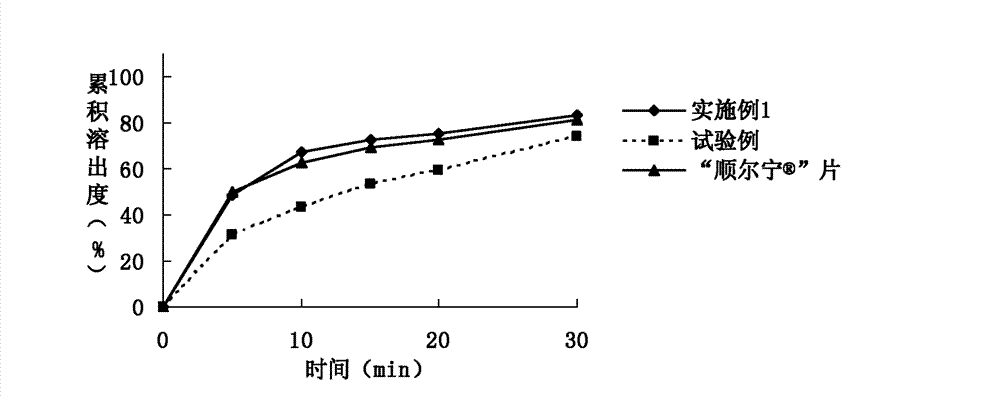
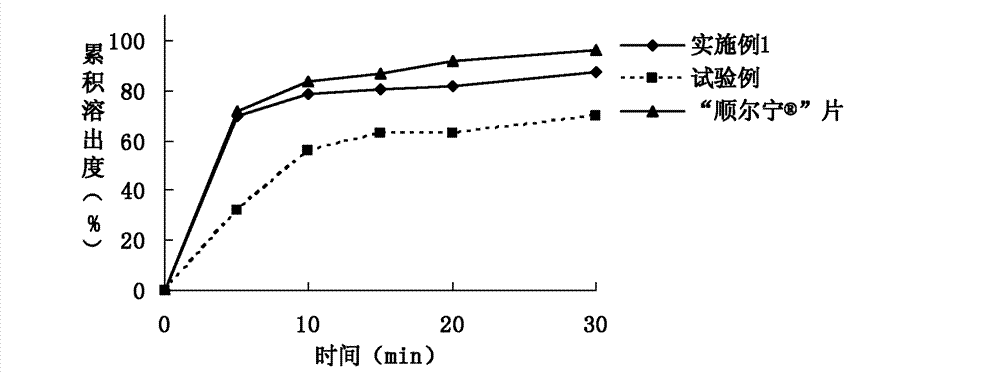
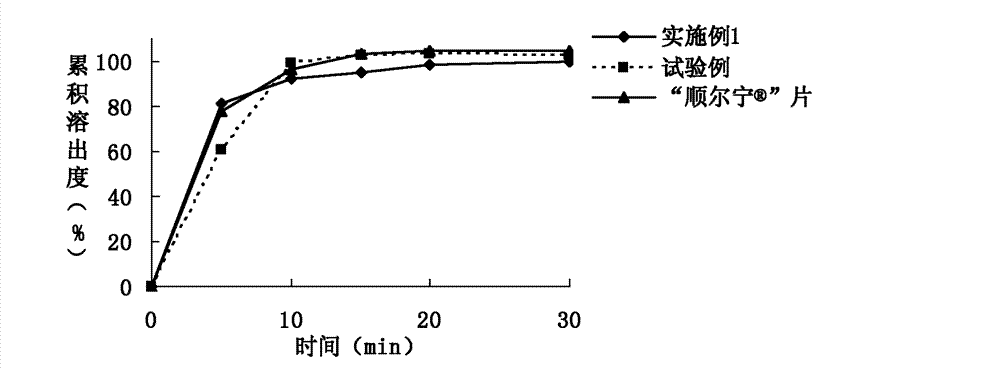
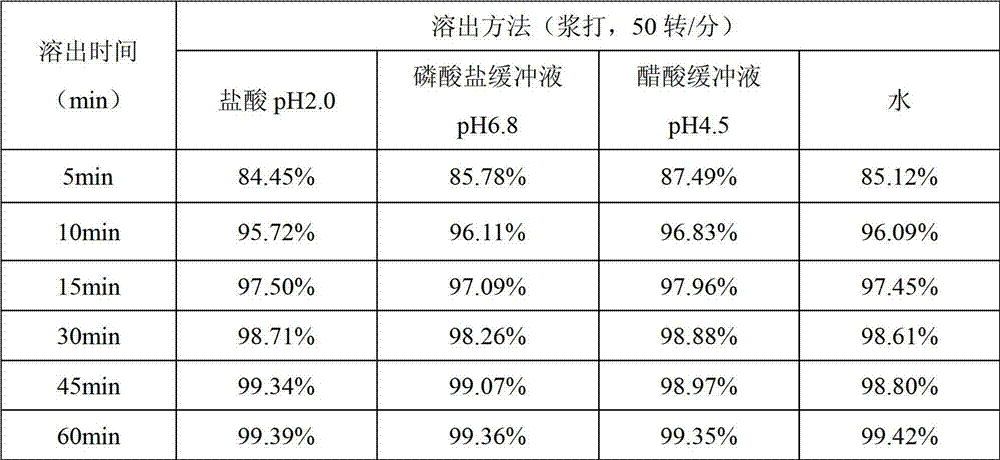
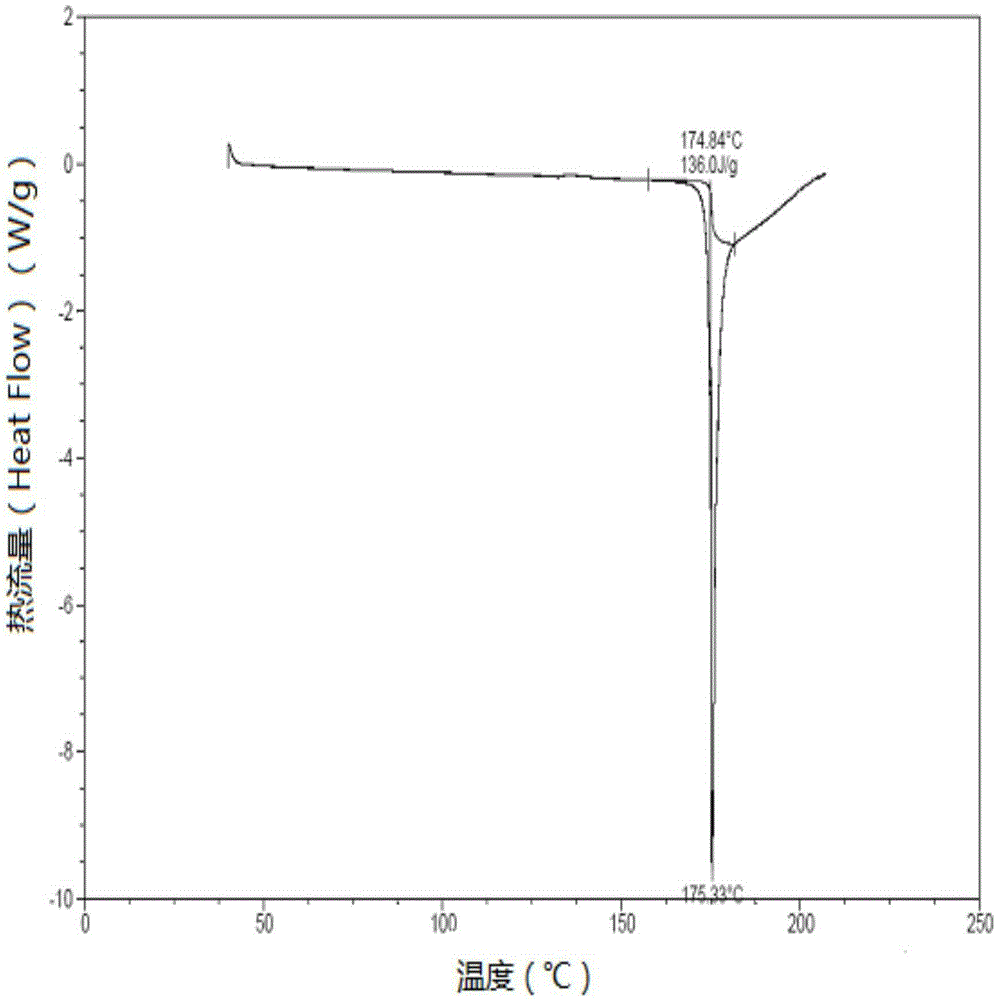
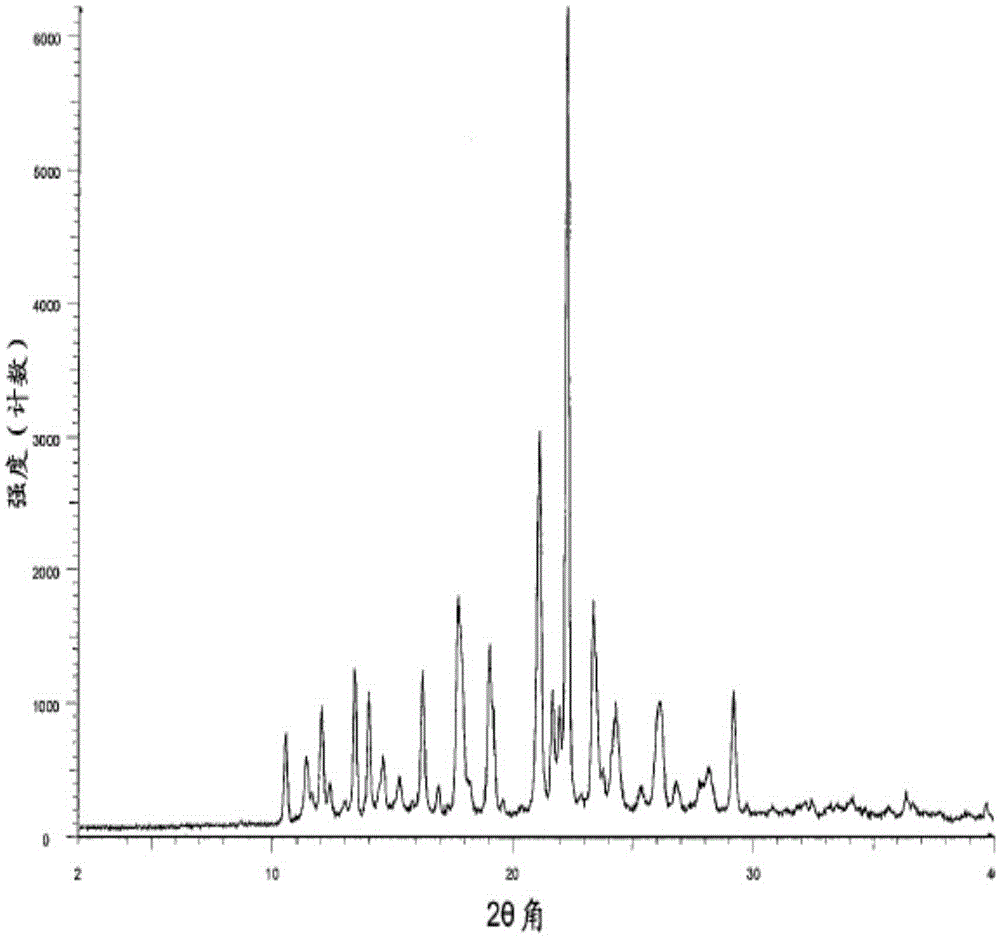
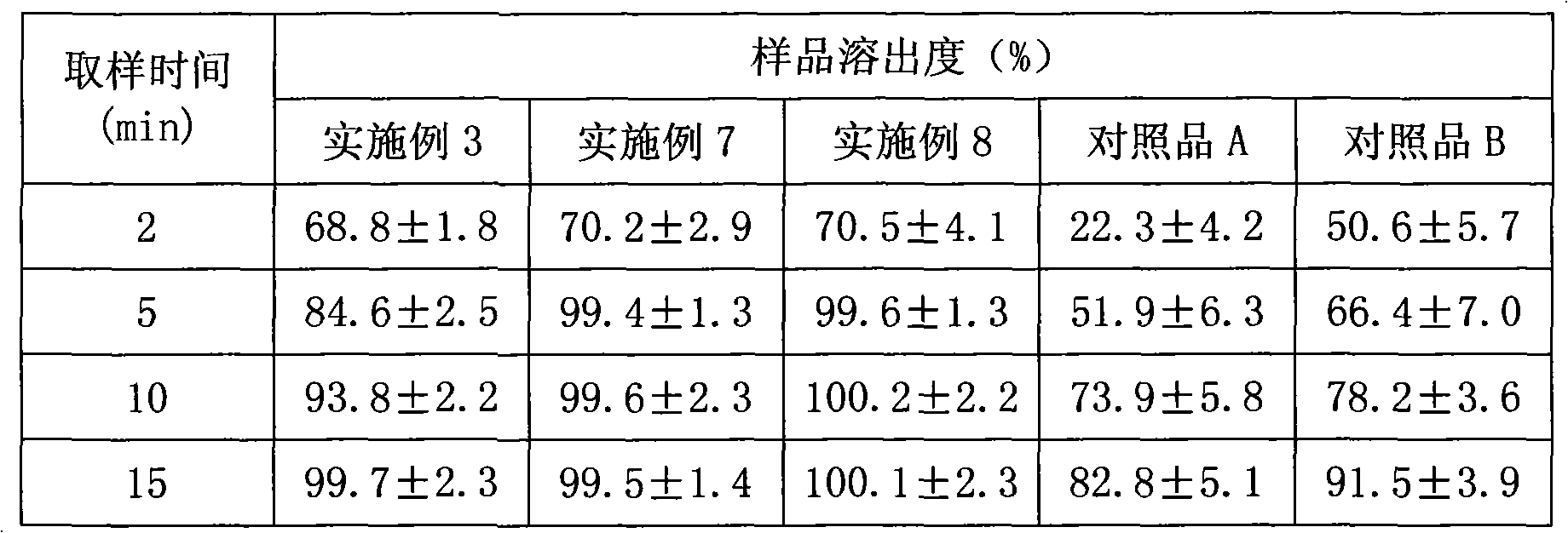
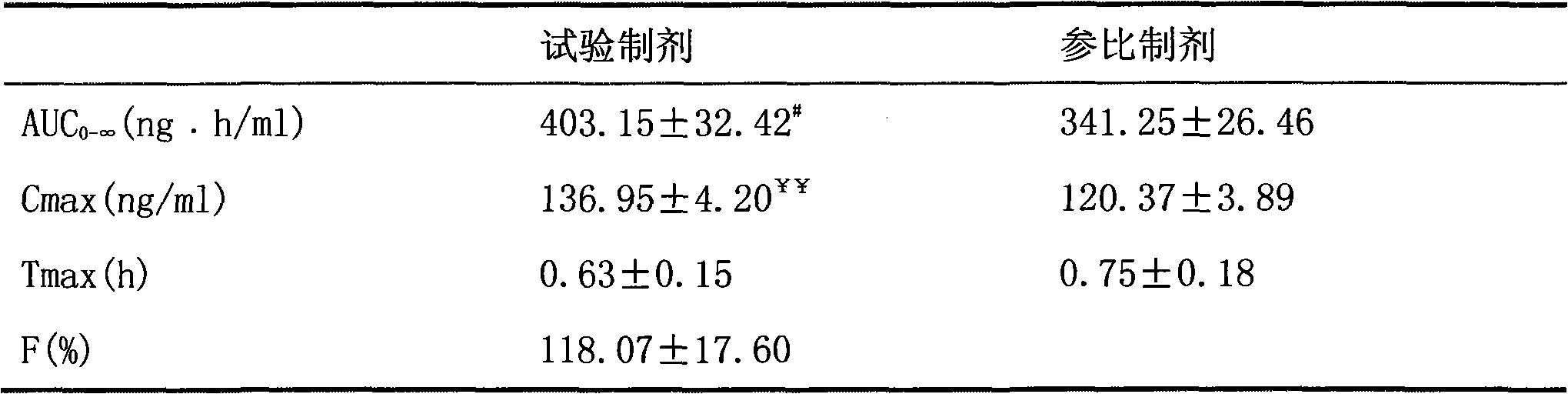
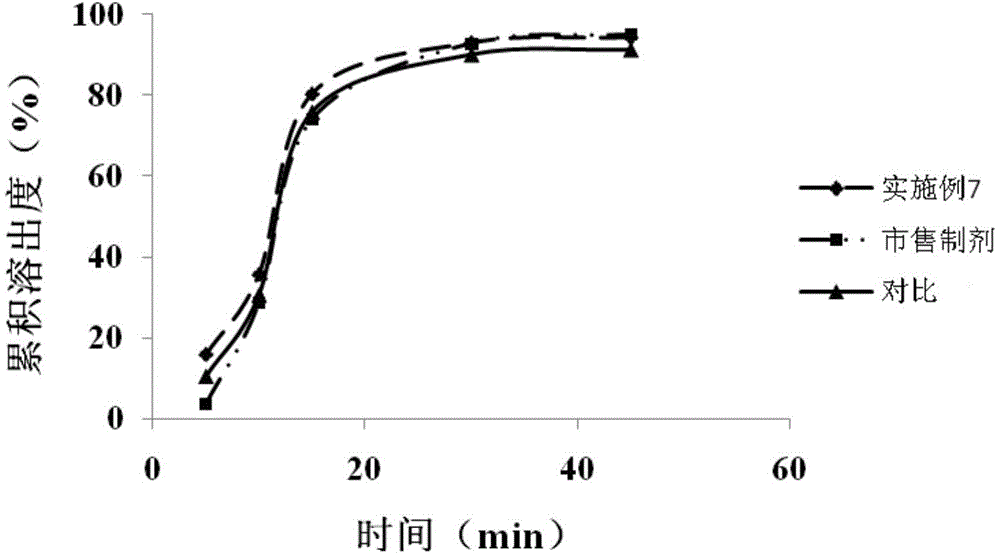
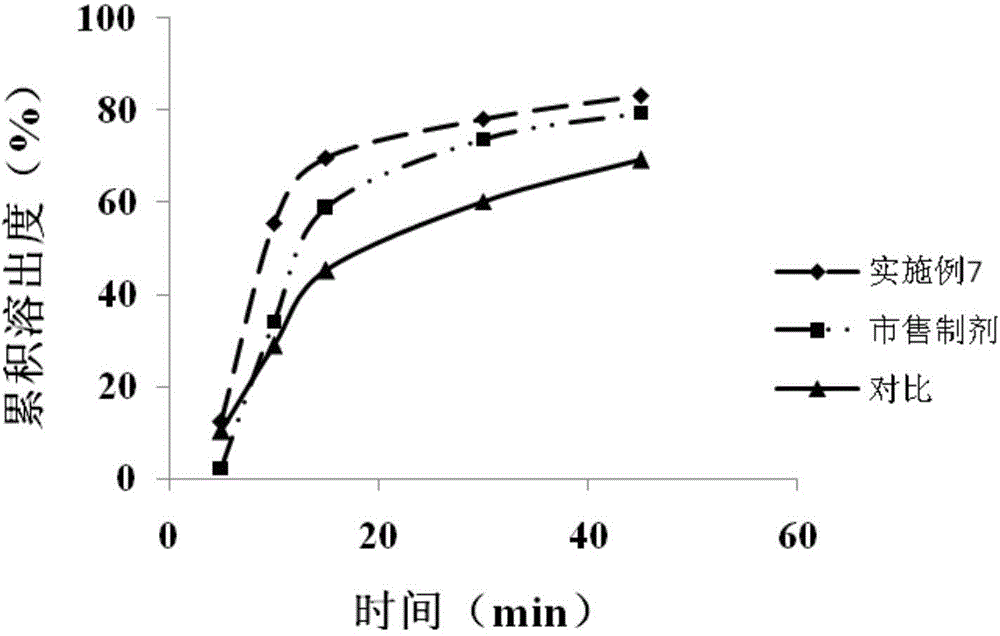
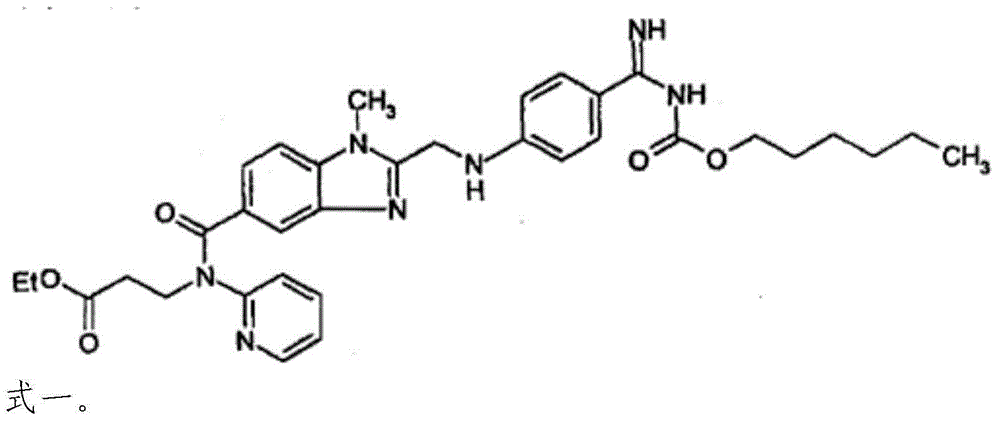
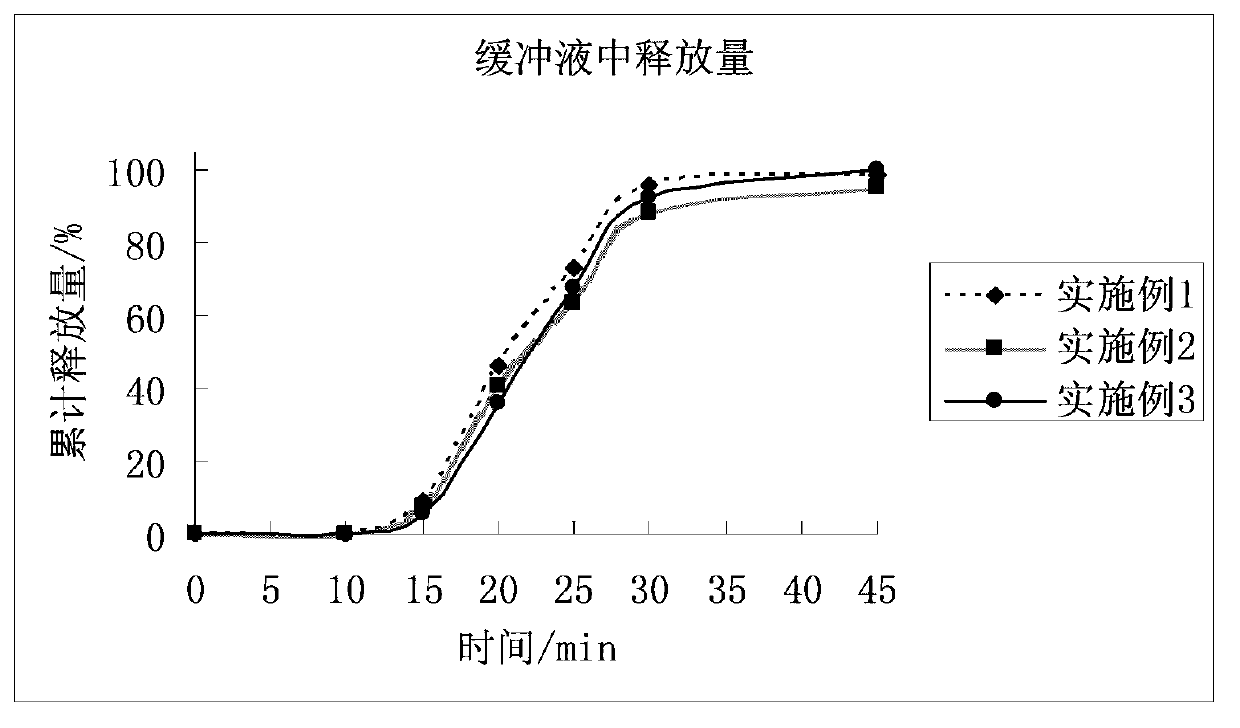
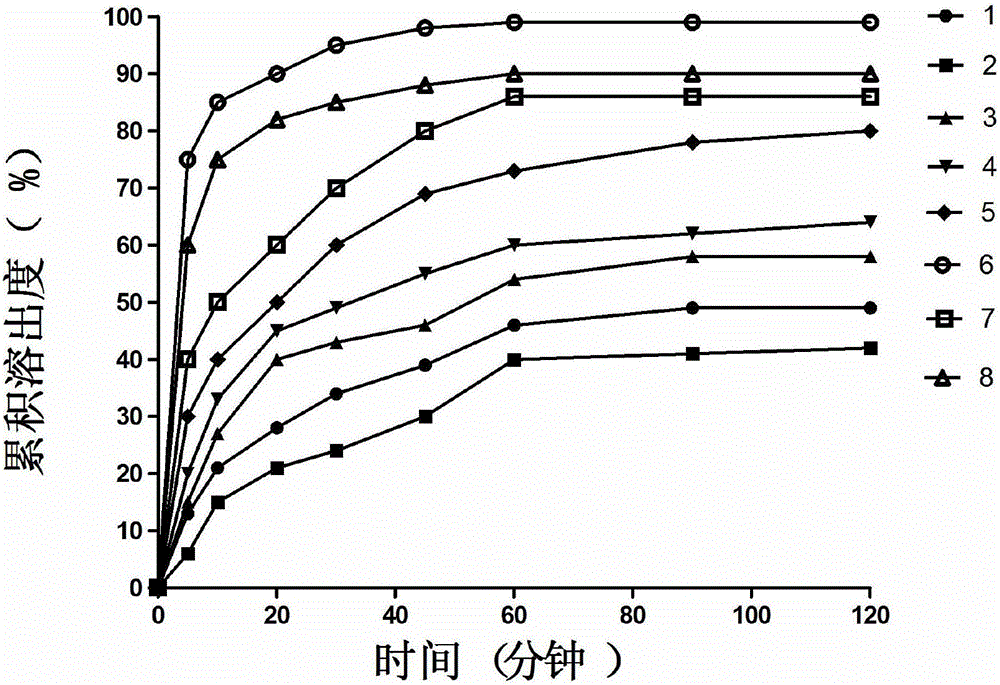
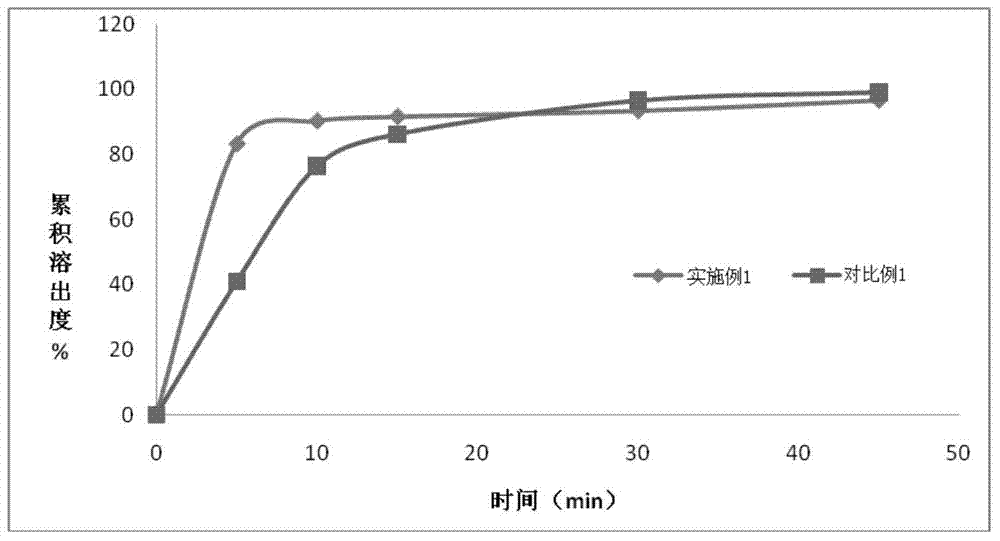
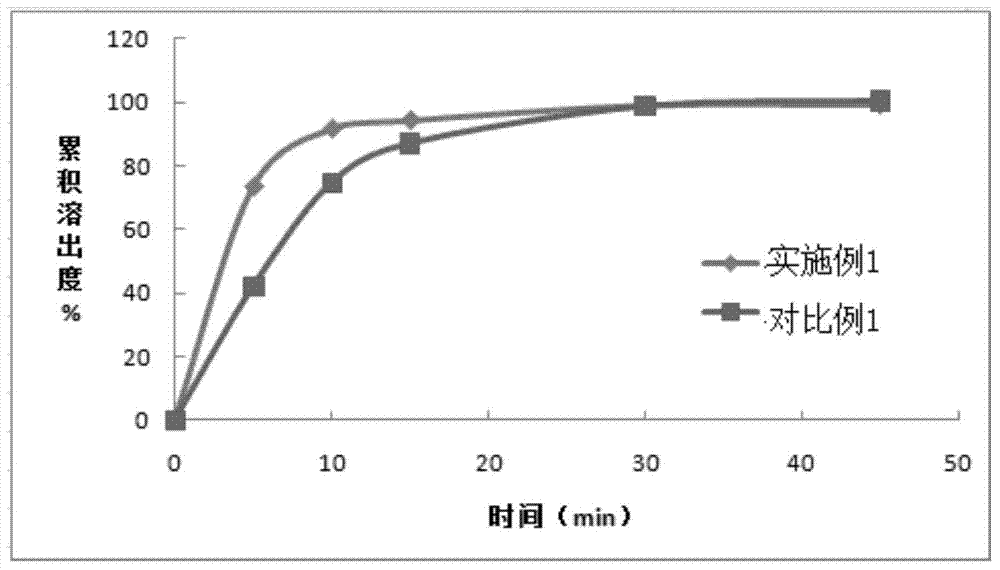
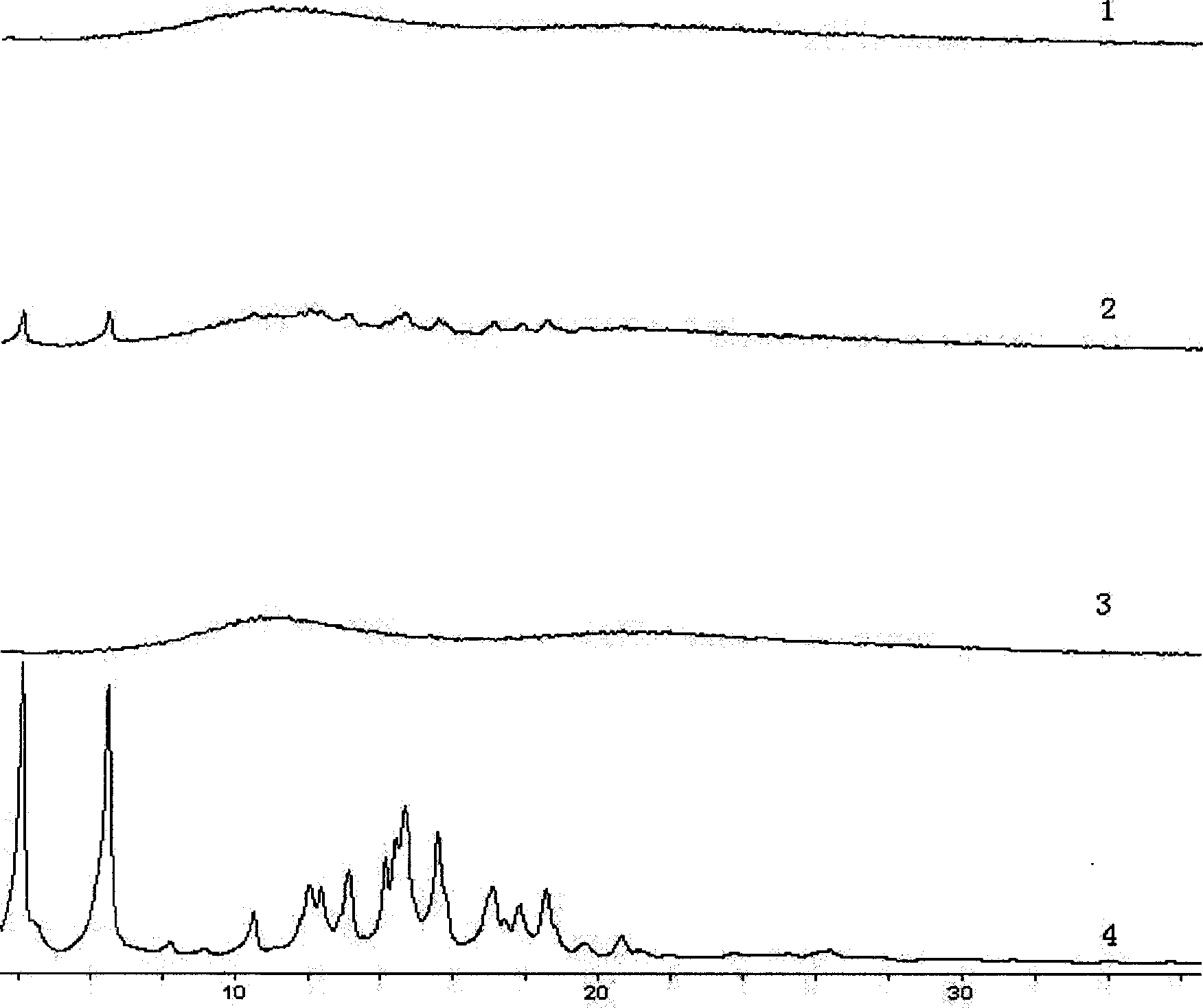
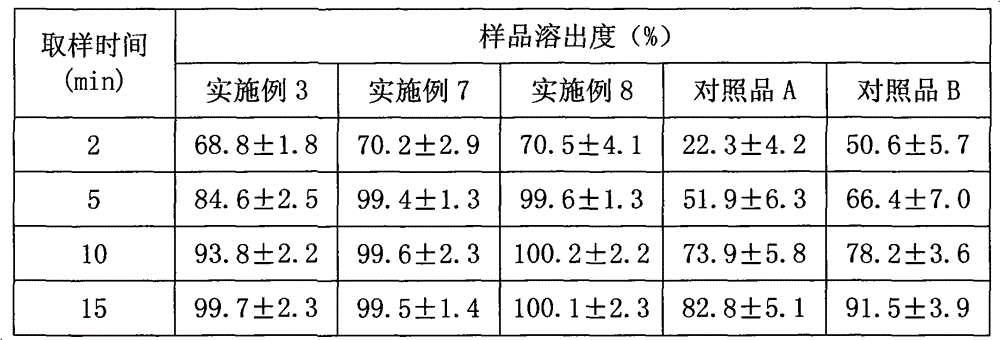
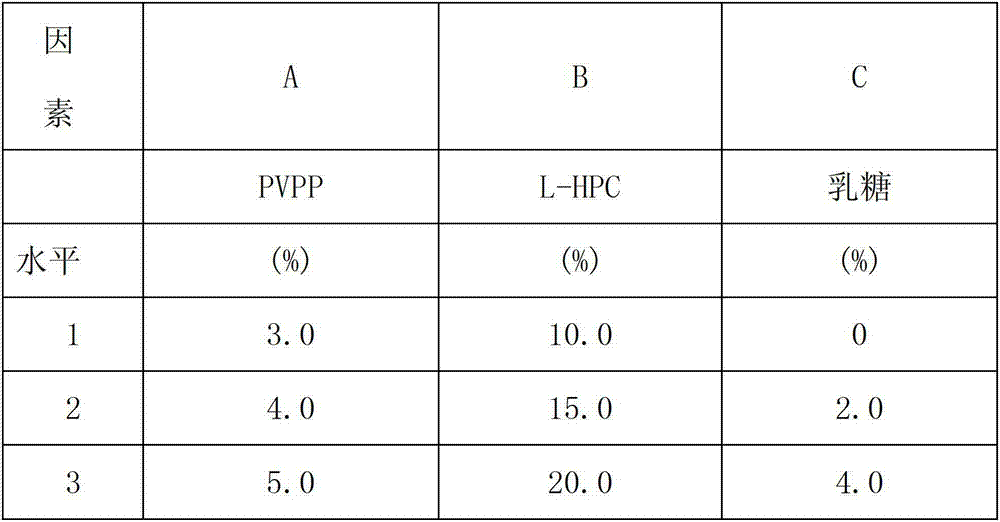
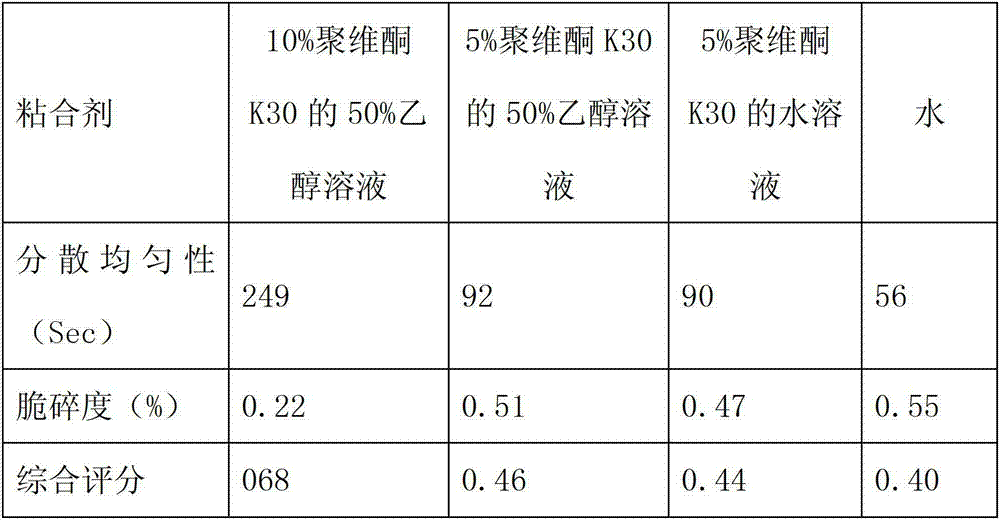
Patents
Literature
490results about How to "Dissolution rate is fast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rapidly-dissolving and stabile montelukast oral solid preparation and preparation method thereof
ActiveCN103239450ADissolution rate is fastImprove stabilityPharmaceutical non-active ingredientsPill deliveryAdhesiveMedicine
The invention relates to a rapidly-dissolving and stabile montelukast oral solid preparation and a preparation method thereof, wherein the preparation uses montelukast as a raw material drug, is added with a diluents, a disintegrating agent, an additive, an adhesive and a lubricant as auxiliary materials, and uses a pharmaceutically acceptable preparation technology. The preparation and the preparation technology can raises a dissolving speed of the oral preparation, enables the oral preparation to substantially raise the dissolving speed in a dissolving mediums of pH1.0 and pH 4.5, and can raises the medicament stability.
Owner:QILU PHARMA HAINAN
Atorvastatin calcium tablet and preparation method thereof
ActiveCN102920675AHigh dissolution rateImprove bioavailabilityMetabolism disorderPharmaceutical non-active ingredientsFiller ExcipientHardness
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet consists of the following components in parts by mass: 7.22 parts of main medicine atorvastatin calcium, 84.55 parts of filler, 6 parts of disintegrating agent croscarmellose sodium, 1.33 parts of adhesive hydroxy propyl cellulose, 800.4 parts of cosolvent polysorbate and 0.5 part of lubricating agent magnesium stearate, wherein the filler comprises the following raw materials in parts by mass: 22.01 parts of calcium carbonate, 21.87 parts of milk sugar and 40.67 parts of microcrystalline cellulose. The atorvastatin calcium tablet has the characteristics of short disintegrating time, fast dissolving-out speed, high bioavailability and small particle diameter, and is convenient to take. Furthermore, the hardness of the tablet can reach 60-70N, so that the tablet is hardly broken, and therefore, the packing and transporting costs are reduced, and the industrialized popularization of the tablet is easily realized.
Owner:HENAN RUNHONG PHARMA
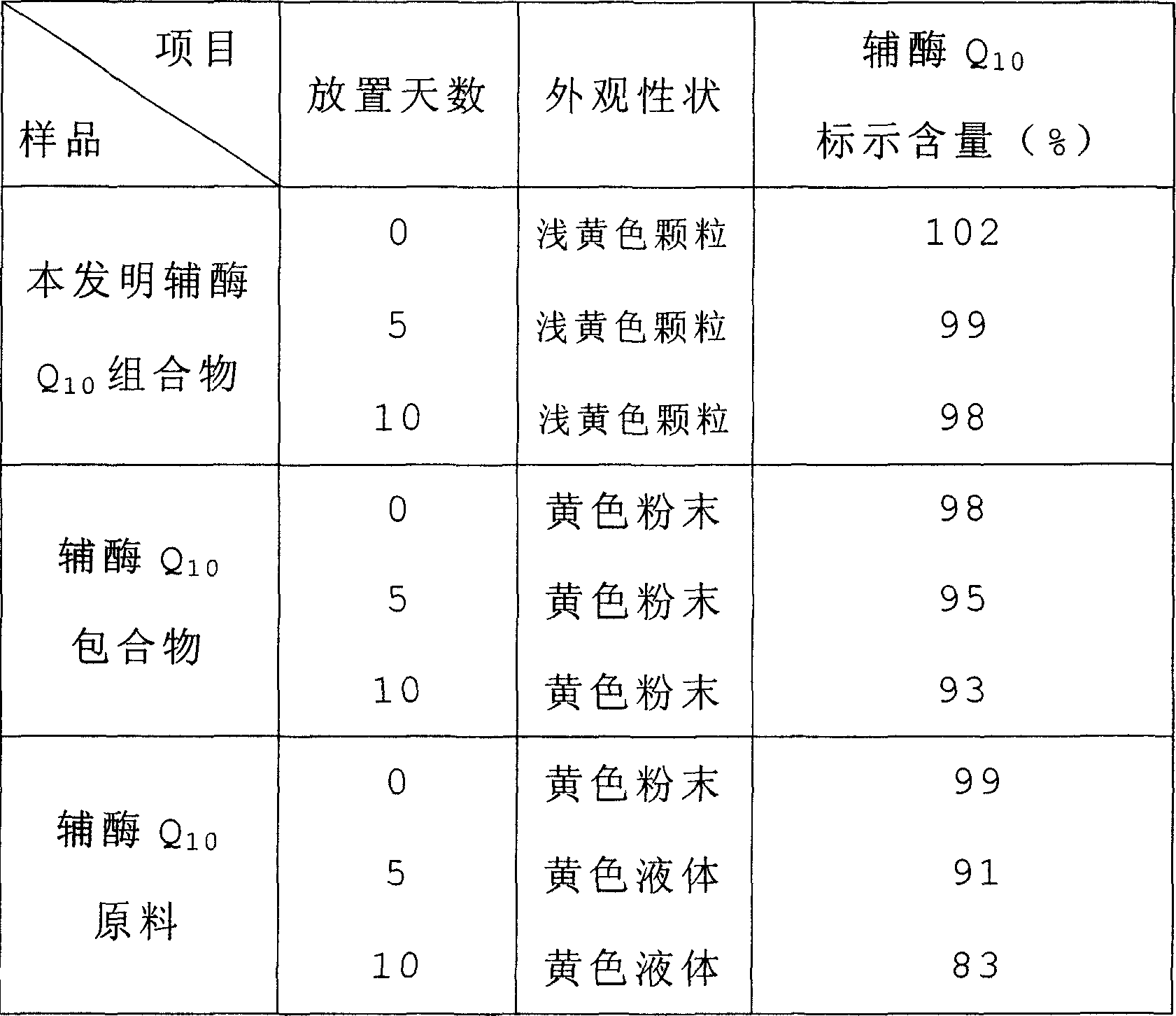
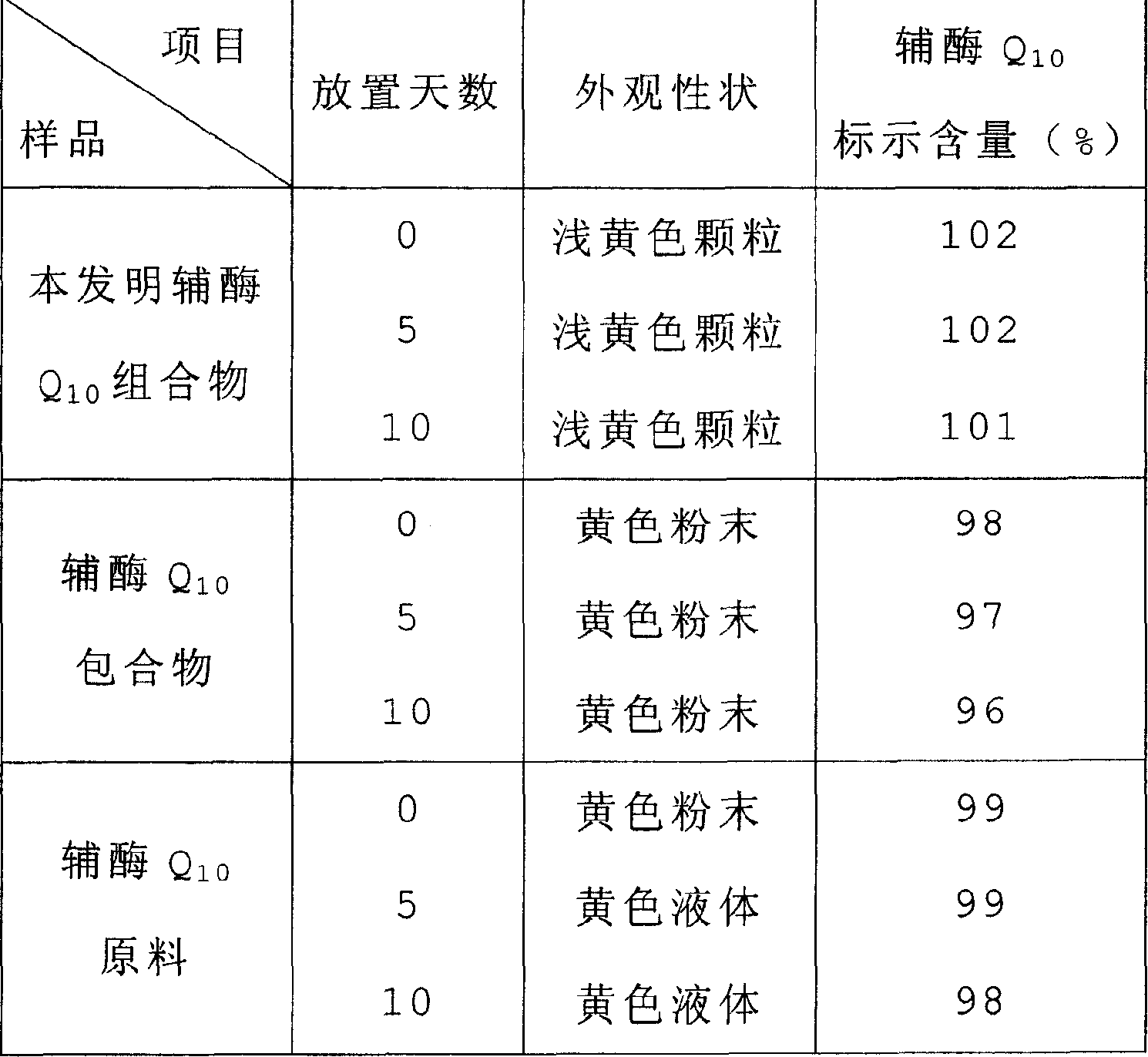
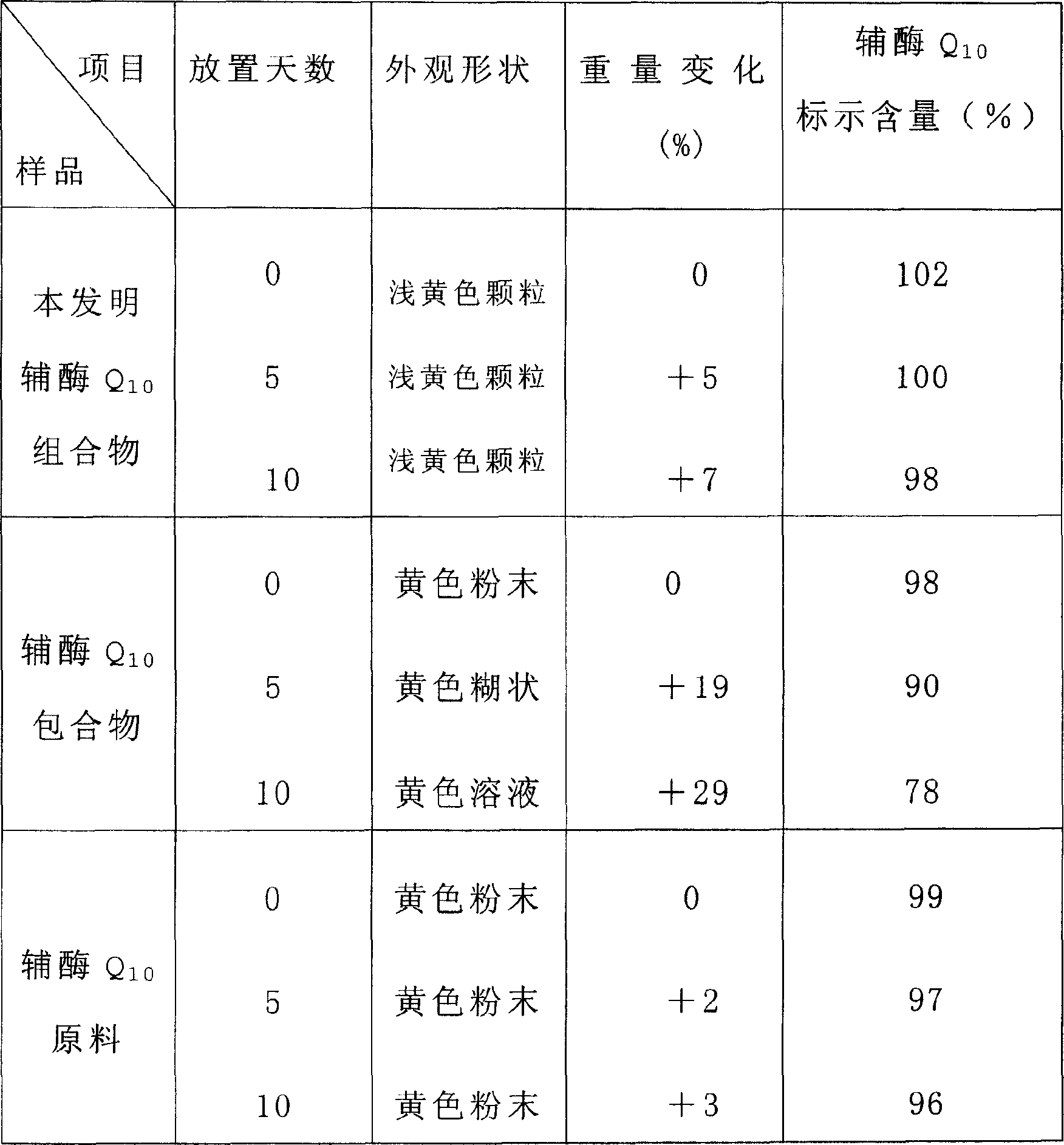
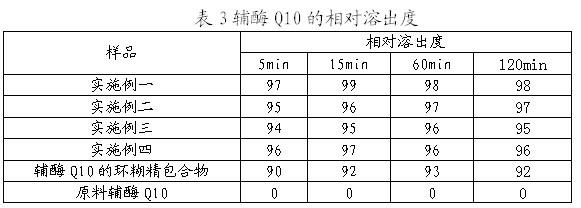
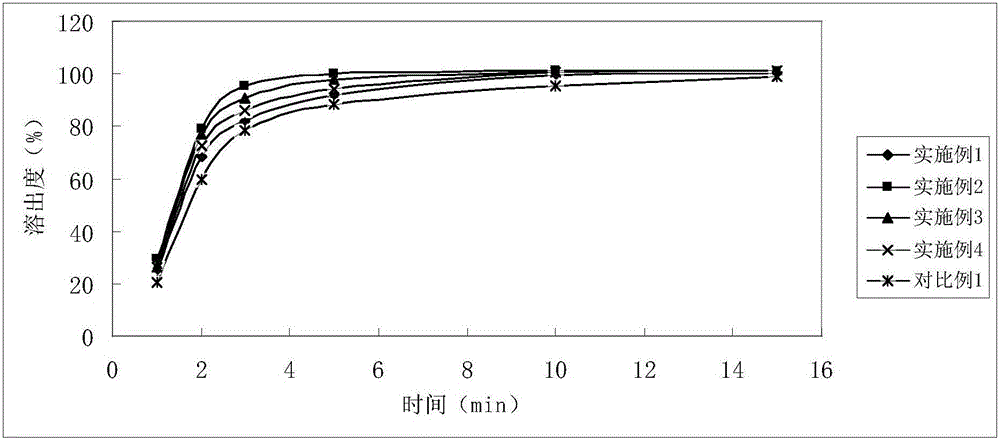
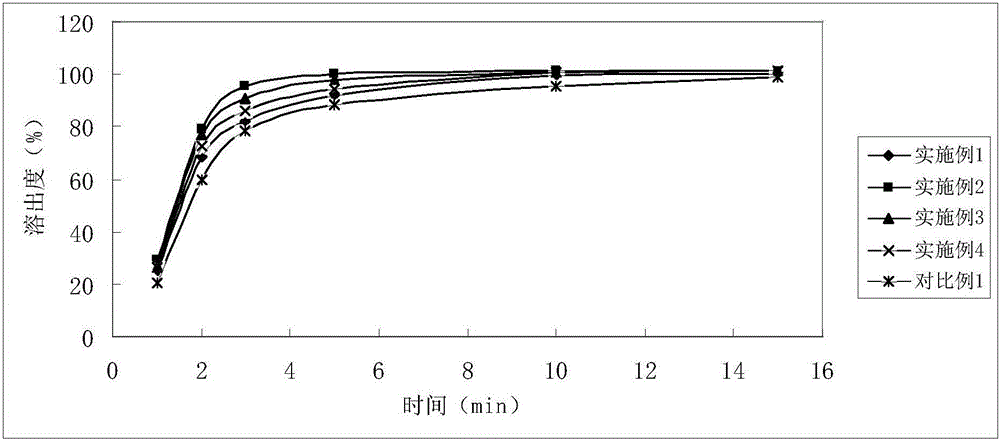
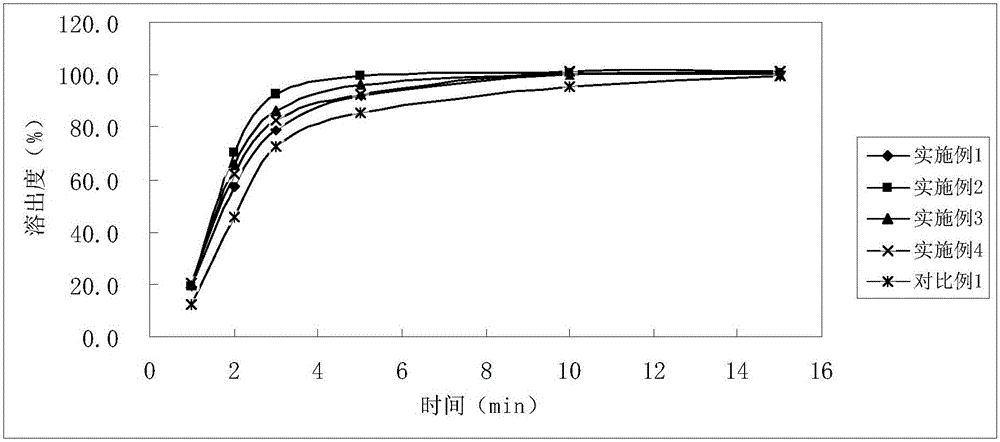
Soluble A-SH CoA-SH Q10 composition and its production
ActiveCN1981750AGood water solubilityDissolution rate is fastOrganic active ingredientsDigestive systemFreeze-dryingHepatitis
A water-soluble coenzyme Q10 composition for treating cardiovascular disease, hepatitis and hypoimmunocompetence is proportionally prepared from coenzyme Q10, gamma-cyclodextrin, alditol and disintegrant through high-speed stirring in water or ultrasonic oscillating, high-pressure homogenizing, freeze-drying, and granulating.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES +1
Water soluble coenzyme Q10 hydroxyl-beta-cyclodextrin inclusion compound and its preparation method
ActiveCN101053556AGood water solubilityDissolution rate is fastPowder deliveryOrganic active ingredientsDissolutionTherapeutic effect
The invention relates to a water-solubility coenzyme Q10 hydroxypropyl-beta-cyclodextrin clathrate and its producing method belonging to medicine and health product field, which has a good treatment effect to the cardiovascular diseases, hepatitis, weak immunity. It contains following material with a weight ratio of coenzyme Q10: hydroxypropyl-beta-cyclodextrin=1:0.5-100 and containing the producing steps of: blending in the water medium by high-speed scattering machine or shears, then in a high pressure homogeneous machine, finally spary drying or freeze-out to prepare the coenzyme Q10 clathrate. The clathrate can be produced to the oral liquid and injection type, which has a high solubility and dissolution, a small wettability, a good stability, and a high relative bioavailability.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES +1
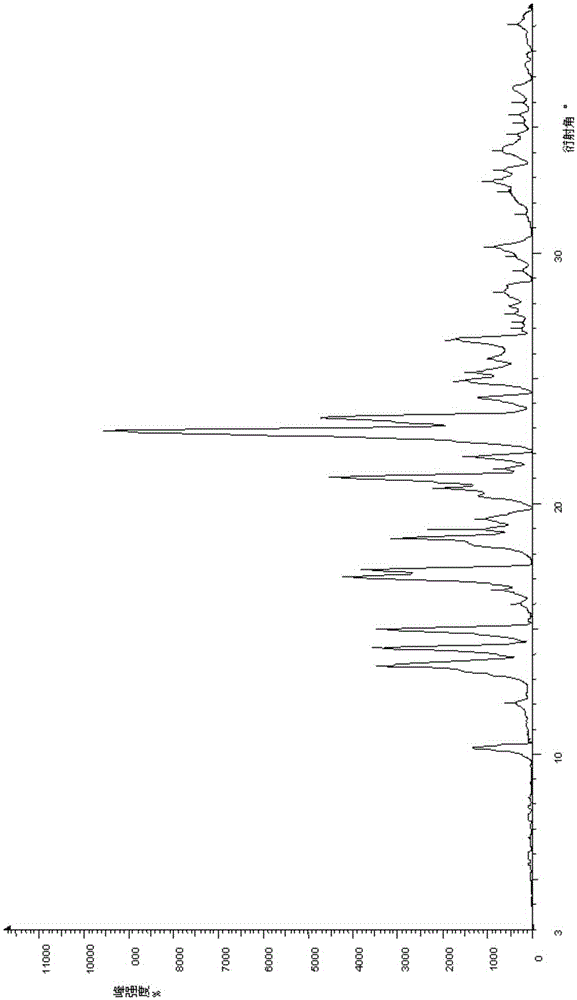
Crystal form, preparing method and application of Olaparib
InactiveCN105254572ADissolution rate is fastImprove efficacyOrganic active ingredientsOrganic chemistry methodsRadiationX-ray
The invention provides a crystal form, preparing method and application of Olaparib. According to the Olaparib with the new crystal form, CuKa radiation is adopted, powder X-ray diffraction represented by 2 theta angle has diffraction peaks at 22.9+ / -0.5, 23.4+ / -0.5, 21.0+ / -0.5, 17.1+ / -0.5, 17.3+ / -0.5, 14.2+ / -0.5, 15.0+ / -0.5, 13.5+ / -0.5, 18.6+ / -0.5, 20.6+ / -0.5, 10.2+ / -0.5, 20.3+ / -0.5, 21.9+ / -0.5, 25.8+ / -0.5 and 26.5+ / -0.5. The granularity D50 of the Olaparib with the crystal form is 2.45 microns which is quite small, so that dissolving-out speed is high when the Olaparib serves as a preparation, and drug efficacy of a product is improved. Furthermore, the preparing method is simple, and the performance of the obtained Olaparib with the crystal form is stable.
Owner:BEIJING COLLAB PHARMA
Tablets of mosapride citrate and preparation method thereof
ActiveCN101816639ADissolution rate is fastReduce solubilityOrganic active ingredientsDigestive systemDissolutionIn vivo
The invention belongs to the field of medicaments and in particular relates to tablets containing mosapride citrate and a preparation method thereof. Because the mosapride citrate is almost insoluble, defects of slow dissolution and low in-vitro and in-vivo bioavailability are present in the mosapride citrate tablets prepared by the common method. In order to improve the dissolution and bioavailability of the mosapride citrate, the invention provides dispersible tablets containing the mosapride citrate and a preparation method thereof. The prepared mosapride citrate dispersible tablets have high dissolution speed and low dissolution difference. The accumulated dissolution in five minutes is close to 100 percent, and the dispersible tablets are almost dissolved completely.
Owner:LUNAN PHARMA GROUP CORPORATION
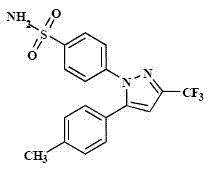
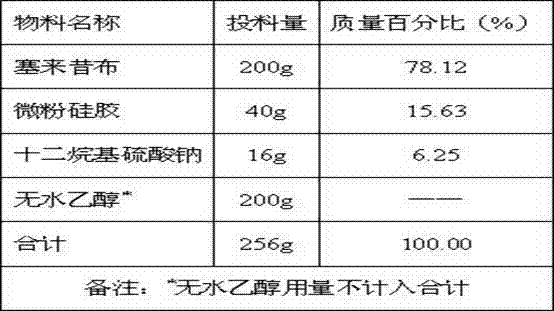
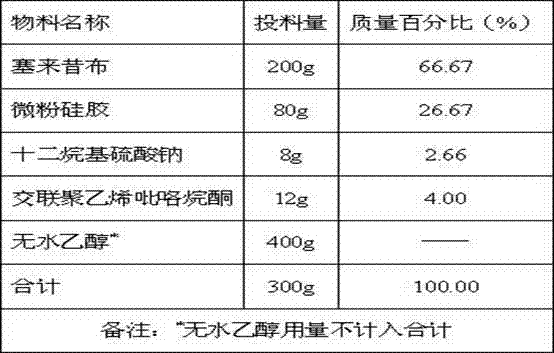
Celecoxib solid composition with high dissolution, preparation method and application
InactiveCN102764264AImprove solubilityDissolution rate is fastOrganic active ingredientsAntipyreticPharmaceutical medicineBioavailability
The invention provides a celecoxib solid composition with high dissolution, a preparation method and an application. The amorphous composition of celecoxib is obtained by dissolving the celecoxib into one or more pharmaceutically acceptable solvents and adsorbing and drying with pharmaceutically acceptable auxiliary materials. The composition can be further prepared into various solid preparation forms such as tablets, capsules and granules according to the actual requirement. Through the prepared celecoxib composition and preparations thereof, the dissolution of the celecoxib can be greatly improved, and the defects of low dissolution and low bioavailability of the celecoxib are overcome.
Owner:HANGZHOU HEZE PHARMA TECH
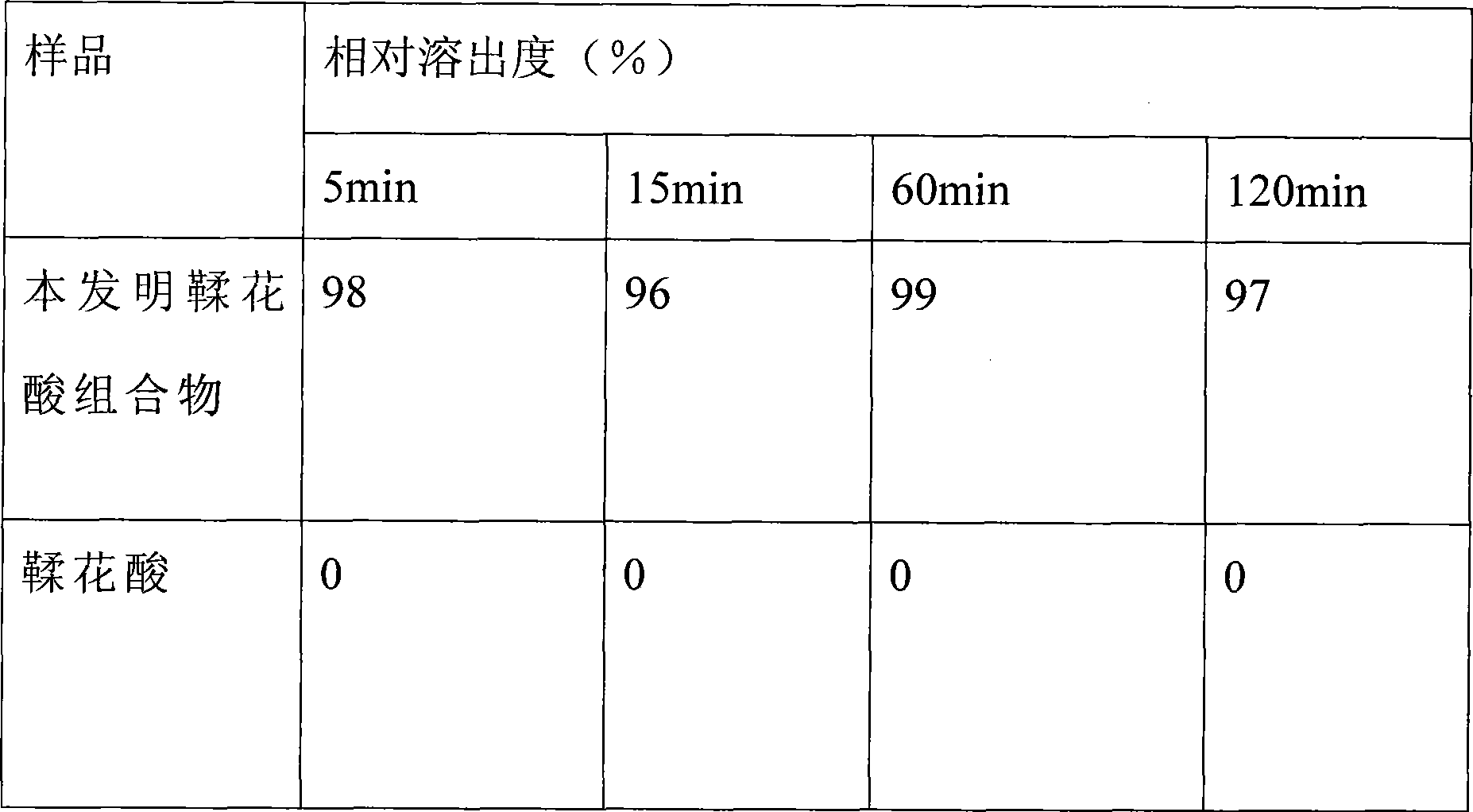
Ellagic acid supermolecule composition and preparation method
ActiveCN101375846AGood water solubilityDissolution rate is fastOrganic active ingredientsAntiviralsDrugChemistry
The invention relates to an ellagic acid supramolecular combination and a preparation method. The substance is an anti-oxidant and has the anti-cancer effect, and the ellagic acid supramolecular combination belongs to the technical field of drugs and health care products. The ellagic acid supramolecular combination is composed of raw materials according to the following weight ratio that ellagic acid: cyclodextrin: emulsifier is equal to 1: (1-50): (0-10). The ellagic acid, the cyclodextrin and the emulsifier are added into a colloid mill, and the ellagic acid supramolecular combination is prepared by grinding and drying. The combination has the advantages of higher solubility, high dissolution rate, good stability and higher relative bioavailability.
Owner:宿迁医美科技有限公司
Method for producing bamboo pomace
ActiveCN1974921AIncreased surface area per unit weightPenetrate fastWashing/displacing pulp-treating liquorsPretreatment with acid reacting compoundsPomaceViscose fiber
The present invention provides a preparation method of bamboo pulp, belonging to the field of chemical fiber technology. Said method includes the following steps: obliquely cutting bamboo strip material into the form of horseshoe, the chip cross-section spacing distance is 20-40 mm, then prehydrolyzing the prepared bamboo strip, adding alkali and cooking, removing sand, making one-stage or two-stage bleaching treatment, making the bleached pulp material undergo the processes of acid treatment, concentration and pulping so as to obtain the invented bamboo pulp. Said bamboo pulp can be used for producing viscose fibre.
Owner:吉林化纤股份有限公司
Water-soluble coenzyme Q10 combination and preparation method thereof
InactiveCN101940564AEasy to getImprove solubilityEther/acetal active ingredientsMacromolecular non-active ingredientsFructoseCarrageenan
The invention relates to a coenzyme Q10 combination and a preparation method thereof and provides a water-soluble coenzyme Q10 combination, which contains the following raw materials in parts by weight: 5-15 parts of coenzyme Q10, 10-120 parts of water-soluble carrier and 0.5-40 parts of emulsifier; the water-soluble carrier is one or more of maltodextrin, CMC, purity gum, soluble starch, lactose, fructose, sorbierite, mannite, Arabic gum, carrageenan, microcrystalline cellulose and sodium carboxymethyl starch; and the emulsifier is non-ionic surfactant. The invention also provides the preparation method of the water-soluble coenzyme Q10 combination. Fat-soluble coenzyme Q10, the water-soluble carrier and the amphoteric emulsifier are mixed and prepared into the water-soluble coenzyme Q10 combination, and the method synthesizes the advantages of an inclusion technique and a solid dispersion technique and solves the problems that the fat-soluble coenzyme Q10 is difficult to dissolve in water and has low bioavailability, and a number of experiments prove that the water-soluble coenzyme Q10 combination has good water solubility, fast dissolution rate, good stability and high content of effective substances.
Owner:杭州华东医药集团康润制药有限公司
Tofacitinib citrate tablets and preparation method thereof
InactiveCN105878202AGood content uniformityDissolution rate is fastOrganic active ingredientsAntipyreticFiller ExcipientLactose
The invention discloses Tofacitinib citrate tablets composed of Tofacitinib citrate and pharmaceutically acceptable auxiliary materials. The auxiliary materials comprise filler, disintegrating agent, flow aid and coating materials. The filler is microcrystalline cellulose lactose premix with the model of Celactose80, and the weight ratio of the Tofacitinib citrate to the microcrystalline cellulose lactose premix with the model of Celactose80 is 1:5-25. The invention further discloses a preparation method. The Tofacitinib citrate tablets has high content uniformity and have the advantages of being high in dissolving-out speed, stable in chemical property and the like.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
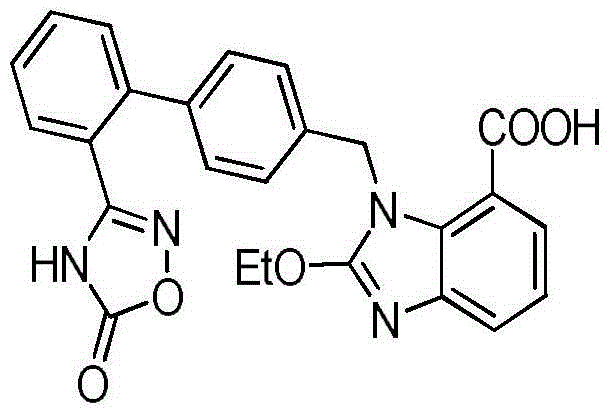
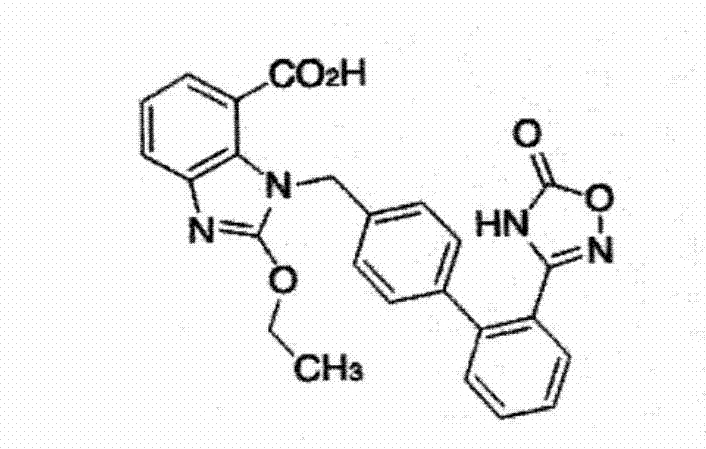
Azilsartan tablet
ActiveCN104523632ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryCelluloseDiethylene glycol monoethyl ether
The invention belongs to the technical field of medicines, and particularly relates to an azilsartan tablet. The azilsartan tablet contains azilsartan, hydroxy propyl cellulose and fumed silica, and is prepared by the following steps: dissolving the azilsartan and the hydroxy propyl cellulose in diethylene glycol monoethyl ether, adding the fumed silica to adsorb, uniformly mixing with pharmaceutically acceptable auxiliary materials and pressing by a direct tableting process. Compared with the prior art, the azilsartan tablet is high in drug dissolution speed and simple in process.
Owner:SHANDONG NEWTIME PHARMA
Acerola cherry preparation and preparation method thereof
The invention provides an acerola cherry preparation which is characterized by comprising 1-50 parts by mass of acerola cherry powder, 20-96 parts by mass of diluent, 2-15 parts by mass of synthetic vitamin C and 0.5-15 parts by mass of additives. The preparation method comprises the following steps of: pulping acerola cherry fruits after pretreatment, adding deastringent liquid into the obtained acerola cherry pulp to agitate, colloid-milling, homogenizing and filtering the mixture to obtain first filter liquid for later use, adding pectinase and cellulose into the obtained first filter residue for enzymolysis treatment, and colloid-milling, homogenizing and filtering the mixture once again to obtain second filter liquid, wherein the deastringent liquid is mixed water solution of citric acid and sodium chloride; and mixing the first filter liquid and the second filter liquid, homogenizing the mixture, carrying out vacuum concentration to obtain concentrated liquid, adding auxiliary materials comprising resistant starch and isomaltooligosaccharide into the concentrated liquid, homogenizing the mixture, and then carrying out vacuum drying to obtain the acerola cherry powder. The acerola cherry preparation provided by the invention has quick vitamin C dissolution speed and high bioavailability.
Owner:三沙南海美源岛生物科技有限公司
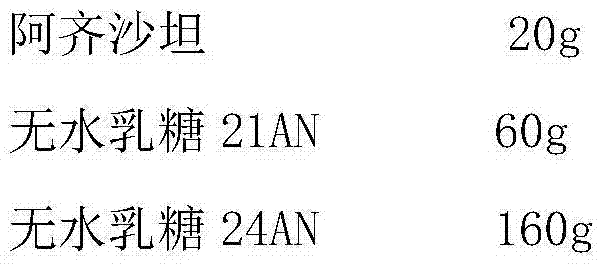
Azilsartan tablet and preparation method thereof
ActiveCN103933000ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryLubricantAcceleration Unit
The invention relates to an Azilsartan tablet and a preparation method thereof. The Azilsartan tablet contains micronized Azilsartan, lactose anhydrous 21AN, lactose anhydrous 24AN, a disintegrating agent, and a lubricant, and is prepared by pressing through adopting tabletting technology. Compared with the prior art, the Azilsartan tablet is fast in dissolving speed, simple in technology, and an acceleration test result shows that the prepared Azilsartan tablet has good stability.
Owner:SHANDONG NEWTIME PHARMA
Dabigatran-containing solid dispersion and preparation method as well as application thereof
InactiveCN104644543AImprove bioavailabilitySimple processOrganic active ingredientsPharmaceutical delivery mechanismPolymer scienceDabigatran ethyl ester
The invention belongs to the technical field of medicines and specifically discloses a dabigatran-containing solid dispersion and a preparation method as well as application thereof. The solid dispersion comprises a dabigatran-containing active substance and a carrier material, wherein the hydrophilic high-molecular polymer accounts for 20%-98% of mass of the solid dispersion and the carrier material is a hydrophilic high-molecular polymer. The preparation of the solid dispersion is low in cost and excellent in technical reproducibility.
Owner:QINGDAO HUANGHAI PHARM CO LTD
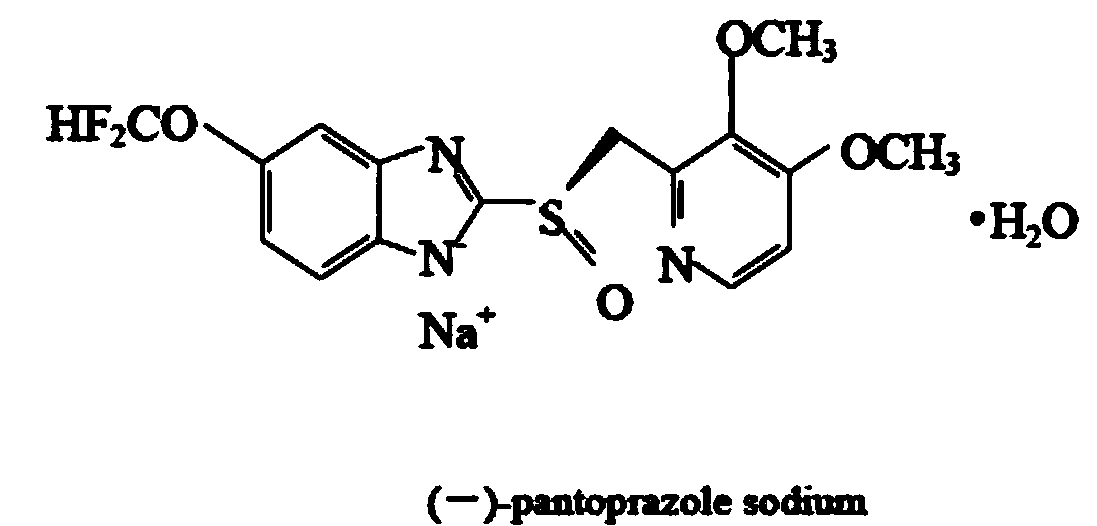
(-)-pantoprazole sodium enteric coated tablet and preparation method thereof
InactiveCN103989655ADissolution rate is fastGood effectOrganic active ingredientsDigestive systemEnteric coated tabletsBioavailability
A provided (-)-pantoprazole sodium enteric coated tablet consists of a tablet core containing (-)-pantoprazole sodium, a separation layer and an enteric layer, the increased weight of the separation layer accounts for 2-9%, and the increased weight of the enteric layer accounts for 7-15%. In dissolution tests using a system with pH of 5.5 or more, 85% or more of the main drug is dissolved in 30 min. The prepared (-)-pantoprazole sodium enteric coated tablet is an oral preparation with stable quality and high bioavailability.
Owner:广东永正药业有限公司
Solid dispersion as well as preparation method and application thereof
ActiveCN106420633AImprove solubilityImprove bioavailabilityPharmaceutical non-active ingredientsGranular deliveryAlpha-TocopherolPolyethylene glycol
The invention relates to solid dispersion as well as a preparation method and an application thereof. The solid dispersion is prepared from indissolvable drugs, a surfactant and a water-soluble polymer material with a spray drying method after mixing and heating dissolution, wherein the surfactant is selected from at least one of sodium dodecyl sulfate, poloxamer, tween, alpha-tocopherol, succinate, polyethylene glycol, sodium cholate and polyethylene glycol / vinyl caprolactam / vinyl acetate copolymer; the water-soluble polymer material is selected from at least one of povidone, copovidone, hydroxypropyl methylcellulose and polyethylene glycol. An organic solvent is not required when the solid dispersion is prepared with the spray drying method, and the problem of organic solvent residues is solved. By means of the solid dispersion, the dissolvability of the indissolvable drugs is increased, the dissolution speed and the dissolubility are remarkably increased, and the bioavailability of the indissolvable drugs is improved.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Apixaban tablet and preparation method thereof
ActiveCN104490841ADissolution rate is fastLow impurity contentOrganic active ingredientsPharmaceutical delivery mechanismCross-linkDissolution
The invention discloses an apixaban tablet and a preparation method thereof, and belongs to the technical field of medicines. The apixaban tablet consists of a tablet core and a coating, wherein the tablet core is composed of apixaban, a fiber-lactose compound, crosslinked carboxy methyl cellulose, lauryl sodium sulfate and magnesium stearate; various components in the tablet core are controlled within limited dosage ranges and a mutual synergistic effect is achieved, so that the dosage of the cross-linked sodium carboxymethyl cellulose as a disintegrating agent is reduced and the dissolution rate of the apixaban tablet is improved; therefore, the average dissolution rate within 10min is more than 90%, the dissolution rate is slightly affected by illumination, temperature and humidity, the performance is stable, and the apixaban tablet is low in contents of impurities. According to the preparation method of the apixaban tablet disclosed by the invention, the tablet core is prepared by a way of directly tabletting a powdery mixture, so that a granulation process is avoided, operation is simple and convenient, and the technological process is simple; and the apixaban, as a crude drug, is subjected to micronization treatment before the mixed powder is prepared, so that the dissolution rate of the apixaban tablet is accelerated.
Owner:HENAN RUNHONG PHARMA
Solid dispersion of protopanaxadiol and preparation method thereof
InactiveCN1879647AImprove solubilityDissolution rate is fastOrganic active ingredientsPowder deliverySolventChemistry
The invention provides a protopanaxadiol solid dispersion and its preparing method, wherein the weight ratio of the protopanaxadiol and carrier material is 1:1-50, and protopanaxadiol solid dispersion can be prepared through melting method, solvent method, solvent-melting method, grinding method, spray-drying method or freeze drying method. The preparation can be made into the dosage forms of capsules, tablets, suppositories and drop pills.
Owner:浙江金石亚药医药科技有限公司
Tablets of mosapride citrate and preparation method thereof
ActiveCN101816639BDissolution rate is fastReduce solubilityOrganic active ingredientsDigestive systemMosapride citrateMedicinal chemistry
The invention belongs to the field of medicaments and in particular relates to tablets containing mosapride citrate and a preparation method thereof. Because the mosapride citrate is almost insoluble, defects of slow dissolution and low in-vitro and in-vivo bioavailability are present in the mosapride citrate tablets prepared by the common method. In order to improve the dissolution and bioavailability of the mosapride citrate, the invention provides dispersible tablets containing the mosapride citrate and a preparation method thereof. The prepared mosapride citrate dispersible tablets have high dissolution speed and low dissolution difference. The accumulated dissolution in five minutes is close to 100 percent, and the dispersible tablets are almost dissolved completely.
Owner:LUNAN PHARMA GROUP CORPORATION
Albendazol dispersible tablets and preparation method thereof
InactiveCN102805736AImprove bioavailabilityGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsPatient complianceCurative effect
The invention relates to albendazol dispersible tablets and a preparation method thereof. The albendazol dispersible tablets comprise, by weight ratio, 200g of albendazol, 50-160g of disintegrant, 18-118g of diluent, 2-12g of surfactant, 6-16g of sweetener, 3-12g of glidant, and 1-4g of lubricant. Bioavailability of the albendazol dispersible tablets made from the proper accessories is improved evidently, and curative effect of the albendazol dispersible tablets is improved. The albendazol dispersible tablets are available to take in multiple ways, and different patients can take the albendazol dispersible tablets in different ways, so that patient compliance is improved. Practice shows that preparation technology of the albendazol dispersible tablets is high in production mechanization level, simple to operate, high in production efficiency and low in cost. The dispersible tablets produced by the preparation technology have the advantages of high dispersing uniformity, short disintegrating time, and fast dissolution, and the product quality completely meets the requirements.
Owner:宁夏启元国药有限公司
Rivaroxaban tablet
ActiveCN104666262ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryDiethylene glycol monoethyl etherMedicine
The invention belongs to the technical field of drugs and in particular relates to a rivaroxaban tablet. The rivaroxaban tablet contains rivaroxaban, hydroxypropyl cellulose and fumed silica and is prepared by dissolving rivaroxaban and hydroxypropyl cellulose in diethylene glycol monoethyl ether, adding fumed silica for adsorption, then mixing the materials with pharmaceutically acceptable auxiliary materials uniformly and pressing the mixture by adopting a direct tabletting process. Compared with the prior art, the rivaroxaban tablet is high in drug dissolution speed and simple in process and dispenses with addition of surfactants and micronization treatment.
Owner:SHANDONG NEWTIME PHARMA
Amorphous Venetoclax and pharmaceutic adjuvant solid dispersoid and preparation method thereof
InactiveCN107648185AGood dispersionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineX-ray
The invention relates to an amorphous Venetoclax and pharmaceutic adjuvant solid dispersoid and a preparation method thereof. The amorphous Venetoclax and pharmaceutic adjuvant solid dispersoid is prepared from Venetoclax and pharmaceutic adjuvants according to the weight ratio of 1:(0.1 to 100), wherein the Venetoclax is in an amorphous state; and in the X-ray powder diffraction spectrum of the solid dispersoid, no Venetoclax crystal feature peaks exist after the background peaks of the pharmaceutic adjuvants are eliminated. The Venetoclax and pharmaceutic adjuvant solid dispersoid provided by the invention has the advantages that the stability and the dispersibility are good; the dissolution rate of the Venetoclax is increased; the improvement of the bioavailability on the medicine preparation and the adsorption of the body on the medicine can be facilitated; and under the acceleration test conditions, high physical stability and chemical stability can be maintained. The preparationmethod of the amorphous Venetoclax and pharmaceutic adjuvant solid dispersoid has the advantages that the operation is simple; the cost is low; the reproducibility is high; the realization is easy; and the method is suitable for industrial production.
Owner:CHANGZHOU AINUOXINRUI PHARMA LTD
Beta-cyclodextrin / amoxicillin inclusion compound and its composition with clavulanic kalium and preparation thereof
InactiveCN1698604AImprove drug stabilityPromote dissolutionAntibacterial agentsHeterocyclic compound active ingredientsCellulosePolyethylene glycol
The invention provides a beta-cyclodextrin / amoxicillin inclusion compound medicinal composition which comprises amoxicillin and beta cyclodextrin by the weight ratio of 1:2.5-1:5. In a preferred embodiment, the composition also comprises clavulanate potassium, wherein the mass ratio of amoxicillin and clavulanate potassium is 14:1-2:1, the supplementary materials are selected from crystalline cellulose, maize starch, citric acid, talcum powder, sodium carboxymethylstarch, stearic acid, calcium stearate, magnesium stearate, amylopectin, lactose, mannitol, crosslinked povidone, talcum powder, polyethylene glycol 4000, and low substituted methylcellulose propylene glycol ether. The invention also discloses the preparing process.
Owner:NANJING J ONE MEDICAL TECH DEV
Rosuvastatin calcium tablet and preparation method thereof
ActiveCN104473899ADissolution rate is fastHigh dissolution rateOrganic active ingredientsMetabolism disorderDissolutionLactose
The invention discloses a rosuvastatin calcium tablet and a preparation method thereof. The rosuvastatin calcium tablet comprises components in parts by weight as follows: 5.0-10.5 parts of rosuvastatin calcium, 20-22 parts of microcrystalline cellulose, 73.5-78.8 parts of lactose, 40 parts of calcium carbonate, 4.5 parts of crosslinked polyvinylpyrrolidone and 1.5 parts of magnesium stearate. The rosuvastatin calcium tablet comprises the rosuvastatin calcium, the microcrystalline cellulose, the lactose, the calcium carbonate, the crosslinked polyvinylpyrrolidone and the magnesium stearate, dosages of all the components are strictly controlled, main medicines and auxiliary materials complement one another to realize a synergistic effect, and the prepared tablet is high in dissolution speed, high in dissolution rate and high in bioavailability; raw materials are easy to obtain, the cost is low, the product equality is controllable, the quality stability and the safety are high, and the rosuvastatin calcium tablet is suitable for popularization and application.
Owner:HENAN RUNHONG PHARMA
Bromine-containing disinfectant
InactiveCN101611717AHas a solubilizing effectImprove bioavailabilityBiocideDisinfectantsDisinfectantDissolution
The invention provides a bromine-containing disinfectant, which is prepared from the following components in percentage by weight: 20 to 60 percent of bromine-containing compounds, 30 to 50 percent of compound cosolvents, 0 to 25 percent of anhydrous sodium carbonate, and 0 to 25 percent of dimethylhydantoin. The bromine-containing disinfectant improves the bioavailability of bromide, wherein the bromine-containing compound has the advantages of high dissolution velocity and strong effects of disinfection and sterilization. The bromine-containing disinfectant is used to prepare thimerosal at a concentration of 250 to 400 ppm to wash and disinfect beer production equipment and pipelines of a beer brewery, can completely kill various bacteria and meets the CRB microbiological standards.
Owner:李新旺 +1
Nimodipine capsule containing semi-solid combination and preparation
InactiveCN101254180AHigh dissolution rateDissolution rate is fastOrganic active ingredientsNervous disorderHard CapsuleSemi solid
The invention discloses a nimodipine capsule with high dissolution and semi-solid composition as content and a preparation method thereof, and belongs to the pharmaceutical preparation field. The method comprises the following steps of mixing micronized nimodipine and a hydrophilic carrier, preparing into solid dispersion by melting extrusion method, cooling, pulverizing, mixing with semi-solid matrix, surfactant, melting point regulator under heating, and filling the content into hard capsule with a capsule filling machine. The invention combines the melting extrusion technique and semi-solid filling hard capsule technique, and after the prepared novel nimodipine capsule is administered into human body, the nimodipine quickly diffuses in molecular form and saturates metabolic enzymes, so as to improve bioavailability. The inventive nimodipine capsule solves the low dissolution and low utilization ratio problems of prior nimodipine preparation; and has the advantages of high dissolution, good stability, simple preparation method, and applicability to industrial production.
Owner:武汉星福海药业有限公司
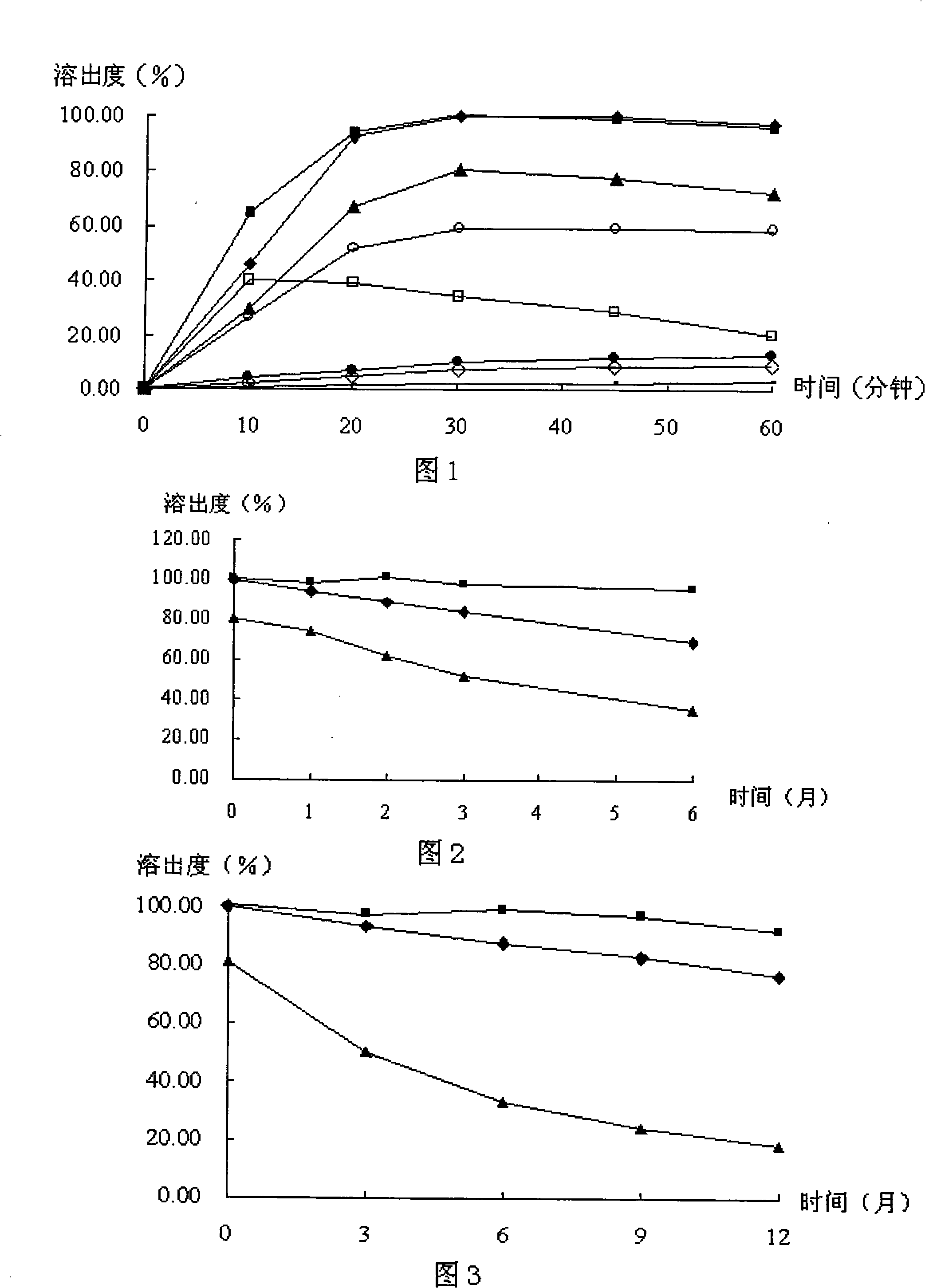
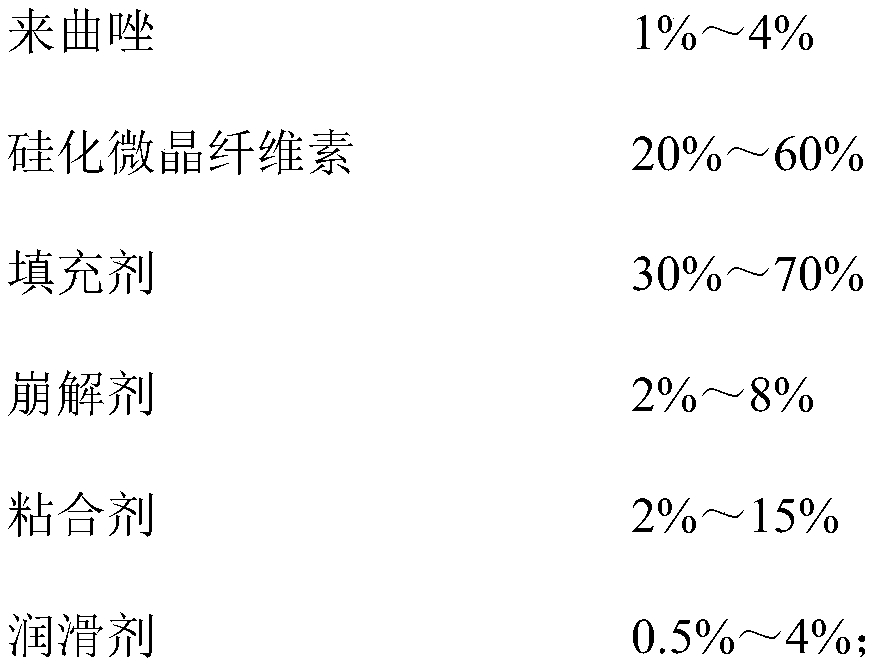
Lelrozol tablet and preparation method thereof
ActiveCN107737112AEvenly dispersedImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineSmallerThan
The invention belongs to the technical field of medicine and particularly relates to a lelrozol tablet and a preparation method thereof. The lelrozol tablet is prepared from lelrozol and silicified microcrystalline cellulose, wherein the content of the silicified microcrystalline cellulose accounts for 20 to 60 percent of total weight of the tablets. The particle size D(v, 0.9) of the lelrozol issmaller than or equal to 60mu m, preferably the particle size D(v, 0.9) of the lelrozol is smaller than or equal to 40mu m and more preferably, the particle size D (v, 0.9) of the lelrozol is smallerthan or equal to 8mu m. According to the preparation method of the lelrozol tablet, disclosed by the invention, the silica microcrystalline cellulose is used, so that the trazodone is uniformly dispersed in the mixed powder; in addition, the lelrozol tablet has good fluidity, and the powder can be directly pressured into tablets, so that the technique is simplified, the time is saved and the laborintensity is low; besides, after a crude drug is crushed, the particle size is obviously reduced; by controlling the content of a disintegrating agent, the dissolution rate of the prepared lelrozol tablet is significantly increased, 85 percent or above can be reached in 15 minutes and thereby the lelrozol tablet is rapidly dissolved out.
Owner:HAINAN JINRUI PHARMA CO LTD
Stable taste-masking levocetirizine medicine composition and preparation method thereof
InactiveCN102860986APleasant tasteGreat tasteOrganic active ingredientsPill deliveryDrugMasking agent
The invention provides a stable taste-masking levocetirizine medicine composition and a preparation method thereof. The medicine composition comprises levocetirizine, a taste masking agent and a stabilizer, the levocetirizine is levocetirizine hydrochloride, the taste masking agent is beta-cyclodextrin or a derivative thereof, and the stabilizer is sodium citrate. Other pharmaceutically acceptable auxiliary materials are selectively added in the medicine composition, the medicine composition can be further prepared into granules, orally disintegrating tablets and chewable tablets, tastes good, is quick in absorption, stable in quality, simple and feasible in preparation process, and can be produced in batches by using the conventional pharmaceutical equipment, and the related medicinal auxiliary materials are easy to purchase and low in cost.
Owner:天津市聚星康华医药科技有限公司
Process for producing high-viscosity cowhide alkaline gelatin by batch and alkaline (BATALK) method
InactiveCN102181234ADissolution rate is fastPromote dissolutionGlue/gelatin preparationIon exchangeChemistry
The invention relates to a process for preparing gelatin, in particular to a process for producing high-viscosity cowhide alkaline gelatin by a batch and alkaline (BATALK) method. The process comprises pretreatment, rubber extraction, filtration, ion exchange, concentration, high temperature sterilization, drying, crushing, mixing, metal detection and formation of a finished product. The process is characterized in that: the pretreatment process is the BATALK method. By the process, the consumption of water and the consumption of sulfur acid can be reduced; and the gelatin obtained by the process has high viscosity.
Owner:罗赛洛(温州)明胶有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com