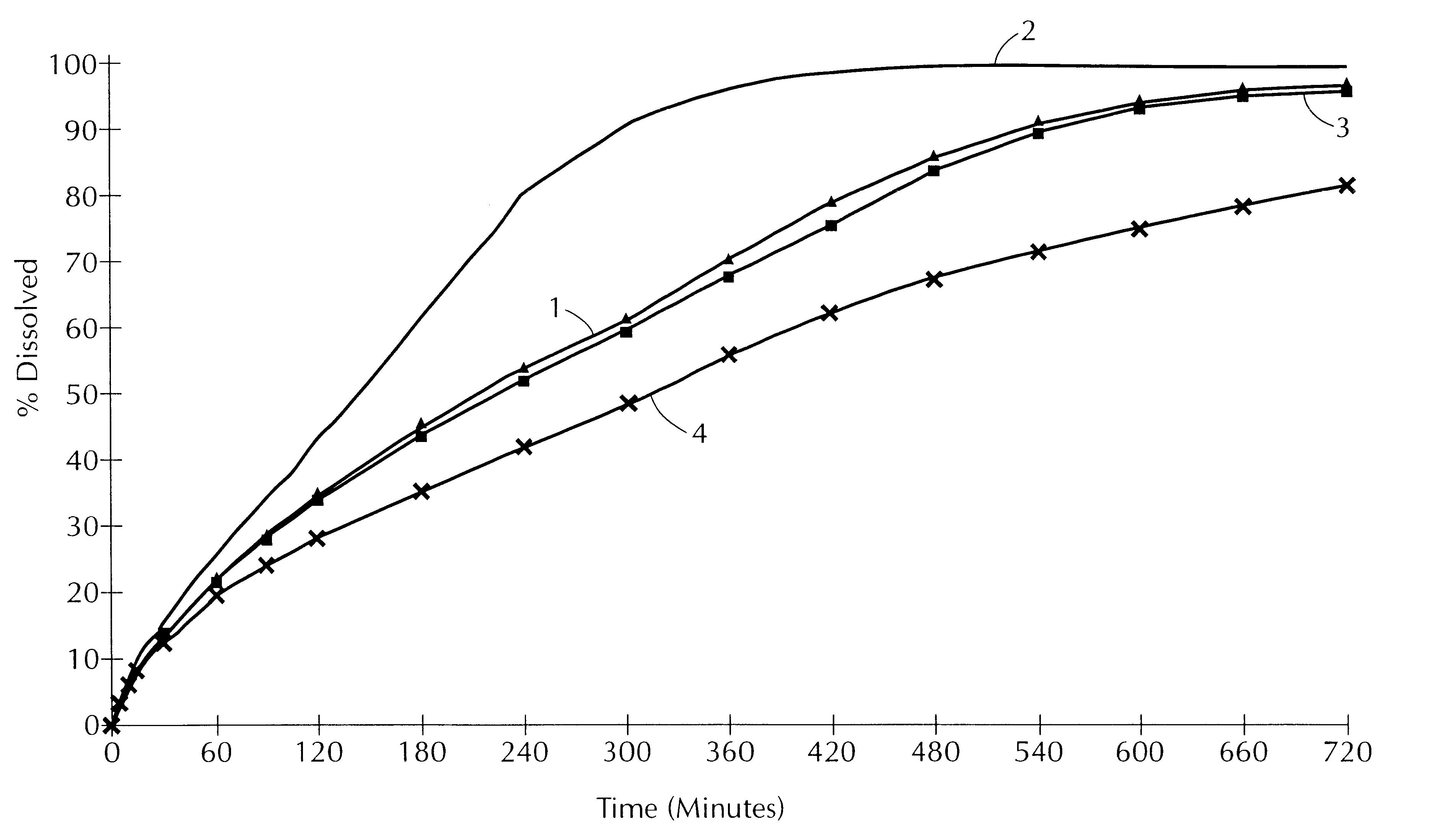
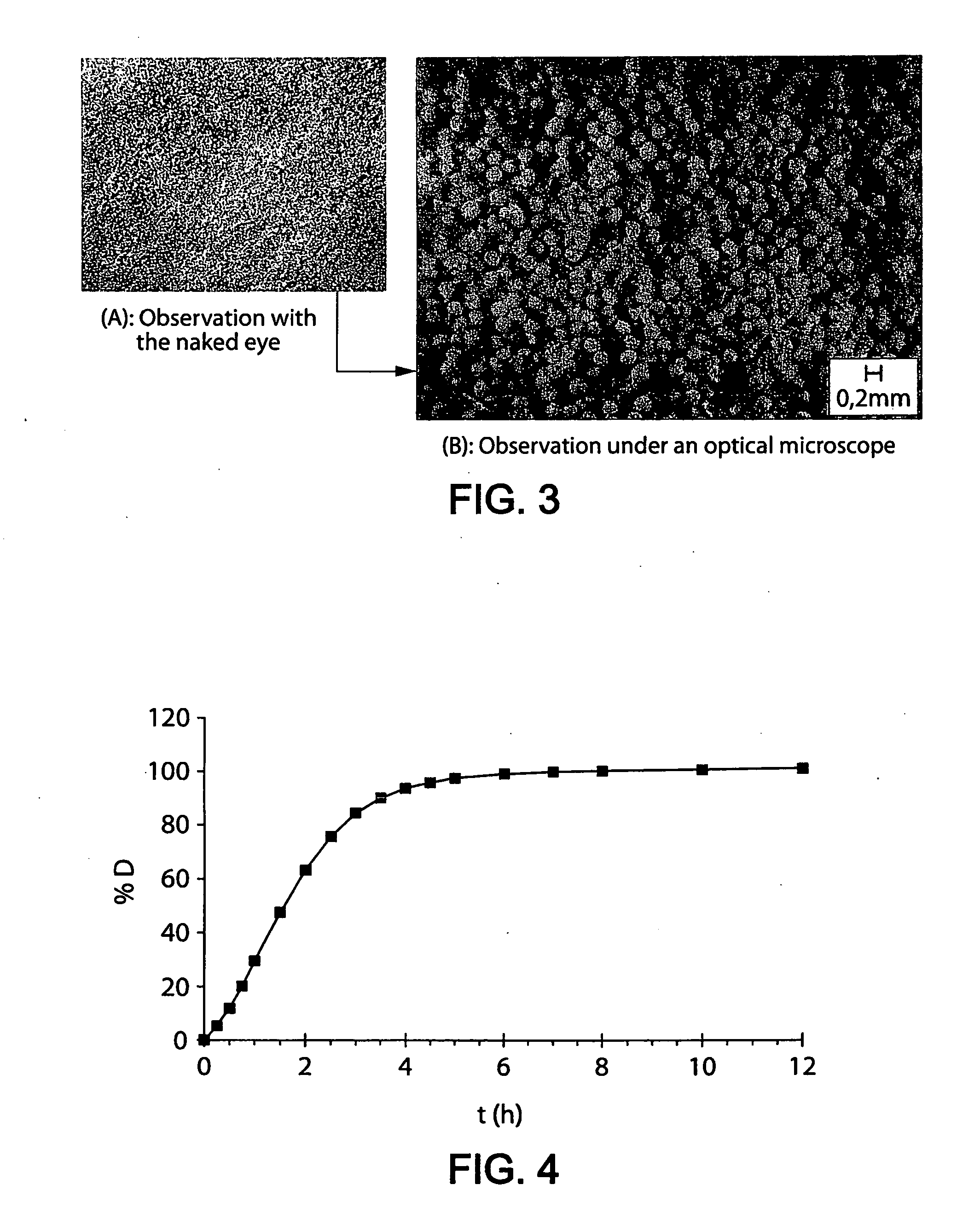
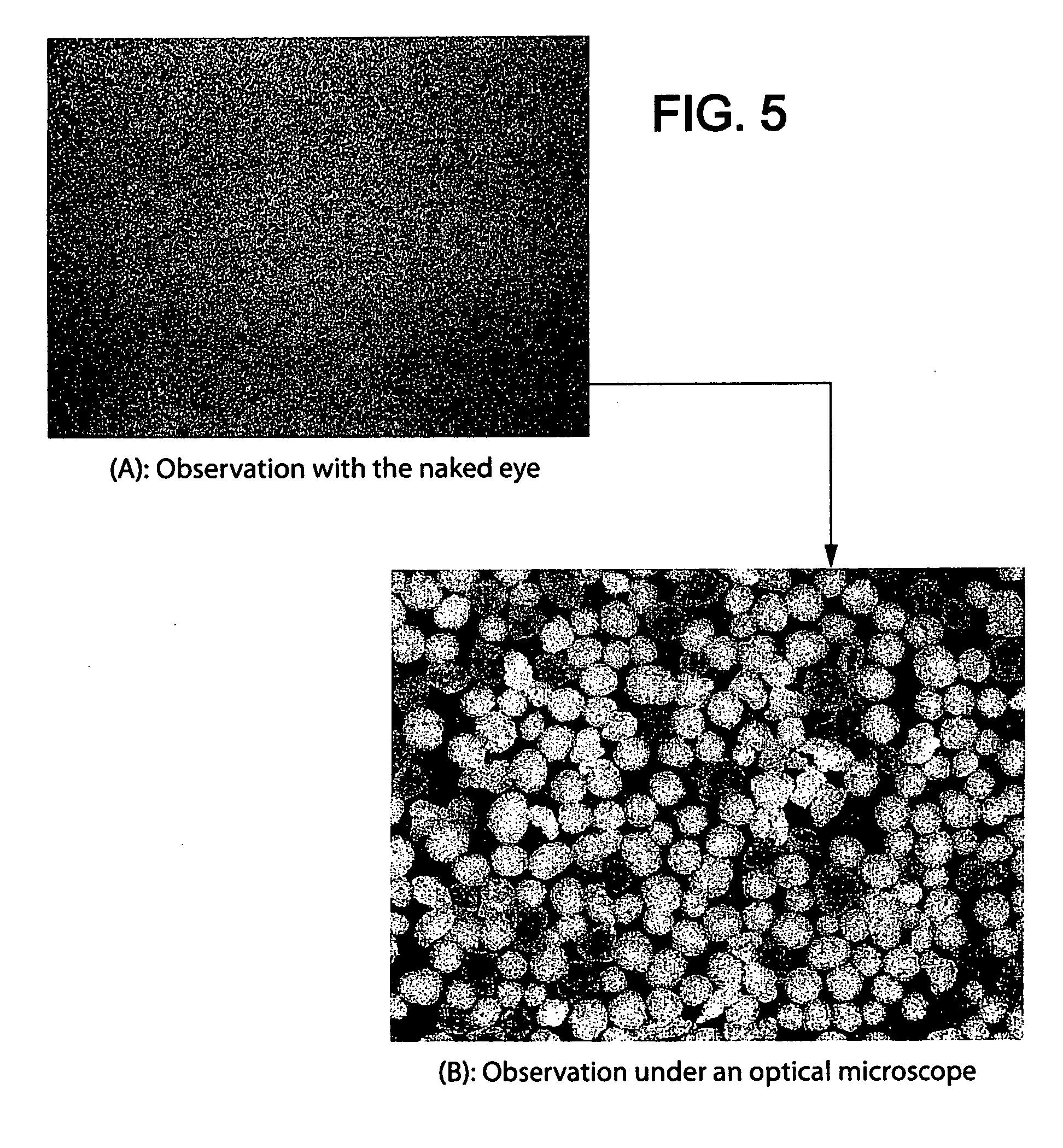
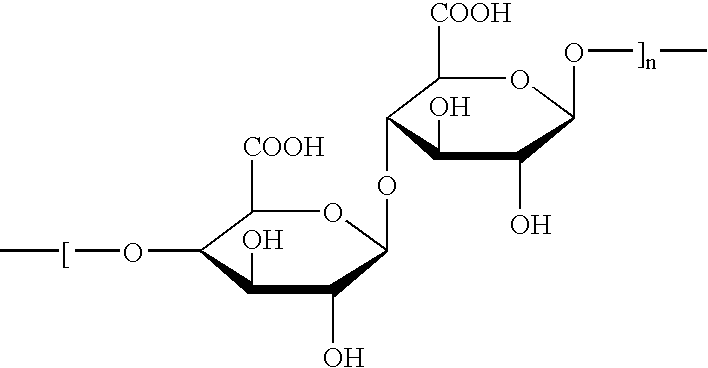
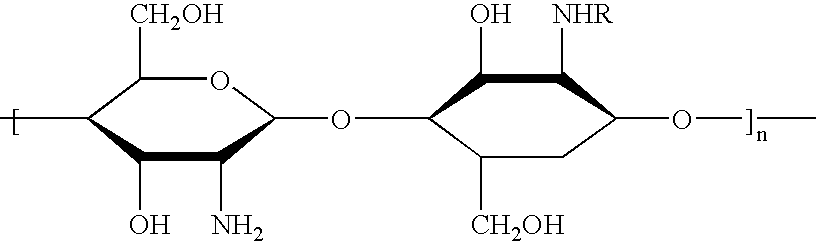
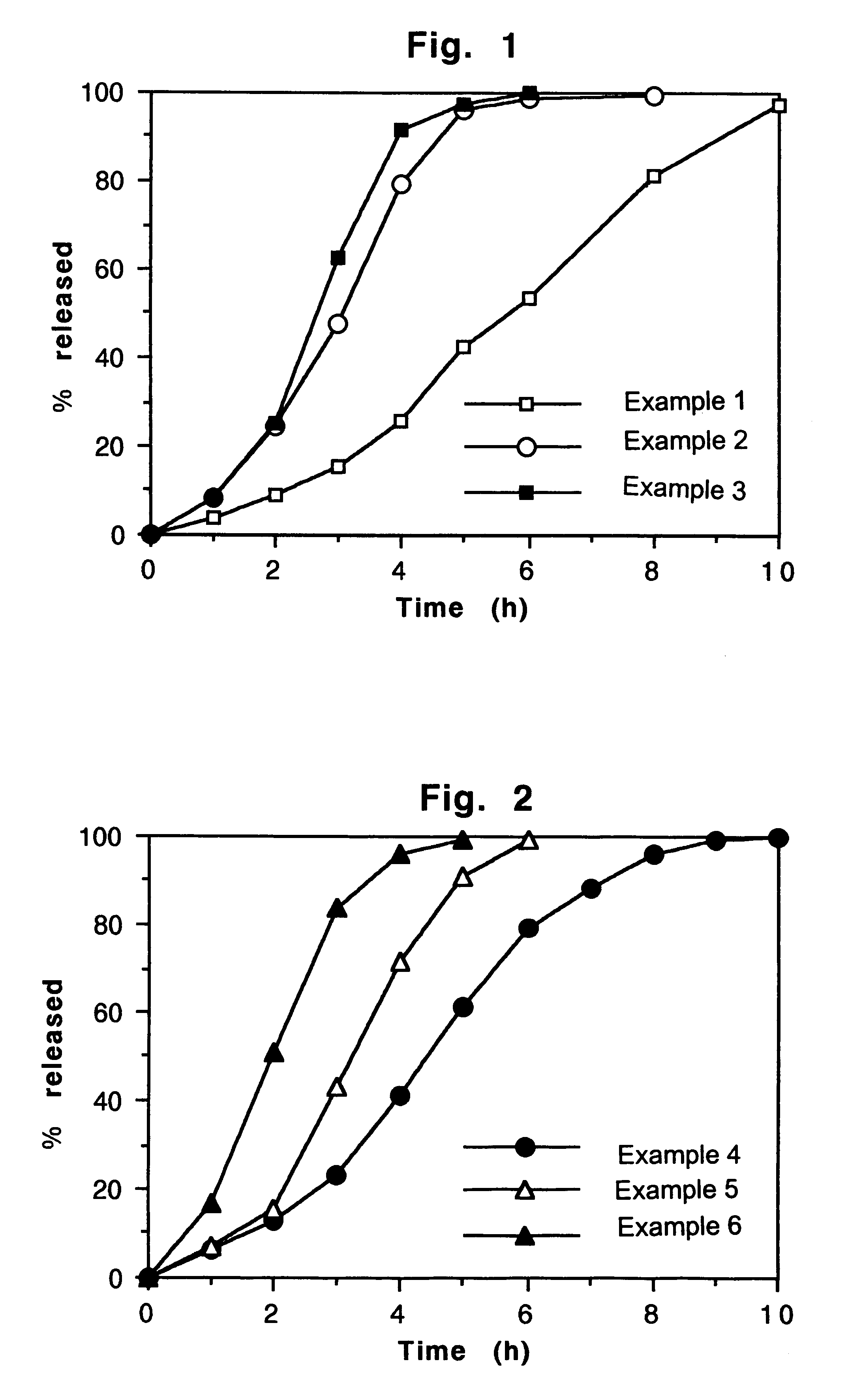
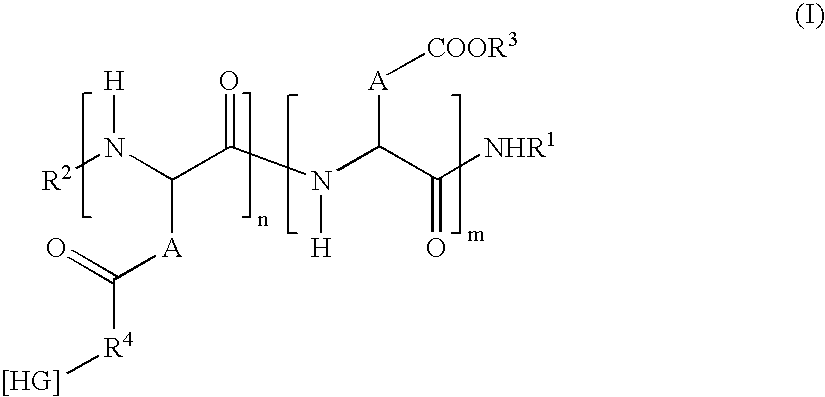
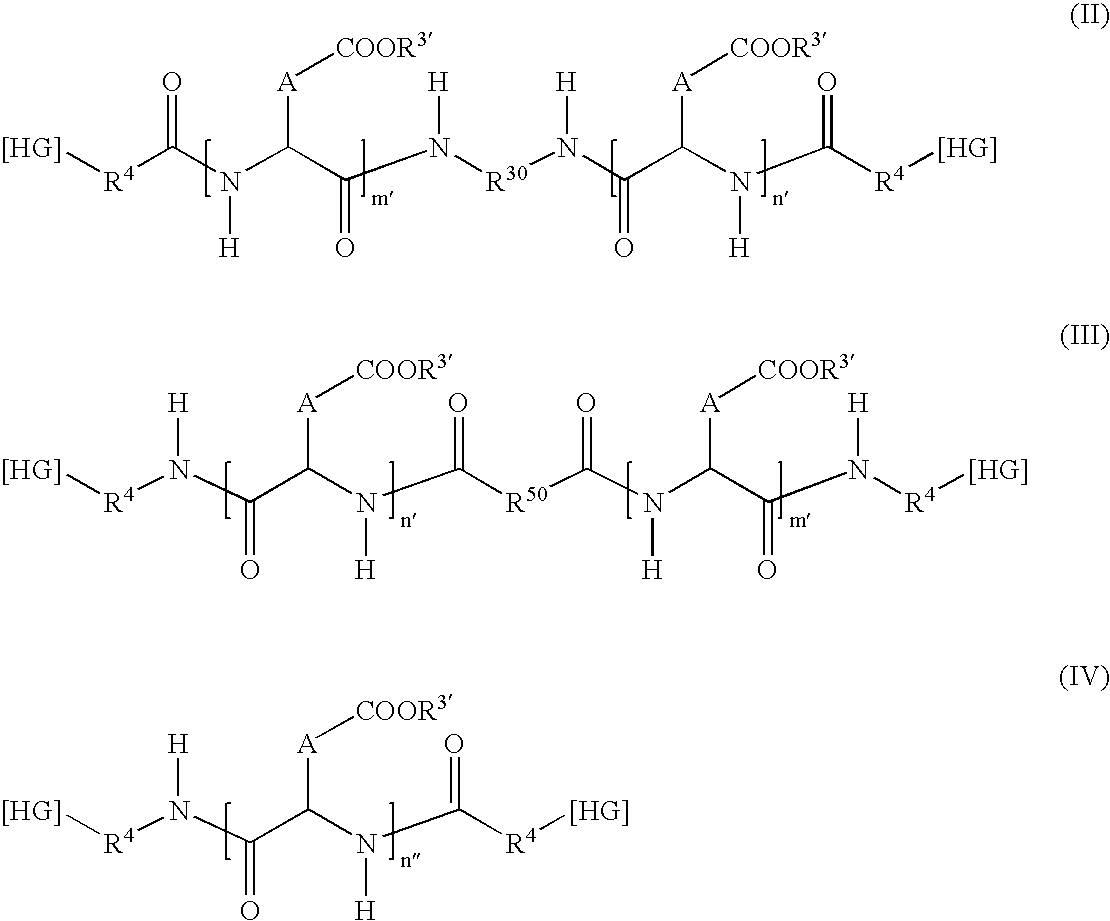
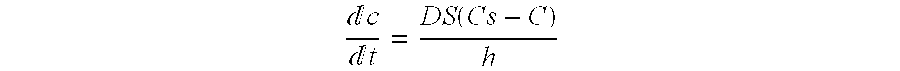Patents
Literature
477 results about "Active principle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical tablet, completely coated, for controlled release of active principles that present problems of bio-availability linked to gastro-intestinal absorption
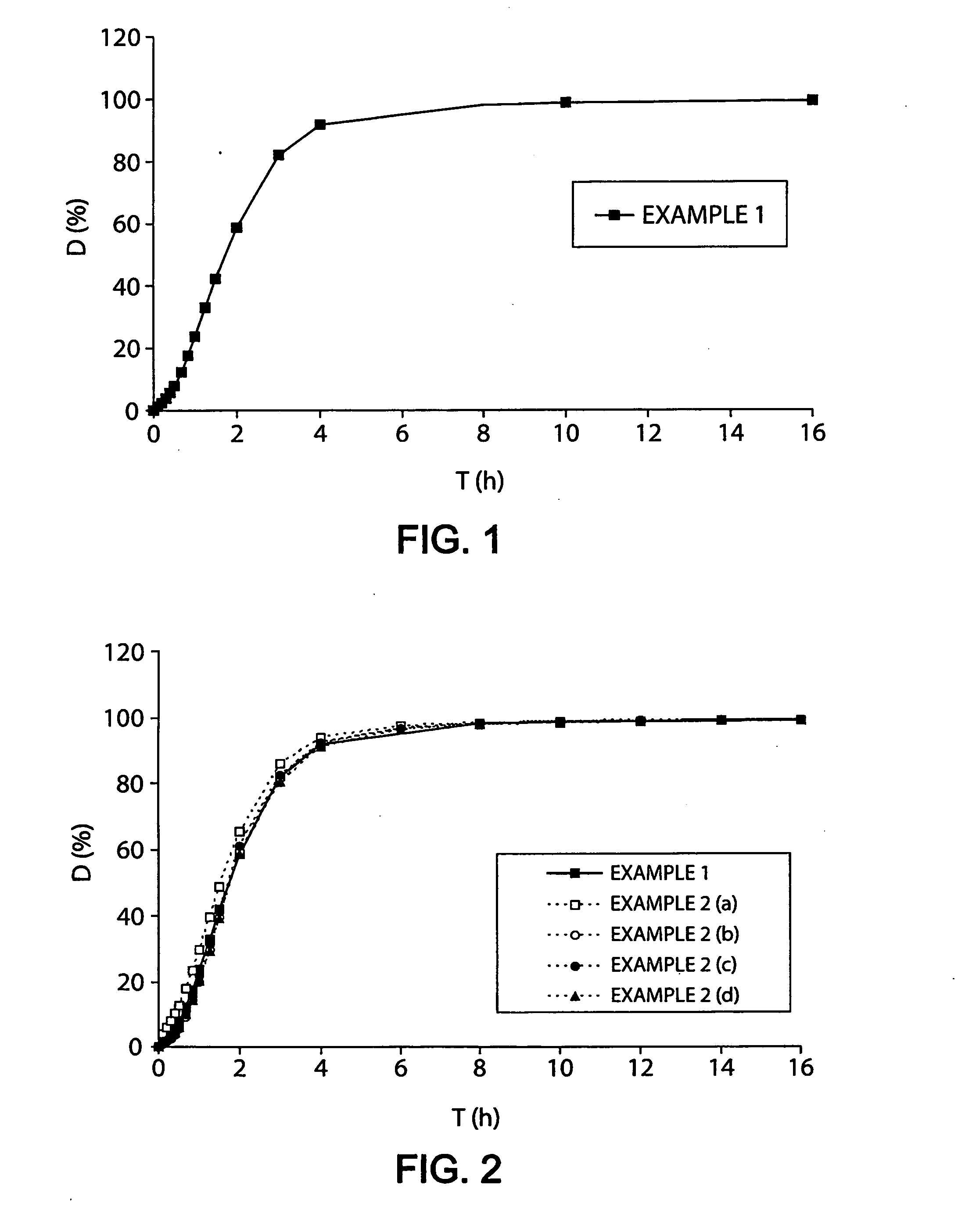
Described herein is a particular type of pharmaceutical tablet, for oral use, which is formed by one or more layers, and is specifically designed for controlled release of active principles that present problems of bio-availability linked to absorption in the gastro-intestinal tract, and in particular active principles that present an erratic and unpredictable absorption linked to the presence or absence of food at the level of the stomach and / or of the first portion of the small intestine, the said pharmaceutical form being characterized in that it is completely coated with one or more films of a biocompatible and biodegradable polymeric material.
Owner:JAGOTEC AG
Process for the manufacture of pharmaceutical composition with modified release of active principle comprising the matrix
The invention relates to a process for the manufacture of a pharmaceutical composition with modified release of active principle comprising at least one active principle, a lipid matrix agent composed of ester of alcohol with at least one fatty acid and at least one adjuvant.This process is characterized in that:a powder composed of at least one component selected in the group comprising the active principle and the adjuvant, is mixed, while heating and fluidizing, in order to obtain individual grains;the said lipid matrix agent is liquefied separately under warm conditions;the said powder is then coated under warm conditions by spraying the said lipid matrix agent over the individual grains;finally, the temperature of the combined product is lowered in order to allow the lipid matrix agent to solidify.
Owner:GATTEFOSSE HLDG
Prolonged-release multimicroparticulate oral pharmaceutical form
InactiveUS20080063725A1Prevent fraudulent abuse of propertyDifficult to administerBiocideNervous disorderProlonged releaseActive principle
Modified-release multimicroparticulate pharmaceutical form capable of maintaining the modified release of the active principle in an alcoholic solution and of resisting attempts at misuse.
Owner:FLAMEL IRELAND
Orally administrable composition capable of providing enhanced bioavailability when ingested
InactiveUS6054136AImprove solubilityImprove bioavailabilityCosmetic preparationsToilet preparationsFatty acid esterIngestion
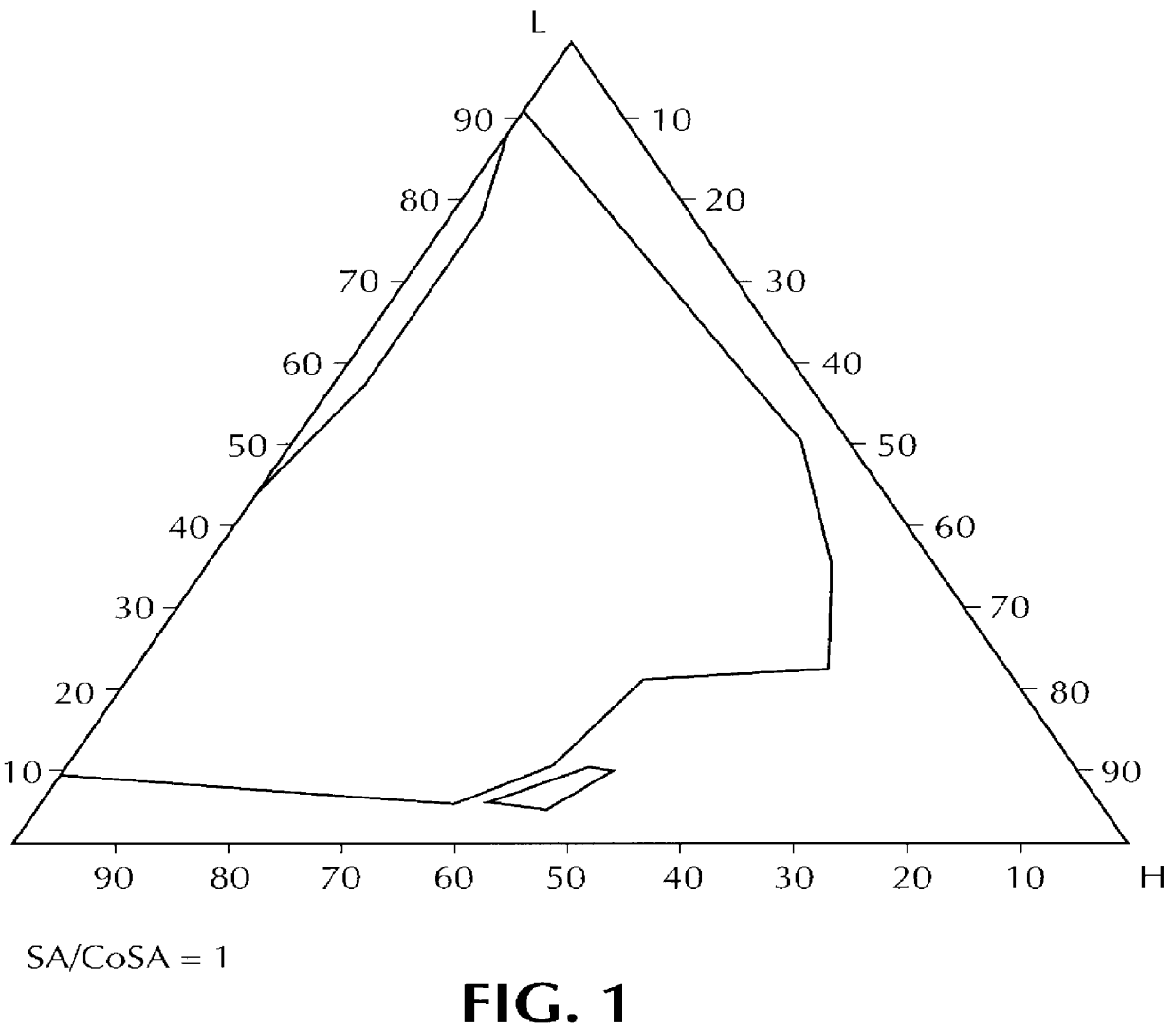
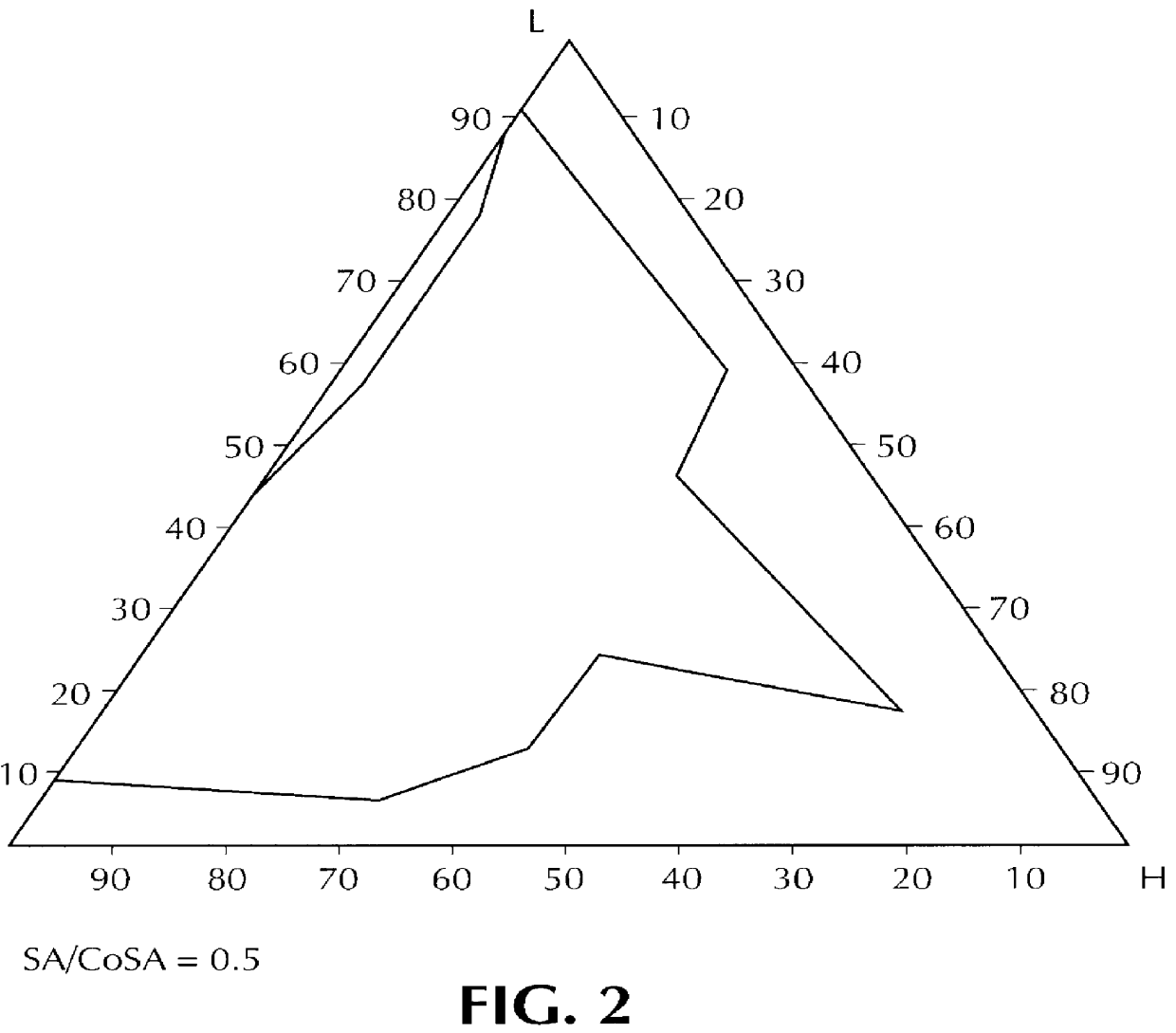
Composition for pharmaceutical or cosmetic use, capable of forming a microemulsion, comprising at least: an active principle, a lipophilic phase consisting of a mixture of fatty acid esters and glycerides, a surfactant (SA), a cosurfactant (CoSA), a hydrophilic phase, characterized: in that the lipophilic phase consists of a mixture of C8 to C18 polyglycolized glycerides having a hydrophilic-lipophilic balance (HLB) of less than 16, this lipophilic phase representing from 30 to 75% of the total weight of the composition; in that the surfactant (SA) is chosen from the group comprising saturated C8-C10 olyglycolized glycerides and oleic esters of polyglycerol, this surfactant having an HLB of less than 16; in that the cosurfactant (CoSA) is chosen from the group comprising lauric esters of propylene glycol, oleic esters of polyglycerol and ethyl diglycol; in that the SA / CoSA ratio is between 0.5 and 6; and in that the hydrophilic phase of the final microemulsion is supplied after ingestion by the physiological fluid of the digestive milieu.
Owner:GATTEFOSSE HLDG
Anti-misuse microparticulate oral pharmaceutical form
InactiveUS20070224129A1Avoid misusePrevent misuse by short liquid extraction and/or crushingOrganic active ingredientsDrug compositionsPublic healthMicroparticle
The present invention relates to solid microparticulate oral pharmaceutical forms whose composition and structure make it possible to avoid misuse of the pharmaceutical active principle (AP) they contain. The object of the present invention is to prevent solid oral drugs from being misappropriated for any use other than the therapeutic use(s) officially approved by the competent public health authorities. In other words, the object is to avoid the voluntary or involuntary misuse of solid oral drugs. The invention relates to a solid oral pharmaceutical form which is characterized in that it contains anti-misuse means, in that at least part of the AP it comprises is contained in coated microparticles for modified release of the AP, and in that the coated microparticles of AP have a coating layer (Ra) which assures the modified release of the AP and simultaneously imparts crushing resistance to the coated microparticles of AP so as to avoid misuse.
Owner:FLAMEL IRELAND
Microcapsules and processes for making the same using various polymers and chitosans
InactiveUS6733790B1Improve stabilityReduce partial pressurePowder deliveryBiocideAnionic polymersAlginic acid
A microcapsule having a mean diameter of from about 0.1 to about 5 mm, a membrane and a matrix containing at least one active principle wherein the microcapsule is the product of the process comprising the steps of (a) forming an aqueous matrix by heating an aqueous solution comprised of a gel former, an anionic polymer selected from the group consisting of a salt of alginic acid and an anionic chitosan derivative and active principle; (b) adding the aqueous matrix to an aqueous solution of chitosan.
Owner:COGNIS IP MANAGEMENT GMBH
Flavonoide esters and their use notably in cosmetics
The invention relates to a flavonoid ester. This flavonoid ester results from the reaction product of at least one flavonoid selected from the group consisting of a flavonoid with a ketone group in the 4-position, a salt, ester or ether of such a flavonoid, and a C-heteroside and / or O-heteroside derivative of such a flavonoid, with the proviso that this flavonoid contains at least one free alcohol group, with an organic monoacid having from 3 to 30 carbon atoms. These flavonoid esters constitute useful active principles for the manufacture of cosmetic, dermopharmaceutical, pharmaceutical, dietetic or agri-foodstuff compositions.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Anti-misuse microparticulate oral pharmaceutical form
InactiveUS20100092553A1Prevent misuse by short liquid extraction and/or crushingPowder deliveryBiocidePublic healthMicroparticle
Owner:FLAMEL IRELAND
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
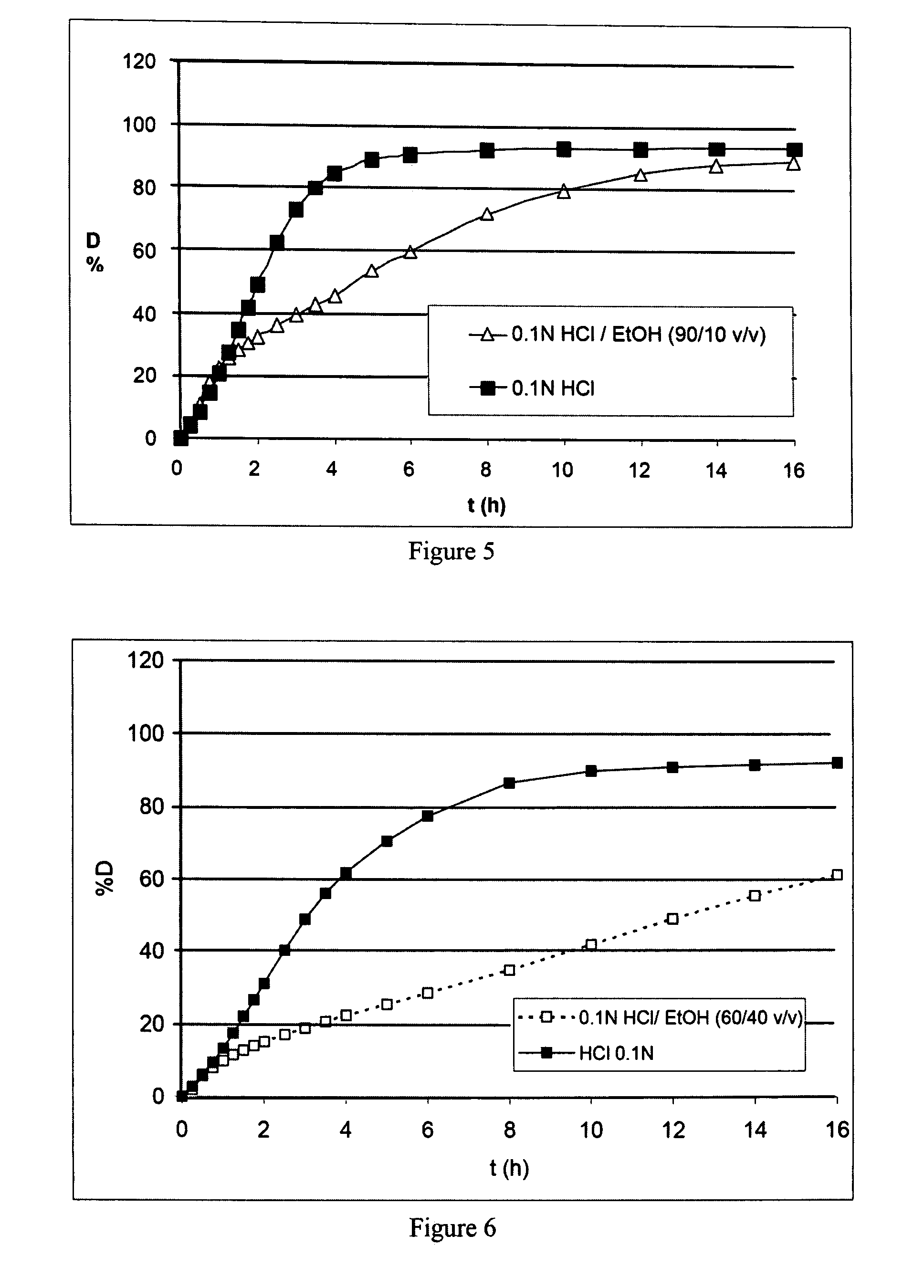
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Controlled release pharmaceutical tablets containing an active principle of low water solubility
It is described a new method for the preparation of pharmaceutical tablets carrying poorly soluble in water principle; this method allows to obtain tablets with fast and / or slow release of the active principle. The peculiar feature is the fact that the poorly soluble in water active principle (es: nifedipine) is treated with a surfactant, during the granulation phase or whatever during the preparation process; the obtained product, subjected to a compression, produces pharmaceutical tablets which show high bioavailability of the carried active principle. This procedure can be used to prepare polymeric matrixes (with modified release), formed by tablets with one or more layers. The procedure of manufacture and the characteristics of the new finished tablet are described.
Owner:JAGOTEC AG
Modified-release microparticles based on amphiphilic copolymer and on active principles(s) and pharmaceutical formulations comprising them
The present invention relates to novel microparticles formed of amphiphilic polyamino acids which transport active principle(s), AP(s), in particular protein and peptide active principle(s), and to novel modified-release pharmaceutical formulations comprising said AP microparticles. The aim of the invention is to develop novel microparticles, charged with AP, obtained by aggregation of nanoparticles of amphiphilic polyamino acids and having improved properties, in particular in the dry solid form, with regard to their ability to be dispersed and, concerning the reconstituted suspension, its stability and its ability to be easily handled and injected. The invention relates firstly to microparticles of amphiphilic polyamino acid (PO) comprising at least one AP (associated noncovalently) which spontaneously form a colloidal suspension of nanoparticles in water, at pH 7.0, under isotonic conditions; which microparticles a. are obtained by atomization of a solution or colloidal suspension of PO comprising at least one AP, b. have a size of between 0.5 and 100 microns, c. and are dispersible in colloidal suspension. The invention also relates to the process for the preparation of these microparticles, to a liquid formulation comprising a suspension of these PO / AP microparticles, to a reconstitution process and kit for this formulation and to a dry form of this formulation.
Owner:FLAMEL TECHNOLOGIES
Microneedle transport device
A transdermal transport device includes a reservoir for holding a formulation of an active principle, and an array of needles which have bores in fluid communication with the reservoir to facilitate transporting the formulation to and from the reservoir through the needles. The device also includes a first actuator which drives the array of needles into the body, and a second actuator which pumps the formulation between the reservoir and the body through the needles. The first actuator is reversible to withdraw the needles from the body.
Owner:MASSACHUSETTS INST OF TECH
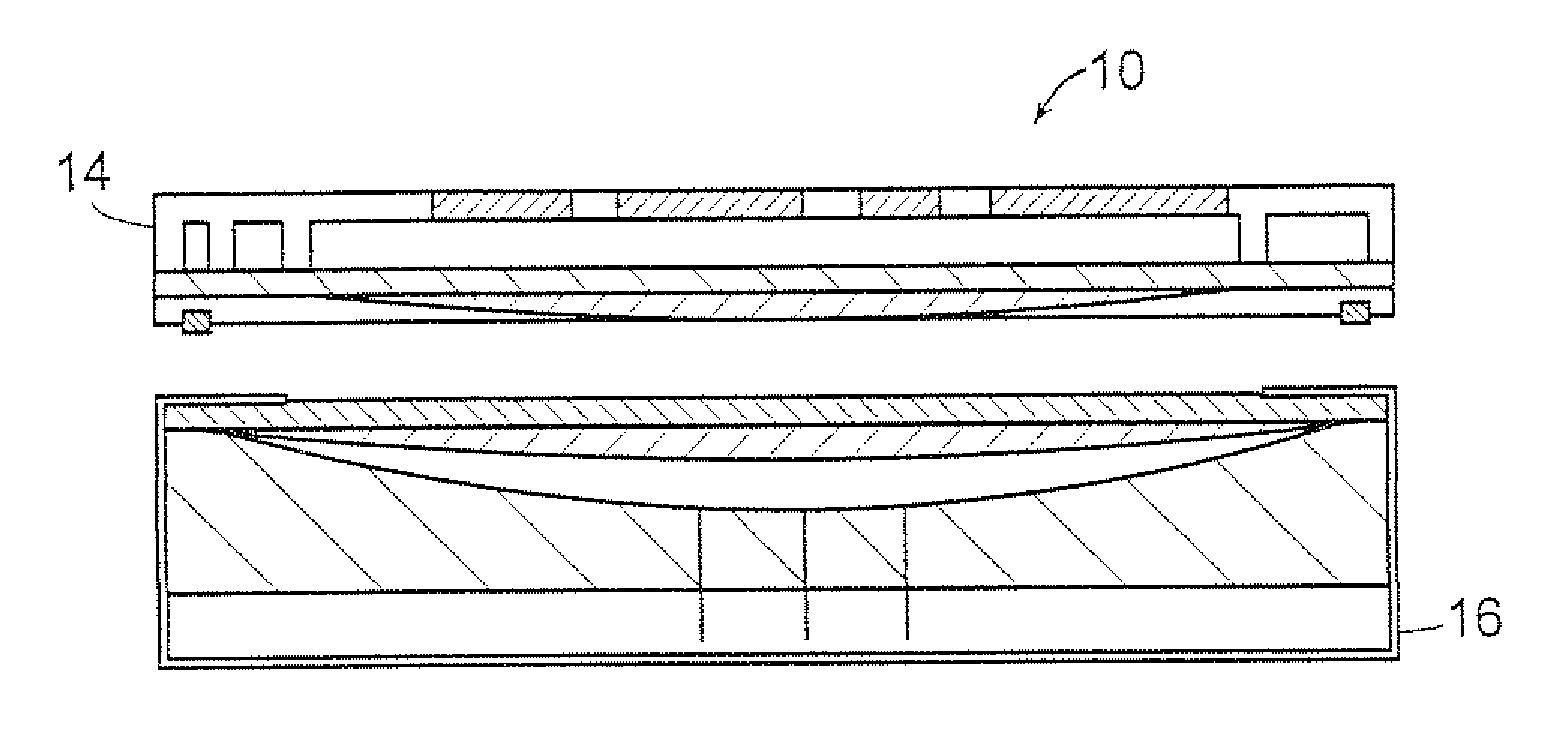
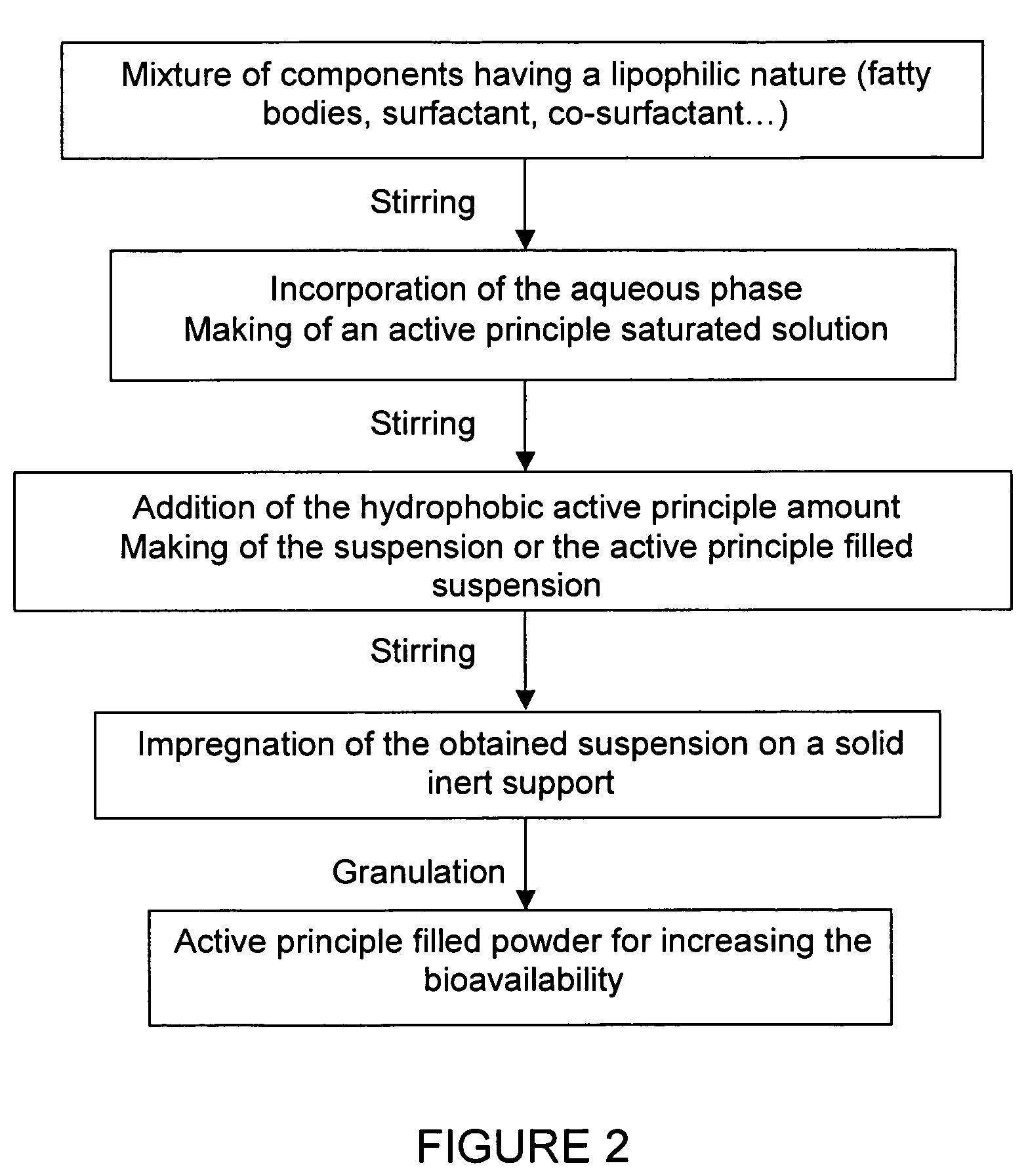
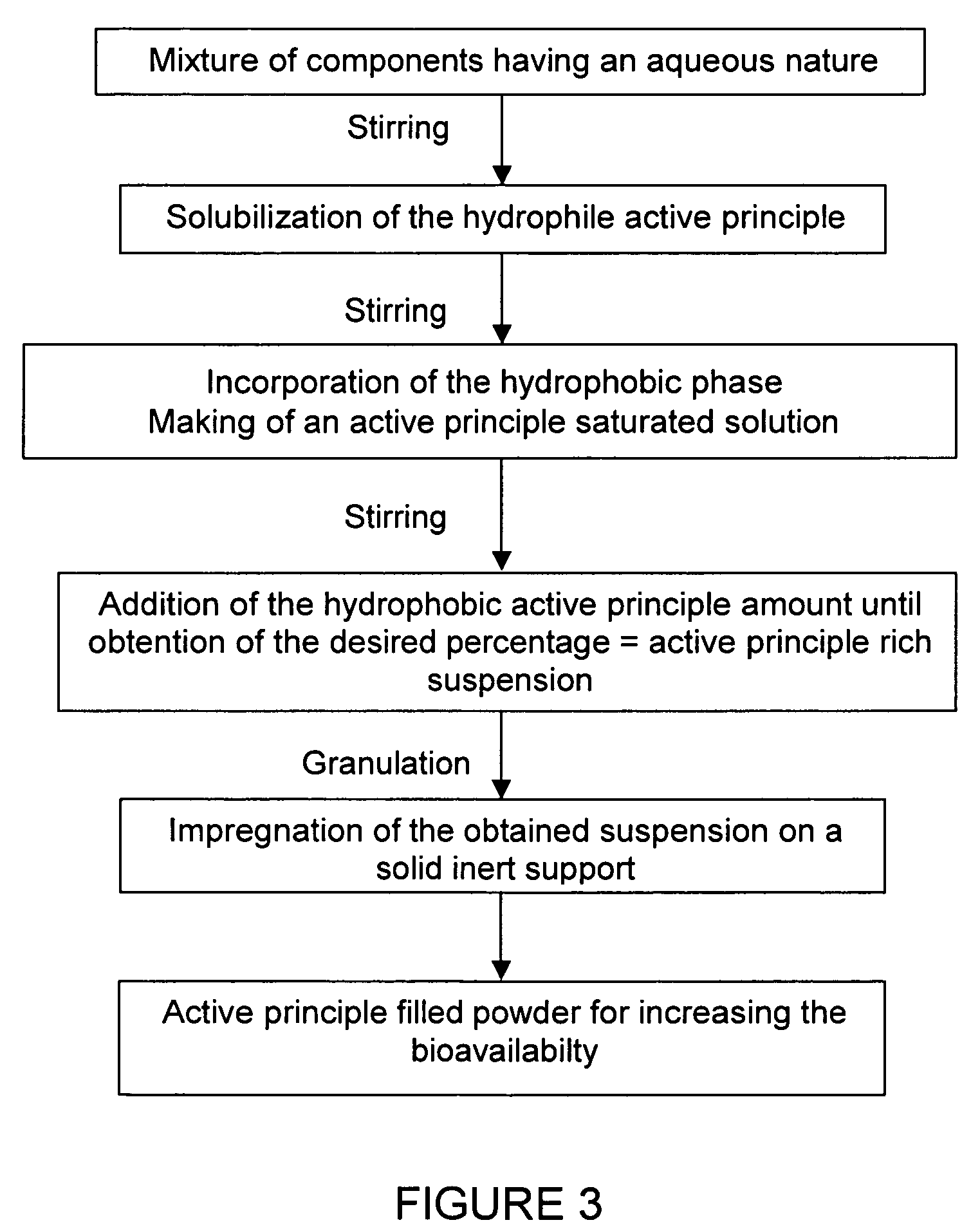
Impregnated powder improving bioavailability and/or solubility and method of production
This invention relates to an impregnated powder for increasing the bioavailabilty and / or the solubility of at least one active principle comprising a solid, inert support in a particle form impregnated by a liquid medium comprising a hydrophobic phase and optionally a hydrophilic phase, at least one surfactant and at least one active principle dissolved in at least one of said phases, wherein said active principle(s) is(are) also present in at least one of said phases in the form of a suspension.Such an impregnated powder is used as a base for various preparations in the pharmaceutical, parapharmaceutical and cosmetic field, in the food complement field and in the food processing industry.
Owner:GALENIX INNOVATIONS
Stent for endoluminal delivery of active principles or agents
InactiveUS20050209681A1Reduce adverse effectsMaintain good propertiesStentsBlood vesselsInsertion stentAnimal body
The present invention provides a stent for implantation at a site within a human or animal body comprising: an expandable body having an inner surface and an outer surface; and treatment agents applied to the outer surface of the expandable body, the treatment agents comprising a combination of Paclitaxel and FK506 or their derivatives or analogues.
Owner:CID CO LTD
Para-coumaric acid or para-hydroxycinnamic acid derivatives and their use in cosmetic or dermatological compositions
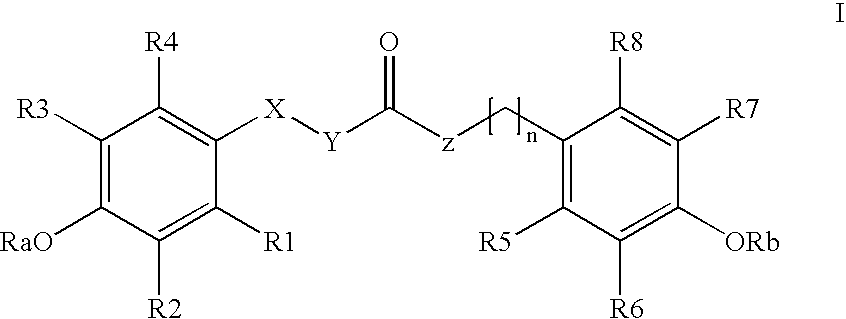
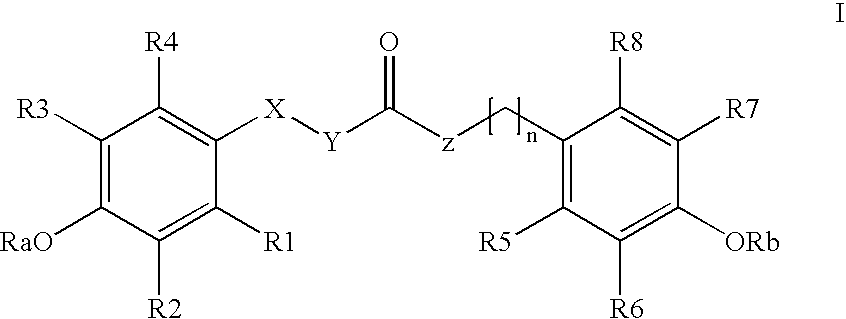
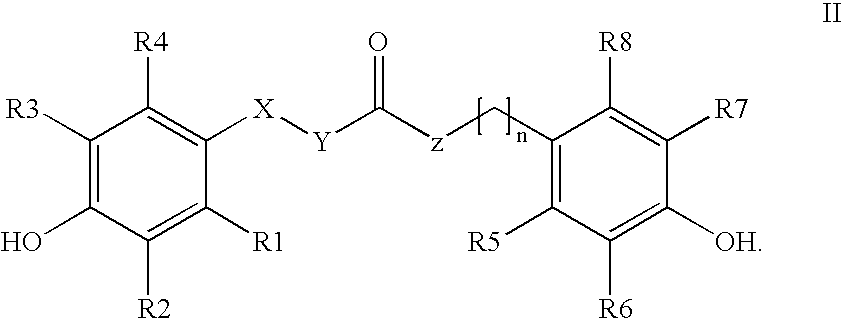
The invention relates to the use of para-coumaric acid or para-hydroxycinnamic acid derivatives in cosmetic or dermatological compositions, specifically to the use of at least one compound derived from para-coumaric acid having a general formula (I) below: in which, especially, Z represents an oxygen or an —NH— group; X and Y are identical and each represent a CH or CH2 group, as an active principle with depigmenting, free-radical-scavenging and / or antiinflammatory activity. The invention also relates to the use of the above compounds for cosmetic care or for the preparation of a pharmaceutical composition, especially for depigmenting an area of skin, having antiradical and / or antiinflammatory activity.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS +1
Active principle which is capable of inducing the conversion of inactive TGFb-latent into active TGFb
The invention relates to active principles which are capable of inducing the conversion of inactive TGFb-Latent into active TGFb. The invention relates in particular to the use of such an extract for the manufacture of a composition for increasing the concentration of active TGFb1 in the skin, notably in the dermis. The present invention also relates to the uses which are derived from said use, as well as to cosmetic or pharmaceutical compositions which comprise said extract.
Owner:LABE DE DERMOCOSMETIQUE ACTIVE DOCTEUR PIERRE RICAUD +1
Composition comprising oil drops
ActiveUS20120141531A1High dissolution rateAntibacterial agentsBiocideActive principleWater soluble polymers
A composition comprises a water-soluble polymer matrix in which are dispersed droplets of oil, the composition comprising an active principle. The invention includes embodiments in which the active principle is included in at least some of the oil droplets as well as embodiments in which the oil droplets are free of active principle. The oil droplets are released as the matrix containing them dissolves in an aqueous medium. In one embodiment, the oil droplets are substantially immobilized in or by the matrix and the immobilizing feature is lost as the matrix dissolves in aqueous media. In certain embodiments, the oil drops may collectively be referred to as the oil phase of the composition of the invention. The product may be in the form of mini-beads. The oil phase and / or the polymer matrix may each include a surfactant.
Owner:SUBLIMITY THERAPEUTICS LTD
Cosmetic composition for greasy skin care, containing a carboxylic fatty acid or a derivative thereof
InactiveUS20050244360A1Inhibit lipogenesisReduction in lipid secretionCosmetic preparationsHair removalMedicineGreasy skin
The invention concerns a cosmetic composition for treating or preventing disorders associated with a greasy skin, in particular by an activity reducing sebum secretion, comprising as active principle at least one dicarboxylic fatty acid or one derivative of such a fatty acid. The invention concerns in particular cosmetic compositions for topical administration. The invention also concerns a cosmetic method for treating or preventing disorders associated with hyperseborrhea.
Owner:LOREAL SA
Formulation of powder containing nanoparticles for aerosol delivery to the lungs
Respirable particles carrying active principles or diagnostics in nanoparticle form are created by mixing the nanoparticles with liquid carrier, then forming the resultant mixture into respirable particles. Spray-drying, freeze spray drying and drying followed by comminution may be used to create the respirable particles, which may be delivered to the lung via a dry powder inhaler. In one example, lactose was used as the excipient and spray-dried with two different types of nanoparticle: gelatin and poly butylcyanoacrylate nanoparticles. The incorporation of nanoparticles did not affect the respirable fraction of the carrier powders.
Owner:FINLAY WARREN HUGH +2
Microcapsules
InactiveUS6818296B1Cosmetic preparationsOrganic detergent compounding agentsOil phaseAnionic polymers
A microcapsule having a mean diameter of from about 0.1 to about 5 mm, a membrane and a matrix containing at least one active principle wherein the microcapsule is the product of the process comprising the steps of (a) forming an aqueous matrix by heating an aqueous solution comprised of a gel former, a chitosan and active principle; (b) forming a dispersed matrix by adding the aqueous matrix in an oil phase; (c) contacting the dispersed matrix with an aqueous solution of an anionic polymer selected from the group consisting of a salt of alginic acid and an anionic chitosan derivative.
Owner:COGNIS IP MANAGEMENT GMBH
Pharmacologic-functioning water and usage of the same
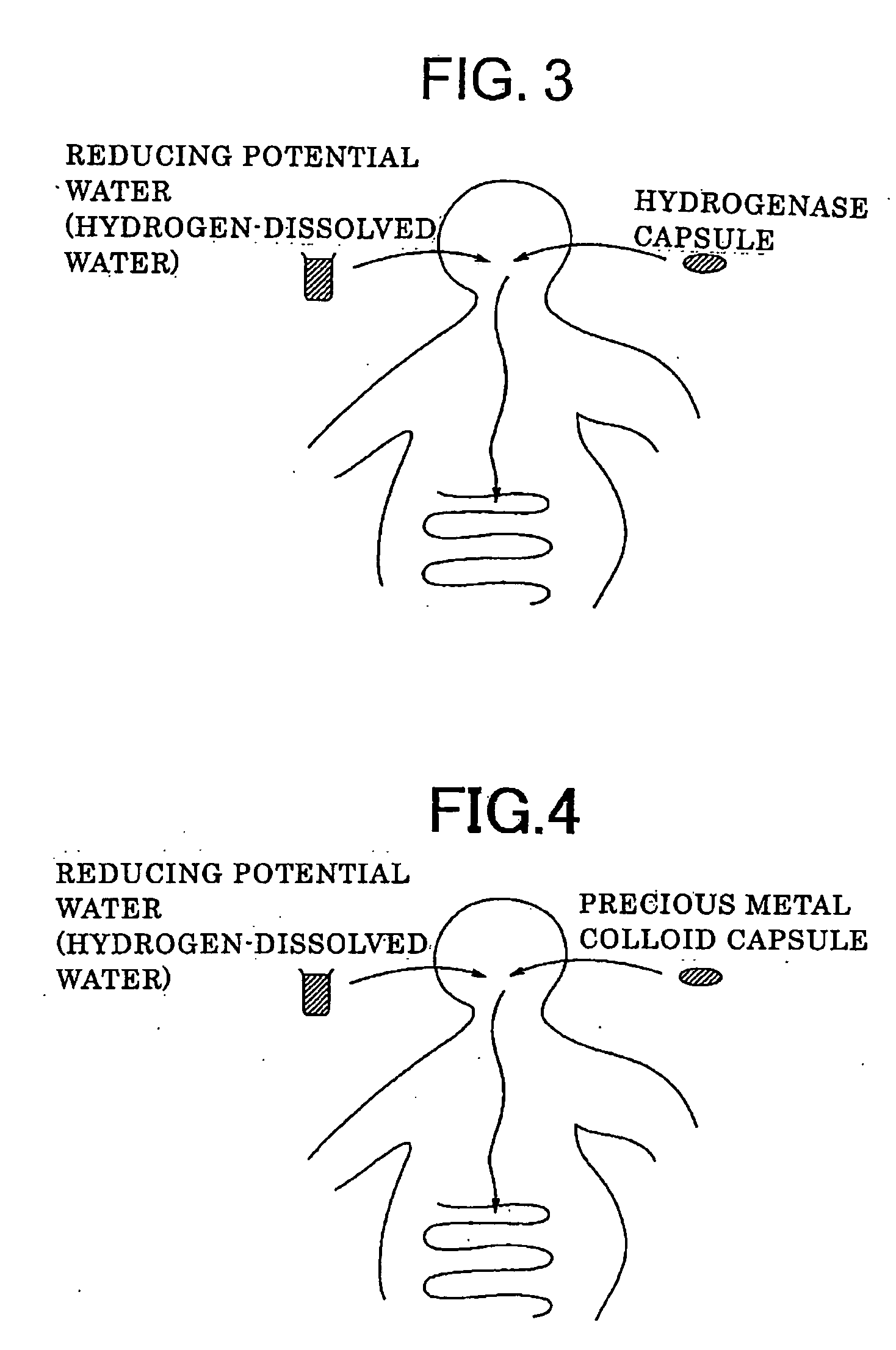
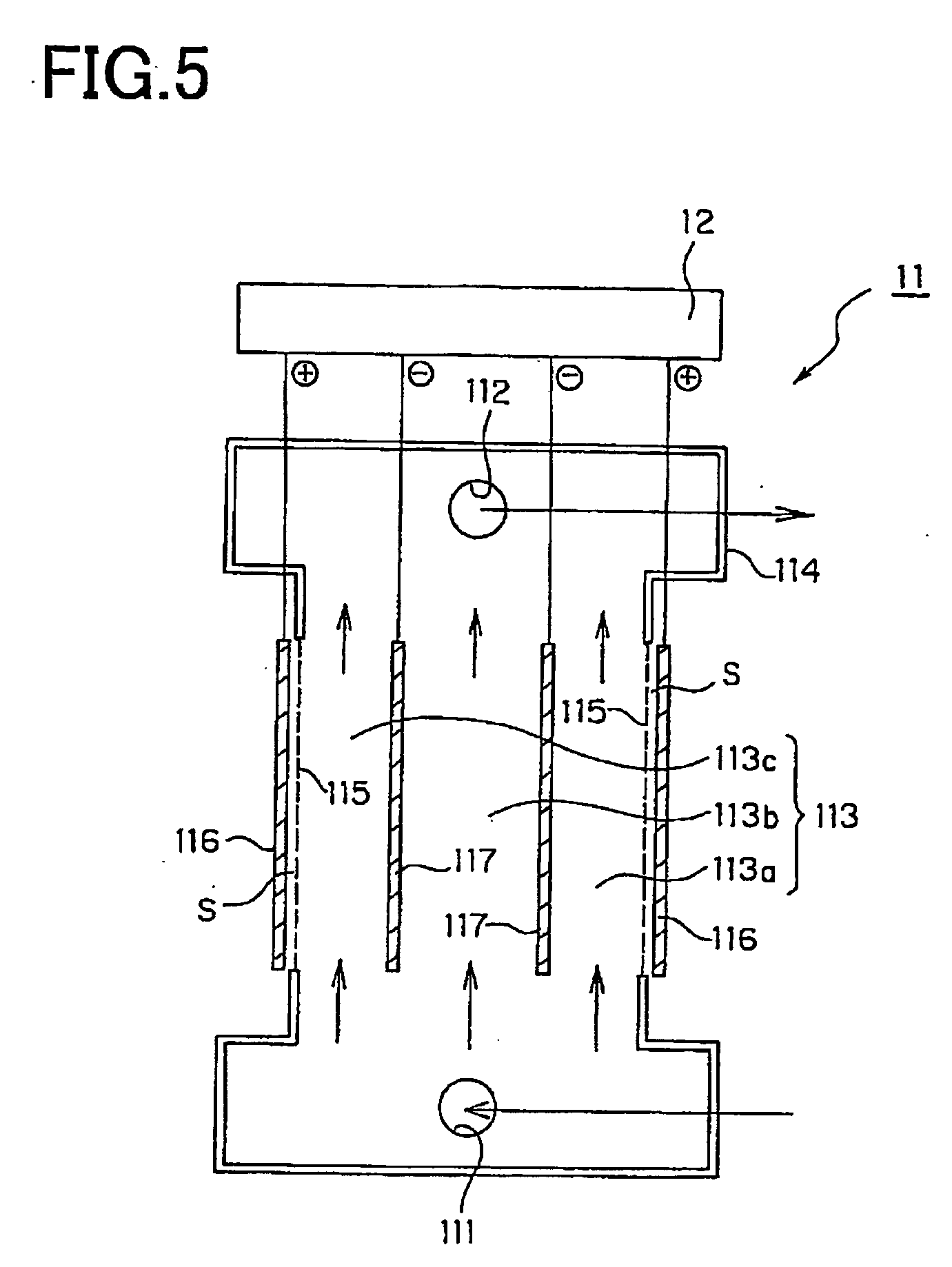
InactiveUS20070148256A1Excellent radical scavenging functionReduced activityOrganic active ingredientsSenses disorderHydrogenSide effect
The present invention provides a new pharmacologic-functioning water demonstrating pharmacologic function without any side effects, and usage of the same. The pharmacologic-functioning water, which demonstrates pharmacologic function without any side effects and includes antioxidant-functioning water as an active principle containing hydrogen-dissolved water, which is made up of molecular hydrogen used as a substrate that is included in raw water, and a precious metal colloid, which is included in the hydrogen-dissolved water and catalyzes the breaking reaction of the molecular hydrogen into a product of atomic hydrogen, is used for preventing and / or treating diseases.
Owner:MIZ CO LTD
Pharmaceutical compositions for gastrointestinal drug delivery
ActiveUS20090011019A1Extended stayOrganic active ingredientsDispersion deliveryControlled releaseImmediate release
A novel pharmaceutical composition, which comprises a therapeutically effective amount of active principle(s) or a pharmaceutically acceptable salt or enantiomer or polymorph thereof, optionally one or more release controlling agent(s) and pharmaceutical acceptable excipient(s) thereof, wherein the composition is formulated to increase the residence time of the said pharmaceutical composition and / or active principle(s) in the gastrointestinal tract. A novel pharmaceutical composition comprising at least two entities wherein one entity is an immediate release / fast release and the other is controlled release. A pharmaceutical composition comprising at least two entities wherein one entity is an immediate release / fast release and the other is bioadhesive. A pharmaceutical composition comprising: at least two entities wherein one entity is controlled release and the other is bioadhesive All the three compositions are formulated to increase the residence time of active principle(s) in the gastrointestinal tract. A multilayered composition with active in a layer which provides immediate release or controlled release of active principles and layer providing increased residence time in the GI tract.
Owner:LUPIN LTD
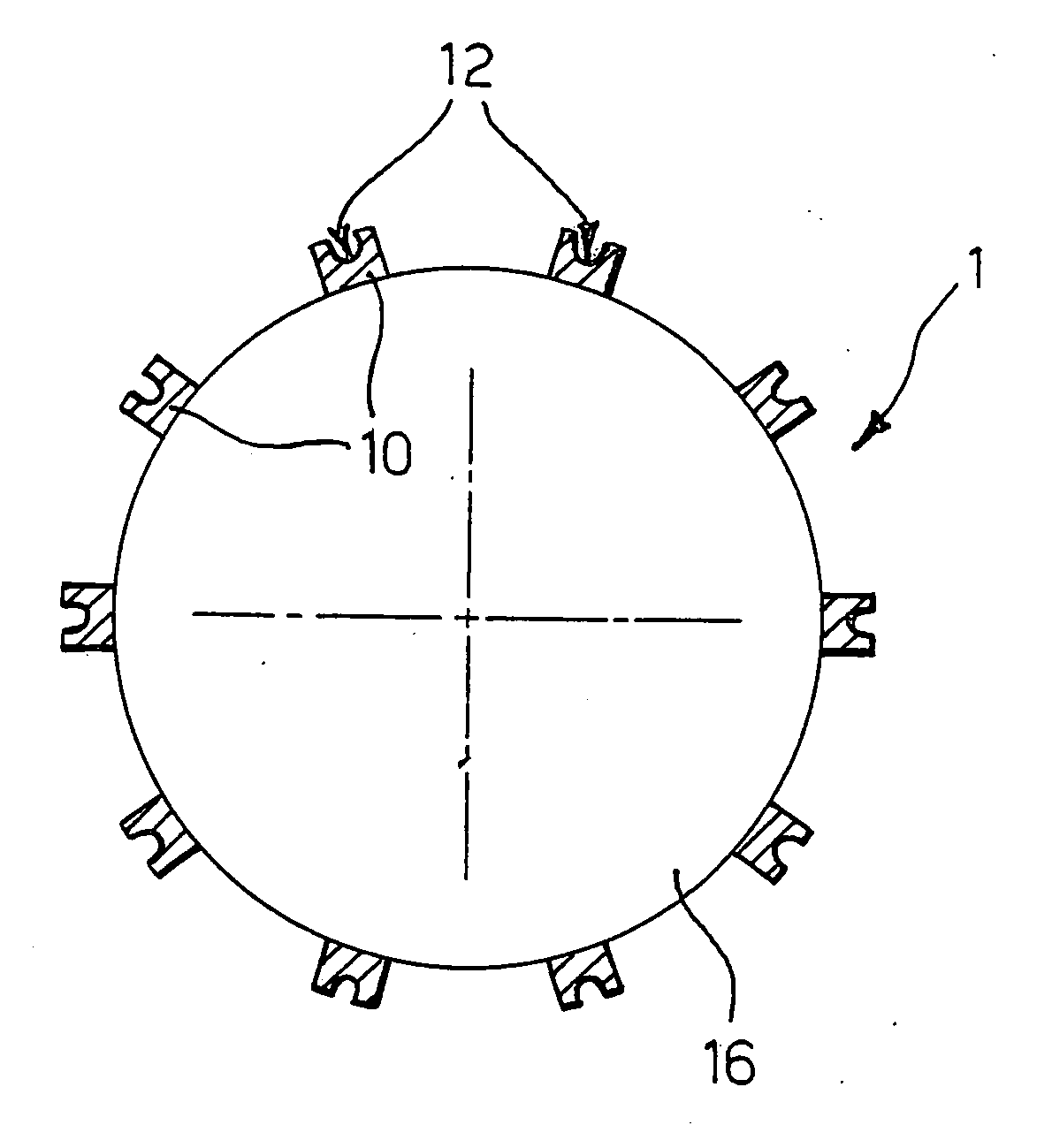
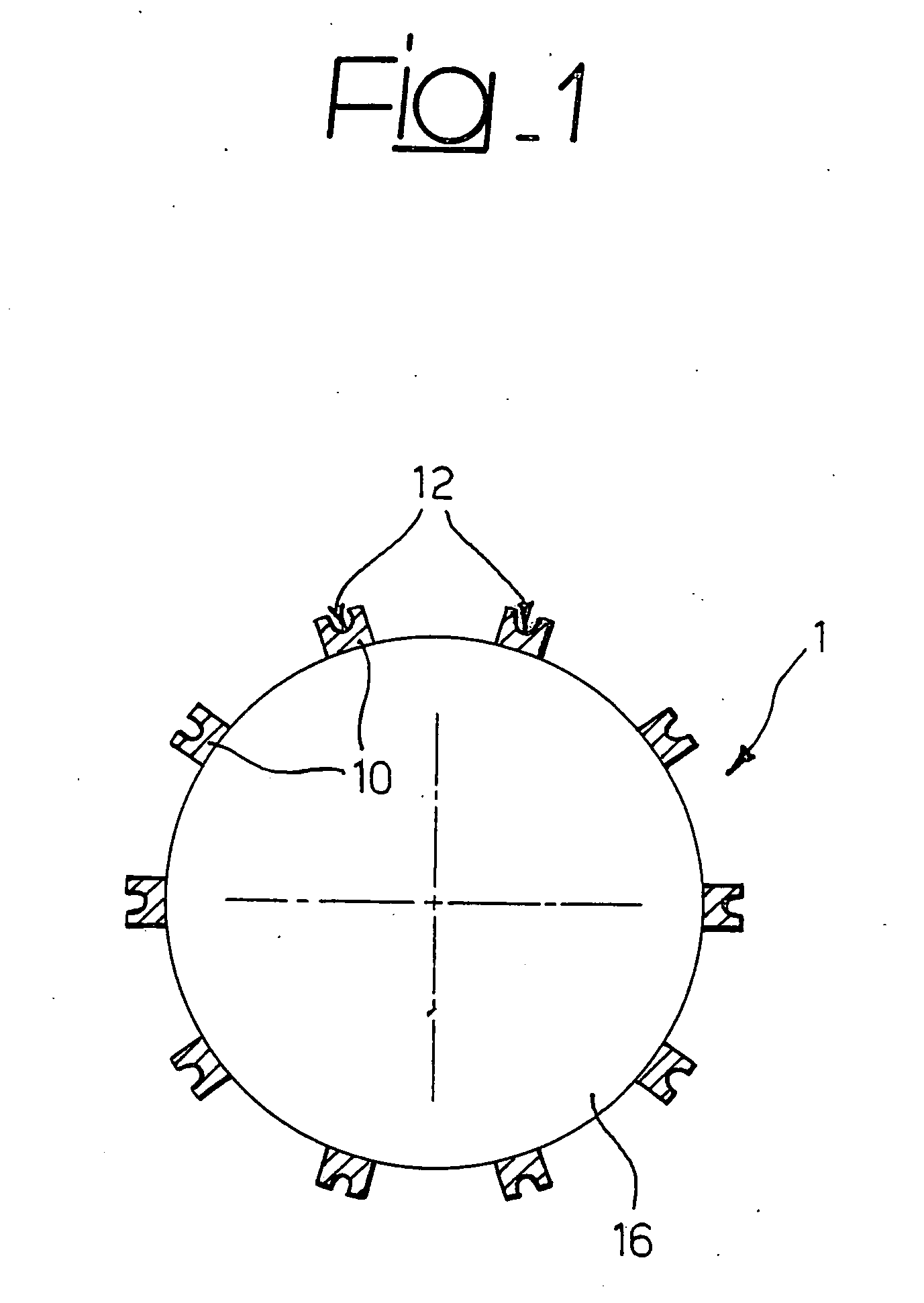
Delivery device, method of using and method of manufacturing
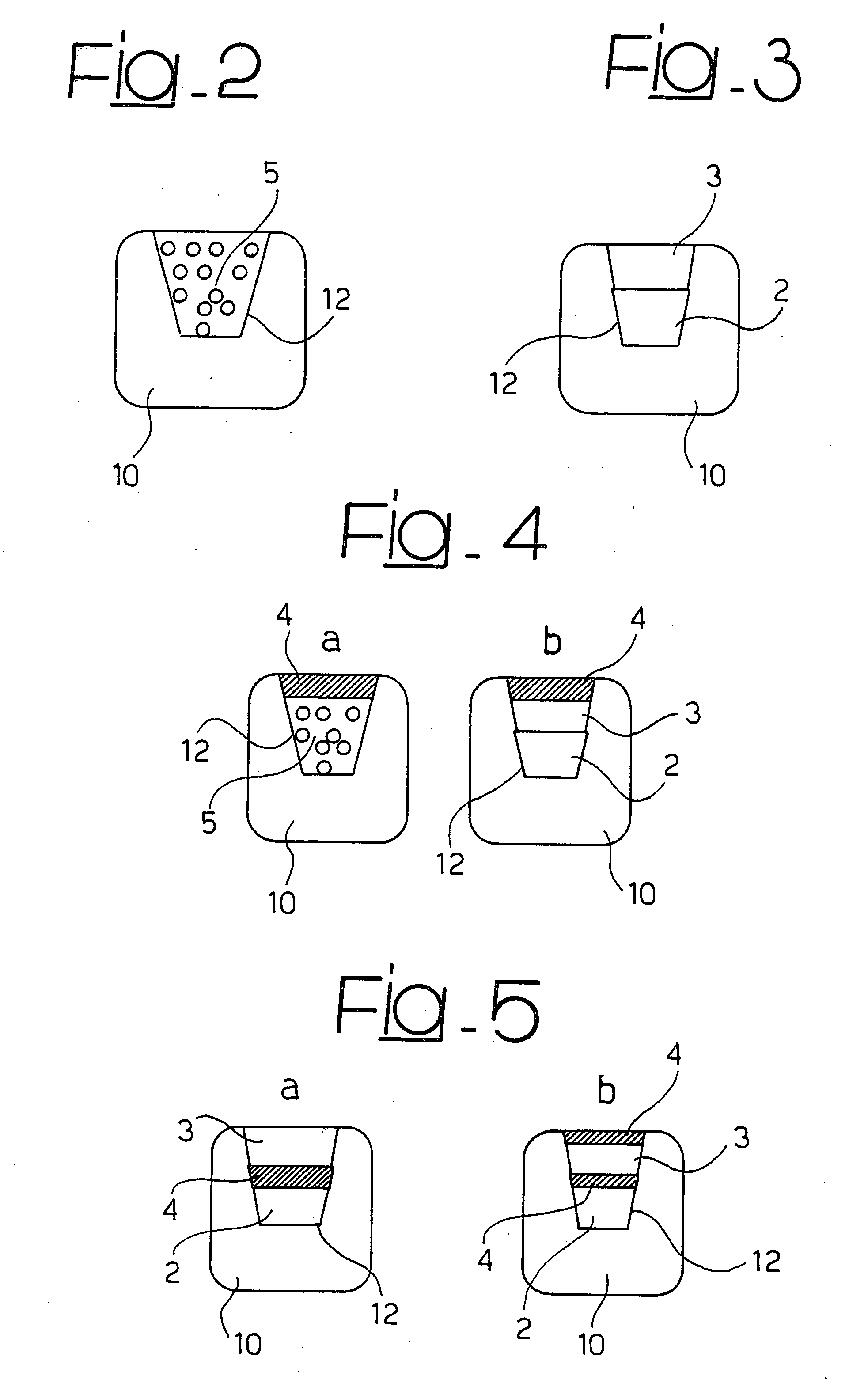
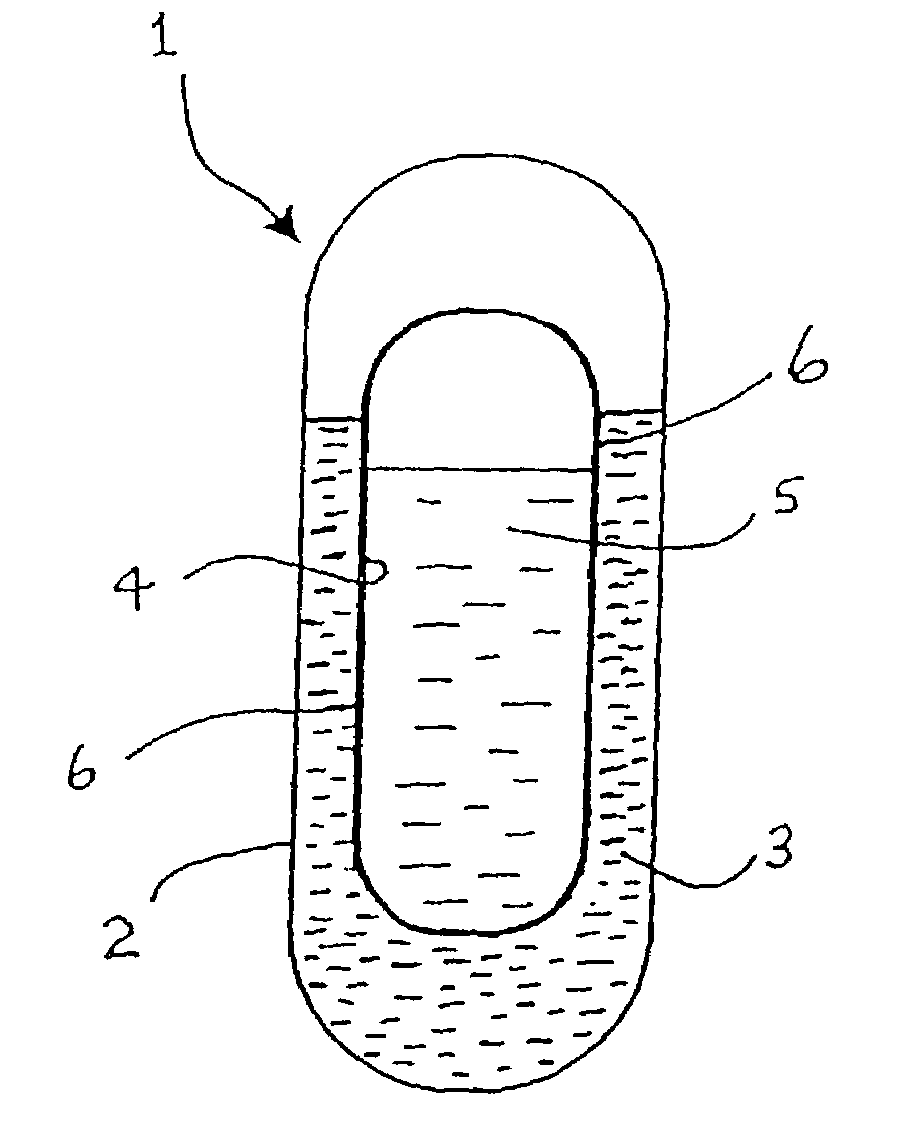
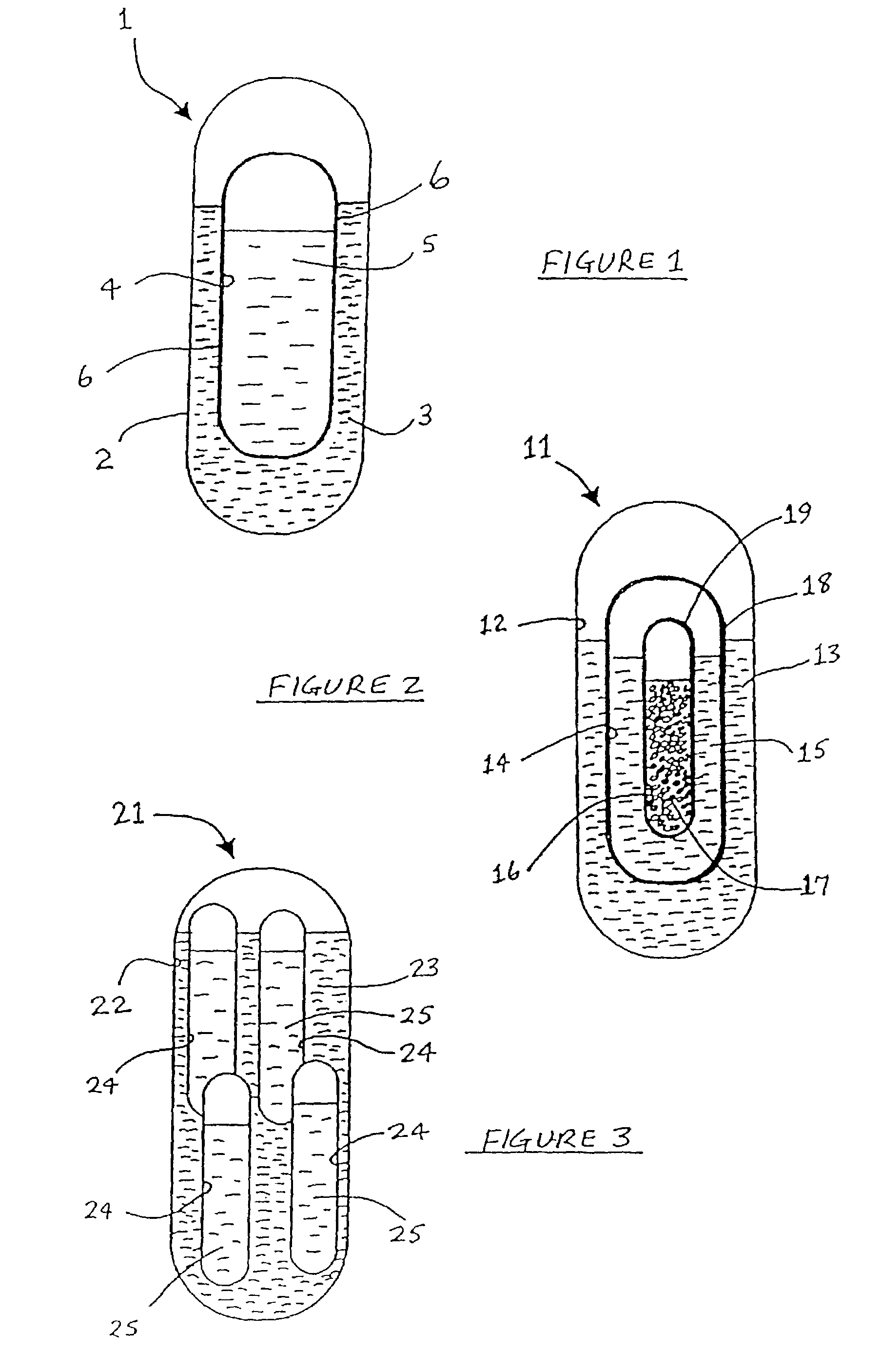
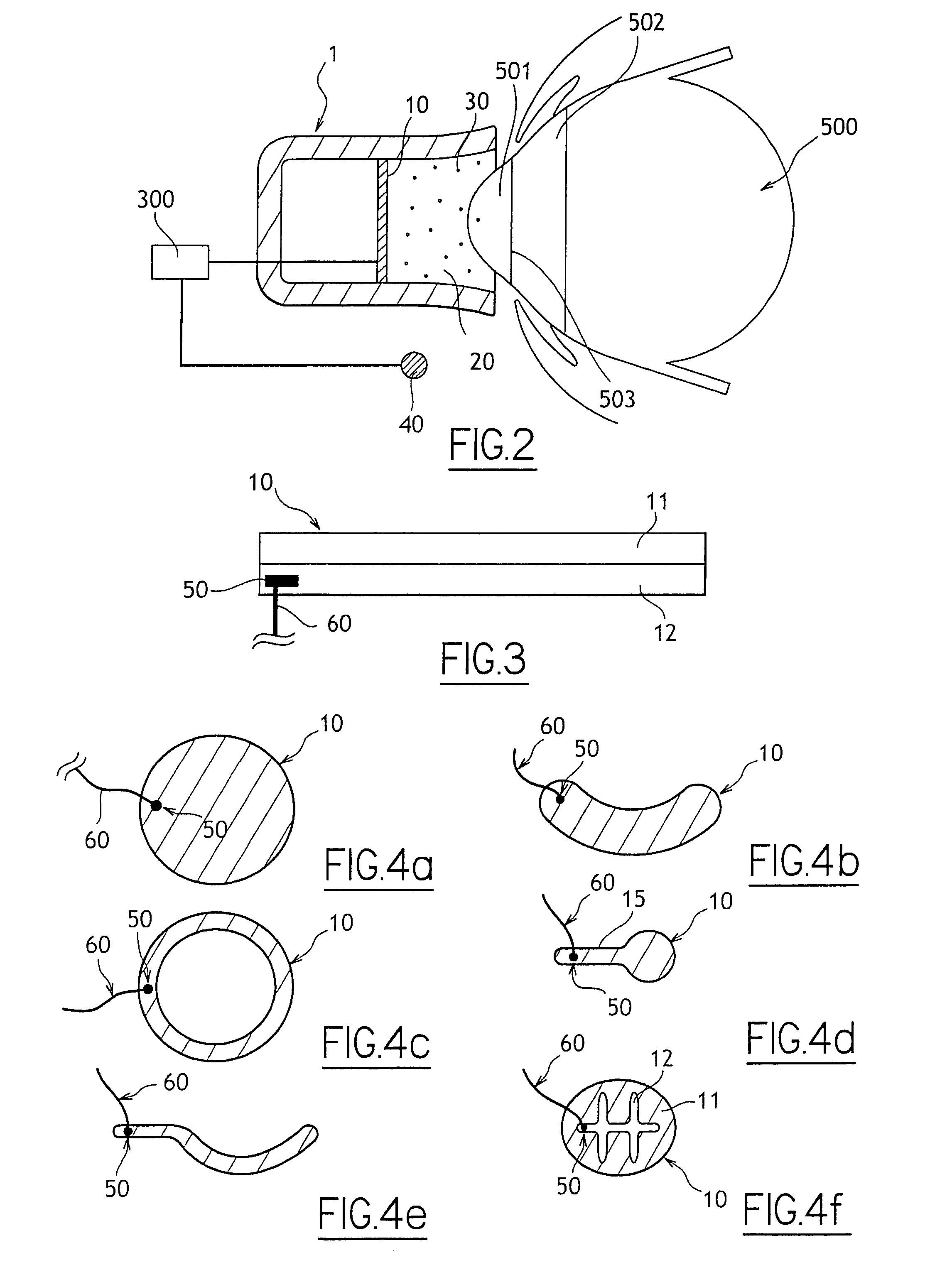
An active principle delivery device (1) comprising an inner capsule (4) within an outer capsule (2), the inner and outer capsules (4,2) containing the same active principle (5,3), with at least the outer capsule (2) being a hard capsule and the active principle (3,5) in at least one of the capsules (2,4), comprising a fluid. Also provided is a method of fabricating such a delivery device (1), as well as a method of controlling the pharmaco-kinetic profile of an active principle.
Owner:MW ENCAP
Stimulation of the activity of an isoform of lysyl oxidase for combating against some pathologies due to an incomplete, absent or disorganized elastogenesis
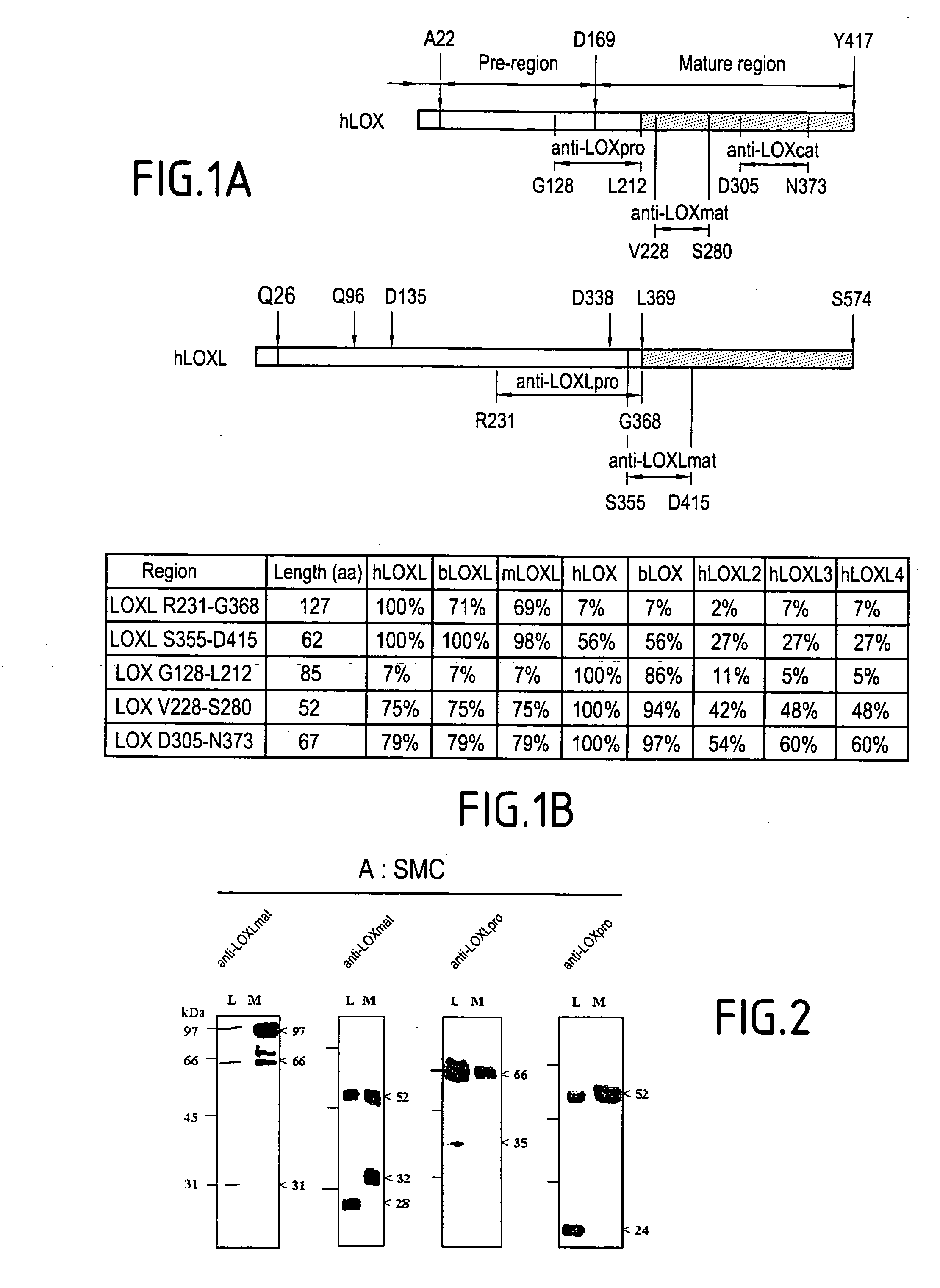
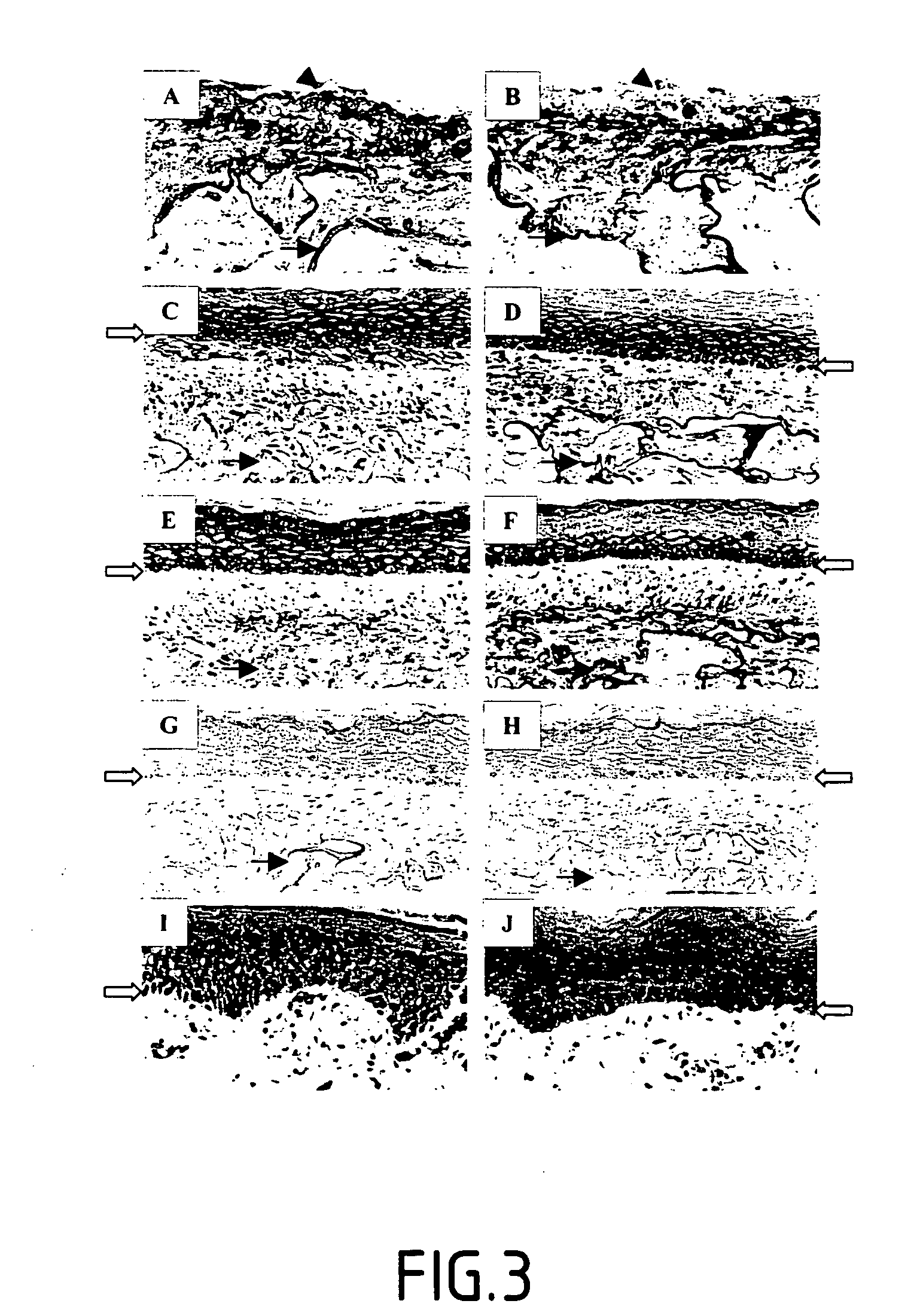
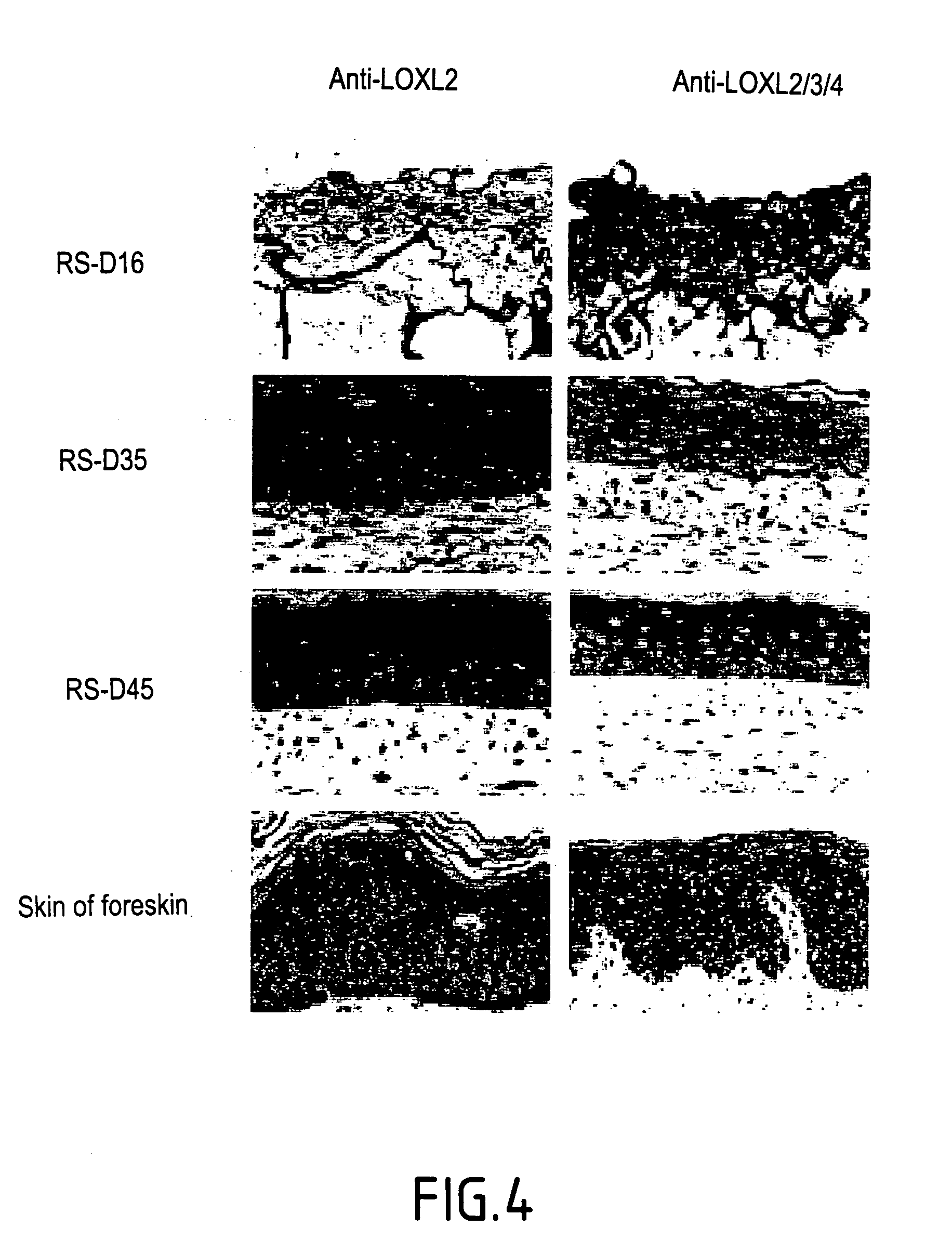
The invention relates to the stimulation of the activity of an isoform of lysyl oxidase, and more particularly of the LOX (lysyl oxidase) isoform. The invention relates notably to a screening method of an active principle which regulates elastogenesis in cases of pathological, disorganized and / or non-functional elastogenesis, as in cases of fibrosis, of solar elastosis, of stretch marks, and / or of dystrophic scars; and / or in cases of some eczematous pathologies. The aim of the invention is mainly to provide such a screening method so as to provide compositions enabling the elastogenesis in the cases cited to be regulated.
Owner:CENT NAT DE LA RECHERCHE SCI +1
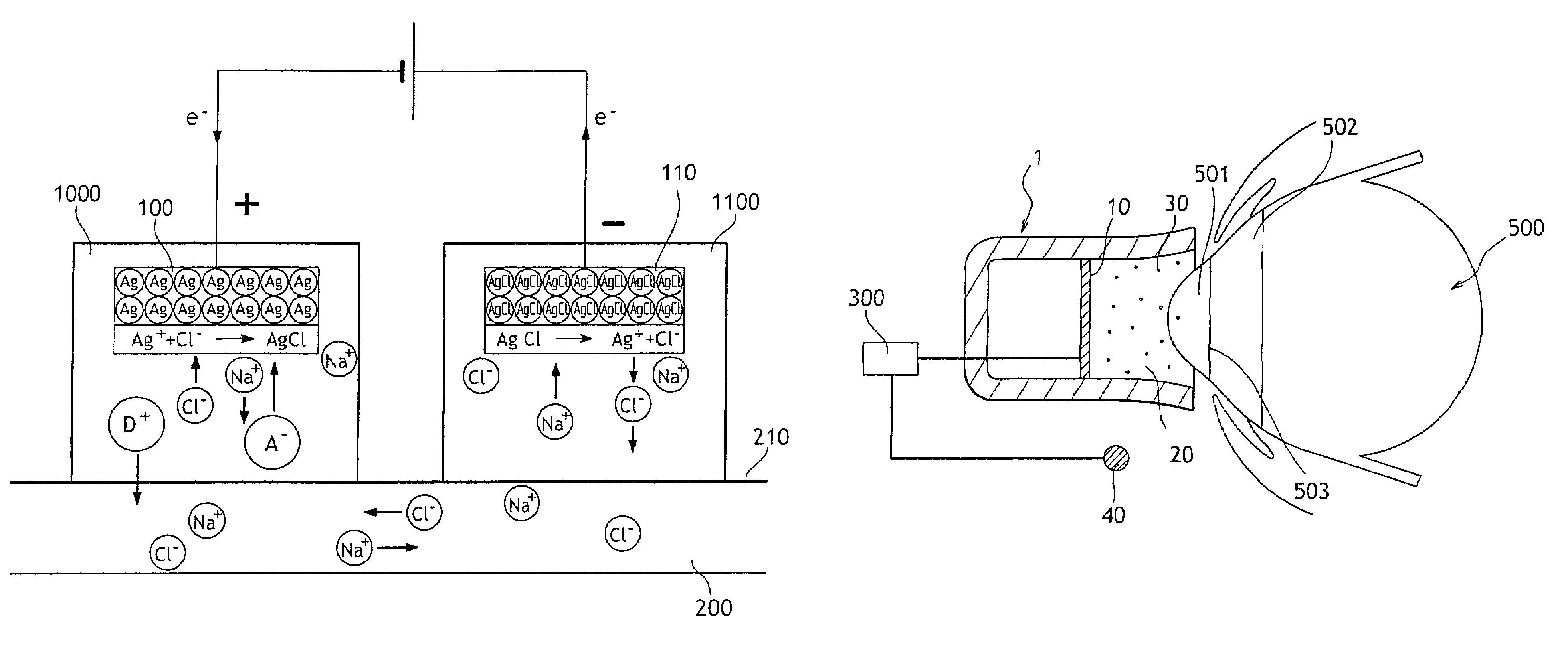
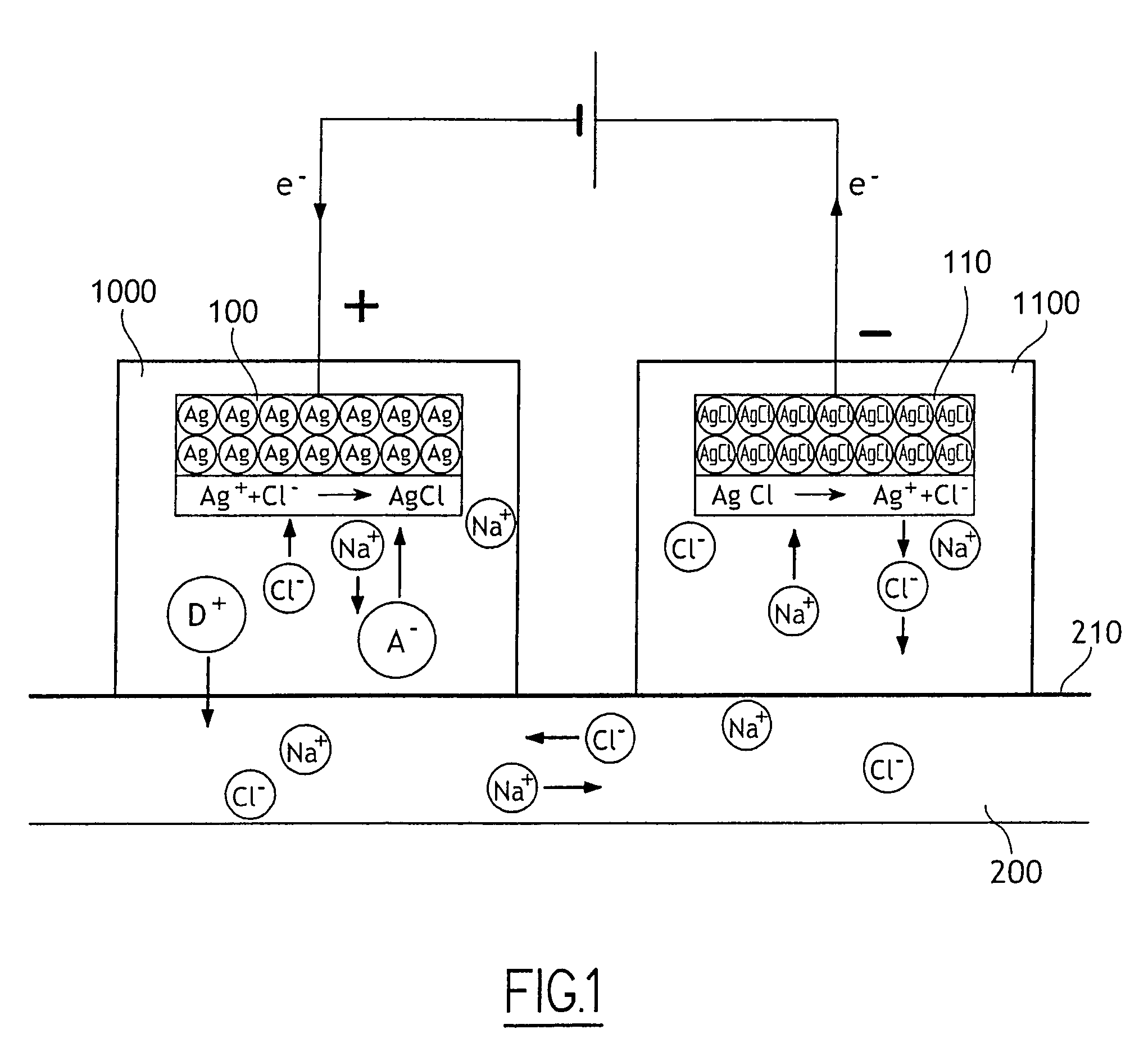
Irritation-reducing ocular iontophoresis device
The invention provides an ocular iontophoresis device for delivering medication, the device comprising a medication reservoir suitable for being positioned on the eye, at least one medication in solution in the reservoir, an active electrode disposed in the reservoir, and a passive electrode, the device including at least one medication dissolved in non-saline water, the said solution having a pH lying in the range 6.5 to 8.5, the medication having a pKa lying in the range about 5.5 to about 9.5 and includes an active principle which is associated with an additive, such as a dendrimer, a polymer, a nanoparticle, a microsphere, a liposome, or an emulsion, and having an ionic form of valency greater than or equal to 1.
Owner:EYEGATE PHARMA SA
Pharmaceutical composition comprising a solid dispersion with a polymer matrix containing a continuous polydextrose phase and a continuous phase of a polymer other than polydextrose
ActiveUS20070243257A1Improve bioavailabilityPowder deliveryPharmaceutical non-active ingredientsPolydextroseAqueous medium
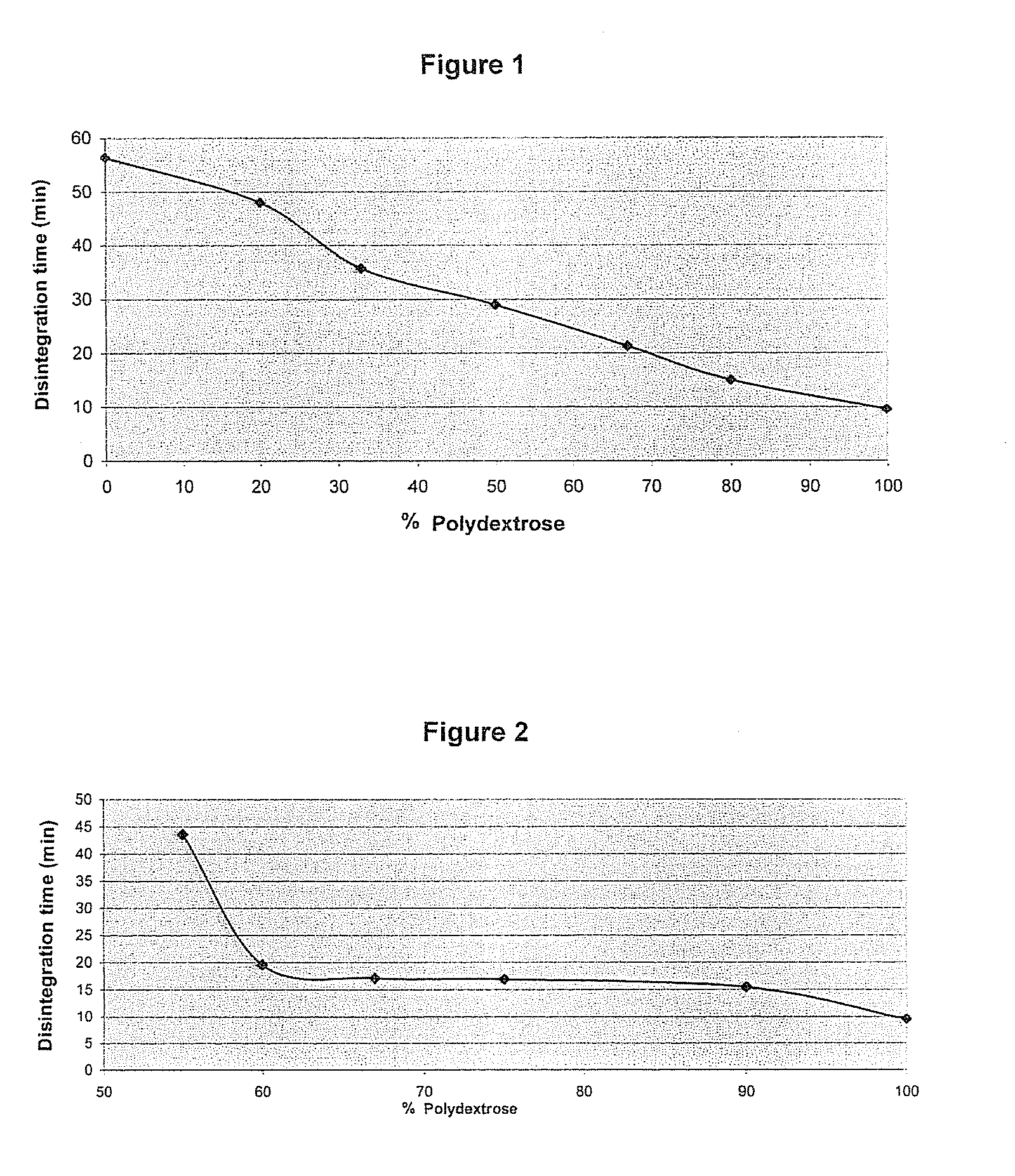
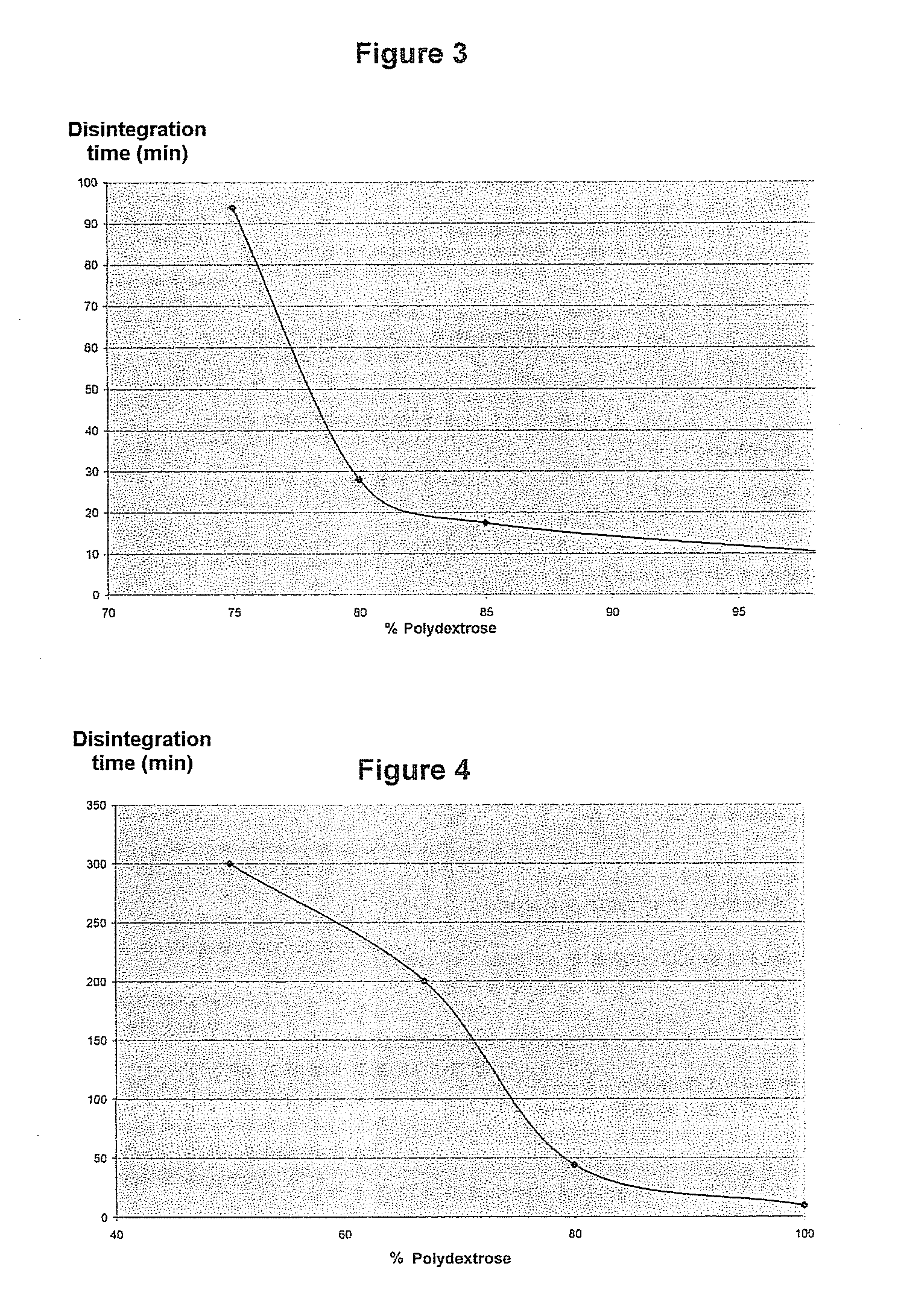
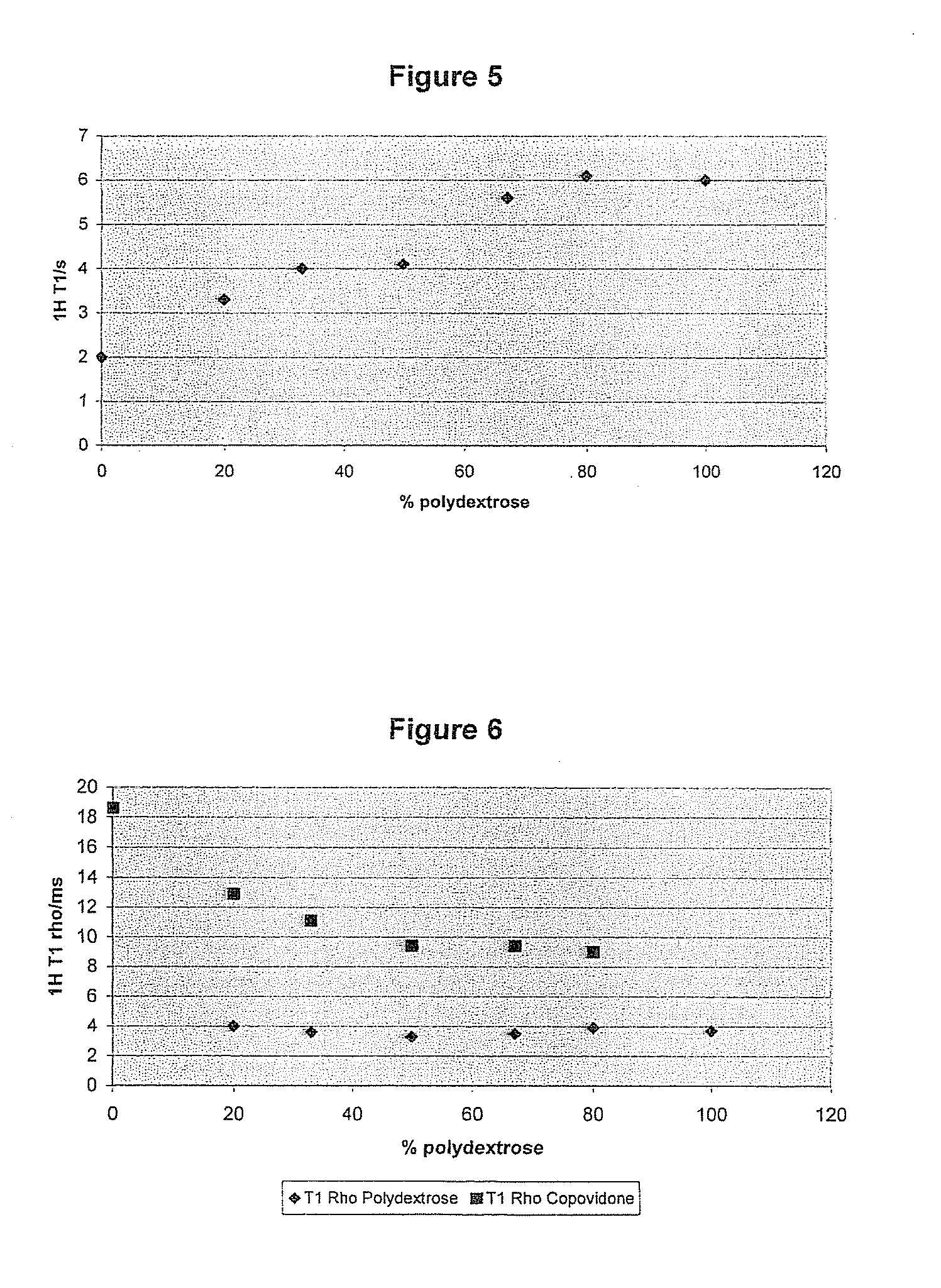
The present invention relates to a solid pharmaceutical composition comprising a solid dispersion containing at least one active principle and a pharmaceutically acceptable polymer matrix, a characterized in that said pharmaceutically acceptable polymer matrix comprises a blend of (i) polydextrose, in the form of a continuous polydextrose phase, in order to promote the disintegration of the composition in an aqueous medium, and (ii) at least one polymer other than polydextrose, in the form of a continuous phase of this polymer, whereby the polydextrose is in a concentration of at least 20 wt % and the at least one polymer other than polydextrose is in a concentration of at least 20 wt % in relation to the total weight of said pharmaceutically acceptable polymer matrix.
Owner:SANOFI SA
Flours produced from fungus myceliated grain
The present patent relates to a method for producing flours from grain myceliated with macroscopic fungi (mushrooms). These flours can be used to prepare food for human consumption, such as bread and biscuits, and for animal consumption, such as fodder. Active principles (ergosterol, beta glucan, linoleic and oleic acids, lectins), enzymes, proteins, amino acids, vitamins, mineral salts, inter alia, can also be extracted from these flours for use in the chemical, foodstuff and cosmetic industries, for producing phytotherapeutic agents, pharmaceuticals, textiles, paper products, pharmaceuticals and fodder for animals.
Owner:EMPRESA BRASILEIRA DE PESQUISA AGROPECUARIA EMBRAPA +2
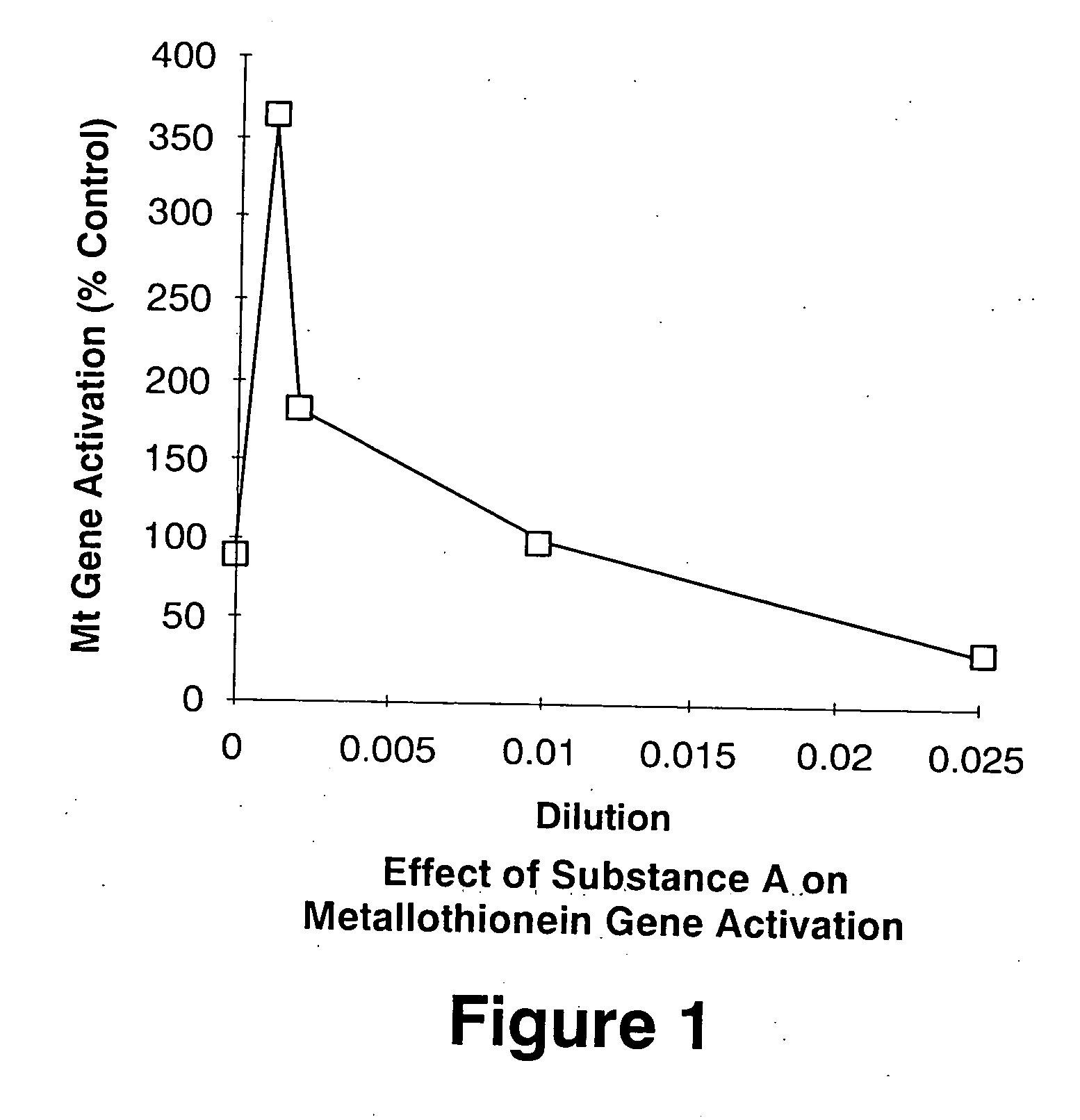
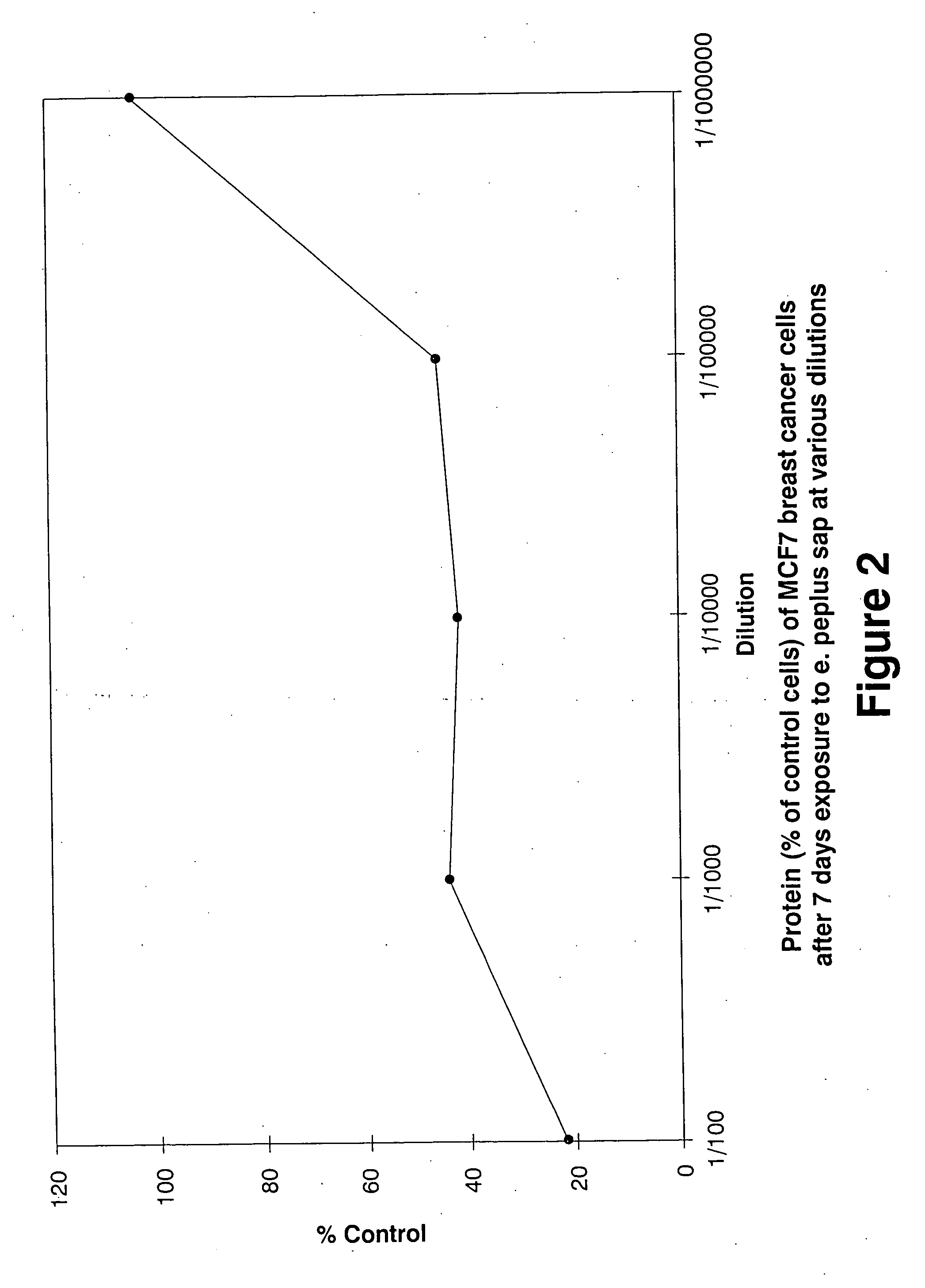
Anti-cancer compounds
This invention relates to a compound or group of compounds present in an active principle derived from plants of species Euphorbia peplus, Euphorbia hirta and Euphorbia drummondii, and to pharmaceutical compositions comprising these compounds. Extracts from these plants have been found to show selective cytotoxicity against several different cancer cell lines. The compounds are useful in effective treatment of cancers, particularly malignant melanomas and squamous cell carcinomas (SCCs). In a preferred embodiment of the invention, the compound is selected from the group consisting of jatrophanes, pepluanes, paralianes and ingenanes, and pharmaceutically-acceptable salts or esters thereof, and more particularly jatrophanes of Conformation II.
Owner:AF 30 APRIL 2003 +1
Device for Delivery of Active Principles
InactiveUS20080292684A1Limit water evaporationEasy to transportAntipyreticAnalgesicsMedicineWater soluble
A device for the delivery of active principles for dermal and, in particular, transdermal application having the form of a two-layered patch consisting of a first layer having a homogeneous composition and comprising at least one active principle, a water-soluble film-forming agent and a hydrophilic adhesive polymer, and of a second layer joined in a permanent manner to the first and having a water vapour permeability of less than 500 g / m2 in 24 hours.
Owner:LAB ITAL BIOCHIM FARM LISAPHARMA
Process of making and using composition containing oxidation-sensitive hydrophilic active principle and maleic anhydride copolymer
The invention relates to the use of a composition for, e.g., promoting the synthesis of the epidermal ceramides and / or for improving the barrier function of the skin, the composition containing at least one oxidation-sensitive hydrophilic active principle selected from the group consisting of ascorbic acid and its compounds and at least one maleic anhydride copolymer. The invention also relates to a cosmetic process for moisturizing the skin comprising the application to the skin of a composition as defined above.
Owner:LOREAL SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com