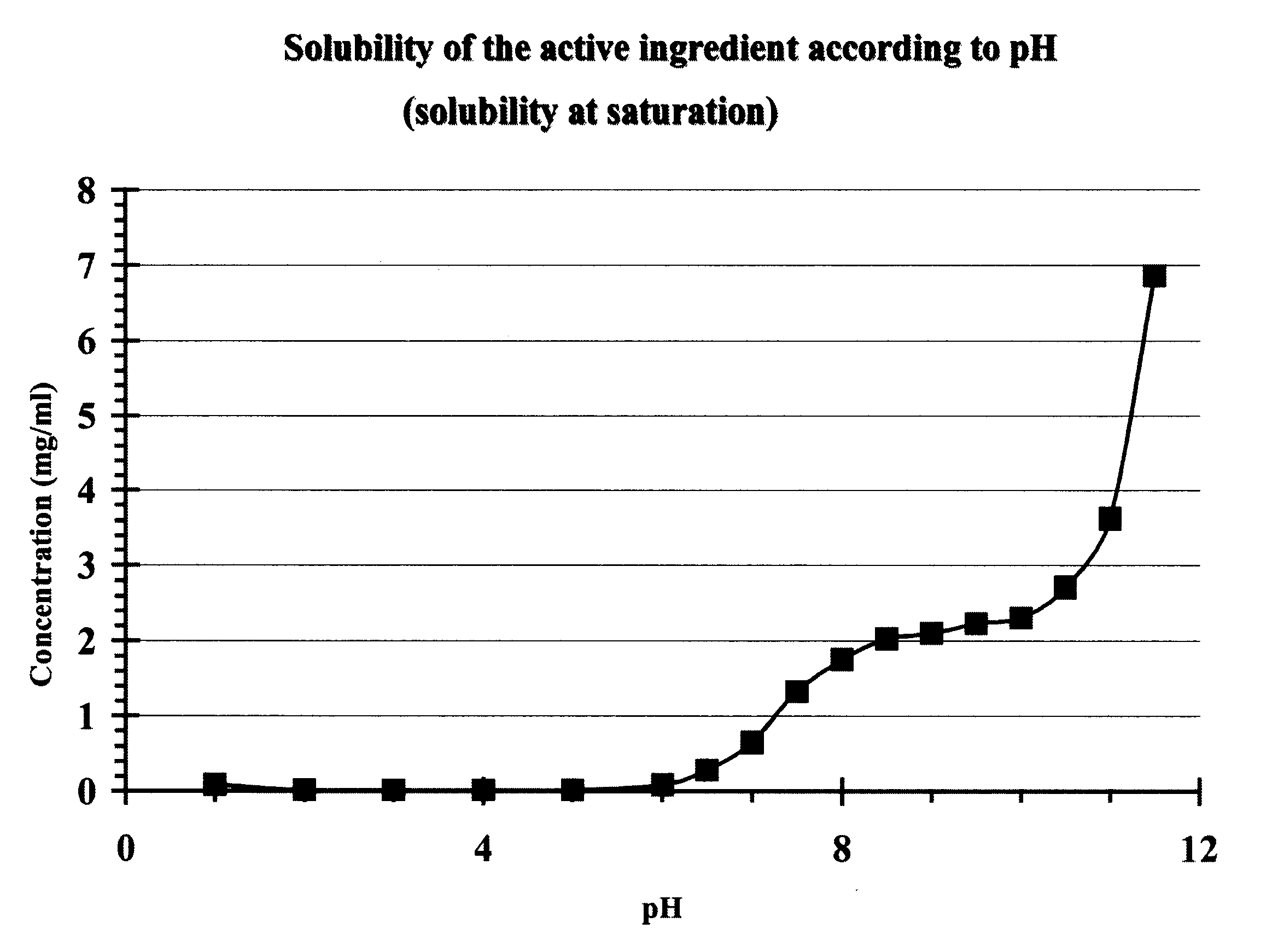
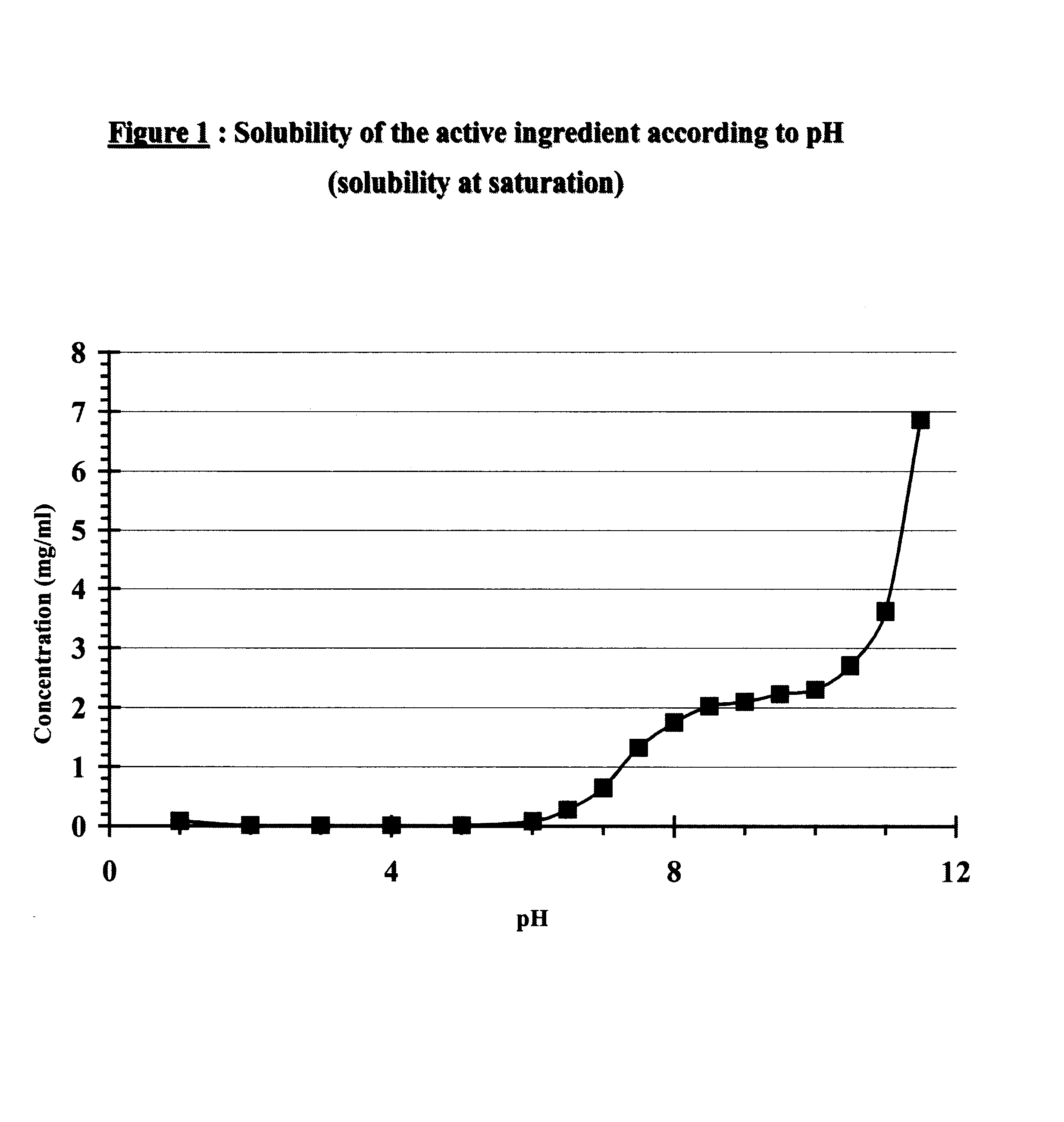
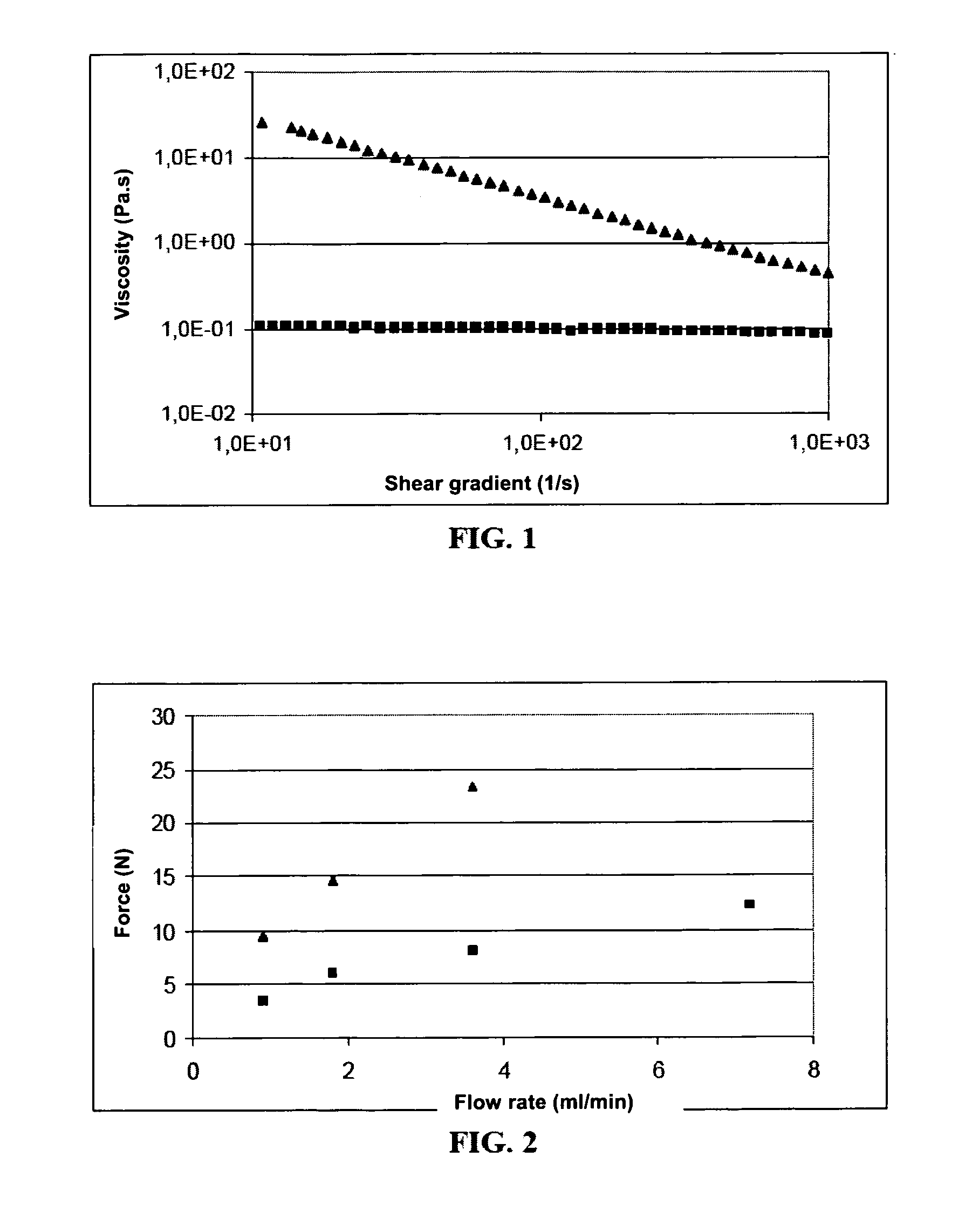
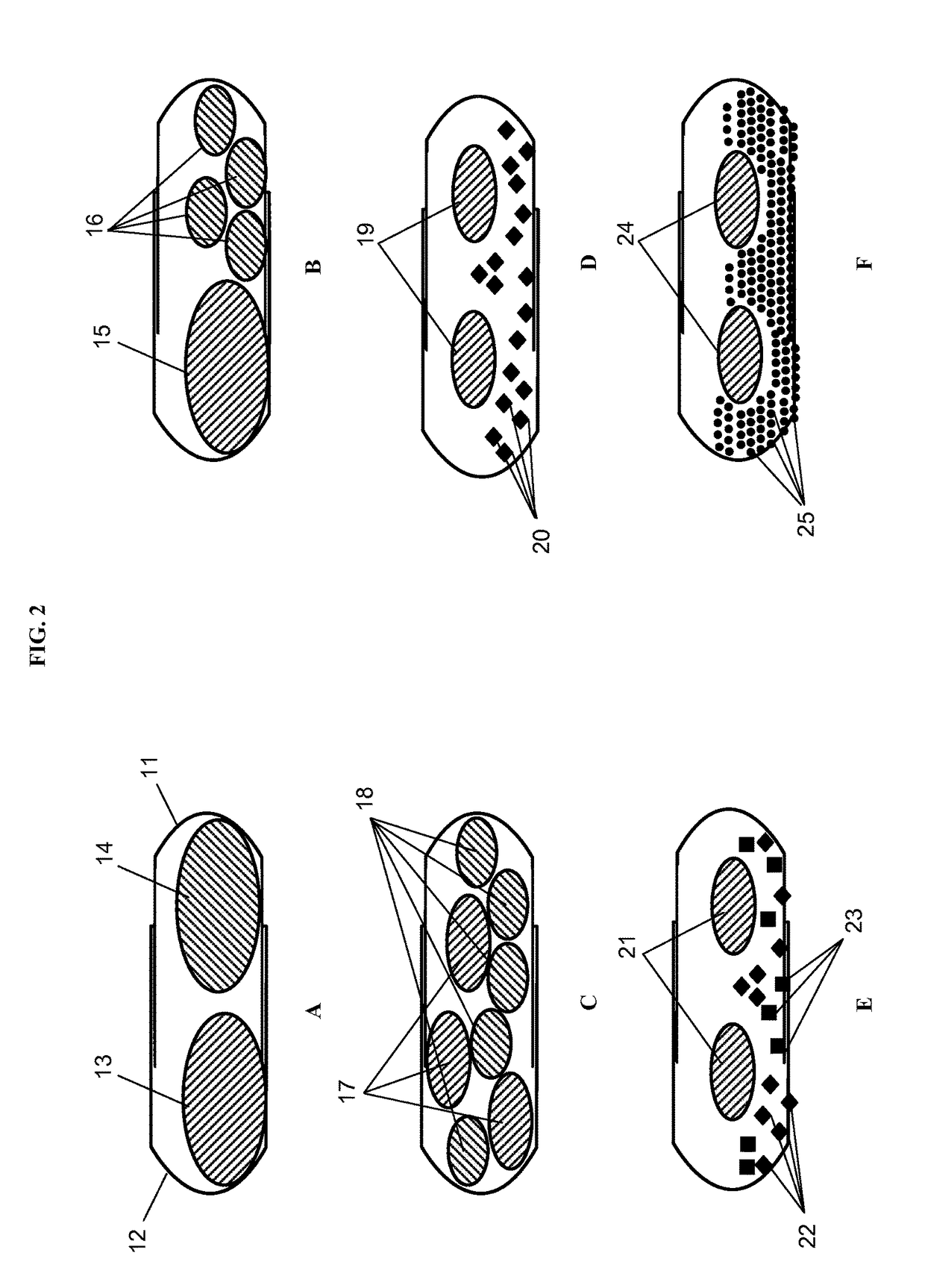
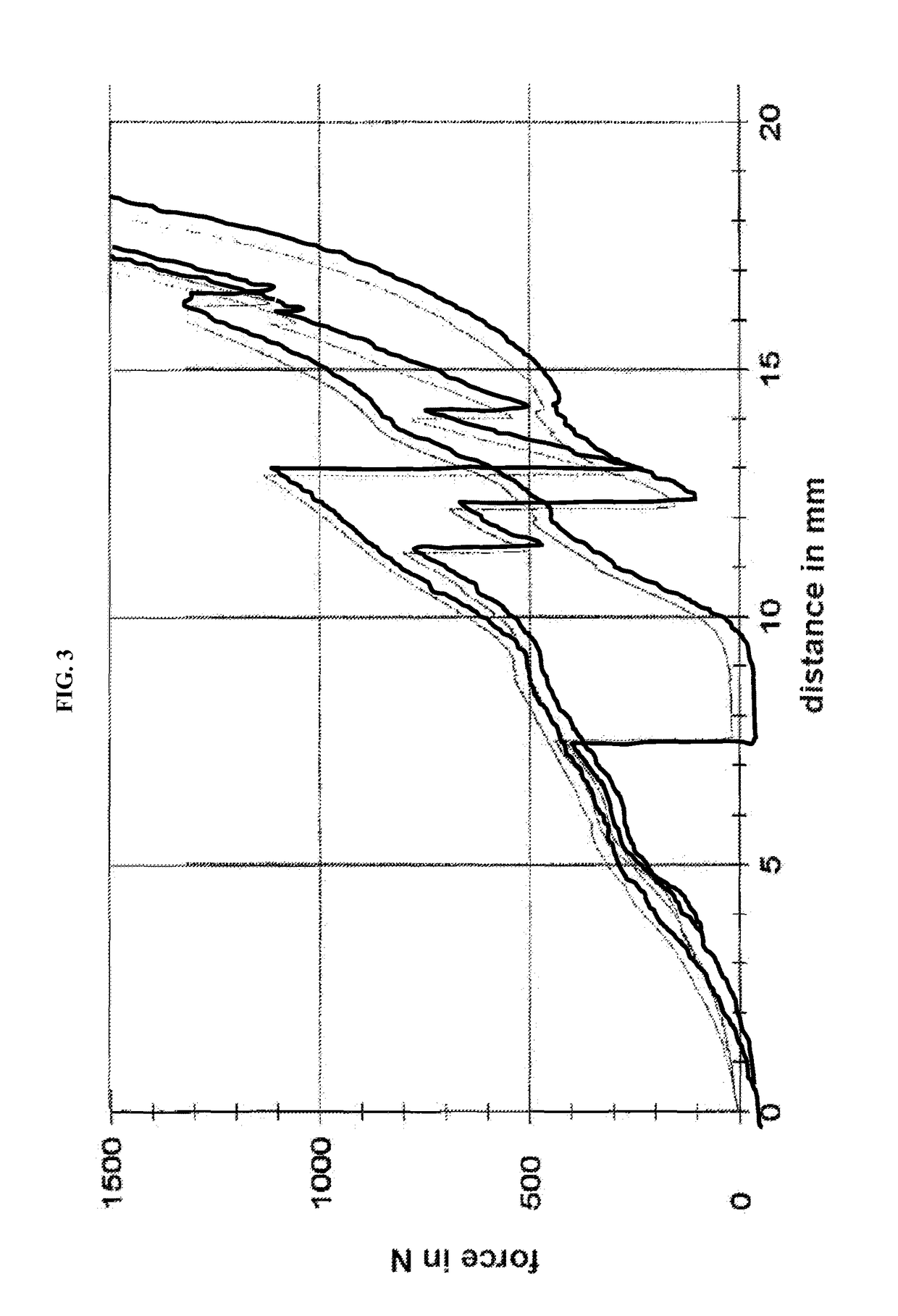
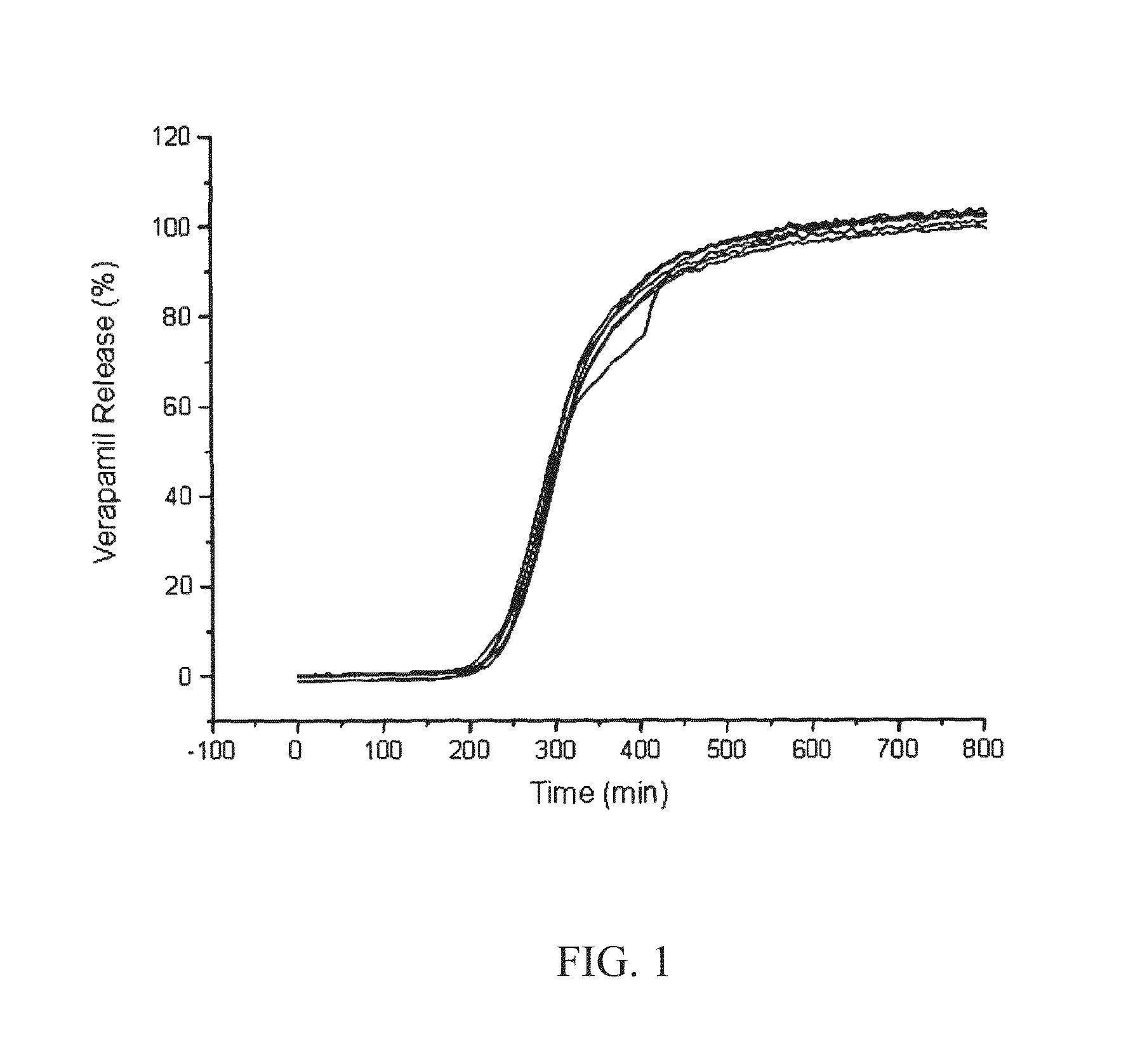
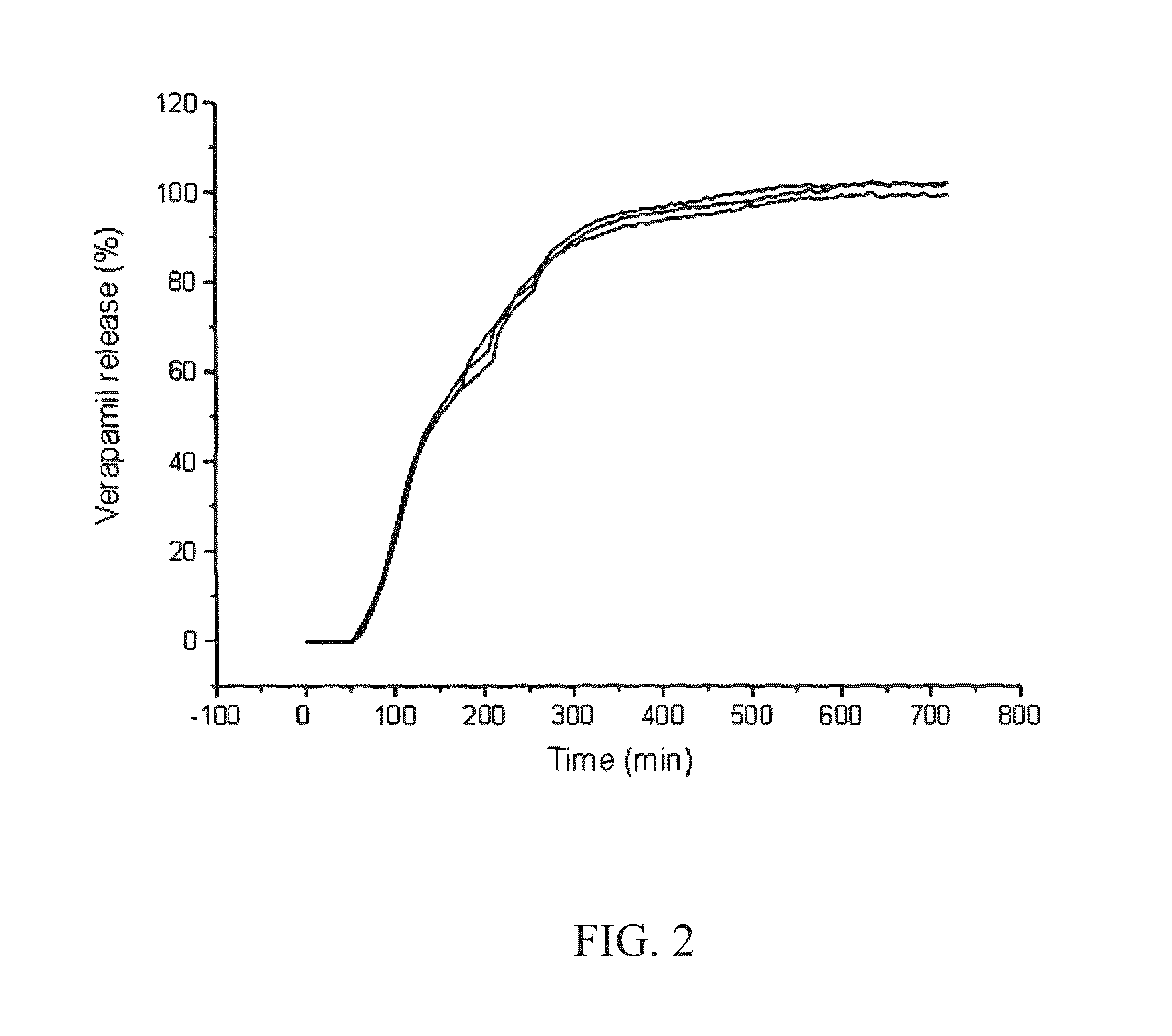
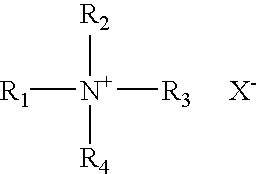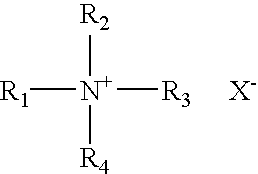Patents
Literature
150 results about "Prolonged release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prolonged release. a term applied to a drug that is designed to deliver a dose of a medication over an extended period.
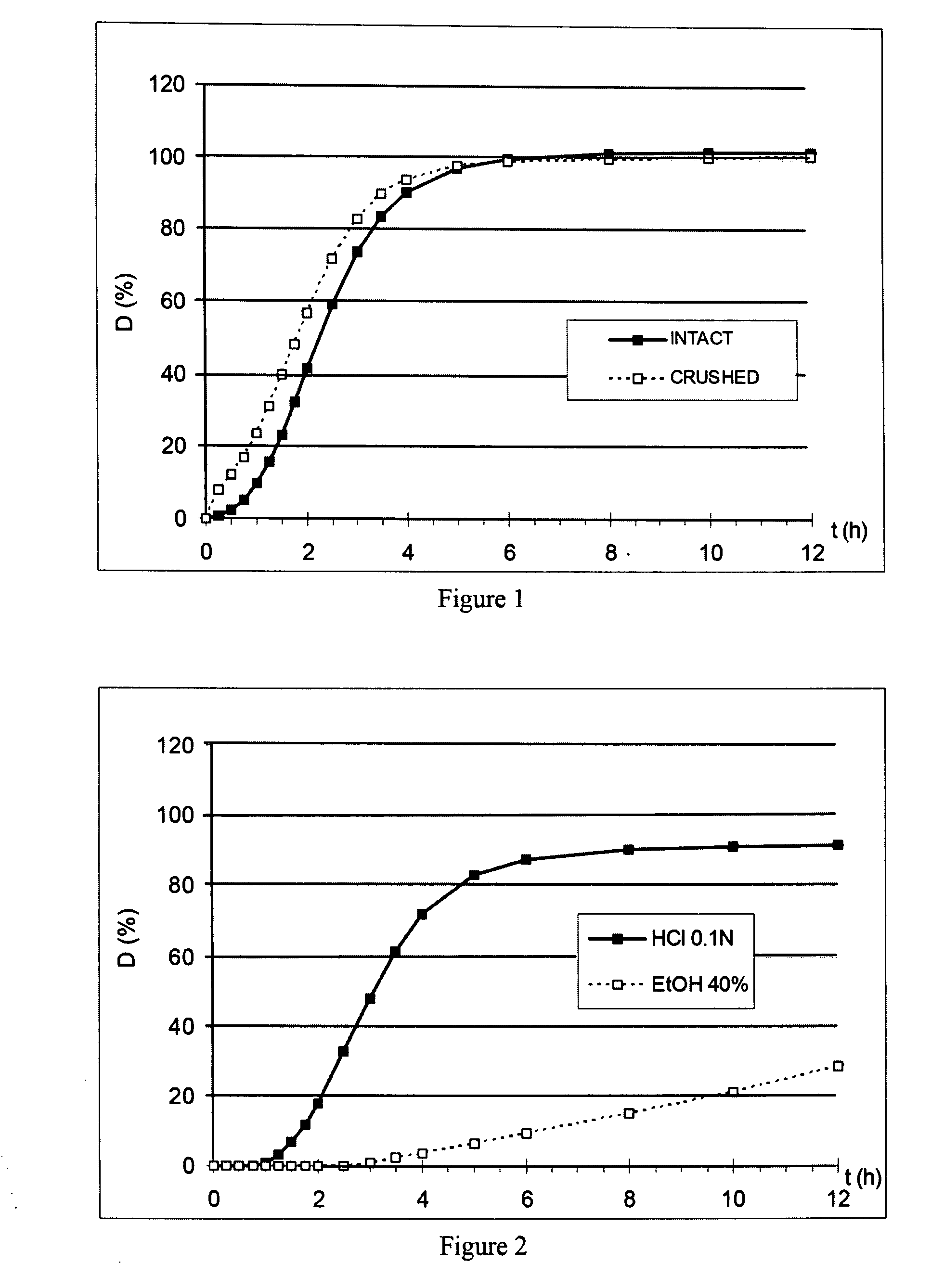
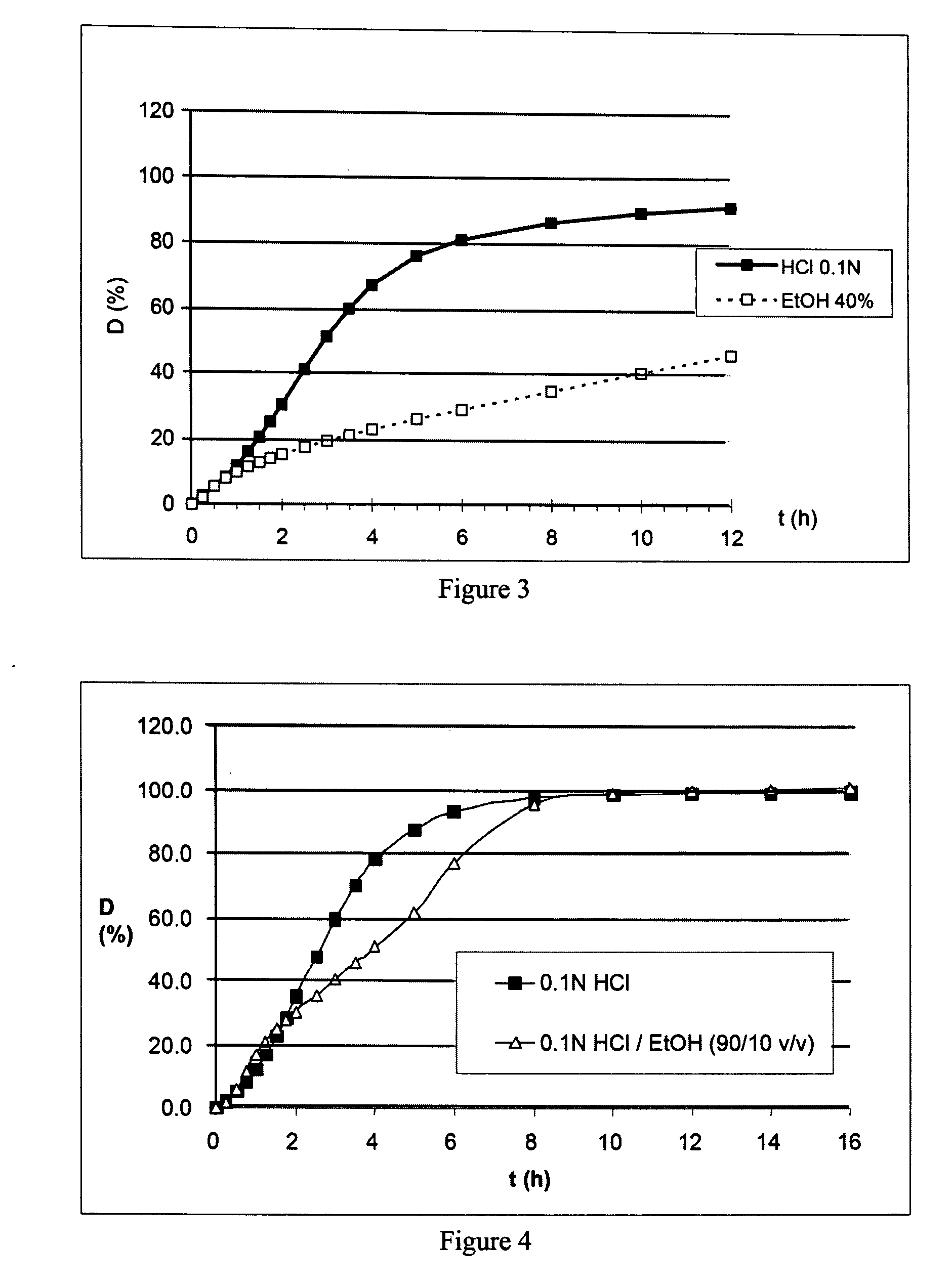
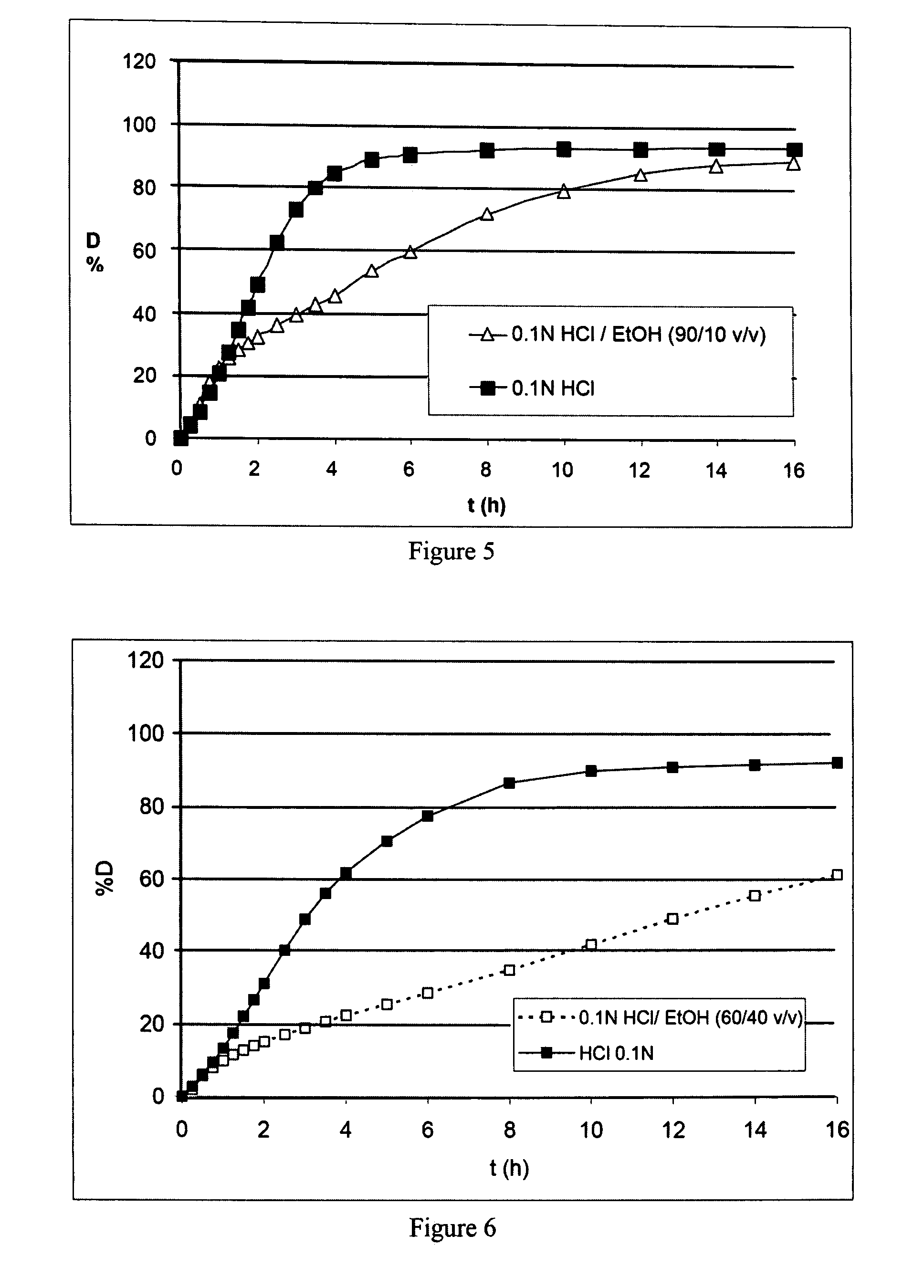
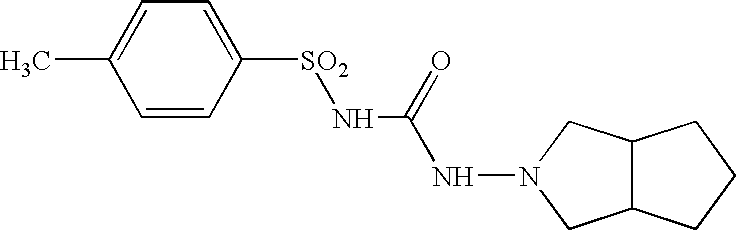
Prolonged-release multimicroparticulate oral pharmaceutical form
InactiveUS20080063725A1Prevent fraudulent abuse of propertyDifficult to administerBiocideNervous disorderProlonged releaseActive principle
Modified-release multimicroparticulate pharmaceutical form capable of maintaining the modified release of the active principle in an alcoholic solution and of resisting attempts at misuse.
Owner:FLAMEL IRELAND
Dual drug dosage forms with improved separation of drugs
InactiveUS20050013863A1Reduces and prevents any migrationReduce penetrationCoatingsBlood disorderMedicineImmediate release
Drug tablets that include a prolonged-release core and an immediate-release layer or shell are prepared with a thin barrier layer of drug-free polymer between the prolonged-release and immediate-release portions of the tablet. The barrier layer is penetrable by gastrointestinal fluid, thereby providing full access of the gastrointestinal fluid to the prolonged-release core, but remains intact during the application of the immediate-release layer, substantially reducing or eliminating any penetration of the immediate-release drug into the prolonged-release portion.
Owner:DEPOMED SYST INC
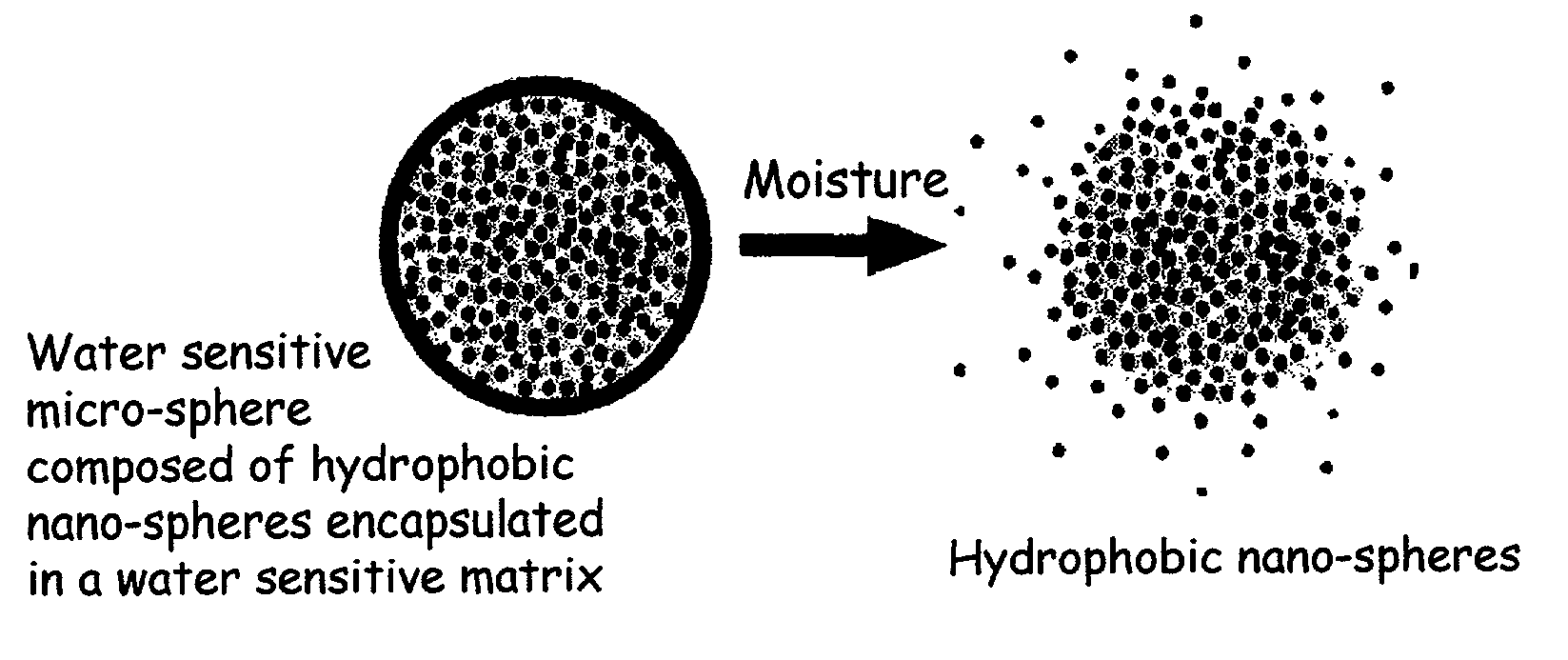
Multi component controlled release system for anhydrous cosmetic compositions
InactiveUS7115282B2Facilitated releaseProcess stepBiocideCosmetic preparationsActive agentProlonged release
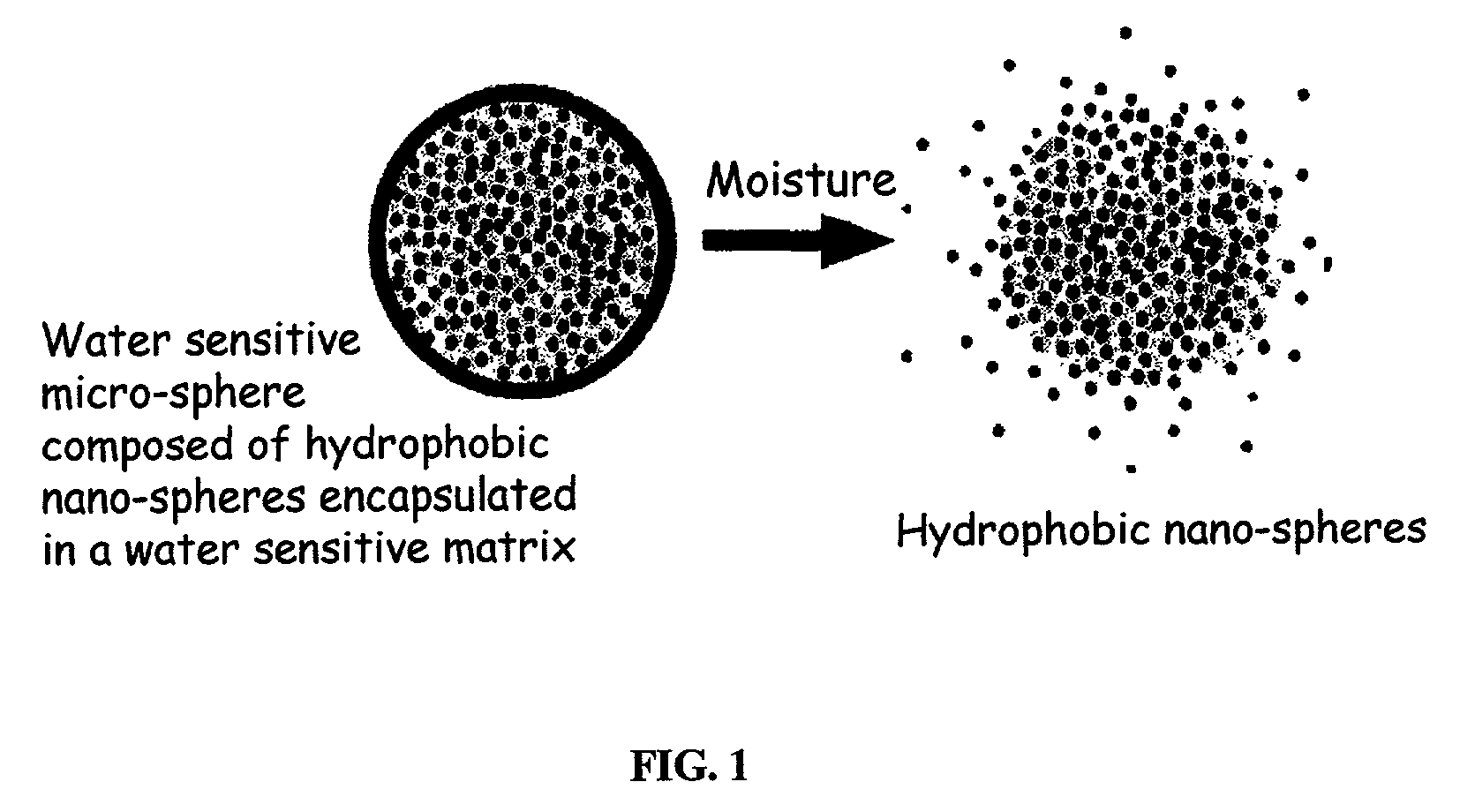
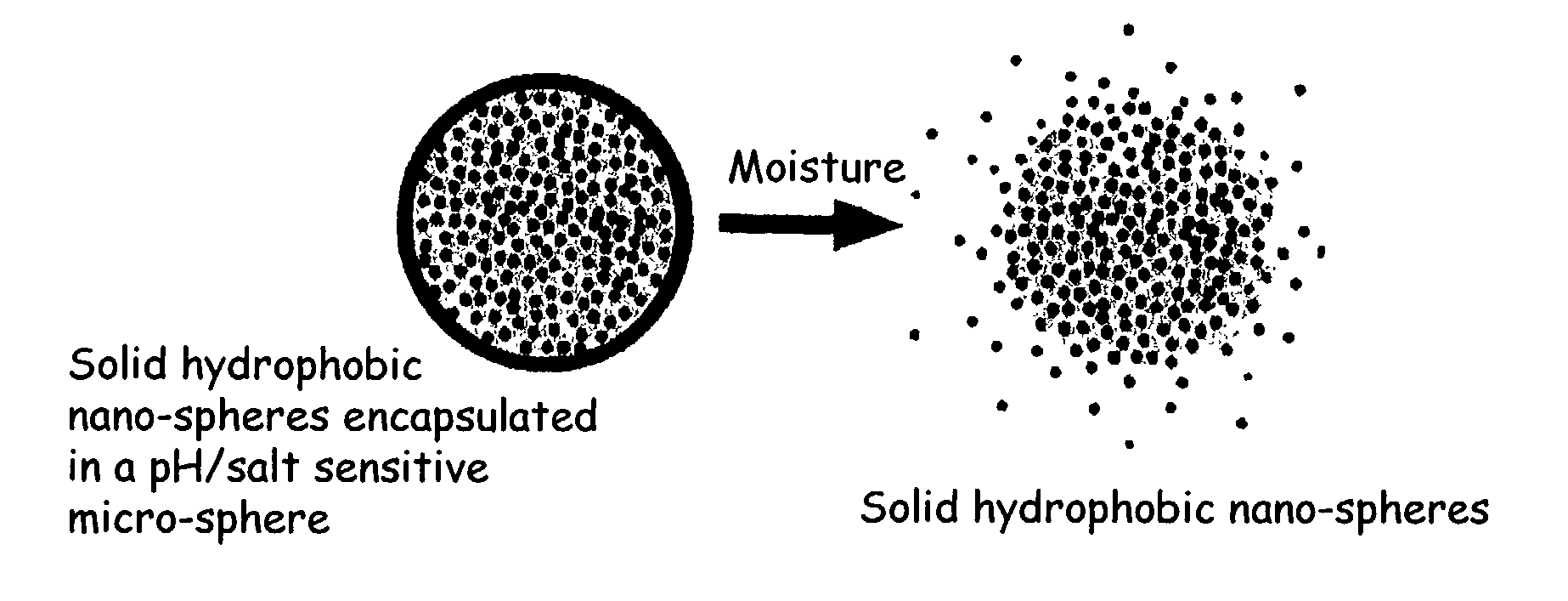
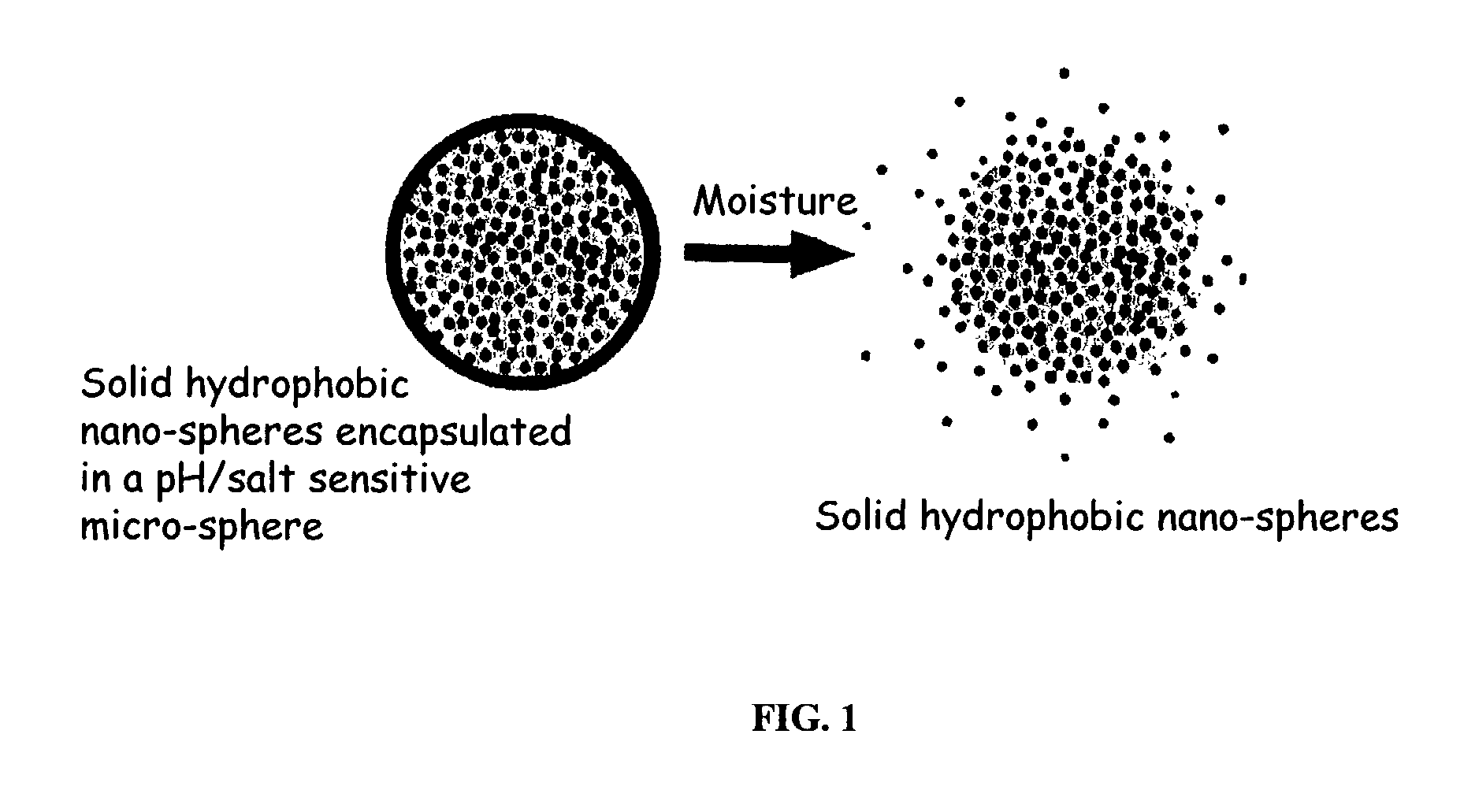
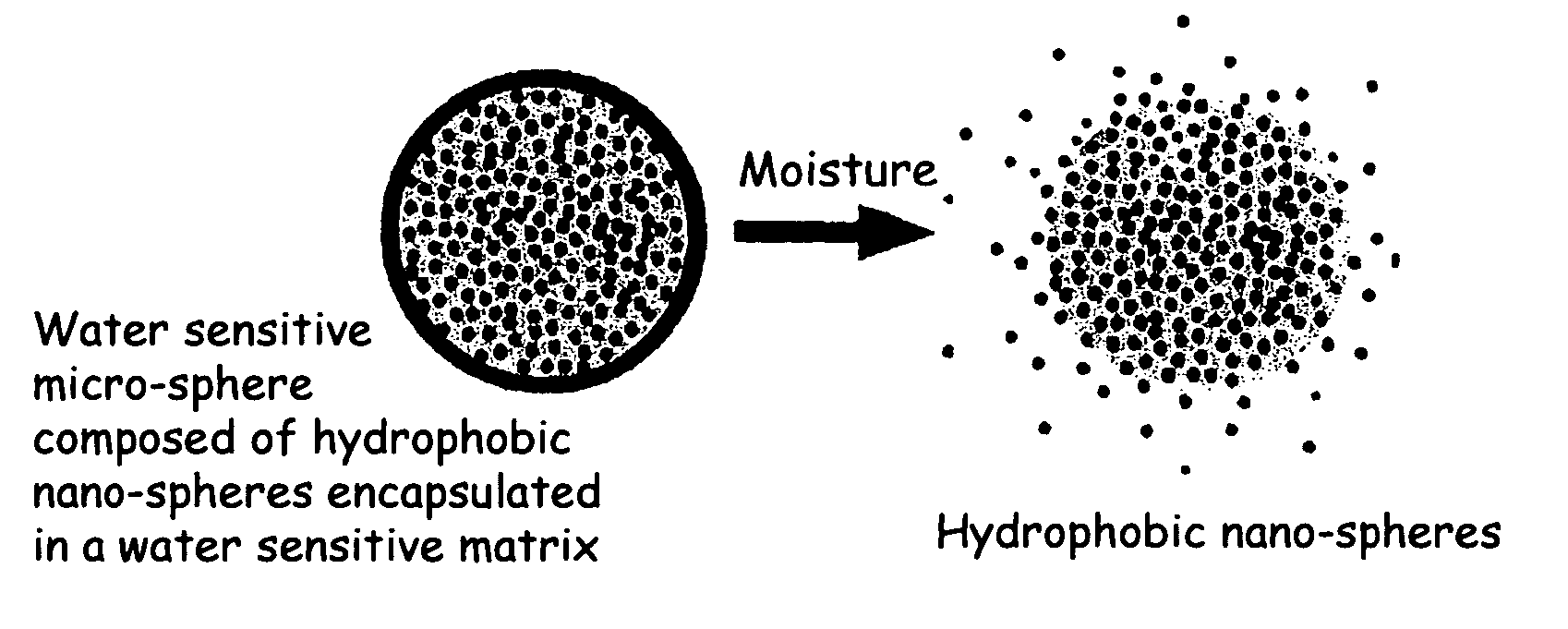
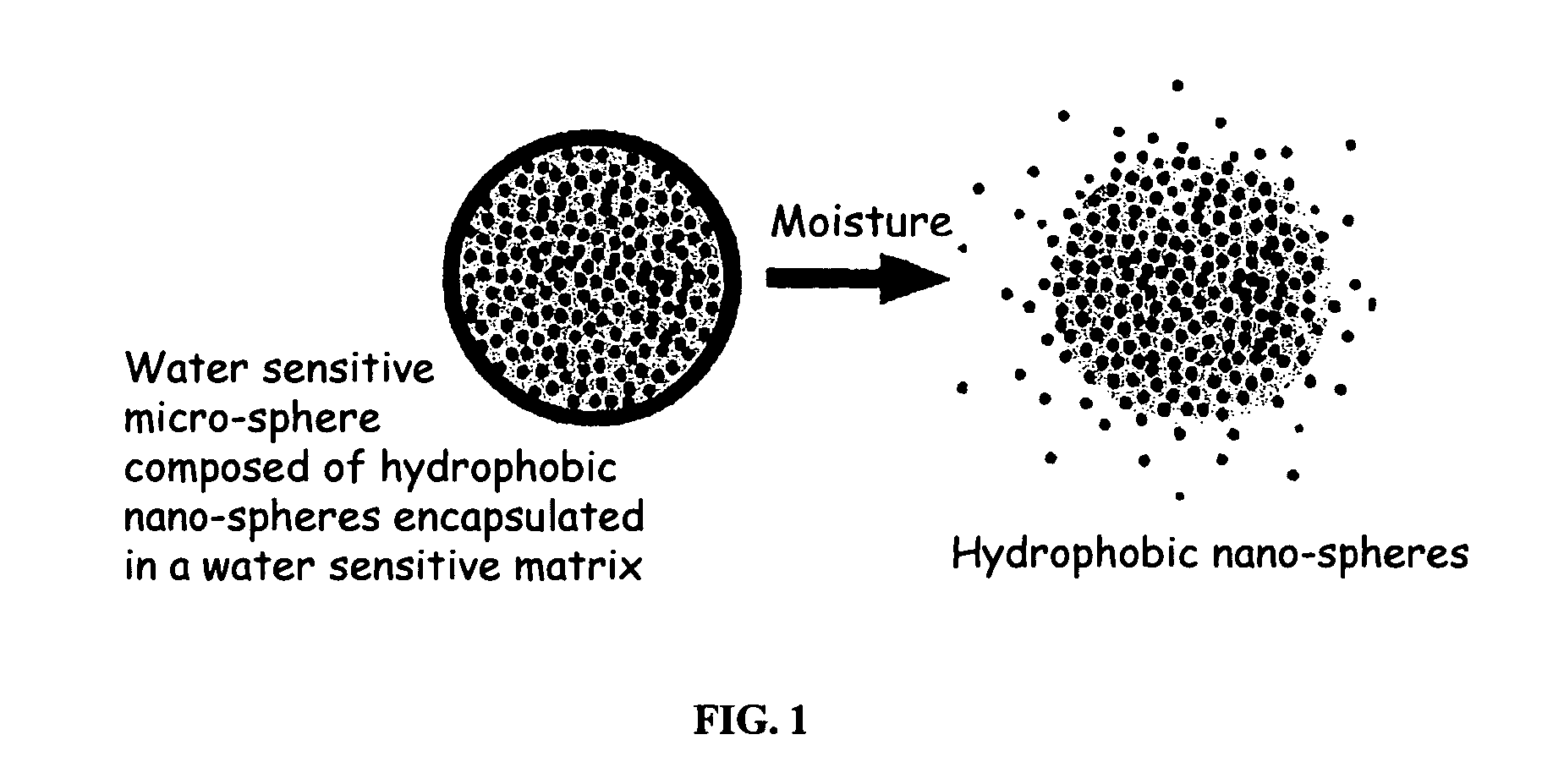
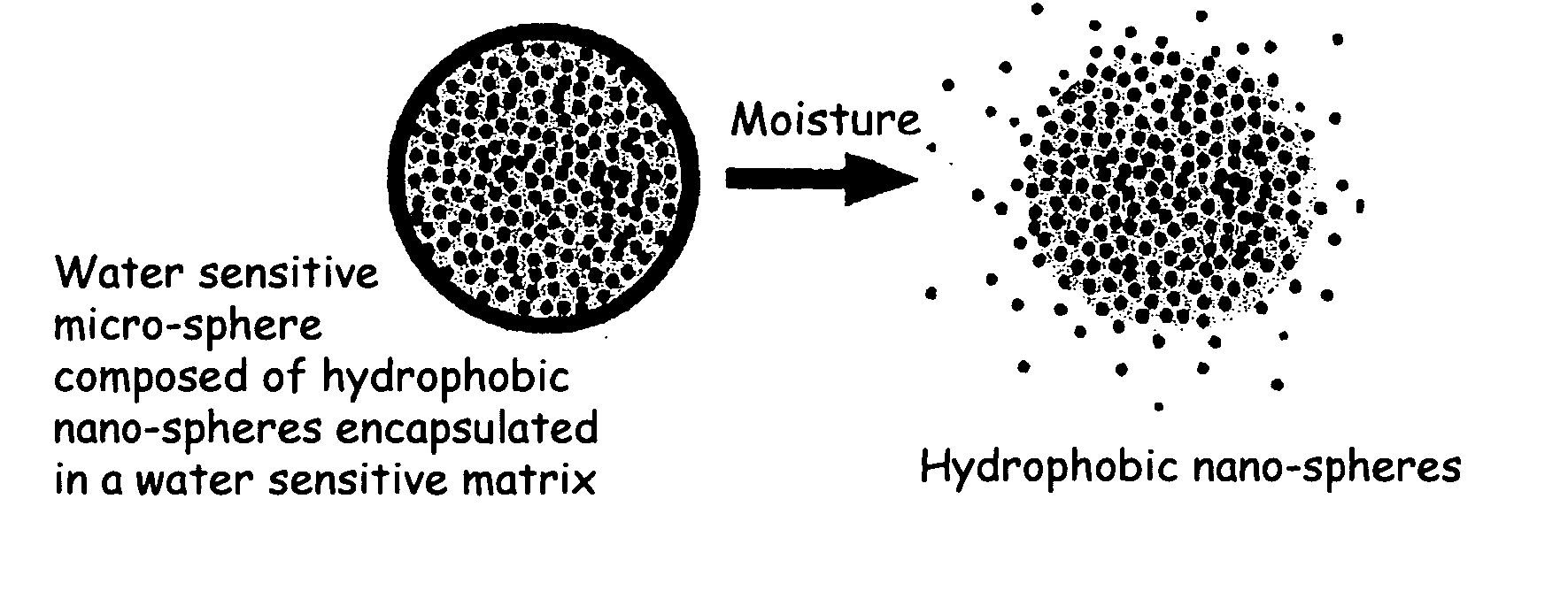
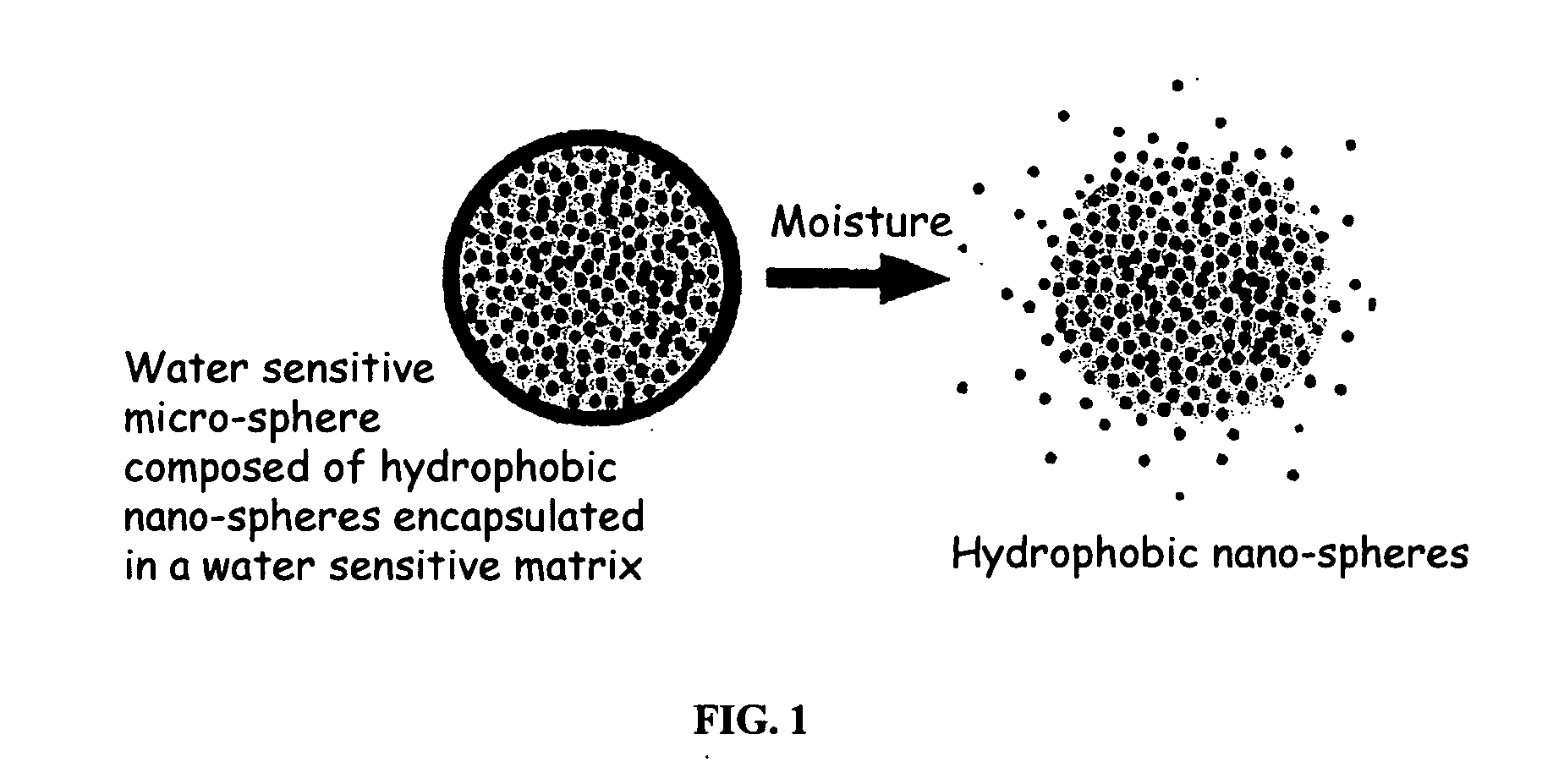
The present invention relates to an improved controlled release system that can be incorporated into anhydrous cosmetic formulations and can encapsulate different types of fragrances, flavors, active ingredients, or combinations of fragrances, flavors, and various active ingredients. The controlled delivery system of the present invention is substantially free-flowing powder formed of solid hydrophobic nano-spheres that are encapsulated in a moisture sensitive micro-spheres. The fragrances, flavors, and active ingredients encapsulated in the nano-spheres can be the same or different from those encapsulated in the micro-sphere. The encapsulation of one or more fragrances, flavors or active agents in the various components of the system, such as nano-spheres and micro-spheres, provides odor or flavor transition (change in odor character or change in flavor character) during the use of the product, in response to moisture, such as wetting the lips, perspiration, and the like. The incorporation of the controlled release system of the present invention into anhydrous cosmetic formulations was found to provide moisture triggered release, as well as prolonged release of the fragrances, flavors, and active ingredients that are encapsulated in the solid hydrophobic nano-spheres over an extended period of time.
Owner:SALVONA
Formulations of a nanoparticulate finasteride, dutasteride or tamsulosin hydrochloride, and mixtures thereof
Described are nanoparticulate compositions of finasteride, dutasteride, tamsulosin hydrochloride, or a combination thereof. The formulations exhibit unexpectedly prolonged release and can be maintained in a depot for release to a patient for a period of up to six months.
Owner:ELAN PHRMA INT LTD
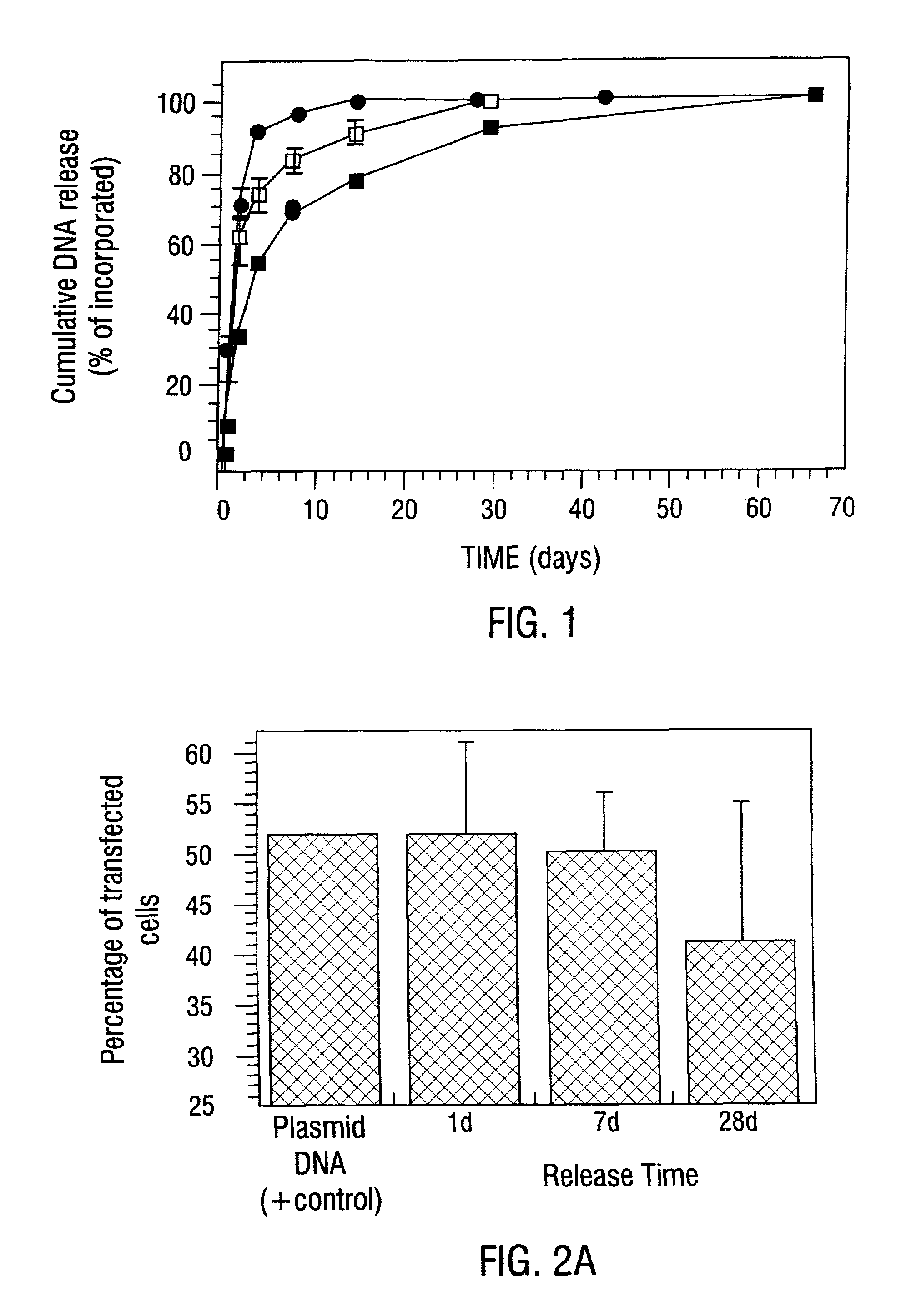
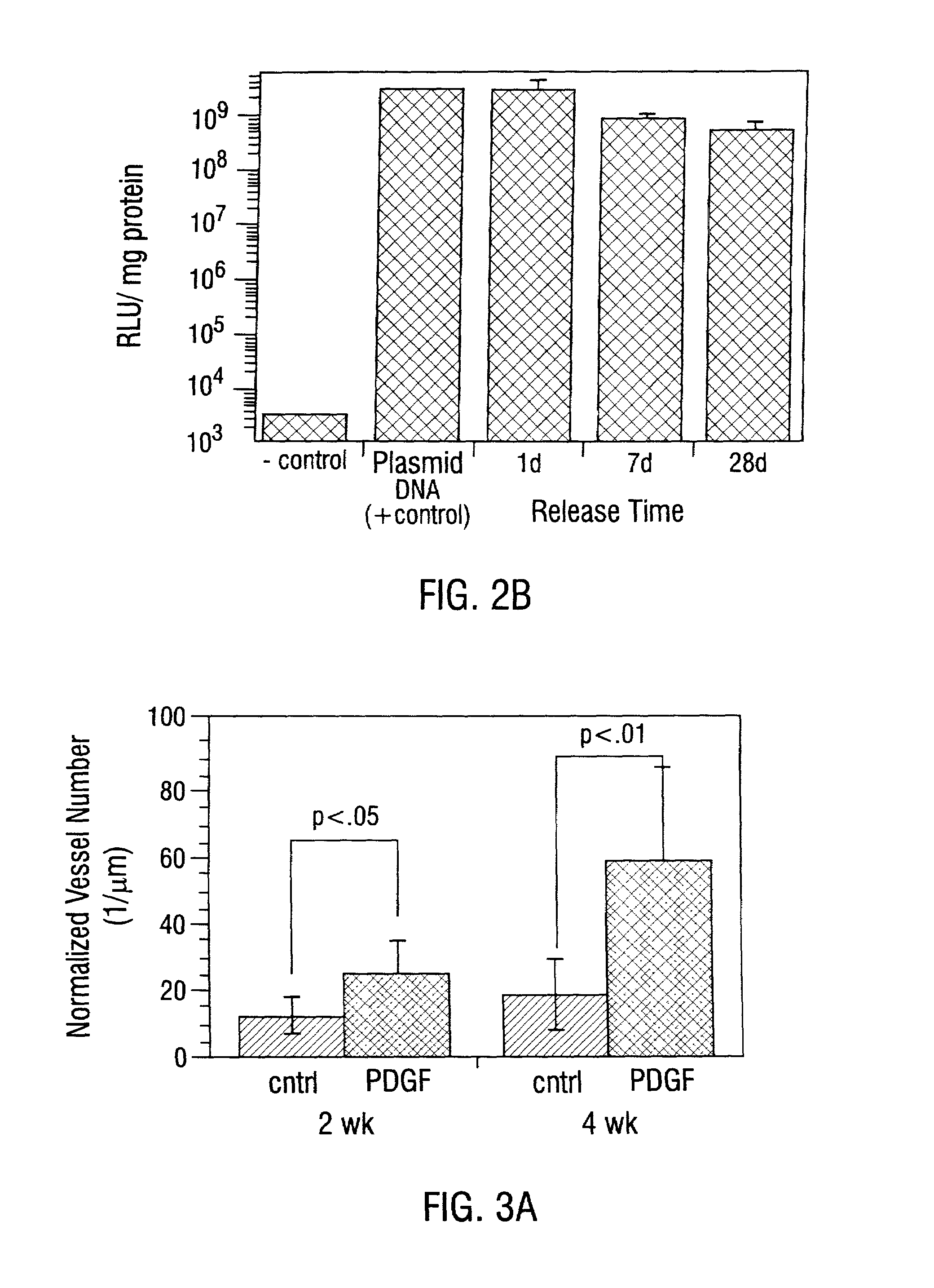
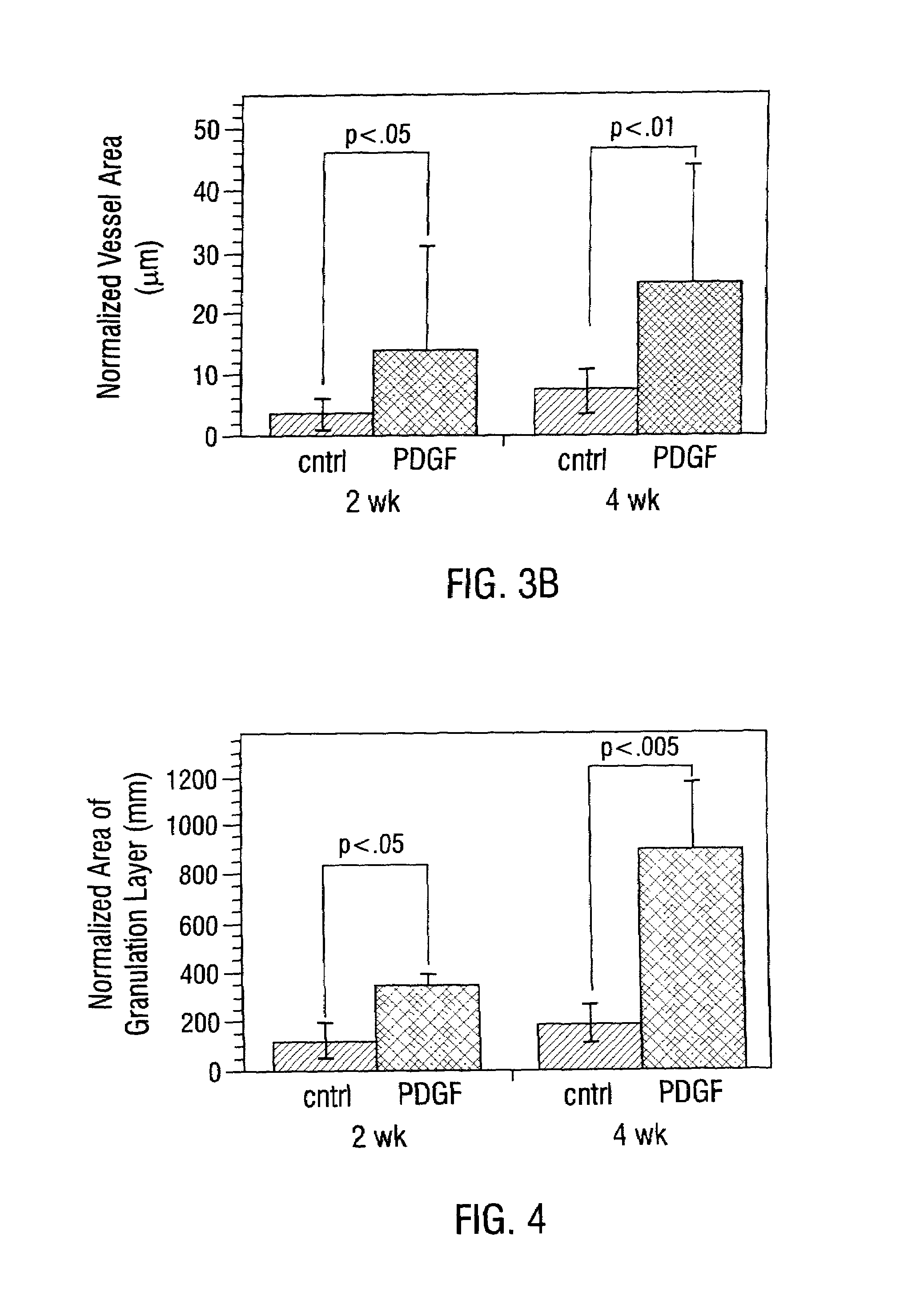
Sustained DNA delivery from structural matrices
InactiveUS7427602B1Improve bioavailabilityPowder deliveryOrganic active ingredientsDna deliveryNucleic acid structure
Disclosed are particular 3-dimensional structural matrices containing nucleic acids, various fabrication processes and methods for the prolonged release of nucleic acids in various biological environments. The nucleic acid-matrix materials are created such that they maintain a defined space, allowing cellular migration, transfection and proliferation to occur in a controlled manner. The fabrication processes provide for both high incorporation efficiencies and control over the sustained nucleic acid release. The resultant nucleic acid-containing structural matrices are thus particularly useful in in vivo cell transfection and gene expression in the context of gene therapy.
Owner:RGT UNIV OF MICHIGAN
Targeted controlled delivery compositions activated by changes in pH or salt concentration
InactiveUS7053034B2Improve impact performanceFacilitated releaseCosmetic preparationsOrganic detergent compounding agentsCarrier systemProlonged release
The present invention relates to a novel controlled release carrier system for pH or salt triggered release and targeted delivery of fragrances and other active ingredients onto fabric, hair, skin, and other biological surfaces and which provides prolonged release of fragrances and other active ingredients over an extended period of time, or yields a high impact fragrance “burst” upon treating the target surface with heat (blow drying the hair, ironing the fabric). The controlled delivery system of the present invention is substantially a free-flowing powder formed of solid hydrophobic nano-spheres comprising the fragrance and other active ingredients that are encapsulated in a pH or salt sensitive micro-spheres. Also described are processes for preparing such compositions and processes for using same. Furthermore, certain components of the aforementioned compositions in combination with one another are novel, and other components have novel uses in increasing fragrance substantivity, particularly in fabric, hair, and skin care preparations. The invention further pertains to consumer and diversified products comprising the controlled release system of the present invention.
Owner:SALVONA
Personal care formulations
InactiveUS6861060B1Increase stickinessIncrease load capacityCosmetic preparationsBiocideLipid formationPersonal care
Personal care and hygiene formulations for topical application to mucosal surfaces. These formulations include an amphiphilic lipid carrier in the form of a colloidal composition which can include a micellar aggregate or mixed micelles dispersed in a continuous aqueous phase, or an emulsion of lipid droplets suspended in a continuous aqueous phase, and an active agent which is an anti-microbial agent. The lipid carrier has high adhesiveness to mucous membranes such as the soft tissues of the oral cavity. The lipid carrier also has a high load capacity for the active agent to be carried to these tissues. These formulations have the desirable properties of carrying a large amount of active agent for controlled and prolonged release thereof at the desired site, such as mucous membrane surfaces and surrounding tissue. Accordingly, the present invention provides a formulation for oral or topical application including an anti-microbial agent and a lipid. The agent is held by the carrier through a hydrophobic interaction and is released from the carrier in a controlled manner over a prolonged period of time. The lipid is also characterized by having a high adhesive capability towards mucous membrane surfaces. The lipid and the agent are preferably present in a ratio in a range of from about 1:10 to 10:1, more preferably from about 1:5 to about 5:1, and most preferably from about 1:3 to about 3:1 in the formulation.
Owner:LURIYA ELENA +1
Multi component controlled delivery system for soap bars
InactiveUS7208460B2Increase depositionImprove skinPowder deliverySoap detergents with organic compounding agentsControlled releaseMicrosphere
Owner:SALVONA IP
Multi component controlled delivery system for soap bars
InactiveUS20050065047A1Increase depositionHigh densitySoap detergents with organic compounding agentsCosmetic preparationsControlled releaseMicrosphere
The present invention relates to an improved controlled delivery system that can be incorporated in soap bars to enhance deposition of active ingredients and sensory markers onto skin. The carrier system also provides controlled release or prolonged release of these actives from the skin over an extended period of time. The controlled delivery system of the present invention comprises substantially free-flowing, powder formed of solid hydrophobic, positively charged, nanospheres of encapsulated active ingredients, that are encapsulated in moisture sensitive microspheres. The high cationic charge density of the nanosphere improves deposition of active ingredients onto skin. The high cationic charge density on the nanosphere surface is created by incorporating a cationic conditioning agent into the solid hydrophobic matrix of the nanospheres, by incorporating a cationic charge “booster” in the moisture sensitive microsphere matrix, or by using a cationic conditioning agent in the nanosphere matrix in conjunction with a cationic charge “booster” in the microsphere matrix. The invention also pertains to soap products comprising the controlled release system of the present invention.
Owner:SALVONA IP
Polymer coatings containing phytochemical agents and methods for making and using same
InactiveUS20080175812A1Reduce and destroy foulingGrowth inhibitionBiocideAntifouling/underwater paintsPhytochemicalControl release
This invention relates to compositions comprising a polymer base incorporating antifouling compositions suitable for use in aquaculture, marine and architectural systems as paints, structures or coatings. In particular, the present invention relates to a polymer based coating incorporating a biocidal antifouling composition suitable for use with aquaculture equipment. The present invention provides polyethylene compositions and latex compounds which may comprise at least one environmentally benign phytochemicals suitable for use in preventing the colonization of a treated surface by a variety of biological species. The compositions of the invention may further comprise control release agents such as, for example, micro-encapsulation of the phytochemicals to maintain sustained and prolonged release of the biocidal agents at the treated surface.
Owner:MAGELLAN
Multilayer tablets containing thiazolidinedione and biguanides and methods for producing them
InactiveUS20060057202A1Facilitated releaseEfficient compressionBiocideOrganic active ingredientsAcute hyperglycaemiaImmediate release
A novel patient-convenient, cost effective pharmaceutical composition, comprising of thiazolidinediones and biguanide for controlling hyperglycemia manufactured as multilayer tablet and its process of manufacturing, for immediate release of thiazolidinediones or thiazolidinediones and biguanide and prolonged release of the biguanide only, the tablet comprising of minimum two layers wherein one outer layer comprises of a mixture of excipients and thiazolidinediones or thiazolidinediones and biguanide allowing immediate release of thiazolidinediones or thiazolidinediones and biguanide respectively and the other layer arranged in contact with the immediate release layer which comprises of a novel composition of excipients and a minimum one or more non-biodegradable, inert polymer(s) and the biguanide allowing pH independent prolonged release of the biguanide up to a period of 8-12 hours. The tablets are for once a day dosing. The tablets may optionally be film coated or enrobed by soft gelatin ribbons for additional protection against oxidation, photodegradation, identification, ease of swallowing, taste masking and for aesthetic appeal without altering the dissolution profile.
Owner:INVENTIA HEALTHCARE LTD
External patch containing estrogen and/or progestogen
InactiveUS8486442B2Stable drug releaseLess irritatingBiocideOrganic active ingredientsIrritationProlonged release
An external patch capable of stable prolonged release and transdermal absorption of active ingredient hormones (estrogens and / or progestogens) contained in a pressure sensitive adhesive layer, which external patch ensures low irritation on the skin. In particular, an external patch comprising a support and, superimposed thereon, a pressure sensitive adhesive layer, characterized in that the pressure sensitive adhesive layer comprises, as indispensable components, 5 to 50 wt. % of styrene / isoprene / styrene block copolymer, 20 to 70 wt. % of tackifier resin, 10 to 60 wt. % of softener and 1 to 20 wt. % of polyvinylpyrrolidone and contains, as an active ingredient, estrogen and / or progestogen.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
Injectable depot compositions and its process of preparation
Novel injectable compositions are provided comprising an active agent which is tamsulosin or letrozole or its pharmaceutically acceptable salts, derivatives, isomers, polymorphs, solvates, hydrates, analogues, enantiomers, tautomeric forms or mixtures thereof and one or more pharmaceutically acceptable excipient(s) wherein the compositions are preferably formulated as biodegradable microparticles or nanoparticles which can optionally be reconstituted with an aqueous, hydro-alcoholic or oily liquid vehicle prior to administration. The novel injectable compositions of the present invention preferably form a depot upon administration in vivo and are in the form of an in situ gelling composition or an implant composition which provides a prolonged release of tamsulosin or letrozole for extended periods of time. Also described are process for preparation of such novel compositions and method of using them.
Owner:PANACEA BIOTEC
Article to be Inserted in a Body Cavity Having Biologically Inhibiting Surfaces and Use and Preparation of the Article
InactiveUS20080140052A1Easy to coverImprove conductivityTracheal tubesSurface reaction electrolytic coatingHigh rateMetallic materials
An article to be inserted in a human or animal body cavity, use of the article and preparation thereof. The article has a biologically inhibiting arrangement of electrically connected electrode materials in direct contact with each other on one or more surfaces of the article The arrangement includes as electrode materials a metallic anode material and a cathode material, where the potential of the cathode material is higher than the potential of the anode material, The cathode material is an electrically conductive material selected among certain non-metallic materials. The arrangement provided on the article releases biological inhibiting ions of the metallic anode material or complexes of such ions when the biologically inhibiting arrangement is contacted with a body fluid. The article can be designed with a controlled release rate suitable for the purpose in question for example an initial high rate of ion release after insertion of a catheter to combat bacteria introduced during the insertion followed by a prolonged release at a lower rate to maintain a low level of bacteria.
Owner:IMPACTCARE
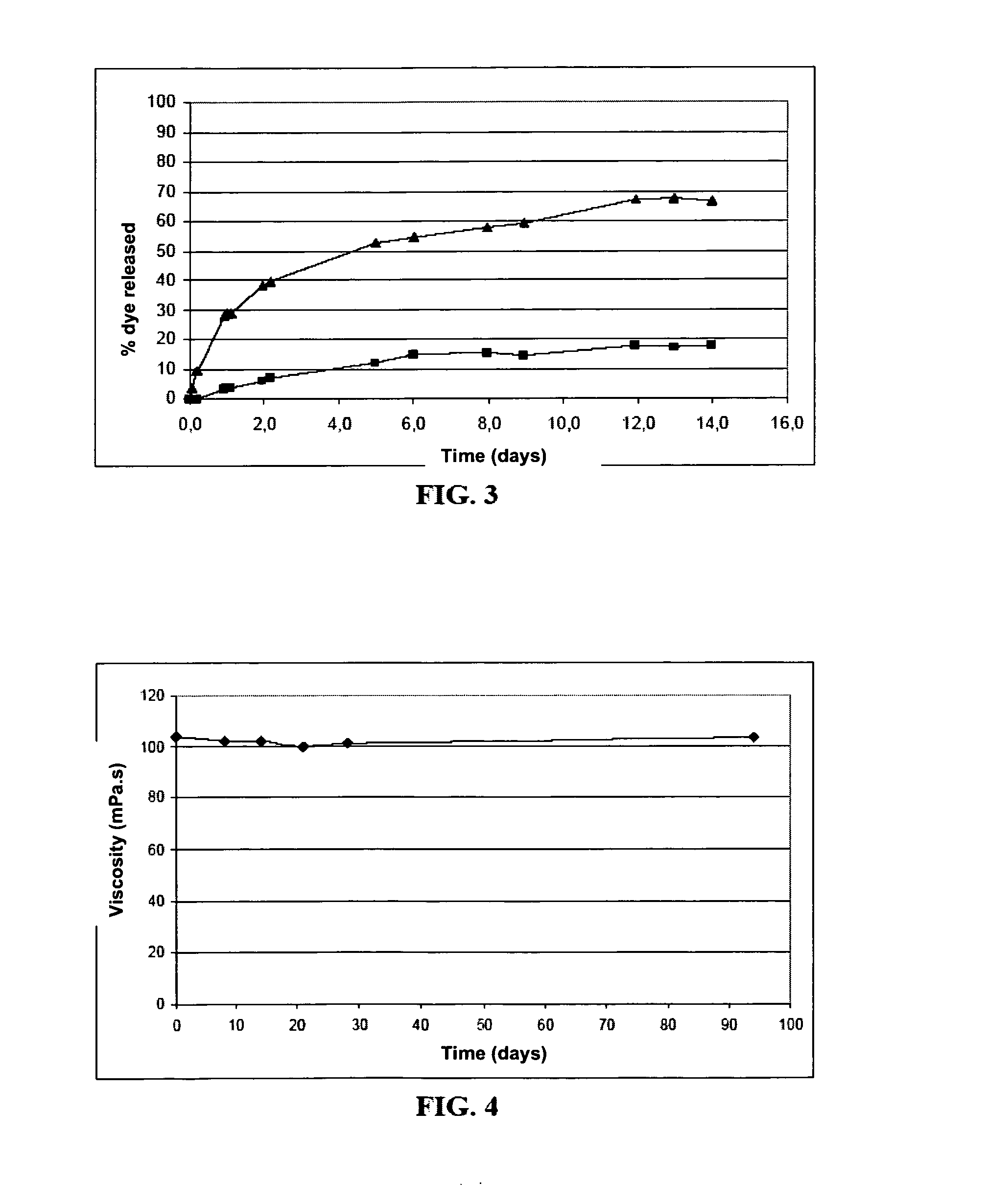
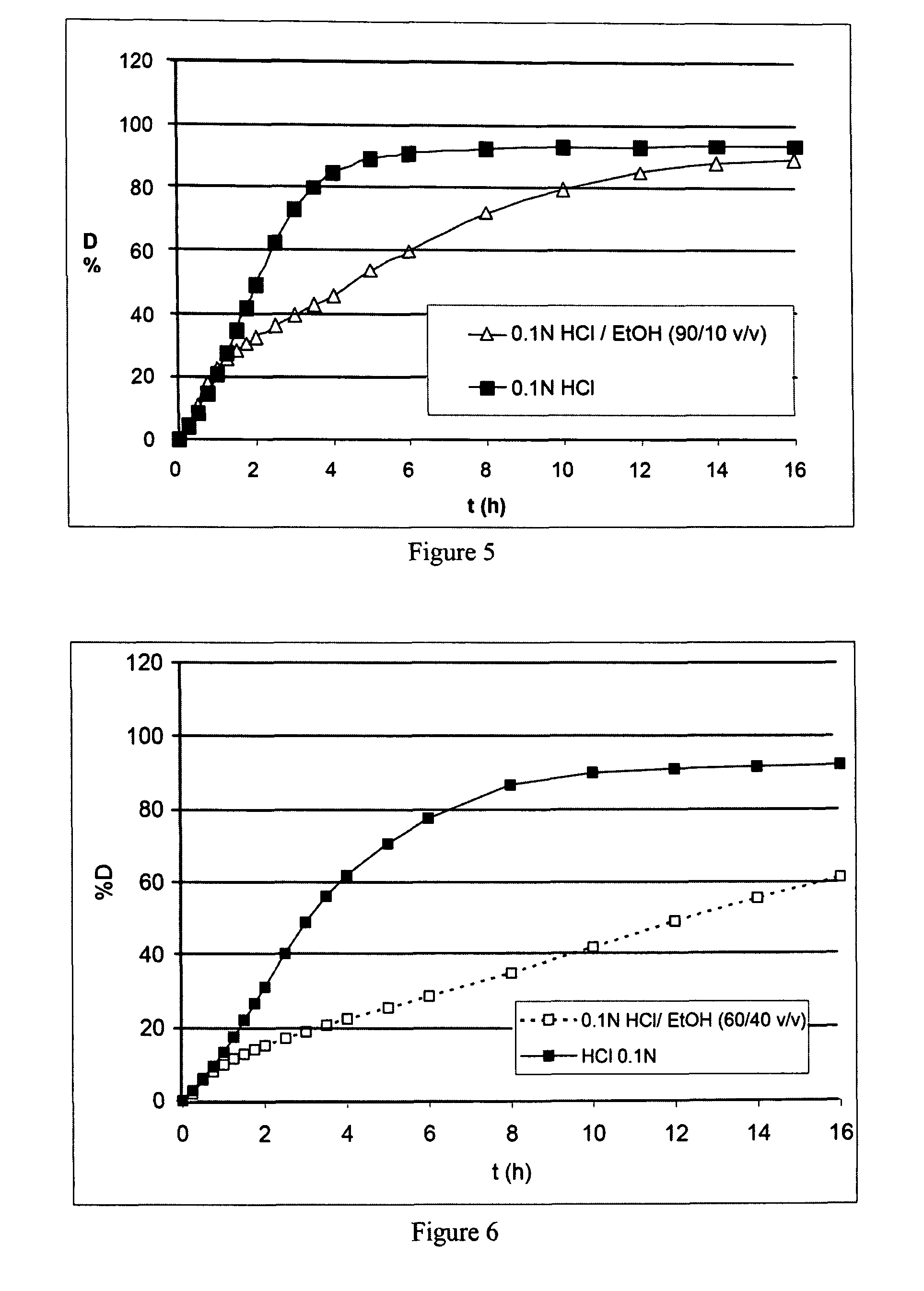
Prolonged release microspheres for injectable administration
The present invention provides microspheres intended to be administered by injection comprising a protein active ingredient and an agent coating the active ingredient intended to prolong its release, wherein they are free of any trace of organic solvent and they can be obtained according to a coating method involving bringing the active ingredient and the coating agent into contact, with stirring, in a supercritical fluid, said coating agent being soluble in this supercritical fluid.
Owner:ETHYPHARM SA
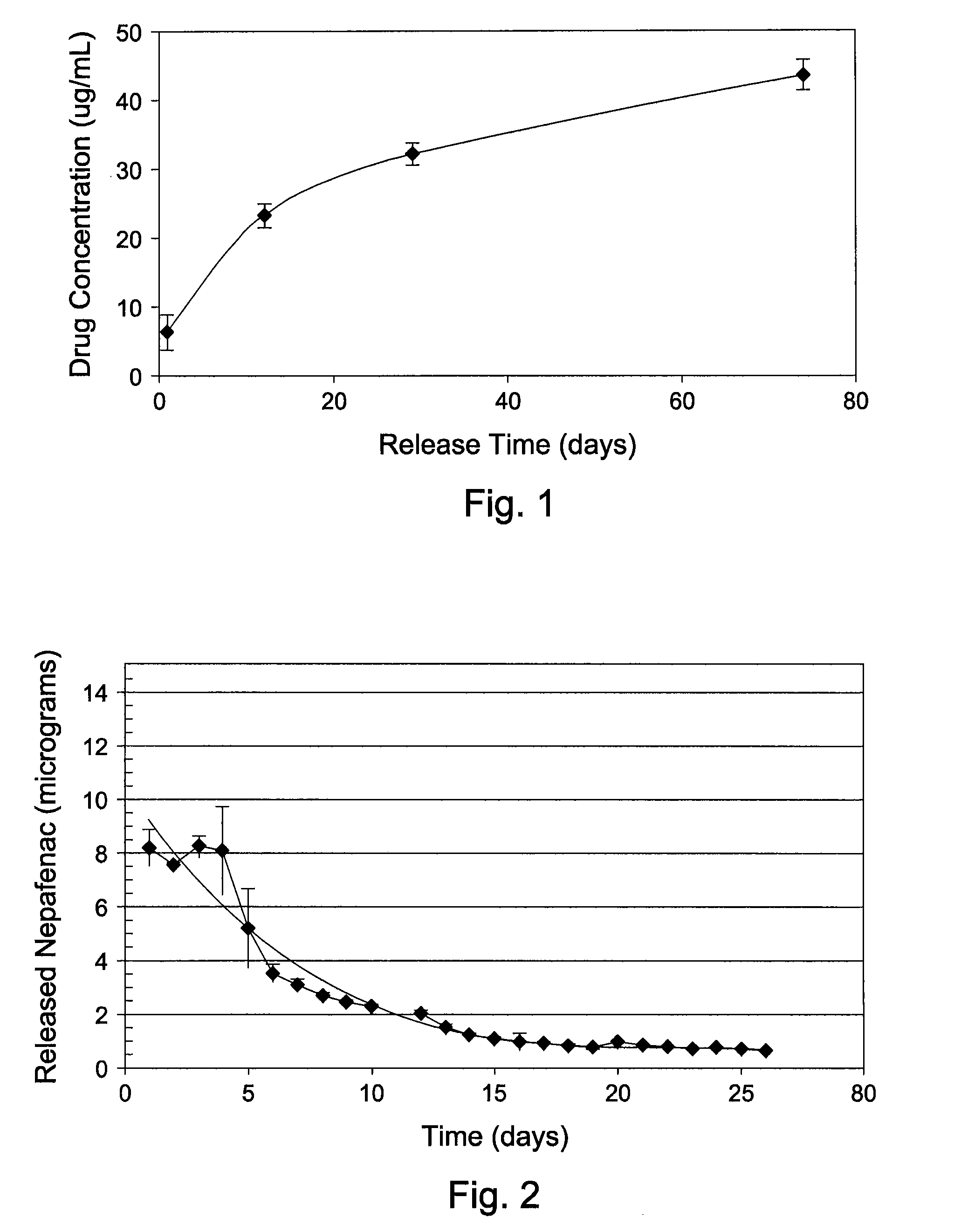
Sustained intraocular delivery of drugs from biodegradable polymeric microparticles
ActiveUS20100261646A1Lower eye pressureSustained releaseHormone peptidesBiocideMicrosphereActive agent
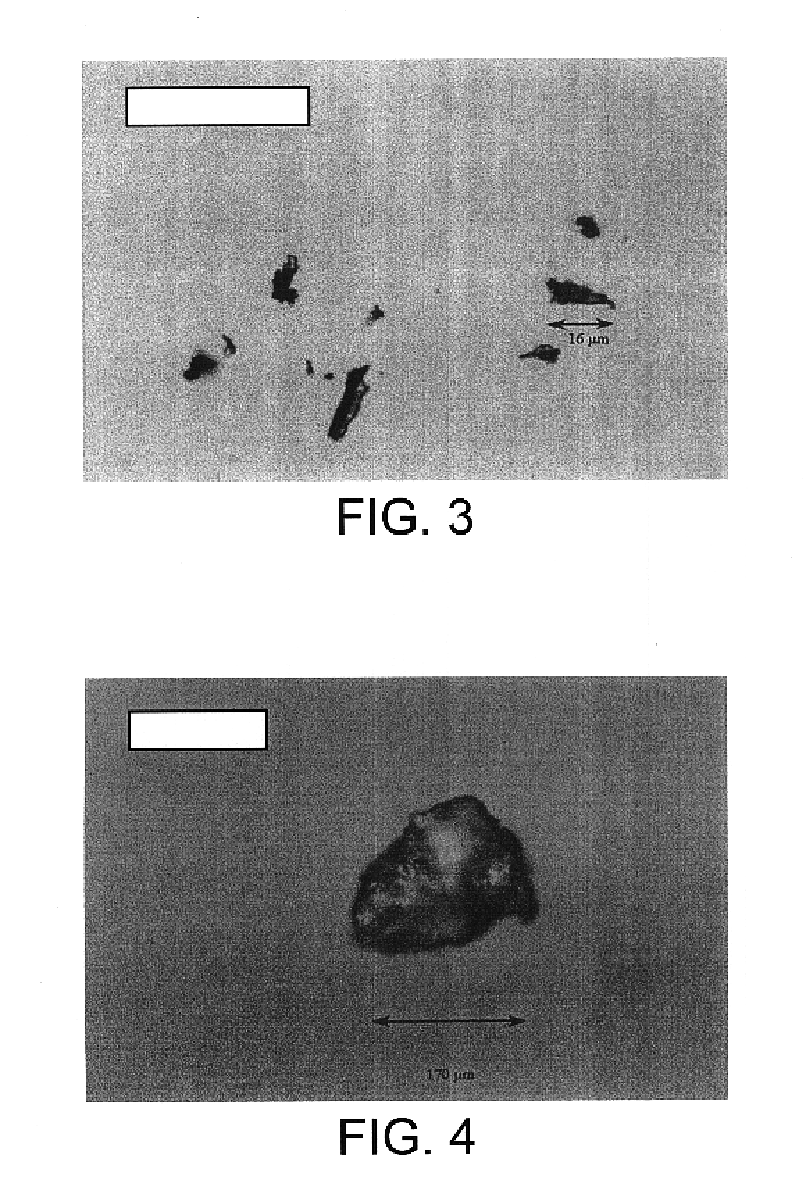
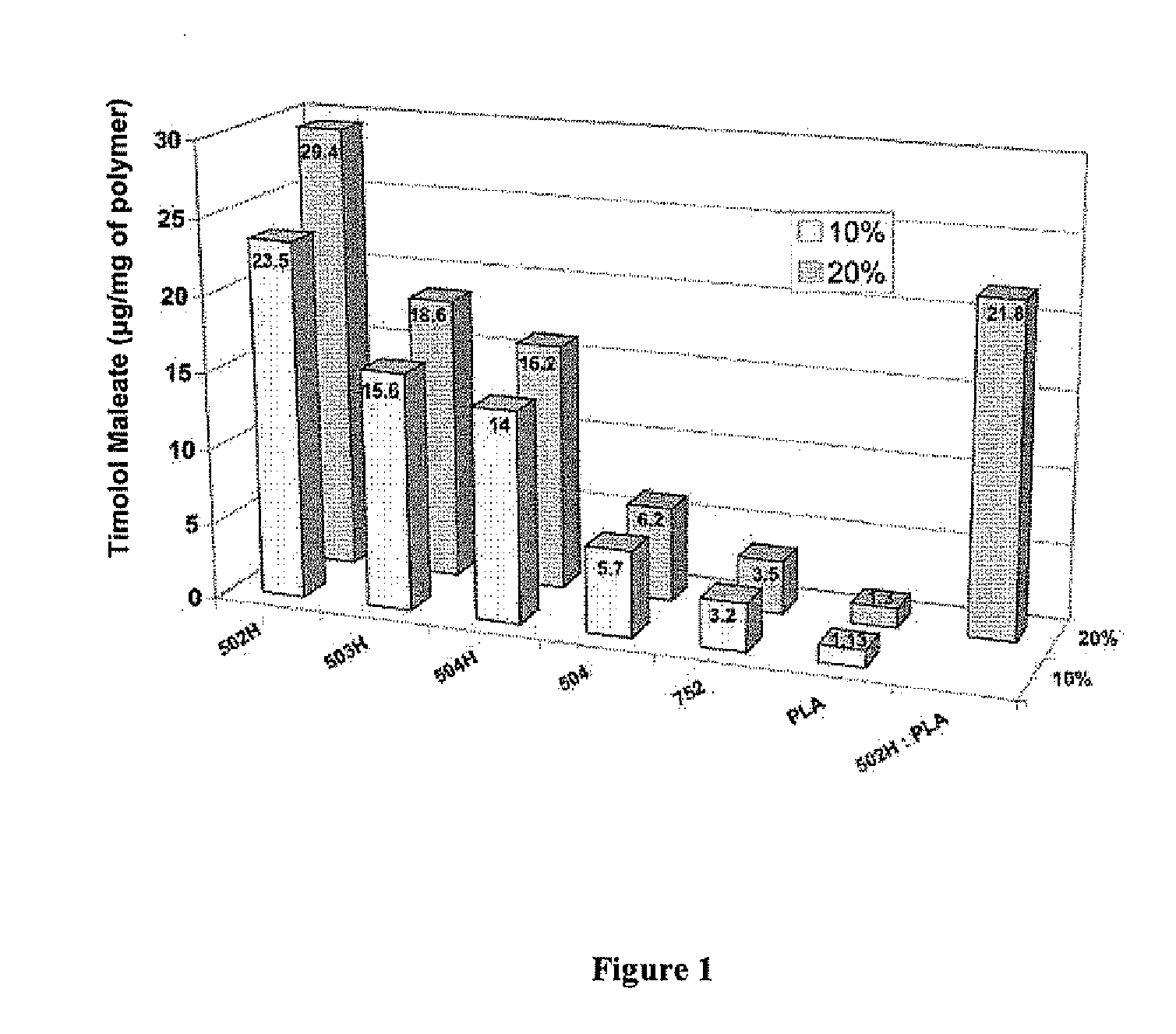
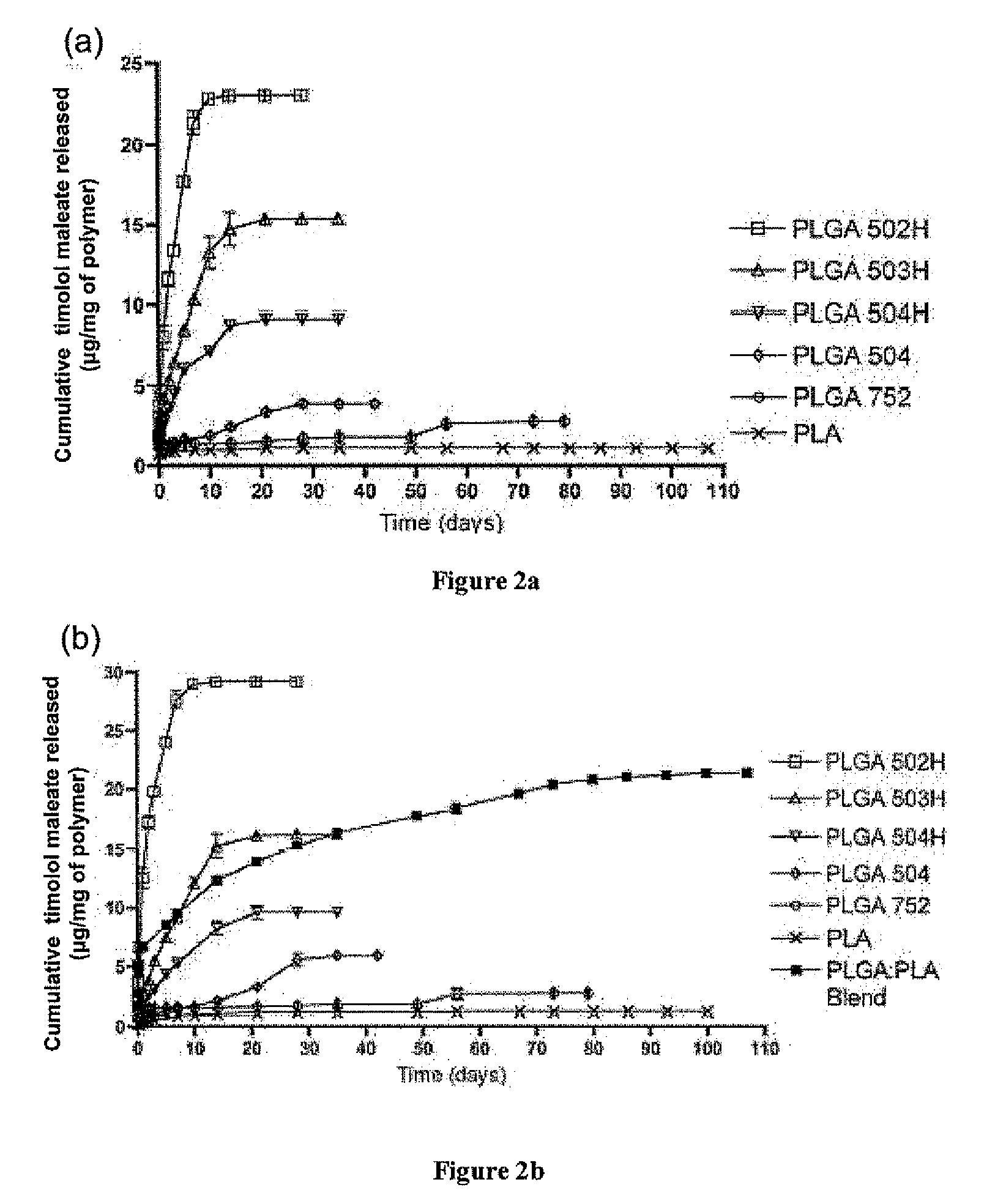
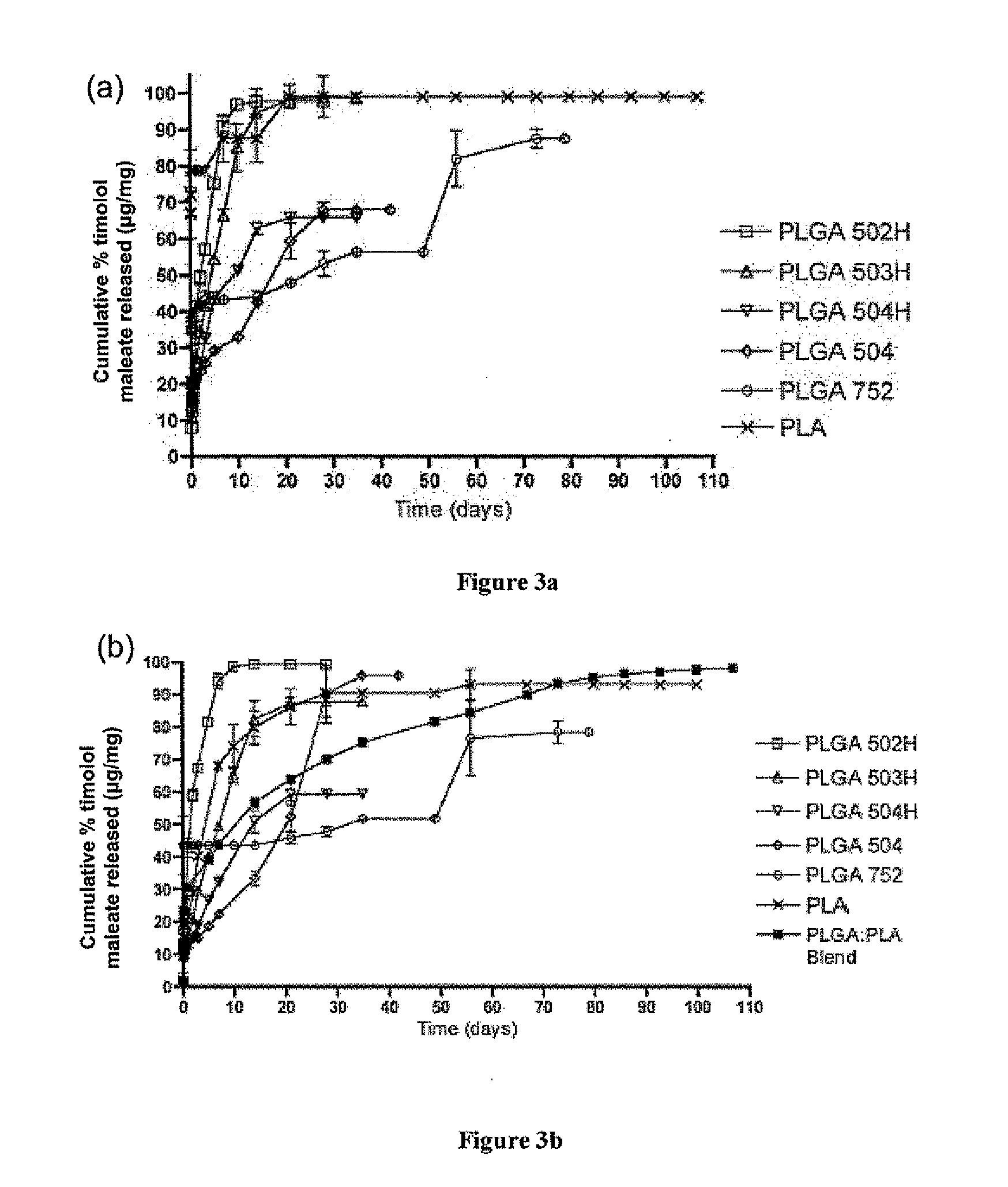
Biodegradable polymeric microparticle compositions containing one or more active agents, especially those useful for treating or preventing or one or more diseases or disorders of the eye, and methods of making and using thereof, are described. The microsphere compositions release an effective amount of the one or more active agents for a period greater than 14 days in vivo, preferably greater than 60 days in vivo, more preferably up to 73 days in vivo, more preferably greater than 90 days in vivo, even more preferably over 100 days in vivo, and most preferably greater than 107 days in vivo. In a preferred embodiment, the microparticle compositions contain one or more active agents useful for managing elevated intraocular pressure (TOP) in the eye. In one embodiment, the microspheres are formed from polylactide-co-glycolide (“PLGA”); in another embodiment, the microspheres are formed from a blend PLGA and poly lactic acid (“PLA”). Relatively hydrophilic, and preferably carboxylated, polymeric materials such as PLGA are used for a drug such as timolol maleate, which is relatively water soluble, to increase drug loading. Higher molecular weight polymers, as well as the ratio of LA (which has a longer degradation time, up to one to two years) to GA (which has a short degradation time, as short as a few days to a week), are used to provide release over a longer period of time. The combination of drug loading and release rate, as well as the minimization of initial burst release, result in prolonged release of a higher amount of drug.
Owner:YALE UNIV +1
Core tablet for controlled release of gliclazide after oral administration
InactiveUS6733782B1Facilitated releaseIncrease concentrationPowder deliveryMetabolism disorderControlled releaseOral medication
The invention relates to a matrix tablet for the prolonged release of gliclazide which ensures continuous and consistent release of the active ingredient after administration by the oral route, the release being insensitive to variations in the pH of the dissolution medium.
Owner:LES LAB SERVIER
Dispersion of polyamino acids in a continuous lipid phase
InactiveUS20080152675A1Extend posting timeImprove protein stabilityBiocidePharmaceutical non-active ingredientsLipid formationWater in oil emulsion
The invention relates to injectable pharmaceutical compositions for the prolonged release of at least one active principle, comprising at least one active principle in an aqueous phase of amphiphilic polymer, said aqueous phase being in the form of a dispersion in a continuous lipid phase. The composition is in the form of a water-in-oil emulsion comprising:a pharmaceutically acceptable, continuous lipid phase,an aqueous disperse phase containing at least one amphiphilic polymer and at least one active principle not covalently bonded to said amphiphilic polymer, andat least one pharmaceutically acceptable surfactant.
Owner:FLAMEL TECHNOLOGIES
Sustained release ophthalmological device and method of making and using the same
InactiveUS7090888B2Eliminate needLow costPretreated surfacesAdhesive dressingsOphthalmological implantVariable thickness
A method of making an ophthalmological implant by applying a layer of pharmaceutical agent and an overlying layer of a bioerodible material, a biodegradable material, a bloavailable material or a mixture thereof, the overlying layer having variable thickness and being dimensioned for prolonged release of the pharmaceutical agent from the implant as the overlying layer degrades.
Owner:SNYDER MICHAEL E +1
Delayed prolonged drug delivery
ActiveUS20130022677A1Slow release rateReduce deliveryBiocideSenses disorderActive agentProlonged release
In one aspect, the present invention is concerned with a treatment where it is desired that an active agent is designed to be released in a prolonged manner at a time point some time after administration of the active agent. The present invention is particularly suited to administering an agent which may be released whilst a subject is sleeping, shortly before waking and continues to administer the drug during the early waking hours. As well as treating certain conditions by a particular regime, the invention also provides novel formulations for a delayed, followed by a prolonged release of drug.
Owner:DRUG DELIVERY INT
Anti-stress, anti-impairment and anti-aging drug and process for manufacturing thereof
The invention relates to a new pharmacotherapeutical strategy, to an anti-stress, anti-impairment and anti-aging drug and to a process for its manufacturing. The drug has an etio-pathogenic and homeostatic action, was preclinically tested and clinically checked up in geriatric, neurologic, psychiatric and stress-dependent pathology. The drug achieves a synergistic biological, neurometabolic and cell-trophic composition, being elaborated by the association of the following active principles: a) against oxidative and catabolic stress; methionine with aminoethanol phenoxyacetates and / or aminoethyl phenoxyacetamides; b) against anabolic stress; hydroxopyrimidine carboxylates and / or oxopyrrolidine acetamides with potassium, zinc and lithium; c) vasodilative and normolipidemic; nicotinic, alcohol and / or acid, or its derivatives, with magnesium and iodine; d) energo-active and e) anti-toxic; aspartate; fructose; vitamin B1; vitamin B6; monoacid phosphate and sulfate. The process for manufacturing the drug stipulates; a) pharmaceutical preparation in two complementary types of capsules or coated tablets, gastrosoluble and enterosoluble, the last being enteric coated; b) prolonged-release of vasodilator from the enterosoluble unit.
Owner:RIGA DAN +1
Green compositions containing synergistic blends of surfactants and linkers
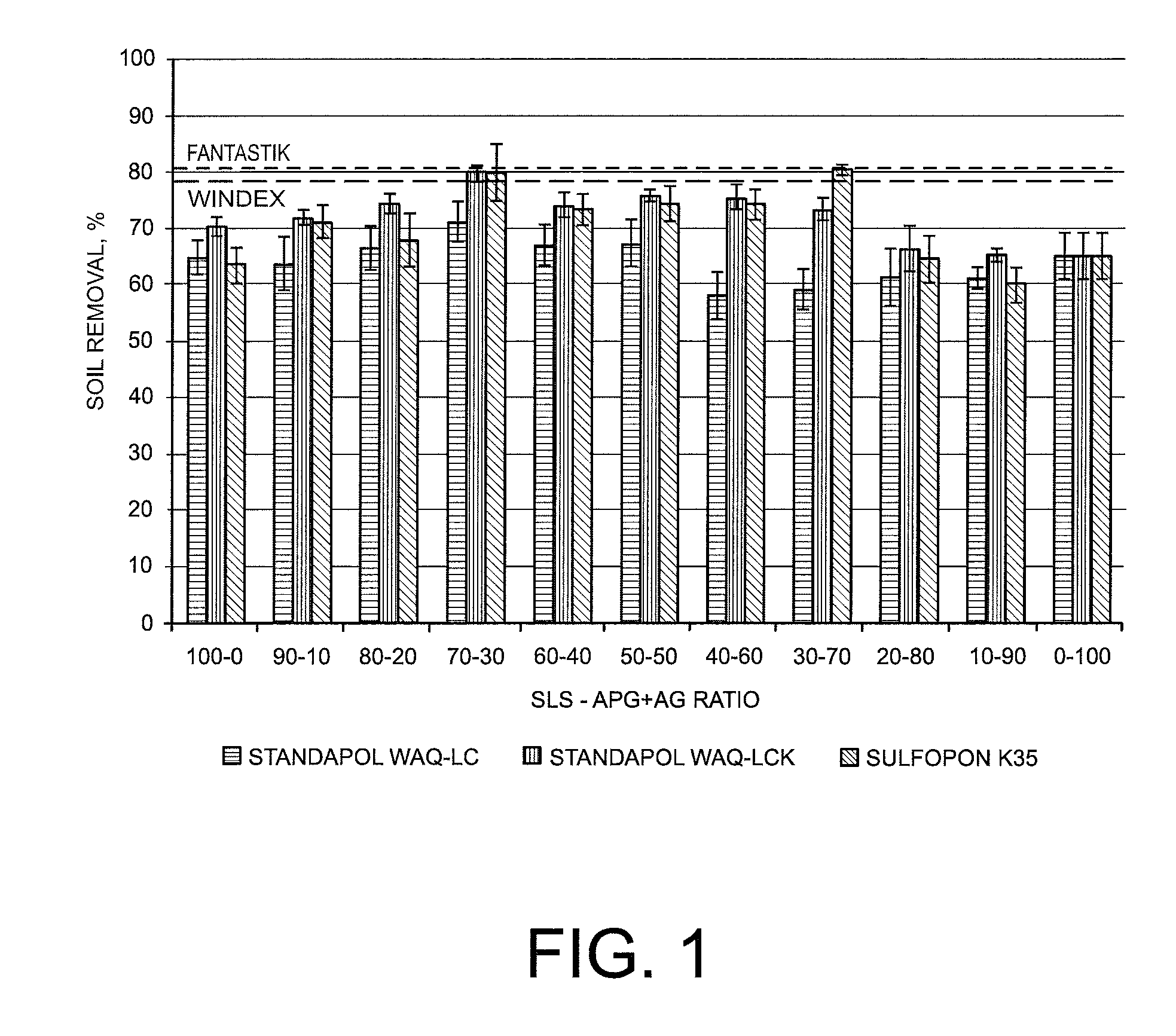
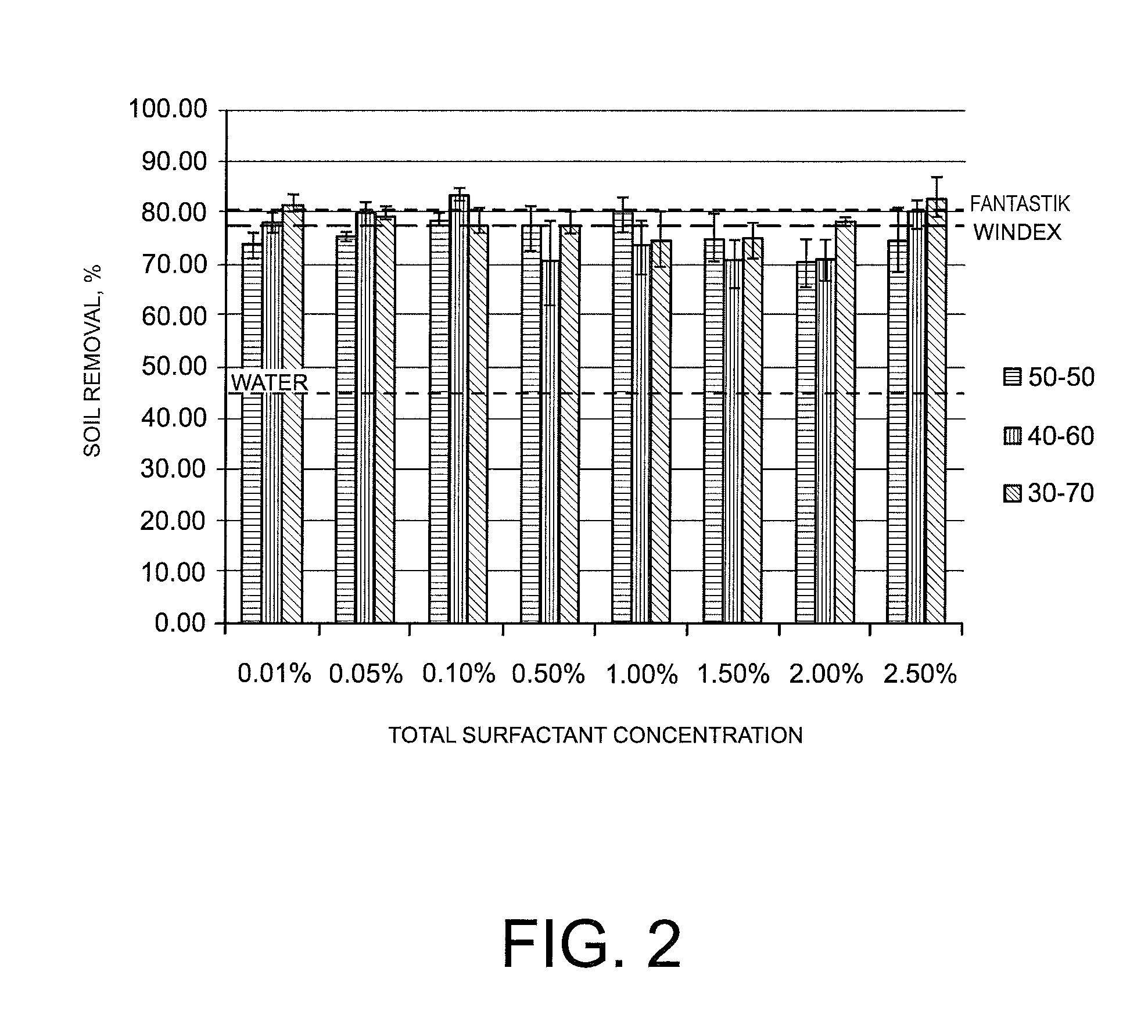
ActiveUS8283304B2Easy to cleanImprove decontamination abilityNon-ionic surface-active compoundsOrganic detergent compounding agentsControl mannerRoom temperature
Cleaning compositions in the form of nano- or micro-emulsions and containing one or more green surfactants are disclosed. The composition may further include one or more green linkers. The presence of two or more green surfactants may synergistically improve the cleaning performance of the composition. Moreover, the presence of the one or more green linkers may also synergistically improve the soil removal or streak reduction performance of the composition. The composition may further include additional natural actives, such as natural fragrances, natural insecticides, natural insect repellants, and natural oils to provide prolonged release of the natural actives in a controlled manner. The green surfactants not only improve the ecological profile of the disclosed compositions but also allow the formation of stable micro- or nano-emulsions at room temperature with good cleaning performances.
Owner:SC JOHNSON & SON INC
Prolonged-release multimicroparticulate oral pharmaceutical form
InactiveUS9023400B2Prevent fraudulent abuse of propertyDifficult to administerBiocideNervous disorderProlonged releaseBULK ACTIVE INGREDIENT
Owner:FLAMEL IRELAND
Ophthalmic device having therapeutic agent delivery capability and method of forming same
ActiveUS20100016439A1Facilitated releaseBiocideOrganic active ingredientsProlonged releaseBiomedical engineering
The present invention is directed to ophthalmic devices that are loaded with therapeutic agent. The devices provide prolonged release of the therapeutic agent to the eye. The ophthalmic devices are typically formed of an ophthalmic material that is particularly desirable for the loading of therapeutic agent and / or the therapeutic agent is typically particularly desirable for loading to the ophthalmic material.
Owner:ALCON INC
Tamper resistant and dose-dumping resistant pharmaceutical dosage form
ActiveUS20130280338A1Facilitated releaseOrganic active ingredientsBiocideAqueous ethanolProlonged release
A tamper-resistant pharmaceutical dosage form comprising a pharmacologically active ingredient embedded in a prolonged release matrix, which comprises a prolonged release matrix material selected from the group consisting of nonionic acrylic polymers and waxy materials and which provides prolonged release of the pharmacologically active ingredient, resistance against solvent extraction, resistance against grinding, and resistance against dose-dumping in aqueous ethanol.
Owner:GRUNENTHAL GMBH
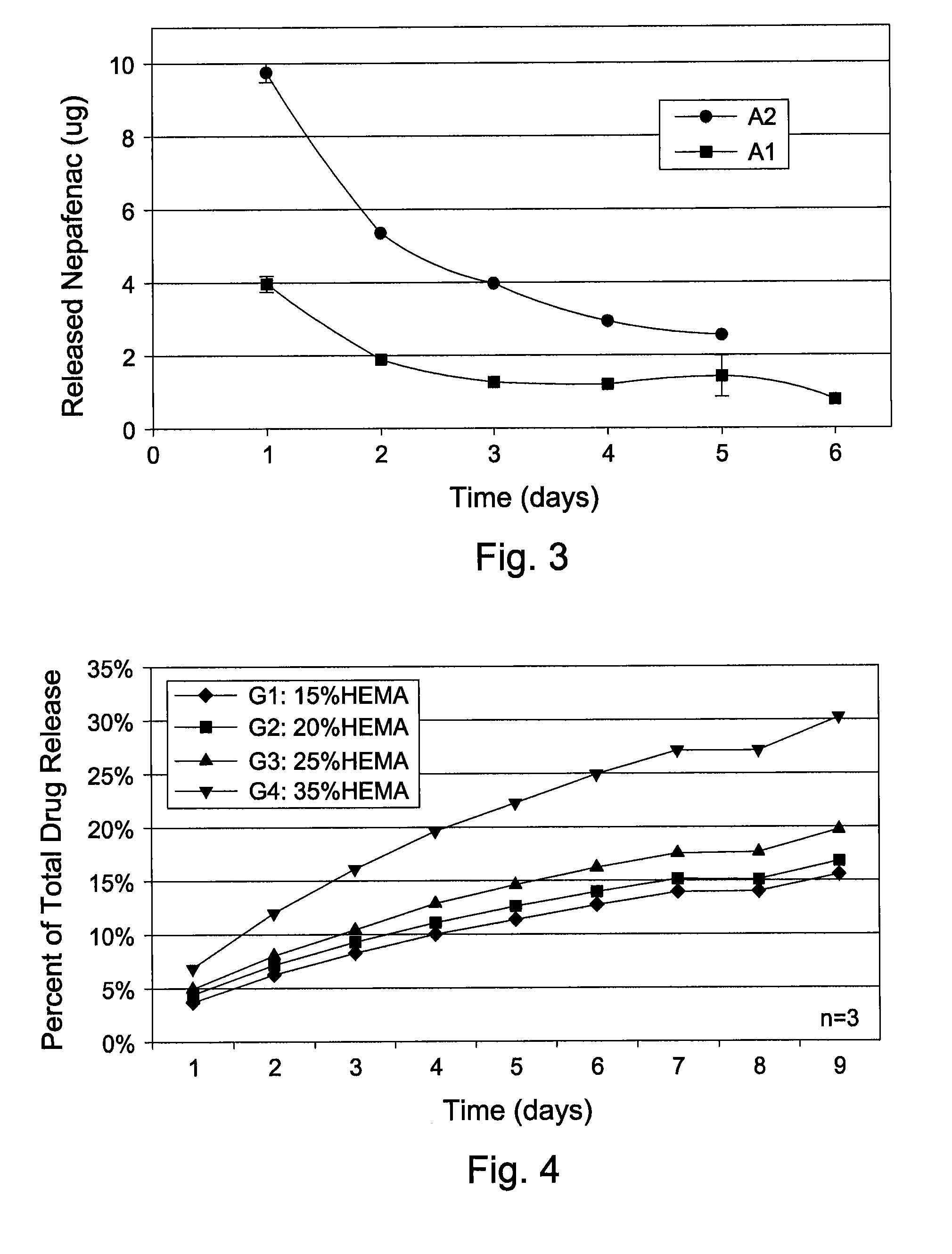
Tamper resistant dosage form with bimodal release profile
InactiveUS9737490B2Patient compliance is goodQuick effectOrganic active ingredientsNervous disorderBreaking strengthImmediate release
The invention relates to a pharmaceutical dosage form comprising(i) at least one formed segment (S1), which contains a first pharmacologically active ingredient (A1) and provides prolonged release thereof, and(ii) at least one further segment (S2), which contains a second pharmacologically active ingredient (A2) and provides immediate release thereof,wherein the at least one formed segment (S1) exhibits a higher breaking strength than the at least one further segment (S2) and the at least one formed segment (S1) exhibits a breaking strength of more than 500 N.
Owner:GRUNENTHAL GMBH
Delayed prolonged drug delivery
In one aspect, the present invention is concerned with a treatment where it is desired that an active agent is designed to be released in a prolonged manner at a time point some time after administration of the active agent. The present invention is particularly suited to administering an agent which may be released whilst a subject is sleeping, shortly before waking and continues to administer the drug during the early waking hours. As well as treating certain conditions by a particular regime, the invention also provides novel formulations for a delayed, followed by a prolonged release of drug.
Owner:DRUG DELIVERY INT
Method and compositions for enhanced delivery of bioactive molecules
Formulations for controlled, prolonged release of bioactive molecules such as therapeutic proteins, peptides and oligonucleotides have been developed. These formulations are based on solid microparticles or nanoparticles formed of the combination of biodegradable, synthetic polymers such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and copolymers thereof. Bioactive molecules are coupled to hydrophilic polymers such as polyethylene glycol or polypropylene glycol and formulated to provide controlled release. The bioactive molecules are more stable, less immunogenic and have improved release rate profiles with lower burst levels and increased drug loading relative to the same bioactive molecules lacking coupled hydrophilic polymers. The controlled release formulations can be administered by injection, by inhalation, nasally, or orally.
Owner:PR药品有限公司
Prolonged release bioadhesive therapeutic systems
InactiveUS7651698B2Reduce in quantityStable levelAntibacterial agentsOrganic active ingredientsMedicineDissolution
Owner:VECTANS PHARMA
Polymer coatings containing phytochemical agents and methods for making and using same
InactiveUS7972635B2Reduce and destroy foulingGrowth inhibitionBiocideAntifouling/underwater paintsControlled releasePhytochemical
This invention relates to compositions comprising a polymer base incorporating antifouling compositions suitable for use in aquaculture, marine and architectural systems as paints, structures or coatings. In particular, the present invention relates to a polymer based coating incorporating a biocidal antifouling composition suitable for use with aquaculture equipment. The present invention provides polyethylene compositions and latex compounds which may comprise at least one environmentally benign phytochemicals suitable for use in preventing the colonization of a treated surface by a variety of biological species. The compositions of the invention may further comprise control release agents such as, for example, micro-encapsulation of the phytochemicals to maintain sustained and prolonged release of the biocidal agents at the treated surface.
Owner:MAGELLAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com