Use of cannabidiol in the treatment of tuberous sclerosis complex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
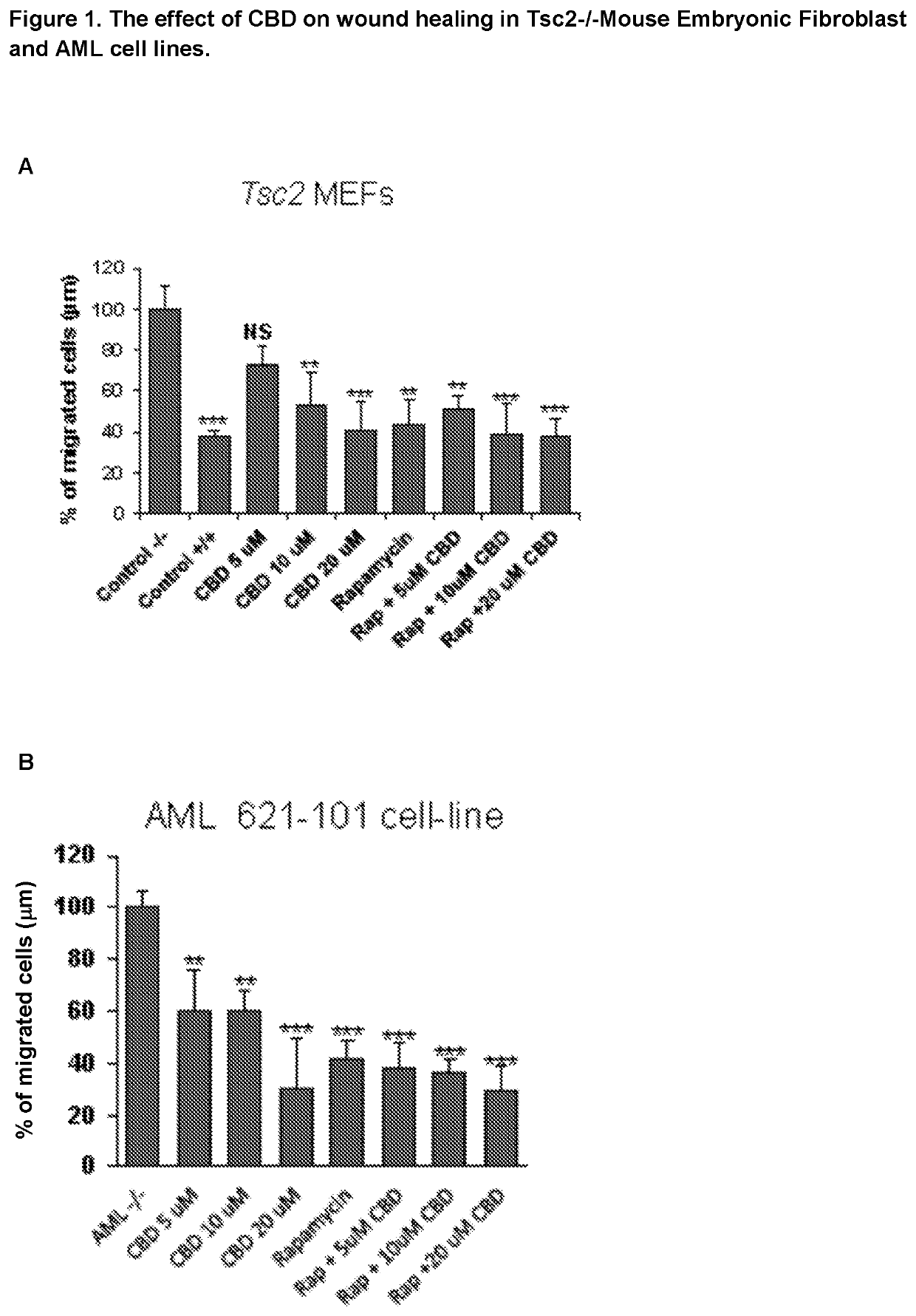
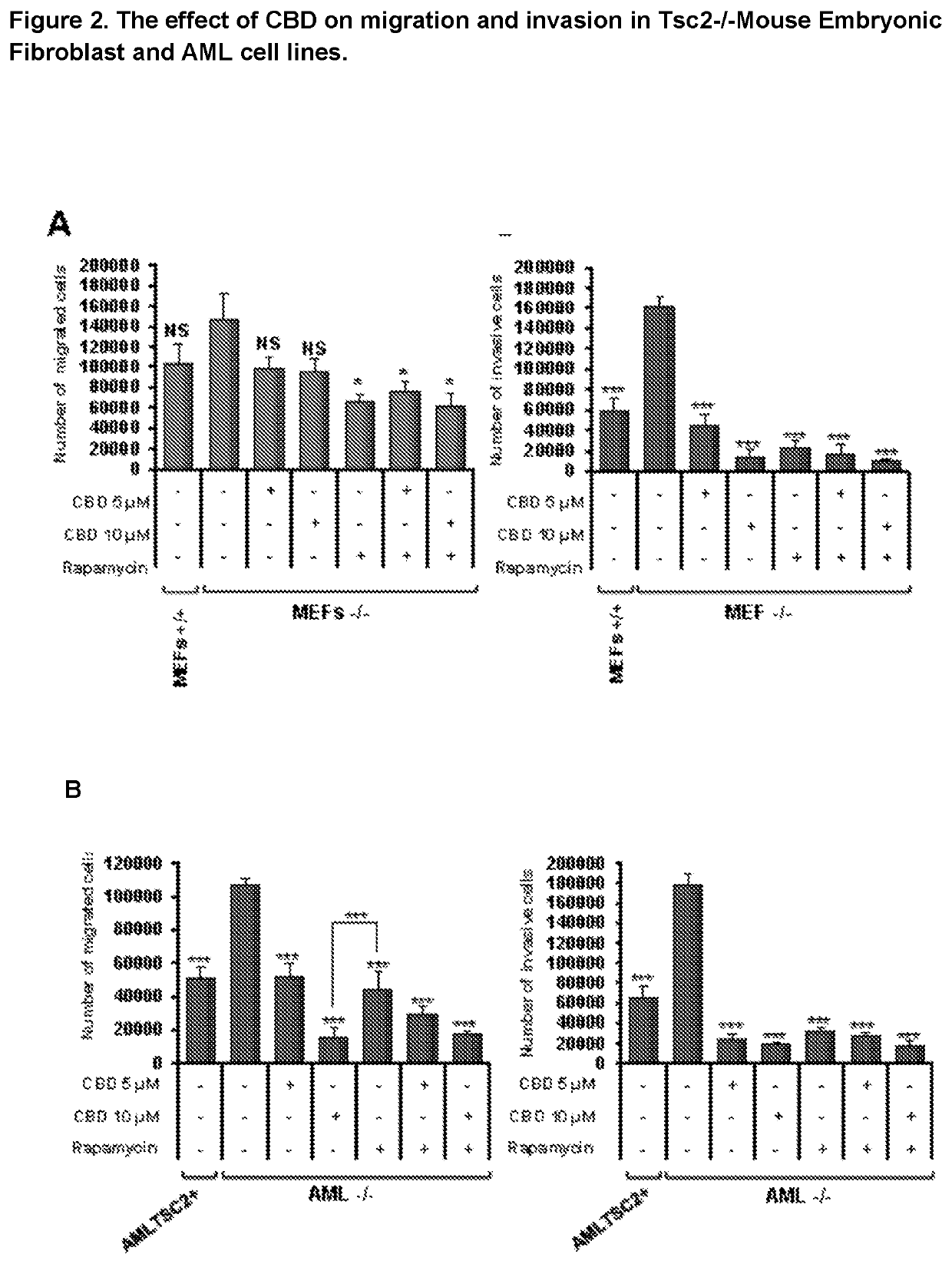
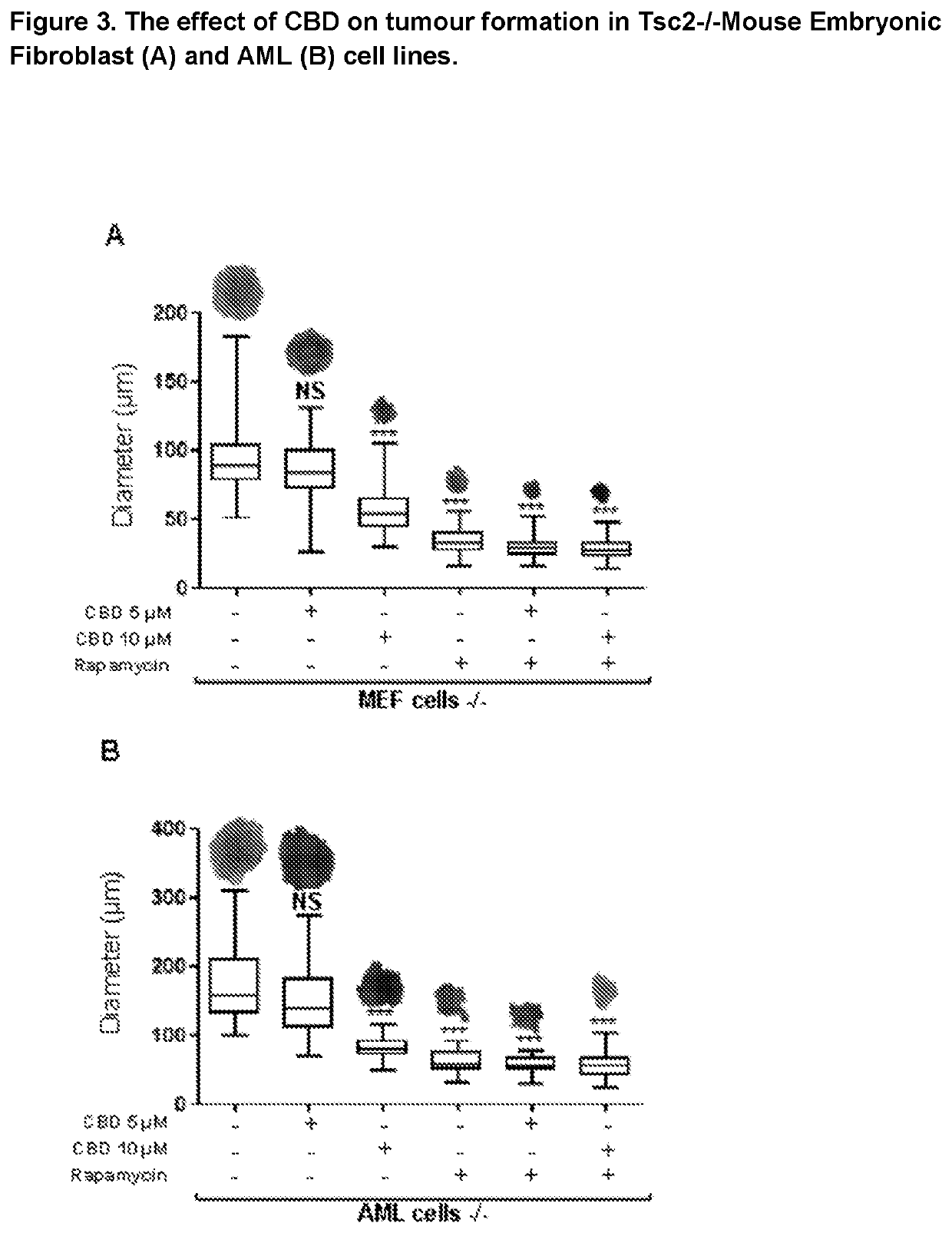
Efficacy of Cannabidiol in Reduction of Tumours in Two Tuberous Sclerosis Complex Cell Lines
Materials and Methods
Cell Lines and Maintenance
[0047](i) Angiomyolipomas (AML) cells derived from a Tuberous Sclerosis Complex patient (621-101 cell-line), with a known loss of function of TSC2. To generate a rescue control cell line, these 621-101 cells were stably transfected with a TSC2-expressing plasmid possessing Zeocin™ resistance (pcDNA3.1zeo-hTSC2). Zeocin™ was purchased from LifeTechnologies (cat no: R25001) and was used to originally generate the stable cell line (by Prof. Lisa Henske) over two weeks of Zeocin™ selection and then for continued maintenance of the cells in tissue culture (at 100 μg / ml).
[0048](ii) Tsc2− / − p53− / − and Tsc2+ / + p53− / − mouse embryonic fibroblast (MEF) cell lines (now referred to as Tsc2− / − and Tsc2+ / +, respectively).
Cell Culture
[0049]Tsc2− / − and Tsc2+ / + cell lines were cultured and maintained in DMEM supplemented with 10% (v / v) FBS and 1% (v / v) penicillin-...
example 2
Efficacy of Cannabidiol in Reduction of Tumours a Zebrafish Model of Tuberous Sclerosis Complex
Materials and Methods
Zebrafish Husbandry
[0071]A zebrafish model of TSC with a tsc2 gene deletion was used. This model has been previously published and validated (Kim et al. 2011). By mating the gene deletion animals with wild-type tsc2+ / + animals, heterozygote (tsc2+ / −) zebrafish were also obtained and tested in this example.
Imaging
[0072]For both pS6 and TUNEL staining, non-consecutive sections were imaged using a Zeiss AxioImager microscope. Exposure time was kept constant during image acquisition and determined by the observation of a slide where primary antibody or enzyme solution was omitted. Pictures were taken with a 20× objective, using the AxioVision software, and coloured using Fiji ImageJ. All counting and measuring of cells was done in the original black and white pictures.
[0073]For TUNEL staining (1:10; Roche, Sigma, UK), 10 μm sections were cut and collect...
example 3
Efficacy of Cannabidiol in Combination With an mTOR Inhibitor in a Tumour Cell Line
Materials and Methods
Cell Lines
[0096]PANC-1 is a human pancreatic carcinoma, epithelial-like cell line; PANC-1 cells are used as an in vitro model of non-endocrine pancreatic cancer for tumorigenicity studies. The cells possess the type B phenotype for glucose-6-phosphate dehydrogenase G6PD and overexpress heregulin / human epidermal growth-factor receptor 2 (HER2 / neu) oncogene.
[0097]MIAPACA is a homo sapiens pancreas carcinoma. This is a hypotriploid human cell line.
Materials
[0098]Everolimus (RAD001, Afinitor, Novartis), is a rapamycin analogue. It is an oral mammalian target of rapamycin (mTOR) inhibitor and belongs to the PI3K related family of protein kinases and is activated by phosphorylation at serine 2448 (S2448).
[0099]Pure CBD was used in this example.
Cell Viability Assay
[0100]Cell viability was analyzed by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. Pancreati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com