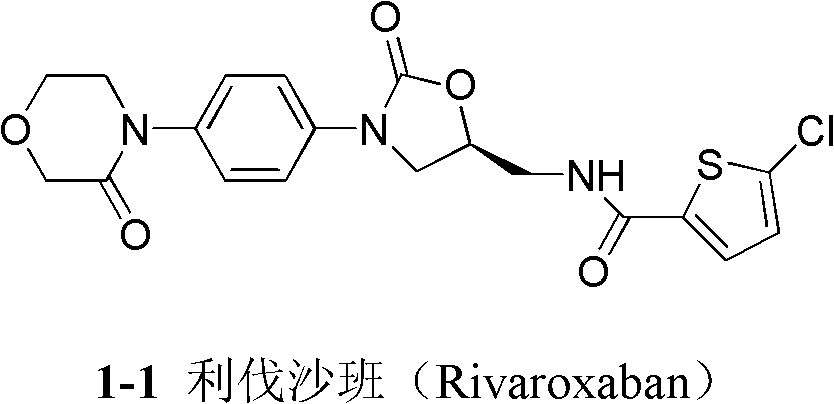
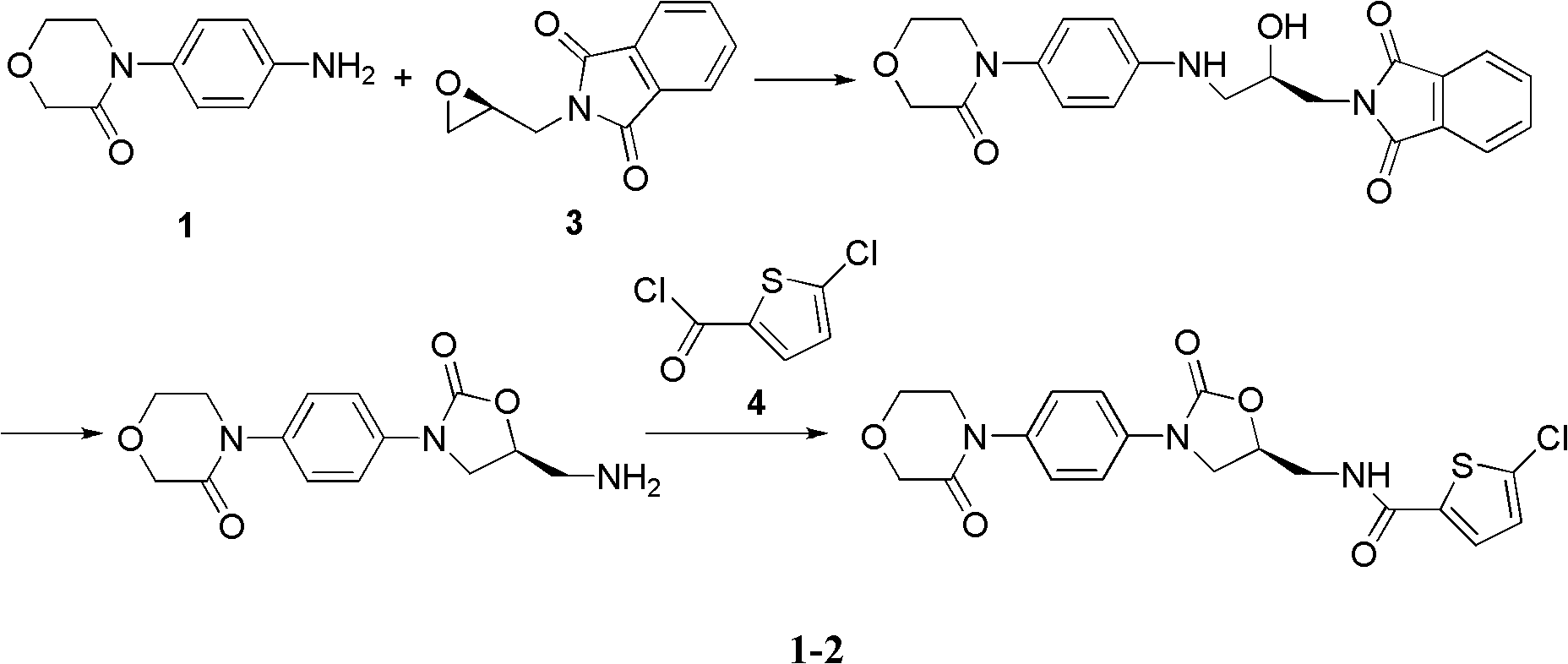
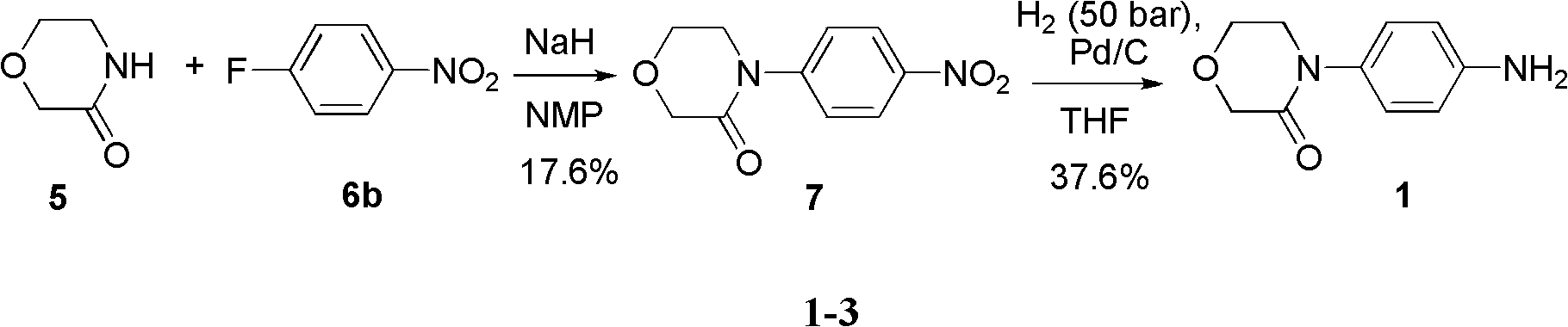
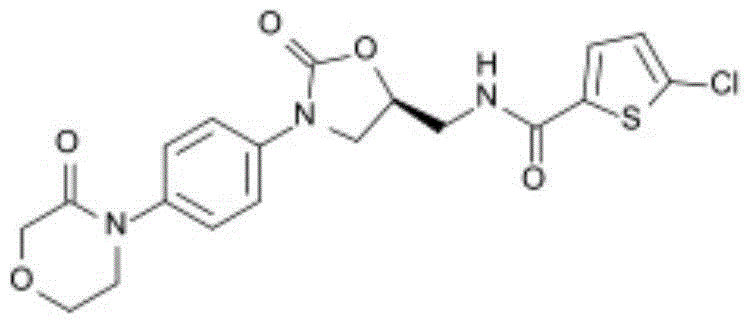
Patents
Literature
314 results about "Rivaroxaban" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rivaroxaban is used along with low-dose aspirin to help prevent heart attack, stroke, and death in people with a certain heart problem (coronary artery disease - CAD) or in people who have reduced blood flow to the arms/legs (peripheral artery disease - PAD).
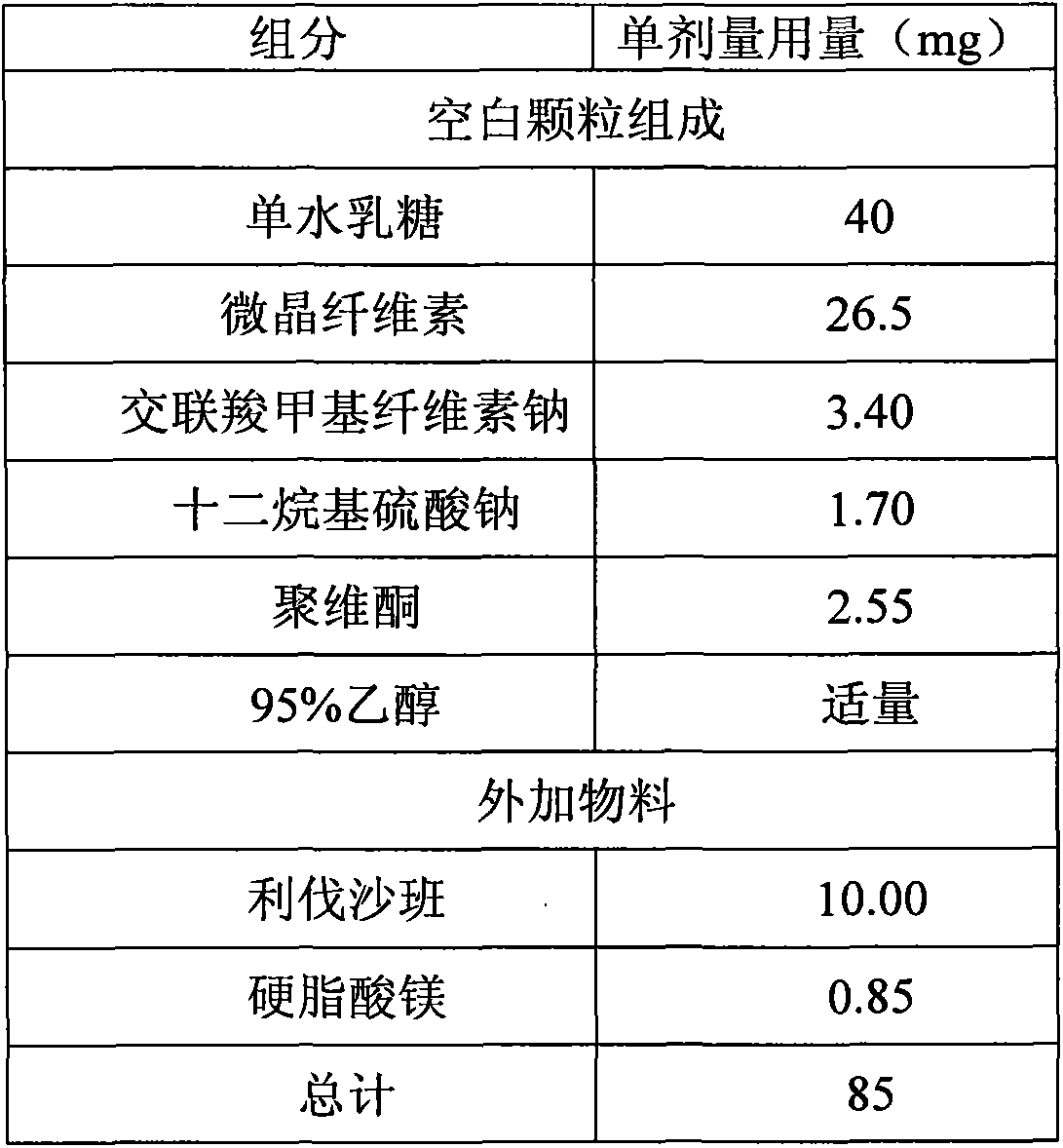
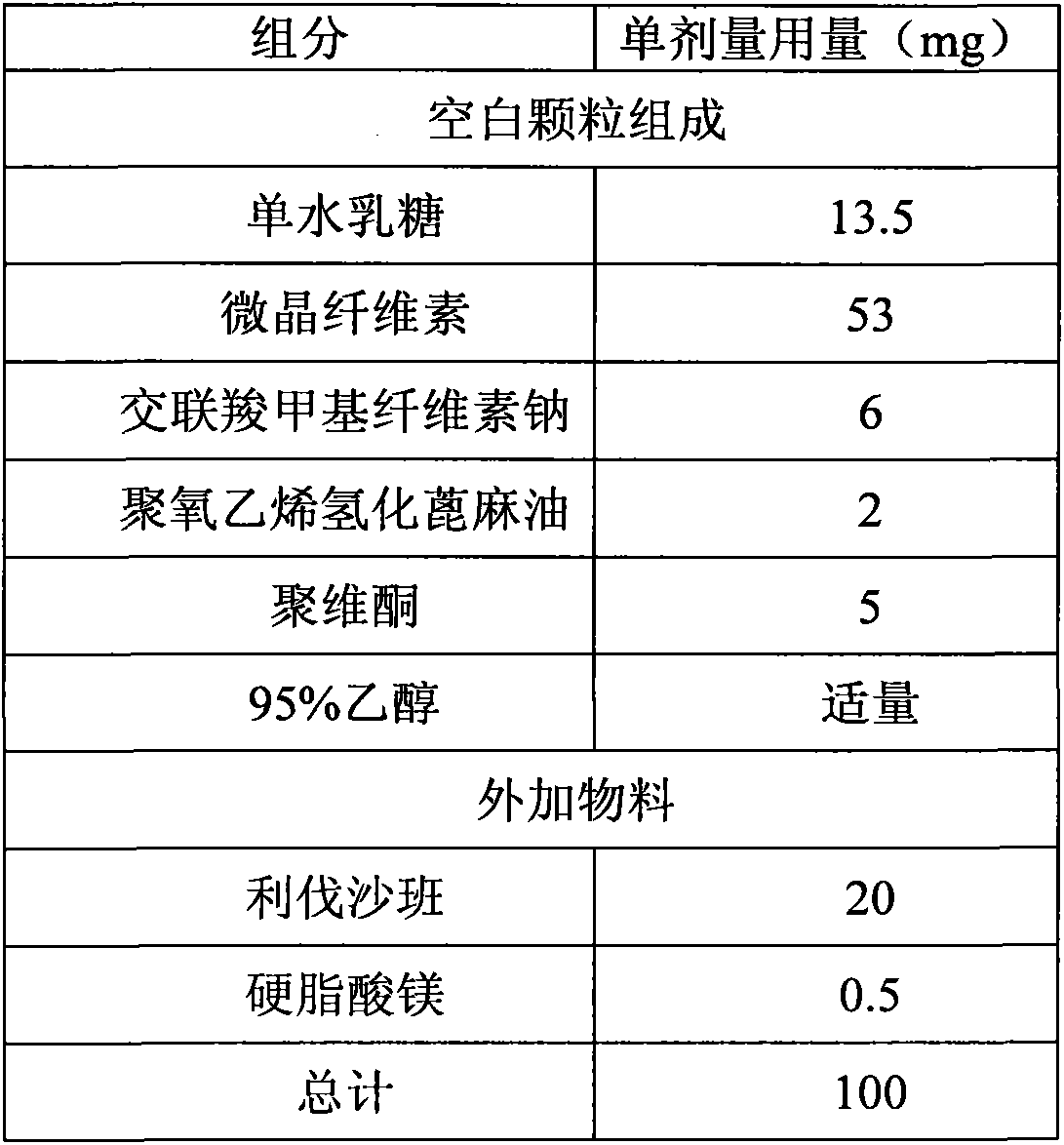
Medicinal composition containing rivaroxaban and preparation method thereof
ActiveCN103550165ASimple compositionEasy to manufactureOrganic active ingredientsPill deliveryActive componentRivaroxaban
The invention relates to a quickly releasing oral solid medicinal composition containing rivaroxaban and a preparation method thereof. Proper medical auxiliaries are treated by a wet granulation process, and are mixed with active components of rivaroxaban to obtain the medicinal composition with remarkably improved dissolution. The preparation method has the characteristics of simple process and industrial production, and solves the problem of complicated preparation process in the prior art.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Preparation method for rivaroxaban intermediate and rivaroxaban
InactiveCN102250076AMild reaction conditionsSimple and fast operationOrganic chemistryOxazolidine EMorpholine
The invention discloses a rivaroxaban intermediate, a structural formula of the rivaroxaban intermediate is represented by the following formula (III). The invention further discloses a preparation method for rivaroxaban. The method is characterized by: carrying out a cyclization reaction for 4-(4-isocyanatophenyl)-3-morpholinone and (R)-epichlorohydrin under a catalytic action of magnesium halide to generate (S)-4- morpholinonephenyloxazolidinone, followed by carrying out functional group conversion to obtain 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl] phenyl}-morpholine-3-ketone, then reacting with 5-chlorothiophene-2-formyl chloride to prepare the rivaroxaban. The preparation method provided by the present invention has advantages of mild reaction conditions, simple operation, high reaction yield and the like. In addition, the use of toxic and harmful reagents is avoided during the synthesis process, such that environmental pollution is reduced, and the method is applicable for the industrial production.
Owner:HENGDIAN GRP JIAYUAN CHEM
Method for preparing rivaroxaban solid composition
InactiveCN103705520ASolve the problem that the particle size cannot be reduced to 5μmIncrease dissolution rateOrganic active ingredientsRespiratory disorderMedicineRivaroxaban
The invention discloses a method for preparing a rivaroxaban solid composition. The method comprises the following steps: crushing rivaroxaban by adopting a wet crushing method, and simultaneously preparing a suspension solution; spraying the suspension solution into other auxiliaries to prepare grains with proper sizes; further preparing minimum medical dosage units. The method has the beneficial effects that: the problem that the grain diameter cannot be reduced to 5mu m when rivaroxaban particles are prepared by adopting a conventional crushing method can be solved, and the dissolution speed of the rivaroxaban preparation can be increased.
Owner:CHINA RESOURCES SAIKE PHARMA
Method for synthesizing rivaroxaban
The invention relates to a method for synthesizing rivaroxaban, in particular to a method for preparing rivaroxaban from 4-(4-aminophenyl)-3-morpholinone. The method is easy to operate for synthesizing the rivaroxaban, does not adopt toxic substances, is high in yield of rivaroxaban and low in cost and is suitable for industrial production.
Owner:HUNAN NORMAL UNIVERSITY
Analysis and detection method for rivaroxaban intermediate
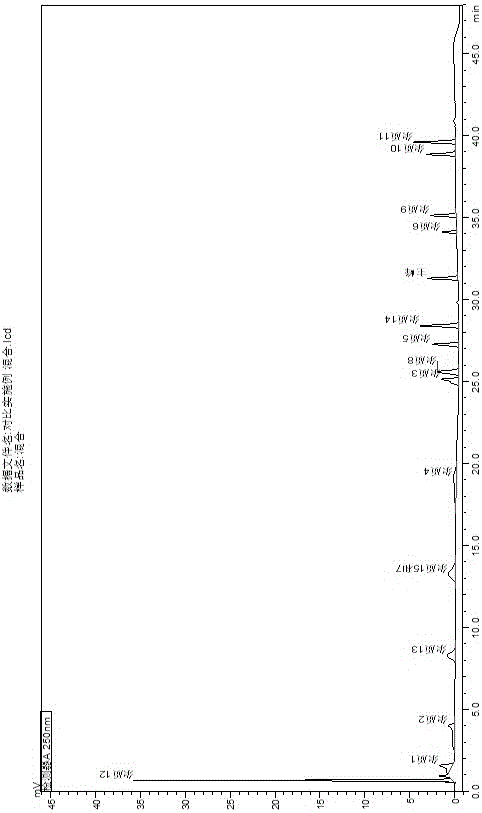
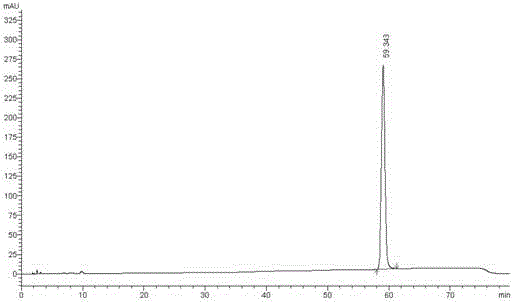
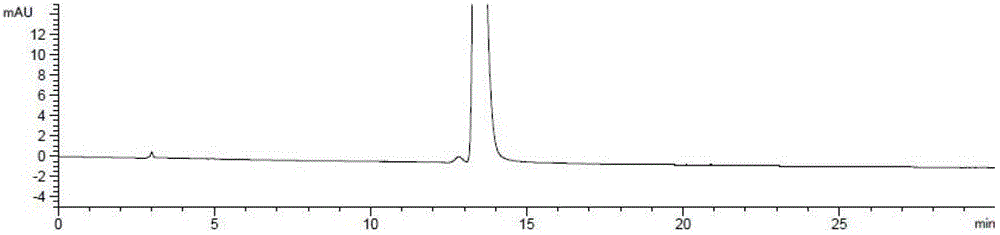
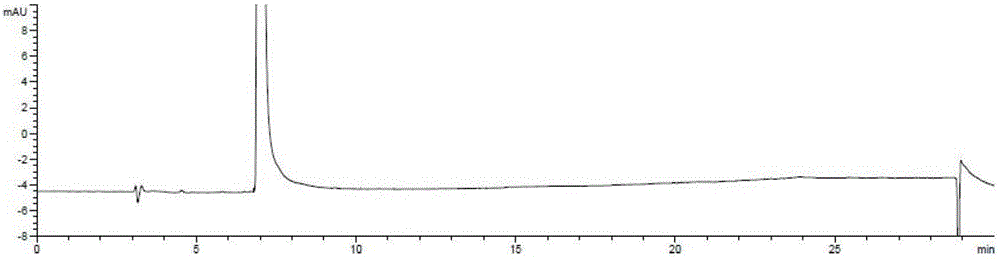
The invention relates to an analysis and detection method for a rivaroxaban intermediate, which is particularly used for mass control on the rivaroxaban intermediate. A chromatographic column (C18, 4.6mm*150mm, 5 microns) which takes octadecyl silane bonded silica gel as a packing and a perchloric acid solution and methanol as flow phases are adopted for gradient elution, and analysis and detection are performed by a high performance liquid chromatography method under the conditions that the detection wavelength is 240nm-250nm, the column temperature is 30-40 DEG C and the velocity is 0.7-1.1 ml / min. By adopting the analysis and detection method provided by the invention, the rivaroxaban intermediate can be effectively separated from impurities of the rivaroxaban intermediate, and moreover, the method has the advantages of high separation degree and sensitivity, good repeatability and durability, short analysis time, simplicity in operation and stable and reliable result.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of rivaroxaban and intermediate thereof and intermediate compound
InactiveCN102408420AShort routeLow costOrganic compound preparationBulk chemical productionProduction rateRivaroxaban
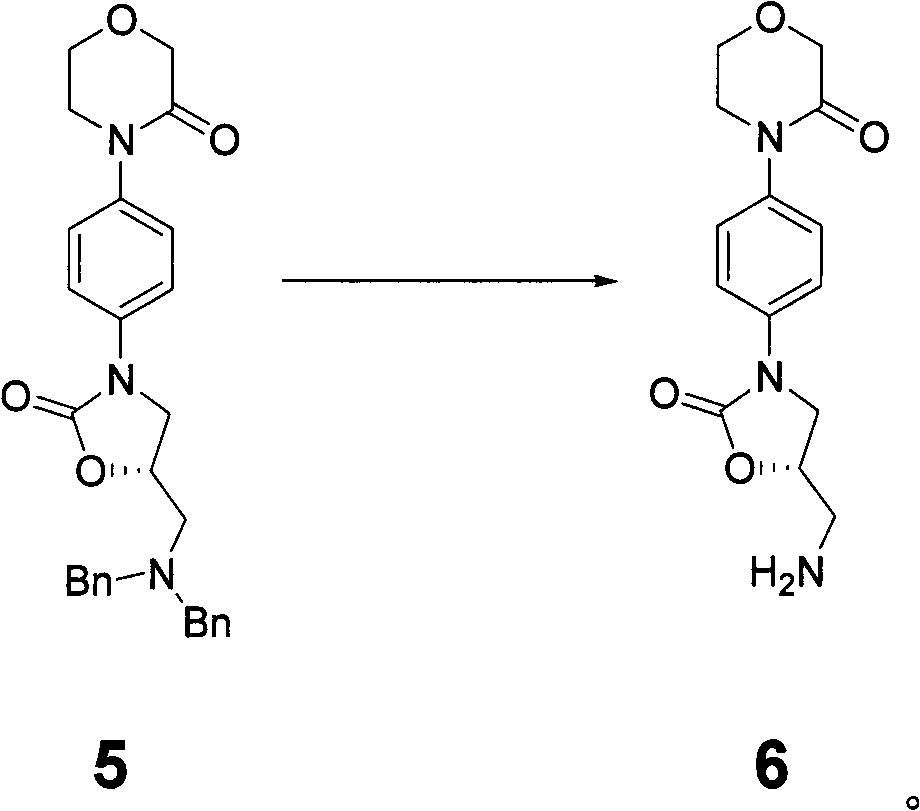
The invention discloses a preparation method of intermediate of rivaroxaban of formula 6, which comprises the following steps of: carrying out reaction of removing benzyl protecting group of amino to the compound 5 in a solvent. The invention also discloses a preparation method of rivaroxaban and an intermediate compound thereof. The preparation method disclosed by the invention has the advantages of shorter process, lower cost, easily acquired material and high production rate, and the prepared product is suitable for industrial production.
Owner:汕头经济特区鮀滨制药厂
Solid pharmaceutical composition containing rivaroxaban
ActiveCN105232488AGood dissolution effectReduce usageOrganic active ingredientsOrganic chemistryRivaroxabanMedical prescription
The invention relates to a pharmaceutical composition containing rivaroxaban, in particular to a solid pharmaceutical composition containing rivaroxaban and a preparation method of the solid pharmaceutical composition. The solid pharmaceutical composition containing the rivaroxaban can be further prepared into a film-coated tablet by a specific preparation technology to serve as a specific administration mode. The solid pharmaceutical composition containing the rivaroxaban and the preparation method of the solid pharmaceutical composition have the advantages that by application of the specific preparation technology, using surfactants in a preparation is avoided and dissolution rate of the tablet is increased effectively; by means of using sodium stearyl fumarate in the composition, marked increase of degraded impurities I during long-term storage of the tablet is avoided effectively; through stability accelerating research, the film-coated tablet containing the rivaroxaban, prepared by the prescription and technological steps, is stable and controllable in quality.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Detection method of Rivaroxaban tablet relevant substances
ActiveCN106442831AEffective controlEfficient detectionOrganic chemistryComponent separationUltraviolet detectorsSilanes
The invention discloses a method for simultaneously detecting Rivaroxaban tablet relevant substances by high performance liquid chromatography. According to the chromatographic conditions, octadecyl silane is used as a filling agent of a chromatographic column; acetonitrile-buffer salts (containing phosphate and ion-pairing agents) are used as a flowing phase to perform gradient elution; an ultraviolet detector is used as a detector. By using the method provided by the invention, impurities in Rivaroxaban tablets can be effectively detected out; the analysis time is short; the efficiency is high; a simple and reliable method is provided for the Rivaroxaban tablets.
Owner:ZEIN BIOTECHNOLOGY CO LTD
Preparation methods for rivaroxaban and intermediate thereof, and intermediate compounds
ActiveCN103012388AEasy to purifyChiral raw materials are readily availableOrganic compound preparationBulk chemical productionChemical compoundRivaroxaban
The present invention discloses a preparation method for a rivaroxaban intermediate compound represented by a formula 5, wherein the preparation method comprises the following steps: carrying out a benzyl group removing reaction of a compound 4 in a solvent to prepare a compound 5. The present invention further discloses a rivaroxaban preparation method and intermediate compounds. According to the preparation method, chiral raw materials are easy to obtain, and have cheap price, the process is simple, the post treatment is simple, the intermediate and the final product are easy to purify, the total yield is high, the purity is high, and industrial production is easily achieved.
Owner:联化科技(上海)有限公司 +1
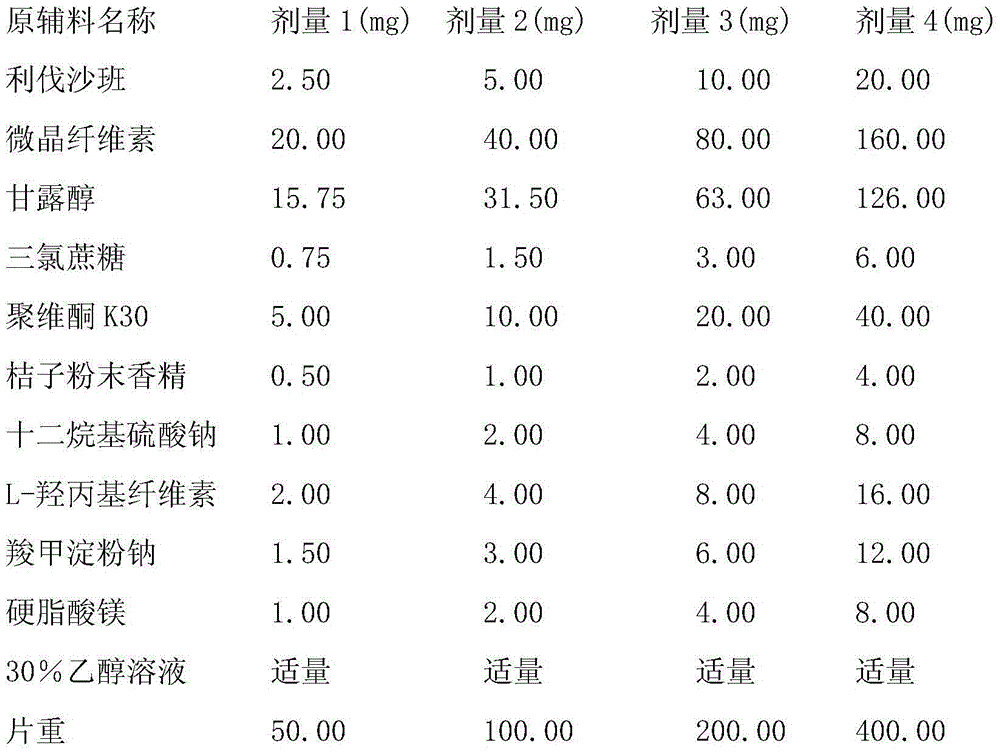
Preparation method of oral preparation containing rivaroxaban
ActiveCN104055743AGood reproducibilityOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseDissolution
The invention relates to an oral tablet containing rivaroxaban. The tablet is prepared by adopting a powder direct mixing and tabletting method. an inventor solves partial problems and solves the problems of pitted surface, sticking, loosening tablet and disintegration and dissolution rate and stability problems through addition and replacement of a proper additives and a screening experiment, so as to obtain a novel formula of rivaroxaban tablets and a preparation method of the rivaroxaban tablets by adopting the powder direct mixing and tabletting method. Each rivaroxaban tablet comprises the following components by weight: 5-20mg of rivaroxaban, 60-80mg of lactose, 4-6mg of mannitol, 2-4mg of croscarmellose sodium, 1-3mg of citric acid, 0.5-2mg of lauryl sodium sulfate, 3-5mg of carbomer, 3-5mg of povidone K90, 3-5mg of hydroxyl propyl cellulose, 0.5-2mg of magnesium stearate and 0.5-2mg of silicon dioxide.
Owner:JILIN BODA PHARMA
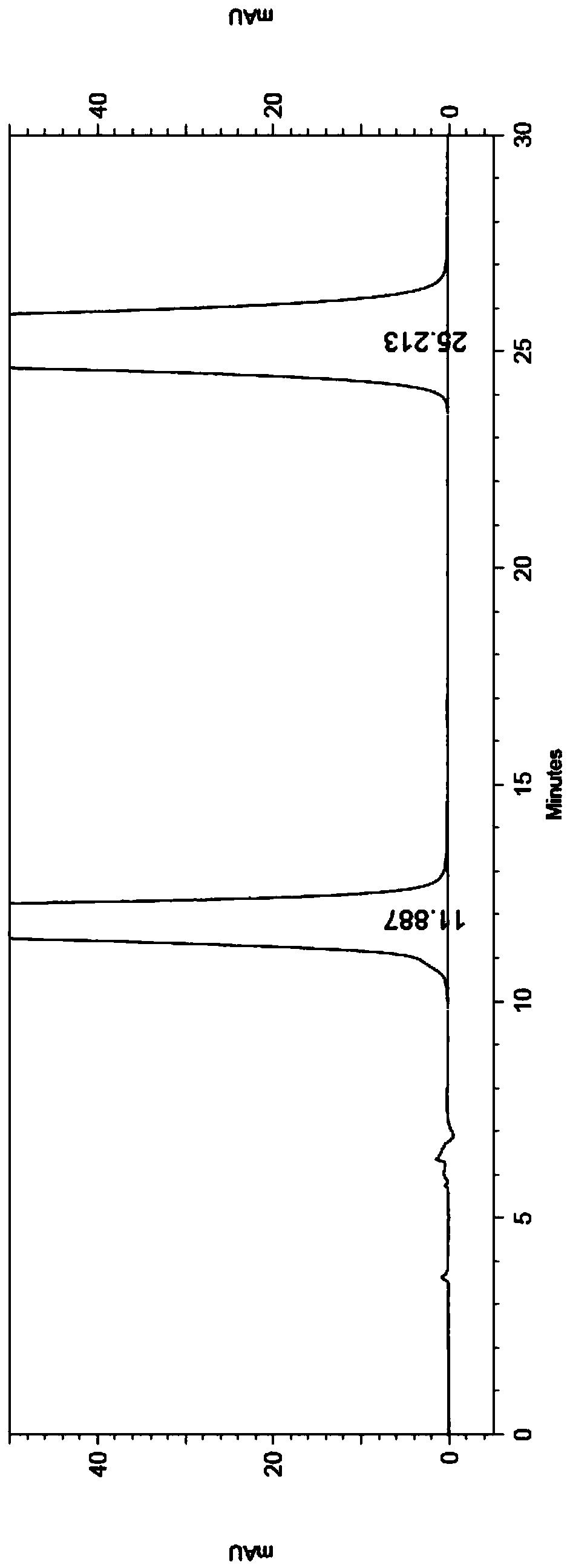
Separation and detection method for anticoagulant drugs
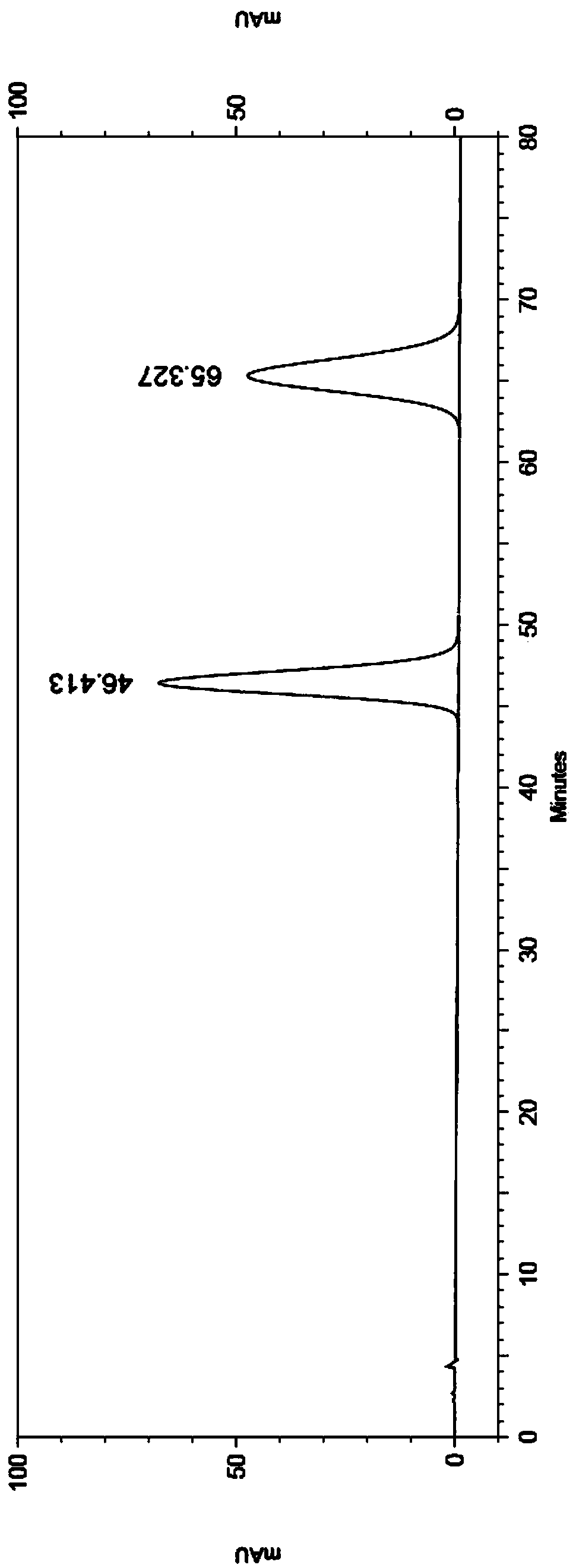
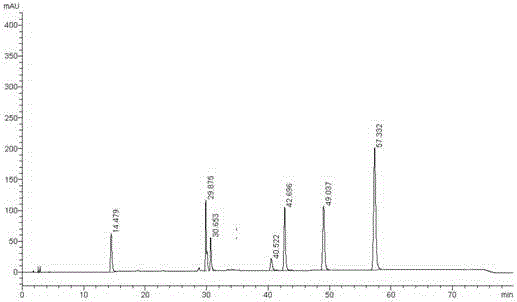
The invention belongs to the field of analytical chemistry and particularly relates to a method of separating and testing (S)-Rivaroxaban and enantiomers thereof by liquid chromatography. The method for separating and testing (S)-Rivaroxaban and enantiomers (impurities) thereof by liquid chromatography is characterized in that a polysaccharide derivative is used as a chiral chromatographic column of filler and a mixed solution of low alcohol or low alkane or low alcohol is used as a moving phase. According to the separation and detection method provided by the invention, (S)-Rivaroxaban can be effectively separated from enantiomers thereof, wherein the separation degree reaches over 1.5 and complete baseline separation is achieved, so that the quality of (S)-Rivaroxaban can be accurately and effectively controlled. The separation method provided by the invention can be used for separating and detecting the (S)-Rivaroxaban and enantiomers thereof within 80 minutes. The method provided by the invention can be used for simply, quickly and accurately separating and detecting (S)-Rivaroxaban and optical isomers thereof.
Owner:SUNSHINE LAKE PHARM CO LTD
Method for separating and measuring rivaroxaban and its impurities, and application thereof
ActiveCN107941936AFast and simultaneous separabilityFast and simultaneous detectionComponent separationRivaroxabanDiluent
The invention belongs to the field of analytical chemistry, and concretely relates to a method for separating and measuring rivaroxaban and its impurities, and an application thereof. A reagent composition for separating and measuring rivaroxaban and its impurities comprises: a diluent which is an aqueous solution of acetonitrile and an acid; a mobile phase A which is a buffer salt solution containing methanol and acetonitrile; and mobile phase B which is a mixture of the buffer salt solution and acetonitrile. The method for separating and measuring rivaroxaban and its impurities by using thereagent composition comprises the following steps: dissolving rivaroxaban in the diluent to obtain a sample solution; preparing the mobile phases; injecting the sample solution into a separation and detection system, and eluting and separating the sample solution with the mobile phases to obtain an eluate; and adding the eluate into a detector, and measuring the eluate. The method has the advantages of realization of complete separation of the rivaroxaban and its impurities, good baseline, high sensitivity, short analysis time, good reappearance, and realization of effective separation and themeasurement of the content of every relevant substance in a rivaroxaban raw medicine and a rivaroxaban preparation.
Owner:CHONGQING HUAPONT PHARMA
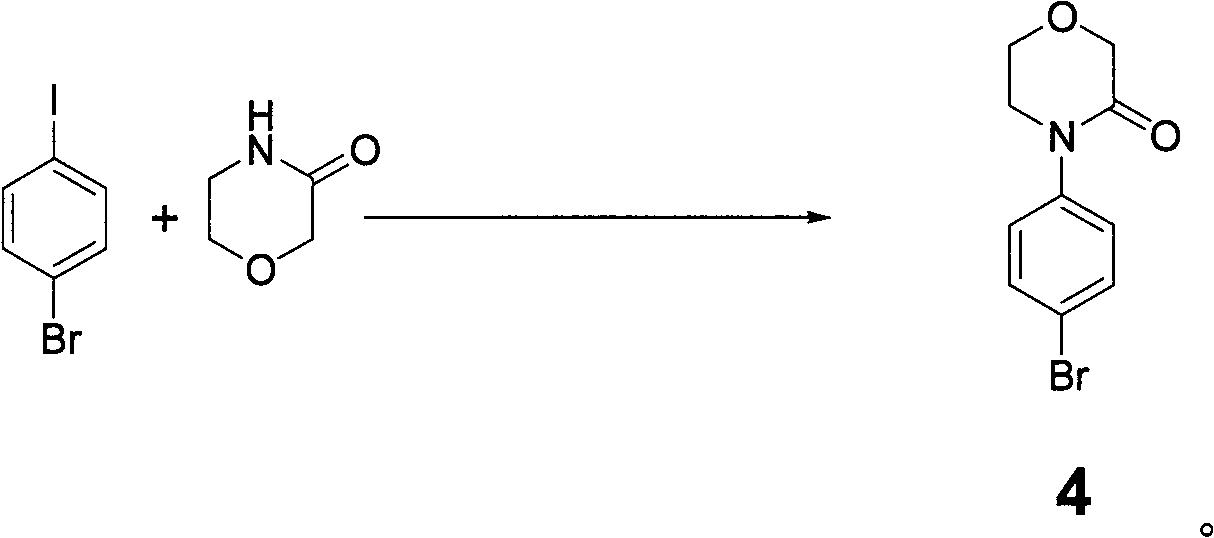
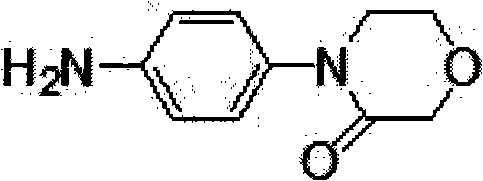
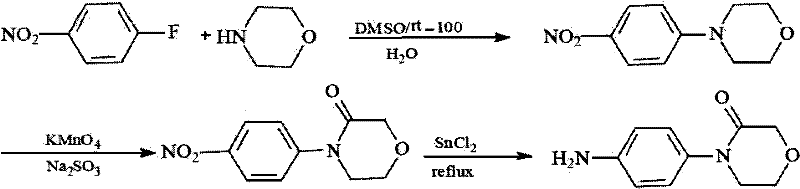
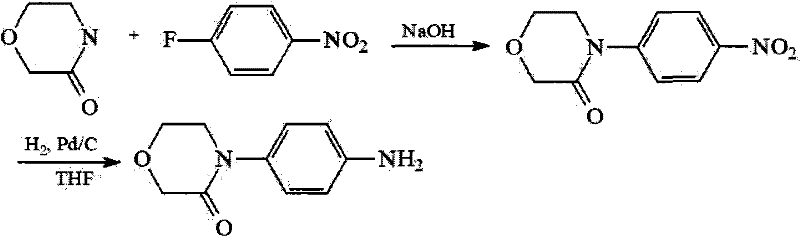
Synthesis method of 4-(4-aminophenyl)-3-morpholone
The invention provides a synthesis method of an important intermediate 4-(4-aminophenyl)-3-morpholone of a new anticoagulant rivaroxaban, belonging to the field of chemical synthesis. The method comprises the following steps: 1) cyclizing nitroaniline and di-halogen aether to obtain 4-(4-nitrophenyl)-morpholine; 2) reacting 4-(4-nitrophenyl)-morpholine and organic hydroperoxide under the action of a catalyst to generate 4-(4-nitrophenyl)-morpholone; and 3) reducing the 4-(4-nitrophenyl)-morpholone into the under the action of a catalyst, wherein the mol yield of the three steps is higher than 49% (on the basis of nitroaniline). The synthesis method provided by the invention has the characteristics of accessible raw materials, low price, simple operation steps, mild reaction environment and the like, and is suitable for industrialized mass production; and the three wastes are easy to process.
Owner:BEIJING GUANHONG TECH
Separation determination method of rivaroxaban optical isomer
InactiveCN103217492ABreak the limitationsSeparation assay is applicableComponent separationRivaroxabanStandard samples
The invention relates to a separation determination method of a rivaroxaban optical isomer. According to the invention, proper amounts of a rivaroxaban optical isomer sample requiring detection and a rivaroxaban racemate standard sample with known component contents are fetched, and are respectively dissolved by using acetonitrile, such that acetonitrile solutions of the rivaroxaban optical isomer sample requiring detection and of the rivaroxaban racemate standard sample with known component contents are prepared; a bonded chiral column CHIRALPAKIC column is adopted, and feeding is carried out; a detection wavelength is 210-260nm, a column temperature is 15-35 DEG C, a feeding volume is 5-20mul, and a mobile phase flow rate is controlled at 0.7-1.0ml / min, such that the separation of the rivaroxaban optical isomer requiring separation is finished; and with an area normalization method, the obtained separation spectrum of the rivaroxaban optical isomer sample requiring detection and the separation spectrum of the rivaroxaban racemate standard sample with known component contents are compared, such that S-configuration and R-configuration rivaroxaban contents in the rivaroxaban optical isomer requiring detection are calculated.
Owner:SHANGHAI INST OF TECH
Rivaroxaban tablet
ActiveCN104666262ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryDiethylene glycol monoethyl etherMedicine
The invention belongs to the technical field of drugs and in particular relates to a rivaroxaban tablet. The rivaroxaban tablet contains rivaroxaban, hydroxypropyl cellulose and fumed silica and is prepared by dissolving rivaroxaban and hydroxypropyl cellulose in diethylene glycol monoethyl ether, adding fumed silica for adsorption, then mixing the materials with pharmaceutically acceptable auxiliary materials uniformly and pressing the mixture by adopting a direct tabletting process. Compared with the prior art, the rivaroxaban tablet is high in drug dissolution speed and simple in process and dispenses with addition of surfactants and micronization treatment.
Owner:SHANDONG NEWTIME PHARMA
Method for synthesizing rivaroxaban intermediate 4-(4-aminophenyl)-3-molindone
ActiveCN103524447AReduce pollutionRaw materials are cheap and easy to getOrganic chemistryIron powderEthylene oxide
The invention discloses a method for synthesizing a rivaroxaban intermediate 4-(4-aminophenyl)-3-molindone. The method comprises the following steps: reacting paranitroaniline with ethylene oxide to obtain a compound II; performing a cyclization reaction on the compound II and bromoacetyl bromide to obtain a compound III; adding the compound III into iron powder to perform a reduction reaction to obtain the 4-(4-aminophenyl)-3-molindone. According to the method, the raw materials are low in cost and easily obtained, the cost is low, the synthesis operation is simple, industrial nitration is avoided, environmental pollution is greatly reduced, and the method is suitable for industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Rivaroxaban-containing tablets
ActiveCN104721156AAdvantages and Notable ImprovementsSignificant progressOrganic active ingredientsPill deliverySucroseFiller Excipient
The present invention discloses rivaroxaban-containing tablets, which are prepared from a raw material containing micronized rivaroxaban and an auxiliary material containing a water soluble filler, wherein a weight ratio of the rivaroxaban to the water soluble filler is 1:0.5-50, and the water-soluble filler is one or a plurality of materials selected from lactose, mannitol, sorbitol and sucrose. According to the present invention, the hydrophilicity of the rivaroxaban is significantly improved, the rapid dissolution the rivaroxaban in the gastrointestinal tract body fluid is easily achieved, the preparation process is simple, the operation is convenient, and the method is suitable for industrial large-scale production.
Owner:SHANDONG NEWTIME PHARMA
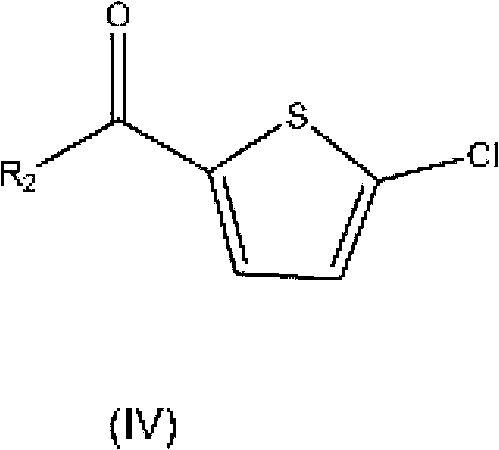
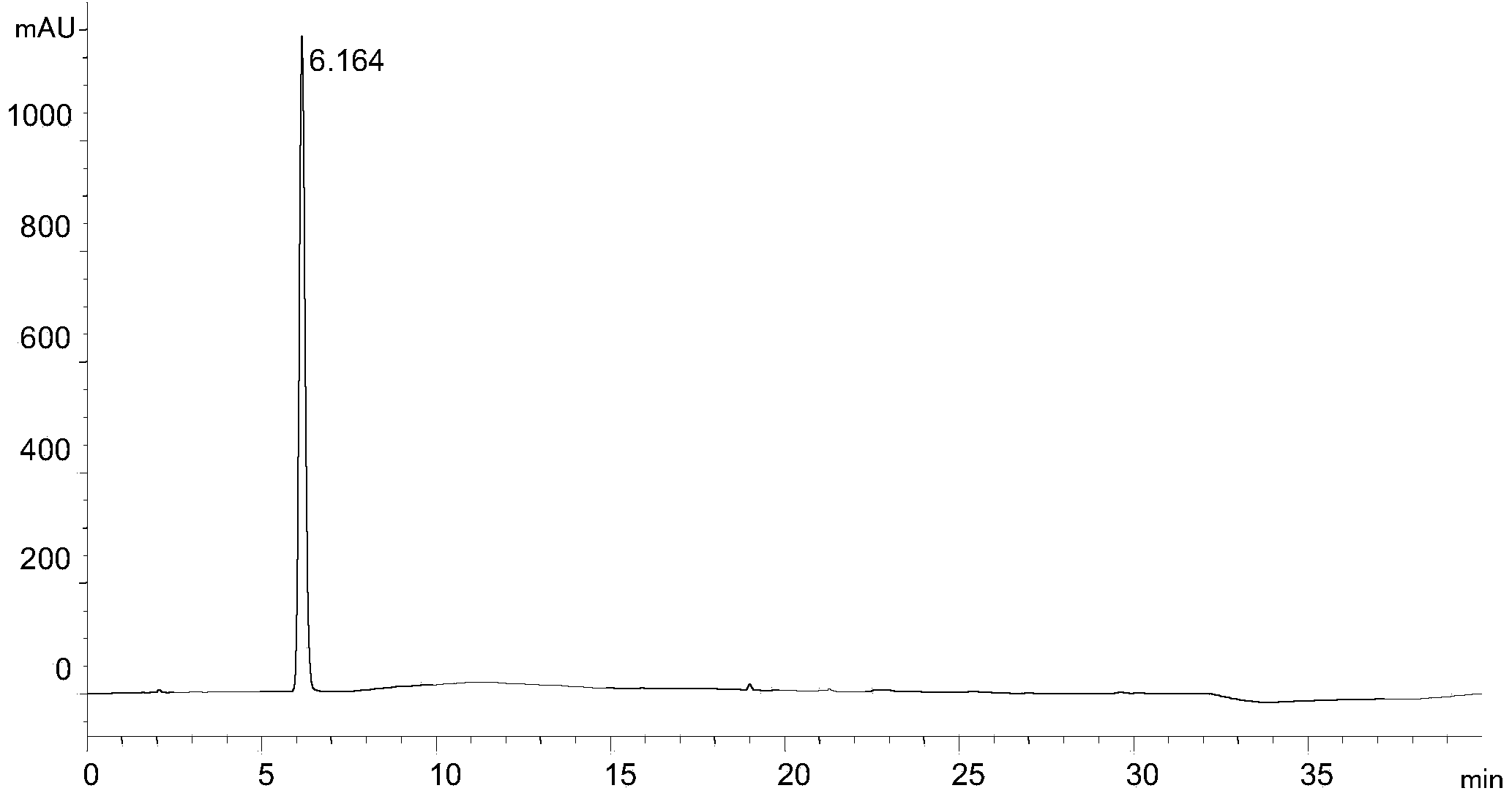
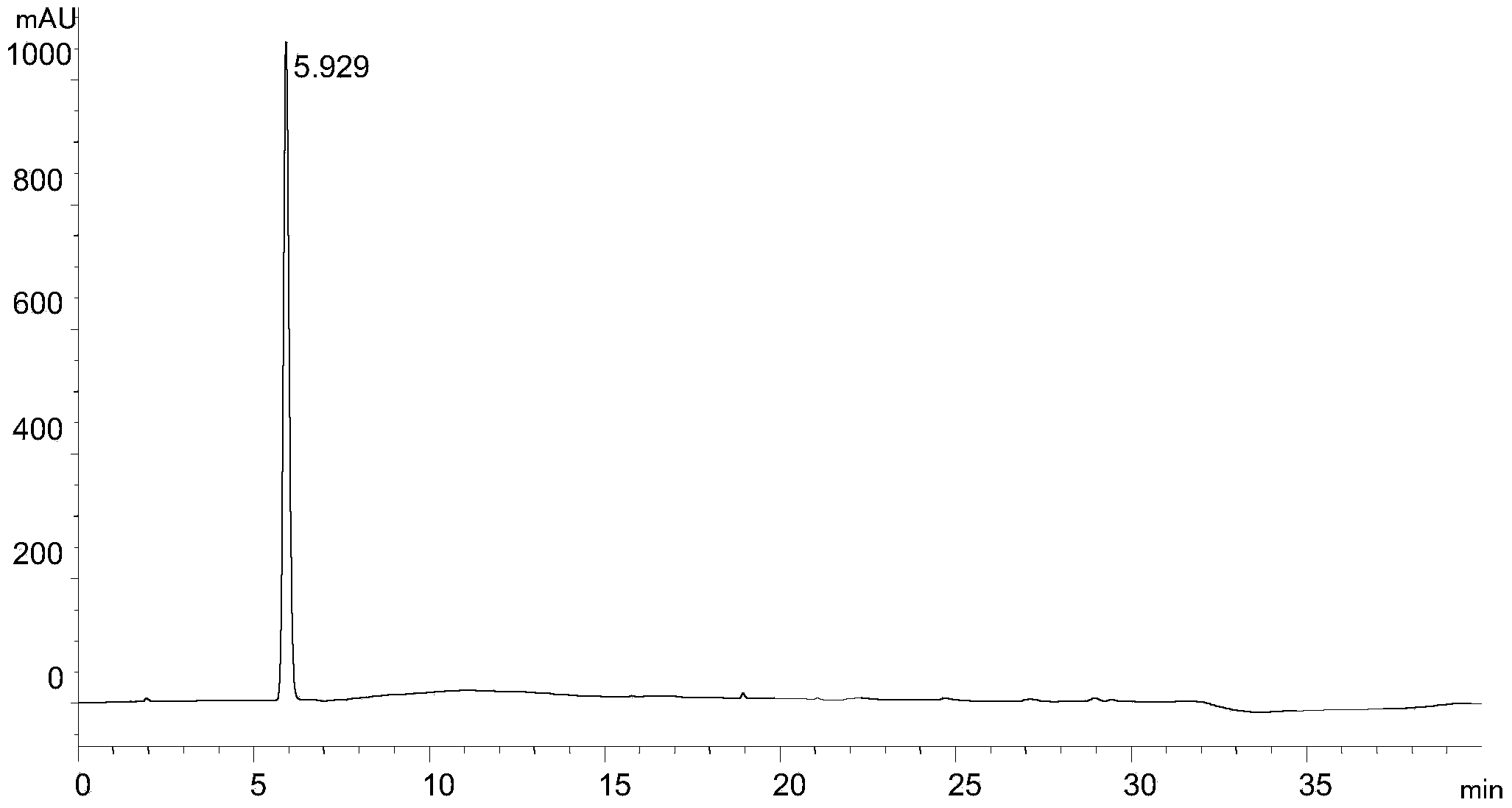
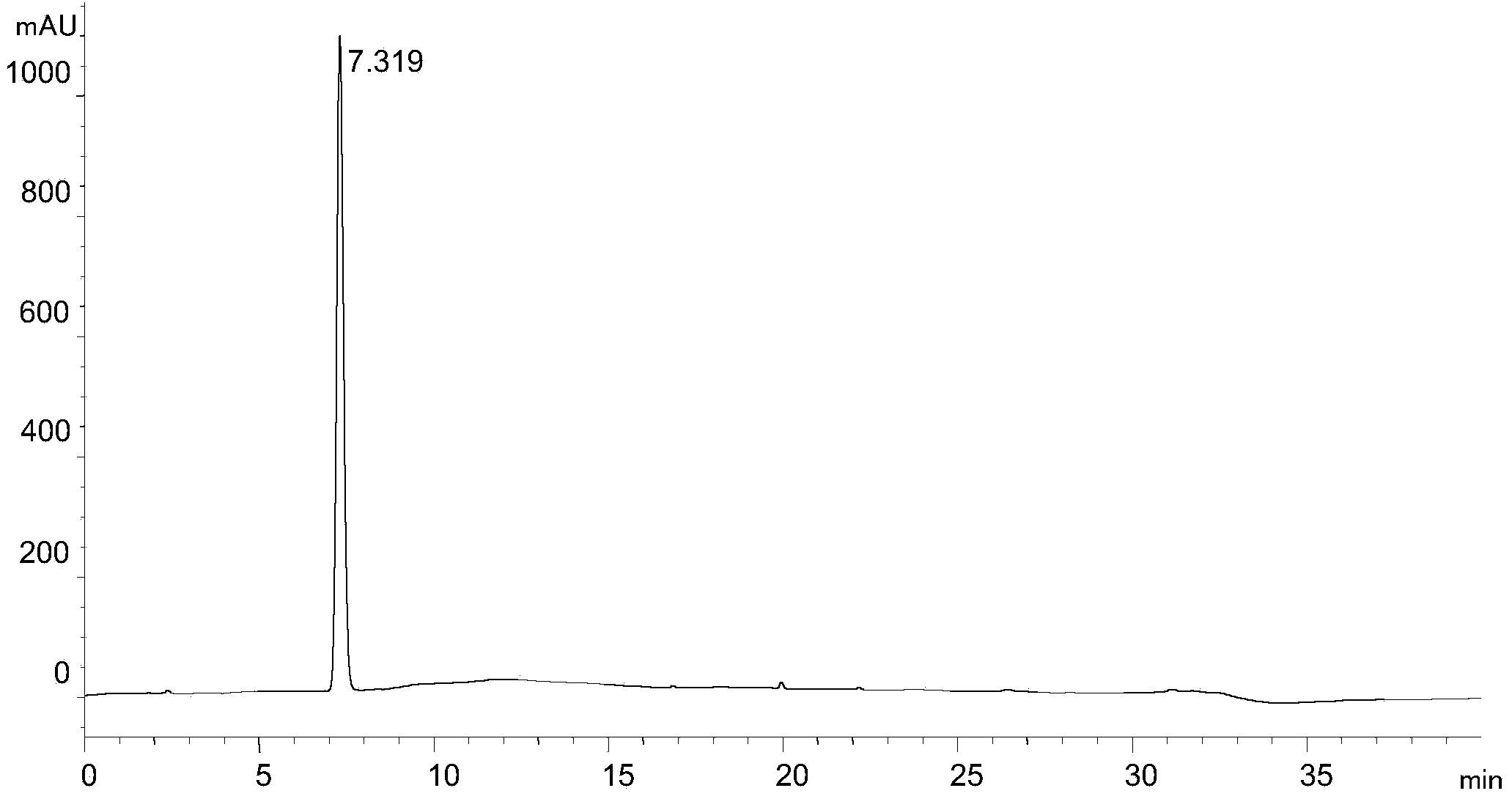
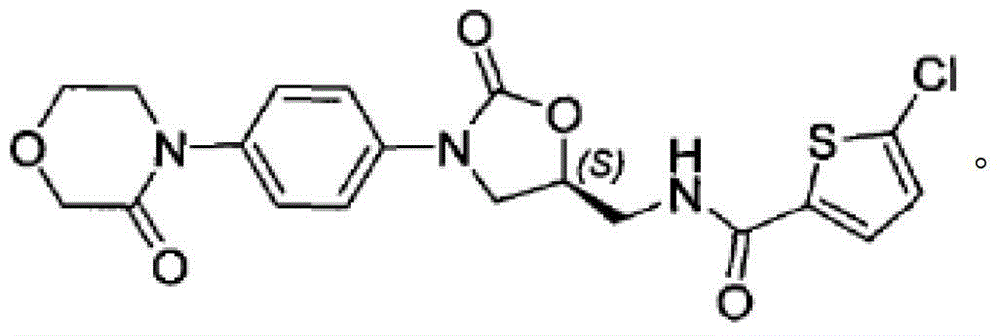
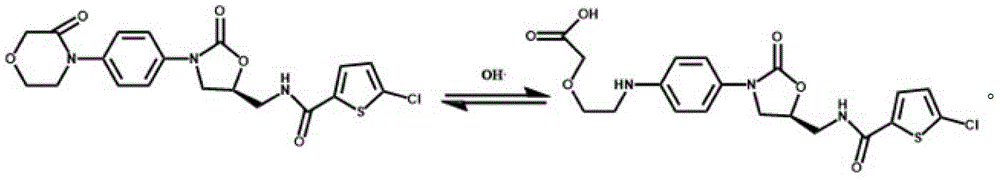
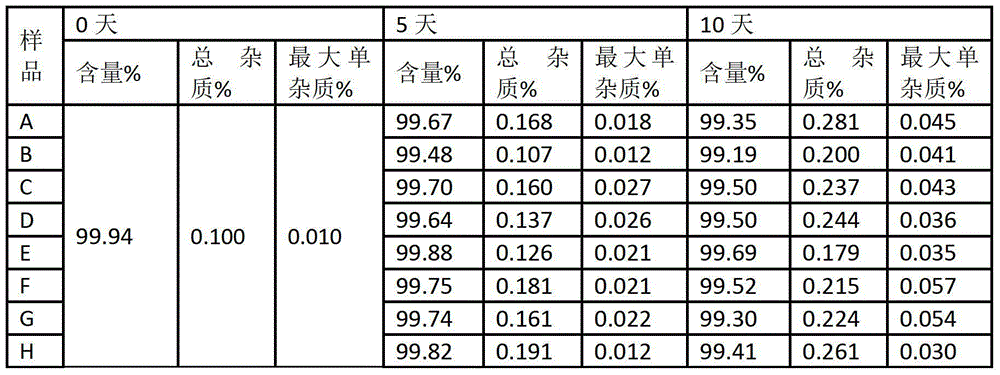
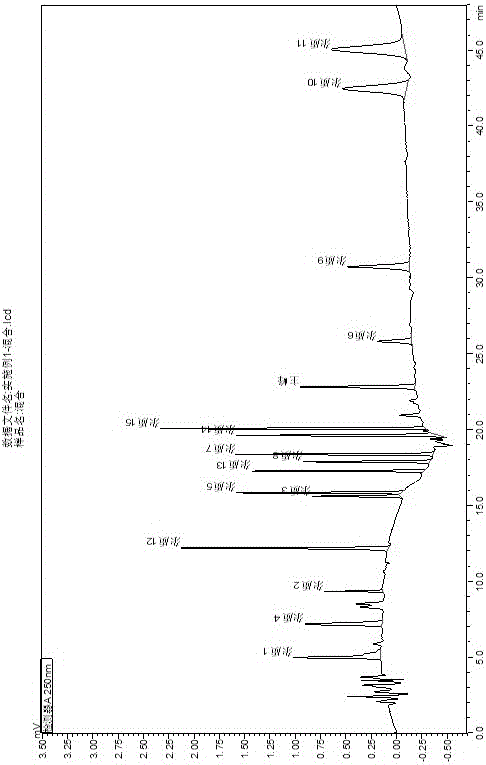
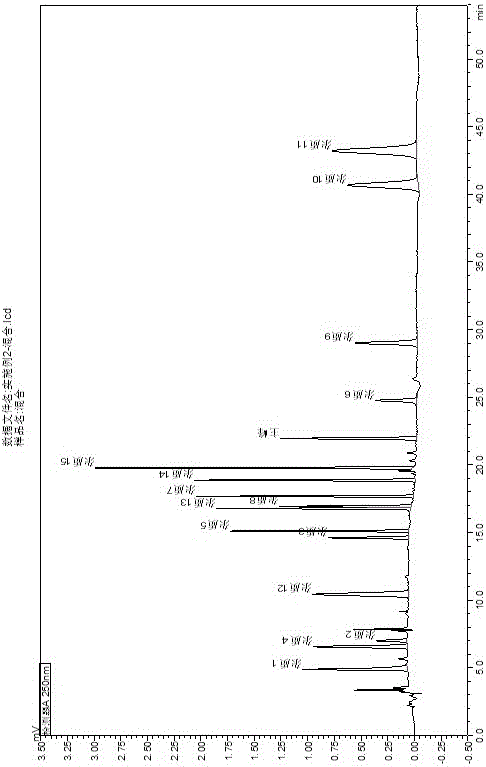
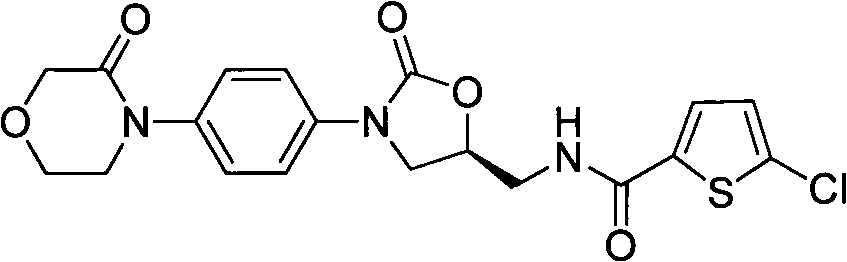
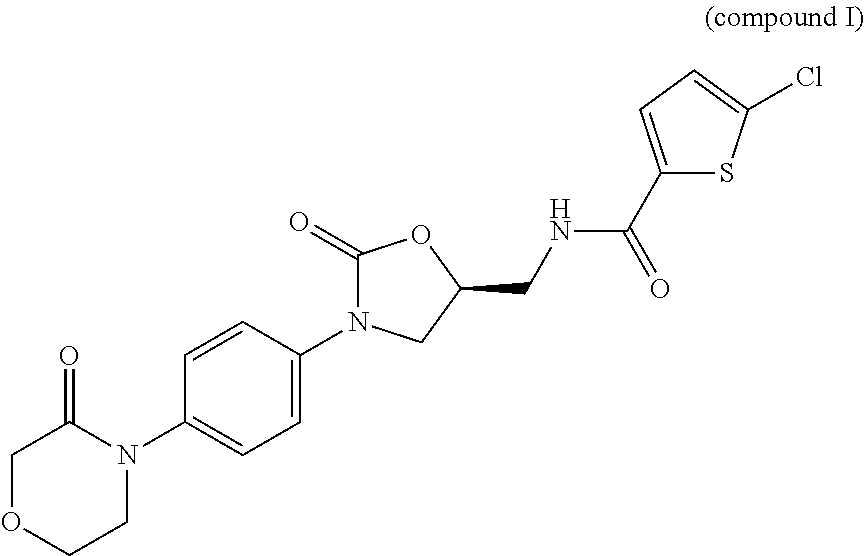
Process for Determining the Suitability for Distribution of a Batch of Thiophene-2-Carboxamide Derivative
The present invention relates to a process for determining the suitability for distribution of a batch of rivaroxaban or of a pharmaceutical composition thereof. In particular, it also relates to two impurities of rivaroxaban, to their use as reference markers to determine the purity of a sample of rivaroxaban or a composition thereof, to analytical methods for determining the purity of a sample of rivaroxaban or a composition thereof and to a process of preparing rivaroxaban or pharmaceutical compositions thereof which are free or substantially free of such impurities.
Owner:MEDICHEM
Rivaroxaban tablets and preparation method thereof
ActiveCN104887633ASimple preparation processImprove medication safetyOrganic active ingredientsPharmaceutical product form changeSucroseRivaroxaban
Owner:SHANDONG NEWTIME PHARMA
Synthesis method of rivaroxaban
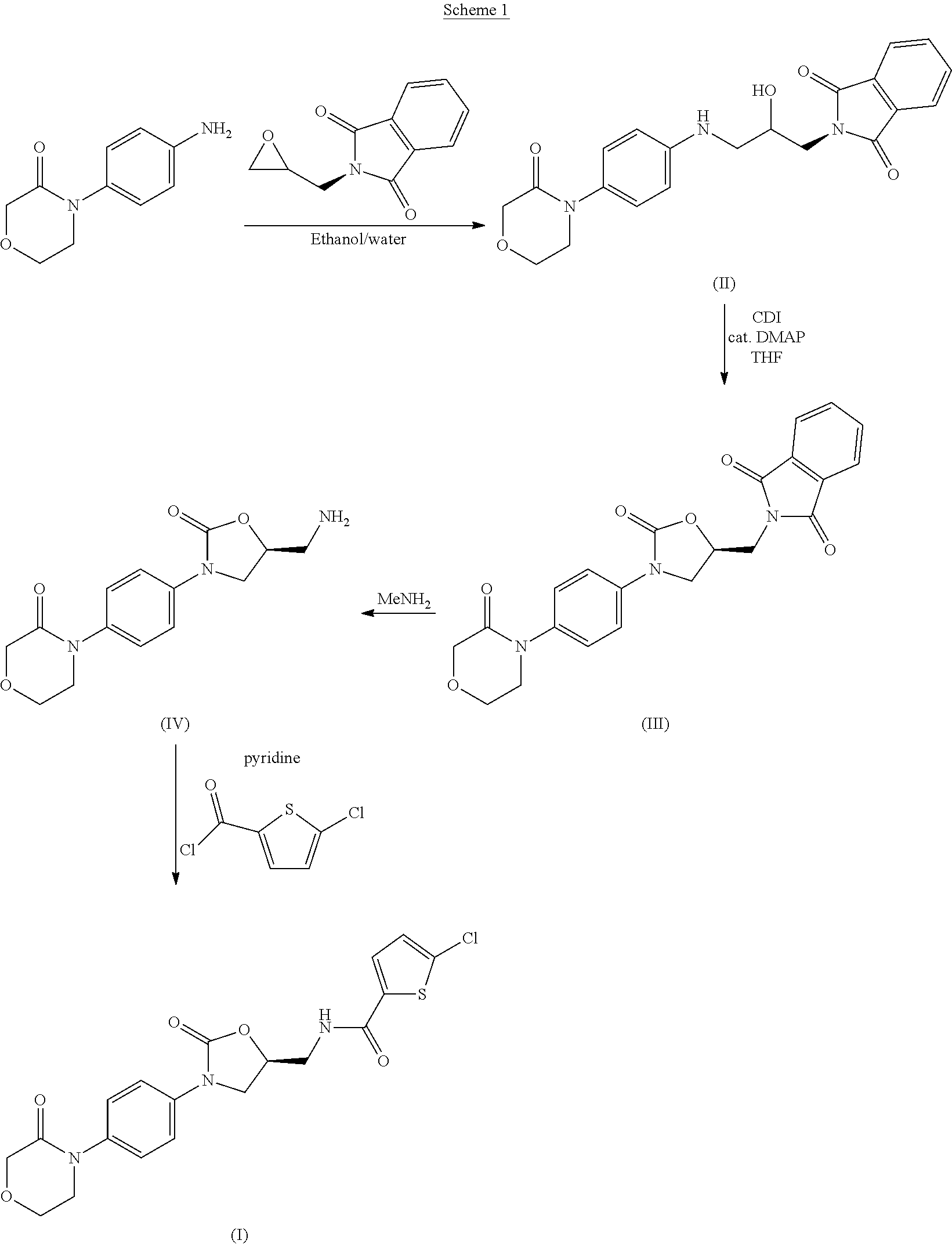
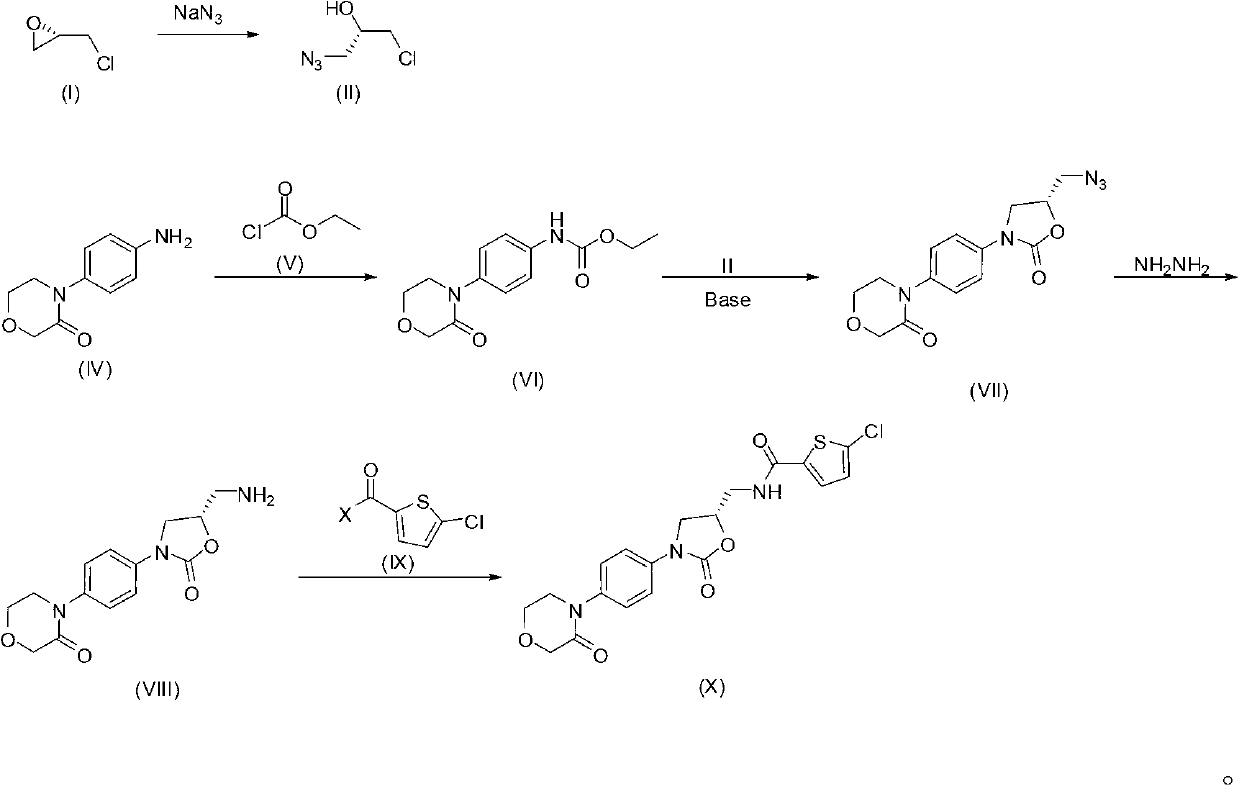
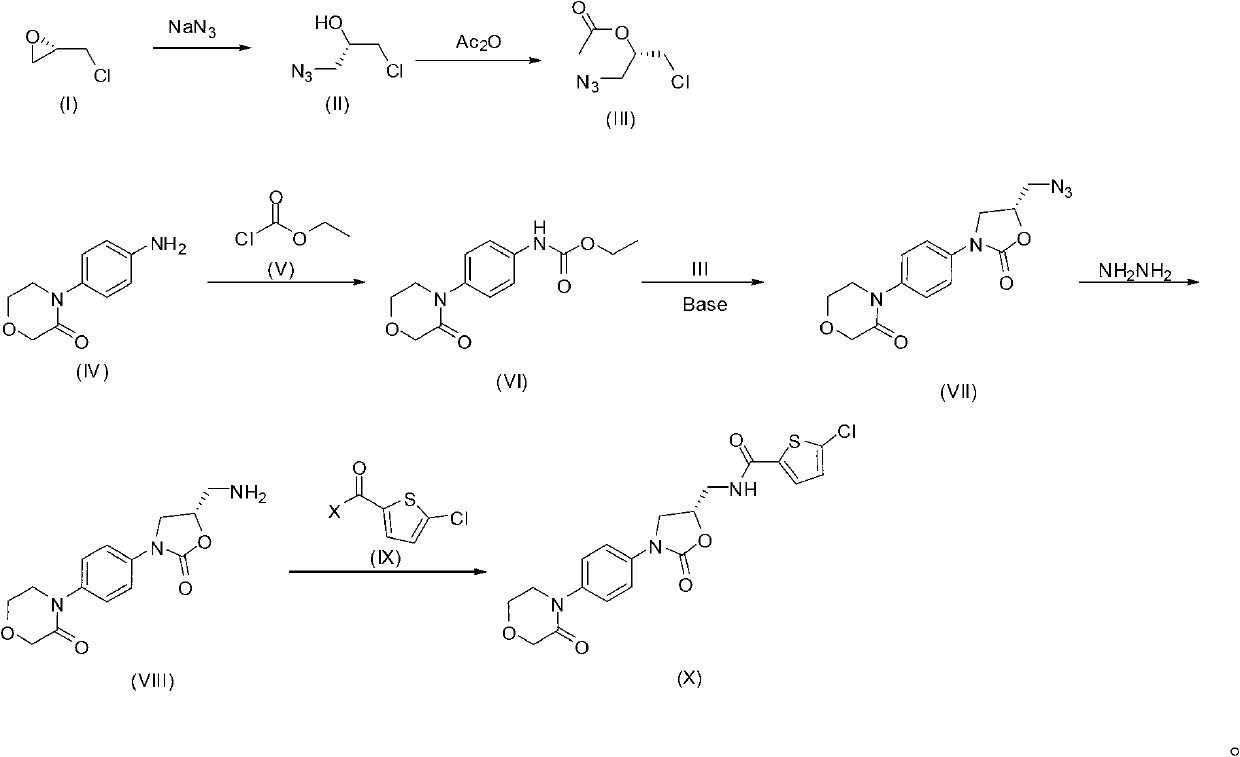
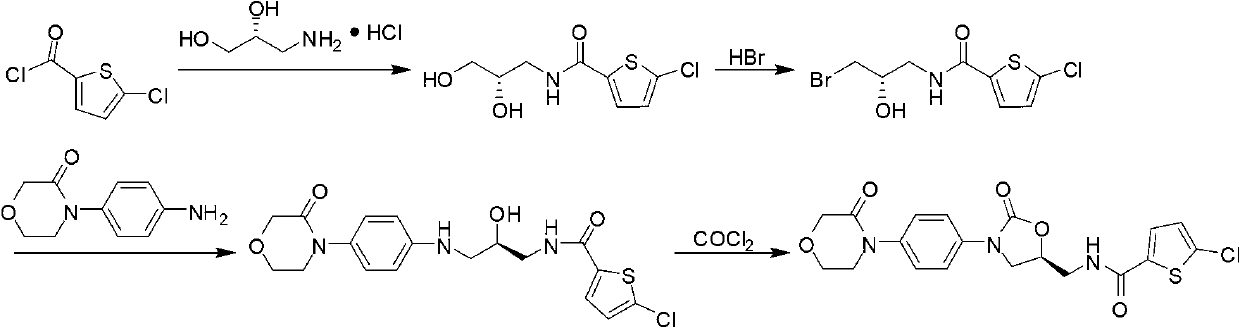
The invention provides a synthesis method of rivaroxaban. The synthesis method comprises the following steps of: (a) reacting a compound shown as a formula (I) with sodium azide to obtain a compound shown as a formula (II), and reacting the compound shown as the formula (II) with acetic anhydride to produce a compound shown as a formula (III); (b) reacting a compound shown as a formula (IV) and a compound shown as a formula (V) to obtain a compound shown as a formula (VI); (c) reacting the compound shown as the formula (VI) with the compound shown as the formula (II) or the compound shown as the formula (III) under the action of an alkali to obtain a compound shown as a formula (VII); (d) reacting the compound shown as the formula (VII) with hydrazine hydrate to obtain a compound shown as a formula (VIII); and (e) reacting the compound shown as the formula (VIII) with a compound shown as a formula (IX) to obtain the compound rivaroxaban shown as a formula (X). The synthesis method has the advantages of fewer reaction steps, mild reaction conditions, simpleness for post-treatment, and suitability for industrial mass production.
Owner:ARROMAX PHARMATECH
Rivaroxaban oral microsphere preparation
InactiveCN103550166AOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMicrosphere
The invention provides a rivaroxaban oral microsphere preparation. The rivaroxaban oral microsphere preparation comprises rivaroxaban and a water-soluble polymer carrier, wherein the water-soluble polymer carrier comprises one or a few of hydroxypropyl methyl cellulose, polyethylene glycol, polyvinylpyrrolidone and cellulose polymer, the weight ratio of the rivaroxaban and water-soluble polymer carrier is 1:(1-10), and the average grain diameter of the microsphere is 38-79 m um. According to the microsphere preparation, the solubility and bioavailability of indissolvable medicines are improved, and the preparation of rivaroxaban medicinal preparation is no longer restricted by crystal form.
Owner:JIANGSU ALPHA PHARM CO LTD
Preparation method for rivaroxaban intermediate
The invention provides a preparation method for a rivaroxaban intermediate. The preparation method is characterized in that a compound represented by formula 1 and triphosgene are used as raw materials and undergo a reaction in an organic solvent at a temperature of 40 to 100 DEG C for 1 to 10 h under the action of an organic amine catalyst, then pressure reduction is carried out to remove the solvent, and an obtained crude product is subjected to refining in the organic solvent so as to prepare a compound represented by formula 2, wherein the formula 1 and the formula 2 are described in the specification. According to the invention, the starting raw materials are easily available on the market and are cheap; triphosgene is used to replace expensive carbonyldiimidazole, CO2 and HCL gas are generated after the reaction, a product is easy to purify, and reaction yield and purity are effectively improved; triphosgene is used to replace expensive carbonyldiimidazole, HCL gas generated after the reaction can be utilized after recovery, so the purpose of green production is achieved; operation process is simple, and rigor conditions like low temperature, no water and no oxygen are not needed.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
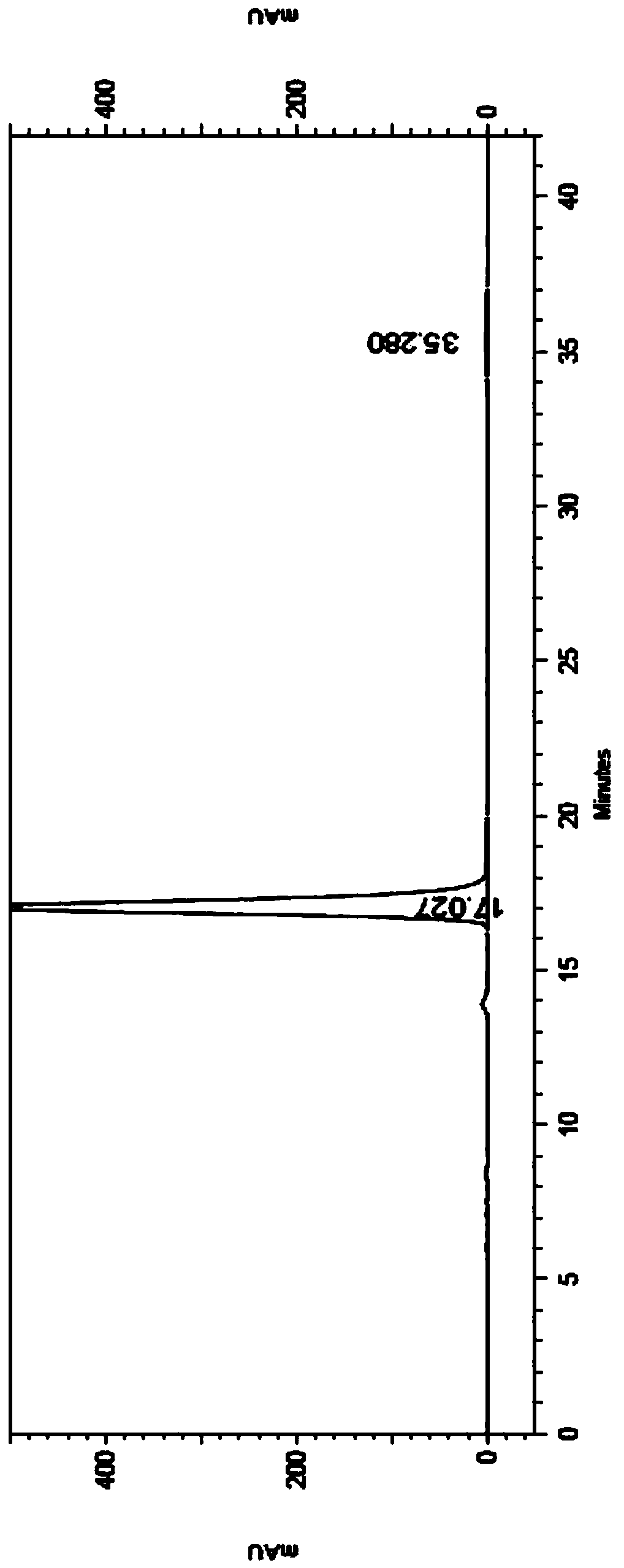
Method for separating and determining rivaroxaban related substances through liquid chromatography
The invention belongs to the field of analytical chemistry, and discloses a method for separating and determining rivaroxaban related substances and the content thereof through liquid chromatography. The method is characterized in that the content of rivaroxaban and related substances thereof can be quantitatively determined through using a chromatographic column with octadecylsilane chemically bonded silica as a packing material and using a certain ratio of buffer salt solution-organic phase as a mobile phase, so the quality of rivaroxaban can be effectively controlled. The method has the advantages of strong specificity, high accuracy and simple operation.
Owner:AVENTIS PHARMA HAINAN
Preparation method of Rivaroxaban midbody and novel synthetic method of Rivaroxaban
The invention discloses a preparation method of (S)-N-glycidol phthalimide in a structural formula 1. The method comprises the following steps of: I, phthalimide salt of a structural formula 9 and a compound of a structural formula 10 are subjected to heating reflux for reaction in an alcohols solvent to obtain the compound of a structural formula 11; II, the compound of the structural formula 11 obtained form the step I is reacted with the compound of a structural formula 12 in an aprotic solvent under the action of alkaline to obtain a (S)-N-glycidol phthalimide crude product of the structural formula 1; and III, the (S)-N-glycidol phthalimide crude product of the structural formula 1 obtained from the step II is refined by ethanol to obtain the (S)-N-glycidol phthalimide with the optical purity larger than or equal to 99.0%. The preparation method is simple to operate and good in safety, the optical purity of the obtained product is high (larger than or equal to 99.0%), and the preparation method is suitable for industrial production. The invention also discloses a novel synthetic method of Rivaroxaban.
Owner:JIANGXI SYNERGY PHARMA
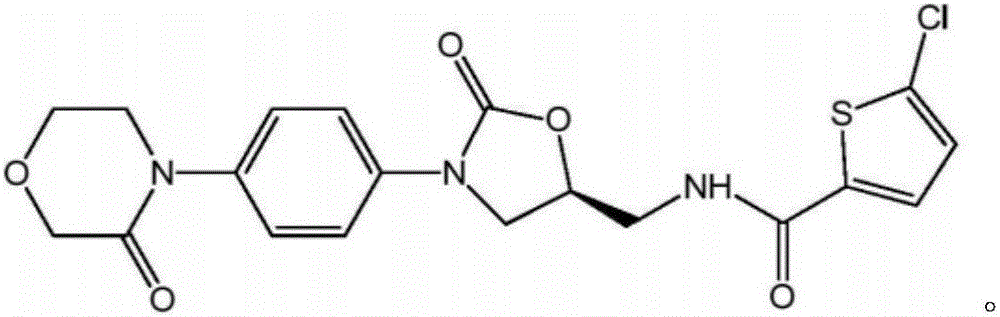
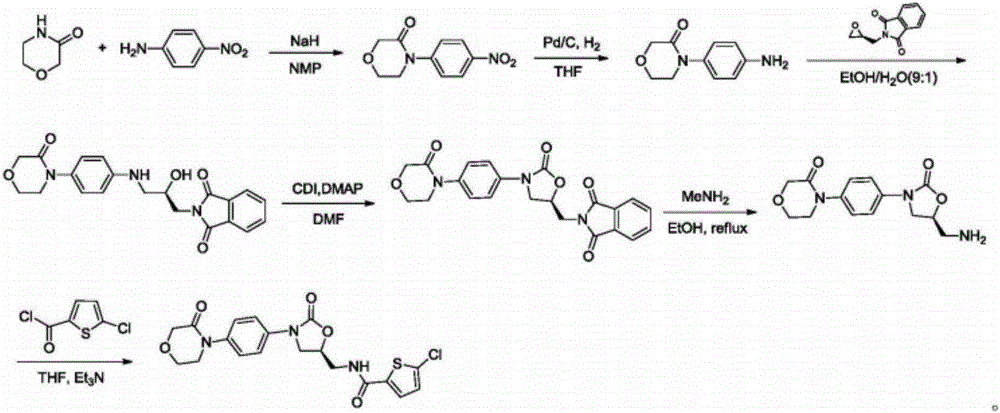
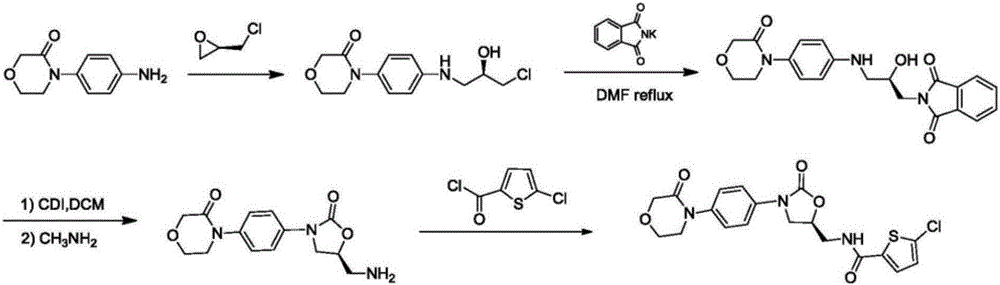
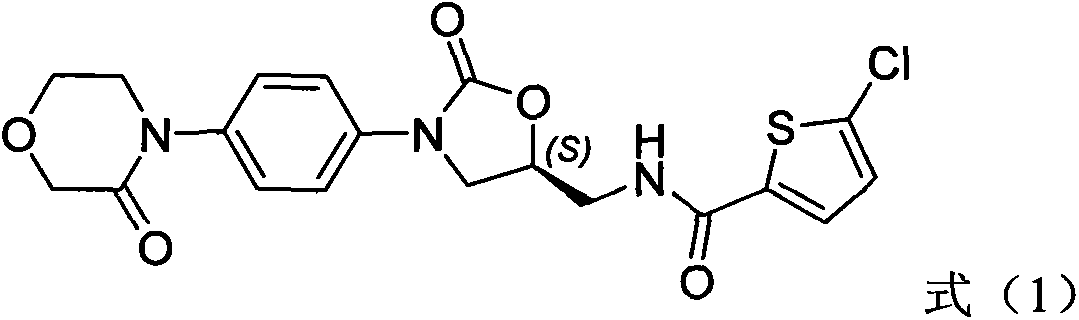
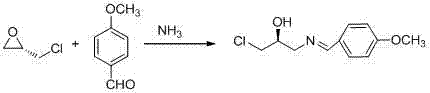
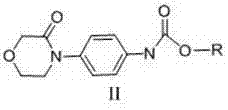
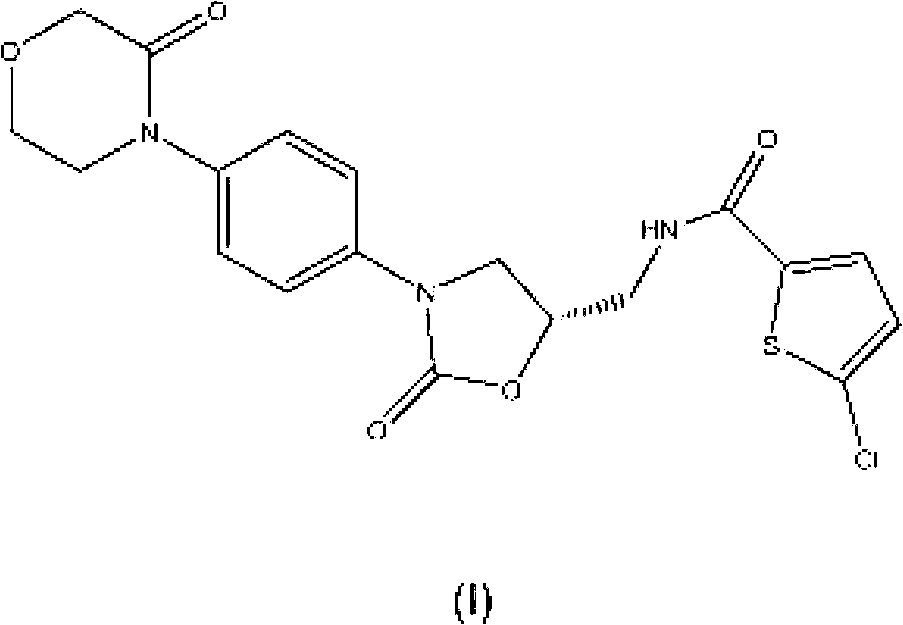
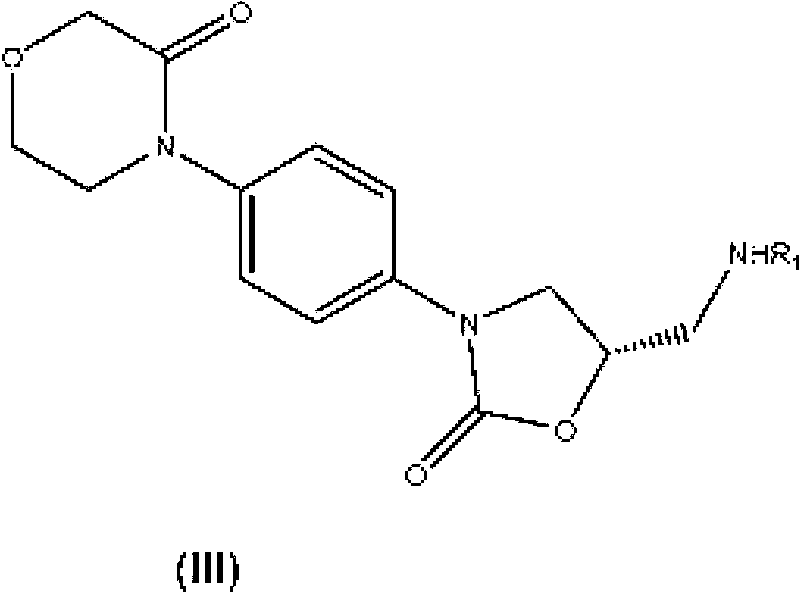
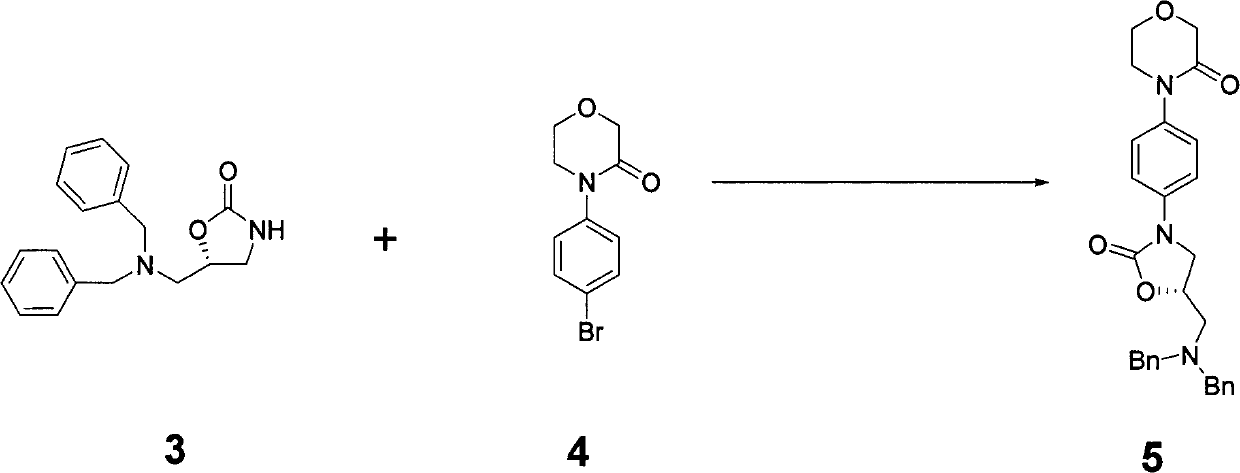
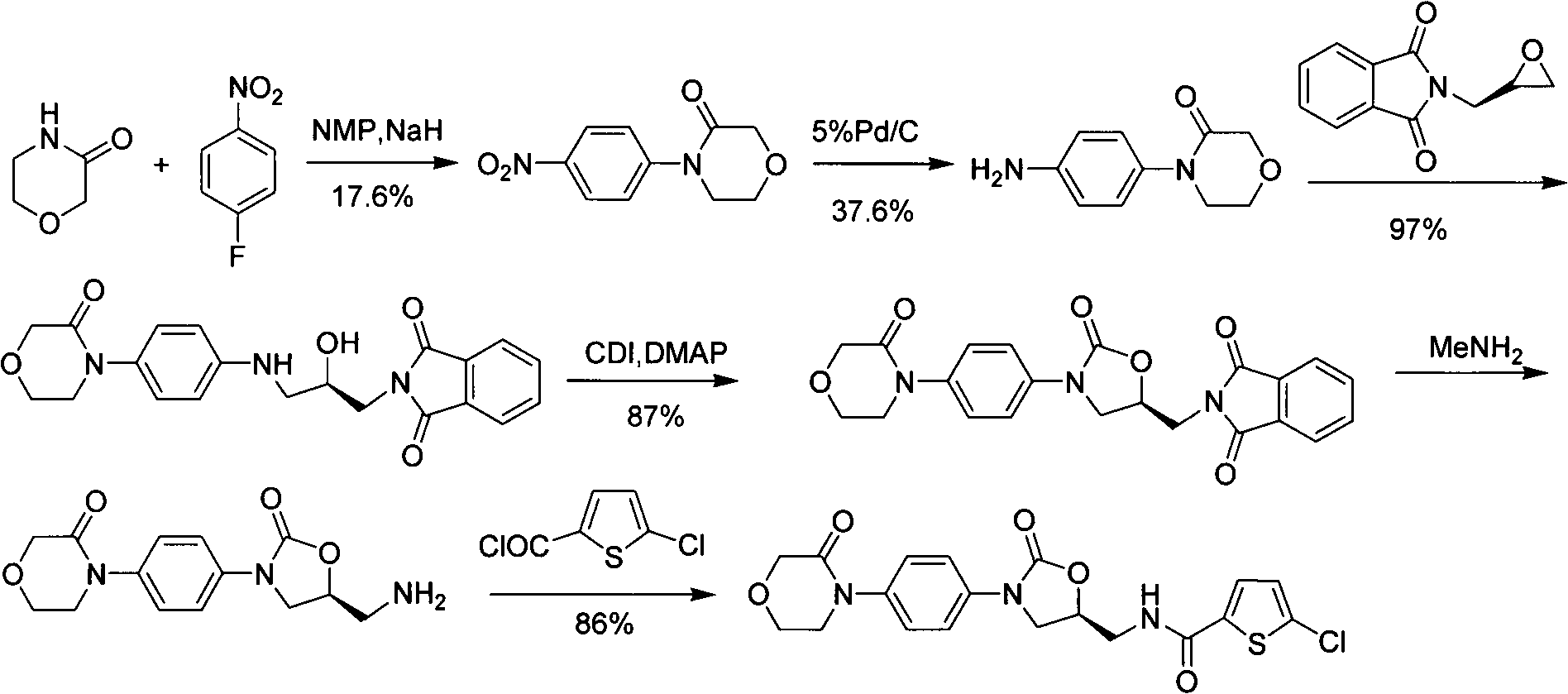
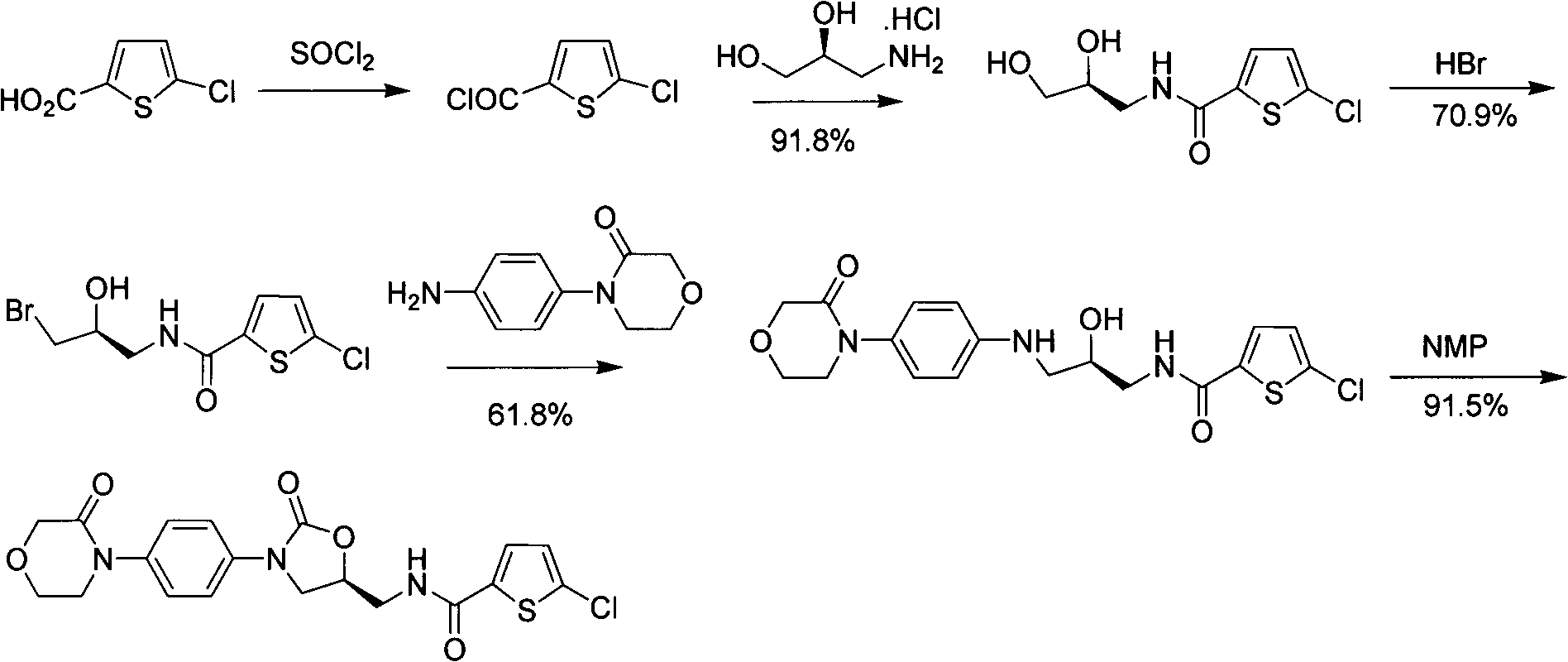
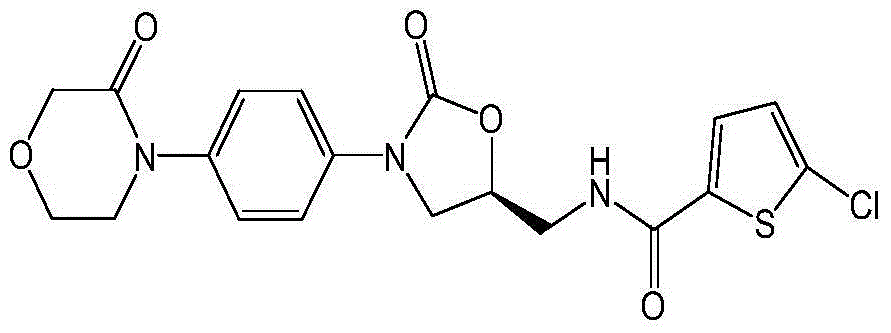
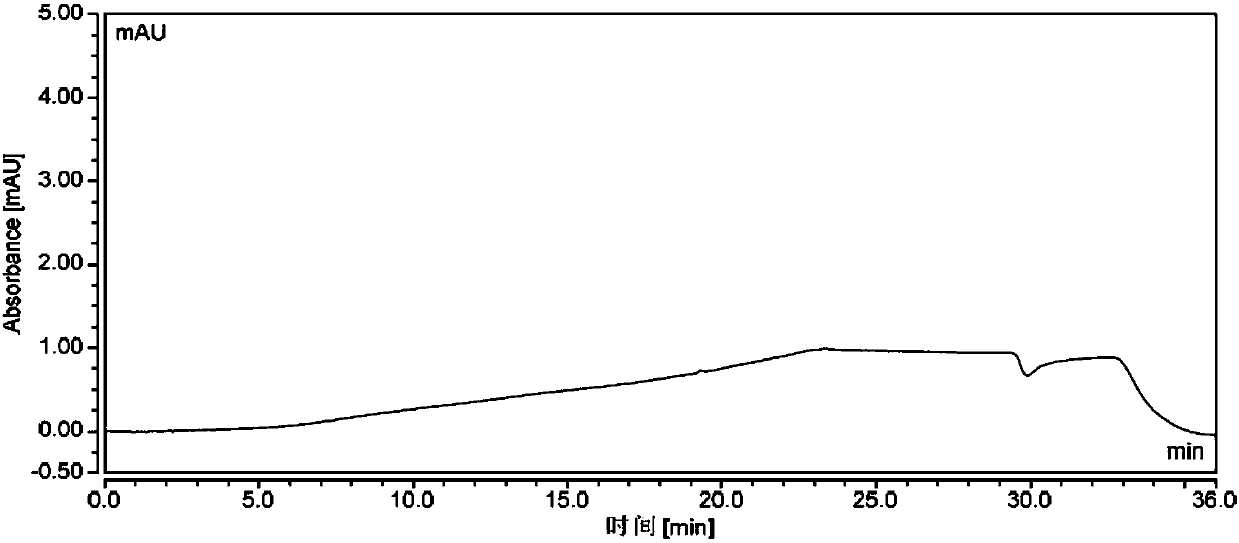
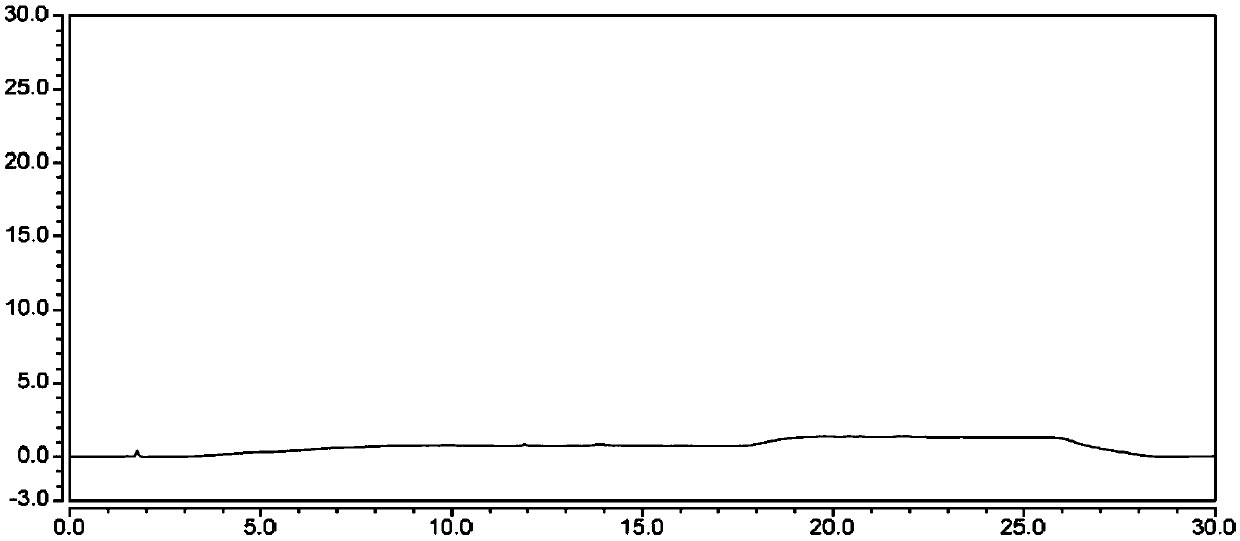
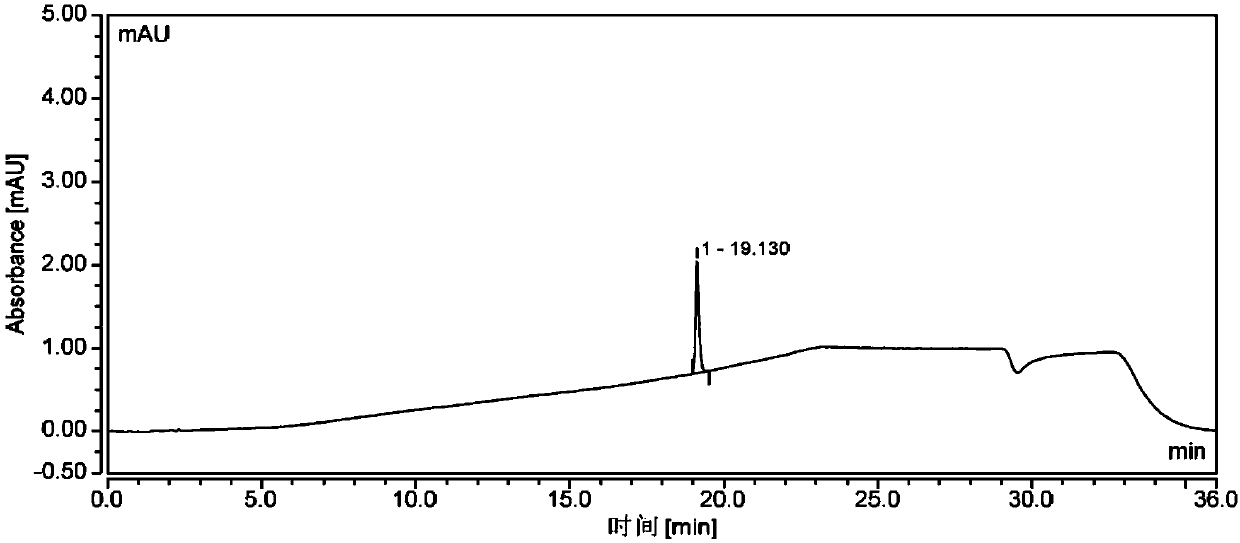
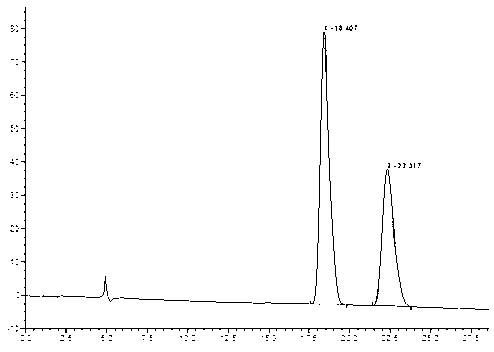
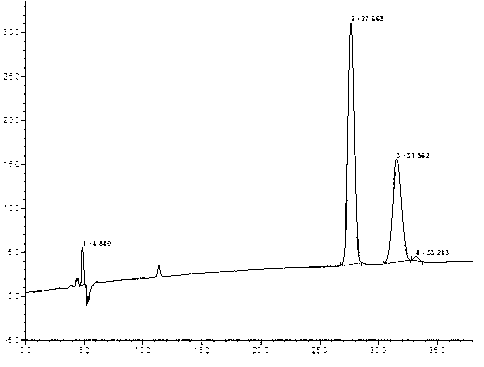
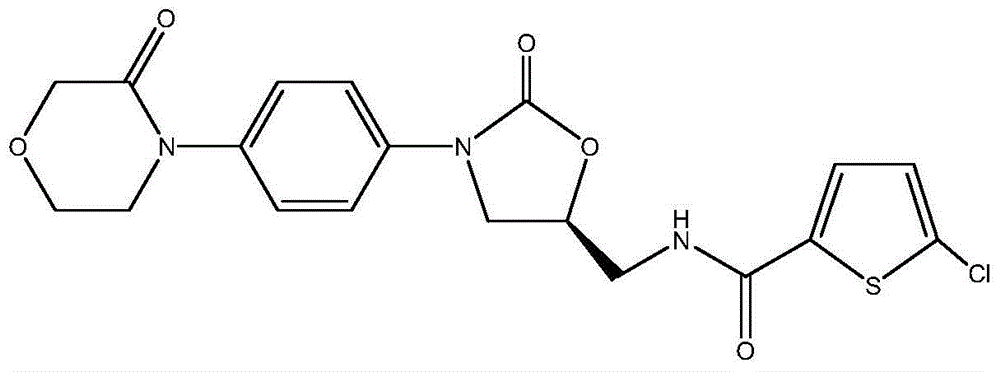
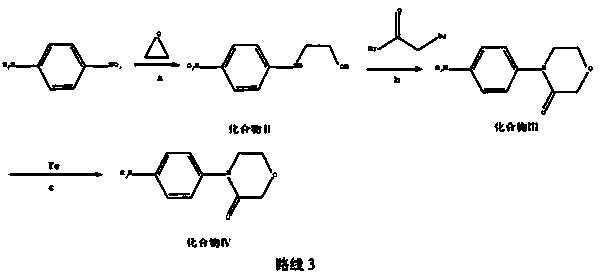
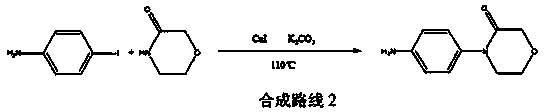
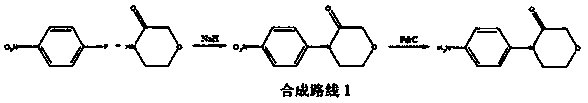
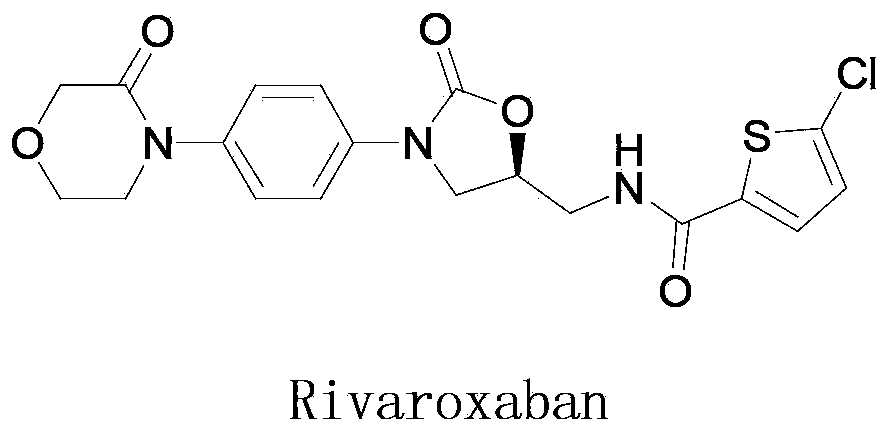
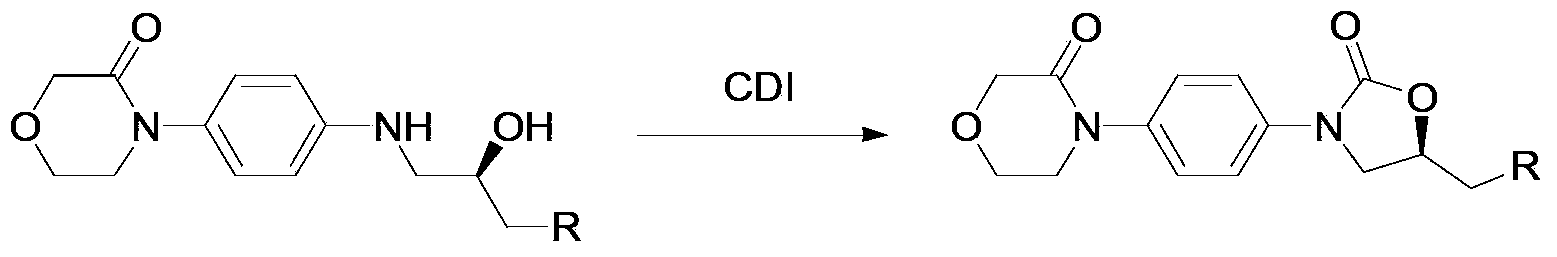
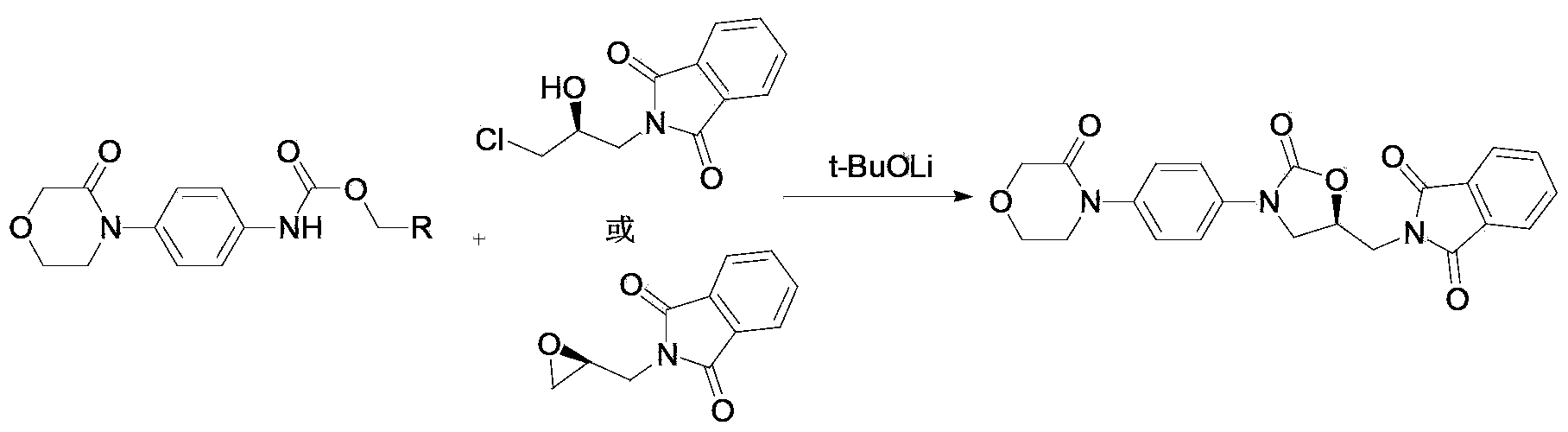
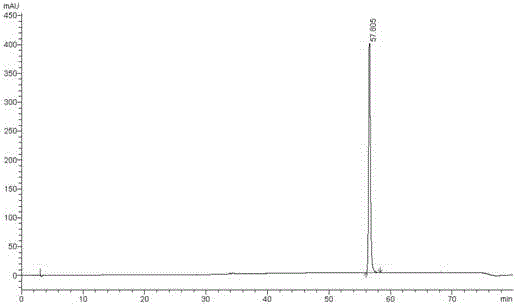
New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone
The invention discloses a new method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone. The method comprises the following steps: reacting a compound 1 and a compound 2 in an inert solvent in the presence of lithium to get a compound 3; and carrying out an ester group removing reaction under the effect of hydrochloric acid or hydrogen chloride to get a compound 4, that is, 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone. The new method provided by the invention has advantages of mild reaction condition, simple operation, easy purification and low production cost, and is environmentally friendly and suitable for industrial production.
Owner:SHANGHAI SYNCORES TECH INC
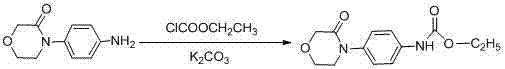
4-(4-amion phenyl)-3-morpholone intermediate amide and synthesis method and application thereof
InactiveCN102320988ARaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
The invention relates to a 4-(4-amion phenyl)-3-morpholone intermediate amide and a synthesis method and application. The method has the advantages of cheap and readily available raw materials, mild reaction condition and high yield; and intermediate amide can be used for synthesizing 4-(4-amion phenyl)-3-morpholone and further synthesizing a rivaroxaban medicament.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Rivaroxaban pharmaceutical composition and preparation method thereof
InactiveCN105078997AHigh dissolution rateImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsRivaroxabanDissolution
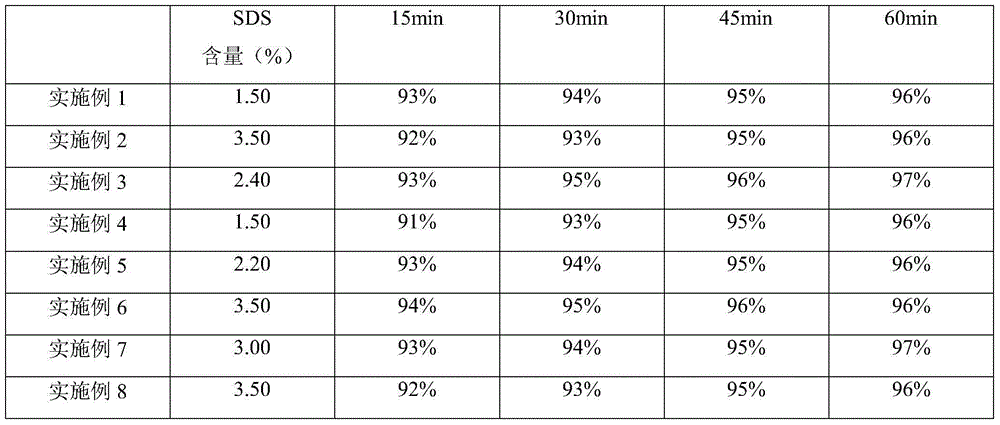
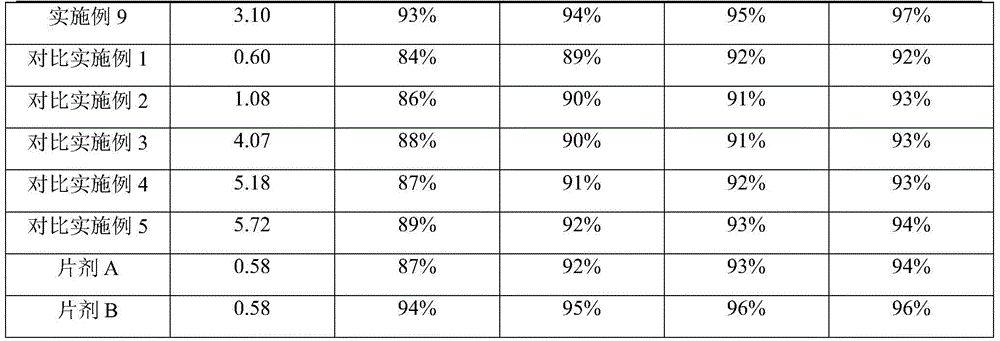
The invention relates to the field of pharmaceutical chemicals, and in particular relates to a rivaroxaban pharmaceutical composition and a preparation method thereof; the pharmaceutical composition consists of a rivaroxaban active substance, a solubilizer, a binding agent, a lubricant, a filling agent and a disintegrating agent, wherein the rivaroxaban active substance is not subject to hydrophilic treatment; and the solubilizer accounts for about 1.5%-3.5% in the entire composition in percentage by mass. The rivaroxaban pharmaceutical composition is stable and good in dissolution; and the preparation method is simple and convenient and is energy-saving, and the preparation method is applicable to industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of rivaroxaban
The invention discloses a preparation method of rivaroxaban. With (S)-4-chloro-3-hydroxybutyronitrile (compound 1) as a raw material, the rivaroxaban is obtained through phthalimide potassium salt substitution, cyano hydrolysis, Hofmann rearranging cyclization, Ullmann coupling, hydrazinolysis and amidation. The rivaroxaban is introduced into a chiral center instead of (S)-epoxy chloropropane which is volatile, high in toxicity and unstable; the safety is relatively high; precious catalyst, raw materials and reagent with large environmental pollution are avoided in the total process in the total synthetic route; the overall synthesis process is small in pollution and easy to treat; the yield and the purity of various steps are high; the preparation method is environmentally friendly, low in production cost and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Preparation method of 4-(nitrobenzophenone)-3-morpholone and method for preparing rivaroxaban by using 4-(nitrobenzophenone)-3-morpholone
ActiveCN103980221ALow priceSimple operation processOrganic chemistryMorpholineTert-Butyloxycarbonyl protecting group
The invention relates to the technical field of preparation of rivaroxaban and an intermediate thereof and particularly relates to a preparation method of 4-(nitrobenzophenone)-3-morpholone which is prepared from halogenated nitrobenzene, ethanolamine and chloroacetyl chloride through a one-pot method. The method for preparing rivaroxaban comprises the steps of reducing 4-(nitrobenzophenone)-3-morpholone into 4-(aminophenyl)-3-morpholone; enabling 4-(aminophenyl)-3-morpholone to react with R-epichlorohydrin to obtain a product; enabling the product to react with N, N-carbonyldiimidazole to obtain a product; enabling the product to react with tert-butyl iminodicarboxylate; preparing hydrochloride; enabling hydrochloride to react with 5-penphene-2-carbonyl chloride. The preparation method of 4-(nitrobenzophenone)-3-morpholone is capable of realizing one-pot production and free of purifying intermediate products in the process, so that the operation process is simplified, the time is saved, and the labor cost is reduced; the preparation method of 4-(nitrobenzophenone)-3-morpholone is low in raw material price, high in obtained product yield and easy to realize large-scale industrial production; in addition, the method for preparing rivaroxaban is cheap, nontoxic and harmless in raw material, simple in process and high in product yield.
Owner:山东康美乐医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com









![New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone](https://images-eureka.patsnap.com/patent_img/f9293812-1233-416c-8f94-176d6c578af2/350714DEST_PATH_IMAGE004.PNG)
![New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone](https://images-eureka.patsnap.com/patent_img/f9293812-1233-416c-8f94-176d6c578af2/380987DEST_PATH_IMAGE001.PNG)
![New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone](https://images-eureka.patsnap.com/patent_img/f9293812-1233-416c-8f94-176d6c578af2/528251DEST_PATH_IMAGE003.PNG)