Patents
Literature
541 results about "Coated tablets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Penn Pharma's chief executive officer, Richard Yarwood, said, "It is a monumental day for Penn Pharma; in less than one year we have built a world-class facility for contained manufacturing and produced our first batch of coated tablets.
Coated tablet formulation and method
ActiveUS20050266080A1Good chemical stabilityEasy to prepareBiocideMetabolism disorderCoated tabletsExcipient
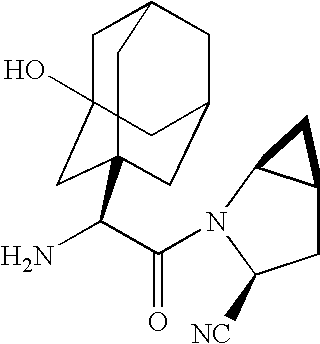
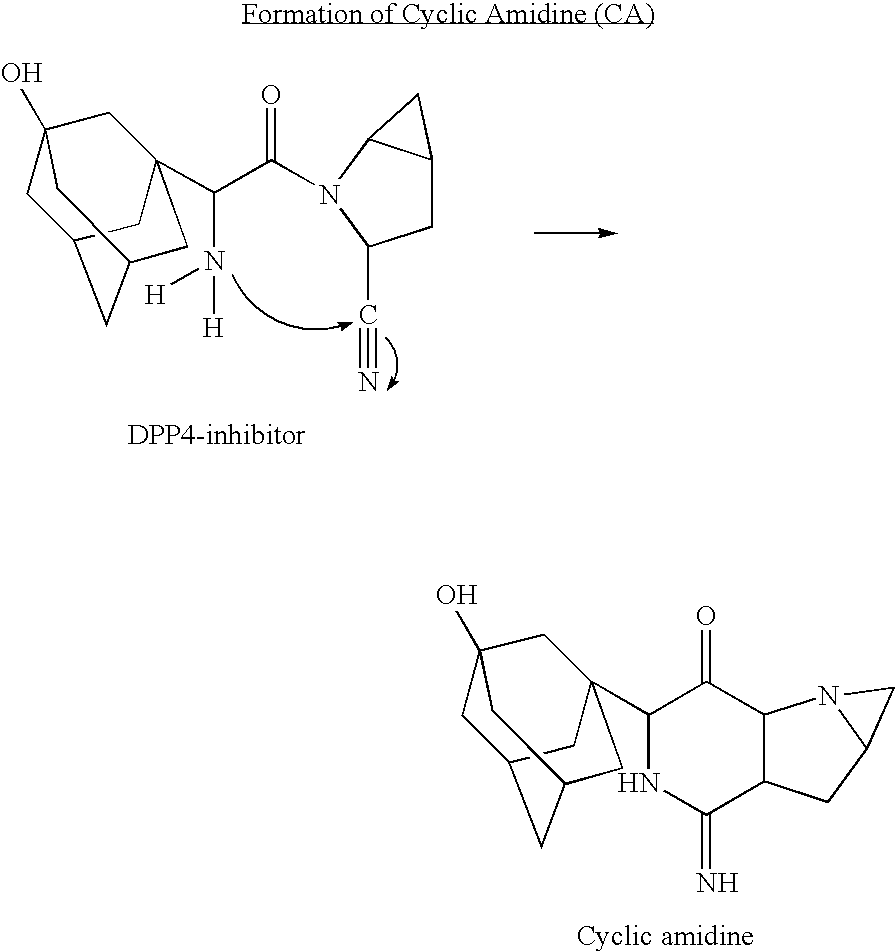
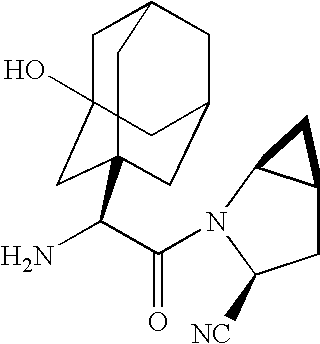
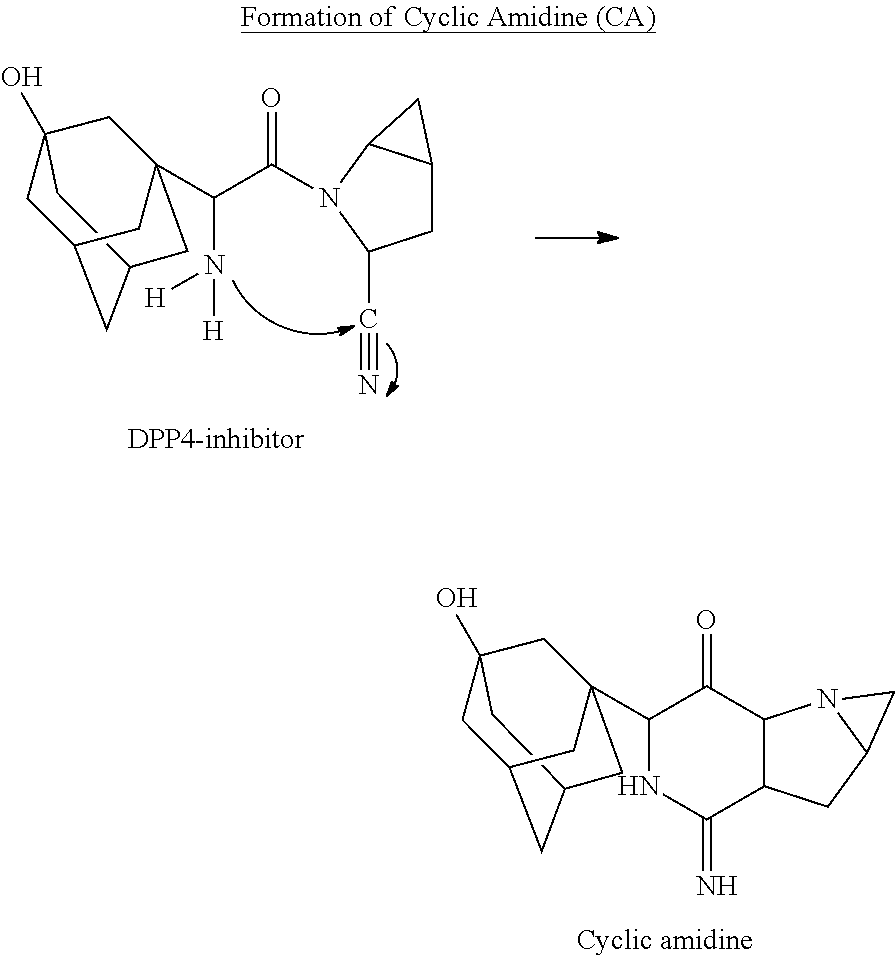
A coated tablet formulation is provided which includes a medicament such as the DPP4-inhibitor, saxaglipitin or its HCl salt, which is subject to intra-molecular cyclization, which formulation includes a tablet core containing one or more fillers, and other conventional excipients, which tablet core includes a coating thereon which may include two or more layers, at least one layer of which is an inner seal coat layer which is formed of one or more coating polymers, a second layer of which is formed of medicament which is the DPP4-inhibitor and one or more coating polymers, and an optional, but preferable third outer protective layer which is formed of one or more coating polymers. A method for forming the coated tablet is also provided.
Owner:ASTRAZENECA AB
Edible holographic products, particularly pharmaceuticals and methods and apparatus for producing same
InactiveUS20060068006A1Preserve product integrityCheap productionPretreated surfacesDiffraction gratingsCoated tabletsPlasticizer
An edible product such as a unit dosage form of a pharmaceutically active substance includes a layer of a material that can receive and retain a high resolution microrelief that can convey information. The microrelief is themo-formable, preferably formed from an aqueous solution of HPMC and / or HPC plus a plasticizer and colorant. Other additives such as strengtheners, surfactants and adherents may be used depending on the application. The materials are selected and proportioned to control the fading or change in color of the visual image or effect produced by the relief to indicate exposure to an unacceptable degree of heat or humidity. The dosage form can be the relief-containing layer itself with the pharmaceutical carried therein. In a preferred form, the layer is an outer coating over a core containing the pharmaceutically active substance. Coated tablets are configured to resist twinning. To produce such dosage forms, the coated core is transported in unison with a flexible mold or transfer plate that can heat-replicate the microrelief on the outer layer of the dosage form, followed by a cooling and release of the transfer plate from the coating.
Owner:EDWARDS ANGELL PALMER & DODGE
Rapidly disintegrating gelatinous coated tablets
The present invention relates to an improved gelatinous coated dosage form having two end regions coated with gelatinous materials and an exposed circumferential band. Openings are provided in at least the exposed band to reveal the core material. The invention also relates to methods for manufacturing such gelatinous coated dosage forms.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Rapidly disintegrating gelatinous coated tablets
The present invention relates to an improved gelatinous coated dosage form having two end regions coated with gelatinous materials and an exposed circumferential band. Openings are provided in at least the exposed band to reveal the core material. The invention also relates to methods for manufacturing such gelatinous coated dosage forms.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Osmotic tablet with a compressed outer coating
The present invention features a method of manufacturing an osmotic tablet including the steps of (i) compressing a tablet core including a first pharmaceutically active agent and a hydrophilic polymer; (ii) applying an osmotic coating to the outer surface of the tablet core to form a coated tablet, wherein the osmotic coating includes at least one opening exposing the tablet core; and (iii) compressing an immediate release coating onto the surface of the coated tablet, wherein the release coating includes a second pharmaceutically active agent.
Owner:MCNEIL PPC INC
Coated tablets with remaining degradation surface over the time
The present invention relates to a pharmaceutical composition for controlled delivery of at least one active ingredient into an aqueous phase, said pharmaceutical composition comprising: a tablet, preferably obtainable by compression, said tablet comprising said at least one active ingredient and optionally excipients; and a coating, applied on said tablet, said coating covering at least part of said tablet to impede the release of said at least one active ingredient from at least part of the surface of said tablet, said coating being applied in a manner allowing the release of said at least one active ingredient from said tablet after contacting said pharmaceutical composition with said aqueous phase, establishing one or more degradation surfaces of said tablet; wherein the first derivative of the area of each degradation surface with respect to time is larger than or equal to zero.
Owner:BIONEER
Edible holographic products, particularly pharmaceuticals and methods and apparatus for producing same
InactiveUS7083805B2Preserve product integrityCheap productionPretreated surfacesDiffraction gratingsCoated tabletsPlasticizer
An edible product such as a unit dosage form of a pharmaceutically active substance includes a layer of a material that can receive and retain a high resolution microrelief that can convey information. The microrelief is themo-formable, preferably formed from an aqueous solution of HPMC and / or HPC plus a plasticizer and colorant. Other additives such as strengtheners, surfactants and adherents may be used depending on the application. The materials are selected and proportioned to control the fading or change in color of the visual image or effect produced by the relief to indicate exposure to an unacceptable degree of heat or humidity. The dosage form can be the relief-containing layer itself with the pharmaceutical carried therein. In a preferred form, the layer is an outer coating over a core containing the pharmaceutically active substance. Coated tablets are configured to resist twinning. To produce such dosage forms, the coated core is transported in unison with a flexible mold or transfer plate that can heat-replicate the microrelief on the outer layer of the dosage form, followed by a cooling and release of the transfer plate from the coating.
Owner:EDWARDS ANGELL PALMER & DODGE
Coated tablet formulation and method
InactiveUS20050214373A1Good chemical stabilityPrevent photolytic degradation and hydrolysisBiocideMetabolism disorderCoated tabletsPeliglitazar
A coated tablet formulation is provided which includes a medicament such as the PPAR α / γ dual agonist peliglitazar or muraglitazar. The coated tablet includes a tablet core containing one or more fillers, one or more binders, one or more disintegrants, and other conventional excipients, and a coating on the tablet core, which coating may include one or more layers, at least one layer of which is formed of medicament and one or more coating polymers, preferably a hydroxypropylmethyl cellulose based polymer. A method for forming the coated tablet via a spray-dried coating technique is also provided.
Owner:BRISTOL MYERS SQUIBB CO
Coated tablet with long term parabolic and zero-order release kinetics
A tablet for the controlled release of an active pharmaceutical ingredient. The tablet comprises a core having a donut-like configuration with a cylindrical hole extending through the center of the core. The core of the body comprises at least one active pharmaceutical agent and at least one hydrophilic, water-soluble, polymeric carrier. The core is coated with a hydrophobic, water-insoluble material covering all of the core except that which is defined by the cylindrical hole. Also included is a method of preparing a tablet for the controlled release of an active ingredient. The method comprises the steps of blending an active pharmaceutical ingredient, a water-soluble hydrophilic, polymeric carrier, and optionally an excipient, to form a mix; compressing the mix; punching a tablet from the mix; coating the tablet in a water insoluble, hydrophobic coating, then drilling a hole through the coated tablet. A second embodiment of this method includes drilling a first hole through the tablet before coating the tablet, placing a rod through the first hole, dipping the tablet into the coating, and then removing the rod and drilling a second hole in the tablet which is larger than the first hole.
Owner:TEMPLE UNIVERSITY
Controlled-release colon targeting drug administration preparation and preparation method thereof
InactiveCN101780055AConvenient treatmentImprove complianceOrganic active ingredientsDigestive systemIntestinal tract diseasesColonic segment
The invention relates to a controlled-release colon targeting drug adminitration preparation. The forms of the preparation are colon site-specific coated tablets or colon targeting pellets. The preparation consists of a tablet core or pellet core, an isolating layer and a controlled-release coating layer, wherein the controlled-release coating layer comprises an internal coating layer and an external coating layer. By adopting the multilayer coating technology, enteric soluble acrylic resin water dispersion and osmotic acrylic resin water dispersion are used as main coating materials for carrying out coating, thereby obtaining the controlled-release colon targeting drug adminitration preparation. The preparation of the invention enables drugs to be released at a constant rate at a colon section, realizes accurate site-specific drug release, increases the concentration of the drugs at some parts of positions with pathological changes, is beneficial to treating ulcerative colitis and carcinoma of colon, avoids the stimulation of the drugs on stomaches and small intestines, achieves the goal of colon site-specific drug release, enhances the targeting site-specific curative effect on colon diseases and reduces the toxic and side effect. Compared with the common oral preparations, under the condition of the same drug adminitration dosage, the preparation of the invention can enhance the curative effect and reduce the incidence rate of untoward reactions. Compared with the enemas or the rectal suppositories, the preparation has the advantages of uniform drug distribution in the colon and good patient compliance.
Owner:ZHEJIANG UNIV
Solid pharmaceutical formulations comprising bibw 2992
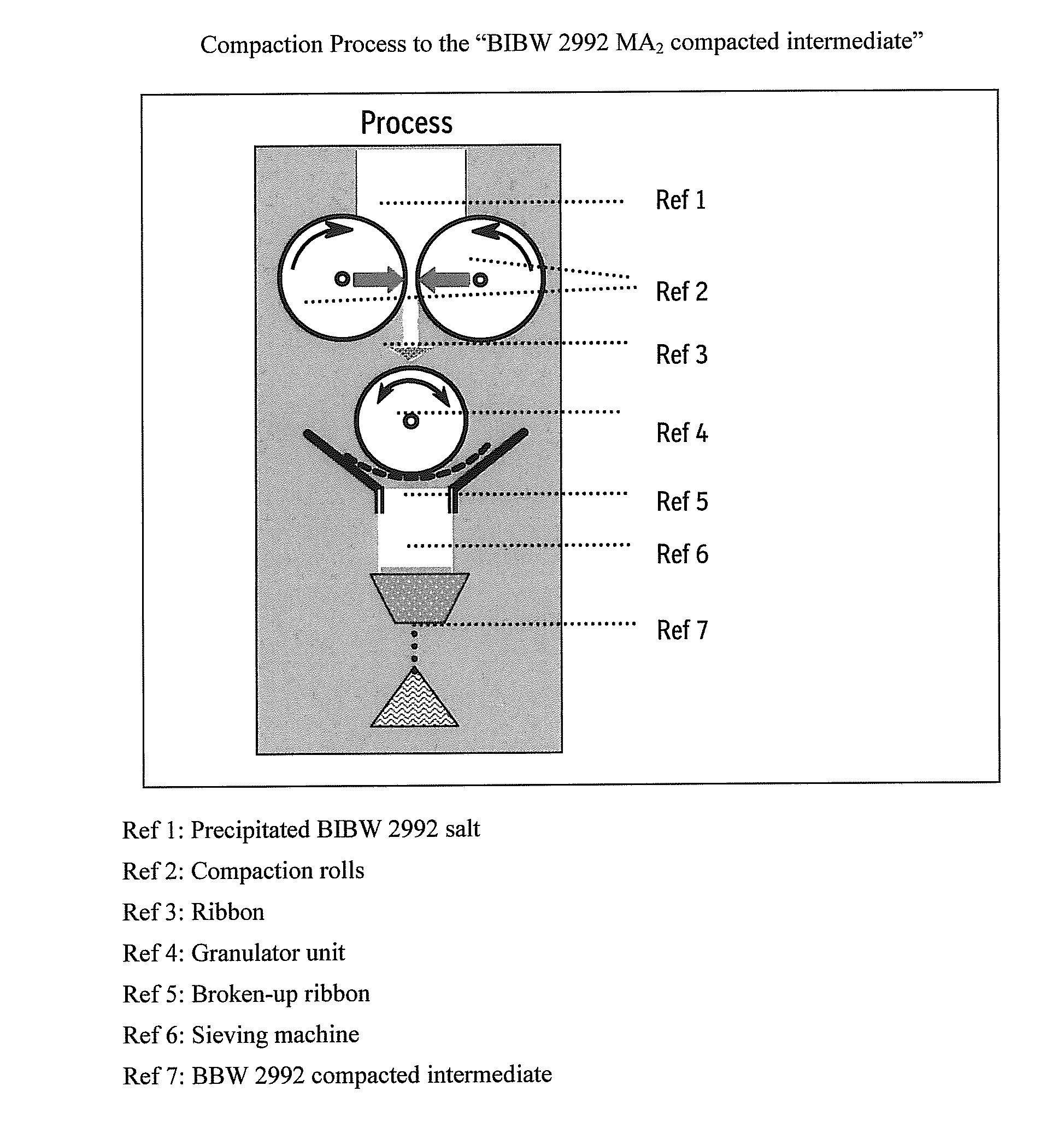
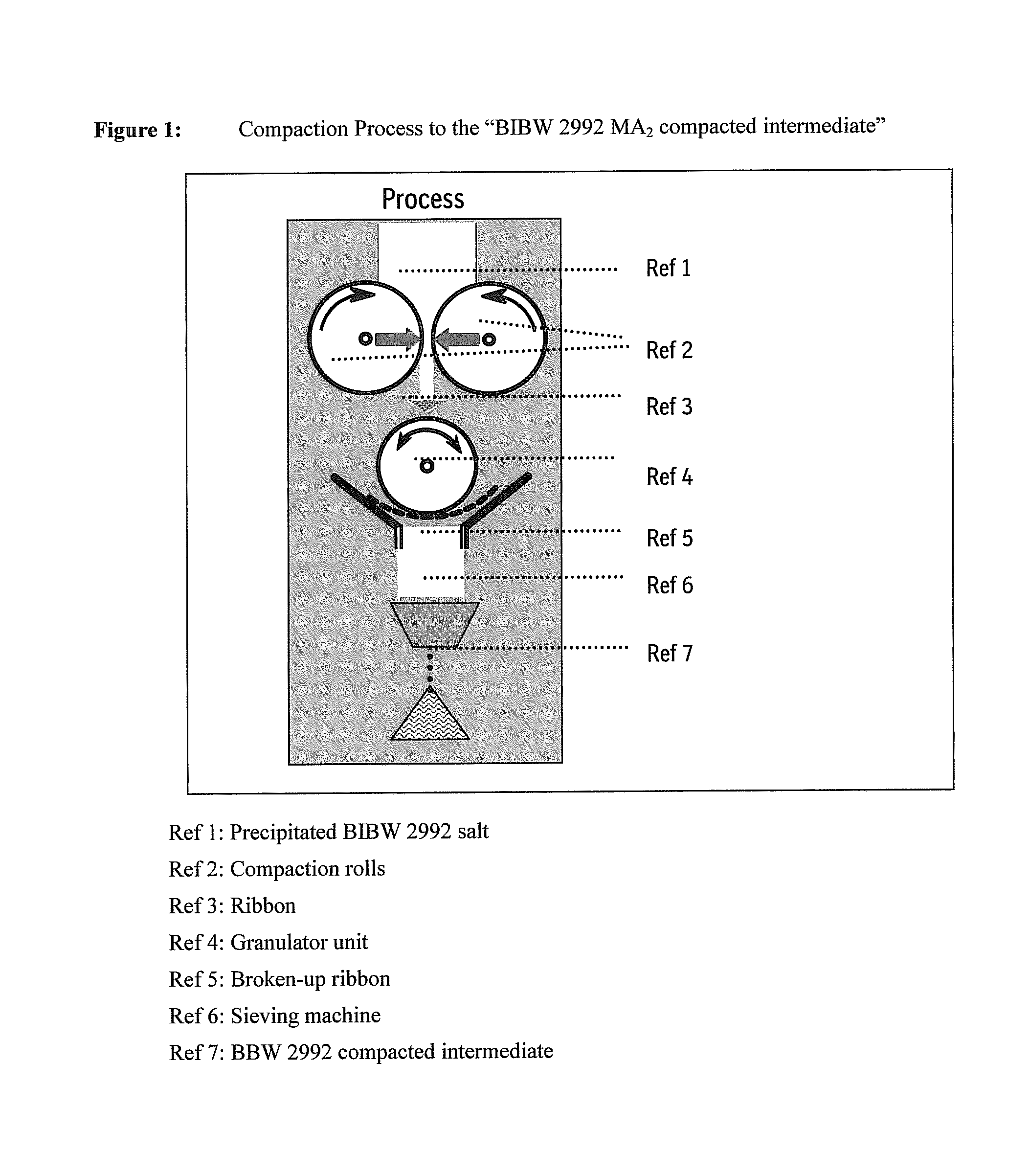
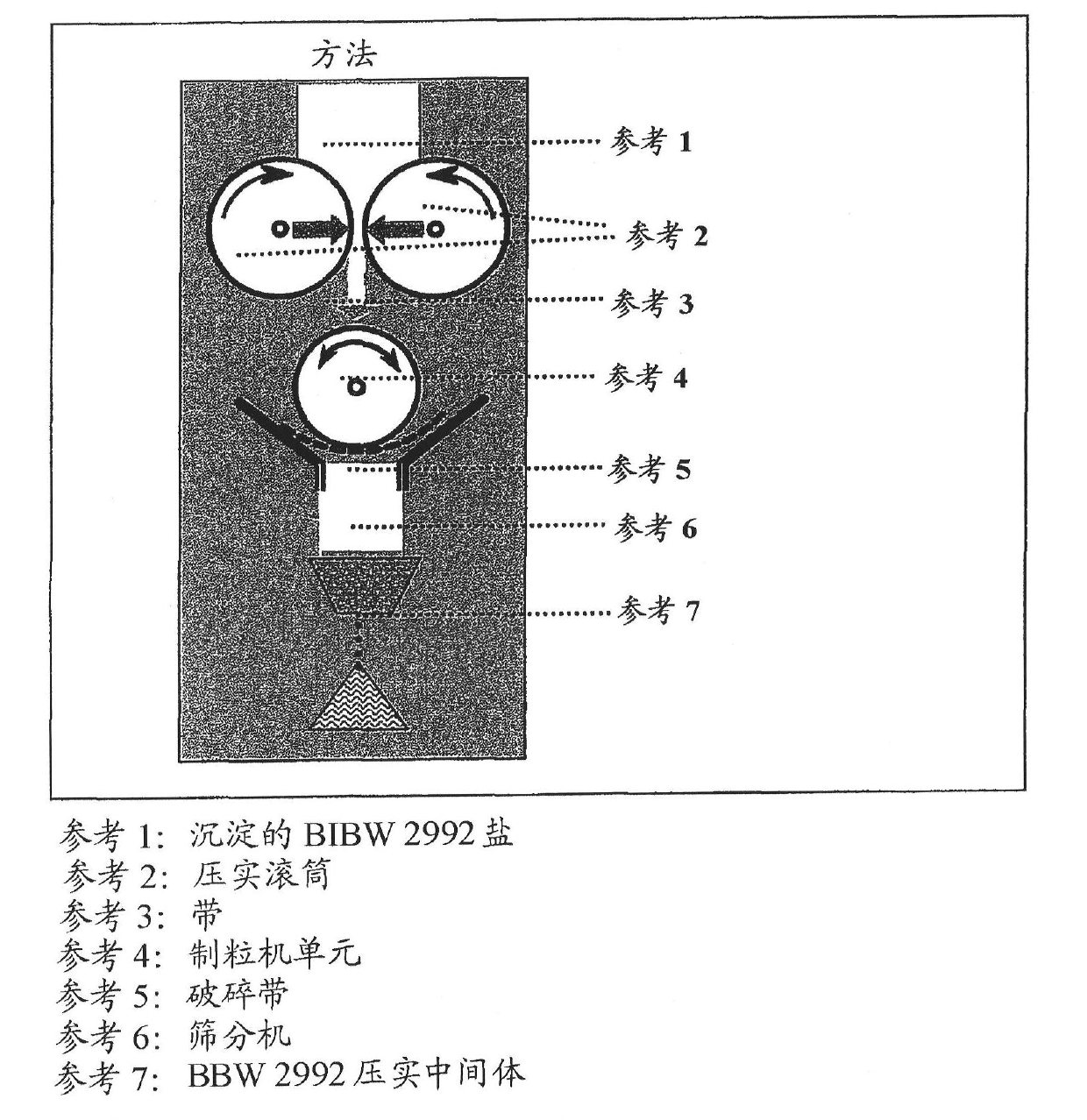
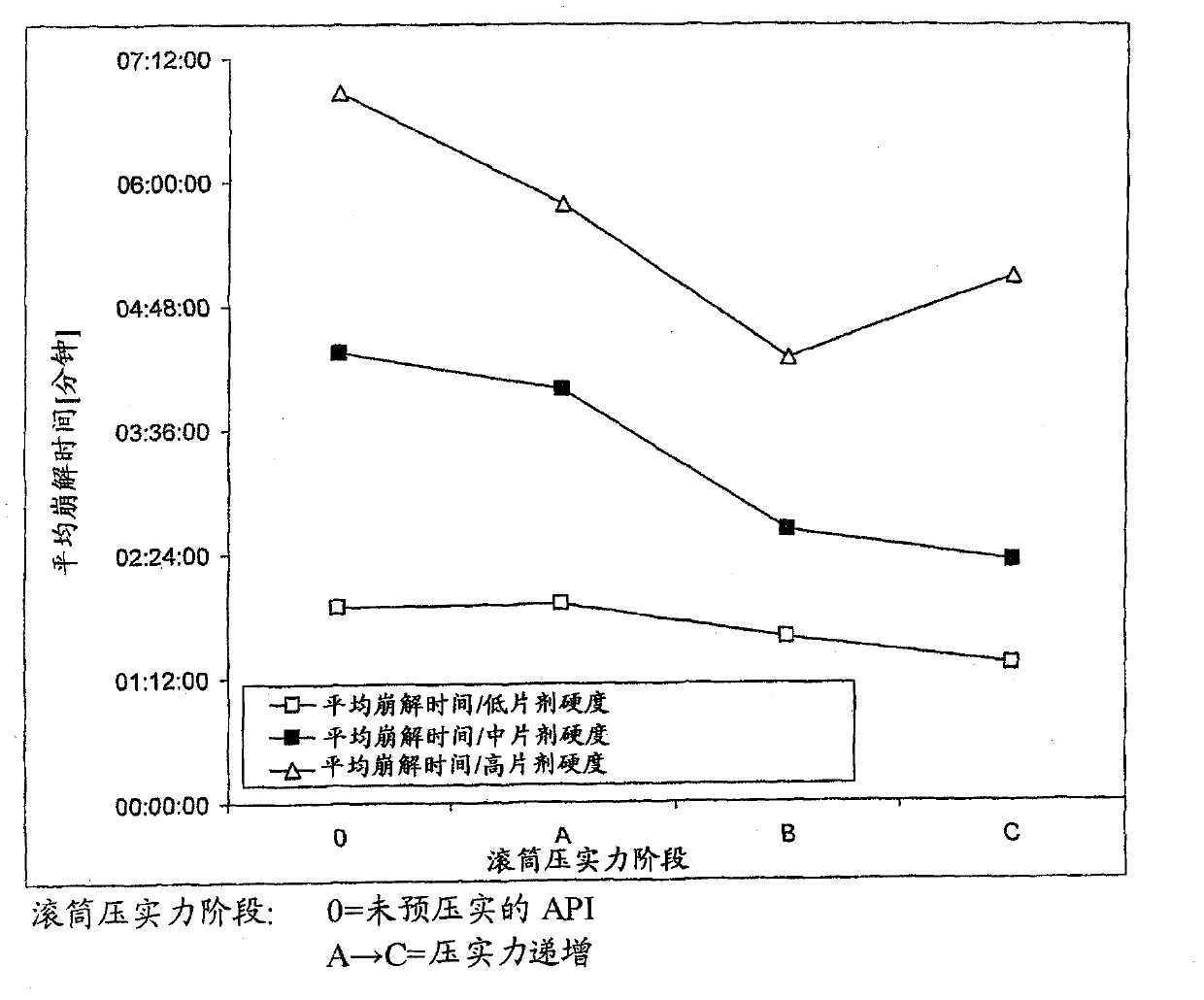
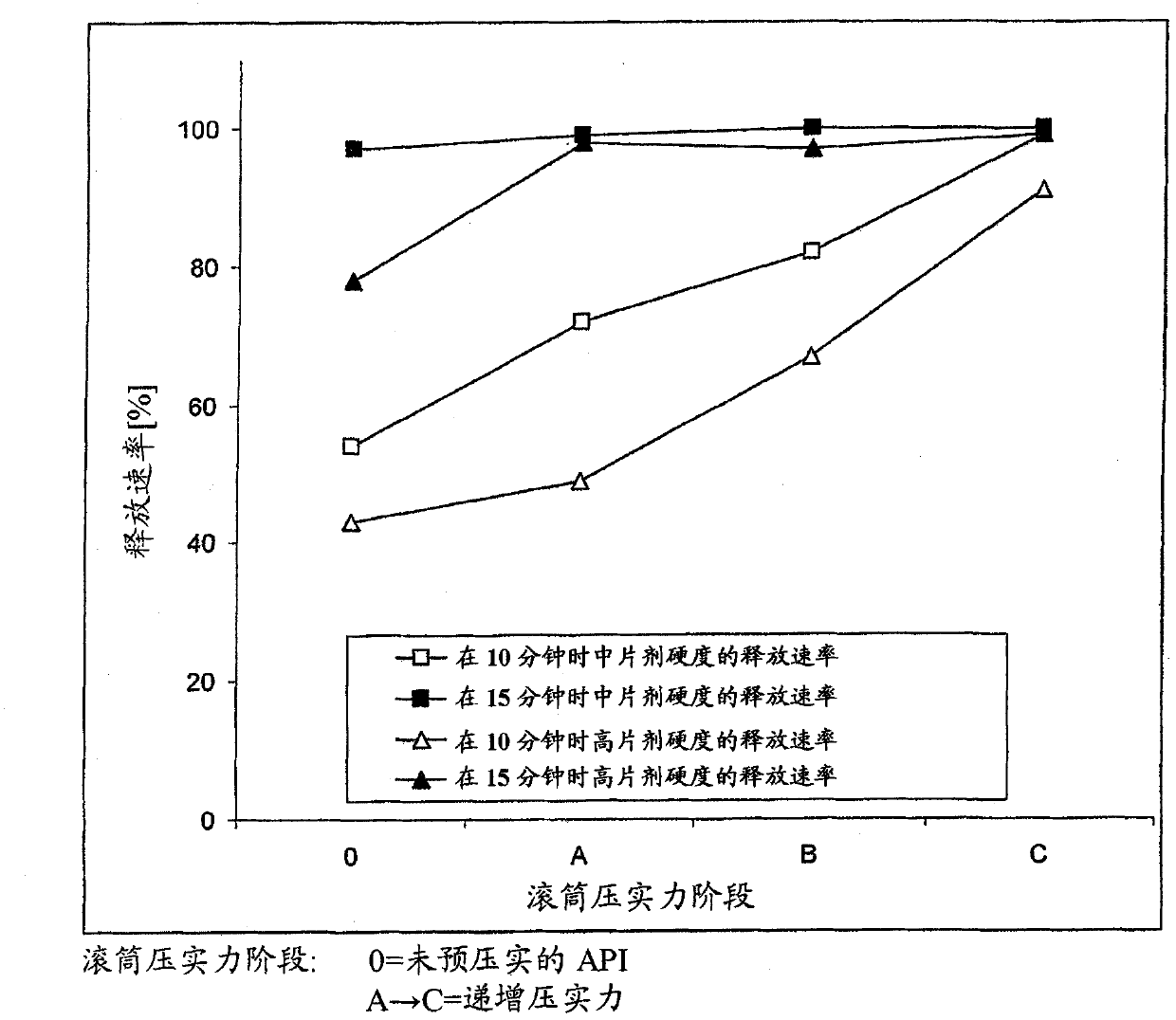
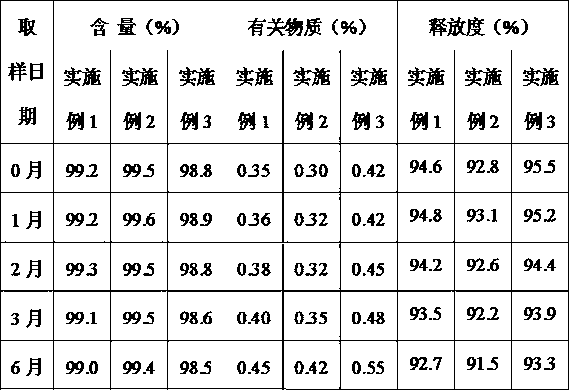
ActiveUS20110142929A1Increase speedImprove liquidityPowder deliveryOrganic active ingredientsCoated tabletsImmediate release
The present invention relates to a pharmaceutical dosage form containing the active substance BIBW 2992 as the dimaleate salt, providing an immediate release profile of the active substance, further, the invention relates to compacted intermediates comprising BIBW 2992 dimaleate salt (BIBW 2992 MA2) in form of a powder prepared using a combined roller compaction and sieving step from BIBW 2992 MA2, intermediate blends prepared from said compacted intermediate as well as solid oral formulations providing an immediate release profile of the active substance, made from said compacted intermediate or from said intermediate blends ready for use / ingestion, e.g. capsule and tablet formulations such as uncoated or film-coated tablets prepared by direct-compression, and methods for their production.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical formulation consisting of a plant dry extract with a calcium coating
InactiveUS7629005B2BiocideInorganic phosphorous active ingredientsVitex agnus-castusBelamcanda chinensis
A pharmaceutical formulation of a calcium salt and a dry plant extract in the form of a coated tablet, in which the formulation has a core of at least one dry plant extract, enveloped by at least one coating of at least one calcium salt. The plant extracts for the core may be selected from: Vitex agnus castus (chaste tree); Belamcanda chinensis (leopard lily); Cimicifuga racemosa (black cohosh); Trifolium pratense L. (purple trefoil); Oenothera biennis hom. (primrose); Glycine soja (soy bean); Serenoa repens (saw-palmetto); Urtica dioica (stinging nettle), in particular its root; Cucurbita pepo (pumpkin), in particular its seed; Pygeum africanum; as well as suitable mixtures of these. Methods for the use of the formulation in treating osteoporosis and for manufacturing the formulation are provided.
Owner:BIONORICA AG
Anti-stress, anti-impairment and anti-aging drug and process for manufacturing thereof
The invention relates to a new pharmacotherapeutical strategy, to an anti-stress, anti-impairment and anti-aging drug and to a process for its manufacturing. The drug has an etio-pathogenic and homeostatic action, was preclinically tested and clinically checked up in geriatric, neurologic, psychiatric and stress-dependent pathology. The drug achieves a synergistic biological, neurometabolic and cell-trophic composition, being elaborated by the association of the following active principles: a) against oxidative and catabolic stress; methionine with aminoethanol phenoxyacetates and / or aminoethyl phenoxyacetamides; b) against anabolic stress; hydroxopyrimidine carboxylates and / or oxopyrrolidine acetamides with potassium, zinc and lithium; c) vasodilative and normolipidemic; nicotinic, alcohol and / or acid, or its derivatives, with magnesium and iodine; d) energo-active and e) anti-toxic; aspartate; fructose; vitamin B1; vitamin B6; monoacid phosphate and sulfate. The process for manufacturing the drug stipulates; a) pharmaceutical preparation in two complementary types of capsules or coated tablets, gastrosoluble and enterosoluble, the last being enteric coated; b) prolonged-release of vasodilator from the enterosoluble unit.
Owner:RIGA DAN +1
Direct compression polymer tablet core
The present invention provides a tablet core which comprises at least about 95% by weight of an aliphatic amine polymer. The invention also provides a method of producing a tablet core comprising at least about 95% by weight of an aliphatic amine polymer resin The method comprises the step of compressing the aliphatic amine polymer to form the tablet core. The tablet core can further include one or more excipients. In this embodiment, the method of producing the tablet core comprises the steps of: (1) hydrating the aliphatic amine polymer to the desired moisture level; (2) blending the aliphatic amine polymer with the excipients in amounts such that the polymer comprises at least about 95% by weight of the resulting blend; and (3) compressing the blend to form the tablet core. The present invention further relates to a coated tablet comprising an aliphatic amine polymer core wherein the coating is a water based coating.
Owner:TYLER JOSEPH +1
Direct compression polymer tablet core
The present invention provides a tablet comprising a compressed tablet core which comprises at least about 80% by weight of an aliphatic amine polymer. The invention also provides a method of producing a tablet core comprising at least about 80% by weight of an aliphatic amine polymer resin. The method comprises the step of compressing the aliphatic amine polymer to form the tablet core. The tablet core can further include one or more excipients. In this embodiment, the method of producing the tablet core comprises the steps of: (1) hydrating the aliphatic amine polymer to the desired moisture level; (2) blending the aliphatic amine polymer with the excipients in amounts such that the polymer comprises at least about 80% by weight of the resulting blend; and (3) compressing the blend to form the tablet core. The present invention further relates to a coated tablet comprising an aliphatic amine polymer core wherein the coating is a water based coating.
Owner:GENZYME CORP
Coated tablet formulations and uses thereof
ActiveUS20110111018A1Good processing characteristicsMaintaining acceptable pharmokinetic propertyBiocideCapsule deliveryCoated tabletsCombinatorial chemistry
The present invention provides coated tablet formulations comprising neratinib maleate, and improved methods for making such coated tablets.
Owner:WYETH LLC
Compound bismuth composition and preparation method thereof
ActiveCN101607086ASimple designGood eradicationAntibacterial agentsPowder deliveryCoated tabletsHelicobacter pylori
The invention discloses a compound bismuth composition which contains antibiotics, a coated tablet of antibacterial agent and bismuth particle or powder, wherein the coated tablet is coated with Opadry II and the coating amount is 3 percent by weight; and the weight ratio of bismuth to the antibiotics to the antibacterial agent is 4-35:5-50:5-50. The invention also discloses a preparation method of the compound bismuth composition. The compound bismuth composition achieves good balance between the mutual action among active ingredients and the action of the active ingredients performed in stomach, and therefore is used for better eliminating the helicobacter pylori of stomach.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
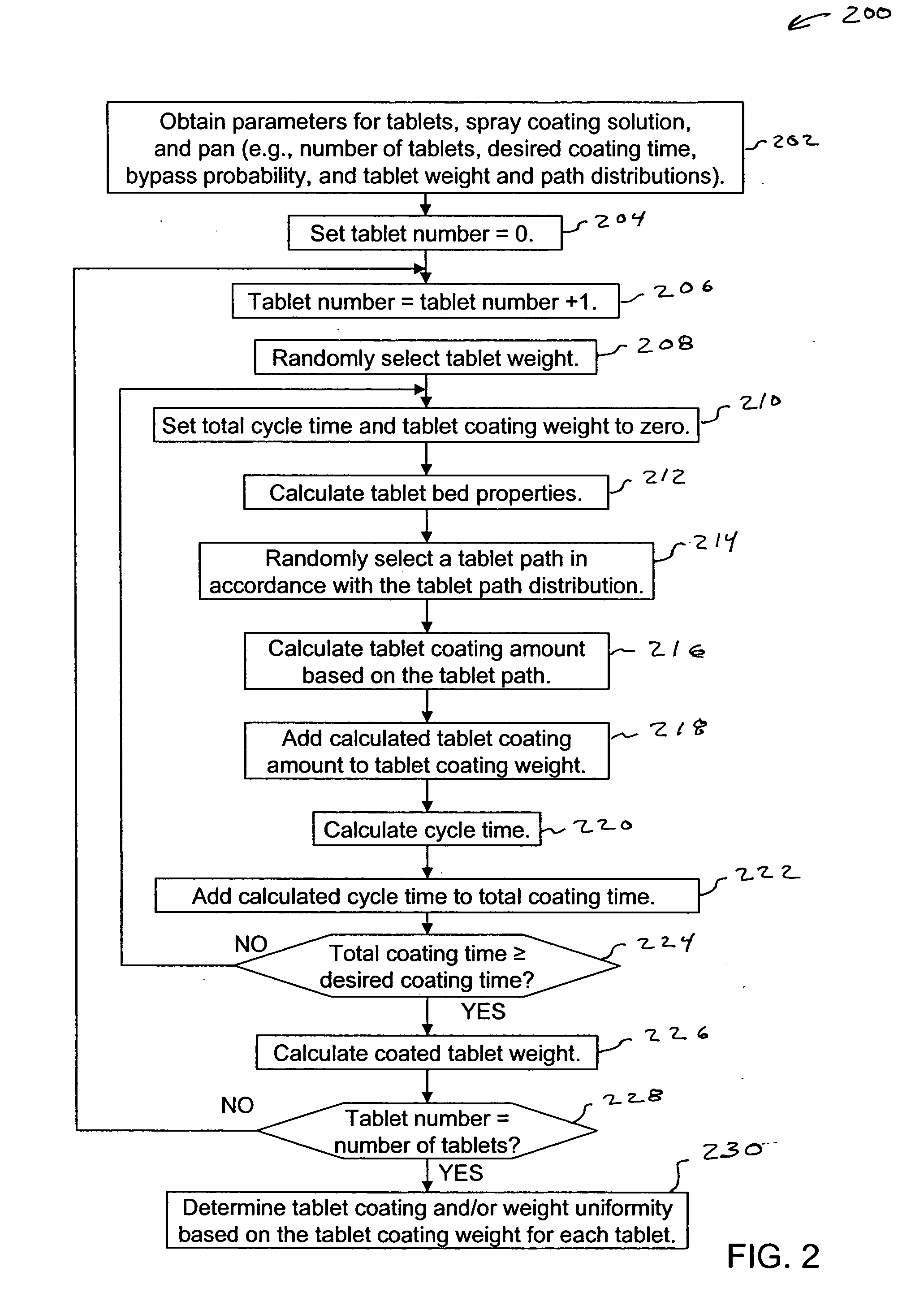
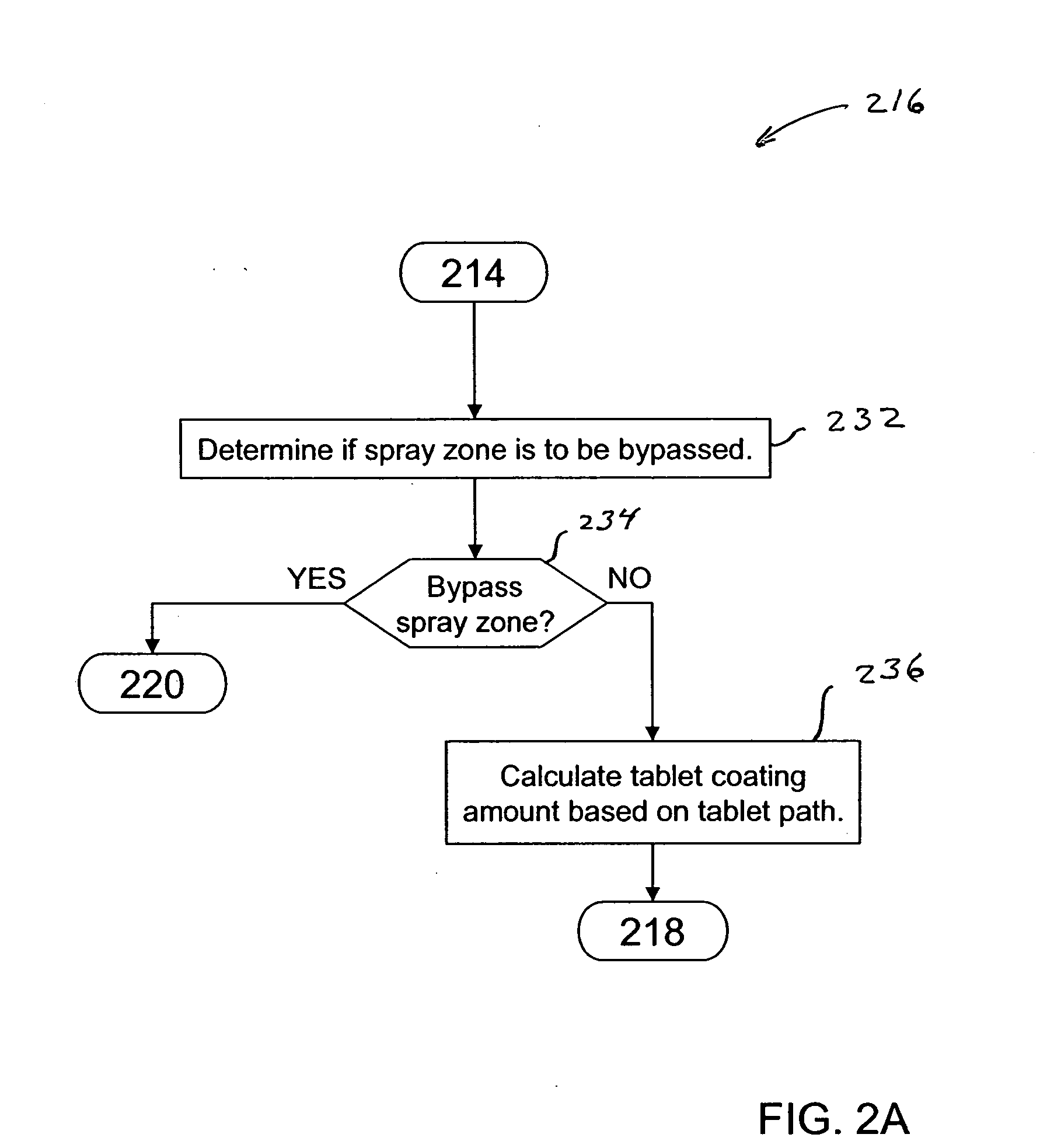
Pan coating simulation for determining tablet coating uniformity
ActiveUS20060100786A1Programme controlAnalogue computers for chemical processesTablet computerCoated tablets
A method and computer program product for simulating a pan coating process estimates at least one of tablet coating uniformity or coated tablet weight uniformity for tablets within a tablet bed of a pan. The simulation includes obtaining a plurality of parameters associated with the pan coating process, selecting tablet paths for each cycle of a simulation tablet in the tablet bed, determining a coating amounts for the tablet based on selected tablet paths, determining cycle times for the tablet, and summing the determined coating amounts and cycle times for the tablet. The simulation is repeated for each of a defined number of simulation tablets representing the tablets within the tablet bed to determine tablet coating uniformity and / or coated tablet weight uniformity for the tablets. Tablet paths and / or cycle times are determined randomly, e.g., in accordance with a Monte Carlo method.
Owner:PARTICLE COATING TECH SOLUTIONS
Sublingual coated tablet of fentanyl
The present invention relates to a fentanyl coated tablet and to the method for the preparation thereof.
Owner:ETHYPHARM SA
Coated tablet formulation and method
A coated tablet formulation is provided which includes a medicament such as the DPP4-inhibitor, saxaglipitinor its HCl salt,which is subject to intra-molecular cyclization, which formulation includes a tablet core containing one or more fillers, and other conventional excipients, which tablet core includes a coating thereon which may include two or more layers, at least one layer of which is an inner seal coat layer which is formed of one or more coating polymers, a second layer of which is formed of medicament which is the DPP4-inhibitor and one or more coating polymers, and an optional, but preferable third outer protective layer which is formed of one or more coating polymers. A method for forming the coated tablet is also provided.
Owner:ASTRAZENECA AB
Ilaprazole enteric-coated tablets and preparation method thereof
InactiveCN102525990AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemActive agentSurface-active agents
The invention provides ilaprazole enteric-coated tablets and a preparation method thereof. Each enteric-coated tablet contains an enteric-coated pellet and pharmaceutically acceptable tablet excipients, wherein the enteric-coated pellet contains a pellet layer, a medicine loading layer, an isolation layer and an enteric coating layer; and the medicine loading layer contains ilaprazole or pharmaceutically acceptable salt thereof and a stabilizer. The enteric-coated pellet tablets prepared from the ilaprazole have good acid resistance; barrier substances such as an antiacid, a surfactant, an organic solvent, a hydrophobic substance and the like are not contained in the prescription, so that the enteric-coated pellet tablets have health benefits and are safe; the preparation method is easy to operate, the organic solvent is not used, and an active substance is quickly and stably released; and in addition, the pellets in the tablets can be widely and uniformly distributed in an intestinal tract after being taken, the dose dumping is dispersed, and the distribution area of a medicine on the surface of the intestinal tract is increased, so the irritation of the medicine to the intestinal tract can be reduced or eliminated, and the bioavailability of the medicine can be improved.
Owner:LIVZON PHARM GRP INC
Direct compression polymer tablet core
The present invention provides a tablet comprising a compressed tablet core which comprises at least about 80% by weight of an aliphatic amine polymer. The invention also provides a method of producing a tablet core comprising at least about 80% by weight of an aliphatic amine polymer resin. The method comprises the step of compressing the aliphatic amine polymer to form the tablet core. The tablet core can further include one or more excipients. In this embodiment, the method of producing the tablet core comprises the steps of: (1) hydrating the aliphatic amine polymer to the desired moisture level; (2) blending the aliphatic amine polymer with the excipients in amounts such that the polymer comprises at least about 80% by weight of the resulting blend; and (3) compressing the blend to form the tablet core. The present invention further relates to a coated tablet comprising an aliphatic amine polymer core wherein the coating is a water based coating.
Owner:TYLER JOSEPH +1
Solid pharmaceutical formulations comprising BIBW 2992
The present invention relates to a pharmaceutical dosage form containing the active substance BIBW 2992 as the dimaleate salt, providing an immediate release profile of the active substance, further, the invention relates to compacted intermediates comprising BIBW 2992 dimaleate salt (BIBW 2992 MA2) in form of a powder prepared using a combined roller compaction and sieving step from BIBW 2992 MA2, intermediate blends prepared from said compacted intermediate as well as solid oral formulations providing an immediate release profile of the active substance, made from said compacted intermediate or from said intermediate blends ready for use / ingestion, e.g. capsule and tablet formulations such as uncoated or film-coated tablets prepared by direct-compression, and methods for their production.
Owner:BOEHRINGER INGELHEIM INT GMBH
Esomeprazole magnesium contained enteric-coated tablet and preparation method thereof
ActiveCN102940611AHigh safety complianceImprove stabilityOrganic active ingredientsDigestive systemCoated tabletsInsulation layer
The invention provides an esomeprazole magnesium contained enteric-coated tablet and a preparation method thereof. The enteric coated tablet is composed of an inner tablet core layer which uses esomeprazole as an active ingredient, an intermediate insulation layer and an enteric coating protection layer. According to the esomeprazole magnesium contained enteric-coated tablet, the conditions that basic remedies are instable and prone to be oxidized and decomposed are overcome, the prepared enteric-coated tablet is even in coating, compact in coating layer, stable and reliable in quality and capable of meeting large-scale production requirements of enterprises at the present stage and has good market development prospects, the releasing rate can reach over 90%, the bioavailability is obviously improved, and safe and effective drug using is guaranteed.
Owner:KAMP PHARMA
Enteric soluble coating slow releasing tablet containing huperzine A and preparing method
InactiveCN1682719AReduce adverse effectsGood curative effectOrganic active ingredientsNervous disorderRelease timeReversible anticholinesterase
The present invention discloses a kind of slow releasing enteric soluble coated tablet containing hyperzine A and its preparation process. Enteric soluble coating technology is adopted to overcome the bad effect of lower pH in stomach on the diffusion of medicine in skeleton tablet to ensure the stable, slow and complete release of medicine. The slow releasing tablet contains hyperzine A as reversible anticholinesterase and has one layer of enteric soluble polymer film coating outside the skeleton slow releasing layer. The active medicine component may be also various pharmaceutically acceptable acid salts of hyperzine A. Each tablet contains hyperzine A I n10-500 microgram and has sustained release time of 20 hr.
Owner:SHENYANG PHARMA UNIVERSITY
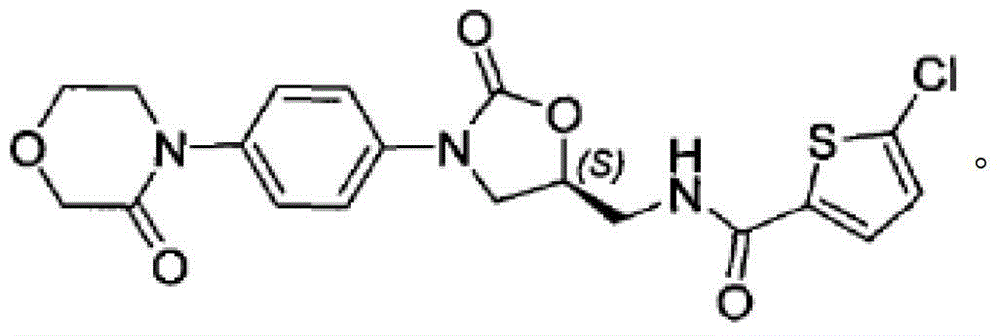
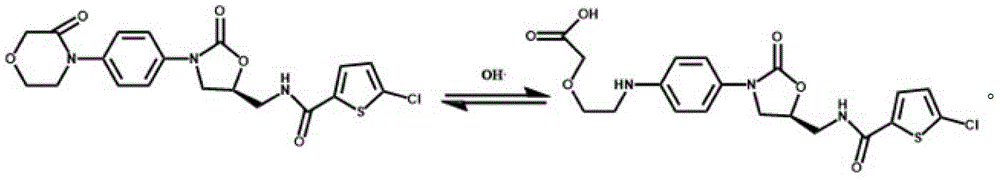
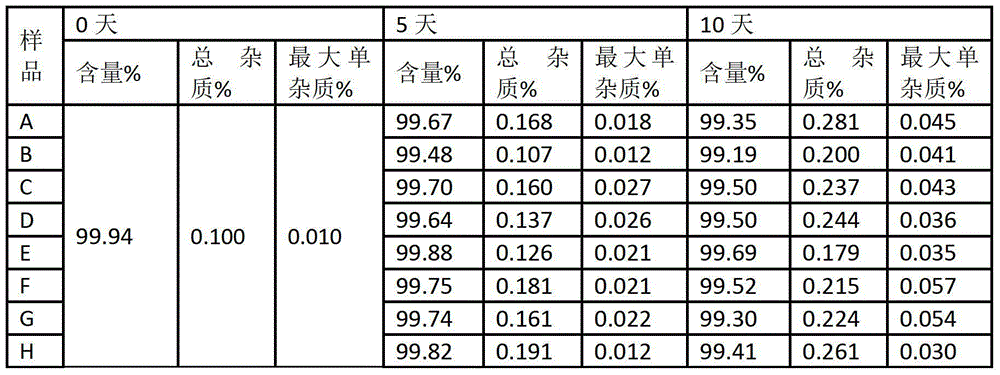
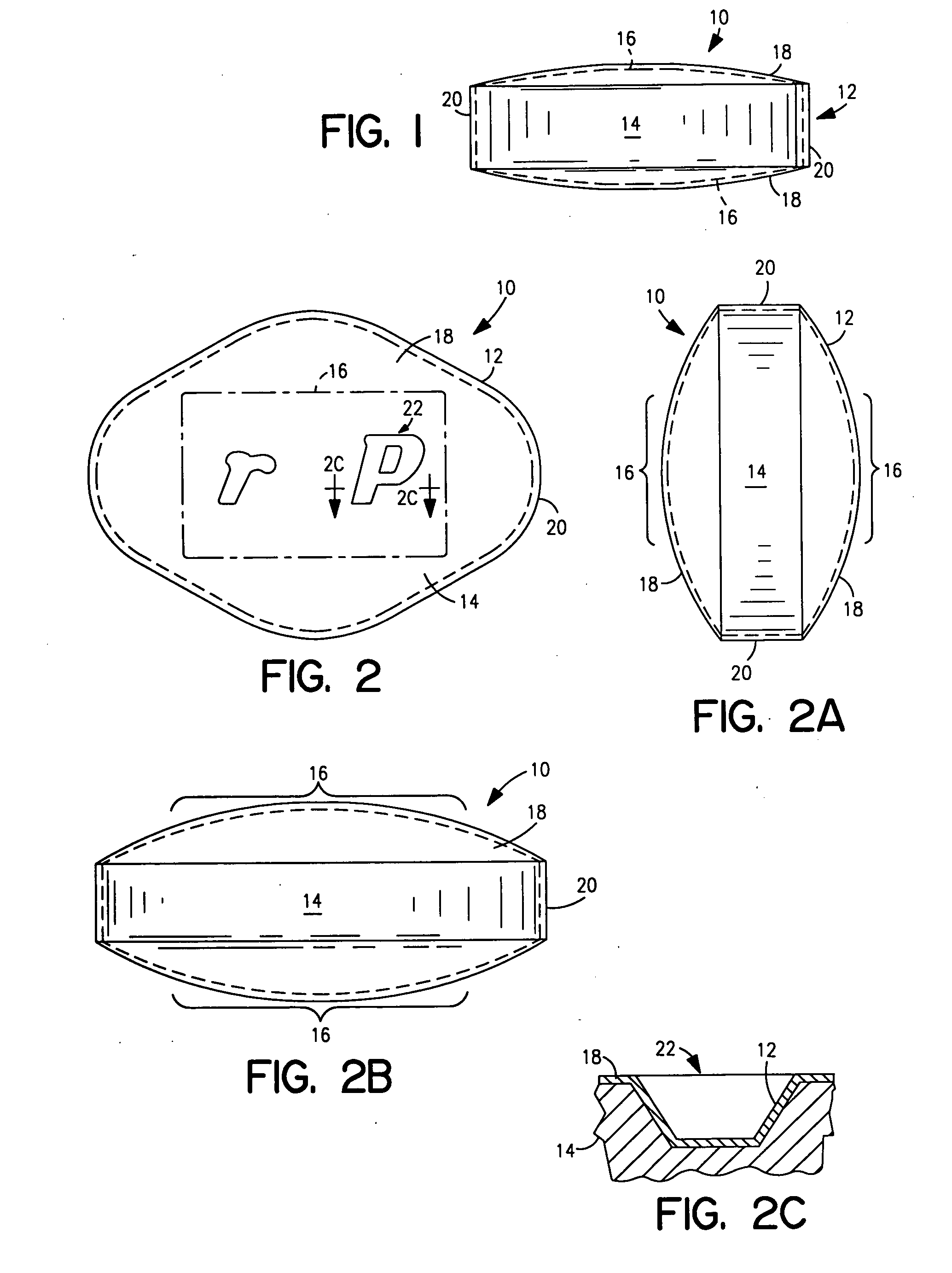
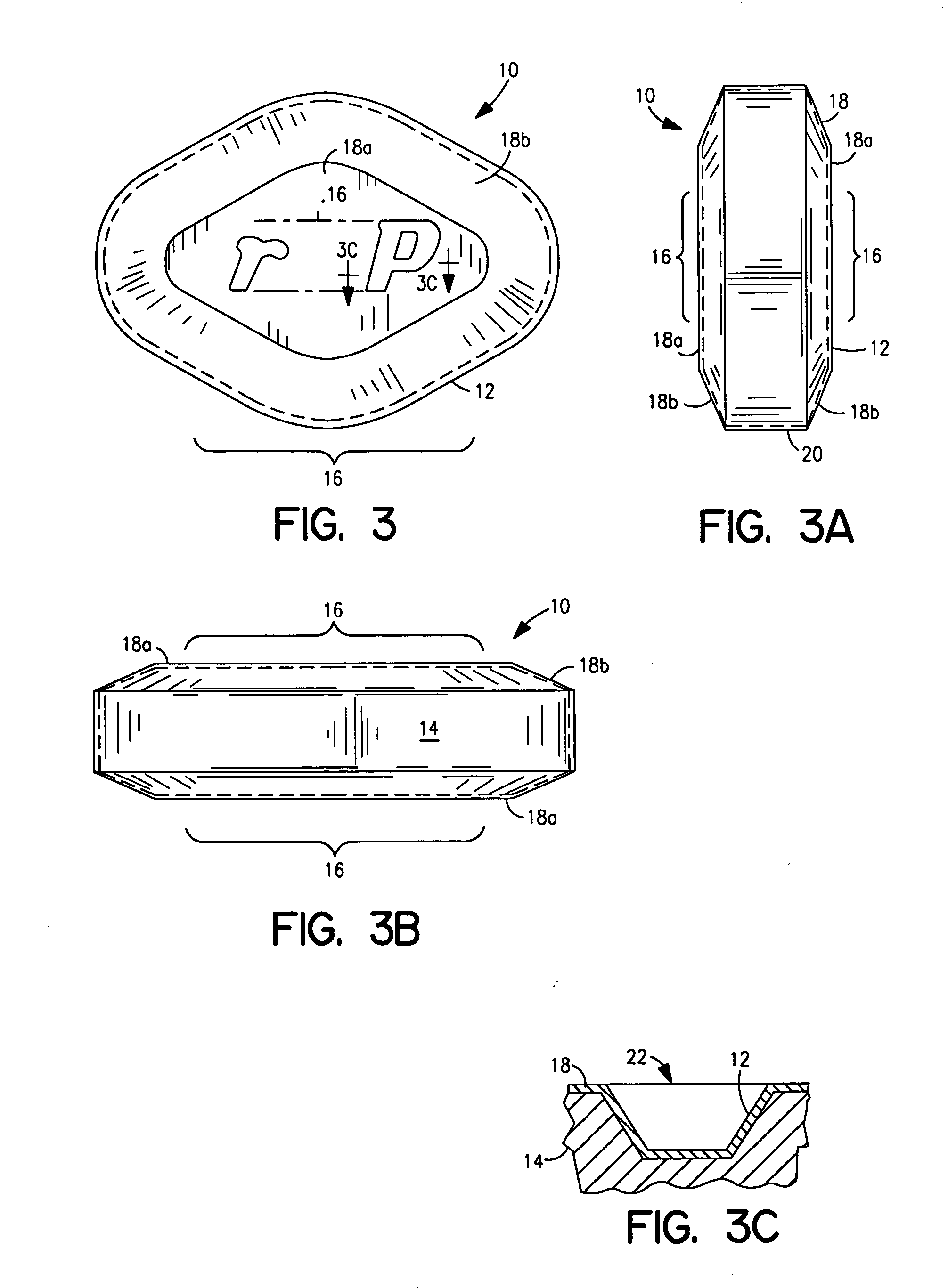
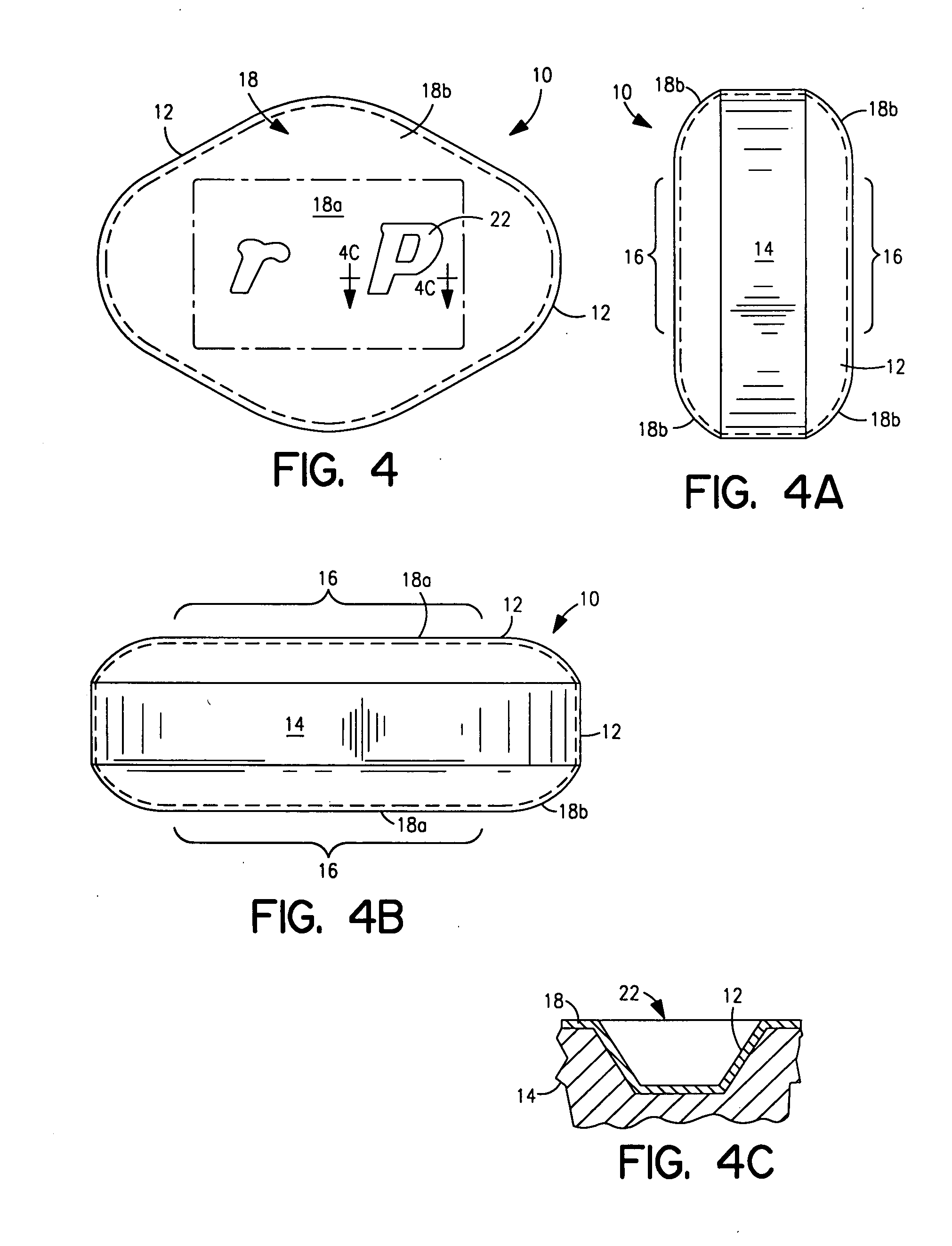
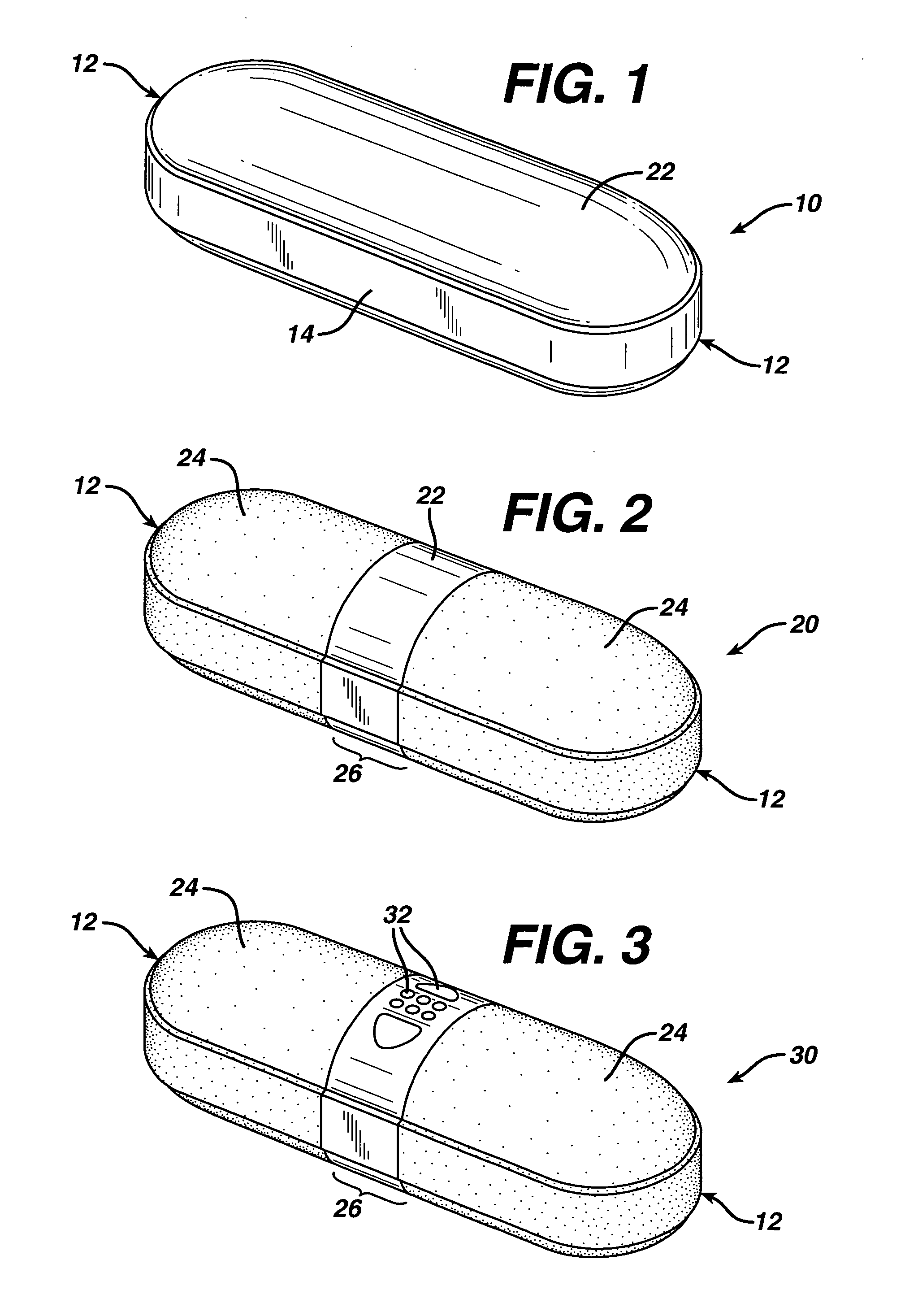
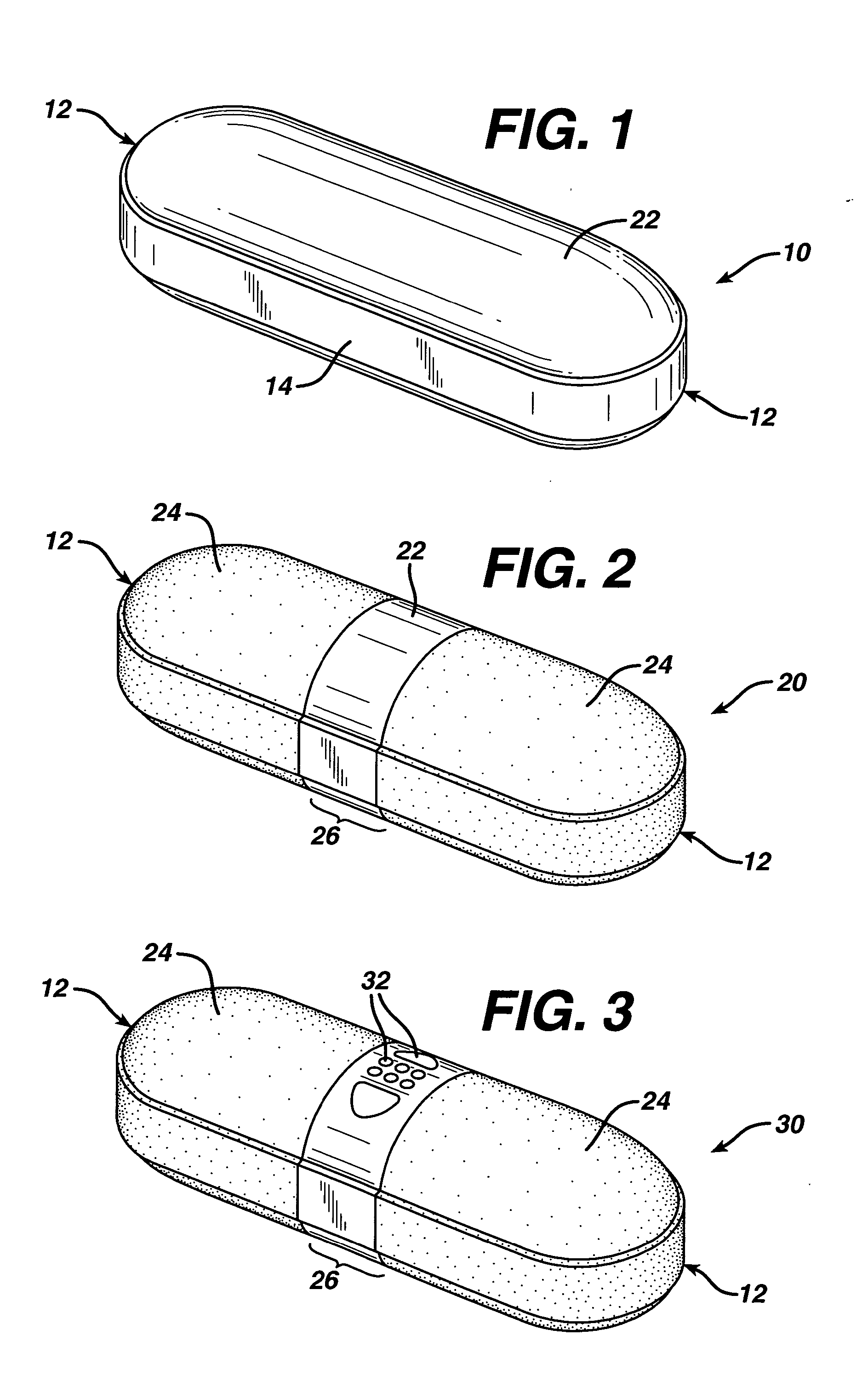
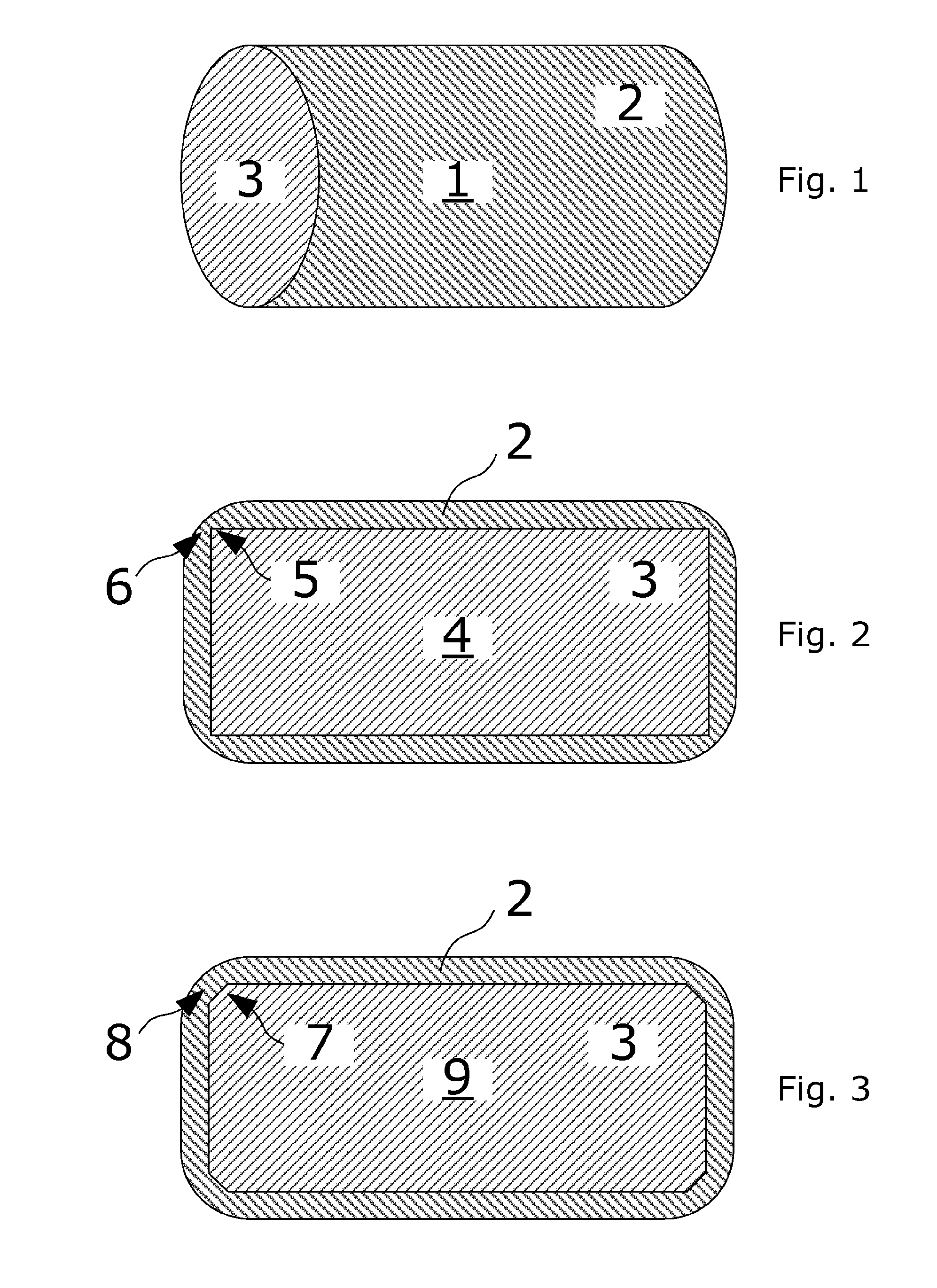
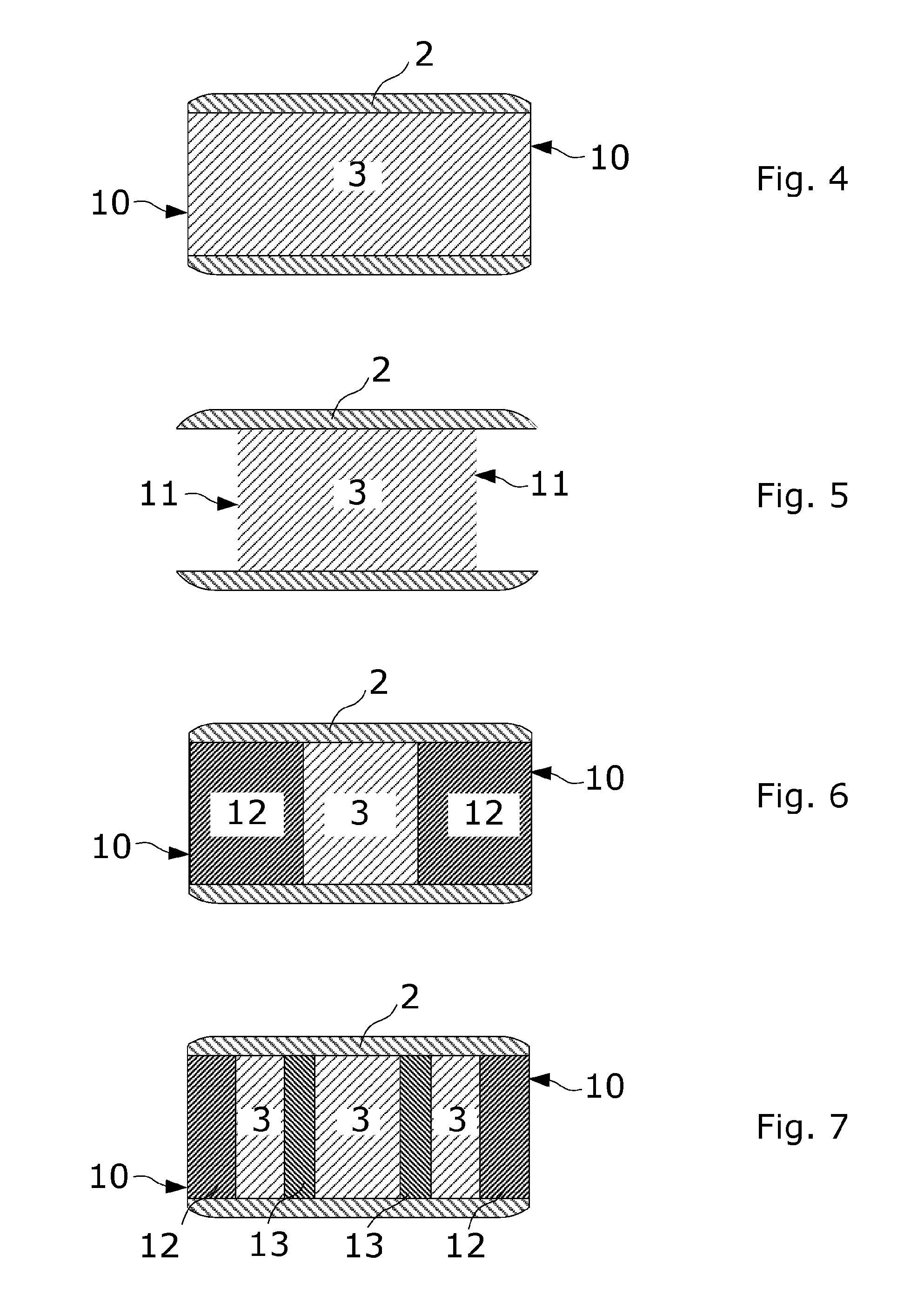
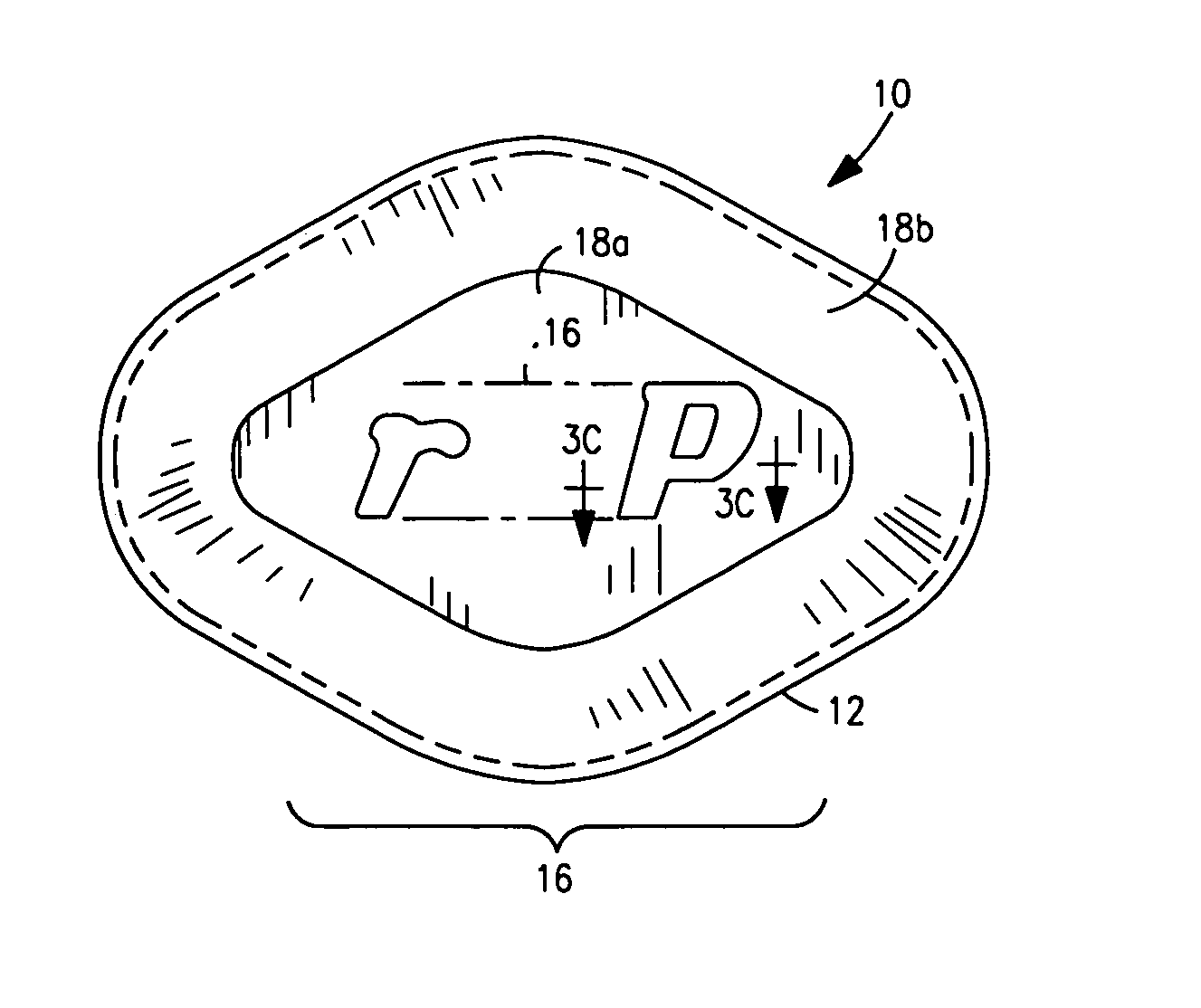
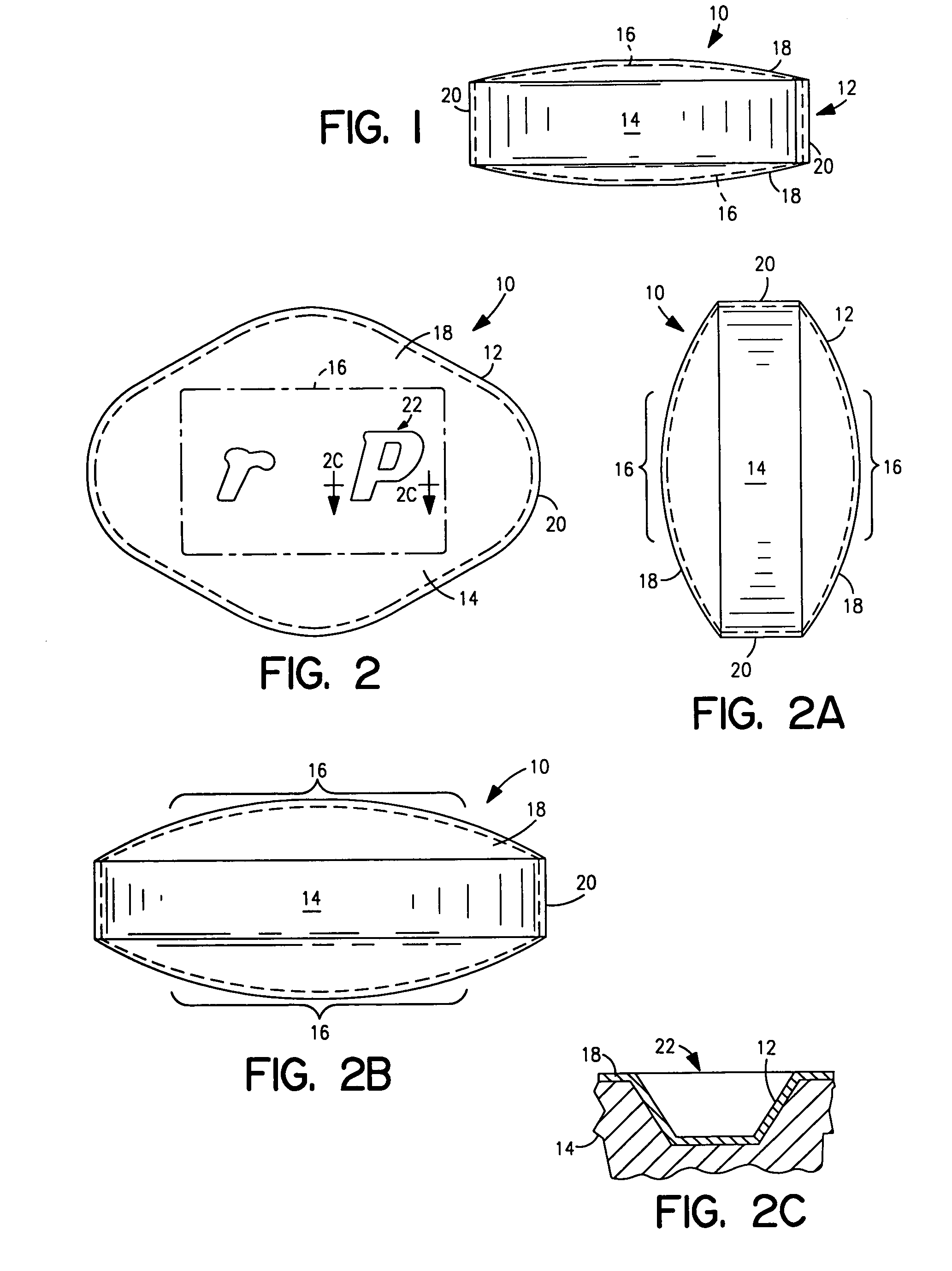
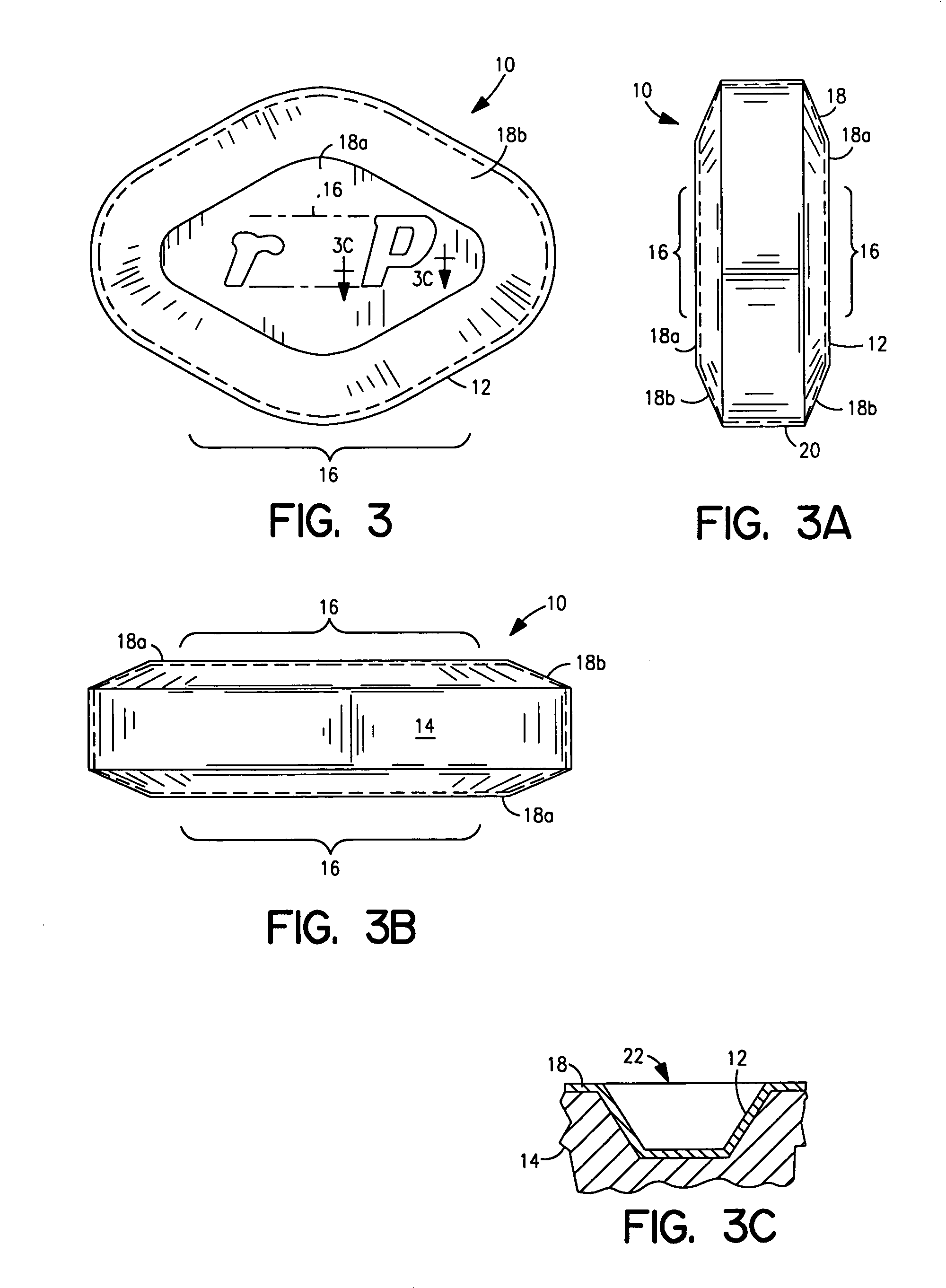
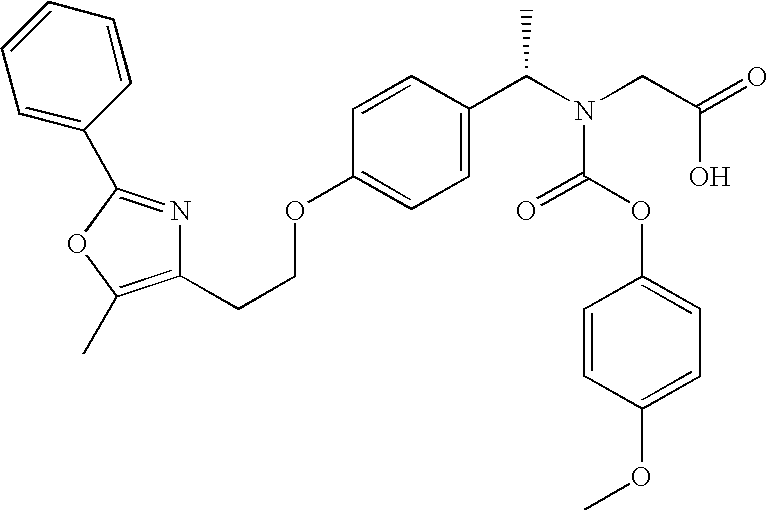
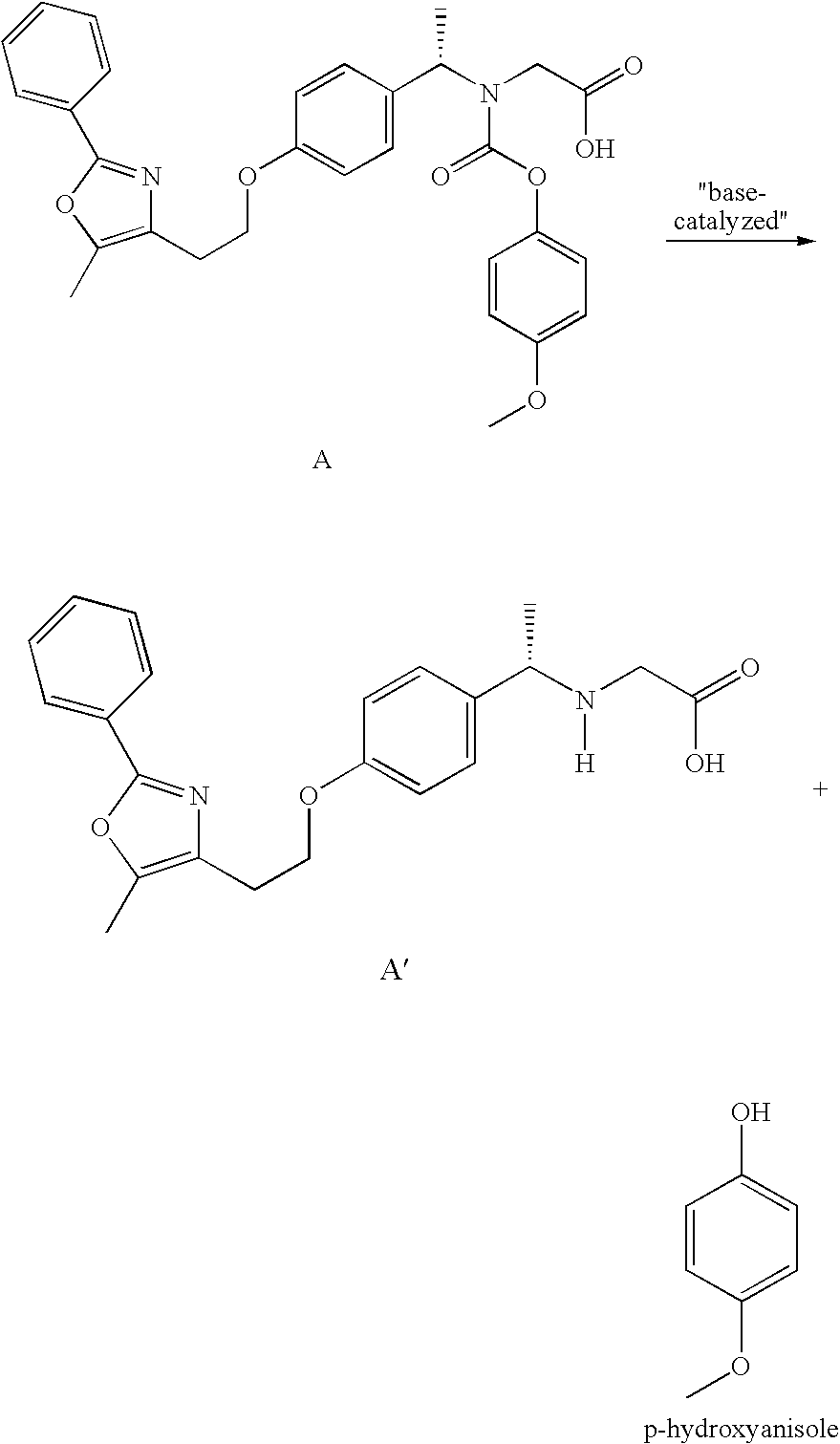
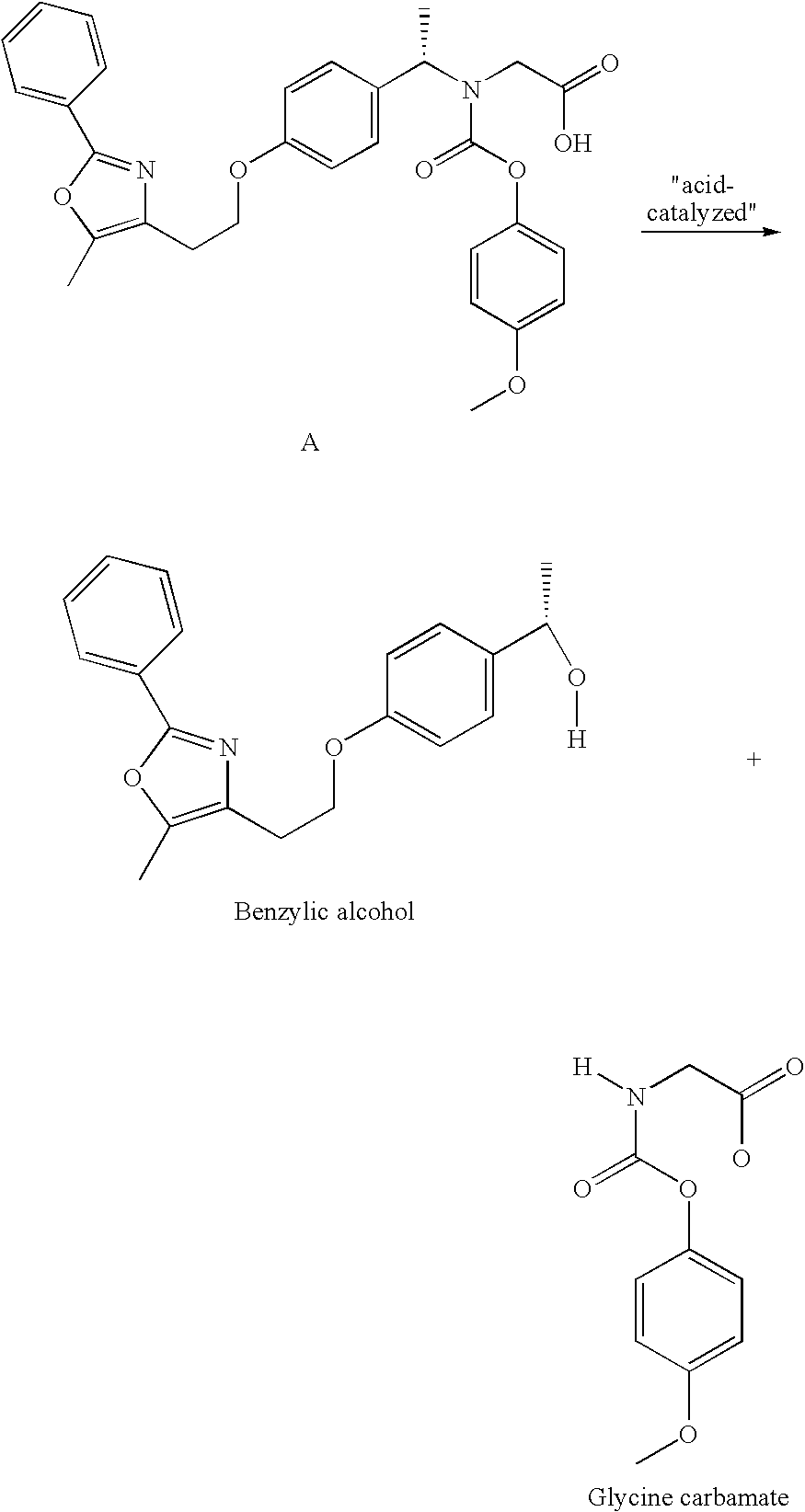
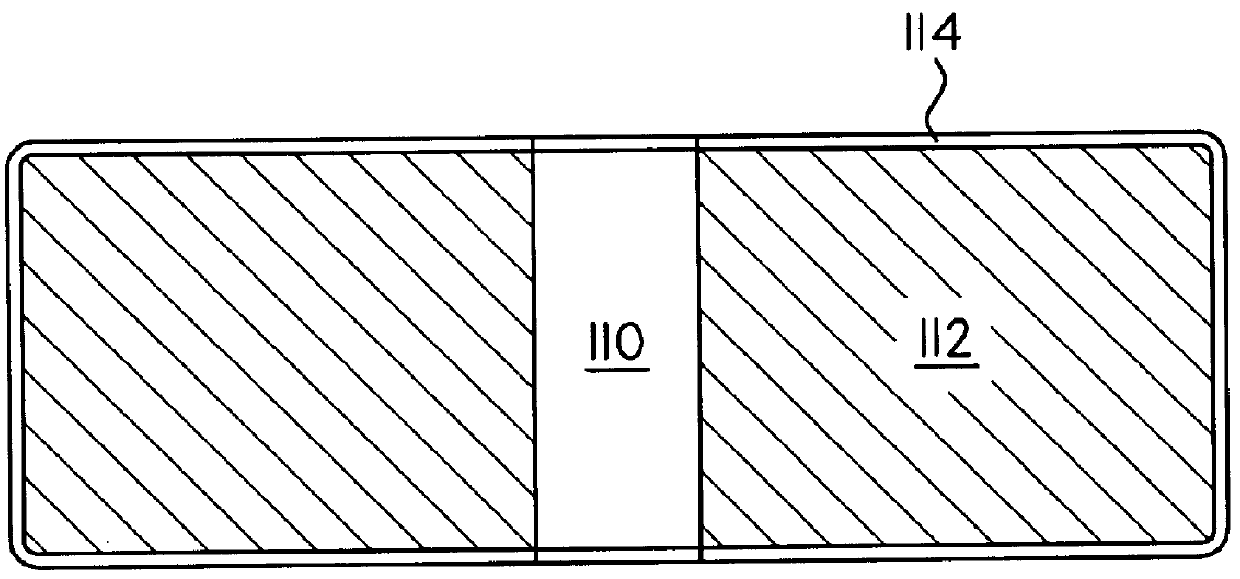
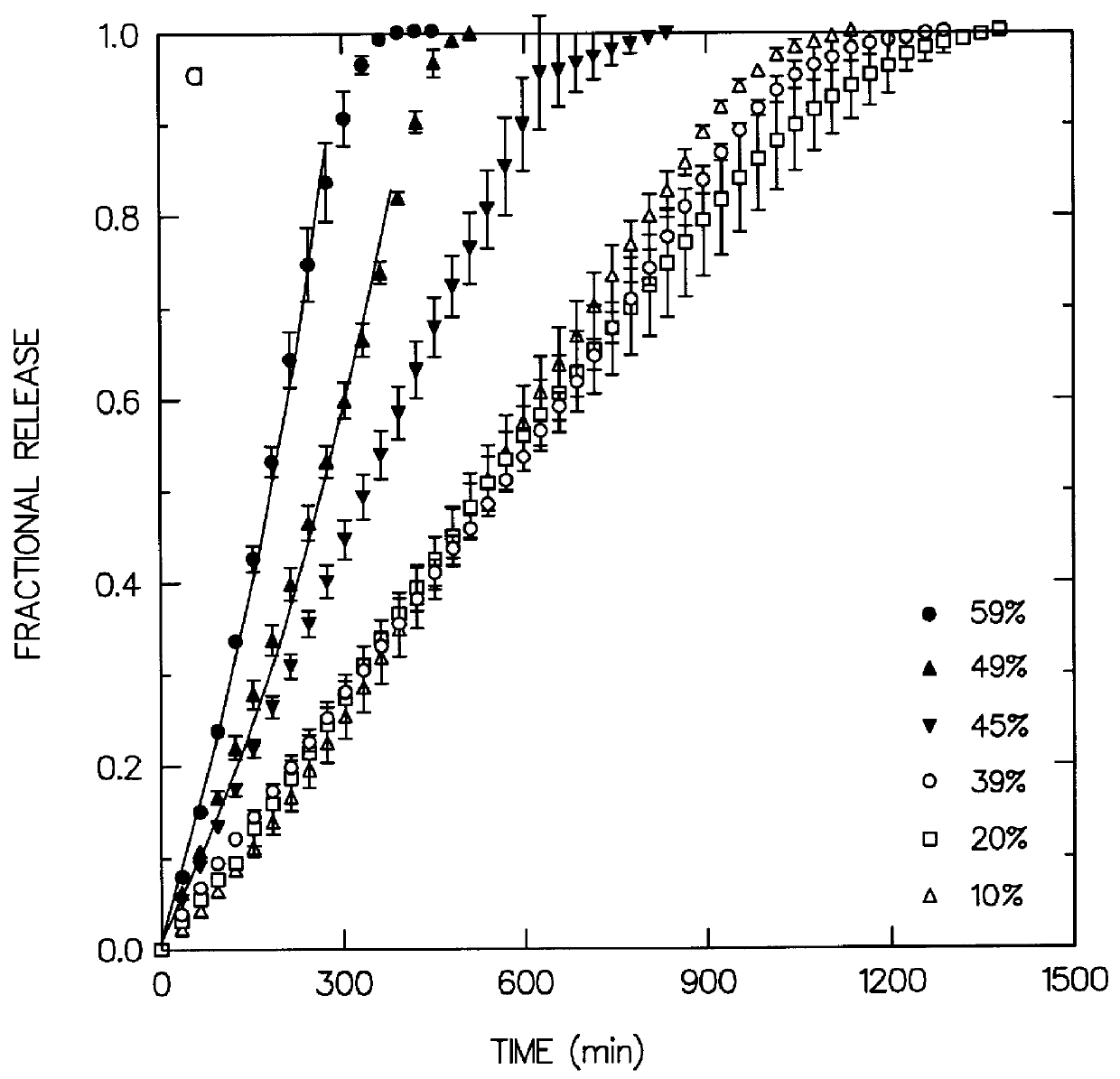
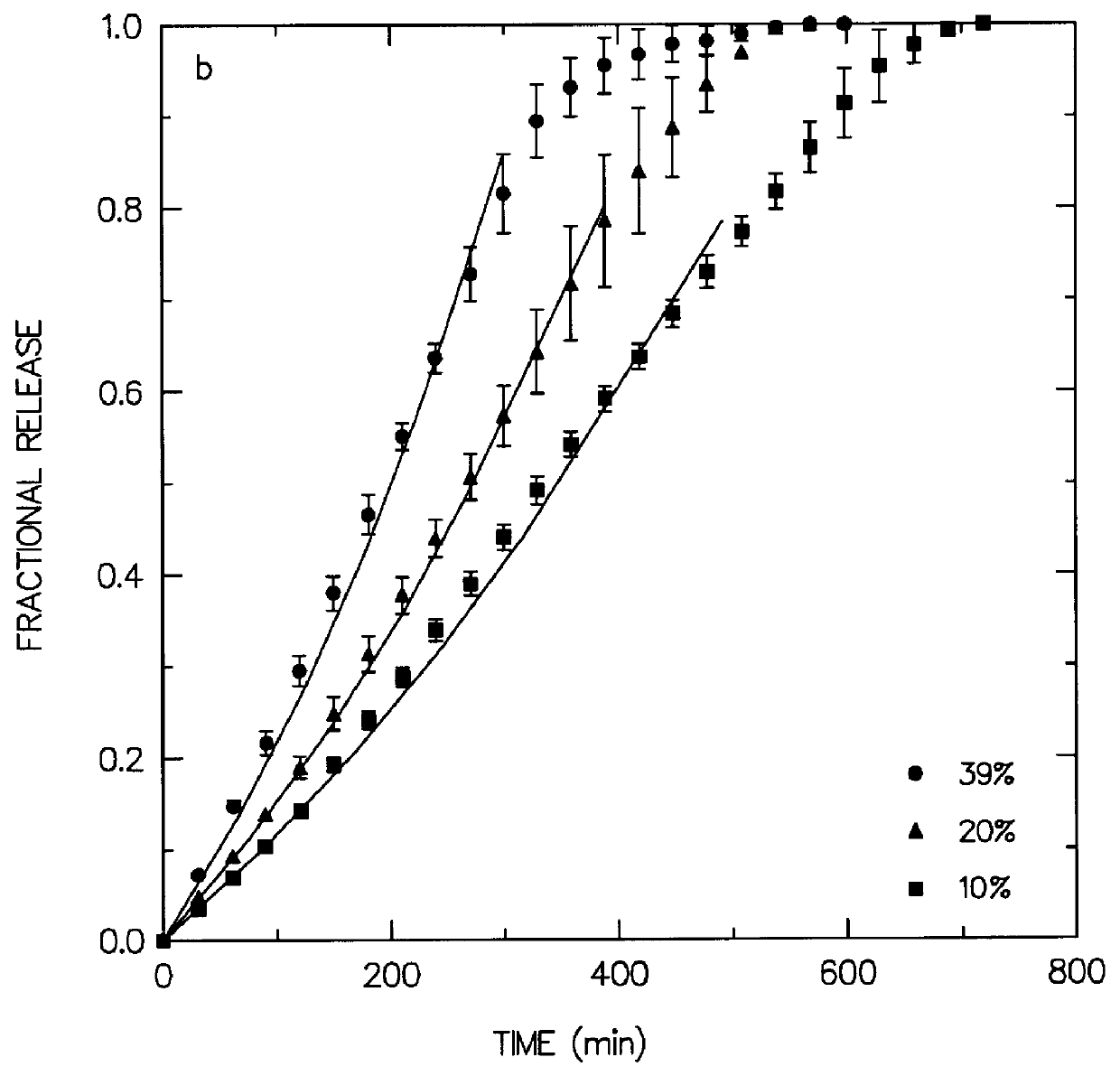
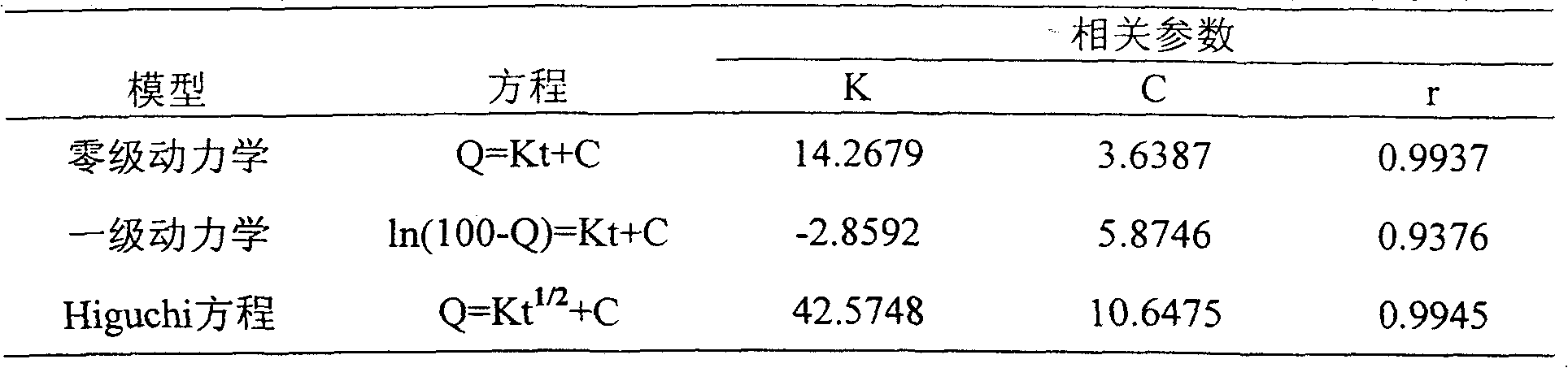
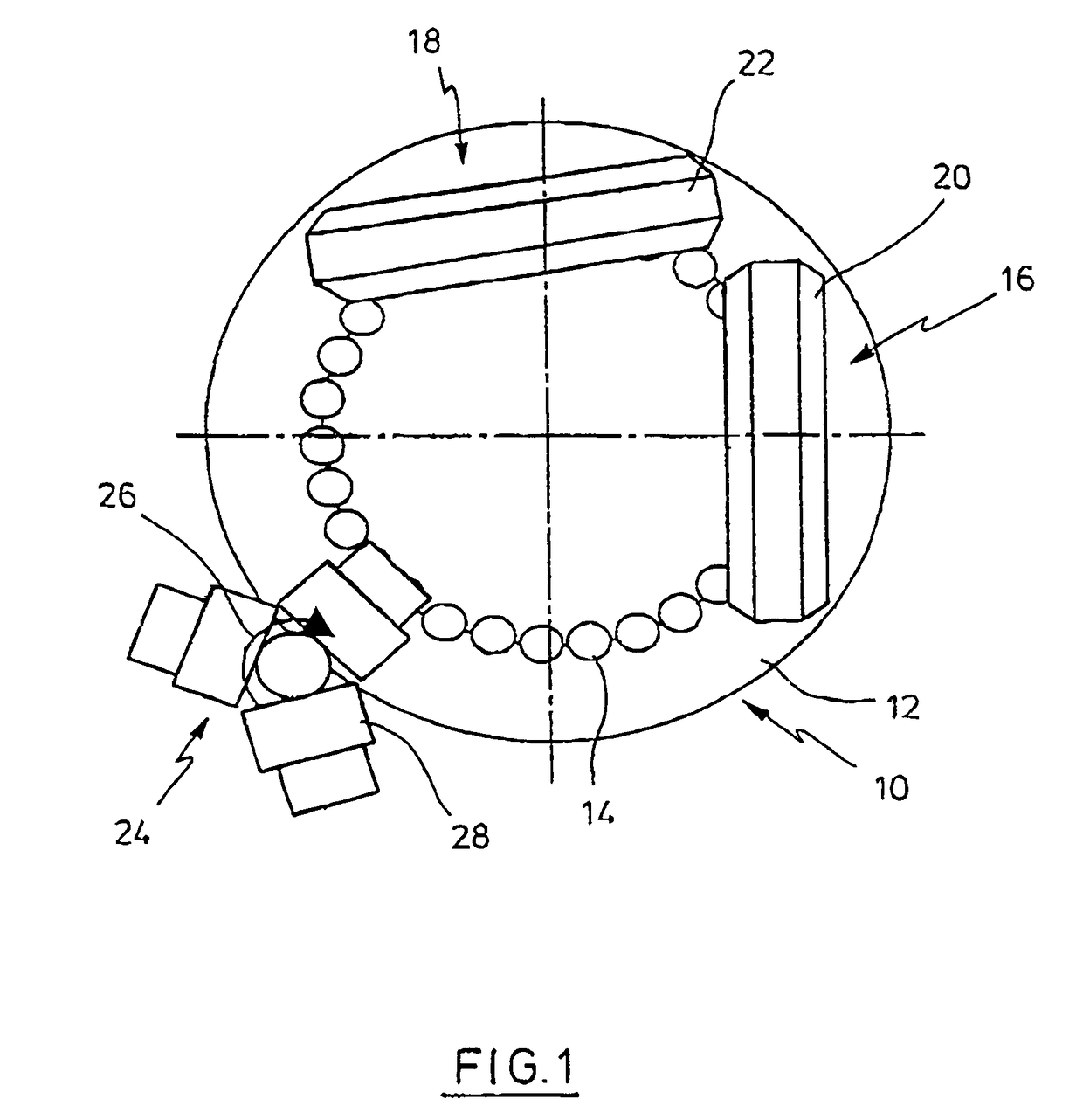
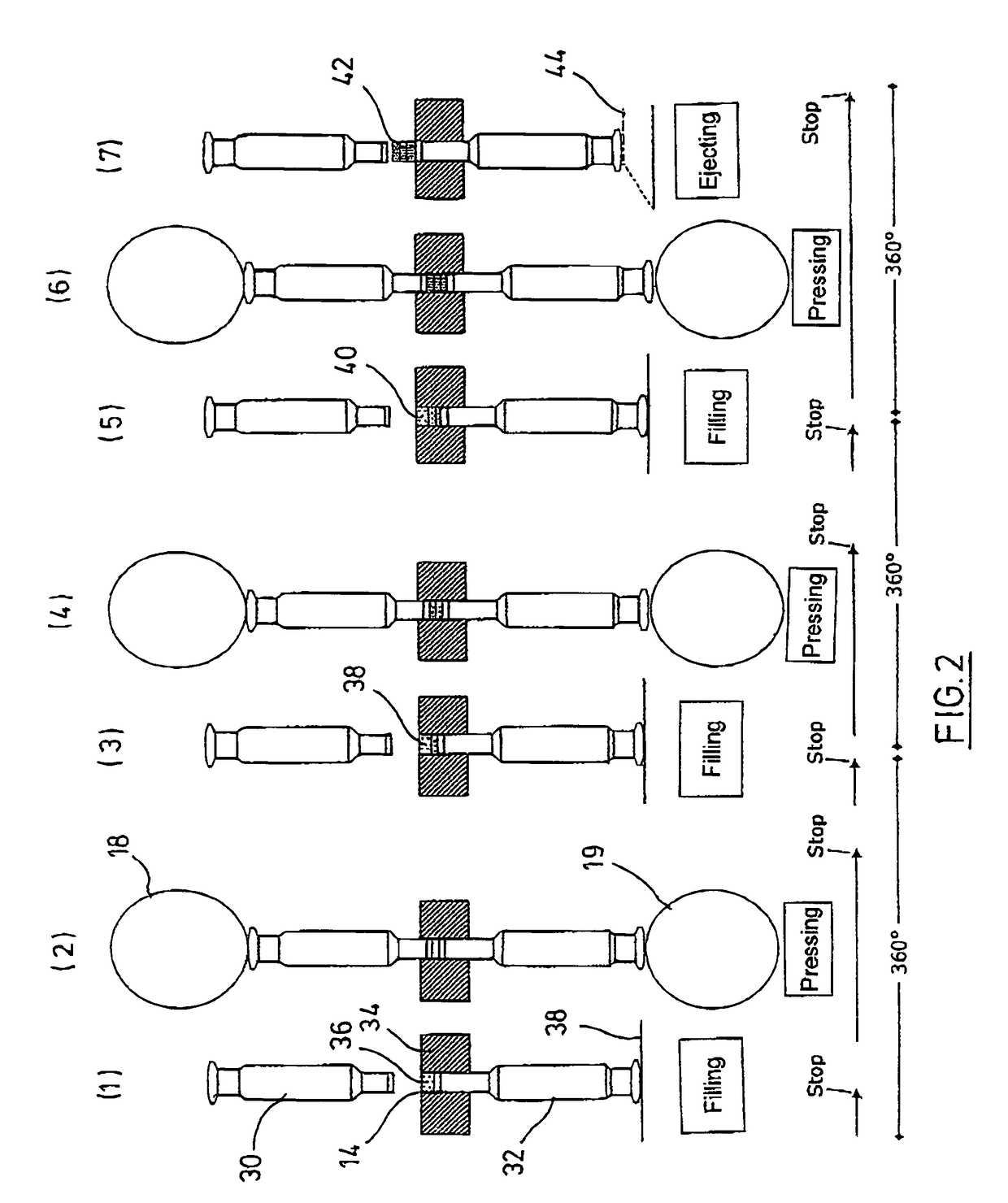
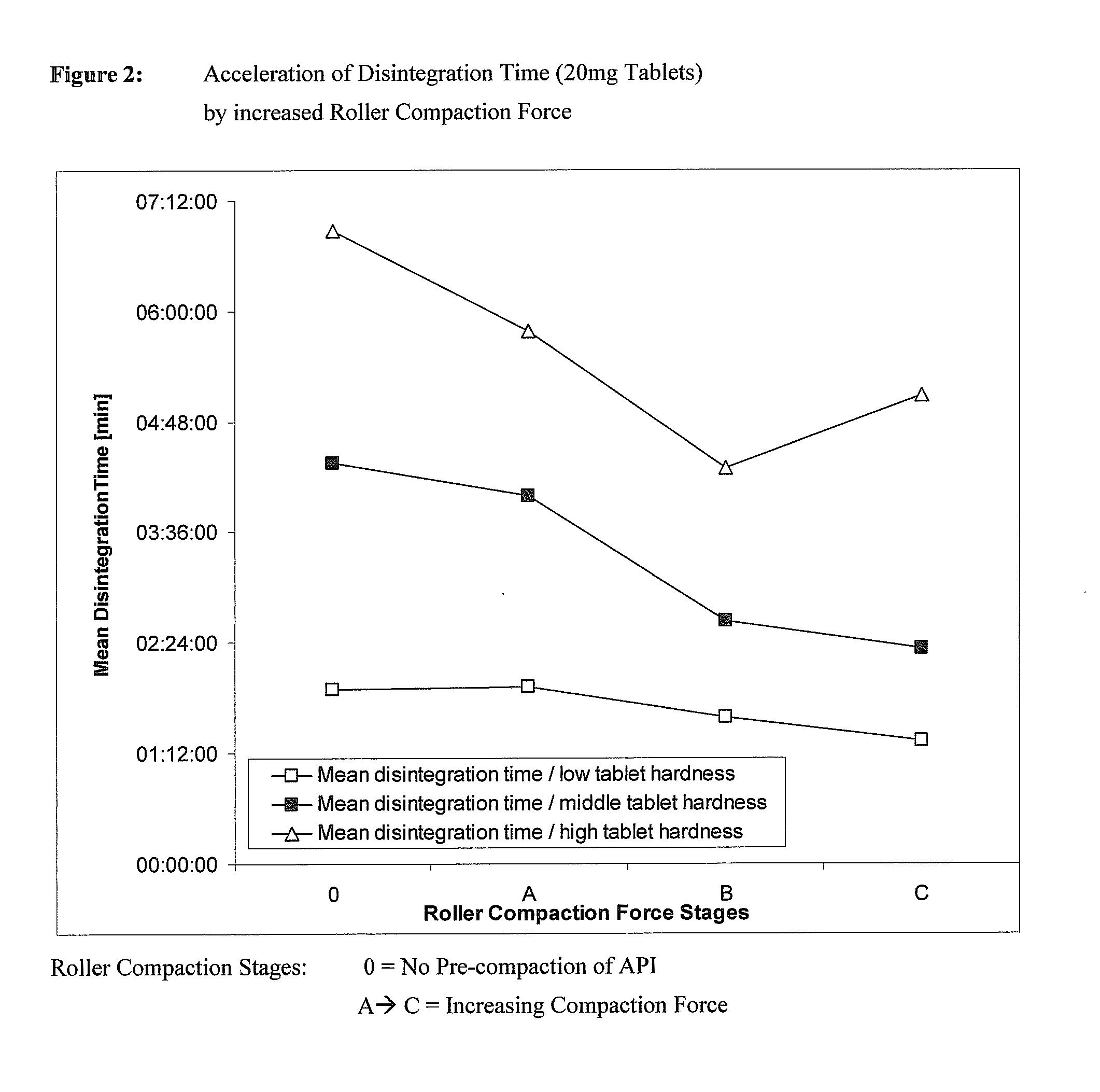
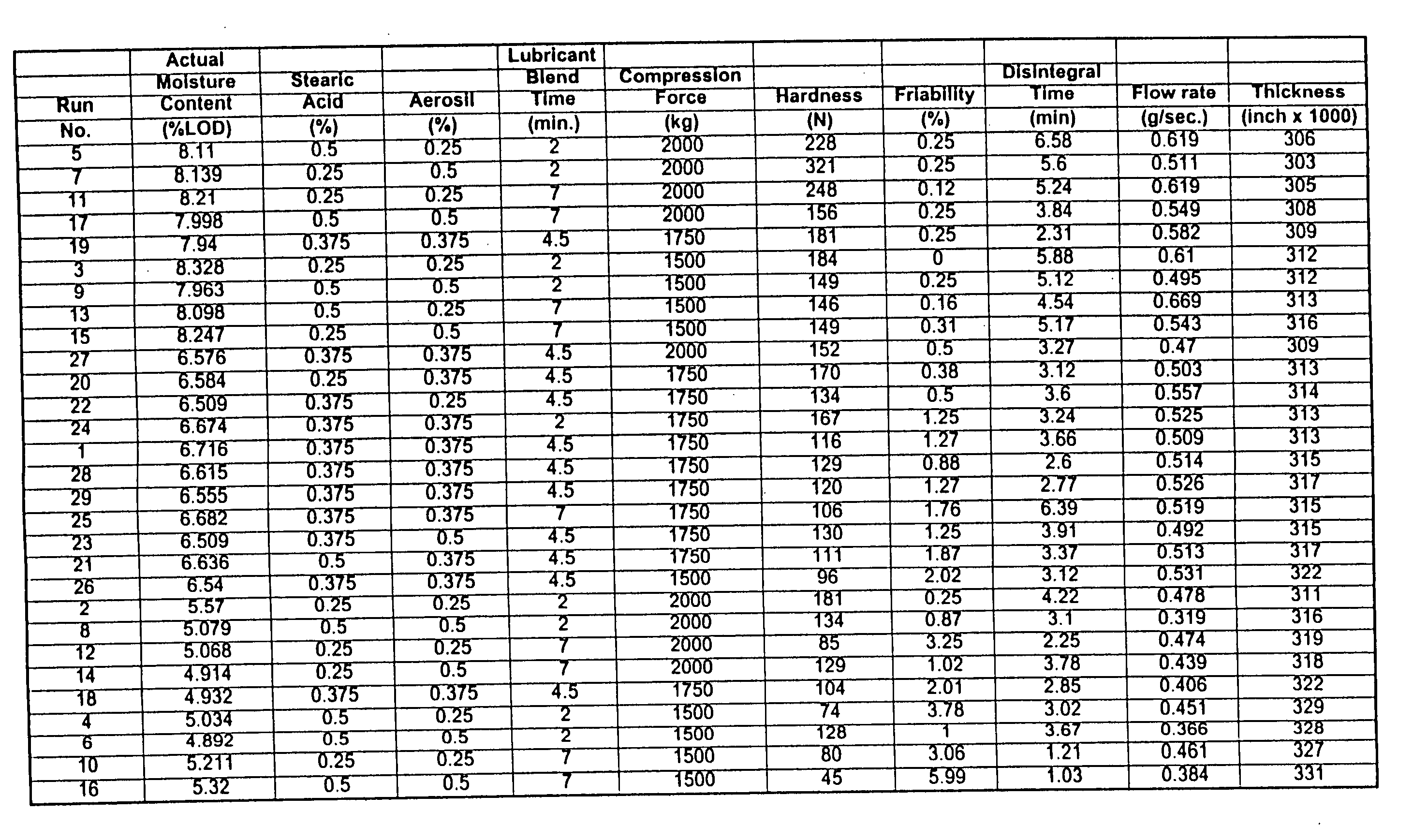
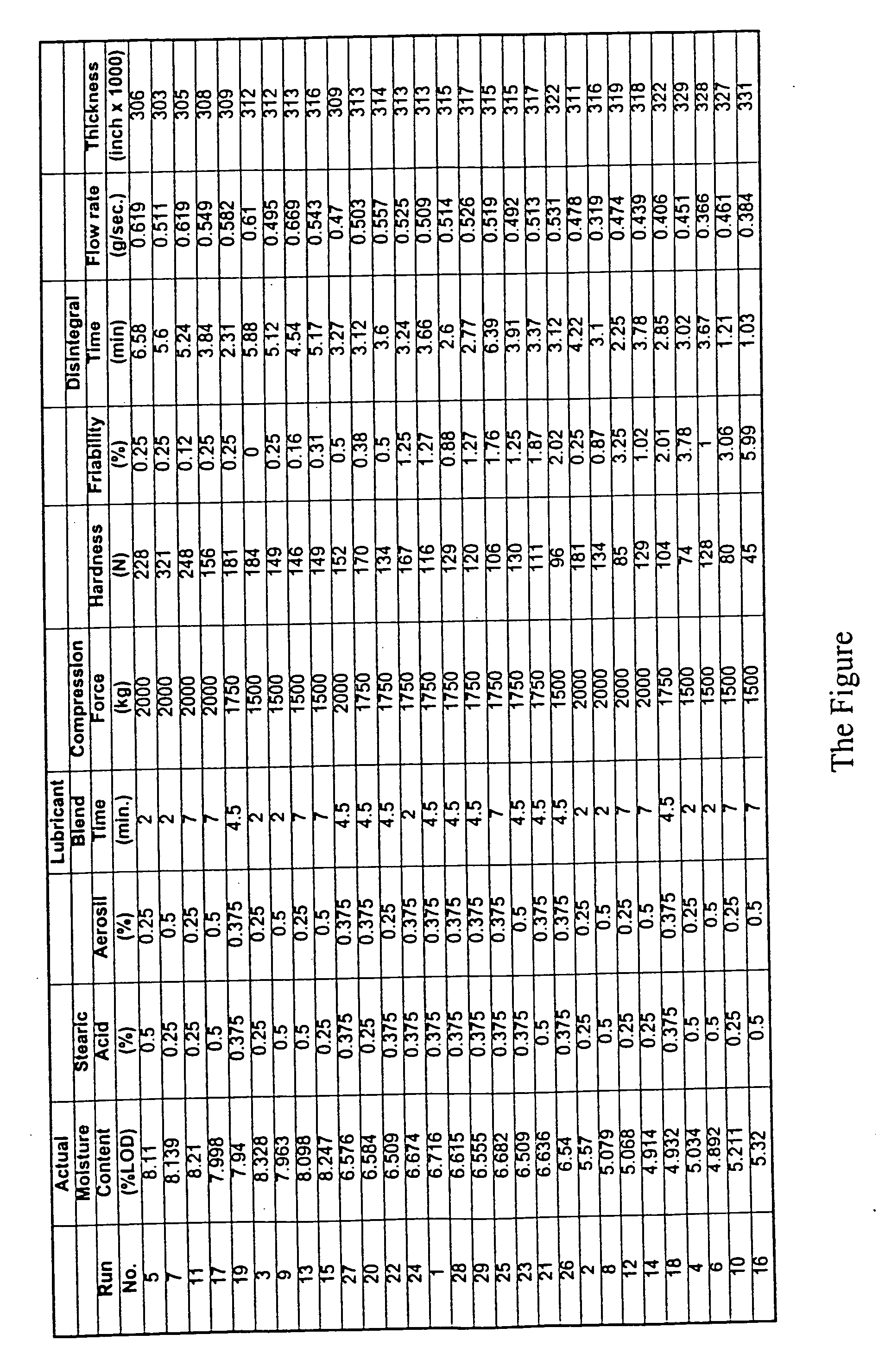
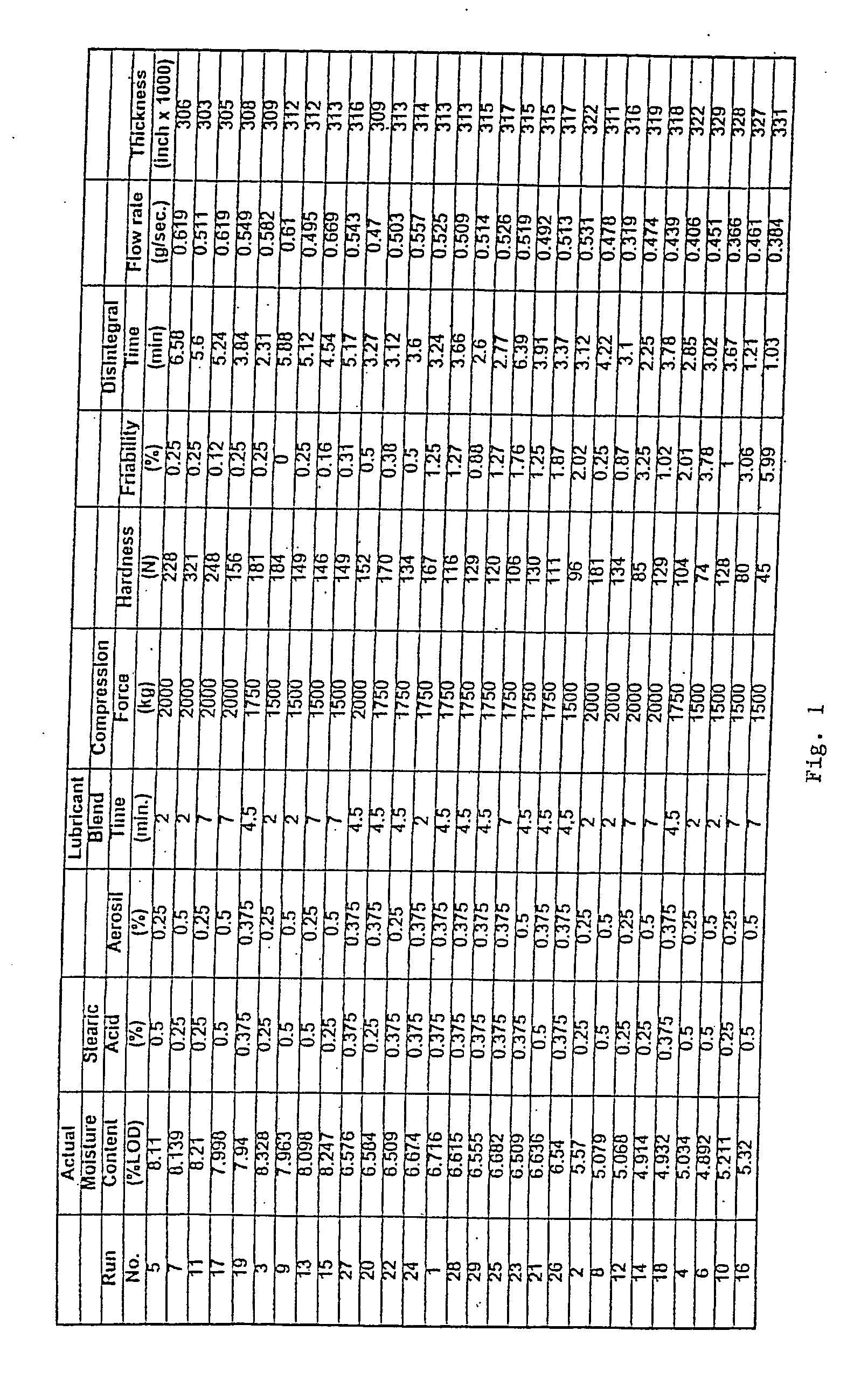
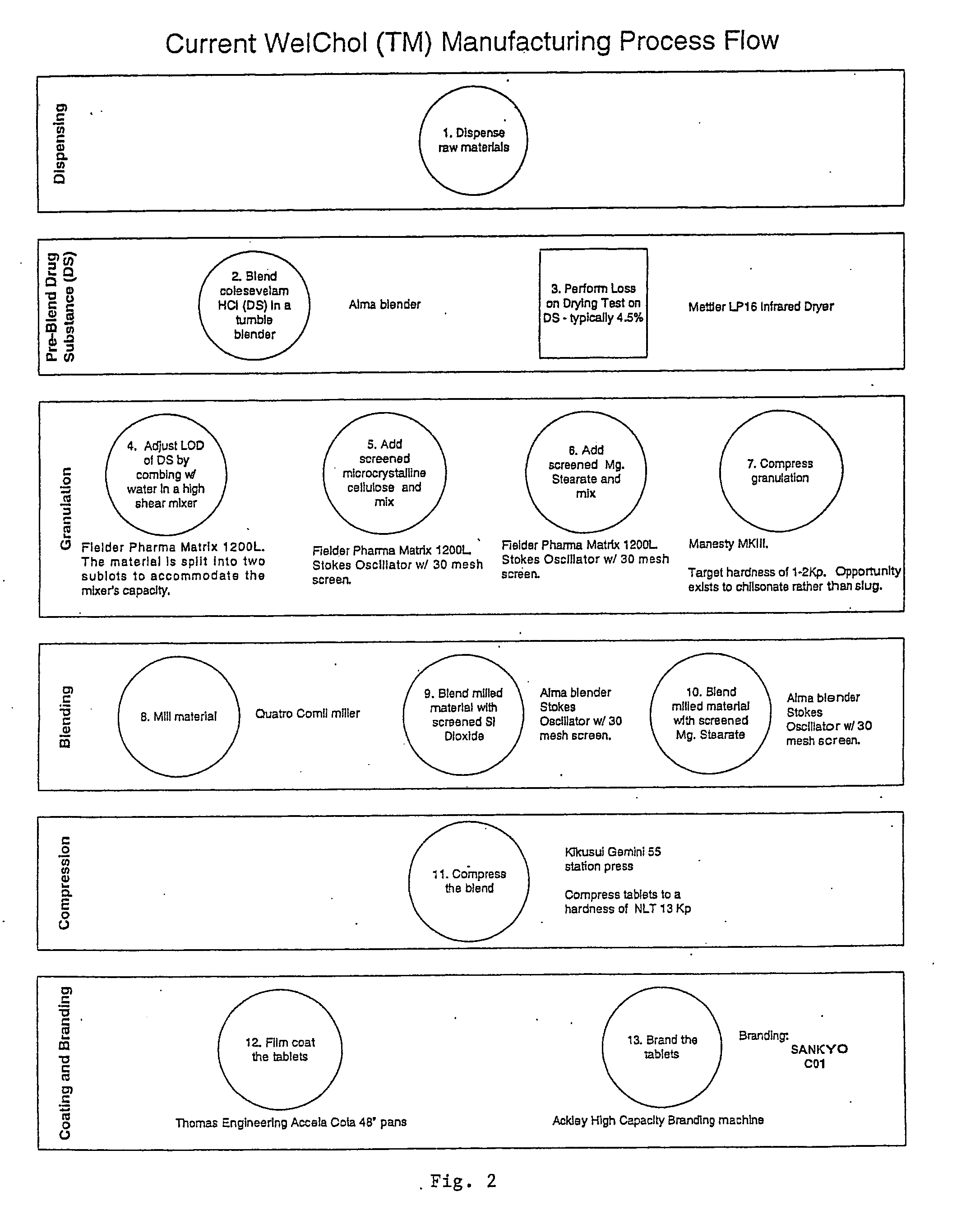
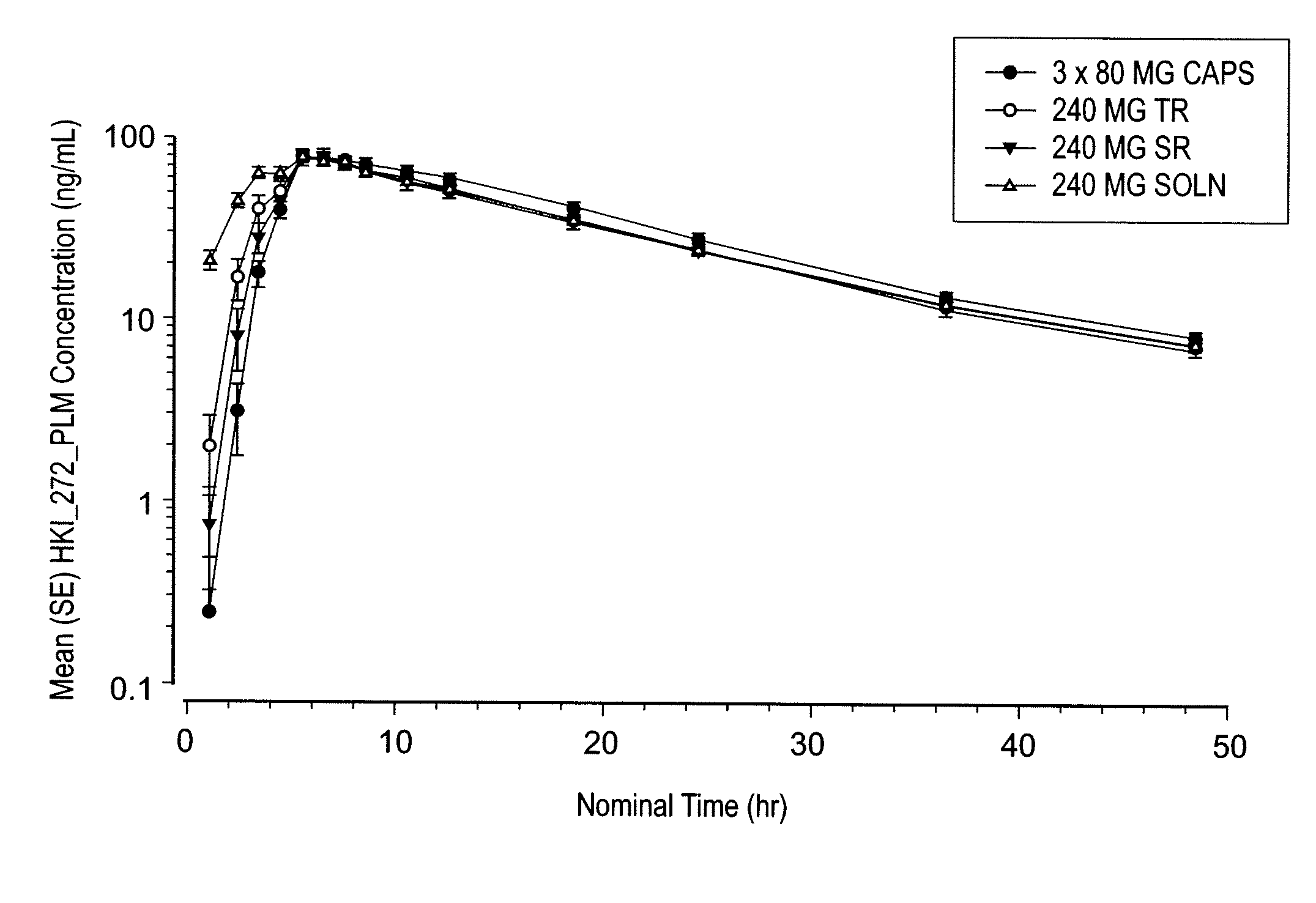
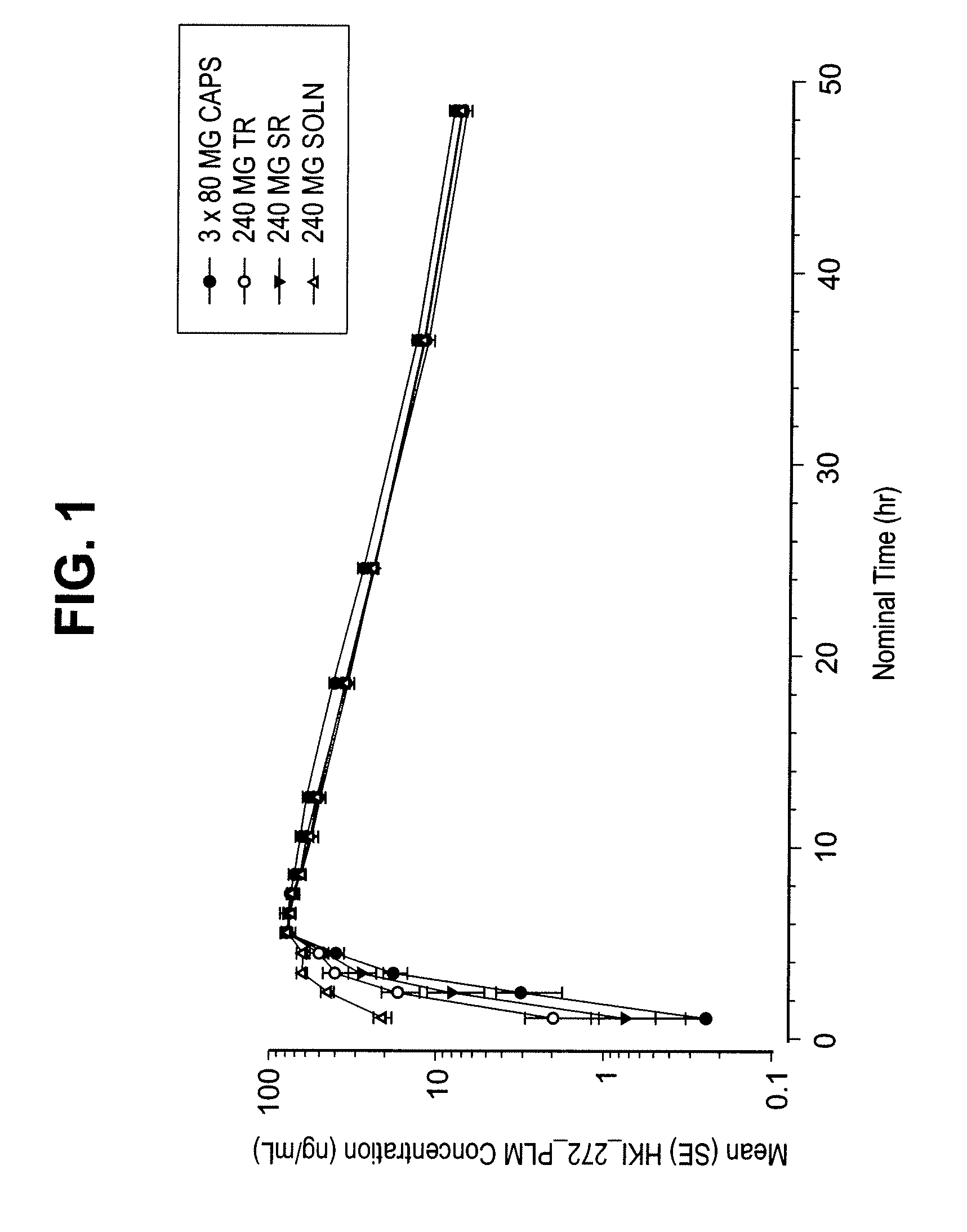
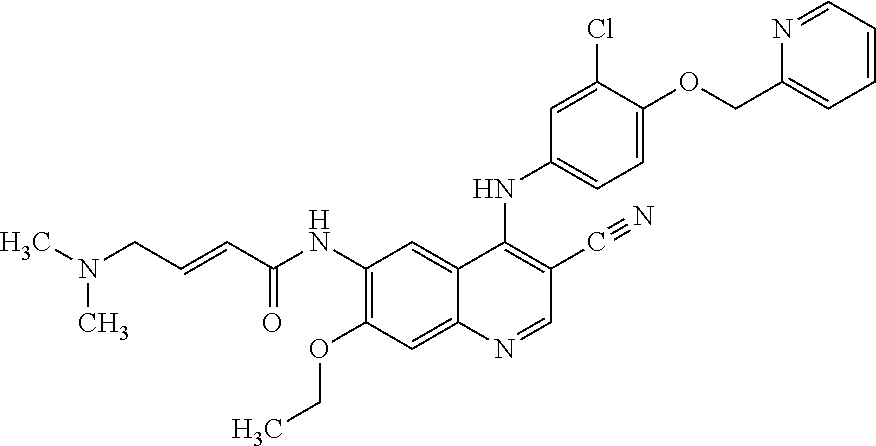
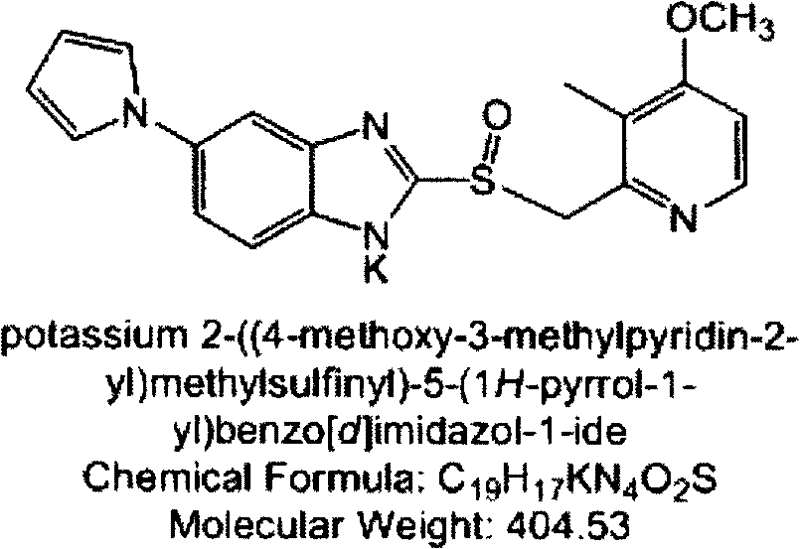
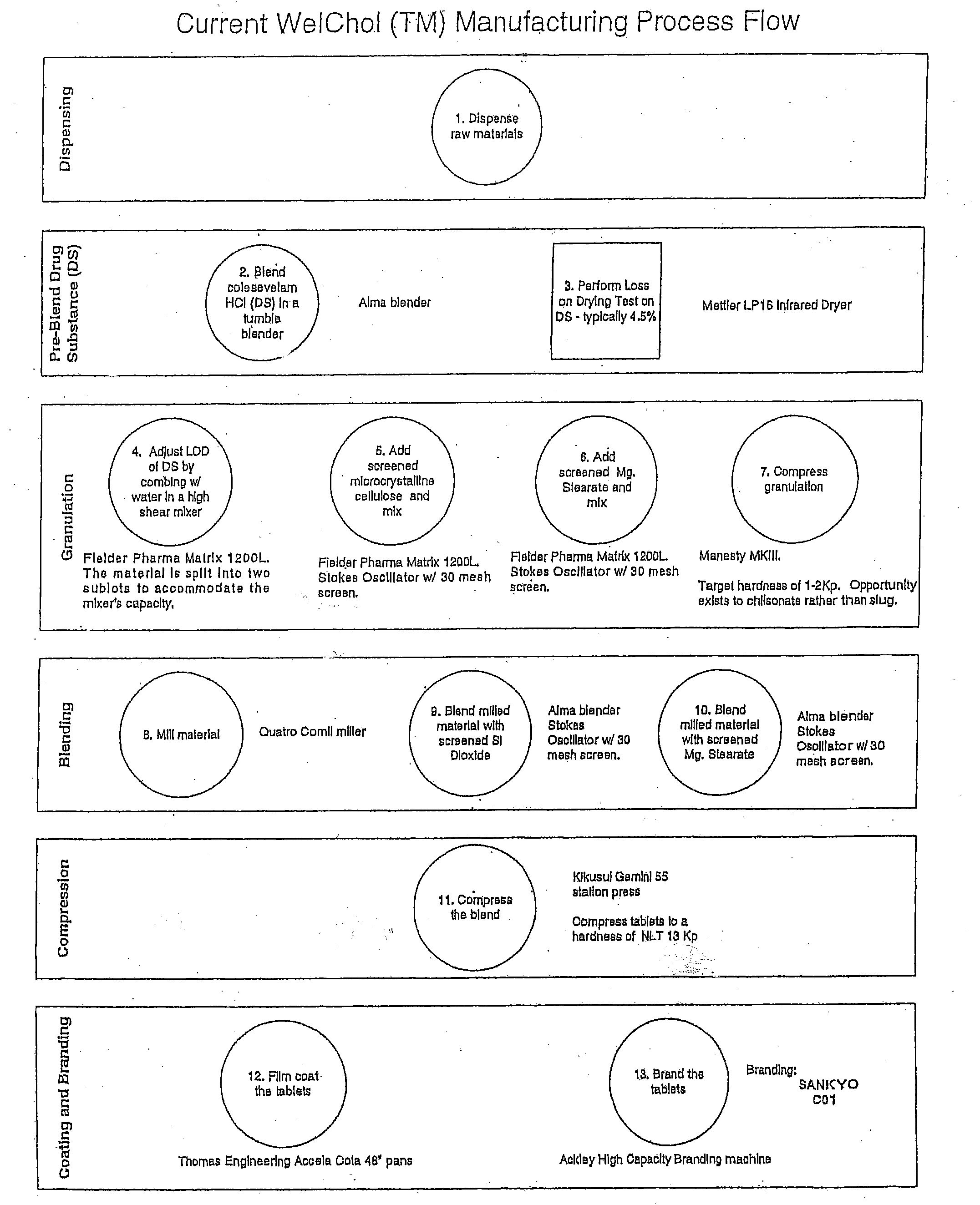
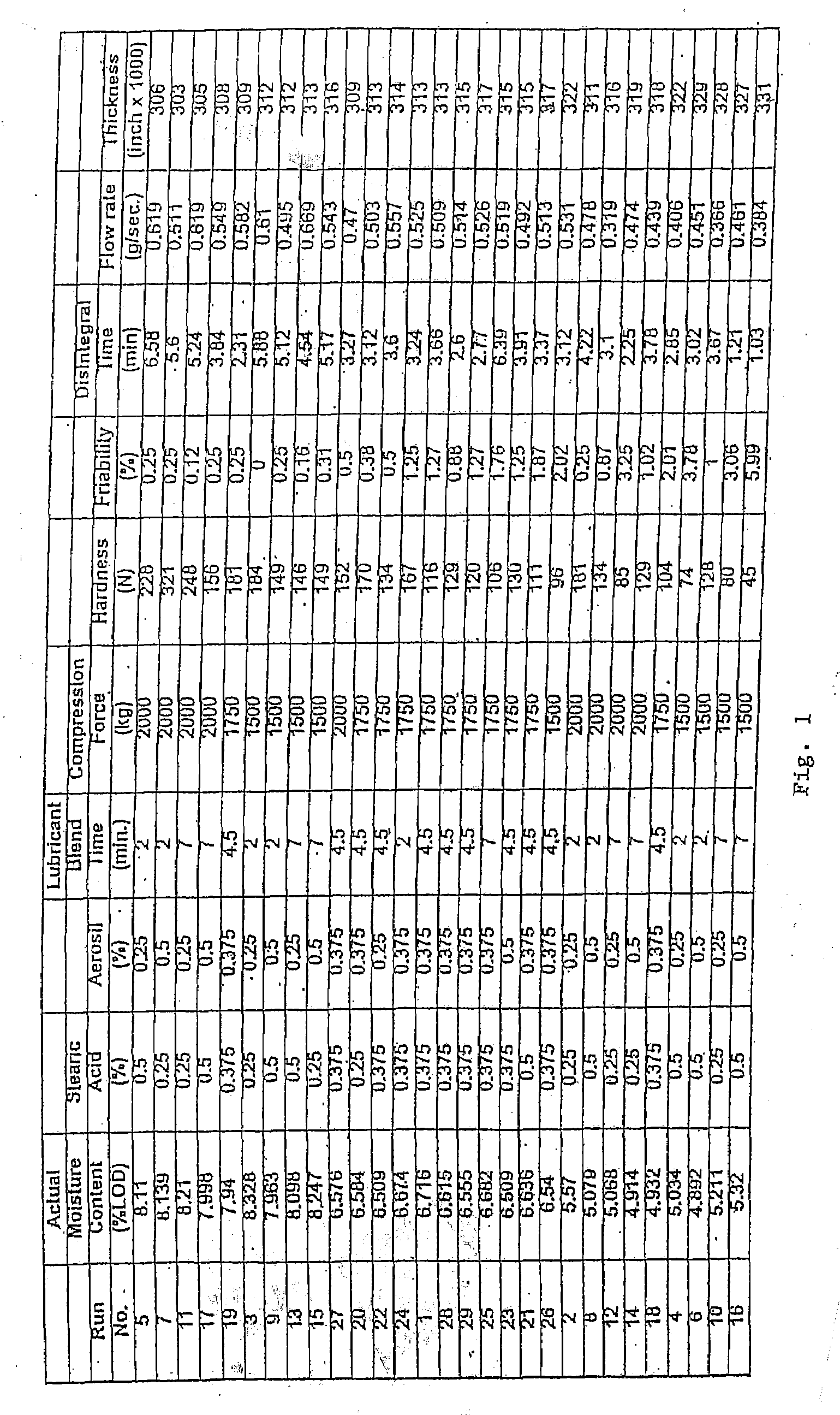
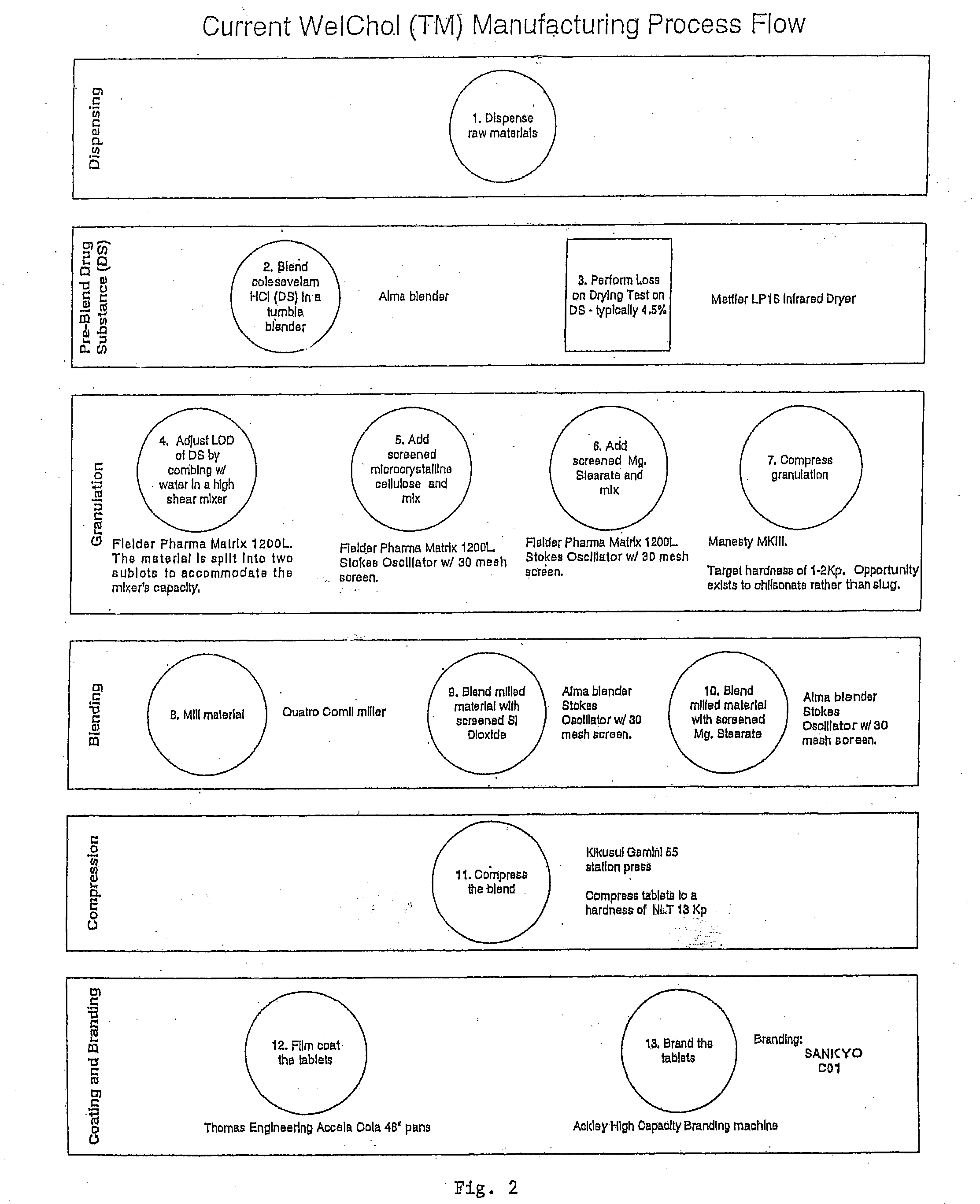
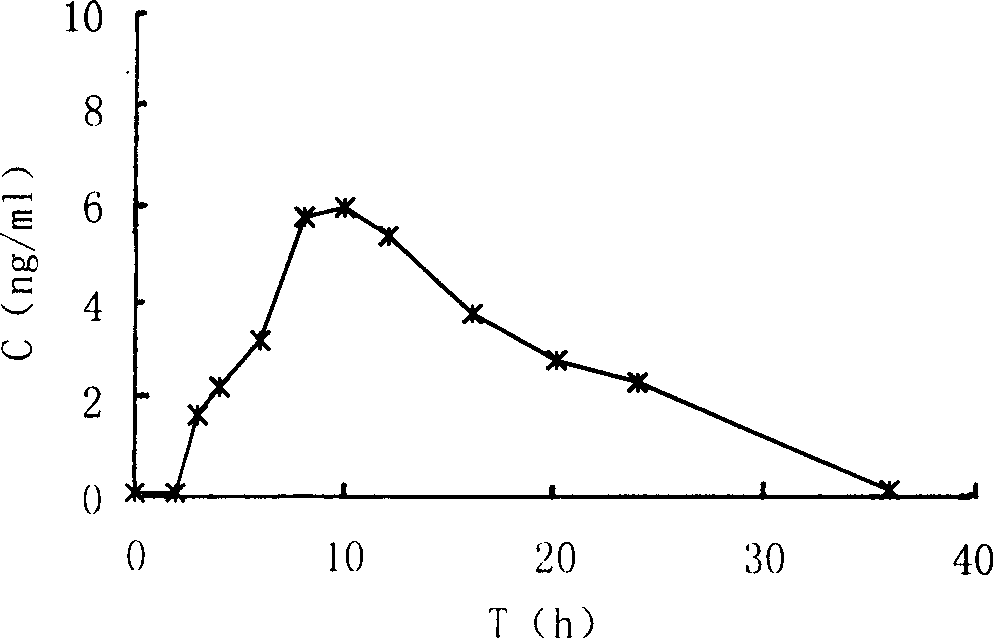
Coated Tablets Of 6-(5-Chloro-2-Pyridyl)-5-[(4-Methyl-1-Piperazinyl)Carbonyloxy]-7-Oxo-6,7-Dihydro-5H-Pyrrolo[3,4-b]Pyrazine And Methods For Measuring Effectiveness Of Coating
InactiveUS20100221338A1Treatment, prevention, or amelioration of a sleep disorderTreatment, prevention, or amelioration of anxietyBiocideNervous disorderCoated tabletsPyrazine
Coated tablets of (6-(5-chloro-2-pyridyl)-5-[(4-methyl-1-piperazinyl)carbonyloxy]-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazine) are provided. The tablets minimize the perceived bitterness of the medicament. A method for analyzing instantaneous dissolution of sub-microgram quantities of core material is also disclosed.
Owner:SUNOVION PHARMA INC
Amino acid salt of (S)-ibuprofen and medicinal composition thereof
InactiveCN101265178AOrganic active ingredientsOrganic chemistryCoated tabletsSustained Release Tablet
The invention provides a preparation and synthesis method of basic amino acid medicine salt of a dextral optical isomer in ibuprofen that is the existing anti-inflammation antipyretic and analgesic, and a medicinal combination thereof. After being mixed with necessary auxiliary material, the medicine salt can be prepared into oral solid preparations such as oral liquid, granules, tablets, dispersible tablets, capsules, coated tablets, sustained release tablets, collocystis, etc. through necessary pharmaceutics operations, and can also be prepared into the preparations for intravenous injection such as hydro-acupuncture, freeze-dried powders, aseptic powders, high volume transfusions, etc.
Owner:FUKANGREN BIO PHARMA
Chinese medicinal preparation for treating liver cancer, hepatitis B cirrhosis and preventing liver fibrillation and preparation thereof
ActiveCN1644213AImprove efficiencyImprove the quality of lifeDigestive systemAntiviralsCoated tabletsGround beetle
A Chinese medicine in the form of coated tablets for treating liver cancer, hepatocirrhosis, hepatitides, breast cancer, etc is prepared from 21 Chinese-medicinal materials including pilose asiabell root, ground beetle, coix seed, peach kernel, etc. Its preparing process is also disclosed.
Owner:康哲(湖南)制药有限公司 +1
Solid pharmaceutical composition containing rivaroxaban
ActiveCN105232488AGood dissolution effectReduce usageOrganic active ingredientsOrganic chemistryRivaroxabanMedical prescription
The invention relates to a pharmaceutical composition containing rivaroxaban, in particular to a solid pharmaceutical composition containing rivaroxaban and a preparation method of the solid pharmaceutical composition. The solid pharmaceutical composition containing the rivaroxaban can be further prepared into a film-coated tablet by a specific preparation technology to serve as a specific administration mode. The solid pharmaceutical composition containing the rivaroxaban and the preparation method of the solid pharmaceutical composition have the advantages that by application of the specific preparation technology, using surfactants in a preparation is avoided and dissolution rate of the tablet is increased effectively; by means of using sodium stearyl fumarate in the composition, marked increase of degraded impurities I during long-term storage of the tablet is avoided effectively; through stability accelerating research, the film-coated tablet containing the rivaroxaban, prepared by the prescription and technological steps, is stable and controllable in quality.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Coated Tablets Of 6-(5-Chloro-2-Pyridyl)-5-[(4-Methyl-1-Piperazinyl)Carbonyloxy]-7-Oxo-6,7-Dihydro-5H-Pyrrolo[3,4-b]Pyrazine And Methods For Measuring Effectiveness Of Coating Coated Tablets Of 6-(5-Chloro-2-Pyridyl)-5-[(4-Methyl-1-Piperazinyl)Carbonyloxy]-7-Oxo-6,7-Dihydro-5H-Pyrrolo[3,4-b]Pyrazine And Methods For Measuring Effectiveness Of Coating](https://images-eureka.patsnap.com/patent_img/90520a30-1d89-4c1a-970e-7cd4fe14d603/US20100221338A1-20100902-D00001.png)
![Coated Tablets Of 6-(5-Chloro-2-Pyridyl)-5-[(4-Methyl-1-Piperazinyl)Carbonyloxy]-7-Oxo-6,7-Dihydro-5H-Pyrrolo[3,4-b]Pyrazine And Methods For Measuring Effectiveness Of Coating Coated Tablets Of 6-(5-Chloro-2-Pyridyl)-5-[(4-Methyl-1-Piperazinyl)Carbonyloxy]-7-Oxo-6,7-Dihydro-5H-Pyrrolo[3,4-b]Pyrazine And Methods For Measuring Effectiveness Of Coating](https://images-eureka.patsnap.com/patent_img/90520a30-1d89-4c1a-970e-7cd4fe14d603/US20100221338A1-20100902-D00002.png)
![Coated Tablets Of 6-(5-Chloro-2-Pyridyl)-5-[(4-Methyl-1-Piperazinyl)Carbonyloxy]-7-Oxo-6,7-Dihydro-5H-Pyrrolo[3,4-b]Pyrazine And Methods For Measuring Effectiveness Of Coating Coated Tablets Of 6-(5-Chloro-2-Pyridyl)-5-[(4-Methyl-1-Piperazinyl)Carbonyloxy]-7-Oxo-6,7-Dihydro-5H-Pyrrolo[3,4-b]Pyrazine And Methods For Measuring Effectiveness Of Coating](https://images-eureka.patsnap.com/patent_img/90520a30-1d89-4c1a-970e-7cd4fe14d603/US20100221338A1-20100902-D00003.png)