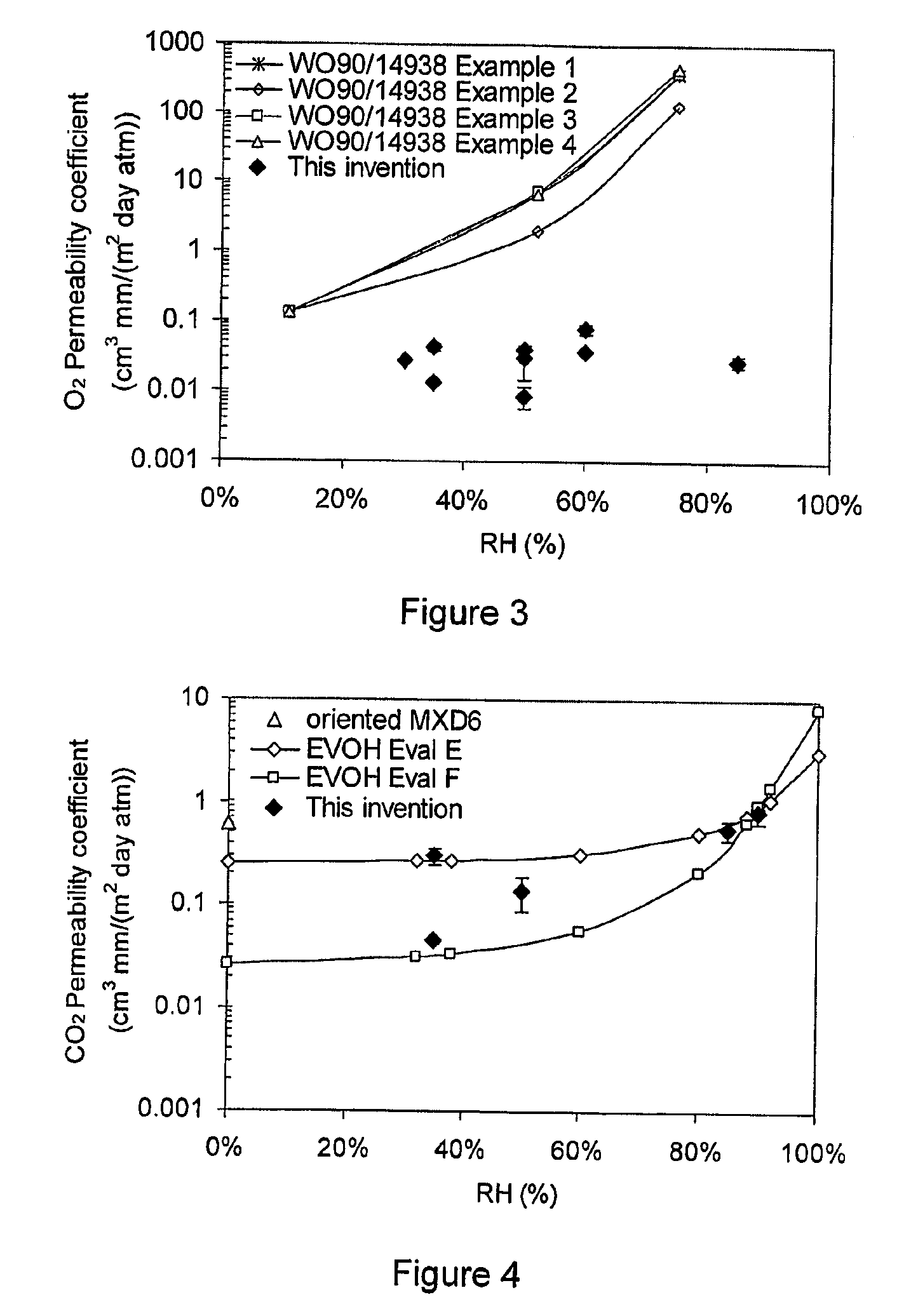
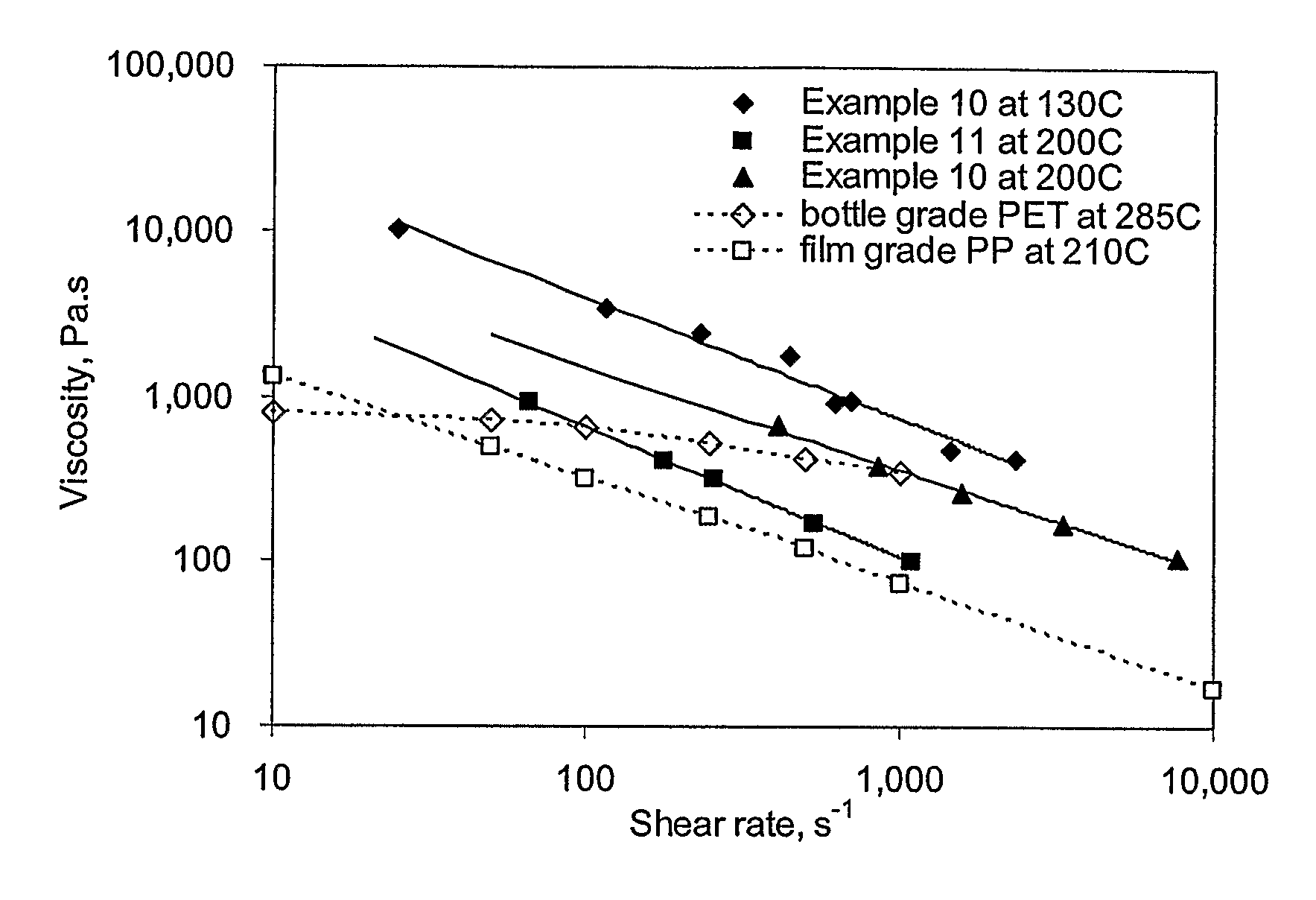
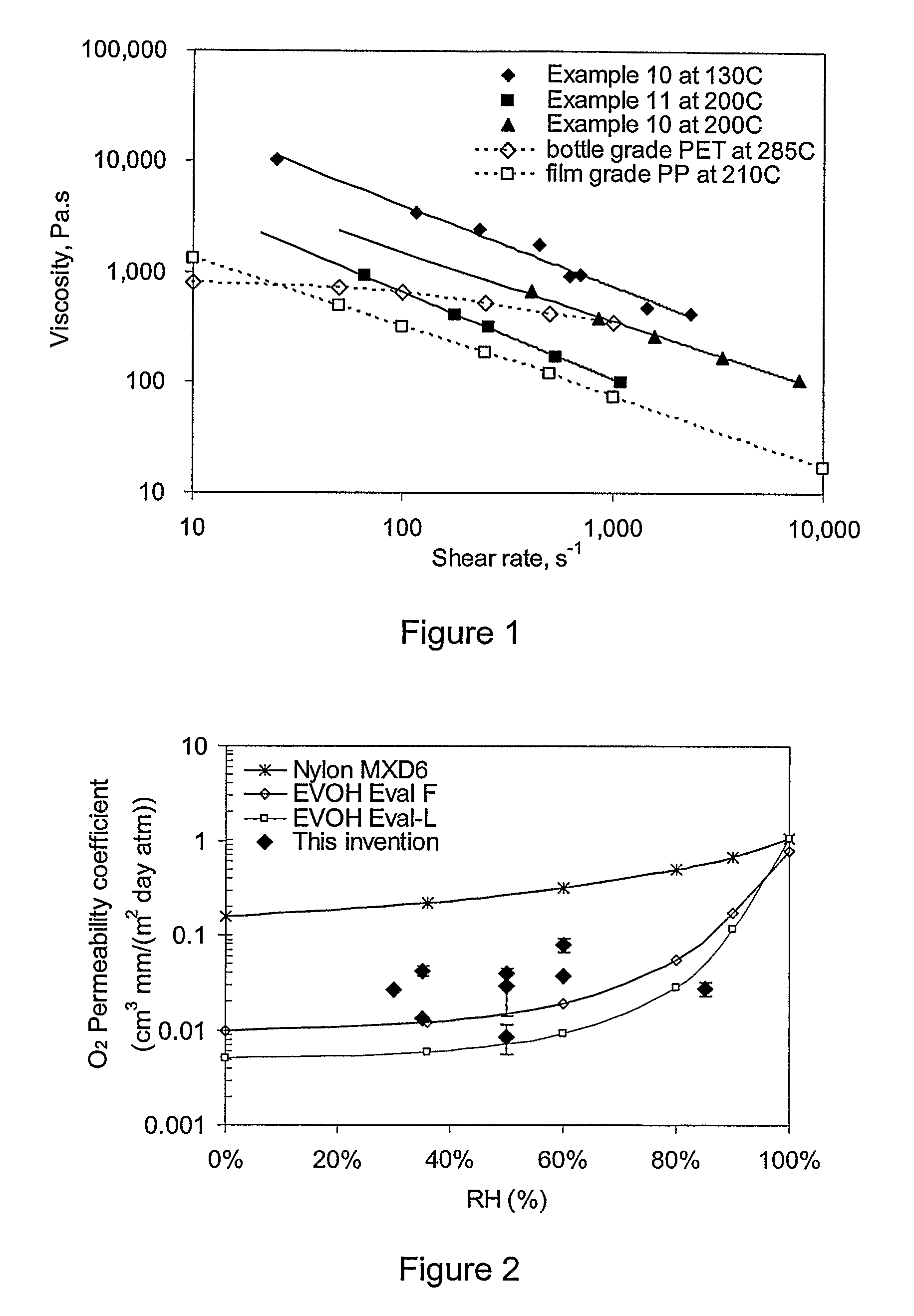
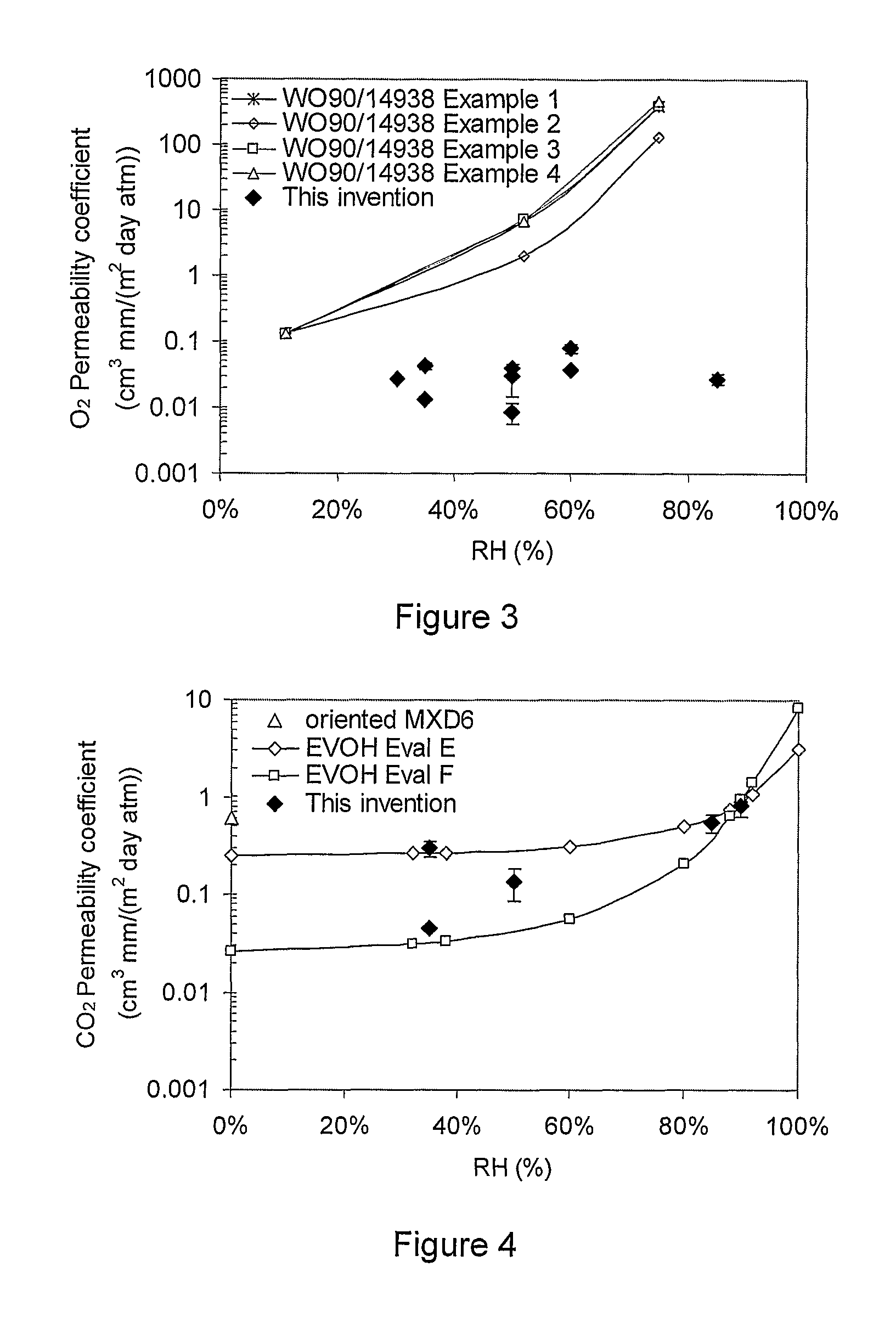
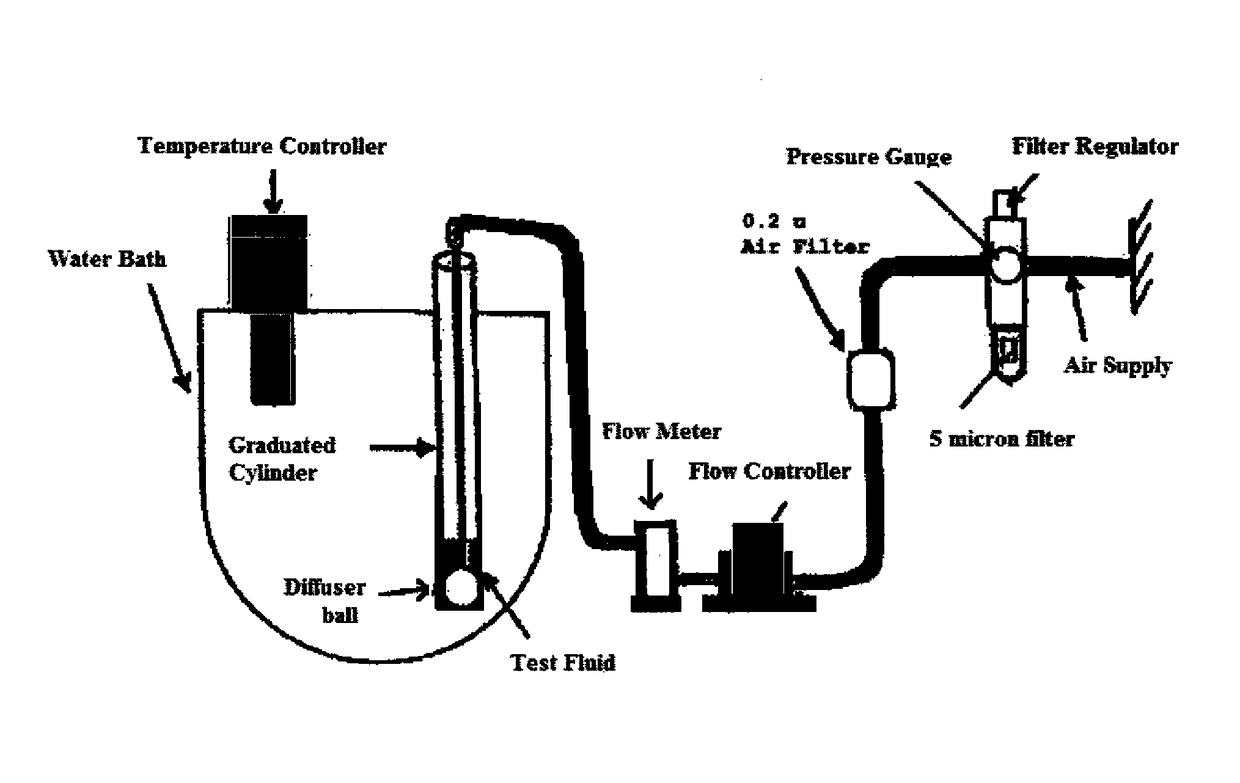
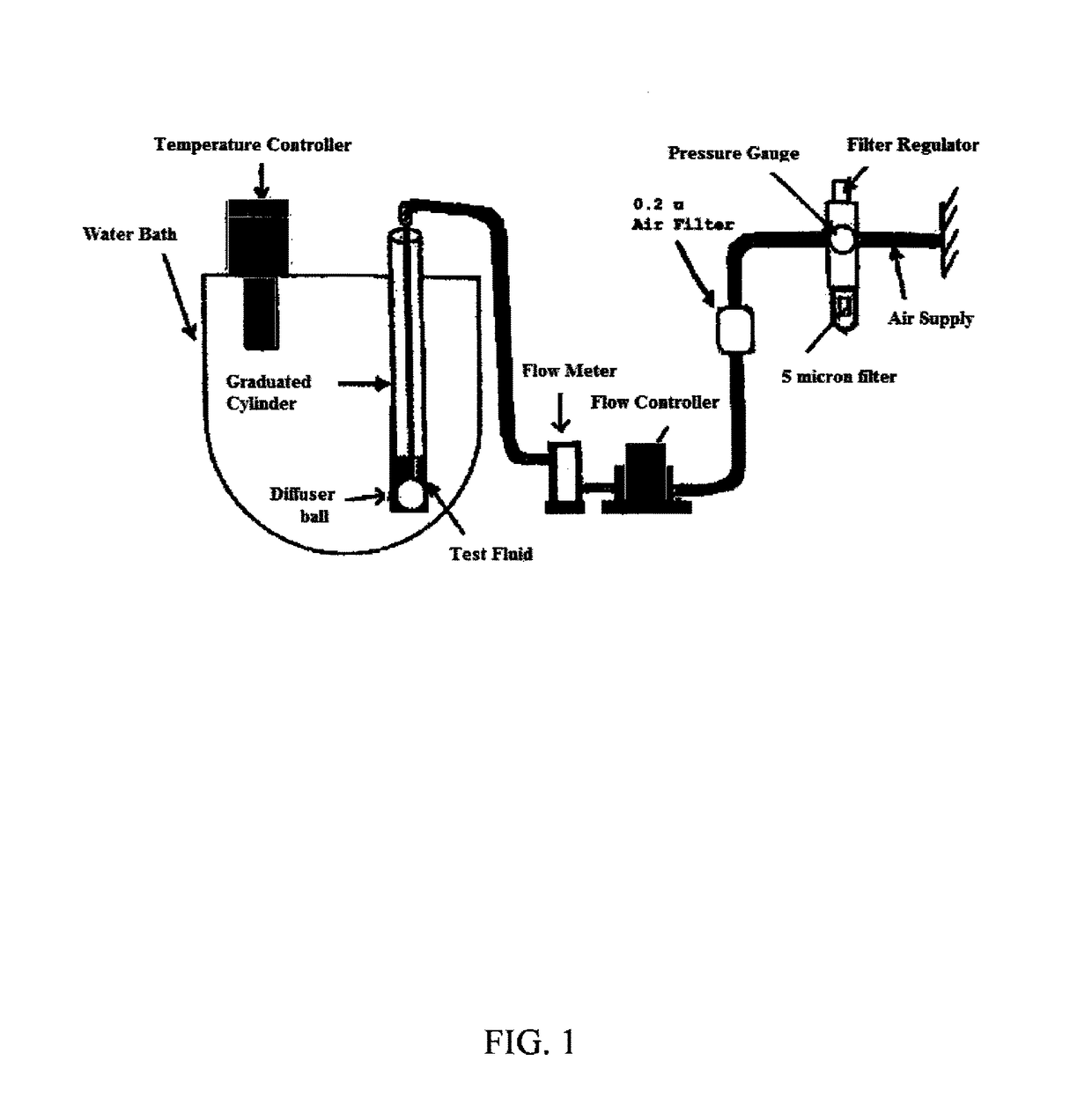
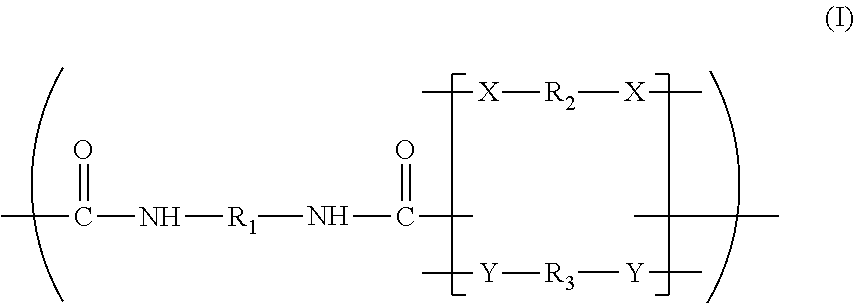
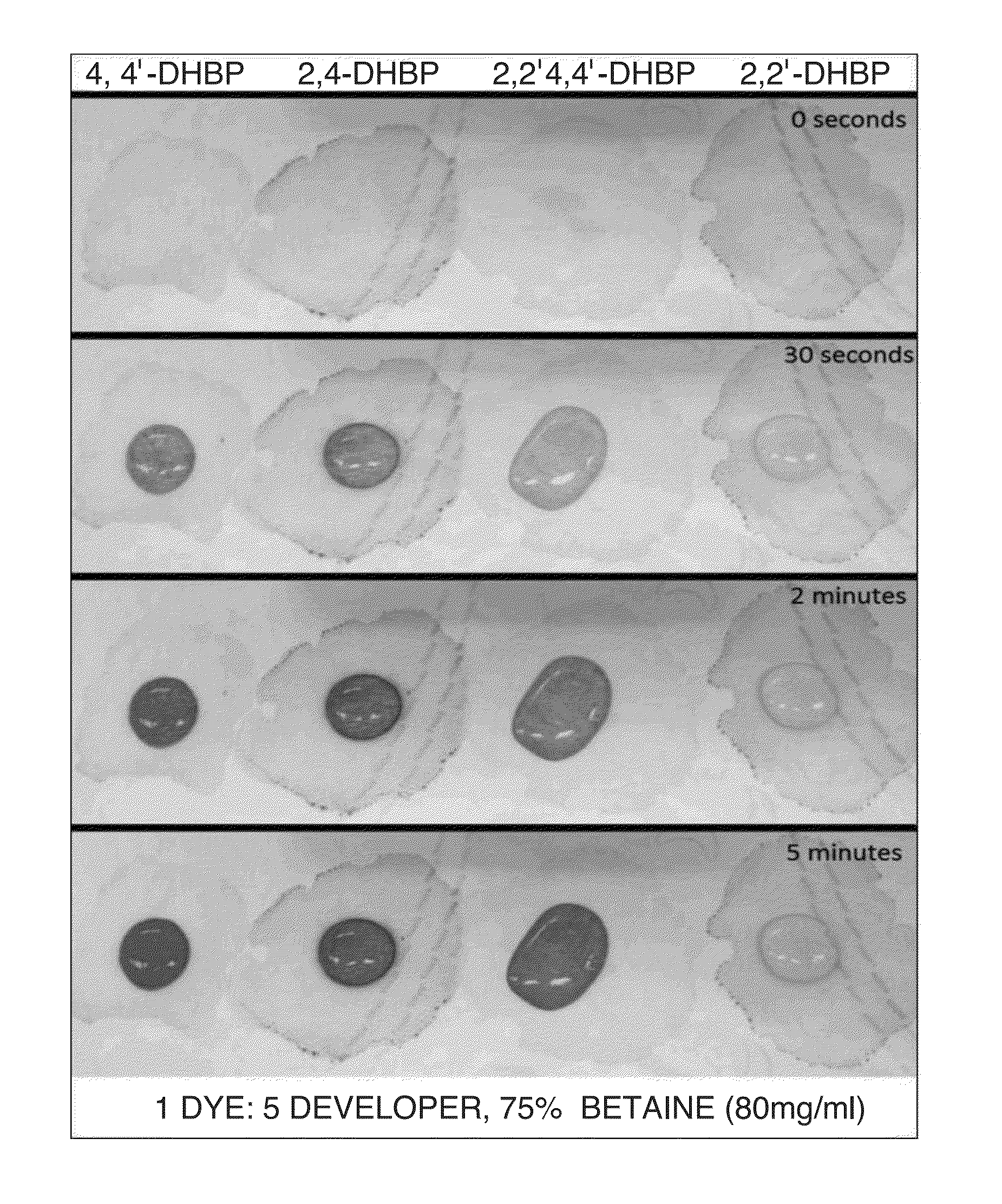
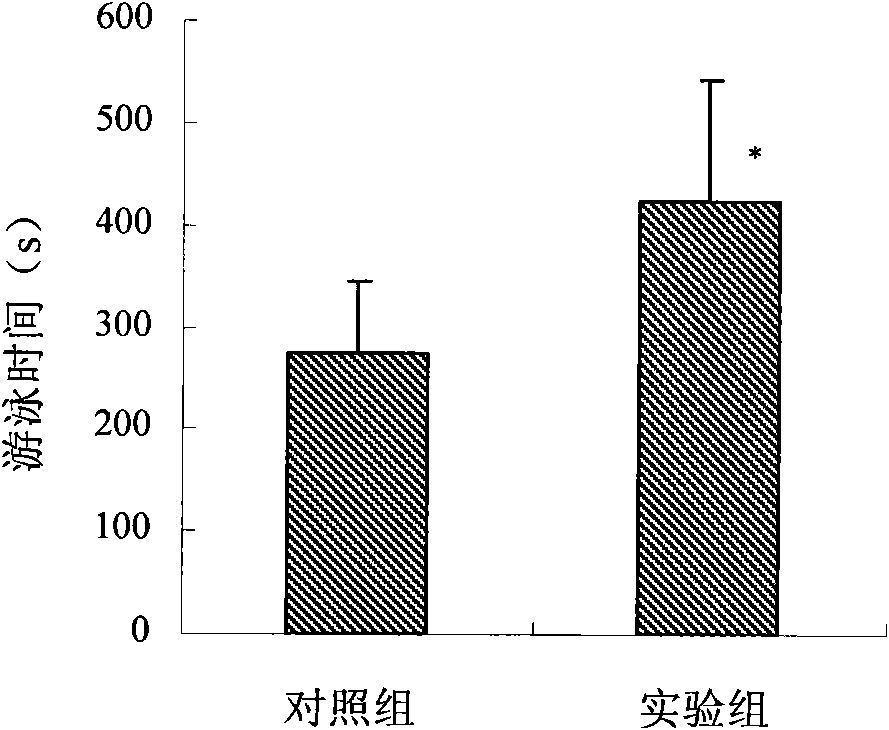
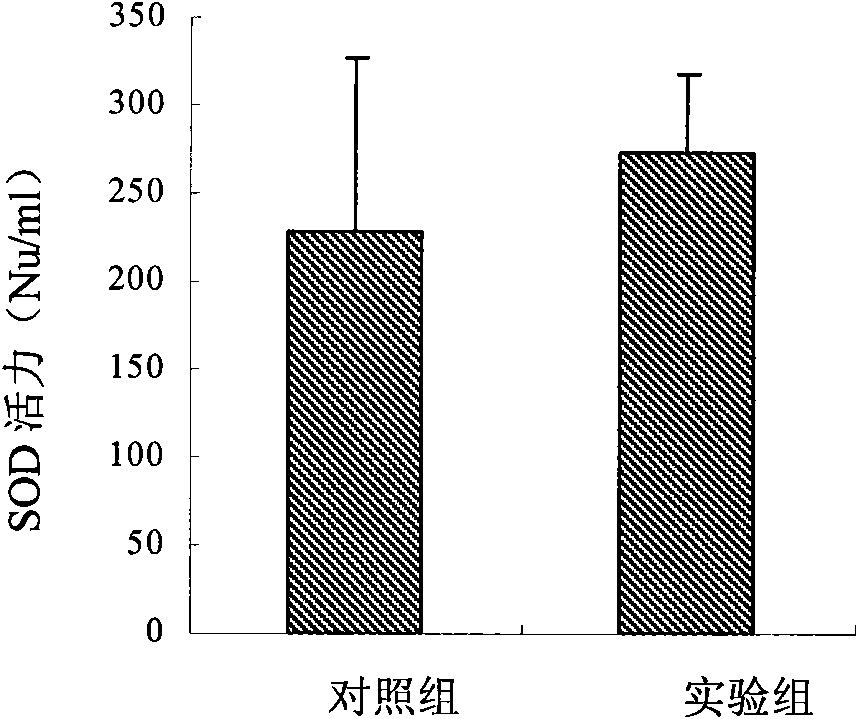
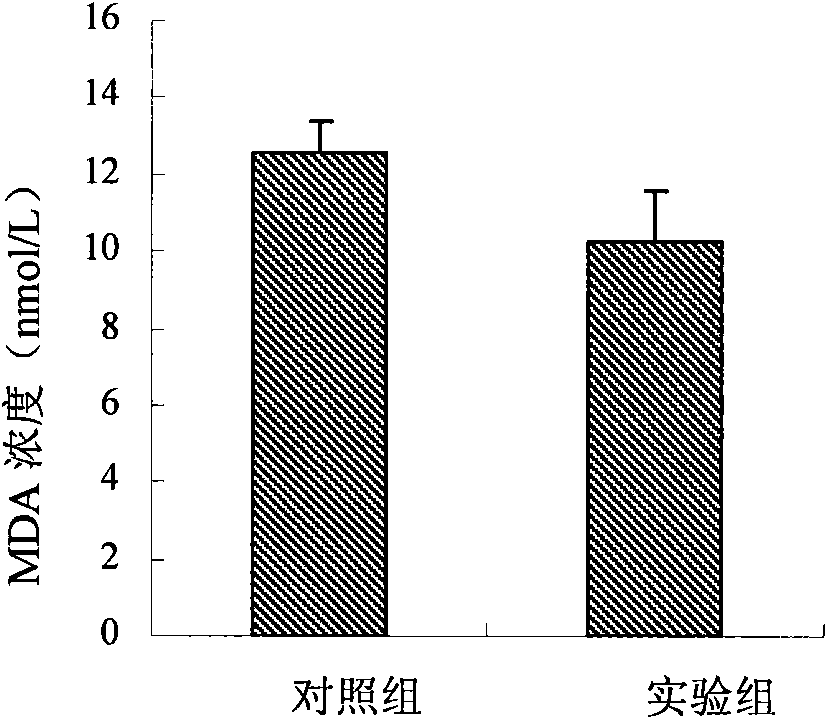
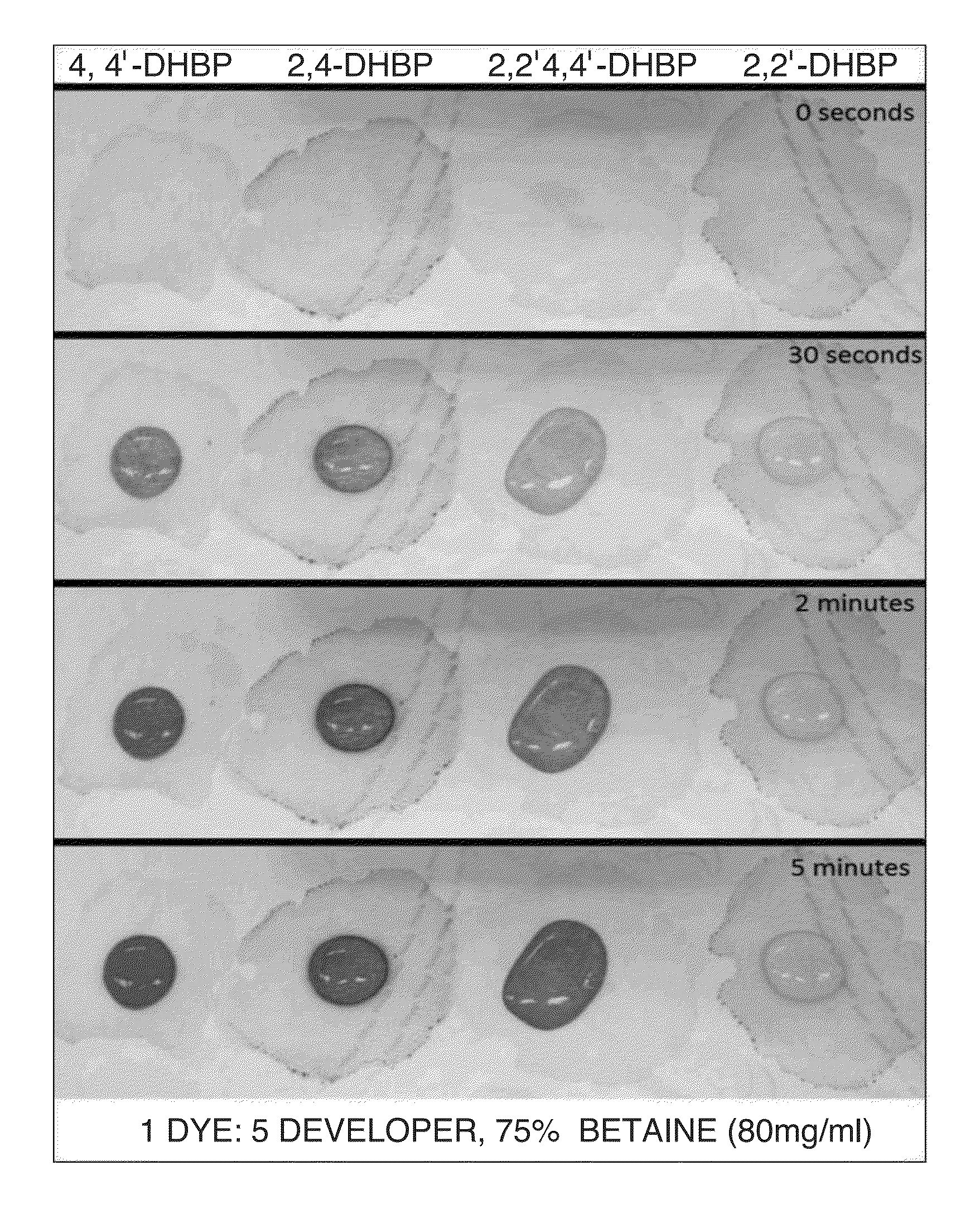
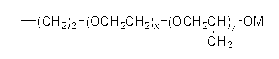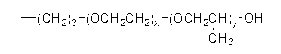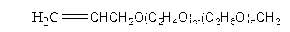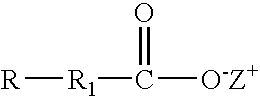Patents
Literature
227 results about "Hydrophilic-lipophilic balance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
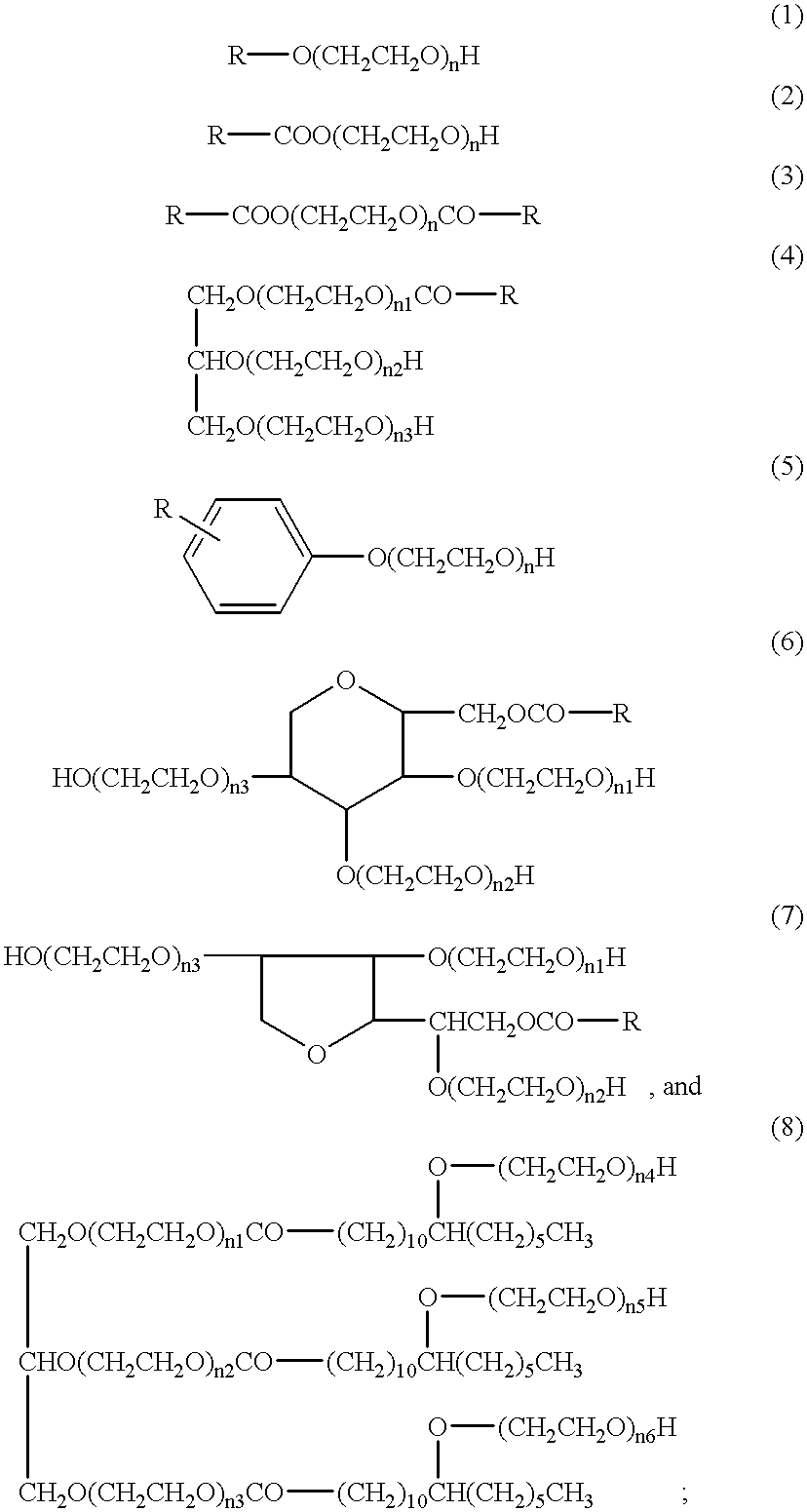
The hydrophilic-lipophilic balance of a surfactant is a measure of the degree to which it is hydrophilic or lipophilic, determined by calculating values for the different regions of the molecule, as described by Griffin in 1949 and 1954. Other methods have been suggested, notably in 1957 by Davies.
Orally administrable composition capable of providing enhanced bioavailability when ingested
InactiveUS6054136AImprove solubilityImprove bioavailabilityCosmetic preparationsToilet preparationsFatty acid esterIngestion
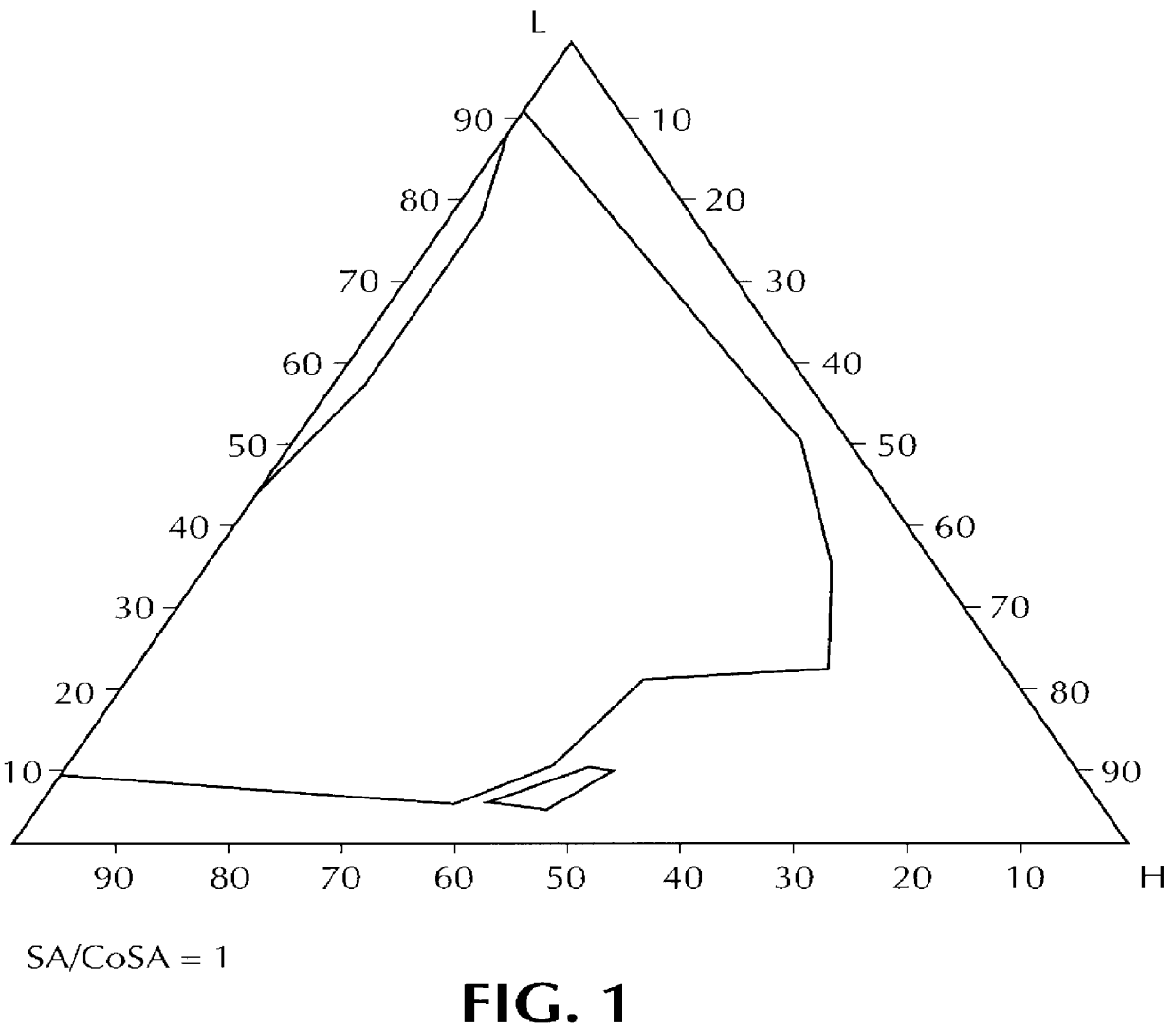
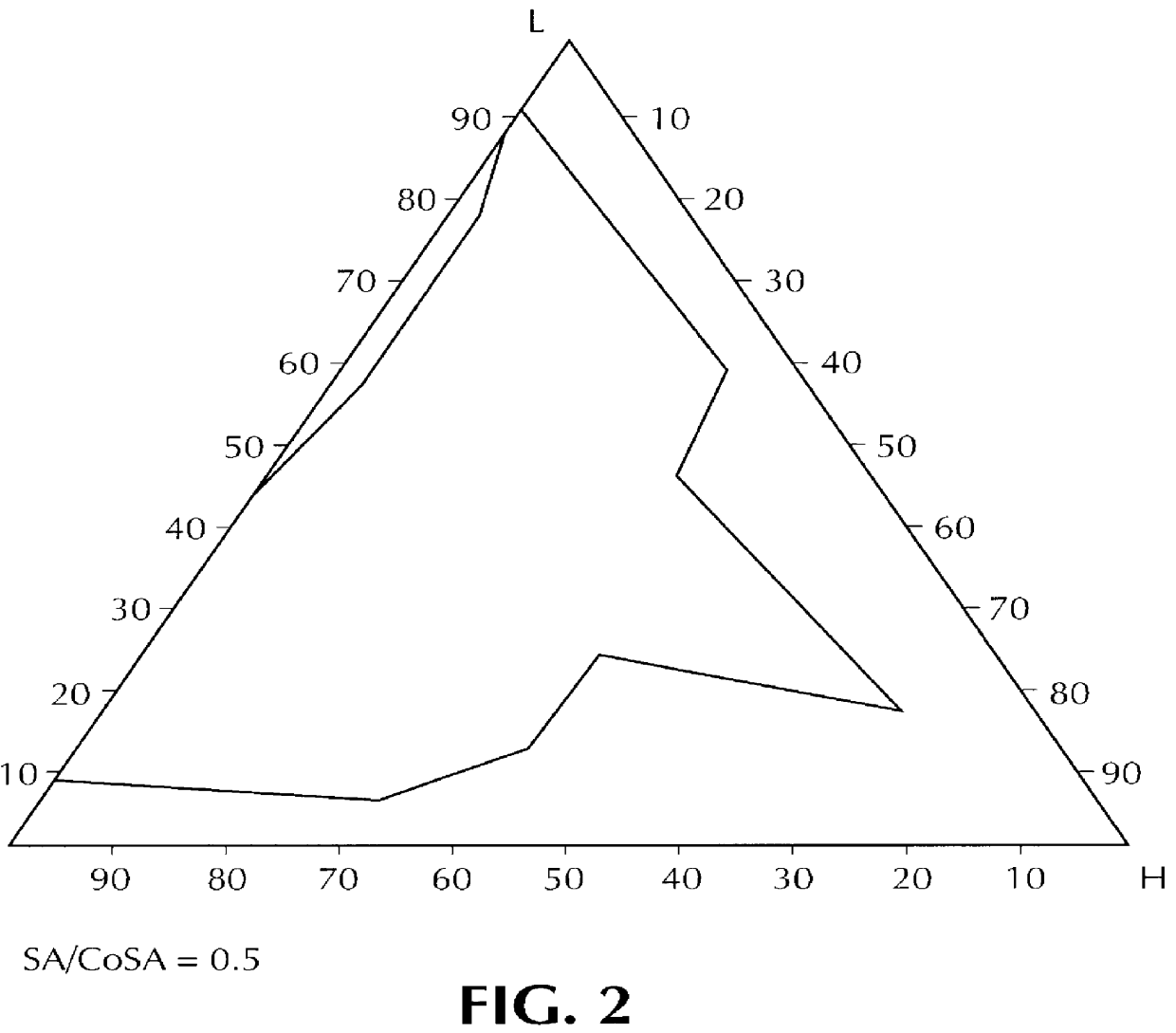
Composition for pharmaceutical or cosmetic use, capable of forming a microemulsion, comprising at least: an active principle, a lipophilic phase consisting of a mixture of fatty acid esters and glycerides, a surfactant (SA), a cosurfactant (CoSA), a hydrophilic phase, characterized: in that the lipophilic phase consists of a mixture of C8 to C18 polyglycolized glycerides having a hydrophilic-lipophilic balance (HLB) of less than 16, this lipophilic phase representing from 30 to 75% of the total weight of the composition; in that the surfactant (SA) is chosen from the group comprising saturated C8-C10 olyglycolized glycerides and oleic esters of polyglycerol, this surfactant having an HLB of less than 16; in that the cosurfactant (CoSA) is chosen from the group comprising lauric esters of propylene glycol, oleic esters of polyglycerol and ethyl diglycol; in that the SA / CoSA ratio is between 0.5 and 6; and in that the hydrophilic phase of the final microemulsion is supplied after ingestion by the physiological fluid of the digestive milieu.
Owner:GATTEFOSSE HLDG
Self-emulsifying composition of OMEGA3 fatty acid
ActiveUS8618168B2Maintain good propertiesAvoid high concentrationsBiocideNervous disorderHydrophilic-lipophilic balanceSelf emulsifying
This invention provides a self-emulsifying composition comprising 50 to 95% by weight in total of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; and 5 to 50% by weight of an emulsifier having a hydrophilic lipophilic balance of at least 10. The composition has no or reduced ethanol content, and exhibits excellent self-emulsifying property, dispersibility in the composition, emulsion stability, and absorption property. The composition is adapted for use as a drug.
Owner:MOCHIDA PHARM CO LTD
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
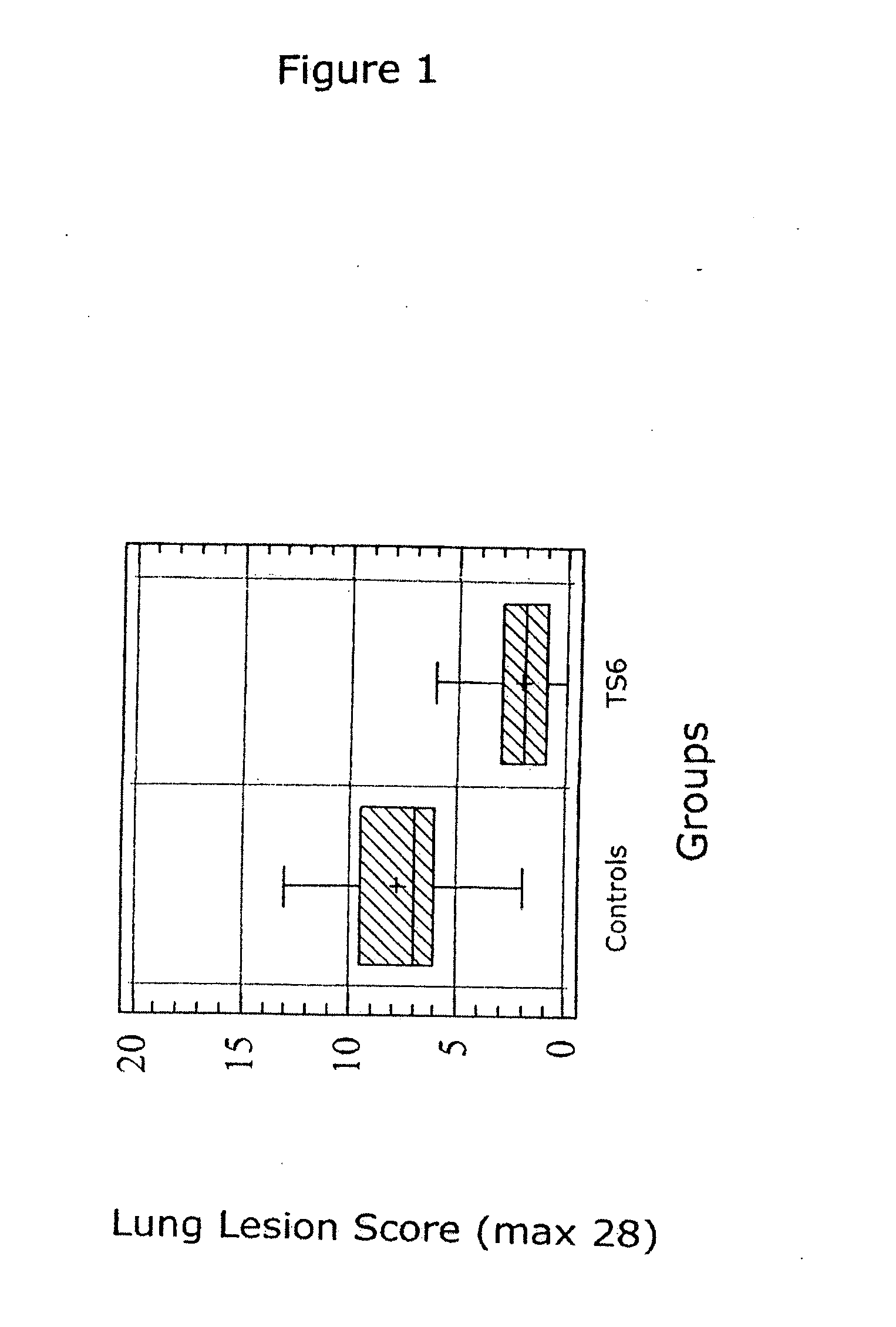
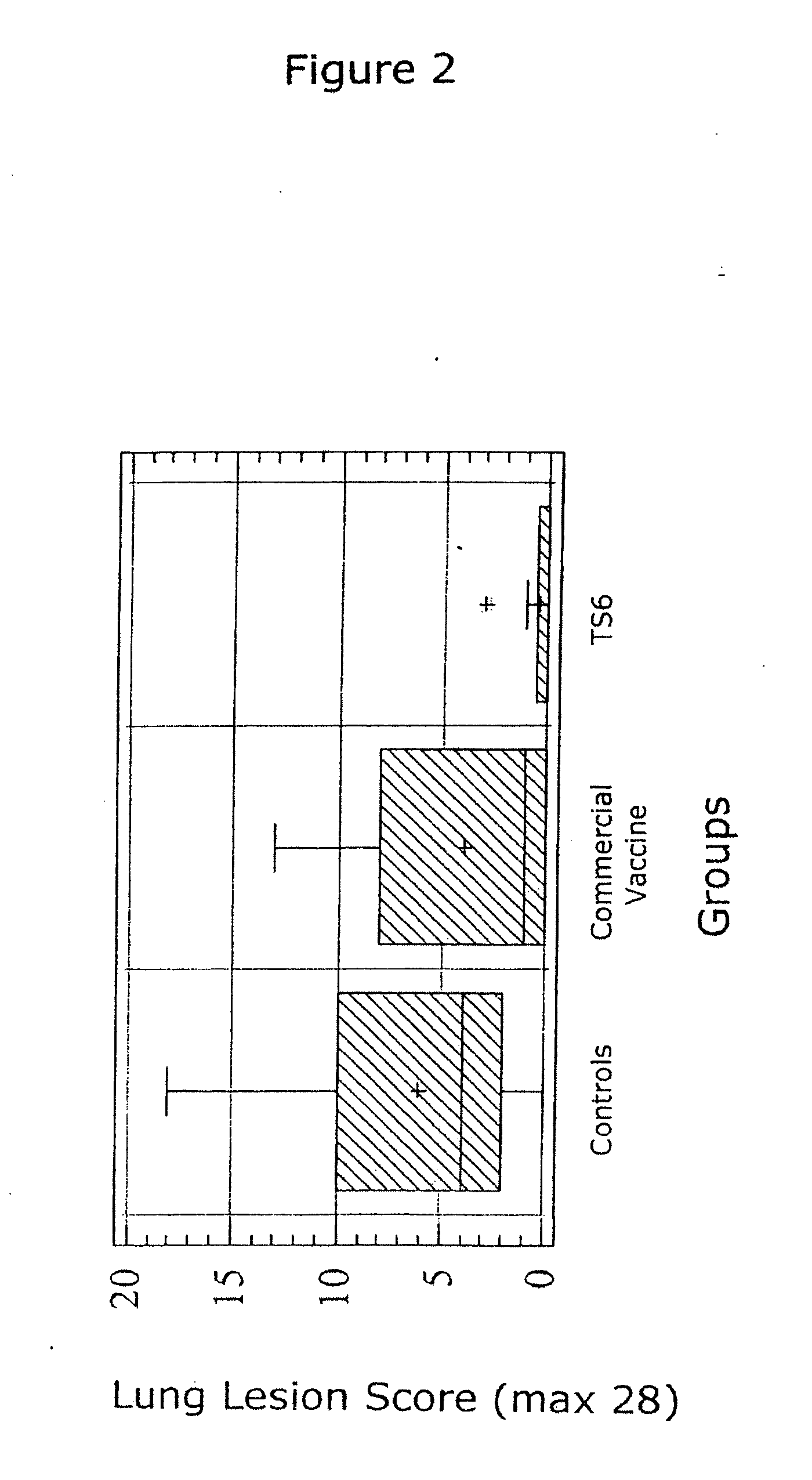
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
Use of nanoparticles for labelling oil field injection waters
InactiveUS20130084643A1Effective sizeEasy to implementOptical radiation measurementSurveyInjection wellOrganosilicon
The present invention relates to the development of tracer fluids, more generally, that of aqueous liquids, intended to be injected under pressure in an oil reservoir, for example from an injection well up to a production well.The object of the invention is to propose a new method of study of a solid medium, i.e. an oil reservoir, by diffusion of a liquid (i.e. injection waters) containing tracers, through said solid medium, which is simple to implement and economical and which remedies the drawbacks of the known tracers for injection waters of oil reservoirs.This method essentially consisting of injecting, in this solid medium, an injection liquid comprising a nanoparticle-based tracer having average dimensions comprised between 20 and 200 nm, detectable by means of one or several S signals at dilutions of less than or equal to 10−7, adapted to form a stable colloidal suspension in a saline medium, at least a portion of which is constituted of a core and a coating provided with an adjustable hydrophilic-lipophilic balance (HLB) and comprising at least one organic and / or organosilicon component; recovering the liquid having diffused; and analyzing this liquid having diffused to measure the quantity of tracer by detection of the signal or signals S.
Owner:TOTAL PUTEAUX FR
Self-emulsifying composition of omega3 fatty acid
ActiveUS20140057981A1Maintain good propertiesAvoid high concentrationsBiocideNervous disorderHydrophilic-lipophilic balanceSelf emulsifying
Owner:MOCHIDA PHARM CO LTD
Linker-Based Lecithin Microemulsion Delivery Vehicles
ActiveUS20080139392A1Improve solubilityPromote absorptionBiocideTransportation and packagingHigh concentrationSide effect
The present invention relates to biocompatible microemulsion systems designed for controlled release drug delivery applications formulated with phospholipids such as lecithin (surfactant), a lipophilic additive (linker) containing 9 or more carbons in their alkyl group and hydrophilic-lipophilic balance (HLB) of 5 or less, and a surfactant-like hydrophilic additive (linker) containing between 6 to 9 carbon atoms in their alkyl tail. The combination of linkers and phospholipids produce formulations capable of delivering high concentrations of poorly soluble drugs into epithelial tissue using low surfactant concentrations, with minimum cytotoxic side effects.
Owner:ACOSTA ZARA EDGAR JOEL +1
Microemulsion flowback aid composition and method of using same
ActiveUS20130261033A1Improve permeabilityImprove breathabilityFlushingDrilling compositionWater dispersibleWater soluble
Disclosed and claimed is a microemulsion flowback aid composition and a method of enhancing recovery of oil or gas during fracturing or stimulation processes. The microemulsion flowback aid composition includes (i) an oil-like phase comprising at least one nonionic surfactant having a hydrophilic-lipophilic balance (HLB) of less than about 9; (ii) a coupling agent capable of stabilizing the microemulsion flowback aid composition; (iii) at least one water-soluble or dispersible nonionic surfactant that is different from the at least one nonionic surfactant in the oil-like phase; (iv) at least one additional surfactant selected from anionic, cationic, amphoteric, and combinations thereof; and (v) water.
Owner:CHAMPIONX USA INC
Water-in-oil microemulsions for oilfield applications
A well treatment microemulsion includes an oil external phase, an internal aqueous phase and a hydrophilic surfactant. The surfactant has a hydrophile lipophile balance of between 8-18. The oil external phase may include d-Limonene, xylenes, light mineral oil, or kerosene. The surfactant is configured to emulsify the water of the internal aqueous phase into the oil of the external (continuous) phase. The surfactant may include polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan tristearate, polyoxyethylene hydrogenated castor oil, polyoxyethylene sorbitan monostearate, polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan monolaurate or mixtures therebetween. The use of hydrophilic surfactants to emulsify an internal aqueous phase within an oil external microemulsion produces unexpected and beneficial results.
Owner:PNC BANK NAT ASSOC
Barrier film
InactiveUS20090110942A1Stable mechanical propertiesImprove homogeneityFireproof paintsFibre treatmentMolten statePolyethylene oxide
A barrier composition which is injection mouldable and able to be made into a transparent film or incorporated (by co-extrusion and / or lamination) into multi-layer film products, the composition on dry basis: a) from 45 to 90% by weight of a starch and / or a modified starch selected from starches modified by reaction with a hydroxyl alkyl group, an acetate or a dicarboxylic acid anhydride or a grafting polymer; b) from 4 to 12% by weight of a water soluble polymer selected from polyvinyl alcohol, polyvinylacetate, and copolymers of ethylene and vinylalcohol which have a melting point compatible with the molten state of the starch components c) from 5 to 45% by weight of a non-crystallising mixture of sorbitol and at least one other plasticizer selected from glycerol, maltitol, xylitol, mannitol, glycerol trioleate, epoxidised linseed or soybean oil, tributyl citrate, acetyl tri-ethyl citrate, glyceryl triacetate, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate; polyethylene oxide or polyethylene glycol; d) from 0.3 to 2.5 % by weight of a C12-22 fatty acid or salt; e) from 0.25% to 3% of an emulsifier system having a hydrophilic lipophilic balance value between 2 and 10. The barrier film may be co-injection moulded with polyethylene terephthalate (PET) or polylactic acid (PLA) for blow moulding into beverage bottles, with polyethylene (PE) or polypropylene (PP) or biodegradable polymers for high gas-barrier containers or closures, or may be co-extruded with polyethylene, polypropylene or polylactic acid for thin film packaging applications or for blow-moulded containers.
Owner:PLANTIC TECH
Barrier film
InactiveUS7854994B2Improve the level ofImprove homogeneityFibre treatmentBottlesPolyethylene terephthalate glycolPolyethylene oxide
A barrier composition which is injection mouldable and able to be made into a transparent film or incorporated (by co-extrusion and / or lamination) into multi-layer film products, the composition on dry basis: a) from 45 to 90% by weight of a starch and / or a modified starch selected from starches modified by reaction with a hydroxyl alkyl group, an acetate or a dicarboxylic acid anhydride or a grafting polymer; b) from 4 to 12% by weight of a water soluble polymer selected from polyvinyl alcohol, polyvinylacetate, and copolymers of ethylene and vinylalcohol which have a melting point compatible with the molten state of the starch components c) from 5 to 45% by weight of a non-crystallising mixture of sorbitol and at least one other plasticizer selected from glycerol, maltitol, xylitol, mannitol, glycerol trioleate, epoxidised linseed or soybean oil, tributyl citrate, acetyl tri-ethyl citrate, glyceryl triacetate, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate; polyethylene oxide or polyethylene glycol; d) from 0.3 to 2.5% by weight of a C12-22 fatty acid or salt; e) from 0.25% to 3% of an emulsifier system having a hydrophilic lipophilic balance value between 2 and 10. The barrier film may be co-injection moulded with polyethylene terephthalate (PET) or polylactic acid (PLA) for blow moulding into beverage bottles, with polyethylene (PE) or polypropylene (PP) or biodegradable polymers for high gas-barrier containers or closures, or may be co-extruded with polyethylene, polypropylene or polylactic acid for thin film packaging applications or for blow-moulded containers.
Owner:PLANTIC TECH
Preparation method of polyether modified polysiloxane foam stabilizer
The invention discloses a preparation method of a polyether modified polysiloxane foam stabilizer and relates to a preparation method of a foam stabilizer for flexible polyurethane foam. The polyether modified polysiloxane foam stabilizer is generated through reaction between allyl epoxyethane and methyloxirane methyl ethers with different molecular weights and silicone oil with low hydrogen content under the conditions that methylbenzene as a solvent and chloroplatinic acid as a catalyst. Through controlling the degree of polymerization of a hydrophilic polyether chain segment and a hydrophobic polysiloxane chain segment of polyether modified polysiloxane, the foam stabilizer has precise hydrophilic-lipophilic balance property, better emulsifying property and a favorable foam stabilizing effect, so that a flexible polyurethane foam mixture has enough stability in a coring stage, a form hole growing stage, a punching stage and an early-stage crosslinking stage, and foam collapse cannot be caused.
Owner:扬州晨化新材料股份有限公司
Method of producing toner
ActiveUS8440382B2Stable productionAvoid separationDevelopersHydrophileHydrophilic-lipophilic balance
Owner:CANON KK
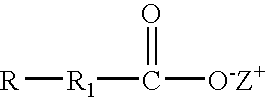
Non-surfactant solubilizing agent
InactiveUS6916773B2Anionic surface-active compoundsDetergent solventsHydrophilic-lipophilic balanceSolvent
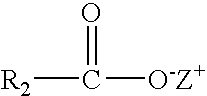
Compositions of the invention include: (a) a solvent having a hydrophilic-lipophilic balance (HLB) value of 5 to 7; and (b) a solubilizing agent having the formula: where R is aryl, cycloaryl, heteroaryl or hetercyclyl; R1 is a bond, (C1-C7)alkyl, substituted (C1-C7)alkyl, (C1-C7)alkoxy, substituted (C1-C7)alkoxy, (C2-C7)alkenyl or substituted (C2-C7)alkenyl; and Z+ is hydrogen, Na, K, NR′4 where R′ is defined herein. Further compositions include: (a) a solvent having a (HLB) value of 5 to 7; and (b) a solubilizing agent having the formula: where R2 is a bond, (C1-C7)alkyl, substituted (C1-C7)alkyl, (C1-C7)alkoxy, substituted (C1-C7)alkoxy, (C2-C7)alkenyl or substituted (C2-C7)alkenyl; and Z+ is hydrogen, Na, K, NR′4 where R′ is defined herein. The compositions are useful in providing stable single phase aqueous solutions when combined with water.
Owner:ECOLAB USA INC
Nanoemulsions having reversible continuous and dispersed phases
A nanoemulsion having reversible continuous and dispersed phases. The nanoemulsion includes an aqueous phase and an oil phase, a weight ratio of the aqueous phase to the oil phase being 1:40-100:1. In the nanoemulsion, the aqueous phase is dispersed as nanosized droplets in the oil phase or the oil phase is dispersed as nanosized droplets in the aqueous phase. The aqueous phase contains water or a water solution and a water-soluble organic nanostructure stabilizer. The oil phase contains an oil or an oil solution, an organic gel thickener, and a hydrophilic surfactant having a hydrophilic-lipophilic balance value greater than 8.0. Also disclosed is a method for preparing the above-described nanoemulsion.
Owner:LG BIONANO LLC
Methods of enhancing fine particle dewatering
InactiveUS6855260B1Increase tonnageGood removal effectWaste water treatment from quariesDrying solid materials with heatFine particulateSlurry
A new method of improving the process of dewatering fine particulate materials is disclosed. In this method, an aqueous slurry of fine particles is treated with appropriate hydrophobizing reagents so that the particulate material becomes moderately hydrophobic with its water contact angle considerably below 90°. A low hydrophile-lipophile balance (HLB) number surfactant is then added to the slurry, so that the surfactant molecules adsorb on the moderately hydrophobic surface primarily by hydrophobic attraction and, thereby, increase its contact angle close to or above 90°. By virtue of the greatly enhanced hydrophobicity, the water molecules adhering to the surface are destabilized and removed more readily by a mechanical dewatering process. Any nonionic surfactant with its HLB number below about 15 may be used for the hydrophobicity enhancement. The surfactants may be used in conjunction with appropriate solvents such as light hydrocarbon oils and short-chain alcohols. The moisture reduction can be further improved by using appropriate electrolytes in conjunction with the low HLB surfactants, spraying surface tension lowering reagents onto the filter cake, subjecting the cake to a suitable vibratory means, and by using combinations thereof.
Owner:YOON ROE HOAN
Methanol diesel fuel and preparation method thereof
InactiveCN101914395AImprove stabilityEasy to prepareLiquid carbonaceous fuelsHydrophileHydrophilic-lipophilic balance
The invention provides methanol diesel fuel, which comprises the following components in percentage by volume: 40 to 85 percent of diesel fuel, 5 to 40 percent of methanol, 0.1 to 2 percent of surfactant with the hydrophile-lipophile balance value of 3 to 6, 5 to 30 percent of cosurfactant and 0.1 to 1 percent of aid. The invention also provides a preparation method for the methanol diesel fuel, which comprises the following steps of: uniformly mixing the diesel fuel and the surfactant with the hydrophile-lipophile balance value of 3 to 6, adding the methanol, and uniformly mixing to prepare microemulsion; and adding the cosurfactant and the aid into the microemulsion, and uniformly mixing to prepare the methanol diesel fuel. In the methanol diesel fuel, the methanol and the diesel fuel form the transparent and stable microemulsion under the action of the surfactant and the cosurfactant, so that the prepared methanol diesel fuel has better stability and no demixing after a long time.
Owner:ZHEJIANG QIANJIANG ENERGY GROUP
Non-foaming aqueous particle-free inkjet ink compositions
ActiveUS20180051184A1Unique balance of solubilityReduce foamingInksPrintingDefoamerHydrophilic-lipophilic balance
Colorless or colored coatings can be ink jetted onto a substrate using an aqueous particle-free inkjet ink composition that has a viscosity of less than 5 centipoises, and that includes a combination of an anionic polyether polyurethane and an anionic acrylic polymer or anionic styrene-acrylic polymer, in a total amount of less than or equal to 20 weight %. The composition can be colorless (free of colorants) or it can contain a suitable colorant to provide a color image. The composition also includes a defoamer that has a hydrophilic-lipophilic balance value of at least 3 and up to and including 5, and which is present in an amount of at least 0.15 weight % and up to and including 1 weight %. Such composition can be used in inkjet printing methods including recirculation apparatus and methods, and can be provided as part of an ink set.
Owner:EASTMAN KODAK CO
Water-Based Wetness-Indicating Composition and Sensor
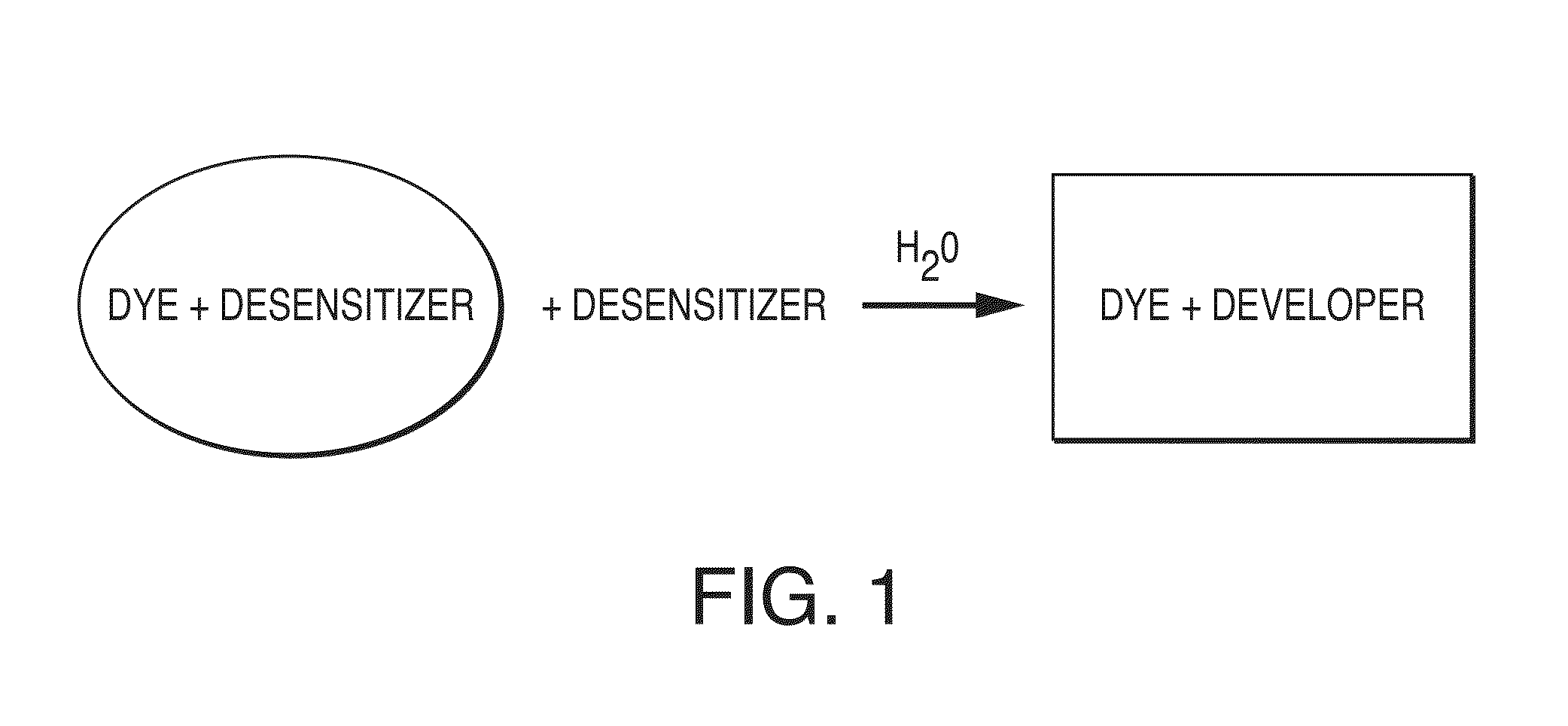
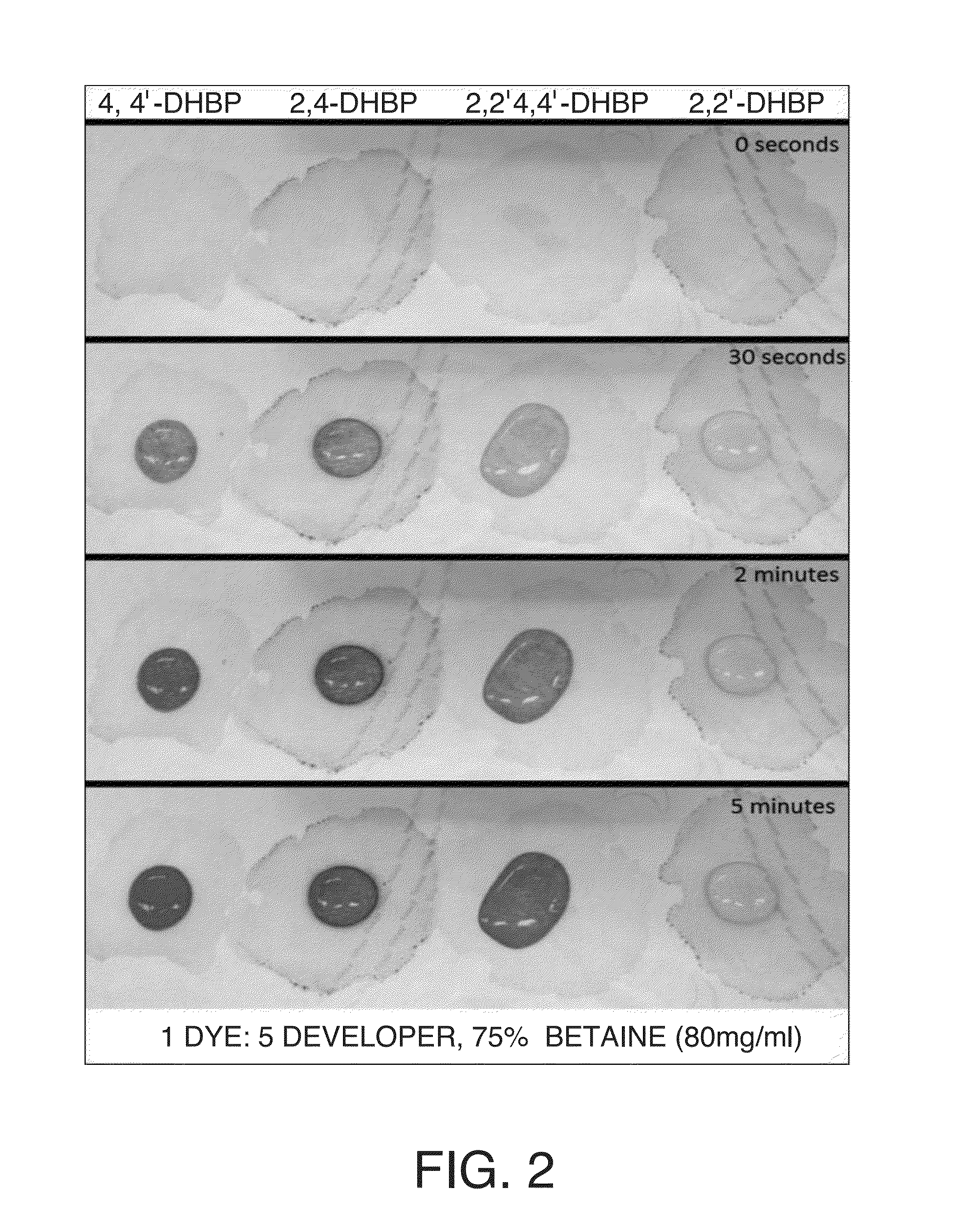
ActiveUS20140241954A1Analysis using chemical indicatorsDuplicating/marking methodsWater basedWater insoluble
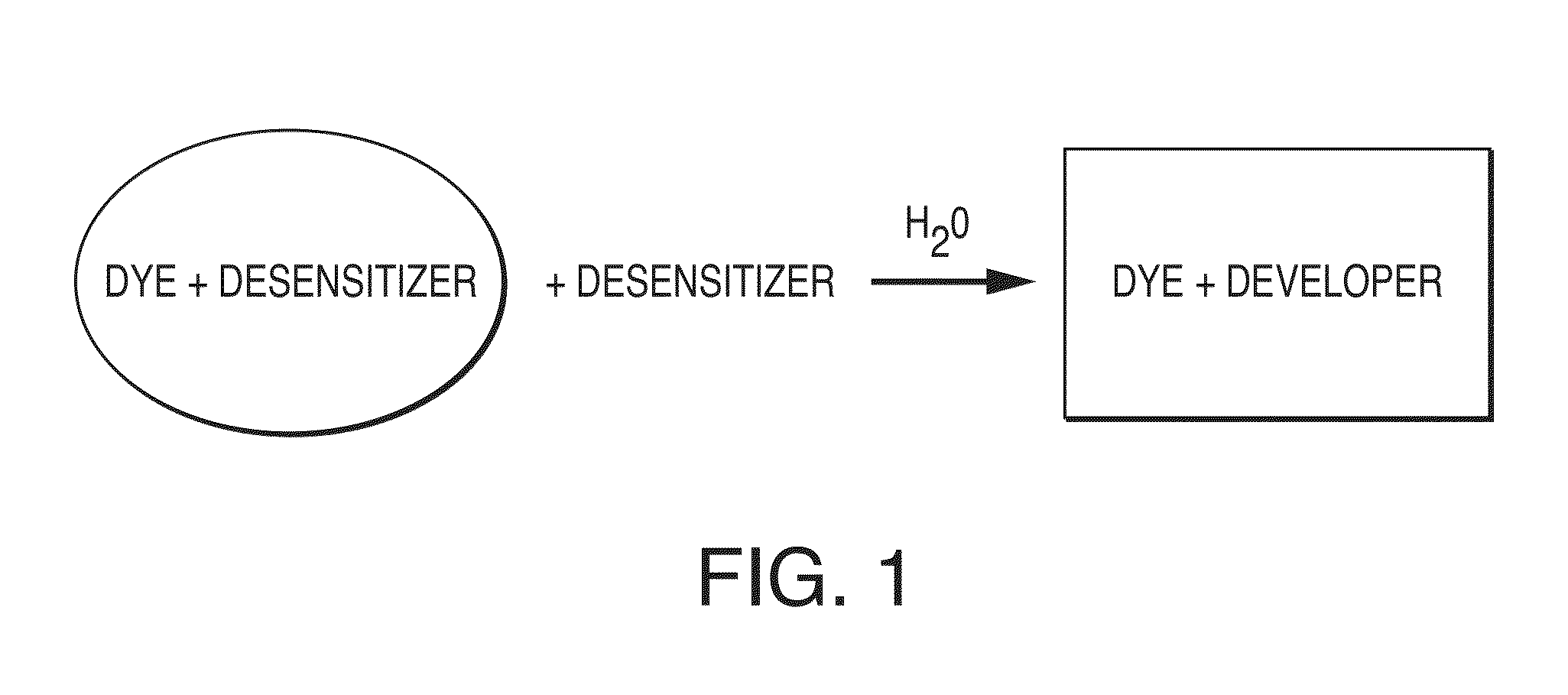
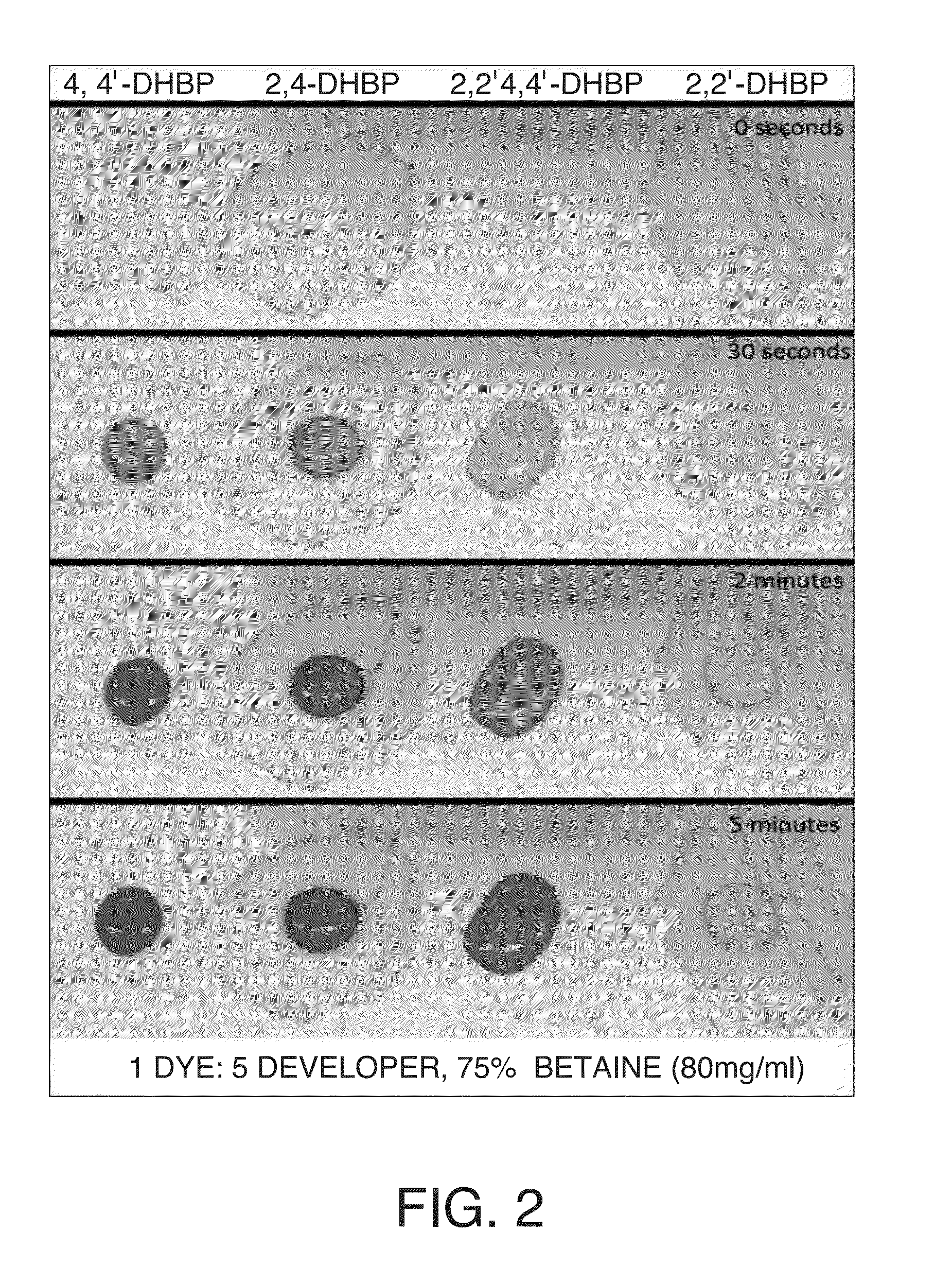
A color-appearing ink composition includes a surfactant blend of at least two non-ionic surfactants having hydrophilic-lipophilic balance values greater than 10, or a non-ionic surfactant having a hydrophilic-lipophilic balance value greater than 10 and a charged surfactant. The composition includes one or more water-insoluble leuco dyes, a developer and a desensitizer. The composition may be applied to a substrate to form a sensor.
Owner:KIMBERLY-CLARK WORLDWIDE INC
SELF-EMULSIFYING COMPOSITION OF w3 FATTY ACID
ActiveUS20120065264A1Low ethanol concentrationEliminate the problemBiocideNervous disorderΩ 3 pufaHydrophilic-lipophilic balance
This invention provides a self-emulsifying composition comprising 50 to 95% by weight in total of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; and 5 to 50% by weight of an emulsifier having a hydrophilic lipophilic balance of at least 10. The composition has no or reduced ethanol content, and exhibits excellent self-emulsifying property, dispersibility in the composition, emulsion stability, and absorption property. The composition is adapted for use as a drug.
Owner:MOCHIDA PHARM CO LTD
Polyurethane coating and preparation method thereof
ActiveCN101985541AWipeability qualifiedImprove degradation efficiencyPolyurea/polyurethane coatingsPolyolHydrophilic-lipophilic balance
The invention provides a polyurethane coating, which comprises the following components: A) surface-modified nanometer titania, B) polyalcohol and C) isocyanate, wherein the surface-modified nanometer titania consists of nanometer titania particles serving as a matrix and a nonionic surfactant which is coated on the surface of the nanometer titania matrix; the grain size of the nanometer titania matrix is 1 to 100nm; the hydrophilic-lipophilic balance value of the nonionic surfactant is 1 to 10; the nonionic surfactant is 1 to 20 percent based on the weight of the nanometer titania matrix; the surface-modified nanometer titania is 2 to 15 percent based on the weight of the polyalcohol; and the weight ratio of the polyalcohol to the isocyanate is 10:1-2:1. The invention also provides a method for preparing the polyurethane coating.
Owner:JIANGSU KAOPULE NEW MATERIALS
Nanowire dispersion compositions and uses thereof
ActiveUS7745498B2Improve solubilityEasy to processMaterial nanotechnologyMixing methodsNanowireHydrophile
Nanowire dispersion compositions (and uses thereof) are disclosed comprising a plurality of inorganic nanowires suspended in an aqueous or non-aqueous solution comprising at least one low molecular weight and / or low HLB (Hydrophile-Lipophile Balance) value dispersant. Methods of further improving the dispersability of a plurality of inorganic nanowires in an aqueous or non-aqueous solution comprise, for example, oxidizing the surface of the nanowires prior to dispersing the nanowires in the aqueous or non-aqueous solution.
Owner:ONED MATERIAL INC
Method for extracting lignin sulfonate using waste liquid from boiling canapina fiber or paper making
InactiveCN101070333AEfficient removalImprove qualityWater/sewage treatment bu osmosis/dialysisLignin derivativesFiberSolubility
This invention relates to a method of using boied hemp or papermaking waste liquor to extract lignosulphonate. Firstly boied hemp or papermaking waste liquor proceed microfiltration and hyperfiltration; take lignin concentrated solution 25 to 50 parts by weight, put at reactor; unlock whipper, add 10% sulfuric acid 5 to 15 parts by weight, at 60 to 80 deg take reaction for 2 to 4hours, then add sulfite 10 to 30 parts by weight, ferric chloride or bluestone 2 to 6 parts by weight, at 80 to 100deg take reaction for 3 to 5hours, adjust pH value 7 to 8, gain lignosulphonate solution. The lignosulphonate has many active group, possess good surface activity and hydrophile-lipophile balance, good solubility and dispersibility, higher yield and good quality.
Owner:武汉海德天物新材料有限公司
Surfactant package for well treatment and method for using same
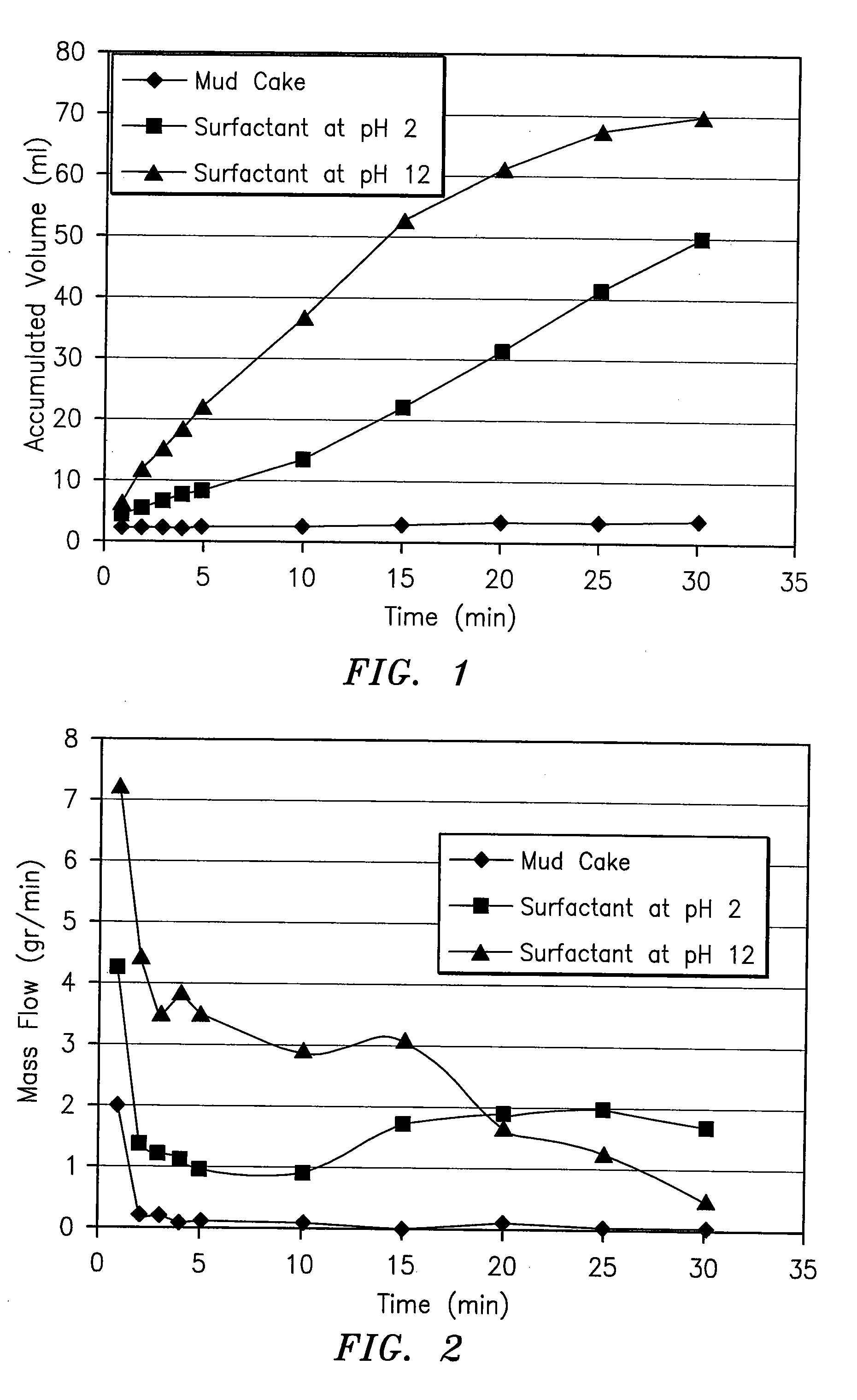
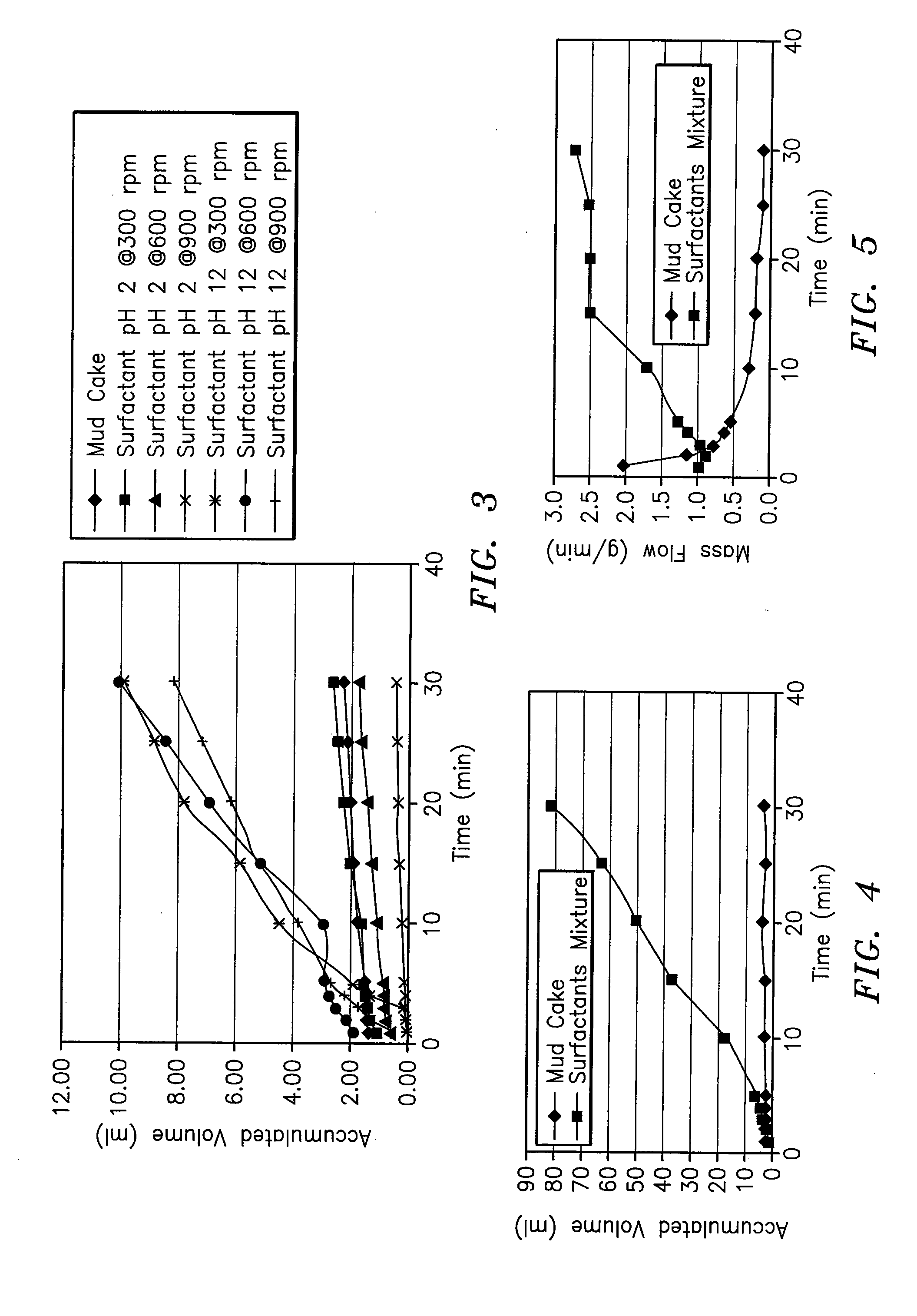
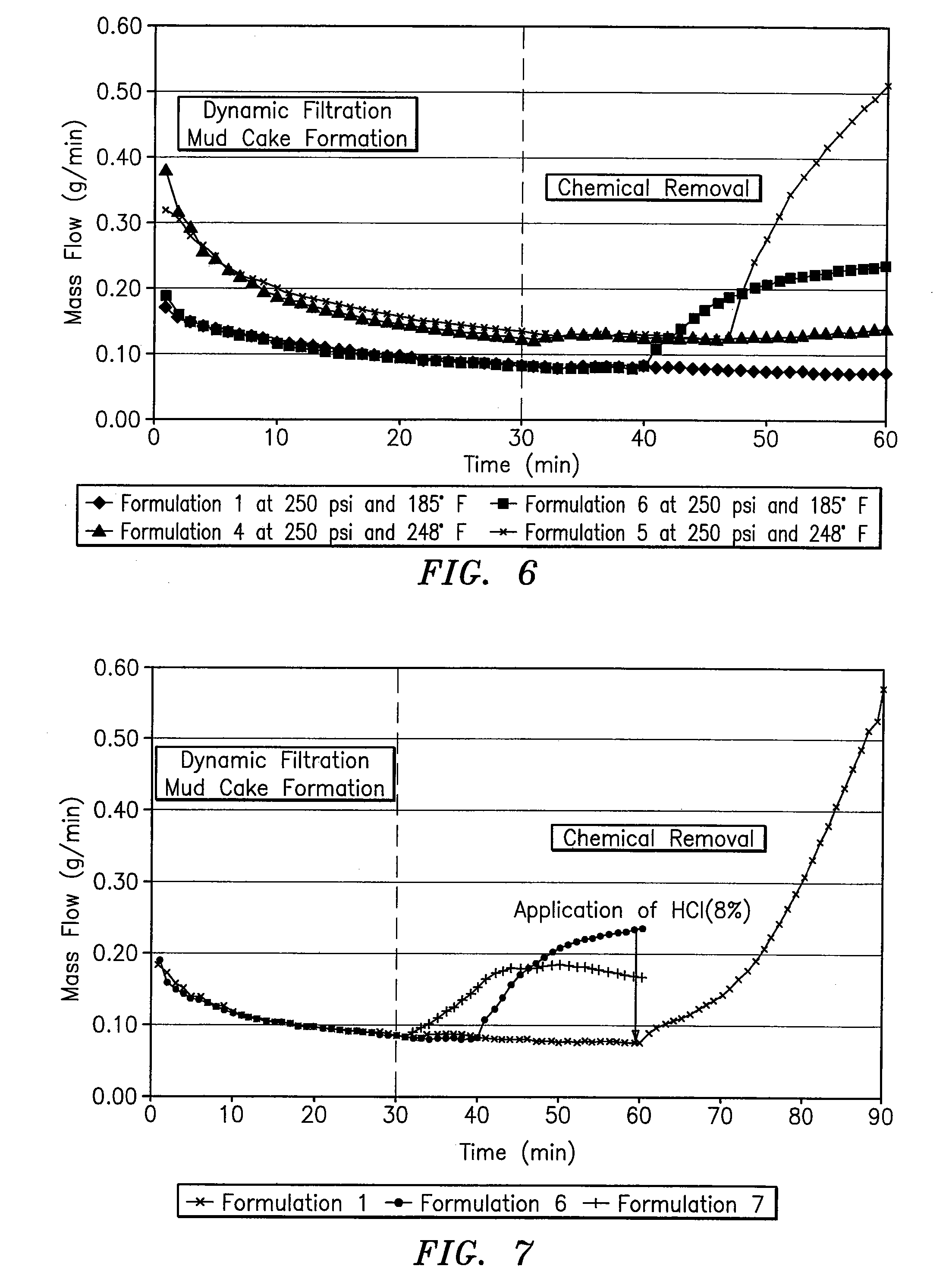
InactiveUS20070191235A1Speed the mud cake removalPerforms betterOther chemical processesMixing methodsHydrophilic-lipophilic balanceNon ionic
A surfactant blend for servicing wells includes a first non-ionic surfactant having a hydrophilic-lipophilic balance of between about 10 and about 15; a second non-ionic surfactant having a hydrophilic-lipophilic balance of between about 2 and about 6; and an anionic surfactant.
Owner:INTREVEP SA
Pigment dispersion and related method of manufacture
InactiveUS7094395B1Inhibition of agglomerationLower potentialCosmetic preparationsMake-upPigment dispersionHydrophile
A method for producing a colored cosmetic composition having inorganic pigments resistant to agglomeration in an oil-in-water emulsion. The method includes the following: preparing an oil dispersion by adding inorganic pigments directly to an oil; preparing an aqueous base that includes water; combining the oil dispersion and aqueous base; and partitioning the inorganic pigments between the oil and the aqueous phase under controlled conditions, for example, hydrophile-lipophile balance, agitation time and speed, and cooling rate. The present invention provides a process to easily disperse iron-oxide pigments, even black iron-oxide pigments, in oil-in-water emulsions. The present invention provides colored cosmetic compositions that have excellent properties, such as smoothness, adhesion to skin, uniform color, ease of removal, smudge resistance and non-oiliness.
Owner:ACCESS BUSINESS GRP INT LLC
Preparation of double-long-chain non-ionic oil-removing surfactant and applications
InactiveCN103215018AGood chemical stabilityImprove salt toleranceEther preparation from oxiranesDrilling compositionAlkali freeNon ionic
The invention relates to a preparation of a double-long-chain non-ionic oil-removing surfactant and applications, belonging to colloid and interface chemistry fields. In the invention, by reacting fatty alcohol with chloropropylene oxide and ethylene oxide, a novel non-ionic surfactant-double-long-chain alkyl glyceryl ether ethoxy compound is prepared. The surfactant has excellent chemical stability, acid and alkali resistance and inorganic electrolyte, wherein the hydrophile lipophile balance can be regulated by controlling alkyl chain length of the raw material fatty alcohol and ethylene oxide number of added on each molecule. The surfactant has big saturation adsorption quantity on a water / air and oil / water interface, thereby high efficiency reducing a tensile force of a crude oil / water interface. The surfactant can be matched to other surfactants, wherein a mol fraction of the surfactant is 0.1-0.5, and a total mass fraction of the surfactant is in a range of 0.05-0.5%, under an oil reservoir temperature 45 DEG C, the interface tension force of Daqing crude oil / stratum water is reduced to 10-3mN / m order of magnitude, without addition of any acid salt, basic salt, neutral electrolyte and cosurfactant. Therefore, the surfactant is suitable for being used as an alkali-free oil displacement agent.
Owner:JIANGNAN UNIV
Application of biodegradable weeding mulching film in potato cultivation
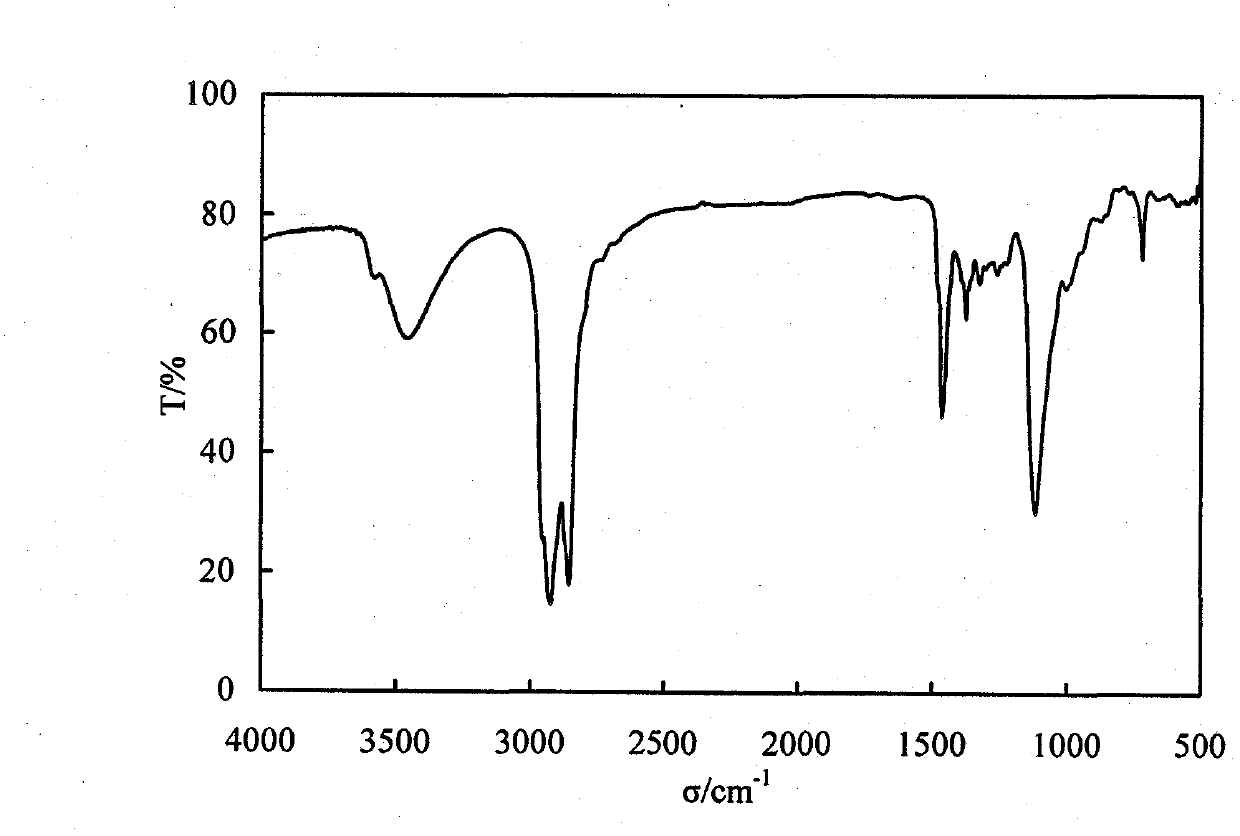
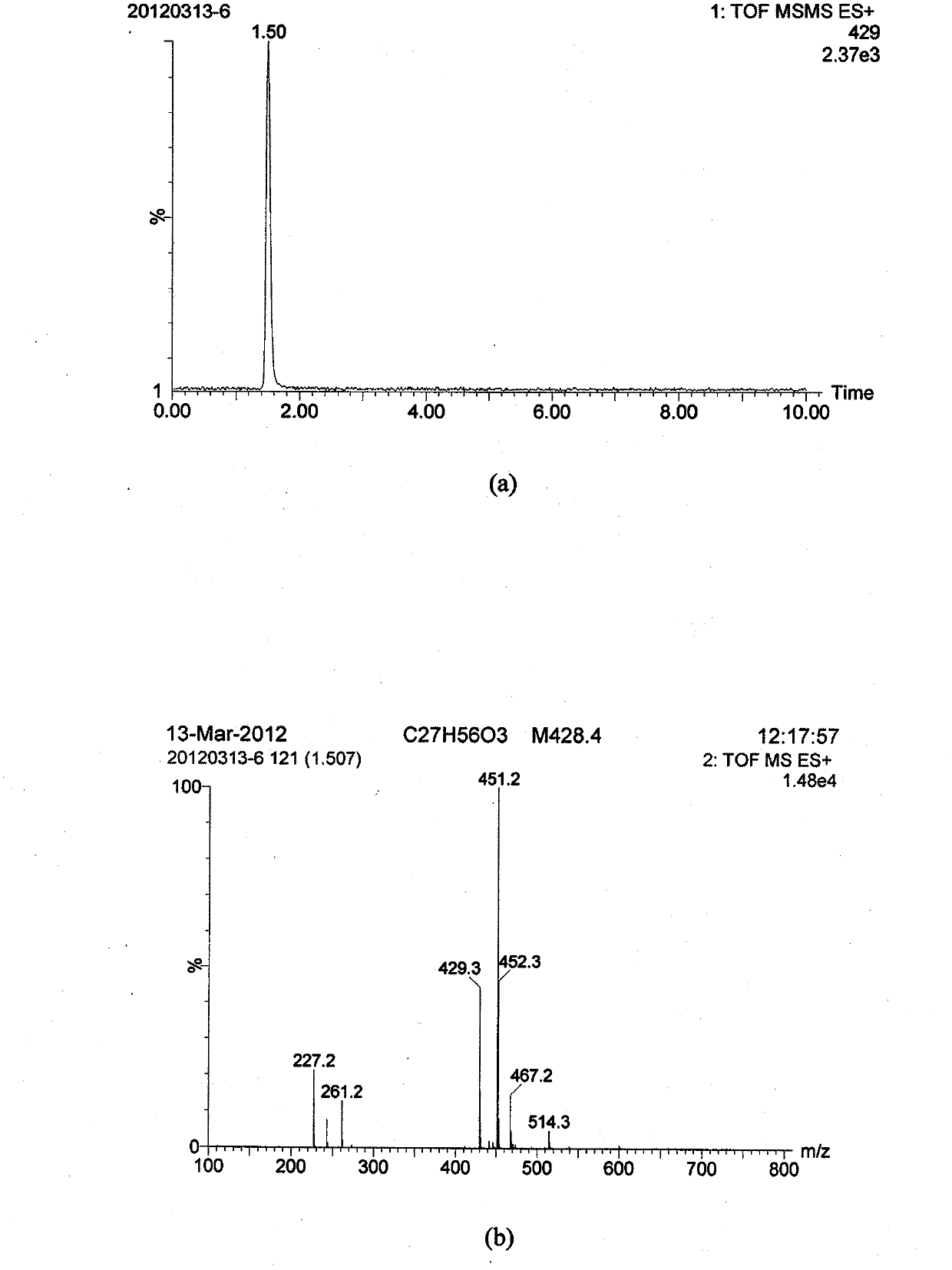
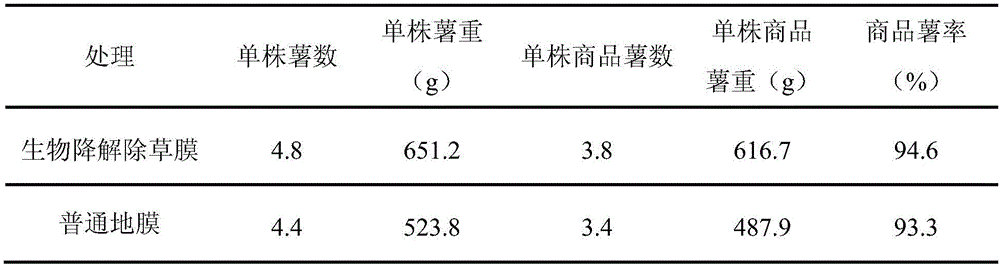
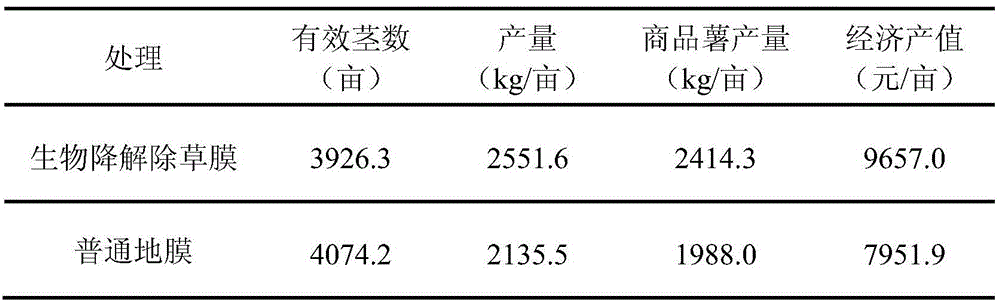
ActiveCN106496968AImprove wettability and dispersibilityGood compatibilityPlant cultivationCultivating equipmentsPolymer scienceHydrophilic-lipophilic balance
The invention belongs to the technical field of potato cultivation, and discloses an application of a biodegradable weeding mulching film in potato cultivation. The mulching film comprises the following components in percentage by mass: 70-97% of biodegradable resin A and 3-30% of biodegradable herbicide master batches, wherein the biodegradable herbicide master batches are prepared by a method comprising the following steps: mixing 10-60% of a herbicide with 0.5-7% of a surfactant A, and performing ultrasonic activation; and mixing 25-75% of biodegradable resin B and 1-10% of a surfactant B at a high speed, and performing extrusion and granulation, thereby obtaining the herbicide master batches, wherein the mass ratio of the surfactant A (the hydrophilic-lipophilic balance value is 7-10) to the surfactant B (the hydrophilic-lipophilic balance value is 3-6) is (1:1)-(1:2). As the biodegradable weeding mulching film is used in potato cultivation, the problems that weeds can grow out from furrow surfaces and environment pollution can be caused by common plastics can be effectively prevented, and the labor cost can be lowered.
Owner:广东省科学院生物与医学工程研究所
Coenzyme Q10 self-emulsifying composition, preparation method and application thereof
InactiveCN101596177AImprove solubilityPromote absorption in the bodyCosmetic preparationsToilet preparationsAdditive ingredientTG - Triglyceride
Owner:苏州国纳生物技术有限公司
Water-Based Wetness-Indicating Composition and Sensor
ActiveUS20140363354A1Analysis using chemical indicatorsPretreated surfacesWater basedWater insoluble
A color-appearing ink composition includes a surfactant blend of at least two non-ionic surfactants having hydrophilic-lipophilic balance values greater than 10, or a non-ionic surfactant having a hydrophilic-lipophilic balance value greater than 10 and a charged surfactant. The composition includes one or more water-insoluble leuco dyes, a developer and a desensitizer. The composition may be applied to a substrate to form a sensor.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Diluting reagent and method compelling time-independent consistency in MCV assay
InactiveUS6225124B1Withdrawing sample devicesDead animal preservationHydrophileHydrophilic-lipophilic balance
Aqueous blood-sample diluting reagent and method of its use for compelling a morphological change in a blood sample to yield an MCV value assayed at elapsed time after the sample is drawn to be consistent within a diagnostically acceptable range with the original, immediate post-drawing MCV value. Selection of a small amount of a predetermined surfactant added within a limited range of concentration, and of a salt for adjusting osmotic pressure of the sample is thereby determined. The blood sample is treated with an anti-coagulant agent immediately post-drawing, and for assay in a particle analyzer at post-drawing elapsed time is diluted with the reagent solution. The reagent has an osmotic pressure (pi) of approximately 150-400 mOsm / kg and a pH of 6.0-8.5. The surfactant is present in a 0.0005% to 0.5% concentration and has a hydrophile-lipophile balance (HLB) of 10-20.
Owner:SYSMEX CORP
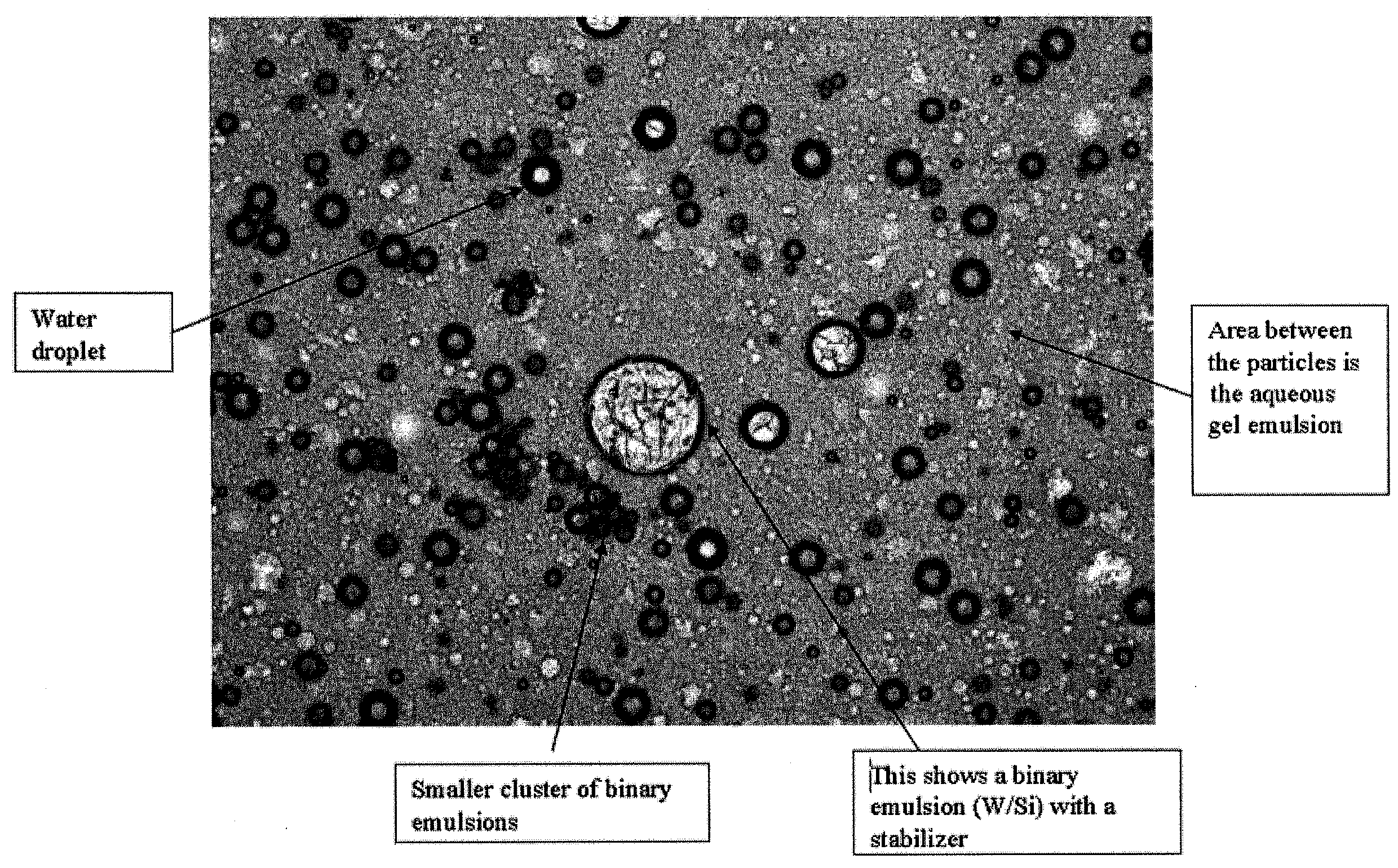
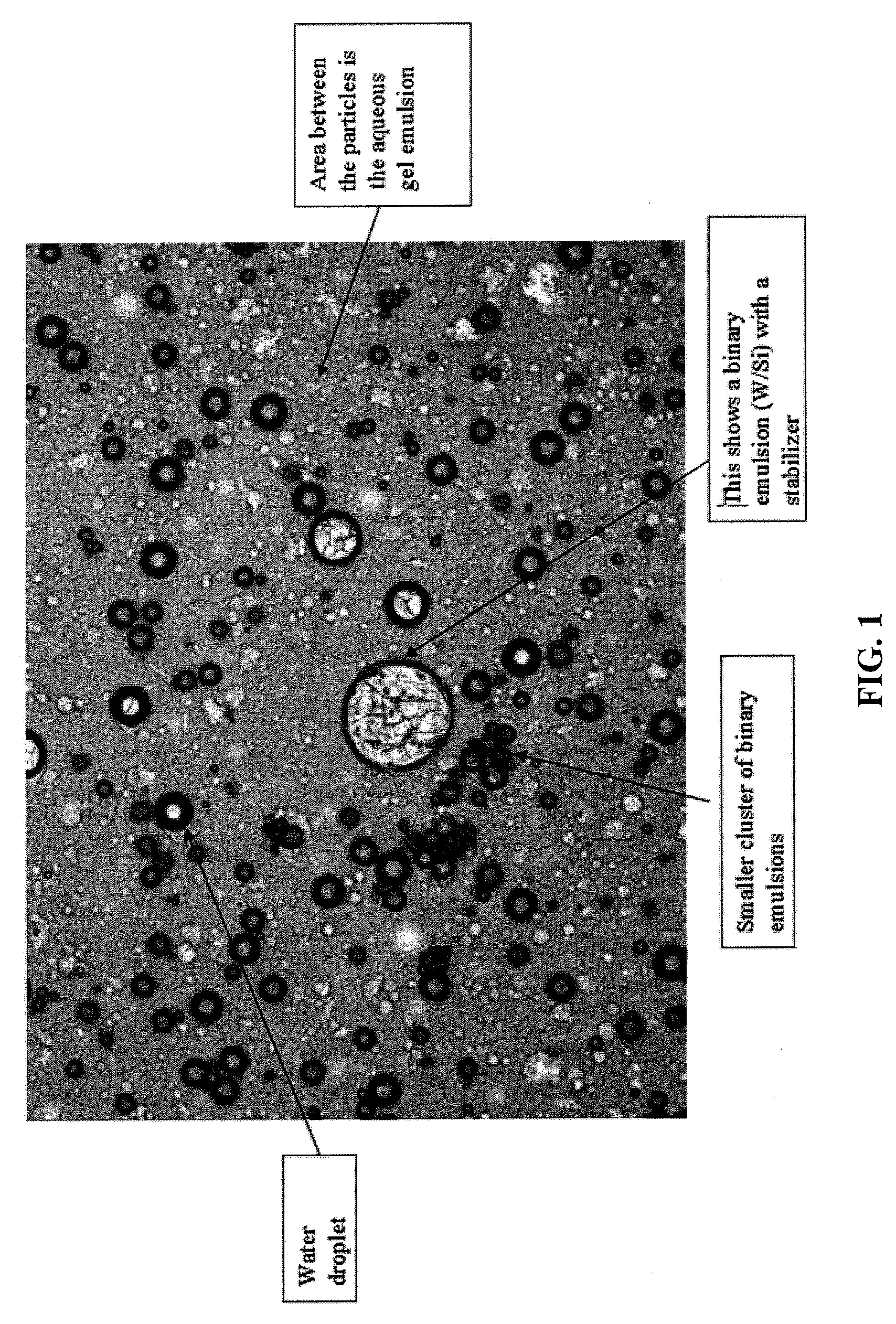
Stable three-phased emulsions
ActiveUS20090232856A1Improve stabilityEasy to spreadCosmetic preparationsBody powdersWater basedThree-phase
Disclosed is a three-phase emulsion comprising an aqueous-gel outer phase; and a water-in-oil inner phase, wherein the emulsion is stable. The aqueous-gel outer phase can include 10% to 50% by weight of the total weight of the emulsion. This phase can also include 70% to 95% by weight of water based on the total weight of the aqueous-gel outer phase, a gelling agent, and an emulsifier having a hydrophilic-lipophilic balance (HLB) value of 10 to 19. The water-in-oil inner phase can include 50% to 95% by weight based on the total weight of the three-phase emulsion. This phase can also include 50% to 80% by weight of water based on the total weight of the water-in-oil inner phase, 20% to 50% by weight of oil based on the total weight of the water-in-oil inner phase, and an emulsifier.
Owner:MARY KAY INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com