Solid pharmaceutical formulations comprising BIBW 2992
一种药物剂型、给药的技术,应用在制备上文提及的压实中间体,立即释放溶出特性,压实中间体,中间体掺合物及固体口服制剂领域,能够解决片剂崩解不令人满意、含有、API含量均一性不够等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
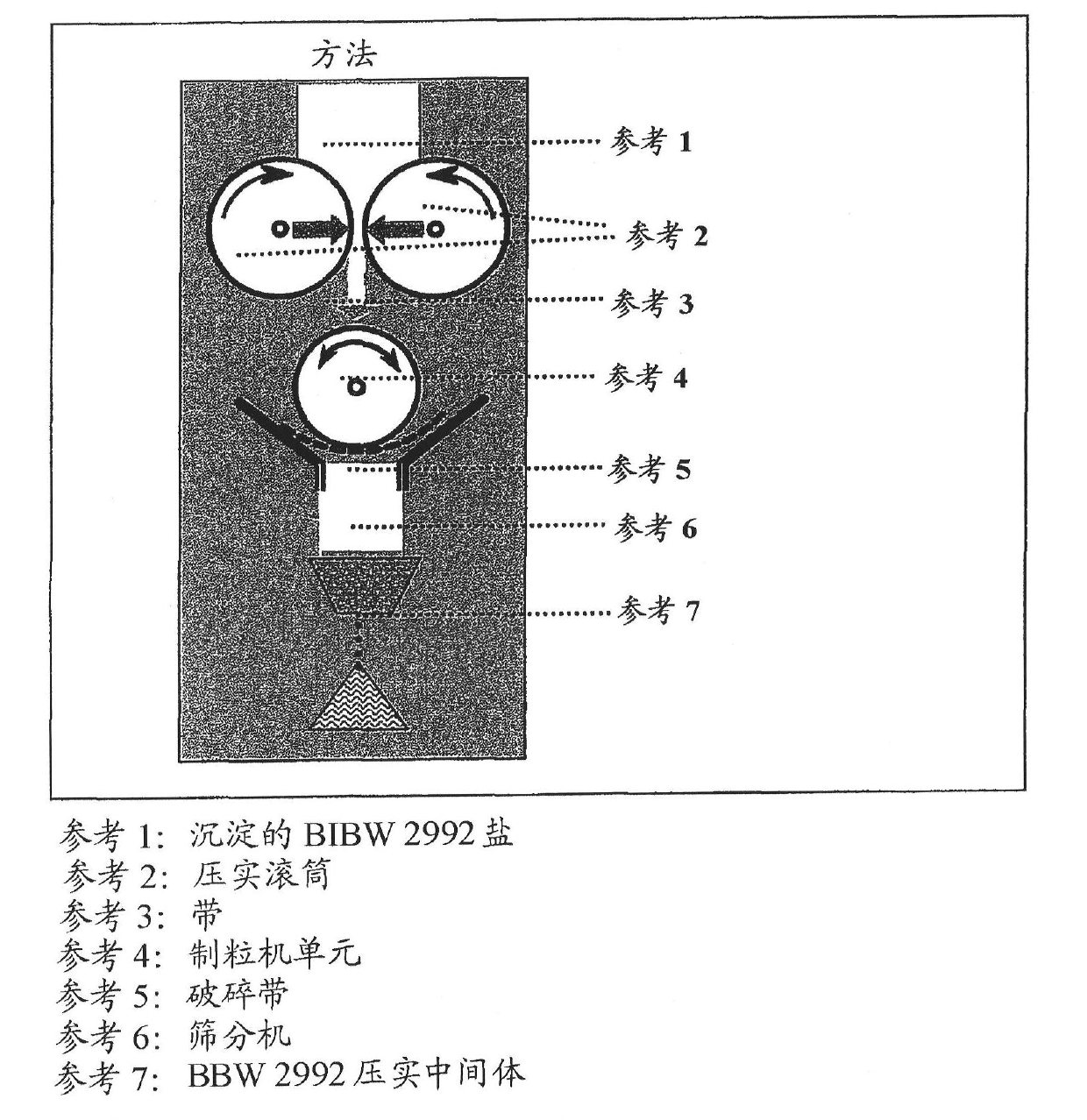
Method used
Image
Examples
Embodiment Construction
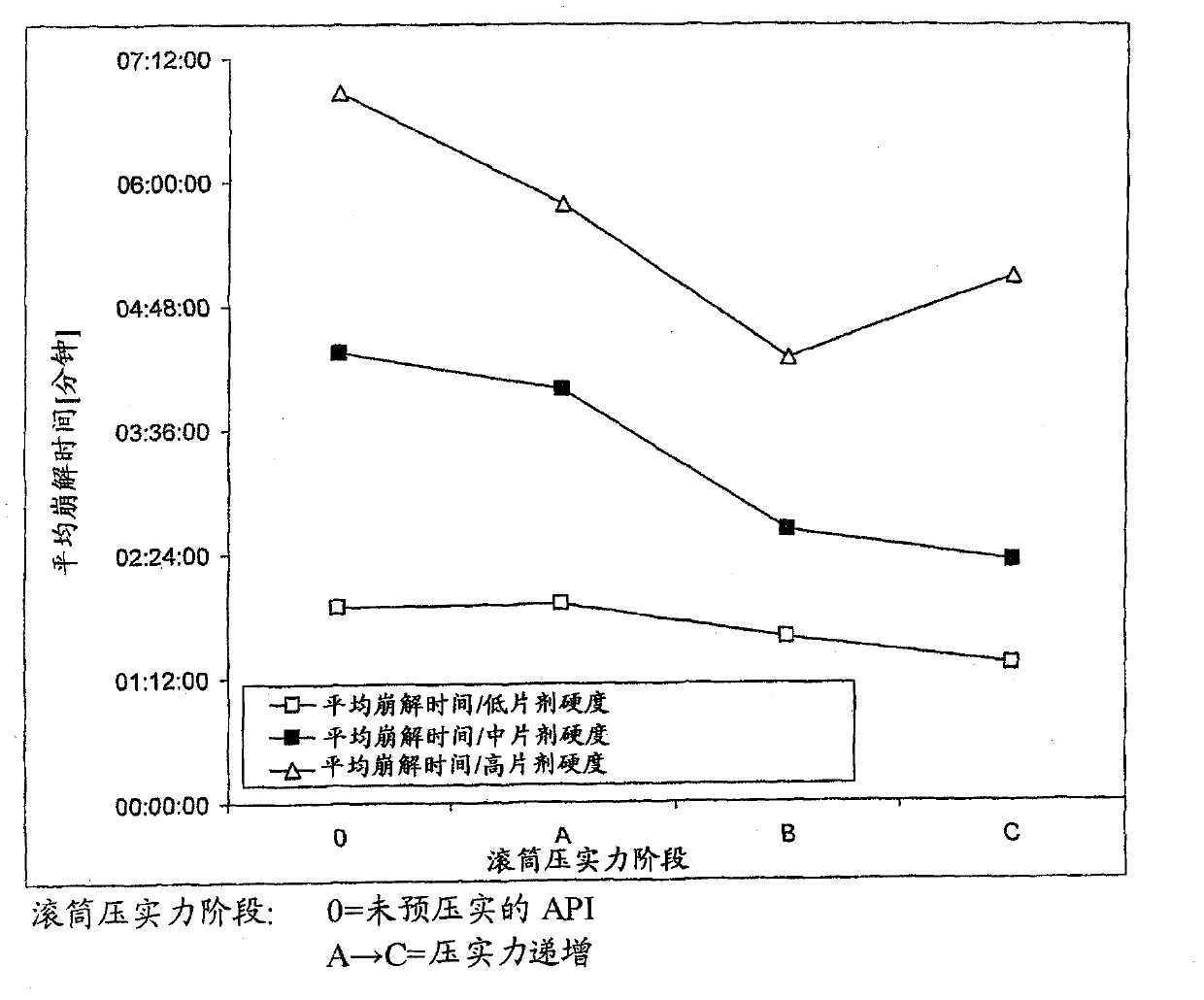
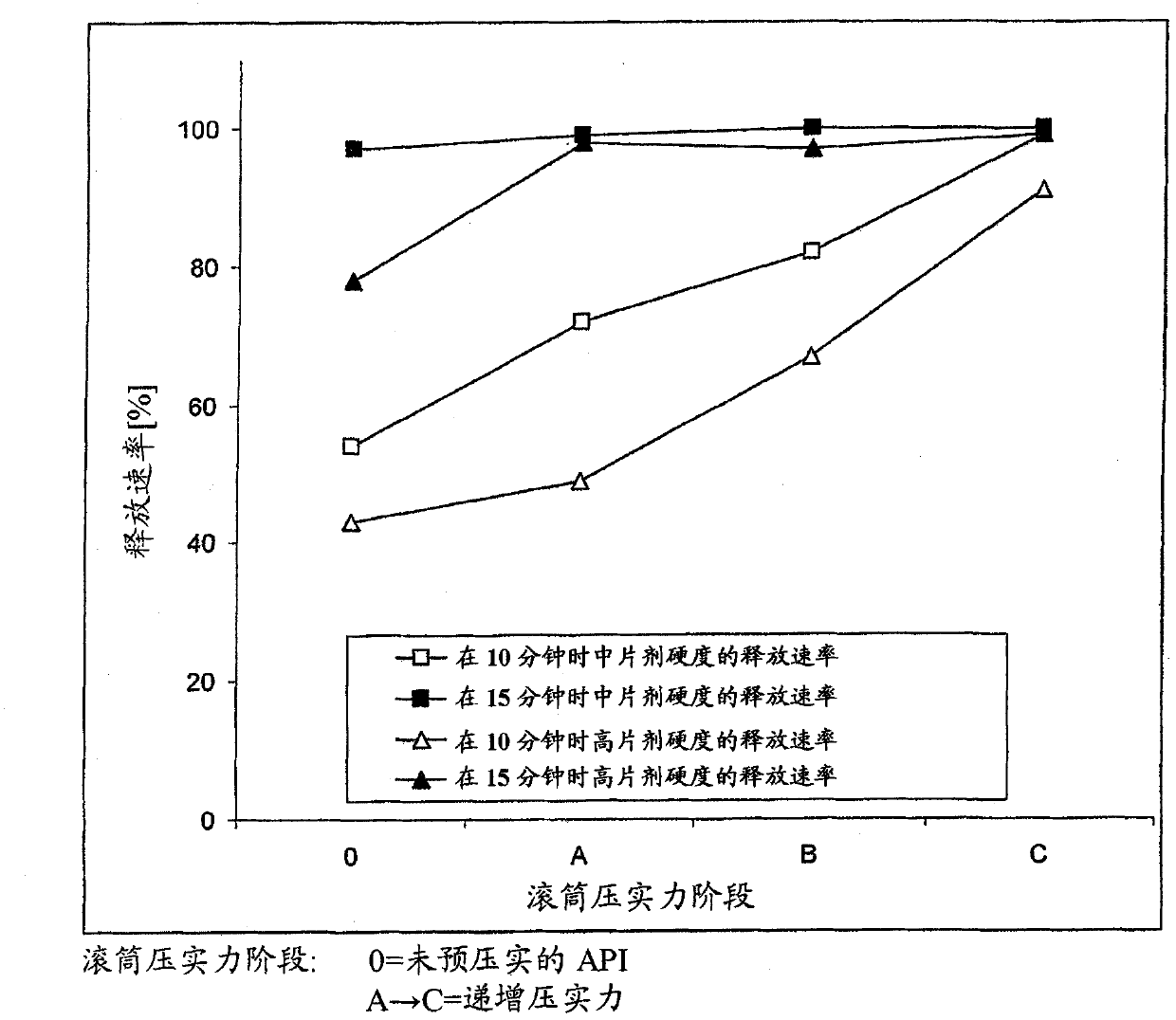
[0082] Dissolution characteristics and pharmacokinetic properties
[0083] BIBW 2992MA described in Examples (Table 4) 2 Four dose strengths (20, 30, 40, 50 mg) of film-coated tablets were subjected to in vitro comparative dissolution testing according to Apparatus 2 (paddle method) of Ph. Eur. 6.2 and described below:
[0084] Apparatus: Apparatus 2 (paddle method)
[0085] Paddle speed: 50 / 75rpm
[0086] Dissolution medium: 0.05M phosphate buffer, pH 6.8
[0087] McIlvaine buffer, pH 4.0
[0088] 0.1M HCl, pH 1
[0089] water (50rpm)
[0090] Volume: 900ml
[0091] Sampling time points: 5, 10, 15, 20, 30 minutes
[0092] Number of Tablets (n): 12 per dosage strength
[0093] Concentration measurements in dissolution vessels were performed with HPLC-UV.
[0094] BIBW 2992MA of the present invention utilizing 20, 30, 40 and 50 mg dose strengths at pH 1.0, 4.0, 6.8 and in water are shown in Figures 6-9, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com