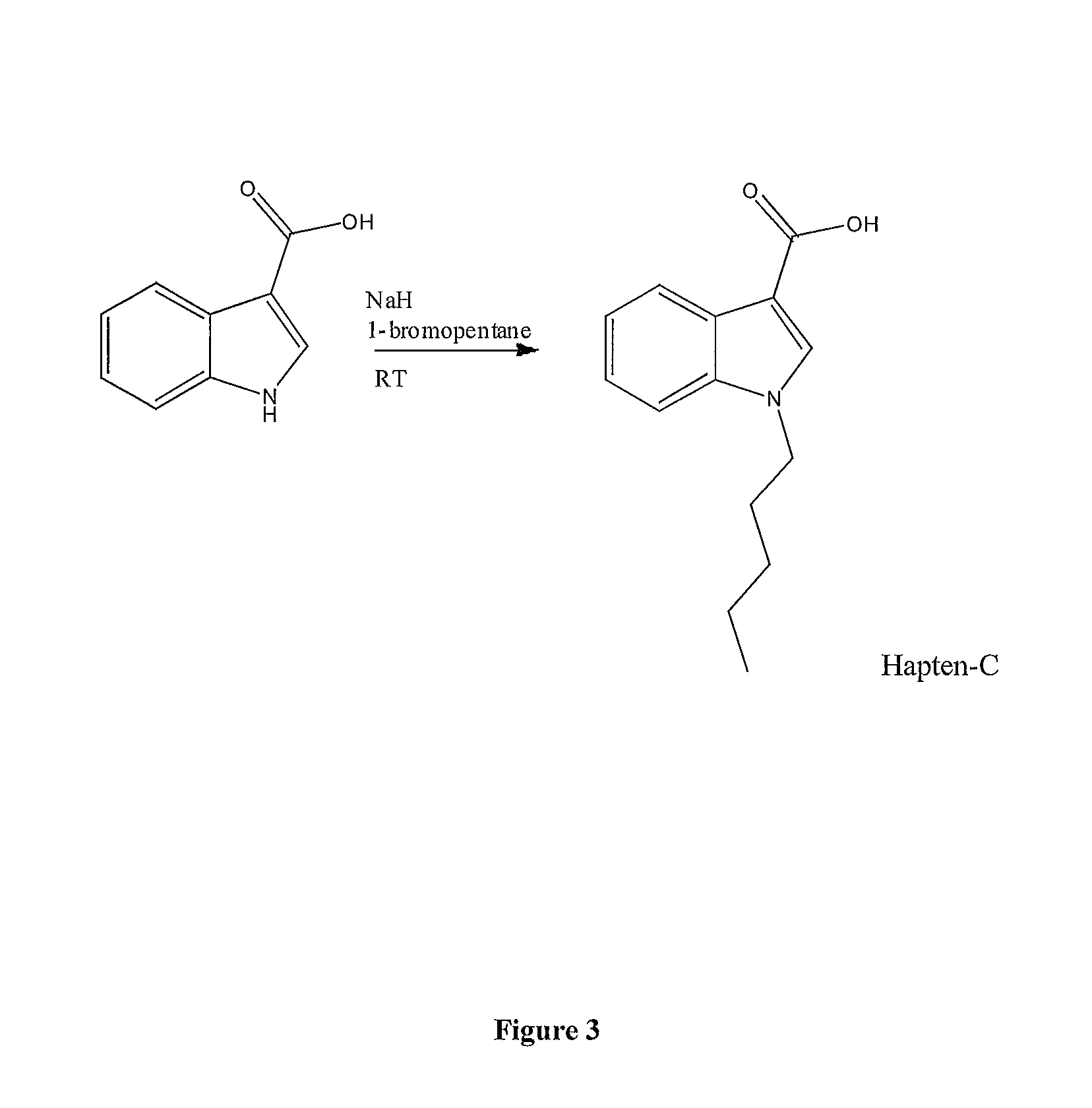
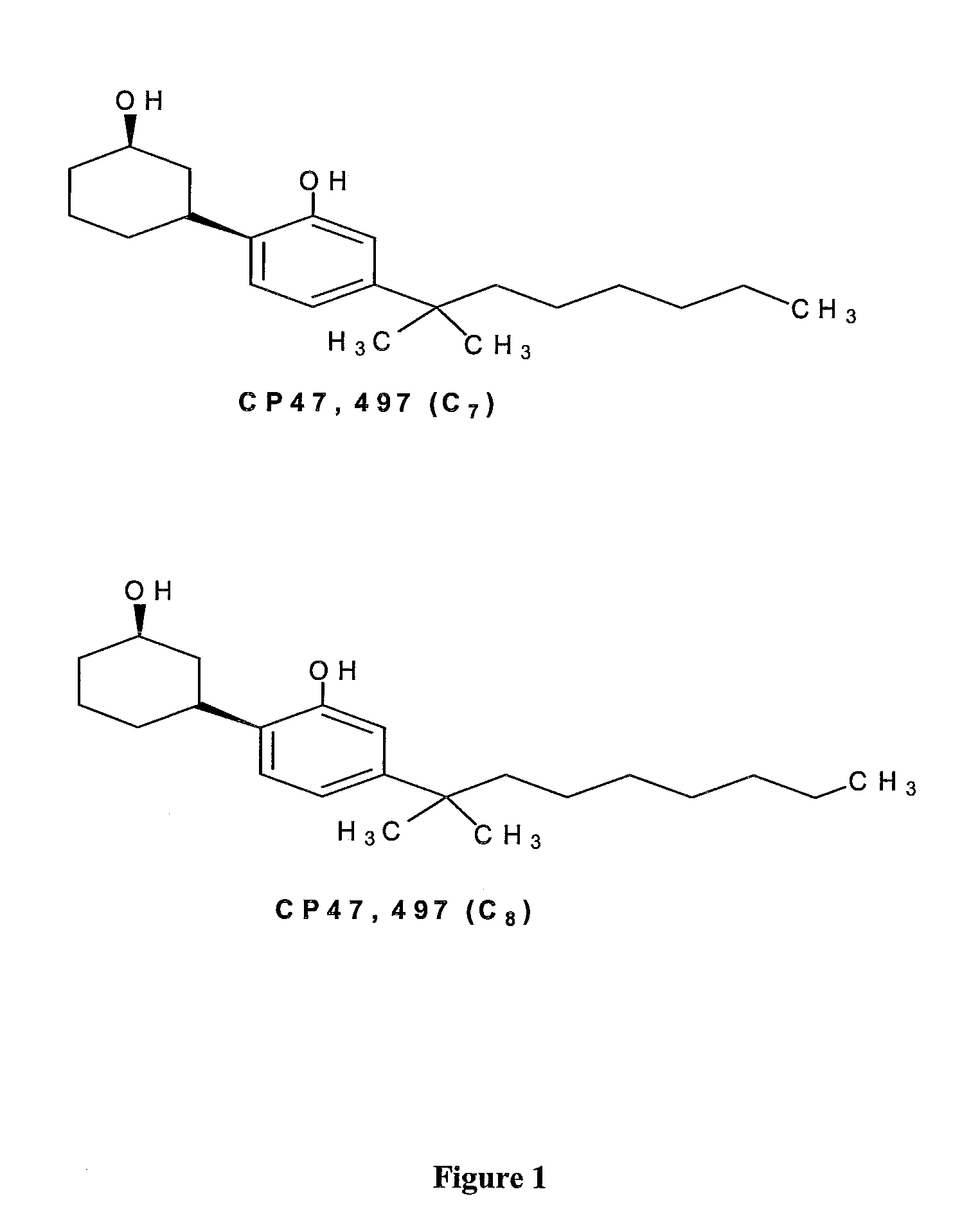
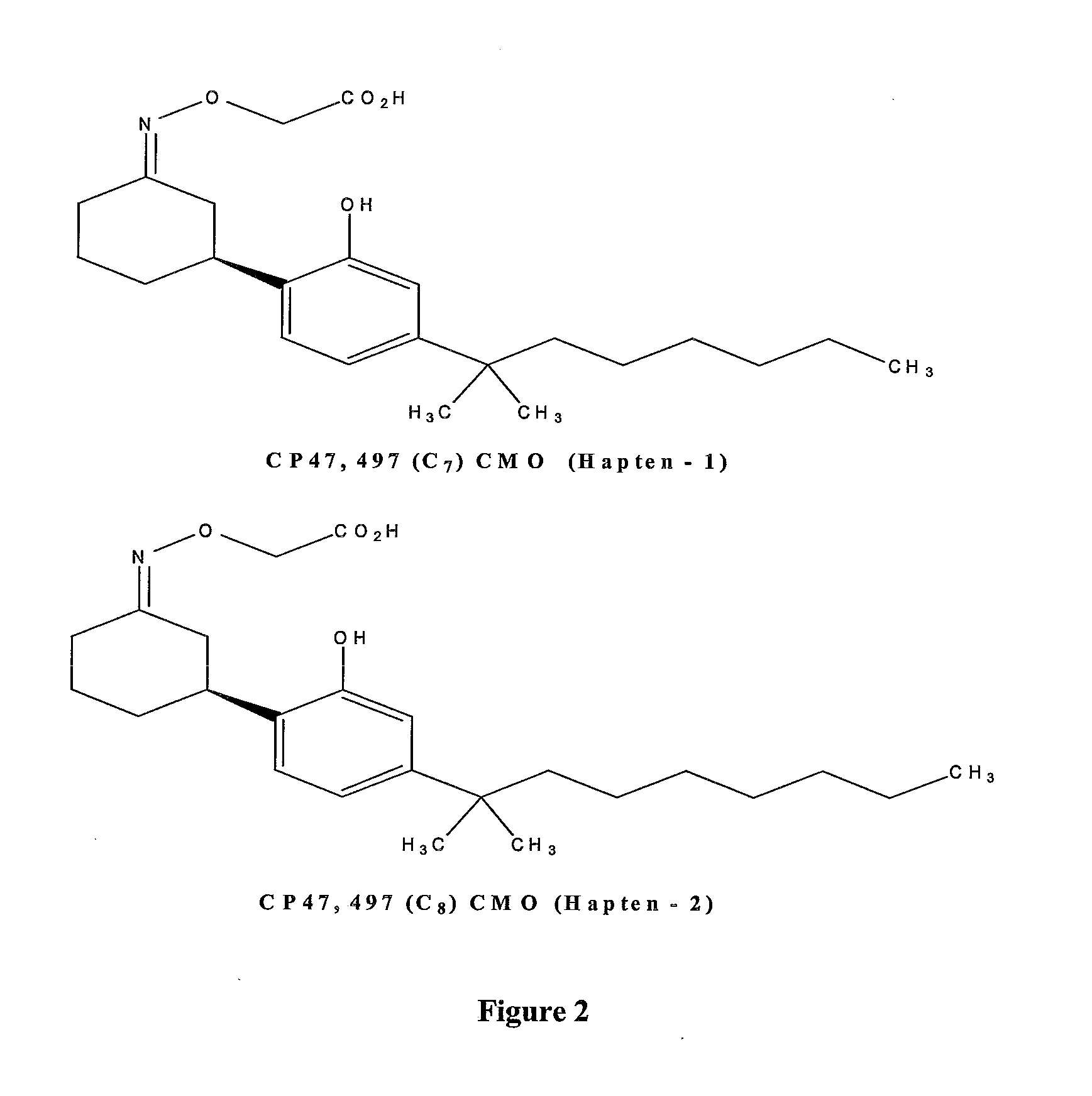
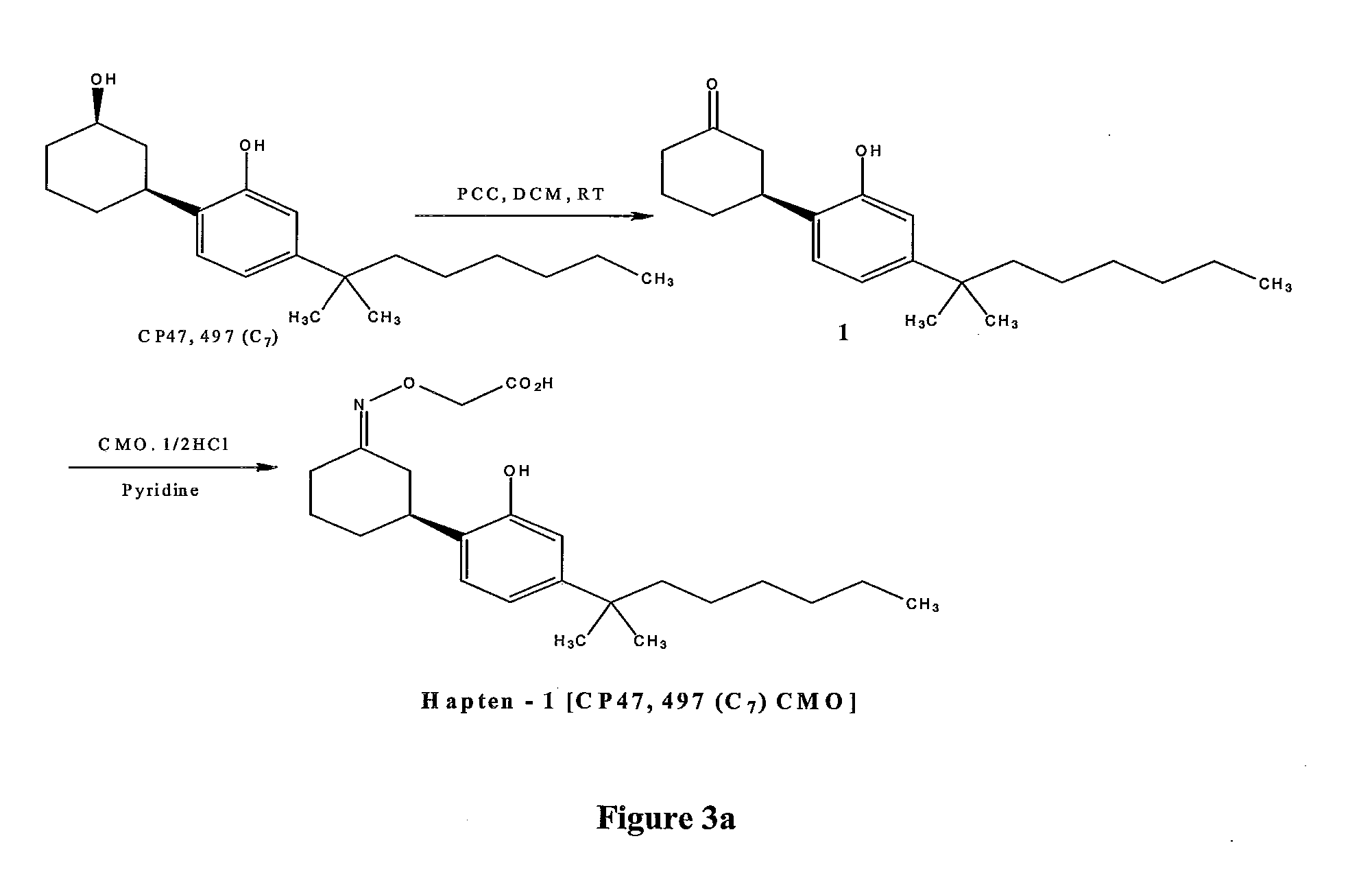
Patents
Literature
52 results about "Synthetic cannabinoids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
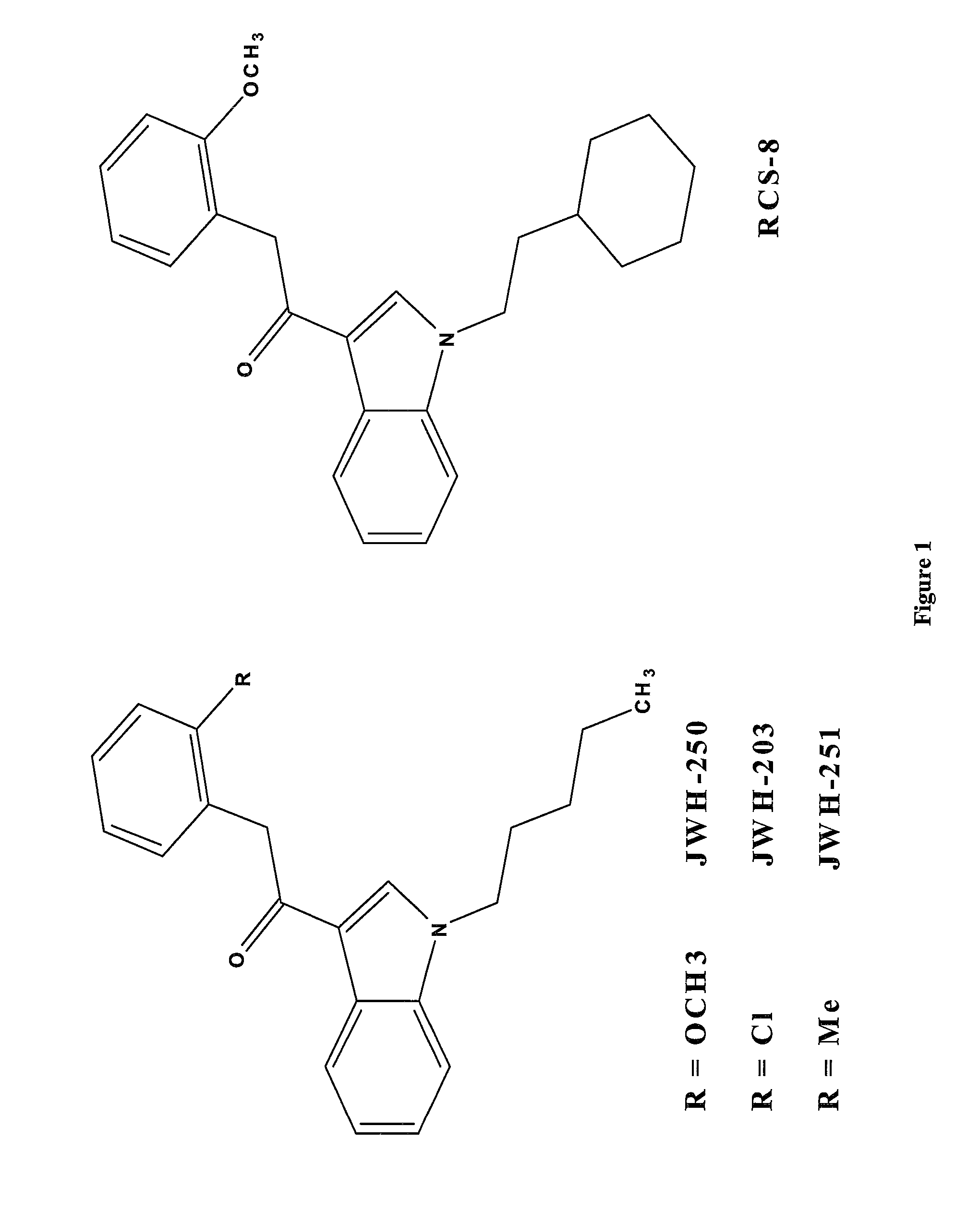
Synthetic cannabinoids are a class of molecules that bind to cannabinoid receptors in the body (the same receptors to which THC and CBD attach, which are cannabinoids in cannabis plants). They are designer drugs that are commonly sprayed onto plant matter and are usually smoked, although they have also been consumed in a concentrated liquid form in the US and UK since 2016. They have been marketed as herbal incense, or “herbal smoking blends” and sold under common names like K2, Spice, and Synthetic Marijuana. They are often labeled “not for human consumption” for liability defense.
Detection of Synthetic Cannabinoids
ActiveUS20130066053A1Immunoglobulins against plantsCarrier-bound/immobilised peptidesSynthetic cannabinoidsAntibody
The invention describes methods and kits for detecting and determining current and future synthetic cannabinoids from the JWH and CP families. Unique antibodies derived from novel immunogens enable said methods and kits.
Owner:NORTHERN BANK LTD
Sustained release cannabinoid formulations
InactiveUS20180085308A1Hydroxy compound active ingredientsGranular deliverySynthetic cannabinoidsPharmaceutical preservatives
The present invention provides modified release pharmaceutical composition comprising one or more natural or synthetic cannabinoids and one or more pharmaceutically acceptable excipients. More specifically, the invention relates to modified release pharmaceutical compositions comprising cannabinoids and a process for preparation thereof. The present invention also provides large scale batches of modified release pharmaceutical composition comprising one or more natural or synthetic cannabinoids and one or more pharmaceutically acceptable excipients.
Owner:CANNTAB THERAPEUTICS LTD
Modified Release Multi-Layer Tablet Cannabinoid Formulations
ActiveUS20180263913A1Nervous disorderHydroxy compound active ingredientsTherapeutic effectDrug release
The present invention provides modified release pharmaceutical compositions, and methods for administering the compositions to a user, including humans. The composition may contain a combination of ingredients in proportions calculated to achieve therapeutic effect, including at least the following ingredients: one or more natural or synthetic cannabinoids, one or more release modifying agent(s), and one or more pharmaceutically acceptable excipient(s). The composition may be in a multi-layered solid dosage form to provide fast, controlled and also sustained release of specific ingredients. More specifically, the invention relates to modified release pharmaceutical compositions comprising cannabinoids and a process for preparation thereof. More specifically, the invention may control drug release in accordance with the therapeutic purpose and pharmacological properties of active substances.
Owner:CANNTAB THERAPEUTICS LTD
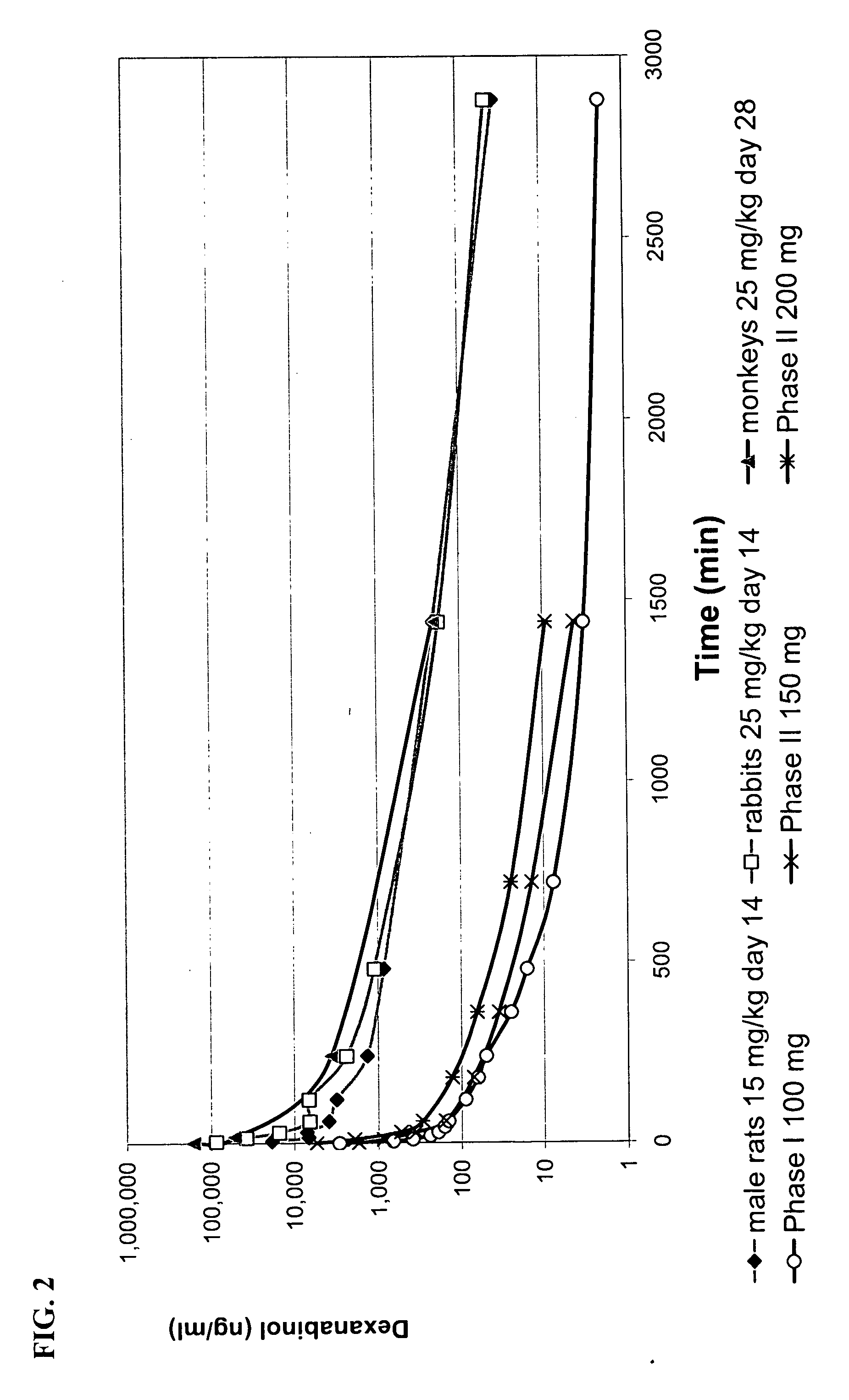
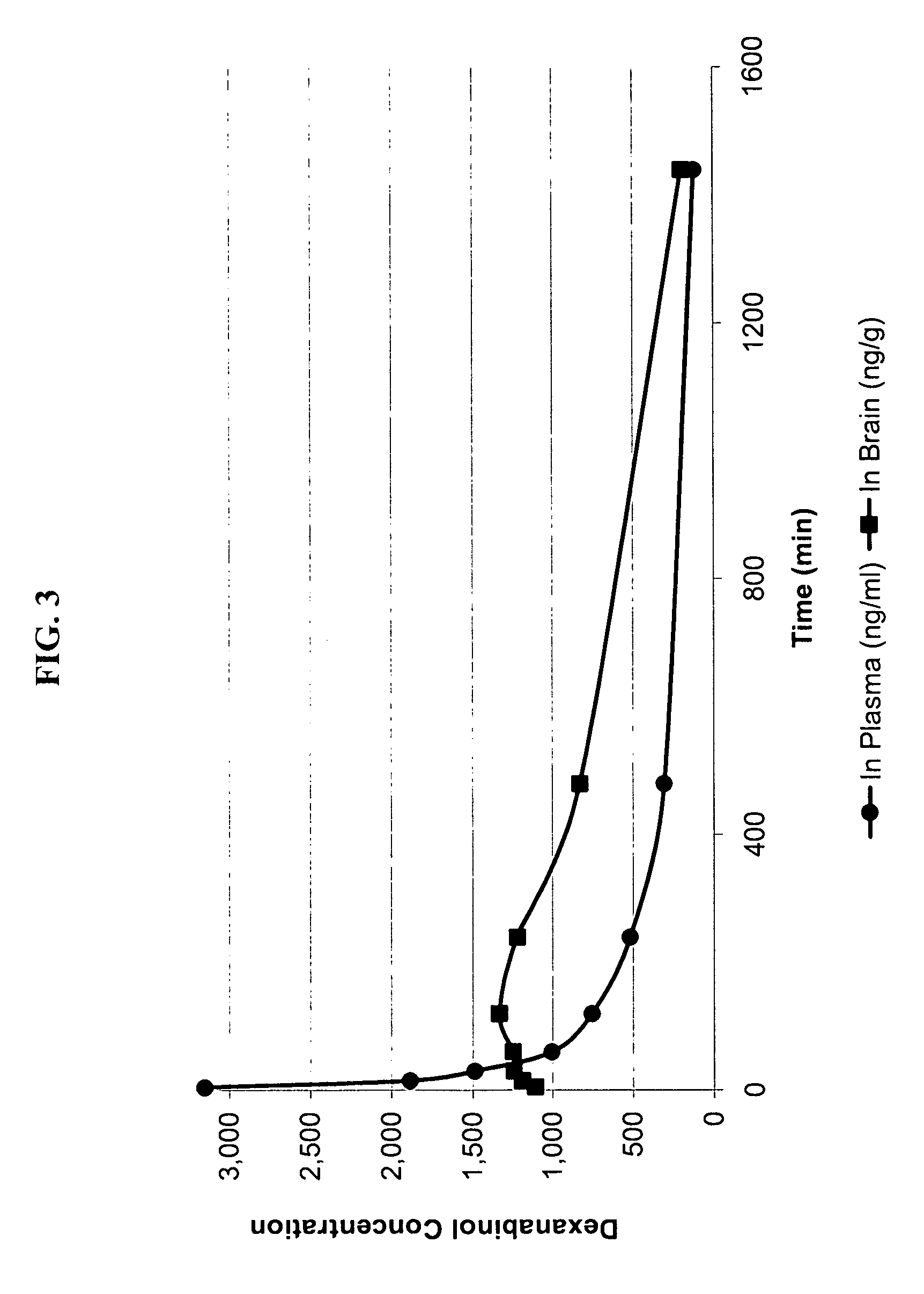
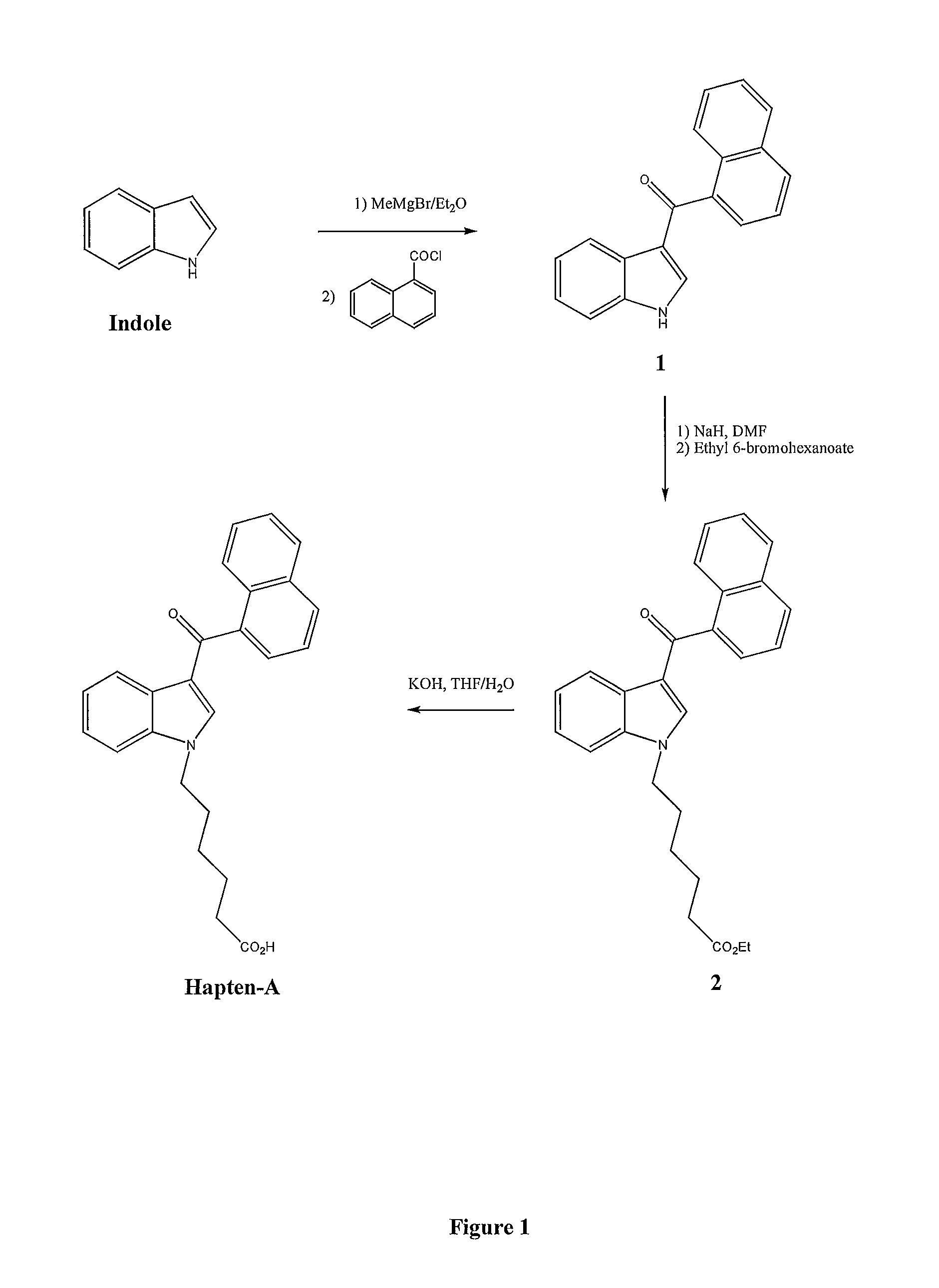
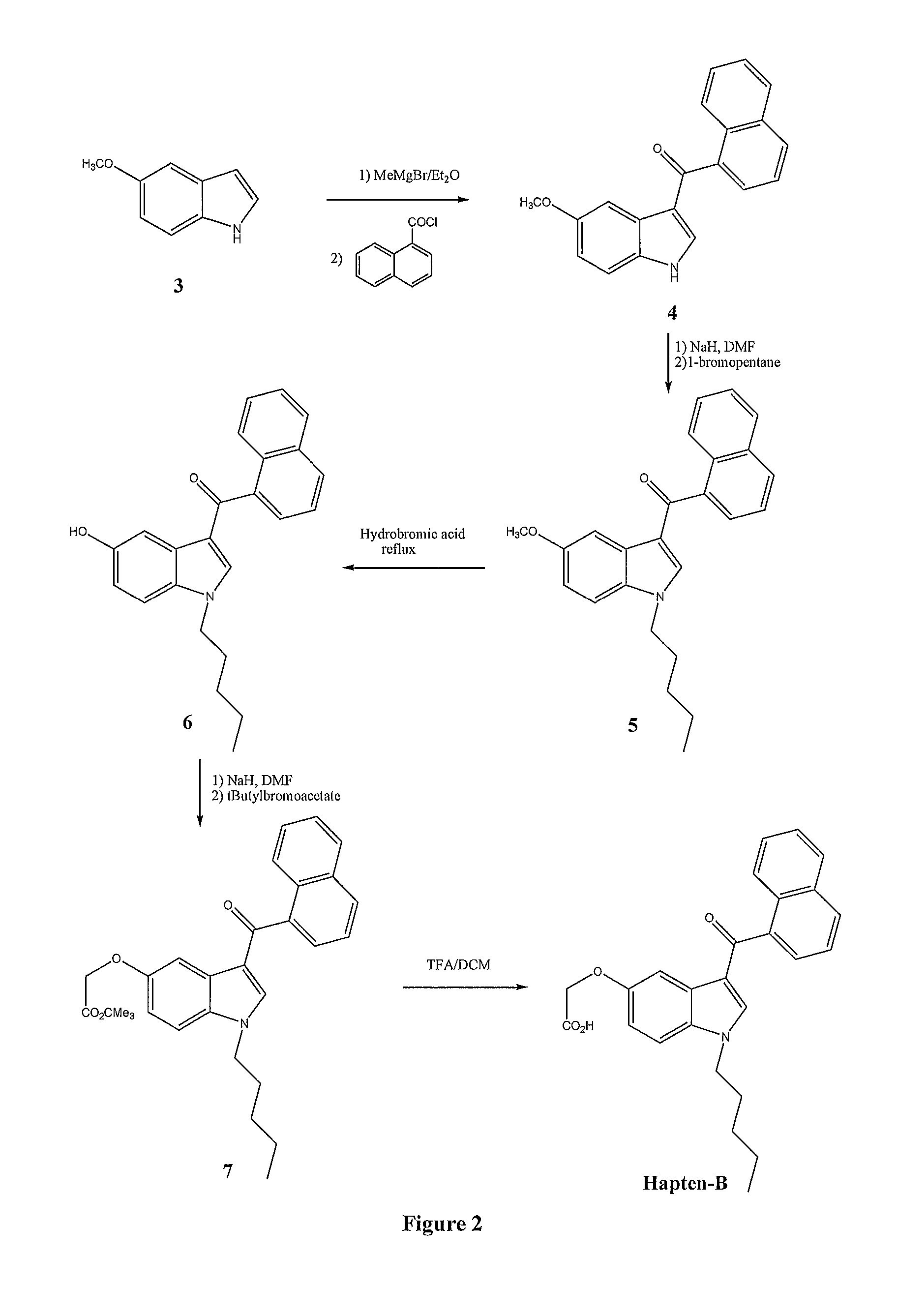
High enantiomeric purity dexanabinol for pharmaceutical compositions
The present invention relates to a synthetic cannabinoid, dexanabinol, of enantiomeric purity in excess of 99.90%, or to a pharmaceutically acceptable salt, ester or solvate of said compound. The present invention also relates to pharmaceutical grade compositions comprising said compound of high enantiomeric purity, and uses thereof for prevention and treatment of neurological disorders, chronic degenerative diseases, CNS poisoning, cognitive impairment, inflammatory diseases or disorders, autoimmune diseases or disorders, pain, emesis, glaucoma and wasting syndromes.
Owner:PHARMOS
Transdermal formulations of synthetic cannabinoids and NANO colloidal silica
InactiveUS20100184848A1Eliminate side effectsPrecise positioningBiocideNervous disorderColloidal silicaNeuropathic pain
The present invention relates to pharmaceutical compositions comprising cannabinoids or mimics thereof. In one aspect, the invention provides transdermal formulations comprising cannabinoids or mimics thereof. In another aspect, the invention provides topical formulations comprising cannabinoids or mimics thereof. In one embodiment the formulations and compositions of the invention comprise nano colloidal silica. The formulations of the invention can be used in the therapeutic treatment of many conditions, including wherein the cannabinoids or mimics thereof are known to reduce the excessive neuronal firing characteristic of neuropathic pain. Muscle spasticity is also benefited by these formulations.
Owner:WINE WILLIAM ABRAHAM +1
Use of cannabis to treat fibromyalgia, methods and compositions thereof
InactiveUS20180116998A1Reduce riskAvoid seizuresNervous disorderHydroxy compound active ingredientsCannabisSynthetic cannabinoids
The present invention discloses a composition comprising cannabis natural extract, or synthetic cannabinoids, for use in treating a subject suffering from fibromyalgia. The present invention further discloses methods and uses of the aforementioned composition.
Owner:ONE WORLD CANNABIS LTD
Sustained Release Cannabinoid Formulations
The present invention provides modified release pharmaceutical composition comprising one or more natural or synthetic cannabinoids and one or more pharmaceutically acceptable excipients. More specifically, the invention relates to modified release pharmaceutical compositions comprising cannabinoids and a process for preparation thereof. The present invention also provides large scale batches of modified release pharmaceutical composition comprising one or more natural or synthetic cannabinoids and one or more pharmaceutically acceptable excipients.
Owner:CANNTAB THERAPEUTICS LTD
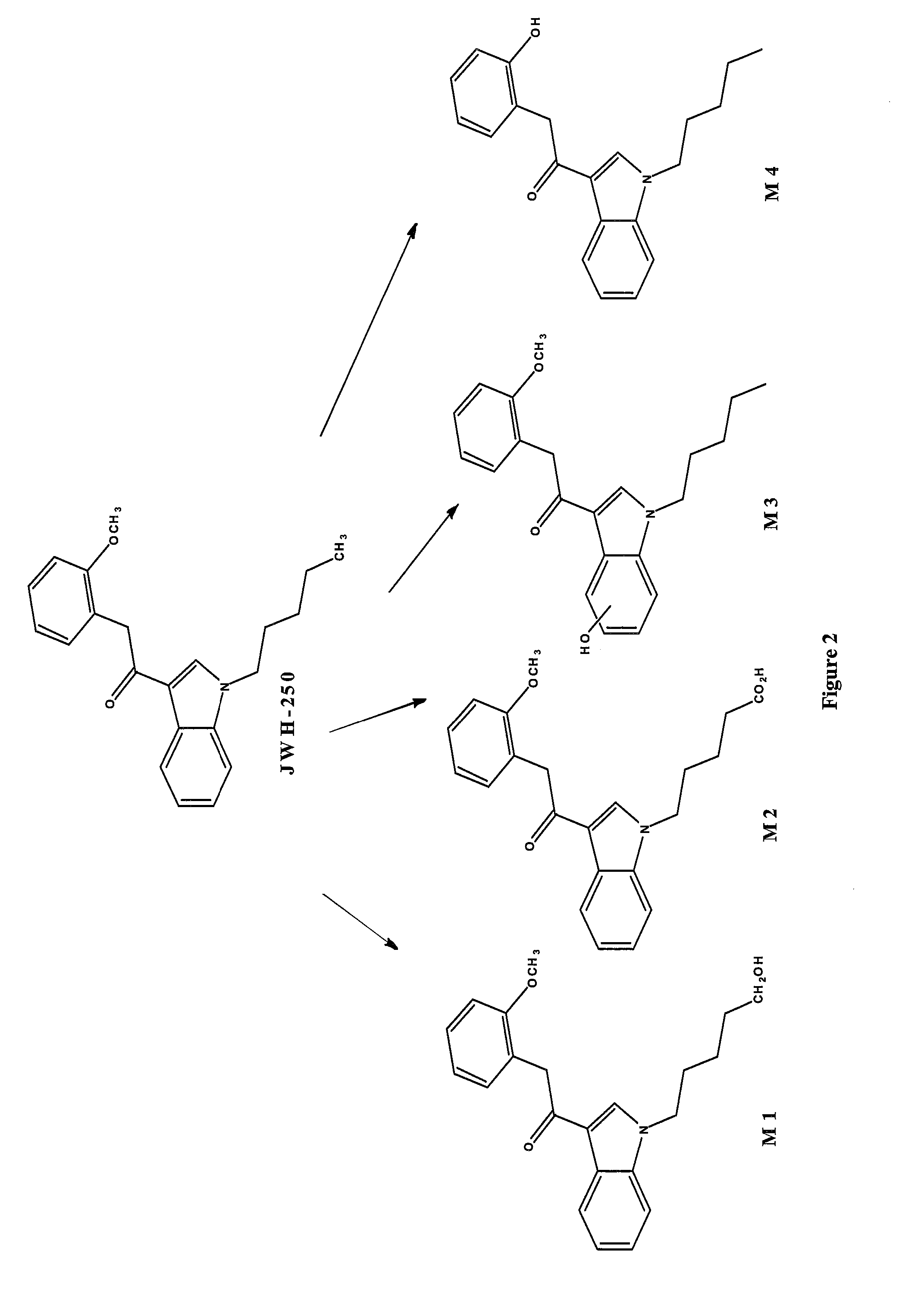
Detection of synthetic cannabinoids
The invention describes methods and kits for detecting and determining current and future synthetic cannabinoids from the JWH and CP families. Unique antibodies derived from novel immunogens enable said methods and kits.
Owner:NORTHERN BANK LTD
Detection of Synthetic Cannabinoids
ActiveUS20130065323A1Immunoglobulins against plantsCarrier-bound/immobilised peptidesSynthetic cannabinoidsAnabasine
The invention describes methods and kits for detecting and determining current and future synthetic cannabinoids from the CP family. Unique antibodies derived from novel immunogens enable said methods and kits.
Owner:NORTHERN BANK LTD
Cannabinoid composition and products including α-tocopherol
InactiveUS10238745B2Improve production stabilityImprove biological activityHydrocarbon active ingredientsHydroxy compound active ingredientsYeastEndogenous cannabinoid
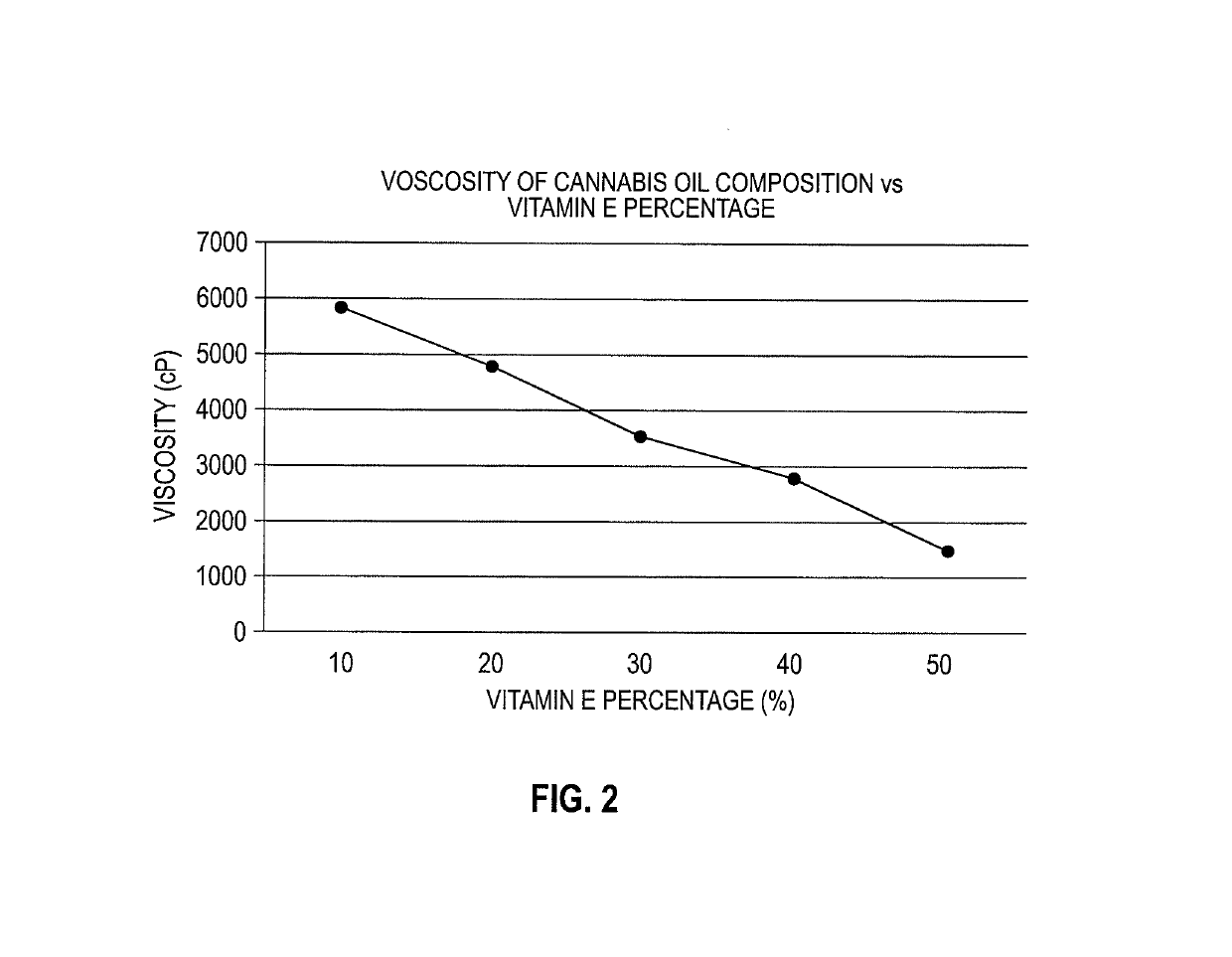
A cannabinoid composition having an excipient including α-tocopherol and at least one isolated cannabinoid having a purity of greater than 80%. The α-tocopherol inhibits oxidation, regulates viscosity of the composition, and improves bioavailability of the cannabinoid. The composition includes a carrier oil mixed with the excipient and cannabinoid. In one embodiment, the at least one cannabinoid is an isolated cannabinoid having a greater than 80% purity, and preferably greater than 90% purity. The at least one cannabinoid is selected from the group consisting of phytocannabinoids, endocannabinoids, synthetic cannabinoids, and combinations thereof. The cannabinoid may be derived from plants other than cannabis sativa, or synthesized from fungi, bacteria, yeast or other synthetic method. Preferably the α-tocopherol comprises at least 10% w / w of the composition.
Owner:CONSTANCE THERAPEUTICS INC
Apparatus, methods and composition for synthesis of cannabinoid compounds
ActiveUS10336978B2Bioreactor/fermenter combinationsBiological substance pretreatmentsChemical compoundCombinatorial chemistry
The disclosure provides systems and methods for producing a cannabinoid product, which comprises contacting a cannabinoid precursor in a first phase with a cannabinoid synthase in a second phase, wherein the first phase and the second phase are substantially immiscible or immiscible. The disclosure also provides a composition comprising the cannabinoid precursor in a first phase and a cannabinoid synthase in a second phase, wherein the first phase and the second phase are substantially immiscible or immiscible.
Owner:TEEWINOT LIFE SCI CORP
Apparatus, methods and composition for synthesis of cannabinoid compounds
ActiveUS20180346866A1Bioreactor/fermenter combinationsBiological substance pretreatmentsChemical compoundCannabinoidergic
The disclosure provides systems and methods for producing a cannabinoid product, which comprises contacting a cannabinoid precursor in a first phase with a cannabinoid synthase in a second phase, wherein the first phase and the second phase are substantially immiscible or immiscible. The disclosure also provides a composition comprising the cannabinoid precursor in a first phase and a cannabinoid synthase in a second phase, wherein the first phase and the second phase are substantially immiscible or immiscible.
Owner:TEEWINOT LIFE SCI CORP
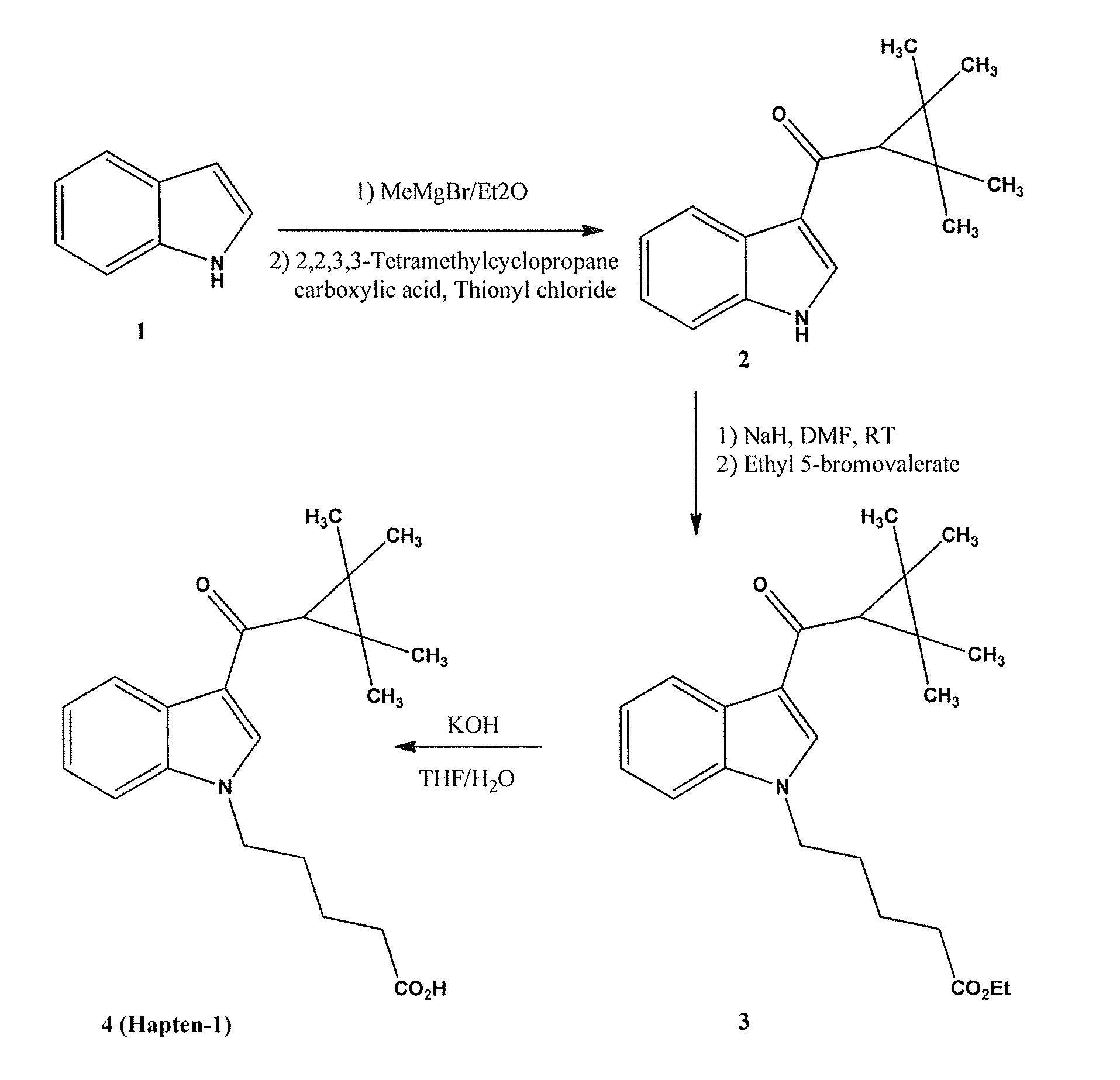
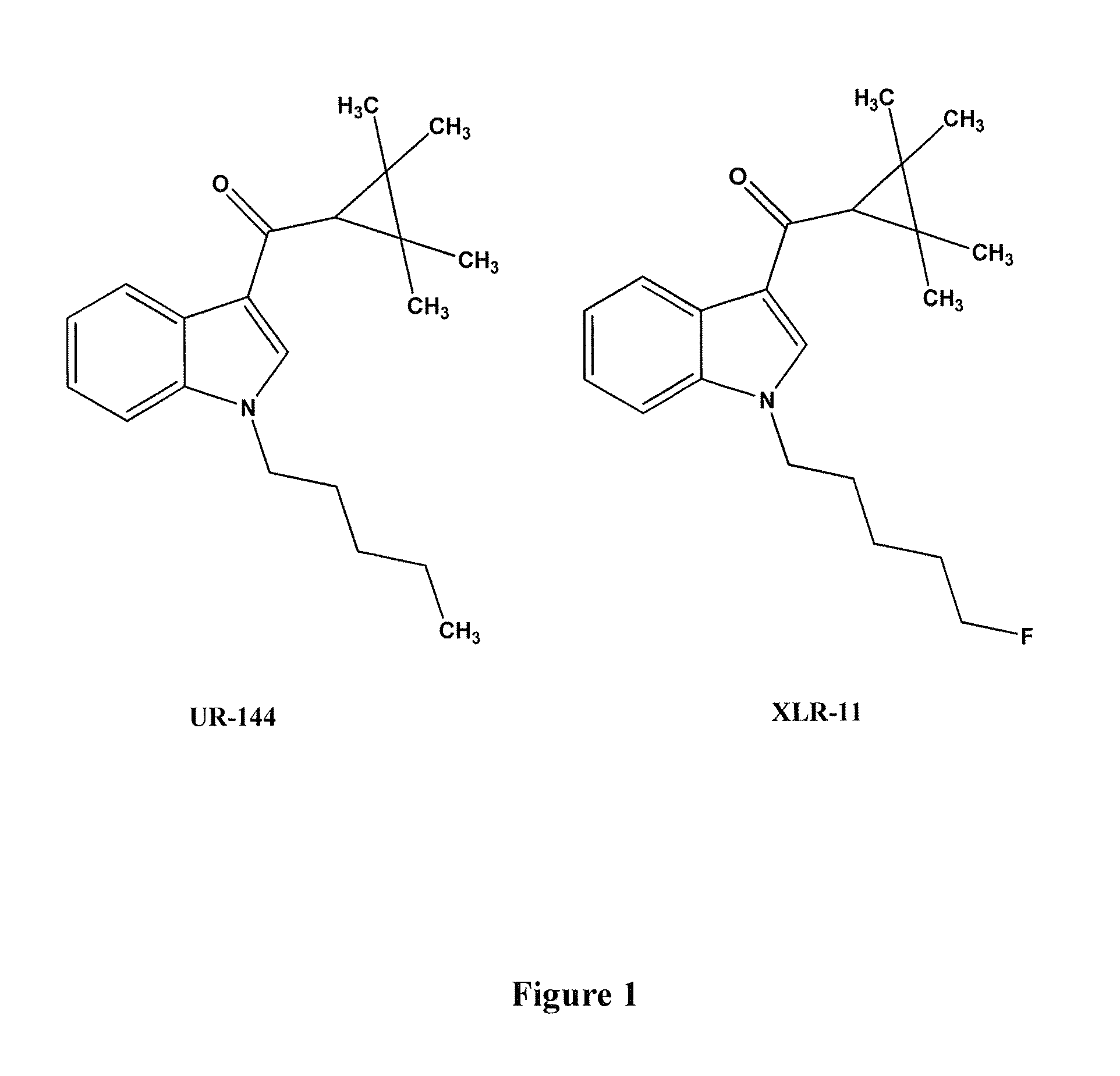
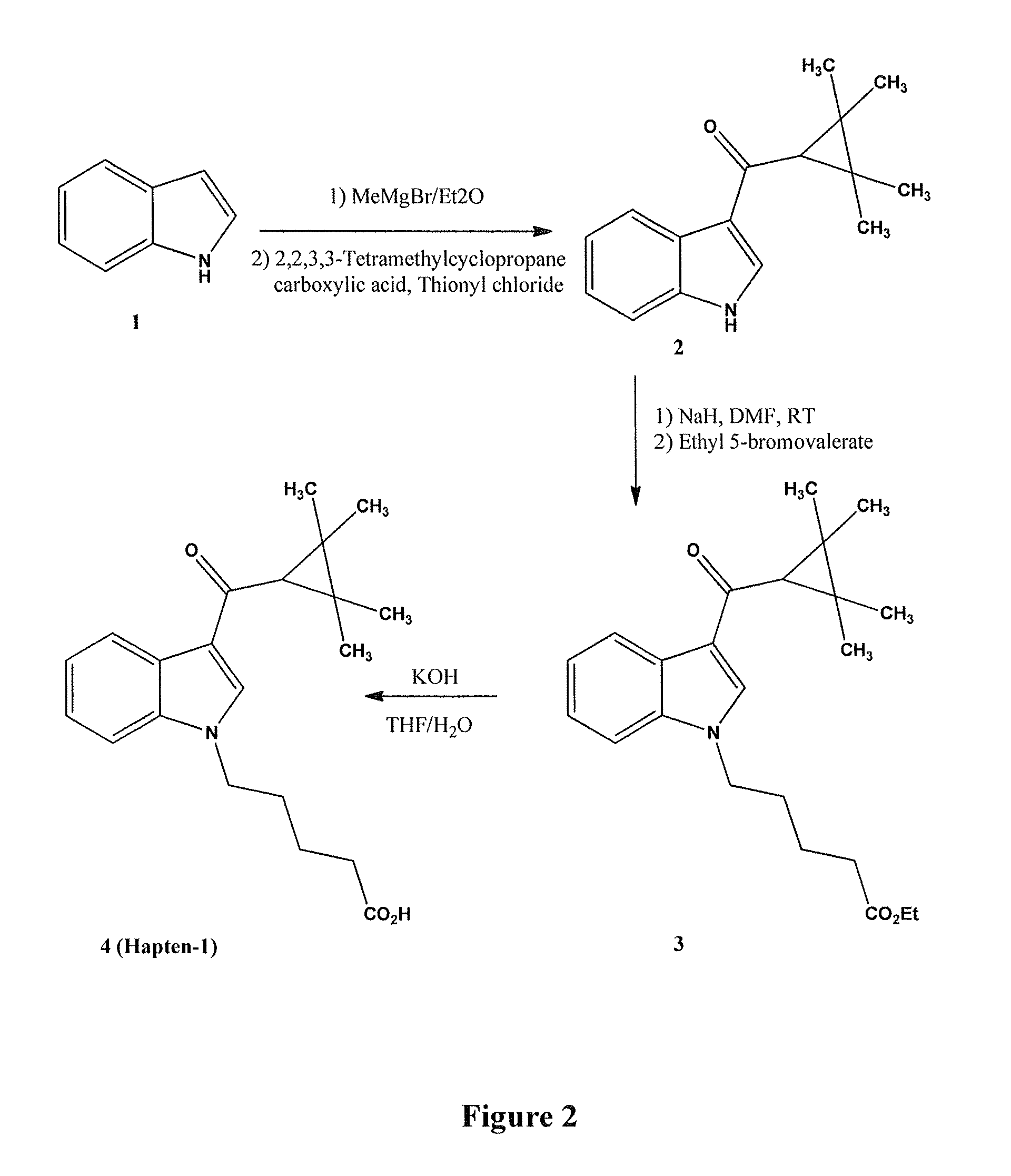
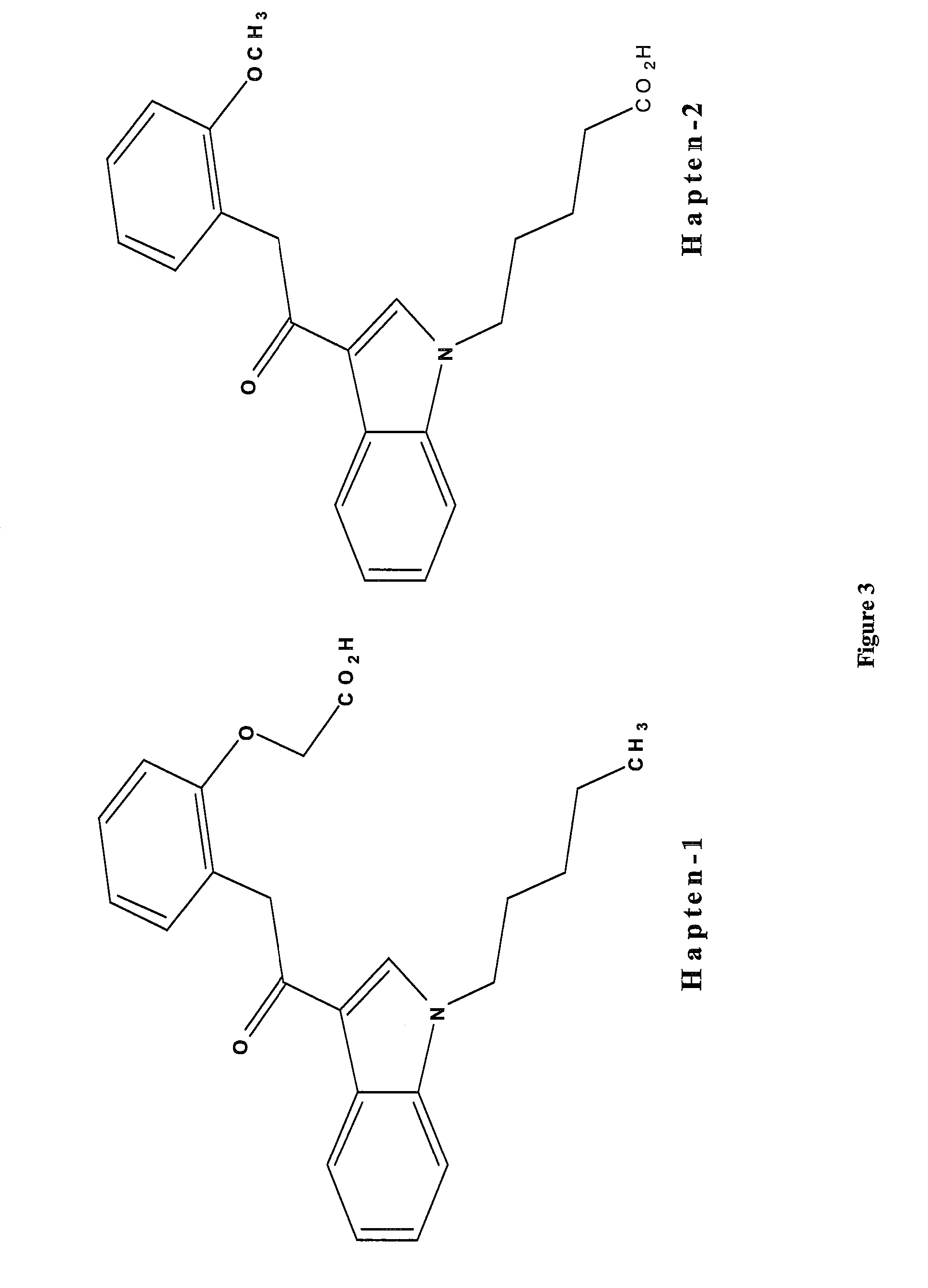
Detection of indazole synthetic cannabinoids
Components for enabling immunodection of indazole synthetic cannabinoids are described including immunogens, haptens, antibodies, methods and kits.
Owner:NORTHERN BANK LTD
Method and apparatus for real-time detection of human cannabinoid intoxication
A method for quantifying the intoxicating affect of natural and synthetic Cannabinoids on Humans. The method includes the capacity to measure a human's psychoactive intoxication as a function of Cannabinoid-Mediated Depolarization-Induced Suppression of Inhibition (C-DISI) relative to the Cannabinoid Receptors found in the Human brain. A Method incorporating the ability to also quantify the affects of Cannabinoids on the various Cannabinoid Receptors found in the Human body. Neurons are used as transistors in a solid state electronic configuration with an apparatus to measure the toxicity of a given aqueous solution comprised of one or more Cannabinoid analytes. Said method incorporates a novel recycling process affiliated with analyte acquisition & testing.
Owner:PEREZ JOHN SCOTT
Topical formulations of cannabinoids and use thereof in the treatment of pain
PendingUS20200289458A1Increase mechanical thresholdReduce and eliminate hyperalgesiaNervous disorderHydrocarbon active ingredientsCannabielsoinPharmaceutical Substances
Pharmaceutical compositions comprising one or more cannabinoids and a pharmaceutically acceptable carrier are disclosed. The compositions are in a form suitable for topical administration, and are useful in the treatment of pain. The cannabinoids suitable for use include cannabidiols, cannabinols, and various other naturally occurring and synthetic cannabinoids. The compositions may also further include anti-inflammatory agents, steroids, or terpenoids. In preferred embodiments, the cannabinoids incorporated in the compositions are cannabidiol (CBD) and cannabinol (CBN) while the carriers are selected from the group consisting of Labrasol™, Transcutol™, lecithin, lysolecithin, isopropyl palmitate, and isopropyl myristate.
Owner:INMED PHARMA INC
Sustained release cannabinoid formulations
InactiveUS20180250262A1Hydroxy compound active ingredientsDrageesDelta-9-tetrahydrocannabinolSynthetic cannabinoids
The present invention provides modified release pharmaceutical composition comprising one or more natural or synthetic cannabinoids and one or more pharmaceutically acceptable excipients. More specifically, the invention relates to modified release pharmaceutical compositions comprising delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), and a process for preparation thereof. The present invention also provides large scale batches of modified release pharmaceutical composition comprising one or more natural or synthetic cannabinoids and one or more pharmaceutically acceptable excipients.
Owner:CANNTAB THERAPEUTICS LTD
Medicinal compounds and nutritional supplements
InactiveUS20190336472A1Promote absorptionAct quicklyNervous disorderDispersion deliveryCannabisMedicine
Synthetic cannabinoid medicinal compounds or nutritional supplements in various carrier combinations are described. The carriers can include N-acylated fatty amino acids, penetration enhancers, and / or various other beneficial carriers. The synthetic cannabinoid composition / carrier combinations can create administration benefits.
Owner:RECEPTOR HLDG INC
Detection of synthetic cannabinoids
ActiveUS9434687B2Immunoglobulins against plantsCarrier-bound/immobilised peptidesSynthetic cannabinoidsAntibody
The invention describes methods and kits for detecting and determining current and future synthetic cannabinoids from the JWH and CP families. Unique antibodies derived from novel immunogens enable said methods and kits.
Owner:NORTHERN BANK LTD
Rapid onset and extended action plant-based and synthetic cannabinoid formulations
InactiveUS20200254041A1Improve practicalityRapid onsetNervous disorderDispersion deliveryNutritionSynthetic cannabinoids
Rapid onset and extended action plant-based medicinal compounds or nutritional supplements and synthetic cannabinoid formulations are described. Rapid onset is provided by N-acylated fatty amino acids and / or penetration enhancers. Extended action can be provided by one or more sustained-release systems.
Owner:RECEPTOR HLDG INC
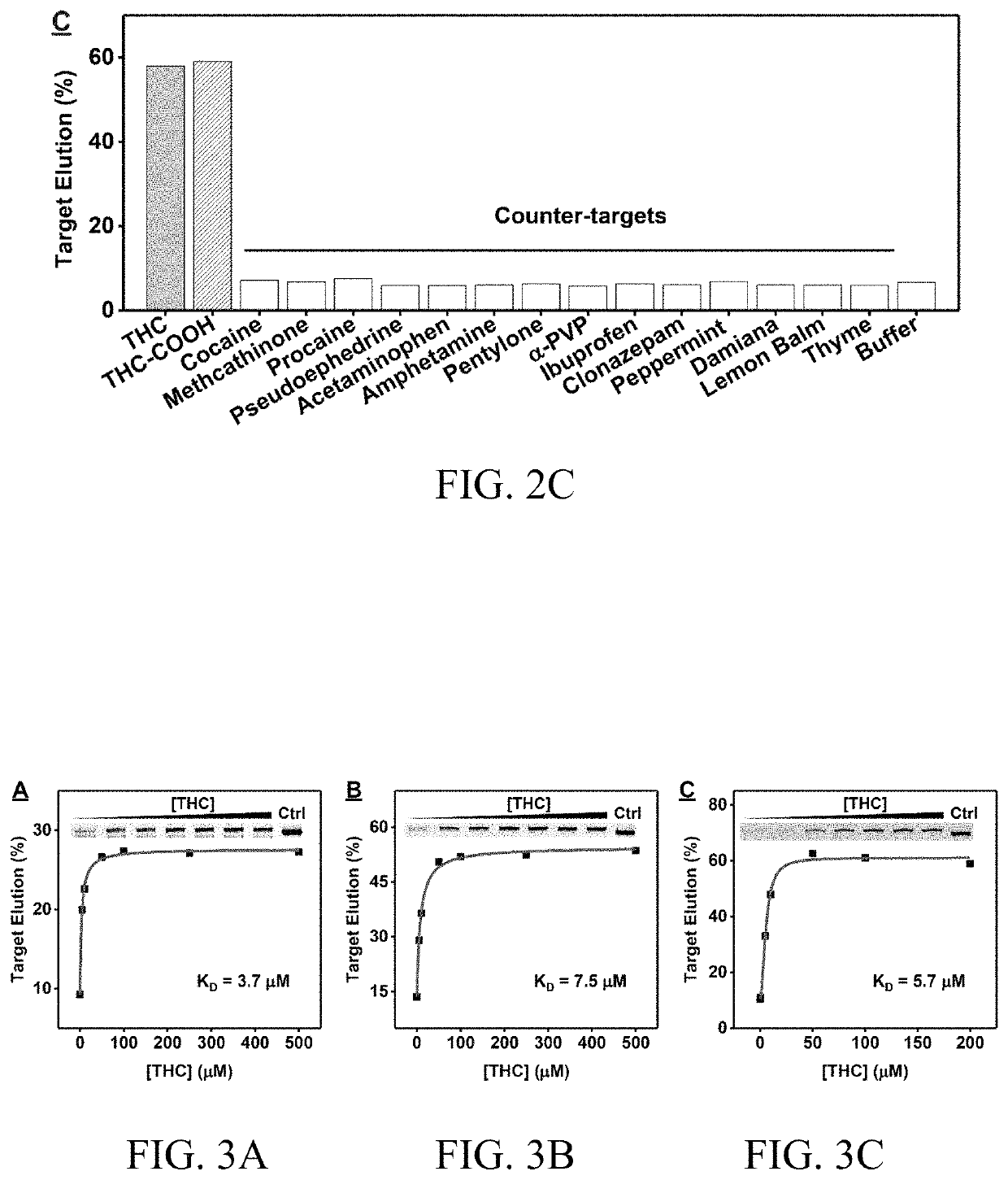
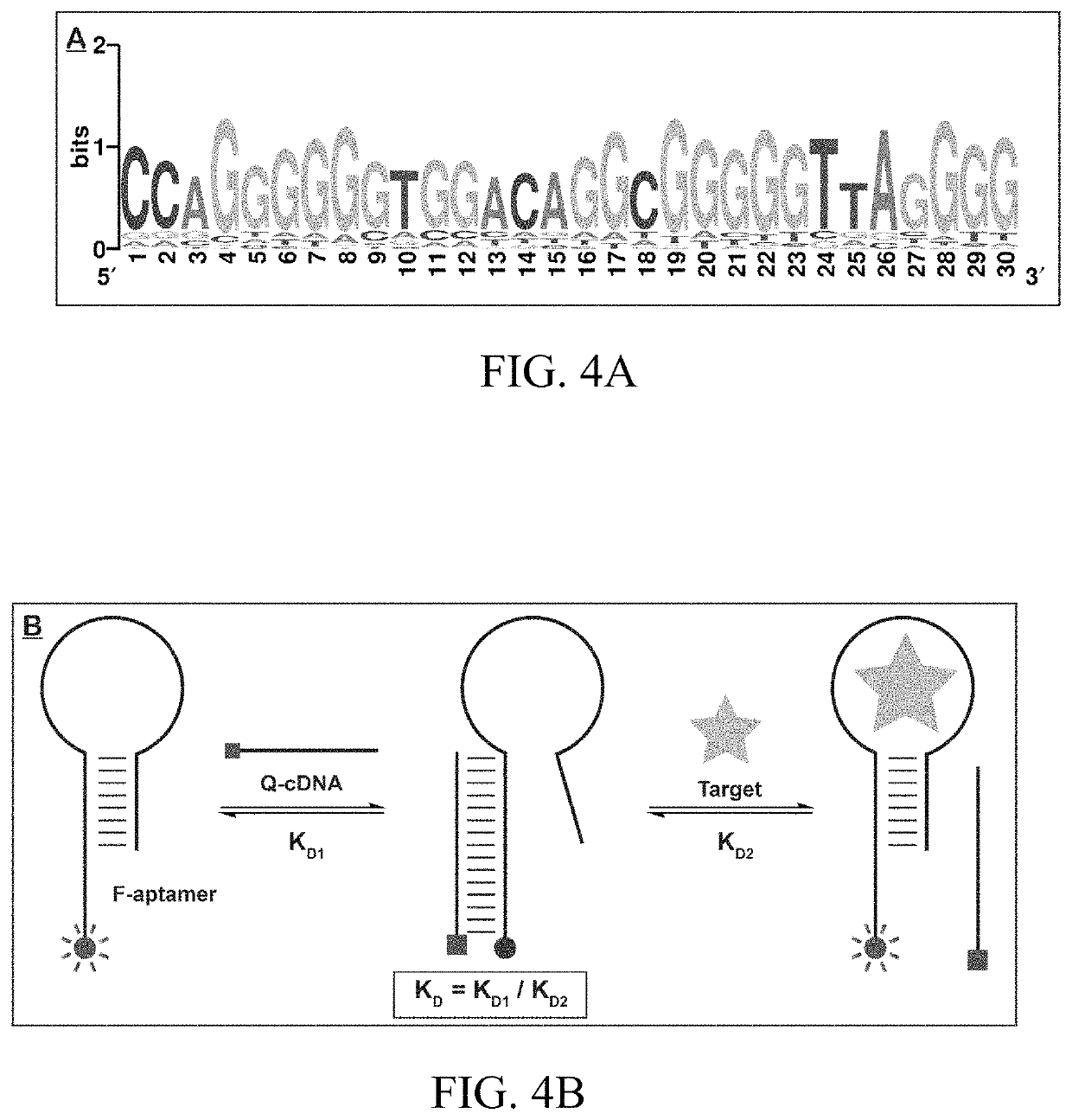
Aptamers that bind to natural and synthetic cannabinoids
ActiveUS10907163B1ConfidenceBiological testingFluorescence/phosphorescenceAptamerSynthetic cannabinoids
The subject invention provides materials and methods for single-step detection of small molecules, e.g., natural and synthetic cannabinoids, in a sample. The subjection invention provides nucleic acids materials, e.g., aptamers (nucleic acid oligonucleotides) that can bind to natural and / or synthetic cannabinoids. The method for detecting a natural or synthetic cannabinoid in a sample comprises contacting the sample with an aptamer-based sensor selective for the natural or synthetic cannabinoid, and sensitively and rapidly detecting the natural or synthetic cannabinoid in the sample. The aptamer-based sensor comprises aptamers that can specifically binds to natural and / or synthetic cannabinoids with nanomolar dissociation constant.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Detection method of indazole derivatives
InactiveCN110161141AMeet the needs of handlingFast wayComponent separationGas chromatography–mass spectrometryGas phase
The invention discloses a detection method of indazole derivatives. The detection method comprises a qualitative detection method of gas chromatography-mass spectrometry, a qualitative detection method of gas chromatography and a quantitative detection method of gas chromatography, and is suitable for qualitative and quantitative detection and identification of indazole derivatives in solid test materials for drug cases. According to the invention, a qualitative and quantitative analysis method for the indazolamide synthetic cannabinoid is established by utilizing the commonly used gas chromatography and gas chromatography-mass spectrometry technology, and the method is quick, simple, convenient and easy to operate, is convenient to popularize and apply, can meet the requirements of judicial identification departments on the handling of such cases, and provides a basis for the smooth litigation of such cases.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Immunoassay for cyclopropylindole based synthetic cannabinoids, metabolites and derivatives thereof
Owner:NORTHERN BANK LTD
Detection of synthetic cannabinoids
ActiveUS9441033B2Immunoglobulins against plantsCarrier-bound/immobilised peptidesSynthetic cannabinoidsAntibody
The invention describes methods and kits for detecting and determining current and future synthetic cannabinoids from the JWH and RCS families. Unique antibodies derived from novel immunogens enable said methods and kits.
Owner:NORTHERN BANK LTD
Modified release multi-layer tablet cannabinoid formulations
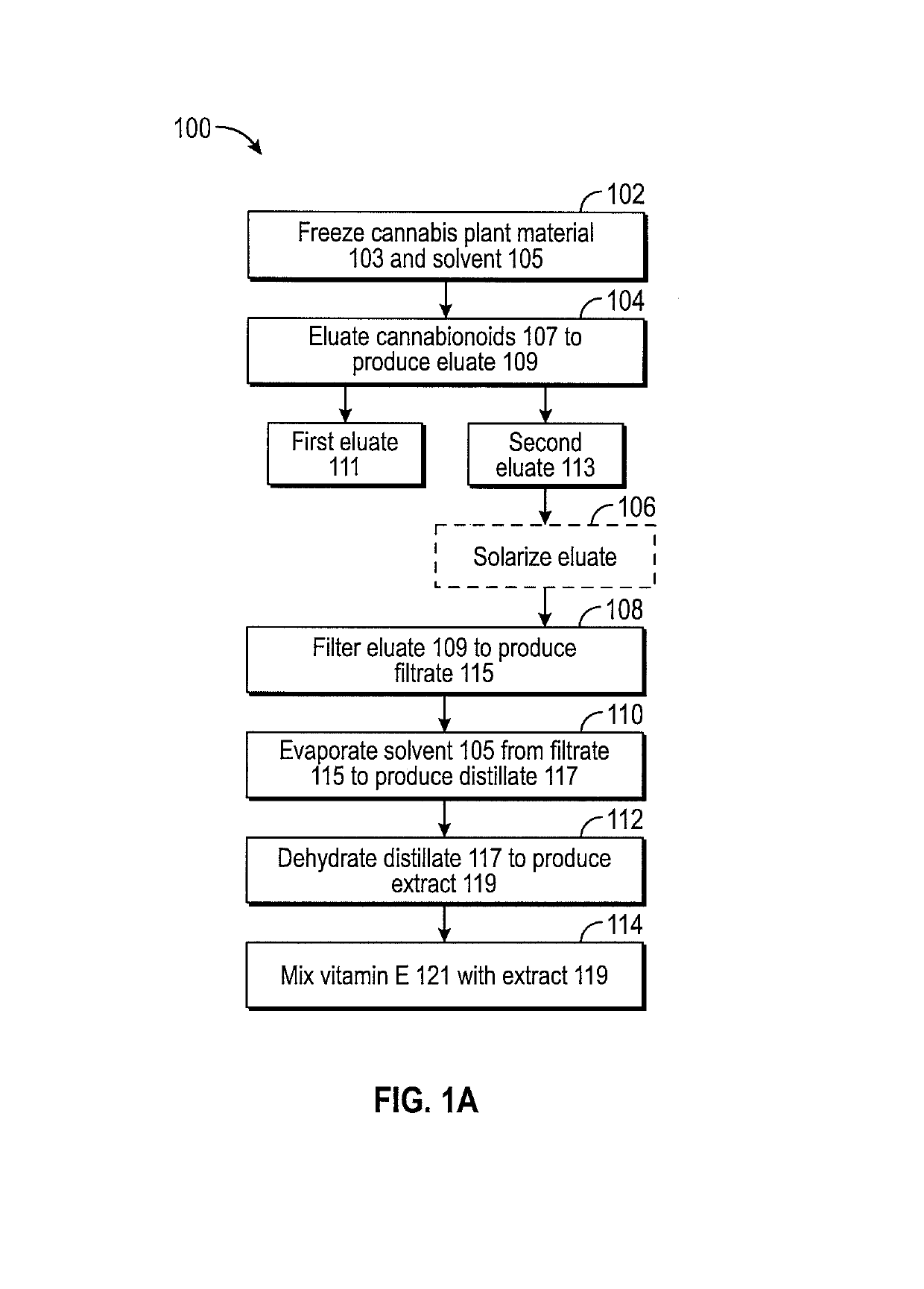
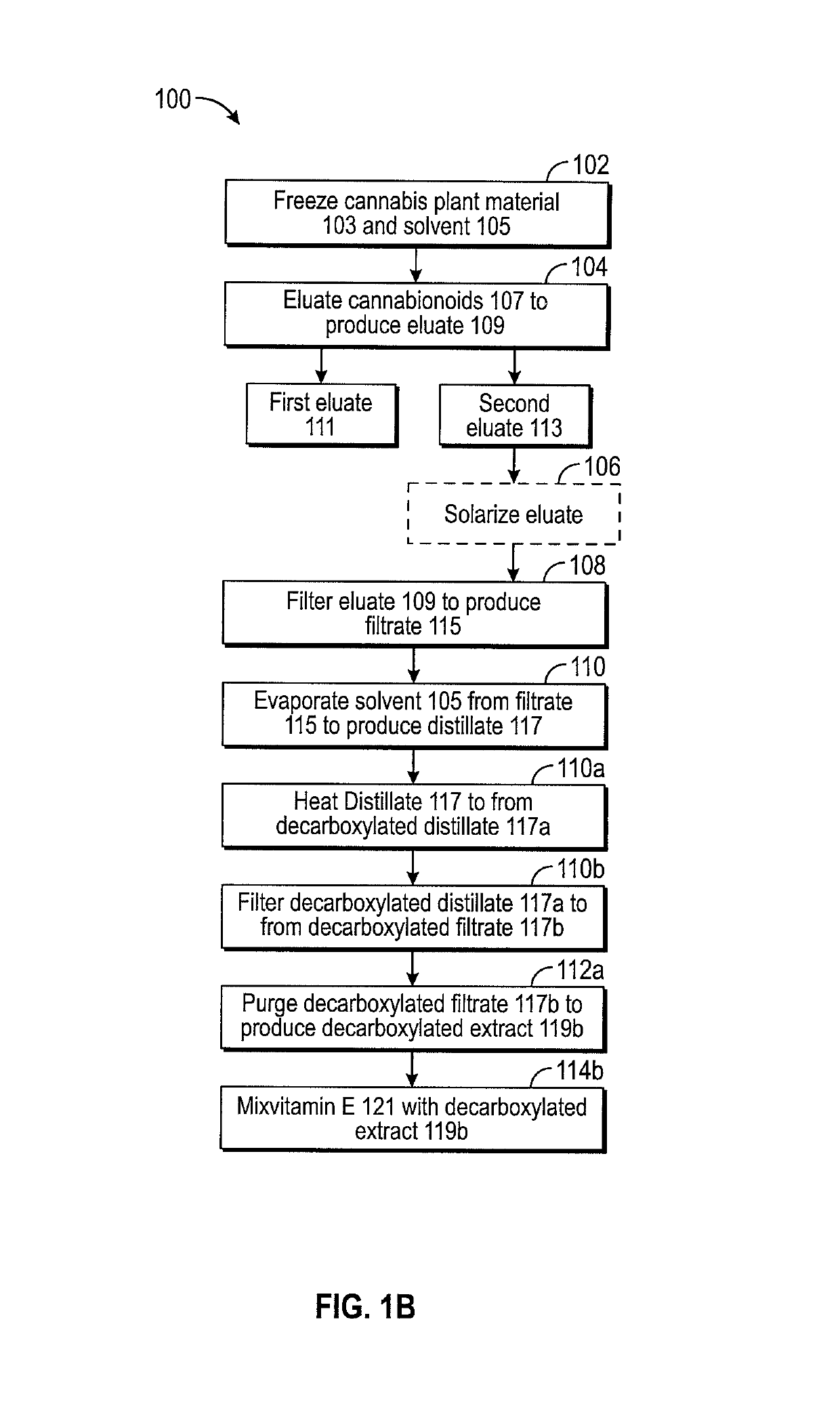
ActiveUS10772837B2Nervous disorderHydroxy compound active ingredientsControlled drugsPharmaceutical drug
The present invention provides modified release pharmaceutical compositions, and methods for administering the compositions to a user, including humans. The composition may contain a combination of ingredients in proportions calculated to achieve therapeutic effect, including at least the following ingredients: one or more natural or synthetic cannabinoids, one or more release modifying agent(s), and one or more pharmaceutically acceptable excipient(s). The composition may be in a multi-layered solid dosage form to provide fast, controlled and also sustained release of specific ingredients. More specifically, the invention relates to modified release pharmaceutical compositions comprising cannabinoids and a process for preparation thereof. More specifically, the invention may control drug release in accordance with the therapeutic purpose and pharmacological properties of active substances.
Owner:CANNTAB THERAPEUTICS LTD
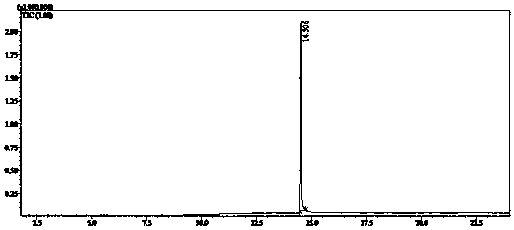
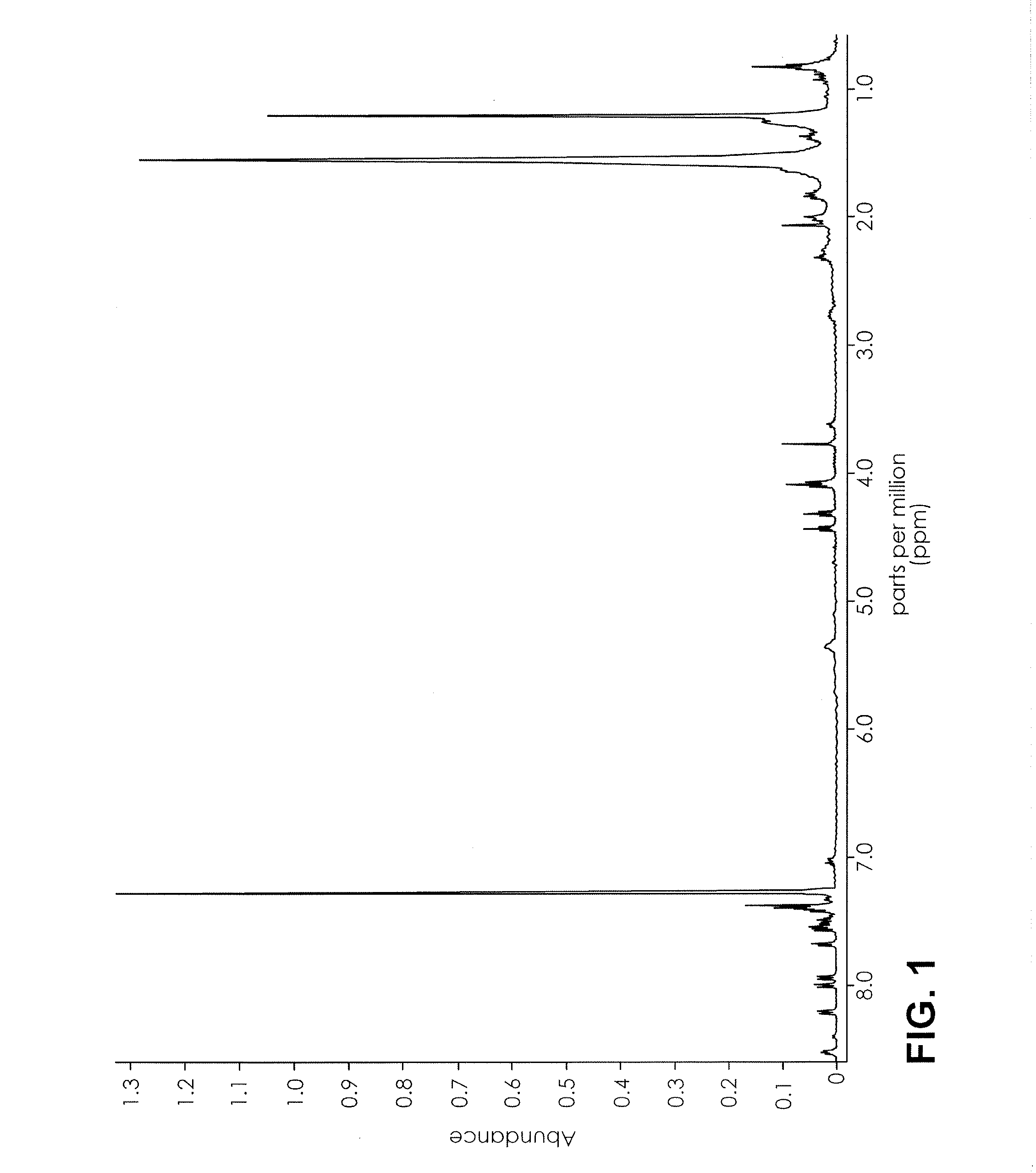
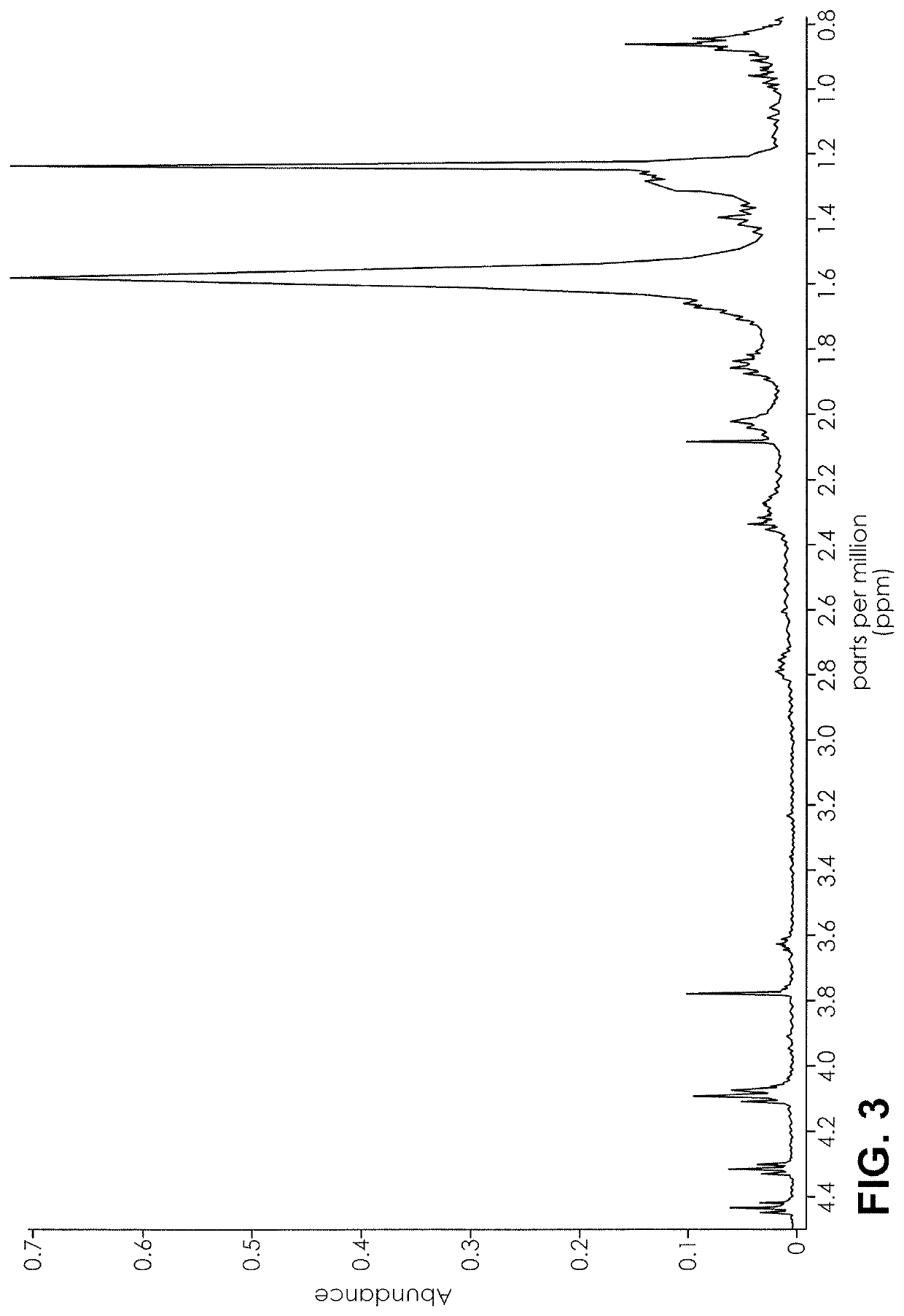
Nuclear magnetic resonance implemented synthetic indole and indazole cannabinoid detection, identification, and quantification
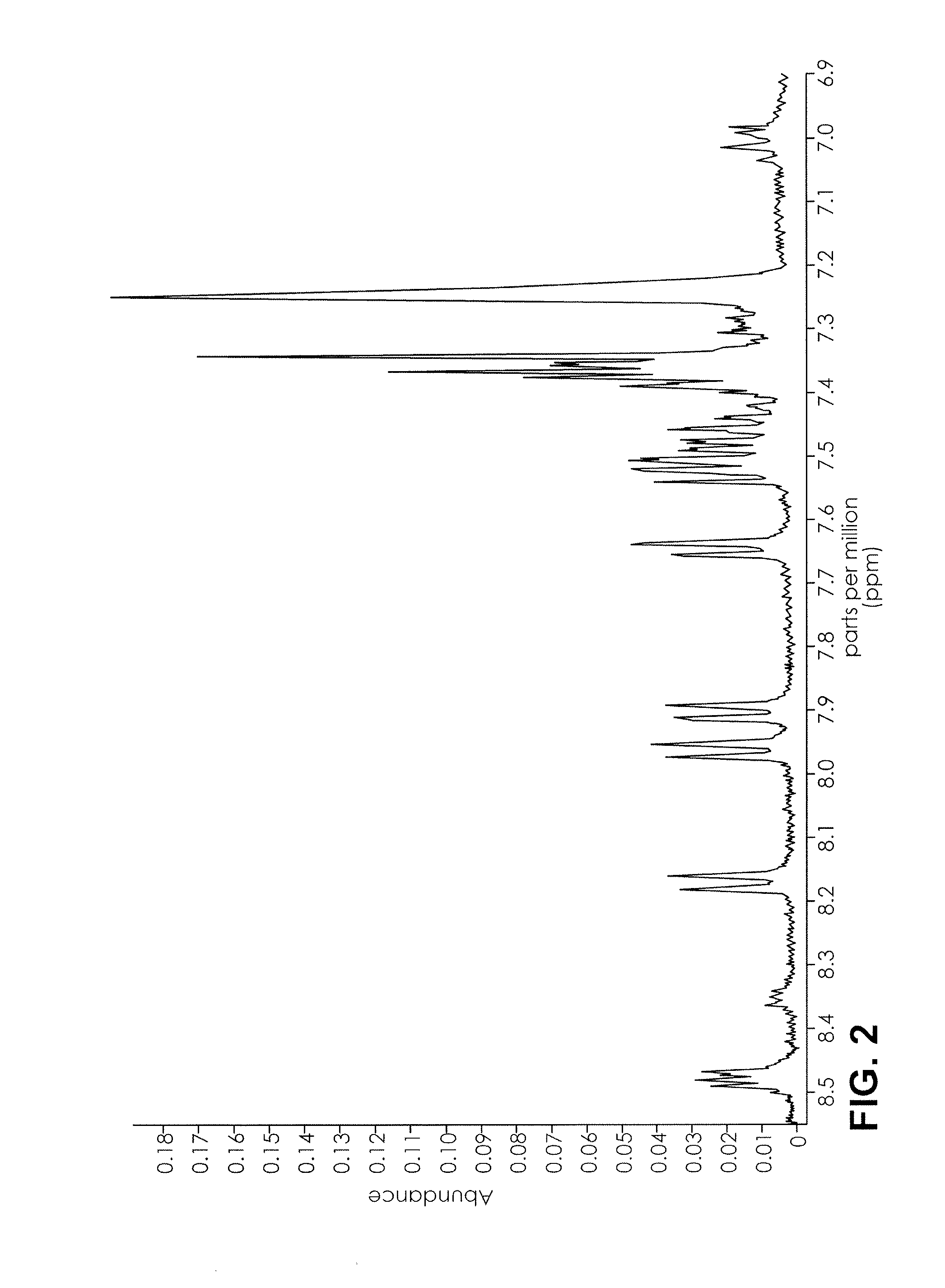
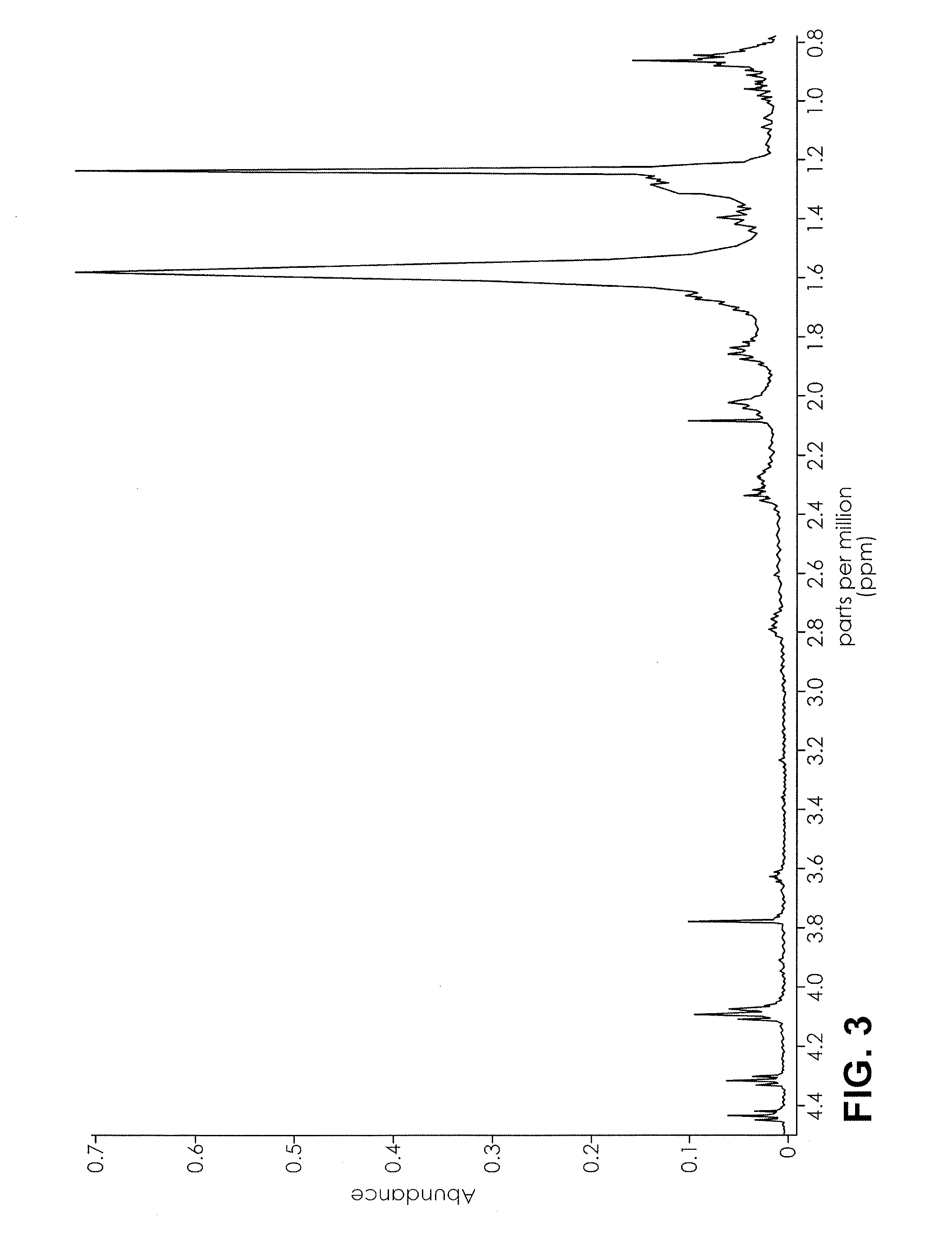
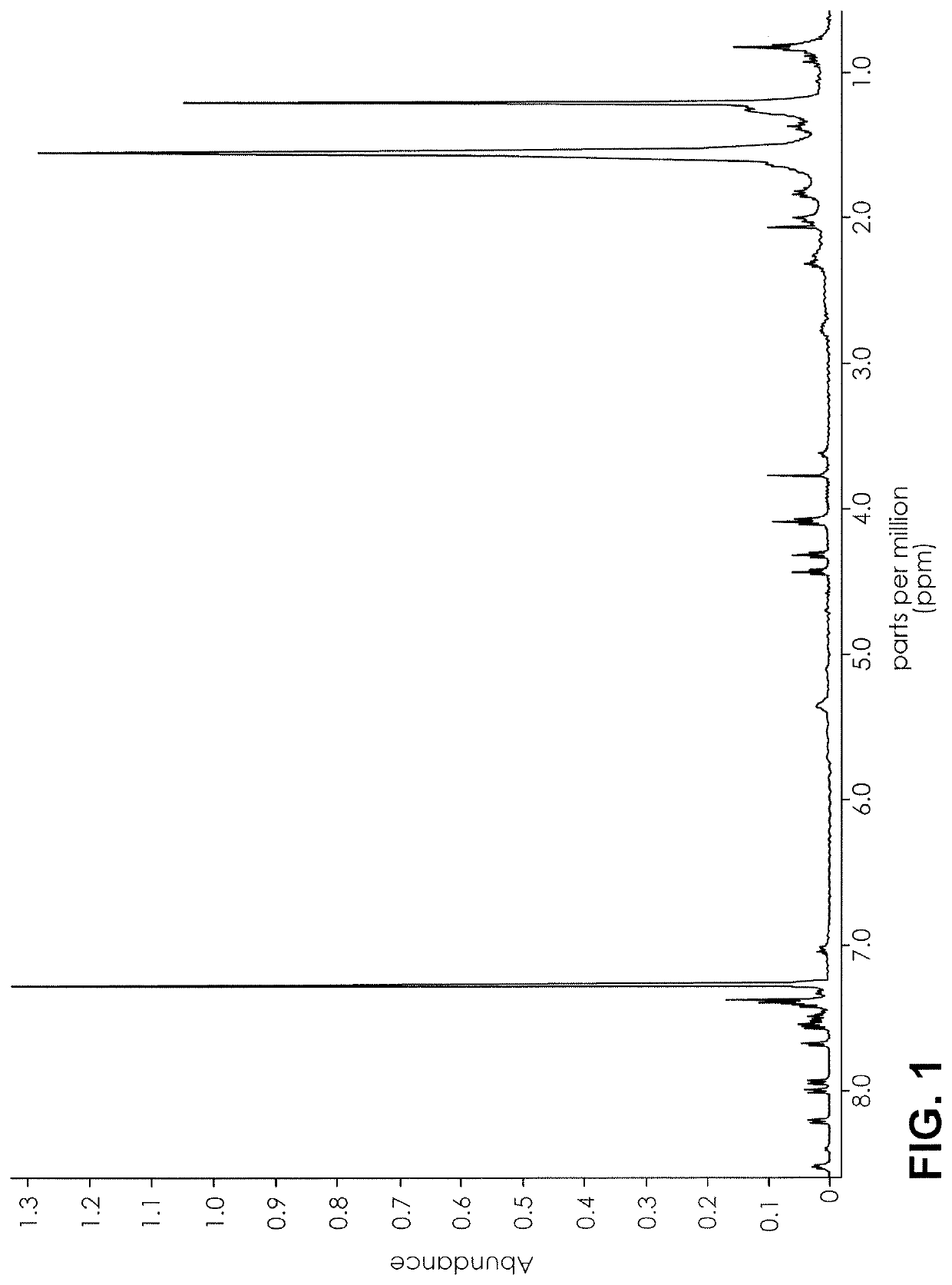
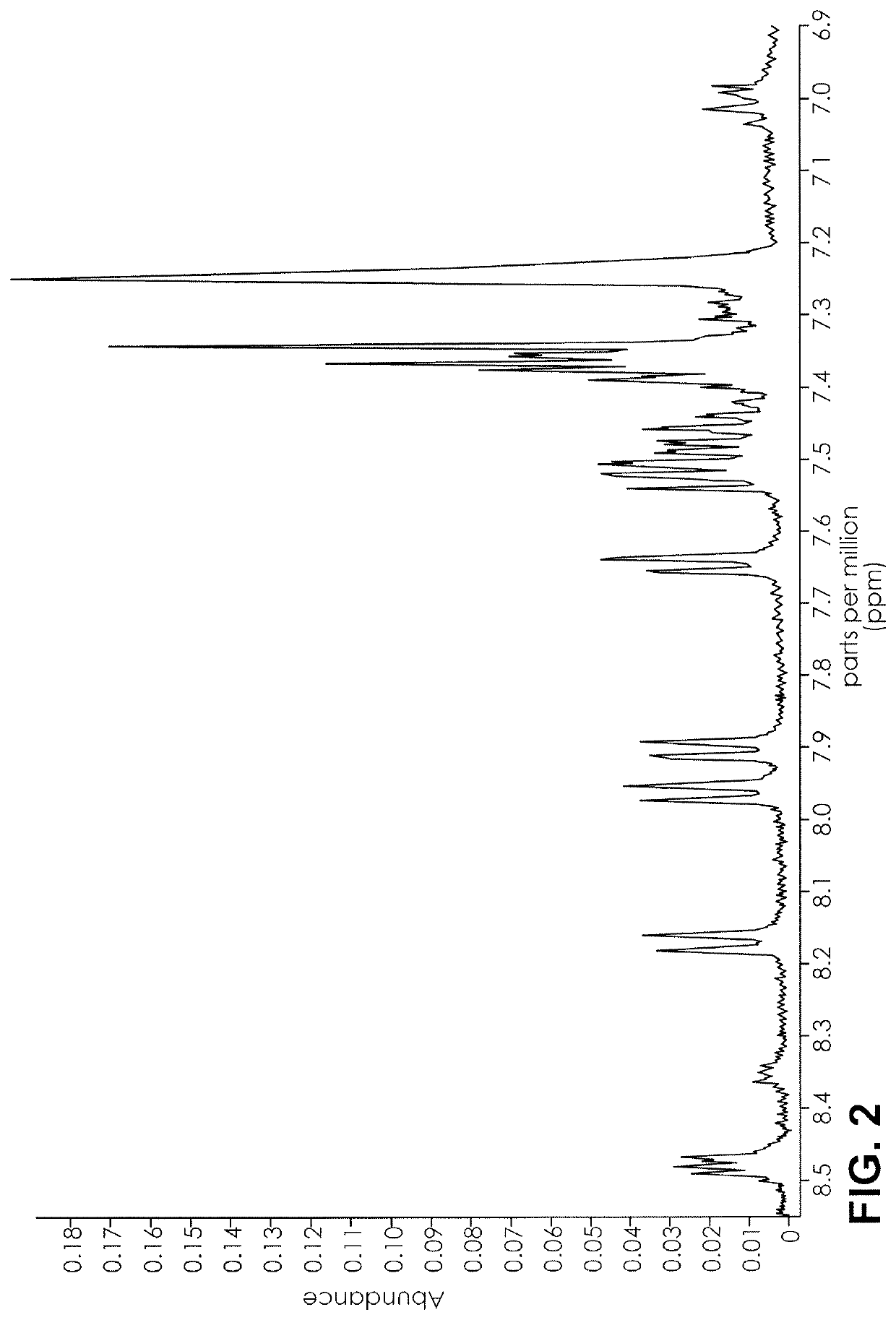
ActiveUS20160084779A1Analysis using nuclear magnetic resonanceElectric/magnetic detectionSolid-state nuclear magnetic resonanceNMR - Nuclear magnetic resonance
The present invention provides a method for detecting synthetic indole and indazole cannabinoids in a sample known or suspected to contain a synthetic indole or indazole cannabinoid. A deuterated solvent is added to the solid sample, creating a suspension. The suspension is mixed to release the cannabinoid from the solid sample. The suspension is subject to a NMR spectroscopy process to produce a sample NMR spectrum. The synthetic cannabinoid is detected in the suspension by analysis of the sample NMR spectrum. When one-dimensional proton NMR is used, detection of a first peak between 8.00 and 8.50 ppm and a second peak between 4.00 and 4.40 ppm, indicates the presence of a synthetic indole or indazole cannabinoid. When two-dimensional Correlation Spectroscopy (COSY) NMR is used, detection of a first spot between 6.50 and 9.00 ppm and a second spot between 1.50 and 4.50 ppm indicates the presence of a synthetic indole or indazole cannabinoid. The method is performed in the absence of chromatography and optionally, may be used to quantify the amount of synthetic cannabinoid.
Owner:HOFSTRA UNIVERSITY
Nuclear magnetic resonance implemented synthetic indole and indazole cannabinoid detection, identification, and quantification
ActiveUS11085891B2Analysis using nuclear magnetic resonanceNMR - Nuclear magnetic resonanceProton NMR
The present invention provides a method for detecting synthetic indole and indazole cannabinoids in a sample known or suspected to contain a synthetic indole or indazole cannabinoid in the absence of chromatography. A deuterated solvent is added to the solid sample, creating a suspension. The synthetic cannabinoid is detected in the suspension by analysis of the sample NMR spectrum. When one-dimensional proton NMR is used, detection of a first peak between 8.00 and 8.50 ppm and a second peak between 4.00 and 4.40 ppm, indicates the presence of a synthetic indole or indazole cannabinoid. When two-dimensional Correlation Spectroscopy (COSY) NMR is used, detection of a first spot between 6.50 and 9.00 ppm and a second spot between 1.50 and 4.50 ppm indicates the presence of a synthetic indole or indazole cannabinoid.
Owner:HOFSTRA UNIVERSITY
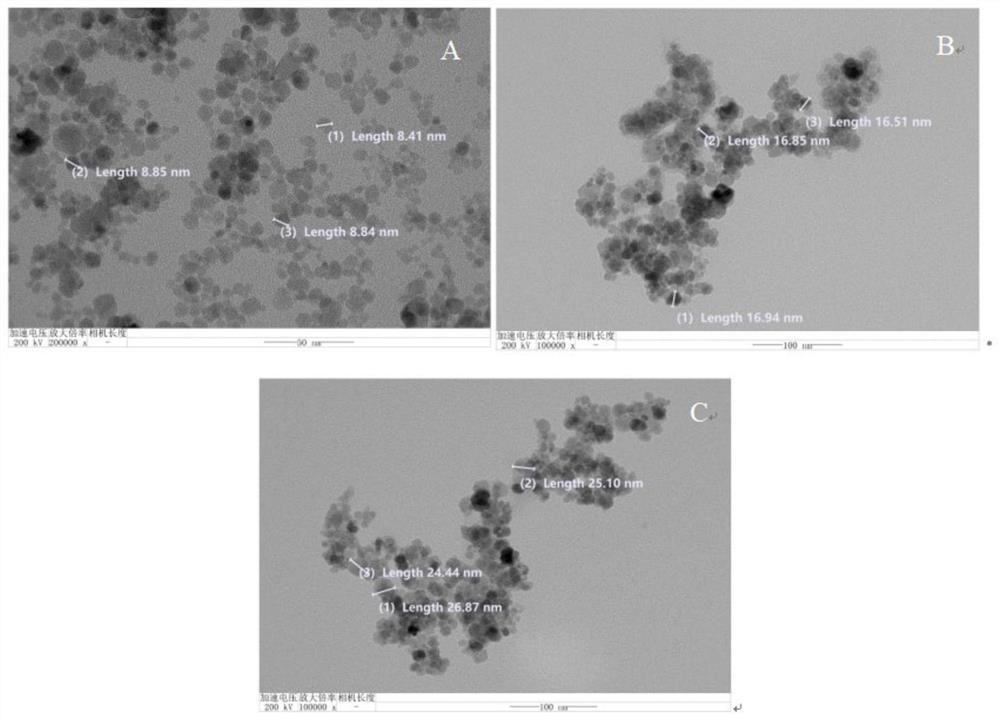
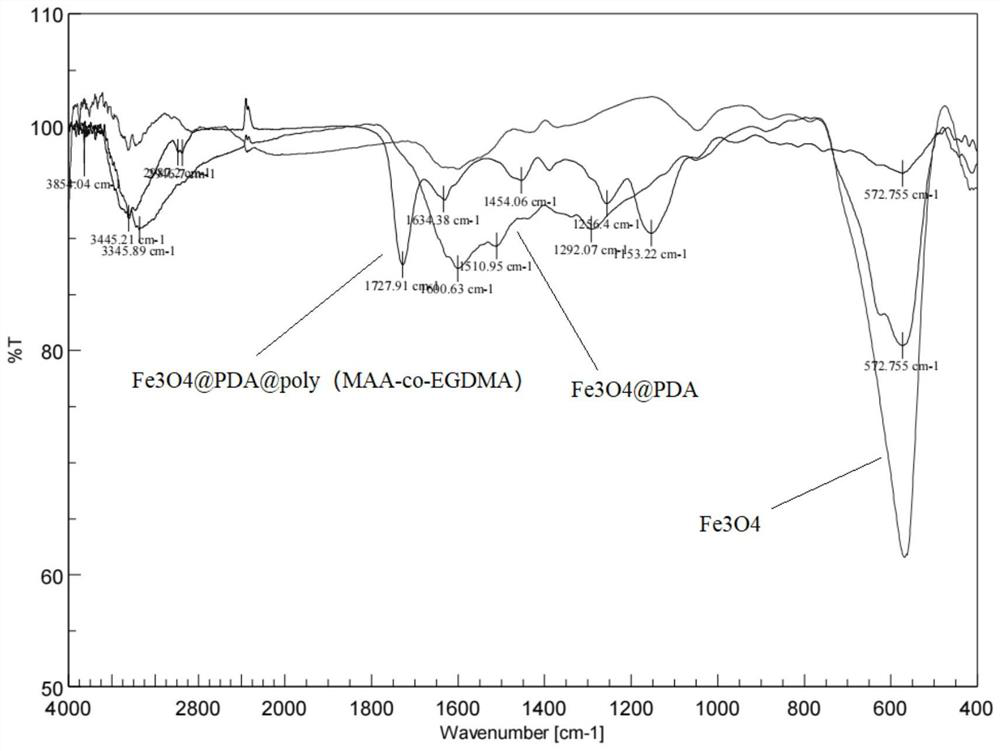
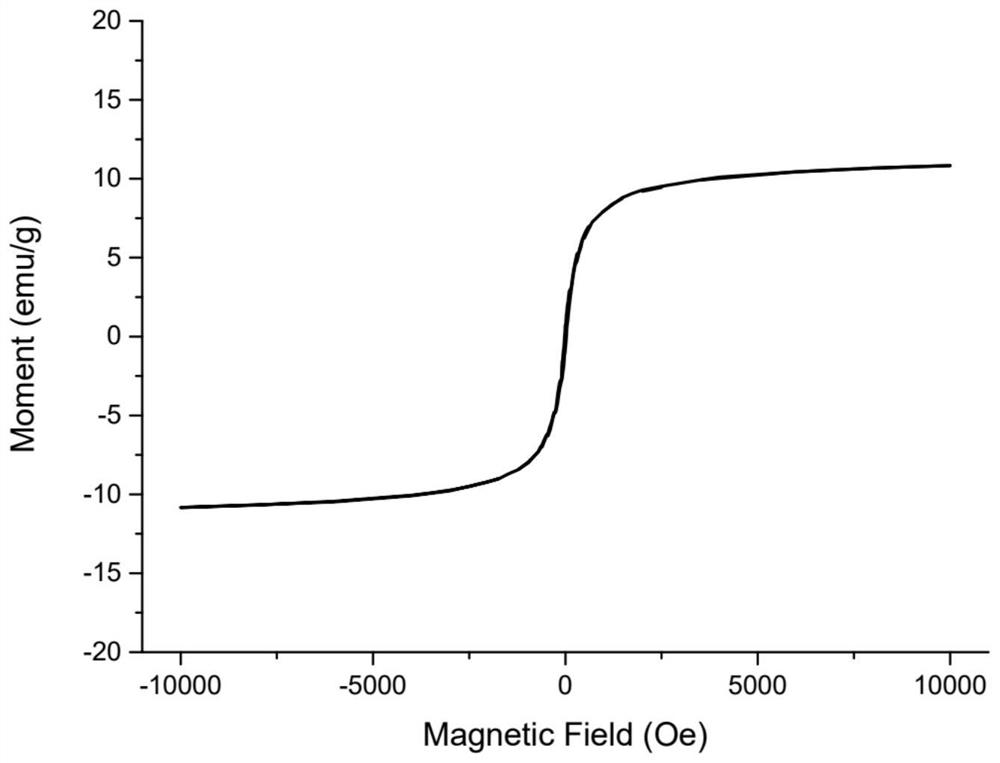
Magnetic nano-material for detecting tetrahydrocannabinoid and synthetic cannabinoid drugs as well as preparation method and application of magnetic nano-material
ActiveCN114832793AIncrease contactGood adsorption and separation performanceComponent separationOther chemical processesEthyleneglycol dimethacrylateDopamine
The invention discloses a magnetic nano-material for detecting tetrahydrocannabinoid and synthetic cannabinoid drugs. The magnetic nano-material is Fe3O4 (at) PDA (at) poly (MAA-co-EGDMA). Wherein the Fe3O4 is located at the core, the outer layer of the Fe3O4 is wrapped with the polydopamine to form a core-shell layer, and the core-shell layer is wrapped with the polymethylacrylic acid crosslinked ethylene glycol dimethacrylate to serve as a loading layer. The prepared Fe3O4 (at) PDA (at) poly (MAA-co-EGDMA) magnetic nano material has the advantages that the complex matrix sample treatment process is simplified, the sensitivity of an instrument for detecting tetrahydrocannabinoic acid and synthetic cannabinoid drugs is greatly improved, the magnetic nano material can be matched with an on-site rapid detection instrument or a laboratory large-scale analysis instrument for use, pretreatment steps are reduced, the cost is reduced, and the detection efficiency is improved. The characteristics of complicated steps and low repeatability in the prior art are avoided, and high-flux automatic detection of trace drugs in sewage and urine is realized.
Owner:CHINA PHARM UNIV
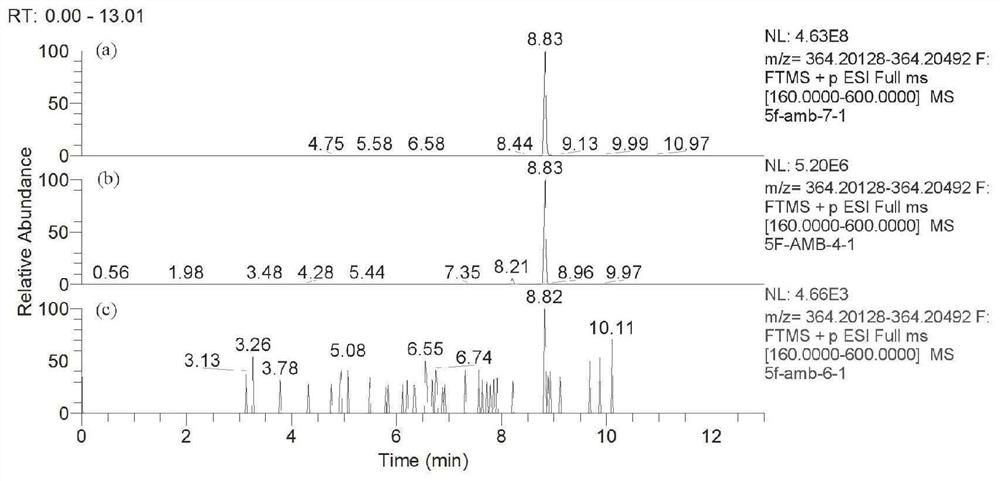
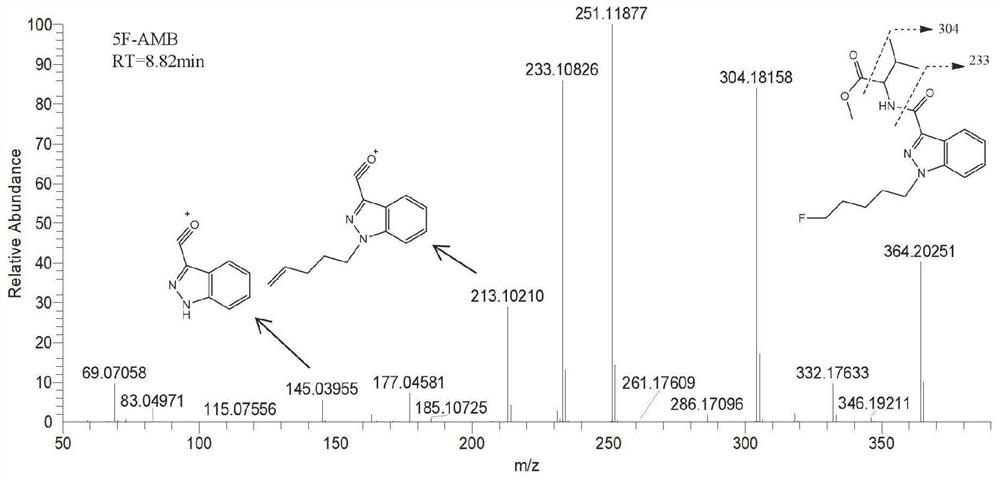
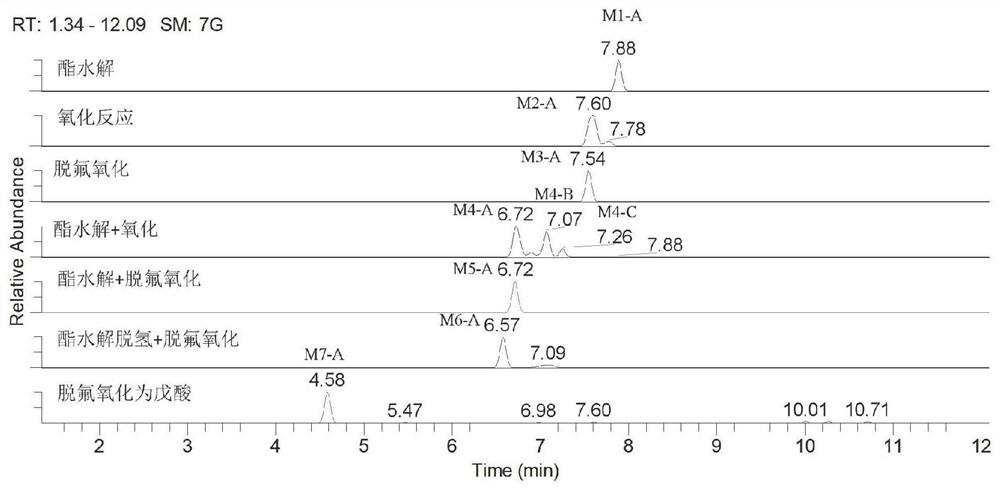
In-vivo biomarker for detecting 5F-AMB and application thereof
The invention discloses an in-vivo biomarker for detecting 5F-AMB and application thereof. The in-vivo biomarker of synthetic cannabinoid 5F-AMB is detected as shown in Table 1. The in-vivo biomarkerfor detecting 5F-AMB and the application thereof overcome the defects of high in-vivo metabolism speed of the synthetic cannabinoid 5F-AMB and generally lower parent drug content in a biological detection material, and the invention provides a method for detecting by using an in-vivo metabolism marker, which has the advantages of high accuracy, high sensitivity and an accurate and reliable result.The biomass of the detection sample required by the invention is less and is only 100 microliters.
Owner:ZHEJIANG POLICE COLLEGE
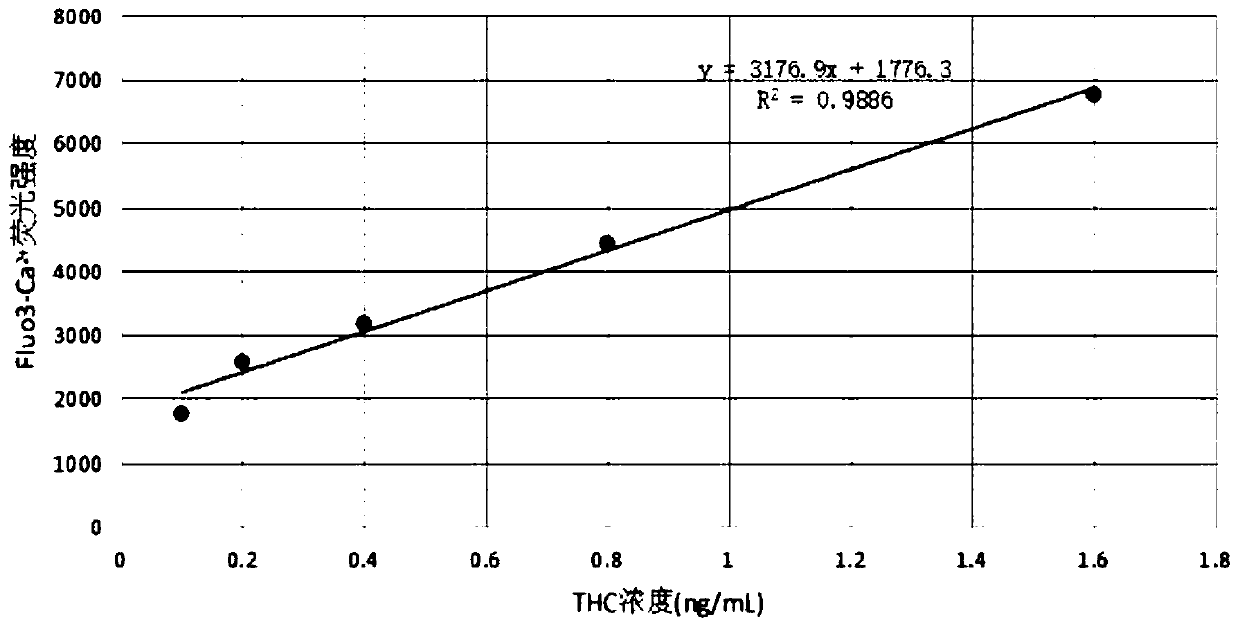
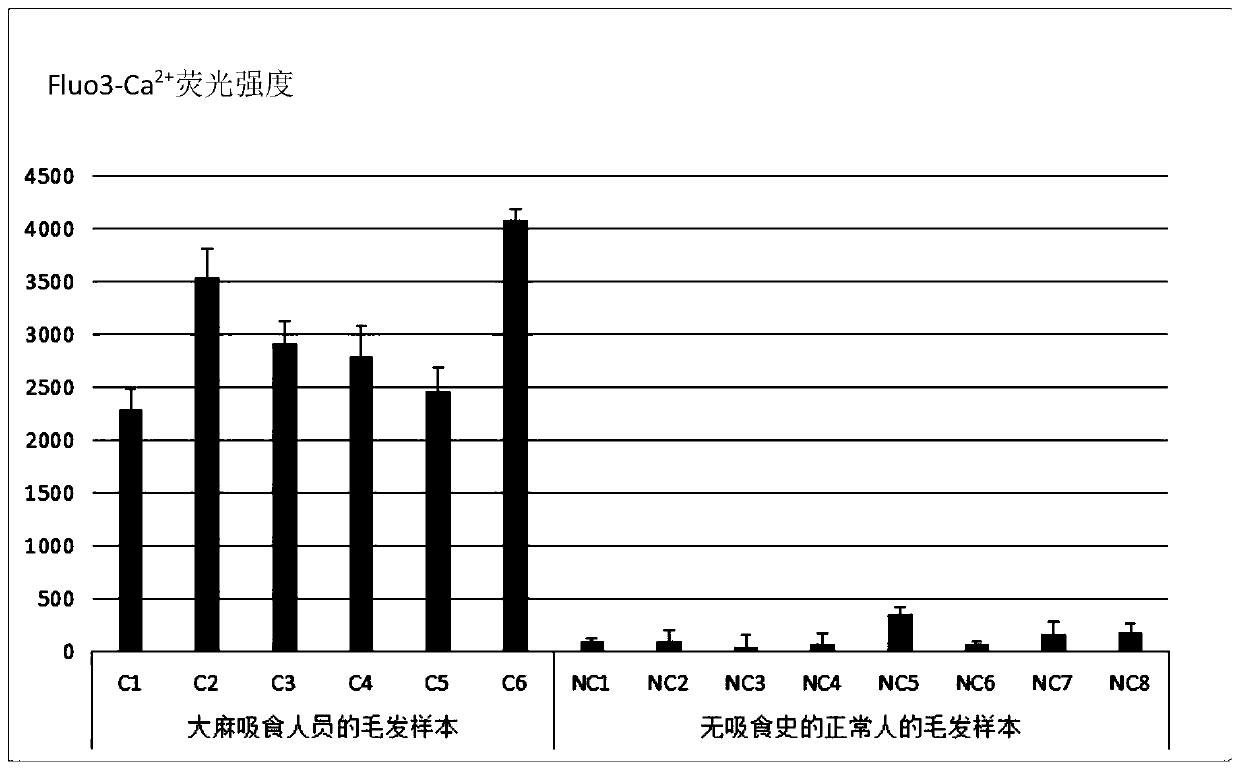
Cannabinoid active substance detection method based on intracellular free Ca<2+> concentration change effect and cannabinoid active substance detection kit
ActiveCN109946451AAccurate detectionHigh sensitivity detectionFluorescence/phosphorescenceCannabisPsychoactive substance
The invention discloses a cannabinoid active substance detection method based on an intracellular free Ca<2+> concentration change effect and a cannabinoid active substance detection kit. The detection kit comprises two components including a detection group reagent and a negative control group reagent, the detection group reagent comprises a monoclonal cell line CB1 / 293 stably expressing a human-derived cannabinoid receptor CB1 gene and an intracellular free Ca<2+> probe reagent Fluo 3-AM; the negative control group reagent comprises the monoclonal cell line CB1 / 293 stably expressing the human-derived cannabinoid receptor CB1 gene, the intracellular free Ca<2+> probe reagent Fluo 3-AM and a cannabinoid receptor antagonist. With the adoption of the detection method, accurate, high-sensitivity and rapid detection of all cannabinoid active substances including natural cannabinoid and synthetic cannabinoid as well as novel synthetic cannabinoid active substances which emerge in endlesslycan be realized, and the problem of difficulty in supervising cannabis psychoactive substances can be solved.
Owner:浙江诺迦生物科技有限公司
Stable cannabinoid compositions
PendingCN111225661AEasy to storeEasy to useHydroxy compound active ingredientsPharmaceutical non-active ingredientsSynthetic cannabinoidsPoloxamer
A composition comprising a cannabinoid, in particular a phytocannabinoid or a synthetic cannabinoid, wherein the cannabinoid is stabilised against oxidation and / or photochemical degradation, characterized in that the composition comprises a micellar solution of poloxamer-based micelles in an aqueous solution and where the poloxamer micelles encapsulate the cannabinoid.
Owner:SOLMIC BIOTECH GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com