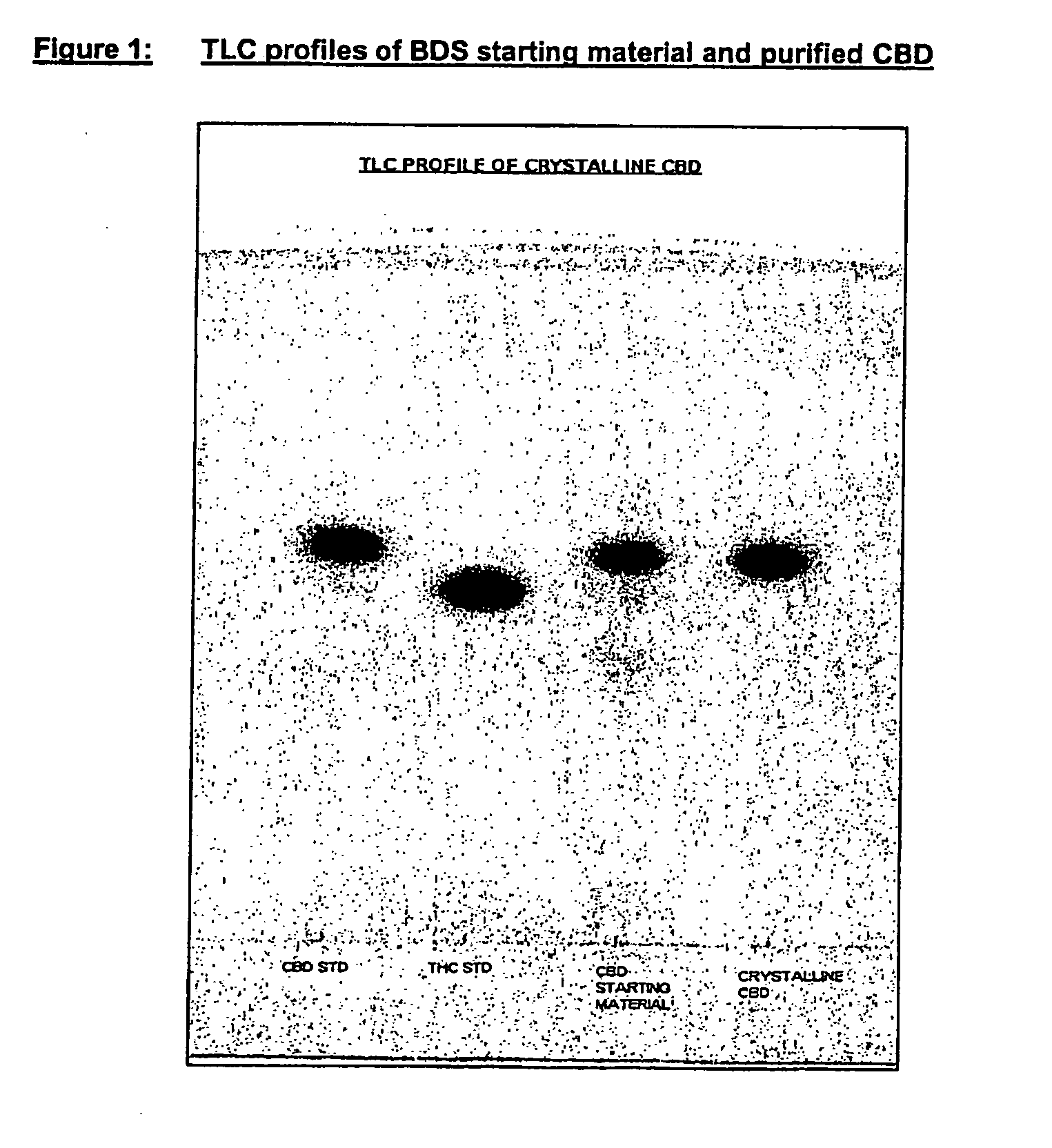
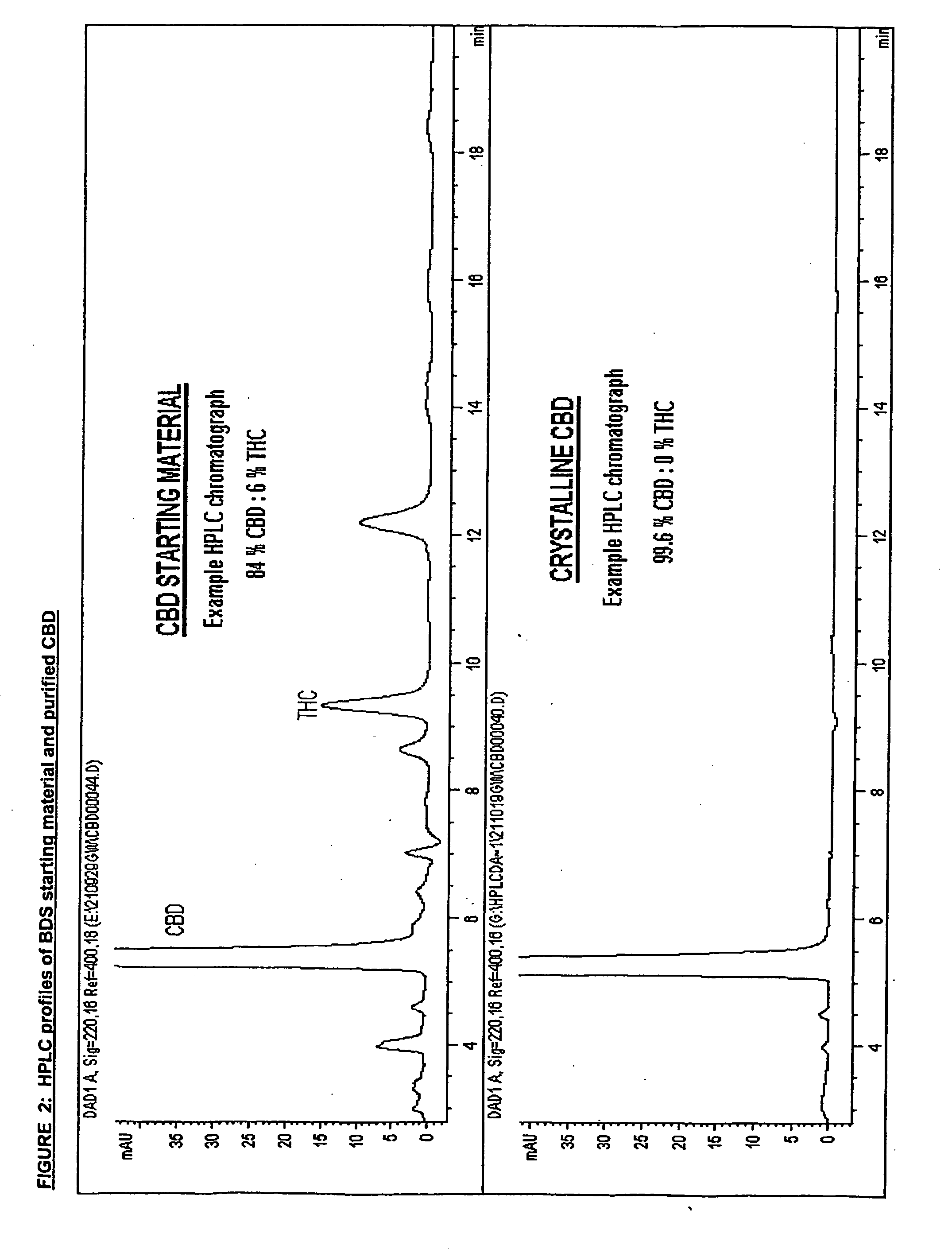
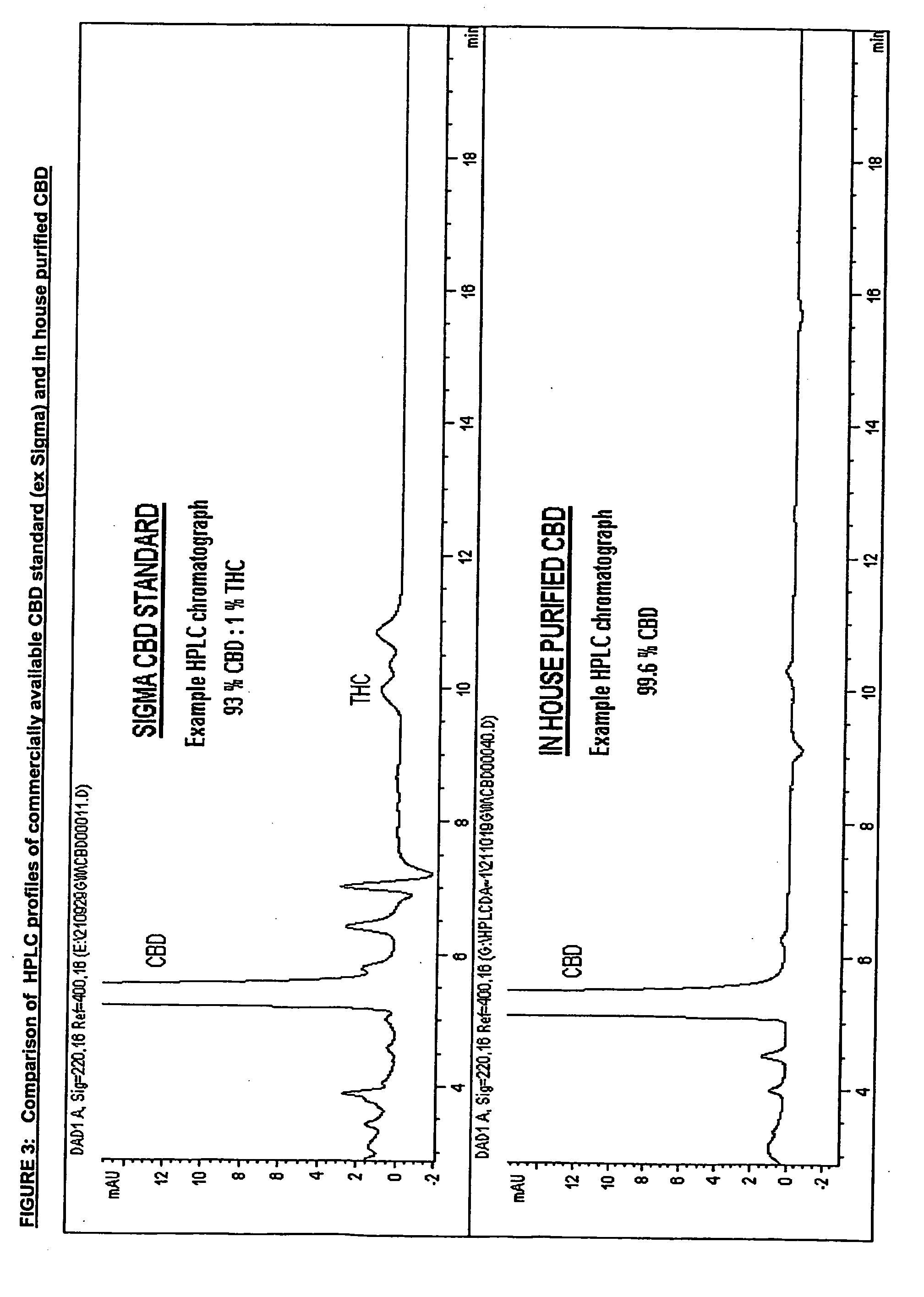
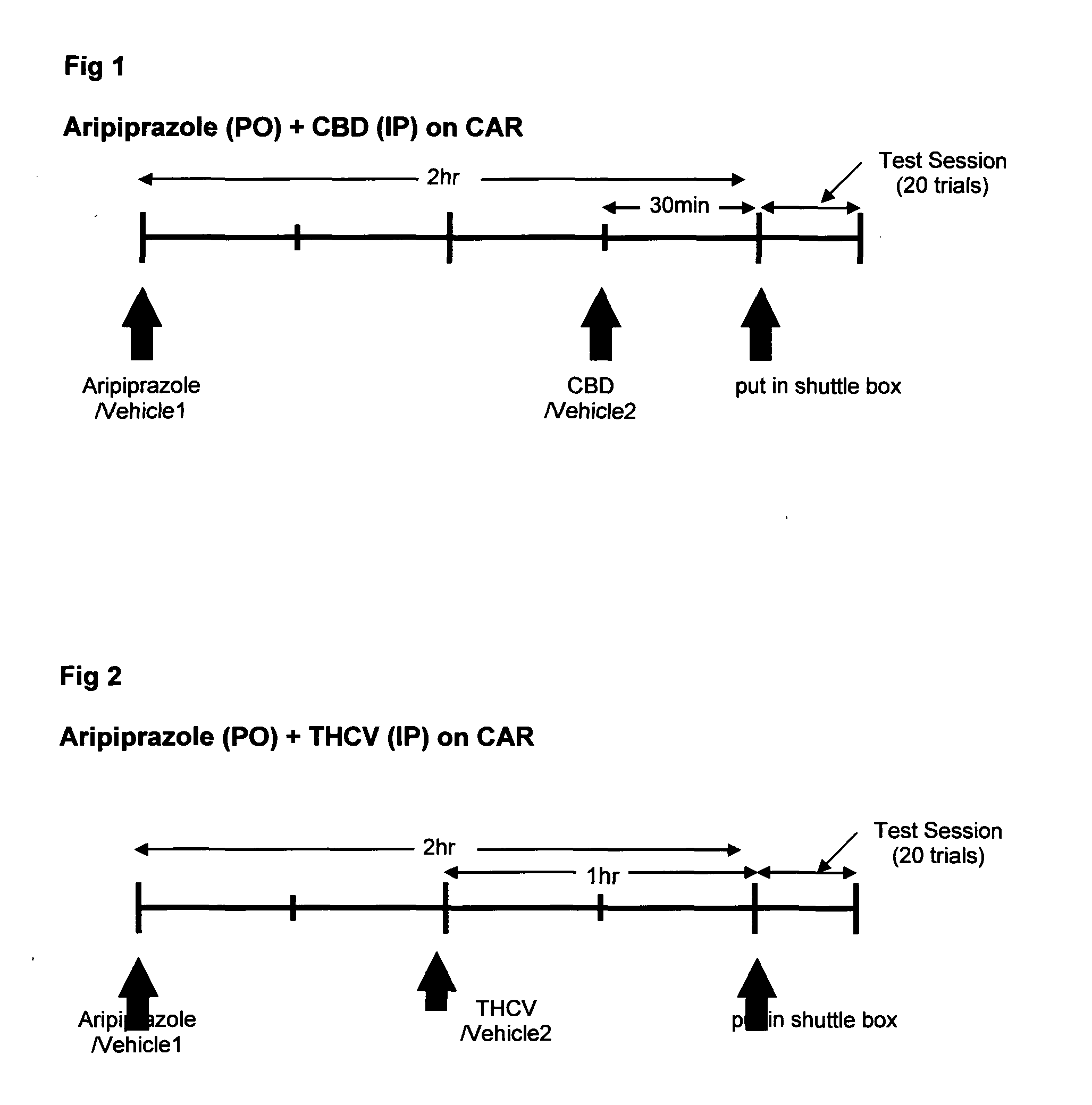
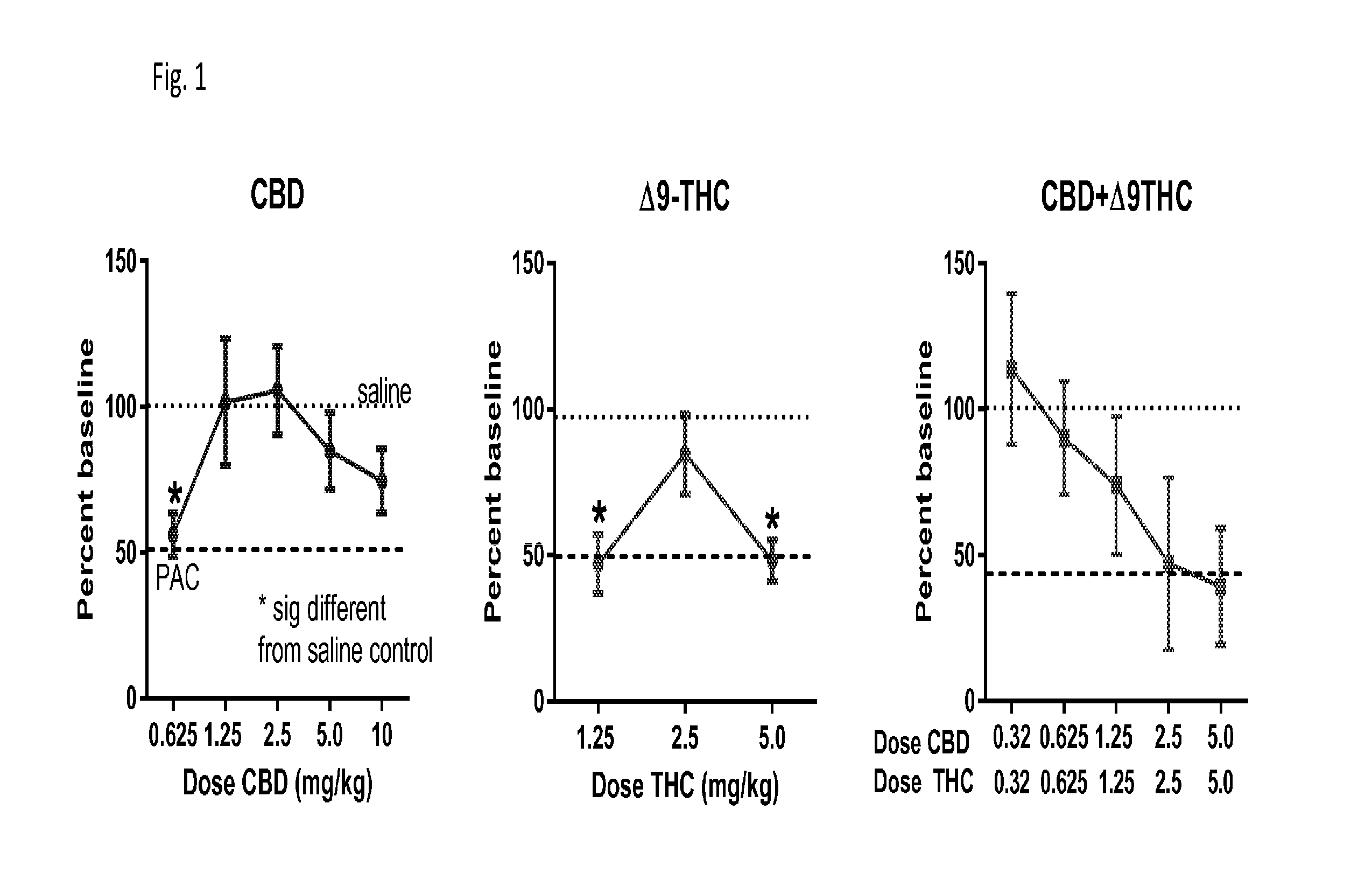
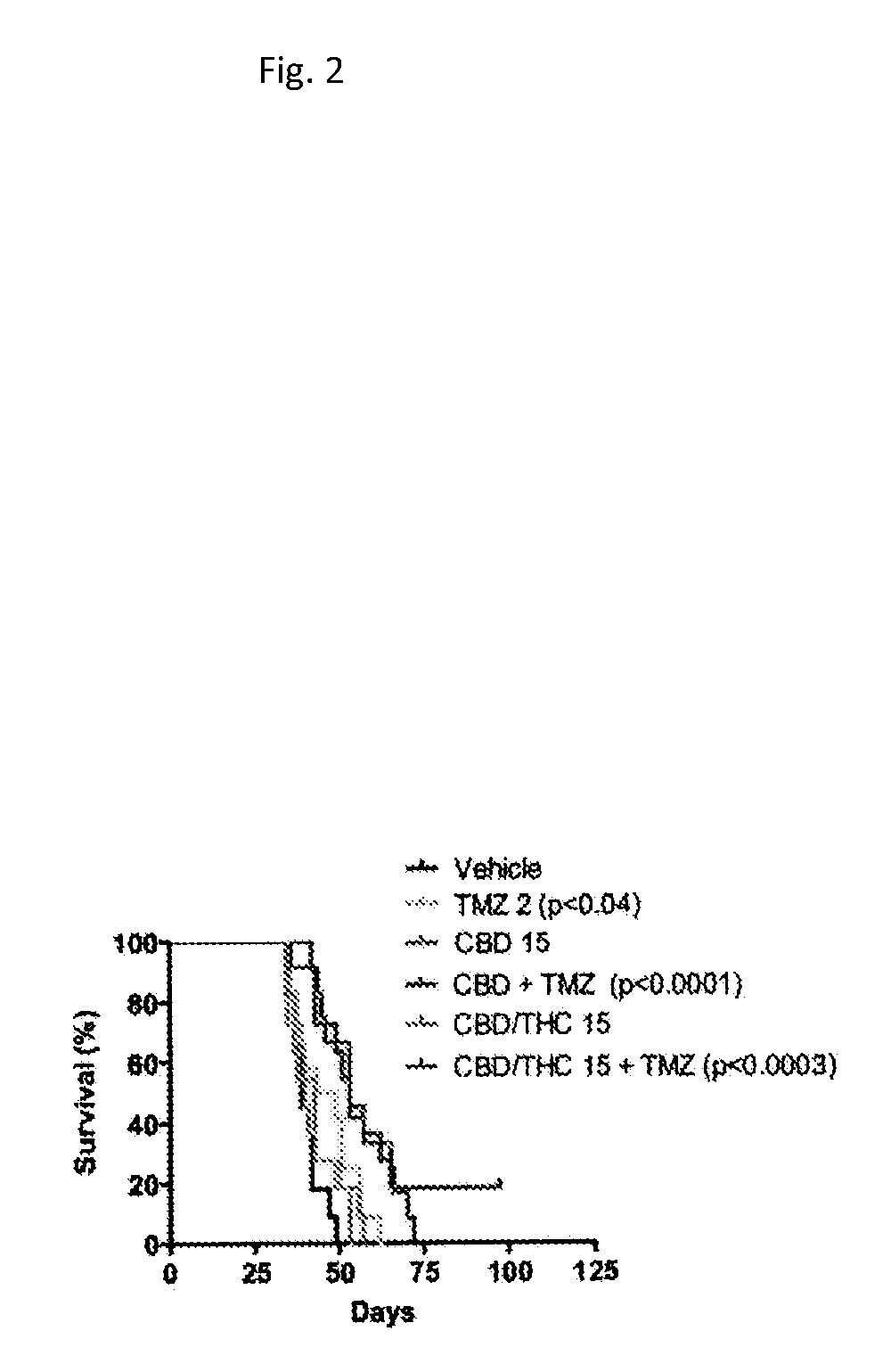
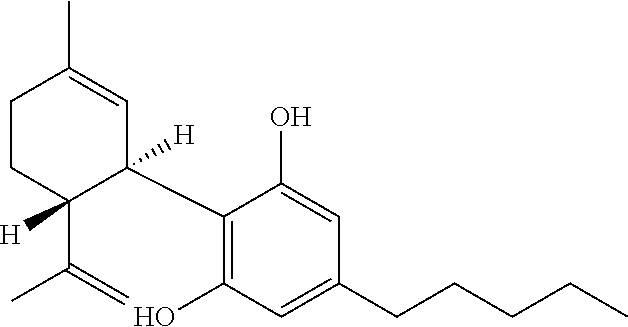
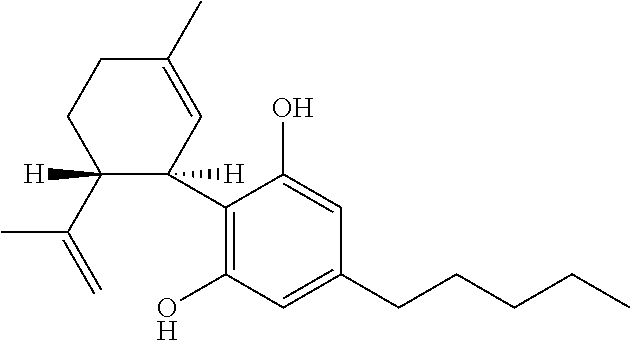
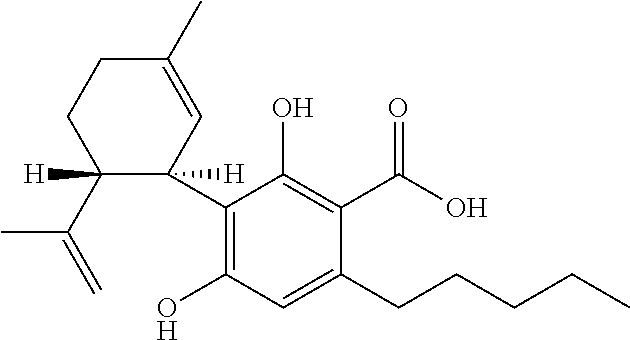
Patents
Literature
742 results about "Cannabidiol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
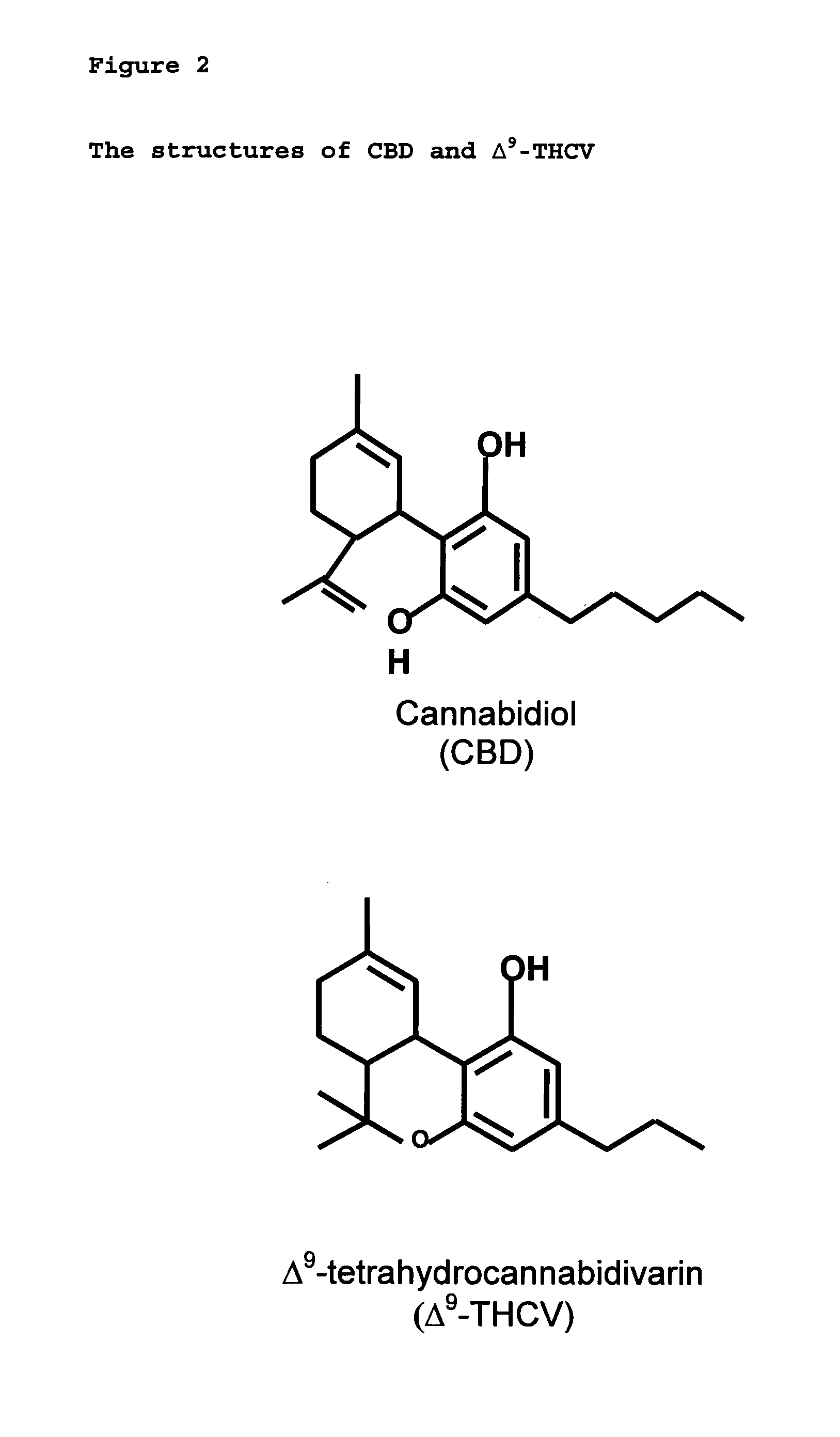
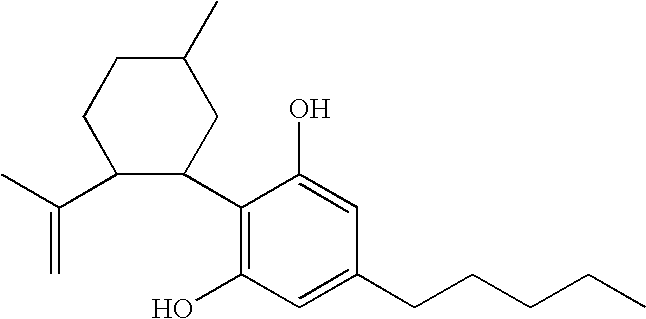
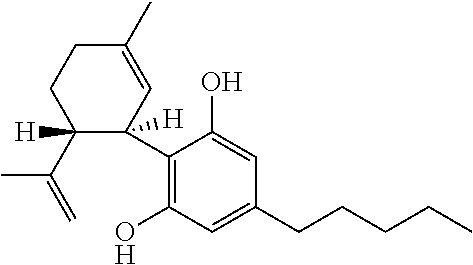
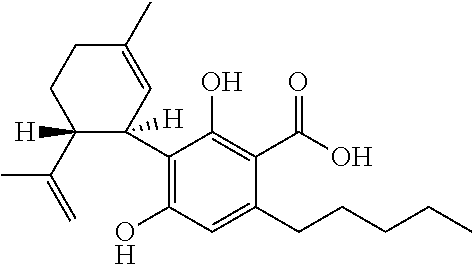
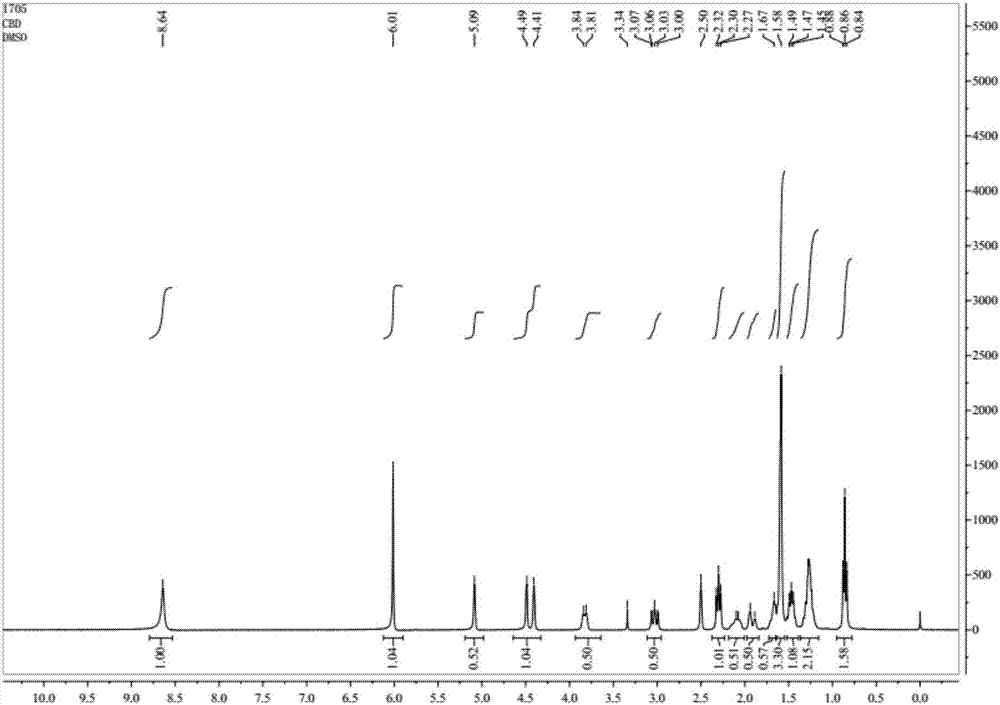
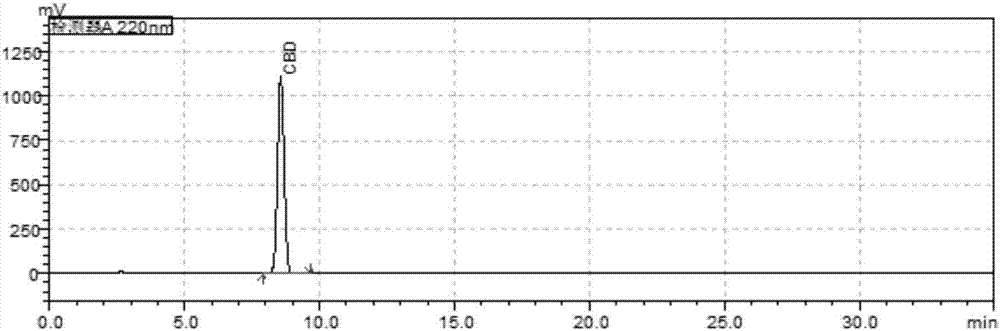
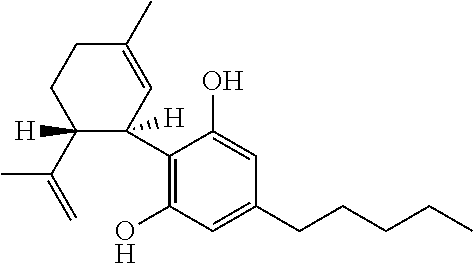
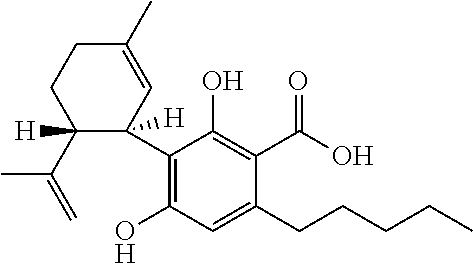
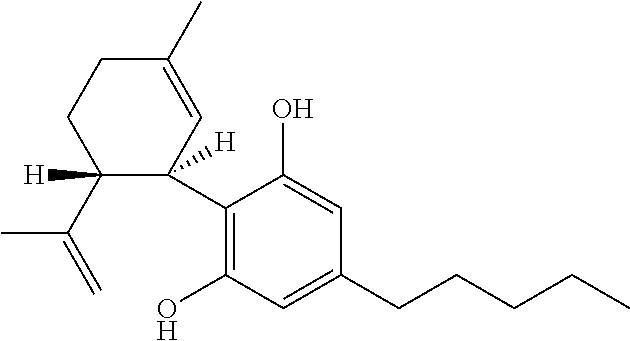
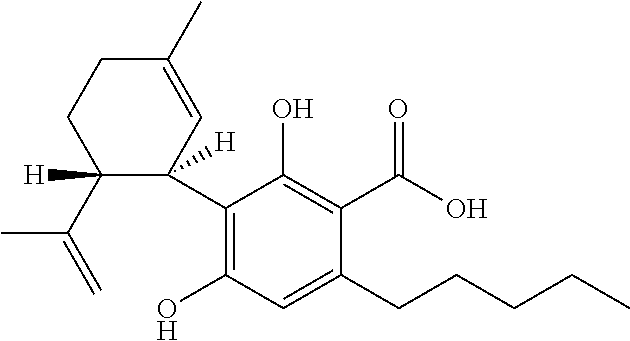
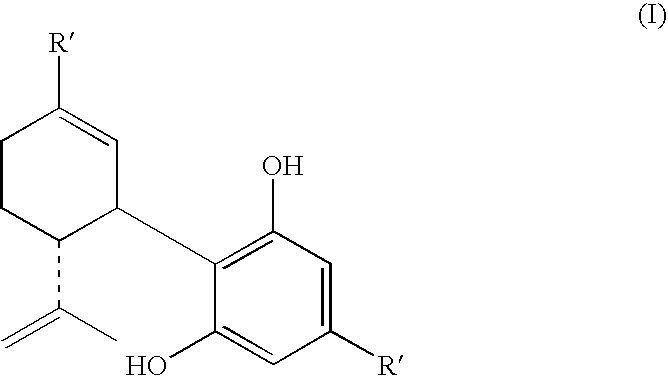
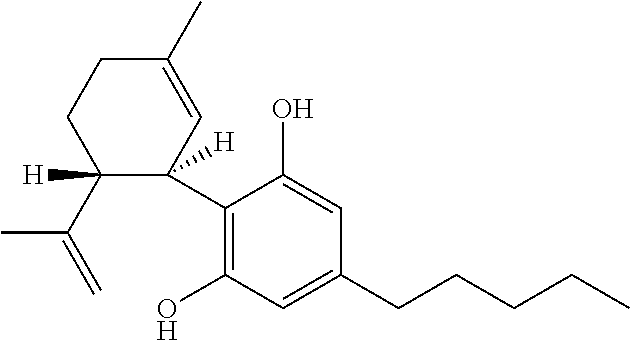
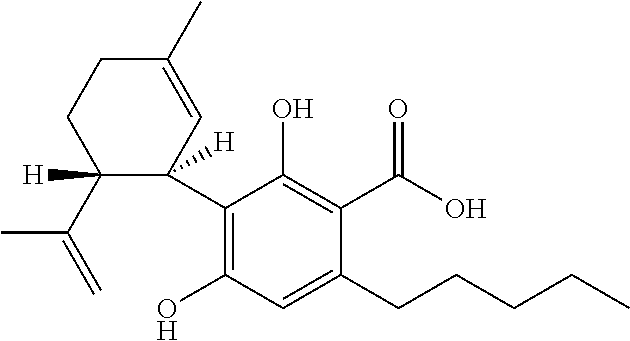
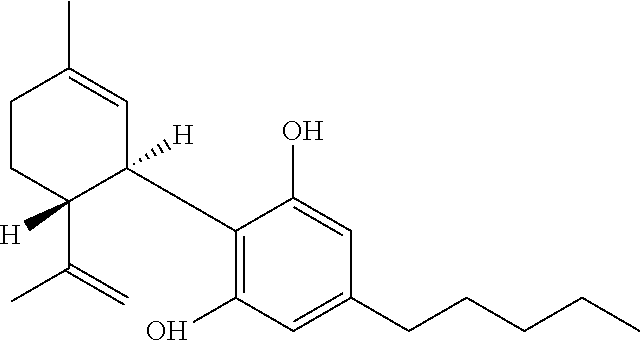
Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants and accounts for up to 40% of the plant's extract. In 2018, clinical research on cannabidiol included preliminary studies of anxiety, cognition, movement disorders, and pain.
Enhanced Delivery of Nicotine, THC, Tobacco, Cannabidiol or Base Alkaloid from an Electronic Cigarette or Other Vapor or Smoke Producing Device Through Use of an Absorption Conditioning Unit
A method for the administration of nicotine, THC, tobacco, cannabidiol or a base alkaloid includes administering in the oral or nasal cavity an absorption conditioning unit having at least two agents selected from the group consisting of (a) a buffer agent, (b) a capturing agent, (c) a penetration agent, and (d) a thermal agent, to the mammal, and then administering by inhalation a bioactive agent selected from the group consisting of nicotine, THC, cannabidiol and a base alkaloid. The absorption conditioning unit may be in a dosage form not containing a drug. The absorption conditioning unit may create a pH in the oral cavity or nasal cavity of 7.8-10 for a period of ten minutes or more after administration, the dosage form not containing an acid and not containing a drug.
Owner:FUISZ RICHARD C +1
Method of preparing cannabidiol from plant material
InactiveUS20060167283A1Reduce the ratioOrganic chemistryHydroxy compound active ingredientsCannabidiolChromatography
The invention relates to methods of preparing cannabidiol in substantially pure form starting from plant material. Also described are substantially pure preparations of cannabidiol having a chromatographic purity of 95% or greater.
Owner:FLOCKHART IAN +3
Cannabidiol liquid composition for smoking
InactiveUS20150181924A1Tobacco preparationTobacco treatmentElectronic cigaretteIntensive care medicine
This application discloses a cannabidiol liquid composition for use in an electronic cigarette smoking device. The cannabidiol liquid composition may be used for pulmonary administration of cannabidiol for more effective absorption. A method of use for the cannabidiol liquid composition is also disclosed.
Owner:HDDC HLDG
Use of cannabinoids in combination with an Anti-psychotic medicament
ActiveUS20110038958A1High activityLess degree of activityBiocideSenses disorderPsychosis drugTypical antipsychotic
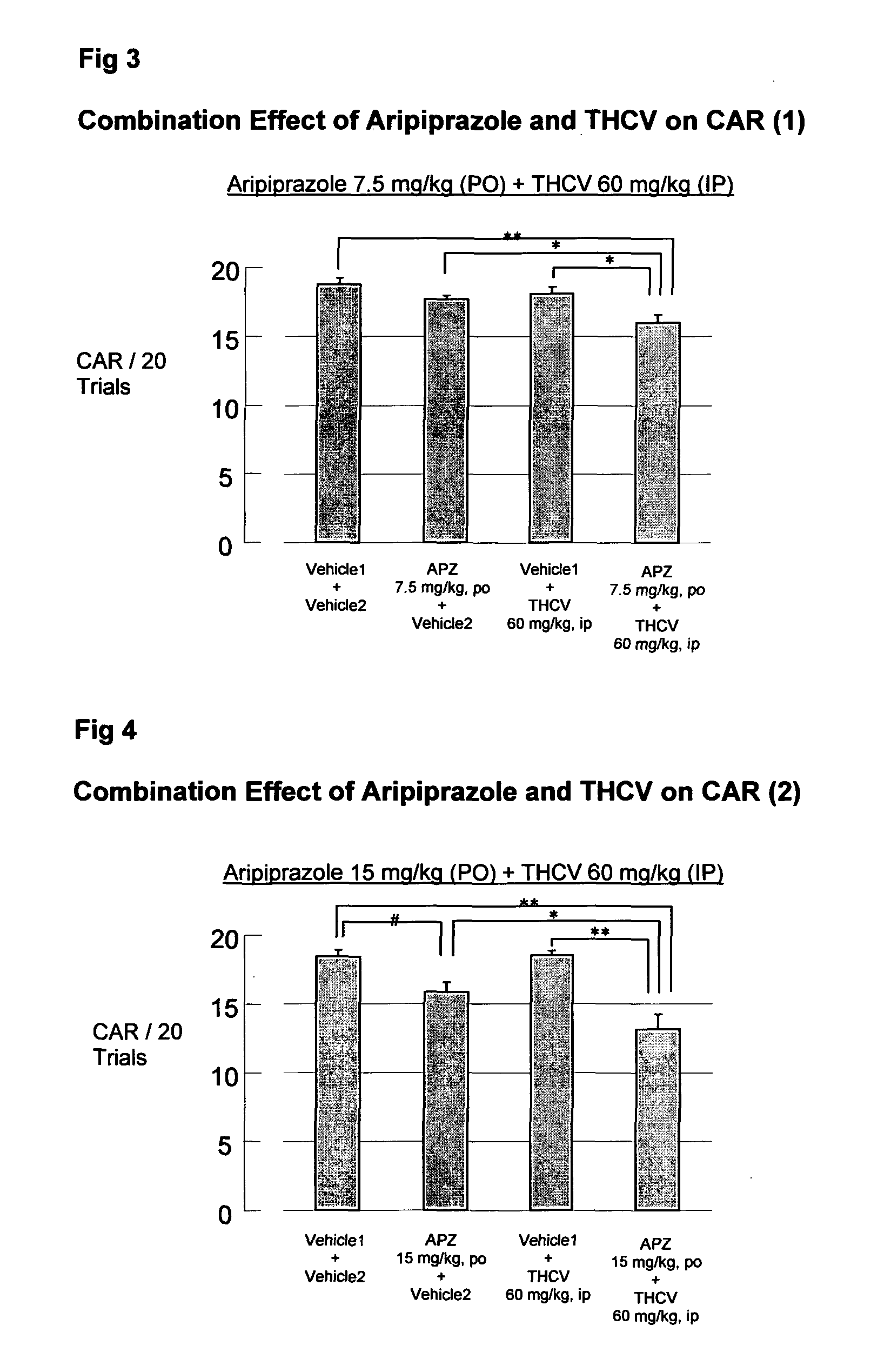
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
Method for extracting cannabidiol from cannabis
ActiveCN106831353AHigh yieldHigh purityOrganic chemistryOrganic compound preparationAlcoholExtraction Fraction
The invention discloses a method for extracting cannabidiol from cannabis. The method comprises the following steps: smashing and drying extraction fractions of cannabis to obtain medicine material powder; extracting the medicine material powder with 30% to 100% (V / V) of ethanol to obtain an extracting solution; concentrating the extracting solution to obtain an extract; carrying out water precipitation on the extract, and removing impurities to obtain a water precipitation solution; centrifuging the water precipitation solution and adding 10% to 100% (V / V) of ethanol into an obtained precipitate, and dissolving the precipitate to obtain an alcoholic solution of the precipitate; carrying out column chromatography on the alcoholic solution of the precipitate; eluting an obtained eluant after the concentration column chromatography, and adding ethanol for supersaturated dissolution to obtain a crystal substance; adding the crystal substance into purified water or ethanol for washing to obtain a primary product; blending the primary product with purified water, and drying to obtain the cannabidiol. Only highly safe ethanol is adopted as a solvent, the purity of the cannabidiol in a finished product is improved, and the psychotoxic component of tetrahydrocannabinol is also removed, so that the product safety is improved, and the method is applicable for industrial production.
Owner:YUNNAN HANSU BIO TECH CO LTD
Stable cannabinoid formulations
The present invention is generally directed to substantially pure cannabidiol, stable cannabinoid pharmaceutical formulations, and methods of their use.
Owner:RADIUS PHARMA INC
Topical regional neuro-affective therapy with cannabinoids
ActiveUS20160256411A1Modulate afferent neural inputGood effectSenses disorderNervous disorderDiseaseCannabinoid
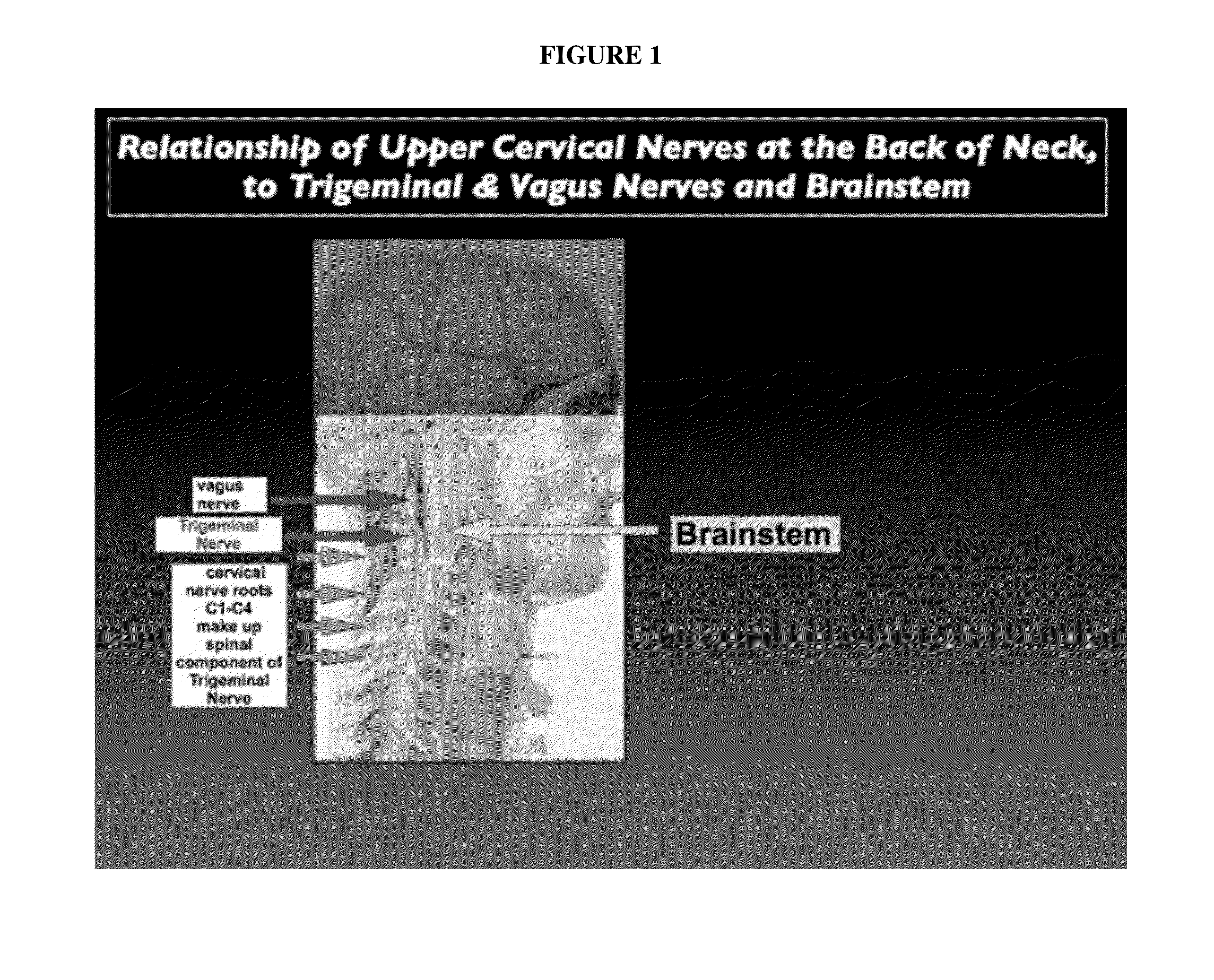
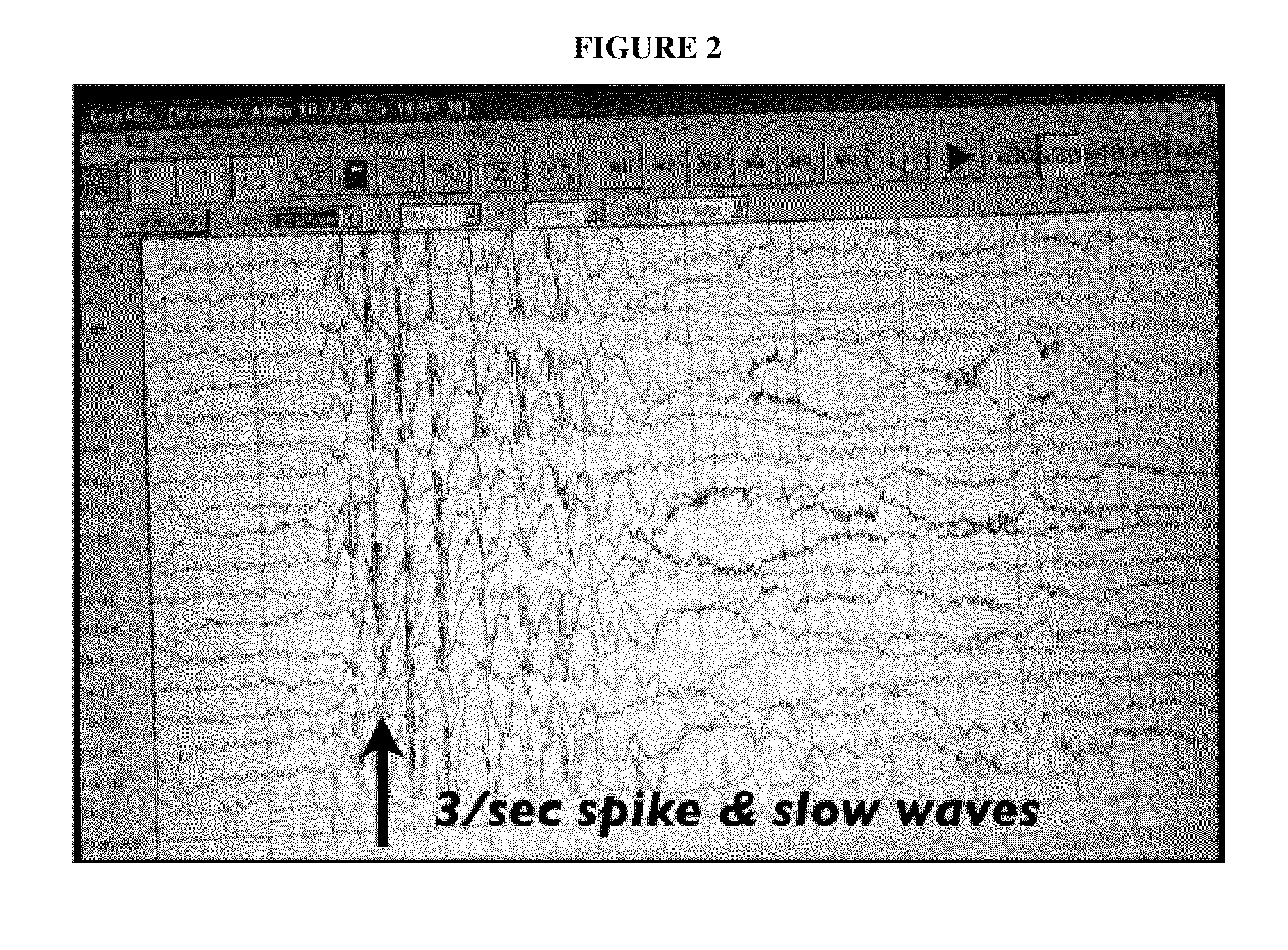
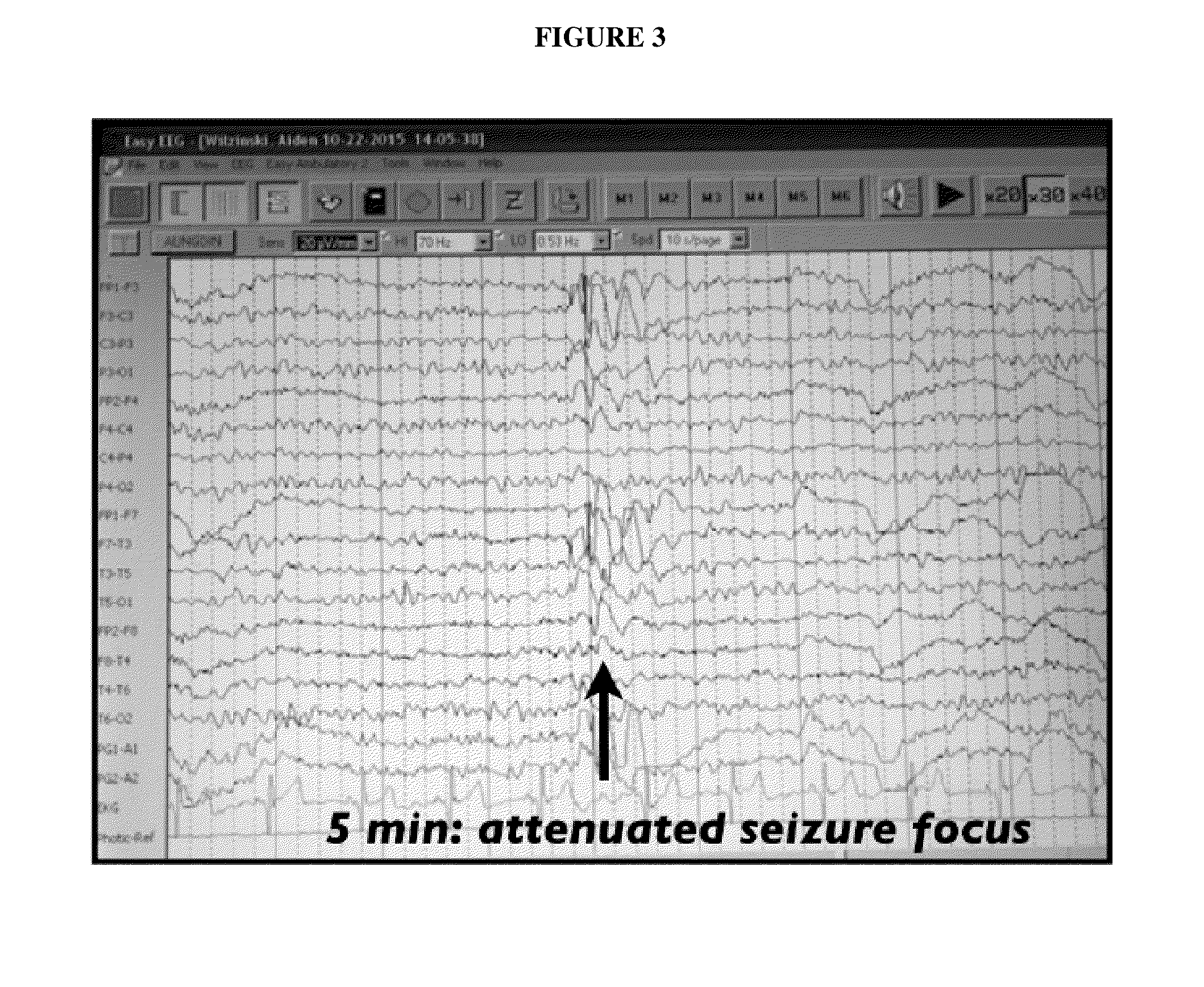
A method of treating a disease state or condition in humans via topical brainstem afferent stimulation therapy via the administration of a cannabinoid drug(s) to the back of the neck of a human patient to provide regional neuro-affective therapy is disclosed. In certain preferred embodiments, the cannabinoid drug(s) are not psychoactive or substantially not psychoactive. In certain embodiments, the cannabinoid drug(s) are incorporated into a pharmaceutically acceptable topical carrier, e.g., a cream. In certain preferred embodiments, the cannabinoid drug(s) comprises cannabidiol.
Owner:DEF LLC
Method for extracting cannabidiol from industrial hemp floral leaves
InactiveCN106278828AHigh purityReduce the impactOrganic chemistryOrganic compound preparationAlcoholSolvent
The invention discloses a method for extracting cannabidiol from industrial hemp floral leaves. The method comprises the following steps: grinding and drying floral leaves of industrial hemp, thereby obtaining medicinal powder; extracting the medicinal powder by adopting 30-100v% of ethanol, thereby obtaining extracting solution; concentrating the extracting solution to obtain extract; performing water precipitation on the extract, removing the impurities, thereby obtaining water precipitation solution; centrifuging the water precipitation solution, adding 10-100v% of ethanol into the obtained precipitate, dissolving to obtain an alcoholic solution of the precipitate; performing column chromatography on the alcoholic solution of the precipitate; concentrating the eluent obtained by eluting after column chromatography, adding ethanol to perform supersaturation dissolving, thereby obtaining a crystal; adding the crystal into purified water or ethanol for washing, thereby obtaining a primary product; uniformly mixing the primary product by using the purified water, drying, thereby obtaining the cannabidiol. According to the method disclosed by the invention, the highly safe ethanol serves as a solvent, so that the purity of the cannabidiol in the finished product is improved, a psychotoxic component, namely tetrahydrocannabinol, is removed, the product safety is improved, and the method is suitable for industrial production.
Owner:YUNNAN HANSU BIO TECH CO LTD
Use of cannabinoids in combination with an anti-psychotic medicament
ActiveUS9017737B2High activityLess degree of activityBiocideSenses disorderTypical antipsychoticPsychosis drug
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
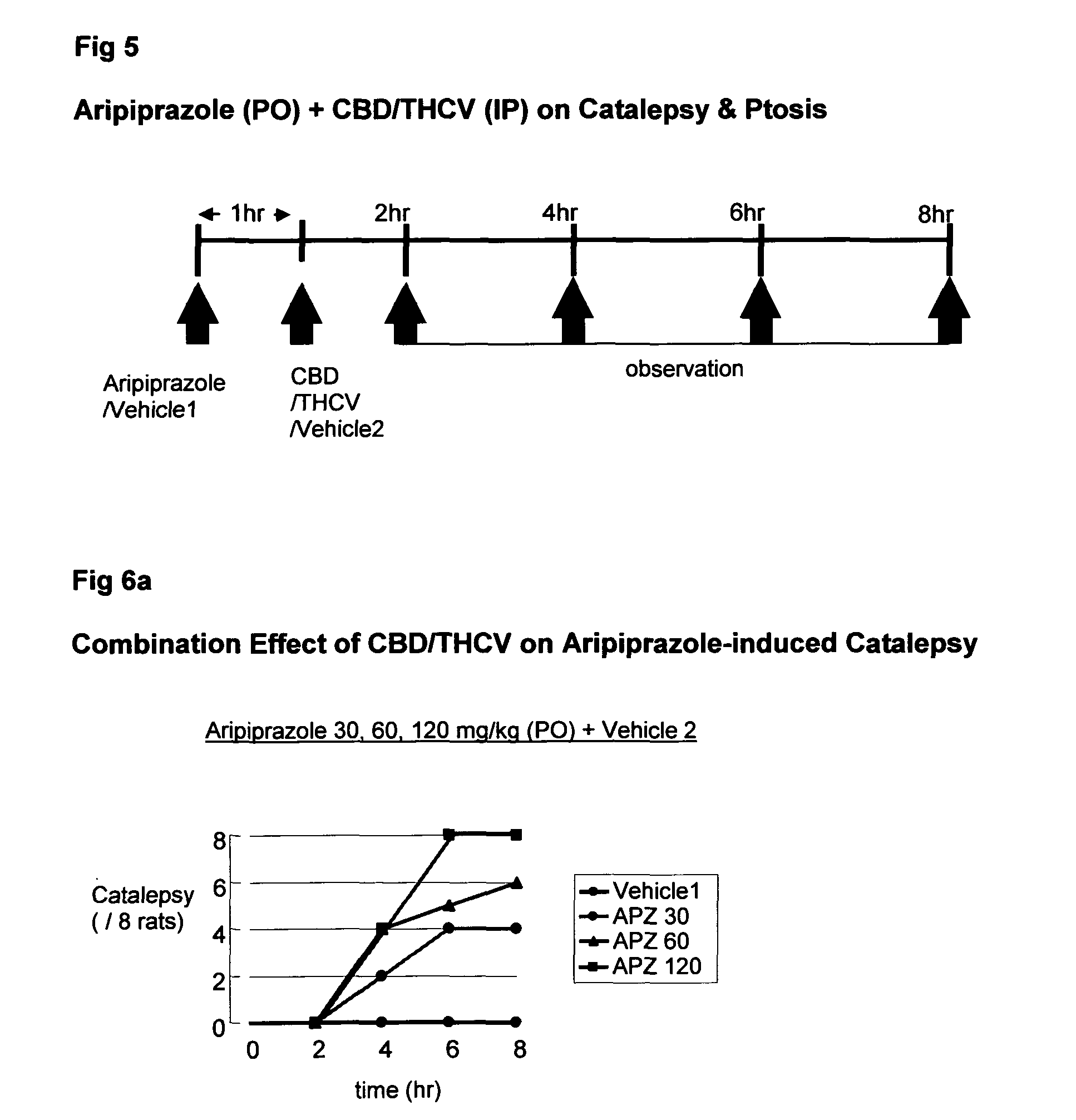
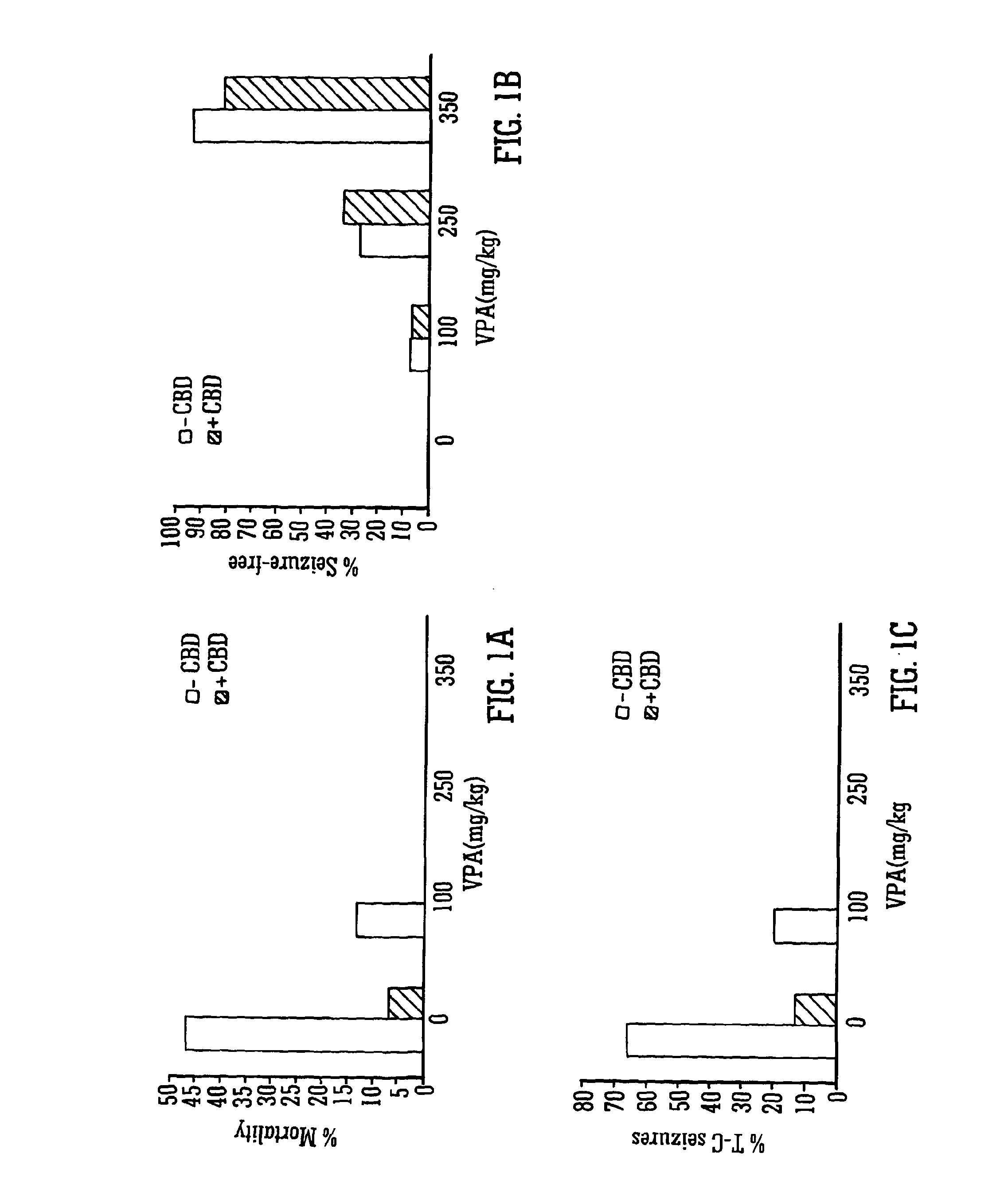
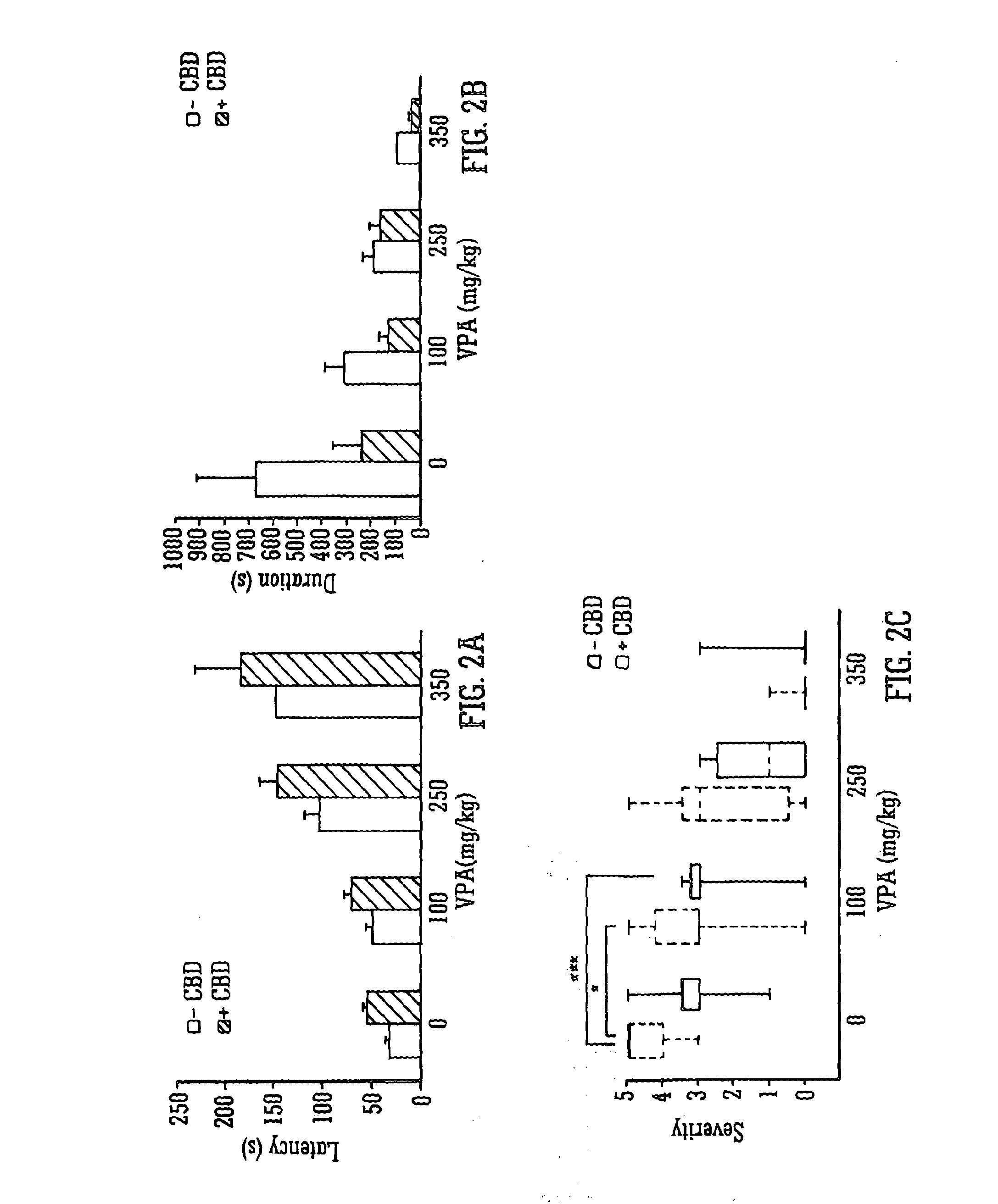
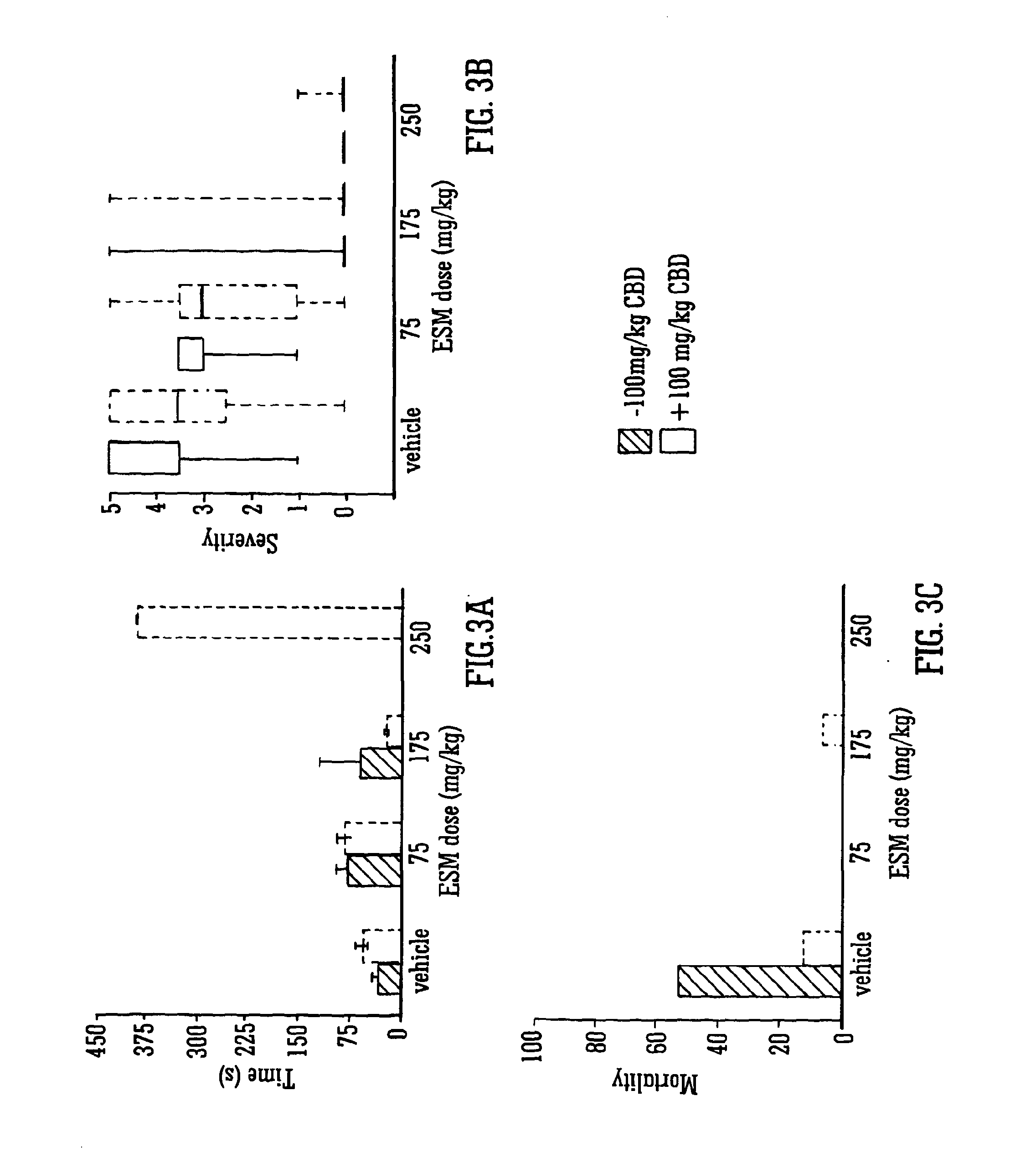
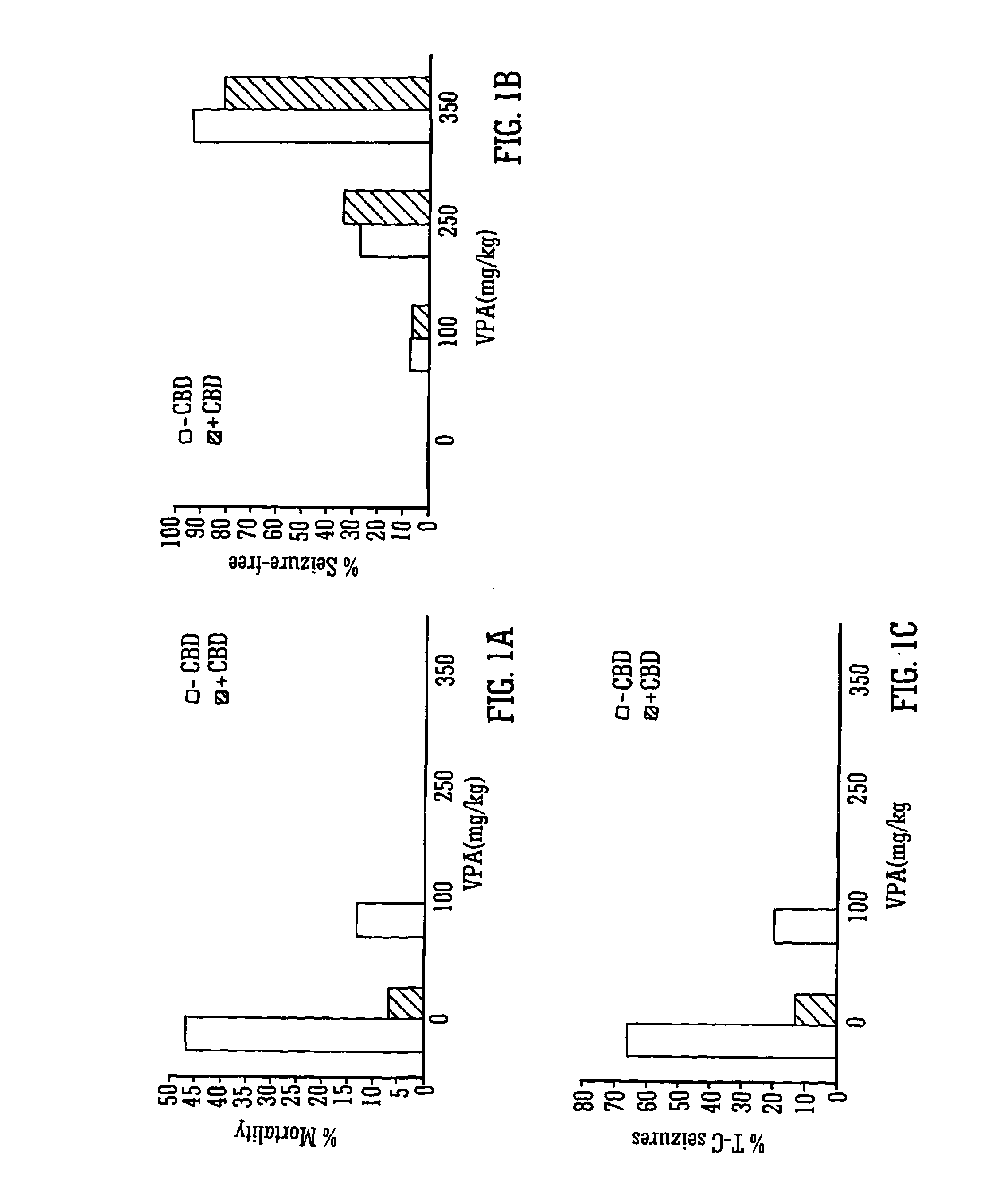
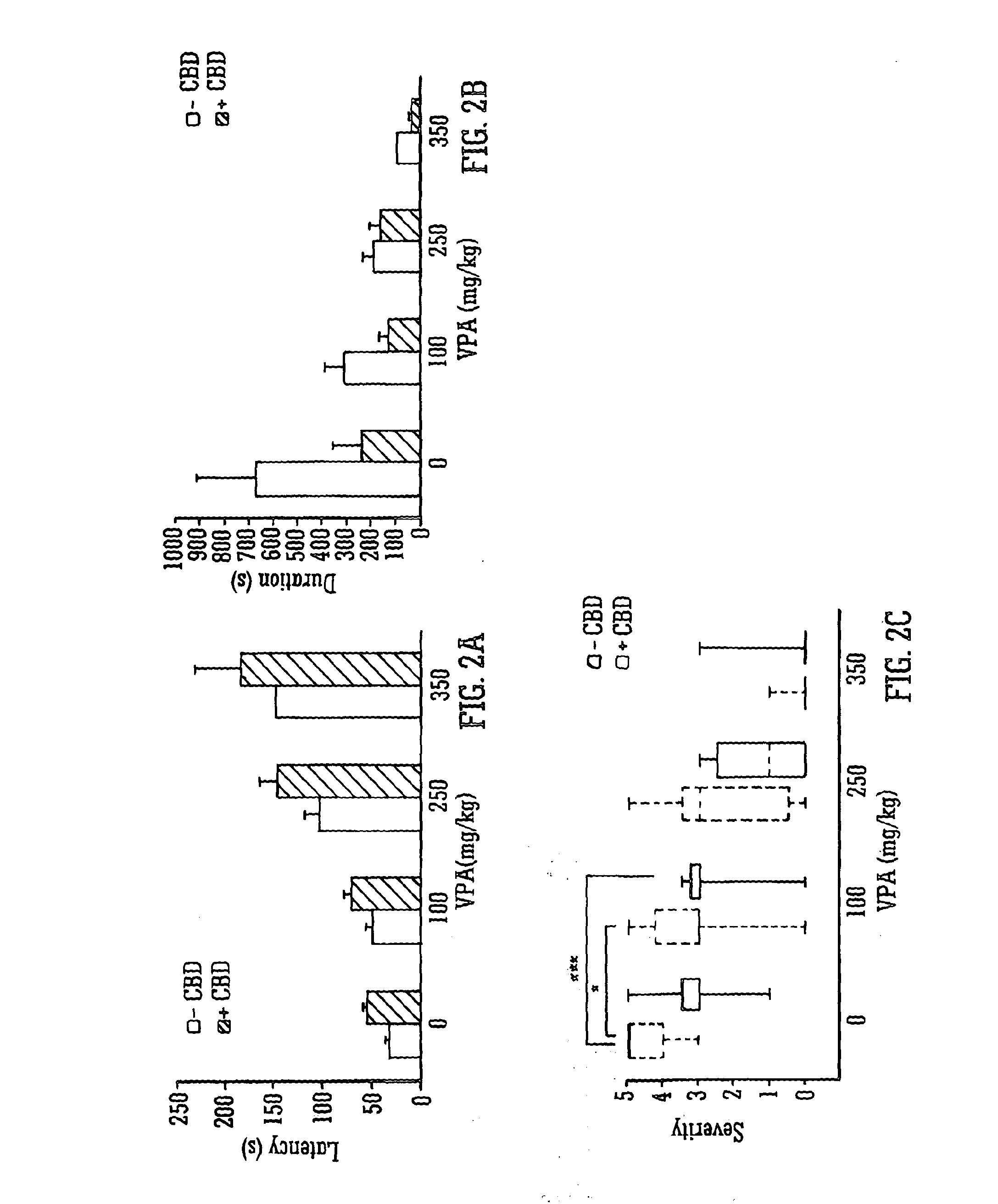
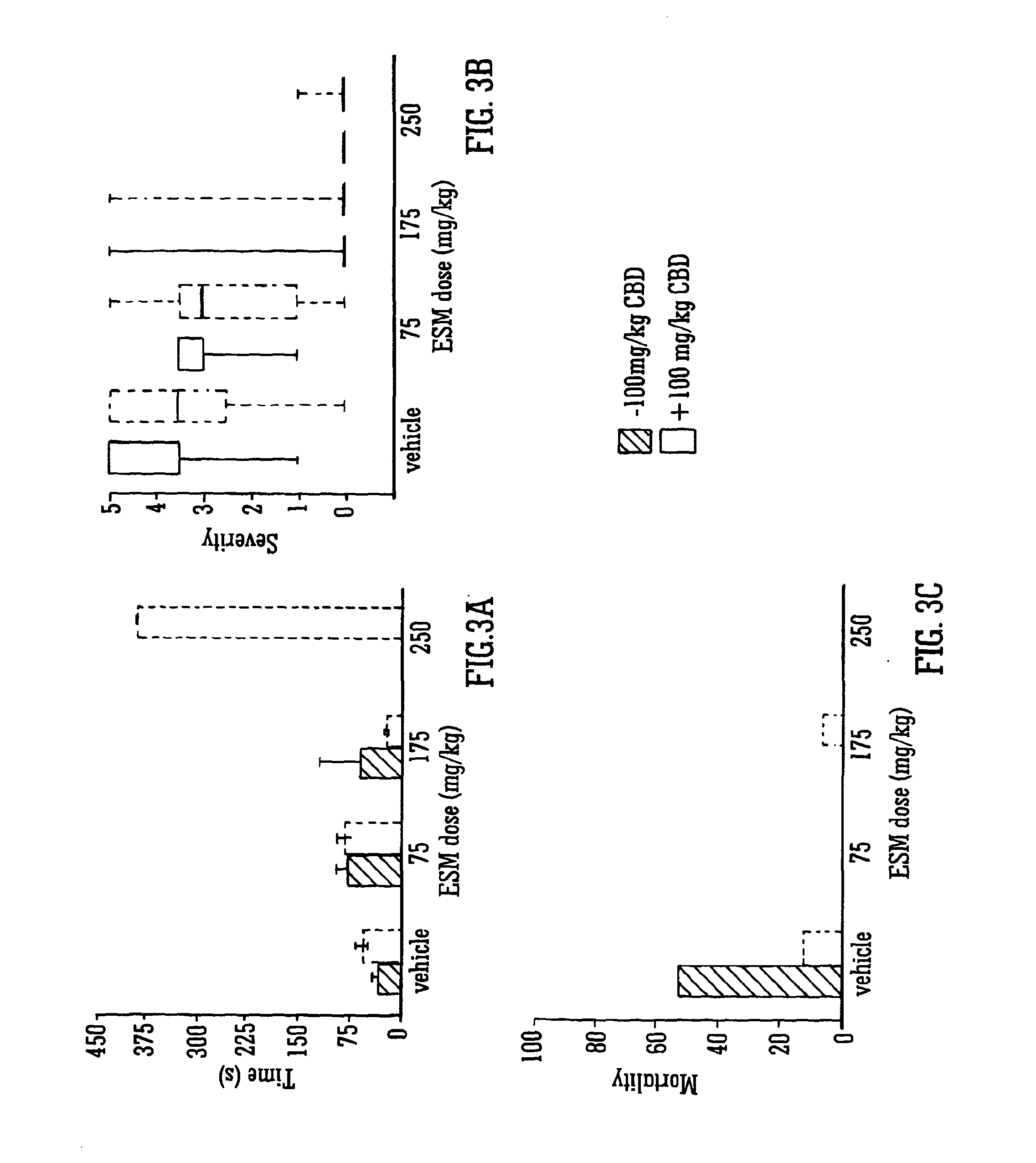
Use of the phytocannabinoid cannabidiol (CBD) in combination with a standard Anti-epileptic drug (SAED) in the treatment of epilepsy
InactiveUS20130296398A1Reduce severityReduce mortalityBiocideNervous disorderValproic AcidCannabidiol
The invention relates to the use of cannabidiol (CBD), at a dose of greater than 300 mg / day, in combination with a standard anti-epileptic drug (SAED) which acts via sodium or calcium channels, for use in the treatment of epilepsy. The SAED is preferably one which•modifies low-threshold or transient neuronal calcium currents,or•reduces high-frequency neuronal firing and sodium-dependent action potentials and enhances GABA effects. Preferred SAEDs are ethosuximide and valproate.
Owner:GW PHARMA LTD +1
New pharmaceutical formulation comprising cannabidiol and tetrahydrocannabidivarin
InactiveUS20100317729A1Enhanced treatment optionGood for weight lossBiocideNervous disorderCannabinoidPharmaceutical formulation
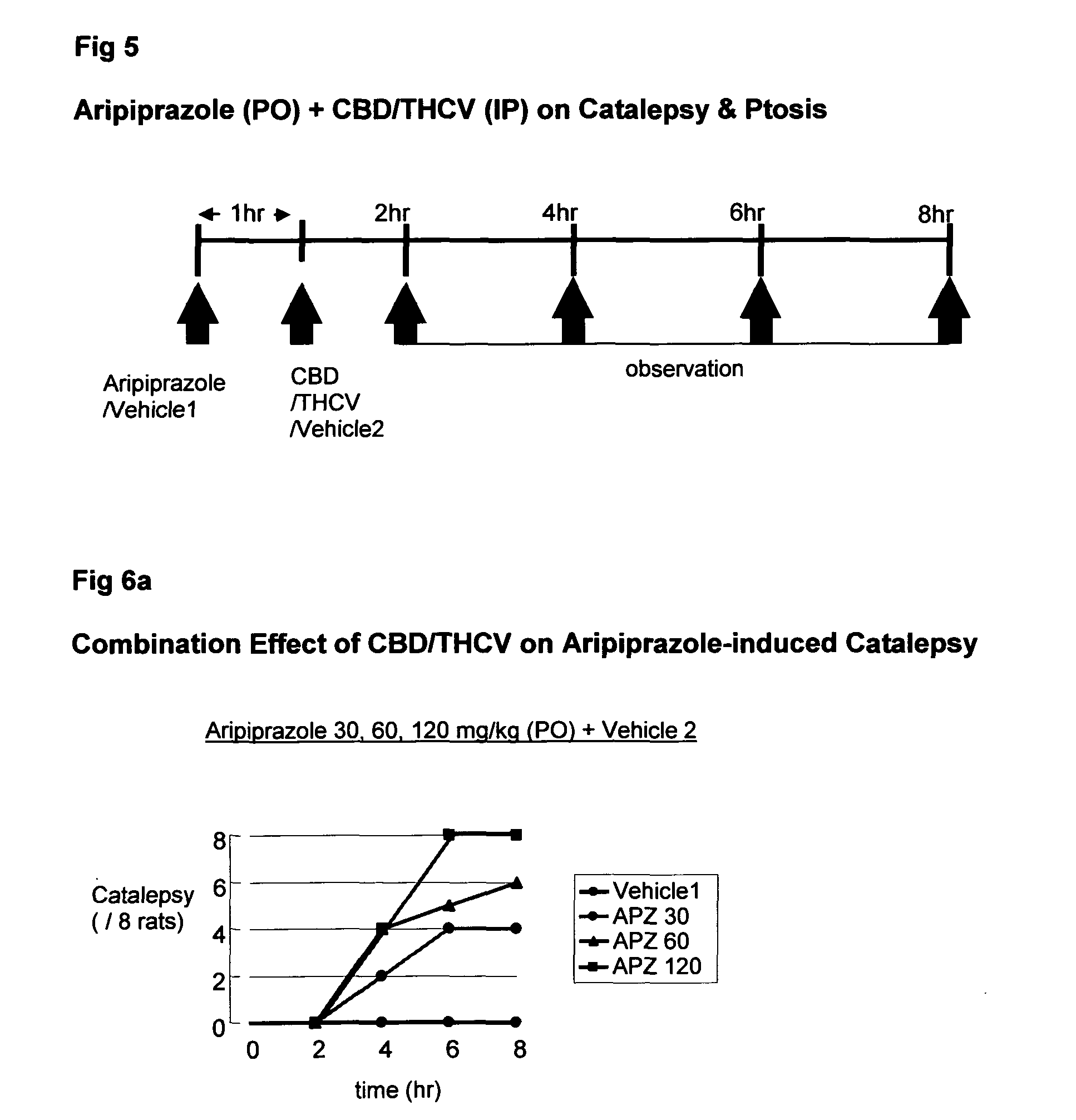
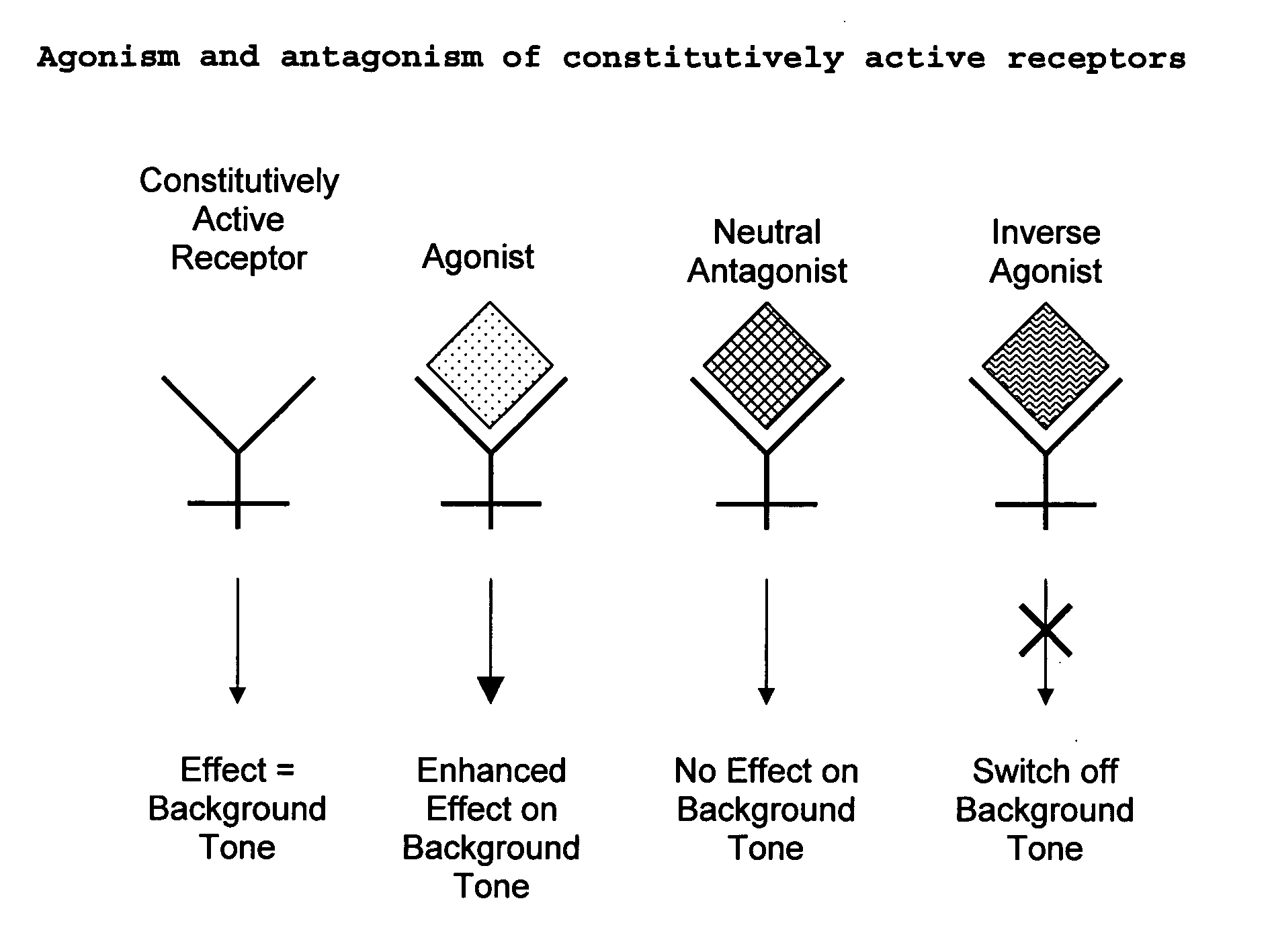
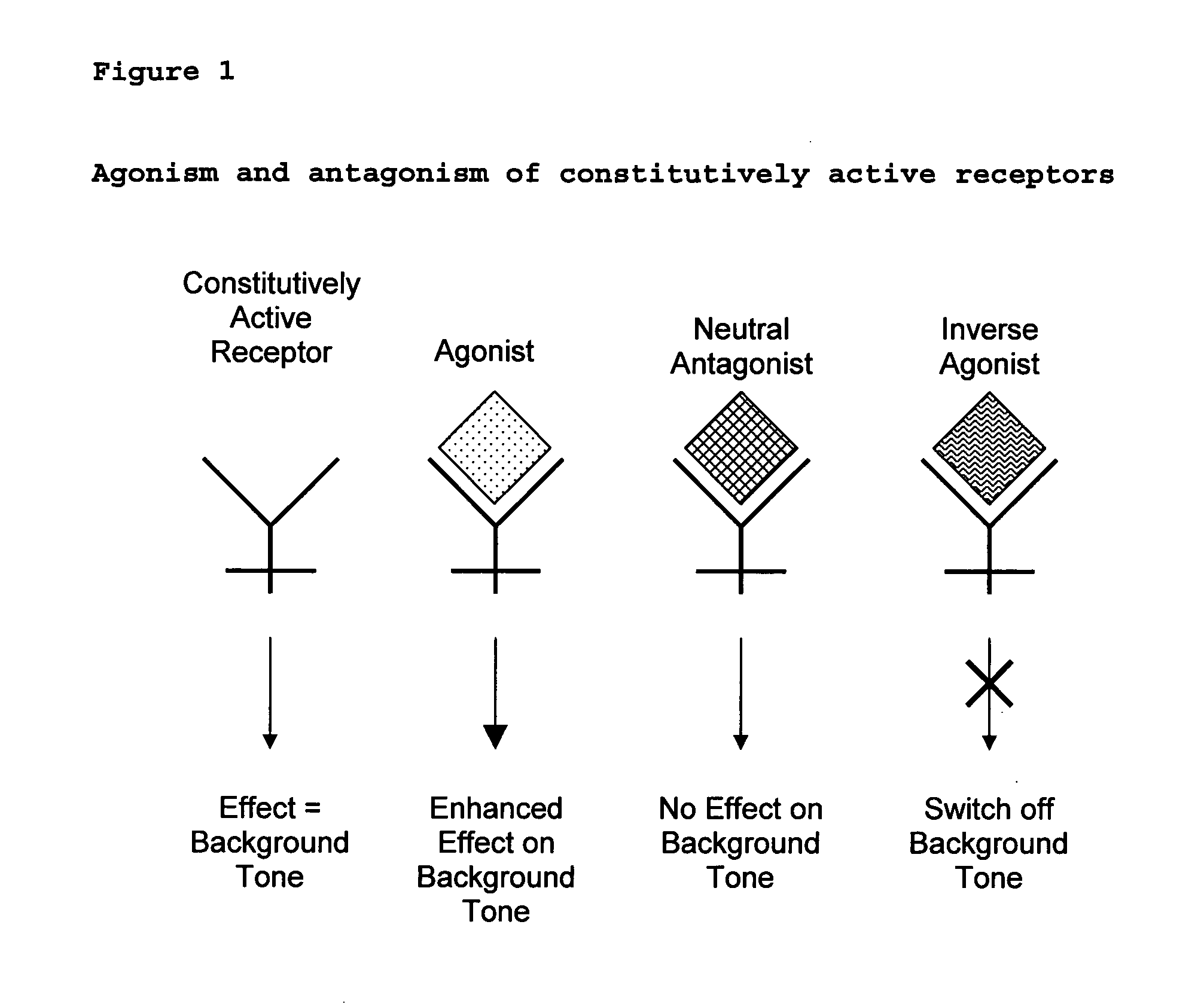
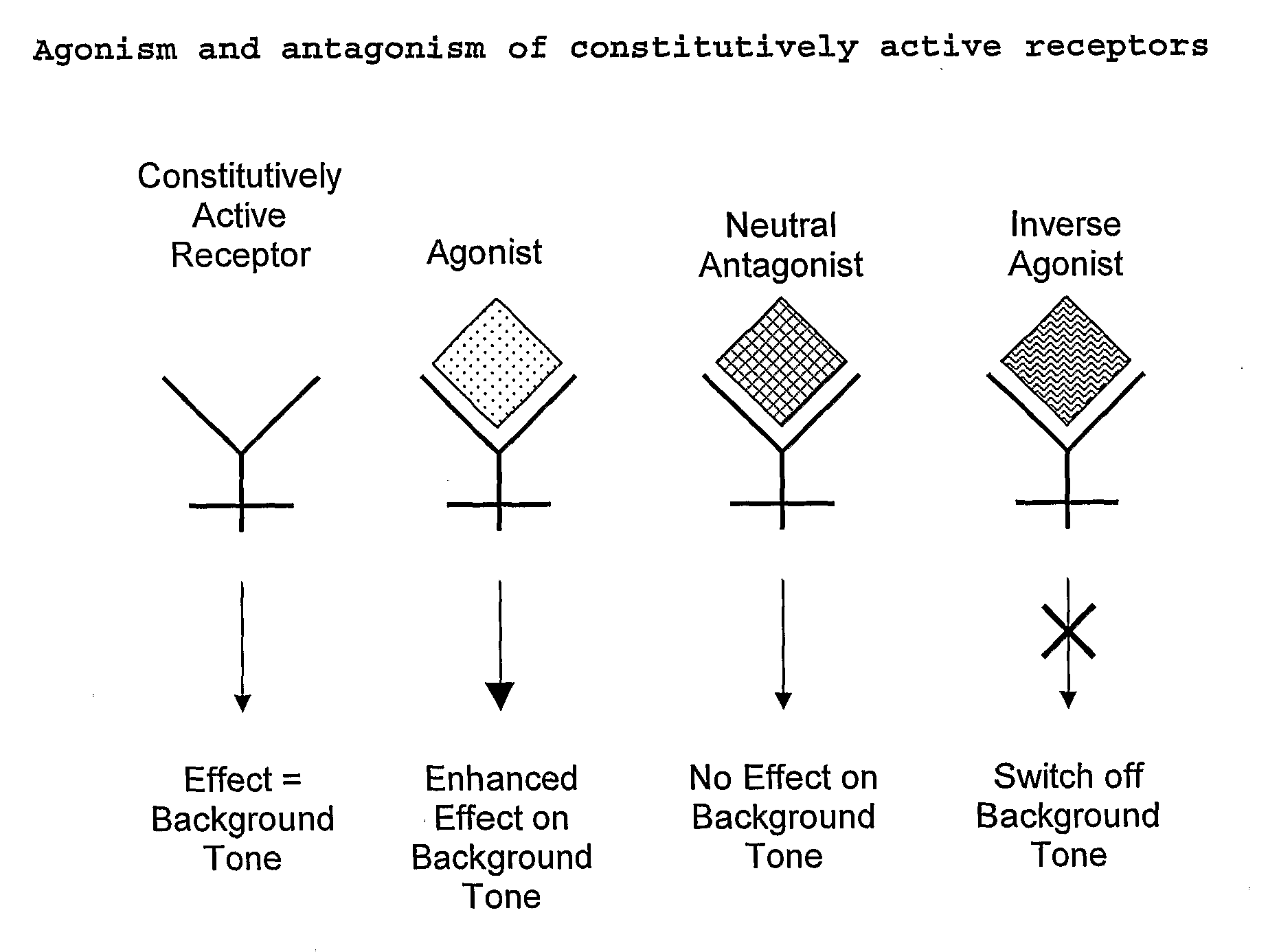
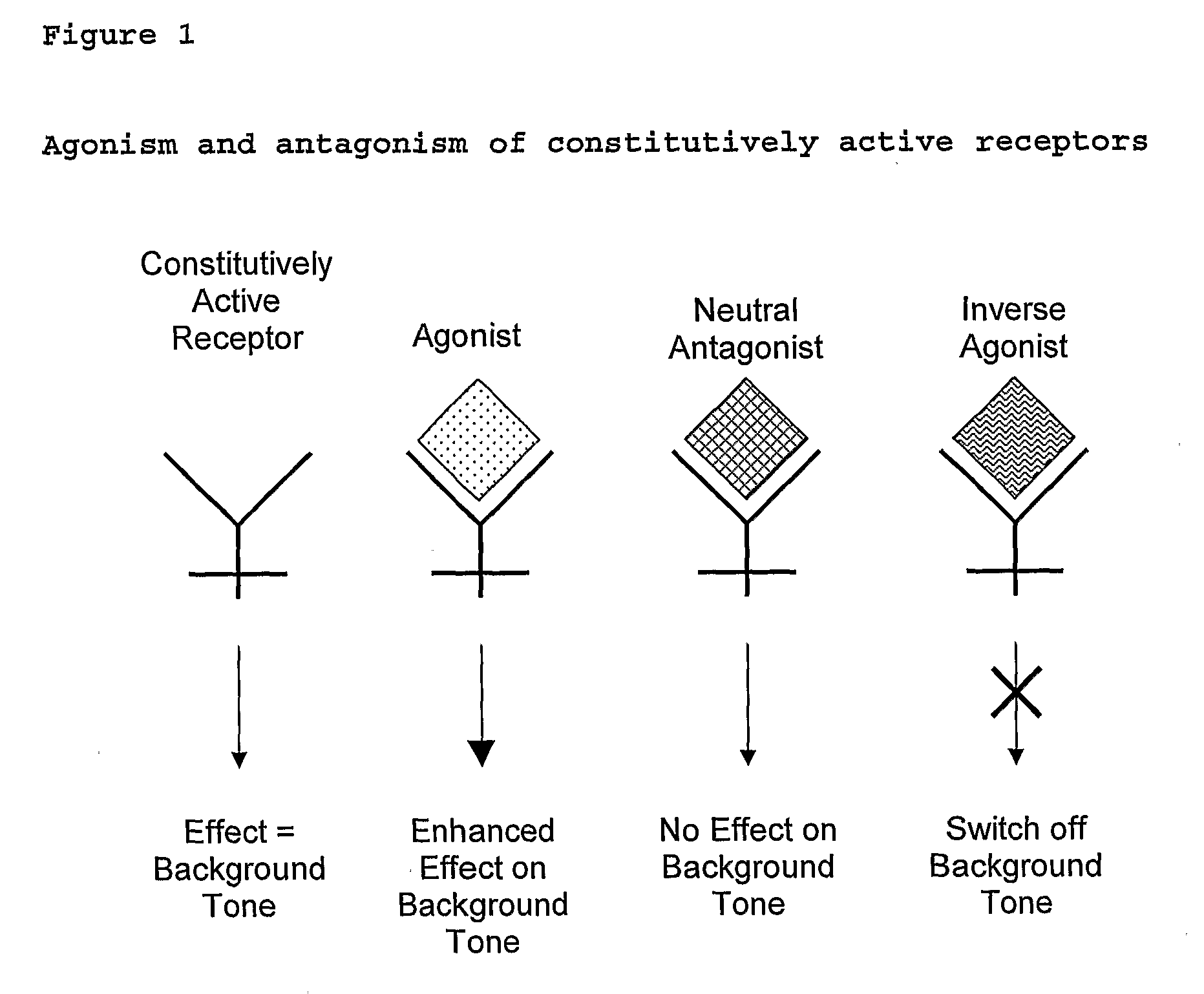
The present invention relates to a novel pharmaceutical formulation comprising a ratioed mix of: (i) one or more compounds that acts as an inverse agonist of the CB1 and / or CB2 receptor; and (ii) one or more compounds that acts as a neutral antagonist of the CB1 and / or CB2 receptor. Preferably both the inverse agonist of the CB1 and / or CB2 receptor and the neutral antagonist of the CB1 and / or CB2 receptor are cannabinoids. Preferably the cannabinoids are tetrahydrocannabidivarin (THCV) and cannabidiol (CBD).
Owner:GW PHARMA LTD
Use for Cannabinoid
InactiveUS20090306221A1High activityLess degree of activityBiocideNervous disorderDiseaseCannabinoid
The present invention relates to the use of one or more cannabinoids in the manufacture of medicaments for use in 0 the treatment of diseases and conditions benefiting from inverse agonism of the CB1 and / or the CB2 cannabinoid receptor. Preferably the cannabinoid is a cannabidiol (CBD) type compound or derivative thereof.
Owner:GW PHARMA LTD
Use of the phytocannabinoid cannabidiol (CBD) in combination with a standard Anti-epileptic drug (SAED) in the treatment of epilepsy
InactiveUS20140155456A9Reduces high-frequency neuronal firingGood effectBiocideNervous disorderValproic AcidCannabidiol
The invention relates to the use of cannabidiol (CBD), at a dose of greater than 300 mg / day, in combination with a standard anti-epileptic drug (SAED) which acts via sodium or calcium channels, for use in the treatment of epilepsy. The SAED is preferably one which•modifies low-threshold or transient neuronal calcium currents,or•reduces high-frequency neuronal firing and sodium-dependent action potentials and enhances GABA effects. Preferred SAEDs are ethosuximide and valproate.
Owner:GW PHARMA LTD +1
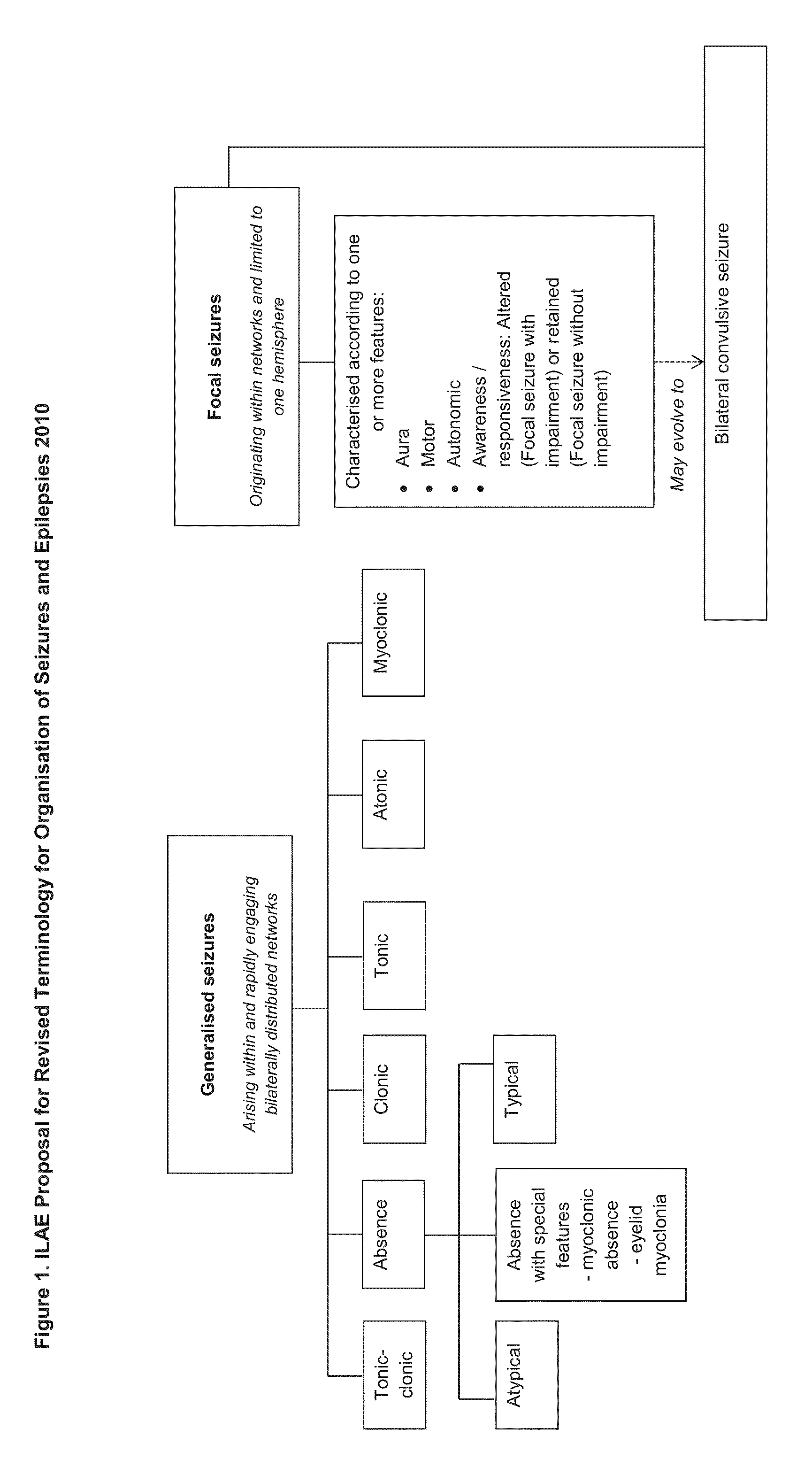
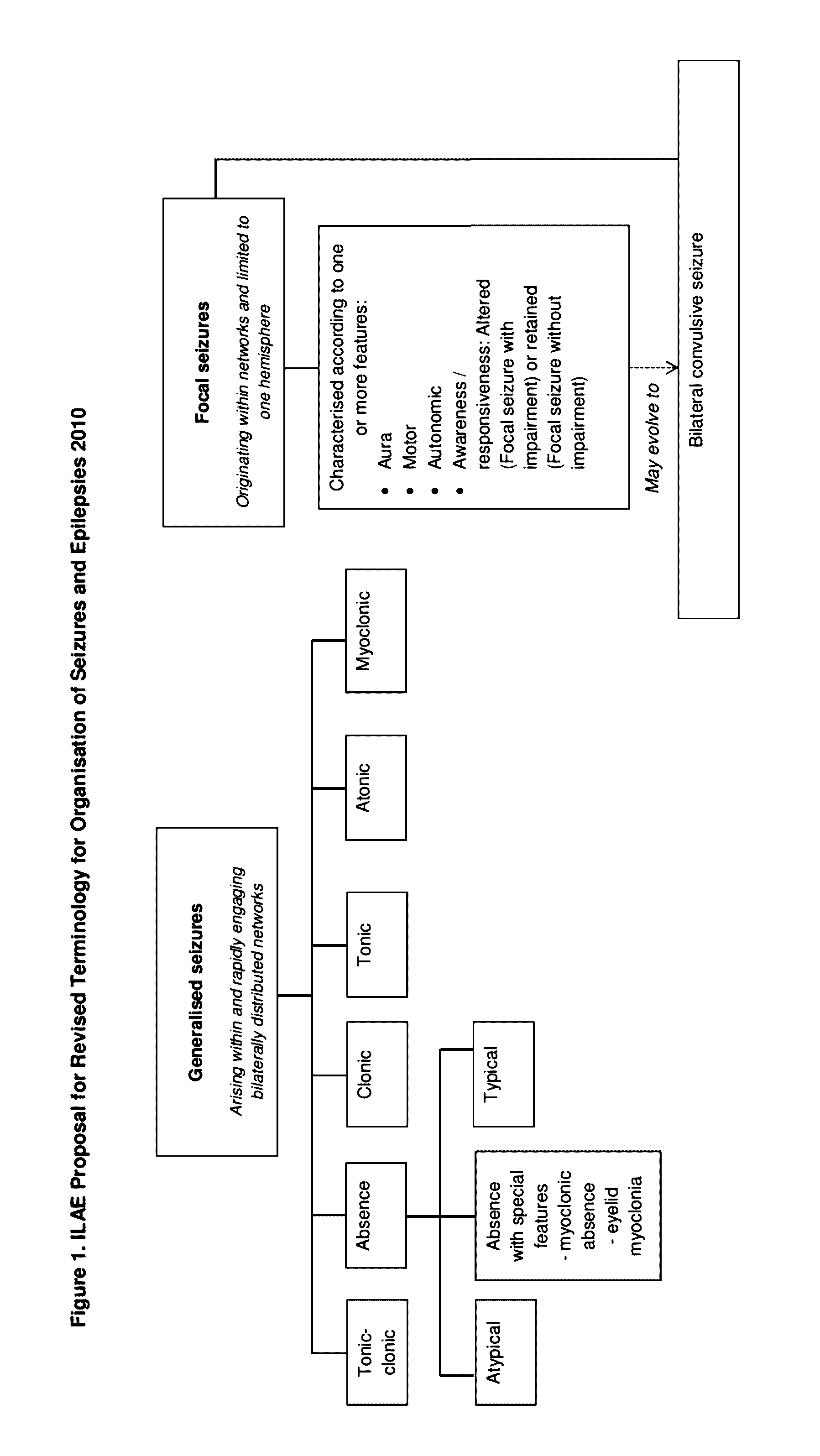
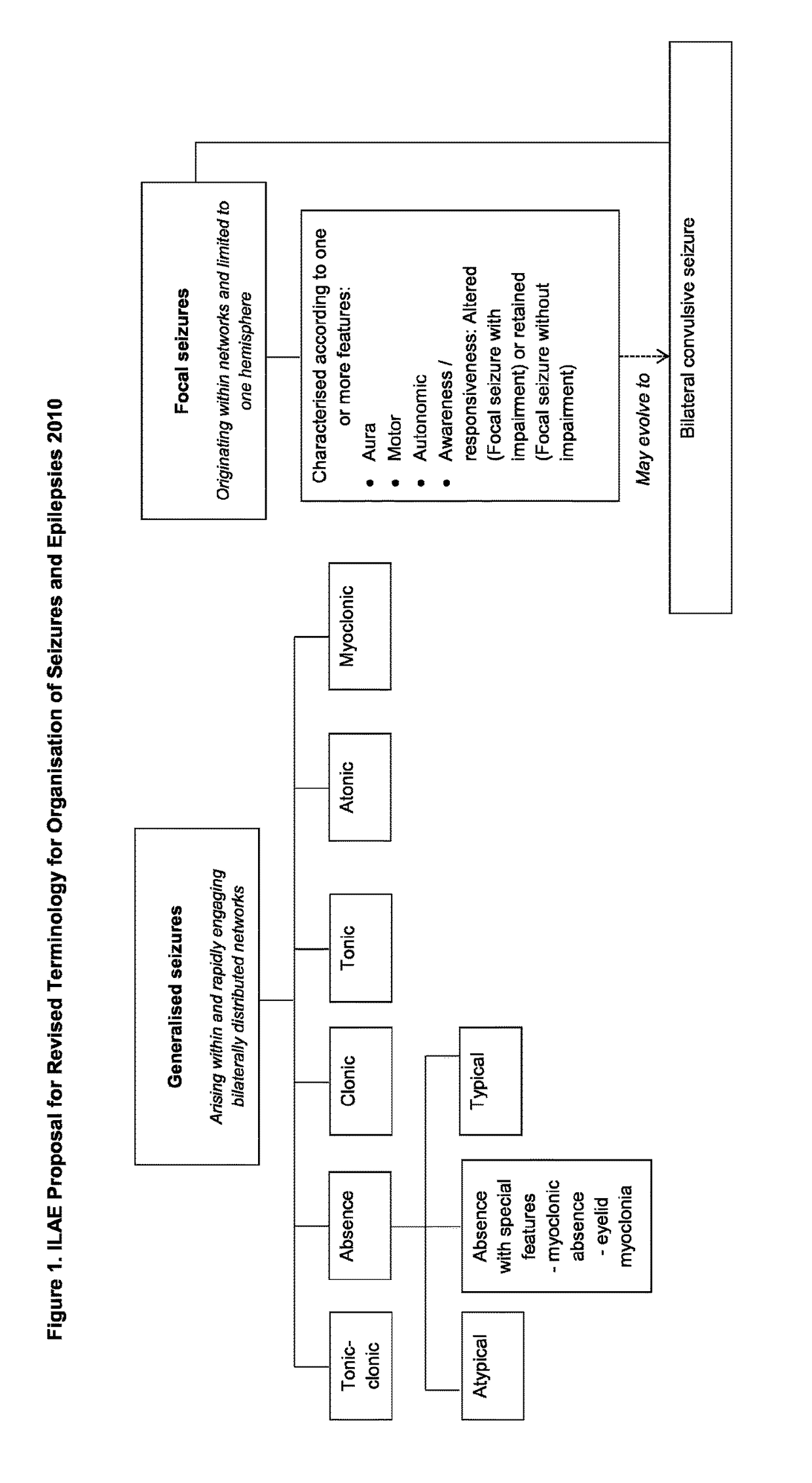
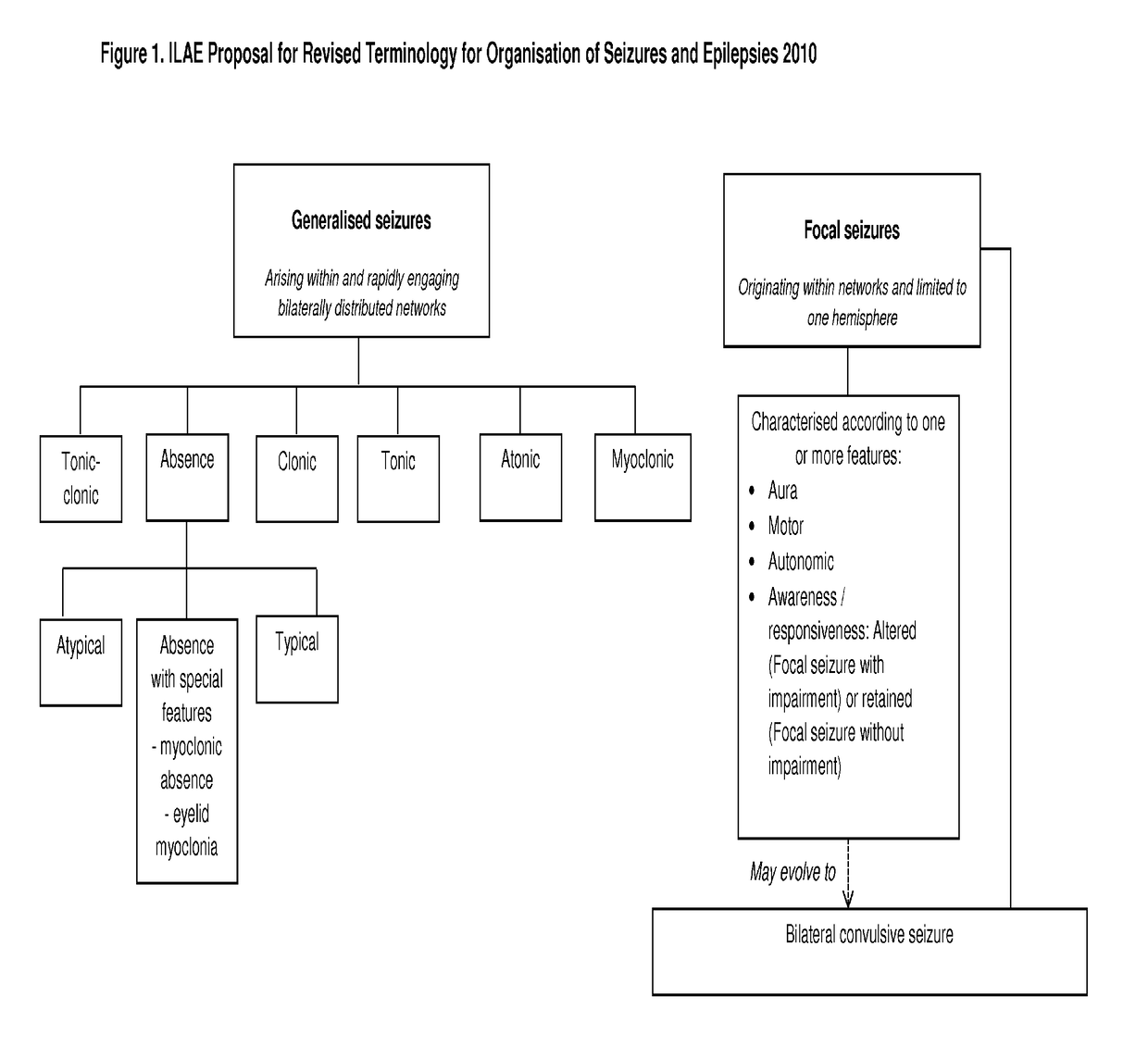
Use of cannabinoids in the treatment of epilepsy
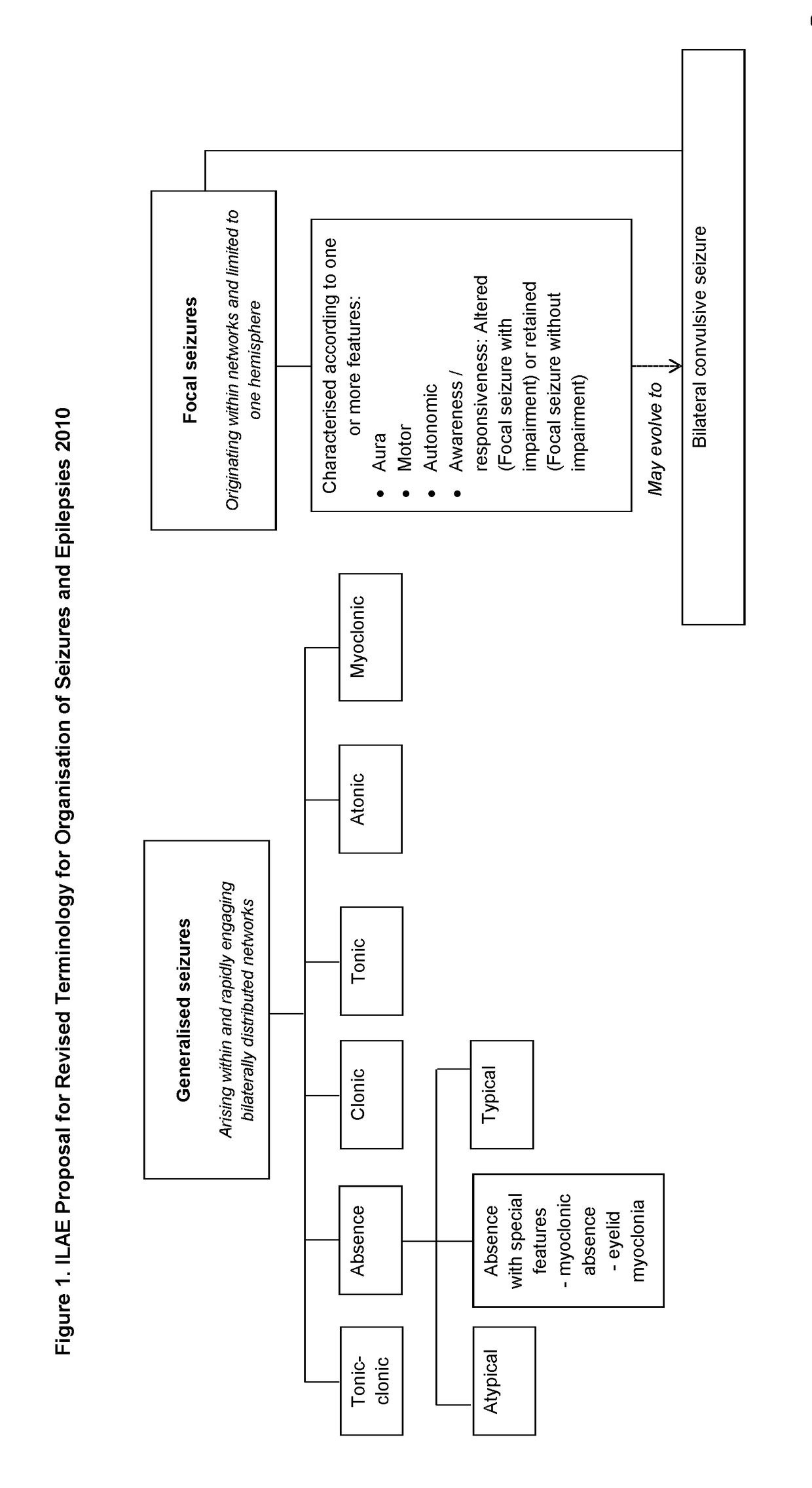
The present disclosure relates to the use of cannabidiol (CBD) in the treatment of absence seizures. In particular, the disclosure relates to the use of CBD for reducing absence seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; CDKL5; Dup15q; Jeavons syndrome; Myoclonic Absence Epilepsy; Neuronal ceroid lipofuscinoses (NCL) and brain abnormalities. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
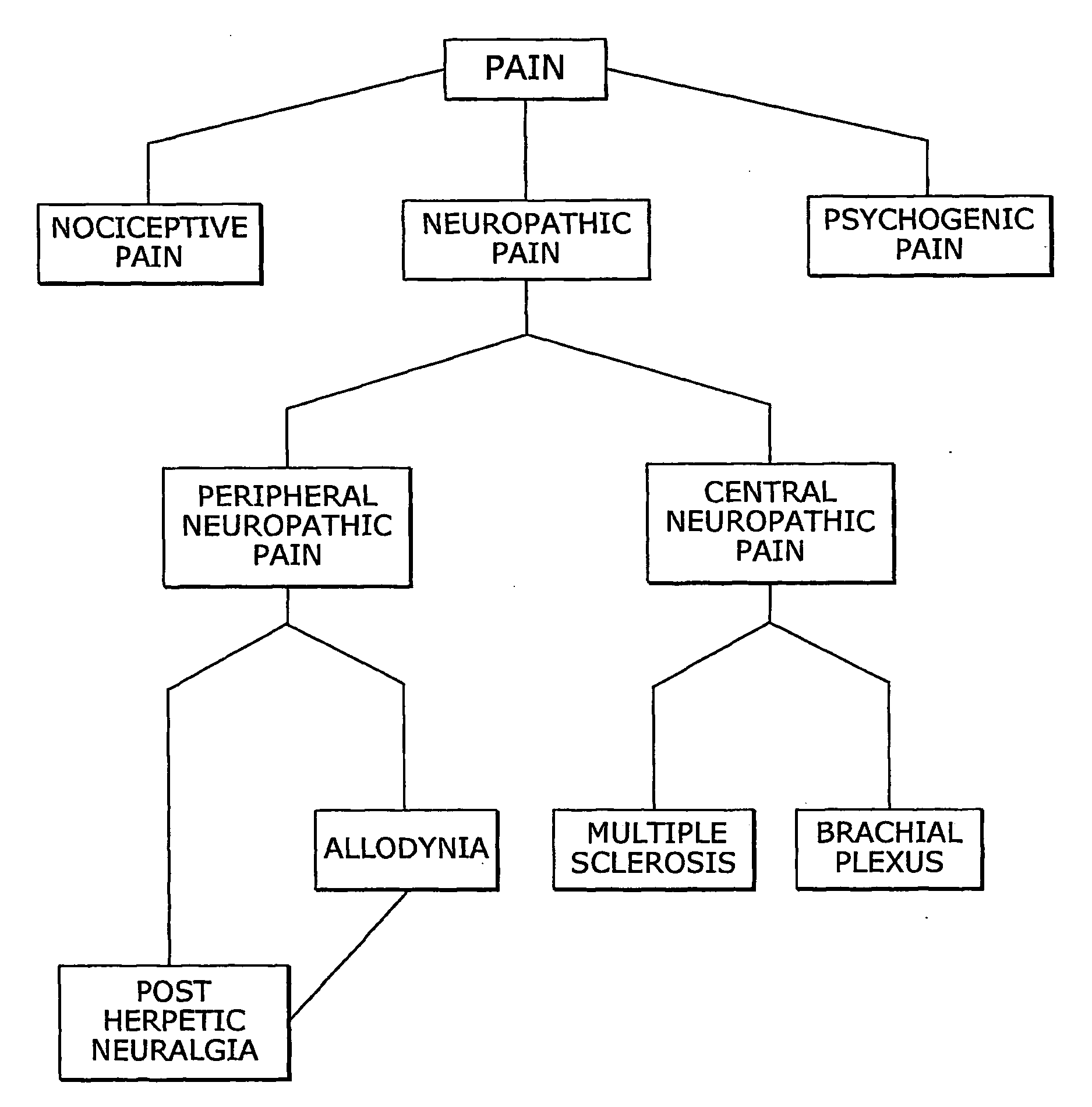
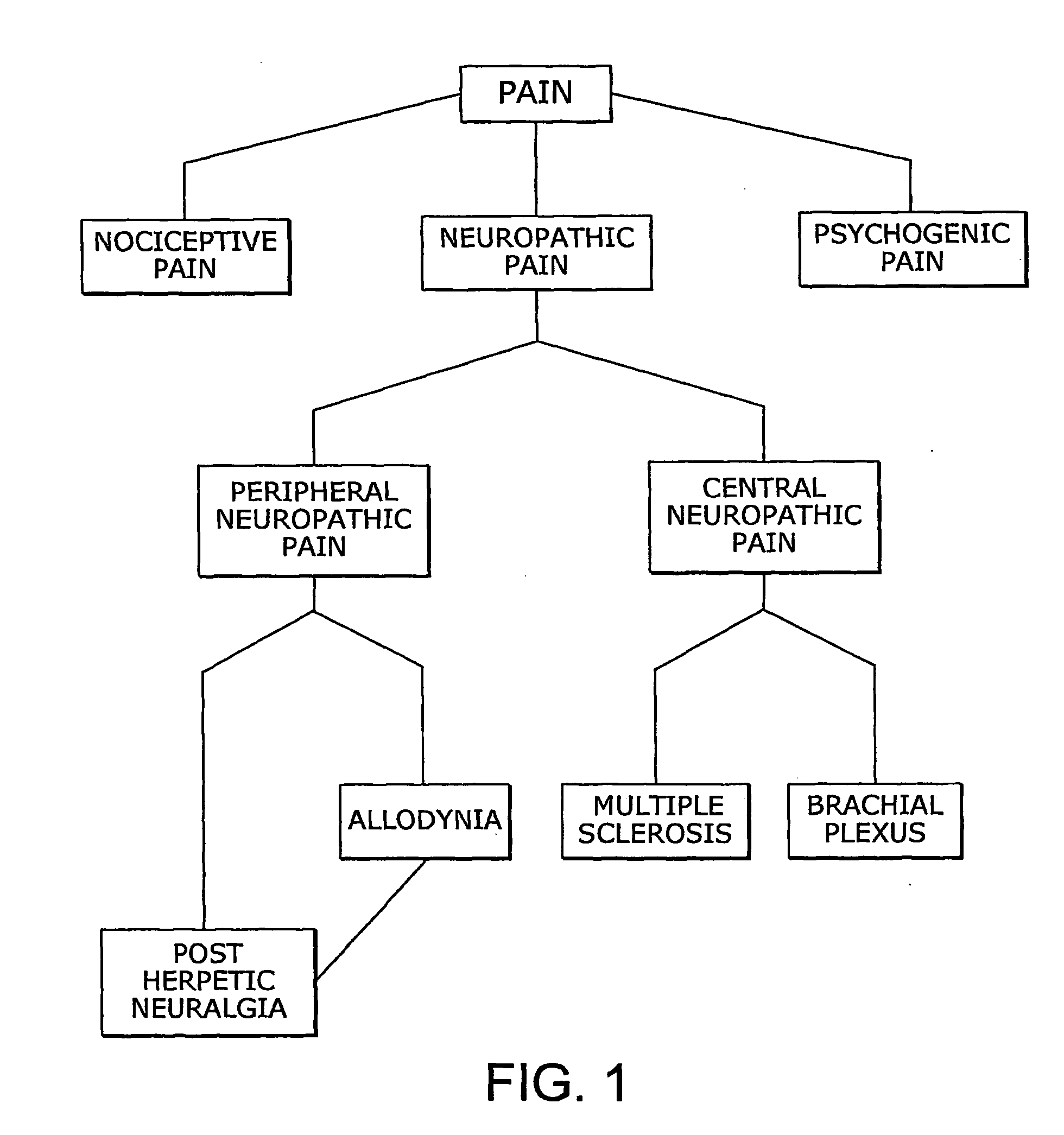
Combination of cannabinoids for the treatment of peripheral neuropathic pain
InactiveUS20100035978A1BiocideNervous disorderPeripheral neuropathic painDelta-9-tetrahydrocannabinol
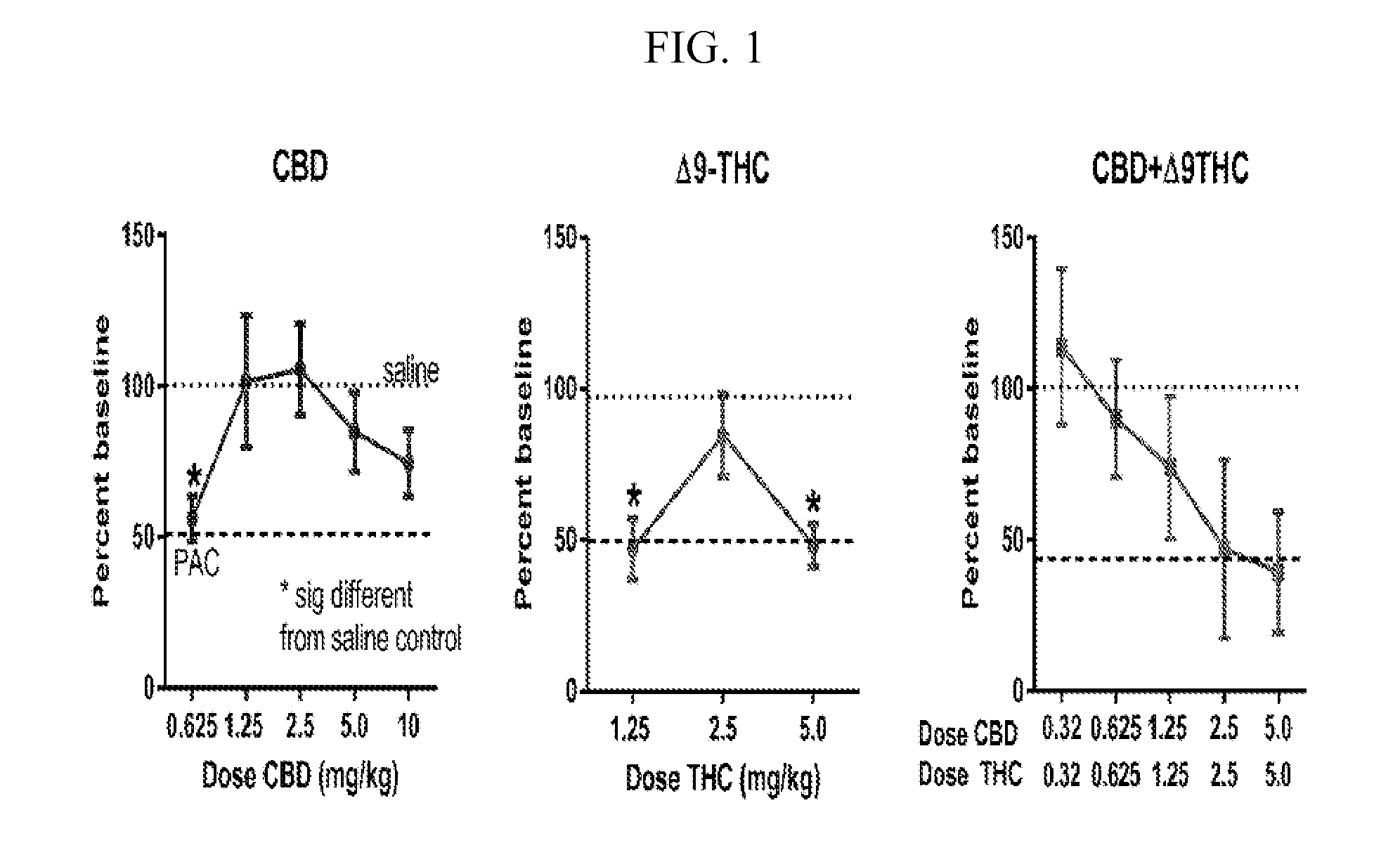
The present invention relates to the use of a combination of cannabinoids in the treatment of neuropathic pain, in particular peripheral neuropathic pain. A combination of cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) may be used, wherein the ratio of CBD:THC by weight is between 10:1 and 1:10.
Owner:GW PHARMA LTD
Method for extracting and purifying cannabidiol from China-hemp
ActiveCN107337586AImprove solubilityHigh extraction rateOrganic chemistryOrganic compound preparationOrganic solventElution
The invention belongs to the technical field of extraction and purification of plant ingredients and particularly relates to a method for extracting and purifying cannabidiol from China-hemp. In the method, an ingredient of cannabidiol is extracted from floral leaves in a mature period of the China-hemp according to a supercritical CO2 extraction technique, a cannabidiol extract is further purified by macroporous adsorbent resin and a rapid purification system, a cannabidiol content of an obtained product is 98% or higher, and the cannabidiol product does not contain a psychotoxic ingredient tetrahydrocannabinol. According to the method, a chemical organic solvent is not used in an extraction process, so that the method is environment-friendly and is low in toxicity and high in efficiency; the loss of cannabidiol in an adsorption elution process is reduced in a purification process, so that the impurity ingredients such as tetrahydrocannabinol and the like are effectively removed, and the use of an organic regent is decreased, so that the purification cost is lowered, and the environmental pollution is reduced. The product is high in yield and purity, and the requirement for development of a value-added product of the China-hemp, in particular to the market requirement of the active ingredient of the cannabidiol product in the China-hemp, can be met, and especially, the cannabidiol product has the great application advantage in the pharmaceutical field.
Owner:DAQING BRANCH OF HEILONGJIANG ACAD OF SCI
Use of cannabidiol in the treatment of epilepsy
ActiveUS20170231923A1Nervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Clonic Seizures
The present disclosure relatesto the use of cannabidiol (CBD) for the treatment of seizures associated with Aicardi Syndrome. In one embodiment the seizures associated with Aicardi Syndrome are convulsive seizures; focal seizures with impairment or infantile spasm. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS20170007551A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
The present invention relates to the use of cannabidiol (CBD) in the treatment of focal seizures. In one embodiment the patients suffering from focal seizures are children and young adults. CBD appears particularly effective in reducing focal seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; CDKL5; Neuronal ceroid lipofuscinoses (NCL); febrile infection related epilepsy syndrome (FIRES); Aicardi syndrome and brain abnormalities in comparison to other seizure types. Significantly CBD additionally is very effective in the reduction of a sub-type of focal seizures, focal seizures with impairment.
Owner:GW RES LTD
Extraction and separation method of cannabidiol
ActiveCN108314608AGood removal effectRemove completelyOrganic chemistryOrganic compound preparationSolubilityOrganic solvent
The invention discloses an extraction and separation method of cannabidiol. According to the method disclosed by the invention, the water solubility of the cannabidiol is enhanced through adopting analkaline solution and the cannabidiol is extracted and enriched by utilizing an organic solvent; then the cannabidiol is purified and enriched through a polyamide resin column, neutral aluminum oxideand a bonded silica gel column; then the cannabidiol is crystallized to obtain high-purity cannabidiol. The method disclosed by the invention is easy to operate, strong in practicability and suitablefor industrial popularization and application.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV +1
Treating or preventing diabetes with cannabidiol
ActiveUS20070099987A1Improve survivalBiocideHydroxy compound active ingredientsDiabetes mellitusInsulitis
Use of a cannabidiol for the manufacture of a medicament identified for the treatment or prevention of diabetes and / or insulitis.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
Use of cannabidiol in the treatment of nocturnal snoring
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of of nocturnal snoring. In particular the CBD appears particularly effective in treating nocturnal snoring in children and young adults with epilepsy the disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
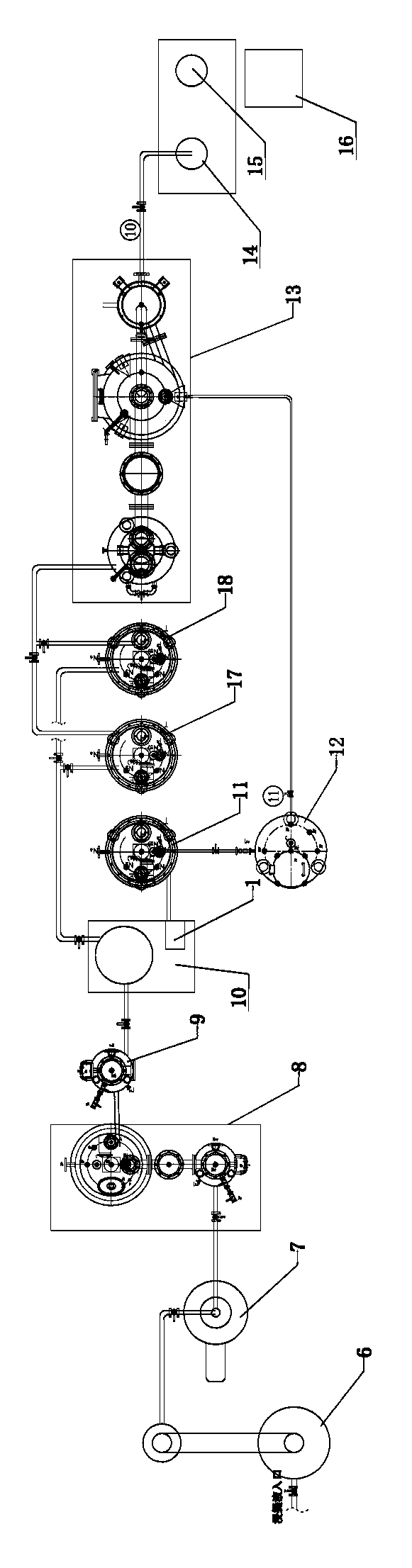
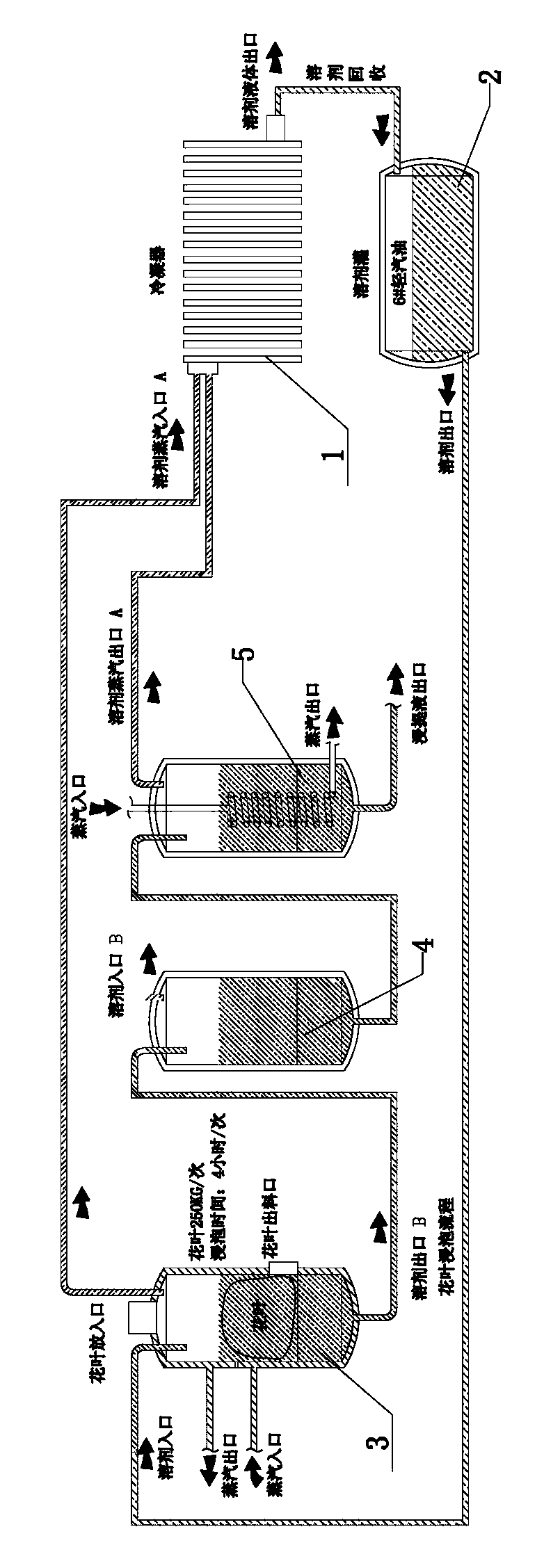
Extraction method and extraction device for cannabidiol-enriched industrial hemp essential oil
ActiveCN104277917AEnsure safetyEnsure normativeEssential-oils/perfumesRotary evaporatorProcess engineering
The invention discloses an extraction method and an extraction device for cannabidiol-enriched industrial hemp essential oil. By adopting devices such as a screening machine, an oven, a soaking tank, a rotary evaporator, an ultrasonic agitation tank, a butterfly centrifuge, a first climbing-film evaporator, a proportioning tank, a pressurized chromatography silica gel column, an eluate tank, a second climbing-film evaporator, a rotary evaporator, a finished product tank and a solvent recovery tank which are sequentially connected by virtue of pipelines, industrial hemps are stepwise subjected to the operation steps comprising screening, baking, extracting, re-treating, filtering, monitoring, sampling, chromatography and concentrating to obtain the finished product and the effective component cannabidiol-enriched industrial hemp essential oil is finally extracted. By virtue of the extraction device disclosed by the invention, the defects of low efficiency, large consumption and inconvenience in scale production of the conventional process are overcome, the semi-finished product containing tetrahydrocannabinol in the processing does not artificially contact and flow, is legal, safe and efficient, the process method is simple and can be easily promoted and the product has high purity, complete active ingredients and low cost.
Owner:HANKANG YUNNAN BIOTECH
Stable cannabinoid formulations
The present invention is generally directed to substantially pure cannabidiol, stable cannabinoid pharmaceutical formulations, and methods of their use.
Owner:RADIUS PHARMA INC
Use of Cannabinoids in the Treatment of Epilepsy
ActiveUS20170172941A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsEtiologyAicardi's syndrome
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of atonic seizures. In particular the CBD appears particularly effective in reducing atonic seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; Aicardi syndrome; CDKL5 and Dup15q in comparison to other seizure types. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
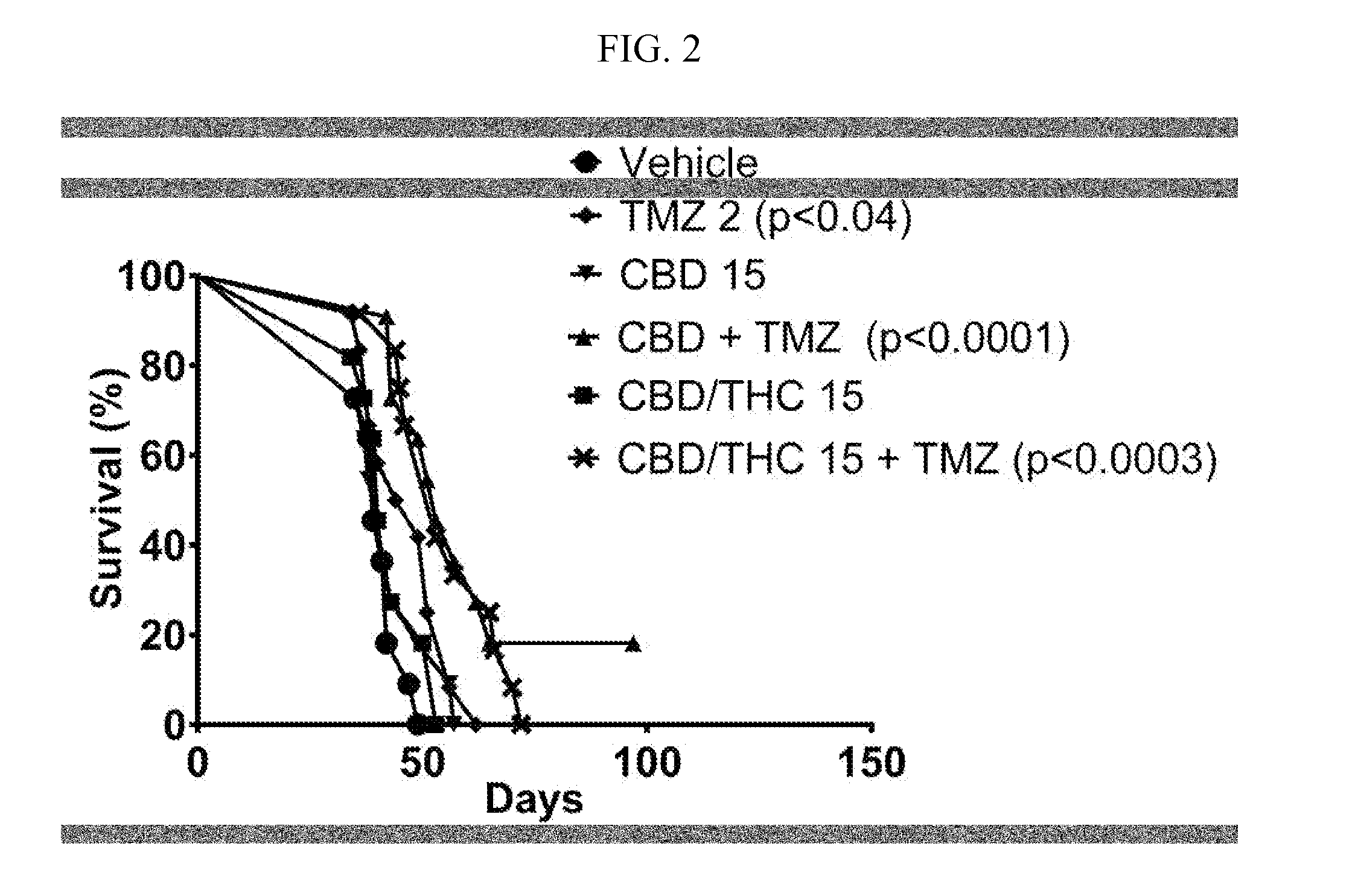
Pharmaceutical compositions for the treatment of pain
The present invention relates to treatment of cancer related pain and constipation. Preferably the subject in need is administered a combination of the cannabinoids cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC). More preferably the cannabinoids are in a predefined ratio by weight of approximately 1:1 of CBD to THC.
Owner:GW RES LTD
Anti-aging dermal composition comprising herbal extracts
This application discloses an anti-aging composition for dermal application comprising cannabidiol hemp oil, chuanxiong extract, mondo grass extract, Chinese foxglove extract, female and panax ginseng extract, dragon's blood resin, lilyturf root, and jojoba oil. Other active ingredients may include licorice root, Astragalus membranceus, tree peony, mulberry bark extraction, longan fruit, wild pansy, rose canina seed powder, and Radix polygoni multiflori. The composition is formulated with other components to serve as a cleanser, a moisturizer, a serum, a gel masque, an eye cream, a toner, or an exfoliant.
Owner:HDDC HLDG
Pharmaceutical Compositons for the Treatment of Chronic Obstructive Pulmonary Disease
InactiveUS20090197941A1BiocideHydroxy compound active ingredientsDelta-9-tetrahydrocannabinolObstructive Pulmonary Diseases
The invention relates to the use of a combination of cannabinoids for the treatment of Chronic Obstructive Pulmonary Disease (COPD). Preferably the combination of cannabinoids are cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC). More preferably the cannabinoids are in a predefined ratio by weight of approximately 1:1 of CBD to THC.
Owner:GW PHARMA LTD
High cannabidiol cannabis strains
InactiveUS20160000843A1Quality improvementImprove uniformityBiocideNervous disorderHigh concentrationMedicine
The invention described herein relates to a cannabis cultivar that produces high concentrations of cannabidiol. The invention further relates to preparations and products derived from the cannabis cultivar. Also provided are methods of treating conditions that are treatable by cannabidiol. by administering a preparation or product derived from the cannabis cultivar.
Owner:MJAR HLDG
Method for extracting compositions from plants
ActiveUS20190241536A1Reduce intensityIncrease concentrationOrganic chemistrySolvent extractionHigh concentrationContinuous flow
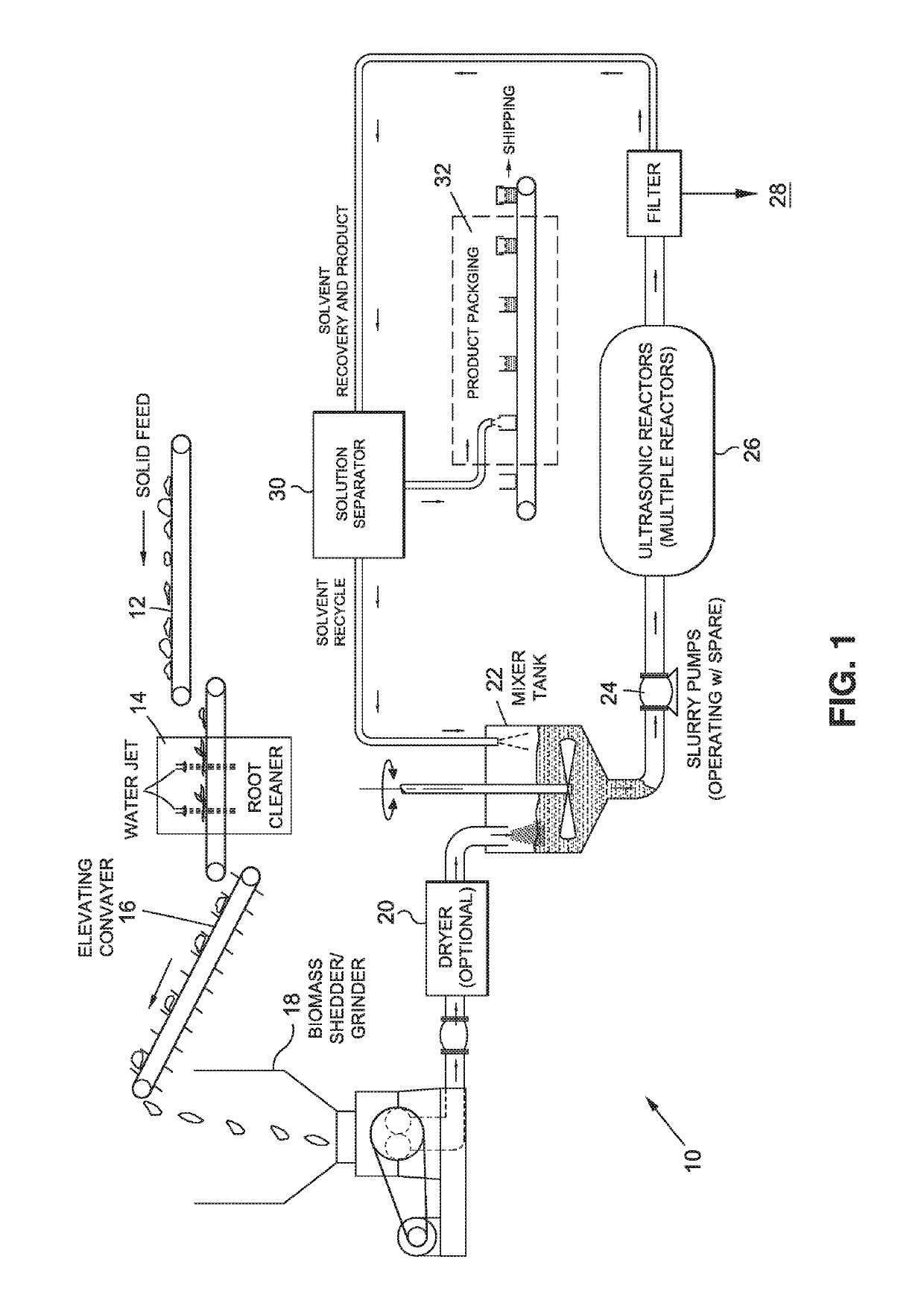
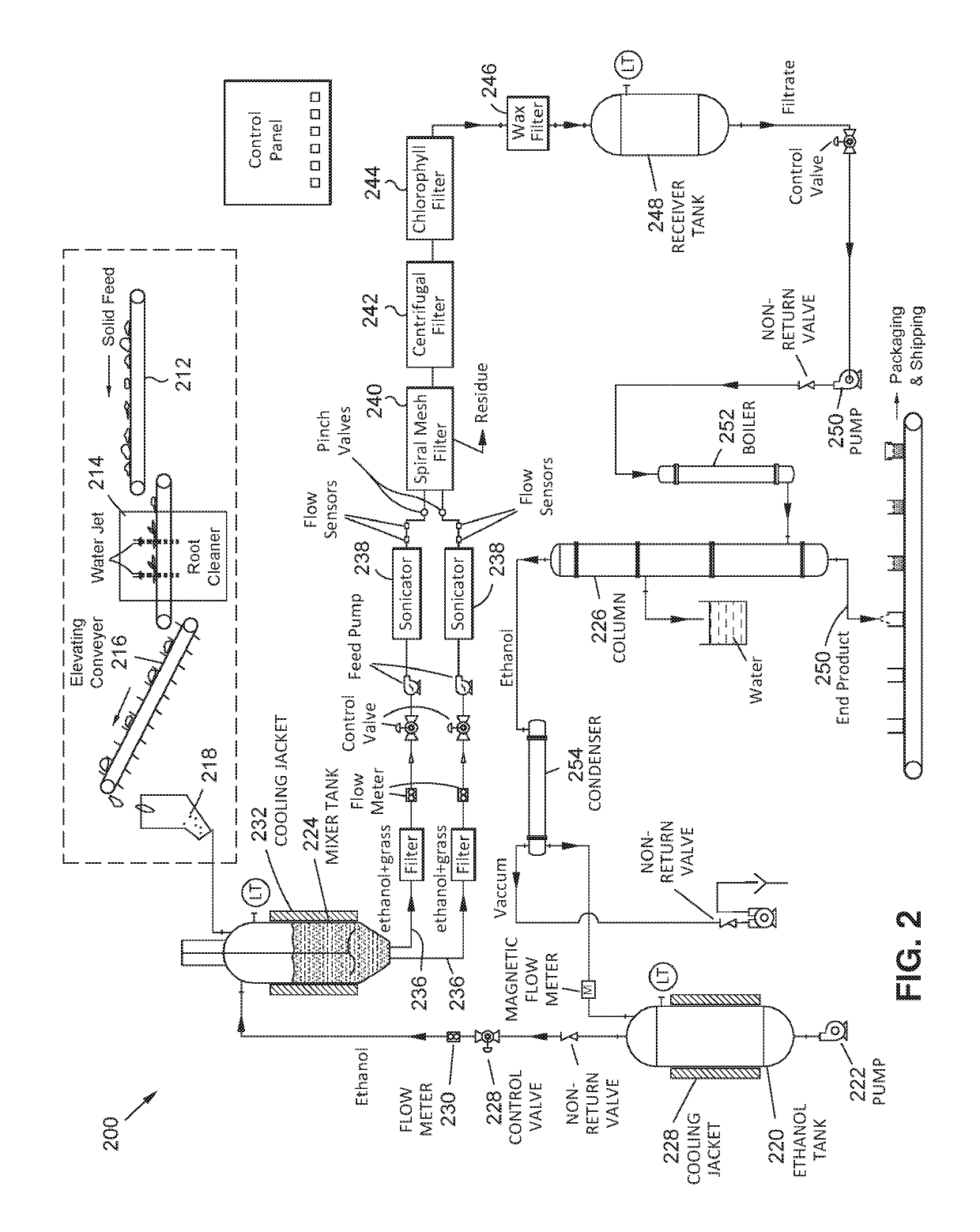
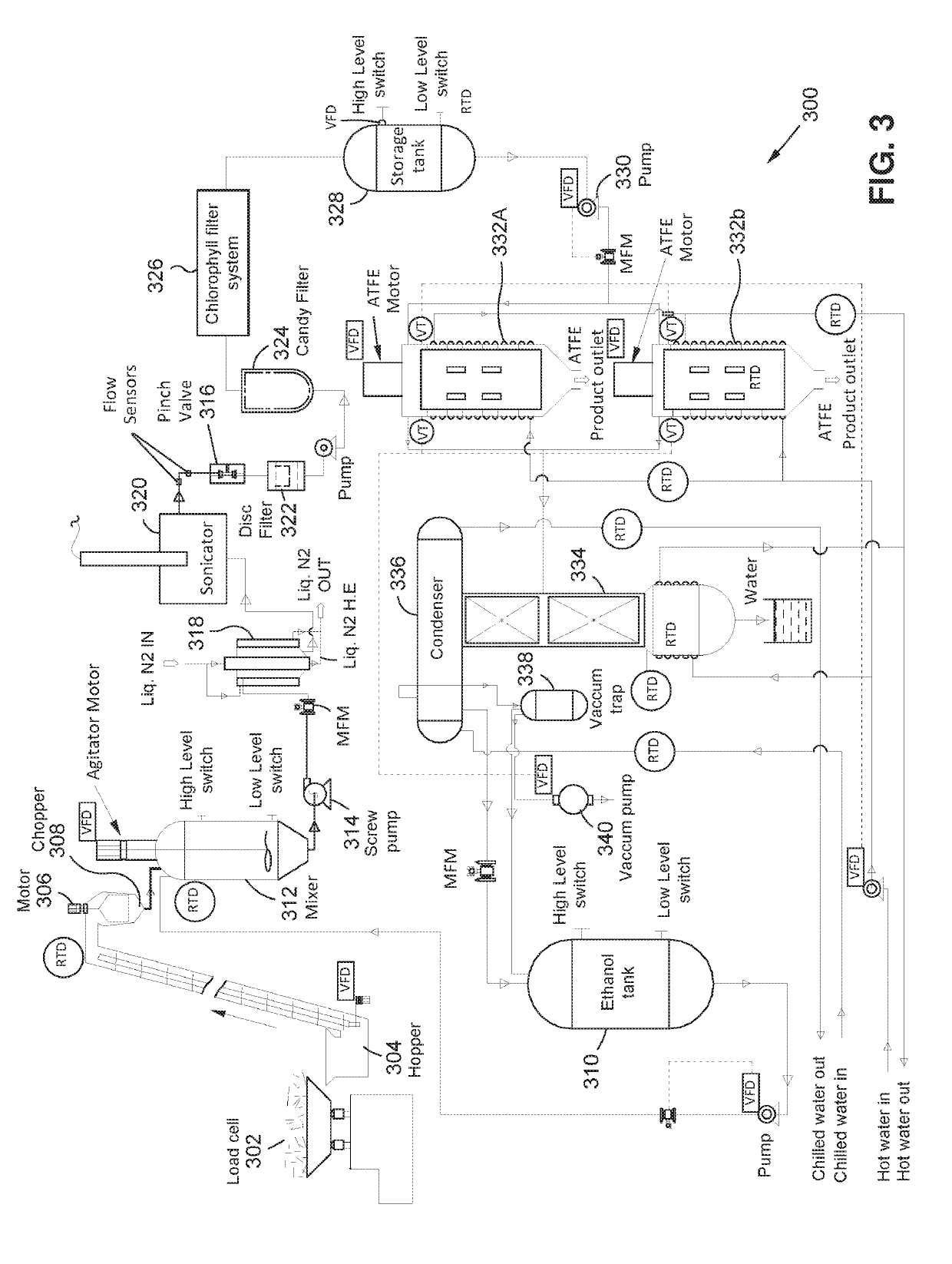
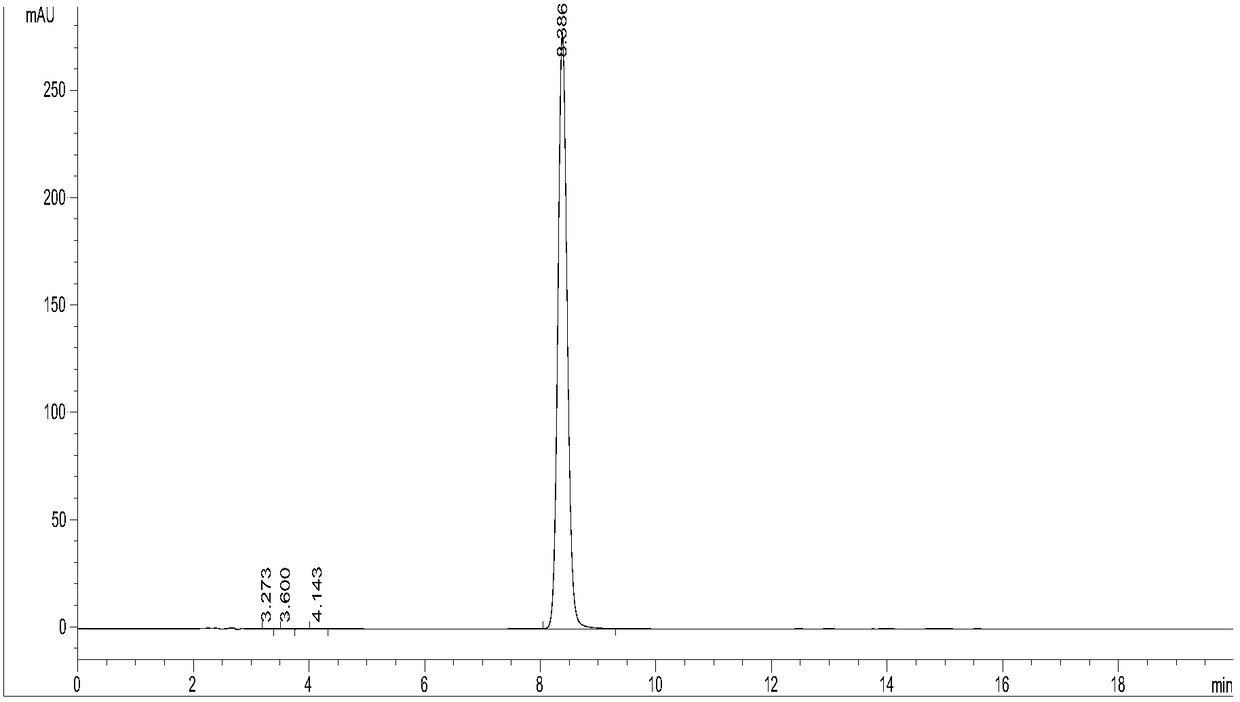
Methods for extracting and concentrating cannabinoids using ultrasound-enhanced solvent extraction. Freshly harvested cannabis plant materials, which may be selectively chosen plant parts or the entire plant itself, are shredded to a particular particle size. The plant material is then mixed with a solvent to form a slurry, and thereafter subjected to ultrasound to release intracellular contents into the solvent. Filtering steps are then applied to remove biomass, waxes and chlorophyll. Water removal and solvent recovery steps are further applied to ultimately derive an extract having high concentrations of target cannabinoids, and in particular cannabidiol (CBD). The methods may be deployed on-site in batch or continuous flow processes, and may further be utilized to derive other types of materials from plants, such as essential oils.
Owner:WORLD CLASS EXTRACTIONS INC
Preparation method of high-purity cannabidiol
ActiveCN108083989AImprove solubilityGuaranteed purityOrganic chemistryHydroxy compound active ingredientsChemical industryPolyamide
The invention belongs to the field of pharmaceutical and chemical industry, and specifically relates to a preparation method of high-purity cannabidiol. The preparation method is characterized by comprising the following steps: taking leaves of cannabis sativa and the plant top occupying one fifth of the whole plant as extraction parts, performing purification by using macroporous absorbent pillarchromatography and polyamide pillar chromatography joint technology, and performing crystallization refining by using a mixed solvent system, thereby maximally improving the yield in the premise of guaranteeing the acquisition of a high-purity product. According to the method provided by the invention, the CBD purity of the obtained product is high, the yield is high, the process is simple, and the industrialization is easy to realized.
Owner:山东析木津健康科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com