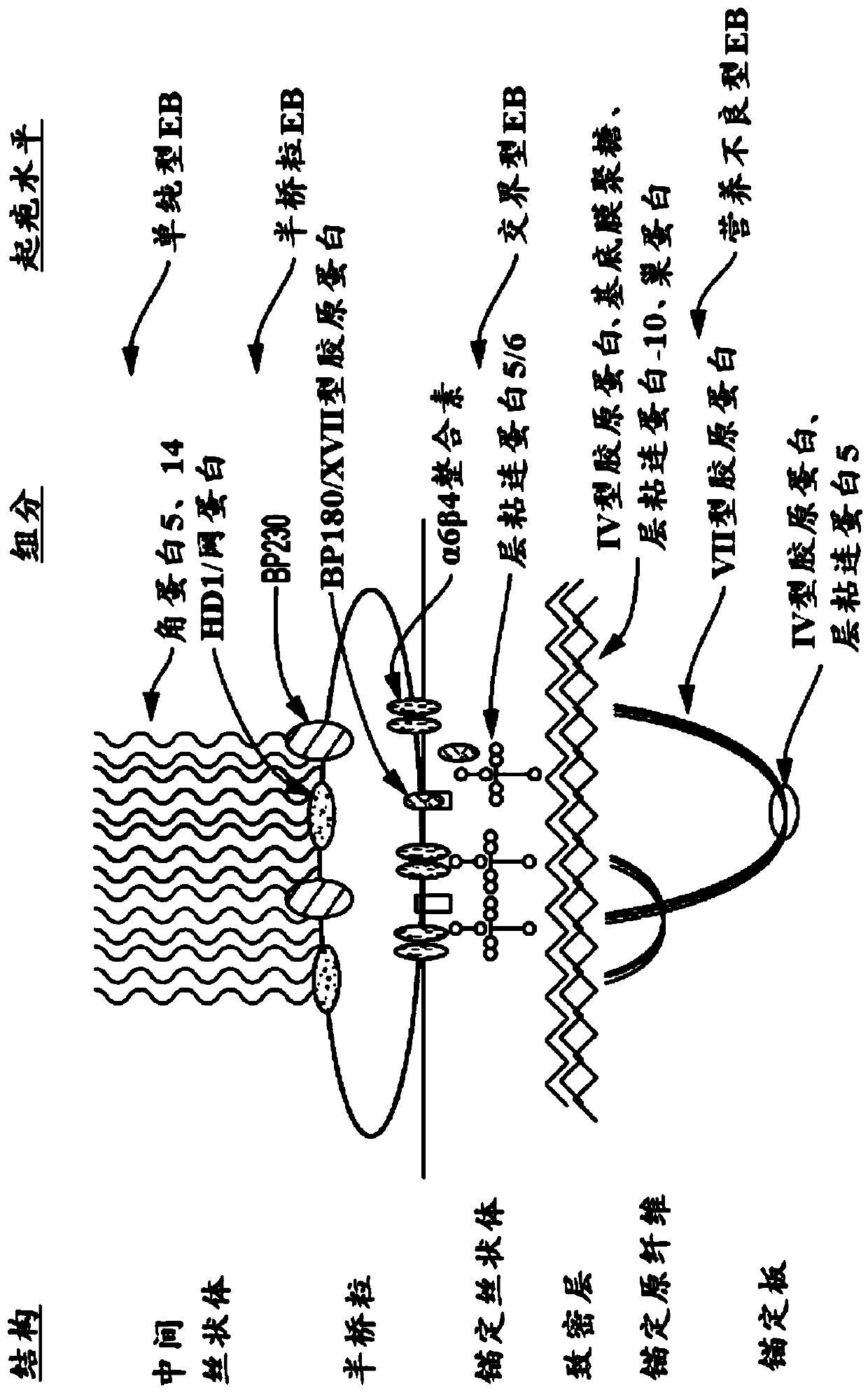
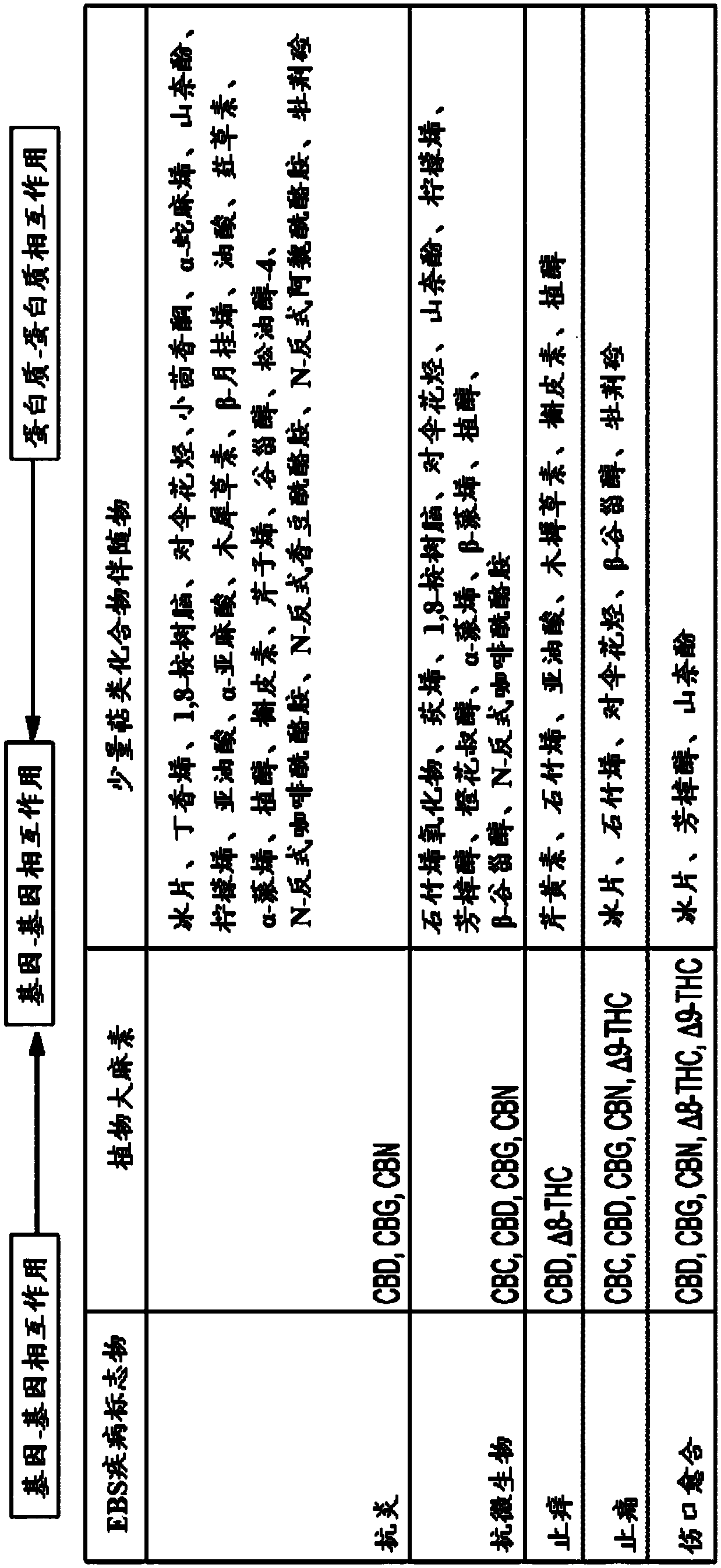
Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders
PendingUS20190142788A1Reduce inflammationPromote regenerationHydroxy compound active ingredientsPharmaceutical delivery mechanismConnective tissueIntermediate filament
Mutations in keratin genes or the genes that regulate keratin expression can result in epithelial cells lacking sufficient structural integrity. The resulting disruption of connective tissue gives rise to inherited disorders such epidermolysis bullosa. It has been found that various cannabinoids (including mixtures of cannabidiols and cannabinol) upregulate expression of various keratins such that loss of function in other keratin genes may be compensated for. By way of this upregulation, these cannabinoids can be used to treat epidermolysis bullosa and other connective tissue disorders arising from intermediate filament dysfunction.
Owner:INMED PHARMA INC
Cannabinoid Composition and Method For Treating Pain
A combination of THC, CBD and Cobalamin (in a ratio of about 63%, 27% and 10%, respectively) is used with a topical carrier such as Shea butter cream to relieve pain. The THC and CBD mixture is extracted from a Cannabis Indica dominant strain using high pressure and carbon dioxide (CO2) as a solvent and comprises: Tetrahydrocannabinol “THC” (9-Tetrahydrocannabinol (delta-9 THC), 8-Tetrahydrocannabinol (Delta-8 THC) and 9-THC Acid), Cannabidiol “CBD”, Cannabinol “CBN”, Cannabichromene (“CBC), Cannabigerol (“CBG”), terpenoids and flavonoids. The Shea butter is an extract from the Shea nut from the Shea tree (Vitellaria paradoxa). The weight of THC, CBD and Cobalamin, excluding other ingredients in the Indica extract, is in the range of about 1.0% to 5% of the total weight of the Shea butter. The cream is applied to joints for indications such as Psoriatic Arthritis and Scleroderma. The mixture is absorbed through the skin and interacts with the peripheral nervous and immune systems via the A Delta and C nerve fibers and CB 2 skin receptors to provide relief from pain without psychotropic or other side effects normally associated with THC.
Owner:INDIA GLOBALIZATION CAPITAL INC
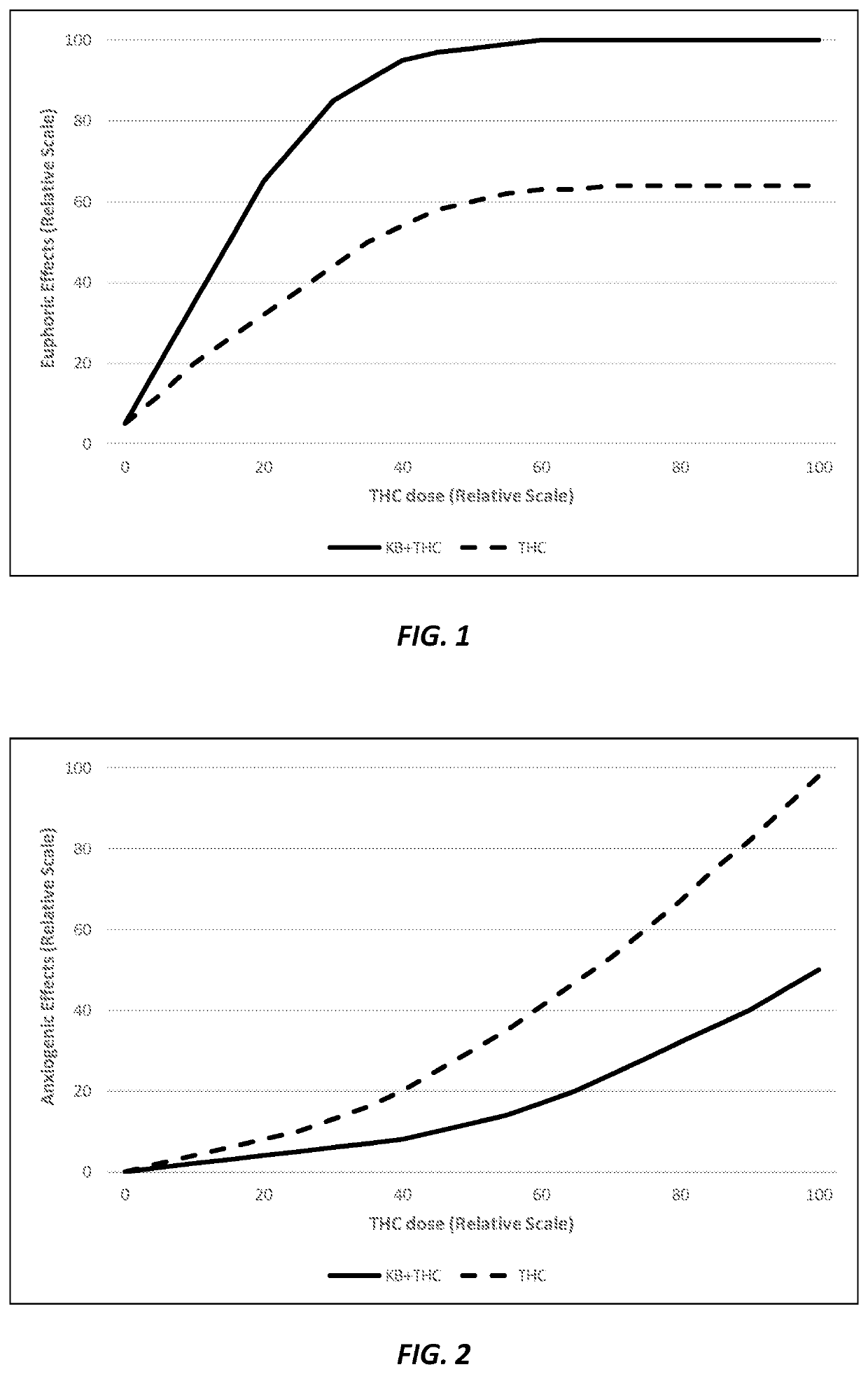
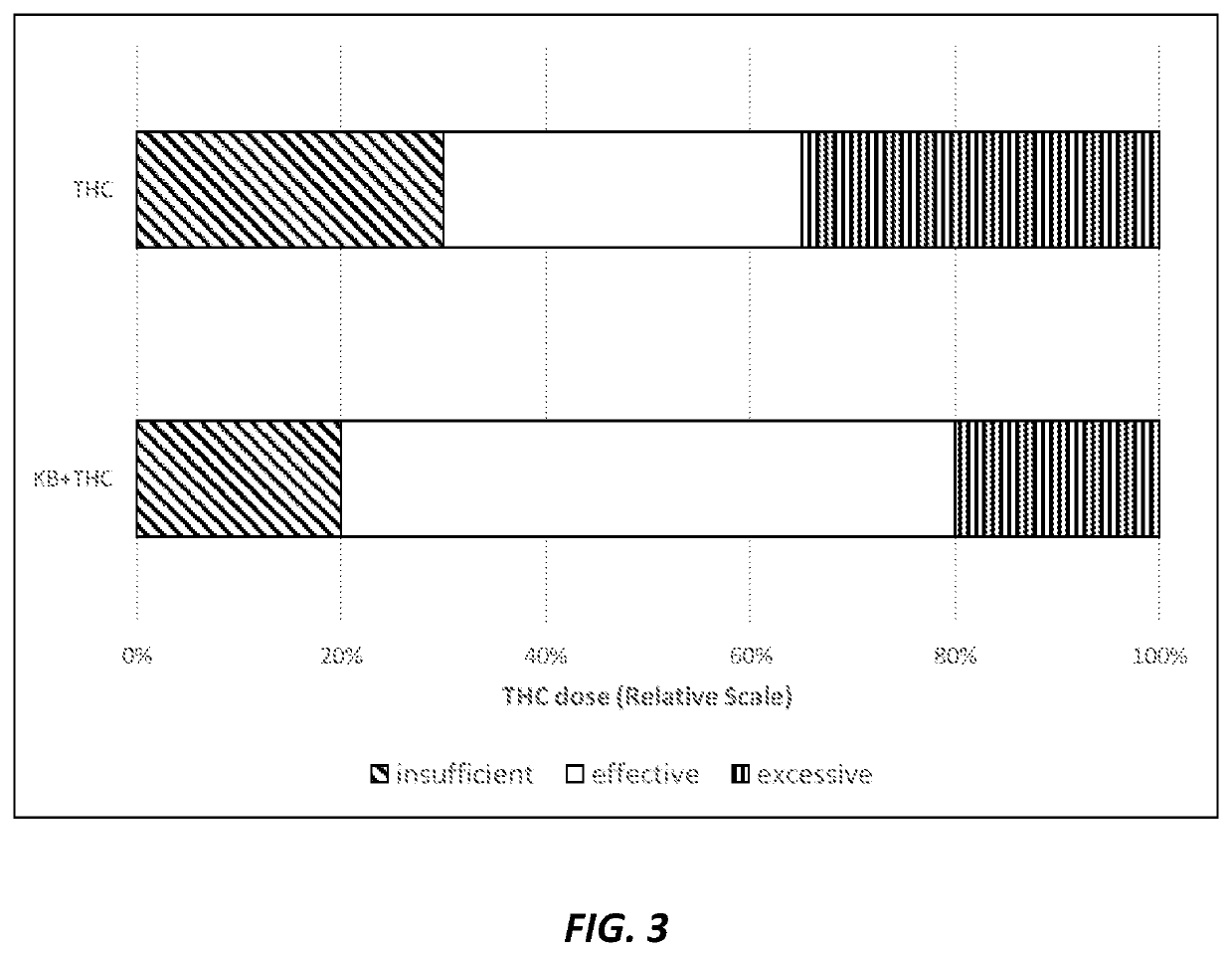
Compositions and methods for delivering tetrahydrocannabinol and ketone bodies
ActiveUS10980772B2Reduce eliminateEliminate the effects ofNervous disorderAntipyreticAcetoacetatesSide effect
Disclosed herein are “ketannabis” (or “ketonnabis”) compositions including a combination of: (1) tetrahydrocannabinol (THC); (2) a ketone body component such as beta-hydroxybutyrate (BHB) and / or acetoacetate; and (3) a dietetically or pharmaceutically acceptable carrier. Also disclosed herein are methods of using such ketannabis compositions for producing desired physiological effects. The ketannabis compositions beneficially enhance the euphoric effects of THC without aggravating common side effects or even acting to reduce common side effects such as anxiety, disruption of short-term memory, appetite increases, heart rate increases, and blood pressure changes.
Owner:AXCESS GLOBAL SCI LLC
Aerosol
PendingCN111000897AHigh acceptanceIncrease awarenessNervous disorderHydroxy compound active ingredientsBiotechnologyCannabielsoin
The invention discloses an aerosol comprising industrial hemp floral leaf essential oil and medicinal components. The industrial hemp floral leaf essential oil added in the invention only contains micromolecular aromatic components and does not contain diterpenoid compounds such as cannabidiol, tetrahydrocannabinol and other medicinal active components, so that the biological activity of an addedobject is not obviously influenced. However, after the industrial hemp floral leaf essential oil is added, all odorless cannabinol products have the symbolic aroma of natural industrial hemp, the sensory deficiency, psychological comfort and implication effects are supplemented, and the market value of the original products and the cognition degree of consumers can be greatly improved. The industrial hemp floral leaf essential oil is added into the odorless cannabinol aerosol, so that the acceptability of patients using natural medical hemp to odorless products can be improved.
Owner:YUNNAN HEMPSON BIO-TECH CO LTD
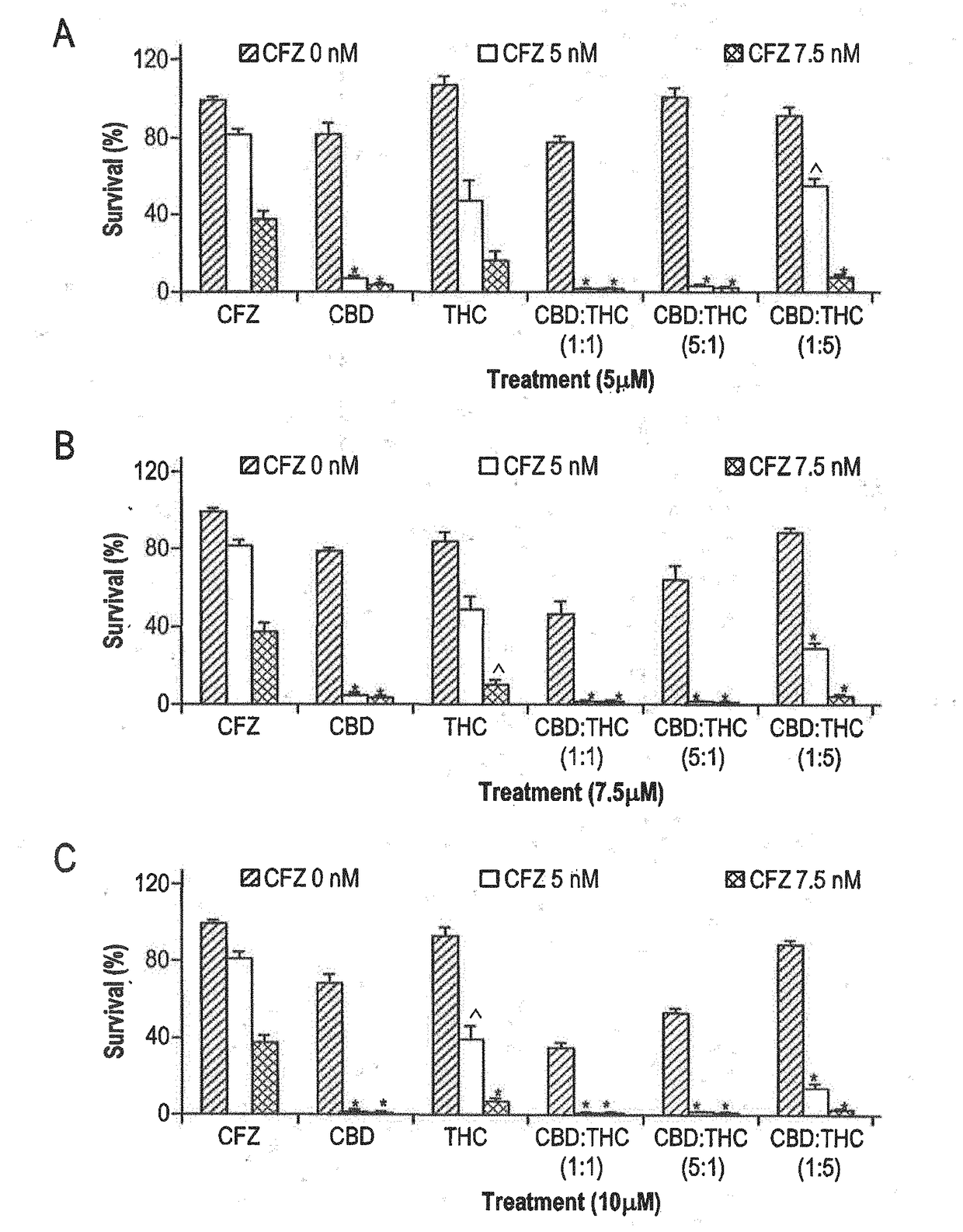
Novel cannabinoid combination therapies for multiple myeloma (MM)
InactiveUS20180185324A1Strong cytotoxicityReduced viabilityHydroxy compound active ingredientsBoron compound active ingredientsCannabinoidCytotoxicity
The present invention discloses a cytotoxic cocktail comprising; (a) a therapeutically effective amount of at least one cannabinoid selected from the group consisting of: cannabidiol (CBD) or a derivative thereof, Tetrahydrocannabinol (THC) or a derivative thereof, and any combination thereof; and (b) at least one therapeutic agent selected from the group consisting of: bortezomib (BTZ), carflizomib (CFZ), lenalidomide (LEN), dexamethasone (DEX), melphalan (MEL) and doxorubicin (DOXO). In a core embodiment the cocktail is conferring a synergistic effect with respect to inhibition or cytotoxicity of multiple myeloma (MM) cells, relative to said at least one therapeutic agent selected from the group consisting of: BTZ, CFZ, LEN, DEX, MEL, DOXO and said CBD and THC, administered separately in a similar concentration.
Owner:ONE WORLD CANNABIS LTD
Process for the Production of Cannabinoids
Owner:BESSOR PHARM LLC
Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders
InactiveCN109689045AHydroxy compound active ingredientsPharmaceutical delivery mechanismConnective tissue fiberMedicine
Mutations in keratin genes or the genes that regulate keratin expression can result in epithelial cells lacking sufficient structural integrity. The resulting disruption of connective tissue gives rise to inherited disorders such epidermolysis bullosa. It has been found that various cannabinoids (including mixtures of cannabidiols and cannabinol) upregulate expression of various keratins such thatloss of function in other keratin genes may be compensated for. By way of this upregulation, these cannabinoids can be used to treat epidermolysis bullosa and other connective tissue disorders arising from intermediate filament dysfunction.
Owner:因美制药公司
Method for simultaneously detecting five cannabinol compounds by using HPLC-MS/MS
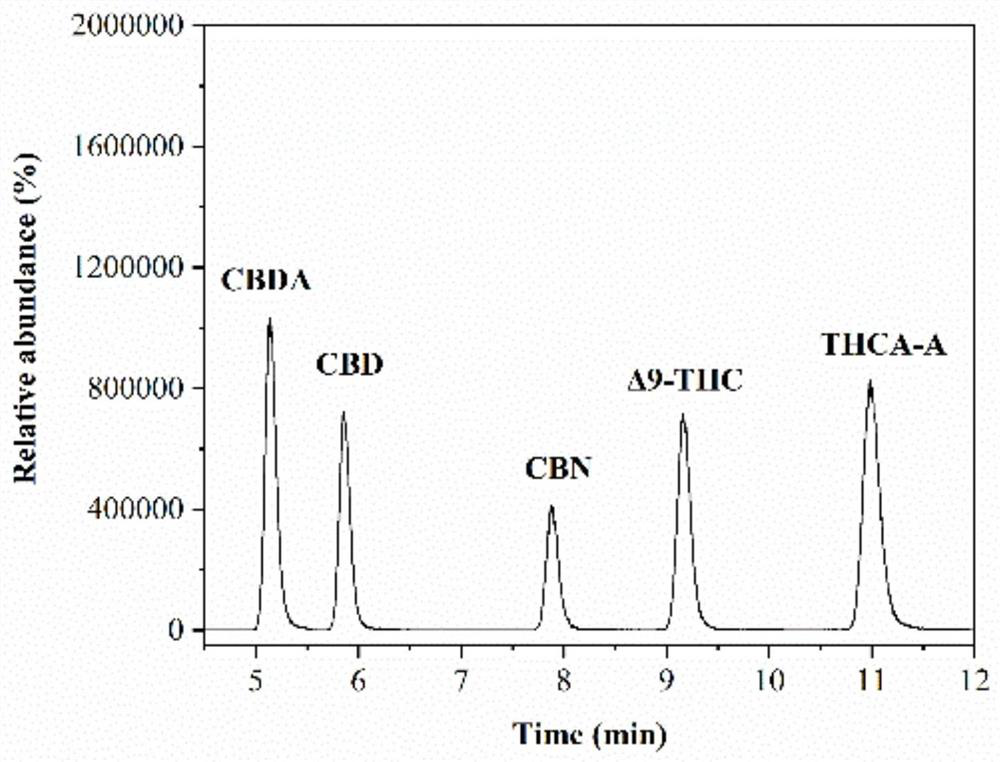
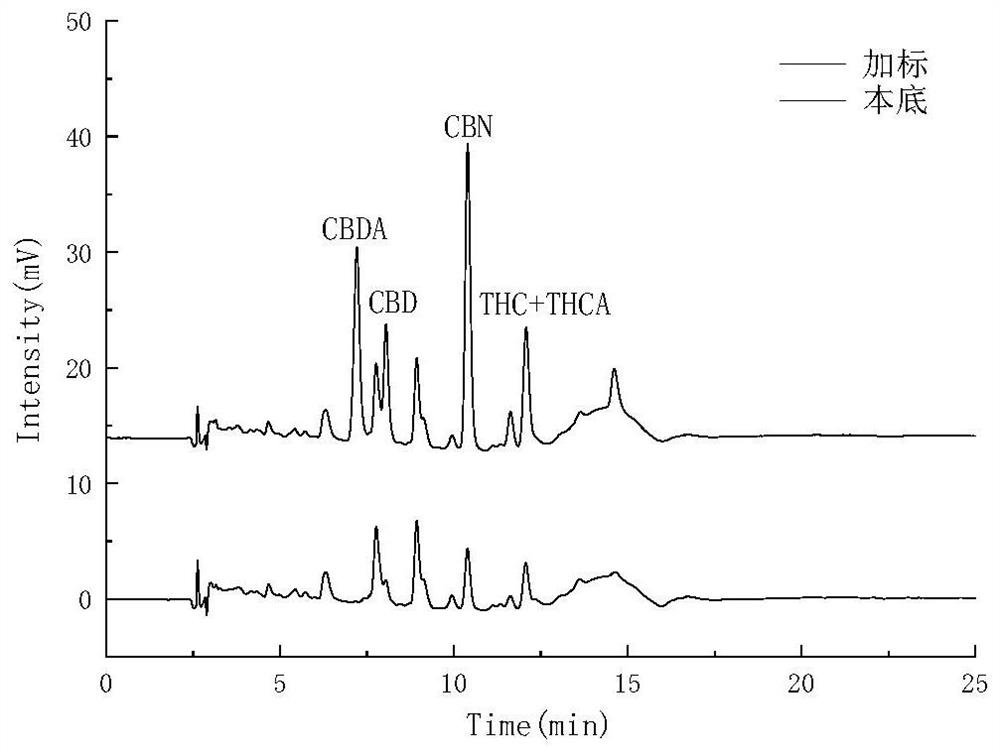
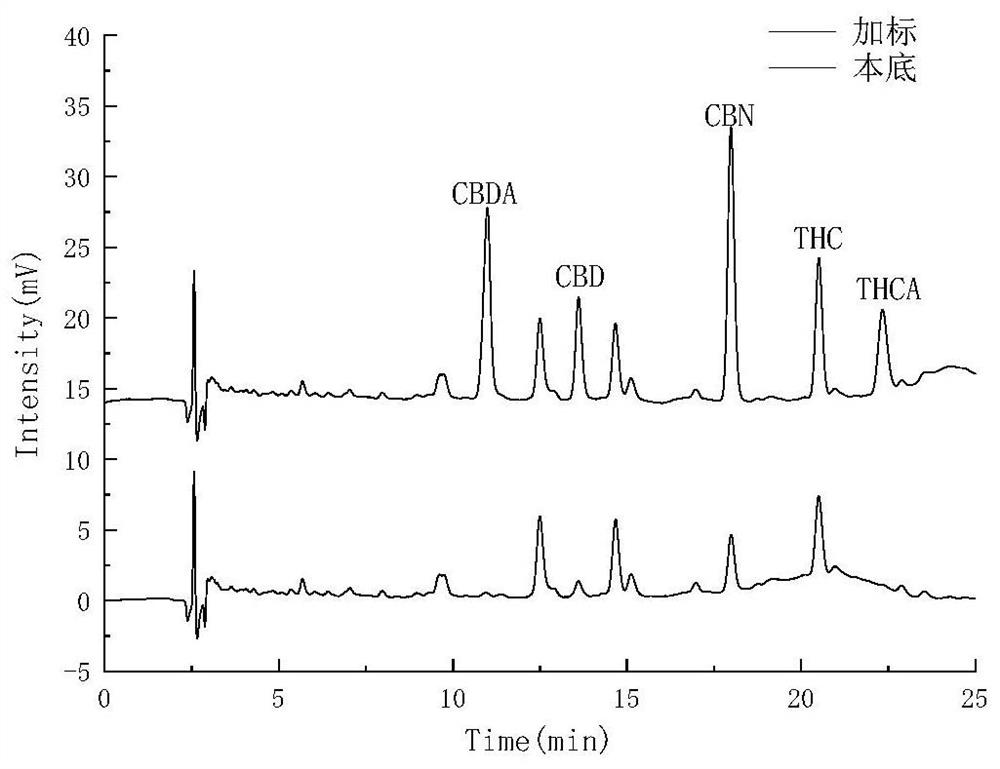
PendingCN112730697AReduce difficultyHigh detection sensitivityComponent separationCannabielsoinGradient elution
The invention belongs to the field of analytical chemistry, and particularly relates to a method for simultaneously detecting five cannabinol compounds by using a high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). According to the method, a special chromatographic column for cannabinol is adopted, a formic acid aqueous solution is used as a mobile phase A, acetonitrile is used as a mobile phase B for gradient elution, and a sample enters a detector for detection. The cannabinol compound is tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabinol (CBN), cannabidiol (CBD) and delta9-tetrahydrocannabinol (THC). The method is high in detection sensitivity, good in recovery rate and easy to operate.
Owner:CHINA NAT INST OF STANDARDIZATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com