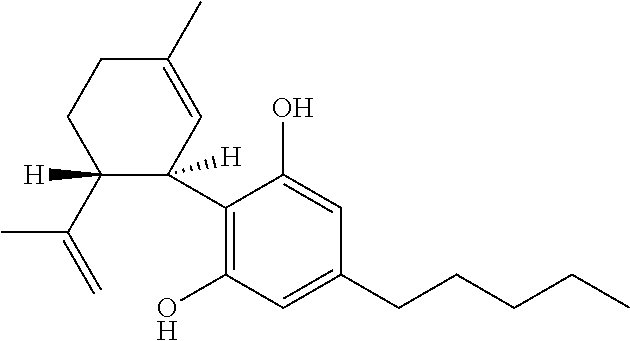
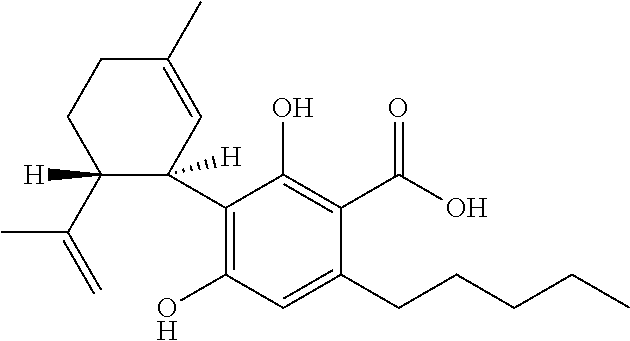
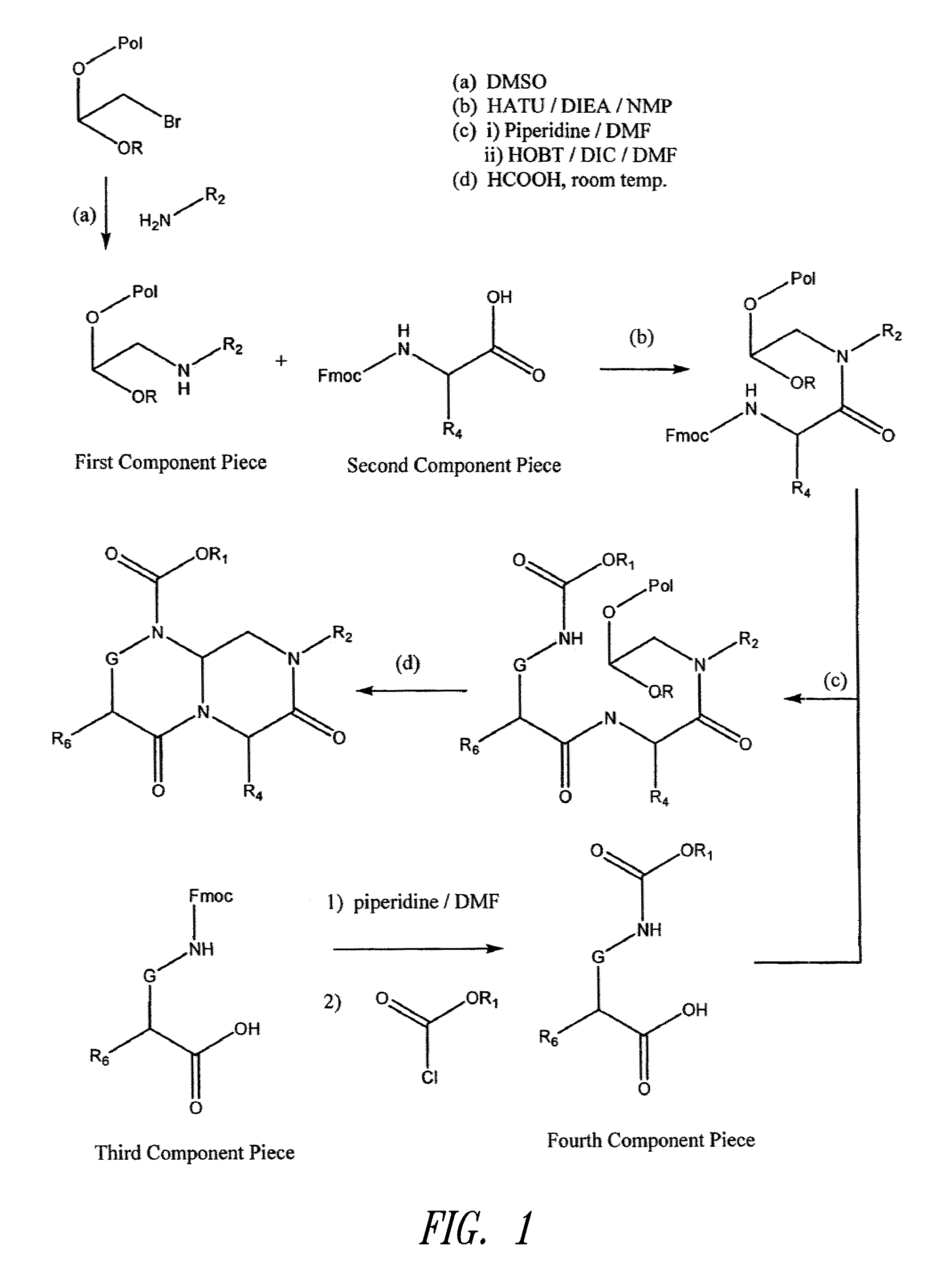
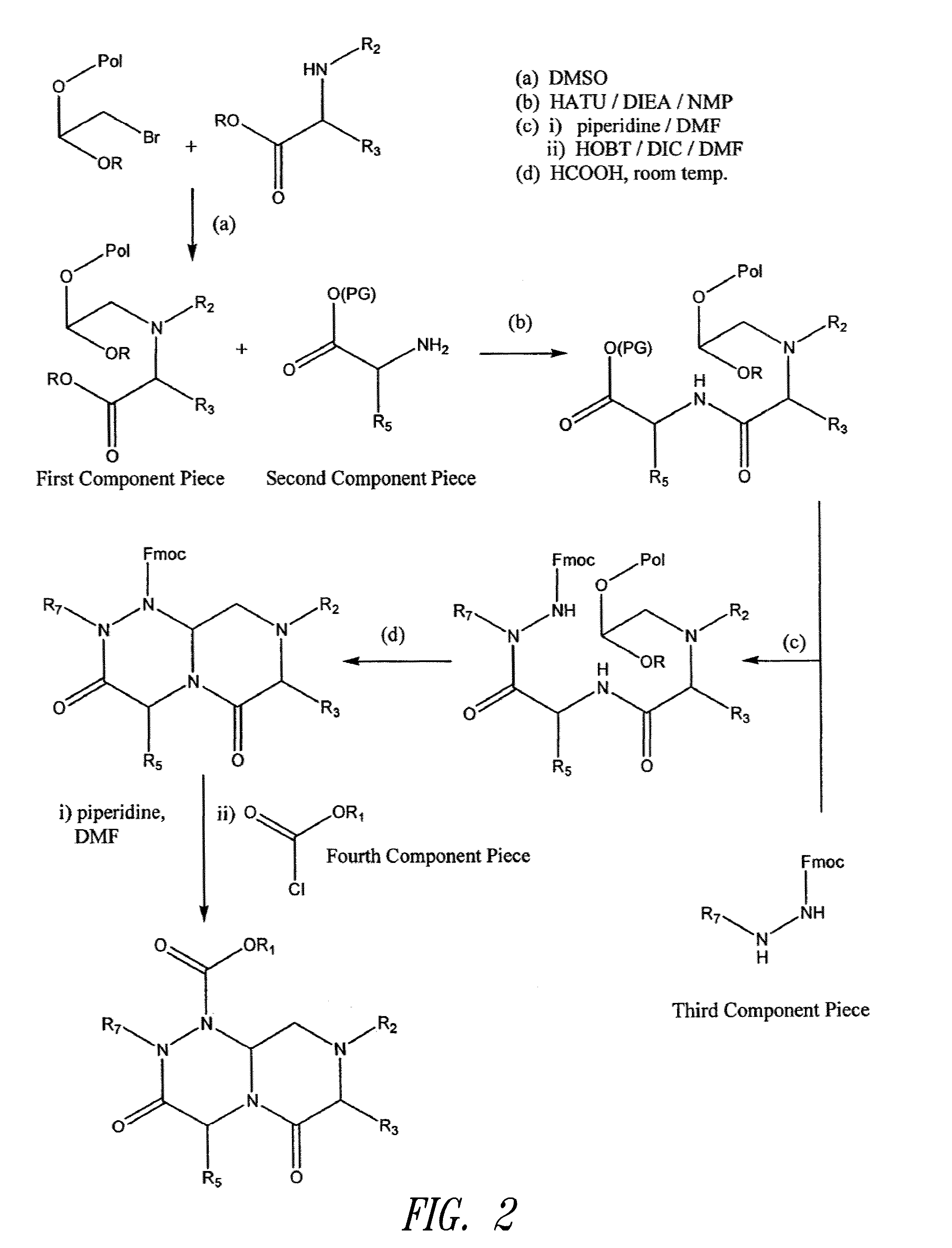
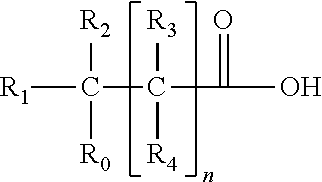Patents
Literature
61 results about "Tuberous sclerosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A rare multisystem genetic disease that causes growth of benign tumors.
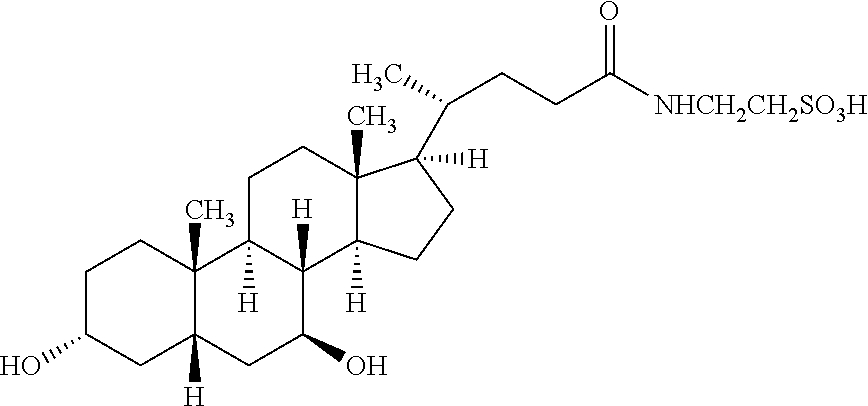
Use of glutaminyl cyclase inhibitors
ActiveUS20090068699A1BiocideOrganic active ingredientsMild cognitive impairment (MCI)Percent Diameter Stenosis
An inhibitor of a glutaminyl peptide cyclotransferase, and use thereof for the treatment and / or prevention of a disease or disorder selected from the group consisting of inflammatory diseases selected froma. neurodegenerative diseases, e.g. mild cognitive impairment (MCI), Alzheimer's disease, neurodegeneration in Down Syndrome, Familial British Dementia, Familial Danish Dementia, multiple sclerosis,b. chronic and acute inflammations, e.g. rheumatoid arthritis, atherosclerosis, restenosis, pancreatitis,c. fibrosis, e.g. lung fibrosis, liver fibrosis, renal fibrosis,d. cancer, e.g. cancer / hemangioendothelioma proliferation, gastric carcinomas,e. metabolic diseases, e.g. hypertension,f. and other inflammatory diseases, e.g. neuropathic pain, graft rejection / graft failure / graft vasculopathy, HIV infections / AIDS, gestosis, tuberous sclerosis.Additionally disclosed are a respective diagnostic method, assay and kit.
Owner:VIVORYON THERAPEUTICS NV
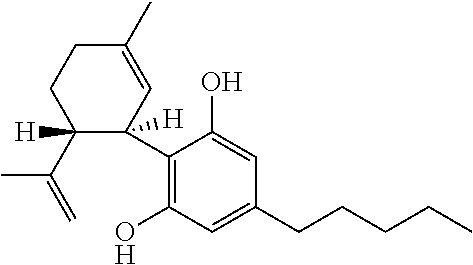
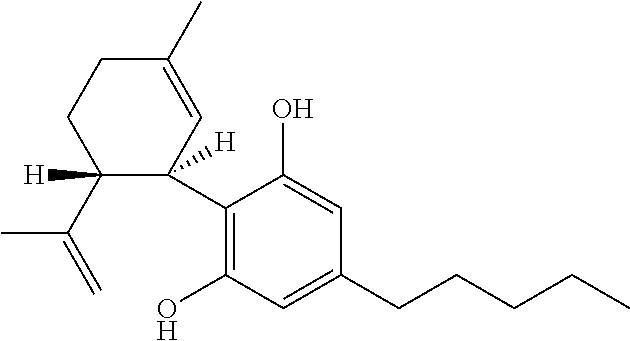
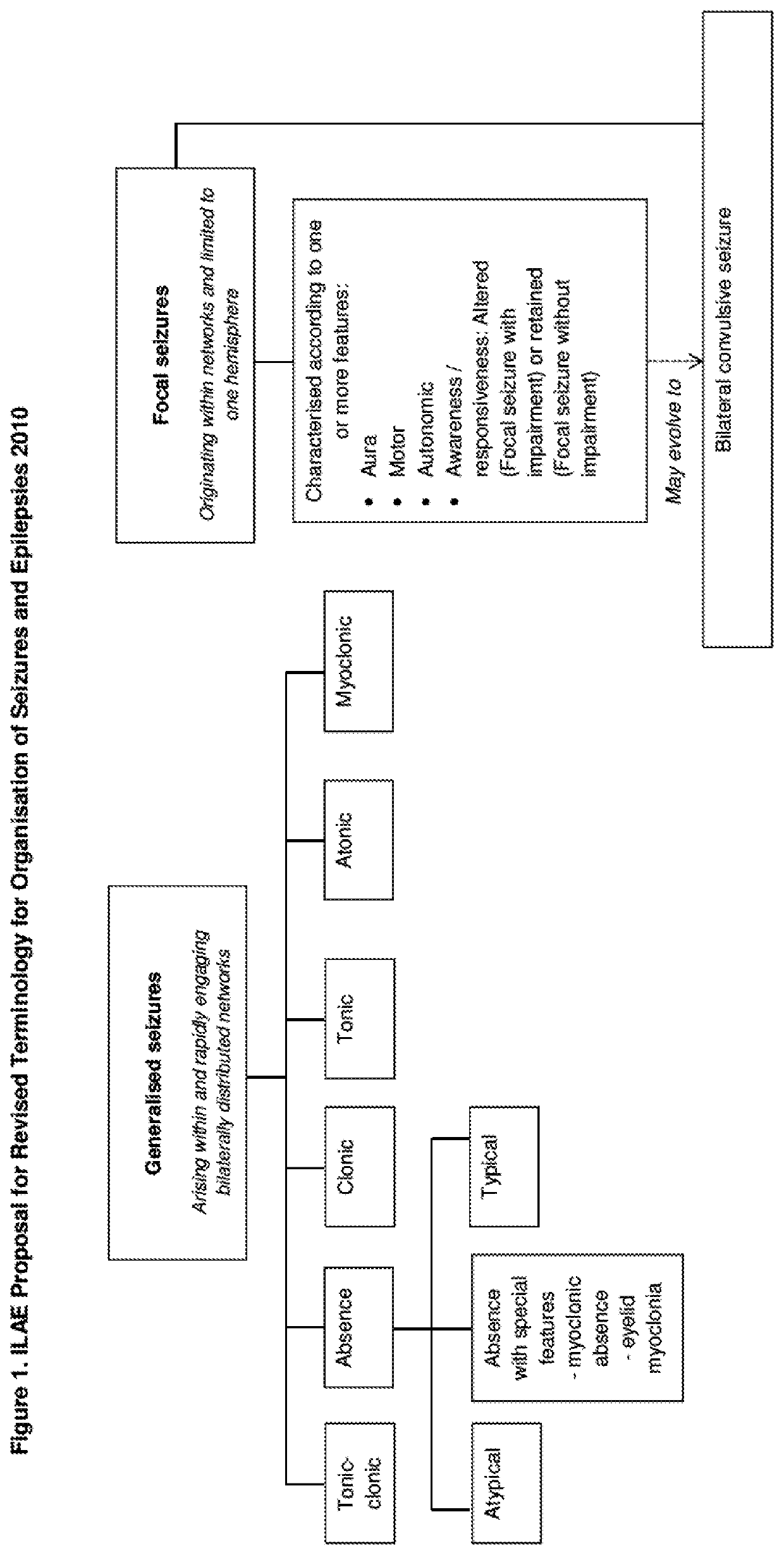
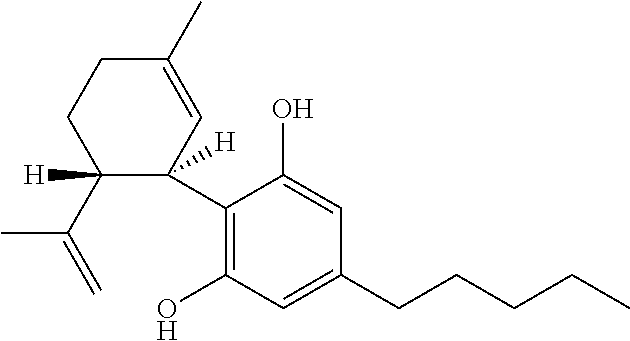
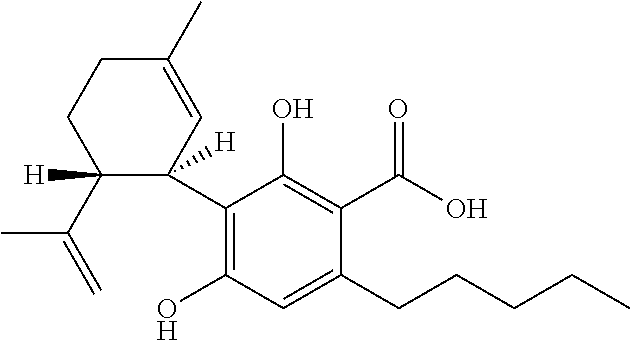
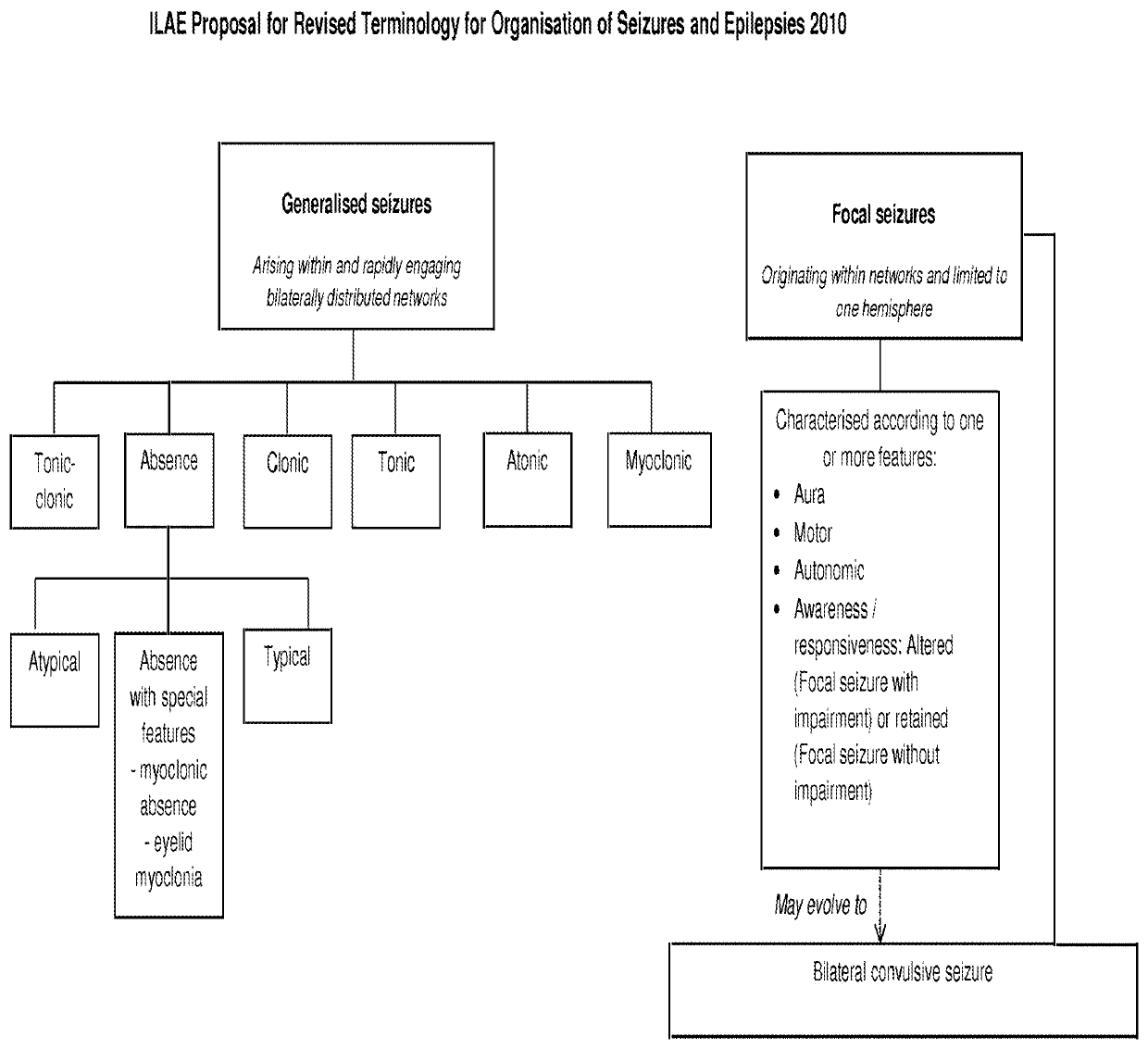
Use of cannabinoids in the treatment of epilepsy
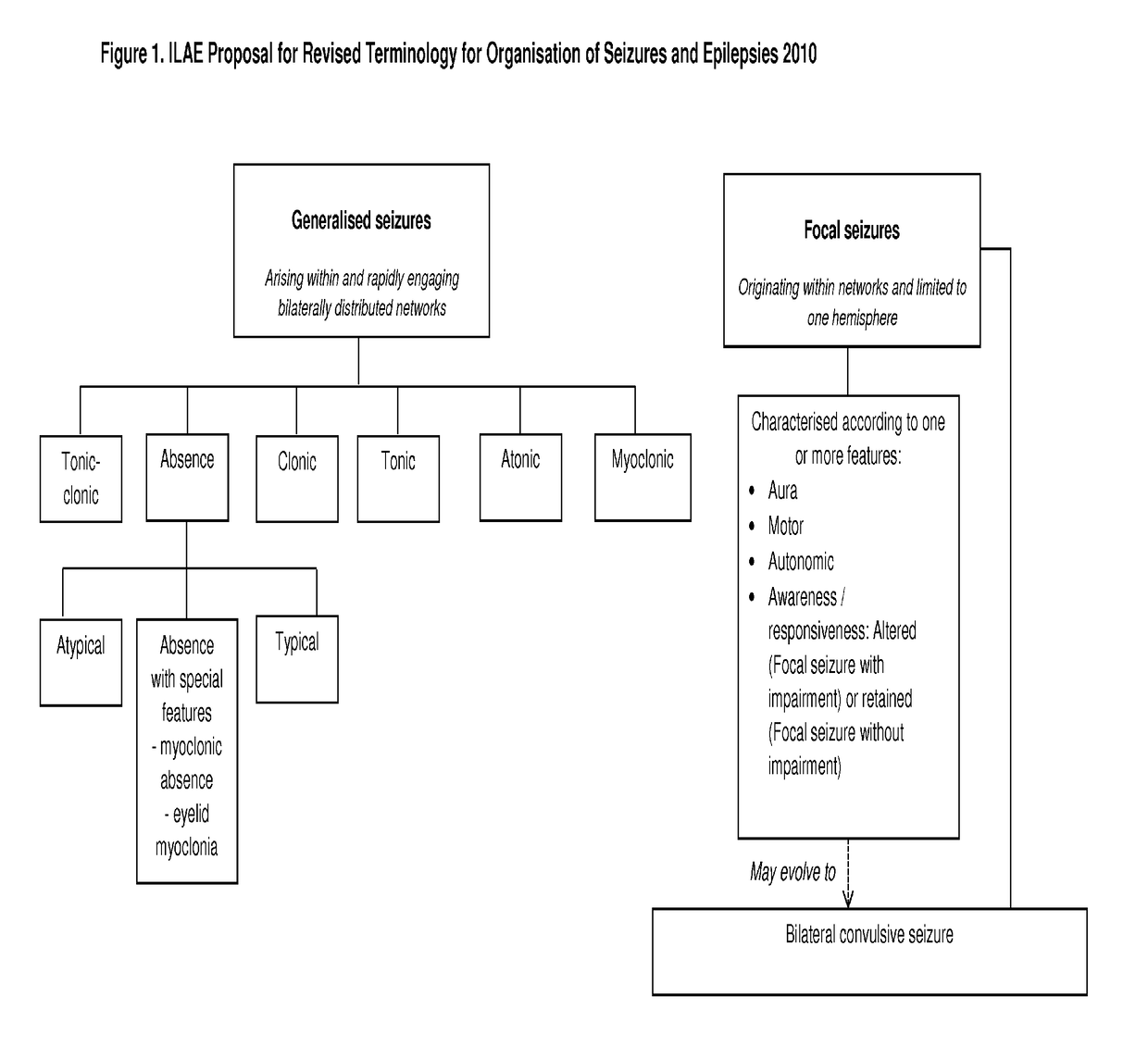
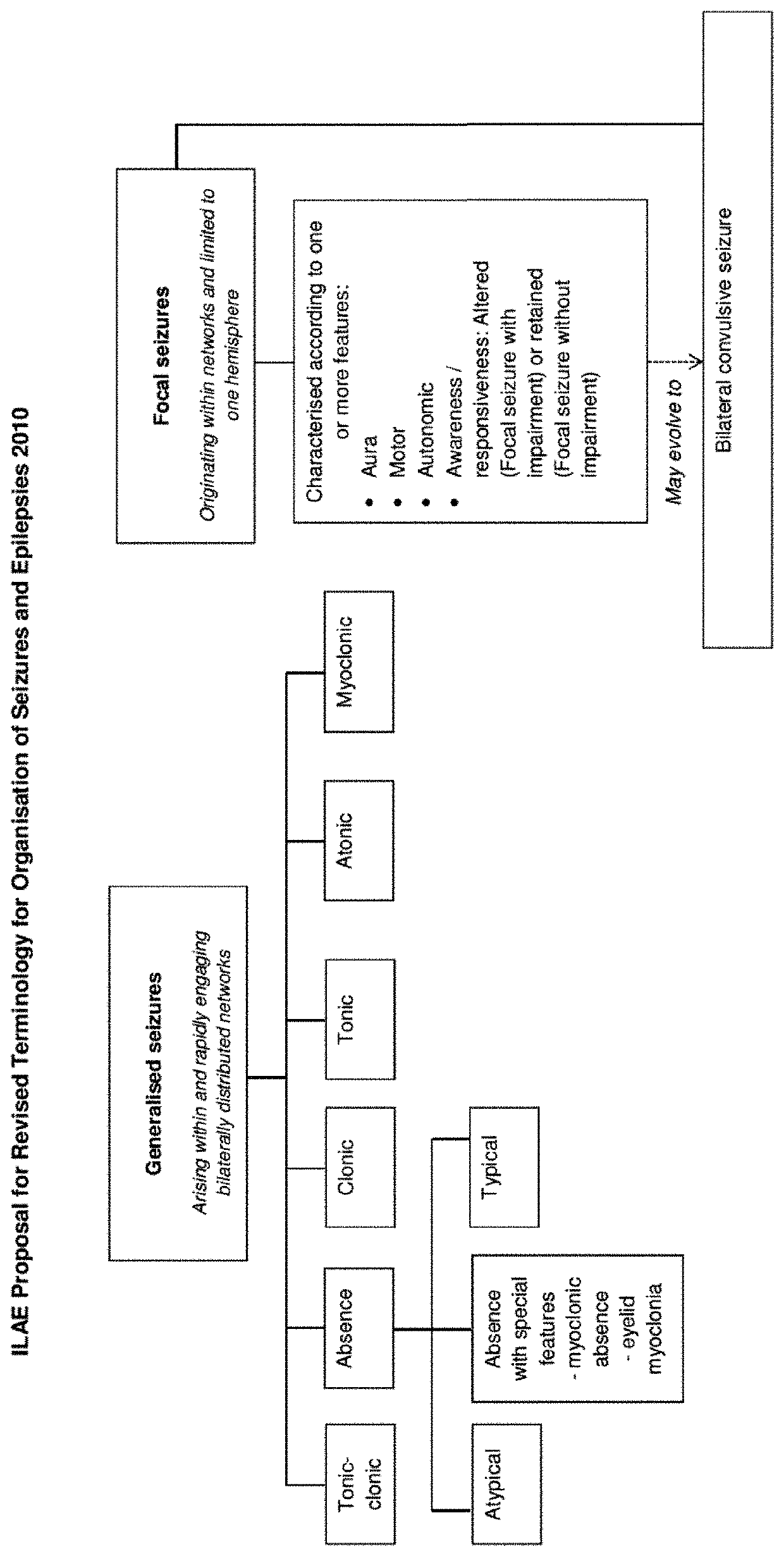
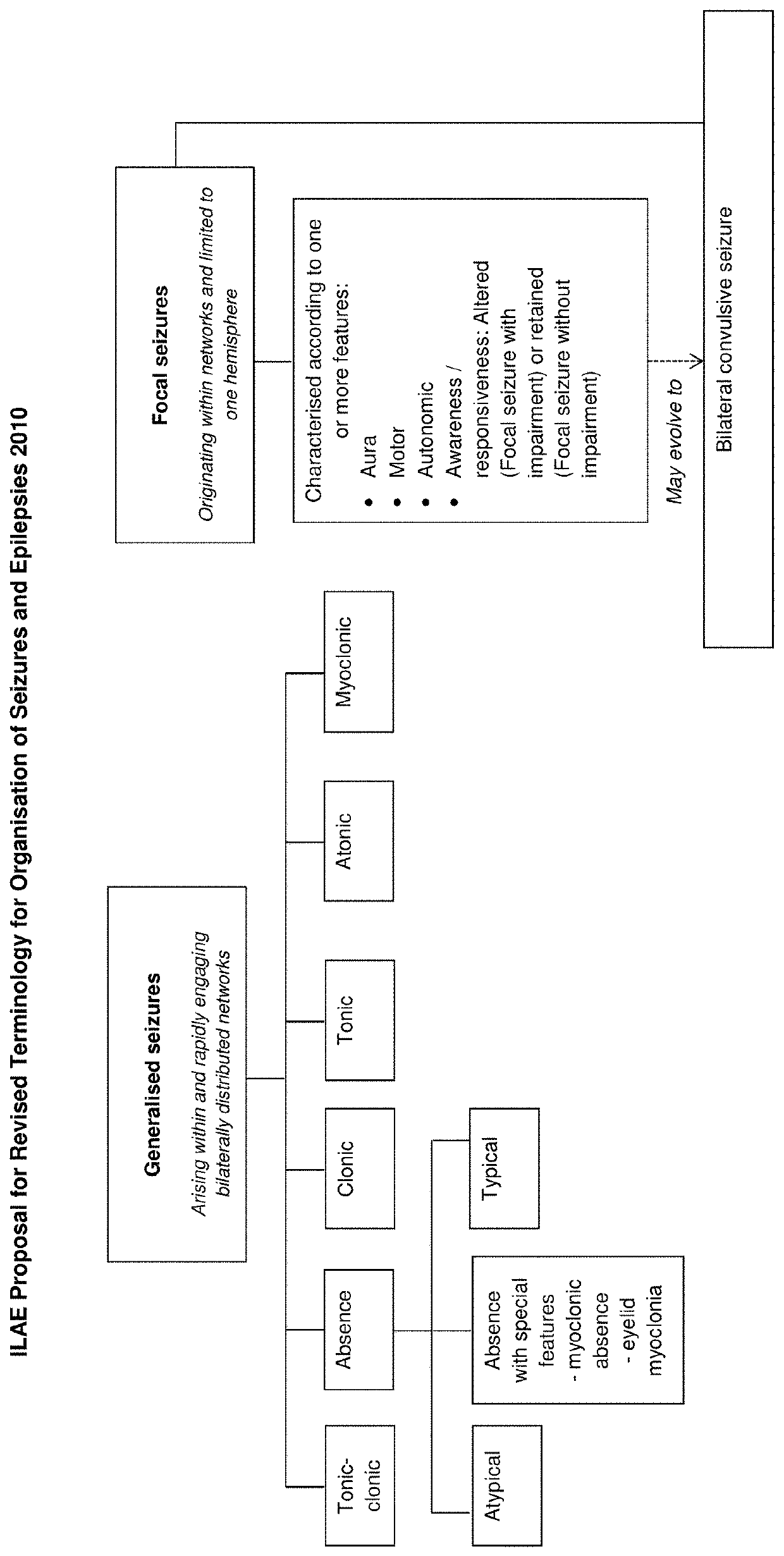
The present disclosure relates to the use of cannabidiol (CBD) in the treatment of absence seizures. In particular, the disclosure relates to the use of CBD for reducing absence seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; CDKL5; Dup15q; Jeavons syndrome; Myoclonic Absence Epilepsy; Neuronal ceroid lipofuscinoses (NCL) and brain abnormalities. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS20170007551A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
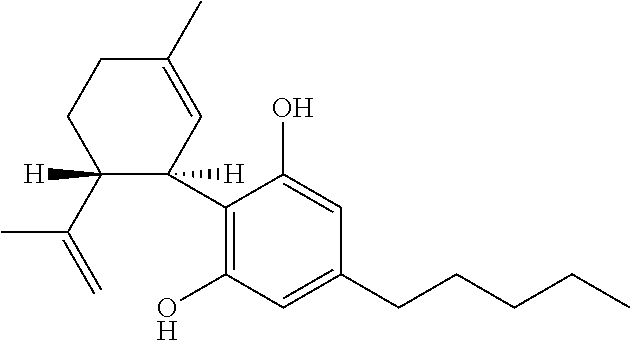
The present invention relates to the use of cannabidiol (CBD) in the treatment of focal seizures. In one embodiment the patients suffering from focal seizures are children and young adults. CBD appears particularly effective in reducing focal seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; CDKL5; Neuronal ceroid lipofuscinoses (NCL); febrile infection related epilepsy syndrome (FIRES); Aicardi syndrome and brain abnormalities in comparison to other seizure types. Significantly CBD additionally is very effective in the reduction of a sub-type of focal seizures, focal seizures with impairment.
Owner:GW RES LTD
Use of Cannabinoids in the Treatment of Epilepsy
ActiveUS20170172941A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsEtiologyAicardi's syndrome
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of atonic seizures. In particular the CBD appears particularly effective in reducing atonic seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; Aicardi syndrome; CDKL5 and Dup15q in comparison to other seizure types. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
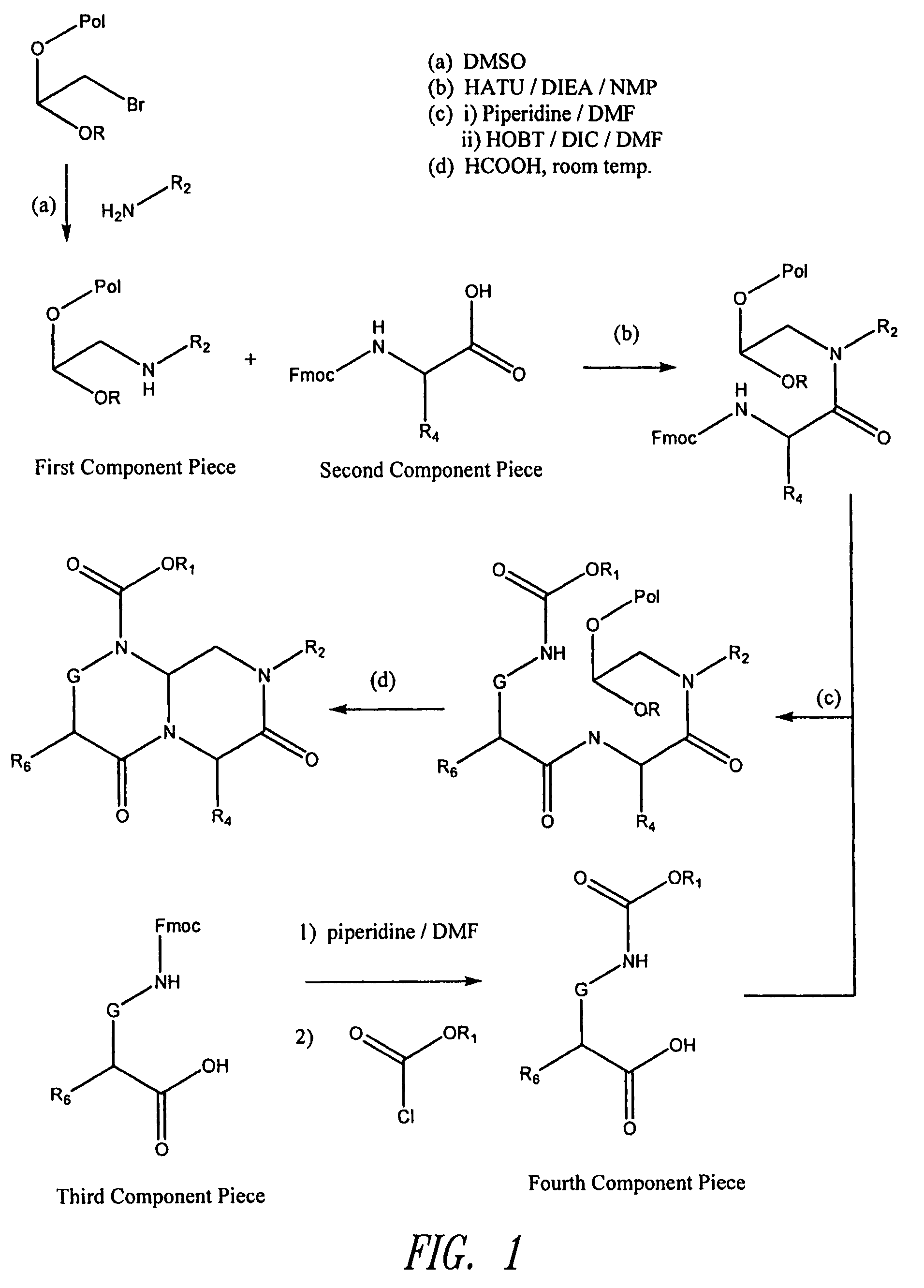
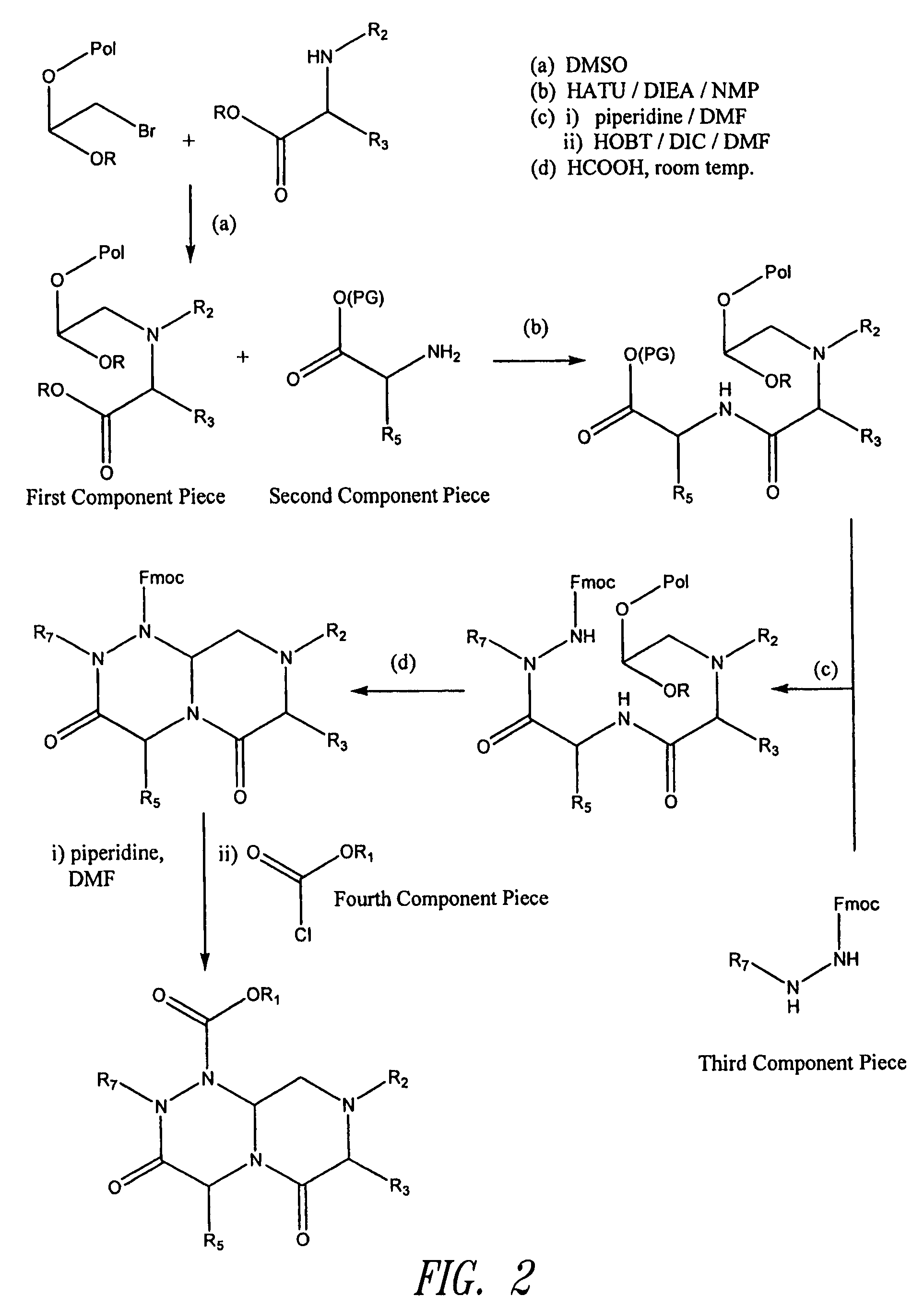
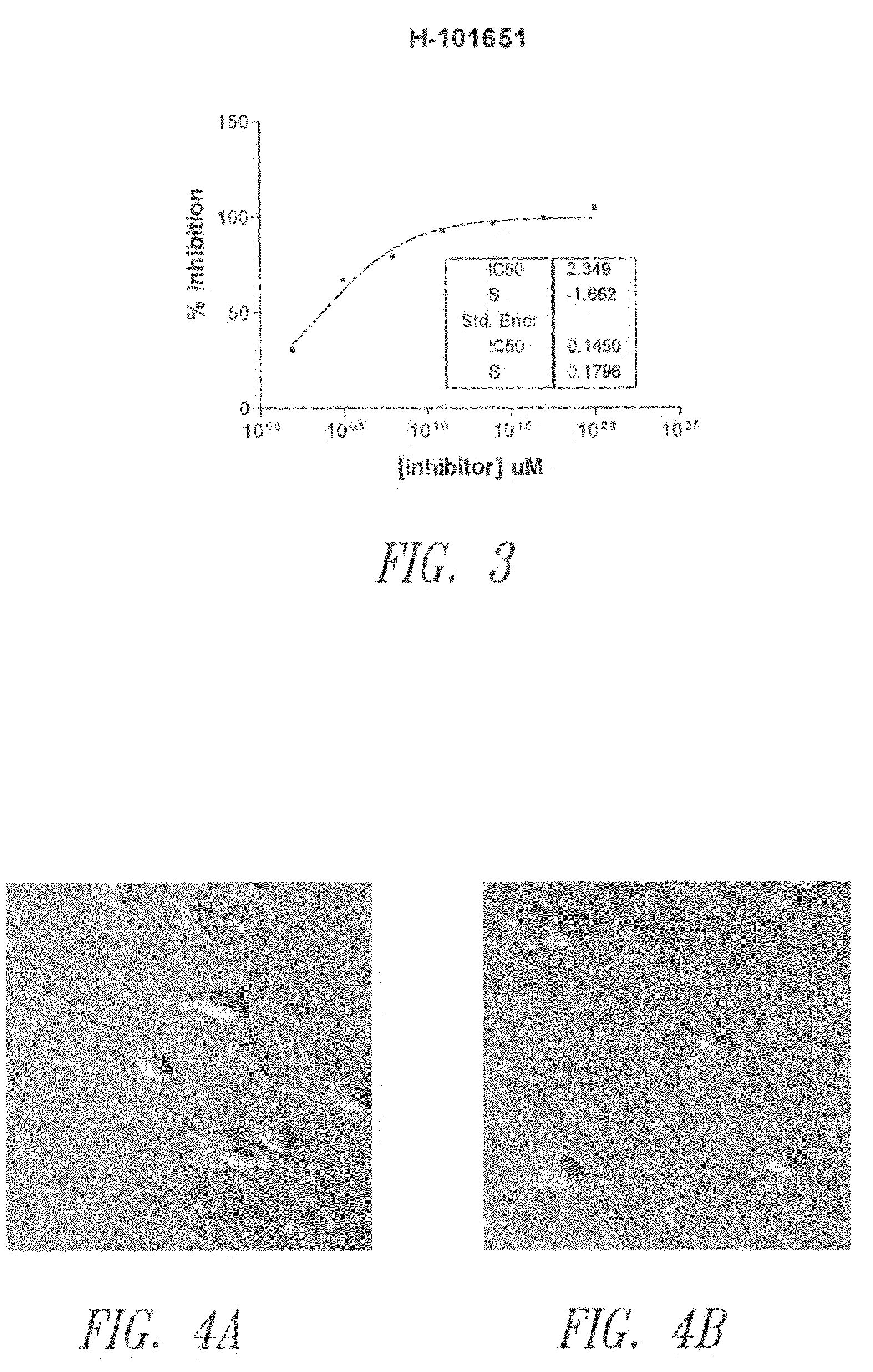
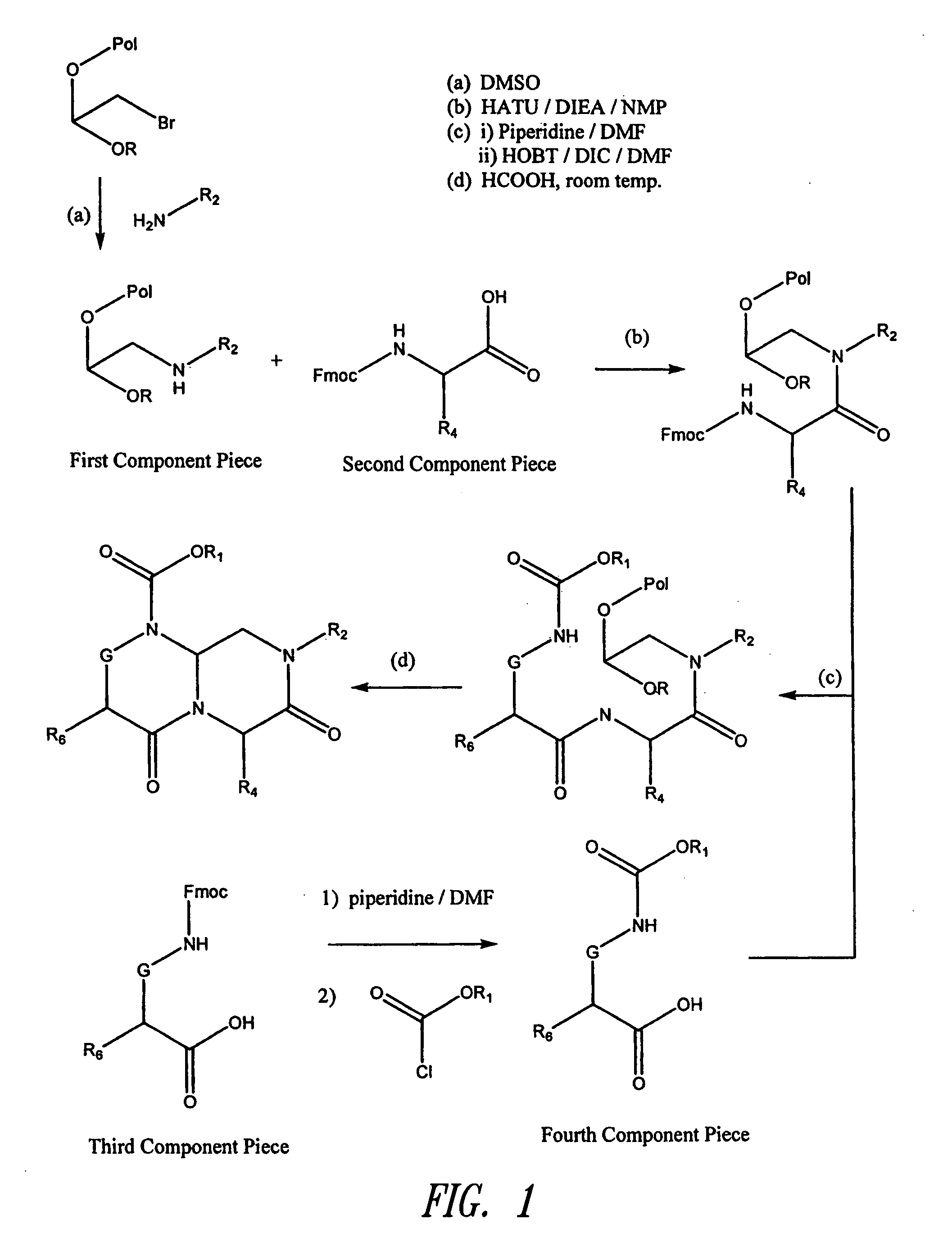
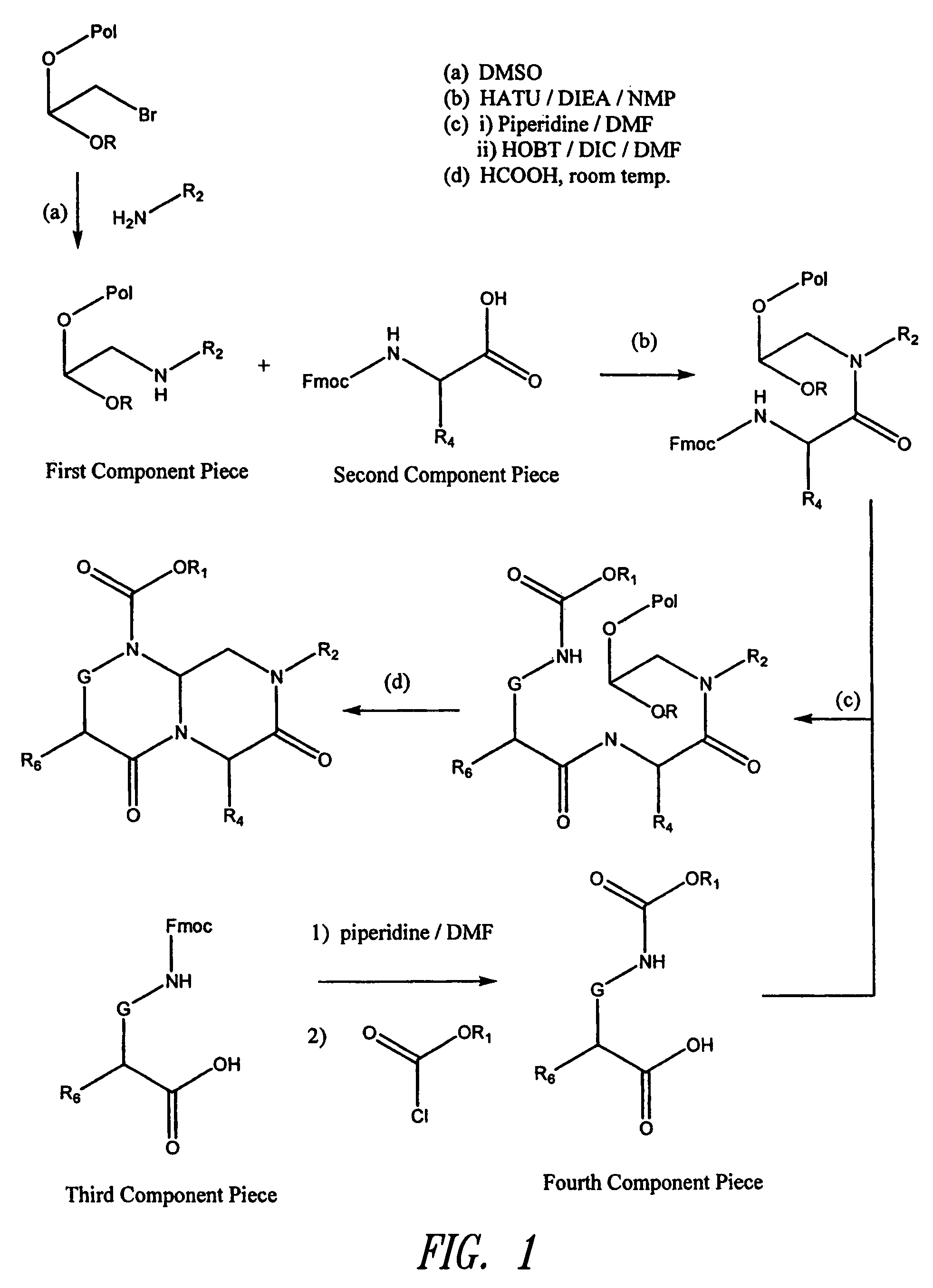
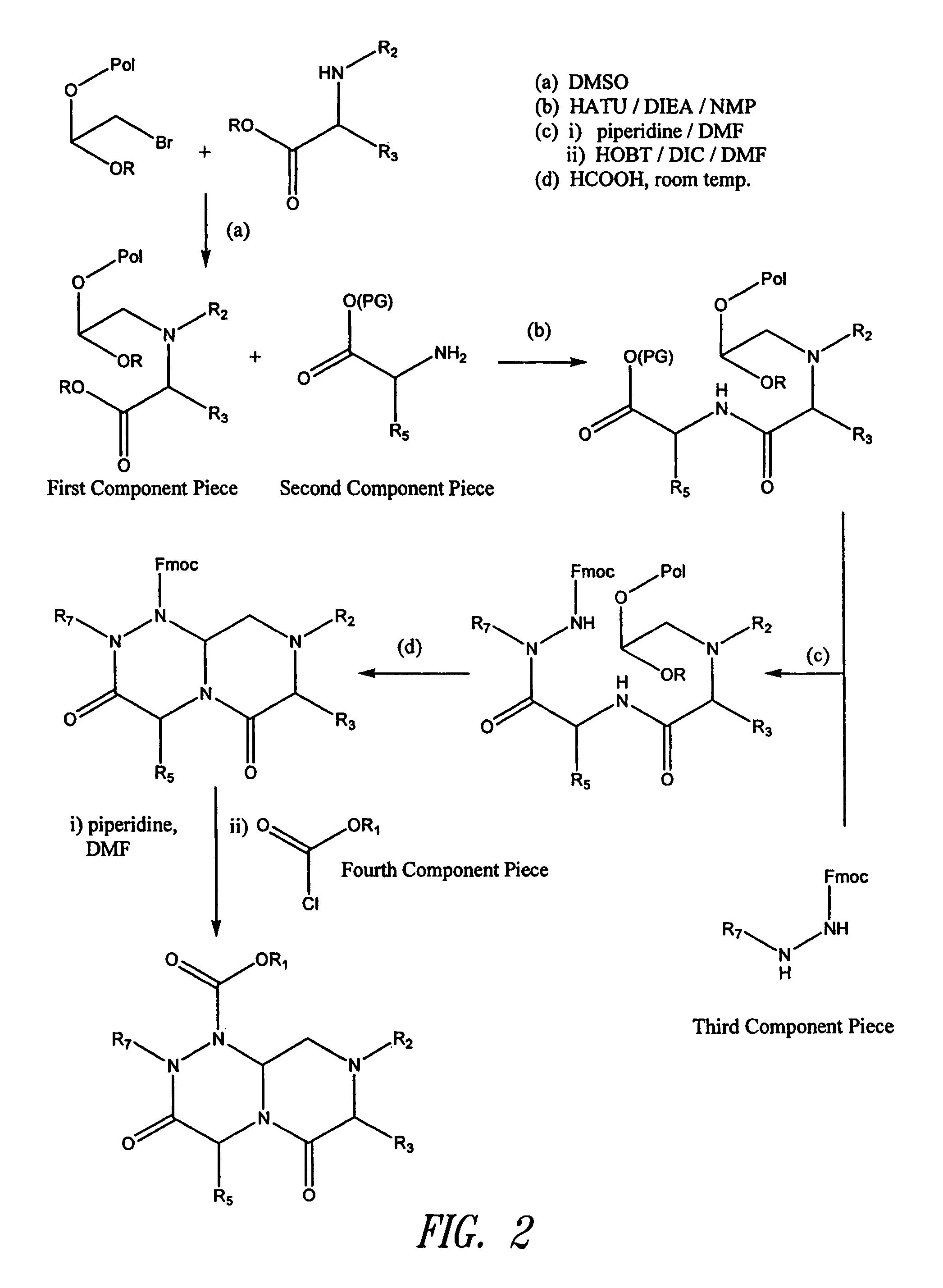
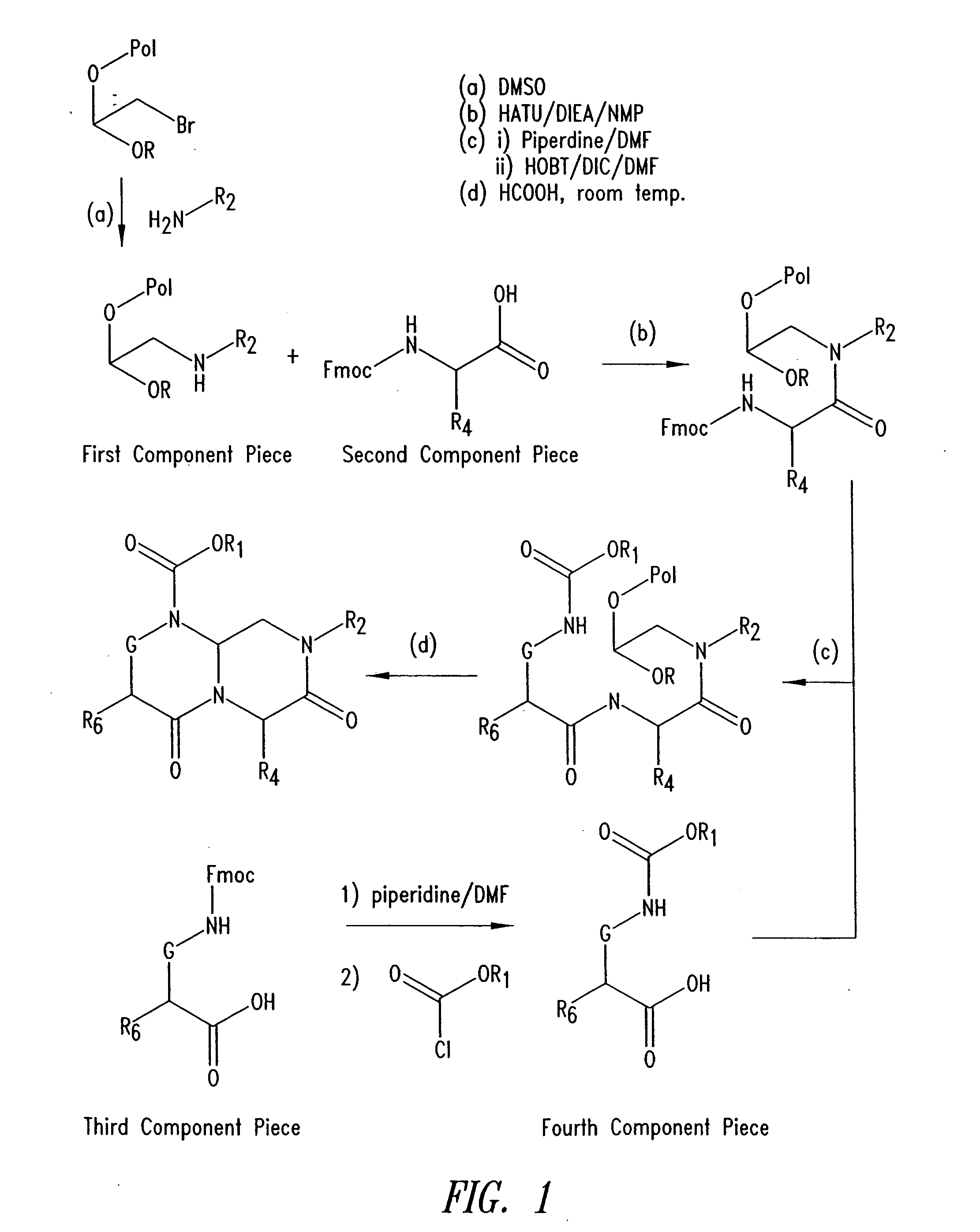
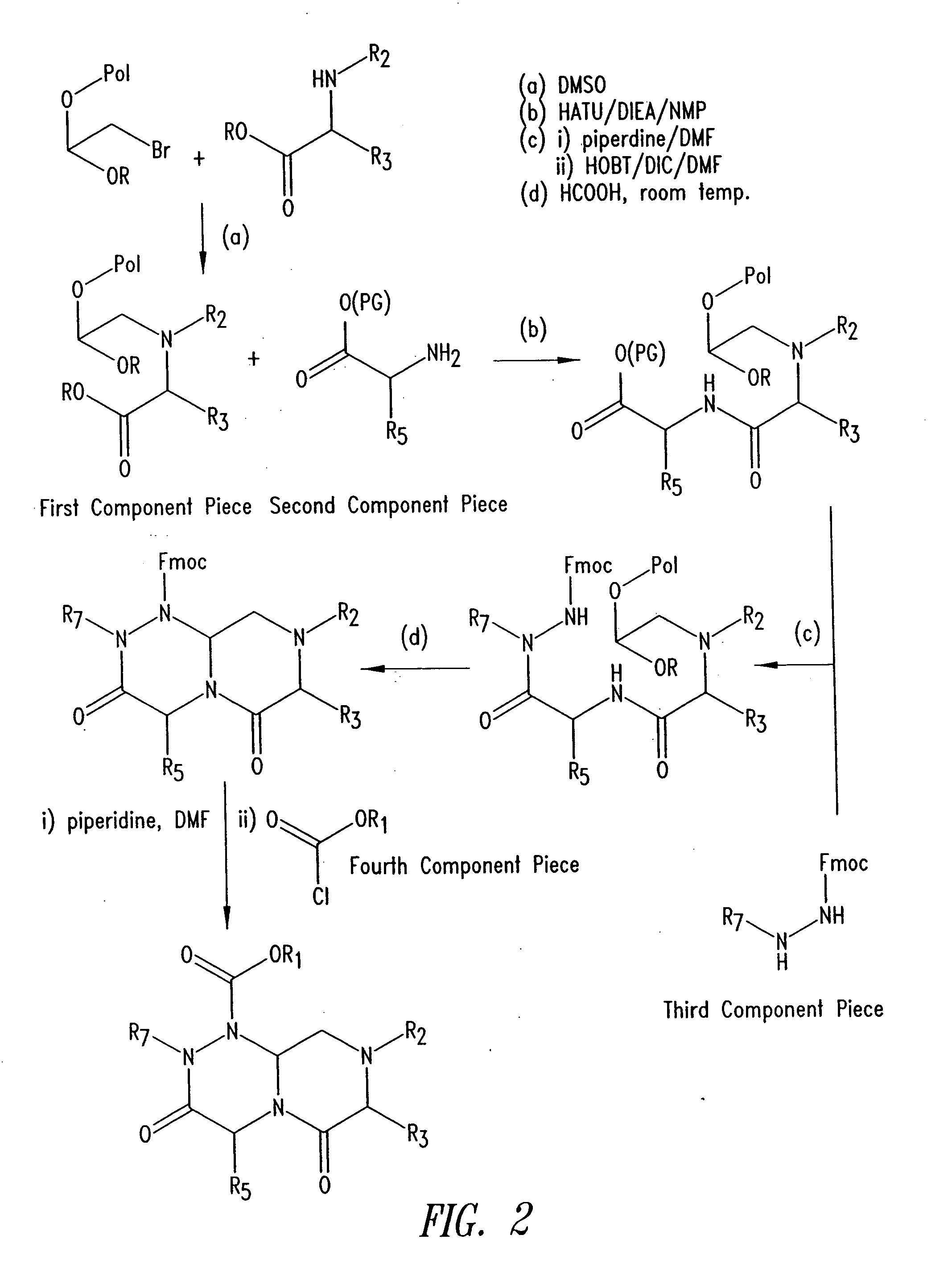
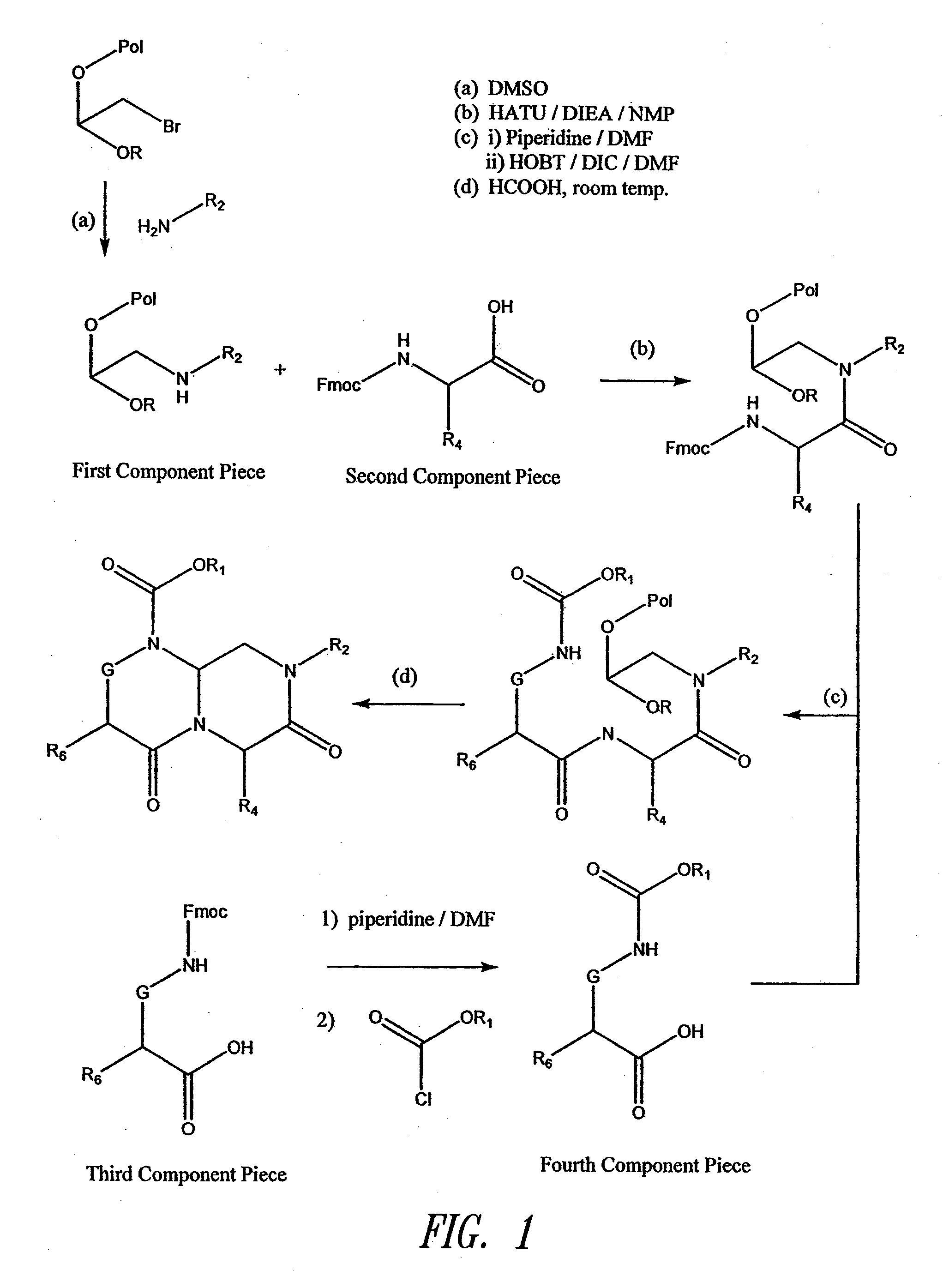
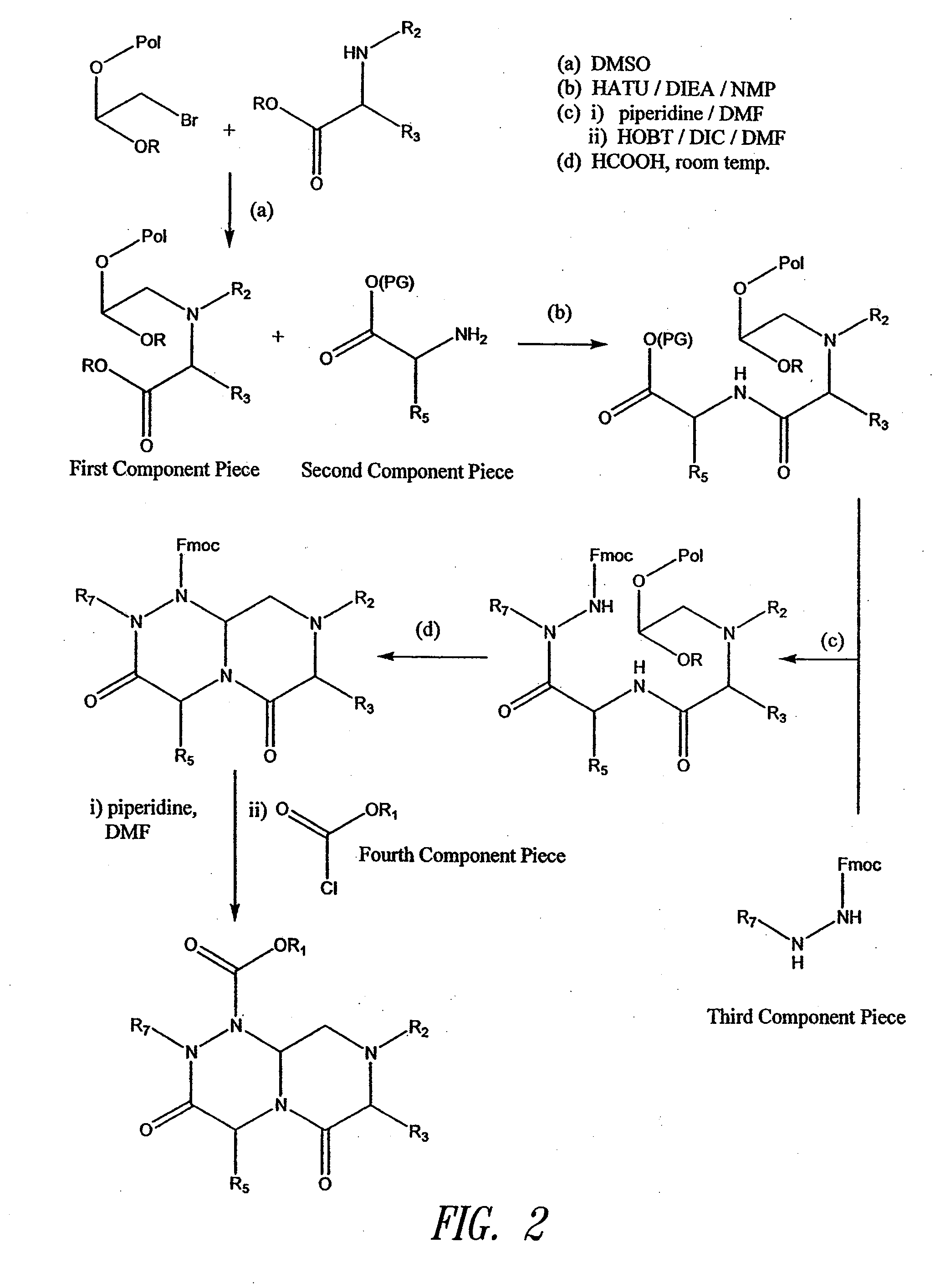
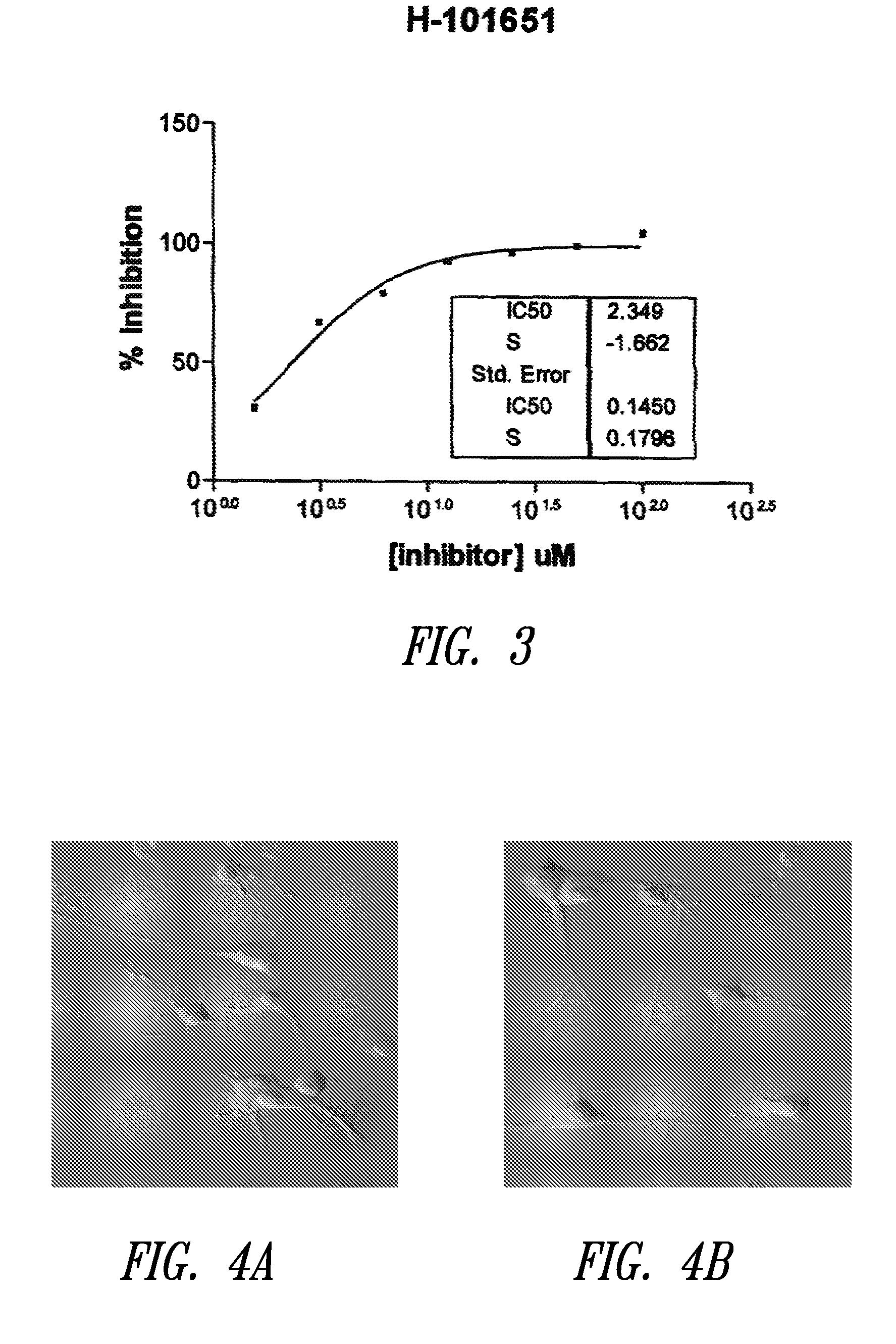
Reverse-turn mimetics and method relating thereto
InactiveUS7671054B1Efficient implementationPromoting neurite outgrowthBiocideOrganic chemistryWide areaUlcerative colitis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins as well as their prodrugs are disclosed. Such reverse-turn mimetic structures and prodrugs have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds and prodrugs for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Reverse-turn mimetics and method relating thereto
InactiveUS20070021425A1Efficient implementationBiocideNervous disorderUlcerative colitisPercent Diameter Stenosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Reverse-turn mimetics and method relating thereto
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Reverse-turn mimetics and method relating thereto
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
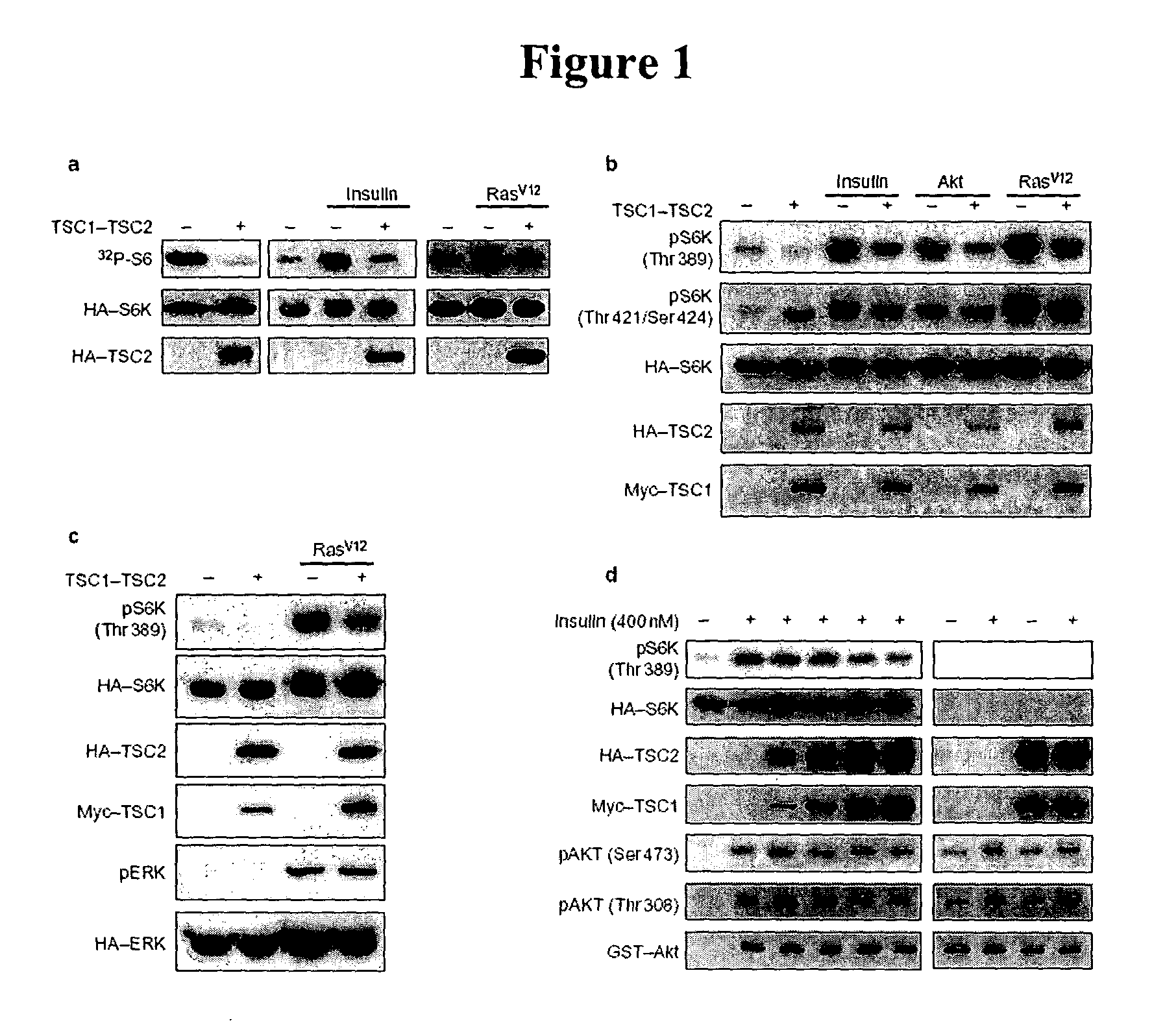
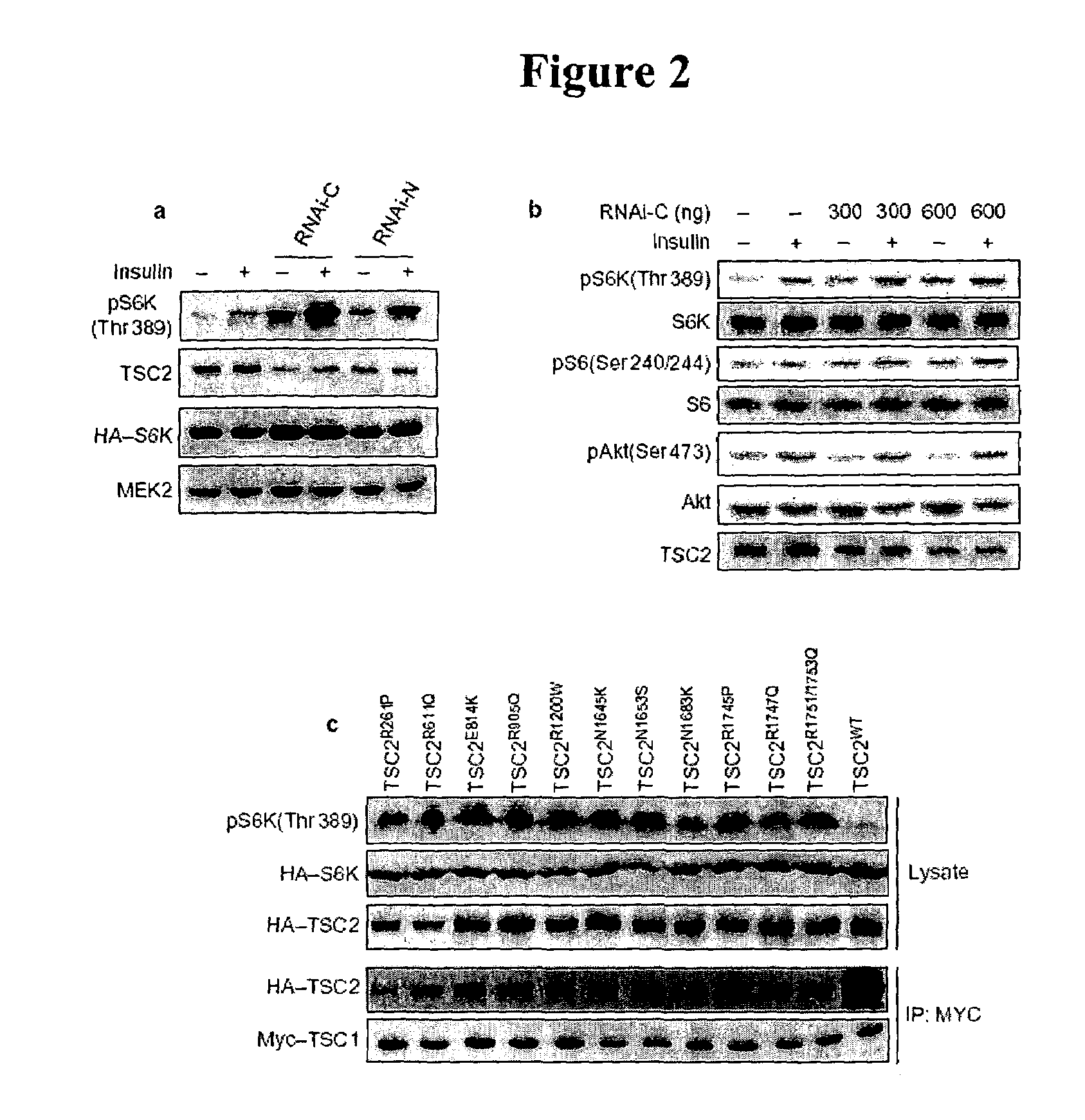
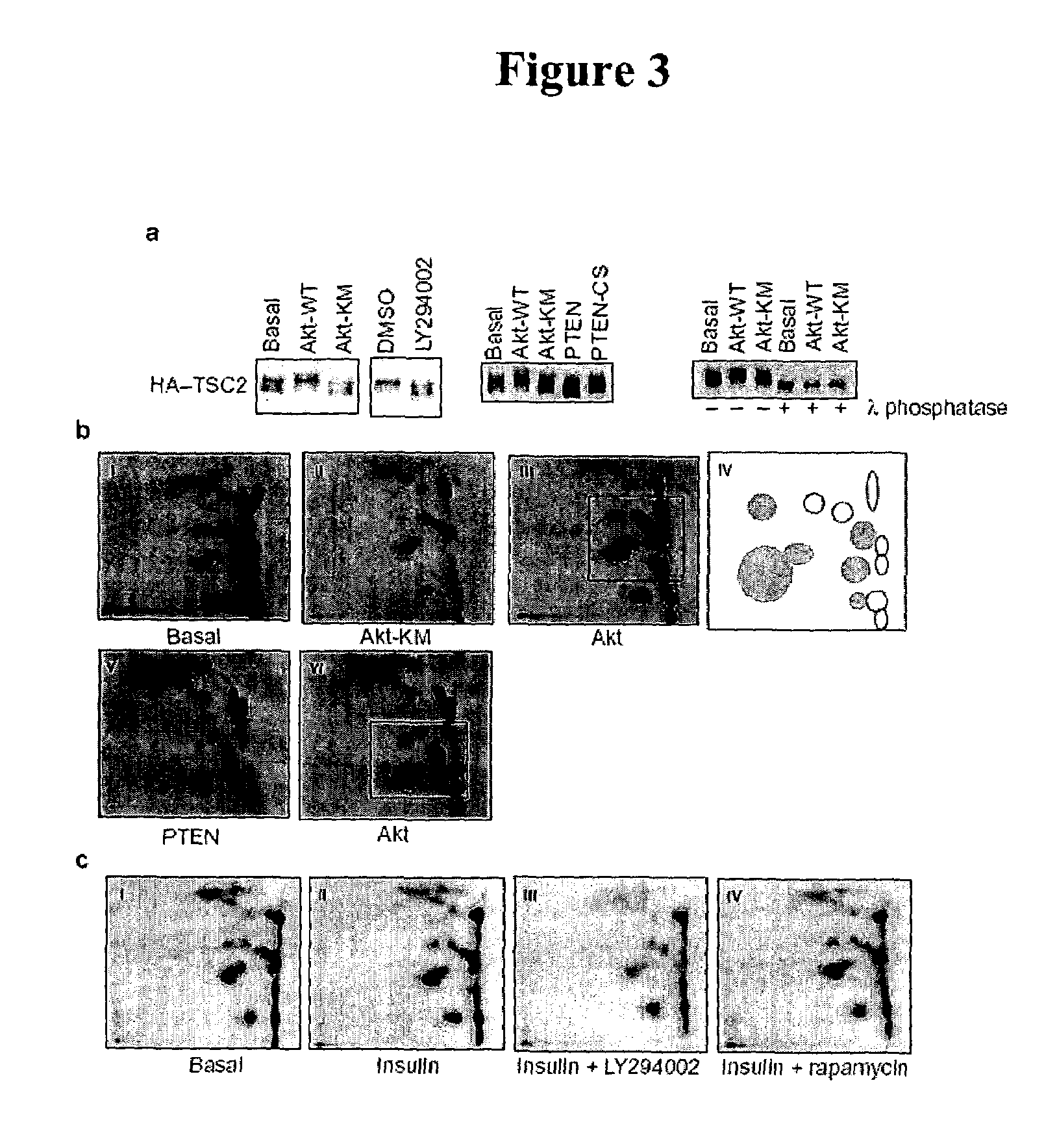
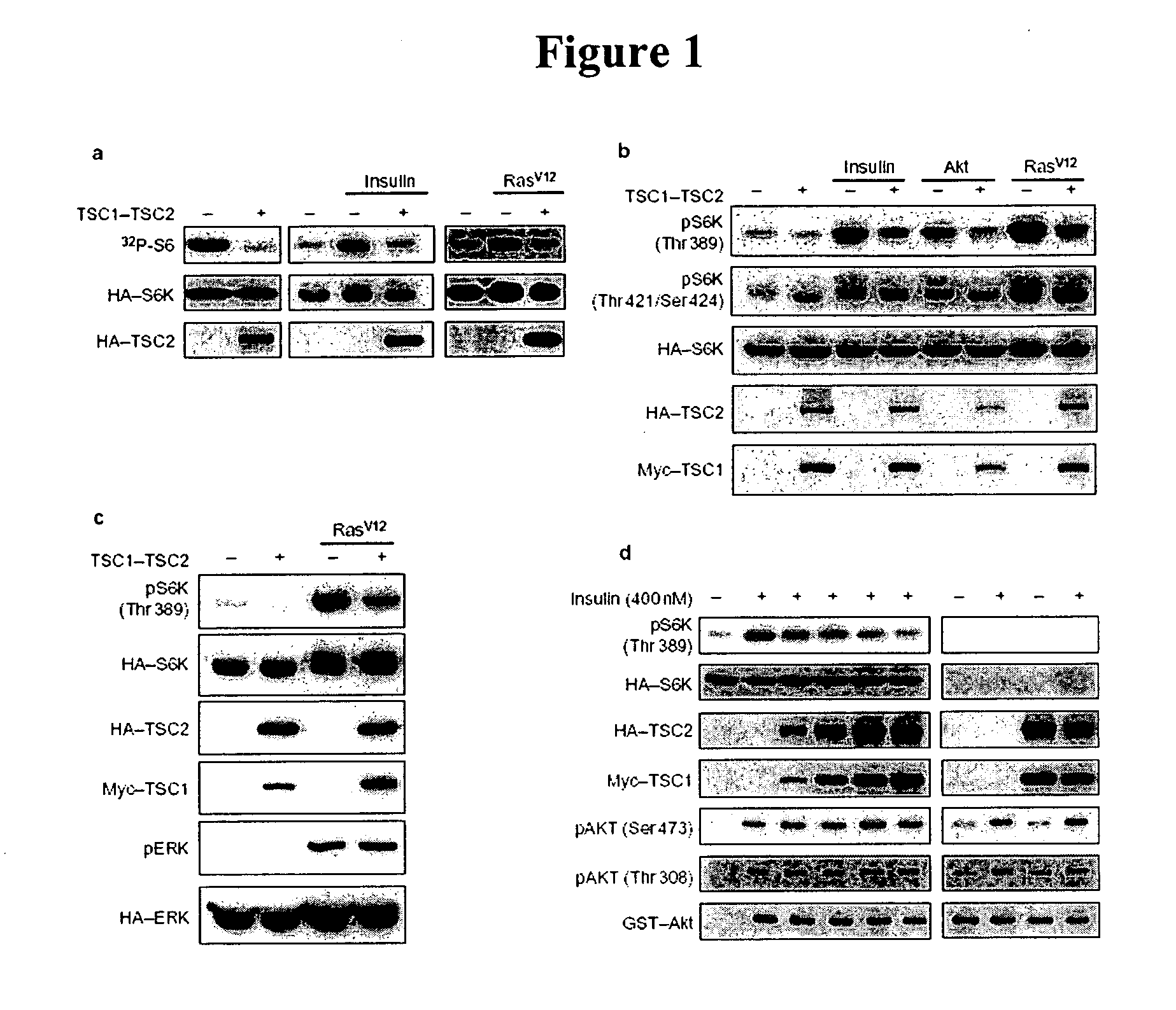
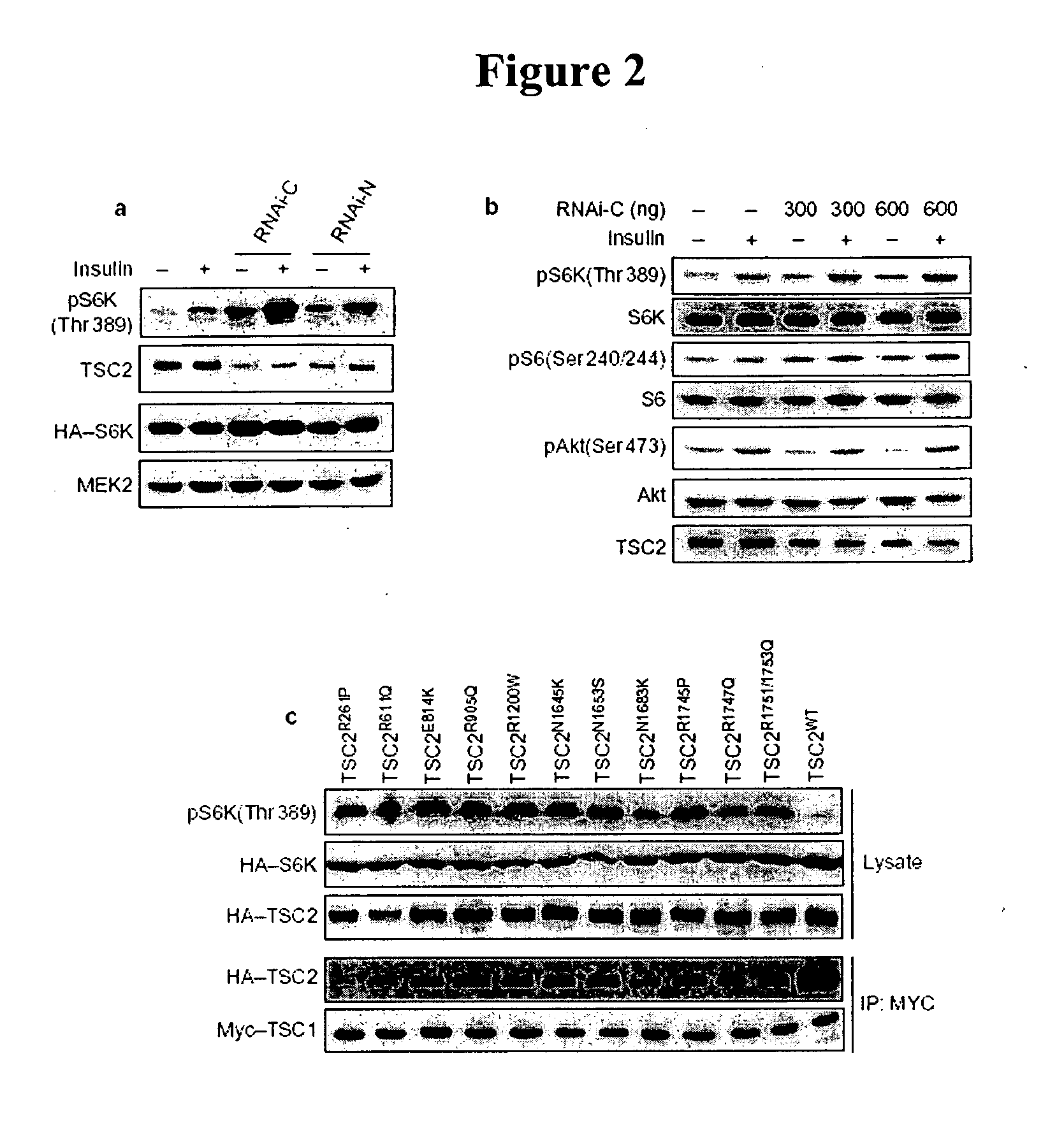
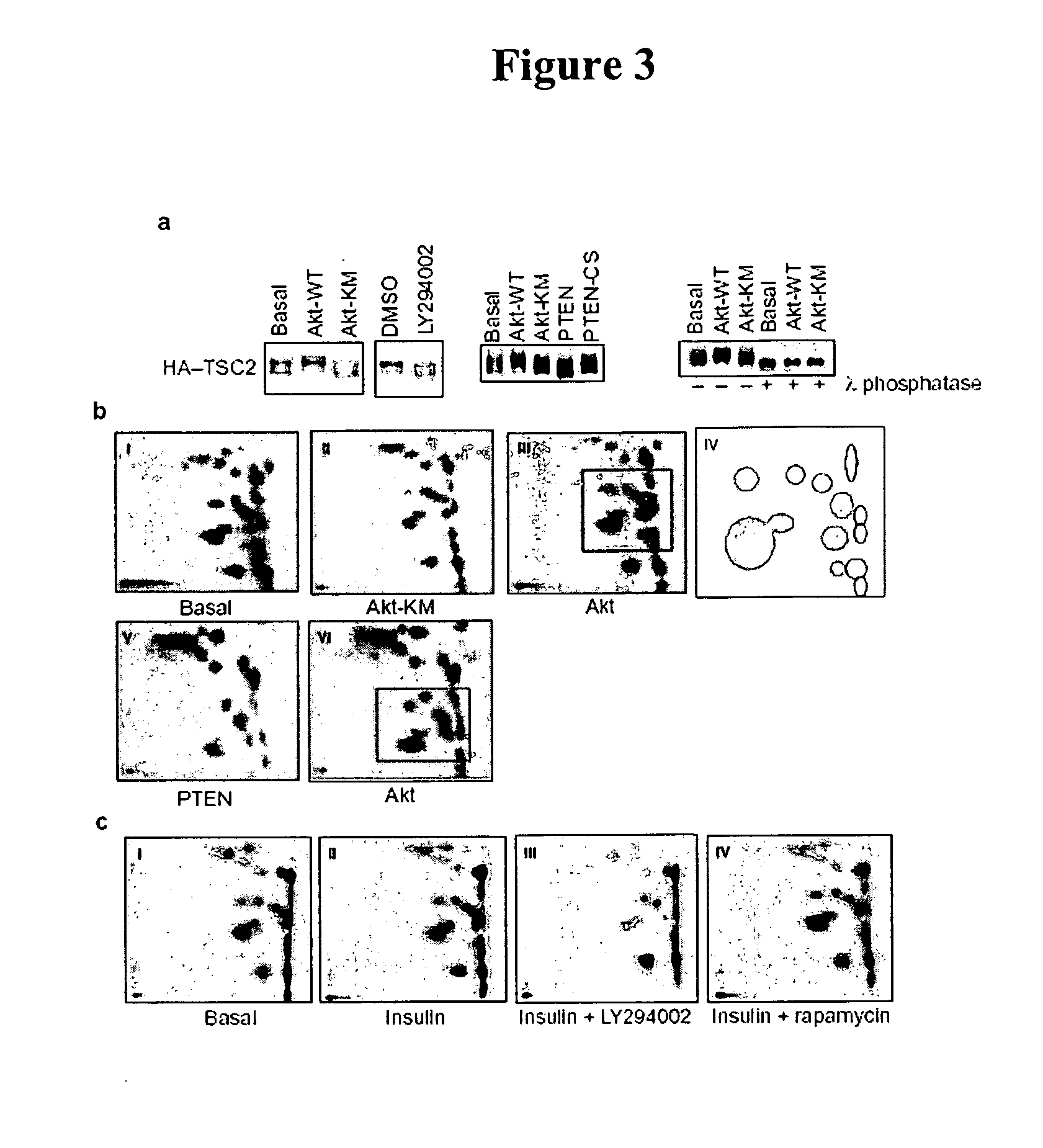
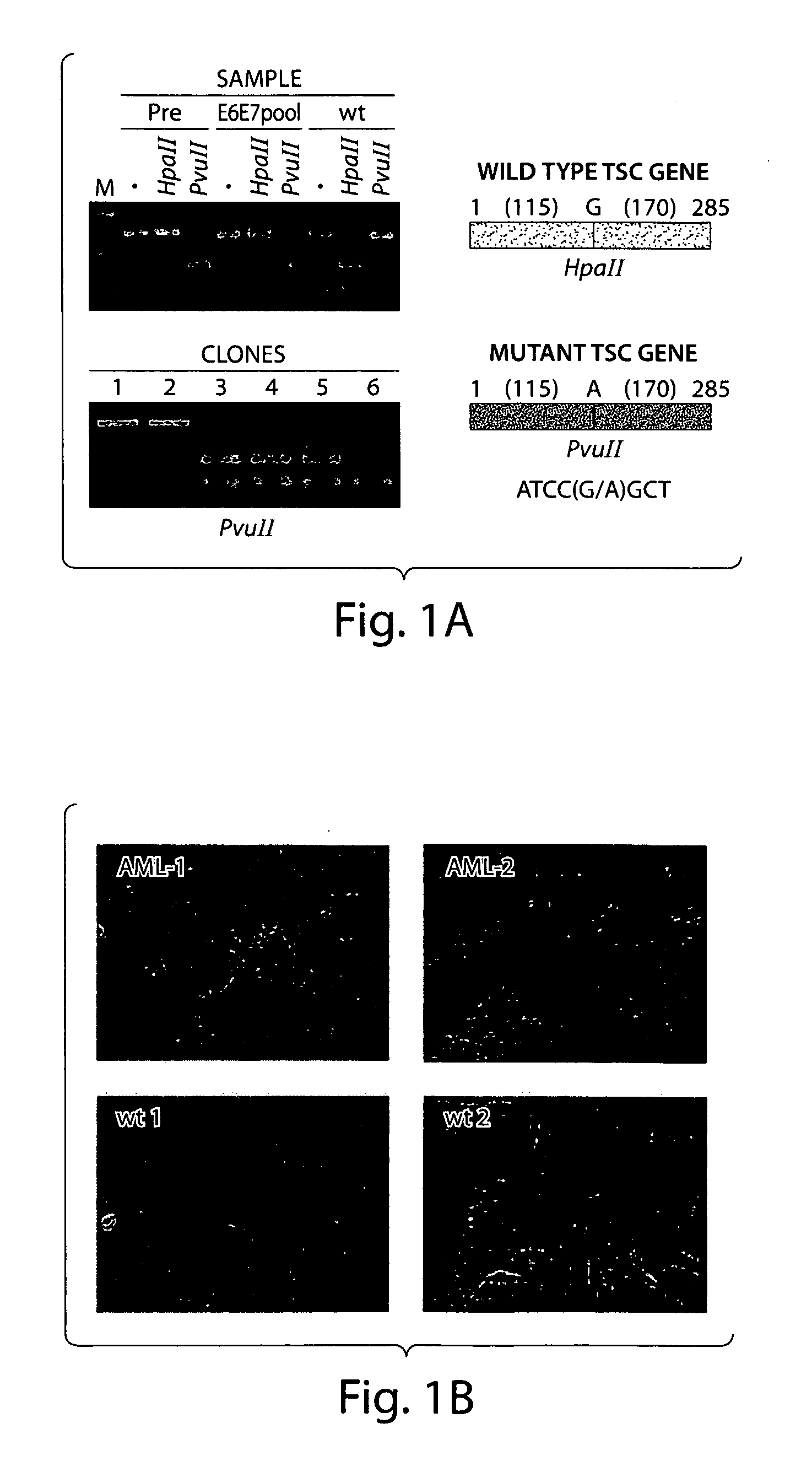
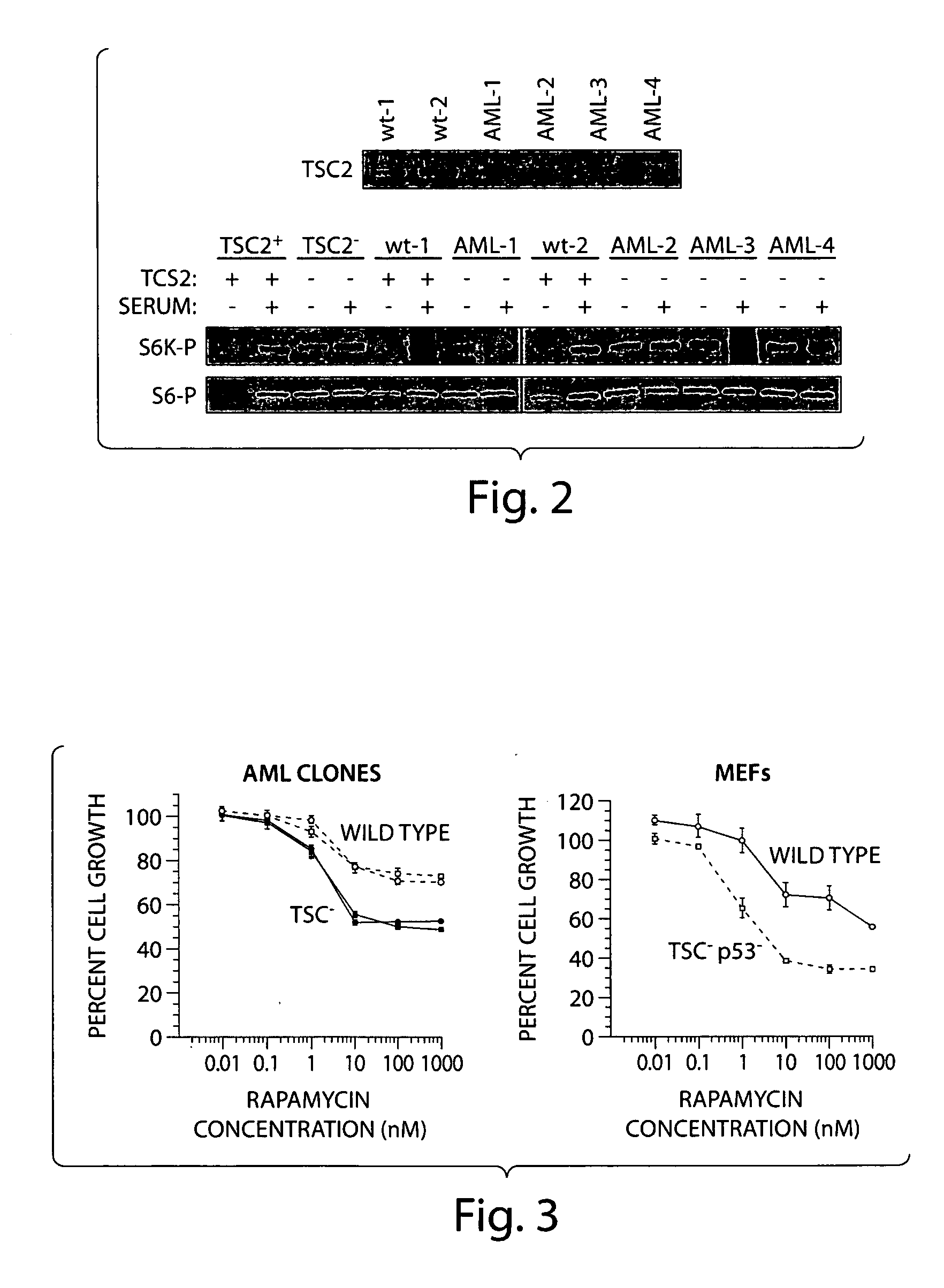
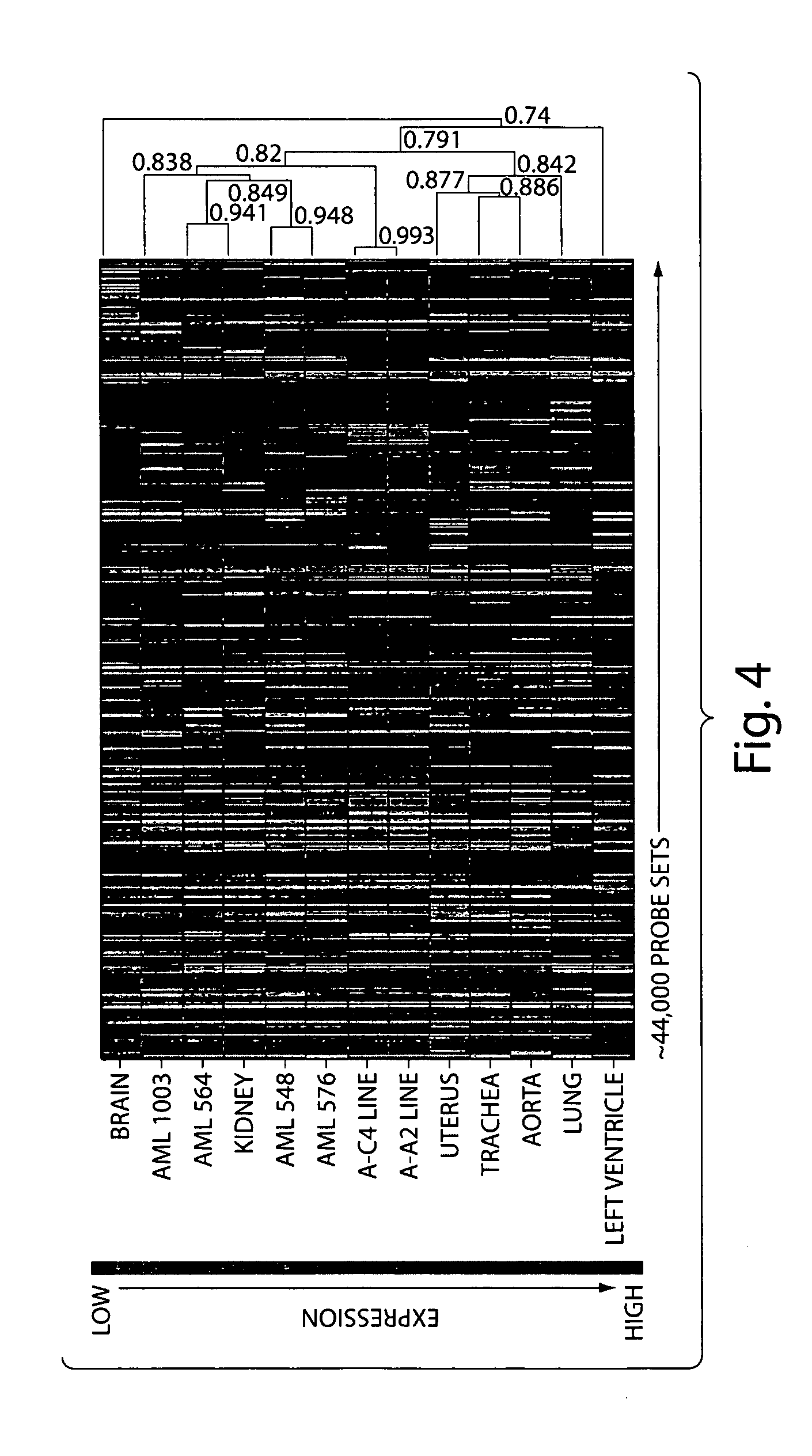
Diagnosis and treatment of diseases arising from defects in the tuberous sclerosis pathway
The present invention relates to compositions and methods for identifying abnormalities in TSC signaling pathways. In particular, the present invention relates to methods of diagnosing and treating disorders such as tuberous sclerosis, which are caused by mutations in the TSC genes. The present invention further relates to methods and compositions for treating cancers mediated by TSC signaling disorders.
Owner:RGT UNIV OF MICHIGAN
Diagnosis and treatment of diseases arising from defects in the tuberous sclerosis pathway
InactiveUS20050070567A1Impact strengthBiocideMicrobiological testing/measurementNodular sclerosisSignal pathway
The present invention relates to compositions and methods for identifying abnormalities in TSC signaling pathways. In particular, the present invention relates to methods of diagnosing and treating disorders such as tuberous sclerosis, which are caused by mutations in the TSC genes. The present invention further relates to methods and compositions for treating cancers mediated by TSC signaling disorders.
Owner:RGT UNIV OF MICHIGAN
Reverse-turn mimetics and method relating thereto
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Modulating endoplasmic reticulum stress in the treatment of tuberous sclerosis
InactiveUS20100022495A1Promote apoptosisReduce and prevent ER stressOrganic active ingredientsBiocideDiseaseHAMARTOMATOUS DISEASES
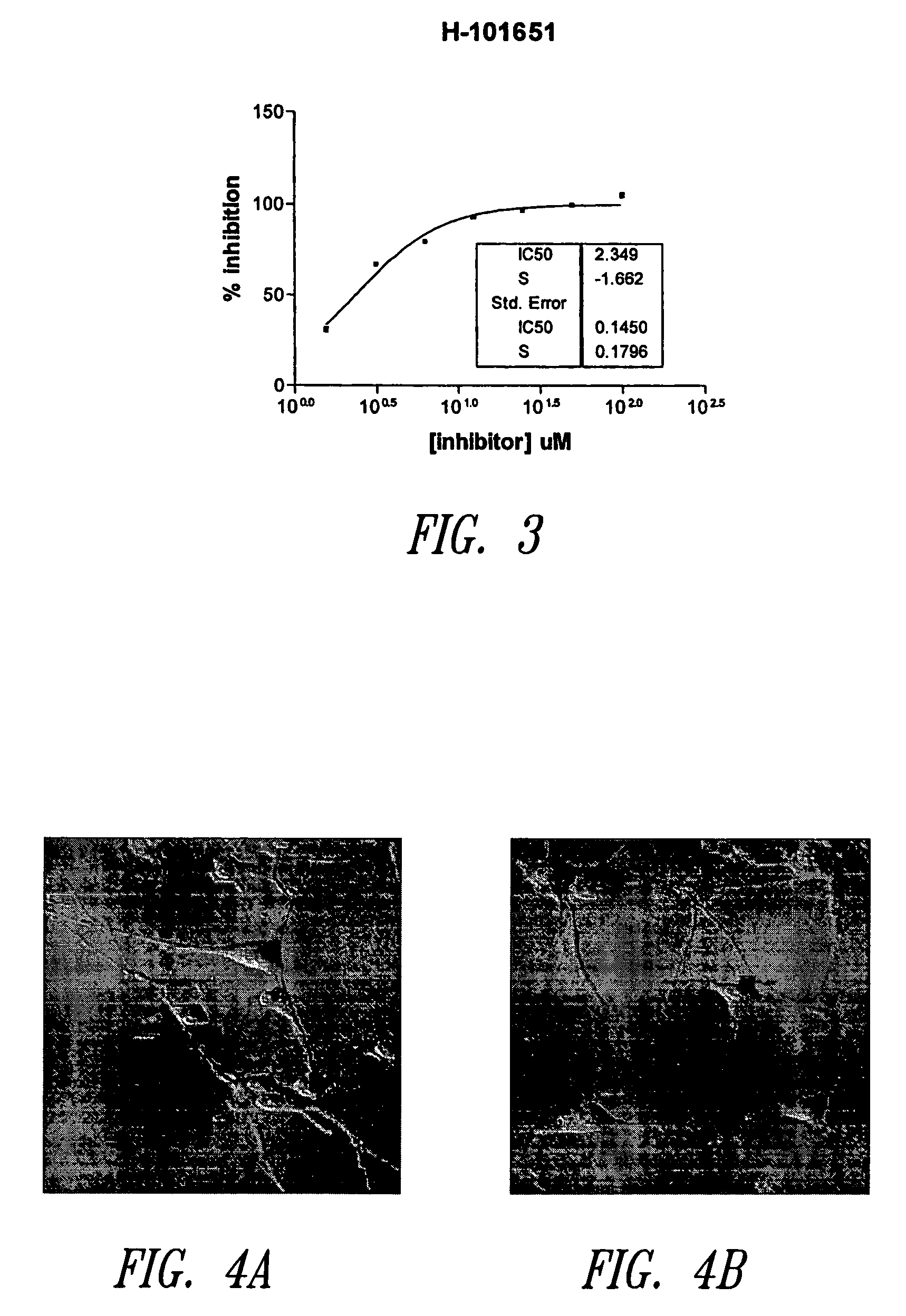
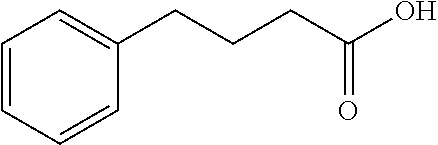
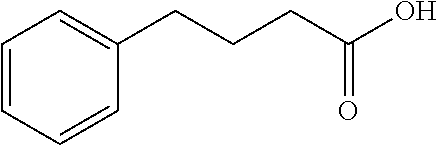
Endoplasmic reticulum stress has been found to be associated with the genetic disease tuberous sclerosis. Tuberous sclerosis is cause by defects in the two genes, TSC1 and TSC2. Agents that modulate ER stress may be used to treat tuberous sclerosis and other hamartomatous diseases. In particular, 4-phenyl butyric acid (PBA) has been shown to reduce ER stress is TSC-deficient cells. Other compounds useful in reducing ER stress are chemical chaperones such as trimethylamine N-oxide arid glycerol may also be useful in treating tuberous sclerosis. The present invention provides methods of treating a subject suffering from tuberous sclerosis using ER stress reducers such as PBA, TUDCA, UDCA, and TMAO. Methods of screening for ER stress reducers by identifying agents that reduce levels of ER stress markers in TSC-deficient cells are also provided. These agents may find use in methods and pharmaceutical compositions for treating tuberous sclerosis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Reverse-turn mimetics and method relating thereto
ActiveUS20060084655A1Efficient implementationPromoting neurite outgrowthBiocideNervous disorderUlcerative colitisNodular sclerosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Modulating Endoplasmic Reticulum Stress in the Treatment of Tuberous Sclerosis
InactiveUS20140011761A1Promote apoptosisReduce and prevent ER stressBiocideCarbohydrate active ingredientsHAMARTOMATOUS DISEASESReticulum cell
Endoplasmic reticulum stress has been found to be associated with the genetic disease tuberous sclerosis. Tuberous sclerosis is cause by defects in the two genes, TSC1 and TSC2. Agents that modulate ER stress may be used to treat tuberous sclerosis and other hamartomatous diseases. In particular, 4-phenyl butyric acid (PBA) has been shown to reduce ER stress is TSC-deficient cells. Other compounds useful in reducing ER stress are chemical chaperones such as trimethylamine N-oxide arid glycerol may also be useful in treating tuberous sclerosis. The present invention provides methods of treating a subject suffering from tuberous sclerosis using ER stress reducers such as PBA, TUDCA, UDCA, and TMAO. Methods of screening for ER stress reducers by identifying agents that reduce levels of ER stress markers in TSC-deficient cells are also provided. These agents may find use in methods and pharmaceutical compositions for treating tuberous sclerosis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Use of cannabinoids in the treatment of epilepsy
ActiveUS20200297656A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsAicardi's syndromeYoung adult
The present invention relates to the use of cannabidiol (CBD) in the treatment of focal seizures. In one embodiment the patients suffering from focal seizures are children and young adults. CBD appears particularly effective in reducing focal seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; CDKLS; Neuronal ceroid lipofuscinoses (NCL); febrile infection related epilepsy syndrome (FIRES); Aicardi syndrome and brain abnormalities in comparison to other seizure types. Significantly CBD additionally is very effective in the reduction of a sub-type of focal seizures, focal seizures with impairment.
Owner:JAZZ PHARM RES UK LTD
Reverse-turn mimetics and method relating thereto
InactiveUS20070043052A1Efficient implementationPromoting neurite outgrowthBiocideOrganic chemistryUlcerative colitisNodular sclerosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
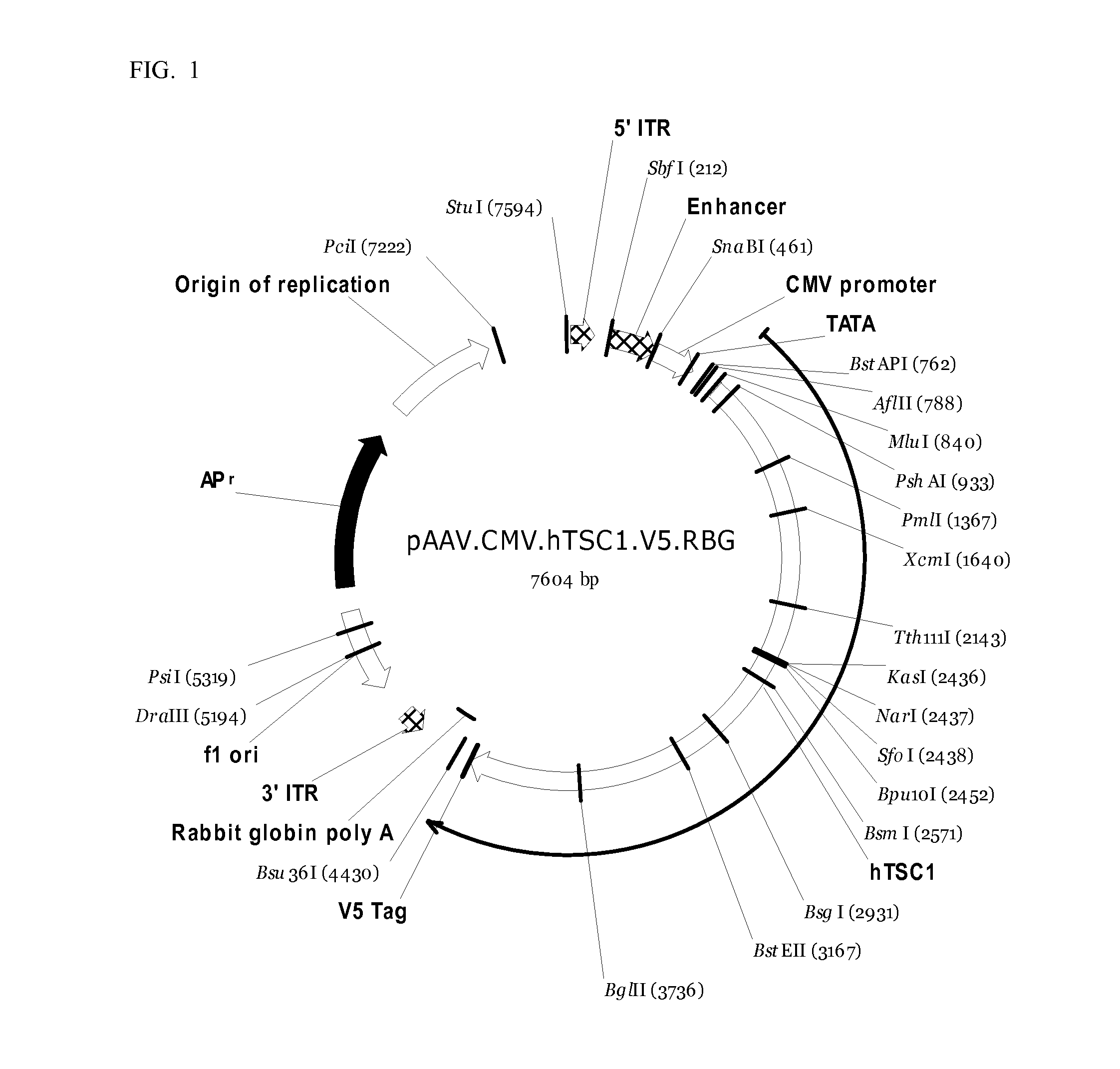
Methods and compositions for treatment of tuberous sclerosis complex
The present disclosure relates to a method of treating Tuberous Sclerosis Complex (TSC). The present disclosure further provides an isolated polynucleotide molecule comprising, a polynucleotide comprising an AAV9 vector; a polynucleotide encoding a TSC1 or a TSC2, or variant thereof; and a promoter. The present disclosure further provides a pharmaceutical composition comprising an isolated polynucleotide molecule.
Owner:GE MEDICAL SYST GLOBAL TECH CO LLC
Immortalized human Tuberous Sclerosis null angiomyolipoma cell and method of use thereof
InactiveUS20050266442A1Microbiological testing/measurementGenetically modified cellsDiseaseEpithelioid angiomyolipoma
Disclosed are immortailized cells and cell lines that do not express the Tuberous Sclerosis Complex(TSC)-2 gene. Also sisclosed are methods of detecting TSC-related disorders using differentially expressed genes.
Owner:THE ROTHBERG INST FOR CHILDHOOD DISEASES
Use of cannabinoids in the treatment of epilepsy
ActiveUS10709671B2Nervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
Owner:GW RES LTD
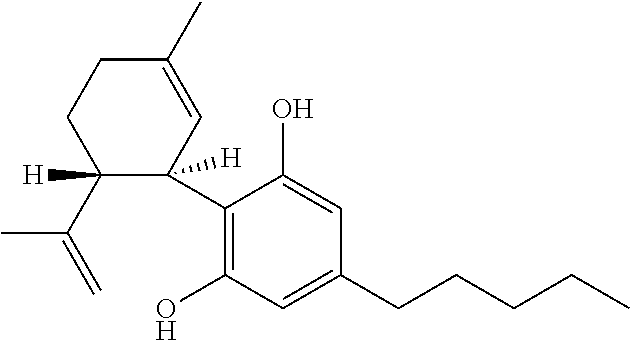
Use of cannabidiol in the treatment of epilepsy
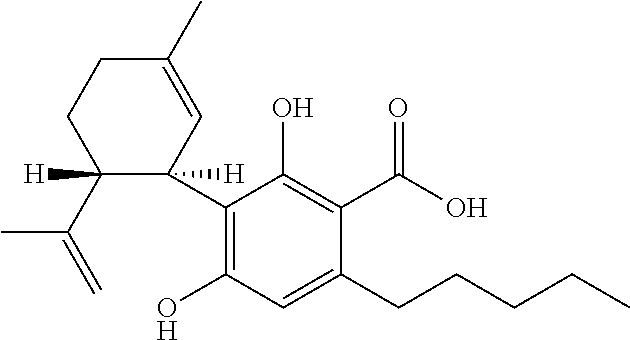
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of Tuberous Sclerosis Complex (TSC). In particular the TSC is treatment resistant and is characterised by generalised seizures or focal seizures with impairment. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
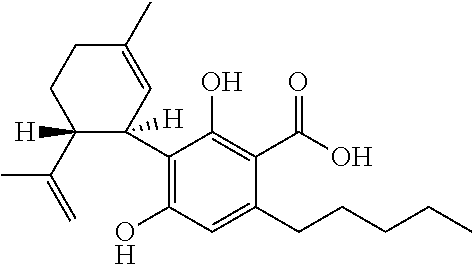
Use of cannabidiol in the treatment of tuberous sclerosis complex
InactiveUS20200206153A1Prevent and reduce tumourReduce doseHydroxy compound active ingredientsAntineoplastic agentsBenign tumoursDisease
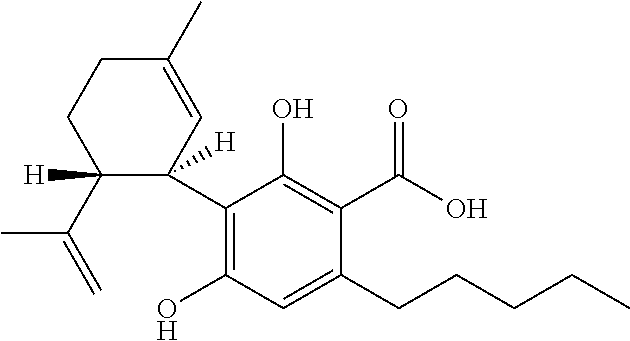
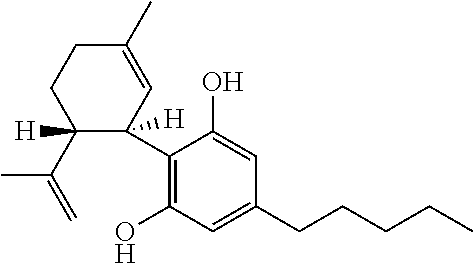
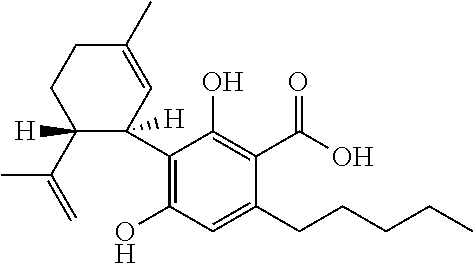
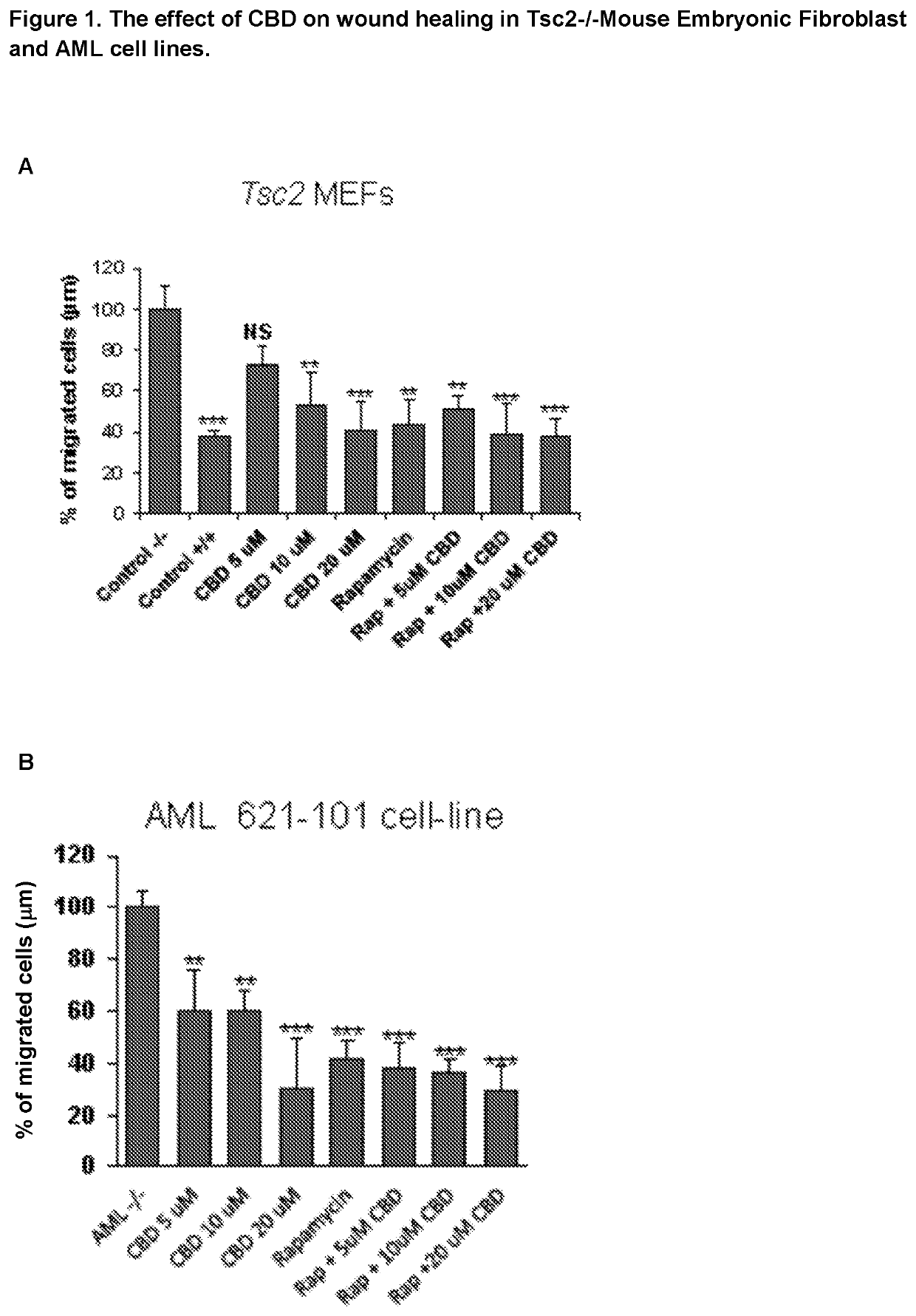
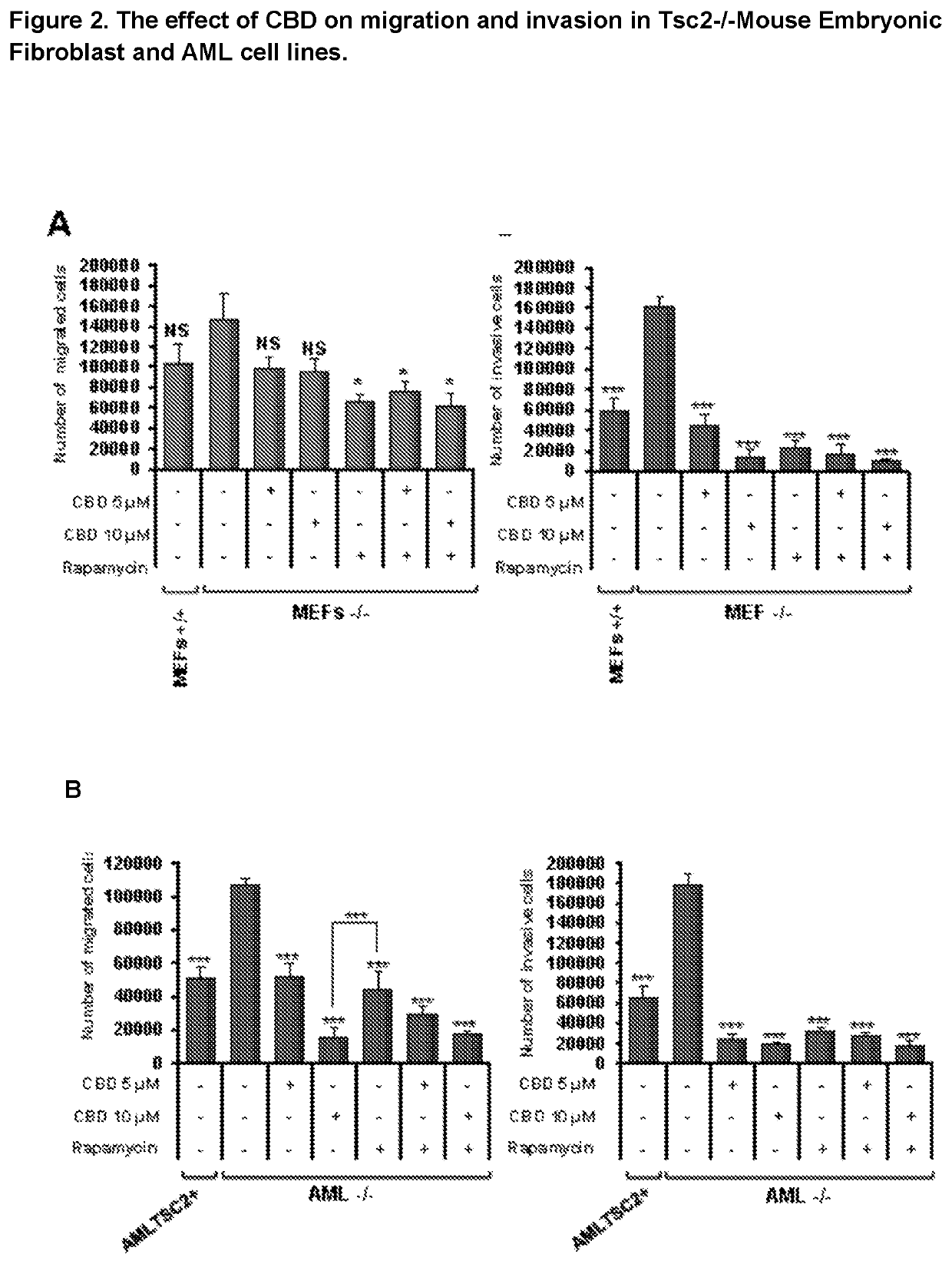
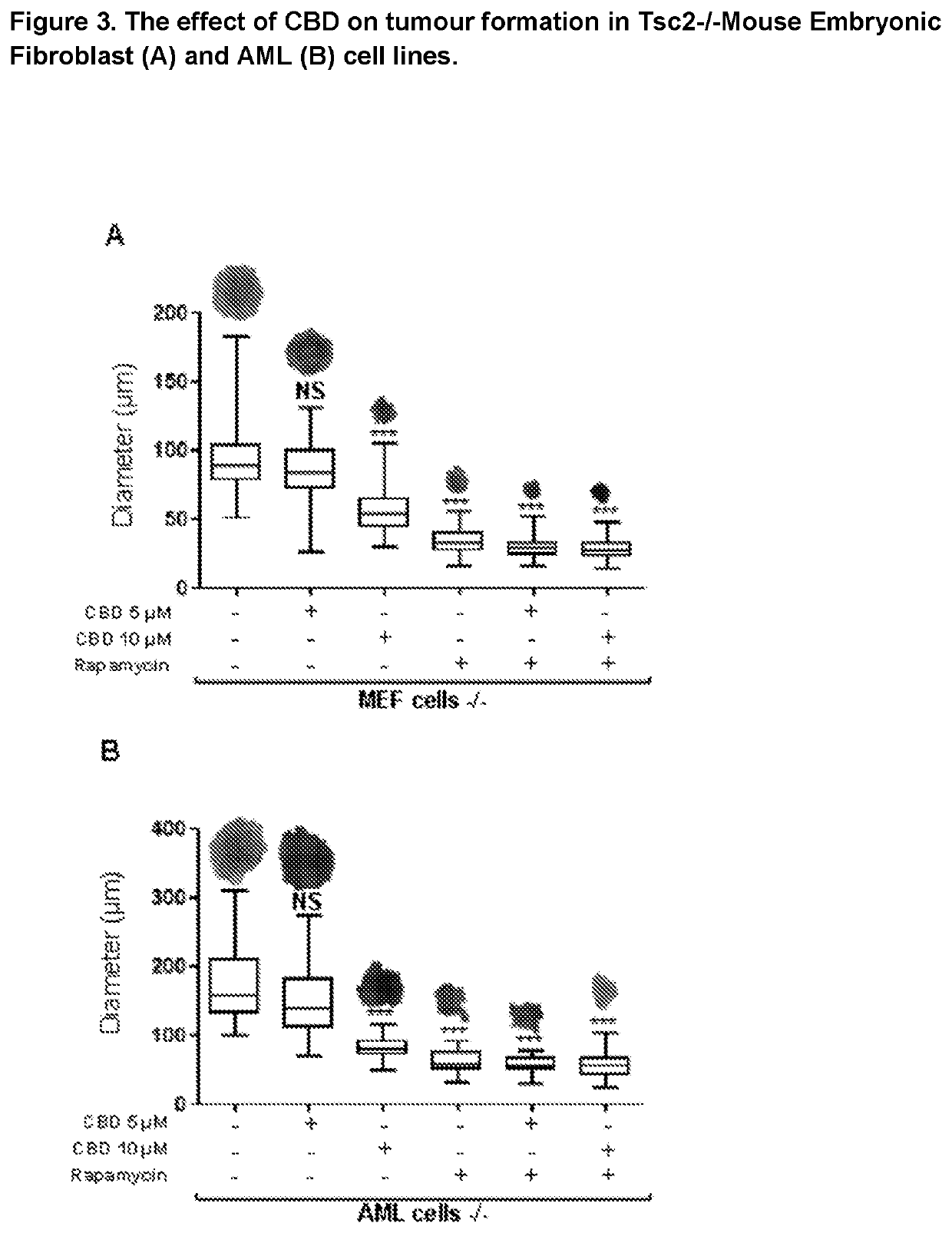
The present invention relates to the use of cannabidiol (CBD) for the treatment of tumours associated with Tuberous Sclerosis Complex (TSC). In particular the CBD was able to decrease the number and size of marker cells, pS6, in a zebrafish model of TSC. This is5 suggestive of a disease modifying effect whereby treatment with CBD could result in the reduction or prevention of the benign tumours that occur in TSC patients. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially 10 removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD. In use the CBD is given concomitantly with one or more other drugs used in the treatment of TSC. Such drugs may include rapamycin and / or everolimus.
Owner:GW RES LTD
Diagnosis and treatment of diseases arising from defects in the tuberous sclerosis pathway
The present invention relates to compositions and methods for identifying abnormalities in TSC signaling pathways. In particular, the present invention relates to methods of diagnosing and treating disorders such as tuberous sclerosis, which are caused by mutations in the TSC genes. The present invention further relates to methods and compositions for treating cancers mediated by TSC signaling disorders.
Owner:RGT UNIV OF MICHIGAN
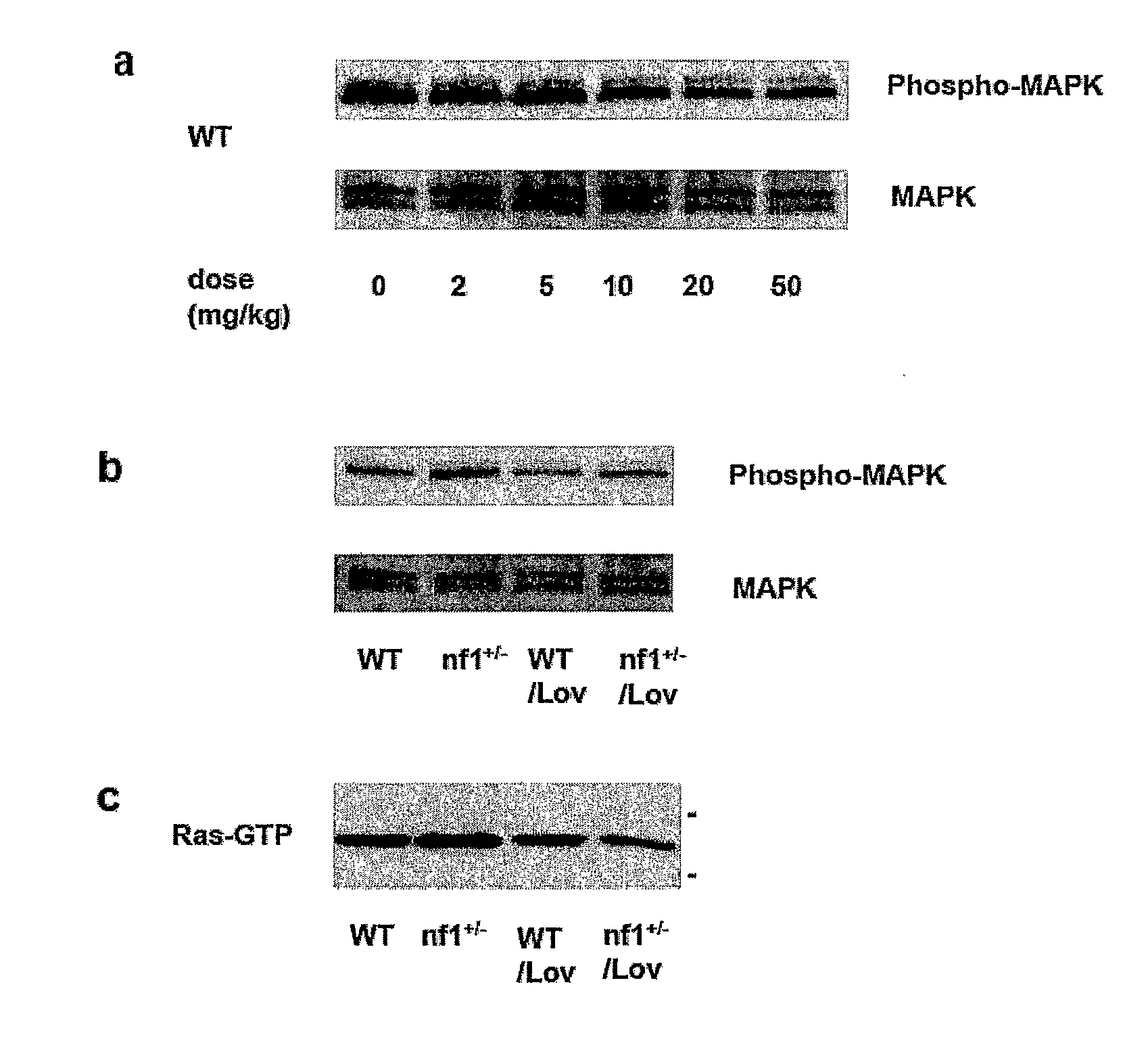
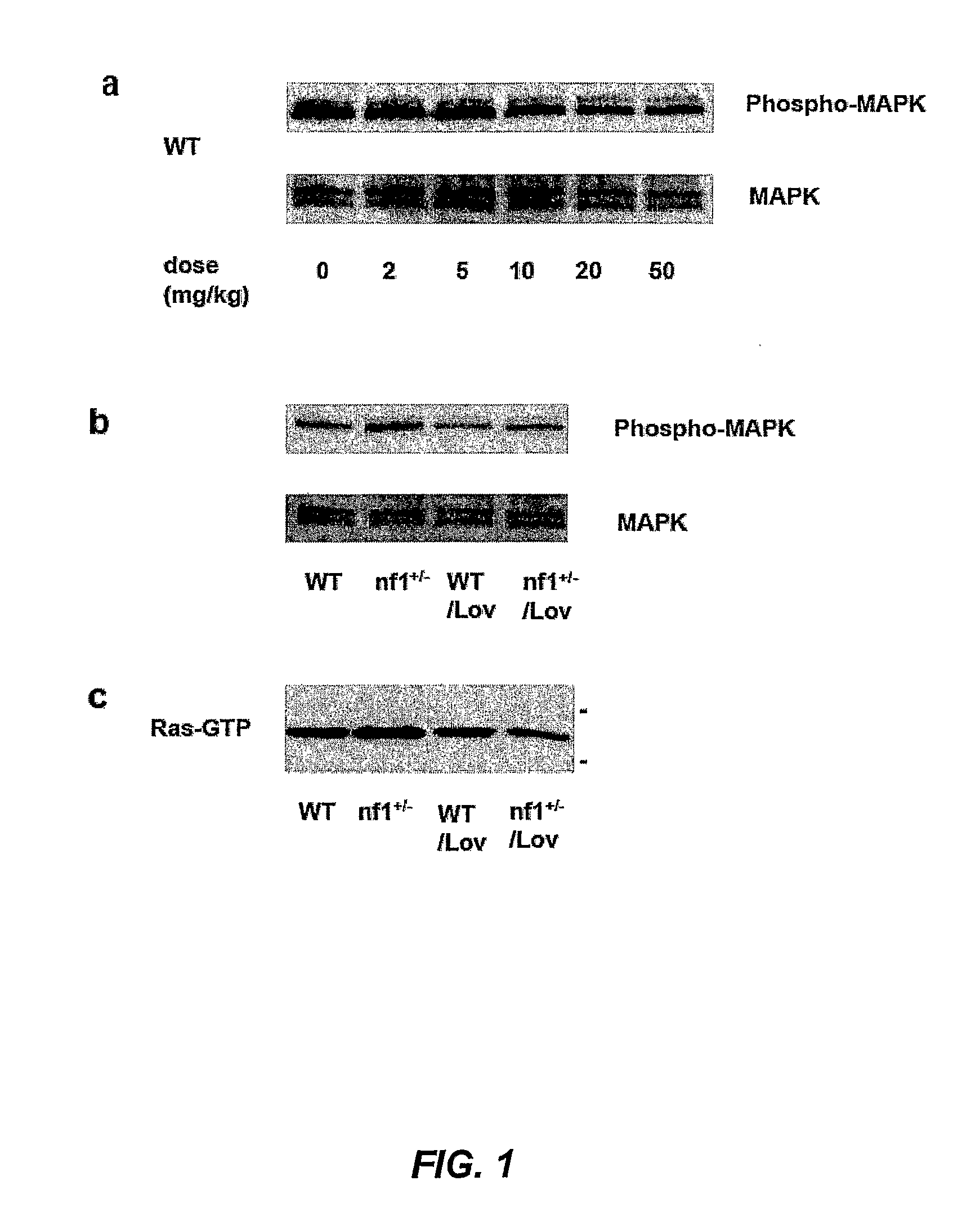
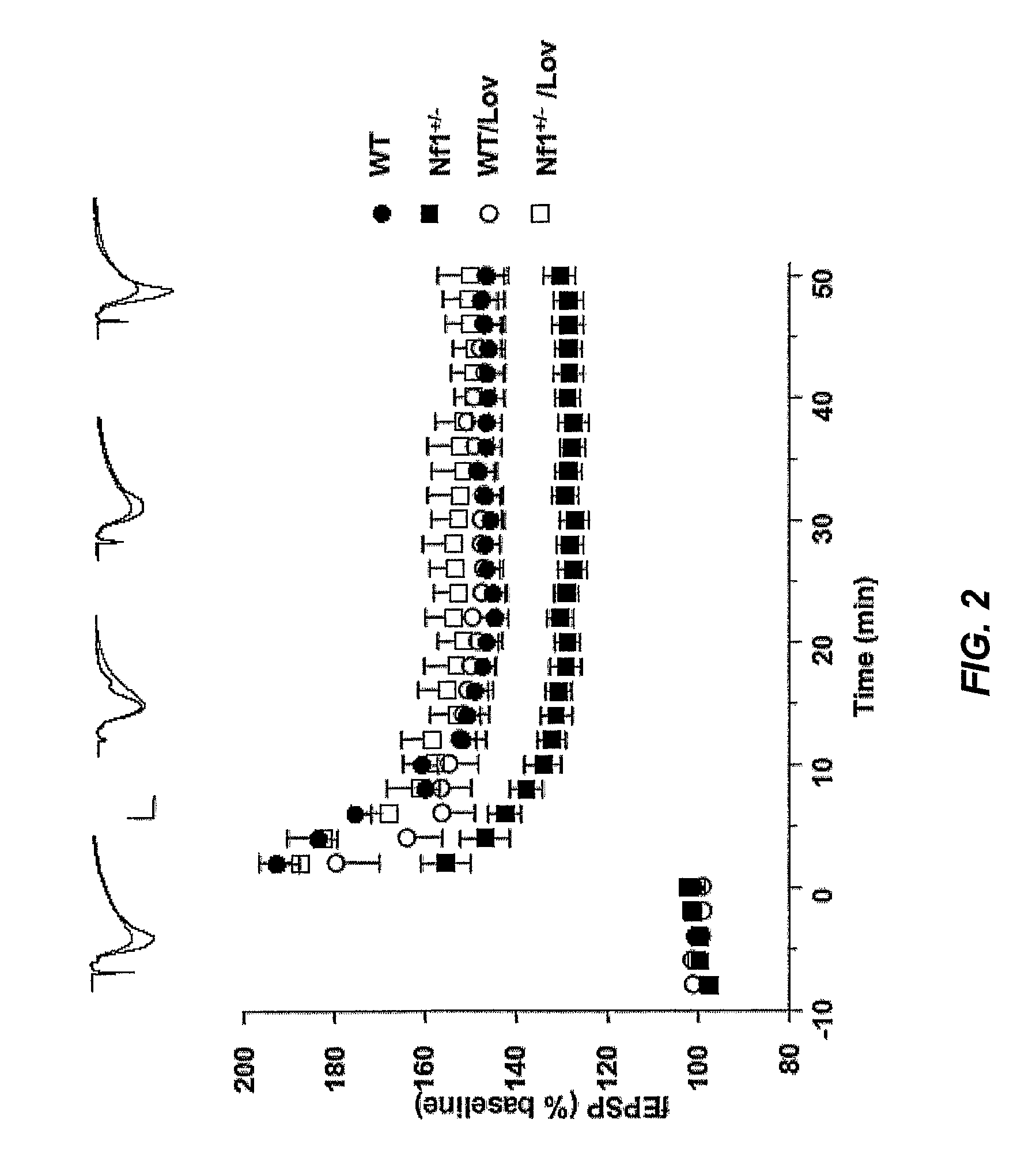
Treating Learning Deficits With Inhibitors of Hmg CoA Reductase
The disclosure provides methods of treating cognitive disorders by administering a HMG CoA reductase inhibitor. Cognitive deficits treatable with the inhibitor compound include those associated with Angelman Syndrome, Neurofibromatosis-1, certain forms of X-linked mental retardation, tuberous sclerosis, Down Syndrome, autism, and attention deficit / hyperactivity disorder.
Owner:RGT UNIV OF CALIFORNIA
Use of cannabidiol in the treatment of epilepsy
ActiveUS11065209B2Reduce in quantityReduce doseNervous disorderDispersion deliveryFocal EpilepsiesAntiepileptic drug
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of Tuberous Sclerosis Complex (TSC). In particular the TSC is treatment resistant and is characterised by generalised seizures or focal seizures with impairment. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Reverse-turn mimetics and method relating thereto
ActiveUS20100222303A1Efficient implementationBiocideOrganic chemistryUlcerative colitisSignalling pathways
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins as well as their prodrugs are disclosed. Such reverse-turn mimetic structures and prodrugs have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds and prodrugs for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
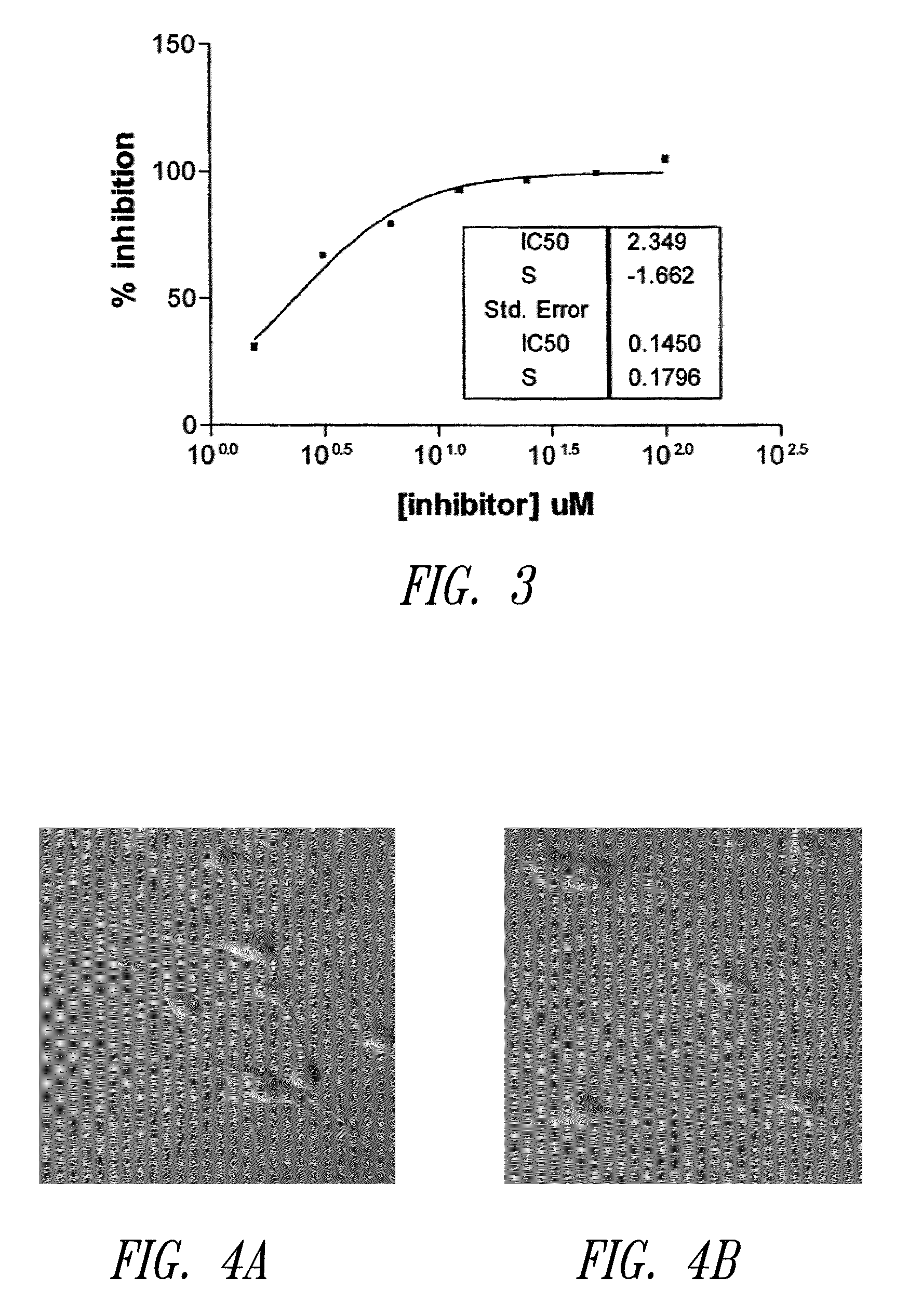
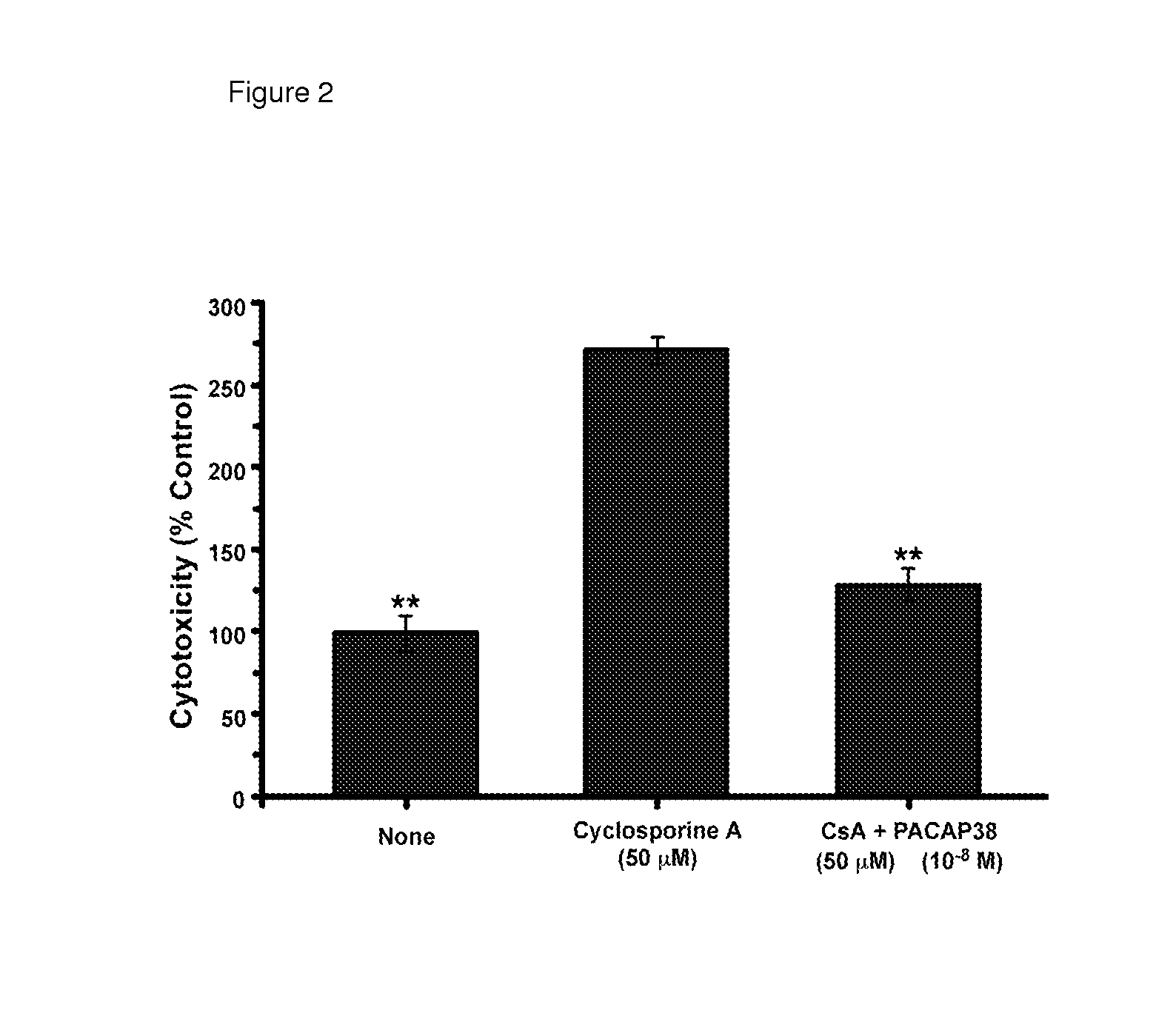
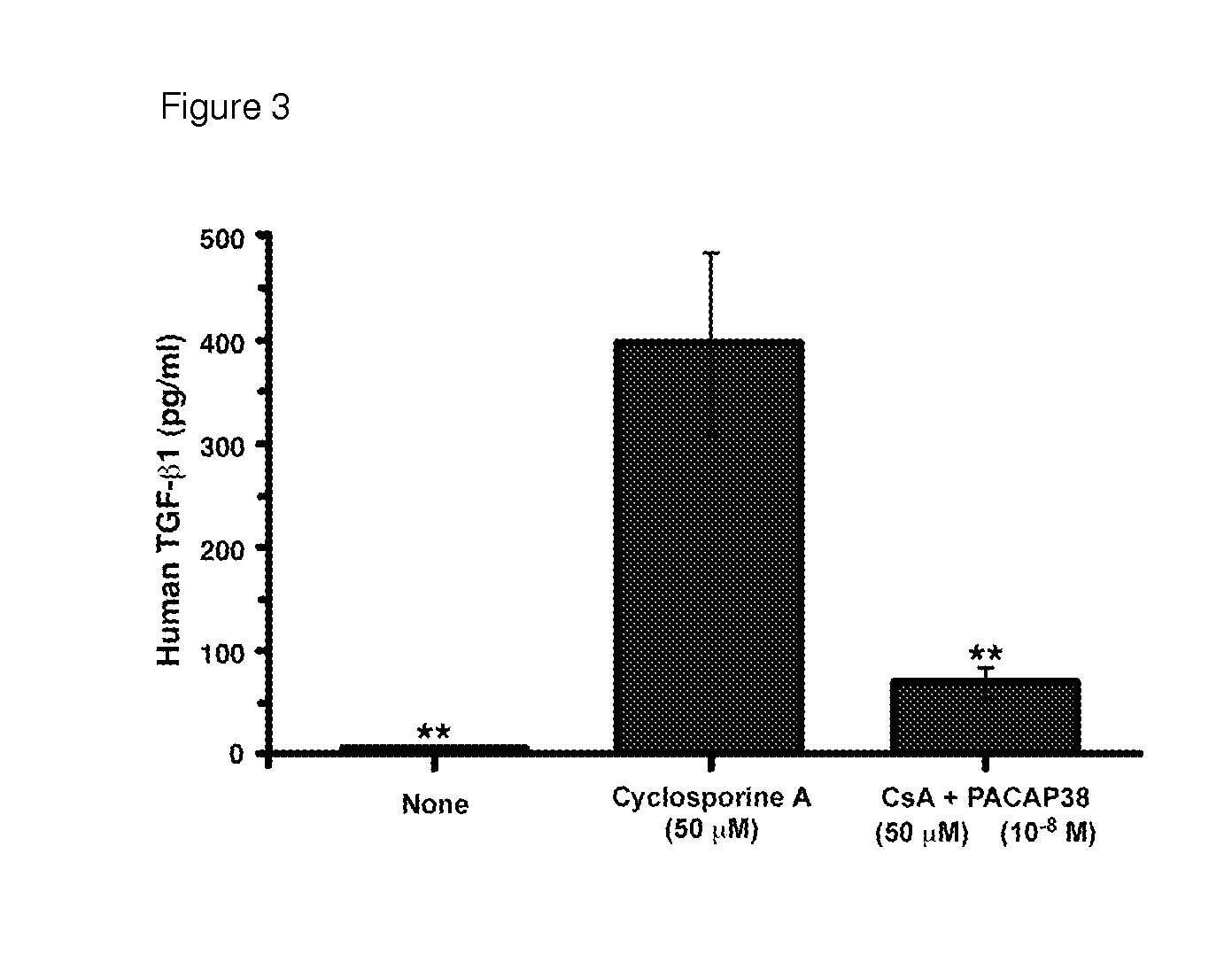
USE OF PITUITARY ADENYLATE CYCLASE-ACTIVATING POLYPEPTIDE (PACAP) AND PACAP ANALOGS AS ADJUNCTIVE TREATMENTS WITH INHIBITORS OF CALCINEURIN OR INHIBITORS OF THE MAMMALIAN TARGET OF RAPAMYCIN (mTOR) COMPLEXES
InactiveUS20120309683A1Protecting the major organsEffective protectionNervous disorderMetabolism disorderUveitisAutoimmune responses
This invention relates to methods and compositions for the treatment, management, reduction, or prevention of injuries to one or more major organs of the body, e.g., the brain, heart, lung, kidneys, liver, and gastrointestinal tract, of a mammal (e.g., a human) caused by one or more calcineurin or mammalian target of rapamycin (mTOR) complex inhibitors. The methods include administering an effective amount of one or more pituitary adenylate cyclase-activating polypeptide (PACAP)-like compounds to the mammal. Combination therapy with one or more PACAP-like compounds, either alone or in combination with one or more other prophylactic / therapeutic agents, plus one or more inhibitors of either calcineurin or the mTOR complexes can be used to treat organ transplantation, autoimmune diseases, graft-versus-host disease, Behçet's disease, hematological cancers, noninfectious uveitis, sarcoidosis, tuberous sclerosis complex, acute neurological diseases, age-related neurodegenerative diseases, Huntington's disease and other CAG codon repeat expansion diseases, keratoconjunctivitis sicca, and restenosis.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
Child tuberous sclerosis treatment drug
The present invention relates to the field of medicines, and in particular discloses a child tuberous sclerosis treatment drug including rapamycin, the child tuberous sclerosis includes child tuberous sclerosis combined intractable epilepsy, child tuberous sclerosis combined cardiac rhabdomyoma, child tuberous sclerosis combined depigmentation, facial steatadenoma, and child tuberous sclerosis combined renal angioleiomyolipoma, and the drug use method is as follows: the initial dose of the rapamycin is 1mg / (m2.d), and the blood drug concentration is maintained at 5-10ng / mL.
Owner:邹丽萍 +1
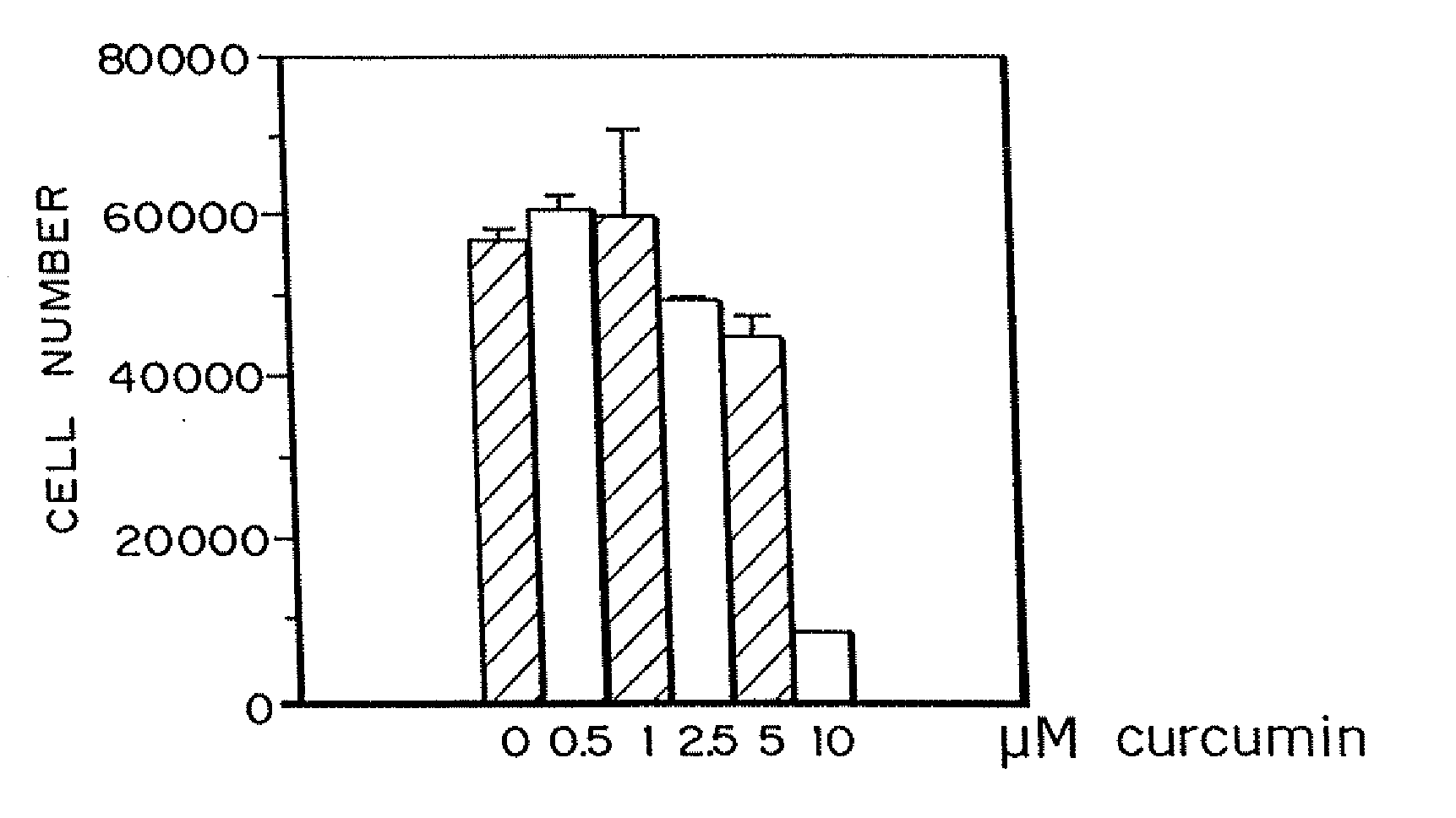
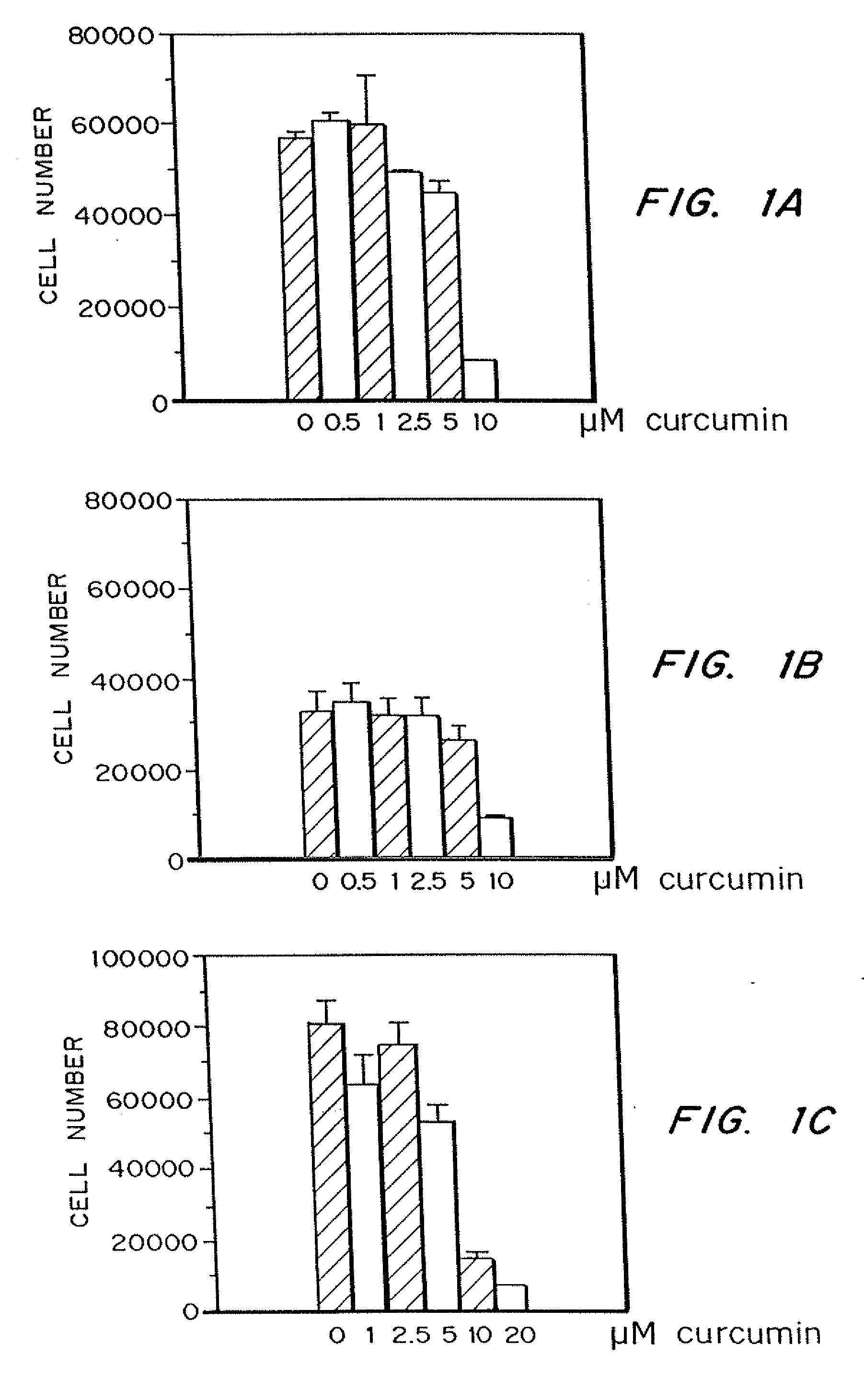
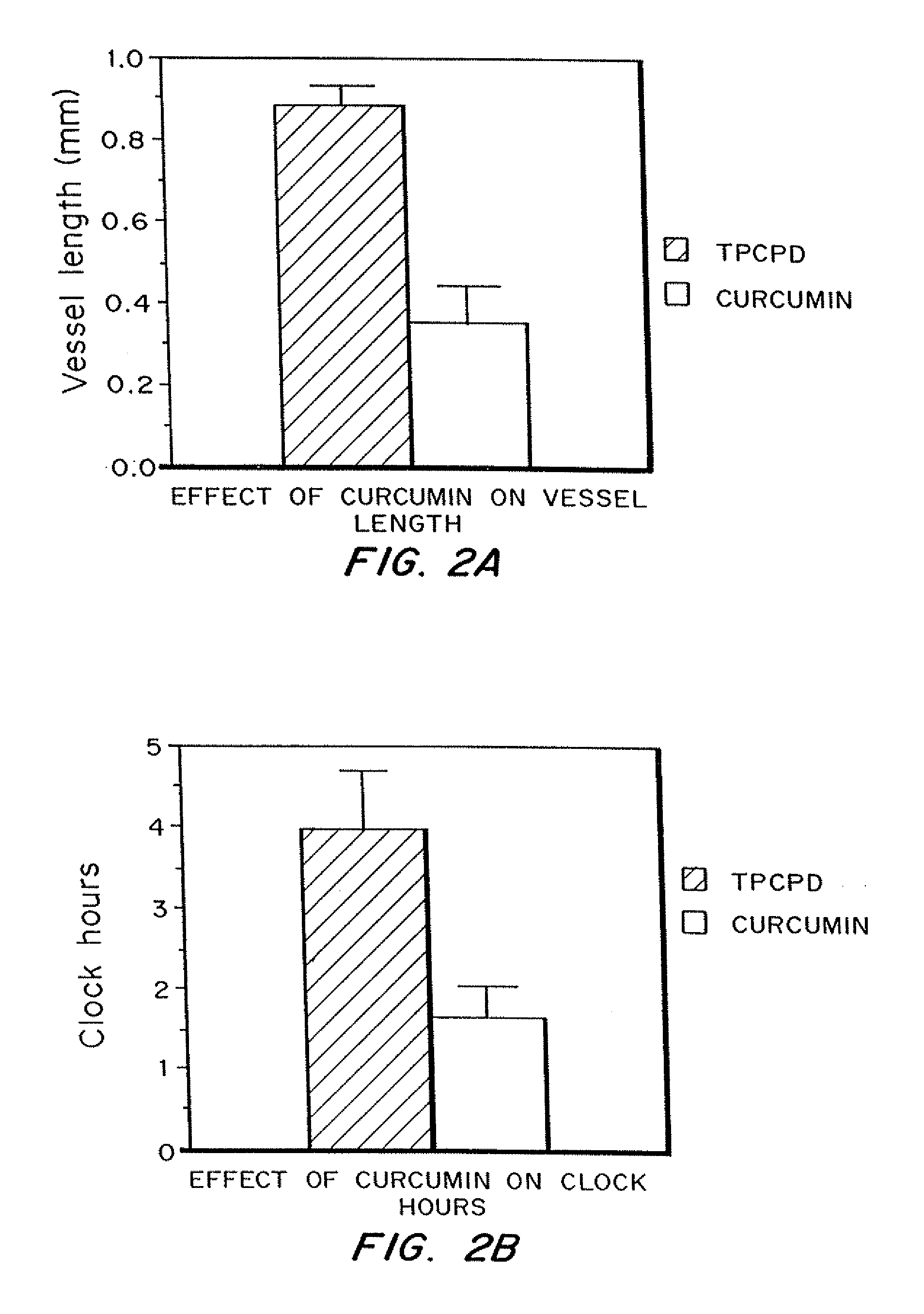
Curcumin and curcuminoid inhibition of angiogenesis
InactiveUS20090018209A1Inhibit angiogenesisImprove the level ofBiocideDigestive systemRecessive dystrophic epidermolysis bullosaSturge–Weber syndrome
Methods for treating diseases or disorders of the skin which are characterized by angiogenesis have been developed using curcumin and curcumin analogs. Based on the results obtained with curcumin, it has been determined that other angiogenesis inhibitors can also be used to treat these skin disorders. It has further been discovered that curcumin acts to inhibit angiogenesis in part by inhibition of basic fibroblast growth factor (bFGF), and thereby provides a means for treating other disorders characterized by elevated levels of bFGF, such as bladder cancer, using curcumin and other analogues which also inhibit bFGF. Representative skin disorders to be treated include the malignant diseases angiosarcoma, hemangioendothelioma, basal cell carcinoma, squamous cell carcinoma, malignant melanoma and Karposi's sarcoma, and the non-malignant diseases or conditions including psoriasis, lymphangiogenesis, hemangioma of childhood, Sturge-Weber syndrome, verruca vulgaris, neurofibromatosis, tuberous sclerosis, pyogenic granulomas, recessive dystrophic epidermolysis bullosa, venous ulcers, acne, rosacea, eczema, molluscum contagious, seborrheic keratosis, and actinic keratosis.
Owner:EMORY UNIVERSITY
Chimeric dystrophin proteins to treat dystrophinopathies
InactiveUS20150329609A1SsRNA viruses negative-sensePolypeptide with localisation/targeting motifMelanomaMarfan syndrome
A chimeric protein that is a fusion construct of a series of functional domains is used to deliver a therapeutic agent to a human subject suffering from disease. In some embodiments, the chimeric protein includes a therapeutic region and a transportation region. The transportation region allows the chimeric protein to be moved across a cellular membrane of an affected cell within the subject. The therapeutic region can be effective in the treatment of, for example, muscular dystrophy, diastrophic dysplasia, malignant melanoma, porphyria, alpha-1 antitrypsin deficiency, Aicardi-Goutieres syndrome, cystic fibrosis, progeria, Marfan syndrome, tuberous sclerosis, adrenoleukodystrophy, and the like.
Owner:SERENDIPITY BIOTECH INC
Reverse-turn mimetics and method relating thereto
InactiveUS20100029630A1Efficient implementationBiocideOrganic chemistryUlcerative colitisNodular sclerosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com